Note: Descriptions are shown in the official language in which they were submitted.
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
COMPOSITIONS AND METHODS FOR TREATMENT OF THROMBOSIS AND FOR PROLONGING
SURVIVAL OF STORED PLATELETS
TECHNICAL FIELD
[0001] This application pertains to methods and compositions, including
pharmaceutical compositions,
for the treatment of vaso-occlusion related to sickle cell anemia, thrombosis,
vascular inflammation and
thrombocytopenia. This application further pertains to methods and
compositions that are useful for improving the
capacity to store platelets. In particular, this invention provides methods
and materials prevent the rapid clearance
of transfused platelets after cold storage or refrigeration, such that the
useful life of cold stored platelets can be
extended beyond 48 hours.
BACKGROUND
[0002] The worldwide demand for platelets is increasing, in large part due to
their prophylactic use to
prevent bleeding in thrombocytopenic cancer patients. The success of new
therapies, especially for patients with
blood cell cancers, is helping to drive up the number of annual cases of
therapy-related thrombocytopenia.
Typically patients receive platelet transfusions when their platelet count
falls below a "trigger" threshold level and
thus the frequency of transfusions in thrombocytopenic patients, in part,
depends on the circulating lifespan of the
transfused platelets. Interestingly, it has been recently reported that the
lifespan of circulating platelets largely
depends on the amount of exposed penultimate residues contained in platelet
surface glycan structures. On nascent
platelets, these residues are normally capped and "masked" by sialic acid. The
terminal sialic acid reidues on glycan
chains can be removed by sialidase enzymes present in blood. Loss of sialic
acid on the platelet surface glycans, in
turn, then leads to a more rapid clearance of platelets by hepatocytes and
macrophages. See Sorenson et al., Blood,
114: 1645-1654 (2009). Accordingly, an agent that could prevent this more
rapid in vivo platelet clearance and
sustain circulating platelet levels for longer periods of time, should provide
a more efficient prophylactic therapy for
thrombocytopenic patients.
[0003] Storage of platelets for transfusions has long been a difficult issue.
According to the Food and
Drug Administration's Blood Products Advisory Committee statement, issued
March 15, 2002, entitled "Review of
Data Supporting Extension of Dating Period for Platelets": "Bacterial
contamination of platelet products continues
to be a problem with a contamination rate estimated at 1/2000 units. Storage
of platelets at room temperature for up
to 5 days allows for proliferation of bacteria in platelet units, and "older"
platelets have been associated with
increased incidence of septic transfusion reactions. Various approaches are
being developed that would either
screen or chemically decontaminate platelet units prior to transfusion. If
such methods are shown to decrease
bacterial contamination of platelet products, storage of platelets out to 7
days may become practical."
[0004] Attempts have been made to reduce the incidence of contamination and
extend the storage life of
platelets by refrigeration or cold storage. See, Snyder and Rinder, N.E.J.
Med. 348:2032-2033 (2003). However,
platelets, unlike other transplantable tissues or cell types, do not tolerate
refrigeration and disappear rapidly from the
circulation if subjected to chilling before transplantation or transfusion.
See Rumjantseva et al., Nature Medicine,
15:1273-80 (2009).
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
[0005] Andrews and Berndt, Current Biology, 13:R282-84 (2003) suggest that
during chilling of platelets
GPIba could be modified in such a way that cold platelet storage may be
feasible by maintaining hemostatic activity
and preventing accelerated clearance. However, attempts to inhibit the rapid
clearance of long-term stored, chilled
platelets have thus far achieved very limited success. For example, Wandall et
al., Blood, 111:324956 (2008) report
the inability to prevent rapid clearance through galactosylation. Hoffmeister
et al., US Patent Application
2008/0138791, report some success in reducing clearance and thereby prolonging
the survival of platelets through
glycan modification of GPIba molecules.
[0006] The useful life of platelets stored at room temperature remains limited
because of the risk of
contamination and loss of function. The current inability to chill platelets
for longer-term storage, thereby allowing
the "stockpiling" of platelets with preserved function, results in chronic
shortages of platelets for clinical
transfusions and adds to the overall costs of clinical platelet transfusions.
[0007] GPIb-IX-V is a multifunctional hetero-complex of four distinct
glycoprotein chains, abundantly
found on the surface of platelets. The GPIba chain is one of the subunits of
GPIb-IX-V and its N-terminal domain
is capable of interacting with several proteins that are either circulating in
or exposed to the bloodstream. These
proteins include von Willebrand Factor (VWF), thrombin, Factor XI, Factor XII,
kininogen, thrombospondin 1
(TSP-1), integrin Mac-1 (CD11b/CD18, amb2 or CR3), P-selectin, as well as
Ashwell-Morell receptors. Because of
this range of interactions, GPIba has a broad role in platelet function with
regard to thrombosis, hemostasis and
inflammation. Specific binding events mediated by GPIba can be separated and
vary in importance to hemostatic
function. For example the importance of regulated binding to VWF is
demonstrated by the finding that a single
amino acid substitution in the N-terminal domain of GPIba can cause gain-of-
function phenotypes resulting in
human platelet-type von Willebrand disease.
[0008] Recent experimental evidence suggests that when platelets are collected
and cooled by
refrigeration during storage prior to transfusion, the GPIba glycoprotein
chain plays a key role in mediating the
subsequent rapid clearance of those transfused platelets from the circulation
of the recipient. This rapid clearance
has been reported to involve surface clustering of the glycans and protein
components of GPIba on stored platelets,
which are observed to form interactions with the recipient's Mac-1 and the
Ashwell-Morell asialoglycoprotein
receptors. See Rumjantseva et al., Nature Medicine, 15:1273-80 (2009).
[0009] Given the role that GPIba plays with regard to multiple platelet
functions, it has been previously
contemplated that a specific antagonist to one or more of the GPIba
interaction domains might have therapeutic
value in treating cases of undesired thrombosis, inflammation,
thrombocytopenia, and rapid platelet clearance.
However, experimental attempts using proteins, including antibodies and
antibody derived fragments, to block the
GPIba interaction domains have typically resulted in undesired
thrombocytopenia. Thus, there exists the need in the
art for a therapeutic agent or drug that will serve as a specific GPIba
binding domain antagonist, without causing
undesired thrombocytopenia. Moreover, a drug that selectively inhibits certain
GPIba binding functions, yet
preserves the capacity of the platelet to maintain its other hemostatic
functions, would have substantial therapeutic
utility in a variety of vascular disease settings.
2
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
[0010] The discovery, using a phage display screening approach, of a ten amino
acid cyclic peptide
termed OS-1, capable of binding to the N-terminal domain of GPIba was recently
reported by Benard et al.,
Biochemistry, 47:4674-82 (2008). The ability of OS-1 to block VWF-mediated
platelet aggregation in vitro, was
reported with this peptide. Two other peptides, designated as PS-4 and OS-2,
were also shown to competitively
inhibit the interaction between VWF-Al-domain and GPIba. However, no in vivo
data was provided in this study
and inhibition of Mac-1 binding to GPIba was not demonstrated. Indeed,
simultaneous inhibition of both VWF and
Mac-1 binding is unexpected for small peptides. In fact, using small peptides
as inhibitors, it has been reported that
the binding sites for VWF and Mac-1 on GPIba are inhibited independently and
therefore distinct binding sites. (See
Munday et al., Blood (ASH Annual Meeting Abstracts) 114:472 (2009) and oral
presentation December 7, 2009).
The N-linked glycans present on GPIba having exposed bGlcNAc and/or galactose
residues represent an entirely
separate point of interaction between either GPIba and Mac-1 or GPIba and the
asialoglycoprotein (Ashwell-
Morell) receptors. Given its small size, it is unlikely the OS-1 peptide is
able to create a steric interference of this
interaction.
[0011] McEwan et al., Blood 114:4883-85 (2009) describes the non-covalent
interaction of 0S-1 peptide
with GPIba and demonstrates that the GPIba-OS-1 complex structure overlaps
with the structure of the GPIba-
VWF Al domain complex. This indicates that the OS-1 peptide directly
interferes with binding of VWF to GPIba.
In commenting on the findings of McEwan, Lopez and Munday, Blood 114:4757-58
(2009) hypothesized that the
OS-1 peptide occupies the site where VWF would interact with GPIba and effects
a conformational change in
GPIba that prevents formation of the GPIba-VWF complex.
[0012] There remains a need for improved methods and materials useful for
extending the useful storage
life of platelets. Such methods should reduce the contamination of platelets,
for example, by reducing bacterial and
viral growth, yet substantially preserve the platelet's hemostatic function
and in vivo half-life after transfusion.
There is a further need for methods and materials for the treatment of
thrombosis, vascular inflammation,
thrombocytopenia, and other platelet-related disorders.
BRIEF SUMMARY OF THE INVENTION
[0013] The present invention describes the creation of novel cyclic peptides,
extending the structure of
OS-1 and enabling their fusion to a second polypeptide, or other moiety, while
retaining the binding activity to
platelet GPIba. These novel GPIba-binding peptide fusions (termed GCPFs) or
conjugates (termed GCPCs)
demonstrate an enhanced activity for blocking specific interactions mediated
by GPIba on the platelet surface.
Moreover, these GCPFs or GCPCs have suitable biological and pharmacokinetic
properties for use as therapeutic
agents in humans. Methods are further described for using GCPFs or GCPCs to
treat humans with thrombotic or
inflammatory disorders. In addition, methods are also described for using
GCPFs or GCPCs to prevent the rapid
clearance of cold stored platelets following transfusion. In a particular
embodiment, a GCPF or GCPC of the
present invention may comprise a GPIba-binding peptide, fused to an Fc domain
from an immunoglobulin to form a
GCPF or GCPC molecule of the present invention. Other peptides that may be
fused to the GPIba-binding peptide
3
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
to form a GCPF or GCPC molecule of the present invention include, but are not
limited to, SolCD39, a Kunitz
domain polypeptide, a fibronectin type III domain, or an XTEN polypeptide,
human serum albumin or MAP-1.
[0014] In addition, the present invention describes the creation of novel
conjugated fusion molecules
comprising GCPFs or GCPCs linked to a polymeric molecule, such as PEG. In a
particular embodiment, GPIba-
binding peptide may be fused to an Fc domain from an immunoglobulin to form a
GCPF molecule, and may then be
fused to PEG to form a GCPC molecule. In preferred embodiments, the
polyethylene glycol has a molecular weight
of between about 300 and 150,000 kilodaltons, preferably between about 300 and
50,000 kilodaltons, and most
preferably between about 1000 and 50,000 kilodaltons. The polymeric molecule
may be branched. For example,
see DeNardo et al., Clinical Cancer Research, 9:3854-64s (2003). The polymeric
molecule may also be releasably
attached to a peptide. For example, see US Patent 8,293,869, which describes
water soluble polymer conjugates.
The disclosure of the above documents are hereby incorporated by reference.
BRIEF DESCRIPTION OF THE FIGURES
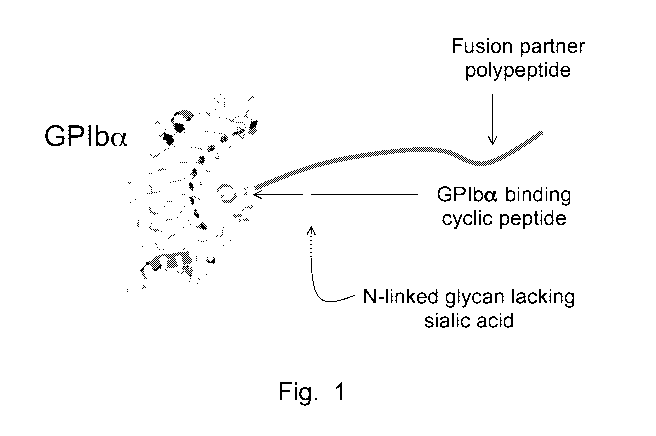
[0015] Figure 1 Schematic depiction of a GCPF binding to GPIba. The GCPF can
inhibit the binding
of N-linked glycans by lectin-like receptors.
[0016] Figure 2 Schematic depiction of the in vivo use of GCPFs to prevent
thrombosis and/or
inflammation. After administration, GCPFs bind in a non-covalent fashion to
GPIba on the surface of circulating
platelets and then prevent binding to Mac-1 and VWF or ULVWF.
[0017] Figure 3 Schematic depiction of the ex vivo use of GCPFs to enable cold
storage of platelets for
greater than 48hours. GCPFs bind in a non-covalent fashion to GPIba on the
surface of isolated platelets and then,
following cold storage and transfusion, block specific platelet binding
functions such as the binding to the lectin-like
Mac-1 integrin receptors on macrophages (Kupffer cells) and/or the Ashwell-
Morrel asialoglycoprotein receptors on
hepatocytes. This prevents rapid platelet clearance while preserving useful
platelet functions.
[0018] Figure 4 Schematic depiction of the deterioration of platelet function
over time when stored at
room temperature.
[0019] Figure 5 Schematic depiction of GCPF monomeric and multimeric
conformations.
