Note: Descriptions are shown in the official language in which they were submitted.
CA 02869553 2014-10-03
WO 2013/151711
PCT/US2013/031189
PROCESS FOR RECOVERY OF IRON/STEEL FROM MILL SCALES AND FINES
Cross-Reference to Related Applications
[0001] This application claims priority to U.S. Provisional Application Ser.
No. 61/620,498
titled "Process for Recovery of Iron/Steel from Mill Scales and Fines," filed
on April 5, 2012,
which is incorporated herein, in its entirety, by this reference.
Technical Field
[0002] The present invention relates to a process for the recovery of
iron/steel from scrap
mill scales and fines, and more particularly to the refining and cooling of
molten steel with
mill scale particles recovered from downstream operations of a steel
manufacturing process.
Background Art
[0003] Steel is produced by refining hot metal from a blast furnace. After
molten steel has
been refined, it is solidified and then subjected to successive rolling
operations during which
the steel may be successively heated and cooled. During the successive heating
and cooling
of steel, or during storage between various rolling operations, surface of the
steel oxidizes to
form mill scale. This mill scale flakes off during handling and rolling of the
steel.
[0004] Large amounts of waste materials are produced in steel-making
operations and mill
scale tend to a large portion of such materials. Mill scales include base
metal chips, platelets
and other fines, which are essentially oxides of iron. Such oxides are
primarily composed of
ferrous/ferric iron (Fe304) surrounding a core of the iron base material.
Thus, mill scale is a
prime candidate for recycling in steel making or blast furnace operations
because mill scale is
a relatively coarse, dense, waste oxide material of relatively high iron
content and low in
impurities such as alumina or silica. In addition, fines are generated from
both the
manufacture and the use of Direct Reduced Iron (DRI) pellets. DRI pellets are
a primary
material used by electric arc furnaces in the making of steel and a secondary
material used by
blast furnaces and cupolas. Many of the pellets are crushed during
transportation to mill sites
from production plants and during normal charging operations.
[0005] Numerous processes have been developed to treat and recycle mill scales
but have
met with varying success and are often costly. Some of these processes use
plasma arc
furnaces, briquetting machines and pelletizing systems. These processes are
very expensive,
CA 02869553 2014-10-03
WO 2013/151711
PCT/US2013/031189
inefficient, and require relatively clean mill scale. Thus, there is a need
for an efficient and
inexpensive process for recovering iron/steel from scrap mill scales.
Summary of the Invention
[0006] The present invention relates to a process for enhancing the efficiency
of iron/steel
reclamation from mill scales and fines. It is an object of the present
invention to recover base
metal iron/steel from mill scale platelets and fines. In one embodiment, the
process uses
byproducts of iron and steel melting operations, such as coke and fines, to
effect iron/steel
recovery. The recovery process in accordance with an embodiment of the present
invention
is performed integral to cupola or blast furnace operations, and the recovered
metal is
collected at the base of the furnace as molten iron/steel. A process in
accordance with an
embodiment of the present invention improves energy utilization of recovery
operations by
taking advantage of the inherent solubility of carbon in iron and other
associated
metallurgical interactions. A process in accordance with an embodiment of the
present
invention generates additional heat energy when coke constituents in a feed
mixture ignite
and burn using oxygen released from the iron/steel mill scale components. The
process in
accordance with an embodiment of the present invention also improves molten
base metal
output from earlier melting of mill scale constituents at the upper levels of
a blast furnace.
Brief Description of Drawings
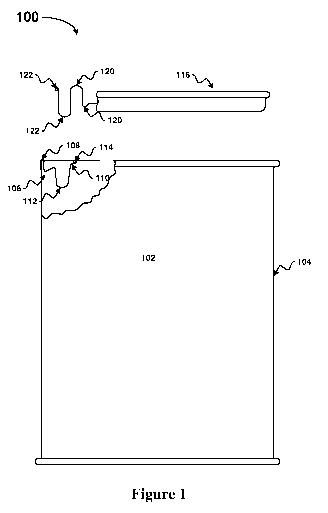
[0007] Figure 1 illustrates an exemplary container used in the process for
recovering
iron/steel from scrap mill scales and fines in accordance with an embodiment
of the present
invention.
Description of Embodiments
[0008] The present invention relates to a process for recovering iron/steel
from scrap mill
scales and fines. In embodiments of the present invention, the process
includes the step of
mixing of components, such as iron/steel chips, platelets and fines, with coke
or other carbon
containing fines to ensure maximum surface contact of all components. In one
embodiment
of the present invention, components are mixed with coke comprising from about
50%
carbon to about 99% carbon. In another embodiment of the present invention,
components
are mixed with coke comprising about 92% carbon. The quantity of coke mixed
with the
components must be sufficient to ensure maximum contact with the surfaces of
iron/steel
component. In some embodiments of the present invention, ratio of carbon to
mill scale is
-2-
CA 02869553 2014-10-03
WO 2013/151711
PCT/US2013/031189
about one carbon to about eight mill scale. Variance of millscale can go
higher depending on
iron content of mill scale. In other embodiments of the present invention,
ratio of DRI fines
to carbon is about one carbon to about twenty five DRI fines. Depending on the
furnace
(electric arc or heat induction versus blast furnace or cupola) variance of
DRI can go higher.
