Note: Descriptions are shown in the official language in which they were submitted.
CA 02872804 2014-11-05
WO 2013/173602
PCT/US2013/041386
PIPELINE REACTION FOR REMOVING HEAVY METALS FROM
PRODUCED FLUIDS
CROSS-REFERENCE TO RELATED APPLICATIONS
[001] This application claims benefit under 35 USC 119 of US Patent
Application
Serial No. 61/647,674 with a filing date of May 16, 2012. This application
claims priority to
and benefits from the foregoing, the disclosures of which are incorporated
herein by
reference.
TECHNICAL FIELD
[002] The invention relates generally to a process, method, and system for
removing
heavy metals including mercury from hydrocarbon fluids such as crude oil and
gases.
BACKGROUND
[003] Pipelines are widely used in a variety of industries, allowing a large
amount of
material to be transported from one place to another. The transport can be for
a short distance
as within a plant or over a long distance such as a continent. A variety of
fluids, such as oil
and/or gas, as well as particulate, and other small solids suspended in
fluids, are transported
cheaply and efficiently using pipelines. Pipelines can be subterranean,
submarine, on the
surface of the earth, and even suspended above the earth. Submarine pipelines
especially
carry enormous quantities of oil and gas products indispensable to energy-
related industries,
often under tremendous pressure and at low temperatures and at high flow
rates.
[004] Oil and gas pipelines, including undersea or submarine pipelines,
typically
carry production fluids from one of the production wells including subsea
wells. These fluids
may be, but are not limited to, a gas, a liquid, an emulsion, a slurry and /
or a stream
comprising solid particles (oil sand). The production fluid can be a single
phase, a two phase
or even a three phase admixture.
[005] Methods have been disclosed to remove heavy metals from produced fluids.
Common approaches utilize treatments for the fluids once the fluids are
recovered from
subterranean reservoirs and brought to a surface production installation. US
Patent No.
4,551,237 discloses the use of an aqueous solution of sulfide materials to
remove arsenic
from oil shale. US Patent No. 4,877,515 discloses a process for removing
mercury from
hydrocarbon streams, gas or liquid. US Patent No. 4,915,818 discloses a method
of removing
mercury from liquid hydrocarbons (natural gas condensate) by contact with a
dilute aqueous
1
CA 02872804 2014-11-05
WO 2013/173602
PCT/US2013/041386
solution of alkali metal sulfide salt. US Patent No. 6,268,543 discloses a
method for
removing elemental mercury with a sulfur compound. US Patent No. 6,350,372
discloses
removing mercury from a hydrocarbon feed by contact with an oil soluble or oil
miscible
sulfur compound U.S. Pat. No. 4,474,896 discloses using polysulfide based
absorbents to
remove elemental mercury (Hg ) from gaseous and liquid hydrocarbon streams.
[006] Given the cost of expensive installations of equipment in production
facilities
for the removal of heavy metals from produced fluids, there is a need for the
efficient
removal of trace levels of heavy metals from hydrocarbon fluids from
production wells,
before reaching refineries, shipping terminals, or upstream oil processing
facilities that
separate and prepare crude oil for sale including land-based oil processing
facilities, and
offshore oil processing platforms including floating production, storage and
offloading
(FPSO) units and others performing similar functions.
SUMMARY OF THE INVENTION
[007] In one aspect, the invention relates to a method for simultaneously
transporting and removing a trace amount of heavy metals from a produced
fluid. The
method comprises: extracting a produced fluid containing heavy metals from a
production
well; injecting into the produced fluid an effective amount of at least fixing
agent and a
dilution fluid forming a mixture; transferring the mixture through a pipeline
from the
production well for a sufficient distance for at least a portion of the heavy
metals to react with
the mixture, at least a fixing agent, and be extracted into the dilution fluid
as complexes; and
separating the dilution fluid containing the heavy metal complexes from the
produced fluid
for a treated produced fluid having a reduced concentration of heavy metals.
