Note: Descriptions are shown in the official language in which they were submitted.
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 FUNCTIONALIZATION OF A SUBSTRATE
2
3 CROSS-REFERENCE TO RELATED APPLICATIONS:
4 [0001] This application claims priority from United States Patent
Application No. 13/446,927
filed on April 13, 2012, U.S. Provisional Patent Application No. 61/673,147
filed on July 18,
6 2012, U.S. Provisional Patent Application No. 61/806,855 filed on March
30, 2013, and
7 Canadian Patent Application No. 2,774,591 filed on April 13, 2012 the
contents of each of which
8 are hereby incorporated by reference in their entirety.
9 TECHNICAL FIELD
[0002] The following relates generally to functionalization of a substrate.
11 BACKGROUND
12 [0003] Organic light emitting diodes (OLEDs) are becoming more
widely used in displays
13 and other optoelectronic applications. Organic electronic displays
typically consist of a matrix of
14 OLEDs, each of which comprises thin films of organic materials that emit
light when excited by
an electric current. The organic thin films are typically sandwiched between
an anode and a
16 cathode, which provide an electric current to the organic thin film to
enable the film to emit light.
17 In a display, the light emitted by the organic thin film must exit the
thin film and penetrate
18 through at least one of the electrodes to be visible to a user. Hence,
at least one of the
19 electrodes in the electrode pair comprises a transparent conductor such
as a transparent
conducting oxide (TCO).
21 [0004] Indium tin oxide (ITO) is the most commonly used TCO due to
its transparency and
22 its high conductivity relative to other TC0s. ITO is used in various
applications requiring
23 transparency and conductivity including liquid crystal displays, plasma
displays, photovoltaics,
24 electronic ink displays, and OLED displays. ITO is typically deposited
as a thin film on a
transparent substrate such as glass.
26 [0005] In the context of OLEDs, an ITO layer is typically formed on
a transparent substrate
27 used as the anode. Holes are injected from the anode into a hole
transport layer (HTL), which
28 carries the holes to the light emitting thin film layer. Concurrently,
electrons are injected via the
29 cathode and are transported through the electron transport layer (ETL)
and recombine with the
holes in the light emitting thin film layer to release a photon. The photon
emitted in the thin film
1
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 layer may then escape the thin film layer, pass through the HTL and exit
the OLED device
2 through the ITO layer and the transparent substrate.
3 [0006] The energy required to inject holes from the anode is
dependent on the hole injection
4 barrier height. The hole injection barrier height depends on the
difference between the work
function of the anode and the highest occupied molecular orbital (HOMO) of the
adjacent
6 organic layer. The hole injection barrier of existing OLEDs is high but
this can be mitigated by
7 providing one or more intermediate organic layers. Each organic layer has
a subsequently
8 deeper HOMO level, enabling holes to pass through a larger number of
smaller injection
9 barriers rather than a single large injection barrier. However, each
additional organic layer
increases the cost of the device and decreases the yield of the manufacturing
process.
11 [0007] It is an object of the present invention to mitigate or
obviate at least one of the above
12 disadvantages.
13 SUMMARY
14 [0008] In a first aspect, there is provided a method of increasing
a work function of an
electrode comprising obtaining an electronegative species from a precursor
using
16 electromagnetic radiation; and reacting a surface of the electrode with
the electronegative
17 species.
18 [0009] The electronegative species may be a halogen. The
electromagnetic radiation may
19 have a wavelength of at least about 100 nm. The electromagnetic
radiation may have a
wavelength of less than about 400 nm. The method may further comprise cleaning
the surface
21 of the electrode. The electrode may be a transparent conducting oxide.
The transparent
22 conducting oxide may be ITO. The electronegative species may be selected
to obtain an
23 electrode of a predetermined work function. The surface coverage of the
species may be
24 selected to obtain an electrode of a predetermined work function. Up to
about a monolayer of
halogen may functionalized to the substrate. The halogen may be chlorine. The
precursor may
26 be a volatile liquid. The precursor may be a gas. The substrate may be
functionalized to
27 increase its stability in air.
28 [0010] In another aspect, an electrode comprising a substrate
functionalized according to
29 the above method is provided. An organic electronic device comprising
the electrode is also
provided.
2
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 [0011] In yet another aspect, there is provided the use of a system
to chemically
2 functionalize a substrate with a species, the system comprising a
reaction chamber; a radiation
3 emitter operable to emit electromagnetic radiation into the reaction
chamber; wherein the
4 reaction chamber is operable to receive a precursor of the species and a
substrate; and wherein
the electromagnetic radiation generates radicals from the precursor of the
species to chemically
6 bond with the substrate. The radiation emitter may emit radiation having
a wavelength of at
7 least about 100 nm. The radiation emitter may emit radiation having a
wavelength of less than
8 about 400 nm. In an example embodiment, the radiation emitter is external
to the reaction
9 chamber; and the reaction chamber is operable to at least partially
transmit ultraviolet radiation
from the radiation emitter.
11 [0012] In yet another aspect, there is provided a method of
increasing a work function of an
12 electrode comprising obtaining chlorine from a precursor using a plasma;
and reacting a surface
13 of the electrode with the chlorine to form at least about 20% of a
chlorine monolayer. In an
14 example embodiment, up to about a monolayer of chlorine may be reacted
to the surface of the
electrode. The substrate may comprise a transparent conducting oxide. The
transparent
16 conducting oxide may be ITO. The surface coverage of the chlorine may be
selected to obtain
17 an electrode of a predetermined work function.
18 [0013] In yet another aspect there is provided an electrode
comprising a substrate
19 functionalized with at least about 20% of a monolayer of halogen. There
is also provided an
organic electronic device comprising the electrode. The organic electronic
device may comprise
21 an organic light emitting diode. The organic light emitting diode may be
phosphorescent. The
22 organic light emitting diode may be fluorescent.
23 BRIEF DESCRIPTION OF THE DRAWINGS
24 [0014] Embodiments will now be described by way of example only
with reference to the
appended drawings wherein:
26 [0015] FIG. 1 is a diagram illustrating a system in accordance with
the present invention
27 comprising a substrate which is functionalized with approximately 0.5 of
a monolayer of a
28 species;
29 [0016] FIG. 2 is a diagram illustrating a system in accordance with
the present invention
wherein the substrate is functionalized with approximately 0.7 of a monolayer
of the species;
3
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 [0017] FIG. 3 is a diagram illustrating a system in accordance with
the present invention
2 wherein the substrate is functionalized with approximately one monolayer
of the species;
3 [0018] FIG. 4 is an X-Ray photoelectron spectroscopy graph showing
that the bonding state
4 of indium-chlorine bonds in InCI3 is equivalent to the bonding state of
indium-chlorine bonds in
chlorine-functionalized ITO;
6 [0019] FIG. 5 is an X-Ray photoelectron spectroscopy graph showing
the relationship
7 between treatment time and chlorine functionalization of an ITO surface;
8 [0020] FIG. 6 is an energy level diagram illustrating the work
function of an example ITO
9 electrode;
[0021] FIG. 7 is an energy level diagram illustrating the work function of
an example
11 chlorine-functionalized ITO electrode;
12 [0022] FIG. 8 is a representative chart showing the relationship
between the approximate
13 surface coverage of chlorine on an ITO substrate and the work function
of the ITO substrate;
14 [0023] FIG. 9 is an X-ray photoelectron spectroscopy graph
comparing the binding energy
of various halogen-functionalized substrates;
16 [0024] FIG. 10 is a representative chart contrasting the change in
work function over time in
17 air for a chlorine functionalized ITO substrate and a bare ITO
substrate;
18 [0025] FIG. 11 is a table showing the work function of various
chlorinated and bare
19 substrates after exposure to air;
[0026] FIG. 12 is a chart comparing the transmittance of chlorine-
functionalized ITO on a
21 glass substrate to the transmittance of a bare ITO electrode on the same
glass substrate;
22 [0027] FIG. 13 is a chart showing a spectrum of the ultraviolet
radiation emitter;
23 [0028] FIG. 14 is an energy level diagram of an example
phosphorescent green OLED
24 construction comprising a bare ITO anode;
[0029] FIG. 15 is an energy level diagram of an example phosphorescent
green OLED
26 construction comprising a chlorinated ITO anode;
27 [0030] FIG. 16 is a representative chart showing the relationship
between the work function
28 of a chlorine functionalized surface and the hole injection barrier
height into a hole transport
29 layer;
4
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 [0031] FIG. 17 is an energy level diagram of an example
phosphorescent green OLED
2 comprising a chlorine-functionalized anode;
3 [0032] FIG. 18 is a current-voltage chart showing a reduction in
required driving voltage with
4 increasing surface chlorination of an ITO anode;
[0033] FIG. 19 is a chart showing the relationship between current
efficiency and luminance
6 of the OLED of FIG. 17 comprising an anode with a monolayer of chlorine;
7 [0034] FIG. 20 is a diagram showing the efficiency of the OLED of
FIG. 17 comprising a
8 monolayer of chlorine on the ITO anode;
9 [0035] FIG. 21 is a table comparing the efficiency of the OLED of
FIG. 17 comprising a
monolayer of chlorine on the ITO anode to phosphorescent green OLED devices in
the prior art;
11 [0036] FIG. 22 is a chart showing the change in luminance over time
for an OLED
12 comprising a chlorine functionalized anode;
13 [0037] FIG. 23 is an energy level diagram of an example fluorescent
green OLED;
14 [0038] FIG. 24 is a diagram showing the current-voltage
characteristics of an example
fluorescent green OLED comprising a chlorine-functionalized ITO anode;
16 [0039] FIG. 25 is a diagram showing the efficiency of an example
fluorescent green OLED
17 comprising a chlorine-functionalized ITO anode;
18 [0040] FIG. 26 is a chart showing the current density with respect
to electric field for chlorine
19 functionalized ITO anode with respect to other anode types;
[0041] FIG. 27 is a diagram illustrating an example plasma
functionalization apparatus in
21 accordance with the present invention;
22 [0042] FIG. 28 is a diagram illustrating another example plasma
functionalization apparatus
23 in accordance with the present invention; and
24 [0043] FIG. 29 is an energy level diagram of an example
phosphorescent green OLED
comprising an ITO anode functionalized with chlorine
26 DETAILED DESCRIPTION
27 [0044] It will be appreciated that for simplicity and clarity of
illustration, where considered
28 appropriate, reference numerals may be repeated among the figures to
indicate corresponding
29 or analogous elements. In addition, numerous specific details are set
forth in order to provide a
5
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 -- thorough understanding of the example embodiments described herein.
