Note: Descriptions are shown in the official language in which they were submitted.
CA 02879712 2015-01-20
WO 2013/170299
PCT/AU2013/000500
1
Electrolytic cell for production of rare earth metals
Field
The present disclosure relates generally to electrolytic cells, in particular
electrolytic
cells adapted to produce rare earth metals, such as neodymium, praseodymium,
cerium, lanthanum and mixtures thereof, by an electrolysis process in a molten
fluoride
or chloride electrolyte bath.
Background
Electrolytic cells for production of aluminium in a molten fluoride or
chloride salt bath
are well known and many of their design features address important
considerations. In
particular, it is important to maintain a stable and low anode-cathode
distance (ACD)
as an energy saving measure in a highly energy intensive process. Maintaining
a
constant ACD may prove difficult where molten aluminium pools on the surface
of the
cathode and is under hydrodynamic forces imposed by strong magnetic fields.
Accordingly, in some cell configurations, the cathodes may be suspended above
the
cell floor onto which the molten aluminium pools. In other configurations, the
cathodes
may be provided with channels into which the molten aluminium may collect,
thereby
draining the molten aluminium from the cathode surface as soon as it forms to
maintain
a constant ACD.
It is also important that the electrolytic cell is configured to liberate
carbon dioxide gas,
which evolves at the anode surface during the electrolysis, from the
interelectrode
space to substantially prevent 'back reaction' with the aluminium metal as it
forms on
the cathode surface, thereby reducing the efficiency of the electrolysis
process.
Neodymium and praseodymium, mixtures thereof, and other rare earth metals, are
also currently made commercially by an electrolysis process in a molten mixed
fluoride
salt bath. In contrast to the electrolytic production of aluminium, the anodes
and
cathodes are disposed in a vertical orientation and the molten metal is
collected into a
receiving vessel on the floor of the cell. The interelectrode space is not
affected by the
molten metal accumulation, but it is nevertheless subject to change by the
continuous
electrolytic consumption of the carbon anode surfaces. The cathodes are
typically
comprised of an inert metal, such as molybdenum or tungsten.
CA 02879712 2015-01-20
WO 2013/170299
PCT/AU2013/000500
2
As the anodes are consumed, there is no effective means to keep anode-cathode
separation distance uniform. As the major part of the process heat is
delivered by the
ohmic resistance of the electrode spacing, the process temperature is highly
variable
and generally controlled by reduction in current supplied to the cell. This is
impractical
in larger scale operations where a number of cells would be connected in
electrical
series. Furthermore, deterioration in current throughout the electrolysis
process is also
undesirable since it decreases the production capacity of the cell. Most
importantly,
failure to closely control the process temperature reduces the process yield,
or
Faraday efficiency, and results in the formation of insoluble sludge which
settles on the
floor of the cell. Consequently, the electrolysis has to be periodically
halted to remove
the sludge from the cell, thereby inhibiting continuous electrolysis.
Poor control of the process temperature also increases the vapour emissions
from the
cell, which are harmful to the working atmosphere and the environment if they
are not
contained.
Additionally, as the anodes are consumed, their displaced volume in the
electrolyte
decreases and the electrolyte level in the cell falls. This reduces the
working area of
the anode immersed in the electrolyte, to the detriment of process efficiency
including
power consumption and increased possibility of 'anode effects' generating
highly
polluting gases.
Moreover, the product rare earth metal is reactive with carbon at the process
temperature. Carbon is a highly undesirable impurity for certain rare earth
metal
product applications. Decreasing the possibility of contact between fugitive
carbon in
the cell and the metal and/or the residence time of product metal in the cell
are
desirable design attributes that are not apparent in the current commercial
cell designs.
This particular problem is not a factor in the design of electrolytic cells
for aluminium
production because aluminium does not react with carbon under these
conditions.
Additionally, in current electrolytic cell designs for rare earth metals, it
is difficult to
maintain the product rare earth metals in a molten state because the operating
temperatures are preferably only 10-30 C above the freezing point of the
product rare
earth metals. This problem is not an issue and is not addressed in
electrolytic cells for
electrolytic production of aluminium because the process temperature is about
300 C
above the freezing point of aluminium.
