Note: Descriptions are shown in the official language in which they were submitted.
CA 02870688 2014-10-16
WO 2013/163116 PCT/US2013/037690
AROMATIZATION OF A METHANE-CONTAINING GAS STREAM
Field of the Invention
This invention relates to a process for the aromatization of a methane-
containing
gas stream in a reactor containing both catalyst and hydrogen acceptor
particles, wherein at
least the hydrogen acceptor particles are in a moving bed state and the
removal of
hydrogen from the reaction zone is accomplished insitu by the hydrogen
acceptor.
Background
The aromatic hydrocarbons (specifically benzene, toluene and xylenes) are the
main high-octane bearing components of the gasoline pool and important
petrochemical
building blocks used to produce high value chemicals and a variety of consumer
products,
for example, styrene, phenol, polymers, plastics, medicines, and others. Since
the late
1930's, aromatics are primarily produced by upgrading of oil-derived
feedstocks via
catalytic reforming or cracking of heavy naphthas. However, occasional severe
oil
shortages and price spikes result in severe aromatics shortages and price
spikes. Therefore,
there is a need to develop new, independent from oil, commercial routes to
produce high
value aromatics from highly abundant and inexpensive hydrocarbon feedstocks
such as
methane or stranded natural gas (which typically containing about 80-90 %vol.
methane).
There are enormous proven reserves of stranded natural gas around the world.
According to some estimates, the world reserves of natural gas are at least
equal to those of
oil. However, unlike the oil reserves that are primarily concentrated in a few
oil-rich
countries and are extensively utilized, upgraded and monetized, the natural
gas reserves are
much more broadly distributed around the world and significantly
underutilized. Many
developing countries that have significant natural gas reserves lack the
proper
infrastructure to exploit them and convert or upgrade them to higher value
products. Quite
often, in such situations, natural gas is flared to the atmosphere and wasted.
Because of the
above reasons, there is enormous economic incentive to develop new
technologies that can
efficiently convert methane or natural gas to higher value chemical products,
specifically
aromatics.
In 1993, Wang et al., (Catal. Lett. 1993, 21, 35-41), discovered a direct, non-
oxidative route to partially convert methane to benzene by contacting methane
with a
catalyst containing 2.0 % wt. Molybdenum on an H-ZSM-5 zeolite support at
atmospheric
pressure and a temperature of 700 C. Since Wang's discovery, numerous
academic and
1
CA 02870688 2014-10-16
WO 2013/163116 PCT/US2013/037690
industrial research groups have become active in this area and have
contributed to further
developing various aspects of the direct, non-oxidative methane to benzene
catalyst and
process technology. Many catalyst formulations have been prepared and tested
and various
reactor and process conditions and schemes have been explored.
Despite these efforts, a direct, non-oxidative methane aromatization catalyst
and
process cannot yet be commercialized. Some important challenges that need to
be
overcome to commercialize this process include: (i) the low, as dictated by
thermodynamic
equilibrium, per pass conversion and benzene yield (for example, 10 % wt. and
6 % wt.,
respectively at 700 C); (ii) the fact that the reaction is favored by high
temperature and
low pressure; (iii) the need to separate the produced aromatics and hydrogen
from
unreacted (mainly methane) hydrocarbon off gas and (iv) the rapid coke
formation and
deposition on the catalyst surface and corresponding relatively fast catalyst
deactivation.
Among these challenges, overcoming the thermodynamic equilibrium limitations
and
significantly improving the conversion and benzene yield per pass has the
potential to
enable the commercialization of an efficient, direct, non-oxidative methane-
containing gas
aromatization process.
The methane aromatization reaction can be described as follows:
Mo/ZSM-5
6CH4 ->" CH 6 + 9H2
According to the reaction, 6 molecules of methane are required to generate a
molecule of benzene. It is also apparent that, the generation of a molecule of
benzene is
accompanied by the generation of 9 molecules of hydrogen. Simple thermodynamic
calculations revealed and experimental data have confirmed that, the methane
aromatization at atmospheric pressure is equilibrium limited to about 10 and
20 % wt. at
reaction temperature of 700 or 800 C, respectively. In addition, experimental
data showed
that the above conversion levels correspond to about 6 and 11.5 % wt. benzene
yield at 700
and 800 C, respectively. The generation of 9 molecules of hydrogen per
molecule of
benzene during the reaction leads to significant volume expansion that
suppresses the
reaction to proceed to the right, i.e. it suppresses methane conversion and
formation of
reaction products, i.e. benzene yield. The aforementioned low per pass
conversions and
benzene yields are not very attractive to provide an economic justification
for scale-up and
commercialization of methane containing gas aromatization process.
