Note: Descriptions are shown in the official language in which they were submitted.
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
INTEGRATED MANUFACTURING AND TEST PROCESS PLATFORM
CROSS-REFERENCE TO RELATED APPLICATIONS
The present specification relies on U.S. Provisional Patent Application Number
61/647,349, filed on May 15, 2012, for priority.
FIELD
The present invention relates to the integration of process and quality
elements into
product realization and/or qualification methods. More particularly, the
present invention
relates to a process platform fully integrated with manufacturing and quality
control
element(s) for ensuring quality control of the manufactured products.
BACKGROUND
During an assembly line manufacturing process, a product is tested for quality
at
multiple points. Usually the enforcement of quality control is performed
manually and hence,
is prone to human errors such as erroneous data transcription, missing steps
or performing
incorrect steps in a testing process, erroneous routing through a process
flow, using tools for
testing that are out of calibration, performing processes for which the user
is not trained,
among other mistakes. These errors may result in inaccurate test results being
obtained.
Further, conveying a product with inaccurate test results to a next
manufacturing stage may
lead to the production of products that suffer from overall inferior quality.
In the later stages of the product development cycle, qualification tests are
conducted
to verify design performance and establish limits of process induced
performance variability.
Creating and implementing these test protocols requires significant time and
resources.
Most of the manufacturing systems employing quality control processes suffer
from
drawbacks, such, as lack of integration of manufacturing management
element(s), lack of
enforcement of manufacturing management element(s) restrictions,
misinterpretation of
written instructions, difficult data analyses of manual records, inexact
process or design
change implementation, change implementation at a remote location requiring
local manual
intervention, and ill-defined traceability between out-of-tolerance calibrated
tools and
manufactured products.
Hence, there is a need for automated systems and methods for executing and
tracking
test results of product units being manufactured during multiple stages of
production.
1
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
There is also a need for a process platform that seamlessly integrates
production
processes with testing processes and allows data exchange between the
processes without
requiring manual intervention.
Further, there is a need for a process platform that replaces paper-based
manual
assembly and test protocols and supports additional processes such as data
definition,
assembly and test execution and report analysis for different types of
products spread across
different business units.
Yet further, there is a need for a reliable process and testing platform
providing a
reduction in time invested in managing in-bound, execution processes and out-
bound data.
Additionally, there is a need for integrating design verification testing and
production
process quality controls which re-use test protocols and routines initially
developed for
verification purposes.
SUMMARY
The present specification describes an automated process platform that
integrates an
automated quality control testing platform with a manufacturing/production
platform for
ensuring quality control of the manufactured products.
The automated platform of the present specification comprises a plurality of
assembly
and test sequences that are executed automatically at predefined stages of the
product
verification or manufacturing process being implemented by the testing or
production
platform. In an embodiment, the process platform may be manually configured by
using a
graphical user interface (GUI). The GUI enables data entry as well as
amendment of pre-
entered data or test sequences. The process platform may be used for
performing quality
control checks even at remote locations that are geographically separated from
a main data
station without requiring an operator for the same at the remote location.
In an embodiment, the present specification provides a new electronic work
environment (NEWE) technical user interface and process platform which
integrates with
existing design and execution platforms, which may, in one embodiment, include
design
verification, validation and/or qualification elements, to automate and assure
quality control
at a plurality of stages in a manufacturing process. Information such as
design specifications,
manufacturing site specifications, manufacturing process variability, testing
sequences and
manufacturing requirements are input into the NEWE. The NEWE electronically
processes
the input information and produces information such as test data, process
induced
performance variation, production results and manufacturing reports. The NEWE
also
2
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
enables equipment tracking throughout the manufacturing process and enforces
quality
control on the equipment used in the production process. In case of a defect
in any of the
equipment used, information regarding the products manufactured using the
defective
equipment may be obtained from an electronic database coupled with the NEWE
technical
user interface and process platform.
A user may interact with the NEWE database by using a comprehensive GUI which
displays required information regarding a manufacturing stage and results of
quality control
checks performed at the stage.
In one embodiment, the present specification describes a process platform for
integrating manufacturing and test process platforms, comprising: a) a
manufacturing
software subsystem; b) a manufacturing database, in data communication with
the
manufacturing software subsystem; c) a process design subsystem, in data
communication
with the manufacturing software subsystem; d) a new electronic work
environment technical
user interface, coupled to the manufacturing software subsystem; e) at least
one display; and
f) at least one processor to control the operation of the entire system and
its components.
