Note: Descriptions are shown in the official language in which they were submitted.
CHEMICAL ADDITIVES TO INHIBIT THE .AER OXIDATION AND SPONTANEOUS
COMBUSTION OF COAL
Statement Regarding Federally Sponsored Research or Development
Not Applicable.
Background of the invention
to This invention relates to methods and compositions for inhibiting
the air oxidation
and spontaneous combustion of coal, low-ranked coal, and in particular sub-
bituminous coal.
Sub-bituminous coal forms within fresh water peat bogs that do not get washed
out into the sea
and therefore have unique chemical properties. Due to the differences in its
formation, sub-
bituminous coal has a loose pore structure and retains high levels of water.
As a result it is a less
.. efficient fuel than other coals such as anthracite or bituminous coal, and
can require as much as
double the amount of coal mass to produce the same amount of energy. Sub-
bituminous coal also
contains large pyrite particles which tend 10 foul and slag furnace walls
before they completely
combust. It is not surprising then that historically, sub-bituminous coal has
been recognized to he
a lower value, less desired feedstock fuel for power generation,
Although all coal dust poses a risk of an airborne explosion, low-ranked and
especially sub-bituminous coal poses additional fire risks even when not-in
the form of airborne
dust. Low-ranked coals are potentially more prone to particle degradation,
thereby increasing the
open surface area. This facilitates oxidation which can lead to spontaneous
combustion. It is
believed that, the autogenous grinding tendencies of shipping, handling and
transfer of low
-
ranked coal creates increasingly small particles, increased surface area, and
a rich environment
CA 2865453 2019-05-27
CA 02865453 2014-08-22
WO 2013/152148
PCT/US2013/035200
for air oxidation to occur resulting in the potential for spontaneous
combustion of that coal.
As a result, as mentioned for example in the article Fire-protection
guidelines for
handling and storing PRB coal, by Edward B. Douberly, Power Magazine (October
2003),
special handling procedures must typically be employed by those low-ranked
coal users to
decrease the risk of unwanted fires but these procedures make it difficult to
simply replace other
higher rank coals with low-ranked coal as a substitute fuel in coal based
power generation. Low-
rank coal users strive to minimize the time allowed to elapse between the
mining and ultimate
burning of sub-bituminous coal, Furthermore when received at its destination,
the coal inventory
must be tightly managed, piles groomed to minimize surface area and organized
in a FIFO
to manner such that the age-order of coal inventory forces utilization of
the "oldest inventory" first.
Also, feeding and handling machinery must be specially designed to minimize
attrition and
aggressive handling lest coal particle size continue to degrade, allowing
finer and finer fragments
to turn to dust, accumulate and spontaneously combust. Finally, thermal and
atmospheric
detectors are employed to constantly analyze piles for the telltale signs of
the early stages of
spontaneous combustion which must then be rapidly treated or consumed for
power production to
prevent further degradation.
These degradation processes present an even greater risk in overseas transport
of
low-rank coal. Most US harbor fitcilities, let alone foreign ones, lack the
special handling
equipment needed to safely handle low-rank coal. In addition the coal placed
in a ship's hold for
extended periods of time creates a situation in which the risk of combustion
is intolerably high.
The relatively lower Btu value as well as the increased risk of fire or have,
for
much of the history of the coal fired power industry, caused sub-bituminous
coal to be removed
from contention as a useful alternative to higher rank coals.
Recently, however, increasing anthracite and bituminous coal mining costs and
2
CA 02865453 2014-08-22
WO 2013/152148
PCT/US2013/035200
rising environmental standards have changed the relative value of sub-
bituminous and low-
ranked coal. lielping to offset the higher moisture content are sub-bituminous
coals' far lower
levels of undesirable constituents such as sulfur, mercury or arsenic. Also,
subbiturninous coal's
high Calcium Oxide and Magnesium Oxide levels cause sub-bituminous coal
combustion to
produce far less boiler slag. As a result, despite its lower inherent energy
and handling
difficulties, in many jurisdictions strict environmental laws have made it
more suitable to use
sub-bituminous coal than other sources of coal, even though they might be of
higher fuel content.
