Note: Descriptions are shown in the official language in which they were submitted.
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
METHODS OF IMPROVING THE YIELD OF 2,4-D RESISTANT CROP PLANTS
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to U. S. Provisional Application
No. 61/656,546,
filed on June 7, 2012, the disclosure of which is hereby expressly
incorporated by reference in
its entirety.
INCORPORATION BY REFERENCE OF MATERIAL SUBMITTED ELECTRONICALLY
[0002] Incorporated by reference in its entirety is a computer-readable
sequence listing
submitted concurrently herewith and identified as follows: one 11,342 byte
ASCII (text) file
named "72747_5T25.txt", created on May 13, 2013.
BACKGROUND OF THE INVENTION
[0001] Weeds can quickly deplete soil of valuable nutrients needed by crops
and other
desirable plants. There are many different types of herbicides presently used
for the control of
weeds. One extremely popular herbicide is glyphosate.
[0002] Crops, such as corn, soybeans, canola, cotton, sugar beets, wheat,
turf, and rice, have
been developed that are resistant to glyphosate. Thus, fields with actively
growing glyphosate
resistant corn, for example, can be sprayed to control weeds without
significantly damaging the
corn plants.
[0003] With the introduction of genetically engineered, glyphosate tolerant
crops (GTCs) in
the mid-1990's, growers were enabled with a simple, convenient, flexible, and
inexpensive tool
for controlling a wide spectrum of broadleaf and grass weeds unparalleled in
agriculture.
Consequently, producers were quick to adopt GTCs and in many instances abandon
many of
the accepted best agronomic practices such as crop rotation, herbicide mode of
action rotation,
tank mixing, incorporation of mechanical with chemical and cultural weed
control. Currently
glyphosate tolerant soybean, cotton, corn, and canola are commercially
available in the United
States and elsewhere in the Western Hemisphere. More GTCs (e.g., wheat, rice,
sugar beets,
turf, etc.) are poised for introduction pending global market acceptance. Many
other glyphosate
resistant species are in experimental to development stages (e.g., alfalfa,
sugar cane, sunflower,
beets, peas, carrot, cucumber, lettuce, onion, strawberry, tomato, and
tobacco; forestry species
like poplar and sweetgum; and horticultural species like marigold, petunia,
and begonias; see
"isb.vt.edu/cfdocs/fieldtestsl.cfm, 2005" website). Additionally, the cost of
glyphosate has
dropped dramatically in recent years to the point that few conventional weed
control programs
1
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
can effectively compete on price and performance with glyphosate GTC systems.
[0004] Glyphosate has been used successfully in burndown and other non-crop
areas for
total vegetation control for more than 15 years. In many instances, as with
GTCs, glyphosate
has been used 1-3 times per year for 3, 5, 10, up to 15 years in a row. These
circumstances
have led to an over-reliance on glyphosate and GTC technology and have placed
a heavy
selection pressure on native weed species for plants that are naturally more
tolerant to
glyphosate or which have developed a mechanism to resist glyphosate's
herbicidal activity.
[0005] Extensive use of glyphosate-only weed control programs is resulting
in the selection
of glyphosate-resistant weeds, and is selecting for the propagation of weed
species that are
inherently more tolerant to glyphosate than most target species (i.e., weed
shifts). (Ng et al.,
2003; Simarmata et al., 2003; Lorraine-Colwill et al., 2003; Sfiligoj, 2004;
Miller et al., 2003;
Heap, 2005; Murphy et al., 2002; Martin et al., 2002.) Although glyphosate has
been widely
used globally for more than 15 years, only a handful of weeds have been
reported to have
developed resistance to glyphosate (Heap, 2005); however, most of these have
been identified
in the past 3-5 years. Resistant weeds include both grass and broadleaf
species¨Lolium
rigidum, Lolium multiflorum, Eleusine indica, Ambrosia artemisiifolia, Conyza
canadensis,
Conyza bonariensis, and Plantago lanceolata. Additionally, weeds that had
previously not
been an agronomic problem prior to the wide use of GTCs are now becoming more
prevalent
and difficult to control in the context of GTCs, which comprise >80% of U.S.
cotton and
soybean acres and >20% of U.S. corn acres (Gianessi, 2005). These weed shifts
are occurring
predominantly with (but not exclusively) difficult-to-control broadleaf weeds.
Some examples
include Ipomoea, Amaranthus, Chenopodium, Taraxacum, and Commelina species.
[0006] In areas where growers are faced with glyphosate resistant weeds or
a shift to more
difficult-to-control weed species, growers can compensate for glyphosate's
weaknesses by tank
mixing or alternating with other herbicides that will control the missed
weeds. One popular and
efficacious tank mix partner for controlling broadleaf escapes in many
instances has been 2,4-
diclorophenoxyacetic acid (2,4-D). 2,4-D has been used agronomically and in
non-crop
situations for broad spectrum, broadleaf weed control for more than 60 years.
Individual cases
of more tolerant species have been reported, but 2,4-D remains one of the most
widely used
herbicides globally. A limitation to further use of 2,4-D is that its
selectivity in dicot crops like
soybean or cotton is very poor, and hence 2,4-D is not typically used on (and
generally not
near) sensitive dicot crops. Additionally, 2,4-D' s use in grass crops is
somewhat limited by the
nature of crop injury that can occur. 2,4-D in combination with glyphosate has
been used to
provide a more robust burndown treatment prior to planting no-till soybeans
and cotton;
2
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
however, due to these dicot species' sensitivity to 2,4-D, these burndown
treatments must occur
at least 14-30 days prior to planting (Agriliance, 2003).
[0007] 2,4-D is in the phenoxy acid class of herbicides, as is MCPA. 2,4-D
has been used
in many monocot crops (such as corn, wheat, and rice) for the selective
control of broadleaf
weeds without severely damaging the desired crop plants. 2,4-D is a synthetic
auxin derivative
that acts to deregulate normal cell-hormone homeostasis and impede balanced,
controlled
growth; however, the exact mode of action is still not known. Triclopyr and
fluroxypyr are
pyridyloxyacetic acid herbicides whose mode of action is as a synthetic auxin,
also.
[0008] These herbicides have different levels of selectivity on certain
plants (e.g., dicots are
more sensitive than grasses). Differential metabolism by different plants is
one explanation for
varying levels of selectivity. In general, plants metabolize 2,4-D slowly, so
varying plant
response to 2,4-D may be more likely explained by different activity at the
target site(s)
(WSSA, 2002). Plant metabolism of 2,4-D typically occurs via a two-phase
mechanism,
typically hydroxylation followed by conjugation with amino acids or glucose
(WSSA, 2002).
[0009] Over time, microbial populations have developed an alternative and
efficient
pathway for degradation of this particular xenobiotic, which results in the
complete
mineralization of 2,4-D. Successive applications of the herbicide select for
microbes that can
utilize the herbicide as a carbon source for growth, giving them a competitive
advantage in the
soil. For this reason, 2,4-D currently formulated has a relatively short soil
half-life, and no
significant carryover effects to subsequent crops are encountered. This adds
to the herbicidal
utility of 2,4-D.
[0010] One organism that has been extensively researched for its ability to
degrade 2,4-D is
Ralstonia eutropha (Streber et al., 1987). The gene that codes for the first
enzymatic step in the
mineralization pathway is tfdA. See U.S. Patent No. 6,153,401 and GENBANK Acc.
No.
M16730. TfdA catalyzes the conversion of 2,4-D acid to dichlorophenol (DCP)
via an cc-
ketoglutarate-dependent dioxygenase reaction (Smejkal et al., 2001). DCP has
little herbicidal
activity compared to 2,4-D. TfdA has been used in transgenic plants to impart
2,4-D resistance
in dicot plants (e.g., cotton and tobacco) normally sensitive to 2,4-D
(Streber et al. (1989),
Lyon et al. (1989), Lyon (1993), and U.S. Patent No. 5,608,147).
[0011] A large number of tfdA-type genes that encode proteins capable of
degrading 2,4-D
have been identified from the environment and deposited into the Genbank
database. Many
homologues are similar to tfdA (>85% amino acid identity) and have similar
enzymatic
properties to tfdA. However, there are a number of homologues that have a
significantly lower
identity to tfdA (25-50%), yet have the characteristic residues associated
with cc-ketoglutarate
3
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
dioxygenase Fe+2 dioxygenases. It is therefore not obvious what the substrate
specificities of
these divergent dioxygenases are.
[0012] One unique example with low homology to tfdA (31% amino acid
identity) is sdpA
from Delftia acidovorans (Kohler et al., 1999, Westendorf et al., 2002,
Westendorf et al.,
2003). This enzyme has been shown to catalyze the first step in (S)-
dichlorprop (and other (S)-
phenoxypropionic acids) as well as 2,4-D (a phenoxyacetic acid) mineralization
(Westendorf et
al., 2003). Transformation of this gene into plants, has not heretofore been
reported.
[0013] Development of new herbicide-tolerant crop (HTC) technologies has
been limited in
success due largely to the efficacy, low cost, and convenience of GTCs.
Consequently, a very
high rate of adoption for GTCs has occurred among producers. This created
little incentive for
developing new HTC technologies.
[0014] Aryloxyalkanoate chemical substructures are a common entity of many
commercialized herbicides including the phenoxyacetate auxins (such as 2,4-D
and
dichlorprop), pyridyloxyacetate auxins (such as fluroxypyr and triclopyr),
aryloxyphenoxypropionates (AOPP) acetyl-coenzyme A carboxylase (ACCase)
inhibitors (such
as haloxyfop, quizalofop, and diclofop), and 5-substituted phenoxyacetate
protoporphyrinogen
oxidase IX inhibitors (such as pyraflufen and flumiclorac). However, these
classes of
herbicides are all quite distinct, and no evidence exists in the current
literature for common
degradation pathways among these chemical classes. A multifunctional enzyme
for the
degradation of herbicides covering multiple modes of action has recently been
described (PCT
US/2005/014737; filed May 2, 2005.
SUMMARY OF THE INVENTION
[0015] This invention is related to methods for improving plant height
and/or yield of crop
plants which are resistant to herbicide 2,4-D by treating the plants with 2,4-
D at application
rates which are not harmful to the plants. In particular, provided is a method
using 2,4-D
application to increase yield of crop plants which express AAD-12 gene for 2,4-
D resistance.
This invention further relates to the use of 2,4-D for improving the yield of
crop plants which
are 2,4-D resistant. The method provided is of particular interest for the
treatment of crops
plants including maize, soybean, spring and winter oil seed rape (canola),
sugar beet, wheat,
sunflower, barley, and rice.
[0016] In some embodiments, the 2,4-D resistant crop plants are transgenic
crop plants
transformed with an aryloxyalkanoate dioxygenase (AAD). In a further
embodiment, the
aryloxyalkanoate dioxygenase (AAD) is AAD-1 or AAD-12. AAD-1 has been
previously
4
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
disclosed in US 2009/0093366 and AAD-12 has been previously disclosed in WO
2007/053482, the contents of which are incorporated by reference in their
entireties.
[0017] The yield-improving effect of the treatment of 2,4-D can be observed
at application
rates from 25 g ae/ha to 5000 g/ha, or 100 g ae/ha to 2500 g ae/ha, or in
particular, 1000 g ae/ha
to 2000 g ae/ha. In one embodiment, 1000 g ae/ha to 1500 g ae/ha of 2,4-D is
used. In another
embodiment, 2000 g ae/ha to 2500 g ae/ha is used. In addition, the yield-
improving effect of
the treatment of 2,4-D is D is particularly pronounced when 2,4-D is applied
in the 2- to 8- leaf
stage of the crop plants before flowering. However, the application rate
and/or leaf-stage of the
crop plant required vary as a function of the plants, their height and the
climate conditions.
[0018] The term increase in yield refers to that the plant yield up to 50%
or more. In one
embodiment, the increase in yield is at least 10%. In another embodiment, the
increase in yield
is at least 20%. In another embodiment, the increase in yield is from 10% to
60%. In another
embodiment, the increase in yield is from 20% to 50%. In another embodiment,
the increase in
yield is statistically significant. The growth-enhancing activity of 2,4-D to
2,4-D resistant crop
plants can be measured in field trials or pot trials. Herbicide having
different mode of action
are generally known to either have an adverse effect on yield or have no
effect on yield.
[0019] In one aspect, provided is a method of improving yield of 2,4-D
resistant crop
plants, comprising treating the plants with a stimulating amount of a
herbicide comprising an
aryloxyalkanoate moiety.
[0020] In one embodiment, the 2,4-D resistant crop plants are transgenic
plants transformed
with an aryloxyalkanoate dioxygenase (AAD). In a further embodiment, the
aryloxyalkanoate
dioxygenase (AAD) is AAD-1 or AAD-12. In another embodiment, the herbicide
comprising
an aryloxyalkanoate moiety is a phenoxy herbicide or phenoxyacetic herbicide.
In a further
embodiment, the herbicide comprising an aryloxyalkanoate moiety is 2,4-D. In a
further
embodiment, the 2,4-D comprises 2,4-D choline or 2,4-D dimethylamine (DMA).
[0021] In one embodiment, the transgenic plants transformed with an
aryloxyalkanoate
dioxygenase (AAD) are selected from cotton, soybean, and canola. In another
embodiment, the
treating is performed at least once at an application rate of 2,4-D as
employed also for weed
control. In another embodiment, the treating is performed twice at an
application rate of 2,4-D
as employed also for weed control. In a further embodiment, 2,4-D is applied
at the V3 and R2
growth stages of soybean with 2,4-D tolerance. In another embodiment, the
treating is
performed at least three times at an application rate of 2,4-D as employed
also for weed control.
In another embodiment, the herbicide comprising an aryloxyalkanoate moiety
reaches the 2,4-D
resistant crop plants via root absorption.
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
[0022] In another embodiment, the 2,4-D resistant crop plants are also
treated with a
herbicide different than 2,4-D for weed control. In a further embodiment, the
herbicide
different than 2,4-D is a phosphor-herbicide or aryloxyphenoxypropionic
herbicide. In a further
embodiment, the phosphor-herbicide comprises glyphosate, glufosinate, their
derivatives, or
combinations thereof. In a further embodiment, the phosphor-herbicide is in
form of
ammonium salt, isopropylammonium salt, isopropylamine salt, or potassium salt.
In another
embodiment, the phosphor-herbicide reaches the 2,4-D resistant crop plants via
root absorption.
In another embodiment, the aryloxyphenoxypropionic herbicide comprises
chlorazifop,
fenoxaprop, fluazifop, haloxyfop, quizalofop, their derivatives, or
combinations thereof. In a
further embodiment, the aryloxyphenoxypropionic herbicide reaches the 2,4-D
resistant crop
plants via root absorption.
[0023] In one embodiment, the 2,4-D resistant crop plants are treated at
least once with 25 g
ae/ha to 5000 g ae/ha 2,4-D. In another embodiment, the 2,4-D resistant crop
plants are treated
at least once with 100 g ae/ha to 2000 g ae/ha 2,4-D. In another embodiment,
the 2,4-D
resistant crop plants are treated at least once with 100 g ae/ha to 2500 g
ae/ha 2,4-D. In another
embodiment, the 2,4-D resistant crop plants are treated at least once with
1000 g ae/ha to 2000
g ae/ha 2,4-D. In a further embodiment, the 2,4-D comprises 2,4-D choline or
2,4-D
dimethylamine (DMA).
[0024] In one embodiment, a method of improving yield of 2,4-D resistant
crop plants is
provided. The method comprises
(a) transforming plant cells with a nucleic acid molecule comprising a
nucleotide
sequence encoding an aryloxyalkanoate dioxygenase (AAD);
(b) selecting transformed cells;
(c) regenerating the plants from the transformed cells; and
(d) treating the plants with a stimulating amount of a herbicide comprising an
aryloxyalkanoate moiety.
[0025] In one embodiment, the aryloxyalkanoate dioxygenase (AAD) is AAD-1
or AAD-
12. In another embodiment, the nucleic acid molecule comprises a selectable
marker which is
not an aryloxyalkanoate dioxygenase (AAD). In a further embodiment or
alternative
embodiment, the selectable marker is phosphinothricin acetyltransferase gene
(pat) or bialaphos
resistance gene (bar). In another embodiment, the nucleic acid molecule is
plant-optimized.
[0026] In another aspect, provided is the use of a herbicide comprising an
aryloxyalkanoate
moiety in the manufacture of transgenic plants with 2,4-D resistance with
increased yield as
compared to its non-transgenic parent plants. In one embodiment, the a
herbicide comprising
6
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
an aryloxyalkanoate moiety is 2,4-D. In a further embodiment, the 2,4-D is
applied at least
once with 25 g ae/ha to 5000 g/ha 2,4-D. In another embodiment, the 2,4-D is
applied at least
once with 100 g ae/ha to 2000 g ae/ha 2,4-D. In another embodiment, the 2,4-D
is applied at
least once with 100 g ae/ha to 2500 g ae/ha 2,4-D. In another embodiment, the
2,4-D is applied
at least once with 1000 g ae/ha to 2000 g ae/ha 2,4-D. In a further
embodiment, the 2,4-D
comprises 2,4-D choline or 2,4-D dimethylamine (DMA). In a further embodiment,
the 2,4-D
resistant crop plants are treated with 2,4-D at least two times before
flowering. In another
embodiment, the 2,4-D resistant crop plants are transgenic plants transformed
with an
aryloxyalkanoate dioxygenase (AAD). In a further embodiment, the
aryloxyalkanoate
dioxygenase (AAD) is AAD-1 or AAD-12.
BRIEF DESCRIPTION OF THE DRAWING AND SEQUENCES
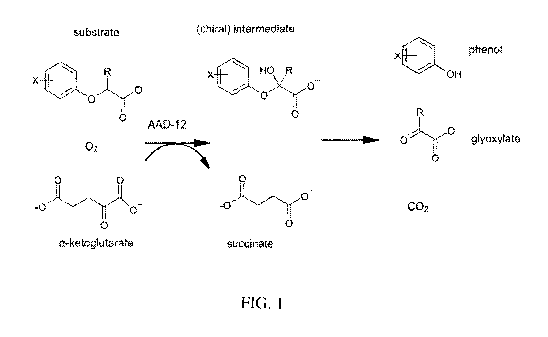
[0027] FIG. 1 illustrates the general chemical reaction that is catalyzed
by AAD-12
enzymes of the subject invention. FIG. 2 shows a representative map for
plasmid pDAB4468.
FIG. 3 shows a representative map for plasmid pDAS1740.
[0028] SEQ ID NO: 1 is the nucleotide sequence of AAD-12 from Delftia
acidovorans.
[0029] SEQ ID NO: 2 is the translated protein sequence encoded by SEQ ID
NO: 1.
[0030] SEQ ID NO: 3 is the plant optimized nucleotide sequence of AAD-12
(v1).
[0031] SEQ ID NO: 4 is the translated protein sequence encoded by SEQ ID
NO: 3.
[0032] SEQ ID NO: 5 is the E. coli optimized nucleotide sequence of AAD-12
(v2).
[0033] SEQ ID NO: 6 is the sequence of the M13 forward primer.
[0034] SEQ ID NO: 7 is the sequence of the M13 reverse primer.
[0035] SEQ ID NO: 8 is the sequence of the forward AAD-12 (v1) PTU primer.
[0036] SEQ ID NO: 9 is the sequence of the reverse AAD-12 (v1) PTU primer.
[0037] SEQ ID NO: 10 is the sequence of the forward AAD-12 (v1) coding PCR
primer.
[0038] SEQ ID NO: 11 is the sequence of the reverse AAD-12 (v1) coding PCR
primer.
[0039] SEQ ID NO: 12 shows the sequence of the "sdpacodF" AAD-12 (v1)
primer.
[0040] SEQ ID NO: 13 shows the sequence of the "sdpacodR" AAD-12 (v1)
primer.
[0041] SEQ ID NO: 14 shows the sequence of the "Ncol of Brady" primer.
[0042] SEQ ID NO: 15 shows the sequence of the "Sacl of Brady" primer.
DETAILED DESCRIPTION OF THE INVENTION
[0043] As used herein, the phrase "transformed" or "transformation" refers
to the
introduction of DNA into a cell. The phrases "transformant" or "transgenic"
refers to plant
7
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
cells, plants, and the like that have been transformed or have undergone a
transformation
procedure. The introduced DNA is usually in the form of a vector containing an
inserted piece
of DNA.
[0044] As used herein, the phrase "selectable marker" or "selectable marker
gene" refers to
a gene that is optionally used in plant transformation to, for example,
protect the plant cells
from a selective agent or provide resistance/tolerance to a selective agent.
Only those cells or
plants that receive a functional selectable marker are capable of dividing or
growing under
conditions having a selective agent. Examples of selective agents can include,
for example,
antibiotics, including spectinomycin, neomycin, kanamycin, paromomycin,
gentamicin, and
hygromycin. These selectable markers include gene for neomycin
phosphotransferase (npt II),
which expresses an enzyme conferring resistance to the antibiotic kanamycin,
and genes for the
related antibiotics neomycin, paromomycin, gentamicin, and G418, or the gene
for hygromycin
phosphotransferase (hpt), which expresses an enzyme conferring resistance to
hygromycin.
Other selectable marker genes can include genes encoding herbicide resistance
including Bar
(resistance against BASTA (glufosinate ammonium), or phosphinothricin (PPT)),
acetolactate
synthase (ALS, resistance against inhibitors such as sulfonylureas (SUs),
imidazolinones
(IMIs), triazolopyrimidines (TPs), pyrimidinyl oxybenzoates (POBs), and
sulfonylamino
carbonyl triazolinones that prevent the first step in the synthesis of the
branched-chain amino
acids), glyphosate, 2,4-D, and metal resistance or sensitivity. The phrase
"marker-positive"
refers to plants that have been transformed to include the selectable marker
gene.
[0045] Various selectable or detectable markers can be incorporated into
the chosen
expression vector to allow identification and selection of transformed plants,
or transformants.
Many methods are available to confirm the expression of selection markers in
transformed
plants, including for example DNA sequencing and PCR (polymerase chain
reaction), Southern
blotting, RNA blotting, immunological methods for detection of a protein
expressed from the
vector, e g., precipitated protein that mediates phosphinothricin resistance,
or other proteins
such as reporter genes 13-g1ucuronidase (GUS), luciferase, green fluorescent
protein (GFP),
DsRed,13-galactosidase, chloramphenicol acetyltransferase (CAT), alkaline
phosphatase, and
the like (See Sambrook, et al., Molecular Cloning: A Laboratory Manual, Third
Edition, Cold
Spring Harbor Press, N.Y., 2001, the content of which is incorporated herein
by reference in its
entirety).
[0046] Selectable marker genes are utilized for the selection of
transformed cells or tissues.
Selectable marker genes include genes encoding antibiotic resistance, such as
those encoding
neomycin phosphotransferase II (NEO) and hygromycin phosphotransferase (HPT)
as well as
8
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
genes conferring resistance to herbicidal compounds. Herbicide resistance
genes generally
code for a modified target protein insensitive to the herbicide or for an
enzyme that degrades or
detoxifies the herbicide in the plant before it can act. See DeBlock et al.
(1987) EMBO J.,
6:2513-2518; DeBlock et al. (1989) Plant Physiol., 91:691-704; Fromm et al.
(1990) 8:833-
839; Gordon-Kamm et al. (1990) 2:603-618). For example, resistance to
glyphosate or
sulfonylurea herbicides has been obtained by using genes coding for the mutant
target enzymes,
5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) and acetolactate synthase
(ALS).
Resistance to glufosinate ammonium, bromoxynil, and 2,4-dichlorophenoxyacetate
(2,4-D)
have been obtained by using bacterial genes encoding phosphinothricin
acetyltransferase, a
nitrilase, or a 2,4-dichlorophenoxyacetate monooxygenase, which detoxify the
respective
herbicides. Enzymes/genes for 2,4-D resistance have been previously disclosed
in US
2009/0093366 and WO 2007/053482, the contents of which are hereby incorporated
by
reference in their entireties.
[0047] Other herbicides can inhibit the growing point or meristem,
including imidazolinone
or sulfonylurea. Exemplary genes in this category code for mutant ALS and AHAS
enzyme as
described, for example, by Lee et al., EMBO J. 7:1241 (1988); and Miki et al.,
Theon. Appl.
Genet. 80:449 (1990), respectively.
[0048] Glyphosate resistance genes include mutant 5-enolpyruvylshikimate-3-
phosphate
synthase (EPSPs) genes (via the introduction of recombinant nucleic acids
and/or various forms
of in vivo mutagenesis of native EPSPs genes), aroA genes and glyphosate
acetyl transferase
(GAT) genes, respectively). Resistance genes for other phosphono compounds
include
glufosinate (phosphinothricin acetyl transferase (PAT) genes from Streptomyces
species,
including Streptomyces hygroscopicus and Streptomyces viridichromogenes), and
pyridinoxy
or phenoxy proprionic acids and cyclohexones (ACCase inhibitor-encoding
genes), See, for
example, U.S. Pat. No. 4,940,835 to Shah, et al. and U.S. Pat. No. 6,248,876
to Barry et al.,
which disclose nucleotide sequences of forms of EPSPs which can confer
glyphosate resistance
to a plant. A DNA molecule encoding a mutant aroA gene can be obtained under
ATCC
accession number 39256, and the nucleotide sequence of the mutant gene is
disclosed in U.S.
Pat. No. 4,769,061 to Comai, European patent application No. 0 333 033 to
Kumada et al., and
U.S. Pat. No. 4,975,374 to Goodman et al., disclosing nucleotide sequences of
glutamine
synthetase genes which confer resistance to herbicides such as L-
phosphinothricin. The
nucleotide sequence of a PAT gene is provided in European application No. 0
242 246 to
Leemans et al. Also DeGreef et al., Bio/Technology 7:61 (1989), describes the
production of
transgenic plants that express chimeric bar genes coding for PAT activity.
Exemplary of genes
9
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
conferring resistance to phenoxy proprionic acids and cyclohexones, including
sethoxydim and
haloxyfop, are the Accl-S1, Accl-S2 and Accl-S3 genes described by Marshall et
al., Theon.
Appl. Genet. 83:435 (1992). GAT genes capable of conferring glyphosate
resistance are
described in WO 2005012515 to Castle et al. Genes conferring resistance to 2,4-
D, fop and
pyridyloxy auxin herbicides are described in WO 2005107437 and U.S. patent
application Ser.
No. 11/587,893.
[0049] Other herbicides can inhibit photosynthesis, including triazine
(psbA and ls+ genes)
or benzonitrile (nitrilase gene). Przibila et al., Plant Cell 3:169 (1991),
describes the
transformation of Chlamydomonas with plasmids encoding mutant psbA genes.
Nucleotide
sequences for nitrilase genes are disclosed in U.S. Pat. No. 4,810,648 to
Stalker, and DNA
molecules containing these genes are available under ATCC Accession Nos.
53435, 67441, and
67442. Cloning and expression of DNA coding for a glutathione S-transferase is
described by
Hayes et al., Biochem. J. 285:173 (1992).
[0050] For purposes of the present invention, selectable marker genes
include, but are not
limited to genes encoding: neomycin phosphotransferase II (Fraley et al.
(1986) CRC Critical
Reviews in Plant Science, 4:1-25); cyanamide hydratase (Maier-Greiner et al.
(1991) Proc.
Natl. Acad. Sci. USA, 88:4250-4264); aspartate kinase; dihydrodipicolinate
synthase (Perl et al.
(1993) Bio/Technology, 11:715-718); tryptophan decarboxylase (Goddijn et al.
(1993) Plant
Mol. Bio., 22:907-912); dihydrodipicolinate synthase and desensitized
aspartade kinase (Perl et
al. (1993) Bio/Technology, 11:715-718); bar gene (Toki et al. (1992) Plant
Physiol., 100:1503-
1507 and Meagher et al. (1996) and Crop Sci., 36:1367); tryptophan
decarboxylase (Goddijn et
al. (1993) Plant Mol. Biol., 22:907-912); neomycin phosphotransferase (NEO)
(Southern et al.
(1982) J. Mol. Appl. Gen., 1:327; hygromycin phosphotransferase (HPT or HYG)
(Shimizu et
al. (1986) Mol. Cell Biol., 6:1074); dihydrofolate reductase (DHFR) (Kwok et
al. (1986) PNAS
USA 4552); phosphinothricin acetyltransferase (DeBlock et al. (1987) EMBO J.,
6:2513); 2,2-
dichloropropionic acid dehalogenase (Buchanan-Wollatron et al. (1989) J. Cell.
Biochem.
13D:330); acetohydroxyacid synthase (Anderson et al., U.S. Pat. No. 4,761,373;
Haughn et al.
(1988) Mol. Gen. Genet. 221:266); 5-enolpyruvyl-shikimate-phosphate synthase
(aroA) (Comai
et al. (1985) Nature 317:741); haloarylnitrilase (Stalker et al., published
PCT application
W087/04181); acetyl-coenzyme A carboxylase (Parker et al. (1990) Plant
Physiol. 92:1220);
dihydropteroate synthase (sul I) (Guerineau et al. (1990) Plant Mol. Biol.
15:127); and 32 kD
photosystem II polypeptide (psbA) (Hirschberg et al. (1983) Science,
222:1346).
[0051] Also included are genes encoding resistance to: chloramphenicol
(Herrera-Estrella et
al. (1983) EMBO J., 2:987-992); methotrexate (Herrera-Estrella et al. (1983)
Nature, 303:209-
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
213; Meijer et al. (1991) Plant Mol Bio., 16:807-820 (1991); hygromycin
(Waldron et al.
(1985) Plant Mol. Biol., 5:103-108; Zhijian et al. (1995) Plant Science,
108:219-227 and Meijer
et al. (1991) Plant Mol. Bio. 16:807-820); streptomycin (Jones et al. (1987)
Mol. Gen. Genet.,
210:86-91); spectinomycin (Bretagne-Sagnard et al. (1996) Transgenic Res.,
5:131-137);
bleomycin (Hille et al. (1986) Plant Mol. Biol., 7:171-176); sulfonamide
(Guerineau et al.
(1990) Plant Mol. Bio., 15:127-136); bromoxynil (Stalker et al. (1988)
Science, 242:419-423);
2,4-D (Streber et al. (1989) Bio/Technology, 7:811-816); glyphosate (Shaw et
al. (1986)
Science, 233:478-481); and phosphinothricin (DeBlock et al. (1987) EMBO J.,
6:2513-2518).
All references recited in the disclosure are hereby incorporated by reference
in their entireties
unless stated otherwise.
[0052] The above list of selectable marker and reporter genes are not meant
to be limiting.
Any reporter or selectable marker gene are encompassed by the present
invention. If necessary,
such genes can be sequenced by methods known in the art.
[0053] The reporter and selectable marker genes are synthesized for optimal
expression in
the plant. That is, the coding sequence of the gene has been modified to
enhance expression in
plants. The synthetic marker gene is designed to be expressed in plants at a
higher level
resulting in higher transformation efficiency. Methods for synthetic
optimization of genes are
available in the art. In fact, several genes have been optimized to increase
expression of the
gene product in plants.
[0054] The marker gene sequence can be optimized for expression in a
particular plant
species or alternatively can be modified for optimal expression in plant
families. The plant
preferred codons may be determined from the codons of highest frequency in the
proteins
expressed in the largest amount in the particular plant species of interest.
See, for example,
EPA 0359472; EPA 0385962; WO 91/16432; Perlak et al. (1991) Proc. Natl. Acad.
Sci. USA,
88:3324-3328; and Murray et al. (1989) Nucleic Acids Research, 17: 477-498;
U.S. Pat. No.
5,380,831; and U.S. Pat. No. 5,436,391, herein incorporated by reference. In
this manner, the
nucleotide sequences can be optimized for expression in any plant. It is
recognized that all or
any part of the gene sequence may be optimized or synthetic. That is, fully
optimized or
partially optimized sequences may also be used.
[0055] In addition, several transformation strategies utilizing the
Agrobacterium-mediated
transformation system have been developed. For example, the binary vector
strategy is based
on a two-plasmid system where T-DNA is in a different plasmid from the rest of
the Ti plasmid.
In a co-integration strategy, a small portion of the T-DNA is placed in the
same vector as the
foreign gene, which vector subsequently recombines with the Ti plasmid.
11
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
[0056] As used herein, the phrase "plant" includes dicotyledons plants and
monocotyledons
plants. Examples of dicotyledons plants include tobacco, Arabidopsis, soybean,
tomato,
papaya, canola, sunflower, cotton, alfalfa, potato, grapevine, pigeon pea,
pea, Brassica,
chickpea, sugar beet, rapeseed, watermelon, melon, pepper, peanut, pumpkin,
radish, spinach,
squash, broccoli, cabbage, carrot, cauliflower, celery, Chinese cabbage,
cucumber, eggplant,
and lettuce. Examples of monocotyledons plants include corn, rice, wheat,
sugarcane, barley,
rye, sorghum, orchids, bamboo, banana, cattails, lilies, oat, onion, millet,
and triticale.
[0057] The subject development of a 2,4-D resistance gene and subsequent
resistant crops
provides excellent options for controlling broadleaf, glyphosate-resistant (or
highly tolerant and
shifted) weed species for in-crop applications. 2,4-D is a broad-spectrum,
relatively
inexpensive, and robust broadleaf herbicide that would provide excellent
utility for growers if
greater crop tolerance could be provided in dicot and monocot crops alike. 2,4-
D-tolerant
transgenic dicot crops would also have greater flexibility in the timing and
rate of application.
An additional utility of the subject herbicide tolerance trait for 2,4-D is
its utility to prevent
damage to normally sensitive crops from 2,4-D drift, volatilization, inversion
(or other off-site
movement phenomenon), misapplication, vandalism, and the like. An additional
benefit of the
AAD-12 gene is that unlike all tfdA homologues characterized to date, AAD-12
is able to
degrade the pyridyloxyacetates auxins (e.g., triclopyr, fluoroxypyr) in
addition to achiral
phenoxy auxins (e.g., 2,4-D, MCPA, 4-chlorophenoxyacetic acid). See Table 1. A
general
illustration of the chemical reactions catalyzed by the subject AAD-12 enzyme
is shown in FIG.
1. (Addition of 0<sub>2</sub> is stereospecific; breakdown of intermediate to phenol
and glyoxylate is
spontaneous.) It should be understood that the chemical structures in FIG. 1
illustrate the
molecular backbones and that various R groups and the like (such as those
shown in Table 1)
are included but are not necessarily specifically illustrated in FIG. 1.
Multiple mixes of different
phenoxy auxin combinations have been used globally to address specific weed
spectra and
environmental conditions in various regions. Use of the AAD-12 gene in plants
affords
protection to a much wider spectrum of auxin herbicides, thereby increasing
the flexibility and
spectra of weeds that can be controlled.
[0058] A single gene (AAD-12) has now been identified which, when
genetically
engineered for expression in plants, has the properties to allow the use of
phenoxy auxin
herbicides in plants where inherent tolerance never existed or was not
sufficiently high to allow
use of these herbicides. Additionally, AAD-12 can provide protection in planta
to
pyridyloxyacetate herbicides where natural tolerance also was not sufficient
to allow selectivity,
expanding the potential utility of these herbicides. Plants containing AAD-12
alone now may be
12
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
treated sequentially or tank mixed with one, two, or a combination of several
phenoxy auxin
herbicides. The rate for each phenoxy auxin herbicide may range from 25 to
4000 g ae/ha, and
more typically from 100 to 2000 g ae/ha for the control of a broad spectrum of
dicot weeds.
Likewise, one, two, or a mixture of several pyridyloxyacetate auxin compounds
may be applied
to plants expressing AAD-12 with reduced risk of injury from said herbicides.
The rate for each
pyridyloxyacetate herbicide may range from 25 to 2000 g ae/ha, and more
typically from 35-
840 g ae/ha for the control of additional dicot weeds.
[0059] Glyphosate is used extensively because it controls a very wide
spectrum of broadleaf
and grass weed species. However, repeated use of glyphosate in GTCs and in non-
crop
applications has, and will continue to, select for weed shifts to naturally
more tolerant species
or glyphosate-resistant biotypes. Tankmix herbicide partners used at
efficacious rates that offer
control of the same species but having different modes of action is prescribed
by most herbicide
resistance management strategies as a method to delay the appearance of
resistant weeds.
Stacking AAD-12 with a glyphosate tolerance trait (and/or with other herbicide-
tolerance traits)
could provide a mechanism to allow for the control of glyphosate resistant
dicot weed species
in GTCs by enabling the use of glyphosate, phenoxy auxin(s) (e.g., 2,4-D) and
pyridyloxyacetates auxin herbicides (e.g., triclopyr)--selectively in the same
crop. Applications
of these herbicides could be simultaneously in a tank mixture comprising two
or more
herbicides of different modes of action; individual applications of single
herbicide composition
in sequential applications as pre-plant, preemergence, or postemergence and
split timing of
applications ranging from approximately 2 hours to approximately 3 months; or,
alternatively,
any combination of any number of herbicides representing each chemical class
can be applied
at any timing within about 7 months of planting the crop up to harvest of the
crop (or the
preharvest interval for the individual herbicide, whichever is shortest).
[0060] It is important to have flexibility in controlling a broad spectrum
of grass and
broadleaf weeds in terms of timing of application, rate of individual
herbicides, and the ability
to control difficult or resistant weeds. Glyphosate applications in a crop
with a glyphosate
resistance gene/AAD-12 stack could range from about 250-2500 g ae/ha; phenoxy
auxin
herbicide(s) (one or more) could be applied from about 25-4000 g ae/ha; and
pyridyloxyacetates auxin herbicide(s) (one or more) could be applied from 25-
2000 g ae/ha.
The optimal combination(s) and timing of these application(s) will depend on
the particular
situation, species, and environment, and will be best determined by a person
skilled in the art of
weed control and having the benefit of the subject disclosure.
[0061] Plantlets are typically resistant throughout the entire growing
cycle. Transformed
13
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
plants will typically be resistant to new herbicide application at any time
the gene is expressed.
Tolerance is shown herein to 2,4-D across the life cycle using the
constitutive promoters tested
thus far (primarily CsVMV and AtUbi 10). One would typically expect this, but
it is an
improvement upon other non-metabolic activities where tolerance can be
significantly impacted
by the reduced expression of a site of action mechanism of resistance, for
example. One
example is Roundup Ready cotton, where the plants were tolerant if sprayed
early, but if
sprayed too late the glyphosate concentrated in the meristems (because it is
not metabolized and
is translocated); viral promoters Monsanto used are not well expressed in the
flowers. The
subject invention provides an improvement in these regards.
[0062] Herbicide formulations (e.g., ester, acid, or salt formulation; or
soluble concentrate,
emulsifiable concentrate, or soluble liquid) and tankmix additives (e.g.,
adjuvants, surfactants,
drift retardants, or compatibility agents) can significantly affect weed
control from a given
herbicide or combination of one or more herbicides. Any combination of these
with any of the
aforementioned herbicide chemistries is within the scope of this invention.
[0063] One skilled in the art would also see the benefit of combining two
or more modes of
action for increasing the spectrum of weeds controlled and/or for the control
of naturally more
tolerant or resistant weed species. This could also extend to chemistries for
which herbicide
tolerance was enabled in crops through human involvement (either
transgenically or non-
transgenically) beyond GTCs. Indeed, traits encoding glyphosate resistance
(e.g., resistant plant
or bacterial EPSPS, glyphosate oxidoreductase (GOX), GAT), glufosinate
resistance (e.g., Pat,
bar), acetolactate synthase (ALS)-inhibiting herbicide resistance (e.g.,
imidazolinone,
sulfonylurea, triazolopyrimidine sulfonanilide, pyrmidinylthiobenzoates, and
other
chemistries=AHAS, Csrl, SurA, et al.), bromoxynil resistance (e.g., Bxn),
resistance to
inhibitors of HPPD (4-hydroxlphenyl-pyruvate-dioxygenase) enzyme, resistance
to inhibitors of
phytoene desaturase (PDS), resistance to photosystem II inhibiting herbicides
(e.g., psbA),
resistance to photosystem I inhibiting herbicides, resistance to
protoporphyrinogen oxidase IX
(PPO)-inhibiting herbicides (e.g., PPO-1), resistance to phenylurea herbicides
(e.g., CYP76B1),
dicamba-degrading enzymes (see, e.g., US 20030135879), and others could be
stacked alone or
in multiple combinations to provide the ability to effectively control or
prevent weed shifts
and/or resistance to any herbicide of the aforementioned classes. In vivo
modified EPSPS can
be used in some preferred embodiments, as well as Class I, Class II, and Class
III glyphosate
resistance genes.
[0064] Regarding additional herbicides, some additional preferred ALS
inhibitors include
but are not limited to the sulfonylureas (such as chlorsulfuron, halosulfuron,
nicosulfuron,
14
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
sulfometuron, sulfosulfuron, trifloxysulfuron), imidazoloninones (such as
imazamox,
imazethapyr, imazaquin), triazolopyrimidine sulfonanilides (such as
cloransulam-methyl,
diclosulam, florasulam, flumetsulam, metosulam, and penoxsulam),
pyrimidinylthiobenzoates
(such as bispyribac and pyrithiobac), and flucarbazone. Some preferred HPPD
inhibitors
include but are not limited to mesotrione, isoxaflutole, and sulcotrione. Some
preferred PPO
inhibitors include but are not limited to flumiclorac, flumioxazin, flufenpyr,
pyraflufen,
fluthiacet, butafenacil, carfentrazone, sulfentrazone, and the diphenylethers
(such as acifluorfen,
fomesafen, lactofen, and oxyfluorfen).
