Note: Descriptions are shown in the official language in which they were submitted.
CA 02877093 2014-12-17
WO 2014/007610 PCT/NL2013/050413
1
USE OF AN ADDITIVE COMPOSITION FOR CEMENTING BORE WELLS
RELATED APPLICATION
This application is a continuation-in-part of U.S. Application No. 13/540,181,
filed July 2, 2012. The entire teaching of the above application is
incorporated
herein by reference.
BACKGROUND OF THE INVENTION
Patent EP 1 349 819 (corresponding to US 7,316,744) of the present inventor
discloses a composition for reinforcing cement, which contains: a) sodium
chloride,
potassium chloride, magnesium chloride, calcium chloride, strontium chloride,
barium
chloride and/or ammonium chloride; b) aluminum chloride; and c) silica and/or
zeolite
and/or apatite. This reference is incorporated herein in its entirety.
The composition for reinforcing cement according to EP 1 349 819 is
commercially available from PowerCem Technologies B.V. under the registered
trade names of PowerCem and RoadCem. In a preferred embodiment of EP 1 349
819 the additive composition comprises a combination of sodium chloride,
potassium
chloride, ammonium chloride, magnesium chloride, calcium chloride, aluminum
chloride, silica, magnesium oxide, magnesium hydrogen phosphate, magnesium
sulphate, sodium carbonate and cement.
The composition for reinforcing cement, according to EP 1 349 819, shows
excellent performances in, for example, the field of road construction, soil
consolidations (i.e. before drilling into the soil) and concrete for flyovers.
SUMMARY OF THE INVENTION
The present inventor has discovered a new cementing composition and a new
use for the cited additive composition.
The present invention relates to the use of an additive composition for
cementing wellbores. Moreover, the present invention relates to a cement
slurry for
cementing a wellbore, comprising: l) cement; II) water; and III) a composition
for
reinforcing cement. In addition, the present invention relates to a method of
cementing a wellbore.
CA 02877093 2014-12-17
WO 2014/007610 PCT/NL2013/050413
2
The present invention is related to the use of a composition for reinforcing
cement, which comprises: a) one or more compounds selected from sodium
chloride,
potassium chloride, magnesium chloride, calcium chloride, strontium chloride,
barium
chloride and ammonium chloride; b) aluminum chloride; and c) one or more
compounds selected from silica, zeolite, and apatite; for cementing a
wellbore.
In an embodiment of the present invention, the composition comprises at least
sodium chloride and calcium chloride from group a).
In an embodiment of the present invention, the composition contains silica
and/or zeolite.
In an embodiment of the present invention, the composition comprises 45 to
90% by weight of the compound or compounds from group a); 1 to 10% by weight
of
the compound from group b); and 1 to 10% by weight of the compound or
compounds from group c); based on the total weight of groups a + b + c.
In an embodiment of the present invention, the composition comprises 45 to
90% by weight of the compound or compounds from group a); 1 to 10% by weight
of
the compound from group b); and 1 to 10% by weight of the compound or
compounds from group c); based on the total weight of the composition.
In an embodiment of the present invention, the composition also comprises
magnesium oxide and/or calcium oxide.
In an embodiment of the present invention, the composition comprises sodium
chloride, potassium chloride, magnesium chloride, calcium chloride, ammonium
chloride, aluminium chloride, magnesium oxide, and silica and/or zeolite.
In an embodiment of the present invention, group c) consists of silica.
In an embodiment of the present invention, the composition further comprises
magnesium hydrogen phosphate, magnesium sulphate and/or sodium carbonate.
Moreover, the present invention relates to a cement slurry for cementing a
wellbore, comprising: l) cement; II) water; and III) a composition for
reinforcing
cement, which comprises: a) one or more compounds selected from sodium
chloride,
potassium chloride, magnesium chloride, calcium chloride, strontium chloride,
barium
chloride and ammonium chloride; b) aluminum chloride; and c) one or more
compounds selected from silica, zeolite, and apatite; for cementing a
wellbore.
A cement slurry is wet cement obtained by mixing dry cement and water and
optionally one or more additives.