In one embodiment, the manufacturing software subsystem comprises a database
that
contains manufacturing process software and core system software for
controlling
manufacturing processes and collecting manufacturing data.
In one embodiment, the manufacturing database is used for housing process
parameters for driving manufacturing processes and data collected from the
manufacturing
processes.
In one embodiment, the process design subsystem is used for storing test
sequences
and providing a series of instructions to the manufacturing software subsystem
to be executed
at one or more stages of the manufacturing process.
In another embodiment, the present specification describes a process platform
for
integrating manufacturing and test process platforms, comprising: a) a
manufacturing
software subsystem, which comprises a database that contains manufacturing
process
software and core system software for controlling manufacturing processes and
collecting
manufacturing data; b) a manufacturing database, in data communication with
the
manufacturing software subsystem, and used for housing process parameters for
driving
manufacturing processes and data collected from the manufacturing processes;
c) a process
design subsystem, in data communication with the manufacturing software
subsystem, used
for storing test sequences and providing a series of instructions to the
manufacturing software
subsystem, executed at one or more stages of the manufacturing process; d) an
electronic
3
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
work environment technical user interface, coupled to the manufacturing
software subsystem;
e) at least one display; and f) at least one processor to control the
operation of the entire
system and its components.
In one embodiment, the new electronic work environment technical user
interface
comprises a main interface coupled with the manufacturing software subsystem
and is
responsible for managing the launching of a manufacturing sequence,
synchronizing with the
manufacturing sequence, and displaying instructions or results for an
operator.
In one embodiment, data is exchanged between the manufacturing software
subsystem
and the manufacturing database via a database API.
In one embodiment, the process parameters and data collected that are housed
within
the manufacturing database include at least one of: product lines and
definitions, station
definitions, user rights definitions, process definitions, or test sequence
definitions.
In one embodiment, the process platform further comprises a traceability GUI
for
handling traceability and tracking definitions and versions of the
manufacturing software
subsystem, exchanged between the manufacturing database and manufacturing
software
subsystem.
In one embodiment, the process platform further comprises a publishing GUI for
enabling a user to define parameters of the process platform, wherein said
parameters include
at least one of: assembly details, test details, traceability requirements, or
tracking
requirements.
In one embodiment, the process platform further comprises an engineering tools
GUI
for enabling a user to define parameters within the manufacturing software
subsystem.
In one embodiment, the process platform further comprises a report viewer for
displaying production report results.
In one embodiment, the process platform further comprises a sequence authoring
GUI
for building and editing test sequences used within the process design
subsystem.
In one embodiment, the present specification describes a method for performing
automated testing of a product being manufactured at multiple sites of the
manufacturing
operation, said method being executed by a process platform having at least
one computing
device executing programmatic instructions stored in non-volatile memory,
comprising: a)
storing data indicative of a product line in a non-volatile memory; b) storing
data indicative
of a process for manufacturing said product line in a non-volatile memory; c)
storing data
indicative of a process for testing said product line in a non-volatile
memory, wherein said
processes coordinate data flows from a manufacturing software subsystem, a
manufacturing
4
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
database, and a process design subsystem; and d) automatically executing
quality control and
process flow procedures stored in a non-volatile memory at predefined stages
of a
manufacturing process being executed by the process platform.
In one embodiment, the manufacturing software subsystem comprises a database
that
contains manufacturing process software and core system software for
controlling
manufacturing processes and collecting manufacturing data.
In one embodiment, the manufacturing database houses process parameters for
driving manufacturing processes and data collected from the manufacturing
processes.
In one embodiment, the process design subsystem stores test sequences and
provides
a series of instructions to the manufacturing software subsystem that are
executed at one or
more stages of the manufacturing process.
In one embodiment, the method further includes tracking equipment used in the
manufacturing process by automatically monitoring quality parameters of the
equipment.
In one embodiment, the method further includes a means for integrating a main
production site with a plurality of remote production sites.
In one embodiment, the method further includes a means for implementing
quality
control procedures at a plurality of remote production sites integrated with a
main production
site.
In on embodiment, the present specification discloses a system for managing a
quality
control process, comprising: a plurality of programmatic instructions stored
in non-volatile
memory, wherein said programmatic instructions, when executed by a processor,
cause a first
graphical user interface to be displayed on a screen; receiving data
indicative of a quality level
of a component; a plurality of programmatic instructions stored in non-
volatile memory,
wherein said programmatic instructions, when executed by a processor,
determine if said
quality level meets a threshold level; a plurality of programmatic
instructions stored in non-
volatile memory, wherein said programmatic instructions, when executed by a
processor,
causes a second graphical user interface to be displayed on a screen, wherein
said second
graphical user interface comprises a rework option; and a plurality of
programmatic
instructions stored in non-volatile memory, wherein said programmatic
instructions, when
executed by a processor, causes said component to be disassembled based upon a
selection of
said rework option.