[0020] Figure 6 Schematic depiction of GCPC molecules wherein the covalent
conjugation to either a
scaffold antibody or Ig fusion protein is achieved using a bifunctional
linker.
BRIEF DESCRIPTION OF THE SEQUENCES
[0021] SEQ ID NO: 1 is the sequence of OS-1, a cyclic peptide useful in the
present invention when
conjugated with a polymeric compound.
[0022] SEQ ID NO: 2 is the sequence of OS-1, modified by the addition of a
lysine residue, which can
be conjugated with a polymeric compound to form a novel conjugated cyclic
peptide useful in the present invention.
[0023] SEQ ID NO: 3 through 17 are the amino acid sequences of novel GCPFs
useful in the present
invention.
4
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
[0024] SEQ ID NO: 18 and 19 are the nucleotide sequence and amino acid
sequences encoding a fusion
protein of the present application. The sequence comprises the GPIba signal
peptide sequence fused to a fusion
protein encoding a GCPF of the present invention in which a CCP is fused to an
Fc fragment of an immunoglobulin.
DETAILED DESCRIPTION OF THE INVENTION
[0025] Thus, the present invention provides methods and materials for
treatment of thrombosis,
thrombocytopenia and other platelet-related disorders using GPIba-binding
peptide fusions ("GCPFs") or GPIba-
binding peptide conjugates ("GCPCs") that are able to bind to GPIba, in a non-
covalent manner, thereby extending
the stability storage of blood plasma components/platelets. In other
embodiments, the present invention provides
methods and materials for extending the useful life for use of platelets and
platelet containing compositions that are
stored in temperatures below about 22 C.
[0026] Although the terms "GCPF" and "GCPC" are essentially interchangeable,
the term GCPF is
typically used to designate a fusion molecule wherein a GPIba-binding peptide
is fused to a second peptidyl moiety,
either directly or through a peptidyl linker; while the term GCPC is typically
used to designate a fusion between a
GPIba-binding peptide and a non-peptidyl moiety; or a fusion in which two
peptidyl moieties are fused by a non-
peptidyl linker or conjugate.
[0027] As used in the present application, the term "fused" is used to mean
that two moieties are "linked"
and/or "conjugated." The first moiety is a cyclic peptide that is able to bind
to GPIba, which is referred to
interchangeably as a cyclic peptide, a GPIba-binding peptide, a GPIba-binding
cyclic peptide or a GPIba-binding
moiety. The second moiety is a peptide or chemical entity that, when fused to
the cyclic peptide, provides an
enhanced activity for blocking specific interactions mediated by GPIba on a
cell surface, and in particular, on the
surface of platelets. As discussed further herein, the enhanced activity may
be derived from biological and
pharmacokinetic properties, such as improved physiological stability; changes
in physical conformation, or from
additional binding or steric hindrance provided by fusion to the second
moiety.
[0028] When the second moiety is a peptide, the fusion of the GPIba-binding
cyclic peptide and the
second moiety forms a GPIba-binding GCPF. The two moieties may be fused
directly to each other, for example,
via one or more covalent bonds, or may be fused by a linker that joins the two
moieties. Useful linkers include for
example, linker peptides and certain non-peptide linkers, which are described
further herein. When the linker is
absent or a peptide, then the entire molecule is a GPIba-binding peptide
fusion, or "GCPF." When the linker is a
non-peptide, or only partially peptidyl, then the molecule is a GPIba-binding
peptide conjugate, or "GCPC."
[0029] When the second moiety is non-peptidyl or partially peptidyl, then the
fusion of the GPIba-
binding cyclic peptide and the second moiety form a GPIba-binding conjugate,
or "GCPC." Again, the two
moieties may be fused directly to each other, for example, via one or more
covalent bonds, or may be fused by a
linker that joins the two moieties.
[0030] The GPIba-binding polypeptide moiety and second moiety of the GCPF or
GCPC of the present
invention may be fused directly to each other, or joined by a linker. A
variety of linkers can be used to join a
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
GPIba-binding polypeptide moiety of an agent to another peptide moiety. The
linker can be a molecule or group of
molecules (such as a monomer or polymer) that connects two molecules and
optionally to place the two molecules
in a particular configuration. Exemplary linkers include polypeptide linkages
between N- and C-termini of proteins
or protein domains, linkage via disulfide bonds, and linkage via chemical
cross-linking reagents.
[0031] In some embodiments, the linker includes one or more peptide bonds,
e.g., generated by
recombinant techniques or peptide synthesis. The linker may contain one or
more amino acid residues that provide
flexibility. Thus, the linker peptide may predominantly include the following
amino acid residues: Gly, Ser, Ala, or
Thr. The linker peptide should have a length that is adequate to link two
molecules in such a way that they assume
the correct conformation relative to one another so that they retain the
desired activity. Suitable lengths for this
purpose include at least one and not more than 30 amino acid residues. For
example, the linker is from about 1 to
about 30 amino acids in length, with linkers of length of about 1 to about 25
amino acids in length being preferred.
Most preferred are linkers of length of about 1,2, 3,4, 5,6, 7, 8,9, 10, 11,
12, 13, 14, 15, 16, 17, 18, 19 and 20
amino acids.
[0032] Exemplary linkers include glycine-serine polymers (including, for
example, (GS)n, (GSGGS)n,
(GGGGS)n and (GGGS)n, where n is an integer of at least one, e.g., one, two,
three, or four), glycine-alanine
polymers, alanine-serine polymers, and other flexible linkers. Glycine-serine
polymers can serve as a neutral tether
between components. Secondly, serine is hydrophilic and therefore able to
solubilize what could be a globular
glycine chain. Third, similar chains have been shown to be effective in
joining subunits of recombinant proteins
such as single chain antibodies. Suitable linkers may also be identified from
three-dimensional structures in
structure databases for natural linkers that bridge the gap between two
polypeptide chains. In some embodiments,
the linker is from a human protein and/or is not immunogenic in a human. Thus,
linkers may be chosen with low
immunogenicity or thought to have low immunogenicity. For example, a linker
may be chosen that exists naturally
in a human. In a preferred embodiment, the linker has the sequence of the
hinge region of an antibody, that is, the
sequence that links the antibody Fab and Fc regions; alternatively the linker
has a sequence that comprises part of
the hinge region, or a sequence that is substantially similar to the hinge
region of an antibody. Another way of
obtaining a suitable linker is by optimizing a simple linker, e.g., (GS)n,
through random mutagenesis.
[0033] Alternatively, once a suitable polypeptide linker is defined,
additional linker polypeptides can be
created to select amino acids that more optimally interact with the domains
being linked. Other types of linkers
include artificial polypeptide linkers and inteins. In another embodiment,
disulfide bonds are designed to link the
two molecules. Other examples include peptide linkers described in US Patent
5,073,627, the disclosure of which is
hereby incorporated by reference. In certain cases, the diagnostic or
therapeutic protein itself can be a linker by
fusing tandem copies of the peptide to the second moiety of the GCPF or GCPC.
In certain embodiments, charged
residues including arginine, lysine, aspartic acid, or glutamic acid may be
incorporated into the linker sequence in
order to form a charged linker.
[0034] In another embodiment, bifunctional protein coupling agents, or
conjugating agents, may serve to
link, or fuse the GPIba-binding polypeptide moiety and second moiety of the
GCPF or GCPC of the present
invention. For example, a variety of bifunctional protein coupling agents may
be used, including but not limited to
6
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
N-succinimidy1-3-(2-pyridyldithiol) propionate (SPDP), succinimidy1-4-(N-
maleimidomethyl)cyclohexane-1-
carboxylate, iminothiolane (IT), bifunctional derivatives of imidoesters (such
as dimethyl adipimidate HCL), active
esters (such as disuccinimidyl suberate), aldehydes (such as glutareldehyde),
bis-azido compounds (such as bis(p-
azidobenzoyl) hexanediamine), bis-diazonium derivatives (such as bis-(p-
diazoniumbenzoy1)-ethylenediamine),
diisocyanates (such as tolyene 2,6-diisocyanate), and bis-active fluorine
compounds (such as 1,5-difluoro-2,4-
dinitrobenzene). See, for example, US Patent 6,669,938). In certain preferred
embodiments, the conjugating agent
may be a bifunctional linker as described in US Patent 7,521,425 and
Doppalapudi et al., PNAS 107:22611-16
(2010). Such conjugating agents may be used to fuse the GPIba-binding
polypeptide moiety to a second moiety,
which may be a complete antibody, or a fusion of the Fc fragment of an
immunoglobulin to another peptide, which
may contribute to targeting of the GCPC. The Fc fragment may, for example, be
fused with a peptide known to
specifically bind to a particular epitope or antigen. In preferred
embodiments, the Fc-fusion molecules may
comprise an Fc fragment of an immunoglobulin fused with an extracellular
portion of a receptor. For example, one
such Fc-receptor fusion molecule is etanercept (Enbre10), in which an Fc
fragment of an immunoglobulin is fused
to a soluble portion of the TNFR receptor.
[0035] The linker or conjugating agent may be cleavable, facilitating release
of a payload, e.g., in the cell
or a particular milieu. For example, an acid-labile linker, peptidase-
sensitive linker, dimethyl linker or disulfide-
containing linker (Chari et al., Cancer Research 52:127-131(1992)) may be
used. In some embodiments, the linker
includes a nonproteinaceous polymer, e.g., polyethylene glycol (PEG),
polypropylene glycol, polyoxyalkylenes, or
copolymers of polyethylene glycol and polypropylene glycol. In other
embodiments, the linker may be a
bifunctional linker.
DESCRIPTION OF EMBODIMENTS OF THE INVENTION:
Compositions
[0036] In one aspect, the present invention provides glycoprotein GPIba-
binding cyclic peptide fusions
(GCPFs) or conjugates (GCPCs) that are able to bind to GPIba in a non-covalent
manner. These GCPFs or GCPCs
may be useful to extend the useful storage life of platelets. In certain
preferred embodiments, the GCPFs or GCPCs
comprising an amino acid sequence selected from the group consisting of SEQ ID
NO: 1 and 2, linked or conjugated
to a second peptide moiety of the invention, and may optionally be further
covalently linked with a polymeric
compound. In other preferred embodiments, the GCPFs or GCPCs comprise amino
acid sequences selected from
the group consisting of SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO: 5, SEQ ID NO:
6, SEQ ID NO: 7, SEQ ID NO:
8 SEQ ID NO: 9, SEQ ID NO: 10, SEQ ID NO: 11, SEQ ID NO: 12, SEQ ID NO: 13,
SEQ ID NO: 14, SEQ ID
NO: 15, SEQ ID NO: 16, or SEQ ID NO: 17. In particular embodiments, the GCPF
comprises an amino acid
sequence encoded by SEQ ID NO: 18, or the amino acid sequence of SEQ ID NO:
19. The resulting GCPFs or
GCPCs are tested to determine that they retain the ability to bind to GPIba in
a non-covalent manner, and that said
GCPFs or GCPCs exhibit enhanced inhibition of platelet binding to Mac-lor
Ashwell-Morell receptors compared to
a cyclic peptide comprising the same amino acid sequence, which lacks a non-
cyclic peptide, or a covalently linked
polymeric compound.
7
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
[0037] In particular embodiments, the fusion peptide is a polypeptide that
increases the in vivo half-life
of the cyclic peptide without causing severe thrombocytopenia. In preferred
embodiments, the fusion polypeptide is
an immunoglobulin Fc domain, SolCD39 peptide (see, for example, Belayev et
al., Stroke, 34:758-63 (2003)); a
Kunitz domain polypeptide (see, for example, Girard et al, Nature, 338:518-20
(1989)), a fibronectin type III domain
(see, for example, Koide et al., J. Molecular Biol., 284:1141-51(1998)), an
XTEN polypeptide (see, for example,
Schellenberger et al., Nat. Biotech. 27:1186-90 (2009); human serum albumin
(see, for example, US Patents
8,143,026 and 7,847,079); or MAP-1 (see, for example, Skjoedt et al., J. Biol.
Chem., 285:8234-43 (2010)). The
disclosure of each of the above publications is hereby incorporated herein by
reference.
[0038] In addition, the present invention describes the creation of novel
conjugated fusion molecules
comprising GCPFs or GCPCs linked to a polymeric molecule, such as PEG. In a
particular embodiment, GPIba-
binding peptide may be fused to an Fc domain from an immunoglobulin to forma a
GCPF or GCPC molecule, and
may then be fused to PEG. In preferred embodiments, the polyethylene glycol
has a molecular weight of between
about 300 and 150,000 kilodaltons, preferably between about 300 and 50,000
kilodaltons, and most preferably
between about 1000 and 50,000 kilodaltons. The polyethylene glycol polymer may
be branched. See, for example,
DeNardo et al., Clinical Cancer Research, 9:3854-64s (2003), the disclosure of
which is hereby incorporated herein
by reference.
