Note: Descriptions are shown in the official language in which they were submitted.
SURGICAL GUIDES FROM SCANNED IMPLANT DATA
CROSS REFERENCE
[0001] This application claims priority to United States Provisional Patent
Application
Serial No. 61/645,890 filed May 11, 2012, United States Provisional Patent
Application Serial
No. 61/642,063 filed May 3, 2012, and also United States Provisional Patent
Application Serial
No. 61/699,938 filed September 12, 2012.
TECHNICAL FIELD
[0002] The present disclosure generally relates to apparatus and methods for
manufacturing a surgical guide, and more particularly, to apparatus and
methods for
manufacturing a patient specific resection guide.
BACKGROUND
[0003] Many surgical procedures require accurate cuts of bone. For example, in
mandibular reconstruction surgery, deficient or infectious portions of the
mandible may be
removed from the patient and replaced with bone graft. In some instances, a
surgeon performing
mandibular reconstruction surgery typically makes several cuts on the mandible
to properly fit a
bone graft. To make an accurate cut, the surgeon may use a resection guide to
guide the motion
of the resection tool toward the bone. The resection guide can also be used to
cut a bone portion
from other anatomic locations of the patient in order to harvest bone grafts.
[0004] As discussed above, resection guides are typically used to make
accurate cuts on
the patient's anatomy. Although many resection guides have been developed over
the years, it is
still desirable to produce resection guides that are specifically designed for
a particular patient in
order to enhance cutting accuracy.
SUMMARY
[0005] The present disclosure relates to methods of making a patient specific
surgical
guide that is configured to guide a movement of a tool toward a tissue body.
In an embodiment,
the method includes the following steps: (1) obtaining a virtual three-
dimensional model of a
fixation member, the obtained virtual three-dimensional model of the fixation
member having a
planned post-operative shape and defining at least one hole that is configured
to receive a
fastener; (2) processing the virtual three-dimensional model of the fixation
member so as to
couple the virtual three-dimensional model of the fixation member to a first
virtual three-
- 1 -
CA 2872397 2019-10-15
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
dimensional model of the tissue body, the first virtual three-dimensional
model of the tissue body
defining a first region, such that a central axis of the at least one hole is
substantially aligned
with a first target location of the first region; (3) creating a virtual three-
dimensional model of a
guide that defines at least one hole; and (4) processing the virtual three-
dimensional model of the
guide so as to couple the virtual three-dimensional model of the guide to a
second virtual three-
dimensional model of the tissue body having a second region that is
substantially identical to the
first region, such that a central axis of the at least one hole is
substantially aligned with a second
target location of the second virtual three-dimensional model of the tissue
body, wherein the
second target location is positioned identically with respect to the first
target location relative to
the respective first and second virtual three-dimensional models of the tissue
body.
[0006] In an embodiment, the method includes the following steps: (1)
processing a
virtual three-dimensional model of a fixation member so as to couple the
virtual three-
dimensional model of the fixation member to a first virtual three-dimensional
model of the tissue
body, the first virtual three-dimensional model of the tissue body defining a
first region, such
that a central axis of the at least one hole is substantially aligned with a
first target location of the
first region; (2) creating a virtual three-dimensional model of a guide that
defines at least one
hole; and (3) processing the virtual three-dimensional model of the guide so
as to couple the
virtual three-dimensional model of the guide to a second virtual three-
dimensional model of the
tissue body having a second region that is substantially identical to the
first region, such that a
central axis of the at least one hole is substantially aligned with a second
target location of the
second virtual three-dimensional model of the tissue body, wherein the second
target location is
positioned identically with respect to the first target location relative to
the respective first and
second virtual three-dimensional models of the tissue body.
[0007] In an embodiment, the method includes the following steps: (1)
obtaining a
virtual three-dimensional model of the tissue body; (2) identifying on the
virtual three-
dimensional model of the tissue body a first region and a second region; (3)
obtaining a virtual
three-dimensional model of a fixation member, the obtained virtual three-
dimensional model of
the fixation member having a planned post-operative shape and defining at
least one first hole
that is configured to receive a fastener; (4) processing the virtual three-
dimensional model of the
fixation member so as to couple the virtual three-dimensional model of the
fixation member to
the virtual three-dimensional model of the tissue body, such that a central
axis of the at least one
first hole is substantially aligned with a first target location of the second
region; (5) creating a
virtual three-dimensional model of a resection guide that defines at least a
pair of cutting guides
and at least one second hole; and (6) processing the virtual three-dimensional
model of the
- 2 -
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
resection guide so as to couple the virtual three-dimensional model of the
resection guide to a
virtual three-dimensional model of a graft portion disposed between the
cutting guides, the graft
portion sized to fit in the second region, such that a central axis of the at
least one second hole is
substantially aligned with a second target location of the three-dimensional
model of the graft
portion, wherein the second target location substantially coincides with
respect to the first target
location when the graft portion is positioned in the second region.
BRIEF DESCRIPTION OF THE DRAWINGS
[0008] The foregoing summary, as well as the following detailed description of
the
preferred embodiments of the application, will be better understood when read
in conjunction
with the appended drawings. For the purposes of illustrating the surgical
instruments and
methods of the present application, there is shown in the drawings preferred
embodiments. It
should be understood, however, that the application is not limited to the
specific embodiments
and methods disclosed, and reference is made to the claims for that purpose.
In the drawings:
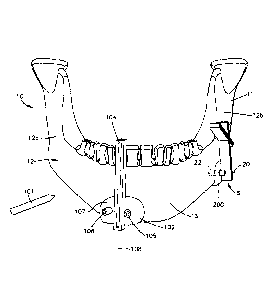
[0009] Fig. IA is a front elevation view of a resection guide coupled to a
patient's
tissue body;
[0010] Fig. 1B is a side elevation view of the resection guide shown in Fig.
1A;
[0011] Fig. 1C is a front elevation view of the tissue body shown in Fig. lA
after a
tissue portion has been removed from the patient;
[0012] Fig. 1D is a side elevation view of a virtual three-dimensional model
of a graft
source;
[0013] Fig. lE is a side elevation view of another resection guide coupled to
the graft
source;
[0014] Fig. 1F is a perspective view of a fixation member coupled to the
patient's tissue
body shown in Fig. 1A;
[0015] Fig. 2 is diagram illustrating the method of making any of the
resection guides
shown in Figs. 1A, 1B, and 1E, in accordance with an embodiment of the present
disclosure;
[0016] Fig. 3A illustrates a physical model of a tissue body in a pre-
operative condition
and a fixation member applied to the physical model, according to an
embodiment of the
disclosure;
[0017] Fig. 3B illustrates a virtual three dimensional model of the physical
model and
fixation member shown Fig. 3B;
[0018] Fig. 3C illustrates a virtual three-dimensional model of a resection
guide
fixation member applied to the tissue body in an ultra- or post-operative
configuration;
- 3 -
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
[0019] Fig. 3D illustrates a virtual three dimensional model of a resection
guide and a
tissue body, in accordance with an embodiment of the disclosure;
[0020] Fig. 4A is a front elevation view of the fixation member shown in Fig.
