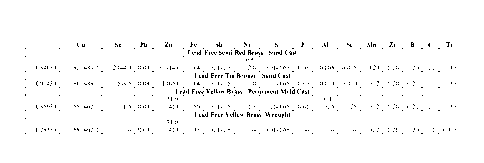Note: Descriptions are shown in the official language in which they were submitted.
=
1
ANTIMONY-MODIFIED LOW-LEAD COPPER ALLOY
Cross-reference to related applications
[0001] This application claims priority from United States Provisional Patent
Application 61/642,260 filed May 3, 2012.
BACKGROUND OF THE INVENTION
[0002] Current plumbing materials are typically made from lead containing
copper
alloys. One standard brass alloy formulation is referred to in the art as
C84400 or the
"81,3,7,9" alloy (consisting of 81% copper, 3% tin, 7% lead, and 9% zinc)
(hereinafter
the "81 alloy"). While there has been a need, due to health and environmental
issues
[as dictated, in part, by the U.S. Environmental Protection Agency (EPA) on
maximum lead content in copper alloys for drinking water applications] and
also for
cost reasons, to reduce lead contained in plumbing fitting, the presence of
lead has
continued to be necessary to achieve the desired properties of the alloy. For
example, the presence of lead in a brass alloy provides for desirable
mechanical
characteristics and to assist in machining and finishing the casting. Simple
removal
of lead or reduction below certain levels substantially degrades the
machinability as
well as the structural integrity of the casting and is not practicable.
[0003] Removal
or reduction of lead from brass alloys has been attempted
previously. Such previous attempts in the art of substituting other elements
in place
of lead has resulted in major machining and finishing issues in the
manufacturing
process, which includes primary casting, primary machining, secondary
machining,
polishing, plating, and mechanical assembly.
[0004] Several low or no lead formulations have previously been described.
See,
for example, products sold under the trade names SeBiLOY or EnviroBrass ,
Federalloy , BiwaliteTM, Eco Brass , Bismuth Red brass (C89833), and Bismuth
CA 2872498 2019-06-12
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
2
Bronze (C89836), as well as U.S. patents 7,056,396 and 6,413,330. Figure 1 is
a
table that includes the formulation of several known alloys based upon their
registration with the Copper Development Association (CDA). The existing art
for low
lead or no lead copper based castings consists of two major categories:
silicon based
materials and bismuth/selenium materials.
[0005] However,
there is a need for a low-lead alloy casting solution providing a
low-cost alloy with similar properties to current copper/lead alloys without
degradation
of mechanical properties or chemical properties, as well as significant
disruption to
the manufacturing process because of lead substitution in the material causing
cutting tool and finishing problems.
SUMMARY OF THE INVENTION
[0006] One
embodiment of the invention relates to a composition of matter
comprising 82% to about 89% copper, about 0.01% to about 0.65% sulfur, greater
than 0.1 to about 1.5% antimony, about 2.0% to about 4.0% tin, less than about
0.09% lead, about 5.0% to about 14.0% zinc, and about 0.5% to about 2.0%
nickel.
[0007] In one embodiment of the invention, the composition comprises 86% to
about
89% copper, about 0.01% to about 0.65% sulfur, greater than 0.1 to about 1.5%
antimony, about 7.5% to about 8.5% tin, less than about 0.09% lead, about 1.0%
to
about 5.0% zinc, and about 1.0% 13/0 nickel.
[0008] In one embodiment of the invention, the composition comprises 58% to
about
62% copper, about 0.01% to about 0.65% sulfur, greater than 0.1 to about 1.5%
antimony, about 1.5% tin, less than about 0.09% lead, about 31.0% to about
41.0%
zinc, and about 1.5% nickel.
[0009] In one embodiment of the invention, the composition comprises 58% to
about
62% copper, about 0.01% to about 0.65% sulfur, greater than 0.1 to about 1.5%
antimony, less than about 0.09% lead, and about 31.0% to about 41.0% zinc.
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
3
[0010]Another embodiment of the invention relates to a method for adding
sulfur to a
brass alloy. A base ingot is heated to a temperature of about 2,100 degrees
Fahrenheit to form a melt. In one embodiment, Zn, Ni, and Sn are added to the
copper the melt at about 2,124 F , stibnite is added at about 2,164 F , and
phosphorous is added at about 2,164 F . Stibnite wrapped in copper foil is
added
and the temperature maintained at about 2164F. In one embodiment, phosphorus
deoxidation is also done at this temperature. Heating of the melt is ceased
and
additives, including tin, zinc, nickel, and carbon, are added at about 2124 F.
At least
a partial amount of slag is skimmed from the melt. Temperature of the melt is
maintained at 2100 F. Slag is removed from the melt.
[0011]Additional features, advantages, and embodiments of the present
disclosure
may be set forth from consideration of the following detailed description,
drawings,
and claims. Moreover, it is to be understood that both the foregoing summary
of the
present disclosure and the following detailed description are exemplary and
intended
to provide further explanation without further limiting the scope of the
present
disclosure claimed.
BRIEF DESCRIPTION OF THE DRAWINGS
[0012]The foregoing and other objects, aspects, features, and advantages of
the
disclosure will become more apparent and better understood by referring to the
following description taken in conjunction with the accompanying drawings, in
which:
[0013] Figure 1 provides a table listing formulations for several known low-
lead
commercial copper alloys.
[0014] Figure 2 provides a table listing formulations of alloys in accordance
with
select embodiments of the invention.
[0015] Figure 3A and 3B is a table of chemical analysis of semi-red brass with
copper
coated graphite, MnS, and sulfur.
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
4
[0016] Figure 4A and 4B is a table of chemical analysis of semi-red brass with
copper
coated graphite and Stibnite in accordance with certain embodiments of the
invention.
[0017] Figure 5A ¨ 5c is a table indicating composition and mechanical
properties of
certain semi-red brass with copper coated graphite, MnS, and Sulfur.
[0018] Figure 6A -6C is a table indicating composition and mechanical
properties of
semi-red brass with copper coated graphite and antimony in accordance with
certain
embodiments of the invention.
[0019] Figure 7 is a table of chemical analysis of yellow brass having
antimony in
accordance with certain embodiments of the invention.
[0020] Figure 8 is a table indicating composition and mechanical properties of
certain
embodiments of yellow brass.
[0021] Figure 9 is an analysis of typical and minimum mechanical properties
for
certain embodiments of the invention and selected prior art alloys.
[0022] Figures 10A illustrates machining chip morphology of a semi-red brass
with
copper coated graphite (1.5%). Figures 10B illustrates machining chip
morphology of
a semi-red brass with copper coated graphite ("CCG") (1.5%) and 1.3% MnS.
Figures 10C illustrates machining chip morphology of a semi-red brass with
0.44%
sulfur. Figures 10D illustrates machining chip morphology of a semi-red brass
with
1.3% MnS. Figures 10E illustrates machining chip morphology of a semi-red
brass
1.64% stibnite and 1.5% calcinated petroleum coke ("CPC").. Figures 1OF
illustrates
machining chip morphology of a semi-red brass with 1.64% stibnite and 1.5%
CPC.
Figures 10G illustrates machining chip morphology of a semi-red brass with
1.64%
stibnite. Figures 10H illustrates machining chip morphology of a semi-red
brass with
1% stibnite. Figures 101 illustrates machining chip morphology of a semi-red
brass
with 0.8% stibnite. Figures 10J illustrates machining chip morphology of a
semi-red
brass with 1.2% stibnite, 1% copper coated graphite, and 0.08% boron.
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
[0023] Figures 11 illustrates machining chip morphology of a yellow brass with
1.5%
copper coated graphite and 0.8% stibnite.
[0024] Figure 12 illustrates the machinability of the C84030 red brass and
C28330
yellow brass in comparison to the commercially available C36000 leaded red
brass.
[0025] Figure 13A is a graph depicting the relationship of amount of stibnite
addition
to three mechanical properties (UTS, YS, and % Elongation); Figure 13B is a
graph
depicting the relationship of antimony concentration to three mechanical
properties
(UTS, YS, and % Elongation); Figure 13C is a graph depicting the relationship
of
sulfur concentration to three mechanical properties (UTS, YS, and "Vo
Elongation).
[0026] Figure 14 is a table of the chemistries for the test samples indicated
in the
metallographic images of Figures 15A-17J.
[0027] Figure 15A: Photomicrograph showing inclusion size of sample 1109319.
Figure 15B: SEM backscatter image of sample 1109319 at low magnification.
Figure
15C: SEM backscatter image of sample 1109319 at higher magnification. Figure
15D: Element map of sample 1109319. Figure 15E: BE image of sample 1109319
showing annotated locations. Figure 15F: EDS spectrum of sample 1109319 -
location 1. Figure 15G: EDS spectrum of sample 1109319- location 2. Figure
15H:
EDS spectrum of sample 1109319 - location 3. Figure 151: EDS spectrum of
sample
1109319 - location 4. Figure 15J Sample 1109319 element map.
[0028] Figure 16A: Photomicrograph showing inclusion size of sample 84XX42-
022812-H20P2-9A. Figure 16B: SEM backscatter image of sample 84XX42-022812-
H20P2-9A at low magnification. Figure 16C: SEM backscatter image of sample
84XX42-022812-H20P2-9A at higher magnification. Figure 16D: Element map of
sample 84XX42-022812-H20P2-9A. Figure 16E: BE image of sample 84XX42-
022812-H20P2-9A showing annotated locations. Figure 16F: EDS spectrum of
sample 84XX42-022812-H20P2-9A - location 1. Figure 16G: EDS spectrum of
sample 84XX42-022812-H20P2-9A - location 2. Figure 16H: EDS spectrum of
sample 84XX42-022812-H20P2-9A - location 3. Figure 161: EDS spectrum of
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
6
sample 84XX42-022812-H20P2-9A - location 4. Figure 16J: Sample 84XX42-
022812-H20P2-9A element map.
[0029]Figure 17A: Photomicrograph showing inclusion size of sample 84XX9-
013112-H18P2-10A. Figure 17B: SEM backscatter image of sample 84XX9-013112-
H18P2-10A at low magnification. Figure 17C: SEM backscatter image of sample
84)0(9-013112-H18P2-10A at higher magnification. Figure 17D: Element map of
sample 84XX9-013112-H18P2-10A. Figure 17E: BE image of sample 84XX9-
013112-H18P2-10A showing annotated locations. Figure 17F: EDS spectrum of
sample 84XX9-013112-H18P2-10A - location 1. Figure 17G EDS spectrum of
sample 84XX9-013112-H18P2-10A - location 2. Figure 17H: EDS spectrum of
sample 84XX9-013112-H18P2-10A - location 3. Figure 171: EDS spectrum of
sample 84XX9-013112-H18P2-10A - location 4. Figure 17J: Sample 84XX9-
013112-H18P2-10A element map.