[0065] Additionally, AAD-12 alone or stacked with one or more additional
HTC traits can
be stacked with one or more additional input (e.g., insect resistance, fungal
resistance, or stress
tolerance, et al.) or output (e.g., increased yield, improved oil profile,
improved fiber quality, et
al.) traits. Thus, the subject invention can be used to provide a complete
agronomic package of
improved crop quality with the ability to flexibly and cost effectively
control any number of
agronomic pests.
[0066] The subject invention relates in part to the identification of an
enzyme that is not
only able to degrade 2,4-D, but also surprisingly possesses novel properties,
which distinguish
the enzyme of the subject invention from previously known tfdA proteins, for
example. Even
though this enzyme has very low homology to tfdA, the genes of the subject
invention can still
be generally classified in the same overall family of a-ketoglutarate-
dependent dioxygenases.
This family of proteins is characterized by three conserved histidine residues
in a
"HX(D/E)X23-26(T/S)X114-183HX10-13R" motif which comprises the active site.
The
histidines coordinate Fe+2 ion in the active site that is essential for
catalytic activity (Hogan et
al., 2000). The preliminary in vitro expression experiments discussed herein
were tailored to
help select for novel attributes. These experiments also indicate the AAD-12
enzyme is unique
from another disparate enzyme of the same class, disclosed in a previously
filed patent
application (PCT US/2005/014737; filed May 2, 2005). The AAD-1 enzyme of that
application
shares only about 25% sequence identity with the subject AAD-12 protein.
[0067] More specifically, the subject invention relates in part to the use
of an enzyme that is
not only capable of degrading 2,4-D, but also pyridyloxyacetate herbicides. No
a-
ketoglutarate-dependent dioxygenase enzyme has previously been reported to
have the ability
to degrade herbicides of different chemical classes and modes of action.
Preferred enzymes and
genes for use according to the subject invention are referred to herein as AAD-
12
(AryloxyAlkanoate Dioxygenase) genes and proteins.
[0068] The subject proteins tested positive for 2,4-D conversion to 2,4-
dichlorophenol
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
("DCP"; herbicidally inactive) in analytical assays. Partially purified
proteins of the subject
invention can rapidly convert 2,4-D to DCP in vitro. An additional advantage
that AAD-12
transformed plants provide is that parent herbicide(s) are metabolized to
inactive forms, thereby
reducing the potential for harvesting herbicidal residues in grain or stover.
[0069] The subject invention also includes methods of controlling weeds
wherein said
methods comprise applying a pyridyloxyacetate and/or a phenoxy auxin herbicide
to plants
comprising an AAD-12 gene.
[0070] In light of these discoveries, novel plants that comprise a
polynucleotide encoding
this type of enzyme are now provided. Heretofore, there was no motivation to
produce such
plants, and there was no expectation that such plants could effectively
produce this enzyme to
render the plants resistant to not only phenoxy acid herbicides (such as 2,4-
D) but also
pyridyloxyacetate herbicides. Thus, the subject invention provides many
advantages that were
not heretofore thought to be possible in the art.
[0071] Publicly available strains (deposited in culture collections like
ATCC or DSMZ) can
be acquired and screened, using techniques disclosed herein, for novel genes.
Sequences
disclosed herein can be used to amplify and clone the homologous genes into a
recombinant
expression system for further screening and testing according to the subject
invention.
[0072] As discussed above in the Background section, one organism that has
been
extensively researched for its ability to degrade 2,4-D is Ralstonia eutropha
(Streber et al.,
1987). The gene that codes for the first enzyme in the degradation pathway is
tfdA. See U.S.
Pat. No. 6,153,401 and GENBANK Acc. No. M16730. TfdA catalyzes the conversion
of 2,4-D
acid to herbicidally inactive DCP via an a-ketoglutarate-dependent dioxygenase
reaction
(Smejkal et al., 2001). TfdA has been used in transgenic plants to impart 2,4-
D resistance in
dicot plants (e.g., cotton and tobacco) normally sensitive to 2,4-D (Streber
et al., 1989; Lyon et
al., 1989; Lyon et al., 1993). A large number of tfdA-type genes that encode
proteins capable
of degrading 2,4-D have been identified from the environment and deposited
into the Genbank
database. Many homologues are quite similar to tfdA (>85% amino acid identity)
and have
similar enzymatic properties to tfdA. However, a small collection of a-
ketoglutarate-dependent
dioxygenase homologues are presently identified that have a low level of
homology to tfdA.
[0073] The subject invention relates in part to surprising discoveries of
new uses for and
functions of a distantly related enzyme, sdpA, from Delftia acidivorans
(Westendorf et al.,
2002, 2003) with low homology to tfdA (31% amino acid identity). This a-
ketoglutarate-
dependent dioxygenase enzyme purified in its native form had previously been
shown to
degrade 2,4-D and S-dichlorprop (Westendorf et al., 2002 and 2003). However,
no a-
16
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
ketoglutarate-dependent dioxygenase enzyme has previously been reported to
have the ability
to degrade herbicides of pyridyloxyacetate chemical class. SdpA has never been
expressed in
plants, nor was there any motivation to do so in part because development of
new HTC
technologies has been limited due largely to the efficacy, low cost, and
convenience of GTCs
(Devine, 2005).
[0074] In light of the novel activity, proteins and genes of the subject
invention are referred
to herein as AAD-12 proteins and genes. AAD-12 was presently confirmed to
degrade a variety
of phenoxyacetate auxin herbicides in vitro. However, this enzyme, as reported
for the first
time herein, was surprisingly found to also be capable of degrading additional
substrates of the
class of aryloxyalkanoate molecules. Substrates of significant agronomic
importance include
the pyridyloxyacetate auxin herbicides. This highly novel discovery is the
basis of significant
Herbicide Tolerant Crop (HTC) and selectable marker trait opportunities. This
enzyme is
unique in its ability to deliver herbicide degradative activity to a range of
broad spectrum
broadleaf herbicides (phenoxyacetate and pyridyloxyacetate auxins).
[0075] Thus, the subject invention relates in part to the degradation of
2,4-
dichlorophenoxyacetic acid, other phenoxyacetic auxin herbicides, and
pyridyloxyacetate
herbicides by a recombinantly expressed aryloxyalkanoate dioxygenase enzyme
(AAD-12).
This invention also relates in part to identification and uses of genes
encoding an
aryloxyalkanoate dioxygenase degrading enzyme (AAD-12) capable of degrading
phenoxy
and/or pyridyloxy auxin herbicides.
[0076] The subject enzyme enables transgenic expression resulting in
tolerance to
combinations of herbicides that would control nearly all broadleaf weeds. AAD-
12 can serve
as an excellent herbicide tolerant crop (HTC) trait to stack with other HTC
traits [e.g.,
glyphosate resistance, glufosinate resistance, ALS-inhibitor (e.g.,
imidazolinone, sulfonylurea,
triazolopyrimidine sulfonanilide) resistance, bromoxynil resistance, HPPD-
inhibitor resistance,
PPO-inhibitor resistance, et al.], and insect resistance traits (Cry1F,
CrylAb, Cry 34/45, other
Bt. Proteins, or insecticidal proteins of a non-Bacillis origin, et al.) for
example. Additionally,
AAD-12 can serve as a selectable marker to aid in selection of primary
transformants of plants
genetically engineered with a second gene or group of genes.
[0077] In addition, the subject microbial gene has been redesigned such
that the protein is
encoded by codons having a bias toward both monocot and dicot plant usage
(hemicot).
Arabidopsis, corn, tobacco, cotton, soybean, canola, and rice have been
transformed with AAD-
12-containing constructs and have demonstrated high levels of resistance to
both the phenoxy
and pyridyloxy auxin herbicides. Thus, the subject invention also relates to
"plant optimized"
17
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
genes that encode proteins of the subject invention.
[0078] Oxyalkanoate groups are useful for introducing a stable acid
functionality into
herbicides. The acidic group can impart phloem mobility by "acid trapping," a
desirable
attribute for herbicide action and therefore could be incorporated into new
herbicides for
mobility purposes. Aspects of the subject invention also provide a mechanism
of creating
HTCs. There exist many potential commercial and experimental herbicides that
can serve as
substrates for AAD-12. Thus, the use of the subject genes can also result in
herbicide tolerance
to those other herbicides as well.
[0079] HTC traits of the subject invention can be used in novel
combinations with other
HTC traits (including but not limited to glyphosate tolerance). These
combinations of traits
give rise to novel methods of controlling weed (and like) species, due to the
newly acquired
resistance or inherent tolerance to herbicides (e.g., glyphosate). Thus, in
addition to the HTC
traits, novel methods for controlling weeds using herbicides, for which
herbicide tolerance was
created by said enzyme in transgenic crops, are within the scope of the
invention.
[0080] This invention can be applied in the context of commercializing a
2,4-D resistance
trait stacked with current glyphosate resistance traits in soybeans, for
example. Thus, this
invention provides a tool to combat broadleaf weed species shifts and/or
selection of herbicide
resistant broadleaf weeds, which culminates from extremely high reliance by
growers on
glyphosate for weed control with various crops.
[0081] The transgenic expression of the subject AAD-12 genes is exemplified
in, for
example, Arabidopsis, tobacco, soybean, cotton, rice, corn and canola.
Soybeans are a preferred
crop for transformation according to the subject invention. However, this
invention can be
utilized in multiple other monocot (such as pasture grasses or turf grass) and
dicot crops like
alfalfa, clover, tree species, et al. Likewise, 2,4-D (or other AAD-12-
substrates) can be more
positively utilized in grass crops where tolerance is moderate, and increased
tolerance via this
trait would provide growers the opportunity to use these herbicides at more
efficacious rates
and over a wider application timing without the risk of crop injury.
[0082] Still further, the subject invention provides a single gene that can
provide resistance
to herbicides that control broadleaf weed. This gene may be utilized in
multiple crops to enable
the use of a broad spectrum herbicide combination. The subject invention can
also control
weeds resistant to current chemicals, and aids in the control of shifting weed
spectra resulting
from current agronomic practices. The subject AAD-12 can also be used in
efforts to
effectively detoxify additional herbicide substrates to non-herbicidal forms.
Thus, the subject
invention provides for the development of additional HTC traits and/or
selectable marker
18
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
technology.
[0083] Separate from, or in addition to, using the subject genes to produce
HTCs, the
subject genes can also be used as selectable markers for successfully
selecting transformants in
cell cultures, greenhouses, and in the field. There is high inherent value for
the subject genes
simply as a selectable marker for biotechnology projects. The promiscuity of
AAD-12 for other
aryloxyalkanoate auxinic herbicides provides many opportunities to utilize
this gene for HTC
and/or selectable marker purposes.
[0084] One cannot easily discuss the term "resistance" and not use the verb
"tolerate" or the
adjective "tolerant." The industry has spent innumerable hours debating
Herbicide Tolerant
Crops (HTC) versus Herbicide Resistant Crops (HRC). HTC is a preferred term in
the industry.
However, the official Weed Science Society of America definition of resistance
is "the
inherited ability of a plant to survive and reproduce following exposure to a
dose of herbicide
normally lethal to the wild type. In a plant, resistance may be naturally
occurring or induced by
such techniques as genetic engineering or selection of variants produced by
tissue culture or
mutagenesis." As used herein unless otherwise indicated, herbicide
"resistance" is heritable
and allows a plant to grow and reproduce in the presence of a typical
herbicidally effective
treatment by a herbicide for a given plant, as suggested by the current
edition of The Herbicide
Handbook as of the filing of the subject disclosure. As is recognized by those
skilled in the art,
a plant may still be considered "resistant" even though some degree of plant
injury from
herbicidal exposure is apparent. As used herein, the term "tolerance" is
broader than the term
"resistance," and includes "resistance" as defined herein, as well an improved
capacity of a
particular plant to withstand the various degrees of herbicidally induced
injury that typically
result in wild-type plants of the same genotype at the same herbicidal dose.
[0085] Transfer of the functional activity to plant or bacterial systems
can involve a nucleic
acid sequence, encoding the amino acid sequence for a protein of the subject
invention,
integrated into a protein expression vector appropriate to the host in which
the vector will
reside. One way to obtain a nucleic acid sequence encoding a protein with
functional activity is
to isolate the native genetic material from the bacterial species which
produce the protein of
interest, using information deduced from the protein's amino acid sequence, as
disclosed herein.
The native sequences can be optimized for expression in plants, for example,
as discussed in
more detail below. An optimized polynucleotide can also be designed based on
the protein
sequence.
[0086] There are a number of methods for obtaining proteins for use
according to the
subject invention. For example, antibodies to the proteins disclosed herein
can be used to
19
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
identify and isolate other proteins from a mixture of proteins. Specifically,
antibodies may be
raised to the portions of the proteins that are most conserved or most
distinct, as compared to
other related proteins. These antibodies can then be used to specifically
identify equivalent
proteins with the characteristic activity by immunoprecipitation, enzyme
linked immunosorbent
assay (ELISA), or immuno-blotting. Antibodies to the proteins disclosed
herein, or to
equivalent proteins, or to fragments of these proteins, can be readily
prepared using standard
procedures. Such antibodies are an aspect of the subject invention. Antibodies
of the subject
invention include monoclonal and polyclonal antibodies, preferably produced in
response to an
exemplified or suggested protein.
[0087] One skilled in the art would readily recognize that proteins (and
genes) of the
subject invention can be obtained from a variety of sources. Since entire
herbicide degradation
operons are known to be encoded on transposable elements such as plasmids, as
well as
genomically integrated, proteins of the subject invention can be obtained from
a wide variety of
microorganisms, for example, including recombinant and/or wild-type bacteria.
[0088] Mutants of bacterial isolates can be made by procedures that are
well known in the
art. For example, asporogenous mutants can be obtained through ethylmethane
sulfonate
(EMS) mutagenesis of an isolate. The mutant strains can also be made using
ultraviolet light
and nitrosoguanidine by procedures well known in the art.
[0089] A protein "from" or "obtainable from" any of the subject isolates
referred to or
suggested herein means that the protein (or a similar protein) can be obtained
from the isolate or
some other source, such as another bacterial strain or a plant. "Derived from"
also has this
connotation, and includes proteins obtainable from a given type of bacterium
that are modified
for expression in a plant, for example. One skilled in the art will readily
recognize that, given
the disclosure of a bacterial gene and protein, a plant can be engineered to
produce the protein.
Antibody preparations, nucleic acid probes (DNA, RNA, or PNA, for example),
and the like
can be prepared using the polynucleotide and/or amino acid sequences disclosed
herein and
used to screen and recover other related genes from other (natural) sources.
[0090] Standard molecular biology techniques may be used to clone and
sequence the
proteins and genes described herein. Additional information may be found in
Sambrook et al.,
1989, which is incorporated herein by reference.
[0091] Polynucleotides and probes: The subject invention further provides
nucleic acid
sequences that encode proteins for use according to the subject invention. The
subject
invention further provides methods of identifying and characterizing genes
that encode proteins
having the desired herbicidal activity. In one embodiment, the subject
invention provides
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
unique nucleotide sequences that are useful as hybridization probes and/or
primers for PCR
techniques. The primers produce characteristic gene fragments that can be used
in the
identification, characterization, and/or isolation of specific genes of
interest. The nucleotide
sequences of the subject invention encode proteins that are distinct from
previously described
proteins.
[0092] The polynucleotides of the subject invention can be used to form
complete "genes"
to encode proteins or peptides in a desired host cell. For example, as the
skilled artisan would
readily recognize, the subject polynucleotides can be appropriately placed
under the control of a
promoter in a host of interest, as is readily known in the art. The level of
gene expression and
temporal/tissue specific expression can greatly impact the utility of the
invention. Generally,
greater levels of protein expression of a degradative gene will result in
faster and more
complete degradation of a substrate (in this case a target herbicide).
Promoters will be desired
to express the target gene at high levels unless the high expression has a
consequential negative
impact on the health of the plant. Typically, one would wish to have the AAD-
12 gene
constitutively expressed in all tissues for complete protection of the plant
at all growth-stages.
However, one could alternatively use a vegetatively expressed resistance gene;
this would allow
use of the target herbicide in-crop for weed control and would subsequently
control sexual
reproduction of the target crop by application during the flowering stage. In
addition, desired
levels and times of expression can also depend on the type of plant and the
level of tolerance
desired. Some preferred embodiments use strong constitutive promoters combined
with
transcription enhancers and the like to increase expression levels and to
enhance tolerance to
desired levels. Some such applications are discussed in more detail below,
before the Examples
section.
[0093] As the skilled artisan knows, DNA typically exists in a double-
stranded form. In
this arrangement, one strand is complementary to the other strand and vice
versa. As DNA is
replicated in a plant (for example), additional complementary strands of DNA
are produced.
The "coding strand" is often used in the art to refer to the strand that binds
with the anti-sense
strand. The mRNA is transcribed from the "anti-sense" strand of DNA. The
"sense" or
"coding" strand has a series of codons (a codon is three nucleotides that can
be read as a three-
residue unit to specify a particular amino acid) that can be read as an open
reading frame (ORF)
to form a protein or peptide of interest. In order to produce a protein in
vivo, a strand of DNA
is typically transcribed into a complementary strand of mRNA which is used as
the template for
the protein. Thus, the subject invention includes the use of the exemplified
polynucleotides
shown in the attached sequence listing and/or equivalents including the
complementary strands.
21
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
RNA and PNA (peptide nucleic acids) that are functionally equivalent to the
exemplified DNA
molecules are included in the subject invention.
[0094] Proteins and genes for use according to the subject invention can be
identified and
obtained by using oligonucleotide probes, for example. These probes are
detectable nucleotide
sequences that can be detectable by virtue of an appropriate label or may be
made inherently
fluorescent as described in International Application No. WO 93/16094. The
probes (and the
polynucleotides of the subject invention) may be DNA, RNA, or PNA. In addition
to adenine
(A), cytosine (C), guanine (G), thymine (T), and uracil (U; for RNA
molecules), synthetic
probes (and polynucleotides) of the subject invention can also have inosine (a
neutral base
capable of pairing with all four bases; sometimes used in place of a mixture
of all four bases in
synthetic probes) and/or other synthetic (non-natural) bases. Thus, where a
synthetic,
degenerate oligonucleotide is referred to herein, and "N" or "n" is used
generically, "N" or "n"
can be G, A, T, C, or inosine. Ambiguity codes as used herein are in
accordance with standard
IUPAC naming conventions as of the filing of the subject application (for
example, R means A
or G, Y means C or T, etc.).
[0095] As is well known in the art, if a probe molecule hybridizes with a
nucleic acid
sample, it can be reasonably assumed that the probe and sample have
substantial
homology/similarity/identity. Preferably, hybridization of the polynucleotide
is first conducted
followed by washes under conditions of low, moderate, or high stringency by
techniques well-
known in the art, as described in, for example, Keller, G. H., M. M. Manak
(1987) DNA
Probes, Stockton Press, New York, N.Y., pp. 169-170. For example, as stated
therein, low
stringency conditions can be achieved by first washing with 2 x SSC (Standard
Saline
Citrate)/0.1% SDS (Sodium Dodecyl Sulfate) for 15 minutes at room temperature.
Two washes
are typically performed. Higher stringency can then be achieved by lowering
the salt
concentration and/or by raising the temperature. For example, the wash
described above can be
followed by two washings with 0.1 x SSC/0.1% SDS for 15 minutes each at room
temperature
followed by subsequent washes with 0.1 x SSC/0.1% SDS for 30 minutes each at
55 C. These
temperatures can be used with other hybridization and wash protocols set forth
herein and as
would be known to one skilled in the art (SSPE can be used as the salt instead
of SSC, for
example). The 2 x SSC/0.1% SDS can be prepared by adding 50 ml of 20 x SSC and
5 ml of
10% SDS to 445 ml of water. 20 x SSC can be prepared by combining NaC1 (175.3
g/0.150
M), sodium citrate (88.2 g/0.015 M), and water, adjusting pH to 7.0 with 10 N
NaOH, then
adjusting the volume to 1 liter. 10% SDS can be prepared by dissolving 10 g of
SDS in 50 ml
of autoclaved water, then diluting to 100 ml.
22
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
[0096] Detection of the probe provides a means for determining in a known
manner
whether hybridization has been maintained. Such a probe analysis provides a
rapid method for
identifying genes of the subject invention. The nucleotide segments used as
probes according
to the invention can be synthesized using a DNA synthesizer and standard
procedures. These
nucleotide sequences can also be used as PCR primers to amplify genes of the
subject
invention.
[0097] Hybridization characteristics of a molecule can be used to define
polynucleotides of
the subject invention. Thus the subject invention includes polynucleotides
(and/or their
complements, preferably their full complements) that hybridize with a
polynucleotide
exemplified herein. That is, one way to define a gene (and the protein it
encodes), for example,
is by its ability to hybridize (under any of the conditions specifically
disclosed herein) with a
known or specifically exemplified gene.
[0098] As used herein, "stringent" conditions for hybridization refers to
conditions which
achieve the same, or about the same, degree of specificity of hybridization as
the conditions
employed by the current applicants. Specifically, hybridization of immobilized
DNA on
Southern blots with 32P-labeled gene-specific probes can be performed by
standard methods
(see, e.g., Maniatis et al. 1982). In general, hybridization and subsequent
washes can be carried
out under conditions that allow for detection of target sequences. For double-
stranded DNA
gene probes, hybridization can be carried out overnight at 20-25 C. below the
melting
temperature (Tm) of the DNA hybrid in 6 x SSPE, 5 x Denhardt's solution, 0.1%
SDS, 0.1
mg/ml denatured DNA.
[0099] Washes can typically be carried out as follows: (1) twice at room
temperature for 15
minutes in 1 x SSPE, 0.1% SDS (low stringency wash); and (2) once at Tm-20 C.
for 15
minutes in 0.2 x SSPE, 0.1% SDS (moderate stringency wash).
[00100] For oligonucleotide probes, hybridization can be carried out overnight
at 10-20 C.
below the melting temperature (Tm) of the hybrid in 6×SSPE, 5 x
Denhardt's solution,
0.1% SDS, 0.1 mg/ml denatured DNA.
[00101] Washes can typically be out as follows: (1) twice at room temperature
for 15
minutes 1 x SSPE, 0.1% SDS (low stringency wash); and (2) once at the
hybridization
temperature for 15 minutes in 1 x SSPE, 0.1% SDS (moderate stringency wash).
[00102] In general, salt and/or temperature can be altered to change
stringency. With a
labeled DNA fragment>70 or so bases in length, the following conditions can be
used: (1) Low:
1 or 2 x SSPE, room temperature; (2) Low: 1 or 2 x SSPE, 42 C.; (3) Moderate:
0.2 x or 1 x
SSPE, 65 C. or (4) High: 0.1 x SSPE, 65 C.
23
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
[00103] Duplex formation and stability depend on substantial complementarity
between the
two strands of a hybrid, and, as noted above, a certain degree of mismatch can
be tolerated.
Therefore, the probe sequences of the subject invention include mutations
(both single and
multiple), deletions, insertions of the described sequences, and combinations
thereof, wherein
said mutations, insertions and deletions permit formation of stable hybrids
with the target
polynucleotide of interest. Mutations, insertions, and deletions can be
produced in a given
polynucleotide sequence in many ways, and these methods are known to an
ordinarily skilled
artisan. Other methods may become known in the future.
[00104] PCR technology: Polymerase Chain Reaction (PCR) is a repetitive,
enzymatic,
primed synthesis of a nucleic acid sequence. This procedure is well known and
commonly used
by those skilled in this art (see Mullis, U.S. Pat. Nos. 4,683,195, 4,683,202,
and 4,800,159;
Saiki et al., 1985). PCR is based on the enzymatic amplification of a DNA
fragment of interest
that is flanked by two oligonucleotide primers that hybridize to opposite
strands of the target
sequence. The primers are preferably oriented with the 3' ends pointing
towards each other.
Repeated cycles of heat denaturation of the template, annealing of the primers
to their
complementary sequences, and extension of the annealed primers with a DNA
polymerase
result in the amplification of the segment defined by the 5' ends of the PCR
primers. The
extension product of each primer can serve as a template for the other primer,
so each cycle
essentially doubles the amount of DNA fragment produced in the previous cycle.
This results
in the exponential accumulation of the specific target fragment, up to several
million-fold in a
few hours. By using a thermostable DNA polymerase such as Tag polymerase,
isolated from
the thermophilic bacterium Thennus aquaticus, the amplification process can be
completely
automated. Other enzymes which can be used are known to those skilled in the
art.
[00105] Exemplified DNA sequences, or segments thereof, can be used as primers
for PCR
amplification. In performing PCR amplification, a certain degree of mismatch
can be tolerated
between primer and template. Therefore, mutations, deletions, and insertions
(especially
additions of nucleotides to the 5' end) of the exemplified primers fall within
the scope of the
subject invention. Mutations, insertions, and deletions can be produced in a
given primer by
methods known to an ordinarily skilled artisan.
[00106] Modification of genes and proteins: The subject genes and proteins can
be fused to
other genes and proteins to produce chimeric or fusion proteins. The genes and
proteins useful
according to the subject invention include not only the specifically
exemplified full-length
sequences, but also portions, segments and/or fragments (including contiguous
fragments and
internal and/or terminal deletions compared to the full-length molecules) of
these sequences,
24
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
variants, mutants, chimerics, and fusions thereof. Proteins of the subject
invention can have
substituted amino acids so long as they retain desired functional activity.
"Variant" genes have
nucleotide sequences that encode the same proteins or equivalent proteins
having activity
equivalent or similar to an exemplified protein.
[00107] The top two results of BLAST searches with the native aad-12
nucleotide sequence
show a reasonable level of homology (about 85%) over 120 base pairs of
sequence.
Hybridization under certain conditions could be expected to include these two
sequences. See
GENBANK Acc. Nos. DQ406818.1 (89329742; Rhodoferax) and AJ6288601.1 (44903451;
Sphingomonas). Rhodoferax is very similar to Delftia but Sphingomonas is an
entirely different
Class phylogenetically.
[00108] The terms "variant proteins" and "equivalent proteins" refer to
proteins having the
same or essentially the same biological/functional activity against the target
substrates and
equivalent sequences as the exemplified proteins. As used herein, reference to
an "equivalent"
sequence refers to sequences having amino acid substitutions, deletions,
additions, or insertions
that improve or do not adversely affect activity to a significant extent.
Fragments retaining
activity are also included in this definition. Fragments and other equivalents
that retain the
same or similar function or activity as a corresponding fragment of an
exemplified protein are
within the scope of the subject invention. Changes, such as amino acid
substitutions or
additions, can be made for a variety of purposes, such as increasing (or
decreasing) protease
stability of the protein (without materially/substantially decreasing the
functional activity of the
protein), removing or adding a restriction site, and the like. Variations of
genes may be readily
constructed using standard techniques for making point mutations, for example.
[00109] In addition, U.S. Pat. No. 5,605,793, for example, describes methods
for generating
additional molecular diversity by using DNA reassembly after random or focused
fragmentation. This can be referred to as gene "shuffling," which typically
involves mixing
fragments (of a desired size) of two or more different DNA molecules, followed
by repeated
rounds of renaturation. This can improve the activity of a protein encoded by
a starting gene.
The result is a chimeric protein having improved activity, altered substrate
specificity, increased
enzyme stability, altered stereospecificity, or other characteristics.
[00110] "Shuffling" can be designed and targeted after obtaining and examining
the atomic
3D (three dimensional) coordinates and crystal structure of a protein of
interest. Thus, "focused
shuffling" can be directed to certain segments of a protein that are ideal for
modification, such
as surface-exposed segments, and preferably not internal segments that are
involved with
protein folding and essential 3D structural integrity.
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
[00111] Specific changes to the "active site" of the enzyme can be made to
affect the
inherent functionallity with respect to activity or stereospecificity. Muller
et. al. (2006). The
known tauD crystal structure was used as a model dioxygenase to determine
active site residues
while bound to its inherent substrate taurine. Elkins et al. (2002) "X-ray
crystal structure of
Escerichia coli taurine/alpha-ketoglutarate dioxygenase complexed to ferrous
iron and
substrates," Biochemistry 41(16):5185-5192. Regarding sequence optimization
and
designability of enzyme active sites, see Chakrabarti et al., PNAS, (Aug. 23,
2005),
102(34):12035-12040.
[00112] Fragments of full-length genes can be made using commercially
available
exonucleases or endonucleases according to standard procedures. For example,
enzymes such
as Ba131 or site-directed mutagenesis can be used to systematically cut off
nucleotides from the
ends of these genes. Also, genes that encode active fragments may be obtained
using a variety
of restriction enzymes. Proteases may be used to directly obtain active
fragments of these
proteins.
[00113] It is within the scope of the invention as disclosed herein that
proteins can be
truncated and still retain functional activity. By "truncated protein," it is
meant that a portion of
a protein may be cleaved off while the remaining truncated protein retains and
exhibits the
desired activity after cleavage. Cleavage can be achieved by various
proteases. Furthermore,
effectively cleaved proteins can be produced using molecular biology
techniques wherein the
DNA bases encoding said protein are removed either through digestion with
restriction
endonucleases or other techniques available to the skilled artisan. After
truncation, said
proteins can be expressed in heterologous systems such as E. coli,
baculoviruses, plant-based
viral systems, yeast, and the like and then placed in insect assays as
disclosed herein to
determine activity. It is well-known in the art that truncated proteins can be
successfully
produced so that they retain functional activity while having less than the
entire, full-length
sequence. For example, B.t. proteins can be used in a truncated (core protein)
form (see, e.g.,
Hofte et al. (1989), and Adang et al. (1985)). As used herein, the term
"protein" can include
functionally active truncations.
[00114] Unless otherwise specified, as used herein, percent sequence identity
and/or
similarity of two nucleic acids is determined using the algorithm of Karlin
and Altschul, 1990,
modified as in Karlin and Altschul 1993. Such an algorithm is incorporated
into the NBLAST
and XBLAST programs of Altschul et al., 1990. BLAST nucleotide searches are
performed
with the NBLAST program, score=100, wordlength=12. Gapped BLAST can be used as
described in Altschul et al., 1997. When utilizing BLAST and Gapped BLAST
programs, the
26
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
default parameters of the respective programs (NBLAST and XBLAST) are used.
See
NCBI/NIH website. To obtain gapped alignments for comparison purposes, the
AlignX
function of Vector NTI Suite 8 (InforMax, Inc., North Bethesda, Md., U.S.A.),
was used
employing the default parameters. These were: a Gap opening penalty of 15, a
Gap extension
penalty of 6.66, and a Gap separation penalty range of 8.
Table 1
Class of Amino Acid Examples of Amino Acids
Nonpolar Ala, Val, Leu, Ile, Pro, Met, Phe,
Trp
Uncharged Polar Gly, Ser, Thr, Cys, Tyr, Asn, Gln
Acidic Asp, Glu
Basic Lys, Arg, His
[00115] Various properties and three-dimensional features of the protein can
also be changed
without adversely affecting the activity/functionality of the protein.
Conservative amino acid
substitutions can be tolerated/made to not adversely affect the activity
and/or three-dimensional
configuration of the molecule. Amino acids can be placed in the following
classes: non-polar,
uncharged polar, basic, and acidic. Conservative substitutions whereby an
amino acid of one
class is replaced with another amino acid of the same type fall within the
scope of the subject
invention so long as the substitution is not adverse to the biological
activity of the compound.
Table 1 provides a listing of examples of amino acids belonging to each class.
In some
instances, non-conservative substitutions can also be made. However, preferred
substitutions
do not significantly detract from the functional/biological activity of the
protein.
[00116] As used herein, reference to "isolated" polynucleotides and/or
"purified" proteins
refers to these molecules when they are not associated with the other
molecules with which they
would be found in nature. Thus, reference to "isolated" and/or "purified"
signifies the
involvement of the "hand of man" as described herein. For example, a bacterial
"gene" of the
subject invention put into a plant for expression is an "isolated
polynucleotide." Likewise, a
protein derived from a bacterial protein and produced by a plant is an
"isolated protein."
[00117] Because of the degeneracy/redundancy of the genetic code, a variety of
different
DNA sequences can encode the amino acid sequences disclosed herein. It is well
within the
skill of a person trained in the art to create alternative DNA sequences that
encode the same, or
essentially the same, proteins. These variant DNA sequences are within the
scope of the
subject invention. This is also discussed in more detail below in the section
entitled
"Optimization of sequence for expression in plants."
[00118] Optimization of sequence for expression in plants: To obtain high
expression of
27
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
heterologous genes in plants it is generally preferred to reengineer the genes
so that they are
more efficiently expressed in (the cytoplasm of) plant cells. Maize is one
such plant where it
may be preferred to re-design the heterologous gene(s) prior to transformation
to increase the
expression level thereof in said plant. Therefore, an additional step in the
design of genes
encoding a bacterial protein is reengineering of a heterologous gene for
optimal expression,
using codon bias more closely aligned with the target plant sequence, whether
a dicot or
monocot species. Sequences can also be optimized for expression in any of the
more particular
types of plants discussed elsewhere herein.
[00119] Transgenic hosts: The protein-encoding genes of the subject invention
can be
introduced into a wide variety of microbial or plant hosts. The subject
invention includes
transgenic plant cells and transgenic plants. Preferred plants (and plant
cells) are corn,
Arabidopsis, tobacco, soybeans, cotton, canola, rice, wheat, turf, legume
forages (e.g., alfalfa
and clover), pasture grasses, and the like. Other types of transgenic plants
can also be made
according to the subject invention, such as fruits, vegetables, ornamental
plants, and trees.
More generally, dicots and/or monocots can be used in various aspects of the
subject invention.
[00120] In preferred embodiments, expression of the gene results, directly or
indirectly, in
the intracellular production (and maintenance) of the protein(s) of interest.
Plants can be
rendered herbicide-resistant in this manner. Such hosts can be referred to as
transgenic,
recombinant, transformed, and/or transfected hosts and/or cells. In some
aspects of this
invention (when cloning and preparing the gene of interest, for example),
microbial (preferably
bacterial) cells can be produced and used according to standard techniques,
with the benefit of
the subject disclosure.
[00121] Plant cells transfected with a polynucleotide of the subject invention
can be
regenerated into whole plants. The subject invention includes cell cultures
including tissue cell
cultures, liquid cultures, and plated cultures. Seeds produced by and/or used
to generate plants
of the subject invention are also included within the scope of the subject
invention. Other plant
tissues and parts are also included in the subject invention. The subject
invention likewise
includes methods of producing plants or cells comprising a polynucleotide of
the subject
invention. One preferred method of producing such plants is by planting a seed
of the subject
invention.
[00122] Although plants can be preferred, the subject invention also includes
production of
highly active recombinant AAD-12 in a Pseudomonas fluorescens (Pf) host
strain, for example.
The subject invention includes preferred growth temperatures for maintaining
soluble active
AAD-12 in this host; a fermentation condition where AAD-12 is produced as more
than 40%
28
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
total cell protein, or at least 10 g/L; a purification process results high
recovery of active
recombinant AAD-12 from a Pf host; a purification scheme which yields at least
10 g active
AAD-12 per kg of cells; a purification scheme which can yield 20 g active AAD-
12 per kg of
cells; a formulation process that can store and restore AAD-12 activity in
solution; and a
lyophilization process that can retain AAD-12 activity for long-term storage
and shelf life.
[00123] Insertion of genes to form transgenic hosts: One aspect of the subject
invention is
the transformation/transfection of plants, plant cells, and other host cells
with polynucleotides
of the subject invention that express proteins of the subject invention.
Plants transformed in
this manner can be rendered resistant to a variety of herbicides with
different modes of action.
[00124] A wide variety of methods are available for introducing a gene
encoding a desired
protein into the target host under conditions that allow for stable
maintenance and expression of
the gene. These methods are well known to those skilled in the art and are
described, for
example, in U.S. Pat. No. 5,135,867.
[00125] Vectors comprising an AAD-12 polynucleotide are included in the scope
of the
subject invention. For example, a large number of cloning vectors comprising a
replication
system in E. coli and a marker that permits selection of the transformed cells
are available for
preparation for the insertion of foreign genes into higher plants. The vectors
comprise, for
example, pBR322, pUC series, M13 mp series, pACYC184, etc. Accordingly, the
sequence
encoding the protein can be inserted into the vector at a suitable restriction
site. The resulting
plasmid is used for transformation into E. coli. The E. coli cells are
cultivated in a suitable
nutrient medium, then harvested and lysed. The plasmid is recovered by
purification away
from genomic DNA. Sequence analysis, restriction analysis, electrophoresis,
and other
biochemical-molecular biological methods are generally carried out as methods
of analysis.
After each manipulation, the DNA sequence used can be restriction digested and
joined to the
next DNA sequence. Each plasmid sequence can be cloned in the same or other
plasmids.
Depending on the method of inserting desired genes into the plant, other DNA
sequences may
be necessary. If, for example, the Ti or Ri plasmid is used for the
transformation of the plant
cell, then at least the right border, but often the right and the left border
of the Ti or Ri plasmid
T-DNA, has to be joined as the flanking region of the genes to be inserted.
The use of T-DNA
for the transformation of plant cells has been intensively researched and
described in EP 120
516; Hoekema (1985); Fraley et al. (1986); and An et al. (1985).
[00126] A large number of techniques are available for inserting DNA into a
plant host cell.
Those techniques include transformation with T-DNA using Agrobacterium
tumefaciens or
Agrobacterium rhizogenes as transformation agent, fusion, injection,
biolistics (microparticle
29
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
bombardment), silicon carbide whiskers, aerosol beaming, PEG, or
electroporation as well as
other possible methods. If Agrobacteria are used for the transformation, the
DNA to be
inserted has to be cloned into special plasmids, namely either into an
intermediate vector or into
a binary vector. The intermediate vectors can be integrated into the Ti or Ri
plasmid by
homologous recombination owing to sequences that are homologous to sequences
in the T-
DNA. The Ti or Ri plasmid also comprises the vir region necessary for the
transfer of the T-
DNA. Intermediate vectors cannot replicate themselves in Agrobacteria. The
intermediate
vector can be transferred into Agrobacterium tumefaciens by means of a helper
plasmid
(conjugation). Binary vectors can replicate themselves both in E. coli and in
Agrobacteria.
They comprise a selection marker gene and a linker or polylinker which are
framed by the right
and left T-DNA border regions. They can be transformed directly into
Agrobacteria (Holsters,
1978). The Agrobacterium used as host cell is to comprise a plasmid carrying a
vir region. The
vir region is necessary for the transfer of the T-DNA into the plant cell.
Additional T-DNA
may be contained. The bacterium so transformed is used for the transformation
of plant cells.
Plant explants can be cultivated advantageously with Agrobacterium tumefaciens
or
Agrobacterium rhizogenes for the transfer of the DNA into the plant cell.
Whole plants can
then be regenerated from the infected plant material (for example, pieces of
leaf, segments of
stalk, roots, but also protoplasts or suspension-cultivated cells) in a
suitable medium, which
may contain antibiotics or biocides for selection. The plants so obtained can
then be tested for
the presence of the inserted DNA. No special demands are made of the plasmids
in the case of
injection and electroporation. It is possible to use ordinary plasmids, such
as, for example, pUC
derivatives.
[00127] The transformed cells grow inside the plants in the usual manner. They
can form
germ cells and transmit the transformed trait(s) to progeny plants. Such
plants can be grown in
the normal manner and crossed with plants that have the same transformed
hereditary factors or
other hereditary factors. The resulting hybrid individuals have the
corresponding phenotypic
properties. In some preferred embodiments of the invention, genes encoding the
bacterial
protein are expressed from transcriptional units inserted into the plant
genome. Preferably, said
transcriptional units are recombinant vectors capable of stable integration
into the plant genome
and enable selection of transformed plant lines expressing mRNA encoding the
proteins.
[00128] Once the inserted DNA has been integrated in the genome, it is
relatively stable
there (and does not come out again). It normally contains a selection marker
that confers on the
transformed plant cells resistance to a biocide or an antibiotic, such as
kanamycin, G418,
bleomycin, hygromycin, or chloramphenicol, inter alia. Plant selectable
markers also typically
CA 02876144 2014-12-05
WO 2013/185036
PCT/US2013/044717
can provide resistance to various herbicides such as glufosinate (e.g.,
PAT/bar), glyphosate
(EPSPS), ALS-inhibitors (e.g., imidazolinone, sulfonylurea, triazolopyrimidine
sulfonanilide, et
al.), bromoxynil, HPPD-inhibitor resistance, PPO-inhibitors, ACC-ase
inhibitors, and many
others. The individually employed marker should accordingly permit the
selection of
transformed cells rather than cells that do not contain the inserted DNA. The
gene(s) of interest
are preferably expressed either by constitutive or inducible promoters in the
plant cell. Once
expressed, the mRNA is translated into proteins, thereby incorporating amino
acids of interest
into protein. The genes encoding a protein expressed in the plant cells can be
under the control
of a constitutive promoter, a tissue-specific promoter, or an inducible
promoter.
[00129] Several techniques exist for introducing foreign recombinant vectors
into plant cells,
and for obtaining plants that stably maintain and express the introduced gene.
Such techniques
include the introduction of genetic material coated onto microparticles
directly into cells (U.S.
Pat. Nos. 4,945,050 to Cornell and 5,141,131 to DowElanco, now Dow
AgroSciences, LLC).
In addition, plants may be transformed using Agrobacterium technology, see
U.S. Pat. Nos.
