Note: Descriptions are shown in the official language in which they were submitted.
81783436
A metal modified Y zeolite, its preparation and use
Technical Field
The present invention relates to a metal modified Y zeolite, its preparation
and use.
Background
Along the catalytic cracking feedstock becomes heavier and heavier, it is
essential for
the catalytic cracking catalyst to have both higher acitivity and higher
thermal and
hydrothermal stabilities to increase the abilities of heavy conversion and
anti-heavy
metal contamination. Therefore, it is required that the main active component
in the
catalytic cracking catalyst, i.e. Y zeolite, has high thermal and hydrothermal
stabilities,
and remains a suitable contribution of acidic active centers.
The rare earth (RE) modified Y zeolite has relative high thermal and
hydrothermal
stabilities, and is widely used in the FCC catalyst. However, the sharp rise
in the rare
earth price results in the remarkable increase in the cost of the FCC
catalyst.
Therefore, it is desirable to introduce other metal ions to the Y zeolite, to
reduce the
rare earth content in the Y zeolite and to ensure a hydrothermal stability
comparable
to the Y zeolite with high rare earth content.
CN1350887A, CN1765492A, and US2007010698A1 propose the preparation
methods for metal-modified Y zeolites. However, with comparison to the rare
earth
modified Y zeolite, the above metal-modified Y zeolites are poor in the
thermal and
hydrothermal stabilities.
CN101898144A and CN101134576A propose the modification to the framework of Y
zeolites to increase the thermal and hydrothermal stabilities of Y zeolites.
However,
the obtained non-rare earth metal-modified Y zeolites produce a low gasoline
yield in
the catalytic cracking.
Summary
Aiming to the problems in the prior art, the present invention proposes a
metal
modified Y zeolite and its preparation method. The metal-modified Y zeolite is
modified with non-rare earth metal elements, and has thermal and hydrothermal
stability comparable to the rare earth modified Y zeolite. And upon being used
in the
catalytic cracking catalyst, the catalyst can show excellent properties in
cracking
activities, gasoline yield, and coke selectivity.
Date Recue/Date Received 2021-05-03
81783436
In one aspect, the present invention provides a metal modified Y zeolite,
characterized
in that: the ratio of the zeolite surface's IVB group metal content to the
zeolite
interior's IVB group metal content is not higher than 0.2; and/or the ratio of
the
distorted tetrahedral-coordinated framework aluminum to the tetrahedral-
coordinated
framework aluminum in the zeolite lattice structure is (0.1-0.8):1.
In another aspect, the present invention provides a process for preparing the
metal-modified Y zeolite, comprising:
(1) a Y-zeolite raw material is subjected to dewatering so that the raw
material has a
water content by weight of not higher than 5%;
(2) the Y zeolite obtained from step (1) is contacted with a mixture of a
compound
containing IVB group metal and an organic solvent, and the resulting mixture
is
optionally filtered and/or dried;
(3) the Y zeolite obtained from step (2) is calcined at 300-700 C, preferably
for at
least 0.5 hour, e.g. 0.5-5 hours;
(4) the Y zeolite obtained from step (3) is contacted with an aqueous acid
solution,
and then calcined at 400-800 C under 1-100% steam condition for 0.5-5 hours to
produce the metal-modified Y zeolite containing the IVB group metal; the acid
concentration, as H+, is 0.1-2.0mol/L.
In another aspect, the present invention provides a process for preparing the
metal-modified Y zeolite, comprising:
(1) a Y zeolite is treated by contacting with an acid solution and/or an
aqueous EDTA
solution; wherein said acid is an organic acid and/or an inorganic acid;
(2) the product obtained from the step (1) is dewatered at a temperature below
400 C,
so that the water content in the zeolite is not higher than 5wt%;
(3) the zeolite obtained from the step (2) is impregnated with a metal in an
organic
solvent;
(4) the metal impregnated Y zeolite obtained from the step (3) and an organic
solvent
are added to a vessel at a solid-to-liquid weight ratio of 1:(5-50) and mixed,
an inert
gas such as one or more of nitrogen and helium is introduced to the veseel,
and the
vessel is kept under a pressure of 0-2.0111Pa (gauge pressure) at a
temperature in the
range from room temperature to 200 C for at least one hour, e.g. 1-48 hours;
filtering
and/or drying are optionally conducted, preferably filtering and drying are
conducted;
(5) the zeolite obtained from the step (4) is calcined; the calcination is
conducted in an
inert gas atmosphere, the calcination temperature is 300-700 C, the
calcination time is
2
Date Recue/Date Received 2021-05-03
81783436
at least 0.5 hour, e.g. 0.5-5 hours.
In another aspect, the present invention also provides a catalytic cracking
catalyst
containing the metal-modified Y zeolite and the preparation method thereof.
Specifically, the present invention involves the following technical
solutions:
1. A metal modified Y zeolite, which contains 1-15wt% of rvB group metal as
oxide,
wherein the metal-modified Y zeolite has a ratio of the distorted
tetrahedral-coordinated framework aluminum to the tetrahedral-coordinqed
framework aluminum in the lattice structure of 0.1-0.8, e.g. 0.2-0.8.
2. The metal modified Y zeolite of any one of the previous solutions, which
has a
specific surface area of 600-850 m2/g or 600-750m2/g, a unit cell size a0 of
2.448-2.458 run or 2.450-2.455nm, a crystallinity of not less than 60%, and
optionally
a SiO2/A1203 molar ratio (framework Si/A1 atom ratio) of 5-50, and the percent
of the
secondary pores (pore diameter of 6-20 nm) to the total secondary pores (pore
diameter of 2-100 urn) being 30-50% or 50%-65%.
3. The metal modified Y zeolite of any one of the previous solutions, wherein
the
modifying metal is Ti and/or Zr, wherein relative to the non-modified Y
zeolite, the
antisymmetric stretching vibration frequency (1050-1150cm-I) and the symmetric
stretching vibration frequency (750-820cm-I) in the infrared spectrum of the
metal-modified Y zeolite do not red-shift in a direction toward the lower
frequency.
4. The metal-modified Y zeolite of any one of the previous solutions, which
has an
2nhydrous chemical composition formula, as oxide and by weight, of
(0-2)Na20-(1-15)M02-(10-25)A1203.(65-75)Si02 or
(0.1-1.2)Na20-(1-10)M02-(20-24)A1203-(67-74)SiO2, wherein M is a IVB group
metal, selected from one or more of Ti, Zr, Hf and RI.
5. The metal modified Y zeolite of any one of the previous solutions, wherein
the TVB
group metal is Ti and/or Zr, and the metal-modified Y zeolite is free of both
framework Ti and framework Zr.
6. The metal modified Y zeolite of any one of the previous solutions, wherein
the ratio
3
Date Recue/Date Received 2021-05-03
81783436
of the zeolite surface's IVB group metal content to the zeolite interior's IVB
group
metal content is not higher than 0.2.
7. The metal modified Y zeolite of any one of the previous solutions, wherein
the
content of the IVB group metal as oxide is 1-10wt%.
8. The metal modified Y zeolite of any one of the previous solutions, wherein
the IVB
group metal comprises Ti and/or Zr.
9. A process for preparing a metal modified Y zeolite, comprising the steps
of:
(1) a Y zeolite is contacted with an acid solution and/or an aqueous EDTA
solution;
wherein said acid is an organic acid and/or an inorganic acid;
(2) the product obtained from the step (1) is dewatered at a temperature below
400 C,
so that the water content in the zeolite is not higher than 5wt%;
(3) the zeolite obtained from the step (2) is impregnated with a metal in an
organic
solvent;
(4) the metal impregnated Y zeolite obtained from the step (3) and an organic
solvent
are added to a vessel at a solid-to-liquid weight ratio of 1:5-50, an inert
gas is
introduced to the vessel, and the vessel is kept under a pressure of 0-2.0MPa,
preferably 0.1-2MPa (gauge pressure) at a temperature in the range from room
temperature to 200 C for at least one hour; filtering and/or drying is
optionally
conducted;
(5) the zeolite obtained from the step (4) is calcined; the calcination is
conducted in an
inert gas atmosphere, the calcination temperature is 300-700 C, the
calcination time is
0.5-5 hours.
10. The process of any one. of the previous solutions, wherein in the step
(1), the Y
zeolite is one or more of NaY, NaHY, NaNH4Y, NH4Y, HY, US'?, DASY zeolite,
once-exchanged-once-calcined Y zeolite, twice-exchanged-twice-calcined Y
zeolite,
and twice-exchanged-once-calcined Y zeolite.
11. The process of any one of the previous solutions, wherein in the step (1),
the Y
zeolite is contacted with the acid solution in a solid-to-liquid weight ratio
of 1:5-1:20
at a temperature in a range from room temperature to 100 C for at least 0.5
hour, then
4
Date Recue/Date Received 2021-05-03
81783436
filtered and washed; the acid solution has an acid concentration, as H+, of
0.1-1moUL.
12. The process of any one of the previous solutions, wherein the contact time
is 0.5-3
hours; the acid is an inorganic acid and/or an organic acid; wherein the
inorganic acid
is one or more of hydrochloric acid, sulfuric acid and nitric acid; and the
organic acid
is one or more of formic acid, acetic acid, oxalic acid, citric acid.
13. The process of any one of the previous solutions, wherein in the step (2),
the
dewatering is to calcine the zeolite obtained from the step (1) at 200-400 C
for 2-10
hours.
14. The process of any one of the previous solutions, wherein in the step (3),
the
impregnation with the metal in the organic solvent comprises the organic
solvent in
which a compound containing IVB group metal is solved is mixed with the
zeolite
obtained from the step (2), and the resulting mixture is kept for at least 0.5
hour,
wherein the solid-to-liquid weight ratio of the Y zeolite and the organic
solvent is
1:(0.5-5).
15. The process of any one of the previous solutions, wherein in the step (3),
the
resulting mixture is kept by standing or being stirred for 0.5-12 hours.
16. The process of any one of the previous solutions, wherein in the step (3),
the
solid-to-liquid weight ratio of the Y zeolite and the organic solvent is 1:1-
2.
17. The process of any one of the previous solutions, wherein the compound
contHining NB group metal is a Ti-containing compound and/or a Zr-containing
compound; the Ti-containing compound is one or more of titanium sulfate,
titanyl
sulfate, titanium tetrachloride, titanium trichloride, tetrabutyl titanate,
and ammonium
fluotitanate, and the Zr-containing compound is one or more of zirconium
tetrachloride, zirconium sulphate, zirconium nitrate, zirconium oxychloride,
zirconium acetate, and zirconium isopropoxide.
18. The process of any one of the previous solutions, wherein in the step (4),
the
vessel is kept for 1-48 hours.
Date Recue/Date Received 2021-05-03
81783436
19. The process of any one of the previous solutions, wherein in the step (4),
the
pressure is 0.5-1.5MPa, the temperature is from room temperature to 150 C, the
time
is 4-24 hours, and the solid-to-liquid weight ratio of the zeolite and the
organic
organic solvent is 1:5-30.
20. The process of any one of the previous solutions, wherein in the step (5),
the
calcination temperature is 450-650 C, and the calcination time is 1-4 hours.
21. The process of any one of the previous solutions, wherein the organic
solvent in
step (3) and/or (4) has a water content of not more than 5wt%.
22. The process of any one of the previous solutions, wherein, the organic
solvent in
step (3) and/or (4) has a water content of not more than 3wt%; and the Y
zeolite
obtained from the step (2) has a water content of not more than 3wt%.
23. The process of any one of the previous solutions, wherein the organic
solvent is
one or more of alkanes, aromatic hydrocarbons, alcohols, ketones, ethers,
esters,
halogenated alkanes such as chloridized alkanes.
24. The process of any one of the previous solutions, wherein the organic
solvent has
a normal boiling point of 40-100 C.
25. The process of any one of the previous solutions, wherein the organic
solvent is
preferably one or more of n-hexane, cyclohexane, heptane, benzene, toluene,
methanol, ethanol, isopropanol, acetone, butanone, and trichloromethane.
26. A process for preparing a metal modified Y zeolite, comprising the steps
of:
(1) a Y-zeolite raw material is subjected to dewatering so that the raw
material has a
water content by weight of not higher than 5%;
(2) the dewatered Y zeolite obtained from step (1) is contacted with a mixture
of a
compound containing IVB group metal and an organic solvent, and the resulting
mixture is optionally filtered and/or dried;
(3) the Y zeolite obtained from step (2) is calcined at 300-700 C;
6
Date Recue/Date Received 2021-05-03
81783436
(4) the Y zeolite obtained from step (3) is contacted with an aqueous acid
solution,
and then calcined at 400-800 C to produce the metal-modified Y zeolite
containing
the NB group metal; the acid concentration, as H+, is 0.1-2.0mol/L.
27. The process of any one of the previous solutions, wherein in the step (2),
the
mixing weight ratio of the compound containing 1VB group metal, the Y zeolite
and
the organic solvent is 0.01-0.15:1:1-50, wherein the weight of the compound
containing IVB group metal is calculated as oxide, and the Y zeolite is
calculated in a
dry basis.
28. The process of any one of the previous solutions, wherein in the step (2),
the
weight ratio of the compound containing IVB 'group metal (as oxide): the Y
zeolite
(dry basis): the organic solvent is 0.01-0.1:1:5-30.
29. The process of any one of the previous solutions, wherein in the step (2),
the
procedure of contacting the dewatered Y zeolite obtained from step (1) with
the
compound containing IVB group metal = and the organic solvent and optionally
filtering and/or drying comprises: the compound containing IVB group metal,
the
organic solvent and the Y zeolite are mixed and contacted at a temperature in
a range
from room temperature to 100 C for at least 0.5 hour, then optionally
filtered, and
then optionally dried.
30. The process of any one of the previous solutions, wherein in the step (2),
the
= procedure of contacting the dewatered Y zeolite obtained from step (1)
with the
mixture of the compound containing IVB group metal and the organic solvent and
optionally filtering and/or drying the resulting mixture is conducted once or
more than
once.
31. The process of any one of the previous solutions, wherein in the step (3),
the
calcination temperature is 350-650 C, the calcination time is 2-4 hours, the
calcination atmosphere is a dried air and/or an inert gas.