CA 02879712 2015-01-20
WO 2013/170299
PCT/AU2013/000500
3
Current commercial activities for electrolytic production of rare earth metals
are small
in scale, labour intensive and operated in a semi-batch manner. Several
deficiencies
prevent the process from being scaled up to allow higher productivity,
continuous
electrolysis, and high standards of environmental performance, occupational
health
and safety to be achieved.
Firstly, the electrolysis cells generally operate in a limited current range
of 5-10
kiloannperes, commensurate with low production capacity.
There is poor control of a rare earth oxide feed material to the cell,
resulting in the
accumulation of insoluble sludges that require frequent cell clean-out thereby
hindering
continuous electrolysis. Additionally, feed material is delivered to the cell
manually,
without a known reference to the current oxide concentration in the cell.
The existing technology uses vertical electrode arrangements. Such
arrangements are
not amenable to achieving a high Faraday efficiency. For example, gas bubbles
which
evolve and rise from the anode surface are likely to be entrained in the
electrolyte
flows and make contact with the product metal forming on the cathode plates,
thereby
reducing the process yield consequent to back-oxidation of the product metal.
Keller in US Patent No. 5,810,993 describes a method of producing neodymium in
an
electrolytic cell designed to operate without the occurrence of anode effects,
therefore
avoiding the generation and release of highly polluting perfluorinated carbon
(PFC)
gases. In this invention, the objectives are achieved firstly by providing a
multitude of
anode plates such that the anodic current density remains well below that at
which the
anode effect may occur, and secondly by physically separating the vertical
cathodes
from the vertical anodes using an inert barrier material which remains porous
to
neodymium ions, such that a higher concentration of dissolved neodymium oxide
can
be maintained in the anode region than in the cathode region. The disclosed
invention
has a number of deficiencies and impracticalities however. There is no
demonstration
in the cited examples that the barrier material (boron nitride) is indeed
permeable to
neodymium ions as would be required for a continuous electrolysis process.
Further,
the proposed anode design is complex and the wear rate of the anode plates may
be
expected to be highly non-uniform and wasteful. The compartmental separation
of the
anodic and cathodic zones further results in a large interelectrode separation
distance,
and a resulting inefficient energy consumption. Further, the invention
proposes use of
carbon as the inert cathode material, while it is well known that carbon will
react with
CA 02879712 2015-01-20
WO 2013/170299
PCT/AU2013/000500
4
and contaminate the product metal.
There is therefore a need for alternative or improved electrolytic cells and
processes
for producing rare earth metals.
The above references to the background art do not constitute an admission that
the art
forms a part of the common general knowledge of a person of ordinary skill in
the art.
The above references are also not intended to limit the electrolytic cell as
disclosed
herein.
Summary of the Disclosure
In a first aspect there is disclosed an electrolytic cell for production of
rare earth metals
comprising:
a cell housing provided with one or more inclined channels disposed in a floor
of the cell housing along which channel(s) the molten rare earth metals
produced in the
electrolytic cell can drain;
one or more cathodes suspended within the cell housing in substantially
vertical
alignment with the one or more channels, respective opposing surfaces of the
one or
more cathodes being downwardly and outwardly inclined at an angle from the
vertical;
one or more pairs of anodes suspended within the cell housing, each anode in
the one or more pairs having a facing surface inclined from the vertical and
spaced
apart in parallel alignment with respective opposing inclined surfaces of the
one or
more cathodes to define a substantially constant anode-cathode distance
therebetween; and,
a sump for receiving molten rare earth metals from the channel, wherein the
sump is spaced apart and isolated from the one or more cathodes and the one or
more
pairs of anodes suspended within the cell housing.
In a second aspect there is disclosed an electrolytic cell for production of
rare earth
metals comprising:
a cell housing for containing an electrolyte bath;
one or more cathodes suspended within the cell housing;
one or more pairs of consumable anodes suspended within the cell housing,
each anode in the one or more pairs being spaced apart from respective
opposing
sides of the cathode; and
a displacement device to control a height of the electrolyte bath contained in
the
CA 02879712 2015-01-20
WO 2013/170299
PCT/AU2013/000500
cell housing.
