Note: Descriptions are shown in the official language in which they were submitted.
CA 02870758 2016-12-01
METHODS OF HYDROLYZING PRETREATED DENSIFIED BIOMASS
PARTICULATES AND SYSTEMS RELATED THERETO
[0001] This application claims priority to U.S. Patent Application Serial
No.
13/458,830filed on April 27, 2012, which application is a continuation-in-part
of U.S.
Patent Application Serial No. 13/202,011 filed on August 17, 2011, which
application is a
U.S. National Stage Filing under 35 U.S.C. 371 from International Application
No.
PCT/US2010/046525, filed August 24, 2010, and published in English as WO
2011/028543 on March 10, 2011, which application claims benefit under 35
U.S.C. 119
(e) of U.S. Provisional Application Serial No. 61/236,403 filed on August 24,
2009.
[0002] Background
[0003] Current attempts to produce cellulosic-based ethanol are cost
prohibitive
and involve a number of steps.
Summary
[0004] In one embodiment, a product is provided comprising at least one
hydrolysable densified biomass particulate having no added binder and
comprised of a
plurality of lignin-coated plant biomass fibers, wherein the at least one
hydrolysable
densified biomass particulate has an intrinsic density substantially
equivalent to a binder-
containing hydrolysable densified biomass particulate and has a substantially
smooth,
non-flakey outer surface. In one embodiment, the novel product contains trace
amounts
of ammonia. In one embodiment, the product comprises one or more hydrolysable
densified biomass particulates, each particulate having no added binder and an
amount of
lignin-coated plant biomass fiber sufficient to form a hydrolysable densified
biomass
particulate which has an intrinsic density substantially equivalent to a
binder-containing
hydrolysable densified biomass particulate.
[0005] In one embodiment, the at least one hydrolysable densified biomass
particulate having no added binder has an increased resistance to deformation,
an
increased hardness, an increased resistance to degradation, an improved shelf
life, or a
1
combination thereof, as compared with a binder-containing hydrolysable
densified
biomass particulate. In one embodiment, the novel product is more able to
resist stress
and is likely less brittle as compared to a binder-containing hydrolysable
densified
biomass particulate.
[00061 In one embodiment, the novel product is harder, such as at least
21%
harder, with at least 20% less variability in hardness than a binder-
containing
hydrolysable densified biomass particulate of the same given mass.
[0007] The novel products described herein can be any suitable shape and
size,
including, for example, substantially rectangular or substantially
cylindrical.
[0008] In one embodiment, each of the plurality of lignin-coated plant
biomass
fibers in the hydrolysable densified particulate is completely coated with
lignin. In one
embodiment, at least some of the plurality of lignin-coated biomass fibers is
also coated
with hemicellulose. In one embodiment, most of the plurality of lignin-coated
plant
biomass fibers in the hydrolysable densified particulate are also coated with
hemicellulose. In one embodiment, substantially all of the plurality of lignin-
coated plant
biomass fibers in the hydrolysable densified particulate are also coated with
hemicellulose, such that the hemicelluloses and lignin appear to come to the
surface in a
"package" rather than as separate components.
[0009] Any suitable plant biomass may be used to produce the novel
products
described herein, including, but not limited to, corn stover, switchgrass,
pine and/or
prairie cord grass.
[0010] In one embodiment, the novel product has an improved shelf life,
increased resistance to degradation, increased flowability, and greater bulk
density as
compared to the binder-containing hydrolysable densified biomass particulate.
[0011] In one embodiment, a packaged product is provided comprising a
container; and a quantity of hydrolysable densified biomass particulates
having no added
binder and located within the container, wherein the quantity of hydrolysable
densified
biomass particulates has a bulk density at greater than a bulk density of an
identical
quantity of binder-containing hydrolysable densified biomass particulates. The
container
may be a rigid container or a flexible bag.
[0012] In one embodiment, an integrated process is provided comprising
subjecting a quantity of biomass fibers to an ammonia treatment, wherein at
least a
portion of lignin contained within each fiber is moved to an outer surface of
each fiber to
produce a
2
CA 2870758 2017-10-26
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
quantity of tacky (i.e., sticky to the touch) biomass fibers; and densifying
the quantity of
tacky biomass fibers to produce one or more hydrolysable densified biomass
particulates,
wherein the quantity of tacky biomass fibers is densified without adding
binder. In one
embodiment the ammonia treatment causes at least a portion of hemicellulose
contained
within each fiber to move to the outer surface of each fiber. In one
embodiment, the
ammonia treatment is an ammonia fiber expansion (AFEXTM) treatment, such as a
gaseous AFEXTM treatment.
[0013] In one embodiment, the integrated process further comprises a
hydrolysis
step in which the hydrolysable densified biomass particulates are hydrolyzed
using high
solids loading, i.e., greater than 12%. Use of high solids loading results in
a cellulosic
sugar stream sufficiently concentrated to allow for conversion of the
liberated sugars
into biofuels through fermentation (e.g., at least about 6 to about 8% by
weight
fermentable sugars) or to an entire suite of other useful bioproducts. In one
embodiment,
the conversion comprises fermentation.
[0014] Various systems for producing the cellulosic sugar stream and/or the
converted cellulosic biomass are also provided.
[0015] In one embodiment, a biofuel is provided comprising at least one
hydrolysable densified biomass particulate of a given mass having no added
binder and
comprised of a plurality of lignin-coated plant biomass fibers, wherein the at
least one
hydrolysable densified biomass particulate has an intrinsic density
substantially
equivalent to a binder-containing hydrolysable densified biomass particulate
of the same
given mass and has a substantially smooth, non-flakey outer surface. Such a
biofuel may
be useful in biomass-burning stoves or boilers.
[0016] In one embodiment, an animal feed is provided, comprising at least
one
hydrolysable densified biomass particulate of a given mass having no added
binder and
comprised of a plurality of lignin-coated plant biomass fibers, wherein the at
least one
hydrolysable densified biomass particulate has an intrinsic density
substantially
equivalent to a binder-containing hydrolysable densified biomass particulate
of the same
given mass and has a substantially smooth, non-flakey outer surface, wherein
the animal
feed has improved digestibility as compared with animal feed containing binder-
containing hydrolysable densified biomass particulates.
[0017] In one embodiment, a solid material is provided, comprising at least
one
hydrolysable densified biomass particulate of a given mass having no added
binder and
3
CA 02870758 2016-12-01
comprised of a plurality of lignin-coated plant biomass fibers, wherein the at
least one
hydrolysable densified biomass particulate has an intrinsic density
substantially
equivalent to a binder-containing hydrolysable densified biomass particulate
of the same
given mass and has a substantially smooth, non-flakcy outer surface, wherein
the solid
material is useful in construction, such as in fiberboard or extruded fibrous
building
materials.
[0017a] According to a further embodiment, we disclose a method of
producing
a sugar-containing stream comprising:
enzymatically hydrolyzing one or more hydrolysable densified
biomass particulates in a stirred vessel to produce the sugar-containing
stream, wherein the stirred vessel is an industrial-scale vessel, wherein
the hydrolysis comprises a solids loading of said densified biomass
particulates within said vessel in a range of 12% to 24%, and wherein
said densified biomass particulates are produced by:
subjecting plant biomass fibers containing lignin and/or
hem icellulose to an ammonia or sodium hydroxide pretreatment to
cause at least a portion of the lignin and/or hemicellulose in said
biomass fibers to move to an outer surface to produce a quantity of
pretreated tacky plant biomass fibers; and
densifying said pretreated tacky plant biomass fibers to produce
the one or more hydrolysable densified biomass particulates, wherein
said densified plant biomass fibers are densified without using added
binder.
[0018] The resulting densified biomass particulates are useful in a variety
of
applications, including, but not limited to, the production of animal feed, an
entire suite
of other bioproducts using chemical catalysis or chemical conversions, other
biochemical
applications, biofuels, including for electricity generating applications
(e.g., burning in a
boiler, biomass-burning stoves, and the like), as a component in solid
materials, such as
fiberboards and extruded fibrous building materials, and the like.
4
CA 02870758 2016-12-01
Brief Description of the Drawings
[0019] FIG. 1 comprises an image showing AFEXTM pretreated corn stover
(AFEXTm-CS), AFEX'm pretreated switchgrass (AFEXTm-SG), AFEXTm-CS briquettes
and AFEXTm-SG briquettes according to various embodiments.
[0020] FIG. 2 comprises an image of a binder-containing non-AFEXTm-CS
briquette and an AFEXTm-CS briquette according to various embodiments.
[0021] FIGS. 3A-3E are images taken at various times of three biomass
samples,
including AFEXTm-CS, AFEXTm-CS briquettes, and soaked AFEXTm-CS briquettes
according to various embodiments.
[0022] FIG. 4 is a graph show % glucan conversion versus biomass at 6 hr,
24 hr
and 72 hr for the biomass samples shown in FIGS. 3C-3E according to various
embodiments.
[0023] FIG. 5 is a graph show % xylan conversion versus biomass at 6 hr, 24
hr
and 72 hr for the biomass samples shown in FIGS. 3C-3Eaccordina to various
embodiments.
[0024] FIG. 6 is a graph showing glucose concentrations for AFEXTm-treated
corn stover pellets produced at 4 different moisture contents according to
various
embodiments.
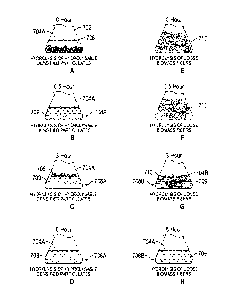
[0025] FIGS. 7A-7H are schematic illustrations which provide a visual
comparison of a hydrolysis process using hydrolysable densified particulates
(7A-7D)
with a conventional hydrolysis process using loose biomass fibers (7E-7H)
according to
various embodiments.
4a
CA 02870758 2015-10-23
[0026] FIG. 8 is a graph showing bulk density for AFEXTm-treated corn
stover
pellets produced at multiple sizes and moisture contents according to various
embodiments.
Detailed Description of the Embodiments
[0027] The scope of the invention should not be limited by the preferred
embodiments set forth in the examples but should be given the broadest
interpretation
consistent with the description as a whole. The claims are not to be limited
to the
preferred or exemplified embodiments of the invention.
[0028] The term "biomass" as used herein, refers in general to organic
matter
harvested or collected from a renewable biological resource as a source of
energy. The
renewable biological resource can include plant materials, animal materials,
and/or
materials produced biologically. The term "biomass" is not considered to
include fossil
fuels, which are not renewable.
[0029] The term "plant biomass" or "ligno-cellulosic biomass (LCB)" as used
herein is intended to refer to virtually any plant-derived organic matter
containing
cellulose and/or hemicellulose as its primary carbohydrates (woody or non-
woody)
available for producing energy on a renewable basis. Plant biomass can
include, but is
not limited to, agricultural crop wastes and residues such as corn stover,
wheat straw, rice
straw, sugar cane bagasse and the like. Plant biomass further includes, but is
not limited
to, woody energy crops, wood wastes and residues such as trees, including
fruit trees,
such as fruit-bearing trees, (e.g., apple trees, orange trees, and the like),
softwood forest
thinnings, bark)/ wastes, sawdust, paper and pulp industry waste streams, wood
fiber, and
the like. Additionally grass crops, such as various prairie grasses, including
prairie cord
grass, switchgrass, big bluestem, little bluestem, side oats grama, and the
like, have
potential to be produced large-scale as additional plant biomass sources. For
urban areas,
potential plant biomass feedstock includes yard waste (e.g., grass clippings,
leaves, tree
clippings, brush, etc.) and vegetable processing waste. Plant biomass is known
to be the
most prevalent form of carbohydrate available in nature and corn stover is
currently the
largest source of readily available plant biomass in the United States. When
used without
a qualifier, the term "biomass" is intended to refer to LCB.
CA 02870758 2019-10-10
WO 2013/163571
PCT/ES2013/038452
[0030] The term "biofuel" as used herein, refers to any renewable solid,
liquid or
gaseous fuel produced biologically and/or chemically, for example, those
derived from
biomass. Most biofuels are originally derived from biological processes such
as the
photosynthesis process and can therefore be considered a solar or chemical
energy
source. Other biofuels, such as natural polymers (e.g., chitin or certain
sources of
microbial cellulose), are not synthesized during photosynthesis, but can
nonetheless be
considered a biofuel because they are biodegradable. There are generally
considered to be
three types of biofuels derived from biomass synthesized during
photosynthesis, namely,
agricultural biofuels (defined below), municipal waste biofuels (residential
and light
commercial garbage or refuse, with most of the recyclable materials such as
glass and
metal removed) and forestry biofuels (e.g., trees, waste or byproduct streams
from wood
products, wood fiber, pulp and paper industries). Biofuels produced from
biomass not
synthesized during photosynthesis include, but are not limited to, those
derived from
chitin, which is a chemically modified form of cellulose known as an N-acetyl
glucosamine polymer. Chitin is a significant component of the waste produced
by the
aquaculture industry because it comprises the shells of seafood.
[0031] The term "agricultural biofuel" as used herein, refers to a biofuel
derived
from agricultural crops, lignocellulosic crop residues, grain processing
facility wastes
(e.g., wheat/oat hulls, corn/bean fines, out-of-specification materials,
etc.), livestock
production facility waste (e.g., manure, carcasses, etc.), livestock
processing facility
waste (e.g., undesirable parts, cleansing streams, contaminated materials,
etc.), food
processing facility waste (e.g., separated waste streams such as grease, fat,
stems, shells,
intet mediate process residue, rinse/cleansing streams, etc.), value-added
agricultural
facility byproducts (e.g., distiller's wet grain (DWG) and syrup from ethanol
production
facilities, etc.), and the like. Examples of livestock industries include, but
are not limited
to, beef, pork, turkey, chicken, egg and dairy facilities. Examples of
agricultural crops
include, but are not limited to, any type of non-woody plant (e.g., cotton),
grains such as
corn, wheat, soybeans, sorghum, barley, oats, rye, and the like, herbs (e.g.,
peanuts), short
rotation herbaceous crops such as switchgrass, alfalfa, and so forth.
