Note: Descriptions are shown in the official language in which they were submitted.
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
DIFFERENTIATION OF HUMAN EMBRYONIC STEM CELLS INTO
PANCREATIC ENDODERM
CROSS REFERENCE TO RELATED APPLICATION
[0001] The present application claims the benefit of U.S. Provisional Patent
Application Serial
No. 61/643,684, filed May 7, 2012, which is incorporated herein by reference
in its entirety for
all purpose.
FIELD OF THE INVENTION
[0002] The present invention is in the field of cell differentiation. More
specifically, the
invention provides ranges of seeding densities of human pluripotent cells
and/or cells expressing
markers indicative of definitive endoderm useful for subsequent efficient
generation of cells
expressing markers indicative of pancreatic endoderm and cells expressing
markers indicative of
pancreatic endocrine.
BACKGROUND
[0003] Advances in cell-replacement therapy for Type I diabetes mellitus and a
shortage of
transplantable islets of Langerhans have focused interest on developing
sources of insulin-
producing cells, or [3. cells, appropriate for engraftment. One approach is
the generation of
functional [3. cells from pluripotent stem cells, such as, for example,
embryonic stem cells.
[0004] In vertebrate embryonic development, a pluripotent cell gives rise to a
group of cells
comprising three germ layers (ectoderm, mesoderm, and endoderm) in a process
known as
gastrulation. Tissues such as, thyroid, thymus, pancreas, gut, and liver, will
develop from the
endoderm, via an intermediate stage. The intermediate stage in this process is
the formation of
definitive endoderm. Definitive endoderm cells express a number of markers,
such as,
HNF3beta, GATA4, MIXL1, CXCR4 and 50X17.
[0005] By the end of gastrulation, the endoderm is partitioned into anterior-
posterior domains
that can be recognized by the expression of a panel of factors that uniquely
mark anterior, mid,
1
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
and posterior regions of the endoderm. For example, Hhex, and Sox2 identify
the anterior region
while Cdxl, 2, and 4 identify the posterior half of the endoderm.
[0006] Migration of endoderm tissue brings the endoderm into close proximity
with different
mesodermal tissues that help in regionalization of the gut tube. This is
accomplished by a
plethora of secreted factors, such as FGFs, Wnts, TGF-Bs, retinoic acid (RA),
and BMP ligands
and their antagonists. For example, FGF4 and BMP promote Cdx2 expression in
the presumptive
hindgut endoderm and repress expression of the anterior genes Hhex and SOX2
(2000
Development, 127:1563-1567). WNT signaling has also been shown to work in
parallel to FGF
signaling to promote hindgut development and inhibit foregut fate (2007
Development,
134:2207-2217). Lastly, secreted retinoic acid by mesenchyme regulates the
foregut-hindgut
boundary (2002 Curr Biol, 12:1215-1220).
[0007] The level of expression of specific transcription factors may be used
to designate the
identity of a tissue. During transformation of the definitive endoderm into a
primitive gut tube,
the gut tube becomes regionalized into broad domains that can be observed at
the molecular level
by restricted gene expression patterns. For example, the regionalized pancreas
domain in the gut
tube shows a very high expression of PDX-1 and very low expression of CDX2 and
SOX2.
Similarly, the presence of high levels of Foxel are indicative of esophagus
tissue; highly
expressed in the lung tissue is NKX2.1; 50X2/0ddl (OSR1) are highly expressed
in stomach
tissue; expression of PROX1/Hhex/AFP is high in liver tissue; 50X17 is highly
expressed in
biliary structure tissues; PDX1, NKX6.1/PTfla, and NKX2.2 are highly expressed
in pancreatic
tissue; and expression of CDX2 is high in intestine tissue. The summary above
is adapted from
Dev Dyn 2009, 238:29-42 and Annu Rev Cell Dev Biol 2009, 25:221-251.
[0008] Formation of the pancreas arises from the differentiation of definitive
endoderm into
pancreatic endoderm (2009 Annu Rev Cell Dev Biol, 25:221-251; 2009 Dev Dyn,
238:2942).
Dorsal and ventral pancreatic domains arise from the foregut epithelium.
Foregut also gives rise
to the esophagus, trachea, lungs, thyroid, stomach, liver, pancreas, and bile
duct system.
[0009] Cells of the pancreatic endoderm express the pancreatic-duodenal
homeobox gene
PDX1. In the absence of PDX1, the pancreas fails to develop beyond the
formation of ventral
and dorsal buds. Thus, PDX1 expression marks a critical step in pancreatic
organogenesis. The
2
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
mature pancreas contains, among other cell types, exocrine tissue and
endocrine tissue. Exocrine
and endocrine tissues arise from the differentiation of pancreatic endoderm.
[00010] D'Amouret al. describes the production of enriched cultures of human
embryonic stem
(ES) cell-derived definitive endoderm in the presence of a high concentration
of activin and low
serum (Nature Biotechnol 2005, 23:1534-1541; U.S. Patent No. 7,704,738).
Transplanting these
cells under the kidney capsule of mice resulted in differentiation into more
mature cells with
characteristics of endodermal tissue (U.S. Patent No. 7,704,738). Human
embryonic stem
cell-derived definitive endoderm cells can be further differentiated into PDX1
positive cells after
addition of FGF-10 and retinoic acid (U.S. Patent Publication No.
2005/0266554A1).
Subsequent transplantation of these pancreatic precursor cells in the fat pad
of immune deficient
mice resulted in formation of functional pancreatic endocrine cells following
a 3-4 month
maturation phase (U.S. Patent No. 7,993,920 and U.S. Patent No. 7,534,608).
[0010] Fisk et al. report a system for producing pancreatic islet cells from
human embryonic
stem cells (U.S. Patent No. 7,033,831). In this case, the differentiation
pathway was divided into
three stages. Human embryonic stem cells were first differentiated to endoderm
using a
combination of sodium butyrate and activin A (U.S. Patent No. 7,326,572). The
cells were then
cultured with BMP antagonists, such as Noggin, in combination with EGF or
betacellulin to
generate PDX1 positive cells. The terminal differentiation was induced by
nicotinamide.
[0011] Small molecule inhibitors have also been used for induction of
pancreatic endocrine
precursor cells. For example, small molecule inhibitors of TGF-B receptor and
BMP receptors
(Development 2011, 138:861-871; Diabetes 2011, 60:239-247) have been used to
significantly
enhance number of pancreatic endocrine cells. In addition, small molecule
activators have also
been used to generate definitive endoderm cells or pancreatic precursor cells
(Curr Opin Cell
Biol 2009, 21:727-732; Nature Chem Biol 2009, 5:258-265).
[0012] Although great strides have been made in improving protocols to
generate pancreatic
cells from human pluripotent stem cells, there exists a great degree of
variability in results
reported by different groups in their efficiency of generating pancreatic
cells from ES cells. This
variability has been attributed to factors, such as differences in ES lines,
duration of the protocol
including the reagents used, and choice of adherent versus suspension
cultures. We demonstrate
3
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
here that whereas the efficiency in directing differentiation of ES cells into
definitive endoderm
is not very sensitive to the cell density, the efficiency to generate
pancreatic endoderm is highly
dependent on the initial seeding density of ES cells. In particular, initial
cell densities in the
range of 0.8-2 X 105 cells/cm2 resulted in highest expression of pancreatic
endoderm and
endocrine markers.