2
CA 02870688 2014-10-16
WO 2013/163116 PCT/US2013/037690
Therefore, there is a need to develop an improved direct, non-oxidative
methane
aromatization process that provides for significantly higher (than these
allowed by the
thermodynamic equilibrium) conversion and benzene yields per pass by
implementing an
insitu hydrogen removal from the reaction zone.
Summary of the Invention
The invention provides a process for the aromatization of a methane-containing
gas
stream comprising: contacting the methane-containing gas stream in a reaction
zone with a
moving bed comprising an aromatization catalyst and a hydrogen acceptor under
methane-
containing gas aromatization conditions to produce a product stream comprising
aromatics
and hydrogen wherein at least a portion of the hydrogen is bound by the
hydrogen acceptor
in the reaction zone and removed from the product and the reaction zone.
The invention further provides a novel process and reactor schemes that employ
single or multiple catalyst and/or hydrogen acceptor moving beds as well as a
reactor that
contains multiple fixed and moving beds of catalyst and hydrogen acceptor
particles.
The invention also provides several catalyst and/or hydrogen acceptor recycle
and
regeneration process schemes. According to these schemes, the catalyst and/or
hydrogen
acceptor particles are regenerated simultaneously or separately in single or
in separate
vessels and then returned back to the reactor for continuous (uninterrupted)
production of
aromatics and hydrogen. The aforementioned insitu hydrogen removal in the
moving bed
state allows for overcoming of the thermodynamic equilibrium limitations and
for shifting
the reaction equilibrium to the right. This results in significantly higher
and economically
more attractive methane-containing gas stream conversion and benzene yields
per pass
relative to the case without hydrogen removal in the reaction zone.
Brief Description of the Drawings
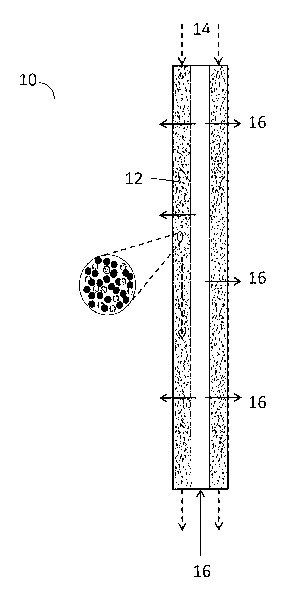
Figure 1 shows a schematic diagram of an embodiment of the invention:
aromatization reactor with a radial flow with catalyst and hydrogen acceptor
particles
intermixed in a single moving bed configuration. The catalyst and hydrogen
acceptor
particles are moving in a direction perpendicular to the gas feed flow.
Figure 2 shows a schematic diagram of another embodiment of the invention:
aromatization reactor with catalyst and hydrogen acceptor particles in
separate stacked
multiple moving beds configuration. The catalyst and hydrogen particles are
moving in
opposite direction to each other but both are perpendicular to the direction
of the gas feed
flow.
3
CA 02870688 2014-10-16
WO 2013/163116 PCT/US2013/037690
Figure 3 shows a schematic diagram of yet another embodiment of the invention:
aromatization reactor with multiple stacked beds of catalyst particles in
fixed bed
configuration and hydrogen acceptor particles in moving bed configuration. The
hydrogen
acceptor particles are moving in a direction perpendicular to the direction of
the gas feed
flow.
Figure 4 shows a schematic diagram of an embodiment of the invention:
regeneration of the mixed catalyst and hydrogen acceptor particles in a single
regeneration
vessel. This regeneration scheme is suitable for the aromatization reactor
shown on Figure
1.
Figure 5 shows a schematic diagram of another embodiment of the invention:
separation, regeneration of each type of particles in a separate vessel
followed by mixing of
particles before feeding back to reactor. This regeneration scheme is also
suitable for the
aromatization reactor shown on Figure 1.