[0032] The term "pretreatment step" as used herein, refers to any step,
i.e.,
treatment intended to alter native biomass so it can be more efficiently and
economically
converted to reactive intermediate chemical compounds such as sugars, organic
acids,
etc., which can then be further processed to a variety of end products such as
ethanol, iso-
6
butanol, long chain alkanes etc. Pretreatment can reduce the degree of
crystallinity of a
polymeric substrate, reduce the interference of lignin with biomass conversion
and by
hydrolyzing some of the structural carbohydrates, thus increasing their
enzymatic
digestibility and accelerating the degradation of biomass to useful products.
Pretreatment
methods can utilize acids of varying concentrations (including sulfuric acids,
hydrochloric acids, organic acids, etc.) and/or alkali such as ammonia,
ammonium
hydroxide, sodium hydroxide, lime, and the like. Pretreatment methods can
additionally
or alternatively utilize hydrothermal treatments including water, heat, steam
or
pressurized steam. Pretreatment can occur or be deployed in various types of
containers,
reactors, pipes, flow through cells and the like. Most pretreatment methods
will cause the
partial or full solubilization and/or destabilization of lignin and/or
hydrolysis of
hemicellulose to pentose sugars.
[0033] The term "moisture content" as used herein, refers to percent
moisture of
biomass. The moisture content is calculated as grams of liquid, such as water
per gram of
wet biomass (biomass dry matter plus liquid times 100%. As such, when used
without
qualification herein, the % moisture content refers to a total weight basis.
[0034] The term "Ammonia Fiber Expansion" (hereinafter "AFEXTm")
pretreatment as used herein, refers to a process for pretreating biomass with
ammonia to
solubilize lignin from plant cell wall and redeposit to the surface of the
biomass. An
AFEXTM pretreatment disrupts the lignocellulosic matrix, thus modifying the
structure of
lignin, partially hydrolyzing hemicellulose, and increasing the accessibility
of cellulose
and the remaining hemicellulose to subsequent enzymatic degradation. Lignin is
the
primary impediment to enzymatic hydrolysis of native biomass, and removal,
relocation
or transformation of lignin is a suspected mechanism of several of the leading
pretreatment technologies, including AFEXTM.
[0035] However, in contrast to many other pretreatments, the lower
temperatures
and non-acidic conditions of the AFEXTM process prevents lignin and/or sugars
from
being converted into furfural, hydroxymethyl furfural, and organic acids that
could
negatively affect microbial activity. The process further expands and swells
cellulose
fibers and further breaks up amorphous hemicellulose in lignocellulosic
biomass. These
structural changes open up the plant cell wall structure enabling more
efficient and
complete conversion of lignocellulosic biomass to value-added products while
preserving
the nutrient value and composition of the material. See, for example, the
methods
7
CA 2870758 2017-10-26
described in U.S. Patent Nos. 6,106, 888; 6,176,176; 5,037,663 and 4,600,590.
[0036] The term "condensed AFEXTM pretreatment" as used herein, refers to
an
AFEXTM pretreatment as defined herein, which uses gaseous ammonia rather than
liquid
ammonia. By allowing hot ammonia gas to condense directly on cooler biomass,
the
biomass heats up quickly and the ammonia and biomass come into intimate
contact.
Such a process is now more commonly referred to as a "GAP" process.
[0037] The term "added binder" as used herein, refers to natural and/or
synthetic
substances and/or energy forms added or applied to pretreated biomass fibers
in an
amount sufficient to improve the stability of a densified biomass particulate.
Examples of
commonly added binders include, but are not limited to, exogenous heat, steam,
water,
corn starch, lignin compounds, lignite, coffee grounds, sap, pitch, polymers,
salts, acids,
bases, molasses, organic compounds, urea, and tar. Specialty additives are
also used to
improve binding and other properties such as color, taste, pH stability, and
water
resistance.
[0038] Added binder in the form of added energy is typically in the form of
heat
which is added outright, i.e., exogenous heat, such as convective or conducted
heat,
although radiated heat may also be used for the same purpose. The intentional
addition
of exogenous heat is in contrast to intrinsic heat which develops as a result
of a material
being processed, such as the heat of friction which develops in densification
equipment
during operation. As such, heat which is inherent to the pretreatment and/or
densification
of biomass is not considered herein to be "added binder." Added binder may be
added to
the pretreated biomass at any time before, during or after a densification
process. The
amount of added binder can vary depending on the substrate being densified.
[0039] The term "particulate" or "biomass particulate" as used herein
refers to
densified (i.e., solid) biomass formed from a plurality of loose biomass
fibers which arc
compressed to form a single particulate product which is dividable into
separate pieces.
A particulate can be hydrolysable or non-hydrolysable and can range in size
from small
microscopic particles (larger than powders) to pellets and briquettes or large
objects, such
as bricks, or larger, such as hay bales or larger, with any suitable mass. The
specific
geometry and mass of a particulate will depend on a variety of factors
including the type
of biomass used, the amount of pressure used to create the particulate, the
desired length
of the particulate, the particular end use, and the like.
[0040] The term "briquette" as used herein refers to a compressed
particulate.
=
8
CA 2870758 2017-10-26
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
[0041] The term "pellet" as used herein refers to an extruded particulate,
i.e., a
compressed particulate formed with a shaping process in which material is
forced through
a die.
[0042] The term "flowability" as used herein refers to the ability of
particulates to
flow out of a container using only the force of gravity. A product having
increased
flowability, therefore, would flow out of the container at a faster rate as
compared to a
product having lower flowability.
[0043] The term "logistical properties" as used herein refers to one or
more
properties of a particulate related to storage, handling, and transportation,
which can
include, but are not limited to stability, shelf life, flowability, high bulk
density, high true
density, compressibility, durability, relaxation, springback, permeability,
unconfined
yield strength, and the like.
[0044] The term "solids loading" as used herein refers to the weight
percent of solids
in a hydrolysis mixture comprising solids, liquid and hydrolyzing
additive (e.g., enzymes). The solids can be loose cellulosic fibers or
densified cellulosic
particulates.
[0045] Cellulosic biofuel production from lignocellulosic biomass has
gained
considerable momentum due to both environmental and social sustainability
benefits.
However, the technology is not yet fully commercialized. One issue impeding
cellulosic
biofuel production using the sugar platform is the hydrolysis-resistant nature
of certain
components in the lignocellulosic biomass.
[0046] Nearly all forms of lignocellulosic biomass, i.e., plant biomass,
such as
monocots, comprise three primary chemical fractions: hemicellulose, cellulose,
and
lignin. Lignin which is a polymer of phenolic molecules, provides structural
integrity to
plants, and is difficult to hydrolyze. As such, after sugars in the biomass
have been
fermented to a bioproduct, such as alcohol, lignin remains as residual
material (i.e., a
recalcitrant lignin matrix).
[0047] Cellulose and hemicelluloses in plant cell walls exist in complex
structures
within the recalcitrant lignin matrix. Hemicellulose is a polymer of short,
highly-
branched chains of mostly five-carbon pentose sugars (xylose and arabinose),
and to a
lesser extent six-carbon hexose sugars (galactose, glucose and mannose).
Because of its
branched structure, hemicellulose is amorphous and relatively easy to
hydrolyze into its
individual constituent sugars by enzyme or dilute acid treatment. Cellulose is
a linear
9
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
polymer comprising of p(1->4) linked D-glucose in plant cell wall, much like
starch with
a linear/branched polymer comprising of a (1¨>4) linked D-glucose, which is
the primary
substrate of corn grain in dry grain and wet mill ethanol plants. However,
unlike starch,
the glucose sugars of cellulose are strung together by 13-glycosidic linkages
which allow
cellulose to form closely-associated linear chains. Because of the high degree
of
hydrogen bonding that can occur between cellulose chains, cellulose forms a
rigid
crystalline structure that is highly stable and much more resistant to
hydrolysis by
chemical or enzymatic attack than starch or hemicellulose polymers. Although
hemicellulose sugars represent the "low-hanging" fruit for conversion to a
biofuel, the
substantially higher content of cellulose represents the greater potential for
maximizing
biofuel yields, on a per ton basis of plant biomass.
[0048] Therefore, a pretreatment process is used to alter and open up the
cell wall
matrix, to hydrolyze the hemicelluloses, and to reduce crystallinity.
Pretreatment disrupts
the recalcitrant portions of lignocellulosic biomass, e.g., cellulose and
lignin, thus
improving its digestibility. After pretreatment, much of the biomass becomes
easily
digestible while a considerable amount remains recalcitrant. Ultimately, the
pretreatment
process makes the cellulose more accessible (during a subsequent hydrolysis
process) for
conversion of the carbohydrate polymer into fermentable sugars (Balan et al.
2008; Sierra
et al. 2008; Sun and Cheng 2002). Ammonia fiber expansion (AFEXTm), for
example, is
capable of opening up the cell wall in agricultural residues with greatly
reduced
degradation products compared to acidic pretreatments (Chundawat et. al.,
2010),
although acidic pretreatments remain a viable option.
[0049] Other pretreatment methods include, for example, ammonia recycled
percolation (ARP), concentrated acid hydrolysis pretreatment, dilute acid
hydrolysis,
two-stage acid hydrolysis pretreatment, high pressure hot water-based methods,
i.e.,
hydrothermal treatments such as steam explosion and aqueous hot water
extraction,
reactor systems (e.g., batch, continuous flow, counter-flow, flow-through, and
the like),
lime treatment and a pH-based treatment, hydrothermal or chemical
pretreatments,
followed by an enzymatic hydrolysis (i.e., enzyme-catalyzed hydrolysis) or
simultaneous
enzymatic hydrolysis and saccharification. As noted above, some methods
generate
nearly complete hydrolysis of the hemicellulose fraction for efficient
recovery of high
yields of the soluble pentose sugars. Recovery of these sugars also
facilitates the physical
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
removal of the surrounding hemicellulose and lignin, thus exposing the
cellulose to later
processing.
[0050] Although the cellulose is more available for conversion into its
component
sugars during hydrolysis after pretreatment, in order for fermentation to
occur
downstream, the resulting sugar concentration needs to be at an appropriate
level (e.g.,
such as at least about 6% fermentable sugars by weight or, in one embodiment
at least
about 7% or about 8% or higher, up to about 9% or higher, such as up to about
18%, or
higher, including any range there between). Some attempts to increase the
sugar stream
concentration include using a lower amount of pretreated biomass to produce a
more
dilute cellulosic sugar stream and then concentrate this stream to achieve
higher sugar
levels. However, concentration of the sugar stream
in this manner is costly.
[0051] Additionally, since pretreated loose biomass fibers rapidly absorb
liquid, use
of higher amounts of loose biomass fibers, i.e., greater than 12% solids
loading of
biomass (e.g., 120 g of pretreated loose biomass fibers per 1 kg total weight
of biomass,
liquid and enzymes), or higher, produces a product which can be difficult to
mix and/or
does not hydrolyze efficiently. Attempts to overcome this problem include
operating in
batch mode by adding pretreated loose biomass fibers in small amounts, with
each
successive load added to the hydrolysis tank only after liquefaction of the
previously
added biomass fibers has been achieved. Even if the batch process comprises
only two or
three batches, the result is a prolonged period of initial liquefaction since
serial
liquefaction phases are required.
[0052] Other options to overcome this problem include using reactors and
impellers
which are currently regarded as "specialized" due to the size of the impellers
in relation
to an inner diameter of the reactor. Such reactors have impellers which have a
diameter
substantially the same length as the inner diameter of the reactor, i.e., an
impeller size to
reactor diameter ratio of greater than about 3:4. Examples include, but are
not limited to,
horizontal paddle mixers, horizontal ribbon blenders, vertical helical
ribbons, anchor-type
impellers, and the like. However, such reactors tend to be more expensive than
those
with smaller impellers. In addition, they are not always suitable for large
vessels
(>500,000 L) due to their weight.
[0053] The various embodiments provide methods for pretreating and
densifying
loose biomass fibers to produce hydrolysable pretreated densified biomass
particulates
11
CA 02870758 2015-10-23
(hereinafter "hydrolysable particulates"). In contrast to conventional
densification
processes, the embodiments described herein do not rely on added binder for
improving
the logistical properties or stability of the resulting hydrolysable
particulates. Rather,
and as discussed herein, the inventors have surprisingly and unexpectedly
determined
that highly stable and high quality hydrolysable particulates can be produced
without
adding binder, i.e., with "no added binder" during the densification stage,
and, in
various embodiments, without adding binder during the pretreatment stage
before
densification or at any point after densification.
[0054] Such particulates have now been shown to improve hydrolysis
efficiency in terms of time and/or yield, and, ultimately, to allow conversion
to occur
downstream. These improvements occur, in part, because the hydrolysable
particulates
described herein unexpectedly allow for higher solids loading during
hydrolysis as
compared to loose biomass fibers, even including pretreated loose biomass
fibers. A
visual comparison of one embodiment of the novel hydrolysis processes
described
herein using hydrolysable densified particulates with a conventional
hydrolysis process
using loose biomass fibers, is shown in the schematic illustrations of FIGS.
7A-7H.
FIGS. 7A-7H are described further in Example 8, as this visual representation
also
correlates with the testing performed in Example 8. Not only is the resulting
sugar
stream at a concentration sufficiently high to provide for effective
conversion, the
downstream bioproducts can now be produced more efficiently and cost
effectively.
[0055] In one embodiment, the hydrolysable particulates are enzymatically
hydrolyzed using a high solids loading, (i.e., a hydrolysable particulate
content of
greater than 12% of a combination of hydrolysable particulates, liquid and
enzymes) up
to about 15% or higher, such as up to about 35%, including any range there
between.
Use of high solids loading of hydrolysable particulates results in a
cellulosic sugar
stream sufficiently concentrated for conversion, such as fermentation.
[0056] Any suitable pretreatment method can be used. In one embodiment, an
ammonia fiber expansion method (AFEXTM) pretreatment is used.
[0057] In one embodiment, loose biomass fibers are heated to a temperature
of
from about 60 C to about 100 C in the presence of concentrated ammonia. See,
for
example, Dale, B.E. et al., 2004, Pretreatment of corn stover using ammonia
fiber
expansion (AFEXT"), Applied Biochem, Biotechnol. 115: 951-963. A rapid
pressure
drop then causes a physical disruption of the
12
biomass structure, exposing cellulose and hemicellulose fibers, without the
extreme sugar
degradation common to many pretreatments.