However, it will be
2 -- understood by those of ordinary skill in the art that the example
embodiments described herein
3 -- may be practised without these specific details. In other instances, well-
known methods,
4 -- procedures and components have not been described in detail so as not to
obscure the
-- example embodiments described herein.
6 [0045] Also, the description is not to be considered as limiting
the scope of the example
7 -- embodiments described herein. For example, reference is made to
functionalizing a transparent
8 -- conducting oxide (TOO) substrate. It will be appreciated that other
substrates may be
9 -- functionalized using the process described herein. Other non-transparent
or non-conducting
-- substrates may also be functionalized according to the process described
herein.
11 [0046] Provided herein is a method of functionalization of a
substrate with a species. In
12 -- particular, the functionalization of an electrode with an
electronegative species to increase the
13 -- work function of the electrode is provided. Also provided is a method of
functionalizing TOO
14 -- electrodes to achieve a higher work function without materially altering
critical properties of the
-- TOO electrode such as conductivity and device stability. In one embodiment,
the substrate is
16 -- functionalized using plasma disassociation of a precursor to release a
reactive species, for
17 -- example, a halogen species. The halogen species is chemically reacted
with a substrate to
18 -- increase the work function of the substrate.
19 [0047] Also provided is a substrate functionalized with up to about
a monolayer of
-- electronegative species. The electronegative species may be a halogen. The
halogen may be
21 -- chlorine. The substrate may be a TOO. An electrode comprising a
functionalized substrate is
22 -- also provided. The substrate may be functionalized to increase the work
function of the
23 -- electrode. In an example embodiment, the substrate is functionalized
with at least about 20
24 -- percent of a monolayer. A functionalization of about 20 percent may be a
significant
-- accomplishment. An organic electronic device employing an electrode
comprising a
26 -- functionalized substrate is also provided.
27 [0048] It has now been found that transparent conducting oxides
including indium tin oxide
28 -- (ITO), which may also be referred to as tin-doped indium oxide, may be
directly used as an
29 -- electrode in an organic electronic device such as an organic light
emitting diode (OLED). The
-- work function of ITO is approximately 4.7 eV in a vacuum. Because 4.7 eV
rarely matches the
31 -- highest occupied molecular orbital (HOMO) level of many common hole
transporting materials,
32 -- the use of ITO as an anode causes a high hole injection barrier and poor
operational stability of
6
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 the device. It is well known that an anode having a work function that is
closer in energy to the
2 HOMO level of the adjacent hole transporting organic material would
reduce the hole injection
3 barrier, thereby reducing the required operating voltage and increasing
the efficiency and
4 operation stability of organic electronic devices.
[0049] In the case of OLEDs, the active light emitting materials typically
have HOMO levels
6 much greater than 4.7 eV. For example the HOMO level of tris(8-
hydroxyquinolinato)aluminium
7 (Alq3), a fluorescent green light emitting compound, is 5.75 eV. Although
some organic light
8 emitting materials may have HOMO levels closer to 4.7 eV, these materials
are typically doped
9 into a host matrix that has a much higher HOMO level than 4.7 eV.
Typically, holes must be
injected into the HOMO of the host in order for the dopant to emit light. For
example, the HOMO
11 level of tris(2-phenylpyridine)iridium(III) [Ir(ppy)3], a phosphorescent
green light emitting
12 compound is 5.4 eV, but is commonly doped into a 4,4'-bis(carbazol-9-
yl)biphenyl (CBP) matrix.
13 CBP has a HOMO level of 6.1 eV, which is much greater than 4.7 eV. In
particular, the HOMO
14 levels of the host materials used in phosphorescent OLEDs are about 6 eV
or greater.
Therefore, there exists a need for a transparent electrode having a work
function that is greater,
16 and preferably slightly greater, than the HOMO level of host materials
used in OLEDs. In
17 particular, there exists a need for a transparent electrode having a
work function of about 6 eV
18 or higher.
19 [0050] One way to increase the work function of a TCO substrate is
to clean the surface of
the substrate to remove contaminants. For example, the surface of the TCO
substrate may be
21 cleaned using ultraviolet (UV) ozone or 02 plasma treatment. Plasma
surface treatment and UV
22 ozone surface treatment are effective in removing organic contaminants
and may leave
23 electronegative species on the surface of the TCO substrate. By way of
example, UV ozone
24 cleaning of the surface of an ITO substrate may increase its work
function to about 5.0 eV.
Cleaning the substrate may cause band bending at the surface of the substrate
and an increase
26 in the surface dipole of the TCO due to electronegative oxygen species
on the surface of the
27 substrate, thereby increasing the work function of the ITO substrate.
Although reference is
28 made to cleaning the TCO substrate using UV ozone or 02 plasma, the
substrate may be
29 cleaned using liquid cleaning methods, for example, using a detergent or
solvent.
[0051] It has been recognized that another way to raise the work function
of ITO, which is
31 an important TCO substrate, is to chemically treat the ITO substrate
with an electronegative
32 halogen, for example, fluorine. In the example of an ITO substrate, a
halogen may be reacted
7
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 with indium atoms or tin atoms on the surface of the substrate to form up
to an approximate
2 layer of indium halide. The process of reacting a surface of the TOO
substrate with a halogen
3 may be referred to as "functionalization".
4 [0052] One way to chemically treat a TOO substrate with halogen is
to react the surface of
the substrate with a halogen-containing acid (e.g. hydrochloric acid). A
halogen gas may also
6 be dissolved in a carrier liquid to be applied to the surface of a TOO
electrode. However, these
7 processes are difficult to control, may etch the surface of the
substrate, and may leave very little
8 halogen functionalized to the surface of the substrate. Hence, the
substrate surface may
9 become rougher, and more contaminated, while the work function of the
electrode may not be
sufficiently increased. Furthermore, the conductivity and transparency of the
substrate may be
11 reduced using this process. Halogenation of a substrate using an
elemental hydrogen
12 containing solution (e.g. NCI) may be combined with UV ozone or 02
plasma treatment.
13 [0053] The work function of a TOO substrate may also be increased
using a halogen
14 containing plasma, which may cause a halide species to react with the
surface of the TOO. For
example, a fluorocarbon plasma such as CFH3, an inorganic fluorine containing
plasma such as
16 SF6, or a pure halogen plasma such as F2 may be used. Multiple plasma
gasses may be used
17 in combination. A carrier gas may also be used, for example, Ar, He, or
N2.
18 [0054] Halogen-containing plasmas are typically used as standard
reactive ion etching (RIE)
19 industrial processes to dry etch substrates including TOO electrodes.
Therefore, halogen-
containing plasmas typically etch the surface of the substrate. This may
decrease the
21 conductivity of the surface and may contaminate the surface with
halocarbons. Halocarbons
22 are molecules comprising one or more carbon atoms covalently bonded to
one or more halogen
23 atoms (e.g. fluorine, chlorine, iodine, and bromine). The chemical bond
between the
24 contaminant and the substrate depends on the materials involved, type of
plasma used and the
processing conditions. The addition of an oxidant (e.g. 02) may reduce the
amount of deposited
26 halocarbons and may also increase the rate at which the substrate is
etched, negatively
27 affecting other properties of the substrate. As is further described
below, an example apparatus
28 is provided for functionalizing species to the surface of a substrate
while reducing the etching of
29 the substrate.
[0055] It may be expected from the electronegativity of each of fluorine,
chlorine, iodine, and
31 bromine that fluorine functionalization provides the highest increase in
work function since it has
32 the highest electronegativity and therefore, would be expected to form
the largest surface
8
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 dipole. Surprisingly, it has now been found that chlorine functionalized
TCO's have a yet higher
2 work function. This has been confirmed from density functional theory
calculations and
3 experimental results as measured by X-ray photoelectron spectroscopy
(XPS) using an ITO
4 substrate that has been functionalized according to the process described
herein. Table 1
summarizes these results.
6 Table 1: Experimental and Theoretical Work Function of Functionalized
ITO
Halogen Functionalized to Experimental Work Density Functional
ITO Surface Function (XPS) [eV] Theory Calculation
[eV]
fluorine 5.7 5.7
chlorine 6.1 6.1
bromine 5.4
iodine 5.2
7 [0056] Therefore, a chlorine-functionalized TCO may have a higher
work function relative to
8 TCO's functionalized with other halogens.
9 [0057] The above mentioned UV ozone and 02 plasma cleaning
treatments are reversible.
