Note: Descriptions are shown in the official language in which they were submitted.
CA 02866305 2014-09-03
WO 2013/134076
PCT/US2013/028728
CHAIN 'MODIFICATION OF GASEOUS METHANE USING AQUEOUS
ELECTROCHEMICAL ACTIVATION AT A THREE-PHASE
INTERFACE
pool] The priority of U.S. Application Serial No. 61/608,583, entitled, "An
Electrochemical Process for Direct one step conversion of methane to Ethylene
on a Three
Phase Gas, Liquid, Solid interface", and filed March 8, 2012, in the name of
the inventor Ed
Chen is hereby claimed pursuant to 35 U.S.C. 019(4 This application is
commonly
assigned herewith and is also hereby incorporated for all purposes as if set
forth verbatim.
herein.
(00021 The priority of U.S. Application Serial No. 61/713,487, entitled, "A
Process for
Electrochemical Fischer Trospcb", filed October 13, 2012, in the name of the
inventor Ed
Chen is hereby claimed pursuant to 35 U.S.C. 119(e). This application is
commonly,
assiened herewith and is also hereby incorporated for all purposes as if set
forth verbaiim
herein.
CROSS-REFERENCE TO RELATED APPLICATIONS
190031 Not applicable.
STATEMENT REGARDING 'FEDERALLY SPONSORED
.RESEARCH OR DEVELOPMENT
10041 Not applicable.
BACKGROUND
100051 This section of this document introduces information about andlor
from the art
that may provide context for or be related to the subject matter described
herein and'or
claimed below. :It provides back.ground information to facilitate a better
understanding of the
various aspects of the claimed subject matter. This is therefore a discussion
of "related" art.
That such art is related in no way implies that it is also "prior" art. The
related art may or
CA 02866305 2014-09-03
WO 2013/134076
PCT/US2013/028728
may not be prior art. The discussion in this section of this document is to be
read in this light,
and not as admissions of prior art.
[0006] Prior
art conunercial processes for converting methane to other hydrocarbons, tbr
example; sometimes include a partial oxidation process that is highly energy
intensive and
operates under high pressures and temperatures. The actuai syngas cleanup step
occurs after
the syngas has been cooled. Tar, oils, phenols, ammonia and water co-products
are
condensed from the gas stream and .purified and sent on. The gas moves to a
cleaning area
where further impurities are removed and finally carbon dioxide is removed.
The syngas is
then passed under high pressures (30 bars) with some more recent "low
pressure" processes
operating at slightly above 10 bars at approximately 200-400 degrees Celsius
to .form
hydrocarbons, oxygenates, and other carbon and hydrogen based species. The
high pressure
reactions utilize iron or nickel as their catalysts, while low pressure
synthesis often uses
cobalt. These processes use solid electrolytes rather than aqueous
electrolytes.
10007] Another
problem with methane activation is catalyst deactivation and
regeneration, temperature control, and high pressures. Catalysts are often
deactivated when
the surface is covered by waxes and coke (carbon black). The high temperatures
also
produce undesirable products such as wax which tends to deactivate the
catalyst. Finally,
water is also a byproduct of this reaction.
(00081 The art
therefore possesses a nwnber of methane activation processes that, even
if satisfactory in sonic respects, have several drawbacks. 'The art
.furtherrnore is always
receptive to improvements or alternative means, methods and configurations.
Therefore the
art will well receive the technique described herein.
SUMMARY
[0009] In a
first aspect, a method for chairs modification of hydrocarbons and organic
compounds comprises: contacting an aqueous electrolyte, a powered electrode
including a
catalyst, and a gaseous methane feedstock in a reaction area and activating
the methane in an
aqueous electrochetnical reaction to generate methyl radicals at the powered
electrode and
yield a long chained hydrocarbon.
2
CA 02866305 2014-09-03
WO 2013/134076
PCT/US2013/028728
NON)
tia..second aspect; method for 'chain modification . of laydrocarbons and mum&
conipounds comprises: contacting an aqueous electrolyte with a catalyst 111 a
reaction area;
introducing a gaseous methane feed.stock directly into the reaction area under
pressure; and
reacting the aqueous electrolyte, the catalyst, and the gaseous methane
feedstock at
temperatures in the range of -10 C to 900 C and at pressures in the range of
.1 .ATM to .100
AT.
