Note: Descriptions are shown in the official language in which they were submitted.
CA 2,869,302
CPST Ref: 11508/00003
1 SYSTEM AND METHOD FOR TREATING WATER
2
3 TECHNICAL FIELD
4 [0001] The presently disclosed embodiments are directed to a
system and method for
treating produced and flow back water and wastewater from processes such as
desalting associated
6 with recovery of crude oil and natural gas from reservoirs and the
preparation of water to be used
7 for enhance oil recovery (EOR) and other requirements such as desalting
and hydrofracturing.
8 BACKGROUND
9 [0002] Petroleum, also commonly referred to as oil, consists of
a complex mixture of
hydrocarbons of various molecular weights, plus other organic compounds.
Petroleum is a
11 naturally occurring liquid found in rock formations. It is generally
accepted that oil is formed
12 mostly from the carbon rich remains of ancient plankton after exposure
to heat and pressure in the
13 Earth's crust over hundreds of millions of years gradually transforming
into oil and natural gas
14 reservoirs. Petroleum is a vital component of the world's supply of
energy as a source of providing
heating and electricity. It is also used as fuel for vehicles when refined,
and as a chemical feedstock
16 in the manufacture of plastics and other commercially important organic
chemicals. Worldwide
17 consumption of oil is approximately thirty billion barrels (4.8 km3) per
year, with developed
18 nations being the largest consumers. For example, the United States
consumed about 25% of the
19 oil produced in 2007. Petroleum is found in deep underground natural
rock formations and may
be associated with other hydrocarbons such as natural gas.
21 [0003] Oil reservoirs may be located deep within the Earth's
crust. As recovery
22 technology advances, oil recovery methods are being performed in deeper
locations within the
23 Earth, most notably in offshore and deep ocean locations. For example,
deep ocean drilling rigs
24 are now drilling in water depths at or in excess of 2,000 meters.
Similarly, there is much activity
at land based locations.
26 [0004] Oil recovery may take a variety of forms and methods.
For example, once a
27 reservoir is identified, an oil well is created by drilling a long hole
into the Earth. A steel pipe,
28 known as a casing, is placed in the hole to provide structural integrity
to the newly drilled well
29 bore. Holes are then made in the base of the well to enable oil to pass
into the bore, which oil is
then removed by various methods. Typically, recovered oil includes various
other secondary
CPST Doc: 316977.2 1
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 byproducts such as natural gas, inorganic compounds and water associated
with it. As wells
2 mature, various techniques are employed to extract as much oil as
possible. These techniques are
3 commonly referred to as enhanced oil recovery (EOR). One of these
techniques injects treated
4 water into a reservoir to displace the oil. This technique requires that
the water is of specific
quality which necessitates treatment prior to injection. Another technology
being used to recover
6 previously unrecovered oil is hydraulic fracturing. This is a technique
used to create fractures in
7 rock with a hydraulic fluid, typically water with additives, under high
pressure to release trapped
8 hydrocarbons.
9 [0005] Crude primary treatment techniques may comprise
multistep processes. For
example, a technique may include first separating byproducts from raw crude
oil, followed by
11 desalting the crude oil. The byproducts and the raw crude are separated
in a device called a
12 separator or dehydrator which removes water. There are several types of
separators depending on
13 the feed stream and the separation objectives. Crude oil, natural gas,
produced water, bottom
14 sludge which is typically sand, and other inert compounds are separated.
The oil is then washed
with water to remove the salts that are trapped within the crude oil, i.e.,
desalting. The washing
16 removes salts and generates a wastewater stream that contains dissolved
salts, suspended material,
17 oil, benzene, ethylbenzene, toluene and xylenes (BETX), and in some
cases heavy metals.
18 [0006] Typical crude oil separation methods generate
substantial quantities of waste. Such
19 systems can generate from as little as 5,000 barrels per day (BPD) to
upwards of 300,000 BPD.
Waste water is generated from the water associated with the recovered
hydrocarbons as well as
21 water used to desalt the crude oil. The characterization of the water
will vary according to its
22 content. As oil and gas production wells mature, there is an increased
percentage of produced
23 water being generated. Produced water limits the capacity of crude oil
transportation by corroding
24 conveyance systems.
[0007] Regulations related to discharge of produced water vary by the
authorities involved
26 and the location receiving the waste stream. Table 1 represents typical
waste water stream and
27 associated regulatory discharge levels.
28
Constituent PPM Range (unless Typical Discharge limits ¨ PPM
otherwise noted) (unless otherwise noted)
Free Oil & grease 100 ¨ 1000 <15
CPST Doc: 316977.2 2
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
BOD 100 ¨ 2000 150
COD 1000 ¨ 5000 Varies
Temperature 25 ¨ 200 deg. C 40 deg. C
Hydrogen Sulfide 0 ¨ 100
Ph 6 ¨ 8 Varies
TDS 50,000 ¨ 300,000 Varies ¨ platform deep ocean
dilution
T SS 100 ¨ 1000 150
Ammonia 0 ¨ 100
Sulfates 500 ¨ 5000
Heavy Metals 10 ¨200 Varies by metal < 1.5
Silica 100 ¨ 2000
Sodium 30,000+
Chloride 30,000+
Hardness 1000+
Iron 10 ¨ 100
Mercury 1 ¨ 10 0.01
Oxygen 5-10
1
2 Table 1
3
4 [0008] As the amount of crude oil recovered increases and
additional water based
enhanced techniques are used, the amount of produced water generated also
increases, thereby
6 creating serious environmental challenges to be addressed. Issues such as
the contamination of
7 water ways such as stream, lakes, groundwater with water containing, oil,
grease, hydrocarbons,
8 metals, etc., must be prevented, some of which contaminants result in
increased levels of chemical
9 oxygen demand (COD) and biochemical oxygen demand (BOD). Moreover,
typical produced
water is extremely high in total dissolved solids (TDS), sometime ten times
that of sea water. TDS
11 can destroy streams, lakes and groundwater by raising salinity levels.
Furthermore, EOR
12 techniques consume large quantities of water. For example, recovery of
hydrocarbons consumes
13 substantial quantities of fresh water for production activities. As oil
recovery activities from
14 reservoirs mature and EOR activities increase, scarce water resources
are taxed at an increasing
rate. Hydrofracturing activities require water treated to specific criteria.
Once the well is fracked,
16 there is substantial water that is removed from the well. This is called
flowback and must be
17 treated in a similar fashion to produced water.
CPST Doc: 316977.2 3
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 [009] Various known methods of treating produced water are
presently utilized. For
2 example, produced water is sent through separate conveyance lines or
combined with oil and
3 transported to shore for treatment. Additionally, produced water is
injected back into deep wells;
4 however, this sometimes results in the water reentering the oil reserves
thereby creating further
problems. Moreover, produced water is treated with conventional technologies
that are large,
6 heavy and generate substantial quantities of sludge while consuming large
amounts of chemicals.
7 Often the resulting sludge is not recoverable into commercial products
and must be disposed of in
8 a land fill.
9 [0010] Water used for desalting must meet certain critical
parameters set within the
industry. Typically the water must be free of suspended solids and low in
oxygen, typically below
11 50 PPB. Depending on the chemical makeup of the crude oil and the amount
of salt contamination
12 the total dissolved solids must be controlled to certain maximum
concentrations. Light crude
13 typically needs wash water with less than 35,000 PPM, while heavy crude
typically need wash
14 water with less than 1,000 PPM. Further processing of the water may
occur as need for preparing
water used for EOR, hydraulic fracturing or other high purity applications.
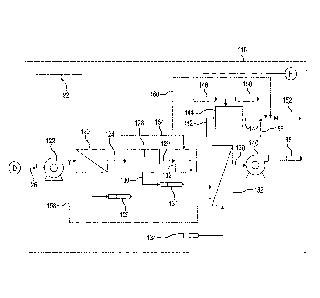
16 [0011] Figure 6 depicts a known system configuration for a
treating water supply 200, e.g.,
17 a fresh water source or a salt water source, for subsequent use for
desalting, EOR and
18 hydrofracturing operations. In this context, the system is described as
if it is arranged on a water
19 based oil platform. Water supply 200 passes through strainer 202 thereby
separating a portion of
the suspended solids from water supply 200 to form water stream 204. Separated
portion of
21 suspended solids 206 is transferred to a platform solids thickening and
handling system via output
22 208. Water stream 204 passes through crude filter 210 thereby further
separating a portion of the
23 suspended solids from water stream 204 to form water stream 212. Crude
filter 210 is a common
24 multimedia, disposable media such as cartridge filters, or sand filter
capable of handling separation
of bulk solids above 10 microns. Separated portion of suspended solids 214 is
transferred to a
26 platform solids thickening and handling system via output 208. Next,
water stream 212 passes
27 through ultrafine filter 216 thereby further separating a portion of
suspended solids from water
28 stream 212 to form water stream 218. Ultrafine filter 216 discharges
secondary stream 220 which
29 can be discharged directly into the original water source, e.g., ocean
or lake, or alternatively to
solids handling operations. Typically ultrafine filters of the type used in
known systems, require
CPST Doc: 316977.2 4
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 constant cleaning with chemicals, e.g., chemicals introduced via cleaning
system 222. Cleaning
2 chemicals of these types cannot be directly discharged and must be
treated or processed onshore.
3 The suspended solids collected at output 208 are transported to shore for
disposal or further
4 processing.
[0012] After filtration of water supply 200, water stream 218 passes to
tower degasifer 224
6 for removal of oxygen. Tower degasifers are well known in the art and may
include multiple
7 stages of packing. Water is trickled through tower 224 while a vacuum is
drawn on the tower via
8 vacuum pump 226 to force oxygen out of water stream 218 to form water
stream 228. Typically,
9 a tower of this type can reduce oxygen levels to less than 50 parts per
billion. To improve tower
224 efficiency, nitrogen generator 230 may feed nitrogen to the tower.
