Note: Descriptions are shown in the official language in which they were submitted.
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
TITLE
EXTENDED-RELEASE FORMULATION FOR REDUCING THE FREQUENCY OF
URINATION AND METHOD OF USE THEREOF
[0001] This application claims priority of U.S. Patent Application Serial No.
13/424,000, filed March 19, 2012, now U.S. Patent No. 8,236,857, and U.S.
Patent
Application Serial No. 13/487,348, filed June 4, 2012. The entirety of the
aforementioned
applications is incorporated herein by reference.
FIELD
[0002] The present application generally relates to methods and compositions
for
inhibiting the contraction of muscles and, in particular, to methods and
compositions for
inhibiting the contraction of smooth muscles of the urinary bladder.
BACKGROUND
[0003] The detrusor muscle is a layer of the urinary bladder wall made of
smooth
muscle fibers arranged in spiral, longitudinal, and circular bundles. When the
bladder is
stretched, this signals the parasympathetic nervous system to contract the
detrusor muscle.
This encourages the bladder to expel urine through the urethra.
[0004] For the urine to exit the bladder, both the autonomically controlled
internal
sphincter and the voluntarily controlled external sphincter must be opened.
Problems with
these muscles can lead to incontinence. If the amount of urine reaches 100% of
the urinary
bladder's absolute capacity, the voluntary sphincter becomes involuntary and
the urine will be
ejected instantly.
[0005] The human adult urinary bladder usually holds about 300-350 ml of urine
(the
working volume), but a full adult bladder may hold up to about 1000 ml (the
absolute
volume), varying among individuals. As urine accumulates, the ridges produced
by folding of
the wall of the bladder (rugae) flatten and the wall of the bladder thins as
it stretches, allowing
the bladder to store larger amounts of urine without a significant rise in
internal pressure.
[0006] In most individuals, the desire to urinate usually starts when the
volume of urine
in the bladder reaches around 200 ml. At this stage it is easy for the
subject, if desired,
to resist the urge to urinate. As the bladder continues to fill, the desire to
urinate becomes
stronger and harder to ignore. Eventually, the bladder will fill to the point
where the urge to
urinate becomes overwhelming, and the subject will no longer be able to ignore
it. In some
individuals, this desire to urinate starts when the bladder is less than 100%
full in relation to its
working volume. Such increased desire to urinate may interfere with normal
activities,
1
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
including the ability to sleep for sufficient uninterrupted periods of rest.
In some cases, this
increased desire to urinate may be associated with medical conditions such as
benign prostate
hyperplasia or prostate cancer in men, or pregnancy in women. However,
increased desire to
urinate also occurs in individuals, both male and female, who are not affected
by another
medical condition.
[0007] Accordingly, there exists a need for compositions and methods for the
treatment of male and female subjects who suffer from a desire to urinate when
the bladder is
less than 100% full of urine in relation to its working volume. Said
compositions and
methods are needed for the inhibition of muscle contraction in order to allow
in said subjects
the desire to urinate to start when the volume of urine in the bladder exceeds
around 100% of
its working volume.
SUMMARY
[0008] One aspect of the present application relates to a method for reducing
the
frequency of urination. The method comprises administering to a subject in
need thereof a
pharmaceutical composition comprising: an active ingredient comprising one or
more
analgesic agents in an amount of 50-400 mg per agent, wherein said one or more
analgesic
agents are selected from the group consisting of aspirin, ibuprofen, naproxen,
naproxen
sodium, indomethacin, nabumetone, and acetaminophen, wherein said
pharmaceutical
composition is formulated for extended-release such that said active
ingredient is released
continuously over a period of 5-24 hours. The method can be used for the
treatment of
nocturia or overactive bladder.
[0009] Another aspect of the present application relates to a method for
reducing the
frequency of urination. The method comprises administering to a subject in
need thereof a
pharmaceutical composition comprising: an active ingredient comprising one or
more
analgesic agents in an amount of 50-400 mg per agent, wherein said one or more
analgesic
agents are selected from the group consisting of aspirin, ibuprofen, naproxen,
naproxen
sodium, indomethacin, nabumetone, and acetaminophen, wherein said
pharmaceutical
composition is formulated for extended release, characterized by a two-phase
release profile
in which 20-60% of said active ingredient is released within two hours of
administration and
remainder of said active ingredient is released continuously over a period of
5-24 hours. The
method can be used for the treatment of nocturia or overactive bladder.
[0010] Another aspect of the present application relates to a method for
reducing the
frequency of urination, comprising administering to a subject in need thereof
an effective
amount of botulinum toxin, wherein said botulinum toxin is administered by
injection into a
2
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
bladder muscle; and orally administering to said subject a pharmaceutical
composition
comprising: an active ingredient comprising one or more analgesic agents in an
amount of
50-400 mg per agent, wherein said one or more analgesic agents are selected
from the group
consisting of aspirin, ibuprofen, naproxen, naproxen sodium, indomethacin,
nabumetone, and
acetaminophen, wherein said pharmaceutical composition is formulated for
extended-release.
The method can be used for the treatment of nocturia or overactive bladder.
[0011] Another aspect of the present application relates to a method for
reducing the
frequency of urination, comprising administering to a subject in need thereof
an effective
amount of one or more analgesic agents, and an effective amount of zolpidem.
The method
can be used for the treatment of nocturia or overactive bladder.
[0012] Another aspect of the present application relates to a method for
reducing the
frequency of urination, comprising administering to said subject a
pharmaceutical
composition comprising: one or more analgesic agents; and an antidiuretic,
wherein said one
or more analgesic agents are formulated for delayed release and wherein said
antidiuretic is
formulated for immediate release. The method can be used for the treatment of
nocturia or
overactive bladder.
[0013] Another aspect of the present application relates to a pharmaceutical
composition comprising an active ingredient comprising one or more analgesic
agents,
zolpidem and a pharmaceutically acceptable carrier.
[0014] Another aspect of the present application relates to a pharmaceutical
composition, comprising one or more analgesic agents and an antidiuretic,
wherein said one
or more analgesic agents are formulated for delayed release and wherein said
antidiuretic is
formulated for immediate release.
BRIEF DESCRIPTION OF DRAWINGS
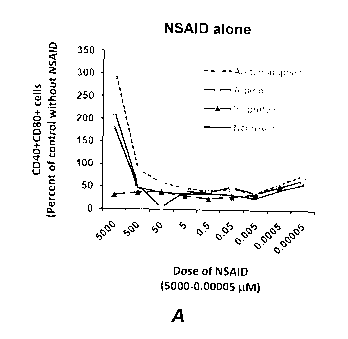
[0015] Figure IA and 1B are diagrams showing that analgesics regulate
expression of
co-stimulatory molecules by Raw 264 macrophage cells in the absence (Figure
1A) or
presence (Figure 1B) of LPS. Cells were cultures for 24 hrs in the presence of
analgesic
alone or together with Salmonella typhimurium LPS (0.05 pg/m1). Results are
mean relative
% of CD4O+CD80+ cells.
DETAILED DESCRIPTION
[0016] The following detailed description is presented to enable any person
skilled in
the art to make and use the invention. For purposes of explanation, specific
nomenclature is
set forth to provide a thorough understanding of the present invention.
However, it will be
3
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
apparent to one skilled in the art that these specific details are not
required to practice the
invention. Descriptions of specific applications are provided only as
representative examples.
The present invention is not intended to be limited to the embodiments shown,
but is to be
accorded the broadest possible scope consistent with the principles and
features disclosed
herein.
[0017] As used herein, the term "an effective amount" means an amount
necessary to
achieve a selected result.
[0018] As used herein, the term "analgesic" refers to agents, compounds or
drugs
used to relieve pain and inclusive of anti-inflammatory compounds. Exemplary
analgesic
and/or anti-inflammatory agents, compounds or drugs include, but are not
limited to, the
following substances: non-steroidal anti-inflammatory drugs (NSAIDs),
salicylates, aspirin,
salicylic acid, methyl salicylate, diflunisal, salsalate, olsalazine,
sulfasalazine, para-
aminophenol derivatives, acetanilide, acetaminophen, phenacetin, fenamates,
mefenamic
acid, meclofenamate, sodium meclofenamate, heteroaryl acetic acid derivatives,
tolmetin,
ketorolac, diclofenac, propionic acid derivatives, ibuprofen, naproxen sodium,
naproxen,
fenoprofen, ketoprofen, flurbiprofen, oxaprozin; enolic acids, oxicam
derivatives, piroxicam,
meloxicam, tenoxicam, ampiroxicam, droxicam, pivoxicam, pyrazolon derivatives,
phenylbutazone, oxyphenbutazone, antipyrine, aminopyrine, dipyrone, coxibs,
celecoxib,
rofecoxib, nabumetone, apazone, indomethacin, sulindac, etodolac,
isobutylphenyl propionic
acid, lumiracoxib, etoricoxib, parecoxib, valdecoxib, tiracoxib, etodolac,
darbufelone,
dexketoprofen, aceclofenac, licofelone, bromfenac, loxoprofen, pranoprofen,
piroxicam,
nimesulide, cizolirine, 3-formylamino-7-methylsulfonylamino-6-phenoxy-4H-1-
benzopyran-
4-one, meloxicam, lornoxicam, d-indobufen, mofezolac, amtolmetin, pranoprofen,
tolfenamic
acid, flurbiprofen, suprofen, oxaprozin, zaltoprofen, alminoprofen,
tiaprofenic acid,
pharmacological salts thereof, hydrates thereof, and solvates thereof
[0019] As used herein, the terms "coxib" and "COX inhibitor" refer to a
composition
of compounds that is capable of inhibiting the activity or expression of COX2
enzymes or is
capable of inhibiting or reducing the severity, including pain and swelling,
of a severe
inflammatory response.
[0020] As used herein, the term "derivative" refers to a chemically modified
compound wherein the modification is considered routine by the ordinary
skilled chemist,
such as an ester or an amide of an acid, protecting groups, such as a benzyl
group for an
alcohol or thiol, and tert-butoxycarbonyl group for an amine.
[0021] As used herein, the term "analogue" refers to a compound which
comprises a
4
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
chemically modified form of a specific compound or class thereof, and which
maintains the
pharmaceutical and/or pharmacological activities characteristic of said
compound or class.
[0022] As used herein, "pharmaceutically acceptable salts" refer to
derivatives of the
disclosed compounds wherein the parent compound is modified by making acid or
base salts
thereof. Examples of pharmaceutically acceptable salts include, but are not
limited to,
mineral or organic acid salts of basic residues such as amines; alkali or
organic salts of acidic
residues such as carboxylic acids; and the like. The pharmaceutically
acceptable salts include
the conventional non-toxic salts or the quarternary ammonium salts of the
parent compound
formed, for example, from non-toxic inorganic or organic acids. For example,
such
conventional non-toxic salts include those derived from inorganic acids such
as hydrochloric,
hydrobromic, sulfuric, sulfamic, phosphoric, nitric and the like; and the
salts prepared from
organic acids such as acetic, propionic, succinic, glycolic, stearic, lactic,
malic, tartaric, citric,
ascorbic, pamoic, maleic, hydroxymaleic, phenylacetic, glutamic, benzoic,
salicylic,
sulfanilic, 2-acetoxybenzoic, fumaric, toluensulfonic, methanesulfonic, ethane
dislfonic,
oxalic, isethionic, and the like.
[0023] As used herein, the phrase "pharmaceutically acceptable" is used with
reference to compounds, materials, compositions, and/or dosage forms which
are, within the
scope of sound medical judgment, suitable for use in contact with the tissues
of human beings
and animals without excessive toxicity, irritation, allergic response, or
other problems or
complications commensurate with a reasonable benefit/risk ratio.
[0024] As used herein "subject" or "patient" encompasses mammals. In one
aspect,
the mammal is a human. In another aspect, the mammal is a non-human primate
such as
chimpanzee, and other apes and monkey species. In one aspect, the mammal is a
domestic
animal such as rabbit, dog, or cat. In another aspect, the mammal is a farm
animal such as
cattle, horse, sheep, goat, or swine. In another aspect, the mammal is a
laboratory animal,
including rodents, such as rats, mice and guinea pigs, and the like.
[0025] The urinary bladder has two important functions: storage of urine and
emptying. Storage of urine occurs at low pressure, which implies that the
detrusor muscle
relaxes during the filling phase. Emptying of the bladder requires a
coordinated contraction of
the detrusor muscle and relaxation of the sphincter muscles of the urethra.
Disturbances of
the storage function may result in lower urinary tract symptoms, such as
urgency, frequency,
and urge incontinence, the components of the overactive bladder syndrome. The
overactive
bladder syndrome, which may be due to involuntary contractions of the smooth
muscle of the
bladder (detrusor) during the storage phase, is a common and underreported
problem, the
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
prevalence of which has only recently been assessed.
[0026] One aspect of the present application relates to a method for reducing
the
frequency of urination by administering to a person in need thereof a
pharmaceutical
composition formulated in an extended-release formulation. The pharmaceutical
composition
comprises one or more analgesic agents and, optionally, one or more
antimuscarinic agents,
one or more antidiuretic agents, one or more spasmolytics and/or zolpidem. The
method can
be used for the treatment of nocturia and/or overactive bladder.
[0027] "Extended-release," also known as sustained-release (SR), sustained-
action
(SA), time-release (TR), controlled-release (CR), modified release (MR), or
continuous-
release (CR), is a mechanism used in medicine tablets or capsules to dissolve
slowly and
release the active ingredient overtime. The advantages of extended-release
tablets or capsules
are that they can often be taken less frequently than immediate-release
formulations of the
same drug, and that they keep steadier levels of the drug in the bloodstream,
thus extending
the duration of the drug action and lowering the peak amount of drug in the
bloodstream. For
example, an extended-release analgesic may allow a person to sleep through the
night without
getting up for the bathroom.
[0028] In one embodiment, the pharmaceutical composition is formulated for
extended-release by embedding the active ingredient in a matrix of insoluble
substance(s) such
as acrylics or chitin. An extended-release form is designed to release the
analgesic compound
at a predetermined rate by maintaining a constant drug level for a specific
period of time.
This can be achieved through a variety of formulations, including, but not
limited to,
liposomes and drug-polymer conjugates, such as hydrogels.
[0029] An extended-release formulation can be designed to release the active
agents
at a predetermined rate so as to maintain a constant drug level for a
specified, extended
period of time, such as up to about 24 hours, about 20 hours, about 16 hours,
about 12 hours,
about 10 hours, about 9 hours, about 8 hours, about 7 hours, about 6 hours,
about 5 hours,
about 4 hours, about 3 hours, about 2 hours, or about 1 hour following
administration or
following a lag period associated with delayed-release of the drug.
[0030] In certain preferred embodiments, the active agents are released over a
time
interval of between about 2 to about 10 hours. Alternatively, the active
agents may be
released over about 3, about 4, about 5, about 6, about 7, about 8, about 9,
about 10 hours,
about 12 hours, about 16 hours, about 20 hours or about 24 hours. In yet other
embodiments,
the active agents are released over a time period between about three to about
eight hours
following administration.
6
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
[0031] In some embodiments, the extended-release formulation comprises an
active
core comprised of one or more inert particles, each in the form of a bead,
pellet, pill, granular
particle, microcapsule, microsphere, microgranule, nanocapsule, or nanosphere
coated on its
surfaces with drugs in the form of e.g., a drug-containing coating or film-
forming
composition using, for example, fluid bed techniques or other methodologies
known to those
of skill in the art. The inert particle can be of various sizes, so long as it
is large enough to
remain poorly dissolved. Alternatively, the active core may be prepared by
granulating and
milling and/or by extrusion and spheronization of a polymer composition
containing the drug
substance.
[0032] The active agents may be introduced to the inert carrier by techniques
known
to one skilled in the art, such as drug layering, powder coating,
extrusion/spheronization,
roller compaction or granulation. The amount of drug in the core will depend
on the dose
that is required, and typically varies from about 5 to 90 weight %. Generally,
the polymeric
coating on the active core will be from about 1 to 50% based on the weight of
the coated
particle, depending on the lag time required and/or the polymers and coating
solvents chosen.
Those skilled in the art will be able to select an appropriate amount of drug
for coating onto
or incorporating into the core to achieve the desired dosage. In one
embodiment, the inactive
core may be a sugar sphere or a buffer crystal or an encapsulated buffer
crystal such as
calcium carbonate, sodium bicarbonate, fumaric acid, tartaric acid, etc. which
alters the
microenvironment of the drug to facilitate its release.
[0033] Extended-release formulations may utilize a variety of extended-release
coatings or mechanisms facilitating the gradual release of active agents over
time. In some
embodiments, the extended-release agent comprises a polymer controlling
release by
dissolution controlled release. In a particular embodiment, the active
agent(s) are
incorporated in a matrix comprising an insoluble polymer and drug particles or
granules
coated with polymeric materials of varying thickness. The polymeric material
may comprise
a lipid barrier comprising a waxy material, such as carnauba wax, beeswax,
spermaceti wax,
candellila wax, shallac wax, cocoa butter, cetostearyl alcohol, partially
hydrogenated
vegetable oils, ceresin, paraffin wax, ceresine, myristyl alcohol, stearyl
alcohol, cetyl alcohol
and stearic acid, along with surfactants, such as polyoxyethylene sorbitan
monooleate. When
contacted with an aqueous medium, such as biological fluids, the polymer
coating emulsifies
or erodes after a predetermined lag-time depending on the thickness of the
polymer coating.
The lag time is independent of gastrointestinal motility, pH, or gastric
residence.
[0034] In other embodiments, the extended-release agent comprises a polymeric
7
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
matrix effecting diffusion controlled release. The matrix may comprise one or
more
hydrophilic and/or water-swellable, matrix forming polymers, pH-dependent
polymers,
and/or pH-independent polymers.
[0035] In one embodiment, the extended-release formulation comprises a water
soluble or water-swellable matrix-forming polymer, optionally containing one
or more
solubility-enhancing excipients and/or release-promoting agents. Upon
solubilization of the
water soluble polymer, the active agent(s) dissolve (if soluble) and gradually
diffuse through
the hydrated portion of the matrix. The gel layer grows with time as more
water permeates
into the core of the matrix, increasing the thickness of the gel layer and
providing a diffusion
barrier to drug release. As the outer layer becomes fully hydrated, the
polymer chains
become completely relaxed and can no longer maintain the integrity of the gel
layer, leading
to disentanglement and erosion of the outer hydrated polymer on the surface of
the matrix.
