Note: Descriptions are shown in the official language in which they were submitted.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
1
ETHYLENE RECOVERY BY ABSORPTION
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application is a continuation-in-part of U.S. Patent Application
Serial No.
12/905,966, filed October 15, 2010, entitled "Improved Ethylene Separation,"
which is hereby
incorporated herein by reference in its entirety for all purposes.
BACKGROUND
Field of the Invention
[0002] This disclosure generally relates to the production of polyethylene.
More specifically this
disclosure relates to systems and processes for improving polyethylene
production efficiency by
decreasing ethylene losses.
Background of the Invention
[0003] The production of polymers such as polyethylene from light gases
requires a high purity
feedstock of monomers and comonomers. Due to the small differences in boiling
points between
the light gases in such a feedstock, industrial production of a high purity
feedstock may require
the operation of multiple distillation columns, high pressures, and cryogenic
temperatures. As
such, the energy costs associated with feedstock purification represent a
significant proportion of
the total cost for the production of such polymers. Further, the
infrastructure required for
producing, maintaining, and recycling high purity feedstock is a significant
portion of the
associated capital cost.
[0004] In order to offset some of the costs and maximize production, it can be
useful to reclaim
and/or recycle any unreacted feedstock gases, especially the light hydrocarbon
reactants, such as
ethylene. Gases comprising unreacted monomers may be separated from the
polymer after the
polymerization reaction. The polymer is processed while the unreacted monomers
are recovered
from the gases that are reclaimed following the polymerization reaction. To
accomplish this, the
reclaimed gas streams have conventionally either been routed through a
purification process or
redirected through other redundant processing steps. In either case,
conventional processes of
recovering monomer have necessitated energetically unfavorable and expensive
processes.
[0005] Consequently, there is a need for high-efficiency separation of
ethylene from a recycle
stream.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
2
BRIEF SUMMARY
[0006] Disclosed herein is a process for recovery of ethylene from a
polymerization product
stream of a polyethylene production system, comprising separating a light gas
stream from the
polymerization product stream, wherein the light gas stream comprises ethane
and unreacted
ethylene, contacting the light gas stream with an absorption solvent system,
wherein the
contacting the light gas stream with the absorption solvent system occurs at a
temperature in a
range of from about 40 F to about 110 F, wherein at least a portion of the
unreacted ethylene
from the light gas stream is absorbed by the absorption solvent system, and
recovering unreacted
ethylene from the absorption solvent system to yield recovered ethylene.
[0007] Further disclosed herein is a polyethylene production process,
comprising contacting
ethylene and a polymerization catalyst in a polymerization reactor under
suitable reaction
conditions to yield a polymerization product stream, separating a light gas
stream from the
polymerization product stream, wherein the light gas stream comprises
unreacted ethylene,
contacting the light gas stream with an absorption solvent system in an
absorption reactor at a
temperature in a range of from about 40 F to about 110 F, wherein at least a
portion of the
unreacted ethylene from the light gas stream is absorbed by the absorption
solvent system to yield
a composition comprising a complex of the absorption solvent system and
unreacted ethylene,
removing unabsorbed gases of the light gas stream from contact with the
absorption solvent
system, recovering unreacted ethylene from the absorption solvent system, and
contacting the
recovered ethylene and the polymerization catalyst.
[0008] Also disclosed herein is a polyethylene production system, comprising a
feed stream
comprising ethylene, wherein the feed stream is characterized by introduction
into a
polymerization reactor, a polymerization product stream, wherein the
polymerization product
stream is characterized by emission from the polymerization reactor and
introduction into a
separator, a light gas stream comprising unreacted ethylene, wherein the light
gas stream is
characterized by emission from the separator, the light gas stream having been
separated from the
polymerization product stream, wherein the light gas stream is characterized
by introduction into
an absorption solvent system, wherein the absorption solvent system has a
temperature in a range
of from about 40 F to about 110 F, an absorbent-ethylene conjugant, wherein
the absorbent-
ethylene conjugant is characterized by formation within the absorption solvent
system by
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
3
absorption of at least a portion of the unreacted ethylene by the absorption
solvent system, and a
waste gas stream comprising ethane, wherein the waste gas stream is
characterized by emission
from the absorption reactor, wherein the waste gas stream comprises components
of the light gas
stream that are not absorbed by the absorption solvent system, and a recovered
unreacted ethylene
stream, wherein the recovered unreacted ethylene stream is characterized by
emission from the
absorption reactor and reintroduction into the polymerization reactor.
[0009] Also disclosed herein is a polyethylene production system, comprising a
polymerization
reactor, wherein the polymerization reactor is configured to receive a feed
stream comprising
ethylene, and wherein the polymerization reactor is configured to emit a
polymerization product
stream, a separator, wherein the separator is configured to receive the
polymerization product
stream and to emit a light gas stream comprising unreacted ethylene, wherein
the light gas stream
has been separated from the polymerization product stream, and an absorption
reactor comprising
an absorption solvent system, wherein the absorption reactor is configured to
receive the light gas
stream, to absorb at least a portion of the unreacted ethylene with the
absorption solvent system at
a temperature in a range of from about 40 F to about 110 F, and to emit a
waste gas stream
comprising components of the light gas stream that are not absorbed by the
absorption solvent
system, and wherein the absorption reactor is further configured to emit a
recovered unreacted
ethylene stream, and wherein the polymerization reactor is further configured
to receive the
recovered unreacted ethylene stream.
[0010] Also disclosed herein is a polyethylene production system, comprising a
polymerization
reactor, wherein the polymerization reactor is configured to receive a feed
stream comprising
ethylene, and wherein the polymerization reactor is configured to emit a
polymerization product
stream, a separator, wherein the separator is configured to receive the
polymerization product
stream and to emit a light gas stream comprising unreacted ethylene, wherein
the light gas stream
has been separated from the polymerization product stream, an absorption
reactor comprising an
absorption solvent system, wherein the absorption reactor is configured to
receive the light gas
stream, to absorb at least a portion of the unreacted ethylene with the
absorption solvent system at
a temperature in a range of from about 40 F to about 110 F and to emit a
waste gas stream
comprising components of the light gas stream that are not absorbed by the
absorption solvent
system, wherein the absorption reactor is further configured to emit a
complexed stream
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
4
comprising ethylene absorbed in the absorbent solvent system, and a solvent
regenerator to
regenerate the absorption solvent system, and to emit a recovered unreacted
ethylene stream,
wherein the polymerization reactor is further configured to receive the
recovered unreacted
ethylene stream.
[0011] The foregoing has outlined rather broadly the features and technical
advantages of the
disclosed inventive subject matter in order that the following detailed
description may be better
understood. The various characteristics described above, as well as other
features, will be readily
apparent to those skilled in the art upon reading the following detailed
description of the preferred
embodiments, and by referring to the accompanying drawings.
DESCRIPTION OF THE DRAWINGS
[0012] For a detailed description of the preferred embodiments of the
disclosed processes and
systems, reference will now be made to the accompanying drawings in which:
[0013] FIGURE 1 illustrates a schematic of a first embodiment of a
polyethylene production
system;
[0014] FIGURE 2 illustrates a schematic of a second embodiment of a
polyethylene production
system;
[0015] FIGURE 3 illustrates a schematic of a third embodiment of a
polyethylene production
system;
[0016] FIGURE 4 illustrates a flow diagram of a first embodiment of a
polyethylene production
process;
[0017] FIGURE 5 illustrates a flow diagram of a second embodiment of a
polyethylene
production process;
[0018] FIGURE 6 illustrates a flow diagram of a third embodiment of a
polyethylene production
process;
[0019] FIGURE 7 is a graph illustrating solubility versus temperature for
ethylene and ethane in
an absorption solvent system;
[0020] FIGURE 8 illustrates a schematic of an embodiment of an absorption
reactor having a
pressure swing absorption configuration;
[0021] FIGURE 9 illustrates a schematic of an embodiment of an absorption
system; and
[0022] FIGURE 10 illustrates a schematic of an embodiment of a simulated
absorption system.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
DETAILED DESCRIPTION
[0023] Disclosed herein are systems, apparatuses, and processes related to the
production of
polyethylene with improved efficiency. The systems, apparatuses, and processes
are generally
related to the separation of a first chemical component or compound from a
composition resulting
from the production of polyethylene and comprising the first chemical
component or compound
and one or more other chemical components, compounds, or the like.
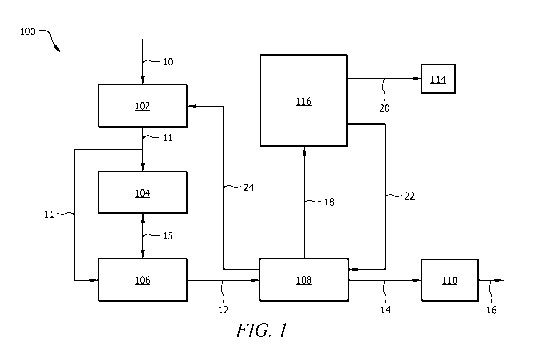
[0024] Referring to Figure 1, a first polyethylene production (PEP) system 100
is disclosed.
PEP system 100 generally comprises a purifier 102, reactors 104, 106, a
separator 108, a processor
110, an absorption reactor 116, and a processing device 114. In the PEP
embodiments disclosed
herein, various system components may be in fluid communication via one or
more conduits (e.g.,
pipes, tubing, flow lines, etc.) suitable for the conveyance of a particular
stream, for example as
show in detail by the numbered streams in Figures 1-3.
[0025] In the embodiment of Figure 1, a feed stream 10 may be communicated to
the purifier
102. A purified feed stream 11 may be communicated from the purifier 102 to
one or more of the
reactors 104, 106. Where such a system comprises two or more reactors, a
reactor stream 15 may
be communicated from reactor 104 to reactor 106. A polymerization product
stream 12 may be
communicated from one or more of the reactors 104, 106 to the separator 108. A
polymer stream
14 may be communicated from the separator 108 to the processor 110. A product
stream 16 may
be emitted from the processor 110. A gas stream 18 may be communicated from
the separator 108
to the absorption reactor 116. A waste gas stream 20 may be communicated from
the absorption
reactor 116 to the processing device 114 and a recycle stream 22 may be
communicated from the
absorption reactor 116 to the separator 108. A reintroduction stream 24 may be
communicated
from the separator 108 to the purifier 102.
[0026] Referring to Figure 2, a second PEP system 200 is disclosed, which has
a number of
system components common with PEP 100. In the alternative embodiment
illustrated by Figure 2,
the second PEP system 200 additionally comprises a deoxygenator 118.
Alternatively to the first
PEP system 100 (as illustrated in Figure 1), in the embodiment illustrated by
Figure 2, the gas
stream 18 may be communicated to the deoxygenator 118. A treated gas stream 26
may be
communicated from the deoxygenator 118 to the absorption reactor 116.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
6
[0027] Referring to Figure 3, a third PEP system 300 is disclosed, which has a
number of system
components common with PEP 100 and PEP 200. In the alternative embodiment
illustrated by
Figure 3, the third PEP system 300 additionally comprises a regenerator 120
(e.g., a desorption
vessel). Alternatively to the first and second PEP systems 100 and 200,
respectively, in the
embodiment illustrated in Figure 3, a complexed stream 28 may be communicated
from the
absorption reactor 116 to the regenerator 120. A recycle stream 22 may be
communicated from the
regenerator 120 to the separator 108, and a regenerated absorbent stream 30
may be communicated
from the regenerator 120 to the absorption reactor 116.
[0028] In Figure 3, a temperature of lean solvent may be taken from stream 30.
The temperature
of the absorption reactor 116 may depend on a temperature of gas stream 18, a
temperature of lean
solvent in stream 30, a heat of solution, and a heat of reaction. In the
disclosed embodiments, the
mass flow rate of lean solvent in stream 30 may be 50 to 300 times greater
than a mass flow rate of
the gas stream 18. Therefore, the temperature of the absorption reactor 116
may highly depend on
the temperature of lean solvent in the disclosed embodiments.
[0029] Various embodiments of suitable PEP systems having been disclosed,
embodiments of a
PEP process are now disclosed. One or more of the embodiments of a PEP process
may be
described with reference to one or more of PEP system 100, PEP system 200,
and/or PEP system
300. Although a given PEP process may be described with reference to one or
more embodiments
of a PEP system, such a disclosure should not be construed as so-limiting.
Although the various
steps of the processes disclosed herein may be disclosed or illustrated in a
particular order, such
should not be construed as limiting the performance of these processes to any
particular order
unless otherwise indicated.
[0030] Referring to Figure 4, a first PEP process 400 is illustrated. PEP
process 400 generally
comprises at block 51 purifying a feed stream, at block 52 polymerizing
monomers of the purified
feed stream to form a polymerization product, at block 53 separating the
polymerization product
into a polymer stream and a gas stream, at block 54 processing the polymer
stream, at block 55
separating at least one gaseous component from the gas stream to form a
recycle stream and a
waste stream, and at block 56 combusting the waste stream.
[0031] In an embodiment, the first PEP process 400 or a portion thereof may be
implemented via
the first PEP system 100 (e.g., as illustrated in Figure 1). Referring to
Figures 1 and 4, in an
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
7
embodiment the feed stream 10 may comprise a gaseous reactant, particularly,
ethylene. In an
embodiment, purifying the feed stream may yield a purified stream 11
comprising substantially
pure monomers (e.g., ethylene monomers), comonomers (e.g., butene-1
comonomers, or
combinations thereof. Polymerizing monomers (optionally, comonomers) of the
purified stream
11 may yield the polymerization product stream 12 generally comprising
unreacted monomer (e.g.,
ethylene), optional unreacted comonomer (e.g., butene-1), by-products (e.g.,
ethane, which may be
by-product ethane formed from ethylene and hydrogen), and a polymerization
product (e.g.,
polymer and optionally, copolymer). Separating the polymerization product
stream 12 may yield
the polymer stream 14 (e.g., polyethylene polymer, copolymer) and the gas
stream 18 generally
comprising unreacted monomer (e.g., ethylene monomer and any optional
comonomer such as
butene-1) and various waste gases (e.g., ethane). Processing the polymer
stream 14 may yield the
product stream 16. Separating at least one gaseous component from the gas
stream 18 may yield a
recycle stream 22, generally comprising unreacted ethylene monomer
(optionally, unreacted
comonomer), and a waste gas stream 20. In an embodiment, separating the gas
stream 18
comprises absorbing ethylene from the gas stream 18 to yield the waste gas
stream 20 and then
releasing the absorbed ethylene to form the recycle stream 22. The recycle
stream 22, comprising
ethylene, may be pressurized (e.g., returned to the separator 108 for
pressurization) and re-
introduced into a PEP process (e.g., PEP process 400) as reintroduction stream
24. Combusting
the waste gas stream 20 may be carried out with a flare as the processing
device 114.
[0032] Referring to Figure 5, a second PEP process 500 is illustrated, which
has a number of
process steps common with PEP process 400. In the alternative embodiment
illustrated by Figure
5, block 55 of Figure 4 is enhanced by at block 57 treating the gas stream to
form a treated gas
stream and at block 55' separating at least one gaseous component from the
treated gas stream to
form a recycle stream and a waste stream.
[0033] In an embodiment, second PEP process 500 or a portion thereof may be
implemented via
the second PEP system 200 (e.g. as illustrated in Figure 2). Alternatively to
the embodiments of
Figures 1 and 4, in the embodiment of Figures 2 and 5 treating the gas stream
18 may yield the
treated gas stream 26. In an embodiment, treating the gas stream 18 comprises
deoxygenating the
gas stream 18. Separating at least one gaseous component from the treated gas
stream 26 may
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
8
yield a recycle stream 22, generally comprising unreacted ethylene monomer
(optionally,
comonomer), and a waste gas stream 20.
[0034] Referring to Figure 6, a third PEP process 600 is illustrated, which
has a number of
process steps common with PEP process 500. In the alternative embodiment
illustrated by Figure
6, block 55' of Figure 5 is enhanced by at block 55" separating at least one
gaseous component
from the treated gas stream to form a complexed stream and a waste gas stream
and at block 58
separating the complexed stream into an absorbent stream and a recycle stream.