Moreover, oxygen
11 scavenger feed 232 may introduce a chemical scavenger, e.g., sodium
sulfite, to reduce oxygen to
12 less than 10 parts per billion.
13 [0013] The foregoing options for treating produced water suffer
from the various defects
14 described above, e.g., expensive, complex, difficult to clean, etc. The
present system and method
for treating produced water provides a variety of benefits that have
heretofore been lacking in
16 known systems. For example, the present system and method recovers
hydrocarbons for
17 commercial value while treating the water for total suspended solids (TS
S), oil, metals, H2S, BOD
18 and other undesirable components. The present invention is sufficiently
flexible to treat different
19 produced water streams and can accommodate changes in those streams that
may occur during
operation. The present system has a small foot print and minimum weight. The
present system
21 and method generates minimum secondary waste and solids while being
simple and easy to
22 operate. The present invention requires minimum consumables and
chemicals while producing
23 treated water of a quality that allows for reuse or discharge. The
present invention provides water
24 for EOR, desalting, hydrofracturing and other production activities
wherein the water is treated for
the removal of contaminants such as sulfates, barium, boron, total dissolved
solids, suspended
26 solids, H2S and oxygen, and agents such as biocides are added to prevent
sulfate reducing bacteria
27 from reducing sulfates to hydrogen sulfide (H2S), for example as need in
EOR use. The present
28 invention provides secondary waste streams from EOR operations that meet
or exceed discharge
29 standards.
SUMMARY
CPST Doc: 316977.2 5
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 [0014] Broadly, the present invention discussed infra provides
a system adapted to
2 condition an initial water feed stream into a treated water stream, the
initial water feed stream
3 including at least one of: a plurality of particles; a sulfate; a
hardness; a dissolved solid; and, an
4 oxygen. The system includes a water cleaning subsystem adapted to treat
the initial water feed
stream to remove the plurality of particles to form a filtered water stream,
and a water reuse
6 subsystem adapted to treat the filtered water stream to remove the
sulfate, the hardness, the
7 dissolved solid and/or the oxygen to form the treated water stream. The
water reuse system
8 includes a sulfate and hardness removal membrane unit adapted to treat
the filtered water stream
9 to remove the sulfate and the hardness, and a high pressure reverse
osmosis unit adapted to treat
the filtered water stream to remove the dissolved solids.
11 [0015] In some embodiments, the water cleaning subsystem
includes at least one of: a
12 gross particle filter adapted to treat the initial water feed stream to
remove the plurality of particles;
13 and, a fine particle filter adapted to treat the initial water feed
stream to remove the plurality of
14 particles. In some embodiments, the water reuse subsystem includes at
least one of: a first oxygen
removal unit adapted to treat the filtered water stream to remove the oxygen;
a second oxygen
16 removal unit adapted to treat the filtered water stream to catalytically
remove the oxygen; and, an
17 oxygen scavenger feeder adapted to blend an oxygen scavenger and the
filtered water stream. In
18 some embodiments, the water reuse subsystem includes the second oxygen
removal unit, wherein
19 the second oxygen removal unit includes a vessel containing a palladium-
doped resin adapted to
catalytically remove the oxygen. In some embodiments, the initial water feed
stream includes
21 ocean water, a fresh water source and/or a pre-treated produced water.
22 [0016] Furthermore, the present invention broadly provides a
system adapted to condition
23 an initial water feed stream into a treated water stream, the initial
water feed stream includes at
24 least one of: a plurality of particles; a sulfate; a hardness; a
dissolved solid; and, an oxygen. The
system includes a water cleaning subsystem adapted to treat the initial water
feed stream to remove
26 the plurality of particles to form a filtered water stream, and a water
reuse subsystem adapted to
27 treat the filtered water stream to remove the sulfate, the hardness, the
dissolved solid and/or the
28 oxygen to form the treated water stream. The water reuse system includes
a first oxygen removal
29 unit adapted to treat the filtered water stream to remove the oxygen,
and a second oxygen removal
unit adapted to treat the filtered water stream to catalytically remove the
oxygen.
CPST Doc: 316977.2 6
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 [0017] In some embodiments, the water cleaning subsystem
includes at least one of: a
2 gross particle filter adapted to treat the initial water feed stream to
remove the plurality of particles;
3 and, a fine particle filter adapted to treat the initial water feed
stream to remove the plurality of
4 particles. In some embodiments, the water reuse subsystem includes at
least one of: a sulfate and
hardness removal membrane unit adapted to treat the filtered water stream to
remove the sulfate
6 and the hardness; a high pressure reverse osmosis unit adapted to treat
the filtered water stream to
7 remove the dissolved solids; and, an oxygen scavenger feeder adapted to
blend an oxygen
8 scavenger and the filtered water stream. In some embodiments, the second
oxygen removal unit
9 includes a vessel containing a palladium-doped resin adapted to
catalytically remove the oxygen.
In some embodiments, the initial water feed stream comprises ocean water, a
fresh water source
11 and/or a pre-treated produced water.
12 [0018] Still yet further, the present invention broadly
provides a system adapted to
13 condition an initial water feed stream into a treated water stream, the
initial water feed stream at
14 includes least one of: a plurality of particles; a sulfate; a hardness;
a dissolved solid; and, an
oxygen. The system includes a water cleaning subsystem adapted to treat the
initial water feed
16 stream to remove the plurality of particles to form a filtered water
stream, and a water reuse
17 subsystem adapted to treat the filtered water stream to remove the
sulfate, the hardness, the
18 dissolved solid and/or the oxygen to form the treated water stream. The
water reuse system
19 includes a sulfate and hardness removal membrane unit adapted to treat
the filtered water stream
to remove the sulfate and the hardness, a high pressure reverse osmosis unit
adapted to treat the
21 filtered water stream to remove the dissolved solids, a first oxygen
removal unit adapted to treat
22 the filtered water stream to remove the oxygen, and a second oxygen
removal unit adapted to treat
23 the filtered water stream to catalytically remove the oxygen.
24 [0019] In some embodiments, the water cleaning subsystem
includes at least one of: a
gross particle filter adapted to treat the initial water feed stream to remove
the plurality of particles;
26 and, a fine particle filter adapted to treat the initial water feed
stream to remove the plurality of
27 particles. In some embodiments, the water reuse subsystem further
includes an oxygen scavenger
28 feeder adapted to blend an oxygen scavenger and the filtered water
stream. In some embodiments,
29 the second oxygen removal unit includes a vessel containing a palladium-
doped resin adapted to
CPST Doc: 316977.2 7
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 catalytically remove the oxygen. In some embodiments, the initial water
feed stream includes
2 ocean water, a fresh water source and/or a pre-treated produced water.
3 [0020] Still further, the present invention broadly provides a
system adapted to condition
4 an initial water feed stream into a treated water stream, the initial
water feed stream including at
least one of: a sulfate; a hardness; a dissolved solid; and, an oxygen. The
system includes a sulfate
6 and hardness removal membrane unit adapted to treat the initial water
feed stream to remove the
7 sulfate and the hardness, a high pressure reverse osmosis unit adapted to
treat the initial water feed
8 stream to remove the dissolved solids, a first oxygen removal unit
adapted to treat the initial water
9 feed stream to remove the oxygen, and a second oxygen removal unit
adapted to treat the initial
water feed stream to catalytically remove the oxygen.
11 [0021] In some embodiments, the initial water feed stream
further includes a plurality of
12 particles and the system includes at least one of: a gross particle
filter adapted to treat the initial
13 water feed stream to remove the plurality of particles; and, a fine
particle filter adapted to treat the
14 initial water feed stream to remove the plurality of particles. In some
embodiments, the system
further includes an oxygen scavenger feeder adapted to blend an oxygen
scavenger and the initial
16 water feed stream. In some embodiments, the second oxygen removal unit
includes a vessel
17 containing a palladium-doped resin adapted to catalytically remove the
oxygen. In some
18 embodiments, the initial water feed stream includes ocean water, a fresh
water source and/or a pre-
19 treated produced water.
[0022] Broadly, the present invention discussed infra provides a system
adapted to
21 condition an initial water feed stream into a treated water stream and
to discharge the treated water
22 stream. The initial water feed stream includes at least one of: a
plurality of particles; an oil; a
23 volatile organic compound; a hydrogen sulfide; a non-volatile compound;
a heavy metal; and, a
24 dissolved ion. The system includes a particle and oil removal subsystem
adapted to treat the initial
water feed stream to remove the plurality of particles and the oil to form a
first partial treated water
26 stream, a chemical oxygen demand reduction subsystem adapted to treat
the first partial treated
27 water stream to remove the volatile organic compound, the hydrogen sulfide
and/or the non-
28 volatile organic compound to form a second partial treated water stream,
and further includes a
29 heavy metal and dissolved ion removal subsystem adapted to treat the
second partial treated water
stream to remove the heavy metal and the dissolved ion to form a treated water
stream.
CPST Doc: 316977.2 8
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 [0023] In some embodiments, the particle and oil removal
subsystem includes at least one
2 of: a gross particle filter adapted to treat the initial water feed
stream to remove the plurality of
3 particles; an oil coalescer unit adapted to treat the initial water feed
stream to remove the oil; a fine
4 particle filter adapted to treat the initial water feed stream to remove
the plurality of particles; and,
an oil removal membrane unit adapted to treat the initial water feed stream to
remove the oil. In
6 some embodiments, the chemical oxygen demand reduction subsystem includes
at least one of: a
7 stripping unit adapted to treat the first partial treated water stream to
remove the volatile organic
8 compound and the hydrogen sulfide and to form a vapor phase comprising
the volatile organic
9 compound and the hydrogen sulfide; and, a hydrocarbon polishing unit
adapted to treat the first
partial treated water stream to remove the non-volatile organic compound. In
some embodiments,
11 the chemical oxygen demand reduction subsystem includes the stripping
unit, and further includes
12 at least one of: a bio scrubber unit adapted to metabolize the volatile
organic compound and the
13 hydrogen sulfide of the vapor phase; and, a flare or a thermal oxidizer
adapted to combust the
14 volatile organic compound and the hydrogen sulfide of the vapor phase.