[008] In another aspect, the invention relates to a method for simultaneously
transporting and removing mercury from a crude. The method comprises:
extracting the
crude containing a trace amount of mercury from a production well; injecting
into the crude
an effective amount of at least fixing agent and a sufficient amount of water
forming a
mixture; transferring the mixture through a pipeline for a sufficient distance
for at least a
portion of mercury to react with the fixing agent forming a soluble mercury
complex in
water; separating the water containing the soluble mercury complex from the
crude for a
treated crude having reduced mercury concentration.
BRIEF DESCRIPTION OF THE FIGURES
2
CA 02872804 2014-11-05
WO 2013/173602
PCT/US2013/041386
[009] FIG. 1 is a diagram of an embodiment of a pipeline conditioning system
from
one or more subsea wells to a floating production, storage and offloading
(FPSO) unit.
[010] FIG. 2 is a diagram of a pipeline conditioning system with one or more
intermediate collection and / or processing facilities.
DETAILED DESCRIPTION
[011] The following terms will be used throughout the specification with
following
meanings unless otherwise indicated.
[012] "Hydrocarbons" refers to hydrocarbon streams such as crude oils and / or
natural gases.
[013] "Produced fluids" refers hydrocarbon gases and / or liquids such as
crude oil
that is removed from a geologic formation via a production well, including
mixtures of
hydrocarbons and water that is typically extracted with the hydrocarbons.
[014] "Crude oil" refers to a hydrocarbon material, including both crude oil
and
condensate, which is typically in liquid form. Under some formation conditions
of
temperature and/or pressure, the crude may be in a solid phase. Under some
conditions, the
oil may be in a very heavy liquid phase that flows slowly, if at all, e.g., as
a slurry phase
comprising oil sand or bitumen flecks. While the description described herein
sometimes
refers to "crude" or "crude oil," the description of "crude oil" also includes
hydrocarbon
gases unless specified otherwise.
[015] "Production well" is a well through which produced fluids are carried
from an
oil-bearing geological formation to the earth's surface, whether the surface
is the seafloor, a
fixed or floating structure on water, or land. Surface facilities are provided
for handling and
processing the produced fluids from the formation upon the surface. Production
well may be
used interchangeably with wellhead or well.
[016] "Produced water" refers to the water generated in the production of oil
and
gas, including formation water (water present naturally in a reservoir), as
well as water
previously injected into a formation either by matrix or fracture injection,
which can be any
of connate water, aquifer water, seawater, desalinated water, industrial by-
product water, and
combinations thereof In one embodiment, produced water is a component of
produced
fluids.
[017] "FPSO" (floating production, storage and offloading unit) is a floating
vessel
for the processing of hydrocarbons and for storage of oil / gas. In one
embodiment, the
FPSO processes an incoming stream of crude oil, water, gas, and sediment, and
produce a
3
CA 02872804 2014-11-05
WO 2013/173602
PCT/US2013/041386
shippable crude oil with acceptable properties including levels of heavy
metals such as
mercury, vapor pressure, basic sediment & water (BS&W) values, etc.
[018] "Pipeline conditioning system" refers to a pipeline that contains
produced
fluids and at least one chemical reagent for the removal of at least a heavy
metal from the
produced fluids.
[019] "Trace amount" refers to the amount of heavy metals in a produced fluid.
The amount varies depending on the source of the fluid and the type of heavy
metal, for
example, ranging from a few ppb to up to 30,000 ppb for mercury and arsenic.
[020] "Heavy metals" refers to gold, silver, mercury, osmium, ruthenium,
uranium,
cadmium, tin, lead, and arsenic. While the description described herein refers
to mercury
removal, in one embodiment, the treatment removes one or more of the heavy
metals from
the produced fluids.
[021] "Mercury sulfide" may be used interchangeably with HgS, referring to
mercurous sulfide, mercuric sulfide, or mixtures thereof Normally, mercury
sulfide is
present as mercuric sulfide with a stoichiometric equivalent of one mole of
sulfide ion per
mole of mercury ion. Mercuric sulfide can be in any of the common crystal
forms, e.g.,
cinnabar, metacinnabar, hypercinnabar, or combinations thereof
[022] "Fixing agent" refers to chemical reagents that are added to the
pipeline to
form complexes with the heavy metals in the produced fluid, or to convert the
heavy metals
into compounds that are soluble in the dilution fluid, e.g., water, that is
added to the pipeline
to assist the flow of the produced fluid in the pipeline.