Water continues to penetrate towards the core through the gel layer, until it
has been
completely eroded. Whereas soluble drugs are released by this combination of
diffusion and
erosion mechanisms, erosion is the predominant mechanism for insoluble drugs,
regardless of
dose.
[0036] Similarly, water-swellable polymers typically hydrate and swell in
biological
fluids forming a homogenous matrix structure that maintains its shape during
drug release
and serves as a carrier for the drug, solubility enhancers and/or release
promoters. The initial
matrix polymer hydration phase results in slow-release of the drug (lag
phase). Once the
water swellable polymer is fully hydrated and swollen, water within the matrix
can similarly
dissolve the drug substance and allow for its diffusion out through the matrix
coating.
[0037] Additionally, the porosity of the matrix can be increased due to the
leaching
out of pH-dependent release promoters so as to release the drug at a faster
rate. The rate of
the drug release then becomes constant and is a function of drug diffusion
through the
hydrated polymer gel. The release rate from the matrix is dependent upon
various factors,
including polymer type and level; drug solubility and dose; polymer: drug
ratio; filler type
and level; polymer to filler ratio; particle size of drug and polymer; and
porosity and shape of
the matrix.
[0038] Exemplary hydrophilic and/or water-swellable, matrix forming polymers
include, but are not limited to, cellulosic polymers, including hydroxyalkyl
celluloses and
carboxyalkyl celluloses, such as hydroxypropylmethylcellulose (HPMC),
hydroxypropylcellulose (HPC), hydroxyethylcellulose (HEC), methylcellulose
(MC),
carboxymethylcellulose (CMC), powdered cellulose such as microcrystalline
cellulose,
8
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
cellulose acetate, ethylcellulose, salts thereof, and combinations thereof;
alginates, gums,
including heteropolysaccharide gums and homopolysaccharide gums, such as
xanthan,
tragacanth, pectin, acacia, karaya, alginates, agar, guar, hydroxypropyl guar,
veegum,
carrageenan, locust bean gum, gellan gum, and derivatives thereofrom; acrylic
resins,
including polymers and copolymers of acrylic acid, methacrylic acid, methyl
acrylate and
methyl methacrylate and cross-linked polyacrylic acid derivatives such as
Carbomers (e.g.,
CARBOPOL , such as including CARBOPOL 71G NF, available in various molecular
weight grades from Noveon, Inc., Cincinnati, OH); carageenan; polyvinyl
acetate (e.g.,
KOLLIDON SR); polyvinyl pyrrolidone and its derivatives such as crospovidone;
polyethylene oxides; and polyvinyl alcohol. Preferred hydrophilic and water-
swellable
polymers include the cellulosic polymers, especially HPMC.
[0039] The extended-release formulation may further comprise at least one
binder
that is capable of cross-linking the hydrophilic compound to form a
hydrophilic polymer
matrix (i.e., a gel matrix) in an aqueous medium, including biological fluids.
[0040] Exemplary binders include homopolysaccharides, such as galactomannan
gums, guar gum, hydroxypropyl guar gum, hydroxypropylcellulose (HPC; e.g.,
Klucel EXF)
and locust bean gum. In other embodiments, the binder is an alginic acid
derivative, EIPC or
microcrystallized cellulose (MCC). Other binders include, but are not limited
to, starches,
microcrystalline cellulose, hydroxypropyl cellulose, hydroxyethyl cellulose,
hydroxypropylmethyl cellulose and polyvinylpyrrolidone.
[0041] In one embodiment, the introduction method is drug layering by spraying
a
suspension of active agent(s) and a binder onto the inert carrier.
[0042] The binder may be present in the bead formulation in an amount of from
about
0.1% to about 15% by weight, and preferably of from about 0.2% to about 10% by
weight.
[0043] In some embodiments, the hydrophilic polymer matrix may further include
an
ionic polymer, a non-ionic polymer, or water-insoluble hydrophobic polymer to
provide a
stronger gel layer and/or reduce pore quantity and dimensions in the matrix so
as to slow
diffusion and erosion rates and concomitant release of the active agent(s).
This may
additionally suppress the initial burst effect and produce a more steady,
"zero order release"
of active agent(s).
[0044] Exemplary ionic polymers for slowing dissolution rate include both
anionic
and cationic polymers. Exemplary anionic polymers include, for example, sodium
carboxymethylcellulose (Na CMC), sodium alginate, polymers of acrylic acid or
carbomers
(e.g., CARBOPOL 934, 940, 974P NF); enteric polymers, such as polyvinyl
acetate
9
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
phthalate (PVAP), methacrylic acid copolymers (e.g., EUDRAGIT L100, L 30D 55,
A, and
FS 30D), hypromellose acetate succinate (AQUAT HPMCAS); and xanthan gum.
Exemplary cationic polymers include, for example, dimethylaminoethyl
methacrylate
copolymer (e.g., EUDRAGIT E 100). Incorporation of anionic polymers,
particularly
enteric polymers, is useful for developing a pH-independent release profile
for weakly basic
drugs as compared to hydrophilic polymer alone.
[0045] Exemplary non-ionic polymers for slowing dissolution rate, include, for
example, hydroxypropylcellulose (HPC) and polyethylene oxide (PEO) (e.g.,
POLYOXTM)
[0046] Exemplary hydrophobic polymers include ethylcellulose (e.g., ETHOCELTm,
SURELEASE ), cellulose acetate, methacrylic acid copolymers (e.g., EUDRAGIT
NE
30D), ammonio-methacrylate copolymers (e.g., EUDRAGIT RL 100 or PO RS100),
polyvinyl acetate, glyceryl monostearate, fatty acids, such as acetyl tributyl
citrate, and
combinations and derivatives thereof.
[0047] The swellable polymer can be incorporated in the formulation in
proportion
from 1% to 50% by weight, preferably from 5% to 40% by weight, most preferably
from 5%
to 20% by weight. The swellable polymers and binders may be incorporated in
the
formulation either prior to or after granulation. The polymers can also be
dispersed in
organic solvents or hydro-alcohols and sprayed during granulation.
[0048] Exemplary release-promoting agents include pH-dependent enteric
polymers
that remain intact at pH value lower than about 4.0 and dissolve at pH values
higher than 4.0,
preferably higher than 5.0, most preferably about 6.0, are considered useful
as release-
promoting agents for this invention. Exemplary pH-dependent polymers include,
but are not
limited to, methacarylic acid copolymers, methacrylic acid-methyl methacrylate
copolymers
(e.g., EUDRAGIT L100 (Type A), EUDRAGIT S100 (Type B), Rohm GmbH, Germany;
methacrylic acid-ethyl acrylate copolymers (e.g., EUDRAGIT L100-55 (Type C)
and
EUDRAGIT L30D-55 copolymer dispersion, Rohm GmbH, Germany); copolymers of
methacrylic acid-methyl methacrylate and methyl methacrylate (EUDRAGIT FS);
terpolymers of methacrylic acid, methacrylate, and ethyl acrylate; cellulose
acetate phthalates
(CAP); hydroxypropyl methylcellulose phthalate (HPMCP) (e.g., HP-55, HP-50, HP-
55S,
Shinetsu Chemical, Japan); polyvinyl acetate phthalates (PVAP) (e.g., COATERIC
,
OPADRY enteric white OY-P-7171); polyvinylbutyrate acetate; cellulose acetate
succinates
(CAS); hydroxypropyl methylcellulose acetate succinate (HPMCAS), e.g., HPMCAS
LF
Grade, MF Grade, HF Grade, including AQOAT LF and AQOAT MF (Shin-Etsu
Chemical, Japan); Shinetsu Chemical, Japan); shellac (e.g., MARCOATTm 125 &
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
MARCOATTm 125N); vinyl acetate-maleic anhydride copolymer; styrene-maleic
monoester
copolymer; carboxymethyl ethylcellulose (CMEC, Freund Corporation, Japan);
cellulose
acetate phthalates (CAP) (e.g., AQUATERIC8); cellulose acetate trimellitates
(CAT); and
mixtures of two or more thereof at weight ratios between about 2:1 to about
5:1, such as, for
instance, a mixture of EUDRAGIT L 100-55 and EUDRAGIT S 100 at a weight
ratio of
about 3:1 to about 2:1, or a mixture of EUDRAGIT L 30 D-55 and EUDRAGIT FS
at a
weight ratio of about 3:1 to about 5:1.
[0049] These polymers may be used either alone or in combination, or together
with
polymers other than those mentioned above. Preferred enteric pH-dependent
polymers are
the pharmaceutically acceptable methacrylic acid copolymers. These copolymers
are anionic
polymers based on methacrylic acid and methyl methacrylate and, preferably,
have a mean
molecular weight of about 135,000. A ratio of free carboxyl groups to methyl-
esterified
carboxyl groups in these copolymers may range, for example, from 1:1 to 1:3,
e.g. around 1:1
or 1:2. Such polymers are sold under the trade name Eudragit such as the
Eudragit L series
e.g., Eudragit L 12.5 , Eudragit L 12.5P , Eudragit L100 , Eudragit L 100-55 ,
Eudragit L-
301D , Eudragit L-30 D-55 , the Eudragit S series e.g., Eudragit S 12.5 ,
Eudragit S 12.5P ,
Eudragit S100 . The release promoters are not limited to pH dependent
polymers. Other
hydrophilic molecules that dissolve rapidly and leach out of the dosage form
quickly leaving
a porous structure can be also be used for the same purpose.
[0050] In some embodiments, the matrix may include a combination of release
promoters and solubility enhancers. The solubility enhancers can be ionic and
non-ionic
surfactants, complexing agents, hydrophilic polymers, pH modifiers, such as
acidifying
agents and alkalinizing agents, as well as molecules that increase the
solubility of poorly
soluble drug through molecular entrapment. Several solubility enhancers can be
utilized
simultaneously.
[0051] Solubility enhancers may include surface active agents, such as sodium
docusate, sodium lauryl sulfate, sodium stearyl fumarate, Tweens and Spans
(PEO modified
sorbitan monoesters and fatty acid sorbitan esters), poly(ethylene oxide)-
polypropylene
oxide-poly(ethylene oxide) block copolymers (aka PLURONICSTm); complexing
agents such
as low molecular weight polyvinyl pyrrolidone and low molecular weight
hydroxypropyl
methyl cellulose; molecules that aid solubility by molecular entrapment such
as
cyclodextrins, and pH modifying agents, including acidifying agents such as
citric acid,
fumaric acid, tartaric acid, and hydrochloric acid; and alkalizing agents such
as meglumine
and sodium hydroxide.
11
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
[0052] Solubility enhancing agents typically constitute from 1% to 80% by
weight,
preferably from 1% to 60%, more preferably from 1% to 50%, of the dosage form
and can be
incorporated in a variety of ways. They can be incorporated in the formulation
prior to
granulation in dry or wet form. They can also be added to the formulation
after the rest of the
materials are granulated or otherwise processed. During granulation,
solubilizers can be
sprayed as solutions with or without a binder.
[0053] In one embodiment, the extended-release formulation comprises a water-
insoluble water-permeable polymeric coating or matrix comprising one or more
water-
insoluble water-permeable film-forming over the active core. The coating may
additionally
include one or more water soluble polymers and/or one or more plasticizers.
The water-
insoluble polymer coating comprises a barrier coating for release of active
agents in the core,
wherein lower molecular weight (viscosity) grades exhibit faster release rates
as compared to
higher viscosity grades.
[0054] In preferred embodiments, the water-insoluble film-forming polymers
include
one or more alkyl cellulose ethers, such as ethyl celluloses and mixtures
thereof, (e.g., ethyl
cellulose grades PR100, PR45, PR20, PR10 and PR7; ETHOCEL , Dow).
[0055] An exemplary water-soluble polymer such as polyvinylpyrrolidone
(POVIDONEED), hydroxypropyl methylcellulose, hydroxypropyl cellulose and
mixtures
thereof.
[0056] In some embodiments, the water-insoluble polymer provides suitable
properties (e.g., extended-release characteristics, mechanical properties, and
coating
properties) without the need for a plasticizer. For example, coatings
comprising polyvinyl
acetate (PVA), neutral copolymers of acrylate/methacrylate esters such as
commercially
available Eudragit NE3OD from Evonik Industries, ethyl cellulose in
combination with
hydroxypropylcellulose, waxes, etc. can be applied without plasticizers.
[0057] In yet another embodiment, the water-insoluble polymer matrix may
further
include a plasticizer. The amount of plasticizer required depends upon the
plasticizer, the
properties of the water-insoluble polymer, and the ultimate desired properties
of the coating.
Suitable levels of plasticizer range from about 1% to about 20%, from about 3%
to about
20%, about 3% to about 5%, about 7% to about 10%, about 12% to about 15%,
about 17% to
about 20%, or about 1%, about 2%, about 3%, about 4%, about 5%, about 6%,
about 7%,
about 8%, about 9%, about 10%, about 15%, or about 20% by weight relative to
the total
weight of the coating, inclusive of all ranges and sub-ranges therebetween.
[0058] Exemplary plasticizers include, but are not limited to, triacetin,
acetylated
12
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
monoglyceride, oils (castor oil, hydrogenated castor oil, rape seed oil,
sesame oil, olive oil,
etc.); citrate esters, triethyl citrate, acetyltriethyl citrate acetyltributyl
citrate, tributyl citrate,
acetyl tri-n-butyl citrate, diethyl phthalate, dibutyl phthalate, dioctyl
phthalate, methyl
paraben, propyl paraben, propyl paraben, butyl paraben, diethyl sebacate,
dibutyl sebacate,
glyceroltributyrate, substituted triglycerides and glycerides, monoacetylated
and diacetylated
glycerides (e.g., MYVACET 9-45), glyceryl monostearate, glycerol tributyrate,
polysorbate
80, polyethyleneglycol (such as PEG-4000, PEG-400), propyleneglycol, 1,2-
propyleneglycol,
glycerin, sorbitol, diethyl oxalate, diethyl malate, diethyl fumarate,
diethylmalonate, dibutyl
succinate, fatty acids, glycerin, sorbitol, diethyl oxalate, diethyl malate,
diethyl maleate,
diethyl fumarate, diethyl succinate, diethyl malonate, dioctyl phthalate,
dibutyl sebacate, and
mixtures thereof The plasticizer can have surfactant properties, such that it
can act as a
release modifier. For example, non-ionic detergents such at Brij 58
(polyoxyethylene (20)
cetyl ether), and the like, can be used.
[0059] Plasticizers can be high boiling point organic solvents used to impart
flexibility to otherwise hard or brittle polymeric materials and can affect
the release profile
for the active agent(s). Plasticizers generally cause a reduction in the
cohesive intermolecular
forces along the polymer chains resulting in various changes in polymer
properties including
a reduction in tensile strength, and increase in elongation and a reduction in
the glass
transition or softening temperature of the polymer. The amount and choice of
the plasticizer
can affect the hardness of a tablet, for example, and can even affect its
dissolution or
disintegration characteristics, as well as its physical and chemical
stability. Certain
plasticizers can increase the elasticity and/or pliability of a coat, thereby
decreasing the coat's
brittleness.
[0060] In another embodiment, the extended-release formulation comprises a
combination of at least two gel-forming polymers, including at least one non-
ionic gel-
forming polymer and/or at least one anionic gel-forming polymer. The gel
formed by the
combination of gel-forming polymers provides controlled release, such that
when the
formulation is ingested and comes into contact with the gastrointestinal
fluids, the polymers
nearest the surface hydrate to form a viscous gel layer. Because of the high
viscosity, the
viscous layer dissolves away only gradually, exposing the material below to
the same
process. The mass thus dissolves away slowly, thereby slowly releasing the
active ingredient
into the gastrointestinal fluids. The combination of at least two gel-forming
polymers enables
properties of the resultant gel, such as viscosity, to be manipulated in order
to provide the
desired release profile.
13
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
[0061] In a particular embodiment, the formulation comprises at least one non-
ionic
gel-forming polymer and at least one anionic gel-forming polymer. In another
embodiment,
the formulation comprises two different non-ionic gel-forming polymers. In yet
another
embodiment, the formulation comprises a combination of non-ionic gel-forming
polymers of
the same chemistry, but having different solubilities, viscosities, and/or
molecular weights
(for example a combination of hydroxyproplyl methylcellulose of different
viscosity grades,
such as HPMC K100 and HPMC K 15M or HPMC K 1 00M).
[0062] Exemplary anionic gel forming polymers include, but are not limited to,
sodium carboxymethylcellulose (Na CMC), carboxymethyl cellulose (CMC), anionic
polysaccharides such as sodium alginate, alginic acid, pectin, polyglucuronic
acid (poly-a-
and -3-1,4-glucuronic acid), polygalacturonic acid (pectic acid), chondroitin
sulfate,
carrageenan, furcellaran, anionic gums such as xanthan gum, polymers of
acrylic acid or
carbomers (Carbopol 934, 940, 974P NF), Carbopol copolymers, a Pemulen
polymer,
polycarbophil, and others.
[0063] Exemplary non-ionic gel-forming polymers include, but are not limited
to,
Povidone (PVP: polyvinyl pyrrolidone), polyvinyl alcohol, copolymer of PVP and
polyvinyl
acetate, HPC (hydroxypropyl cellulose), HPMC (hydroxypropyl methylcellulose),
hydroxyethyl cellulose, hydroxymethyl cellulose, gelatin, polyethylene oxide,
acacia, dextrin,
starch, polyhydroxyethylmethacrylate (PHEMA), water soluble nonionic
polymethacrylates
and their copolymers, modified cellulose, modified polysaccharides, nonionic
gums, nonionic
polysaccharides and/or mixtures thereof.
[0064] The formulation may optionally comprise an enteric polymer as described
above, and/or at least one excipient, such as a filler, a binder (as described
above), a
disintegrant, and/or a flow aid or glidant.
[0065] Exemplary fillers include but are not limited to, lactose, glucose,
fructose,
sucrose, dicalcium phosphate, sugar alcohols also known as "sugar polyol" such
as sorbitol,
manitol, lactitol, xylitol, isomalt, erythritol, and hydrogenated starch
hydrolysates (a blend of
several sugar alcohols), corn starch, potato starch, sodium
carboxymethycellulose,
ethylcellulose and cellulose acetate, enteric polymers, or a mixture thereof.
[0066] Exemplary binders, include but are not limited to, water-soluble
hydrophilic
polymers, such as Povidone (PVP: polyvinyl pyrrolidone), copovidone (a
copolymer of
polyvinyl pyrrolidone and polyvinyl acetate), low molecular weight HPC
(hydroxypropyl
cellulose) low molecular weight HPMC (hydroxypropyl methylcellulose), low
molecular
weight carboxy methyl cellulose, ethylcellulose, gelatin, polyethylene oxide,
acacia, dextrin,
14
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
magnesium aluminum silicate, starch, and polymethacrylates such as Eudragit NE
30D,
Eudragit RL, Eudragit RS, Eudragit E, polyvinyl acetate, and enteric polymers,
or mixtures
thereof.