In some embodiments,
the heavy metal and dissolved ion removal subsystem includes at least one of:
a heavy metal and
16 dissolved ion removal unit adapted to treat the second partial treated
water stream to adsorb the
17 heavy metal and the dissolved ion and to form a plurality of adsorbed
heavy metals and a plurality
18 of adsorbed dissolved ions; a heavy metal and dissolved ion
precipitation unit adapted to
19 precipitate the plurality of adsorbed heavy metals as a plurality of
insoluble metal hydroxides and
the plurality of adsorbed dissolved ions as a plurality of insoluble
compounds; and, a filter press
21 adapted to form at least one cake comprising the plurality of insoluble
metal hydroxides and the
22 plurality of insoluble compounds.
23 [0024] In some embodiments, the present invention system is
further adapted to prepare
24 the treated water stream for an enhanced oil recovery operation, the
treated water stream including
at least one of: a sulfate; a hardness; a dissolved solid; and, an oxygen. In
those embodiments, the
26 system further includes a water reuse subsystem adapted to treat the
treated water stream to remove
27 the sulfate, the hardness, the dissolved solid and/or the oxygen to form
an enhanced oil recovery
28 feed stream. In some embodiments, the water reuse subsystem includes at
least one of: a sulfate
29 and hardness removal membrane unit adapted to treat the treated water
stream to remove the sulfate
and the hardness; a high pressure reverse osmosis unit adapted to treat the
treated water stream to
CPST Doc: 316977.2 9
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 remove the dissolved solid; an oxygen removal unit adapted to treat the
treated water stream to
2 remove the oxygen; and, an oxygen scavenger feeder adapted to blend an
oxygen scavenger and
3 the treated water stream.
4 [0025] In some embodiments, the present invention system
further includes a water
cleaning subsystem adapted to treat an unconditioned cleaning water feed
stream to remove a
6 plurality of particles to form a conditioned cleaning water feed stream,
wherein the conditioned
7 cleaning water feed stream is used by at least one of: the particle and
oil removal subsystem; the
8 chemical oxygen demand reduction subsystem; and, the heavy metal and
dissolved ion removal
9 subsystem. In some embodiments, the water cleaning subsystem includes at
least one of: a gross
particle filter adapted to treat the unconditioned cleaning water feed stream
to remove the plurality
11 of particles; and, a fine particle filter adapted to treat the
unconditioned cleaning water feed stream
12 to remove the plurality of particles. In some embodiments, the
unconditioned cleaning water feed
13 stream includes ocean water or a fresh water source.
14 [0026] According to aspects illustrated herein, there is
provided a method for conditioning
an initial water feed stream into a treated water stream. The initial water
feed stream includes at
16 least one of: a plurality of particles; an oil; a volatile organic
compound; a hydrogen sulfide; a non-
17 volatile compound; a heavy metal; and a dissolved ion. The method
includes: a) treating the initial
18 water feed stream to remove the plurality of particles and the oil to
form a first partial treated water
19 stream; b) treating the first partial treated water stream to remove the
volatile organic compound,
the hydrogen sulfide and/or the heavy metal to form a second partial treated
water stream; and, c)
21 treating the second partial treated water stream to remove the heavy
metal to for the treated water
22 stream.
23 [0027] In some embodiments, the step of treating the produced
water feed stream is
24 performed using at least one of: a gross particle filter adapted to
treat the initial water feed stream
to remove the plurality of particles; an oil coalescer unit adapted to treat
the initial water feed
26 stream to remove the oil; a fine particle filter adapted to treat the
initial water feed stream to remove
27 the plurality of particles; and, an oil removal membrane unit adapted to
treat the initial water feed
28 stream to remove the oil. In some embodiments, the step of treating the
first partial treated water
29 stream is performed using at least one of: a stripping unit adapted to
treat the first partial treated
water stream to remove the volatile organic compound and the hydrogen sulfide
and to form a
CPST Doc: 316977.2 10
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 vapor phase comprising the volatile organic compound and the hydrogen
sulfide; and, a chemical
2 oxygen demand polishing unit adapted to treat the first partial treated
water stream to remove the
3 non-volatile organic compound. In some embodiments, the step of treating
the first partial treated
4 water stream is performed using the stripping unit and at least one of: a
bio scrubber unit adapted
to metabolize the volatile organic compound and the hydrogen sulfide of the
vapor phase; and, a
6 flare or a thermal oxidizer adapted to combust the volatile organic
compound and the hydrogen
7 sulfide of the vapor phase. In some embodiments, the step of treating the
second partial treated
8 water stream is performed using at least one of: a heavy metal and
dissolved ion removal unit
9 adapted to treat the second partial treated water stream to adsorb the
heavy metal and the dissolved
ion and to form a plurality of adsorbed heavy metals and a plurality of
adsorbed dissolved ions; a
11 heavy metal and dissolved ion precipitation unit adapted to precipitate
the plurality of adsorbed
12 heavy metals as a plurality of insoluble metal hydroxides and the
plurality of adsorbed dissolved
13 ions as a plurality of insoluble compounds; and, a filter press adapted
to form at least one cake
14 comprising the plurality of insoluble metal hydroxides and the plurality
of insoluble compounds.
[0028] In some embodiments, the treated water stream includes at least one
of: a sulfate; a
16 hardness; a dissolved solid; and, an oxygen, and the method further
includes: d) treating the treated
17 water stream to remove the sulfate, the hardness, the dissolved solid
and/or the oxygen to form an
18 enhanced oil recovery feed stream. In some embodiments, the step of
treating the treated water
19 stream is performed using at least one of: a sulfate and hardness
removal membrane unit adapted
to treat the treated water stream to remove the sulfate and the hardness; a
high pressure reverse
21 osmosis unit adapted to treat the treated water stream to remove the
dissolved solids; an oxygen
22 removal unit adapted to treat the treated water stream to remove the
oxygen; and, an oxygen
23 scavenger feeder adapted to blend an oxygen scavenger and the treated
water stream.
24 [0029] In some embodiments, the method further include:
treating an unconditioned
cleaning water feed stream to remove a plurality of particles to form a
conditioned cleaning water
26 feed stream, wherein the conditioned cleaning water feed stream is used
in at least one of: steps
27 a), b) and c). In some embodiments, the step of treating an
unconditioned cleaning water feed
28 stream is performed using at least one of: a gross particle filter
adapted to treat the unconditioned
29 cleaning water feed stream to remove the plurality of particles; and, a
fine particle filter adapted
to treat the unconditioned cleaning water feed stream to remove the plurality
of particles. In some
CPST Doc: 316977.2 11
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 embodiments, the unconditioned cleaning water feed stream includes ocean
water or a fresh water
2 source.
3 [0030] Other objects, features and advantages of one or more
embodiments will be readily
4 appreciable from the following detailed description and from the
accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
6 [0031] Various embodiments are disclosed, by way of example
only, with reference to the
7 accompanying drawings in which corresponding reference symbols indicate
corresponding parts,
8 in which:
9 Figure 1 is a first portion of a schematic diagram of a present
invention system for
treating produced water showing a clean water production subsystem;
11 Figure 2 is a second portion of a schematic diagram of a present
invention system
12 for treating produced water showing a particle and oil subsystem;
13 Figure 3 is a first portion of a schematic diagram of a present
invention system for
14 treating produced water showing a chemical oxygen demand reduction
subsystem;
Figure 4 is a first portion of a schematic diagram of a present invention
system for
16 treating produced water showing a heavy metal removal subsystem;
17 Figure 5 is a first portion of a schematic diagram of a present
invention system for
18 treating produced water showing a water reuse subsystem;
19 Figure 6 is a schematic diagram of a known desalting system; and,
Figure 7 is a schematic diagram of an alternate embodiment of present
invention
21 system for treating a water supply for subsequent use.
22 DETAILED DESCRIPTION
23 [0032] At the outset, it should be appreciated that like
drawing numbers on different
24 drawing views identify identical, or functionally similar, structural
elements of the embodiments
set forth herein. Furthermore, it is understood that these embodiments are not
limited to the
26 particular methodology, materials and modifications described and as
such may, of course, vary.
27 It is also understood that the terminology used herein is for the
purpose of describing particular
28 aspects only, and is not intended to limit the scope of the disclosed
embodiments.
29 [0033] Unless defined otherwise, all technical and scientific
terms used herein have the
same meaning as commonly understood to one of ordinary skill in the art to
which these
CPST Doc: 316977.2 12
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 embodiments belong. As used herein, the term "average" shall be construed
broadly to include
2 any calculation in which a result datum or decision is obtained based on
a plurality of input data,
3 which can include but is not limited to, weighted averages, yes or no
decisions based on rolling
4 inputs, etc. The term "produced water", as used herein, is intended to
mean water that is produced
when oil and gas are extracted from the ground. Oil and gas reservoirs have a
natural water layer,
6 i.e., formation water, that lies under the hydrocarbons. Oil reservoirs
frequently contain large
7 volumes of water, while gas reservoirs tend to have smaller quantities.
To achieve maximum oil
8 recovery additional water is often injected into the reservoirs to help
force the oil to the surface.
9 Both the formation water and the injected water are eventually produced
along with the oil and
therefore as the field becomes depleted the produced water content of the oil
increases.
11 Additionally, "produced water" is intended to include water commonly
known as flow back water
12 used in hydrofracturing operations, as well as water used in desalting
operations. "Pre-treated
13 produced water" is intended to mean produced water that has been treated
by some or all of the
14 subsystems described infra which occur prior to the water reuse
subsystem. Furthermore, as used
herein, the phrase "to treat...to remove" is intended to mean performing an
operation on a
16 component to remove all or some of a constituent within the component,
wherein the extent of
17 partial removal is further described infra, while the phrase "to treat..
to adsorb" is intended to
18 mean performing an operation on a component to adsorb all or some of a
constituent within the
19 component, wherein the extent of partial adsorption is further described
infra.
