Note: Descriptions are shown in the official language in which they were submitted.
CA 02867422 2014-09-15
WO 2013/137899 PCT/US2012/029347
1
Probiotic/Antioxidant Composition
FIELD OF THE INVENTION
[0001] The invention is in the field of compositions that can benefit from use
of a
proboiotic/antioxidant composition with improved antioxidant capabilities.
BACKGROUND OF THE INVENTION
[0002] Probiotic microorganisms are believed to provide many advantages when
used in
topical compositions or in food compositions. Probiotic microorganisms are
known to have
beneficial properties such as sooth inflammation, balance skin microflora,
inhibit growth of
harmful bacteria, and so on.
[0003] The potential for antioxidants and probiotics to inhibit oxidation is
directly dose
dependent, with higher amounts of antioxidants and probiotics exerting a
greater inhibitory
effect.
10004] The use of a low concentration of a probiotic/antioxidant composition
containing at
least one probiotic and at least one antioxidant in a probiotic/antioxidant
combination which
acts synergistically as an antioxidant in an amount sufficient to inhibit one
or more of the
pathways that contribute to skin inflammation would reduce costs in food and
cosmetic
preservation and enhance antioxidant performance.
SUMMARY OF THE INVENTION
100051 An embodiment of this invention is composition comprising a probiotic
and an
antioxidant in a probiotic/antioxidant wt/wt ratio of about 0.1/2.5 to about
1.0/95 and wherein
said probiotic/antioxidant composition act synergistically as an inhibitor at
a concentration of
< about 10 pg/mL and is lost at concentrations of > about 10 pg/mL.
[0006] In an embodiment the probiotic is lactobacillus.
10007] In an embodiment the antioxidant is Thermus thermophillus.
100081 In an embodiment the composition contains at least one additive chosen
from an
antioxidant, a filler, a preserving agent, a fragrance, a neutralizing agents,
a thickener, a
cosmetic active agent, an emollient, and mixtures thereof.
[0009] In and embodiment the composition added to a cosmetic taking the form
of a
mascara, an eyeliner, a product for the eyebrows, a product for the lips, a
face powder, an
CA 02867422 2014-09-15
WO 2013/137899 PCT/US2012/029347
2
eye shadow, a foundation, a make-up product for the body, a concealer product,
a nail
varnish, a skincare product, and a hair care product.
[00101 In an embodiment the composition is added to a food.
100111 The composition of claim 1, wherein the composition further comprises
an at least one
pigment, dye, or stain.
10012] In an embodiment the probiotic/antioxidant wt/wt ratio is 0.5/2.85.
[0013] In an embodiment the probiotic/antioxidant wt/wt ratio is 1/95.
[00141 in an embodiment the composition is 70% alpha-glucan oligosaccharide,
about 19%
polyminia sonchifolia root juice, about 10% maltodextrix, about 1%
lactobacillus, about 95%
thermus thermophillus ferment, about 5% glycerin, a methylsilanol
hydroxyproline aspartate,
and a Triticum monococcum [wheat] seed extract.
DETAILED DESCRIPTION OF THE DRAWINGS
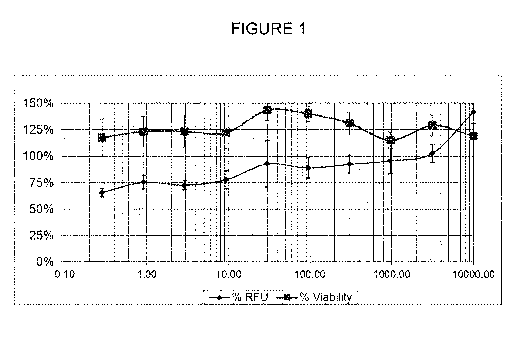
[0015] Figure 1 depicts testing of probiotic and antioxidant blend
composition. Viability is
the measure of toxicity of the cells in culture. RFU is the measure of the
dose dependent
antioxidant effect of the probiotic/antioxidant composition CYO RFU).