[0067] Exemplary disintegrants include but are not limited to low-substituted
carboxymethyl cellulose sodium, crospovidone (cross-linked polyvinyl
pyrrolidone), sodium
carboxymethyl starch (sodium starch glycolate), cross-linked sodium
carboxymethyl
cellulose (Croscarmellose), pregelatinized starch (starch 1500),
microcrystalline cellulose,
water insoluble starch, calcium carboxymethyl cellulose, low substituted
hydroxypropyl
cellulose, and magnesium or aluminum silicate.
[0068] Exemplary glidants include but are not limited to, magnesium, silicon
dioxide,
talc, starch, titanium dioxide, and the like.
[00691 In yet another embodiment, the extended-release formulation is formed
by
coating a water soluble/dispersible drug-containing particle, such as a bead
or bead
population therein (as described above), with a coating material, and,
optionally, a pore
former and other excipients. The coating material is preferably selected from
a group
comprising cellulosic polymers, such as ethylcellulose (e.g., SURELEASO,
methylcellulose, hydroxypropyl cellulose, hydroxypropylmethyl cellulose,
cellulose acetate,
and cellulose acetate phthalate; polyvinyl alcohol; acrylic polymers such as
polyacrylates,
polymethacrylates and copolymers thereof, and other water-based or solvent-
based coating
materials. The release-controlling coating for a given bead population may be
controlled by
at least one parameter of the release controlling coating, such as the nature
of the coating,
coating level, type and concentration of a pore former, process parameters and
combinations
thereof. Thus, changing a parameter, such as a pore former concentration, or
the conditions
of the curing, allows for changes in the release of active agent(s) from any
given bead
population, thereby allowing for selective adjustment of the formulation to a
pre-determined
release profile.
[0070] Pore formers suitable for use in the release controlling coating herein
can be
organic or inorganic agents, and include materials that can be dissolved,
extracted or leached
from the coating in the environment of use. Exemplary pore forming agents
include, but are
not limited to, organic compounds such as mono-, oligo-, and polysaccharides
including
sucrose, glucose, fructose, mannitol, mannose, galactose, sorbitol, pullulan,
dextran;
polymers soluble in the environment of use such as water-soluble hydrophilic
polymers,
hydroxyalkylcelluloses, carboxyalkylcelluloses, hydroxypropylmethylcellulose,
cellulose
ethers, acrylic resins, polyvinylpyrrolidone, cross-linked
polyvinylpyrrolidone, polyethylene
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
oxide, Carbowaxes, Carbopol, and the like, diols, polyols, polyhydric
alcohols, polyalkylene
glycols, polyethylene glycols, polypropylene glycols, or block polymers
thereof, polyglycols,
poly(a-Q)alkylenediols; inorganic compounds such as alkali metal salts,
lithium carbonate,
sodium chloride, sodium bromide, potassium chloride, potassium sulfate,
potassium
phosphate, sodium acetate, sodium citrate, suitable calcium salts, combination
thereof, and
the like.
[0071] The release controlling coating can further comprise other additives
known in
the art, such as plasticizers, anti-adherents, glidants (or flow aids), and
antifoams.
[0072] In some embodiments, the coated particles or beads may additionally
include
an "overcoat," to provide, e.g., moisture protection, static charge reduction,
taste-masking,
flavoring, coloring, and/or polish or other cosmetic appeal to the beads.
Suitable coating
materials for such an overcoat are known in the art, and include, but are not
limited to,
cellulosic polymers such as hydroxypropylmethylcellulose,
hydroxypropylcellulose and
microcrystalline cellulose, or combinations thereof (for example, various
OPADRY coating
materials).
[0073] The coated particles or beads may additionally contain enhancers that
may be
exemplified by, but not limited to, solubility enhancers, dissolution
enhancers, absorption
enhancers, permeability enhancers, stabilizers, complexing agents, enzyme
inhibitors, p-
glycoprotein inhibitors, and multidrug resistance protein inhibitors.
Alternatively, the
formulation can also contain enhancers that are separated from the coated
particles, for
example in a separate population of beads or as a powder. In yet another
embodiment, the
enhancer(s) may be contained in a separate layer on coated particles either
under or above the
release controlling coating.
[0074] In other embodiments, the extended-release formulation is formulated to
release the active agent(s) by an osmotic mechanism. By way of example, a
capsule may be
formulated with a single osmotic unit or it may incorporate 2, 3, 4, 5, or 6
push-pull units
encapsulated within a hard gelatin capsule, whereby each bilayer push pull
unit contains an
osmotic push layer and a drug layer, both surrounded by a semi-permeable
membrane. One
or more orifices are drilled through the membrane next to the drug layer. This
membrane
may be additionally covered with a pH-dependent enteric coating to prevent
release until
after gastric emptying. The gelatin capsule dissolves immediately after
ingestion. As the
push pull unit(s) enter the small intestine, the enteric coating breaks down,
which then allows
fluid to flow through the semi-permeable membrane, swelling the osmotic push
compartment
to force to force drugs out through the orifice(s) at a rate precisely
controlled by the rate of
16
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
water transport through the semi-permeable membrane. Release of drugs can
occur over a
constant rate for up to 24 hours or more.
[0075] The osmotic push layer comprises one or more osmotic agents creating
the
driving force for transport of water through the semi-permeable membrane into
the core of
the delivery vehicle. One class of osmotic agents includes water-swellable
hydrophilic
polymers, also referred to as "osmopolymers" and "hydrogels," including, but
not limited to,
hydrophilic vinyl and acrylic polymers, polysaccharides such as calcium
alginate,
polyethylene oxide (PEO), polyethylene glycol (PEG), polypropylene glycol
(PPG), poly(2-
hydroxyethyl methacrylate), poly(acrylic) acid, poly(methacrylic) acid,
polyvinylpyrrolidone
(PVP), crosslinked PVP, polyvinyl alcohol (PVA), PVA/PVP copolymers, PVA/PVP
copolymers with hydrophobic monomers such as methyl methacrylate and vinyl
acetate,
hydrophilic polyurethanes containing large PEO blocks, sodium croscarmellose,
carrageenan,
hydroxyethyl cellulose (HEC), hydroxypropyl cellulose (HPC), hydroxypropyl
methyl
cellulose (HPMC), carboxymethyl cellulose (CMC) and carboxyethyl, cellulose
(CEC),
sodium alginate, polycarbophil, gelatin, xanthan gum, and sodium starch
glycolate.
[0076] Another class of osmotic agents includes osmogens, which are capable of
imbibing water to effect an osmotic pressure gradient across the semi-
permeable membrane.
Exemplary osmogens include, but are not limited to, inorganic salts, such as
magnesium
sulfate, magnesium chloride, calcium chloride, sodium chloride, lithium
chloride, potassium
sulfate, potassium phosphates, sodium carbonate, sodium sulfite, lithium
sulfate, potassium
chloride, and sodium sulfate; sugars, such as dextrose, fructose, glucose,
inositol, lactose,
maltose, mannitol, raffinose, sorbitol, sucrose, trehalose, and xylitol;
organic acids, such as
ascorbic acid, benzoic acid, fumaric acid, citric acid, maleic acid, sebacic
acid, sorbic acid,
adipic acid, edetic acid, glutamic acid, p-toluenesulfonic acid, succinic
acid, and tartaric acid;
urea; and mixtures thereof
[0077] Materials useful in forming the semipermeable membrane include various
grades of acrylics, vinyls, ethers, polyamides, polyesters, and cellulosic
derivatives that are
water-permeable and water-insoluble at physiologically relevant pHs, or are
susceptible to
being rendered water-insoluble by chemical alteration, such as crosslinking.
[0078] In some embodiments, the extended-release formulation may comprise a
polysaccharide coating that is resistant to erosion in both the stomach and
intestine. Such
polymers can be only degraded in the colon, which contains a large microflora
containing
biodegradable enzymes breaking down, for example, the polysaccharide coatings
to release
the drug contents in a controlled, time-dependent manner. Exemplary
polysaccharide
17
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
coatings may include, for example, amylose, arabinogalactan, chitosan,
chondroitin sulfate,
cyclodextrin, dextran, guar gum, pectin, xylan, and combinations or
derivatives therefrom.
[0079] In some embodiments, the pharmaceutical composition is formulated for
delayed extended-release. As used herein, the term "delayed-release" refers to
a medication
that does not immediately disintegrate and release the active ingredient(s)
into the body. In
some embodiments, the term "delayed extended-release" is used with reference
to a drug
formulation having a release profile in which there is a predetermined delay
in the release of
the drug following administration. In some embodiments, the delayed extended-
release
formulation includes an extended-release formulation coated with an enteric
coating, which is
a barrier applied to oral medication that prevents release of medication
before it reaches the
small intestine. Delayed-release formulations, such as enteric coatings,
prevent drugs having
an irritant effect on the stomach, such as aspirin, from dissolving in the
stomach. Such
coatings are also used to protect acid-unstable drugs from the stomach's
acidic exposure,
delivering them instead to a basic pH environment (intestine's pH 5.5 and
above) where they
do not degrade, and give their desired action.
[0080] The term "pulsatile release" is a type of delayed-release, which is
used herein
with reference to a drug formulation that provides rapid and transient release
of the drug
within a short time period immediately after a predetermined lag period,
thereby producing a
"pulsed" plasma profile of the drug after drug administration. Formulations
may be designed
to provide a single pulsatile release or multiple pulsatile releases at
predetermined time
intervals following administration, or a pulsatile release (e.g., 20-60% of
the active
ingredient) followed with extended release over a period of time (e.g., a
continuous release of
the remainder of the active ingredient).
[0081] A delayed-release or pulsatile release formulation generally comprises
one or
more elements covered with a barrier coating, which dissolves, erodes or
ruptures following a
specified lag phase. In some embodiments, the pharmaceutical composition of
the present
application is formulated for extended-release or delayed extended-release and
comprises
100% of the total dosage of a given active agent administered in a single unit
dose. In other
embodiments, the pharmaceutical composition comprises an extended/delayed-
release
component and an immediate-release component. In some embodiments, the
immediate-
release component and the extended/delayed-release component contain the same
active
ingredient. In other embodiments, the immediate-release component and the
extended/delayed-release component contain different active ingredients (e.g.,
an analgesic in
one component and an antimuscarinic agent in another component). In some
embodiments,
18
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
the first and second components each contains an analgesic selected from the
group
consisting of aspirin, ibuprofen, naproxen sodium, indomethacin, nabumetone,
and
acetaminophen. In other embodiments, the extended/delayed-release component is
coated
with an enteric coating. In other embodiments, the immediate-release component
and/or the
extended/delayed-release component further comprises an antimuscarinic agent
selected from
the group consisting of oxybutynin, solifenacin, darifenacin and atropine. In
other
embodiments, the analgesic agent in each component is administered orally at a
daily dose of
mg - 2000 mg, 20 mg - 1000 mg, 50 mg - 500 mg or 250-1000 mg. In other
embodiments,
the immediate-release component and/or the extended/delayed-release component
further
comprises an antidiuretic agent, an antimuscarinic agent or both. In other
embodiments, the
treatment method includes administering to a subject a diuretic at least 8 or
7 hours prior to a
target time, such as bedtime, and administering to the subject the
pharmaceutical composition
comprising the immediate-release component and/or the extended/delayed-release
component
within 2 hours prior to the target time.
[0082] In other embodiments, the "immediate-release" component provide about 5-
50% of the total dosage of the active agent(s) and the "extended-release"
component provides
50-95% of the total dosage of the active agent(s) to be delivered by the
pharmaceutical
formulation. For example, the immediate-release component may provide about 20-
60%, or
about 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60% of the total dosage of the
active
agent(s) to be delivered by the pharmaceutical formulation. The extended-
release component
provides about 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75% or 80% of the total
dosage of
the active agent(s) to be delivered by the formulation. In some embodiments,
the extended-
release component further comprises a barrier coating to delay the release of
the active agent.
[0083] A barrier coating for delayed-release may consist of a variety of
different
materials, depending on the objective. In addition, a formulation may comprise
a plurality of
barrier coatings to facilitate release in a temporal manner. The coating may
be a sugar
coating, a film coating (e.g., based on hydroxypropyl methylcellulose,
methylcellulose,
methyl hydroxyethylcellulose, hydroxypropylcellulose, carboxymethylcellulose,
acrylate
copolymers, polyethylene glycols and/or polyvinylpyrrolidone), or a coating
based on
methacrylic acid copolymer, cellulose acetate phthalate, hydroxypropyl
methylcellulose
phthalate, hydroxypropyl methylcellulose acetate succinate, polyvinyl acetate
phthalate,
shellac, and/or ethylcellulose. Furthermore, the formulation may additionally
include a time
delay material such as, for example, glyceryl monostearate or glyceryl
distearate.
[0084] In some embodiments, the delayed, extended-release formulation includes
an
19
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
enteric coating comprised one or more polymers facilitating release of active
agents in
proximal or distal regions of the gastrointestinal tract. As used herein, the
term "enteric
polymer coating" is a coating comprising of one or more polymers having a pH
dependent or
pH-independent release profile. An enteric coated pill will not dissolve in
the acidic juices of
the stomach (pH ¨3), but they will in the alkaline (pH 7-9) environment
present in the small
intestine or colon. An enteric polymer coating typically resists releases of
the active agents
until some time after a gastric emptying lag period of about 3-4 hours after
administration.
[0085] pH dependent enteric coatings comprises one or more pH-dependent or pH-
sensitive polymers that maintain their structural integrity at low pH, as in
the stomach, but
dissolve in higher pH environments in more distal regions of the
gastrointestinal tract, such as
the small intestine, where the drug contents are released. For purposes of the
present
invention, "pH dependent" is defined as having characteristics (e.g.,
dissolution) which vary
according to environmental pH. Exemplary pH-dependent polymers include, but
are not
limited to, methacarylic acid copolymers, methacrylic acid-methyl methacrylate
copolymers
(e.g., EUDRAGIT L100 (Type A), EUDRAGIT S100 (Type B), Rohm GmbH, Germany;
methacrylic acid-ethyl acrylate copolymers (e.g., EUDRAGIT L100-55 (Type C)
and
EUDRAGIT L30D-55 copolymer dispersion, Rohm GmbH, Germany); copolymers of
methacrylic acid-methyl methacrylate and methyl methacrylate (EUDRAGIT FS);
terpolymers of methacrylic acid, methacrylate, and ethyl acrylate; cellulose
acetate phthalates
(CAP); hydroxypropyl methylcellulose phthalate (HPMCP) (e.g., HP-55, HP-50, HP-
55S,
Shinetsu Chemical, Japan); polyvinyl acetate phthalates (PVAP) (e.g., COATERIC
,
OPADRY enteric white OY-P-7171); cellulose acetate succinates (CAS);
hydroxypropyl
methylcellulose acetate succinate (HPMCAS), e.g., HPMCAS LF Grade, MF Grade,
HF
Grade, including AQOAT LF and AQOAT MF (Shin-Etsu Chemical, Japan); Shinetsu
Chemical, Japan); shellac (e.g., MarcoatTM 125 & MarcoatTM 125N);
carboxymethyl
ethylcellulose (CMEC, Freund Corporation, Japan), cellulose acetate phthalates
(CAP) (e.g.,
AQUATERIC ); cellulose acetate trimellitates (CAT); and mixtures of two or
more thereof
at weight ratios between about 2:1 to about 5:1, such as, for instance, a
mixture of
EUDRAGIT L 100-55 and EUDRAGIT S 100 at a weight ratio of about 3:1 to about
2:1,
or a mixture of EUDRAGIT L 30 D-55 and EUDRAGIT FS at a weight ratio of
about 3:1
to about 5:1.
[0086] pH-dependent polymers typically exhibit a characteristic pH optimum for
dissolution. In some embodiments, the pH-dependent polymer exhibits a pH
optimum
between about 5.0 and 5.5, between about 5.5 and 6.0, between about 6.0 and
6.5, or between
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
about 6.5 and 7Ø In other embodiments, the pH-dependent polymer exhibits a
pH optimum
of >5.0, of >5.5, of >6.0, of >6.5, or of >7Ø
[0087] These polymers may be used either alone or in combination, or together
with
polymers other than those mentioned above. Preferred enteric pH-dependent
polymers are
the pharmaceutically acceptable methacrylic acid copolymers. These copolymers
are anionic
polymers based on methacrylic acid and methyl methacrylate and, preferably,
have a mean
molecular weight of about 135,000. A ratio of free carboxyl groups to methyl-
esterified
carboxyl groups in these copolymers may range, for example, from 1:1 to 1:3,
e.g. around 1:1
or 1:2. Such polymers are sold under the trade name Eudragit such as the
Eudragit L series
e.g., Eudragit L 12.5 , Eudragit L 12.5P , Eudragit L100 , Eudragit L 100-55 ,
Eudragit L-
30D , Eudragit L-30 D-55 , the Eudragit S series e.g., Eudragit S 12.5 ,
Eudragit S 12.5P ,
Eudragit S100 . The release promoters are not limited to pH dependent
polymers. Other
hydrophilic molecules that dissolve rapidly and leach out of the dosage form
quickly leaving
a porous structure can be also be used for the same purpose.
[0088] In certain embodiment, the coating methodology employs the blending of
one
or more pH-dependent and one or more pH-independent polymers. The blending of
pH-
dependent and pH-independent polymers can reduce the release rate of active
ingredients
once the soluble polymer has reached its optimum pH of solubilization.
[0089] In some embodiments, a "time-controlled" or "time-dependent" release
profile
can be obtained using a water insoluble capsule body containing one or more
active agents,
wherein the capsule body closed at one end with an insoluble, but permeable
and swellable
hydrogel plug. Upon contact with gastrointestinal fluid or dissolution medium,
the plug
swells, pushing itself out of the capsule and releasing the drugs after a pre-
determined lag
time, which can be controlled by e.g., the position and dimensions of the
plug. The capsule
body may be further coated with an outer pH-dependent enteric coating keeping
the capsule
intact until it reaches the small intestine. Suitable plug materials include,
for example,
polymethacrylates, erodible compressed polymers (e.g., HPMC, polyvinyl
alcohol),
congealed melted polymer (e.g., glyceryl mono oleate) and enzymatically
controlled erodible
polymers (e.g., polysaccharides, such as amylose, arabinogalactan, chitosan,
chondroitin
sulfate, cyclodextrin, dextran, guar gum, pectin and xylan).