SUMMARY
[0013] In an embodiment, the present invention concerns a method of culturing
pluripotent
stem cells comprising seeding the pluripotent stem cells on a surface, wherein
the pluripotent
stem cells are seeded at a density of from about 0.8 X 105 cells/cm2 to about
3.0 X 105 cells/cm2.
In some embodiments, the pluripotent stem cells cultured are embryonic stem
cells. In some
embodiments, the pluripotent stem cells cultured are human embryonic stem
cells. In some
embodiments, the surface where the pluripotent stem cells are seeded comprises
MatrigelTM.
[0014] In an embodiment, the present invention relates to a method of
differentiating
pluripotent stem cells comprising seeding the pluripotent stem cells, at a
density of from about
0.8 x 105 cells/cm2 to about 3.0 x 105 cells/cm2, on a surface, and
differentiating the pluripotent
stem cells into cells expressing markers indicative of definitive endoderm. In
some
embodiments, the pluripotent stem cells differentiated are embryonic stem
cells. In some
embodiments, the pluripotent stem cells differentiated are human embryonic
stem cells. In some
embodiments, the surface where the pluripotent stem cells are seeded comprises
MatrigelTM. In
some embodiments, the cells expressing markers indicative of definitive
endoderm are human.
[0015] In an embodiment, the invention relates to a method of obtaining cells
expressing
markers indicative of definitive endoderm comprising differentiating
pluripotent stem cells
seeded on a surface at a seeding density of from about 0.8 x 105 cells/cm2 to
about 3.0 x 105
cells/cm2. In some embodiments, the pluripotent stem cells used in the method
of obtaining cells
expressing markers indicative of definitive endoderm are embryonic stem cells.
In some
embodiments, the embryonic stem cells used in the method of obtaining cells
expressing markers
characteristic of definitive endoderm are human embryonic stem cells. In some
embodiments,
the pluripotent stem cells seeded on a surface which comprises MatrigelTM. In
some
embodiments, the cells expressing markers indicative of definitive endoderm
are human.
4
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
[0016] In an embodiment, the present invention provides a method of
differentiating cells
expressing markers indicative of definitive endoderm comprising
differentiating pluripotent stem
cells that have been seeded on a first surface at a seeding density sufficient
to maximize
differentiation efficiency of the pluripotent stem cells into cells expressing
markers indicative of
definitive endoderm, and differentiating the cells expressing markers
indicative of definitive
endoderm into cells expressing markers indicative of pancreatic endoderm by
seeding the cells
expressing markers indicative of definitive endoderm on a second surface at a
seeding density
sufficient to maximize differentiation efficiency of the cells expressing
markers indicative of
definitive endoderm into cells expressing markers characteristic of pancreatic
endoderm. In
some aspects of the invention, the seeding density sufficient to maximize
differentiation
efficiency of the pluripotent stem cells into cells expressing markers
indicative of definitive
endoderm is from about 0.8 x 105 cells/cm2 to about 3.0 x 105 cells/cm2. In
some aspects of the
invention, the seeding density sufficient to maximize differentiation
efficiency of the cells
expressing markers indicative of definitive endoderm into cells expressing
markers characteristic
of pancreatic endoderm is from about 1.5 x 105 cells/cm2 to about 5.0 x 105
cells/cm2. In some
embodiments, the pluripotent stem cells used in the method of differentiating
cells are embryonic
stem cells. In some embodiments of the invention, the embryonic stem cells
used in the method
of differentiating cells are human embryonic stem cells. In some embodiments
of the invention,
the first surface where the pluripotent stem cells are seeded comprises
MatrigelTM. In some
embodiments of the invention, the second surface, where the cells expressing
markers indicative
of definitive endoderm are seeded comprises MatrigelTM. In some embodiments of
the invention,
the cells expressing markers indicative of definitive endoderm are human. In
some embodiments
of the invention, the cells expressing markers indicative of pancreatic
endoderm are human.
[0017] In an embodiment, the invention relates to a method of differentiating
cells expressing
markers indicative of definitive endoderm into cells expressing markers
indicative of pancreatic
endocrine comprising differentiating pluripotent stem cells that have been
seeded on a surface at
a seeding density of from about 0.8 X 105 cells/cm2 to about 3.0 X 105
cells/cm2 into cells
expressing markers indicative of definitive endoderm, and differentiating the
cells expressing
markers of definitive endoderm into cells expressing markers indicative of
pancreatic endocrine.
In some aspects of the invention, the pluripotent stem cells used to
differentiate into cells
expressing markers indicative of definitive endoderm are embryonic stem cells.
In some
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
embodiments, the embryonic stem cells used to differentiate into cells
expressing markers
indicative of definitive endoderm are human embryonic stem cells. In some
embodiments, the
pluripotent stem cells used to differentiate into cells expressing markers
indicative of definitive
endoderm are seeded on a surface which comprises MatrigelTM.
[0018] In an embodiment, the invention relates to a method of obtaining cells
expressing
markers indicative of pancreatic endoderm comprising seeding pluripotent stem
cells on a
surface, differentiating the pluripotent stem cells into cells expressing
markers indicative of
definitive endoderm, seeding the cells expressing markers indicative of
definitive endoderm on a
surface, and differentiating the cells expressing markers indicative of
definitive endoderm into
cells expressing markers indicative of pancreatic endoderm. In some aspects of
the invention,
the pluripotent stem cells used in the method of obtaining cells expressing
markers indicative of
pancreatic endoderm are seeded on the surface at a density of from about 0.8 x
105 cells/cm2 to
about 3.0 x 105 cells/cm2. In some aspects of the invention, the cells
expressing markers
indicative of definitive endoderm are seeded on a surface at a density of from
about 1.5 x 105
cells/cm2 to about 5.0 x 105 cells/cm2. In some aspects of the invention, the
pluripotent stem
cells differentiated into cells expressing markers indicative of definitive
endoderm are embryonic
stem cells. In some aspects of the invention, the embryonic stem cells
differentiated into cells
expressing markers indicative of definitive endoderm are human embryonic stem
cells. In some
aspects of the invention the pluripotent stem cells are seeded on a surface
comprising MatrigelTM.
In some aspects of the invention, the cells expressing markers indicative of
definitive endoderm
are seeded on a surface comprising MatrigelTM.
[0019] In one embodiment, the invention relates to a method of obtaining cells
expressing
markers indicative of pancreatic endocrine comprising seeding pluripotent stem
cells on a
surface; differentiating the pluripotent stem cells into cells expressing
markers indicative of the
definitive endoderm; and differentiating the cells expressing markers
indicative of definitive
endoderm into cells expressing markers indicative of pancreatic endocrine. In
some aspects of
the invention, the pluripotent stem cells used in the method of obtaining
cells expressing markers
indicative of pancreatic endoderm are seeded on the surface at a density of
from about 0.8 x 105
cells/cm2 to about 3.0 x 105 cells/cm2. In some aspects of the invention, the
cells expressing
markers indicative of definitive endoderm are seeded on a surface at a density
of from about 1.5
6
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
x 105 cells/cm2 to about 5.0 x 105 cells/cm2. In some aspects of the
invention, the pluripotent
stem cells differentiated into cells expressing markers indicative of
definitive endoderm are
embryonic stem cells. In some aspects of the invention, the embryonic stem
cells differentiated
into cells expressing markers indicative of definitive endoderm are human
embryonic stem cells.
