Note: Descriptions are shown in the official language in which they were submitted.
ELECTROLESS NICKEL COATINGS AND COMPOSITIONS AND METHODS
FOR FORMING THE COATINGS
TECHNICAL FIELD
[0002] This application relates to electroless nickel coatings, methods
for forming the
electroless nickel coating, and electroless nickel baths for forming the
electroless nickel
coatings.
BACKGROUND
[0003] Electroless nickel plating is a widely utilized plating process,
which provides a
continuous deposit of a nickel metal or nickel/alloy coating on metallic or
non-metallic
substrates without the need for external electric plating current. Electroless
plating has been
described as a controlled autocatalytic chemical reduction process for
depositing metals. The
process involves a continuous buildup of a nickel coating on a substrate by
immersion of the
substrate in a nickel plating bath under appropriate electroless plating
conditions. The plating
baths generally comprise an electroless nickel salt and a reducing agent. Some
electroless
nickel baths use hypophosphite ions as a reducing agent, and during the
process, the
hypophosphite ions are oxidized to orthophosphite ions, and the nickel cations
in the plating
bath are reduced to form a nickel phosphorous alloy as a deposit on the
desired substrate
surface. As the reaction proceeds, the level of orthophosphite ions in the
bath increases, and
the orthophosphite ions often are precipitated from the plating solutions as
insoluble metal
orthophosphites. Typically, the source of nickel ions in the electroless
plating baths
described in the prior art has included nickel chloride, nickel sulfate,
nickel bromide, nickel
fluoroborate, nickel sulfonatc, nickel sulfamatc, and nickel alkyl sulfonates.
[0004] In order to have a continuous and consistent electroless plating
process, the
reactants must be replenished. The frequency at which additions of the
reactants are made to
the bath depends on how far the concentrations of the reacting species can be
allowed to vary
from their optimum concentrations without adversely affecting the plating
process, or
concurrently the deposit. The electroless plating reaction not only yields a
nickel alloy
CA 2879315 2018-04-24
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-2-
deposit; it also generates by-products, which accumulate in solution. As the
concentration of
the by-products increase, their influence on the plating reaction also
increases.
[0005] Electroless nickel-phosphorous coatings can be chemically treated,
e.g., etched,
to produce black coatings (Ni-P black). These black electroless nickel
coatings can act as
efficient absorbers and be used as very low reflectance coatings in optical
instruments and
sensors. Chemical etching of electroless nickel-phosphorous coatings typically
involves acid
etching of low (1-3% phosphorous) or medium-low (3-6% phosphorous) nickel
phosphorous
alloys. Higher phosphorous content alloys are not suitable because they are
too corrosion
resistant to blacken as result of acid etching.
SUMMARY
[0006] An embodiment described herein relates to an aqueous electroless
nickel plating
bath for forming electroless nickel coatings. The aqueous electroless nickel
plating bath can
include nickel, a hypophosphorous reducing agent, zinc, at least one of a
complexing agent,
chelating agent, and/or pH buffer, and a bismuth stabilizer wherein the bath
is free of a sulfur
compound.
[0007] In some enthodiments, the hypophosphorous reducing agent is selected
from the
group consisting of sodium hypophosphite, potassium hypophosphite, ammonium
hypophosphite, and combinations thereof.
[0008] In other embodiments, the at least one pH buffer, complexing agent,
or chelating
agent can be selected from the group consisting of acetic acid, fonnic acid,
succinic acid,
malonic acid, an ammonium salt, lactic acid, malic acid, citric acid, glycine,
alanine, glycolic
acid, lysine, aspartic acid, ethylene diamine tetraacetic acid (EDTA), and
combinations
thereof. In some embodiments, mixtures of 2 or more of the above pH buffers,
complexing
agents, and/or chelating agents can be used in the electroless nickel plating
bath described
herein.
[0009] In still other embodiments, the nickel can be provided in the bath
in the form of
a water soluble nickel salt. The nickel salt can be selected from the group
consisting of
nickel chloride, nickel bromide, nickel iodide, nickel acetate, nickel malate,
a nickel
hypophosphite and combinations thereof.
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-3-
[0010] In other embodiments, the pII of the electroless nickel plating bath
can be
maintained at about 4.5 to about 5.0, and the bath temperature can be
maintained at about
175 F to about 200 F during plating.
[0011] In still other embodiments, the electroless nickel plating bath can
include about
2 g/1 to about 10 g/1 of nickel, about 20 g/1 to about 35 g/1 of a
hypophosphorous reducing
agent, about 1 g/1 to about 75 g/1 each of the complexing agent, chelating
agent, and/or pH
buffer, about 40 ppm to about 120 ppm zinc, and about 5 ppm to about 30 ppm of
a bismuth
stabilizer.
[0012] In yet other embodiments, the electroless nickel plating bath can
include lactic
acid, acetic acid, malic acid, succinic acid, sodium hypophosphite, ammonium
hydroxide,
nickel, zinc, and ethylenediamine tetraacetic acid (EDTA).
[0013] The electroless nickel plating bath can be used to form an
electroless nickel
deposit or coating on the surface of a substrate by contacting or immersing a
surface of the
substrate in the bath. The pH of the electroless nickel plating bath can be
maintained at about
4.5 to about 5.0, and the bath temperature can be maintained at about 175 F to
about 200 F
during electroless nickel plating of the substrate. The deposit or coating can
have a
phosphorous content of about 8% to about 11%.
[0014] In some embodiments, the electroless nickel coating can be a top
coating that is
plated over a mid-phosphorous (e.g., about 7% to about 9% phosphorous) or a
high
phosphorous (about 9% to about 13% phosphorous) electroless nickel under
coating to form a
duplex or multilayer deposit or coating.