1F;
[0021] Fig. 4B is a top view of the a fixation member rand a marker shown in
Fig. 4A,
according to an embodiment of the disclosure;
[0022] Figs. 5A and 5B illustrate a virtual three-dimensional model of the
fixation
member applied to the tissue body, and a virtual three-dimensional model of a
resection guide
applied to the graft source, respectively, illustrating how the virtual three-
dimensional model of
the resection guide includes elements that correspond to the virtual three-
dimensional model of
the fixation member;
[0023] Fig. 6 is a flowchart that describes a method of making a resection
guide in
accordance with an embodiment of the present disclosure;
[0024] Fig. 7 is a flowchart that describes a method of making a resection
guide in
accordance with another embodiment of the present disclosure; and
[0025] Fig. 8 is a flowchart that describes a method of making a resection
guide in
accordance with another embodiment of the present disclosure.
DETAILED DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
[0026] Certain terminology is used in the following description for
convenience only
and is not limiting. The words "right", "left", "lower" and "upper" designate
directions in the
drawings to which reference is made. The words "proximally" and "distally"
refer to directions
toward and away from, respectively, the surgeon using the surgical device. The
words,
"anterior", "posterior", "superior", "inferior" and related words and/or
phrases designate
preferred positions and orientations in the human body to which reference is
made and are not
meant to be limiting. The terminology includes the above-listed words,
derivatives thereof, and
words of similar import.
[0027] With reference to Figs. 1A-1C, a surgical system 8 can include one or
more
resection guides 100 and 200 that can be coupled to a tissue body 10 to guide
one or more tools
101 toward the tissue body 10 in order to prepare the tissue body 10 for
receiving a graft. For
instance the resection guides 100 and 200 can guide a tool 101 that cuts the
tissue body 10 so as
to create a void 14 (Fig. 1C) in the tissue body 10. The tissue body 10 can
define spaced apart
first and second tissue portions 12a and 12b. The first and second tissue
portions 12a and 12b
can be any particular portions or segments of the tissue body and are used
herein to refer to tissue
portions that define the void 14. Further, the resection guides 100 and 200
can be used to guide a
- 4 -
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
drill bit that form anchoring locations 22 (Fig. 1B), for instance bores or
holes, in the tissue body
10. Anchoring locations are used to allow an anchor or screw to couple a bone
fixation member,
such as plate, to the tissue body 10 as detailed below. It should be
appreciated that the cutting
tool 101 may be a saw, blade, a drill bit, or any other tool capable of
cutting or otherwise
preparing tissue. As used herein, the tissue body 10 can include a patient's
bone, such as the
mandible 12, and can include the first and second tissue portions 12a and 12b.
The tissue body
can also include anatomical tissue, synthetic tissue, or both. Although the
drawings illustrate
a mandible 12, the tissue body 10 can be other parts of the patient's anatomy
such as a maxilla.
[0028] Referring to Fig. 1A, the resection guide 100 is configured to be
coupled to the
tissue body 10 and can include a resection guide body 102 that is configured
to abut at least a
portion of the tissue body 10, for instance tissue portion 12a. The resection
guide body 102 can
define an inner surface (not shown) that is contoured to match the contour of
a particular outer
surface of the tissue body 10 so that the resection guide 100 can only fit
over the that particular
outer surface of the tissue body 10. The resection guide 100 can define one or
more slots 104
that are configured and sized to receive the cutting tool 101 therein. The
slot 104 can extend
through the resection guide body 102, and can be elongate along a first
resection axis 108. The
tissue body 10 can be cut by inserting the cutting tool 101 through the slot
104 when the
resection guide 100 is coupled to the tissue body 10. In particular, the slot
104 guides the
movement of the cutting tool 101 toward the tissue body 10 along the first
resection axis 108.
[0029] In addition to the slot 104, the resection guide 100 can further
include one or
more drill holes 106 that extend through the resection guide body 102. Each of
the drill holes
106 is configured and sized to receive a drill bit or any other suitable tool
capable of making
holes into and/or through the tissue body 10. The drill holes 106 can be
elongate along an
anchoring location axis 20. The anchoring location axis 20 thus extends
through the drill hole
106 into alignment with then anchoring location 22, for instance a hole or
bore, formed in the
tissue body by the drill bit inserted through the drill hole 106. The
anchoring location 22 is
configured and sized to receive an anchor or fastener.
[0030] The resection guide 100 can further define one or more fastener holes
107 that
are configured and sized to receive a fastener, such as a pin, a wire, or a
screw therethrough.
Each of the fastener holes 107 extends through the resection guide body 102
and is configured
to guide the movement of the fastener through the resection guide body 102 in
order to
temporarily couple the resection guide 100 to the tissue body 10.
[0031] When resection guide 100 is coupled to the tissue body 10, the cutting
tool 101
can be inserted through the slot 104 and into the tissue body 10 to make a cut
on the tissue body
- 5 -
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
at the desired anatomical location. Further, the drill bit can be inserted
through the drill holes
106 to form the anchoring locations in the tissue body 10. The fasteners
inserted through the
fastener holes 107 can then be withdrawn from the tissue body 10 and the
resection guide body
102 to decouple the resection guide 100 from the tissue body 10. Although the
present
disclosure mostly refers to resection guides, any of the resection guides
described herein may
alternatively be positioning guides, drill guides, or any other guide defining
at least one hole that
is configured to receive a cutting tool such as a drill bit.
[0032] With reference to Fig. 1B, the resection guide 200 is configured to be
coupled to
the tissue body 10 to guide the movement of one or more tools 101 toward the
tissue body 10 in
order to prepare the tissue body 10. The resection guide 200 is configured
similarly to the
resection guide 100, however, the resection guide 200 can be coupled to the
tissue body 10 at a
location spaced from the resection guide 100. The resection guides 100 and 200
can be used to
guide a tool 101 to resect tissue from the tissue body 10 so as to create the
void 14 (Fig. IC).
The resection guide 200 can include a resection guide body 202 that is
configured to abut at least
a portion of the tissue body 10, for instance tissue portion 12b. The
resection guide body 202
can define an inner surface that is contoured to match the contoured of a
particular outer surface
of the tissue body 10 so that the resection guide 200 can only fit over the
that particular outer
surface of the tissue body 10. The resection guide 200 can define one or more
slots 204 that are
configured to receive the cutting tool 101. In the depicted embodiment, the
resection guide 200
can define a first slot 204 and a second slot 205. Each of the first slot 204
and the second slot
205 extends through the resection guide body 202, and each can be configured
to receive the
cutting tool 101. The first slot 204 can be elongate along a first resection
axis 208 such that the
first slot 204 can guide the movement of the cutting tool 101 into the tissue
body 10 along the
first resection axis 208. The second slot 205 can be elongate along a second
resection axis 209
such that the second slot 205 can guide the movement of the cutting tool 101
into the tissue body
10. The first resection axis 208 can be oriented at an oblique angle relative
to the second
resection axis 209. In operation, the cutting tool 101 can be inserted through
slot 204 and 205
and into the tissue body 10 to cut the tissue body 10.