[0034] Moreover, as used herein, the phrases "comprises at least one of'
and "comprising
21 at least one of' in combination with a system or element is intended to
mean that the system or
22 element includes one or more of the elements listed after the phrase.
For example, a device
23 comprising at least one of: a first element; a second element; and, a
third element, is intended to
24 be construed as any one of the following structural arrangements: a
device comprising a first
element; a device comprising a second element; a device comprising a third
element; a device
26 comprising a first element and a second element; a device comprising a
first element and a third
27 element; a device comprising a first element, a second element and a
third element; or, a device
28 comprising a second element and a third element. A similar
interpretation is intended when the
29 phrase "used in at least one of:" is used herein. Furthermore, as used
herein, "and/or" is intended
to mean a grammatical conjunction used to indicate that one or more of the
elements or conditions
CPST Doc: 316977.2 13
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 recited may be included or occur. For example, a device comprising a
first element, a second
2 element and/or a third element, is intended to be construed as any one of
the following structural
3 arrangements: a device comprising a first element; a device comprising a
second element; a device
4 comprising a third element; a device comprising a first element and a
second element; a device
comprising a first element and a third element; a device comprising a first
element, a second
6 element and a third element; or, a device comprising a second element and
a third element.
7 [0035] Moreover, although any methods, devices or materials
similar or equivalent to those
8 described herein can be used in the practice or testing of these
embodiments, some embodiments
9 of methods, devices, and materials are now described.
[0036] Broadly, the present invention recovers hydrocarbons of commercial
value by
11 limiting use of chemicals that would prevent such recovery. The present
invention treats produced
12 water for TSS, oil, metals, H2S, BOD, COD and other contaminates that
would prevent discharge
13 of the treated water to the environment. The present invention is
flexible in that it can be adjusted
14 as needed to treat different streams and changes within a given stream.
The present invention has
a small foot print with minimum weight when compared to known systems of
gravity and induced
16 gas separation, nut shell filtration, metals precipitation, and
biological treatment. The present
17 invention causes minimum secondary and solids waste generation, while
utilizing a minimum
18 amount of consumables and chemicals. The present invention produced
treated water of a quality
19 that allows for reuse and/or discharge. Additionally, the present
invention provides water for EOR,
desalting, hydrofracturing or other production clean water requirements where
the water is treated
21 for the removal of sulfates, suspended solids, dissolved solids, H2S,
and oxygen, while agents such
22 as biocides are added to prevent sulfate reducing bacteria from reducing
sulfates to hydrogen
23 sulfide (H2S) where required.
24 [0037] The present system and method broadly comprises:
filtration pretreatment;
hydrocarbon removal; volatile organic compound (VOC) and H2S removal; metals
and specific
26 ion contaminate removal; solids dewatering; and, discharge. In some
embodiments, the present
27 invention may also prepare water for use in EOR, clean water production
uses or hydrofracturing,
28 and thus may broadly comprise: nano filtration; low and high pressure
reverse osmosis; and,
29 degasification.
[0038] Filtration pretreatment
CPST Doc: 316977.2 14
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 [0039] The present invention comprises a two step, high rate,
compact filtration stage. The
2 first step provides for crude filtration for sand and solids removal
while the second step removes
3 finer particles that may cause fouling of oil recovery membranes or
reduced recovery rates. The
4 second step is performed after the primary oil recovery step. Crude
filtration is performed in using
a gross particle filter, while fine filtration is performed in a fine particle
filter. Both filtration steps
6 may include a pneumatic assist for water flush cleaning. Filtration media
types and sizes are
7 adjusted as required for process optimization. The first filtration step
may be a slotted duplex
8 stainless steel wedge wire with openings of 200 microns to remove fine
sand. After the water
9 leaves the primary oil separation device any entrained particles will be
removed by the polishing
filter using a 20 micron cleanable filter cloth media made from Teflon .
Pneumatic gas assist is
11 used to dislodge any sticky material from the filter surface. A cleaning
water stream is returned
12 to a dehydrator, i.e., an upstream system forming part of the crude oil
processing system, wherein
13 oil recovery is accomplished, or the cleaning water stream can be sent
to a stilling tank for
14 separation of oil and solids. The solids can then be sent to a
dewatering system, such as a J-Press
filter press sold by Siemens located in Alpharetta, Georgia.
16 [0040] Hydrocarbon removal
17 [0041] The present invention comprises a two step process for
the efficient removal of the
18 majority of hydrocarbons present in the produced water stream. This
inventive process is highly
19 efficient within a small foot print or surface area consumed.
Hydrocarbons are recovered for
commercial use since chemicals are not used that would prevent recovery and
use of oil. It should
21 be appreciated that the present two step process allows for high
recoveries, typically in excess of
22 95%.
23 [0042] The first step of the hydrocarbon removal stage
comprises the primary oil
24 separation and recovery operation. This step utilizes a vessel filled
with a resin bead packing that
attracts fine oil droplets on its surface. As the droplets grow in size, they
release and float to the
26 top of vessel. The layer of oil forming at the top of the vessel is
removed and recovered for
27 commercial value. Recovery of oil can be as high as 95% with residual
oil of less than 5 parts per
28 million (PPM) in the water stream. In view of the efficiency of the
resin separator system, the
29 water exiting the separator can be directly discharged to the
environment where conditions allow.
However, it should be appreciated that whether the water is directly
discharged or further
CPST Doc: 316977.2 15
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 processed is based on the quality of the feed stream and the discharge
standards for the application
2 or location.
3 [0043] The resin separator system can be run at higher
temperatures, e.g., 70 C or higher,
4 to prevent paraffin and asphaltene from coating the vessel or resin
beads. The high surface area
afforded by the resin beads permits the overall size of the separator system
to be relatively small
6 and light weight. The separator system may comprise one or more vessels,
and the feed stream
7 may be introduced under pressure or gravity fed through the separator.
Moreover, the feed stream
8 may flow upwardly or downwardly through the separator. The present resin
separator system
9 permits fouled media, i.e., fouled resin beads, to be cleaned in place or
removed for external
cleaning. Cleaning is accomplished by up flow fluidizing the resin bed and/or
the addition of hot
11 water to remove waxes and particulates. The layer of oil forming at the
top of the vessel is removed
12 and recovered for commercial value.
13 [0044] The second step of the hydrocarbon removal stage, also
referred to as the oil
14 removal stage, comprises at least one membrane separation operation. It
should be appreciated
that the filtration pretreatment stage and primary oil recovery stage increase
and/or optimize the
16 performance of the membrane separation operation. The membranes used in
the second step of
17 the hydrocarbon removal stage remove the balance of free oil and
additional hydrocarbons such as
18 BETX compounds from the feed stream. The foregoing is accomplished by
allowing oil to build
19 up to over 1,000 PPM within the membrane. As oil levels increase within the
membrane,
additional hydrocarbons are removed thus decreasing the BOD and COD load on
additional
21 treatment devices and providing for the additional recovery of
hydrocarbons.
22 [0045] Suitable membranes may be selected from but not limited
to hydrophilic
23 membranes, polyacrylonitrile (PAN) polymer, polyvinylidene fluoride
(PVDF) and polyvinyl
24 chloride (PVC). Membranes having pore sizes between 0.01 ¨ .05 microns
with an approximate
molecular weight cutoff between 10K ¨ 60K Daltons are suitable for use in the
present invention.
26 It should be appreciated that suitable membranes are hydrophilic which
attract water and repel oil,
27 and the level of hydropholicity may be specifically selected based on
the requirements of the
28 membrane separation operation. The hydrophilic characteristic of the
membrane allows for low
29 tangential surface velocities thereby saving on pumping horse power.
Some membranes, e.g.,
PVDF, can be run at hot temperatures, i.e., 70 C or higher, to prevent
paraffin and asphaltene from
CPST Doc: 316977.2 16
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 coating the membranes. These membranes are design so that TDS will not be
affected by the
2 process. In short, by minimizing rejection of TDS, low pressure and high
flux rate can be achieved
3 through the membranes without introducing scaling issues.
4 [0046] VOC and H2S removal
[0047] The VOC and H2S removal stage comprises air stripping of these
components using
6 conventional, known techniques. For example, the water feed stream flows
down though
7 packaging material or a series of trays while air is introduced via a
counter current up flow. This
8 arrangement removes a substantial amount of VOCs and H2S from the water
feed stream thereby
9 reducing the load to the final semi volatile organic compound (SVOC)
removal device. It should
be appreciated that a tray type air stripper as described is compact in size
and provides VOC and
11 H2S removal to acceptable levels. Other gas/liquid contacting devices
known in the art may be
12 utilized for the VOC and H2S removal stage, e.g., columns with random or
structured packing.
13 [0048] In some embodiments, the air stripping device may
comprise a biogas filter adapted
14 to destroy VOCs and H2S in the off gas from the stripper, thus
permitting direct air discharge as
the exiting air stream meets acceptable air discharge standards. In some
embodiments, the biogas
16 filter uses a high surface ceramic media that is hydrophilic. The media
provides high surface area
17 and flow throughput for the air, which provides increased contact with
bacteria specifically
18 selected to destroy, in an aerobic process, VOCs using chemoheterotophic
bacteria and H2S using
19 sulfur-oxidizing bacteria. Biofilters are reactors in which waste gases
are allowed to pass through
a porous packed bed material immobilized with suitable microbial cultures. As
the waste gas
21 passes through the filter medium, the contaminants in the gas transverse
to the liquid phase
22 surrounding the microbial biofilm in the media. The contaminants are
subsequently converted to
23 CO2, H20, 504, inorganic salts and biomass by microorganisms. The high
surface area of the
24 media permits the unit to have a small overall footprint and low weight.