[0090] In other embodiments, capsules or bilayered tablets may be formulated
to
contain a drug-containing core, covered by a swelling layer, and an outer
insoluble, but semi-
permeable polymer coating or membrane. The lag time prior to rupture can be
controlled by
the permeation and mechanical properties of the polymer coating and the
swelling behavior
21
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
of the swelling layer. Typically, the swelling layer comprises one or more
swelling agents,
such as swellable hydrophilic polymers that swell and retain water in their
structures.
[0091] Exemplary water swellable materials to be used in the delayed-release
coating
include, but are not limited to, polyethylene oxide (having e.g., an average
molecular weight
between 1,000,000 to 7,000,000, such as POLY0X ), methylcellulose,
hydroxypropyl
cellulose, hydroxypropyl methylcellulose; polyalkylene oxides having a weight
average
molecular weight of 100,000 to 6,000,000, including but not limited to
poly(methylene
oxide), poly(butylene oxide); poly(hydroxy alkyl methacrylate) having a
molecular weight of
from 25,000 to 5,000,000; poly(vinyl)alcohol, having a low acetal residue,
which is cross-
linked with glyoxal, formaldehyde or glutaraldehyde and having a degree of
polymerization
of from 200 to 30,000; mixtures of methyl cellulose, cross-linked agar and
carboxymethyl
cellulose; hydrogel forming copolymers produced by forming a dispersion of a
finely divided
copolymer of maleic anhydride with styrene, ethylene, propylene, butylene or
isobutylene
cross-linked with from 0.001 to 0.5 moles of saturated cross-linking agent per
mole of maleic
anyhydride in the copolymer; CARBOPOL acidic carboxy polymers having a
molecular
weight of 450,000 to 4,000,000; CYANAMER polyacrylamides; cross-linked water
swellable indenemaleicanhydride polymers; GOODRITE polyacrylic acid having a
molecular weight of 80,000 to 200,000; starch graft copolymers; AQUA-KEEPS
acrylate
polymer polysaccharides composed of condensed glucose units such as diester
cross-linked
polyglucan; carbomers having a viscosity of 3,000 to 60,000 mPa as a 0.5%4%
w/v aqueous
solution; cellulose ethers such as hydroxypropylcellulose having a viscosity
of about 1000-
7000 mPa s as a 1% w/w aqueous solution (25 C); hydroxypropyl methylcellulose
having a
viscosity of about 1000 or higher, preferably 2,500 or higher to a maximum of
25,000 mPa as
a 2% w/v aqueous solution; polyvinylpyrrolidone having a viscosity of about
300-700 mPa s
as a 10% w/v aqueous solution at 20 C; and combinations thereof.
[0092] Alternatively, the release time of the drugs can be controlled by a
disintegration lag time depending on the balance between the tolerability and
thickness of a
water insoluble polymer membrane (such as ethyl cellulose, EC) containing
predefined
micropores at the bottom of the body and the amount of a swellable excipient,
such as low
substituted hydroxypropyl cellulose (L-HPC) and sodium glycolate. After oral
administration, GI fluids permeate through the micropores, causing swelling of
the swellable
excipients, which produces an inner pressure disengaging the capsular
components, including
a first capsule body containing the swellable materials, a second capsule body
containing the
drugs, and an outer cap attached to the first capsule body.
22
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
[0093] The enteric layer may further comprise anti-tackiness agents, such as
talc or
glyceryl monostearate and/or plasticizers. The enteric layer may further
comprise one or
more plasticizers including, but not limited to, triethyl citrate, acetyl
triethyl citrate,
acetyltributyl citrate, polyethylene glycol acetylated monoglycerides,
glycerin, triacetin,
propylene glycol, phthalate esters (e.g., diethyl phthalate, dibutyl
phthalate), titanium dioxide,
ferric oxides, castor oil, sorbitol and dibutyl sebacate.
[0094] In another embodiment, the delayed release formulation employs a water-
permeable but insoluble film coating to enclose the active ingredient and an
osmotic agent.
As water from the gut slowly diffuses through the film into the core, the core
swells until the
film bursts, thereby releasing the active ingredients. The film coating may be
adjusted to
permit various rates of water permeation or release time.
[0095] In another embodiment, the delayed release formulation employs a water-
impermeable tablet coating whereby water enters through a controlled aperture
in the coating
until the core bursts. When the tablet bursts, the drug contents are released
immediately or
over a longer period of time. These and other techniques may be modified to
allow for a pre-
determined lag period before release of drugs is initiated.
[0096] In another embodiment, the active agents are delivered in a formulation
to
provide both delayed-release and extended-release (delayed-sustained). The
term "delayed-
extended-release" is used herein with reference to a drug formulation
providing pulsatile
release of active agents at a pre-determined time or lag period following
administration,
which is then followed by extended-release of the active agents thereafter.
[0097] In some embodiments, immediate-release, extended-release, delayed-
release,
or delayed-extended-release formulations comprises an active core comprised of
one or more
inert particles, each in the form of a bead, pellet, pill, granular particle,
microcapsule,
microsphere, microgranule, nanocapsule, or nanosphere coated on its surfaces
with drugs in
the form of e.g., a drug-containing film-forming composition using, for
example, fluid bed
techniques or other methodologies known to those of skill in the art. The
inert particle can be
of various sizes, so long as it is large enough to remain poorly dissolved.
Alternatively, the
active core may be prepared by granulating and milling and/or by extrusion and
spheronization of a polymer composition containing the drug substance.
[0098] The amount of drug in the core will depend on the dose that is
required, and
typically varies from about 5 to 90 weight %. Generally, the polymeric coating
on the active
core will be from about 1 to 50% based on the weight of the coated particle,
depending on the
lag time and type of release profile required and/or the polymers and coating
solvents chosen.
23
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
Those skilled in the art will be able to select an appropriate amount of drug
for coating onto
or incorporating into the core to achieve the desired dosage. In one
embodiment, the inactive
core may be a sugar sphere or a buffer crystal or an encapsulated buffer
crystal such as
calcium carbonate, sodium bicarbonate, fumaric acid, tartaric acid, etc. which
alters the
microenvironment of the drug to facilitate its release.
[0099] In some embodiments, for example, delayed-release or delayed-extended-
release compositions may formed by coating a water soluble/dispersible drug-
containing
particle, such as a bead, with a mixture of a water insoluble polymer and an
enteric polymer,
wherein the water insoluble polymer and the enteric polymer may be present at
a weight ratio
of from 4:1 to 1:1, and the total weight of the coatings is 10 to 60 weight %
based on the total
weight of the coated beads. The drug layered beads may optionally include an
inner
dissolution rate controlling membrane of ethylcellulose. The composition of
the outer layer,
as well as the individual weights of the inner and outer layers of the
polymeric membrane are
optimized for achieving desired circadian rhythm release profiles for a given
active, which
are predicted based on in vitro/in vivo correlations.
[0100] In other embodiments the formulations may comprise a mixture of
immediate-
release drug-containing particles without a dissolution rate controlling
polymer membrane
and delayed-extended-release beads exhibiting, for example, a lag time of 2-4
hours
following oral administration, thus providing a two-pulse release profile.
[01011 In some embodiments, the active core is coated with one or more layers
of
dissolution rate-controlling polymers to obtain desired release profiles with
or without a lag
time. An inner layer membrane can largely control the rate of drug release
following
imbibition of water or body fluids into the core, while the outer layer
membrane can provide
for a desired lag time (the period of no or little drug release following
imbibition of water or
body fluids into the core). The inner layer membrane may comprise a water
insoluble
polymer, or a mixture of water insoluble and water soluble polymers.
[0102] The polymers suitable for the outer membrane, which largely controls
the lag
time of up to 6 hours may comprise an enteric polymer, as described above, and
a water
insoluble polymer at 10 to 50 weight %. The ratio of water insoluble polymer
to enteric
polymer may vary from 4:1 to 1:2, preferably the polymers are present at a
ratio of about 1:1.
The water insoluble polymer typically used is ethylcellulose.
[0103] Exemplary water insoluble polymers include ethylcellulose, polyvinyl
acetate
(Kollicoat SR#OD from BASF), neutral copolymers based on ethyl acrylate and
methylmethacrylate, copolymers of acrylic and methacrylic acid esters with
quaternary
24
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
ammonium groups such as EUDRAGIT NE, RS and RS30D, RL or RL3OD and the like.
Exemplary water soluble polymers include low molecular weight HPMC, HPC,
methylcellulose, polyethylene glycol (PEG of molecular weight>3000) at a
thickness ranging
from 1 weight % up to 10 weight % depending on the solubility of the active in
water and the
solvent or latex suspension based coating formulation used. The water
insoluble polymer to
water soluble polymer may typically vary from 95:5 to 60:40, preferably from
80:20 to 65:35.
[0104] In some embodiments, AMBERLITETm IRP69 resin is used as an extended-
release carrier. AMBERLITETm IRP69 is an insoluble, strongly acidic, sodium
form cation
exchange resin that is suitable as carrier for cationic (basic) substances. In
other
embodiments, DUOLITETm AP143/1093 resin is used as an extended-release
carrier.
DUOLITETm AP143/1093 is an insoluble, strongly basic, anion exchange resin
that is
suitable as a carrier for anionic (acidic) substances.
[0105] When used as a drug carrier, AMBERLITE IRP69 or/and DUOLITETm
AP143/1093 resin provides a means for binding medicinal agents onto an
insoluble polymeric
matrix. Extended-release is achieved through the formation of resin-drug
complexes (drug
resinates). The drug is released from the resin in vivo as the drug reaches
equilibrium with
the high electrolyte concentrations, which are typical of the gastrointestinal
tract. More
hydrophobic drugs will usually elute from the resin at a lower rate, owing to
hydrophobic
interactions with the aromatic structure of the cation exchange system.
[0106] In some embodiments, the pharmaceutical composition is formulated for
oral
administration. Oral dosage forms include, for example, tablets, capsules,
caplets, and may
also comprise a plurality of granules, beads, powders or pellets that may or
may not be
encapsulated. Tablets and capsules represent the most convenient oral dosage
forms, in
which case solid pharmaceutical carriers are employed.
[0107] In a delayed-release formulation, one or more barrier coatings may be
applied
to pellets, tablets, or capsules to facilitate slow dissolution and
concomitant release of drugs
into the intestine. Typically, the barrier coating contains one or more
polymers encasing,
surrounding, or forming a layer, or membrane around the therapeutic
composition or active
core.
[0108] In some embodiments, the active agents are delivered in a formulation
to
provide delayed-release at a pre-determined time following administration. The
delay may
be up to about 10 minutes, about 20 minutes, about 30 minutes, about 1 hour,
about 2 hours,
about 3 hours, about 4 hours, about 5 hours, about 6 hours, or longer.
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
[0109] Various coating techniques may be applied to granules, beads, powders
or
pellets, tablets, capsules or combinations thereof containing active agents to
produce different
and distinct release profiles. In some embodiments, the pharmaceutical
composition is in a
tablet or capsule form containing a single coating layer. In other
embodiments, the
pharmaceutical composition is in a tablet or capsule form containing multiple
coating layers.
[0110] In some embodiments, the pharmaceutical composition comprises a
plurality
of active ingredients selected from the group consisting of analgesics,
antimuscarinic agents,
antidiuretics, spasmolytics and zolpidem. Examples of antimuscarinic agents
include, but are
not limited to, oxybutynin, solifenacin, darifenacin and atropine. Examples of
antidiuretics
include, but are not limited to, antidiuretic hormone (ADH), angiotensin II,
aldosterone,
vasopressin, vasopressin analogs (e.g., desmopressin argipressin, lypressin,
felypressin,
ornipressin, terlipressin; vasopressin receptor agonists, atrial natriuretic
peptide (ANP) and C-
type natriuretic peptide (CNP) receptor (i.e., NPR1, NPR2, NPR3) antagonists
(e.g., HS-142-
1, isatin, [Asu7,23']b-ANP-(7-28)], anantin, a cyclic peptide from
Streptomyces coerulescens,
and 3G12 monoclonal antibody); somatostatin type 2 receptor antagonists (e.g.,
somatostatin), and pharmaceutically-acceptable derivatives, analogs, salts,
hydrates, and
solvates thereof Examples of spasmolytics include, but are not limited to,
carisoprodol,
benzodiazepines, baclofen, cyclobenzaprine, metaxalone, methocarbamol,
clonidine,
clonidine analog, and dantrolene. In some embodiments, the pharmaceutical
composition
comprises one or more analgesics. In other embodiments, the pharmaceutical
composition
comprises (1) one or more analgesics, and (2) one or more other active
ingredients selected
from the group consisting of antimuscarinic agents, antidiuretics and
spasmolytics. In another
embodiment, the pharmaceutical composition comprises (1) one or more
analgesics and (2)
one or more antimuscarinic agents. In another embodiment, the pharmaceutical
composition
comprises (1) one or more analgesics and (2) one or more antidiuretics. In
another
embodiment, the pharmaceutical composition comprises (1) one or more
analgesics and (2)
one or more spasmolytics. In another embodiment, the pharmaceutical
composition
comprises (1) one or more analgesics and (2) zolpidem. In another embodiment,
the
pharmaceutical composition comprises (1) one or two analgesics, (2) one or two
antimuscarinic agents, and (3) one or two antidiuretics. In another
embodiment, the
pharmaceutical composition comprises (1) one or more analgesics, (2) one or
more
spasmolytics agents, and (3) one or more antidiuretics. In yet another
embodiment, the
pharmaceutical composition comprises (1) one or more analgesics, (2) one or
more
antidiuretics, and (3) zolpidem.
26
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
[0111] In one embodiment, the plurality of active ingredients are formulated
for
immediate-release. In other embodiment, the plurality of active ingredients
are formulated
for extended-release. In other embodiment, the plurality of active ingredients
are formulated
for both immediate-release and extended-release (e.g., a first portion of each
active ingredient
is formulated for immediate-release and a second portion of each active
ingredient is
formulated for extended-release). In yet other embodiment, some of the
plurality of active
ingredients are formulated for immediate-release and some of the plurality of
active
ingredients are formulated for extended-release (e.g., active ingredients A,
B, C are
formulated for immediate-release and active ingredients C and D are formulated
for
extended-release). In some other embodiments, the immediate-release component
and/or the
extended-release component is further coated with a delayed-release coating,
such as an
enteric coating.
[0112] In certain embodiments, the pharmaceutical composition comprises an
immediate-release component and an extended-release component. The immediate-
release
component may comprise one or more active ingredients selected from the group
consisting
of analgesics, antimuscarinic agents, antidiuretics and spasmolytics. The
extended-release
component may comprise one or more active ingredients selected from the group
consisting
of analgesics, antimuscarinic agents, antidiuretics and spasmolytics. In some
embodiments,
the immediate-release component and the extended-release component have
exactly the same
active ingredients. In other embodiments, the immediate-release component and
the
extended-release component have different active ingredients. In yet other
embodiments, the
immediate-release component and the extended-release component have one or
more
common active ingredients. In some other embodiments, the immediate-release
component
and/or the extended-release component is further coated with a delayed-release
coating, such
as an enteric coating.
[0113] In one embodiment, the pharmaceutical composition comprises two or more
active ingredients (e.g., two or more analgesic agents, or a mixture of one or
more analgesic
agent and one or more antimuscarinic agents or antidiuretics or spasmolytics
or zolidem),
formulated for immediate-release at about the same time. In another
embodiment, the
pharmaceutical composition comprises two ore more active ingredients,
formulated for
extended-release at about the same time. In another embodiment, the
pharmaceutical
composition comprises two or more active ingredients formulated as two
extended-release
components, each providing a different extended-release profile. For example,
a first
extended-release component releases a first active ingredient at a first
release rate and a
27
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
second extended-release component releases a second active ingredient at a
second release
rate. In another embodiment, the pharmaceutical composition comprises two or
more active
ingredients, both formulated for delayed release. In another embodiment, the
pharmaceutical
composition comprises two or more active ingredients formulated for delayed
release. In
another embodiment, the pharmaceutical composition comprises two or more
active
ingredients formulated as two delayed-release components, each providing a
different
delayed-release profile. For example, a first delayed-release component
releases a first active
ingredient at a first time point and a second delayed-release component
releases a second
active ingredient at a second time point. In another embodiment, the
pharmaceutical
composition comprises two or more active ingredients, one or more of which are
formulated
for immediate-release and the others are formulated for extended-release. In
another
embodiment, the pharmaceutical composition comprises two or more active
ingredients, a
fraction of which is formulated for immediate-release and the remainder is
formulated for
extended-release.
[0114] In other embodiments, the pharmaceutical composition comprises two
active
ingredients (e.g., two analgesic agents, or a mixture of one analgesic agent
and one
antimuscarinic agent or antidiuretic or spasmolytic or zolpidem) formulated
for immediate-
release, and (2) two active ingredients (e.g., two analgesic agents, or a
mixture of one
analgesic agent and one antimuscarinic agent or antidiuretic or spasmolytic or
zolpidem)
formulated for extended-release. In other embodiments, the pharmaceutical
composition
comprises three active ingredients formulated for immediate-release, and (2)
three active
ingredients formulated for extended-release. In other embodiments, the
pharmaceutical
composition comprises four active ingredients formulated for immediate-
release, and (2) four
active ingredients formulated for extended-release. In these embodiments, the
active
ingredient(s) in the immediate-release component can be the same as, or
different from, the
active ingredient(s) in the extended-release component. In some other
embodiments, the
immediate-release component and/or the extended-release component is further
coated with a
delayed-release coating, such as an enteric coating.
[0115] In some embodiments, the pharmaceutical composition comprises one or
more
analgesic agents; and an antidiuretic, wherein the one or more analgesic
agents are
formulated for delayed release and wherein the antidiuretic is formulated for
immediate
release. In other embodiments, the pharmaceutical composition further
comprises an
additional agent selected from the group consisting of an antimuscarinic
agent, an antidiuretic
agent, a spasmolytic and zolpidem, wherein the additional agent is formulated
for delayed
28
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
release. In some embodiments, The delayed release formulation delays the
release of the
active ingredient (e.g., the analgesic agent, antimuscarinic agent,
antidiuretic agent,
spasmolytic and/or zolpidem) for a period of 1, 2, 3, 4 or 5 hours.
[0116] The term "immediate-release" is used herein with reference to a drug
formulation that does not contain a dissolution rate controlling material.