5,177,010 to University of Toledo; 5,104,310 to Texas A&M; European Patent
Application
0131624B1; European Patent Applications 120516, 159418B1 and 176,112 to
Schilperoot; U.S.
Pat. Nos. 5,149,645, 5,469,976, 5,464,763 and 4,940,838 and 4,693,976 to
Schilperoot;
European Patent Applications 116718, 290799, 320500, all to Max Planck;
European Patent
Applications 604662 and 627752, and U.S. Pat. No. 5,591,616, to Japan Tobacco;
European
Patent Applications 0267159 and 0292435, and U.S. Pat. No. 5,231,019, all to
Ciba Geigy, now
Syngenta; U.S. Pat. Nos. 5,463,174 and 4,762,785, both to Calgene; and U.S.
Pat. Nos.
5,004,863 and 5,159,135, both to Agracetus. Other transformation technology
includes
whiskers technology. See U.S. Pat. Nos. 5,302,523 and 5,464,765, both to
Zeneca, now
Syngenta. Other direct DNA delivery transformation technology includes aerosol
beam
technology. See U.S. Pat. No. 6,809,232. Electroporation technology has also
been used to
transform plants. See WO 87/06614 to Boyce Thompson Institute; U.S. Pat. Nos.
5,472,869 and
5,384,253, both to Dekalb; and WO 92/09696 and WO 93/21335, both to Plant
Genetic
Systems. Furthermore, viral vectors can also be used to produce transgenic
plants expressing
the protein of interest. For example, monocotyledonous plants can be
transformed with a viral
vector using the methods described in U.S. Pat. No. 5,569,597 to Mycogen Plant
Science and
Ciba-Geigy (now Syngenta), as well as U.S. Pat. Nos. 5,589,367 and 5,316,931,
both to
Biosource, now Large Scale Biology.
[00130] As
mentioned previously, the manner in which the DNA construct is introduced
into the plant host is not critical to this invention. Any method that
provides for efficient
31
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
transformation may be employed. For example, various methods for plant cell
transformation
are described herein and include the use of Ti or Ri-plasmids and the like to
perform
Agrobacterium mediated transformation. In many instances, it will be desirable
to have the
construct used for transformation bordered on one or both sides by T-DNA
borders, more
specifically the right border. This is particularly useful when the construct
uses Agrobacterium
tumefaciens or Agrobacterium rhizogenes as a mode for transformation, although
T-DNA
borders may find use with other modes of transformation. Where Agrobacterium
is used for
plant cell transformation, a vector may be used which may be introduced into
the host for
homologous recombination with T-DNA or the Ti or Ri plasmid present in the
host.
Introduction of the vector may be performed via electroporation, tri-parental
mating and other
techniques for transforming gram-negative bacteria which are known to those
skilled in the art.
The manner of vector transformation into the Agrobacterium host is not
critical to this
invention. The Ti or Ri plasmid containing the T-DNA for recombination may be
capable or
incapable of causing gall formation, and is not critical to said invention so
long as the vir genes
are present in said host.
[00131] In some cases where Agrobacterium is used for transformation, the
expression
construct being within the T-DNA borders will be inserted into a broad
spectrum vector such as
pRK2 or derivatives thereof as described in Ditta et al. (1980) and EPO 0 120
515. Included
within the expression construct and the T-DNA will be one or more markers as
described herein
which allow for selection of transformed Agrobacterium and transformed plant
cells. The
particular marker employed is not essential to this invention, with the
preferred marker
depending on the host and construction used.
[00132] For transformation of plant cells using Agrobacterium, explants may be
combined
and incubated with the transformed Agrobacterium for sufficient time to allow
transformation
thereof. After transformation, the Agrobacteria are killed by selection with
the appropriate
antibiotic and plant cells are cultured with the appropriate selective medium.
Once calli are
formed, shoot formation can be encouraged by employing the appropriate plant
hormones
according to methods well known in the art of plant tissue culturing and plant
regeneration.
However, a callus intermediate stage is not always necessary. After shoot
formation, said plant
cells can be transferred to medium which encourages root formation thereby
completing plant
regeneration. The plants may then be grown to seed and said seed can be used
to establish
future generations. Regardless of transformation technique, the gene encoding
a bacterial
protein is preferably incorporated into a gene transfer vector adapted to
express said gene in a
plant cell by including in the vector a plant promoter regulatory element, as
well as 3' non-
32
CA 02876144 2014-12-05
WO 2013/185036
PCT/US2013/044717
translated transcriptional termination regions such as Nos and the like.
[00133] In addition to numerous technologies for transforming plants, the type
of tissue that
is contacted with the foreign genes may vary as well. Such tissue would
include but would not
be limited to embryogenic tissue, callus tissue types I, II, and III,
hypocotyl, meristem, root
tissue, tissues for expression in phloem, and the like. Almost all plant
tissues may be
transformed during dedifferentiation using appropriate techniques described
herein.
[00134] As mentioned above, a variety of selectable markers can be used, if
desired.
Preference for a particular marker is at the discretion of the artisan, but
any of the following
selectable markers may be used along with any other gene not listed herein
which could
function as a selectable marker. Such selectable markers include but are not
limited to
aminoglycoside phosphotransferase gene of transposon Tn5 (Aph II) which
encodes resistance
to the antibiotics kanamycin, neomycin and G41; hygromycin resistance;
methotrexate
resistance, as well as those genes which encode for resistance or tolerance to
glyphosate;
phosphinothricin (bialaphos or glufosinate); ALS-inhibiting herbicides
(imidazolinones,
sulfonylureas and triazolopyrimidine herbicides), ACC-ase inhibitors (e.g.,
ayryloxypropionates
or cyclohexanediones), and others such as bromoxynil, and HPPD-inhibitors
(e.g., mesotrione)
and the like.
[00135] In
addition to a selectable marker, it may be desirous to use a reporter gene. In
some instances a reporter gene may be used with or without a selectable
marker. Reporter
genes are genes that are typically not present in the recipient organism or
tissue and typically
encode for proteins resulting in some phenotypic change or enzymatic property.
Examples of
such genes are provided in Weising et al., 1988. Preferred reporter genes
include the beta-
glucuronidase (GUS) of the uidA locus of E. coli, the chloramphenicol acetyl
transferase gene
from Tn9 of E. coli, the green fluorescent protein from the bioluminescent
jellyfish Aequorea
victoria, and the luciferase genes from firefly Photinus pyralis. An assay for
detecting reporter
gene expression may then be performed at a suitable time after said gene has
been introduced
into recipient cells. A preferred such assay entails the use of the gene
encoding beta-
glucuronidase (GUS) of the uidA locus of E. coli as described by Jefferson et
al., (1987) to
identify transformed cells.
[00136] In
addition to plant promoter regulatory elements, promoter regulatory elements
from a variety of sources can be used efficiently in plant cells to express
foreign genes. For
example, promoter regulatory elements of bacterial origin, such as the
octopine synthase
promoter, the nopaline synthase promoter, the mannopine synthase promoter;
promoters of viral
origin, such as the cauliflower mosaic virus (35S and 195), 35T (which is a re-
engineered 355
33
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
promoter, see U.S. Pat. No. 6,166,302, especially Example 7E) and the like may
be used. Plant
promoter regulatory elements include but are not limited to ribulose-1,6-
bisphosphate (RUBP)
carboxylase small subunit (ssu), beta-conglycinin promoter, beta-phaseolin
promoter, ADH
promoter, heat-shock promoters, and tissue specific promoters. Other elements
such as matrix
attachment regions, scaffold attachment regions, introns, enhancers,
polyadenylation sequences
and the like may be present and thus may improve the transcription efficiency
or DNA
integration. Such elements may or may not be necessary for DNA function,
although they can
provide better expression or functioning of the DNA by affecting
transcription, mRNA stability,
and the like. Such elements may be included in the DNA as desired to obtain
optimal
performance of the transformed DNA in the plant. Typical elements include but
are not limited
to Adh-intron 1, Adh-intron 6, the alfalfa mosaic virus coat protein leader
sequence, osmotin
UTR sequences, the maize streak virus coat protein leader sequence, as well as
others available
to a skilled artisan. Constitutive promoter regulatory elements may also be
used thereby
directing continuous gene expression in all cells types and at all times
(e.g., actin, ubiquitin,
CaMV 35S, and the like). Tissue specific promoter regulatory elements are
responsible for
gene expression in specific cell or tissue types, such as the leaves or seeds
(e.g., zein, oleosin,
napin, ACP, globulin and the like) and these may also be used.
[00137] Promoter regulatory elements may also be active (or inactive)
during a certain stage
of the plant's development as well as active in plant tissues and organs.
Examples of such
include but are not limited to pollen-specific, embryo-specific, corn-silk-
specific, cotton-fiber-
specific, root-specific, seed-endosperm-specific, or vegetative phase-specific
promoter
regulatory elements and the like. Under certain circumstances it may be
desirable to use an
inducible promoter regulatory element, which is responsible for expression of
genes in response
to a specific signal, such as: physical stimulus (heat shock genes), light
(RUBP carboxylase),
hormone (Em), metabolites, chemical (tetracycline responsive), and stress.
Other desirable
transcription and translation elements that function in plants may be used.
Numerous plant-
specific gene transfer vectors are known in the art.
[00138] Plant RNA viral based systems can also be used to express bacterial
protein. In so
doing, the gene encoding a protein can be inserted into the coat promoter
region of a suitable
plant virus which will infect the host plant of interest. The protein can then
be expressed thus
providing protection of the plant from herbicide damage. Plant RNA viral based
systems are
described in U.S. Pat. No. 5,500,360 to Mycogen Plant Sciences, Inc. and U.S.
Pat. Nos.
5,316,931 and 5,589,367 to Biosource, now Large Scale Biology.
[00139] Means of further increasing tolerance or resistance levels. It is
shown herein that
34
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
plants of the subject invention can be imparted with novel herbicide
resistance traits without
observable adverse effects on phenotype including yield. Such plants are
within the scope of
the subject invention. Plants exemplified and suggested herein can withstand 2
x, 3 x, 4 x, and
x typical application levels, for example, of at least one subject herbicide.
Improvements in
these tolerance levels are within the scope of this invention. For example,
various techniques
are know in the art, and can forseeably be optimized and further developed,
for increasing
expression of a given gene.
[00140] One such method includes increasing the copy number of the subject AAD-
12 genes
(in expression cassettes and the like). Transformation events can also be
selected for those
having multiple copies of the genes.
[00141] Strong promoters and enhancers can be used to "supercharge"
expression.
Examples of such promoters include the preferred 35T promoter which uses 35S
enhancers.
35S, maize ubiquitin, Arabidopsis ubiquitin, A.t. actin, and CSMV promoters
are included for
such uses. Other strong viral promoters are also preferred. Enhancers include
4 OCS and the
35S double enhancer. Matrix attachment regions (MARs) can also be used to
increase
transformation efficiencies and transgene expression.
[00142] Shuffling (directed evolution) and transcription factors can also
be used for
embodiments according to the subject invention.
[00143] Variant proteins can also be designed that differ at the sequence
level but that retain
the same or similar overall essential three-dimensional structure, surface
charge distribution,
and the like. See e.g. U.S. Pat. No. 7,058,515; Larson et al., Protein Sci.
2002 11: 2804-2813,
"Thoroughly sampling sequence space: Large-scale protein design of structural
ensembles";
Crameri et al., Nature Biotechnology 15, 436-438 (1997), "Molecular evolution
of an arsenate
detoxification pathway by DNA shuffling"; Stemmer, W. P. C. 1994. "DNA
shuffling by
random fragmentation and reassembly: in vitro recombination for molecular
evolution" Proc.
Natl. Acad. Sci. USA 91: 10747-10751; Stemmer, W. P. C. 1994. "Rapid evolution
of a protein
in vitro by DNA shuffling" Nature 370: 389-391; Stemmer, W. P. C. 1995.
Searching sequence
space. Bio/Technology 13: 549-553; Crameri, A., et al. 1996. "Construction and
evolution of
antibody-phage libraries by DNA shuffling" Nature Medicine 2: 100-103; and
Crameri, A., et
al. 1996. "Improved green fluorescent protein by molecular evolution using DNA
shuffling"
Nature Biotechnology 14: 315-319.
[00144] The activity of recombinant polynucleotides inserted into plant cells
can be
dependent upon the influence of endogenous plant DNA adjacent the insert.
Thus, another
option is taking advantage of events that are known to be excellent locations
in a plant genome
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
for insertions. See e.g. WO 2005/103266 Al, relating to crylF and crylAc
cotton events; the
subject AAD-12 gene can be substituted in those genomic loci in place of the
cryl F and/or
crylAc inserts. Thus, targeted homologous recombination, for example, can be
used according
to the subject invention. This type of technology is the subject of, for
example, WO 03/080809
A2 and the corresponding published U.S. application 20030232410, relating to
the use of zinc
fingers for targeted recombination. The use of recombinases (cre-10 x and flp-
frt for example)
is also known.
[00145] AAD-12 detoxification is believed to occur in the cytoplasm. Thus,
means for
further stabilizing this protein and mRNAs (including blocking mRNA
degradation) are
included in aspects of the subject invention, and art-known techniques can be
applied
accordingly. The subject proteins can be designed to resist degradation by
proteases and the
like (protease cleavage sites can be effectively removed by re-engineering the
amino acid
sequence of the protein). Such embodiments include the use of 5' and 3' stem
loop structures
like UTRs from osmotin, and per5 (AU-rich untranslated 5' sequences). 5' caps
like 7-methyl
or 2'-0-methyl groups, e.g., 7-methylguanylic acid residue, can also be used.
See, e.g.: Proc.
Natl. Acad. Sci. USA Vol. 74, No. 7, pp. 2734-2738 (July 1977) Importance of
5'-terminal
blocking structure to stabilize mRNA in eukaryotic protein synthesis. Protein
complexes or
ligand blocking groups can also be used.
[00146] Computational design of 5' or 3' UTR most suitable for AAD-12
(synthetic hairpins)
can also be conducted within the scope of the subject invention. Computer
modeling in
general, as well as gene shuffling and directed evolution, are discussed
elsewhere herein. More
specifically regarding computer modeling and UTRs, computer modeling
techniques for use in
predicting/evaluating 5' and 3' UTR derivatives of the present invention
include, but are not
limited to: MFold version 3.1 available from Genetics Corporation Group,
Madison, Wis. (see
Zucker et al., Algorithms and Thermodynamics for RNA Secondary Structure
Prediction: A
Practical Guide. In RNA Biochemistry and Biotechnology, 11-43, J. Barciszewski
& B. F. C.
Clark, eds., NATO ASI Series, Kluwer Academic Publishers, Dordrecht, NL,
(1999); Zucker et
al., Expanded Sequence Dependence of Thermodynamic Parameters Improves
Prediction of
RNA Secondary Structure. J. Mol. Biol. 288, 911-940 (1999); Zucker et al., RNA
Secondary
Structure Prediction. In Current Protocols in Nucleic Acid Chemistry S.
Beaucage, D. E.
Bergstrom, G. D. Glick, and R. A. Jones eds., John Wiley & Sons, New York,
11.2.1-11.2.10,
(2000)), COVE (RNA structure analysis using covariance models (stochastic
context free
grammar methods)) v. 2.4.2 (Eddy & Durbin, Nucl. Acids Res. 1994, 22: 2079-
2088) which is
freely distributed as source code and which can be downloaded by accessing the
website
36
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
genetics.wustl.edu/eddy/software/, and FOLDALIGN, also freely distributed and
available for
downloading at the website bioinf.au.dk. FOLDALIGN/ (see Finding the most
significant
common sequence and structure motifs in a set of RNA sequences. J. Gorodkin,
L. J. Heyer and
G. D. Stormo. Nucleic Acids Research, Vol. 25, no. 18 pp 3724-3732, 1997;
Finding Common
Sequence and Structure Motifs in a set of RNA Sequences. J. Gorodkin, L. J.
Heyer, and G. D.
Stormo. ISMB 5;120-123, 1997).
[00147] Embodiments of the subject invention can be used in conjunction with
naturally
evolved or chemically induced mutants (mutants can be selected by screening
techniques, then
transformed with AAD-12 and possibly other genes). Plants of the subject
invention can be
combined with ALS resistance and/or evolved glyphosate resistance.
Aminopyralid resistance,
for example, can also be combined or "stacked" with an AAD-12 gene.
[00148] Traditional breeding techniques can also be combined with the subject
invention to
powerfully combine, introgress, and improve desired traits.
[00149] Further improvements also include use with appropriate safeners to
further protect
plants and/or to add cross resistance to more herbicides. Safeners typically
act to increase
plants immune system by activating/expressing cP450. Safeners are chemical
agents that
reduce the phytotoxicity of herbicides to crop plants by a physiological or
molecular
mechanism, without compromising weed control efficacy.
[00150] Herbicide safeners include benoxacor, cloquintocet, cyometrinil,
dichlormid,
dicyclonon, dietholate, fenchlorazole, fenclorim, flurazole, fluxofenim,
furilazole, isoxadifen,
mefenpyr, mephenate, naphthalic anhydride, and oxabetrinil. Plant activators
(a new class of
compounds that protect plants by activating their defense mechanisms) can also
be used in
embodiments of the subject invention. These include acibenzolar and
probenazole.
[00151] Commercialized safeners can be used for the protection of large-seeded
grass crops,
such as corn, grain sorghum, and wet-sown rice, against preplant-incorporated
or
preemergence-applied herbicides of the thiocarbamate and chloroacetanilide
families. Safeners
also have been developed to protect winter cereal crops such as wheat against
postemergence
applications of aryloxyphenoxypropionate and sulfonylurea herbicides. The use
of safeners for
the protection of corn and rice against sulfonylurea, imidazolinone,
cyclohexanedione,
isoxazole, and triketone herbicides is also well-established. A safener-
induced enhancement of
herbicide detoxification in safened plants is widely accepted as the major
mechanism involved
in safener action. Safeners induce cofactors such as glutathione and herbicide-
detoxifying
enzymes such as glutathione S-transferases, cytochrome P450 monooxygenases,
and glucosyl
transferases. Hatzios K K, Burgos N (2004) "Metabolism-based herbicide
resistance: regulation
37
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
by safeners," Weed Science: Vol. 52, No. 3 pp. 454-467.
[00152] Use of a cytochrome p450 monooxygenase gene stacked with AAD-12 is one
preferred embodiment. There are P450s involved in herbicide metabolism; cP450
can be of
mammalian or plant origin, for example. In higher plants, cytochrome P450
monooxygenase
(P450) is known to conduct secondary metabolism. It also plays an important
role in the
oxidative metabolism of xenobiotics in cooperation with NADPH-cytochrome P450
oxidoreductase (reductase). Resistance to some herbicides has been reported as
a result of the
metabolism by P450 as well as glutathione S-transferase. A number of
microsomal P450
species involved in xenobiotic metabolism in mammals have been characterized
by molecular
cloning. Some of them were reported to metabolize several herbicides
efficiently. Thus,
transgenic plants with plant or mammalian P450 can show resistance to several
herbicides.
[00153] One preferred embodiment of the foregoing is the use cP450 for
resistance to
acetochlor (acetochlor-based products include Surpass , Keystone , Keystone
LA, FulTime
and TopNotch herbicides) and/or trifluralin (such as Treflani0). Such
resistance in soybeans
and/or corn is included in some preferred embodiments. For additional guidance
regarding
such embodiments, see e.g. Inui et al., "A selectable marker using cytochrome
P450
monooxygenases for Arabidopsis transformation," Plant Biotechnology 22, 281-
286 (2005)
(relating to a selection system for transformation of Arabidopsis thaliana via
Agrobacterium
tumefaciens that uses human cytochrome P450 monooxygenases that metabolize
herbicides;
herbicide tolerant seedlings were transformed and selected with the herbicides
acetochlor,
amiprophos-methyl, chlorpropham, chlorsulfuron, norflurazon, and
pendimethalin); Siminszky
et al., "Expression of a soybean cytochrome P450 monooxygenase cDNA in yeast
and tobacco
enhances the metabolism of phenylurea herbicides," PNAS Vol. 96, Issue 4, 1750-
1755, Feb.
16, 1999; Sheldon et al, Weed Science: Vol. 48, No. 3, pp. 291-295, "A
cytochrome P450
monooxygenase cDNA (CYP71A10) confers resistance to linuron in transgenic
Nicotiana
tabacum"; and "Phytoremediation of the herbicides atrazine and metolachlor by
transgenic rice
plants expressing human CYP 1A1, CYP2B6, and CYP2C19," J Agric Food Chem. 2006
Apr.
19; 54(8):2985-91 (relating to testing a human cytochrome p450 monooxygenase
in rice where
the rice plants reportedly showed high tolerance to chloroacetomides
(acetochlor, alachlor,
metoachlor, pretilachlor, and thenylchlor), oxyacetamides (mefenacet),
pyridazinones
(norflurazon), 2,6-dinitroanalines (trifluralin and pendimethalin),
phosphamidates (amiprofos-
methyl, thiocarbamates (pyributicarb), and ureas (chlortoluron)).
[00154] There is also the possibility of altering or using different 2,4-D
chemistries to make
the subject AAD-12 genes more efficient. Such possible changes include
creating better
38
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
substrates and better leaving groups (higher electronegativity). Auxin
transport inhibitors (e.g.
diflufenzopyr) can also be used to increase herbicide activity with 2,4-D.
[00155] Unless specifically indicated or implied, the terms "a," "an," and
"the" signify "at
least one" as used herein. All patents, patent applications, provisional
applications, and
publications referred to or cited herein are incorporated by reference in
their entirety to the
extent they are not inconsistent with the explicit teachings of this
specification.
[00156] Following are examples that illustrate procedures for practicing the
invention.
These examples should not be construed as limiting. All percentages are by
weight and all
solvent mixture proportions are by volume unless otherwise noted.
EXAMPLES
Example 1
Method for Identifying Genes That Impart Resistance to 2,4-D In Planta
[00157] As a way to identify genes which possess herbicide degrading
activities in planta, it
is possible to mine current public databases such as NCBI (National Center for
Biotechnology
Information). To begin the process, it is necessary to have a functional gene
sequence already
identified that encodes a protein with the desired characteristics (i.e., a-
ketoglutarate
dioxygenase activity). This protein sequence is then used as the input for the
BLAST (Basic
Local Alignment Search Tool) (Altschul et al., 1997) algorithm to compare
against available
NCBI protein sequences deposited. Using default settings, this search returns
upwards of 100
homologous protein sequences at varying levels. These range from highly
identical (85-98%)
to very low identity (23-32%) at the amino acid level. Traditionally only
sequences with high
homology would be expected to retain similar properties to the input sequence.
In this case,
only sequences with .gtoreq.50% homology were chosen. As exemplified herein,
cloning and
recombinantly expressing homologues with as little as 31% amino acid
conservation (relative to
tfdA from Ralstonia eutropha) can be used to impart commercial levels of
resistance not only
to the intended herbicide, but also to substrates never previously tested with
these enzymes.
[00158] A single gene (sdpA) was identified from the NCBI database (see the
ncbi.nlm.nih.gov website; accession #AF516752) as a homologue with only 31%
amino acid
identity to tfdA. Percent identity was determined by first translating both
the sdpA and tfdA
DNA sequences deposited in the database to proteins, then using ClustalW in
the VectorNTI
software package to perform the multiple sequence alignment.
39
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Example 2
Optimization of Sequence for Expression in Plants and Bacteria
[00159] To obtain higher levels of expression of heterologous genes in plants,
it may be
preferred to reengineer the protein encoding sequence of the genes so that
they are more
efficiently expressed in plant cells. Maize is one such plant where it may be
preferred to re-
design the heterologous protein coding region prior to transformation to
increase the expression
level of the gene and the level of encoded protein in the plant. Therefore, an
additional step in
the design of genes encoding a bacterial protein is reengineering of a
heterologous gene for
optimal expression.
Table 2: Compilation of G + C contents of protein coding regions of maize
genes
Protein Classa Range % G + C Mean % G + Cb
Metabolic Enzymes (76) 44.4-75.3 59.0 (±8.0)
Structural Proteins (18) 48.6-70.5 63.6 (±6.7)
Regulatory Proteins (5) 57.2-68.8 62.0 (±4.9)
Uncharacterized Proteins (9) 41.5-70.3 64.3 (±7.2)
All Proteins (108) 44.4-75.3 60.8 (±5.2)c
a Number of genes in class given in parentheses.
b Standard deviations given in parentheses.
c Combined groups mean ignored in mean calculation.
[00160] One reason for the reengineering of a bacterial protein for expression
in maize is due
to the non-optimal G+C content of the native gene. For example, the very low
G+C content of
many native bacterial gene(s) (and consequent skewing towards high A+T
content) results in
the generation of sequences mimicking or duplicating plant gene control
sequences that are
known to be highly A+T rich. The presence of some A+T-rich sequences within
the DNA of
gene(s) introduced into plants (e.g., TATA box regions normally found in gene
promoters) may
result in aberrant transcription of the gene(s). On the other hand, the
presence of other
regulatory sequences residing in the transcribed mRNA (e.g., polyadenylation
signal sequences
(AAUAAA), or sequences complementary to small nuclear RNAs involved in pre-
mRNA
splicing) may lead to RNA instability. Therefore, one goal in the design of
genes encoding a
bacterial protein for maize expression, more preferably referred to as plant
optimized gene(s), is
to generate a DNA sequence having a higher G+C content, and preferably one
close to that of
maize genes coding for metabolic enzymes. Another goal in the design of the
plant optimized
gene(s) encoding a bacterial protein is to generate a DNA sequence in which
the sequence
modifications do not hinder translation.
[00161] Table 2 illustrates how high the G+C content is in maize. For the data
in Table 2,
coding regions of the genes were extracted from GenBank (Release 71) entries,
and base
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
compositions were calculated using the MacVectorTM program (Accelerys, San
Diego, Calif.).
Intron sequences were ignored in the calculations.
[00162] Due to the plasticity afforded by the redundancy/degeneracy of the
genetic code
(i.e., some amino acids are specified by more than one codon), evolution of
the genomes in
different organisms or classes of organisms has resulted in differential usage
of redundant
codons. This "codon bias" is reflected in the mean base composition of protein
coding regions.
For example, organisms with relatively low G+C contents utilize codons having
A or T in the
third position of redundant codons, whereas those having higher G+C contents
utilize codons
having G or C in the third position. It is thought that the presence of
"minor" codons within an
mRNA may reduce the absolute translation rate of that mRNA, especially when
the relative
abundance of the charged tRNA corresponding to the minor codon is low. An
extension of this
is that the diminution of translation rate by individual minor codons would be
at least additive
for multiple minor codons. Therefore, mRNAs having high relative contents of
minor codons
would have correspondingly low translation rates. This rate would be reflected
by subsequent
low levels of the encoded protein.
Table 3. Preferred amino acid codons for proteins expressed
in maize
Amino Acid Codon*
Alanine GCC/GCG
Cysteine TGC/TGT
Aspartic Acid GAC/GAT
Glutamic Acid GAG/GAA
Phenylalanine TTC/TTT
Glycine GGC/GGG
Histidine CAC/CAT
Isoleucine ATC/ATT
Lysine AAG/AAA
Leucine CTG/CTC
Methionine ATG
Asparagine AAC/AAT
Proline CCG/CCA
Glutamine CAG/CAA
Arginine AGG/CGC
Serine AGC/TCC
Threonine ACC/ACG
Valine GTG/GTC
Tryptophan TGG
Tryrosine TAC/TAT
Stop TGA/TAG
[00163] In engineering genes encoding a bacterial protein for maize (or other
plant, such as
41
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
cotton or soybean) expression, the codon bias of the plant has been
determined. The codon bias
for maize is the statistical codon distribution that the plant uses for coding
its proteins and the
preferred codon usage is shown in Table 3. After determining the bias, the
percent frequency of
the codons in the gene(s) of interest is determined. The primary codons
preferred by the plant
should be determined, as well as the second, third, and fourth choices of
preferred codons when
multiple choices exist. A new DNA sequence can then be designed which encodes
the amino
sequence of the bacterial protein, but the new DNA sequence differs from the
native bacterial
DNA sequence (encoding the protein) by the substitution of the plant (first
preferred, second
preferred, third preferred, or fourth preferred) codons to specify the amino
acid at each position
within the protein amino acid sequence. The new sequence is then analyzed for
restriction
enzyme sites that might have been created by the modification. The identified
sites are further
modified by replacing the codons with first, second, third, or fourth choice
preferred codons.
Other sites in the sequence which could affect transcription or translation of
the gene of interest
are the exon:intron junctions (5' or 3'), poly A addition signals, or RNA
polymerase termination
signals. The sequence is further analyzed and modified to reduce the frequency
of TA or GC
doublets. In addition to the doublets, G or C sequence blocks that have more
than about four
residues that are the same can affect transcription of the sequence.
Therefore, these blocks are
also modified by replacing the codons of first or second choice, etc. with the
next preferred
codon of choice.
[00164] It is preferred that the plant optimized gene(s) encoding a bacterial
protein contain
about 63% of first choice codons, between about 22% to about 37% second choice
codons, and
between about 15% to about 0% third or fourth choice codons, wherein the total
percentage is
100%. Most preferred the plant optimized gene(s) contains about 63% of first
choice codons, at
least about 22% second choice codons, about 7.5% third choice codons, and
about 7.5% fourth
choice codons, wherein the total percentage is 100%. The method described
above enables one
skilled in the art to modify gene(s) that are foreign to a particular plant so
that the genes are
optimally expressed in plants. The method is further illustrated in PCT
application WO
97/13402.
[00165] Thus, in order to design plant optimized genes encoding a bacterial
protein, a DNA
sequence is designed to encode the amino acid sequence of said protein
utilizing a redundant
genetic code established from a codon bias table compiled from the gene
sequences for the
particular plant or plants. The resulting DNA sequence has a higher degree of
codon diversity,
a desirable base composition, can contain strategically placed restriction
enzyme recognition
sites, and lacks sequences that might interfere with transcription of the
gene, or translation of
42
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
the product mRNA. Thus, synthetic genes that are functionally equivalent to
the proteins/genes
of the subject invention can be used to transform hosts, including plants.
Additional guidance
regarding the production of synthetic genes can be found in, for example, U.S.
Pat. No.
5,380,831.
[00166] AAD-12 Plant Rebuild Analysis: Extensive analysis of the 876 base
pairs (bp) of the
DNA sequence of the native AAD-12 coding region (SEQ ID NO: 1) revealed the
presence of
several sequence motifs that are thought to be detrimental to optimal plant
expression, as well
as a non-optimal codon composition. The protein encoded by SEQ ID NO: 1 (AAD-
12) is
presented as SEQ ID NO: 2. To improve production of the recombinant protein in
monocots as
well as dicots, a "plant-optimized" DNA sequence AAD-12 (v1) (SEQ ID NO: 3)
was
developed that encodes a protein (SEQ ID NO: 4) which is the same as the
native SEQ ID NO:
2 except for the addition of an alanine residue at the second position
(underlined in SEQ ID
NO: 4). The additional alanine codon (GCT; underlined in SEQ ID NO: 3) encodes
part of an
NcoI restriction enzyme recognition site (CCATGG) spanning the ATG
translational start
codon. Thus, it serves the dual purpose of facilitating subsequent cloning
operations while
improving the sequence context surrounding the ATG start codon to optimize
translation
initiation. The proteins encoded by the native and plant-optimized (v1) coding
regions are
99.3% identical, differing only at amino acid number 2. In contrast, the
native and plant-
optimized (v1) DNA sequences of the coding regions are only 79.7% identical.
[00167] Table 4 shows the differences in codon compositions of the native
(Columns A and
D) and plant-optimized sequences (Columns B and E), and allows comparison to a
theoretical
plant-optimized sequence (Columns C and F).
[00168] It is clear from examination of Table 4 that the native and plant-
optimized coding
regions, while encoding nearly identical proteins, are substantially different
from one another.
The Plant-Optimized version (v1) closely mimics the codon composition of a
theoretical plant-
optimized coding region encoding the AAD-12 protein.
43
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Table 4. Codon composition comparisons of coding regions of Native AAD-/2,
Plant-Optimized version
(v1) and a Theoretical Plant-Optimized version.
A B C D E F
Amino Native Plant Opt Theor. Plant Amino Native Plant Opt Theor.
Plant Opt.
Acid Codon # vl # Opt. # Acid Codon # vl # #
ALA GCA 1 10 11 LEU (L) CTA 0 0 0
(A)
GCC 35 16 15 CTC 1 8 8
GCG 7 0 0 CTG 23 0 0
GCT 0 18 17 CTT 0 8 8
ARG AGA 0 4 5 TTA 0 0 0
(R)
AGG 0 4 6 TTG 0 8 8
CGA 0 0 0 LYS (K) AAA 1 1 2
CGC 15 6 4 AAG 5 5 4
CGG 3 0 0 MET ATG 10 10 10
(M)
CGT 0 4 3 PHE (F) TTC 7 5 5
ASN AAC 3 2 2 TTT 1 3 3
(N)
AAT 1 2 2 PRO (P) CCA 0 5 6
ASP (D) GAC 15 9 9 CCC 9 4 4
GAT 2 8 8 CCG 5 0 0
CYS TGC 3 2 2 CCT 0 5 5
(C)
TGT 0 1 1 SER (S) AGC 5 4 3
END TAA 1 0 1 AGT 0 0 0
TAG 0 0 TCA 0 3 3
TGA 0 1 TCC 2 3 3
GLN CAA 1 8 7 TCG 6 0 0
(Q)
CAG 13 6 7 TCT 0 3 3
GLU GAA 3 4 4 THR (T) ACA 1 4 5
(E)
GAG 8 7 7 ACC 11 7 7
GLY GGA 0 8 7 ACG 5 0 0
(G)
GGC 24 7 7 ACT 1 7 6
GGG 1 3 4 TRP (W) TGG 8 8 8
GGT 0 7 7 TYR (Y) TAC 4 3 3
HIS (H) CAC 8 9 9 TAT 1 2 2
CAT 8 7 7 VAL (V) GTA 0 0 0
ILE (I) ATA 0 2 2 GTC 6 8 7
ATC 10 4 5 GTG 18 8 9
ATT 1 5 4GTT 0 8 8 ,
Totals 163 164 163 ' Totals 130 130 130
[00169] Rebuild for E. coli Expression: Specially engineered strains of
Escherichia coli and
associated vector systems are often used to produce relatively large amounts
of proteins for
biochemical and analytical studies. It is sometimes found that a native gene
encoding the
desired protein is not well suited for high level expression in E. coli, even
though the source
organism for the gene may be another bacterial genus. In such cases it is
possible and desirable
44
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
to reengineer the protein coding region of the gene to render it more suitable
for expression in
E. coli. E. coli Class II genes are defined as those that are highly and
continuously expressed
during the exponential growth phase of E. coli cells. (Henaut, A. and Danchin,
A. (1996) in
Escherichia coli and Salmonella typhimurium cellular and molecular biology,
vol. 2, pp. 2047-
2066. Neidhardt, F., Curtiss III, R., Ingraham, J., Lin, E., Low, B.,
Magasanik, B., Reznikoff,
W., Riley, M., Schaechter, M. and Umbarger, H. (eds.) American Society for
Microbiology,
Washington, D.C.). Through examination of the codon compositions of the coding
regions of
E. coli Class II genes, one can devise an average codon composition for these
E. coli-Class II
gene coding regions.
[00170] It is thought that a protein coding region having an average codon
composition
mimicking that of the Class II genes will be favored for expression during the
exponential
growth phase of E. coli. Using these guidelines, a new DNA sequence that
encodes the AAD-
12 protein (SEQ ID NO: 4); including the additional alanine at the second
position, as
mentioned above), was designed according to the average codon composition of
E. coli Class II
gene coding regions. The initial sequence, whose design was based only on
codon
composition, was further engineered to include certain restriction enzyme
recognition
sequences suitable for cloning into E. coli expression vectors. Detrimental
sequence features
such as highly stable stemloop structures were avoided, as were intragenic
sequences
homologous to the 3' end of the 16S ribosomal RNA (L e. Shine Dalgarno
sequences). The E.
coli-optimized sequence (v2) is disclosed as SEQ ID NO: 5 and encodes the
protein disclosed
in SEQ ID NO: 4.
[00171] The native and E. coli-optimized (v2) DNA sequences are 84.0%
identical, while the
plant-optimized (v1) and E. coli-optimized (v2) DNA sequences are 76.0%
identical. Table 5
presents the codon compositions of the native AAD-12 coding region (Columns A
and D), an
AAD-12 coding region optimized for expression in E. coli (v2; Columns B and E)
and the
codon composition of a theoretical coding region for the AAD-12 protein having
an optimal
codon composition of E. coli Class II genes (Columns C and F).
[00172] It is clear from examination of Table 6 that the native and E. coli-
optimized coding
regions, while encoding nearly identical proteins, are substantially different
from one another.
The E. coli-Optimized version (v2) closely mimics the codon composition of a
theoretical E.
coli-optimized coding region encoding the AAD-12 protein.
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Table 5. Codon composition comparisons of coding regions of Native AAD-12, E.
coli-Optimized
version (v2) and a Theoretical E. coli Class II-Optimized version.
A B C D E F
Amino Native E. coli Theor. Amino Native E. coli
Theor, Class
Acid Codon # Opt v2 # Class II #
Acid Codon # Opt v2 # II #
ALA (A) GCA 1 13 13 LEU (L) CTA 0 0 0
GCC 35 0 0 CTC 1 2 0
GCG 7 18 17 CTG 23 20 24
GCT 0 13 14 CTT 0 1 0
ARG (R) AGA 0 0 0 TTA 0 1 0
AGG 0 0 0 TTG 0 0 0
CGA 0 0 0 LYS (K) AAA 1 4 5
CGC 15 6 6 AAG 5 2 1
CGG 3 0 0 MET ATG 10 10 10
(M)
CGT 0 12 12 PHE (F) TTC 7 6 6
ASN (N) AAC 3 4 4 TTT 1 2 2
AAT 1 0 0 PRO (P) CCA 0 3 2
ASP (D) GAC 15 10 9 CCC 9 0 0
GAT 2 7 8 CCG 5 11 12
CYS (C) TGC 3 2 2 CCT 0 0 0
TGT 0 1 1 SER (S) AGC 5 4 4
END TAA 1 1 1 AGT 0 0 0
TAG 0 0 0 TCA 0 0 0
TGA 0 0 0 TCC 2 5 4
GLN (Q) CAA 1 3 3 TCG 6 0 0
CAG 13 11 11 TCT 0 4 5
GLU (E) GAA 3 8 8 THR (T) ACA 1 0 0
GAG 8 3 3 ACC 11 12 12
GLY (G) GGA 0 0 0 ACG 5 0 0
GGC 24 12 11 ACT 1 6 6
GGG 1 0 0 TRP (W) TGG 8 8 8
GGT 0 13 14 TYR (Y) TAC 4 3 3
HIS (H) CAC 8 11 11 TAT 1 2 2
CAT 8 5 5 VAL (V) GTA 0 6 6
ILE (I) ATA 0 0 0 GTC 6 0 0
ATC 10 7 7 GTG 18 8 7
ATT 1 4 4 GTT 0 10 11
Totals 163 164 164 Totals 130 130 130
[00173] Design of a soybean-codon-biased DNA sequence encoding a soybean EPSPS
having mutations that confer glyphosate tolerance. This example teaches the
design of a new
DNA sequence that encodes a mutated soybean 5-enolpyruvoylshikimate 3-
phosphate synthase
(EPSPS), but is optimized for expression in soybean cells. The amino acid
sequence of a triply-
mutated soybean EPSPS is disclosed as SEQ ID NO: 5 of WO 2004/009761. The
mutated
amino acids in the so-disclosed sequence are at residue 183 (threonine of
native protein
replaced with isoleucine), residue 186 (arginine in native protein replaced
with lysine), and
residue 187 (proline in native protein replaced with serine). Thus, one can
deduce the amino
46
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
acid sequence of the native soybean EPSPS protein by replacing the substituted
amino acids of
SEQ ID NO:5 of WO 2004/009761 with the native amino acids at the appropriate
positions.
Such native protein sequence is disclosed as SEQ ID NO: 20 of
PCT/U52005/014737 (filed
May 2, 2005). A doubly mutated soybean EPSPS protein sequence, containing a
mutation at
residue 183 (threonine of native protein replaced with isoleucine), and at
residue 187 (proline in
native protein replaced with serine) is disclosed as SEQ ID NO: 21 of
PCT/U52005/014737.
[00174] A codon usage table for soybean (Glycine max) protein coding
sequences,
calculated from 362,096 codons (approximately 870 coding sequences), was
obtained from the
"kazusa.or.jp/codon" World Wide Web site. Those data were reformatted as
displayed in Table
6. Columns D and H of Table 6 present the distributions (in % of usage for all
codons for that
amino acid) of synonymous codons for each amino acid, as found in the protein
coding regions
of soybean genes. It is evident that some synonymous codons for some amino
acids (an amino
acid may be specified by 1, 2, 3, 4, or 6 codons) are present relatively
rarely in soybean protein
coding regions (for example, compare usage of GCG and GCT codons to specify
alanine).
[00175] A biased soybean codon usage table was calculated from the data in
Table 6.
Codons found in soybean genes less than about 10% of total occurrences for the
particular
amino acid were ignored. To balance the distribution of the remaining codon
choices for an
amino acid, a weighted average representation for each codon was calculated,
using the
formula:
Weighted % of C 1 =1/(%C1 + %C2 + %C3 + etc.) x %Cl x 100
where Cl is the codon in question, C2, C3, etc. represent the remaining
synonymous codons,
and the % values for the relevant codons are taken from columns D and H of
Table 6 (ignoring
the rare codon values in bold font).
