Note: Descriptions are shown in the official language in which they were submitted.
CA 02867364 2014-09-15
Sep.'15, 2014 9:13AM No. 0522
P. 7
METHOD UTILIZING INDUSTRIAL FLUE GAS FOR REMOVING METAL
IONS FROM RICE HULLS
FIELD OF THE INVENTION
[0001] The invention relates to the recycling of rice hull, and more
particularly to a
method for removal of metal ions from rice hull by utilizing industrial flue
gas.
BACKGROUND OF THE INVENTION
[0002] China stands first in the world for rice production.. The rice planting
area was
about 430 million mu in 2008, and the total output was about 189 million tons,
which can
produce nearly 40 million tons of rice hull. In the world, the annual total
output of the =
rice hull is more than 68 million tons. The rice hull includes water, organic
matter (lignin,
cellulose, and hernicellulose), amorphous silica, and a small amount of metal
ions.
Conventionally, the rice hull is ground into chaff for use as feeds. With the
development
of science and technology, the rice hull is used for power generation, for
producing oil
and nano products, particularly, for preparation of nano silica.
[0003] A typical method for producing silica from the rice hull includes:
boiling the rice
hull with strong acid (e. g. hydrochloric acid, sulfuric acid, nitric acid,
and so on), and
washing in ultrapure water; calcining the rice hull to remove most of
impurities; soaking
the rice hull in the strong acid and rewashing using the high pure water, for
further
reducing the impurity content; drying the rice hull, transporting to a biomass
power plant
for combustion or chemically thermal decomposition, to yield nano silica.
[0004] The above method for removing metal ions from rice hull involves strong
acids.
1
PAGE 7124 RCVD AT 911512014 919:28 AM [Eastern Daylight Timer SVR:F0000314
DNIS:3905 CSID:9057470606 DURATION (mms):03.11
CA 02867364 2014-09-15
Sep.'15. 2014 9:13AM No. 0522
P. 8
Strong acids are harmful to human body and pollutes environment. In addition,
the waste
water resulting from the acid treatment cannot be drained off directly,
thereby increasing
the disposal cost.
SUMMARY OF THE INVENTION
[0005] In view of the above-desaibed problems, it is one objective of the
invention to a
method for removal of metal ions from rice hull by utilizing industrial flue
gas that
features high efficiency, environmental friendliness, and economy.
[0006] To achieve the above objective, in accordance with one embodiment of
the
invention, there is provided a method for removal of metal ions from rice hull
by utilizing
industrial flue gas. The method comprises the following steps: 1) providing a
water
storage reactor, and disposing a gas dispersion device at a bottom of the
water storage
reactor; 2) bagging rice hull and placing in the water storage reactor, and
pressing down
the bagged rice hull below a water surface of the water storage reactor; 3)
spraying
industrial flue gas into the water storage reactor via the gas dispersion
device; controlling
the amount of the industrial flue gas such that the amount of carbon dioxide
dissolved in
per 100 g of water is about 1 g whereby generating a carbonic acid solution;
4) allowing
the carbonic acid solution to react with a metal ion of the rice hull to yield
a precipitate; 5)
washing the rice hull collected in step 4), washing again with desalinated
water, and
squeezing the rich hull, whereby removing the metal ion from the rice hull.
[0007] Preferably, the water storage reactor has a depth of between 6 and 10
m.
Appropriate depth of the water storage reactor can ensure the carbon dioxide
in the
industrial flue gas is sufficiently dissolved in the water to yield a carbonic
acid solution
with a certain concentration.
2
PAGE 8124 RCVD AT 911512014 9:19:28 AM !Eastern Daylight Timer SVR:F0000314
DNIS:3905 31D:9057470608 DURATION (mmis):03.11
CA 02867364 2014-09-15
Sep:15. 2014 9:13AM No. 0522
P. 9
[0008] Preferably, the gas dispersion device comprises a gas orifice
configured to
horizontally or vertically agitate water to form vortexes, and the industrial
flue gas is
sprayed from the gas orifice. The vortexes of the water can improve the degree
of
dispersion of carbon dioxide in the water thereby facilitating the formation
of carbonic
acid. The gas orifice can be multiple in annular distribution, and sprays gas
outward along
a tangential direction.