[0058] Nearly all of the ammonia can be recovered and reused while the
remaining ammonia serves as nitrogen source for microbes in fermentation. In
one
embodiment, about one (1) to two (2) wt% of ammonia remains on the pretreated
biomass.
[0059] Additionally, since there is no wash stream in the process, dry
matter
recovery following an AFEXTM treatment is essentially quantitative. This is
because
AFEXTM is basically a dry to dry process.
[0060] AFEXTM treated biomass is also stable for longer periods (e.g., up
to at
least a year) than non-AFEXTm-treated biomass and can be fed at very high
solids
loadings (such as at least about 40%) in enzymatic hydrolysis or fermentation
process as
compared with dilute acid or other aqueous pretreatments that cannot easily
exceed 20%
solids.
[0061] Cellulose and hemicellulose are also well-preserved in an AFEXTM
process, showing little degradation. As such, there is no need for
neutralization prior to
enzymatic hydrolysis of AFEXTM treated biomass. Enzymatic hydrolysis of AFEXTm-
treated biomass also produces clean sugar streams for subsequent fermentation.
[0062] Degradation products from AFEXTm-treated biomass have also been
identified and quantified. One such study compared AFEXTM and acid-pretreated
corn
stover using LC-MS/GC-MS techniques. In acid-pretreated feedstock, over 40
major
compounds were detected, including organic acids, furans, aromatic compounds,
phenolics, amides and oligosaccharides. AFEXTM pretreatment performed under
mild
alkaline condition produced very little acetic acid, HMF, and furfural. See,
Dale, B.E. et
al., 2004, supra, and Dale, B.E. et al, 2005b, Pretreatment of Switchgrass
Using
Ammonia Fiber Expansion (AFEXTm), Applied Biochemistry and Biotechnology. Vol.
121-124. pp. 1133¨ 1142. See also Dale, B.E. et al., 2005a. Optimization of
the
Ammonia Fiber Explosion (AFEXTM) Treatment Parameters for Enzymatic Hydrolysis
of
Corn Stover, Bioresource Technology. Vol. 96, pp. 2014-2018.
[0063] In one embodiment, a modified AFEXTM pretreatment process, i.e.,
gaseous AFEXTM pretreatment, is used, as described in Example 1. In this
method,
gaseous ammonia is used, which condenses on the biomass itself.
[0064] In one embodiment, AFEXTM pretreatment conditions are optimized
for
a particular biomass type. Such conditions include, but are not limited to,
ammonia
13
CA 2870758 2017-10-26
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
loading, moisture content of biomass, temperature, and residence time. In one
embodiment, corn stover is subject to an AFEXTM pretreatment at a temperature
of
about 90 "C, ammonia: dry corn stover mass ratio of 1:1, moisture content of
corn
stover of 37.5%, and residence time (holding at target temperature), of five
(5) mm. In
one embodiment, switchgrass is subjected to an AFEXTm pretreatment at a
temperature
of about 100 C, ammonia loading of 1:1 kg of ammonia: kg of dry matter, and
45%
moisture content (total weight basis) at five (5) mm residence time.
[0065] Hydrolysis results of AFEXTm-treated and untreated samples show 93%
vs.
16% glucan conversion, respectively. The ethanol yield of optimized AFEXTm-
treated
switchgrass was measured to be about 0.2 g ethanol/g dry biomass, which is 2.5
times
more than that of the untreated sample. See Dale, B.E. et al., 2005b, supra.
[0066] In one embodiment, approximately 98% of the theoretical glucose
yield is
obtained during enzymatic hydrolysis of an AFEXTm-treated corn stover using 60
filter
paper units (FPU) of cellulase enzyme/g of glucan (equal to 22 FPU/g of dry
corn
stover).
[0067] Ethanol yield has been shown to increase by up to 2.2 times over
that of an
untreated sample. In one embodiment, lower enzyme loadings of 15 and 7.5 FPU/g
of
glucan do not significantly affect the glucose yield, as compared with 60
_ETU. In this
embodiment, differences between effects at different enzyme levels decreased
as the
treatment temperature increased. See, for example, Dale, B.E. et al., 2004,
supra; and
Dale, B.E. et al., 2004, supra.
[0068] Optimal AFEXTm pretreatment conditions for hydrolysis and
fermentation
of switchgrass and corn stover are also discussed in Dale, B.E. et al., 2004,
supra; Dale,
B.E. et al, 2005b, supra; and Dale, B.E. et al., 2005b, supra.
[0069] In one embodiment, a modified AFEXTM pretreatment with significantly
reduced ammonia loadings and lower required concentrations of ammonia is used.
See
Elizabeth (Newton) Sendich, et al., Recent process improvements for the
ammonia fiber
expansion (AFEXTM) process and resulting reductions in minimum ethanol selling
price, 2008, Bioresource Technology 99: 8429-8435 and U.S. Patent Application
Publication No. 2008/000873 to Dale, B.E.
[0070] In one embodiment, steam is used as a pretreatment instead of or in
addition to an AFEXTM treatment. However, steam tends to reduce availability
of
14
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
sugars, thus reducing the overall quality of animal feed. Regardless, steam
remains a
viable optional embodiment for pretreatment.
[0071] When biomass fibers are being densified, the fibers themselves
typically
become hot as they are being foimed into hydrolysable particulates. Such
intrinsic heat
can include the heat of friction which develops during an extrusion or
compaction
process, as is known in the art. As defined herein, such heat is not
considered to be
"added binder."
[0072] Although added binder is not used during the densification process
as
described herein, in one embodiment, added binder can be added or applied to
loose
biomass fibers prior to densification. Addition of liquid, such as water,
during
pretreatment can raise the moisture content of the hydrolysable particulates
to between
about 10 and about 50%,
[0073] Steam can be used in the reaction vessel prior to and/or during
pretreatment, such as an AFEXTM pretreatment. Adding steam to loose biomass
fibers
during pretreatment may allow water to be distributed more evenly throughout
the
hydrolysable particulates during hydrolysis. In one embodiment, added binder
is
applied or added to hydrolysable particulates (i.e., after densification),
although such a
step can increase processing costs.
When the densification process is complete, steam evaporates off the
hydrolyzed
particulates, leaving a product that is sufficiently dry, i.e., typically
about five (5) to
about 20% moisture content, although the embodiments are not so limited.
[0074] It is to be noted that minimal amounts of the various substances and
energy
sources noted in the definition of "added binder" may be added at any point in
the
pretreatment and/or densification process and/or after the densification
process in
amounts that do not improve the logistical properties and/or stability of the
biomass
particulate, and therefore do not technically function as "added binder," as
defined
herein. However, such additions can increase processing costs.
[0075] Although a non-volatile base, such as sodium hydroxide, may also be
used
to move the lignin to the surface, the sodium hydroxide which remains after
evaporation
may negatively impact further application of the treated material, such as for
animal
feed and other applications.
[0076] Due to temperatures reaching the glass transition temperature of the
oligomers within the fiber (e.g., lignin, hemicelluloses), pretreatments, such
as AFEXTM
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
(and/or steam) also transfers these oligomers (primarily lignin), and in some
embodiments, an amount of hemicellulose, to the surface. Once on the surface,
the
lignin and hemicellulose are tacky. Surprisingly, these oligomers (lignin or
lignin and
hemicellulose) contain sufficient tackiness to provide properties at least
comparable to
that of a hydrolysable particulate which was densified with added binder (as
the term is
defined herein). In various embodiments, no added binder is used at any point
of the
process, including prior to, during or after densification.
[0077] As such, the inventors have discovered there is not only no need to
apply or
add "added binder" (which can also be referred to as "curing," typically
through use of
added steam) to the pretreated biomass (e.g., using exogenous heat) prior to
forming
them into hydrolysable particulates. Additionally surprising and unexpected is
the
discovery that there is no need to apply or add "added binder" in any form
during
densification (and in various embodiments, no need to apply or add "added
binder"
before or after densification) to produce hydrolysable particulates having
logistical
properties at least as good as, if not better than, conventional hydrolysable
particulates
containing added binder. The ability to omit the step of adding and/or
applying an
added binder anytime during the process, and particularly during
densification, further
provides significant costs savings during production, leading to a product
which is not
only environmentally green but highly economical and transportable, including
transportable by conventional means.
[0078] In one embodiment, the densification device utilizes a gear mesh
system to
compress biomass through a tapering channel between adjacent gear teeth. This
densification device operates at temperatures less than 60 C. (See Example
2). Such a
densification device can be used to make briquettes, as the term is defined
herein. In
one embodiment energy consumption is minimized and physical and downstream
processing characteristics are optimized.
[0079] In one embodiment, the densification device is an extrusion device
which
can form conventional substantially cylindrically-shaped particulates, now
commonly
referred to as pellets (See Example 4).
[0080] In one embodiment, an integrated biomass pretreatment and
densification
process is provided. In a particular embodiment, an ammonia treatment, such as
an
ammonia fiber expansion (AFEXTM) pretreatment or condensed AFEXTm pretreatment
16
is used in conjunction with a compaction process to produce hydrolysable
particulates,
in a process requiring no added binder.
[0081] In one embodiment, the hydrolysable particulates are hydrolysable
briquettes having a bulk density of at least ten (10) times that of chopped
biomass
(which is about 50 kg/m3). In one embodiment, the hydrolysablc particulates
are
hydrolysable pellets having a bulk density of about 550 kg/m3. Use of an
integrated
process as described herein eliminates the need for further pretreatment at
the
processing plant and further minimizes the distance that low density feedstock
bales
need to be transported.
[0082] In one embodiment, hydrolysable particulates are transported to
centralized processing facilities using existing transportation and handling
infrastructure
used for grains for further processing, such as hydrolyzing and/or converting
(e.g.,
fermenting) and/or further processing, to produce various bioproducts.
[0083] In one embodiment, AFEXTM conditions are optimized according to the
type
of biomass being processed to enhance inherent binding properties of the loose
biomass
particles and increase hydrolysis efficiency following densification and
storage.
[0084] It is further expected that downstream processing characteristics
for
briquettes will be at least as good as, or better than non-densified biomass
in terms of
conversion rates (e.g., fermentation rates), yields, and so forth. Indeed, and
as noted
herein, the improvement to hydrolysis for pellets is, unexpectedly, at least
partially the
result of the decreased ability of the hydrolysable particulate to absorb
water.
[0085] Conventional wisdom would suggest that poor water absorption would
decrease the efficiency of enzyme hydrolysis. Rather, with the decreased
ability of the
hydrolysable pellet to absorb water, the hydrolysable particulates are capable
of moving
freely within the liquid and enzyme solution at high solid loading, even after
the
hydrolysable pellets are fully disintegrated. In one embodiment, the
hydrolysable
particulates improve hydrolysis as a result of their ability to promoting
mixing of the
material, even at high solid loading.
[0086] In one embodiment, hydrolysis occurs in a vertically stirred reactor
with an
impeller size to tank diameter ratio of between 1:4 and 1:2. In one
embodiment, the
hydrolysis occurs in a vertically stirred reactor with an impeller size to
tank diameter
ratio of about 1:3, although the various embodiments are not so limited. In
one
embodiment, downstream conversion, such as fermentation, can also occur in
such a
17
CA 2870758 2017-10-26
CA 02870758 2015-10-23
reactor. Examples of reactors with impellers having such a ratio between
impeller
length and reactor diameter, include, but are not limited to, marine
impellers, pitched
blade turbines, Rushton impellers, and the like. This is in contrast to
conventional
operations not involving solid suspensions which require specialized and more
expensive reactors throughout the hydrolysis and/or conversion steps.
[0087] In one embodiment, enzymatic hydrolysis is used. Any suitable enzyme
capable of hydrolyzing the selected biomass can be used, including
endoglucanases,
cellobiohydrolases, xylanases, pectinases, ligninases, swollenins, and the
like.
[0088] In one embodiment, AFEXTM treated hydrolysable particulates having
no
added binder are provided. In contrast to conventional binder-containing
particulates,
the novel AEEXTm-treated hydrolysable particulates described herein have a
substantially smooth, non-flakey outer surface, likely due to the presence of
lignin and,
in some embodiments, hemicellulose, on the outer surface of the hydrolysable
particulate, which essentially serve as a type of coating. As such, AFEXTm-
treated
hydrolysable particulates are not susceptible to flaking (loss of mass) as
with a
conventional binder-containing particulate, which has no coating and contains
removable flakes on its outer surface.
[0089] In some embodiments, the presence of lignin and/or hemicellulose is
not
restricted to the surface only, but also is found deeper inside the
microscopic pores of
the hydrolysable particulate. Therefore, the AFEXTm-treated hydrolysable
particulates
may have added benefits, such as more efficient burning/co-firing with lignite
coal than
a conventional binder-containing particulate having added binder which is
chemically
restricted to the surface of the binder-containing particulate only.
[0090] The AF'EXTm-trcated hydrolysable particulates are also less bendable
and
therefore tend to be straighter than conventional non-pretreated particulates.
Surprisingly, the novel AFEXTm-treated hydrolysable particulates have a harder
"feel"
to them (and are likely less brittle) as compared with the softer feel of a
conventional
non-pretreated particulate.
[0091] Hardness tests (e.g., Example 4) reveal that an AFEXTm-treated
pellet is
stronger initially before suddenly breaking. In contrast, a conventional
pellet, while
maintaining strength for a longer time, is essentially more "squeezable" or
"squishier"
than the novel AFEXTm-treated hydrolysable pellets described herein (more
comparable
to softness of a "cigar"). In one embodiment, an AFEXTm-trcated corn stover
(CS)
18
hydrolysable pellet is at least 21% harder and demonstrates at least 20% less
variability
in hardness as compared with a non-pretreated CS hydrolysable pellet. In one
embodiment, the novel AFEXTm[[ --]]-treated hydrolysable pellet exhibit less
deformation than conventional non-pretreated CS hydrolysable pellet (See, for
example,
Table 7). It is likely that AFEXTM -treated hydrolysable pellets, as well as
AFEXTM -
treated hydrolysable briquette and other particulates made from other types of
biomass
will demonstrate similar or better results.
[0092] Lignin is generally darker than other components in plant material,
so the
resulting material is noticeably darker in appearance than a material not
substantially
surrounded by lignin.