[0030] Figure 18A: BE image of Perm Mold sample at low magnification. Figure
18B: BE image of Perm Mold sample at high magnification. Figure 18C: EDS
spectrum of Perm Mold sample - location 1. Figure 18D: EDS
spectrum of Perm
Mold sample - location 2. Figure 18E: EDS spectrum of Perm Mold sample -
location 3. Figure 18F: EDS spectrum of Perm Mold sample - location 4. Figure
18G: EDS spectrum of Perm Mold sample - location 5. Figure 18H: EDS spectrum
of Perm Mold sample - location 6. Figure 181: Element map of Perm Mold sample.
[0031] Figure 19A: BE image of Annealed sample at low magnification. Figure
19B:
BE image of Annealed sample at high magnification. Figure 19C: EDS
spectrum of Annealed sample - location 1. Figure 19D: EDS spectrum of Annealed
sample - location 2. Figure 19E: EDS spectrum of Annealed sample - location 3.
Figure 19F: EDS spectrum of Annealed sample - location 4. Figure 19G: EDS
spectrum of Annealed sample - location 5. Figure 19H: EDS spectrum
of
Annealed sample - location 6. Figure 191: EDS spectrum of Annealed sample -
location 7. Figure 19J: Element map of Annealed sample.
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
7
[0032] Figure 20A: BE image of Cold Rolled sample at low magnification. Figure
20B: BE image of Cold Rolled sample at high magnification. Figure 20C: EDS
spectrum of Cold Rolled sample - location 1. Figure 20D: EDS
spectrum of Cold
Rolled sample - location 2. Figure 20E: EDS spectrum of Cold Rolled sample -
location 3. Figure 20F: EDS spectrum of Cold Rolled sample - location 4.
Figure
20G: EDS spectrum of Cold Rolled sample - location 5. Figure 20H: Element map
of
Cold Rolled sample.
[0033] Figures 21A-C illustrate phase diagrams for semi-red brass and Alloy
C84030.
Figure 21A is a phase diagram for a red brass without antimony. Figure 21B is
a
phase diagram for semi-red brass with 0.8 wt% antimony. Figure 21C is a phase
diagram for semi-red brass with 1.3 wt% antimony.
[0034] Figure 22A is a phase assemblage diagram of Semi-Red Brass with 0.8 Sb.
Figure 22B is a magnified part of the phase assemblage diagram of Semi-Red
Brass
with 0.8 Sb. Figure 22C is a magnified part of the phase assemblage diagram of
Semi-Red Brass with 1.3 Sb. Figure 22D is a magnified part of the phase
assemblage diagram of Semi-Red Brass with 0.8 Sb ¨ Scheil Cooling. Figure 22E
is
a magnified part of the phase assemblage diagram of Semi-Red Brass with 1.3 Sb
¨
Scheil Cooling.
[0035] Figure 23 is phase diagram showing the location of the yellow brass
alloy
61/38/0.3/0 Cu/Zn/Sn/Sb wt%.
[0036] Figure 24A is an equilibrium phase assemblage diagram of yellow brass
with 0
wt% Sb. Figure 24B is an equilibrium phase assemblage diagram of yellow brass
with 0.6 wt% Sb. Figure 24C is an equilibrium phase assemblage diagram of
yellow
brass with 1 wt% Sb. Figure 24D is a Scheil phase assemblage diagram of yellow
brass with 0 wt% Sb. Figure 24E is a Scheil phase assemblage diagram of yellow
brass with 0.6 wt% Sb. Figure 24F is a Scheil phase assemblage diagram of
yellow
brass with 1 wt% Sb.
[0037] Figure 25 is a free energy diagram.
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
8
[0038] Figure 26A shows dezincification corrosion (between lines) extends to a
maximum depth of 0.0012" (31.2 microns) from the exposed surface (towards top)
in
the metallographic section prepared through the edge of the "MBAF 180" sample.
Unetched. (494X). Figure 26B shows dezincification corrosion (between lines)
extends to a maximum depth of 0.0113" (287.0 microns) from the exposed surface
(towards top) in the metallographic section prepared through the core of the
"MBAF
180" sample. Unetched. (201X).
[0039] Figure 27A shows dezincification corrosion (between lines) extends to a
maximum depth of 0.04830" (1,228.1 microns) from the exposed surface (towards
top) in the metallographic section prepared through the thin walled section of
the
"C36000 Ht# 1-Yeager" sample. Unetched. (50X). Figure 27B shows
dezincification
corrosion (between red lines) extends to a maximum depth of 0.05133" (1,303.8
microns) from the exposed surface (towards top) in the metallographic section
prepared through the thick walled section of the "C36000 Ht# 1-Yeager" sample.
Unetched. (50X).
[0040] Figure 28A shows no dezincification corrosion is present at the exposed
surface (towards top) in the metallographic section prepared through the edge
of the
"28330-Lab# 358050 P4 H2a" sample. Unetched. (494X). Figure 28B shows
dezincification corrosion (between lines) extends to a maximum depth of
0.0033"
(82.8 microns) from the exposed surface (towards top) in the metallographic
section
prepared through the core of the "28330-Lab# 358050 P4 H2a" sample. Unetched.
(494X).
[0041] Figure 29A shows no dezincification corrosion is present at the exposed
surface (towards top) in the metallographic section prepared through the edge
of the
"84030-62412-H3P2-9" sample. Unetched. (494X). Figure
29B shows no
dezincification corrosion is present at the exposed surface (towards top) in
the
metallographic section prepared through the edge of the "84030-62412-H3P2-9"
sample. Unetched. (494X).
9
[0042]Figure 30 illustrates the chemical compositions of various alloys tested
based
on Design of Experiments (DOE).
[0043] Figure 31 illustrates the relation of alloy properties between C84030
red brass
and two commercial brasses which were used as the base for the DOE.
[0044]Figure 32 illustrates the composition and mechanical properties of
various
tested alloys based on Design of Experiments (DOE).
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0045] In the following detailed description, reference is made to the
accompanying
drawings, which form a part hereof. In the drawings, similar symbols typically
identify
similar components, unless context dictates otherwise. The illustrative
embodiments
described in the detailed description, drawings, and claims are not meant to
be
limiting. Other embodiments may be utilized, and other changes may be made,
without departing from the spirit or scope of the subject matter presented
here. It will
be readily understood that the aspects of the present disclosure, as generally
described herein, and illustrated in the figures, can be arranged,
substituted,
combined, and designed in a wide variety of different configurations, all of
which are
explicitly contemplated and made part of this disclosure.
[0046]Brass alloys typically utilize lead as a chip breaker and to generally
improve
the qualities desirable in brass alloys for use in a wide range of situations,
including
plumbing. The use of sulfides as a replacement for lead has been previously
taught
in U.S. Patent Publication U.S. 2012/0121455 Al March 17, 2012.
[0047]It has been observed that the addition of elemental sulfur in place of
lead in a
"standard" brass alloy may not result in the sulfur becoming integrated into
the final
alloy, but rather loss to the dross. As further described below, the brass
alloys of the
present invention utilize antimony for improved properties. In one embodiment,
of the
present invention a sulfur containing mineral, stibnite, is utilized as a
source of sulfur
and to provide antimony to the alloy.
CA 2872498 2019-06-12
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
[0048]Stibnite is a naturally occurring sulfide mineral in the form of Sb2S3.
Stibnite
typically contains 26.7% sulfur , 69.2% antimony and 0.4% moisture. Apparent
density is 1.19 g/cc. Particle size is 325 mesh or 44 microns. One embodiment,
utilized in the examples noted below, contains 27% S and 69% Sb.
[0049]Figure 2 illustrates the nominal ranges for four alloy embodiments, each
including antimony. C84030 is a red brass having sulfur and antimony. C90430
is a
tin bronze having sulfur and antimony. C85930 is a yellow brass for permanent
mold
casting applications having sulfur and antimony. C28330 is a yellow brass for
wrought applications having sulfur and antimony. For easy of reference, the
respective embodiments will be referred to by these numbers throughout. The
specific materials used for such formulations may be specified in certain
embodiments.
Alloy components
[0050]The alloys of the present invention comprise copper, zinc, tin, sulfur,
nickel,
phosphorus, and antimony. In certain embodiments, one or more of manganese,
zirconium, boron, titanium and/or carbon are included.
[0051]The alloys, comprise as a principal component, copper. Copper provides
basic properties to the alloy, including antimicrobial properties and
corrosion
resistance. Pure copper has a relatively low yield strength, and tensile
strength, and
is not very hard relative to its common alloy classes of bronze and brass.
Therefore,
it is desirable to improve the properties of copper for use in many
applications
through alloying. The copper will typically be added as a base ingot. The base
ingot's composition purity will vary depending on the source mine and post-
mining
processing. The copper may also be sourced from recycled materials, which can
vary widely in composition. Therefore, it should be appreciated that ingot
chemistry
can vary, so, in one embodiment, the chemistry of the base ingot is taken into
account. For example, the amount of zinc in the base ingot is taken into
account
when determining how much additional zinc to add to arrive at the desired
final
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
11
composition for the alloy. The base ingot should be selected to provide the
required
copper for the alloy while considering the secondary elements in the base
ingot and
their intended presence in the final alloy since small amounts of various
impurities,
such as iron, are common and have no material effect on the desired
properties.
[0052] Lead has typically been included as a component in copper alloys,
particularly
for applications such as plumbing where machinability is an important factor.
Lead
has a low melting point relative to many other elements common to copper
alloys. As
such, lead, in a copper alloy, tends to migrate to the interdendritic or grain
boundary
areas as the melt cools. The presence of lead at interdendritic or grain
boundary
areas can greatly improve machinability and pressure tightness. However, in
recent
decades the serious detrimental impacts of lead have made use of lead in many
applications of copper alloys undesirable. In particular, the presence of the
lead at
the interdendritic or grain boundary areas, the feature that is generally
accepted to
improve machinability, is, in part, responsible for the unwanted ease with
which lead
can leach from a copper alloy.