In another embodiment, the present specification discloses a system for
managing a
quality control process, comprising: a plurality of programmatic instructions
stored in non-
volatile memory, wherein said programmatic instructions, when executed by a
processor, cause
5
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
a first graphical user interface to be displayed on a screen; receiving data
indicative of a quality
level of a component, wherein said quality level comprises a qualitative
quality level and a
quantitative quality level; a plurality of programmatic instructions stored in
non-volatile
memory, wherein said programmatic instructions, when executed by a processor,
determine if
said quality level meets a threshold level; a plurality of programmatic
instructions stored in
non-volatile memory, wherein said programmatic instructions, when executed by
a processor,
causes a second graphical user interface to be displayed on a screen, wherein
said second
graphical user interface comprises a retry option, wherein said retry option
is only selectable or
displayed if said component is not assembled; and a plurality of programmatic
instructions
stored in non-volatile memory, wherein said programmatic instructions, when
executed by a
processor, causes said quality level checks to be performed again on said
component based
upon a selection of said retry option.
The aforementioned and other embodiments of the present shall be described in
greater depth in the drawings and detailed description provided below.
BRIEF DESCRIPTION OF THE DRAWINGS
These and other features and advantages of the present invention will be
further
appreciated, as they become better understood by reference to the detailed
description when
considered in connection with the accompanying drawings:
FIG. 1 illustrates a block diagram of a Process Platform, in accordance with
an
embodiment of the present invention;
FIG. 2 illustrates a New Electronic Work Environment (NEWE) Graphical User
Interface (GUI) context for the process platform described in FIG. 1 and in
accordance with an
embodiment of the present invention;
FIG. 3A illustrates an exemplary graphic of a NEWE GUI, in accordance with an
embodiment of the present invention;
FIG. 3B illustrates another exemplary graphic of a NEWE GUI, in accordance
with an
embodiment of the present invention;
FIG. 4A illustrates the stages of quality control conventionally implemented
conventionally during a product manufacturing process;
FIG. 4B illustrates the stages of quality control implemented during a product
manufacturing process, in accordance with an embodiment of the present
invention;
FIG. 5A illustrates the steps of a manufacturing process implementing
conventional
methods of quality control;
6
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
FIG. 5B illustrates the steps of a manufacturing process implementing the NEWE
process platform in accordance with an embodiment of the present invention;
FIG. 5C is a diagrammatic representation of input to and output from the NEWE
process platform, in accordance with an embodiment of the present invention;
FIG. 6 is a diagrammatic illustration of the integrated process platform
interfacing with
a main location and a plurality of remote locations, in accordance with an
embodiment of the
present invention; and
FIG. 7 is a block diagram illustrating a hardware configuration of the NEWE
technical
user interface in which a main site is integrated with a remote site, in
accordance with an
embodiment of the present invention.
DETAILED DESCRIPTION
The present specification provides a novel process platform for replacing
paper-based
manual test platforms, paper-based work instructions, travellers, and data
collection used for
manufacturing products. Additionally, the present specification enables the
close integration
of verification or qualification tests with the application and enforcement of
quality control
steps in the execution process. In various embodiments, the architecture of
the process
platform supports data definition, product assembly and test execution and
report analysis for
different types of products spread across a single or multiple business
unit(s). Further, in
various embodiments, operators interact with the process platform through a
touch-screen
based graphical user interface (GUI) and a barcode or RFID scanner. This
functionality
replaces operator interaction with work instructions and travellers of a paper
based system,
thereby reducing errors in quality testing and enforcing a strict quality
control.
The process platform described in the present specification addresses the
drawbacks of
a conventional assembly and test process used in conjunction with a
manufacturing process by
providing integration of quality and manufacturing management element(s), and
enforcing
quality and manufacturing management element(s) restrictions. The process
platform also
allows transfer of quality and manufacturing data and test sequences between a
data center and
one or more remote production sites, providing implementation of a change at a
remote
location without any local manual intervention.