32. The process of any one of the previous solutions, wherein in the step (4),
the
7
Date Recue/Date Received 2021-05-03
81783436
condition for contacting the Y zeolite obtained from step (3) and the aqueous
acid
solution comprises: the weight ratio (solid-to-liquid ratio) of the Y zeolite
obtained
from step (3) to the aqueous acid solution is 1:5-20, the contact temperature
is in a
range from room temperature .to 100 C, the contact time is at least 0.5 hour;
the
aqueous acid solution has an acid concentration, as H+, of 0.1-2mol/L.
33. The process of any one of the previous solutions, wherein the aqueous acid
solution has an acid concentration, as H+, of 0.5-2 mol/L.
34. The process of any one of the previous solutions, wherein the organic
solvent is
one or more of alkanes, aromatic hydrocarbons, alcohols, ketones, ethers,
esters,
halogenated alkanes such as chloridized alkanes.
35. The process of any one of the previous solutions, wherein the organic
solvent is
selected from one or more of n-hexane, cyclohexane, heptane, benzene, toluene,
methanol, ethanol, isopropanol, acetone, butanone, tichloromethane.
36. The process of any one of the previous solutions, wherein the organic
solvent has
a normal boiling point of 40-100 C.
37. The process of any one of the previous solutions, wherein the organic
solvent has
a water content of not more than 5wt%.
38. The process of any one of the previous solutions, wherein the organic
solvent has
a water content of not more than lwt%.
39. The process of any one of the previous solutions, wherein in the step (2),
the
temperature for contacting the dewatered Y zeolite obtained from step (1) and
the
mixture of the compound containing IVB group metal and the organic solvent is
such
a temperature that allows the organic solvent in a liquid state.
40. The process of any one of the previous solutions, wherein the compound
containing WB group metal comprises a Ti-containing compound and/or a
Zr-containing compound.
8
Date Recue/Date Received 2021-05-03
81783436
41. The process of any one of the previous solutions, wherein the Ti-
containing
compound is one or more of titanium sulfate, titanyl sulfate, titanium
tetrachloride,
titanium trichloride, tetrabutyl titanate, ammonium fluotitanate, and the Zr-
containing
compound is one or more of zirconium tetrachloride, zirconium sulphate,
zirconium
nitrate, zirconium oxychloride, ZirCODiUM acetate, and zirconium isopropoxide.
42. The process of any one of the previous solutions, wherein in the step (1),
the Y
zeolite raw material is one or more of NaY zeolite, NaHY zeolite, NaNH4Y
zeolite,
NH4Y zeolite and HY zeolite.
43. The process of any one of the previous solutions, wherein in the step (1),
the Y
zeolite raw material has a water content, after dewatering, of not more than
lwt%.
44. The process of any one of the previous solutions, wherein in the step (4),
the
calcination is conducted in a 1-100% steam atmosphere.
45. The metal-modified Y zeolite of any one of Solutions 1-8, which is
obtainable or
obtained by the process of any one of Solutions 9-44.
46. A catalytic cracking catalyst, based on the total weight of the catalyst,
containing
10-60wt% of a metal-modified Y zeolite, 10-60wt% of a clay and 5-50wt% of a
binder, wherein said metal-modified Y zeolite is the metal-modified Y zeolite
of any
one of Solutions 1-8.
47. The catalytic cracking catalyst of Solution 46, wherein the catalytic
cracking
catalyst contains 20-55wt% of the IVB group metal-modified Y zeolite, 15-60wt%
of
the clay and 10-40wt% of the binder.
48. The catalytic cracking catalyst of Solution 46 wherein the catalyst
further contains
other molecular sieves commonly used in the catalytic cracking catalyst, said
other
molecular sieves include Y-type molecular sieves, WI-structured molecular
sieves,
and SAPO molecular sieves.
9
Date Recue/Date Received 2021-05-03
81783436
49. The catalytic cracking catalyst of Solution 46, wherein, the content of
other
molecular sieves commonly used in the catalytic cracking catalyst is not more
than
40wt%, such as 1-35wt%.
50. A method for preparing the catalytic cracking catalyst, which comprises
the steps
of preparing a metal-modified Y zeolite, mixing and slurrying the metal-
modified Y
zeolite and a clay and a binder, and spray-drying the resulting mixture,
wherein said
metal-modified Y zeolite is prepared according to the process of any one of
Solutions
9-44.
51. The method of Solution 50, wherein the clay is selected from one or more
of
kaolin, halloysite, rectorite, diatomite, montmorillonite, bentonite and
sepiolite; and
the binder is selected from one or more of hydrated alumina, alumina sol,
pseudobohenaite, bohemite, alumina monohydrate, alumina trihydrate, and
amorphous
aluminum hydroxyde.
52. A solution according to any one of the above Solution 1-51, wherein the
metal-modified Y zeolite substantially does not contain one or more of V, Nb,
Ta,
Cr, Mo, W., Mn, Tc, Re, Fe-. Ru, Jr.. Ni, Pd., Pt, Cu-, Zn, Ag, Au, Cd,
Hg, Sc and Y.
Brief Description of the Drawings
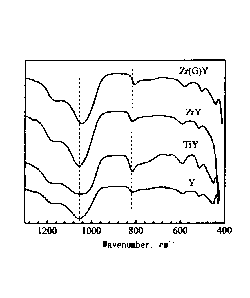
Figure 1 is a spectra
for a standard Y zeolite (Y) and the metal-modified Y
zeolites prepared in Examples B.1.1.2, B.1.1.6 and B.1.2.4.
Figure 2 is a 27A1-NMR spectra for a standard Y zeolite (Y) and the metal-
modified Y
zeolites prepared in Examples B.1.1.2, B.1.2.2 and B.1.2.5.
Detailed Description
Modified Zeolite
The first metal modified Y zeolite according to the present invention is
characterized
in that the ratio of the zeolite surface's IVB group metal content to the
zeolite
interior's IVB group metal content is not higher than 0.2; e.g. 0.001-0.2; or
0.02-0.18.
The second metal modified Y zeolite according to the present invention is
Date Recue/Date Received 2021-05-03
81783436
characterized in that the ratio of the distorted tetrahedral-coordinated
framework
alnminum to the tetrahedral-coordinated framework aluminum in the zeolite
lattice
structure is (0.1-0.8):1; (0.2O.8):1; (0.2-0.6):1; (0.1-0.6):1; or (0.2-
0.5):1.
The third metal modified Y zeolite according to the present invention is
characterized
in that the ratio of the zeolite surface's IVB group metal content to the
zeolite
interior's IVB group metal content is not higher than 0.2; e.g. 0.001-0.2; or
0.02-0.18;
and the ratio of the distorted tetrahedral-coordinated framework aluminum to
the
tetrahedral-coordinated framework aluminum in the zeolite lattice structure is
(0.1-0.8):1; (0.2-0.8):1; (0.2-0.6):1; (0.1-0.6):1; or (0.2-0.5):1.
The above three metal-modified Y zeolites are further characterized by one or
more of
the following features:
(1) the content of IVB group metal, as oxide and based on the metal-modified Y
zeolite: 1-15wt%, or 1-10wt%;
(2) the specific surface area: 600-850m2/g, 600-750m2/g, or 630-730m2/g;
(3) the unit cell size (expressed as a0): 2.448-2.458nm, 2.450-2.455run;
2.449-2.455nm; or 2.449-2.452nm;
(4) the crystallinity: not less than 60%, e.g. 60-120%, or 60-95%; and
(5) the SiO2/A1203 molar ratio: 5-50, 5-20, 5-8, 5-6
(6) the percent of the secondary pores (pore diameter of 6-20 urn) to the
total
secondary pores (pore diameter of 2-100 urn) being 30-50% or 50%-65%, e.g.35%,
40%, 45%, 50%, 55%, 60%.
According to the present invention, the 1VB group metal is selected from one
or more
of Ti, Zr, Hf, and Rf, e.g. one or more of Ti, Zr and Hf, preferably Ti and/or
Zr.
According to .the present invention, as oxide and by weight, the metal-
modified Y
zeolite has an anhydrous chemical composition formula, as oxide and by weight,
of:
(0-2)Na20-(1-15)M02-(10-25)A1203.(65-75)SiO2, or
(0.1 -1.2)Na20=(1 -10)M02-(20-24)A1203.(67-74) S i02;
wherein M is a NB group metal, selected from one or more of Ti, Zr, Hf and RE
In the metal-modified Y zeolite according to the present invention, most of
the NB
group metal ions are located in the interior of the zeolite, and a small
amount of ions
are present on the surface of the zeolite. The ratio of the zeolite surface's
NB group
metal content to the zeolite interior's NB group metal content is not higher
than 0.2.
11
Date Recue/Date Received 2021-05-03
81783436
In the present invention, including the following Examples, the analysis
methods for
the zeolite are as follows:
The zeolite surface's IVB group metal content refers to the content of the IVB
group
metal which can be measured in the depth of 2-5 urn from the zeolite's surface
by
using X-ray Photoelectron Spectroscopy ()CPS). The zeolite interior's IVB
group
metal content refers to the difference between the zeolite bulk's IVB group
metal
content and the zeolite surface's IVB group metal content. The zeolite bulk's
IVB
group metal content is the content of IVB group metal in the zeolite that can
be
obtained through chemical analysis method.
The ratio of the distorted tetrahedral-coordinated framework aluminum to the
tetrahedral-coordinated framework aluminum refers to the ratio of the spectrum
peak
area at a chemical shift of 40 to that at a chemical shift of 60, as measured
by 27A1
MAS NMER.
Secondary pores are determined and measured according to the standard method
RIPP151-90. A reference can be made to Analytical Methods in Petrochemical
Industry (RIPP Experiment Techniques), Yang Cuiding et.al, Science Press,
1990.
The element content is determined by X-ray fluorescence spectrometry.
The specific surface area is determined by the BET method.
The unit cell size and the crystallinity are determined by X-ray diffraction
according
to the standard methods RIPP145-90 and RIPPI46-90 respectively. A reference
can be
made to Analytical Methods in Petrochemical Industry (RIPP Experiment
Techniques),
Yang Cuiding et.al, Science Press, 1990.
The SiO2/A1203 molar ratio (i.e. framework Si/AI atom ratio) is determined
according
to the standard method SH/T0339-92.
Modified Zeolite Preparation Process
The present invention provides a process for preparing the metal-modified Y
zeolite,
comprising:
(1) a Y-zeolite raw material is subjected to dewatering so that the raw
material has a
water content by weight of not higher than 5%;
(2) the dewatered Y zeolite obtained from step (1) is contacted with a mixture
of a
compound containing IVB group metal and an organic solvent, and the resulting
mixture is optionally filtered and/or dried;
(3) the Y zeolite obtained from step (2) is calcined at 300-700 C, preferably
for at
12
Date Recue/Date Received 2021-05-03
81783436
least 0.5 hours, e.g. 0.5-5 hours;
(4) the Y zeolite obtained from step (3) is contacted with an aqueous acid
solution,
and then calcined at 400-800 C in a 1-100% steam condition for 0.5-5 hours to
produce the metal-modified Y zeolite containing the IVB group metal; the acid
concentration, as H+, is 0.1-2.0mol/L.
The metal-modified Y zeolite prepared by the above-mentioned process is
characterized by one or more features selected from:
(1) most of the IVB group metal ions are located in the interior of the
zeolite, while a
small amount of ions are present on the zeolite surface;
(2) the ratio of the zeolite surface's IVB group metal content to the zeolite
interior's
IVB group metal content is not higher than 0.2; .
(3) the ratio of the zeolite surface's IVB group metal content to the zeolite
interior's
IVB group metal content is 0.001-0.2;
(4) the ratio of the zeolite surface's IVB group metal content to the zeolite
interior's
IVB group metal content is 0.02-0.18;
(5) the ratio of the distorted tetrahedral-coordinated framework aluminum to
the
tetrahedral-coordinated framework aluminum in the zeolite lattice structure is
(0.2-0.8):1;
(6) the ratio of the distorted tetrahedral-coordinated framework aluminum to
the
tetrahedral-coordinated framework aluminum in the zeolite lattice structure is
(0.2-0.6):1;
(7) the ratio of the distorted tetrahedral-coordinated framework aluminum to
the
tetrahedral-coordinated framework aluminum in the zeolite lattice structure is
(0.1-0.6):1; and
(8) the ratio of the distorted tetrahedral-coordinated framework aluminum to
the
tetrahedral-coordinated framework aluminum in the zeolite lattice structure is
(0.2-0.5):1.
Step (1): Dewatering
The Y zeolite raw material can be one or more of NaY zeolite, NH4Y zeolite, HY
zeolite, NaNH4Y zeolite and NaHY zeolite, preferably NaY zeolite.
The NaY zeolite can by synthesized by crystallization. After removing the
mother
liquor, the crystallized zeolite can be used in the present invention directly
or after
being washed. The NaY zeolite can be commercially available or can be prepared
according to the method in the prior art, e.g. the method disclosed in
USP3671191.
13
Date Recue/Date Received 2021-05-03
81783436
The NaNH4Y zeolite is one obtained by exchanging NaY zeolite with NH4 to a
certain extent.
The NaHY zeolite can be obtained by calcining the NaNH4Y zeolite or by
exchanging
the NaY zeolite with Er to a certain extent.
The dewatering is preferably conducted at a temperature of not more than 400
C. The
clewatering can be done by drying or calcining. The drying can be a
conventional
drying method or a vacuum drying method. Upon using the calcination to
dewater, the
calcination temperature is preferably not more than 400 C, e.g. 200-400 C,
usnnlly
250-350 C. The conventional drying method includes heat-drying, air-drying,
flash-drying, or spray-drying. The drying temperature is usually not higher
than
200 C, e.g. 80-200 C. The dewatered zeolite preferably has a water content
of not
higher than 3wt%, preferably not higher than 1wt%.
Step (2): contact -optional filtering -optional drying
In the step (2), the dewatered Y zeolite obtained from the step (1) is
contacted with a
mixture of a compound containing NB group metal and an organic solvent to
introduce the modifying metal into the zeolite.