[023] The invention relates to a method for simultaneously transporting and
removing heavy metals contained in produced fluids such as crude oil, gases
and the like. In
the course of being transferred through a pipeline with a sufficient amount of
dilution fluid,
e.g., water including produced water and / or lighter hydrocarbon, sufficient
mixing occurs in
the pipeline for reactions to take place between the fixing agent and heavy
metals such as
mercury, arsenic, etc. to be extracted into the dilution fluid or to
precipitate out of the crude.
[024] Produced Fluids for Removal of Heavy Metals: Heavy metals such as lead,
zinc, mercury, silver, arsenic and the like can be present in trace amounts in
all types of
hydrocarbon streams such as crude oils and natural gases. Some crude oils
contain trace
amounts of heavy mercury and/or arsenic. The amount of mercury and / or
arsenic can range
from below the analytical detection limit to several thousand ppb depending on
the feed
source.
4
CA 02872804 2014-11-05
WO 2013/173602
PCT/US2013/041386
[025] Arsenic species can be present in produced fluids in various forms
including
but not limited to trimethylarsine, arsine (AsH3), triphenylarsine (Ph3As),
triphenylarsine
oxide (Ph3As0), arsenic sulfide minerals (e.g., As4S4 or AsS or As2S3), metal
arsenic sulfide
minerals (e.g., FeAsS; (Co, Ni, Fe)AsS; (Fe, Co)AsS), arsenic selenide (e.g.,
As25e5, As25e3),
arsenic-reactive sulfur species, organo-arsenic species, and inorganic arsenic
held in small
water droplets.
[026] Mercury can be present in produced fluids as elemental mercury Hg ,
ionic
mercury, inorganic mercury compounds, and / or organic mercury compounds.
Examples
include but are not limited to: mercuric halides, mercurous halides, mercuric
oxides,
mercuric sulfide, mercuric sulfate, mercurous sulfate, mercury selenide
mercury hydroxides,
organo-mercury compounds and mixtures of thereof. Mercury can be present as
particulate
mercury, which can be removed by filtration or centrifugation. The particulate
mercury in
one embodiment is predominantly non-volatile.
[027] In one embodiment, the produced fluid is a crude oil containing at
least50
pbbw mercury. In another embodiment, the mercury level is at least 100 pbbw.
In one
embodiment of a mercury-containing crude, less than 50% of the mercury can be
removed by
stripping (or more than 50% of the mercury is non-volatile). In another
embodiment, at least
65% of the mercury in the crude is non-volatile. In a third embodiment, at
least 75% of the
mercury is of the particulate or non-volatile type.
[028] In one embodiment, the produced fluid for transporting in the pipeline
is in the
form of a mixture of crude oil and water. For some production wells, the
amount of produced
water in the crude can be as much as 98% of the crude / water mixture
transported in the
pipeline.
[029] Pipeline Reaction: The pipeline reaction system effectively reduces
levels of
heavy metals such as mercury and / or arsenic from produced fluids with the
addition of at
least a chemical reagent as a fixing agent to the pipeline. The fixing agent
can be introduced
into the pipeline along with a dilution fluid or separately by itself without
a dilution fluid,
into the production well at the well head, into a manifold, into a location
downhole in the
wellbore, an intermediate location into a pipeline between the production well
and a
processing facility, or combinations of the above. In one embodiment, the
dilution fluid is
produced water in the production fluids.
[030] In one embodiment, the fixing agent is introduced into the pipeline at
an entry
point at the wellhead or close to the well head, e.g., within 1000 ft of the
well head, and
separate from the dilution fluid. In another embodiment, the fixing agent is
introduced into
5
CA 02872804 2014-11-05
WO 2013/173602
PCT/US2013/041386
the production well along with a dilution fluid. In yet another embodiment,
the fixing agent
is introduced into a pipeline carrying a crude in a processing facility for
the reaction to take
place in the pipeline before the crude reaches its destination such as a piece
of equipment in
the facility.