There is substantially
no delay in the release of the active agents following administration of an
immediate-release
formulation. An immediate-release coating may include suitable materials
immediately
dissolving following administration so as to release the drug contents
therein. Exemplary
immediate-release coating materials include gelatin, polyvinyl alcohol
polyethylene glycol
(P VA-PEG)copolymers (e.g., KOLLICOAT ) and various others materials known to
those
skilled in the art.
[0117] An immediate-release composition may comprise 100% of the total dosage
of
a given active agent administered in a single unit dose. Alternatively, an
immediate-release
component may be included as a component in a combined release profile
formulation that
may provide about 1% to about 60% of the total dosage of the active agent(s)
to be delivered
by the pharmaceutical formulation. For example, the immediate-release
component may
provide about 5%-60%, about 10% to about 60%, about 10% to about 50%, about
10% to
about 40%, about 10% to about 30%, about 10% to about 20%, about 20% to about
60%,
about 20% to about 50%, about 20% to about 30%, about 30% to about 60%, about
30% to
about 50%, about 40% to about 60%, about 40% to about 50%, about 45% to about
60% or
about 45% to about 50% of the total dosage of the active agent(s) to be
delivered by the
formulation. In alternate embodiments, the immediate-release component
provides about 2,
4, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55or 60% of the total dosage of the
active agent(s) to
be delivered by the formulation.
[0118] In some embodiments, the immediate-release or delayed-release
formulation
comprises an active core comprised of one or more inert particles, each in the
form of a bead,
pellet, pill, granular particle, microcapsule, microsphere, microgranule,
nanocapsule, or
nanosphere coated on its surfaces with drugs in the form of e.g., a drug-
containing film-
forming composition using, for example, fluid bed techniques or other
methodologies known
to those of skill in the art. The inert particle can be of various sizes, so
long as it is large
enough to remain poorly dissolved. Alternatively, the active core may be
prepared by
granulating and milling and/or by extrusion and spheronization of a polymer
composition
containing the drug substance.
29
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
[0119] The amount of drug in the core will depend on the dose that is
required, and
typically varies from about 5 to 90 weight %. Generally, the polymeric coating
on the active
core will be from about 1 to 50% based on the weight of the coated particle,
depending on the
lag time and type of release profile required and/or the polymers and coating
solvents chosen.
Those skilled in the art will be able to select an appropriate amount of drug
for coating onto
or incorporating into the core to achieve the desired dosage. In one
embodiment, the inactive
core may be a sugar sphere or a buffer crystal or an encapsulated buffer
crystal such as
calcium carbonate, sodium bicarbonate, fumaric acid, tartaric acid, etc. which
alters the
microenvironment of the drug to facilitate its release.
[0120] In some embodiments, the delayed-release formulation is formed by
coating a
water soluble/dispersible drug-containing particle, such as a bead, with a
mixture of a water
insoluble polymer and an enteric polymer, wherein the water insoluble polymer
and the
enteric polymer may be present at a weight ratio of from 4:1 to 1:1, and the
total weight of
the coatings is 10 to 60 weight % based on the total weight of the coated
beads. The drug
layered beads may optionally include an inner dissolution rate controlling
membrane of
ethylcellulose. The composition of the outer layer, as well as the individual
weights of the
inner and outer layers of the polymeric membrane are optimized for achieving
desired
circadian rhythm release profiles for a given active, which are predicted
based on in vitro/in
vivo correlations.
[0121] In other embodiments the formulations comprise a mixture of immediate-
release drug-containing particles without a dissolution rate controlling
polymer membrane
and delayed-release beads exhibiting, for example, a lag time of 2-4 hours
following oral
administration, thus providing a two-pulse release profile. In yet other
embodiments the
formulations comprise a mixture of two types of delayed-release beads: a first
type that
exhibits a lag time of 1-3 hours and a second type that exhibits a lag time of
4-6 hours.
[0122] Preferably, the formulations are designed with release profiles to
limit
interference with restful sleep, wherein the formulation releases the medicine
when the
individual would normally be awakened by an urge to urinate. For example,
consider an
individual who begins sleeping at 11 PM and is normally awakened at 12:30 AM,
3:00 AM,
and 6:00 AM to urinate. A delayed, extended-release vehicle could be taken at
10 PM and
start delivering the medicine at 12 AM and gradually release the medicine over
a period of 5-
8 hours,thereby delaying or eliminate the need to urinate.
[0123] In other embodiments, the formulations are designed with a release
profile
such that a fraction of the medicine (e.g., 20-60%) is released immediately or
within 2 hours
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
of administration and the rest is released over an extended period of time.
The
pharmaceutical composition may be administered daily or administered on an as
needed basis.
In certain embodiments, the pharmaceutical composition is administered to the
subject prior
to bedtime. In some embodiments, the pharmaceutical composition is
administered
immediately before bedtime. In some embodiments, the pharmaceutical
composition is
administered within about two hours before bedtime, preferably within about
one hour before
bedtime. In another embodiment, the pharmaceutical composition is administered
about two
hours before bedtime. In a further embodiment, the pharmaceutical composition
is
administered at least two hours before bedtime. In another embodiment, the
pharmaceutical
composition is administered about one hour before bedtime. In a further
embodiment, the
pharmaceutical composition is administered at least one hour before bedtime.
In a still
further embodiment, the pharmaceutical composition is administered less than
one hour
before bedtime. In still another embodiment, the pharmaceutical composition is
administered
immediately before bedtime. Preferably, the pharmaceutical composition is
administered
orally.
[0124] The appropriate dosage ("therapeutically effective amount") of the
active
agent(s) in the immediate-release component or the extended-release component
will depend,
for example, on the severity and course of the condition, the mode of
administration, the
bioavailability of the particular agent(s), the age and weight of the patient,
the patient's
clinical history and response to the active agent(s), discretion of the
physician, etc.
[0125] As a general proposition, the therapeutically effective amount of the
active
agent(s) in the immediate-release component, the extended-release component or
the
delayed-extended-release component is administered in the range of about 100
ug/kg body
weight/day to about 100 mg/kg body weight/day whether by one or more
administrations. In
some embodiments, the range of each active agent administered daily in a
single dose or in
multiple does is from about 100 ug/kg body weight/day to about 50 mg/kg body
weight/day,
100 ug/kg body weight/day to about 10 mg/kg body weight/day, 100 ug/kg body
weight/day
to about 1 mg/kg body weight/day, 100 ug/kg body weight/day to about 10 mg/kg
body
weight/day, 500 ug/kg body weight/day to about 100 mg/kg body weight/day, 500
jig/kg
body weight/day to about 50 mg/kg body weight/day, 500 jig/kg body weight/ day
to about 5
mg/kg body weight/ day, 1 mg/kg body weight/day to about 100 mg/kg body
weight/day, 1
mg/kg body weight/day to about 50 mg/kg body weight/ day, 1 mg/kg body
weight/day to
about 10 mg/kg body weight/day, 5 mg/kg body weight/dose to about 100 mg/kg
body
weight/day, 5 mg/kg body weight/dose to about 50 mg/kg body weight/day, 10
mg/kg body
31
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
weight/day to about 100 mg/kg body weight/day, and 10 mg/kg body weight/day to
about 50
mg/kg body weight/day.
[0126] The active agent(s) described herein may be included in an immediate-
release
component or an extended-release component, a delayed-extended-release
component or
combinations thereof for daily oral administration at a single dose or
combined dose range of
1 mg to 2000 mg, 5 mg to 2000 mg, 10 mg to 2000 mg, 50 mg to 2000 mg, 100 mg
to 2000
mg, 200 mg to 2000 mg, 500 mg to 2000 mg, 5 mg to 1800 mg, 10 mg to 1600 mg,
50 mg to
1600 mg, 100 mg to 1500 mg, 150 mg to 1200 mg, 200 mg to 1000 mg, 300 mg to
800 mg,
325 mg to 500 mg, 1 mg to 1000 mg, 1 mg to 500 mg, 1 mg to 200 mg, 5 mg to
1000 mg, 5
mg to 500 mg, 5 mg to 200 mg, 10 mg to 1000 mg, 10 mg to 500 mg, 10 mg to 200
mg, 50
mg to 1000 mg, 50 mg to 500 mg, 50 mg to 200 mg, 250 mg to 1000 mg, 250 mg to
500 mg,
500 mg to 1000 mg, 500 mg to 2000 mg. As expected, the dosage will be
dependent on the
condition, size, age and condition of the patient.
[0127] In some embodiments, the pharmaceutical composition comprises a single
analgesic agent. In one embodiment, the single analgesic agent is aspirin. In
another
embodiment, the single analgesic agent is ibuprofen. In another embodiment,
the single
analgesic agent is naproxen or naproxen sodium. In another embodiment, the
single
analgesic agent is indomethacin. In another embodiment, the single analgesic
agent is
nabumetone. In another embodiment, the single analgesic agent is
acetaminophen.
[0128] In some embodiments, the single analgesic agent is given at a daily
dose of 1
mg to 2000 mg, 5 mg to 2000 mg, 20 mg to 2000 mg, 5 mg to 1000 mg, 20 mg to
1000 mg,
50 mg to 500 mg, 100 mg to 500 mg, 250 mg to 500 mg, 250 mg to 1000 mg or 500
mg to
1000 mg. In certain embodiments, the pharmaceutical composition comprises
acetylsalicylic
acid, ibuprofen, naproxen, naproxen sodium, indomethancin, nabumetone or
acetaminophen
as a single analgesic agent and the analgesic agent is administered orally at
a daily dose in the
range of 5 mg to 2000 mg, 20 mg to 2000 mg, 5 mg to 1000 mg, 20 mg to 1000 mg,
50 mg to
500 mg, 100 mg to 500 mg, 250 mg to 500 mg, 250 mg to 1000 mg or 500 mg to
1000 mg.
In some embodiments, a second analgesic agent is given at a daily dose of 1 mg
to 2000 mg,
mg to 2000 mg, 20 mg to 2000 mg, 5 mg to 1000 mg, 20 mg to 1000 mg, 50 mg to
500 mg,
100 mg to 500 mg, 250 mg to 500 mg, 250 mg to 1000 mg or 500 mg to 1000 mg.
[0129] In other embodiments, the pharmaceutical composition comprises a pair
of
analgesic agents. Examples of such paired analgesic agents include, but are
not limited to,
acetylsalicylic acid and ibuprofen, acetylsalicylic acid and naproxen sodium,
acetylsalicylic
acid and nabumetone, acetylsalicylic acid and acetaminophen, acetylsalicylic
acid and
32
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
indomethancin, ibuprofen and naproxen sodium, ibuprofen and nabumetone,
ibuprofen and
acetaminophen, ibuprofen and indomethancin, naproxen, naproxen sodium and
nabumetone,
naproxen sodium and acetaminophen, naproxen sodium and indomethancin,
nabumetone and
acetaminophen, nabumetone and indomethancin, and acetaminophen and
indomethancin. The
paired analgesic agents are mixed at a weight ratio in the range of 0.1:1 to
10:1, 0.2:1 to 5:1
or 0.3:1 to 3:1, with a combined dose in the range of 5 mg to 2000 mg, 20 mg
to 2000 mg,
100 mg to 2000 mg, 200 mg to 2000 mg, 500 mg to 2000 mg, 5 mg to 1500 mg, 20
mg to
1500 mg, 100 mg to 1500 mg, 200 mg to 1500 mg, 500 mg to 1500 mg, 5 mg to 1000
mg, 20
mg to 1000 mg, 100 mg to 1000 mg, 250 mg to 500 mg, 250 mg to 1000 mg, 250 mg
to
1500 mg, 500 mg to 1000 mg, 500 mg to 1500 mg, 1000 mg to 1500 mg, and 1000 mg
to
2000 mg. In one embodiment, the paired analgesic agents are mixed at a weight
ratio of 1:1.
[0130] In some other embodiments, the pharmaceutical composition of the
present
application further comprises one or more antimuscarinic agents. Examples of
the
antimuscarinic agents include, but are not limited to, oxybutynin,
solifenacin, darifenacin,
fesoterodine, tolterodine, trospium and atropine. The daily dose of
antimuscarinic agent is in
the range of 0.01 mg to 100 mg, 0.1 mg to 100 mg, 1 mg to 100 mg, 10 mg to 100
mg, 0.01
mg to 25 mg, 0.1 mg to 25 mg, 1 mg to 25 mg, 10 mg to 25 mg, 0.01 mg to 10 mg,
0.1 mg to
mg, 1 mg to 10 mg, 10 mg to 100 mg and 10 mg to 25 mg.
[0131] In certain embodiments, the pharmaceutical composition comprises an
analgesic agent selected from the group consisting of cetylsalicylic acid,
ibuprofen, naproxen,
naproxen sodium, nabumetone, acetaminophen and indomethancin, and an
antimuscarinic
agent selected from the group consisting of oxybutynin, solifenacin,
darifenacin and atropine.
[0132] Another aspect of the present application relates to a method for
reducing the
frequency of urination by administering to a person in need thereof a
pharmaceutical
composition formulated in an immediate-release formulation. The pharmaceutical
composition comprises a plurality of analgesic agents and/or antimuscarinic
agents.
[0133] In certain embodiments, the pharmaceutical composition comprises two or
more analgesic agents. In other embodiments, the pharmaceutical composition
comprises
one or more analgesic agents and one or more antimuscarinic agents. The
pharmaceutical
composition may be formulated into a tablet, capsule, dragee, powder,
granulate, liquid, gel
or emulsion form. Said liquid, gel or emulsion may be ingested by the subject
in naked form
or contained within a capsule.
[0134] In certain embodiments, the analgesic agent is selected from the group
consisting of salicylates, aspirin, salicylic acid, methyl salicylate,
diflunisal, salsalate,
33
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
olsalazine, sulfasalazine, para-aminophenol derivatives, acetanilide,
acetaminophen,
phenacetin, fenamates, mefenamic acid, meclofenamate, sodium meclofenamate,
heteroaryl
acetic acid derivatives, tolmetin, ketorolac, diclofenac, propionic acid
derivatives, ibuprofen,
naproxen sodium, naproxen, fenoprofen, ketoprofen, flurbiprofen, oxaprozin;
enolic acids,
oxicam derivatives, piroxicam, meloxicam, tenoxicam, ampiroxicam, droxicam,
pivoxicam,
pyrazolon derivatives, phenylbutazone, oxyphenbutazone, antipyrine,
aminopyrine, dipyrone,
coxibs, celecoxib, rofecoxib, nabumetone, apazone, nimesulide, indomethacin,
sulindac,
etodolac, diflunisal and isobutylphenyl propionic acid. The antimuscarinic
agent is selected
from the group consisting of oxybutynin, solifenacin, darifenacin and
atropine.
[0135] In some embodiments, the pharmaceutical composition comprises a single
analgesic agent and a single antimuscarinic agent. In one embodiment, the
single analgesic
agent is aspirin. In another embodiment, the single analgesic agent is
ibuprofen. In another
embodiment, the single analgesic agent is naproxen or naproxen sodium. In
another
embodiment, the single analgesic agent is indomethacin. In another embodiment,
the single
analgesic agent is nabumetone. In another embodiment, the single analgesic
agent is
acetaminophen. The analgesic agent and anti-muscarinic agent may be given at
doses in the
ranges described above.
[0136] In some embodiments, the pharmaceutical composition comprises one or
more
analgesic agents, individually or in combination, in an amount between 50-2000
mg, 50-1500
mg, 50-1200 mg, 50-1000 mg, 50-800 mg, 50-600 mg, 50-500 mg, 50-400 mg, 50-300
mg,
50-250 mg, 50-200 mg, 50-150 mg, 50-100 mg, 100-2000 mg, 100-1500 mg, 100-1200
mg,
100-1000 mg, 100-800 mg, 100-600 mg, 100-400 mg, 100-250 mg, 250-2000 mg, 250-
1500
mg, 250-1200 mg, 250-1000 mg, 250-800 mg, 250-600 mg, 250-400 mg, 400-2000 mg,
400-
1500 mg, 400-1200 mg, 400-1000 mg, 400-800 mg, 400-600 mg, 600-2000 mg, 600-
1500
mg, 600-1200 mg, 600-1000 mg, 600-800 mg, 800-2000 mg, 800-1500 mg, 800-1200
mg,
800-1000 mg, 1000-2000 mg, 1000-1500 mg, 1000-1200 mg, 1200-2000 mg, 1200-1500
mg
or 1500-2000 mg, wherein the composition is formulated for extended release
with a release
profile in which the one or more analgesic agents are released continuously
over a period of
5-24 hours, 5-8, 8-16 hours or 16-24 hours.
[0137] In some embodiments, the composition is formulated for extended release
with a release profile in which at least 90% of the one or more analgesic
agents are released
continuously over a period of 5-24 hours, 5-8, 8-16 hours or 16-24 hours.
[0138] In some embodiments, the composition is formulated for extended release
with a release profile in which the one or more analgesic agents are released
continuously
34
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
over a period of 5, 6, 7, 8, 10, 12, 14, 16, 18, 20, 22 or 24 hours. In some
embodiments, the
pharmaceutical composition further comprises an antimuscarinic agent, an
antidiuretic agent
or a spasmolytic.
[0139] In other embodiments, the composition is formulated for extended
release with
a release profile in which the analgesic agent is released at a steady rate
over a period of 5-24
hours, 5-8, 8-16 hours or 16-24 hours. In other embodiments, the composition
is formulated
for extended release with a release profile in which the analgesic agent is
released at a steady
rate over a period of 5, 6, 7, 8, 10, 12, 14, 16, 18, 20, 22 or 24 hours. As
used herein, "a
steady rate over a period of time" is defined as a release profile in which
the release rate at
any point during a given period of time is within 30% - 300% of the average
release rate over
that given period of time. For example, if 80 mg of aspirin is released at a
steady rate over a
period of 8 hours, the average release rate is 10 mg/hr during this period of
time and the
actual release rate at any time during this period is within the range of 3
mg/hr to 30 mg/hr
(i.e., within 30% - 300% of the average release rate of 10 mg/hr during the 8
hour period). In
some embodiments, the pharmaceutical composition further comprises an
antimuscarinic
agent, an antidiuretic agent or a spasmolytic.
[0140] In some embodiments, the analgesic agent is selected from the group
consisting of aspirin, ibuprofen, naproxen sodium, naproxen, indomethacin,
nabumetone and
acetaminophen. The pharmaceutical composition is formulated to provide a
steady release of
small amount of the analgesic agent to maintain an effective drug
concentration in the blood
such that the overall amount of the drug in a single dosage is reduced
compared to the
immediate release formulation.