[00176] The Weighted % value for each codon is given in Columns C and G of
Table 6.
TGA was arbitrarily chosen as the translation terminator. The biased codon
usage frequencies
were then entered into a specialized genetic code table for use by the
OptGeneTM gene design
program (Ocimum Biosolutions LLC, Indianapolis, Ind.).
47
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Table 6. Synonymous codon representation in soybean protein coding sequences,
and
calculation of a biased codon representation set for soybean-optimized
synthetic gene
design.
A B C D E F G H
Amino Weighted Soybean Amino Weighted
Soybean
Acid Codon % % Acid Codon % %
ALA GCA 33.1 30.3 LEU (L) CTA DNU 9.1
(A)
GCC 24.5 22.5 CTC 22.4 18.1
GCG DNU* 8.5 CTG 16.3
13.2
GCT 42.3 38.7 CTT 31.5 25.5
ARG AGA 36.0 30.9 TTA DNU 9.8
(R)
AGG 32.2 27.6 TTG 29.9 24.2
CGA DNU 8.2 LYS (K) AAA 42.5
42.5
CGC 14.8 12.7 AAG 57.5 57.5
CGG DNU 6.0 MET (M) ATG 100.0
100
CGT 16.9 14.5 PHE (F) TTC 49.2 49.2
ASN AAC 50.0 50.0 TTT 50.8 50.8
(N)
AAT 50.0 50.0 PRO (P) CCA 39.8
36.5
ASP (D) GAC 38.1 38.1 CCC 20.9 19.2
GAT 61.9 61.9 CCG DNU 8.3
CYS (C) TGC 50.0 50.0 CCT 39.3 36.0
TGT 50.0 50.0 SER (S) AGC 16.0 15.1
END TAA DNU 40.7 AGT 18.2 17.1
TAG DNU 22.7 TCA 21.9 20.6
TGA 100.0 36.6 TCC 18.0 16.9
GLN CAA 55.5 55.5 TCG DNU 6.1
(Q)
CAG 44.5 44.5 TCT 25.8 24.2
GLU (E) GAA 50.5 50.5 THR (T) ACA 32.4
29.7
GAG 49.5 49.5 ACC 30.2 27.7
GLY GGA 31.9 31.9 ACG DNU 8.3
(G)
GGC 19.3 19.3 ACT 37.4 34.3
GGG 18.4 18.4 TRP (W) TGG 100.0
100
GGT 30.4 30.4 TYR (Y) TAC 48.2
48.2
HIS (H) CAC 44.8 44.8 TAT 51.8 51.8
CAT 55.2 55.2 VAL (V) GTA 11.5
11.5
ILE (I) ATA 23.4 23.4 GTC 17.8 17.8
ATC 29.9 29.9 GTG 32.0 32.0
ATT 46.7 46.7 GTT 38.7 38.7
[00177] To derive a soybean-optimized DNA sequence encoding the doubly mutated
EPSPS
protein, the protein sequence of SEQ ID NO: 21 from PCT/U52005/014737 was
reverse-
48
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
translated by the OptGeneTM program using the soybean-biased genetic code
derived above.
The initial DNA sequence thus derived was then modified by compensating codon
changes
(while retaining overall weighted average representation for the codons) to
reduce the numbers
of CG and TA doublets between adjacent codons, increase the numbers of CT and
TG doublets
between adjacent codons, remove highly stable intrastrand secondary
structures, remove or add
restriction enzyme recognition sites, and to remove other sequences that might
be detrimental to
expression or cloning manipulations of the engineered gene. Further
refinements of the
sequence were made to eliminate potential plant intron splice sites, long runs
of A/T or C/G
residues, and other motifs that might interfere with RNA stability,
transcription, or translation
of the coding region in plant cells. Other changes were made to eliminate long
internal Open
Reading Frames (frames other than +1). These changes were all made within the
constraints of
retaining the soybean-biased codon composition as described above, and while
preserving the
amino acid sequence disclosed as SEQ ID NO: 21 of PCT/US2005/014737.
[00178] The soybean-biased DNA sequence that encodes the EPSPS protein of SEQ
ID NO:
21 is disclosed as bases 1-1575 of SEQ ID NO: 22 of PCT/U52005/014737.
Synthesis of a
DNA fragment comprising SEQ ID NO: 22 of PCT/U52005/014737 was performed by a
commercial supplier (PicoScript, Houston Tex.).
EXAMPLE 3
Cloning of Expression and Transformation Vectors
[00179] Construction of E. coli, pET Expression Vector: Using the restriction
enzymes
corresponding to the sites added with the additional cloning linkers (Xba 1,
Xho 1) AAD-12
(v2) was cut out of the picoscript vector, and ligated into a pET280
streptomycin/spectinomycin
resistant vector. Ligated products were then transformed into TOP1OF' E. coli,
and plated on to
Luria Broth + 50 lug/m1 Streptomycin & Spectinomycin (LB S/S) agar plates.
[00180] To differentiate between AAD-12 (v2): pET280 and pCR2.1: pET280
ligations,
approximately 20 isolated colonies were picked into 6 ml of LB-S/S, and grown
at 37 C. for 4
hours with agitation. Each culture was then spotted onto LB + Kanamycin 50
lug/m1 plates,
which were incubated at 37 C. overnight. Colonies that grew on the LB-K were
assumed to
have the pCR2.1 vector ligated in, and were discarded. Plasmids were isolated
from the
remaining cultures as before, and checked for correctness with digestion by
XbaI/XhoI. The
final expression construct was given the designation pDAB3222.
[00181] Construction of Pseudomonas Expression Vector: The AAD-12 (v2) open
reading
frame was initially cloned into the modified pET expression vector (Novagen),
"pET280 S/S,"
49
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
as an XbaI-XhoI fragment. The resulting plasmid pDAB725 was confirmed with
restriction
enzyme digestion and sequencing reactions. The AAD-12 (v2) open reading frame
from
pDAB725 was transferred into the Pseudomonas expression vector, pMYC1803, as
an XbaI-
XhoI fragment. Positive colonies were confirmed via restriction enzyme
digestion. The
completed construct pDAB739 was transformed into the MB217 and MB324
Pseudomonas
expression strains.
[00182] Completion of Binary Vectors: The plant optimized gene AAD-12 (v1) was
received
from Picoscript (the gene rebuild design was completed (see above) and out-
sourced to
Picoscript for construction) and sequence verified (SEQ ID NO: 3) internally,
to confirm that
no alterations of the expected sequence were present. The sequencing reactions
were carried
out with M13 Forward (SEQ ID NO: 6) and M13 Reverse (SEQ ID NO: 7) primers
using the
Beckman Coulter "Dye Terminator Cycle Sequencing with Quick Start Kit"
reagents as before.
Sequence data was analyzed and results indicated that no anomalies were
present in the plant
optimized AAD-12 (v1) DNA sequence. The AAD-12 (v1) gene was cloned into
pDAB726 as
an Nco I-Sac I fragment. The resulting construct was designated pDAB723,
containing:
[AtUbil0 promoter: Nt OSM 5'UTR: AAD-12 (v1): Nt 05M3'UTR: ORF1 polyA 3'UTR]
(verified with a PvuII and a Not I restriction digests). A Not I-Not I
fragment containing the
described cassette was then cloned into the Not I site of the binary vector
pDAB3038. The
resulting binary vector, pDAB724, containing the following cassette [AtUbil0
promoter: Nt
OSM5'UTR: AAD-12 (v1): Nt OSM 3'UTR: ORF1 polyA 3'UTR: CsVMV promoter: PAT:
ORF25/26 3'UTR] was restriction digested (with Bam HI, Nco I, Not I, SacI, and
Xmn I) for
verification of the correct orientation. The verified completed construct
(pDAB724) was used
for transformation into Agrobacterium.
[00183] Cloning of Additional Transformation Constructs: All other constructs
created for
transformation into appropriate plant species were built using similar
procedures as previously
described herein, and other standard molecular cloning methods (Maniatis et
al., 1982).
Example 4
Transformation into Arabidopsis and Selection
[00184] Arabidopsis thaliana Growth Conditions: Wild type Arabidopsis seed was
suspended in a 0.1% Agarose (Sigma Chemical Co., St. Louis, Mo.) solution. The
suspended
seed was stored at 4 C. for 2 days to complete dormancy requirements and
ensure synchronous
seed germination (stratification).
[00185] Sunshine Mix LP5 (Sun Gro Horticulture, Bellevue, Wash.) was covered
with fine
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
vermiculite and sub-irrigated with Hoagland's solution until wet. The soil mix
was allowed to
drain for 24 hours. Stratified seed was sown onto the vermiculite and covered
with humidity
domes (KORD Products, Bramalea, Ontario, Canada) for 7 days.
[00186] Seeds were germinated and plants were grown in a Conviron (models
CMP4030 and
CMP3244, Controlled Environments Limited, Winnipeg, Manitoba, Canada) under
long day
conditions (16 hours light/8 hours dark) at a light intensity of 120-150
[tmo1/m2 sec under
constant temperature (22 C.) and humidity (40-50%). Plants were initially
watered with
Hoagland's solution and subsequently with deionized water to keep the soil
moist but not wet.
[00187] Agrobacterium Transformation: An LB + agar plate with erythromycin
(Sigma
Chemical Co., St. Louis, Mo.) (200 mg/L) or spectinomycin (100 mg/L)
containing a streaked
DH5a colony was used to provide a colony to inoculate 4 ml mini prep cultures
(liquid LB +
erythromycin). The cultures were incubated overnight at 37 C. with constant
agitation.
Qiagen (Valencia, Calif.) Spin Mini Preps, performed per manufacturer's
instructions, were
used to purify the plasmid DNA.
[00188] Electro-competent Agrobacterium tumefaciens (strains Z707s, EHA101s,
and
LBA4404s) cells were prepared using a protocol from Weigel and Glazebrook
(2002). The
competent Agrobacterium cells were transformed using an electroporation method
adapted
from Weigel and Glazebrook (2002). 50 jai of competent agro cells were thawed
on ice and 10-
25 ng of the desired plasmid was added to the cells. The DNA and cell mix was
added to pre-
chilled electroporation cuvettes (2 mm). An Eppendorf Electroporator 2510 was
used for the
transformation with the following conditions, Voltage: 2.4 kV, Pulse length: 5
msec.
[00189] After electroporation, 1 ml of YEP broth (per liter: 10 g yeast
extract, 10 g Bacto-
peptone, 5 g NaC1) was added to the cuvette, and the cell-YEP suspension was
transferred to a
15 ml culture tube. The cells were incubated at 28 C. in a water bath with
constant agitation
for 4 hours. After incubation, the culture was plated on YEP + agar with
erythromycin (200
mg/L) or spectinomycin (100 mg/L) and streptomycin (Sigma Chemical Co., St.
Louis, Mo.)
(250 mg/L). The plates were incubated for 2-4 days at 28 C.
[00190] Colonies were selected and streaked onto fresh YEP + agar with
erythromycin (200
mg/L) or spectinomycin (100 mg/L) and streptomycin (250 mg/L) plates and
incubated at 28
C. for 1-3 days. Colonies were selected for PCR analysis to verify the
presence of the gene
insert by using vector specific primers. Qiagen Spin Mini Preps, performed per
manufacturer's
instructions, were used to purify the plasmid DNA from selected Agrobacterium
colonies with
the following exception: 4 ml aliquots of a 15 ml overnight mini prep culture
(liquid YEP +
erythromycin (200 mg/L) or spectinomycin (100 mg/L)) and streptomycin (250
mg/L)) were
51
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
used for the DNA purification. An alternative to using Qiagen Spin Mini Prep
DNA was lysing
the transformed Agrobacterium cells, suspended in 10 [t.1 of water, at 100 C.
for 5 minutes.
Plasmid DNA from the binary vector used in the Agrobacterium transformation
was included as
a control. The PCR reaction was completed using Taq DNA polymerase from Takara
Mirus
Bio Inc. (Madison, Wis.) per manufacturer's instructions at 0.5 x
concentrations. PCR reactions
were carried out in a MJ Research Peltier Thermal Cycler programmed with the
following
conditions; 1) 94 C. for 3 minutes, 2) 94 C. for 45 seconds, 3) 55 C. for
30 seconds, 4) 72 C.
for 1 minute, for 29 cycles then 1 cycle of 72 C. for 10 minutes. The
reaction was maintained
at 4 C. after cycling. The amplification was analyzed by 1% agarose gel
electrophoresis and
visualized by ethidium bromide staining. A colony was selected whose PCR
product was
identical to the plasmid control.
[00191] Arabidopsis Transformation: Arabidopsis was transformed using the
floral dip
method. The selected colony was used to inoculate one or more 15-30 ml pre-
cultures of YEP
broth containing erythromycin (200 mg/L) or spectinomycin (100 mg/L) and
streptomycin (250
mg/L). The culture(s) was incubated overnight at 28 C. with constant
agitation at 220 rpm.
Each pre-culture was used to inoculate two 500 ml cultures of YEP broth
containing
erythromycin (200 mg/L) or spectinomycin (100 mg/L) and streptomycin (250
mg/L) and the)
cultures were incubated overnight at 28 C. with constant agitation. The cells
were then
pelleted at approx. 8700 x g for 10 minutes at room temperature, and the
resulting supernatant
discarded. The cell pellet was gently resuspended in 500 ml infiltration media
containing: 1/2 x
Murashige and Skoog salts/Gamborg's B5 vitamins, 10% (w/v) sucrose, 0.044 [t.M
benzylamino
purine (10 p1/liter of 1 mg/ml stock in DMSO) and 300 p1/liter Silwet L-77.
Plants
approximately 1 month old were dipped into the media for 15 seconds, being
sure to submerge
the newest inflorescence. The plants were then laid down on their sides and
covered
(transparent or opaque) for 24 hours, then washed with water, and placed
upright. The plants
were grown at 22 C., with a 16-hour light/8-hour dark photoperiod.
Approximately 4 weeks
after dipping, the seeds were harvested.
[00192] Selection of Transformed Plants: Freshly harvested T1 seed [AAD-12
(v1) gene]
was allowed to dry for 7 days at room temperature. T1 seed was sown in 26.5 x
51-cm
germination trays (T.O. Plastics Inc., Clearwater, Minn.), each receiving a
200 mg aliquots of
stratified T1 seed (.about.10,000 seed) that had previously been suspended in
40 ml of 0.1%
agarose solution and stored at 4 C. for 2 days to complete dormancy
requirements and ensure
synchronous seed germination.
[00193] Sunshine Mix LP5 (Sun Gro Horticulture Inc., Bellevue, Wash.) was
covered with
52
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
fine vermiculite and subirrigated with Hoagland's solution until wet, then
allowed to gravity
drain. Each 40 ml aliquot of stratified seed was sown evenly onto the
vermiculite with a pipette
and covered with humidity domes (KORD Products, Bramalea, Ontario, Canada) for
4-5 days.
Domes were removed 1 day prior to initial transformant selection using
glufosinate
postemergence spray (selecting for the co-transformed PAT gene).
[00194] Seven days after planting (DAP) and again 11 DAP, T1 plants (cotyledon
and 2-4-1f
stage, respectively) were sprayed with a 0.2% solution of Liberty herbicide
(200 g ai/L
glufosinate, Bayer Crop Sciences, Kansas City, Mo.) at a spray volume of 10
ml/tray (703 L/ha)
using a DeVilbiss compressed air spray tip to deliver an effective rate of 280
g ai/ha glufosinate
per application. Survivors (plants actively growing) were identified 4-7 days
after the final
spraying and transplanted individually into 3-inch pots prepared with potting
media (Metro Mix
360). Transplanted plants were covered with humidity domes for 3-4 days and
placed in a 22
C. growth chamber as before or moved to directly to the greenhouse. Domes were
subsequently removed and plants reared in the greenhouse (22 5 C., 50 30%
RH, 14 h
light:10 dark, minimum 500 [tE/m2 s1 natural + supplemental light) at least 1
day prior to
testing for the ability of AAD-12 (v1) (plant optimized gene) to provide
phenoxy auxin
herbicide resistance.
[00195] T1 plants were then randomly assigned to various rates of 2,4-D. For
Arabidopsis,
50 g ae/ha 2,4-D is an effective dose to distinguish sensitive plants from
ones with meaningful
levels of resistance. Elevated rates were also applied to determine relative
levels of resistance
(50, 200, 800, or 3200 g ae/ha).
[00196] All auxin herbicide applications were made using the DeVilbiss sprayer
as described
above to apply 703 L/ha spray volume (0.4 ml solution/3-inch pot) or applied
by track sprayer
in a 187 L/ha spray volume. 2,4-D used was either technical grade (Sigma, St.
Louis, Mo.)
dissolved in DMSO and diluted in water (<1% DMSO final concentration) or the
commercial
dimethylamine salt formulation (456 g ae/L, NuFarm, St Joseph, Mo.).
Dichlorprop used was
commercial grade formulated as potassium salt of R-dichlorprop (600 g ai/L, AH
Marks). As
herbicide rates increased beyond 800 g ae/ha, the pH of the spray solution
became exceedingly
acidic, burning the leaves of young, tender Arabidopsis plants and
complicating evaluation of
the primary effects of the herbicides. It became standard practice to apply
these high rates of
herbicides in 200 mM HEPES buffer, pH 7.5.
[00197] Some T1 individuals were subjected to alternative commercial
herbicides instead of
a phenoxy auxin. One point of interest was determining whether the
pyridyloxyacetate auxin
herbicides, triclopyr and fluoroxypyr, could be effectively degraded in
planta. Herbicides were
53
CA 02876144 2014-12-05
WO 2013/185036
PCT/US2013/044717
applied to T1 plants with use of a track sparyer in a 187 L/ha spray volume.
T1 plants that
exhibited tolerance to 2,4-D DMA were further accessed in the T2 generation.
[00198] Results of Selection of Transformed Plants: The first Arabidopsis
transformations
were conducted using AAD-12 (v1) (plant optimized gene). T1 transformants were
first
selected from the background of untransformed seed using a glufosinate
selection scheme.
Over 300,000 T1 seed were screened and 316 glufosinate resistant plants were
identified (PAT
gene), equating to a transformation/selection frequency of 0.10% which lies in
the normal range
of selection frequency of constructs where PAT + Liberty are used for
selection. T1 plants
selected above were subsequently transplanted to individual pots and sprayed
with various rates
of commercial aryloxyalkanoate herbicides.
Table 7. AAD-12 vl (plant optimized)-transformed T1 Arabidopsis response to a
range of
2,4-D rates applied postemergence compared to or AAD-1 v3 (T4) homozygous
resistant
population, Pat-CrylF transformed, auxin-sensitive control.
% Injury % Injury
AAD-12 vl gene T1 transformants Std
Averages <20% 20-40% >40% Ave Dev
Untreated control-buffer 6 0 0 0 0
50 g ae/ha 2,4-D 6 0 2 16 24
200 g ae/ha 2,4-D 6 1 1 11 18
800 g ae/ha 2,4-D 5 2 1 15 20
3200 g ae/ha 2,4-D 8 0 0 6 6
% Injury % Injury
PAT/CrylF (transformed control) Ave Std
Averages <20% 20-40% >40% Dev
Untreated control-buffer 10 0 0 0 0
50 g ae/ha 2,4-D 4 1 5 31 16
200 g ae/ha 2,4-D 0 0 10 70 2
800 g ae/ha 2,4-D 0 0 10 81 8
3200 g ae/ha 2,4-D 0 0 10 91 2
Homozygous AAD-1 (v3) gene T4 % Injury % Injury
plants Ave Std
Averages <20% 20-40% >40% Dev
Untreated control-buffer 10 0 0 0 0
50 g ae/ha 2,4-D 10 0 0 0 0
200 g ae/ha 2,4-D 10 0 0 0 0
800 g ae/ha 2,4-D 10 0 0 0 0
3200g ae/ha 2,4-D 9 1 0 2 6
[00199] Table 7 compares the response of AAD-12 (v1) and control genes to
impart 2,4-D
resistance to Arabidopsis T1 transformants. Response is presented in terms of
% visual injury 2
WAT. Data are presented as a histogram of individuals exhibiting little or no
injury (<20%),
54
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
moderate injury (20-40%), or severe injury (>40%). Since each T1 is an
independent
transformation event, one can expect significant variation of individual T1
responses within a
given rate. An arithmetic mean and standard deviation is presented for each
treatment. The
range in individual response is also indicated in the last column for each
rate and
transformation. PAT/Cry1F-transformed Arabidopsis served as an auxin-sensitive
transformed
control. The AAD-12 (v1) gene imparted herbicide resistance to individual T1
Arabidopsis
plants. Within a given treatment, the level of plant response varied greatly
and can be attributed
to the fact each plant represents an independent transformation event.
Table 8. T1 Arabidopsis response to a range of R-dichlorprop rates
applied postemergence.
AAD-12 vl gene % Injury % Injury
Averages <20% 20-40% >40% Ave Std Dev
Untreated control 6 0 0 0 0
50 g ae/ha R-dichlorprop 0 0 8 63 7
200 g ae/ha R-dichlorprop 0 0 8 85 10
800 g ae/ha R-dichlorprop 0 0 8 96 4
3200 g ae/ha R-dichlorprop 0 0 8 98 2
PAT/CrylF % Injury % Injury
Averages <20% 20-40% >40% Ave Std Dev
Untreated control 10 0 0 0 0
50 g ae/ha R-dichlorprop 0 10 0 27 2
200 g ae/ha R-dichlorprop 0 0 10 69 3
800 g ae/ha R-dichlorprop 0 0 10 83 6
3200 g ae/ha R-dichlorprop 0 0 10 90 2
Homozygous AAD-1 (v3) % Injury % Injury
gene T4 plants <20% 20-40% >40% Ave Std Dev
Untreated control 10 0 0 0 0
50 g ae/ha R-dichlorprop 10 0 0 0 0
200 g ae/ha R-dichlorprop 10 0 0 0 0
800 g ae/ha R-dichlorprop 10 0 0 0 0
3200 g ae/ha R-dichlorprop 10 0 0 0 0
[00200] Of important note, at each 2,4-D rate tested, there were
individuals that were
unaffected while some were severely affected. An overall population injury
average by rate is
presented in Table 7 simply to demonstrate the significant difference between
the plants
transformed with AAD-12 (v1) versus the wild type or PAT/Cry1F-transformed
controls.
Injury levels tend to be greater and the frequency of uninjured plants was
lower at elevated rates
up to 3,200 g ae/ha (or ¨6 x field rate). Also at these high rates, the spray
solution becomes
highly acidic unless buffered. Arabidopsis grown mostly in the growth chamber
has a very thin
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
cuticle and severe burning effects can complicate testing at these elevated
rates. Nonetheless,
many individuals have survived 3,200 g ae/ha 2,4-D with little or no injury.
[00201] Table 8 shows a similarly conducted dose response of T1 Arabidopsis to
the
phenoxypropionic acid, dichlorprop. The data shows that the herbicidally
active (R-) isomer of
dichlorprop does not serve as a suitable substrate for AAD-12 (v1). The fact
that AAD-1 will
metabolize R-dichlorprop well enough to impart commercially acceptable
tolerance is one
distinguishing characteristic that separates the two genes. (Table 8). AAD-1
and AAD-12 are
considered R- and S-specific a-ketoglutarate dioxygenases, respectively.
[00202] AAD-12 (v1) as a Selectable Marker: The ability to use AAD-12 (v1) as
a selectable
marker using 2,4-D as the selection agent was analyzed initially with
Arabidopsis transformed
as described above. Approximately 50 T4 generation Arabidopsis seed
(homozygous for A
AD-12 (v1)) were spiked into approximately 5,000 wild type (sensitive) seed.
Several
treatments were compared, each tray of plants receiving either one or two
application timings of
2,4-D in one of the following treatment schemes: 7 DAP, 11 DAP, or 7 followed
by 11 DAP.
Since all individuals also contained the PAT gene in the same transformation
vector, AAD-12
selected with 2,4-D could be directly compared to PAT selected with
glufosinate.
[00203] Treatments were applied with a DeVilbiss spray tip as previously
described. Plants
were identified as Resistant or Sensitive 17 DAP. The optimum treatment was 75
g ae/ha 2,4-D
applied 7 and 11 days after planting (DAP), was equally effective in selection
frequency, and
resulted in less herbicidal injury to the transformed individuals than the
Liberty selection
scheme. These results indicate AAD-12 (v1) can be effectively used as an
alternative selectable
marker for a population of transformed Arabidopsis.
[00204] Heritability: A variety of T1 events were self-pollinated to produce
T2 seed. These
seed were progeny tested by applying 2,4-D (200 g ae/ha) to 100 random T2
siblings. Each
individual T2 plant was transplanted to 7.5-cm square pots prior to spray
application (track
sprayer at 187 L/ha applications rate). Seventy-five percent of the T1
families (T2 plants)
segregated in the anticipated 3 Resistant:1 Sensitive model for a dominantly
inherited single
locus with Mendelian inheritance as determined by Chi square analysis
(P>0.05).
[00205] Seed were collected from 12 to 20 T2 individuals (T3 seed). Twenty-
five T3
siblings from each of eight randomly-selected T2 families were progeny tested
as previously
described. Approximately one-third of the T2 families anticipated to be
homozygous (non-
segregating populations) have been identified in each line. These data show
AAD-12 (v1) is
stably integrated and inherited in a Mendelian fashion to at least three
generations.
56
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Table 9. Comparison of T2 AAD-12 (v1) and transformed control Arabidopsis
plant
response to various foliar-applied auxinic herbicides.
Pyridyloxyacetic auxins
Ave % Injury 14DAT
Segregating T2 AAD-12 (v/)plants
Herbicide Treatment (pDAB724.01.120) Pat/Crylf -
Control
280 g ae/ha Triclopyr 0 52
560 g ae/ha Triclopyr 3 58
1120 g ae/ha Triclopyr 0 75*
2240 g ae/ha Triclopyr 3 75*
280 g ae/ha Fluroxypyr 0 75*
560 g ae/ha Fluroxypyr 2 75*
1120 g ae/ha Fluroxypyr 3 75*
2240 g ae/ha Fluroxypyr 5 75*
Inactive DCP metabolite
280 g ae/ha 2,4-DCP 0 0
560 g ae/ha 2,4-DCP 0 0
1120 g ae/ha 2,4-DCP 0 0
2240 g ae/ha 2,4-DCP 0 0
[00206] Additional Foliar Applications Herbicide Resistance in AAD-12
Arabidopsis: The
ability of AAD-12 (v1) to provide resistance to other aryloxyalkanoate auxin
herbicides in
transgenic Arabidopsis was determined by foliar application of various
substrates. T2
generation Arabidopsis seed was stratified, and sown into selection trays much
like that of
Arabidopsis. A transformed-control line containing PAT and the insect
resistance gene Cryl F
was planted in a similar manner. Seedlings were transferred to individual 3-
inch pots in the
greenhouse. All plants were sprayed with the use of a track sprayer set at 187
L/ha. The plants
were sprayed with a range of pyridyloxyacetate herbicides: 280-2240 g ae/ha
triclopyr (Garlon
3A, Dow AgroSciences) and 280-2240 g ae/ha fluoroxypyr (Starane, Dow
AgroSciences); and
the 2,4-D metabolite resulting from AAD-12 activity, 2,4-dichlorophenol (DCP,
Sigma) (at a
molar equivalent to 280-2240 g ae/ha of 2,4-D, technical grade DCP was used).
All
applications were formulated in water. Each treatment was replicated 3-4
times. Plants were
evaluated at 3 and 14 days after treatment.
[00207] There is no effect of the 2,4-D metabolite, 2,4-dichlorophenol (DCP),
on transgenic
non-AAD-12 control Arabidopsis (Pat/Cry1F). AAD-12-transformed plants were
also clearly
protected from the triclopyr and fluoroxypyr herbicide injury that was seen in
the transformed
non-resistant controls (see Table 9). These results confirm that AAD-12 (v1)
in Arabidopsis
provides resistance to the pyridyloxyacetic auxins tested. This is the first
report of an enzyme
57
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
with significant activity on pyridyloxyacetic acid herbicides. No other 2,4-D
degrading enzyme
has been reported with similar activity.
[00208] Molecular Analysis of AAD-12 (v1) Arabidopsis: Invader Assay (methods
of Third
Wave Agbio Kit Procedures) for PAT gene copy number analysis was performed
with total
DNA obtained from Qiagen DNeasy kit on multiple AAD-12 (v1) homozygous lines
to
determine stable integration of the plant transformation unit containing PAT
and AAD-12 (v1).
Analysis assumed direct physical linkage of these genes as they were contained
on the same
plasmid.
[00209] Results showed that all 2,4-D resistant plants assayed, contained PAT
(and thus by
inference, AAD-12 (v1)). Copy number analysis showed total inserts ranged from
1 to 5 copies.
This correlates, too, with the AAD-12 (v1) protein expression data indicating
that the presence
of the enzyme yields significantly high levels of resistance to all
commercially available
phenoxyacetic and pyridyloxyacetic acids.
[00210] Arabidopsis Transformed with Molecular Stack of AAD-12 (v1) and a
Glyphosate
Resistance Gene: T1 Arabidopsis seed was produced, as previously described,
containing the
pDAB3759 plasmid (AAD-12 (v1) + EPSPS) which encodes a putative glyphosate
resistance
trait. T1 transformants were selected using AAD-12 (v1) as the selectable
marker as described.
T1 plants (individually transformed events) were recovered from the first
selection attempt and
transferred to three-inch pots in the greenhouse as previously described.
Three different control
Arabidopsis lines were also tested: wild type Columbia-0, AAD-12 (v1) + PAT T4
homozygous
lines (pDAB724-transformed), and PAT + CrylF homozygous line (transformed
control). The
pDAB3759 and pDAB724 transformed plants were pre-selected at the seedling
stage for 2,4-D
tolerance. Four days after transplanting, plants were evenly divided for
foliar treatment by
track sprayer as previously described with 0, 26.25, 105, 420, or 1680 g ae/ha
glyphosate
(Glyphomax Plus, Dow AgroSciences) in water. All treatments were replicated 5
to 20 times.
Plants were evaluated 7 and 14 days after treatment.
58
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Table 10. T1 Arabidopsis response to a range of glyphosate rates
applied postemergence (14 DAT).
AAD-12 v 1 gene + EPSPS + % Injury % Injury
HptII (pDAB3759)
(Averages) <20% 20-40% >40% Ave Std Dev
Untreated control 5 0 0 0 0
26.25 g ae/ha glyphosate 13 2 1 11 16
105 g ae/ha glyphosate 10 1 5 34 38
420 g ae/ha glyphosate 5 6 5 44 37
1680 g ae/ha glyphosate 0 0 16 85 9
PAT/CrylF % Injury % Injury
Averages <20% 20-40% >40% Ave Std Dev
Untreated control 5 0 0 0 0
26.25 g ae/ha glyphosate 0 0 5 67 7
105 g ae/ha glyphosate 0 0 5 100 0
420 g ae/ha glyphosate 0 0 5 100 0
1680 g ae/ha glyphosate 0 0 5 100 0
Wild type (Col-0) % Injury % Injury
Averages <20% 20-40% >40% Ave Std Dev
Untreated control 5 0 0 0 0
26.25 g ae/ha glyphosate 0 0 5 75 13
105 g ae/ha glyphosate 0 0 5 100 0
420 g ae/ha glyphosate 0 0 5 100 0
1680 g ae/ha glyphosate 0 0 5 100 0
pDAB724 T4 (PAT + AAD- % Injury % Injury
12) Averages <20% 20-40% >40% Ave Std Dev
Untreated control 5 0 0 0 0
26.25 g ae/ha glyphosate 0 0 5 66 8
105 g ae/ha glyphosate 0 0 5 100 0
420 g ae/ha glyphosate 0 0 5 100 0
1680 g ae/ha glyphosate 0 0 5 100 0
[00211] Initial resistance assessment indicated plants tolerant to 2,4-D were
subsequently
tolerant to glyphosate when compared to the response of the three control
lines. These results
indicate that resistance can be imparted to plants to two herbicides with
differing modes of
action, including 2,4-D and glyphosate tolerance, allowing application of both
herbicides
postemergence. Additionally, AAD-12 + 2,4-D was used effectively as a
selectable marker for
a true resistance selection.
[00212] AAD-12 Arabidopsis Genetically Stacked with AAD-1 to Give Wider
Spectrum of
Herbicide Tolerance: AAD-12 (v1) (pDAB724) and AAD-1 (v3) (pDAB721) plants
were
59
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
reciprocally crossed and Fl seed was collected. Eight Fl seeds were planted
and allowed to
grow to produce seed. Tissue samples were taken from the eight Fl plants and
subjected to
Western analysis to confirm the presence of both genes. It was concluded that
all 8 plants
tested expressed both AAD-1 and AAD-12 proteins. The seed was bulked and
allowed to dry
for a week before planting.
[00213] One hundred F2 seeds were sown and 280 g ai/ha glufosinate was
applied. Ninety-
six F2 plants survived glufosinate selection fitting an expected segregation
ration for two
independently assorting loci for glufosinate resistance (15 R:1 S).
Glufosinate resistant plants
were then treated with 560 g ae/ha R-dichlorprop + 560 g ae/ha triclopyr,
applied to the plants
under the same spray regimen as used for the other testing. Plants were graded
at 3 and 14
DAT. Sixty-three of the 96 plants that survived glufosinate selection also
survived the
herbicide application. These data are consistent with an expected segregation
pattern (9R: 6S)
of two independently assorting dominant traits where each gene gives
resistance to only one of
the auxinic herbicides (either R-dichlroprop or triclopyr). The results
indicate that AAD-12
(pDAB724) can be successfully stacked with AAD-1 (pDAB721), thus increasing
the spectrum
herbicides that may be applied to the crop of interest [(2,4-D+R-dichlorprop)
and (2,4-
D+fluoroxypyr+triclopyr), respectively]. This could be useful to bring 2,4-D
tolerance to a
very sensitive species through conventional stacking of two separate 2,4-D
resistance genes.
Additionally, if either gene were used as a selectable marker for a third and
fourth gene of
interest through independent transformation activities, then each gene pair
could be brought
together through conventional breeding activities and subsequently selected in
the F 1
generation through paired sprays with herbicides that are exclusive between
the AAD-1 and
AAD-12 enzymes (as shown with R-dichlorprop and triclopyr for AAD-1 and AAD-
12,
respectively).
[00214] Other AAD stacks are also within the scope of the subject invention.
The TfdA
protein discussed elsewhere herein (Streber et al.), for example, can be used
together with the
subject AAD-12 genes to impart spectrums of herbicide resistance in transgenic
plants of the
subject invention.
Example 5
WHISKERS-Mediated Transformation of Corn Using Imazethapyr Selection
[00215] Cloning of AAD-12 (v1): The AAD-12 (v1) gene was cut out of the
intermediate
vector pDAB3283 as an Ncol/Sacl fragment. This was ligated directionally into
the similarly
cut pDAB3403 vector containing the ZmUbil monocot promoter. The two fragments
were
ligated together using T4 DNA ligase and transformed into DH5a cells.
Minipreps were
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
performed on the resulting colonies using Qiagen's QIA Spin mini prep kit, and
the colonies
were digested to check for orientation. This first intermediate construct
(pDAB4100) contains
the ZmUbil:AAD-12 (v1) cassette. This construct was digested with Notl and
Pvul to liberate
the gene cassette and digest the unwanted backbone. This was ligated to Notl
cut pDAB2212,
which contains the AHAS selectable marker driven by the Rice Actin promoter
OsActl. The
final construct was designated pDAB4101 or pDAS1863, and contains ZmUbil/AAD-
12
(v1)/ZmPer5::OsActl/AHAS/LZmLip.
[00216] Callus/Suspension Initiation: To obtain immature embryos for callus
culture
initiation, Fl crosses between greenhouse-grown Hi-II parents A and B
(Armstrong et al. 1991)
were performed. When embryos were 1.0-1.2 mm in size (approximately 9-10 days
post-
pollination), ears were harvested and surface sterilized by scrubbing with
Liqui-Nox soap,
immersed in 70% ethanol for 2-3 minutes, then immersed in 20% commercial
bleach (0.1%
sodium hypochlorite) for 30 minutes.
[00217] Ears were rinsed in sterile, distilled water, and immature zygotic
embryos were
aseptically excised and cultured on 15Ag10 medium (N6 Medium (Chu et al.,
1975), 1.0 mg/L
2,4-D, 20 g/L sucrose, 100 mg/L casein hydrolysate (enzymatic digest), 25 mM L-
proline, 10
mg/L AgNO3, 2.5 g/L Gelrite, pH 5.8) for 2-3 weeks with the scutellum facing
away from the
medium. Tissue showing the proper morphology (Welter et al., 1995) was
selectively
transferred at biweekly intervals onto fresh 15Ag10 medium for about 6 weeks,
then transferred
to 4 medium (N6 Medium, 1.0 mg/L 2,4-D, 20 g/L sucrose, 100 mg/L casein
hydrolysate
(enzymatic digest), 6 mM L-proline, 2.5 g/L Gelrite, pH 5.8) at bi-weekly
intervals for
approximately 2 months.
[00218] To initiate embryogenic suspension cultures, approximately 3 ml packed
cell
volume (PCV) of callus tissue originating from a single embryo was added to
approximately 30
ml of H9CP + liquid medium (MS basal salt mixture (Murashige and Skoog, 1962),
modified
MS Vitamins containing 10-fold less nicotinic acid and 5-fold higher thiamine-
HC1, 2.0 mg/L
2,4-D, 2.0 mg/L a-naphthaleneacetic acid (NAA), 30 g/L sucrose, 200 mg/L
casein hydrolysate
(acid digest), 100 mg/L myo-inositol, 6 mM L-proline, 5% v/v coconut water
(added just before
subculture), pH 6.0). Suspension cultures were maintained under dark
conditions in 125 ml
Erlenmeyer flasks in a temperature-controlled shaker set at 125 rpm at 28 C.
Cell lines
typically became established within 2 to 3 months after initiation. During
establishment,
suspensions were subcultured every 3.5 days by adding 3 ml PCV of cells and 7
ml of
conditioned medium to 20 ml of fresh H9CP+ liquid medium using a wide-bore
pipette. Once
the tissue started doubling in growth, suspensions were scaled-up and
maintained in 500 ml
61
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
flasks whereby 12 ml PCV of cells and 28 ml conditioned medium was transferred
into 80 ml
H9CP+ medium. Once the suspensions were fully established, they were
cryopreserved for
future use.
[00219] Cryopreservation and Thawing Of Suspensions: Two days post-subculture,
4 ml
PCV of suspension cells and 4 ml of conditioned medium were added to 8 ml of
cryoprotectant
(dissolved in H9CP+ medium without coconut water, 1 M glycerol, 1 M DMSO, 2 M
sucrose,
filter sterilized) and allowed to shake at 125 rpm at 4 C. for 1 hour in a
125 ml flask. After 1
hour 4.5 ml was added to a chilled 5.0 ml Corning cryo vial. Once filled
individual vials were
held for 15 minutes at 4 C. in a controlled rate freezer, then allowed to
freeze at a rate of -0.5
C./minute until reaching a final temperature of -40 C. After reaching the
final temperature,
vials were transferred to boxes within racks inside a Cryoplus 4 storage unit
(Form a Scientific)
filled with liquid nitrogen vapors.
[00220] For thawing, vials were removed from the storage unit and placed in a
closed dry ice
container, then plunged into a water bath held at 40-45 C. until "boiling"
subsided. When
thawed, contents were poured over a stack of ¨8 sterile 70 mm Whatman filter
papers (No. 4) in
covered 100 x 25 mm Petri dishes. Liquid was allowed to absorb into the
filters for several
minutes, then the top filter containing the cells was transferred onto GN6
medium (N6 medium,
2.0 mg/L 2,4-D, 30 g/L sucrose, 2.5 g/L Gelrite, pH 5.8) for 1 week. After 1
week, only tissue
with promising morphology was transferred off the filter paper directly onto
fresh GN6
medium. This tissue was subcultured every 7-14 days until 1 to 3 grams was
available for
suspension initiation into approximately 30 ml H9CP+ medium in 125 ml
Erlenmeyer flasks.
Three milliliters PCV was subcultured into fresh H9CP+ medium every 3.5 days
until a total of
12 ml PCV was obtained, at which point subculture took place as described
previously.
[00221] Stable Transformation: Approximately 24 hours prior to transformation,
12 ml PCV
of previously cryopreserved embryogenic maize suspension cells plus 28 ml of
conditioned
medium was subcultured into 80 ml of GN6 liquid medium (GN6 medium lacking
Gelrite) in a
500 ml Erlenmeyer flask, and placed on a shaker at 125 rpm at 28 C. This was
repeated 2
times using the same cell line such that a total of 36 ml PCV was distributed
across 3 flasks.
After 24 hours the GN6 liquid media was removed and replaced with 72 ml GN6
S/M osmotic
medium (N6 Medium, 2.0 mg/L 2,4-D, 30 g/L sucrose, 45.5 g/L sorbitol, 45.5 g/L
mannitol,
100 mg/L myo-inositol, pH 6.0) per flask in order to plasmolyze the cells. The
flasks were
placed on a shaker shaken at 125 RPM in the dark for 30-35 minutes at 28 C.,
and during this
time a 50 mg/ml suspension of silicon carbide whiskers was prepared by adding
the appropriate
volume 8.1 ml of GN6 S/M liquid medium to ¨405 mg of pre-autoclaved, sterile
silicon carbide
62
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
whiskers (Advanced Composite Materials, Inc.).