CA 02877093 2014-12-17
WO 2014/007610 PCT/NL2013/050413
3
In one embodiment, the inventive composition for reinforcing cement is first
dispersed or dissolved in water to obtain an reinforcing dispersion or
reinforcing
solution. This dispersion or solution is subsequently added to a wet cement
that is
prepared by mixing cement, optionally additives and water.
In another embodiment, the inventive composition for reinforcing cement is
added to the cement in dry form and subsequently water is added.
All embodiments described above for the use also apply to the cement slurry
and method for cementing a wellbore and vice versa.
In an embodiment of the cement slurry, said slurry comprises between 50 and
85 wt%, preferably between 65 and 75 wt% of: l) cement, and between 20 and 40
wt%, preferably between 25 and 30wt% of; II) water, and between 0.1 and 10
wt%,
preferably between 1 and 3 wt%, more preferably between 1.5 and 2.5 wt% of
composition III).
In addition, the present invention relates to a method of cementing a
wellbore,
comprising the steps of: i) drilling a wellbore; ii) introducing a casing
string into the
wellbore; iii) preparing a cement slurry based on a combination of cement and
the
composition for reinforcing cement, which comprises: a) one or more compounds
selected from sodium chloride, potassium chloride, magnesium chloride, calcium
chloride, strontium chloride, barium chloride and ammonium chloride; b)
aluminum
chloride; and c) one or more compounds selected from silica, zeolite, and
apatite, for
cementing a wellbore; iv) pumping said cement slurry into the wellbore; and v)
allowing said cement slurry to set.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows a Scanning Electron Microscopic photograph of hardened
cement slurry according to the present invention showing nanoscopic
crystalline
structure.
Figure 2 shows a Scanning Electron Microscopic photograph of hardened
cement slurry according to the prior art without the presences of the
additive.
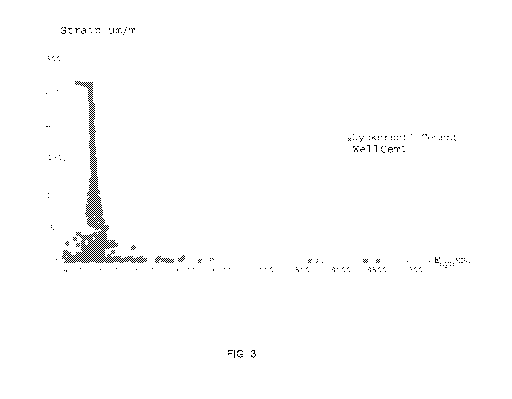
Figure 3 shows a graph of the strain (in micrometers per meter) on the
ordinate (y-axis) and the dynamic modulus of elasticity or Edyn (in Mega
Pascal) on
the abscissa (x-axis) for a sample according to the present invention and a
reference
sample.
CA 02877093 2014-12-17
WO 2014/007610 PCT/NL2013/050413
4
DETAILED DESCRIPTION OF THE INVENTION
One important use of concrete or cement in the oil and gas field is so-called
"well cementing" or the cementing of the drilling or oil well. For this use
deep bores
are drilled into the ground or soil. The inside of these bores are covered by
a metallic
layer or pipe that is used to guide the oil from the oil field up to the
surface. These
metallic layers should adhere to surrounding environment (i.e. soil or rock).
In order
to obtain this adhesion between the metallic layer (casing or casing string)
and the
surroundings cement is often used.
Wel!bores are protected and sealed by cementing, i.e. for shutting off water
penetration into the well, to seal the annulus after a casing string (viz. a
long section
of connected oilfield pipe) has been introduced down the wellbore, or to plug
a
wellbore to abandon it.
Cementing is carried out using a cement slurry that is pumped into the well.
In
this method, usually the drilling fluids that are present inside the well are
replaced by
cement. The cement slurry fills the space between the casing and the actual
wellbore, and hardens to create a seal. This prevents external materials
entering the
well flow. This cementing also positions the casing string into place
permanently.