[0141] In some embodiments, the pharmaceutical composition comprises 50-250
mg,
250-400 mg or 400-600 mg of an analgesic agent formulated for extended release
with a
release profile in which at least 90% of the analgesic agent is released
continuously, or at a
steady rate, over a period of 5-24, 5-8, 8-16 or 16-24 hours.
[0142] In one particular embodiment, the pharmaceutical composition comprises
50-
250 mg of acetaminophen formulated for extended release with a release profile
in which at
least 90% of acetaminophen is released continuously, or at a steady rate, over
a period of 5-
24, 5-8, 8-16 or 16-24 hours.
[0143] In another particular embodiment, the pharmaceutical composition
comprises
250-400 mg of acetaminophen formulated for extended release with a release
profile in which
90% of acetaminophen is released continuously, or at a steady rate over a
period of 5-24, 5-8,
8-16 or 16-24 hours.
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
[0144] In another particular embodiment, the pharmaceutical composition
comprises
400-600 mg of acetaminophen formulated for extended release with a release
profile in which
90% of acetaminophen is released continuously, or at a steady rate over a
period of 5-24, 5-8,
8-16 or 16-24 hours.
[0145] In another particular embodiment, the pharmaceutical composition
comprises
600-800 mg of acetaminophen formulated for extended release with a release
profile in which
90% of acetaminophen is released continuously, or at a steady rate over a
period of 5-24, 5-8,
8-16 or 16-24 hours.
[0146] In yet another embodiment, the pharmaceutical composition comprises 800-
1000 mg of acetaminophen formulated for extended release with a release
profile in which at
least 90% of acetaminophen is released continuously, or at a steady rate over
a period of 5-
24, 5-8, 8-16 or 16-24 hours.
[0147] In some other embodiments, the pharmaceutical composition comprises one
or
more analgesic agent(s), individually or in combination, in an amount between
50-2000 mg,
50-1500 mg, 50-1200 mg, 50-1000 mg, 50-800 mg, 50-600 mg, 50-500 mg, 50-400
mg, 50-
300 mg, 50-250 mg, 50-200 mg, 100-2000 mg, 100-1500 mg, 100-1200 mg, 100-1000
mg,
100-800 mg, 100-600 mg, 100-500 mg, 100-400 mg, 100-300 mg, 100-200 mg, 200-
2000
mg, 200-1500 mg, 200-1200 mg, 200-1000 mg, 200-800 mg, 200-600 mg, 200-400 mg,
400-
2000 mg, 400-1500 mg, 400-1200 mg, 400-1000 mg, 400-800 mg, 400-600 mg, 600-
2000
mg, 600-1500 mg, 600-1200 mg, 600-1000 mg, 600-800 mg, 800-2000 mg, 800-1500
mg,
800-1200 mg, 800-1000 mg, 1000-2000 mg, 1000-1500 mg, 1000-1200 mg, 1200-2000
mg,
1200-1500 mg or 1500-2000 mg, wherein the analgesic agent(s) are formulated
for extended
release, characterized by a two-phase release profile in which 20-60% of the
analgesic
agent(s) are released within 2 hours of administration and the remainder are
released
continuously, or at a steady rate, over a period of 5-24 hours. In yet another
embodiment, the
analgesic agent(s) is formulated for extended release with a two-phase release
profile in
which 20, 30, 40, 50 or 60% of the analgesic agent(s) are released within 2
hours of
administration and the remainder are released continuously, or at a steady
rate, over a period
of 5-8, 8-16 or 16-24 hours. In one embodiment, the analgesic agent(s) are
selected from the
group consisting of aspirin, ibuprofen, naproxen sodium, naproxen,
indomethacin,
nabumetone and acetaminophen. In another embodiment, the analgesic agent is
acetaminophen. In some embodiments, the pharmaceutical composition further
comprises an
antimuscarinic agent, an antidiuretic agent, a spasmolytic, or zolpidem.
36
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
[0148] In another embodiment, the pharmaceutical composition comprises 50-400
mg
of acetaminophen formulated for extended release with a two-phase release
profile in which
20%, 30%, 40%, 50% or 60% of the acetaminophen is released within 2 hours of
administration and the remainder is released continuously, or at a steady
rate, over a period of
5-24, 5-8, 8-16 or 16-24 hours.
[0149] In another embodiment, the pharmaceutical composition comprises 100-300
mg of acetaminophen formulated for extended release with a two-phase release
profile in
which 20%, 30%, 40% 50% or 60% of the acetaminophen is released within 2 hours
of
administration and the remainder is released at a steady rate over a period of
5-24, 5-8, 8-16
or 16-24 hours.
[0150] In another embodiment, the pharmaceutical composition comprises 400-600
mg of acetaminophen formulated for extended release with a two-phase release
profile in
which 20%, 30%, 40% 50% or 60% of the acetaminophen is released within 2 hours
of
administration and the remainder is released continuously, or at a steady
rate, in a period of 5-
24, 5-8, 8-16 or 16-24 hours.
[0151] In another embodiment, the pharmaceutical composition comprises 600-800
mg of acetaminophen formulated for extended release with a two-phase release
profile in
which 20%, 30%, 40% 50% or 60% of the acetaminophen is released within 2 hours
of
administration and the remainder is released continuously, or at a steady
rate, in a period of 5-
24, 5-8, 8-16 or 16-24 hours.
[0152] In another embodiment, the pharmaceutical composition comprises 800-
1000
mg of acetaminophen formulated for extended release with a two-phase release
profile in
which 20%, 30%, 40% 50% or 60% of the acetaminophen is released within 2 hours
of
administration and the remainder is released continuously, or at a steady
rate, in a period of 5-
24, 5-8, 8-16 or 16-24 hours.
[0153] In another embodiment, the pharmaceutical composition comprises 1000-
1200
mg of acetaminophen formulated for extended release with a two-phase release
profile in
which 20%, 30%, 40% 50% or 60% of the acetaminophen is released within 2 hours
of
administration and the remainder is released continuously, or at a steady
rate, in a period of 5-
24, 5-8, 8-16 or 16-24 hours.
[0154] Another aspect of the present application relates to a method for
treating
nocturia by administering to a person in need thereof a first pharmaceutical
composition
comprising a diuretic, followed with a second pharmaceutical composition
comprising one or
more analgesic agents. The first pharmaceutical composition is dosed and
formulated to have
37
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
a diuretic effect within 6 hours of administration and is administered at
least 8 or 7 hours
prior to bedtime. The second pharmaceutical composition is formulated for
extended-release
or delayed, extended-release, and is administered within 2 hours prior to
bedtime.
[0155] Examples of diuretics include, but are not limited to, acidifying
salts, such as
CaC12 and NH4C1; arginine vasopressin receptor 2 antagonists, such as
amphotericin B and
lithium citrate; aquaretics, such as Goldenrod and Junipe; Na-H exchanger
antagonists, such
as dopamine; carbonic anhydrase inhibitors, such as acetazolamide and
dorzolamide; loop
diuretics, such as bumetanide, ethacrynic acid, furosemide and torsemide;
osmotic diuretics,
such as glucose and mannitol; potassium-sparing diuretics, such as amiloride,
spironolactone,
triamterene, potassium canrenoate; thiazides, such as bendroflumethiazide and
hydrochlorothiazide; and xanthines, such as caffeine, theophylline and
theobromine.
[0156] In some embodiments, the second pharmaceutical composition further
comprises one or more antimuscarinic agents. In some other embodiments, the
second
pharmaceutical composition further comprises one or more antidiuretic agents.
In some other
embodiments, the second pharmaceutical composition further comprises one or
more
spasmolytics. In some other embodiments, the second pharmaceutical composition
further
comprises zolpidem. The second pharmaceutical composition may be formulated in
immediate-release formulation or delayed-release formulation.
[0157] Another aspect of the present application relates to a method for
reducing the
frequency of urination by administering to a subject in need thereof, two or
more analgesic
agents alternatively to prevent the development of drug resistance. In one
embodiment, the
method comprises administering a first analgesic agent for a first period of
time and then
administering a second analgesic agent for a second period of time. In another
embodiment,
the method further comprises administering a third analgesic agent for a third
period of time.
The first, second and third analgesic agents are different from each other and
at least one of
which is formulated for extended-release or delayed, extended-release. In one
embodiment,
the first analgesic agent is acetaminophen, the second analgesic agent is
ibuprofen and the
third analgesic agent is naproxen sodium. The length of each period may vary
depending on
the subject's response to each analgesic agent. In some embodiments, each
period lasts from
3 days to three weeks. In another embodiment, the first, second and third
analgesic are all
formulated for extended-release or delayed, extended-release.
[0158] Another aspect of the present application relates to a pharmaceutical
composition comprising a plurality of active ingredients and a
pharmaceutically acceptable
carrier, wherein at least one of the plurality of active ingredients is
formulated for extended-
38
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
release or delayed, extended-release. In some embodiments, the plurality of
active
ingredients comprises one or more analgesics, and one or more antidiuretic
agents. In other
embodiments, the plurality of active ingredients comprises one or more
analgesics and one or
more antimuscarinic agents. In other embodiments, the plurality of active
ingredients
comprises one or more analgesics and zolpedim. In other embodiments, the
plurality of
active ingredients comprises one or more analgesics, one or more antidiuretic
agents and one
or more antimuscarinic agent. In other embodiments, the plurality of active
ingredients
comprises one or more analgesics, zopedim and one or more antidiuretic agents
or one or
more antimuscarinic agents. The antimuscarinic agent may be selected from the
group
consisting of oxybutynin, solifenacin, darifenacin and atropine. In other
embodiments, the
pharmaceutical composition comprises two different analgesics selected from
the group
consisting of cetylsalicylic acid, ibuprofen, naproxen sodium, naproxen,
nabumetone,
acetaminophen and indomethancin. In yet other embodiments, the pharmaceutical
composition comprises one analgesic selected from the group consisting of
cetylsalicylic
acid, ibuprofen, naproxen sodium, nabumetone, acetaminophen and indomethancin;
and an
antimuscarinic agent selected from the group consisting of oxybutynin,
solifenacin, darifenacin
and atropine.
[0159] In other embodiments, the pharmaceutical composition of the present
application further comprises one or more spasmolytics. Examples of
spasmolytics include,
but are not limited to, carisoprodol, benzodiazepines, baclofen,
cyclobenzaprine, metaxalone,
methocarbamol, clonidine, clonidine analog, and dantrolene. In some
embodiments, the
spasmolytics is used at a daily dose of 1 mg to 1000 mg, 1 mg to 100 mg, 10 mg
to 1000 mg,
mg to 100 mg, 20 mg to 1000 mg, 20 mg to 800 mg, 20 mg to 500 mg, 20 mg to 200
mg,
50 mg to 1000 mg, 50 mg to 800 mg, 50 mg to 200 mg, 100 mg to 800 mg, 100 mg
to 500
mg, 200 mg to 800 mg, and 200 mg to 500 mg. The spasmolytics may be
formulated, alone
or together with other active ingredient(s) in the pharmaceutical composition,
for immediate-
release, extended-release, delayed-extended-release or combinations thereof.
[0160] In some embodiments, the pharmaceutical composition comprises one or
more
analgesic agents selected from the group consisting of aspirin, ibuprofen,
naproxen, naproxen
sodium, indomethacin, nabumetone and acetaminophen in an amount of 50-400 mg
per
agent, and one or more antimuscarinic agents selected from the group
consisting of
oxybutynin, solifenacin, darifenacin and atropine in a total amount of 1-25
mg, wherein the
pharmaceutical composition is formulated for extended release with a two-phase
release
profile in which 20-60% of the active ingredients are released within 2 hours
of
39
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
administration, and the remainder of the active ingredients are released
continuously, or at a
steady rate, in a period of 5-24 hours, 5-8 hours, 8-16 hours or 16-24 hours.
[0161] In some embodiments, the pharmaceutical composition comprises one or
more
analgesic agents selected from the group consisting of aspirin, ibuprofen,
naproxen, naproxen
sodium, indomethacin, nabumetone and acetaminophen in an amount of 50-400 mg
per
agent, and one or more antidiuretic agents selected from the group consisting
of antidiuretic
hormone (ADH), angiotensin II, aldosterone, vasopressin, vasopressin analogs
(e.g.,
desmopressin argipressin, lypressin, felypressin, ornipressin, terlipressin);
vasopressin
receptor agonists, atrial natriuretic peptide (ANP) and C-type natriuretic
peptide (CNP)
receptor (i.e., NPR1, NPR2, NPR3) antagonists (e.g., HS-142-1, isatin,
[Asu7,23'1b-ANP-(7-
28)], anantin, a cyclic peptide from Streptomyces coerulescens, and 3G12
monoclonal
antibody); somatostatin type 2 receptor antagonists (e.g., somatostatin), and
pharmaceutically-acceptable derivatives, analogs, salts, hydrates, and
solvates thereof,
wherein the pharmaceutical composition is formulated for extended release with
a two-phase
release profile in which 20-60% of the active ingredients are released within
2 hours of
administration, and the remainder are released continuously, or at a steady
rate, in a period of
5-24 hours, 5-8 hours, 8-16 hours or 16-24 hours.
[0162] In some embodiments, the pharmaceutical composition comprises one or
more
analgesic agents selected from the group consisting of aspirin, ibuprofen,
naproxen,
naproxen sodium, indomethacin, nabumetone and acetaminophen in an amount of 50-
400 mg
per agent, and one or more spasmolytics selected from the group consisting of
carisoprodol,
benzodiazepines, baclofen, cyclobenzaprine, metaxalone, methocarbamol,
clonidine,
clonidine analog, and dantrolene in a total amount of 50-500 mg, wherein the
pharmaceutical
composition is formulated for extended release with a two-phase release
profile in which 20-
60% of the active ingredients are released within 2 hours of administration,
and the remainder
are released continuously, or at a steady rate, in a period of 5-24 hours, 5-8
hours, 8-16 hours
or 16-24 hours.
[0163] The present invention is further illustrated by the following example
which
should not be construed as limiting. The contents of all references, patents
and published
patent applications cited throughout this application are incorporated herein
by reference.
EXAMPLE 1: INHIBITION OF THE URGE TO URINATE
[0164] Twenty volunteer subjects, both male and female were enrolled, each of
which
experienced premature urge or desire to urinate, interfering with their
ability to sleep for a
sufficient period of time to feel adequately rested. Each subject ingested 400-
800 mg of
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
ibuprofen as a single dose prior to bedtime. At least 14 subjects reported
that they were able
to rest better because they were not being awakened as frequently by the urge
to urinate.
[0165] Several subjects reported that after several weeks of nightly use of
ibuprofen,
the benefit of less frequent urges to urinate was no longer being realized.
However, all of
these subjects further reported the return of the benefit after several days
of abstaining from
taking the dosages.
EXAMPLE 2: EFFECT OF ANALGESIC AGENTS, BOTULINUM NEUROTOXIN AND
ANTIMUSCARINIC AGENTS ON MACROPHAGE RESPONSES TO
INFLAMMATORY AND NON-INFLAMMATORY STIMULI
Experimental Design
[0166] This study is designed to determine the dose and in vitro efficacy of
analgesics
and antimuscarinic agents in controlling macrophage response to inflammatory
and non-
inflammatory stimuli mediated by COX2 and prostaglandins (PGE, PGH, etc.). It
establishes
baseline (dose and kinetic) responses to inflammatory and non-inflammatory
effectors in
bladder cells. Briefly, cultured cells are exposed to analgesic agents and/or
antimuscarinic
agents in the absence or presence of various effectors.
[0167] The effectors include: lipopolysaccharide (LPS), an inflammatory agent
and
Cox2 inducer, as inflammatory stimuli; carbachol or acetylcholine, a
stimulator of smooth
muscle contraction, as non-inflammatory stimuli; botulinum neurotoxin A, a
known inhibitor
of acetylcholine release, as positive control; and arachidonic acid (AA),
gamma linolenic acid
(DGLA) or eicosapentaenoic acid (EPA) as precursors of prostaglandins, which
are produced
following the sequential oxidation of AA, DGLA or EPA inside the cell by
cyclooxygenases
(COX1 and COX2) and terminal prostaglandin synthases.
[0168] The analgesic agents include: Salicylates such as aspirin, iso-butyl-
propanoic-
phenolic acid derivative (ibuprofen) such as Advil, Motrin, Nuprin, and
Medipren, naproxen
sodium such as Aleve, Anaprox, Antalgin, Feminax Ultra, Flanax, Inza, Midol
Extended
Relief, Nalgesin, Naposin, Naprelan, Naprogesic, Naprosyn, Naprosyn
suspension, EC-
Naprosyn, Narocin, Proxen, Synflex and Xenobid, acetic acid derivative such as
indomethacin (Indocin),1-naphthaleneacetic acid derivative such as nabumetone
or relafen,
N-acetyl-para-aminophenol (APAP) derivative such as acetaminophen or
paracetamol
(Tylenol) and Celecoxib.
[0169] The antimuscarinic agents include: oxybutynin, solifenacin, darifenacin
and
atropine.
41
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
[0170] Macrophages are subjected to short term (1-2 hrs) or long term (24-48
hrs)
stimulation with:
1) Each analgesic agent alone at various doses.
(2) Each analgesic agent at various doses in the presence of LPS.
(3) Each analgesic agent at various doses in the presence of carbachol or
acetylcholine.
(4) Each analgesic agent at various doses in the presence of AA, DGLA, or EPA.
(5) Botulinum neurotoxin A alone at various doses.
(6) Botulinum neurotoxin A at various doses in the presence of LPS.
(7) Botulinum neurotoxin A at various doses in the presence of carbachol or
acetylcholine.
(8) Botulinum neurotoxin A at various doses in the presence of AA, DGLA, or
EPA.
(9) Each antimuscarinic agent alone at various doses.
(10) Each antimuscarinic agent at various doses in the presence of LPS.
(11) Each antimuscarinic agent at various doses in the presence of carbachol
or acetylcholine.
(12) Each antimuscarinic agent at various doses in the presence of AA, DGLA,
or EPA.
[0171] The cells are then analyzed for the release of PGH2, PGE, PGE2,
Prostacydin,
Thromboxane, IL-6, TNF-a, the COX2 activity, the production of cAMP and
cGMP,
the production of IL-113, IL-6, TNF-a and COX2 mRNA, and surface expression of
CD80,
CD86 and MHC class II molecules.
Materials and Methods
Macrophage cells
[0172] Murine RAW264.7 or J774 macrophage cells (obtained from ATCC) were
used in this study. Cells were maintained in a culture medium containing RPMI
1640
supplemented with 10 % fetal bovine serum (FBS), 15 mM HEPES, 2 mM L-
glutamine, 100
11/m1 penicillin, and 100 lag / ml of streptomycin. Cells were cultured at 37
C in a 5 % CO2
atmosphere and split (passages) once a week.