[00222] After incubation in GN6 S/M, the contents of each flask were pooled
into a 250 ml
centrifuge bottle. Once all cells settled to the bottom, all but ¨44 ml of GN6
S/M liquid was
drawn off and collected in a sterile 1-L flask for future use. The pre-wetted
suspension of
whiskers was vortexed for 60 seconds on maximum speed and 8.1 ml was then
added to the
bottle, to which 170 lug DNA was added as a last step. The bottle was
immediately placed in a
modified Red Devil 5400 commercial paint mixer and agitated for 10 seconds.
After agitation,
the cocktail of cells, media, whiskers and DNA was added to the contents of
the 1-L flask along
with 125 ml fresh GN6 liquid medium to reduce the osmoticant. The cells were
allowed to
recover on a shaker at 125 RPM for 2 hours at 28 C. before being filtered
onto Whatman #4
filter paper (5.5 cm) using a glass cell collector unit that was connected to
a house vacuum line.
[00223] Approximately 2 ml of dispersed suspension was pipetted onto the
surface of the
filter as the vacuum was drawn. Filters were placed onto 60 x 20 mm plates of
GN6 medium.
Plates were cultured for 1 week at 28 C. in a dark box.
[00224] After 1 week, filter papers were transferred to 60 x 20 mm plates of
GN6 (3P)
medium (N6 Medium, 2.0 mg/L 2,4-D, 30 g/L sucrose, 100 mg/L myo-inositol, 3
[t.M
imazethapyr from Pursuit DG, 2.5 g/L Gelrite, pH 5.8). Plates were placed in
boxes and
cultured for an additional week.
[00225] Two weeks post-transformation, the tissue was embedded by scraping all
cells on
the plate into 3.0 ml of melted GN6 agarose medium (N6 medium, 2.0 mg/L 2,4-D,
30 g/L
sucrose, 100 mg/L myo-inositol, 7 g/L Sea Plaque agarose, pH 5.8, autoclaved
for only 10
minutes at 121 C.) containing 3 [t.M imazethapyr from Pursuit DG. The tissue
was broken up
and the 3 ml of agarose and tissue were evenly poured onto the surface of a
100 x 15 mm plate
of GN6 (3P). This was repeated for all remaining plates. Once embedded, plates
were
individually sealed with Nescofilm or Parafilm M , and then cultured until
putative isolates
appeared.
[00226] Protocol for Isolate Recovery and Regeneration: Putatively transformed
events were
isolated off the Pursuit -containing embedded plates approximately 9 weeks
post-
transformation by transferring to fresh selection medium of the same
concentration in 60 x 20
mm plates. If sustained growth was evident after approximately 2-3 weeks, the
event was
deemed to be resistant and was submitted for molecular analysis.
63
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Table 11. Characterization of TO corn plants transformed with AAD-12
AAD-12 AAD-12 AAD-12 AHAS
E vent Spray % Injury ELISA PCR PCR Copy #
Treatment (14 DAT) (ppm (cloning (PTU) (Invader)
TSP) Region)
4101(0)003.001 2240g 0 146.9 + + 1
ae/ha 2,4-D
4101(0)003.003 2240g 0 153.5 + + 1
ae/ha 2,4-D
4101(0)005.001 2240g 0 539.7 + + 9
ae/ha 2,4-D
0 g ae
4101(0)005.0012 /ha 0 562.9 + + 7
2,4-D
4101(0)001.001 70 g ae/ha. 5 170.7 + + 6
imazethapyr
4101(0)002.001 0 g ae/ha. 0 105.6 + - 2
imazethapyr
4101(0)002.002 70 g ae/ha. 0 105.3 + - 2
imazethapyr
Band
70 g ae/ha smaller
4101(0)003.002 . 0 0 + 15
imazethapyr than
expected
[00227] Regeneration was initiated by transferring callus tissue to a
cytokinin-based
induction medium, 28 (3P), containing 3 [tM imazethapyr from Pursuit® DG,
MS salts and
vitamins, 30.0 g/L sucrose, 5 mg/L BAP, 0.25 mg/L 2,4-D, 2.5 g/L Gelrite; pH
5.7. Cells were
allowed to grow in low light (13 [tEm-2 s-1) for one week, then higher light
(40 [tEm-2 s-1) for
another week, before being transferred to regeneration medium, 36 (3P), which
was identical to
28 (3P) except that it lacked plant growth regulators. Small (3-5 cm)
plantlets were removed
and placed into 150 x 25-mm culture tubes containing selection-free SHGA
medium (Schenk
and Hildebrandt basal salts and vitamins, 1972; 1 g/L myo-inositol, 10 g/L
sucrose, 2.0 g/L
Gelrite, pH 5.8). Once plantlets developed a sufficient root and shoot system,
they were
transplanted to soil in the greenhouse.
[00228] From 4 experiments, full plantlets, comprised of a shoot and root,
were formed in
vitro on the embedded selection plates under dark conditions without
undergoing a traditional
callus phase. Leaf tissues from nine of these "early regenerators" were
submitted for coding
region PCR and Plant Transcription Unit (PTU) PCR for the AAD-12 gene and gene
cassette,
respectively. All had an intact AAD-12 coding region, while 3 did not have a
full-length PTU
(Table 11). These "early regenerators" were identified as 4101 events to
differentiate them
64
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
from the traditionally-derived events, which were identified as "1283" events.
Plants from 19
additional events, obtained via standard selection and regeneration, were sent
to the greenhouse,
grown to maturity and cross-pollinated with a proprietary inbred line in order
to produce T1
seed. Some of the events appear to be clones of one another due to similar
banding patterns
following Southern blot, so only 14 unique events were represented. TO plants
from events
were tolerant 70 g/ha imazethapyr. Invader analysis (AHAS gene) indicated
insertion
complexity ranging from 1 to >10 copies. Thirteen events contained the compete
coding region
for AAD-12; however, further analysis indicated the complete plant
transformation unit had not
been incorporated for nine events. None of the compromised 1863 events were
advanced
beyond the T1 stage and further characterization utilized the 4101 events.
[00229] Molecular Analysis - Maize Materials and Methods: Tissue harvesting
DNA
isolation and quantification. Fresh tissue is placed into tubes and
lyophilized at 4 C. for 2
days. After the tissue is fully dried, a tungsten bead (Valenite) is placed in
the tube and the
samples are subjected to 1 minute of dry grinding using a Kelco bead mill. The
standard
DNeasy DNA isolation procedure is then followed (Qiagen, DNeasy 69109). An
aliquot of the
extracted DNA is then stained with Pico Green (Molecular Probes P7589) and
read in the
fluorometer (BioTek) with known standards to obtain the concentration in ng/
1.
[00230] Invader assay analysis: The DNA samples are diluted to 20 ng/p.1 then
denatured by
incubation in a thermocycler at 95 C. for 10 minutes. Signal Probe mix is
then prepared using
the provided oligo mix and MgC12 (Third Wave Technologies). An aliquot of 7.5
1 is placed
in each well of the Invader assay plate followed by an aliquot of 7.5 1 of
controls, standards,
and 20 ng/p.1 diluted unknown samples. Each well is overlaid with 15 1 of
mineral oil (Sigma).
The plates are then incubated at 63 C. for 1 hour and read on the fluorometer
(Biotek).
Calculation of % signal over background for the target probe divided by the %
signal over
background internal control probe will calculate the ratio. The ratio of known
copy standards
developed and validated with Southern blot analysis is used to identify the
estimated copy of
the unknown events.
[00231] Polymerase chain reaction: A total of 100 ng of total DNA is used as
the template.
20 mM of each primer is used with the Takara Ex Taq PCR Polymerase kit (Mirus
TAKRROO1A). Primers for the AAD-12 (v1) PTU are Forward-GAACAGTTAG
ACATGGTCTA AAGG (SEQ ID NO: 8) and Reverse-GCTGCAACAC TGATAAATGC
CAACTGG (SEQ ID NO: 9). The PCR reaction is carried out in the 9700 Geneamp
thermocycler (Applied Biosystems), by subjecting the samples to 94 C. for 3
minutes and 35
cycles of 94 C. for 30 seconds, 63 C. for 30 seconds, and 72 C. for 1
minute and 45 seconds
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
followed by 72 C. for 10 minutes.
[00232] Primers for AAD-12 (v1) Coding Region PCR are Forward-ATGGCTCAGA
CCACTCTCCA AA (SEQ ID NO: 10) and Reverse-AGCTGCATCC ATGCCAGGGA (SEQ
ID NO: 11). The PCR reaction is carried out in the 9700 Geneamp thermocycler
(Applied
Biosystems), by subjecting the samples to 94 C. for 3 minutes and 35 cycles
of 94 C. for 30
seconds, 65 C. for 30 seconds, and 72 C. for 1 minute and 45 seconds
followed by 72 C. for
minutes. PCR products are analyzed by electrophoresis on a 1% agarose gel
stained with
EtBr.
[00233] Southern Blot Analysis: Southern blot analysis is performed with
genomic DNA
obtained from Qiagen DNeasy kit. A total of 2 lug of genomic leaf DNA or 10
lug of genomic
callus DNA is subjected to an overnight digestion using BSM I and SWA I
restriction enzymes
to obtain PTU data.
[00234] After the overnight digestion an aliquot of ¨100 ng is run on a 1% gel
to ensure
complete digestion. After this assurance the samples are run on a large 0.85%
agarose gel
overnight at 40 volts. The gel is then denatured in 0.2 M NaOH, 0.6 M NaC1 for
30 minutes.
The gel is then neutralized in 0.5 M Tris HC1, 1.5 M NaC1 pH of 7.5 for 30
minutes. A gel
apparatus containing 20 x SSC is then set up to obtain a gravity gel to nylon
membrane
(Millipore INYC00010) transfer overnight. After the overnight transfer the
membrane is then
subjected to UV light via a crosslinker (Stratagene UV stratalinker 1800) at
1200 x 100
microjoules. The membrane is then washed in 0.1% SDS, 0.1 SSC for 45 minutes.
After the 45
minute wash, the membrane is baked for 3 hours at 80 C. and then stored at 4
C. until
hybridization. The hybridization template fragment is prepared using the above
coding region
PCR using plasmid DNA. The product is run on a 1% agarose gel and excised and
then gel
extracted using the Qiagen (28706) gel extraction procedure. The membrane is
then subjected
to a pre-hybridization at 60 C. step for 1 hour in Perfect Hyb buffer (Sigma
H7033). The
Prime it RmT dCTP-labeling rxn (Stratagene 300392) procedure is used to
develop the p32
based probe (Perkin Elmer). The probe is cleaned up using the Probe Quant. G50
columns
(Amersham 27-5335-01). Two million counts CPM are used to hybridize the
southern blots
overnight. After the overnight hybridization the blots are then subjected to
two 20 minute
washes at 65 C. in 0.1% SDS, 0.1 SSC. The blots are then exposed to film
overnight,
incubating at -80 C.
[00235] Postemergence Herbicide Tolerance in AAD-12 Transformed TO Corn: Four
TO
events were allowed to acclimate in the greenhouse and were grown until 2-4
new, normal
looking leaves had emerged from the whorl (i.e., plants had transitioned from
tissue culture to
66
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
greenhouse growing conditions). Plants were grown at 27 C. under 16 hour
light: 8 hour dark
conditions in the greenhouse. Plants were then treated with commercial
formulations of either
Pursuit (imazethapyr) or 2,4-D Amine 4. Pursuit was sprayed to demonstrate
the function of
the selectable marker gene present within the events tested. Herbicide
applications were made
with a track sprayer at a spray volume of 187 L/ha, 50-cm spray height. Plants
were sprayed
with either a lethal dose of imazethapyr (70 g ae/ha) or a rate of 2,4-D DMA
salt capable of
significant injury to untransformed corn lines (2240 g ae/ha). A lethal dose
is defined as the
rate that causes >95% injury to the Hi-II inbred. Hi-II is the genetic
background of the
transformants of the present invention.
[00236] Several individuals were safened from the herbicides to which the
respective genes
were to provide resistance. The individual clone '001' from event "001"
(a.k.a., 4101(0)-001-
001), however, did incur minor injury but recovered by 14 DAT. Three of the
four events were
moved forward and individuals were crossed with 5XH751 and taken to the next
generation.
Each herbicide tolerant plant was positive for the presence of the AAD-12
coding region (PCR
assay) or the presence of the AHAS gene (Invader assay) for 2,4-D and
imazethapyr-tolerant
plants, respectively. AAD-12 protein was detected in all 2,4-D tolerant TO
plants events
containing an intact coding region. The copy number of the transgene(s) (AHAS,
and by
inference AAD-12) varied significantly from 1 to 15 copies. Individual TO
plants were grown
to maturity and cross-pollinated with a proprietary inbred line in order to
produce T1 seed.
[00237] Verification of High 2,4-D Tolerance in T1 Corn: T1 AAD-12 (v1) seed
were
planted into 3-inch pots containing Metro Mix media and at 2 leaf stage were
sprayed with 70 g
ae/ha imazethapyr to eliminate nulls. S urviving plants were transplanted to 1-
gallon pots
containing Metro Mix media and placed in the same growth conditions as before.
At V3-V4
stage the plants were sprayed in the track sprayer set to 187 L/ha at either
560 or 2240 g ae/ha
2,4-D DMA. Plants were graded at 3 and 14 DAT and compared to 5XH751 x Hi II
control
plants. A grading scale of 0-10 (no injury to extreme auxin injury) was
developed to
distinguish brace root injury. Brace Root grades were taken on 14DAT to show
2,4-D
tolerance. 2,4-D causes brace root malformation, and is a consistent indicator
of auxinic
herbicide injury in corn. Brace root data (as seen in the table below)
demonstrates that 2 of the
3 events tested were robustly tolerant to 2240 g ae/ha 2,4-D DMA. Event
"pDAB4101(0)001.001" was apparently unstable; however, the other two events
were robustly
tolerant to 2,4-D and 2,4-D + imazethapyr or 2,4-D + glyphosate (see Table
12).
67
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Table 12. Brace Root injury of AAD-12 (v1) transformed T1 plants and
untransformed control corn
plants: Average Brace Root Injury (0-10 Scale)
Herbicide Untransformed AAD-12 (v1) AAD-12 (v1) AAD-12 (v1)
Control pDAB4101(0) pDAB4101(0) pDAB4101(0)
003.003 001.001 005.001
0 g ae/ha 2,4-D DMA 0 0 0 0
2240 g ae/ha 2,4-D
9 1 8 0
DMA
A scale of 0-10, 10 being the highest, was used for grading the 2,4-D DMA
injury. Results are a
visual average of four replications per treatment.
[00238] AAD-12 (v1) Heritability in Corn: A progeny test was also conducted on
seven
AAD-12 (v1) T1 families that had been crossed with 5XH751. The seeds were
planted in
three-inch pots as described above. At the 3 leaf stage all plants were
sprayed with 70 g ae/ha
imazethapyr in the track sprayer as previously described. After 14 DAT,
resistant and sensitive
plants were counted. Four out of the six lines tested segregated as a single
locus, dominant
Mendelian trait (1R:1S) as determined by Chi square analysis. Surviving plants
were
subsequently sprayed with 2,4-D and all plants were deemed tolerant to 2,4-D
(rates > 560 g
ae/ha). AAD-12 is heritable as a robust aryloxyalkanoate auxin resistance gene
in multiple
species when reciprocally crossed to a commercial hybrid.
[00239] Stacking of AAD-12 (v1) to Increase Herbicide Spectrum: AAD-12 (v1)
(pDAB4101) and elite Roundup Ready inbred (BE1146RR) were reciprocally crossed
and Fl
seed was collected. The seed from two F1 lines were planted and treated with
70 g ae/ha
imazethapyr at the V2 stage to eliminate nulls. To the surviving plants, reps
were separated and
either treated with 1120 g ae/ha 2,4-D DMA + 70 g ae/ha imazethapyr (to
confirm presence of
AHAS gene) or 1120 g ae/ha 2,4-D DMA+1680 g ae/ha glyphosate (to confirm the
presence of
the Round Up Ready gene) in a track sprayer calibrated to 187 L/ha. Plants
were graded 3 and
16 DAT. Spray data showed that AAD-12 (v1) can be conventionally stacked with
a
glyphosate tolerance gene (such as the Roundup CP4-EPSPS gene) or other
herbicide tolerance
genes to provide an increased spectrum of herbicides that may be applied
safely to corn.
Likewise imidazolinone + 2,4-D + glyphosate tolerance was observed in F1
plants and showed
no negative phenotype by the molecular or breeding stack combinations of these
multiple
transgenes.
68
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Table 13. Data demonstrating increase herbicide tolerance spectrum resulting
from an F 1 stack of
AAD-12 (v1) and BE1146RR (an elite glyphosate tolerant inbred abbreviated as
AF): Average %
Injury 16DAT
Herbicide Untransformed 2P782 AAD-12 (v1) AAD-12 (v1)
Control (Roundup pDAB4101(0) pDAB4101(0)
Ready Control) 003.R003.AF 005.R001.AF
0 g ae/ha 2,4-D DMA 0 0 0 0
1120 g ae/ha 2,4-D DMA 21 19 0 0
1120 g ae/ha 2,4-D DMA +
100 100 5 1
70 g ae/ha imazethapyr
1120 g ae/ha 2,4-D DMA +
100 71 2 5
1680 g ae/ha glyphosate
[00240] Field Tolerance of pDAB4101 Transformed Corn Plants to 2,4-D,
Triclopyr and
Fluoroxypyr Herbicides: Field level tolerance trials were conducted on two AAD-
12 (v1)
pDAB4101 events (4101(0)003.R.003.AF and 4101(0)005.R001.AF) and one Roundup
Ready
(RR) control hybrid (2P782) at Fowler, Ind. and Wayside, Miss. Seeds were
planted with cone
planter on 40-inch row spacing at Wayside and 30 inch spacing at Fowler. The
experimental
design was a randomized complete block design with 3 replications. Herbicide
treatments were
2,4-D (dimethylamine salt) at 1120, 2240 and 4480 g ae/ha, triclopyr at 840 g
ae/ha,
fluoroxypyr at 280 g ae/ha and an untreated control. The AAD-12 (v1) events
contained the
AHAS gene as a selectable marker. The F2 corn events were segregating so the
AAD-12 (v1)
plants were treated with imazethapyr at 70 g ae/ha to remove the null plants.
Herbicide
treatments were applied when corn reached the V6 stage using compressed air
backpack sprayer
delivering 187 L/ha carrier volume at 130-200 kpa pressure. Visual injury
ratings were taken at
7, 14 and 21 days after treatment. Brace root injury ratings were taken at
28DAT on a scale of
0-10 with 0-1 being slight brace root fusing, 1-3 being moderate brace root
swelling/wandering
and root proliferation, 3-5 being moderate brace root fusing, 5-9 severe brace
root fusing and
malformation and 10 being total inhibition of brace roots.
[00241] AAD-12 (v1) event response to 2,4-D, triclopyr, and fluoroxypyr at 14
days after
treatment are shown in Table 14. Crop injury was most severe at 14 DAT. The RR
control
corn (2P782) was severely injured (44% at 14 DAT) by 2,4-D at 4480 g ae/ha,
which is 8 times
(8 x) the normal field use rate. The AAD-12 (v1) events all demonstrated
excellent tolerance to
2,4-D at 14 DAT with 0% injury at the 1, 2 and 4 x rates, respectively. The
control corn
(2P782) was severely injured (31% at 14 DAT) by the 2 x rate of triclopyr (840
g ae/ha). AAD-
12 (v1) events demonstrated tolerance at 2 x rates of triclopyr with an
average of 3% injury at
14 DAT across the two events. Fluoroxypyr at 280 g ae/ha caused 11% visual
injury to the
wild-type corn at 14 DAT. AAD-12 (v1) events demonstrated increased tolerance
with an
69
CA 02876144 2014-12-05
WO 2013/185036
PCT/US2013/044717
average of 8% injury at 5 DAT.
Table 14. Visual injury of AAD-12 events and wild-type corn following foliar
applications
of 2,4-D, triclopyr and fluroxypyr under field conditions: % Visual Injury 14
DAT
AAD-12 4101(0) AAD-12
Treatment Rate (g ae/ha) 003.R.003.AF
4101(0) 2P782 control
005.001.AF
Untreated 0 0 0 0
2,4-D 1120 0 0 9
2,4-D 2240 0 1 20
2,4-D 4480 0 1 34
Fluroxypyr 280 1 5 11
Triclopyr 840 3 4 31
Dicamba 840 8 8 11
[00242] Applications of auxinic herbicides to corn in the V6 growth stage can
cause
malformation of the brace roots. Table 15 shows the severity of the brace root
injury caused by
2,4-D, triclopyr, and fluoroxypyr. Triclopyr at 840 g ae/ha caused the most
severe brace root
fusing and malformation resulting in an average brace root injury score of 7
in the 2P782
control-type corn.
Table 15. Brace root injury ratings for AAD-12 and wild-type corn plants in
response to
2,4-D, triclopyr and fluroxypyr under field conditions: Brace toot injury
rating (0-10 scale)
28 DAT
AAD-12 4101(0) AAD-12
Treatment Rate (g ae/ha) 003.R.003.AF
4101(0) 2P782 control
005.001.AF
Untreated 0 0 0 0
2,4-D 1120 0 0 3
2,4-D 2240 0 0 5
2,4-D 4480 0 0 6
Fluroxypyr 280 0 0 2
Triclopyr 840 0 0 7
Dicamba 840 1 1 1
[00243] Both AAD-12 (v1) corn events showed no brace root injury from the
triclopyr
treatment. Brace root injury in 2P782 corn increased with increasing rates of
2,4-D. At 4480 g
ae/ha of 2,4-D, the AAD-12 events showed no brace root injury; whereas, severe
brace root
fusing and malformation was seen in the 2P782 hybrid. Fluoroxypyr caused only
moderate
brace root swelling and wandering in the wild-type corn with the AAD-12 (v1)
events showing
no brace root injury.
[00244] This data clearly shows that AAD-12(v1) conveys high level tolerance
in corn to
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
2,4-D, triclopyr and fluoroxypyr at rates far exceeding those commercially
used and that cause
non-AAD-12 (v1) corn severe visual and brace root injury.
Example 6
Tobacco Transformation
[00245] Tobacco transformation with Agrobacterium tumefaciens was carried out
by a
method similar, but not identical, to published methods (Horsch et al., 1988).
To provide
source tissue for the transformation, tobacco seed (Nicotiana tabacum cv.
KY160) was surface
sterilized and planted on the surface of TOB-medium, which is a hormone-free
Murashige and
Skoog medium (Murashige and Skoog, 1962) solidified with agar. Plants were
grown for 6-8
weeks in a lighted incubator room at 28-30 C. and leaves collected sterilely
for use in the
transformation protocol. Pieces of approximately one square centimeter were
sterilely cut from
these leaves, excluding the midrib. Cultures of the Agrobacterium strains
(EHA101S
containing pDAB3278, aka pDAS1580, AAD-12 (v1)+PAT), grown overnight in a
flask on a
shaker set at 250 rpm at 28 C., were pelleted in a centrifuge and resuspended
in sterile
Murashige & Skoog salts, and adjusted to a final optical density of 0.5 at 600
nm. Leaf pieces
were dipped in this bacterial suspension for approximately 30 seconds, then
blotted dry on
sterile paper towels and placed right side up on TOB+ medium (Murashige and
Skoog medium
containing 1 mg/L indole acetic acid and 2.5 mg/L benzyladenine) and incubated
in the dark at
28 C. Two days later the leaf pieces were moved to TOB+ medium containing 250
mg/L
cefotaxime (Agri-Bio, North Miami, Fla.) and 5 mg/L glufosinate ammonium
(active ingredient
in Basta, Bayer Crop Sciences) and incubated at 28-30 C. in the light. Leaf
pieces were moved
to fresh TOB+ medium with cefotaxime and Basta twice per week for the first
two weeks and
once per week thereafter. Four to six weeks after the leaf pieces were treated
with the bacteria,
small plants arising from transformed foci were removed from this tissue
preparation and
planted into medium TOB-containing 250 mg/L cefotaxime and 10 mg/L Basta in
PhytatrayTM
II vessels (Sigma). These plantlets were grown in a lighted incubator room.
After 3 weeks,
stem cuttings were taken and re-rooted in the same media. Plants were ready to
send out to the
greenhouse after 2-3 additional weeks.
[00246] Plants were moved into the greenhouse by washing the agar from the
roots,
transplanting into soil in 13.75 cm square pots, placing the pot into a Ziploc
bag (SC Johnson
& Son, Inc.), placing tap water into the bottom of the bag, and placing in
indirect light in a 30
C. greenhouse for one week. After 3-7 days, the bag was opened; the plants
were fertilized
and allowed to grow in the open bag until the plants were greenhouse-
acclimated, at which time
71
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
the bag was removed. Plants were grown under ordinary warm greenhouse
conditions (30 C.,
16 hour day, 8 hour night, minimum natural + supplemental light =500 [tE/m2
s1).
[00247] Prior to propagation, TO plants were sampled for DNA analysis to
determine the
insert copy number. The PAT gene which was molecularly linked to AAD-12 (v1)
was assayed
for convenience. Fresh tissue was placed into tubes and lyophilized at 4 C.
for 2 days. After
the tissue was fully dried, a tungsten bead (Valenite) was placed in the tube
and the samples
were subjected to 1 minute of dry grinding using a Kelco bead mill. The
standard DNeasy
DNA isolation procedure was then followed (Qiagen, DNeasy 69109). An aliquot
of the
extracted DNA was then stained with Pico Green (Molecular Probes P7589) and
read in the
fluorometer (BioTek) with known standards to obtain the concentration in ng/
1.
[00248] The DNA samples were diluted to 9 ng/p.1 and then denatured by
incubation in a
thermocycler at 95 C. for 10 minutes. Signal Probe mix was then prepared
using the provided
oligo mix and MgC12 (Third Wave Technologies). An aliquot of 7.5 pi was placed
in each well
of the Invader assay plate followed by an aliquot of 7.5 pi of controls,
standards, and 20 ng/p.1
diluted unknown samples. Each well was overlaid with 15 pi of mineral oil
(Sigma). The
plates were then incubated at 63 C. for 1.5 hours and read on the fluorometer
(Biotek).
Calculation of % signal over background for the target probe divided by the %
signal over
background internal control probe will calculate the ratio. The ratio of known
copy standards
developed and validated with southern blot analysis was used to identify the
estimated copy of
the unknown events.
[00249] All events were also assayed for the presence of the AAD-12 (v1) gene
by PCR
using the same extracted DNA samples. A total of 100 ng of total DNA was used
as template.
20 mM of each primer was used with the Takara Ex Taq PCR Polymerase kit.
Primers for the
Plant Transcription Unit (PTU) PCR AAD-12 were (SdpacodF: ATGGCTCATG
CTGCCCTCAG CC) (SEQ ID NO: 12) and (SdpacodR: CGGGCAGGCC TAACTCCACC
AA) (SEQ ID NO: 13). The PCR reaction was carried out in the 9700 Geneamp
thermocycler
(Applied Biosystems), by subjecting the samples to 94 C. for 3 minutes and 35
cycles of 94 C.
for 30 seconds, 64 C. for 30 seconds, and 72 C. for 1 minute and 45 seconds
followed by 72
C. for 10 minutes. PCR products were analyzed by electrophoresis on a 1%
agarose gel
stained with EtBr. Four to 12 clonal lineages from each of 18 PCR positive
events with 1-3
copies of PAT gene (and presumably AAD-12 (v1) since these genes are
physically linked)
were regenerated and moved to the greenhouse.
72
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Table 16. Tobacco TO events transformed with pDAS1580 (AAD-12 (v1) + PAT)
PTU Full PTU Full PTU Relative
# Plant ID Copy # PCR and
and Herbicide
Tube PAT AAD12 Under 2 1 copy Tolerance*
1580[1]-
1 001 6 + Not
tested
1580[1]-
2 002 8 + Not
tested
1580[1]-
3 003 10 + Not
tested
1580[1]-
4 004 1 + * * High
1580[1]-
005 2 + * Variable
1580[1]-
6 006 6 + Not
tested
1580[1]-
7 007 4 + Not
tested
1580[1]-
8 008 3 + Variable
1580[1]-
9 009 4 + Not
tested
1580[1]-
010 8 + Not tested
1580[1]-
11 011 3 + High
1580[1]-
12 012 12 + Not
tested
1580[1]-
13 013 13 + Not
tested
1580[1]-
14 014 4 + Not
tested
1580[1]-
015 2 + * High
1580[1]-
16 016 1? + * * High
1580[1]-
17 017 3 + High
1580[1]-
18 018 1 + * * Variable
1580[1]-
19 019 1 + * * Variable
1580[1]-
020 1 + * * Not tested
1580[1]-
21 021 1 + * * Not
tested
1580[1]-
22 022 3 + Variable
1580[1]-
23 023 1 + * * Variable
73
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
1580[1]-
24 024 1 + * * Variable
1580[1]-
25 025 5 + Not tested
1580[1]-
26 026 3 + Variable
1580[1]-
27 027 3 + Low
1580[1]-
28 028 4 + Not tested
1580[1]-
29 029 3 + Variable
1580[1]-
30 030 1 + * * High
1580[1]-
31 031 1 + * * High
1580[1]-
32 032 2 + * High
@Distinguishing herbicide tolerance performance of events required assessment
of
relative tolerance when treated with 560 g ae/ha fluroxypyr where tolerance
was variable
across events.
[00250] Postemergence Herbicide Tolerance in AAD-12 (v1) Transformed TO
Tobacco: TO
plants from each of the 19 events were challenged with a wide range of 2,4-D,
triclopyr, or
fluoroxypyr sprayed on plants that were 3-4 inches tall. Spray applications
were made as
previously described using a track sprayer at a spray volume of 187 L/ha. 2,4-
D dimethylamine
salt (Riverside Corp) was applied at 0, 140, 560, or 2240 g ae/ha to
representative clones from
each event mixed in deionized water. Fluoroxypyr was likewise applied at 35,
140, or 560 g
ae/ha. Triclopyr was applied at 70, 280, or 1120 g ae/ha. Each treatment was
replicated 1-3
times. Injury ratings were recorded 3 and 14 DAT. Every event tested was more
tolerant to
2,4-D than the untransformed control line KY160. In several events, some
initial auxinic
herbicide-related epinasty occurred at doses of 560 g ae/ha 2,4-D or less.
Some events were
uninjured at 2,4-D applied at 2240 g ae/ha (equivalent to 4 x field rate). On
the whole, AAD-12
(v1) events were more sensitive to fluoroxypyr, followed by triclopyr, and
least affected by 2,4-
D. The quality of the events with respect to magnitude of resistance was
discerned using TO
plant responses to 560 g ae/ha fluoroxypyr. Events were categorized into "low"
(>40% injury
14 DAT), "medium" (20-40% injury), "high" (<20% injury). Some events were
inconsistent in
response among replicates and were deemed "variable."
[00251] Verification of High 2,4-D Tolerance in T1 Tobacco: Two to four TO
individuals
surviving high rates of 2,4-D and fluoroxypyr were saved from each event and
allowed to self
74
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
fertilize in the greenhouse to give rise to T1 seed. The T1 seed was
stratified, and sown into
selection trays much like that of Arabidopsis, followed by selective removal
of untransformed
nulls in this segregating population with 560 g ai/ha glufosinate (PAT gene
selection).
Survivors were transferred to individual 3-inch pots in the greenhouse. These
lines provided
high levels of resistance to 2,4-D in the TO generation. Improved consistency
of response is
anticipated in T1 plants not having come directly from tissue culture. These
plants were
compared against wild type KY160 tobacco. All plants were sprayed with a track
sprayer set at
187 L/ha. The plants were sprayed from a range of 140-2240 g ae/ha 2,4-D
dimethylamine salt
(DMA), 70-1120 g ae/ha triclopyr or 35-560 g ae/ha fluoroxypyr. All
applications were
formulated in water. Each treatment was replicated 2-4 times. Plants were
evaluated at 3 and
14 days after treatment. Plants were assigned injury rating with respect to
stunting, chlorosis,
and necrosis. The T1 generation is segregating, so some variable response is
expected due to
difference in zygosity.
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Table 17. Segregating AAD-12 T1 tobacco plants' response to phenoxy and
pyridyloxy
auxin herbicides.
1580(1)-018
KY160 ¨ 1580(1)-004 (high (high tolerance
Wild type tolerance in To in To
generation) generation)
Herbicide Average % Injury of Replicates 14 DAT
140 g ae/ha 2,4-D DMA 45 0 0
560 g ae/ha 2,4-D DMA 60 0 0
2240 g ae/ha 2,4-D DMA 73 0 0
70 g ae/ha triclopyr 40 0 5
280 g ae/ha triclopyr 65 0 5
1120 g ae/ha triclopyr 80 0 8
35 g ae/ha fluroxypyr 85 0 8
140 g ae/ha fluroxypyr 93 0 10
560 g ae/ha fluroxypyr 100 3 18
[00252] No injury was observed at 4 x field rate (2240 g ae/ha) for 2,4-D or
below. Some
injury was observed with triclopyr treatments in one event line, but the
greatest injury was
observed with fluoroxypyr. The fluoroxypyr injury was short-lived and new
growth on one
event was nearly indistinguishable from the untreated control by 14 DAT (Table
17). It is
important to note that untransformed tobacco is exceedingly sensitive to
fluoroxypyr. These
results indicated commercial level 2,4-D tolerance can be provided by AAD-12
(v1), even in a
very auxin-sensitive dicot crop like tobacco. These results also show
resistance can be
imparted to the pyridyloxyacetic acid herbicides, triclopyr and fluoroxypyr.
Having the ability
to prescribe treatments in an herbicide tolerant crop protected by AAD-12 with
various active
ingredients having varying spectra of weed control is extremely useful to
growers.
[00253] AAD-12 (v1) Heritability in Tobacco: A 100 plant progeny test was also
conducted
on seven T1 lines of AAD-12 (v1) lines. The seeds were stratified, sown, and
transplanted with
respect to the procedure above with the exception that null plants were not
removed by Liberty
selection. All plants were then sprayed with 560 g ae/ha 2,4-D DMA as
previously described.
After 14 DAT, resistant and sensitive plants were counted. Five out of the
seven lines tested
segregated as a single locus, dominant Mendelian trait (3R:1S) as determined
by Chi square
analysis. AAD-12 is heritable as a robust aryloxyalkanoate auxin resistance
gene in multiple
species.
[00254] Field Tolerance of pDAS1580 Tobacco Plants to 2,4-D, Dichloprop,
Triclopyr and
Fluoroxypyr Herbicides: Field level tolerance trials were conducted on three
AAD-12 (v1) lines
76
CA 02876144 2014-12-05
WO 2013/185036
PCT/US2013/044717
(events pDAS1580-[1]-018.001, pDAS1580-[1]-004.001 and pDAS1580-[11-020.016)
and one
wild-type line (KY160) at field stations in Indiana and Miss. Tobacco
transplants were grown
in the greenhouse by planting T1 seed in 72 well transplant flats (Hummert
International)
containing Metro 360 media according to growing conditions indicated above.
The null plants
were selectively removed by Liberty selection as previously described. The
transplant plants
were transported to the field stations and planted at either 14 or 24 inches
apart using industrial
vegetable planters. Drip irrigation at the Mississippi site and overhead
irrigation at the Indiana
site were used to keep plants growing vigorously.
[00255] The experimental design was a split plot design with 4 replications.
The main plot
was herbicide treatment and the sub-plot was tobacco line. The herbicide
treatments were 2,4-
D (dimethylamine salt) at 280, 560, 1120, 2240 and 4480 g ae/ha, triclopyr at
840 g ae/ha,
fluoroxypyr at 280 g ae/ha and an untreated control. Plots were one row by 25-
30 ft. Herbicide
treatments were applied 3-4 weeks after transplanting using compressed air
backpack sprayer
delivering 187 L/ha carrier volume at 130-200 kpa pressure. Visual rating of
injury, growth
inhibition, and epinasty were taken at 7, 14 and 21 days after treatment.
Table 18. AAD-12 tobacco plants response to 2,4-D, triclopyr, and fluroxypyr
under field
conditions.
Herbicide Treatment Average % Injury across locations at 14 DAT
Active Wild
PDAS1580- PDAS1580- PDAS1580-
Ingredient Rate type
[1]-004.001 [1]-020.016 [1]-018.001
2,4-D 280 GM AE/HA 48 0 0 0
2,4-D 560 GM AE/HA 63 0 0 2
2,4-D 1120 GM AE/HA 78 1 1 2
2,4-D 2240 GM AE/HA 87 4 4 4
2,4-D 4480 GM AE/HA 92 4 4 4
Triclopyr 840 GM AE/HA 53 5 5 4
Fluroxypyr 280 GM AE/HA 99 11 11 12
[00256] AAD-12 (v1) event response to 2,4-D, triclopyr, and fluoroxypyr are
shown in Table
18. The non-transformed tobacco line was severely injured (63% at 14 DAT) by
2,4-D at 560 g
ae/ha which is considered the 1× field application rate. The AAD-12 (v1)
lines all
demonstrated excellent tolerance to 2,4-D at 14 DAT with average injury of 1,
4, and 4% injury
observed at the 2, 4 and 8× rates, respectively. The non-transformed
tobacco line was
severely injured (53% at 14 DAT) by the 2 x rate of triclopyr (840 g ae/ha);
whereas, AAD-12
(v1) lines demonstrated tolerance with an average of 5% injury at 14 DAT
across the three
77
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
lines. Fluoroxypyr at 280 g ae/ha caused severe injury (99%) to the non-
transformed line at 14
DAT. AAD-12 (v1) lines demonstrated increased tolerance with an average of 11%
injury at
14 DAT.
[00257] These results indicate that AAD-12 (v1) transformed event lines
displayed a high
level of tolerance to 2,4-D, triclopyr and fluoroxypyr at multiples of
commercial use rates that
were lethal or caused severe epinastic malformations to non-transformed
tobacco under
representative field conditions.
[00258] AAD-12 (v1) Protection Against Elevated 2,4-D Rates: Results showing
AAD-12
(v1) protection against elevated rates of 2,4-D DMA in the greenhouse are
shown in Table 19.
T1 AAD-12 (v1) plants from an event segregating 3R:1S when selected with 560 g
ai/ha
Liberty using the same protocol as previously described. T1 AAD-1 (v3) seed
was also planted
for transformed tobacco controls (see PCT/US2005/014737). Untransformed KY160
was
served as the sensitive control. Plants were sprayed using a track sprayer set
to 187 L/ha at 140,
560, 2240, 8960, and 35840 g ae/ha 2,4-D DMA and rated 3 and 14 DAT.
[00259] AAD-12 (v1) and AAD-1 (v3) both effectively protected tobacco against
2,4-D
injury at doses up to 4 x commercial use rates. AAD-12 (v1), however, clearly
demonstrated a
marked advantage over AAD-1 (v3) by protecting up to 64 x the standard field
rates.
Table 19. Results demonstrating protection provided by AAD-12 (v1) and AAD-1
(v3)
against elevated rates of 2,4-D.
KY160
AAD-1 (v3) AAD-12 (v1)
control
Treatment Average % Injury of Replicates 14 DAT
2240 g ae/ha 2,4-D 95 4 0
8960 g ae/ha 2,4-D 99 9 0
35840 g ae/ha 2,4-D 100 32 4
[00260] Stacking of AAD-12 to Increase Herbicide Spectrum: Homozygous AAD-
12 (v1)
(pDAS1580) and AAD-1 (v3) (pDAB721) plants (see PCT/U52005/014737 for the
latter) were
both reciprocally crossed and Fl seed was collected. The Fl seed from two
reciprocal crosses
of each gene were stratified and treated 4 reps of each cross were treated
under the same spray
regimine as used for the other testing with one of the following treatments:
70, 140, 280 g ae/ha
fluoroxypyr (selective for the AAD-12 (v1) gene); 280, 560, 1120 g ae/ha R-
dichloroprop
(selective for the AAD-1 (v3) gene); or 560, 1120, 2240 g ae/ha 2,4-D DMA (to
confirm 2,4-D
tolerance). Homozygous T2 plants of each gene were also planted for use as
controls. Plants
were graded at 3 and 14 DAT. Spray results are shown in Table 20.
[00261] The results confirm that AAD-12 (v1) can be successfully stacked with
AAD-1 (v3),
78
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
thus increasing the spectrum herbicides that may be applied to the crop of
interest
(phenoxyactetic acids + phenoxypropionic acids vs penoxyacetic acids +
pyridyloxyacetic acids
for AAD-1 and AAD-12, respectively). The complementary nature of herbicide
cross
resistance patterns allows convenient use of these two genes as complementary
and stackable
field-selectable markers. In crops where tolerance with a single gene may be
marginal, one
skilled in the art recognizes that one can increase tolerance by stacking a
second tolerance gene
for the same herbicide. Such can be done using the same gene with the same or
different
promoters; however, as observed here, stacking and tracking two complementary
traits can be
facilitated by the distinguishing cross protection to phenoxypropionic acids
[from AAD-1 (v3)]
or pyidyloxyacetic acids [AAD-12 (v1)].