[0049] In some embodiments, a final oil recovery step may comprise passing
the feed
26 stream through a specially design adsorptive resin or granular activated
carbon which adsorb final
27 traces of hydrocarbons. Such resins, e.g., macroporous styrene-DVB, and
activated carbon have
28 a high infinity for organic compounds and thus readily adsorb organic
compounds. Depending on
29 the concentration of and types of hydrocarbons and the desire to recover
additional organic
compounds, the foregoing oil recovery unit can be in upstream or downstream of
the air stripper
CPST Doc: 316977.2 17
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 described above. The final oil recovery unit may be regenerated with
steam to desorb
2 hydrocarbons and any captured hydrocarbons can be recovered therefrom.
Typically, the
3 foregoing media, i.e., resins and/or activated carbon, are held in
pressure vessels in various series
4 and/or parallel configurations as required by particular system needs.
[0050] Heavy metals ion and dissolved specific ion contaminates removal
6 [0051] Produced water typically comprises various types of
heavy metals and/or other
7 dissolved contaminate ions that have restrictions for discharge, e.g.,
lead, copper, cadmium,
8 mercury, strontium and barium. Specific resins used for the removal of
metals and other dissolved
9 ion contaminants, e.g., boron, are designed to work in concentrated brine
salt streams, e.g.,
macroporous styrene divinylbenzene with iminodiacetic acid functional groups
and a
11
macroreticular polymer with thiol functional groups. For boron
contaminants, a N-
12 methylglucimine functional group can be used. Typically, the foregoing
media, i.e., resins, are
13 held in pressure vessels in various series and/or parallel
configurations as required by particular
14 system needs. In view of inherent resin properties, metals and dissolved
specific ion contaminates
can be removed from the produced water stream and then during resin
regeneration using acids
16 and hydroxides, a very concentrated stream of metals and contaminates
can be generated. The
17 metals in the regeneration stream can be precipitated using hydroxide
which is added to adjust the
18 pH of collected solution to the appropriate range for heavy metal
precipitation as insoluble metal
19 hydroxides along with other insoluble contaminates. Heavy metal
precipitates may be
subsequently dewatered into a cake as described infra. Other chemistry which
is common to the
21 art can be used and additives such as filtering acids can be used. The
present invention process is
22 very efficient relative to conventional precipitation clarification
processes, and generates far less
23 sludge while maintaining a smaller overall footprint. After removal of
heavy metal ions, the
24 resulting water can be further treated with the addition of an oxidizing
agent to remove traces of
BOD, COD and H25. Examples of oxidizing agents can include but are not limited
to, chlorine,
26 ozone and peroxide, which agents can be generated locally or supplied
from an outside source.
27 [0052] Solids dewatering
28 [0053] A reaction tank, reactor mixer, polymer, chemical feed
for pH adjustment and filter
29 press is included in the present system for precipitation of solids and
removal of water prior to
CPST Doc: 316977.2 18
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 transportation to a disposal location. The reaction tank in combination
with the filter press can
2 also be used for dewatering solids from filtration stages describe supra.
3 [0054] Discharge
4 [0055] One benefit of the present invention is that water from
the process can be directly
discharged to an ocean or other body of water. If the TDS level or temperature
is not within
6 discharge requirements, the wastewater is discharged below surface, and
depending on the
7 wastewater chemistry, discharged in a deep distribution/dilution pipeline
or deep well waste
8 injection.
9 [0056] Preparation for water for injection for enhanced oil
recovery, for desalting feed
water and/or for hydrofracturing (optional)
11 [0057] One of the aspects of the present invention is that
water from a fresh water or
12 seawater treatment system can be fed directly to this subsystem for
preparation for enhanced oil
13 recovery (EOR), desalting, water for production activities and/or
hydrofracturing in whole or in
14 part depending on the subsystems demands. For example, should the volume
of produced water
decrease or stop entirely, processing of water for the foregoing operations
can continue.
16 [0058] In some embodiments, the processed water goes through a
secondary treatment
17 stage to allow for reuse in EOR, desalting, water for production
activities and/or hydrofracturing.
18 It should be appreciated that water used for EOR must be free of
suspended solids, sulfates,
19 oxygen, boron, barium and strontium. Additionally, a biocide is often
added to prevent biological
attack on any recovered oil. In some embodiments, the processed water goes
through an additional
21 treatment stage to allow for reuse in low total dissolved solids (TDS)
EOR, desalting, water for
22 production activities and/or hydrofracturing. It should be appreciated
that water used for these
23 operations must be free of various contaminated such as suspended
solids, sulfates, oxygen, barium
24 and strontium, and must have low TDS. Additionally, a biocide is often
added to prevent
biological attack on any recovered oil.
26 [0059] Preparation of water for use in EOR comprises nano
filtration of the water stream.
27 Nano filtration is accomplished using a low pressure nano filtration
process with a membrane.
28 Suitable membranes, e.g., reverse osmosis membrane elements designed to
allow monovalent ions
29 such as sodium and chloride to pass through as permeate, are designed
specifically for the removal
CPST Doc: 316977.2 19
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 of large ions, typically divalent such as sulfates, hardness, barium and
strontium. The membranes
2 are run at low pressures and do not remove general TDS such as sodium and
chloride.
3 [0060] In some embodiments, further filtration of the water is
necessary prior to use in
4 EOR, desalting and/or hydrofracturing, i.e., where low TDS water is
required. In these
embodiments, alternate forms of reverse osmosis filtration occur. Salinity
removal from the water
6 is accomplished using a high pressure, high rejection membrane designed
for streams comprising
7 monovalent ions such as sodium and chloride, e.g., high pressure reverse
osmosis membrane
8 elements, as described in greater detail infra. Where feasible, energy is
recovered from the reverse
9 osmosis brine stream and returned to the feed stream.
[0061] In addition to nano filtration, preparation for use of water in EOR,
desalting or
11 hydrofracturing comprises a degasification stage. A special membrane is
used to remove oxygen
12 from the water. The membrane is a hydrophobic thin film composite of
polydimethysilicon
13 (PDMS) on a polysulfone base. The membrane has a bubble point of
approximately 300 psi. The
14 membrane does not let water pass through, i.e., the membrane only allows
gases to pass. Under
typical operating conditions, the membrane can remove up to 99% of the oxygen
in the feed stream,
16 while operating at a feed pressure of 50-200 psi and temperature of up
to 77 C. The combination
17 of using a vacuum pump on the gas side of the membrane and nitrogen
sweep gas causes the
18 removal of 02 to less than 50 parts per billion (PPB).
19 [0062] In some embodiments, the system comprises further oxygen
removal from the
water stream. Depending on the feed oxygen concentration and the desired
removal efficiency, a
21 polishing resin device can be used to further reduced oxygen in the
water. The water is passed
22 through a pressurized vessel at approximately 50-150 psi holding a
weakly basic, macroporous,
23 palladium-doped, polymer based resin in the form of spherical beads. The
oxygen is catalytically
24 removed from the water in the presence of a suitable reducing agent such
as hydrogen. Hydrogen
is introduced to the vessel under pressure and is dissolved in the water to be
treated and then passed
26 through the resin bed. Residual oxygen concentrations of less than 20
ppb can be obtained at flow
27 rates up to a superficial velocity of 80 meters per hour at temperatures
up to 120 C. Optionally,
28 an oxygen removal agent such as hydrazine may be used for final
polishing and removal of 02.
29 [0063] In view of the foregoing, it should be appreciated that
the present invention
comprises groupings of elements into subsystems. The present invention broadly
comprises the
CPST Doc: 316977.2 20
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 following grouped subsystems in various combinations: water cleaning;
particle and oil removal;
2 chemical oxygen demand reduction; heavy metal removal; and, water reuse.
3 [0064] Figures 1 through 5 depict a typical embodiment of a
present invention system for
4 treating produced water. It should be noted that in order to depict the
present invention with
sufficient detail in the figures, the system was broken in to portions and
distributed across Figures
6 1 through 5. The connections between the separate portions are
represented by encircled letters.
7 For example, one connection between Figure 1 and Figure 2 is shown by the
encircled 'A'.
8 [0065] Water cleaning subsystem
9 [0066] Water cleaning subsystem 10 receives unconditioned
cleaning water feed stream
12, e.g., ocean water or other water supply such as a fresh water, and passes
water feed stream 12
11 through gross particle filter 14 thereby removing large particulate
matter. In some embodiments,
12 water feed stream 12 is pumped through water cleaning subsystem 10 as
described infra relative
13 to particle and oil removal subsystem 16. In some embodiments of the
invention, gross particle
14 filter 14 is a self-cleaning tubular backwash filter such as the
strainers manufactured by SAMCO
Technologies, Kinney, RP Adams, DOW TequaticIm plus. Gross particle filter 14
is fitted with a
16 100-200 micron wedgewire filter element. Other filtration or solids
liquid separation technologies
17 may also be used for large particulate removal, such as a disc filter or
centrifuge. Gross particle
18 filter 14 is arranged to be backwashed with filtered water from the
unit. The backwash wastewater
19 is returned to the source of water feed stream 12, e.g., an ocean, via
outlet 18.
[0067] Subsequently, gross filtered water stream 20 exits filter 14 and is
passed through
21 fine particle filter 22 wherein particulate matter with sizes ranging
from 10-100 microns is
22 removed. In some embodiments of the invention, fine particle filter 22
is a self-cleaning tubular
23 backwash filter such as the tubular filter manufactured by SAMCO
Technologies. Fine particle
24 filter 22 is fitted with a 10-20 micron media which may be a Teflon ,
polypropylene, nylon or
metal cloth filter element. Other filtration or solids liquid separation
technologies may be used for
26 fine particulate removal, such as a disc filter, cartridge filter, or
bag filter. Fine particle filter 22
27 is designed to be backwashed with filtered water from the unit.