In some aspects of the invention the pluripotent stem cells are seeded on a
surface comprising
MatrigelTM. In some aspects of the invention, the cells expressing markers
indicative of
definitive endoderm are seeded on a surface comprising MatrigelTM.
[0020] In an embodiment, the invention relates to a method of differentiating
cells expressing
markers indicative of definitive endoderm comprising seeding cells expressing
markers
indicative of definitive endoderm on a surface at a seeding density of from
about 1.5 x 105
cells/cm2 to about 5.0 x 105 cells/cm2, and differentiating the cells
expressing markers indicative
of definitive endoderm into cells expressing markers indicative of pancreatic
endoderm. In some
aspects of the invention, the cells used are human.
[0021] The present invention provides a population of cells expressing markers
indicative of
the pancreatic endoderm lineage obtained in vitro by the stepwise
differentiation of 0.8 x 105
pluripotent cells/cm2 to 3 x 105 pluripotent cells/cm2.
[0022] In an embodiment of the present invention, cells expressing markers
indicative of
pancreatic endocrine lineage are obtained in vitro by the stepwise
differentiation of 0.8 x 105
pluripotent cells/cm2 to 3 x 105 pluripotent cells/cm2.
[0023] In an embodiment of the present invention, cells expressing markers
indicative of
pancreatic endoderm lineage are obtained in vitro by the stepwise
differentiation of cells
expressing markers indicative of the definitive endoderm seeded on a surface
at a density of 1.5
x 105 cells/cm2 to 5 x 105 cells/cm2.
[0024] In an embodiment of the present invention, cells expressing markers
indicative of
pancreatic endocrine lineage are obtained in vitro by the stepwise
differentiation of cells
expressing markers indicative of definitive endoderm seeded on a surface at
1.5 x 105 to 5 X 105
cells/cm2.
7
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
BRIEF DESCRIPTION OF THE DRAWINGS
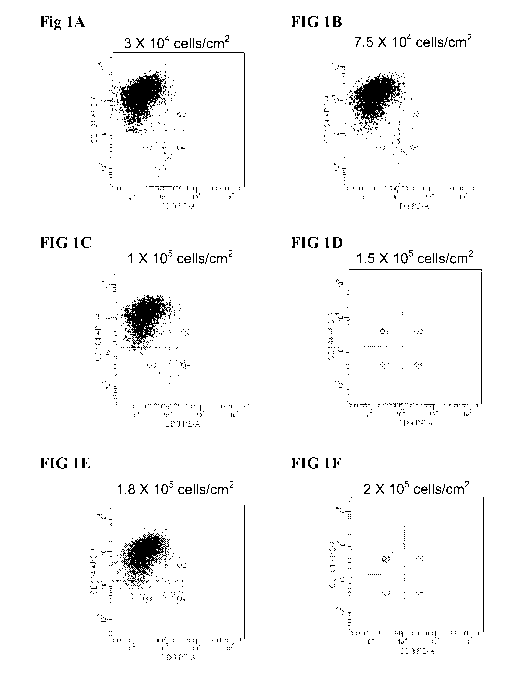
[0025] Figure lA to Figure 1F shows FACS histogram expression profiles of
CXCR4 (Y-axis,
marker of DE) and CD-9 (X-axis, marker of undifferentiated ES cells) for H1
cells seeded at 0.3
X 105 cells/cm2 (FIG 1A), 0.75 X 105 cells/cm2 (FIG 1B), 1 X 105 cells/cm2
(FIG 1C), 1.5 X 105
cells/cm2 (FIG 1D), 1.8 X 105 cells/cm2 (FIG 1E), and 2 X 105 cells/cm2 (FIG
1F).
[0026] Figure 2A to Figure 2G show data from real-time PCR analyses of the
expression of the
following genes in cells of the human embryonic stem cell line H1 seeded at
various densities
and subsequently differentiated to DE as outlined in Example 1: 50X7 (FIG 2A),
NANOG (FIG
2B), OCT4 (FIG 2C), AFP (FIG 2D), SOX17 (FIG 2E), FOXA2 (FIG 2F), and CXCR4
(FIG
2G).
[0027] Figure 3A-3H show phase contrast images of cultures prior to induction
of DE that were
seeded at various cell densities: 0.3 X 105 cells/cm2 (FIG 3A), 0.5 X 105
cells/cm2 (FIG 3B), 0.75
X 105 cells/cm2 (FIG 3C), 0.9 X 105 cells/cm2 (FIG 3D), 1 X 105 cells/cm2 (FIG
3E), 1.1 X 105
cells/cm2 (FIG 3F), 1.2 X 105 cells/cm2 (FIG 3G) and 1.5 X 105 cells/cm2 (FIG
3H).
[0028] Figure 4A-4G show phase contrast images of DE day 4 cultures that were
initially
seeded at various cell densities of ES cells: 0.3 X 105 cells/cm2 (FIG 4A),
0.5 X 105 cells/cm2
(FIG 4B), 0.75 X 105 cells/cm2 (FIG 4C), 1 X 105 cells/cm2 (FIG 4D), 1.1 X 105
cells/cm2 (FIG
4E), 1.2 X 105 cells/cm2 (FIG 4F) and 1.5 X 105 cells/cm2 (FIG 4G).
[0029] Figure 5A-5F show phase contrast images of stage 5 cultures that were
initially seeded
at various cell densities of ES cells: 5 X 104 cells/cm2 (FIG 5A), 7.5 X 104
cells/cm2 (FIG 5B), 1
X 105 cells/cm2 (FIG 5C), 1.5 X 105 cells/cm2 (FIG 5D), 1.8 X 105 cells/cm2
(FIG 5E) and 2.0 X
105 cells/cm2 (FIG 5F).
[0030] Figure 6A to Figure 6J depict data from real-time PCR analyses of the
expression of the
following genes in cells of the human embryonic stem cell line H1 seeded at
various densities
and subsequently differentiated to stage 5 as outlined in Example 2: ZIC1 (FIG
6A), CDX2 (FIG
6B), PDX-1 (FIG 6C), NKX6.1 (FIG 6D), NKX2.2 (FIG 6E), NGN3 (FIG 6F), NEUROD
(FIG
6G), insulin (FIG 6H) HNF4a (FIG 61), and PTFla (FIG 6J).
8
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
DETAILED DESCRIPTION
[0031] For clarity of disclosure, and not by way of limitation, the detailed
description of the
invention is divided into the following subsections that describe or
illustrate certain features,
embodiments or applications of the present invention.
Definitions
[0032] Stem cells are undifferentiated cells defined by their ability, at the
single cell level, to
both self-renew and differentiate. Stem cells may produce progeny cells,
including self-
renewing progenitors, non-renewing progenitors, and terminally differentiated
cells. Stem cells
are also characterized by their ability to differentiate in vitro into
functional cells of various cell
lineages from multiple germ layers (endoderm, mesoderm and ectoderm). Stem
cells also give
rise to tissues of multiple germ layers following transplantation and
contribute substantially to
most, if not all, tissues following injection into blastocysts.