[0093] In one embodiment, the AFEXTm-treated CS pellets have a specific
gravity
of up to 1.16 as compared with a non-pretreated CS pellet, which can have a
specific
gravity of no more than 0.87, although the various embodiments are not so
limited. As
the AFEXTm-treated hydrolysable pellets appear to be less porous and further
demonstrate superior hardness properties as compared with conventional non-
pretreated
pellets, AFEXTm-treated hydrolysable pellet are likely to show improved short
and long
term storage properties including, flowability, compression strength, water
solubility,
absorption, and overall shelf life, with reduced susceptibility to degradation
due to heat,
bugs, and the like.
[0094] It is also expected that the AFEXTm-treated hydrolysable
particulates will
have an improved flowability. Further testing, as noted in prophetic examples
will
quantify the amount of improvement.
[0095] In one embodiment, some or all of the above noted features are also
present
in hydrolysable particulates other than pellets (e.g., briquettes). In one
embodiment,
some or all of the above-noted features are additionally or alternatively
present in
hydrolysable particulates pretreated by methods other than AFEX1 m, such as
with other
ammonia treatments or other pretreatment methods described herein. See also
Examples
6-11.
[0096] In one embodiment, a method is provided comprising hydrolyzing
(e.g.,
enzymatically hydrolyzing) one or more hydrolysable densified cellulosic
biomass
particulates at a solids loading greater than about 12% up to about 35% (such
as about
18% and about 24%) to produce a convertible sugar-containing stream. In one
embodiment, the converting comprises fermenting the sugar-containing stream to
19
CA 2870758 2017-10-26
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
produce a bioproduct. In one embodiment, biomass in the hydrolysable densified
cellulosic biomass particulates is corn stover, switchgrass, wood, prairie
cord grass, or
combinations thereof.
[0097] In one embodiment, the hydrolysable densified cellulosic biomass
particulates are produced by subjecting a quantity of loose cellulosic fibers
to a
pretreatment (e.g., ammonia pretreatment) wherein at least a portion of lignin
contained
within each fiber is moved to an outer surface of each fiber to produce a
quantity of
tacky loose cellulosic biomass fibers; and densifying the quantity of tacky
loose
cellulosic biomass fibers to produce the one or more hydrolysable densified
cellulosic
biomass particulates wherein the quantity of tacky biomass fibers is densified
without
use of added binder. In one embodiment, the pretreating step and the
densifying step
form an integrated process. In one embodiment, the ammonia pretreatment is an
ammonia fiber expansion (AFEXTm) treatment, such as a gaseous AFEX-rm
treatment.
In one embodiment, the method further comprises adding water and/or steam
during the
pretreating step.
[0098] The method the bioproducts is a biofuel (e.g., ethanol or butanol).
[0099] In one embodiment, a system is provided comprising a hydrolyzing
facility
for hydrolyzing one or more hydrolysable densified cellulosic biomass
particulates at a
solids loading greater than about 12% up to about 35% to produce a convertible
sugar-
containing stream. The hydrolyzing facility can be part of a bioproduct
production
facility, such as.an ethanol production facility. In one embodiment, biomass
in the
biomass particulates is corn stover.
[00100] In one embodiment, the system further comprises a pretreatment
facility for
subjecting a quantity of loose cellulosic biomass fibers to a pretreatment
wherein at
least a portion of lignin contained within each fiber is moved to an outer
surface of each
fiber to produce a quantity of tacky loose cellulosic biomass fibers; and a
densifying
facility for densifying the quantity of tacky loose cellulosic biomass fibers
to produce
the one or more hydrolysable densified cellulosic biomass particulates wherein
the
quantity of tacky biomass fibers is densified without use of added binder. In
one
embodiment, the pretreatment facility and densifying facility are co-located.
[00101] The resulting hydrolysable particulates are useful in a variety of
applications, including, but not limited to, the production of animal feed, an
entire suite
of other bioproducts using chemical catalysis or chemical conversions (e.g.,
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
fermentation), other biochemical applications, biofuels, including for
electricity
generating applications (e.g., burning in a boiler, biomass-burning stoves,
and the like),
as a component in solid materials, such as fiberboards and extruded fibrous
building
materials, and the like.
[00102] The ammonia pretreatment in the various AFEXTM processes described
herein dissolves a certain amount of lignin and further brings a significant
amount of
lignin from the interior of a plant material to the outer surface or outer
edges of the
fiber. As a result, the material is more easily digested by animals. In one
embodiment,
a combination of pretreated hydrolysable particulates, such as AFEXTm-treated
briquettes or pellets, as described herein, together with suitable additives
and fillers as is
known in the art produces a novel animal feed.
[00103] In one embodiment, a blending of the pretreated hydrolysable
particulates,
such as AFEXTm-treated briquettes or pellets with coal provides a novel feed
material in
power plants.
[00104] The logistics of harvesting, handling, transporting, and storing low
bulk
density feedstocks pose a significant challenge to the developing bioeconomy.
Assuming a yield of 70 gal/ton, biomass baled at a density 120 kg/m3 would
require
over ten times the volume of material for a given volume of ethanol compared
with corn
grain. This lower bulk density will not allow trucks to reach maximum weight
capacity,
further increasing the number of trucks required for feedstock supply.
[00105] As the bioeconomy for alternative bioproducts develops, individual
producers will need the flexibility to sell their biomass into the bioenergy
market as
economics warrant. For example, with use of regional biomass processing
centers
(RBPCs) (within a 5 to 10 mile area, for example), round bales may be
transported
using the existing infrastructure and equipment of the trucking industry.
Because the
RBPCs will be scaled appropriately, trucking distances for round bales can be
minimized. Moreover, the presence of multiple, distributed RBPCs can minimize
need
for long term storage of round bales. Shorter term storage can use bale wraps
and other
current methods to minimize expense. With use of the novel integrated
pretreatment
(e.g., AFEXTM pretreatment)/densification system described herein,
hydrolysable
particulates can be more efficiently transported to centralized processing
sites.
[00106] The various embodiments will be further described by reference to the
following examples, which are offered to further illustrate various
embodiments. It
should be understood, however, that many variations and modifications may be
made
while remaining within the scope of the various embodiments.
EXAMPLE 1
[00107] Corn stover (CS) (everything remaining after grain is harvested,
typically including stalks and leaves w/o cobs) from a hybrid corn plant (Zea
mays L.)
grown at the Michigan State University (MSU) Agronomy Center Field was
harvested
in October 2007, and stored at room temperature in individual five (5) kg bags
which
were housed in a 30-gal trash bin. Switchgrass (SG) from the "Alamo" lowland
variety
of seed, Panicum virgatum L. grown at the Thelen Field located on Farm Lane at
MSU,
was harvested in October, 2005, and stored in sealed Ziploc brand plastic
bags in a
freezer at four (4) C.
[00108] The CS and SG were each subjected to an AFEXTM treatment
comparable to the methods described in U.S. Patent Nos. '888, '176, '663, and
'590
noted above, but with certain modifications. Specifically, rather than
applying liquid
ammonia to the biomass and allowing the ammonia and biomass to react as in
conventional AFEXTM treatment, gaseous ammonia was used instead. By allowing
hot
ammonia gas to condense directly on cooler biomass, the ammonia and biomass
become well-mixed.
[00109] The gaseous AFEXTM pretreatment was performed in the Biomass
Conversion Research Laboratory at Michigan State University, East Lansing,
Michigan.
Unless otherwise noted, standard laboratory equipment available in
conventionally
stocked laboratories was used. The AFEXTM pretreatment was performed in an
approved ventilation hood with protective glass sash minimum face velocity of
75
feet/minute.
[00110] A Parr Instruments Model 4524 bench top reactor (hereinafter "4254
reactor") was used for this testing. The reaction chamber was first placed
into the
heating mantle of the 4254 reactor. A J-type T-couple temperature probe was
connected to a Parr Instruments Model 4843 Modular (heat) controller
(hereinafter
"4843 controller") on one end and to the reaction chamber on the other end by
placing
the temperature probe against the internal wall of (about half-way down) the
reaction
chamber. The reaction chamber was then covered with a custom-fabricated
circular
stainless sheet metal piece having an approximately 12.7 cm (about five (5)
in) diameter
relief cut out for the temperature probe. The controller was turned on to low
(with a red
22
CA 2870758 2017-10-26
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
heater switch) and a J- type temperature (blue) controller showed a room
temperature
reading of about 25 C + 5 C.
[00111] A (yellow) K-type thermocouple (red display) and (green) Omega
brand
CX105 pressure connector (having offices in Stamford, CT) (green display) from
the
controller were briefly connected to test the 4254 reactor cover probes. The
red display
showed a room temperature reading of about 25 C + 5 C. The green display
showed a
one (1) atm gauge pressure reading of -0.34 to about 0.34 atm (about -5 to
about 5
psig). The yellow and green connecters and 4254 reactor cover were then set
aside and
the blue preheat temperature was turned on to preheat the 4254 reactor to a
target
temperature of room temperature +20 C. The blue display was observed for
about five
(5) minutes to ensure that the blue temperature increased at a rate of about
three (3)
C/minute.
[00112] A Sartorius MA35 moisture analyzer (Goettingen, Germany) was used
to
determine the moisture content of each of the biomass samples. Initial
moisture
measurements for the samples were typically five (5) to ten (10) %. The weight
of each
sample added to the 4254 reactor was 150 g dry weight, i.e., "dry biomass." An
amount
of biomass was then weighed out to result in 150 g of dry biomass (as given by
the total
moisture calculation). For example, for a biomass sample containing five (5) %
moisture content, the following calculation would be made: x (g) of water in
biomass =
(150 g dry biomass/(1-0.05) ¨ 150 g dry biomass). Solving for "x" results in
7.9 g of
water present in the biomass. Thus, in this example, adding 150 g dry weight
of
biomass would include weighing and adding 157.9 g of the biomass sample at 5%
moisture content.
[00113] A calculation was then made to determine the amount of deionized
water to
be added to each sample. For corn stover, the desired moisture content was
37.5%.
For switchgrass, the desired moisture content 45%. These values were selected
because
they represent the optimal respective biomass moistures for maximum glucose
and
xylose yields from enzymatic hydrolysis after AFEXTm.
[00114] Therefore, for a corn stover sample with 7.9 g of water already
present, but
requiring 37.5% moisture content, the following calculation would be made: x
(g)
water to be added to biomass = (150 g dry biomass/(1-0.375)-150g -7.9 g water
already
in biomass. Solving for "x" would result in 82.1 g of water to be added. The
total
weight of a 150 g dry weight corn stover sample in this instance would be 82.1
+g + 7.9
23
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
g + 150 g = 240 g. Water was misted onto each biomass sample with a water
bottle until
the total weight (dry biomass (g) + water desired (g)) was achieved. The
biomass was
evenly coated with water by stirring the biomass.
[00115] An empty 500 ml ammonia cylinder having a 208 g maximum fill level
(Parker 500 ml spun 316 Stainless steel pressure vessel (hereinafter "Parker
cylinder")
with high-pressure Swagelok0 Series 83 two-way ball valves installed at both
ends,
made by Swagelok Co. (having offices in Chicago, IL) was weighed. Since eight
(8) g
was determined to be the approximate residual ammonia left in the cylinder
after
completion of this step, the total weight of the cylinder and ammonia required
for
AFEXTM pretreatment was determined by adding eight (8) g to the weight of the
amount
of ammonia needed.
[00116] The Parker cylinder was attached to an AirgasTm brand stock ammonia
tank
(with siphon tube) made by Airgas, Inc. (Radnor, PA), by opening the inlet
valve on the
ammonia tank, followed by opening the inlet valve on the Parker cylinder. The
Parker
cylinder was allowed to fill until it was cold and no more filling noise from
the cylinder
could be heard (elapsed time was about one (1) min). The exit valve on the
ammonia
tank was opened about 1/4 way. After a few trials, it was determined that it
took about
20 seconds to add 158 g of ammonia to the Parker cylinder. Thereafter, all
valves were
closed, starting with the exit valve of the Parker cylinder and finally the
exit valve on
the ammonia tank. The Parker cylinder was weighed to make sure the total
weight was
equal to the expected weight. Some ammonia was released under the hood if the
weight
was too great. When it was not enough, the above step was repeated.
[00117] The Parker cylinder, now containing ammonia, was heated by first
wrapping it in BH Thermal brand Briskheat (Columbus, OH) heat tape and
plugging in
the BH Thermal brand Briskheat (Columbus, OH) heat tape controller. Cylinder
pressure started at 0-125 psig (depending on the temperature of the ammonia
inside the
cylinder, as it became cold during the filling step). The Parker cylinder was
heated to
600 psig (40 bar), adjustable from 400 psig (27 bar) for "colder" reactions
(80 C) to
1000 psig (70 bar) for hot reactions (160 C). The pressure increased slowly,
but always
at a rate less than 0.034 atm/sec (five (5) psig/sec).
[00118] The desired biomass was then added to the reaction chamber. The
(black)
temperature probe was removed from the reaction chamber and placed into the
slot on
the side of the heater mantle that allowed the outside surface temperature of
the reaction
24
chamber to be measured. The (blue) display temperature was adjusted (using
arrow
keys) +20 degrees more than the original preheat to allow for the continued
heating of
the reaction chamber.
[00119] The cover of the reaction chamber was replaced and a funnel was
added.
The selected biomass sample was then poured down the funnel into the reaction
chamber. Once added, the (yellow) temperature probe tip was completely covered
with
biomass and was observed to be about 2.54 cm (about one (1) in) from the
ammonia
input nozzle of the cover. The funnel was then removed, the cover returned on
top of
the 4254 reactor and brackets were tightened with bolts to seal it in place.
[00120] The Parker cylinder was then attached to the reaction chamber. A
Welch
Model 8803 vacuum pump. (Niles, Illinois) was also attached to the reaction
chamber.
The vacuum valve on the 4524 reactor was opened and the vacuum was turned on
to
pump air from the 4254 reactor for one (1) minute. The vacuum valve was closed
and
the vacuum was turn off. The (yellow) temperature probe and (green) pressure
connector was plugged into the 4843 controller. The valve on ammonia cylinder
(only)
leading towards reaction chamber was opened.