[0033] In addition to the first slot 204 and the second slot 205, the
resection guide 200
can define one or more drill holes 206 that extend through the resection guide
body 202. Each of
the drill holes 206 is configured and sized to receive a drill bit or any
other suitable tool capable
of making boles into and/or through the tissue body 10. The drill boles 206
can be elongate
along an anchoring location axis 24. The anchoring location axis 24 thus
extends through the
drill hole 106 into alignment with anchoring location 22, for instance a hole
or bore, formed in
- 6 -
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
the tissue body by the drill bit inserted through the drill hole 206. The
anchoring location 22 is
configured and sized to receive an anchor or fastener.
[0034] The resection guide 200 can further define one or more fastener holes
207 that
extend through the resection guide body 202 that are configured and sized to
receive a fastener,
such as a pin, a wire, or a screw, that is used to temporarily couple the
resection guide 200 to the
tissue body 10. Once the resection guide 200 is coupled to the tissue body 10,
the cutting tool
101 can be inserted through the slot 204 and into the tissue body 10 to make a
cut on the tissue
body 10 at the desired anatomical location. Further, the cutting tool 101 can
be inserted through
the slot 205 and into the tissue body 10 to make a cut on the tissue body 10
at the desired
anatomical location. A drill bit can inserted through the drill guide holes
206 to form an
anchoring location 22 in the tissue body 10. When cuts have been made on the
tissue body 10
along the resection axes 108, 208, and 209, a portion of the tissue body 10
can be removed from
the patient. The fasteners inserted through the fastener holes 207 can be
withdrawn from the
tissue body 10 to decouple the resection guide 200 from the tissue body 10.
[0035] With reference to Fig. 1C, as discussed above, cuts can be made on the
tissue
body 10 along the resection axes 108, 208, and 209 to allow removal of a
tissue portion from the
tissue body 10, thereby defining a void 14 in the tissue body 10. The void 14
extends between
the cut, exposed surfaces of the tissue portion 12a and 12b. The removed
tissue portion can be
damaged or diseased tissue. The void 14 of the tissue body 10 can be filled
with the graft, and
the graft coupled to the tissue portions 12a and 12b with the bone fixation
element, or plate, as
discussed in detail below.
[0036] With reference to Figs. 1D-E, as discussed above, the removed tissue
portion
can be replaced with the graft, such as graft 320 (Fig. 1F). The graft can be
harvested from any
suitable graft source 300, such as a vascularized bone graft source. Further,
the graft can be an
autologous graft. Examples of suitable graft sources include, but are not
limited to, the scapula,
hip, rib, forearm, among others. The graft source 300 can also be a fibula
302. Regardless of the
kind of graft source selected, the graft source 300 can be cut at appropriate
locations and
orientation to obtain a graft that properly fits in the void 14 (Fig. 1C)
defined by the cut exposed
surfaces of the tissue portions 12a and 12b. To define size and shape of the
desired graft, a
virtual three-dimensional model 301 of the graft source 300 can be obtained to
determine the
appropriate location and orientation of the cuts to be made to harvest a graft
from the waft
source 300. The virtual three-dimensional model 301 of the graft source 300
can be obtained by
scanning the graft source 300 using any suitable technology such as x-ray
computed tomography
(CT), or any suitable mapping technology for instance, laser, optical, CT,
magnetic resonance
- 7 -
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
imaging (MRI) and coordinate measuring machines. In an embodiment, an imaging
machine,
such as CT scan machine, can be used to scan the graft source 300. The imaging
machine can
include or be in electronic communication with a computer, such a computer
530, that includes a
computer memory in electronic communication with a processor. The computer 530
can be any
computing device and can include a smart phone, tablet or any other computer.
The data
obtained by scanning the graft source 300 can transmitted to or stored in the
computer memory.
The scanned data can be processed, via the processor, and in accordance with
software
instructions running on the computer 530, to create the virtual three-
dimensional model 301 of
the graft source 300. Alternatively, the scanned data can be downloaded or
transferred
wirelessly or via a hardwire connection over an electronic communications
network to a different
computing device at a location that is remote from the imaging machine, in
order to create the
virtual three-dimensional model 301 of the graft source 300.
[0037] When the virtual three-dimensional model 301 of the graft source 300
has been
obtained, the surgical operation can be planned. The surgical operation can be
planned using any
suitable software program that is configured to process, edit and manipulate
data that is
representative of the image of the scanned graft source, for instance scanned
image data. The
software operate over networked computing architecture that includes host and
client computing
devices. Further, the software can be a web based application configured to
process instructions
based on inputs from a graphical user interface running on a computer, for
instance computer
530. In an embodiment, one suitable software program configured to process,
manipulate and or
edit images or image data, is sold or licensed under the trademark PROPLAN CMF
(.t by
Synthes. PROPLAN CMF can be used to process and manipulate the virtual three-
dimensional model 301.
[0038] The graft 320 that replaces the removed tissue portion should be
configured and
sized to fit properly in the void 14 (Fig. 1C). For instance, a plurality of
graft portions 304, 306,
and 308 can be harvested from the graft source 300 and then interconnected to
from a complete
graft for insertion in the void 14. As such, resection axes can be defined as
so the form the
plurality of graft portions 305, 306, and 308. Using the virtual three-
dimensional model 301 of
the graft source 300, the resection can be planned via the computer running
software that is
configured to process, manipulate and edit images, such as the scanned image
data described
above. The user can input instructions that causes the processor to carry out
the desired edits or
manipulations to the virtual three-dimensional model 301 of the graft source
300. The user can
determine the location and the orientation of the resections to be made on the
graft source 300 to
obtain graft portions 304, 306, and 308 that can later be interconnected to
form the graft 320. To
- 8 -
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
harvest the graft portions 304, 306, and 308, the user can determine that cuts
have to be made
along the resection axes 310, 312, 314, 316, and 318. It should be appreciated
that patient
anatomy and shape and size of the removed tissue portion, resections can be
made along other
resection axes to form the properly sized graft portions.
[0039] With continuing reference to Figs. 1D-E, after planning the desired
resections to
be made on the graft source 300 using the virtual three-dimensional model 301
in the computer,
the resection guide 400 configured in accordance with the planned surgical
procedure and
manufacturing using rapid production technology as described below can be
placed on the graft
source 300 to guide the movement of the cutting tool 101 into the graft source
300. The
resection guide 400 can include a resection guide body 402 that is configured
and adapted to abut
at least a portion of the graft source 300. The resection guide body 402 can
define an inner
surface that can be contoured to match a particular outer surface of the graft
source 300 so that
the resection guide 400 can only fit over the that particular outer surface of
the graft source 300.
[0040] The resection guide 400 defines a plurality of slots that are each
configured to
receive the cutting tool 101 to guide the movement of the cutting tool 101
toward the graft
source 300. In the depicted embodiment, the resection guide 400 can define a
first slot 410, a
second slot 412, a third slot 416, and a fourth slot 418 that are spaced from
one another. Each of
the slots 410, 412, 416, and 418 extend through the resection guide body 402.
The resection
guide 400 can be configured so that the slots 410, 412, 416, and 418 are
substantially aligned
with the predetermined resection axes 310, 312, 314, 316, and 318 when the
resection guide 400
is placed over the graft source 300. For example, the first slot 410 can be
substantially aligned
with the first resection axis 310 when the resection guide 400 is placed over
the graft source 300.
