Note: Descriptions are shown in the official language in which they were submitted.
CA 02871003 2014-10-20
5'FIC11 3088FC - 1 - ,
Intrauterine application of 18-methyl-15ss,16ss-methylene-19-nor-20-spirox-4-
en-3-
one systems, intrauterine systems containing 18-methyl-15ss,16ss-methylene-19-
nor-
20-spirox-4-en-3-one, as well as the use thereof in contraception and
gynaecological
therapy
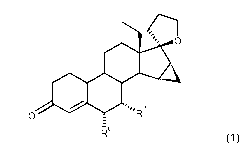
[0001] The present invention relates to the subject matter characterized in
the patent
claims, i.e. the intrauterine use of 18-methyl-1511,1611-methylene-19-nor-20-
spirox-4-en-
3-ones of the formula (I), and to an intrauterine system comprising 18-methyl-
1511,1611-
methylene-19-nor-20-spirox-4-en-3-ones of the general formula I,
/i¨-
0 . R
=,
R-
formula (1)
wherein R6 and R7 may be a hydrogen atom or together may be an a-methylene
group.
[0002] The invention therefore relates to the intrauterine use of 18-
methyl-1511,1611-
methylene-19-nor-20-spirox-4-en-3-one (compound A) or 18-methyl-
6a,7a,1511,1611-
dimethylene-19-nor-20-spirox-4-en-3-one (cornpound B)
0 ...
0
O.A i6011A
00 OW
0 0
(A) (B)
and to an intrauterine system (IUS) comprising one of the said compounds.
[0003] The invention further relates to the use of an !US comprising the
substance (A)
or (B) in contraception and in gynaecological treatment.
CA 02871003 2014-10-20
BHC113088FC
= [0004] Gynaecological treatment means, for example, treatment of
endometriosls,
endometrial hyperplasia, inflammation of the endometriurn (endometritis),
uterine-based
pain and dysmenorrhoea, except use in the treatment of menorrhagia [also known
as
hypermenorrhoea or heavy menstrual bleeding (HMB)] and other forms of uterine
bleeding disorders.
[0005] The invention also relates to the use of 18-methyl-153,16f3-methylene-
19-nor-
20-spirox-4-en-3-one (compound A) in contraception and gynaecological
treatment.
[0006] The progestins employable according to the invention, 18-
methyl-1511,1611-
methylene-19-nor-20-spirox-4-en-3-one (A) or 18-methyl-6a,7a,15R,1611-
dimethylene-
19-nor-20-spirox-4-en-3-one (B), and preparation thereof, are described in
WO 2008/000521, with the former compound (A) being disclosed there only as
intermediate.
[0007] Compound B and further substances described in WO 2008/000521, are used
in pharmaceutical preparations for contraception and in the therapeutical
treatment of
premenstrual complaints such as headaches, depressive moods , water retention
and
mastodynia. W02008/000521 discloses in addition to oral and transdermal dosage
forms also parenteral oily injection solutions. However, WO 2008/000521 does
not
describe an intrauterine use, nor the compounds being employed in an
intrauterine
system (IUS).
[0008] Approaches with hormone-based contraceptives are widely accepted by
users
owing to easy application and high contraceptive reliability. Among them, oral
contraceptives (pill) are the most frequently used contraceptive method of all
in many
countries. Nevertheless, there is also time and again a critical discussion of
hormone-
containing, more specifically oestrogen-containing, contraceptives taking
place both
publicly and in the literature, owing to potential risks (such as a slightly
increased risk of
thrombosis, loss of libido, nausea, and headache)1.
[0009] A promising new method of contraception involves the intrauterine
administration of hormones by means of an appropriate intrauterine system. At
the
forefront is Mirene, a levonorgestrel-containing intrauterine system (IUS)
which
continuously releases the active ingredient over a period of up to five years.
The product
is used in contraception and in the treatment of increased menstrual bleeding
1 Biter et al. Contraception 84 (2011) 342-356
CA 02871003 2014-10-20
B1-1C113a88FC 73-
(menorrhagia or hypermenorrhoea). This product is described in, inter alia,
EP 0652738 B1 and EP 0652737 B1.
[0010] The basis of the contraceptive effect of Mirena is essentially the
thickening of
the cervical mucus and the local action of levonorgestrel which results in a
strong anti-
proliferative effect on the endometrium. Furthermore, levonorgestrel alters
the utero-
tubal environment and impairs the motility and function of sperm.
