Note: Descriptions are shown in the official language in which they were submitted.
CA 02862349 2014-07-23
WO 2013/113777 1
PCT/EP2013/051837
Device for the cathodic protection of a metal wall
against corrosion in a saline environment
The invention relates to the cathodic protection
of installations submerged in a briny or saline
environment.
Protection of the metal hulls of boats or of metal
parts against corrosion by seawater is often provided,
conventionally, by a sacrificial anode, consisting of a
metal that is more electronegative than that to be
protected. When the boat and its sacrificial anode are
immersed in seawater, polarization occurs, the hull
becoming the cathode of an electrochemical cell and the
sacrificial anode becoming the anode of this same cell.
As a result, it is the metallic constituents of the
sacrificial anode that are subjected to the effects of
corrosion by the seawater and not those of the hull.
These constituents M are released in the seawater in
the form of cations according to the reaction M Mn+ +
n e-.
The sacrificial anodes protecting the submerged
steel parts of a boat or of any other seagoing vessel
or fixed installation are generally of aluminum-zinc
(2-6%)-indium (0.01-0.05%), or of aluminum-gallium
(0.01%) or of zinc-Al alloy (0.1-0.5%). The drawback of
this protection is that it releases the ions of the
metals constituting the sacrificial anodes into the
marine environment. Although this drawback is
relatively minimal in the case of boats at sea, it has
to be taken much more seriously when boats are berthed
in a port, as the metals of the anode will accumulate
in the water and in the sea bed of the berthing zones,
where they will be absorbed by the creatures living
there. The problem also arises for fixed installations
such as oil rigs and off-shore wind turbines. It is
therefore imperative to find effective and economical
CA 02862349 2014-07-23
WO 2013/113777 2
PCT/EP2013/051837
solutions for avoiding this release of harmful metals
into the environment as far as possible, especially as
developments in environmental protection legislation
might make the use of solutions for controlling wastes
from sacrificial anodes obligatory in certain
circumstances.
An alternative solution to the use of a
sacrificial anode as has just been described is to make
this anode from a material that is not necessarily more
electropositive than the material to be protected
(which may be steel, cast iron, graphite, metal oxides,
etc.), but apply an electric potential to it,
constantly or cyclically, by means of a generator of
direct or rectified current. This potential makes the
anode more corrodible than the wall to be protected.
This technique is onerous to apply, especially in zones
of the installation with difficult access, but it is
effective mainly for large installations. This
technique is known as "impressed current cathodic
protection" (abbreviated to TCCP).
The aim of the invention is to propose a solution
for avoiding release of the cations resulting from the
dissolution of an anode of a device for cathodic
protection into the environment.
For this purpose, the invention relates to a
device for the cathodic protection of a metal wall
against corrosion in a saline environment, comprising
an anode and means for connecting said anode to said
wall, said anode being at a higher electrochemical
potential than said wall, characterized in that said
anode is placed in a compartment delimited by a wall
that is permeable to electrons and, optionally, to
water, comprising:
- a porous outer layer of a material selected
from: polymer materials, ceramic materials or hydrated
inorganic materials;
CA 02862349 2014-07-23
WO 2013/113777 3
PCT/EP2013/051837
- and at least one porous layer able to capture
the cations emitted by the anode during dissolution
thereof, the material constituting said at least one
layer being selected from osmotic membranes, activated
charcoal, a cation exchange resin such as a zeolite, a
cation capture polymer with nanofillers, cation capture
mineral compounds such as phyllosilicates and
inosilicates, semipermeable organic microporous
nanofiltration membranes of a type that retains
cations.
Said wall may also comprise a membrane that traps
negatively charged pollutants.
The anode may be a sacrificial anode whose
electrochemical potential is naturally higher than that
of the metal wall.
Otherwise, the device may comprise means by which
an electrochemical potential higher than that of the
metal wall can be applied to the anode.
The means for connecting the anode to the metal
wall may be in contact with the wall outside the
compartment and may pass through the porous wall of the
compartment hermetically.
The means for connecting the anode to the metal
wall may be in contact with the metal wall inside the
compartment, and the porous wall of the compartment is
connected hermetically to the metal wall.
The device may comprise means for keeping the
anode at a distance from the metal wall.