Table 20. Comparison of auxinic herbicide cross tolerance of AAD-12 (v1)
(pDAS1580)
and AAD-1 (v3) (pDAB721) T2 plants compared to AAD-12 x AAD-1 Fl cross and to
wild type
Treatment Average % Injury 14 DAT
KY160 AAD-12 AAD-1 AAD-12 (v1)
Wild type (v1) (v3) x AAD (v3)
control (pDAS1580) (pDAB721) Fl
560 g ae/ha 2,4-D 63 0 0 0
1120 g ae/ha 2,4-D 80 0 4 0
2240 g ae/ha 2,4-D 90 0 9 0
280 g ae/ha R-dichloprop 25 15 0 0
560 g ae/ha R-dichloprop 60 50 0 0
1120 g ae/ha R-dichloprop 80 70 3 0
70 g ae/ha fluroxypyr 40 0 40 0
140 g ae/ha fluroxypyr 65 0 60 0
280 g ae/ha fluroxypyr 75 3 75 3
Example 7
Soybean Transformation
[00262] Soybean improvement via gene transfer techniques has been accomplished
for such
traits as herbicide tolerance (Padgette et al., 1995), amino acid modification
(Falco et al., 1995),
and insect resistance (Parrott et al., 1994). Introduction of foreign traits
into crop species
requires methods that will allow for routine production of transgenic lines
using selectable
marker sequences, containing simple inserts. The transgenes should be
inherited as a single
functional locus in order to simplify breeding. Delivery of foreign genes into
cultivated
soybean by microprojectile bombardment of zygotic embryo axes (McCabe et al.,
1988) or
79
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
somatic embryogenic cultures (Finer and McMullen, 1991), and Agrobacterium-
mediated
transformation of cotyledonary explants (Hinchee et al., 1988) or zygotic
embryos (Chee et al.,
1989) have been reported.
[00263] Transformants derived from Agrobacterium-mediated transformations tend
to
possess simple inserts with low copy number (Birch, 1991). There are benefits
and
disadvantages associated with each of the three target tissues investigated
for gene transfer into
soybean, zygotic embryonic axis (Chee et al., 1989; McCabe et al., 1988),
cotyledon (Hinchee
et al., 1988) and somatic embryogenic cultures (Finer and McMullen, 1991). The
latter have
been extensively investigated as a target tissue for direct gene transfer.
Embryogenic cultures
tend to be quite prolific and can be maintained over a prolonged period.
However, sterility and
chromosomal aberrations of the primary transformants have been associated with
age of the
embryogenic suspensions (Singh et al., 1998) and thus continuous initiation of
new cultures
appears to be necessary for soybean transformation systems utilizing this
tissue. This system
needs a high level of 2,4-D, 40 mg/L concentration, to initiate the
embryogenic callus and this
poses a fundamental problem in using the AAD-12 (v1) gene since the
transformed locus could
not be developed further with 2,4-D in the medium. So, the meristem based
transformation is
ideal for the development of 2,4-D resistant plant using AAD-12 (v1).
[00264] Gateway Cloning of Binary Constructs: The AAD-12 (v1) coding sequence
was
cloned into five different Gateway Donor vectors containing different plant
promoters. The
resulting AAD-12 (v1) plant expression cassettes were subsequently cloned into
a Gateway
Destination Binary vector via the LR Clonase reaction (Invitrogen Corporation,
Carlsbad Calif.,
Cat #11791-019).
[00265] An NcoI-SacI fragment containing the AAD-12 (v1) coding sequence was
digested
from DASPIC012 and ligated into corresponding NcoI-SacI restriction sites
within the
following Gateway Donor vectors: pDAB3912 (attL1//CsVMV promoter//AtuORF23
3'UTR//attL2); pDAB3916 (attL1//AtUbil0 promoter//AtuORF23 3'UTR//attL2);
pDAB4458
(attL1//AtUbi3 promoter//AtuORF23 3'UTR//attL2); pDAB4459 (attL1//ZmUbil
promoter//AtuORF23 3'UTR//attL2); and pDAB4460 (attL1//AtAct2
promoter//AtuORF23
3'UTR//attL2). The resulting constructs containing the following plant
expression cassettes
were designated: pDAB4463 (attL1//CsVMV promoter//AAD-12 (v1)//AtuORF23
3'UTR//attL2); pDAB4467 (attL1//AtUbil0 promoter//AAD-12 (v1)//AtuORF23
3'UTR//attL2); pDAB4471 (attL1//AtUbi3 promoter//AAD-12 (v1)//AtuORF23
3'UTR//attL2);
pDAB4475 (attL1//ZmUbil promoter//AAD-12 (v1)//AtuORF23 3'UTR//attL2); and
pDAB4479 (attL1//AtAct2 promoter//AAD-12 (v1)//AtuORF23 3'UTR//attL2). These
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
constructs were confirmed via restriction enzyme digestion and sequencing.
[00266] The plant expression cassettes were recombined into the Gateway
Destination
Binary vector pDAB4484 (RB7 MARv3//attR1-ccdB-chloramphenicol resistance-
attR2//CsVMV promoter//PATv6//AtuORF1 3'UTR) via the Gateway LR Clonase
reaction.
Gateway Technology uses lambda phage-based site-specific recombination instead
of
restriction endonuclease and ligase to insert a gene of interest into an
expression vector.
Invitrogen Corporation, Gateway Technology: A Universal Technology to Clone
DNA
Sequences for Functional Analysis and Expression in multiple Systems,
Technical Manual,
Catalog #'s 12535-019 and 12535-027, Gateway Technology Version E, Sep. 22,
2003, #25-
022. The DNA recombination sequences (attL, and attR,) and the LR Clonase
enzyme mixture
allows any DNA fragment flanked by a recombination site to be transferred into
any vector
containing a corresponding site. The attL1 site of the donor vector
corresponds with attR1 of
the binary vector. Likewise, the attL2 site of the donor vector corresponds
with attR2 of the
binary vector. Using the Gateway Technology the plant expression cassette
(from the donor
vector) which is flanked by the attL sites can be recombined into the attR
sites of the binary
vector. The resulting constructs containing the following plant expression
cassettes were
labeled as: pDAB4464 (RB7 MARv3//CsVMV promoter//AAD-12 (v1)//AtuORF23
3'UTR//CsVMV promoter//PATv6 AtuORF1 3'UTR); pDAB4468 (RB7 MARv3//AtUbil0
promoter//AAD-12 (v1)//AtuORF23 3'UTR//CsVMV promoter//PATv6//AtuORF1 3'UTR);
pDAB4472 (RB7 MARv3//AtUbi3 promoter//AAD-12 (v1)//AtuORF23 3'UTR//CsVMV
promoter//PATv6//AtuORF1 3'UTR); pDAB4476 (RB7 MARv3//ZmUbi1 promoter//AAD-12
(v1)//AtuORF23 3'UTR//CsVMV promoter//PATv6 AtuORF1 3'UTR); and pDAB4480 (RB7
MARv3//AtAct2 promoter//AAD-12 (v1)//AtuORF23 3'UTR//CsVMV
promoter//PATv6//AtuORF1 3'UTR). These constructs were confirmed via
restriction enzyme
digestion and sequencing.
[00267] Transformation Method 1 - Agrobacterium-mediated Transformation: The
first
reports of soybean transformation targeted meristematic cells in the
cotyledonary node region
(Hinchee et al., 1988) and shoot multiplication from apical meristems (McCabe
et al., 1988). In
the A. tumefaciens-based cotyledonary node method, explant preparation and
culture media
composition stimulate proliferation of auxiliary meristems in the node
(Hinchee et al., 1988). It
remains unclear whether a truly dedifferentiated, but totipotent, callus
culture is initiated by
these treatments. The recovery of multiple clones of a transformation event
from a single
explant and the infrequent recovery of chimeric plants (Clemente et al., 2000;
Olhoft et al.,
2003) indicates a single cell origin followed by multiplication of the
transgenic cell to produce
81
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
either a proliferating transgenic meristem culture or a uniformly transformed
shoot that
undergoes further shoot multiplication. The soybean shoot multiplication
method, originally
based on microprojectile bombardment (McCabe et al., 1988) and, more recently,
adapted for
Agrobacterium-mediated transformation (Martine11 et al., 2002), apparently
does not undergo
the same level or type of dedifferentiation as the cotyledonary node method
because the system
is based on successful identification of germ line chimeras. Also, this is a
non 2,4-D based
protocol which would be ideal for 2,4-D selection system. Thus, the
cotyledonary node method
may be the method of choice to develop 2,4-D resistant soybean cultivars.
[00268] Plant transformation production of AAD-12 (v1) tolerant phenotypes.
Seed derived
explants of "Maverick" and the Agrobacterium mediated cot-node transformation
protocol was
used to produces AAD-12 (v1) transgenic plants.
[00269] Agrobacterium Preparation and Inoculation: Agrobacterium strain EHA101
(Hood
et al. 1986), carrying each of five binary pDAB vectors (Table 8) was used to
initiate
transformation. Each binary vector contains the AAD-12 (v1) gene and a plant-
selectable gene
(PAT) cassette within the T-DNA region. Plasmids were mobilized into the
EHA101 strain of
Agrobacterium by electroporation. The selected colonies were then analyzed for
the integration
of genes before the Agrobacterium treatment of the soybean explants. Maverick
seeds were
used in all transformation experiments and the seeds were obtained from
University of
Missouri, Columbia, Mo.
[00270] Agrobacterium-mediated transformation of soybean (Glycine max) using
the PAT
gene as a selectable marker coupled with the herbicide glufosinate as a
selective agent was
carried out. The seeds were germinated on B5 basal medium (Gamborg et al.
1968) solidified
with 3 g/L Phytagel (Sigma-Aldrich, St. Louis, Mo.). Selected shoots were then
transferred to
the rooting medium. The optimal selection scheme was the use of glufosinate at
8 mg/L across
the first and second shoot initiation stages in the medium and 3-4 mg/L during
shoot elongation
in the medium.
[00271] Prior to transferring elongated shoots (3-5 cm) to rooting medium, the
excised end
of the internodes were dipped in 1 mg/L indole 3-butyric acid for 1-3 min to
promote rooting
(Khan et al. 1994). The shoots struck roots in 25 x 100 mm glass culture tubes
containing
rooting medium and then they were transferred to soil mix for acclimatization
of plantlets in
Metro-mix 200 (Hummert International, Earth City, Mo.) in open Magenta boxes
in Convirons.
Glufosinate, the active ingredient of Liberty herbicide (Bayer Crop Science),
was used for
selection during shoot initiation and elongation. The rooted plantlets were
acclimated in open
Magenta boxes for several weeks before they were screened and transferred to
the greenhouse
82
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
for further acclimation and establishment.
[00272] Assay of Putatively Transformed Plantlets, and Analyses Established TO
Plants in
the Greenhouse: The terminal leaflets of selected leaves of these plantlets
were leaf painted
with 50 mg/L of glufosinate twice with a week interval to observe the results
to screen for
putative transformants. The screened plantlets were then transferred to the
greenhouse and after
acclimation the leaves were painted with glufosinate again to confirm the
tolerance status of
these plantlets in the GH and deemed to be putative transformants.
[00273] Plants that are transferred to the greenhouse can be assayed for the
presence of an
active PAT gene further with a non-destructive manner by painting a section of
leaf of the TO
primary transformant, or progeny thereof, with a glufosinate solution [0.05-2%
v/v Liberty
Herbicide, preferably 0.25-1.0% (v/v),=500-2000 ppm glufosinate, Bayer Crop
Science].
Depending on the concentration used, assessment for glufosinate injury can be
made 1-7 days
after treatment. Plants can also be tested for 2,4-D tolerance in a non-
destructive manner by
selective application of a 2,4-D solution in water (0.25-1% v/v commercial 2,4-
D
dimethylamine salt formulation, preferably 0.5% v/v=2280 ppm 2,4-D ae) to the
terminal
leaflet of the newly expanding trifoliolate one or two, preferably two, nodes
below the youngest
emerging trifolioate. This assay allows assessment of 2,4-D sensitive plants 6
hours to several
days after application by assessment of leaf flipping or rotation >90 degrees
from the plane of
the adjacent leaflets. Plants tolerant to 2,4-D will not respond to 2,4-D. TO
plants will be
allowed to self fertilize in the greenhouse to give rise to T1 seed. T1 plants
(and to the extent
enough TO plant clones are produced) will be sprayed with a range of herbicide
doses to
determine the level of herbicide protection afforded by AAD-12 (v1) and PAT
genes in
transgenic soybean. Rates of 2,4-D used on TO plants will typically comprise
one or two
selective rates in the range of 100-1120 g ae/ha using a track sprayer as
previously described.
T1 plants will be treated with a wider herbicide dose ranging from 50-3200 g
ae/ha 2,4-D.
Likewise, TO and T1 plants can be screened for glufosinate resistance by
postemergence
treatment with 200-800 and 50-3200 g ae/ha glufosinate, respectively.
Glyphosate resistance
(in plants transformed with constructs that contain EPSPS) or another
glyphosate tolerance gene
can be assessed in the T1 generation by postemergence applications of
glyphosate with a dose
range from 280-2240 g ae/ha glyphosate. Individual TO plants were assessed for
the presence
of the coding region of the gene of interest (AAD-12 (v1) or PAT v6) and copy
number.
Determination of the inheritance of AAD-12 (v1) will be made using T1 and T2
progeny
segregation with respect to herbicide tolerance as described in previous
examples.
[00274] A subset of the initial transformants were assessed in the TO
generation according to
83
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
the methods above. Any plant confirmed as having the AAD-12 (v1) coding
region, regardless
of the promoter driving the gene did not respond to the 2,4-D leaf painting
whereas wild type
Maverick soybeans did. PAT-only transformed plants responded the same at wild
type plants
to leaf paint applications of 2,4-D.
[00275] 2,4-D was applied to a subset of the plants that were of similar size
to the wild type
control plants with either 560 or 1120 g ae 2,4-D. All AAD-12 (v1)-containing
plants were
clearly resistant to the herbicide application versus the wild type Maverick
soybeans. A slight
level of injury (2 DAT) was observed for two AAD-12 (v1) plants, however,
injury was
temporary and no injury was observed 7 DAT. Wild type control plants were
severely injured
7-14 DAT at 560 g ae/ha 2,4-D and killed at 1120 g ae/ha. These data are
consistent with the
fact that AAD-12 (v1) can impart high tolerance (>2× field rates) to a
sensitive crop like
soybeans. The screened plants were then sampled for molecular and biochemical
analyses for
the confirmation of the AAD12 (v1) genes integration, copy number, and gene
expression
levels.
[00276] Molecular Analyses - Soybean: Tissue harvesting DNA isolation and
quantification.
Fresh tissue is placed into tubes and lyophilized at 4 C. for 2 days. After
the tissue is fully
dried, a tungsten bead (Valenite) is placed in the tube and the samples are
subjected to 1 minute
of dry grinding using a Kelco bead mill. The standard DNeasy DNA isolation
procedure is then
followed (Qiagen, DNeasy 69109). An aliquot of the extracted DNA is then
stained with Pico
Green (Molecular Probes P7589) and read in the fluorometer (BioTek) with known
standards to
obtain the concentration in ng/ L.
[00277] Polymerase chain reaction: A total of 100 ng of total DNA is used as
the template.
20 mM of each primer is used with the Takara Ex Taq PCR Polymerase kit (Mirus
TAKRROO1A). Primers for the AAD-12 (v1) PTU are (Forward-ATAATGCCAG
CCTGTTAAAC GCC) (SEQ ID NO: 8) and (Reverse-CTCAAGCATA TGAATGACCT
CGA) (SEQ ID NO: 9). The PCR reaction is carried out in the 9700 Geneamp
thermocycler
(Applied Biosystems), by subjecting the samples to 94 C. for 3 minutes and 35
cycles of 94 C.
for 30 seconds, 63 C. for 30 seconds, and 72 C. for 1 minute and 45 seconds
followed by 72
C. for 10 minutes. Primers for Coding Region PCR AAD-12 (v1) are (Forward-
ATGGCTCATG CTGCCCTCAG CC) (SEQ ID NO: 10) and (Reverse-CGGGCAGGCC
TAACTCCACC AA) (SEQ ID NO: 11). The PCR reaction is carried out in the 9700
Geneamp
thermocycler (Applied Biosystems), by subjecting the samples to 94 C. for 3
minutes and 35
cycles of 94 C. for 30 seconds, 65 C. for 30 seconds, and 72 C. for 1
minute and 45 seconds
followed by 72 C. for 10 minutes. PCR products are analyzed by
electrophoresis on a 1%
84
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
agarose gel stained with EtBr.
[00278] Southern blot analysis: Southern blot analysis is performed with total
DNA obtained
from Qiagen DNeasy kit. A total of 10 lug of genomic DNA is subjected to an
overnight
digestion to obtain integration data. After the overnight digestion an aliquot
of ¨100 ng is run
on a 1% gel to ensure complete digestion. After this assurance the samples are
run on a large
0.85% agarose gel overnight at 40 volts. The gel is then denatured in 0.2 M
NaOH, 0.6 M NaC1
for 30 minutes. The gel is then neutralized in 0.5 M Tris HC1, 1.5 M NaC1 pH
of 7.5 for 30
minutes. A gel apparatus containing 20 x SSC is then set up to obtain a
gravity gel to nylon
membrane (Millipore INYC00010) transfer overnight. After the overnight
transfer the
membrane is then subjected to UV light via a crosslinker (Stratagene UV
stratalinker 1800) at
1200 x 100 microjoules. The membrane is then washed in 0.1% SDS, 0.1 SSC for
45 minutes.
After the 45 minute wash, the membrane is baked for 3 hours at 80 C. and then
stored at 4 C.
until hybridization. The hybridization template fragment is prepared using the
above coding
region PCR using plasmid DNA. The product is run on a 1% agarose gel and
excised and then
gel extracted using the Qiagen (28706) gel extraction procedure. The membrane
is then
subjected to a pre-hybridization at 60 C. step for 1 hour in Perfect Hyb
buffer (Sigma H7033).
The Prime it RmT dCTP-labeling rxn (Stratagene 300392) procedure is used to
develop the p32
based probe (Perkin Elmer). The probe is cleaned up using the Probe Quant. G50
columns
(Amersham 27-5335-01). Two million counts CPM are used to hybridize the
southern blots
overnight. After the overnight hybridization the blots are then subjected to
two 20 minute
washes at 65 C. in 0.1% SDS, 0.1 SSC. The blots are then exposed to film
overnight,
incubating at -80 C.
[00279] Biochemical Analyses - Soybean: Tissue Sampling and Extracting AAD-12
(v1)
protein from soybean leaves. Approximately 50 to 100 mg of leaf tissue was
sampled from the
N-2 leaves that were 2,4-D leaf painted, but after 1 DAT. The terminal N-2
leaflet was
removed and either cut into small pieces or 2-single-hole-punched leaf discs (-
0.5 cm in
diameter) and were frozen on dry ice instantly. Protein analysis (ELISA and
Western analysis)
was completed accordingly.
[00280] T1 Progeny evaluation: TO plants will be allowed to self fertilize to
derive T1
families. Progeny testing (segregation analysis) will be assayed using
glufosinate at 560 g ai/ha
as the selection agent applied at the V1-V2 growth stage. Surviving plants
will be further
assayed for 2,4-D tolerance at one or more growth stages from V2-V6. Seed will
be produced
through self fertilization to allow broader herbicide testing on the
transgenic soybean.
[00281] AAD-12 (v1) transgenic Maverick soybean plants have been generated
through
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Agrobacterium-mediated transformation system. The TO plants obtained tolerated
up to 2 x
levels of 2,4-D field applications and developed fertile seeds. The frequency
of fertile
transgenic soybean plants was up to 5.9%. The integration of the AAD1-12 (v1)
gene into the
soybean genome was confirmed by Southern blot analysis. This analysis
indicated that most of
the transgenic plants contained a low copy number. The plants screened with
AAD-12 (v1)
antibodies showed positive for ELISA and the appropriate band in Western
analysis.
[00282] Transformation Method 2 - Aerosol-Beam Mediated Transformation of
Embryogenic Soybean Callus Tissue: Culture of embryogenic soybean callus
tissue and
subsequent beaming can be accomplished as described in U.S. Pat. No. 6,809,232
(Held et al.)
to create transformants using constructs provided herein.
[00283] Transformation Method 3 - Biolistic Bombardment of Soybean: This can
be
accomplished using mature seed derived embryonic axes meristem (McCabe et al.
(1988)).
Following established methods of biolistic bombardment, one can expect
recovery of
transformed soybean plants.
[00284] Transformation Method 4 - Whiskers Mediated Transformation: Whisker
preparation and whisker transformation can be performed according to methods
described
previously by Terakawa et al. (2005)). Following established methods of
biolistic
bombardment, one can expect recovery of transformed soybean plants.
[00285] Maverick seeds were surface-sterilized in 70% ethanol for 1 min
followed by
immersion in 1% sodium hypochlorite for 20 minutes and then rinsed three times
in sterile
distilled water. The seeds were soaked in distilled water for 18-20 hours. The
embryonic axes
were excised from seeds, and the apical meristems were exposed by removing the
primary
leaves. The embryonic axes were positioned in the bombardment medium [BM: MS
(Murashige and Skoog 1962) basal salts medium, 3% sucrose and 0.8% phytagel
Sigma, pH
5.7] with the apical region directed upwards in 5-cm culture dishes containing
12 ml culture
medium.
[00286] Transformation Method 5 - Particle bombardment-mediated transformation
for
embryogenic callus tissue can be optimized for according to previous methods
(Khalafalla et
al., 2005; El-Shemy et al., 2004, 2006).
Example 8
AAD-12 (v1) in Cotton
[00287] Cotton Transformation Protocol: Cotton seeds (Co310 genotype) are
surface-
sterilized in 95% ethanol for 1 minute, rinsed, sterilized with 50% commercial
bleach for
twenty minutes, and then rinsed 3 times with sterile distilled water before
being germinated on
86
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
G-media (Table 21) in Magenta GA-7 vessels and maintained under high light
intensity of 40-
60 [tE/m2, with the photoperiod set at 16 hours of light and 8 hours dark at
28 C.
[00288] Cotyledon segments (-5 mm) square are isolated from 7-10 day old
seedlings into
liquid M liquid media (Table 21) in Petri plates (Nunc, item #0875728). Cut
segments are
treated with an Agrobacterium solution (for 30 minutes) then transferred to
semi-solid M-media
(Table 21) and undergo co-cultivation for 2-3 days. Following co-cultivation,
segments are
transferred to MG media (Table 21). Carbenicillin is the antibiotic used to
kill the
Agrobacterium and glufosinate-ammonium is the selection agent that would allow
growth of
only those cells that contain the transferred gene.
[00289] Agrobacterium preparation: Inoculate 35 ml of Y media (Table 21)
(containing
streptomycin (100 mg/ml stock) and erythromycin (100 mg/ml stock)), with one
loop of
bacteria to grow overnight in the dark at 28 C., while shaking at 150 rpm.
The next day, pour
the Agrobacterium solution into a sterile oakridge tube (Nalge-Nunc, 3139-
0050), and
centrifuge for in Beckman J2-21 at 8,000 rpm for 5 minutes. Pour off the
supernatant and
resuspend the pellet in 25 ml of M liquid (Table 21) and vortex. Place an
aliquot into a glass
culture tube (Fisher, 14-961-27) for Klett reading (Klett-Summerson, model 800-
3). Dilute the
new suspension using M liquid media to a Klett-meter reading of 108 colony
forming units per
ml with a total volume of 40 ml.
[00290] After three weeks, callus from the cotyledon segments is isolated and
transferred to
fresh MG media. The callus is transferred for an additional 3 weeks on MG
media. In a side-
by-side comparison, MG media can be supplemented with dichlorprop (added to
the media at a
concentration of 0.01 and 0.05 mg/L) to supplement for the degradation of the
2,4-D, since
dichlorprop is not a substrate for to the AAD-12 enzyme, however dichlorprop
is more active
on cotton than 2,4-D. In a separate comparison, segments which were plated on
MG media
containing no growth regulator compared to standard MG media, showed reduced
callusing, but
there still is callus growth. Callus is then transferred to CG-media (Table
21), and transferred
again to fresh selection medium after three weeks. After another three weeks
the callus tissue is
transferred to D media (Table 21) lacking plant growth regulators for
embryogenic callus
induction. After 4-8 weeks on this media, embryogenic callus is formed, and
can be
distinguished from the non-embryogenic callus by its yellowish-white color and
granular cells.
Embryos start to regenerate soon after and are distinct green in color. Cotton
can take time to
regenerate and form embryos, one of the ways to speed up this process is to
stress the tissue.
Dessication is a common way to accomplish this, via changes in the
microenvironment of the
tissue and plate, by using less culture media and/or adopting various modes of
plate enclosure
87
CA 02876144 2014-12-05
WO 2013/185036
PCT/US2013/044717
(taping versus parafilm).
Table 21. Media for Cotton Transformation
Ingredients
G M liquid M MG CG D DK Y
in 1 liter
LS Salts
200 ml 200 ml 200 ml 200 ml 200 ml
(5X)
Glucose 30 grams 30 grams 30 grams 30 grams 20 grams
modified
B5 vit 1 ml 1 ml 1 ml 1 ml 1 ml 10 ml 1 ml
(1000x)
kinetin
lml 1 ml 1 ml 4.6 ml 0.5ml
(1mM)
2,4-D
lml 1 ml 1 ml
(1mM)
agar 8 grams 8 grams 8 grams 8 grams 8 grams 8 grams
DKW salts 1 1
(D190) package
package
MY0-
Inositol 1 ml 10 ml
(100x)
Sucrose
30 grams 30
grams 10 grams
3%
NAA
Carbenicilli
n(250 2m1 0.4 ml
mg/ml)
GLA
0.5 ml 0.3 ml
(10mg/m1)
Peptone
grams
Yeast 10
Extract
grams
NaC1 5
grams
[00291] Larger, well-developed embryos are isolated and transferred to DK
media (Table 21)
for embryo development. After 3 weeks (or when the embryos have developed),
germinated
embryos are transferred to fresh media for shoot and root development. After 4-
8 weeks, any
well-developed plants are transferred into soil and grown to maturity.
Following a couple of
months, the plant has grown to a point that it can be sprayed to determine if
it has resistance to
2,4-D.
[00292] Cell Transformation: Several experiments were initiated in which
cotyledon
segments were treated with Agrobacterium containing pDAB724. Over 2000 of the
resulting
segments were treated using various auxin options for the proliferation of
pDAB724 cotton
88
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
callus, either: 0.1 or 0.5 mg/L R-dichlorprop, standard 2,4-D concentration
and no auxin
treatment. The callus was selected on glufosinate-ammonium, due to the
inclusion of the PAT
gene in the construct. Callus line analysis in the form of PCR and Invader
will be used to
determine if and to be sure the gene was present at the callus stage; then
callus lines that are
embryogenic will be sent for Western analysis. Embryogenic cotton callus was
stressed using
dessication techniques to improve the quality and quantity of the tissue
recovered. Almost 200
callus events have been screened for intact PTU and expression using Western
analysis for the
AAD-12 (v1) gene.
[00293] Plant Regeneration: AAD-12 (v1) cotton lines that have produced plants
according
to the above protocol will be sent to the greenhouse. To demonstrate the AAD-
12 (v1) gene
provides resistance to 2,4-D in cotton, both the AAD-12 (v1) cotton plant and
wild-type cotton
plants will be sprayed with a track sprayer delivering 560 g ae/ha 2,4-D at a
spray volume of
187 L/ha. The plants will be evaluated at 3 and 14 days after treatment.
Plants surviving a
selective rate of 2,4-D will be self pollinated to create T1 seed or
outcrossed with an elite cotton
line to produce Fl seed. The subsequent seed produced will be planted and
evaluated for
herbicide resistance as previously described. AAD-12 (v1) events can be
combined with other
desired HT or IR trants.
Example 9
Agrobacterium Transformation of Other Crops
[00294] In light of the subject disclosure, additional crops can be
transformed according to
the subject invention using techniques that are known in the art. For
Agrobacterium-mediated
trans-formation of rye, see, e.g., Popelka and Altpeter (2003). For
Agrobacterium-mediated
transformation of soybean, see, e.g., Hinchee et al., 1988. For Agrobacterium-
mediated
transformation of sorghum, see, e.g., Zhao et al., 2000. For Agrobacterium-
mediated
transformation of barley, see, e.g., Tingay et al., 1997. For Agrobacterium-
mediated
transformation of wheat, see, e.g., Cheng et al., 1997. For Agrobacterium-
mediated
transformation of rice, see, e.g., Hiei et al., 1997.
[00295] The Latin names for these and other plants are given below. It should
be clear that
these and other (non Agrobacterium)transformation techniques can be used to
transform AAD-
12 (v1), for example, into these and other plants, including but not limited
to Maize (Zea mays),
Wheat (Triticum spp.), Rice (Oryza spp. and Zizania spp.), Barley (Hordeum
spp.), Cotton
(Abroma augusta and Gossypium spp.), Soybean (Glycine max), Sugar and table
beets (Beta
spp.), Sugar cane (Arenga pinnata), Tomato (Lycopersicon esculentum and other
spp., Physalis
ixocarpa, Solanum incanum and other spp., and Cyphomandra betacea), Potato
(Solanum
89
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
tubersoum), Sweet potato (Ipomoea betatas), Rye (Secale spp.), Peppers
(Capsicum annuum,
sinense, and frutescens), Lettuce (Lactuca sativa, perennis, and pulchella),
Cabbage (Brassica
spp), Celery (Apium graveolens), Eggplant (Solanum melongena), Peanut (Arachis
hypogea),
Sorghum (all Sorghum species), Alfalfa (Medicago sativua), Carrot (Daucus
carota), Beans
(Phaseolus spp. and other genera), Oats (Avena sativa and strigosa), Peas
(Pisum, Vigna, and
Tetragonolobus spp.), Sunflower (Helianthus annuus), Squash (Cucurbita spp.),
Cucumber
(Cucumis sativa), Tobacco (Nicotiana spp.), Arabidopsis (Arabidopsis
thaliana), Turfgrass
(Lolium, Agrostis, Poa, Cynadon, and other genera), Clover (Tifolium), Vetch
(Vicia). Such
plants, with AAD-12 (v1) genes, for example, are included in the subject
invention.
[00296] AAD-12 (v1) has the potential to increase the applicability of key
auxinic herbicides
for in-season use in many deciduous and evergreen timber cropping systems.
Triclopyr, 2,4-D,
and/or fluoroxypyr resistant timber species would increase the flexibility of
over-the-top use of
these herbicides without injury concerns. These species would include, but not
limited to:
Alder (Alnus spp.), ash (Fraxinus spp.), aspen and poplar species (Populus
spp.), beech (Fagus
spp.), birch (Betula spp.), cherry (Prunus spp.), eucalyptus (Eucalyptus
spp.), hickory (Carya
spp.), maple (Acer spp.), oak (Quercus spp), and pine (Pinus spp). Use of
auxin resistance for
the selective weed control in ornamental and fruit-bearing species is also
within the scope of
this invention. Examples could include, but not be limited to, rose (Rosa
spp.), burning bush
(Euonymus spp.), petunia (Petunia spp), begonia (Begonia spp.), rhododendron
(Rhododendron
spp), crabapple or apple (Malus spp.), pear (Pyrus spp.), peach (Prunus spp),
and marigolds
(Tagetes spp.).
Example 10
Further Evidence of Surprising Results: AAD-12 vs. AAD-2
[00297] AAD-2 (v1) Initial Cloning: Another gene was identified from the NCBI
database
(see the ncbi.nlm.nih.gov website; accession #AP005940) as a homologue with
only 44%
amino acid identity to tfdA. This gene is referred to herein as AAD-2 (v1) for
consistency.
Percent identity was determined by first translating both the AAD-2 and tfdA
DNA sequences
(SEQ ID NO: 12 of PCT/U52005/014737 and GENBANK Accession No. M16730,
respectively) to proteins (SEQ ID NO: 13 of PCT/U52005/014737 and GENBANK
Accession
No. M16730, respectively), then using ClustalW in the VectorNTI software
package to perform
the multiple sequence alignment.
[00298] The strain of Bradyrhizobium japonicum containing the AAD-2 (v1) gene
was
obtained from Northern Regional Research Laboratory (NRRL, strain #B4450). The
lyophilized strain was revived according to NRRL protocol and stored at -80
C. in 20%
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
glycerol for internal use as Dow Bacterial strain DB 663. From this freezer
stock, a plate of
Tryptic Soy Agar was then struck out with a loopful of cells for isolation,
and incubated at 28
C. for 3 days. A single colony was used to inoculate 100 ml of Tryptic Soy
Broth in a 500 ml
tri-baffled flask, which was incubated overnight at 28 C. on a floor shaker
at 150 rpm. From
this, total DNA was isolated with the gram negative protocol of Qiagen's
DNeasy kit (Qiagen
cat. #69504). The following primers were designed to amplify the target gene
from genomic
DNA, Forward: 5' ACT AGT AAC AAA GAA GGA GAT ATA CCA TGA CGA T 3' [(brj ap
5'(speI) SEQ ID NO: 14 of PCT/U52005/014737 (added Spe I restriction site and
Ribosome
Binding Site (RBS))] and Reverse: 5' TTC TCG AGC TAT CAC TCC GCC GCC TGC TGC
TGC 3' [(br jap 3' (xhoI) SEQ ID NO: 15 of PCT/US2005/014737 (added a Xho I
site)].
[00299] Fifty microliter reactions were set up as follows: Fail Safe Buffer 25
0, ea. primer 1
0 (50 ng/0), gDNA 1 0 (200 ng/0), H<sub>20</sub> 21 0, Taq polymerase 1 0 (2.5
units/0).
Three Fail Safe Buffers-A, B, and C-were used in three separate reactions. PCR
was then
carried out under the following conditions: 95 C. 3.0 minutes heat denature
cycle; 95 C. 1.0
minute, 50 C. 1.0 minute, 72 C. 1.5 minutes, for 30 cycles; followed by a
final cycle of 72 C.
minutes, using the FailSafe PCR System (Epicenter cat. #F599100). The
resulting ¨1 kb PCR
product was cloned into pCR 2.1 (Invitrogen cat. #K4550-40) following the
included protocol,
with chemically competent TOP1OF' E. coli as the host strain, for verification
of nucleotide
sequence.
[00300] Ten of the resulting white colonies were picked into 3 0 Luria Broth +
1000 lug/m1
Ampicillin (LB Amp), and grown overnight at 37 C. with agitation. Plasmids
were purified
from each culture using Nucleospin Plus Plasmid Miniprep Kit (BD Biosciences
cat. #K3063-
2) and following included protocol. Restriction digestion of the isolated
DNA's was completed
to confirm the presence of the PCR product in the pCR2.1 vector. Plasmid DNA
was digested
with the restriction enzyme EcoRI (New England Biolabs cat. #R0101S).
Sequencing was
carried out with Beckman CEQ Quick Start Kit (Beckman Coulter cat. #608120)
using M13
Forward [5' GTA AAA CGA CGG CCA G 31 (SEQ ID NO: 6) and Reverse [5' CAG GAA
ACA GCT ATG AC 31 (SEQ ID NO: 7) primers, per manufacturers instructions. This
gene
sequence and its corresponding protein was given a new general designation AAD-
2 (v1) for
internal consistency.
[00301] Completion of AAD-2 (v1) Binary Vector: The AAD-2 (v1) gene was PCR
amplified from pDAB3202. During the PCR reaction alterations were made within
the primers
to introduce the AflIII and SacI restriction sites in the 5' primer and 3'
primer, respectively. See
PCT/U52005/014737. The primers "NcoI of Brady" [5' TAT ACC ACA TGT CGA TCG CCA
91
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
TCC GGC AGC TT 31 (SEQ ID NO:14) and "SacI of Brady" [5' GAG CTC CTA TCA CTC
CGC CGC CTG CTG CTG CAC 31 (SEQ ID NO:15) were used to amplify a DNA fragment
using the Fail Safe PCR System (Epicentre). The PCR product was cloned into
the pCR2.1
TOPO TA cloning vector (Invitrogen) and sequence verified with M13 Forward and
M13
Reverse primers using the Beckman Coulter "Dye Terminator Cycle Sequencing
with Quick
Start Kit" sequencing reagents. Sequence data identified a clone with the
correct sequence
(pDAB716). The AflIII/SacI AAD-2 (v1) gene fragment was then cloned into the
NcoI/SacI
pDAB726 vector. The resulting construct (pDAB717); AtUbil0 promoter: Nt OSM
5'UTR:
AAD-2 (v1): Nt 05M3'UTR: ORF1 polyA 3'UTR was verified with restriction
digests (with
NcoI/SacI). This construct was cloned into the binary pDAB3038 as a NotI-NotI
DNA
fragment. The resulting construct (pDAB767); AtUbil0 promoter: Nt 05M5'UTR:
AAD-2
(v1): Nt OSM 3'UTR: ORF1 polyA 3'UTR: CsVMV promoter: PAT: 0RF25/26 3'UTR was
restriction digested (with Nod, EcoRI, HinDIII, NcoI, PvuII, and SalI) for
verification of the
correct orientation. The completed construct (pDAB767) was then used for
transformation into
Agrobacterium.
[00302] Evaluation of Transformed Arabidopsis: Freshly harvested T1 seed
transformed
with a plant optimized AAD-12 (v1) or native AAD-2 (v1) gene were planted and
selected for
resistance to glufosinate as previously described Plants were then randomly
assigned to various
rates of 2,4-D (50-3200 g ae/ha). Herbicide applications were applied by track
sprayer in a 187
L/ha spray volume. 2,4-D used was the commercial dimethylamine salt
formulation (456 g
ae/L, NuFarm, St Joseph, Mo.) mixed in 200 mM Tris buffer (pH 9.0) or 200 mM
HEPES
buffer (pH7.5).
[00303] AAD-12 (v1) and AAD-2 (v1) did provide detectable 2,4-D resistance
versus the
transformed and untransformed control lines; however, individual constructs
were widely
variable in their ability to impart 2,4-D resistance to individual T1
Arabidopsis plants.
Surprisingly, AAD-2 (v1) and AAD-2 (v2) transformants were far less resistant
to 2,4-D than
the AAD-12 (v1) gene, both from a frequency of highly tolerant plants as well
as overall
average injury. No plants transformed with AAD-2 (v1) survived 200 g ae/ha 2,4-
D relatively
uninjured (<20% visual injury), and overall population injury was about 83%
(see
PCT/U52005/014737). Conversely, AAD-12 (v1) had a population injury average of
about 6%
when treated with 3,200 g ae/ha 2,4-D. Tolerance improved slightly for plant-
optimized AAD-
2 (v2) versus the native gene; however, comparison of both AAD-12 and AAD-2
plant
optimized genes indicates a significant advantage for AAD-12 (v1) in planta.
[00304] These results are unexpected given that the in vitro comparison of AAD-
2 (v1) (see
92
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
PCT/US2005/014737) and AAD-12 (v2) indicated both were highly efficacious at
degrading
2,4-D and both shared an S-type specificity with respect to chiral
aryloxyalkanoate substrates.
AAD-2 (v1) is expressed in individual T1 plants to varying levels; however,
little protection
from 2,4-D injury is afforded by this expressed protein. No substantial
difference was evident
in protein expression level (in planta) for the native and plant optimized AAD-
2 genes (see
PCT/US2005/014737). These data corroborate earlier findings that make the
functional
expression of AAD-12 (v1) in planta, and resulting herbicide resistance to 2,4-
D and
pyridyloxyacetate herbicides, unexpected.
Example 11
In-Crop Use of Phenoxy Auxins Herbicides in Soybeans, Cotton, and Other Dicot
Crops
Transformed Only with AAD-12 (v1)
[00305] AAD-12 (v1) can enable the use of phenoxy auxin herbicides (e.g., 2,4-
D and
MCPA) and pyridyloxy auxins (triclopyr and fluoroxypyr) for the control of a
wide spectrum of
broadleaf weeds directly in crops normally sensitive to 2,4-D. Application of
2,4-D at 280 to
2240 g ae/ha would control most broadleaf weed species present in agronomic
environments.
More typically, 560-1120 g ae/ha is used. For triclopyr, application rates
would typically range
from 70-1120 g ae/ha, more typically 140-420 g ae/ha. For fluoroxypyr,
application rates
would typically range from 35-560 g ae/ha, more typically 70-280 ae/ha.
[00306] An advantage to this additional tool is the extremely low cost of the
broadleaf
herbicide component and potential short-lived residual weed control provided
by higher rates of
2,4-D, triclopyr, and fluoroxypyr when used at higher rates, whereas a non-
residual herbicide
like glyphosate would provide no control of later germinating weeds. This tool
also provides a
mechanism to combine herbicide modes of action with the convenience of HTC as
an integrated
herbicide resistance and weed shift management strategy.
[00307] A further advantage this tool provides is the ability to tankmix broad
spectrum
broadleaf weed control herbicides (e.g., 2,4-D, triclopyr and fluoroxypyr)
with commonly used
residual weed control herbicides. These herbicides are typically applied prior
to or at planting,
but often are less effective on emerged, established weeds that may exist in
the field prior to
planting. By extending the utility of these aryloxy auxin herbicides to
include at-plant,
preemergence, or pre-plant applications, the flexibility of residual weed
control programs
increases. One skilled in the art would recognize the residual herbicide
program will differ
based on the crop of interest, but typical programs would include herbicides
of the
chloracetmide and dinitroaniline herbicide families, but also including
herbicides such as
clomazone, sulfentrazone, and a variety of ALS-inhibiting PPO-inhibiting, and
HPPD-
93
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
inhibiting herbicides.