For example, the surface of the cleaned TCO substrate may be re-contaminated,
11 electronegative species on the surface of the TCO may desorb, and the
surface of the substrate
12 is prone to hydrolysis. The above-described halogenation treatments
offer greater stability than
13 the UV ozone or 02 plasma treatments, however, typical application of
these treatments are
14 prone to etching the surface of the TCO substrate. Furthermore, these
halogenations
treatments may affect other critical properties including the surface
roughness, conductivity and
16 transparency of the TCO. Also, handling halogen-containing gases for
plasma processes
17 requires special safety precautions due to the toxicity and reactive
nature of the materials
18 involved.
19 [0058] The above-mentioned techniques may be unable to increase the
work function of
TCO substrates to a level enabling efficient injection of holes into hole
transporting organic
21 materials with deep HOMO levels (e.g. 6 eV or greater). As a result,
additional hole injection
22 layers (HILs) and hole transport layers (HTLs) with HOMO levels between
the work function of
23 the TCO substrate and the HOMO level of the active organic layer are
typically required in
24 practical organic optoelectronic devices to facilitate charge injection
from the anode. For
example, a number of intermediate organic layers may be used, each having a
subsequently
26 deeper HOMO level. This enables holes to pass through a larger number of
smaller injection
9
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 barriers rather than a single large injection barrier. Each additional
layer increases the cost of
2 the device and decreases the yield of the manufacturing process.
3 [0059] Other methods to incorporate a TOO electrode with an
insufficiently high work
4 function into a device involve coating the TOO with a high work function
polymer (e.g. PEDOT),
a self-assembled monolayer (SAM), or a metal oxide (e.g. W03). Such methods,
however, may
6 increase impedance, device complexity and fabrication cost, while
introducing additional
7 problems related to device stability.
8 [0060] The example embodiments described herein are, in one aspect,
directed to the
9 functionalization of TOO thin films with halogens to modify their work
function. In particular,
example embodiments are described with reference to halogens and/or
halocarbons released
11 from a halogen-containing precursor compound under ultraviolet
radiation. However, it can be
12 recognized that functionalization of other substrates using the methods
described herein falls
13 within the scope of the invention. In one embodiment, the substrate is
functionalized using
14 plasma dissociation of a precursor to release an electronegative
species, for example, a
halogen. The halogen is chemically reacted with a substrate to increase the
work function of
16 the substrate. For example, functionalizing a substrate with a halogen
using a halogen-
17 containing plasma, and in particular, a chlorine-containing plasma,
falls within the scope of the
18 invention, as is further described below.
19 [0061] In another embodiment, a method of functionalizing the
surface of a substrate with a
species is provided, wherein a precursor containing the species is dissociated
using
21 electromagnetic (EM) radiation. The species is then reacted with the
substrate to increase the
22 work function of the substrate. In particular, a TOO substrate may be
functionalized with a
23 halogen by dissociating the halogen atom from a precursor using EM
radiation. Any wavelength
24 of electromagnetic radiation that breaks the bond between the species
and the precursor may
be used, however, ultraviolet (UV) radiation, has been found to be
particularly effective. In
26 particular, UV radiation having a wavelength of between 100 nm and 400
nm was found to be
27 effective. A catalyst may assist in breaking the bond between the
halogen and the precursor.
28 The catalyst may comprise the chemical surface of the substrate.
29 [0062] The chemical bond between the species and the surface may
increase the stability of
the functionalized material in air, as will be further described herein.
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 [0063] In some embodiments, and in examples that will be described
herein, the halogen-
2 containing compound is a volatile halogen-containing organic precursor.
It will be appreciated
3 that inorganic precursors may also be used. Organic precursors that
release halogen atoms
4 include halocarbons. The precursor may comprise two different halogens,
for example, fluorine
and chlorine. For example, the halogen-containing precursor may comprise a
mixture of a
6 fluoride and a chloride.
7 [0064] Example precursors include, for example, haloalkanes,
haloakenes, and
8 haloaromatics. Common chlorinated precursors include chloromethane,
dichloromethane
9 tetrachloromethane, perchloroethylene, tetrachloroethylene, 1,1,2,2-
tetrachloroethane, 1,1,2-
trichloroethane, carbon tetrachloride, chloroform, methylene chloride,
trichloroethylene, methyl
11 chloroform, 1,1,1-trichloroethane, 1,2,3-trichloropropane, ethylene
dichloride, dichloropropane,
12 dichlorobenzene, trichlorobenzene, propylene dichloride, 1,2-
dichloroethylene, 1,1
13 dichloroethane, etc. The precursor may comprise a halogen-containing
polymer such as
14 polytetrafluoroethylene (PTFE). The precursor may comprise metal
halides, for example,
indium halides, zinc halides, and tin halides. In an example, the metal halide
species is
16 preferably a constituent metallic element of the substrate. A
constituent metallic element will
17 typically not substantially alter the surface chemistry of the substrate
if the metal component of
18 the precursor remains on the surface of the substrate. For example, an
indium halide may be
19 used as a halogen-containing species for ITO whereas a tin halide may be
used as a precursor
for tin oxide substrates.
21 [0065] Upon functionalizing the substrate, residual contamination
may be removed by
22 additional treatment with EM radiation of an appropriate wavelength.
Contaminants may be
23 removed using a UV ozone treatment and/or using an appropriate plasma
cleaning treatment,
24 such as 02 plasma. The cleaning process is performed at a low energy to
reduce the likelihood
of the surface of the substrate being etched. When using an organic precursor,
oxygen reacts
26 with the remnants of organic precursor molecules to form volatile
molecules (e.g. CO2 and H20)
27 which may be advantageously flushed from the surface of the substrate.
Volatile molecules
28 may also evaporate from the surface of the substrate. Hence, in some
embodiments, organic
29 precursors may leave less contamination in comparison with inorganic
precursors after a
cleaning step.
11
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 [0066] However, inorganic precursors may be used in the methods
described herein.
2 Examples of these precursors include pure halogen gases, hydrogen
halides, boron halides,
3 sulphur halides, and phosphorus halides.
4 [0067] In some embodiments, the substrate may be functionalized
with other elements, for
example, sulphur, boron, or phosphorus using appropriate volatile precursors.
For example,
6 ammonium sulphide can be used to functionalize a substrate with sulphur.
Other species that
7 may be functionalized to the surface of a substrate to alter the work
function may be used.
8 [0068] The process of treating the substrate involves obtaining a
transparent conducting
9 (TC) substrate, for example, an ITO film deposited on glass. Other
example TOO substrates
include TCOs deposited on glass, such as tin oxide, indium oxide, cadmium
oxide, FTO, ZnO,
11 NiO, Mo03, W03, AuOx (oxidized gold), cadmium tin oxide (CTO), zinc tin
oxide (ZTO),
12 antimony tin oxide (ATO), aluminum zinc oxide (AZO), titanium zinc oxide
(TZO), gallium zinc
13 oxide (GZO), aluminum gallium zinc oxide (AGZO), indium gallium zinc
oxide (IGZO), gallium
14 indium oxide (G10), zinc indium oxide (Z10), gallium indium tin oxide
(GITO), zinc indium tin
oxide (ZITO), titanium indium oxide (T10), tin cadmium oxide (TOO), indium
cadmium oxide
16 (100), zinc cadmium oxide (ZOO), aluminum cadmium oxide (AC0). It will
be appreciated that
17 other substrates including transparent conducting (TO) substrates may be
used.
18 [0069] The TO substrate may be deposited on a transparent
mechanical supporting layer,
19 for example, glass. The mechanical supporting layer may be rigid,
flexible, planar, curved, or
any other geometry that may be functionalized using the method described
herein.
21 [0070] The substrate may be comprised of a plurality of different
layers. For example, the
22 substrate may comprise multiple layers of different TC0s, a metal film
on top of a TOO, a metal
23 film sandwiched between two TOO layers, or a thin layer of a high work
function material such
24 as a transition metal oxide on top of a metal or TOO layer. Various
layers in the substrate may
be conducting, semiconducting, or insulating.
26 [0071] The substrate may comprise a plurality of layers of
different metals, metal oxides,
27 TCO's, polymers and carbon based materials. The electrode may be a metal
coated with a layer
28 of metal oxide, including its native metal oxide. The electrode may be
solid or porous. One or
29 more layers of the substrate may comprise nano-material building blocks,
for example nano-
particles, nano-rods, nano-tubes or other nano-scale materials. One or more
layers of the
12
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 substrate may comprise a composite of different materials, for example
nano-particles in a
2 polymer matrix. One or more layers of the substrate may comprise micron-
scale particles.
3 [0072] The substrate may be patterned with nano-scale or micron-
scale features, for
4 example, features to enhance the out-coupling of light from an
optoelectronic device. One or
more layers of the substrate may be patterned. The substrate me be comprised
of a plurality of
6 layers with different refractive index, for example to form a Bragg
mirror or photonic crystal.
7 [0073] The substrate may be transparent, semi-transparent, opaque
or reflective. The
8 substrate may include a mechanical support layer, such as a piece of
glass, flexible plastic, or
9 semiconductor wafer. The substrate and mechanical support layer may be
the same material.
The substrate may be mechanically self-supporting, for example a metal foil or
silicon wafer.
11 [0074] Although reference is made to functionalizing a substrate
with a halogen, it will be
12 appreciated that the substrate may be functionalized with other species.
For example, the
13 substrate may be functionalized with a halocarbon to affect the surface
energy of the
14 functionalized surface. Typically, halocarbon treatments erode the
surface of TOO substrates
less than halogenations treatments, however, the equipment required to perform
the halocarbon
16 treatments is specialized. Additionally, the conductivity of certain
halocarbons is strongly
17 dependent on processing conditions, and therefore, difficult to control.