[0016] Figure 2 depicts testing of the antioxidant blend composition.
Viability is the measure
of toxicity of the cells in culture. RFU is the measure of the dose dependent
antioxidant
effect of the probiotic/antioxidant composition (% RFU).
[0017] Figure 3 is the proposed testing of the probiotic blend composition.
Viability is the
measure of toxicity of the cells in culture. RFU is the measure of the dose
dependent
antioxidant effect of the probiotic/antioxidant composition ( /0 RFU).
DETAILED DESCRIPTION
[0018] The terms used in this specification generally have their ordinary
meanings in the
art, within the context of the invention, and in the specific context where
each term is used.
Certain terms are discussed below, or elsewhere in the specification, to
provide additional
guidance to the practitioner in describing the compounds, compositions, and
methods of
the invention and how to make and use them. Moreover, it will be appreciated
that the
CA 02867422 2014-09-15
WO 2013/137899 PCT/US2012/029347
3
same thing can be said in more than one way. Consequently, alternative
language and
synonyms may be used for any one or more of the terms discussed herein, nor is
any
special significance to be placed upon whether or not a term is elaborated or
discussed
herein. The use of examples anywhere in this specification, including examples
of any
terms discussed herein, is illustrative only, and in no way limits the scope
and meaning of
the invention or of any exemplified term. Likewise, the invention is not
limited to the
examples presented.
[0019] The present disclosure is directed to a composition comprising a
probiotic and an
antioxidant in a probiotic/antioxidant wt/wt ratio of about 0.1/2.5 to about
1.0/95 and
wherein said probiotic/antioxidant composition act synergistically as an
inhibitor at a
concentration of < about 10 pg/mL and is lost at concentrations of > about 10
pg/mL.
[0020] It is well established in the dermatological literature that various
pathways are
responsible for inflammatory human skin, degradation of food product, and
aging. These
pathways are triggered by any number of perturbations (UV light, chemical
irritants,
mechanical trauma). The processes are often oxidative in nature, and
scientific literature
has demonstrated that topical and/or systemic antioxidants are effective at
inhibiting these
oxidative steps.
[0021] A sampling of pathways responsible for inflammation, aging, and
degradation follow.
100221 "Adhesion Pathway" is the pathway by which cells adhere to blood
vessels and
other skin tissues when injury or immune challenge has occurred.
[0023] "Chemotaxis Pathway" means the pathway where chemical signals cause
inflammatory cells to migrate toward a site, such as skin or tissue, where
immune challenge
has occurred. If such inflammatory cells are prevented from migrating to the
site of immune
challenge the resulting damage that such cells provide to skin or tissues can
be mitigated.
[0024] "Collagenase Pathway" means the pathway by which the enzyme collagenase
breaks down the peptide bonds in collagen and destroys extracellular
structures such as
those found in bacteria or infiltrating lymphocytes at the sites of
inflammation. The
collagenases released will cause tissue damage by breaking down collagen
fibrils in the
extra cellular matrix.
[0025] "COX Pathway" means the pathway by which the cyclooxygenase (COX)
enzyme
(including but not limited to cyclooxygenase-2 or COX-2) converts arachidonic
acid and/or
CA 02867422 2014-09-15
WO 2013/137899 PCT/US2012/029347
4
other fatty acids to prostaglandin or prostanoids which ultimately contributes
to
inflammation or pain in immune challenged tissue such as skin.
[0026] "PDE Pathway" means that pathway by which PDE (phosphodiesterase)
including
phosphodiesterase-4 (PDE4) cleaves the phosphodiester bond that may be found
in
proteins and other molecules present in bacteria, viruses, and other molecules
that
contribute to skin inflammation. PDE4, in particular; is a member of a family
of enzymes
that catalyze the degradation of CAMP to the corresponding 5'-nucleotide
monophosphate.