In one embodiment said displacement device controls the height of the
electrolyte bath
contained in the cell housing in response to anode consumption and a volume of
rare
5 earth metal product contained in the cell housing.
In a third aspect there is disclosed an electrolytic cell for production of
rare earth
metals comprising:
a cell housing;
one or more cathodes suspended within the cell housing;
one or more pairs of consumable anodes suspended within the cell housing,
each anode in the one or more pairs being spaced apart from respective
opposing
sides of the cathode; and,
a device operatively associated with the one or more pairs of consumable
anodes to control a distance between the anodes and the cathode in response to
anode consumption.
In a further aspect there is disclosed a system for electrolytically producing
rare earth
metals comprising:
an electrolytic cell in accordance with any one of the first, second or third
aspects as defined above;
a feed material comprising one or more rare earth metal compounds capable of
undergoing electrolysis to produce rare earth metals;
an electrolyte in which molten state the feed material is soluble; and,
a source of direct current configured to pass a current between an anode and a
cathode of the electrolytic cell to electrolyse the feed material and thereby
produce
molten rare earth metal product.
In another aspect there is disclosed a process for electrolytically producing
rare earth
metals comprising:
providing an electrolytic cell in accordance with the second aspect;
charging the electrolytic cell with a feed material comprising one or more
rare
earth metal compounds capable of undergoing electrolysis to produce rare earth
metals and an electrolyte bath comprising molten electrolyte in which the feed
material
is soluble;
passing a direct current between at least one consumable anode and a cathode
in the electrolytic cell to electrolyse the feed material and thereby produce
molten rare
CA 02879712 2015-01-20
WO 2013/170299
PCT/AU2013/000500
6
earth metal product; and,
displacing the molten electrolyte in the electrolytic cell to maintain a
height of
the electrolyte bath in the electrolytic cell.
In one embodiment, the step of displacing is performed in response to a rate
of anode
consumption and/or a change in a volume of rare earth metal product contained
in the
electrolytic cell.
In a still further aspect there is disclosed a process for electrolytically
producing rare
earth metals comprising:
providing an electrolytic cell in accordance with the third aspect;
charging the electrolytic cell with a feed material comprising one or more
rare
earth metal compounds capable of undergoing electrolysis to produce rare earth
metals and a molten electrolyte in which the feed material is soluble;
passing a direct current between at least one consumable anode and a cathode
in the electrolytic cell to electrolyse the feed material and thereby produce
molten rare
earth metal product; and,
translating the or each consumable anode toward the cathode in response to a
rate of anode consumption to maintain a constant anode-cathode distance in the
electrolytic cell.
Embodiments disclosed allow improved control capability for anode-cathode
distance
(ACD) and consequently process temperature, improved control of electrolyte
bath
height in the electrolytic cell and anode immersion, better mixing of the
electrolyte to
enhance dissolution of the feed material, and higher Faraday efficiency by
limiting
opportunity for back reaction of anode gas with produced metal.
Brief Description of the Figures
Notwithstanding any other forms which may fall within the scope of the
disclosure as
set forth in the Summary, specific embodiments will now be described, by way
of
example only, with reference to the accompanying drawings in which:
Figure 1 is side view of an electrolytic cell in accordance with one specific
embodiment; and
Figure 2 is a cross-sectional view of the electrolytic cell shown in Figure 1.
CA 02879712 2015-01-20
WO 2013/170299
PCT/AU2013/000500
7
Detailed Description of Specific Embodiments
The description broadly relates to an electrolytic cell arranged to produce
rare earth
metals by an electrolysis process in a molten electrolytic salt bath.
The rare earth metals produced in the electrolytic cell disclosed herein
include those
rare earth metals having a melting point less than 1100 C. Exemplary rare
earth
metals include, but are not limited to, Ce, La, Nd, Pr, Snn, Eu, and alloys
thereof
including didymium and nnischmetal. The electrolytic cell disclosed herein is
also
suitable for the production of alloys of rare earth metals with iron.
The molten electrolytic salt bath behaves as a solvent for the feed material.