Methods
[0039] In another aspect, the present invention provides methods for
increasing the useful storage life of
platelets. The methods comprise storing platelets with a GCPF or GCPC
comprising the amino acid sequence of or
SEQ ID NO: 1 or 2, fused with a second peptide moiety and optionally a
polymeric compound. In particular
embodiments, the GCPF or GCPC may comprise the amino acid sequence of SEQ ID
NO: 3 to 17. The GCPF or
GCPC has the ability to bind to GPIba in a non-covalent manner, and said
cyclic conjugated peptide exhibits
enhanced inhibition of platelet binding to Mac-lor Ashwell-Morell receptors
compared to or relative to a cyclic
peptide comprising the same amino acid sequence, without fusion to a fusion
peptide or that is not covalently linked
polymeric compound. In certain embodiments, the platelets are stored at
temperatures below 22 C. In preferred
embodiments, the platelets are stored at temperatures of from about 0 C to
about 4 C.
[0040] In some embodiments, the platelets may be stored for 2 to 7 days at
temperatures below 22 C. In
preferred embodiments, the platelets may be stored for 5 to 7 days at
temperatures below 22 C. In other preferred
embodiments, the platelets may be stored for 2 to 7 days at temperatures of
from about 0 C to about 4 C. In
preferred embodiments, the platelets may be stored for 5 to 7 days at
temperatures of from about 0 C to about 4 C.
[0041] The present inventors theorized that treatment of platelets with GCPFs
or GCPCs can result in
significantly enhanced useful storage life for platelets that have been
refrigerated or subject to cold-storage.
Additional benefits may include the improved available supply of platelets and
other plasma products, as well as
improved safety as platelets and other fresh plasma products may be stored at
colder temperatures to reduce the risk
of pathogenic contamination. Characterization and biological investigations of
stored platelets and use of current
storage technologies may be employed. (See, for example, Devine, Clin Lab Med
30:475-87 (2010)).
8
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
[0042] Together, these establish the potential use of the present invention
for extending useful half-
life/storage of platelets and platelet containing serum or blood plasma. The
compositions and methods of the
present invention may also be used in a clinical setting. For example, the
methods and compositions of the present
invention may be useful for the treatment and/or prevention of vaso-occlusion
related to sickle-cell anemia
thrombosis, vascular inflammation, thrombocytopenia and other platelet-related
disorders.
[0043] Thus, in other aspects, the present invention comprises methods of
treating a subject who is
suffering from vaso-occlusion related to sickle-cell anemia, thrombosis,
vascular inflammation, thrombocytopenia
or other platelet-related disorders, the method comprising treating ex vivo
isolated platelets from one or more donors
with a GCPF or GCPC of the present invention, and then administering to said
subject these allogeneic platelets that
have been treated with GCPF or GCPC (allogeneic platelet treatment and
transfusion). Such treatment and
transfusions may be useful for reduction of poor responses or refractoriness
to allogeneic platelet transfusion (See,
for example, Slichter, Hematology 2007:172-78 (2007)).
[0044] In other embodiments, the methods of the present invention comprise
preventing, avoiding or
reducing vaso-occlusion related to sickle-cell anemia, thrombosis, vascular
inflammation, thrombocytopenia or
other platelet-related disorders in a subject, the method comprising treating
ex vivo the same subject's platelets with
a GCPF or GCPC of the present invention; and administering to said subject the
platelets that have been treated with
GCPF or GCPC said same subject the autologous platelets that have been treated
with GCPF or GCPC (autologous
platelet treatment and transfusions). Such treatment and transfusions may be
useful for reduction of poor responses
or refractoriness to autologous platelet transfusion (See, for example,
Slichter, Hematology 2007).
[0045] In other embodiments, the present invention comprises methods of
preventing or treating, or a
method of avoiding or reducing vaso-occlusion related to sickle-cell anemia ,
thrombosis, vascular inflammation, or
another platelet related disorder or disease in a subject, comprising
administering to said subject an effective amount
of a composition comprising a GCPF or GCPC of the present invention.
[0046] In other embodiments, the present invention comprises methods of
preventing or treating diseases
or disorders related to von Willebrand Factor (VWF) or ADAMTS-13, or
preventing or treating diseases or
disorders characterized by dysregulation of VWF or ADAMTS-13 levels. For
example, methods of avoiding
dengue hemorrhagic fever ("DHF") and/or dengue shock syndrome ("DSS").
Individuals with DHF and DSS have
been demonstrated to exhibit high levels of VWF and VWF activating factor, and
low levels of ADAMTS-13, which
functions as a regulator of VWF. See, for example, Djamiatum et al., PLoS Negl
Trop Dis; 6:e1628 (2012).
[0047] In preferred embodiments, the GCPF or GCPC is administered in
combination with one or more
selectin antagonists. The selectin antagonist is selected from the group
consisting of rPSGL-Ig (Myers et al.,
Thrombosis and Haemostasis, 85:423-29 (2001)) or GMI-1070 (Chang et al.,
Blood, 116:1179-86 (2010)).
[0048] In preferred embodiments, the GCPF or GCPC is administered in
combination with one or more
VWF antagonists. The VWF antagonist is preferably selected from the group
consisting of AJW200 (Kageyama et
al., Arteriosclerosis, Thrombosis and Vascular Biology, 22:187 (2002)), ARC-
1779 (Cosmi, Curr Opin Mol Ther,
11:322-28 (2009)), ALX-0081 (Bartunek et al., Circulation, 118:S656 (2008)),
ALX-0681 (Majidi et al., Human
Antibodies, 18:81-100 (2009)) or GPG-290 (Wadanoli et al., Thromb. Haemost.,
98:397-405 (2007)) or GPIba-Ig
9
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
variant fusion polypeptides. (Shaw et al., US patent 7,727,535). The
disclosure of these documents is hereby
specifically incorporated by reference into the specification.
[0049] In other preferred embodiments, the GCPF or GCPC is administered in
combination with one or
more thrombopoietin (TPO) mimetics or TPO receptor agonists. Suitable TPO
mimetics and/or TPO receptor
agonists include the approved drugs PROMACTAO (eltrombopag) (GlaxoSmithKline,
Inc.); and NPLATEO
(romiplostim) (Amgen, Inc.). Other TPO mimetics and/or TPO receptor agonists
that may be useful in the present
invention also include those described in United States Patents: US 7,169,931
(Takemoto et al., Shionogi & Co.);
US 7,488,590 (Feige et al., Amgen, Inc.) (and related PCT patent publications
W02001/83525 and
W02002/024782); US 6,498,155 (Luengo and Lamb, GlaxoSmithKline); US 7,786,159
(Spencer and Punnonen,
Strategics, Inc.); and in PCT patent publication W02004/026332 (Kaushansky and
MacDonald; Johnson &
Johnson); PCT patent publication W02009/148954 (Yurklow and Shukla; Johnson &
Johnson). The disclosure of
these documents is hereby specifically incorporated by reference into the
specification.
GCPF Peptides
[0050] In certain embodiments, the pharmaceutical compositions comprise a full-
length GCPF-014
peptide[Ac-ACTERMALHNLCGG-NH2(SEQIDNO: 1)orAc-ACTERMALHNLCGGK-
NH2 (SEQ ID NO: 2)] and a pharmaceutically acceptable carrier suitable for
administration to an individual. In
certain embodiments, the pharmaceutical compositions comprise full-length GCPF-
014 peptide (SEQ ID NO: 1 or
SEQ ID NO: 2) and a pharmaceutically acceptable carrier suitable for
administration to an individual. In certain
embodiments, the pharmaceutical compositions comprise a biologically-active
fragment of GCPF-014 peptide
comprising and a pharmaceutically acceptable carrier suitable for
administration to an individual. In certain
embodiments, the pharmaceutical compositions comprise a biologically-active
fragment of a full-length GCPF-014
peptide comprising Ac- AC TERMALHNLCGG -NH2 (SEQ ID NO: 1) or Ac- ACTERMALHNLC
G G K -NH2 (SEQ ID NO: 2) with linker amino acids and fusion polypeptide and a
pharmaceutically acceptable
carrier suitable for administration to an individual.
[0051] In certain embodiments, the present invention relates to fusion
molecules, comprising a GCPF or
GCPC of the present invention, in which a GPIba protein binding moiety is
directly fused or linked to a second
peptide moiety. The GPIba protein binding moiety is preferably a CCP. The
second peptide moiety preferably
provides improved characteristics, such as increased stability, increased half-
life, or better pharmacokinetic
properties. In certain cases, the GCPF- or GCPC- fusion molecule may be able
to form dimers or further oligomers
of GCPF- or GCPC- fusion molecules. A GCPF- or GCPC- fusion molecule of the
present invention may further be
linked to a polymeric molecule, such as PEG. In a particular embodiment, a
GPIba protein binding moiety of the
present invention may be fused to an Fc domain from an immunoglobulin to forma
a GCPF or GCPC fusion
molecule (see, for example, US Patent 6,835,809). Other peptide moieties that
may be linked or fused to GPIba
protein binding moiety (such as a CCP) to form a GCPF or GCPC fusion molecule
of the present invention include
SolCD39 (see, for example, Belayev et al., Stroke, 34:758-63 (2003)), a Kunitz
domain polypeptide (see, for
example, Girard et al, Nature, 338:518-20 (1989)), a fibronectin type III
domain (see, for example, Koide et al., J.
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
Molecular Biol., 284:1141-51(1998)), an XTEN polypeptide (see, for example,
Schellenberger et al., Nat. Biotech.
27:1186-90 (2009); human serum albumin (see, for example, US Patents 8,143,026
and 7,847,079); or MAP-1 (see,
for example, Skjoedt et al., J. Biol. Chem., 285:8234-43 (2010)). The
disclosure of each of the above publications is
hereby incorporated herein by reference.
[0052] In certain embodiments, the invention comprises molecules in which the
GCPF-014 peptide is
modified by the addition of physiologically compatible polymeric compounds,
such as polyethylene glycol. The
polymeric compound is covalently bound to an amino acid residue of the GCPF
peptide, generally a lysine residue.
If desired, the amino acid sequence of a peptide can be modified by
substitution of one or more amino acid residues
with lysine, and/or by the addition of one or more lysine residues, to provide
a convenient site for conjugation of the
polymeric compound or for a bifunctional linker.
[0053] Preferred polymeric compounds for use in the present invention include
monofunctional amino-
reactive polyethylene glycol (PEG) polymers having molecular weights between
about 3-5,000 Daltons, and can be
chemically conjugated to reactive amine sites on GCPF peptides. PEG reagents
that form stable amide linkages
such as N-Hydroxy-succinimide or propionaldehyde PEG derivatives, are known in
the art, and can be conjugated
to GCPF peptides. PEG polymers suitable for use in the present invention, and
methods for their preparation and
conjugation to peptides are described, for example, in US 7,030,278; US
6,956,135; US 6,916,962; US 6,541,543;
US 5,990,237; US 5,252,714; US Patent Application US 2010/0010194; PCT Patent
Application W02009/114151;
and PCT Patent Application W02001/024831. The polymeric molecule may also be
releasably attached to a peptide.
For example, see US Patent 8,293,869, which describes water soluble polymer
conjugates. The disclosure of each
of the above publications is hereby incorporated herein by reference.
[0054] Bifunctional linkers useful in the present invention, and methods for
their conjugation to peptides
are described, for example, in US Patent 7,521,425 and Doppalapudi et al.,
PNAS, 107:22611-16 (2010). The
disclosure of each of the above publications is hereby incorporated herein by
reference
[0055] Examples of GCPFs or GCPCs useful in the present invention include:
C T/S ERZALHNLC (X)N K ¨ Fus (SEQ ID NO: 3)
(J)N C T/S ERZALHNLC (X)N K - Fus (SEQ ID NO: 4)
Fus - (J)N C T/S ERZALHNLC (X)N (SEQ ID NO: 5)
Fus - (J)N C T/S ERZALHNLC (X)N - Fus (SEQ ID NO: 6)
Fus - (J)N C T/S ERZALHNLC (X)N K¨ Peg (SEQ ID NO: 7)
Peg - (J)N C T/S ERZALHNLC (X)N - Fus (SEQ ID NO: 8)
ACT/SERMALHNLCGGG-Fus (SEQIDNO: 9)
Fus-GCT/SERDALHNLCGGGG (SEQIDNO: 10)
11
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
Fus-ACT/SERMALHNLCSSG-Fus (SEQIDNO: 11)
Peg-ACT/SERDALHNLCGRG-Fus (SEQIDNO: 12)
Fus¨AKCT/SERZALHNLCRSK¨Peg(SEQIDNO: 13)
(J)N C T/S ERZ AL HNLC (X)N K - Bf (SEQ ID NO: 14)
Bfl - (J)N C T/S ERZ ALHNLC (X)N K (SEQ ID NO: 15)
(DN C T/S ERZ ALHNLC (X)N K - Bfl ¨ Fus (SEQ ID NO: 16)
Fus ¨ Bfl - (J)N C T/S ERZALHNLC (X)N (SEQ ID NO: 17)
J = any amino acid except C
Z = M, D, L, W or A
X = any amino acid except C
N = 0 ¨ 10
Fus = fusion polypeptide (such as an IgG, etc.)