[00091 Preferably, the gas orifice is at least 1.5 m higher than the bottom of
the water
storage reactor. The carbonic acid reacts with the rice hull to yield the
precipitate. To
prevent the precipitate from blocking the gas orifice, the gas orifice is at
least 1.5 in
higher than the bottom of the water storage reactor.
[0010] Preferably, the gas orifice has a pore size of between 0.005 and 0.012
nun. The
spray of the gas from the gas orifice satisfies the Laplace's equation, that
is to say,
additional pressure on the spherical surface is proportional to the surface
tension
coefficient and is inversely proportional to the radius of the spherical
radius. When the
surface tension coefficient is constant, the smaller the radius, the greater
the additional
pressure. The smaller the gas orifice, the smaller the sprayed bubbles. When
small
bubbles of carbon dioxide are sprayed from the gas orifice, the bubbles
expand, and the
surface tension decreases rapidly, the bubbles burst, thus increasing the
contact area of
the carbon dioxide with the water, and accelerating the formation of carbonic
acid.
[0011] Preferably, the gas dispersion device further comprises a plurality of
microporous
aerators, and the industrial flue gas is sprayed from the microporous
aerators. The
microporous aerators are configured to improve the dissolution of carbon
dioxide in the
water.
[0012] The present disclosure also provides another method for removal of
metal ions
from rice hull by utilizing industrial flue gas. The method comprises the
following steps:
3
PAGE 9124 RCVD AT 911512014 9:19:28 AM [Eastern Daylight Time] SVR:F0000314
DNIS:3905 CSID:9051410600 DURATION (mmis):03.11
CA 02867364 2014-09-15
Sep:15, 2014 9:13AM No. 0522
P. 10
1) providing a reaction tank, the reaction tank comprising a gas distributor
disposed at a
lower part thereof and a liquid distributor disposed at an upper part thereof,
wherein, the
gas distributor comprises microporous aerators, a recycle liquid outlet is
disposed on a
wall of the reaction tank below the gas distributor, a gas outlet is disposed
at a top of the
reaction tank, and a precipitate outlet is disposed at a bottom of the
reaction tank; 2)
filling the reaction tank with rice hull and water, closing the gas outlet,
and allowing the
industrial flue gas to be sprayed from the microporous aerators of the gas
distributor; 3)
allowing carbon dioxide in the industrial flue gas to be dissolved in the
water, 4 g of
carbon dioxide per 100 g of water, to yield a carbonic acid solution; 4)
allowing the
carbon acid solution to react with a metal ion of the rice hull to yield a
precipitate; and 5)
washing the rice hull collected in step 4), wasbing again with desalinated
water, and
squeezing the rich hull, whereby removing the metal ion from the rice hull.
[0013] The principle of the method for removal of metal ions from rice hull by
utilizing
industrial flue gas is summarized as follows. Carbon dioxide in the industrial
flue gas is
dissolved in water to yield carbonic acid which is used to acidify rice hull
where carbonic
acid reacts with metal ions such as aluminum, calcium, magnesium, iron,
manganese to
yield corresponding insoluble salts. The precipitates are a carbonate or an
oxide of the
metal. Thus, the metal ions of the rice hull can be efficiently removed.
Carbon dioxide is
a nonpolar molecule, but it is soluble in a strong polar solvent, and the
dissolubility
thereof is related to the temperature, the pressure, and the properties of the
solvent. With
the increase of temperature, the dissolubility of carbon dioxide decreases. At
normal
temperature and pressure, the carbon dioxide volume and the water volume in a
saturated
aqueous solution is almost 1: 1. Most of the carbon dioxide is weakly bound to
water
molecule to form a hydrate molecule, and only a small part of the carbon
dioxide
participates in the formation of carbonic acid. Low concentration of carbonic
acid cannot
treat a large amount of rice hull. When the pressure of carbon dioxide is less
than 0.5
4
PAGE 10124 RCVD AT 011512014 0:1018 AM [Eastern Daylight Time SVR:F0000314
DNIS:3005 CS10:0051410606" DURATION (mmis):03.11
CA 02867364 2014-09-15
Sep. 15. 2014 9:13AM No, 0522
P. 11
=
MPa, the dissolubility is proportional to the pressure; when the pressure
exceeds 0.5 MPa,
due to the formation of carbonic acid, with the increase of the pressure, the
dissolubility
of carbon dioxide increases rapidly. Thus, to improve the concentration of
carbonic acid
in water so as to remove the metal ions in the rice hull, it is a key element
to increase the
carbon dioxide pressure.