[0033] Stem cells are classified by their developmental potential as: (1)
totipotent, meaning
able to give rise to all embryonic and extraembryonic cell types; (2)
pluripotent, meaning able to
give rise to all embryonic cell types; (3) multipotent, meaning able to give
rise to a subset of cell
lineages but all within a particular tissue, organ, or physiological system
(for example,
hematopoietic stem cells (HSC) can produce progeny that include HSC (self-
renewal), blood
cell restricted oligopotent progenitors, and all cell types and elements
(e.g., platelets) that are
normal components of the blood); (4) oligopotent, meaning able to give rise to
a more restricted
subset of cell lineages than multipotent stem cells; and (5) unipotent,
meaning able to give rise to
a single cell lineage (e.g., spermatogenic stem cells).
[0034] Differentiation is the process by which an unspecialized
("uncommitted") or less
specialized cell acquires the features of a specialized cell such as, for
example, a nerve cell or a
muscle cell. A differentiated cell or a differentiation-induced cell is one
that has taken on a more
specialized ("committed") position within the lineage of a cell. The term
"committed", when
applied to the process of differentiation, refers to a cell that has proceeded
in the differentiation
pathway to a point where, under normal circumstances, it will continue to
differentiate into a
9
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
specific cell type or subset of cell types, and cannot, under normal
circumstances, differentiate
into a different cell type or revert to a less differentiated cell type. "De-
differentiation" refers to
the process by which a cell reverts to a less specialized (or committed)
position within the
lineage of a cell. As used herein, the lineage of a cell defines the heredity
of the cell, i.e., which
cells it came from and what cells it can give rise to. The lineage of a cell
places the cell within a
hereditary scheme of development and differentiation. A lineage-specific
marker refers to a
characteristic specifically associated with the phenotype of cells of a
lineage of interest and can
be used to assess the differentiation of an uncommitted cell to the lineage of
interest.
[0035] "Markers", as used herein, are nucleic acid or polypeptide molecules
that are
differentially expressed in a cell of interest. In this context, differential
expression means an
increased level for a positive marker and a decreased level for a negative
marker as compared to
an undifferentiated cell. The detectable level of the marker nucleic acid or
polypeptide is
sufficiently higher or lower in the cells of interest compared to other cells,
such that the cell of
interest can be identified and distinguished from other cells using any of a
variety of methods
known in the art.
[0036] As used herein, a cell is "positive for" a specific marker or
"positive" when the specific
marker is detected in the cell. Similarly, the cell is "negative for" a
specific marker, or
"negative" when the specific marker is not detected in the cell.
[0037] As used herein, "Cell density" and "Seeding Density" are used
interchangeably herein
and refer to the number of cells seeded per unit area of a planar or curved
substrate.
[0038] As used herein, "stage 1" and "Si" are used interchangeably to identify
cells expressing
markers characteristic of the definitive endoderm (DE).
[0039] "Definitive endoderm", as used herein, refers to cells which bear the
characteristics of
cells arising from the epiblast during gastrulation and which form the
gastrointestinal tract and its
derivatives. Definitive endoderm cells express at least one of the following
markers: HNF3 beta,
GATA4, 50X17, CXCR4, Cerberus, OTX2, goosecoid, C-Kit, CD99, and MIXL1.
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
[0040] "Gut tube", as used herein, refers to cells derived from definitive
endoderm that express
at least one of the following markers: HNF3-beta, HNF1-beta, or HNF4-alpha.
Gut tube cells can
give rise to all endodermal organs, such as lungs, liver, pancreas, stomach,
and intestine.
[0041] Used herein interchangeably are "stage 2" and "S2" which identify cells
expressing
markers characteristic of the primitive gut tube.
[0042] "Foregut endoderm" refers to endoderm cells that give rise to
esophagus, lungs,
stomach, liver, pancreas, gall bladder, and a portion of the duodenum.
[0043] "Posterior foregut" refers to endoderm cells that can give rise to
posterior stomach,
pancreas, liver, and a portion of the duodenum.
[0044] "Mid-gut endoderm" refers to endoderm cells that can give rise to the
intestines,
portions of the duodenum, appendix, and ascending colon.
[0045] "Hind-gut endoderm" refers to endoderm cells that can give rise to the
distal third of the
transverse colon, the descending colon, sigmoid colon and rectum.
[0046] Both "stage 3" and "S3" are used interchangeably to identify cells
expressing markers
characteristic of the foregut endoderm. . "Cells expressing markers
characteristic of the foregut
lineage", as used herein, refers to cells expressing at least one of the
following markers: PDX-1,
FOXA2, CDX2, SOX2, and HNF4 alpha.
[0047] Used interchangeably herein are "stage 4" and "S4" to identify cells
expressing markers
characteristic of the pancreatic foregut precursor. "Cells expressing markers
characteristic of the
pancreatic foregut precursor lineage", as used herein, refers to cells
expressing at least one of the
following markers: PDX-1, NKX6.1, HNF6, FOXA2, PTFla, Proxl and HNF4 alpha.
[0048] As used herein, "stage 5" and "S5" are used interchangeably to identify
cells expressing
markers characteristic of the pancreatic endoderm and pancreatic endocrine
precursor cells.
"Cells expressing markers characteristic of the pancreatic endoderm lineage",
as used herein,
refers to cells expressing at least one of the following markers: PDX1,
NKX6.1, HNF1 beta,
PTF1 alpha, HNF6, HNF4 alpha, SOX9, HB9 or PROX1. Cells expressing markers
characteristic of the pancreatic endoderm lineage do not substantially express
CDX2 or SOX2.
11
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
[0049] "Pancreatic endocrine cell", or "Pancreatic hormone expressing cell",
or "Cells
expressing markers characteristic of the pancreatic endocrine lineage" as used
herein, refers to a
cell capable of expressing at least one of the following hormones: insulin,
glucagon,
somatostatin, ghrelin, and pancreatic polypeptide.
[0050] "Pancreatic endocrine precursor cell" or "Pancreatic endocrine
progenitor cell" refers to
pancreatic endoderm cells capable of becoming a pancreatic hormone expressing
cell. Such a
cell can express at least one of the following markers: NGN3, NKX2.2, NeuroD,
ISL-1, Pax4,
Pax6, or ARX.
[0051] Used interchangeably herein are "dl", "d 1", and "day 1"; "d2", "d 2",
and "day 2";
"d3", "d 3", and "day 3", and so on. These number letter combinations specify
the day of
incubation in the different stages during the stepwise differentiation
protocol of the instant
application.
[0052] "Glucose" and "D-Glucose" are used interchangeably herein and refer to
dextrose, a
sugar commonly found in nature.
[0053] Used interchangeably herein are "NeuroD" and "NeuroD 1" which identify
a protein
expressed in pancreatic endocrine progenitor cells and the gene encoding it.
[0054] Used interchangeably herein are "LDN" and "LDN-193189" to indicate a
BMP receptor
inhibitor available from Stemgent, CA, USA.
Isolation, Expansion and Culture of Pluripotent Stem Cells
[0055] Pluripotent stem cells may express one or more of the stage-specific
embryonic antigens
(SSEA) 3 and 4, and markers detectable using antibodies designated Tra-1-60
and Tra-1-81
(Thomson et al. 1998, Science 282:1145-1147). Differentiation of pluripotent
stem cells in vitro
results in the loss of SSEA-4, Tra-1-60, and Tra-1-81 expression.