In vitro treatment of macrophage cells with analgesics
[0173] RAW264.7 macrophage cells were seeded in 96-well plates at a cell
density of
1.5x105 cells per well in 100 ill of the culture medium. The cells were
treated with (1)
various concentrations of analgesic (acetaminophen, aspirin, ibuprophen or
naproxen), (2)
various concentrations of lipopolysaccharide (LPS), which is an effector of
inflammatory
stimuli to macrophage cells, (3) various concentrations of carbachol or
acetylcholine, which
are effectors of non-inflammatory stimuli, (4) analgesic and LPS or (5)
analgesic and
carbachol or acetylcholine. Briefly, the analgesics were dissolved in FBS-free
culture
42
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
medium (i.e., RPMI 1640 supplemented with 15 mM HEPES, 2 mM L-glutamine, 100 U
/ ml
penicillin, and 100 lig / ml of streptomycin), and diluted to desired
concentrations by serial
dilution with the same medium. For cells treated with analgesic in the absence
of LPS, 50
of analgesic solution and 50 of FBS-free culture medium were added to each
well. For
cells treated with analgesic in the presence of LPS, 50 1.11 of analgesic
solution and 50 ill of
LPS (from Salmonella typhimurium) in FBS-free culture medium were added to
each well.
All conditions were tested in duplicates.
[0174] After 24 or 48 hours of culture, 150111 of culture supernatants were
collected,
spun down for 2 min at 8,000 rpm at 4 C to remove cells and debris and stored
at -70 C for
analysis of cytokine responses by ELISA. The cells were collected and washed
by
centrifugation (5 min at 1,500 rpm at 4 C) in 500 ill of Phosphate buffer
(PBS). Half of the
cells were then snap frozen in liquid nitrogen and stored at -70 C. The
remaining cells were
stained with fluorescent monoclonal antibodies and analyzed by flow cytometry.
Flow cytometry analysis of co-stimulatory molecule expression
[0175] For flow cytometry analysis, macrophages were diluted in 100 of FACS
buffer (phosphate buffered saline (PBS) with 2% bovine serum albumin (BSA) and
0.01%
NaN3) and stained 30 min at 4 C by addition of FITC-conjugated anti-CD40, PE-
conjugated
anti-CD80, PE-conjugated anti-CD86 antibody, anti MHC class II (I-Ad ) PE (BD
Bioscience). Cells were then washed by centrifugation (5 min at 1,500 rpm at 4
C) in 300 pi
of FACS buffer. After a second wash, cells were re-suspended in 200 ill of
FACS buffer and
the percentage of cells expressing a given marker (single positive), or a
combination of
markers (double positive) were analyzed with the aid of an Accuri C6 flow
cytometer (BD
Biosciences).
Analysis of cytokine responses by ELISA
[0176] Culture supernatants were subjected to cytokine-specific ELISA to
determine
IL-1[3, IL-6 and TNF-cc responses in cultures of macrophages treated with
analgesic, LPS
alone or a combination of LPS and analgesic. The assays were performed on Nunc
MaxiSorp
Immunoplates (Nunc) coated overnight with 100 ill of anti-mouse IL-6, TNF-cc
mAbs (BD
Biosciences) or IL-1I3 mAb (R&D Systems) in 0.1 M sodium bicarbonate buffer
(pH 9.5).
After two washes with PBS (200 ill per well), 200 ill of PBS 3% BSA were added
in each
well (blocking) and the plates incubated for 2 hours at room temperature.
Plates were washed
again two times by addition of 2001.il per well, 100 1.11 of cytokine
standards and serial
dilutions of culture supernatants were added in duplicate and the plates were
incubated
43
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
overnight at 4 C. Finally, the plates were washed twice and incubated with 100
ill of
secondary biotinylated anti-mouse IL-6, TNFec mAbs (BD Biosciences) or IL-113
(R&D
Systems) followed by peroxidase-labelled goat anti-biotin mAb (Vector
Laboratories). The
colorimetric reaction was developed by the addition of 2,2'-azino-bis (3)-
ethylbenzylthiazoline-6-sulfonic acid (ABTS) substrate and LI2(1302 (Sigma)
and the absorbance
measured at 415 nm with a Victor V multilabel plate reader (PerkinElmer).
Determination of COX2 activity and the production of cAMP and cGMP
[0177] The COX2 activity in the cultured macrophages is determined by
sequential
competitive ELISA (R&D Systems). The production of cAMP and cGMP is determined
by
the cAMP assay and cGMP assay. These assays are performed routinely in the
art.
Results
[0178] Table 1 summarizes the experiments performed with Raw 264 macrophage
cell line and main findings in terms of the effects of analgesics on cell
surface expression of
costimulatory molecules CD40 and CD80. Expression of these molecules is
stimulated by
COX2 and inflammatory signals and thus, was evaluated to determine functional
consequences of inhibition of COX2.
[0179] As shown in Table 2, acetaminophen, aspirin, ibuprophen and naproxen
inhibit basal expression of co-stimulatory molecules CD40 and CD80 by
macrophages at all
the tested doses (i.e., 5x 105 nM, 5x 104 nM, 5x 103 nM, 5x 102 nM, 50 nM and
5 nM),
except for the highest dose (i.e., 5x 106 nM), which appears to enhance,
rather than inhibit,
expression of the co-stimulatory molecules. As shown in Figures 1A and 1B,
such inhibitory
effect on CD40 and CD50 expression was observed at analgesic doses as low as
0.05 nM
(i.e., 0.00005 uM). This finding supports the notion that a controlled release
of small doses
of analgesic may be preferable to acute delivery of large doses. The
experiment also revealed
that acetaminophen, aspirin, ibuprophen and naproxen have a similar inhibitory
effect on LPS
induced expression of CD40 and CD80.
Table 1. Summary of experiments
LPS
Control Salmonella
typhimurium Acetaminophen Aspirin Ibuprophen Naproxen
TESTS
1 X
2 X Dose
44
CA 02866853 2014-09-08
WO 2013/142197 PCT/US2013/030901
responses
(0, 5, 50,
1000)
ng/mL
3 X Dose responses
(0, 5, 50, 500, 5x103, 5x104, 5x105, 5x106) nM
4 X X (5 ng/mL) Dose responses
X (50 ng/mL (0, 5 , 50, 500, 5x103, 5x104, 5x105, 5x106) nM
X (1000
ng/mL)
ANALYSIS
a Characterization of activation/stimulatory status: Flow cytometry
analysis of
CD40, CD80, CD86 and MHC class II
Mediators of inflammatory responses: ELISA analysis of IL-113, IL-6, TNF-cc
Table 2. Summary of main findings
Effectors % Positive Negative LPS Dose analgesic (nM)
Control 5
ng/ml
5x106 5x105 5x104 5x103 500 50 5
CD40+CD80+ 20.6 77.8
Acetaminophen CD40+CD80+ 63 18 12 9.8 8.3
9.5 7.5
Aspirin CD40+CD80+ 44 11 10.3 8.3 8
10.5 7.5
Ibuprophen CD40+CD80+ ND* 6.4 7.7 7.9 6.0 4.9 5.8
Naproxen CD40+CD80+ 37 9.6 7.7 6.9 7.2 6.8 5.2
Analgesic plus LPS
Acetaminophen CD40+CD80+ 95.1 82.7 72.4 68.8 66.8 66.2 62.1
Aspirin CD40+CD80+ 84.5 80 78.7 74.7 75.8 70.1 65.7
Ibuprophen CD40+CD80+ ND 67 77.9 72.9 71.1 63.7 60.3
Naproxen CD40+CD80+ 66.0 74.1 77.1 71.0 68.8 72 73
* ND: not done (toxicity)
[0180] Table 3 summarizes the results of several studies that measured serum
levels
of analgesic after oral therapeutic doses in adult humans. As shown in Table
3, the maximum
serum levels of analgesic after an oral therapeutic dose are in the range of
104 to 105 nM.
CA 02866853 2014-09-08
WO 2013/142197 PCT/US2013/030901
Therefore, the doses of analgesic tested in vitro in Table 2 cover the range
of concentrations
achievable in vivo in humans.
Table 3. Serum levels of analgesic in human blood after oral therapeutic doses
Maximum serum
Analgesic drug Molecular levels after oral References
weight therapeutic doses
mg/L nM
Acetaminophen 151.16 11-18 7.2x104- * BMC Clinical
Pharmacology.2010, 10:10
(Tylenol) 1.19x105 * Anaesth Intensive Care. 2011,
39:242
Aspirin 181.66 30-100 1.65x105- * Disposition of Toxic Drugs and
Chemicals in
(Acetylsalicylic acid) 5.5x105 Man, 8th Edition, Biomedical
Public, Foster City,
CA, 2008, pp. 22-25
* J Lab Clin Med. 1984 Jun;103:869
Ibuprofen 206.29 24-32 1.16x105- * BMC Clinical Pharmacology2010,
10:10
(Advil, Motrin) 1.55 x105 * J Clin Phannacol. 2001, 41:330
Naproxen 230.26 Up to Up to * J Clin Pharmacol. 2001, 41:330
(Aleve) 60 2.6x105
EXAMPLE 3: EFFECT OF ANALGESIC AGENTS, BOTULINUM NEUROTOXIN AND
ANTIMUSCARINIC AGENTS ON MOUSE BLADDER SMOOTH MUSCLE CELL
RESPONSES TO INFLAMMATORY AND NON-INFLAMMATORY STIMULI
Experimental Design
[0181] This study is designed to characterize how the optimal doses of
analgesics
determined in Example 2 affect bladder smooth muscle cells in cell culture or
tissue cultures,
and to address whether different classes of analgesics can synergize to more
efficiently
inhibit COX2 and PGE2 responses.
[0182] The effectors, analgesic agents and antimuscarinic agents are described
in
Example 2.
[0183] Primary culture of mouse bladder smooth muscle cells are subjected to
short
term (1-2 hrs) or long term (24-48 hrs) stimulation with:
(1) Each analgesic agent alone at various doses.
(2) Each analgesic agent at various doses in the presence of LPS.
46
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
(3) Each analgesic agent at various doses in the presence of carbachol or
acetylcholine.
(4) Each analgesic agent at various doses in the presence of AA, DGLA, or EPA.
(5) Botulinum neurotoxin A alone at various doses.
(6) Botulinum neurotoxin A at various doses in the presence of LPS.
(7) Botulinum neurotoxin A at various doses in the presence of carbachol or
acetylcholine.
(8) Botulinum neurotoxin A at various doses in the presence of AA, DGLA, or
EPA.
(9) Each antimuscarinic agent alone at various doses.
(10) Each antimuscarinic agent at various doses in the presence of LPS.
(11) Each antimuscarinic agent at various doses in the presence of carbachol
or
acetylcholine.
(12) Each antimuscarinic agent at various doses in the presence of AA, DGLA,
or
EPA.
[0184] The cells are then analyzed for the release of PGH2, PGE, PGE2,
Prostacydin,
Thromboxane, IL-113, IL-6, TNF-a, the COX2 activity, the production of cAMP
and cGMP,
the production of IL-113, IL-6, TNF-a and COX2 mRNA, and surface expression of
CD80,
CD86 and MHC class II molecules.
Materials and Methods
Isolation and purification of mouse bladder cells
[0185] Bladder cells were removed from euthanized animals C57BL/6 mice (8-12
weeks old) and cells were isolated by enzymatic digestion followed by
purification on a
Percoll gradient. Briefly, bladders from 10 mice were minced with scissors to
fine slurry in
ml of digestion buffer (RPMI 1640, 2% fetal bovine serum, 0.5 mg/ml
collagenase, 30
ug/m1DNase). Bladder slurries were enzymatically digested for 30 minutes at 37
C.
Undigested fragments were further dispersed through a cell-trainer. The cell
suspension was
pelleted and added to a discontinue 20%, 40% and 75% Percoll gradient for
purification on
mononuclear cells. Each experiment used 50-60 bladders.
[0186] After washes in RPMI 1640, bladder cells were resuspended RPMI 1640
supplemented with 10 % fetal bovine serum, 15 mM HEPES, 2 mM L-glutamine, 100
U/ml
penicillin, and 100 ,g / ml of streptomycin and seeded in clear-bottom black
96-well cell
culture microculture plates at a cell density of 3x104 cells per well in 100
1. Cells were
cultured at 37 C in a 5 % CO2 atmosphere.
47
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
In vitro treatment of cells with analgesics
[0187] Bladder cells were treated with analgesic solutions (50 [t1/ well)
either alone or
together with carbachol (10-Molar, 50 pi/ well), as an example of non-
inflammatory stimuli,
or lipopolysaccharide (LPS) of Salmonella typhimurium (1 jig/ml, 50 ii1/
well), as an example
of non-inflammatory stimuli. When no other effectors were added to the cells,
50 p.1 of
RPMI 1640 without fetal bovine serum were added to the wells to adjust the
final volume to
200 pl.
[0188] After 24 hours of culture, 150 [11 of culture supernatants were
collected, spun
down for 2 min at 8,000 rpm at 4 C to remove cells and debris and stored at -
70 C for
analysis of Prostaglandin E2 (PGE2) responses by ELISA. Cells were fixed,
permeabilized
and blocked for detection of Cyclooxygenase-2 (COX2) using a fluorogenic
substrate. In
selected experiment cells were stimulated 12 hours in vitro for analysis of
COX2 responses
Analysis of COX2 responses
[0189] COX2 responses were analyzed by a Cell-Based ELISA using Human/mouse
total COX2 immunoassay (R&D Systems), following the instructions of the
manufacturer.
Briefly, after cells fixation and permeabilization, a mouse anti-total COX2
and a rabbit anti-
total GAPDH were added to the wells of the clear-bottom black 96-well cell
culture
microculture plates. After incubation and washes, an HRP-conjugated anti-mouse
IgG and an
AP-conjugated anti-rabbit IgG were added to the wells. Following another
incubation and set
of washes, the HRP- and AP-fluorogenic substrates were added. Finally, a
Victor V
multilabel plate reader (PerkinElmer) was used to read the fluorescence
emitted at 600 nm
(COX2 fluorescence) and 450 nm (GAPDH fluorescence). Results are expressed as
relative
levels of total COX2 as determined by relative fluorescence unit (RFUs) and
normalized to
the housekeeping protein GAPDH.
Analysis of PGE2 responses
[0190] Prostaglandin E2 responses were analyzed by a sequential competitive
ELISA
(R&D Systems). More specifically, culture supernatants or PGE2 standards were
added to
the wells of a 96-well polystyrene microplate coated with a goat anti-mouse
polyclonal
antibody. After one hour incubation on a microplate shaker, an HRP-conjugated
PGE2 was
added and plates incubated for an additional two hours at room temperature.
The plates were
then washed and HRP substrate solution added to each well. The color was
allowed to
develop for 30 min and the reaction stopped by addition sulfuric acid before
reading the plate
at 450 nm with wavelength correction at 570 nm. Results are expressed as mean
pg/ml of
PGE2.
48
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
Other assays
[0191] The release of PGH2, PGE, Prostacydin, Thromboxane, IL-113, IL-6, and
TNF-
a, the production of cAMP and cGMP, the production of IL-1P, IL-6, TNF-a and
COX2
mRNA, and surface expression of CD80, CD86 and MHC class II molecules are
determined
as described in Example 2.
Results
Analgesics inhibit COX2 responses of mouse bladder cells to an inflammatory
stimulus
[0192] Several analgesics (acetaminophen, aspirin, ibuprofen and naproxen)
were
tested on mouse bladder cells at the concentration of 5 uM or 50 [EM to
determine whether
the analgesics could induce COX2 responses. Analysis of 24-hour cultures
showed that none
of the analgesics tested induced COX2 responses in mouse bladder cells in
vitro.
[0193] The effect of these analgesics on the COX2 responses of mouse bladder
cells
to carbachol or LPS stimulation in vitro was also tested. As indicated in
Table 1, the dose of
carbachol tested has no significant effect on COX2 levels in mouse bladder
cells. On the
other hand, LPS significantly increased total COX2 levels. Interestingly,
acetaminophen,
aspirin, ibuprofen and naproxen could all suppress the effect of LPS on COX2
levels. The
suppressive effect of the analgesic was seen when these drugs were tested at
either 5 [EM or
50 [EM (Table 4).
Table 4. COX2 expression by mouse bladder cells after in vitro stimulation and
treatment
with analgesic
Stimulus Analgesic Total COX2 levels
(Normalized RFUs)
None None 158 + 18
Carbachol (mM) None 149 21
LPS (1 g/m1) None 420 26
LPS (1[1g/m1) Acetaminophen (5 [EM) 275 12
LPS (1[1g/m1) Aspirin (5 [EM) 240 17
LPS (1 ug/ml) Ibuprofen (5 [EM)) 253 32
LPS (1 tig/m1) Naproxen (5 M) 284 11
LPS (In/me Acetaminophen (50 [EM) 243 15
LPS (1[1g/m1) Aspirin (50 [EM) 258 21
LPS (1[1g/m1) Ibuprofen (50 [EM) 266 19
LPS (1 g/m1) Naproxen (50 [EM) 279 23
49
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
Analgesics inhibit PGE2 responses of mouse bladder cells to an inflammatory
stimulus
[0194] The secretion of PGE2 in culture supernatants of mouse bladder cells
was
measured to determine the biological significance of the alteration of mouse
bladder cell
COX2 levels by analgesics. As shown in Table 5, PGE2 was not detected in the
culture
supernatants of unstimulated bladder cells or bladder cells cultured in the
presence of
carbachol. Consistent with COX2 responses described above, stimulation of
mouse bladder
cells with LPS induced the secretion of high levels of PGE2. Addition of the
analgesics
acetaminophen, aspirin, ibuprofen and naproxen suppressed the effect of LPS on
PGE2
secretion and no difference was seen between the responses of cells treated
with the 5 or 50
M dose of analgesic.
Table 5. PGE2 secretion by mouse bladder cells after in vitro stimulation and
treatment with
analgesic.