[0014] There are three ways to improve the balance pressure of carbon dioxide
on the
liquid surface. The first is to make use of water pressure, the second is to
dispose a gas
dispersion device, and the third is to increase the gas pressure on the liquid
surface in an
enclosed tank. One method of the present disclosure is to dispose a water
storage reactor
which makes use of the water pressure and the gas dispersion device to improve
the
dissolubility of carbon dioxide in water. Another method of the present
disclosure is to
dispose a reaction tank which makes use of an enclosed space to improve the
carbon
dioxide pressure on the liquid surface, thereby facilitating the dissolution
of carbon
dioxide.
[0015] The method of the present disclosure involving the water storage
reactor can treat
a large amount of rice hull for one cycle, and the involved equipment is
simple and easy
for operation. The method can remove the metal ions and dusts of the rice
hull, so it is
particularly suitable for primary tough treatment.
[0016] The method of the present disclosure involving the reaction tank has
controllable
reaction conditions, high carbon dioxide solubility, and high efficiency,
which is
particularly suitable for secondary fine treatment.
[0017] Compared with conventional methods for removal of metal ions using a
strong
acid, the present disclosure has the following advantages:
[0018] 1. The carbonic acid has weak acidity, and poses little pollution to
environment.
PAGE 11124 RCVD AT 911512014 9:19:28 AM [Eastern Daylight Timer SVR:F0000314
DNIS:3905: MD:9051410606 DURATION (mmis):03.11
CA 02867364 2014-09-15
Sep.' 15. 2014 9:14AM No. 0522
P. 12
=
=
Carbon dioxide from the industrial flue gas comprising power plant flue gas or
exhaust
gas is utilized for the removal of metal ions from the rice hull, which saves
the costs and
prevents the environmental pollution. A. biomass power plant equipped with 12
MW plant
unit produces 678 thousand tons of industrial flue gas annually, based on the
carbon
dioxide percentage of about at least 10%, the carbon oxide content is about
67.8 thousand
tons. Making full use of the carbon dioxide will produce considerable economic
value.
[0019] 2. The acidification of the rice hull produces a precipitate and a
solution
comprising soluble substances such as sodium, potassium, nitrogen, phosphorus,
sulfur.
The solution can be used as a direct nutrient solution for plant cultivation.
The precipitate
can be used as a construction material or an additive, which poses no
pollution. Strong
acid used in conventional methods tends to pollute environment, and the
recycling cost is
pretty high.
[0020] 3. After being washed twice, squeezed using desalinated water, and
dried, the
acidified rice hull can be directly transported to a biomass power plant for
combustion or
thermal decomposition to yield nano silica. lithe rice hull is treated by
strong acid, the
high temperature calcination is required, followed by soaking in strong acid
and washing
by high purity water, and then drying, which is very complex.
[0021] Thus, in the present disclosure, the industrial flue gas is introduced
to water where
carbon dioxide is dissolved in the water to yield carbonic acid. The carbonic
acid reacts
with the rice hull soaked in the water whereby removing the metal ions. After
being
washed and dried, the treated rice hull can be directly transported to a
biomass power
plant for combustion or thermal decomposition, no need of high temperature
calcination,
to yield nano silica_ The method reuses the industrial flue gas, reduces the
emissions, so it
is environmentally friendly, and has low pollution, low energy consumption,
high
efficiency.