[0011] Although the contraceptive effect of Mirena is mainly a result of a
local effect,
the comparatively high systemic stability of levonorgestrel (active ingredient
in Mirena )
means that Mirena also exhibits plasma levels of active ingredient of on
average about
206 pg/m12. Although this value is below that of orally administered
levonorgestrel-
containing contraceptives, it is still high enough for it to inhibit ovulation
in about 20% of
users in the first year of use and for it to be able to cause the known
systemic adverse
events, for example acne, depressed moods, chest pain or reduced libido3.
[0012] Some first-time users also have a problem with irregular bleeding
patterns in the
initial phase, i.e. immediately after inserting the IUS (so called
"spotting"). This spotting
may last for up to a few months before either no or only very small and
infrequent
haemorrhages occur4.
[0013] Benign ovarian cysts are described as a further common adverse event of
Mirene5.
[0014] Besides the aforementioned hormonal (contraceptive) methods, there is a
broad range of non-hormone-based approaches and products, such as natural
contraceptive methods (e.g. hormone level measurement, or temperature method),
mechanical methods (e.g. condom or diaphragm) or chemical methods (e.g.
spermicides). Unfortunately, none of the alternative methods (apart from
irreversible
sterilization) provides contraception with close to the same level of
reliability as achieved
by hormone-based methods.
[0015] It is therefore an object of the present invention to provide a
contraceptive
method which not only provides a comparatively high level of reliability in
contraception
2 See information sheet Mirena March 2011 ¨ DE/9
3 Lahteenmaki P. et al. Steroids 2000 65: 693-697
4 Suvisaari J, Lahteenmaki P.-Contraception 1996 Oct; 54(4): 207-8
5 Product monograph ¨ Mirena 8th edition August 2009; Finland: Schering AG and
Leiras Oy,
CA 02871003 2014-10-20
BHC113088FC -,4-
as achieved by the known hormone-based contraceptive methods, but also
exhibits
even better compatibility.
[00161 Another object of the invention is to achieve a regular bleeding
pattern, i.e. less
spotting, more quickly6.
[0017] The object is achieved according to the invention by the intrauterine
use of
compounds of formula (I)
P.A
OW'', 7
'R
formula (1)
wherein R6 and R7 are a hydrogen atom or together are an a-methylene group,
namely by the intrauterine use of 18-methyl-1511,1611-methylene-19-nor-20-
spirox-4-en-
3-one or 18-methy1-6a,7a,1511,1611-dimethylene-19-nor-20-spirox-4-en-3-one.
[0018] Surprisingly in the intrauterine use of 18-methy1-1511,1611-methylene-
19-nor-20-
spirox-4-en-3-one or 18-methy1-6a,7a,1511,1611-bis-methylene-19-nor-20-spirox-
4-en-3-
one in rats, we were able to demonstrate a differentiating action between
local (uterus)
and systemic (peripheral tissue) effects.
[0019] This effect was demonstrated by comparing the local effects in the
uterus
(weight increase, see Example 1; Fig. 1/8 and 2/8) and by the systemic effect
such as,
for example, lowering of the LH level in ovary-resected rats (Fig. 3/8 and
4/8).
[0020] Compared with LNG, the substances also have increased local
potency, as
shown by the strong induction of corresponding marker genes in the gene
expression
experiment. Thus, the anti-oestogenic effect of gestagens on the uterus is
mediated inter
alia by 1GFBP-1. Fig. 5/8 and 6/8 shows that1GFBP-1 gene expression is induced
by
compound A, even at a rate of release from the IUS that is approx. 7x lower
than with
levonorgestrel.
6 Andersson et al. Contraception 1994, 49:56-71
CA 02871003 2014-10-20
BHC113088FC 75-
[0021] The marked strong dissociation of local vs. systemic and the high
gestagenic
efficacy of the substances are sufficient for causing a contraceptive action
only due to
the local effects. Systemically caused side effects, such as those occurring
with the use
of other gestagens, may thus be prevented or at least greatly reduced. Owing
to the
possible higher local gestagen concentration, a more rapidly commencing and
better
bleeding control can also be expected.
