Note: Descriptions are shown in the official language in which they were submitted.
CA 02876265 2014-12-10
1
Electrode unit
Description
The invention relates to an electrode unit for an electrochemical device for
storing
electrical energy, comprising a solid electrolyte and a porous solid
electrode, the solid
electrolyte dividing a compartment for molten cathode material and a
compartment for
molten anode material and the porous electrode being connected via a non-
electron-
conductive interlayer to the solid electrolyte and the molten cathode material
flowing
along the electrode during charging or discharging.
Generating electrical energy by means of fossil fuel-fired power stations is
associated
with the production of CO2 and thus has a considerable impact on the
greenhouse
effect. Generating energy from renewable energy sources, for example wind,
sun,
geothermal energy or hydroelectric power, avoids this disadvantage. However,
these
renewable energy sources are not always available at the times they are
required by
load profiles. In addition, the energy is often generated at locations which
differ from
the places where the energy demand is located. If this systemic disadvantage
is to be
overcome, the energy generated must be stored, buffered and possibly even
transported.
Against this background, it is not possible to base a stable power grid solely
on
renewable energy sources. There is accordingly a need to compensate and buffer
such
fluctuations by highly effective systems which are inexpensive and energy-
efficient.
Electrical energy is currently stored on an industrial scale using pumped-
storage power
stations in which the potential energy arising from the geodetic difference in
height of
the water is utilized for conversion into electricity. However, the
construction of such
pumped-storage power stations is restricted by topographic and environmental
considerations. Pressure storage power plants, which make use of air
compression for
storing energy, are limited by their comparatively low efficiency. Other forms
of energy
storage, such as supercapacitors or flywheels are intended for other target
markets, in
particular short-term storage. Electrical energy may in particular be stored
using
batteries, various designs of which have been implemented industrially. In
particular, it
is necessary to use batteries which are rechargeable for this purpose.
Corresponding batteries which function on the basis of a molten alkali metal
as anode
and a cathodic reaction partner, generally sulfur, are known for example from
DE-A 26
35 900 or DE-A 26 10 222. The molten alkali metal and the cathodic reaction
partner
are divided by a solid electrolyte which is permeable to cations. The alkali
metal reacts
with the cathodic reaction partner on the cathode. For example when using
sodium as
CA 02876265 2014-12-10
2
the alkali metal and sulfur as the cathodic reaction partner, this is sodium
polysulfide.
The battery is charged by splitting the sodium polysulfide on the electrode
back into
sodium and sulfur by applying electrical energy.
The storage capacity of batteries based on a molten alkali metal and a
cathodic
reaction partner is increased by using batteries in which the quantity of
reactants used
in increased by additional storage vessels. The liquid sodium is supplied to
the solid
electrolyte for discharging. The liquid sodium simultaneously acts as anode
and forms
cations which are transported to the cathode through the cation-conductive
solid
electrolyte. At the cathode, the sulfur flowing to the cathode is reduced to
polysulfide,
i.e. is reacted with the sodium ions to yield sodium polysulfide. The
corresponding
sodium polysulfide may be collected in a further vessel. It is alternatively
also possible
to collect the sodium polysulfide together with the sulfur in the vessel
around the
cathode compartment. Due to the difference in density, the sulfur rises up and
the
sodium polysulfide settles out. This difference in density may also be
exploited to bring
about flow along the cathode. A corresponding battery design is described, for
example, in WO 2011/161072.
In batteries which operate using a redox-system based on sodium and sulfur,
electrical
energy may be obtained on reacting sodium and sulfur to yield sodium
polysulfide at a
high level of efficiency of roughly 90%. The battery is charged by reversing
the
procedure by introducing electricity and cleaving the sodium polysulfide into
sulfur and
sodium. Since all the electrochemical reactants are in molten form and the
ideal
conductivity range of the ion-conductive ceramic membrane is not achieved
until
relatively high temperatures, the operating temperature of such a battery is
conventionally approx. 300 C.
The solid, sodium ion-conductive electrolyte used in the battery is
conventionally 6"-
alumina. Mechanical failure cannot be ruled out with such a ceramic. In such
an event,
an uncontrolled reaction between sodium and sulfur may occur, which due to its
exothermic nature may lead to an undesired temperature rise in the battery. In
order to
keep the temperature rise as small as possible in such a case, it is known,
for example
from JP-A 10270073, to use a displacer of aluminum with which the compartment
for
the sodium on the sodium side of the solid electrolyte is restricted to a gap
with a width
of 0.01 to 0.2 mm. The gap is here produced by a combination of plastic
deformation
and elastic rebound during heat treatment of the displacer which was
introduced into
the solid electrolyte which is conventionally of tubular construction.
In order to keep the internal resistance of such battery systems as low as
possible,
current is supplied on both the positive and the negative sides by metallic
supply lines
which must be not only corrosion-resistant in the surrounding medium but also
highly
=
CA 02876265 2014-12-10
3
electrically conductive. The prior art therefore prefers electrical conductors
made of
aluminum or aluminum alloys, since for example copper, which is highly
conductive,
has inadequate corrosion-resistance to sulfur and polysulfide.
The disadvantage of the anode and current conductor design described here is
the
elevated labile reaction potential of aluminum and sulfur and in particular of
aluminum
and sodium polysulfide. In the event of mechanical failure of the ceramic
membrane,
not only is an exothermic sodium-sulfur reaction to be anticipated, but also
an
exothermic aluminum-sulfur reaction, the metals reacting similarly violently
with sodium
polysulfide as they do with sulfur.