100111 The
above presents a sill-whiled sun-unary of thepresently disclosed subject
illattef
in order to provide a basic tinderstauding.of some aspects thereof. The
summary is not an
exhaustive overview, nor is it intended to identify key or critical elements
to delineate the
scope of the subject inatter claimed below. Its sole purpose is to present
some concepts in a
simplified form as a prelude to the more detailed description set forth Mow,
BRIEF DESCRIPTION CIF TIIE DRAWINGS
100121 The
claimed subject matter may be better understood by reference to the
followitk:,!:
description taken in conjunction with the accompanying drawings, in which like
reference
nimierals identify like elements, and in which:
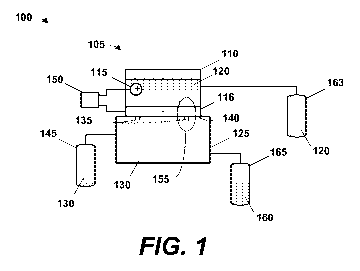
100131 Figure 1
depicts one particular embodiment of an electrolytie cell in accordance
with. some aspects of the presently disclosed technique,
100141 Figure 2
graphically illustrates one particular embodiment of a process in
aecordance.with other aspects of the presently disclosed technique,
100151 Figure
3A-figure 3B depict a copper mesh reaction electrode as may be used in
some embo di men is.
10016] Figure
4A-Figure 4E3 depict a gas difftision. electrode as may be used in some
embo di men is,
100171 Figure
5A-Figure-53 depicts a gas diffusion electrode 'as may be used in some
embodiments_
3
CA 02866305 2014-09-03
WO 2013/134076
PCT/US2013/028728
1001.8) .Figure
( depicts a portion of an embodiment in. 3xilich the eleetrodes are
electrically short circuited,
1001.9l While
the invention is susceptible to various modifications and alternative forms,
the drawings illustrate specific. embodiments herein described in detail by
way of example, It
should 'be understood, however, that the description herein of specific
embodiments is not
intended to limit the invention to the particular forms disclosed, but on the
contrary, the
intention is to cover all modificatic*, equivalents, and alternatives falling
.within the: spirit
and scope of the itINvitiort as defined by the appended claims.
DETAILED DESCRIPTION
1.00201
Illustrative embodiments Of the subject matter claimed below kvill now be
disclosed.. In the interest. of clarity, not all features of an actual
implementation are described
in this specification,. It will be appreciated that in the :development of any-
such actual.
embodiment, numerous implementation-specific decisions must be made to achieve
the
developers' specific goals., such as compliance with system-related and
business-related
constraints, winch ili 'vary .from one implementation to another. Moreover, it
Nvii1 be
appreciated that such a development effort, even if complex and time-
consuming, would be a
routine undertaking for those of ordinary skill in the art having the benefit
of this disclosure.
[00211 The
presently disclosed technique is a process. for converting gaseous
hydrocarbons to longer...chained liquid hydrocarbons, longer chained. gaseous
hyl,frocarbons,.
branched-ch.ain liquid hydrocarbons, branched.-chain gaseous hydrocarbons, as
well as
Chained and. branched-chain organic compounds, in t:!eneral, the method is for
chain
modification of hydrocarbons and organic compounds, including chain
lengthening.. This
.process more particularly -uses aqueous electrolytes to act as a reducing
atmosphere and
hydrogen and. oxygen source for hydrocarbon uses. The process in the disclosed
technique
is Aqueous Electrochemical Activation of Methane (AEA/VI) on three phase
interface of gas-
liquid-solid electrode. AEAM directly turns natural gas and other sources of
tiled-lane (Cl-
into C2+ hydrocarbons and other organic compounds, One exemplary product is
ethylene
(CAL) and alcohols such as methanol, ethanol, prop.anol, andlor butanol.
4
CA 02866305 2014-09-03
WO 2013/134076
PCT/US2013/028728
100221 The
reaction of hydrocarbon gases may be successfully achieved with an aqueous
electrochemical solution serving as a liquid ion source along with the supply
for hydrogen or
singlet oxygen being provided by the aqueous electrolyte through acids and/or
bases of the
aqueous electrolyte. The gaseous hydrocarbon is balanced with the aqueous
electrolyte at a
solid phase thin film catalyst which is connected to the reaction electrode of
an electrolytic
cell. The reaction may also be adjusted with different pHs or any kind of
additive in the
electrolytic solution,
(00231 The
reaction works by utilizing a 3 phase interface which defines a reaction area.