The term cement is understood to refer to a salt hydrate consisting of a fine-
ground material which, after mixing with water, forms a more or less plastic
mass,
which hardens both under water and in the outside air and which is capable of
bonding materials suitable for that purpose to form a mass that is stable also
in
water. The cement standards according to European standard NEN-EN-197-1 are as
follows: CEM I is Portland cement; CEM II is composite Portland cement; CEM
III is
blast furnace slag cement; CEM IV is pozzolan cement and CEM V is composite
cement.
The wet cement (viz. cement slurry) is obtained by the use of mixers (e.g.
hydraulic jet mixers, re-circulating mixers or batch mixers) from water and
dry
cement and one or more additives.
For wellbore cementing Portland cement is most frequently used (calibrated
with additives to 8 different API classes). Examples of additives are
accelerators,
which shorten the setting time required for the cement, as well as retarders,
which do
the opposite and make the cement setting time longer. In order to decrease or
increase the density of the cement, lightweight and heavyweight additives are
added.
Additives can be added to transform the compressive strength of the cement, as
well
CA 02877093 2014-12-17
WO 2014/007610 PCT/NL2013/050413
as flow properties and dehydration rates. Extenders can be used to expand the
cement in an effort to reduce the cost of cementing, and antifoam additives
can be
added to prevent foaming within the well. In order to plug lost circulation
zones,
bridging materials are added, as well.
5 The
present invention provides a very special additive for cement to be used
for wellbores.
A method for well cementing is known in the art. After the casing string has
been run into the well, a cementing head is attached to the top of the
wellhead to
receive the slurry from the pumps. A so-called bottom plug and top plug are
present
inside the casing and prevent mixing of the drilling fluids from the cement
slurry.
First, the bottom plug is introduced into the well, and cement slurry is
pumped into
the well behind it, viz. within the casing and not yet between the casing and
its
surroundings. Then the pressure on the cement being pumped into the well is
increased until a diaphragm is broken within the bottom plug, permitting the
cement
slurry to flow through it and up the outside of the casing string, viz.
outside of the
casing and hence between the casing and its surroundings. After the proper
volume
of cement is pumped into the well, a top plug is pumped into the casing
pushing the
remaining slurry through the bottom plug. Once the top plug reaches the bottom
plug, the pumps are turned off, and the cement is allowed to set.
Since wellbores are very deep, setting or hardening at deep depths and under
conditions of high temperature and/or high pressure, and optionally corrosive
environments, there are stringent requirements for the cement.
A few of the challenges today with respect to well cementing are discussed
below.
Despite recent technological advances with elastomers, polymers, fibres and
reactive components that self-heal micro fissures, the cement sheath between
the
casing string and the surrounding rock and/or soil is not always able to
deliver an
acceptable long-term solution for today's demanding drilling environment.
Changes
in down hole conditions with pressure and temperature fluctuations impose
stresses
on the cement sheath. Consequently, shrinking and de-bonding of the cement
sheath
creates very small micro cracks allowing fluid migration which is undesirable.
Besides these external forces that cause cement sheath damage an evaluation of
conventional oil well cement sheath on the nanoscopic scale from 1 ¨ 100 nm
CA 02877093 2014-12-17
WO 2014/007610 PCT/NL2013/050413
6
reveals that the chemical bond between components within the cement itself is
relatively brittle.
Examples of the challenges are: i) micro cracks occurring because of
fluctuations in pressure and/or temperature inside the well; ii) undesired gas
migration due to shrinkage or expansion of the cement; iii) corrosion of the
protective
casing, which costs hundreds of millions and which reduces longevity.
There are several demands required in the field of well cementing, viz. with
respect to density, permeability, shrinkage, bonding, chemical resistance,
setting
time, viscosity, flexibility, and durability. Moreover, downhole temperature
can
exceed 200 C.