PDE4 is abundant and is the major regulator of CAMP metabolism in almost every
pro-
inflammatory and immune cell. PDE4 inhibitors exert their anti-inflammatory
effects by
inhibiting the breakdown of cAMP (leading to an increased concentration of
CAMP in
immune cells) which will ultimately lead to a decrease in the production and
release of pro-
inflammatory cytokines such as Interleukin 1-.beta. (IL-1 .beta.) and Tumor
Necrosis Factor
.alpha. (TNF.alpha.).
[0027] "Elastase Pathway" means the pathway by which the enzyme elastase
degrades
proteins including elastin that are found in bacteria and other molecules.
When the
Elastase Pathway is triggered the cascade of reactions contributes to
inflammation or pain
in immune challenged tissue such as skin. Elastase, a peptidase released from
infiltrating
neutrophils at the site of inflammation, will break down elastin, an elastic
fiber that, together
with collagen, helps determine the mechanical properties of skin and other
tissues.
Inhibition of elastase will minimize the damage that may be caused by
infiltrating
neutrophils which in turn will help preserve the integrity of the extra
cellular matrix.
[0028] "Histamine Pathway" means the pathway where the amino acid histidine is
decarboxylated to form histamine in response to immune challenge or other
injury to tissue
or skin. Histamine is a biogenic amine that is synthesized and stored in mast
cells which
reside primarily in the skin. Histamine plays a major role in the initiation
of the inflammatory
cascade. Upon stimulation, mast cells (and basophils) will release their
stored histamine
which will bind to H1 receptors on a vanety of cells (including smooth muscle
cells and
endothelial cells in blood vessels) exerting its biologic effects. These
effects include
vasodilation, separation of endothelial cells (causing abnormal vascular
permeability), pain
and itching. Inhibition of histamine release provides amelioration from many
of the adverse
effects of inflammation.
CA 02867422 2014-09-15
WO 2013/137899 PCT/US2012/029347
[0029] "Histamine Receptor Pathway" means that pathway by which cellular
receptors for
histamine are activated to bind to histamine, which in turn contributes to the
inflammatory
condition of tissues or skin.
[0030] "LO Pathway" means the pathway by which the enzyme lipooxygenase,
preferably
5-lipooxygenase, catalyzes the conversion of arachidonic acid to 5-
hydroperoxyeicosatetraenoic acid and then to leukotriene A4, which ultimately
contributes
to inflammation or pain in immune challenged tissue such as skin.
[0031] "PLA-2 Pathway" means the pathway by which the phospholipase A2 (PLA-2)
enzyme hydrolyzes phospholipids to form fatty acid lysophospholipid products
such as
arachidonic acid, which ultimately converts to leukotrienes and
prostaglandins, which
contribute to the inflammatory response in immune challenged tissue such as
skin.
[0032] "VEGF Pathway" means the pathway by which VEGF (vascular endothelial
growth
factor) causes angiogensis (the formation of blood vessels) in immune
challenged skin. In
addition to inducing angiogenesis, VEGF also is responsible for increasing
vascular
leakage which will lead to increased edema in damaged tissue or skin.
[00331 "Immune challenged" means tissues or skin subjected to environmental,
bacterial or
viral assaults and where any one or more of the Pathways that contribute to
inflammation
have been triggered.
[0034] "Inflammation" means, when used to describe skin, that the skin has
been subjected
to moderate to severe environmental or chemical assault and is moderately to
severely
immune challenged. Examples of inflammation include sunburn, windburn, acne,
insect
bites, cuts, burns, rosacea, and the like. Inflammation typically produces one
or more of
redness, pain, and heat in the skin. An anti-inflammatory reduces the
inflammation
response.
[0035] "Inhibitor" or "inhibit" means, when used with a particular Pathway, an
ingredient or
combination of ingredients that inhibits the Pathway in whole or in part. For
example,
Histamine Pathway Inhibitor means an ingredient or combination of ingredients
that inhibits
the Histamine Pathway in whole or in part.