[00121] The AFEXTM reaction was started by opening the 4254 reactor valve
connected to the Parker cylinder. When the pressure between the Parker ammonia
cylinder and the reaction chamber was equalized, the valves between the
ammonia
cylinder and the reaction chamber were closed (i.e., after about one (1) min).
The heat
tape on the Parker cylinder was also turned off. The 4843 reactor heater was
left on a
low setting at 20 C above the original temperature used at pre-heat. After
about one
(1) minute the peak (red) display temperature and (green) pressure were
recorded.
When the (red) display temperature did not get >100 C within 1 minute, it
meant the
feedstock was not touching the temperature probe. The temperature and pressure
were
recorded approximately every five (5) minutes thereafter.
[00122] Starting approximately five (5) minutes before expansion step
noted
below, the' vacuum was detached from the 4524 reaction chamber cover. The
ammonia
cylinder pipe was removed from the reaction chamber cover. The reaction
chamber
was rotated so that the 4524 pressure release valve was facing toward the back
of the
fume ventilation hood. The ventilation hood sash was adjusted for maximum face
velocity (75 feet/minute recommended). Expansion step: Ear protection was
worn. The
=
CA 2870758 2017-10-26
ammonia pressure in the 4524 was released by opening the pressure release
valve
quickly.
[00123] The reaction chamber cover was removed. The biomass was removed
and placed in a tray and left under the ventilation hood to allow ammonia
vapor to
volatilize. The AFEXTM biomass was allowed to air-dry over-night. The Parker
cylinder was weighed to determine residual grams of ammonia applied to the
biomass
and the weight was recorded. The remaining ammonia (approximately 8 g) was
released from the Parker cylinder inside of ventilation hood.
EXAMPLE 2
Starting Materials and Sample Preparation
[00124] Corn stover (CS) obtained from the same source as described in
Example
1 was used. Two samples, two (2) kg each, of each type of biomass were then
subjected to the AFEXTM pretreatment according to the method described in
Example 1.
After pretreatment, samples were densified using a briquetting device (Federal
Machine
Co. d/b/a ComPAKco, LLC, Fargo, ND) to produce AFEXTM corn stover (AFEXTm-
CS) briquettes and AFEXTM switchgrass (AFEXTm-SG) briquettes.
[00125] FIG. 1 shows an image of the four resulting products, which
include
seven (7) g of AFEXTm-CS 102, 12 g of AFEXTm-SG 104, a 22 g AFEXTm-CS 106
briquette and a 23 g AFEXTm-SG briquette 108. The AFEXTm-CS and AFEXTM SG
briquettes, 106 and 108, respectively, had a substantially rectangular shape.
Both
briquettes 106 and 108 were about 2.54 cm (about one (1) in) wide, about 1.27
(0.5 in)
depth and about 10.16 to about 12.7 cm (about four (4) to about five (5) in)
in length.
(Briquette length is dependent on the particular setting use on the ComPAKco
machine).
[00126] This image illustrates that just seven (7) to 12 grams of
unbriquetted
(i.e., loose) biomass, such as AFEXTm-CS 102 and AFEXTm-SG 104, occupies more
space than a 22 or 23 g briquette, such as AFEXTm-CS briquette 106 and AFEXTm-
SG
briquette 108. In this instance, the unbriquetted biomass (102 and 104)
occupies about
570 to about 980% more space than the briquetted biomass (106 and 108).
[00127] FIG. 2 comprises an image of a binder-containing non-AFEXTm-CS
briquette and an AFEXTm-CS briquette according to various embodiments.
Testing Performed
[00128] Several additional samples were prepared in the manner described
above
and subjected to preliminary physical tests such as Angle of Repose ( )
according to the
26
CA 2870758 2017-10-26
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
method described in Carr. R. L. Jr. 1965. Evaluating flow properties of
solids. Chemical
Engineering 72(3): 163-168.
[00129] Thermal Conductivity (W/m C) was determined with a thermal
properties
meter (KD2, Decagon Devices, Pullman, WA) that utilized the line heat source
probe
technique described in Baghe-Khandan, M., S. Y Choi, and M.R. Okos. 1981,
Improved
line heat source thermal conductivity probe, J. of Food Science 46(5):1430-
1432.
[00130] Water activity was measured using a calibrated water activity meter
(AW
Sprint TH 500. Novasina, Talstrasse, Switzerland).
[00131] Bulk density (kg/m3), true density (kg/m3) and porosity were
determined
using a multivolume pycnometer (Micromeritics model 1305, Norcross, GA) as
described in Sahin, S. and S. G. Sumnu. 2006, Physical properties of foods,
New York,
NY: Springer Science Media. TLC.
[00132] Moisture Content was determined by ASAE standard method S352.1
using ISOTEMP laboratory scale (model no: 838F, Fisher Scientific, Pittsburg,
PA) as
described in ASAE Standards. 51st ed. 2004. S352.1: Moisture measurement --
Grain
and seeds, St. Joseph, Mich.: ASABE.
[00133] Color properties (L*, a*, b*) were measured using a
spectrocolorimeter
(LabScan XE, Hunter Associates Laboratory, Reston, VA).
[00134] Roundness and sphericity were determined using an Olympus SZH10
stereo microscope with a DP digital camera, followed by image analysis of the
particles
by Image Pro Plus software.
[00135] Water Solubility Index (%) and Water Absorption Index (-) were
calculated using the method described in Anderson, R. A., H. F. Conway, V. F.
Pfeifer,
and E. L. Griffin. 1969, Gelatinization of corn grits by roll and extrusion
cooking,
Cereal Science Today 14 (1): 4.
[00136] Results are shown in Table 1 below:
27
Table 1: Physical properties of AFEXTm-CS and SG vs. AFEXTm-CS and AFEXTm-SG
Briquettes* ts.)
Biomass type AoR TC aw BD Porosit TD
MC Round Sphericity WA1 WSI
Uri
(0) (W/M (-) (kg/m3 Color (kg/m3) (%) -
ness
C) (-) L* a* b*
AFEXTM 57.4a 0.035b 0.575c 547.2a 0.487
918a 12.2b 21.7b 2.21c 6.47b 0.56a 0.64a 6.30b 6.74a
switchgrass
briquettes
APEX"( 56a 0.055a 0.787a b 0.640a c
22.7a 17.8c 2.20c 5.94b 0.635a 0.52c 6.17b 6.14a
switchgrass
biomass
AFEXTm
60.6a 0.04ab 0.451b 549.2a 0.376b 722b 6.9c 21.5b 3.14b 6.70b
0.45b 0.6b 7.14ab 4.36a
Corn stover
briquettes
AFEXTM 54.4a 0.045ab 0.672b b 0.657a
c 14.3b 24.2a 3.69a 8.81a 0.56a 0.61ab 8.03a 5.63a
corn stover
biomass
o
* Similar letters for a given property is not significantly different at
a=0.05 o
n=2 for all the properties analyzed
AoR ¨ Angle of Repose (0); TC ¨ Thermal Conductivity (W/m C); aw ¨ Water
activity (-); BD ¨ Bulk density (kg/m3); TD ¨ True
Density (kg/nal); MC ¨ Moisture Content (% db); L5 - Brightness or luminosity;
a5 - redness or greenness; b5 - yellowness or
blueness; WAI ¨ Water Absorption Index (-); WSI ¨ Water Solubility Index (%)
oe
JI
tsJ
8
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
Conclusion
[00137] The AFEXTm-CS briquettes (e.g., 106) and AFEXTm-SG briquettes
(e.g.,
108), had a relatively smooth surface and held together well during handling.
The
AFEXTM briquettes of both the corn stover and switchgrass possess lower
porosity, water
adsorption index, water activity, and moisture content as compared to the non-
briquetted
AFEXTM samples. Such properties are an indication of improved storability for
the
briquetted biomass. Lower porosity, higher bulk density and higher true
density of the
briquettes are also indicative of reduced shipping costs.
[00138] The briquettes exhibited other desirable properties as shown in
'fable 1. In
particular, the briquettes demonstrated a high angle of repose. A briquette's
angle of
repose is defined as the angle between the horizontal and the plane of contact
between two
briquettes when the upper briquette is just about to slide over the lower.
This is also
known as angle of friction. Therefore, particles have an expected value of 45
degrees.
Both the corn stover briquettes and switchgrass briquettes tested herein
exhibited higher
than expected angles of repose of 57.4 and 60.6, respectively, as shown in
Table 1. These
values are likely related to the briquettes' substantially rectangular
geometry.
EXAMPLE 3
[00139] The purpose of this experiment was to compare hydrolysis properties
of
AFEXTm-CS briquettes as compared with AFEXTm-CS biomass (i.e., unbriquetted).
Starting Materials
[00140] Corn stover (CS) obtained from the same source as described in
Example 1
was used. An AFEXTm pretreatment was performed on the CS in the same manner as
described in Example 1. Briquettes were made according to the method described
in
Example 2.
[00141] Tested samples included 1.7 g of AFEXTm-CS biomass, a 1.6 g AFEXTm-
CS briquette, and a 2.2 g AFEXTm-CS soaked in 100 ml amount of de-ionized
water at 25
C for five (5) minutes before hydrolysis to produce a soaked AFEXTm-CS
briquette.
Procedure
[00142] After being placed in a 500 ml beaker, an enzymatic hydrolysis was
performed on each sample according to a standard laboratory protocol at one
(1)% solids
loading. See, for example, Shishir P.S. Chundawat , Balan Venkatesh, Bruce E.
Dale,
2005, Effect of particle size based separation of milled corn stover on AFEXTM
29
pretreatment and enzymatic digestibility, Biotechnology and Bioengineering,
Vol. 96,
Issue 2, pp 219-231.
[00143] Fifteen Filter Paper Units (FPU) of an enzyme, specifically
Spezyme CP
Genencor , a Danisco Division, having offices in Rochester, NY whole
cellulose, was
added. The samples were incubated at 50 C in a New Brunswick incubator Innova
44,
(Edison, NJ) while being shaken at 150 RPM within the incubator. Observations
and
samples were taken at 6 hrs, 24 hrs and 72 hrs incubation time.
Results
[00144] A visual inspection of the resulting hydrolysates indicates that
each of the
three samples completely dissolved immediately upon water addition. (FIG. 3B).
Therefore, it is apparent that all three samples hydrolyzed to substantially
the same extent
in substantially the same amount of time
[00145] Approximately two (2) ml samples were taken from the incubator
were
filtered and run through a Shimadzu high pressure liquid chromatographer
(HPLC) Model
LC-2010HT w/ELSD-LT to determine glucan and xylan conversions.
[00146] FIGS. 3A-3E are images taken at various times of three biomass
samples,
including AFEX-CS, AFEX-CS pellets, and soaked AFEX-CS pellets. FIGS. 4A and
4B
are comparative hydrolysis graphs showing glucan conversions of the samples
shown in
FIGS. 3A-3E. As can be seen, the glucan conversions remain substantially the
same
across each sample.
[00147] Table 2 shows percent of glucan converted to glucose at various
times in
each of the samples.
Table 2. Percent of Glucan converted to Glucose
% glucan % glucan
% glucan conversion conversion
conversion (to glucose) (to glucose)
(to glucose)
Biomass type 6h 24h 72h
AFEXTm CS 44.3 61.7 71.4
AFEXTM CS-Briquette 48.3 65.9 73.7
Soaked AFEXTM CS
Briquette 47.5 64.0 71.3
[00148] Table 3 shows the percentage of total glucose produced between
samplings.
CA 2870758 2017-10-26
CA 02870758 2019-10-10
WO 2013/163571 PCT/US2013/038452
Table 3. Percentage of total glucose produced between samplings
% total % total % total
glucose glucose glucose
Biomass type 6h 24h 72h
AFEXTm CS 44.3 17.4 9.7
AFBXTM CS-
Briquette 48.4 17.5 7.8
Soaked AFEXTM 47.5 16.5 7.3
CS-Briquette
[00149] Table 4 shows percentage of total xylan converted to xylose and total
xylan in
each sample before hydrolysis.
Table 4. Percentage of total xylan converted to xylose
Biomass % xylan % xylan % xylan Total
Type conversion conversion conversion Xylan (g)
(to xylose) (to xylose) (to xylose)
6h 24h 72h
AFEXTM CS 16.5 29.7 37.9 0.42
AFEXTM CS-
Briquette 24.1 39.6 48.0 0.38
Soaked
AFEXTM CS-
Briquette 11.8 19.3 23.4 0.72
[00150] Table 5 shows the percentage of total xylose produced between
samplings.
Table 5. Percentage of total xylose produced between samplings
% total % total % total
xylose xylose xylose
Biomass type 6h 24h 72h
AFEXTM CS 16.5 13.2 8.1
AFEXTM CS-
Briquette 24.1 15.5 8.4
Soaked AFEXTM
CS-Briquette 11.8 7.5 4.0
31
Conclusion
[00151] The substantially instantaneous hydrolyzing (e.g., wetting and
dispersion)
in the AFEXTM CS briquette demonstrates that briquetting of corn stover
biomass does
not affect hydrolysis. It is likely that other AFEXTM briquettes made from
other biomass
materials will behave in a similar manner. Indeed, as FIG. 3B shows, most of
the biomass
in each briquette is converted to sugar within six (6) hrs, which compares
favorably with
the unbriquetted AFEXTm-CS biomass sample. Additionally, both briquettes
(AFEXTm-
CS briquette and the soaked AFEXTm-CS briquette) hydrolyzed to nearly the same
extent
as the unbriqucttcd sample. This determination was made by observing the lack
of solids
remaining after 72 hours (FIG. 3E). Since the three samples had virtually the
same
conversions, the test was concluded at 72 hours. These results are confirmed
in FIGS. 4A
and 4B.
EXAMPLE 4
[00152] This test was performed to determine the comparative hardness
between
AFEXTm-CS pellets and non AFEXTm-CS pellets, i.e., pellets exposed to no
pretreatment.
Starting Materials
[00153] CS obtained from the same source as described in Example 1 was used
in
this testing. Some of the CS was subjected to the AFEXTM pretreatment as
described in
Example 1. No additional treatment was performed on the AFEXTm-treated biomass
prior
to pelleting, including no added binder and no artificial drying (any
evaporation occurring
in open air at room temperature is considered to be negligible during the
course of the
testing procedure).