[0053]Sulfur is added to the alloys of the present invention to overcome
certain
disadvantages of using leaded copper alloys. Sulfur present in the melt will
typically
react with transition metals also present in the melt to form transition metal
sulphides.
For example, copper sulfide and zinc sulfide may be formed, or, for
embodiments
where manganese is present, it can form manganese sulfide. Figure 25
illustrates a
free-energy diagram for several transition metal sulphides that may form in
embodiments of the present invention. The melting point for copper sulfide is
1130
Celsius, 1185 Celsius for zinc sulfide, 1610 Celsius for manganese sulfide,
and 832
Celsius for tin sulfide. Thus, without limiting the scope of the invention, in
light of the
free energy of formation, it is believed that a significant amount of the
sulfide
formation will be zinc sulfide for those embodiments having no manganese. It
is
believed that sulphides that solidify after the copper has become to solidify,
thus
forming dendrites in the melt, aggregate at the interdendritic areas or grain
boundaries.
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
12
[0054]Sulfur provides similar properties as lead would impart to a copper
alloy,
without the health concerns associated with lead. Sulfur forms sulphides which
it is
believed tend to aggregate at the interdendritic or grain boundary areas. The
presence of the sulphides provides a break in the metallic structure and a
point for
the formation of a chip in the grain boundary region and improve machining
lubricity,
allowing for improved overall machinability. The sulphides predominate in the
alloys
of the present invention provide improved lubricity. Good distribution of
sulphides
improves pressure tightness, as well as, machinability.
[0055] It is believed that the presence of tin in some embodiments increases
the
strength and hardness but reduces ductility by solid solution strengthening
and by
forming Cu-Sn intermetallic phase such as Cu3Sn. It also increases the
solidification
range. Casting fluidity increases with tin content. Tin also increases
corrosion
resistance. However, currently Sn is very expensive relative to other
components.
[0056]With respect to zinc, it is believed that the presence of Zn is similar
to that of
Sn, but to a lesser degree, in certain embodiments approximately 2% Zn is
roughly
equivalent to 1 % Sn with respect to the above mentioned improvements to
characteristics noted above. Zn increases strength and hardness by solid
solution
hardening. However, Cu-Zn alloys have a short freezing range. Zn is much less
expensive than Sn.
[0057]With respect to certain embodiments, iron can be considered an impurity
picked up from stirring rods, skimmers, etc during melting and pouring
operations, or
as an impurity in the base ingot. Such categories of impurity have no material
effect
on alloy properties.
[0058] [0059] In some embodiments, nickel is included to increase strength and
hardness. Further, nickel aids in distribution of the sulphide particles in
the alloy. In
one embodiment, adding nickel helps the sulfide precipitate during the cooling
process of the casting. The precipitation of the sulfide is desirable as the
suspended
sulfides act as a substitute to the lead for chip breaking and machining
lubricity
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
13
during the post casting machining operations. With the lower lead content, it
is
believed that the sulfide precipitate will minimize the effects of lowered
machinability.
[0060] Phosphorus may be added to provide deoxidation. The
addition of
phosphorus reduces the gas content in the liquid alloy. Removal of gas
generally
provides higher quality castings by reducing gas content in the melt and
reducing
porosity in the finished alloy. However, excess phosphorus can contribute to
metal-
mold reaction giving rise to low mechanical properties and porous castings.
[0061]Aluminum is, in some embodiments, such as semi-red brasses and tin
bronzes, treated as an impurity. In such embodiments, aluminum has harmful
effects
on pressure tightness and mechanical properties. However, aluminum in yellow
brass castings can selectively improve casting fluidity. It is believed that
aluminum
encourages a fine feathery dendritic structure in such embodiments which
allows for
easy flow of liquid metal.
[0062]Silicon is also considered an impurity. In foundries with multiple
alloys, silicon
based materials can lead to silicon contamination in non silicon containing
alloys. A
small amount of residual silicon can contaminate semi red brass alloys, making
production of multiple alloys near impossible. In addition, the presence of
silicon can
reduce the mechanical properties of semi-red brass alloys.
[0063]Manganese may be added in certain embodiments. The manganese is
believed to aid in the distribution of sulphides. In
particular, the presence of
manganese is believed to aid in the formation of and retention of zinc sulfide
in the
melt. In one embodiment, a small amount of manganese is added to improve
pressure tightness. In one embodiment, manganese is added as MnS.
[0064] Either zirconium or boron may be added individually (not necessarily in
combination) to produce a fine grained structure which improves surface finish
of
castings during polishing.
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
14
[0065] Carbon may be added in certain embodiments to improve pressure
tightness,
reduce porosity, and improve machinability. In one embodiment, carbon may be
added to the alloy as copper coated graphite ("CCG"). One type of copper
coated
graphite product is available from Superior Graphite and sold under the name
DesulcoMC TM. One embodiment of the copper coated graphite utilizes graphite
that
contains 99.5% min carbon, 0.5% max ash, and 0.5% max moisture. US mesh size
of particles is 200 or 125 microns. This graphite is coated with 60% Cu by
weight
and has very low S.
[0066] In another embodiment, carbon may be added to the alloy as calcinated
petroleum coke ("CPC") also known as thermally purified coke. CPC may be
screened to size. In one aspect, 1% sulfur is added and the CPC is coated with
60%
Cu by weight. CPC, because of its relatively higher and coarser S content
compared
to copper coated graphite, imparts slightly higher S to the alloy and hence,
better
machinability.
[0067] It is believed that a majority of the carbon is not present in the
final alloy.
Rather, it is believed that carbon particles are formed that float to the
surface as
dross or reacting to form carbon dioxide (around 2100 F) that is released from
the
melt as a gas. It has been observed that final carbon content of alloy is
about
0.005% with a low density of 2.2 g/cc. Carbon particles float and form CO2 at
2100F
(like a carbon boil) and purify the melt. Thus, the alloys utilizing carbon
may be more
homogeneous and pure compared with other additions such as S, MnS, stibnite
etc.
Further, the atomic radius of carbon is 0.91X10 -10 M, which is smaller than
that of
copper (1.57X-1 M). Without limiting the scope of the invention, it is
believed that
carbon because of its low atomic volume remains in the face centered cubic
crystal
lattice of copper, thus contributing to strength and ductility.
[0068] Titanium may be added in combination with carbon, such as in graphite
form.
Without limiting the scope of the invention, it is believed that the titanium
aids in
bonding the carbon particles with the copper matrix, particularly for raw
graphite. For
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
embodiments utilizing copper coated with carbon, titanium may not be useful to
distribute the carbon.
[0069] Brass alloys having antimony have been shown to exhibit dezincification
resistance. In addition, antimony may aid in chip breaking by segregating to
the grain
boundaries. This provides for improved machinability. Sb forms compounds with
Cu
(Cu2Sb) and Zn (ZnSb). As discussed further below in regard to the back
scattered
electron images (18B-F and 20B-F) in the alloy materials, Sb, if added as
Stibnite,
separates from the S and interacts with Sn and Cu. Antimony may be provided in
the
form of Stibnite, which has the benefit of also providing sulfur and avoiding
certain
issues that arise with the use of elemental sulfur.
Alloy Formulations
[0070] Figure 2 illustrates a table listing four embodiment corresponding to
semi-red
brass, tin bronze, yellow brass (permanent mold cast) and yellow brass (sand
cast).
Figure 3 is a table comparing the content of various alloy heats, with the
components
noted in the comments. Figure 3 provides a comparison, for example, of the
sulfur
content of alloys having various components. Figure 4
provides chemical
compositions of embodiments of a semi-red brass having antimony and copper
coated graphite. Figure 7 provides chemical compositions of embodiments of a
yellow brass having antimony.
[0071] It can be observed that the use of stibnite provides sulfur to the
finished alloy
in a similar amount as some other components, such as the much more expensive
MnS. As has been noted above, the use of sulfur in the alloy formation
provides
several problems, including environmental concerns due to the amount of sulfur
dust
and sulfur dioxide released, often violently, into the environment rather than
integrated into the alloy melt. The use of sources of sulfur that provide for
a better
"yield" with regard to the amount of sulfur added and that retained in the
finished
alloy has been observed to be beneficial. Further, various sulfur sources
could be
utilized, but any non-sulfur component may have a negative impact on the
properties
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
16
of the finished alloy. Further, cost and availability are a consideration for
selecting a
sulfide for use as a sulfur source in the alloy. It has been observed that
stibnite in the
range of 0.4 to 1.6% provides sulfur in the amount desired and the antimony
that
remains in the alloy does not have an unacceptable impact on the properties of
the
finished alloys. A discussion of the impact of the antimony on the alloy
mechanical
properties is discussed further below.
[0072]One embodiment of the invention relates to a composition of matter
comprising 82% to about 89% copper, about 0.01% to about 0.65% sulfur, greater
than 0 to about 1.5% antimony, about 2.0% to about 4.0% tin, less than about
0.09%
lead, about 5.0% to about 14.0% zinc, and about 0.5 to about 2.0% nickel. In
one
embodiment, less than 0.65% sulfur is utilized to minimize the formation of
gases
such as sulfur dioxide, which negatively impact the mechanical properties of
the
finished product made from the alloy.
[0073] In one embodiment of the invention, the composition comprises 86% to
about
89% copper, about 0.1% to about 0.65% sulfur, greater than 0 to about 1.5%
antimony, about 7.5% to about 8.5% tin, less than about 0.09% lead, about 1.0%
to
about 5.0% zinc, and about 1.0% `)/0 nickel.
[0074] In one embodiment of the invention, the composition comprises 58% to
about
62% copper, about 0.01% to about 0.65% sulfur, greater than 0 to about 1.5%
antimony, about 1.5% tin, less than about 0.09% lead, about 31.0% to about
41.0%
zinc, and about 1.5% % nickel.
[0075] In one embodiment of the invention, the composition comprises 58% to
about
62% copper, about 0.01% to about 0.65% sulfur, greater than 0 to about 1.5%
antimony, less than about 0.09% lead, and about 31.0% to about 41.0% zinc.
[0076] Figure 3 is a chemical analysis table showing examples of various semi-
red
brasses with copper coated graphite, MnS, and Sulfur. Figure 4 is a chemical
analysis table showing examples of various semi-red brass with copper coated
graphite and antimony in accordance with certain embodiments of the invention.
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
17
Figure 7 is a chemical analysis table showing embodiments of yellow brass with
antimony in accordance with certain embodiments of the invention.