Undifferentiated pluripotent
stem cells typically have alkaline phosphatase activity, which can be detected
by fixing the cells
with 4% paraformaldehyde, and then developing with Vector Red as a substrate,
as described by
the manufacturer (Vector Laboratoriesõ CA, USA). Undifferentiated pluripotent
stem cells also
typically express OCT4 and TERT, as detected by RT-PCR.
12
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
[0056] Another desirable phenotype of propagated pluripotent stem cells is a
potential to
differentiate into cells of all three germinal layers: endoderm, mesoderm, and
ectoderm tissues.
Pluripotency of stem cells can be confirmed, for example, by injecting cells
into SCID mice,
fixing the teratomas that form using 4% paraformaldehyde, and then examining
them
histologically for evidence of cell types from the three germ layers.
Alternatively, pluripotency
may be determined by the creation of embryoid bodies and assessing the
embryoid bodies for the
presence of markers associated with the three germinal layers.
[0057] Propagated pluripotent stem cell lines may be karyotyped using a
standard G-banding
technique and compared to published karyotypes of the corresponding primate
species. It is
desirable to obtain cells that have a "normal karyotype," which means that the
cells are euploid,
wherein all human chromosomes are present and not noticeably altered.
Pluripotent cells may be
readily expanded in culture using various feeder layers or by using matrix
protein coated vessels.
Alternatively, chemically defined surfaces in combination with defined media
such as mTesrt1
media (StemCell Technologies, Vancouver, Canada) may be used for routine
expansion of the
cells. Pluripotent cells may be readily removed from culture plates using
enzymatic, mechanical
or use of various calcium chelators such as EDTA (Ethylenediaminetetraacetic
acid).
Alternatively, pluripotent cells may be expanded in suspension in the absence
of any matrix
proteins or a feeder layer.
Sources of Pluripotent Stem Cells
[0058] The types of pluripotent stem cells that may be used include
established lines of
pluripotent cells derived from tissue formed after gestation, including pre-
embryonic tissue (such
as, for example, a blastocyst), embryonic tissue, or fetal tissue taken any
time during gestation,
typically but not necessarily, before approximately 10 to 12 weeks gestation.
Non-limiting
examples are established lines of human embryonic stem cells (hESCs) or human
embryonic
germ cells, such as, for example the human embryonic stem cell lines H1, H7,
and H9 (WiCell
Research Institute, Madison, WI, USA). Also suitable are cells taken from a
pluripotent stem
cell population already cultured in the absence of feeder cells. Also suitable
are inducible
pluripotent cells (IPS) or reprogrammed pluripotent cells that can be derived
from adult somatic
cells using forced expression of a number of pluripotent related transcription
factors, such as
13
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
OCT4, NANOG, Sox2, KLF4, and ZFP42 (Annu Rev Genomics Hum Genet 2011, 12:165-
185).
The human embryonic stem cells used in the methods of the invention may also
be prepared as
described by Thomson et al. (U.S. Patent No. 5,843,780; Science, 1998,
282:1145-1147; Curr
Top Dev Biol 1998, 38:133-165; Proc Natl Acad Sci U.S.A. 1995, 92:7844-7848).
Formation of Cells Expressing Markers Characteristic of the Pancreatic
Endoderm
Lineage from Pluripotent Stem Cells
[0059] Characteristics of pluripotent stem cells are well known to those
skilled in the art, and
additional characteristics of pluripotent stem cells continue to be
identified. Pluripotent stem cell
markers include, for example, the expression of one or more of the following:
ABCG2, cripto,
FOXD3, CONNEXIN43, CONNEXIN45, OCT4, 50X2, NANOG, hTERT, UTF1, ZFP42,
SSEA-3, SSEA-4, Tra 1-60, Tra 1-81.
[0060] Pluripotent stem cells suitable for use in the present invention
include, for example, the
human embryonic stem cell line H9 (NIH code: WA09), the human embryonic stem
cell line H1
(NIH code: WA01), the human embryonic stem cell line H7 (NIH code: WA07), and
the human
embryonic stem cell line 5A002 (Cellartis, Sweden). Also suitable for use in
the present
invention are cells that express at least one of the following markers
characteristic of pluripotent
cells: ABCG2, cripto, CD9, FOXD3, CONNEXIN43, CONNEXIN45, OCT4, 50X2, NANOG,
hTERT, UTF1, ZFP42, SSEA-3, SSEA-4, Tra 1-60, and Tra 1-81.
[0061] Markers characteristic of the definitive endoderm lineage are selected
from the group
consisting of 50X17, GATA4, HNF3 beta, GSC, CER1, Nodal, FGF8, Brachyury, Mix-
like
homeobox protein, FGF4, CD48, eomesodermin (EOMES), DKK4, FGF17, GATA6, CXCR4,
C-Kit, CD99, and OTX2. Suitable for use in the present invention is a cell
that expresses at least
one of the markers characteristic of the definitive endoderm lineage. In one
aspect of the present
invention, a cell expressing markers characteristic of the definitive endoderm
lineage is a
primitive streak precursor cell. In an alternate aspect, a cell expressing
markers characteristic of
the definitive endoderm lineage is a mesendoderm cell. In an alternate aspect,
a cell expressing
markers characteristic of the definitive endoderm lineage is a definitive
endoderm cell.
14
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
[0062] Markers characteristic of the pancreatic endoderm lineage are selected
from the group
consisting of PDX1, NKX6.1, HNF1 beta, PTF1 alpha, HNF6, HNF4 alpha, SOX9, HB9
and
PROX1. Suitable for use in the present invention is a cell that expresses at
least one of the
markers characteristic of the pancreatic endoderm lineage. In one aspect of
the present
invention, a cell expressing markers characteristic of the pancreatic endoderm
lineage is a
pancreatic endoderm cell wherein the expression of PDX-1 and NKX6.1 are
substantially higher
than the expression of CDX2 and 50X2.
[0063] Markers characteristic of the pancreatic endocrine lineage are selected
from the group
consisting of NGN3, NEUROD, ISL1, PDX1, NKX6.1, PAX4, ARX, NKX2.2, and PAX6.
In
one embodiment, a pancreatic endocrine cell is capable of expressing at least
one of the
following hormones: insulin, glucagon, somatostatin, and pancreatic
polypeptide. Suitable for
use in the present invention is a cell that expresses at least one of the
markers characteristic of
the pancreatic endocrine lineage. In one aspect of the present invention, a
cell expressing
markers characteristic of the pancreatic endocrine lineage is a pancreatic
endocrine cell. The
pancreatic endocrine cell may be a pancreatic hormone-expressing cell.
Alternatively, the
pancreatic endocrine cell may be a pancreatic hormone-secreting cell.
[0064] In one aspect of the present invention, the pancreatic endocrine cell
is a cell expressing
markers characteristic of the 13 cell lineage. A cell expressing markers
characteristic of the 13 cell
lineage expresses PDX1 and at least one of the following transcription
factors: NKX2.2,
NKX6.1, NEUROD, ISL1, HNF3 beta, MAFA, PAX4, and PAX6. In one aspect of the
present
invention, a cell expressing markers characteristic of the 13 cell lineage is
a 13 cell.