Even with precise control
18 over processing conditions, the most conductive halocarbons, for
example, conductive
19 fluorocarbons, are much less conductive than many TCO's including ITO.
However, EM
dissociation of a halocarbon precursor to deposit a halocarbon film on a
substrate may be
21 achieved, as is described below with reference to FIG 1.
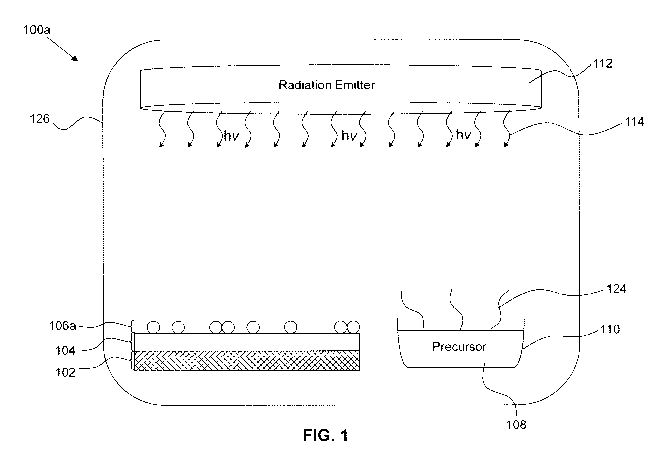
22 [0075] Turning to FIG. 1, a system for functionalizing a substrate
is provided. The system
23 may comprise a reaction chamber 126, in which a substrate 104 can be
placed. A species may
24 be deposited on a substrate 104 in the reaction chamber 126. The
precursor compound 108
can be placed, or fed into, the reaction chamber 126. The precursor compound
108 may be a
26 volatile liquid or solid. The dissociation of the precursor 108 may take
place in the vapour
27 phase, liquid phase, or solid phase. A functionalization reaction with
the surface of the
28 substrate 104 may take place on the surface of the substrate 104 in
contact with the vapour
29 phase. The precursor compound 108 may also be a gas, in which case no
evaporation of a
volatile precursor compound 108 is required to render the precursor compound
108 into the
31 vapour phase. A gas comprising the precursor compound 108 may be
provided into the
32 reaction chamber 126 through a tube (not shown).
13
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 [0076] A radiation emitter 112 emits EM radiation into the chamber
126. The radiation
2 emitter 112 may emit UV radiation of, for example, between 100 nm and 400
nm. The radiation
3 emitter 112 may be located within the reaction chamber 126. The radiation
emitter 126 may
4 alternately be external to the reaction chamber 126 if the walls of the
reaction chamber are at
least partially transparent to the radiation.
6 [0077] In an example embodiment, the precursor 108 is applied
directly to the surface of the
7 substrate 104, for example, in the form of a liquid or fine particulate
(e.g. powder or
8 nanoparticulate). The dissociation reaction of the precursor compound 108
and the subsequent
9 functionalization of the substrate 104 reaction may proceed directly on
the surface of the
substrate 104. The reaction may be catalyzed. For example, the reaction may be
catalyzed by
11 the surface of the substrate 104. In an example embodiment, a catalyst
is disposed in the
12 system to facilitate or enable the functionalization reaction.
13 [0078] Specifically, in the embodiment shown in FIG. 1, the
precursor compound 108 is a
14 volatile liquid contained in an open reservoir 110. The precursor
compound 108 evaporates into
its vapour phase.
16 [0079] The substrate 104 may itself be deposited on a mechanical
supporting layer 102.
17 For example, the substrate may comprise a TCO thin film (e.g. ITO)
deposited on a glass
18 substrate. The reaction chamber 126 isolates the substrate 104 from
external contaminants
19 and retains the precursor vapour and the reactive species in the
vicinity of the substrate 104.
[0080] The radiation emitter 112 is operable to emit EM radiation 114 into
the reaction
21 chamber 126 to disassociate halogen species from the halogen-containing
precursor 108. The
22 disassociation may be achieved in the vapour phase, in the liquid phase
(i.e. in the reservoir
23 110 or on the surface of the walls of the reaction chamber 126), in the
solid phase, or on the
24 surface of a substrate. An example halogen containing volatile precursor
compound is
dihalobenzene.
26 [0081] As the halogen species chemically bonds with the substrate,
a monolayer 106a
27 begins to form. As can be seen from FIG. 1, a partial monolayer 106a
corresponding to
28 approximately half of the surface of the substrate 104 has been formed.
As will be explained in
29 further detail below, the surface properties of the substrate 104 may be
tuned based on the
surface coverage of the substrate 104 by the functionalizing species 106a.
14
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 [0082] As used herein, the term "monolayer" refers to a coating
having approximately one
2 layer of atoms. It is understood that a layer having slightly more or
less than a monolayer would
3 be considered a monolayer. It is also understood that a monolayer
containing impurities, for
4 example residual carbon, would be considered a monolayer.
[0083] Although the system of FIG. 1 is described in terms of
functionalizing a substrate
6 with a halogen, in some embodiments, the species being deposited is a
halocarbon. The
7 halocarbon molecule may form a polymeric structure when functionalized to
the surface of the
8 substrate. For example, a fluorocarbon film may be deposited on the
surface of the substrate.
9 Fluorocarbon films comprising a C:F ratio controllably set between 1:3
and 3:1 have been
achieved and confirmed via X-ray photoelectron spectroscopy (XPS). Higher or
lower ratios of
11 carbon to halogen are possible. XPS results have indicated the presence
of CF3, CF2, OF, C-
12 OF, and C-H bonds. Some species, for example, halocarbons may be able to
react to form
13 multiple layers of a halocarbon film that may be several nanometres
thick. The halocarbon film
14 may be conductive or may be insulating. The work function of the surface
depends on the
amount and type of halocarbon.
16 [0084] Other properties may be changed, including surface energy,
to increase or decrease
17 the hydrophobicity of the surface. A surface may be functionalized using
a template to adjust
18 the surface energy at particular areas on the surface. A surface with a
modified surface energy
19 may interact more favourably with certain species and resist interaction
with other species. For
example, a hydrophobic surface would bead water while a hydrophilic surface
could be wetted
21 with water. Functionalizing particular areas of a surface may enable the
functionalized regions
22 to react with a species and the unfunctionalized regions to be resistant
to reaction and vice-
23 versa. Although reference is made to about half a monolayer being formed
on the substrate
24 104, less than a monolayer may be formed. For example, at least about 20
percent of a
monolayer may be formed on the substrate.
26 [0085] Turning now to FIG. 2, the system of FIG. 1 is shown,
however, the partial monolayer
27 106a in FIG. 1 has become more populated with chemically bonded species,
as is shown by
28 106b. The functionalization reaction may be controlled by varying the
wavelength of the
29 electromagnetic radiation used to dissociate the halogen from the
precursors, the intensity of
the EM radiation, the temperature at which the reaction takes place, the
precursor being used,
31 the presence of any catalysts, the substrate, and the halogen being
functionalized to the
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 substrate. The monolayer 106b continues to form on the substrate 104 as
long as halogens
2 continue to react with the substrate 104.
3 [0086] Referring now to FIG. 3, the substrate 104 is shown with a
monolayer 106c formed
4 on its surface. As described above, the monolayer 106c may have
imperfections (not shown).
It may be desirable to cease the functionalization of the substrate 104 during
the
6 functionalization process prior to forming a monolayer. The
disassociation of precursors 116
7 may be ceased by removing, blocking, or otherwise interrupting radiation
from the radiation
8 emitter 112. Once the release of halogen atoms from the precursors has
ceased, the surface
9 coverage remains substantially constant. The ability to stop the
functionalization reaction
almost instantaneously enables control over the degree to which the substrate
104 is
11 functionalized.
12 [0087] As is known, a functionalized substrate may include
contaminants. Removing
13 organic contaminants from the surface may increase the work function of
the substrate 104.
14 After functionalizing the desired portion of the substrate 104, the
substrate 104 may be cleaned.
Specifically, the substrate 104 may be cleaned to remove contaminants
deposited during the
16 functionalization reaction. For example, the contaminants may comprise
organic compounds
17 originating from the precursor 108. In the case of organic precursors,
the contaminants may be
18 reacted with UV generated ozone to produce volatile compounds which may
be flushed from the
19 reaction chamber 126.
[0088] The functionalization process may not significantly increase the
surface roughness of
21 the substrate 104. In an example embodiment, an ITO substrate was
functionalized with
22 chlorine. An atomic force microscope (AFM) was used to characterize the
surface of a bare UV
23 ozone treated ITO substrate and a chlorine-functionalized ITO substrate.
The surface
24 roughness, expressed in terms of the arithmetic mean value, Ra, was
found to be 2.2 nm for the
bare surface and 1.9 nm after being functionalized with a monolayer of
chlorine atoms. It can
26 be appreciated that the monolayer was not a perfect monolayer and there
may be some
27 variability in coverage and contamination.
28 [0089] Referring now to FIG. 4, an XPS chart showing the 2p core-
level energy spectrum of
29 chlorine-functionalized ITO overlaid on the 2p core-level spectrum of an
InCI3 reference is
provided. The similarities between the InCI3 curve and the chlorine-
functionalized ITO curve
31 suggest that the indium-chlorine bonds on the surface of the
functionalized ITO substrate are in
32 the same chemical state as the indium-chlorine bonds in InC13.
16
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 [0090] Turning now to FIG. 5, a chart of the approximate surface
functionalization (as
2 estimated by the 2p peak intensity of chlorine) with respect to reaction
time is provided. Several
3 ITO substrates were functionalized with chlorine using the EM radiation
dissociation method.