As a result, sub-bituminous coal tonnage has greatly increased over the past
decade and
subbituminous coal producers are actively seeking methods for safe shipment in
massive ocean-
in going vessels and acknowledge the urgent need for technology to address
the dangers in
spontaneous combustion of this coal.
Thus there is a clear utility in novel methods and compositions for inhibiting
the
spontaneous combustion of coal piles. The art described in this section is not
intended to
constitute an admission that any patent, publication or other information
referred to herein is
"Prior Art" with respect to this invention, unless specifically designated as
such. In addition, this
section should not be construed to mean that a search has been made or that no
other pertinent
information as defined in 37 CFR 1.56(a) exists.
Brief Summary of the Invention
At least one embodiment of the invention is directed towards a method of
inhibiting the spontaneous combustion of a pile of low-ranked coal. The method
comprises the
step of applying to the coal an inhibitor composition. The composition
comprises crude glycerin
and a VAE copolymer or a PVA copolymer in a ratio of between 90:10 and 10:90.
The low-ranked coal, may be lefi undisturbed for a period of time in which it
is
3
CA 02865453 2014-08-22
WO 2013/152148
PCT/US2013/035200
probable that but for the presence of the composition, the pile would have
spontaneously
combusted. The low-ranked coal may be exposed to air or an oxidizing
atmosphere for a period
of at least 5 days prior to the coal being positioned into a pile. The coal
may he handled by an
apparatus comprising at least pinch point through which the coal will pass and
wherein portions
$ of the coal will accumulate and persist. The coal may be loaded into a
ship's hold and remain
undisturbed in a pile of at least 30,000 tons for at least 10 days. The coal
may be sub- bituminous
coal. The composition may inhibit the spontaneous combustion at a rate greater
than that of a
mixture of VAE mixed with pure glycerin. The pile may be within a ship's hold.
The method
may exclude the use of a FIFO method in handling the pile. l'he composition
may prevent the
oxidation of carbonyl groups within the coal for at least 60 days and but for
the composition the
carbonyl groups would have undergone at least a 50% increase in the oxidation
of the coal's
carbonyl groups.
Brief Description of the Drawings
A detailed description of the invention is hereafter described with specific
reference being made to the drawings in which:
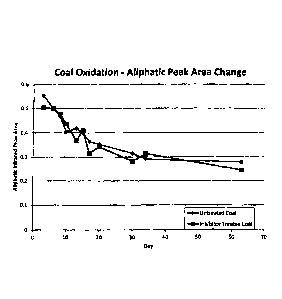
FIG. I is a graph illustrating how the invention affects the oxidation of
aliphatic
portions of coal.
FIG. 2 is a graph illustrating how the invention affects the formation and
oxidation
zo of carbonyl containing portions of coal.
FIG. 3 is a graph illustrating how the invention affects the adiabatic
temperature of
coal.
4
CA 02865453 2014-08-22
WO 2013/152148
PCT/US2013/035200
Detailed Description of the invention
The following definitions are provided to determine how terms used in this
application, and in particular how the claims, are to be construed. The
organization of the
definitions is for convenience only and is not intended to limit any of the
definitions to any
.. particular category.
"Arching" means an obstruction in the flow of coal material through a portion
of
a coal handling process formed out of coal material which has agglomerated
into the fimnn of an
arch, the arching can be cohesive (formed by particle to particle bonds),
interlocking (formed by
particles which are large relative to the size of an outlet it passes through
and are compacted
to together by mechanical force suach as a collapsing rathole), or both,
"As-skipped" means a rocky composition of matter which has been removed from
the ground, substantially all of which has been ground into particles having a
volume of no more
than 3 inches3, and has not had removed from it the naturally occurring
moisture present when
the composition was in the ground.
"Crude glycerin" means a by-product derivative from a transesterification
reaction
involving triglycerides including transesterification reactions involving
biodiesel manufacturing
processes, in which the by-product comprises glycerin and at least one
component selected from
the list consisting of: fatty acids, esters, salt, methanol, tocopherol,
sterol, mono-glycerides, di-
glycerides, and tri-glycerides.