[0065] The present invention recites a method of culturing human pluripotent
stem cells
comprising seeding human pluripotent stem cells on a surface at a density of
from about 0.8 x
105 cells/cm2 to about 3.0 x 105 cells/cm2. In one aspect of the invention,
the human pluripotent
stem cells are human embryonic stem cells. In some aspects of the invention
the surface where
the cells are seeded comprises MatrigelTM.
[0066] In one aspect, the invention refers to a method of differentiating
pluripotent stem cells.
The method comprises seeding the pluripotent stem cells at a density of from
about 0.8 x 105
cells/cm2 to about 3.0 x 105 cells/cm2 on a surface and then differentiating
the pluripotent cells
CA 02872770 2014-11-05
WO 2013/169769
PCT/US2013/039940
into cells expressing markers indicative of definitive endoderm. In some
aspects of the
invention, the pluripotent cells are embryonic stem cells. In some aspects of
the invention, the
embryonic stem cells are human embryonic stem cells. In some aspects of the
invention the
surface where the cells are seeded comprises MatrigelTM.
[0067] The invention refers to a method of obtaining cells expressing markers
indicative of
definitive endoderm by differentiating human embryonic pluripotent stem cells
that have been
seeded on a surface at a seeding density of from about 0.8 x 105 cells/cm2 to
about 3.0 x 105
cells/cm2. In some aspects of the invention the surface where the cells are
seeded comprises
MatrigelTM.
[0068] In one aspect, the invention refers to a method of differentiating
cells expressing
markers indicative of the human definitive endoderm comprising differentiating
human
embryonic pluripotent stem cells, that have been seeded on a first surface at
a seeding density
sufficient to maximize differentiation of the pluripotent cells, into cells
expressing markers
indicative of the definitive endoderm; and differentiating the cells
expressing markers indicative
of definitive endoderm, seeded on a second surface at a seeding density
sufficient to maximize
the differentiation efficiency, into cells expressing markers indicative of
pancreatic endoderm.
In some embodiments, the pluripotent stem cells are seeded at a seeding
density of from about
0.8 x 105 cells/cm2 to about 3.0 x 105 cells/cm2. In some embodiments, the
cells expressing
markers indicative of definitive endoderm are seeded on the surface at a
seeding density of from
about from about 1.5 x 105 cells/cm2 to about 5.0 x 105 cells/cm2. In some
aspects, the
pluripotent cells in the method of differentiating cells expressing markers
indicative of the
human definitive endoderm comprises using embryonic stem cells. In some
aspects of the
invention, the embryonic stem cells are human embryonic stem cells. In some
aspects of the
invention the surfaces where the cells are seeded comprise MatrigelTM.
[0069] The invention refers to a method of differentiating cells expressing
markers indicative
of definitive endoderm that have been produced by the differentiation of
pluripotent stem cells
into cells expressing markers indicative of pancreatic endocrine. Where the
pluripotent stem
cells have been seeded on a surface at a seeding density of from about 0.8 x
105 cells/cm2 to
about 3.0 x 105 cells/cm2. In some aspects of the invention the pluripotent
stem cells used are
16
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
embryonic stem cells. In some aspects of the invention, the embryonic stem
cells used are
human embryonic stem cells. In some aspects of the invention the surfaces
where the cells are
seeded comprise MatrigelTM.
[0070] In one aspect, the invention refers to a method of obtaining cells
expressing markers
indicative of pancreatic endoderm comprising seeding pluripotent stem cells on
a surface;
differentiating the pluripotent stem cells into cells expressing markers
indicative of the definitive
endoderm; and differentiating the cells expressing markers indicative of the
definitive endoderm
into cells expressing markers indicative of pancreatic endoderm. In some
aspects of the
invention, the pluripotent stem cells are seeded at density of from about 0.8
x 105 cells/cm2 to
about 3.0 x 105 cells/cm2. In some aspects of the invention, the cells
expressing markers
indicative of definitive endoderm are seeded at a density of from about 1.5 x
105 cells/cm2 to
about 5.0 x 105 cells/cm2. In some aspects of the invention, the pluripotent
stem cells are
embryonic stem cells. In some aspects of the invention, the embryonic stem
cells are human
embryonic stem cells. In some aspects of the invention the surfaces where the
cells are seeded
comprise MatrigelTM.
[0071] In one aspect, the invention relates to a method of obtaining cells
expressing markers
indicative of pancreatic endocrine lineage, comprising seeding pluripotent
stem cells on a
surface; differentiating the pluripotent stem cells into cells expressing
markers indicative of
definitive endoderm; and differentiating the cells expressing markers
indicative of definitive
endoderm into cells expressing markers indicative of pancreatic endocrine. In
some aspects of
the invention, the pluripotent stem cells used to obtain cells expressing
markers indicative of
pancreatic endocrine lineage are seeded at a density of from about 0.8 x 105
cells/cm2 to about
3.0 x 105 cells/cm2. In some aspects of the invention, the cells expressing
markers indicative of
definitive endoderm are seeded at a density of from about 1.5 x 105 cells/cm2
to about 5.0 x 105
cells/cm2. In some aspects of the invention, the pluripotent stem cells are
embryonic stem cells.
In some aspects of the invention, the embryonic stem cells are human embryonic
stem cells. In
some aspects of the invention the surfaces where the cells are seeded comprise
MatrigelTM.
[0072] In one aspect, the invention refers to a method of differentiating
cells expressing
markers indicative of definitive endoderm comprising seeding cells expressing
markers
17
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
indicative of definitive endoderm on a surface at a seeding density of from
about 1.5 x 105
cells/cm2 to about 5.0 x 105 cells/cm2 and then differentiating the cells
expressing markers
indicative of definitive endoderm into cells expressing markers indicative of
pancreatic
endoderm. In some aspects of the invention, the cells expressing markers
indicative of definitive
endoderm used in the method are human cells expressing markers indicative of
definitive
endoderm. In some aspects of the invention, the cells expressing markers
indicative of
pancreatic endoderm are human.
[0073] In one aspect, the invention relates to a method of differentiating
cells expressing
markers indicative of definitive endoderm seeded on a surface at a seeding
density of from about
1.5 x 105 cells/cm2 to about 5.0 x 105 cells/cm2 and then differentiating the
cells expressing
markers indicative of definitive endoderm into cells expressing markers
indicative of pancreatic
endocrine. In some aspects, the cells expressing markers indicative of the
definitive endoderm
are human. In some aspects, the cells expressing markers indicative of the
pancreatic endocrine
are human.
[0074] This invention describes a range of ES cell densities that can be
efficiently
differentiated to pancreatic endoderm and endocrine lineages.
[0075] Another aspect of this invention describes a range of DE cell densities
that can be
efficiently differentiated to pancreatic endoderm and endocrine lineages.
[0076] Publications cited throughout this document are hereby incorporated by
reference in
their entirety. The present invention is further illustrated, but not limited,
by the following
examples.
EXAMPLES
Example 1
Seeding Density of Embryonic Stem Cells does not Significantly Affect
Expression of
Definitive Endoderm Markers
[0077] This example was carried out to understand if the initial seeding
density of ES cells
would significantly impact production of cells of the definitive endoderm
lineage.