[0035] In an embodiment, third PEP process 600 or a portion thereof may be
implemented via
the third PEP system 300 (e.g. as illustrated in Figure 3). Alternatively to
the embodiments of
Figures 1&4 and 2&5, in the embodiment of Figures 3 and 6 separating at least
one gaseous
component from the treated gas stream 26 may yield an unreacted monomer-
absorbent (e.g., an
ethylene-absorbent) in complexed stream 28. In an embodiment, separating the
unreacted
monomer-absorbent complexed stream 28 comprises releasing the absorbed
ethylene to form a
recycle stream 22 and a regenerated absorbent stream 30. In the embodiment of
Figures 3 and 6,
separating at least one gaseous component from the treated gas stream 26 may
yield an unreacted
comonomer-absorbent (e.g., a butene- 1-absorbent) in complexed stream 28. In
an embodiment,
separating the unreacted comonomer-absorbent in complexed stream 28 comprises
releasing the
absorbed comonomer to form a recycle stream 22 and a regenerated absorbent
stream 30.
[0036] In one or more of the embodiments disclosed herein, purifying a feed
stream (e.g., at
block 51) may comprise separating unwanted compounds and elements from a feed
stream
comprising ethylene to form a purified feed stream. In an embodiment, the feed
stream may
comprise ethylene and various other gases, such as but not limited to methane,
ethane, acetylene,
propylene, various other hydrocarbons having three or more carbon atoms, or
combinations
thereof. In an embodiment, purifying a feed stream may comprise any suitable
method or process,
including the non-limiting examples filtering, membrane screening, reacting
with various
chemicals, absorbing, adsorbing, distillation(s), or combinations thereof.
[0037] In embodiments as illustrated by Figures 1-3, purifying a feed stream
may comprise
routing the feed stream 10 to the purifier 102. In one or more of the
embodiments disclosed herein,
the purifier 102 may comprise a device or apparatus suitable for the
purification of one or more
reactant gases in a feed stream comprising a plurality of potentially unwanted
gaseous compounds,
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
9
elements, contaminants, or the like. Non-limiting examples of a suitable
purifier 102 may
comprise a filter, a membrane, a reactor, an absorbent, a molecular sieve, one
or more distillation
columns, or combinations thereof. The purifier 102 may be configured to
separate ethylene from a
stream comprising methane, ethane, acetylene, propane, propylene, water,
oxygen various other
gaseous hydrocarbons, various contaminants, and/or combinations thereof.
[0038] In an embodiment, purifying a feed stream may yield a purified feed 11
comprising
substantially pure ethylene. In an embodiment, the purified feed stream may
comprise less than
25% by total weight of the stream, alternatively, less than about 10%,
alternatively, less than about
1.0% of any one or more of nitrogen, oxygen, methane, ethane, propane, or
combinations thereof.
As used herein "substantially pure ethylene" refers to a fluid stream
comprising at least about 60%
ethylene, alternatively, at least about 70% ethylene, alternatively, at least
about 80% ethylene,
alternatively, at least about 90% ethylene, alternatively, at least about 95%
ethylene, alternatively,
at least about 99% ethylene by total weight of the stream, alternatively, at
least about 99.5%
ethylene by total weight of the stream. In an embodiment, the feed stream 11
may further
comprise trace amounts of ethane, for example, as from a recycle stream as
will be discussed.
[0039] In one or more of the embodiments disclosed herein, polymerizing
monomers of the
purified feed (e.g., at block 52) may comprise allowing a polymerization
reaction between a
plurality of monomers by contacting a monomer or monomers with a catalyst
system under
conditions suitable for the formation of a polymer. In one or more of the
embodiments disclosed
herein, polymerizing comonomers (e.g., at block 52) may comprise allowing a
polymerization
reaction between a plurality of comonomers by contacting a comonomer or
comonomers with a
catalyst system under conditions suitable for the formation of a copolymer. In
an embodiment, any
suitable catalyst system may be employed. A suitable catalyst system may
comprise a catalyst
and, optionally, a co-catalyst and/or promoter. Nonlimiting examples of
suitable catalyst systems
include Ziegler Natta catalysts, Ziegler catalysts, chromium catalysts,
chromium oxide catalysts,
chromocene catalysts, metallocene catalysts, nickel catalysts, or combinations
thereof. Catalyst
systems suitable for use in this disclosure have been described, for example,
in U.S. Patent No.
7,619,047 and U.S. Patent Application Publication Nos. 2007/0197374,
2009/0004417,
2010/0029872, 2006/0094590, and 2010/0041842, each of which is incorporated by
reference
herein in its entirety.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
[0040] In embodiments as illustrated by Figures 1-3, polymerizing monomers of
the purified
feed may comprise routing the feed stream 11 to the polymerization reactors or
"reactors" 104,
106. In one or more of the embodiments disclosed herein, the reactors 104, 106
may comprise any
vessel or combination of vessels suitably configured to provide an environment
for a chemical
reaction (e.g., a contact zone) between monomers (e.g., ethylene) and/or
polymers (e.g., an
"active" or growing polymer chain), and optionally comonomers (e.g., butene-1)
and/or
copolymers, in the presence of a catalyst to yield a polymer (e.g., a
polyethylene polymer) and/or
copolymer. Although the embodiments illustrated in Figures 1, 2, and 3,
illustrate various PEP
systems having two reactors in series, one of skill in the art viewing this
disclosure will recognize
that one reactor, alternatively, any suitable number and/or configuration of
reactors may be
employed.
[0041] As used herein, the terms "polymerization reactor" or "reactor" include
any
polymerization reactor capable of polymerizing olefin monomers or comonomers
to produce
homopolymers or copolymers. Such homopolymers and copolymers are referred to
as resins or
polymers. The various types of reactors include those that may be referred to
as batch, slurry, gas-
phase, solution, high pressure, tubular or autoclave reactors. Gas phase
reactors may comprise
fluidized bed reactors or staged horizontal reactors. Slurry reactors may
comprise vertical or
horizontal loops. High pressure reactors may comprise autoclave or tubular
reactors. Reactor
types can include batch or continuous processes. Continuous processes could
use intermittent or
continuous product discharge. Processes may also include partial or full
direct recycle of
unreacted monomer, unreacted comonomer, and/or diluent.
[0042] Polymerization reactor systems of the present disclosure may comprise
one type of
reactor in a system or multiple reactors of the same or different type.
Production of polymers in
multiple reactors may include several stages in at least two separate
polymerization reactors
interconnected by a transfer device making it possible to transfer the
polymers resulting from the
first polymerization reactor (e.g., reactor 104) into the second reactor
(e.g., reactor 106). The
desired polymerization conditions in one of the reactors may be different from
the operating
conditions of the other reactors. Alternatively, polymerization in multiple
reactors may include the
manual transfer of polymer from one reactor to subsequent reactors for
continued polymerization.
Multiple reactor systems may include any combination including, but not
limited to, multiple loop
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
11
reactors, multiple gas reactors, a combination of loop and gas reactors,
multiple high pressure
reactors or a combination of high pressure with loop and/or gas reactors. The
multiple reactors
may be operated in series or in parallel.
[0043] According to one aspect, the polymerization reactor system may comprise
at least one
loop slurry reactor comprising vertical or horizontal loops. Monomer, diluent,
catalyst, and
optionally any comonomer, may be continuously fed to a loop reactor where
polymerization
occurs. Generally, continuous processes may comprise the continuous
introduction of a monomer,
an optional comonomer, a catalyst, and a diluent into a polymerization reactor
and the continuous
removal from this reactor of a suspension comprising polymer particles and the
diluent. Reactor
effluent may be flashed to remove the solid polymer from the liquids that
comprise the diluent,
monomer and/or comonomer. Various technologies may be used for this separation
step including
but not limited to, flashing that may include any combination of heat addition
and pressure
reduction; separation by cyclonic action in either a cyclone or hydrocyclone;
or separation by
centrifugation.
[0044] In one or more embodiments, a comonomer may comprise unsaturated
hydrocarbons
having 3 to 12 carbon atoms. For example, a comonomer may comprise propene,
butene-1,
hexene-1, octenes, or combinations thereof.
[0045] A typical slurry polymerization process (also known as the particle
form process), is
disclosed, for example, in U.S. Patent Nos. 3,248,179, 4,501,885, 5,565,175,
5,575,979, 6,239,235,
6,262,191 and 6,833,415, each of which is incorporated by reference in its
entirety herein.
[0046] In embodiments, suitable diluents used in slurry polymerization
include, but are not
limited to, the monomer, and optionally, the comonomer, being polymerized and
hydrocarbons that
are liquids under reaction conditions. Examples of suitable monomer diluents
include, but are not
limited to, hydrocarbons such as propane, cyclohexane, isobutane, n-butane, n-
pentane, isopentane,
neopentane, and n-hexane. In embodiments, comonomer diluents may comprise
unsaturated
hydrocarbons having 3 to 12 carbon atoms. Examples of suitable comonomer
diluents include, but
are not limited to propene, butene-1, hexene-1, octenes, or combinations
thereof. Some loop
polymerization reactions can occur under bulk conditions where no diluent is
used. An example is
polymerization of propylene monomer as disclosed in U.S. Patent Nos.
5,455,314, which is
incorporated by reference herein in its entirety.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
12
[0047] According to yet another aspect, the polymerization reactor may
comprise at least one gas
phase reactor. Such systems may employ a continuous recycle stream containing
one or more
monomers continuously cycled through a fluidized bed in the presence of the
catalyst under
polymerization conditions. A recycle stream may be withdrawn from the
fluidized bed and
recycled back into the reactor. Simultaneously, polymer product may be
withdrawn from the
reactor and new or fresh monomer may be added to replace the polymerized
monomer. Likewise,
copolymer product may optionally be withdrawn from the reactor and new or
fresh comonomer
may be added to replace polymerized comonomer, polymerized monomer, or
combinations
thereof Such gas phase reactors may comprise a process for multi-step gas-
phase polymerization
of olefins, in which olefins are polymerized in the gaseous phase in at least
two independent gas-
phase polymerization zones while feeding a catalyst-containing polymer formed
in a first
polymerization zone to a second polymerization zone. One type of gas phase
reactor is disclosed
in U.S. Patent Nos. 5,352,749, 4588,790 and 5,436,304, each of which is
incorporated by reference
in its entirety herein.
[0048] According to still another aspect, a high pressure polymerization
reactor may comprise a
tubular reactor or an autoclave reactor. Tubular reactors may have several
zones where fresh
monomer (optionally, comonomer), initiators, or catalysts may be added.
Monomer (optionally,
comonomer) may be entrained in an inert gaseous stream and introduced at one
zone of the reactor.
Initiators, catalysts, and/or catalyst components may be entrained in a
gaseous stream and
introduced at another zone of the reactor. The gas streams may be intermixed
for polymerization.
Heat and pressure may be employed appropriately to obtain optimal
polymerization reaction
conditions.
[0049] According to yet another aspect, the polymerization reactor may
comprise a solution
polymerization reactor wherein the monomer (optionally, comonomer) may be
contacted with the
catalyst composition by suitable stirring or other means. A carrier comprising
an inert organic
diluent or excess monomer (optionally, comonomer) may be employed. If desired,
the monomer
and/or optional comonomer may be brought in the vapor phase into contact with
the catalytic
reaction product, in the presence or absence of liquid material. The
polymerization zone is
maintained at temperatures and pressures that will result in the formation of
a solution of the
polymer in a reaction medium. Agitation may be employed to obtain better
temperature control
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
13
and to maintain uniform polymerization mixtures throughout the polymerization
zone. Adequate
means are utilized for dissipating the exothermic heat of polymerization.
[0050] Polymerization reactors suitable for the disclosed systems and
processes may further
comprise any combination of at least one raw material feed system, at least
one feed system for
catalyst or catalyst components, and/or at least one polymer recovery system.
Suitable reactor
systems may further comprise systems for feedstock purification, catalyst
storage and preparation,
extrusion, reactor cooling, polymer recovery, fractionation, recycle, storage,
loadout, laboratory
analysis, and process control.
[0051] Conditions that are controlled for polymerization efficiency and to
provide resin
properties include temperature, pressure and the concentrations of various
reactants.
Polymerization temperature can affect catalyst productivity, polymer molecular
weight and
molecular weight distribution. Suitable polymerization temperature may be any
temperature below
the de-polymerization temperature according to the Gibbs Free energy equation.
Typically this
includes from about 60 C to about 280 C, for example, and from about 70 C to
about 110 C,
depending upon the type of polymerization reactor.
[0052] Suitable pressures will also vary according to the reactor and
polymerization type. The
pressure for liquid phase polymerizations in a loop reactor is typically less
than 1000 psig.
Pressure for gas phase polymerization is usually at about 200 to 500 psig.
High pressure
polymerization in tubular or autoclave reactors is generally run at about
20,000 to 75,000 psig.
Polymerization reactors can also be operated in a supercritical region
occurring at generally higher
temperatures and pressures. Operation above the critical point of a
pressure/temperature diagram
(supercritical phase) may offer advantages. In an embodiment, polymerization
may occur in an
environment having a suitable combination of temperature and pressure. For
example,
polymerization may occur at a pressure in a range from about 550 psi to about
650 psi,
alternatively, about 600 psi to about 625 psi and a temperature in a range
from about 170 F to
about 230 F, alternatively, from about 195 F to about 220 F.
[0053] The concentration of various reactants can be controlled to produce
resins with certain
physical and mechanical properties. The proposed end-use product that will be
formed by the resin
and the method of forming that product determines the desired resin
properties. Mechanical
properties include tensile, flexural, impact, creep, stress relaxation and
hardness tests. Physical
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
14
properties include density, molecular weight, molecular weight distribution,
melting temperature,
glass transition temperature, temperature melt of crystallization, density,
stereoregularity, crack
growth, long chain branching and rheological measurements.
[0054] The concentrations and/or partial pressures of monomer, comonomer,
hydrogen, co-
catalyst, modifiers, and electron donors are important in producing these
resin properties.
Comonomer may be used to control product density. Hydrogen may be used to
control product
molecular weight. Cocatalysts can be used to alkylate, scavenge poisons and
control molecular
weight. Modifiers can be used to control product properties and electron
donors affect
stereoregularity, the molecular weight distribution, or molecular weight. In
addition, the
concentration of poisons is minimized because poisons impact the reactions and
product properties.
[0055] In an embodiment, polymerizing monomers of the purified feed may
comprise
introducing a suitable catalyst system into the first and/or second reactor
104, 106, respectively, so
as to form a slurry. Alternatively, a suitable catalyst system may reside in
the first and/or second
reactor 104, 106, respectively.
[0056] As explained above, polymerizing monomers of the purified feed may
comprise
selectively manipulating one or more polymerization reaction conditions to
yield a given polymer
product, to yield a polymer product having one or more desirable properties,
to achieve a desired
efficiency, to achieve a desired yield, the like, or combinations thereof Non-
limiting examples of
such parameters include temperature, pressure, type and/or quantity of
catalyst or co-catalyst, and
the concentrations and/or partial pressures of various reactants. In an
embodiment, polymerizing
monomers of the purified feed 52 may comprise adjusting one or more
polymerization reaction
conditions.
[0057] In an embodiment, polymerizing monomers of the purified feed may
comprise
maintaining a suitable temperature, pressure, and/or partial pressure(s)
during the polymerization
reaction, alternatively, cycling between a series of suitable temperatures,
pressures, and/or partials
pressure(s) during the polymerization reaction.
[0058] In an embodiment, polymerizing monomers of the purified feed may
comprise
circulating, flowing, cycling, mixing, agitating, or combinations thereof, the
monomers (optionally,
comonomers), catalyst system, and/or the slurry within and/or between the
reactors 104, 106. In an
embodiment where the monomers (optionally, comonomers), catalyst system,
and/or slurry are
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
circulated, circulation may be at a velocity (e.g., slurry velocity) of from
about 1 m/s to about 30
m/s, alternatively, from about 2 m/s to about 17 m/s, alternatively, from
about 3 m/s to about 15
m/s.
