Note: Descriptions are shown in the official language in which they were submitted.
CA 02868603 2014-10-23
UNFURLING ELECTRODE DEVICES WITH THE MULTIPLE
LONGITUDINAL ELECTRODE SEGMENTS
CROSS REFERENCES
[0001] This application claims the benefit of and priority to U.S. Patent
Application No.
14/519,409, filed October 21, 2014, entitled "UNFURLING ELECTRODE DEVICES WITH
THE MULTIPLE LONGITUDINAL ELECTRODE SEGMENTS," U.S. Patent Application No.
14/519,950, filed October 21, 2014, entitled "UNFURLING ELECTRODE DEVICES WITH
SPRING," and U.S. Patent Application No. 14/520,028, filed October 21, 2014,
entitled,
"UNFURLING ELECTRODE DEVICES WITH THE PROTECTION ELEMENT," each which
claim priority to U.S. Provisional Application No. 61/895,501, filed on
October 25, 2013,
entitled, "UNFURLING ELECTRODE DEVICES WITH THE MULTIPLE LONGITUDINAL
ELECTRODE SEGMENTS," U.S. Provisional Application No. 61/895,514, filed
October 25,
2013, entitled, "UNFURLING ELECTRODE DEVICES WITH SPRING," and U.S.
Provisional
Application No. 61,895,530, filed on October 25, 2013, entitled, "UNFURLING
ELECTRODE
DEVICES WITH THE PROTECTION ELEMENT," each of which are incorporated by
reference in their entirety for all purposes.
BACKGROUND
[0002] The human body has a number of internal body lumens or cavities located
within, such
as the differing parts of the gastro-intestinal tract, many of which have an
inner lining or layer.
Body lumens may include, for example, the esophagus, small and large
intestines, stomach,
remnant after bariatric surgery, rectum and anus. These inner linings may be
susceptible to
disease. In some cases, different ablation techniques have been utilized with
respect to the inner
lining in order to prevent the spread of disease to otherwise healthy tissue
located nearby.
[0003] Internal body lumens may have different sizes with respect to each
other or with respect
to different patients. As a result, different sized devices may have been
utilized to accommodate
these different sized lumens. However, this may involve utilizing multiple
devices such as
multiple sizing and/or treatment devices, which may not be as efficient, cost
effective, or safe as
a device that can both size and treat with a single intubation.
1
CA 02868603 2014-10-23
[0004] Another problem may exist when treating a target site larger than the
surface area of the
treatment device. Conventional ablation approaches often involved three or
more repositioning
steps in order to treat a target site. Such repositioning activities may be
susceptible to decreased
accuracy of treatment, over or under ablation of subregions of the target
site, or both. In
addition, repositioning activities may be ad hoc with respect to the number of
repositioning steps
and the physical processes associated with the actual repositioning of the
device. Such lack of
consistency may further decrease accuracy of treatment, efficiency of
treatment, or both.
[0005] There may thus be a need for systems, devices and methods that may
overcome the
above and/or other disadvantages of known systems, devices, and methods.
SUMMARY
[0006] Methods, systems, and devices are described for providing treatment to
a target site,
such as a site within a body lumen. Systems may include an expansion member
coupled with a
distal portion of a catheter, and an ablation structure support coupled to an
ablation structure
configured to at least partially furl and unfurl around the expansion member
as the expansion
member expands and contracts. The expansion member may include a non-
compliant,
compliant, or highly-compliant balloon. The ablation structure support may
include one or more
longitudinal electrodes, longitudinal electrode zones, and/or longitudinal
electrode regions. In
some embodiments, the system may include one or more protection elements
positioned along
the catheter distal to the ablation structure, proximal to the ablation
structure, or both.
[0007] According to some embodiments of the disclosure, an ablation device for
treatment of
tissue in body lumens with varying sizes is provided. The ablation device
generally includes an
expansion member coupled with a distal portion of a catheter, and an ablation
structure including
a number of longitudinal electrode regions. An ablation structure support may
be coupled with
the ablation structure and the ablation structure and the ablation structure
support may be
configured to at least partially unfurl or furl around the expansion member as
the expansion
member expands or contracts.
100081 In some embodiments, the ablation device further includes one or more
springs coupled
with the ablation structure support configured to furl the ablation structure
support at least
2
CA 02868603 2014-10-23
partially around the expansion member. The one or more springs may include one
or more
constant force springs. The ablation device may also include one or more
protection elements
positioned along the catheter at least distal or proximal to the ablation
structure.
[0009] In some embodiments, each of the longitudinal electrode regions of the
ablation device
is configured to be selectively enabled or disabled. Each of the longitudinal
electrode regions
may be controlled or wired separately. In certain embodiments, the
longitudinal electrode
regions include at least two longitudinal electrode regions with different
widths. The ablation
device may include at least one bipolar electrode array in some embodiments.
In certain
instances, the expansion member is a balloon, and the balloon may be compliant
in some
embodiments.
[0010] According to some embodiments of the disclosure, a method for treatment
of tissue in
body lumens with varying sizes is provided. The method generally includes
inserting an ablation
structure coupled with an ablation structure support and an expansion member
into a body
lumen. The ablation structure and the ablation structure support may be
configured to at least
partially unfurl or furl around the expansion member as the expansion member
expands or
contracts. The method further includes expanding the expansion member to at
least partially
unfurl the ablation structure to engage a circumferential section of the body
lumen, and
delivering energy through one or more of a plurality of longitudinal electrode
regions of the
ablation structure to the circumferential section of the body lumen.
[0011] In some instances, delivering energy through one or more of the
plurality of
longitudinal electrode regions of the ablation structure to the
circumferential section of the body
lumen includes selectively enabling each of the longitudinal electrode
regions. The method may
further include contracting the expansion member to facilitate removing the
ablation structure
from the body lumen. In some embodiments, one or more springs cause the
ablation structure to
furl at least partially around the expansion member as the expansion member
contracts. The one
or more springs may include one or more constant force springs in some
instances.
[0012] In certain embodiments, the method further includes utilizing one or
more protection
elements with respect to at least a distal portion or a proximal portion of
the ablation structure
while inserting the ablation structure coupled with the ablation structure
support and the
expansion member into the body lumen. In some embodiments, the method further
includes
3
CA 02868603 2014-10-23
moving the one or more protection elements away from the ablation structure
after positioning
the ablation structure in the body lumen. In certain instances, the method
further includes
determining an impedance for each of the plurality of longitudinal electrode
regions of the
ablation structure, and comparing the determined impedances to determine
whether one or more
of the longitudinal electrode regions is at least partially covered by an
electrode segment.
[0013] In some embodiments, the longitudinal electrode regions of the ablation
structure
include at least two longitudinal electrode regions with different widths. The
ablation structure
may include at least a bipolar electrode array in certain instances. The
expansion member may
be a balloon in some embodiments.
[0014] According to some embodiments of the disclosure, an ablation device for
treatment of
tissue in body lumens with varying sizes is provided. The ablation device
generally includes an
expansion member coupled with a distal portion of a catheter and an ablation
structure
configured to at least partially unfurl or furl around the expansion member as
the expansion
member expands or contracts. The ablation device further includes one or more
springs, coupled
with the ablation structure, and configured to provide a force to cause the
ablation structure to
unfurl or furl around the expansion member as the expansion member expands or
contracts.
[0015] In some embodiments, the one or more springs of the ablation device
includes one or
more strips of material coupled with the ablation structure laterally with
respect to a longitudinal
axis of the ablation structure. In certain instances, the one or more springs
of the ablation device
includes one or more strips of material such that a density of the one or more
strips of material
proximal to a free end of the ablation structure is less than a density of the
one or more strips of
material distal to the free end of the ablation structure. In yet other
embodiments, the one or
more springs of the ablation device include one or more strips of material
such that a density of
the one or more strips of material proximal to a free end of the ablation
structure and proximal to
a mounted end of the ablation structure is less than a density of the one or
more strips of material
at a middle portion of the ablation structure. The one or more strips of
material may include a
metallic material or a polymer material. The one or more strips of material
may include a shape
memory polymer material in some embodiments.
[0016] In certain instances, the one or more springs of the ablation device
includes at least a
first spring with a first length and a second spring with a second length
different from the first
4
CA 02868603 2014-10-23
length. The first length may be greater than the second length and the second
spring with the
second length may be positioned distal to the free end of the ablation
structure.
[0017] In some embodiments, the one or more strips of material of the one or
more springs
includes one or more rectangular strips of material. In certain instances, at
least one of the
rectangular strips of material includes one or more openings configured into
at least one end of
the at least one rectangular strips. In some embodiments, at least one of the
strips of material
includes at least a tapered portion. In yet other embodiments, at least one of
the strips of material
includes one or more slots configured into at least one end of the at least
one strip of material.
[0018] In certain embodiments, the ablation structure of the ablation device
includes a number
of longitudinal electrode regions where the longitudinal electrode regions are
configured to be
sequentially activated. In some embodiments, the ablation structure includes a
number of
longitudinal electrode regions where the longitudinal electrode regions are
configured to be
sequentially activated beginning with a first electrode region adjacent to a
free end of the
ablation structure. In yet some other embodiments, the ablation structure
includes a number of
longitudinal electrode regions where each longitudinal electrode region is
configured to be
selectively enabled or disabled. The longitudinal electrode regions may
include at least two
longitudinal electrode regions with different widths. In certain instances,
the at least two
longitudinal electrode regions of different widths include a first
longitudinal electrode region
adjacent to a free end of the ablation structure and a second longitudinal
electrode region, where
the first longitudinal electrode region has a width greater than the width of
the second
longitudinal electrode region.
[0019] In some embodiments, the longitudinal electrode regions of the ablation
device include
at least three longitudinal electrode regions with different widths comprising
a first longitudinal
electrode region proximal to a free end of the ablation structure, a second
longitudinal electrode
region adjacent to the first longitudinal electrode region, and a third
longitudinal electrode region
distal to the free end of the ablation structure. The first longitudinal
electrode region may have a
width greater than the width of the second longitudinal electrode region and
the second
longitudinal electrode region may have a width greater than the width of the
third longitudinal
electrode region.
5
CA 02868603 2014-10-23
[0020] In certain embodiments, the ablation structure of the ablation device
includes at least a
bipolar electrode array. In some embodiments, the expansion member of the
ablation device is a
balloon.
[0021] According to embodiments of the disclosure, an ablation device for
treatment of tissue
in body lumens with varying sizes is provided. The ablation device generally
includes an
ablation structure coupled with a catheter including one or more protection
elements positioned
along the catheter distal to the ablation structure, proximal to the ablation
structure, or both. In
some embodiments, the ablation structure includes a furled bi-polar electrode
array.
[0022] In some instances, the one or more protection elements include one or
more cones
configured to protect one or more edges of the ablation structure. The one or
more cones may
each have a base circumference greater than a circumference of the collapsed
ablation structure.
Furthermore, the one or more cones may be configured to move away from the
ablation structure
when the ablation structure is deployed to engage the tissue. In some
embodiments, the ablation
device may further include one or more tethers configured to facilitate moving
the one or more
cones with respect to the ablation structure.
[0023] In certain instances, the one or more protection elements are
configured to prevent the
ablation structure from distending along the catheter during at least
deployment into a body
lumen or removal from the body lumen. The one or more protection elements may
be
configured to prevent the ablation structure from damaging a surface of a body
lumen during at
least deployment into the body lumen or removal from the body lumen.
[0024] In some embodiments, the one or more protection elements include one or
more
bumpers coupled with one or more edges of the ablation structure. The one or
more bumpers
may overhang the edge of the ablation structure inwards towards the catheter.
[0025] According to various embodiments, the one or more protection elements
proximal to
the ablation structure may include a raised bump coupled with the catheter.
The raised bump
may be configured to prevent the ablation structure from distending proximally
along the
catheter during insertion or deployment into a body lumen.