[0077] In one embodiment, the brass alloy includes stibnite. The stibnite
may be
added in the range of greater than zero but less than 1.2%. In one embodiment,
the
preferred range is about 0.4 to about 1.2%. In one embodiment, the stibnite is
1.64%. In an alternative embodiment, the stibnite is 0.6%. In an
alternative
embodiment, the stibnite is 0.4%. The preferred embodiment utilizes about 1%
stibnite. Addition of elemental S contributes to environmental problems due to
release of sulfur dust and SO2 to the atmosphere. The use of stibnite provides
a
source of antimony and a source of sulfur, without the drawbacks associated
with
working with elemental sulfur in alloy melts. The preferred range for Sb ,S
and
stibnite, in the final alloy, are 0.3 to 0.8%, 0.1 to 0.35% and 0.4 to 1%
respectively.
(This is evident from Figures 13B and 13C)
[0078] In certain embodiments, the brass alloy may include stibnite in
combination
with carbon. In one embodiment, the alloy includes 1.0% CCG or CPC and 1%
stibnite. In a further embodiment, an additional 0.2% sulphur is provided for
better
machinability. In one embodiment, 1% carbon and 1% stibnite is utilized. In
one
embodiment, the stibnite is 0.6% and the carbon is 1. In an alternative
embodiment,
the stibnite is 1.64% and the carbon is 1.5%. In one embodiment, the carbon is
copper coated graphite. In an alternative embodiment, the carbon is CPC.
[0079] It should be appreciated that the total amount of stibnite utilized
in the melt
can be varied to alter the amount of sulfur and the amount of antimony in the
final
alloy. For example, using a 27% S/69% Sb: 0.4 % Stibnite gives 0.071% S and
0.27% Sb; 0.6% Stibnite gives 0.12% S and 0.4 % Sb; 0.8% stibnite gives 0.2% S
and 0.64% Sb; 1% stibnite gives 0.25% S and 0.77% Sb; 1.2% stibnite gives
0.278
% S and 0.859% Sb, and 1.64% Stibnite gives 0.4% S and 1.35 % Sb.
[0080] In one embodiment, about 0.5 to about 1.0 CCG (or CPC) together with
about
0.8 to about 1.0 Stibnite provides desirable mechanical properties and
machinability.
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
18
The use of the stibnite provides benefits of sulfur while avoiding many of the
issues
with using pure sulfur in an alloy melt, including flaring and excess dross.
As shown
in the SEM (Figures 15J, 16J, 17J, 181, 19J, and 20H) the stibnite breaks down
to Sb
and S. Sb reacts with Cu and Sn to form intermetallic compounds; whereas S
reacts
with Cu and Zn to form their sulfides.
[0081]The addition of stibnite to provide sulfur and antimony provides several
advantages over the use of elemental sulfur. The use of sulfur results in
undesired
consequences, including environmental impact. For example, sulfur addition to
the
melt may cause flaring that results in the loss of sulfur as well as dangerous
conditions during the addition. Further, the use of sulfur directly results in
a lower
yield with respect to the percentage of added sulfur that enters the melt and
the final
alloy, as much of the sulfur is lost to dross. The increase in dross can cause
other
problems with the alloying.
[0082] In one embodiment, the stibnite is wrapped in copper foil prior to
addition to
the melt. The wrapped stibnite may be added after melting the ingots and
bringing
temperature to about 2000F.
[0083] In on embodiment, about 0.5 to about 1.0 CCG (or CPC) is utilized with
about
0.8 to about 1.0 Stibnite to provide the best combination of mechanical
properties
and machinability. In a further embodiment, additional sulfur may be added to
further
increase the amount of sulfur in the alloy.
Alloy Characteristics
[0084] In one embodiment, an alloy of the present invention solidifies in a
manner
such that a multitude of discrete particles of sulfur/sulfide are distributed
throughout
in a generally uniform manner throughout the casting. These nonmetallic sulfur
particles serve to improve lubricity and break chips developed during the
machining
of parts cast in this new alloy, thereby improving machinability with a
significant or
complete reduction in the amount of lead. Without limiting the scope of the
invention,
the sulfides are believed to improve lubricity. The presence of antimony
further
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
19
improves properties of the C84030 red brass as described below. Embodiments
utilizing stibnite provide for a source of antimony and sulfur while also
delivering the
sulfur in a form more readily compatible with the alloy melt process.
[0085]The preferred embodiments of the described alloy retain machinability
advantages of the current leaded alloys. Further, it is believed that due to
the relative
scarcity of certain materials involved, the preferred embodiments of the ingot
alloy
will cost considerately less than that of the bismuth and/or selenium alloyed
brasses
that are currently advocated for replacement of leaded brass alloys. The
sulfur is
present in certain embodiments described herein as a sulfide which is soluble
in the
melt, but is precipitated as a sulfide during solidification and subsequent
cooling of
the alloy in a piece part. This precipitated sulfur enables improved
machinability by
serving as a chip breaker similar to the function of lead in alloys and in
bismuth and
selenium alloys. In the case of bismuth and/or selenium alloys the formation
of
bisnnuthides or selenides, along with some metallic bismuth, accomplishes a
similar
objective as this new sulfur containing alloy. The improvement in
machinability may
show up as increased tool life, improved machining surfaces, reduced tool
forces,
etc. This new idea also supplies the industry with a low lead brass/bronze
which in
today's environment is seeing any number of regulatory authorities limit by
law the
amount of lead that can be contained in plumbing fittings.
Melt Process
[0086] In one embodiment, graphite is placed on the bottom of the crucible
prior to
heating. In one embodiment, silicon carbide or clay graphite crucibles may be
used
in the melts. It is believed that the use of graphite reduces the loss of zinc
during the
heat without substantially becoming incorporated into the final alloy. In
one
embodiment, approximately two cups of graphite are used for a 90 to 95 lbs
capacity
crucible. For the examples used herein, a B-30 crucible was used for the
melts,
which has a capacity of 90 to 95 lbs of alloy. For embodiments using CPC or
CCG,
the carbon is wrapped in copper foil, preheated in oven at 150 C to drive off
moisture
and plunged into the melt followed by stirring.
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
[0087] Based upon the desired end alloy's formulation, the required base ingot
is
placed in the crucible and the furnace started. The base ingot, is brought to
a
temperature of about 1,149 degrees Celsius to form a melt. In one embodiment a
conventional gas-fired furnace is used, and in another an induction furnace is
used.
The furnace is then turned off, i.e. the melt is no longer heated. Then the
additives,
except, in one embodiment, for sulfur and phosphorus, are then plunged into
the melt
between 15 to 20 seconds to achieve desired levels of Zn, Ni and Sn. The
additives
comprise the materials needed to achieve the final desired alloy composition
for a
given base ingot. In one embodiment, the additives comprise elemental forms of
the
elements to be present in the final alloy. Then a partial amount of slag is
skimmed
from the top of the melt.
[0088]The furnace is then brought to a temperature of about 1,171 Celsius. The
furnace is then shut off and the sulfur additive is plunged in, such as in the
form of
stibnite. For certain embodiments having phosphorus added, such as for
degassing/deoxidizing of the melt, the furnace is then reheated to a
temperature of
about 1,177 degrees Celsius and phosphorous is plunged into the melt as a Cu-P
master alloy. Next, preferably all of the slag is skimmed from the top of the
crucible.
Tail castings for pressure testing and evaluation of machinability and
plating, buttons,
wedges and mini ingots for chemical analysis, and web bars for tensile testing
are
poured at about 1,149, about 1,116, and about 1,093 degrees Celsius
respectively.
Mechanical Properties
[0089]Mechanical properties of various embodiments of the present alloys were
tested as well as those for red brass without antimony added (as stibnite or
otherwise). Sample heats, prepared in accordance with the process above and
the
resultant alloys were tested for ultimate tensile strength ("UTS"), yield
strength ("YS"),
percent elongation ("E%"), Brinnell hardness ("BHN"), and Modulus of
Elasticity
("MoE").
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
21
[0090] Figure 5 shows composition and mechanical properties of a with one
or
more of copper coated graphite, MnS, and Sulfur. Table 1 (below in the section
regarding machinability) provides an analysis of select properties of certain
embodiments of the alloys of semi-red brass with copper coated graphite, MnS,
and
Sulfur, including machinability, mechanical properties, cost, etc. Figure 6
shows
Composition and Mechanical Properties of Semi-red Brass C84030, illustrating
semi-
red brass with stibnite added and with various other combinations of MnS and
copper
coated graphite. As can be seen in Figures 5-6, the mechanical properties of
semi-
red Brass (SRB) with low amounts of Stibnite are generally around 40.5 ksi
UTS,
18.3 ksi Ys and 41.0% elongation. The addition of stibnite in a yellow brass
alloy,
either for wrought or permanent mold casting, with all variations of antimony
of
Stibnite are generally around 49.83 ksi UTS, 29.0 ksi Ys and 7% elongation.
The 1%
stibnite alloy of C84030 provided 42.9 ksi UTS, 20.3 ksi YS, and 32%
Elognation.
[0091] It has been observed that the sulfur content of SRB increases when MnS
is
added along with 0.4 and 0.6 A) Stibnite. When 1.64% stibnite is utilized,
the sulfur
level goes to 0.4%, but Sb level also increases to 1.35%.
[0092] Antimony is observed to improve or not be detrimental to certain
desirable
properties of the brass alloy at low levels. Above 1.5% the antimony's
presence
begins to negatively impact mechanical properties. However, sufficient
antimony is
necessary to provide the improved characteristics. Thus, one embodiment of
C84030 includes 0.1% to 1.5% antimony.
[0093] The use of carbon in a brass alloy provides beneficial results. SRB
with
CCG or CPC have UTS around 43.5ksi, YS of 18.1 ksi, but elongation in the
range
59% (49-61%). However, adding MnS to CCG decreases UTS slightly to (42.9 ksi),
YS remains almost unchanged (17.95 ksi), elongation decreases to 33-51% (47%
avg). High levels of stibnite, 1.64%, in combination with CCG or CPC decreases
UTS to 38.5 ksi, YS is around 19.8 ksi and elongation drops to 17-22% (20%
Avg).
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
22
[0094] The use of MnS in a brass alloy gives 42.3 ksi UTS, 18.08 ksi YS and
45%
elongation. Adding MnS to CCG and CPC does give good combination of UTS, YS
and % elongation. However, sulfur level is not high enough to produce
desirable
machinability. Besides, addition of MnS increases ingot cost significantly.