The second slot 412 can be substantially aligned with the second resection
axis 312 when the
resection guide 400 is placed over the graft source 300. The third slot 414
can be substantially
aligned with the third resection axis 314 when the resection guide 400 is
placed over the graft
source 300. The fourth slot 416 can be substantially aligned with the fourth
resection axis 316
when the resection guide 400 is placed over the graft source 300. The fifth
slot 418 can be
substantially aligned with the fifth resection axis 318 when the resection
guide 400 is placed over
the graft source 300.
[0041] In addition to the slots, the resection guide 400 can further define
one or more
drill holes 406 that are configured and sized to receive at least one drill
bit or any other apparatus
that is capable of making anchoring locations 303, such as a hole or bore, in
the graft source 300.
In operation, the drill bit can be inserted through some or all of the drill
holes 406 to make a hole
in the graft source 300. The anchoring locations formed in the graft source
300 are configured
- 9 -
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
and sized to receive an anchor, such as screw, rivet, nail or an suitable bone
fixation device. The
anchoring locations 303 can correspond to openings formed in a fixation
member, such as plate,
such that the anchor can be inserted through fixation member openings into the
respective
anchoring locations 303 in the graft source 300, as discussed below.
[0042] The resection guide 400 can further define one or more fastener holes
407 that
are configured and sized to receive a fastener, such as a pin, a wire, or a
screw. The fastener can
be inserted through the fastener holes 407 and into the graft source 300 to
temporarily couple the
resection guide 400 to the graft source 300. The resection guide 400 can be
coupled to the graft
source 300 by inserting fasteners through the fastener holes 407. Then, the
cutting tool 101 can
be inserted sequentially through the slots 410, 412, 416, and 418 and advanced
into the graft
source 300 to so as to cut and harvest the graft portions 304, 306, and 308. A
drill bit can
inserted in the drill holes 406 to form anchoring locations 303 (not shown) in
the graft source
portions 304, 306, and 308 The resection guide 400 can then be decoupled from
the graft source
300 by removing the fastener from the fastener holes 407 and the graft source
300.
[0043] With reference to Fig. IF, the graft portions 304, 306, and 308 can
then be
placed in the void 14 (Fig. 1C) in order to replace the tissue portion removed
from the tissue
body 10. The graft portions 304, 306, and 308 can then be coupled to each
other to form the
graft 320. Any suitable fixation member 322, such as a fixation plate 324, and
a plurality of
anchors, such as screws can be used to couple the graft portions 304, 306, and
308 can together
to form the graft 320. The graft 320 can be a bone graft, and can be connected
to the tissue body
using the fixation member 322, such as the fixation plate 324.
[0044] In an embodiment, the fixation member 322 can be configured as a bone
fixation implant. The fixation member 322 can be bent so that its contour
matches the contour of
the tissue body 10 and the interconnected graft portions 304, 306, and 308.
For instance, the
fixation member 322 can be countered along the tissue portion 12a, the graft
320 and tissue
portion 12b. Further, the fixation member 322 defines one or more holes 326
that are configured
to receive a anchors discussed above. The holes 326 can be threaded holes or
partially threaded
depending on the selected anchor type. When the fixation member 322 is placed
against the
tissue body 10 and the graft portions 304, 306, and 308, one or more anchors
can be inserted
through at least one fastener hole 326 and into anchoring locations 22 in the
tissue body 10 or the
anchoring locations 303 formed in the graft 320 so as to couple the graft
portions 304, 306, and
308 to one another and to couple the graft 320 to the tissue body 10. The
fixation member 322
can be formed from a variety of biocompatible materials, such as cobalt
chromium molybdenum
(CoCrMo), titanium, and titanium alloys, stainless steel, ceramics, or
polymers such as
- 10-
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
polyetheretherketone (PEEK), polyetherketoneketone (PEKK), and bioresorbable
materials. A
coating may be added or applied to the bone fixation implant 410 to improve
physical or
chemical properties or to provide medications. Examples of coatings include
plasma-sprayed
titanium coating or Hydroxyapatite. In accordance with an alternative
embodiment, the fixation
member 322 can be patient specific bone fixation plate.
[0045] Referring to Figs. 2 and 3A-3D, 5A and 5B, a method of making a patient
specific surgical resection guide, for instance any of the resection guides
100, 200 and/or 400
described above disclosure or any other suitable resection guide. The method
can include all or
some of the steps schematically represented as steps A, B, C, D, E, and F in
Fig. 2, some of
which are carried out using one or more computing devices, or computers 530
running suitable
software used to manipulate or edit images and or three-dimensional models. In
accordance with
the embodiment illustrated in Fig. 2, the method of making a patient specific
surgical guide can
include in step A obtaining a physical model of a tissue body and a fixation
member, for instance
fixation member 322. Step B can include scanning the physical model of the
tissue body and the
fixation member using a scanning and or mapping machine 508. Step C can
include creating a
virtual three-dimensional model of the physical model and the fixation member
on a computer
530. Step D can include creating a virtual three-dimensional model of the
fixation member
applied to the tissue body in an intra- or post-operative configuration. Intra-
or post- operative
configuration means the desired or intended shape of the tissue body and
fixation member when
the tissue body 10 has been surgically reconstructed with graft an fixation
member. Step E can
include creating a virtual three-dimensional model of a resection guide based
on the intra- or
post-operative virtual three-dimensional model of the tissue body and the
fixation member. Step
F can include making a surgical resection guide based on the virtual three-
dimensional model of
the resection guide.
[0046] Referring Figs. 2 and 3B, in step A the user obtains a physical model
500 of the
tissue body 10. The tissue body 10 can be a native tissue body or a
reconstructed tissue body.
The physical model 500 of the tissue body 10 can be created by scanning the
tissue body 10
using any suitable technology and then forming a three-dimensional model based
on the scanned
data. For instance, a virtual three-dimensional model 510 of the tissue body
10 can be obtained
by scanning the tissue body 10 using any suitable technology, such as CT scan
machine, laser
scanning machine, optical scanning machine, MRI machine, and coordinate
measure machine.
In an embodiment, a scanning machine can be used to scan a tissue body 10 so
as to obtain
scanned data of the tissue body 10. The scanned data is then downloaded or
transferred to a
computer in electrical communication with the scanning machine. For instance
the scanned data
- 11 -
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
can be transmitted wirelessly or via hard connection through a LAN, WAN or any
suitable
communications network to the computer. In the computer, a virtual three-
dimensional model
510 of the tissue body 10 is created using a computer running suitable
software capable of
processing and editing, or manipulating images and/or image data. The virtual
three-dimensional
model 510 of the tissue body 10 is a representation of the tissue body 10 in
its pre-operative
condition. As further detailed below, the virtual three-dimensional model 510
of the tissue body
can be manipulated in accordance with a surgical plan in order to obtain a
virtual three-
dimensional model 520 (Fig. 3C) of the tissue body 10 in its intra- or post-
operative
configuration. In other words, the virtual three-dimensional model 510 can be
manipulated such
that the model represents the desired or intended shape and configuration of
the tissue body 10
when the resected tissue has be replaced by the graft 320. The virtual three-
dimensional model
520 of the tissue body 10 is downloaded or transferred via a communications
network to a
manufacturing machine or machines. Then, using the virtual three-dimensional
520 model of the
tissue body 10, the manufacturing machine can create a physical model 500
(Fig. 3A of the tissue
body 10 in its intra- or post-operative condition. For instance, a rapid
prototyping device or
process can be used to create the physical model 500 of the tissue body 10
using the virtual three
dimensional model of the tissue body 10. In rapid prototyping manufacturing
processes, a virtual
design, such as a computer aided design model, is transformed into a physical
model. Examples
of rapid prototyping devices and processes include, but are not limited to,
selective laser
sintering (SLS), fused deposition modeling (FDM), stereolithography (SLA), and
3D printing. A
computer numerical control (CNC) machine can also be used to create the
physical model 500 of
the tissue body 10 in its pre-operative or post-operative condition.