[0059] In an embodiment, polymerizing monomers of the purified feed may
comprise
configuring reactors 104, 106 to yield a multimodal (e.g., a bimodal) polymer
(e.g., polyethylene).
For example, the resultant polymer may comprise both a relatively high
molecular weight, low
density (HMWLD) polyethylene polymer and a relatively low molecular weight,
high density
(LMWHD) polyethylene polymer. For example, various types of suitable polymers
may be
characterized as having a various densities. For example, a Type I may be
characterized as having
a density in a range of from about 0.910 g/cm3 to about 0.925 g/cm3,
alternatively, a Type II may
be characterized as having a density from about 0.926 g/cm3 to about 0.940
g/cm3, alternatively, a
Type III may be characterized as having a density from about 0.941 g/cm3 to
about 0.959 g/cm3,
alternatively, a Type IV may be characterized as having a density of greater
than about 0.960
g/cm3.
[0060] In an embodiment, polymerizing monomers may comprise polymerizing
comonomers in
one or more of polymerization reactors 104, 106.
[0061] In the embodiments illustrated in Figures 1-3, polymerizing monomers of
the purified
feed may yield a polymerization product stream 12. Such a polymerization
product stream 12 may
generally comprise various solids, semi-solids, volatile and nonvolatile
liquids, gases and
combinations thereof. In an embodiment, the polymerization product stream 12
may comprise
hydrogen, nitrogen, methane, ethylene, ethane, propylene, propane, butane,
isobutane, pentane,
hexane, hexene-1 and heavier hydrocarbons. In an embodiment, ethylene may be
present in a
range of from about 0.1% to about 15%, alternatively, from about 1.5% to about
5%, alternatively,
about 2% to about 4% by total weight of the stream. Ethane may be present in a
range of from
about 0.001% to about 4%, alternatively, from about 0.2% to about 0.5% by
total weight of the
stream. Isobutane may be present in a range from about 80% to about 98%,
alternatively, from
about 92% to about 96%, alternatively, about 95% by total weight of the
stream.
[0062] The solids and/or liquids may comprise a polymer product (e.g., a
polyethylene polymer),
often referred to at this stage of the PEP process as "polymer fluff." The
gases may comprise
unreacted, gaseous reactant monomers or optional comonomers (e.g., unreacted
ethylene
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
16
monomers, unreacted butene-1 monomers), gaseous waste products, gaseous
contaminants, or
combinations thereof.
[0063] In one or more of the embodiments disclosed herein, separating the
polymerization
product into a polymer stream and a gas stream (e.g., at block 53) may
generally comprise
removing any gases from liquids and/or solids (e.g., the polymer fluff) by any
suitable process.
[0064] In embodiments as illustrated by Figures 1-3, separating the
polymerization product into
a polymer stream and a gas stream may comprise routing the polymerization
product steam 12 to
the separator 108. In one or more of the embodiments disclosed herein, the
separator 108 may be
configured to separate a stream (e.g., polymerization product comprising
polyethylene) into gases,
liquids, solids, or combinations thereof. The reaction product may comprise
unreacted, gaseous
monomers or optional comonomers (e.g., unreacted ethylene monomers, unreacted
butene-1
monomers), gaseous waste products, and/or gaseous contaminants. As used
herein, an "unreacted
monomer," for example, ethylene, refers to a monomer that was introduced into
a polymerization
reactor during a polymerization reaction but was not incorporated into a
polymer. As used herein,
an "unreacted comonomer," for example, butene-1, refers to a comonomer that
was introduced into
a polymerization reactor during a polymerization reaction but was not
incorporated into a polymer.
[0065] In an embodiment, the separator 108 may comprise a vapor-liquid
separator. Suitable
examples of such a separator may include a distillation column, a flash tank,
a filter, a membrane, a
reactor, an absorbent, an adsorbent, a molecular sieve, or combinations
thereof. In an embodiment,
the separator comprises a flash tank. Not seeking to be bound by theory, such
a flash tank may
comprise a vessel configured to vaporize and/or remove low vapor pressure
components from a
high temperature and/or high pressure fluid. The separator 108 may be
configured such that an
incoming stream may be separated into a liquid stream (e.g., a condensate
stream) and a gas (e.g.,
vapor) stream. The liquid or condensate stream may comprise a reaction product
(e.g.,
polyethylene, often referred to as "polymer fluff'). The gas or vapor stream
may comprise volatile
solvents, gaseous, unreacted monomers and/or optional comonomers, waste gases
(secondary
reaction products, such as contaminants and the like), or combinations
thereof. The separator may
be configured such that the feed stream is flashed by heat, pressure
reduction, or both such that the
enthalpy of the stream is increased. This may be accomplished via a heater, a
flashline heater,
various other operations commonly known in the art, or combinations thereof.
For example, a
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
17
flash line heater comprising a double pipe may exchange heat by hot water or
steam. Such a
flashline heater may increase the temperature of the stream while reducing its
pressure.
[0066] In an embodiment, separating the polymerization product into a polymer
stream and a gas
stream may comprise distilling, vaporizing, flashing, filtering, membrane
screening, absorbing,
adsorbing, or combinations thereof, the polymerization product. In the
embodiments illustrated in
Figures 1-3, separating the polymerization product into a polymer stream and a
gas stream yields a
gas stream 18 and a polymer stream 14. In an embodiment, the gas stream 18 may
comprise
hydrogen, nitrogen, methane, ethylene, ethane, propylene, propane, butane,
isobutane, pentane,
hexane, hexene-1 and heavier hydrocarbons. In an embodiment, ethylene may be
present in a
range of from about 0.1% to about 15%, alternatively, from about 1.5% to about
5%, alternatively,
about 2% to about 4% by total weight of the stream. Ethane may be present in a
range of from
about 0.001% to about 4%, alternatively, from about 0.2% to about 0.5% by
total weight of the
stream. Isobutane may be present in a range from about 80% to about 98%,
alternatively, from
about 92% to about 96%, alternatively, about 95% by total weight of the
stream.
[0067] In one or more one or more of the embodiments disclosed herein,
processing the polymer
stream (e.g., at block 54) comprises any suitable process or series of
processes configured to
produce a polymer product as may be suitable for commercial or industrial
usage, storage,
transportation, further processing, or combinations thereof.
[0068] In embodiments as illustrated by Figures 1-3, processing the polymer
stream may
comprise routing the polymer stream 14 to the processor 110. The processor 110
may be
configured for the performance of a suitable processing means, nonlimiting
examples of which
include cooling, injection molding, melting, pelletizing, blow molding,
extrusion molding,
rotational molding, thermoforming, cast molding, the like, or combinations
thereof. Various
additives and modifiers may be added to the polymer to provide better
processing during
manufacturing and for desired properties in the end product. Nonlimiting
examples of such
additives may include surface modifiers such as slip agents, antiblocks,
tackifiers; antioxidants
such as primary and secondary antioxidants; pigments; processing aids such as
waxes/oils and
fluoroelastomers; and special additives such as fire retardants, antistats,
scavengers, absorbers,
odor enhancers, and degradation agents.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
18
[0069] In an embodiment, the processor 110 may be configured to form a
suitable polymer
product. Nonlimiting examples of suitable polymer products as may result from
such processing
include films, powders, pellets, resins, liquids, or any other suitable form
as will be appreciated by
those of skill in the art. Such a suitable output may be for use in, for
examples, one or more of
various consumer or industrial products. For example, the polymer product may
be utilized any
one or more of various articles, including, but not limited to, bottles,
drums, toys, household
containers, utensils, film products, drums, fuel tanks, pipes, geomembranes,
and liners. In a
particular embodiment, the processor is configured to form pellets for
transportation to a consumer
product manufacturer. For example, in the embodiments illustrated in Figures 1-
3, processing the
polymer stream yields a polymer product 16 (e.g., pelletized polyethylene).
[0070] In one or more one or more of the embodiments disclosed herein,
treating the gas stream
(e.g., at block 57) comprises any suitable process or reaction for removing
oxygen, oxygenated
compounds, oxidizing compounds, or combinations thereof (cumulatively referred
to herein as
"oxygen") from the gas stream. Suitable processes or reactions will be
appreciated by those of
skill in the art viewing this disclosure. Nonlimiting examples of suitable
processes for removing
oxygen include various catalyzed reactions, contacting with a chemical species
known to react
with oxygen, filtering, absorbing, adsorbing, heating, cooling, or
combinations thereof.
[0071] In embodiments as illustrated by Figures 2-3, treating the gas stream
may comprise
routing the gas stream 18 to the deoxygenator 118. In one or more one or more
of the
embodiments disclosed herein, the deoxygenator 118 may comprise a device or
apparatus
configured for the removal oxygen, from a gas stream. Nonlimiting examples of
a suitable
deoxygenator include various reactors (e.g., a fluidized bed reactor or a
fixed bed), a filter, or
combinations thereof. A suitable deoxygenator 118 may be configured to reduce,
prevent, or
exclude compounds and/or elements (e.g., oxygen) that may have the effect of
poisoning an
absorption solvent from reaching the absorption reactor (e.g., as will be
disclosed herein).
[0072] In the embodiments illustrated by Figures 2-3, treating the gas stream
yields a treated gas
stream 26 being substantially free of oxygen. As used herein "substantially
free of oxygen" refers
to a fluid stream comprising no more than least about 5% oxygen,
alternatively, no more than
about 1% oxygen, alternatively, no more than about 0.1% oxygen, alternatively,
no more than
about 0.01% oxygen by total weight of the stream.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
19
[0073] In one or more one or more of the embodiments disclosed herein,
separating at least one
gaseous component from the gas stream and/or the treated gas stream,
collectively referred to as a
gas stream, (e.g., at block 55, 55', or 55") generally comprises any suitable
method of selectively
separating at least a first chemical component or compound from a stream
comprising the first
chemical component or compound and one or more other chemical components,
compounds, or
the like. In various embodiments, the gaseous component separated from the gas
stream may
comprise one or more hydrocarbons. Nonlimiting examples of such hydrocarbons
include alkanes
(e.g., butane, particularly, isobutane) and alkenes or olefin monomers (e.g.,
ethylene) or optional
comonomers (e.g., butene-1). In an embodiment, the gaseous component separated
from the gas
stream may comprise an unreacted hydrocarbon monomer, e.g., ethylene.
Optionally, the gaseous
component separated from the gas stream may comprise an unreacted hydrocarbon
comonomer,
e.g., propene. In an embodiment, the gaseous component separated from the gas
stream may
comprise an unreacted hydrocarbon monomer (e.g., ethylene, alone or in
combination with other
hydrocarbons, such as, isobutane), or optionally, hydrocarbon comonomer (e.g.,
propene, alone or
in combination with other hydrocarbons, such as, isobutane). In an embodiment,
the gaseous
component separated from the gas stream may comprise ethylene, alone or in
combination with
isobutane. In an embodiment, capturing isobutane may result in a savings of
the cost of the
captured isobutane and reduce the presence of isobutane in flare emissions.
Nonlimiting examples
of suitable separating means include distilling, vaporizing, flashing,
filtering, membrane screening,
absorbing, adsorbing, molecular weight exclusion, size exclusion, polarity-
based separation, or
combinations thereof.
[0074] In an embodiment, separating at least one gaseous component from the
gas stream may
comprise contacting the gas stream with the absorbent (e.g., an absorption
solvent system, as will
be disclosed herein), for example, so as to allow the gaseous component to be
absorbent by the
absorbent. In such an embodiment, separating at least one gaseous component
from the gas stream
comprises selectively absorbing the at least one gaseous component from a gas
stream. In such an
embodiment, absorbing the at least one gaseous component from the gas stream
generally
comprises contacting the gas stream with a suitable absorbent, allowing the at
least one component
to be absorbed by the absorbent, and, optionally, removing a waste stream
comprising unabsorbed
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
gases. In an additional embodiment, separating at least one gaseous component
from the gas
stream may further comprise liberating the absorbed gaseous component from the
absorbent.
[0075] In an embodiment, contacting the gas stream with the absorbent may
comprise any
suitable means of ensuring sufficient contact between the gas stream and the
absorbent.
Nonlimiting examples of suitable means by which to provide sufficient contact
between the gas
stream and the absorbent include the use of various reactor systems, such as
those disclosed above
(e.g., an absorption column or sparged or mixed tank). Not intending to
limited by theory, a
suitable reactor system may ensure contact between a two or more gaseous,
liquid, and or solid
compositions by agitating or mixing the two components in the presence of each
other, circulating,
dispersing, or diffusing a first composition through or within a second
composition, or various
other techniques known to those of skill in the art. In an embodiment, the gas
stream and the
absorbent may be brought into contact in a suitable ratio. Such a suitable
ratio of gas stream to
absorbent may be in a range of from about 1,000 lb/hr :1000 gpm to about 2,500
lb/hr :25 gpm,
alternatively, from about 1000 lb/hr :250 gpm to about 2500 lb/hr :100 gpm,
alternatively, about
1875 lb/hr :250 gpm.
[0076] In an embodiment as illustrated by Figures 1-3, separating at least one
gaseous
component from the gas stream (e.g., gas stream 18 of Figure 1 or treated gas
stream 26 of Figures
2-3) may comprise routing the gas stream to the absorption reactor 116. In one
or more of the
embodiments disclosed herein, the absorption reactor 116 may comprise a
reactor configured to
selectively absorb at least a first chemical component or compound from a
stream comprising the
first chemical component or compound and one or more other chemical
components, compounds,
or the like. Non-limiting examples of suitable absorption reactors and/or
absorption reactor
configurations include an absorption (distillation) tower, a pressure-swing
absorption (PSA)
configuration, a sparger tank, an agitation reactor, one or more compressors,
one or more recycle
pumps, or combinations thereof
[0077] In an embodiment, the absorption reactor may be configured to dissipate
a gas within a
liquid (e.g., by bubbling the gas through the liquid). For example, in an
embodiment, the
absorption reactor 116 may include a solvent circulation system configured to
circulate solvent
through the absorption reactor 116. The solvent circulation flow rate may be
determined by the
operating conditions of the absorption system, as is disclosed herein below.
In an embodiment, the
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
21
absorption reactor 116 may comprise and/or be in fluid communication with one
or more pumps
configured to recirculate solvent via and/or within the absorption reactor
116. In an additional
and/or alternative embodiment, the absorption reactor 116 may comprise a
packed bed or column
configured to maintain smaller bubble sizes (e.g., of the gas being dissipated
within the liquid), for
example, so as to maintain a relatively large surface area of contact between
the gas and the liquid,
for example, so as to maintain an efficiency of mass transfer and/or
absorption of the gas into the
liquid. In an embodiment, the packing material of the packed bed or column may
comprise a
polymeric material, metallic material, or combinations thereof In an
embodiment, the absorption
reactor 116 may have multiple packed beds or columns. In an embodiment, only a
section of the
absorption reactor 116 may have a packing material. In an embodiment, the
packing material of a
packed absorption reactor 116 may have a random packing or may have a
structured packing. An
example of a suitable absorption reactor is illustrated in the Gas Processors
Association,
"Engineering Data Book" 10th ed. at Fig 19-16.
[0078] In an embodiment where the absorption reactor 116 comprises a solvent
reactor, the
absorption reactor may comprise a suitable absorption solvent system, as will
be disclosed herein.
Such an absorption reactor 116 may be configured to retain the absorption
solvent system. For
example, the absorption solvent system may be retained within the reactor as a
liquid, as a fixed
bed, or as a fluidized bed.
[0079] In an embodiment, a suitable absorption solvent system may be capable
of reversibly
complexing with the ethylene and/or isobutane. Such an absorption solvent
system may generally
comprise a complexing agent and a solvent. In an embodiment, an absorption
solvent system may
be characterized as having a selectivity of ethylene to ethane where ethylene
and ethane are present
at the same partial pressure of about 40:1 at approximately 14 psi, about 12:1
at approximately 20
psi, about 6:1 at approximately 40 psi, and about 3:1 at approximately 180 psi
partial pressure. In
an embodiment, the solvent system may be further characterized as having a
high contaminant
tolerance and as exhibiting high stability at increased and/or fluctuating
temperatures and/or
pressures, or combinations thereof.