6
PAGE 12124 RCVD AT 011512014 0:10:28 AM (Eastern Daylight Timer SVR:F0000314
DMS:3905 CSID:9057410606 DURATION (mmis):03.11
CA 02867364 2014-09-15
SeP:15, 2014 9:14AM No, 0522
P, 13
=
BRIEF DESCRIPTION OF THE DRAWINGS
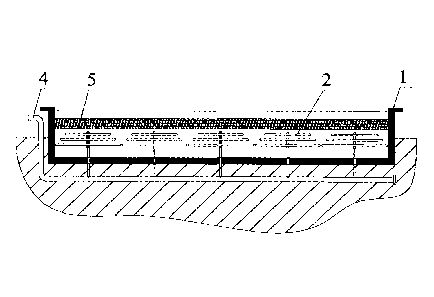
[0022] FIG. 1 is a sectional view of a water storage reactor in according to
one
embodiment of the invention;
[0023] FIG. 2 is a top view of a water storage reactor in FIG. 1; and
[0024] FIG. 3 is a sectional view of a reaction tank in according to one
embodiment of
the invention.
DETAILED DESCRIPTION OF THE EMBODIMENTS
[0025] For further illustrating the invention, experiments detailing a method
for removal
of metal ions from rice hull by utilizing industrial flue gas are described
below. It should
be noted that the following examples are intended to describe and not to limit
the
invention.
Example 1
[0026] As shown in FIGS. 1-2, a method for removal of metal ions from rice
hull by
utilizing industrial flue gas is described as follows,
[0027] 1. A water storage reactor 1 having a depth of 7 ra and a length and
width
respectively of 100 m was provided. 25 gas dispersion devices 2 for
introducing
industrial flue gas were disposed at the bottom of the water storage reactor
1. The gas
dispersion devices 2 comprised a gas orifice (not shown in the drawings)
configured to
horizontally or vertically agitate water to form vortexes. The industrial flue
gas was
sprayed from the gas orifice. The gas orifice was 1.5 m higher than the bottom
of the
water storage reactor, and had a pore size of 0.01 mm. The gas dispersion
devices 2
7
PAGE 13124 ROO AT 0115/2014 9:10:28 AM [Eastern Daylight Timer SVR:F0000314
DMS:3905* CSID:9051410606* DURATION (mmis):03.11
Sep. 15. 2014 9:14AM CA 02867364 2014-09-15
No. 0522
P. 14
further comprised a plurality of microporous aerators, and the industrial flue
gas was
sprayed from the microporous aerators.
[0028] 2. The rice hull 5 was bagged, placed in the water storage reactor 1,
and pressed
down using press bars to be lower than the water surface of the water storage
reactor.
[0029] 3. The industrial flue gas released from a biomass power plant was
filtered by dust
collecting equipment, and received by a gas main 4 which was connected to the
gas
dispersion devices. The industrial flue gas was sprayed into the water with
depth of 5.5 in
via the gas dispersion devices 2. Under such conditions, the dissolution
amount of carbon
dioxide in the water was increased by 5 folds compared with that under normal
temperature and pressure, that is, 1 g of carbon dioxide was dissolved in per
100 g of
water. Thus, a carbonic acid solution was obtained, which was adapted to
acidify the rice
hull 5. The metal ion of the rice hull 5 reacted with the carbonic acid
solution to yield a
precipitate. Thereafter, the rice hull 5 was washed, and squeezed with
desalinated water,
whereby removing the metal ion of the rice hull 5.
[0030] Besides the precipitate, the acidification of the rice hull also
produced a solution.
The solution was rich in nitrogen, phosphorus, potassium, sodium, and small
organic
molecules. The precipitate WILS mainly a carbonate and oxide of a metal such
as
aluminum, calcium, magnesium, iron, and manganese. The insoluble substances
and the
dust in the industrial flue gas precipitated in the bottom of the water
storage reactor, The
treatment period for the rice hull lasted for 6 days. Then, the rice hull was
washed twice,
and then desalinated water added, and squeezed. After such steps, between 60
and 75% of
metal ions were removed. For each cycle, the treatment amount of the rice hull
can
reached about 2500 tons.