[0015] In some embodiments, the duplex or multilayer, deposit or coating
can then be
etched with an etching agent to provide the coated substrate with a black
surface. The
etchant agent can include an iron blackening agent and an acid. In some
embodiments, the
etchant agent can include ferric sulfate and hydrochloric acid.
[0016] In other embodiments, the duplex or multilayer, deposit or coating
can be
contacted with an electroless copper plating bath to provide an electroless
copper coating
over the duplex or multilayer coating.
[0017] Other embodiments described herein relate to a method for preparing
a
multilayer black electroless nickel coating on a substrate. The method
includes contacting
the substrate with a first electroless nickel plating bath to form a first
electroless nickel
coating on the substrate. The substrate is then contacted with a second
electroless nickel
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-4-
plating bath to form a second electroless nickel coating over the first
electroless coating. The
second electroless nickel plating bath can be different from the first
electroless nickel plating
bath and can include nickel, a hypophosphorous reducing agent, zinc, a bismuth
stabilizer,
and at least one of a complexing agent, chelating agent, or pH buffer. The
second electroless
nickel plating bath is free of a sulfur compound. The second electroless
nickel coating is then
etched with an etchant agent to provide the coated substrate with a black
surface.
[0018] In some embodiments, the first electroless nickel coating can have a
phosphorous content about 7% to about 13% by weight. In other embodiments, the
second
electroless nickel coating can have a phosphorous content of about 8% to about
11%.
[0019] In some embodiments, the first electroless nickel plating bath can
include nickel,
a hypophosphorous reducing agent, at least one of a complexing agent,
chelating agent, or pH
buffer.
[0020] In other embodiments, the at least one pH buffer, complexing agent,
or chelating
agent of the second electroless nickel plating bath can be selected from the
group consisting
of an acetic acid, formic acid, succinic acid, malonic acid, an ammonium salt,
lactic acid,
malic acid, citric acid, glycine, alanine, glycolic acid, lysine, aspartic
acid, ethylene diamine
tetraacetic acid (EDTA), and combinations thereof. In some embodiments,
mixtures of 2 or
more of the above pH buffers, complexing agents, and/or chelating agents can
be used in the
second electroless nickel plating bath described herein.
[0021] In still other embodiments, the nickel can be provided in the second
electroless
plating bath in the form of a water soluble nickel salt. The nickel salt can
be selected from
the group consisting of nickel chloride, nickel bromide, nickel iodide, nickel
acetate, nickel
malate, a nickel hypophosphite and combinations thereof.
[0022] In other embodiments, the pII of the second electroless nickel
plating bath can
be maintained at about 4.5 to about 5.0, and the bath temperature can be
maintained at about
175 F to about 200 F during electroless nickel plating with the second
electroless plating
bath.
BRIEF DESCRIPTION OF DRAWINGS
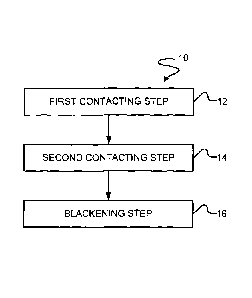
[0023] Fig. 1 illustrates a flow diagram showing a black electroless nickel
plating
process in accordance with an embodiment.
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-5-
[0024] Fig. 2 illustrates a flow diagram showing an electroless copper-
nickel plating
process in accordance with another embodiment.
[0025] Fig. 3 illustrates a graph comparing the rates of deposition per
metal turnover
(MTO) of electroless nickel coatings formed using an electroless nickel
plating bath in
accordance with an embodiment of the application and using a commercially
available
electroless nickel plating bath.
[0026] Fig. 4 illustrates a graph showing the percent phosphorous content
per metal
turnover (MTO) of electroless nickel deposits formed using an electroless
nickel plating bath
in accordance with an embodiment of the application.
[0027] Fig. 5 illustrates a photograph comparing black electroless nickel
coatings
subjected to neutral salt spray formed using an electroless nickel plating
bath in accordance
with an embodiment of the application and using a commercially available
electroless nickel
plating bath.
[0028] Fig. 6 illustrates a photograph showing an electroless copper-nickel
multilayer
coating prepared in accordance with an embodiment.
[0029] Fig. 7 illustrates a photograph comparing various electroless nickel
deposits
coated with an electroless copper coating.
DETAILED DESCRIPTION
[0030] In the specification and the claims, which follow, reference will be
made to a
number of terms, which shall be defined to have the following meanings.
[0031] The singular forms "a", "an" and "the" include plural referents
unless the context
clearly dictates otherwise.
[0032] "Optional" or "optionally" means that the subsequently described
event or
circumstance may or may not occur, and that the description includes instances
where the
event occurs and instances where it does not.
[0033] As used herein, the term "solvent" can refer to a single solvent or
a mixture of
solvents.
[0034] It is also understood that terms such as "top," "bottom," "outward,"
"inward,"
and the like are words of convenience and are not to be construed as limiting
terms.
Furthermore, whenever a particular feature of the invention is said to
comprise or consist of
at least one of a number of elements of a group and combinations thereof, it
is understood
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-6-
that the feature may comprise or consist of any of the elements of the group,
either
individually or in combination with any of the other elements of that group.
[0035] Approximating language, as used herein throughout the specification
and
claims, may be applied to modify any quantitative representation that could
permissibly vary
without resulting in a change in the basic function to which it is related.
Accordingly, a value
modified by a term or totals, such as "about", is not to be limited to the
precise value
specified. In some instances, the approximating language may correspond to the
precision of
an instrument for measuring the value.
[0036] Embodiments described herein relate to electroless nickel plating
baths used to
form electroless nickel coatings on a substrate, methods of foiming multilayer
electroless
nickel coatings on a substrate, and methods of forming black electroless
nickel coatings.