Adding S
to SRB gives 39.7 ksi UTS, 18.67 YS and 29% elongation.
[0095] Figure 8 is a table indicating composition and mechanical properties of
certain
embodiments of C28330 a yellow brass. Properties of certain embodiments of
yellow
brass C28330 have been compared with known leaded yellow brass alloys C26000
and C35600. Zr and B were added to heat P4H2A to see if grain refinement would
produce any beneficial effect. There appears to have some benefits. UTS and
hardness of the grain refined one are relatively higher in the cold rolled
condition. A
elongation and UTS of cold rolled and annealed at 1290 F are also relatively
higher.
It is believed that the grain size of this annealed sample is finer than that
of heat
P4H1. Figure 8 also includes information regarding annealing and cold working.
[0096]Overall, the tested embodiments of yellow brass C28330 had comparable
results to the two leaded alloys C26000 and C35600 mentioned above. It is
believed that quarter hard, half hard, hard and extra hard correspond to
different
stages of cold rolling such as 10%, 30%, 45% cold reduction etc. The hardness
depends on the work hardening behaviour of the alloy. Embodiments of C28330
were cold rolled from 0.150 to 0.040 inch. This corresponds to 73% cold
reduction.
This is equivalent to extra hard condition.
[0097] Figure 9 summarizes the properties for several commercially available
alloy
compounds and for embodiments tested of the C84030 red brass and the C85930
yellow brass.
Machinability
[0098] Machinability was tested for certain embodiments of semi-red brass
C84030
and yellow brass C28330. Machinability testing described in the present
application
was performed using the following method. The piece parts were machined by a
CA 02872498 2014-11-03
WO 2013/166454 PCMJS2013/039567
23
coolant fed, 2 axis, CNC Turning Center. The cutting tool was a carbide
insert. The
machinability is based on a ratio of energy that was used during the turning
on the
above mentioned CNC Turning Center. The calculation formula can be written as
follows:
CF = (F1 / E2) X 100
CF = Cutting Force
El = Energy used during the turning of a "known" alloy C 36000 (CDA).
E2 = Energy used during the turning of the New Alloy.
Feed rate = .005 IPR
Spindle Speed = 1,500 RPM
Depth of Cut = Radial Depth of Cut = 0.038 inches
[0099]An electrical meter was used to measure the electrical pull while the
cutting
tool was under load. This pull was captured via milliamp measurement. Table 1
below lists the chemical composition and machinability rating for several
tested
samples. Table 2 below lists the chemical composition and machinability rating
for
several tested samples of a C84030 red brass in accordance with the present
invention. Table 3 below lists the chemical composition and machinability
rating for
several tested samples of a C28330 yellow brass in accordance with the present
invention.
Table 1 MACHINABILITY RATING FOR SEMI-RED BRASS
Heat No Cu Sn Zn S Mn Pb Sb C
Mach Addition
Ratin
g(%)
C84XX1- 86.80 2.92
9.00 0.012 .001 .019 .005 .00 37 Only
012412- 2
CCG(1.
H1P1-7C 5%)
C84XX2- 86.83 3.00
8.81 0.087 .046 .017 .004 .00 36 1.5
012412- 2
(VoCCG
H3P3-7C +1.3
%MnS
C84XX5- 86.96 2.99 8.54 0.298 .000 .019 .011 48 0.44
012512- 5
%Sulfur
H8P4-7X
C84XX6- 86.90 3.04 8.62 0.162 .023 .017 .006 49 1.3
012612-
%MnS
Hi 0P2-7X
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
24
Table 2 MACHINABILITY RATING FOR SEMI-RED BRASS C84030
Heat No Cu Sn Zn S Mn Pb Sb C Mach
Addition
Ratin
1109319 82.47 2.94 12.98 .169 .004 .019 .266 63
0.4%Sti
bnite+0.
6 %MnS
1109308 82.77 2.81 12.94 .071 .0003 .020 269 0.4
cY0Stibnit
e
1109330 82.10 2.95 13.26 .121 .0005 .034 .395 60 0.6
%Stibnit
1109341 83.00 2.79 12.29 .251 .007 .022 .418 62 0.6
%Stibnit
e + 1
%MnS
1109352 82.57 2.92 12.57 .264 .007 .024 .423 61 0.6%
Stibnite+
1 %MnS
84XX9- 85.28 3.07 8.76 .379 .001 .035 1.32 .005 53
1.64 %
013112-
Stibnite+
H17P1-7-X- 1.5%
CPC
84XX9- 85.13 3.06 8.91 .386 .001 .033 1.34 .005 53 1.64%
013112-
Stibnite+
H18P2-7-X- 1.5%
CPC
84XX41- 86.59 2.91 8.72 .212 .001 .019 .641 .002
57 0.8 %
022812-
Stibnite
H19P1-7X
84XX42- 86.41 2.92 8.68 .249 .0008 .02 .77 .002
59 1.0 %
022812-
Stibnite
H20P2-7X
84030- 84.49 2.80 10.10 .373 .001 .019 1.01 .002 49 1.2%
062912-
Stibnite,
H7P1-7-C- B 1%
CCG,
0.08 B
84XX4- 85.39 2.87 8.81 .428 .001 .050 1.26 39 1.64%
012512-
Stibnite
H6P2
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
Table 3 Yellow Brass C28330 Machinability Properties
Heat No Cu Sn Zn S Mn Pb Sb C
Mach Addition
Ratin
g(%)
28330- 61.00 1.45 36.9 .016 .032 .005 .336 .00 61
1.5%
030813- 2 2
CCG,
P4H2b*
0.8%
Stibnite
*0.036% B, 0.010% Zr
[0100] Figures 10A-J illustrates the machinability of the 084030 red brass and
Figure
11 illustrates C28330 yellow brass in comparison to the commercially available
C36000 leaded red brass.
[0101]Machinability testing of embodiments of 084030 red brass indicate the
addition of CCG does not improve machinability. There is some improvement in
machinability when CCG and MnS are added together. The addition of sulfur
improves machinability; however, addition of sulfur creates a lot of fumes in
the
melting area which is not environmentally friendly. The addition of MnS
improves
machinability; however, MnS is very expensive and increases ingot cost
significantly.
The addition of antimony as stibnite improves machinability. However, the
benefits to
machinability of embodiments of the C84030 red brass are lessened above 1%
stibnite (for example, providing 0.8% antimony) as machinability decreases
when
stibnite content exceeds 1%. Further, it has been observed that antimony, for
example provided as stibnite, in combination with MnS or COP improves
machinability. In one embodiment, a red-brass alloy includes 0.4-1% stibnite.
In one
embodiment, 0.3-0.8% antimony is included.
[0102]Machinability index of wrought alloy 028330 was 61%, which compares with
C84030 containing Sb under 1%. It was observed that tail castings produced by
permanent mold casting were used for machinability evaluation. These have a
fine
grain structure compared with sand cast 084030 tail castings. Chip morphology
of
028330 was not good in comparison with C84030. However, it should be noted
that
the machined surfaces looked good. It should be appreciated that the
possibility
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
26
exists that the machinability rating could change if different speeds, feeds
and tool
geometry were to be used and samples can be machined well with proper use of
tools and appropriate feed rate and speed.
Effect of Stibnite, Antimony, and Sulfur content on Mechanical Properties of
Semi Red Brass
[0103] Initially, the impact of sulfur upon the alloy's mechanical properties
can be
studied. Table 4 shows the effect of sulfur addition on mechanical properties
(UTS,
YS, and %Elongation) of four alloys from Figure 5. Two different target sulfur
contents were tested to compare the impact of sulfur. As can be seen, the
lower
sulfur content of alloys 84XX5-H8P4-9A-X and 84XX5-H9P1-9A-X exhibited lower
YS, but higher UTS and substantially higher %Elongation over the higher sulfur
content alloys 84XX51-H21P3-9A-X and 84XX52-H22P1-9A-X. High S levels make
the alloy drossy and less clean leading to inclusions and porosity in the
castings and
hence, lower UTS and %elongation. YS, however, remains unaffected.
Table 4 Effect of Sulfur Addition on Mechanical Properties
Alloy Number Sulfur, UTS, ksi YS, ksi "Yo Elongation
Wt%
84XX5-H8P4-9A-X 0.298 39.0 18.87 26.5
84XX5-H9P1-9A-X 0.289 40.45 18.48 31
84XX51-H21P3-9A-X 0.473 37.4 20.2 18
84XX52-H22P1-9A-X 0.487 34.0 19.98 14
[0104] The impact of various components in the alloys of the present
invention
was tested. Table 5 lists the seven alloys of the present invention from
Figure 6.
The mechanical properties of these alloys were tested. Specifically, UTS, YS,
and
%elongation. Table 5 also lists the weight percent for each of the sample
alloys of
stibnite, antimony, and sulfur. The UTS and YS exhibit a trend of improving
(increasing) with a maximum at alloy 84XX42-H20P2-7X, which has 1.0 %
stibnite,
(0.770 antimony, and 0.249 sulfur). %Elongation exhibits a trend of decreasing
as
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
27
the weight percent of each of stibnite, antimony, and sulfur increase, with
the
decrease between more pronounced at the higher percentages.
[0105]The back scattered electron images (Figures 15B-D and 15J; 16 B-D and
16H-
J; and 17 B-J) show that Sb is associated with Sn and Cu. It is possible that
a ternary
compound of Cu-Sn-Sb forms. These compounds together with Sb in solid solution
with Cu contribute to strength and ductility and also machinability. However,
excess
amount of Sb can have adverse effect on strength and ductility which has been
observed in this investigation, for example as in case of 1.64 Stibnite
addition (1.3%
Sb in the alloy)
Table 5 Effect of Stibnite Content, Antimony and Sulfur on Mechanical
Properties of Semi-Red Brass
Alloy Stibnite, Antimony, Sulfur, UTS, ksi YS, ksi %
Number Wt% Wt% Wt% Elongation
11093122A 0.4 0.269 0.071 40.2 16.1 43.5
1109332 1A 0.6 0.395 0.121 40.5 17.15 39.5
84XX41- 0.8 0.661 0.212 41.75 20.82 31
H19P1-7X
84XX42- 1.0 0.770 0.249 42.30 20.35 33
H20P2-7X
84XX4- 1.20 0.859 0.278 40.4 19.20 29.5
032212-7X
84XX9- 1.64 1.260 0.428 34.85 19.44 15.5
H17P1-7-X-
C
84XX9- 1.64 1.320 0.424 39.3 19.86 25.5
Hl 8P2-7-X-
C
Micrographical Analysis
[0106]Micrographical analysis was done on certain embodiments of C84030 red
brass as indicated in Figure 14. Sample 1109319 was from a button. The other
two
were from grip area of the tensile test bar.