Another disadvantage of the battery design described in WO 2011/161072 is,
however,
also that when a porous electrode is used, sulfur enters the upper region of
the
electrode during discharging and reacts to form sodium polysulfide. This
remains in the
electrode. As discharge operation continues, the sodium polysulfide is reduced
further
and thus takes up more sodium, so reducing the electrochemical potential and
the
voltage in the electrode thus falls. Under certain circumstances, sodium
uptake may go
as far as complete conversion into sodium sulfide (Na2S) with formation of a
solid, the
solid clogging the electrode or sodium and sulfur at least being removed from
the
system for further charging cycles so leading to a decline in charge/discharge
life.
A further disadvantage of the batteries known from the prior art is that the
sulfur which
arises during charging is electrically insulating, which may lead to an
uncontrolled
increase in internal resistance and thus in charging voltage.
The object of the present invention was accordingly to provide an electrode
unit which
does not exhibit the disadvantages of the electrodes known from the prior art.
Said object is achieved by an electrode unit for an electrochemical device for
storing
electrical energy, comprising a solid electrolyte and a porous solid
electrode, the solid
electrolyte dividing a compartment for molten cathode material and a
compartment for
molten anode material and the porous electrode being extensively connected via
a
non-electron-conductive interlayer to the solid electrolyte and the molten
cathode
material flowing along the porous electrode during charging or discharging,
wherein, on
the side remote from the solid electrolyte, the porous electrode is covered
towards the
compartment for the molten cathode material with an extensive tube or sheet
metal
wall, the extensive tube or sheet metal wall comprising inlet openings in the
direction of
flow of the cathode material, through which the cathode material penetrates
into the
porous electrode, reacts electrochemically in the porous electrode and emerges
back
out of the porous electrode through outlet openings downstream in the
direction of flow.
CA 02876265 2014-12-10
4
The device according to the invention provides uniform flow through the porous
electrode, so ensuring not only more uniform distribution of substances but
also
uniform electrical power from the battery during the discharge process.
Battery power
does not decline as the discharge process progresses.
For the purposes of the present invention, an anode material should be taken
to mean
a liquid reactant which is supplied to the anode side during discharging. The
anode
material is preferably electrically conductive, in particular a liquid alkali
metal is used as
the anode material. Suitable anode materials are for example lithium, sodium,
potassium, in particular sodium or potassium.
The cathode material is a liquid reactant which is electrochemically reacted
with the
anode material. The cathode material conventionally forms a salt by chemical
reaction
with the anode material. Suitable cathode materials are for example sulfur or
polysulfides. The cathode material is here used in liquid form.
Transport through the porous electrode proceeds solely by convection and
diffusion. In
this way it is possible to dispense with pumps or similar devices providing
forced
transport. The disadvantage of such devices is generally that they require
electricity
which is then no longer available. A further disadvantage of forced transport
devices is
the wear they undergo.
In order to achieve uniform functioning of the entire electrode, in particular
in large
electrochemical devices for storing electrical energy, hereinafter also
designated
"battery", in a preferred embodiment for large devices for electrochemical
energy
storage, the extensive tube or sheet metal wall has the structure of
corrugated sheet
metal, such that alternate, perpendicularly oriented lengthwise channels are
formed
between the boundary of the porous electrode material and the corrugated sheet
metal-like tube or sheet metal wall, which lengthwise channels are, however,
capable
of communicating with the cavities of the electrode material. Convective flow
driven by
the difference in density, for example between polysulfide and sulfide, can
develop in
these lengthwise channels, said flow being directed upwards during charging
and
downwards during discharging.
In a further particularly preferred embodiment, the porous electrode material
is
subdivided into lengthwise segments, flow barriers being arranged between the
lengthwise segments in order to force mass transfer of liquid cathode material
between
porous electrode and lengthwise channel.
In a further embodiment, the porous electrode segments are additionally
encased by
means of segment walls closed at the sides in order to force purposeful inflow
into and
CA 02876265 2014-12-10
outflow from the porous electrode. In this preferred embodiment, the segment
wall
comprises a plurality of rows of inlet openings and outlet openings oriented
transversely of the direction of flow, the inlet openings and outlet openings
alternating
in the direction of flow and flow barriers being accommodated in the porous
electrode,
5 in each case in the direction of flow, upstream of the inlet openings and
downstream of
the outlet openings.
During discharging of the battery, i.e. when releasing electrical energy, the
cathode
material enters through the inlet openings into the porous electrode and is
electrochemically reacted with the anode material. The reaction product then
emerges
through the outlet openings. The flow barriers in the porous electrode ensure
that the
reaction product is forced to emerge at the outlet openings, such that the
reaction
product cannot flow onwards in the porous electrode. This allows cathode
material to
enter through inlet openings downstream of the outlet openings into the porous
electrode where it can be reacted. In this way, the entire length of the
electrode may be
uniformly used for electrochemical reaction of the anode material with the
cathode
material.
In order to be able to utilize the entire surface area of the porous
electrode, it is
furthermore preferred for a row of outlet openings in each case immediately to
be
followed by a row of inlet openings. In this case, the optionally unreacted
cathode
material and the reaction product flow up to a flow barrier and pass out from
the
electrode through the outlet openings, and fresh cathode material, which is
reacted
with the anode material, is supplied to the porous electrode directly below
the flow
barrier.