Figure 6 shows a schematic diagram of another embodiment of the invention:
regeneration (without separation) of catalyst and hydrogen acceptor particles
in separated
vessels. This regeneration scheme is suitable for the aromatization reactor
shown on Figure
2.
Figure 7 shows a schematic diagram of another embodiment of the invention:
regeneration of catalyst and hydrogen acceptor particles in separate vessels.
The catalyst
particles are regenerated insitu in the reactor (in fixed bed mode) whereas
the hydrogen
acceptor particles are regenerated in a separate vessel. This regeneration
scheme is suitable
for the aromatization reactor shown on Figure 3.
Detailed Description
The conversion of a methane-containing gas stream to aromatics is typically
carried
out in a reactor comprising a catalyst, which is active in the conversion of
the methane-
containing gas stream to aromatics. The methane-containing gas stream that is
fed to the
reactor comprises more than 50 % vol. methane, preferably more than 70 % vol.
methane
and more preferably of from 75 % vol. to 100 % vol. methane. The balance of
the methane-
containing gas may be other alkanes, for example, ethane, propane and butane.
The
methane-containing gas stream may be natural gas which is a naturally
occurring
hydrocarbon gas mixture consisting primarily of methane, with up to about 30 %
vol.
concentration of other hydrocarbons (usually mainly ethane and propane), as
well as small
amounts of other impurities such as carbon dioxide, nitrogen and others.
4
CA 02870688 2014-10-16
WO 2013/163116 PCT/US2013/037690
The conversion of a methane-containing gas stream is carried out at a gas
hourly
space velocity of from 100 to 60,000 h-1, a pressure of from 0.5 to 10 bar and
a temperature
or from 500 to 900 C. More preferably, the conversion is carried out at gas
hourly space
velocity of from 300 to 30,000 h-1, a pressure of from 0.5 to 5 bar and a
temperature of
from 700 to 875 C. Even more preferably, the conversion is carried out at gas
hourly
space velocity of from 500 to 10,000 h-1, a pressure of from 0.5 to 3 bar and
a temperature
of from 700 to 850 C. Various co-feeds such as CO, CO2 or hydrogen or
mixtures thereof
that react with coke precursors or prevent their formation during methane
aromatization
can be added at levels of < 10 % vol. to the methane-containing feed to
improve the
stability performance or regenerability of the catalyst. The methane
aromatization is then
carried out until conversion falls to values that are lower than those that
are economically
acceptable. At this point, the aromatization catalyst has to be regenerated to
restore its
aromatization activity to a level similar to its original activity. Following
the regeneration,
the catalyst is again contacted with a methane-containing gas stream in the
reaction zone of
the aromatization reactor for continuous production of aromatics.
Any catalyst suitable for methane-containing gas aromatization can be used in
the
process of this invention. The catalyst typically comprises one or more active
metals on an
inorganic oxide support and optionally comprises promoters and other
beneficial
compounds. The active metal or metals, promoters, compounds and the inorganic
support
all contribute to the overall aromatization activity, mechanical strength and
performance of
the aromatization catalyst.
The active metal component(s) of the catalyst may be any metal that exhibits
catalytic activity when contacted with a methane-containing gas stream under
methane
aromatization conditions. The active metal may be selected from the group
consisting of:
vanadium, chromium, manganese, zinc, iron, cobalt, nickel, copper, gallium,
germanium,
niobium, molybdenum, ruthenium, rhodium, silver, tantalum, tungsten, rhenium,
platinum
and lead and mixtures thereof. The active metal is preferably molybdenum.
The promoter or promoters may be any element or elements that, when added in a
certain preferred amount and by a certain preferred method during catalyst
synthesis,
improve the performance of the catalyst in the methane aromatization reaction.