18
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
[0078] Cells of the human embryonic stem cell line H1 (hESC H1) were harvested
at various
passages (passage 40 to passage 52) and were seeded as single cells at the
following densities:
0.3 X 105 cells/cm2, 0.5 X 105 cells/cm2, 0.75 X 105 cells/cm2, 0.9 X 105
cells/cm2, 1 X 105
cells/cm2, 1.25 X 105 cells/cm2, 1.5 X 105 cells/cm2, 1.8 X 105 cells/cm2, and
2 X 105 cells/cm2
on MatrigelTM (1:30 dilution; BD Biosciences, Franklin Lakes, NJ) coated
dishes in either
mTeSRCD1 media (StemCell Technologies, Vancouver, Canada) or MEF-CM
(conditioned
media) supplemented with 10 iiiM of Y27632 (Rock inhibitor, Catalog No. Y0503,
SigmaAldrich, St. Louis, MO). Forty-eight hours post seeding, cultures were
washed and
incubated in incomplete PBS (phosphate buffered saline without Mg or Ca) for
approximately 30
seconds. Cultures were differentiated into definitive endoderm (DE) lineage as
follows:
[0079] Stage 1 (Definitive Endoderm (DE)- 4 days): Cells were cultured for one
day in stage 1
media: MCDB-131 medium (Catalog No. 10372-019, Invitrogen, Carlsbad, CA)
supplemented
with 2% fatty acid-free BSA (Catalog No. 68700, Proliant, Ankeny, IA), 0.0012
g/ml sodium
bicarbonate (Catalog No. S3187, SigmaAldrich), lx GlutaMaxTm (Catalog No.
35050-079,
Invitrogen), 2.5 mM D-Glucose (Catalog No. G8769, SigmaAldrich), 1:50000X ITS-
X
(Invitrogen), 100 ng/ml GDF8 (R&D Systems, Minneapolis, MN) and 2.5 iiiM MCX
compound
(a GSK3B inhibitor, 1 4-Prop-2-en- 1 -y1-3 ,5 ,7,1 4,1 7,23 ,27-
heptaazatetracyclo
[19.3.1.1-2,6¨.1-8,12¨]heptacosa-1(25),2(27),3,5,8(26),9,11,21,23-nonaen-16-
one, US Patent
Application Publication No. 2010-0015711; incorporated herein by reference in
its entirety).
Cells were then cultured for additional three days in MCDB-131 medium
supplemented with 2%
fatty acid-free BSA, 0.0012 g/ml sodium bicarbonate, lx GlutaMaxTm, 2.5 mM D-
Glucose, 100
ng/ml GDF8, and 1:50000X ITS-X.
[0080] At end of DE stage, samples were collected and analyzed by real-time
PCR and
fluorescent activated cell sorting (FACS). Cell hESC-derived cells were
released into single-cell
suspension by incubation in TrypLE Express (Invitrogen Catalog No. 12604) at
37 C for 3-5
minutes and subsequently counted in duplicates using a hemocytometer. Cells
were then washed
twice in staining buffer (PBS containing 0.2% BSA) (BD Biosciences Catalog No.
554657). For
surface marker staining, lx105 to lx106 cells were re-suspended in 100 jul
blocking buffer (0.5%
human gamma-globulin diluted 1:4 in staining buffer). Directly conjugated
primary antibodies
CD184 APC (Allophycocyanin, BD Biosciences Catalog No. 555976), and CD9 PE (BD
19
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
Biosciences Catalog No. 555372) were added to the cells at a final dilution of
1:20 and incubated
for 30 minutes at 4 C. Stained cells were washed twice in BD staining buffer,
re-suspended in
200 jul staining buffer, followed by incubation in 15 jul of 7AAD for
live/dead discrimination
prior to analysis on the BD FACS Canto.
[0081] Total RNA was extracted with the RNeasy Mini Kit (Qiagen; Valencia, CA)
and
reverse-transcribed using a High Capacity cDNA Reverse Transcription Kit
(Applied
Biosystems, Foster City, CA) according to manufacturer's instructions. cDNA
was amplified
using Taqman Universal Master Mix and Taqman Gene Expression Assays which were
pre-
loaded onto custom Taqman Arrays (Applied Biosystems). Data were analyzed
using Sequence
Detection Software (Applied Biosystems) and normalized to undifferentiated
human embryonic
stem (hES) cells using the AACt method. All primers were purchased from
Applied Biosystems.
[0082] Figure lA to Figure 1F shows FACS histogram expression profiles of
CXCR4 (Y-axis,
marker of DE) and CD-9 (X-axis, marker of undifferentiated ES cells) for H1
cells seeded at 0.3
X 105 cells/cm2 (FIG 1A), 0.75 X 105 cells/cm2 (FIG 1B), 1 X 105 cells/cm2
(FIG 1C), 1.5 X 105
cells/cm2 (FIG 1D), 1.8 X 105 cells/cm2 (FIG 1E), and 2 X 105 cells/cm2 (FIG
1F). Percentage
expression of CXCR4 and CD9 is summarized in Table I. As shown in Figure 1 and
Table I, the
initial seeding density of undifferentiated ES cells had no significant impact
on subsequent
differentiation to definitive endoderm as measured by upregulation of CXCR4
and down
regulation of CD9.
Table I
Effect of Seeding Density of ES Cells on Expression of Definitive Endoderm
Marker CXCR4
Seeding density of DE day 0 DE day 4
ES cells Cell density Cell density % CXCR4 %
CD9
(cells/cm2) (cells/cm2) (cells/cm2)
0.5 X 104 1.1 2.6 93.3 4.9
0.75 X 104 1.25 2.8 93.1 5.6
1.0X 105 2.23 3.95 93.1 5.3
1.5 X 105 2.87 3.75 90.9 6.5
1.8 X 105 2.58 4.4 93.1 4.7
2.0 X 105 2.8 5.2 92.2 6.1
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
[0083] Figure 2A to Figure 2G show data from real-time PCR analyses of the
expression of the
following genes in cells of the human embryonic stem cell line H1 seeded at
various densities
and subsequently differentiated to DE as outlined in Example 1: SOX7 (FIG 2A),
NANOG (FIG
2B), OCT4 (FIG 2C), AFP (FIG 2D), SOX17 (FIG 2E), FOXA2 (FIG 2F), and CXCR4
(FIG
2G). Consistent with FACS data, there was no significant difference between
genes commonly
expressed at DE stage (CXCR4, SOX17, FOXA2) for H1 cells seeded at various
densities on
MatrigelTm-coated surfaces. Moreover, initial seeding density did not have a
significant impact
on genes associated with extra embryonic endoderm (AFP, SOX7) and
pluripotentcy related
genes (OCT4, Nanog).
[0084] Figures 3 and 4 depict phase contrast images of cultures prior to
induction of DE (Fig
3A to Fig 3G) and 4 days after initiation of differentiation to DE (Fig 4A to
Fig 4G) for H1 cells
seeded at various seeding densities: 3 X 104 cells/cm2 (Fig 3A and Fig 4A); 5
X 104 cells/cm2
(Fig 4A and Fig 4B); 7.5 X 104 cells/cm2 (Fig 4A and Fig 4C); 1 X 105
cells/cm2, Fig 4D, ; Fig
4E, 1.1 X 105 cells/cm2;Fig 4F, 1.2 X 105 cells/cm2; Fig 4G, 1.5 X 105
cells/cm2. Figure 4
clearly shows that there was a significant morphological difference for
cultures seeded at <1 X
105 cells/cm2 as compared to cultures seeded at higher cell densities.