[031] In one embodiment, the dilution fluid is non-potable water, e.g.,
connate
water, aquifer water, seawater, desalinated water, oil field produced water,
industrial by-
product water, or combinations thereof In another embodiment, the dilution
fluid is a lighter
hydrocarbon, e.g., pentane, diesel oil, gas oil, kerosene, gasoline, benzene,
toluene, heptane,
and the like. Depending on the produced fluids to be transported and the type
of dilution
fluid employed, the volume ratio of dilution fluid to the produced fluid in
the pipeline may
range from 20:1 to 1:20 in one embodiment, 5:1 to 1:5 in another embodiment,
and 4:1 to 1:1
in a yet another embodiment.
[032] In the pipeline, the fixing agent effectively extracts heavy metals from
the
produced fluid into a dilution fluid such as water. The pipeline is of
sufficient length so that,
in the course of transferring produced fluid through it, sufficient mixing of
produced fluids
and water occurs for reactions to take place between the fixing agent and the
heavy metals,
for heavy metals such as mercury to form insoluble complexes, or be extracted
from the
produced fluid into the water phase. In one embodiment wherein mercury reacts
with the
fixing agent to form insoluble complexes, the heavy metals can then be removed
by filtration,
settling, or other methods known in the art, e.g., removal of solids from a or
gas liquid stream
to produce a hydrocarbon product with reduced mercury content. In another
embodiment
wherein mercury reacts with the fixing agent and is extracted into the
dilution fluid as a
soluble compound, the Hg-enriched water phase can be separated from the crude
by means
known in the art, e.g., gravity settler, coalescer, separator, etc., at a
processing facility at the
destination of the pipeline to produce a hydrocarbon product with reduced
mercury content.
[033] The pipeline is sufficiently long for a residence time of at least one
minute in
one embodiment, at least 10 minutes in another embodiment, at least 30 minutes
in yet
another embodiment, at least 10 hours in a fourth embodiment. The pipeline can
be in the
range of 20-200 hours that extends for hundreds if not thousands of
kilometers. In one
embodiment, the reaction takes place over a relatively short pipeline, e.g.,
at least 10 m but
less than 50 meters for intra-facility transport. In yet another embodiment,
the reaction takes
place in pipeline sections for a long distance transport of at least 0.5 km,
at least 50 km, at
least 500 km and less than 10,000 km in another embodiment. In one embodiment
the flow
in the pipeline is turbulent, and in another embodiment the flow is laminar.
6
CA 02872804 2014-11-05
WO 2013/173602
PCT/US2013/041386
[034] For effective removal of mercury from the produced fluids with
sufficient
mixing to create a dispersion of water in a produced fluid such as crude oil,
or oil in the
water, the pipeline has a minimum superficial liquid velocity (based on
combined oil and
water phases) of at least 0.1 m/s in one embodiment; at least 0.5 m/s in a
second embodiment;
and at least 5 m/s in a third embodiment. In one embodiment with the transport
of certain
produced fluids or under certain transport conditions, e.g., heavy oil and /
or at or low
superficial velocities, the natural mixing in the pipeline can be augmented
with the use of
mixers at the point of introduction of the fixing agent, or at intervals
downstream in the
pipeline. Examples include static or in-line mixers as described in Kirk-
Othmer
Encyclopedia of Chemical Technology, Mixing and Blending by David S. Dickey,
Section
10, incorporated herein by reference.
[035] Depending on the produced fluid being carried in the pipeline, e.g., oil
sand
with low viscosity, crude oil, etc., the temperature of the pipeline is
maintained at a
temperature of at least 5 C in one embodiment, at least 10 C in a second
embodiment, and at
least 10 C in a second embodiment. The produced fluid can be mixed with a
heated dilution
fluid at the production site before being pumped through the pipeline for the
mixture in the
pipeline to have a temperature in the range of 5-70 C at the entry point of
the fixing agent. In
one embodiment, steam or hot water containing fixing agents is injected at the
entry point, or
at intervals along the pipeline for the desired chemistry and temperature for
the pipeline
reaction to take place.