In order to prevent reaction product which has formed in the porous electrode
and
emerges through the outlet openings from immediately entering the porous
electrode
again through the downstream inlet openings, it is furthermore preferred for
the inlet
openings which follow the outlet openings in the direction of flow of the
cathode
material to be arranged in a staggered manner relative to the outlet openings.
It is for example here possible to construct the inlet openings and the outlet
openings in
each case with a rectangular cross-section and to provide a web of the
extensive
electrode in each case between two adjacent inlet openings or two adjacent
outlet
openings in the width of the inlet openings or outlet openings respectively.
In the case
of the staggered arrangement, an inlet opening is in each case followed by the
web
between two outlet openings while an outlet opening is followed by the web
between
two inlet openings.
CA 02876265 2014-12-10
6
In addition to a design with rectangular inlet openings and outlet openings,
it is also
possible to construct the inlet openings and outlet openings in any desired
other shape.
These may accordingly be constructed for example in a circular, semicircular,
elliptical,
oval, triangular or polygonal shape with as many vertices as desired. A
circular,
semicircular or rectangular shape of the inlet openings and outlet openings is
here
preferred. It is also possible to provide inlet openings and outlet openings
of different
shapes, an identical shape of inlet openings and outlet openings being
preferred.
According to the invention, the segment wall is electrically conductively
connected to
the porous electrode. During discharging of the device, the electrical voltage
released
during the electrochemical reaction of the anode material with the cathode
material is
conducted via the porous electrode to the segment wall and can be picked off
from the
segment wall. It is particularly preferred for this purpose for the segment
wall to be
electrically conductively connected to one or more bus conductors. In order to
avoid the
risk, arising from the nature of the material, of an undesired exothermic
reaction of the
current conductor with sulfur or polysulfide, in a preferred embodiment the
bus
conductors are made from highly conductive materials such as aluminum, copper
or
sodium jacketed or encased in special steel. The bus conductor may here also
be
provided in the form of a cover, which is preferably constructed such that the
cover
forms flow channels along the electrode. It is alternatively also possible to
provide a
cover which is constructed such that flow channels are formed along the
electrode, to
make the electrical contact through the cover and to arrange the bus conductor
outside
the cover. It is preferred, however, to construct the cover electrically
conductively as a
bus conductor. It is here furthermore preferred for the cover additionally to
accommodate rod electrodes which are preferably made from an electrically
highly
conductive material which differs from the material of the cover. The rod
electrodes
may here for example lie outside on the cover or are enclosed by the material
of the
cover. The individual rod electrodes are here preferably arranged
equidistantly in the
cover. The rod electrodes may, for example, be arranged in each case between
two
flow channels. Alternatively, however, the rod electrodes may for example also
be
arranged in each case in the region of a flow channel.
In one particularly preferred embodiment, the cover is of corrugated
construction in
order to form the flow channels, the troughs in each case resting against the
extensive
electrode and the flow channels being formed by the corresponding peaks. In
addition
to a corrugated design, it is alternatively also possible, for example, to
make the cover
flat with webs, the flow channels in each case being formed between two webs
and the
webs resting against the extensive electrode to form the flow channels.
In one particularly preferred embodiment, the solid electrolyte is of
cylindrical
construction and the porous electrode encloses the solid electrolyte. In this
case, the
CA 02876265 2014-12-10
7
anode material is located in the interior of the cylindrically shaped solid
electrolyte and
the cathode material flows outside along the porous electrode. In a
cylindrical design of
the solid electrolyte, the segment wall is preferably formed by at least one
sleeve which
encloses the porous electrode. In this case, the inlet openings and outlet
openings are
formed in the sleeve. In order to ensure current flow from the porous
electrode to the
sleeve-type extensive electrode, the external diameter of the porous electrode
here
corresponds to the internal diameter of the sleeve. In this way, the sleeve
rests
extensively against the porous electrode.
If the segment wall is formed by just one sleeve, it is possible to form a
plurality of rows
of inlet openings and outlet openings in the sleeve. A design with a plurality
of sleeves
is preferred, however, the inlet openings and outlet openings in this case
being formed
in each case at the ends of a sleeve. To this end, it is for example possible
in each
case to shape the ends of the sleeve as a rectangular profile. In this case,
the opposite
rectangular profiles in a sleeve are constructed such that the recesses are in
each case
opposite one another. A plurality of sleeves are then successively placed on
the
electrode, the sleeves each being rotated relative to one another such that
the
recesses of one sleeve are opposite the projecting regions located
therebetween. In
this way, the inlet openings and outlet openings are formed by the recesses
and the
adjacent sleeve.
In the case of a cylindrically shaped solid electrolyte, the cover likewise
preferably
takes the form of a sleeve and is configured such that the channels are
oriented in the
axial direction along the porous electrode. It is here possible on the one
hand to
construct the cover as an annular sleeve which is provided with webs which
divide the
individual flow channels or the sleeve is of corrugated construction such that
the peaks
and troughs form the channels, the peaks in each case resting against the
sleeves
which form the segment walls. During discharging of the battery, the cathode
material
flows through the flow channels and in each case enters via inlet openings
into the
porous electrode and, after electrochemical reaction, emerges back out of the
outlet
openings as the reaction product. Due to the staggered arrangement of inlet
openings
and outlet openings, the material emerging from the outlet openings cannot
immediately enter the porous electrode again through downstream inlet
openings. In
this way it is ensured that in each case sufficient unreacted cathode material
reaches
the porous electrode through the inlet openings.