It may comprise a plurality of layers able to
capture the cations emitted by the anode during
dissolution of the latter, each layer preferentially
capturing cations different from those captured
preferentially by the other layers.
As will have been understood, the invention
consists of placing the anode in a compartment
delimited by a series of porous membranes permeable at
least to electrons, or even also to water, arranged in
CA 02862349 2014-07-23
WO 2013/113777 4
PCT/EP2013/051837
layers. The outer layer consists of a nonmetallic
porous membrane, intended to reduce the hydraulic flow
in the vicinity of the anode. The other porous layer or
layers play(s) the role of cation barrier or trap,
which prevents the cations resulting from dissolution
of the anode to escape from the compartment into the
environment.
If the membranes are all permeable to water, the
space separating the anode from the wall of the
compartment becomes filled with water naturally. If at
least one of the membranes is not permeable to water
but only to electrons, it is necessary, during
installation of the device, to fill the compartment
with water, preferably seawater to obtain good
electrical conductivity, in order to bathe the anode in
an electron-conducting medium and to endow the
compartment with its operational form, with internal
and external pressures that are balanced.
The invention will be better understood on reading
the description given below, referring to the following
appended figures:
Fig. 1, which shows schematically, in
longitudinal section, a first example of carrying out
the invention;
Fig. 2, which shows schematically, in
longitudinal section, a second example of carrying out
the invention.
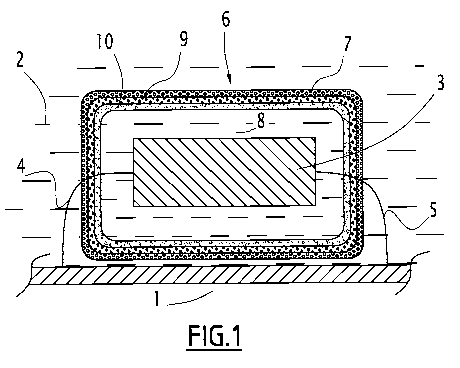
Fig. 1 shows a metal wall 1 belonging to equipment
installed in a marine environment 2, such as an oil rig
or a wind turbine. However, this wall 1 could also be
the hull of a boat, or of some other seagoing vessel.
In a known manner, a sacrificial anode 3 is arranged in
the vicinity of the wall 1, to which it is connected by
electrical connectors 4, 5. In general, as in the prior
art, the sacrificial anode 3 is constructed around a
single steel connector, and in this case the connectors
4, 5 shown are in fact the two ends of this single
CA 02862349 2014-07-23
A
WO 2013/113777 5
PCT/EP2013/051837
connector. The material constituting the sacrificial
anode 3 is conventional for this purpose (Al-In, Al-Ga
or Zn alloy for example), and selection thereof is not
a characteristic feature of the invention.
According to the invention, the sacrificial anode
3 is enclosed in a compartment 6 that is delimited by a
set of membranes forming layers, and surrounds the
anode 3 at a distance, for example of the order of
1 cm.
The outermost layer 7 is a porous layer permeable
to electrons, and preferably also to water, intended to
reduce the hydraulic flow between the external
environment and the internal space 8 of the
compartment. The material of which it is constituted is
selected from polymer materials, ceramic materials or
hydrated inorganic materials.
As examples of such materials, we may mention,
nonexhaustively, thermoplastic polymers of the
polyethylene or high-density polyethylene type, or
porcelains of the industrial mullite or alumina type.
If this material is electrically insulating, it
must be porous. In fact, polarization of the anode
corresponds to establishment of a small electrochemical
circuit, which can only function if electrons are
circulating. The open porosity allows the electrons to
pass into the liquid even if layer 7 is insulating.
This outermost layer 7, whose thickness is
generally of the order of a millimeter, has the
function of protecting the anode and the other
membranes of compartment 6 against hydraulic abrasion.
It must have suitable properties of wear resistance and
impact strength, and resistance to deformation in the
presence of a moving fluid.
The permeability of layer 7 is for example of the
order of 10 ml/min per 1 cm2 of anode surface.