Pneumatic gas assist can be used
28 to dislodge any sticky material from the filter surface. Air is
compressed with line pressure into a
29 dome on the device and expanded when the pressure is relieved during
backwash. The backwash
wastewater is returned to the source of water feed stream 12, e.g., an ocean,
via outlet 24. The
CPST Doc: 316977.2 21
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 water stream exiting fine particle filter 22 is conditioned cleaning
water feed stream 26 which, in
2 some embodiments, is used in particle and oil removal subsystem 16,
chemical oxygen demand
3 reduction subsystem 28, heavy metal and dissolved ion removal subsystem
30, water reuse
4 subsystem 118 and/or oxidizing agent feed stream 94. It should be
appreciated that chemical and
biological oxygen demand levels are reduced by a reduction or removal of
hydrocarbons, volatile
6 gases, non-volatile gases and H2S, and that chemical oxygen demand
reduction subsystem 28 is
7 used to reduce or remove all or some of these contaminants.
8 [0068] Particle and oil removal subsystem
9 [0069] Particle and oil removal subsystem 16 receives initial
water feed stream 32 from a
plant oil dehydrator, desalter (not shown) or other produced water generators
such as flow back
11 from a hydrofracturing operation. Feed stream 32 is pumped to gross
particle filter 34 at a pressure
12 of approximately 50 to 150 pounds per square inch gauge (psig) thereby
removing large particulate
13 matter. In some embodiments of the invention, gross particle filter 34
is a self-cleaning tubular
14 backwash filter such as the strainers manufactured by SAMCO
Technologies, Kinney, RP Adams,
DOW Tequatic' plus. Gross particle filter 34 is fitted with a 100-200 micron
wedgewire filter
16 element. Other filtration or solids liquid separation technologies may
also be used for large
17 particulate removal, such as a disc filter or centrifuge. Gross particle
filter 34 is arranged to be
18 backwashed with filtered water from the unit. The backwash wastewater is
returned via outlet 36
19 to the dehydrator or stilling tank where solids and attached
hydrocarbons settle out and can be
removed as sludge or recovered. Depending on the nature of the solids,
backwash wastewater may
21 be sent back to precipitation unit 104, separated and the solids
dewatered.
22 [0070] Filtered water 38 from gross particle filter 34
comprises very small oil droplets.
23 Filtered water 38 flows to coalescer unit 40 for oil removal. Due to the
small size of oil droplets,
24 the oil will not separate from filtered water 38 in a traditional
gravity separator without assistance.
Coalescer unit 40 comprises a multi chamber vessel or pair of single chamber
vessels, designed
26 for either atmospheric or pressure operation. These units are custom
designed for each application.
27 A suitable coalescer unit can be obtained from SAMCO Technologies
located in Buffalo, New
28 York. The first chamber or vessel holds a bed of coalescing media. In
some embodiments of the
29 invention, the coalescing media is AmberliteTM ROC110 manufactured by Dow
Chemical
Company. The coalescing media attracts the very small oil droplets and allows
them to
CPST Doc: 316977.2 22
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 agglomerate into larger oil droplets that then float to the top of the
water in the second chamber or
2 vessel, similar to a traditional gravity separator. The floating oil
phase is returned to the dehydrator
3 or other suitable collection device for recovery via outlet 42. Depending
on the characteristics of
4 filtered water 38 cleaning may be required. Fouling by contaminants such
as particles and paraffin
can occur. Removal of contaminants is accomplished by internal or external
reverse
6 flow/fluidizing of the coalescing media bed and/or hot water stripping of
the media bed. Moreover,
7 in some embodiments, coalescer unit 40 may require the use of additional
water, i.e., conditioned
8 cleaning water feed stream 26. It should be appreciated that recovery of
oil in coalescer unit 40
9 may be as high as 95% with residual oil as low as 5 ppm in the water
stream. In view of the
efficiency of the resin separator system, the water exiting the separator can
be directly discharged
11 to the environment where conditions allow. However, it should be
appreciated that whether the
12 water is directly discharged or further processed is based on the
quality of the feed stream and the
13 discharge standards for the application or location.
14 [0071] Water feed 44 exiting the separating chamber or vessel
of coalescer unit 40 is
transferred to fine particle filter 46 at a pressure of approximately 50 to
150 psig using pump 48,
16 if required, wherein particulate matter with sizes ranging from 10-100
microns is removed. In
17 some embodiments of the invention, fine particle filter 46 is a self-
cleaning tubular backwash filter
18 such as the tubular filter manufactured by SAMCO Technologies. Fine
particle filter 46 is fitted
19 with a 10-50 micron media which may be a Teflon , polypropylene, nylon
or metal cloth filter
element. Other filtration or solids liquid separation technologies may be used
for fine particulate
21 removal, such as a disc filter, cartridge filter, or bag filter. Fine
particle filter 46 is designed to be
22 backwashed with filtered water from the unit. The backwash wastewater is
returned via outlet 50
23 to the dehydrator or stilling tank where solids settle out and can be
removed as sludge. Fine particle
24 filter 46 comprises a dome for trapping and compression of air to allow
for pneumatic cleaning
assist.
26 [0072] Water stream 52 exiting fine particle filter 46 may
containing trace amounts of free
27 and emulsified oil. Water stream 52 flows to oil removal membrane unit
54 for further removal
28 of oil. In some embodiments, water stream 52 is transferred to membrane
unit 54 using pump 56.
29 It should be appreciated that depending system needs, only one of pumps
48 and 56 will be
included, e.g., a single pump may be sized to be sufficient for the needs of
the removal subsystem
CPST Doc: 316977.2 23
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 16. Oil removal membrane unit 54 is an array of pressure rated housings
filled with spiral wound
2 or hollow fiber membrane modules, the number and arrangement of which are
dictated by the
3 water flow rate to yield a permeate water flow rate of 5-20
gallons/minute/square foot of
4 membrane. In some embodiments of the invention, the membrane elements are
hydrophilic
polyacrylonitrile (PAN) polymer, PVDF, or PVC. A suitable example of a
membrane element is
6 General Electric's MW Series membrane. Feed water is pressurized to
approximately 100-300 psi
7 prior to entering the membrane array. Water, dissolved ionic species, and
small molecular weight
8 hydrocarbons below approximately 50K Dalton and approximately 0.01 micron
cutoff pass
9 through the membrane and are collected as permeate stream 58 resulting in
85% or more of the
volume of the feed water entering the unit. The remaining water and higher
molecular weight
11 above 50K Dalton molecular weight hydrocarbons are substantially
rejected by the membrane and
12 returned via outlet 60 to the dehydrator for oil recovery. As oil
concentrates in the membrane
13 separator, the oil adsorbs BETX compounds thus reducing the COD and BOD
in the water. If the
14 membrane requires cleaning, hot water and/or caustic compounds may be
flushed through the
membranes or externally clean the membranes in place within system.
16 [0073] Chemical oxygen demand (COD) and biological oxygen
demand (BOD) reduction
17 sub system
18 [0074] Chemical oxygen demand reduction subsystem 28 receives
low pressure permeate
19 stream 58 from oil removal membrane unit 54 which stream 58 flows to
stripping unit 62 for
removal of volatile organic compounds (VOC) and hydrogen sulfide (H2 S) to
reduce chemical
21 oxygen demand (COD) of the water. Stripping unit 62 is a conventional
countercurrent gas/liquid
22 contacting tower filled with random or structured packing well known to
those skilled in the art,
23 e.g., Jaeger Tri-Packs or a low profile tray type. Water stream 58,
rich in VOC and H25, enters
24 the top of stripping unit 62 and flows downward through the packing or
trays. Clean atmospheric
air stream 64 is introduced into the bottom of stripping unit 62 via blower 66
and flows upwardly.
26 As water stream 58 contacts air stream 64, VOC and H25 transfer from the
liquid phase to the gas
27 phase yielding vapor phase stream 68 rich in VOC and H25. Vapor phase
stream 68 exits the top
28 of the unit, i.e., stripping unit 62, and water stream 70, lean in VOC
and H25, exits the bottom of
29 the unit, i.e., stripping unit 62. An example of a suitable stripping
unit 62 is a convention tower
type stripping unit such as the stripping unit sold by Delta Cooling Towers,
inc. located in
CPST Doc: 316977.2 24
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 Rockaway, New Jersey, or the shallow tray compact type sold by Bisco
Environmental located in
2 Taunton, Massachusetts.
3 [0075] In some embodiments of the invention, the VOC and H2S
rich vapor phase, i.e.,
4 vapor phase stream 68, flows to gas flare 72. Gas flare 72 is also
commonly known in the art as a
flare and a flare stack, and may, in some embodiments, be a thermal oxidizer.
In some
6 embodiments of the invention, vapor phase stream 68 flows to bio scrubber
unit 74 for treatment,
7 e.g., reducing carbon content, to allow discharge to atmosphere meeting
regulatory limits. In some
8 embodiments of the invention, bio scrubber unit 74 is a conventional
contacting tower filled with
9 BioVastTm or 1ViP2C, a porous, hydrophilic, high surface area flow through
ceramic packing
manufactured by CerMediaTm LLC located in Buffalo, New York. The media
provides high
11 surface area and flow throughput for the air, which provides increased
contact with
12 microorganisms that are generally indigenous to the region where the
unit resides and may include
13 any species of heterotrophic bacteria that inoculate the media and adapt
to destroy, in an aerobic
14 process, VOCs and H2S. Biofilters are reactors in which waste gases are
allowed to pass through
a porous packed bed material immobilized with suitable microbial cultures. As
the waste gas
16 passes through the filter medium, the contaminants in the gas transverse
to the liquid phase
17 surrounding the microbial biofilm in the medium where they are degrade
to CO2, H20, SO4,
18 inorganic salts and biomass by microorganisms. The high surface area of
the media permits the
19 unit to have a small overall footprint and low weight.
[0076] Conditioned cleaning water stream 26, obtained from an ocean or
other available
21 clean water source as described supra, is trickled across the top of the
packing material for
22 humidity and water wetting control. Water stream 26 is sprayed over the
packing material at a rate
23 necessary to maintain saturated packing material while flushing away
treated byproducts. Packing
24 material size is selected to cause an empty bed contact of between 5 ¨
60 seconds. VOC and H2S
rich vapor, i.e., vapor phase stream 68, flows into the bottom of the tower of
bio scrubber unit 74
26 and upwardly through the packing material. The preferred packing
material is a very porous
27 ceramic media which has extremely high surface area. The surface of the
packing material is
28 covered with a biofilm of naturally occurring microorganisms that
metabolize VOCs and H2S to
29 carbon dioxide, water, and sulfates which can be safely discharged to
the atmosphere or ocean.