[036] The pipeline reaction system can be either land-based or located subsea,
extending from a production site to a crude processing facility and receiving
production flow
from a surface wellhead or other sources. Examples include subsea pipelines,
where the great
depth of the pipeline can make the pipeline relatively inaccessible, and where
the pipelines
include a header or vertical section that forms a substantial pressure head.
The pipeline
system can be on-shore, off-shore (as a platform, FPSO, etc), or combinations
thereof For
off-shore locations, the pipeline system can be a structure rising above the
surface of the
water (well platform) or it can be sub-surface (on the sea bed).
[037] In one embodiment where the production site is at a sufficient distance
from
the processing facility, the pipeline system includes intermediate separation,
collection and /
or processing facilities. The intermediate facilities contain one or more
supply tanks to
dispense fixing agents and / or other process aids, e.g., foamants, NaOH,
diluents, etc., to
facilitate the flow of produced fluids in into the pipeline. The intermediate
facilities may
also include equipment such as gravity separator, plate separator, hydroclone,
coalescer,
7
CA 02872804 2014-11-05
WO 2013/173602
PCT/US2013/041386
centrifuge, filter, collection tanks, etc. for the separation, storage, and
treatment of recovered
water after separation from the crude. The separation is carried out at the
destination in one
embodiment, and at intervals along the pipeline in another embodiment.
[038] In one embodiment for a pipeline system within a production or
processing
facility, the pipeline may extend from a first equipment to another equipment
located at a
different location or section of the facility. The first equipment can be a
vessel where the
fixing agent is first introduced or mixed with the produced fluid. The second
equipment can
be a separator for the oil / water separation or another vessel. In one
embodiment, additional
chemical reagents such as complexing agents can be added to the second
equipment to
facilitate the oil / water separation to recover treated crude oil and waste
water for subsequent
water treatment or discharge.
[039] The wastewater after being separated from the treated crude is injected
back
into the oil or gas reservoir (in production or depleted) in one embodiment.
In another
embodiment, the wastewater is further treated being injected into the
reservoir prior to being
discharged. In another embodiment the wastewater is treated to meet
environmental
regulations for water quality and discharged.
[040] In one embodiment after the pipeline reaction, at least 50% of mercury
is
removed from the produced fluid for a mercury concentration of less than 100
ppbw in the
treated hydrocarbon. In another embodiment, at least 50% of arsenic is removed
from a
produced fluid such as shale oil for an treated shale oil having less than 100
ppbw arsenic in
the treated hydrocarbon. In yet another embodiment after the pipeline
reaction, at least 50%
of mercury is removed from the produced fluid for a mercury concentration of
less than 50
ppbw in the treated hydrocarbon. In another embodiment, at least 50% of
arsenic is
removed from a produced fluid such as shale oil for an treated shale oil
having less than 50
ppbw arsenic in the treated hydrocarbon. A least 75% of the heavy metals such
as mercury
and / or arsenic is removed from a produced fluid such as crude oil in one
embodiment; and
at least 90% in a second embodiment.
[041] Fixing Agent: In one embodiment for the removal of arsenic and / or
mercury, the fixing agent is a sulfur-based compound for forming sulfur
complexes with the
heavy metals. Examples include organic and inorganic sulfide materials
(including
polysulfides), which in some embodiments, convert the heavy metal complexes
into a form
which is more soluble in an aqueous dilution fluid than in a produced fluid
such as shale oil.
In one embodiment, the sulfur based compounds are selected from sodium
polysulfide,
ammonium polysulfide, and mixtures thereof
8
CA 02872804 2014-11-05
WO 2013/173602
PCT/US2013/041386
[042] In one embodiment, the fixing agent is a water-soluble monatomic sulfur
species, e.g., sodium sulfides and alkali sulfides such as hydrosulfides or
ammonium sulfides,
for the extraction of mercury into an aqueous dilution fluid as soluble
mercury sulfur
complexes. In another embodiment, the sulfur-based compound is any of hydrogen
sulfide,
bisulfide salt, or a polysulfide, for the formation of precipitates which
require separation from
the treated produced fluid by filtration, centrifugation, and the like. In yet
another
embodiment, the fixing agent is an organic polysulfide such as di-tertiary-
nonyl-polysulfide.