Stimulus Analgesic PGE2 levels (pg/ml)
None None <20.5
Carbachol (mM) None <20.5
LPS (1 g/m1) None 925 55
LPS (1 g/m1) Acetaminophen (5 M) 619 32
LPS (1 g/m1) Aspirin (5 M) 588 21
LPS (1 g/m1) Ibuprofen (5 M)) 593 46
LPS (1 g/m1) Naproxen (5 M) 597 19
LPS (1 g/m1) Acetaminophen (50 M) 600 45
LPS (1 g/m1) Aspirin (50 M) 571 53
LPS (1 g/m1) Ibuprofen (50 M) 568 32
LPS (1 g/m1) Naproxen (50 M) 588 37
[0195] In summary, these data show that the analgesics alone at 5 M or 50 M
do
not induce COX2 and PGE2 responses in mouse bladder cells. The analgesics at 5
M or 50
M, however, significantly inhibit COX2 and PGE2 responses of mouse bladder
cells
stimulated in vitro with LPS (1 jig/ml). No significant effect of analgesics
was observed on
COX2 and PGE2 responses of mouse bladder cells stimulated with carbachol (1
mM).
EXAMPLE 4: EFFECT OF ANALGESIC AGENTS, BOTULINUM NEUROTOXIN AND
ANTIMUSCARINIC AGENTS ON MOUSE BLADDER SMOOTH MUSCLE CELL
CONTRACTION.
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
Experimental Design
[0196] Cultured mouse or rat bladder smooth muscle cells and mouse or rat
bladder
smooth muscle tissue are exposed to inflammatory stimuli and non-inflammatory
stimuli in
the presence of analgesic agent and/or antimuscarinic agent at various
concentrations. The
stimulus-induced muscle contraction is measured to evaluate the inhibitory
effect of the
analgesic agent and/or antimuscarinic agent.
[0197] The effectors, analgesic agents and antimuscarinic agents are described
in
Example 2.
[0198] Primary cultures of mouse bladder smooth muscle cells are subjected to
short
term (1-2 hrs) or long term (24-48 hrs) stimulation with:
(1) Each analgesic agent alone at various doses.
(2) Each analgesic agent at various doses in the presence of LPS.
(3) Each analgesic agent at various doses in the presence of carbachol or
acetylcholine.
(4) Each analgesic agent at various doses in the presence of AA, DGLA, or EPA.
(5) Botulinum neurotoxin A alone at various doses.
(6) Botulinum neurotoxin A at various doses in the presence of LPS.
(7) Botulinum neurotoxin A at various doses in the presence of carbachol or
acetylcholine.
(8) Botulinum neurotoxin A at various doses in the presence of AA, DGLA, or
EPA.
(9) Each antimuscarinic agent alone at various doses.
(10) Each antimuscarinic agent at various doses in the presence of LPS.
(11) Each antimuscarinic agent at various doses in the presence of carbachol
or
acetylcholine.
(12) Each antimuscarinic agent at various doses in the presence of AA, DGLA,
or
EPA.
Materials and Methods
[0199] Primary mouse bladder cells are isolated as described in Example 3. In
selected experiments, cultures of bladder tissue are used. Bladder smooth
muscle cell
contractions are recorded with a Grass polygraph (Quincy Mass, USA).
EXAMPLE 5: EFFECT OF ORAL ANALGESIC AGENTS AND ANTIMUSCARINIC
AGENTS ON COX2 AND PGE2 RESPONSES OF MOUSE BLADDER SMOOTH
MUSCLE CELLS.
Experimental design:
51
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
[0200] Normal mice and mice with over active bladder syndrome are given oral
doses
of aspirin, naproxen sodium, ibuprofen, Indocin, nabumetone, Tylenol,
Celecoxib,
oxybutynin, solifenacin, darifenacin, atropine and combinations thereof
Control groups
include untreated normal mice and untreated OAB mice with over active bladder
syndrome.
Thirty (30) minutes after last doses, the bladders are collected and
stimulated ex vivo with
carbachol or acetylcholine. In selected experiments, the bladders are treated
with botulinum
neurotoxin A before stimulation with carbachol. Animals are maintained in
metabolic cages
and frequency (and volume) of urination are evaluated. Bladder outputs are
determined by
monitoring water intake and cage litter weight. Serum PGH2, PGE, PGE2,
Prostacydin,
Thromboxane, IL-6, TNF-a, cAMP, and cGMP levels are determined by ELISA.
CD80, CD86, MHC class II expression in whole blood cells are determined by
flow
cytometry.
[0201] At the end of the experiment, animals are euthanized and ex vivo
bladder
contractions are recorded with a Grass polygraph. Portions of bladders are
fixed in formalin,
and COX2 responses are analyzed by immunohistochemistry.
EXAMPLE 6: EFFECT OF ANALGESIC AGENTS, BOTULINUM NEUROTOXIN AND
ANTIMUSCARINIC AGENTS ON HUMAN BLADDER SMOOTH MUSCLE CELL
RESPONSES TO INFLAMMATORY AND NON-INFLAMMATORY STIMULI
Experimental Design
[02021 This study is designed to characterize how the optimal doses of
analgesic
determined in Examples 1-5 affect human bladder smooth muscle cells in cell
culture or
tissue cultures, and to address whether different classes of analgesics can
synergize to more
efficiently inhibit COX2 and PGE2 responses.
[0203] The effectors, analgesic agents and antimuscarinic agents are described
in
Example 2.
[0204] Human bladder smooth muscle cells are subjected to short term (1-2 hrs)
or
long term (24-48 hrs) stimulation with:
(1) Each analgesic agent alone at various doses.
(2) Each analgesic agent at various doses in the presence of LPS.
(3) Each analgesic agent at various doses in the presence of carbachol or
acetylcholine.
(4) Each analgesic agent at various doses in the presence of AA, DGLA, or EPA.
(5) Botulinum neurotoxin A alone at various doses.
(6) Botulinum neurotoxin A at various doses in the presence of LPS.
52
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
(7) Botulinum neurotoxin A at various doses in the presence of carbachol or
acetylcholine.
(8) Botulinum neurotoxin A at various doses in the presence of AA, DGLA, or
EPA.
(9) Each antimuscarinic agent alone at various doses.
(10) Each antimuscarinic agent at various doses in the presence of LPS.
(11) Each antimuscarinic agent at various doses in the presence of carbachol
or
acetylcholine.
(12) Each antimuscarinic agent at various doses in the presence of AA, DGLA,
or
EPA.
[0205] The cells are then analyzed for the release of PGH2, PGE, PGE2,
Prostacydin,
Thromboxane, IL-1f3, IL-6, TNF-a, the COX2 activity, the production of cAMP
and cGMP,
the production of IL-1f3, IL-6, TNF-a and COX2 mRNA, and surface expression of
CD80,
CD86 and MHC class II molecules.
EXAMPLE 7: EFFECT OF ANALGESIC AGENTS, BOTULINUM NEUROTOXIN AND
ANTIMUSCARINIC AGENTS ON HUMAN BLADDER SMOOTH MUSCLE CELL
CONTRACTION.
Experimental Design
[0206] Cultured human bladder smooth muscle cells are exposed to inflammatory
stimuli and non-inflammatory stimuli in the presence of an analgesic agent
and/or
antimuscarinic agent at various concentrations. The stimuli-induced muscle
contraction is
measured to evaluate the inhibitory effect of the analgesic agent and/or
antimuscarinic agent.
[0207] The effectors, analgesic agents and antimuscarinic agents are described
in
Example 2.
[0208] Human bladder smooth muscle cells are subjected to short term (1-2 hrs)
or
long term (24-48 hrs) stimulation with:
(1) Each analgesic agent alone at various doses.
(2) Each analgesic agent at various doses in the presence of LPS.
(3) Each analgesic agent at various doses in the presence of carbachol or
acetylcholine.
(4) Each analgesic agent at various doses in the presence of AA, DGLA, or EPA.
(5) Botulinum neurotoxin A alone at various doses.
(6) Botulinum neurotoxin A at various doses in the presence of LPS.
(7) Botulinum neurotoxin A at various doses in the presence of carbachol or
acetylcholine.
53
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
(8) Botulinum neurotoxin A at various doses in the presence of AA, DGLA, or
EPA.
(9) Each antimuscarinic agent alone at various doses.
(10) Each antimuscarinic agent at various doses in the presence of LPS.
(11) Each antimuscarinic agent at various doses in the presence of carbachol
or
acetylcholine.
(12) Each antimuscarinic agent at various doses in the presence of AA, DGLA,
or
EPA.
[0209] Bladder smooth muscle cell contractions are recorded with a Grass
polygraph
(Quincy Mass, USA).
EXAMPLE 8: EFFECT OF ANALGESIC AGENTS ON NORMAL HUMAN BLADDER
SMOOTH MUSCLE CELL RESPONSES TO INFLAMMATORY AND NON
INFLAMMATORY SIGNALS
EXPERIMENTAL DESIGN
Culture of normal human bladder smooth muscle cells
[0210] Normal human bladder smooth muscle cells were isolated by enzymatic
digestion from macroscopically normal pieces of human bladder. Cells were
expended in
vitro by culture at 37 C in a 5 % CO2 atmosphere in RPMI 1640 supplemented
with 10 %
fetal bovine serum, 15 mM HEPES, 2 mM L-glutamine, 100 U/ml penicillin, and
100 mg /
ml of streptomycin and passage once a week by treatment with trypsin to detach
cells
followed by reseeding in a new culture flask. The first week of culture, the
culture medium
was supplemented with 0.5 ng/ml epidermal growth factor, 2 ng/ml fibroblast
growth factor,
and 5 g/m1 insulin.
Treatment of normal human bladder smooth muscle cells with analgesics in vitro
[0211] Bladder smooth muscle cells trypsinized and seeded in microculture
plates at a
cell density of 3x104 cells per well in 100 I were treated with analgesic
solutions (50 1/
well) either alone or together carbachol (10-Molar, 50 1/ well), as an
example of non-
inflammatory stimuli, or lipopolysaccharide (LPS) of Salmonella typhimurium (1
g/ml, 50
1/ well), as an example of non-inflammatory stimuli. When no other effectors
were added to
the cells, 50 1 of RPMI 1640 without fetal bovine serum were added to the
wells to adjust
the final volume to 200111.
[0212] After 24 hours of culture, 150 I of culture supernatants were
collected, spun
down for 2 mM at 8,000 rpm at 4 C to remove cells and debris and stored at -70
C for
analysis of Prostaglandin E2 (PGE) responses by ELISA. Cells were fixed,
permeabilized
54
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
and blocked for detection of COX2 using a fluorogenic substrate. In selected
experiment cells
were stimulated 12 hours in vitro for analysis of COX2, PGE2 and cytokine
responses.
Analysis of COX2, PGE2 and cytokine responses
[0213] COX2 and PGE2 responses were analyzed as described in Example 3.
Cytokine responses were analyzed as described in Example 2.
RESULTS
[0214] Analgesics inhibit COX2 responses of normal human bladder smooth muscle
cells to inflammatory and non- inflammatory stimuli - Analysis of cells and
culture
supernatants after 24 hours of cultures showed that none of the analgesics
tested alone
induced COX2 responses in normal human bladder smooth muscle cells. However,
as
summarized in Table 6, carbachol induced low, but significant COX2 responses
in normal
human bladder smooth muscle cells. On the other hand, LPS treatment resulted
in higher
levels of COX2 responses in normal human bladder smooth muscle cells.
Acetaminophen,
aspirin, ibuprofen and naproxen could all suppress the effect of carbachol and
LPS on COX2
levels. The suppressive effect of the analgesics was seen on LPS-induced
responses when
these drugs were tested at either 5 viM or 50 iAM.
Table 6. COX2 expression by normal human bladder smooth muscle cells after in
vitro
stimulation with inflammatory and non- inflammatory stimuli and treatment with
analgesic
Total COX2 levels# Total COX2 levels
Stimulus Analgesic (Normalized RFUs) (Normalized RFUs)
subject 1 subject 2
None None 230 199
Carbachol 10-3M None (50 M) 437 462
Carbachol 10-3 Acetaminophen (50 viM) 298 310
Carbachol 10-3M Aspirin (50 [I,M) 312 297
Carbachol 10-3M Ibuprofen (50 i,J,M) 309 330
Carbachol 10-3M Naproxen (50 [I,M) 296 354
LPS (10 gimp None 672 633
LPS (10 vig/m1) Acetaminophen (5 M) 428 457
LPS (10 vig/m1) Aspirin (5 i,J,M) 472 491
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
LPS (10 g/m1) Ibuprofen (5 M) 417 456
LPS (10 gime Naproxen (5 M 458 501
LPS (10 g/m1) Acetaminophen (50 M) 399 509
LPS (10 g/m1) Aspirin (5011M) 413 484
LPS (10 g/m1) Ibuprofen (50 M) 427 466
LPS (10 g/m1) Naproxen (50 M) 409 458
#Data are expressed as mean of duplicates
[0215] Analgesics inhibit PGE2 responses of normal human bladder smooth muscle
cells to inflammatory and non- inflammatory stimuli - Consistent with the
induction of COX2
responses described above, both carbachol and LPS induced production of PGE2
by normal
human bladder smooth muscle cells. Acetaminophen, aspirin, ibuprofen and
naproxen were
also found to suppress the LPS-induced PGE2 responses at either 5 !AM or 50 M
(Table 7).
Table 7. PGE2 secretion by normal human bladder smooth muscle cells after in
vitro
stimulation with inflammatory and non- inflammatory stimuli and treatment with
analgesic
Stimulus Analgesic PGE2 levels!' PGE2 levels (pg/ml)
(pg/ml) Subject 2
Subject 1
None None <20.5 <20.5
Carbachol 10-3M None 129 104
Carbachol le Acetaminophen (50 M) 76 62
Carbachol 10-3M Aspirin (50 M) 89 59
Carbachol 10-3M Ibuprofen (50 M) 84 73
Carbachol 10-3M Naproxen (50 M) 77 66
LPS (10 gime None 1125 998
LPS (10 g/me Acetaminophen (5 M) 817 542
LPS (10 gimp Aspirin (5 M) 838 598
LPS (10 g/m1) Ibuprofen (5 M) 824 527
LPS (10 g/m1) Naproxen (5 M 859 506
LPS (10 gimp Acetaminophen (50 M) 803 540
LPS (10 g/m1) Aspirin (50 M) 812 534
56
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
LPS (10 jug/m1) Ibuprofen (50 OA) 821 501
LPS (10 gimp Naproxen (50 i.tIVI) 819 523
#Data are expressed as mean of duplicates
[0216] Analgesics inhibit cytokine responses of normal human bladder cells to
inflammatory stimuli - Analysis of cells and culture supernatants after 24
hours of culture
showed that none of the analgesics tested alone induced IL-6 or TNFa secretion
in normal
human bladder smooth muscle cells. As shown in Tables 8 and 9, the doses of
carbachol
tested induced low, but significant TNFa and IL-6 responses in normal human
bladder
smooth muscle cells. On the other hand, LPS treatment resulted in massive
induction of
these proinflammatory cytokines. Acetaminophen, aspirin, ibuprofen and
naproxen suppress
the effect of carbachol and LPS on TNFa and IL-6 responses. The suppressive
effect of the
analgesics on LPS-induced responses was seen when these drugs were tested at
either 51,IM
or 50 IAM.
Table 8. TNFa secretion by normal human bladder smooth muscle cells after in
vitro
stimulation with inflammatory and non- inflammatory stimuli and treatment with
analgesic
Stimuli Analgesic TNFa (pg/ml) TNFa (pg/ml)
Subject! Subject 2
None None <5 <5
Carbachol 10-3M None 350 286
Carbachol 10-3 M Acetaminophen (50 viM) 138 164
Carbachol 10-3M Aspirin (50 p,M) 110 142
Carbachol 10-3M Ibuprofen (50 p,M) 146 121
Carbachol 10-3M Naproxen (50 p,M) 129 137
_
LPS (10 vig/m1) None 5725 4107
LPS (10 jig/m1) Acetaminophen (5 viM) 2338 2267
LPS (10 vig/m1) Aspirin (5 viM) 2479 2187
LPS (10 jig/m1) Ibuprofen (5 p,M) 2733 2288
LPS (10 rig/m1) Naproxen (5 viM 2591 2215
LPS (10 jig/m1) Acetaminophen (50 p,M) 2184 2056
LPS (10 is/m1) Aspirin (50 p,M) 2266 2089
LPS (10 pg/m1) Ibuprofen (50 viM) 2603 1997
LPS (10 rig/ml) Naproxen (50 viM) 2427 2192
57
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
14Data are expressed as mean of duplicates.
Table 9. IL-6 secretion by normal human bladder smooth muscle cells after in
vitro
stimulation with inflammatory and non- inflammatory stimuli and treatment with
analgesic
Stimulus Analgesic IL-6 (pg/m1)# IL-6 (pg/ml)
Subject 1 Subject 2
None None <5 <5
Carbachol 10-3M None 232 278
Carbachol 10-3 M Acetaminophen (50 ;AM) 119 135
Carbachol 10-3M Aspirin (50 M) 95 146
Carbachol 10-3M Ibuprofen (50 M) 107 118
Carbachol 10-3M Naproxen (50 }AM) 114 127
LPS (10 g/m1) None 4838 4383
LPS (10 g/m1) Acetaminophen (5 M) 2012 2308
LPS (10 ps/m1) Aspirin (5 I_LM) 2199 2089
LPS (10 g/m1) Ibuprofen (5 M) 2063 2173
LPS (10 g/m1) Naproxen (5 ;AM 2077 2229
LPS (10 g/m1) Acetaminophen (50 M) 2018 1983
LPS (10 g/m1) Aspirin (50 M) 1987 2010
LPS (10 g/ml) Ibuprofen (50 M) 2021 1991
LPS (10 .ig/m1) Naproxen (50 M) 2102 2028
#Data are expressed as mean of duplicates
[0217] Primary normal human bladder smooth muscle cells were isolated,
cultured
and evaluated for their responses to analgesics in the presence of non-
inflammatory
(carbachol) and inflammatory (LPS) stimuli. The goal of this study was to
determine
whether or not normal human bladder smooth muscle cells recapitulate the
observations
previously made with murine bladder cells.
[0218] The above-described experiment will be repeated with analgesic agents
and/or
antimuscarinic agents in delayed-release, or extended-release formulation or
delayed-and-
extended-release formulations.
[0219] The above description is for the purpose of teaching the person of
ordinary
skill in the art how to practice the present invention, and it is not intended
to detail all those
obvious modifications and variations of it which will become apparent to the
skilled worker
upon reading the description. It is intended, however, that all such obvious
modifications and
58
CA 02866853 2014-09-08
WO 2013/142197
PCT/US2013/030901
variations be included within the scope of the present invention, which is
defined by the
following claims. The claims are intended to cover the claimed components and
steps in any
sequence which is effective to meet the objectives there intended, unless the
context
specifically indicates the contrary.
59