[0080] In an embodiment, the complexing agent may comprise a metallic salt. In
such an
embodiment, the metallic salt may comprise a salt of one or more transition
metals and a weakly-
ionic halogen. Non-limiting examples of suitable transition metals include
silver, gold, copper,
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
22
platinum, palladium, or nickel. Non-limiting example of suitable weakly-ionic
halogens include
chlorine and bromine. In an embodiment, a suitable transition metal salt may
be characterized as
having a high specificity for olefins. Non-limiting examples of suitable
transition metal-halogen
salts include silver chloride (AgC1) and copper chloride (CuC1). In a
particular embodiment, the
salt employed in the absorption solvent system comprises CuCl. Not seeking to
be bound by
theory, such a metallic salt may interact with the double carbon bonds of
olefins (e.g., ethylene).
[0081] In an embodiment, the complexing agent may comprise a copper (I)
carboxylate. In such
an embodiment, suitable copper (I) carboxylates may comprise salts of copper
(I) and mono-, di-,
and/or tri-carboxylic acids containing 1-20 carbon atoms. The carboxylic acid
component of the
salt may comprise an aliphatic constituent, a cyclic constituent, an aryl
constituent, or
combinations thereof. Other suitable examples of copper (I) carboxylates
include Cu(I) formate,
Cu(I) acetate, Cu(I) propionate, Cu(I) butyrate, Cu(I) pentanoate, Cu(I)
hexanoate, Cu(I) octanoate,
Cu(I) decanoate, Cu(I) 2-ethyl-hexoate, Cu(I) hexadecanoate, Cu(I)
tetradecanoate, Cu(I) methyl
formate, Cu(I) ethyl acetate, Cu(I) n-propyl acetate, Cu(I) n-butyl acetate,
Cu(I) ethyl propanoate,
Cu(I) octoate, Cu(I) benzoate, Cu(I) p-t-butyl benzoate, and the like. In an
additional embodiment,
the complexing agent may comprise an adduct of a copper (I) carboxylate, for
example, as
disclosed herein, and boron trifluoride (BF3).
[0082] In an additional and/or alternative embodiment, the complexing agent
may comprise a
copper (I) sulfonate. Non-limiting examples of suitable copper (I) sulfonates
include the copper (I)
salts of sulfonic acids having 4 to 22 carbon atoms. The sulfonic acid
component of the salt may
comprise an aliphatic constituent, a cyclic constituent, an aryl constituent,
or combinations thereof.
The aliphatic sulfonic acids can be straight chain or branched. Examples of
suitable aliphatic
sulfonic acids include, but are not limited to, n-butanesulfonic acid, 2-ethyl-
1 -hexanesulfonic acid,
2-methylnonanesulfonic acid, dodecanesulfonic acid, 2-ethyl-5-n-
pentyltridecanesulfonic acid, n-
eicosanesulfonic acid, and the like. Examples of suitable aromatic sulfonic
acids include
benzenesulfonic acid, alkylbenzenesulfonic acids wherein the alkyl member
contains from 1 to 16
carbon atoms, such as p-toluenesulfonic acid, dodecylbenzenesulfonic acid (o-,
m-, and p-), p-
hexadecylbenzenesulfonic acid, and the like, naphthalenesulfonic acid,
phenolsulfonic acid,
naphtholsulfonic acids, and halobenzenesulfonic acids, such as p-
chlorobenzenesulfonic acid, p-
bromobenzenesulfonic acid, and the like.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
23
[0083] In an embodiment where the complexing agent may further comprise a
hindered olefin.
For example, the complexing agent may comprise such a hindered olefin in an
embodiment
wherein the complexing agent forms a copper complex with insufficient
solubility. An example of
such a hindered olefin is a propylene tetramer (i.e. dodecene). Not intending
to be bound by
theory, the hindered olefin may increase the solubility of the copper complex
while being easily
displaced by ethylene.
[0084] In various embodiments, the complexing agent may comprise one or more
of the
complexing agents disclosed in U.S. Patent Nos. 5,104,570; 5,191,153;
5,259,986; and 5,523,512,
each of which is incorporated by reference in its entirety.
[0085] In an embodiment, the solvent may comprise an amine or an amine
complex, an aromatic
hydrocarbon, an olefin, or combinations thereof. Non-limiting examples of
solvent amines include
pyridine, benzylamine, and aniline. For examples, the amine may comprise an
aniline
(phenylamine, aminobenzene); alternatively, aniline combined with
dimethyleformamide (DMF),
and in embodiments, aniline and N-methylpyrrolidone (NMP). In an embodiment
where the
solvent comprises an aromatic hydrocarbon, the aromatic hydrocarbon may
comprise an
unsubstituted or alkyl substituted aryl groups. In such an embodiment, the
aromatic hydrocarbon
may be in the liquid phase under normal, ambient conditions. Suitable non-
limiting examples
include toluene, xylene, and the like. In embodiments where the solvent
comprises an olefin, non-
limiting examples include olefins having 10 to 16 carbon atoms. For example,
the olefin may
comprise propylene tetramer, dodecene, tetradecene, hexadecene, or
combinations thereof.
[0086] In an embodiment, the solvent may be characterized as aprotic, that is,
as not including a
dissociable hydrogen atom. Not intending to be bound by theory, a dissociable
hydrogen solvent
may result in the hydrogenation of the double bond between carbons in an
olefin such as ethylene.
Further, the solvent may be characterized as polar, as having a slight
polarity, or as having
unidirectional, electric charge. Not intending to be bound by theory, a polar
solvent may interact
with and at least partially solubilize the salt.
[0087] In an embodiment, the solvent may be characterized as a liquid produced
industrially in
relatively high volumes, having a relatively low cost, being easily
transportable, or combinations
thereof The solvent may be further characterized as capable of retaining a
complexed olefin-metal
salt or retaining a weakly ionic metal salt despite fluctuations in
temperature and/or pressure.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
24
[0088] In an embodiment, the absorption solvent system may comprise copper
chloride, aniline,
and dimethyleformamide (CuCl/aniline/DMF). In an alternative embodiment, the
absorption
solvent system may comprise copper chloride, aniline, and N-methylpyrrolidone
(CuCl/aniline/NMP). In such an embodiment, a CuCl/aniline/NMP solvent system
may be
characterized as having increased volatile stability at lower pressures and
higher temperatures. In
alternative embodiments, the absorption solvent system may comprise copper (I)
carboxylate and
an aromatic solvent such as toluene or xylene. In alternative embodiments, the
absorption solvent
system may comprise copper (I) sulfonate and an aromatic solvent such as
toluene or xylene. In
alternative embodiments, the absorption solvent system may comprise an adduct
of copper (I)
carboxylate and BF3 in an aromatic solvent such as toluene or xylene.
[0089] In an embodiment, the absorption solvent system may comprise copper (I)
2-ethyl-
hexanoate and propylene tetramer. In an embodiment, the absorption solvent
system may
comprise copper (I) 2-ethyl-hexanoate and dodecene. In an embodiment, the
absorption solvent
system may comprise copper (I) hexadecanoate and hexadecene. In an embodiment,
the absorption
solvent system may comprise copper (I) tetradecanoate and tetradecene.
[0090] In an embodiment, allowing the at least one component to be absorbed by
the absorbent
may comprise allowing the at least one component to become reversibly bound,
linked, bonded or
combinations thereof to the absorbent or a portion thereof, for example, via
the formation of
various links, bonds, attractions, complexes, or combinations thereof. For
example, in an
embodiment where the absorbent comprises an absorption solvent system (e.g., a
CuCl/aniline/DMF solvent system or a CuCl/aniline/NMP solvent system),
allowing absorption of
the at least one component may comprise allowing a complex to form between the
absorbent and
the at least one component, referred to as an absorbed component complex
(e.g., an absorbed
ethylene complex).
[0091] Allowing absorption of the at least one component may further comprise
providing
and/or maintaining a suitable pressure of the environment in which the gas
stream and absorbent
are brought into contact, providing and/or maintaining a suitable partial
pressure of a gas,
providing and/or maintaining a suitable temperature in the environment in
which the gas stream
and absorbent are brought into contact, catalyzing the absorption, or
combinations thereof Not
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
intending to be bound by theory, the absorption of the at least one component
by the absorbent
may be improved at a suitable temperature and/or pressure.
[0092] In an embodiment, the absorption reactor 116 may be capable of
selectively inducing
thermal and/or pressure fluctuations, variations, or cycles. In an embodiment,
the absorption
reactor 116 may be configured to selectively absorb and/or induce the
absorption of an unreacted
ethylene monomer (and optionally, comonomer) from a composition comprising
various other
gases (e.g., ethane). In another embodiment, the absorption reactor 116 may be
configured to
selectively absorb and/or induce the absorption of butane, particularly,
isobutane, from a
composition comprising various other gases. In still another embodiment, the
absorption reactor
116 may be configured to selectively absorb both unreacted ethylene and
butane, particularly,
isobutane, from a composition comprising various other gases (e.g., ethane).
[0093] In an embodiment, the absorption reactor 116 may be configured to
provide or maintain a
suitable temperature, for example, as may be dependent upon the phase in which
the absorption
reactor operates at a given time. For example, the absorption reactor 116 may
be configured to
provide or maintain a suitable temperature, for example, for the purpose of
increasing absorption of
a desired chemical species, decreasing absorption of a desired chemical
species, flashing an
unabsorbed gas from the reactor 116, recovering unreacted ethylene from the
absorption reactor
116, regenerating absorbent in the absorption reactor 116, or combinations
thereof. In an
embodiment, such a suitable temperature may be in a range of from about 40 F
to about 110 F,
alternatively, from about 40 F to about 60 F, alternatively, from about 45
F to about 55 F,
alternatively, from about 50 F to about 55 F, alternatively about 50 F. For
example, it has been
found the operating temperature of the absorption reactor 116 (and absorption
solvent system) in a
temperature range of from about 40 F to about 110 F, alternatively, from
about 40 F to about 60
F, alternatively about 50 F may yield an unexpected increase in the
absorption of ethylene relative
to the absorption of ethane. Not intending to be bound by theory, one skilled
in the art will
appreciate (for example, based on partial pressure concepts from Raoult's law)
the expectation for
solubility of ethylene and ethane in an absorbent solvent to increase at
decreasing temperatures.
However, contrary to such expectations, it has been found that the amount of
ethylene absorbed in
the absorbent solvent and/or the absorbent solvent system of the disclosed
embodiments decreases
as the temperature decreases below 50 F. Because of this unexpected
phenomenon, absorption of
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
26
ethylene may be greatest for temperatures in a range of from about 40 F to
about 110 F,
alternatively, in a range of from about 40 F to about 60 F, alternatively,
at a temperature of about
50 F. Figure 7 is graph showing the solubility at varying temperatures for
ethylene and ethane in
a copper chloride, aniline, NMP absorbent solvent system. The graph
illustrates the expected
solubility trend of ethane and the unexpected solubility trend of ethylene
across the temperatures
discussed above.
[0094] In an embodiment, the absorption reactor 116 may be configured to
provide or maintain a
suitable temperature in a range from about 40 F to about 110 F during
absorption of one or more
components of the gas stream (e.g., ethylene and/or isobutane). As disclosed
above, it has been
found that ethylene solubility is unexpectedly greatest at temperature in a
range of from about 40
F to about 60 F. In an embodiment, the absorption reactor 116 may be operated
at a temperature
of from about 40 F to about 60 F, alternatively a temperature of about 50 F
during absorption of
ethylene and/or isobutene from a gas stream. In an alternative embodiment, the
absorption reactor
may be operated at a temperature of from about 60 F to about 110 F, or from
about 70 F to about
90 F during absorption of ethylene and/or isobutene from a gas stream. For
example, such
absorption temperatures of the absorption reactor 116 may be suitable as an
economic alternative
to operating at a lower temperature (which may require energy expenditure with
cooling, for
example). For example, operating an absorption reactor, like absorption
reactor 116, at
temperatures in a range of from about 60 F to about 110 F, or from about 70
F to about 90 F
may require less energy, which may create a cost savings, by allowing the
absorption reactor to be
operated at the ambient temperature of a given geographic location.
[0095] In an embodiment, the absorption reactor 116 may be configured to
provide or maintain a
suitable pressure during operation. Such a suitable pressure may be in a range
of from about 5 psig
to about 500 psig, alternatively, from about 50 psig to about 450 psig,
alternatively, from about 75
psig to about 400 psig. In an additional embodiment, the absorption reactor
116 may be
configured to provide or maintain a suitable partial pressure of ethylene
during operation. Such a
suitable ethylene partial pressure may be in a range of from about 1 psia to
about 400 psia,
alternatively, from about 30 psia to about 200 psia, alternatively, from about
40 psia to about 250
psia, alternatively, from about 40 psia to about 75 psia, alternatively, from
about 40 psig to about
60 psig, alternatively about 40 psig, alternatively, about 60 psig. Not
intending to be bound by
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
27
theory, pressurizing the absorption reactor 116 may facilitate absorption of
ethylene and/or the
formation of a complex of ethylene and the absorption solvent system (e.g.,
the CuCl/aniline/NMP
system). Also, not intending to be bound by theory, the selectivity of the
absorption solvent
system for ethylene may increase with a decrease in the pressure of the
absorption reactor.
[0096] In an embodiment, the absorption reactor 116 may be configured for
batch and/or
continuous processes. For example, in an embodiment, a PEP system may comprise
two or more
absorption reactors (e.g., such as absorption reactor 116), each of which may
be configured for
batch operation. For example, by employing two or more absorption reactors,
such a system may
be configured to allow for continuous operation by absorbing a component of a
gas stream into a
"first batch" in the first absorption reactor while a "second batch" is
prepared for absorption in the
second absorption reactor. As such, by cycling between two or more suitable
reactors, a system
may operate continuously.
[0097] For example, in an embodiment two or more absorption reactors (e.g., an
absorption
reactor system) may be configured for pressure swing absorption (PSA) of
ethylene using a liquid
solvent, for example, the absorption solvent system or absorption solvent as
disclosed herein. In
such an embodiment, the absorption reactor 116 may include two or more
absorption reactors
configured for PSA (e.g., an absorption reactor system). Figure 8, shows an
absorption reactor
system 800 with four absorption reactors 810, 820, 830, and 840 configured for
PSA. Although
the embodiment of Figure 8 illustrates four absorption reactors (e.g.,
absorption reactors 810, 820,
830, and 840), one of skill in the art, upon viewing this disclosure, will
recognize that two, three,
five, six, seven, eight, or more absorption reactors may be similarly
employed. In such an
embodiment, the each of the absorption reactors may be configured
substantially as disclosed
herein. In an embodiment, one or more of the reactors 810, 820, 830, and 840
may be connected
via a circulation system (for example, comprising one or more pumps, valves,
conduits, and the
like) to circulate the liquid solvent in the reactors 810, 820, 830, and 840
during absorption. The
absorption reactors 810, 820, 830, and 840 may cycle between an absorption
phase (in which a
gaseous component, such as ethylene and/or isobutane, is absorbed by the
absorption solvent
and/or absorption solvent system) and a regeneration phase (in which the
absorbed and/or
complexed gaseous component is liberated from the absorption solvent system
and/or the
absorption solvent system is prepared for reuse, as will be disclosed herein).
For example, the
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
28
reactors 810, 820, 830, and 840 may be cycled between the absorption and
regeneration phases
(e.g., via one or more intermediate phases) on a coordinated basis so that not
all reactors 810, 820,
830, 840 are undergoing absorption or regeneration at the same time. In an
embodiment where
absorption reactors 810, 820, 830, and 840 are configured to operate in PSA,
the reactors 810, 820,
830, and 840 serve as both absorbers and as regenerators. In such an
embodiment, separate vessels
for regeneration may not be required (e.g., as disclosed herein).
[0098] As an example of PSA operation on a coordinated basis, at a given phase
during such
operation, reactor 810 may operate in the absorption phase, for example, at
absorption conditions
as disclosed herein. At substantially the same time, reactor 820 may be
pressurized to an
intermediate pressure, for example, below that of the absorption pressure.