Peg = polyethyleneglycol
Bfl = bifunctional linker
[0056] Munday et al. mapped a critical binding site for Mac-1 to GPIba
sequence Arg 218 to 224, a
distinct site from VWF binding site. Munday et al., Blood (ASH Annual Meeting
Abstracts) 114:472 (2009).
Munday et al. proposed that a peptide corresponding to this region would
therefore inhibit GPIba binding to Mac-1,
but block neither platelet adhesion to immobilized VWF nor thrombin-induced
platelet aggregation, and could
therefore specifically inhibit leukocyte-platelet complexes that promote
vascular inflammation. Thus, the GPIba
binding sites to Mac-1 and VWF are distinct and can be independently blocked.
[0057] Without being bound by any specific theory, it is believed that there
are multiple mechanism by
which GCPFs and GCPCs prevent platelet clearance. The first mechanism is
likely through preventing the protein-
protein binding of Mac-lvia allosteric effects caused by the GCPF or GCPC at
the GPIba protein binding site. The
second mechanism is likely by steric hindrance or shielding, caused by the
presence of a conjugated PEG polymer,
preventing the interaction between components of the N-linked glycans on GPlba
and the lectin-like receptors such
as the Ashwell-Morell or Mac-1 receptors. A third mechanism by which GCPFs or
GCPCs can prevent platelet
clearance is through the inhibition of the binding of VWF and GPIba. VWF
binding has been been implicated as a
contributing event in the rapid clearance of cold stored platelets. See
Rumjantseva et al., Transfusion and Apheresis
Science 42:63-70 (2010). A fourth mechanism by which GCPFs or GCPCs can
prevent platelet clearance is by
preventing morphological changes and microaggregation of platelets (Maurer et
al., US Patent application
2009/0041737). The disclosure of each of the above publications is hereby
incorporated herein by reference.
Host Cells and GCPF Protein Expression
[0058] Vector DNAs encoding GCPFs can be introduced into prokaryotic or
eukaryotic cells (host cells)
via conventional transformation or transfection techniques.
12
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
[0059] A host cell can be any prokaryotic or eukaryotic cell. For example,
GCPFs can be expressed in
bacterial cells such as E. coil, insect cells, yeast or mammalian cells (such
as human, Chinese hamster ovary cells
(CHO) or COS cells). Other suitable host cells are known to those skilled in
the art.
[0060] Mammalian host cell, such as a Chinese hamster ovary cells (CHO) or COS
cells can be
transfected with expression vectors to enable, via posttranslational
modification, the generation of the sialyl Lewisx
epitope on the N-linked and 0-linked glycans of CCP fusion polypeptides. In
the case of CHO cells this requires the
co-expression of an alpha-1,3/1,4 fucosyltranseferase (Kukowska-Latallo et
al., Genes Dev. 4:1288-303, 1990) and
Core2 beta-1,6-N-acetylglucosaminyltransferase enzymes. (Kumar et al., Blood
88:3872-79,1996). The presence of
the sialyl Lewisx epitopes on the N-linked and 0-linked glycans of non-CCP
fusion and/or immunoglobulin
polypeptides may enhance the binding to selectin family of adhesion molecules.
[0061] For stable transfection of mammalian cells, it is known that, depending
upon the expression vector
and transfection technique used, only a small fraction of cells may integrate
the foreign DNA into their genome. In
order to identify and select these integrants, a gene that encodes a
selectable marker (e.g., resistance to antibiotics) is
generally introduced into the host cells along with the gene of interest.
Various selectable markers include those that
confer resistance to drugs, such as G418, hygromycin and methotrexate. Nucleic
acid encoding a selectable marker
can be introduced into a host cell on the same vector as that encoding GCPFs
or can be introduced on a separate
vector. Cells stably transfected with the introduced nucleic acid can be
identified by drug selection (e.g., cells that
have incorporated the selectable marker gene will survive, while the other
cells die).
[0062] A host cell of the invention, such as a prokaryotic or eukaryotic host
cell in culture, can be used to
produce (i.e., express) GCPFs. Accordingly, the invention further provides
methods for producing GCPFs using the
host cells of the invention. In one embodiment, the method comprises culturing
the host cell of invention (into which
a recombinant expression vector encoding GCPFs has been introduced) in a
suitable medium such that GCPF is
produced. In another embodiment, the method further comprises isolating GCPF
from the medium or the host cell.
[0063] The fusion polypeptides may be isolated and purified in accordance with
conventional conditions,
such as extraction, precipitation, chromatography, affinity chromatography,
electrophoresis or the like.
COMPOSITIONS AND FORMULATIONS:
[0064] In certain embodiments, the composition further comprises one or more
surfactants. Exemplary
surfactants include, but are not limited to, natural emulsifiers (e.g. acacia,
agar, alginic acid, sodium alginate,
tragacanth, chondrux, cholesterol, xanthan, pectin, gelatin, egg yolk, casein,
wool fat, cholesterol, wax, and lecithin),
colloidal clays (e.g. bentonite [aluminum silicate] and Veegum [magnesium
aluminum silicate]), long chain amino
acid derivatives, high molecular weight alcohols (e.g. stearyl alcohol, cetyl
alcohol, oleyl alcohol, triacetin
monostearate, ethylene glycol distearate, glyceryl monostearate, and propylene
glycol monostearate, polyvinyl
alcohol), carbomers (e.g. carboxy polymethylene, polyacrylic acid, acrylic
acid polymer, and carboxyvinyl
polymer), carrageenan, cellulosic derivatives (e.g. carboxymethylcellulose
sodium, powdered cellulose,
hydroxymethyl cellulose, hydroxypropyl cellulose, hydroxypropyl
methylcellulose, methylcellulose), sorbitan fatty
acid esters (e.g. polyoxyethylene sorbitan monolaurate [Tween 20],
polyoxyethylene sorbitan [Tween 60],
13
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
polyoxyethylene sorbitan monooleate [Tween 80], sorbitan monopalmitate [Span
40], sorbitan monostearate [Span
60], sorbitan tristearate [Span 65], glyceryl monooleate, sorbitan monooleate
[Span 80]), polyoxyethylene esters
(e.g. polyoxyethylene monostearate [Myrj 45], polyoxyethylene hydrogenated
castor oil, polyethoxylated castor oil,
polyoxymethylene stearate, and Solutol), sucrose fatty acid esters,
polyethylene glycol fatty acid esters (e.g.
Cremophor), polyoxyethylene ethers, (e.g. polyoxyethylene lauryl ether [Brij
30]), poly(vinyl¨pyrrolidone),
diethylene glycol monolaurate, triethanolamine oleate, sodium oleate,
potassium oleate, ethyl oleate, oleic acid,
ethyl laurate, sodium lauryl sulfate, Pluronic F 68, Poloxamer 188,
cetrimonium bromide, cetylpyridinium chloride,
benzalkonium chloride, docusate sodium, etc. and/or combinations thereof In
certain embodiments, the surfactant
is a Tween surfactant (e.g., Tween 60, Tween 80, etc.).
[0065] In certain embodiments, the composition further comprises one or more
preservatives.
Exemplary preservatives may include antioxidants, chelating agents,
antimicrobial preservatives, antifungal
preservatives, alcohol preservatives, acidic preservatives, and other
preservatives.
[0066] In certain embodiments, the one or more preservative comprises an
antioxidant. Exemplary
antioxidants include, but are not limited to, phosphites, dibutyl phosphite,
alpha tocopherol, ascorbic acid, acorbyl
palmitate, butylated hydroxyanisole, butylated hydroxytoluene,
monothioglycerol, potassium metabisulfite,
propionic acid, propyl gallate, sodium ascorbate, sodium bisulfite, sodium
metabisulfite, sodium sulfite, cysteine
hydrochloride, thioglycerol, sodium mercaptoacetate, sodium formaldehyde
sulfoxylate (SFS), lecithin, and alpha-
tocopherol. In certain embodiments, the antioxidant is dibutyl phosphite or
sodium bisulfite (NaHS03).
[0067] In certain embodiments, the one or more preservative comprises a
chelating agent. Exemplary
chelating agents include, but are not limited to, ethylenediaminetetraacetic
acid (EDTA), citric acid monohydrate,
disodium edetate, dipotassium edetate, edetic acid, fumaric acid, malic acid,
phosphoric acid, sodium edetate,
tartaric acid, and trisodium edetate.
[0068] In certain embodiments, the one or more preservative comprises an
antimicrobial preservative.
Exemplary antimicrobial preservatives include, but are not limited to,
benzalkonium chloride, benzethonium
chloride, benzyl alcohol, bronopol, cetrimide, cetylpyridinium chloride,
chlorhexidine, chlorobutanol, chlorocresol,
chloroxylenol, cresol, ethyl alcohol, glycerin, hexetidine, imidurea, phenol,
phenoxyethanol, phenylethyl alcohol,
phenylmercuric nitrate, propylene glycol, and thimerosal.
[0069] In certain embodiments, the one or more preservative comprises an
antifungal preservative.
Exemplary antifungal preservatives include, but are not limited to, butyl
paraben, methyl paraben, ethyl paraben,
propyl paraben, benzoic acid, hydroxybenzoic acid, potassium benzoate,
potassium sorbate, sodium benzoate,
sodium propionate, and sorbic acid.
[0070] In certain embodiments, the one or more preservative comprises an
alcohol preservative.
Exemplary alcohol preservatives include, but are not limited to, ethanol,
polyethylene glycol, phenol, phenolic
compounds, bisphenol, chlorobutanol, hydroxybenzoate, and phenylethyl alcohol.
[0071] In certain embodiments, the one or more preservative comprises an
acidic preservative.
Exemplary acidic preservatives include, but are not limited to, vitamin A,
vitamin C, vitamin E, beta¨carotene, citric
acid, acetic acid, dehydroacetic acid, ascorbic acid, sorbic acid, and phytic
acid.
14
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
[0072] Other preservatives include, but are not limited to, tocopherol,
tocopherol acetate, deteroxime
mesylate, cetrimide, butylated hydroxyanisol (BHA), butylated hydroxytoluened
(BHT), ethylenediamine, sodium
lauryl sulfate (SLS), sodium lauryl ether sulfate (SLES), sodium bisulfite,
sodium metabisulfite, potassium sulfite,
potassium metabisulfite, Glydant Plus, Phenonip, methylparaben, Germall 115,
Germaben II, Neolone, Kathon, and
Euxyl.
[0073] In certain embodiments, the composition further comprises one or more
diluents. Exemplary
diluents include, but are not limited to, calcium carbonate, sodium carbonate,
calcium phosphate, dicalcium
phosphate, calcium sulfate, calcium hydrogen phosphate, sodium phosphate
lactose, sucrose, cellulose,
microcrystalline cellulose, kaolin, mannitol, sorbitol, inositol, sodium
chloride, dry starch, cornstarch, powdered
sugar, etc., and combinations thereof
[0074] In certain embodiments, the composition further comprises one or more
granulating and/or
dispersing agents. Exemplary granulating and/or dispersing agents include, but
are not limited to, potato starch,
corn starch, tapioca starch, sodium starch glycolate, clays, alginic acid,
guar gum, citrus pulp, agar, bentonite,
cellulose and wood products, natural sponge, cation¨exchange resins, calcium
carbonate, silicates, sodium
carbonate, cross¨linked poly(vinyl¨pyrrolidone) (crospovidone), sodium
carboxymethyl starch (sodium starch
glycolate), carboxymethyl cellulose, cross¨linked sodium carboxymethyl
cellulose (croscarmellose),
methylcellulose, pregelatinized starch (starch 1500), microcrystalline starch,
water insoluble starch, calcium
carboxymethyl cellulose, magnesium aluminum silicate (Veegum), sodium lauryl
sulfate, quaternary ammonium
compounds, etc., and combinations thereof
[0075] In certain embodiments, the composition further comprises one or more
binding agents.
Exemplary binding agents include, but are not limited to, starch (e.g.
cornstarch and starch paste); gelatin; sugars
(e.g. sucrose, glucose, dextrose, dextrin, molasses, lactose, lactitol,
mannitol, etc.); natural and synthetic gums (e.g.
acacia, sodium alginate, extract of Irish moss, panwar gum, ghatti gum,
mucilage of isapol husks,
carboxymethylcellulose, methylcellulose, ethylcellulose,
hydroxyethylcellulose, hydroxypropyl cellulose,
hydroxypropyl methylcellulose, microcrystalline cellulose, cellulose acetate,
poly(vinyl¨pyrrolidone), magnesium
aluminum silicate (Veegum), and larch arabogalactan); alginates; polyethylene
oxide; polyethylene glycol; inorganic
calcium salts; silicic acid; polymethacrylates; waxes; water; alcohol; etc.;
and combinations thereof
[0076] In certain embodiments, the composition further comprises one or more
buffering agents.