In another embodiment, the sulfur based compound is an organic compound
containing at
least a sulfur atom that is reactive with mercury as disclosed in US Patent
No. 6,685,824; the
relevant disclosure is included herein by reference. Examples include but are
not limited to
dithiocarbamates, sulfurized olefins, mercaptans, thiophenes, thiophenols,
mono and dithio
organic acids, and mono and dithiesters.
[043] In one embodiment for the treatment / removal of heavy metals such as
elemental mercury in the gas phase, the fixing agent is a polysulfide (organic
or inorganic)
which converts the elemental Hg into a species that is dissolved in the
dilution fluid, e.g.,
HgS2H-.
[044] In another embodiment, the fixing agent is an oxidizing agent which
converts
the heavy metal to an oxidation state that is soluble in water. Examplary
fixing agents
include elemental halogens or halogen containing compounds, e.g., chlorine,
iodine, fluorine
or bromine, alkali metal salts of halogens, e.g., halides, chlorine dioxide,
etc; iodide of a
heavy metal cation; ammonium iodide; an alkaline metal iodide;
etheylenediamine
dihydroiodide; hypochlorite ions (0cr such as Na0C1, Na0C12, Na0C13, Na0C14,
Ca(0C1)2,
NaC103,NaC102, etc.); vanadium oxytrichloride; Fenton's reagent; hypobromite
ions;
chlorine dioxine; iodate 103 (such as potassium iodate KI03 and sodium iodate
NaI03);
monopersulfate; alkali salts of peroxide like calcium hydroxide; peroxidases
that are capable
of oxidizing iodide; oxides, peroxides and mixed oxides, including oxyhalites,
their acids and
salts thereof In one embodiment, the fixing agent is selected from KMn04,
K2S208,
K2Cr02, and C12. In another embodiment, the fixing agent is selected from the
group of
persulfates. In yet another embodiment, the fixing agent is selected from the
group of
sodium perborate, potassium perborate, sodium carbonate perhydrate, potassium
peroxymonosulfate, sodium peroxocarbonate, sodium peroxodicarbonate, and
mixtures
thereof
[045] In one embodiment in addition to at least a fixing agent, a complexing
agent is
also added to the fixing agent to form strong complexes with the heavy metal
cations in the
9
CA 02872804 2014-11-05
WO 2013/173602
PCT/US2013/041386
produced fluids, e.g., Hg2+, extracting heavy metal complexes from the oil
phase and / or the
interface phase of the oil-water emulsion into the water phase by forming
water soluble
complexes. Examples of complexing agents to be added to an oxidizing fixing
agent include
hydrazines, sodium metabisulfite (Na2S205), sodium thiosulfate (Na2S203),
thiourea,
thiosulfates (such as Na2S203), ethylenediaminetetraacetic acid, and
combinations thereof
In one embodiment with the addition of a complexing agent to a fixing agent,
the fixing agent
is added to the pipeline first to oxidize the heavy metal, then the complexing
agent is
subsequently added to form a complex that is soluble in water. The complexing
agent can be
injected at intervals along the pipeline, or it can be subsequently added
after the introduction
lo of the fixing agent.