The other layer or layers of the wall of
compartment 6 (there are two of them, 9, 10, in the
CA 02862349 2014-07-23
WO 2013/113777 6
PCT/EP2013/051837
example shown) consist(s) of one or more materials
serving as cation trap, which trap the cations emitted
by the sacrificial anode 3 to prevent them entering the
marine environment 2. Various types of materials may be
suitable for this purpose: osmotic membranes, activated
carbon in the form of powder or granules, a cation
exchange resin such as a zeolite, a cation capture
polymer with negative nanofillers attracting the
cations, cation capture mineral compounds such as
phyllosilicates and inosilicates. Such materials are
included among those commonly used in water treatment
and softening for cation capture or exchange. They may
be supplemented with a membrane of activated alumina or
of a functionally equivalent compound, which for its
part traps the negatively charged pollutants, such as
As and fluorides, which could reduce the efficacy of
the membranes trapping the cations.
Semipermeable membranes employed in electrolytic
processes of ion exchange may also be used.
Semipermeable organic microporous nanofiltration
membranes of a type that retains cations may also be
suitable.
The number of layers of cation capture materials
is arbitrary, to be chosen by the user. These layers
may advantageously be of multiple kinds, and each
species of layer may, for example, preferentially
absorb one or more of the chemical species that the
sacrificial anode 3 is likely to release.
For example, we may envisage:
- an outer layer 9 permeable to the chemical
elements with radius below 1.5 A and impermeable to the
chemical elements with radius above 1.5 A such as Ca,
K, Mg, Na;
- and an inner layer 10 permeable to the chemical
elements with radius below 1.1 A such as 0, Cl, N
(which will therefore be able to penetrate into the
internal space 8 of compartment 6 or leave it, which
CA 02862349 2014-07-23
WO 2013/113777 7
PCT/EP2013/051837
does not have drawbacks), and impermeable to the
chemical elements with radius above 1.1 A such as Al,
Zn, Ga, In, thus the principal elements that the anode
3 may emit in the form of cations, and whose release
into the environment is undesirable; the thickness of
the layers, and notably of layer 10, may vary from
about 1 mm to some cm, as a function of the size of the
anode 3 and therefore of the quantity of cations to be
trapped.
In the case when the whole of the wall delimiting
compartment 6 is permeable to water, water penetrates
into compartment 6 and a balance of pressures is
attained between the interior and the exterior of
compartment 6. Compartment 6 therefore assumes its
nominal shape permanently and its wall is not subjected
to crushing, which could lead to rupture thereof.
As has been mentioned, it is not obligatory for
the whole of the wall defining compartment 6 to be
permeable to water. It may only be permeable to
electrons, but then prefilling of compartment 6 with
water, preferably seawater, is necessary when
installing the device according to the invention.
Owing to the invention, gradual dissolution of the
sacrificial anode 3 takes place without pollution of
the environment by the cations resulting from said
dissolution, as they are captured by the layer or
layers 9, 10. The latter must advantageously have a
total capacity for absorption of the various cations
and an absorption volume that are sufficient so that
saturation does not occur before the end of the life of
the sacrificial anode 3.
In the example shown in Fig. 1, the electrical
conductors 4, 5 are in contact with the metal wall 1 in
zones located outside of compartment 6. It is therefore
necessary to ensure hermeticity of the wall of
compartment 6 in the zones where the conductors 4, 5
pass through. However, as a variant, as shown in Fig.
CA 02862349 2014-07-23
J64
WO 2013/113777 8
PCT/EP2013/051837
2, the contacts between conductors 5, 6 and the metal
wall 1 may be located within compartment 6. The wall of
compartment 6 is then in hermetic contact with the
metal wall 1 to be protected.
As shown in the figures, it is preferable that the
sacrificial anode 3 is not in direct contact with the
wall 1 to be protected. This avoids the creation of
short-circuits between anode 3 and at least the zone of
wall 1 that is opposite it. In this way, a larger part
of the surface of wall 1 can be protected in the best
conditions by one and the same anode 3. Means for
keeping the anode 3 at a distance from wall 1 are
therefore preferably provided (not shown in the
figures). In practice, however, they can often consist
of the connectors 4, 5, which are generally made of
steel and have, owing to their material and their
dimensions, sufficient rigidity to keep anode 3 at a
distance from wall 1.
As a variant, the invention is also applicable to
the case when the anode is not a sacrificial anode in
the sense that it naturally has an electrochemical
potential higher than that of the wall 1 to be
protected, but is placed at this potential by a
generator of direct or rectified current to which it is
connected by conductors that pass through the wall of
compartment 6 hermetically.