[0047] Once the user obtains the physical model 500 of the tissue body 10, the
fixation
member 322, such as a fixation plate 324 or any other bone fixation implant,
can be coupled to
the physical model 500. In the depicted embodiment, the fixation plate 324 can
bent to conform
to the shape of the physical model 500. That is, the fixation member 322, such
as the fixation
plate 324, can be shaped in accordance with a planned post-operative shape.
The fixation plate
324 can be coupled to the physical model 500 at the same location and in the
same orientation on
the physical model 500 as it would be placed on the tissue body 10. One or
more markers 502
can be at least partially inserted at least one of the holes 326 of the
fixation member 322 to mark
the location and angulation of that fastener hole 326. Each marker 502 can
include a handle 504
and a rod 506 that extends from the handle 504. At least a portion of the rod
506 can be
configured and sized to be received by one of the holes 326. The rod can
define a length and in
some embodiments, Some markers 502 can have rods 506 with shorter lengths than
others. The
- 12-
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
markers 502 with the shorter length rods 506 can be positioned between markers
502 with longer
rods 506 to accommodate the maximum number of markers 502 in the fastener
boles 326.
[0048] With reference to Figs. 4A and 4B, the fixation member 322 can include
a
fixation member body 321. The fixation member body 321 extends between a first
end 321a and
a second end 321b opposite the frist end 321a along a longitudinal direction
L. The fixation
body 321 defines an outer surface 323 and an inner surface 325 spaced from the
outer surface
323 along a transverse direction T that is transverse to the longitudinal
direction L. The inner
surface 325 is configured so contour to the surface of the graft source or
tissue body 10. The
fixation member 322 has a thickness defined as the distance between the outer
surface 323 and
the inner surface 325.The fixation member body 321 define a plurality of holes
326 that extend
through the fixation member body 321 along a central hole axis X. The holes
326 space apart
from each other along longitudinal direction L. Each hole 326 is configured
and sized to receive
at least an anchor therethrough. The holes 326 can be as threaded or partially
threaded. The
holes 326 can be configured in any suitable manner or orientation to receive
an anchor therein.
The central hole axis X can thus be angulated respect to the direction T. In
an embodiment, the
central axes X of some or all of the holes 326 can be angularly offset
relative to the direction T.
The fixation member 322 is configured to be bent to conform to the shape of a
portion of the
tissue body 10 or a portion of the physical model 500 of the tissue body as
shown in step A of
Fig. 3A. Before bending the fixation member 322, small screw inserts (not
shown) can be placed
in the holes 326 to help maintain the shape of the holes 326 during the
bending process.
Furthermore, the fixation member 322 is generally not bent or deformed at
positions where the
holes 326 are located to avoid, or at least minimize significantly changing
the shape of the holes
326 during bending.
[0049] The markers 502 can be used to accurately create the holes 326 in a
virtual
three-dimensional model of the fixation member 322. As discussed above in step
A, markers
502 can be inserted through the holes 326 after the fixation member 322 has
been bent to
conform to the shape of at least a portion of the physical model 500 and
coupled to the physical
model 500. A portion of the marker 502, such as a portion of the rod 506, can
be inserted in one
of the holes 326 such that that rod 506 extends along the respective central
hole axis X. Hence,
the rod 506 can be elongate along the central hole axis X of one of the holes
326 when at least a
portion of the rod 506 is inserted in that specific hole 326. Accordingly,
markers 502 can be
inserted in one or more holes 326 to identify the angulation of the respective
hole 326 .
[0050] Referring to Fig. 2, in step B the physical model 500, the fixation
member 322,
and the markers 502 can be scanned using any suitable scanning or imaging
technology as
- 13 -
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
described above to obtain scanned image data for the physical model 500, the
fixation member
322, and the markers 502. For instance a scanning machine can be used to scan
the physical
model 500, the fixation member 322, and the markers 502, and using the scanned
image data
can be used, via a computer 530 to create a virtual three-dimensional model
512 of the physical
model 500, the fixation member 322, and the markers 502. In accordance with an
alternative
embodiment, only physical model 500 and the fixation member 322 are scanned,
and a virtual
three-dimensional model is created of the physical model 500 and the fixation
member 322 such
that the markers 502 are not scanned. In a further embodiment, only the
fixation member 322,
which has been shaped in accordance with a planned intra- or post-operative
configuration, is
scanned. In particular, the fixation member 322 can be bent to a shape to its
planned intra- or
post-operative shape and then scanned to obtained the scanned image data.
[0051] Referring to Figs. 2 and 3B, in step C, once the three-dimensional
image of the
fixation member 322 coupled to the physical model 500 is obtained with the
scanning machine,
the scanned image data is loaded onto a computer 530 to create a virtual three-
dimensional
model 512 of the physical model 500, the fixation member 322, and the markers
502.
Alternatively, a virtual three-dimensional model of at least the fixation
member 322 can be
created with the computer 530 without the need of scanned image data of the
physical model 500
of the fixation member 322. The computer 530 can include a processor and a non-
transitory
computer readable storage medium configured to store data, such as scanned
image data, and
suitable software. The computer 530 may be local, for instance in the same
general area as the
scanning machine, or remote and the scanned image data is transferred to the
computer 530 via a
communications network. Thus the obtain or stored scanned image data can be
manipulated by a
user via software running on the computer that is local to the scanning
machine and/or surgery
location or remote to the scanning machine and/or surgery location. For
example, the scanned
image data can be manipulated remotely by the surgeon who will be performing
the surgery.
The virtual three-dimensional model 512 is typically composed of data in
different formats. For
instance, the three-dimensional model 512 can contain data in a Standard
Tessellation Language
(STL) format. Regardless of the data format, the virtual three-dimensional
model 512 includes
data that maps and represents the shape, contour, and size of at least the
physical model 500 and
the fixation member 322 as coupled to the physical model 500.
[0052] Continuing with reference Figs. 2 and 3B, in step C the virtual three-
dimensional
model 512 can include data representing the markers 502 position in the
fixation members 322
so as to enhance the accuracy of the orientation of the holes 326 of the
fixation member 322.