Also, at substantially the
same time, reactor 830 may depressurize from an intermediate pressure to a
regeneration pressure,
and while reactor 840 may depressurize from an absorption pressure (from
previously being in an
absorption phase) to an intermediate pressure. Not intending to be bound by
theory,
depressurization (e.g., from the absorption pressure to the intermediate
pressure and from the
intermediate pressure to the regeneration pressure) of each of reactors 810,
820, 830, and/or 840
following absorption may allow the absorbed gaseous components (e.g., ethylene
and/or isobutane)
to be liberated from the absorbent and/or the absorbent to be regenerated
(e.g., prepared for re-use,
as disclosed herein). In an embodiment, the pressure from one or more of the
reactors (e.g.,
reactors 810, 820, 830, and/or 840) may be utilized to pressurize another of
these reactors. For
example, in the embodiment of Figure 8, the pressure of gas in reactor 840 may
be used to
pressurize reactor 820 to the intermediate pressure through line 850, with
valves 858 and 884 being
in an open position and valves 882 and 856 being in a closed position. Valves
862, 864, 866, and
868 may be switched between an open position and a closed position to allow
product nitrogen in
stream 860 to flow in and out of reactors 810, 820, 830, and 840. Valves 852,
854, 856, 858 may
be switched between an open position and a closed position to allow
pressurization and
depressurization of reactors 810, 820, 830, and 840 through stream 850. Valves
882, 884, 886, 888
may be switched between an open position and a closed position to allow light
gas stream 880 to
feed to reactors 810, 820, 830, and 840 when in the absorption phase. Valves
892, 894, 896, and
898 may be switched between an open position and a closed position to remove
any purge gas
from reactors 810, 820, 830, and 840 through stream 890.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
29
[0099] In an embodiment, a stripping gas, such as isobutane or nitrogen, may
be added to the
absorption reactors 810, 820, 830, and 840, for example, through stream 870
during the
regeneration phase. Stream 870 may be positioned at a bottom of reactors 810,
820, 830, and 840
so the stripping gas may bubble through the reactor 810, 820, 830, or 840 (and
through any
packing materials therein). Valves 872, 874, 876, and 878 may be switched
between open and
closed positions to add the stripping gas to the reactors 810, 820, 830, and
840 during regeneration.
Not intending to be bound by theory, the stripping gas may lower the partial
pressure of ethylene in
the absorption reactors 810, 820, 830, and 840 during regeneration.
[00100] In an embodiment, one or more of the absorption reactors 810, 820,
830, and 840 may
comprise internals to distribute the gas through the liquid absorption solvent
and prevent
channeling. Suitable internals may include distillation packing that
distributes gas and reduces
axial mixing of the liquid. Internals may prevent liquid absorption solvent in
the absorption
reactors 810, 820, 830, and 840 from mixing so that solvent flow would be
first saturated and then
a saturation front may move vertically upward through the absorption reactors
810, 820, 830, and
840.
[00101] In an embodiment, separating at least one gaseous component from the
gas stream
comprises removing a waste stream. In an embodiment, the remaining unabsorbed
gas stream
components form the waste stream. In an embodiment where the absorbed
component comprises
ethylene and the absorbent comprises a CuCl/aniline/DMF or a CuCl/aniline/NMP
solvent system,
such a waste stream may comprise methane, ethane, acetylene, propylene,
various other
hydrocarbons, volatile contaminants, or combinations thereof. Further, such a
waste stream may
be substantially free of unreacted ethylene monomers or, optionally,
comonomers. As used herein,
"substantially free of unreacted ethylene monomers" means that the waste gases
comprise less than
50% unreacted ethylene monomers, alternatively, less than 10% unreacted
ethylene monomers,
alternatively, less than 1.0% unreacted ethylene monomers, alternatively, less
than 0.1 unreacted
ethylene monomers, alternatively, less than 0.01% unreacted ethylene monomers
by total weight of
the stream.
[00102] In an embodiment, removing the waste stream may comprise cooling the
waste stream,
and/or reducing or increasing the waste stream pressure such that the waste
stream flows to the
processing device 114. For example, in an embodiment, the waste stream may be
"swept away"
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
by conveying a suitable sweep gas (e.g., an inert or unreactive gas, as
disclosed above) through the
vessel containing the waste gas (e.g., the absorption reactor 116) at a
sufficient pressure, at
velocity, or combinations thereof to expel the waste gases therefrom. For
example, in the
embodiments illustrated by Figures 1-3, separating at least one gaseous
component from the gas
stream yields a waste gas stream 20 being substantially free of unreacted
ethylene monomers
(optionally, comonomers), alternatively, a waste gas stream having a reduced
concentration of
unreacted ethylene monomers (optionally, comonomers). For example, the waste
gas stream may
comprise less than about 30%, alternatively, less than about 25%,
alternatively, less than about
20%, alternatively, less than about 15%, alternatively, less than about 10%
unreacted ethylene
monomers by total weight of the stream. In an additional embodiment, the
ethylene may be
decreased by a percentage of the ethylene present in the gas stream prior to
separating at least one
gaseous component therefrom. For example, the waste gas stream may comprise
less than about
40%, alternatively, less than about 30%, alternatively, less than about 20% by
total weight of the
stream of the unreacted ethylene monomers present in the gas stream prior to
separation.
[00103] In an embodiment, separating at least one gaseous component from the
gas stream may
further comprise liberating the absorbed gaseous component from the absorbent
(e.g., in situ within
absorption reactor 116 and/or in another vessel such as regenerator 120).
Liberating the absorbed
gaseous component from the absorbent generally comprises any suitable means of
reversing the
various links, bonds, attractions, complexes, or combinations thereof by which
the at least one
gaseous component is bound, linked, bonded or combinations thereof to the
absorbent or a portion
thereof. Nonlimiting examples of a suitable means by which to liberate the
absorbed gaseous
component include altering absorption kinetics or the absorption equilibrium
of the absorbent,
heating or depressurizing the absorbent, altering the partial pressure of the
absorbed gas, or
combinations thereof.
[00104] In an embodiment, the absorbed gaseous component may be liberated
(e.g., desorbed
and/or decomplexed) from the absorbent within the one or more of such
absorption reactors in a
regeneration and/or desorption phase. For example, in the embodiment of
Figures 1 and 2 (and/or,
in an embodiment where the absorption reactor 116 is configured in a PSA
configuration, as
disclosed herein with respect to Figure 8), the absorption reactor 116 may be
configured to induce
the release of the gas absorbed or complexed by the absorption solvent
therefrom (e.g., desorption
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
31
and/or decomplexation of the absorbed and/or complexed ethylene and/or
isobutane), as disclosed
in detail herein. Not intending to be bound by theory, inducing the release of
the absorbed or
complexed gas may comprise altering the reaction kinetics or the gas-solvent
equilibrium of the
absorption solvent system, the temperature of the absorption reactor 116, the
pressure of the
absorption reactor 116, the partial pressure of the absorbed gas, or
combinations thereof. In such
an embodiment, the absorption reactor 116 may comprise controls, thermal
conduits, electric
conduits, compressors, vacuums, the like, or combinations thereof configured
to alter the reaction
kinetics, the gas-solvent equilibrium, the temperature of the absorption
reactor 116, the pressure of
the absorption reactor 116, or combinations thereof.
[00105] For example, in an embodiment, liberating the absorbed gaseous
component may
comprise depressurizing the solution comprising the complexed ethylene to a
suitable partial
pressure. In an additional embodiment, liberating the absorbed gaseous
component may comprise
heating he solution comprising the complexed ethylene within the absorption
reactor 116
(alternatively, within a regenerator 120, as disclosed herein below) to a
suitable temperature. Such
a suitable temperature may be in a range of from about 110 F to about 200 F,
alternatively, from
about 140 F to about 160 F, alternatively, from about 160 F to about 200
F, alternatively, from
about 180 F to about 200 F, to encourage release of the absorbed compound
(e.g., ethylene and/or
isobutane) from the absorption solvent. For example, in a particular
embodiment, the absorption
reactor 116 (alternatively, the regenerator 120) may be operated at a
temperature of from about
160 F to about 200 F, alternatively, from about 180 F to about 200 F
during the liberation of the
absorbed component (e.g., ethylene and/or isobutene) from the absorption
solvent. In an
alternative embodiment, the absorption reactor 116 (alternatively, the
regenerator 120) may be
operated at a temperature of from about 140 F to about 160 F during the
liberation of the
absorbed component (e.g., ethylene and/or isobutene) from the absorption
solvent. For example,
such liberation temperatures may be suitable as an economic alternative. For
example, operation
an absorption reactor like absorption reactor 116 (alternatively, a
regenerator like regenerator 120)
at temperatures in a range of from about 140 F to about 160 F during the
liberation of the
absorbed component may require less energy, which may create a cost savings,
by allowing heat
derived from other sources (e.g., polymerization reactor coolant, low pressure
stream, heat-
exchangers upstream of regenerators, heat-exchangers in the absorbent recycle
line, polymerization
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
32
reactors, flash-line heaters, flash vessels, or the like, or combinations
thereof) to be utilized to heat
the absorption reactor and/or the regenerator.
[00106] Additionally, in such an embodiment, the absorption reactor 116 may be
configured to
evacuate gases (e.g., a previously absorbed and then released gas, such as
ethylene) and/or to
facilitate the release of the absorbed gas via a pressure differential. The
absorption reactor 116
may be configured to provide or maintain a suitable partial pressure. Such a
suitable partial
pressure may be in a range of from about 0.1 psig to about 40 psig,
alternatively, from about 5 psig
to about 30 psig, alternatively, from about 5 psig to about 15 psig. In an
embodiment, the
absorption reactor 116 may be configured to provide or maintain an ethylene
partial pressure in a
range of from about 0 psia to about 5 psia.
[00107] In an alternative embodiment, separating at least one gaseous
component from the gas
stream may further comprise removing the solution comprising the absorbed
component complex
(e.g., the absorbed ethylene complex) for further processing. In such an
alternative embodiment,
the absorption complex comprising the absorbed gaseous component may be
removed from the
absorption reactor 116 to the regenerator 120 for liberation of the absorbed
gaseous component
and/or regeneration of the absorption complex as a complexed stream 28. In
such an embodiment,
the complexed stream 28 may comprise ethylene, ethane, and/or isobutane.
Ethylene may be
present in a range of from about 0.1% to about 10%, alternatively, from about
0.4% to about 5%,
alternatively, from about 0.5% to about 2.5% by total weight of the stream.
Ethane may be present
in a range of from about 0.1% to about 1%, alternatively, from about 0.2% to
about 0.5% by total
weight of the stream. Isobutane may be present in a range of from about 0.1%
to about 1%,
alternatively, from about 0.2% to about 0.5% by total weight of the stream.
[00108] In one or more of the embodiments disclosed herein, separating a
complexed stream into
a recycle stream and an absorbent stream (e.g., at block 58) comprises
liberating the absorbed
gaseous component from the absorbent. As explained above, liberating the
absorbed gaseous
component from the absorbent generally comprises any suitable means for
reversing the various
links, bonds, attractions, complexes, or combinations thereof by which the at
least one gaseous
component is bound, linked, bonded or combinations thereof to the absorbent or
a portion thereof.
Various processes and/or parameters for liberating an absorbed gaseous
component were disclosed
above with respect to liberation within the absorption reactor.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
33
[00109] In the embodiment illustrated by Figure 3, separating a complexed
stream into a recycle
stream and an absorbent stream may comprise routing the complexed stream 28 to
the regenerator
120. In one or more one or more of the embodiments disclosed herein, a
regenerator 120 may
comprise a device or apparatus configured to recover, regenerate, recycle,
and/or purify an
absorption solvent and/or to liberate an absorbed gas. Non-limiting examples
of a suitable
regenerator include a flash reactor, a depressurization reactor, a solvent
regeneration reactor, or
combinations thereof.
[00110] In an embodiment, regenerator 120 may be configured to operate on the
basis of a
pressure differential. In such an embodiment, the regenerator 120 may be
configured to provide or
maintain a suitable internal pressure. Such a suitable internal pressure may
be in a range of from
about 0 psig to about 150 psig, alternatively, from about 5 psig to about 30
psig, alternatively, from
about 5 psig to about 15 psig, alternatively, from about 0 psig to about 10
psig. In an embodiment,
the regenerator 120 may be configured to provide or maintain a suitable
partial pressure. Such a
suitable partial pressure may be in a range of from about 0 psia to about 50
psia.
[00111] In an embodiment, regenerator 120 may be configured to operate on the
basis of an
elevated temperature. Such a regenerator 120 may be configured to provide or
maintain a suitable
temperature. Such a suitable temperature may be in a range of from about 110
F to about 200 F,
alternatively, from about 140 F to about 200 F, alternatively, from about
140 F to about 160 F,
alternatively, from about 160 F to about 200 F, alternatively, from about
180 F to about 200 F,
to vaporize and/or release an absorbed compound (e.g., ethylene and/or
isobutane) from the
absorption solvent. In an embodiment, regenerator 120 (e.g., like the
absorption reactor 116) may
be heated to desorb, or regenerate, the absorption solvent system using heat
sources comprising
cooling water, low-pressure steam, or combinations thereof. Cooling water, low
pressure steam, or
a combination thereof may be suitable for heating regenerator 120 (or the
absorption reactor 116,
as disclosed above) to a temperature of from about 140 F to about 200 F.
[00112] In an embodiment, the regenerator 120 may be configured for batch
and/or continuous
processes. For example, in an embodiment, a PEP system may comprise two or
more absorption
regenerators (e.g., such as regenerator 120), each of which may be configured
for batch operation.
As explained above, by employing two or more absorption reactors, such a
system may operate to
regenerate the absorbent continuously.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
34
[00113] In an embodiment, separating a complexed stream into a recycle stream
and an absorbent
stream may yield a regenerated absorbent steam which may be reused in an
absorption reaction and
a recycle stream comprising unreacted monomers (optionally, comonomers) which
may be
reintroduced into or reused in a PEP process. For example, in the embodiment
illustrated by
Figure 3, separating a complexed stream into a recycle stream and an absorbent
stream 58 yields a
recycle stream 22 which may be returned to the purifier 102 and a regenerated
absorbent stream 30
which may be returned to the absorption reactor 116.
[00114] In an embodiment, liberating the absorbed gas may also yield a recycle
stream
comprising unreacted monomers (optionally, comonomers) which may be returned
to the separator
108 for pressurization (e.g., via one or more compressors located at the
separator 108). For
example, in the embodiments illustrated by Figures 1-3, liberating the
absorbed gas yields a recycle
stream 22 which may be returned to the separator 108. Pressurizing the recycle
stream 22 may
yield a reintroduction stream 24 which may be reintroduced into or reused in a
PEP process. For
example, in the embodiments illustrated by Figures 1-3, a reintroduction
stream 24 is introduced
into the purifier 102. In an alternative embodiment, a recycle stream (such as
recycle stream 22)
may be pressurized and/or reintroduced into a PEP process without being
returned to the separator
108. In an embodiment, the recycle stream 22 may comprise substantially pure
ethylene;
alternatively, the recycle stream 22 may comprise ethylene and butane,
particularly, isobutane. In
an embodiment, the gas stream may comprise may comprise nitrogen, ethylene,
ethane, and/or
isobutane. Ethylene may be present in a range of from about 65% to about 99%,
alternatively,
from about 70% to about 90%, alternatively, about 75% to about 85% by total
weight of the
stream. Ethane may be present in a range of from about 1% to about 20%,
alternatively, from
about 5% to about 15%, alternatively, from about 7.5% to about 12.5% by total
weight of the
stream. Isobutane may be present in a range of from about 1% to about 20%,
alternatively, from
about 5% to about 15%, alternatively, from about 7.5% to about 12.5% by total
weight of the
stream.
[00115] In one or more one or more of the embodiments disclosed herein,
combusting a waste gas
stream (e.g., at block 56) may generally comprise burning or incinerating one
or more gaseous
components of the waste gas stream 20. In one or more of the embodiments
disclosed herein,
combusting the waste gas stream 20 may further or alternatively comprise
cracking, catalytic
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
cracking, pyrolysis, dehydrogenating, scrubbing, converting, treating, or
combinations thereof, of
the waste gas stream 20 or combustion products.