[0022] As furthermore demonstrated in comparative transactivation
studies (see
Example 2), the substances employed according to the invention have an
androgenic
effect that is at least 10 times lower compared to LNG. This property, still
enhanced by
the marked dissociation of local vs. systemic, shows that, even with local
uterine uses of
very high doses in comparison with levonorgestrel, no systemic androgenic
effects (e.g.
acne) are expected, even if systemic concentrations comparable to
levonorgestrel with
Mirena uses were present.
[0023] Owing to the properties mentioned of substances A and B, the
latter are very
well suited to intrauterine use in gynaecological treatment, but in particular
also in
contraception. Preference is given here to an intrauterine administration by
means of an
intrauterine system.
[0024] An intrauterine system which may be utilized is a polymer system,
as is
employed, for example, with Mirena .
[0025] A person skilled in the art is familiar with the preparation of an IUS
which is
carried out as described, for example in EP 0 652 738
[0026] Thus the active ingredient (A) or (B) is first made with a polymeric
support
material into a central rod (core). The active ingredient may be admixed with
the
polymeric support material, for example polydimethylsiloxane (PDMS), at any
ratio.
[0027] After the shaping process, i.e. after vulcanization, the core prepared
in this way
is normally surrounded in a second step by a polymer-based membrane which
ensures
uniform dosing over a long period. The desired release rate can be controlled
via the
choice of polymer and via the thickness of the membrane.
[0028] Suitable polymers for the membrane are in principle the same
polymers as
those for the core (the central rod). Mention must be made here, for example,
of
CA 02871003 2014-10-20
BHC113088FC 76-
polydimethylsiloxane which may optionally be fluorinated, or else other
mixtures of
polymers. Membrane thickness is preferably around 0.5 mm.
[0029] The membrane is applied by firstly swelling a tubing (membrane)
prepared
from the desired polymer in a solvent and then pressing the core containing
the active
ingredient into the still swollen tubing. The ends of the tubing are then
preferably also
sealed by a stopper, preferably consisting of the same material as the
tubing/membrane,
in order to counteract "bleeding" of the active ingredient at the ends of the
tubing, which
may result in a "burst effect" during use. The tubing may also be bonded with
silicone in
place of the stoppers.
[0030] Systems releasing a daily dose in the range of 1 ¨ 500 pg of the
particular
active ingredient (A) or (B) may be employed according to the invention.
[0031] The release rate of active ingredient (A) may be chosen here to be
half of that
of active ingredient (B), owing to the higher efficacy of the former.
[0032] Thus, the resulting preferred dose range for the active ingredient (A)
is 1-
200 pg/day, with particular preference being given to 1-100 pg/day in
particular 2-
50 pg/day. The preferred dose range of active ingredient (B) is 2-500 pg/day,
with
particular preference being given to 2-200 pg/day, in particular 5-100 pg/day.
[0033] The invention therefore also relates to an intrauterine system
comprising the
active ingredient (A) or (B), and to the use of the intrauterine system in
contraception.
[0034] The examples below serve to illustrate the invention.
[0035] The progestins employable according to the invention, 18-methyl-
1511,16II-
methylene-19-nor-20-spirox-4-en-3-one (compound A) or 18-methyl-
6a,7a,1511,1611-
dimethylene-19-nor-20-spirox-4-en-3-one (compound B), are prepared as
described in
WO 2008/000521 (compound A: example 14 f; compound B: example 2).
[0036] The process of preparing the active ingredient-charged rods used in the
rat
experiment described below was carried out similarly to the process of
preparing the
active ingredient reservoirs, as described for an IUS usable in humans, for
example (see
for example EP 0 652 738 B1). Polymers which may be used for preparing the rod
are
polysiloxanes and modified polysiloxane polymers (see for example EP 0652738
B1,
WO 00/29464 and WO 00/00550).
CA 02871003 2014-10-20
BHC113088FC -7-
[0037] Specifically, first an active ingredient-charged core was prepared by
vulcanizing a mixture of polyethylene oxide block-polydimethylsiloxane
copolymer (PEO
-b-PDMS), polydimethylsiloxane and 10 per cent by weight of the active
ingredient (in
this case the particular progestin A or B), using a Pt (0)-
divinyltetramethyldisiloxane
catalyst.
It is also possible to use polydimethylsiloxane (PDMS) rather than PEO-b-PDMS,
with
bis(2,4-dichlorobenzoyl) peroxide having been used here as the vulcanization
catalyst.