The contacting process comprises mixing and slurrying a mixture of a compound
containing NB group metal and an organic solvent and the Y zeolite, and being
subjected to an ion exchange at the exchange temperature (or the contact
temperature).
After the contact, a filtering is optionally conducted. Then, a drying is
optionally
conducted.
The contact can be done once or more than once. The so-called "more than once"
contact means the zeolite obtained from the previous treatment is contacted
with a
mixture of an organic solvent and a compound of the modifying metal; and after
each
contact, the filtering is optionally done, and the drying is optionally done.
In the case
of "more than once" contact, it is preferable to dry after the last contact.
In the case of
"more than once" contact, the Y zeolite obtained through filtering can be
treated
directly with a compound containing IVB group metal and an organic solvent, or
can
be dried and/or calcined, and then treated with a compound contsining NB group
metal and an organic solvent.
There can be at least one temperature point in the exchange temperature range,
at
which point the solvent can be present in a liquid state.
In each contact, the weight ratio of the compound containing NB group metal
(as
14
Date Recue/Date Received 2021-05-03
81783436
oxide) : Y zeolite (dry basis) : the organic solvent is (0.01-0.15):1:(1-50),
or
(0.01-0.14):1:(5-30), or (0.02-0.11):1:(5-25), or (0.01-0.1):1:(5-30).
The contact time for example is at least 0.5 hour, e.g. 0.5-5 hours, or 1.5-
3.5 hours.
The contact temperature can be such a temperature, at which the organic
solvent is in
a liquid state. The exchange temperature can be such a temperature range, in
which
the organic solvent is in a liquid state. Usually, the exchange temperature
can be a
temperature range, the lower point of which is higher than the solidifying
point of the
organic solvent, and the upper point of which is lower than the boiling point
of the
organic solvent. For example, the the exchange temperature is, from the room
temperature to a temperature which is 20 C lower than the normal boiling
point of
the organic solvent; from 0 to 100 C; from room temperature to 100 C; from 0
to
100 C and 20 C lower than the normal boiling point of the organic solvent;
and
from room temperature to 100 C and 20 C lower than the normal boiling point
of
the organic solvent. The room temperature is 15-40 C. The normal boiling
point
means the boiling point at 1 atm.
The drying temperature is usually not more than 200 C, e.g. 0-200 C, from room
temperature to 150 C, from room temperature to I20 C, from 100 to 120 C.
The drying time can be 4-48 hours, 12-48 hours.
The IVB group metal can be one or more of Ti, Zr and Hf, preferably Ti and/or
Zr.
The compound contqining IVB group metal can be one or more of compounds
containing Ti and/or Zr, e.g. a Ti-containing compound, a Zr-containing
compound or
a Ti- and Zr-containing compound. The compound containing IVB group metal is
preferably soluble in the used organic solvent, for example, its solubility in
the
organic solvent is not less than 0.1g of the compound containing IVB group
metal/100g of the organic solvent. The Ti-contsining compound can be one or
more of
=
titnnium sulfate, titanyl sulfate, titanium tetrachloride, titanium
trichloride, tetrabutyl
titPnste, and ammonium fluotitanate, the Zr-containing compound can be one or
more
of zirconium tetrachloride, zirconium sulphate, zirconium nitrate, zirconium
oxychloride, zirconium acetate, and zirconium isopropoxide.
The organic solvent has a water content of not more than 5wt%, preferably not
more
than lwt%, e.g. not more than 0.1wt%, not more than 0.01wt%, or not more than
0.001wt%. Preferably, in the organic solvent, the content of the organic
substance as
solvent is not less than 95wt%, preferably not less than 99wt%. The organic
solvent
can be one or more of alkanes, aromatic hydrocarbons, alcohols, ketones,
ethers,
Date Recue/Date Received 2021-05-03
81783436
esters, halogenated alkalies such as chloridized alkanes. The normal boiling
point of
the organic solvent (latm) is preferably 40-100 C, which is both favorable for
the
dispersion of the metal component, and favorable for removing the organic
solvent.
The organic solvent can be, for example, one or more of n-hexane, cyclohexane,
heptane, benzene, toluene, methanol, ethanol, isopropanol, acetone, butanone
and
trichloromethane.
Step (3): Calcination
The calcination temperature can be for example 300-700 C, 350-650 C, 400-620
C,
or 450-600 C.
The calcination time can be for example 0.5-5 hours, 1-5 hours, 2-4 hours.
The calcination atmosphere can be for example a dried air, and/or an inert gas
atmosphere, preferably an inert gas atmosphere.
The inert gas can be nitrogen and/or helium. In case of the dried air, the
water content
therein is below I vol%, e.g. below 0.5vo1%.
Step (4): Acid treatment-calcination
The temperature for contacting the aqueous acid solution with the Y-zeolite
obtained
from the step (3) is in a range from room temperature to 100 C, e.g. 75-95 C.
The contact time is not less than 0.2 hour, e.g. 0.5-5 hours,
The contart solid-to-liquid ratio (the weight ratio of zeolite to the aqueous
acid
solution) is 1:5-20, e.g. 1:6-14.
The concentration of the used aqueous acid solution, as 1-1+, is 0.1-2mol/L,
0.5-2mol/L,
0.5-1.5mol/L. After contact, the filtering is done. After filtering, the acid-
contacted
zeolite is washed with water to remove free acid, and then dried and calcined.
The
calcination temperature is 400-800 C, 500-600 C. The calcination atmosphere is
a
1-100% steam condition. The calcination time is 0.5-5 hours, or 1-3 hours. The
acid
used in the step (4) is selected from one or more of hydrochloric acid,
sulfuric acid,
nitric acid, oxalic acid, acetic acid, formic acid, preferably one or more of
hydrochloric acid, oxalic acid, and formic acid.
In another aspect, the present invention provides a process for preparing the
metal-modified Y zeolite, which comprises:
(1) a Y zeolite is treated by contacting with an acid solution and/or an
aqueous EDTA
solution; wherein said acid is an organic acid and/or an inorganic acid;
(2) the product obtained from the step (1) is dewatered at a temperature below
400 C,
16
Date Recue/Date Received 2021-05-03
81783436
so that the water content in the zeolite is not higher than 5wt%;
(3) the zeolite obtained from the step (2) is impregnated with a metal in an
organic
solvent;
(4) the metal impregnated Y zeolite obtained from the step (3) and an organic
solvent
are added to a vessel at a solid-to-liquid weight ratio of 1:5-50, an inert
gas such as
nitrogen and/or helium is introduced to the vessel, and the vessel is kept
under a
pressure of 0-2.0MPa (gauge pressure) at a temperature in the range from room
temperature to 200 C for at least one hour, e.g. 1-48 hours; filtering and/or
drying is
optionally conducted, and filtering and drying are preferably conducted;
(5) the zeolite obtained from the step (4) is calcined; the calcination is
conducted in an
inert gas atmosphere, the calcination temperature is 300-700 C, the
calcination time is
0.5-5 hours, or more than 0.5 hour.
The metal-modified Y zeolite prepared by the above-mentioned process is
characterized by one or more features selected from:
(1) most of the IVB group metal ions are located in the interior of the
zeolite, while a
small amount of ions are present on the zeolite surface;
(2) the ratio of the zeolite surface's IVB group metal content to the zeolite
interior's
IVB group metal content is not higher than 0.2;
(3) the ratio of the zeolite surface's IVB group metal content to the zeolite
interior's
IVB group metal content is 0.001-0.2;
(4) the ratio of the zeolite surface's IVB group metal content to the zeolite
interior's
TVB group metal content is 0.02-0.18;
(5) the ratio of the distorted tetrahedral-coordinated framework aluminum to
the
tetrahedral-coordinated framework aluminum in the zeolite lattice structure is
(0.2-0.8):1;
(6) the ratio of the distorted tetrahedral-coordinated framework aluminum to
the
tetrahedral-coordinated framework aluminum in the zeolite lattice structure is
(0.2-0.6):1;
(7) the ratio of the distorted tetrahedral-coordinated framework aluminum to
the
tetrahedral-coordinated framework aluminum in the zeolite lattice structure is
(0.1-0.6):1; and
(8) the ratio of the distorted tetrahedral-coordinated framework aluminum to
the
tetrahedral-coordinated framework aluminum in the zeolite lattice structure is
(0.2-0.5): 1.
17
Date Recue/Date Received 2021-05-03
81783436
Step (1): Contact
The Y zeolite as starting material can be one or more of NaY, NaHY, NaNH4Y,
NH4Y,
BY, USY, once-exchanged-once-calcined Y zeolite, DASY zeolite,
twice-exchanged-twice-calcined Y zeolite, twice-exchanged-once-calcined Y
zeolite.
The once-exchanged-once-calcined Y zeolite for example can be the Y zeolite
obtained by subjecting a NaY zeolite to once-exchanging-once-calcination; DASY
zeolite for example can be the Y zeolite obtained by subjecting a Y zeolite to
calcination in presence of steam; The twice-exchanged-twice-calcined Y zeolite
for
example can be the Y zeolite obtained by subjecting a NaY zeolite to
twice-exchanging-twice-calcination; The twice-exchanged-once-calcined Y
zeolite for
example can be the Y zeolite obtained by subjecting a =NaY zeolite to
twice-exchanging-once-calcination; and preferably, the exchange is conducted
with
11+ and/or NH4+.
The weight ratio between the Y zeolite (dry basis) and the acid solution (the
aqueous
acid solution) or the aqueous EDTA solution (the solid-to-liquid ratio) is 1:5-
20. The
contact temperature is in a range from room temperature to 100 C. The contact
time is
at least 0.5 hour, e.g. 0.5-3 hours. After the contact, the filtering and
washing can be
done. The acid concentration of the acid solution, as II+, is 0.1-1mon, 0.2-
0.5mol/L
or 0.5-1mol/L. The acid can be an inorganic acid and/or an organic acid. The
inorganic acid can be one or more of hydrochloric acid, sulfuric acid and
nitric acid;
the organic acid can be one or more of formic acid, acetic acid, oxalic acid,
citric acid.
In the step (1), the washing can be done with water such as deionized water
and ,
distillated water to remove the acid in the zeolite. For example, the weight
ratio of
water to zeolite can be 5-20:1. The treated zeolite obtained from the step (1)
has a
Na2O content of not higher than 4.0wt% and preferably not higher than 2.0wt%.
Step (2)-Calcination
The treated zeolite obtained from the step (1) can be calcined to remove the
adsorbed
water. Through the calcination, the water content in the zeolite is not higher
than
5wt%, for example not higher than 3wt%.
The calcination temperature can be 200-400 C, e.g. 300-350 C.
The calcination time can be 2-10 hours, e.g. 2-4 hours.
The solid content of the calcined zeolite is not less than 95wt%, not less
than 97wt%,
or 97-99.9wt%.
Step (3)
18
Date Recue/Date Received 2021-05-03
81783436
The zeolite being impregnated with a metal in an organic solvent comprises
mixing
the compound contAining IVB group metal in the organic solvent and the
zeolite, and
keeping the mixture for at least 0.5 hours, e.g. 0.5-12 hours with stirring or
without
stirring (by standing). For example, the mixture was kept with stirring for
0.5-12
hours. Then, the next step can be conducted, for example, by proceeding with
the step
(4), or repeating the step (3). The introduction of modifying metal(s) into
the Y zeolite
can be done through one or more than one impregnations. The solid-to-liquid
weight
ratio of the Y zeolite to the organic solvent can be 1:(0.5-5), 1:(1-2), 1:(1-
4), or
1:(1.1-1.6). The impregnation temperature is one that can make the organic
solvent be
in a liquid state. The impregnation can be done in a manner of isometric
impregnation
or excessive impregnation. The impregnation temperature is not particularly
limited,
for example, the impregnation can be done at room temperature.
The IVB group metal is selected from one or more of Ti, Zr, Hf and Rf,
preferably Ti
and/or Zr. The compound containing TVB group metal can be one or more of a
Ti-containing compound, a Zr-containing compound, a Hf-containing compound, a
Rf-containing compound, e.g. a Ti-containing compound and/or a Zr-containing
compound. The compound containing IVB group metal can be an inorganic salt
and/or an organometallic compound of IVB group metal, for example, the
Ti-containing compound can be one or more of titnnium sulfate, titanyl
sulfate,
titanium tetrachloride, titanium trichloride, tetrabutyl titanate, and
ammonium
fluotitanate. The Zr-containing compound can be one or more of zirconium
tetrachloride, zirconium sulphate, zirconium nitrate, zirconium oxych_loride,
zirconium acetate, and zirconium isopropmdcle.
The organic solvent has a water content of not higher than 5wt%, or not higher
than
3wt%, or not higher than lwt%. The organic solvent can be one or more of
alkanes,
aromatic hydrocarbons, alcohols, ketones, ethers, esters, halogenated alkanes
such as
chloridized alkanes. The organic solvent can have a normal boiling point of 40-
100 C.
The organic solvent is preferably one or more of n-hexane, cyclohexane,
heptane,
benzene, toluene, methanol, ethanol, isopropanol, acetone, butanone,
trichloromethane.
Step (4) Introducing an inert gas-optional filtering - optional drying
The impregnated zeolite and the organic solvent are placed in a reaction
vessel such
as autoclave. The solid-to-liquid weight ratio of the zeolite to the organic
solvent is
1:(5-50), e.g. 1:(5-30) or 1:(5-10). Generally, the organic solvent used in
the step (4) is
19
Date Recue/Date Received 2021-05-03
81783436
identical to that used in the step (3). An inert gas such as nitrogen and
helium can be
introduced to the reaction vessel. The rftsrtion vessel pressure. (gauge
pressure) is
0.0-2.01v1Pa, or 0.5-1.5MPa. The reaction vessel temperature is from room
temperature to 200 C, from room temperature to 150 C, or from room temperature
to
90 C. The substances in the reaction vessel can be kept by stsnding or under
stirring
for at least 1 hour, usually 1-48 hours, 2-24 hours, or 4-24 hours.
Then, the filtering and/or drying can be optionally conducted. The filtering
and the
drying are preferably conducted so that the zeolite can be separated from the
organic
solvent. The filtering and the drying can be conventionally conducted.. The
existing
drying process can be adopted, such as air-drying, flash-drying and spray-
drying. For
example, the drying temperature can be 100-200 C. For example, the drying
time can
be 1 second to 2 days, e.g. 6-24 hours.