4 The duration of the functionalization reaction of each substrate was
selected from between 0
and 10 minutes. XPS was used to measure the approximate surface coverage of
chlorine on
6 the functionalized substrates. As the reaction time of the
functionalization process increases
7 from 0 to 10 minutes, there is a proportional increase in the intensity
of the 2p peak,
8 demonstrating that the functionalization of substrate can be increased by
increasing the reaction
9 time. Conversely, with a shorter reaction time, the substrate is less
functionalized, i.e., less than
a monolayer is formed on the surface of the substrate. By selecting an
appropriate duration of
11 the functionalization reaction, the surface coverage may be tuned, for
example, the surface
12 coverage may be tuned to a predetermined fraction of a monolayer.
13 [0091] FIG. 6 shows a band diagram of the work function of a
standard ITO substrate with a
14 bare surface. The work function of bare ITO is approximately 4.7 eV (5
eV after cleaning),
which is significantly lower than the approximately 6 eV that is desired to
efficiently inject holes
16 from the anode into the light emitting layer of typical organic
electronic devices.
17 [0092] Turning now to FIG. 7, an energy level diagram is shown for
an ITO substrate that
18 has been functionalized with a monolayer of chlorine is provided. Each
chlorine atom in the
19 monolayer is chemically bonded to an indium atom in the ITO substrate,
as was evidenced by
the XPS chart of FIG. 4, above. The work function at the surface of the
functionalized ITO
21 electrode is significantly higher than the work function of bare UV
ozone treated ITO. For
22 example, the work function of ITO functionalized with a monolayer of
chlorine may be
23 approximately 6.1 eV in comparison with approximately -5 eV for bare, UV
ozone treated ITO.
24 [0093] The increase in work function of the chlorinated substrate
with respect to the bare
substrate may be attributable to the surface dipole induced by the chlorine
atoms on the surface
26 of the ITO. Therefore, functionalizing species increase the work
function of the ITO in
27 proportion to their dipole moment with the surface of the substrate. A
desired increase in the
28 work function of an electrode can be obtained by selecting an
appropriate functionalization
29 species. Surprisingly, as was described above, chlorine achieves the
highest dipole despite
being less electronegative than fluorine. Density functional theory
calculations indicate that the
31 In-CI bond length is greater than the In-F bond length, resulting in a
larger net dipole moment for
32 chlorine in comparison with fluorine.
17
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 [0094] The work function of the TOO substrate may be tuned within a
range by controlling
2 the reaction time, for example, by interrupting or blocking radiation 114
from the radiation
3 emitter 112. The concentration of the precursor compound may also be
selected to tune the
4 range. For example, by depositing less than a monolayer on the surface of
a substrate, the
work function may be set to be lower than the work function of a substrate
that has been
6 functionalized with a full monolayer of species but higher that of a bare
substrate surface.
7 [0095] Referring to FIG. 8, a chart illustrating the relationship
between work function and
8 surface coverage of chlorine on an ITO substrate as approximated from the
chlorine 2p core-
9 level XPS results is provided. The work function is approximately
linearly related to the surface
functionalization of the ITO substrate. It will be appreciated that the chart
of FIG. 8 is a rough
11 approximation and is a representation of the relationship only.
12 [0096] By way of example, a functionalization of about 15% of a
monolayer corresponds to
13 a work function of approximately 5.65 eV. A functionalization of
approximately 95% of a
14 monolayer corresponds to a work function of approximately 6.15 eV.
Hence, the work function
may be tuned depending on the application by functionalizing the surface with
up to a
16 monolayer. When a higher work function is desired, for example, above
6.1 eV, the surface
17 may be functionalized with about a monolayer of chlorine. As stated
above, it will be
18 appreciated that the monolayer, or portions of the monolayer, may be
imperfect.
19 [0097] In the context of OLEDs, ITO is commonly used as an anode.
The work function of a
functionalized ITO anode may be tuned to match the HOMO level of the organic
hole
21 transporting material, as is further described below.
22 [0098] Referring to FIG. 9, XPS core-level spectra of ITO
functionalized with iodine,
23 bromine, and fluorine are provided. As was described above, chlorine
induces the largest
24 dipoles in the halogen-indium bond on a functionalized ITO surface,
thereby providing the
maximum increase in work function relative to ITO functionalized with other
halogens.
26 [0099] As can be seen in Table 1 below, the functionalization of
various TOO surfaces is
27 possible. UPS refers to ultraviolet photoelectron spectroscopy.
28
18
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 Table 2: Experimental work function from XPS/UPS of Various
Functionalized Substrates
Substrate Functionalized Work Function of Work Function of
Species Clean Substrate Functionalized
[eV] Substrate [eV]
ITO chlorine 4.7 6.1
FTO fluorine 4.9 5.6
ZnO chlorine 4.7 5.3
Au chlorine 5.2 6.2
2
3 [00100] In addition to increasing the work function of the
electrode, halogen functionalization
4 increases the stability of the work function of the electrode relative to
that of a bare UV ozone or
02 plasma treated TOO electrode. Turning to FIG. 10, a chart showing the
stability of the work
6 function of an ITO substrate functionalized with a monolayer of chlorine
is compared with a bare
7 ITO substrate in the presence of air. The surface of the bare substrate
was treated with UV
8 ozone for 15 minutes. As can be seen from Fig. 10, the work function of
the bare electrode
9 drops by approximately 0.1 eV over about three hours in the presence of
air. In contrast, there
is no substantial change in the work function of the functionalized substrate.
This demonstrates
11 the increased stability provided to functionalized substrates. This may
be advantageous in a
12 production environment, as a functionalized substrate may be left in
atmospheric conditions for
13 a period of time without impacting the work function of the substrate.
Higher stability may
14 enable substrates to be stored in air, rather than in a vacuum or under
an inert gas. The
stability of the functionalized substrate depends on the ambient environment
including the
16 ambient temperature and humidity.
17 [00101] Turning to FIG. 11, a table is provided showing the work
function of various
18 substrates after being exposed to air for a period. It can be
appreciated that under the same
19 conditions and after exposure to air, the work function of the
functionalized substrate is
substantially higher than the work function of the bare substrate under the
same conditions.
21 [00102] Referring now to FIG. 12, a chart is provided to illustrate
that the transmittance
22 characteristics of a chlorine-functionalized ITO substrate are not
substantially inferior to the
23 transmittance characteristics of a bare ITO substrate. The ITO layers
were deposited on
24 transparent substrates. As can be seen from the chart, the transmittance
curves are very
similar over a wide range of wavelengths. Importantly, the curves are almost
indistinguishable
26 over the visible spectrum, illustrating that a chlorine-functionalized
ITO anode may be used in
19
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 an organic optoelectronic device with no increase in the attenuation of
light transmitted through
2 the anode relative to that of a bare ITO anode.
3 [00103] Turning to FIG. 13, a spectrum of the ultraviolet lamp used
to functionalize the ITO
4 substrates in the examples above is provided. Specifically, the spectrum
corresponds to that of
a PL16-110 Photo Surface Processing Chamber (Sen LightsTm). The wavelength
6 corresponding to the cut-off wavelength of PyrexTm glass, which may be
used as a reaction
7 chamber, is also provided.
8 [00104] The conductivity of a chlorine-functionalized ITO substrate
is also not substantially
9 inferior to the conductivity of a bare ITO substrate. As measured with a
4-point probe the sheet
resistance of an example chlorine-functionalized ITO substrate is 18.2 Ohms
per square,
11 compared to 18.1 Ohms per square for a bare ITO substrate.
12 [00105] One application of a transparent conducting substrate, for
example, an ITO substrate
13 that has been functionalized to have a high work function, is the use of
the substrate in an
14 organic electronic device. Functionalizing the surface of an ITO
substrate with a halogen
species to increase the work function of the ITO substrate can reduce the hole
injection barrier.
16 Reducing the hole injection barrier improves the efficiency of hole
injection in an OLED, thereby
17 decreasing the amount of voltage required to induce a current in the
device.
18 [00106] It will be appreciated that although a functionalized TCO
substrate is shown in an
19 example OLED construction, other OLED constructions may use
functionalized TCO substrates.
Furthermore, other types of electronic devices may comprise functionalized TCO
substrates.
21 [00107] FIG. 14 shows an example energy diagram of an embodiment of an
OLED using a
22 transparent conducting substrate from the prior art. An ITO layer 1280
is typically formed on a
23 transparent substrate used as the anode. Holes are injected from the
anode 1280 into a hole
24 injection layer (HIL) 1282, then to a hole transport layer (HTL) 1284,
through an electron
blocking layer (EBL) 1286 and into to the light emitting thin film layer 1292.
Concurrently,
26 electrons are injected via the cathode 1298 and are transported through
the electron transport
27 layer (ETL) 1296, through the hole blocking layer (HBL) 1294, and
recombine with holes in the
28 light emitting thin film layer to release photons. The photons emitted
in the thin film layer may
29 then escape through ITO layer 1280 and any transparent substrate
supporting the ITO layer
1280. A ghost line 1290 is provided to show the relative work function of a
chlorine-
31 functionalized ITO layer, which is significantly better aligned with the
emitting layer.
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 [00108] FIG. 15 is an energy level diagram for a simplified
phosphorescent OLED comprising
2 a chlorine functionalized ITO electrode 1380. At lower barrier heights,
holes can be injected
3 more efficiently from the anode. As can be seen from the diagram, the
height of the hole
4 injection barrier, which is dependent on the difference between the HOMO
of the emitting layer
and the work function of the ITO electrode 1380, is relatively low for the
chlorine functionalized
6 electrode. This lower hole injection barrier enables the electrode to
inject directly into the host
7 1284, thereby enabling the host and the HTL 1283 to be the same material.