Exemplary buffering agents include, but are not limited to, citrate buffer
solutions, acetate buffer solutions,
phosphate buffer solutions, ammonium chloride, calcium carbonate, calcium
chloride, calcium citrate, calcium
glubionate, calcium gluceptate, calcium gluconate, D¨gluconic acid, calcium
glycerophosphate, calcium lactate,
propanoic acid, calcium levulinate, pentanoic acid, dibasic calcium phosphate,
phosphoric acid, tribasic calcium
phosphate, calcium hydroxide phosphate, potassium acetate, potassium chloride,
potassium gluconate, potassium
mixtures, dibasic potassium phosphate, monobasic potassium phosphate,
potassium phosphate mixtures, sodium
acetate, sodium bicarbonate, sodium chloride, sodium citrate, sodium lactate,
dibasic sodium phosphate, monobasic
sodium phosphate, sodium phosphate mixtures, tromethamine, magnesium
hydroxide, aluminum hydroxide, alginic
acid, pyrogen¨free water, isotonic saline, Ringer's solution, ethyl alcohol,
etc., and combinations thereof
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
[0077] In certain embodiments, the composition further comprises one or more
lubricating agents.
Exemplary lubricating agents include, but are not limited to, magnesium
stearate, calcium stearate, stearic acid,
silica, talc, malt, glyceryl behanate, hydrogenated vegetable oils,
polyethylene glycol, sodium benzoate, sodium
acetate, sodium chloride, leucine, magnesium lauryl sulfate, sodium lauryl
sulfate, etc., and combinations thereof
[0078] In certain embodiments, the composition further comprises one or more
solubilizing or
suspending agents. Exemplary solubilizing or suspending agents include, but
are not limited to, water, organic
solvents, oils, and mixtures thereof Exemplary oils include, but are not
limited to, almond, apricot kernel, avocado,
babassu, bergamot, black current seed, borage, cade, camomile, canola,
caraway, carnauba, castor, cinnamon, cocoa
butter, coconut, cod liver, coffee, corn, cotton seed, emu, eucalyptus,
evening primrose, fish, flaxseed, geraniol,
gourd, grape seed, hazel nut, hyssop, isopropyl myristate, jojoba, kukui nut,
lavandin, lavender, lemon, litsea
cubeba, macademia nut, mallow, mango seed, meadowfoam seed, mink, nutmeg,
olive, orange, orange roughy,
palm, palm kernel, peach kernel, peanut, poppy seed, pumpkin seed, rapeseed,
rice bran, rosemary, safflower,
sandalwood, sasquana, savoury, sea buckthorn, sesame, shea butter, silicone,
soybean, sunflower, tea tree, thistle,
tsubaki, vetiver, walnut, and wheat germ oils, butyl stearate, caprylic
triglyceride, capric triglyceride,
cyclomethicone, diethyl sebacate, dimethicone 360, isopropyl myristate,
mineral oil, octyldodecanol, oleyl alcohol,
silicone oil, and combinations thereof In certain embodiments, the oil is
mineral oil.
[0079] In some embodiments, the pharmaceutically acceptable excipient is at
least 95%, 96%, 97%,
98%, 9noz/0,
or 100% pure. In some embodiments, the excipient is approved for use in humans
and for veterinary
use. In some embodiments, the excipient is approved by United States Food and
Drug Administration. In some
embodiments, the excipient is pharmaceutical grade. In some embodiments, the
excipient meets the standards of the
United States Pharmacopoeia (USP), the European Pharmacopoeia (EP), the
British Pharmacopoeia, and/or the
International Pharmacopoeia.
[0080] The formulations of the pharmaceutical compositions described herein
may be prepared by any
method known or hereafter developed in the art of pharmacology. In general,
such preparatory methods include the
step of bringing the active ingredient (i.e., a glycosylated deltorphin
variant) into association with a carrier and/or
one or more other accessory ingredients, and then, if necessary and/or
desirable, shaping and/or packaging the
product into a desired single¨ or multi¨dose unit.
[0081] A pharmaceutical composition of the invention may be prepared,
packaged, and/or sold in bulk,
as a single unit dose, and/or as a plurality of single unit doses. As used
herein, a "unit dose" is discrete amount of
the pharmaceutical composition comprising a predetermined amount of the active
ingredient. The amount of the
active ingredient is generally equal to the dosage of the active ingredient
which would be administered to a subject
and/or a convenient fraction of such a dosage such as, for example, one¨half
or one¨third of such a dosage.
[0082] The relative amounts of the active ingredient, the pharmaceutically
acceptable carrier, and/or any
additional ingredients in a pharmaceutical composition of the invention will
vary, depending upon the identity, size,
and/or condition of the subject treated and further depending upon the route
by which the composition is to be
administered. By way of example, the composition may comprise between 0.1% and
100% (w/w) of the active
ingredient.
16
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
[0083] Preferred dosage forms include oral and parenteral dosage forms. Liquid
dosage forms for oral
and parenteral administration include, but are not limited to,
pharmaceutically acceptable emulsions,
microemulsions, solutions, suspensions, syrups and elixirs. In addition to the
active ingredients, the liquid dosage
forms may comprise inert diluents commonly used in the art such as, for
example, water or other solvents,
solubilizing agents and emulsifiers such as ethyl alcohol, isopropyl alcohol,
ethyl carbonate, ethyl acetate, benzyl
alcohol, benzyl benzoate, propylene glycol, 1,3¨butylene glycol,
dimethylformamide, oils (in particular, cottonseed,
groundnut, corn, germ, olive, castor, and sesame oils), glycerol,
tetrahydrofurfuryl alcohol, polyethylene glycols and
fatty acid esters of sorbitan, and mixtures thereof Besides inert diluents,
the oral compositions can include
adjuvants such as wetting agents, emulsifying and suspending agents,
sweetening, flavoring, and perfuming agents.
In certain embodiments for parenteral administration, the conjugates of the
invention are mixed with solubilizing
agents such as Cremophor, alcohols, oils, modified oils, glycols,
polysorbates, cyclodextrins, polymers, and
combinations thereof
[0084] Injectable preparations, for example, sterile injectable aqueous or
oleaginous suspensions may be
formulated according to the known art using suitable dispersing or wetting
agents and suspending agents. The
sterile injectable preparation may be a sterile injectable solution,
suspension or emulsion in a nontoxic parenterally
acceptable diluent or solvent, for example, as a solution in 1,3¨butanediol.
Among the acceptable vehicles and
solvents that may be employed are water, Ringer's solution, U.S.P. and
isotonic sodium chloride solution. In
addition, sterile, fixed oils are conventionally employed as a solvent or
suspending medium. For this purpose any
bland fixed oil can be employed including synthetic mono¨ or diglycerides. In
addition, fatty acids such as oleic
acid are used in the preparation of injectables.
[0085] The injectable formulations can be sterilized, for example, by
filtration through a bacterial¨
retaining filter, or by incorporating sterilizing agents in the form of
sterile solid compositions which can be
dissolved or dispersed in sterile water or other sterile injectable medium
prior to use.
[0086] In order to prolong the effect of a drug, it is often desirable to slow
the absorption of the drug
from subcutaneous or intramuscular injection. This may be accomplished by the
use of a liquid suspension of
crystalline or amorphous material with poor water solubility. The rate of
absorption of the drug then depends upon
its rate of dissolution which, in turn, may depend upon crystal size and
crystalline form. Alternatively, delayed
absorption of a parenterally administered drug form is accomplished by
dissolving or suspending the drug in an oil
vehicle.
[0087] Compositions for rectal or vaginal administration are typically
suppositories which can be
prepared by mixing the conjugates of this invention with suitable
non¨irritating excipients or carriers such as cocoa
butter, polyethylene glycol or a suppository wax which are solid at ambient
temperature but liquid at body
temperature and therefore melt in the rectum or vaginal cavity and release the
active ingredient.
[0088] Compositions for oral administration are typically liquid or in solid
dosage forms. Compositions
for oral administration may include protease inhibitors, including organic
acids such as citric acid, in order to inhibit
pancreatic and brush border proteases. Compositions for oral administration
may additionally include absorption
enhancers, such as acylcarnitine and lauroylcarnitine, to facilitate the
uptake of the peptide through the lumen of the
17
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
intestine into the systemic circulation by a paracellular transport mechanism.
Compositions for oral administration
may additionally include detergents to improve the solubility of the peptides
and excipients and to decrease
interactions with intestinal mucus. Solid form compositions for oral
administration, such as tablets or capsules, may
typically comprise an enteric coating, which further protects the peptides
from stomach proteases and permits
passage of the tablet or capsule into the small intestine. The solid form
composition may additionally comprise a
subcoat such as a non-ionic polymer. Examples of preparation of such orally
available formulations are disclosed in
US Patent 5,912,014, US Patent 6,086,918 and US Patent 6,673,574. The
disclosure of each of these documents is
hereby incorporated herein by reference.
[0089] Solid dosage forms for oral administration include capsules, tablets,
pills, powders, and granules.
In such solid dosage forms, the active ingredient is mixed with at least one
inert, pharmaceutically acceptable
excipient or carrier such as sodium citrate or dicalcium phosphate and/or a)
fillers or extenders such as starches,
lactose, sucrose, glucose, mannitol, and silicic acid, b) binders such as, for
example, carboxymethylcellulose,
alginates, gelatin, polyvinylpyrrolidinone, sucrose, and acacia, c) humectants
such as glycerol, d) disintegrating
agents such as agar, calcium carbonate, potato or tapioca starch, alginic
acid, certain silicates, and sodium carbonate,
e) solution retarding agents such as paraffin, f) absorption accelerators such
as quaternary ammonium compounds,
g) wetting agents such as, for example, cetyl alcohol and glycerol
monostearate, h) absorbents such as kaolin and
bentonite clay, and i) lubricants such as talc, calcium stearate, magnesium
stearate, solid polyethylene glycols,
sodium lauryl sulfate, and mixtures thereof In the case of capsules, tablets
and pills, the dosage form may comprise
buffering agents.
[0090] Solid compositions of a similar type may be employed as fillers in soft
and hard¨filled gelatin
capsules using such excipients as lactose or milk sugar as well as high
molecular weight polyethylene glycols and
the like. The solid dosage forms of tablets, dragees, capsules, pills, and
granules can be prepared with coatings and
shells such as enteric coatings and other coatings well known in the
pharmaceutical formulating art. They may
optionally comprise opacifying agents and can be of a composition that they
release the active ingredient(s) only, or
preferentially, in a certain part of the intestinal tract, optionally, in a
delayed manner. Examples of embedding
compositions which can be used include polymeric substances and waxes. Solid
compositions of a similar type may
be employed as fillers in soft and hard¨filled gelatin capsules using such
excipients as lactose or milk sugar as well
as high molecular weight polethylene glycols and the like.
[0091] The active ingredients can be in micro¨encapsulated form with one or
more excipients as noted
above. The solid dosage forms of tablets, dragees, capsules, pills, and
granules can be prepared with coatings and
shells such as enteric coatings, release controlling coatings and other
coatings well known in the pharmaceutical
formulating art. In such solid dosage forms the active ingredient may be
admixed with at least one inert diluent such
as sucrose, lactose or starch. Such dosage forms may comprise, as is normal
practice, additional substances other
than inert diluents, e.g., tableting lubricants and other tableting aids such
a magnesium stearate and microcrystalline
cellulose. In the case of capsules, tablets and pills, the dosage forms may
comprise buffering agents. They may
optionally comprise opacifying agents and can be of a composition that they
release the active ingredient(s) only, or
18
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
preferentially, in a certain part of the intestinal tract, optionally, in a
delayed manner. Examples of embedding
compositions which can be used include polymeric substances and waxes.
[0092] A pharmaceutical composition of the invention may be prepared,
packaged, and/or sold in a
formulation suitable for pulmonary administration via the buccal cavity. Such
a formulation may comprise dry
particles which comprise the active ingredient and which have a diameter in
the range from about 0.5 to about 7
nanometers or from about 1 to about 6 nanometers. Such compositions are
conveniently in the form of dry powders
for administration using a device comprising a dry powder reservoir to which a
stream of propellant may be directed
to disperse the powder and/or using a self propelling solvent/powder
dispensing container such as a device
comprising the active ingredient dissolved and/or suspended in a low¨boiling
propellant in a sealed container. Such
powders comprise particles wherein at least 98% of the particles by weight
have a diameter greater than 0.5
nanometers and at least 95% of the particles by number have a diameter less
than 7 nanometers. Alternatively, at
least 95% of the particles by weight have a diameter greater than 1 nanometer
and at least 90% of the particles by
number have a diameter less than 6 nanometers. Dry powder compositions may
include a solid fine powder diluent
such as sugar and are conveniently provided in a unit dose form.
[0093] Low boiling propellants generally include liquid propellants having a
boiling point of below 65 F
at atmospheric pressure. Generally the propellant may constitute 50 to 99.9%
(w/w) of the composition, and the
active ingredient may constitute 0.1 to 20% (w/w) of the composition. The
propellant may further comprise
additional ingredients such as a liquid non¨ionic and/or solid anionic
surfactant and/or a solid diluent (which may
have a particle size of the same order as particles comprising the active
ingredient).