A catalyst, a liquid, and a gas a positioned in the sante location and an
electric potential is
applied to make electrons available to the reaction site. When methane is used
as the gas it is
possible to create methane radicals which then join with other molecules or
parts of
molecules or themselves to create longer chained. hydrocarbons and/or organic
molecules.
The reaction site can also cause branched chain production by reacting with a
newly created
molecule and building on that or continuous chain building. Thus from the
simple molecule
of methane, C114, chains of molecules can be built. 'Existing chained
molecules can be
lengthened, and existing chained molecules can be branched: A simple example
is methane
can be converted to methanol, CEI3(011). Different voltages create different
reaction
product distribation.s or fadlitate different reaction. types.
(00241 This
aqueous electrochemical reaction includes a reaction that proceeds at room
temperature and pressure, although higher temperatures and pressures may be
used. In
general, temperatures may range from -IOC to 240C, or from -IOC to 1000C, and
pressures
may range from .1 ATM to 10 AT, or from .1 ATM to 100 ATM. The process
generates
motive methyl .radicals through. the reaction on the reaction electrodes. On
the reaction
electrode, th.e production of methyl radicals occurs.
100251 In at
least some embodiments, the reactants need no pre-treatment. Typically
methanol front methane must first go through steam reforming to produce swims
CO and
H2). The presently disclosed technique can perform the production of methanol
without
reforming to produce synaas. Similarly, as described further below, the
gaseous methane
feedstock may be introduced "directly" into the chamber of an electrochemical
cell,
CA 02866305 2014-09-03
WO 2013/134076
PCT/US2013/028728
(0026) In
general, the method introduces a liquid ion source into a first chamber into
contact with. a catalyst supporting reaction electrode while a. counter
electrode is disposed in
the liquid ion source. The reaction electrode is powered. A gaseous methane
feedstock is then
introduced directly into a second chamber under enough pressure to overcome
the
gravitational pressure of the column of electrolyte, which depends on the
height of the water,
to induce a reaction among the liquid ion source, the catalyst, and the
gaseous methane
feedstock when the electrodes are powered.
10027) In the
embodiments illustrated herein, the technique employs an electrochemical
cell such as the one illustrated in Figure I. The electrochemical cell 100
generally comprises
a reactor 105 in one chamber 110 of which are positioned two electrodes 1.15,
116, a cathode
and an anode, separated by a liquid i0T1 source, i.e., an. electrolyte 120.
Those in the art will
appreciate that the identity of the electrodes 115, 116 as cathode and anode
is a matter of
polarity that can vary by implementatim In the illustrated embodiment, the
counter electrode
115 is die anode and the reaction electrode 116 is the cathode. The reaction
electrode .116
shall be referred to as the "reaction" electrode and the counter electrode 115
the "counter"
electrode for reasons discussed further below.
100281 There is
also a second chamber 125 into -which a gaseous methane feedstock 130
is introduced as described below. 'The two chambers are joined by apertures
135 through the
wall 140 separating the two chambers 110, 125. The reactor 105 may be
constructed in
conventional fashion except as noted herein. For example, materials selection,
fabrication
techniques, and assembly processes in light of the operational parameters
disclosed herein
will be readily ascertainable to those skilled in the art.
(00291
Catalysts will be implementation specific depending, at least in part, on the
implementation of the reaction electrode 116. Depending on the embodiment,
suitable
catalysts may include, but are not limited to, nickel, copper, iron, tin,
zinc, mthenium,
palladium, rheniunt, or any of the other transition or lanthanide metals, or a
noble metal such
as platinum, palladium, gold, or silver. They may also include products
thereof, including for
example cuprous chloride or cuprous oxide, other compounds of catalytic
metals, as well as
organometalic compotmds. Exem.plary organometallic compounds include, but are
not
limited to, tetracarbonyl nickel, lithiumdiphenylcuprate,
pentamesitylpentacopper, and
etharatedimer.