The coke plays a central role in the recovery process because of the
interaction between the
carbon present in the coke with oxygen and iron atoms.
[0009] In a cupola or blast furnace, coke reacts with oxygen in the air to
produce carbon
monoxide:
2 C + 02 ¨> 2 CO
The carbon monoxide reduces iron oxide (Fe203) to molten iron, and transforms
to carbon
dioxide in the process:
Fe203 + 3 CO ¨> 2 Fe + 3 CO2
During operation of cupolas and blast furnaces, elevated temperatures existing
at the upper
levels of cupolas and blast furnaces increase the rotational and vibrational
energies of carbon,
oxygen and iron atoms and their electrons causing accelerated interaction
rates. Carbon has
four electrons available in its 2sp4 orbitals. Oxygen in iron oxide
(FeO.Fe203) shares iron's
two valence electrons, but readily transfers to carbon to share its higher
energy electrons to
form carbon monoxide (CO). This release of oxygen from iron oxide results in
the formation
of elemental iron. CO retains the two additional electrons to breakdown
additional iron oxide
to elemental iron, and forms carbon dioxide (CO2). Excess carbon dissolves in
the elemental
iron to form delta and austenitic phases of the iron/carbon alloy system. In
one embodiment
of the present invention, an addition of 4.3% carbon lowers the melting point
of the
liquid/solid iron/carbon mixture from about 2780 C to about 2090 C. The
austenite phase
is derived from the peritectic reaction of delta ferrite and molten iron-
carbon solution.
During cooling to room temperature, austenite undergoes a eutectoid reaction,
which at room
temperature produces a variety of useful steel structures. Higher carbon
contents of the
iron/carbon alloy system increases the melting point of the system and results
in formation of
cementite (Fe3C). Such increase in the melting point is undesirable for an
economical
recovery of iron/steel from mill scales and fines. However, additional heat
energy can be
derived from the ignition and burning of coke as appropriate furnace
temperatures are
attained. Molten iron/steel is produced in the upper levels of the furnace,
thereby permitting
-3-
CA 02869553 2014-10-03
WO 2013/151711
PCT/US2013/031189
frequent loading of recoverable scrap/coke mix and a resultant increase in
production output.
[0010] In a process in accordance with an embodiment of the present invention,
mill scales
components are mixed with coke or other carbon containing fines to form a
mixture. The
mixture provides all components with maximum contact surface necessary for
efficient
reaction. The mixture is poured into a container and sealed. Referring now to
the drawing,
and more particularly, to Figure 1, there is shown an exemplary container,
generally
designated 100, used in the process for recovering iron/steel from scrap mill
scales and fines
in accordance with an embodiment of the present invention. In container 100 of
Figure 1, a
container body 102 is provided with a side wall 104 leading to a body end 106
which is
covered, in the depicted embodiment, with a ring 106. Methods of forming
container bodies
and container ends and of attaching or coupling the two, to form the depicted
device, are well
known in the art. Ring 106 forms a flange over body end such that a cross
section shaped as
an inverted "U" defines a container rim 108. Ring 106 extends inward from
container rim
108 to form a ring edge 110. Diameter of ring edge 110 is smaller than the
diameter of
container rim 108 such that it forms a concentric ring edge 110 from container
rim 110. Ring
106 forms a curl depression 112 having a U-shaped cross-section as it extends
inward from
container rim 108. Ring edge 110 curls inward to form a ring lip 114.
[0011] The opening at or near the container rim 108 is covered by a container
plug 116
generally extending laterally across the container opening up to container rim
110 such that it
resembles a disc covering the container opening. Viewing from the center
towards the edge,
container plug 116 forms a series of alternating depressions and ridges.
First, container plug
116 forms a curl depression 118 having a shallow U-shaped cross-section.
Immediately
following curl depression 118, container plug 116 forms a ridge 120 having an
inverted U-
shaped cross section. Following ridge 120, container plug 116 forms a second
curl
depression 122 having a U-shaped cross-section. The edge following curl
depression 122
curls outward to form plug lip 124. When container plug 116 is placed over the
container
opening, container plug 116 covers the entire container opening and curl
depression 122
contacts the inner surface of curl depression 112 such that curl depression
122 forms a tight
fit with curl depression 112. The tight fit between curl depression 122 and
curl depression
112 provides a gas-tight seal between container body 102 and container plug
116. In some
embodiments of the present invention, 0.33g of tin solder paste is placed in
the curl
depression prior to placement of the container plug to ensure a gas tight
seal. Post closure,
-4-
CA 02869553 2014-10-03
WO 2013/151711
PCT/US2013/031189
the mini-furnace is placed such that a heat induction system can heat the top
1/16 inch of the
mini-furnace to 550 degrees F in 4.5 seconds. The tin solder flows through the
curl
depression and upon cooling assures a tight and very secure seal.
[0012] Container 100 is made from a material having a composition that is
compatible with
the core components of the mill scales. In one embodiment of the present
invention,
container 100 is a flat rolled steel can having a composition of about 0.15%
by weight.