In addition to the above-described embodiment, in which the inlet openings and
downstream outlet openings are arranged directly one above the other and the
flow
channels are formed in the axial direction along the porous electrode, it is
also possible
for the flow channels to extend spirally. In the case of a flat electrode
unit, the flow
channels may extend obliquely. In this case, the arrangement of the inlet
openings and
CA 02876265 2014-12-10
8
outlet openings is preferably such that in each case in one flow channel an
outlet
opening follows an inlet opening and the inlet opening immediately downstream
of the
outlet opening is located in an adjacent flow channel. In this case too, a
corresponding
arrangement of the inlet openings and outlet openings ensures that the
reaction
product emerging from the outlet openings does not immediately enter the
porous
electrode again via downstream inlet openings.
In order to reduce the quantity of liquid anode material, it is furthermore
preferred, in
particular in the case of a cylindrical configuration of the solid
electrolyte, for a displacer
to be accommodated in the solid electrolyte. A gap into which the anode
material can
flow is here formed between the displacer and the solid electrolyte. The anode
material
may here for example be introduced via an annular gap at the end of the solid
electrolyte or alternatively be supplied through a flow channel in the
displacer. The
anode material is preferably fed through a flow channel in the displacer.
As is known from the prior art, the displacer may for example be made from
aluminum.
It is, however, preferred to make the displacer from special steel. Suitable
special
steels are in particular molybdenum-stabilized special steels 1.4571, 1.4401,
1.4404,
1.4405 and 1.4539. If the displacer is made from special steel, it is
preferably made
from special steel sheet. The advantage of using special steel over aluminum
is that
while special steel does indeed corrode at relatively high temperatures, which
may for
example occur in the event of failure of the solid electrolyte, an
uncontrollably rapid
reaction with sulfur and polysulfide does not proceed, unlike with aluminum.
Since the mechanical strength of special steel is greater than that of
aluminum and
thus very much less plastic deformation occurs at the battery operating
temperature,
the special steel cannot adapt to the shape of the solid electrolyte in the
case of a
cylindrical design of the displacer. Thermal expansion may thus lead to
failure of the
solid electrolyte if the displacer made from special steel is pressed
nonuniformly
against the solid electrolyte. Such nonuniform contact pressure by the
displacer against
the solid electrolyte is the result, for example, of manufacturing
inaccuracies in the
production of the ceramic solid electrolyte. In order to equalize longitudinal
expansion
due to temperature changes, it is therefore preferred to construct the
displacer in such
a manner that it rests resiliently against the internal geometry of the solid
electrolyte.
Loading of the solid electrolyte is additionally minimized by using special
steel sheet
with a thickness in the range from 0.05 to 0.5 mm, preferably in the range
from 0.07 to
0.15 mm, for example 0.1 mm, to make the displacer.
In order to obtain a shape which rests resiliently against the internal
geometry, the
displacer preferably comprises an outer contour with projections and recesses.
The
CA 02876265 2014-12-10
9
projections and recesses may be obtained, for example, by a corrugated or zig-
zag-
shaped design of the displacer.
Since special steel is only a moderately good electrical conductor, it is
furthermore
preferred for the displacer additionally to comprise current conducting means.
The
current conducting means ensure a uniform current supply both during charging
and
during discharging. Suitable current conducting means are for example
preferably
current collectors arranged uniformly over the circumference of the displacer,
which
current collectors are made in a preferred embodiment from a special steel
tube closed
at both ends in which a core of an electrically highly conductive material is
introduced.
The surface of the special steel tube here rests continuously against the core
of
electrically highly conductive material. The special steel tube protects the
electrically
highly conductive core from attack by sulfur and polysulfide in the event of
failure of the
solid electrolyte.
In an alternative embodiment, the current conducting means comprise a
continuous or
patterned coating of an electrically highly conductive material on the inside
of the
displacer.
Suitable electrically highly conductive materials for the current collector or
the coating
are for example copper, aluminum, silver or gold. If a current collector with
a special
steel tube is used, the electrically highly conductive material may also be
sodium.
While the sodium will indeed be liquid at a conventional operating temperature
300 C,
it cannot escape due to the special steel tube. The electrically highly
conductive
material is particularly preferably copper or aluminum.
In order to ensure proper functioning of the electrode unit, the current
conducting
means must be connected highly electrically conductively to the displacer.
When using
current collectors, this may for example be achieved by in each case welding
the
current collectors to the displacer. It is preferred, however, to clamp the
current
collectors in recesses of the displacer. The current collectors are here
preferably
arranged on the outside of the displacer.
The current collectors may be firmly connected to the displacer for example by
making
the recesses omega-shaped with the diameter of the omega matching the external
diameter of the wire. Given an appropriate design of the receSses, the wires
may in
each case be clamped in the recesses with a stable connection and form a
uniform
contact with the displacer over their entire length.