6
CA 02866305 2014-09-03
WO 2013/134076
PCT/US2013/028728
[00301 The
electrolyte 120 will also be implementation specific depending, at least in
part, on the implementation of the reaction electrode 116. Exemplary liquid
ionic substances
include, but are not limited to, Alkali or alkaline Earth. salts, such as
halides, sulfates, sulfites,
carbonates, nitrates, or nitrites. The electrolyte 120 may therefore be,
depending upon the
embodiment, magnesium sulfate (MgS), sodium chloride (NaCI), sulfuric acid
(H2S0.4),
potassium chkvide (KCl), hydrogen chloride (HCI), hydrogen bromide (HBO,
hydrogen
fluoride (HE), potassium chloride (MI), potassium bromide (KBr), and potassium
iodide
(KI), or any other suitable electrolyte and acid or base known to the art,
(00311 The pH
of the electrolyte 120 may range from 0 to 3 and concentrations of
between 0.1M and 3M may be used. Some embodiments may use water to control pH
and
concentration, and such water may be industrial grade water, brine, sea water,
or even tap
water. The liquid ion source, or electrolyte 120, may comprise essentially any
liquid ionic
substance. In some embodiments, the electrolyte 120 is a halide to benefit
catalyst lifetime.
100321 In
addition to the reactor 105, the electrochemical cell 100 includes a gas
source
145 and a power source 150, and an electrolyte source 163. The gas source 145
provides the
gaseous methane feedstock 130 while the power source 150 is powering the
electrodes 115,
116 under enough pressure to balance and overcome the gravitational press= of
the column
of electrolyte, which depends on the height of the water, sufficient to
maintain the reaction at
the three phase interface 155. The three phase interface 155 defines a
reaction area. In some
embodiments, this pressure might be, for example, 10000 pascals, or from 0.1
ATM to 10
Am or from 0.1 ATM to 100 ATM. The electrolyte source 163 provides adequate
levels of
the electrolyte 120 to ensure proper operations. The three phases at the
interface .155 are the
liquid. electrolyte 120, the solid catalyst of the reaction electrode 116, and
the gaseous
methane feedstock 130. The product 160 is collected in a vessel 165 of some
kind in any
suitable manner known to the art. In some embodiments, the products 160 may be
forwarded
to yet other processes either after collection or without ever being collected
at all. In these
embodiments, the products 160 may be streamed directly to downstream processes
using
techniques well known in the att.
00331 The
embodiment of Figure 1 includes only a single reactor 105. However, in
alternative embodiments, multiple units of these may be arranged for greater
efficiencies. In
7
CA 02866305 2014-09-03
WO 2013/134076
PCT/US2013/028728
larger single Chamber, pressure would more likely have to be adjusted with
electrolyte level
rather than changes in gaseous .methane feedstock 130 pressure in the chamber
125.
[00341 Those in
the art will appreciate that some implementation specific details are
omitted from Figure 1. For example, various instrumentation such as flow
regulators, mass
regulators, a pH regulator, and sensors for temperatures and pressures are not
shown but will
typically be found in most embodiments. Such instrumentation is used in
conventional
fashion to achieve, monitor, and maintain various operational parameters of
the process.
Exemplary operational parameters include, but are not limited to, pressures,
temperatures,
pH, and the like that will become apparent to those skilled in the art.
However, this type of
detail is omitted from the present disclosure because it is routine and
conventional so as not
to obscure the subject matter claimed below.
100351 The
reaction is conceptually illustrated in FIG, 2. in this embodiment, the
feedstock 130' is natural gas and the electrolyte 1120' is Sodium Chloride.
Reactive hydrogen
ions (In are fed to the natural gas stream 130' through the electrolyte 120'
with an applied
cathode potential. The molecules may also ill MED react with water on the
interface to form
alcohols, oxygenates, and ketones. .Exemplary alcohols include but are not
limited to
methanol, ethanol, propanol, butanol. In one example of this reaction, the
reaction occurs at
room temperature and with an applied cathode potential of 0,01V versus SHE to
1.99V
versus SHE. The voltage level can be used to control the resulting product. A
voltage of
0.1V may result in a methanol product whereas a 0.5V voltage may result in
butanol.
100361 Still
further, very little catalyst deactivation MCUM in some ern1)odiments because
the catalyst is protected by a layer of chloride, which also acts as an
absorbent for the
reactants, and the electrolyte is saturated with CI 7 preventing typical
catalyst poisons from
bonding with the catalyst and deactivating it, as this would force the release
of a Cf ion into
the liquid. ln addition, this process further prevents the deposition of
impurities in water,
which could deactivate the catalyst. These aspects will be explored further
below.