[0037] The electroless nickel plating bath used to form the electroless
nickel coatings,
multilayer electroless nickel coatings, and/or black electroless nickel
coatings described
herein is free of sulfur compounds, such as organic sulfur compounds, and can
form a sulfur-
free electroless nickel coatings, which can be uniformly etched or blackened
to provide a
black electroless nickel coating. The electroless nickel plating bath replaces
sulfur
compounds, which have been typically employed in solutions for forming black
electroless
nickel coatings, with metallic containing compounds, such as zinc and bismuth.
Replacement
of the sulfur compounds with the metallic containing compound allows for
improvements in
operability, stability, and unifoimity over current black electroless nickel
plating methods.
[0038] Sulfur containing electroless nickel plating baths used to form
black electroless
nickel coatings require the addition of spent components (e.g., nickel) at
precise moments
(e.g., additions made at last 5-10 minutes of plating to insure sulfur
codeposition) in order to
achieve a repeatable coloring effect. If the timing is not precise, the
resulting effect will alter
the black coloring to the point of possibly failing to produce the desired
color. The proposed
compositions, baths, and methods require no such addition, besides the normal
addition to
replenish spent components, as per the nounal operation of an electroless
nickel plating bath.
[0039] Electroless nickel plating baths having a sulfur based chemistry
also rely on the
codeposition of the sulfur directly into the electroless nickel deposit. The
result is often an
uneven distribution of the sulfur across the substrate surface, resulting in a
streaky, non-
uniform coloring. The electroless nickel plating baths described herein do not
rely on the
presence of sulfur for co-deposition, and instead rely on the concentration of
the available
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-7-
bismuth to allow for blackening of the electroless nickel plating deposit when
exposed to an
etchant. The presence of organic-sulfur compounds can cause decomposition of
the sulfur
compound at cathode surfaces forming very small particles of nickel sulfide
which in turn
adversely affects ductility, internal stress, electrical conductivity and
corrosion resistance of
the deposit. The breakdown of the organic-sulfur compounds can result in
byproducts that
accumulate within the electroless nickel plating bath, which interferes with
the deposition of
the sulfur. This limits the potential age of the bath to 2 metal turnovers
(MT0s) before issues
begin to arise. The sulfur-free or non-sulfur containing electroless nickel
plating baths have
no such limitation, and can be plated out for at least 4 MT0s. During this
time, there is no
loss of uniformity or depth of color from makeup of electroless nickel deposit
formed on a
substrate from the electroless nickel plating bath.
[0040] Advantageously the inclusion of zinc in the electroless nickel
plating bath
causes electroless nickel deposit to be formed with an increased phosphorous
content.
Electroless nickel plating baths that include zinc can plate at a slower rate.
Plating at a
slower rate causes a black electroless nickel deposit to be formed with an
increased overall
phosphorous content (e.g., at least 5% increase) compared to electroless
nickel deposits
formed using typical sulfur based black electroless nickel chemistry, which
contains on
average 1-5% phosphorous. Black electroless nickel deposits with an increased
phosphorous
content prepared using the sulfur-free electroless nickel plating baths
described herein are
more corrosion resistant than electroless nickel deposits of other available
sulfur based
chemistry.
[0041] In some embodiments, the electroless nickel plating bath used to
form the
electroless nickel coating, multilayer coating, or black coating is free of a
sulfur compound
and can include an aqueous solution of nickel, a hypophosphorous reducing
agent, zinc, at
least of one of a complexing agent, chelating agent, and/or pH buffer, and a
bismuth
stabilizer.
[0042] The nickel can be provided in the bath in the form of a water
soluble nickel salt.
The water-soluble nickel salts can include those which are soluble in the
plating bath and
which can yield an aqueous solution of a predetermined concentration. The
nickel salt can be
selected from the group consisting of nickel chloride, nickel bromide, nickel
iodide, nickel
acetate, nickel malate, a nickel hypophosphite and combinations thereof. The
water-soluble
nickel salts may be used alone or as a mixture.
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-8-
[0043] In some embodiments, the concentration of nickel in the electroless
nickel
plating bath can be from about 1 g/L to 70 g/L. In other embodiments, the
concentration of
nickel in the electroless nickel plating bath can be about 4 g/L to about
6g/L. Electroless
nickel coatings formed from sulfur-free electroless nickel baths that include
about 4 g/1
showed decrease in blackening; whereas electroless nickel coatings formed from
sulfur-free
electroless nickel baths with 8 g/1 showed no increase in the resulting black
deposit.
[0044] The hypophosphorous reducing agent used in the bath can include any
of a
variety of hypophosphorous reducing agents used in known types of the
electroless nickel
plating baths. In some embodiments, the hypophosphorous reducing agent is
selected from
the group consisting of sodium hypophosphite, potassium hypophosphite,
ammonium
hypophosphite, and combinations thereof.
[0045] The concentration of the hypophosphorous reducing agent in the
electroless
nickel plating can differ with the respective types of hypophosphorous
reducing agent and
can be adjusted to vary the concentration of the phosphorous in the
electroless nickel coating
that is formed using the bath. In some embodiments, the concentration of the
hypophosphorous reducing agent in the electroless nickel plating bath can be
about 15 g/L to
about 40 g/L. In other embodiments, the concentration of the hypophosphorous
reducing
agent in the electroless nickel plating bath can be about 20 to about 35 g/L.
A decrease in
concentration of the hypophosphorous reducing agent from about 30 g/1 to about
25 g/l can
result in a decrease in phosphorous in the electroless nickel coating so
fondled by 2%. This
decrease in phosphorous content can result in a deeper black being produced
upon blackening
of the coating.