The process platform of the present specification is coupled to at least one
display,
which displays information about each component within the system and the
functioning of
the system, by means of a GUI. The GUI also presents various menus that allow
users to
configure settings according to their requirements. The platform further
comprises at least
7
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
one processor to control the operation of the entire system and its
components. It should
further be appreciated that the at least one processor is capable of
processing programmatic
instructions, has a memory capable of storing programmatic instructions, and
employs
software comprised of a plurality of programmatic instructions for performing
the processes
described herein. In one embodiment, the at least one processor is a computing
device
capable of receiving, executing, and transmitting a plurality of programmatic
instructions
stored on a volatile or non-volatile computer readable medium.
The present specification is directed towards multiple embodiments. The
following
disclosure is provided in order to enable a person having ordinary skill in
the art to practice
the invention. Language used in this specification should not be interpreted
as a general
disavowal of any one specific embodiment or used to limit the claims beyond
the meaning of
the terms used therein. The general principles defined herein may be applied
to other
embodiments and applications without departing from the spirit and scope of
the invention.
Also, the terminology and phraseology used is for the purpose of describing
exemplary
embodiments and should not be considered limiting. Thus, the present invention
is to be
accorded the widest scope encompassing numerous alternatives, modifications
and
equivalents consistent with the principles and features disclosed. For purpose
of clarity,
details relating to technical material that is known in the technical fields
related to the
invention have not been described in detail so as not to unnecessarily obscure
the present
invention.
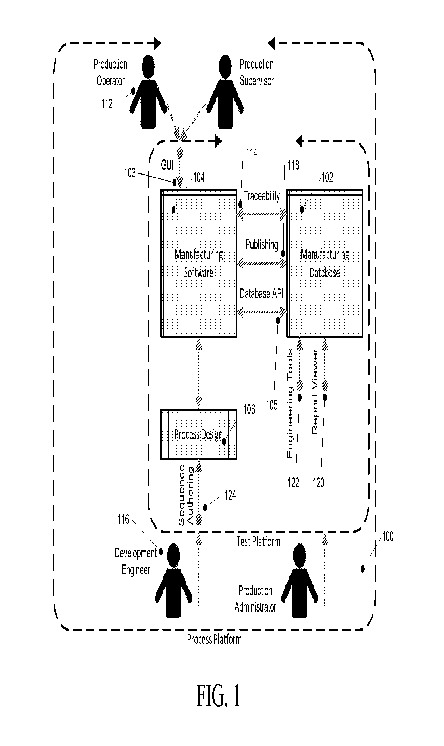
FIG. 1 illustrates a block diagram of the novel process platform, in
accordance with an
embodiment of the present invention. As illustrated, the Process Platform 100
is a test platform
that comprises a database subsystem 102, which in one embodiment is a
Manufacturing
Database, a Manufacturing Software subsystem 104 and a Process Design software
subsystem
106.
In an embodiment, the Manufacturing Database subsystem 102 is a database which
contains process parameters used to drive manufacturing processes as well as
data collected
from manufacturing processes. Thus, the Manufacturing Database 102 is a
clearinghouse for
storing information related to manufacturing processes. The Manufacturing
Software
subsystem 104 is a database which contains manufacturing process software;
graphics used in a
new electronic work environment technical user interface (NEWE GUI); and the
core system
software allowing the manufacturing processes to be controlled and
manufacturing data to be
collected. Communication between the two subsystems occurs by means of an
abstract
software communication layer. The Process Design subsystem 106 is a commercial
off-the-
8
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
shelf (COTS) programming environment, and communicates to the Manufacturing
Software
subsystem 104 by means of uploading its native format files.
A plurality of interfaces is provided to enable user interaction within the
subsystems of
the process platform 100 of the present invention. In one embodiment, sequence
authoring is
employed where data is manually input into a software application, such as a
word processing
or spreadsheet application. In one embodiment, a graphical user interface is
employed which
involves the exchange of XML files, driven by events such as commencing a
manufacturing
process, publishing a new process revision, etc. Specific embodiments of
interfaces are
described with greater detail below.
In an embodiment, at least one user interface of the Process Platform 100
comprises a
graphical user interface (GUI) 103, coupled to the Manufacturing Software
subsystem 104, and
is principally used by an operator 112 of the Process Platform 100. In one
embodiment, GUI
103 is a New Electronic Work Environment (NEWE) technical user interface which
is
responsible for managing the launching of a Manufacturing Sequence, which
includes both
assembly and test processes; synchronizing with the Manufacturing Sequence;
and displaying
instructions or results for the operator, and is described in greater detail
below with respect to
FIG. 2.