100371
Returning now to Figure I, additional attention will now be directed to the
electrochemical cell 100. As noted above, the reactor 105 can be fabricated
from
conventional materials using conventional fabrication techniques. Notably, the
presently
disclosed technique operates at room temperatures and pressures whereas
conventional
8
CA 02866305 2014-09-03
WO 2013/134076
PCT/US2013/028728
processes are performed at temperatures and pressures much higher. Design
considerations
.pertaining to temperature and pressure therefore can be relaxed relative to
conventional
practice. However, conventional reactor designs may neventieless be used in
some
embodiments.
100381 The
presently disclosed technique admits variation in the implementation of the
electrode at which the reaction occurs, hereafter referred to as the "reaction
electrode". The
other electrode will be referred to as the "counter electrode". In the
embodiment of Figure!,
the reaction electrode 116 is the reaction electrode and the counter electrode
115 is the
counter electrode. As noted above, those in the art will appreciate that the
identity of the
electrodes 115, 116 as cathode and anode is a limner of polarity that can vary
by
implementation.
(00391 One such
modification is that the copper mesh used in the illustrated embodiment
is an 80 mesh rather than a. 40 mesh. This mesh may be plated with high
current densities to
produce fractal foam structures with high surface areas which may be utilized
as catalysts in
this reaction.
100401 More
.particularly, the catalyst 305 is supported on a. copper mesh 310 embedded
in an ion exchange resin 300 as shown in Figure 3A. The catalyst 305 can be a
plated
catalyst or powdered catalyst. The metal catalyst 305 is a catalyst capable of
reducing
methane to a long chained hydrocarbon or organic compound and alcohol.
Exemplary
-metals include, but are not limited to, metals such as copper, silver, gold,
iron, tin, zinc,
ruthenium, platinum, palladium, rhenium, or any of the other transition or
lanthanide
metals. in one embodiment, the metal catalyst is silver, copper, copper
chloride or copper
oxide. Ion exchange resins are well known in the art and any suitable ion
exchange resin
.known to the art may be used. In one particular embodiment, the ion exchange
resin is
NAFION 117 by Dupont
10041j The
copper wire mesh 310 can be used to structure the catalyst 305 within the
resin 300. The assembly 315 containing the catalyst 305 can be deposited onto
or otherwise
structurally associated with a hydrophilic paper 320, as shown in Figure 3B.
Electrical leads
not shown) can then be attached to the copper wire mesh 310 in conventional
fashion. The
9
CA 02866305 2014-09-03
WO 2013/134076
PCT/US2013/028728
reaction electrode 320 is but one implementation of the reaction electrode 116
in Figure 1.
Alternative implementations will be discussed below.
100421 The
counter electrode 115, the reaction electrode 116 is disposed within a reactor
105 so that, in use, it is submerged in the electrolyte 120 and the catalyst
305 forms one part
of the three-phase interface 155. When electricity is applied to electrodes
1.15, 116,
electrochemical reduction discussed above takes place to produce hydrocarbons
and organic
chemicals. The reaction electrode 320 receives the electrical power and
catalyzes a reaction
between the hydrogen in the electrolyte 120 and the gaseous methane feedstock
130.
10043j As
Mentioned above, the copper mesh 310 in the illustrated embodiment is an
mesh in the range of 1- 400 mesh.
100441 In a
second embodiment shown in Figure 4A-.Figure 4B, a gas diffusion electrode
400 comprises a hydrophobic layer 405 that is porous to methane but
impermeable or nearly
impemieable to aqueous electrolytes. In one embodiment of the electrode 400, a
!mil thick
advcarb carbon paper 410 treated with TEFLON* (i.e., polytetrafluoroethylene)
dispersion
(not separately shown) is coated with activated carbon 415 with copper 420
deposited in the
pores of the activated carbon 415. The copper 420 may be deposited through a
wet
impregnation method, electrolydc reduction, or other means of reduction of
copper, silver
other transition metals into the porous carbon material.
(0045) This
material is then mix.ed with a hydrophilic binding agent (not shown), such as
-polyvinyl alcohol (PVA), polyvinyl acetate (PVAc), or Nafton. An ink is made
from the
mixture of impregnated graphite., binding agent, and alcohol or other organic
solvent. The
ink is painted onto the hydrophobic layer 405 and then bonded through any
means, such as
atmospheric drying, heat press, or other means of application of heat.