[0026] In various embodiments, the ablation device further includes an
expansion member
coupled with the catheter wherein the ablation structure is furled around the
expansion member.
6
CA 02868603 2014-10-23
In such embodiments, the one or more protection elements distal to the
ablation structure may
include a portion of the expansion member configured to bunch up when the
expansion member
is unexpanded such that a diameter of the bunched up portion exceeds a
diameter of the furled
ablation structure. The bunched up portion of the expansion member may be
configured to
prevent the ablation structure from distending distally along the catheter
during removal from a
body lumen.
[0027] In certain instances, the one or more protection elements include a
first protection
element positioned distal to the ablation structure with respect to the
catheter and a second
protection element positioned proximal to the ablation structure with respect
to the catheter.
[0028] In accordance with some embodiments of the present disclosure, a method
for treatment
of tissue in body lumens with varying sizes is provided. The method generally
includes inserting
an ablation structure coupled with a catheter into a body lumen. One or more
protection
elements may be positioned along the catheter at least distal or proximal to
the ablation structure.
The method further includes expanding an expansion member to at least
partially unfurl the
ablation structure to engage the body lumen. The protection elements may
include one or more
cones configured to protect one or more edges of the ablation structure. In
some instances, the
method further includes displacing the one or more cones away from the
ablation structure when
the ablation structure is deployed to engage the body lumen. The method may
further include
utilizing one or more tethers coupled with the one or more protection elements
to facilitate
moving the one or more protection elements with respect to the ablation
structure.
[0029] In some instances, the one or more protection elements are configured
to prevent the
ablation structure from distending along the catheter during deployment into
the body lumen. In
certain embodiments, the one or more protection elements include one or more
bumpers coupled
with one or more edges of the ablation structure. The one or more bumpers may
overhang the
edge of the ablation structure inwards towards the catheter.
[0030] According to various embodiments, the one or more protection elements
proximal to
the ablation structure may include a raised bump coupled with the catheter.
The raised bump
may be configured to prevent the ablation structure from distending proximally
along the
catheter during deployment or insertion into the body lumen.
7
CA 02868603 2014-10-23
[0031] In certain instances, the one or more protection elements distal to the
ablation structure
may include a portion of the expansion member configured to bunch up when the
expansion
member is unexpanded such that a diameter of the bunched up portion exceeds a
diameter of the
furled ablation structure. The bunched up portion of the expansion member may
be configured
to prevent the ablation structure from distending distally along the catheter
during removal from
a body lumen.
[0032] In some embodiments, the one or more protection elements include a
first protection
element positioned distal to the ablation structure with respect to the
catheter and a second
protection element positioned proximal to the ablation structure with respect
to the catheter.
[0033] The foregoing has outlined rather broadly the features and technical
advantages of
examples according to the disclosure in order that the detailed description
that follows may be
better understood. Additional features and advantages will be described
hereinafter. The
conception and specific examples disclosed may be readily utilized as a basis
for modifying or
designing other structures for carrying out the same purposes of the present
disclosure. Such
equivalent constructions do not depart from the spirit and scope of the
appended claims.
Features which are believed to be characteristic of the concepts disclosed
herein, both as to their
organization and method of operation, together with associated advantages will
be better
understood from the following description when considered in connection with
the
accompanying figures. Each of the figures is provided for the purpose of
illustration and
description only, and not as a definition of the limits of the claims.
BRIEF DESCRIPTION OF THE DRAWING
[0034] A further understanding of the nature and advantages of the embodiments
may be
realized by reference to the following drawings. In the appended figures,
similar components or
features may have the same reference label. Further, various components of the
same type may
be distinguished by following the reference label by a dash and a second label
that distinguishes
among the similar components. If only the first reference label is used in the
specification, the
description is applicable to any one of the similar components having the same
first reference
label irrespective of the second reference label.
8
CA 02868603 2014-10-23
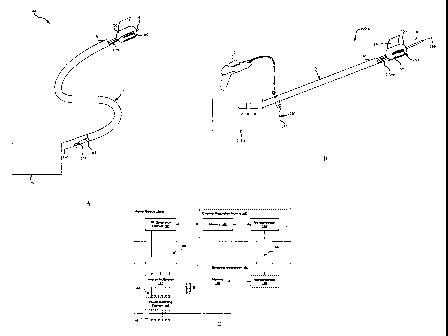
[0035] FIG. lA is a schematic diagram of a system for delivering treatment to
a target
treatment area including components configured according to various
embodiments.
[0036] FIG. 1B is schematic diagram of one specific embodiment of the system
shown in FIG.
1A.
[0037] FIG. 1C is a schematic diagram of a power source and a switching
mechanism of the
system shown in FIG. lA and FIG. 1B.
[0038] FIG. 2 is a schematic view of portions of an upper digestive tract in a
human.
[0039] FIG. 3A is a schematic view of an ablation device, in a
furled/collapsed mode, within
an esophagus.
[0040] FIG. 3B is a schematic view of an ablation device, in an
unfurled/expanded mode,
within an esophagus
[0041] FIG. 4 is a perspective view of an ablation device, in a
furled/collapsed mode, coupled
with multiple separately-wired longitudinal electrode zones.
[0042] FIG. 5 is a perspective view of an ablation device, in an
unfurled/expanded mode,
coupled with multiple separately-wired longitudinal electrode zones.
[0043] FIG. 6 is a perspective view of the partially unfurled ablation device
of FIG. 5.
[0044] FIG. 7 is a perspective view of the partially unfurled distal portion
of the ablation
device of FIG. 2 with a single spring and multiple longitudinal electrode
regions of uniform
widths.
[0045] FIG. 8 is a perspective view of the partially unfurled distal portion
of the ablation
device of FIG. 2 with a single spring and multiple longitudinal electrode
regions of uniform
narrow widths.
[0046] FIG. 9 is a perspective view of the partially unfurled distal portion
of the ablation
device of FIG. 2 with a single spring and multiple variable-length
longitudinal electrode regions
of successively decreasing widths.
9
CA 02868603 2014-10-23
[0047] FIG. 10A is a top cross-sectional view of an ablation structure of the
ablation device of
FIG. 2 in an unfurled/expanded mode with a partially overlapped electrode
region and fully
overlapped electrode regions.
[0048] FIG. 10B is a top cross-sectional view of an ablation structure of the
ablation device of
FIG. 2 in an unfurled/expanded mode with fully overlapped electrode regions.
[0049] FIG. 11 is a cross section view of a linear electrode zone array
pattern of the ablation
device of FIG. 2.
[0050] FIG. 12 is a schematic view of the electrode patterns of the ablation
device of FIG. 2.
[0051] FIG. 13 is a cross section view of an ablation structure of the
ablation device of FIG. 2.
[0052] FIG. 14 is a plan view of an ablation structure of the ablation device
of FIG. 2 with
variable-length springs.
[0053] FIG. 15 is a plan view of a slotted spring of the ablation structure of
FIG. 15.
[0054] FIG. 16A is a plan view of a tapered spring of the ablation structure
of FIG. 15.
[0055] FIG. 16B is a plan view of an ablation structure of the ablation device
of FIG. 2 with
tapered springs.
[0056] FIG. 17 is a plan view of a metal spring including a pinning hole of
the ablation
structure of FIG. 15.
[0057] FIG. 18 is a plan view of an ablation structure of the ablation device
of FIG. 2 with
pinned variable-length metal springs.
[0058] FIG. 19A is a perspective view of the ablation device of FIG. 4 in a
furled/collapsed
mode with proximal and distal protective cone elements.
[0059] FIG. 19B is a perspective view of the ablation device of FIG. 5 in an
unfurled/expanded
mode with proximal and distal protective cone elements.
100601 FIG. 20A is a perspective view of the ablation device of FIG. 4 in a
furled/collapsed
mode with a distal protective bumper element.
CA 02868603 2014-10-23
[0061] FIG. 20B is a perspective view of the ablation device of FIG. 5 in an
unfurled/expanded
mode with a distal protective bumper element.
[0062] FIG. 21 is a perspective view of the ablation device of FIG. 20B in an
unfurled/expanded mode with a tethered distal protective cone element.
[0063] FIG. 22 is a perspective view of the ablation device of FIG. 20B in an
expanded mode
with tethered proximal and distal protective cone elements.
[0064] FIG. 23A is a perspective view of the ablation device of FIG. 4 in a
furled/collapsed
mode with a proximal raised bump element.
[0065] FIG. 23B is a perspective view of the ablation device of FIG. 5 in an
unfurled/expanded
mode with a proximal raised bump element.
[0066] FIG. 24 is a perspective view of the ablation device of FIG. 4 in a
furled/collapsed
mode.
[0067] FIG. 25 is a flow diagram illustrating a method for providing treatment
to a target site
area according to various embodiments.
[0068] FIG. 26 is a flow diagram illustrating a method for providing treatment
to a target site
area according to various embodiments.
[0069] FIG. 27 is a flow diagram illustrating a method for providing treatment
to a target site
area using an expansion member including one or more protection elements
according to various
embodiments.
[0070] FIG. 28 is a flow diagram illustrating a method for providing treatment
to a target site
area using an expansion member including one or more movable protection
elements according
to various embodiments.
[0071] FIG. 29 is a flow diagram illustrating a method for providing treatment
to a target site
area according to various embodiments.
[0072] FIG. 30 is a flow diagram illustrating a method for providing treatment
to a target site
area using an expansion member including one or more protection elements
according to various
embodiments.
11
CA 02868603 2014-10-23
[0073] FIG. 31 is a flow diagram illustrating a method for providing treatment
to a target site
area using an expansion member including one or more protection elements
according to various
embodiments.
[0074] FIG. 32 is a flow diagram illustrating a method for providing treatment
to a target site
area using an expansion member including one or more tethered movable
protection elements
according to various embodiments.
DETAILED DESCRIPTION
[0075] Methods, systems, and devices are described for providing treatment to
a target site,
such as a site within a body lumen. Systems may include an expansion member
that may be
coupled with a distal portion of a catheter. An ablation structure may be
wrapped around the
expansion member such that expanding the expansion member may engage body
lumens of
varying sizes.
[0076] An ablation structure support coupled to an ablation structure may be
positioned at a
distal end of a catheter. The ablation structure may include a flexible
circuit capable of furling
and unfurling around an expansion member upon which it may be disposed.
Various aspects of
the flexible circuit may be similar to typical integrated circuits and
microelectronic devices. The
flexible circuit may include multiple separately wired or separately
controlled longitudinal
electrodes, longitudinal electrode zones, or both, aligned in parallel to an
axis about which the
ablation structure transitions between a furled configuration and an unfurled
configuration.
[0077] The ablation structure may include longitudinal electrodes of varying
widths,
longitudinal electrode zones of varying widths, or both. Each longitudinal
electrode or
longitudinal electrode zone may be selectively enabled or selectively
disabled. For purposes of
this application, enabling an electrode or electrode zone has the same meaning
as activating an
electrode or electrode zone. In some instances, the ablation structure
includes an electrode array,
such as, for example, a bipolar electrode array.
[0078] The ablation structure support may be coupled to one or more springs.
Springs may be
made of various materials such as, for example, a metallic material or a
polymer material. The
positional density of the springs relative to the ablation structure support
may vary such that
12
CA 02868603 2014-10-23
s
clawing effects are reduced at one or more ablation structure locations.
Spring density may be
varied by structures that include, for example, slotted springs, tapered
springs, and/or variable-
length springs.
[0079] One or more protective elements may be positioned along the catheter
distal to the
ablation structure and/or proximal to the ablation structure. Slidably movable
conical protection
elements may be positioned such that they cover the edges of the ablation
structure support,
preventing scraping of the lumen or ablation support structure distension
during insertion and
removal. The distal end of a tethering structure may be mounted to the conical
protection
elements such that the protection elements may be moved upon deployment of the
expansion
member, removing the cones from obstructing the furling and unfurling
transitions. In some
cases, a flexible distal protective bumper element less than the circumference
of the furled
ablation structure is coupled with the longitudinal edge of the distal lateral
portion of the ablation
structure. The furled ablation structure including the protective bumper
element may resemble
the familiar tubular shape of an endoscope. Additionally, the catheter may
include a raised bump
positioned proximal to the ablation structure configured to prevent distention
of the ablation
structure along the catheter during insertion of the ablation structure into a
body lumen.
