Note: Descriptions are shown in the official language in which they were submitted.
CA 02866168 2014-09-02
WO 2013/163088 PCT/US2013/037597
Atty. Docket: ZGNX-128W0
PISTON CLOSURES FOR DRUG DELIVERY CAPSULES
FIELD OF THE INVENTION
[0001] The present invention relates to a piston comprised of
polytetrafluoroethylene
(PTEE) modified with perfluoro(propyl vinyl ether) (PPVE) to form a copolymer.
The
piston is used in a drug delivery system such as a pre-filled syringe, an auto-
injector, or
especially a needle-free injector, for delivery of liquid formulations
contained in drug
capsules. Delivery is preferably by needle free injection, wherein the piston
is both a
mechanical system for delivery and a closure seal for the formulation
container. The
material used to construct the piston is selected so that the piston will have
properties
such that the container closure system maintains integrity over the range of
storage and
stability testing temperatures expected for the device.
BACKGROUND OF THE INVENTION
[0002] Many drugs need to be delivered outside of the physician's office,
for
example due to the need for acute treatment or frequent administration, such
as
continuously, daily, twice daily, four times daily, weekly, bi-weekly, or
monthly.
For this reason, the drugs often need to be delivered by someone who is not a
skilled medical service provider such as the patient or a family member of the
patient. Passive systems such as oral dosage forms, simple nasal sprays, or
passive
transdermal patches can be used, but auto-injectors, automated pumps, bolus
injectors, active transdermal systems, or sophisticated pulmonary delivery
systems
are often preferred for these products, because of features chosen from their
relative
ease of use, high dose control and repeatability, ability to titrate the dose
or control
infusion rate, compliance monitoring features, dose reminders, etc.
[0003] Oral drugs have the advantage that they are easy to self
administer and are
generally accepted by the patient. However, many drugs, especially peptide and
protein drugs, have very limited oral bioavailability, due to digestion and
first pass
liver metabolism. Additionally, absorption following oral delivery is delayed,
with
time to peak plasma concentrations (T.) of ¨40 minutes or longer. Thus, a
dosage
form and/ or drug delivery device that is easy and fast to self administer can
be
crucial for acute, debilitating conditions, for example migraine and cluster
headache, hypoglycemia, hyperglycemia, seizure, allergic reaction including
anaphylaxis, drug overdose, acute asthma, exposure to warfare agents such as
toxins or bioweapons, acute pain, erectile dysfunction, snake, insect, and
spider
1
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
bite, heart conditions, fainting, anxiety, psychotic episodes, insomnia, leg
cramps,
and other acute conditions. .
[0004] Many patients and unskilled care givers have difficulties
administering
drugs, including but not limited to inability of lack of desire to follow
complex
directions, fear of self administration or administering drug to another, etc.
Ensuring treatment compliance and proper delivery can be problematic,
especially
with complex systems that require filling, reconstitution, and other
preparation
steps. Thus there is a great advantage to a delivery system that is easy and
quick to
use, with minimal steps required for preparation and delivery, such as a
prefilled
syringe, an auto-injector including but not limited to a prefilled
autoinjector or an
autoinjector with a prefilled, replaceable drug capsule, prefilled pump, a
pump with
a replaceable prefilled drug capsule, a prefilled transdermal system, a
transdermal
system with a replaceable drug capsule, a prefilled inhaler, or an inhaler
with a
preplaceable drug capsule. A preferred drug delivery system is a prefilled,
single
dose, disposable autoinjector, more preferably a needle free injector. The
drug
delivery system should require a minimal number of steps for preparation and
delivery, preferably less than ten steps, more preferably less than five, most
preferably three, two, or one step.
[0005] Many pharmaceutically active compounds need to be delivered
parenterally
by injection or infusion for reasons of low bioavilability when delivered via
other
routes such as oral, buccal, nasal, pulmonary, or transdermal, or the need for
more
rapid onset than can be achieved by other routes. Most injectors, including
prefilled syringes and autoinjectors, comprise an injection needle. Many
patients,
however, are needle-averse or suffer from needle-phobia. In addition,
injectors
with needles entail danger of needle stick injury and cross contamination, and
require special sharps and biohazard disposal systems which are in general not
available outside of hospitals, laboratories, or doctors offices. In addition,
it is a
problem that patients may need to be trained to self administer an injection,
although for some indications the number of injections they would self
administer
is only a few. In addition, a needle and syringe in general needs to be
filled, and for
some formulations dried drug requires reconstitution, which further
complicates
self administration and reduces compliance. These issues often rule out the
possibility of treatment in a home setting, either self treatment or by a
relatively un-
2
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
trained care giver such as a family member. The inability to dose at home can
lead
to higher costs of therapy, delay in treatment, reduced compliance, reduced
comfort, and potential exposure to hospital acquired infections.
[0006] In addition, in a hospital, clinic, or doctor's office setting,
there is a large
advantage to easy to use drug delivery devices and dosage forms, to reduce
cost,
time, training requirements, risk of injury, and dosing errors. Therefore,
there is a
significant need for simple, easy to use drug capsules for such systems as
pole
mounted and table top pump systems, injectors, aerosol delivery systems, and
the
like.
[0007] Some drug delivery systems have drug capsules which are factory
prefilled
with a liquid formulation, to minimize the amount of preparation required for
delivery. Alternatively, capsules may be multi compartment and contain a
powdered formulation and a diluent for reconstitution. These capsules can
either be
integrated into a device which is disposed of when the formulation is
exhausted, or
multiple capsules can be supplied with a durable device to which they are
integrated
prior to use, and the capsule is disposed of after delivery. Drug capsules may
comprise a polymer or metal, but preferably have a glass component in direct
contact with the formulation, more preferably a borosilicate glass component.
[0008] Drug capsules which are pre-filled function as the primary
container closure
system which ensures stability and sterility of the formulation during
storage. The
drug capsule components must be made of materials that are compatible with the
formulation when in contact during storage, and not cause degradation of the
formulation components. They also must not leach unacceptable levels of
materials
into the formulation during storage. The materials and design of the drug
capsule
must isolate the formulation during storage, not allowing ingress of
contaminants,
air, water vapor, bacteria, or viruses. The materials and design of the drug
capsule
must also ensure that there is no egress of formulation components, especially
liquid components such as water for injection. The stability and sterility of
the
formulation must in general be maintained for storage periods of 6 months,
preferably for 1 year, more preferably for 2 years, still more preferably for
a period
of 3 years or more.
[0009] In many prefilled drug delivery systems or dosage forms, the drug
capsule
functions as a syringe. The capsule of this type of injector will have a
polymer,
3
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
metal, or preferably glass syringe body. The syringe body will have in general
an
exit orifice leading to, for example, a needle, a system for connecting a
needle such
as a luer fitting, a needle free injector injection orifice, an aerosol
generator, a
transdermal applicator, an infusion set, a secondary dose chamber for
multidose
systems, or the like. The syringe body will also in general be sealed in
another
region by a stopper which also functions as the syringe piston during
delivery.
[0010] Prefilled drug capsules must be tested to demonstrate that they
will provide
adequate stability and sterility of the formulation during storage. This
testing is
called container/closure integrity testing. They must also be tested to ensure
that
capsule components in contact with the formulation have sufficient low levels
of
components that will leach into the formulation that will leach into the
formulation
during storage, generally called leachable and extractable testing. Often
testing is
done at elevated or reduced temperatures, to ensure that container closure
integrity
is maintained over the range of temperatures expected in the storage of the
device.
Elevated temperature testing is also done to estimate the effects of longer
term
storage, called accelerated stability testing. Temperature testing may also be
done
by cycling the temperature of the drug capsule between predetermined high and
low
temperatures for a predetermined number of cycles, and holding the capsule at
the
high and low temperature for predetermined times. This type of testing is
referred
to as temperature cycling or thermal cycling. Temperature testing is often
combined
with drug stability, dye ingress, water vapor transmission rate, microbial
challenge,
or other tests to demonstrate stability and sterility. Thus the drug capsule
components, including syringe body, piston, and exit orifice sealing
feature(s), must
be designed and made of components that will maintain container closure
integrity
at elevated temperatures, reduced temperatures, and during temperature
cycling.
[0011] The drug capsule, and especially the piston and syringe body of a
syringe
type drug capsule, are subject to very high stresses to ensure a sufficient
seal during
storage. These stresses, especially of the piston, are in general even higher
during
piston insertion. In general, the index of thermal expansion of the piston and
syringe body of a prefilled syringe will be different, which can further
increase
stresses during elevated temperature or thermal cycling. This problem is
especially
acute when the drug capsule comprises borosilicate glass. Borosilicate glass
is a
preferred material because of its wide application in drug containers and
laboratory
4
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
glassware. Borosilicate glass has a very low index of thermal expansion,
greatly
reducing its propensity to break when exposed to elevated temperatures and
temperature gradients. However, this property of low thermal expansion can
lead
to high stresses at elevated temperatures if other components, such as a
syringe
piston, do not have similarly low thermal expansion coefficients. When a
component such as a piston is fabricated from a polymer, such as rubber,
plastic or
PTFE, high stresses can lead to permanent deformation due to yield, or over
longer
periods, creep. This can lead to a significant problem during temperature
changes
during storage or testing. For example, if a syringe piston yields or creeps
when in
a borosilicate glass capsule at high temperature, when the temperature is
subsequently reduced, the piston may no longer have sufficient sealing
properties in
the syringe body, leading to loss of container closure integrity. This problem
is
especially acute during thermal cycling, when the drug capsule is exposed to
elevated and then reduced temperatures, as creep or yield at the elevated
temperature is more likely to lead to loss of container closure integrity at
the
reduced temperature. It is an additional problem that the reduction in sealing
combined with thermal cycling can cause the piston to move over time in the
syringe body, potentially impacting dosing performance and dose uniformity.
[0012] Thus it can be seen that the material and design of prefilled
syringe drug
capsule components much be selected very carefully to ensure container closure
integrity and injector performance over shelf life and during testing.