100461 The
copper 420 impregnated into the ion. electrode 400 is then made into a
caprous halide through any suitable procedure. One embodiment of the procedure
to make
the cuprous halide is to submerge the electrode in a solution of hydrochloric
acid and cupric
chloride, heat to 100 C for 2 hours. Another embodiment submerges the
impregnated
electrode 400 in 3 M K13r or 3 M K1 and. nm a 4 V pulse of electricity to the
electrode 400 in
CA 02866305 2014-09-03
WO 2013/134076
PCT/US2013/028728
order to form a thin film of cuprous halide 425, shown in cross-section Figure
48, in the
electrode 400.
100471 In
another emtxxliment, the copper panicles in the electrode are first plated
with
silver by electroless plating or another method, creating a thin film of
silver over the copper.
Copper may then be plated onto the silver and transfonned into a halide
through procedure
previously described. In another embodiment, silver particles are deposited
into the
hydrophilic layer, coate41 with copper electrolytically, and then the same
procedure for the
conversion of the copper layer to a copper halide layer is conducted.
100481 in
another embodiment, the gas diffusion electrode uses nanoparticles reduced
from a solution of Cupric Chloride with an excess of ascorbic acid and 10
grams of carbon
graphite. The amalgam was heated to 1 00C for eight hours. It is then mixed
with equal
amounts ill weight of a hydrophilk binder.
100491 In
another enibodiment, a high mesh copper of 200 mesh is allowed to form
cuprous chloride in a solution of cupric chloride and hydrochloric acid. This
layer of halide
on the surface of the catalyst material allows for catalyst regeneration. This
accounts for the
abnormally high lifetime of the three phase reaction. The result is then
treated in a 1M
solution of Cupric Chloride heated to 10(PC.
1905)1 The
electrode 400 therefore -includes a covering or coating 425 of cuprous
chloride to prevent "poisoning" or fouling of the electrode 400 during
operation. The
electrodes in this embodiment must be copper so that no other metals foul the
reaction by
creating intermediate products which ruin the efficacy of the surface of the
copper. Some
embodiments also treat the copper with a high surface area powder by
electroplating, which
will allow for the generation of greater microturbulenee, thereby creating
more contact and
release between the three phase reaction surface. Furthermore, contrary to
conventional
practice, rather than separate the cathode and anode, the cathode and anode
are al .lowed to
remain in the same electrolyte in this embodiment. (The electrolyte is
filtered through a pump
not shown.) The electrolyte is therefore contacted directly to the gas
diffusion electrode 400
rather than through the intercession of a polymer exchange membrane.
11
CA 02866305 2014-09-03
WO 2013/134076
PCT/US2013/028728
(0051)
Catalysts in this particular embodiment may include copper, silver, gold,
iron, tin,
zinc, ruthenium, platinum, palladium, rhenium, or any of the other transition
or lanthanide
metals. In addition, the catalysts may be formed into a metal foam or
alternatively it may be
deposited through electroless or electrolytic deposition onto a porous support
with a
hydrophobic and hydrophilic layer.
1.00521 In
electrochemical systems, it is often difficult to make a good electrical
contact
between gas diffusion medium and the current collector. The need for a solid
polymer
electrolyte to some degree is the first order solution to the problem. at
hand. Carbon paper has
a significant resistance across of up to 2fl that impedes the effective
application of gas
diffusion electrodes to electrochemical applications. By pressing a wire made
from a metal
such as nickel, copper, iron, steel, or a noble metal such as platinum, gold,
or silver directly
into the carbon paper, gas diffusion media may be extended into applications
such as
'hydrocarbon processing and fuel cell applications. The production of such
papers is
relatively straight forward though requires a few enabling aspects .fur it to
work. A small
amount: of adhesive material is mixed in with activated carbon particles with
a high internal
porosity, for example a BET of 50m21gram, This serves as the binder which may
be applied
between existing conductive gas diffusion medium such as a carbon paper, a
toray paper, or
other conductive gas diffusion electrodes. Figure M. shows one embodiment 500
of the
pressed wire mesh 505 in. paper 510. The wire 505 is first submerged in a
slurry of activated
carbon and adhesive (not shown), which is mixed. in a ratio by weight of 1:1
that provides for
full conductivity of the thin binding layer. This layer than presses the wire
mesh 505 into the
surface of the carbon paper 510, providing uniform conductivity.