Container 100 wall is of sufficient thickness so as to withstand the weight of
mill scales, fines
and coke. The container wall is of sufficient thickness so as to withstand
high pressures from
gases generated with the container. In one embodiment of the present
invention, container
100 wall thickness is from about 0.12 inches to about 0.015 inches. The
internal surface of
container 100 is coated with a material that assists in sealing container 100
and enhances the
mill scale conversion reaction. The material used for coating the internal
surface of container
100 has low melting point, attaches or bonds to the internal surface of
container 100, is
ductile, and resistant to corrosion from oils present in contaminants in the
mill scales and
coke. Container 100 internal surface coating material combines with sulfur,
phosphorous,
silicon and other contaminants present in the mill scales and coke to form
compounds that
minimize the dissolution of the contaminants in the recovered molten
iron/steel. The
contaminants combine with coating material to form compounds or eutectics that
limit the
solubility of the contaminants in molten iron/steel. The resulting slag
segregates from the
molten iron/steel, and the coating material can be recovered from the slag.
Exemplary
materials that can be used to coat the internal surface of container 100
include tin, zinc,
aluminum, and the like. In some embodiments of the present invention, internal
surface of
container 100 is coated with tin having a thickness of from about 0.00003
inches to about
0.0001 inches. Container 100 capacity is chosen such that it facilitates
melting and recovery
of mill scale base metal. For example, containers of smaller size are useful
in furnaces
having a fixed opening. Containers of smaller capacity are also useful for
controlling the
quantity of mill scale fed into the furnace without disrupting furnace
operating conditions.
Containers of smaller capacity minimize production losses resulting from
damages to a
container. In one embodiment of the present invention, the volume of container
100 is from
about 0.01 gallons to about 1.5 gallons. In another embodiment of the present
invention, the
volume of container 100 is from about 0.01 gallons to about 1 gallon.
Container 100 include
a plurality of vents to release carbon monoxide and/or carbon dioxide gases
generated by
reactions between components within container 100. In one embodiment of the
present
-5-
CA 02869553 2014-10-03
WO 2013/151711
PCT/US2013/031189
invention, vents are located on the top surface of container 100. In
embodiments of the
present invention where vents located on the top surface of container 100, the
diameters of
the vents are about 0.1875 inches. In another embodiment of the present
invention, vents are
located along the sides of container 100.
[0013] In a process for recovering iron/steel from scrap mill scales, DRI
fines and fines in
accordance with an embodiment of the present invention, containers 100 are fed
into the
furnace from the top of a furnace. Containers 100 comprising the mill scale-
coke mixture are
brought to the top of the furnace along with raw mateirals via a mechanical
device, such as a
crane, a skip car powered by winches or conveyor belts, and the like.
Containers 100 are
charged into the furnace in a manner similar to charging raw materials into
the furnace. In
some embodiments of the process in accordance with the present invention,
containers 100
can be used in blast furnaces having a double bell system, where two bells are
used to control
the entry of raw material into the blast furnace. In such blast furnaces,
containers 100 are
placed into the upper or small bell. The small bell is then rotated to
position container 100
more accurately, and then opens to drop container 100 into the large bell. The
small bell then
closes, to seal the blast furnace, while the large bell dispenses container
100 into the blast
furnace. In other embodiments of the process in accordance with the present
invention,
container 100 can be used in blast furnaces having a bell-less system, where
multiple hoppers
containing multiple containers are discharged into the blast furnace through
valves. In such
blast furnaces having bell-less systems, a chute can also be implemented in
order to precisely
control where container 100 is placed.
[0014] As container 100 travel towards the bottom of the furnace, its contents
and the pallet
are consumed. Elevated temperatures existing at the upper levels of furnaces
and additional
heat obtained from furnace operations permit the contents of container 100 to
react and form
molten metal while containers 100 are still at the upper levels of the
furnaces. Charge levels
are monitored using instruments which determine whether a flat surface is
reestablished at the
bottom. Once a reasonably flat surface is reestablished at the bottom,
additional containers
can be charged into the furnace. In one embodiment of the present invention,
additional
containers can be charged into a cupola at one hour intervals. In another
embodiment of the
present invention, additional containers can be charged into a blast furnace
at every 12 hour
intervals. Thus, the formation of molten iron/steel at the upper levels of the
furnace permit
frequent loading of recoverable scrap/coke mix and a resultant increase in
production output.
-6-
CA 02869553 2014-10-03
WO 2013/151711
PCT/US2013/031189
Molten metal collects at the base of the furnace and withdrawn into ladles.
The molten metal
in ladles are then transferred for downstream processing. For example, the
ladles contents are
poured into ingots or other product molds, allowed to solidify and prepared
for processing
into desired configurations.
[0015] As such, those skilled in the art will appreciate that the conception
upon which this
disclosure is based may readily be utilized as a basis for the designing of
other structures,
methods and systems for carrying out the several purposes of the present
invention. It is
important, therefore, that equivalent constructions insofar as they do not
depart from the spirit
and scope of the present invention, are included in the present invention.
-7-