[0013] Some issues are particularly acute in the context of elevated
viscosity
formulations, including but not limited to controlled release formulations,
and
formulations of biologic drugs, such as Monoclonal AntiBodies (MABs). Elevated
viscosity leads to many delivery difficulties, such as high required hand
strength for
a needle and syringe, long delivery times, and additional pain and fear
associated
with a large bore needle. Thus there is a need to deliver these compounds
without a
needle, preferably in a rapid, automated fashion using a system that does not
require
filling, reconstitution, or other complex procedures.
[0014] One particularly preferred drug delivery device is the needle free
injector.
Needle free injectors have many advantages over other drug delivery systems,
particularly for home use. They have advantages similar to needle injectors,
such
as high bioavailability, rapid onset, and high reproducibility. They also have
many
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
of the advantages of other delivery methodologies, such as avoidance of needle
phobia, avoidance of needle stick injury, reduced or no pain, and no
requirement
for sharps disposal.
[0015] Needle-free injectors are available using many different types of
energy
storage. The energy may be supplied by the user, for example where a spring is
manually compressed and latched to temporarily store the energy until it is
required
to actuate the injector. Alternatively, the injector may be supplied having
the
energy already stored--for instance by means of a pre-compressed spring
(mechanical or compressed gas), or by pyrotechnic charge.
[0016] Some injectors are intended for disposal after a single use,
whereas others
have a re-loadable and/or multidose energy storage means and a single or multi-
dose medicament cartridge, and there are many combinations to suit particular
applications and markets. For the purposes of the present disclosure, the term
"actuator" will be used to describe the energy storage and release mechanism,
whether or not it is combined with a medicament cartridge. In all cases, it is
necessary to arrange for sufficient force at the end of the delivery to
deliver the
entire dose of medicament at the required pressure.
[0017] EP 0 063 341 and EP 0 063 342 disclose a needle-free injector
which
includes a piston pump for expelling the liquid to be injected, which is
driven by a
motor by means of a pressure agent. The liquid container is mounted laterally
to the
piston pump. The amount of liquid required for an injection is sucked into the
pump chamber by way of an inlet passage and a flap check valve when the piston
is
retracted. As soon as the piston is moved in the direction of the nozzle body
the
liquid is urged through the outlet passage to the nozzle and expelled. The
piston of
the piston pump is a solid round piston.
[0018] EP 0 133 471 describes a needle-free vaccination unit which is
operated
with carbon dioxide under pressure, from a siphon cartridge by way of a
special
valve.
[0019] EP 0 347 190 discloses a vacuum compressed gas injector in which
the
depth of penetration of the injected drug can be adjusted by means of the gas
pressure and the volume of the drug can be adjusted by way of the piston
stroke.
6
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
[0020] EP 0 427 457 discloses a needle-free hypodermic syringe which is
operated
by means of compressed gas by way of a two-stage valve. The injection agent is
disposed in an ampoule which is fitted into a protective casing secured to the
injector housing. The ampoule is fitted on to the end of the piston rod.
Disposed at
the other end of the ampoule is the nozzle whose diameter decreases towards
the
end of the ampoule.
[0021] WO 89/08469 discloses a needle-free injector for one-off use. WO
92/08508 sets forth a needle-free injector which is designed for three
injections.
The ampoule containing the drug is screwed into one end of the drive unit,
with the
piston rod being fitted into the open end of the ampoule. At its one end, the
ampoule contains the nozzle through which the drug is expelled. A displaceable
closure plug is provided approximately at the center of the length of the
ampoule.
The dose to be injected can be adjusted by changing the depth of the ampoule.
The
piston rod which projects from the drive unit after actuation of the injector
is
pushed back by hand. Both units are operated with compressed gas.
[0022] WO 93/03779 discloses a needle-free injector with a two-part
housing and a
liquid container which is fitted laterally to the unit. The drive spring for
the piston
is stressed by means of a drive motor. The spring is released as soon as the
two
parts of the housing are displaced relative to each other by pressing the
nozzle
against the injection location. Respective valves are provided in the intake
passage
for the liquid and in the outlet of the metering chamber.
[0023] WO 95/03844 discloses a further needle-free injector. It includes
a liquid-
filled cartridge which at one end includes a nozzle through which the liquid
is
expelled. At the other end the cartridge is closed by a cap-type piston which
can be
pushed into the cartridge. A piston which is loaded by a prestressed spring,
after
release of the spring, displaces the cap-type piston into the cartridge by a
predetermined distance, with the amount of liquid to be injected being
expelled in
that case. The spring is triggered as soon as the nozzle is pressed
sufficiently firmly
against the injection location. This injector is intended for one-off or
repeated use.
The cartridge is arranged in front of the spring-loaded piston and is a fixed
component of the injector. The position of the piston of the injector which is
intended for a plurality of uses is displaced after each use by a distance in
a
7
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
direction towards the nozzle. The piston and the drive spring cannot be reset.
The
pre stressing of the spring is initially sufficiently great to expel the
entire amount of
liquid in the cartridge all at once. The spring can only be stressed again if
the
injector is dismantled and the drive portion of the injector assembled with a
fresh,
completely filled cartridge.
[0024] U.S. patent No. 5,891,086 describes a needle-free injector,
combining an
actuator and a medicament cartridge. The cartridge is pre-filled with a liquid
to be
injected in a subject, and having a liquid outlet and a free piston in contact
with the
liquid, the actuator comprising an impact member urged by a spring and
temporarily restrained by a latch means, the impact member being movable in a
first direction under the force of the spring to first strike the free piston
and then to
continue to move the piston in the first direction to expel a dose of liquid
through
the liquid outlet, the spring providing a built-in energy store and being
adapted to
move from a higher energy state to a lower energy state, but not vice versa.
The
actuator may comprise trigger means to operate the said latch, and thus
initiate the
injection, only when a predetermined contact force is achieved between the
liquid
outlet of the said cartridge and the subject.
[0025] In U.S. Pat. No. 3,859,996, Mizzy discloses a controlled leak
method to
ensure that the injector orifice is placed correctly at the required pressure
on the
subject's skin at the correct normal to the skin attitude. When placement
conditions
are met, controlled leak is sealed off by contact pressure on the subject's
skin, the
pressure within the injector control circuit rises until a pressure sensitive
pilot valve
opens to admit high pressure gas to drive the piston and inject the
medicament.
[0026] In WO Patent 82/02835, Cohen and Ep-A-347190 Finger, disclose a
method to improve the seal between the orifice and the skin and prevent
relative
movement between each. This method is to employ a vacuum device to suck the
epidermis directly and firmly onto the discharge orifice. The discharge
orifice is
positioned normal to the skin surface in order to suck the epidermis into the
orifice.
This method for injection of the medicament into the skin and the injector
mechanism are different and do not apply to the present invention because of
its
unique ampoule design.
8
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
[0027] In U.S. Pat. No. 3,859,996 Mizzy discloses a pressure sensitive
sleeve on
the injector which is placed on the subject, whereby operation of the injector
is
prevented from operating until the correct contact pressure between orifice
and the
skin is achieved. The basic aim is to stretch the epidermis over the discharge
orifice
and apply the pressurized medicament at a rate which is higher than the
epidermis
will deform away from the orifice.
[0028] In U.S. Pat. No. 5,480,381, T. Weston discloses a means of
pressuring the
medicament at a sufficiently high rate to pierce the epidermis before it has
time to
deform away from the orifice. In addition, the device directly senses that the
pressure of the discharge orifice on the subject's epidermis is at a
predetermined
value to permit operation of the injector. The device is based on a cam and
cam
follower mechanism for mechanical sequencing, and contains a chamber provided
with a liquid outlet for expelling the liquid, and an impact member, to dispel
the
liquid.
[0029] In U.S. Pat. No. 5,891,086, T. Weston describes a needle-free
injector that
contains a chamber that is pre-filled with a pressurized gas which exerts a
constant
force on an impact member in order to strike components of a cartridge and
expulse
a dose of medicament. This device contains an adjustment knob which sets the
dose
and the impact gap, and uses direct contact pressure sensing to initiate the
injection.
Further examples and improvements to this needle-free injector are found in
U56620135, U56554818, U56415631, U56409032, U56280410, U56258059,
U56251091, U56216493, U56179583, U56174304, U56149625, U56135979,
U55957886, US5891086, and U55480381, incorporated herein by reference.
[0030] A number of biologically-active agents in viscous formulations
would
benefit from being delivered using the needle-free injector. This group could
consist of (but not limited to) anti-inflammatory agents, antibacterial
agents,
antiparasitic agents, antifungal agents, antiviral agents, anti-neoplastic
agents,
analgesic agents, anaesthetics, vaccines, central nervous system agents,
growth
factors, hormones, antihistamines, osteoinductive agents, cardiovascular
agents,
anti-ulcer agents, bronchodilators, vasodilators, birth control agents and
fertility
enhancing agents, interferon alpha, growth hormone, osteoporosis drugs
including
PTH and PTH analogs and fragments, obesity drugs, psychiatric drugs, anti-
9
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
diabetes, female infertility, AIDS, treatment of growth retardation in
children,
hepatitis, multiple sclerosis, migraine headaches, and allergic reactions.
[0031] The easiest to use drug delivery systems comprise a liquid drug
formulation
that is prefilled in a drug capsule at the factory. This has the distinct
advantage that
the patient or care provider does not have to fill the capsule, making it
easier and
faster to use. Ease of use and rapid delivery can be critical for acute
conditions,
including but not limited to migraine and cluster headache. However, being
prefilled has the disadvantage that the drug formulation container must
maintain the
required properties of the formulation over the shelf life of the system.
These
properties include, but are not limited to, the formulation concentration,
which can
change if water or other carriers are lost to the atmosphere, or if the active
pharmaceutical ingredient is absorbed by drug contact surfaces, purity, which
can
change if the drug is exposed to contaminants from the environment or the drug
container components themselves, stability (i.e. the chemical and
conformational
properties of the drug molecules) which can be adversely affected by contact
with
poorly chosen drug container materials or contaminants, and sterility, which
can be
impacted if the drug formulation is exposed to microbial or viral
contamination.