An example of preferred product criteria for cement for wells are the
following:
Density: value < 1300 kg/m3
Permeability: material has to be impermeable
Shrinkage: material may not shrink, expansion is preferred
Bonding: good bond required with steel
Chemical resistance: high chemical resistance required
Thickening time: materials needs to be workable up to 6 hours
Viscosity: preferably 300 CP
Flexibility: stretch of 2% without fracturing
Known Portland cement consists of five major compounds and a few minor
compounds. The composition of a typical Portland cement is as follows: 50 wt%
of
tricalcium silicate (Ca3Si05 or 3CaO.Si02); 25 wt% of dicalcium silicate
(Ca25iO4 or
2CaO.5i02); 10 wt% of tricalcium aluminate (Ca3A1406 or 3CaO.A1203); 10 wt% of
tetracalcium aluminoferrite (Ca4Al2Fe2010 or 4CaO.A1203.Fe203); 5 wt% of
gypsum
(Ca504.2H20).
Without wishing to be tied to any specific theory, experimental results
indicate that the components which are present in the composition for
reinforcing
cement used in the present application form crystalline structures when added
to
cement material which crystalline structures are well bonded together and are
homogeneously distributed, in between the cement particles, and thereby bind
the
cement particles. This is clearly visible in Figure 1. Figure 1 shows a
Scanning
Electron Microscopic photograph of hardened cement slurry according to the
present
invention showing nanoscopic crystalline structure. A cement mixture has been
CA 02877093 2014-12-17
WO 2014/007610 PCT/NL2013/050413
7
prepares and allowed to set. Samples of this hardened cement were prepared and
measured using SEM by the Nanolab of the Radboud University Nijmegen.
Hardened cement which is prepared without this binder or with known binders
has a relatively open structure when viewed on a microscopic scale, with
crystalline
agglomerations which are not homogeneously distributed. This is clearly
visible in
Figure 2. Consequently, the interaction between the crystalline agglomerations
and
also between the cement particles and the crystalline agglomerations is poor.
The crystalline compounds which are formed by this additive (and which are
shown in Figure 1) are surprisingly homogeneously distributed and may be in
the
form of acicular (viz. needle-like) structures. The homogeneous distribution
of the
crystalline structures results in an optimum strength and stability. The water
in the
cement is bound in, and to, the crystalline structures. Consequently, there
are no
local concentrations of water, and therefore the formation of potential weak
spots is
avoided. The crystalline structures comprise, inter alia, zeolite and/or
apatite
compounds. Zeolites are a widespread group of silicate crystals of, inter
alia,
hydrated alkali metal and alkaline earth metal aluminosilicates. Apatites
belong to
the group of strontium, barium or calcium halophosphates, the halogen ion
usually
being a chloride or fluoride, but which may also be substituted by a hydroxyl
group.
The formation of these structures is one of the reasons why silicon, aluminum
and/or
phosphate compounds are added to the composition.
Without wishing to be bound to a theory, the following is observed. When
water is added to cement, each of the compounds undergoes hydration and
contributes to the final product. Only the calcium silicates contribute to
strength.
Tricalcium silicate is responsible for most of the early strength during first
7 days.
Dicalcium silicate, which reacts more slowly, contributes only to the strength
at later
times. Upon the addition of water, tricalcium silicate rapidly reacts to
release calcium
ions, hydroxide ions, and a large amount of heat. The pH quickly rises over 12
because of the release of alkaline hydroxide (OH-) ions. This initial
hydrolysis slows
down quickly with a corresponding decrease in heat.
The reaction slowly continues producing calcium and hydroxide ions until the
system becomes saturated. Once this occurs, the calcium hydroxide starts to
crystallize. Simultaneously, calcium silicate hydrate begins to form. Ions
precipitate
out of solution accelerating the reaction of tricalcium silicate to calcium
and
CA 02877093 2014-12-17
WO 2014/007610 PCT/NL2013/050413
8
hydroxide ions, also called Le Chatelier's principle. The evolution of heat is
then
dramatically increased again.
The formation of the calcium hydroxide and calcium silicate hydrate crystals
provide "seeds" upon which more calcium silicate hydrate can form. The calcium
silicate hydrate crystals grow thicker which makes it more difficult for water
molecules to reach the anhydrate tricalcium silicate. The speed of the
reaction is
controlled by the rate at which water molecules diffuse through the calcium
silicate
hydrate coating. This coating thickens over time causing the production of
calcium
silicate hydrate to become slower and slower. The majority of space is filled
with
calcium silicate hydrate, what is not filled with the hardened hydrate is
primarily
calcium hydroxide solution. The hydration will continue as long as water is
present
and there are still anhydrate compounds in the cement paste.
