Note: Descriptions are shown in the official language in which they were submitted.
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
1
PRODUCTION OF AMMONIUM PHOSPHATES
TECHNICAL FIELD
The present invention relates in general to production of ammonium phosphates
from phosphorus-
containing solutions and in particular to production of ammonium phosphates
from a feed liquid
comprising phosphoric acid.
BACKGROUND
All water-soluble phosphate salts such as soluble fertilizers are derived from
phosphoric acid, Phosphoric
acid is produced commercially by either a 'wet' or a thermal process. Wet
digestion of phosphate rock is
the most common process. Thermal processing is energy intensive and therefore
expensive. For that
reason, quantities of acid produced thermally are much smaller and mainly used
for production of
industrial phosphates.
Phosphoric acid for fertilizer production is almost solely based on wet
digestion of rock phosphate. The
process is mainly based on dissolution of apatite with sulfuric acid, After
dissolution of the rock, calcium
sulfate (gypsum) and phosphoric acid are separated by filtration. To produce
merchant-grade phosphoric
acid, high acid concentrations are required and water is evaporated. Calcium
sulfate exists in a number of
different crystal forms depending on the prevailing conditions such as
temperature, phosphorus
concentration in the slurry, and level of free sulfate. Calcium sulfate is
either precipitated as di-hydrate
(CaS042H20) or as hemi-hydrate (CaSO4.Y2H20). Phosphoric acid produced through
this process is
characterized by a relatively low purity.
For deriving ammonium phosphate salts, merchant-grade phosphoric acid, having
a concentration of
about 54% P205, is neutralized with ammonia to form either mono-ammonium
phosphate (MAP) or di-
ammonium phosphate (DAP) by controlling the ammonia-to-phosphoric acid mole
ratio during the
neutralization process. Ammonia is used in liquid or gaseous form. Liquid
anhydrous ammonia is usually
preferred since surplus heat from other systems in necessary for vaporizing
liquid ammonia into a
gaseous form.
The neutralization of merchant-grade phosphoric acid with ammonia is usually
performed in several
stages using several reaction vessels. The mole ratio of ammonia to phosphoric
acid in the pre-reactor/s
is normally held at a level which gives the maximum solubility for the slurry
(between 1.4 and 1.45 for
production of DAP and usually less than 1 for production of MAP). For
operation control, the ammonia to
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
2
phosphoric acid mole ratio is determined by monitoring the pH of the slurry.
Excess heat of reaction is
removed from the pre-neutralizer/s by adding water to the reactor/s.
Evaporation of the water cools the
slurry. As the mole ratio of ammonia to phosphoric acid is increased over 1,
un-reacted ammonia escapes
from the reactor and the gaseous vapors released must be scrubbed with an
acid.
The slurry from the pre-neutralization reactor/s which usually contain between
16 to 23% water is usually
fed into an ammoniator-granulator to complete the addition of ammonia for the
desired product.
Completion of the neutralization and additional evaporation of water results
in solid particles being formed.
It is necessary to recover the un-reacted ammonia from the gaseous vapors by
scrubbing with an acid.
Thereafter, the solid ammonium phosphates are usually dried in a separate
reactor to reduce moisture
content. Loss of ammonia from the dryer is usually recovered by scrubbing with
acid. The solid ammonium
phosphates are normally cooled by passing air through a cooling reactor.
For several applications such as fertigation, i.e. the application of water-
soluble fertilizers in the irrigation
water, and foliar fertilization, i.e. spraying fertilizers on leaves, there is
a need for fully-soluble ammonium
phosphates to avoid clogging of the fertigation equipment by non-dissolved
solids. Wet-process
phosphoric acid contains a substantial amount of impurities such as iron,
aluminum, calcium, magnesium,
cadmium, etc. which form water-insoluble solids upon neutralization with
ammonia and thus fertilizer-
grade ammonium phosphates are not completely water-soluble. Therefore, fully-
soluble P fertilizers for
fertigation purposes must be specially produced from purified phosphoric acid
which means additional
processing.
The current technology for phosphoric acid purification is based on extraction
of impure wet-process
phosphoric acid into an organic solvent (ketones, tri-alkyl phosphates,
alcohols, etc.) followed by back
extraction with water forming a pure phosphoric acid but with a lower
concentration, which is thereafter
concentrated by water evaporation. Purified phosphoric acid is thereafter
neutralized with ammonia
forming fully-soluble ammonium phosphate products according to the procedure
described above.
The disadvantages of the state-of-the art technologies for production of
ammonium phosphates are
numerous. The phosphoric acid as produced from the gypsum filter, in a
dihydrate process, is not suitable
for direct manufacture of ammonium phosphate salts. The acid must be further
concentrated by water
evaporation to a suitable phosphoric acid concentration (usually about 54%
P205). Normally,
concentration of phosphoric acid is done in three stages. The acid from the
filter (28% P205) is evaporated
to 40% P205 in a single stage vacuum evaporator. The acid is then clarified to
remove precipitated solids
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
3
and the clarified acid is then concentrated to 54% P205 in two stages. The
inter-stage concentration is
about 48% P205. The 54% P205 acid is used for ammonium phosphate production
according to the
procedure described above.
To concentrate acids through evaporation is a very energy-intensive process.
The amount of steam
required for concentrating phosphoric acid usually varies between 2.5 ¨ 5 tons
of steam per ton of
phosphorus, depending on production conditions. If the phosphoric acid is
purified by solvent extraction
the energy demand is about 7 tons steam per ton of phosphorus. The energy
demand for concentration of
phosphoric acid is a major production cost. Expensive equipment such as steam
distribution systems,
evaporators, effluent gas scrubbers, condensation systems, cooling water
systems, liquid effluent
treatment systems and acid storage facilities are necessary for production of
merchant-grade phosphoric
acid. About 50 tons of cooling water is required in order to condense one ton
of vapor. In a barometric
condenser the vapor is directly contacted by the water and as a result
impurities in the vapor contaminate
the cooling water which results in large quantities of contaminated effluents.
Furthermore, additional
equipment is needed for the neutralization of phosphoric acid with ammonia in
several stages, drying,
cooling and scrubbing of ammonia from gaseous vapors. Production of ammonium
phosphate of technical
quality requires additional processing steps as described above.
US patent 3,894,143 describes a process for obtaining crystallized ammonium
phosphate from wet-
process phosphoric acid and ammonia. The process consists of a) forming a
mixture of aqueous
phosphoric acid and acetone in which all components are miscible with water,
b) precipitating impurities
by addition of ammonia and separating the precipitated impurities to form a
purified mixture, c) contacting
the purified mixture with ammonia to produce ammonium phosphate crystals and a
supernatant liquid, and
d) separating the ammonium phosphate crystals from the supernatant liquid and
distilling the supernatant
to separate the acetone for recycling. The disadvantages of this method
include distillation of large
quantities of acetone, limited yield of ammonium phosphates, and production of
large quantities of dilute
aqueous ammonium phosphate effluents. A further main limitation of this
procedure is the insufficient
selectivity. Water miscible solvents are approaching the properties of water
which results in that cationic
and anionic contaminants are co-extracted to a large extent.
US patents 3,975,178 and 4,236,911 are similar to US patent 3,894,143 and
consist of forming a mixture
of aqueous phosphoric acid and acetone or methanol in which all components are
miscible with water
followed by addition of ammonia in order to precipitate impurities and
thereafter ammonium phosphate.
The disadvantages are similar to that reported for US patent 3,894,143.
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
4
US patent 4,132,540 describes a process for removing solvent from the
raffinate of extracted phosphoric
acid. Residual solvent is removed from the raffinate by addition of ammonium
or alkali or alkaline earth
metal cations in an atomic ratio to phosphorus of between 0.1:1 and 0.6:1.
US patent 4,311,681 describes a process for separation of impurities such as
silica and organic impurities
from an organic solvent by washing with an aqueous alkali orthophosphate
solution sufficient to maintain
the pH of the solvent-aqueous mixture at from about 9.5 to about 12.5
US patent 4,678,650 describes a process for production of an aqueous alkali
phosphate solution by
mixing an aqueous phase containing an alkali compound with an organic phase
containing phosphoric
acid in a volume ratio larger than 1:1, and thereafter separating the
resulting aqueous alkali phosphate
solution from the organic phase.
US patent 4,751,066 describes a process for the alkaline stripping of wet
process phosphoric acid from a
water immiscible organic solvent in order to produce a sodium phosphate
solution.
In the published international patent application WO 2008/115121, a method and
an arrangement for
phosphorus recovery are disclosed. Phosphorus ions are extracted from
solutions by adsorbing
phosphorus ions in a scavenger and by releasing the phosphorus ions into an
eluate during regeneration
of the scavenger. The regeneration is performed by ammonia. Phosphate anions
are precipitated in form
of tri-ammonium phosphate upon introduction of excess amounts of ammonia. The
ammonia remaining in
solution after the precipitation of tri-ammonium phosphate is reused for
regenerating the scavenger.
Unfortunately, tri-ammonium phosphate is unstable at ambient temperature and
atmospheric pressure
resulting in the decomposition of the crystal accompanied with release of
ammonia which requires further
processing into stable forms of ammonium phosphate. Tri-ammonium phosphate is
not suitable for direct
use in agriculture.
GB 636,035 discloses improvements of processes of producing diammonium
phosphate. Crystals of
mono-ammonium phosphate are introduced into a solution of diammonium phosphate
in a reactor and
anhydrous ammonia is fed into the reactor. Diammonium phosphate crystals are
collected at the chamber
bottom.
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
In the US patent 3,415,619, a process for making ammonium phosphate is
disclosed. Water-soluble
ammonium phosphate is achieved by extracting a substantially iron-free aqueous
phosphoric acid, derived
from the reaction of calcium phosphate-containing ore and a strong mineral
acid, into a water-immiscible
extractant, separating the phosphoric acid-laden extractant from the residual
aqueous phase, removing
5 the calcium impurities therefrom, contacting the phosphoric acid-laden
extractant with anhydrous
ammonia at a temperature of between about 20 and 90 C, and separating solid,
water-soluble ammonium
phosphate from the extractant. The solid ammonium phosphate is indicated to be
washed with low boiling
hydrocarbon solvents to remove organic extractant adhesive thereto. The
process according to this
disclosure has several drawbacks. In experiments performed by the applicant of
the present invention, it
was concluded that it is very difficult to produce di-ammonium phosphate
according to the presented ideas
even with a large excess of ammonia. The main difficulty of this approach is
thus that large amounts of
solvent remain adhering to the precipitated ammonium phosphate crystals and
this loss of expensive
solvent would be economically unacceptable. Removal of adhering solvent by
distillation is difficult since
the boiling point for solvents such as tributyl phosphate (289 C) exceeds the
melting point for mono-
ammonium phosphate (190 C). Washing the adhering solvent with another
hydrocarbon solvent was
proved to be incomplete, and considerable amounts of extractant remain even
after extensive washing.