Step (5) Calcination
The zeolite obtained from the step (4) is calcined; the calcination is
conducted in an
inert gas atmosphere, the calcination temperature is 300-700 C, the
calcination time is
0.5-5 hours, or more than 0.5 hour.
The calcination is conducted in an inert gas atmosphere. The calcination
temperature
is 300-700 C, 450-650 C or 500-600 C. The calcination time is 0.5-5 hours, or
1-4
hours. The inert gas comprises one or more of nitrogen and helium.
Catalytic cracking catalyst
The present invention further provides a catalytic cracking catalyst, based on
the total
weight of the catalyst, containing 20-60wt% of the NB group metal modified Y
zeolite according to the present invention, 10-60wt% of a clay and 5-50wt% of
a
binder.
Method for preparing catalytic cracking catalyst
The present invention also provides a method for preparing the catalytic
cracking
catalyst, which comprises the steps of mixing and slurrying the metal-modified
Y
zeolite according to the present invention and a clay and a binder, and spray-
drying
the resulting mixture. For example, deionized water , the clay and the binder
can be
mixed and slurried, and to the resulting slurry is added the modified Y
zeolite. The
technology of spray-drying and calcination are well known in the prior art,
and
therefore would not be discussed in detail.
Date Recue/Date Received 2021-05-03
81783436
According to the present invention, the clay is selected from one or more of
kaolin,
halloysite, rectorite, diatomite, montmorillonite, bentonite, and sepiolite,
which are
well known in the art.
According to the present invention, the binder refers to a substance, which
can form a
heat-resistant inorganic oxide after calcination. The heat resistant inorganic
oxide
comprises one or more of alumina, silica, amorphous silica-alumina, preferably
alumina. The binder is preferably selected from one or more of hydrated
alumina,
alumina sol, pseudobohemite, bohemite, alumina trihydrate, alumina
monohydrate,
and amorphous aluminum hydroxide. These different binders can transfer to the
form
of-A1,03 after calcination. These binders are well known in the art.
The NB metal-modified Y zeolite according to the present invention has high
crystallinity, large specific surface area, and high thermal and hydrothermal
stability.
According to the present invention, Y zeolite is modified with the IV group
metal,
without using the costly rare earth material. And the modified Y zeolite
according to
the present invention can provide a comparable or better thermal and
hydrothermal
stability than the rare earth modified Y zeolite.
The modified Y zeolite according to the present invention can be used in the
catalytic
cracking catalyst to substitute the rare-earth modified Y zeolite. The
catalyst cost can
be remarkably reduced.
The catalytic cracking catalyst according to the present invention shows
excellent
performance in cracking activities, gasoline yield, and coke selectivity.
Example
In the following Examples, the used room temperature is 15-40 C such as 26 C.
The light oil micro-activity (MA) is measured according to the standard method
RIPP92-90, wherein 5g of the catalyst is used. The reaction temperature is 460
C.
The feedstock is a straight run light diesel having a distillation range of
235-337 C.
The product composition is analyzed by gas chromatography. According to the
product composition, the micro-activity is calculated, as follows:
light oil micro-activity (MA) ¨(the gasoline output (<216 C) + gas output
+coke
output)/feedstock inputx 100%
In the Examples and Comparative Examples, the used starting materials are
21
Date Recue/Date Received 2021-05-03
81783436
commercially available and their detailed specifications are as follows.
Zeolite raw materials, industrial product, available from Sinopec Catalyst
Company,
Qilu Division.
ReCI3 (Mixed rare earth chloride), industrial grade, available from Sinopee
Catalyst
Company, Qilu
Other Agents: Chemir-ally pure, unless indicated to the contrary
The secondary pore volume is measured according to the standard method
REPP151-90. A reference can be made to Analytical Methods in Petrochemical
Industry (RIPP Experiment Techniques), Yang Cuiding et.al, Science Press,
1990.
According to the adsorption isotherm, the total pore volume of the zeolite is
measured.
Then the micro-pore volume of the zeolite is measured from the adsorption
isotherm
according to the T plotting method. The total pore volume minus the micro-pore
volume leaves the secondary pore volume, and the percent of the secondary
pores
(pore diameter of 6-20 rim) to the total secondary pores (pore diameter of 2-
100 nna)
is calculated from the secondary pore distribution of the zeolite.
A.1 Zeolite Modification
Example A.1.1.1
200g of NaY zeolite was calcined at 300 C for 3 hours (after calcination, the
water
content was lwt%). After being cooled to room temperature, it was placed in
2000g of
ethanol (the ethanol content ¨ 99.9wt%). The resulting slurry was stirred
homogenously. To the slurry was added 10.5g of zirconium nitrate
(Zr(NO3)4=5H20).
Then, the resulting mixture was stirred at room temperature for 2 hours, and
filtered.
The filter cake was dried in a baker at 100 C for 24 hours, and then calcined
at 600 C
for 2 hours.
The above calcined Y zeolite was added to 2000g of an aqueous inorganic acid
solution with an acid concentration of 1.0mol/L (a diluted hydrochloric acid
solution).
The resulting mixture was mixed homogenously, stirred at 80 C for 3 hours,
then
filtered, washed with deionized water (the weight of washing water was 15
times
larger than the dry basis weight of the zeolite), and filtered. The filter
cake was
22
Date Recue/Date Received 2021-05-03
81783436
removed, and calcined in a 100% steam at 600 C for 1 hour. Finally, the Zr-
modified
zeolite was obtained and named as Zr(2)Y, the properties of which were shown
in
Table Al.
Example A.1.1.2
200g of NaY zeolite was vacunmi zed at 200 C under 0.001Pa for 4 hours. After
being
cooled to room temperature (water content = 0.5wt%), it was placed in 1500g of
ethanol (ethanol content = 99.9wt%). The resulting slurry was stirred
homogenously.
To the shiny was added 15.7g of zirconium oxychloride (ZrOC12-8H20). Then, the
resulting mixture was stirred at room temperature for 3 hours, and filtered.
The filter
cake was dried in a baker at 100 C for 24 hours, and then calcined at 500 C
for 3
hours.
The above calcined Y zeolite was added to 1500g of an aqueous oxalic acid
solution
with an acid concentration of 2.0mol/L. The resulting mixture was mixed
homogenously, warmed upto 90 C, stirred for 1 hour, then filtered, and washed
with
deionized water (the weight of washing water was 15 times larger than the dry
basis
weight of the zeolite). The filter cake was removed, and calcined in a 100%
steam at
500 C for 2 hour. Finally, the Zr-modified zeolite was obtained and named as
Zr(4)Y,
the properties of which were shown in Table Al.
Example A.1.1.3
200g of NaY zeolite was calcined at 300 C for 3 hours. After being cooled to
room
temperature (water content = 1 wt%), it was placed in 1000g of n-hexane (n-
hexane
content --- 99.5wt%). The resulting slurry was stirred homogenously. To the
slurry was
added 37.8g of zirconium isopropoxide. The resulting mixture was stirred at
room
temperature for 3 hours, and filtered. The filter cake was dried in a baker at
120 C for
48 hours, and then calcined at 500 C (in a dried air atmosphere, the water
content in
the air was not more than 0.2vo1%) for 4 hours.
The above calcined Y zeolite was added to 1000g of an aqueous inorganic acid
(sulfuric acid) solution with an acid concentration of 0.5mol/L. The resulting
mixture
23
Date Recue/Date Received 2021-05-03
81783436
was stirred at 80 C for 3 hours, then filtered, and washed with deionized
water (the
weight of washing water was 20 times larger than the dry basis weight of the
zeolite).
The filter cake was removed, and calcined at 500 C, under a 100% steam
atmosphere
for 3 hours. Finally, the Zr-modified zeolite was obtained and named as
Zr(8)Y, the
properties of which were shown in Table Al.
Example A.1.1.4
200g of NaY zeolite was calcined at 300 C for 3 hours. After being cooled to
room
temperature, it was placed in 1000g of butanone (butanone content = 99.5wt%).
The
resulting slurry was stirred homogenously. To the slurry was added 14.2g of
titnnium
tetrachloride. The resulting mixture was stirred at room temperature for 2
hours, and
filtered. The filter cake was dried in a baker at 120 C for 24 hours, and then
calcined
at 450 C in a nitrogen atmosphere for 4 hours.
The above calcined Y zeolite was added to 1000g of an aqueous inorganic acid
(hydrochloric acid) solution with an acid concentration of 0.5mol/L. The
mixture was
mixed homogenously, stirred at 80 C for 2 hours, then filtered, and washed
with
deionized water (the weight of washing water was 10 times larger than the dry
basis
weight of the zeolite). The filter cake was removed, and calcined at 500 C in
a 100%
steam atmosphere for 2 hours. Finally, the Ti-modified zeolite was obtained
and
named as Ti(4)Y, the properties of which were shown in Table Al.
Example A.1.1.5
200g of NaY zeolite was vacuumized. at 300 C under 0.001Pa for 4 hours. After
being
cooled to room temperature, it was placed in 2000g of cyclohexane (cyclohexane
content=99.9wt%). The resulting slurry was stirred homogenously. To the slurry
was
added 63.9g of tetrabutyl titanate. The resulting mixture was stirred at room
temperature for 3 hours, and filtered. The filter cake was dried at 100 C in a
baker for
48 hours, and then calcined at 600 C in a nitrogen atmosphere for 2 hours.
The above calcined Y zeolite was added to 2000g of an aqueous oxalic acid
solution
with an acid concentration of 1.5mol/L. The mixture was mixed homogenously,
stirred at 90 C for 1 hour, then filtered, and washed with deionized water
(the weight
of washing water was 20 times larger than the dry basis weight of the
zeolite). The
24
Date Recue/Date Received 2021-05-03
81783436
filter cake was removed, and calcined at 600 C in a 100% steam atmosphere for
2
hours. Finally, the Ti-modified zeolite was obtained and named as Ti(10)Y, the
properties of which were shown in Table Al.
Example A.1.1.6
200g of NaY zeolite was vacuurnized at 300 C under 0.001Pa for 4 hours. After
being
cooled to room temperature, it was placed in 3000g of ethanol (ethanol content
=
99.9wt%). The resulting slurry was stirred homogenously. To the slurry were
added
3.6g of titanium tetrachloride and 31.5g of zirconium nitrate. The resulting
mixture
was stirred at room temperature for 3 hours, and filtered. The filter cake was
dried at
100 C in a baker for 48 hours, and then calcined at 550 C in a nitrogen
atmosphere
for 3 hours.
The above calcined Y zeolite was added to 3000g of an aqueous inorganic acid
(nitric
acid) solution with an acid concentration of 1.0mol/L. The mixture was mixed
homogenously, stirred at 80 C for 2 hours, then filtered, and washed with
deionized
water (the weight of washing water was 20 times larger than the dry basis
weight of
the zeolite). The filter cake was removed, and calcined at 550 C under a 100%
steam
atmosphere for 3 hours. Finally, the Ti- and Zr-modified zeolite was obtained
and
named as Ti-Zr-Y, the properties of which were shown in Table Al.
Example A.1.1.7
A modified zeolite was prepared according to Example A.1.1.4, except that
after being
treated with the inorganic acid, the filter cake was firstly dried, and then
calcined at
500 C in an air atmosphere to produce the modified zeolite named as Ti(4)Y-1.
Example A.1.2.1
200g of NaY zeolite and 2000g of deionized water were mixed and slurried. To
the
resulting slurry was added 45mL of a solution of 270g/1 REC13. The mixture was
adjusted with a diluted hydrochloric acid to pH=3.8, and warmed upto 80 C to
exchange for 1 hour. After filtering and washing, the resulting filter cake
was calcined
at 500 C for 3 hours. Then, the resulting Y zeolite and 2000g of deionized
water were
mixed and slurried. To the slurry was added 45g of ammonium sulfate. The
mixture
was adjusted with a diluted hydrochloric acid to pH=4.0, and warmed upto 80 C
to
exchange for 1 hour. After filtering and washing, the resulting filter cake
was calcined
Date Recue/Date Received 2021-05-03
81783436
at 600 C, under a 100% steam atmosphere for 3 hours. Finally, a RE-modified
zeolite
was obtained and named as RE(8)Y, the properties of which were shown in Table
Al.
Example A.1.2.2
200g of NaY zeolite was placed in 2000g of deionized water. The resulting
slurry was
stirred homogenously. To the slurry was added 31.4g of zirconium oxychloride
ZrOC12.8H20. The mixture was warmed upto 90 C, stirred for 3 hours, and
filtered.
The filter cake was dried at 100 C in a baker for 12 hours, and calcined at
500 C for 3
hours. Then the calcined Y zeolite and 2000g of deionized water were mixed and
slurried. To the resulting slurry was added 45g of ammonium sulfate. The
mixture was
adjusted with a diluted hydrochloric acid to pH=4.0, and warmed upto 80 C to
exchange for 1 hour. After filtering and washing, the filter cake was calcined
at 500 C
in a 100% steam atmosphere for 2 hours. Finally, the Zr-modified zeolite was
obtained and named as Zr(W)Y, the properties of which were shown in Table Al.
Example A.1.2.3
A modified zeolite was prepared according to Example A.1.2.2, except that
I4.2g of
titanium 'tetrachloride was used in place of 31.4g of zirconium oxychloride
ZrOC12.8H20. Finally, the Ti-modified zeolite was obtained and named as
Ti(W)Y,
the properties of which were shown in Table Al.
Example A.1.2.4
A Zr-modified Y zeolite was prepared according to Example 1 of CN101134576A.
Finally, the Zr-modified zeolite was obtained and named as Zr(G)Y, the
properties of
which were shown in Table Al.
Example A.1.2.5
200g of NH4USY (Si/AI atom ratio=5.2) was added to 500 g of absolute ethanol
under
a violent stirring to form a suspension, to which was added 50 g/L of a
solution of
butyl titanate-absolute ethanol (as TiO2) under a violent stirring. The
mixture was
air-dried overnight under stirring. The resulting sample was calcined at 500
C for 5
hours to produce Ti-modified zeolites with titanium content of 2.4wt% and
9.1wt%,
named as DT2 and DT9.