The inorganic oxide support can be any support that, when combined with the
active metal or metals and optionally the promoter or promoters contributes to
the overall
catalyst performance exhibited in the methane aromatization reaction. The
support has to
5
CA 02870688 2014-10-16
WO 2013/163116 PCT/US2013/037690
be suitable for treating or impregnating with the active metal compound or
solution thereof
and a promoter compound or solution thereof. The inorganic support preferably
has a
well-developed porous structure with a sufficiently high surface area and pore
volume and
suitable for aromatization surface acidity. The inorganic oxide support may be
selected
from the group consisting of zeolites, non-zeolitic molecular sieves, silica,
alumina,
zirconia, titania, yttria, ceria, rare earth metal oxides and mixtures
thereof. The inorganic
oxide support of this invention preferably contains zeolite as the primary
component. The
zeolite is selected from the group consisting of ZSM-5, ZSM-22, ZSM-8, ZSM-11,
ZSM-
12 or ZSM-35 zeolite structure types. The zeolite is preferably a ZSM-5
zeolite. The
ZSM-5 zeolite further may have a Si02/A1203 ratio of 10 to 100. Preferably,
the
Si02/A1203 ratio of the zeolite is in the range of 20-50. Even more preferably
the
Si02/A1203 ratio is from 20 to 40 and most preferably about 30. The support
may
optionally contain about 15-70% wt of a binder that binds the zeolite powder
particles
together and allows for shaping of the catalyst in the desired form and for
achieving the
desired high catalyst mechanical strength. More preferably the support
contains from 15-
30 % wt. binder. The binder is selected from the group consisting of silica,
alumina,
zirconia, titania, yttria, ceria, rare earth oxides or mixtures thereof.
The final shaped catalyst could be in the form of cylindrical pellets, rings
or spheres.
The preferred catalyst shape of this invention is spherical (for moving bed
operation) or
pellets (for fixed bed operation). The spherical or pelletized catalyst of
this invention could
be prepared by any method known to those skilled in the art. Preferably, the
spherical
catalyst of this invention is prepared via spray drying of zeolite containing
sols of
appropriate concentration and composition. The zeolite containing sol may
optionally
contain binder. The spherical catalyst has predominant particle size or
diameter that makes
it suitable for fluidization. The spherical particle diameter of the catalyst
of this invention
is preferably selected to be in the range of 20-500 microns. More preferably,
the spherical
catalyst of this invention has particle diameter in the range of 50-200
microns. More
preferably, the spherical catalyst of this invention has particle diameter in
the range of 50-
200 microns. The pelletized catalyst of this invention is prepared by
extrusion of suitable
extrusion mix containing appropriate concentration of zeolite powder and
optionally binder.
The hydrogen acceptor used in this reaction can be any metal-containing alloy
or a
compound that has the ability, when subjected to aromatization operating
conditions, to
selectively accept or react with hydrogen to form a sufficiently strong
hydrogen-acceptor
6
CA 02870688 2014-10-16
WO 2013/163116 PCT/US2013/037690
bond. The hydrogen acceptor preferably reversibly binds the hydrogen in such a
way that
during operation in the moving bed reactor the hydrogen is strongly bound to
the acceptor
under the methane containing gas aromatization conditions. In addition, the
hydrogen
acceptor is preferably able to release the hydrogen when transported to the
regeneration
section where it is subjected to regeneration conditions that favor release of
the previously
bound hydrogen and regeneration of the hydrogen acceptor.
Suitable hydrogen acceptors include Ti, Zr, V, Nb, Hf, Co, Mg, La, Pd, Ni, Fe,
Cu,
Ag, Cr, Th as well as other transition metals, elements or compounds or
mixtures thereof.
The hydrogen acceptor may comprise metals that exhibit magnetic properties,
for example
Fe, Co or Ni or various ferro-, para- or dimagnetic alloys of these metals.
One or more
hydrogen acceptors that exhibit appropriate particle sizes and mass for moving
bed
operation may be used in the reaction zone to achieve the desired degree of
hydrogen
separation and removal.
The aromatization reaction of this invention is carried out in a moving bed
reactor.
To enable this, suitably shaped and sufficiently robust catalyst and hydrogen
acceptor
particles that are able to sustain the rigors of high severity moving or
moving and fixed bed
operation are prepared and used for the reaction. According to the present
invention, the
use of the catalyst and hydrogen acceptor in a moving bed reactor provides
several
advantages over prior art. The most significant advantage of the process of
this invention is
that it provides for insitu removal of hydrogen from the reaction zone and as
a consequence,
an increase of both methane-containing gas stream conversion and benzene yield
per pass
to values that are significantly higher relative to these dictated by the
methane
aromatization reaction equilibrium. This is enabled by mixing and placing the
catalyst and
hydrogen acceptor particles in a moving-bed state in the reaction zone or the
aromatization
reactor (see Figures 1-3). The usage of hydrogen acceptor particles moving bed
reactors
when operating under aromatization conditions provides for the quick removal
of the
produced hydrogen from the reaction zone and for shifting the aromatization
reaction
equilibrium toward greater methane conversion and benzene yield per pass.