"Low Ranked Coal" means coal which has a gross calorific value limit of no
greater than 9,500 BTU/lb on a moist mineral-included as-shipped basis. Low
ranked coal
includes sub-bituminous coals, lignite coals, and high volume or highly
oxidized bituminous
coals.
5
CA 02865453 2014-08-22
WO 2013/152148
PCT/US2013/035200
"Along" means non glycerol organic material and typically consists of soaps,
free
fatty acids, and other impurities.
"Particulate material" means a material that has a tendency to form dust
particles
when handled, processed, or contacted, which includes but is not limited to
coal, dirt, wood
chips, agricultural products, fruits, fertilizers, ores, mineral ores, fine
materials, sand, gravel, soil,
fertilizers, or other dust generating material, and any combination thereof.
"Pinch .Point" means a piece of equipment or portion thereof present in an
industrial process through which there is a general flow of coal material but
due to the shape of
the piece of equipment or portion thereof, the flow of a portion of the
material becomes impinged
lo and that portion remains stationary for a period of time, exemplary
industrial processes include
but are not limited to coal processing, coal refining, coal handling, coal
grinding, coal
transporting, coal loading, coal storing, and coal unloading, exemplary types
of equipment
include but arc not limited to chutes, bent or curved pipes, channels, or
ducts (elbows), or spaces
small enough such that bridges of agglomerated materials collect. Pinch points
can cause arching
and ratholing of the flowing coal material.
"Powder River Basin" means the geological region (approximately 190 km east-
west by 320 km north-south) which is a rich source of naturally occurring Sub-
Bituminous Coal
located in in southeastern Montana and north eastern Wyoming in the vicinity
of the cities of
Gillette, Wyoming, Sheridan Wyoming, and Miles City Montana.
"PTA" means polyvinyl acetate polymer.
"Ratholing" means the obstructing of the flow of coal material through a
portion
of a coal handling process formed out of coal material which has cohesive
strength (formed by
particle to particle bonds) such that while some of the material flows along a
channel within the
mass of material, material which is outside of the channel becomes stagnant
and does not flow.
6
CA 02865453 2014-08-22
WO 2013/152148 PCT/US2013/035200
Rath les may collapse in the presence of external force such as vibration and
when they collapse
they may reform into arches.
"Sub-Bituminous Coal" means the compositions of matter bearing this name as
defined in ASTM 1)388-05, it includes naturally occurring coal compositions
which have a gross
calorific value limit of between :11,500 BTU/lb and 8,300 BTU/lb on a moist,
mineral-matter-free
basis, it typically has an. as-shipped gross calorific value limit of 84004800
BRE/lb, it includes
but is not limited to Powder River Basin Coal.
"ME' means vinyl acetate ethylene co-polymer. En at least one embodiment the
repeating units of VAE are selected from one of formula 1, II, 111, IV, and
any combination
to thereof wherein:
H CI 3HC
0-C
10 1
H 0
1 -C-C----
-o
=
M C-H
H-C-H
1
H-C-H H-C-H
J
c-c.- -c-C
HO 1 1
H
1
0=C
H3C jrõ H3C
Formula (I) Formula OD
7
CA 02865453 2014-08-22
WO 2013/152148
PCT/US2013/035200
3HC
0=C
H 0
1
õõõ..
1
H
HC 4i
.............................................. n
1 1
H
0=C
H3C m
Formula ( 3 I 3)
, CH2-C
2 CH2
=
0
HC
Formula (IV)
wherein n is the number of cross linking units, m is the number of first chain
units,
.. and a is the number of second chain units, either, some, or all of n, m,
and o can be I or more,
although m and o will frequently he 2 or 3 or 4 or more, either or both of the
first and second
chain units can be left side end (terminal) units of a polymer chain and/or
right side end
(terminal) units of a polymer chain, \JAE can also comprise co-polymers
containing additional
cross linking units and can comprise additional polymer chains,
to In the event that the above definitions or a description stated
elsewhere in this
application is inconsistent with a meaning (explicit or implicit) which is
commonly used, in a
dictionary, the application
and the claim terms in particular are understood to he construed according to
the definition or
description in this application, and not according to the common definition or
dictionary
definition. In light ofthe above, in the event that a term
can only be understood if it is construed by a dictionary, if the term is
defined by the Kirk-Oihnzer
Encyclopedia of Chemical Technology; 5th Edit-ion, (2005), (Published by
Wiley, John & Sons,
Inc.) this definition shall control how the term is to be defined in the
claims.