A Traceability API/GUI 114 is provided for handling traceability (association
of part
identification and part data with the product data); tracking (association of
unique part
identification with the product data) definitions; and creating versions of
the Manufacturing
Software subsystem 102 and is principally used by an engineer 116 for defining
the traceability
and the tracking of parts and products being manufactured and tested by the
Process Platform
100. The Traceability API/GUI 114 enables data communication between
Manufacturing
Software subsystem 104 and Manufacturing Database 102 and ensures that unique
part data is
linked with the product data to form a complete product data set representing
all quality
elements of the product.
A Publishing API/GUI 118 is provided for enabling an engineer to define
parameters of
the Process Platform 100, such as assembly details, test details, traceability
and tracking
requirements. It constitutes the action of launching the new process design
into operation,
accomplished by replacement of the existing parameters with new parameters by
defining data
exchange between the Manufacturing Software subsystem 104 and Manufacturing
Database
102 .
A database API 105 forms the abstract software communication layer through
which
data is exchanged; thus, via database API 105, process parameters are
collected from the
9
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
Manufacturing Database 102 and test results get stored back into the
Manufacturing Database
102 .
An Engineering Tools GUI 122 is provided for defining parameters within the
manufacturing database 102, typically used by an engineer.
A Report Viewer 120 is an application provided for displaying production
report results
that are generated by the Manufacturing Database 102.
A Sequence Authoring GUI 124 is provided to build and edit process design, for
process design subsystem 106, typically used by an engineer. Sequence
authoring involves
manual data input into a commercial off-the-shelf (COTS) software application.
Process
Design subsystem 106 provides a series of instructions to the manufacturing
software
subsystem 104, each containing a different set of data elements such as, but
not limited to, TQC
type, control limits, and Units of Measure.
Process Design subsystem 106 is in data communication with manufacturing
software
104, by means of manual uploading of its native format files.
In various embodiments, the manufacturing database 102 may be obtained as a
software
package from those that may be well-known to those of skill in the art. In an
embodiment the
Manufacturing Database 102 comprises definitions such as product lines and
definitions,
station definitions, user rights definitions, process definitions and test
sequences definitions that
are required for the functioning of the process platform 100.
In various embodiments, the process platform 100, and more specifically, the
Manufacturing Database 102 comprises a list of valid products within an
organization that are
manufactured and subsequently tested, along with their definitions. In an
embodiment, the valid
products are divided into various product lines, as may be appropriate to the
organization that is
implementing the process platform. By way of example only, in one embodiment,
the product
lines may include product lines such as, but not limited to anesthesia
delivery and ventilation
(AD&V), patient monitoring and connectivity (PM&C), and diagnostic cardiology
(DC), each
having their specific products associated with that product line.
In an embodiment, process definitions are provided by using a set of
structures and
rules. In an embodiment, processes are separated by line, group, and sub-
group. A sub-group
comprises valid product types that share the same process. In an embodiment,
the processes are
defined in a Sequence of Execution (SOE) module. The SOE module also comprises
operation
definitions. By way of example only, operations defined in the SOE may include
a "Ready"
and a "Rework" operation. Each operation defined in the SOE has various
characteristics which
provide enforcement of manufacturing process. In an embodiment, each operation
has a "Fail -
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
transition to the Rework operation" and "Pass - transition between operations"
which further
enforces the process flow defined in the SOE.
In an embodiment, a publishing repository, housed within Manufacturing
Database 102,
manages data to be deployed on any remote station where a product requiring
the
implementation of process(es) described herein is being manufactured. The
Manufacturing
Database 102 also contains test results and an analysis module which includes
formatted
information for producing one or more manufacturing and testing reports. The
traceability
module defines assembly rules that permit multiple products being manufactured
to be linked.
In one embodiment, the process platform of the present specification comprises
a New
Electronic Work Environment (NEWE) technical user interface. In various
embodiments, the
NEWE Graphical User Interface (GUI) is responsible for managing the launching
of a
Manufacturing Sequence, synchronizing with the Manufacturing Sequence, and
displaying
instructions or results for the operator. Design specifications may be input
into the NEWE
interface whereby work instructions and work orders may be electronically
produced thereby
eliminating a plurality of manual errors.
FIG. 2 illustrates an NEWE User Interface flow context, in accordance with an
embodiment of the present invention. As illustrated, the NEWE Graphical User
Interface (GUI)
200 is an executable available on the process platform of the present
invention, and is the main
interface used by operators. Thus, a process operator, as shown in FIG. 1, can
use the NEWE
GUI of the present invention to interface with manufacturing software
subsystem 104.
Referring back to FIG. 2, in an embodiment, the NEWE GUI 200 is deployed via a
publishing
module, which enables an engineer to define parameters of the Process
Platform, such as
assembly details, test details, traceability and tracking requirements.