Furthermore, separation of extractant and hydrocarbon solvent become a complex
and expensive task
due to the need for distillation.
The published international patent application WO 2010/138045 describes a
process comprising addition
of ammonia to a phosphorus-loaded water immiscible liquid phase in order to
precipitate ammonium
phosphates. The precipitated ammonium phosphates are washed with saturated
aqueous solution of
ammonium phosphate and the washed crystals are dried. Residual scavenger
washed form the crystals is
separated by a phase separation of the scavenger and the saturated aqueous
solution of ammonium
phosphate and the separated residual scavenger is reused for further adsorbing
of phosphorus to be
reused for further extraction. The washing liquid depleted from residual
scavenger is reused for further
washing of the crystals. A disadvantage is the need for three phase
separation.
US patent 3,518,071 describes a process for production of nitrophosphate
fertilizer and ammonium-nitrate
calcium-carbonate fertilizers. The process consist of a) digesting phosphate
rock with nitric acid to
produce a solution of calcium nitrate, phosphoric acid and nitric acid, b)
extracting phosphoric acid and
nitric acid from the leach solution with amyl alcohol, c) re-extracting
phosphoric acid and nitric acid with a
concentrated solution of mainly ammonium nitrate containing some ammonium
phosphate, with a NIP
molar ratio of about 95:1, d) evaporating the strip solution to form a slurry
of crystallized ammonium
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
6
phosphate and ammonium nitrate, e) separating the crystals from the liquor, f)
recycling at least a portion
of the liquor to be used in step c, g) treating the raffinate from step b with
ammonia and carbon dioxide to
form ammonium nitrate solution and precipitated calcium carbonate.
The main purpose of US patent 3,518,071 is to use liquid-liquid extraction for
separation of calcium nitrate
instead of separating calcium nitrate by precipitation at low temperatures
which is the common practice at
the nitrophosphate process. There are a number of disadvantages with this
method. The final product is
generally a mixture of ammonium phosphate and ammonium nitrate and not well-
defined mono-
ammonium phosphate or di-ammonium phosphate. Furthermore, co-extraction of
nitric acid results in
precipitation of calcium phosphates which render the liquid-liquid extraction
process non-operational. This
limits the yield of extracted phosphoric acid. Moreover, a high concentration
of ammonium nitrate is
required in the solution used for stripping in order to decrease the amount of
ammonium phosphate that
remains dissolved in the ammonium nitrate liquor. Also, the method requires
processing of ammonium
nitrate into a final product by water evaporation. Furthermore, the phosphate
product contains ammonium
nitrate via mother liquor adhering to the precipitate. Moreover,
crystallization is obtained by exceeding the
solubility of dissolved salts by evaporation of water which requires equipment
such as evaporators, steam
distribution systems, etc., as well as, an energy source. Finally, amyl
alcohol and other solvents suitable
for this process have a high water solubility which makes the process complex
as the solvent has to be
recovered from the aqueous streams by extraction and distillation.
The patent GB 1,238,188 describes a process for production of ammonium
phosphates by digesting
phosphate rock with nitric acid. The process consist of a) treating the leach
solution with a solvent
composed of aliphatic alcohols containing 4 ¨ 6 carbon atoms to extract nitric
acid and phosphoric acid, b)
purifying the organic extractant by contacting the extractant with a solution
composed of mainly
ammonium nitrate, c) ammoniating the purified extractant to form a precipitate
of ammonium phosphate, a
light phase containing the solvent, and a heavy aqueous phase containing
ammonium nitrate and
ammonium phosphate, d) separating the light phase by decanting and recycling
the decanted light phase
to the extraction step, e) separating the ammonium phosphate precipitate from
the heavy aqueous phase,
f) washing the separated ammonium phosphate with water, g) the washing water
of ammonium phosphate
and part of the heavy aqueous phase containing ammonium nitrate and ammonium
phosphate is recycled
to the extractant purification step, h) the aqueous solution from the
purification step is being recycled into
the extraction step, i) the raffinate from the extraction step and part of the
heavy aqueous phase
containing ammonium nitrate and ammonium phosphate is treated by distillation
to recover the solvent
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
7
from the aqueous phase, and j) treating the distilled aqueous phase with
ammonia and carbon dioxide to
form ammonium nitrate solution and precipitated calcium carbonate.
Similar to US patent 3,518,071, there are a number of disadvantages with the
method according to the
patent GB 1,238,188. Co-extraction of nitric acid results in precipitation of
calcium phosphates which limits
the yield of extracted phosphoric acid from the leach solution. Furthermore, a
high concentration of
ammonium nitrate is required during neutralization with ammonia in order to
decrease the amount of
ammonium phosphate that remains dissolved in the ammonium nitrate liquor.
Also, the method requires
processing of ammonium nitrate into a final product by water evaporation.
Furthermore, the precipitated
ammonium phosphate contains ammonium nitrate via mother liquor adhering to the
precipitate and
washing of the adhering ammonium nitrate with water results in substantial
dissolution of the precipitated
ammonium phosphate. This requires acidification of the wash water with nitric
acid (to convert dissolved
ammonium phosphate into ammonium nitrate and phosphoric acid) and re-
extraction of phosphoric acid
from the wash water which is costly. Finally, solvents suitable for this
process have a high water solubility
which makes the process complex as the solvent has to be recovered from the
aqueous stream by
distillation.
US patent 4,112,118 describes a process for stripping phosphoric acid from an
organic solvent with a
basic compound of ammonia, sodium or potassium being anhydrous or with water
in an amount of up to
10 moles or with a solid dihydrogen phosphate salt, to give a liquid phase
mixture comprising an organic
solvent phase substantially free of phosphoric acid and an aqueous phase
comprising dissolved
phosphoric acid and dissolved dihydrogen phosphate of the base. One main
disadvantage of the process
is that the stripping process requires that the organic solvent is loaded with
a highly concentrated
phosphoric acid solution of > 60% weight. The process thus requires
concentration of phosphoric acid by
water evaporation, equipment such as evaporators, steam distribution systems,
etc., as well as, an energy
source. Another main disadvantage of the process is that the product is an
aqueous solution which
requires further treatment.
Russian patent 424849 describes a method for production of mono-ammonium
phosphate by using a
solvent composed of tributyl phosphate in a kerosene diluent. The method
comprises contacting the
tributyl phosphate solvent, which is loaded with phosphoric acid, with a
solution of di-ammonium
phosphate at an organic to aqueous phase ratio of about 3:1 to form a depleted
solvent and mono-
ammonium phosphate crystals in a solution of mono-ammonium phosphate. It is
further mentioned that
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
8
the mother liquor can be separated and treated with ammonia to produce a di-
ammonium phosphate
solution which can be recycled.
It was, however, found by many experiments performed by the applicant of the
present invention that
stripping a solvent composed of tributyl phosphate in kerosene with a di-
ammonium phosphate solution
according to the procedure described in Russian patent 424849 results in
severe emulsion formation
when the stripped solvent is re-contacted with phosphoric acid for further
phosphate extraction. The
formation of emulsion makes the process non-operational in industrial
applications where efficient
recirculating of solvent is required.
SUMMARY
A general object of the present invention is to produce fully soluble, pure
and well defined mono or di-
ammonium phosphate avoiding problems with the prior art. An additional object
is to enable production of
solid ammonium phosphates without any need for evaporation of water with
associated equipment such
as evaporators, steam distribution systems, etc., as well as, an energy source
for heat production. Another
object is to enable production of solid ammonium phosphates by use of liquid-
liquid extraction in a robust
way enabling efficient recirculation of process liquids. Additional objects
are discussed in connection with
the different embodiments presented further below.
The above objects are achieved by methods and devices according to the
enclosed independent patent
claims. Preferred embodiments are defined by the dependent patent claims. In
general words, in a first
aspect, an arrangement for production of pure ammonium phosphates comprises an
extraction section, a
stripping section and end treatment arrangements. The extraction section is
configured for performing a
liquid-liquid extraction of phosphate between a feed liquid comprising
phosphoric acid and a solvent. The
solvent has a solubility in water of less than 2%, and preferably below 1%.
The feed liquid is essentially
free from nitrate ions. The extraction section has a first extraction inlet
for provision of the feed liquid, a
second extraction inlet for provision of the solvent, a first extraction
outlet for delivering of the feed liquid at
least partly depleted in phosphate and a second extraction outlet for
delivering of the solvent loaded with
phosphate. The stripping section is configured for performing a liquid-liquid
extraction of phosphate
between the solvent loaded with phosphate and a strip solution. The stripping
section has a first stripping
inlet, connected to the second extraction outlet, for provision of the solvent
loaded with phosphate, a
second stripping inlet for provision of input strip solution, a first
stripping outlet for delivering the solvent at
least partly depleted in phosphate and a second stripping outlet for
delivering output strip solution. The
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
9
first stripping outlet is connected to the second extraction inlet for
recirculating the solvent at least partly
depleted in phosphate for further extraction of phosphate. The strip solution
is an aqueous ammonium
phosphate solution, wherein at least 80% of the ammonium phosphate in the
input strip solution is
monoammonium phosphate and/or wherein the solvent is a water-immiscible
alcohol. The end treatment
arrangements comprise a source of ammonia, an adding arrangement, a cooling
arrangement, a
precipitate remover and a recirculation system. The adding arrangement is
connected to the source of
ammonia and is configured for adding ammonia from the source of ammonia into
at least a partial stream
of the strip solution. The cooling arrangement is configured for cooling off
heat generated from the
chemical reaction when the ammonia from the source of ammonia is added into
the at least a partial
stream of the strip solution. The precipitate remover is configured for
separating crystals from the loaded
strip solution. The recirculating system is connected between an outlet from
the precipitate remover and
the second stripping inlet of the stripping section. The recirculating system
is configured for reusing strip
solution from the precipitate remover as input strip solution.
In a second aspect, a method for production of pure ammonium phosphates
comprises extracting of
phosphate from a feed liquid comprising phosphoric acid by a liquid-liquid
extraction into a solvent. The
solvent has a solubility in water of less than 2% and preferably less than 1%.
The feed liquid is essentially
free from nitrate ions, The solvent is stripped of at least a part of the
phosphate by a liquid-liquid extraction
into a strip solution. The strip solution is an aqueous ammonium phosphate
solution. The strip solution
loaded with stripped phosphate and the solvent at least partially depleted in
phosphate are separated.
The solvent at least partly depleted in phosphate is recirculated for further
extraction of phosphate in the
extracting. At least 80% of the ammonium phosphate in the input strip solution
is monoammonium
phosphate and/or the solvent is a water-immiscible alcohol. Ammonia is added
into at least a partial
stream of the strip solution. Heat generated when said ammonia is added into
said at least a partial
stream of said strip solution is cooled off. Crystals are removed from the
loaded strip solution. The strip
solution is recirculated after the step of removing crystals for use as strip
solution input in the stripping
process.