With the visual representation of the markers 502, the user can better
determine the orientation of
- 14-
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
the holes 326 of the fixation member 322. As discussed above with respect to
Figs. 4A and 4B,
the markers 502 can help determine the angulation of the hole 326 with respect
to the transverse
on T of the fixation member 322. Using the scanning process in step B, the
location of the
opposed ends 327 of each hole 326 can be obtained. However, the path of each
hole 326 from a
first hole end 327 to a second hole end 329 may not be necessarily obtained by
the scanning
process described in step B. Hence, the virtual three-dimensional model 512
can be manipulated
to virtually create each of the holes 326 virtual model of the fixation member
322. To do so, the
central hole axis X can be developed in the virtual model so to extend through
the a center of the
first hole end 327 and the center of the second hole end 329. Then, the hole
326 is created so
that it has a path along the previously drawn central axis X' of that
particular hole 326. This
process does not entail the use of the markers 502. Alternatively, the visual
representation of the
markers 502 can be used obtain a more accurate path for the holes 326. To do
so, the central axis
X' is drawn from the second hole end 329 to an end 507 of the rod 506 that is
attached to the
handle 504. Then, the hole 326 that follows the central axis X is created in
the virtual three-
dimensional model 512. This process can be repeated for each hole 326.
[0053] In step C, the virtual three-dimensional model 512 can include models
of each
component. That is, the virtual three-dimensional model 512 can include a
virtual three-
dimensional model 514 of the physical model 500, a virtual three-dimensional
model 516 of the
fixation member 322, such as the fixation plate 324, and a virtual three-
dimensional model 518
of the markers 502. The virtual three-dimensional models 512 (or any virtual
model described
herein) can be manipulated by a user using conventional software typical in
the art. For
example, a software program that is configured to process and edit images,
sold under the
trademark PROPLAN CMFA) by Synthes, may be used to process and manipulate the
virtual
models obtain from the scanning machine 508. The software allows the user to
analyze the
tissue body 10 and pre-operatively plan the patient's surgery including the
shape and design of a
resection guide, such as a resection guide 600 discussed below.
[0054] Referring to Figs. 2 and 3C, in step D, the virtual three-dimensional
model 520
of the tissue body 10 can be manipulated into the intra- or post-operative
shape and configuration
in accordance with a planned surgical procedure. Specifically, the virtual
three-dimensional
model 516 of the fixation member 322 can be imported into a previously
obtained three-
dimensional model 520 of the tissue body 10, and manipulated using a computer
create a virtual
three-dimensional model 520 of the tissue body 10 in the intra- or post-
operative shape and
configuration. In other words, using the virtual three-dimensional model 520
of the tissue body
10, the user may pre-plan a surgery, such as a mandibular reconstruction
surgery, in the
- 15 -
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
computer 530 using a suitable software such as the software sold under the
trademark
PROPLAN CMF by Synthes. In the computer 530, the virtual three-dimensional
model 516 of
the fixation member 322 can be coupled to the virtual three-dimensional model
520 of the tissue
body 10 in the intra- or post-operative configuration in accordance with a
predetermined surgical
plan as discussed in detail above with respect to Fig. 1F. Thus, the virtual
three-dimensional
model 516 of the fixation member 322 can be aligned with the virtual three-
dimensional model
520 of the tissue body 10 according to a desired surgical plan. As discussed
above, the three-
dimensional model 520 can represent a native tissue body 10 or a reconstructed
tissue body 10
that includes the graft 320. The virtual three-dimensional model 516 of the
fixation member
322 coupled to the three-dimensional model 520 of the tissue body 10 are
collectively referred to
as the virtual three-dimensional model 526.
[0055] Referring to Fig. 2 and 3D, in step E, a virtual three-dimensional
model 522 of a
resection guide 600 can be created and designed based on the virtual three-
dimensional model
526 of the fixation member 322 coupled to the tissue body 10. Thus, the
resection guide 600 (or
any other suitable resection guide) can be designed and manufactured based on
the virtual three-
dimensional model 526 of the fixation member 322 coupled to the virtual model
520 of the tissue
body 10. In accordance with an alternate embodiment, the virtual three-
dimensional model 522
of the resection guide 600 can be created using a virtual three-dimensional
model 521 of the
tissue body 10 that has been previously obtained via a scanning machine. The
virtual three-
dimensional model 521 of the tissue body 10 can be substantially identical to
the virtual three-
dimensional model 520 of the tissue body 10 used in step D. However, in some
embodiments,
the virtual three-dimensional model 521 of the tissue body 10 represents the
tissue body 10 in a
pre-operative shape or condition. .
[0056] Continuing with reference to Fig. 2 and 3D, in step E a virtual three-
dimensional
model 522 of the resection guide 600 is can be configured to or designed to
allow a surgeon to
guide movement of the cutting tool 101 toward the tissue body 10, for instance
when the
resection guide is formed as detailed below. In the depicted embodiment, the
resection guide
600, or the model of the resection guide, can include a resection guide body
602 that is
configured to abut at least a portion of the tissue body 10. The resection
guide body 602 can
define at least one slot 604 that extends through the resection guide body
602. The slot 604 can
be configured and sized to receive the cutting tool 101, and guide the cutting
tool 101 toward the
tissue body 10 when the resection guide 600 is coupled to the tissue body 10,
as represented in a
three-dimensional virtual model. In addition to the slot 604, the resection
guide 600 can define
one or more drill holes 606 that are each configured and sized to receive a
drill bit or any other
- 16-
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
apparatus capable of making holes or anchoring locations in the tissue body
10. Each of the drill
holes 606 can extend through the resection guide body 602. In addition to the
drill holes 606, the
resection guide 600 can define one or more fastener holes 607 that are each
configured and sized
to receive a fastener such as a screw. Each of the fastener holes 607 can
extend through the
resection guide body 602. At least one fastener can be inserted through each
fastener hole 607
and into the tissue body 10 to couple the resection guide 600 to the tissue
body 10.
[0057] Continuing with Fig. 2 and 3D, in step E, the virtual three-dimensional
model
522 of the resection guide 600 can be designed such that the location and
orientation of the drill
holes 606 in the virtual three-dimensional model 522 relative to the tissue
body 10 are
substantially aligned with the location and orientation of the same number of
holes 326 of the
fixation member 322. For instance, as show in Fig. 3C, in step C, the fixation
member 322
includes first hole 326 and a second hole 326b positioned at a location and
orientation G and H,
respectively, relative to the tissue body 10. Accordingly, the virtual three-
dimensional model 522
of the resection guide 600 can be designed, for example in the computer 530,
such that at least
one hole 606a and second hole 606b has substantially the same location and
orientation relative
to the tissue body 10 as one of the holes 326, for instance holes 326a and
326b, of the fixation
member 322, relative to the location and orientations G and H on the tissue
body 10. The
location G can be referred to as the first position relative to the virtual
three-dimensional model
520, and the location identified H can be referred to as the second position
relative to the virtual
three-dimensional model 520. The holes 326a and 326b are located and oriented
relative to the
tissue body 10 such that the insertion of anchors through the holes 326a and
326b into the anchor
locations do not impinge upon nerves of the tissue body 10. Also, the holes
326 are located and
oriented relative to the tissue body 10 such that anchors are inserted through
tissue that is not
damaged or diseased.