[00308] Further benefits could include tolerance to 2,4-D, triclopyr or
fluoroxypyr required
before planting following aryloxyacetic acid auxin herbicide application (see
previous
example); and fewer problems from contamination injury to dicot crops
resulting from
incompletely cleaned bulk tanks that had contained 2,4-D, triclopyr or
fluoroxypyr. Dicamba
(and many other herbicides) can still be used for the subsequent control of
AAD-12 (v1)-
transformed dicot crop volunteers.
[00309] Those skilled in the art will also recognize that the above example
can be applied to
any 2,4-D-sensitive (or other aryloxy auxin herbicide) crop that would be
protected by the
AAD-12 (v1) gene if stably transformed. One skilled in the art of weed control
will now
recognize that use of various commercial phenoxy or pyridyloxy auxin
herbicides alone or in
combination with a herbicide is enabled by AAD-12 (v1) transformation.
Specific rates of
other herbicides representative of these chemistries can be determined by the
herbicide labels
compiled in the CPR (Crop Protection Reference) book or similar compilation or
any
commercial or academic crop protection references such as the Crop Protection
Guide from
Agriliance (2005). Each alternative herbicide enabled for use in HTCs by AAD-
12 (v1),
whether used alone, tank mixed, or sequentially, is considered within the
scope of this
invention.
Example 12
In-Crop Use of Phenoxy Auxin and Pyridyloxy Auxin Herbicides in AAD-12 (v1)
Only
Transformed Corn, Rice, and Other Monocot Species
[00310] In an analogous fashion, transformation of grass species (such as, but
not limited to,
corn, rice, wheat, barley, or turf and pasture grasses) with AAD-12 (v1) would
allow the use of
highly efficacious phenoxy and pyridyloxy auxins in crops where normally
selectivity is not
certain. Most grass species have a natural tolerance to auxinic herbicides
such as the phenoxy
auxins (i.e., 2,4-D.). However, a relatively low level of crop selectivity has
resulted in
diminished utility in these crops due to a shortened window of application
timing or
unacceptable injury risk. AAD-12 (v1)-transformed monocot crops would,
therefore, enable the
use of a similar combination of treatments described for dicot crops such as
the application of
2,4-D at 280 to 2240 g ae/ha to control most broadleaf weed species. More
typically, 560-1120
g ae/ha is used. For triclopyr, application rates would typically range from
70-1120 g ae/ha,
more typically 140-420 g ae/ha. For fluoroxypyr, application rates would
typically range from
35-560 g ae/ha, more typically 70-280 ae/ha.
[00311] An advantage to this additional tool is the extremely low cost of the
broadleaf
94
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
herbicide component and potential short-lived residual weed control provided
by higher rates of
2,4-D, triclopyr, or fluoroxypyr. In contrast, a non-residual herbicide like
glyphosate would
provide no control of later-germinating weeds. This tool would also provide a
mechanism to
rotate herbicide modes of action with the convenience of HTC as an integrated-
herbicide-
resistance and weed-shift-management strategy in a glyphosate tolerant
crop/AAD-12 (v1)
HTC combination strategy, whether one rotates crops species or not.
[00312] A further advantage this tool provides is the ability to tankmix broad
spectrum
broadleaf weed control herbicides (e.g., 2,4-D, triclopyr and fluoroxypyr)
with commonly used
residual weed control herbicides. These herbicides are typically applied prior
to or at planting,
but often are less effective on emerged, established weeds that may exist in
the field prior to
planting. By extending the utility of these aryloxy auxin herbicides to
include at-plant,
preemergence, or pre-plant applications, the flexibility of residual weed
control programs
increases. One skilled in the art would recognize the residual herbicide
program will differ
based on the crop of interest, but typical programs would include herbicides
of the
chloracetmide and dinitroaniline herbicide families, but also including
herbicides such as
clomazone, sulfentrazone, and a variety of ALS-inhibiting PPO-inhibiting, and
HPPD-
inhibiting herbicides.
[00313] The increased tolerance of corn, rice, and other monocots to the
phenoxy or
pyridyloxy auxins shall enable use of these herbicides in-crop without growth
stage restrictions
or the potential for crop leaning, unfurling phenomena such as "rat-tailing,"
crop leaning,
growth regulator-induced stalk brittleness in corn, or deformed brace roots.
Each alternative
herbicide enabled for use in HTCs by AAD-12 (v1), whether used alone, tank
mixed, or
sequentially, is considered within the scope of this invention.
Example 13
AAD-12 (v1) in Rice
[00314] Media Description: Culture media employed were adjusted to pH 5.8 with
1 M KOH
and solidified with 2.5 g/L Phytagel (Sigma). Embryogenic calli were cultured
in 100 x 20 mm
Petri dishes containing 40 ml semi-solid medium. Rice plantlets were grown on
50 ml medium
in Magenta boxes. Cell suspensions were maintained in 125-ml conical flasks
containing 35
ml liquid medium and rotated at 125 rpm. Induction and maintenance of
embryogenic cultures
took place in the dark at 25-26 C., and plant regeneration and whole-plant
culture took place in
a 16-hour photoperiod (Zhang et al. 1996).
[00315] Induction and maintenance of embryogenic callus took place on NB basal
medium
as described previously (Li et al. 1993), but adapted to contain 500 mg/L
glutamine.
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Suspension cultures were initiated and maintained in SZ liquid medium (Zhang
et al. 1998)
with the inclusion of 30 g/L sucrose in place of maltose. Osmotic medium (NBO)
consisted of
NB medium with the addition of 0.256 M each of mannitol and sorbitol.
Hygromycin-B-
resistant callus was selected on NB medium supplemented with 50 mg/L
hygromycin B for 3-4
weeks. Pre-regeneration took place on medium (PRH50) consisting of NB medium
without
2,4-dichlorophenoxyacetic acid (2,4-D), but with the addition of 2 mg/L 6-
benzylaminopurine
(BAP), 1 mg/L a-naphthaleneacetic acid (NAA), 5 mg/L abscisic acid (ABA) and
50 mg/L
hygromycin B for 1 week. Regeneration of plantlets followed via culture on
regeneration
medium (RNH50) comprising NB medium without 2,4-D, and supplemented with 3
mg/L BAP,
0.5 mg/L NAA, and 50 mg/L hygromycin B until shoots regenerated. Shoots were
transferred
to rooting medium with half-strength Murashige and Skoog basal salts and
Gamborg's B5
vitamins, supplemented with 1% sucrose and 50 mg/L hygromycin B (1/2MSH50).
[00316] Tissue Culture Development: Mature desiccated seeds of Oryza sativa L.
japonica
cv. Taipei 309 were sterilized as described in Zhang et al. 1996. Embryogenic
tissues were
induced by culturing sterile mature rice seeds on NB medium in the dark. The
primary callus
approximately 1 mm in diameter, was removed from the scutellum and used to
initiate cell
suspension in SZ liquid medium. Suspensions were then maintained as described
in Zhang
1995. Suspension-derived embryogenic tissues were removed from liquid culture
3-5 days
after the previous subculture and placed on NBO osmotic medium to form a
circle about 2.5 cm
across in a Petri dish and cultured for 4 hous prior to bombardment. Sixteen
to 20 h after
bombardment, tissues were transferred from NBO medium onto NBH50 hygromycin B
selection medium, ensuring that the bombarded surface was facing upward, and
incubated in
the dark for 14-17 days. Newly formed callus was then separated from the
original bombarded
explants and placed nearby on the same medium. Following an additional 8-12
days, relatively
compact, opaque callus was visually identified, and transferred to PRH50 pre-
regeneration
medium for 7 days in the dark. Growing callus, which became more compact and
opaque was
then subcultured onto RNH50 regeneration medium for a period of 14-21 days
under a 16-hour
photoperiod. Regenerating shoots were transferred to Magenta boxes containing
1/2 MSH50
medium. Multiple plants regenerated from a single explant are considered
siblings and were
treated as one independent plant line. A plant was scored as positive for the
hph gene if it
produced thick, white roots and grew vigorously on 1/2 MSH50 medium. Once
plantlets had
reached the top of Magenta boxes, they were transferred to soil in a 6-cm pot
under 100%
humidity for a week, then moved to a growth chamber with a 14-h light period
at 30 C. and in
the dark at 21 C. for 2-3 weeks before transplanting into 13-cm pots in the
greenhouse. Seeds
96
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
were collected and dried at 37 C. for one week prior to storage.
[00317] Microprojectile Bombardment: All bombardments were conducted with the
Biolistic
PDS-1000/HeTM system (Bio-Rad, Laboratories, Inc.). Three milligrams of 1.0
micron
diameter gold particles were washed one with 100% ethanol, twice with sterile
distilled water
and resuspended in 50 [1.1 water in a siliconized Eppendorf tube. Five
micrograms plasmid
DNA representing a 1:6 molar ratio of pDOW3303 (Hpt-containing vector) to
pDAB4101
(AAD-12 (v1)+AHAS), 20 jai spermidine (0.1 M) and 50 jai calcium chloride (2.5
M) were
added to the gold suspension. The mixture was incubated at room temperature
for 10 min,
pelleted at 10000 rpm for 10 s, resuspended in 60 jai cold 100% ethanol and 8-
9 jai was
distributed onto each macrocarrier. Tissue samples were bombarded at 1100 psi
and 27 in of
Hg vacuum as described by Zhang et al. (1996).
[00318] Postemergence Herbicide Tolerance in AAD-12 (v1) Transformed TO Rice:
Rice
plantlets at the 3-5 leaf stage were sprayed with a lethal dose of 0.16% (v/v)
solution of Pursuit
(to confirm the presence of the AHAS gene) containing 1% Sunit II (v/v) and
1.25% UAN (v/v)
using a track sprayer calibrated to 187 L/ha. Rating for sensitivity or
resistance was performed
at 36 days after treatment (DAT). Ten of the 33 events sent to the greenhouse
were robustly
tolerant to the Pursuit; others suffered varying levels of herbicide injury.
Plants were sampled
and molecular characterization was performed that identified seven of these 10
events as
containing both the AAD-12 (v1) PTU and the entire AHAS coding region.
[00319] Heritability of AAD-12 (v1) in T1 Rice: A 100-plant progeny test was
conducted on
five T1 lines of AAD-12 (v1) lines that contained both the AAD-12 (v1) PTU and
AHAS
coding region. The seeds were planted with respect to the procedure above and
sprayed with
140 g ae/ha imazethapyr using a track sprayer as previously described. After
14 DAT, resistant
and sensitive plants were counted. Two out of the five lines tested segregated
as a single locus,
dominant Mendelian trait (3R:1S) as determined by Chi square analysis. AAD-12
coseregated
with the AHAS selectable marker as determined by 2,4-D tolerance testing
below.
[00320] Verification of High 2,4-D Tolerance in T1 Rice: The following T1 AAD-
12 (v1)
single segregating locus lines were planted into 3-inch pots containing Metro
Mix media:
pDAB4101(20)003 and pDAB4101(27)002. At 2-3 leaf stage were sprayed with 140 g
ae/ha
imazethapyr. Nulls were eliminated and individuals were sprayed at V3-V4 stage
in the track
sprayer set to 187 L/ha at 1120, 2240 or 4480 g ae/ha 2,4-D DMA (2 x, 4 x, and
8 x typical
commercial use rates, respectively). Plants were graded at 7 and 14 DAT and
compared to
untransformed commercial rice cultivar, 'Lamont,' as negative control plants.
97
CA 02876144 2014-12-05
WO 2013/185036
PCT/US2013/044717
Table 22. T1 AAD-12 (v1) and untransformed control response to varying levels
of 2,4-D
DMA: Average % injury 14 DAT
Lemont
Herbicide Untransformed pDAB4101(20)003 pDAB4101(27)002
Control
1120 g ae/ha 2,4-D
20 10 10
DMA
2240 g ae/ha 2,4-D
35 15 30
DMA
4480 g ae/ha 2,4-D
50 23 40
DMA
[00321] Injury data (Table 22) shows that the AAD-12 (v1)-transformed lines
are more
tolerant to high rates of 2,4-D DMA than the untransformed controls. The line
pDAB4101(20)003 was more tolerant to high levels of 2,4-D than the line
pDAB4101(27)002.
The data also demonstrates that tolerance of 2,4-D is stable for at least two
generations.
[00322] Tissue Harvesting, DNA Isolation and Quantification: Fresh tissue was
placed into
tubes and lyophilized at 4 C. for 2 days. After the tissue was fully dried, a
tungsten bead
(Valenite) was placed in the tube and the samples were subjected to 1 minute
of dry grinding
using a Kelco bead mill. The standard DNeasy DNA isolation procedure was then
followed
(Qiagen, Dneasy 69109). An aliquot of the extracted DNA was then stained with
Pico Green
(Molecular Probes P7589) and scanned in the florometer (BioTek) with known
standards to
obtain the concentration in ng/ 1.
[00323] AAD-12 (v1) Expression: All 33 TO transgenic rice lines and 1 non-
transgenic
control were analyzed for AAD-12 expression using ELISA blot. AAD-12 was
detected in the
clones of 20 lines, but not in line Taipai 309 control plant. Twelve of the 20
lines that had some
of the clones tolerant to imazethapyr were expressing AAD-12 protein, were AAD-
12 PCR
PTU positive, and AHAS coding region positive. Expression levels ranged from
2.3 to 1092.4
ppm of total soluble protein.
[00324] Field Tolerance of pDAB4101 Rice Plants to 2,4-D and Triclopyr
Herbicides: A
field level tolerance trial was conducted with AAD-12 (v1) event pDAB4101[20]
and one wild-
type rice (Clearfield 131) at Wayside, Miss. (a non-transgenic imidazolinone-
resistant variety).
The experimental design was a randomized complete block design with a single
replication.
Herbicide treatments were 2 x rates of 2,4-D (dimethylamine salt) at 2240 g
ae/ha and triclopyr
at 560 g ae/ha plus an untreated control. Within each herbicide treatment, two
rows of T1
generation pDAB4101[20] and two rows of Clearfield rice were planted using a
small plot drill
with 8-inch row spacing. The pDAB4101 [20] rice contained the AHAS gene as a
selectable
98
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
marker for the AAD-12(v1) gene. Imazethapyr was applied at the one leaf stage
as selection
agent to remove any AAD-12 (v1) null plants from the plots. Herbicide
treatments were
applied when the rice reached the 2 leaf stage using compressed air backpack
sprayer delivering
187 L/ha carrier volume at 130-200 kpa pressure. Visual ratings of injury were
taken at 7, 14
and 21 days after application.
[00325] AAD-12 (v1) event response to 2,4-D and triclopyr are shown in Table
23. The
non-transformed rice line (Clearfield) was severely injured (30% at 7DAT and
35% at 15DAT)
by 2,4-D at 2240 g ae/ha which is considered the 4 x commercial use rate. The
AAD-12 (v1)
event demonstrated excellent tolerance to 2,4-D with no injury observed at 7
or 15DAT. The
non-transformed rice was significantly injured (15% at 7DAT and 25% at 15DAT)
by the 2 x
rate of triclopyr (560 g ae/ha). The AAD-12 (v1) event demonstrated excellence
tolerance to
the 2 x rates of triclopyr with no injury observed at either 7 or 15DAT.
[00326] These results indicate that the AAD-12 (v1) transformed rice displayed
a high level
of resistance to 2,4-D and triclopyr at rates that caused severe visual injury
to the Clearfield
rice. It also demonstrates the ability to stack multiple herbicide tolerance
genes with AAD-12 I
multiple species to provide resistance to a wider spectrum of effective
chemistries.
Table 23. AAD-12 T1 generation rice plants response to 2,4-D and triclopyr
under field
conditions
% Visual Injury
Herbicide Treatment
7 DAT 15 DAT
Active Rate AAD-12 event Wild-type AAD-12 event Wild-type
Ingredient pDAB4101[20] Clearfield pDAB4101[20] Clearfield
2,4-D 2240 GM
0 15 0 35
AE/HA
Triclopyr 840 GM
0 30 0 25
AE/HA
Untreated 0 0 0 0
Example 14
AAD-12 (v1) in Canola
[00327] Canola Transformation: The AAD-12 (v1) gene conferring resistance to
2,4-D was
used to transform Brassica napus var. Nexera*710 with Agrobacterium-mediated
transformation and plasmid pDAB3759. The construct contained AAD-12 (v1) gene
driven by
CsVMV promoter and Pat gene driven by AtUbil0 promoter and the EPSPS
glyphosate
resistance trait driven by AtUbil0 promoter.
[00328] Seeds were surface-sterilized with 10% commercial bleach for 10
minutes and
rinsed 3 times with sterile distilled water. The seeds were then placed on one
half concentration
99
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
of MS basal medium (Murashige and Skoog, 1962) and maintained under growth
regime set at
25 C., and a photoperiod of 16 hours light/8 hours dark.
[00329] Hypocotyl segments (3-5 mm) were excised from 5-7 day old seedlings
and placed
on callus induction medium K1D1 (MS medium with 1 mg/L kinetin and 1 mg/L 2,4-
D) for 3
days as pre-treatment. The segments were then transferred into a petri plate,
treated with
Agrobacterium Z7075 or LBA4404 strain containing pDAB3759. The Agrobacterium
was
grown overnight at 28 C. in the dark on a shaker at 150 rpm and subsequently
re-suspended in
the culture medium.
[00330] After 30 min treatment of the hypocotyl segments with Agrobacterium,
these were
placed back on the callus induction medium for 3 days. Following co-
cultivation, the segments
were placed on K 1D1TC (callus induction medium containing 250 mg/L
Carbenicillin and 300
mg/L Timentin) for one week or two weeks of recovery. Alternately, the
segments were placed
directly on selection medium K1D1H1 (above medium with 1 mg/L Herbiace).
Carbenicillin
and Timentin were the antibiotics used to kill the Agrobacterium. The
selection agent Herbiace
allowed the growth of the transformed cells.
[00331] Callused hypocotyl segments were then placed on B3Z1H1 (MS medium, 3
mg/L
benzylamino purine, 1 mg/L Zeatin, 0.5 gm/L MES [2-(N-morpholino) ethane
sulfonic acid], 5
mg/L silver nitrate, 1 mg/L Herbiace, Carbenicillin and Timentin) shoot
regeneration medium.
After 2-3 weeks shoots started regenerating. Hypocotyl segments along with the
shoots are
transferred to B3Z1H3 medium (MS medium, 3 mg/L benzylamino purine, 1 mg/L
Zeatin, 0.5
gm/L MES [2-(N-morpholino) ethane sulfonic acid], 5 mg/L silver nitrate, 3
mg/L Herbiace,
Carbenicillin and Timentin) for another 2-3 weeks.
[00332] Shoots were excised from the hypocotyl segments and transferred to
shoot
elongation medium MESH5 or MES10 (MS, 0.5 gm/L MES, 5 or 10 mg/L Herbiace,
Carbenicillin, Timentin) for 2-4 weeks. The elongated shoots are cultured for
root induction on
MSI.1 (MS with 0.1 mg/L Indolebutyric acid). Once the plants had a well
established root
system, these were transplanted into soil. The plants were acclimated under
controlled
environmental conditions in the Conviron for 1-2 weeks before transfer to the
greenhouse.
[00333] Molecular Analysis - Canola Materials and Methods: Tissue harvesting
DNA
isolation and quantification. Fresh tissue was placed into tubes and
lyophilized at 4 C. for 2
days. After the tissue was fully dried, a tungsten bead (Valenite) was placed
in the tube and the
samples were subjected to 1 minute of dry grinding using a Kelco bead mill.
The standard
DNeasy DNA isolation procedure was then followed (Qiagen, DNeasy 69109). An
aliquot of
the extracted DNA was then stained with Pico Green (Molecular Probes P7589)
and read in the
100
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
fluorometer (BioTek) with known standards to obtain the concentration in ng/
1.
[00334] Polymerase chain reaction: A total of 100 ng of total DNA was used as
the template.
20 mM of each primer was used with the Takara Ex Taq PCR Polymerase kit (Mirus
TAKRROO1A). Primers for Coding Region PCR AAD-12 (v1) were (SEQ ID NO: 10)
(forward) and (SEQ ID NO: 11) (reverse). The PCR reaction was carried out in
the 9700
Geneamp thermocycler (Applied Biosystems), by subjecting the samples to 94 C.
for 3
minutes and 35 cycles of 94 C. for 30 seconds, 65 C. for 30 seconds, and 72
C. for 2 minutes
followed by 72 C. for 10 minutes. PCR products were analyzed by
electrophoresis on a 1%
agarose gel stained with EtBr. 35 samples from 35 plants with AAD-12 (v1)
events tested
positive. Three negative control samples tested negative.
[00335] ELISA: Using established ELISA described in previous section, AAD-12
protein
was detected in 5 different canola transformation plant events. Expression
levels ranged from
14 to over 700 ppm of total soluble protein (TSP). Three different
untransformed plant samples
were tested in parallel with no signal detected, indicating that the
antibodies used in the assay
have minimal cross reactivity to the canola cell matrix. These samples were
also confirmed
positive by Western analysis. A summary of the results is presented in Table
24.
Table 24. Expression of AAD-12 (v1) in Canola plants
[TSP] [AAD-12] Expression
Sample #(ppm TSP) Western
g//mL) (ng/mL)
(ELISA)
31 5614.96 1692.12 301.36 ++++
33 4988.26 2121.52 425.30 ++++
38 5372.25 3879.09 722.06 ++++
39 2812.77 41.36 14.71 +
40 3691.48 468.74 126.98 +++
Control 1 2736.24 0.00 0.00
Control 2 2176.06 0.00 0.00 -
Control 3 3403.26 0.00 0.00 -
[00336] Postemergence Herbicide Tolerance in AAD-12(v1) Transformed TO Canola:
Forty-
five TO events from the transformed with the construct pDAB3759, were sent to
the greenhouse
over a period of time and were allowed to acclimate in the greenhouse. The
plants were grown
until 2-4 new, normal looking leaves had emerged (i.e., plants had
transitioned from tissue
culture to greenhouse growing conditions). Plants were then treated with a
lethal dose of the
commercial formulations of 2,4-D Amine 4 at a rate of 560 g ae/ha. Herbicide
applications
were made with a track sprayer at a spray volume of 187 L/ha, 50-cm spray
height. A lethal
dose is defined as the rate that causes >95% injury to the untransformed
controls.
101
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
[00337] Twenty-four of the events were tolerant to the 2,4-D DMA herbicide
application.
Some events did incur minor injury but recovered by 14 DAT. Events were
progressed to the
T1 (and T2 generation) by selfpollination under controlled, bagged,
conditions.
[00338] AAD-12 (v1) Heritability in Canola: A 100 plant progeny test was also
conducted
on 11 T1 lines of AAD-12 (v1). The seeds were sown and transplanted to 3-inch
pots filled
with Metro Mix media. All plants were then sprayed with 560 g ae/ha 2,4-D DMA
as
previously described. After 14 DAT, resistant and sensitive plants were
counted. Seven out of
the 11 lines tested segregated as a single locus, dominant Mendelian trait
(3R:1S) as determined
by Chi-square analysis. AAD-12 is heritable as a robust aryloxyalkanoate auxin
resistance gene
in multiple species and can be stacked with one or more additional herbicide
resistance genes.
[00339] AAD-12 (v1) Heritability in Canola: A 100 plant progeny test was also
conducted
on 11 T1 lines of AAD-12 (v1). The seeds were sown and transplanted to 3-inch
pots filled
with Metro Mix media. All plants were then sprayed with 560 g ae/ha 2,4-D DMA
as
previously described. After 14 DAT, resistant and sensitive plants were
counted. Seven out of
the 11 lines tested segregated as a single locus, dominant Mendelian trait
(3R:1S) as determined
by Chi-square analysis. AAD-12 is heritable as a robust aryloxyalkanoate auxin
resistance gene
in multiple species and can be stacked with one or more additional herbicide
resistance genes.
[00340] Verification of High 2,4-D Tolerance in T1 Canola: For T1 AAD-12 (v1),
5-6 mg
of seed were stratified, sown, and a fine layer of Sunshine Mix #5 media was
added as a top
layer of soil. Emerging plants were selected with 560 g ae/ha 2,4-D at 7 and
13 days after
planting.
Table 25. T1 AAD-12 (v1) and untransformed control response to varying rates
postemergence 2,4-
D DMA applications: Average % injury 14 DAT
Herbicide Untransformed pDAB3759 pDAB3759 pDAB3759 pDAB3759 pDAB3759
2,4-D DMA Control (33) (18) (18) (18) (18)
013.001 009.001 022.001 030.001 023.001
1120 g ae/ha 90 0 0 13 5 3
2240 g ae/ha 95 1 5 83 31 6
[00341] Surviving plants were transplanted into 3-inch pots containing Metro
Mix media.
Surviving plants from T1 progenies, that were selected with 560 g ae/ha 2,4-D,
were also
transplanted into 3-inch pots filled with Metro Mix soil. At 2-4 leaf stage
plants were sprayed
with either 280, 560, 1120, or 2240 g ae/ha 2,4-D DMA. Plants were graded at 3
and 14 DAT
and compared to untransformed control plants. A sampling of T1 event injury
data 14DAT
may be seen in Table 25. Data suggests that multiple events are robustly
resistant to 2240 g
ae/ha 2,4-D, while other events demonstrated less robust tolerance up to 1120
g ae/ha 2,4-D.
102
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Surviving plants were transplanted to 51/4" pots containing Metro Mix media
and placed in the
same growth conditions as before and self-pollinated to produce only
homozygous seed.
[00342] Field Tolerance of pDAB3759 Canola Plants to 2,4-D, Dichloprop,
Triclopyr and
Fluoroxypyr Herbicides: Field level tolerance trial was conducted on two AAD-
12 (v1) events
3759(20)018.001 and 3759(18)030.001 and a wild-type canola (Nex710) in Fowler,
Ind. The
experimental design was a randomized complete block design with 3
replications. Herbicide
treatments were 2,4-D (dimethylamine salt) at 280, 560, 1120, 2240 and 4480 g
ae/ha, triclopyr
at 840 g ae/ha, fluoroxypyr at 280 g ae/ha and an untreated control. Within
each herbicide
treatment, single 20 ft row/event for event 3759(18)030.0011, 3759(18)018.001
and wild-type
line (Nex710) were planted with a 4 row drill on 8 inch row spacing. Herbicide
treatments
were applied when canola reached the 4-6 leaf stage using compressed air
backpack sprayer
delivering 187 L/ha carrier volume at 130-200 kpa pressure. Visual injury
ratings were taken at
7, 14 and 21 days after application.
Table 26. AAD-12 (pDAB3759) canola plants response to 2,4-D, triclopyr, and
fluroxypyr under field conditions.
Herbicide Treatment % Visual Injury at 14 DAT
Active Rate AAD-12 event AAD-12 event Wild Type
Ingredient 3759(20)018.001 3759(18)030.001 (Nex710)
2,4-D 280 GM AE/HA 0 a 0 b 0 c
2,4-D 560 GM AE/HA 0 a 0 b 15 d
2,4-D 1120 GM AE/HA 2 a 2 ab 33 bc
2,4-D 2240 GM AE/HA 3 a 3 ab 48 a
Triclopyr 840 GM AE/HA 6 a 6 ab 25 cd
Fluroxypyr 280 GM AE/HA 7 a 8 a 37 ab
[00343] Canola response to 2,4-D, triclopyr, and fluoroxypyr are shown in
Table 26. The
wild-type canola (Nex710) was severely injured (72% at 14DAT) by 2,4-D at 2240
g ae/ha
which is considered the 4 x rate. The AAD-12 (v1) events all demonstrated
excellent tolerance
to 2,4-D at 14DAT with an average injury of 2, 3 and 2% observed at the 1, 2
and 4 x rates,
respectively. The wild-type canola was severely injured (25% at 14DAT) by the
2 x rate of
triclopyr (840 g ae/ha). AAD-12 (v1) events demonstrated tolerance at 2 x
rates of triclopyr
with an average of 6% injury at 14DAT across the two events. Fluoroxypyr at
280 g ae/ha
caused severe injury (37%) to the non-transformed line at 14DAA. AAD-12 (v1)
events
demonstrated increased tolerance with an average of 8% injury at 5DAT.
[00344] These results indicate that AAD-12 (v1) transformed events displayed a
high level
of resistance to 2,4-D, triclopyr and fluoroxypyr at rates that were lethal or
caused severe
epinastic malformations to non-transformed canola. AAD-12 has been shown to
have relative
103
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
efficacy of 2,4-D > triclopyr > fluoroxypyr.
Example 15
Transformation and Selection of the AAD-12 Soybean Event DAS-68416-4
[00345] Transgenic soybean (Glycine max) Event DAS-68416-4 was generated
through
Agrobacterium-mediated transformation of soybean cotyledonary node explants.
The disarmed
Agrobacterium strain EHA101 (Hood et al., 2006), carrying the binary vector
pDAB4468 (FIG.
2) with the selectable marker (pat) and the gene of interest (AAD-12) within
the T-strand DNA
region, was used to initiate transformation.
[00346] Agrobacterium-mediated transformation was carried out. Briefly,
soybean seeds (cv
Maverick) were germinated on basal media and cotyledonary nodes were isolated
and infected
with Agrobacterium. Shoot initiation, shoot elongation, and rooting media were
supplemented
with cefotaxime, timentin and vancomycin for removal of Agrobacterium.
Glufosinate
selection was employed to inhibit the growth of non-transformed shoots.
Selected shoots were
transferred to rooting medium for root development and then transferred to
soil mix for
acclimatization of plantlets.
[00347] Terminal leaflets of selected plantlets were leaf painted with
glufosinate to screen
for putative transformants. The screened plantlets were transferred to the
greenhouse, allowed
to acclimate and then leaf-painted with glufosinate to reconfirm tolerance and
deemed to be
putative transformants. The screened plants were sampled and molecular
analyses for the
confirmation of the selectable marker gene and/or the gene of interest were
carried out. TO
plants were allowed to self fertilize in the greenhouse to give rise to T1
seed.
[00348] The T1 plants were backcrossed and introgressed into elite germplasm
(Maverick).
This event, soybean Event DAS-68416-4, was generated from an independent
transformed
isolate. The event was selected based on its unique characteristics such as
single insertion site,
normal Mendelian segregation and stable expression, and a superior combination
of efficacy,
including herbicide tolerance and agronomic performance in broad genotype
backgrounds and
across multiple environmental locations. Additional description of soybean
Event DAS-68416-
4 has been disclosed in WO 2011/066384, which is incorporated by reference in
its entirety.
Example 16
Generation of 2008 Agronomic Data
[00349] An agronomic study with Event DAS-68416-4 soybean and a non-transgenic
control
(var. Maverick) was conducted in 2008 at six sites located in Iowa, Illinois,
Indiana, Nebraska
and Ontario, Canada (2 sites). Agronomic determinants, including
stand/population count,
104
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
seedling/plant vigor, plant height, lodging, disease incidence, insect damage,
and days to
flowering were evaluated to investigate the equivalency of the soybean Event
DAS-68416-4
(with and without herbicide treatments) as compared to the control line
Maverick. This study is
referred to as Agronomic Experiment Sl.
Table 27. Agronomic parameters evaluated in Agnomic Experiment Sl.
Trait Evaluation Timing Description of Data Scale
Early population VC-V2 Number of plants Actual count per plot
emerged in rows of
each plot
Seedling vigor VC-V2
Visual estimate of 1-10 scaled based on
average vigor of growth of the non-
emerged plants per transformed soybeans
plot 10
= Growth equivalence
to non-transformed
9 = Plant health is 90% as
compared to
non-
transformed, etc.
Plant vigor / injury After post-emergent Injury
from 1-10 scale based on growth
herbicide herbicide of
the non-transformed
applications applications soybeans
= Growth equivalence
to non-transformed
9 = Plant health is 90% as
compared to
non-
transformed, etc.
Plant height Approximately R6 Height from soil Height in cm
surface to the tip of (average of 10 plants per
the highest leaf plot)
when extended by
hand
Lodging Approximately R8
Visual estimate of Visual estimate on 0-100%
lodging severity
scale based on the number
of plants lodged
Final population
Approximately R8 The number of Actual count per plot,
plants remaining in including plants removed
rows of each plot during previous sampling
[00350] The test and control soybean seed were planted at a seeding rate of
approximately
112 seeds per 25 ft row with a row spacing of approximately 30 inches (75 cm).
At each site,
three replicate plots of each treatment were established, with each plot
consisting of 2-25 ft
rows. Plots were arranged in a randomized complete block (RCB) design, with a
unique
randomization at each site. Each soybean plot was bordered by two rows of a
non-transgenic
soybean of similar maturity. The entire trial site was surrounded by a minimum
of 10 ft of a
non-transgenic soybean of similar relative maturity.
105
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
[00351] Herbicide treatments were applied with a spray volume of approximately
20 gallons
per acre (187 L/ha). These applications were designed to replicate maximum
label rate
commercial practices. 2,4-D was applied as three broadcast over-the-top
applications for a
seasonal total of 3 lb ae/A. Individual applications of 1.0 lb ae A (1,120
g/ha) were made at
pre-emergence and approximately V4 and R2 growth stages. Glufosinate was
applied as two
broadcast over-the-top applications for a seasonal total of 0.74 lb ai/A (828
g ai/ha). Individual
applications of 0.33 lb ai/A and 0.41 lb ai/A (374 and 454 g ai/ha) were made
at approximately
V6 and R1 growth stages.
[00352] Analysis of variance was conducted across the field sites for the
agronomic data
using a mixed model (SAS Version 8; SAS Institute 1999). Entry was considered
a fixed
effect, and location, block within location, location-by-entry, and entry-by-
block within
location were designated as random effects. The significance of an overall
treatment effect was
estimated using an F-test. Paired contrasts were made between the control and
unsprayed
soybean Event DAS-68416-4 (unsprayed), soybean Event DAS-68416-4 sprayed with
glufosinate (soybean Event DAS-68416-4 + glufosinate), soybean Event DAS-68416-
4 sprayed
with 2,4-D (soybean Event DAS-68416-4 + 2,4-D) and soybean Event DAS-68416-4
sprayed
with both glufosinate and 2,4-D (soybean Event DAS-68416-4 + both) transgenic
entries using
t-tests. Adjusted P-values were also calculated using the False Discovery Rate
(FDR) to control
for multiplicity (Benjamini and Hochberg, 1995).
[00353] An analysis of the agronomic data collected from the control, soybean
Event DAS-
68416-4 unsprayed, soybean Event DAS-68416-4 + 2,4-D, soybean Event DAS-68416-
4 +
glufosinate, and soybean Event DAS-68416-4 + both herbicides was conducted. No
statistically significant differences were observed for stand count, early
population, seedling
vigor, injury after application, lodging, final stand count or days to
flowering (Table 28). For
height, a significant paired t-test was observed between the control and the
soybean Event
DAS-68416-4 + 2,4-D spray. However, no significant overall treatment effect
was observed,
differences were very small between the soybean Event DAS-68416-4 treatment
and the
control, and differences were not shared among the different soybean Event DAS-
68416-4
treatments. Based on these results, soybean Event DAS-68416-4 was
agronomically equivalent
to the near-isogenic non-transgenic control.
106
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Table 28. Analysis of agronomic characteristics from Agronomic Experiment Sl.
Overall Sprayed Sprayed 2,4- Sprayed
Unsprayed
Treatment
Control (P-value,b Glufosinate D Both
Analyte
Effect
Adj. 1_,)e (P-value, (P-value, (P-
value,
(Pr>F)a Adj. P) Adj. P) Adj.
P)
Stand Count 0.774 170 172 175 173 175
(no. of plants)
(0.709,0.824) (0.311,0.575) (0.476,0.672) (0.269,0.575)
Early Population 0.714 76.7 77.4 79.1 79.0 79.4
(% emergence)d
(0.738,0.824) (0.301,0.575) (0.327,0.575) (0.256,0.575)
Seedling Vigor' 0.547 9.72 9.39 9.50 9.44 9.39
(0.146,0.575) (0.326,0.575) (0.222,0.575) (0.146,0.575)
Vigor/Injury 0.511 10.0 9.86 9.89 9.83 9.67
App. 2e
(0.461,0.671) (0.555,0.718) (0.378,0.611) (0.087,0.575)
Vigor/Injury 0.462 10.0 10.0 9.89 9.83 9.89
App. 3e
(1.000,1.000) (0.320,0.575) (0.141,0.575) (0.320,0.575)
Vigor/Injury 0.431 9.94 9.89 9.78 9.67 9.78
App. 5e
(0.721,0.824) (0.289,0.575) (0.085,0.575) (0.289,0.575)
Height (cm) 0.144 101 98.1 99.2 96.1 97.2
(0.145,0.575) (0.390,0.611) (0.020,0.575) (0.062,0.575)
Lodging (%) 0.948 17.2 18.2 21.3 20.7 21.7
(0.885,0.904) (0.551,0.718) (0.606,0.746) (0.511,0.700)
Final Stand
0.268 156 154 161 155 163
Count
(no. of plants) ...
(0.770,0.840) (0.335,0.575) (0.817,0.853) (0.127,0.575)
Flowering Daysr 0.452 49.0 49.5 49.4 48.7 49.2
(0.261,0.575) (0.395,0.611) (0.568,0.718) (0.668,0.801)
a Overall treatment effect estimated using an F-test.
b
Comparison of the sprayed and unsprayed treatments to the control using a t-
test.
c P-values adjusted using a False Discovery Rate (FDR) procedure.
d
0-100% scale; (Stand count divided by the no. of seeds planted) * 100.
e Visual estimate on 1-10 scale; 10 = growth equivalent to non-transformed
plants.
f
Visual estimate on 0-100% scale; 0% = no damage.
f The number of days from the time of planting until flowering.
Bolded P-values are significant (<0.05).
Example 17
Generation of 2009 Agronomic Data
[00354] An agronomic study with soybean Event DAS-68416-4 and a non-transgenic
control
(var. Maverick) was conducted in 2009 at 8 sites located in Arkansas, Iowa,
Illinois, Indiana,
Missouri, and Nebraska. Agronomic determinants, including stand/population
count,
seedling/plant vigor, plant height, disease incidence, insect damage, and days
to flowering were
evaluated to investigate the equivalency of the soybean Event DAS-68416-4
soybeans (with
107
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
and without herbicide treatments) to the control (Table 29).
Table 29. Data collected in agronomic and yield trials, 2009.
Evaluation
Characteristic Descripti on Units reported Test *
Timing
Emergence VC - V2 Stand count in 1 meter section of row %
divided by number of seeds planted
per meter
Seedling vigor V1 - V3 General seedling vigor 1 (low) to 10 B
(high)
Visual injury Post V3 Visual injury 1 day post herbicide %
application application at V3 stage
Visual injury Post V3 Visual injury 7 days post herbicide %
application application at V3 stage
Visual injury Post V3 Visual injury 14 days post herbicide %
application application at V3 stage
Days to Flower Number of days from planting to days
when 50% of plants are at R1
Stand count R2 Number of plants in one meter section
of row
Visual injury Post R2 Visual injury 1 day post herbicide %
application application at R2 stage
Visual injury Post R2 Visual injury 7 days post herbicide %
application application at R2 stage
Visual injury Post R2 Visual injury 14 days post herbicide %
application application at R2 stage
Disease ¨R6 Opportunistic note on any disease that %
incidence occured at a location
Insect damage ¨R6 Opportunistic note on any insect %
damage that occured at a location
Plant Height R8 Final height of plot at R8 cm
Maturity R8 Number of days from planting to days
when 95% of plants in plot have
reached their mature color
Lodging R8 Degree of lodging in a plot 1 (none) - 5 B
(flat)
Yield R8 Weight of seed produced by the plot bu/acre
100 seed weight R8 Weight of 100 random seeds from the g
harvested plot
* B ¨ Sprayed and Unsprayed tests, S ¨ Sprayed tests only
[00355] A randomized-complete-block design was used for trials. Entries were
soybean
Event DAS-68416-4, a Maverick control line, and commercially available non-
transgenic
soybean lines. The test, control and reference soybean seed were planted at a
seeding rate of
approximately 112 seeds per row with row spacing of approximately 30 inches
(75 cm). At
each site, 4 replicate plots of each treatment were established, with each
plot consisting of 2-25
108
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
ft rows. Each soybean plot was bordered by 2 rows of a non-transgenic soybean
(Maverick).
The entire trial site was surrounded by a minimum of 4 rows (or 10 ft) of non-
transgenic
soybean (Maverick). Appropriate insect, weed, and disease control practices
were applied to
produce an agronomically acceptable crop.
[00356] Herbicide treatments were applied to replicate maximum label rate
commercial
practices. Treatments consisted of a non-sprayed control and herbicide
applications of 2,4-D,
glufosinate, 2,4-D/glufosinate applied at the specified growth stages. For the
2,4-D
applications, the herbicide was applied at a rate of 1.0 lb ae /A (1,120 g
ae/ha) at the V4 and R2
growth stages. For the glufosinate treatments, applications were made to
plants at the V4 and
V6-R2 growth stages. For both applications, glufosinate was applied at a rate
of 0.33 lb ai/A
(374 g ai/ha) and 0.41 lb ai/A (454 g ai/ha) for the V4 and V6-R2
applications, respectively.
Entries for both herbicide applications were soybean Event DAS-68416-4 and the
controls
including non-transgenic Maverick. Maverick plots were expected to die after
herbicide
application.