[0036] "Irritant", when used to describe skin, means that the skin has been
aggravated by
environmental assaults or toxins, or application of products containing one or
more
ingredients to which the skin is sensitized or otherwise incompatible.
Irritation may result in
CA 02867422 2014-09-15
WO 2013/137899 PCT/US2012/029347
6
redness, itchiness, dryness, blemishes, enlarged pores, and so on. Irritated
skin may also
exhibit one or more of redness, pain, and heat.
[0037] "Pathway", when used with respect to inflammation, means a cascade of
reactions
that occurs when skin or tissue is exposed to immune challenge, and which
ultimately
contributes to skin inflammation.
[0038] Percentages mentioned herein shall mean percentage by weight unless
otherwise
indicated and are represented as wt/wt.
[0039] As used herein, "about" or "approximately" shall generally mean within
20 percent,
preferably within 10 percent, and more preferably within 5 percent of a given
value or
range. Other than in the operating examples, or where otherwise indicated, all
numbers
expressing quantities of ingredients and/or reaction conditions are to be
understood as
being modified in all instances by the term "about".
[00401 "Film former" or "film forming agent" or "film forming resin" as used
herein means a
polymer which, after dissolution in at least one solvent (such as, for
example, water and
organic solvents), leaves a film on the substrate to which it is applied, for
example, once
the at least one solvent evaporates, absorbs and/or dissipates on the
substrate.
[0041] "Keratinous substrates", as used herein, include but are not limited
to, skin, hair,
lashes and nails.
[0042] As used herein, the term ratio is used to express the relationship in
quantity or
amount of probiotic and antioxidant.
[0043] As used herein, the expression "at least one" means one or more and
thus includes
individual components as well as mixtures/combinations.
100441 The term "probiotic" means bacteria belonging to the order
Lactobacillales, including
but not limited to those from the genus Lactobacillus, Leuconostoc,
Pediococcus,
Lactococcus, Enterococcus, Oenococcus, Sporolactobacillus, Teragenococcus, and
so on;
or a yeast belonging to the order Saccharomyces.
[0045] The term "antioxidant" means bacteria belonging to the order
Thermophillus,
including but not limited to Thermus Thermophillus, Geobacillus Thermophilus,
Talaromyces Thermophilus, L. Bulgaricus S. Thermophilus, Lactococcus
Thermophilus,
Sphaerobacter Thermophilus.
CA 02867422 2014-09-15
WO 2013/137899 PCT/US2012/029347
7
100461 The term "cosmetic" includes a composition in the form of aqueous gels
or
dispersions, emulsions, or anhydrous compositions and will generally be
suitable for
applying color to skin, hair, or lashes. Suitable aqueous gels contain from
about 0.1 to 99%
water from about 1-99.9% of other cosmetic ingredients. Emulsions may be in
the oil in
water or water in oil form, or silicon and water, or water and silicon and
generally comprise
from about 0.1 to 99% water and from about 0.1 to 99% oil. Anhydrous
compositions
generally contain less than about 1% water, in addition to 0.1 to 90% oils,
and optionally
other ingredients.
[0047] The compositions of the invention may contain particulate materials in
the form of
pigments, inert particulates, or mixtures thereof. If present, suggested
ranges are from
about 0.01-75%, preferably about 0.5-70%, more preferably about 0.1-65% by
weight of the
total composition. In the case where the composition may comprise mixtures of
pigments
and powders, suitable ranges include about 0.01-75% pigment and 0.1-75%
powder, such
weights by weight of the total composition.
100481 In one embodiment, the composition may also comprise at least one
coloring agent
chosen from pigments, natural colorant, and dyes or a combination of pigments,
natural
colorants, and dyes. As used herein, pigments refer to colored solid particles
at 25 C. that
are not soluble in the liquid fatty phase. Pigments may include nacreous
pigments (i.e.,
nacres), and pearling agents.