Since the chlorine-
8 functionalized anode is closely aligned with the HOMO level of the HTL
1283, there is no need
9 for the HIL layer 1282. In contrast, a bare ITO electrode 1280 has a high
injection barrier,
making it inefficient to inject holes without the intermediate HIL, as was
shown in FIG. 14. If the
11 HTL and ETL are selected to have appropriate energy levels, as
understood by one skilled in
12 the art, the EBL and HBL may also be eliminated.
13 [00109] Referring now to FIG. 16, a UPS chart showing the
relationship between the work
14 function of the anode and the barrier height for holes in an OLED device
is provided. It can be
seen that increasing the work function of the electrode using halogen
functionalization reduces
16 the hole injection barrier height.
17 [00110] In an example embodiment, a chlorine-functionalized ITO
anode was prepared for
18 use in a phosphorescent green bottom emitting OLED. An OLED comprising a
chlorine-
19 functionalized ITO anode and another OLED comprising a bare UV ozone
treated ITO anode
were fabricated in a Kurt J. Lesker LUMINOSTm cluster tool with a base
pressure of 10-8 Torr on
21 commercially patterned ITO coated glass (25 mm 25 mm). ITO substrates
were ultrasonically
22 cleaned with a standard regiment of AICOnOXTM dissolved in deionized
(DI) water, acetone, and
23 methanol. The ITO substrates were then treated using UV ozone treatment
for 3 minutes in a
24 PL16-110 Photo Surface Processing Chamber (Sen Lights).
[00111] Chlorine-functionalized ITO was prepared by functionalizing the
surface of the ITO
26 substrate for 10 minutes according to the method described in FIG. 1 and
in a Pyrexm" Petri dish
27 with 0.2 ml 1,2-dichlorobenzene as the precursor compound. A PyrexTM
reservoir was used as
28 the chamber and the UV source was located outside of the chamber. A
transmission spectrum
29 of Pyrex is provided in FIG. 24a and the spectrum of the UV lamp is
provided in FIG. 24b.
Once the functionalization reaction was complete, the ITO substrate was
treated in UV ozone
31 for 3 minutes.
21
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 [00112] The organic layers and the LiF cathode were thermally deposited
from alumina
2 crucibles in dedicated organic chamber. The Al layer was deposited in a
separate dedicated
3 metal deposition chamber from a boron nitride crucible without breaking
vacuum. All layers were
4 patterned using stainless steel shadow masks to define the device
structure. The active area for
all devices was 2 mm2.
6 [00113] The standard device structure is as follows: anode/CBP (35
nm)/CBP:Ir(ppy)2(acac)
7 (15 nm, 8c)/0)/TPBi (65 nm)/LiF (1 nm)/AI (100 nm), where Ir(ppy)2(acac)
is bis(2-phenylpyridine)
8 (acetylacetonate)iridium(III), and TPBi is 2,2,2" -(1,3,5-benzinetriyI)-
tris(1-phenyl-1-H-
9 benzimidazole).
[00114] An energy level diagram 1200 of the example phosphorescent OLED
structure is
11 provided in FIG. 17. The chlorine-functionalized ITO anode 1202 has a
significantly higher work
12 function than a bare UV ozone treated ITO anode 1206. Hence, the
chlorine-functionalized
13 anode is better able to inject holes into the CBP layer 1204, as the
HOMO level of the CBP
14 layer 1204 is well aligned with the work function of the chlorine-
functionalized ITO anode. The
Ir(ppy)2(acac) layer 1208 may be doped into the CBP layer 1204. The TPBi layer
1210 is in
16 electrical communication with the LiF/AI cathode layer 1212 and the
Ir(ppy)2(acac) layer 1208.
17 [00115] FIG. 18 is a diagram showing the current-voltage
characteristics of the example
18 device of FIG. 17. As can be seen, as the treatment time increases to a
point where a
19 monolayer is formed, the voltage required to drive current decreases.
Hence, if a monolayer of
chlorine is functionalized to the surface of the ITO anode used in the example
OLED device, the
21 voltage required to operate the OLED may be significantly reduced. As
can be seen from FIG.
22 18, the voltage may be reduced by approximately 4 V at an equivalent
current density.
23 [00116] FIG. 19 is a chart of the current efficiency of the example
OLED device of FIG. 17
24 comprising a chlorine-functionalized anode with respect to the luminance
being output from an
OLED reference device from the prior art. Specifically, the OLED device
comprises a UV ozone
26 treated anode with the structure: anode/PEDOT:PSS (5 nm)/a-NPD (35
nm)/CBP:Ir(ppy)2(acac)
27 (15 nm, 8`)/0)/TPBi (65 nm)/LiF (1 nm)/AI (100 nm), where a-NPD is N, N'-
bis(naphthalen-l-yI)-
28 N,N'-bis(phenyI)-benzidine. It will be appreciated that the PEDOT:PSS (5
nm)/a-NPD (35 nm)
29 layers in the reference device are required to inject holes into the
CBP:Ir(ppy)2(acac) emission
layer from the bar UV ozone treated ITO anode. It can be seen from FIG. 19
that the chlorine-
31 functionalized anode increases the current efficiency with respect to
the reference OLED
22
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 comprising a bare UV ozone treated electrode. In particular, at high
luminance, the OLED
2 comprising the functionalized anode is significantly more efficient.
3 [00117] Turning to FIG. 20, the current efficiency and external
quantum efficiency (EQE) of
4 the phosphorescent OLEDs comprising a chlorine functionalized anode is
provided. The
phosphorescent OLED has a high maximum current efficiency of 93.5 cd/A at 400
cd/m2, which
6 corresponds to a maximum EQE of 24.7%. At 10,000 cd/m2 the current
efficiency and EQE are
7 still relatively high at 79.6 cd/A and 21% respectively. Turning to FIG.
21, the example OLED of
8 FIG. 17 comprising a chlorinated ITO anode is compared with devices
constructed using
9 methods found in the prior art. As can be seen, the OLED comprising the
chlorine-
functionalized anode may be constructed to be significantly more simple in
terms of device
11 layers and materials and may further exhibit a significantly higher
external quantum efficiency.
12 [00118] Referring now to FIG. 22, a chart is provided showing the
change in luminance
13 measured in vacuum for the example OLED with the structure:
electrode/CuPc (25nm)/a-NPD
14 (45 nm)/CBP:Ir(ppy)2(acac) (15 nm, 8 /0)/TPB1 (10 nm)/A1q3 (45 nm)/LiF
(1 nm)/AI (100 nm),
where CuPc is copper phthalocyanine. As can be seen, the luminance of an OLED
comprising
16 an ITO anode that has been functionalized with chlorine is higher than
an OLED comprising a
17 bare UV ozone treated ITO anode after being in operation for several
hours. This demonstrates
18 that the OLED comprising an ITO anode maintains a relatively higher
luminance over time.
19 [00119] In another example embodiment, a fluorescent green OLED was
fabricated following
the same procedure as for the phosphorescent OLED outlined above. The standard
device
21 structure of the OLED is as follows: anode/CBP (50 nm)/A1q3:C545T (30
nm, 1%)/A1q3 (15
22 nm)/LiF (1 nm)/A1 (100 nm), where CBP is 4,4'-bis(carbazol-9-
yl)biphenyl, A1q3 is tris(8-hydroxy-
23 quinolinato)aluminium, and C545T is 2,3,6,7-tetrahydro-1,1,7,7,-
tetramethy1-1H, 5H,11H-10-(2-
24 benzothiazolyl)quinolizino[9,9a,1gh]coumarin.
[00120] An energy level diagram 900 of the fluorescent OLED structure is
provided in FIG.
26 23. Numeral 902 refers to the chlorine-functionalized ITO anode, which
has a significantly
27 higher work function than the bare ITO anode 906. Hence, the chlorine-
functionalized anode
28 902 is better able to inject holes into the CBP 904, as the HOMO level
of the CBP 904 is well
29 aligned with the work function of the chlorine functionalized ITO anode
902. The OLED further
comprises an Alq3:C545T layer 908 and an A1q3 layer 910, which is in
communication with the
31 LiF/AI cathode 912.
23
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 [00121] The HOMO level of the CBP layer in contact with the anode is
approximately 6.1 eV.
2 The work function of the functionalized anode is approximately 6.1 eV and
the work function of
3 the bare anode is approximately 5.0 eV, after being treated with ozone,
as measured by
4 ultraviolet photoelectron spectroscopy (UPS). The work function of the
bare anode is too low to
efficiently inject holes into the OLED whereas the work function of the
functionalized anode is
6 more aligned with the HOMO level of the CBP layer.
7 [00122] FIG. 24 is a chart showing the current-voltage
characteristics of a fluorescent green
8 OLED comprising a chlorine-functionalized ITO anode with respect to a
fluorescent green OLED
9 of identical construction comprising a bare UV ozone treated ITO anode.
As can be seen from
FIG. 24, the voltage required to achieve a particular current density is
significantly lower for the
11 OLED comprising the chlorine-functionalized anode.
12 [00123] Specifically, the current density of the OLED device
comprising the functionalized
13 electrode dramatically increases with a driving voltage of more than 6
volts. At 10 volts, the
14 current density of the OLED comprising the chlorine-functionalized ITO
anode is approximately
300 mA/cm2. In contrast, the current density of the OLED comprising the bare
ITO electrode is
16 insignificant. The higher current density of the OLED comprising the
chlorine-functionalized ITO
17 anode demonstrates that the higher work function enables more efficient
injection of holes into
18 organic hole transporting materials with deep HOMO levels.