[00154] The remaining portion underwent a different (non-AFEXTM) procedure,
which included adding approximately five (5) to ten (10) g of water per 100 g
of CS to
bring the moisture content of the biomass to 15% prior to pelleting.
[00155] Lodgepole pine biomass from the Driftmier Engineering Laboratory at
the
University of Georgia (Athens, GA) also underwent a similar nonAFEXTM
procedure,
and because the biomass moisture was measured to be greater than 15%, it was
put in a
dryer until it was at 12-15% moisture content.
[00156] Ten (10) AFEXTm-CS pellets and ten (10) non-AFEXTm-CS pellets were
formed with a Yankee Pellet Machine Model 400 (Yankee Pellet Mill, Effingham,
NH), a
centrifugal die mill which produces pellets currently considered the industry
standard. Ten
32
CA 2870758 2017-10-26
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
(10) nonAFEXTM pine pellets were pelletized using a California Pellet Machine,
Model
CL (CPM, Crawfordsville. IN).
[00157] Pellets produced on both these machines have a substantially
cylindrical shape
and are about six (6) mm in diameter. Length can be varied as desired, but is
generally
more uniform than the device used above in Example 2. For purposes of testing,
the
pellets were about one (1) inch.
Procedure
[00158] The pellets were tested for hardness using a 12T Carver Laboratory
Hydraulic
Press/Hardness testing apparatus with 400PSI gauge (Carver, Wabash, IN).
Specifically,
this test measured the amount of force needed to crush each pellet beyond its
yield
strength. The determination of "yield strength" was made through trained
observation and
"feel." Specifically, pressure was applied to each pellet until the tester
observed and felt
the pellet "give." Multiple pellets were tested and an average hardness, i.e.,
pressure
required causing pellets to yield (Table 6), and average deformation (Table 7)
was
determined.
Results
[00159] Comparative hardness results are shown below in Table 6:
Table 6. Comparative Pellet Hardness for AFEXTM and nonAFEXTM
Pellets
nonAFEXTM AFEXTM pellets NonAFEXTM
pellets (psi) (psi) Pine pellet
140 120 125
130 120 125
70 100 75
100 140 90
90 140 90
70 110 110
120 130 130
70 130 75
90 120 80
[00160] Measurements of the final diameter of each pellet after it "gave"
were also made. These measurements are shown in Table 7. (Note that the data
is randomized as compared with Table 6).
33
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
Table 7. Comparative Pellet Deformation for AFEXTM and nonAFEXTM
Pellets (initial diameter: 6 mm)
nonAFEXTM NonAFEXTM
CS pellets AFEXTM pellets Pine pellet
(mm) CS (mm) (mm)
5.26 4.66 5.08
4.67 5.28 5.07
4.96 5.28 5.13
4.84 4.98 5.1
5.2 4.73 5.28
5.08 5.18 4.59
4.76 5 4.75
4.15 5.12 4.61
5.39 5.36 4.98
[00161] The untreated, binder-added corn stover pellets average yield point
was 98 psi
+25 psi. The AFEXTM, no binder added corn stover pellets average yield point
was 119 psi
+20 psi, and the nonAFEXTM binder-added pine pellet average yield point was 98
psi +23
psi.
[00162] All cylindrical pellets had a beginning diameter of 6.00 mm. The
untreated,
binder-added corn stover pellets average deformation at yield was 1.06 mm
+0.36 mm.
The AFEXTm. no binder added corn stover pellets average deformation at yield
was 0.95
nun +0.24 nun, and the nonAFEXTM, binder-added pine pellet average deformation
at
yield was 1.06 mm +0.23 mm.
Conclusion
[00163] The AFEXTM pellets showed greater durability as compared to non-AFEXTm
pellets. AFEXTM pellet quality is also more consistent than the non-AFEXTm
pellets. As
such, it is expected that any given AFEXTM pellet is less likely to be
deformed or
disfigured (not a cylindrical shape) as compared with a nonAFEXTM pellet.
EXAMPLE 5
[00164] This test was performed to determine the bulk density of AFEXTm-CS
pellets
as compared to nonAFEXTM CS pellets.
[00165] AFEXTm-CS pellets and nonAFEXTM CS produced according to the method
described in Example 4 (about six (6) mm in diameter and about one (1) inch in
length)
were added to a 500 ml beaker and weighed.
34
CA 02870758 2015-10-23
[00166] The non-AFEXTM CS pellets had a bulk density of about 36 lb/ft3
(553
g/L), while the AFEXTm-CS pellets had a bulk density of about bout 38 lb/ft3
(578g/L).
[00167] As this preliminary test indicates, the AFEXTm-CS pellets showed a
higher
bulk density than the nonAFEXTM CS pellets. This is likely due to their smooth
non-
flaky outer surface (which also is expected to improve their flowability), as
compared to
the rough flaky outer surface of the nonAFEXTM pellets. It is expected that a
test
performed on a larger scale would demonstrate an even greater difference in
bulk density.
Likely, the edge effects caused by the small size of the container were a
significant factor
in this preliminary testing.
[00168] It is also possible that pellets which are longer than the one (1)
inch pellets
may weigh each other down to create a hider mass at a higher density.
Alternatively,
shorter pellets may pack better. Additional testing (including in larger
containers) will be
performed to optimize pellet size, and therefore, overall bulk density, for a
given
application.
EXAMPLE 6
[00169] In this testing, various properties of untreated corn stover
briquettes were
compared with AFEXTm-treated corn stover briquettes.
Starting Materials
[00170] Corn stover (CS) obtained from the same source as described in
Example 1
was used. An AFEXTm pretreatment was performed on the CS in the same manner as
described in Example 1. Briquettes were made according to the method described
in
Example 2.
Procedure
[00171] Standard procedures were followed to obtain the results shown in
Tables 8
and 9. Specifically, Moisture Total: ASTM E871; Ash Content: ASTM D1102;
Sulfur
Content: ATSM 1)4239; Gross Caloric Value at Constant Volume: ASTM E711;
Chlorine
Content: ASTM D6721; Bulk Density: ASTM E873; Fines (Particles less than 0.32
cm
(0.125 in): Twin Peaks Test CH-P-06; Durability Index: Kansas State Method;
Sample
above 3.8 cm (1.5 in): Twin Peaks Test CH-P-06; Maximum Length: Twin Peaks
Test
CH-P-06; Diameter, Range: Twin Peaks Test CH-P-05. The tumbling method used to
arrive at the durability indices noted herein is known as the "Kansas State
Method."
CA 02870758 2019-10-10
WO 2013/163571 PCT/US2013/038452
Results
[00172] The results are shown below in Tables 8 and 9:
Table 8. Corn Stover Briquettes, Untreated
METHOD UNITS MOISTURE AS
FREE RECEIVED
Moisture Total ASTM E871 wt% 12.08
Ash ASTM D1102 wt% 4.13 3.63
Sulfur ASTM D4239 wt% 0.095 0.084
Gross Cal. Value at ASTM E711 Btu/lb 8017 7048
Const. (Btu/kg) (17,638) (15,506)
Chlorine ASTM D6721 mg/kg 4218 3709
Bulk Density ASTM E873 lbs/fe 44.08
(kg/m3) (706)
Fines < 0.125 in (< 0.32 TPT CH-P-06 wt% 0.57
cm)
Durability Index Kansas State PDI 97.9
Sample >1.5 in (3.8 cm) TPT CH-P-06 wt% 4
Maximum Length TPT CH-P-06 in (cm) 1.6 (4.1)
(Single Briquette)
Diameter, Range TPT CH-P-05 in (cm) 0.235-0.241
(0.597-0.612)
Diameter, Average TPT CH-P-05 in (cm) 0.239 (0.607
Bag Weight lbs (kg) 3.5 (1.6)
Table 9. Corn Stover Briquettes, AFEXTM
METHOD UNII'S MOISTURE AS
FREE RECEIVED
Moisture Total ASTM E871 wt% 7.39
Ash ASTM D1102 wt% 4.03 3.73
Sulfur ASTM D4239 wt% 0.087 0.08
Chlorine ASTM D6721 mg/kg 3484 3226
Bulk Density ASTM E873 lbs/fC 47.15
(kg/m3) (765)
Fines < 0.125 in TPT CH-P-06 wt% 0.2
(<0.32 cm)
Durability Index Kansas State PDT 97.9
Sample >1.5 in (3.8 TPT CH-P-06 wt% 3.9
cm)
Maximum Length TPT CH-P-06 in (cm) 1.85(4.7)
(Single Briquette)
Diameter, Range TPT CH-P-05 in (cm) 0.232-0.242
(0.589-0.615)
Bag Weight lbs (kg) 3.5 (1.6)
36
Conclusion
[00173] As the results in Tables 8 and 9 show, the AFEXTM briquette has an
increased
gross caloric value, i.e., an AFEXTM briquette burns about 4.8% more
efficiently due to the
presence of less moisture in the AFEXTM briquette as compared with an
untreated briquette.
Specifically, the caloric increase, nonAFEXTM to AFEXTM was calculated as
follows: 7388
Btu/lb ¨ 7048 Btu/lb = 340 Btu/lb (or 748 Btu/kg); therefore % increase, non
AFEXTM to
AFEXTM is (340 Btu/lb)/(7048 Btu/lb) * 100% = 4.8%. Additionally, bulk density
increased
by an average of seven (7)% and there is an approximately 65% reduction in the
amount of
fines (i.e., broken pieces having a diameter less than 0.125 cm) in an AFEXTM
briquette beg
weighing about 3.5 lb (1.6 kg) as compared with a briquette bag of untreated
corn stover
having approximately the same weight.
[00174] Additionally, although the "durability indices" between AFEXTM and
non-
AFEXTM briquettes are substantially the same in this testing, the method of
testing
durability was a simple tumbling experiment ("Kansas State Method"), as
compared with
the destructive testing described in the above examples. As such, insufficient
energy is
provided to create the separation required to be able to properly distinguish
between the
briquettes. Regardless, a high durability indicc shows that the AFEXTM
briquettes are
suitable for use in the briquette industry.
EXAMPLE 7
[00175] This test was performed to determine the water absorption capacity
of
pelleted AFEXTm-treated corn stover compared to non-pelletized AFEXTm-treated
corn
stover.
[00176] Conventional multi-pass, low cob corn stover was harvested and
baled by
Iowa State University (ISU) on October 23, 2011. The stover was sourced from a
field
located at the GPS coordinates of 42.21 North, -93.74 West. Following grain
harvest, the
corn stover was windrowed using a Hiniker 5600 Series side discharge
windrowing stalk
chopper, and baled using a Massey Ferguson MF2170XD large square baler. The
bales
were stored under tarps and then milled to an approximately one-inch particle
size using a
Vermeer BG 480 mill. The baled corn stover was then dried to a less than 5%
moisture
content.
[00177] Corn stover was also obtained from a blend of multiple sources,
with the
predominant source being the National Renewable Energy Laboratory as provided
by a
farm in Wray, Colorado, in 2002 as chopped corn stover. The corn stover was
dried and
37
CA 2870758 2017-10-26
then ground in a Wiley Mill (Thomas Scientific, Swedesboro, NJ) to an
approximately 5
mm particle size prior to use.
[00178] AFEXTM pretreatment was performed on the two corn stover samples
by
packing each at a density of 100 g dry matter per L into a vertical pressure
vessel
(hereinafter "vessel") having an inner diameter of 10 cm and a height of 90
cm. The
moisture level with was adjusted by adding distilled water to increase the
moisture content
to about 25%. The resulting bed of corn stover was heated by introducing
saturated steam
at 10-15 psig and a mass flow rate of 1 gram per second into the top of the
vessel and
venting at the bottom for approximately 10 minutes. The final moisture content
of the
corn stover was approximately 40%.
[00179] The bottom of the vessel was sealed while compressed anhydrous
ammonia
vapor was introduced into the top. Maximum pressure during this ammoniation
step
reached 200 psig. Ammonia was added until a ratio of 1:1 ammonia:dry corn
stover was
achieved. The temperature of the corn stover was about 80 to about100 C
initially and
gradually decreased to about 30 to about 50 C.
[00180] After a residence time of approximately 30 minutes, the pressure
was
released from the vessel by allowing vapor to flow out through the bottom. The
residual
ammonia was then removed from the corn stover by introducing steam at a mass
flow rate
of 1 gram per second into the top of the vessel while venting from the bottom.
After
approximately 20 minutes, the steam flow was stopped and the corn stover
removed from
the vessel. The AFEXTm-treated corn stover was then dried in a 50 C
convection oven
(Blue M Electric Company Class A Batch Oven, Blue Island, IL).
[00181] Pelletization was performed using a Buskirk Engineering (Ossian,
IN)
PM610 flat die pellet mill (hereinafter "pellet mill"). A die with 0.25 in
diameter circular
holes was used. Tap water was added to the AFEXTm-treated corn stover and
mixed by
hand until the desired moisture content was obtained. Three samples of corn
stover
weighing between about 3 and about 5 kg were manually added to the pellet mill
at a rate
sufficient to keep a mat of corn stover on the die. A roller then pressed the
corn stover
through the die, producing pellets. The pellets were collected and dried in
the Blue M
convection oven.
[00182] Samples Nos. 1 and 2 comprised the corn stover supplied from
Colorado,
milled to 5 mm particle size, and pelletized at 12% moisture and 50% moisture,
38
CA 2870758 2017-10-26
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
respectively. Samples Nos. 3 and 4 were the 1-inch corn stover obtained from
ISU and
pelletized at 20% moisture and not pelletized, respectively.
[00183] Samples were added to distilled water at 250 g total weight in a 500
mL
Erlenmeyer baffled flask and placed in a 50 C shake flask incubator overnight
to absorb
water and disrupt the pellet shape. The moisture content for pelletized and
loose biomass
was measured using an Haus (Parsipanny, NJ) MB25 moisture analyzer. For the
pelletized samples (Nos. 1-3), 37.5 g dry weight of the corn stover Samples
was added to
each flask, while 25 g dry weight of corn stover was added for Sample No. 4.
Distilled
water was added to each flask to increase the total weight to 250 g. After
soaking
overnight, the samples were removed and filtered through a Whatman #1
cellulose filter
via vacuum filtration.