[00116] As disclosed herein, the waste gas stream 20 may comprise volatilized
solvents,
unreacted gases, secondary products, contaminants, hydrocarbons, or
combinations thereof. In an
embodiment, the waste gas stream 20 may comprise hydrogen, nitrogen, methane,
ethylene,
ethane, propylene, propane, butane, isobutane, heavier hydrocarbons, or
combinations thereof
Ethylene may be present in a range of from about 1% to about 40%,
alternatively, from about 2.5%
to about 20 % by total weight of the stream. Ethane may be present in a range
of from about 5% to
about 50%, alternatively, from about 30% to about 40% by total weight of the
stream. Isobutane
may be present in a range from about 1% to about 20%, alternatively, from
about 1.5% to about
5%, alternatively, from about 2% to about 3% by total weight of the stream.
Nitrogen may be
present in a range from about 10% to about 80%, alternatively, from about 35%
to about 50%,
alternatively, from about 40% to about 45% by total weight of the stream.
[00117] In embodiments as illustrated by Figures 1-3, combusting waste gas
stream may comprise
routing the waste gas stream 20 to the processing device 114. In one or more
of the embodiments
disclosed herein, the processing device 114 may comprise a combustion device
or apparatus, such
as a flare. Nonlimiting examples of a suitable flare include a torch,
incinerator, the like, or
combinations thereof. A flare may suitably comprise one or more controllable
nozzles, an ignition
source, a bypass valve, a pressure relief valve, or combinations thereof. The
flare may be
configured to provide an environment for the combustion of various waste
products, for example,
atomic gases (e.g. nitrogen, oxygen), oxides (e.g. carbon monoxide, oxides of
nitrogen or sulfur),
various unwanted gaseous products, or combinations thereof In an embodiment,
the flare may
additionally comprise a device or apparatus configured to selectively remove
one or more of
contaminants prior to, during, and/or after combustion (e.g., such that a
given combustion product
is not released into the atmosphere).
[00118] In one or more of the embodiments disclosed herein, the processing
device 114 may
comprise a cracker, catalytic cracker, scrubber, converter, treater,
dehydrogenator, deoxygenator,
or combinations thereof, for example. In an embodiment, processing device 114
may comprise an
ethylene cracker. In the processing device 114, one or more gaseous
components, such as ethane,
from waste gas stream 20 may be converted to a desired product, such as
ethylene monomer. The
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
36
desired product formed in the processing device 114 may be recycled to one or
more of purifier
102, reactor 104, reactor 106, for example.
[00119] In other alternative embodiments, waste gas stream 20 may be used as
fuel (for example
for steam generation or co-gen operations, and/or may be used as fuel and/or a
feed to a thermal
cracking unit to form ethylene (e.g., to form feed stream 10). In another
alternative embodiment,
the waste gas from waste gas stream 20 may be exported from the plant to a
monomer plant.
[00120] In an embodiment, implementation of one or more of the disclosed
systems (e.g., PEP
systems 100, 200, and/or 300) and/or processes (e.g., PEP processes 400, 500,
and/or 600) may
allow for the recovery of a substantial portion of the ethylene monomers that
would otherwise be
lost due to the operation of such systems or processes, for example, by
flaring. In an embodiment,
one or more of the disclosed systems may allow for the recovery of up to about
75%, alternatively,
up to about 85%, alternatively, up to about 90%, alternatively, up to about
95% by total weight of
the stream of the ethylene monomers that would otherwise be lost. In an
embodiment, one or more
of the disclosed systems may allow for the recovery of up to about 75%,
alternatively, up to about
85%, alternatively, up to about 90%, alternatively, up to about 95% by total
weight of the stream of
the isobutane that would otherwise be lost. The recovery of such a portion of
the unreacted
ethylene monomers may yield a significant economic benefit, for example, by
improving the
efficiency of usage of ethylene monomers and decreasing capital inputs
associated with the
acquisition of ethylene monomers. Similarly, the recovery of such a portion of
isobutane may
yield a significant economic benefit, for example, by decreasing capital
inputs associated with the
acquisition of isobutane and/or by reducing the presence of isobutane in flare
emissions.
[00121] In an embodiment, implementation of one or more of the disclosed
systems and/or
processes may decrease the amount of ethane that is returned to a
polymerization reactor (such as
reactors 104 and/or 106) via a recycle stream. By decreasing the amount of
ethane contained in a
stream recycled to a polymerization reactor, the overall efficiency of the
polyethylene production
may be improved (for example, by increasing the ethylene concentration without
reaching the
bubble point in the loop reactor). For example, decreasing the amount of
ethane in a recycled
stream may improve polymerization reactor efficiency, improve catalyst
efficiency, reduce
polymer fouling, reduce polymerization downtime, or combinations thereof
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
37
[00122] A skilled artisan will recognize that industrial and commercial
polyethylene
manufacturing processes may necessitate one or more, often several,
compressors or similar
apparatuses. Such compressors are used throughout polyethylene manufacturing,
for example to
pressurize reactors 104, 106 during polymerization. Further, a skilled artisan
will recognize that a
polyethylene manufacturing process includes one or more deoxygenators and/or
similar de-
oxidizing apparatuses, for instance purifying solvents or reactants and/or for
purging reactors of
oxygen. Because the infrastructure and the support therefore, for example to
provide power and
maintain the compressors and/or deoxygenators, already exists within a
commercial polyethylene
manufacturing plant, reallocating a portion of these available resources for
use in the disclosed
systems may necessitate little, if any, additional capital expenditure in
order to incorporate the
disclosed systems and or processes.
[00123] Further, because compressors, deoxygenators, and various other
components are already
employed in various polyethylene processes and systems, the opportunity for
increased operation
of such apparatuses may improve the overall efficiency of polyethylene
production systems and
processes. For example, when a portion of a PEP process or system is taken off-
line for
maintenance and/or repair, other portions of the system (e.g., a compressor, a
deoxygenator, a
reactor, etc.) may continue to provide service according to the current
processes. Operating and/or
reallocating resources for operation of the disclosed PEP systems and/or
processes may thereby
increase the efficiency with which conventional systems are used.
ADDITIONAL DESCRIPTION
[00124] A process and system for the production for polyethylene has been
described. The
following clauses are offered as further description:
[00125] Embodiment A. A process for recovery of ethylene from a
polymerization
product stream of a polyethylene production system, comprising:
separating a light gas stream from the polymerization product stream, wherein
the light
gas stream comprises ethane and unreacted ethylene;
contacting the light gas stream with an absorption solvent system, wherein the
contacting
the light gas stream with the absorption solvent system occurs at a
temperature in a range of from
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
38
about 40 F to about 110 F, wherein at least a portion of the unreacted
ethylene from the light gas
stream is absorbed by the absorption solvent system; and
recovering unreacted ethylene from the absorption solvent system to yield
recovered
ethylene.
[00126] Embodiment B. The process of claim 1, wherein the absorption
solvent
system comprises copper chloride, aniline, and N-methylpyrrolidone.
[00127] Embodiment C. The process of claim 1, wherein the contacting
the light gas
stream with the absorption solvent system occurs at a temperature in a range
of from about 40 F
to about 60 F.
[00128] Embodiment D. The process of claim 3, wherein the contacting
the light gas
stream with the absorption solvent system occurs at a temperature of about 50
F.
[00129] Embodiment E. The process of claim 1, wherein the contacting
the light gas
stream with the absorption solvent system occurs at a temperature in a range
of from about 60 F
to about 90 F.
[00130] Embodiment F. The process of claim 5, further comprising:
introducing a stripping gas into the absorption solvent system, wherein at
least a portion of
the stripping gas is absorbed by the absorption solvent system.
[00131] Embodiment G. The process of claim 6, wherein the stripping
gas is selected
from the group consisting of nitrogen and isobutane.
[00132] Embodiment H. The process of claim 1, wherein the contacting
the light gas
stream with the absorption solvent system comprises bubbling the light gas
stream through a
packed bed in the absorption solvent system.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
39
[00133] Embodiment I. The process of claim 1, wherein the contacting
the light gas
stream with the absorption solvent system comprises pressurizing the light gas
stream and the
absorption solvent system to a pressure in a range of from about 40 psig to
about 60 psig.
[00134] Embodiment J. The process of claim 1, wherein the recovering
unreacted
ethylene from the absorption solvent system comprises depressurizing the
absorption solvent
system having absorbed unreacted ethylene at a temperature in a range of from
about 110 F to
about 200 F.
[00135] Embodiment K. The process of claim 10, wherein the
depressurizing the
absorption solvent system occurs at a pressure in a range of from about 0 psig
to about 10 psig.
[00136] Embodiment L. The process of claim 10, wherein the
depressurizing the
absorption solvent system having absorbed unreacted ethylene occurs at a
temperature in a range
of from about 140 F to about 160 F.
[00137] Embodiment M. The process of claim 10, wherein the
depressurizing the
absorption solvent system having absorbed unreacted ethylene occurs at a
temperature in a range
of from about 160 F to about 200 F.
[00138] Embodiment N. The process of claim 1, further comprising:
removing at least a portion of elemental oxygen or oxygen-containing compounds
from the
light gas stream before contacting the light gas stream with the absorption
solvent system.
[00139] Embodiment 0. A polyethylene production process, comprising:
contacting ethylene and a polymerization catalyst in a polymerization reactor
under suitable
reaction conditions to yield a polymerization product stream;
separating a light gas stream from the polymerization product stream, wherein
the light gas
stream comprises unreacted ethylene;
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
contacting the light gas stream with an absorption solvent system in an
absorption reactor at
a temperature in a range of from about 40 F to about 110 F, wherein at least
a portion of the
unreacted ethylene from the light gas stream is absorbed by the absorption
solvent system to yield
a composition comprising a complex of the absorption solvent system and
unreacted ethylene;
removing unabsorbed gases of the light gas stream from contact with the
absorption solvent
system;
recovering unreacted ethylene from the absorption solvent system; and
contacting the recovered ethylene and the polymerization catalyst.
[00140] Embodiment P. The process of claim 15, further comprising:
introducing a stream comprising the composition comprising the complex of the
absorption
solvent system and unreacted ethylene into a solvent regenerator at a
temperature in a range of
about 50 F to about 200 F;
recovering unreacted ethylene from the composition comprising the complex of
the
absorption solvent system and unreacted ethylene to yield recovered ethylene
and a regenerated
absorption solvent system;
introducing a stream comprising the recovered ethylene into the polymerization
reactor;
and
introducing a stream comprising the regenerated absorption solvent system into
the
absorption reactor.
[00141] Embodiment Q. The process of claim 16, wherein the introducing
a stream
comprising the composition comprising the complex of the absorption solvent
system and
unreacted ethylene into a solvent regenerator occurs at a pressure in a range
of about 0 psig to
about 10 psig.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
41
[00142] Embodiment R. The process of claim 15, wherein the recovering
unreacted
ethylene from the absorption solvent system comprises depressurizing the
absorption reactor to a
pressure in a range of from about 0 psig to about 10 psig.
[00143] Embodiment S. The process of claim 15, further comprising:
removing unabsorbed gases of the light gas stream from contact with the
absorption
solvent system to form a waste gas stream.
[00144] Embodiment T. The process of claim 15, wherein the absorption
solvent
system comprises copper chloride, aniline, and N-methylpyrrolidone.
[00145] Embodiment U. The process of claim 15, wherein the contacting
the light gas
stream with the absorption solvent system in an absorption reactor comprises
pressurizing the
absorption reactor to a pressure in a range of from about 40 psig to about 60
psig.
[00146] Embodiment V. The process of claim 15, further comprising:
removing at least a portion of elemental oxygen or oxygen-containing compounds
from the
light gas stream before introducing the light gas stream into the absorption
reactor.
[00147] Embodiment W. A polyethylene production system, comprising:
a feed stream comprising ethylene, wherein the feed stream is characterized by
introduction
into a polymerization reactor;
a polymerization product stream, wherein the polymerization product stream is
characterized by emission from the polymerization reactor and introduction
into a separator;
a light gas stream comprising unreacted ethylene, wherein the light gas stream
is
characterized by emission from the separator, the light gas stream having been
separated from the
polymerization product stream, wherein the light gas stream is characterized
by introduction into
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
42
an absorption solvent system, wherein the absorption solvent system has a
temperature in a range
of from about 40 F to about 110 F;
an absorbent-ethylene conjugant, wherein the absorbent-ethylene conjugant is
characterized
by formation within the absorption solvent system by absorption of at least a
portion of the
unreacted ethylene by the absorption solvent system; and
a waste gas stream comprising ethane, wherein the waste gas stream is
characterized by
emission from the absorption reactor, wherein the waste gas stream comprises
components of the
light gas stream that are not absorbed by the absorption solvent system; and
a recovered unreacted ethylene stream, wherein the recovered unreacted
ethylene stream is
characterized by emission from the absorption reactor and reintroduction into
the polymerization
reactor.
[00148] Embodiment X. The system of claim 23, wherein recovery of the
recovered
unreacted ethylene from the absorbent-ethylene conjugant occurs via a pressure
reduction from a
pressure of the absorption reactor to a pressure in a range of from about 0
psig to about 10 psig.
[00149] Embodiment Y. The system of claim 23, wherein recovery of the
recovered
unreacted ethylene from the absorbent-ethylene conjugant occurs at a
temperature in a range of
about 110 F to about 200 F.
[00150] Embodiment Z. The system of claim 23, wherein the absorption
solvent
system comprises copper chloride, aniline, and N-methylpyrrolidone.
[00151] Embodiment AA. A polyethylene production system, comprising:
a polymerization reactor, wherein the polymerization reactor is configured to
receive a feed
stream comprising ethylene, and wherein the polymerization reactor is
configured to emit a
polymerization product stream;
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
43
a separator, wherein the separator is configured to receive the polymerization
product
stream and to emit a light gas stream comprising unreacted ethylene, wherein
the light gas stream
has been separated from the polymerization product stream; and
an absorption reactor comprising an absorption solvent system, wherein the
absorption
reactor is configured to receive the light gas stream, to absorb at least a
portion of the unreacted
ethylene with the absorption solvent system at a temperature in a range of
from about 40 F to
about 110 F, and to emit a waste gas stream comprising components of the
light gas stream that
are not absorbed by the absorption solvent system, and wherein the absorption
reactor is further
configured to emit a recovered unreacted ethylene stream, and wherein the
polymerization reactor
is further configured to receive the recovered unreacted ethylene stream.
[00152] Embodiment AB. The system of claim 27, wherein the recovered
unreacted
ethylene is recovered from the absorption solvent system via a pressure
reduction from a pressure
of the absorption reactor to a pressure in a range of from about 0 psig to
about 10 psig.
[00153] Embodiment AC. The system of claim 27, wherein the recovered
unreacted
ethylene is recovered from the absorption solvent system via a temperature
increase from the
absorption temperature to a temperature in a range of from about 110 F to
about 200 F.
[00154] Embodiment AD. The system of claim 27, wherein the absorption
reactor
comprises two or more packed-bed reactors, wherein the recovered unreacted
ethylene is recovered
from the absorption solvent system via a pressure reduction of one of the two
or more packed-bed
reactors while another of the packed bed reactors operates at a pressure in a
range of from about 40
psig to about 60 psig.
[00155] Embodiment AE. The system of claim 27, wherein the absorption
solvent
system comprises copper chloride, aniline, and N-methylpyrrolidone.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
44
[00156] Embodiment AF. The system of claim 27, further comprising a
second
absorption reactor, wherein the absorption reactors are configured to absorb
ethylene in a liquid
solvent through pressure swing absorption.