Figure 1 shows a reactor 10 with a single moving bed 12 that comprises a
mixture
of catalyst and hydrogen acceptor particles. The catalyst and hydrogen
acceptor particles
flow downward as shown by arrow 14, and the process gas flows upward through
the
center section and outward through the moving bed 12 as shown by arrows 16.
7
CA 02870688 2014-10-16
WO 2013/163116 PCT/US2013/037690
Figure 2 shows a reactor 110 with multiple separate moving beds comprising
catalyst or hydrogen acceptor particles. The reactor contains catalyst moving
beds 120 and
hydrogen acceptor moving beds 122. The catalyst and hydrogen acceptor
particles move
through each bed and the process gas flows upward as shown by arrow 116.
Figure 3 shows a reactor 210 with multiple moving beds 222 comprising hydrogen
acceptor particles and multiple fixed beds 220 comprising catalyst. The
process gas flows
upward as shown by arrow 216.
Another advantage of the present invention is that it allows for volume
expansion
of the hydrogen acceptor particles during the process of binding of hydrogen
to take place
under moving bed operation conditions. Hydrogen acceptors undergo significant
volume
expansion in the process of binding hydrogen and at some point in the process
the
hydrogen acceptor will bind so much hydrogen that it reaches its maximum
hydrogen
binding capacity. If the acceptor were used in a fixed bed reactor
configuration it would
expand and agglomerate in the confined bed volume. This would cause
agglomeration of
the hydrogen acceptor particles, plugging and significant reactor pressure
drop, and
suppression of the aromatization reaction.
Another advantage of the present invention is that, the particle shapes, sizes
and
mass of both hydrogen acceptor and catalyst particles can be designed and
selected in such
a way so that they can be combined together in the reactor to form the desired
moving bed.
Also, the invention provides for two or more different by chemical formula
and/or physical
properties hydrogen acceptors to be simultaneously used with the catalyst in
the moving
bed reactor to achieve the desired degree of hydrogen separation from the
aromatization
reaction zone.
Another advantage of the process of this invention is that it provides for the
catalyst
and the hydrogen acceptor particles to be simultaneously and continuously
withdrawn from
the reaction zone, regenerated in a separate vessel or vessels according to
one of the
schemes illustrated in Figures 4-7 and then continuously returned back to the
reactor for
continuous aromatics and hydrogen production. The hydrogen acceptor and
catalyst
regeneration can be accomplished either simultaneously or stepwise in the same
vessel as
illustrated in Figure 4 or separately in separate vessels as illustrated in
Figures 5-7. These
later operation schemes provide for maximum flexibility to accomplish the
hydrogen
release or regeneration of the acceptor and catalyst under different and
suitable for the
purpose operating conditions. The regeneration of catalyst and hydrogen
acceptor can be
8
CA 02870688 2014-10-16
WO 2013/163116 PCT/US2013/037690
accomplished in fixed, moving or fluidized bed reactor vessels schematically
shown in
Figures 4-7. In the specific case of separate regeneration as illustrated in
Figure 5, the
hydrogen acceptor particles can be separated from the catalyst on the basis of
(but not
limited to) differences in mass, particle size, density or on the basis of
difference in
magnetic properties between the acceptor and the catalyst particles. In the
later case, the
hydrogen acceptor of this invention can be selected from the group of
materials exhibiting
ferro-, para-or diamagnetic properties and comprising Fe, Co or Ni. In the
case of separate
regenerations illustrated in Figures 6 and 7, the hydrogen acceptor particles
are separated
from the catalyst particles in the reactor or reactor zone and therefore do
not need to be
separated before entering their regeneration vessel.
Figure 4 shows a regenerator vessel 300 that is used to regenerate the
catalyst and
regenerate the hydrogen acceptor. The catalyst and hydrogen acceptor particles
are
introduced via inlet 302 and are then removed via outlet 304. Hydrogen removed
from the
hydrogen acceptor and gases produced by catalyst regeneration are removed from
the
regenerator via one or more outlets (not shown).