However, this difference
did not translate into significant difference in genes/protein associated with
DE. Data from this
example highlight that the initial seeding density did not significantly
impact expression of
markers associated with DE. Cultures of ES cells seeded at densities in the
range of 0.3-2 X 105
cells/cm2 showed similar efficiencies in differentiation to DE.
Example 2
Seeding Density of Embryonic Stem Cells Significantly Affect Expression of
Pancreatic
Endoderm and Pancreatic endocrine Markers
[0085] This example was carried out to understand if the initial seeding
density of ES
significantly impacts generation of pancreatic endoderm/endocrine cultures.
[0086] Cells of the human embryonic stem cell line H1 (hESC H1) were harvested
at various
passages (passage 40 to passage 52) and were seeded as single cells at the
following densities:
0.5 X 105 cells/cm2, 0.75 X 105 cells/cm2, 1 X 105 cells/cm2, 1.5 X 105
cells/cm2, 1.8 X 105
21
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
cells/cm2, and 2 X 105 cells/cm2 on MATRIGELTm (1:30 dilution; BD Biosciences,
NJ) coated
dishes in MEF-CM (conditioned media) supplemented with 10 iiiM of Y27632.
Forty-eight
hours post seeding, cultures were washed and incubated in incomplete PBS
(phosphate buffered
saline without Mg or Ca) for approximately 30 seconds.
[0087] Cultures were differentiated into pancreatic endoderm/endocrine
lineages as follows:
a) Stage 1 (Definitive Endoderm (DE)- 4 days): Cells were cultured for one day
in stage
1 media: MCDB-131 medium (Invitrogen Catalog No.10372-019) supplemented
with 2% fatty acid-free BSA (Proliant Catalog No. 68700), 0.0012 g/ml sodium
bicarbonate (SigmaAldrich Catalog No. S3187), lx GlutaMaxTm (Invitrogen
Catalog
No. 35050-079), 2.5 mM D-Glucose (SigmaAldrich Catalog No. G8769), 1:50000X
ITS-X (Invitrogen), 100 ng/ml GDF8 (R&D Systems) and 2.5 iiiM MCX compound.
Cells were then cultured for additional three days in MCDB-131 medium
supplemented with 2% fatty acid-free BSA, 0.0012 g/ml sodium bicarbonate, lx
GlutaMaxTm, 2.5 mM D-Glucose, 100 ng/ml GDF8, and 1:50000X ITS-X.
b) Stage 2 (Primitive gut tube- 2 days): Cells were treated for two days with
MCDB-131
medium supplemented with 1:50000X ITS-X, 0.1% ALBUMAX BSA (Invitrogen);
0.0012 g/ml sodium bicarbonate; 1X GlutaMaxTm; 2.5 mM D-Glucose; and 50 ng/ml
FGF7, then
c) Stage 3 (Foregut- 3 days): Cells were treated with MCDB-131 medium
supplemented
with a 1:200 dilution of ITS-X; 20 mM Glucose; 1X GlutaMaxTm; 0.0015 g/ml
sodium bicarbonate; 0.1% ALBUMAX BSA; 0.25 04 SANT-1; 20 ng/ml of
Activin-A; 2 04 RA; 50 ng/ml FGF7; and 200 nM LDN (BMP receptor inhibitor;
Catalog No. 04-0019; Stemgent, CA) for three days.
d) Stage 4 (Pancreatic foregut precursor- 3 days): Cells were treated with
MCDB-131
medium supplemented with a 1:200 dilution of ITS-X; 20 mM Glucose; 1X
GlutaMaxTm; 0.0015 g/ml sodium bicarbonate; 0.1% ALBUMAX BSA; 0.25 04
SANT-1; 50 nM TPB (PKC activator; Catalog No. 565740; EMD Chemicals,
Gibstown, NJ); 200 nM LDN-193189; 2 04 ALk5 inhibitor (SD-208, disclosed in
22
CA 02872770 2014-11-05
WO 2013/169769 PCT/US2013/039940
Molecular Pharmacology 2007, 72:152-161); and 100 nM CYP26A inhibitor (N-{4-
[2-Ethy1-1-(1H-1, 2, 4-triazol-1-yl)butyl]phenylf -1, 3-benzothiazol-2-amine,
Janssen,
Belgium) for three days.
e) Stage 5 (Pancreatic endoderm/endocrine -3 days): Stage 4 cells were treated
with
MCDB-131 medium supplemented with a 1:200 dilution of ITS-X; 20 mM Glucose;
1X GlutaMaxTm; 0.0015 g/ml sodium bicarbonate; 0.1% ALBUMAX BSA; 200 nM
LDN-193189; 100 nM CYP26A inhibitor, and 2 04 ALk5 for three days.
[0088] At end of stage 5, phase contrast images were collected for all tested
cell densities along
with mRNA for PCR analysis of relevant pancreatic endoderm genes. Figure 5A-5F
show phase
contrast images of stage 5 cultures that were initially seeded at various cell
densities of ES cells:
X 104 cells/cm2 (FIG 5A), 7.5 X 104 cells/cm2 (FIG 5B), 1 X 105 cells/cm2 (FIG
5C), 1.5 X 105
cells/cm2 (FIG 5D), 1.8 X 105 cells/cm2 (FIG 5E) and 2.0 X 105 cells/cm2 (FIG
5F). Dramatic
heterogeneity of cultures differentiated from cultures seeded at densities
less than 1 X 105
cells/cm2 indicates that initial cell density of ES cells significantly
impacts morphology of later
stage cultures. In particular, cells differentiated from cultures initially
seeded at a density higher
than 1.5 X 105 cells/cm2 showed a uniform morphology throughout the area of
the culture dish.
[0089] Figure 6A to Figure 6J depict data from real-time PCR analyses of the
expression of the
following genes in cells of the human embryonic stem cell line H1 seeded at
various densities
and subsequently differentiated to stage 5 as outlined in Example 2: ZIC1 (FIG
6A), CDX2 (FIG
6B), PDX-1 (FIG 6C), NKX6.1 (FIG 6D), NKX2.2 (FIG 6E), NGN3 (FIG 6F), NEUROD
(FIG
6G), insulin (FIG 6H) HNF4a (FIG 61), and PTFla (FIG 6J). Unlike the effects
observed in
Example 1, initial seeding density dramatically affected expression of
pancreatic
endoderm/endocrine markers. In particular, cells differentiated from cultures
with an initial
seeding density of less than 1-1.5 X 105 cells/cm2 showed a significant drop
in expression of
PDX-1, NKX6.1, NGN3, NKX2.2, NeuroD, and insulin while showing upregulation of
ectoderm
marker ZIC1 and posterior gut marker, CDX2. This data along with data from
Example 1 clearly
highlight that a high expression of CXCR4 and other DE related genes are not
predictive of
production of pancreatic endoderm/endocrine genes. Initial seeding density
appears to be an
important variable in controlling the efficiency of pancreatic
endoderm/endocrine cells.
23