[00157] Embodiment AG. A polyethylene production system, comprising:
a polymerization reactor, wherein the polymerization reactor is configured to
receive a feed
stream comprising ethylene, and wherein the polymerization reactor is
configured to emit a
polymerization product stream;
a separator, wherein the separator is configured to receive the polymerization
product
stream and to emit a light gas stream comprising unreacted ethylene, wherein
the light gas stream
has been separated from the polymerization product stream;
an absorption reactor comprising an absorption solvent system, wherein the
absorption
reactor is configured to receive the light gas stream, to absorb at least a
portion of the unreacted
ethylene with the absorption solvent system at a temperature in a range of
from about 40 F to
about 110 F and to emit a waste gas stream comprising components of the light
gas stream that are
not absorbed by the absorption solvent system, wherein the absorption reactor
is further configured
to emit a complexed stream comprising ethylene absorbed in the absorbent
solvent system; and
a solvent regenerator to regenerate the absorption solvent system, and to emit
a recovered
unreacted ethylene stream, wherein the polymerization reactor is further
configured to receive the
recovered unreacted ethylene stream.
[00158] Embodiment AH. The system of claim 33, wherein the solvent
regenerator is
configured to operate at a pressure in a range of from about 0 psig to about
10 psig.
[00159] Embodiment Al. The system of claim 33, wherein the solvent
regenerator is
configured to operate at a temperature in a range of from about 110 F to
about 200 F.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
EXAMPLES
[00160] The disclosure having been generally described, the following examples
are given as
particular embodiments of the disclosure and to demonstrate the practice and
advantages thereof
It is understood that these examples are given by way of illustration and is
not intended to limit the
specification or the claims in any manner.
[00161] A computerized commercial process simulator was employed to generate
an output from
a model in accordance with the systems and/or processes disclosed herein. The
model employed is
illustrated at Figure 9, which shows an embodiment of a system 900, as
disclosed herein, and shall
be used to describe the examples below. In the embodiment shown in Figure 9, a
light gas stream
18, which was separated from a polymerization product stream of a polyethylene
reactor, feeds to
an absorption reactor 116. The total molar and mass flows and component molar
and mass flows
of the light gas stream 18 are shown in Table 1 below:
Table 1
Total Molar Flow (lbmol/hr) 52.9 Total Mass Flow (lb/hr)
1127
Component Molar Flow Component Mass Flow
(lbmol/hr) (lb/hr)
Hydrogen 15.4 Hydrogen 31
Nitrogen 4.9 Nitrogen
137
Ethylene 26 Ethylene
729
Ethane 5.6 Ethane
169
Isobutane 1.1 Isobutane 62
Component Molar Component Mass
Fraction Fraction
Hydrogen 0.291 Hydrogen
0.028
Nitrogen 0.092 Nitrogen
0.121
Ethylene 0.491 Ethylene
0.646
Ethane 0.106 Ethane
0.150
Isobutane 0.020 Isobutane
0.055
[00162] Unreacted ethylene that enters the absorption reactor 116 is absorbed
in the absorption
solvent system within the absorption reactor 116. Absorbed unreacted ethylene
flows, as
complexed stream 28, to a first regenerator 120. In stream 28, the absorbed
ethylene is heated by
heat exchanger REG1HEAT before entering the first regenerator 120. Ethylene
desorbs from the
solvent from the absorption solvent system in first regenerator 120 and flows
through stream 29 to
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
46
a second regenerator 122. Stream 29 may be cooled with heat exchanger REG2COOL
before
entering the second regenerator 122. Ethylene is recovered in stream 20.
Absorption solvent in
streams 32 and 34 combine in heat exchanger FEEDCOOL to recycle to the
absorption reactor 116
in stream 30.
[00163] Table 2 shows operating conditions for examples 1-44 of ethylene
recovery using the
system 900 of Figure 9. For the examples shown in Table 2, the absorption
solvent system
comprises a copper chloride, aniline, and NMP system, as disclosed herein, and
composition of the
purified product is based on 90% ethylene recovery. The composition of the
purified product
recovered in Figure 9 comprises ethylene, ethane, nitrogen, hydrogen, and
isobutane. The wt % of
each of these components in the purified product is shown in Table 2. The
purified product
compositions shown in Table are compositions of stream 20 in Figure 9. Select
examples from
Table 2 are discussed in detail below.
EXAMPLE 3
[00164] In Example 3 of Table 2, the absorption reactor 116 in Figure 9
operates at a temperature
of 15 F, with a lean solvent temperature of 14 F and pressure of 40 psig.
The first regenerator
120 operates at a temperature of 150 F and pressure of 0 psig. The second
regenerator 122
operates at a temperature of 50 F and pressure of 0 psig. Under these
conditions, system 900
recovers 90 % of the ethylene and the solvent circulation flow rate to 344,776
lb/hr and the amount
of ethylene in the purified product to 64.5%.
EXAMPLE 4
[00165] In Example 4 of Table 2, the operating conditions are the same as
Example 3, except the
first regenerator 120 operates at a temperature of 200 F and pressure of 0
psig. Under these
conditions, system 900 recovers 90% of ethylene for a solvent circulation flow
rate of 143,736
lb/hr, and the purified product contains 77.5% ethylene.
EXAMPLE 7
[00166] In Example 7 of Table 2, the absorption reactor 116 in Figure 9
operates at a temperature
of 53 F, with a lean solvent temperature of 50 F. Absorption reactor 116
also operates at a
pressure of 40 psig. The first regenerator 120 operates at a temperature of
150 F and a pressure of
0 psig. The second regenerator 122 operates at a temperature of 50 F and a
pressure of 0 psig.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
47
Under these conditions, system 900 recovers 90% of the ethylene for a solvent
circulation flow rate
of 53,920 lb/hr. The purified product composition for Example 7 is shown in
Table 2.
[00167] When comparing Example 7 with the Examples 3 and 4, the solvent
circulation flow rate
of 53,920 lb/hr in Example 7 is less than the flow rates of 143,736 lb/hr and
344,776 lb/hr in the
Examples 3 and 4. Thus, Example 7 shows the solvent circulation flow rate
required to absorb
ethylene in a copper chloride aniline NMP absorption solvent system is much
less for an
absorption temperature of 53 F than for an absorption temperature of 15 F
because of the
unexpected drop in solubility for ethylene in the absorption solvent system
for temperatures below
about 50 F.
EXAMPLE 8
[00168] In Example 8 of Table 2, the absorption reactor 116 in Figure 9
operates at a temperature
of 55 F, with a lean solvent temperature of 50 F. Absorption reactor 116
also operates at a
pressure of 40 psig. The first regenerator 120 operates at a temperature of
200 F and a pressure of
0 psig. The second regenerator 122 operates at a temperature of 50 F and a
pressure of 0 psig.
Under these conditions, system 800 recovers 90% of the ethylene for a solvent
circulation flow rate
of 47,785 lb/hr. The purified product composition for Example 8 is shown in
Table 2.
[00169] Example 8 confirms the results shown in Example 7 that lower solvent
circulation flow
rates are required when the absorption reactor 116 operates at a temperature
of 55 F instead of
temperatures below 50 F. Example 2 additionally shows varying the temperature
of the
regenerators 120 from 150 F to 200 F does not affect the solvent circulation
flow rate to a
significant degree.
EXAMPLE 19
[00170] In Example 19 of Table 2, the absorption reactor 116 in Figure 9
operates at a
temperature of 53 F, with a lean solvent temperature of 50 F. Absorption
reactor 116 also
operates at a pressure of 40 psig. The first regenerator 120 operates at a
temperature of 200 F and
a pressure of 10 psig. The second regenerator 122 operates at a temperature of
50 F and a
pressure of 10 psig. Under these conditions, system 900 recovers 90% of the
ethylene for a solvent
circulation flow rate of 59,272 lb/hr. The purified product composition is
shown in Table 2.
[00171] Example 19 confirms the lower solvent circulation rates discussed in
Examples 7 and 8
when compared to Examples 3 and 4. Example 19 also shows varying the pressure
of the first and
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
48
second regenerators 120 and 122 between 0 psig and 10 psig does not
significantly alter results.
Operation of the regenerators 120 and 122 at 0 psig may provide a lower
solvent circulation rate as
well as enhanced product purity, and operation of the regenerators 120 and 122
at 10 psig may
provide a safer design because a positive pressure in the regenerators 120 and
122 reduces a chance
of air and water infiltration via leaks in the system and process, which may
react with copper
chloride in the absorption solvent system and inhibit performance.
EXAMPLE 28
[00172] In Example 28 of Table 2, the absorption reactor 116 in Figure 9
operates at 52 F, with a
lean solvent temperature of 50 F. Absorption reactor 116 also operates at a
pressure of 60 psig.
The first regenerator 120 operates at a temperature of 100 F and a pressure
of 0 psig. The second
regenerator 122 operates at a temperature of 50 F and a pressure of 0 psig.
Under these
conditions, system 900 recovers 90% of the ethylene for a solvent circulation
flow rate of 58,613
lb/hr. The purified product composition is shown in Table 2.
[00173] Under the conditions in Example 28, the solvent circulation flow rate
is less than that of
the Examples 3 and 4, and the amount of ethylene in the purified product is
significantly higher.
EXAMPLE 29
[00174] In Example 29 of Figure 2, the absorption reactor 116 in Figure 9
operates at 55 F, with
a lean solvent temperature of 50 F. Absorption reactor 116 also operates at a
pressure of 60 psig.
The first regenerator 120 operates at a temperature of 150 F and a pressure
of 0 psig. The second
regenerator 122 operates at a temperature of 50 F and a pressure of 0 psig.
Under these
conditions, system 900 recovers 90% of the ethylene for a circulation flow
rate of solvent of
51,106 lb/hr. The purified product composition is shown in Table 2.
[00175] Under the conditions in Example 29, the solvent circulation flow rate
is less than that of
the Examples 3 and 4, and the amount of ethylene in the purified product is
significantly higher.
EXAMPLE 30
[00176] In Example 30 of Table 2, the absorption reactor 116 in Figure 9
operates at 56 F, with a
lean solvent temperature of 50 F. Absorption reactor 116 also operates at a
pressure of 60 psig.
The first regenerator 120 operates at a temperature of 200 F and a pressure
of 0 psig. The second
regenerator 122 operates at a temperature of 50 F and a pressure of 0 psig.
Under these
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
49
conditions, system 900 recovers 90% of the ethylene for a circulation flow
rate of solvent of
46,744 lb/hr. The purified product composition is shown in Table 2.
[00177] Under the conditions in Example 30, the solvent circulation flow rate
is less than that of
the Examples 3 and 4, and the amount of ethylene in the purified product is
significantly higher.
EXAMPLE 33
[00178] In Example 33 of Table 2, the absorption reactor 116 in Figure 9
operates at a
temperature of 102 F, with a lean solvent temperature of 100 F. Absorption
reactor 116 also
operates at a pressure of 60 psig. The first regenerator 120 operates at a
temperature of 200 F and
a pressure of 0 psig. The second regenerator 122 operates at a temperature of
50 F and a pressure
of 0 psig. Under these conditions, system 900 recovers 90% of the ethylene for
a solvent
circulation flow rate of 63,435 lb/hr. The purified product composition for
Example 33 is shown in
Table 2.
[00179] Under the conditions in Example 33, the solvent circulation flow rate
is less than that of
the Examples 3 and 4, and the amount of ethylene in the purified product is
significantly higher.
Moreover, Example 33 show that operation of the absorption reactor 116 at
temperatures higher
than the temperatures of maximum solubility shown in Figure 7, for example, at
102 F as shown
in Example 33, may still prove economically feasible because, for example,
solvent circulation
flow rates remain low compared with conditions of Examples 3 and 4.
EXAMPLE 40
[00180] In Example 40 of Table 2, the absorption reactor 116 in Figure 9
operates at a
temperature of 52 F, with a lean solvent temperature of 50 F. Absorption
reactor 116 also
operates at a pressure of 60 psig. The first regenerator 120 operates at a
temperature of 150 F and
a pressure of 10 psig. The second regenerator 122 operates at a temperature of
50 F and a
pressure of 10 psig. Under these conditions, system 900 recovers 90% of the
ethylene for a solvent
circulation flow rate of 57,441 lb/hr. The purified product composition for
Example 40 is shown in
Table 2.
[00181] Under the conditions in Example 40, the solvent circulation flow rate
is less than that of
the Examples 3 and 4, and the amount of ethylene in the purified product is
significantly higher.
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
EXAMPLE 41
[00182] In Example 41 of Table 2, the absorption reactor 116 in Figure 9
operates at 55 F, with a
lean solvent temperature of 50 F. Absorption reactor 116 also operates at a
pressure of 60 psig.
The first regenerator 120 operates at a temperature of 200 F and a pressure
of 10 psig. The second
regenerator 122 operates at a temperature of 50 F and a pressure of 10 psig.
Under these
conditions, system 900 recovers 90% of the ethylene for a circulation flow
rate of solvent of
51,482 lb/hr. The purified product composition is shown in Table 2.
[00183] Under the conditions in Example 41, the solvent circulation flow rate
is less than that of
the Examples 3 and 4, and the amount of ethylene in the purified product is
significantly higher.
EXAMPLE SIMULATION
[00184] A computerized commercial process simulator was employed to generate
an output from
a model in accordance with the systems and/or processes disclosed herein. The
model employed is
illustrated at Figure 10, wherein a gaseous stream, designated VAP FEED (e.g.,
the light gas
stream disclosed herein) feeds to absorption reactor ASORB1. The output
generated by the
commercial process simulator is a material balance and a heat balance, shown
in Table 3. The
names designating the various streams listed in Table 3 correspond to streams
illustrated in Figure
10. In Figure 10, ASORB1 is the absorption reactor, which is shown as a four
stage absorber
operating at 90 F.
Table 2
Flow rate of
0
Lean Absorber top REG1 REG2 Absorber REG1 REG2
Ethylene circulation Ethylene Ethane Nitrogen Hydrogen Isobutane N
Example Solvent temperature temperature temperature pressure temperature
temperature
Recovery
solvent (wt %) (wt 0
%) (wt %) (wt %) (wt %)
Temp. (SF) (SF) (sF) (sF) (psig) (psig)
(psig) 1-,
(lb/hr) W
_
1 14 15 50 50 40 0 0 90% 1704044
48.9% 11.6% 10.0% 27.2% 2.2 A,
(A
2 14 15 100 50 40 0 0 90%
731337 57.7% 13.0% 11.1% _ 18.9% 2.3 A, cot
3 14 15 150 50 40 0 0 90% 344776
64.5% 15.2% 8.5% 9.5% 2.2% pe
4 14 15 200 50 40 0 0 90%
143736 77.5% 16.3% 2.1% _ 3.3% 0.8% N
50 50 50 50 40 0 0 90% 672565 62.1% 13.3%
7.0% 15.1% 2.5%
6 50 51 100 50 40 0 0 90%
158735 81.9% 11.2% 1.8% _ 4.1% 1.0%
7 50 53 150 50 40 0 0 90% 53920 95.9%
2.3% 0.5% 1.1% 0.3%
8 50 55 200 50 40 0 0 90%
47785 96.5% 1.9% 0.4% _ 1.0% 0.3%
9 100 100 100 50 40 0 0 90% 921807
62.1% 13.0% 7.1% 15.3% 2.5%
100 100 150 50 40 0 0 90% 343211
78.4% 9.5% 3.2% _ 7.1% 1.9%
11 100 101 200 50 40 0 0 90%
88403 95.6% 1.9% 0.7% _ 1.4% 0.4%
12 14 14 50 50 40 10 10 N/A
_
13 14 15 100 50 40 10 10 90% 1321719 50.3%
12.0% 10.2% 25.3% 2.2%
_
14 14 14 150 50 40 10 10 90% 685442 56.2%
13.3% 10.6%2.3%
_ 17'7%
14 15 200 50 40 10 10 90% 420362 64.1% 14.8%
7.1% 11.7% 2.4% P
_
16 50 50 50 50 40 10 10 90% 1367657 54.8%
11.8% 9.0% 22.2% 2.2% .
17 50 50 100 50 40 10 10 90% 463945 66.9%
14.6% 5.1% 11.1% 2.3% 1.3
03
o,
18 50 51 150 50 40 10 10 90%
121635 86.6% 8.1% 1.3% . 3.1% 0.8% up
..]