[00184] Once all liquid was drained, the vacuum was turned off. The volume of
liquid
was then measured. The water absorption capacity was measured as the
difference
between the final volume of recovered liquid and the total volume of water
added. This
measurement allowed for the calculation of free liquid (as a percentage of the
total weight
of components) present at 15% solids in the initial stage of hydrolysis
assuming complete
mixing. The results are shown in Table 10.
Table 10: Water absorption capacity of AFEXTm-treated corn stover
Sample Type/moisture Water absorbed per g Free liquid at 15%
No. content biomass solids loading
1 Pellet/12% 4.5g 18%
3 Pellet/20% 3.9g 26%
4 Non-pelletized/5% 5.7g Trace
moisture
[00185] These results demonstrate that pelletized corn stover at varying
moisture
contents can be added to water at 15% solids loading and allow the water to
retain
between about 18 and about 26% of its total mass as liquid. The amount of free
liquid is
considerably increased in the pellet produced using 1 inch particle size corn
stover
(Sample No. 3) compared to pellets produced at the 5 mm particle size. This
may be due
to increased compression of larger particle size corn stover through the die,
which
decreases capillary volume within the corn stover and thus decreases moisture
absorption
capacity. This amount of free liquid can ensure that the solids remain in
suspension,
which will allow for even mixing for downstream processes, such as hydrolysis.
39
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
EXAMPLE 8
[00186] This testing was performed to determine the bulk density and shelf
life of
pelletized, AFEXTm-treated corn stover as well as the impact of mixing on the
initial rate
of hydrolysis.
Storability and Bulk Density
[00187] Corn stover was sourced, AFEXTm-treated, and densified in the manner
described in Example 7. In addition to the previously described pellets,
pellets were also
produced at a moisture content of 25% and 35% from the AFEXTm-treated corn
stover
obtained from Wray, Colorado, and milled through a 5 mm screen.
[00188] After pelletization, about 10 g of the pellets were placed in a sealed
plastic bag
and observed over the course of one month. In addition, pellets dried to less
than 15%
moisture content were sealed in plastic containers and also observed over the
course of
one month. Samples were considered to have sufficient shelf life if no visible
fungal
growth occurred. The remaining pellets were dried in the 50 C convection oven
described in Example 7 until a moisture content of less than 15% was obtained.
[00189] Bulk density was measured by placing the dried pellets in a 1000 mL
beaker.
The beaker was lightly shaken to ensure even settling of pellets and weighed
using a
balance with a sensitivity of 0.01 g (OHaus GT 4000). Bulk density of the
pellets was
calculated as (total weight ¨ beaker weight) * (1 ¨ moisture content)/1 L.
[00190] Pellets produced at 50% moisture content and placed in the plastic bag
began
to show signs of fungal growth after 24 hours. Within 7 days, the pellets were
completely
coated in a white fungus. Pellets produced at 35% moisture content and placed
in plastic
bags began showing fungal growth within 3 days. Within 7 days, the pellets
were
completely coated in a white fungus. In comparison, pellets produced at 12%,
20%, and
25% moisture contents did not appear to have any fungal growth occur for at
least one
month. Likewise, when pellets were dried to less than 20% moisture content,
all samples
appeared to have no fungal growth for at least one month.
[00191] Bulk density of the pellets, together with untreated loose corn stover
and
AFEXTm-treated loose corn stover as controls, are shown in FIG. 6. As FIG. 6
shows, the
bulk density of the pellets increased from 50 g/L for untreated corn stover to
nearly 600
g/L for material pelleted at 12% moisture content. Corn stover pelleted at
higher moisture
contents saw a significant decrease in bulk density, although the bulk density
was still
CA 02870758 2015-10-23
greater than for conventional bales (120 kg/m3) and the loose AFEXTm-treated
corn stover
which had a bulk density of ¨80 kg/m3.
[00192] With respect to hulk density, AFEXTm-treated corn stover pellets
can be
produced at any moisture content between 12 and 50% total weight basis, and
can be
produced at particle sizes ranging from 2 mm to 25 mm (1 inch), and maintain a
bulk
density above 200 kg/m3. It is possible that pellets can be produced at even
higher and/or
lower moisture contents. However, dryer pellets provide a higher bulk density
and longer
term storability.
Impact of Mixing on Rate of Hydrolysis
[00193] The one-inch corn stover obtained from ISU was used. In addition,
identical corn stover was obtained and AFEXTm-treated, but not pelletized.
[00194] For Samples No. 1, 2, and 3, enzymatic hydrolysis was performed at
18%
solids loading. Hydrolysis was performed in 2.8 L baffled Erlenmeyer flasks.
To each
flask, 500 mL of 0.1 M sodium citrate/ citric acid buffer (Sigma Aldrich, St.
Louis, MO)
at pH 4.5 was added. Novozymes CTec2 cellulosic enzyme and Novozymes HTec2
hemicellulosic enzyme was added to each flask at a protein level of 1260 mg
and 540 mg,
respectively (7 mg and 3 mg per g corn stover). Distilled water was added to
bring the
total weight of the solution up to 1000 g minus the weight of 180 g dry weight
of corn
stover.
[00195] For Sample No. 4, enzymatic hydrolysis was performed at 24% solid
loading. Hydrolysis was performed in a 125 mL baffled Erlenmeyer flask. To
each flask,
25 mL of 0.1 M sodium citrate/ citric acid buffer (Sigma Aldrich, St. Louis,
MO) at a pH
of 4.5 was added. Novozymes CTec2 cellulosic enzyme and Novozymes HTec2
hemicellulosic enzyme was added to each flask at a protein level of 84 mg and
36 mg,
respectively (7 mg and 3 mg per g corn stover). Distilled water was added to
bring the
total weight of the solution up to 50 g minus the weight of 12 g dry weight of
corn stover.
[00196] In Sample No. 1, unpelletized AFEXTm-treated corn stover was added
in a
fed batch manner, with half (90 g dry weight) of the material added at the
beginning of
hydrolysis and half (90 g dry weight) added after 3 hours. In Sample No. 2,
unpelletized
AFEXTm-treated corn stover was all added immediately (180 g dry weight). In
Sample
No. 3, pelletized AFEXT"Ltreated corn stover was all added immediately (180 g
dry
weight). In Sample No. 4, pelletized AFEXTM-treated corn stover was added in a
fed
41
batch manner, with half (6 g dry weight) added at the beginning of hydrolysis
and half (6
g dry weight) after 3 hours. After the first biomass addition, the flasks were
placed in a
shake flask incubator at 50 C and rotated at 200 RPM. The samples were
inspected
visually every hour and manually swirled to determine the flowability of the
liquid
medium and the ability to suspend biomass particulates.
[00197] A 1 mL sample was obtained at 6 hours and 24 hours after enzyme
addition
and analyzed for sugar production via HPLC. A Biorad (Hercules, CA) Aminex HPX
87P
column was used to separate individual sugars at a flow rate of 0.6 mL/min and
with the
column heated at 85 C. A Waters 2414 refractive index detector (Milford, MA)
was used
to quantify the sugars.
[00198] A visual representation of an exemplary hydrolysis that can be
performed
according to the various embodiments described herein, such as the hydrolysis
performed
in this example, is shown in FIGS. 7A-7H. Hydrolysis of hydrolysable densified
particulates 706 (e.g., Sample No. 3) is shown in FIGS. 7A-7D. The hydrolysis
begins at
0 hrs, as shown in FIG. 7A with a number of hydrolysable densified
particulates 706
placed in a container 702 with an amount of liquid, such as water, having a
water line
704A. Within 0.5 hours, as shown in FIG. 7B, a suspension 708A is formed
containing
particles 709, with no hydrolysable densified particulates 706 visible above
the water line
704A. The particles remain in suspension throughout the first 6 hours of
hydrolysis and
beyond, as shown in FIGS. 7C and 7D. If desired, additional hydrolysable
densified
particulates 706 can optionally be added at the 3 hr point to increase the
solid loading
further (e.g., Sample No. 4), as shown in FIG. 7C.
[00199] In contrast, during a conventional hydrolysis of loose biomass
fibers (e.g.,
Sample No. 2), as shown in FIGS. 7E-7H, the loose biomass fibers and liquid,
such as
water, immediately combine to form wet loose biomass fibers 710 as shown in
FIG. 7E,
with no mixing occurring, even at the 0.5 hr point, as shown in FIG. 7F. By
the 3 hr point
as shown in FIG. 7G, a water line 704B is visible for the first time. For a
comparable
amount of starting materials, this water line 704B is lower than the water
line 704A shown
in FIGS. 7A-7D, i.e., when hydrolysable densified particulates 706 are used as
the
substrate.
[00200] Despite the eventual presence of free water as shown in FIG. 7G,
the
suspension 708B containing particles 709 is impeded by the presence of the
unmixed wet
42
CA 2870758 2017-10-26
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
loose biomass fibers 710 present both above and below the water line 704B. At
the 6 hr
point, however, as shown in FIG. 7H, the wet loose biomass fibers 710 have
become
sufficiently hydrolyzed such that all solids (710) have now been converted to
particles 709
which remain in the suspension 708B, comparable to HG. 7D, although the sugar
concentration in the suspension 708B is lower.
[00201] As these schematics demonstrate, not only does hydrolysis occur faster
initially
with the hydrolysable densified particulates 706, but additional hydrolysable
densified
particulates 706 can optionally be added after a relatively short time period,
such as no
more than about half-way through a hydrolysis cycle, i.e., a higher solids
loading is
possible, such that the resulting suspension 708A of FIG. 7D has a higher
sugar
concentration as compared to the sugar concentration of suspension 708B of
FIG. 7H.
[00202] Table 11 displays visual observations of the dissolution of biomass
during the
first 6 hours after enzyme addition for Samples No. 1, 2, and 3.
Table 11: Observations on mixing ability during the first 6 hours of enzymatic
hydrolysis for pelleted and non-pelleted AFEXTm-treated corn stover.
Time Sample 1 Sample 2 Sample 3
0 hour Standing water Very large pile of Pellets completely
observed, but pile of biomass, no standing submerged
biomass was not free water was observed.
floating Biomass could not be
mixed.
1 hour Slurry was very thick No visible change in
Mixture of pellets and
and difficult to mix, appearance. No free biomass. Easily
but all biomass was standing water swirled and not viscous
submerged in water observed
2 hour Material is easily Small amount of Pellets completely
mixed, but more standing water disrupted. Easily
viscous than with observed. Biomass swirled and not viscous
pellets. All biomass pile shrank slightly in
submerged. size as particles are
liquefied. Cannot be
mixed by swirling.
3 hour Easily mixed before Biomass pile continues No visible change
second addition of to shrink in size.
biomass. After Small amount of
biomass addition, some standing water
biomass was above the observed. Mixing is
water line. Very thick not possible by
and not flowable when swirling.
swirled.
4 hour All biomass was below Most of the biomass is No visible change
43
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
the water line, but still now below the water
thick. Reasonably line, which is now at
flowable and can be the same height as the
mixed by swirling, other two samples.
Still very viscous and
difficult to swirl.
hour Hydrolysate is in All biomass is below No visible change
suspension and easily the water line. Sample
mixed. can be mixed by
swirling, but still
viscous compared to
the fed batch sample
6 hour No visible change. Hydrolysate is in No visible change.
Glucose concentration suspension and easily Glucose concentration
is 36.7 g/L mixed. Glucose is 40.7 g/L.
concentration is 31.1
g/L.
24 hours Easily mixable. Easily mixable. Easily mixable.
Glucose concentration Glucose concentration Glucose concentration
is 49.5 g/L is 46.4 g/L is 54.4 g/L
[00203] These results show that use of densified corn stover significantly
improves the
initial phase of hydrolysis. The glucose released in the first six hours was
31% higher
than for the loose biomass without fed batch addition and 11% higher than the
loose
biomass with fed batch addition. The improved hydrolysis performance continued
through 24 hours. Furthermore, the pellet hydrolysate remained at a low
apparent
viscosity and was easily mixed throughout the first 6 hours, suggesting that a
standard
impeller could keep the biomass in suspension. Because the biomass was able to
easily
stay in suspension, the solid loading could easily be increased. In Sample No.
4, the
biomass stayed in suspension and was easily mixed throughout the first 6 hours
despite the
increased solid loading. A glucose concentration of 71 g/L was obtained after
24 hours, a
30% increase over pellets at 18% solid loading.
[00204] In comparison, the fed batch hydrolysis was not easily mixable in the
first hour
of hydrolysis as well as the first hour after a second addition of enzymes.
The loose
biomass without fed batch addition remained unmixable for up to 5 hours.
EXAMPLE 9
[00205] This test was performed to deteimine if an 18% solid loading
hydrolysis with
AFEXTm-treated corn stover pellets could be performed in a vertical stirred
tank reactor
with impeller size to tank diameter ratio of 1:3.
44
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
[00206] Corn stover was AFEXTm-treated and pelletized in the manner described
for
Sample No. 1 in Example 7. A glass 6 liter Microferm reactor (New Brunswick
Scientific, Enfield, CT) equipped with a six bladed Rushton impeller and a
three blade
marine impeller was used. The impeller diameter was about 7.5 cm and the tank
inner
diameter was about 21.5 cm, for an impeller size to tank diameter ratio of
0.35, or about
1:3. Four evenly spaced vertical baffles were also present in the reactor.
Distilled water
and enzymes were added to a total weight of 4.60 kg. Enzymes used were
Novozymes
CTec2 at 7,000 mg and HTec2 at 3,000 mg. Approximately 1 kg dry weight of
pellets
was added to the solution. Temperature was maintained at 50 C and pH was
manually
adjusted to 5 using 4 M NaOH (Sigma Aldrich, St. Louis, MO). The impellers
were spun
at 400 rpm. Visual observations were recorded throughout the first 30 minutes
of
hydrolysis, and 20 mL samples were obtained at 1, 4, and 6 hours after the
addition of
pellets. These samples were quantified for sugar analysis according to the
previous
example.
[00207] After 48 hours of hydrolysis, the hydrolysate broth was centrifuged to
remove
the biomass particulates. The supernatant was then fermented using Zymomonas
mobilis
AX101 as the fermenting organism. The pH was adjusted to 6 and the temperature
decreased to 30 C. Z mobilis was grown on yeast extract and added to the
hydrolysate at
an initial OD at 600 nm of 1. Corn steep liquor at 1% (v/v) loading and
potassium
phosphate at 2 g/L were also added as nutrients. Samples were taken at 24
hours after
inoculation to assess ethanol production and sugar utilization. Samples were
analyzed for
ethanol production and sugar consumption via HPLC as described in Example 8.