26
Date Recue/Date Received 2021-05-03
o 00
w
.6
'---:i
x
oc
0
u.)
.,
.4.
c
(J.)
O 0,
o Table Al Physical and chemical properties of metal-modified Y zeolites
.6
x Example A.1.1.X 1 2
3 4 5 6 7
0
0
0
O Sample
Zr(2)Y Zr(4)Y Zr(8)Y Ti(4)Y Ti(10)Y Ti-Zr-Y Ti(4)Y-1
0.
r..)
0
r..)
a0,nm 2.452 2.450
2.449 2.451 2.449 2.449 2.455
cb
9'
O
erystallinity,% 77.6 73.7 70.3 72.6 69.1 68.8 73.0
(...)
Na20,wt% 1.0 0.9
0.7 0.9 0.8 1.0 0.9
A1203,wt% 22.8 22.5
21.8 22.8 21.3 20.9 22.8
Si02,wt% 73.6 72.5
69.7 72.1 68.8 70.5 72.0
Zr02,wt% 2.1 3.8
7.3 0 0 5.5 0
t\.) Ti02,wt% 0 0
0 3.7 8.6 1.9 3.7
--.)
RE203,wt% 0 0
0 0 0 0 0
specific surface area, in2/g 676 710
690 726 702 640 722
lattice collapse temperature, C 1048 1044
1051 1046 1050 1051 - 1045
the ratio of the zeolite surface's IVB group metal content to the zeolite
0.05 0.10
0.12 0.08 0.16 0.15 0.10
interior's IVB group metal content
the ratio of the distorted tetrahedral-coordinated framework aluminum
0.4 0.3
0.5 0.4 0.6 0.5 0.3
to the tetrahedral-coordinated framework aluminum
_
The percent of the secondary pores (pore diameter of 6-20 mn) to
48.2 50.6
57.8 52.4 62.4 64.5 51.8
the total secondary pores (pore diameter of 2-100 nm)
o 00
w
---:i
rp'
oc
x
u.)
0 .4. .,
c
(J.)
O 0,
o
w
rp'
x Table Al (continued)
0
0
0
' Example A.1.2.X
1 2 3 4 5
0
0.
r..)
c) Sample RE(8)Y Zr(W)Y Ti(W)Y
Zr(G)Y DT2 DT9
r..)
cb
9' a0,nm 2.451 2.450
2.452 2.451 2.458 2.448
0
(...)
crystallinity,% 62.7 50.6
53.9 64.2 69.9 63.0
Na20,wt% 1.0 0.8
1.0 0.8 1.1 1.2
A1203,wt% 21.7 21.9
21.8 20.8 21.2 21.8
Si02,wt% 70.5 69.9
72.8 70.7 74.8 67.2
Zr02,wt% 0 7.0
0 7.5 0 0
t.)
_______________________________________________________________________________
________________________________
Ti02,wt% 0 0
3.9 - 0 2.4 9.1
RE203,wt% 6.6 0
0 0 0 0
specific surface area, m2/g 640 548
526 624 535 520
lattice collapse temperature, C 1043 1028
1015 1030 1036 1036
the ratio of the zeolite surface's IVB group metal content to
- 1.8
1.2 .. 1.0 1.3
the zeolite interior's IVB group metal content
the ratio of the distorted tetrahedral-coordinated framework
- 0.05
0.06 0.02 0.01 0.04
aluminum to the tetrahedral-coordinated framework aluminum
81783436
A.2 Stability of the modified zeolite
Modified Y zeolites prepared according to Examples A.1.1.1-A.1.1.7 and
A.1.2.1-A.1.2.5 were aged at 800 C under a 100% steam condition for 8 hours to
determine the crystallinity and the specific surface area, and the
crystallinity retention
and the specific surface area retention were calculated. The results were
listed in Table
A2. The aged zeolites were subjected to the light oil micro-activity (MA)
test. The
results were listed in Table A2.
Table A2 Physical and chemical properties of the metal-modified Y zeolites
after the
hydrothermal aging
Example A.2.1 .X 1 2 3 4 5 6 7
Zr(2) Zr(4) Zr(8) Ti(4) Ti(10) Ti(4)Y-
Y zeolite
Y Y Y Y Y Y 1
Crystallinity retention,% 60.5 62.1 64.7 63.9 65.9 66.3 63.2
Specific surface area
62.4 65.0 66.7 64.1 67.8 68.0 65.2
retention, %
800 C/8h, activity 81 84 86 85 86 87 84
Table A2 (continued)
Example A.2.2.X 1 2 3 4 5
Y zeolite RE(8)Y Zr(W)Y Ti(W)Y Zr(G)Y DT2 DT9
Crystallinity retention,% 63.2 45.5 50.2 60.8 60.2 62.0
Specific surface area retention, % 65.5 50.8 51.6 64.9 -61.6
62.9
800 C/8h, activity 84 75 75 80 .70 72
1
Example A.2.1.8
According to Example A.1.1.5, Ti-modifed Y zeolites having Ti contents (as
TiO2) of
lwt%, 2wt%, 7wt%, 12wt%, 15wt% were prepared. The physical and chemical
properties were listed in Table A3. These Ti-modifed Y zeolites were aged at
800 C
under a 100% steam condition for 8 hours to determine the crystallinity and
the
specific surface area, and the crystallinity retention and the specific
surface area
retention were calculated. The aged zeolites were subjected to the light oil
micro-activity (MA) test. The results were listed in Table A3.
29
Date Recue/Date Received 2021-05-03
81783436
Example A.2.2.6
According to Example A.1.2.1, RE-modifed Y zeolites having RE contents of
lwt%,
2wt%, 12wt%, 15wt% were prepared. The physical and chemical properties were
listed in Table A3. These RE-modifed Y zeolites were aged at 800 C under a
100%
steam condition for 8 hours to determine the crystallinity and the specific
surface area,
and the crystallinity retention and the specific surface area retention were
calculated.
The aged zeolites were subjected to the light oil micro-activity (MA) test The
results
were listed in Table A3.
Date Recue/Date Received 2021-05-03
O oc
cT
oc
I
0
Table A3
rp'
O
Example A.2.1.8 Example A.2.2.6
Ti02,wt% 1.0 2.0 7.0
12.0 15.0 0 0 0 0
0
RE203,wt% 0 0 0 0
0 1,0 2.0 12.0 15.0
9' Na20,wt% 1.1 0.9 1.1
0.9 0.9 0.8 1.0 1.0 0.9
0
A1203,wt% 23.2 22.8 21.4 19.2
16.5 22.0 21.8 20.2 17.2
Si02,wt% 73.3 72.8 68.7 66.9
67.1 74.5 73.5 66.0 66.2
a0, nm 2.449 2.450 2.452
2.455 2.455 2.455 2.457 2.458 2.460
Crystallinity, % 71.2 70.6 72.8
71.1 69.8 70.2 68.4 62.2 60.8
specific surface area, m2/g 674 680 690
666 645 620 653 560 490
(4, Lattice collapse temperature, C 1042 1045 1050
1046 1046 1030 1044 1045 1045
the ratio of the zeolite surface's IVB group metal
0.02 0.05 0.12
0.15 0.17
content to the zeolite interior's IVB group metal content
Zeolite No. Ti(1)Y Ti(2)Y Ti(7)Y
Ti(12)Y Ti(15)Y
After the hydrothermal aging
Crystallinity retention,% 62.2 61.2 63.6
66.0 64.5 60.0 63.4 66.2 65.0
Specific surface area retention, % 61.9 63.8 63.4
67.7 66.6 62.2 64.5 67.0 65.9
800 C/8h, activity 82 85 87
85 85 80 82 83 83
81783436
A.3 Catalyst
Example A.3.1.1
The moriified Y zeolite prepared according to the present invention, Zr(8)Y,
was used
as active component to prepare the catalyst according to the conventional
preparation
method of the catalytic cracking catalyst The preparation was as follows.
According
to the ratio of zeolite (dry basis):kaolin (dry basis):pseudobohemite (as
A1203):alumina sol (as A1203) being 38:34:20:8, kaolin and decationized water
were
mixed and slurried. To the resulting slurry was added alumina sol, and further
added
pseudoboehmite under a continuous stirring. After 30 minutes of stirring, a
liquor
containing 'zeolite was added to the colloid. The resulting mixture was mixed
homogenously, spray-dried and shaped to produce a catalyst, named as Cl
The catalyst was pretreated at 800 C in a 100% steam condition for 17 hours.
Then
the pretreated catalyst was tested on a small-scale fixed fluidised bed (ACE)
for
catalyst evaluation. The feedstock for evaluation was Wuhun HI, the properties
of
which were shown in Table A4. Reaction temperature, catalyst-to-oil ratio,
WHSV
and evaluation result were listed in Table A5.
wherein,
conversion=gasoline yield+liquefied gas yield+Dry gas yield+coke yield
coke selectivity =coke yield*100/conversion
Example A.3.2.1
A catalyst was prepared according to Example A.3.1.1, except that the same
amount
of RE(8)Y zeolite was used in place of Zr(8)Y zeolite to produce a catalyst
named as
DC1. Then DC1 was evaluated according to Example A.3.1.1. The evaluation
result
was listed in Table AS.
Example A.3.2.2
A catalyst was prepared according to Example A.3.1.1, except that the same
amount
of Zr(W)Y zeolite was used in place of Zr(8)Y zeolite to produce a catalyst
named as
DC2. Then DC2 was evaluated according to Example A.3.1.1. The evaluation
result
was listed in Table A5.
32
Date Recue/Date Received 2021-05-03
81783436
Table A4
Feedstock Wuhun HI
Density (2000), g/cm3 0.9044
Refraction (20 C) 1.5217
Viscosity (100 C), mni2/s 9.96
Freezing point, C 40
Aniline point, C 95.8
C,wt% 85.98
H,wt% 12.86
S,wt% 0.55
N,wt% 0.18
Residual Carbon, wt% 3.0
Distillation range, C
Initial distillation point
5% 243
10% 294
30% 316
50% 395
70% 429
90% 473
Table A5
Example A.3.1.1 A.3.2.1 A.3.2.2
(mtatyst Cl DC1 DC2
Reaction Temp, C 500 500 500
Catalyst-to-oil weight ratio 5 5 5
WHSV,11-1 16 16 16
Production Distribution, wt%
Dry gas 1.15 1.10 1.18
liquefied gas 11.15 11.66 12.03 -
coke 4.02 5.45 4.65
33
Date Recue/Date Received 2021-05-03
81783436
gasoline 56.40 53.08 50.30
diesel 20.17 21.46 23.02
Heavy oil 7.11 7.25 8.82
Conversion, wt% 72.72 71.29 68.16
Coke selectivity 5.53 7.64 6.82
Example A.3.1.2
According to Example A.1.1.1, the used amount of zirconium nitrate was
adjusted to
prepare a Zr-modified Y zeolite, named as Zr(6)Y, wherein the ratio of the
used
amount of zirconium nitrate (as ZrO2) to the weight amount of the zeolite was
6:100
by weight.
323g of pseudobohemite (having a solid content of 62wt%) and 1343g of
deionized
water were mixed. The mixture was stirred for 15 minutes and mixed
homogenously
to produce a pseudohoehmite slurry, the pH value of which was adjusted with
diluted
hydrochloric acid to 3.5. The resulting slurry was aged at room temperature
for 6
hours. To the aged slurry were added 447g of kaolin (having a solid content of
76wt%)
and 372g of alumina sol (having an alumina content of 21.5wt%). The resulting
slurry
was stirred for 60 minutes. To the above slurry was added a slurry formed by
slurrying 380g (dry basis) of the above modified Zr(6)Y zeolite and 880g of
deionized
water. The resulting mixture was stirred for 60 minutes to produce a catalyst
slurry,
which was spray-dried and shaped, and calcined at 550 C for 1 hour to produce
a
catalytic cracking catalyst, named as Cll. The ZrO2 content of the catalyst
C11,
measured by NRF, was 2.2wt%.
Example A.3.1.3
A Zr-modified Y zeolite was prepared according to Example A.1.1.2, named as
Zr(10)Y, wherein Zr02:zeolite = 10:100.
421g of kaolin (having a solid content of 76wt%), 465g of alumina sol (having
an
alumina content of 21.5wt%) and 732g of deionized water were added to and
slurried
34
Date Recue/Date Received 2021-05-03
81783436
in a slurry vessel, to which was added 1667g of an acidified pseudoboehmite
(being
acidified with hydrochloric acid, the mole ratio of hydrochloric acid /
aiiirriini-= 0.15,
and having a solid content of 12wt%). After stirring for 60 minutes, to the
vessel was
added a slurry formed by slurrying 380g (dry basis) of the above modified
Zr(10)Y
zeolite and 880g of deionized water. The resulting mixture was stirred for 60
minutes
to produce a catalyst slurry, which was spray-dried and shaped, and calcined
at 550 C
for 1 hour to produce a catalytic cracking catalyst, named as C21. The ZrO2
content of
the catalyst C21, measured by KU, was 3.5wt%.
Example A.3.1.4
447g of kaolin, 372g of alumina sol and 800g of deionized water were mixed and
slurried for 60 minutes. After adding 1667g of an acidified pseudobohemite,
the
resulting slurry was further stirred for 60 minutes. To the resulting mixture
was added
a slurry formed by slurrying 380g (dry basis) of the above modified Ti(2)Y
zeolite
and 880g of deionized water. The resulting mixture was stirred for 60 minutes
to
produce a catalyst slurry, which was spray-dried and shaped, and calcined at
650 C
for 2 hours to produce a catalytic cracking catalyst, named as C31. The TiO2
content
of the catalyst C31, measured by XR_F, was 0.75wt%.
Example A.3.1.5
447g of kaolin, 372g of alumina sol and 800g of deionized water were mixed and
slurried for 60 minutes. After adding 1667g of an acidified pseudobohemite,
the
resulting slurry was further stirred for 60 minutes. To the resulting mixture
was added
a slurry formed by slurrying 380g (dry basis) of the above modified Ti(4)Y
zeolite
and 880g of deionized water. The resulting mixture was stirred for 60 minutes
to
produce a catalyst slurry, which was spray-dried and shaped, and calcined at
650 C
for 2 hours to produce a catalytic cracking catalyst, named as 41. The TiO2
content of
the catalyst C41, measured by XRF, was 1.5wt%.