19 50 53 200 50 40 10 10 90%
59272 95.6% 2.5% 0.5% _ 1.2% 0.3%
100 100 100 50 40 10 10 90% 1828349 54.8% 11.6%
9.0% 22.3% 2.2% 1.3
21 100 100 150 50 40 10 10 90% 880270 64.5%
12.7% 5.7% 14.7% 2.4% o
/
Ø
22 100 100 200 50 40 10 10 90%
415884 77.5% 9.6% 2.3% 8.4% 2.1% 1
/
23 14 15 50 50 60 0 0 90%
858069 50.7% 12.0% 10.4% _ 24.7% 2.2%
o0,
24 14 15 100 50 60 0 0 90%
384021 58.5% 13.9% 11.4% _ 13.7% 2.5% o,
14 15 150 50 60 0 0 90% 159379
71.6% 16.9% 4.5% _ 5.6% 1.4%
26 14 16 200 50 60 0 0 90% 93956
82.2% 12.7% 1.7% 2.8% 0.7%
27 50 50 50 50 60 0 0 90%
296859 68.5% 14.6% 4.6% _ 9.8% 2.5%
28 50 52 100 50 60 0 0 90%
58613 93.9% 3.4% 0.7% , 1.6% 0.4%
29 50 55 150 50 60 0 0 90%
51106 94.7% 2.9% 0.6% , 1.4% 0.4%
50 56 200 50 60 0 0 90% 46744
95.3% 2.6% 0.5% , 1.3% 0.3%
31 100 100 100 50 60 0 0 90% 428830
68.5% 13.3% 5.0% 10.6% 2.6%
32 100 100 150 50 60 0 0 90%
111161 90.5% 4.1% 1.5% , 3.0% 0.9%
33 100 102 200 50 60 0 0 90%
63435 95.7% 1.8% 0.7% 1.4% 0.4%
34 14 14 50 50 60 10 10 N/A
IV
14 15 100 50 60 10 10 90% 693610 52.5% 12.5%
10.6% 22.1% 2.3% n
36 14 15 150 50 60 10 10 90%
346101 60.5% 14.3% 10.5% . 12.3% 2.4%
37 14 15 200 50 60 10 10 90% 181196 71.0%
16.3% 4.4%6.7% 1.6%
.
CP
38 50 50 50 50 60 10 10 90% 669442 58.7%
12.6% 8.2% 18.2% 2.3% N
39 50 51 100 50 60 10 10 90% 179196 75.0%
15.2% 2.6%5.8% 1.4%
,
0
1-,
50 52 150 50 60 10 10 90% 57441 94.1% 3.3%
0.7% 1.6% 0.4% W
41 50 55 200 50 60 10 10 90%
51482 94.8% 2.9% 0.5% , 1.4% 0.4% 7:-8
42 100 100 100 50 60 10 10 90% 896707 58.7%
12.4% 8.3% 18.3% 2.4% Uvi
1-,
43 100 100 150 50 60 10 10 90% 378813
71.8% 12.1% 4.1% , 9.6% 2.4%
, 44 100 100 200 50 60 10 10
90% 130215 89.1% 4.8% 14.3% 3.7% 1.1% W:::'
0
k...)
Table 3
o
1-.
(...)
1-.
col
.6.
L1CUCLL L2CUCLR L3CUCLR2 L4CUCLR3 L5CUCLL L6CUCLL LKO1 LKO2 LKO3 V1
V1FLARE V2 V3 VAP-REC VAPFEED Ce
oe
t..)
Substream:
MIXED
Mole Flow
lbmol/hr
C2=
1.949416 41.85801 41.85801 41.85801 1.949413
1.949413 2.02E-04 4.41E-03 9.89E-04 3.172776 3.172573 39.91302 39.9096
39.90861 43.08116
C2 0.9764562 5.916248
5.916248 5.916248 0.9764532 0.9764532 6.17E-04 8.14E-
04 2.10E-04 5.654325 5.653708 4.940621 4.940017 4.939807 10.5935
N2 1.15E-03 0.1711679 0.1711679 0.1711679 1.15E-03
1.15E-03 8.35E-06 8.99E-07 6.78E-08 7.187729
7.187721 0.1700194 0.1700186 0.1700185 7.357739
1C4 0.8615088 3.112527
3.112527 3.112527 0.8615092 0.8615092 2.14E-04
2.23E-03 1.08E-03 0.1670439 0.1668301 2.253242 2.252096 2.251014 2.417848
CUCL 131.4402 131.4402 131.4402 131.4402 131.4402 131.4402 1.50E-13 2.85E-
13 0 1.50E-13 4.78E-22 2.85E-13 2.86E-24 0 0 P
ANILINE 580.5749 580.5749
580.5749 580.5749 580.5748 580.5748 2.47E-03 0.2059512 0.0219362 2.48E-03
9.19E-06 0.20608 0.022065 1.29E-04 0 0
n,
00
NMP 789.7864 789.7864 789.7864
789.7864 789.7864 789.7864 2.38E-03 0.1961199 9.42E-03 2.38E-
03 3.17E-06 0.1961404 9.44E-03 2.06E-05 0 .
Mole Frac
...1
kNa)
A.
C2=
1.29E-03 0.0269554 0.0269554 0.0269554 1.29E-03
1.29E-03 0.0343637 0.0107758 0.0294004 0.1960108 0.1960697 0.8371173 0.843697
0.8442764 0.6789755 n,
C2 6.49E-04 3.81E-03
3.81E-03 3.81E-03 6.49E-04 6.49E-04 0.1047838
1.99E-03 6.25E-03 0.3493185 0.3494075 0.1036223 0.104433 0.1045028 0.1669576
0
1-
a.
'
N2 7.64E-07 1.10E-04
1.10E-04 1.10E-04 7.64E-07 7.64E-07 1.42E-03
2.19E-06 2.02E-06 0.4440506 0.4442117 3.57E-03 3.59E-03 3.60E-03 0.1159608
1-
0
1C4 5.72E-04 2.00E-03
2.00E-03 2.00E-03 5.72E-04 5.72E-04 0.0362971
5.44E-03 0.032152 0.0103198 0.0103103 0.0472584 0.0476097 0.0476207 0.0381062
1
0
CUCL 0.0873014 0.0846439 0.0846439 0.0846439 0.0873014 0.0873014 2.54E-11
6.97E-13 0 9.24E-15 2.95E-23 5.99E-15 6.04E-26 0 0 ..)
ANILINE 0.3856129
0.3738747 0.3738747 0.3738747 0.3856128 0.3856128 0.4198489 0.5029009
0.6521121 1.53E-04 5.68E-07 4.32E-03 4.66E-04 2.73E-06 0
NMP 0.5245694
0.5086013 0.5086013 0.5086013 0.5245694 0.5245694 0.4032882 0.4788945
0.2800845 1.47E-04 1.96E-07 4.11E-03 2.00E-04 4.36E-07 0
Mass Flow
lb/hr
C2= 54.68846 1174.274 1174.274
1174.274 54.68837 54.68837 5.68E-03 0.1238009 0.027745 89.00829
89.00261 1119.71 1119.614 1119.587 1208.589
C2 29.36169 177.8994 177.8994
177.8994 29.3616 29.3616 0.0185606 0.0244775 6.32E-03
170.0235 170.005 148.5627 148.5445 148.5382 318.5427
N2 0.0322059 4.795009
4.795009 4.795009 0.0322058 0.0322058 2.34E-04 2.52E-05
1.90E-06 201.3533 201.3531 4.762835 4.762812 4.76281 206.1159
1C4 50.07382 180.9106
180.9106 180.9106 50.07384 50.07384 0.0124277 0.129461 0.0628636 9.709158
9.69673 130.9661 130.8995 130.8366 140.5336
CUCL 13012.41 13012.41 13012.41 13012.41 13012.41 13012.41 1.48E-11 2.83E-
11 0 1.48E-11 4.73E-20 2.83E-11 2.83E-22 0 0 IV
ANILINE 54067.97 54067.97
54067.97 54067.97 54067.96 54067.96 0.2303272
19.17989 2.042886 0.2311832 8.56E-04 19.19188 2.054883 0.0120247 0 n
NMP 78293.58 78293.58
78293.58 78293.58 78293.58 78293.58 0.2355062 19.44187
0.9339974 0.2358215 3.14E-04 19.4439 0.9360284 2.04E-03 0
Mass Frac
(/)
t..)
C2= 3.76E-04 7.99E-03
7.99E-03 7.99E-03 3.76E-04 3.76E-04 0.0112959 3.18E-
03 9.03E-03 0.1891534 0.1893437 0.7761549 0.7958521 0.797575 0.645 o
1-,
C2 2.02E-04 1.21E-03
1.21E-03 1.21E-03 2.02E-04 2.02E-04 0.0369193 6.29E-
04 2.06E-03 0.3613207 0.3616676 0.1029799 0.1055895 0.1058162 0.17
(....)
N2 2.21E-07 3.26E-05
3.26E-05 3.26E-05 2.21E-07 2.21E-07 4.66E-04 6.47E-
07 6.18E-07 0.4279003 0.4283574 3.30E-03 3.39E-03 3.39E-03 0.11 Ci5
(....)
1C4 3.44E-04 1.23E-03
1.23E-03 1.23E-03 3.44E-04 3.44E-04 0.0247203 3.33E-
03 0.0204513 0.0206331 0.0206287 0.0907823 0.0930468 0.0932058 0.075 col
1-,
CUCL 0.0894273 0.0885729 0.0885729 0.0885729 0.0894273 0.0894273 2.95E-11
7.26E-13 0 3.15E-14 1.01E-22 1.96E-14 2.01E-25 0 0 O
(....)
ANILINE 0.3715804 0.36803 0.36803
0.36803 0.3715804 0.3715804 0.4581486 0.4930622 0.6646093 4.91E-04 1.82E-
06 0.0133033 1.46E-03 8.57E-06 0
NMP 0.5380702 0.532929 0.532929
0.532929 0.5380702 0.5380702 0.4684502 0.4997972 0.3038561
5.01E-04 6.69E-07 0.013478 6.65E-04 1.46E-06 0
0
t..)
o
1-,
(....)
1-,
c.n
.P.
oe
oe
L1CUCLL L2CUCLR L3CUCLR2 L4CUCLR3 L5CUCLL L6CUCLL LKO1 LKO2 LKO3 V1
V1FLARE V2 V3 VAP-REC VAPFEED t..)
Total Flow
lbmol/hr 1505.59 1552.86 1552.86 1552.86
1505.59 1505.59 5.89E-03 0.4095263 0.0336387 16.18674 16.18085
47.67913 47.30324 47.2696 63.45025
Total Flow
lb/hr 145508 1.47E+05 1.47E+05
1.47E+05 1.46E+05 1.46E+05 0.5027346 38.89952 3.073815 470.5613
470.0586 1442.638 1406.812 1403.738 1873.781
Total Flow
cuft/hr 2000 2063.515 9563.191
13833 2058.304 2058.521 8.16E-03 0.6204605 0.04765 825.9148 634.7071
12547.34 11089.96 8812.544 1155.656
Temperature F 90 105.0961 95.53801 140 158 158.2431 -20
90 -20 96.94405 -20 158 90 -20 0
Pressure psia 117.6959 114.6959 25 25 25 118.6959
114.6959 24.9 24.8 114.6959 114.6959 25 24.9 24.8 226.6959
Vapor Frac 0 0 0.020591 0.0297334 0 0 0 0
0 1 1 1 1 1 0.9823996
Liquid Frac 1 1 0.9794089 0.9702665 1 1 1
1 1 0 0 0 0 0 0.0176004 P
Solid Frac 0 0 0 0 0 0 0 0 0 0
0 0 0 0 0 0
n,
00
Enthalpy
.
Btu/lbmol
-60439.71 -58273.13 -58273.13 -56229.58 -57622.43 -
57585.8 -49592.05 -47471.61 -28177.63 -8659.402 -9795.256 13137.72 12629.88
11470.01 5793.013 ...1
Enthalpy
n,
Btu/lb
-625.377 -615.9475 -615.9475 -594.3472 -596.2263 -595.8472 -581.0902 -
499.7715 -308.3662 -297.8729 -337.1825 434.201 424.6725 386.242 196.1639
0
1-
a.
,
Enthalpy
1-
Btu/hr
-9.10E+07 -9.05E+07 -9.05E+07 -8.73E+07 -8.68E+07 -
8.67E+07 -292.1342 -19440.87 -947.8608 -1.40E+05 -1.59E+05 6.26E+05 5.97E+05
5.42E+05 3.68E+05 0
1
0
Entropy
..)
Btu/lbmol-R -112.3696 -109.6524 -109.5691
-106.0242 -107.4881 -107.4788 -111.4813 -112.727 -110.4079 -19.64671 -
21.92954 -18.274 -19.22263 -21.55552 -25.0739
Entropy
Btu/lb-R -1.162701 -1.159027
-1.158146 -1.120676 -1.112192 -1.112096 -
1.306271 -1.186767 -1.208266 -0.6758228 -0.7548814 -0.603955 -0.6463497 -
0.7258623 -0.8490563
Density
lbmol/cuft
0.75276 0.7525312 0.1623788 0.1122576 0.7314713 0.731394 0.7221323
0.6600361 0.7059546 0.0195985 0.0254934 3.80E-03 4.27E-03 5.36E-03
0.0549041
Density
lb/cull 72.75067 71.19494 15.36222
10.62039 70.69322 70.68575 61.62902 62.6946 64.50811
0.5697456 0.7405913 0.1149756 0.1268545 0.1592887 1.6214
Average MW 96.64524 94.6073 94.6073
94.6073 96.64524 96.64524 85.34311 94.98663 91.37714
29.0708 29.05031 30.25722 29.74029 29.69643 29.5315
Liq Vol 60F
IV
cull/hr 2474.029 2538.765 2538.765
2538.765 2474.029 2474.029 8.78E-03 0.6165612 0.0501975
18.42872 18.41994 65.35257 64.78621 64.73601 83.15566 n
c 4
k ... )
c : ,
,.... ,
,.... ,
u ,
c : ,
,.... ,
CA 02869744 2014-10-06
WO 2013/154882 PCT/US2013/035103
54
[00185] At least one embodiment is disclosed and variations, combinations,
and/or modifications
of the embodiment(s) and/or features of the embodiment(s) made by a person
having ordinary skill
in the art are within the scope of the disclosure. Alternative embodiments
that result from
combining, integrating, and/or omitting features of the embodiment(s) are also
within the scope of
the disclosure. Where numerical ranges or limitations are expressly stated,
such express ranges or
limitations should be understood to include iterative ranges or limitations of
like magnitude falling
within the expressly stated ranges or limitations (e.g., from about 1 to about
10 includes, 2, 3, 4,
etc.; greater than 0.10 includes 0.11, 0.12, 0.13, etc.). For example,
whenever a numerical range
with a lower limit, RI, and an upper limit, Ru, is disclosed, any number
falling within the range is
specifically disclosed. In particular, the following numbers within the range
are specifically
disclosed: R=Ri+k* (Re-R1), wherein k is a variable ranging from 1 percent to
100 percent with a
1 percent increment, i.e., k is 1 percent, 2 percent, 3 percent, 4 percent, 5
percent, ..... 50 percent,
51 percent, 52 percent... 95 percent, 96 percent, 97 percent, 98 percent, 99
percent, or 100 percent.
Moreover, any numerical range defined by two R numbers as defined in the above
is also
specifically disclosed. Use of the term "optionally" with respect to any
element of a claim means
that the element is required, or alternatively, the element is not required,
both alternatives being
within the scope of the claim. Use of broader terms such as comprises,
includes, and having
should be understood to provide support for narrower terms such as consisting
of, consisting
essentially of, and comprised substantially of. Accordingly, the scope of
protection is not limited
by the description set out above but is defined by the claims that follow,
that scope including all
equivalents of the subject matter of the claims. Each and every claim is
incorporated as further
disclosure into the specification and the claims are embodiment(s) of the
disclosed inventive
subject matter. The discussion of a reference in the disclosure is not an
admission that it is prior
art, especially any reference that has a publication date after the priority
date of this application.
The disclosure of all patents, patent applications, and publications cited in
the disclosure are hereby
incorporated by reference, to the extent that they provide exemplary,
procedural or other details
supplementary to the disclosure.