In an embodiment, exemplary features of the NEWE GUI 200 include network
accessibility between the Manufacturing Software subsystem 224 and the Main
Station
Interface 201 on the station where it runs, which are easily usable with a
touch screen display
and a size of 1920 x 1080 pixels.
In an embodiment, a Main Station Interface 201 of the NEWE GUI 200 is coupled
with
a module for controlling execution of manufacturing sequences 202 and at least
one module
204 for message display 204, which allows for process platform messaging to
the user. Also
included are image display 206, video display 208, and display of .pdf files
210 which allow
graphic images to be retrieved from the test package and displayed to the
operator. In one
embodiment, the NEWE also includes a module 212 for displaying a menu; a
login/logout
module 214 which verifies operator identification and ensures that the system
is accessed with
11
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
permission only; an assembly module 216, which provides assembly instructions
for a
particular part; a rework routing module 218 which routes specific parts or
assemblies to a
rework function if the quality step or functional test does not meet
specification; a rework
assembly/disassembly module 220 allowing tracking of sub-assemblies or parts
during a
disassembly or repair process; and an auto test display module 222 which
initiates or displays
execution results from an automated testing sub-routine. In one particular
embodiment, all of
the above-mentioned modules are created through Manufacturing Software control
224.
FIG. 3A illustrates an exemplary instance of the NEWE GUI, in accordance with
an
embodiment of the present invention. As illustrated, section 302 of the GUI
provides a back
button 306, a next button 304 and a stop button 308. These buttons allow the
operator to go
back and forth through multiple sections of a production sequence. The back
button 306 may be
disabled while configuring an automated test sequence. In an embodiment, the
next button 304
is enabled only when an 'End Section' step is executed in the test sequence.
Upon pressing the
next button 304, the system executes a verification step to validate that the
result of any total
quality check (TQC) performed earlier is 'pass'. If the result is a "no pass",
a pop-up is
displayed to a user to indicate that the product is failing the test and ask
for confirmation if the
user wants to continue. If the user clicks on a 'Yes' button, the sequence
fails and the number
of retry attempts is logged. The maximum number of times a user is permitted
to retry is
predefined. In an embodiment, upon pressing the back button 306, a previous
set of TQC
values are saved in the Test Database but are not displayed on the NEWE GUI,
thereby
prompting a user to enter new values for the page. Further, in an embodiment,
if a component
has already been assembled, the user is not permitted to disassemble it by
using the NEWE
GUI. The disassembly may be performed by initiating a 'Rework' operation. The
stop button
308 allows the operator to stop the execution of a test sequence with a
'Terminated' which, in
an embodiment, is not considered as a 'Fail' state. Further, in an embodiment,
a lock button
310 allows an operator to stop and lock the execution of the NEWE GUI when the
operator has
to leave the station. The locked test sequence may then be unlocked only upon
entering a
predefined valid password.
In an embodiment, when a new product unit is scanned by using the process
platform of
the present invention, the NEWE GUI creates the unit record in the
Manufacturing Database,
and commences the execution of a predefined sequence corresponding to the
unit. As
illustrated the NEWE GUI may display an image (jpg file), a video (.avi file)
or a .pdf file in
section 312. Section 314 of the NEWE GUI displays information such as work
station
identification, operator identification, a serial number and a product number
of the unit being
12
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
tested, an operation identification code, a published package identification
and a page number.
In an embodiment, a Sales Order number and Sales Order options value are
obtained from an
operator and are also displayed in section 314. In an embodiment, the NEWE GUI
also
provides a display to allow assembly and disassembly of already assembled
subcomponents
during a 'Rework' operation.
In an embodiment of the present invention, five types of TQCs are predefined.
A
Qualitative TQC is displayed in section 316 with a description and two buttons
depicting a pass
and a fail result. A Quantitative TQC is displayed in section 318 with a
description and a text
box to enter a value of Numeric Limit Test result. A Tracked Component
Assembly TQC is
displayed in section 320 with a description as well a text box to scan a unit
(the bar code
containing the serial number and the part number). In an embodiment, the
result is 'Pass' if the
unit exists in the Manufacturing Database, is in a 'Ready' operation, is not
already assembled,
and if both a Top (parent) product/component and a child product/component of
the unit have
the same Sales Order number. A Non-Tracked Component Assembly TQC is displayed
in
section 322 for entering a serial number and the part number of the unit being
tested. A Remote
Instrument Calibration TQC is displayed in section 324 with a description and
a text box to
enter an Asset number of the unit being tested.