9
CA 2865453 2019-05-27
CA 02865453 2014-08-22
WO 2013/152148
PCT/US2013/035200
In at least one embodiment the surface of a pile of low-ranked coal is treated
with
an inhibiting composition to inhibit the spontaneous combustion of the pile.
The inhibitor is a
composition comprising crude glycerin and VAE copolymer and/or a PVA
copolymer. The crude
glycerin is derived from a transesterification reaction involving
triglyeerides.
When handling low-ranked coals people need to be conscious of two distinct
fire-
based dangers. Coal dust (which can result from all coal not just low-ranked
coal) is highly
mixed with atmospheric oxygen and can be a cause of a spontaneous explosion.
Unique to low-
ranked coal is its high propensity to oxidize which can cause fires within the
coal piles itself.
Low-ranked coal can oxidize and suffer from non-explosion tires within the
pile even when
substantially all airborne dust has been removed from the presence of the mass
of coal.
In at least one embodiment the pile is in the proximity of substantially no
dust so it
is substantially not at risk of an airborne explosion, but the low ranked-coal
is at risk of an
oxidation induced fire. In at least one embodiment the presence of dust is
excluded. In at least
one embodiment the presence of coal which is non-low grade is excluded. In at
least one
embodiment a substantial risk of an airborne explosion is excluded while the
risk of oxidation
and oxidation induced combustion is substantial.
Biocliesel is typically made through a chemical process called
transesterification in
which vegetable oil or animal fats are converted to fatty acid alkyl esters
and crude glycerin by-
product. Fatty acids and fatty acid alkyl esters can be produced from oils and
fats by base-
catalyzed transesterification of the oil, direct acid-catalyzed esterifie-
ation of the oil and
conversion of The oil to fatty acids and subsequent esterification to
biodiesei.
The majority of fatty acid alkyl esters are produced by the base-catalyzed
method.
In general, any base may be used as the catalyst used for transesterification
of the oil to produce
CA 02865453 2014-08-22
WO 2013/152148
PCT/US2013/035200
biodiesel, however sodium hydroxide or potassium hydroxide are used in most
commercial
processes.
Suitable examples of crude glycerin and its manufacture can be found in among
other places in US Patent Application 12/246,975. In the biodiesel
manufacturing process, the
oils and fats can be filtered and preprocessed to remove water and
contaminants. If free fatty
acids are present, they can be removed or transformed into biodiesel using
special pretreatment
technologies, such as acid catalyzed esterification. The pretreated oils and
fats can then be mixed
with an alcohol and a catalyst (e.g. base). The base used for the reaction is
typically sodium
hydroxide or potassium hydroxide, being dissolved in the alcohol used
(typically ethanol or
methanol) to form the corresponding alkoxide, with standard agitation or
mixing. It should be
appreciated that any suitable base can be used. The alkoxide may then be
charged into a closed
reaction vessel and the oils and fats are added. The system can then be
closed, and held at about
71 degrees C (160 degrees F) for a period of about I. to 8 hours, although
some systems
recommend that the reactions take place at room temperature.
I 5 Once the reactions are complete the oil molecules (e.g.
triglycerides) are
hydrolyzed and two major products are produced: I) a crude fatty acid alkyl
esters phase (i.e.
biodiesel phase) and 2) a crude glycerin phase. Typically, the crude fatty
acid alkyl ester phase
forms a layer on top of the denser crude glycerin phase. Because the crude
glycerin phase is
denser than the biodiesel phase, the two can be gravity separated. For
example, the crude
glycerin phase can be simply drawn off the bottom of a settling vessel. In
some cases, a
centrifuge may be employed to speed the separation of the two phases.
The crude glycerin phase typically consists of a mixture of glycerin, methyl
esters,
methanol, mong and inorganic salts and water. Methyl esters are typically
present in an amount
of about 0.01 to about 5 percent by weight.