One advantage with the present invention is that well defined fully soluble,
pure and well defined mono or
di-ammonium phosphate are possible to produce in an industrially applicable
process in an efficient and
economic manner. Additional advantages are discussed in connection with the
different embodiments
presented further below.
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
BRIEF DESCRIPTION OF THE DRAWINGS
The invention, together with further objects and advantages thereof, may best
be understood by making
reference to the following description taken together with the accompanying
drawing and tables in which:
5 FIG. 1 is a flow diagram of steps of an embodiment of a method for
production of ammonium
phosphates;
FIG. 2 is a block scheme of an embodiment of an arrangement for production of
mono-ammonium
phosphate;
FIG. 3 is a block scheme of an embodiment of an arrangement for production of
di-ammonium
10 phosphate;
FIG, 4 is a block scheme of an embodiment of an arrangement for production of
both mono-
ammonium phosphate and di-ammonium phosphate;
FIG. 5 is a block scheme of another embodiment of an arrangement for
production of both mono-
ammonium phosphate and di-ammonium phosphate;
FIG. 6 is a block scheme of another embodiment of an arrangement for
production of di-
ammonium phosphate;
FIG. 7 is a block scheme of another embodiment of an arrangement for
production of mono-
ammonium phosphate; and
FIG. 8 is a block scheme of yet another embodiment of an arrangement for
production of both
zo mono-ammonium phosphate and di-ammonium phosphate.
DETAILED DESCRIPTION
Some often used terminology in the present disclosure is to be interpreted as
follows:
Solvent ¨ A liquid phase, typically organic, which preferentially dissolves
extractable solute species from
an aqueous solution.
Extractant¨ An active component, typically organic, of a solvent enabling
extraction.
Solvent extraction (liquid liquid extraction) ¨ The separation of one or more
solutes from a mixture by
mass transfer between immiscible phases in which at least one phase typically
is an organic liquid.
Stripping ¨ The displacement from the solvent of the ions or acids removed
from the process solution to
make the solvent ready for reuse.
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
11
Diluent ¨ A liquid, typically organic, in which an extractant is dissolved to
form a solvent.
Raffinate ¨ An aqueous phase from which a solute has been removed by
extraction.
The present disclosure is based on extraction of phosphoric acid with a
water¨immiscible or at least
substantially water-immiscible solvent. Several water-immiscible solvents are
suggested in the literature
as suitable for extraction of phosphoric acid.
The suggested solvents can generally be divided into the following groups: a)
alkyl phosphates such as
tributyl phosphate, b) amines such as tri-n-octylamine, c) alcohols such as
isoamyl alcohol, n-amyl
alcohol, cyclohexanol, methyl cyclohexanol, tertiary amyl alcohol, isobutanol,
n-butanol, heptanol, d)
ketones such as methyl-isobutyl ketone, methyl propyl ketone, diethyl ketone,
methyl ethyl ketone, methyl-
n-butyl ketone, e) amides such as butyl acetamide, f) aldehydes such as
benzaldehyde, g) esters such as
ethyl acetate, butyl acetate, amyl acetate, cyclohexanone, h) ethers such as
diethyl ether, di-n-amyl ether,
and glycol ethers such as di-ethylene glycol.
All the above mentioned solvents are classified as water-immiscible. However,
most of the mentioned
solvents have in fact a relatively high solubility in water which may result
in contamination of both aqueous
streams and the final product with traces of the solvent. High water
solubility usually requires recovery of
dissolved solvent from aqueous steams by distillation which is costly and
complex. For example, n-butanol
has a water solubility of ca 90 grams per liter at room temperature. Several
of the mentioned solvents
have other disadvantages in addition to high solubility such as flammability
and subjection to explosion
risk, e.g. di-isopropyl ether.
Furthermore, several of the mentioned solvents show very little extraction
capacity for phosphoric acid
below a specific threshold concentration. This means that the feed phosphoric
acid must initially have a
high concentration, which usually requires concentration of the acid by water
evaporation. In addition, only
partial extraction of phosphoric acid is possible with such solvents. A
typical example for a solvent with
threshold concentration for phosphoric acid extraction is methyl isobutyl
ketone. In general, ethers, esters
and selected ketones have a threshold concentration for phosphoric acid
extraction and are therefore
unsuitable for extracting phosphate from low concentration sources.
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
12
Tributyl phosphate is commonly used in the phosphate industry for purification
of phosphoric acid by
liquid-liquid extraction. Tributyl phosphate is non-flammable, has low
toxicity and very low solubility in
water of ca 0.4 grams per liter at room temperature. Furthermore, the
solubility decreases with increasing
temperature. Tributyl phosphate has also a reasonably constant distribution
coefficient, i.e. ability for
extracting phosphoric acid, down to low phosphoric acid concentration. Due to
the relatively high density
of tributyl phosphate it is commonly mixed with a diluent, such as, aliphatic
kerosene in order to improve
the physical separation of the immiscible phases.
Tributyl phosphate enables extraction of phosphoric acid in preference to
dissolved salts such as chlorides
or sulfates, and in preference to dissolved acids such as hydrochloric acid or
sulfuric acid. The presence
of dissolved salts or acids enhances extraction of phosphoric acid by a
salting out mechanism which can
enable almost complete extraction of phosphoric acid.
However, tributyl phosphate extracts nitric acid in preference to phosphoric
acid which makes selective
extraction of phosphoric acid from nitric acid impossible. In general,
solvents, which have a low solubility
in water, extract nitric acid in preference to phosphoric acid. Solvents with
increased selectivity for
phosphoric acid such as amyl alcohol have high water solubility and still co-
extract considerable amounts
of nitric acid. The main advantage of using tributyl phosphate as a solvent
for extraction of phosphoric
acid is that the low water solubility enables to operate without a need for
distilling the solvent from
aqueous streams which is costly and complex.
It was mentioned above that most of the suitable solvents have a relatively
high solubility in water, e.g.
most alcohols. However, alcohols having relatively long carbon chains also
have a relatively low water
solubility. An alternative solvent for extracting phosphoric acid is therefore
long carbon chain alcohols, e.g.
heptanol, having a solubility in water of less than 2%, and preferably less
than 1%. If the solubility is lower
than such a level, the amount of solvent following the water stream becomes
reasonably low to be taken
care of by not too expensive and complex arrangements.
The Russian patent 424849 mentioned in the background section proposed the use
of a solvent
composed of tributyl phosphate in a kerosene diluent. However, in many
experiments performed by the
present inventors, it was concluded that the process according to Russian
patent 424849 has major
drawbacks which makes it practically non-operational and thus not industrially
applicable in an economical
aspect. As mentioned further above, it was found that stripping a solvent
composed of tributyl phosphate
in kerosene with a di-ammonium phosphate solution according to the procedure
described in Russian
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
13
patent 424849 results in severe emulsion formation when the stripped solvent
is re-contacted with
phosphoric acid solution, e.g. reused in a repeated extraction-stripping
process.
The cause for the emulsion formation was further investigated. It was found
that after contacting a solvent
composed of tributyl phosphate in kerosene with solutions of di-ammonium
phosphate of varying
concentrations, very small crystals were found in the solvent. The size of the
crystals was so small that
they are only visible with a microscope. The tested concentrations were
according to the Russian patent
424849, as well as higher or lower concentrations, and at a wide range of
phase ratios.
It was further found that it took generally more than 24 hours for the micro-
crystals to settle and
sometimes more than 48 hours. After the crystals have settled, the solvent can
again be reused for
extraction of phosphoric acid without formation of any emulsion. The existence
of the crystals in the
stripping section thus caused the emulsion problems in the extraction section,
when the solvent was
recycled. In other words, a surprising formation of micro-crystals in a later
stage manufacturing process
induced problems in an earlier stage manufacturing process, mediated by the
recycling of the solvent. The
separation time in an operational liquid-liquid extraction system is usually
less than 20 minutes. The very
long separation time required for settling of the micro-crystals makes the
process practically non-
operational, at least for economically interesting and efficient industrial
applications.
It should be emphasized that the emulsion is not observable in the stripping
section and it is not until the
stripped solvent is reused for further extraction of phosphate that the
emulsion occurs.
Furthermore, testing experiments to remove the micro-crystals by washing with
water, phosphoric acid or
mono-ammonium phosphate solutions were unsuccessful. It was not possible to
dissolve the crystals with
the mentioned aqueous solutions and an emulsion was formed when the solvent
again was contacted with
phosphoric acid, also after extensive washing.
It was further found that phosphoric acid probably was an ingredient in the
micro-crystals since contacting
tributyl phosphate in kerosene which was depleted in phosphoric acid with a di-
ammonium phosphate
solution did not result in any formation of micro-crystals and emulsion was
furthermore not formed upon a
subsequent contact with phosphoric acid. It is therefore believed that the
micro-crystals probably are
composed of ammonium phosphates.
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
14
Addition of dodecanol as a modifier to a tributyl phosphate in kerosene
solvent did not solve the emulsion
problem. Changing the diluent from aliphatic kerosene to an aromatic diluent
or using alcohol such as
heptanol as a diluent did not solve the emulsion problem either.
It was, however, surprisingly found that a solvent composed of tributyl
phosphate in kerosene which is
loaded with phosphoric acid can be stripped with a saturated solution of mono-
ammonium phosphate
without any formation of micro-crystals in the solvent. In the tests solvent
loading of 0.4 ¨ 2 molar H3PO4
was used and the mono-ammonium phosphate was about 3.5 molar at room
temperature. The solvent
can thus immediately after stripping be reused for phosphoric acid extraction
without formation of
emulsion.
In contrast to stripping with a di-ammonium phosphate solution, which is based
on conversion of
phosphoric acid in the solvent to mono-ammonium phosphate, it was found that
when the strip solution is
composed of mono-ammonium phosphate stripping becomes based on an extraction
equilibrium. In other
words, the concentration of residual phosphoric acid in the solvent is
dependent on the concentration of
stripped phosphoric acid in the ammonium phosphate solution. In table 1,
equilibrium concentrations of
phosphoric acid in a solvent composed of 80 volume percent tributyl phosphate
and 20 volume percent
kerosene are shown as a function of phosphoric acid content in saturated mono-
ammonium phosphate
solution. The concentrations are determined during stripping at room
temperature and at an organic to
aqueous phase ratio of 1.
Since stripping with a mono-ammonium phosphate solution is being based on
extraction equilibrium,
complete stripping of phosphoric acid from the solvent will require more than
a single contact stage.
However, from table 1 it can be seen that stripping with a mono-ammonium
phosphate solution is efficient.