Metal lograph ic Procedure
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
28
[0107]A portion of each sample was mounted, metallographically prepared and
then
examined optically using an inverted metallograph and a scanning electron
microscope equipped with energy dispersive spectroscopy (SEM/EDS) in
backscatter
electron (BE) mode for semi-quantitative chemical content and elemental
mapping.
BE mode achieves greater contrast between elements of differing atomic weight
percentages.
Results -Red Brass
[0108]The observed microstructures consist of dispersed particles throughout
the
copper-rich matrix. As polished metallograph photomicrographs were taken at
500X.
Image analysis was then performed to determine the particle size. The minimum,
maximum and average measurements are reported in the following table. As
polished photomicrographs are provided in Figures 15A, 16A, and 17A for each
of
the three tested sample alloys respectively.
[0109]A micrographical analysis of certain embodiments was undertaken to
characterize the alloy and provide information regarding the microstructure
and
positioning of various elements within the alloy's structure. Figure 14 lists
the
chemistries for the alloys whose micrographs are shown in Figures 15-17. It
appears
stibnite breaks down to Sb and S. S forms ZnS and Cu2S. Sb is in solid
solution with
the copper matrix and also appears to form a compound with Sn. Looking at the
Sb-
Sn phase diagram, there is an intermetallic compound 5n35b2 which forms over a
composition range of 43.6 to 61 % Sb.
[0110] Figures 15A, 16A, and 17A, are a photographs of Sample 1109337, Sample
84XX42-022812-H20P2-9A, and 84XX9-013112-H18P2-10A, respectively, showing
inclusions. Figures 15B and 15C are a SEM images of Sample 1109337. Figures
16B and 16C are a SEM images of Sample 84XX42-022812-H20P2-9A. Figures 17B
and 17C are a SEM images of Sample 84XX9-013112-H18P2-10A. The dark
materials illustrate sulfur distribution within the alloy. As can be seen, the
sulfur
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
29
distribution as copper sulfides and zinc sulfides are present in dendritic and
interdendritic areas.
[0111] Figure 15D illustrates elemental mapping of Sample 1109337 for sulfur,
manganese, iron, nickel, copper, zinc, tin, and antimony. Figure 15J
illustrates
elemental mapping of Sample 1109337 for sulfur, iron, nickel, copper, zinc,
tin, and
antimony. The distribution of elements is indicative of the stibnite breaking
down into
antimony and sulfur. In particular, the antimony distribution of 15D and the
sulfur
distribution of 15D indicate that the antimony is not isolated to the regions
with sulfur,
i.e. still present as a sulfide. As can be seen in Figure 25, at the
temperatures
involved in the alloy melt (2164 degrees Fahrenheit), the observed elemental
distribution is in agreement with the expected formation based upon the free
energies
of the involved reactions.
[0112] Figure 16D illustrates elemental mapping of Sample 84XX42-022812-H20P2-
9A for sulfur, manganese, iron, nickel, copper, zinc, tin, and antimony.
Figures 16J
illustrates elemental mapping of Sample 84XX42-022812-H20P2-9A for sulfur,
iron,
nickel, copper, zinc, tin, antimony, phosphorous, and lead. The distribution
of
elements is indicative of the stibnite breaking down into antimony and sulfur.
In
particular, the antimony distribution of 16F and the sulfur distribution of
16D indicate
that the antimony is not isolated to the regions with sulfur, i.e. still
present as a
sulfide. As can be seen in Figure 25, at the temperatures involved in the
alloy melt
(2164 degrees Fahrenheit), the observed elemental distribution is in agreement
with
the expected formation based upon the free energies of the involved reactions.
[0113] Figure 17D illustrates elemental mapping of Sample 84XX9-013112-H18P2-
10A for sulfur, manganese, iron, nickel, copper, zinc, tin, and antimony.
Figure 17J
illustrates elemental mapping of Sample 84XX9-013112-H18P2-10A for sulfur,
iron,
nickel, copper, zinc, tin, antimony, phosphorous, and lead. The distribution
of
elements is indicative of the stibnite breaking down into antimony and sulfur.
In
particular, the antimony distribution of 18F and the sulfur distribution of
18D indicate
that the antimony is not isolated to the regions with sulfur, i.e. still
present as a
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
sulfide. As can be seen in Figure 25, at the temperatures involved in the
alloy melt
(2164 degrees Fahrenheit), the observed elemental distribution is in agreement
with
the expected formation based upon the free energies of the involved reactions.
[0114] The micrograph information supports the improved mechanical
properties discussed above. Because some antimony remains in solid solution, a
good %elongation is observed. The intermetallic compound and the solid
solution
contribute to strength. However, if there is too much intermetallic compound,
strength
and % elongation could gradually decrease. A decrease in UTS and % elongation
is
observed at 1.64% Stibnite addition.
[0115]SEM /EDS element analysis reveals dispersed particles primarily
consisting of
sulfur, zinc, tin, or antimony. SEM backscatter images taken at 200X and 1000X
along with element maps at 1500X showing the requested element intensities are
provided in Figures 15E-J, 16E-J, and 17E-J. Table 6 sets froth the particle
size
information for the tested alloys. The average particle size increases with Sb
and S
contents, shown in Figure 14
TABLE 6 PARTICLE SIZES FOR THE THREE SAMPLES
Minimum Maximum
Average
Sample ID (pm) (pm) (pm)
1109319 0.1 16.6 1.2
84XX9-013112-H18P2-
0.1 22.8 1.8
10A
84XX42-022812-
0.2 18.1 3.8
H20 P2-9A
[0116]SEM EDS spectra results of the base material from sample 1109320 consist
of
significant amounts of copper with lesser amounts of tin, nickel, and zinc
(see
location 1, Figure 15F). The light colored phase reveals significant amounts
of
copper, tin, and antimony with lesser amounts of nickel zinc (see location 2
and 4,
Figure 15G and 151). The dark colored phase reveals significant amounts of
zinc and
CA 02872498 2014-11-03
WO 2013/166454 PCMJS2013/039567
31
sulfur with lesser amounts of nickel (see location 3, Figure 15H). Although
the EDS
spectra did not show any peaks for Sb, presence of Sb in the microstructure
(matrix
as well as some internnetallic compounds) is evident from the elemental maps
(see
Fig. 15D). Note, because Mn content of this sample is very low there was Mn
detected in the area tested for Figure 15D but not in Figure 15J and the EDS
spectra
do now show a peak for Mn. This indications a very non-uniform distribution of
Mn.
A semi-quantitative chemical analysis data is reported in Table 7 for the
sample
1109320 locations indicated in Figure 15E.
TABLE 7 SEMI-QUANTITATIVE CHEMICAL ANALYSIS OF SAMPLE 1109319
SpectrumS Ni Cu Zn Sn
Location 1 4.6 84.1 9.8 1.5
Location 2 2.1 65.6 4.6 27.6
Location 3 6.5 3.1 69.2 21.2
Location 4 2.3 65.9 4.1 27.8
[0117] SEM EDS spectra results of the base material from sample 84XX42-022812-
H20P2-9A consist of significant amounts of copper with lesser amounts of tin,
nickel,
and zinc (see location 1, Figure 16E). The dark colored phase reveals
significant
amounts of zinc and sulfur with lesser amounts of copper (see location 2,
Figure
16G). The light colored phase reveals significant amounts of copper, tin, and
antimony with lesser amounts of phosphorous, lead, iron, nickel, and zinc (see
location 3 and 4, Figures 16H, 161).
[0118] Semi-quantitative chemical analysis data is reported in table 8 for the
above
locations. Sb is present in the matrix in solid solution and also in the
intermetallic
compounds.
CA 02872498 2014-11-03
WO 2013/166454 PCMJS2013/039567
32
TABLE 8 SEMI-QUANTITATIVE CHEMICAL ANALYSIS OF SAMPLE 84XX42-
022812-H20P2-9A
õ....
Spectrum P S Fe Ni Cu Zn Sn Sb Pb
Location 1 --- --- --- 1.1 87.0 9.3 2.6 ---
Location 2 --- 29.2 --- --- 2.3 68.4 ---
Location 3 1.3 --- 0.3 12.2 54.5 1.4 10.7 13.4
6.2
Location 4 --- 0.1 4.5 80.3 5.4 6.0 2.4 1.2
[0119]SEM EDS spectra results of the base material from sample 84XX9-011312-
H18P2-10A consist of significant amounts of copper with lesser amounts of tin,
antimony, nickel and zinc (see location 1, Figure 17F). The light colored
phase
reveals significant amounts of copper, tin, and antimony with lesser amounts
of nickel
zinc (see location 2, Figure 17G). The dark colored phase reveals significant
amounts of zinc and sulfur with lesser amounts of iron and copper (see
location 3
and 4, Figures 17H, 171). Location 4 (Figure 17J) reveals lesser a mounts of
nickel.
[0120]Semi-quantitative chemical analysis data is reported In Table 9 for the
above
locations. Sb is present in the matrix in solid solution and also in the
intermetallic
compounds.
TABLE 9 SEMI-QUANTITATIVE CHEMICAL ANALYSIS OF SAMPLE 8000-
011312-H18P2-10A
Spectrum S K Fe Ni Cu Zn Sn Sb
Location 1 0.9 86.4 8.6 3.3 0.8
Location 2 4.4 57.7 1.5 16.2 20.1
Location 3 28.2 0.1 0.3 6.0 65.4
Location 4 17.7 --- 0.3 0.6 29.6 50.6 1.1
CA 02872498 2014-11-03
WO 2013/166454
PCT/US2013/039567
33
RESULTS - Yellow Brass
[0121] Metallography work was also done on embodiments of yellow brass C28330.
Chemistry of this alloy (28330-030613-P4H2A) is given in Figure 7. A section
from
each sample was hot mounted in conductive epoxy, metallographically prepared
to a
0.04 micron final polish, and examined a SEM in backscatter mode to identify
the
observed particles. Backscatter mode achieves greater contrast between
elements of
different weight percentages. Evaluation consisted of backscatter electron
(BE) beam
images taken at low and high magnification, SEM/EDS spectra with semi-
quantitative
chemical content, and elemental mapping.