To maintain these properties, it is essential that the drug formulation
container be
properly designed, especially in the selection of the materials that are to be
in
contact with drug formulation during storage. Many materials have been found
to
be excellent drug contact materials in the sense that they do not impact the
purity of
the drug formulation by having volatile components that can extract into the
formulation, and do not further impact the chemical or conformational
properties of
the drug due to the drug being stored in contact with them. These materials
include
glasses, and selected polymers, including but not limited to fluoropolymers
such as
polytetrafluoroethylene (PTFE). Borosilicate glass is a preferred glass in
that it's
very low thermal expansion coefficient allows exposure to elevated
temperatures,
such as may be seen during sterilization, without creating stresses that lead
to
breakage. For example, ome needle free injectors, such as that described in U.
S.
Patent 5,891,086, utilize a glass drug container, which is sealed at one end
by a
PTFE piston.
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
[0032] It is a problem that prefilled drug capsules must maintain their
integrity,
including a barrier to contamination and water vapor transmission, over the
range
of temperatures expected during storage of the device. In the case where the
drug
capsule comprises a piston and syringe body, a difference in thermal expansion
coefficient (CTE) between the piston and the syringe body can create a gap at
low
or high temperature, allowing loss and/or contamination of the formulation.
One
way to avoid this is to use very soft rubber that is compressed sufficiently
such that
no gap will occur. However, this soft rubber may not be consistent with other
required properties of the piston. Specifically, for needle free injectors of
the type
described in US 5,891,086, wherein an impact member flies across a gap and
subsequently strikes the piston, creating a pressure spike that creates a hole
through
the skin, the piston must be sufficiently rigid under this high stress
condition that a
sufficient amount of the energy is transferred to the formulation, a condition
that
rubber in general does not satisfy. Unfortunately, more rigid materials cannot
in
general be compressed sufficiently such that container closure integrity is
maintained over the range of expected storage temperatures.
[0033] PTFE has intermediate properties in that it is soft enough to be
inserted into
a glass drug container, but rigid enough to transfer energy to the drug
formulation.
In fact it has the highly beneficial property that it is substantially non-
resilient
when subjected to a slowly applied force, such as might be seen during
insertion
into a glass drug container, but is highly resilient when subjected to a
rapidly
applied force, such as might be seen during a drug delivery event.
[0034] If one looks only at the thermal expansion coefficient and
compressibility of
PTFE, it would be expected that it would be able to maintain a seal in a
borosilicate
glass drug container over the temperatures to be expected during shelf life,
and over
the temperatures it would be exposed to during the temperature cycling
required for
stability testing. However, if one exposes such a system to temperature
cycling, for
example between 40 C and 2 C for 12 hours at each temperature for 30 days
(i.e.
30 cycles), one finds that leakage does occur. The reason for this is the
large
difference in thermal expansion coefficient between PTFE (CTE = 1.5 x 10-4 /
C)
and Borosilicate glass (CTE = 3 x 10-6/ C) causes the PTFE at elevated
temperatures, already under significant stress after insertion into the drug
container,
11
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
to be exposed to even higher stresses, causing it to yield, and effectively
causing it
to have a smaller unstressed diameter. When it is subsequently subjected to 2
C, it
is no longer compressed enough to maintain a seal.
[0035] In general syringes type drug capsules, including but not limited
to prefilled
syringes or auto-injectors with elastomeric pistons, require the use of
silicone oil or
some other lubricant to prevent the piston from binding to the inner surface
of the
syringe barrel. In addition, silicone oil or another lubricant is required to
maintain
acceptable sliding friction during travel down the barrel. However, the
problem
with the use of these types of lubricants is that they cause aggregation of
many
recombinant proteins and biological molecules over time. These aggregates tend
to
be immunogenic.
SUMMARY OF THE INVENTION
[0036] A drug capsule comprising a piston and syringe body for use in a
drug
delivery device, including but not limited to an injector, pump, transdermal
system,
spray system which creates an aerosol for particular types of treatment
including
but not limited to pulmonary, nasal, dermal, and ocular, preferably a
prefilled
syringe or an auto-injector, more preferably for use in a needle-free injector
system
is disclosed. This component may be a substantially cylindrical container
comprised of glass which may be ion exchange strengthened borosilicate glass.
The cylindrical glass container is open at one end, and the opening is sealed
with a
piston comprised of materials which allow for maintaining a tight seal between
the
piston and the glass during temperature changes expected to occur during
sterilization, testing, and storage, e.g. 0 C to 50 C, or 10 C to 40 C. The
piston
may be comprised of one or more polymers which polymers may be linked and
may form copolymers. The polymers may be polytetrafluoroethylene (PTFE) alone
or in combination with perfluoro(propyl vinyl ether) (PPVE). The capsule may
be
specifically designed for single use, and may be factory filled and sealed
with a
liquid formulation comprising a pharmaceutically active drug sealed inside
using
the piston as a seal for an open end of a glass capsule.
[0037] An aspect of the invention is a needle-free drug delivery system
which
comprises a cylindrical syringe body opened at a first end, the body being
comprised of a material which does not readily react with the formulation such
as a
12
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
non-reactive high density polymeric material or a glass such as borosilicate
glass
strengthened with ion exchange. The syringe body may be pre-filled at the
factory
with a liquid formulation comprised of a pharmaceutically acceptable carrier
and a
pharmaceutically acceptable drug. The formulation may be specifically designed
for injection from a needle-free injector. The system includes a piston which
has an
external diameter substantially equal to the internal diameter of the syringe
body
opened at a first end and as such being configured such that the piston seals
the first
end of the syringe body and prevents the formulation from leaking out of the
syringe body. In particular, the piston prevents leakage out of the container
over a
range of temperature changes which might occur during storage which can
include
temperature cycling over a range of 0 C to 50 C. The piston may be comprised
of
a copolymer. The copolymer may be polytetrafluoroethylene (PTFE) (modified
with perfluoro(propyl vinyl ether) (PPVE)).
[0038] Another aspect of the invention is a piston sealed drug capsule
comprised of
a cylindrical syringe body opened at a first end. The body is prefilled at a
factory
with a single dose of a liquid formulation comprised of a pharmaceutically
acceptable carrier and a pharmaceutically active drug. The opened end of the
cylindrical syringe body is sealed with a piston comprised of a non-reactive
polymeric material such as a copolymer of polytetrafluoroethylene (PTFE) and
perfluoro(propyl vinyl ether) (PPVE). The composition of the piston and the
internal diameter of the syringe body are comprised of materials and sized so
as to
maintain the integrity of the formulation inside the syringe body over a
period of
time of one year or more during temperature cycling which might normally be
expected to occur during storage such as temperature ranges of from 0 C to 50
C.
[0039] An object of the invention is to provide a drug capsule for a drug
delivery
system that enables drug administration in a setting outside of a hospital,
clinic, or
doctors office, by simplifying the preparation and administration of the drug
using
the delivery system, reducing fear and anxiety related to drug administration
by the
patient or unskilled care giver, and reducing the number of steps associated
with
and the complexity of drug administration.
[0040] A further object of the invention is to provide a drug capsule for
use in a
hospital, clinic, or doctor office setting that reduces costs, improves
outcomes, and
13
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
improves safety by reducing the steps required and the complexity of
preparation of
a drug delivery system and drug administration.
[0041] An objective of the invention is to provide a method for
delivering
therapeutics that limits the possibility of needle stick and cross
contamination, for
example with the HIV virus; improves patient compliance; reduces needle
phobia,
and improves efficacy of drug delivery.
[0042] The invention is carried out using a prefilled drug capsule,
preferably a drug
capsule that functions as a piston and syringe. Preferably the drug may be
removably attached to an actuator to for a drug delivery system, whereby the
drug
capsule can be disposed of and replaced after the drug contents are exhausted.
Preferably the drug capsule is permanently attached to a drug delivery system,
and
the entire system is disposed of when the drug contents are exhausted. The
invention can be carried out using any drug delivery methodology whereby the
drug
formulation is contained in and delivered from the drug capsule, including but
not
limited to parenteral, dermal, transdermal, buccal, oral, ocular, pulmonary,
vaginal,
or enteral delivery. Preferably, the invention is carried out using a
prefilled syringe
or auto-injector, more preferably using a needle free injector. Most
preferably, the
invention is carried out utilizing a pre-filled, self contained, single use,
portable
needle free injector.
[0043] In a particularly preferred embodiment, the invention is carried
out using a
needle free injector that is powered by a self contained compressed gas
charge,
elements of which are described in U.S. Patent No. 5,891,086 (incorporated by
reference in its entirety). This embodiment includes a device for delivering
formulations by needle-free injection, for example Sub-Cutaneously (SC), Intra-
Dermally (ID) or Intra-Muscularly (IM). An actuator is used in conjunction
with a
drug cartridge to form a needle-free injector. The cartridge is pre-filled
with a
liquid to be injected in a subject, the cartridge having at least one liquid
outlet and a
free piston inward of the liquid outlet in contact with the liquid.
[0044] The actuator comprises:
(a) a housing having a forward portion adapted to be connected with the
cartridge;
(b) impact member mounted within said housing inward of the forward
portion so as to be movable from a first position toward the forward portion
to
14
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
strike the free piston when a cartridge is connected and to continue to move
the
piston toward the liquid outlet whereby a dose of the liquid is expelled
through the
liquid outlet in the cartridge;
(c) an element within said housing which prevents movement of the impact
member, wherein upon actuation the element allows movement of the impact
member. The element may prevent movement by engaging said impact member to
prevent movement of the impact member until actuation, or more preferably
prevents the energy source from applying force to the impact member. In a
preferred embodiment, the energy source is a source of compressed gas, and the
element is a gas valve which is opened when the device is actuated. The
element
may be actuated in many ways including buttons, levers, and the like, but
preferably actuation occurs by pressing the liquid outlet against the desired
injection site.
[0045] The current invention describes various formulations that can be
delivered
using drug delivery systems comprising a drug capsule, including but not
limited to
the injector of 5,891,086. These formulations comprise active ingredients, and
may
include various polymers, carriers, etc.