[0046] The zinc or zinc ions can be incorporated into the electroless
nickel plating bath
by introducing a zinc compound into the bath. Examples of the zinc compound
are zinc
carbonate, zinc oxide, zinc chloride, zinc benzoate, zinc nitrate, zinc
phosphate, zinc stcarate,
and zinc salicylate.
[0047] In some embodiments, the concentration of zinc in the electroless
nickel plating
bath can be about 40 ppm to about 120 ppm. A concentration of zinc in the
electroless nickel
plating bath below 40 ppm can result in a loss of color of the electroless
nickel coating
formed from the bath. A concentration of zinc in the electroless nickel
plating bath above
100 ppm did not adversely affect color of the electroless nickel coating
formed from the bath,
but the plating rate substantially decreased.
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-9-
[0048] The bismuth stabilizer can be incorporated into the electroless
nickel plating
bath by introducing a bismuth salt into the bath, such as bismuth trichloride
or bismuth
nitrate. The concentration of the bismuth stabilizer in the electroless nickel
plating bath can
be about 5 ppm to about 30 ppm. The higher the concentration of bismuth
stabilizer provided
in the electroless nickel plating bath the deeper the black color that can be
produced from an
electroless nickel coating formed from the bath. At concentration of the
bismuth stabilizer
over about 30 ppm, the bath can become over-stabilized and further plating is
inhibited.
[0049] In some embodiments, a complexing agent or a mixture of complexing
agents
may be included in the electroless nickel plating bath. Complexing agents as
used herein can
also include chelating agents. The complexing agents and/or chelating agents
generally
retard the precipitation of nickel ions from the plating solution as insoluble
salts, such as
phosphites, by forming a more stable nickel complex with the nickel ions and
provide for a
moderate rate of the reaction of nickel precipitation.
[0050] The complexing agents and/or chelating agents can be included in the
plating
bath in amounts sufficient to complex the nickel ions present in the bath and
to further
solubilize the hypophosphite degradation products 'butted during the plating
process.
Generally complexing agents and/or chelating agents are employed in amounts of
up to about
200 g/1 with amounts of about 1 to about 75 g/1 being more typical. In other
embodiments,
the complexing agents and/or chelating agents are provided in the electroless
nickel plating
bath at amounts from about 20 to about 40 g/1.
[0051] A variety of complexing agents, used in known electroless nickel
plating
solutions, may be used. Specific examples of the complexing agents may include
monocarboxylic acids, such as glycolic acid, lactic acid, gluconic acid or
propionic acid,
dicarboxylic acids, such as malic acid, malonic acid, succinic acid, tartaric
acid, oxalic acid
or adipic acid, aminocarboxylic acids, such as glycine or alanine, ethylene
diamine
derivatives, such as ethylenediamine tetraacetate, versenol (N-hydroxyethyl
ethylenediamine-
N,N',N'-triacetic acid) or quadrol (N,N,N', N'-tetrahydroxyethyl ethylene
diamine), phosphnic
acids, such as 1-hydroxyethane-1,1-diphosphonic acid, ethylene diamine
tetramethylene
phosphonic acid and water-soluble salts thereof. The complexing agents may be
used either
alone or in combination.
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-10-
[0052] Some complexing agents, such as acetic acid or succinic, for
example, may also
act as a pH buffering agent, and the appropriate concentration of such
additive components
can be optimized for any plating bath after consideration of their dual
functionality.
[0053] In some embodiments, at least one pH buffer, complexing agent, or
chelating
agent can be selected from the group consisting of an acetic acid, formic
acid, succinic acid,
malonic acid, an ammonium salt, lactic acid, malic acid, citric acid, glycine,
alanine, glycolic
acid, lysine, aspartic acid, ethylene diamine tetraacetic acid (EDTA), and
combinations
thereof. In some embodiments, mixtures of 2 or more of the above pH buffers,
complexing
agents, and/or chelating agents can be used in the electroless nickel plating
bath described
herein, with each pII buffer, complexing agent, and/or chelating agent being
provided at a
concentration of about of about 1 to about 75 g/1.
[0054] The plating bath may also contain, in addition to the above
components,
additives with various kinds of purposes so long as the properties of the
plating bath are not
deteriorated.
[0055] The aqueous electroless nickel plating baths can be operated or
maintained at a
pH of about 4.5 to about 5.0 during electroless nickel plating of the
substrate. With this range
of pH, the reducing reaction by the hypophosphorous reducing agent is allowed
to occur
efficiently to prevent decomposition of the hypophosphorous reducing agent as
well as to
prevent the performance of precipitation for plating from being deteriorated
and to prevent
the plating bath from being decomposed. Moreover, with this range of pH, it is
possible to
prevent the plating bath from being lowered in stability as a result of the
excessively high
reducing potential of the reducing agent.
[0056] At least one pH adjustment agent can be used to adjust the pH to the
above
range. When the pII of the bath is too high, it can be adjusted by adding, for
example, an
acid. When the pH of the bath is too low, it can be adjusted by adding, for
example,
ammonium hydroxide.
[0057] The stability of the operating pH of the plating bath can be
controlled by the
addition of various buffer compounds such as acetic acid, propionic acid,
boric acid, or the
like, in amounts up to about 30 g/1 with amounts of from about 2 to about 30
g/1 being
typical. As noted above, some of the buffering compounds such as acetic acid
and succinic
acid may also function as complexing agents.
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-11-
[0058] In accordance with the methods described herein, a substrate can be
plated with
the electroless nickel plating bath to provide an electroless nickel deposit
or coating on the
substrate. The substrate can be any substrate capable of supporting the
electroless nickel
coating but is typically a material for which the electroless nickel coating
displays sufficient
affinity to form a stable coating thereupon. Substrates may be inorganic
materials, such as
metals, or organic materials such as plastics, or composite materials, for
example, organic
polymer comprising inorganic filler. In one embodiment, the substrate is a
metal substrate.