Example A.3.1.6
A catalyst was prepared according to Example A.3.1.5, except that the same
amount
Date Recue/Date Received 2021-05-03
81783436
of Ti(4)Y-1 was used in place of the Ti(4)Y zeolite to produce the catalyst,
named as
C41-1.
Example A.3.1.7
A catalyst was prepared according to Example A.3.1.5, except that a REY
zeolite
prepared according to the prior method (SiO2/A1203 molar ratio=5.1, rare earth
content=3.8wt%, Na2O content=0.4-wt%) was used in place of a part of the
Ti(4)Y
zeolite. The weight ratio of the REY zeolite prepared according to the prior
method to
the Ti(4)Y zeolite was 1:1 to produce a catalyst, named as C41-2.
Example A.3.1.8
According to Example A.1.1.4, the used amount of titanium tetrachloride was
adjusted to prepare a Ti-modified Y zeolite, named as Ti(8)Y, wherein the
ratio of the
used amount of titanium tetrachloride (as TiO2) to the weight amount of the
zeolite
was 8:100 by weight.
421g of kaolin, 698g of alumina sol and 900g of deionized water were mixed and
slurried for 60 minutes. After adding 1250g of an acidified pseudobohemite,
the
resulting slurry was further stirred for 60 minutes.
To the resulting mixture was added a slurry formed by slurrying 380g (dry
basis) of
the above modified Ti(8)Y zeolite and 800g of deionized water. The resulting
mixture
was stirred for 60 minutes to produce a catalyst slurry, which was spray-dried
and
shaped, and calcined at 700 C for 2 hours to produce a catalytic cracking
catalyst,
named as C51. The TiO2 content of the catalyst C51, measured by XRF, was
3.0wt%.
Example A.3.1.9
The same starting materials as those used in Example A.3.1.2 were used, except
for
the metal-modified Y zeolite.
355g of pseudoboehmite and 1478g of deionized water were mixed and stirred for
30
minutes to produce a pseudoboehmite slurry, the pH of which was adjusted with
a
suitable amount of diluted hydrochloric acid to 3.8. The resulting slurry was
aged at
36
Date Recue/Date Received 2021-05-03
81783436
60 C for 2 hours. To the aged slurry were added 395g of kaolin and 465g of
alumina
sol. The resulting mixture was stirred for 60 minutes. Then to the slurry was
added a
slurry formed by slurrying 380g (dry basis) of the above modified Ti-Zr-Y
zeolite and
880g of deionized water. The resulting mixture was stirred for 60 minutes to
produce
a catalyst slurry, which was spray-dried and shaped, and calcined at 600 C for
3 hours
to produce a catalytic cracking catalyst, named as C61.
Example A3.2.3
A catalyst was prepared according to Example A.3.1.8, except that the same
amount
of RE(8)Y zeolite was used in place of Ti(8) Y zeolite to produce a catalyst,
named as
DC11. The RE203 content of the catalyst DC11, measured by XRF, was 2.32w0/0.
Example A.3.2.4
A catalyst was prepared according to Example A.3.1.8, except that the same
amount
of Ti(W)Y zeolite was used in place of Ti(8)Y zeolite to produce a catalyst,
named as
DC21. The 1102 content of the catalyst DC21, measured by XRF, was 2.40wt%.
Example A.3.2.5
A catalyst was prepared according to Example A.3.1.2, except that the same
amount
of Zr(W)Y zeolite was used in place of Zr(6)Y zeolite to produce a catalyst,
named as
DC31. The Zr02 content of the catalyst DC31, measured by XRF, was 2.18wt%.
The catalysts C11-C61 and DC11-DC31 were pretreated at 800 C in a 100% steam
condition for 8 hours. Then the pretreated catalyst was tested on a small-
scale fixed
fluidised bed (ACE) for catalyst evaluation. The feedstock for evaluation was
Wuhun
the properties of which were shown in Table A4. Reaction temperature,
catalyst-to-oil ratio, WHSV and evaluation result were listed in Table A6.
wherein, conversion¨gasoline yield+liquefied gas yield+Dry gas yield+coke
yield
37
Date Recue/Date Received 2021-05-03
oc 0
cTCD
oc
(J.)
(J.)
0 Table A6 Evaluation Result
Example A.3.1.2 A.3.1.3 A.3.1.4 A.3.1.5 A.3.1.6
A.3.1.7 A.3.1.8 A.3.1.9 A.3.2.3 A.3.2.4 A.3.2.5
0
Catalysts C11 C21 C31 C41 C41-1 C41-2 C51 C61 DC11 DC21
DC31
0 Ti(4)Y
Zeolites Zr(6)Y Zr(10)Y Ti(2)Y Ti(4)Y Ti(4)Y-1
Ti(8)Y Ti-Zr-Y RE(8)Y Ti(W)Y Zr(W)Y
9' REY
0
Reaction Temp, C.: 500 500 500 500 500 500
500 500 500 500 500
Catalyst-to-oil weight ratio 8.04 8.04 8.04 8.04 8.04 8.04
8.04 8.04 8.04 8.04 8.04
WHSV, 16 16 16 16 16 16
16 16 16 16 16
Production Distribution, wt%
Dry gas 1.35 1.32 1.33 1.34 1.33 1.3
1.32 1.35 1.34 1.34 1.35
(4.)
00
liquefied gas 14.04 13.61 13.30 14.43 14.21
13.96 14.52 14.26 13.58 13.35 13.72
coke 6.71 6.86 7.52 7.05 7.13 7.42
6.76 6.71 8.02 7.29 7.12
gasoline 54.11 53.52 52.98 53.44 53.25
53.51 53.24 53.87 52,22 49.87 50.55
diesel 16.76 16.84 16.96 16.65 16.94
16.71 16.94 16.84 16.89 17.19 17.37
Heavy oil 7.03 7.85 7.91 7.09 7.14 7.1
7.22 6.97 7.95 10.96 9.89
Total 100 100 100 100 100 100
100 100 100 100 100
Conversion, wt% 76.21 75.31 75.13 76.26 75.92
76.19 75.84 76.19 75.16 71.85 72.74
coke /conversion 0.08805 0.09109 0.10009 0.09245 0.09391
0.09739 0.08914 0.08807 0.10671 0.10146 0.09788
81783436
B.1 Zeolite Modification
Example B.1.1.1
(1) At room temperature, 200g of NaY zeolite (dry basis, 75wt%) and 1500m1 of
a
hydrochloric acid solution having a molar concentration of 0.5mol/L were mixed
and
stirred for 30 minutes. After filtering, the filter cake was washed with
1500m1
deionized water to produce an acid-treated NaY zeolite, which had a Na2O
content of
3.5wt%;
(2) The acid-treated NaY zeolite was calcined at 300 C for 3 hours to produce
a
zeolite having a solid content of 96wt%, named as Fl;
(3) 5.23 g of zirconium nitrate Zr(NO3)4=5H20 was dissolved in 200g of ethanol
(analytically pure, ethanol content = 99.9wt%) to produce an impregnation
liquor. The
impregnation liquor and the treated Y zeolite Fl were mixed homogenously and
kept
by standing at room temperature for 1 hour.
(4) The product from the step (3) and 800m1 of ethanol were mixed and
transferred to
an autoclave, to which nitrogen was introduced. The pressure was kept at
0.5MF'a.
Then the mixture was kept by standing at room temperature for 12 hours. After
filtering, the filter cake was heat-dried at 100 C for 24 hours.
(5) The product from the step (4) was calcined in a nitrogen atmosphere at 500
C for
4 hours to produce a Zr-modified Y zeolite, named as ZrY(1), the properties of
which
were shown in Table B2.
Example B.1.1.2 to Example B.1.1.7
With reference to Example B.1.1.1, modified zeolites were prepared according
to the
process of the present invention. The operation conditions and the product
properties
were shown in Table D2.
Example B.1.1.8
A modified zeolite was prepared according to Example B.1.1.1, except that in
the step
(4), nitrogen was introduced and the pressure (gauge pressure) was kept at 0
MPa.
The obtained modified zeolite was named as ZrY(1)-1, the properties of which
were
shown in Table B2_
39
Date Recue/Date Received 2021-05-03
81783436
Example B.1.1.9
A modified zeolite was prepared according to Example B.1.1.1, except that in
the step
(1):
(1) At room temperature, 200g of NaY zeolite (dry basis, 75wt%) and 1500m1 of
a
EDTA solution having a molar concentration of 0.5mol/L were mixed and stirred
for
30 minutes. After filtering, the filter cake was washed with 15001n1 deionized
water to
produce an acid-treated NaY zeolite, which had a Na2O content of 3.5wt%;
Steps (2)-(5) were identical to those in Example B.1.1.1. The obtained
modified
zeolite was named as ZrY(1)-2, the properties of which were shown in Table B2.
Example B.1.2.1
200g of NaY zeolite (the same as that in Example B.1.1.1) and 2000g of
deionized
water were slurried. To the slurry was added 60g of Prnm onium sulfate. The
resulting
slurry was adjusted with diluted hydrochloric acid to pli=4.0, warmed upto to
80 C
and exchanged for 1 hour. After filtering and washing with water, the filter
cake was
calcined at 550 C in a 100% steam atmosphere for 2 hours. The above procedure
was
repeated twice to produce a modified Y zeolite.
Then, the resulting Y zeolite and 2000g of deionized water were slurried. To
the slurry
was added 45m1 of a REC13 solution (270g/1). The resulting slurry was adjusted
with
diluted hydrochloric acid to pH=3.8, warmed upto to 80 C and exchanged for 1
hour.
To the mixture was added 45g of ammonium sulfate, and the resulting mixture
was
stirred for 1 hour. After filtering and washing, the filter cake was calcined
at 550 C in
a 100% steam atmosphere for 2 hours. Finnlly, a rare earth modified REY
.zeolite was
obtained and named as REY, the properties of which were shown in Table B3.
Example B.1.2.2
The modified zeolite was prepared according to B.1.1.2, except that the same
amount
of zirconium oxychloride ZrOC12-8H20 was dissolved in 200g of deionized water.
Finally, a Zr-modified zeolite was obtained and named as Zr(W)Y, the
properties of
which were shown in Table B3.
Date Recue/Date Received 2021-05-03
81783436
Example B.1.2.3
200g of NaY zeolite and 2000g of deionized water were slurried. To the
resulting
slurry was added 60g NH401. The slurry was adjusted to a pH of 3.8, warmed
upto
80 C, and exchanged for 2 hours. After filtering and washing with water, the
filter
cake was calcined at 600 C in a 100% steam condition for 2 hours.
The calcined zeolite and 2000g of deionized water were slurried. To the
resulting
slurry was added 45g NH4C1 and 31.4g zirconium oxychloride ZrOC12-8H20 to
conduct a second ion-exchange substantially at the same temperature and for
the same
time as the first exchange. After filtering and washing with water, the filter
cake was
calcined at 600 C in a 100% steam condition for 2 hours. Finally, a Zr-
modified
zeolite was obtained and named as Zr(J)Y, the properties of which were shown
in
Table B3.
Example B.1.2.4
A Zr-modified Y zeolite was prepared according to Example 1 of CN101134576A.
Finally, the Zr-modified zeolite was obtained and named as Zr(G)Y, the
properties of
which were shown in Table B3.
Example B.1.2.5
200g of DASY0.0 zeolite was added to 500 g of absolute ethanol under a violent
stirring to form a suspension, to which was added 50 g/L of a solution of
butyl
titanate-absolute ethanol (as TiO2) under a violent stirring. The mixture was
air-dried
overnight under stirring. The resulting sample was calcined at 500 C for 5
hours to
produce Ti-modified zeolites with titanium content of 7.9wt%, named as Ti(D)Y.
It could be seen from the FT-ER.. spectra of modified Y zeolites in Fig. 1
that the
antisynunetric stretching vibration frequency (1050-1150cm-1) and the
symmetric
stretching vibration frequency (750-820cm4) of the Zr(G)Y zeolite provided in
the
prior art (e.g. Example B.1.2.4) had a red-shift in a direction toward the
lower
frequency, showing that Zr entered the framework structure of the Y zeolite.
The
modified Y zeolites provided by the present invention (e.g. ZrY and TiY) did
not
41
Date Recue/Date Received 2021-05-03
81783436
show the red shift, showing that Zr and Ti did not enter the framework
structure of the
zeolite.
It could be seen from the 27.A1-NNIR spectra of modified Y zeolites in Fig. 2
that the
modified Y zeolite had much tetrahedral-coordinated framework aluminum
(chemical
shift 60) and little hexahedral-coordinated framework aluminum (chemical shift
0).
Compared to the Y zeolite, the peak of tetrahedral-coordinated framework
aluminum
of the ZrY zeolite prepared by organic solution impregnation method became
wider
and moved towards the lower chemical shift, showing that Zr entering the
interior of
the zeolite interacted with the zeolite framework [A104]. This interaction
caused the
spectrum peak of tetrahedral-coordinated framework aluminum moves to higher
field.,
meanwhile the spectrum geak of the distorted tetrahedral-coordinated framework
aluminum was remarkable (chemical shift 40). The ratio of the distorted
tetrahedral-coordinated framework aluminum to the tetrahedral-coordinated
framework alnmimun could be e.g. 0.1-0.6. Zr(W)Y and Ti(D)Y zeolites, prepared
according to Example B.1.2.2 (aqueous solution impregnation) and Example
B.1.2.5,
had no remarkable change in the peak of distorted tetrahedral-coordinated
framework
aluminum, showing that Zr or Ti had little interaction with the zeolite's
framework
[A104], and the ratio of the distorted tetrahedral-coordinated framework
aluminum to
the tetrahedral-coordinated framework alnminum was less than 0.1. It could be
seen
that the modification method according to the present invention was more
favorable
for the metal ions to enter the interior of the zeolite, interact with the
zeolite's
framework [A104], and have an effect of stabilizing the zeolite framework
structure.
42
Date Recue/Date Received 2021-05-03
o
ea
oc
CD
'---:i
x
oc
(D
C
.4.