For
ethanol production, a BioRad Aminex 8711 column was used instead of Aminex
87P.
[00208] The corn stover pellets were immediately suspended when agitation was
initiated, and rapidly broke down to individual particulates within 10
minutes. As the
pellets were disrupted, a layer of corn stover was deposited along the surface
of the vessel.
This layer appeared to be thin and not pennanent, as sections were continually
breaking
off and re-entering suspension. Within 20 minutes, all of the corn stover was
suspended
and remained suspended for the 48 hour duration of hydrolysis. Glucose
concentration
was 21.9 g/L, 34.2 g/L, and 44.1 g/L after 1, 4, and 6 hours, consistent with
the
performance in shake flasks.
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
[00209] Glucose and xylose titer were 51.6 g/L and 24.3 g/L at the onset of
fermentation. After 24 hours, glucose was completely consumed, and xylose was
partially
consumed to a final concentration of 13.1 g/L. This partial consumption is
common for
fermentation of ALEXTm-treated corn stover with this microbe, see Lau MW et
al.,
Biotechnology for Biofuels 3:11(2010) as an example. Final ethanol
concentration was
32.3 g/L.
[00210] As demonstrated, enzymatic hydrolysis and fermentation can be
performed at
levels as high as 18% solids loading, while still achieving final ethanol
concentrations in
excess of 30 g/L. An impeller size to tank diameter ratio of about 1:3 was
sufficient to
keep the solids in suspension and allow even mixing. It is likely that even
higher solids
loading can be used, although further testing will be performed to confirm
this hypothesis.
EXAMPLE 10
[00211] In this test, pellets produced at different moisture contents were
hydrolyzed at
high solids to determine its impact on resulting glucose yields.
[00212] Corn stover was obtained from multiple sources but predominantly Wray,
CO,
as described in Example 7. This corn stover was milled to a 5 mm particle
size, AFEXTm-
treated, and pelleted as described in Example 7. Pellets were produced at 12%
moisture.
25% moisture, 35% moisture, and 50% moisture content. Enzymatic hydrolysis was
performed at 18% solid loading in 250 mL Erlenmeyer flasks at 100 g total
weight.
Eighteen grams (dry weight) of pellets were added to each flask, with water
added in an
amount to result in a total weight of 100 g for all components added.
[00213] Tetracycline and cycloheximide were added at final concentrations
of 20 mg/L
and 15 mg/L, respectively, to control fungal contamination. A citrate buffer
was used to
control pH as described in Example 8. Novozymes CTec2 and HTec2 enzyme were
added
at a protein loading of 7 mg and 3 mg per g pellet, respectively. After enzyme
addition,
the flasks were sealed and placed in a shake flask incubator set at 50 C and
200 rpm
rotation. A 1 mL sample was obtained at 1, 6, 24, 48, and 72 hours after
enzyme was
added and analyzed for sugar content as described in Example 9. The results
are shown in
FIG. 8. (Note that the line for 50% moisture is shifted 0.5 hours to the left
for clarity.
[00214] As FIG. 8 shows, a glucose concentration above 60 g/L was obtained for
all
AFEXTm-treated corn stover pellets within 48 hours. This concentration is
sufficient for
effective fermentation to ethanol or other value added products. The pellets
also
46
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
hydrolyze at a rapid rate, producing over 50% of the total sugars within the
first 6 hours.
Pellets produced at a higher moisture content tended to have greater sugar
yields than
pellets produced at low moisture content. However, the pellets produced at 50%
moisture
did not appreciably release more glucose than pellets produced at 35%
moisture.
[00215] As demonstrated, AFEXTm-treated biomass can be pelletized over a wide
range
of moisture contents and still be viable as a feedstock for fermentable sugar
production.
Depending on the economics and desires of the customers it may be possible to
customize
moisture content to provide a suitable combination of storability versus sugar
concentration for any number of applications.
EXAMPLE 11 (PROPHETIC)
[00216] Samples of biomass, such as switchgrass and prairie cord grass will be
collected at various maturities, and corn stover will be collected following
grain harvest.
Biomass composition will be determined at harvest, during storage in round
bales, after
initial AFEXTM processing and densification, and after storage of densified
pellets.
AFEXTm pretreatment will be statistically optimized for hydrolysis and binding
properties
based on parameters of time, temperature, biomass moisture, and ammonia to
biomass
ratio. AFEXTM conditions providing at least 90% of glucan conversion and 80%
xylan
conversion will be used to prepare materials for densification.
[00217] Densification will be performed using any suitable method, including
the
methods used in Examples 2, 3, or 8.
[00218] The resulting pellets will be subjected to various environmental
conditions to
simulate long-term storage, and then evaluated for flowability, compression
strength, etc.
Downstream processing characteristics will be evaluated using a standardized
set of
hydrolysis and fermentation conditions, including separate hydrolysis and
fermentation
(SHF) vs. simultaneous saccharification and fermentation (SSF). In one
embodiment a
comparison of these properties will be made between freshly prepared pellets
(i.e., within
about one (1) month), stored pellets and non-densified biomass.
EXAMPLE 12 (PROPHETIC)
[00219] AFEXTM pretreatment of prairie cord grass will be statistically
optimized for
time, temperature, biomass moisture, and ammonia to biomass ratio. A fairly
broad range
of AFEXTm pretreatment conditions gives similar hydrolysis results, giving us
confidence
that there are sets of pretreatment conditions that also enhance binding
properties.
47
CA 02870758 2019-10-10
WO 2013/163571
PCT/US2013/038452
AFEXTm pretreatment conditions providing at least 90% of glucan conversion and
80%
xylan conversion will be identified and used to prepare materials for
densification. We
will characterize these pretreated materials for surface properties using
various methods
developed in our lab (ESCA, Prussian blue staining, SEM), and will correlate
those
properties with the pellet density and durability.
EXAMPLE 13 (PROPHETIC)
[00220] Operating variables will be investigated to optimize operating
conditions for
converting pretreated biomass into densified pellets. These variables include
AFEXTM
pretreatment conditions, moisture content, particle size, die temperature
versus bond
strength, rate of compaction versus quality of output, energy usage, existing
surface
chemistry and variations, compaction ratios and resultant density, and
compacted package
size and shape. Attrition and wear of mechanical components will also be
assessed.
EXAMPLE 14 (PROPHETIC)
[00221] Biomass pretreated using any known AFEXTM procedure or according to
the
procedure in Example 1 or with any other appropriate modification of an AFEXTM
procedure will be densified using any suitable method, including the methods
described in
Examples 2 and 3.
[00222] The densified biomass will then be subjected to various environmental
conditions, including temperature (25 to 40 C), relative humidity (60 to 90%),
consolidation stress (0 to 120 kPa), and storage time (0 to 6 mo). Following
storage,
physical characteristics will be evaluated as described below:
[00223] Flowability may be evaluated with a simple test in which a number of
AFEXTm-pellets are placed in a container, such as the bed of a truck and
tipped to about
45 degrees. A comparison with conventional pellets may be made by noting the
time it
takes for the pellets to flow out of the container.
[00224] Flowability will also be evaluated using Can Indices. See ASTM D6393.
1999, Standard test method for bulk solids characterization by Can indices,
ASTM
Standards, W. Conshohocken. PA. Flowability is comprehensively defined as the
ability
of a material to flow un-abruptly under a given environmental condition. The
flowability
measurement is most often done by Carr Indices, by calculating the total
flowability index
and total floodability index. Can, R. L. Jr. 1965, Evaluating flow properties
of solids,
Chemical Engineering 72(3): 163-168.
48
[00225] A higher value to total flowability index and lower value to total
floodability index will yield an ideal material with low or no flow problems.
Another way
to quantify flowability is by measuring the Jenike Shear Stress properties.
See Jenike, A.
W. 1964, Storage and flow of Bulletin No. 123, Utah Engineering station,
Bulletin of
University of Utah. Jenike's method will also be used to determine particle
cohesion,
yield locus, angle of internal friction, yield strength, and flow function,
and particle size
distribution. See ASTM D6128. 2000, Standard Test Method for Shear Testing of
Bulk
Solids Using the Jenike Shear Cell, ASTM Standards, W. Conshohocken. PA, and
ASAE
S19.3. 2003, Method of determining and expressing fineness of feed materials
by sieving,
ASAE Standards. St Joseph, MI: ASABE.
[00226] Additionally, glucan, xylan, galactan, arabinan, mannan, lignin,
ash and
fiber levels will be evaluated to determine their effect on storage and
flowability behavior.
Furthermore, several other physical properties will be measured as indicators
of poor
flowability (i.e., particle size, particle shape, thermal properties, moisture
properties, and
color). See Selig, M, et al., 2008, Enzymatic saccharification of
lignocellulosic biomass,
Technical report NRELITP-510-42629; Sluiter, A, B. Hames, R. Ruiz, C.Scarlata,
J.
Sluiter, and D. Templeton, 2008a, Determination of ash in biomass, Technical
report
NRELITP-510-42622; Sluiter, A, B. Hames, R. Ruiz, C.Scarlata, J. Sluiter, D.
Templeton,
and D. Crocker. 2008b, Determination of structural carbohydrates and lignin in
biomass,
Technical report NRELITP-510-42618.
[00227] Rheological material properties that affect the ability of biomass
to he
handled pre- and post-densification will be established. Such properties
include, but arc
not limited to, bulk density, true density, compressibility, relaxation,
springback,
permeability, unconfined yield strength, and frictional qualities. These
properties are a
function of the feedstock particle size and distribution, shape factor,
moisture condition,
and consolidation pressure and time. Since commercial rheological testers are
typically
designed for use with small grains and fine powders; and consequently, do not
accommodate particulate that is greater than 1/4 inch in diameter, we will
develop new
measurement systems for characterizing larger feedstock particles. Systems
include
compaction and shear cells that can be scaled for various material sizes,
integrated with
commercial load frames, and operated over a range of consolidation pressures.
49
CA 2870758 2017-10-26
[00228] Data will be analyzed to determine conditions which lead to
improved (or
optimized) flowability, using formal statistical methods such as general
linear models,
regression, response surface analysis, multivariate analysis, and other
techniques as
appropriate. See Myers, H. R. 1986, Classical and modern regression
applications, 2n1
edition. Duxbury publications, CA. USA. Draper, N. R., and Smith, H. 1998,
Applied
Regression Analysis, New York, NY: John Wiley and Sons, Inc.
EXAMPLE 15 (PROPHETIC)
[00229] At least three types of biomass will be evaluated, namely corn
stover,
switchgrass, and prairie cord grass. For each of these feedstocks, samples of
raw ground
biomass, AFEXTm-pretreated biomass, and AFEXTm-pretreated and densified
biomass
(before and after storage) will be collected. Thus, 3 x 4 = 12 total biomass
sample types
will be evaluated. Separate hydrolysis and fermentation (SHF) will be
evaluated. For
saccharification, flasks will be incubated for 48 h at 50 C and 250 rpm in an
orbital
shaker. Samples will be removed at 0, 2, 4, 6, 8, 18, 24, 30, 36, and 48 hr.
Flasks will then
be cooled to 30 C and inoculated with 2 ml of a 12-18 hr culture of a
recombinant strain
of Saccharomyces cerevisiae which possesses pentose-fermenting capabilities
grown in a
medium containing two (2) g/I glucose and two (2) g/1 yeast extract. Flasks
will be
incubated for an additional 96 h at 30 C and 150 rpm in an orbital shaker.
Samples will
be removed at 0, 3, 6, 9, 18, 24, 36, 48, 60, 72, 84, and 96 hr during
fermentation.
[00230] Simultaneous saccharification and fermentation (SSF) will also be
performed to evaluate conversion. The main difference will be that flasks will
be dosed
with enzyme and immediately inoculated with yeast as noted above, then
incubated for
144 hr at 30 C. Samples will be removed at 0, 2, 4, 6, 8, 18, 24, 36, 48, 60,
72, 96, 120,
and 144 hr. Enzyme and biomass loadings and other conditions will be identical
to those
listed above.
[00231] Novel densified biomass products and methods for making and using
same
are described herein. In one embodiment, a conventional pretreatment is used
to produce a
tacky biomass which, surprisingly, is easily convertible to a solid
hydrolysable particulate
without the use of added binder. The hydrolysable particulates are also
surprisingly at
least as dense and demonstrate superior hardness properties as compared with
conventional densified particulates produced with and/or containing added
binder(s).
In one embodiment, hydrolysable particulates comprising more than one type of
biomass
material (e.g., corn stover, grasses, and/or wood, and the like) are provided.
In
CA 2870758 2017-10-26
CA 02870758 2015-10-23
this way, a commodity hydrolysable solid biomass product having relatively
uniform
properties is provided which may be more easily adopted into the biomass
processing
industry. Such properties may include, but are not limited to, BTU content,
sugar content,
and so forth.
[00233] Any suitable type of densification process may be used to produce
products
having a variety of sizes and shapes. In one embodiment, the densification
process device
uses a gear mesh system to compress biomass through a tapering channel between
adjacent gear teeth, forming high density hydrolysable particulates. In one
embodiment,
the system operates at lower temperature, pressure, and energy requirements
than
conventional processes.
[00234] In one embodiment, the pretreated hydrolysable particulates "hold
up"
better, i.e., are more resistant to physical forces, during shipping, handling
and/or storing
as compared to particulates which are not pretreated. In one embodiment, the
resulting
products have an increased flowability as compared with conventional biomass
solids,
which allow for automated loading and unloading of transport vehicles and
storage
systems, as well as transport through the processing facility.
[00235] Although specific embodiments have been illustrated and described
herein,
it will be appreciated by those of ordinary skill in the art that any
procedure that is
calculated to achieve the same purpose may be substituted for the specific
embodiments
shown. For example, although the process has been discussed using particular
types of
plant biomass, any type of plant biomass or other types of biomass or
biofuels, such as
agricultural biofuels, for example, may he used. This application is intended
to cover any
adaptations or variations of the present subject matter. Therefore, it is
manifestly intended
that embodiments of this invention be limited only by the claims and the
equivalents
thereof.
51