Non-limiting examples of metal substrates include iron, chromium, nickel,
cobalt, copper,
aluminum, titanium, and the like. In another embodiment, the substrate
comprises steel. In
one embodiment, the substrate comprises low alloy steel, for example low alloy
carbon steel.
[0059] The substrate can be plated by contacting the substrate with or
immersing the
substrate in the plating bath for a duration time effective to form an
electroless nickel coating
or deposit on a desired surface of the substrate. In some embodiment, the
substrate can be
cleaned or pre-processed prior to plating. During plating, the bath can be
maintained at a
bath temperature about 175 F to about 200 F. The duration of contact of the
electroless
nickel plating bath with the substrate being plated will determine the
thickness of the
electroless nickel coating. Typically, a contact time can range from as little
as about one
minute to several hours or even several days.
[0060] During the deposition of the electroless nickel deposit or coating,
mild agitation
can be employed. The mild agitation can be, for example, a mild air agitation,
mechanical
agitation, bath circulation by pumping, rotation of a barrel for barrel
plating, etc. The
electroless nickel plating bath also may be subjected to a periodic or
continuous filtration
treatment to reduce the level of contaminants therein. Replenishment of the
constituents of
the bath may also be performed, in some embodiments, on a periodic or
continuous basis to
maintain the concentration of constituents, and in particular, the
concentration of nickel ions
and hypophosphite ions, as well as the pH level within the desired limits.
[0061] The electroless nickel coated substrate so formed can be removed
from the
electroless nickel plating bath and rinsed, for example, with deionized water.
[0062] The electroless nickel coating foimed on a surface of the substrate
using the
electroless nickel plating bath can have of relatively uniform thickness. In
one embodiment,
the electroless nickel coating can have an average thickness in a range from
about 1 micron to
about 250 microns. In another embodiment, the electroless nickel coating can
have an
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-12-
average thickness in a range from about 1 micron to about 100 microns. In yet
another
embodiment, the electroless nickel coating can have an average thickness in a
range from
about 1 micron to about 10 microns. The electroless nickel coating can also
have a
phosphorous content of about 8% to about 11%.
[0063] In some embodiments, the electroless nickel coating can be a top
coating that is
plated over a mid-phosphorous (e.g., about 7% to about 9% phosphorous) or a
high
phosphorous (about 9% to about 13% phosphorous) electroless nickel under
coating to form a
duplex or multilayer electroless nickel deposit or coating. The duplex or
multilayer
electroless nickel coating can advantageously be blackened to form a black
electroless nickel
coating.
[0064] Fig. 1 illustrates a flow diagram showing a method 10 of preparing a
black
electroless nickel coating on a substrate. In the method 10, at step 12 a
substrate can be
contacted with a first electroless nickel plating bath, by, for example,
immersing the substrate
in the first electroless nickel plating bath, to foim a first electroless
nickel coating on the
substrate. The first electroless nickel plating bath can include nickel, a
hypophosphorous
reducing agent, at least one of a complexing agent, chelating agent, or pH
buffer, and
optionally a sulfur compound, such as thiosulfates, thionic acid, or thiourea
to provide a mid-
phosphorous or high-phosphorous under coating.
[0065] An example of an electroless nickel plating bath that can be used to
produce a
high phosphorous electroless nickel coating can include about 6 g/1 nickel,
about 36 g/1
sodium hypophosphite, about 20 g/lmalic acid, about 15 g/1 lactic acid, about
5 g/1 succinic
acid, and about 0.4 ppm lead.
[0066] An example of an electroless nickel plating bath that can be used to
produce a
mid phosphorous electroless nickel coating can include about 6 g/1 nickel,
about 30 g/1
sodium hypophosphite, about 12 g/lmalic acid, about 18 g/1 lactic acid, about
14 g/1 acetic
acid, 1.0 ppm thiourea, and about 1.0 ppm lead.
[0067] The electroless nickel coating fondled on a surface of the substrate
using the first
electroless nickel plating bath can have of relatively unifonn thickness. In
one embodiment,
the electroless nickel under coating can have an average thickness in a range
from about 5
micron to about 250 microns, or about 5 microns to about 100 microns. In some
embodiments, the thickness of the electroless nickel under coating be at least
two times, three
times, four times, or five times greater than the thickness of the top coat.
In yet another
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-13-
embodiment, the electroless nickel under coating can have an average thickness
in a range
from about 5 micron to about 15 microns. The electroless nickel coating can
also have a
phosphorous content of about 7% to about 13%.
[0068] After formation of the first electroless nickel under coating on the
surface of the
substrate, at step 14, the coated substrate can be removed from the first
electroless nickel
plating bath, optionally rinsed, and then contacted with the second ekctroless
nickel plating
bath to form a second electroless nickel coating over the first electroless
coating. The coated
substrate can be contacted with the second electroplating bath by, for
example, immersing the
coated substrate in the second electroless nickel plating bath for a duration
a time effective to
form the second electroless nickel coating or top coating.
[0069] The second electroless nickel plating bath can be different that the
first
electroless nickel plating bath and be formulated such that it is free of a
sulfur compound as
described above. In some embodiments, the second electroless nickel plating
bath includes
nickel, a hypophosphorous reducing agent, zinc, a bismuth stabilizer, and at
least one of a
complexing agent, chelating agent, or pII buffer, and is free of a sulfur
compound. In other
embodiments, the electroless nickel plating bath can include lactic acid,
acetic acid, malic
acid, succinic acid, sodium hypophosphite, ammonium hydroxide, nickel, zinc,
and
ethylenediamine tetraacetic acid. In still other embodiments, the electroless
nickel plating
bath can include about 2 g/1 to about 10 g/1 nickel, about 20 g/1 to about 35
g/1 of a
hypophosphorous reducing agent, about 1 g/1 to about 75 g/1 each of the
complexing agent,
chelating agent, and/or pH buffer, about 40 ppm to about 120 ppm zinc, and
about 5 ppm to
about 30 ppm of a bismuth stabilizer.