100531 The
binder slurry both binds the metal of the wire mesh. 505 to the surface of the
conductive paper 510, while providing conductivity itself, and holds the wire
mesh 505 firm
against the conductive paper 510, which overcomes the contact resistance. The
surface of the
wire mesh 505 is cleaned with a solvent before being applied to the carbon
paper 510 to
remove any oils from the surface of the contact region, as this may cause
unwanted resistance
to build up. The wire should be thick enough that the wire mesh 505 forms a
slight
indentation into the paper 510 as to provide .maximurn contact area.
po541 In
another embodiment 500', the production of the paper 510 is conducted and
deposited directly onto the wire mesh 505, the result of which is shown in
Figure 5B.
12
CA 02866305 2014-09-03
WO 2013/134076
PCT/US2013/028728
Conductive carbon paper is often made by pyrolyzing carbon containing
compounds. Thus,
by using a conductive -material with high corrosion resistance in a low oxygen
environment, it
-would be possible to convert carbon containing material directly onto the
wire mesh
conductor, providing for a single step process to deposit. The process may
otherwise be in
accordance with conventional practice .for producing and pyrolyzing carbon
based materials
to form carbon paper such as polyanaline based carbon .fiber paper.
1.00551 The
technique illustrated in Figure 5A-Figure 5B can improve the conductivity of
the carbon papers 510 and significantly reduce the resistance thereof by up to
an ohm or
more. In the embodiment 500 of Figure 5A, more particularly, a carbon paper
510 has a I-
400 mesh pure copper mesh 505 embedded halfway into the carbon paper 510. In
the
embodiment 500' of Figure 5B, the carbon paper 510 has the copper wire mesh
505
einbedded in therein such that no metal is showing. Spacing between the wires
of the mesh
505 can be from 1mm to lcm. The carbon paper 510 should. generally be as thin
as possible
while still being sturdy enougl'i to withstand handling in both embodiments.
MIMI In one
particular ernbodinient, the electrodes are electrically short circuited
within
the electrolyte while maintaining a three phase interface. Figure 6 depicts a
portion 600 of an
embodiment in which the electrodes are electrically short circuited. In this
drawing, only a
single electrode 605 is shown but the potential is electric potential is drawn
across the
electrode 605. The com.panion electrode (not shown) is similarly electrically
short: circuited.
10057j So,
turning now to the process again arid referring to Figure 1, a methane gas or
gaseous mixture including methane 130 is .introduced into the second chamber
125 of the
reactor 105 under pressure. The exemplary embodiments discussed below all
include the
following design characteristics: (1) a three-phase catalytic interface 155
for solid catalyst,
gaseous methane feedstock 130, and liquid ion source (e.g., a liquid
electrolyte) 120, (2) a
cathode 116 and anode 115 in the same, .filtered electrolyte 120, and (3) an
electrolyte 120
contacted directly to the reaction electrode, which is the cathode 116.
100581 The
inethod of operation generally comprises introducing the electrolyte 120 into
the first chamber 110 into direct contact with the powered electrode surfaces
115 and .116.
The gaseous tnethane feedstock .130 is then introduced. into the second.
chamber- .125 under
enough pressure to over come the gravitational pressure of the column of
electrolyte, which
13
CA 02866305 2014-09-03
WO 2013/134076
PCT/US2013/028728
depends on the height of the water, to induce the reaction. During the
reaction, the
electrolyte 120 is filtered, the gaseous methane feedstock 130 is maintained
at a selected
pressure to ensure its presence at the three phase interface 155, and the
product 165 is
collected. Within this general context, the following examples are
implemented.
100591 Above
the second Chamber 125, but attached to it., is an area for the introduction
of a cathode reaction electrode 116 where the three-phase interface 155 will
form. Catalysts
supported by the reaction electrode .1.16 include copper, silver, gold, iron,
tin, zinc,
ruthenium, platinum, palladium, rhenium, or any of the other transition or
lanthanide metals.
In addition, the catalysts may be fonned into a metal foam or alternatively it
may be
deposited through electroless or electrytic deposition onto a porous support
with a
hydrophobic and hydrophilic layer as previously described above. The
electrolyte 120 may
comprise, for example, potassium chloride (KCI), potassium bromide (KBr),
potassium
iodide (Kl), or any other suitable electrolyte known to the art.
100601 This
particular embodiment implements the reaction electrode 116 as the gas
diffusion electrode described above with the cuprous halide coating.
Alternative
embodiments may use another cuprous halide coating the surface of the .metal..
Cuprous
Oxide, Cupric Oxide, and other varying valence states of copper will also work
in the
reaction.