There are many types of naturally occurring bacteria that provide biochemical
destruction of VOCs
CPST Doc: 316977.2 25
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 and H2S. Examples include but are not limited to chemoheterotophic
bacteria and sulfur oxidizing
2 bacteria. Water stream 76 is discharged to an ocean or other suitable
discharge location and treated
3 air stream 78 is discharged to the atmosphere.
4 [0077] Water stream 70, which exits from the bottom of
stripping unit 62, flows to
hydrocarbon polishing unit 80 for removal of dissolved, non-volatile organic
compounds, e.g.,
6 phenols and polycyclic aromatic hydrocarbons (PHA), to recover additional
hydrocarbons and to
7 further reduce COD of water stream 70 to make it suitable for disposal or
reuse. In some
8 embodiments, water stream 70 is transferred to polishing unit 80 using
pump 82. In some
9 embodiments of the invention, hydrocarbon polishing unit 80 comprises two
standard AS1ViE
pressure vessels filled with synthetic adsorbent resin. The pressure vessels
may be arranged in
11 various series and parallel configurations. An example of a suitable
synthetic adsorbent resin is
12 the styrene-DVB macroporous material DoweXI'm Optiporelm L493 manufactured
by Dow
13 Chemical Company located in Midland, Michigan. Other adsorptive media
such as activated
14 carbon, e.g., activated carbon sold by Calgon Carbon located in
Pittsburgh, Pennsylvania, may be
utilized in hydrocarbon polishing unit 80. During normal service, the vessels
that form
16 hydrocarbon polishing unit 80 are arranged in series or parallel with
the first vessel removing the
17 largest portion, and possibly all, of the COD load from water stream 70,
with the second vessel
18 acting as a polishing unit for any trace materials passing through the
first vessel. As water stream
19 70 flows through the adsorbent, i.e., synthetic adsorbent resin,
dissolved non-volatile organic
compounds transfer to and are bound to active sites on the adsorbent surface
while treated water
21 stream 84, having COD levels meeting discharge limits, exits hydrocarbon
polishing unit 80 via
22 outlet 88.
23 [0078] In some embodiments of the invention, the synthetic
adsorbent resin used in
24 hydrocarbon polishing unit 80 can be regenerated. During a regeneration
event, one vessel remains
online treating water stream 70 while the other vessel is regenerated. The
vessel to be regenerated
26 is taken offline and the saturated synthetic adsorbent resin is
contacted with 50-150 psig 87 steam
27 to desorb the bound organics. Vapor containing the desorbed organic
material exits the vessel
28 being regenerated and is then condensed and recycled to the dehydrator
for recovery via outlet 88.
29 It should be appreciated that in some embodiments, all hydrocarbons are
recovered in polishing
CPST Doc: 316977.2 26
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 unit 80 thereby eliminating the need to include stripping unit 62, gas
flare 72 and bio filter scrubber
2 unit 74.
3 [0079] Heavy metals and dissolved ion removal subsystem
4 [0080] Heavy metals removal subsystem 30 receives water stream
84 exiting hydrocarbon
polishing unit 80 contains dissolved heavy metal ions, such as lead, copper,
cadmium, mercury,
6 barium and strontium, and other ions such as boron which must be removed
to a level suitable for
7 discharge, e.g., discharge in an ocean. In some embodiments of the
invention, heavy metals and
8 dissolved ion removal unit 90 comprises two standard ASME pressure
vessels filled with ion
9 exchange resin such as AmberliteTM lRA748, AmbersepTM GT74 or AmberliteTM
lRA743 (for
boron removal), all sold by Dow Chemical Company located in Midland, Michigan.
Other ion
11 exchange resins may be used, i.e., ion exchange resins designed for
selective removal of trace
12 metal compounds from high salinity solutions. During normal service, the
vessels are arranged in
13 series with the first vessel removing the largest portion, and possibly
all, of the heavy metals and/or
14 dissolved ions load from water stream 84 with the second vessel acting
as a polishing unit for any
trace material passing through the first vessel. It should be appreciated that
vessels may also be
16 arranged in parallel depending on the needs of the system, spatial
constraints, etc. As water stream
17 84 flows through the ion exchange resin bed, dissolved heavy metal ions
and other dissolved ions
18 transfer to and are bound to active sites on the adsorbent surface of
the ion exchange resin material
19 while treated water stream 92, having contaminant levels meeting
discharge limits, exits heavy
metals and dissolved ion removal unit 90. Treated water stream 92 is blended
with oxidizing agent
21 feed stream 94, which may include oxidizing agents such as chlorine,
ozone or hydrogen peroxide,
22 for trace COD removal and H2S destruction, after which treated water
stream 92 is discharged,
23 e.g., discharged to an ocean via outlet 96. Alternatively, treated water
stream 92 can be further
24 treated for use in the water recovery stream for enhanced oil recovery
(EOR), desalting and
hydrofracturing operations as described infra.
26 [0081] In some embodiments of the invention, the ion exchange
resin used in heavy metals
27 and dissolved ion removal unit 90 can be regenerated. During a
regeneration event, one vessel
28 remains online treating water stream 84 while the other vessel is being
regenerated. The vessel to
29 be regenerated is taken offline and saturated ion exchange resin is
contacted with concentrated
acidic stream 98, e.g., sulfuric or hydrochloric acid, to desorb the heavy
metals and other dissolved
CPST Doc: 316977.2 27
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 ions such as boron. The ion exchange resin is then contacted with
concentrated basic stream 100,
2 e.g., sodium hydroxide, to restore exchange capacity to the ion exchange
resin. Some resins, e.g.,
3 Ambersep' GT74, do not require the sodium hydroxide restoration step.
4 [0082] Regeneration solutions stream 102 exiting the ion
exchanged resin bed of heavy
metals and dissolved ion removal unit 90 is rich in heavy metals, and is
collected for subsequent
6 processing in heavy metals and dissolved ion precipitation unit 104.
Concentrated basic stream
7 106, e.g., sodium hydroxide or calcium hydroxide (lime), is added to
regeneration solutions stream
8 102 to adjust the pH of stream 102 to the appropriate range for heavy
metal precipitation as
9 insoluble metal hydroxides. In some embodiments of the invention, the
metal hydroxide
precipitate and dissolved ion precipitate is transferred from precipitation
unit 104 to filter press
11 108 using pump 110. The metal hydroxide precipitate is filtered from
regeneration solutions
12 stream 102 using filter press 108, i.e., a standard filter press well
known to those skilled in the art.
13 Alternative solid/liquid separating devices, such as a centrifuge, may
also be used. Precipitated
14 solids cake 112 are collected for offsite disposal. Filtrate stream 114
is recycled to heavy metals
and dissolved ion removal unit 90 while final filtered waste water stream 116
may be returned to
16 the dehydrator or discharged.
17 [0083] Water reuse subsystem
18 [0084] In many applications it is advantageous to utilize
produced water as a supply for
19 EOR and other production operations. For EOR operations, pressurized
water is injected into an
oil reservoir to increase reservoir pressure and oil output. Water used for
this purpose must have
21 low concentrations of sulfate, salts contributing to hardness, metals,
boron, TDS and oxygen which
22 otherwise would degrade the oil or yield high concentration of H2S,
react with down hole
23 chemistry, or plug the oil recovery collection system in recovered oil
or natural gas. In some
24 embodiments, water reuse subsystem 118 is used to prepare water for
subsequent EOR operations,
hydrofracturing or other production activities. It should be appreciated that
"TDS" is intended to
26 include but not be limited to salts that contribute to water hardness.
27 [0085] In some embodiments of the invention, water stream 92
exiting heavy metals and
28 dissolved ion removal unit 90 flows to sulfate and hardness removal
membrane unit 120. Sulfate
29 and hardness removal membrane unit 120 comprises an array of pressure
rated housings filled with
spiral wound nanofiltration membrane modules, the number and arrangement of
which are dictated
CPST Doc: 316977.2 28
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 by the flowrate of water stream 92 needed to yield a permeate water
flowrate of 10-15
2 gallons/minute/square foot of membrane. In some embodiments of the
invention, the membrane
3 elements are FilmtecTM SR90 sold by Dow Chemical Company located in
Midland, Michigan.
4 Water stream 92 is pressurized to 100-300 psi prior to entering the
membrane array of sulfate and
hardness removal membrane unit 120 using pump 122. Water and some dissolved
ionic species,
6 namely sodium and chloride ions, pass through the membrane and are
collected as permeate water
7 stream 124 amounting to 75-85% volume of water stream 92 entering unit
120. The remaining
8 water and higher molecular weight ions, e.g., divalents such as sulfate
and hardness, are rejected
9 by the membrane array and are discharged to the ocean via outlet 126.
[0086] In some embodiments, low TDS water is required for various
operations, e.g., EOR,
11 desalting and/or hydrofracturing. As described above, low TDS water is
produced using high
12 pressure, high rejection reverse osmosis membranes. In these
embodiments, permeate water
13 stream 124 flows to TDS removal unit 128. TDS removal unit 128 comprises
high pressure, high
14 rejection reverse osmosis membrane elements, e.g., FilmtecTM SW30 sold by
Dow Chemical
located in Midland, Michigan. Permeate water stream 124 is pressurized to 700-
1500 psi prior to
16 entering the membrane array by a high pressure pump integral to or
incorporated within TDS
17 removal unit 128. The membrane rejects low weight ionic compounds like
sodium and chloride.