[0070] The second electroless nickel coating formed on first electroless
nickel coating
using the second electroless nickel plating bath can have of relatively
uniform thickness and
an average thickness in a range from about 1 micron to about 100 microns. In
some
embodiments, the average thickness can be less than the thickness of the first
electroless
nickel coating and be in a range from about 1 micron to about 10 microns. The
second
electroless nickel coating can also have a phosphorous content of about 8% to
about 11%.
[0071] Following formation of the second electroless nickel top coating
over the first
electroless nickel coating, at step 16, the multilayer or duplex coated
substrate can be
removed from the second electroless nickel plating bath, optionally rinsed,
and then etched
with an etching agent to provide the coated substrate with a black surface.
The etchant agent
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-14-
can include an aqueous solution of an iron blackening agent and an acid. In
some
embodiments, the etchant agent can include an aqueous solution of ferric
sulfate and
hydrochloric acid. In still other embodiments, the etchant agent can include
ferric sulfate,
hydrochloric acid, and reaction enhancer, such as potassium iodate. The
temperature of the
etchant agent can be about 70 F to 90 F and the coated substrate can be
immersed in the
etchant agent for a duration of time effective to blacken the coating, for
example, for about 1
minute to about 3 minutes.
[0072] On removal black electroless nickel coated substrate from the
etchant agent, the
substrate can be rinsed and dried. The black electroless coating so formed has
a consistent
uniform deposit of black electroless nickel with a unifoini thickness and
black coloring,
which is streakless.
[0073] In other embodiments, the duplex or multilayer electroless nickel
coating can
advantageously be further plated with another material to modify the coating.
Fig. 2
illustrates a flow diagram showing a method 20 of preparing an electroless
copper-nickel
coating on a substrate. In the method 20, at step 22 a substrate can be
contacted with a first
electroless nickel plating bath, by, for example, immersing the substrate in
the first electroless
nickel plating bath, to form a first electroless nickel coating on the
substrate. The first
electroless nickel plating bath can include nickel, a hypophosphorous reducing
agent, at least
one of a complexing agent, chelating agent, or pH buffer, and optionally a
sulfur compound,
such as thiosulfates, thionic acid, or thiourea to provide a mid-phosphorous
or high-
phosphorous under coating.
[0074] The electroless nickel coating fondled on a surface of the substrate
using the first
electroless nickel plating bath can have of relatively unifomt thickness. In
one embodiment,
the electroless nickel under coating can have an average thickness in a range
from about 5
micron to about 250 microns, or about 5 microns to about 100 microns. In some
embodiments, the thickness of the electroless nickel under coating be at least
two times, three
times, four times, or more greater that the thickness of the top coat. In yet
another
embodiment, the electroless nickel under coating can have an average thickness
in a range
from about 5 micron to about 15 microns. The electroless nickel coating can
also have a
phosphorous content of about 7% to about 13%.
[0075] After formation of the first electroless nickel under coating on the
surface of the
substrate, at step 24, the coated substrate can be removed from the first
electroless nickel
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-15-
plating bath, optionally rinsed, and then contacted with the second
electroless nickel plating
bath to form a second electroless nickel coating over the first electroless
coating. The coated
substrate can be contacted with the second electroplating bath by, for
example, immersing the
coated substrate in the second electroless nickel plating bath for a duration
a time effective to
form the second electroless nickel coating or top coating.
[0076] The second electroless nickel plating bath can be different that the
first
electroless nickel plating bath and be formulated such that it is free of a
sulfur compound as
described above. In some embodiments, the second electroless nickel plating
bath is includes
nickel, a hypophosphorous reducing agent, zinc, a bismuth stabilizer, and at
least one of a
complexing agent, chelating agent, or pII buffer, and is free of a sulfur
compound. In other
embodiments, the electroless nickel plating bath can include lactic acid,
acetic acid, malic
acid, succinic acid, sodium hypophosphite, ammonium hydroxide, nickel, zinc,
and
ethylenediamine tetraacetic acid. In still other embodiments, the electroless
nickel plating
bath can include about 2 g/1 to about 10 g/1 of nickel, about 20 g/1 to about
35 g/l
hypophosphorous reducing agent, about 1 g/1 to about 75 g/1 each of the
complexing agent,
chelating agent, and/or pH buffer, about 40 ppm to about 120 ppm zinc, and
about 5 ppm to
about 30 ppm bismuth stabilizer.
[0077] The second electroless nickel coating formed on first electroless
nickel coating
using the second electroless nickel plating bath can have of relatively undo,
in thickness and
an average thickness in a range from about 1 micron to about 100 microns. In
some
embodiments, the average thickness can be less than the thickness of the first
electroless
nickel coating and be in a range from about 1 micron to about 10 microns. The
second
electroless nickel coating can also have a phosphorous content of about 8% to
about 11%.
[0078] Following formation of the second electroless nickel top coating
over the first
electroless nickel coating, at step 26, the multilayer or duplex coated
substrate can be
removed from the second electroless nickel plating bath, optionally rinsed,
and then
immersed in an acid solution, such as a hydrochloric acid solution, to
reactivate the surface of
the coating. Reactivation of the surface using an acid solution was found to
advantageously
enhance copper deposition in the subsequent copper coating step.