In Figure 5, regenerator system 400 comprises a separation step 402 to
separate the
catalyst from the hydrogen acceptor that is fed from the reactor via line 404.
The catalyst
is fed to catalyst regeneration vessel 406, and the hydrogen acceptor is fed
to hydrogen
acceptor regeneration vessel 408. The catalyst and hydrogen acceptor are then
mixed back
together in mixing step 410 and then fed back to the reactor via line 412.
Figure 6 shows a regeneration system 500 that comprises a regeneration vessel
for
the catalyst 502 and a regeneration vessel for the hydrogen acceptor 504. No
separation
step is required because this regeneration scheme is used for a reaction
system like that
shown in Figure 2 where the catalyst and hydrogen acceptor are kept separate.
Figure 7 shows that the catalyst is regenerated insitu in the fixed catalyst
beds 620
shown in Figure 3. The hydrogen acceptor is transported from the moving beds
622 to a
regeneration vessel 630 for removing the hydrogen from the hydrogen acceptor.
The methane aromatization catalyst forms coke during the reaction. An
accumulation of coke on the surface of the catalyst gradually covers the
active
aromatization sites of the catalyst resulting in gradual reduction of its
activity. Therefore,
the coked catalyst has to be removed at a certain carefully chosen frequency
from the
reaction zone of the aromatization reactor and regenerated in a regeneration
vessel(s) as
illustrated in Figures 4-6. In the case of an aromatization reactor as shown
in Figure 3,
9
CA 02870688 2014-10-16
WO 2013/163116 PCT/US2013/037690
where the catalyst is in a fixed bed and hydrogen acceptor in a moving bed
configuration,
the coked catalyst is regenerated insitu in the reactor. The regeneration of
the catalyst could
be conducted by any of the methods known to those skilled in the art while the
hydrogen
acceptor particles are completely withdrawn or still moving through the
reaction zone of
the reactor.
The regeneration of the catalyst can be carried out by any method known to
those
skilled in the art. For example, two possible regeneration methods are hot
hydrogen
stripping and oxidative burn at temperatures sufficient to remove the coke
from the surface
of the catalyst. If hot hydrogen stripping is used to regenerate the catalyst,
then at least a
portion of the hydrogen used for the catalyst regeneration may come from the
hydrogen
released from the hydrogen acceptor. Additionally, fresh hydrogen may be fed
to the
catalyst regeneration vessel as needed to properly supplement the hydrogen
released from
hydrogen acceptor and to complete the catalyst regeneration. If the
regeneration is carried
out in the same vessel (see Figure 4), then the hydrogen removed from the
hydrogen
acceptor insitu or exsitu can at least partially hydrogen strip and regenerate
the catalyst.
If the regeneration of catalyst and hydrogen acceptor particles is carried out
in
different vessels, the operating conditions of each vessel can be selected and
maintained to
favor the regeneration of the catalyst or the hydrogen acceptor respectively.
Hydrogen
removed from the hydrogen acceptor can be used to at least partially hydrogen
strip and
regenerate the catalyst.
Yet another advantage of the process of this invention is that it provides for
the
release of the hydrogen that is bound to the hydrogen acceptor when the
saturated acceptor
is subjected to the regeneration conditions in the regeneration vessel(s).
Furthermore, the
released hydrogen can be utilized to regenerate the catalyst or subjected to
any other
suitable chemical use or monetized to improve the overall aromatization
process
economics.
Another advantage of the present invention is that, it allows for different
regeneration conditions to be used in the different regeneration vessels to
optimize and
minimize the regeneration time required for the catalyst and hydrogen acceptor
and to
improve performance in the aromatization reaction.
The aforementioned advantages of the process of the present invention provide
for
an efficient removal of hydrogen from the reaction zone of methane-containing
gas
aromatization reactor operating in moving bed mode and for shifting the
reaction
CA 02870688 2014-10-16
WO 2013/163116
PCT/US2013/037690
equilibrium towards higher methane-containing gas stream conversion and
benzene yields
per pass. Therefore, the present invention has the potential to allow for the
commercialization of an economically attractive direct, non-oxidative methane-
containing
gas stream aromatization process.
11