[0038] To prepare the active ingredient-containing core, a vertical piston
unit with a
corresponding nozzle head was used. The dimensions of the nozzle head were
such
that the outer diameter of the active ingredient-containing core is about 1
mm.
[0039] The active ingredient-containing core prepared in this way is then
coated with a
membrane consisting of PDMS, polytrifluoropropylmethylsiloxanes (PTFPMS) or a
PTFPMS / PDMS elastomeric mixture (75% PTFPMS, 25% PDMS). The inner diameter
of the membrane material was ¨ 1 mm, with an outer diameter of ¨1.5 mm.
[0040] The coating was carried out by cutting the core and the membrane
to a length
of 10-15 mm, with the membrane being slightly longer (respectively approx. 1
mm at
either end) than the core, in order to enable the ends of the membrane to be
sealed with
a small stopper after the core has been inserted. In order to enable the core
to be
inserted into the membrane, the latter was first made to swell in a
cyclohexane or
acetone-hexane mixture. The active ingredient-containing core was then pushed
into the
swollen membrane. Finally, the ends of the tubing were either bonded with
silicone or
sealed with a small stopper made of PTFPMS.
[0041] Example 1
The local uterine action of the progestin compared to systemic side effects
(dissociation)
was investigated on the basis of studies using rats. The uterus of ovary-
resected rats
responds to implantation of progestin-containing IUS (rods) with
decidualization and
weight gain. The local progestin effects were also determined on the basis of
changes in
gene expression.
CA 02871003 2014-10-20
BHC113088FC
[0042] Serum levels of luteinizing hormone (LH) are used for detecting
systemic
effects of the locally administered progestin. Basal serum-LH levels of ovary-
resected
rats are elevated compared to the levels of intact control animals. Undesired
systemic
efficacy of the uterine-administered progestin can be detected by a decrease
in the LH
level.
[0043] Method:
Ovary-resected female rats were treated with estradiol (E2) for three days
(0.2 pg/day/animal, subcutaneous dosing). On day 4, an IUS (rod) was implanted
into
the right uterine horn of each animal. The left uterine horn remained
untreated for
internal comparison. Administration of E2 was continued with a daily dose of
0.1 pg/animal to ensure responsiveness of the uterus (maintaining progesterone-
receptor expression) to progestins. Blood was taken for LH level measurements
on days
4, 10 and 17.
[0044] Performing the gene expression analyses:
The uterine tissue was homogenized in 800 pl of RLT lysis buffer (Qiagen,
Hilden,
Germany; #79216) using a Precellys24 homogenizer (Peqlab, Erlangen, Germany;
2.8 mm ceramic beads; #91-PCS-CK28, 2x 6000 rpm). 400 pl of the homogenate
obtained were used for isolating total RNA, using the QIAsymphony RNA kit
(Qiagen,
#931636) on a QIAsymphony SP robot for automated sample preparation. Reverse
transcription of from 1 pg to 4 pg of total RNA was carried out using the
SuperScript III
first-strand synthesis system (Invitrogen, Carlsbad, USA; #18080-051)
according to the
random hexamer procedure.
Gene expression analysis was carried out with from 50 ng to 200 ng of cDNA per
reaction on an SDS7900HT Real.time PCR system (Applied Biosystems, Carlsbad,
USA) using TaqMan probes (Applied Biosystems; 1GFBP-1 Rn00565713_m1, Cyp26a1
Rn00590308_m1, PPIA Rn00690933_m1) and the Fast Blue qPCR MasterMix Plus
(Eurogentec, Liege, Belgium; #RT-QP2X-03-FFB). For relative quantification,
cyclophilin
A (PPIA) was used as an endogenous control. Relative expression levels were
calculated according to the comparative delta delta CT method.
[0045] Results:
CA 02871003 2014-10-20
BHC113068FC --,9-
= 18-Methyl-1511,1611-methylene-19-nor-20-spirox-4-en-3-one (compound A)
and 18-
methyl-6a,7a,15I1,1611-dimethylene-19-nor-20-spirox-4-en-3-one (compound B)
exhibited
dose-dependent local efficacy by way of weight gain in the IUS-carrying
uterine horn
(Fig. 1/8 and 2/8).
[0046] Within the release range tested (for compound A: 0.6 -10 pg per animal
and
day, and for compound B: 1-45 pg/animal and day) both progestins surpirisingly
exhibited no LH decrease and therefore no systemic side effect, with the
exception of
the 10 pg/animal and day dose of compound A (Fig. 3/8 and 4/8).