18 Water stream 129, i.e., water collected from permeate water stream 124,
amounts to 30-70%
19 volume of the feed water entering TDS removal unit 128. The remaining
water and lower
molecular weight ions such as sodium, i.e., waste stream 130, the material
rejected by the
21 membranes, are discharged to the ocean or other suitable receiving body
via outlet 131. Where
22 feasible, the high pressure, i.e., energy, of waste stream 130 can be
recovered using an energy
23 recovery device and returned to the feed stream through reduction of
power to the main feed pump.
24 Suitable energy recovery units include but are not limited to work
exchangers and turbines, e.g.,
DWEERTM and Calder ERT sold by Flowserve located in Irving, Texas.
26 [0087] After sulfate removal, permeate water stream 129 flows
to oxygen removal unit
27 132. Oxygen removal unit 132 comprises an array of pressure rated
housings filled with gas
28 permeable hollow fibers or spiral wound, the number and arrangement of
which are dictated by
29 the flowrate of water stream 129 needed to maintain pressure loss at
less than 25 psi and to reduce
oxygen concentration from saturation to less than 50-100 PPB. In some
embodiments of the
CPST Doc: 316977.2 29
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 invention, the membrane elements are Liqui-Cel membrane contactors as
sold by Membrana
2 located in Wuppertal, Germany, or alternatively, may be MDS-32502 as sold by
Membrane
3 Development Specialist located in Solana, California. It has been found
that the spiral wound
4 configuration of MDS-32502 is easily cleaned. A booster pump integral to
or incorporated within
oxygen removal unit 132 boosts water stream 129 to 50-150 psi prior to
entering the membrane
6 array. Water and dissolved gases, namely oxygen, flow through the shell
side of the contactor. A
7 liquid ring vacuum pump pulls a 50 tor vacuum on the tube side to drive
gas transfer from the
8 liquid to the gas phase across the membrane. Additionally, nitrogen gas
stream 134 from a pressure
9 or thermal swing nitrogen generator flows through the hollow fibers to
lower oxygen partial
pressure and further drive oxygen from the liquid phase to the gas phase.
Sweep gas stream 136
11 is discharged to atmosphere 138 via vacuum pump 140.
12 [0088] Deoxygenated water stream 142 may be further polished
with the use of an catalytic
13 reaction in pressurized vessel 144. As described supra, pressurized
vessel 144 is filled with a
14 palladium-doped resin. Deoxygenated water stream 142 receives
pressurized hydrogen stream
146 which is dissolved therein. Subsequently, the water stream passes through
the resin bed and
16 exits as deoxygenated water stream 148. Suitable resins include Lewatit
K 3433 (a crosslinked
17 polystyrene resin with tertiary amine functional groups) sold by
Lenntech by located in Rotterdam,
18 Netherlands. It should be appreciated that in some embodiments, oxygen
may be removed from
19 feed water 129 by using an ion exchange resin.
[0089] Deoxygenated water stream 148, which may be blended with oxygen
scavenger
21 stream 150 such as hydrazine to further reduce dissolved oxygen
concentration below 5 PPB, is
22 then sent to EOR injection equipment for subsequent use via outlet 152.
23 [0090] In view of the foregoing, it should be appreciated that
a variety of configurations
24 are possible depending on the requirements for the treated water stream.
For example, as shown
in Figure 7, water stream 26 exiting water cleaning system 10 flows to sulfate
and hardness
26 removal membrane unit 120. Water stream 26 may be ocean water, a fresh
water source and/or a
27 pre-treated produced water. The structure and function of sulfate and
hardness removal membrane
28 unit 120 is described above. Water stream 26 is pressurized to 100-300
psi prior to entering the
29 membrane array of sulfate and hardness removal membrane unit 120 using
pump 122. Water and
some dissolved ionic species, namely sodium and chloride ions, pass through
the membrane and
CPST Doc: 316977.2 30
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 are collected as permeate water stream 124 amounting to 75-85% volume of
water stream 26
2 entering unit 120. The remaining water and higher molecular weight ions,
e.g., divalents such as
3 sulfate and hardness, are rejected by the membrane array and are
discharged to the ocean via outlet
4 126.
[0091] In some embodiments, water stream 124 passes directly to oxygen
removal unit
6 132 (as shown by dashed line 154). In other embodiments, low TDS water is
required for various
7 operations, e.g., EOR, desalting and/or hydrofracturing. As described
above, low TDS water is
8 produced using high pressure, high rejection reverse osmosis membranes.
In these embodiments,
9 permeate water stream 124 flows to TDS removal unit 128. The structure
and operation of TDS
removal unit 128 is described above. Water stream 129, i.e., water collected
from permeate water
11 stream 124, amounts to 30-70% volume of the feed water entering TDS
removal unit 128. The
12 remaining water and lower molecular weight ions such as sodium, i.e.,
waste stream 130, the
13 material rejected by the membranes, are discharged to the ocean or other
suitable receiving body
14 via outlet 131. Where feasible, the high pressure, i.e., energy, of
waste stream 130 can be
recovered using an energy recovery device and returned to the feed stream
through reduction of
16 power to the main feed pump. Suitable energy recovery units include but
are not limited to work
17 exchangers and turbines, e.g., DWEERTM and Calder ERT sold by Flowserve
located in Irving,
18 Texas. Thus, it should be appreciated that the various embodiments
comprise single and double
19 stage reverse osmosis systems.
[0092] After sulfate removal, permeate water stream 124 or 129 flows to
oxygen removal
21 unit 132. The structure and operation of oxygen removal unit 132 is
described above.
22 [0093] Deoxygenated water stream 142 may pass directly out of
the system via outlet 152
23 (as shown by dashed line 156), or alternatively may be further polished
with the use of an catalytic
24 reaction in pressurized vessel 144, as described supra.
[0094] Deoxygenated water stream 148, may pass directly out of the system
via outlet 152,
26 or alternatively may be blended with oxygen scavenger stream 150 such as
hydrazine to further
27 reduce dissolved oxygen concentration below 5 PPB, is then sent to EOR
injection equipment,
28 desalting equipment, hydraulic fracturing equipment, and other suitable
uses, for subsequent use
29 via outlet 152. Thus, it should be appreciated that the foregoing oxygen
removal system may
CPST Doc: 316977.2 31
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 comprise a single or double stage removal system and may further include
incorporation of a
2 chemical oxygen scavenger.
3 [0095] Moreover, some embodiments may pass the water stream
exiting pump 122 directly
4 to oxygen removal unit 132, as shown by dashed line 158, thereby
bypassing both sulfate and
hardness removal membrane unit 120 and TDS removal unit 128. Additionally,
permeate water
6 stream 129 may pass directly to outlet 152 for subsequent use, as shown
by dashed line 160,
7 thereby bypassing oxygen removal unit 132 and pressurized vessel 144.
8 [0096] In some embodiments, removal of boron is required.
Hardness removal membrane
9 systems do not remove boron as it is a monovalent ion (only at higher pH)
so a separate boron
removal step is required following sulfate and hardness removal membrane unit
120 or following
11 TDS removal unit 128. This is accomplished by including boron removal
unit 162 (shown in
12 dashed lines to indicate the optional nature of its inclusion in the
overall system) which includes a
13 boron selective resin such as DOW AMBERLITETm 743. The resin has a
selective affinity for
14 boron over other salts in the water stream. When the resin is saturated
with boron it is regenerated
using a multiple step process of feeding specific dosages of sulfuric acid and
caustic soda and then
16 returned removal unit 162 to service. In the embodiments that include
boron removal unit 162,
17 the boron resin is held in a least one pressure or gravity vessel in
series with sulfate and hardness
18 removal membrane unit 120 or following TDS removal unit 128, as
described above. It should be
19 appreciated that the nature and operation of boron removal unit 162 is
substantially similar to the
nature and operation of heavy metals and dissolved ion removal unit 90
described supra.
21 [0097] It should be further appreciated that brine waste stream
131, from TDS removal
22 unit 128 is typically discharge to a surface body of water or deep well
injected. Alternatively,
23 waste stream 131 can be further concentrated by high pressure sea water
reverse osmosis
24 membranes running at pressures up to 1,000-1,500 psi. At these
pressures, a reverse osmosis
concentrator can produce sodium chloride brines is the range of 8-10%. A
traditional chloralkali
26 process, also known as chlor-alkali and chlor alkali, is an industrial
process for the electrolysis of
27 sodium chloride solution, i.e., brine. This process requires
concentrations of sodium chloride of
28 approximately 300 grams/liter for efficient process performance. As a
final step before sending
29 the brine to a traditional chloralkali process, the brine solution from
the reverse osmosis
concentrator must be concentrated in an evaporation process where additional
water is evaporated
CPST Doc: 316977.2 32
Date Recue/Date Received 2020-11-12
CA 2,869,302
CPST Ref: 11508/00003
1 from the brine to concentrate it to approximately 300 g/L. A typical
piece of equipment to
2 accomplish this step is an evaporator/brine concentrator such as those
sold by General Electric.
3 With the brine conditioned and concentrated, it can be fed to a standard
pretreatment for a known
4 chloralkali process.
[0098] The present invention provides: hydrocarbon recovery; compact
layout; light
6 weight overall structure; minimum chemical use; and, minimum waste
generation. Unique aspects
7 of the present invention include but are not limited to: use of a resin
for oil removal in conjunction
8 with a membrane; use of small foot print filters staged around the resin
unit; an air striper with bio
9 filter for volatile organic compounds and H2S removal; an air striper
with COD resin trap; a heavy
metals removal unit in place of conventional precipitation; blending with a
sea, fresh or treated
11 water waste stream; water conditioned for use in hydrofracturing,
desalting and EOR having high
12 or low TDS; use of degasification membranes; and, degasification
membranes in combination with
13 resin degasification.
14 [099] It will be appreciated that various of the above-
disclosed and other features and
functions, or alternatives thereof, may be desirably combined into many other
different systems or
16 applications. Various presently unforeseen or unanticipated
alternatives, modifications, variations
17 or improvements therein may be subsequently made by those skilled in the
art.
18
CPST Doc: 316977.2 33
Date Recue/Date Received 2020-11-12