Example 2
8
PAGE 14124 RCVD AT 911512014 9:19:28 AM (Eastern Daylight Timer SVR:F0000314
DNIS:3905' CSID:9051470606 DURATION (mmis):03.11
CA 02867364 2014-09-15
Sep. 15. 2014 9:14AM No. 0522
P. 15
[0031] As shown in FIG. 3, a method for removal of metal ions from rice hull
by utilizing
industrial flue gas is described as follows.
[0032] A reaction tank 13, having a height of 15 m and an inside capacity of
1000 m3,
was provided. A gas distributor 7 comprising microporous aerators was disposed
at the
lower part of the reaction tank 13. A recycle liquid outlet 6 was disposed on
the wall of
the reaction tank 13 below the gas distributor 7. A gas outlet 10 was disposed
at the top of
the reaction tank 13, and a cone 9 was disposed at the bottom of the reaction
tank 13 for
collecting precipitates. At the bottom of the cone, a precipitate outlet 8 was
disposed. A
demister 12 and a liquid distributor 11 were disposed at the upper part of the
reaction tank
13. The demister 12 was disposed above the liquid distributor 11.
[0033] Firstly, the reaction tank 13 was filled with rice hull 5 and water.
The rice hull 5
was floated on the water surface, and was below the liquid distributor 11. The
gas outlet
was closed. The industrial flue gas was sprayed from the microporous aerators
of the
gas distributor 7. Under such conditions, the pressure of the industrial flue
gas in the
reaction tank 13 increased rapidly. The dissolution amount of carbon dioxide
in the water
was increased by 20 folds compared with that under normal temperature and
pressure,
that is, 4 g of carbon dioxide was dissolved in per 100 g of water. Thus, a
carbonic
acid solution was obtained, which was adapted to acidify the rice hull 5. The
carbonic
acid solution was sprayed on. the rice hull 5 from the liquid distributor 11,
so that the
metal ion of the rice hull reacted with the carbonic acid solution to yield a
precipitate.
Thereafter, the rice bull 5 was washed, and squeezed with desalinated water,
whereby
removing the metal ion from the rice hull 5.
[0034] After such steps, 80% of metal ions were removed. For each cycle, the
treatment
amount of the rice hull can reached about 100 tons.
[0035] If the water storage reactor 1 in Example 1 and the reaction tank in
Example 2
9
PAGE 15124 RCVD AT 9115120149:19:28 AM !Eastern Daylight Timer SVR:F0000314
DNIS:3905 CSID:9057470606 DURATION (mmis):03.11
CA 02867364 2014-09-15
Sep, 15, 2014 9:14AM No. 0522
P. 16
were combined for use, that is, the water storage reactor 1 is used for
primary treatment,
and the reaction tank 13 is used for secondary treatment, and the resulting
rice hull is
washed and squeezed using desalinated water thrice, then 90% of metal ions are
removed.
[0036] Metal residues in the rice hull after being treated by method in
example 1,
example 2, and a combination thereof are listed in Table 1.
Table 1
Componeras (VG)
Metal
Sample Elements
Si02 Al Fe Mn Mg Ca Cu Zn Ti K Na iris content
Rawiicehull
80.5 3.80 0.44 02 271 3360.027 0.003 0312,8 1.53 4,12 1538
MethodinExample 1 Content 88.3 13 02 0.1 1.0 1.1 0.025 0.002 02 1.4 1 2.4
633
_
MethodinExample2 (wt.%) 94.7 0.7 0.18 0.06 0.5 0.6 0.015 0.001 0.1505 0.6
2 330
Combioalicnthereof 975 0.4 0.1 0.03 0.2 0.2 0.01 0 0.1 02 03
1 1.54
[00371 As shown in the above table, the method of the invention exhibits the
same
removal effect of metal ions from rice hull by utilizing industrial flue gas
as by utilizing
strong acid, and the resulting rice hull can absolutely meet the requirement
for
preparation of silica for a biomass power plant.
PAGE 16124 RCVD AT 9/1512014 9:10:28 AM [Eastern Daylight Timer SVR:F0000314
DNIS:3905 CSID:9051410606 DURATION (mmis):03.11