[0047] The pharmacokinetic profile of 18-methyl-1511,16R-methylene-19-nor-20-
spirox-4-en-3-one and 18-methyl-6a,7a,1511,1611-dimethylene-19-nor-20-spirox-4-
en-3-
one, respectively, indicated a very fast break-down rate in all in-vitro
metabolic studies
(liver) as well as in all animal species studied in vivo.
[0048] With local administration by means of IUS (rods) in rats, compound A
exhibited
a 4- to 7-fold higher potency in inducing gene expression than levonorgestrel,
with
identical release rates (Fig. 5/8 and 6/8). This higher local potency
additionally supports
the possibility of achieving more rapid and stronger local gestagenic effects
on the
uterus without causing systemic side effects in the process.
[0049] As a result, these progestins can be dosed with local
efficacy in such a way
that the side effects described for levonorgestrel do not occur in the woman.
[0050] Likewise, very rapid break-down rates have also been found in vitro
(liver) for
humans. The rapid in-vitro breakdown in the liver may also indicate rapid in-
vivo
breakdown, resulting in a very low systemic exposition of 18-methyl-15R,1611-
methylene-
19-nor-20-spirox-4-en-3-one and 18-Methyl-6a,7a,15a,1611-dimethylene-19-nor-20-
spirox-4-en-3-one after administration through an IUS being calculated. The
expected
substance levels (Css = concentration at steady state) are calculated from the
rate of
release from the IUS divided by the clearance. Using a dose of 20 pg per day
and
woman, which corresponds to that of Mirena, gives a calculated systemic
exposition
(load) for 18-methyl-1511,1611-methylene-19-nor-20-spirox-4-en-3-one and 18-
methyl-
6a,7a,15f1,1611-dimethylene-19-nor-20-spirox-4-en-3-one, which is over 30
times lower in
comparison with Mirena .
CA 02871003 2014-10-20
BHC113088FC
r0051) Example 2
The action on the human androgen receptor (hAR) was studied by means of
transactivation analyses. For this, different concentrations of the test
substances were to
cells with stable expression of the human androgen receptor, and activation of
the
androgen receptor can be detected via a reporter gene.
[0052] Method:
For transactivation studies, P03 (human prostate carcinoma) cells which have
been
stably transfected with hAR and the MTV-luc reporter gene were used. The
culture
medium used was RPM! medium (without L-glutamine; without Phenol Red) #E15-49
PAA L-glutamine 200mM #25030-024 Gibco BRL 100 U /100 pg/ml
penicillin/streptomycin Gibco # 15140-122, with 10% foetal calf serum (FCS).
The cells
were cultured at 37 C and 5% CO2. The test medium corresponded to the culture
medium, except that 10% FCS was replaced with 5% activated carbon-treated FCS
(GCS). Cells were seeded in wells of a 96 well plate ("CulturPlate" from
Packard
#6005180) with 2x104 cells / well / 200 pl of test medium. The cells were
incubated with
different concentrations of the test substances, and 80 pl of substrate were
measured
using the "steadylite HTS Reporter Gene Assay System" from Perkin Elmer.
[0053] Results:
The results show that compound A (18-methy1-1511,1611-methylene-19-nor-20-
spirox-4-
en-3-one) and compound B (18-methy1-6a,7a,1511,1611-bis-methylene-19-nor-20-
spirox-
4-en-3-one) have an EC50 in hAR transactivation which is more than 10 times
higher
than that of levonorgestrel: While the EC50 values are 6.9 nM for compound A
and 56
= 25 nM for compound B, levonorgestrel has an EC50 of only 0.5 nM.
This >10-fold
dissociation over levonorgestrel means that no systemic androgenic effects are
expected
when the compounds are used, even if local uterine uses were to produce
systemic
active ingredient levels as those observed for levonorgestrel with Mirena
uses.
[0054] Example 3
The amounts of active ingredient (A) or (B) released were determined by means
of
reversed-phase liquid chromatography with UV detection in a 1% strength 2-
hydroxypropyl-fl-cyclodextrin (2-HPBCD) solution.
CA 02871003 2014-10-20
BHC113088FC
The in-vitro release rates stated in Figures 7/8 and 8/8 were determined for a
rod
enveloped by a PTFPMS membrane.