In various embodiments, a test sequences module can be launched from the NEWE
GUI using an Execution Control component (202 in Fig 2). In an embodiment, a
test sequence
may be an automated test sequence, containing calls to instruments and
algorithm or manual
instruction sequences, calling the NEWE GUI Control API. This system also
includes
interaction with the Manufacturing Database as it implies rules for the Test
Sequences creation.
FIG. 3B illustrates an instance of the NEWE GUI, in accordance with an
embodiment
of the present invention. The illustrated GUI 326 displays information
regarding total quality
control (TQC) 328 of a unit of product being manufactured. Thus, TQC
information that is
generally presented in the form of a spreadsheet is transferred to and
presented in the form of a
GUI 326. Buttons bearing graphical images 330 and 332 enable a user to confirm
or deny
presented information, respectively. For example, as illustrated, a user is
required to confirm if
a torque value is set at 3 N-m. The user may click on button 330 to confirm
the torque value or
button 332 to deny the torque setting. A user may enter information regarding
a voltage value
corresponding to the torque value by using text box 334 and a scan PCBA value
using text box
336. Buttons 338, 340 bearing a cross or a tick mark is provided adjacent the
text boxes 334,
336, respectively, to enable a user to obtain a fail or a pass test value.
13
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
FIG. 4A illustrates the stages of quality control conventionally implemented
during
product manufacturing. As illustrated, a manufacturing process is initiated
with the production
of a design document 402 which is usually produced by a Research and
Development (R&D)
team. As is known in the art, especially in cases of manufacturing medical
devices, a Design
History File (DHF) 404 is created with respect to each new design and this DHF
serves as a
means of defining quality control of the manufactured medical device at a
design stage. As a
next stage of product manufacture, materials predefined at the design stage
402 are obtained
from suppliers 408 who in turn obtain it from other suppliers 406 (suppliers'
supplier). A
quality check at this stage may be applied by using a past performance record
or a supplier's
score card 410 in conjunction with a quality management system (QMS) 412 which
may be
described as an organizational structure, and includes procedures, processes
and resources
needed to implement quality. Usually the QMS 412 is a manual and paper-based
system and is
hence prone to human errors. Once a first article is manufactured, a quality
check is performed
on the first article 414 as part of a regular manual inspection 416 by using
existing
paper/manual QMS 412. At a next stage where a larger volume production line,
418,
manufacturing of the product is performed, one or more paper/manual based
quality checks 420
are performed as part of the predefined production process 422. Further,
conventionally, the
paper or manual based QMS 412 is also used to implement quality control when
an out of box
failure 424 of the product occurs at a customer site 426 or a customers'
customer site 428.
Next, to deal with subsequent product complaints 430 made by users a
reliability/performance
process 432 which may be a part of the QMS 412 is conventionally implemented.
A business
collaboration program such as SharePoint 432 may also be used to aid, among
other aspects,
quality control management in the manufacturing process.
FIG. 4B illustrates the stages of quality control implemented during
manufacturing of a
product, in accordance with an embodiment of the present invention. As
illustrated, the process
platform provided by the present invention comprising NEWE 434 GUI as
described above is
implemented within the existing quality control framework illustrated in FIG.
4A. Hence, by
using the NEWE 434, the manual/paper based quality control checks are replaced
with
automated testing sequences and electronically defined process instructions,
process flow and
unit data collection. In various embodiments, initial design specifications
along with desired
quality checks and controls may be established at a commencement stage of the
manufacturing
process. Additionally, NEWE may be used to create or establish limits for the
quality checks
based upon process variation found during the verification tests found during
the design phase.
Further, the NEWE 434 may iteratively evolve and re-design the quality checks
based on input
14
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
received at each stage of the manufacturing process. Such inputs may be fed
manually or via
electronic means to the NEWE 434 for automating the quality control management
of the
manufacturing process. Further, the NEWE 434 enables integration of quality
control test
sequences with the entire design and manufacturing process.
FIG. 5A illustrates the steps of a manufacturing process implementing
conventional
methods of quality control. Product travellers 508 are created using
configuration tools 502
and work instructions 504 on a development platform such as Agile 506, which
comprises
software and methods based on iterative and incremental development where
requirements and
solutions evolve through collaboration between self-organizing, cross-
functional teams.