Moreover, the expansion member may include a portion distal to the ablation
structure that
bunches up when the expansion member is unexpanded such that the bunched up
expansion
member material prevents distension of the ablation structure along the
catheter during removal
of the ablation structure from a body lumen.
[0080] With reference to FIG. IA, a general system 100 for delivering
treatment to a target
treatment area is shown in accordance with various embodiments. The system 100
may be
designed for providing treatment to a target area inside of a body, such as
the wall of an organ or
lumens in the gastrointestinal tract, for example. The system 100 may include
a power source
105, a catheter 115, and an expansion member 120. The expansion member 120 may
generally
be configured to support an ablation structure 160 that may be used to supply
therapy to the
target treatment site. The system 100 may operate by positioning a guide
assembly 165 inside a
body and passing the expansion member 120 over the guide assembly 165 such
that the
expansion member 120 may be delivered to a target treatment site inside the
body. The power
13
CA 02868603 2014-10-23
source 105 may then be used to supply power to an ablation structure 160
disposed on the
expansion member 120 so that therapy may be applied to the target treatment
site.
[0081] The expansion member 120 may be an inflatable device capable of
transitioning
between a collapsed or unexpanded configuration and an expanded configuration
with the use of
a supplementary expansion mechanism. Suitable expansion members 120 include
but are not
limited to a balloon, compliant balloon, balloon with a tapered geometry,
bladder, and the like.
In some embodiments, the power source 105 is configured to inflate the
expansion member 120
by, for example, incorporating the supplementary expansion mechanism
internally. The
collapsed configuration may be generally used when the expansion member 120 is
inserted into
and removed from the body lumen. When the expansion member 120 obtains a
desired ablation
position, the expansion member 120 may expand, such as by inflating from a
deflated state (i.e.
the collapsed configuration) to a substantially inflated state (i.e., the
expanded configuration).
[0082] The expansion member 120 may be configured to support an ablation
structure 160. In
some embodiments, the ablation structure 160 is a therapeutic or diagnostic
instrument, such as
an ablation element that may provide ablative energy to the target treatment
area. Some ablation
structures 160 may be designed so that they make direct contact with a target
treatment area,
including pressing of the ablation structure 160 against the target site.
[0083] The expansion member 120 may be coupled with the catheter 115 such that
the
expansion member 120 may be maneuvered through a channel of the body, such as
the
esophagus, and at the target treatment area. The catheter 115 may be coupled
with the power
source/inflation device 105 at the proximal end 145 of catheter 115. The
expansion member 120
may be positioned between the distal end 140 of the catheter 115 and a portion
150 of the
catheter 115. In some embodiments, the catheter 115 includes an opening 175
configured to
allow the entry and exit of the guide assembly 165 such that the catheter 115
is slidably movable
relative to the guide assembly 165. The guide assembly entry point 175 may
typically be located
outside of the catheter 115 and proximate the power source 105.
[0084] The power source 105 may provide power to the ablation structure 160
disposed on the
expansion member 120. In some embodiments, power is provided from the power
source 105 to
the ablation structure 160 via one or more transmission lines 170 extending
between the power
source 105 and the ablation structure 160 and housed within a channel of the
catheter 115.
14
. CA 02868603 2014-10-23
[0085] FIG. IB illustrates a system 100-a that may be an example of the system
100 shown in
FIG. lA according to various embodiments. The system 100-a may include a
generator 105-a, a
hand-held air compressor 112, a guide assembly 165 with a distal end 166 and a
proximal end
167, a catheter 115, an expansion member 120, an ablation structure 160,
and/or an ablation
structure support 180 coupled with the expansion member 120.
[0086] The expansion member 120 may include a balloon on which the ablation
structure
support 180 may be supported. The expansion member 120 may be a flexible
material capable
of being curved or folded that, when expanded, generally may have an elongated
cylindrical
shape, including a rounded distal end. The expansion member 120 may taper at
its proximal end
and couple with the catheter 115 near portion 150 of catheter 115.
[0087] Disposed on a portion of the surface of the expansion member 120 may be
an ablation
structure 160 configured to provide treatment to the target treatment area.
The ablation structure
160 may include a single electrode including multiple electrode zones or a
series of electrodes
169 laterally adjacent to one another and parallel to the longitudinal axis of
the ablation structure
160 and expansion member 120. The one or more electrodes 169 may be
interlaced, with
approximately half of the electrodes extending from a first bus and
approximately half of the
electrodes extending from a second bus. The first bus or the second bus may be
connected to a
positive terminal and the other of the first bus or the second bus may be
connected to a negative
or ground terminal to thereby provide a bipolar electrode configuration. When
connected to the
power source 105-a, the one or more electrodes 169 may provide ablative energy
to the target
treatment area.
[0088] The expansion member 120 may be coupled with a portion 150 of the
catheter 115 that
is proximate to the distal end 140 of catheter 115. The ablation structure
support 180 may be
furled at least partially around the outside circumference of the expansion
member 120 such that
when the expansion member 120 expands, the ablation structure support 180
adapts to the
changing circumference while the ablation structure 160 maintains a constant
electrode density
per unit area. A set of transmission wires 170-a may extend from the power
source 105-a to the
ablation structure 160 through the channel of the catheter 115. Zone
activation may be
controlled from the power source 105-a and/or from a switching printed circuit
board configured
to drive one or more additional channels.
CA 02868603 2014-10-23
[0089] With reference now to FIG. 1C, a power source 105-b is schematically
illustrated in
accordance with various embodiments. Power source 105-b may be an example of
power source
105 or 105-a described with reference to FIGS. lA or 1B. In general, the power
source 105-b
may include a power switching mechanism 190 which may be configured to switch
on and
switch off longitudinal electrode regions, thus controlling, at least in part,
the order, timing, and
duration of energy delivery at the treatment site. For example, the power
switching mechanism
190 may include an RF generation element 181, which may be configured to
transmit RF energy
on one or more output channels 186 to a power switching element 192 where such
power
switching element 192 then reroutes the RF energy 198 to multiple longitudinal
electrode
regions. In some implementations, the power switching mechanism 190 is an
external
component communicatively coupled to the power source 105-b and the catheter
115. In other
cases, the power switching mechanism 190 is integral to and/or attached to the
catheter 115. In
certain instances, the combined catheter 115 and switching mechanism 190
components are
single-use disposable components.
[0090] In some cases, the number of defined longitudinal electrode regions of
the ablation
structure 160 is less than or equal to the number of RF channels 186 supported
by the power
source 105-b, with each defined longitudinal electrode region coupled to a
single RF channel
186. In such a configuration, the switching mechanism 190 may be
communicatively coupled
with a channel regulation module 183 integrated with the power source 105-b.
The channel
regulation module 183 may include a microprocessor 184 and a memory 182. The
switching
mechanism 190 may also include a microprocessor 195 and a memory 194. The
channel
regulation module 183 may direct the switching mechanism 190 to either enable
or disable the
RF channel 186 associated with one or more electrode regions in accordance
with one or more
algorithms stored in memory 182. In some instances, the switching mechanism
190 may
communicate ablation parameters such as, for example, impedance to the power
source 105-b for
use in algorithmic determinations.
[0091] In certain implementations, the number of defined electrode regions
exceeds the
number of RF channels 186 supported by the power source 105-b. For example, an
RF
generation element 181 may support a maximum of 3 RF channels 186, where the
ablation
structure 160 (see e.g., FIG. 1) may include 6 separately-wired electrode
regions. In such cases,
16
CA 02868603 2014-10-23
,
the RF generation element 181 may be configured to transmit RF energy on only
one output
channel 186 to the power switching element 192, where such power switching
element 192 then
reroutes the RF energy to multiple longitudinal electrode regions.
Alternatively, the RF
generation element 181 may be configured to transmit RF energy over multiple
output channels
186 to an inverse multiplexer 191, where such inverse multiplexer 191 expands
the number of
channels 197 by, for example, re-routing the common return of the bipolar
system.
100921 Additionally, or alternatively, the power source 105-b may be
configured to transmit
RF energy across one or more channels 186 concurrently or in a defined
sequence. In some
embodiments, the switching mechanism 190 switches RF output channels 186 on or
off by
blocking the transmission from the RF generation element 181. The switching
mechanism 190
may include a power-switching element 192 such as, for example, a
metal¨oxide¨semiconductor
field-effect transistor or a relay. In some instance, an isolation element 193
is positioned
between the power switching element 192 and the logic element or
microprocessor 195 and
memory 194. In some instances, the channel regulation module 183 communicates
a
longitudinal electrode region activation sequence to the power switching
element 192 that either
blocks or allows RF transmission in accordance with the received sequence,
thus controlling the
activation, timing, and or duration of energy delivery at the longitudinal
electrode regions
associated with the RF channels 186. Additionally, or alternatively, the
switching mechanism
190 may determine the longitudinal electrode region activation sequence
independent of the
power source 105-b based, at least in part, on an algorithm stored in memory
194.
100931 In some instances, the switching mechanism 190 monitors current and/or
interprets
other signals communicated from the power source 105-b to determine, in part,
when to switch a
channel on or off. Additionally, or alternatively, the power source 105-b may
control the
switching behavior of the switching mechanism 190 via a one-way or two-way
communication
channel 185 coupling the power source logic element 184 and the switching
mechanism logic
element 195. In certain implementations, the power source 105-b may receive
feedback from the
switching mechanism 190, such as, for example, an acknowledgment that
switching instructions
were received and/or that the directed switching behavior was executed.
Communication
between the logic elements 184, 195 may implement an established communication
protocol
such as, for example, I2C or SPI.
17
CA 02868603 2014-10-23
[0094] The ablation of tissue may result in a variation to the impedance of
that tissue as
compared to unablated tissue. A probe sensor may be used to determine the
ablation condition
of regions of the circumferential treatment site by, for example, comparing
the impedance of a
region of a treatment site with previous impedance data for the same and/or
different regions of a
treatment site. This data may then be used to select the activation state
and/or activation duration
for one or more longitudinal electrode regions. It will be appreciated by one
skilled in the art
that these and other automated selection algorithms may be implemented on one
or more
communicatively coupled computer devices external to the power source 105-b.
For example,
additional computer software, such as image analysis software, may be used to
identify
previously ablated regions and/or overlapping electrode segments as part of an
algorithm that
regulates the activation and/or energy delivery profile of the associated
electrode regions.
[0095] Referring now to FIG. 2, certain disorders may cause the retrograde
flow of gastric or
intestinal contents from the stomach 212, into the esophagus 214, as shown by
arrows A and B.
Although the causes of these problems are varied, this retrograde flow may
result in secondary
disorders, such as Barrett's esophagus, which require treatment independent of
and quite
different from treatments appropriate for the primary disorder ¨ such as
disorders of the lower
esophageal sphincter 216. Barrett's esophagus is an inflammatory disorder in
which the stomach
acids, bile acids and enzymes regurgitated from the stomach and duodenum enter
into the lower
esophagus causing damage to the esophageal mucosa. When this type of
retrograde flow occurs
frequently enough, damage may occur to esophageal epithelial cells 218. In
some cases the
damage may lead to the alteration of the squamous cells, causing them to
change into taller
specialized columnar epithelial cells 220. This metaplastic change of the
mucosal epithelium
from squamous cells to columnar cells is called Barrett's esophagus. Although
some of the
columnar cells may be benign, others may result in adenocarcinoma.
[0096] In some embodiments, the methods, systems, and devices described are
configured to
treat columnar epithelium of selected sites of the esophagus through the
ablation of the tissue.