In the above description, the directions of flow and transport paths of the
anode
material and cathode material were in each case stated for the discharge
process in
CA 02876265 2014-12-10
which current is generated. Transport proceeds in the opposite direction for
charging
the device for storing electrical energy. In this case, the reaction product
arising during
discharging is passed into the porous electrode through the outlet openings,
reacted in
the porous electrode to yield the anode material and cathode material and the
cathode
5 material passes through the inlet openings back out of the porous
electrode and flows
into a storage vessel. The cations formed during the charging process are
transported
through the solid electrolyte, take up an electron and are transported as
neutral anode
material through the flow channel in the displacer or through the annular feed
device,
through which the anode material flows during charging, back into a storage
vessel.
The electrode unit according to the invention is in particular suitable for
use in devices
for storing electrical energy which are operated with an alkali metal as anode
material.
A suitable anode material is here for example lithium, sodium or potassium,
preferably
sodium or potassium. The device for storing electrical energy is here operated
at a
temperature at which the alkali metal used is in liquid form. Corresponding
temperatures may be provided, for example, by simultaneously constructing the
displacer present in the cylindrical solid electrolyte as a heating element,
such that the
latter may be used to keep the temperature in the electrode unit within a
range in which
the anode material is in liquid form. Since the anode material is a liquid
metal, it is
electrically conductive and may thus directly be used as an anode. All that is
required
for...this purpose is to contact an electrical conductor through which the
current can flow
with the liquid anode material.
The cathode material used is a material which is capable of reacting
chemically with
the anode material. Sulfur or polysulfide is preferably used as the cathode
material.
In a preferred embodiment, a ceramic is used as the solid electrolyte. 13-
Alumina or 13"-
alumina is particularly suitable as a material for the solid electrolyte. This
is preferably
stabilized, for example with MgO or L120.
As an alternative to 13-alumina or p"-alumina, other ceramic materials may
also be used
as the solid electrolyte. The ceramic known as NASCION , the composition of
which is
stated in EP-A 0 553 400, may for example be used. The ceramic known in
everyday
language as "ceramic" is also particularly preferred. Sodium ion-conductive
glasses or
zeolites and feldspas may also be used as an alternative to ceramics. In
particular,
however, sodium 13"-alumina, sodium 13-alumina or sodium 13/13"-alumina are
preferred.
The sodium ion-conductive ceramics are preferably thin-walled tubes closed at
one end
at the bottom and open at the top if the solid electrolyte is of cylindrical
construction. In
this case, it is furthermore preferred for the tubes to have a diameter of 20
to 50 mm
and a length in the range from 0.5 m to 2 in. The wall thickness is preferably
in the
range from 0.5 mm to 3 mm, in particular in the range from 1.5 mm to 2 mm.
CA 02876265 2014-12-10
11
The porous electrode is made from a material which is inert towards the
substances
used in the electrochemical reaction. Carbon, in particular in the form of
graphite, is for
example suitable as the electrode material.
According to the invention, the electrode is porous so that the substances
participating
in the electrochemical reaction can flow through it. This is achieved, for
example, in
that the material of the porous electrodes assumes the form of a felt or
nonwoven. The
electrode is very particularly preferably a graphite felt electrode.
In order to avoid the electrode coming into direct contact with the solid
electrolyte, a
porous liquid electrolyte-filled layer which is insulating in terms of
electron conduction is
arranged between the porous electrode and the solid electrolyte. For the
purposes of
the present invention, "insulating in terms of electron conduction" should be
taken to
mean a material which exhibits a specific resistance of at least 108 Ohm*cm
and in
particular of at least 109 Ohm*cm. The material for the insulating layer
should here be
selected such that cations which are transported through the solid electrolyte
can also
pass through the insulating layer to the porous electrode while electron
conductivity is
negligibly small. Examples of a suitable electrically insulating material
which is
arranged between the solid electrolyte and the electrode are anodized or
sulfide-
passivated aluminum fabric, ceramic fibers, glass fibers or carbon fabric.
Using the
electrically nonconductive material ensures that nonconductive cathode
material, for
example sulfur, is not deposited on the solid electrolyte, so limiting current
flow during
charging.
The segment wall, via which the porous electrode is current-conductively
connected to
the bus conductor, is preferably made from a metallic material, in particular
from steel.
Suitable steels are the same as those which may also be used for the
displacer.
If the segment wall is made from a steel, it is preferred, as with the
displacer, for
additional current conducting means to be included. Since the segment wall is
in
contact with sulfur and polysulfide on both sides, coating with an
electrically highly
conductive material is not possible here. The current conducting means used
here are
therefore preferably current collectors made from a special steel tube closed
at both
ends and with an electrically highly conductive core, as were described above
in
relation to the displacer. Using the current conducting means improves the
electrical
conductivity of the electrode. In a particularly preferred embodiment, the
special steel
tube of the bus conductor is additionally chromium-plated.
CA 02876265 2014-12-10
12
As likewise with the displacer, it is preferred to arrange the electrically
conductive wires
with a clamp connection in troughs of the extensive electrode which is of
corrugated
construction.
The cover which forms the channels is likewise preferably made from an
electrically
conductive material and, in a particularly preferred embodiment,
simultaneously serves
as the bus conductor. It is alternatively also possible to provide the bus
conductor
outside the cover. The cover is preferably likewise made from a metallic
material, for
example from steel. The same material as for the segment wall is preferably
used here.
Exemplary embodiments of the invention are illustrated in the figures and
explained in
more detail in the following description.