[0122]SEM/EDS spectra analysis was taken at several locations including the
base
material and dispersed inclusions throughout the base material on all three
samples.
Figures 18A-I show images and spectra results for the Perm Mold sample.
Figures
19A-J show images and spectra results for the cold rolled and annealed sample.
Figures 20A-H show images and spectra results for the Cold Rolled sample. Semi-
quantitative data for each sample location are reported in the following table
(Table
10).
Table 10
Sample ID Location S Ti Mn Fe Cu Zn Zr Sn
1 64.5 35.6
2 56.0 42.3 1.7
Perm Mold
3 56.7 36.2 7.1
Figure
18B 4 27.9 2.0 4.4 65.8
61.2 38.8
6 8.1 0.1 1.5
54.0 34.0 1.2 1.2
Annealed 1 57.9 39.8 2.4
Figure 2 65.7 34.3
19B 3 93.5 4.2 2.3
4 61.5 36.6 1.9
CA 02872498 2014-11-03
WO 2013/166454
PCT/US2013/039567
34
25.6 3.5 10.8 60.1
6 59.6 36.5 3.9
7 4.4 0.1 2.4 59.8 32.6
0.7
1 57.8 39.9 2.4
Cold
2 63.3 36.1 0.6
Rolled
3 53.3 37.5 9.2
Figure
4 25.4 2.8 13.5 58.4
20B
5 4.4 0.6 55.7 36.9 2.5
[0123] Results are semi-quantitative, the spectra results are in weight
percent unless
otherwise indicated and the method used was SEM/EDS
[0124]The results indicate that grain size of permanent mold cast sample is
about 50
microns (Fig.18A). Grains and second phase particles get elongated during cold
rolling(Figs. 20A and 20B). Annealing at 1290 F has produced a recrystallized
microstructure. Grains are equiaxed. (Fig.19B) Average grain size is about 70
microns. This is relatively coarser than the as-cast grain size. It is
believed that for
alloys of C28330 annealed at 1100 and 1200 F. there would be finer than 70
microns
grains as evident from the elongation values. The large grain size of 1290 F
annealing reduces the % elongation. Antimony content of the two alloys was in
the
range 0.3 to 0.4%. Although the tested regions did not show any antimony peaks
it is
believed to be due to the low levels. Antimony is believed to be in solid
solution with
Cu.
Analysis of Additives for Red Brass
[0125] As discussed above, copper may utilize a number of elements to alloy
with.
The use of stibnite as disclosed herein was tested in comparison to two forms
of
carbon, CCG and CPC, sulfur, manganese sulfide, and combinations thereof as
indicated in Table 11.
Table 11 Analysis of mechanical properties and cost for red brass alloys
CA 02872498 2014-11-03
WO 2013/166454 PCMJS2013/039567
Additiv S C Sb Mn UTS YS % Machin Comments
es Elong -ability
CCG 0.002 - 43.3 18.1 57 37 1.5 CCG
CCG + .087- 0.002 - 0.046- 42.3 17.8 48 36 1.5CCG+
MnS .108 0.053 1.3 MnS
CCG + 0.417 0.006 1.35 - 38.2 19.6 23 48 1.5CCG + 1.64
Stibnite Stibnite.
Stibnite 0.428- - 1.32- - 38.3 19.7 23 39 1.64
Stibnite
0.424 1.26
Stibnite 0.249 - 0.77 - 42.3 20.35 33 1.0 Stibnite
Stibnite 0.212 - 0.64 - 41.7 20.82 31 0.8 Stibnite
5
Stibnite 0.071 0.269 40.5 16.25 45 0.4 Stibnite
Stibnite 0.121 0.395 40.0 17.08 36 60 0.6 Stibnite
Sulfur 0.298- - 39.7 18.7 29 48 0.4S
0.289
MnS 0.162- - 0.023- 42.1 18.1 45 49 1.3 MnS
0.123 0.033
CPC + 0.121- 0.005- 0.047- 41.4 17.9 46 51 1.5 CPC + 1.33
MnS 0.122 0.007 0.052 MnS
CPC 0.012- 0.004- - 43.5 18.1 60 52 1.5 CPC
0.009 0.002
CPC + 0.379- - 0.005 0.001 38.7 19.87 21 53 1.5 CPC+1.64
Stibnite 0.386 Stibnite
Phase Diagram
[0126] Phase information was gathered for red brass C84030 (Figures 21A-C, 22A-
E)
and yellow brass C28330 alloys (Figures 23 and 24A-F).
[0127]The base composition: 87 Cu, 9 Zn, 3 Sn, 1 Ni, 0.4 S for the red brass,
plus
the indicated amount of antimony. It is generally observed that Sb forms
stable
compounds with Cu (Cu2Sb), with Mn (MnSb and Mn2Sb) with Zn (ZnSb) and with S
(Sb2S3). Among these, it is believed that only Cu2Sb forms when Sb is added in
the
range of 0.4 to 1.3 wt%. The addition of Sb did not change the liquidus or the
solidus
temperatures. Figures 21A and 21B illustrate the phase diagrams of alloys
having
0% antimony, 0.8%, and 1.3% antimony respectively.
[0128] Figure 22A is a phase assemblage diagram of Semi-Red Brass with 0.8 Sb.
Less than 2 wt% Cu2Sb formed, as can be seen in the magnified Figure 22B.
CA 02872498 2014-11-03
WO 2013/166454
PCT/US2013/039567
36
Magnified part of the phase assemblage diagram of Semi-Red Brass with 0.8 Sb.
Figure 22C is a magnified part of a phase assemblage diagram of semi-red brass
C84030 with 1.3% antimony. When the Sb content is increased to 1.3, the amount
of
Cu2Sb increased to around 3 wt%. Similar amounts of Cu2Sb form during Scheil
cooling as well, as can e seen in Figures 19D (0.8% Sb)and 22E (1.3% Sb).
Scheil
cooling shows that one can expect the FCC solid solution phase (Cu containing
Zn,
Ni, some Sn and some Sb in solid solution), the beta (13') phase with Zn,
Cu3Sn
intermetallic compound, Cu2S and Cu2Sb. Melting point is not affected by the
addition of Sb and is about 1025 C which is close to equilibrium temp of 1030
C.
Solidus temp under Scheil cooling is 825 C.
[0129]Microstructural analysis shows that there are Zn, Sn and Ni in solid
solution
with Cu. In view of the microstructure and the phase analysis, it is believed
that
stibnite breaks down to Sb and S. Some Sb is in solid solution with Cu and
some
forms Cu2Sb compound. S combines with Zn and also Cu to form ZnS and Cu2S.
The high level of Sn and Cu in some phases indicates that it is Cu3Sn phase.
Based on the observed phases described above, a 100 kg overall alloy will
contain
the following amounts of each phase in kg.
Table 12 Equilibrium phases
Equilibrium Scheil Cooling
Compositio
FCC Cu2S Cu3S Zn FCC Cu2S Cu2 Cu3S y 13(BCC
i)
Semi-red 91 0 7.7 1.2
87.4 0 2.0 1.4 0. 8.5
brass 4
+ 0.8 wt% 89.5 1.6 7.7 1.2 85.7 1.6 2.0 1.5 0.
8.6
Sb 4
+ 1.3 wt% 88.4 2.5 7.7 1.2 84.8 2.7 1.8 1.5 0.
8.6
Sb 4
Table 13 Liquidus and solidus temperatures:
Equilibrium Scheil Cooling
Composition Liquidu Solidus Liquidus Solidus
Semi-red 1030 C 878 C 1026 C 825 C
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
37
brass
+ 0.8 wt% Sb 1029 C 875 C 1025 C
825 C
+ 1.3 wt% Sb 1028 C 874 C 1025 C
825 C
[0130] Figure 23 illustrates a phase diagram showing the location of the
yellow brass
alloy C28330 (61/38/0.3/0 Cu/Zn/Sn/Sb wt%). Figures 24A illustrates
Equilibrium
phase assemblage diagram of yellow brass with 0 wt% Sb Figure 24B illustrates
Equilibrium phase assemblage diagram of yellow brass with 0.6 wt% Sb. The
impact
of the antimony can be seen in Figure 24B and is further seen in Figure 24C is
an
equilibrium phase assemblage diagram of yellow brass with 1 wt% Sb. Figure 24D
is
a Scheil phase assemblage diagram of yellow brass with 0 wt% Sb. Figure 24E is
a
Scheil phase assemblage diagram of yellow brass with 0.6 wt% Sb. Figure 24F is
a
Scheil phase assemblage diagram of yellow brass with 1 wt% Sb. The Scheil
cooling
shows that expected phases the beta (P) Phase with Zn, some ZnS and Cu2Sb.
Observed melting point with Sb is about 900 C and solidus temp is 894 C.
A 100 kg overall alloy will contain the following amounts of each phase in kg.
Table 14 Relative amount of the phases present at room temperature:
Equilibrium Scheil Cooling
Compositio
FCC Cu2S Zn fr(BCC FC Cu2S Zn 13(BCC fr(BCC
b S 2) C b S 1) 2)
Yellow 51.9 0 0.9 47.2 4.9 0 0.9 94.2 0
brass
+ 0.6 wt% 47.6 1.2 0.9 50.3 0 1.2 0.9 97.9 0
Sb
+ 1 wt% Sb 44.7 2.0 0.9 52.3 0 2 0.9
97.1 0
Table 15 Relative Liquidus and solidus temperatures:
Compositio Equilibrium Scheil Cooling
Liquidus Solidus Liquidus Solidus
Yellow 904 C 897 C 904 C 877 C
brass
+ 0.6 wt% 901 C 896 C 902 C 877 C
Sb
+ 1 wt% Sb 905 C 894 C 901 C 877 C
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
38
Liquidus and Solidus Temperatures
[0131]Thermal investigation of the systems was performed using a DSC-2400
Setaram Setsys Differential Scanning Calorimetry. Temperature calibration of
the
DSC was done using 7 pure metals: In, Sn, Pb, Zn, Al, Ag, and Au spanning the
temperature range from 156 to 1065 C. The samples were cut and mechanically
polished to remove any possible contaminated surface layers. Afterwards, they
were
cleaned with ethanol and placed in a graphite crucible with a lid cover to
limit possible
evaporation and protect the apparatus. To avoid oxidation, the analysis
chamber
was evacuated to 10-2 mbar and then flooded with argon. The DSC measurements
were carried out under flowing argon atmosphere. Three replicas of each sample
were tested. The weight of the sample was 62-78 mg.