The term "ablation" as used herein means thermal damage to the tissue causing
tissue or cell
necrosis. It will be appreciated by one skilled in the art that some
therapeutic procedures may
have a desired treatment effect that falls short of ablation, such as, for
example, some level of
agitation or damage that may be imparted to the tissue to insure a desired
change in the cellular
18
CA 02868603 2014,-10-23
makeup of the tissue, rather than necrosis of the tissue. In some instances, a
variety of different
energy delivery devices are utilized to create a treatment effect in a
superficial layer of tissue,
while preserving intact the function of deeper layers, as described hereafter.
[0097] Cell or tissue necrosis may be achieved with the use of energy, such as
RF energy, at
appropriate levels to accomplish ablation of mucosal or submucosal level
tissue, while
substantially preserving muscularis tissue. Such ablation may be utilized to
remove the
columnar growths 220 from the portions of the esophagus 214 so affected.
[0098] Referring now to FIG. 3A and FIG. 3B, the expansion member 120 may be
inserted
into the body in any of various ways including, for example, guide assembly
165 placement,
endoscopic placement, surgery, or by other means. Expansion member 120 may be
an example
of expansion member 120 of FIG. lA and/or FIG. 1B. Referring now to FIG. 3A,
the expansion
member 120 is shown in a collapsed configuration in accordance with various
embodiments.
The expansion member 120 may be configured for transitioning between the
collapsed
configuration shown and an expanded configuration as shown in FIG. 3B. In the
expanded
configuration, at least one dimension of the expansion member 120 may have
increased. In
various embodiments, the expanded configuration is significantly larger than
the collapsed
configuration and allows the ablation structure 160 to contact a treatment
surface such as
columnar epithelial cells 220-a and/or 220-b. The ablation structure 160 may
be delivered to the
treatment site within the body lumen while in a collapsed state. This low-
profile configuration
may allow for ease-of-access to the treatment site without discomfort or
complications to the
patient. When an endoscope (not shown) is used, the portion 150 of catheter
115 may be
positioned along the outside of the endoscope. Alternately, an endoscope may
be used to
visualize the pathway that expansion member 120 should follow during
placement. The distal
end 166 of the guide assembly 165 may be positioned along the outside of an
endoscope and left
in the body lumen after removal of the endoscope. The proximal end 167 (see
e.g., FIG. 1B) of
the guide assembly 165 may be inserted into the distal end 140 of the catheter
115 and the
catheter 115 inserted into the esophagus following the path determined by the
guide assembly
165.
[0099] An ablation structure 160 may be provided and may be coupled to the
expansion
member 120 and positioned near portion 150 of catheter 115. In some instances,
the expansion
19
CA 02868603 2014:10-23
member 120 is bonded to the portion 150 of catheter 115. The ablation
structure 160 may
include one or more electrodes 169. The one or more electrodes 169 may be
arranged into
multiple longitudinal electrodes zones 161, 162 of equal or varying widths.
The one or more
electrodes 169 may be coupled to a power source 105 (see e.g., FIG. 1A)
configured for
powering the one or more electrodes and/or longitudinal electrode zones 161,
162 at levels
appropriate to provide the ablation of tissue to a predetermined depth of
tissue.
[0100] In some embodiments, the ablation structure 160 includes a flexible,
non-distensible
backing. For example, the ablation structure 160 may include a thin,
rectangular sheet of
polymer materials such as polyimide, polyester or other flexible thermoplastic
or thermosetting
polymer film. The ablation structure 160 may also include polymer covered
materials, or other
nonconductive materials. Additionally, the backing may include an electrically
insulating
polymer, with an electro-conductive material, such as copper, deposited onto a
surface so that an
electrode pattern may be etched into the material to create an electrode
array.
[0101] The ablation structure 160 may be operated in direct contact with the
wall of the tissue
site. This may be achieved by coupling the ablation structure 160 to an
expansion member 120,
which has a configuration that may be expandable in a shape that conforms to
the dimensions of
the inner lumen of the treatment site, such as the human lower esophageal
tract. The ablation
structure 160 may be positioned so that energy may be uniformly applied to the
inner
circumference of the lumen where treatment is desired. This may be
accomplished by first
positioning the expansion member 120 at the treatment site in a collapsed
configuration with the
ablation structure 160 furled around the expansion member 120. Once the
apparatus is advanced
to the appropriate site, the expansion member 120 may be expanded,
transitioning the ablation
structure 160 from a furled state to an unfurled state, thus engaging the
internal wall of the
lumen.
[0102] With reference to FIG. 3B, the ablation structure 160 uniformly engages
the inner wall
of the lumen with one or more electrode zones 161, 162 having a constant
density so that the
energy may be uniformly applied to all or a portion of the circumference of
the inner lumen of
the esophagus or other tissue site. An expansion member 120 may include, for
example, a
balloon, such as a compliant balloon and/or a balloon with a tapered geometry
that expands to an
expanded configuration when inflated.
CA 02868603 2014-10-23
[0103] In some embodiments, as the expansion member 120 expands and the
ablation structure
160 unfurls, additional electrodes or electrode zones 163 are exposed from
beneath an
overlapping portion 181 of the ablation structure 160. Selective enabling of
one or more
longitudinal electrodes 169 and/or longitudinal electrode zones 161, 163
allows the total surface
area of the ablation structure 160 to be divided, thus accommodating certain
power limitations of
a power source, and thereby providing appropriate energy density to the
tissue. The ablation
structure 160 may extend an arc length distance greater than the circumference
of the expansion
member 120 such that when the expansion member 120 expands, gapless
circumferential
ablation is effected for various sized body lumens.
[0104] The ablation structure 160 may be positioned and energy applied to the
inner
circumference of a body lumen treatment site. This may be accomplished by
first positioning the
expansion member 120 to the treatment site in a collapsed configuration. Once
the ablation
structure 160 is advanced to the appropriate treatment site, expansion member
120 may be
expanded, advancing the ablation structure 160 to engage the internal wall of
the body lumen.
The desired treatment energy may then be delivered to the tissue by
selectively enabling one or
more longitudinal electrodes and/or longitudinal electrode zones 161, 162,
163. In some
instances, all longitudinal electrodes and/or longitudinal electrode zones
161, 162, 163 are
sequentially enabled starting with the longitudinal electrode and/or
longitudinal electrode zone
161 adjacent the free or overlapping edge 181 of ablation structure 160. The
detection of a fully-
shielded longitudinal electrode and/or longitudinal electrode zone may stop
energy delivery to
additional longitudinal electrodes and/or longitudinal electrode zones in the
sequence as the
additional longitudinal electrodes and/or longitudinal electrode zones are not
in contact with the
tissue of the body lumen.
[0105] In certain embodiments, the ablation structure 160 delivers a variety
of different types
of energy including but not limited to, radio frequency, microwave,
ultrasonic, resistive heating,
chemical, a heatable fluid, optical including without limitation, ultraviolet,
visible, infrared,
collimated or non-collimated, coherent or incoherent, or other light energy,
and the like.
[0106] Referring now to FIG. 4, the distal portion of a general system 400 for
delivering
treatment to a target treatment area is shown in a collapsed configuration in
accordance with
various embodiments. System 400 may be an example of system 100 or 100-a
described with
21
CA 02868603 2014:10-23
reference to FIGS. lA or 1B and may include a catheter 115, an expansion
member 120 coupled
with the catheter 115, and an ablation structure 160 furled around the
expansion member 120.
To engage the inner surface of a body lumen that is larger than the collapsed
diameter of the
expansion member 120, expansion member 120 may be expanded (see e.g., FIG 5)
until the
desired pressure is exerted on the inside wall of the lumen. The expansion
member 120 may be
expanded such that pressure applied to the body lumen is less than a pressure
that would
otherwise result in damage such as, for example, by lacerating or perforating
the lumen.
Although the exposed surface area of the ablation structure 160 increases as
the expansion
member 120 expands, the individually and sequentially enabled electrodes or
electrode zones
may maintain a constant electrode density across the surface of ablation
structure 160. Energy,
including but not limited to an RF signal, delivered to the electrodes or
electrode zones may
provide a uniform treatment within each region to a precise depth of tissue.
After the treatment
has been administered, the expansion member 120 may be collapsed and the
system removed
from the body lumen.
[0107] In some embodiments, the ablation structure 160 includes a large single
electrode
divided into adjacent longitudinal electrode zones 161, 162 of either uniform
or varying widths,
configured to reduce the degree of ablation-region overlap and thus reduce the
degree of over
ablation. Longitudinal electrode zones 161, 162 may be selectively enabled via
multiple
transmission lines 170 extending between the power source 105 (see e.g., FIG.
1A) and the
longitudinal electrode zones 161, 162. The ablation structure 160 may include
an electrode array
163 etched on its surface, and may be aligned between the distal end 404 and
proximal end 402
of the expansion member 120.
[0108] Referring now to FIG. 5, the system 400 described with reference to
FIG. 4 is shown in
an expanded configuration in accordance with various embodiments. When the
expansion
member 120 is inflated or otherwise expanded, ablation structure 160 unfurls,
exposing
electrodes 504, 506. The longitudinal electrodes 502, 504, 506 of ablation
structure 160 may be
selectively enabled via multiple transmission lines 170 extending between the
power source 105
(see e.g., FIG. 1A) and the longitudinal electrodes 502, 504, 506. For
example, isolated
transmission wires 170-a, 170-b, 170-c may be individually wired to electrodes
or electrode
zones 502, 504, 506. The wires may be individually insulated with heat shrink
tubing along the
22
CA 02868603 2014-10-23
entire length of the catheter 115. Each of the wires may terminate to a mini
connector plug of
the power source 105 or a switching printed circuit board controller
configured to drive
transmission channels in addition to the channels of the power source 105.
Under this
configuration, power may be delivered to one or more electrodes or electrode
zones selectively
such that treatment may be administered to a specific area along the ablation
structure 160. The
switching printed circuit board may be included on the catheter 115, external
to the power source
105, or within the power source 105.
[0109] In some embodiments, the ablation structure 160 is configured such that
one or more of
the longitudinal electrodes or longitudinal electrode segments positioned at
the free end 181 of
the ablation structure 160 have a width greater than the width of the
remaining longitudinal
electrodes or longitudinal electrode segments nearer the bonded end (not
shown) of the ablation
structure 160. When a minimum lumen size may be anticipated, for example,
about 16 mm in
the case of an esophagus, the arc length corresponding to the circumference of
this minimum size
may be used as the width for one or more wider longitudinal electrodes or
longitudinal electrode
regions positioned adjacent to the free end 181 of the ablation structure 160.
For example, if the
maximum lumen size may be 37 mm, the total arc length of the one or more
electrodes may be
calculated as 37*pi=116 mm. In the case of a single electrode with multiple
longitudinal
electrode zones, the electrode area equal to the arc length of the minimum
lumen size (16*pi=50
mm) may be configured as two electrode regions with a width of 25 mm each. The
remaining
regions may include a set of narrow width electrode zones, for example, seven
electrode zones of
10 mm each. This may result in a reduced number of total regions as compared
to using a set of
fixed width regions, while continuing to provide narrow width regions for
potential areas of
ablation structure overlap.
101101 With reference now to FIG. 6, the distal portion of a general system
600 for delivering
treatment to a target treatment area is shown in a collapsed configuration in
accordance with
various embodiments. System 600 may be an example of systems 100, 100-a, or
400 described
with reference to FIGS. 1A, 1B, 4, or 5, and may include a catheter 115, an
expansion member
120 coupled with the catheter 115, and an ablation structure 160. The ablation
structure 160 is
shown in a partially unfurled configuration to illustrate the underside of
ablation structure 160.
The ablation structure 160 may be coupled with an ablation structure support
180 that is bonded
23
CA 02868603 2014710-23
,
,
or otherwise attached to the expansion member 120. For example, a first end
612 of the ablation
structure 160 may be bonded or otherwise attached to the expansion member 120.