0
01
m Table B1
.6 .
x
m Raw Material NaY DASY(0.0)
Once-exchanged-once-calcined
0
m
m Elemental composition, wt%
0.
r..)
0
r..) Na2O 12.8 1.2
3.7
cb
9' A1203 21.9 23.6
23.4
0
(...)
SiO2 64.4 71.7
72.0
Unit cell size, run 2.466 2.448
2.453
crystallinity, % 81.6 65.6
78.0
Total specific surface area, m2/g 762 620
644
Total pore volume, ml/g 0.377 0.353
0.352
.4.
(4.)
rp
oc
ea
.--=
x
cc
CD
(.4.)
)
.4.
c
(,4,)
a) co= o Table B2
sl,
11'
X
a)
o Example B.1.I.1
B.I.1.2 B.1.1.3 B.1.1.4 B.1.1.5
CD
CD
o. Once-
exchanged- Once-exchanged-
NJ Y zeolite raw material NaY DASY(0.0)
NaY
0
NJ once-
calcined once-calcined
O
9' Acid solution concentration, mon.. 0.5 0.1
1.9 0.2 0.5
o
(...) Step (I) Hydrochloric
hydrochloric Oxalic Sulfuric Hydrochloric
Acid
acid acid
acid acid acid
Acid solution:zeolite weight ratio 10 10 10
10 10
The content of Na2O after treatment, wt% 3.5 0.6 1.2
0.8 3.5
Calcination Temperature, C 300 300 350
350 300
,
Step (2) Calcination time, hours 3 3 3
3 3 =
Water content, wt% 4 3 1.5
= 1.5 4
.4.
.4.
Compound containing IVB group metal zirconium nitrate zirconium
oxychloride zirconium isopropoxide zirconium isopropoxide titanium
tetrachloride
_
Solvent ethanol ethanol " n-
hexane n-hexane butanone
Step (3) Water content in solvent, wt% . 0.1 0.1
' 0.2 0.2 0.3
Solvent:Zeolite weight ratio 1.3 1.5 1.5
1.2 1.3
Metal salt (as oxide):zeolite weight ratio 0.01 0.03
0.06 0.10 0.02
Used solvent ethanol ethanol n-
hexane n-hexane butanone
Solvent:Zeolite weight ratio 5.1 10.0
10.0 10.0 5.1
Introduced Inert Gas nitrogen nitrogen
nitrogen nitrogen nitrogen
Step (4) Pressure, MPa - 0.5 1.0 0.5
0.8 0.5
_
.
Temperature, C 26 25 r-60
25 25
Time, hour 12 a 5
a 5
Manner By standing Being stirred
Being stirred By standing. being stirred
00
0
a):ri
5'
oc
u.)
X
.4.
co
K,
u.)
c
cs=
co
0 Drying Temperature, C 100 120 120
120 100
o) Step (4)
5' Drying Time, hour 24 6 6
6 12
X
co
o Inert Gas nitrogen
nitrogen nitrogen nitrogen nitrogen
CD
Z
co Step (5) Calcination Temperature, C
500 , 550 550 550 500
o.
NJ
0 Calcination Time, hour 4 2 3
3 3
NJ
- ,
O Metal-modified Y zeolite, No. ZrY(1) ZrY(3)
ZrY(6) ZrY(10) TiY(2)
9'
o
(...) Na20,wt% 1.0 0.5 0.8
0.6 1,2
A1203,wt% 23.3 23.5
22.2 20.5 22.8
Si02,wt% 73.8 72.8
70.5 67.8 73.1
Zr02,wt% 0.9 2.8 6.1
10.5
T102,wt%
2.2
a0, rim 2.456 2.448
2.450 2.452 2.451
_
.4, Crystallinity, % 78.8 64.7
70.6 72.2 76.9
Specific surface area ,m2/g 710 616 624
635 702
lattice collapse temperature, C 1048 1050
1045 1047 1044
The ratio of the distorted tetrahedral-coordinated framework
0.2 0.3 0.5
0.6 0.2
aluminum to the tetrahedral-coordinated framework aluminum
The percent of the secondary pores (pore diameter of 6-20 nm) to the
32.5 42.8
48.7 50.3 38.6
total secondary pores (pore diameter of 2-100 nm)
0
00
--A
CT
oc
x
u.)
CD .4. ,r)
u.) c
CD
co=
o Table 1712 (continued)
sv
5'
X
a) Example B.1.1.6 B.1.1.7
B.1.1.8 B.1.1.9
o
CD
a) Y zeolite raw material Once-exchanged once-calcined Once-
exchanged- once-calcined NaY NaY
o..
NJ
0 Acid solution concentration, mol/L 1.0
1.0 0.5 0.5
NJ
cb Step (I) Acid acetic acid oxalic acid
hydrochloric acid EDTA
9'
o
(...) Acid solution:zeolite weight ratio 10
10 10 10
The content of Na2O after treatment, wt% 1.0 1.0
3.5 3.5
Calcination Temperature, C 350 350
300 300
Step (2) Calcination time, hours 3 3
3 3
Water content, wt% 1.8 1.5
4 4
Compound containing IVB group metal butyl titanate butyl
titanate+zirconium nitrate zirconium nitrate zirconium nitrate
Solvent cyclohexane ethanol
ethanol ethanol
.4.
co=
Step (3) Water content in solvent,wt% 0.2 0.1
0.1 0.1
SoIvent:Zeolite weight ratio 1.5 1.5
1.3 1.3
Metal salt (as oxide):zeolite weight ratio 0.10 0.08
0.01 0.01
Used solvent cyciohexane Ethanol
ethanol ethanol
Solvent:Zeolite weight ratio 10.0 8.0
5.1 5.1
Introduced Inert Gas nitrogen nitrogen
nitrogen nitrogen
Step (4) Pressure, MPa 0.5 1.5
0.0 0.5
Temperature, C 25 80
26 26
Time, hour. 10 24
12 12
Manner Being stirred Being stirred
By standing By standing
,
.,
Drying Temperature, C 120 120
100 100
Step (4)
Drying Time, hour 6 24
24 24
. .
=
00
0
--,
sv
---.)
CT
oc
x
u.)
co
.4.
c
co=
co
Inert Gas nitrogen nitrogen nitrogen
nitrogen
o)
5'
X Step (5) Calcination Temperature, C 550
550 500 500
co _
o
a) Calcination Time, hour 2 3
4 4
R=
co
a. Metal-modified Y zeolite, No. TiY(10) Ti-Zr-Y
ZrY(1)-1 ZrY(1)-2
NJ
0
NJ Na20,wt% 1.0 0.8
1.0 0.9
O A1203,wt% 21.5 21.9
- 23.2 23.4
9'
o _
(...) Si02,wt% 67.1 69.0
73.9 73.6
Zr02,wt% 3.8
0.9 0.9
Ti02,wt% 9.8 4.0
a0, nm 2.455 2.455
2.454 2.455
crystallinity, % 73.5 72.9
71.5 77.7
specific surface area ,m2/g 642 639
700 699
lattice collapse temperature, C 1045 1050
1045 1048
.4.
---.)
the ratio of the distorted tetrahedral-coordinated
framework aluminum to the tetrahedral-coordinated 0.5 0.5
0.2 0.2
framework aluminum
The percent of the secondary pores (pore diameter
a 6-20 nm) to the total secondary pores (pore 49.8 44.4
35.4 33.9
diameter of 2-100 nrn)
,
00
oc
U.)
CD
(J.)
co
Table B3
Example B.1.2.1 B.1.2.2
B.1.2.3 B.1.2.4 B.1.2.5
0
Y zeolite No. KEY Zr(W)Y Zr(1)Y
Zr(G)Y Ti(D)Y
Na20,wt% 1.2 0.8 0.6
0.5 1
9' A1203,wt% 21.4 20.6 21.8
20.8 21.5
Si02,wt% 70.5 70.2 71
70.7 69.3
Zr02,wt% 8 6.2
7.5
Ti02,wt%
7.9
RE203,wt5 6.6
ct0, um 2.451 2.45
2.452 2.451 2.453
.4. crystallinity, % 62.7 51.9 55.7
60.2 59.6
00
specific surface area ,1n2/g 640 536 548
624 610
lattice collapse temperature, C 1043 1027 1028
1041 1033
the ratio of the distorted tetrahedral-coordinated
0.05 0.01
0.02 0.01
framework aluminum to the tetrahedral-coordinated framework aluminum
B.2 Stability of the modified zeolite
Modified Y zeolites prepared according to Example B.2.1.1-B.2.1.9 and B.2.2.1-
B.2.2.5 were aged at 800 C under a 100% steam condition for 8
hours and 17hours respectively to determine the crystallinity and the specific
surface area, and the crystallinity retention and the specific surface
area retention were calculated. The aged zeolites were subjected to the light
oil micro-activity (MA) test. The results were listed in Table B4.
O Oc
sv
Ei
'----1
X
00
CD
(j.)
c
(J.)
co
CN
O Table B4
sv
Ei
x Example B.2.1.X 1 2 3 4 5
6 7 8 9
0
0
0
Y zeolite ZrY(1) ZrY(3) ZrY(6) ZrY(10)
TiY(2) TiY(10) Ti-Zr-Y ZrY(1)-1 ZrY(1)-2
0
0.
r..) 800 C/8h crystallinity retention, % 61.2 62.8 63.7 68.4
63.1 67.5 68.6 62.0 61.8
.:0
(5 800 C/17h crystallinity retention, % 55.5 57.0 56.7
62.5 57.6 60.1 60 56.5 56.1
.:0 800 C/8h specific surface area
0.)
62.0 61.3 62.9 69.6 66.1
68 69.6 62.8 61.0
retention,%
800 C/17h specific surface area
57.2 56.7 57.6 63.9 59.6
62.2 62.8 57.0 56.4
retention,%
800 C/8h activity,% 80 82 84 87 85
86 87 80 80
800 C/171i activity,% 76 77 79 82 79
80 81 75 77
.4. _
Z)
Table B4 (continued)
Example B.2.2.X 1 2 3
4 5
Y zeolite REY Zr(W)Y
Zr(J)Y Zr(G)Y Ti(D)Y
800 C/8h crystallinity retention, % 63.2 50.4 50.5
60.8 61.6
..
800 C/17h crystallinity retention, % 56.5 45.8 42.5
52.9 53
800 C/8h specific surface area retention,% 67.1 58 54.2
64.9 62.4
800 C/17h specific surface area retention,% 61.2 52.6 49.8
58.2 59.1
800 C/8h activity,% 85 75 72
80 72
800 C/17h activity,% 79 68 65
75 66
_
81783436
B.3 catalyst
Example B.3.1.1
The modified Y zeolite prepared according to the present invention, Zr(6)Y,
was used
as active component to prepare the catalyst according to the conventional
preparation
method of the catalytic cracking catalyst. The preparation was as follows.
According to the ratio of zeolite (dry basis):kaolin (dry
basis):pseudobohemite (as
A1203):alumina sol (as A1203) being 38:34:20:8, kaolin and decationized water
were
mixed and slurried. To the resulting slurry was added alumina sol, and further
added
pseudoboehmite under a continuous stirring. After 30 minutes of stirring, a
liquor
containing zeolite was added to the colloid. The resulting mixture was mixed
homogenously, spray-dried and shaped to produce a catalyst, named as Cl.
Evaluation of heavy oil cracking performance:
The catalyst was pretreated at 800 C in a 100% steam condition for 8 hours.
Then the
pretreated catalyst was tested on a small-scale fixed fluidised bed (ACE) for
catalyst
evaluation. The feedstock for evaluation was a mixed oil of ZhengHai VGO and
DaQing atmospheric residue (80:20 by weight), the properties of which were
shown
in Table B5. Reaction Temp=500 C, WHSV-16h-1, Catalyst-to-oil weight ratio-45.
The evaluation result was listed in Table B6.
wherein,
conversion = gasoline yield+liquefied gasyield+Dry gasyield+coke yield
coke selectivity ¨coke yieldx100/conversion
Date Recue/Date Received 2021-05-03
81783436
Table B5
Feedstock ZhengHai VG() DaQing
Atmospheric
Residue
Density (20 C), g/cm3 0.9154 0.8906
Refraction (70 C) 1.4926 1.4957(20 C)
Viscosity (100 C) mm2/s 6.962 24.84
SARA composition, %
Saturated hydrocarbons 64.0 51.2
Aromatic hydrocarbons 32.0 29.7
Resin 4.0 18.3
Asphaltene 0.0 0.8
Freezing point, C 35 43
Aniline point, C 82.0 >105
C, wt% 85.38 86.54
H ,wt% 12.03 13.03
S ,wt% 2.0 0.13
N ,wt% 0.16 0.3
Residual Carbon % 0.18 4.3
Distillation range, C
Initial distillation point 329 282
5% 363 351
10% 378 370
30% 410 482
50% 436 553
70% 462
90% 501
Example B.3.1.2
A catalyst was prepared according to Example B.3.1.1, except that the same
amount
of TiY(2) zeolite was used in place of ZrY(6) zeolite to produce a catalyst
C2. Then
the evaluation of C2 was done according to Example B.3.1.1. The evaluation
result
51
Date Recue/Date Received 2021-05-03
81783436
was listed in Table B6.
Example B.3.2.1 to Example B.3.2.3
A series of catalysts were prepared according to Example B.3.1.1, except that
the
same amount of REY zeolite, the same amount of Zr(W)Y zeolite, and the same
amount of Ti(D)Y zeolite were respectively used in place of ZrY(6) zeolite to
produce
the catalysts DC1, DC2 and DC3. Then the evaluation of DC1-DC3 was done
according to Example B.3.1.1. The evaluation result was listed in Table B6.