At an organic to aqueous phase ratio of 1:1 and a phosphoric acid loading of
1.65 molar, stripping of 86%
of the phosphoric acid content is possible in a single contact stage. From
table 1 it can be further seen
that the stripping process can be used for concentrating phosphoric acid in
the strip solution by using an
organic to aqueous phase ratio larger than 1 during the stripping process. In
preferred embodiments, the
phosphoric acid concentration during the stripping process can be increased
between 2 times up to more
than 5 times of the original concentration.
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
Molar H3PO4 in water-immiscible solvent Molar H3PO4 in saturated NH4H2PO4
0,23 1,42
0,46 2,39
0,78 3,49
1 4,33
Table 1. Equilibrium concentrations of phosphoric acid as a function of
phosphoric
acid content in saturated mono-ammonium phosphate solution.
5 A general advantage of using a mono-ammonium phosphate solution compared
with the use of di-
ammonium phosphate solution is that mono-ammonium phosphate has a lower
viscosity compared to di-
ammonium phosphate, which makes the phase separation more rapid and more
complete. In general, due
to a higher density, stripping with mono-ammonium phosphate was found to be
considerably superior to
conventional stripping with water with regard to separation time and
completeness of the separation.
It was furthermore found that stripping phosphoric acid from a loaded tributyl
phosphate solvent with a
solution of mono-ammonium phosphate of any concentration can be done in any
phase ratio without
formation of precipitates as long as the initial temperature of the mono-
ammonium phosphate solution is
below a level in which the solubility of mono-ammonium phosphate is decreased
by the stripping of
phosphoric acid. This means that the liquid-liquid extraction in most
embodiments is a two-phase
extraction. The temperature of the initial mono-ammonium phosphate solution,
if saturated, should
therefore preferably be below 50 C and most preferably below 40 C.
Furthermore, the temperature of the
mono-ammonium phosphate solution should not be decreased during the stripping
process below a level
set by the difference between the solubility of mono-ammonium phosphate and
its concentration in
solution in order not to form precipitates. However, if the mono-ammonium
phosphate solution is not
= saturated the temperature can also be higher than 50 C.
One advantage of using mono-ammonium phosphate for stripping is that stripping
in most embodiments
involves only two phases, one organic and one aqueous, without formation of
precipitates. This enables to
operate with conventional liquid-liquid extraction equipment such as pulsed-
columns, mixer settlers or any
other liquid-liquid extraction equipment such as, agitated columns, non-
agitated columns, inline mixers,
centrifugal contactors, etc.
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
16
Further investigations were performed on the use of mixtures of mono- and di-
ammonium phosphate for
stripping a solvent composed of tributyl phosphate in kerosene. The results
are summarized in table 2.
Percent of (NI-14)2HPO4 Percent of NH4H2PO4 Emulsion formation
(on molar basis) (on molar basis)
50 50 Yes
33 67 Yes
25 75 Yes
20 80 No
Table 2. Emulsion formation during stripping with mixtures of NH4H2PO4 and
(NH4)2H PO4.
Table 2 shows whether emulsion is formed or not during the stripping of a
solvent composed of 80 volume
percent tributyl phosphate and 20 volume percent kerosene and with a loading
of 0.9 ¨ 1.6 molar H3PO4,
with mixtures of NH4H2PO4 and (NH4)2HPO4. The phosphate concentration in all
solutions used for
stripping was set to 3.5 molar, It can be seen that stripping with mixtures of
mono- and di-ammonium
phosphate results in formation of emulsions, when the stripped solvent
subsequently is reused for
extraction of phosphoric acid, at a di-ammonium phosphate content higher than
25% on molar basis.
Stripping with a solution composed of mono-ammonium phosphate with a di-
ammonium phosphate
content of 20% on molar basis does not result in formation of micro-crystals
in the solvent and an
emulsion is not formed during subsequent extraction of phosphoric acid.
According to some observations
it is believed that at low di-ammonium phosphate content, precipitation takes
place in the aqueous phase.
In other words, phosphoric acid is initially stripped from the solvent to the
aqueous phase and thereafter
crystals of mono-ammonium phosphate are formed in the aqueous phase and
therefore micro-crystals are
not formed in the solvent. At high di-ammonium phosphate content, micro-
crystals are formed directly in
the solvent even if large mono-ammonium phosphate seed crystals are
artificially added during the
stripping process.
Amines such as tri-n-octylamine are water-immiscible solvents with very low
water solubility (< 50 ppm)
which are suitable for extraction of phosphoric acid. Testing tri-n-octylamine
(20% dodecanol as a
modifier, 55% aliphatic kerosene as a diluent) as a solvent for extraction of
phosphoric acid and stripping
with ammonium phosphate gave results which were similar to the results
obtained for tributyl phosphate.
Stripping a loaded amine solvent with a di-ammonium phosphate solution
resulted in severe emulsion
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
17
formation which renders the process non-operational. Stripping a loaded amine
solvent with a mono-
ammonium phosphate solution did not result in any emulsion formation upon
reusing the solvent. Stripping
a loaded amine solvent with a mixture of 50% NH4H2PO4 and 50% (NH4)2HPO4
resulted in emulsion
formation which renders the process non-operational. Whereas stripping a
loaded amine solvent with 80%
NH4H2PO4 and 20% (NH4)2HPO4 did not result in emulsion formation upon reusing
the solvent.
Heptanol is a water-immiscible solvent with relatively low water solubility
which has a reasonably constant
distribution coefficient down to low phosphoric acid concentration. The
solubility in water of heptanol is
about 1 gram per liter which is more than double the water solubility of
tributyl phosphate. In general,
alcohols with larger number of carbon atoms have a lower solubility in water,
but extraction of phosphoric
acid decrease with increased number of carbon atoms. However, the water
solubility of heptanol is still
sufficiently low to enable operation without requirement for distilling the
solvent. A further disadvantage of
heptanol in comparison to tributyl phosphate is that extraction of phosphoric
acid with heptanol is
considerably less efficient. The phosphoric acid loading obtained with
heptanol is about half that obtained
with tributyl phosphate. However, even though heptanol is inferior to tributyl
phosphate it can still be used
for phosphate extraction.
It has also surprisingly been found that stripping a heptanol solvent, loaded
with phosphoric acid, with a
di-ammonium phosphate solution does not result in micro-crystals remaining in
the solvent. The heptanol
solvent can therefore be directly reused for extraction of phosphoric acid
without formation of any
emulsion. There was no correlation between viscosity or density and the
formation of micro-crystals
remaining in the solvent. Amines have a lower density compared to tributyl
phosphate and still emulsion
forms upon stripping with a di-ammonium phosphate solution. Heptanol has a
higher viscosity compared
to tributyl phosphate and emulsion is not formed upon stripping with a di-
ammonium phosphate solution
and re-contacting with phosphoric acid.
Based on the above presented surprising results, an advantageous arrangement
for production of pure
ammonium phosphates can be outlined. The arrangement involves an extraction
section, a stripping
section and end treatment arrangements. The different parts are intimately
interdependent regarding
composition of the used liquids. The extraction section performs a liquid-
liquid extraction of phosphate
between a feed liquid comprising phosphoric acid and a solvent. The solvent
has a solubility in water of
less than 2%. This enables production of ammonium phosphate precipitates with
satisfying levels of
adhering solvent. The feed liquid has furthermore to be essentially free from
nitrate ions, since the suitable
solvents in general extract nitric acid in preference to phosphoric acid. The
stripping section performs a
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
18
liquid-liquid extraction of phosphate between the solvent loaded with
phosphate and a strip solution. The
phosphate depleted solvent is connected back to the extraction section for
further extraction of phosphate.
The strip solution is an aqueous ammonium phosphate solution. According to the
above findings, at least
one of two conditions has to be fulfilled. A first condition is that at least
80% of the ammonium phosphate
in the input strip solution is monoammonium phosphate. The second condition is
that the solvent is a
water-immiscible alcohol.
By such arrangements, a strip solution loaded with phosphate is provided in a
manner suitable for
industrial production. The loaded strip solution is then treated in different
manners in order to obtain well
defined MAP and/or well-defined DAP. Such end treatment arrangements utilize a
source of ammonia. An
adding arrangement is connected to the source of ammonia and adds the ammonia
into at least a partial
stream of the strip solution. Heat is generated by such addition of ammonia
and a cooling arrangement is
therefore provided for removing that heat. The arrangement also comprises a
precipitate remover which
separates crystals of MAP or DAP from the loaded strip solution. Finally, a
recirculating system is
connected between the precipitate remover and the stripping section for
enabling reuse of strip solution
from the precipitate remover as input strip solution in the stripping section.
The present approach disclosed here above enables production of clean and well
defined mono- or di-
ammonium phosphate in one and the same plant without a need for concentrating
phosphoric acid by
water evaporation. This is obtained by a combination of liquid-liquid
extraction and chemical precipitation
by which emulsions and crude formation due to non-settling precipitates are
avoided due to specific
properties of the strip solution and/or solvent. In preferred embodiments,
operational problems such as
formation of precipitates during stripping of the solvent, production of
ammonium phosphate precipitates
with unsatisfying levels of adhering solvent, are additionally avoided.
Fig. 1 illustrates a flow diagram of steps of a method for production of pure
ammonium phosphates. The
procedure begins in step 200. In step 210, phosphate is extracted from a feed
liquid comprising
phosphoric acid by a liquid-liquid extraction into a solvent. The solvent has
a solubility in water of less than
2%. The feed liquid is furthermore essentially free from nitrate ions. The
solvent is in step 212 stripped of
at least a part of the phosphate content by a liquid-liquid extraction into a
strip solution. At least 80% of the
ammonium phosphate in the input strip solution is monoammonium phosphate
and/or the solvent is a
water-immiscible alcohol. The strip solution loaded with stripped phosphate
and the solvent at least
partially depleted in phosphate are separated in step 214. In step 216, the
solvent, at least partly depleted
in phosphate, is recirculated for further extraction of phosphate in the
extracting step 210.
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
19
In step 220, ammonia is added into at least a partial stream of the strip
solution. In step 222, heat
generated when the ammonia is added into the at least a partial stream of the
strip solution is cooled off,
entirely or partly. Crystals from the loaded strip solution are removed in
step 224. In the figure, the steps
220-224 are illustrated as being subsequent steps. However, since they
describe different circulating
processes, their relative dependencies in time and process liquids may differ
from embodiment to
embodiment. All steps 220-224 are, however, performed in all embodiments in
one way or another. In step
226, the strip solution is recirculated after the step of removing the
crystals 224 for use as strip solution
input in the step of stripping 212. The procedure ends in step 299.
A number of embodiments of the present ideas will be presented here below in
order to describe the
advantages and variety possibilities.