Dicalcium silicate also affects the strength of concrete through its
hydration.
Dicalcium silicate reacts with water in a similar manner as tricalcium
silicate, but
much more slowly. The heat released is less than that by the hydration of
tricalcium
silicate because the dicalcium silicate is much less reactive. The other major
components of Portland cement, tricalcium aluminate and tetracalcium
aluminoferrite
also react with water. Heat is evolved with cement hydration. This is due to
the
breaking and making of chemical bonds during hydration.
The strength of cement bound products is very much dependent upon the
hydration reaction just discussed. Water plays a critical role, particularly
the amount
used. The strength of the product increases, when a lower amount of water is
used.
The hydration reaction itself consumes a specific amount of water. The empty
space
(porosity) is determined by the water to cement ratio. The water to cement
ratio is
also called the water to cement factor (abbreviated by wcf) which is the ratio
of the
weight of water to the weight of cement used in the slurry. The wcf has an
important
influence on the quality of the cement produced.
Low water to cement ratio leads to high strength but low workability. High
water to cement ratio leads to low strength, but good workability. Time is
also an
important factor in determining product strength. The product hardens as time
passes. The hydration reactions get slower and slower as the tricalcium
silicate
hydrate forms. It takes a great deal of time up to several years for all of
the bonds to
form, which eventually determines the product's strength for the life of the
well.
CA 02877093 2014-12-17
WO 2014/007610 PCT/NL2013/050413
9
When the composition according to the present invention is used as additive,
moisture remains necessary for hydration and hardening. The five major
compounds
of the hydration process of cement still remain the most important hydration
products
but the minor products of hydration probably change. Furthermore, the rate at
which
important hydration reactions occur and the relative distribution of hydration
products
changes as a result of the addition of the present inventive composition. In
addition,
the crystallization of calcium hydroxide accordingly occurs at different rates
and the
reduction of heat generation from the hydration reactions occurs. There are
more
crystals formed during the reactions and the relevant crystalline matrix is
much more
extensive.
When adding the present composition, the water changes chemically in
sphere, electrical load, surface tension and reaches a chemical/physical
equilibrium
in the matrix. This complex process depends of the type and mass of materials
involved in the cement slurry. Similar to the chemical processes physical
aspects are
part of the equilibrium process in the matrix when the amount of water,
trapped as
free water is reduced and the crystals grow into the empty void space. This
makes
the product less permeable to water and more resistant to all types of attack
that are
either water dependant or water influenced. A bigger fraction of the water is
converted to crystalline water than is the case with the reactions in the
absence of
the present inventive composition. The reduced porosity and increased
crystalline
structural matrix increases compressive, flexural and breaking strength of the
product and change the relative ratio between these strengths.
As before, the strength of the product increases when less water is used to
make a product. The hydration reaction itself now tends to consume a different
amount of water. When the present inventive composition is mixed with oil well
cement it is also possible to use salt water and achieve a good end result.
The empty space (porosity) is still determined by the water to cement ratio
but
is affected to a lesser extent as a result of the increased rate and extent of
the
crystallization process.
The extended crystallization process changes significantly with the present
inventive composition. The present inventive composition causes a physio-
chemical
equilibrium in the oil well cement slurry based on synergy between water
percentage
and API Class G oil well cement. This is followed by changes in the chemical
and
physical properties of the cement slurry, first from hydrophilic then into
hydrophobic.
CA 02877093 2014-12-17
WO 2014/007610 PCT/NL2013/050413
As a result, strong hydrogen bonds form which make a significant contribution
to the
bonding forces. The binding mechanism changes from "glue" to "wrapping" and
the
cement slurry exhibits a crystalline structure that is able to partially block
capillary
pores. Because of this fiber-like structure, it becomes flexible and prevents
micro
5 __ cracking from occurring.