[0094] Pharmaceutical compositions of the invention formulated for pulmonary
delivery may provide the
active ingredient in the form of droplets of a solution and/or suspension.
Such formulations may be prepared,
packaged, and/or sold as aqueous and/or dilute alcoholic solutions and/or
suspensions, optionally sterile, comprising
the active ingredient, and may conveniently be administered using any
nebulization and/or atomization device. Such
formulations may further comprise one or more additional ingredients
including, but not limited to, a flavoring agent
such as saccharin sodium, a volatile oil, a buffering agent, a surface active
agent, and/or a preservative such as
methylhydroxybenzoate. The droplets provided by this route of administration
may have an average diameter in the
range from about 0.1 to about 200 nanometers.
[0095] The formulations described herein as being useful for pulmonary
delivery are useful for intranasal
delivery of a pharmaceutical composition of the invention. Another formulation
suitable for intranasal
administration is a coarse powder comprising the active ingredient and having
an average particle from about 0.2 to
500 micrometers. Such a formulation is administered in the manner in which
snuff is taken, i.e. by rapid inhalation
through the nasal passage from a container of the powder held close to the
nares.
[0096] Formulations suitable for nasal administration may, for example,
comprise from about as little as
0.1% (w/w) and as much as 100% (w/w) of the active ingredient, and may
comprise one or more of the additional
ingredients described herein. A pharmaceutical composition of the invention
may be prepared, packaged, and/or
sold in a formulation suitable for buccal administration. Such formulations
may, for example, be in the form of
tablets and/or lozenges made using conventional methods, and may, for example,
comprise 0.1 to 20% (w/w) active
19
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
ingredient, the balance comprising an orally dissolvable and/or degradable
composition and, optionally, one or more
of the additional ingredients described herein. Alternately, formulations
suitable for buccal administration may
comprise a powder and/or an aerosolized and/or atomized solution and/or
suspension comprising the active
ingredient. Such powdered, aerosolized, and/or aerosolized formulations, when
dispersed, may have an average
particle and/or droplet size in the range from about 0.1 to about 200
nanometers, and may further comprise one or
more of the additional ingredients described herein.
[0097] A pharmaceutical composition of the invention may be prepared,
packaged, and/or sold in a
formulation suitable for ophthalmic administration. Such formulations may, for
example, be in the form of eye
drops including, for example, a 0.1/1.0% (w/w) solution and/or suspension of
the active ingredient in an aqueous or
oily liquid carrier. Such drops may further comprise buffering agents, salts,
and/or one or more other of the
additional ingredients described herein. Other opthalmically¨administrable
formulations which are useful include
those which comprise the active ingredient in microcrystalline form and/or in
a liposomal preparation. Ear drops
and/or eye drops are contemplated as being within the scope of this invention.
[0098] General considerations in the formulation and/or manufacture of
pharmaceutical agents may be
found, for example, in Remington: The Science and Practice of Pharmacy 21st
ed., Lippincott Williams & Wilkins,
2005.
[0099] The skilled clinician will be able to determine the appropriate dosage
amount and number of
doses of an agent to be administered to subject, dependent upon both the age
and weight of the subject, the
underlying condition, and the response of an individual patient to the
treatment. In addition, the clinician will be able
to determine the appropriate timing for delivery of the agent in a manner
effective to treat the subject.
Preferably, the agent is delivered within 48 hours prior to exposure of the
patient to an amount of a thrombosis or
thrombocytopenia provoking stimulus effective to induce thrombosis or
thrombocytopenia, and more preferably,
within 36 hours, and more preferably within 24 hours, and more preferably
within 12 hours, and more preferably
within 6 hours, 5 hours, 4 hours, 3 hours, 2 hours, or 1 hour prior to
exposure of the patient to an amount of
thrombosis or thrombocytopenia provoking stimulus effective to induce
thrombosis or thrombocytopenia. In one
embodiment, the agent is administered as soon as it is recognized (i.e.,
immediately) by the subject or clinician that
the subject has been exposed or is about to be exposed to a thrombosis or
thrombocytopenia provoking stimulus, and
especially a thrombosis or thrombocytopenia provoking stimulus to which the
subject is sensitized. In another
embodiment, the agent is administered upon the first sign of development of
thrombosis or thrombocytopenia, and
preferably, within at least 2 hours of the development of symptoms of
thrombosis or thrombocytopenia, and more
preferably, within at least 1 hour, and more preferably within at least 30
minutes, and more preferably within at least
minutes, and more preferably within at least 5 minutes of development of
symptoms of thrombosis or
thrombocytopenia. Symptoms of thrombosis or thrombocytopenia and methods for
measuring or detecting such
symptoms have been described and are well known in the art. Preferably, such
administrations are given until signs
of reduction of thrombosis or thrombocytopenia appear, and then as needed
until the symptoms of thrombosis or
thrombocytopenia are gone.
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
[0100] Although the descriptions of pharmaceutical compositions provided
herein are principally
directed to pharmaceutical compositions, which are suitable for administration
to humans, it will be understood by
the skilled artisan that such compositions are generally suitable for
administration to animals of all sorts.
Modification of pharmaceutical compositions suitable for administration to
humans in order to render the
compositions suitable for administration to various animals is well
understood, and the ordinarily skilled veterinary
pharmacologist can design and/or perform such modification with merely
ordinary, if any, experimentation.
[0101] Still further encompassed by the invention are kits that comprise one
or more inventive
complexes and/or compositions. Kits are typically provided in a suitable
container (e.g., for example, a glass, foil,
plastic, or cardboard package). In certain embodiments, an inventive kit may
include one or more pharmaceutical
excipients, pharmaceutical additives, therapeutically active agents, and the
like, as described herein. In certain
embodiments, an inventive kit may include means for proper administration,
such as, for example, graduated cups,
syringes, needles, cleaning aids, and the like. In certain embodiments, an
inventive kit may include instructions for
proper administration and/or preparation for proper administration.
EXAMPLES
[0102] PEG polymers can be modified by various functional groups (see Harris
et al., Clin
Phamacokinet, 40:539-551 (2001) and the amino terminal end of GCPFs or other
linking amino acids, such as lysine
residues present in the GCPF, can be linked thereto. By "pegylated GCPF" is
meant a GCPF having a polyethylene
glycol moiety covalently bound to an amino acid residue or linking group of
the peptide backbone of the GCPF.
Isolation Of Platelets for Labeling with CM-Orange
[0103] Blood is drawn from consenting normal human volunteers by venipuncture
into 0.1 volume of
Aster-Jandl citrate-based anticoagulant (Hartwig and DeSisto, J. Cell Biol.,
407-425 (1991)) and platelet rich plasma
(PRP) is prepared by centrifugation of the anticoagulated blood at 300xg for
20 min at room temperature. Platelets
are separated from plasma proteins by gel-filtration at room temperature
through a small Sepharose 2B column
(Hoffmeister et al., Biol Chem. 276, 24751-24759 (2001)). Platelets used in
the in vitro THP-1 cell or HepG2
binding & phagocytosis assays described below are labeled with 1.8 uM
CellTrackerTm Orange CMTMR (CM-
Orange) for 20 min at 37 C (Brown et al., J. Biol. Chem. 275, 5987-5995
(2000)), and unincorporated dye was
removed by centrifugation (850xg, 5 min) with 5 volumes of washing buffer
containing 140 mM NaCk 5 mM KO,
12 mM trisodium citrate, 10 mM glucose, and 12.5 mM sucrose, 1 ug/ml PGE1, pH
6.0 (buffer A). Platelets were
resuspended at 3x108/m1 in a solution containing 140 mM NaCk 3 mM KO, 0.5 mM
MgC12, 5 mM NaHCO3, 10
mM glucose and 10 mM Hepes, pH 7.4 (buffer B).
Platelet Preparation
[0104] Human venous blood is collected from healthy volunteers by venipuncure
into one-tenth of the
blood volume of Aster Jandl citrate-based anticoagulant (85 mM sodium citrate,
69 mM citric acid, 111 mM
glucose, pH 4.6). See Rumjantseva et al., Nature Medicine, 15:1273-80 (2009).
21
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
[0105] Platelet-rich plasma (PRP) is prepared by centrifugation at 268 g for
20 min at 22 C. For long-
term storage experiments, human platelet concentrates are stored at 4 C for up
to 10 days without agitation. PRP
samples (3 ml) are obtained under sterile conditions at days 0, 2, 5 and 10.
Platelets are collected from the PRP by
centrifugation at 834 g for 5 mi, washed in platelet buffer (140 mM NaC1, 5 mM
KO, 12 mM trisodium citrate, 10
mM glucose, 12.5 mM sucrose 1 ug/ml PGE1, pH 6.0)(buffer A) and resuspended in
10 mM HEPES, 140 mM NaC1,
3 mM KO, 0.5 mM MgC12, 10 mM glucose and 0.5 mM NaHCO3, pH 7.4 (buffer B). See
Rumjantseva et al.,
Nature Medicine, 15:1273-80 (2009)
Platelet Temperature And Storage Protocols
[0106] Isolated platelets are chilled for 2 hrs at ice-bath temperatures, a
process designated as short term
cooling (or as 0 C), or are resuspended in platelet-poor plasma and stored for
48 hr at 4 C, designated as long-term
refrigeration (or as 4 C). All cooled and refrigerated platelets are rewarmed
for 15 min at 37 C before use. With
the exception of Indiumill labeling, all labeling procedures and enzymatic
digestion of platelets are performed
before storage. Freshly isolated platelets, maintained for a maximum of 2 hrs
at 22 C are used as controls for all
survival experiments, and are designated as fresh platelets (or as 22 C).
Rumjantseva et al., Nature Medicine,
15:1273-80 (2009).
Histology
[0107] Mice are infused with 3 x 109 biotinylated platelets. Organs are
collected 5, 15 and 30 mins and
24 hrs afterward, fixed in paraformaldehyde and embedded in paraffin and
sectioned every 3 um. Distribution of
biotinylated platelets is visualized with streptavidin-peroxidase conjugate
and the ImmunoHisto Peroxidase
Detection Kit (Pierce). Sections are counterstained with H&E according to the
manufacturer's recommendations.
Quantitative analysis of staining is done in blinded samples. Ten tissue
sections from mice having similar levels of
injected platelets are selected and scored for hepatocytes and macrophages
containing biotinylated platelets. See
Rumjantseva et al., Nature Medicine, 15:1273-80 (2009)
Macrophage Depletion
[0108] Mice are depleted of phagocytic cells by a single injection of
liposomes containing
dichloromethylene bisphosphonate (clodronate liposomes). Control liposomes are
prepared with PBS in place of
condronate. Mice are injected intravenously with 0.02 ml of clodronate
liposomes or control liposomes per 10 g
body weight 24 hrs before platelet transfusion. This treatment depletes 99% of
Kupffer cells and 95% of splenic
macrophages. Macrophages are stained with an antibody to mouse F4/80 (SEROTEC,
clone CI:A3-1) in tissue
sections of clodronate-treated and untreated mice. See Rumjantseva et al.,
Nature Medicine, 15:1273-80 (2009).
Multi-Distance Spatial Cluster Analysis (Ripley's K-Function)
[0109] K-function analysis is used to determine whether GPIba distribution is
random, clustered or
dispersed on the membrane of cold versus room-temperature platelets. GPIba is
visualized with immunogold, x and
22
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
y coordinates are measured and L(r)-r versus the radius r is plotted. Values
less than -1 indicate significant
dispersal, whereas values greater than 1 indicate significant clustering. See
Rumjantseva et al., Transfusion and
Apheresis Science 42:63-70 (2010).
In Vitro Platelet Phagocytic Assay Using Stimulated THP-1 Cells (Mac-1
Mediated Binding)
[0110] THP-1 cells are obtained from ATCC (ATCC #TIB-202). Differentiated THP-
1 phagocytic cells
(1 x 106 cells/m1) are activated by the addition of 150 pg/ml phorbol 12-
myristate 13-acetate for 15 min at 37 C and
plated onto human albumin (1 mg/m1)-coated 24-well plates (1 x106 cells/well)
and allowed to adhere for 45 min at
37 C in RPMI 1640 medium. The cells are washed and maintained in Hanks'
balanced salt solution (HBSS)
(Cellgro, Mediatech) containing Ca2'Mg2 or alternatively HBSS without Ca2'Mg2'
containing 2 mM EGTA and 2
mM EDTA to determine the effects of divalent cations on platelet phagocytosis.