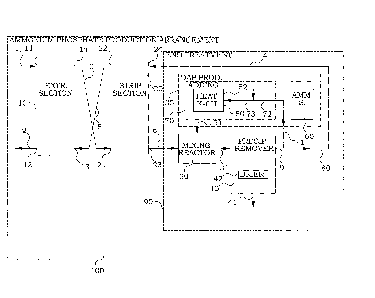
An embodiment of an arrangement 100 for production of pure ammonium
phosphates, in this embodiment
mono-ammonium phosphate, is illustrated in detail in Fig. 2. A phosphoric acid
containing feed liquid 1 is
fed to an extraction section 10 configured for performing a liquid-liquid
extraction of phosphate between
the feed liquid 1 and a solvent 5. The solvent 5 is water-immiscible and has a
solubility in water of less
than 2%. The water-immiscible solvent 5 is in this particular embodiment
tributyl phosphate in aliphatic
kerosene.
The phosphoric acid feed liquid 1 is typically obtained by digesting a
phosphorus containing material with
a mineral acid. The phosphorus containing material can be rock phosphate or
other phosphorus
containing material such as phosphorus rich ashes such as ash of incinerated
sewage sludge, ash of
incinerated slaughterhouse waste, ash of manure, etc. The mineral acid used
for digestion should
preferably be of sulfuric acid, hydrochloric acid or phosphoric acid in order
to obtain a selective extraction
of phosphoric acid.
The concentration of phosphoric acid in the leach solution can be very low,
such as below 7% P205 or
even below 4% P205. Dilute phosphoric acid solutions are typical for sludge
ash leach solutions. When
processing dilute leach solutions it is an advantage that the solution also
contains dissolved salts or acids
which are not extracted in preference to phosphoric acid but have a salting
out effect which enables
almost complete extraction of phosphoric acid at low concentrations. Leach
solutions with higher
phosphoric acid concentration can, of course, be processed according to the
invention. There is no limit
on the maximum concentration of phosphoric acid.
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
In alternative embodiments, the phosphoric acid feed liquid 1 can be provided
in other ways. The
particular way in which the phosphoric acid feed liquid 1 is provided does not
substantially influence the
main ideas in the present disclosures, as long as the feed liquid is
essentially free from nitrate ions, as
5 was discussed further above.
Since the main object of the ideas presented in the present disclosure is to
enable production of
ammonium phosphates without any need for concentrating phosphoric acid by
water evaporation, it is
obvious and most beneficial that the concentration of phosphoric acid is the
maximum concentration
10 practically possible to obtain by digestion with an acid. Phosphate rock
digestion with sulfuric acid
according to the di-hydrate process typically results in a phosphoric acid
concentration of about 28%
P205. The aqueous leach solution is optionally pretreated to remove ionic
compounds such as iron,
fluorine, etc.
15 The extraction section 10 has a first extraction inlet 11 for provision of
the feed liquid 1, and a second
extraction inlet 13 for provision of the solvent 5. The extraction section 10
has also a first extraction outlet
12 for delivering of the raffinate or feed liquid 2 at least partly depleted
in phosphate, and a second
extraction outlet 14 for delivering of the solvent 3 loaded with phosphate.
20 As previously discussed, any organic solvent capable of removing phosphorus
from aqueous solutions
can be used. The mechanism of phosphorus extraction can be solvation of
phosphoric acid or both ion
association and solvation. The composition of the organic solvent should be
selected according to the
concentration of the phosphoric acid feed, presence of additional acids or
salts, etc. in order to obtain a
high loading capacity and an effective operational extraction process.
Tributyl phosphate in aliphatic
kerosene is used as a preferred solvent in the present embodiment.
The temperature of the water-immiscible solvent 5 is preferably below 60 C
since lower temperatures
generally favor phosphoric acid extraction.
The liquid-liquid extraction process in the extraction section 10 is
preferably a continuous liquid-liquid
extraction process using preferably liquid-liquid extraction equipment such as
pulsed-columns. However,
any other liquid-liquid extraction equipment can be used such as, agitated
columns, non-agitated columns,
mixer settlers, inline mixers, centrifugal contactors, etc.
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
21
The raffinate 2, i.e. the feed liquid which is at least partially depleted in
phosphate, is led to further
treatment such as reuse for dissolution, etc.
The water-immiscible solvent 3 which is loaded with phosphoric acid is
optionally scrubbed with an
aqueous solution to remove co-extracted impurities.
The loading of the solvent 3 depends on the concentration of phosphoric acid
in the feed liquid 1,
concentration of dissolved salts and acids, phase ratio of input water-
immiscible solvent 5 to feed liquid 1,
as well as, number of contact stages during extraction in the extraction
section 10. A special advantage
with the present embodiment is that high yield of ammonium phosphate
production can be obtained even
with very low loading of phosphoric acid in the solvent 3. The phosphoric acid
loading in the solvent 3 can
be below 2% P205 and still high yield of solid ammonium phosphate can be
obtained by increasing the
concentration of phosphoric acid during the stripping process. Of course, the
loading of the solvent 3 can
be higher. A typical feed solution obtained from digestion of rock phosphate
with sulfuric acid according to
the di-hydrate process usually results in a phosphoric acid loading of ca 6%
P2O5 in the water-immiscible
solvent 3.
Returning to Fig. 2, the phosphorus-loaded water-immiscible solvent 3 is
thereafter provided to a stripping
section 20. The stripping section 20 is configured for performing a liquid-
liquid extraction of phosphate
between the solvent 3 loaded with phosphate and a strip solution 4. The strip
solution 4 is an aqueous
ammonium phosphate solution. In the present embodiment, substantially all the
ammonium phosphate in
the input strip solution 4 is monoammonium phosphate.
The stripping section 20 has a first stripping inlet 21, connected to the
second extraction outlet 14, for
provision of the solvent 3 loaded with phosphate. The stripping section 20
also has a second stripping
inlet 24 for provision of input strip solution 4. The stripping section 20
furthermore has a first stripping
outlet 22 for delivering the solvent 5 at least partly depleted in phosphate,
and a second stripping outlet 23
for delivering output strip solution 6. The first stripping outlet 22 is
connected to the second extraction inlet
13 for recirculating the solvent 5 at least partly depleted in phosphate for
further extraction of phosphate in
the extraction section 10.
The stripping section 20 is preferably a continuous liquid-liquid extraction
process using preferably liquid-
liquid extraction equipment such as pulsed-columns or mixer settlers. However,
any other liquid-liquid
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
22
extraction equipment can be used such as, agitated columns, non-agitated
columns, inline mixers,
centrifugal contactors, etc.
When producing mono-ammonium phosphate according to the present embodiment,
the ammonium
phosphate solution 4 is preferably a recycled mono-ammonium phosphate
solution, e.g. having a
concentration of about 30 percent by weight and a pH of about 3.5.
In cases in which the phosphoric acid loading in the solvent is low, the
phosphoric acid concentration is
increased during the stripping process by using an organic to aqueous phase
ratio larger than 1. This
requires several contact stages for obtaining complete stripping of phosphoric
acid. The phosphoric acid
concentration during the stripping process can usually be increased between 2
times up to more than 5
times of the original concentration. The output strip solution 6 is in the
present embodiment composed of a
mixture of NH4H2PO4 and H3PO4 in the case of using a solution of NH4H2PO4 for
stripping. The output
strip solution 6 is provided to a number of end treatment arrangements 90.
The end treatment arrangements 90 of the present embodiment comprise a source
of ammonia 60.
Furthermore, an adding arrangement 70 is connected to the source of ammonia
60. The adding
arrangement 70 is configured for adding ammonia from the source of ammonia 60
into at least a partial
stream 71 of the strip solution. In this embodiment, a part of a strip
solution 9 exiting a precipitate remover
40, which will be further described below, is deviated and the ammonia from
the source of ammonia 60 is
added to that partial stream 71. In the present embodiment, the partial stream
71 comprises a solution of
mono-ammonium phosphate 72, and by adding the ammonia, the solution is
transferred into a solution of
di-ammonium phosphate 73.
Addition of ammonia to the partial stream 71 of aqueous ammonium phosphate
solution results in heat
generation. This process is thus an exothermic process and in the present
embodiment used for
production of mono-ammonium phosphate, the resulting ammoniated solution 73 is
composed of a
mixture of NH4H2PO4 and (NH4)2HPO4, which furthermore becomes heated. A
cooling arrangement 50 is
therefore provided, configured for cooling off heat generated when the ammonia
from the source of
ammonia 60 is added into the partial stream 71 of the strip solution. This
heat of neutralization is
preferably removed by a heat exchanger 52. A cooled solution of a mixture of
mono-ammonium
phosphate and di-ammonium phosphate 51 leaves the cooling arrangement 50.
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
23
As will be discussed further below, the solution of a mixture of mono-ammonium
phosphate and di-
ammonium phosphate 73 is preferably cooled more than what is given by the
addition of ammonia.
Addition of ammonia to a saturated NH4H2PO4 solution 72 results in
considerable increase in the solubility
limit of the ionic species in solution as long as the ammonia addition is up
to a certain level. This enables
to cool the ammonium phosphate solution 51 to temperature below the
temperature of the strip solution 6
without formation of any precipitates. Heat exchange can thus be performed
without scale formation on
heat exchangers.
According to the literature it is known that neutralization of phosphoric acid
to di-ammonium phosphate
results in a higher heat release (1510 kcal/kg gaseous NH3 reacted, or 990
kcal /kg liquid NH3 reacted)
compared with neutralization of mono-ammonium phosphate to di-ammonium
phosphate (1130 kcal/kg
gaseous NH3 reacted, or 610 kcal /kg liquid NH3 reacted). This means that by
adding ammonia to a
saturated mono-ammonium phosphate solution, total heat generation is lower
compared to production of
mono-ammonium phosphate by direct neutralization of phosphoric acid with
ammonia.
In addition, according to a preferred embodiment according to Fig. 2, heat
exchange can be performed by
using ammonia refrigeration. In that manner the cooling effect of converting
liquid ammonia into gaseous
ammonia can be used for removing heat of neutralization. Obtained gaseous
ammonia can then be used
as the ammonia feed. Of course, heat exchange can be performed by exchanging
heat with other liquids
such as water or gases such as air.
Addition of ammonia should preferably be proportional to the phosphate loading
in the water immiscible
solvent 3. Conductivity decreases and pH level increases with decreasing
concentration of phosphoric
acid in the solvent. Addition of ammonia can thereby be controlled by
monitoring pH and/or conductivity in
the solvent 3 with a suitable sensor. Another alternative is to monitor pH
and/or conductivity in the strip
solution 6 or in the reuse circuit before or after addition of ammonia with
suitable sensors.