Further research indicated that only part of the present composition is
involved in the chemical reaction. The remaining part is buffered in the pore
structure
and remains active, even after 90 days, still able to actively react within
the silicate-
containing matrix. During this period the remaining active composition of the
10 __ invention subsequently participates in the continued formation of a
crystalline
structure, improving durability.
Tests from independent laboratories have indicated special properties that
could not be attributed to conventional cement. The special properties are
improved
fatigue values, higher compressive strength, chemical durability and even fire
__ resistance. The process continues for up to 180 days, further improving the
physical
properties until the matrix is fully saturated with the durable crystalline
structure
(Figure 1) (Picture taken by Nanolab, Radboud University of Nijmegen).
The compressive strength of set cement is an indication of the cement's
resistance to failure in compression. Cement must be strong enough to support
the
__ casing in the hole, withstand the shocks of drilling and perforating, and
support high
hydraulic pressure without fracturing. The compressive strength test
determines the
strength of set cement under downhole conditions. This property is measured in
pounds per square inch (psi). The compression strength of conventional cement
decreases in time with an increase in permeability. This is not observed with
a
__ cement obtained by the cement slurry of the present invention.
A low density cement is particularly preferred to be able to pump cement
slurry, especially at higher temperatures. The additive composition lowers the
density of cement slurry, which is an advantage. Moreover, the present
composition
improves the bonding with water, which is an advantage over traditional oil
well
__ cement. The crystallization process actually results in an expansion of the
cement
since it obtains a higher volume with same mass.
With respect to the permeability, it can be observed that the cement slurry
when mixed with the present composition obtains, after curing, a higher
density due
to crystallization of water. Based on the fact that the water content in the
slurry
CA 02877093 2014-12-17
WO 2014/007610 PCT/NL2013/050413
11
bonds much better in the modified crystal matrix that is obtained due to the
presence
of the present composition. Over time the remaining part of the present
composition
is buffered in the pore structure and is even after months still able to
actively react
within the matrix. This results in a reduction in capillary forces while the
crystalline
structure keeps growing.
The presence of the nanostructures (i.e. modified crystal matrix) by use of
the
present composition also has been observed to lead to higher chemical
resistance
values (i.e. that chemicals in the soils are not damaging the cement), and to
a
reduced shrinkage during the expansion process.
The crystallization process of the cement sheath at a scale of 1 -100
nanometers shows that elements cross-link and create long needle crystalline
structures that interlock, block the capillary pores, and enhance dynamics and
chemistry of the cement hydration process. As a result, the molecular
structure
changes with hydrogen bridges in a stable, locked position. It is important to
recognize that the mechanical properties of a cement bound material are
determined
during the first hours of the binding and during the first 48 hours of the
hardening
stage. Consequently, if a modification of the cement hydration process is
required to
enhance the structural, mechanical and chemical resistance behaviour of the
cement
sheath, it has to take place within 72 hours.
The above is in sharp contrast with conventional oil well cement slurries
which
still depend on the calcium silicate hydrate (C-S-H) matrix and cement
hydration
process where only a few chemical reactions take place.
To prevent excessive shrinkage of cement, it is important to design a cement
slurry that is able to absorb pull and tensile forces. The inventor has
succeeded in
this by using the present composition.
As discussed above a good bonding is required to steel. The present inventor
has proven increased binding to a steel casing using the composition according
to
the present invention. Without wanting to be bound to a theory, it is proposed
that
this is due to the effect that crystals are allowed to grow under normal
circumstances
of e.g. temperature in the formation (due to the available moisture in
formation).
An additional advantage of the composition used in the present invention is
that it is easy to handle and can be provided in ready-to-mix bags.
An additional advantage of the present composition is that it allows more
moisture to be mixed in the cement slurry than with traditional cement which
ensures
CA 02877093 2014-12-17
WO 2014/007610
PCT/NL2013/050413
12
a higher viscosity. The present composition affects the viscosity of the
cement bound
material. Normal cement shows a lower viscosity and therefore has more
character.
With the present composition a higher viscosity will be achieved which results
in a
higher flexural behaviour.
When the composition of the present invention is used in well cement, it is
possible to add fine cohesive material to the cement slurry. The use of the
present
composition can increase the flexibility up to for example 2000 mm/m compared
to
normal cement having a value of only 150 mm/m. A person skilled in the art can
custom engineer the slurry in order to optimize the flexibility and stiffness.