[0079] Following reactivation of the duplex coating, at step 28, the
multilayer or duplex
coated substrate can be removed from the acidic solution, optionally rinsed,
and then
contacted with an electroless copper plating bath by, for example, immersing
the duplex
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-16-
coated substrate in the electroless copper plating bath. The electroless
copper plating bath
can include an aqueous solution of copper sulfate pentahydrate and sulfuric
acid. The coated
substrate can be immersed in the electroless copper plating bath for a
duration of time
effective to form a copper coating, for example, for about 1 minute to about 3
minutes.
[0080] On removal electroless copper-nickel coated substrate from the
electroless
copper plating bath, the substrate can be rinsed and dried. The electroless
copper-nickel
coating so formed had a consistent uniform deposit with a uniform thickness
and copper
coloring.
[0081] The following examples illustrate the electroless nickel plating
solutions of the
invention. Unless otherwise indicated in the following examples, in the
written description
and in the claims, all parts and percentages are by weight, temperatures are
in degrees
centigrade and pressure is at or near atmospheric pressure.
Example 1
[0082] A black electroless coating was prepared using a high phosphorous
nickel
undercoating, a sulfur free electroless nickel top coating, and an acidic iron
sulfate etchant.
[0083] The high-phosphorous electroless nickel plating bath was prepared
with the
following foimulation:
High Phosphorus electroless nickel plating bath
Nickel Metal 6 g/1
Sodium Hypophosphite 36 g/1
Malic Acid 20 g/1
Lactic Acid 15 g/1
Succinic Acid 5 g/1
Lead 0.4 ppm
PH 4.6-4.8
Temperature 190 F
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-17-
[0084] The sulfur-free electroless nickel plating bath was prepared with
the following
formulation.
Sulfur-free electroless nickel plating bath
Lactic Acid 20 g/1
Acetic Acid 28 g/1
Malic Acid 3 g/1
Succinic Acid 8 g/1
Sodium Hypophosphite 25 g/1
Ammonium hydroxide 34 g/1
Nickel 6 g/1
Zinc 100 ppm
Bismuth Trichloride 15 ppm
EDTA 100 ppm
Temperature (EN Bath) = 192 F
pH= 4.8
[0085] The etchant agent for blackening the sulfur-free electroless nickel
plating bath
was prepared with the following formulation.
Etchant agent
50% Ferric Sulfate 50% of total volume of
12M Hydrochloride Acid 2.5% of total Volume of
Potassium Iodate 50 ppm
Temperature (Blackening Agent) = 70 F
[0086] An undercoat of the high phosphorous electroless nickel was plated
on a steel
substrate for a time of about 1 hour, to provide a high phosphorous
electroless nickel coating
having a thickness of about 0.50 mils. The steel substrate was then rinsed and
placed in the
sulfur-free electroless nickel plating bath for 30 minutes, to provide an
electroless nickel top
coat with a thickness of about 0.15 mils. The steel substrate was then removed
from the
sulfur-free electroless nickel bath, rinsed for 30 seconds, then submerged in
the etchant
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-18-
solution for about 1 minute. The steel substrate was again removed, rinsed,
and allowed to
set for 24 hours for maximum hardness. The chemistry of the process allowed
the electroless
nickel coated steel substrate to be stored following the application of the
undercoating for a
later date, if the user desires. If this is the case, the electroless nickel
coated steel substrate
would need to be electrocleaned and reactivated in 50% HCl acid before the
steel substrate
can be submerged
[0087] A summary of the black electroless nickel plating process is shown
below.
Process:
1.) Appropriate clean cycle for substrate being plated.
2.) EN High Phosphorous bath ¨ 60 mins.
3.) Rinse- 30 secs
4.) Electroclean-2 mins*
5.) Activation- 10 secs*
6.) Proposed Sulfur-free EN Bath- 30 mins
7.) Rinse- 30 secs
8.) Ferric Sulfate Solution-1 min
9.) Rinse 30 secs
10.) Blow dry parts
* These steps only need to be done if part is allowed to set for 1 hour or
more before top coat
is applied.
Example 2
[0088] Each component of the electroless solution was modified in an
attempt to
increase the black produced.
Lactic Acid
[0089] 20% increase showed no benefit, while a 20% decrease seemed to
result in
slight increase in black. Further removal of the Lactic acid, 40% and 60%
decrease, resulted
in no increase of the depth of the black, but the bath began to display
stability issues.
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-19-
Acetic Acid
[0090] 20% decreased had no effect on the final product. 20% increase
caused slight
increase in black. Further increase of Acetic Acid showed no benefit in
overall deposit
quality.
Malic Acid
[0091] Any increase of in Malic resulted in a decrease in the black
coloring. When
complete removal was trialed, the result was the best produce black coloring,
but stability
issues were seen when the levels dropped to under 3 g/l.
Glycine
[0092] Showed no effect on the black when the levels were increased or
decreased. For
this reason, Glycine was removed completely from the fomiul a to lower
potential cost.
Sodium Hypophosphite
[0093] Tested reduction in solution in an attempt to reduce the %P in the
deposit. From
the initial amount, the concentration was reduced to 25 g/1 from 30 g/l, which
resulted a drop
in percent phosphorous by 2% in the final product. This drop resulted in a
deeper black being
produced.
Ammonium hydroxide
[0094] The concentration was adjusted in order to compensate for the
increased Acetic
Acid in solution. Trials to judge the effect that ammonia played in the
solution, resulted in
attempting to produce an ammonia-free chemistry, but the resulting solution
would not
blacken.
Nickel
[0095] Trials at 4 g/1 showed decrease in blacking, where as a solution
with 8 g/1
showed no increase in the resulting black deposit
Succinic Acid
[0096] The Succinic acid was added to assist in controlling the phosphorus
content and
deepening the final color. Trialed concentrations that went up to 12 g/l. The
result was a
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-20-
darker overall deposit up to 8 g/l, which gradually decrease as the
concentrations exceed this
amount.