[0046] An aspect of the invention is a desirable delivery time,
especially for high
viscosity formulations. Desirable delivery times may include any delivery
times
wherein the formulation is successfully delivered. Preferred delivery times
include
those less than the reaction time of a human, for example less than ¨600 ms,
more
preferably less than 100 ms.
[0047] Another aspect of the invention is acceptable pain associated with
injection.
[0048] Another aspect of the invention relates to alleviation of fear of
needles
associated with injection of formulations.
[0049] Another aspect of the invention relates to the elimination of the
danger of
needle stick injury and cross-contamination associated with injection of
formulations.
[0050] Another aspect of the invention relates to the simplification of
preparation
associated with delivery of formulations, by supplying a pre-filled, single
use or
multi dose, disposable drug capsules.
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
[0051] Another aspect of the invention relates to the drug release
profile associated
with injection of high viscosity depot formulation, especially surface eroding
systems.
[0052] Another aspect of the invention is to supply a piston for use in a
drug
capsule of a drug delivery devicedevice, preferably a drug capsule that
functions as
a piston and syringe, wherein the piston material is sufficiently lubricious
as to not
require additional lubricant.
[0053] Another aspect of the invention is a container closure system that
is
compatible with drug formulations, especially comprising at least one active
pharmaceutical ingredient chosen from a list including but not limited to: a
biologic
or nucleic acid, a polynucleic acid, a small molecule therapeutic, a protein,
a
peptide, and an antibody, preferably a monoclonal antibody.
[0054] Another aspect of the invention is to supply a prefilled container
closure
system comprising a piston and syringe body for adrug delivery device,
preferably a
prefilled syringe or auto injector, more preferably a needle free injector,
wherein
the coefficient of thermal expansion of the piston and the syringe body are
sufficiently close together that container closure integrity is maintained
over the
range of temperatures expected during storage and testing of the device.
[0055] A further aspect of the invention is to supply a container closure
system for
an drug delivery device, preferably a prefilled injection device, more
preferably a
needle free injector, comprising a formulation container that further
comprises a
glass capsule, preferably a borosilicate glass capsule, sealed by a piston,
wherein
the piston properties, including but not limited to thermal expansion, yield
strength,
and recovery after deformation under load (creep), especially at elevated
temperatures, are such that ability to maintain container closure integrity is
maintained over the range of temperatures expected during storage,
sterilization,
and testing of the container closure system.
[0056] A further aspect of the invention is to supply a prefilled
container closure
system for a drug delivery device that comprises a glass capsule, sealed by a
piston,
wherein the piston is naturally sufficiently lubricious that it does not
require
additional lubricant for insertion, or to deliver the drug formulation.
[0057] It is a further aspect of the invention to supply a piston for a
needle free
injector that is sufficiently compliant that it can be pressed into a syringe
body with
16
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
enough compression to maintain a tight seal over the range of storage and
testing
temperatures expected, and yet is rigid enough when struck by an impact member
that a sufficient fraction of the energy of the impact member is transmitted
to the
formulation such that a successful needle free injection can be achieved.
[0058] It is a further aspect of the invention to provide a container
closure system
comprising a piston and syringe body for a drug delivery system such that the
movement of a piston is sufficiently low over the temperature excursions that
are
expected during storage and testing as to not impact the functioning of the
injector.
[0059] It is a further aspect of the invention to provide a method of
modifying a
PTFE piston of a drug capsule to improve the shelf life, reliability, and
container
closure integrity of the drug capsule.
[0060] It is a further aspect of the invention to provide a piston for a
drug capsule,
which piston is fabricated from a PTFE material which is modified in such a
way
that the drug capsule, when the piston is sealingly placed in the drug
capsule,
preferably in a glass syringe body, more preferably a borosilicate glass
syringe
body, is better able to maintain container closure integrity and device
reliability
after the drug capsule is exposed to the range of temperatures and the
temperature
cycling that is experienced during storage and testing.
[0061] It is a further aspect of the invention to provide a piston that
seals a
container/closure system, said piston having one or more circumferential
raised ribs
of triangular cross section, preferably of triangular cross section with the
top of the
triangle where it contacts the syringe body removed to form a frustum, in
order to
supply high sealing contact sealing pressure while minimizing creep.
[0062] These and other objects, advantages, and features of the invention
will become
apparent to those persons skilled in the art upon reading the details of the
formulations
and methodology as more fully described below.
BRIEF DESCRIPTION OF THE DRAWINGS
[0063] The invention is best understood from the following detailed
description
when read in conjunction with the accompanying drawings. It is emphasized
that,
according to common practice, the various features of the drawings are not to-
scale.
17
CA 02866168 2014-09-02
WO 2013/163088 PCT/US2013/037597
Atty. Docket: ZGNX-128W0
On the contrary, the dimensions of the various features are arbitrarily
expanded or
reduced for clarity. Included in the drawings are the following figures:
[0064] Fig. 1 is a schematic diagram of a needle free injector that
utilizes the
invention.
[0065] Fig. 2 shows another embodiment of a needle free injector that
utilizes the
invention.
[0066] Fig. 2a show an embodiment of a latch used in the triggering
mechanism of
the invention, in the "safe" configuration.
[0067] Fig. 2b shows the embodiment of figure 2a, in the ready to fire
configuration.
[0068] Fig. 2c shows the embodiment of figure 2a, in the triggered
configuration.
[0069] Fig. 3 shows another embodiment of a needle free injector that
uses the invention.
[0070] Fig. 4 shows an embodiment of a drug capsule that can be used with
the above
and other embodiments of the invention.
[0071] Fig. 5 shows the improvement in deformation after an applied load
of one
preferred material used in the invention vs. PTFE, modified by the inclusion
of less than
1% PPVE.
[0072] Fig. 6 shows the reduced deformation under load at elevated
temperature of a
preferred material used in the invention vs. PTFE.
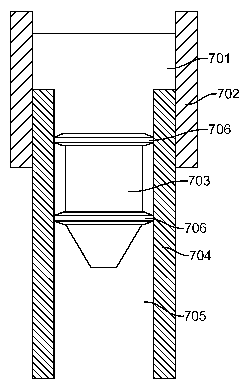
[0073] Fig. 7 shows a schematic of the apparatus used to test the
integrity of the drug
cartridge via dye ingress.
[0074] Fig. 8 shows the results of temperature cycling with a PTFE piston
previously
used in an injector.
[0075] Fig. 9 show the results of a measurement of piston movement during
temperature cycling utilizing a glass filled PTFE piston previously evaluated
for use
in an injector.
[0076] Fig. 10 shows the results of temperature cycling with a modified
PTFE piston
used in the invention, where the PTFE has been modified by the inclusion of
less than 1%
PPVE by weight.
[0077] Fig. 11 shows the results of temperature cycling with a modified
PTFE piston,
modified with the inclusion of less PPVE than that shown in figure 9.
18
CA 02866168 2014-09-02
WO 2013/163088 PCT/US2013/037597
Atty. Docket: ZGNX-128W0
DETAILED DESCRIPTION OF THE INVENTION
[0078] Before the present formulations and methods are described, it is
to be understood
that this invention is not limited to particular devices, components,
formulations and
methods described, as such may, of course, vary. It is also to be understood
that the
terminology used herein is for the purpose of describing particular
embodiments only,
and is not intended to be limiting, since the scope of the present invention
will be limited
only by the appended claims.
[0079] Where a range of values is provided, it is understood that each
intervening value,
to the tenth of the unit of the lower limit unless the context clearly
dictates otherwise,
between the upper and lower limits of that range is also specifically
disclosed. Each
smaller range between any stated value or intervening value in a stated range
and any
other stated or intervening value in that stated range is encompassed within
the invention.
The upper and lower limits of these smaller ranges may independently be
included or
excluded in the range, and each range where either, neither or both limits are
included in
the smaller ranges is also encompassed within the invention, subject to any
specifically
excluded limit in the stated range. Where the stated range includes one or
both of the
limits, ranges excluding either or both of those included limits are also
included in the
invention.
[0080] Unless defined otherwise, all technical and scientific terms used
herein have the
same meaning as commonly understood by one of ordinary skill in the art to
which this
invention belongs. Although any methods and materials similar or equivalent to
those
described herein can be used in the practice or testing of the present
invention, the
preferred methods and materials are now described. All publications mentioned
herein
are incorporated herein by reference to disclose and describe the methods
and/or
materials in connection with which the publications are cited.
[0081] It must be noted that as used herein and in the appended claims,
the singular
forms "a", "an", and "the" include plural referents unless the context clearly
dictates
otherwise. Thus, for example, reference to "a formulation" includes a
plurality of such
formulations and reference to "the method" includes reference to one or more
methods
and equivalents thereof known to those skilled in the art, and so forth.
[0082] The publications discussed herein are provided solely for their
disclosure prior to
the filing date of the present application. Nothing herein is to be construed
as an
admission that the present invention is not entitled to antedate such
publication by virtue
19
CA 02866168 2014-09-02
WO 2013/163088 PCT/US2013/037597
Atty. Docket: ZGNX-128W0
of prior invention. Further, the dates of publication provided may be
different from the
actual publication dates which may need to be independently confirmed.
DEFINITIONS
[0083] Active Pharmaceutical Ingredient, API, active drug substance,
medicament,
or the like: A component of a pharmaceutical formulation that is
pharmaceutically
active and is delivered for a desired effect.
[0084] Actuator: A mechanical device for moving or controlling a
mechanism or
system. An example of an actuator is a lever that a patient uses to ready an
autoinjector for delivery. Alternatively, an actuator can refer to the
mechanical
portion of an drug delivery device that optionally includes a safety that must
be set
prior to delivery, triggers the device, and ensures the proper pressure
profile during
delivery. The device may be triggered by many means, such as by pressing a
button, pressing the device against a desired injection site, inhaling through
the
device, etc.
[0085] Aggregation: formation of linked molecules held together by Van
der Waals
forces or chemical bonds.
[0086] AUC: Area under the curve, or the integral, of the plasma
concentration of
delivered drug over time.