In alternative embodiments, it is possible to add ammonia from the source of
ammonia directly to the strip
solution 6 in order to form mono-ammonium phosphate from the remaining
phosphoric acid. However,
neutralization of phosphoric acid with ammonia is highly exothermic and
results in significant heat
formation which then must be removed in the process. The heat generation is
about 20 C per
neutralization of 1 molar phosphoric acid to mono-ammonium phosphate with
liquid ammonia and about
25 C per neutralization of 1 molar phosphoric acid to mono-ammonium phosphate
with gaseous
ammonia. Direct neutralization of a strip solution 6, containing about 3.5
molar phosphoric acid, results in
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
24
a temperature increase of about 88 C when using gaseous ammonia. The
solubility of ammonium
phosphates is very temperature dependent. For example, the solubility of mono-
ammonium phosphate at
20 C is about 40 g per 100 ml water and at 100 C the solubility increase to
about 170 g per 100 ml water.
This implies that if ammonia is added directly to the strip solution 6
containing phosphoric acid, there is no
precipitation of ammonium phosphates due to a higher solubility at a higher
temperature. It is indeed
possible to cool the strip solution 6 after addition of ammonia by heat
exchange. However, since the
solubility of ammonium phosphates is temperature dependent this procedure may
result in crystallization
of ammonium phosphates upon heat exchangers which reduces heat exchange
efficiency and requires
frequent scale removal which is unwanted and makes it difficult to operate
continuously.
Therefore, preferably, according to the embodiment of Fig. 2, spontaneous
precipitation of mono-
ammonium phosphate is instead caused upon mixing the strip solution 6 with the
recycled and cooled di-
ammonium phosphate solution 51 in a mixing reactor 30. It was found that if
the strip solution 6 and the
recycled di-ammonium phosphate solution 51 have the same temperature, the
temperature increase
during precipitation of mono-ammonium phosphate is only about 2 C per molar
precipitated phosphate.
For a phosphoric acid concentration of 3.5 molar in the strip solution 6, the
temperature increase is only
about 7 C in the mixing reactor 30. Compared with a temperature increase of
about 88 C for neutralizing
the strip solution directly with gaseous ammonia, such a temperature increase
is easily handled. As
mentioned above, the recycled and cooled di-ammonium phosphate solution 51 can
be controlled to have
a temperature which is lower than the temperature of the strip solution 6,
which can compensate for the
temperature increase during precipitation of mono-ammonium phosphate, obtained
by combining the strip
solution 6 with the recycled di-ammonium phosphate solution 51.
In other words, the cooling arrangement is configured for maintaining a
temperature of the loaded strip
solution below a saturation temperature for monoammonium phosphate, whereby
crystals of
monoammonium phosphate are precipitated. The cooling is preferably performed
in a stream of
ammonium phosphate in which precipitation does not occur. In a preferred
embodiment, the adding
arrangement 70 comprises the cooling arrangement 50. The actual cooling can
also be performed in
different ways. The cooling arrangement 50 can e.g. operate on the liquid
stream into which the ammonia
is going to be added, i.e. before the mixing. The cooling arrangement 50, can
alternatively or in
combination also operate on the liquid stream into which the ammonia has been
added. Finally, also the
ammonia that is going to be added may be cooled. The cooling arrangement 50 is
typically a heat
exchange device 52. However, any other alternative cooling arrangements 50 can
also be utilized, such
as Peltier elements, cooling by heat conduction etc.
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
In this way, according to the present embodiment, precipitation of mono-
ammonium phosphate occurs in
the mixing reactor 30 without any requirement of cooling the mixing reactor 30
itself. In such a way,
crystallization of ammonium phosphate upon heat exchangers is avoided, which
improves the heat
5 exchange efficiency of the process and makes continuous operation easy and
robust without requirement
for frequent scale removal from heat exchangers.
The slurry 31 exiting the mixing reactor 30 is composed of NH4H2PO4 crystals
in a saturated NH4H2PO4
solution. The slurry 31 exiting the mixing reactor 30 is fed to the earlier
mentioned precipitate remover 40.
10 The precipitate remover 40 is configured for separating crystals 41 from
the loaded strip solution 31 exiting
the mixing reactor 30. Separation can be done by any solid-liquid separation
technique such as filtration,
settling, centrifugation, etc.
In other words, end treatment arrangements 90 comprise a diammonium phosphate
supplying
15 arrangement 35 and a mixing reactor 30. The mixing reactor 30 is connected
to the second stripping outlet
23 of the stripping section 20 and the diammonium phosphate supplying
arrangement 35. The mixing
reactor 30 is configured for mixing a diammonium phosphate solution into the
output strip solution 6 from
the stripping section 20. In this embodiment, the cooling arrangement 50 is
configured for maintaining a
temperature of the solution 31 leaving the mixing reactor 30 below a
saturation temperature for
20 monoammonium phosphate. This thereby causes crystals of monoammonium
phosphate to precipitate
from a saturated monoammonium phosphate solution. The precipitate remover 40
is therefore in this
embodiment configured for separating crystals of monoammonium phosphate. The
diammonium
phosphate supplying arrangement 35 comprises in this embodiment the adding
arrangement 70. The
adding arrangement 70 comprises in this embodiment an inlet connected to the
outlet from said
25 precipitate remover 40 for supply of the partial stream 71 of the strip
solution 9 exiting the precipitate
remover 40. The adding arrangement is configured for adding the ammonia from
the source of ammonia
60 into the partial stream 71 of the strip solution exiting the precipitate
remover 40, thereby forming a
solution comprising diammonium phosphate. The adding arrangement is further
configured for returning
the solution comprising diammonium phosphate to the diammonium phosphate
mixing reactor 30. The
cooling arrangement is configured for maintaining a temperature of the loaded
strip solution below a
saturation temperature for monoammonium phosphate, whereby crystals of
monoammonium phosphate
are precipitated.
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
26
The separated crystals can be dried in a drier 42 and/or granulated according
to known processes forming
a final mono-ammonium phosphate product. Since the adhering solution is a
saturated mono-ammonium
phosphate solution, the water content in the separated crystals is low. The
separated crystals can also be
mixed with other ingredients such as nitrogen and potassium forming different
fertilizer products.
It was found that residual solvent in the precipitated crystals, according to
the present embodiment, is very
low since it only corresponds to dissolved solvent in the solution adhering to
the separated crystals. The
water solubility of water-immiscible solvents such as tributyl phosphate is
low which results in solvent
levels below 20 ppm in the precipitated crystals after separation.
Furthermore, according to a further embodiment, it is possible to remove
traces of dissolved solvent from
the aqueous solution before the precipitation of ammonium phosphates. This can
be done by adding an
oxidizer such as hydrogen peroxide, etc. in order to oxidize traces of solvent
to carbon dioxide and
phosphoric acid. In such a manner it is possible even to eliminate the
presence of residual solvent in the
product. In addition, also if other contaminants such as fluorine etc. are co-
extracted with phosphoric acid
they can be removed from the strip solution by precipitation, extraction, etc.
before precipitation of
ammonium phosphates.
Returning to the embodiment of Fig. 2, the aqueous ammonium phosphate solution
9 exiting the
precipitate remover 40 is split into two parts. One partial stream of the
aqueous mono-ammonium
phosphate solution 4 is recycled back to the second stripping inlet 24 of
stripping section 20. The
arrangement thereby comprises a recirculating system 80 connected between an
outlet from the
precipitate remover 40 and said second stripping inlet 24 of said stripping
section 20, where the
recirculating system 80 is configured for reusing strip solution 4 from the
precipitate remover 40 as input
strip solution. A second partial stream 71 of the aqueous ammonium phosphate
solution is as mentioned
above ammoniated by adding gaseous or liquid ammonia.
The amount of ammonia that is introduced into the system is entirely used for
creation of the ammonium
phosphate crystals. The losses of ammonia during these processes are very
small. In order to achieve an
equilibrium in the process, the adding arrangement 70 is preferably configured
to add an amount of
substance of ammonia to the adding arrangement 70 that is in dependence of an
amount of substance of
stripped phosphate in the strip solution 6 exiting said second stripping
outlet 23 of the stripping section 20.
The added amount of substance of ammonia is also dependent on the relative
amounts of
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
27
monoammonium phosphate and/or diammonium phosphate that are produced, see
embodiments
described further below.
The following is an example of production of ammonium phosphate according to
the embodiment of Fig.
2. Flows correspond to mono-ammonium phosphate production from sewage sludge
ash in a capacity of
20,000 tons ash per year.
A flow of 12.3 cubic meters per hour of a water-immiscible solvent 5 composed
of 80 volume percent
tributyl phosphate and 20 volume percent kerosene and a flow of 5.3 cubic
meter per hour of a treated
ash leach solution 1 containing 155 kg H3PO4 per cubic meter are fed into a
liquid-liquid extraction section
10 having six contact stages. The outflows from the extraction section are
12.3 cubic meter per hour of a
water-immiscible solvent 3 containing 65 kg H3PO4 per cubic meter and 5.3
cubic meter per hour of a
phosphate depleted raffinate 2. The flow of 12.3 cubic meter per hour of the
loaded water-immiscible
solvent 3 and a flow of 4.1 cubic meter per hour of recycled solution 4
containing 402 kg dissolved
NH4H2PO4 per cubic meter are fed into a second liquid-liquid extractor, the
stripping section 20, having
five contact stages. The outflows from the stripping section 20 are 12.3 cubic
meter per hour of a
phosphate depleted water-immiscible solvent 5 and 4.1 cubic meter per hour of
an aqueous solution 6
containing 191 kg H3PO4 and 402 kg NR4H2PO4 per cubic meter all in dissolved
form.
The flow of 4.1 cubic meter per hour of strip solution 6 and a flow of 2.3
cubic meter per hour of an
ammonium phosphate solution 51 containing 462 kg (NH4)2HPO4 per cubic meter
are fed into the mixing
reactor 30. The outflow from the mixing reactor 30 is 6.4 cubic meters per
hour of slurry composed of
14.4% solid NH4H2PO4 in a solution containing 402 kg dissolved NH4H2PO4 per
cubic meter. The slurry is
fed to a solid-liquid separator as a precipitate remover 40, which separates
920 kg of solid NH4H2PO4
crystals 41 per hour for further processing. Water is added to the separated
liquids in order to compensate
for water adhering to separated crystals.
A flow of 6.4 cubic meters per hour of separated liquids 9 containing 402 kg
dissolved NH4H2PO4 per
cubic meter is divided into two flows. One flow 4 of 4.1 cubic meter per hour
containing 402 kg dissolved
NH4H2PO4 per cubic meter is recycled and used for stripping a water-immiscible
solvent loaded with
phosphoric acid 3. A second flow of 2.3 cubic meters per hour containing 402
kg dissolved NH4H2PO4 per
cubic meter is ammoniated by mixing 114 kg per hour of anhydrous ammonia.
After removal of heat of
neutralization by heat exchange, the flow of 2.3 cubic meters per hour 51 now
containing 462 kg dissolved
(NR4)2HPO4 per cubic meter is fed into the mixing reactor 30 as described
above.
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
28
According to the example above, even though the loading of the solvent 3 is
only 65 kg phosphoric acid
per cubic meter, a yield of 402 kg of solid ammonium phosphate per cubic meter
of strip solution 6 is
obtained at room temperature without cooling the mixing reactor with a heat
exchanger.