In the drawings:
Figure 1 shows a sectional representation through an electrode unit
constructed
according to the invention,
Figure 2 shows a plan view of an electrode unit constructed according to the
invention with a segment wall,
Figure 3 shows a three-dimensional representation of the electrode unit
according to
the invention with a segment wall,
Figure 4 shows a sectional representation of a displacer constructed according
to
the invention,
Figure 5 shows a sectional representation of an extensive electrode configured
according to the invention.
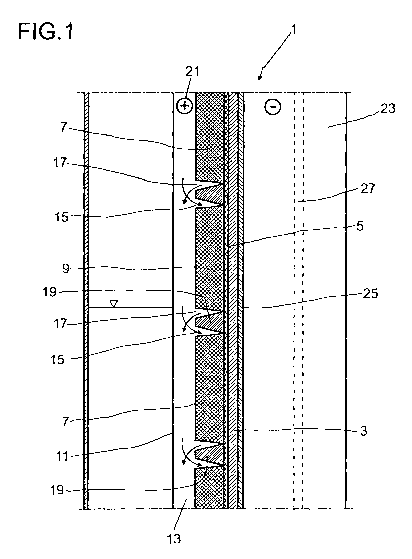
Figure 1 shows an electrode unit according to the invention in the form of a
longitudinal
section.
An electrode unit 1 comprises a solid electrolyte 3, which in the embodiment
shown
here is of cylindrical construction and is closed at one end. The solid
electrolyte 3 is
generally a ceramic membrane which is permeable to specific cations. As
described
above, 13"-alumina is for example suitable as a material for the solid
electrolyte 3.
The solid electrolyte 3 is adjoined by an interlayer 5 which is insulating in
terms of
electron conduction. The interlayer 5 which is insulating in terms of electron
conduction
is for example a passivated aluminum fabric, for example an anodized or
sulfide-
CA 02876265 2014-12-10
13
passivated aluminum fabric, or carbon fabric or is made up of ceramic fibers
or glass
fibers. It is alternatively also possible to apply a special coating to the
porous electrode
as the interlayer 5 which is insulating in terms of electron conduction. In
the present
context, insulating in terms of electron conduction means that the specific
resistance of
the layer is greater than 108 Ohm*cm, preferably greater than 109 Ohm*cm and
in
particular greater than 2 x 10 Ohm*cm.
The interlayer 5 which is insulating in terms of electron conduction is
enclosed by a
porous electrode 7. The porous electrode 7 is for example made from a graphite
felt. In
a preferred embodiment, as shown in Figures 2 and 3, the porous electrode 7 is
enclosed by a segment wall 9. In the embodiment shown here with a cylindrical
solid
electrolyte 3, the segment wall 9 takes the form of a sleeve.
The segment wall 9 is adjoined by a cover 11. In the embodiment shown here,
the
cover 11 takes the form of a cladding tube which has a corrugated cross-
section. In this
way, the cover 11 constructed in the form of a cladding tube rests in each
case with the
troughs against the segment wall 9 and, with the peaks, forms channels 13
along the
segment wall 9. During charging or discharging, cathode material flows through
the
channels 13. As has already been described above, the cathode material is for
example sulfur or an alkali metal polysulfide.
In the embodiments shown in Figures 2 and 3, when the electrode unit 1 is in
operation, during discharging the cathode material flows out of the flow
channel 13
through inlet openings 15 into the porous electrode 7 where it is reduced
electrochemically to the anion. The anion reacts with the cations likewise
transported
through the solid electrolyte 3 into the porous electrode 7 to form a salt.
The cations
are particularly preferably alkali metal ions, such that an alkali metal salt,
in particular
an alkali metal polysulfide, very particularly preferably sodium polysulfide,
is formed in
the porous electrode 7. The reaction product formed in the porous electrode 7,
for
example the alkali metal polysulfide, emerges via outlet openings 17 back out
of the
porous electrode 7 into the flow channel 13.
The number of sleeves used for the segment wall 9 here depends on the height
of the
sleeve and the length of the electrode unit and may also be greater than the
number
shown here. It is also possible to provide only one sleeve and to form a
plurality of rows
of inlet openings 15 and outlet openings 17 in the sleeve.
In order to be able to output a constant electrical power irrespective of the
state of
discharge, the porous electrode 7 is segmented by flow barriers 19. The flow
barrier 19
prevents the reaction product which has formed in the porous electrode 7 from
continuing to flow onwards through the porous electrode 7 in the region of the
outlet
CA 02876265 2014-12-10
14
openings 17. The flow barrier 19 ensures that all the material emerges from
the porous
electrode 7 into the flow channel 13 in the region of the outlet opening 17.
This ensures
fresh cathode material is supplied to the porous electrode 7 in a downstream
segment,
so improving the performance of the electrode unit 1. So that material which
has
emerged from an outlet opening 17 does not immediately enter the next segment
of the
porous electrode, the inlet openings 15 downstream from the outlet openings 17
are
arranged in a staggered manner relative to the outlet openings 17.
The current released during discharging is picked off via a current terminal
21. To this
end, the respective segments of the porous electrode 7 are contacted with the
current
terminal 21. Contact is made, for example, via the segment wall 9 and the
cover 11.