[0087] Auto-injector: a drug delivery system which is an injector,
wherein the
important parameters of the dosing, including but not limited to the dose
delivered,
the rate of delivery, the formulation pressure or pressure profile, the
duration of the
delivery, the depth of delivery, are controlled automatically by the device
without
any input from the user during the delivery event. In some cases the user may
program certain parameters, such as the dose, into the device prior to
delivery.
Autoinjectors may be electronically controlled or all mechanical. They may be
prefilled, or be filled with formulation by the user prior to the delivery
event.
Autoinjectors are preferably portable. They may have an external power source
such as mains power, but preferably have a self contained power source.
Autoinjectors, especially electronic autoinjectors may have additional
features such
as dosing reminders, compliance monitors, time and date stamps for dosing
events,
and may include a wired or wireless means of downloading these data. A
particularly preferred autoinjector is a portable, self contained, prefilled,
single dose
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
disposable, all mechanical needle free injector comprising a pressurized gas
power
source and a drug capsule comprising borosilicate glass and a modified PTFE
piston.
[0088] Biodegradable: capable of chemically breaking down or degrading
within
the body to form nontoxic components. The rate of degradation of a depot can
be
the same or different from the rate of drug release.
[0089] Biologic: A medicinal products created by biological processes (as
opposed
to chemically). Examples include such as vaccines, blood and blood components,
allergenics,l 1] somatic cells, gene therapy, tissues, stem cells, immune
globulins,
and recombinant therapeutic proteins Biologics may be isolated from natural
sources such as humans, animals, plants, or microorganism - or may be produced
by biotechnology methods.
[0090] Borosilicate glass: a type of glass comprising silica and boron
that is
commonly used in chemical and medical applications. Borosilicate glass has a
very
low coefficient of expansion (-3 x 10-6) making is less susceptible to
breakage
when exposed to heat, for example when heat sterilized.
[0091] Bulk erosion :The rate of water penetration into the depot exceeds
the rate at
which the depot is eroded (i.e. transformed into water soluble
products)¨leading to
an erosion process that occurs throughout the entire volume of the depot---
true with
most hydrophilic polymers used in drug delivery currently.
[0092] Carrier: a non-active portion of a formulation which may be a
liquid and
which may act as a solvent for the formulation, or wherein the formulation is
suspended. Useful carriers do not adversely interact with the active
pharmaceutical
ingredient and have properties which allow for delivery, for example needle
free
injection. Preferred carriers include water, saline, and mixtures thereof.
Other
carriers can be used provided that they can be formulated to create a suitable
solution and do not adversely affect the drug thereof or human tissue.
[0093] Centipoise and centistokes: different measurements of viscosity,
which are
not just different units. Centipoise is a dynamic measurement of viscosity
whereas
centistokes is a kinematic measurement of viscosity. The conversion from
centistokes and centipoise to s.i. units is given below:
lcS = 0.0001m2/s 1cP = 0.001Ns/m2
21
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
[0094] Coefficient of Thermal Expansion, Thermal Expansion Coefficient,
CTE,
and the like: The fractional change in a dimension of a material (AL/L), per
degree
C.
[0095] Coefficient of Friction: a constant of proportionality relating
the normal
force between two materials, and the frictional force between those materials.
Generally friction is considered to be independent of other factors, such as
the area
of contact. The coefficient of static friction characterizes the frictional
force
between to materials when at rest. This force is generally what is required to
start
relative movement. The coefficient of dynamic friction characterizes the
frictional
force between to materials that are moving relative to one another. In
general, the
coefficient of static friction is higher than the coefficient of dynamic
friction.
[0096] Container Closure, Container Closure System, and the like: A drug
container that is designed to maintain sterility and eliminate the possibility
of
contamination of the drug formulation. For container closure systems that
contain
aqueous formulations, the container closure system must have sufficiently low
water vapor transmission rate such that the concentration of the formulation
does
not change appreciably over the product shelf life. Preferred materials have
sufficiently low extractable materials such that they do not contaminate the
formulation. For multi component container closure systems, the interface(s)
between the components must be such that liquid carriers, contaminants,
including
but not limited to microbial and viral contaminants, and gasses such as air
cannot
appreciably pass through over the shelf life of the system and over the
expected
temperature range. Container closure system materials that are in contact with
the
drug formulation must have properties such said contact does not lead to
unacceptable levels of degradation of the drug formulation. Preferred
materials for
container closures include glass, more preferably borosilicate glass, or
fluorinated
polymers such as polytetrafluoroethylene (PTEE), including modified PTFEs,
preferably modified by the inclusion of a PPVE copolymer, more preferably by
the
inclusion of PPVE in an amount less than I% by weight.
[0097] Container Closure Integrity: The ability of a container closure
system to
maintain sterility, eliminate the possibility of contamination, and minimize
loss of
carrier during storage.
22
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
[0098] Deformation Under Load, Creep, Cold Flow, and the like: Changes in
the
dimensional properties of a material, especially a polymer, when placed under
a
load. The load may be externally applied, as when the piston of the current
invention is inserted into the glass drug capsule, and may be increased by
subjecting the drug formulation container of the current invention to elevated
temperatures.
[0099] Depot Injection, Depot, and the like: an injection, usually
subcutaneous,
intravenous, or intramuscular, of a pharmacological agent which releases its
active
compound in a consistent way over a long period of time. Depot injections may
be
available as certain forms of a drug, such as decanoate salts or esters.
Examples of
depot injections include Depo Provera and haloperidol decanoate. Depots can
be,
but are not always, localized in one spot in the body.
[00100] DosePro or Intraject: a single use, prefilled, disposable, needle
free injector
currently manufactured by Zogenix Corporation. A cartridge is pre-filled with
a
liquid to be injected in a subject, and having a liquid outlet and a free
piston in
contact with the liquid, the actuator comprising an impact member urged by a
compressed gas spring and temporarily restrained until the device is actuated,
the
impact member being movable in a first direction under the force of the spring
to
first strike the free piston and then to continue to move the piston in the
first
direction to expel a dose of liquid through the liquid outlet, the spring
providing a
built-in energy store and being adapted to move from a higher energy state to
a
lower energy state, but not vice versa. The actuator may comprise a trigger
means
to actuate the device, and thus initiate the injection, only when the device
is pressed
against the skin. Elements of DosePro are described in U.S. Patent No.
5,891,086,
and additional description and improvements can be found in US6620135,
U56554818, U56415631, U56409032, U56280410, U56258059, U56251091,
U56216493, U56179583, U56174304, U56149625, U56135979, U55957886,
US5891086, and U55480381, incorporated herein by reference. Although many
delivery systems and techniques may be used with the current invention,
DosePro is
the preferred method.
[00101] Drug Cartridge, Drug Capsule, and the like: a container closure
system
utilized in an drug delivery system, and is preferably prefilled and
disposable.. In a
preferred embodiment, the Drug Capsule comprises a glass container, preferably
a
23
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
borosilicate glass container, which forms a syringe body, and is closed on one
end
by a modified PTFE piston. The glass container comprises at least one delivery
orifice, preferably opposite the piston, which is sealed, for example by an
end cap,
prior to preparation for use. Preferably the glass container is contained in a
polymeric sleeve, which comprises a feature such as screw threads for
attachment
to an actuator. The glass container may be strengthened to avoid breakage upon
actuation by ion exchange strengthening. The drug capsule may contain multiple
doses, or preferably contains a single dose and is disposed of after a
delivery event.
[00102] Drug Delivery System, Drug Delivery Device, and the like: a system
for
delivery of a formulation to an animal or preferably a human subject.
Preferred
drug delivery systems include a prefilled drug capsule which functions as a
container closure system and also comprises a piston and syringe body to
deliver
the formulation from the drug capsule, either directly to the subject, or to
an
additional component or subsystem that delivers the formulation. The drug
capsule
may be disposed of and replaced after the drug is exhausted, or preferably
permanently integrated with the actuator, whereby the entire drug delivery
system
is disposed of after the drug is exhausted. Drug delivery systems may be
parenteral, transdermal, pulmonary, buccal, enteral, oral, ocular, vaginal, or
deliver
by any other route of delivery. Preferred drug delivery systems are prefilled
syringes, pumps, or auto-injectors, most preferably the drug delivery system
is a
needle free injector, preferably a portable, self contained, prefilled, single
use
disposable needle free injector.
[00103] Dye Ingress, Dye Penetration, and the like: A test of
container/closure
integrity, wherein the drug capsule of the current invention is exposed to a
dye, and
then inspected to see if any of the dye has penetrated to the liquid
formulation.
Figure 6 shows schematically a dye ingress apparatus. This test is preferably
performed after temperature cycling in an environmental chamber, wherein the
temperature of the drug capsule is cycled up and down in a predetermined
manner
(see "thermal cycling")
[00104] Excipient: Any substance, including a carrier, added to an active
drug
substance to permit the mixture to achieve the appropriate physical
characteristics
necessary for effective delivery of the active drug.
24
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
[00105] Formulation: Any liquid, solid, powder, or other state of matter
that can be
delivered from a drug delivery device. Preferred formulations are liquid
formulations, including but not limited to solutions, suspensions including
nano-
suspensions, emulsions, polymers and gels. Formulations include but are not
limited to those containing excipients that are suitable for administration to
a
human, and contain one or more active pharmaceutical ingredients.
[00106] Immunogenicity: Immunogenicity is the ability of a substance (an
antigen)
to provoke an immune response. Aggregated biologic drugs can be immunogenic
even when the unaggregated molecule is not immunogenic.
[00107] Needle free Injector, Needle-less injector, and the like: a drug
delivery
system delivers a subcutaneous, intramuscular, or intradermal injection
without the
use of a hypodermic needle. Injection is achieved by creating at least one
high
velocity liquid jet with sufficient velocity to penetrate the skin, stratum
subcutaneum, or muscle to the desired depth. Needle free injection systems
include, but are not limited to, the DosePro system manufactured by Zogenix
Corporation, the Bioject 2000, Iject or Vitaject devices manufactured by
Bioject
Medical Technologies, Incorporated, the Mediject VISION and Mediject VALE
devices manufactured by Antares, the PenJet device manufactured by Visionary
Medical, the CrossJect device manufactured by Crossject, the MiniJect device
manufactured by Biovalve, the Implaject device manufactured by Caretek
Medical,
the PowderJect device manufactured by AlgoRx, the J-tip device manufactured by
National Medical Products, the AdvantaJet manufactured by Activa Systems, the
Injex 30 device manufactured by Injex-Equidyne, and the Mhi-500 device
manufactured by Medical House Products.