[00357] Analysis of variance was conducted across the field sites for the
agronomic data
using a mixed model (SAS Version 8; SAS Institute 1999). Entry was considered
a fixed
effect, and location, block within location, location-by-entry, and entry-by-
block within
location were designated as random effects. Analysis at individual locations
was done in an
analogous manner with entry as a fixed effect, and block and entry-by-block as
random effects.
Data were not rounded for statistical analysis. Significant differences were
declared at the 95%
confidence level, and the significance of an overall treatment effect was
estimated using an F-
test. Paired contrasts were made between unsprayed AAD-12 (unsprayed), AAD-12
sprayed
with glufosinate (AAD- 12 + glufosinate), AAD- 12 sprayed with 2,4-D (AAD- 12
+ 2,4-D)
and AAD- 12 sprayed with both glufosinate and 2,4-D (AAD-12 + 2,4-D +
glufosinate)
transgenic entries and the control entry using T-tests.
[00358] Due to the large number of contrasts made in this study, multiplicity
was an issue.
Multiplicity is an issue when a large number of comparisons are made in a
single study to look
for unexpected effects. Under these conditions, the probability of falsely
declaring differences
based on comparison-wise p-values is very high (1-0.95nuber of compansoils..
) In this study there were
four comparisons per analyte (16 analyzed observation types for agronomics),
resulting in 64
comparisons for agronomics. Therefore, the probability of declaring one or
more false
differences based on unadjusted p-values was 99% for agronomics (1-0.9564.)
[00359] An analysis of the agronomic data collected from the control, AAD-12
unsprayed,
AAD- 12 + glufosinate, AAD-12 + 2,4-D, and AAD-12 + 2,4-D + glufosinate
entries was
109
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
conducted. For the across-site analysis, no statistically significant
differences were observed
for seedling vigor, final population, plant vigor/injury (V4, R1), lodging,
disease incidence,
insect damage, days to flowering, days to maturity, number of pods, number of
seeds, yield, and
plant height. For stand count and early population, a significant paired t-
test was observed
between the control and the AAD-12 + glufosinate entry, but was not
accompanied by a
significant overall treatment effect or FDR adjusted p-value. For plant
vigor/injury (R2),
significant paired t-tests and a significant overall treatment effect were
observed between the
control and both the AAD-12 + glufosinate and AAD-12 + 2,4-D + glufosinate
entries, but were
not accompanied by a significant FDR adjusted p-value. The mean results for
all of these
variables were also within the range found for the reference lines tested in
this study.
Example 18
Transformation and Selection of the AAD1 Event pDAS 1740-278
[00360] The AAD1 event, pDAS 1740-278, was produced by WHISKER - mediated
transformation of maize line Hi-II. The transformation method used is
described in US Patent
Application # 20090093366. An Fspl fragment of plasmid pDAS1740 (FIG. 3), also
referred to
as pDAB3812, was transformed into the maize line. This plasmid construct
contains the plant
expression cassette containing the RB7 MARv3 : : Zea mays Ubiquitin 1 promoter
v2 // AAD1
v3 // Zea mays PERS 3'UTR :: RB 7 MARv4 plant transcription unit (PTU).
[00361] Numerous events were produced. Those events that survived and produced
healthy,
haloxyfop-resistant callus tissue were assigned unique identification codes
representing putative
transformation events, and continually transferred to fresh selection medium.
Plants were
regenerated from tissue derived from each unique event and transferred to the
greenhouse.
[00362] Leaf samples were taken for molecular analysis to verify the presence
of the AAD-I
transgene by Southern Blot, DNA border confirmation, and genomic marker
assisted
confirmation. Positive TO plants were pollinated with inbred lines to obtain
T1 seed. T1 plants
of Event pDAS 1470-278-9 (DAS-40278-9) was selected, self-pollinated and
characterized for
five generations. Meanwhile, the T1 plants were backcrossed and introgressed
into elite
germplasm (XHH 13) through marker-assisted selection for several generations.
This event
was generated from an independent transformed isolate. The event was selected
based on its
unique characteristics such as single insertion site, normal Mendelian
segregation and stable
expression, and a superior combination of efficacy, including herbicide
tolerance and
agronomic performance in broad genotype backgrounds and across multiple
environmental
locations. Additional description regarding the corn Event pDAS-1740-278-9 has
been
disclosed in WO 2011/022469, which is incorporated by reference in its
entirety.
110
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Example 19
Herbicide Application and Agronomic Data
[00363] Herbicide treatments were applied with a spray volume of approximately
20 gallons
per acre (187 L/ha).
[00364] These applications were designed to replicate maximum label rate
commercial
practices. Weedar 64 (026491-0006) at concentration 39%, 3,76 lb ae/gal, 451 g
ae/1 and
Assure II (106155) at concentration 10.2%, 0.87 lb ai/gal, 104 g ai/g were
used.
[00365] 2,4-D (Weedar 64) was applied as 3 broadcast over-the-top applications
to Test
Entries 4 and 5 (seasonal total of 3 lb ae/A). Individual applications were at
pre-emergence and
approximately V4 and V8 -V8.5 stages. Individual target application rates were
1.0 lb ae/A for
Weedar 64, or 1120 g ae/ha. Actual application rates ranged from 1096 - 1231 g
ae/A.
[00366] Quizalofop (Assure II) was applied as a single broadcast over-the-top
application to
Test Entries 3 and 5. Application timing was at approximately V6 growth stage.
The target
application rate was 0.0825 lb ai/A for Assure II, or 92 g ai/ha. Actual
application rates ranged
from 90.8 - 103 g ai/ha. Agronomic characteristics were recorded for all test
entries within
Blocks 2, 3, and 4 at each location. Table 30 lists characteristics that were
measured.
111
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Table 30. Agronomic data for corn Event pDAS-1740-278-9
Trait Evaluation Timing Description of Data
Early Population V1 and V4 Number of plants emerged per plot
Seedling Vigor V4 Visual estimate of average vigor of emerged
plants per plot
Plant Approximately 1-2 Injury from herbicide applications
Vigor/Injury weeks after
applications
Time to Silking Approximately 50% The number of accumulated heat units from
the
Silking time of planting until approximately 50% of
the
plants have emerged silks.
Time to Pollen Approximately 50% The number of accumulated heat units from
the
Shed Pollen Shed time of planting until approximately 50% of
the
plants are shedding pollen
Pollen Viability Approximately 50% Evaluation of pollen color and shape over
time
Plant Height Approximately R6 Height to the tip of the tassel
Ear Height Approximately R6 Height to the base of the primary ear
Stalk Lodging Approximately R6 Visual estimate of percent of plants in
the plot
with stalks broken below the primary ear
Root Lodging Approximately R6 Visual estimate of percent of plants in
the plot
leaning approximately 30 or more in the first
¨1/2 meter above the soil surface
Final Population Approximately R6 The number of plants remaining per plot
Days to Approximately R6 The number of accumulated heat units from
the
Maturity time of planting until approximately 50% of
the
plants have reached physiological maturity
Stay Green Approximately R6 Overall plant health
Disease Approximately R6 Visual estimate of foliar disease
incidence
Incidence
Insect Damage Approximately R6 Visual estimate of insect damage
Note: Heat Unit = ((MAX temp + MIN temp) / 2) ¨ 50 F
[00367] An analysis of the agronomic data collected from the control, aad-1
unsprayed, aad-1
+ 2,4-D, aad-\ + quizalofop, and aad-\ + both entries was conducted. For the
across-site
analysis, no statistically significant differences were observed for early
population (V1 and V4),
vigor, final population, crop injury, time to silking, time to pollen shed,
stalk lodging, root
lodging, disease incidence, insect damage, days to maturity, plant height, and
pollen viability
(shape and color) values in the across location summary analysis. For stay
green and ear
height, significant paired t-tests were observed between the control and the
aad-1 + quizalofop
entries, but were not accompanied by significant overall treatment effects or
False Discovery
Rates (FDR) adjusted p-values (Table 31).
112
CA 02876144 2014-12-05
WO 2013/185036
PCT/US2013/044717
Table 31. Summary analysis of agronomic characteristics results across
locations for the DAS-40278-9
aad-1 corn (sprayed and unsprayed) and control.
Analyte Overall Control Unsprayed (P- Sprayed Sprayed
Sprayed
Trt. value,b Adj. Quizalofop (P- 2,4-D
(P- Both (P-value,
Effect P)e value, Adj. P) value,
Adj. P) Adj. P)
(pr>F)a
Early (0.351) 42.8 41.3 41.7 41.9 44.1
population (0.303, 0.819) (0.443, 0.819)
(0.556, 0.819) (0.393, 0.819)
V1 (no. of
plants)
Early (0.768) 43.1 43.3 43.7 44.3 44.8
population (0.883, 0.984) (0.687, 0.863)
(0.423, 0.819) (0.263, 0.819)
V4 (no. of
plants)
Seedling (0.308) 7.69 7.39 7.36 7.58 7.78
Vigord (0.197, 0.819) (0.161, 0.819)
(0.633, 0.819) (0.729, 0.889)
Final (0.873) 40.1 39.6 39.7 39.9 41.1
population (0.747, 0.889) (0.802, 0.924)
(0.943, 1.00) (0.521, 0.819)
(no. of
plants)
Crop Injury NA' 0 0 0 0 0
- 1st app.e
Crop Injury (0.431) 0 0 0 0 0.28
- 2nd app.e (1.00, 1.00) (1.00,
1.00) (1.00, 1.00) (0.130,0.819)
Crop Injury NA 0 0 0 0 0
- 3rd app.e
Crop Injury NA 0 0 0 0 0
- 4th app.e
Time to (0.294) 1291 1291 1293 1304 1300
Silking (0.996, 1.00) (0.781, 0.917)
(0.088, 0.819) (0.224, 0.819)
(heat units)
Time to (0.331) 1336 1331 1342 1347 1347
Pollen Shed (0.564, 0.819) (0.480, 0.819)
(0.245, 0.819) (0.245, 0.819)
(heat units)
Pollen Shape (0.872) 10.9 10.9 11.3 11.4 11.3
0 minutes (0.931, 1.00) (0.546, 0.819)
(0.439, 0.819) (0.605, 0.819)
(%)g
Pollen Shape (0.486) 49.2 50.8 46.4 48.1 51.9
30 minutes (0.618, 0.819) (0.409, 0.819)
(0.739, 0.889) (0.409, 0.819)
(%)
Pollen Shape (0.724) 74.4 74.7 73.6 73.9 75.0
60 minutes (0.809, 0.924) (0.470, 0.819)
(0.629, 0.819) (0.629, 0.819)
(%)
Pollen Shape (0.816) 82.6 82.6 82.6 82.6 82.5
120 minutes (1.00, 1.00) (1.00, 1.00) (1.00,
1.00) (0.337, 0.819)
(%)
Pollen Color (0.524) 51.9 52.5 48.9 50.3 53.6
30 minutes (0.850, 0.960) (0.306, 0.819)
(0.573, 0.819) (0.573, 0.819)
(v)n
Pollen Color (0.332) 75.3 75.9 74.2 74.2 75.9
113
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
60 minutes (0.612, 0.819) (0.315, 0.819)
(0.315, 0.819) (0.612, 0.819)
(%)
Pollen Color NA 84.0 84.0 84.0 84.0 84.0
120 minutes
(%)
Stalk (0.261) 5.11 5.22 5.00 5.00 5.00
Lodging (%) (0.356, 0.819) (0.356, 0.819)
(0.356, 0.819) (0.356, 0.819)
Root (0.431) 0.44 0.17 0.72 0.17 0.11
Lodging (%) (0.457, 0.819) (0.457, 0.819)
(0.457, 0.819) (0.373, 0.819)
Stay Green (0.260) 4.67 4.28 3.92 4.17 4.11
(0.250, 0.819) (0.034m, (0.144, 0.819) (0.106, 0.819)
0.819)
Disease (0.741) 6.42 6.22 6.17 6.17 6.17
Incidencel (0.383, 0.819) (0.265, 0.819)
(0.265, 0.819) (0.265, 0.819)
Insect (0.627) 7.67 7.78 7.78 7.72 7.56
Damagek (0.500, 0.819) (0.500, 0.819)
(0.736, 0.889) (0.500, 0.819)
Days to (0.487) 2411 2413 2415 2416 2417
Maturity (0.558, 0.819) (0.302, 0.819)
(0.185, 0.819) (0.104, 0.819)
(heat units)f
Plant Height (0.676) 294 290 290 291 291
(cm) (0.206, 0.819) (0.109, 0.819)
(0.350, 0.819) (0.286, 0.819)
Ear Height (0.089) 124 120 118 121 118
(cm) (0.089, 0.819) (0.018m, (0.214,
0.819) (0.016m,
0.786) 0.186)
a Overall treatment effect estimated using an F-test.
Comparison of the sprayed and unsprayed treatments to the control using a t-
test.
c P-values adjusted using a False Discovery Rate (FDR) procedure.
d Visual estimate on 1-9 scale; 9 = tall plants with large robust leaves.
e 0-100% scale; with 0 = no injury and 100 = dead plant.
f The number of heat units that have accumulated from the time of planting.
g 0-100% scale; with % pollen grains with collapsed walls.
0-100% scale; with % pollen grains with intense yellow color.
i Visual estimate on 1-9 scale with 1 no visible green tissue.
J
Visual estimate on 1-9 scale with 1 being poor disease resistance.
k Visual estimate on 1-9 scale with 1 being poor insect resistance.
NA = statistical analysis not performed since no variability across replicates
or treatment.
m Statistical difference indicated by P- Value <0.05.
Example 20
Additional Argonomic Trials
[00368] Agronomic characteristics of corn line 40278 compared to a near-
isoline corn line
were evaluated across diverse environments. Treatments included 4 genetically
distinct hybrids
114
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
and their appropriate near-isoline control hybrids tested across a total of 21
locations.
[00369] The four test hybrids were medium to late maturity hybrids ranging
from 99 to 113
day relative maturity. Experiment A tested event DAS-40278-9 in the genetic
background
Inbred C x BC3S1 conversion. This hybrid has a relative maturity of 109 days
and was tested
at 16 locations (Table 32). Experiment B tested the hybrid background Inbred E
x BC3S1
conversion, a 113 day relative maturity hybrid. This hybrid was tested at 14
locations, using a
slightly different set of locations than Experiment A. Experiments C and D
tested hybrid
backgrounds BC2S1 conversion x Inbred D and BC2S1 conversion x Inbred F,
respectively.
Both of these hybrids have a 99 day relative maturity and were tested at the
same 10 locations.
[00370] For each trial, a randomized complete block design was used with two
replications
per location and two row plots. Row length was 20 feet and each row was seeded
at 34 seeds
per row. Standard regional agronomic practices were used in the management of
the trials.
[00371] Data were collected and analyzed for eight agronomic characteristics;
plant height,
ear height, stalk lodging, root lodging, final population, grain moisture,
test weight, and yield.
The parameters plant height and ear height provide information about the
appearance of the
hybrids. The agronomic characteristics of percent stalk lodging and root
lodging determine the
harvestability of a hybrid. Final population count measures seed quality and
seasonal growing
conditions that affect yield. Percent grain moisture at harvest defines the
maturity of the
hybrid, and yield (bushels/acre adjusted for moisture) and test weight (weight
in pounds of a
bushel of corn adjusted to 15.5% moisture) describe the reproductive
capability of the hybrid.
[00372] Analysis of variance was conducted across the field sites using a
linear model.
Entry and location were included in the model as fixed effects. Mixed models
including
location and location by entry as random effects were explored, but location
by entry explained
only a small portion of variance and its variance component was often not
significantly
different from zero. For stock and root lodging a logarithmic transformation
was used to
stabilize the variance, however means and ranges are reported on the original
scale. Significant
differences were declared at the 95% confidence level. The significance of an
overall treatment
effect was estimated using a t-test.
[00373] Results from these agronomic characterization trials can be found in
Table 32. No
statistically significant differences were found for any of the four 40278
hybrids compared to
the isoline controls (at p<0.05) for the parameters of ear height, stalk
lodging, root lodging,
grain moisture, test weight, and yield. Final population count and plant
height were statistically
different in Experiments A and B, respectively, but similar differences were
not seen in
comparisons with the other 40278 hybrids tested. Some of the variation seen
may be due to low
115
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
levels of genetic variability remaining from the backcrossing of the DAS-40278-
9 event into
the elite inbred lines. The overall range of values for the measured
parameters are all within the
range of values obtained for traditional corn hybrids and would not lead to a
conclusion of
increased weediness. In summary, agronomic characterization data indicate that
40278 corn is
biologically equivalent to conventional corn.
Table 32. Analysis of agronomic characteristics.
Experiment A
Parameter Treatment Mean Range P-value
(units) Min Max
Plant Height AAD-1 96.31 94.00 99.00 0.6174
(inches)
Control 95.41 95.00 98.00
Ear Height AAD-1 41.08 30.00 48.00 0.4538
(inches)
Control 44.42 40.00 47.00
Stalk Lodging AAD-1 3.64 0.00 27.70 0.2020
0) Control 2.49 0.00 28.57
Root Lodging AAD-1 1.00 0.00 7.81 0.7658
0) Control 0.89 0.00 28.33
Final AAD-1 31.06 27.00 36.00 0.0230
Population
Control 32.17 27.00 36.00
(plants/acre in
1000's)
Grain Moisture AAD-1 22.10 14.32 27.80 0.5132
0) Control 21.84 14.52 31.00
Test Weight AAD-1 54.94 51.10 56.80 0.4123
(lb/bushel)
Control 54.66 51.00 56.80
Yield AAD-1 193.50 138.85 229.38 0.9712
(bushels/acre)
Control 187.05 99.87 256.72
Experiment B
Parameter Treatment Mean Range P-value
(units) Min Max
Plant Height AAD-1 106.92 104.00 108.00 0.0178
(inches)
Control 100.79 95.00 104.00
Ear Height AAD-1 51.75 49.00 50.00 0.1552
(inches)
Control 45.63 38.00 50.00
Stalk Lodging AAD-1 1.24 0.00 15.07 0.1513
0) Control 0.72 0.00 22.22
Root Lodging AAD-1 0.64 0.00 6.15 0.2498
0) Control 0.40 0.00 9.09
Final AAD-1 31.30 26.00 37.00 0.4001
Population
Control 30.98 25.00 35.00
(plants/acre in
1000's)
116
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Grain Moisture AAD-1 23.71 14.34 28.70 0.9869
0) Control 23.72 13.39 31.10
Test Weight AAD-1 56.96 50.90 59.50 0.2796
(lb/bushel)
Control 56.67 52.00 60.10
Yield AAD-1 200.08 102.32 258.36 0.2031
(bushels/acre)
Control 205.41 95.35 259.03
Experiment C
Parameter Treatment Mean Range P-value
(units) Min Max
Plant Height AAD-1 95.92 94.00 96.00 0.1262
(inches)
Control 90.92 90.00 90.00
Ear Height AAD-1 47.75 41.00 50.00 0.4630
(inches)
Control 43.75 37.00 46.00
Stalk Lodging AAD-1 6.74 0.00 27.47 0.4964
0) Control 5.46 0.00 28.12
Root Lodging AAD-1 0.3512 0.00 7.58 0.8783
0) Control 0.3077 0.00 33.33
Final AAD-1 32.78 29.00 36.00 0.0543
Population
Control 31.68 24.00 35.00
(plants/acre in
1000's)
Grain Moisture AAD-1 19.09 13.33 25.90 0.5706
0) Control 19.36 13.66 26.50
Test Weight AAD-1 54.62 42.10 58.80 0.1715
(lb/bushel)
Control 55.14 52.80 58.40
Yield AAD-1 192.48 135.96 243.89 0.2218
(bushels/acre)
Control 200.35 129.02 285.58
Experiment D
Parameter Treatment Mean Range P-value
(units) Min Max
Stalk Lodging AAD-1 7.29 0.00 9.26 0.4364
0) Control 4.17 0.00 39.06
Final AAD-1 29.93 27.00 34.00 0.0571
Population
Control 31.86 29.00 35.00
(plants/acre in
1000's)
Grain Moisture AAD-1 18.74 19.40 24.40 0.4716
0) Control 19.32 13.35 25.70
Test Weight AAD-1 56.59 54.80 58.30 0.0992
(lb/bushel)
Control 55.50 52.70 57.40
Yield AAD-1 203.55 196.51 240.17 0.7370
(bushels/acre)
Control 199.82 118.56 264.11
[00374] Agronomic characteristics of hybrid corn containing event DAS-40278-9
compared
117
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
to near- isoline corn were collected from multiple field trials across diverse
geographic
environments for a growing season. The results for hybrid corn lines
containing event DAS-
40278-9 as compared to null plants are listed in Table 33.
Table 33. Yield, percent moisture, and final population results for hybrid
corn containing
evnt DAS-40278-9 as compared to the near-isoline control.
Final Population
Name Yield Grain Moisture (%)
(Plants/acre
reported in 1000's)
Hybrid Corn Contianing
218.1 21.59 31.69
DAS-40278-9
Control Hybrid Corn 217.4 21.91 30.42
[00375] Agronomic characteristics for the hybrid corn lines containing event
DAS-40278-9
and null plants sprayed with the herbicides quizalofop (280 g ae/ha) at the V3
stage of
development and 2,4-D (2,240 g ae/ha) sprayed at the V6 stage of development
are in Table 34.
Table 34. Agronomic data for event DAS 40278-9 as compared to the near-isoline
control.
Trial Yield Grain Moisture Stock Lodge Root
Lodge Final Population
(%) (%) (plants/acre reported
in
1000's)
Spray Trial
Hybrid Corn 214.9 23.4 0.61 2.19 30
#1 containing
DAS-40278-9
Control Hybrid 177.9 23.46 0.97 36.32 28.36
Corn #1
LSD (0.5) 13.3 1.107 0.89 10.7 1.1
Non Spray
Hybrid Corn 219.6 22.3 0.95 1.78 30.8
#1 containing
DAS-40278-9
Control Hybrid 220.3 22.51 0.54 1.52 30.55
Corn #1
LSD (0.5) 6.9 0.358 0.98 1.65 0.7
Spray Trial
Hybrid Corn 198.6 26.76 0.38 2.08 29.29
#2 containing
DAS-40278-9
Control Hybrid 172.3 23.76 1.5 39.16 28.86
Corn #2
LSD (0.5) 13.3 1.107 0.89 10.7 1.1
Non Spray
Hybrid Corn 207.8 24.34 0.22 0.59 31
#2 containing
DAS-40278-9
Control Hybrid 206.2 24.88 0.35 0.12 30.94
Corn #2
LSD (0.5) 8.0 0.645 0.55 1.79 0.9
118
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Example 21
2,4-D Increases Growth of 2,4-D Resistant Soybean
[00376] Transgenic soybean with AAD-12 transgene provides protection to the
soybean
plant while weeds are destroyed by application of 2,4-D. It has been
unexpectedly observed
that 2,4-D also increase growth in 2,4-D tolerant soybean. This increased
growth has resulted
in increases in plant height and/or yield of sprayed plots compared to non-
sprayed plots.
[00377] Increase in plant growth and/or yield resulting from the application
of 2,4-D is
described for soybean plants genetically engineered to the tolerant to 2,4-D.
Trials were grown
across multiple locations covering the North American soybean growing region.
Entries
included elite lines into which event DAS-68416-4 (which conditions tolerance
to 2,4-D) had
been introgressed. Treatments consisted of non-sprayed and 2,4-D sprayed
treatment applied at
both the V3 and R2 growth stages. Plots were measured for various agronomic
characteristics
throughout the season including plant height and grain yield. Weeds were
controlled
throughout the season in both sprayed and non-sprayed plots to eliminate any
competition
effect. At the conclusion of the trial, data analysis measured a significant
increase in both
height and yield for those entries which had been sprayed with 2,4-D compared
with those
which received no treatment. An increase in yield is an additional benefits to
the weed control
delivered by 2,4-D on 2,4-D resistant soybeans.
[00378] Field trials were run in 2011 to compare the agronomic characteristics
of soybean
event DAS-68416-4 (International Patent Application No. 2011/066384) that had
been sprayed
with 2,4-D, with the agronomic characteristics of unsprayed soybean event DAS-
68416-4. The
field trials contained entries of 4 elite soybean lines into which soybean
event DAS-68416-4
had been introgressed, and the respective null isolines of the 4 elite soybean
lines which did not
contain soybean event DAS-68416-4. The trials were planted across differing
geographical
locations (ten locations in total). The experiment was set up as a modified
split plot with two
replications per location. Whole plots were treatments and subplots were
entries. Each plot
consisted of two rows, 12.5 feet long, planted 30 inches apart. The sprayed
plots were treated
with 2,4-D (1120 g ae/ha) sprayed at the V3 and R2 growth stages. Throughout
the season,
field plots were maintained under normal agronomic practices and kept free
from weeds.
Various agronomic characteristics were measured for the soybean plants to
determine how the
application of 2,4-D affected the performance of the soybean agronomic
characteristics. The
tested agronomic characteristics and the growth stage when the data were
collected are listed in
Table 35.
119
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Table 35. List of agronomic characteristics measured in 2011 yield trials to
compare
2,4-D sprayed and unsprayed soybean event DAS-68416-4.
Characteristic Measured Growth Stage of
Measurement
1. Emergence: Stand count (above) divided by the Calculated based on early
number of seeds planted in a one meter section stand count
multiplied by 100.
2. Seedling vigor: Percent vigor with 0% representing V1 ¨ V3
a plot with all dead plants and 100% representing
plots that look very healthy.
3. Days to Flowering: Days from planting when 50% R1
of the plants in the plot began to flower.
4. Stand count at R2: Number of plants in a R2
representative one meter section of row at the R2
growth stage.
5. Disease incidence: Severity of disease in the plot R6
rated on a scale of 0-100%.
6. Insect damage: Percentage of plant tissue in the R6
plot damaged by insects.
7. Plant height: Average height in centimeters of the R8
plants in each plot measured from the soil surface
to the tip after leaves have fallen.
8. Lodging: Percent lodging at harvest time with 0% R8
= no lodging and 100% = all plants in a plot flat on
the ground.
9. Days to maturity. Days from planting when 95% of R8
the pods in a plot reached their dry down color.
10. Shattering: Percentage of pods shattered per plot. R8
11. Yield: Bushels per acre adjusting to 13% moisture. R8
12. 100 seed weight: For each plot count out 100 seeds R8
and record the weight in grams.
[00379] At the end of the soybean growing season, data from all locations were
combined
and an across location analysis was performed. Data analysis was carried out
using JMP Pro
9Ø3 (SAS, Cary, NC). Least square means from the analysis are reported in
Table 28. The
application of 2,4-D on soybean event DAS-68416-4 containing the AAD-12
transgene resulted
in a conditioning effect of increased growth. The increased growth culminated
in significantly
greater yield and plant height measurements in field plots sprayed with 2,4-D
as compared to
field plots not sprayed with 2,4-D. These increases were ascertainable when
the data was
analyzed cumulatively across all locations. In contrast, the increased yield
for soybean event
DAS-68416-4 sprayed with 2,4-D was diminished by a location by treatment
interaction. Both
average height and yield were increased about 5% by applications of 2,4-D in
Table 36.
120
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Table 36. Least square means from the across location analysis comparing
soybean
event DAS-68416-4 that was sprayed with 2,4-D to unsprayed plants. Levels not
connected by the same letter are significantly different.
Treatments Applied 2,4-D at 1,120 g ae/ha Unsprayed
(at V3 and R2 stages)
Emergence (%) 77 (A) 74 (A)
Vigor V1-V3 (%) 87 (A) 87 (A)
Days to Flowering (days from 44 (A) 44 (A)
planting)
Stand Count at R2 (plants/ m) 21 (A) 22 (A)
Disease Incidence R6 (%) 1 (A) 1 (A)
Insect Damage R6 (%) 2 (A) 2 (A)
Height (cm) 81 (A) 77 (A)
Maturity (days from planting) 109 (A) 109 (A)
Lodging (%) 10 (A) 8 (B)
Shattering (%) 0 (A) 1 (A)
Yield (bu/acre) 56.4 (A) 53.7 (B)
100 Seed Weight (g) 14.8 (A) 14.8 (A)
[00380] As shown in Table 37, at least one of the ten locations (Location #a3)
reported
significantly higher yield harvests for the unsprayed soybean event DAS-68416-
4 plants as
compared to the 2,4-D sprayed soybean event DAS-68416-4 plants. When the
results for all of
the locations were accumulated the application of 2,4-D on soybean event DAS-
68416-4
containing the AAD-12 transgene indicated a conditioning effect resulting in
increased growth.
For instance, the yield of soybean event DAS-68416-4 plants sprayed with 2,4-D
was 56.4
bu/acre which is considerably greater than the yield of unsprayed soybean
event DAS-68416-4
plants which was 53.7 bu/acre. Likewise, the height of soybean event DAS-68416-
4 plants
sprayed with 2,4-D was 81 cm which is considerably greater than the height of
unsprayed
soybean event DAS-68416-4 plants which was 77 cm.
121
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Table 37. Least square means for yield from specific locations comparing
soybean
event DAS-68416-4 that was sprayed with 2,4-D to unsprayed plants. Levels not
connected by the same letter are significantly different.
Yield
Location Number Treatment Yield %
(bu/acre)
Location #al Sprayed 51 A 121.5
Unsprayed 42 B 100
Location #a2 Sprayed 67 A 115.6
Unsprayed 58 B 100
Location #a3 Sprayed 44 B 88
Unsprayed 50 A 100
Location #a4 Sprayed 68 A 97
Unsprayed 70 A 100
Location #a5 Sprayed 75 A 102.8
Unsprayed 73 A 100
Location #a6 Sprayed 57 A 132.6
Unsprayed 43 B 100
Location #a7 Sprayed 48 A 102.2
Unsprayed 47 A 100
Location #a8 Sprayed 39 A 91
Unsprayed 43 A 100
Location #a9 Sprayed 57 A 101.8
Unsprayed 56 A 100
Location #a10 Sprayed 59 A 107.3
Unsprayed 55 A 100
Average Sprayed 106
Example 22
2,4-D Increases Growth of 2,4-D Resistant Soybean in 2,4-D/Glyphosate
Combination
[00381] Similar field trials as in the previous Example were run in 2010 but
with two
applications of 2,4-D in combination with glyphosate. Results show that
increased growth of
2,4-D resistant soybean, in plant height and/or yield of sprayed plots
compared to non-sprayed
plots, is due to application of 2,4-D.
[00382] Significant treatment effects were observed for a number of parameters
measured.
Both 2,4-D and glyphosate were sprayed at the V3 and R2 growth stages. The
trials were
planted across differing geographical locations (six locations in total). The
tested agronomic
characteristics and the growth stage when the data were collected are listed
in Table 30. The
average height was increased 6% and average yield was increased 17% for
sprayed soybean in
Table 38. In addition, average seed weight was increased 6% for sprayed
soybean.
122
CA 02876144 2014-12-05
WO 2013/185036
PCT/US2013/044717
Table 38. Least square means from the across location analysis comparing 2,4-D
tolerant soybean that was sprayed with 2,4-D plus glyphosate to unsprayed
plants.
Levels not connected by the same letter are significantly different.
Treatments Applied 2,4-D plus glyphosate
Unsprayed
Both at 1,120 g ae/ha
(at V3 and R2 stages)
Emergence (%) 54 (A) 54 (A)
Vigor V1-V3 (%) 7 (A) 7 (A)
Days to Flowering (days from 41 (A) 41 (A)
planting)
Stand Count at R2 (plants/ m) 15 (A) 15 (A)
Disease Incidence R6 (%) 4 (A) 4 (A)
Insect Damage R6 (%) 17 (A) 14 (B)
Height (cm) 109 (A) 103 (B)
Maturity (days from planting) 117 (A) 116 (B)
Lodging (%) 17 (A) 9 (B)
Shattering (%) 0 (A) 0 (A)
Yield (bu/acre) 43.4 (A) 37.0 (B)
100 Seed Weight (g) 12.2 (A) 11.5 (B)
[00383] As shown in Table 39, certain geographical variations were also
observed in this
Example. The average yield was increased 21.6% for sprayed soybean in Table
39.
Table 39. Least square means for yield from specific locations 2,4-D tolerant
soybean that was sprayed with 2,4-D plus glyphosate to unsprayed plants.
Yield
Location Number Treatment Yield %
(bu/acre)
Location #b1 Sprayed 39 A 162.5
Unsprayed 24 B 100
Location #b2 Sprayed 51 A 104.1
Unsprayed 49 A 100
Location #b3 Sprayed 56 A 155.5
Unsprayed 36 B 100
Location #b4 Sprayed 35 A 106.1
Unsprayed 33 A 100
Location #b5 Sprayed 48 A 104.3
Unsprayed 46 A 100
Location #b6 Sprayed 32 A 97.0
Unsprayed 33 A 100
Average Sprayed 121.6
Example 23
Yield Trial Results Comparing Sprayed and Non-sprayed Treatments
[00384] 2,4-D resistant transgenic crop plants transformed with an
aryloxyalkanoate
dioxygenase (AAD) resulted in increased yield when treated with a stimulating
amount of
123
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
herbicide comprising an aryloxyalkanoate moiety. Soybean events comprising an
AAD-12
gene expression cassette were tested in replicated yield trials under sprayed
and non-sprayed
conditions. There was one series of experiments which contained early soybeans
adapted to
northern latitudes and another series of experiments which contained late
soybeans adapted to
more southern latitudes. In previous experiments there were instances where
soybean entries
comprising an AAD-12 gene expression cassette were treated with 2,4-D during
the growing
season exhibited and increased yield relative to the unsprayed checks.
[00385] A modified split plot design with 2 replications was used for the
trials. Each plot
was 2 rows wide with 30 inch row spacing and 12.5 feet long. There was a 2.5
to 3 foot
alleyway between plots planted end to end to allow for movement within the
trial during the
season. The sprayed blocks were sprayed sequentially (twice) during the
growing season with
2,4-D choline + glyphosate (premix) at 2185 g ae/ha + AMS at 2% weight per
weight.
Table 40. List of analysis locations for yield trials
comparing sprayed verses non-sprayed treatments.
Location Trial
Atlantic, IA early
Brookings, SD early
Cherry Grove, MN early
Deerfield, MI early
Kirklin, IN early
Otterbein, IN early
Richland, IA early
Wyoming, IL early
Atlantic, IA late
Carlyle, IL late
Fisk, MO late
Otterbein, IN late
Seymour, IL late
Stewardson, IL late
Sycamore, GA late
Tallas see, AL late
[00386] The first application was at the V3 growth stage and the second
application at R2
growth stage. Both the experimentatal and control field trials were kept weed
free throughout
124
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
the season by use of conventional herbicides or hand weeding. Data were
collected on
emergence, seedling vigor, crop injury, flowering date, stand count at R2,
disease incidence,
insect damage plant height, maturity date, lodging, shattering 100 seed weight
and yield. Data
were analyzed using JMP Pro 9Ø3. Table 40 lists the locations that were
used in the final
analysis. Some locations which were planted were not included in the analysis
due to within
plot variability.
[00387] Across location analysis were performed for both the early and late
trials. Tables 41
and 42 show the yield analysis of variance for the early and late trials
respectively.
Table 41. Across location (8 locations) analysis of variance for yield
in the early variety sprayed vs non-sprayed trials.
Source Nparm DF DFDen F Ratio Prob > F
NAME 8 8 57.030 3.780 0.001
TRT 1 1 5.989 12.409 0.013
NAME*TRT 8 8 183.000 0.530 0.833
[00388] For both the early and late trials there was a significant (P=0.05)
name effect. This
was expected since each elite soybean line into which an event had been
introgressed was from
a different genetic background.
Table 42. Across location (8 locations) analysis of variance for yield
in the late variety sprayed vs non-sprayed trials.
Source Nparm DF DFDen F Ratio Prob > F
NAME 11 11 76.020 3.096 0.002
TRT 1 1 7.039 3.050 0.124
NAME*TRT 11 11 257.700 0.499 0.903
[00389] A significant treatment effect was measured for the early trial
indicating that the
sprayed and non-sprayed treatments differed for yield. For the late trial
there was not a
significant treatment effect which indicates that sprayed and non-sprayed
plots did not differ for
yield.
125
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Table 43. Table of least squares yield means from early yield trial.
Treatment number Yield (bu/acre)
289-1(HOMO),Non-sprayed 42.0 A
289-1(HOMO),Sprayed 46.0 A
289-2(HOMO),Non-sprayed 41.8 A
289-2(HOMO),Sprayed 45.7 A
7471638-26(HOMO),Non-sprayed 38.2 B
7471638-26(HOMO),Sprayed 42.9 A
76983-1(HOMO),Non-sprayed 38.4 B
76983-1(HOMO),Sprayed 42.5 A
76983-2(HOMO),Non-sprayed 39.6 A
76983-2(HOMO),Sprayed 42.9 A
75209(HOMO),Non-sprayed 46.4 A
75209(HOMO),Sprayed 47.6 A
75209[11(HOMO),Non-sprayed 48.1 B
75209[11(HOMO),Sprayed 52.7 A
75357-71(HOMO),Non- sprayed 46.2 A
75357-71(HOMO),Sprayed 49.5 A
99345-31[4](HOMO),Non-sprayed 40.1 B
99345-31[4] (HOMO),Sprayed 46.0 A
[00390] For both the early and late trials the name by treatment interaction
effect was not
significant indicating that the effect of the treatment (or lack of an effect)
was the same for each
entry in a particular trial.
[00391] Table 43 shows average yield for each entry by treatment combination
in the early
trial, where HOMO stands for homozygous. Values followed by the same letter
(within a given
variety) are not different according to Student's t at P=0.05. There were four
entries which
exhibited higher yield when sequentially sprayed at V3 and R3 with 2,4-D
choline + glyphosate
(premix) at 2185 g ae/ha + AMS.
[00392] Table 44 shows average yield for each entry by treatment combination.
Values
followed by the same letter (within a given variety) are not different
according to Student's t at
P=0.05. As reported above there was not a significant treatment effect or
treatment by entry
effect for the late trial so mean separation was not carried out. Letters in
the table indicate that
126
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
there was no difference between sprayed and non-sprayed treatments in the late
test.
Table 44. Table of least squares yield means from the 2012 late yield trial.
Treatment number Yield (bu/acre)
348-1(HOMO),Non-sprayed 54.5 A
348-1(HOMO),Sprayed 54.7 A
348[3] (HOMO),Non- sprayed 51.1 A
348[3] (HOMO),Sprayed 54.5 A
4075433-15 (HOMO),Non- sprayed 59.6 A
4075433-15 (HOMO),Sprayed 60.4 A
75226-1(HOMO),Non-sprayed 52.1 A
75226-1(HOMO),Sprayed 55.2 A
75226-2(HOMO),Non-sprayed 51.1 A
75226-2(HOMO),Sprayed 52.2 A
75505 (HOMO),Non- sprayed 50.1 A
75505 (HOMO),Sprayed 54.6 A
99753-81 (HOMO),Non- sprayed 56.1 A
99753-81(HOMO),Sprayed 55.4 A
75358-72(HOMO),Non-sprayed 50.7 A
75358-72(HOMO),Sprayed 53.8 A
75358-72[1] (HOMO),Non- sprayed 48.4 A
75358-72[1] (HOMO),Sprayed 50.1 A
99753-75[4] (HOMO),Non- sprayed 52.1 A
99753-75[4] (HOMO),Sprayed 53.4 A
Control-1,Non-sprayed 49.2 A
Control-1,Sprayed 51.4 A
Control-2,Non-sprayed 49.6 A
Control-2,Sprayed 52.0 A
[00393] Results from yield trials in this example once again show that in some
environments
for some soybean genotypes there may be an increase in yield following
application of 2,4-D.
In the past two years such yield increase has been observed in yield trials
that have been run in
MG 2 growing region.
127
CA 02876144 2014-12-05
WO 2013/185036 PCT/US2013/044717
Example 24
Comparison Between Soybean and Corn
[00394] The yield results from the field trials in soybean comprising an AAD-
12 transgene
indicate that an application of 2,4-D may increase the yield of soybeans in
certain environments
for certain soybean genotypes. These results are surprising when compared to
the transgenic
corn events which comprise an AAD-1 transgene. The yield of AAD-1 transgenic
corn plants
did not consistently show a statistically singnificant increase in yield after
sprayed with 2,4-D.
These AAD-1 transgenic corn plants are biologically equivalent to conventional
corn.
Additional field studies in diverse geographical locales were completed from
2010 through
2012 on hybrid corn lines. Throughout these field studies the yield of the
corn lines sprayed
with 2,4-D (2,185 g ae/ha and 4,370 g ae/ha) were compared to untreated
control corn lines
(e.g., not sprayed with 2,4-D). The results of these experiments further
substantiate that corn
plants containing the AAD-1 transgene do not result in a significant increase
in yield as a result
of treatment with a 2,4-D spray. Comparatively, a yield increase has been
shown in some
soybean genotypes following an application of 2,4-D. The observed yield
increase in soybean
genotypes which is shown following an application of 2,4-D is an unexpected
improvement that
is applicable for increasing the yield of crop plants. The disclosed method
can be deployed for
using a 2,4-D treatment to increase the yield of transgenic crop plants, for
example expressing
an AAD-12 gene.
[00395] Although the foregoing invention has been described in some detail by
way of
illustration and example for purposes of clarity of understanding, it will be
obvious that certain
changes and modifications may be practiced within the scope of the appended
claims.
128