100491 Pigments, dyes, and coloring agents also include encapsulation of the
pigments,
dyes, and coloring agents with a material such as a polymer, a pigment, a wax,
a sugar
derivative, or a combination of the proceeding material. Encapsulation of
pigments, dyes,
and coloring agents are disclosed in patent application US 20110229536 which
processes
and definitions are hereby incorporated by reference.
[0050] In one aspect, the composition may include fillers including, but not
limited to, talc,
kaolin, silica, barium sulfate, aluminum hydroxide, calcium silicate, silica,
silicone powders,
and acrylic acid copolymers.
100511 In addition, the compositions may include one or more of the following
components:
sunscreen agent, SPF booster, secondary emulsifier, emollient, moisturizer,
humectant,
film former/waterproofing agent, bio-active (functional) ingredient,
fragrance, a metal
chelating agent such as edetic acid, an antibacterial agent, an antiseptic
agent such as
methyl-p-hydroxybenzoate, a stabilizer, such as phenacetin, etidronic acid, or
oxyquinoline
sulfate, an organic solvent, such as ethanol, benzyl alcohol, or benzyloxy
ethanol, a water-
CA 02867422 2014-09-15
WO 2013/137899 PCT/US2012/029347
8
soluble polymer such as hydroxyethyl cellulose, and a humectant or any
combinations
thereof. The liquid mixture of the first and second parts contains preferably
a medium
composed mainly of water. Further, a persulfate such as ammonium persulfate
may be
added in the liquid mixture as the third part in order to improve the
bleaching activity.
100521 It is, of course, understood that the composition may further include
optional
ingredients generally known by one skilled the art to be suitable for use in
cosmetic
compositions. Lists of carriers and optional ingredients, which are well known
in the art,
are disclosed, for example, in 'Cosmetics: Science and Technology," edited by
M. S.
Balsam and E. Sagarin, 2nd Edition, 1972, Wiley Pub. Co.; The Chemistry and
Manufacture of Cosmetics" by M. G. DeNavasse; and "Harry's Cosmeticology," J.
B.
Wilkinson et al., 7th Edition, 1982, Chem. Pub. Co.; the disclosures of each
of the above
being incorporated herein by reference.
EXAMPLE
[0053] Antioxidant/Probiotic composition
Ratio tested
INCI Name of all components
Probiotic Blend:
70% Alpha-glucan oligosaccharide
19% Polyrninia sonchifolia root juice
10% Maltodextrix
1% Lactobacillus
Antioxidant Blend:
60 95% Thermus thermophillus ferment
5% Glycerin
Methylsilanol hydroxyproline aspartate
10 Triticum monococcum [wheat] seed extract
[0054] Inflamation was measured by the magnitude of cytokine release after
exposure to a
single dose of irritant. The anti-inflammatory potential of the skin model is
measured by the
tissue viability. The tissue viability is determined by measuring the relative
conversion of
MTT (3[4,5-dimethylthiazol-2-y1]-2,5-diphenyltetrazolium bromide) in the skin
model treated
with the antioxidant/probiotic composition, probiotic alone, antioxidant
alone, and a
negative/solvent control (sterile, deionized water) and a positive control
(hydrocortisone
cream).
[00551 The skin model is the EpiDerm TM skin model (MatTek Corporation,
Ashland, MA).
The skin model was exposed to antioxidant/probiotic composition, probiotic
alone,
antioxidant alone, and a negative/solvent control, applied topically to the
skin model, for six
CA 02867422 2014-09-15
=
WO 2013/137899 PCT/US2012/029347
9
hours in the presence and absence of phorbol-12-myristate 13-acetate (PMA).
The toxicity
of the test article was determined by the NAD(P)H-dependent microsomal enzyme
reduction of MTT.