[00108] Perfluoropropyl Vinyl Ether, PPVE, and the like: a polymer used in
the
manufacture of fluoropolymers and other specialty agrochemical and
pharmaceutical applications. In the context of the present invention, PPVE is
used
to modify PTFE to improve its properties for use in injection pistons.
Preferably,
the PTFE is modified by the inclusion of less than I% PPVE by weight.
[00109] Polytetrafluoroethylene, PTFE, Teflon, and the like: a synthetic
fluoropolymer of tetrafluoroethylene. PTFE is most well known by the DuPont
brand name Teflon. PTFE is a high molecular weight fluorocarbon solid,
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
consisting wholly of carbon and fluorine. PTFE has one of the lowest
coefficients
of friction against any solid.
[00110] Portable: easily carried by a person, possibly by hand or in a
back pack, but
preferably in a purse, pocket or the like. A portable drug delivery device had
a
longest dimension which is less than 30 cm, preferably less than 25 cm, more
preferably less than 20 cm, most preferably less than or about 15 cm. Portable
drug delivery devices are preferably self contained.
[00111] Prefilled: Filled with formulation prior to being received by the
end user,
i.e. patient or care giver. A drug capsule can be prefilled at a pharmacy, but
preferably will be prefilled at a factory prior to being packaged and shipped.
Prefilled capsules will require testing to demonstrate they will be able to
maintain
stability and sterility of the drug formulation over the shelf life, and over
the range
of storage conditions, especially temperature, that are expected during
storage and
use. In general, prefilled drug capsules will require testing at elevated
termperatures, and temperature cycling.
[00112] Prophylaxis: The administration of a drug used to prevent the
occurrence or
development of an adverse condition or medical disorder.
[00113] Self Contained: Including all of the components and functionality
required
to effect drug delivery. A self contained drug delivery system may be a kit
which
comprises an actuator and one or a multiplicity of replaceable, prefilled drug
capsules, but will not require any additional components. A self contained
drug
delivery system comprises an energy source, such as a battery, mechanical
spring,
compressed gas source, chemical reaction, or the like. The energy source may
contain enough energy for a multiplicity of drug delivery events, and when
exhausted may be replaced, recharged, or the entire device may be disposed of.
The energy source may also be energized by the user or care giver prior to
delivery,
for example a mechanical spring that is compressed but do not require the user
to
input energy during the delivery event. Preferably, a self contained drug
delivery
device contains sufficient energy for a single drug delivery event, after
which is
cannot be re-used, and must be disposed of. Self contained drug delivery
systems
do not require the use of mains power during the delivery event, although they
may
comprise rechargeable batteries that recharged using mains power prior to the
drug
delivery event.
26
CA 02866168 2014-09-02
WO 2013/163088 PCT/US2013/037597
Atty. Docket: ZGNX-128W0
[00114] Surface Erosion: The rate of water penetration into the depot is
slower than
the rate at which the depot is eroded. The depot erodes from the surface
before
water has penetrated the entire volume of the device.
[00115] Specific gravity: ratio of a compound's density to that of water.
[00116] Spring: a mechanism capable of storing energy for use in
propelling the
medicament in the syringe out of the drug capsule, through an optional drug
delivery
component or sub assembly, and into or onto a body, wherein the force provided
by the
energy store is proportional to a displacement. This mechanism may be
mechanical, e.g.
compressible metal component such as a coil spring or Belleville washer stack.
Preferably, the mechanism is a compressed gas spring in which the energy is
stored, and
when released the gas expands.
[00117] Strain: the deformation of a body, especially the piston of the
current invention,
when subjected to an external load. Deformation can be elastic, wherein the
body returns
to its previous configuration after the external load is removed. It can also
be inelastic,
wherein the body is permanently changed by the load.
[00118] Stress, load, and the like: An applied force or pressure that
tends to deform a
body, especially the piston of the current invention. See also Strain.
[00119] Modified PTFE: PTFE that has been modified to improve its
performance, for
example when used as a material for injection pistons. Preferably, the PTFE is
modified
by the inclusion of a perfluoropropyl vinyl ether (PPVE) modifier, more
preferably by the
inclusion of less than 1% by weight of PPVE. PTFE modified in this way it has
lower
(<1/3) deformation under load than un-modified PTFE under similar conditions
of load
and temperature.
[00120] Thermal Cycling, Temperature Cycling, and the like: a method of
testing
properties of a drug delivery system, and specifically the container/closure
integrity of
the drug capsule, of the current invention wherein the object under test is
placed in an
environmental chamber and exposed to a prespecified set of temperatures that
change
over time in a prespecified way. In one embodiment of the test, the inside
diameter of the
glass capsules and the outside diameter of the pistons are measured, the
capsules are
assembled and are filled with normal saline, placed in an environmental
chamber nozzle
down and cycled between 40 C and 2 C for 12 hours at each temperature for 30
days (i.e.
30 cycles). Movement of the piston relative to the glass capsule is measured
at
27
CA 02866168 2014-09-02
WO 2013/163088 PCT/US2013/037597
Atty. Docket: ZGNX-128W0
prespecified intervals. At the end of the test, the capsules are exposed to a
dye (see "Dye
Ingress" and Figure 6), and checked for leakage.
[00121] Water Vapor Transmission Rate (WVTR)) is the steady state rate at
which water
vapor permeates through a material or out of a drug capsule. Values are
expressed in
g/100 in2/24 hr in US standard units and g/m2/24 hr in metric units.
INVENTION IN GENERAL
[00122] In general, the container closure system, or drug capsule of a
drug delivery device
comprises a cylinder, preferably a right circular cylinder, which forms a
syringe body.
The syringe body generally comprises one or more outlet orifices. The syringe
body is
closed on one end by a stopper, which preferably during delivery acts as a
piston. The
outlet orifice either delivers the drug directly, as when it is a needle free
injector injection
orifice or an aerosolization nozzle, or it may lead to an additional drug
delivery
component or sub-system, such as a needle, infusion set, or the like. During
storage the
outlet orifice(s), or the additional component or subsystem, are sealed by a
valve, stopper,
end cap or the like.. Upon triggering of the drug delivery device, the piston
slides down
the barrel of the cylinder and forces the formulation out of the exit orifice.
It is thus
required that the friction between the piston and syringe body be sufficiently
low such
that the available force is sufficient to achieve delivery. To achieve this,
lubricant can be
used. However, this lubricant will be in contact with the drug formulation,
and can have
adverse impact on the stability of the formulation. For example, most standard
needle
and syringe injectors have a rubber stopper lubricated with oil, such as
silicone oil, which
can lead to issues such as aggregation of protein drugs and other biologics,
potentially
causing immunogenicity. Thus it is preferred that the piston be made of a
material that is
sufficiently lubricious that no additional lubricant is required.
[00123] One particularly preferred compound for use in a piston is
Polytetrafluoroethylene, or PTFE. PTFE is an excellent material for drug
formulation
contact, as it is very non-reactive, partly because of the strength of
carbon¨fluorine
bonds. PTFE is also very lubricious, having one of the lowest coefficients of
friction
against most solids. In general, the use of PTFE for a piston obviates the
need for a
separate lubricant.
[00124] Although plastics, for example polycycloolefin, are used for some
syringe bodies,
including prefilled injectors, the gold standard material for syringe bodies
and other drug
28
CA 02866168 2014-09-02
WO 2013/163088 PCT/US2013/037597
Atty. Docket: ZGNX-128W0
contact surfaces is glass, more preferably borosilicate glass. However, it is
problem that
glass and PTFE have significantly different coefficients of thermal expansion,
with PTFE
having a fairly high thermal expansion coefficient of approximately 10-16 * 10-
5 /deg C. ,
and borosilicate glass having a much lower coefficient, 0.5 * 10-5 /deg C.
This
difference in expansion can lead to loss of container closure integrity upon a
reduction in
temperature. For example, a 10 degree reduction in temperature would lead to a
10 pm
difference in contraction for a 1 cm PTFE piston in a borosilicate glass
syringe body.
Depending on the amount of preload on the PTFE when it is forced into the
syringe body
and the amount of creep of the PTFE during storage, this differential thermal
expansion
could lead to as much as a 5 p.m gap around the piston, leading to a loss of
container
closure integrity and potentially leading to loss of sterility, contamination,
and/or
evaporation of carrier. This problem can be exacerbated if prior to being
exposed to low
temperature, the drug cartridge is exposed to elevated temperature, for
example 40 C
which is often used in accelerated stability and temperature cycling studies.
Exposure of
the piston to elevated temperature causes it to want to expand. Because it is
constrained
by the syringe body, this can cause the piston to yield or creep, leading to a
smaller
effective outside diameter. When subsequently exposed to a reduced
temperature, there is
a much larger likelihood of loss of container closure integrity.
[00125] PTFE can be modified to improve its properties for use in pistons
for drug
delivery systems. Preferably, the modified PTFEs are Tetrafluoroethylene-
Perfluoro(Propyl Vinyl Ether) (PPVE) copolymers, comprising less than 1% PPVE
by
weight. PTFE modified by the inclusion of PPVE have many properties that make
them
well suited for injection drug delivery piston, including low deformation
under load (see
figure 5), especially at elevated temperatures (see figure 6), low coefficient
of friction ( ,
= 0.2), low extractables and leachables, high tensile strength (-40 MPa), wide
temperature range (-200 to 260 C), low permeation, no water absorption,
almost
universal chemical resistance, good light and weathering resistance, and high
purity.
[00126] In the needle free injector embodiment of FIG. 1, the injection
force is provided
by a compressed gas spring. This is in the form of a cylinder 130 which is
closed at its
upper end and which contains gas, typically air, under a pressure which is
typically in the
range 5.5 MPa (800 psi) to 20.7 MPa (3000 psi). The cylinder houses a ram 111.