An adhesive
bond may be implemented along the full length or partial length of the contact
zone between the
first end 612 of the ablation structure 160 and the expansion member 120. In
certain instances,
adhesion occurs when the expansion member 120 is in an expanded configuration.
The internal
surface of the ablation structure 160 may include four variable-width springs
602, 604, 606, 608
that extend perpendicularly from the bonded first edge 612 of the ablation
structure 160 and
perpendicular to the longitudinal axis of the expansion member 120, configured
such that
localized spring density increases linearly toward the free end 181 of the
ablation structure 160.
The ablation structure 160 may include a single electrode segregated into
longitudinal segments
502, 504, 506, 610 of one or more varying widths. The longitudinal electrode
segment 502
adjacent to the free edge 181 of the ablation structure 160 may have a width
greater than the
width of the other longitudinal electrode segments 504, 506, 610. Each
longitudinal electrode
segment 502, 504, 506, 610 may be coupled to a power source 105 (see e.g.,
FIG. 1A) by a
wiring structure isolated from the wiring structure for the other longitudinal
electrode segments
170-a, 170-b, 170-c, 170-d.
[0111] In some embodiments, an ablation structure 160 includes a large single
electrode
divided into adjacent longitudinal electrode zones of uniform width configured
to reduce the
degree of ablation-region overlap and thus reduce the degree of over ablation.
Such a uniform
width configuration may be useful when a minimum size lumen is not known, such
as when the
ablation system is used for a variety of different body lumens. With reference
now to FIG. 7,
the distal portion of a general system 700 for delivering treatment to a
target treatment area is
shown in a collapsed configuration in accordance with various embodiments.
System 700 may
be an example of systems 100, 100-a, 400, or 600 described with reference to
FIGS. 1A, 1B, or
4-6, and may include a catheter 115, an expansion member 120 coupled with the
catheter 115,
and an ablation structure 160. The ablation structure 160 is shown in a
partially unfurled
configuration to illustrate the underside of ablation structure 160. The
ablation structure 160
may be coupled with an ablation structure support 180 that is bonded or
otherwise attached to the
expansion member 120 along bonded edge 722. The underside surface of the
ablation structure
160 may include a single spring 720 extending from the bonded edge 722 of the
ablation
structure 160 perpendicular to the longitudinal axis of the expansion member
120. A single
24
CA 02868603 2014710-23
electrode may be segregated into longitudinal zones 702, 704, 706, 708, 710,
712, 714, 716, 718
of uniform widths. Each longitudinal electrode zone 702, 704, 706, 708, 710,
712, 714, 716, 718
may be coupled to a power source 105 (see e.g., FIG. 1A) by a wiring structure
isolated from the
wiring structure for the other longitudinal electrode segments 170-a, 170-b,
170-c, 170-d
(additional isolated wiring structures not shown).
10112] In some embodiments, an ablation structure 160 includes a large single
electrode
segregated into adjacent longitudinal electrode zones of uniform narrow width,
for example, with
width less than 10 mm, configured to reduce the degree of ablation-region
overlap and thus
reduce the degree of over ablation in those regions where overlap is present.
With reference now
to FIG. 8, the distal portion of a general system 800 for delivering treatment
to a target treatment
area is shown in a collapsed configuration in accordance with various
embodiments. System 800
may be an example of systems 100, 100-a, 400, 600, or 700 described with
reference to FIGS.
1A, 1B, or 4-7, and may include a catheter 115, an expansion member 120
coupled with the
catheter 115, and an ablation structure 160. The ablation structure 160 is
shown in a partially
unfurled configuration to illustrate the underside of ablation structure 160.
The ablation structure
160 may be coupled with an ablation structure support 180 that is bonded or
otherwise attached
to the expansion member 120 along bonded edge 812. The underside surface of
the ablation
structure 160 may include a single spring 820 extending from the bonded edge
812 of the
ablation structure 160 perpendicular to the longitudinal axis of the expansion
member 120. A
single electrode may be segregated into longitudinal zones 802, 804, 806, 808,
810 of uniform
widths. Each longitudinal electrode zone 802, 804, 806, 808, 810 may be
coupled to a power
source 105 (see e.g., FIG. 1A) by a wiring structure isolated from the wiring
structure for other
longitudinal electrode zones 170-a, 170-b, 170-c, 170-d (additional isolated
wiring structures not
shown).
[0113] In some embodiments, an ablation structure 160 includes a large single
electrode
segregated into adjacent longitudinal electrode zones of varying widths
configured to reduce the
degree of ablation-region overlap and thus reduce the degree of over ablation.
With reference
now to FIG. 9, the distal portion of a general system 900 for delivering
treatment to a target
treatment area is shown in a collapsed configuration in accordance with
various embodiments.
System 900 may be an example of systems 100, 100-a, 400, 600, 700, or 800
described with
CA 02868603 2014710-23
reference to FIGS. 1A, 1B, or 4-8, and may include a catheter 115, an
expansion member 120
coupled with the catheter 115, and an ablation structure 160. The ablation
structure 160 is shown
in a partially unfurled configuration to illustrate the underside of ablation
structure 160. The
ablation structure 160 may be coupled with an ablation structure support 180
that is bonded or
otherwise attached to the expansion member 120 along bonded edge 914. The
underside surface
of the ablation structure 160 may include one spring 916 extending from the
bonded edge 914 of
the ablation structure 160 perpendicular to the longitudinal axis of the
expansion member 120. A
single electrode may be segregated into longitudinal zones 902, 904, 906, 908,
910, 912 of
varying widths. The adjacent longitudinal electrode zones 912 extending from
the free edge 181
of the ablation structure 160 decrease in width linearly until reaching a
minimum width such as,
for example, 10 mm. All subsequent adjacent longitudinal electrode zones 902,
904, 906, 908,
910 may have a width equal to the minimum width. Each longitudinal electrode
segment 902,
904, 906, 908, 910, 912 may be coupled to a power source 105 (see e.g., FIG.
1A) by a wiring
structure isolated from the wiring structure for other longitudinal electrode
zones 170-a, 170-b,
170-c, 170-d (additional isolated wiring structures not shown).
[0114] Referring now to FIG. 10A and FIG. 10B, a cross sectional view of an
ablation
structure 160 and expansion member 120 is shown in accordance with various
embodiments.
The ablation structure 160 may be an example of the ablation structure 160
described in
connection with FIGS. 1A, 1B, 4 and/or 5. As shown, the ablation structure 160
may be attached
at a first end 1012 to the expansion member 120 (adhesion not shown). The free
end 181 of the
ablation structure 160 may be furled around the expansion member 120,
overlapping the bonded
end 1012 one or more times. As the expansion member 120 expands, the ablation
structure 160
unfurls and further exposes additional electrodes or electrode zones that had
previously been
shielded by the overlapping portion 181 of the ablation structure 160.
[0115] At any given inflation diameter, one or more electrodes or electrode
zones may be in
contact with a region of the treatment tissue and one or more electrode
segments may be in
contact with the insular backing of the ablation structure 160 or ablation
structure support 180.
Electrode traces may be bridged with conductive material, such as, for
example, saline, mucous,
or tissue coagulum. In the absence of mitigating structures or procedures,
these materials may
come in contact with the insular backing of the ablation structure 160. This
can drain the energy
26
CA 02868603 2014710-23
intended for tissue treatment both where a single electrode is used and/or
where ablation zones
are oriented circumferentially, thus reducing the intended energy delivery and
thereby reducing
the density/tissue ablation depth below a threshold level. In some
embodiments, each
longitudinal electrode or longitudinal electrode zone is separately
controlled, separately wired, or
both such that each longitudinal electrode or longitudinal electrode zone may
be unaffected by
the presence or absence of electrode regions covered by the ablation structure
and/or coated by
conductive matter such as fluids or tissue. The total energy delivered to each
longitudinal
electrodes or longitudinal electrode zone may be calculated based on the
longitudinal electrode's
or longitudinal electrode zone's total active surface area according to
ablation parameters
developed in the art such as, for example, the parameters used with ablation
catheter systems.
[0116] Often the free end 181 of the ablation structure 160 fully or partially
overlaps one or
more longitudinal electrode regions. When an electrode segment 1006 is only
partially exposed
(i.e., partially covered up with an overlapping portion 181 of the ablation
structure 160), as
shown in FIG. 10A, and therefore only in partial contact with a tissue
treatment area, energy
1002 may be delivered to the treatment area and energy 1004 may be delivered
to the backing of
the ablation structure 160, which may result in an energy delivery profile
that deviates from an
intended energy delivery profile, delivering an excessive amount of energy to
the treatment
tissue region. Configuring the longitudinal electrodes or longitudinal
electrode segments such
that the width of the inner-most partially exposed longitudinal electrode or
longitudinal electrode
zone does not exceed a predetermined percentage of the circumference of the
treatment area,
such as, for example, about twenty percent, may reduce this effect.
[0117] Various methods may be used to limit the extent to which energy may be
over-
delivered to tissue treatment areas in contact with partially shielded
longitudinal electrodes or
longitudinal electrode zones, such as electrode zone 1006 of FIG. 10A. A
channel regulation
module (see e.g., FIG. 1C) may include computer instructions configured to
enable each adjacent
longitudinal electrode or longitudinal electrode zone sequentially starting
with the longitudinal
electrode or longitudinal electrode zone 1008 adjacent to the free edge 181 of
the ablation
structure 160. Computer instructions may be further provided to execute
impedance
measurements and calculations such that the degree of ablation may be
controlled in real time. A
partially shielded longitudinal electrode or longitudinal electrode zone 1006
may often have
27
CA 02868603 2014710-23
higher impedance than unshielded longitudinal electrodes or longitudinal
electrode zones such as
zone 1010 shown in FIG. 10B. The impedance of each longitudinal electrode or
longitudinal
electrode zone may be compared to the impedance measurements obtained for
previous zones in
advance of enabling each longitudinal electrode or longitudinal electrode
zone. Detection of
higher impedance may be indicative of a partially occluded (e.g., 1006 of FIG.
10A) or a fully
occluded (e.g., 1007 of FIG. 10B) electrode or electrode zone. Computer
instructions may be
further provided to reduce ablation time and/or lower voltage delivered to the
partially/fully
occluded electrode or electrode zone 1006, 1007 as determined by the detection
of higher
starting impedance. Computer instructions may be further provided that cease
sequential
enablement of electrodes or electrode zones 1014, 1016 subsequent to the
detection of a first
partially or fully occluded electrode or electrode zone 1006, 1007. In
addition to having starting
impedance greater than the impedance of unshielded longitudinal electrodes or
longitudinal
electrode zones 1010, occluded electrodes or electrode zones 1006, 1007, may
often experience
an increased rate of change in impedance as compared to unshielded
longitudinal electrodes or
longitudinal electrode zones 1010. Computer instructions may be provided that
compare the
impedance change rate during ablation with the impedance change rate from
prior ablations in
the same patient or previous patients, where detection of a higher rate of
impedance change
indicates the currently enabled electrode or electrode zone may be partially
shielded. Computer
instructions may be further provided to reduce ablation time and/or lower the
voltage delivered
to the occluded electrode or electrode zone.