19 [00124] A major advantage of aligning the work function of the ITO anode
with the HOMO of
the CBP layer is that the power efficiency of the OLED is increased; that is
to say, the light
21 output per unit of electrical input in increased. Referring to FIG. 25,
a chart showing the current
22 and power efficiencies of the OLED devices discussed above is provided.
The device with bare
23 ITO anode has a lower power efficiency and a lower current efficiency
due to the poor injection
24 of holes from bare ITO anode into the deep 6.1 eV HOMO of CBP. The
device with chlorine-
functionalized ITO anode has a much higher efficiency, with a maximum current
efficiency at a
26 luminance of approximately 1000 cd/m2 of 23 cd/A versus the
approximately 18cd/A for the bare
27 ITO anode.
28 [00125] Similarly, the power efficiency of the OLED comprising a
chlorine-functionalized ITO
29 anode is approximately 12 Im/VV at a luminance of 1000 cd/m2, whereas
the power efficiency of
the OLED comprising the bare ITO anode is approximately 5 Im/W at a luminance
of 1000
31 cd/m2. The increased power efficiency suggests that the chlorine
functionalization of the ITO
32 anode has a significant effect on power efficiency.
24
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 [00126] Given the improved alignment of the work function of the
chlorinated ITO anode and
2 the HOMO of the CBP layer, it may be possible to forego the several HILs
and HTLs that are
3 typically required in such a device construction without an unacceptable
loss of efficiency.
4 Foregoing the requirement for HTLs is advantageous, as the number of
processing steps
required to construct the OLED device may be reduced, thereby increasing the
manufacturing
6 yield of OLED devices and reducing costs associated with their
production.
7 [00127] The halogen functionalized electrode in the examples above
contributes little series
8 impedance to the device. For example, a chlorine-functionalized ITO anode
was prepared for
9 use in a single-carrier hole-only organic device. The structure of the
device is as follows:
anode/a-NPD (536 nm)/Ag (50 nm). A first device comprising a bare UV ozone
treated anode
11 was compared to a second device having a UV ozone treated ITO anode
coated with 1 nm of
12 vacuum deposited Mo03, and a third device comprising a chlorine-
functionalized ITO anode.
13 The hole injection barrier height between the anode and the a-NPD
organic layer was measured
14 for each device using UPS. The hole injection barrier height was 0.6 eV
for bare UV ozone
treated ITO, 0.45 eV for UV ozone treated ITO coated with 1 nm of vacuum
deposited Mo03
16 and 0.45 eV for chlorine-functionalized ITO. The performance of the
device with the UV ozone
17 treated ITO coated with 1 nm Mo03 may initially be expected to be the
same as the device with
18 the chlorine-functionalized ITO since the barrier height for holes is
the same for both devices.
19 [00128] FIG. 26 is a diagram showing the current-voltage
characteristics of the example
single-carrier hole-only organic devices described above. The current density
at a given voltage
21 is highest for the device with the chlorine-functionalized ITO anode.
The device with the UV
22 ozone treated ITO anode coated with 1 nm Mo03 is nevertheless exhibiting
a higher current
23 density at any given voltage than the device with the bare UV ozone
treated ITO anode due its
24 lower hole injection barrier height. Unexpectedly, the current density
for the device with the
chlorine-functionalized ITO anode is higher at a given voltage than for the
device with the UV
26 ozone treated ITO anode coated with 1 nm Mo03, despite the same barrier
height for holes. The
27 lower current density in the device with the UV ozone treated ITO anode
coated with 1 nm Mo03
28 shows that the Mo03 layer introduces a series impedance into the device.
29 [00129] As was described above, a substrate may also be functionalized
using a plasma.
FIG. 27 is a plasma system for functionalizing a substrate. The system
comprises a reaction
31 chamber 2608, which is grounded 2612. The system may comprise a
plurality of rods 2620
32 which support a substrate support 2626 upon which the substrate 2652 may
be placed. The
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 substrate 2652 is placed in electrical communication with the substrate
support 2626. The
2 substrate 2652 is deposited on a non-conductive mechanical support 2650,
for example, glass.
3 A high energy plasma shield 2624 is also provided. The plasma shield 2624
may also be
4 supported by the rods 2620. The plasma shield 2624 is also grounded.
[00130] A radio frequency (RF) power source 2610 provides power to the powered
electrode
6 2611. The reaction chamber 2608 comprises an inlet 2614 through which a
gas comprising a
7 precursor may be pumped and an outlet 2616 through which the vacuum
chamber can be
8 evacuated by a vacuum pump. The precursor may be a liquid or a gas. When
the powered
9 electrode 1611 is powered by an RF power source 2610, a plasma is
generated between the
powered electrode 1611 and the grounded portions of the system including the
reaction
11 chamber 2608. In particular, the highest energy plasma is generated in
the region of the
12 highest electric field, which may be between the powered electrode 2611
and the plasma shield
13 2624. However, plasma may also be generated elsewhere in the chamber
2608.
14 [00131] It will be appreciated that various plasma methods for
generating halogen-containing
plasmas may be used, including glow discharged based plasmas. It will also be
appreciated
16 that plasma may be used in combination with UV light treatment to
functionalize a substrate.
17 For example, dichlorobenzene diluted in argon gas may be used with ultra-
violet light or with
18 radio frequency electromagnetic radiation to functionalize a substrate
with a halogen species.
19 [00132] The plasma causes the dissociation of any precursors in the
reaction chamber. The
dissociated precursors may then react with the surface of the substrate 2652
to begin to form a
21 monolayer 2654. As is well known, particles in the plasma may have a
substantial kinetic
22 energy. The plasma shield 2624 prevents the plasma having the highest
kinetic energy from
23 directly impinging on the surface of the substrate 2652, thereby
reducing the etching effects on
24 the substrate 2652.
[00133] By way of example, the chamber may be pumped down to about 250 mTorr
and 1,2-
26 dichlorobenzene may be leaked in as a precursor for an ITO substrate.
The substrate may be
27 treated for approximately 5 minutes. Etching of the substrate was
minimized due to the
28 positioning of the substrate behind the plasma shield 2624. The
functionalized substrate may
29 be cleaned to remove residual contaminates.
[00134] Another example plasma system for functionalizing a substrate is
provided in FIG.
31 28. In the example of FIG. 28, the RF power source 2610 is connected to
the substrate support
26
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 2626, and hence, to the substrate 2652 itself. A grounded electrode 2711
is positioned in a
2 parallel arrangement with the substrate 2652. When the power source 2610
is activated, a
3 plasma is generated between the grounded electrode 2711 and the substrate
2652. As outlined
4 above, the plasma causes the dissociation of any precursors in the
reaction chamber. The
dissociated precursors may then react with the surface of the substrate 2652
to begin to form a
6 monolayer 2654. To mitigate any etching effects of the plasma, the gas
comprising the
7 precursor may be diluted with a carrier gas, as is outlined below.
8 [00135] It will be appreciated that various known precursor-
containing gases may be used.
9 For example, other halogen-containing precursors or fluorocarbon-
containing precursors may
be used. Example precursors include bromine, chlorine, tri-chloroethane,
dichlorobenzene,
11 haloalkanes, haloakenes, and haloaromatics. Common chlorinated
precursors include
12 chloromethane, dichloromethane, trichloromethane, tetrachloroethane,
perchloroethylene,
13 tetrachloroethylene, 1,1,2,2-tetrachloroethane, 1,1,2-trichloroethane,
carbon tetrachloride,
14 chloroform, methylene chloride, trichloroethylene, methyl chloroform,
1,1,1-trichloroethane,
1,2,3-trichloropropane, ethylene dichloride, dichloropropane, dichlorobenzene,
propylene
16 dichloride, 1,2-dichloroethylene, 1,1 dichloroethane, etc. The precursor
may also comprise a
17 halogen-containing polymer. Inorganic precursors may also be used.
Examples of inorganic
18 precursors include pure halogen gases, hydrogen halides, boron halides,
sulphur halides, and
19 phosphorus halides.
[00136] A difference in ionization energy between the precursor and carrier
gas can be
21 advantageously used to affect the amount of halogen radicals or ions
generated using
22 electromagnetic radiation. For example, in a plasma-based process using
RF electromagnetic
23 radiation chlorine gas may ionize more readily than argon gas.
Therefore, although the
24 concentration of chlorine in argon may be relatively low, for example,
1%, the concentration of
active ionized chlorine species in the plasma may be much higher.
26 [00137] The precursor may comprise a mixture of various halogen-
containing precursors. An
27 oxidizing agent, for example oxygen, may also be added to the diluted
precursor mixture to
28 increase the removal of carbon impurities from the surface of the
substrate.
29 [00138] Plasma functionalization of the substrate may be employed in
procedures whereby a
UV treatment may be damaging to the substrate, or otherwise adversely affect
the substrate.
31 For example, in some cases, thin film transistors (TFTs) on the
substrate may be damaged by
27
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 UV radiation. Specifically, UV radiation may degrade a semiconductor
substrate including
2 silicon substrates and indium gallium zinc oxide (IGZO) substrates.
3 [00139] Functionalization of a substrate using plasma may be
advantageous, as the plasma
4 treatment is typically conducted under vacuum, as are many other steps of
OLED fabrication.