The end
of the ram 111 has a frusto-conical portion 131 and a flange 132 between which
is
29
CA 02866168 2014-09-02
WO 2013/163088 PCT/US2013/037597
Atty. Docket: ZGNX-128W0
situated an 0-ring seal 133. Prior to use, the ram 111 is held in the
illustrated position by
a latch 108 engaging in a groove in the ram, the upper surface of the groove
forming a
cam surface 109. The latch 108 is shown on a larger scale in FIG. 2a. In the
position
shown in FIG. 1 the latch is unable to move leftwards, because it bears
against the inner
wall of a sleeve 102.
[00127] The lower end of the cylinder 130 has an outwardly directed flange
130a, which
enables the cylinder to be held by crimping the flange 130a beneath an
outwardly
directed flange 140a at the upper end of a coupling 140. The sleeve 102 is
formed of an
upper sleeve portion 102a within which the clinder is situated, and a lower
sleeve portion
102b. The sleeve portion 102b is connected to the coupling by the inter-
engaging screw
threads 141 formed on the inner and outer walls of the sleeve portion 102b and
coupling
140 respectively.
[00128] The injector contains a drug capsule 103 which is preferably
glass, more
preferably borosilicate glass. drug capsule 103 has a piston 104 slidingly and
sealingly
located therein, in contact with medicament 105. The properties of piston 104
must be
consistent with contact with the formulation 105 over the shelf life of the
device, and
must ensure stability and sterility of formulation 105 by maintaining a seal
over the shelf
life and over all temperatures to be seen during storage and during testing.
PTFE is a
preferred material for piston 104, more preferably a modified PTFE, more
preferably
PTFE modified by the addition of Perfluoro (Propyl Vinyl Ether) (PPVE)
copolymer,
most preferably in an amount less than 1%. As considered from the upper end of
FIG. 1,
piston 104 may comprise a cylindrical portion encircled by a larger diameter
sealing
portion 146, more preferably with two larger diameter sealing features 146.
Larger
diameter sealing features 146 function to create the required compression that
will
maintain sealing over the life of the device without creating too high an
insertion force
when piston 104 is inserted into glass cartridge 103. Piston 104 further
comprises a
frusto-conical portion, designed to mate with the lower end of drug capsule
103 at the end
of delivery to ensure that essentially all medicament is delivered. The drug
capsule 103
has a discharge orifice 106. The orifice 106 is sealed by a resilient seal 134
which is held
in place by a seal carrier 135. The seal carrier 135 is connected to the lower
sleeve
portion 102b by a frangible joint 136.
[00129] As a precaution against accidental firing, a removable blocking
element 137 is
provided between the lower part of the upper sleeve portion 102a. The lower
edge of
CA 02866168 2014-09-02
WO 2013/163088 PCT/US2013/037597
Atty. Docket: ZGNX-128W0
blocking element 137 bears against lower sleeve portion102a. The function of
blocking
element 137 is to inhibit relative movement of the upper and lower sections,
and thus
inhibit triggering of the device, until blocking element 137 is removed.
Blocking element
137 may be a tear off band, but is preferably a separate element that is
removed by radial
displacement.
[00130] An annular space 138 is formed in the inside wall of the sleeve
102, where the
sleeve is adjacent the cylinder 130, and the space is filled with a damping
grease
(indicated diagrammatically by a succession of black bands), so that the
grease is in
intimate contact both with the sleeve 102 and the cylinder 130. It should be
noted that
although a defined annular space is convenient from the point of view of
providing a
particular location for the grease, it could be omitted and the grease simply
smeared over
all or part of the outside of cylinder 130 and/or inside of sleeve 102.
[00131] When the embodiment of FIG. 1 is to be operated, the user snaps
off seal carrier
135 at frangible joint 136, which takes seal 134 with it and exposes orifice
106. The user
then removes blocking element 137, and grasping the upper part of sleeve 102
urges the
orifice against the substrate (e.g. the user's own skin) which is to be
injected. This moves
upper sleeve portion 102a downwardly, with respect to lower sleeve portion
102b. This
brings aperture 139 in the wall of upper sleeve portion 102a into alignment
with latch
108, which is thus able to move sideways into aperture 139 under the influence
of the
force of the gas within cylinder 130 acting on latch 108 via cam surface 109
formed in
ram 111. The injector is thus caused to fire. The resulting recoil is damped
by the
damping grease.
[00132] FIG. 2 illustrates an embodiment of the needle-free injector with
setting means
30 for disengaging the blocking element 38. In this figure, the means for
disengaging the
blocking element 38 comprises cap 31 enclosing, and holding rigidly, seal
carrier 20;
lever 32; and collar 33. The lever contains lip 34 at the far end, over which
cap 31 is
positioned. This ensures that lever 32 cannot be moved before the outer cap 31
is
removed, which in turn ensures that the user cannot move the latch or
disengage the
safety mechanism until the cap has been removed. This is important because if
blocking
element 38 can be removed before removing cap 31, as is possible in the
embodiment
shown in figure 1, the act of removing cap 31 can cause the device to fire.
Lever 32 is
pivoted around pivot axis 35, with the pivoted surface in contact with
injector being a
cam surface 36. The force required to pivot lever 32 is in the range from
about 2N to
31
CA 02866168 2014-09-02
WO 2013/163088 PCT/US2013/037597
Atty. Docket: ZGNX-128W0
about 30N. Collar 33 contains pin 37 which extends into the device through
opening 28
in upper sleeve 12 to impinge on the far side of latch 6. The force required
to move latch
6 is in the range from about 20N to about 120N. To stop the upper sleeve
section 12
moving with respect to lower sleeve section 13, there is blocking element 38
between the
upper and lower sleeves, which form part of collar 33. Blocking element 38
takes the
place of the tear off band of the embodiment shown in figure 1.
[00133] To deliver the device contents, cap 31 is removed, exposing
injection orifice 18.
With outer cap 31 removed, lip 34 is exposed, enabling lever 32 to rotate
about the pivot
axis 35. Only when the outer cap 31 is removed can lever 32 be rotated. At
this point
latch 6 is on flat (non-camming) surface 27 of ram 2, as shown in figure 2a.
As lever 32
rotates, cam surface 36 forces collar 33 to move in the direction Q, pushing
pin 37 against
latch 6. When lever 32 has rotated through a complete cycle, approximately 180
degrees,
latch 6 moves to the second position, onto ram camming surface 7, as shown in
FIG. 2b.
Blocking element 38 no longer restricts the movement of upper sleeve 12 with
respect to
lower sleeve 13 and the device can trigger as described above.
[00134] Figure 3 shows another embodiment of the injector device. In this
embodiment,
the latch of the previous embodiments is replaced by a spool valve comprising
spool 16,
valve block 17, and spool retaining cage 15. The operation of this embodiment
is as
follows: The user removes cap 2, which also removes rubber seal 4 and spin cap
3. Spin
cap 3 is provided to ensure that the act of screwing cap 2 onto capsule sleeve
6 doesn't
create stresses in rubber seal 4, which can lead to loss of seal. Cap 2 is
threaded onto
both capsule sleeve 6 and case 1, ensuring that as cap 2 is removed capsule
sleeve 6 is
biased downward, preventing accidental actuation. Nozzle 20 is then pressed
against the
desired injection site. This causes the internal components to move upward
relative to
case 1, sliding body 14, and spool retaining cage 15. When the motion is
sufficient, spool
16 is forced into spool retaining cage 15 by the pressure of the gas in gas
cylinder 18,
allowing the gas to pressurize ram head 11, and the injection proceeds as
above.
[00135] All of the embodiments in figures 1 ¨ 3 have in common a drug
capsule like that
shown in figure 4, which can be used with many types of drug delivery systems.
The
drug capsule comprises a syringe body 5 that is preferably comprised of glass,
more
preferably comprised of borosilicate glass. Syringe body 5 is contained within
capsule
sleeve 6. Syringe body 5 is sealed on one end by piston 7, forming a reservoir
for drug
formulation 19 which is preferably a liquid drug. Piston 7 comprises larger
diameter
32
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
sealing ribs 22. At the opposite end of capsule 5 from piston 7 is outlet
orifice 20, which
forms the liquid injection jet in the case of needle free injection, can be an
aerosolization
nozzle in the emobodiment where the drug delivery system is a aerosol drug
delivery
system, or may lead to an additional drug delivery component or sub-assembly
such as a
needle, infusion set, transdermal technology, or the like. A single outlet
orifice is shown
in Figure 4, but the capsule may comprise 2, 3, 4, or more outlet orifices. In
the case of
the outlet orifice being aerosolization nozzle, the system may comprise more
than 100 or
more than 1000 outlet orifices. Prior to injection, injection orifice 20 is
closed by a seal
(not shown). Threads 21 are provided to facilitate attachment to an actuator,
such as
those disclosed in figures 1 ¨ 3 or similar systems appropriate to the rate
and force
required for other delivery methodologies. Sealing ribs 22 function to create
the required
compression that will maintain sealing over the life of the drug capsule and
the
temperatures the drug capsule will be exposed to during storage,
sterilization, and/or
testing, without creating too high of an insertion force when piston 104 is
inserted into
glass syringe body 5. Sealing ribs 22 have a triangular shape, or preferably a
triangular
shape with the vertex in contact with the syringe body 5 flattened or
truncated to form a
frustum. This shape serves to focus the stress into the contact zone with
syringe body 5,
enabling sealing ribs 22 to maintain the contact pressure at the interface
with syringe
body 5 while maintaining a lower shear stress in the surrounding material. The
high
stress contact area is encapsulated by the surrounding material of sealing
ribs 22 at a
lower stress as the distance from syringe body 5 increases, creating
essentially
compressive stress at the contact region, making this region not subject to
creep.
[00136] Use of a
prefilled drug delivery device, has many benefits over non prefilled
devices such as a standard needle and syringe, including:
= No need to draw formulation into the drug capsule prior to use
= Fewer steps
= Simpler instructions
= Minimal amount of equipment required (especially important for acute
indications
wherein the injection system must be carried around by the user.)