[0111] CM-Orange-labeled RT or chilled platelets (5 x108 cells/m1) are pre-
incubated for 5min with
GCPFs diluted to concentrations ranging from 0.1 nM to 100 nM, then added to
the THP-1 cells for 30 min at 37 C
under gentle agitation. Surface-associated platelets are removed through
digestion with 0.05% trypsin-EDTA
(Invitrogen) followed by the addition of trypsin inhibitors for 5 min. THP-1
cells are detached from the wells and
incubated with FITC-anti-CD61 mAb, which recognizes the platelet-specific b3
integrin (Product # BYA9203-1
Accurate Scientific Corp., Westbury, NY). Platelet ingestion is determined and
quantified by flow cytometry on a
FACSCalibur flow cytometer (BD Biosciences). Data is acquired in log10
fluorescence. The percentage of
phagocytes positive for CM-Orange fluorescence when incubated with RT
platelets is set to one in order to calculate
the ratio of the phagocytic increase for the chilled platelet population.
Construction of Mac-1 Transfected Mammalian Cells and their Cultivation:
[0112] Chinese hamster ovary (CHO) or HEK-293 cell lines (ATCC Manassas, VA)
are generated that express
functional recombinant human Mac-1 receptor on the cell surface. Isolated cDNA
encoding am chain (CD1 1 b) is
cloned into the expression vector pcDNA3, isolated cDNA encoding 132integrin
(CD18) is cloned into pZeoSV.
SuperfectTM transfection reagent (Qiagen, Hilden, Germany) is used for cell
transfection. Clones are selected for
resistance against 700 Kg/m1 G418 (Geneticin , Gibco, Eggenstein, Germany) and
250 Kg/m1 Zeocin (Invitrogen,
Karlsruhe, Germany) and by the flow cytometric detection of CD ii b and CD18
epitopes. Clones used in further
experiments should contain identical expression levels of CD1 1 b or CD18 as
determined by flow cytometry and can
be further examined by RT-PCR and immunoprecipitation to prove the correct
expression of Mac-1. Transfected
cells are maintained in Dulbecco's modified Eagle medium (DMEM), 10% (voUvol)
fetal calf serum (FCS), 100
U/ml penicillin and 100 Kg/m1 streptomycin. All culture media are from GIBCO
(Eggenstein, Germany), and the
cell culture plastic was from Nunc (Roskilde, Denmark).
Adhesion assay of platelets to Mac-1 expressing cells
[0113] CHO cells expressing Mac-1 are seeded on a VenaFuxTm platform (Cellix
LTD, Dublin).
Approximately 2.5 x106 human platelets in PRP are incubated for 5min with
control buffer or buffer with GCPFs
23
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
diluted to concentrations ranging from 0.1 nM to 100 nM. Treated platelets are
then infused into VenaFuxTm
platform according to the manufacturer's protocol and platelet adhesion events
are quantified by the Cellix image
analysis software.
Adhesion assay of Mac-1 bearing cells to immobilized GPIba
[0114] THP-1 cells or alternatively CHO or 293 cells expressing Mac-1 are
harvested with cell-
dissociating buffer (Life Technologies) for 1 minute at 22 C, washed twice,
resuspended in serum-free media, and
loaded with BCECF AM [2',7'-bis-(2-carboyethyl)-5-(and-6)-carboxyfluorescein,
acetoxymethyl ester] (1 umoUL)
according to the manufacturer's protocol (Molecular Probes). Cells (105 per
well) are placed in 48-well tissue
culture plates (Costar) coated with 200 uL of 5 nmoUL fibrinogen or 50 nmoUL
sGPIb (R&D Systems, Minneapolis
MN Cat #4067-GP) overnight at 4 C and then blocked with 0.5%
polyvinylpyrrolidone for 1 hour at room
temperature. Coated plates are then incubated for 5min with GCPFs diluted to
concentrations ranging from 0.1 nM
to 100 nM. Adhesion to cells with Mac-1 is stimulated with phorbol 12-
myristate 13-acetate (PMA) (20 ng/mL) in
the presence of 2 mmoUL Mg2. Plates are washed with 0.9% NaC1 (3 to 5 times),
and adhesion was quantified by
measuring the fluorescence of BCECF AM¨loaded cells with a Cytofluor II
fluorescence multiwell microplate
reader (PerSeptive Biosystems).
In vitro HepG2-Based Platelet Ingestion Assay (Ashwell-Morrell Mediated
Binding)
[0115] Human HepG2 hepatocarcinoma cells (ATCC HB-8065) are maintained in aMEM
(GIBCO
Invitrogen), 2% heat-inactivated bovine calf serum (BCS), 3% standard fetal
bovine serum (FBS), and 1% of a
penicillin and streptomycin solution composed of 10,000 U m1-1 penicillin G
and 10 mg ml-lstreptomycin sulphate.
HepG2 cells express Ashwell-Morell receptors similar to human hepatocytes, but
do not express Mac-1 receptors.
HepG2 cells are not passaged > 2 times before use.
[0116] For assays, the HepG2 cells are transferred to 24-well plates (106 per
well), allowed to adhere for
24 h, and starved for 30 min by replacing aMEM media without serum.
Cytochalasin D (SIGMA) is diluted into
aMEM media at the indicated concentrations and added to the HepG2 cells. DMSO
is used as control. GCPFs at 10
fold diluted concentrations from 0.1 nM to 100 nM are mixed lx108 CM-Orange-
labeled platelets (fresh, at 22 C
platelets or platelets cooled at 0 C or 4 C) per well, with or without
cytochalasin D. HepG2 cells and platelets are
then incubated for 5-30 min at 37 C with gentle agitation. After the
incubation period, the HepG2 monolayers are
washed 3 times by removing and changing the buffer. HepG2 cells are
dissociated from the wells with 0.05%
trypsin, 0.53 mM EDTA in HBSS (GIBCO Invitrogen) at 37 C for < 10 min. CM-
Orange-labeled platelet ingestion
(phagocytosis) is quantified by flow cytometry. HepG2 cells are gated
according to their forward and side scatter
characteristics. HepG2 cells with ingested platelets acquired orange
fluorescence. Platelets adherent to HepG2 cell
surfaces are labeled with the FITC conjugated antibody to human CD61 (Beckon-
Dickinson). CM-Orange-labeled
cells are counted. Approximately 10,000 events can be acquired for each
sample.
Von Willebrand Factor Binding Assay
24
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
[0117] Platelet bound VWF was detected by incubating 3 ill of FITC-conjugated
antibody to human or
mouse VWF, or with non-immune rabbit polyclonal IgG (control), with 2.5 x106
human platelets in PRP for 20 min
at room temperature after platelets have been incubated for 5 min with control
buffer or buffer with GCPFs diluted
to concentrations ranging from 0.1 nM to 100 nM. Labeled samples were analyzed
by flow cytometry after dilution
into buffer. Data is expressed as % positive cells determined for VWF compared
to the appropriate IgG negative
control.
Human Platelet Studies
[0118] The following human platelet studies are conducted using procedures
from Wandall et al., Blood
111:3249-56 (2008) are adapted as follows.
Optimization of the GCPF-treatment of platelets.
[0119] Aliquots (2 mL) of nonwashed human apheresis platelets (1- 2 x 109
platelets/mL) are incubated
with increasing concentrations (0.00 mM to 1.50 mM at 0.25 mM increments) of
GCPFs for time periods of 0 to 90
minutes at 15 minute increments at 37 C. Platelets are washed, and the
platelets are assessed for maximal RCA-1
binding and minimal sWGA binding using varying concentrations of GCPFs and
time of incubation.
In vivo studies.
[0120] Human studies are conducted with the approval of an institutional
review board and a radiation
safety committee after obtaining informed consent from all volunteers in
accordance with the Declaration of
Helsinki. Volunteers must meet standard donation criteria, and must not have
taken any medication known to alter
platelet function for 14 days before platelet donation.
[0121] A PFA-100 assay (Siemans USA, Deerfield IL), is performed with human
blood collected into
vacutainer tubes, containing 3.2% sodium citrate as the anticoagulant, from
volunteers who had not taken any
platelet inhibitory medications over the previous two weeks. A 5 IA aliquot of
GCPF dissolved in 25% DMSO is
added to 1 ml of whole human blood to give a final GCPF concentration ranging
from 0.1 nM to 100 nM and a final
DMSO concentration of 0.125%. Tubes are inverted 10 times to mix, and allowed
to sit at room temperature for 5
m prior to analysis with the PFA-100 instrument. Collagen/epinephrine
cartridges (Siemans USA, Deerfield, IL) are
used for the PFA-100 assay following the manufacturer's protocol. Closure
times of 80 4 seconds are typically
obtained with 0.125% DMSO alone in whole blood.
[0122] The study is an open-label, controlled phase 1 study using standard
radiolabeled autologous
platelet transfusion protocols to determine platelet recoveries and survivals.
Holmes et al., Brit. J. Haematol. 84:717-
23 (1993). Three platelet products - - each stored for 48 hours - - are
evaluated: (1) platelets stored at 4 C that were
treated with GCPF ("GCPF-treated cooled"); (2) control platelets stored at 4 C
without GCPF treatment ("untreated
cooled"); and (3) control platelets stored at 22 C without GCPF treatment
("untreated room temperature" or
[0123] Healthy volunteers are enrolled, and each donatee apheresis platelets
on a Haemonetics MCS+
apheresis machine (Haemonetics, Braintree, MA). Platelets are collected into
one bag, and then divided into 2 bags
of approximately 120 mL. GCPFs are supplied in 0.9% saline to produce a 40 mM
sterile filtered solution filled into
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
5-mL plastic syringes. Aseptic media fill validation is conducted as a part of
the controls on the fill and finish
operation. The stock solution is kept at 4 C throughout the process and
storage and stability of the CCP fusion
molecule is verified. Immediately after collection, one bag of platelets from
each donor is treated with GCPF by
sterile docking the GCPF fusion molecule container onto a platelet storage
bag. After addition of GCPFs, the
GCPF-treated platelets are incubated for 1 hour at 37 C with agitation and
then stored at 4 C for 36 to 48 hours
without agitation. The other bag of platelets serves as a control and is
incubated without GCPF treatment for 1 hour
at 37 C with agitation, followed by storage for 36 to 48 hours either without
agitation at 4 C or with agitation at
22 C.
[0124] The bags of platelets from each individual donor are radiolabeled as
described in Holmes et al., Br
J Haematol, 84:717-23 (1993) with a different radioactive isotope, either
51Chromium or 111Indium, and 5 mL to 10
mL of both the radiolabeled test and control platelets are simultaneously
transfused back to their donor.
Radioisotopes used for labeling are alternated between test and control
platelets to avoid bias related to the isotope
used for radiolabeling. Blood samples are drawn before and at 2 hours, 1, 2,
3, 5, 7, and 10 days after transfusion,
and the posttransfusion recovery and survival of the platelets are determined
using the COST program, described in
Lotter et al., Comput Biol Med; 18:305-15 (1988). Samples are obtained to
correct for elution of either
radioisotopic label and for any residual radioactivity bound to red cells. The
recovery and survival data are reported
both uncorrected for label elution or residual activity, as well as corrected
for these 2 parameters.
[0125] During and for 2 hours after each platelet infusion, subjects are
carefully monitored for vital signs
and potential adverse reactions. Follow-up visits are conducted at days 1, 2,
3, 5, 7, 10, 14, and 90. Vital signs are
obtained at each visit, and the subjects are queried about the occurrence of
any adverse events. Additional telephone
interviews to document any long-term adverse events are conducted on days 28,
42, 56, and 70 after infusion.
In vitro testing of the human platelet preparations.
[0126] Baseline and at days 14 and 90 after infusion, samples are taken from
each volunteer to detect IgG
and IgM antiplatelet antibodies. Platelets with and without GCPF treatment are
incubated with each volunteer's
plasma and with the Fab'2 fraction of FITC-conjugated goat antibody to the Fc
chain of human IgG and IgM
(Jackson Laboratories, Bar Harbor, ME). Binding of conjugated Fab'2 fragments
is monitored by FACS analysis
(FACScan; Becton Dickinson Biosciences). Plasma with known HLA antibodies is
used as a positive control.
[0127] Samples are collected on days 0 and 2 from the stored platelets for the
following measurements.
Blood gas and pH measurements using a blood gas analyzer (Bayer, East Walpole,
MA). Glucose and lactate are
measured using an Abbott Aeroset Analyzer (Abbott, Round Lake, IL). Platelet
counts and mean platelet volume
(MPV) are performed on an ABX Micros particle counter (ABX, Montpellier,
France). Morphology score is
performed by the method of Kunicki et al., Transfusion; 15:414-21 (1975).
Hypertonic shock response (HSR) and
extent of shape change (ESC) are performed as described in Murphy et al.,
Transfus Med Rev; 8:29-36 (1994).
CD62P expression is measured by FITC-labeled CD62P-specific monoclonal
antibody S-12 using FACS analysis.
Annexin V binding is determined by FACS analysis using fluorescently labeled
annexin-V (Vybrant Assay Kit [V-
13240]; Molecular Probes, Eugene, OR). GCPF-treatment is verified using
fluorescently labeled RCA-1 and sWGA
lectins with FACS analysis as described in Hoffmeister et al., Science,
301:1531-34 (2003).