[046] The fixing agent can be added as in a solid form, or slurried /
dissolved in a
diluent, e.g., water, alcohol (such as methanol, ethanol, propanol), a light
hydrocarbon
diluent, or combinations thereof, in an effective amount for the treated
produced fluid to have
a mercury concentration of less than 100 ppbw. Effective amount means a
sufficient amount
for a molar ratio of fixing agent to heavy metals ranging from 1:1 to
100,000:1 in one
embodiment, 5:1 to 20,000:1 in a second embodiment; from 50:1 to 10,000:1 in a
third
embodiment; from 100:1 to 5,000:1 in a fourth embodiment; and from 150:1 to
500:1 in a
fifth embodiment. If a complexing agent is to be added to the pipeline
reaction to effectively
stabilize (forming complexes with) soluble heavy metals, e.g., mercury, in the
oil-water
mixture, the amount as molar ratio of complexing agent to soluble mercury
ranges from 2:1
to about 100,000:1 in one embodiment; from 5:1 to about 3,000:1 in a second
embodiment;
and from 20:1 to 500:1 in a third embodiment.
[047] The fixing agent can be injected into the pipeline or into a location
downhole
using conventional equipment known in the art such as metering pumps or jet
pumps. In one
embodiment with the addition of both an oxidant as a fixing agent and a
complexing agent,
the oxidant can be added to the pipeline and then mixed by a first static
mixer. The
complexing agent can be added and mixed with a second static mixer, then
allowed to enter
the pipeline for the reaction to go to sufficient conversion.
[048] Some of the fixing agents may require special handling, e.g., corrosion
resistant equipment and / or safety procedures. In one embodiment with the use
of sodium
hypochlorite as a fixing agent, the solution can be generated on-site with the
use of
commercially available electro-chlorination system, allowing the generation of
sodium
hypochlorite on-site for injection directly into the pipeline. In another
embodiment, the
pipeline reaction is allowed to take place in a section that provides
sufficient residence time
CA 02872804 2014-11-05
WO 2013/173602
PCT/US2013/041386
for the removal of the target heavy metals from the produced fluids. For
example, the
pipeline reaction section requiring special handling can run from the
production well to an
intermediate processing facility located a short distance from the production
well, for the
collection and separation of the treated produced fluids from waste water
containing heavy
metals and corrosive fixing agents. Additional aqueous dilution fluid can be
injected into the
pipeline for the transport of the treated produced fluids from the
intermediate processing
facility to the final destination, e.g., shipping terminal or FPSO.
[049] Figures Illustrating Embodiments: Reference will be made to the figures
to
further illustrate embodiments of the invention.
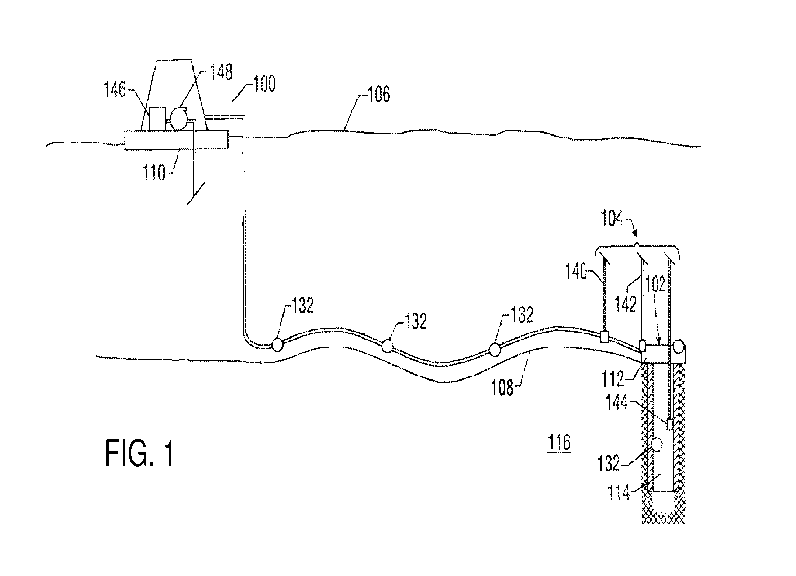
[050] FIG. 1 is a diagram of an exemplary floating production, storage and
offloading (FPSO) unit with a pipeline conditioning system for removing heavy
metals from
hydrocarbons such as oil and gas from one or more subsea wells 102. In one
embodiment, a
system 104 for dispensing at least a fixing agent into the pipeline deployed
in conjunction
with the facility 100 is located at a water surface 106. The dispensing system
104 services
one or more subsea production wells 102 residing in a seabed 108.