11
CA 02865453 2014-08-22
WO 2013/152148
PCT/US2013/035200
In at least one embodiment, methanol can be present in the crude glycerin in
an
amount greater than about 5 weight percent to about 30 weight percent. In at
least one
embodiment, the crude glycerin comprises about 30 to about 95 weight percent
of glycerin.
VAE is a copolymer in which multiple vinyl acetate polymers contain ethylene
side branches which form cross linkages and connect the polymers to each other
forming
copolymer networks.
In at least one embodiment the composition comprises between 90:10 and 10:90
of VAE copolymer to crude glycerin by mass. In at least one embodiment the
composition
further comprises water. in at least one embodiment the composition comprises
water and the
to crude glycerin both prevents the freezing of the water and prevents its
evaporation thereby
reducing the tendency of oxidation to mom
In at least one embodiment the composition is applied according to any one of
the
methods or apparatuses of US 5,441,566.
The components of the inhibiting composition may be mixed immediately before
is addition to the low-grade coal or may be pm-mixed or some components may
be pre-mixed and
other components may be mixed immediately before addition. The material may be
applied in
liquid form by a spray boom having one or more spray heads. In at least one
embodiment the
composition is applied to the material to be coated by at least one of the
methods disclosed in US
Patent 5,622,561.
20 In at
least one embodiment the composition is applied as the pile is being formed.
This can occur for example when material is loaded into a rail car, dump
truck, storage facility,
silo, or ship's hold. The composition can be applied to the material before
and/or as it is poured
or dumped into a pile. In at least one embodiment the material passes along a
conveyer belt
before it is poured or dumped arid the composition is applied to the material
as it travels along the
12
CA 02865453 2014-08-22
WO 2013/152148
PCT/US2013/035200
belt. In at least one embodiment the composition functions as a tackifier
which helps to hold
together the material in the form of larger clumps that are less likely to
launch as airborne dust.
In at least one embodiment the inhibitor is applied such that one or more of
the
standard safety protocols can be omitted from the handling of Sub-Bituminous
Coal or low-rank
coal. For example the inhibitor treated sub-bitumous or low rank coal can be
safely processed
through a legacy coal handling apparatus having one or more pinch points. Or
tbr example the
inhibitor treated low-rank coal can be allowed to remain undisturbed for
longer than is allowed
for untreated low-ranked coal or it is handicd in a non-FIFO manner even
though this will result
in a pile of low-ranked coil remaining in inventory longer than the safety
protocols recommend.
to In at least one embodiment the sub-bitumous coal is mined from the
Powder River
Basin and is handled, stored, and transported according to a legacy coal
handling method.
In at least one embodiment the inhibitor treated low-ranked coal is loaded
into the
hold of a ship where it will sit for at least II days undisturbed.
In at least one embodiment the coal is loaded onto the ship after it has sat
within a
is railcar for 1-20 days also undisturbed.
The inventive composition is quite effective and displays a number of
unexpected
and beneficial results. Prior art combustion inhibiting formulations such as
US Patent 5,576,056,
Japanese Patents 56133392, 4032149, and 4597922, and Japanese Patent
Applications
2000080356, 2006328413, and 1998265757 focus on reducing the formation of dust
clouds from
20 coal piles and thereby reduce the spontaneous combustion of those dust
clouds, They however do
nothing to prevent the degradation/oxidation of coal within the piles.
Moreover they do not
adequately reduce the potential involuntary combustibility of low-rank or sub-
bitumous coal but
rather only bitumous coal.
Without limitation to theory and in particular the scope of the claims, it is
believed
13
CA 02865453 2014-08-22
WO 2013/152148
PCT/US2013/035200
that the crude glycerin forms hydrogen bonds with the coal's hydroxyl groups
and carbonyl
groups which reduces the reactivity of these groups with oxygen while
simultaneously the VAE
and/or PVA. copolymer serves to seal the surfaces of the treated coal. In
addition, the
"impurities" within the crude glycerin (such as but not limited to fatty acid
methyl esters, partially
hydrolyzed fatty acid methyl esters, and inorganic salts) perform better than
pure glycerin because
they produce a physical barrier along the surface of the coal piles which
further bars the oxidation
processes of the treated coal.