101181 Referring now to FIG. 11A through FIG. 11C, the electrode patterns may
be varied
depending on the length of the site to be treated, the depth of the mucosa and
submucosa, in the
case of the esophagus, at the site of treatment, and other factors. The
electrode patterns 1102 ¨
1208, may be examples of electrode patterns included with the electrode array
163 of FIG. 4 and
FIG. 5. An electrode array pattern may be composed of particular electrode
elements that may
be arranged in various configuration, such as, for example, a circumferential
orientation or a
longitudinal orientation. An electrode element is a conductive element of an
electrode array. In
some instances, electrode elements are aligned parallel to one another. The
density of the
electrode elements may affect the depth of an ablation treatment. The
longitudinal electrode or
longitudinal electrode zone patterns may be aligned in an axial or transverse
direction across the
one or more electrodes, or formed in a linear or non-linear parallel matrix or
series of bipolar
28
CA 02868603 2014-10-23
=
pairs or monopolar electrodes. One or more different patterns may be coupled
to various
locations of the ablation structure 160. For example, an electrode array, as
shown in FIG. 11A
through FIG. 11C, may comprise a pattern of bipolar axial interlaced finger
electrodes 1102,
monopolar rectangles 1104 with 1 mm separation, or six bipolar rings 1106 with
2 mm
separation. Other suitable RF electrode patterns may be used including,
without limitation, those
patterns shown in FIG. 12A through FIG. 12D. Patterns may include, for
example, bipolar axial
interlaced finger electrodes 1202 with 0.3 mm separation, monopolar bands 1204
with 0.3 mm
separation, bipolar rings 1208 with 0.3 mm separation, and/or undulating
electrodes 1206 with
0.2548 mm separation.
[0119] The depth of treatment may be controlled by the selection of
appropriate treatment
parameters by the operator as described in the examples set forth herein. One
parameter that
may affect the depth of treatment is the density of electrode elements. As the
spacing between
electrode elements decreases, the depth of treatment of the affected tissue
also decreases when
RF energy is delivered in bipolar fashion across the electrodes. Very close
spacing of the
electrode elements may limit the current and resulting ohmic heating to a
shallow depth such that
injury and heating of the submucosal layer are minimized. For treatment of
esophageal tissue
using RF energy, it may be desirable to have a spacing between adjacent
electrode elements be
no more than, (i) 3 mm, (ii) 2 mm, (iii) 1 mm (iv) 0.5 mm or (v) 0.3 mm (vi)
0.1 mm and the
like.
[0120] In various embodiments, the dimensions of the electrodes and spacing
between the
electrode elements are selected to enable controlled depth ablation. Examples
of electrode
configurations for controlled depth ablation are described in U.S. Patent Nos.
6,551,310 (Ganz et
al.), 7,150,745 (Stern et al.), 7,344,535 (Stern et al.), 7,530,979 (Ganz et
al.), 7,993,336 (Jackson
et al.), 8,012,149 (Jackson et al.), 8,192,426 (Stern et al.), 8,439,908
(Utley et al.), and 8,398,631
(Ganz et al.), the entire contents of each which are incorporated herein for
all purposes. In
various embodiments, the power generator and/or a channel regulation module
(see e.g., FIG.
1C) are configured to control the application of energy using the ablation
structure 160 to effect
ablation of tissue to a controlled depth.
[0121] Although described in terms of an electrode array for RF ablation,
those skilled in the
art will appreciate that the ablation structure suitable for use with the
embodiments described
29
CA 02868603 2014-10-23
herein may be configured for administering other forms of therapy or
diagnosis. For example,
the techniques described above may be applied to form an antenna for microwave
ablation. In
another example, the operative member may include sensor elements overlaying
the expandable
support device. Monopolar RF configurations may also be used in some
embodiments. Some
embodiments may utilize bipolar RF configurations.
[0122] In various embodiments, the ablation structures described herein are
ablation devices,
and in some embodiments, RF ablation devices. In various embodiments, the
ablation structures
described herein are configured for thermal ablation. In some embodiments, the
ablation
structures described herein are configured to heat surrounding tissue by
resistive heating or
conduction. Embodiments of ablation structures described herein may be
configured to treat or
diagnose the surrounding tissue by other modalities.
[0123] In various embodiments, the ablation structures described herein are
configured for
ablation of abnormal tissue in the esophagus. In some instances, the ablation
structures
described herein are configured for ablation of abnormal tissue in the lower
esophageal
sphincter. In certain implementations, the ablation structures described
herein are configured for
ablation of Barrett's esophagus and/or pre-cancerous tissue in the epithelium
without injuring the
underlying muscalaris. In some embodiments, the ablation structures described
herein are
configured for use in a variety of body lumens and organs including, but not
limited to, the
gastrointestinal (GI) tract (e.g. the esophagus or duodenum), the alimentary
tract, the digestive
system (e.g. the bile duct), the cardiovascular system, the endocrine system
(e.g. the pancreas),
and the respiratory system.
[0124] In various embodiments, the ablation structures described herein are
configured to
ablate tissue to a predetermined depth. In some cases, the ablation structures
described herein
are configured to ablate mucosal tissue without injuring the underlying
submucosal tissue. In
certain instances, the ablation structures described herein are configured to
ablate mucosal tissue
without injuring the underlying muscalaris. In some implementations, the
ablation structures
described herein are configured to apply the appropriate level of energy to
the tissue to achieve
an ablation depth that does not extend beyond the submucosa layer of the
esophagus. In some
embodiments, the ablation structures described herein are configured to
control the depth of
ablation to the epithelium. In some instances, the ablation structures
described herein are
CA 02868603 2014-10-23
configured for superficial ablation. For example, various embodiments of an
ablation structure
may be configured to sear the tissue surface. In certain cases, the ablation
structures described
herein are configured to deliver sufficient energy to initiate regrowth of
tissue, for example, in a
muco sal layer.
[0125] Controlling the depth of ablation may be based on several factors such
as power and
treatment time. In various embodiments, the power source activates the
longitudinal electrodes
or longitudinal electrode zones with sufficient power and for a sufficient
amount of time to
ablate tissue to a predetermined depth. In an exemplary embodiment, the power
source activates
one or more longitudinal electrodes or longitudinal electrode zones with
sufficient power and for
a length of time necessary to deliver between about 1 Esq.-cm and about 50
J/sq.-cm, between
about 10 J/sq.-cm and about 40 J/sq.-cm, between about 15 J/sq.-cm and about
105 J/sq.- cm,
between about 25 J/sq.-cm and about 105 J/sq.-cm, between about 30 J/sq.-cm
and about 105
J/sq.-cm, between about 35 Esq.-cm and about 105 J/sq.-cm, or between about 40
J/sq.-cm and
about 105 J/sq.-cm. Other energy per unit area amounts may be utilized in some
embodiments.
[0126] In various embodiments, the power source 105-a (see e.g., FIG. 1B) may
be configured
to deliver between about 10 Watts/sq.-cm and about 50 Watts/sq.-cm, between
about 10
Watts/sq.-cm and about 40 Watts/sq.- cm, between about 10 Watts/sq.-cm and
about 30
Watts/sq.-cm, between about 15 Watts/sq.-cm and about 30 Watts/sq.-cm, or
between about 15
Watts/sq.-cm and about 40 Watts/sq.-cm. Other energy per unit area amounts may
be utilized in
some embodiments.
[0127] In some instances, the power source 105-a is configured to activate the
longitudinal
electrodes or longitudinal electrode zones for between about 10 ms and about 5
minutes, between
about 100 ms and about 1 minute, between about 100 ms and about 30 seconds,
between about
10 ms and about 1 second, between about 100 ms and about 1 second, or between
about 300 ms
and about 800 ms. In certain embodiments, the power generator is configured to
activate the
electrodes for less than 1 second, less than 500 ms, or less than 300 ms. In
some
implementations, the power source is configured to deliver about 40 W/sq.-cm
for a duration of
about 300 ms to about 800 ms. In some embodiments, the power source is
configured to deliver
between about 12 J/sq.-cm to about 15 J/sq.-cm for a duration of about 300 ms
to about 800 ms.
Other energy per unit area amounts and time amounts may be utilized in some
embodiments.
31
CA 02868603 2014-10-23
[0128] In certain cases, the ablation structure support 180 is spirally furled
about a longitudinal
axis of the expansion member 120. The electrode pattern may be aligned in
axial or traverse
direction across the backing, formed in a linear or non-linear parallel array
or series of bipolar
pairs, or other suitable pattern. Referring now to FIG. 13, a cross sectional
view of an expansion
member 120, an ablation structure support 180, and an ablation structure 160
is shown in
accordance with various embodiments. In some embodiments, the ablation
structure support 180
includes a flexible, non-distensible backing. For example, the backing may
include a thin,
rectangular sheet of a polymer material such as polyimide, polyester or other
flexible
thermoplastic or thermosetting polymer film, polymer covered materials, or
other nonconductive
materials. The backing may also include an electrically insulating polymer,
with an electro-
conductive material, such as copper, deposited onto a surface. The ablation
structure 160 may be
formed from a metallic layer 1310 that may be etched to include a pattern of
electrodes using any
known technique, such as etching using masks.
[0129] One or more constant force springs 1304, 1306 may be attached to a
flexible backing
1302 by application of an adhesive substance 1308. A polytetrafluoroethylene
film may be
etched on one side and adhered to the spring side of the ablation structure
160. The entire
ablation structure 160 may be laminated as a flat sheet, providing for
friction reduction during
the transition between furled configurations and unfurled configurations, thus
reducing the
pressure required to transition to an unfurled configuration. Other methods of
constructing an
ablation structure 160 and ablation structure support 180 may also be
utilized. In some
instances, the ablation structure support 180 includes memory shape polymer
springs made from
polymer thermoplastics such as, for example, amorphous thermoplastic
polyetherimide or
organic polymer thermoplastic from the polyaryletherketone family. The entire
laminated
ablation structure support 180 may be heat treated to achieve a persistent
final spiral
conformation. In an alternate embodiment, metallic springs, such as springs
made from surgical
stainless steel, may be used. The metallic springs are set in coils prior to
lamination such that a
persistent spiral conformation may be retained.
[0130] Referring now to FIG. 14 through FIG. 17, various structures for
localized reduction of
spring density are shown in accordance with various embodiments. With
reference now to FIG.
14, four springs of varying lengths 1402, 1404, 1406, 1408, are attached to
the electrically
32
CA 02868603 2014-10-23
insulated inside surface of the ablation structure support 180. The ablation
structure support 180
may be an example of the ablation structure support 180 of FIGS. 1B, 6, 7,
8,9, and/or 13. The
variable-length springs 1402, 1404, 1406, 1408 are arranged such that the
distal ends 1410, 1412
of one or more of the springs 1404, 1406 are not coterminous with the free end
181 of the
ablation structure support 180. In some embodiments, a set of four springs
includes two longer
springs 1402, 1408 of equal length, one spring 1406 of shorter length, and one
spring 1404 of a
length less than the length of the longer springs 1402, 1408, but greater than
the length of the
shorter spring 1406. The spring density near the free end 181 of the ablation
structure support
180 may be half that of the spring density near the bonded end 1415. In some
instances, the
spring density declines linearly from the bonded end 1415 to the free end 181
of the ablation
structure support 180. Spring density at or near the free end 181 of the
ablation structure support
180 may be directly related to the degree of clawing exhibited, where clawing
refers to the
tendency of the free end 181 of the ablation structure support 180 to return
towards the initial
spring radius.
[0131] With reference now to FIG. 15 through FIG. 17, localized spring density
reduction may
be obtained by reducing the amount of material at one or more locations along
one or more
springs. The spring 1506, 1602, 1702 may be examples of one or more of the
springs described
with reference to FIGS. 6-9, and/or 13-14. For example, referring to FIG. 15,
a slot 1504 may
be included at a distal portion of spring 1506, reducing the clawing force of
the spring 1506 at or
near the slotted region 1504. With reference to FIG. 16A, in some instances,
localized spring
density reduction is accomplished by tapering the distal portion 1604 of the
spring 1602.
Material may also be removed from the spring by techniques such as, for
example, hole
punching. Referring to FIG. 17, in some instances, one or more holes 1704 are
punched at or
near the distal end of one or more springs 1702. One or more of these
techniques (i.e., variable
width springs, tapered springs, slotted springs, and hole punched springs) may
be combined such
that a particular localized spring density is achieved.
[0132] Referring now to FIG. 16B, in certain embodiments, multiple polymeric
springs 1600-
a, 1600-b, each with a tapered portion, may be attached to the electrically
insulated internal
surface of the ablation structure support 180. The ablation structure support
180 may be an
example of the ablation structure support 180 of FIGS. 1B, 6, 7, 8, and/or 9.