Conventionally, each well
102 includes a wellhead 112 and related equipment positioned over a wellbore
114 formed in
a subterranean formation 116. Production fluid is conveyed to a surface
collection facility
such as the FPSO 100 or separate structure, such as an intermediate collection
and / or
processing facility (not shown), via a pipeline 120. The fluid may be conveyed
to the surface
facility 100 in an untreated state or after being processed, at least
partially, by an intermediate
collection and / or processing facility (not shown). The line 120 extends
directly from the
wellhead 112 or from a manifold (not shown) that receives production flow from
a plurality
of wellheads 112.
[051] The flow line 120 includes a vertical section or riser 124 (not shown)
that
terminates at the FP SO 100. The dispensing system 104 continuously or
intermittently
injects at least a fixing agent into the flow line 120 or the well 102 for the
removal of heavy
metals.
[052] In one embodiment, the dispensing system 104 can be utilized with one or
more sensors 132 positioned along selected locations along the flow line 120
and the well
102. During production operations, the dispensing system 104 supplies (or
pumps) one or
more fixing agents to the flow line 120. This supply of fixing agents may be
continuous,
intermittent or actively controlled in response to sensor measurements. In one
mode of
controlled operation, the dispensing system 104 receives signals from the
sensors 132
regarding a parameter of interest relating to a characteristic of the produced
fluid, e.g.,
11
CA 02872804 2014-11-05
WO 2013/173602
PCT/US2013/041386
temperature, pressure, flow rate, amount of water, concentration of heavy
metals in the
produced fluids based on the formation of intermediate complexes, etc. Based
on the data
provided by the sensors 132, the dispensing system 104 determines the
appropriate type and /
or amount of fixing agents needed for the pipeline reactions to take place to
reduce the
concentration of mercury, arsenic, and the like.
[053] In embodiments, the dispensing system 104 can include one or more supply
lines 140, 142, 144 that dispense fixing agents, e.g., fixing agents such as
sodium
hypochlorite, etc., into the pipeline 120 at a location close to the wellhead,
or right at the
wellhead 102, in a manifold (not shown) or into a location downhole in the
wellbore 114,
respectively. The supply tank or tanks 146 and injection units 148 can be
positioned on the
surface facility 110 for continuous supply to the dispensing system 104. In
other
embodiments, one or more of the supply lines 140, 142, 144 can be inside or
along the
pipeline 120, for intermittent dispensing of fixing agents into the pipeline
120 for the removal
of heavy metals.
[054] While multiple dispensation points are shown in FIG. 1, it should be
understood that a single dispensation point may be adequate. Moreover, the
above-discussed
locations are merely representative of the locations at which the fixing
agents can be
dispensed into the production fluid for the pipeline reactions. The pipeline
120 can extend
on land between a production well at a remote location to a facility 100
located in a refinery
or a shipping terminal. Lastly, the dispensing system 104 is not limited to
the dispensing of
fixing agents for the removal of heavy metals. It can also be used for the
addition of other
process aids into the pipeline.
[055] In one embodiment as shown in FIG. 2, the pipeline reaction system
further
includes intermediate collection and / or processing facilities. As shown, oil
platform 2 is
connected to receive production fluid from a wellhead 4 via pipeline 10, and
pipeline 12 for
the supply of a dilution fluid needed for the removal of heavy metals. The
wellhead tree 4 is
connected by an output pipeline 6 to a first processing facility 8, which is
connected by
pipeline 10 and pipeline 12 to a second processing facility 14 situated
remotely therefrom.
The facilities 8 and 14 may be floating and/or tethered to the seabed. In one
embodiment,
the facilities contain one or more supply tanks to dispense fixing agents or
other process aids
into the pipeline 10. In another embodiment, the facility may include
equipment such as
gravity separator, plate separator, hydroclone, coalescer, centrifuge, filter,
etc., for the
collection and separation of crude oil from water containing heavy metals, and
the discharge
of waste water containing removed mercury into pipeline 86 to a reservoir
under wellhead 78.
12