= Fast administration
= Improved patient compliance
= Improved disease outcomes.
33
CA 02866168 2014-09-02
WO 2013/163088 PCT/US2013/037597
Atty. Docket: ZGNX-128W0
[00137] Self contained drug delivery devices systemsare preferred as the
energy for the
delivery comes from the device rather than the patient or caregiver that is
administering
the medication. This can be very important, for example, in the delivery of
high viscosity
formulations that require high hand strength and long delivery times with a
standard
needle and syringe.
[00138] Prefilled drug delivery systems are preferred as they require
fewer or no steps to
prepare the device for delivery. This can be very important in the case of
self
administration or administration by an un-skilled care giver such as a family
member.
This can also be very important for acute episodes that require rapid
intervention, such as
migraine and other pain, anaphylaxis, seizure, and the like.
[00139] Portable drug delivery devices are preferred, as they can be
carried by the user or
care giver and be available when treatment is required. This feature can be
very
important for acute episodes that require rapid intervention, such as migraine
and other
pain, anaphylaxis, seizure, and the like.
[00140] Prefilled portable drug delivery systems, Prefilled self contained
drug delivery
systems, and portable, self contained drug delivery systems are particularly
preferred.
The most preferred drug delivery systems are prefilled, portable, and self
contained.
These systems are the most likely to have the best outcomes for a wide range
of
conditions, due to being easy to use, requiring miminal training, being small
and discrete,
being readily available when needed, requiring miminal steps for preparation
and
delivery, and reducing the amount of time skill required of a care giver. All
of these
features reduce time and cost of therapy, increase compliance, and increase
positive
outcomes.
[00141] A preferred embodiment of the drug delivery system is an
autoinjector. Injection
is preferred because of high bioavailability, reproducibility, ability to
control and titrate
dose, and rapid onset. Most pharmaceutically acceptable compounds can be
injected,
preferably in liquid form, although injection of solids and liquids is also
known in the art.
[00142] A preferred embodiment of the autoinjector is the needle free
injector. Needle
free injectors are preferred because of:
= No danger of needle stick injury and related exposure to disease
= No needle phobia
34
CA 02866168 2014-09-02
WO 2013/163088 PCT/US2013/037597
Atty. Docket: ZGNX-128W0
= Small diameter liquid jets result in little or no pain sensation
= No requirement for sharps disposal
= Very short flow path (as compared to a hypodermic needle) reduces viscous
losses and enables delivery of high viscosity formulations.
[00143] Autoinjectors including needle free injectors can deliver any
injection including
intradermal, subcutaneous, intravenous, or intramuscular injections.
Preferably, for the
embodiment where the drug delivery system is an autoinjector, the injection is
a sub-
cutaneous injection.
[00144] In the most preferred embodiment, the drug delivery system is a
prefilled, single
dose, disposable, self contained, portable needle free injector comprising a
borosilicate
glass piston strengthened with ion exchange with a single injection orifice
and a PTFE
piston modified by the inclusion of less than 1% of PPVE and comprising two
sealing
ribs with the cross sectional shape of a frustrum.
[00145] Prefilled drug capsules must maintain container closure integrity
over the labeled
shelf life of the system. Preferred shelf lives include 1 year, preferably
greater than one
year, more preferably 2 years or more, most preferably 3 years or more.
Container
closure integrity must be maintained over the range of allowed storage
temperatures,
testing temperatures, and after sterilization of the components or terminal
sterilization of
the drug capsule. Storage, sterilization, and testing temperatures are
preferably 15 to 30
degrees C, more preferably 2 ¨ 40 degrees C, most preferably -10-50 degrees C,
may be
always above -10, 0, 2, 5, 10, 15, or 20 degrees C, and may be always below
100, 85, 75,
60, 50, 40, 30, or 25 degrees C.
[00146] Figure 5 shows the results of a test of deformation of piston
materials comparing
PTFE to a PTFE modified by the inclusion of less than 1% by weight PPVE. After
a 24
hours recovery from a 15 MPa load applied for 100 hours, it can be seen that
the
modified PTFE had significantly less deformation, 4% vs. 11% for the un-
modified
PTFE.
[00147] Figure 6 shows how the resistance of PTFE modified with less than
1% PPVE to
deformation under load is also seen at elevated temperatures.
[00148] Figure 7 shows schematically the apparatus used for dye ingress
tests. Dye
container 602 is placed sealingly about capsule 604, and is filled with dye
601. Liquid
605, usually normal saline, is contained within capsule 604. Piston 603 seals
liquid 605
CA 02866168 2014-09-02
WO 2013/163088 PCT/US2013/037597
Atty. Docket: ZGNX-128W0
into capsule 604. Dye ingress is observed when the dye is seen to traverse one
or both of
the ribs of piston 603.
EXAMPLES
[00149] The following examples are put forth so as to provide those of
ordinary skill in
the art with a complete disclosure and description of how to make and use the
present
invention, and are not intended to limit the scope of what the inventors
regard as their
invention nor are they intended to represent that the experiments below are
all or the only
experiments performed. Efforts have been made to ensure accuracy with respect
to
numbers used (e.g., amounts, temperature, etc.) but some experimental errors
and
deviations should be accounted for. Unless indicated otherwise, parts are
parts by
weight, molecular weight is weight average molecular weight, temperature is in
degrees
Centigrade, and pressure is at or near atmospheric.
EXAMPLE 1
[00150] Drug capsules were constructed using borosilicate glass syringe
bodies, and
unmodified PTFE pistons. Before assembly the inside diameter of the syringe
body
and outside diameter of the piston ribs were measured and recorded. Twenty
drug
capsules were assembled and filled with normal saline.
[00151] Water-filled drug capsules were placed in an incubator and
subjected to five
thermal cycles between 40 C and 2 C. The drug capsules were maintained for at
least 12 hours at each temperature extreme. Following the thermal cycling, the
pistons were subjected to a continuous dye ingress test for 24 hours at room
temperature (20 C).
[00152] The results of the test are shown in figure 8. Notably, 12 of the
drug
capsules exhibited leakage, suggesting these capsules would have difficulty
maintaining container closure integrity over the shelf life of the product.
EXAMPLE 2
[00153] 20 drug capsules containing pistons made from glass filled PTFE
were
subjected to a thermal cycling test wherein they were cycled between 40 C and
2 C
for 12 hours at each temperature for 30 days (i.e. 30 cycles). The piston
movement
was measured at regular intervals throughout the life cycle of the test. For
this test,
36
CA 02866168 2014-09-02
WO 2013/163088
PCT/US2013/037597
Atty. Docket: ZGNX-128W0
the maximum acceptable piston movement, based on previously determined
requirements, was 0.5 mm.
[00154] A graph of piston movement is shown in Figure 9. As can be seen
from this
figure, the maximum acceptable movement was reached at 20 cycles, and was
exceeded after 30 cycles.
EXAMPLE 3
[00155] Drug capsules were constructed using borosilicate glass syringe
bodies, and
modified PTFE pistons. The PTFE was modified by the introduction of less than
1% PPVE. Before assembly the inside diameter of the syringe body and outside
diameter of the two piston ribs were measured and recorded. Twenty five drug
capsules were assembled and filled with normal saline. The assembled drug
capsules were then placed in an environmental chamber, and subjected to a 34
temperature cycles. Each cycle lasted one day and consisted of 12 hours at 40
C,
followed by 12 hours at 2 C. After 8, 14, 20 and 34 cycles, the movement of
the
piston in the direction of the injection orifice was measured. Following the
last
cycle, the drug capsules were placed in a dye ingress apparatus (see figure 7)
and
tested for leakage.
[00156] The results of these tests are shown in figure 10. Notably, as can
be seen in
the last column of figure 10, none of these cartridges exhibited leakage,
leading to
the expectation that cartridges assembled with pistons fabricated from this
modified
PTFE will maintain container closure integrity over the shelf life of the
product.
With a single exception, movement of the pistons did not exceed 0.5 mm,
significantly better results than those seen with glass filled PTFE pistons,
see
example 2 above.
EXAMPLE 4
[00157] Drug cartridges were constructed using borosilicate glass syringe
bodies,
and modified PTFE pistons. The PTFE was modified by the inclusion of less than
1% PPVE, and differs from that presented in example 3 in that it had less PPVE
to
improve extrusion properties. Before assembly, the inside diameter of the
syringe
37
CA 02866168 2014-09-02
WO 2013/163088 PCT/US2013/037597
Atty. Docket: ZGNX-128W0
body and outside diameter of the two piston ribs were measured and recorded.
Twenty cartridges were assembled and filled with normal saline. The assembled
cartridges were then placed in an environmental chamber, and subjected to 29
temperature cycles. Each cycle lasted one day and consisted of 12 hours at 40
C,
followed by 12 hours at 2 C. After 1, 4, 8, 12, 15, 21, and 29 cycles, the
movement of the piston in the direction of the nozzle was measured. Following
the
last cycle, the cartridges were placed in a dye ingress apparatus (see figure
7) and
tested for leakage.
[00158] The results of these tests are shown in figure 11. Notably, as can
be seen in
the last column of figure 11, none of these cartridges exhibited leakage,
leading to
the expectation that cartridges assembled with pistons with this modified PTFE
will
maintain container closure integrity over the shelf life of the product. Again
with
only a single exception, movement of the pistons did not exceed 0.5mm.
[00159] The instant invention is shown and described herein in a manner
which is
considered to be the most practical and preferred embodiments. It is
recognized,
however, that departures may be made therefrom which are within the scope of
the
invention and that obvious modifications will occur to one skilled in the art
upon reading
this disclosure.
[00160] While the present invention has been described with reference to
the specific
embodiments thereof, it should be understood by those skilled in the art that
various
changes may be made and equivalents may be substituted without departing from
the true
spirit and scope of the invention. In addition, many modifications may be made
to adapt
a particular situation, material, composition of matter, process, process step
or steps, to
the objective, spirit and scope of the present invention. All such
modifications are
intended to be within the scope of the claims appended hereto.
38