[0132]Two samples, one from the semi-red brass, C84030 and the other from the
yellow brass, C28330 were used to measure the liquidus and solidus
temperatures.
Their compositions are given in Table 16
Table 16 Samples for Liquidus and Solidus Study
Alloy Cu Ni Zn Mn S Sb Sn Fe Al P Pb Si C
84XX42-
86.41 .762 8.68 .0008 .249 0.77 2.92 .159 .001 .014 .02 .002 .002
022812-
H20P2-7X
28330-
61.50 .019 36.76 .012 .009 .324 1.22 .092 .002 .001 .007 .001 .002
030613-
P4H2a-2*
*0.013% B, 0.020% Zr
[0133]To find out the solidus and liquidus temperature the samples were heated
from
room temperature up to 1100 C, then cooled to 800 C, then heated to 1100 C and
cooled to 800 C again. Finally the apparatus was brought down to room
temperature.
These experiments were conducted under an Argon atmosphere which was
preceded by vacuum pump evacuation of the DSC chamber. Thus data from two
cycles were collected. The heating was done at 10 C/rnin and the cooling at 15
C/min, as agreed. The solidus and the liquidus temperatures, obtained from
both
cycles are provided in the table below. Data from the first cycle is more
representative of the alloys because of the Zn loss that occurs in the second
cycle
CA 02872498 2014-11-03
WO 2013/166454
PCT/US2013/039567
39
which was 7.3% for C84030 ( low-Zn alloy) and 35.4% for the C28330 (high Zn)
alloy.
The measured values are shown in Table 17.
Table 17 Solidus and Liquidus Temperatures
1st Cycle 2nd Cycle
Sample No.
Solidus T, Liquidus T, Solidus T, Liquidus T,
84XX42-022812-H20P2-
872 1034 903 1041
7X
28330-030613-P4H2a-2 849 1050 980 1055
Dezincification Study
[0134]Four brass samples, two commercial brasses and embodiments of the
C28330 yellow brass and C84030 red brass, were evaluated for the resistance to
dezincification corrosion in accordance with ISO 6590, "Corrosion of Metals
and
Alloys ¨ Determination of the Dezincification Resistance of Brass." In this
test, ground
cross sections are immersed in a 1% copper chloride solution at 75 5 C for 24
hours. At the end of this immersion period, polished cross sections are
prepared
perpendicular to the exposed surfaces, and the depth of any dezincification
corrosion
is measured. This analysis was performed in both a thin area and a thick area
of the
casting per the ISO specification. Where possible the sections were prepared
from
thick and thin walled sections. The samples that exhibited a uniform cross
section
samples were taken from the edge and the core. More than 100 microns of
dezincification penetration was considered to exceed the allowable
dezincification.
Table 18 Alloys used for Dezincification Work
Cu Ni Zn Mn S Sb Sn Fe Al P Pb Si C
84030- 83.62 1.07 11.06 .003 .314 0.854 2.81 .210 .001 .028 .018 .004
062712-
H3P2-7-X
28330- 61.50 .019 36.76 .012 .009 .324 1.22 .092 .002 .001 .007 .001 .002
030613-
P4H2a-2*
MBAF 180, 63 5.0 17.0 12.0 - - 1.0 - 1.0 -
White Metal**
C36000 61.5 35.5 3.0
*0.013% B, 0.020% Zr
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
** Nominal compositions, with 1.0% Bi
*** Nominal compositions
[0135]Figure 26A shows dezincification corrosion (between lines) extends to a
maximum depth of 0.0012" (31.2 microns) from the exposed surface (towards top)
in
the metallographic section prepared through the edge of the "MBAF 180" sample
Unetched. (494X). Figure 26B shows dezincification corrosion (between lines)
extends to a maximum depth of 0.0113" (287.0 microns) from the exposed surface
(towards top) in the metallographic section prepared through the core of the
"MBAF
180" sample. Unetched. (201X).
[0136]Figure 27A shows dezincification corrosion (between lines) extends to a
maximum depth of 0.04830" (1,228.1 microns) from the exposed surface (towards
top) in the metallographic section prepared through the thin walled section of
the
"C36000 Ht# 1-Yeager" sample. Unetched. (50X). Figure 27B shows
dezincification
corrosion (between red lines) extends to a maximum depth of 0.05133" (1,303.8
microns) from the exposed surface (towards top) in the metallographic section
prepared through the thick walled section of the "C36000 Ht# 1-Yeager" sample.
Unetched. (50X).
[0137]Figure 28A shows no dezincification corrosion is present at the exposed
surface (towards top) in the metallographic section prepared through the edge
of the
"28330-Lab# 358050 P4 H2a" sample of a C28330 yellow brass alloy Unetched.
(494X)). Figure 28B shows dezincification corrosion (between lines) extends to
a
maximum depth of 0.0033" (82.8 microns) from the exposed surface (towards top)
in
the metallographic section prepared through the core of the "28330-Lab# 358050
P4
H2a" sample. Unetched. (494X).
[0138]Figure 29A shows no dezincification corrosion is present at the exposed
surface (towards top) in the metallographic section prepared through the edge
of the
"84030-62412-H3P2-9" sample. Unetched. (494X). Figure
29B shows no
dezincification corrosion is present at the exposed surface (towards top) in
the
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
41
metallographic section prepared through the edge of the "84030-62412-H3P2-9"
sample. Unetched. (494X).
[0139]This dezincification results show that embodiments of yellow brass
C28330 of
the present invention provide for superior resistance to dezincification in
comparison
with commercial alloys, including C36000 yellow brass. Specifically, the
results
indicate that the section from the core of the sample identified as "MBAF 180"
and
both the thick and thin walled sections from the sample identified as "C36000
Ht# 1-
Yeager" exhibit significant dezincification corrosion when tested in
accordance with
ISO 6509, "Corrosion of Metals and Alloys ¨ Determination of the
Dezincification
Resistance of Brass." ISO 6509 does not include any acceptance criteria,
however,
the dezincification depth of these samples exceed the 100 micron maximum
dezincification depth included in the similar Australian Standard AS 2345,
"Dezincification Resistance of Copper Alloys." These results indicate these
samples
are susceptible to dezincification corrosion. This investigation indicates
that the
section from the edge of the sample identified as "MBAF 180" and the sections
from
both the core and the edge of the samples identified as "28330-Lab# 358050 P4
H2a" and "84030-62412-H3P2-9" exhibit dezincification corrosion which is in
conformance with the 100 micron maximum dezincification depth when tested in
accordance with ISO 6509.
Parameters of Properties Experiments.
[0140]A series of alloys were created and tested to determine the properties
of alloys
having a composition outside of that set forth for the C84030 red brass alloy
in Figure
2. Figure 30 illustrates the chemical compositions of these variances on
C84030 with
the component in variance shown in italics. The compositions were selected by
software to be outside of the nominal C84030 composition such as Cu, Sn, Zn,
Ni,
and Sb except a few cases in case of sulfur.
[0141] In order to provide a "figure of merit" to quantify the properties of
the variance
alloys in comparison to C84030, UTS, YS and EL % properties were utilized.
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
42
Sufficient results for each of those three property values had to occur
together for
one of the pours to create further investigation. These three property values
are:
1) The Ultimate Tensile Strength has to be greater than the maximum limit of C
84030 ( > than 42.9)
2) The Yield Strength has to be greater than the maximum limit of C 84030 ( >
than 20.3)
3) The Elongation % has to be greater than the typical limit of C 84400 ( >
than
26)
[0142] Table 19 below provides a summary of the results of the variance
testing. The
design of experiment (DOE) was conceptually structured based on a statistical
Taguchi method. The defining elements to the alloy were brought both above and
below their defined limits. Table 19 below shows this logic always with the
end
result being 100%. The end goal being to see if better properties existed by
going
both above and below the defined limits to the nominal range for C84030.
Figure 31
illustrates the relation of alloy properties between C84030 red brass and two
commercial brasses which were used as the base for the DOE. Figure 32 includes
composition information for several tested variance alloys along with the
mechanical
properties. None of the variance alloys provided superior results for the
three
properties. The mechanical properties of the eight alloys show that although
there
are some alloys outside the range for C84030 that can meet some of the minimum
and typical mechanical properties shown in Figure 9 for C84030, there is no
single
alloy that can exceed the requirnnents set out above for UTS, YS and %
elongation.
CA 02872498 2014-11-03
WO 2013/166454 PCT/US2013/039567
43
Table 19: Design of Experiment factor formulations based on Taguchi method
L 8 (27) = 2 Levels with 7 Factors Total of Factors The Total
of
1 thru 6 the 7 Factors
/ (C) 2 (5) 3 (5b) 4 (-Ni) 5 WV 6 (in) 7 (Cu)
1 1 1 1 1 1 1
4.90% 100.00%
0.05% 0.05% 0.05% 0.25% 1.00% 3.50% 95.10%
2 1 1 1 2 2 2
25.15% 100.00%
0.05% 0.05% 0.05% 3.00% 6.00% 16.00% 74.85%
3 1 2 2 1 1 2
20.15% 100.00%
0.05% 0.85% 2.00% 0.25% 1.00% 16.00% 79.85%
4 1 2 2 2 2 1
15.40% 100.00%
0.05% 0.85% 2.00% 3.00% 6.00% 3.50% 84.60%
2 1 2 1 2 1
13.50% 100.00%
1.70% 0.05% 2.00% 0.25% 6.00% 3.50% 86.50%
6 2 1 2 2 1 2
23.75% 100.00%
1.70% 0.05% 2.00% 3.00% 1.00% 16.00% 76.25%
7 2 2 1 1 2 2
23.15% 100.00%
1.70% 0.85% 0.05% 0.25% 6.00% 16.00% 76.85%
8 2 2 1 2 1 1
8.40% 100.00%
1.70% 0.85% 0.05% 3.00% 1.00% 3.50% 91.60%
[0143]The foregoing description of illustrative embodiments has been presented
for
purposes of illustration and of description. It is not intended to be
exhaustive or
limiting with respect to the precise form disclosed, and modifications and
variations
are possible in light of the above teachings or may be acquired from practice
of the
disclosed embodiments. It is intended that the scope of the invention be
defined by
the claims appended hereto and their equivalents.