100611 By
maintaining a three phase interface between gaseous methane feedstock 130
and the electrolyte 120, the methane will form organic chemicals and form a
nearly complete
conversion when there is continuous contact to the gaseous methane feedstock
.130 on the
three phase interface 155 between the liquid electrolyte 120, the solid
catalyst, and the
gaseous methane feedstock 130. Another means of maintaining the three phase
interface is to
use a separation membrane which selectively allows hydrogen and water to
penetrate. One
such membnute is Nation. Another means is to use a fuel cell type set up but
instead of
generating a current, a current is introduced to generate a chemical.
100621 Other
reaction mechanism also produces organic compounds such as ethers,
epox.ides, and alcohols, a:mong other compounds.
14
CA 02866305 2014-09-03
WO 2013/134076
PCT/US2013/028728
10063l The
electrolyte 120 should be relatively concentrated at..-11M-W and should be a
halide electrolytes discussed aboVe to increase cataly.st lifetime. The higher
the surface.area.
between the reaction electrode 116 and .the gaseous. chamber. 1-25 on one side
and the liquid
electrolyte 120 on the other side, the higher the conversion rates. Operating -
pressures could
he .ranged from. only 10000 pascals, or from atm to 100 atm, or from .1 atm to
100 atm.:
though Standard Temperature and Pressures (STP) were sufficient for the
reaction.
[00641 in one
embodiment of the gas diffusion electrode (GI)E) an antioxidant layer of
a.scorbic acid is .mixed with the GDE high porosity carbon. The -high porosity
carbon
includes nanotubes, fullerines, and other specialized formations of carbon as
described above
The high porosity carbon is impregnated throtmli reduction of cupric chloride,
or other ibrrn
of cathon. it is then made into a halide by treatment with a chloride solution
under the proper
and temperature of EISI.F conditions. it also includes a reaction in the solid
-polymer
phase. A .pasteis made from the impregnated carbon, astorbie kid, and a
hydrophilic binding
gent. . This paste is painted onto a hydrophobic layer. Sorne embodiments
include
antioxidants in the laver as described above.
1-)0651 Note
that not a.li embodiments wili manifeSt all these characteristies and, to the
extent they do, they will not neceaSarily manifest Them to the same extent
Thus, some
embodiments may omit one or more of these Characteristics entirely.
'Furthermore, some.
embodiments may exhibit other characteristics in addition to., or in lieu of, -
those described:
herein.
100661 The
phrase "capable of' as used herein is a recognition of the fact that some
functions described for the various parts of the disclosed apparatus are
performed only svhen
the apparatus is powered andfor in operation. Those in the art having the
benefit of this
disclosure will appreciate that the embodiments illustrated herein include a
number of
electronic or eleetro-inechanical parts that, to operate, require electrical
power. 'Even when
provided with power, some functions described herein only cwt. when ìn
operation. 'Thus,
at nines, some embodiments of-the apparatus of the invention are. "capable of'
performing the
recited ftinctions even when they ate not actually performing thetri-----4.e.,
when there is no
power or when they are powered but not in operation.
CA 02866305 2014-09-03
WO 2013/134076
PCT/US2013/028728
(0067) The
following patent, applications, and publications are hereby incorporated by
reference for all purposes as i.f set forth verbatim herein:
10068j U.S.
Application Serial No. 61/608,583, entitled, "An Electrochemical Process fbr
Direct one step conversion of methane to Ethylene on a Three Phase Gas,
Liquid, Solid
Interface," and filed March 8, 2012, in the name of the inventor Ed Chen and
commonly
assigned here w ith
1.0069] U.S.
Application Serial "No. 61/713,487, entitled, "A Process for Electrochemical
Fischer Trospcb," filed October 13, 2012, in the name of the inventor Ed Chen
and
commonly assigned herewith..
100701 To the
extent that any patent, patent application, or other reference incorporated
herein by reference conflicts with the present disclosure set forth herein,
the present
disclosure controls.
(00711 This
concludes the detailed description. The particular embodiments disclosed
above are illustrative only, as the invention may be modified and practiced in
different but
equivalent manners apparent to those skilled in the art having the benefit of
the teachings
herein. Furthermore, no limitations are intended to the details of
construction or design herein
shown, other than as described in the claims below. It is therefore evident
that the particular
-embodiments disclosed above may be altered or modified and all such
variations are
considered within the scope and spirit of the invention. Accordingly, the
protection sought
herein is as set forth in the claims below.
16