Conventionally, product travellers 508 contain a set of products that need to
be created and the
steps that are to be followed to create those products, and hard copies of
such travellers 508 are
passed from one workstation to another during execution of a manufacturing
process. The
product traveller 508 is created and iteratively revised upon receiving inputs
from the work
instruction 504, the Agile platform 506, and a work order 510 which, in turn,
is created as a
consequence of a sales order 512 developed as part of predefined manufacturing
requirements
514. Further, as illustrated a backflush 516 of raw materials, etc. obtained
from the work order
510 are re-processed by the ERP system 514 and incorporated in to the sales
order 512. A first
pass yield tracker 518 is used to monitor the quality of the manufactured
products for creating a
production quality report 520 by using manual and paper based tools. Further,
equipment
calibration 522, equipment tracking 524 and maintaining training records 526
are all performed
as independent activities with independent quality control procedures, which
are not integrated
with the entire manufacturing process.
FIG. 5B illustrates the steps of a manufacturing process implementing the NEWE
process platform, in accordance with an embodiment of the present invention.
As illustrated, a
NEWE process platform 528 interfaces with the Agile development platform 506,
the
manufacturing process, as well as calibration and training processes, thereby
producing
electronic work orders 530, product travellers 532, sales orders 533,
backflush 534, equipment
calibration 535, production quality reports 536, and training records 538. The
use of the NEWE
process platform 528 ensures automatic enforcement of quality standards as
quality control test
sequences are fully integrated with design as well as implementation stages of
the
manufacturing process. Design specifications may be input into the NEWE
process platform
528, whereby work instructions and work orders may be electronically produced
thereby
eliminating a plurality of manual errors. Further, by using the NEWE process
platform 528 of
the present invention, equipment used in the manufacturing process may be
tracked
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
electronically so that if there is a defect in the equipment, an exact number
and identification
codes of the products that have been manufactured by using the defective
equipment may be
made automatically.
FIG. 5C is a diagrammatic representation of the input and output of the NEWE
test
platform, in accordance with an embodiment of the present invention. As
illustrated, desired
product specification 540, desired station/site parameters 542, test software
544, and other
required manufacturing documentation 546 is input to the NEWE platform 555.
The input
information is processed electronically by the NEWE platform 555 to
automatically output test
data 548, production results 550 and manufacturing reports 552.
FIG. 6 is a diagrammatic illustration of the integrated process platform
interfacing with
a main location and a plurality of remote locations, in accordance with an
embodiment of the
present invention. As illustrated, a main location running a manufacturing
database center 636
with a plurality of remote manufacturing locations such as remote site 1 638
and remote site 2
640, by using the integrated process platform of the present invention. Each
remote site has at
least one process platform unit operating using the NEWE GUI.
FIG. 7 is a block diagram illustrating a hardware configuration of the NEWE
integrating a main site with a remote site, in accordance with an embodiment
of the present
invention. A main site 702 employing the NEWE test platform 704 comprises a
Microsoft SQL
standard cluster 706 comprising a data warehouse 708 and an online transaction
processing
(OLTP) module 710. The main site 702 also comprises an application server 712
for coupling a
plurality of fixed work stations 714 at the main site 702 with the Microsoft
SQL standard
cluster 706. The data warehouse 708 stores manufacturing data such as design
specification,
work instructions etc., and the OLTP module 710 processes the stored data. The
data
warehouse 708 is also coupled with a plurality of reporting stations 716 at
the main site 702 as
well as a plurality of reporting stations 718 deployed at a remote site 720.
The remote site 720
also comprises an OLTP server 722 coupled with an application server 724 which
in turn is
coupled with a plurality of fixed work stations 726. The application server
712 deployed at the
main site 702 is coupled with the application server 724 deployed at the
remote site 720,
thereby allowing automatic updating of any changes implemented locally at the
remote site 720
to the main site 702.
The present specification describes a novel process platform that replaces
paper-based
work instructions, travellers, and data collection used for manufacturing
products. The present
specification provides a method and platform for performing automated testing
of a product
being manufactured at multiple sites of the manufacturing operation. The
process platform of
16
CA 02873759 2014-11-14
WO 2013/173521
PCT/US2013/041247
the present invention may be deployed at multiple locations and be integrated
with existing
quality control systems. The process platform comprises a plurality of pre-
defined instructions
and is programmed to execute these instructions automatically at different
stages for
performing desired quality checks on the product being manufactured at
multiple
manufacturing stages.
The above examples are merely illustrative of the many applications of the
system of
present invention. Although only a few embodiments of the present invention
have been
described herein, it should be understood that the present invention might be
embodied in
many other specific forms without departing from the spirit or scope of the
invention.
Therefore, the present examples and embodiments are to be considered as
illustrative and not
restrictive, and the invention may be modified within the scope of the
appended claims.
17