The present applicant has carried out the following tests in a laboratory:
energy absorption, flexibility, tensile strength, and compressive strength.
The results
are provided below.
Figure 3 shows the flexibility after 24 hours of hardening. Figure 3 shows a
graph of the strain (in micrometers per meter) on the ordinate (y-axis) and
the
dynamic modulus of elasticity or Edyn (in Mega Pascal) on the abscissa (x-
axis) for a
sample according to the present invention and a reference sample. A reference
sample comprising only cement and water (Dyckerhoff cement) is shown in dark
grey
color and a sample according to the present invention comprising cement, water
and
the present composition (Wellcem1) (PowerCem of Powercem Technologies B.V.) is
shown in light grey color. It is clearly visible that the ratio of dynamic
modulus of
elasticity to strain (Edyn*strain) is much higher for the slurry according to
the present
invention (viz. 1.74x106) than that for the reference slurry (1.3x106).
Table 1 below shows the makeup of the reference cement and the slurry
according to the present invention.
Table 1:
Material
Reference cement (in kg) Slurry according to the
invention in kg)
Cement (API Class G by 12.5 12.5
Dyckerhoff)
Additive composition 0.375
Water 75 4.75
CA 02877093 2014-12-17
WO 2014/007610 PCT/NL2013/050413
13
The components of the additive composition are set forth in Table 2:
Component Quantity
(% by weight of total additive
composition)
Naa
(ten. pure) 32.4
NH4C1(techn. pure)
AlC13.6H20 (extra pure) 3.2
KCI (techn. pure) 17.3
CaC11.211,0 (team. pure) 16.2
Mgt:12.61120 (techn. pure) 17.3
Mg0 (pure) 2,2
MgHPO4.3H20 (tedin. pure) 3.2
MgS0420 (tectin_ pure) 2.7
Na2C0?,(techn. pure) 3.2
Amorphous Si02 (5-40 m)=
The wcf (water cement factor or water cement ratio) for the slurry according
to the
present invention is 0.38. This wcf is the ratio of the weight of water to the
weight of
cement used in the slurry and has an important influence on the quality of the
cement produced. The water cement factor can be higher depending on the type
of
cement used. It should be ensured that th amount of free water that remains
complies with the requirements as stated for the API cement types.
The test results after 24 hours are provided below in Table 3.
Table 3:
Test Reference cement Slurry according to Difference (A)
the invention
Betiding force 3_ 1 NIMM2 4,5 NInim2 45'.%
Compressive 13,4 1\41mm 20.9 Nimm2 4- 56%
strength
Average density .1947 kgim3 1917 kg/n3 %
The result of this comparative research shows major differences between the
samples of API Class G and nano-engineered API Class G cement (viz. the slurry
CA 02877093 2014-12-17
WO 2014/007610 PCT/NL2013/050413
14
according to the invention). Test data show higher bending forces from the
nano
enhanced oil well cement as well as significantly stronger values for
compressive
strength.
It can be concluded that the addition of the present composition to a cement
slurry for well cementing provides a higher Dynamic Elastic modulus (Edyn)
(measured according to ASTM E1875 ¨ 08) and a higher flexibility
(Edyn/Breaking
strain). Thus, there is less chance of the formation of cracks on both short
and long
term. Moreover, the compression strength and the breaking strength are
increased
with the use of the present composition as well as the chemical resistance,
viscosity,
and permeability after hardening.
Mechanical properties tests of the newly created oil well cement show a
fundamental change in the molecular structure. The creation of a dense
crystalline
mineral fibre structure tightly knits the materials together. Resulting into
the
introduction of significant ductility and tensile strength increase, thus
greatly
reducing cracking and blocking the capillary pores of the cement sheath. The
present
invention is further explained in the appended claims.
While this invention has been particularly shown and described with
references to preferred embodiments thereof, it will be understood by those
skilled in
the art that various changes in form and details may be made therein without
departing from the scope of the invention encompassed by the appended claims.