Table.B6
Example B.3.1.1 B.3.1.2 B.3.2.1 B.3.2.2
B.3.2.3
Zeolite No. ZrY(6) TiY(2) REY Zr(W)Y Ti(D)Y
Catalyst Cl C2 DC1 DC2 DC3
Reaction Temp, C 500 500 500 500 500
Catalyst-to-oil weight
5 5 5 5
ratio
WHSV,11-1 16 16 16 16 16
Production
Distribution, wt%
Dry gas 1.40 L39 1.38 1.39 1.37
liquefied gas 17.15 16.92 16.53 16.44 16.98
coke 4.52 4.75 5.35 5.16 5.33
gasoline 50.80 49.98 49.70 45.62 48.29
diesel 17.85 18.76 18.64 19.97 18.73
Heavy oil 8.28 8.20 8.40 11.42 9.30
Conversion, wt% 73.87 73.04 72.96 68.61 71.97
Total liquid yield, wt% 85.80 85.66 84.87 82.03 84.00
Coke selectivity 6.12 6.50 - 7.33 7.52 7.41
Example B.3.1.3
A Zr-modified Y zeolite was prepared according to Example B.1.1.1, named as
Zr(2)Y,
52
Date Recue/Date Received 2021-05-03
81783436
wherein the weight ratio of zirconium nitrate(as ZrO2) ;Y zeolite¨ 0.02:1.
323g of pseudoboehmite and 1343g of deionized water were mixed and stirred for
15
minutes to produce a pseudoboehmite slurry. The slurry was adjusted with
diluted
hydrochloric acid (having a concentration of 15wt%) to a pH of 3.5, and aged
at room
temperature for 6 hours. To the aged slurry were added 421g of kaolin and 465g
of
alumina sol. After stirring for 60minutes, to the resulting slurry was added a
slurry
formed by slurrying 380g (dry basis) of the above modified ZrY(2) zeolite and
800g
of deionized water. The resulting mixture was stirred for 60 minutes to
produce a
catalyst slurry, which was spray-dried and shaped, and calcined at 550 C for 2
hours
to produce a catalytic cracking catalyst, named as C17. The ZrO2 content of
the
catalyst C17, measured by XRF, was 0.75wt%.
Example B.3.1.4
421g of kaolin, 465g of alumina sol and 732g of deionized water were added to
and
slurried in a slurry vessel, to which was added 1667g of an acidified
pseudoboehmite.
After stirring for 60 minutes, to the vessel was added a slurry formed by
slurrying
380g (dry basis) of the above modified Zr(6)Y zeolite and 800g of deionized
water.
The resulting mixture was stirred for 60 minutes to produce a catalyst slurry,
which
was spray-dried and shaped, and calcined at 550 C for 1 hour to produce a
catalytic
cracking catalyst, named as C18. The ZrO2 content of the catalyst C18,
measured by
XRF, was 2.18wt%.
Example B.3.1.5
421g of kaolin, 558g of alumina sol and 800g of deionized water were mixed and
slurried for 60 minutes. After adding 1500g of an acidified pseudobohemite,
the
resulting slurry was further stirred for 60 minutes. To the resulting mixture
was added
a slurry formed by slurrying 380g (dry basis) of the above modified ZrY(10)
zeolite
and 800g of deionized water. The resulting mixture was stirred for 60 minutes
to
produce a catalyst slurry, which was spray-dried and shaped, and calcined at
650 C
for 2 hours to produce a catalytic cracking catalyst, named as C19. The ZrO2
content
of the catalyst C19, measured by XRF, was 3.52wt%.
53
Date Recue/Date Received 2021-05-03
81783436
Example B.3.1.6
According to Example B.2.1.5, a Ti-modified Y zeolite was prepared and named
as
TiY(8), wherein during the impregnation of the step (3), the weight ratio of
titanium
tetrachloride (as Ti02) to zeolite was 0.08:1.
421g of kaolin and 380g of deionized water were mixed and slurried for 60
minutes.
After adding 1667g of an acidified pseudobohemite, the resulting slurry was
further
stirred for 30 minutes. To the resulting mixture was added a slurry formed by
slurrying 380g (dry basis) of the above modified TiY(8) zeolite and 800g of
deionized
water. After stirring for 60 minutes, to the resulting slurry was added 465g
of alumina
sol. The resulting mixture was stirred for 30 minutes to produce a catalyst
slurry,
which was spray-dried and shaped, and calcined at 700 C for 2 hours to produce
a
catalytic cracking catalyst, named as C20. The TiO2 content of the catalyst
C20,
measured by XR.F, was 3.02wt%.
Example B.3.1.7
According to Example B.2.1.5, a Ti-modified Y zeolite was prepared and named
as
TiY(4), wherein during the impregnation of the step (3), the weight ratio of
titanium
tetrachloride (as Ti02) to zeolite was 0.04:1.
323g of pseudoboehmite and 1478g of deionized water were mixed. The mixture
was
stirred for 30 minutes to produce a pseudoboehmite slurry, the pH value of
which was
adjusted with a suitable amount of diluted hydrochloric acid to 3.8. The
resulting
slurry was aged at 60 C for 2 hours. To the aged slurry were added 421g of
kaolin and
465g of alumina sol. The resulting slurry was stirred for 60 minutes. To the
above
slurry was added a slurry formed by slurrying 380g (dry basis) of the above
modified
TiY(4) zeolite and 800g of deionized water. The resulting mixture was stirred
for 60
minutes to produce a catalyst slurry, which was spray-dried and shaped, and
calcined
at 600 C for 3 hours to produce a catalytic cracking catalyst, named as C21.
The TiO2
content of the catalyst C21, measured by XRF, was 1.48wt%.
Example B.3.1.8
According to Example B.2.1.5, a Hf-modified Y zeolite was prepared and named
as
54
Date Recue/Date Received 2021-05-03
81783436
HfY(6), wherein during the impregnation of the step (3), the weight ratio of
hafnium
nitrate (as Hf02) to zeolite was 0.06:1.
421g of kaolin and 380g of deionized water were mixed and slurried for 60
minutes.
After adding 1667g of an acidified pseudobohemite, the resulting slurry was
further
stirred for 30 minutes. To the resulting mixture was added a slurry formed by
slurrying 380g (dry basis) of the above modified HfY(6) zeolite and 800g of
deionized water. After stirring for 60 minutes, to the resulting slurry was
added 465g
of alumina sol. The resulting mixture was stirred for 30 minutes to produce a
catalyst
slurry, which was spray-dried and shaped, and calcined at 700 C for 2 hours to
produce a catalytic cracking catalyst, named as C22. The Hf02 content of the
catalyst
C22, measured by XRF, was 2.11wt%.
Example B.3.1.9
421g of kaolin and 380g of deionized water were mixed and slurried for 60
minutes.
After adding 1667g of an acidified pseudobohemite, the resulting slurry was
further
stirred for 30 minutes. To the resulting mixture was added a slurry formed by
slurrying 380g (dry basis) of the above modified Ti-Zr-Y zeolite and 800g of
deionized water. After stirring for 60 minutes, to the resulting slurry was
added 465g
of alumina sol. The resulting mixture was stirred for., 30 minutes to produce
a catalyst
slurry, which was spray-dried and shaped, and calcined at 700 C for 2 hours to
produce a catalytic cracking catalyst, named as C23. The TiO2 and ZrO2
contents of
the catalyst C23, measured by XRF, were 1.50wt% and. 1.48wt% respectively.
Example B.3.1.10 to Example B.3.1.12
A series of catalysts were prepared according to Example B.3.1.3, except that
the
same amount of ZrY(1), the same amount of ZrY(1)-1 and the same amount of
ZrY(1)-2 were respectively used in place of ZrY(2) zeolite to produce the
catalysts
C24, C25 and C26.
=
Example B.3.2.4
A catalyst was prepared according to Example B.3.1.4, except that the same
amount
of Zr(W)Y zeolite was used in place of ZrY(6) zeolite to produce the catalyst
DC14.
The ZrO2content of the catalyst DC14, measured by xtu, was 2.19wt%.
Date Recue/Date Received 2021-05-03
81783436
Example B.3.2.5
500g of NaY zeolite (dry basis) and 6000g of deionized water were mixed and
slurried. To the resulting slurry was added 200g of NHIC1. The mixture was
adjusted
to pH=3.8, warmed upto 80 C to exchange for 2 hours, filtered and washed with
water.
The above procedure was repeated for three times. The resulting filter cake
was
calcined at 600 C under a 100% steam atmosphere for 2 hours. Then, the
calcined Y
zeolite and 6000g of deionized water were mixed and slurried. To the slurry
was
added 150g NH4C1 and 95.1g of titanium tetrachloride. The mixture was warmed
upto 80 C to exchange for 3 hours. After filtering and washing, the resulting
filter
cake was calcined at 600 C, under a 100% steam atmosphere for 2 hours.
Finally, a
zeolite was obtsined and named as Ti(J)Y zeolite.
A catalyst was prepared according to Example B.3.1.6, except that the same
amount
of Ti(J)Y zeolite was used in place of TiY(8) zeolite to produce the catalyst
DCI5.
The TiO2content of the catalyst DC15, measured by XR_F, was 1.72wt%.
Example B.3.2.6
200g of NaY zeolite and 2000g of deionized water were mixed and slurried. To
the
resulting slurry was added 45mL of a solution of 270g/1 RECI3. The mixture was
adjusted with a diluted hydrochloric acid to pli=3.8, and warmed upto 80 C to
exchange for 1 hour. After filtering and washing, the resulting filter cake
was calcined
at 500 C for 3 hours. Then, the resulting Y zeolite and 2000g of deionized
water were
mixed and slurried. To the slurry was added 45g of ammonium sulfate. The
mixture
was adjusted with a diluted hydrochloric acid to pH=4.0, and warmed upto 80 C
to
= exchange for 1 hour. After filtering and washing, the resulting filter
cake was calcined
at 600 C, under a 100% steam atmosphere for 3 hours. Finally, a RE-modified
zeolite
was obtained and named as REY(8).
According to the ratio of zeolite (dry basis):kaolin (dry
basis):pseudobohemite (as
A1203):aluraina sol (as A1203) being 38:34:20:8, kaolin and decationized water
were
mixed and slurried. To the resulting slurry was added alumina sol, and further
added
pseudoboehmite under a continuous stirring. After about 30 minutes of
stirring, a
56
Date Recue/Date Received 2021-05-03
81783436
liquor containing zeolite was added to the colloid. The resulting mixture was
mixed
homogenously, spray-dried and shaped to produce a catalyst, named as DC16.
The catalysts C17-C26 and DC14-DC16 were pretreated at 800 C in a 100% steam
condition for 17 hours. Then the pretreated catalysts were tested on a small-
scale
fixed fluidised bed (ACE) for catalyst evaluation. The feedstock for
evaluation was
Wuhun III, the properties of which were shown in Table B7. Reaction
temperature =
500 C, and the catalyst-to-oil weight ratio = 5. The evaluation result was
listed in
Table )38.
wherein,
conversion=gasoline yield + liquefied gas yield + Dry gas yield + coke yield
coke selectivity =coke yield/conversion
57
Date Recue/Date Received 2021-05-03
0
oc
cT
oc
(D
(J.)
Table B7 Feedstock's properties
v.)
0
Feedstock Wuhun HI
density (20 C), g/cm3 0.9044
0
Refraction (20 C) 1.5217
0
Viscosity (100 C), nun2/s 9.96
9'
0 Freezing point, C 40
Aniline point, C 95.8
C,wt% 85.98
H,wt% 12.86
S,wt% 0.55
N,wt% 0.18
00
Residual Carbon, wt% 3.0
Distillation range, C
Initial distillation point 243
5% 294
10% 316
30% 395
50% 429
70% 473
90%
00
0
CD
oc
Table B8 Evaluation Result
0
Example B.3.1.3 B.3.1.4 B.3.1.5 B.3.1.6
B.3.1.7 B.3.1.8 B.3.1.9
Catalyst No. - C17 C18 C19 C20 C21
C22 C23
0
Modified Y Zeolite No. ZrY(2) ZrY(6) ZrY(10) TiY(8) TiY(4)
HfY(6) Ti-Zr-Y
0
Reaction Temp, C 500 500 500 500 500
500 500
9' Catalyst-To-Oil Ratio (Weight) 5 5 5 5 5
5 5
0
WHSV, 16 16 16 16 16
16 16
Production Distribution,wt%
Dry Gas 1.25 1.12 1.29 1.22 1.10
1.18 1.20
Liquefied Gas 12.04 13.05 11.53 12.09
12.41 12.48 12.35
Coke 5.09 4.26 4.95 4.96 5.01
4.85 4.99
Gasoline 53.99 54.61 54.59 54.44
54.07 53.95 . 54.11
Diesel 21.41 20.98 21.58 21.34
21.84 21.69 21.36
Heavy Oil 6.22 5.98 6.06 5.95 "5.57
5.85 5.99
Total 100 100 100 100 100
100 100
Conversion, wt % 72.37 73.04 72.36 72.71
72.59 72.46 72.65
Coke /Conversion 0.07033 0.05832 0.06841 0.06822
0,06902 0.06693 0.06869
O 00
w
.6
'---:i
oc
x
u.)
0 .4. .,
Table B8: Evaluation Result
(continued) c u.)
O 0,
o
Da Example B.3.1.10 B.3.1.11 B.3.1.12
B.3.2.4 B.3.2.5 B.3.2.6
.6
x
O Catalyst No. C24
C25 C26 DC14 DC15 DC16
0
0
O Modified Y Zeolite No.
ZrY(1) ZrY(1)-1 ZrY(1)-2 Zr(W)Y Ti(J)Y RE(8)Y
0.
r..)
0
" Reaction Temp, C 500 500 500
500 500 500
cb
9'
0 Catalyst-To-Oil Ratio (Weight) 5 5 5 5
5 5
(...)
WHSV, Ifl 16 16 16
16 16 16
Production Distribution, wt%
Dry Gas 1.21 1.22 1.23
1.32 1.18 1.10
Liquefied Gas 11.81 11.79 11.89
11.78 12.03 11.66
cr, Coke 4.62 4.52 4.71
5.88 5.25 5.45
Gasoline 54.15 54.21 54.48
50.57 50.30 53.08
__.
Diesel 21.88 21.91 21.28
22.01 22.12 21.46
Heavy Oil 6.33 6.35 6.41
8.44 9.12 7.25
Total 100 100 100
100 100 - 100
Conversion,wt% 71.79 71.74 72.31
69.55 68.76 . 71.29
Coke/Conversion 0.06435 0.06301 0.06514
0.08454 0.07635 0.0764