EDTA
[0097] Increasing or decreasing the amount in solution showed no final
effect on the
black color. Amounts were left at initial level to ensure proper chelation of
addition metals.
Zinc
[0098] When trials dropped this amount below 40 ppm, the result was a loss
of color.
Trials in which the amount was increased above 100 ppm, there was no effect on
the black
produced, but the plating rate drops sharply.
Bismuth Trichloride
[0099] Trials showed that higher the concentration that can be maintain,
deeper the
black that is produced. Although if you exceed 30ppm, the bath would become
over-
stabilized and further plating would be impossible. 15 ppm would allow for 1
hour of plating
without any replenishment before an effect in the black color can be observed.
Example 3
[00100] Initial Testing on the life of the bath, showed that the bath is
able to age out to at
least 4 metal turnovers (MTOs) with no loss in color or uniformity compared to
conventional
electroless nickel baths, such as a sulfur-compound containing, mid-
phosphorous electroless
nickel plating bath. The rate of deposition per metal turnover (MTO) of
electroless nickel
coatings formed using the sulfur-free electroless nickel plating bath was
compared with the
rate of deposition per metal turnover (MTO) using a commercially available
electroless
nickel plating bath. (Fig. 3) The test was repeated in triplicate to validate
this information.
Solutions were also tested for percent Phosphorous at 0 and 4 MTOs. The result
showed that
their respective phosphorous concentrations were 10.8% and 8.5% respectively.
(Fig. 4)
Intrinsic Stress was also measured at 0 and 4 MTOs to determine the effect the
higher levels
of Bismuth would have on the bath. The values were measured to be 1500 PSI
compressive
in both cases. Further testing was also perfoimed to determine proper
adhesion. In this case,
the deposit at 0 and 4 MTOs were subjected to the Quench Test (the rapid
heating then
cooling of the deposit), bend test (the deposit was bent to 90' and examined
to look for poor
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
adhesion for deposit to substrate), and the Scribe test (In which the
deposit's surface is
marred and then examined for cracks due to stress). The proposed chemistry
passed all the
tests. When deposit is allowed 24 hrs to set, the color passes the Eraser test
(>10 swipes of
an eraser across the surface and examined for abrasion). Moreover, compared to
black,
electroless nickel coatings prepared using commercially available black
electroless plating
processes, a black electroless nickel coating prepared by the process
described herein when
subjected 116 hours of neutral salt spray exposure maintains a higher level of
black (Fig. 5).
Example 4
[00101] The duplex coated substrate with the sulfur-free electroless nickel
top coating
prepared by process described above (ebENi process) was further studied by
subjecting the
duplex coating to a copper immersion process for bare steel surfaces. The
duplex coated steel
substrate described above was immersed in an electroless copper bath having
the following
formulation.
Electroless copper plating bath
g/L Copper Sulfate Pentahydrate
5 mL/L Sulfuric Acid
[00102] A summary of electroless copper plating process is shown below:
1. Panel was plated to a thickness of 0.4 mils in EN High Phosphorous
bath
2. Rinse: 1 min
3. Panel was plated in proposed Sulfur-free EN Bath to a thickness of 0.2
mils
4. Rinse: 30 secs
5. 50% HCl: 20 secs
6. Rinse: 30 secs
7. Electroless CuSO4 solution 2 mills
8. Rinse 30 secs
9. Dried
CA 02879315 2015-01-15
WO 2014/015063
PCT/US2013/050932
-92-
[00103] An example of the deposit so formed is illustrated in Fig. 6.
Testing showed
than an intermediate step is need to reactivate the surface before exposure to
the electroless
copper plating bath.
[00104] In order to fully assess the deposit, the immersion properties
needed to be
compared against the traditional EN processes.
[00105] Four EN baths were plated then exposed to a cycle in an attempt to
facilitate
copper immersion over the EN deposit:
En Chemistry:
1. Traditional low phosphorous electroless nickel plating bath
60 mIJI, 6% ENS
150 mL/L Enova EF-163B
Temperature: 190 F
pH: 4.9
2. Traditional mid phosphorous electroless nickel plating bath
60 mIJI, 6% ENS
150 mL/L Enova MS-9
Temperature: 190 F
pH :4.9
3. Traditional high phosphorous electroless nickel plating bath 60 mL/L 6%
LNS
150 mL/L Enova EF-949B.
Temperature: 190 F
pH :4.8
4. Proposed ebENi process:
60 mL/L Enova 949
150 ml,/T, proposed Sulfur-free EN Bath
Temperature: 190 F
pH :4.9
-23-
Plating Cycle
Cycle for Low, Mid, and High Phos system:
1. Panel was plated to a thickness of 0.4 mils
2. Rinse: 30 secs
3. 50% IIC1: 20 secs
4. Rinse: 30 secs
5. CuSai solution: 2 mins
6. Rinse: 30 secs
7. Dried
Cycle for ebENi system:
1. Panel was plated to a thickness of 0.4 mils in Enova 949
2. Rinse: 1 min
3. Panel was plated in ebENi to a thickness of 0.2 mils
4. Rinse: 30 sec
5. 50% HC1 20 secs
6. Rinse: 30 secs
7. CuSO4 solution 2 mins
8. Rinse: 30 secs
9. Dried
[00106] As illustrated in Fig. 6, all three traditional processes show
the copper did not
fully cover the panel. The ebENi system shows complete, uniform coverage of
copper over
the top of the EN deposit.
[00107] From the above description, those skilled in the art will
perceive improvements,
changes and modifications. Such improvements, changes and modifications within
the skill
of the art are intended to be covered by the appended claims.
CA 2879315 2018-04-24