En addition the composition inhibits combustion caused by the re-filling of
the
voids within the coal particles. Because moisture does not only flow in one
direction voids
to tbrmed by evaporation sometimes become re-filled by condensing moisture.
The process of re-
filling the moisture, however, generates heat which accelerates oxidation and
thus may in its own
right, cause spontaneous combustion. In at least one embodiment the invention
retains the
moisture and thereby prevents the re-filling of the coal with heat laden
moisture.
In at least one embodiment the composition is used on low ranked coal passing
through a funnel shaped piece of processing equipment or other pinch point
containing
equipment. Funnels are characterized as having sloped side walls which slope
down to a small
opening. Because of the frictional properties of the sloped walls, the innate
autogenous/cohesive
properties of the low ranked coal, and/or the magnitude of the slope, coal
particles adjacent to the
walls will have a different flow rate than particles farther from the walls
and closer to a region
directly over the opening. In at least one embodiment, one of the: frictional
properties, slope
magnitude, size of the opening, size of the coal material particles, and
autogenouskohesive
properties of the coal particles, and any combination thereof is such that but
for the presence of
the composition the coal particles would form a rathole and only flow through
a channel and
would oxidize but with the composition such oxidation does not occur.
14
CA 02865453 2014-08-22
WO 2013/152148
PCT/US2013/035200
As previously mentioned in some cases ratholes, arches, and other pinch points
can be dislodged or broken up by applying energy such as vibrations. These
vibrations however
can be dangerous, expensive, and may cause explosions or damage the equipment
and as a result
they are undesirable. In at least one embodiment the method excludes the
application of energy
(including but not limited to vibration) to break up a rathole or arch in the
piece of equipment that
forms a rathole and/or arch for a period of time over which otherwise a user
would have applied.
In at least one embodiment the application of energy is excluded for a period
of time extending
from between I week to 12 months.
Rathole and arching effects are known to be exacerbated by increasing moisture
to contents. As previously stated low-ranked coals typically contain higher
moisture contents than
higher ranked coals and are therefore expected to be more likely to form
ratholes, arches or
manifest other pinch points than higher ranked coals. In at least one
embodiment the low-ranked
coal is passed through a piece of equipment in which in the absence of the
composition, due to its
moisture content the (ow ranked coal would form an arch, rathole, or pinch
point, but a higher
ranked coal would not form an arch, rathole, or pinch point, and because of
the presence of the
composition, the moisture laden low ranked coal does not oxidize or is
inhibited from oxidizing.
In at least one embodiment the coal processing equipment contains a crack in
which low ranked coal particles collect, become stagnant and may oxidize but
in the presence of
the composition such oxidation is inhibited and/or does not occur.
In at least one embodiment the low ranked coal passes through the coal
processing
equipment in the absence of one added item selected from the list consisting
of: water, wetting
agents, foams, micelle encapsulating agents, CO2, N2, and any combination
thereof.
CA 02865453 2014-08-22
WO 2013/152148
PCT/US2013/035200
EXAMPLES
The foregoing may be better understood by reference to the following
examples, which are presented for purposes of illustration and are not
intended to limit
the scope of the invention.
Coal oxidation takes place in a series of steps including the oxidation of
functional groups of the coal by oxygen, the build-up of oxygen containing
oxidation
product groups within the coal and the formation of gaseous carbon monoxide
and carbon
dioxide as the ultimate oxidation products. Using infrared spectroscopy it is
possible to
monitor the first two processes over time as a sample of coal is oxidized in
air. In FIGs. I
to and 2 there are shown samples of treated and untreated sub-bituminous
coal which are
compared using Fourier Transform Infiared Spectroscopy (FriR). Both samples
were
placed in a controlled temperature oven and representative portions collected
over time as
oxidation of the sub-bituminous coal took place. FTIR spectra of each sub-
bituminous
coal portion was then obtained and the area of the peaks in the aliphatic
hydrocarbon
region and the area of the peaks in the carbon-oxygen double bond region
(carbonyl) were
determined. As shown in FIG I, the aliphatic hydrocarbon groups of the
untreated and
the treated sub-bituminous coal samples are oxidized at approximately the same
rate over
the course of the test. This is demonstrated by the similarity of the rate of
disappearance
of these functional groups as measured by the change in the aliphatic peak
area of each.