[0058] Referring to Fig. 2, in step F, once the virtual three-dimensional
model 522 of
the resection guide 600 has been completed, the resection guide 600 can be
made based on the
three-dimensional virtual model 522 using any suitable technology, such as the
rapid prototyping
technology. For instance, the virtual three-dimensional model 522 of the
resection guide 600 can
be downloaded or transferred from the computer 530 to a machine such as a
CAD/CAM
manufacturing machine, or to a computer coupled to such a machine. The
resection guide 600
can be made using a rapid prototyping manufacturing devices or process. In
rapid prototyping
manufacturing process, a virtual design, such as a computer aided design
model, is transformed
into a physical model or construct. Examples of rapid prototyping technologies
include, but are
not limited to, selective laser sintering (SLS), fused deposition modeling
(FDM),
- 17-
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
stereolithography (SLA), and 3D printing, as well as a computer numerical
control (CNC)
machine. The manufacturing machine 532 makes the resection guide 600 out of
any desired
material. For example, the resection guide 600 can be partly or entirely made
of a suitable
polymer or metallic material. Then, the user can perform any desired surgical
operation on a
patient using the resection guide 600. All or some of the steps shown in Fig.
2A can be executed
by a processor or a computer. In addition, all or some of the data involved in
the method
described above, such as the virtual models, can be stored on non-transitory
computer readable
storage medium to a local computer or a remote computer.
[0059] Aside from the resection guide 600, the method described above can be
used to
make any other suitable resection guide. For example, the resection guides 100
and 200 can be
made using the method described above. It should be appreciated that all the
virtual three-
dimensional models mentioned in the present disclosure can be created and
manipulated using a
computer aided software that is run in computer 530. The method described in
the present
application can be used to manufacture resection guides for use in mandibular
reconstruction
surgery as described above. However, the method described in the present
application can be
used to make resection guides for use in orthognatic surgery or
craniomaxillofacial surgery that
may include distraction of bone segments.
[0060] With reference to Figs 5A and 5B, the method described above can also
be used
to construct the resection guide 400 used to harvest the graft. In this
method, the resection guide
400 can include one or more slots 403 and a plurality of drill holes 406a-
406f. The resection
guide 400 can be virtually designed so that the location and orientation of
the that the drill holes
406a-f relative to the graft 320 are in substantial alignment with the
fastener holes 326a-f and
tissue locations Y when the graft 320 is positioned in the void 14 (Fig. 1C)
and the fixation
member 322 is positioned against the graft 320 and the tissue body 10. For
instance, the virtual
three-dimensional model 512 of the tissue body 10 is obtained as described
above with respect to
steps A-C discussed above and show in Figs. 2, 3A and 3B. Then, on a virtual
three-dimensional
model of the tissue body 10, a first resection region 11 (Fig. 1A) and a
second resection region
13 (Fig. 1A) are identified. The first resection region 11 is also referred to
as the first region 11,
and the second resection region 13 is also referred to as the second region
13. The virtual three-
dimensional model 516 of the fixation member 322 is obtained as described
above with respect
to Fig. 2. The obtained three-dimensional model 516 can have a planned post-
operative shape,
and can define at least one first bole 326 that is configured to receive a
fastener. The virtual
three-dimensional model 516 of the fixation member 322 is processed (in a
processor) so as to
obtain the virtual three-dimensional model 516 of the fixation member 322,
such that a central
- 18-
axis of the at least one first hole 326a is substantially aligned with a first
target location K of the
second resection region 13 of the tissue body. The virtual three-dimensional
model 401 of the
resection guide 400 is created by, for example, scanning the resection guide
400 as described
above in steps B and C of Fig. 2. The virtual three-dimensional model 401 of
the resection guide
400 can be processed (in a processor) so as to couple the virtual three-
dimensional model 401 of
the resection guide 400 to the virtual three-dimensional model 301 of the
graft portion disposed
between at least two cutting guides 403. The graft portion can be graft
portion 304, graft portion
306, graft portion 308, or a combination thereof. Thus, the graft portion can
be the graft 320.
The graft portion, such as the graft 320, can be sized to fit in the second
region 13 or void 14.
The virtual three-dimensional model 401 of the resection guide 400 can be
processed via a
processor on a computer so as to couple the virtual three-dimensional model
401 of the
resection guide 400 to the virtual three-dimensional model 301 of the graft
portion, such that the
central axis of one of the drill holes 406 is substantially aligned with one
of the target locations L
of the graft source. At least one of the target locations L substantially
coincides with the target
location K when the graft 320 is positioned in the void 14.
[0061] With reference to Fig. 6, a method 700 of making a resection guide can
include
steps 701, 702, 703 and 704. Step 701 includes obtaining a virtual three-
dimensional model 516
of a fixation member 322, wherein the obtained virtual three-dimensional model
516 of the
fixation member 322 has a planned post-operative shape and defines at least
one hole 326 that is
configured to receive a fastener. Step 702 includes processing the virtual
three-dimensional
model of the fixation member 322 so as to couple the virtual three-dimensional
model 516 of the
fixation member 322 to a first virtual three-dimensional model 520 of the
tissue body 10, the first
virtual three-dimensional model 520 of the tissue body 10 defining a first
region 11, such that a
central axis X of the at least one hole 326 is substantially aligned with a
first target location M of
the first region 11. The first region 11 can correspond to the tissue portion
12b. Step 703
includes creating a virtual three-dimensional model 522 of a resection guide
600 that defines at
least one cutting guide 603 and at least one hole 606. Alternatively, step 703
includes creating a
virtual three-dimensional model 522 of a guide 600, such as a positioning
guide or a drill guide,
that defines at least one hole 606. Step 704 includes processing the virtual
three-dimensional
model 522 of the resection guide 600 so as to couple the virtual three-
dimensional model 522 of
the resection guide 600 to a second virtual three-dimensional model 521 of the
tissue body 10
having a second region 15 that is substantially identical to the first region
11, such that a central
axis of the at least one hole 606 is substantially aligned with a second
target location N of the
second virtual three-dimensional model 521 of the tissue body 10, wherein the
second target
- 19 -
CA 2872397 2019-10-15
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
location N is positioned identically with respect to the first target location
M relative to the
respective first and second virtual three-dimensional models 520, 521 of the
tissue body 10.
[0062] The second processing step 704 can further include aligning the cutting
guide
603 with a preoperatively planned interface between the first region lithe
second region 13 of
the tissue body 10. The obtaining step 701 can further include scanning the
fixation member 322
to obtain an image of the fixation member 322, transferring via communication
network, the
image data to a computer and manipulating the image of the fixation member 322
to define the at
least one hole 326 of the fixation member 322 in the virtual three-dimensional
model 516 of the
fixation member 322. The manipulating step includes identifying the central
axis X of the at least
one hole 326. The method can further include constructing the resection guide
600 identical to
the virtual three-dimensional model 522 of the resection guide 600 using a
rapid prototyping
manufacturing process. The step of constructing the resection guide 600 can
include transferring
the virtual three-dimensional model 522 of the resection guide 600 from the
computer to a
manufacturing machine 532.
The obtaining step 701 can include scanning the fixation member 322 using a
scanning
machine 508. The obtaining step 701 can include scanning the fixation member
322 using any
one of the following scanning machines, namely: CT scan machine, laser
scanner, optical
scanner, MRI machine, or coordinate measure machine. The obtaining step 701
can further
include coupling the fixation member 322 to a physical model 500 of the tissue
body 10. The
obtaining step 701 can further include bending the fixation member to the post-
operative shape.