The springs 1600-a,
33
CA 02868603 2014710-23
1600-b may be arranged such that the first set of springs 1600-a are aligned
adjacent to one
another with the ends of the tapered portions 1606 abutting the free end of
the ablation structure
support 181, and the second set of springs 1600-b are aligned adjacent to one
another with the
ends of the tapered portions 1608 abutting the mounted end of the ablation
structure support
1610. In some instances, the ends of the non-tapered spring portions 1612 of
the first set of
springs 1600-b are joined with ends of the non-tapered portion 1614 of the
second set of springs
1600-b. In some embodiments, the location where the non-tapered ends join 1616
is at or near a
middle portion of the ablation structure support 180. The spring density near
the free end 181
and the mounted end 1610 of the ablation structure support 180 may each be
less than half the
spring density at or near the middle portion 1616 of the ablation structure
support 180. In some
instances, the spring density declines linearly in the direction of the free
end 181 and the
mounted end 1610 of the ablation structure support 181. In certain
embodiments, the tapered
portions 1608 of each of springs 1600-b taper to a defined density and extend
to the mounted end
1610 of the ablation structure support 180 at the defined density. Spring
density at or near the
free end 181 and mounted end 1610 of the ablation structure support 180 may be
directly related
to the degree of clawing exhibited.
101331 Referring now to FIG. 18, an ablation structure support 180 including
metal springs
1802, 1804, 1806 is shown in accordance with various embodiments. The three
metal springs
1802, 1804, 1806 shown may each include a hole 1814, 1816, 1818 at the distal
end of the
springs near the free edge 181 of the ablation structure support 180. The
metal springs 1802,
1804, 1806 may be examples of one or more springs 602, 604, 606, 608 of FIG.
6, 720 of FIG. 7,
820 of FIG. 8, 916 of FIG. 9, 1304, 1305 of FIG. 13, and/or 1402, 1404, 1406,
1408 of FIG. 14.
Fixture pins 1808, 1810, 1812 may pass through the spring holes 1814, 1816,
1818 and may each
have a shaft circumference less than the spring holes 1814, 1816, 1818 and, in
some instances, an
external head circumference greater than the internal circumference of the
spring holes 1814,
1816, 1818. The fixture pins 1808, 1810, 1812 may pass through the spring
holes 1814, 1816,
1818 such that the ablation structure support 180 may be stretched into a flat
configuration
during lamination procedures. Such procedures are generally not utilized for
shape memory
polymer springs that are heat treated subsequent to lamination.
34
CA 02868603 2014710-23
[0134] Referring now to FIG. 19A and FIG. 19B the distal portion of a general
system 1900
for delivering treatment to a target treatment area is shown in a collapsed
configuration (FIG.
19A) and in an expanded configuration (FIG. 19B) in accordance with various
embodiments.
System 1900 may be an example of systems 100, 100-a, 400, 600, 700, 800, or
900 described
with reference to FIGS. 1A, 1B, or 4-9, and may include a catheter 115, an
expansion member
120 (shown in FIG. 19B only) coupled with the catheter 115, and an ablation
structure 160.
Conical protection elements 1902, 1904 may be positioned along the catheter
115 at the lateral
edges 1906, 1908 of the ablation structure 160. The conical structures 1902,
1904 may be made
of, for example, a flexible smooth polymer such as a high-density
polyethylene. The larger
openings 1910, 1912 of the conical structures 1902, 1904 are positioned facing
the lateral edges
1906, 1908 of the ablation structure 160. The circumference at the large-
diameter ends 1910,
1912 of the conical structures 1902, 1904 may be greater than the
circumference of the collapsed
ablation structure 160 such that the entire edge 1906, 1908 of the ablation
structure 160 may be
insertable in the larger openings 1910, 1912 of the conical structures 1902,
1904, thus preventing
distention of the ablation structure 160 during removal and/or scraping of the
lumen during
insertion. The small-diameter end 1914 of the conical structure 1904 may have
an internal
circumference slightly greater than the external circumference of the distal
portion 140 of the
catheter 115 such that the conical structure 1904 may be slidably movable
along the distal
portion 140 of the catheter 115. In some embodiments, the small diameter end
1916 of the
conical structure 1902 has an internal circumference less than the external
circumference of
portion 150 of catheter 115 such that the conical structure 1902 may not be
slidably movable
along the portion 150 of catheter 115. Referring now to FIG. 19B, the distal
portion of system
1900 for delivering treatment to a target treatment area is shown in an
expanded configuration in
accordance with various embodiments. The conical structure 1904 may be moved
slightly away
from the ablation structure 160 such that the ablation structure 160 unfurls
in response to the
expansion of the expansion member 120 without being obstructed by the conical
structures 1902,
1904.
[0135] Referring now to FIG. 20A and FIG. 20B, the distal portion of a general
system 2000
for delivering treatment to a target treatment area is shown in a collapsed
configuration (FIG.
20A) and in an expanded configuration (FIG. 20B) in accordance with various
embodiments.
System 2000 may be an example of systems 100, 100-a, 400, 600, 700, 800, 900,
or 1900
CA 02868603 2014710-23
described with reference to FIGS. 1A, 1B, 4-9, or 19 and may include a
catheter 115, an
expansion member 120 coupled with the catheter 115, and an ablation structure
160. A bumper
overhang structure 2002 may be bonded to a portion of the distal lateral edges
2004 of the
ablation structure 160. The bumper overhang structure 2002 may be made of a
highly flexible
material such that the structure does not significantly impede the unfurling
of the ablation
structure 160 in response to the expansion of the expansion member 120. The
overhang bumper
structure 2002 may consist of an arcuate portion 2008 coplanar with, and
bonded to, the surface
of the ablation structure 160, and multiple adjacent trapezoid shaped
structures 2006 extending
perpendicularly from the arcuate portion 2008 radially towards the distal
portion 140 of the
catheter 115. The arc length of the arcuate portion 2008 may be such that a
sufficient portion of
the edge 2004 of the ablation structure 160 may be covered to prevent
distention during removal
and/or scraping of the lumen during insertion.
[0136] Referring now to FIG. 21, the distal portion of a general system 2100
for delivering
treatment to a target treatment area is shown in an expanded configuration in
accordance with
various embodiments. System 2100 may be an example of systems 100, 100-a, 400,
600, 700,
800, 900, 1900, or 2000 described with reference to FIGS. 1A, 1B, 4-9, 19 or
20 and may
include a catheter 115, an expansion member 120 coupled with the catheter 115,
and an ablation
structure 160. In some embodiments, a tethering structure 2102 extends
internally all or a
portion of the length of the catheter 115, out through distal portion 140,
with the distal end 2106
of the tether 2102 attached to the distal, slidably movable, protection
element 1904-a. The
conical protection element 1904-a may be an example of the conical protection
element 1904
described in connection with FIG. 19. The proximal end 2110 of the tethering
structure 2102
extends out an opening 165 near the proximal portion 145 of the catheter 115
such that an
operator can manipulate the tether 2102 and control the re-positioning of the
slidably movable
protection element 1904-a.
[0137] With reference now to FIG. 22, the distal portion of a general system
2200 for
delivering treatment to a target treatment area is shown in an expanded
configuration in
accordance with various embodiments. System 2200 may be an example of systems
100, 100-a,
400, 600, 700, 800, 900, 1900, 2000, or 2100 described with reference to FIGS.
1A, 1B, 4-9, or
19-21 and may include a catheter 115, an expansion member 120 coupled with the
catheter 115,
36
CA 02868603 2014-10-23
and an ablation structure 160. In some embodiments, a tethering structure 2202
extends
externally all or part of the length of the of the proximal portion of the
catheter 115 with the
distal end 2204 of the tether 2202 attached to the proximal, slidably movable,
protection element
1902-a. The conical protection element 1902-a may be an example of the conical
protection
element 1902 described with reference to FIG. 19. The tethering structure 2202
may be secured
to the catheter 115 by, for example, one or more ring shaped structures 2206
mounted to the
catheter 115 where such ringed structures 2206 have an internal circumference
slightly greater
than the circumference of the tethering structure 2202 allowing the tethering
structure 2202 to
slide freely in the one or more rings 2206. The distal end 2204 of the
tethering structure 2202
may be accessible to the operator at the proximal portion 145 (not shown) of
the catheter 115
such that an operator can manipulate the tether 2202 and control the re-
positioning of the
slidably movable protection element 1902-a.
[0138] With reference to FIG. 23A, the distal portion of a general system 2300
for delivering
treatment to a target treatment area is shown in a collapsed/furled
configuration in accordance
with various embodiments. System 2300 may be an example of systems 100, 100-a,
400, 600,
700, 800, 900, 1900, 2000, 2100, or 2200 described with reference to FIGS. 1A,
1B, 4-9, or 19-
22 and may include a catheter 115, an expansion member 120 coupled with the
catheter 115, and
an ablation structure 160. As shown, in some embodiments, a raised bump 2305
is coupled with
the catheter 115 and positioned near the proximal end 2310 of the expansion
member 120. The
raised bump 2305 may be made from any suitable polymeric material and may be
adhered to or
otherwise attached to the catheter 115. The raised bump 2305 may be configured
to prevent
proximal distension of ablation structure 160 along catheter 115 during
insertion of the ablation
structure 160 into a body lumen such as the esophagus. Accordingly, the height
of the raised
bump 2305 may be sufficiently large such that it protrudes away from catheter
115 further than
the ablation structure 160 when the expansion member 120 is in a collapsed or
unexpanded
configuration. The raised bump 2305 may be an example of a protection element
as described
with reference to FIGS. 19-22. Referring to FIG. 23B, the expansion member 120
and ablation
structure 160 illustrated in FIG. 23A is shown in an expanded/unfurled
configuration in
accordance with various embodiments.
37
CA 02868603 2014-10-23
,
[0139] With reference to FIG. 24, the distal portion of a general system 2400
for delivering
treatment to a target treatment area is shown in a collapsed/furled
configuration in accordance
with various embodiments. System 2400 may be an example of systems 100, 100-a,
400, 600,
700, 800, 900, 1900, 2000, 2100, 2200, or 2300 described with reference to
FIGS. 1A, 1B, 4-9,
or 19-23 and may include a catheter 115, an expansion member 120 coupled with
the catheter
115, and an ablation structure 160. As shown, the expansion member 120 may
include a
bunched up portion 2405 positioned near the distal end 140 of the catheter
115. The bunched up
portion 2405 of expansion member 120 may be configured to prevent distension
of the ablation
structure 160 in a distal direction along the catheter 115 while the ablation
structure 160 is being
removed from a body lumen. Accordingly, the bunched up portion 2405 may be
configured to
have an average diameter that is larger than the diameter of the ablation
structure 160 in an
unexpanded or collapsed configuration. The bunched up portion 2405 may be an
example of a
protection element as described with reference to FIGS. 19-22.
[0140] The expansion member 120 may be modified in a variety of ways to create
the bunched
up portion 2405. For example, the expansion member 120 may be designed to have
a steep taper
angle on the distal end of the expansion member 120. The steep taper angle
will cause the distal
portion of the expansion member 120 to bunch up while unexpanded, thus forming
a bunched up
portion 2405. Additionally or alternatively, the expansion member 120 may
include multiple
layers of material near the distal end to form the bunched up portion 2405.
Accordingly, the
distal portion of the expansion member 120 may be thicker than the rest of
expansion member
120. The multiple layers of expansion member 120 may be thermally fused
together or may be
joined through adhesives or mechanical fastening elements.
[0141] For example, in various embodiments the expansion member 120 is a
balloon formed
from a two-step blow-molding process. The first step of the blow-molding
process may include
forming a first balloon and then cutting off the distal end of the balloon.