26
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
[0128] Platelet aggregation and agglutination experiments are performed with a
PLT aggregation profiler
(Model PAP-; Bio/Data, Horsham, PA). Platelets are washed and resuspended as
described in Babic et al.,
Transfusion; 47:442-51 (2007) and activated by adding 0.1 U to 1 U thrombin
(Sigma-Aldrich, St Louis, MO) per
mL; platelet-rich plasma (PRP) is mixed with platelet-poor plasma (PPP) in the
ratio 1:1 and is then activated
through the addition of 1.5 mg/mL ristocetin (Sigma-Aldrich) for 3 minutes at
37 C under constant stirring (1000
rpm). Resuspension buffer for washed PLTs and PPP for PRP are set as maximum
of light transmission.
In vivo safety of infusion of cold-stored human platelets treated with GCPFs.
[0129] The safety of transfused chilled GCPF-treated platelets, along with any
residual GCPF, can be
evaluated by regular clinical assessments, follow-up phone interviews,
monitoring the subjects' platelet counts, and
testing for the presence of antibodies against both GCPF-treated and untreated
platelets.
[0130] Platelet count in the storage bags is assesed before and after storage.
Platelets are assessed at 1, 2,
4 days for GCPF-treated 4 C-storage, control 4 C-stored, and control 22 C-
stored platelets. Overall, pH, PCO2,
HCO3, glucose, P-selectin, and annexin V binding is measured.
[0131] The following procedured are adapted from Hoffmeister et al., US Patent
Application
2008/0138791. Modest cooling primes platelets for activation, but
refrigeration causes shape changes and rapid
clearance, compromising storage of platelets for therapeutic transfusions. It
has preveiously been shown that shape
change inhibition does not normalize cold-induced clearance. It has also been
shown that cooling platelets
rearranges the surface configuration of the von Willebrand factor (VWF)
receptor complex alpha subunit (GPIba),
such that it becomes targeted for recognition by complement receptor 3
receptors (CR3) predominantly expressed on
liver macrophages, leading to platelet phagocytosis and clearance. GPIba
removal prolongs survival of unchilled
platelets. Chilled platelets bind VWF and function normally in vitro and ex
vivo after transfusion into CR3-deficient
mice. Cooled platelets, however, are not "activated" like platelets exposed to
thrombin or ADP, and their VWF-
receptor complex reacts normally with activated VWF.
[0132] As the temperature falls below 37 C, platelets become more susceptible
to activation by
thrombotic stimuli, a phenomenon known as "priming" (Faraday and Rosenfeld,
Anesthesiology, 88:1579-1585
(1998); Hoffmeister et al., J Biol Chem 276:24751-24759 (2001)). Priming may
be an adaptation to limit bleeding
at lower temperatures of body surfaces where most injuries occur. It has been
proposed that the hepatic clearance
system's purpose is to remove repeatedly primed platelets, and that
conformational changes in GPIba that promote
this clearance do not affect GPIba's hemostatically important binding to VWF.
Therefore, selective modification of
GPIba may accommodate cold storage of platelets for transfusion.
[0133] This example compares the in vitro and in vivo hemostatic function of
chilled, unmodified and
chilled, GCPF-treated platelets. Chilled platelets are not "activated" in the
sense of agonist-stimulated platelets.
Patients undergoing surgery under hypothermic conditions may develop
thrombocytopenia or show severe
hemostatic post-operative impairments. It is believed that under these
hypothermic conditions, platelets might lose
their functionality. However, when patients undergo hypothermic surgery, the
whole organism is exposed to
hypothermia leading therefore to changes in multiple tissues. Adhesion of non-
chilled platelets to hepatic sinusoidal
endothelial cells is a major mechanism of cold preservation injury (Takeda et
al., Transplantation 27:820-28 (1999)).
27
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
Therefore, it is likely that it is the interaction between cold hepatic
endothelium and platelets, not platelet chilling
per se, that leads to deleterious consequences under hypothermic conditions of
surgery or transplantation of cold
preserved organs (Upadhya et al, Transplantation 73:1764-70 (2002)).
[0134] Two approaches show that chilled platelets have hemostatic function. In
one approach, the
circulation of chilled platelets in amb2 deficient mice facilitates studies of
platelet function after cooling. In the
other approach, the function of modified chilled and (presumably) circulating
platelets is tested.
[0135] Murine platelets that express the human GPIba chain are generated from
transgenic mice (Ware
et al., PNAS 97:2803-08 (2000)) and are herein referred to as "murineTG"
platelets. Human and murineTG unmodified
and GCPF-treated chilled platelets are tested for functionality, including in
vitro aggregation to agonists, P-selectin
exposure and fibrinogen binding.
[0136] amb2-deficient or wild-type mice are transfused with murine chilled or
room temperature platelets,
GCPF-treated or not, and allowed to circulate for 30 min, 2 and 24 hours. It
is determined whether chilled platelets
contribute to clotting reactions caused by tail vein bleeding and if these
platelets bind agents such as fibrinogen after
activation. It is further determined how chilled platelets, GCPF-treated or
not, contribute to clotting on ferric
chloride injured and exteriorized mouse mesenteries, an in vivo thrombus-
formation model. This method detects the
number of platelets adherent to injured vessels and has documented impaired
platelet vessel wall interactions of
platelets lacking glycoprotein V or 133-integrin function (Ni et al, Blood
98:368-73 (2001); Andre et al, Nat Med
8:247-52 (2002)). Finally, the storage parameters of the modified platelets
are determined.
[0137] In vitro platelet function is compared using aggregation with thrombin
and ADP and botrocetin
induced VWF-binding to murineTG platelets. MurineTG and human chilled
platelets GCPF-treated or untreated
platelets are normalized to a platelet concentration of 0.3x109/mm3, and
aggregation is induced using the various
agonists according to standard protocols (Bergmeier et al., J. Biol. Chem.,
276: 25121-26 (2001)). To study VWF-
binding, murine VWF is activated using botrocetin and the binding of
fluorescently labeled VWF to chilled platelets
modified or not in PRP is analyzed (Bergmeier, Ibid). To evaluate whether
degranulation of platelets occurs during
GCPF-treatment, P-selectin exposure of chilled murineTG and human platelets,
GCPF-treated or not, is measured
using fluorescent labeled anti-P-selectin antibodies by flow cytometry as
described in Michelson et al., Proc Natl
Acad Sci, USA 93:11877-82 (1996).
[0138] 109 CMFDA-labeled platelets are transfused into mice, first verifying
that these platelets are
functional in vitro. It is determined whether chilled platelets contribute to
aggregation by transfusing chilled or room
temperature CMFDA-labeled platelets into amb2 deficient mice. At 30 min, 2
hours and twenty-four hours after the
infusion of platelets, a standard tail vein bleeding test is performed as
described in Denis et al, Proc Natl Acad Sci
USA 95:9524-29 (1998). The emerging blood is fixed immediately in 1%
formaldehyde and platelet aggregation is
determined by whole blood flow cytometry. Platelet aggregates appear as bigger
sized particles in the dot plot
analysis. To verify that the transfused platelets do not aggregate in the
normal circulation, the mice are also bled
through the retroorbital eye plexus into an anticoagulant. Platelets do not
form aggregates under these bleeding
conditions. The emerging blood is fixed immediately and platelets are analyzed
by flow cytometry in whole blood
as described above. Platelets are identified through binding of a
phycoerythrin-conjugated 11'11,133 specific
28
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
monoclonal antibody. The infused platelets in the blood sample are identified
by their CMFDA-fluorescence. Non-
infused platelets are identified by their lack of CMFDA fluorescence, per
Michelson et al, Proc Natl Acad Sci USA
93:11877-82 (1996). The same set of tests is performed with CMFDA modified
GCPF-treated chilled platelets
transfusing these platelets into am132 and wild-type. This experiment tests
aggregation of chilled platelets modified
or not in shed blood.
[0139] 109 CM-orange labeled unmodified chilled or room temperature platelets
are transfused into am132
deficient mice to verify that these platelets are functional in vitro. At 30
min, 2 h and twenty-four hours after the
infusion of CM-orange labeled platelets, PRP is isolated as described and
analyzed by flow cytometry. P-selectin
exposure is measured using an anti FITC-conjugated anti P-selectin antibody as
described in Berger et al, Blood
92:4446-52 (1998). Non-infused platelets are identified by their lack of CM-
orange fluorescence. The infused
platelets in the blood sample are identified by their CM-orange fluorescence.
CM-orange and P-selectin positive
platelets appear as double positive fluorescently (CM-orange/FITC) stained
platelets. To verify that chilled platelets
still expose P-selectin after thrombin activation, PRP is activated through
the addition of thrombin (1 U/ml, 2 min at
37 C) and P-selectin exposure is measured as described. To analyze the binding
of fibrinogen to 1143, isolated
platelets are activated through the addition of thrombin (1 U/ml, 2 min, 37 C)
and Oregon-green coupled fibrinogen
(20 ug/ml) added for 20 min at 37 C (Hellmann et al, Cytometry 17:287-93
(1994)). The samples are analyzed
immediately by flow cytometry. The infused platelets in the PRP sample are
identified by their CM-orange
fluorescence. CM-orange and Oregon-green positive platelets appear as double
positive fluorescently stained (CM-
orange/Oregon green) platelets. The same sets of experiments are performed
with CM-orange labeled CCP-treated
chilled platelets transfused into aM132 deficient and WT mice.
In Vivo Anti-Thrombotic Activity of GCPF-014.
[0140] Initial in vivo experiments administering GCPF-014 into both wild-type
mice and mice expressing human
GPIba demonstrate that GCPF-014 is active in transgenic mice (described in
Ware et al., PNAS 97:2803-08 (2000))
in which the human GPIba replaces the mouse platelet GPIba. GCPF-014 extended
tail bleeding time, as measured
in Ware et al., Id. As expected, GCPF-014 is not active in wild-type mice,
demonstrating that GCPF-014 is specific
for the human platelet GPIba. Treatment with GCPF-014 demonstrated no overt
toxicity at 50 ug per mouse (avg
wt = 20 gm; dose = approximately 2.5 mg/kg GCPF-014.
In Vivo Thrombosis Model [See Hoffmeister etal., US 2008/0138791]
[0141] The delivery of room temperature and unmodified chilled platelets to
injured endothelium of
amb2 deficient mice can be demonstrated using double fluorescently labeled
platelets. The resting blood vessel is
monitored for 4 min, then ferric chloride (301 of a 250-mM solution) (Sigma,
St Louis, Mo.) is applied on top of the
arteriole by superfusion, and video recording resumed for another 10 min.
Centerline erythrocyte velocity (Vrbc) is
measured before filming and 10 min after ferric chloride injury. The shear
rate is calculated on the basis of
Poiseuille's law for a Newtonian fluid (Denis, et al, Proc Natl Acad Sci USA
95:9524-29 (1998). These experiments
show if chilled platelets have normal hemostatic function. These experiments
are repeated in wild-type mice
29
CA 02871057 2014-06-20
WO 2013/096932 PCT/US2012/071530
comparing room temperature and GCPF-treated chilled murineTG platelets using
two different, fluorescently labeled
platelet populations injected into the same mouse and analyze thrombus
formation and incorporation of both platelet
populations.
[0142] In vitro platelet functions and survival and in vivo hemostatic
activity are measured in untreated
chilled and GCPF-treated chilled murineTG platelets stored for 1, 5, 7 and 14
days under refrigeration as described
above. Recovery and circulation times of these stored untreated chilled and
GCPF-treated chilled platelets are
compared in order to determine that: 1) the modification through GCPF-
treatment onto chilled murineTG platelets is
stable after long-term refrigeration; and 2) the GCPF-treated chilled
platelets function normally. Survival
experiments are performed as described above. As an ultimate test that GCPF-
treated, stored platelets are
functionally intact and contribute to hemostasis, the platelets are transfused
into total-body-irradiated mice (Hoyer et
al, Oncology 49:166-72 (1992)). To obtain sufficient numbers of platelets,
mice are injected with commercially
available murine thrombopoietin for seven days to increase their platelet
count (Lok et al, Nature 369:565-68
(1994)). Isolated platelets are modified using the optimized GCPF-treated
protocol, stored under refrigeration,
transfused, and tail vein bleeding times measured. Since untreated chilled
platelets do not persist in the circulation,
a comparison of GCPF-treated cooled platelets with room temperature stored
platelets is not necessary at this point.
The murineTG platelets are stored under refrigeration in standard test tubes.
If a comparison with room temperature
stored murineTG platelets is desired, primate platelets can be used. Rather
than engineer special down-scale, gas-
permeable storage containers to accommodate mouse platelets, such comparisons
are more appropriate for primates
(including humans) for which room temperature storage bags have been designed.
* * *
[0143] Although the foregoing invention has been described in some detail by
way of illustration and
example for purposes of clarity of understanding, it is apparent to those
skilled in the art that numerous minor
changes and modifications to the methods and materials described may be
practiced while remaining within the
scope of the invention. Therefore, the description and examples should not be
construed as limiting the scope of the
invention.
[0144] The disclosures of all publications, patents, patent applications and
published patent applications
referred to herein are hereby incorporated herein by reference in their
entirety.