Both the segment wall 9 and the cover 11 are here of electrically conductive
construction. It is alternatively also possible in each case to connect the
porous
electrodes 7, which are enclosed by the segment walls 9, to a central
conductor which
is contacted with the current terminal 21. Any other possible way known to a
person
skilled in the art of electrically contacting the porous electrode 7 is also
possible.
In the simplified embodiment shown in Figure 5, the structure comprises no
segment
walls. The porous electrodes 7 are in direct contact with the corrugated cover
11, such
that in this case too vertically directed flow channels 13 are formed. The
current supply
lines are in direct electrical contact with the porous electrode 7.
During discharging, the porous electrode 7 is the cathode. The anode is formed
by the
anode material which is located on the opposite side of the solid electrolyte
3 from the
porous electrode 7. In the embodiment shown here with a cylindrical solid
electrolyte 3,
the anode material is located in the interior of the solid electrolyte 3. In
order to be able
to keep the quantity of anode material small, a displacer 23 is located in the
solid
electrolyte 3. The displacer 23 is here constructed such that a gap 25 is
provided
between the solid electrolyte 3 and the displacer 23. The anode material is
located in
the gap 25. If an alkali metal is used as the anode material, the anode
material is itself
electrically conductive and may be used directly as an electrode, during
discharging, as
the anode. To this end, it is for example possible for the displacer 23 to be
electrically
conductive and the displacer 23 to form the current terminal.
A channel 27 is constructed in the displacer 23 for feeding the anode
material. The
anode material flows through the channel 27 into the gap 25 and, on
electrochemical
reduction, forms cations which pass through the cation-conductive solid
electrolyte 3
into the porous electrode 7 where the cations enter into a neutralization
reaction with
the anions formed therein.
CA 02876265 2014-12-10
It is furthermore possible to heat the displacer 23 in order to establish the
temperature
required for operation so that the anode material and cathode material remain
molten.
Heating may be provided electrically for example with a heating rod.
5 in one particular embodiment, heating is provided with variable heating
power
distributed over the length of the electrode unit, such that more heating is
provided at
the top and the least at the bottom. This means that alkali metal which has
cooled to
below the melting point and the surrounding cathode material melt from above
downwards in the form of a melt cone so ensuring that destructive pressures
cannot
10 arise due to entrapped melt.
For charging, the salt, for example sodium polysulfide, is supplied via the
channels 13,
enters through the outlet openings 17 into the porous electrode and is split
by an
applied voltage into sodium ions and sulfur, wherein the sodium ions can flow
through
15 the solid electrolyte 3 into the gap 25 and emerge through the channel
27. The sulfur
passes out of the porous electrode 7, through the inlet openings 15 in the
segment wall
9, into the flow channel 13. Flow is initiated by the difference in density
between
sodium polysulfide and sulfur. Since the sodium polysulfide has a higher
density than
sulfur, the sodium polysulfide sinks downwards and forms a flow, such that the
electrode unit 1 may be continuously operated provided that a supply of alkali
metal
and sulfur is available.
The sulfur and alkali metal are stored in storage vessels arranged separately
from one
another, wherein the storage vessel for the sulfur may for example also
enclose the
cover 11 and flows via the channels 13 formed by the cover 11 to the porous
electrode
7. The resultant salt is then likewise collected in the storage vessels for
sulfur. Due to
the difference in density, a biphasic system forms, the sodium polysulfide
being located
below and the sulfur on top.
Figure 2 shows a plan view of the electrode unit 1 constructed according to
the
invention. The plan view shown in Figure 2 in particular reveals the
corrugated design
of the cover 11 in the form of a cladding tube. The corrugated cover 11 here
rests with
troughs 29 against the segment wall 9 and individual channels 13 are formed by
the
peaks 31, which alternate with the troughs 29. During discharging, the cathode
material
flows into the channels 13, which are formed by the peaks 31, and then enters
through
inlet openings 15 into the porous electrode. The material which does not pass
into the
porous electrode 7 flows onward through the flow channel 13. At the outlet
openings
17, the material flowing through the channel mixes with the emerging material,
such
that a mixture enters into downstream inlet openings 15 in the same flow
channel 13,
which mixture comprises a higher proportion of unreacted cathode material than
the
material emerging from the outlet openings.
CA 02876265 2014-12-10
16
Figure 3 shows the electrode unit according to the invention in three
dimensions, the
cover 11 having a cutaway to show the underlying components. Said cutaway is
not
present in the installed electrode unit 1. The representation in Figure 3
reveals that the
outlet openings 17 are arranged in a staggered manner relative to the
downstream inlet
openings 15. This prevents material from an outlet opening 17 from being able
to flow
directly into the downstream inlet opening 15. In the embodiment shown here,
the inlet
openings 15 and outlet openings 17 are in each case constructed with a
rectangular
cross-section, an extension 33 of the extensive electrode 9 constructed as a
sleeve in
each case being located between two inlet openings 15 or two outlet openings
17,
which extension is respectively of the same width as the downstream inlet
opening 15
or the preceding outlet opening 17.