This cut-off portion
may be then added back into the balloon mold while a second balloon is being
formed. In
particular, the cut-off portion is placed in the mold such that it overlaps
the distal portion of the
second balloon as it is being formed. By overlapping the cut-off portion of
the first balloon with
the distal portion of the second balloon, the distal portion of the second
balloon will be thicker
than the rest of the balloon material. Accordingly, when the balloon is in a
deflated or
38
CA 02868603 2014-.10-23
unexpanded state, the distal portion may form a bunched up portion 2405 due to
the excess
material. It may be appreciated that the size of the bunched up portion 2405
may be tailored by
modifying the taper angle of the distal portion of expansion member 120 in
addition to the
number and thickness of additional layers of material near the distal portion
of expansion
member 120.
[0142] With reference to FIG. 25, a general method 2500 of using various
embodiments of the
systems and/or devices described herein is shown in accordance with various
embodiments. For
example, method 2500 may be implemented utilizing the various embodiments of
system 100,
power source 105, hand-held compressor 112, expansion member 120, ablation
structure 160,
ablation structure support 180, protection elements 1902, 1904, 2002 and/or
other devices and/or
components. At block 2505, the ablation structure 160 coupled with the
ablation structure
support 180 and the expansion member 120 may be inserted into the body lumen.
The ablation
structure 160 coupled with the ablation structure support 180, in combination,
may unfurl in
response to the expansion of the expansion member 120, and furl in response to
the contraction
of the expansion member 120. A guide assembly 165 may be used such that the
expansion
member 120 may be passed over the guide assembly 165 delivering the ablation
structure 160 to
a target treatment area inside the body lumen.
[0143] At block 2510, the expansion member 120 may be expanded such that the
ablation
structure 160 coupled with the ablation structure support 180, in combination,
unfurl and engage
a circumferential section of the body lumen. In some instances, the expansion
member 120
includes a compliant balloon. In some embodiments, the power source 105 and/or
the hand-held
compressor 112 may be used to expand the expansion member 120.
[0144] At block 2515, energy may be delivered through the ablation structure
160 to first part
of a circumferential treatment area of the body lumen. In some embodiments,
the ablation
structure 160 includes two or more longitudinal electrodes or longitudinal
electrode zones of
varying widths. In some embodiments, the ablation structure 160 includes two
or more
longitudinal electrodes or longitudinal electrode zones configured to be
selectively enabled or
selectively disabled. In certain instances, the ablation structure 160
includes a bipolar electrode
array.
39
CA 02868603 2014-.10-23
,
101451 With reference to FIG. 26, a general method 2600 of using various
embodiments of the
systems and/or devices described herein is shown in accordance with various
embodiments.
Method 2600 may be an example of method 2500 described with reference to FIG.
25. For
example, blocks 2605, 2610, and 2615 may be examples of the methods described
in blocks
2505, 2510, and 2515 of method 2500. Furthermore, at block 2620, the expansion
member 120
may be contracted such that the ablation structure 160 coupled with the
ablation structure support
180 including one or more springs, in combination, furls and disengages a
circumferential
section of the body lumen. For example, method 2600 may be implemented
utilizing the various
embodiments of system 100, power source 105, hand-held compressor 112,
expansion member
120, ablation structure 160, ablation structure support 180, springs 1506,
1602, 1702, protection
elements 1902, 1904, 2002, and/or other devices and/or components. In some
instances, the
expansion member 120 includes a compliant balloon. In some embodiments, a
vacuum is used
to fully contract the expansion member 120. In some instances, the one or more
springs include
a constant force spring.
101461 With reference to FIG. 27, a general method 2700 of using various
embodiments of the
systems and/or devices described herein is shown in accordance with various
embodiments.
Method 2700 may be an example of method 2500 described with reference to FIG.
25. For
example, blocks 2705, 2710, and 2715 may be examples of the methods described
in blocks
2505, 2510, and 2515 of method 2500. Furthermore, at block 2720, one or more
protection
elements may be utilized during the insertion of the ablation structure
coupled with the ablation
structure support 180 and the expansion member 120 into the body lumen. For
example, method
2700 may be implemented utilizing the various embodiments of system 100, power
source 105,
hand-held compressor 112, expansion member 120, ablation structure 160,
ablation structure
support 180, springs 1506, 1602, 1702, protection elements 1902, 1904, 2002,
and/or other
devices and/or components. One or more protection elements are positioned at
the distal portion,
proximal portion or both of the ablation structure 160. These protection
elements may, for
example include conical shaped structures, bumper shaped structures, a raised
bump 2305
coupled with the catheter 115, or a portion 2405 of the expansion member 120
that is bunched
up, each positioned such that they prevent distension of the ablation
structure 160 and/or prevent
the leading edge of the ablation structure from scraping the lumen wall during
insertion. In some
CA 02868603 2014-10-23
embodiments of the method, one or more of the protection elements are moved
away from the
ablation structure after the ablation structure is positioned.
[0147] With reference to FIG. 28, a general method 2800 of using various
embodiments of the
systems and/or devices described herein is shown in accordance with various
embodiments.
Method 2800 may be an example of method 2500 described with reference to FIG.
25. For
example, blocks 2805, 2810, and 2815 may be examples of the methods described
in blocks
2505, 2510, and 2515 of method 2500. Furthermore, at block 2820, one or more
protection
elements are moved distally from the lateral edge of the ablation structure
160 subsequent to
positioning the ablation structure 160 in the body lumen. For example, method
2800 may be
implemented utilizing the various embodiments of system 100, power source 105,
hand-held
compressor 112, expansion member 120, ablation structure 160, ablation
structure support 180,
springs 1506, 1602, 1702, protection elements 1902, 1904, tethers 2102, 2202,
and/or other
devices and/or components. Moving one or more protection elements after
positioning of the
ablation structure 160 facilitates unobstructed unfurling of the ablation
structure 160 coupled
with the ablation structure support 180, in combination, while continuing to
prevent distension of
the ablation structure 160 and/or preventing the leading edge of the ablation
structure 160 from
scraping the lumen wall during insertion.
[0148] With reference to FIG. 29, a general method 2900 of using various
embodiments of the
systems and/or devices described herein is shown in accordance with various
embodiments.
Method 2900 may be an example of method 2500 described with reference to FIG.
25. For
example, blocks 2905, 2910, and 2915 may be examples of the methods described
in blocks
2505, 2510, and 2515 of method 2500. Furthermore, at block 2920, an impedance
level may be
determined for each longitudinal electrode or longitudinal electrode zone. For
example, method
2900 may be implemented utilizing the various embodiments of system 100, power
source 105,
hand-held compressor 112, expansion member 120, ablation structure 160,
ablation structure
support 180, springs 1506, 1602, 1702, protection elements 1902, 1904, 2002,
and/or other
devices and/or components. In some instances, measurements are taken
sequentially prior to
enabling a given longitudinal electrode or longitudinal electrode zone,
starting with the
longitudinal electrode or longitudinal electrode zone adjacent to the free
edge of the ablation
structure. In another embodiment, the rate of impedance change is measured
during ablation. In
41
CA 02868603 2014-10-23
some instances, at block 2925, the starting impedance of the current
longitudinal electrode or
longitudinal electrode zone in the sequence of electrodes or electrode zones
is compared to
previously obtained impedance starting impedance levels for prior longitudinal
electrodes or
longitudinal electrode zones in the sequence of electrodes or electrode zones.
In some instances,
the rate of impedance change during ablation for the current longitudinal
electrode or
longitudinal electrode zone is compared to previously obtained impedance
change rates for the
current patient and/or from previous patients.
[0149] With reference to FIG. 30 a general method 3000 of using various
embodiments of the
systems and/or devices described herein is shown in accordance with various
embodiments. For
example, method 3000 may be implemented utilizing the various embodiments of
system 100,
expansion member 120, ablation structure 160, ablation structure support 180,
protection
elements 1902, 1904, 2002 and/or other devices and/or components. At block
3005, the ablation
structure 160 coupled with the ablation structure support 180 and the
expansion member 120 are
inserted into the body lumen. The one or more protection elements may be
positioned at the
distal portion, proximal portion or both of the ablation structure 160. These
protection elements
may, for example, include conical shaped structures, overhanging bumper shaped
structures, a
raised bump 2305 coupled with the catheter 115, or a portion 2405 of the
expansion member 120
that is bunched up, each positioned such that they prevent distension of the
ablation structure 160
and/or prevent the leading edge of the ablation structure 160 from scraping
the lumen wall
during insertion. In certain instances, the ablation structure 160 includes a
furled bipolar
electrode array.
[0150] At block 3010, the expansion member 120 may be expanded such that the
ablation
structure 160 coupled with the ablation structure support 180, in combination,
may unfurl and
engage a circumferential section of the body lumen. In some instances, the
expansion member
120 includes a compliant balloon. In some embodiments, the power source 105
and/or the hand-
held compressor 112 are used to expand the expansion member 120.
[0151] With reference to FIG. 31, a general method 3100 of using various
embodiments of the
systems and/or devices described herein is shown in accordance with various
embodiments.
Method 3100 may be an example of method 3000 described with reference to FIG.
30. For
example, blocks 3105 and 3110 may be examples of the methods described in
blocks 3005, and
42
CA 02868603 2014-10-23
3010 of method 3000. Furthermore, at block 3115, one or more protection
elements may be
displaced distally from the lateral edge of the ablation structure 160
subsequent to positioning the
ablation structure in the body lumen. For example, method 3100 may be
implemented utilizing
the various embodiments of system 100, expansion member 120, ablation
structure 160, ablation
structure support 180, protection elements 1902, 1904 and/or other devices
and/or components.
Moving of one or more protection elements after positioning of the ablation
structure 160
facilitates unobstructed unfurling of the ablation structure 160 coupled with
the ablation structure
support 180, in combination, while continuing to prevent distension of the
ablation structure 160
and/or preventing the leading edge of the ablation structure from scraping the
lumen wall during
insertion.
[0152] With reference to FIG. 32, a general method 3200 of using various
embodiments of the
systems and/or devices described herein is shown in accordance with various
embodiments.
Method 3200 may be an example of method 3000 described with reference to FIG.
30. For
example, blocks 3205 and 3210 may be examples of the methods described in
blocks 3005, and
3010 of method 3000. Furthermore, at block 3215, one or more protection
elements may be
moved relative to the ablation structure 160 through the use of one or more
tethering structures
coupled to one or more of the protective elements. For example, method 3200
may be
implemented utilizing the various embodiments of system 100, expansion member
120, ablation
structure 160, ablation structure support 180, protection elements 1902, 1904,
tethers 2102, 2202,
and/or other devices and/or components. In some embodiments, the tethering
structures extend
upward from the protection elements along the length of the catheter such that
an operator may
manipulate the tether and control the re-positioning of one or more of the
protective elements.
[0153] The foregoing description provides examples, and is not intended to
limit the scope,
applicability or configuration of the various embodiments. Rather, the
description and/or figures
provide those skilled in the art with an enabling description for implementing
various
embodiments. Various changes may be made in the function and arrangement of
elements.
[0154] Thus, various embodiments may omit, substitute, or add various
procedures or
components as appropriate. For instance, it should be appreciated that the
methods may be
performed in an order different than that described, and that various steps
may be added, omitted
or combined. Also, aspects and elements described with respect to certain
embodiments may be
43
CA 02868603 2014-10-23
combined in various other embodiments. It should also be appreciated that the
following
systems, methods, and devices, may individually or collectively be components
of a larger
system, wherein other procedures may take precedence over or otherwise modify
their
application.
[0155] The foregoing descriptions of specific embodiments have been presented
for purposes
of illustration and description. They are not intended to be exhaustive or to
limit the invention to
the precise forms disclosed, and obviously many modifications and variations
are possible in
light of the above teaching. The embodiments were chosen and described in
order to explain the
principles of the various embodiments and its practical application, to
thereby enable others
skilled in the art to utilize the various embodiments with various
modifications as are suited to
the particular use contemplated. It is intended that the scope of the various
embodiments be
defined by the Claims appended hereto and their equivalents.
44