As such, the functionalization process can be conducted in the same line of
operations as other
6 steps of OLED fabrication without requiring a substantial increase in
pressure, thereby reducing
7 the time required to perform the functionalization step. Furthermore,
existing plasma equipment
8 may be used to functionalize substrates. Existing plasma equipment may be
used even for
9 large substrates including so-called "Generation 8" substrates, which are
2.2 m by 2.5 m.
[00140] In another example, the plasma may make use of a carrier gas to reduce
the
11 concentration of the halogen-containing species. A gas having a lower
concentration of
12 halogen-containing species may be safer to handle and may further reduce
the etching effects
13 of the plasma. For example, pure chlorine gas is extremely toxic and
corrosive and is typically
14 stored in pressurized cylinders. Chlorine gas that has been diluted with
a carrier gas, for
example, 1% chlorine gas in an argon carrier, is less toxic and corrosive.
16 [00141] By way of example, the carrier gas may comprise a noble gas, for
example, argon.
17 Other example carrier gases include helium, neon, krypton, and xenon.
The carrier gas may
18 comprise a mixture of these gases in various proportions.
19 [00142] The carrier gas may comprise up to about 99.9% of the total gas
volume. For
example, the halogen-containing precursor may be introduced at concentrations
of about 0.1%,
21 1%, 5%, or 10% with one or more carrier gases comprising the balance.
The concentration of
22 the halogen-containing precursor may be selected depending on the
desired processing time,
23 fractional coverage of the substrate, reactivity of the halogen and
substrate, or various other
24 processing parameters. Lower concentrations of halogen-containing
precursor plasmas have
comparatively lower etching rates than higher concentrations of the same
species.
26 [00143] For example, the concentration of the gas containing the
halogen-containing
27 precursor may be 5% in a 95% carrier gas. In a specific example, a 5%
dichlorobenzene and
28 95% argon mixture may be used.
29 [00144] By way of example, an ITO substrate coated on a glass sheet was
functionalized
with chlorine gas diluted in an argon carrier gas at a concentration of about
1%, as out below.
28
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 [00145] ITO substrates were cleaned with detergent, acetone and
methanol and loaded into
2 a commercial Advanced Energy reactive ion etching system. The processing
chamber was
3 pumped down to a base pressure of 10-2 Torr using a scroll-pump. Chlorine
gas diluted in
4 argon at 1% concentration was leaked into the processing chamber using a
mass flow controller
to a pressure of between 1 and 10 mTorr. A forward radio frequency power of
about 50W at
6 13.56 MHz was applied to the processing chamber, resulting the formation
of a chlorine and
7 argon plasma. The substrate was treated for 10 seconds. It will be
appreciated that other
8 treatment times, operating pressures and plasma powers may be used. In
this example, a
9 relatively low power was chosen to minimize etching of the sample.
[00146] The work function of the treated sample was measured using x-ray
photoelectron
11 spectroscopy and was found to be > 6.0 eV. The CI 2p core-level of
chlorine on the surface of
12 the sample suggests the formation of In-CI bonds. The surface roughness
of the sample
13 measured using atomic force microscopy was found to be about 2 nm, which
is substantially the
14 same as the bare substrate prior to the plasma treatment.
[00147] Organic light emitting diodes with the structure of CBP (35
nm)/CBP:Ir(ppy)2(acac)
16 (8%, 15 nm)/TPBi (65 nm)/LiF (1 32 nm)/AI (100 nm) were fabricated on
the plasma
17 functionalized ITO substrates. These OLEDs exhibited a relatively high
external quantum
18 efficiency of 24%, demonstrating that such processes can be used to
prepare electrodes for
19 organic optoelectronic devices, such as OLEDs. It will be appreciated
that although ITO was
used, other substrates may be functionalized with halogens using a similar
process, for
21 example, other transparent conducting oxides (TC0s), metal oxides or
metals may be
22 functionalized.
23 [00148] A process similar to that described above may also be used for
functionalization
24 using other halogens or for functionalizing a surface with
chalcogenides, for example sulfur from
a sulfur containing precursor diluted in a carrier gas. Functionalizing a
substrate with chlorine
26 using RIE is also possible.
27 [00149] As described above, metal halides may be used as precursors.
Example processes
28 for functionalizing an ITO substrate using metal halide precursors are
set out below.
29 Specifically, an ITO substrate was functionalized with chlorine from a
metal chloride precursor in
the vapour phase in Example 1, in the liquid phase in Example 2, and using an
organic
31 precursor in Example 3.
29
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 EXAMPLE 1
2 [00150] An ITO coated glass substrate was ultrasonically cleaned with a
standard regiment
3 of AlconoxTM dissolved in deionized (DI) water, acetone, and methanol.
The ITO substrates
4 were then treated using UV ozone treatment for 3 minutes in a PL16-110
Photo Surface
Processing Chamber (Sen Lights).
6 [00151] The surface of the ITO substrate was exposed to InCI3 in the
vapour phase in a
7 vacuum chamber by subliming InCI3 powder from an alumina crucible. The
exposure was
8 equivalent to depositing about a 5 A thick layer of InCI3 on the surface
of the substrate as
9 determined by a quartz crystal microbalance.
[00152] The ITO substrate was treated using UV ozone treatment for 3 minutes
in a PL16-
11 110 Photo Surface Processing Chamber.
12 EXAMPLE 2
13 [00153] An ITO coated glass substrate was ultrasonically cleaned with a
standard regiment
14 of AlconoxTM dissolved in deionized (DI) water, acetone, and methanol.
The ITO substrates
were then treated using UV ozone treatment for 3 minutes in a PL16-110 Photo
Surface
16 Processing Chamber.
17 [00154] The surface of the ITO substrate was exposed to a dilute
solution of InCI3 dissolved
18 in ethanol by spin-coating the solution onto the surface of the
substrate at 2000 rpm.
19 [00155] The ITO substrate was treated using UV ozone treatment for 3
minutes in a PL16-
110 Photo Surface Processing Chamber.
21 EXAMPLE 3
22 [00156] An ITO coated glass substrate was ultrasonically cleaned with a
standard regiment
23 of AlconoxTM dissolved in deionized (DI) water, acetone, and methanol.
The ITO substrates
24 were then treated using UV ozone treatment for 3 minutes in a PL16-110
Photo Surface
Processing Chamber.
26 [00157] The surface of the ITO substrate was treated with 1,2-
dichlorobenzene (DCB) vapour
27 in a closed Pyrex Petri dish under UV irradiation in a PL16-110 Photo
Surface Processing
28 Chamber for 10 minutes.
29 [00158] The ITO substrate was treated using UV ozone treatment for 3
minutes in a PL16-
110 Photo Surface Processing Chamber.
CA 02870236 2014-10-09
WO 2013/152446 PCT/CA2013/050291
1 [00159] The chemical composition and work function of all of the
substrates were
2 characterized using x-ray photoelectron spectroscopy (XPS) in a PHI 5500
Multi-Technique
3 System using monochromatic Al Ka (hv = 1486.7 eV). An x-ray photoelectron
spectroscopy
4 (XPS) spectrum is provided in FIG. 28 to show the chemical state of the
chlorine bonded to the
surface using the three example procedures set out above. Specifically, the
XPS spectrum
6 shows the 2p core-level energy spectrum of the three different chlorine
functionalized ITO
7 substrates, functionalized using InCI3 vapour (Example 1), InCI3 in
solution (Example 2), and
8 DCB vapour using UV light. The similarities between the Cl 2p core-level
spectrum for the three
9 different chlorine functionalized substrates suggests that the surface
chemical state of the three
samples is similar.
11 [00160] As such, FIG. 28 indicates that the surface of ITO can be
reacted with InCI3 in the
12 vapour phase and InCI3 dissolved in solution to functionalize the
surface with chlorine. The
13 work function of each of the three chlorine functionalized ITO
substrates can be estimated to be
14 6.05 0.05 eV. Since the surface chemical state and work function of
the ITO substrates
functionalized with InCI3 vapour and InCI3 dissolved in solution are similar
to the ITO substrate
16 functionalized using dichlorobenzene and UV light.
17 [00161] It will be appreciated that the substrates functionalized
using a metal halide are
18 expected to function in the same way when used in a device, for example
an OLED, as the ITO
19 substrates described above, given their similar chemical structure.
[00162] It can be appreciated that potential applications of organic
optoelectronic devices
21 comprising substrates functionalized according to the method described
herein comprise
22 organic photovoltaics, OLEDs, organic thin film transistors, and
biomedical devices. It will be
23 appreciated that although reference is made to organic electronic
devices comprising TCO
24 functionalized electrodes, inorganic electronic devices may comprise
functionalized TOO
electrodes. For example, LCD electrodes may be functionalized using the
process as described
26 herein.
27 [00163] Other potential applications of substrates functionalized
according to the methods
28 described above comprise functionalizing a substrate to adjust surface
energy, and templating
29 growth of materials on a substrate that has been selectively
functionalized.
[00164] It will be appreciated that although examples were provided
disclosing the
31 functionalization of TOO substrates, other types of substrates may be
functionalized. For
31
CA 02870236 2014-10-09
WO 2013/152446
PCT/CA2013/050291
1 example, metals, polymers (including conductive polymers), and ceramics
may be
2 functionalized with a species using the process as described herein.
3 [00165] Although example methods of functionalizing substrates are
provided above, it will
4 be appreciated that substrates may be functionalized using methods other
than those
described. For example, it will be appreciated that electrodes comprising TOO
substrates may
6 be functionalized with up to about a monolayer of a halogen species using
methods other than
7 those described above.
8 [00166] Although the above has been described with reference to certain
specific example
9 embodiments, various modifications thereof will be apparent to those
skilled in the art without
departing from the scope of the claims appended hereto.
11
12
32