The objects with the present embodiment were many. One object was to avoid
operational problems such
as emulsions and crude formation. In particular, the object was to form a
regenerated solvent without
contamination with micro-crystals. An additional object is to provide pure
ammonium phosphates with very
low contamination of adhering water-immiscible solvent. Another object of the
present invention is to
provide a high yield of ammonium phosphates by spontaneous precipitation
without requirement for
cooling during precipitation. Yet another object of the present invention is
to enable improved phase
separation during stripping of phosphoric acid from the solvent. Another
object of the present invention is
to enable recycling of the solvent without need for further treatment by
liquid-liquid extraction or distillation.
Yet another object of the present invention is to provide a cost effective
method for production of
ammonium phosphates without the need for scrubbing ammonia from effluent
vapors. All these objects
were achieved by the embodiment of Fig. 2.
In a comparison with the method according to the Russian patent 424849, the
present embodiment has
additional advantages. Precipitation of mono-ammonium phosphate according to
the Russian patent
424849 is based on formation of a mixture of three phases: solvent, crystals
and aqueous solution.
Formation of precipitates during the stripping process requires the use of
special equipment that can
handle three phases. Conventional liquid-liquid extraction equipment can
therefore not be used.
Furthermore, operation with three phases can promote crude formation which can
lead to operational
problems. In addition, in performed experiments, it was found that separation
of residual solvent from
mono-ammonium phosphate crystals by feeding a mixture of the above mentioned
three phases to a
commercial centrifuge results in too high levels of residual solvent in
crystals (>3000 ppm tributyl
phosphate). Separation of the three phases by gravity results in somewhat
lower residual solvent in
crystals but the purity of the crystals was found to be dependent on the
organic to aqueous volume ratio in
the three phase mixture. With the organic to aqueous volume ratio required
according to Russian patent
424849 for obtaining stoichiometric stripping/precipitation of mono-ammonium
phosphate, the level of
residual solvent in crystals is still too high even when gravity separation is
used (>1000 ppm tributyl
phosphate).
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
29
If di-ammonium phosphate is the desired end product, precipitated mono-
ammonium phosphate can be
converted to di-ammonium phosphate by feeding the precipitated mono-ammonium
phosphate and
ammonia into a reactor containing a di-ammonium phosphate solution according
to the process described
in GB 636,035.
Furthermore, an embodiment of an arrangement for direct production of di-
ammonium phosphate is
illustrated in Fig. 3. The extraction section 10 and the stripping section 20
are the same as presented in
Fig. 2, however, the end treatment arrangements 90 are somewhat modified. The
diammonium phosphate
supplying arrangement 35 provides as previous a solution comprising di-
ammonium phosphate to the
mixing reactor 30 for precipitation of mono-ammonium phosphate. However, the
details of the
diammonium phosphate supplying arrangement 35 are now somewhat different,
which will be discussed
further below. Precipitated mono-ammonium phosphate 41, possibly together with
some remaining
solution of mono-ammonium phosphate, is provided as a slurry to a DAP
conversion reactor 32. Ammonia
from the source of ammonia 60 is fed into the DAP conversion reactor 32. In
other words, a diammonium
phosphate production portion is configured for adding ammonia to a stream
originating from the loaded
strip solution, e.g. via the precipitate remover 40 as in this embodiment, or
direct from the stripping section
20, as discussed in later embodiment. The addition of ammonia results in
precipitation of diammonium
phosphate and provides a slurry 33 of di-ammonium phosphate crystals in a di-
ammonium phosphate
solution. The slurry 33 is cooled by the cooling arrangement 50 by heat
exchange to a temperature
preferably below 90 C. Di-ammonium phosphate crystals 44 are separated from
the mother liquid 45 in a
DAP precipitate remover 43. A first part 46 of the separated di-ammonium
phosphate solution is recycled
to the DAP conversion reactor 32. A second part 51 of the separated di-
ammonium phosphate solution 45
is recycled to the mixing reactor 30 to form precipitated intermediate mono-
ammonium phosphate. In such
a manner, ammonia addition and heat exchange during conversion of mono-
ammonium phosphate into
di-ammonium phosphate is utilized for the production of the intermediate mono-
ammonium phosphate.
The embodiments of Fig. 2 and Fig. 3 can also be combined in such a way that
only a part of the MAP
crystals separated in the precipitate remover 40 is brought into the DAP
conversion reactor 32. The
remaining part remains as MAP. In such a way, it is possible to produce well-
defined mono-ammonium
phosphate and well-defined di-ammonium phosphate in the same plant.
Another embodiment for simultaneous production of well-defined mono-ammonium
phosphate and well-
defined di-ammonium phosphate is illustrated in Fig. 4. Here a partial stream
81 of the strip solution 6
loaded with mono-ammonium phosphate and phosphoric acid is entered into a DAP
mixing reactor 32. A
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
solution or slurry of tri-ammonium phosphate 83 is added. With appropriate
mixing ratios and with an
appropriate temperature, a di-ammonium phosphate slurry 33 will be formed. The
cooling arrangement is
preferably configured for maintaining a temperature of the loaded strip
solution below a saturation
temperature for diammonium phosphate, whereby crystals of diammonium phosphate
are precipitated.
5 The slurry 33 comprises precipitated di-ammonium phosphate crystals as well
as a saturated di-
ammonium phosphate solution. The di-ammonium phosphate crystals 44 are removed
in the DAP
precipitation remover 43, leaving a saturated di-ammonium phosphate solution
45. The saturated di-
ammonium phosphate solution 45 is led to a second adding arrangement 70, in
which ammonium from a
second ammonium source 60 (or from the ammonium source used for the mono-
ammonium phosphate
10 production) is added. A slurry of tri-ammonium phosphate 82 is formed,
which is cooled down in a heat
exchanger 52, to provide the slurry of tri-ammonium phosphate 83 to be used in
the DAP mixing reactor
32.
A drawback with this embodiment is that the di-ammonium phosphate production
part successively will
15 collect volumes of di-ammonium phosphate solutions that are not re-
circulated back to the stripping
section. At the same time, the mono-ammonium phosphate production part will
loose the corresponding
volumes, which have to be replaced. Another embodiment, at least partly
solving that problem is illustrated
in Fig. 5. Here, the diammonium phosphate supplying arrangement 35 connected
to the stream of mono-
ammonium phosphate is omitted. Instead, the diammonium phosphate supplying
arrangement 35 of the
20 di-ammonium phosphate production is used for the supply of diammonium
phosphate 51 to the mixing
reactor 30. To this end, a partial stream 84 of the ammonium phosphate
solution 45, in this embodiment a
solution of diammonium phosphate, leaving a precipitation remover, in this
embodiment the DAP
precipitation remover 43, is re-circulated to be used in the production of
mono-ammonium phosphate.
25 In other words, the diammonium phosphate production portion is configured
for adding ammonia to a
stream comprising monoammonium phosphate. This causes crystals of diammonium
phosphate to
precipitate from a saturated diammonium phosphate solution. The diammonium
phosphate supplying
arrangement comprises a diammonium phosphate reconnection, connecting a part
stream of the
saturated diammonium phosphate solution from the diammonium phosphate
production portion as the
30 supplied diammonium phosphate to the production of monoammonium phosphate.
In a further embodiment, the embodiments of Figs 4 and 5 can be combined, such
that the mixing reactor
30 can have a supply of diammonium phosphate solution from either of the
diammonium phosphate
supplying arrangements 35. The amount of diammonium phosphate solution from
the different parts can
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
31
then be controlled to suit a requested ratio of produced monoammonium
phosphate crystals and
diammonium phosphate crystals.
An additional embodiment of an arrangement for production of di-ammonium
phosphate according to the
present invention is hereby described with reference to Fig. 6. Most parts are
similar as in Fig. 2 and the
differences consist mainly of the type of solutions used in the different
parts of the arrangement. The
phosphoric acid containing feed solution 1 is fed to a liquid-liquid
extraction section 10. The water-
immiscible solvent 5A is in this embodiment a long-chain alcohol, preferably n-
heptanol, in order not to
form severe emulsion during re-use of the solvent for extraction of phosphoric
acid as described earlier.
The temperature of the water-immiscible solvent 5A is preferably below 60 C
since lower temperatures
favor phosphoric acid extraction.
The liquid-liquid extraction section 10 is preferably configured for executing
a continuous liquid-liquid
extraction process using preferably liquid-liquid extraction equipment such as
pulsed-columns. However,
any other liquid-liquid extraction equipment can be used such as, agitated
columns, non-agitated columns,
mixer settlers, inline mixers, centrifugal contactors, etc. The raffinate 2,
which is depleted in phosphate, is
led to further treatment such as reuse for dissolution, etc. The water-
immiscible solvent 3A which is loaded
with phosphoric acid is optionally scrubbed with an aqueous solution to remove
co-extracted impurities.
The phosphorus-loaded water immiscible liquid phase 3A is thereafter mixed
with a recycled di-ammonium
phosphate solution as strip solution 4A in the stripping section 20. As
before, the stripping section 20 is
preferably configured for a continuous liquid-liquid extraction process using
preferably liquid-liquid
extraction equipment such as a mixer settler. However, any other liquid-liquid
extraction equipment can be
used such as, pulsed-columns, agitated columns, non-agitated columns, inline
mixers, centrifugal
contactors, etc.
In contrast to stripping with a solution of mono-ammonium phosphate, which was
found to be based on
extraction equilibrium, stripping with a solution of di-ammonium phosphate is
being based on conversion
of phosphoric acid into a dissolved salt.
The reaction is believed to be the following:
(NH4)2HPO4 + H3PO4 = 2 NH4H2PO4
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
32
Since stripping with a di-ammonium phosphate solution is not based on
extraction equilibrium, complete
stripping can be obtained in a single contact stage. Therefore when using a di-
ammonium phosphate
solution for stripping, the preferred liquid-liquid extraction equipment is a
single mixer settler unit.
Stripping of phosphoric acid from the loaded water-immiscible solvent
according to the present
embodiment is done with recycled di-ammonium phosphate solution 4A preferably
in such a way that the
solubility of mono-ammonium phosphate or di-ammonium phosphate is not
exceeded. This enables to
operate with only two phases during stripping in a mixer settler.
According to the present embodiment, stripping of phosphoric acid from the
loaded water-immiscible
solvent 3A is done with a solution of di-ammonium phosphate 4A by which
precipitation of mono-
ammonium phosphate or di-ammonium phosphate does not occur. It was found that
by controlling the
phase ratio of the solvent 3A to di-ammonium phosphate solution 4A during
stripping to a certain level
precipitation of mono-ammonium phosphate can be completely omitted. The phase
ratio of solvent 3A to
di-ammonium phosphate solution 4A in which precipitation of mono-ammonium
phosphate does not occur
depends on the phosphoric acid loading in the water-immiscible solvent 3A and
the concentration of di-
ammonium phosphate solution 4A. This means that the phase ratio preferably has
to be adapted for each
operational condition.