In the embodiment shown here, the extensive electrodes 9 are made as separate
sleeves which in each case comprise the inlet openings 15 at one end and the
outlet
openings 17 at the other end. Design as individual sleeves facilitates
installation and
production. Alternatively, however, it is also possible to provide only one
sleeve in
which inlet openings 15 and outlet openings 17 are formed. The design with
separate
sleeves, the ends of which respectively comprise inlet openings 15 and outlet
openings
17, is preferred however. In one particularly preferred embodiment, the inlet
openings
15 and the outlet openings 17 on one sleeve are in each case in axial
alignment with
one another. It is furthermore also possible, in addition to the rectangular
inlet openings
15 and outlet openings 17 shown here, to construct the inlet openings and
outlet
openings in any other desired shape. The openings may accordingly, for
example, be
constructed in the form of a semicircle or a semiellipse or even as a
triangle, if the
openings are in each case at the end of the sleeve. If only one extensive
electrode is
provided, in which a plurality of rows of inlet openings 15 and outlet
openings 17 are
constructed, said openings may also be constructed in any other desired shape,
for
example elliptical, circular, triangular or polygonal with as many vertices as
desired.
In addition to the embodiment shown here with a cylindrical solid electrolyte
3 and thus
likewise cylindrical porous electrodes 7, it is also possible to construct the
electrode
unit 1 with any other desired cross-section and also as an extensive electrode
unit.
Preferably, however, the electrode unit 1 is cylindrical, as shown here.
In order to create a longer electrode unit 1, more than the two segment walls
9
constructed as a sleeve which are shown here may be provided.
Figure 4 shows a sectional representation through a displacer constructed
according to
the invention.
CA 02876265 2014-12-10
17
The displacer 23 is preferably made from special steel. In order to avoid
damaging the
solid electrolyte 3 by thermal expansion of the displacer 23, the displacer 23
is
preferably configured such that it rests resiliently against the solid
electrolyte 3. Resting
in a resilient manner against the solid electrolyte may for example be
achieved by a
design with projections 35 and recesses 37. This results for example in a
corrugated
design of the displacer 23. It is furthermore also possible, in particular, if
additional
current conductors 39 are provided, to make the recesses 37 omega-shaped, into
which current conductors 39 with a circular cross-section are clamped.
In the embodiment shown here, the current conductors 39 comprise a jacket in
the
form of a tube 41 closed at both ends and a core 43 of an electrically highly
conductive
material. The entire circumference of the core 43 here rests against the tube
41. As
has already been described above, the tube is preferably made from a special
steel
and the core is of aluminum, copper, silver, gold or sodium. Using the current
conductors 39 improves the electrical conductivity of the displacer 23 made
from
special steel which has comparatively poor conductivity.
The displacer is conventionally hollow on the inside. The internal region 45
of the
displacer may for example be used to accommodate a vessel comprising sodium.
The
vessel is here preferably likewise made from special steel.
Figure 5 shows a sectional representation of the extensive electrode in one
embodiment of the invention.
The solid electrolyte 3 is enclosed by an electrically insulating layer .5 and
a porous
electrode 7. The porous electrode 7 is adjoined by the cover 11 which, in the
embodiment shown here, is of corrugated construction. Flow channels 13,
through
which sulfur and polysulfide flow, are formed by the corrugated design of the
cover 11.
If the cover 11 is made from steel, additional current conductors 47 should be
provided
to improve electrical characteristics. The current conductors 47 are here
preferably
arranged on the side of the cover 11 which faces towards the solid electrolyte
3. In the
embodiment shown here, the current conductors 47 are accommodated in flow
channels 23 of the extensive electrode. The geometries of the flow channels 23
and
current conductors 47 are here adapted to one another such that a current
conductor
47 in each case rests continuously against the wall of a flow channel 13. In
order to
avoid an unwanted reaction of the current conductor with the sulfur or
polysulfide, the
current conductor 47, like the current conductor 39 arranged on the displacer
side, is
made with a jacket of a special steel tube 49 closed at both ends and a core
51 of an
electrically highly conductive material. The electrically highly conductive
material is
preferably copper, aluminum, silver or gold, particularly preferably copper or
aluminum.
CA 02876265 2014-12-10
18
In addition to being arranged in every other flow channel 13, as shown here,
any other
desired uniform or nonuniform distribution of the current conductor 39 is
possible. For
example, in the case of a uniform distribution it is accordingly also possible
to provide
the current conductors only in every third or every fourth flow channel 13.
In addition to the embodiment shown here, it is furthermore also possible to
arrange
current conductors on the side of the cover 11 remote from the solid
electrolyte 3. In
this case, it is preferred to contact the electrically highly conductive
material directly
with the material of the cover 11, for example by a coating or also by a clamp
connection of a wire made from the electrically highly conductive material in
a trough of
the cover 11 of corrugated construction that is remote from the solid
electrolyte 3. In
order to prevent reaction of the electrically highly conductive material with
sulfur or
polysulfide, a cover, which is not shown here, in this case encloses the
extensive
electrode and thus also the electrically highly conductive material. The same
cover
material is here preferably selected as for the cover 11.
CA 02876265 2014-12-10
19-
List of reference numerals
1 Electrode unit
3 Solid electrolyte
5 Layer insulating in terms of electron conduction
7 Porous electrode
9 Segment wall
11 Cover
13 Flow channel
Inlet opening
17 Outlet opening
19 Flow barrier
21 Current terminal
15 23 Displacer
Gap
27 Channel
29 Trough
31 Peak
20 33 Extension
Projection
37 Recess
39 Current conductor
41 Tube
25 43 Core
Internal region of the displacer 23
47 Current conductor
49 Special steel tube
51 Core