The obtaining step 701 can further include inserting at least a portion of a
marker 502 into the at
least one hole 326 of the fixation member 322 to identify a path of the at
least one hole 326
relative to a thickness of the fixation member 322. The obtaining step 701 can
further include
scanning the physical model 500 of the tissue body 10, the marker 502 that is
inserted into at
least one hole 326 of the fixation member 322, and the fixation member 322
that is coupled to
the physical model 500 of the tissue body 10.
The processing step 704 can include manipulating via a processor, according to
software
stored in a computer readable medium, the virtual three-dimensional model 522
of the resection
guide 600 so that the resection guide 600 is contoured to fit over a
particular portion of the
virtual the second virtual three-dimensional model 521 of the tissue body 10.
All or some of the
steps shown in Fig. 6 or described above can be executed by a processor
running on a computer.
The virtual three-dimensional models described in the present disclosure can
be stored on a non-
transitory computer readable storage medium. The processor and the computer
readable storage
medium can be part of the same computer or different computers.
- 20 -
[0063] With reference to Fig. 6, a method 800 of making a patient specific
surgical
resection guide 600 can include the steps 801, 802, and 803. The step 802
includes processing a
virtual three-dimensional model 516 of a fixation member 322 so as to couple
the virtual three-
dimensional model 516 of the fixation member 322 to a first virtual three-
dimensional model 520
of the tissue body 10, the first virtual three-dimensional model 520 of the
tissue body 10 defining
a first region 11, such that a central axis X of the at least one hole 326 is
substantially aligned
with a first target location M of the first region 11. The step 802 includes
creating a virtual
three-dimensional model 522 of a resection guide 600 that defines at least one
cutting guide 603
and at least one hole 606. Alternatively, the step 802 includes creating a
virtual three-
dimensional model 522 of a guide, such as a positioning guide or a drill
guide, that defines at
least one hole 606. The step 803 includes processing the virtual three-
dimensional model 522 of
the resection guide 600 so as to couple the virtual three-dimensional model
522 of the resection
guide 600 to a second virtual three-dimensional model 521 of the tissue body
10 having a second
region 15 that is substantially identical to the first region 11, such that a
central axis X of the at
least one hole 326 is substantially aligned with a second target location N of
the second virtual
three-dimensional model 521 of the tissue body 10, wherein the second target
location N is
positioned identically with respect to the first target location M relative to
the respective first and
second virtual three-dimensional models 520, 521 of the tissue body 10.
100641 In accordance with an alternate embodiment, the method 800 illustrated
in Fig. 6
can further include the step obtaining the virtual three-dimensional model 516
of the fixation
member 322 in a computer 530. The obtaining step can include scanning the
fixation member
322 using a scanning machine 508. The obtaining step can further include
scanning the fixation
member 322 using any of the following scanning machines, namely CT scan
machine, laser
scanner, optical scanner, MRI machine, or coordinate measure machine. The
method illustrated
in Fig. 4 can further include constructing the resection guide 600 identical
to the virtual three-
dimensional model 522 of the resection guide 600 using a rapid prototyping
manufacturing
process. The constructing step can further include transferring the virtual
three-dimensional
model 522 of the resection guide 600 from the computer 530 to a manufacturing
machine 532 via
a communications network. The obtaining step can include coupling the fixation
member to a
physical model of the tissue body. The obtaining step can include bending
fixation member to
the post-operative shape. The obtaining step can include inserting a marker
into the at least one
hole of the fixation member to identify a path of the at least one hole
relative to a thickness of the
fixation member. The obtaining step can include scanning the physical model of
the tissue body,
the marker that is inserted into at least one hole of the fixation member, and
the fixation member
- 21 -
CA 2872397 2019-10-15
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
that is coupled to the physical model of the tissue body. The obtaining step
can include scanning
the physical model of the tissue body, and the fixation member that is coupled
to the physical
model of the tissue body. The processing step 803 can include manipulating the
virtual three-
dimensional model of the resection guide so that the resection guide is
contoured to fit over a
particular portion of the virtual the second virtual three-dimensional model
of the tissue body.
All or some of the steps shown in Fig. 7 or described above can be executed by
a processor as a
computer.
[0065] With reference to Fig. 8, a method 900 of making a patient specific
surgical
resection guide 600 can include the steps 901, 902, 903, 904, 905 and 906. The
step 901
includes obtaining a virtual three-dimensional model 521 of the tissue body
10. The step 902
includes identifying on the virtual three-dimensional model 522 of the tissue
body 10 a first
retention region 11 and a second resection region 13. The first resection
region 11 is also
referred to as the first region 11, and the second resection region 13 is also
referred to as the
second region 13. The step 903 includes obtaining a virtual three-dimensional
model 516 of a
fixation member 322, the obtained virtual three-dimensional model 516 of the
fixation member
322 having a planned post-operative shape and defining at least one first hole
326 that is
configured to receive a fastener. The step 904 includes processing the virtual
three-dimensional
model 516 of the fixation member 322 so as to couple the virtual three-
dimensional model 516 of
the fixation member 322 to the virtual three-dimensional model of the tissue
body 10, such that a
central axis X of the at least one first hole 326 is substantially aligned
with a first target location
K of the second resection region 13. The step 905 includes creating a virtual
three-dimensional
model 401 of a resection guide 400 that defines at least a pair of cutting
guides 403 and at least
one second hole 406. The step 906 includes processing the virtual three-
dimensional model 401
of the resection guide 400 so as to couple the virtual three-dimensional model
401 of the
resection guide 400 to a virtual three-dimensional model 301 of a graft
portion 320 disposed
between the cutting guides 403, the graft portion 320 sized to fit in the
second region 13, such
that a central axis of the at least one second hole 406 is substantially
aligned with a second target
location L of the three-dimensional model 301 of the graft portion 320,
wherein the second target
location L substantially coincides with respect to the first target location K
when the graft
portion 320 is positioned in the second resection region 13. All or some of
the steps shown in
Fig. 5 or described above can be executed by a processor. The obtaining step
901 can further
include scanning the fixation member to obtain an image of the fixation
member, and
manipulating the image of the fixation member to define the at least one first
hole of the fixation
member in the virtual three-dimensional model of the fixation member. The
manipulating step
- 22 -
CA 02872397 2014-10-31
WO 2013/165558 PCT/US2013/030131
can further include identifying the central axis of the at least first one
hole. The method can
further comprise the step of constructing the resection guide identical to the
virtual three-
dimensional model of the resection guide using a rapid prototyping
manufacturing process.
[0066] It should be noted that the illustrations and discussions of the
embodiments
shown in the figures are for exemplary purposes only, and should not be
construed limiting the
disclosure. One skilled in the art will appreciate that the present disclosure
contemplates various
embodiments. For example, although the present disclosure refers to virtual
three-dimensional
models, it is envisioned that any of the virtual models described in the
present disclosure can be
two-dimensional. It should be further appreciated that the features and
structures described and
illustrated in accordance one embodiment can apply to all embodiments as
described herein,
unless otherwise indicated. Additionally, it should be understood that the
concepts described
above with the above-described embodiments may be employed alone or in
combination with
any of the other embodiments described above.
- 23 -