The ammonium phosphate solution, i.e. the input strip solution 4A, is in a
test setup of this embodiment
composed of recycled di-ammonium phosphate solution having a concentration of
about 40 percent by
weight and a pH of about 8.
Similar to stripping with a solution of mono-ammonium phosphate, stripping
with a solution of di-
ammonium phosphate can be used for increasing the concentration of phosphate
in the strip solution 6
compared to the original concentration in the water-immiscible solvent 3A.
This is done by having an
organic to aqueous phase ratio larger than 1 during the stripping process.
Generally, a higher
concentration of di-ammonium phosphate 4A will enable a higher organic to
aqueous phase ratio during
stripping. A higher di-ammonium phosphate concentration can be obtained by
operating at a higher
temperature. The organic to aqueous phase ratio must not be increased to
levels in which precipitation of
mono-ammonium phosphate occurs during the stripping process.
The phase ratio is calculated for specific operational conditions and
temperature according to the solubility
of the ionic species involved, For a phosphoric acid loading in the water-
immiscible solvent 3A of 1 molar
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
33
and a di-ammonium phosphate concentration 4A of 4 molar, an organic to aqueous
phase ratio of 2:1 is
sufficient for not forming any precipitates.
Returning to Fig. 6, the strip solution 6 is composed of a mixture of NH4H2PO4
and (NH4)2HPO4. The ratio
of (NH4)2HPO4 to NH4H2PO4 in the obtained strip solution 6 is therefore
important since sufficient amount
of (NH4)2HPO4 is required in order not to form precipitates of mono-ammonium
phosphate during the
stripping process in the stripping section 20. The strip solution 6 is mixed
with an ammonium phosphate
slurry comprising (NH4)3PO4 51A. The pH in the mixing reactor 30 is typically
maintained at about pH=8.
The slurry 31A exiting the mixing reactor 30 is composed of (NH4)2HPO4
crystals in a saturated
(NH4)2HPO4 solution. The slurry 31A exiting the mixing reactor 30 is fed to
the precipitate remover 40. In
this embodiment, the precipitate remover 40 is configured for separating the
precipitated di-ammonium
phosphate crystals 44 from the di-ammonium phosphate solution 9A. Separation
can be done by any
solid-liquid separation technique such as filtration, settling,
centrifugation, etc.
The separated crystals can be dried and/or granulated according to known
processes forming a final di-
ammonium phosphate product. The separated crystals can also be mixed with
other ingredients such as
nitrogen and potassium forming different fertilizer products.
The aqueous di-ammonium phosphate solution 9A exiting the precipitate remover
40 is split into two parts
4A and 72A. One part of the aqueous di-ammonium phosphate solution 4A is
recycled back to the
stripping section 20. A second part 72A of the aqueous di-ammonium phosphate
solution is ammoniated
by adding gaseous or liquid ammonia, Addition of ammonia to the aqueous di-
ammonium phosphate
solution 72A results in heat generation and formation of a slurry 73A. The
heat of neutralization is
removed by the heat exchanger 52. The ammoniated slurry 73A is composed of a
mixture of (NH4)2HPO4
and (NH4)3PO4. Addition of ammonia should be proportional to the phosphate
loading in the water
immiscible solvent 3A. The cooled off slurry 51A is provided to the mixing
reactor 30 as described further
above.
In another embodiment, the input strip solution is a mixture of NH4H2PO4 and
(NH4)2HPO4. The input strip
solution has a ratio of N/P and a concentration of phosphate ions prohibiting
crystals of monoammonium
phosphate or diammonium phosphate to precipitate when brought in contact with
the solvent loaded with
phosphate in the stripping section. If the input strip solution mixture has
more than 20% (NH4)2HPO4, the
solvent has to be a water-immiscible alcohol in order to avoid emulsion
formation, as described further
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
34
above. If the input strip solution mixture contains less than 20% (NH4)2HPO4,
any water-immiscible solvent
can be used.
If the input strip solution has a ratio of N/P and a concentration of
phosphate ions causing crystals of
monoammonium phosphate to precipitate when brought in contact with the solvent
loaded with phosphate
in the stripping section, the stripping section has to be designed as a three
phase stripping section. The
precipitate remover is then configured for separating the crystals of
monoammonium phosphate from the
loaded strip solution exiting the stripping section. This is typically the
situation when only (NH4)2HPO4 is
used in the input strip solution. In such a case, a water-immiscible alcohol
has to be used as solvent.
When the input strip solution is a mixture of NH4H2PO4 and (NH4)2HPO4, the
arrangement preferably
comprises a mixing control unit, configured to control the composition of the
input strip solution 4.
Another embodiment which enables production of mono-ammonium phosphate is
illustrated in Fig. 7. In
this embodiment the strip solution 4A is composed of (NH4)2HPO4 and the water-
immiscible solvent 3A is
composed of a long change alcohol such as n-heptanol in order not to form
emulsions during reuse of the
solvent for extraction of phosphoric acid as discussed earlier.
The loaded water-immiscible solvent 3A is stripped with the strip solution 4A
and results in formation of
three phases; stripped solvent 5A, loaded strip solution and precipitated
NH4H2PO4 crystals. The stripped
solvent 5A is separated and is reused for extraction of phosphoric acid. The
aqueous phase leaving the
stripping section, i.e. the strip solution 6B is now composed of the NH4H2PO4
crystals in saturated
NH4H2PO4 solution. A small problem with this embodiment is that the so formed
NH4H2PO4 crystals
contain too high levels of residual solvent, In order to remove such residual
solvent, the strip solution 6B is
mixed with recycled mono-ammonium phosphate solution 26 in a washing volume
25. This washing
enables separation of residual solvent at a higher aqueous to organic phase
ratio and the residual solvent
15 can be re-cycled into the extraction section 10. The slurry of NH4H2PO4
crystals in saturated NH4H2PO4
solution 27 after separation of residual solvent 15 is fed to the precipitate
remover 40. In the precipitate
remover 40 mono-ammonium phosphate crystals 41 are separated from the mother
liquid.
A first part 28 of the separated mono-ammonium phosphate solution 9 is
provided to the adding
arrangement 70 for being ammoniated by addition of ammonia from the source of
ammonia 60. A solution
composed of (NH4)2HPO4 4A is formed. Also this neutralization reaction
generates heat, which is cooled
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
off by the heat exchanger 52 of the cooling arrangement 50. After cooling in
heat exchanger 52, the di-
ammonium phosphate solution 4A is reused for stripping.
A second part 26 of the separated mono-ammonium phosphate solution 9 is reused
for separation of
5 residual solvent, as was described here above.
The three phase stripping section is also applicable in systems where a
mixture of NH4H2PO4 and
(NH4)2HPO4 is used as input strip solution, and where the ratio of N/P and the
concentration of phosphate
ions causing crystals of monoammonium phosphate to precipitate when brought in
contact with the
10 solvent loaded with phosphate in the stripping section. If the molar amount
of NH4H2PO4 is equal or larger
than 4 times the molar amount of (NH4)2HPO4, any water-immiscible solvent can
be used, e.g. tributyl
phosphate in a suitable diluent. Otherwise, a water-immiscible alcohol has to
be used as solvent. In other
words, the loaded water-immiscible solvent 3a is stripped with a strip
solution 4A composed of a mixture
of NH4H2PO4 and (NH4)2HPO4 in which the molar concentration of NH4H2PO4 is 80%
in relation to total
15 molar phosphate if a non-alcohol is utilized as solvent. The stripping
process results in formation of three
phases.
Minor changes may then be made to the arrangement. These are indicated with
dotted lines in Fig. 7. To
this end, a third part 29 of the separated mono-ammonium phosphate solution 9
is allowed to short-cut the
20 adding arrangement, which means that after cooling in heat exchanger 52,
the di-ammonium phosphate
solution is mixed with the third part 29 of the separated mono-ammonium
phosphate solution, forming a
solution 4A composed of a mixture of NH4H2PO4 and (NH4)2HPO4. Preferably, the
adding arrangement
comprises an adding control configured to add a stream of the strip solution
direct from the outlet of the
precipitate remover 40 with a stream of the solution comprising diammonium
phosphate from the heat
25 exchanger 52 to obtain the required N/P ratio at the stripping section 20.
As mentioned above, in case the molar concentration of NI-14H2PO4 is 80% in
relation to total molar
phosphate which is reused for stripping, the solvent may be a non-alcohol
solvent. In such a case, the
problems with residual solvent in the mono-ammonium phosphate crystals are
significantly reduced and
30 the provision of the washing of the crystals can thereby be omitted.
The use of a three phase stripping section 20 can also be combined with
production of either or both of
MAP and DAP. One embodiment is illustrated in Fig. 8. A slurry 85 of
monoammonium phosphate crystals
in a saturated monoammonium solution is taken from the precipitate remover 40
and entered into a DAP
CA 02877271 2014-12-18
WO 2013/191639 PCT/SE2013/050736
36
mixing reactor 32. Ammonia is added and the neutralization heat is cooled off,
in analogy with the
embodiment of Fig. 3. However, in the embodiment of Fig. 8, the diammonium
phosphate solution 45 from
the DAP precipitate remover 43 is here instead returned to be used as strip
solution. If the amount of
diammonium phosphate crystals is too low to maintain the input strip solution,
a stream of
monoammonium phosphate 86 can be taken from the precipitate remover 40, and
ammonia can be added
for turning the solution into a diammonium phosphate solution. Optionally, a
cooling arrangement could be
connected also to such a stream.
If the strip solution is composed of a mixture of NH4H2PO4 and (NH4)2HPO4, a
part 87 of the
monoammonium phosphate solution from the precipitate remover 40 can be mixed
into the input strip
solution without passing the adding arrangement 70. Also here, an adding
control can be provided and
configured to add a stream of the strip solution direct from the outlet of the
precipitate remover 40 with a
stream of the solution comprising diammonium phosphate to obtain the required
N/P ratio at the stripping
section 20.
If the solvent is a non-alcohol, the arrangement for washing the crystals can
also be omitted.
The embodiments described above are to be understood as a few illustrative
examples of the present
invention. It will be understood by those skilled in the art that various
modifications, combinations and
changes may be made to the embodiments without departing from the scope of the
present invention. For
example, parallel production of both mono-ammonium phosphate and di-ammonium
phosphate according
to the principles described above, production of di-ammonium phosphate by
reacting tri-ammonium
phosphate slurry with a strip solution composed of mono-ammonium phosphate and
phosphoric acid, and
production of di-ammonium phosphate in combination with stripping with a di-
ammonium phosphate
solution in which the intermediate is mono-ammonium phosphate. The scope of
the present invention is,
however, defined by the appended claims.