As shown in FIG 2, the treated sub-bituminous coal displays a steady increase
in carbonyl
species as expected from the oxidation of the aliphatic groups. The untreated
sub-
bituminous coal, however, displays a relatively unchanged level of carbonyl
species over
the course of the test. The latter is an indication that the untreated sub-
bituminous coal
oxidation process is producing gaseous carbon monoxide and carbon dioxide at a
much
16
CA 02865453 2014-08-22
WO 2013/152148
PCT/US2013/035200
faster rate than in the treated sub-bituminous coal. Clearly, overall
oxidation of the
treated sub-bituminous coal takes place more slowly than the untreated sub-
bituminous
coal.
Referring now to FIG. 3 there is shown the results of testing the semi-
adiabatic oxidation levels of treated and untreated sub-bitumous coal. in this
test,
selected sub-bitumous coal samples were prepared under an inert atmosphere
prior to use
and placed in a dewar flask equipped with a thermocouple for measuring the
temperature
of the coal and with a gas inlet tube to deliver gas directly to the coal and
a gas outlet tube
to allow gas to escape from the flask. One flask contained treated sub-
bituminous coal
le .. and another flask contained an untreated portion of the same sub-
bituminous coal. The
flasks were sealed and placed in a controlled temperature oven under constant
nitrogen
flow within the flask. When the temperature of the contents of the flasks had
reached a
steady state the nitrogen flow was stopped and air flow was initiated to both
flasks. The
same air flow rate from the same air source was applied to each flask. With
the
.. application of air to the samples the sub-bituminous coal began to oxidize
and the
temperature within the flasks began to climb.
The rate of heat build-up within the flasks is related to the rate of
oxidation
of the material within the flask. Thus, the change in temperature within the
flasks
provides an indirect measure of the tendency of the contents to resist
oxidation
(spontaneous combustion) in air. A good spontaneous combustion inhibitor
should limit
the build-up of heat within the flask compared to untreated coal under
comparable
conditions. As shown in Figure 3, the treated sub-bituminous coal yielded a
much lower
temperature change compared with the untreated coal.
17
While this invention may be embodied in many different fonns, there
described in detail herein specific preferred embodiments of the invention.
The present
disclosure is an exemplification of the principles of the invention and is not
intended to
limit the invention to the particular embodiments illustrated.
.Furthermore, the invention encompasses any possible combination of some or
all of the
various embodiments described herein. In addition the
invention encompasses any possible combination that also specifically excludes
any one
lo or some of the various embodiments described herein,
The above disclosure is intended to be illustrative and not exhaustive. This
description will suggest many variations and alternatives to one of ordinary
skill in this art, All
these alternatives and variations are intended to be included within the scope
of the claims where
the term "comprising" means "including, but not limited to". Those familiar
with the art may
15 recognize other equivalents to the specific embodiments described herein
which equivalents are
also intended to be encompassed by the claims.
All ranges and parameters disclosed herein are understood to encompass any and
all subranges subsumed therein, and every number between the endpoints. For
example, a stated
range of "1 to 10" should be considered to include any and all subranges
between (and inclusive
20 00 the minimum value of 1 and the maximum value of 10; that is, all
subranges beginning with a
minimum value of 1 or more, (e.g. Ito 6.1), and ending with a maximum value of
10 or less,
(e.g. 2.3 to 9.4, 3 to 8.4 to 7), and finally to each number 1,2, 3, 4, 5, 6,
7, 8, 9, and 10 contained
within the range
18
CA 2865453 2019-05-27
CA 02865453 2014-08-22
WO 2013/152148
PCT/US2013/035200
This completes the description of the preferred and alternate embodiments of
the
invention. Those skilled in the art may recognize other equivalents to the
specific embodiment
described herein which equivalents are intended to be encompassed by the
claims attached hereto.
19