The
electrolyte for use in the molten electrolytic salt bath may comprise halide
salts, in
particular fluoride salts. Examples of 'fluoride salts' include, but are not
limited to,
metal fluoride salts including rare earth metal fluorides such as LaF3, CeF3,
NdF3, and
PrF3, alkali metal fluorides such as LiF, KF, and alkaline earth metal
fluorides such as
CaF2, BaF2.
Selection of a feed material for the electrolysis process will depend on the
desired rare
earth metal product and the composition of the electrolyte. Where the
electrolyte is
composed of fluoride salts, the feed material that is subjected to the
electrolysis
process may comprise oxides of the rare earth metals.
The term 'rare earth metal oxide' broadly refers to any oxide or any
precursors of such
oxides of a rare earth metal, including rare earth metal hydroxides,
carbonates or
oxalates. Rare earth metals are a set of seventeen chemical elements in the
periodic
table, specifically the fifteen lanthanides plus scandium and yttrium.
Scandium and
yttrium are considered rare earth metals since they tend to occur in the same
ore
deposits as the lanthanides and exhibit similar chemical properties. The
lanthanides
include lanthanum, cerium, praseodymium, neodymium, promethium, samarium,
europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium,
ytterbium, and
lutetium.
Suitable examples of feed material for electrolytic production of neodymium or
praseodymium include neodymium oxide (Nd203) or praseodymium oxide (Pr6011).
Where an alloy, such as didymium, is the desired product the feed material may
CA 02879712 2015-01-20
WO 2013/170299
PCT/AU2013/000500
8
comprise two or more oxides of rare earth metals (e.g. Nd203and Pr6011) in the
desired
stoichionnetric ratio of the desired alloy. Mischnnetal may be prepared from
oxides of
several rare earth metals, such as Ce, La, Nd, Pr, wherein the ratio of rare
earth
metals in the nnischnnetal corresponds to the ratio of rare earth metal oxides
in the feed
material.
Alternatively, where the electrolyte is composed of chloride salts, the feed
material may
comprise chloride salts of the rare earth metals.
In one embodiment the electrolyte comprises one or more rare earth metal
fluorides
and lithium fluoride. The one or more rare earth metal fluorides may be
present in the
electrolyte in a range of about 70-95 wt% with the balance as lithium
fluoride.
Optionally, the electrolyte may further comprise up to 20 wt% calcium fluoride
and/or
barium fluoride.
It will be appreciated by persons skilled in the art that the operating
temperature of the
electrolytic cell will depend on the target rare earth metal product or rare
earth metal
alloy, the composition of the electrolyte, and consequently the respective
freezing
points of the rare earth metal, alloy and electrolyte. In one embodiment, the
operating
temperature of the electrolytic cell may be in the range of 5 ¨ 50 C above
the freezing
point of the electrolyte, and preferably 10 ¨ 20 C above the melting point of
the
electrolyte. The composition of the electrolyte is selected so that the
liquidus of the
electrolyte may be in a range of 5 ¨ 50 C above the freezing point of the
metal.
In some embodiments, where the target rare earth metal product is nnischnnetal
(a
mixture of cerium, lanthanum, neodymium and praseodymium), the freezing point
is
variable depending on the composition of the mischnnetal and the relative
ratios of the
rare earth metals therein, but nonetheless is around 800 C. In these
embodiments,
the electrolyte may include barium or calcium fluorides as described above to
achieve
an electrolyte liquidus in the range of 5 ¨ 50 C above the freezing point of
the
nnischnnetal.
In other embodiments, where the freezing points of the rare earth metal alloys
or
mixtures are 800 C or lower, the electrolyte may optionally comprise one or
more rare
earth metal chloride and lithium chloride salts.
Referring to Figures 1 and 2, where like numerals refer to like parts
throughout, there is
CA 02879712 2015-01-20
WO 2013/170299
PCT/AU2013/000500
9
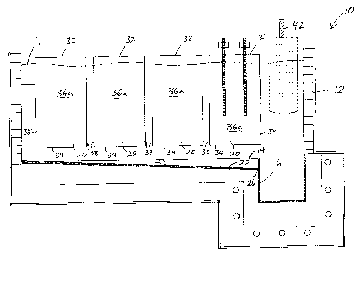
shown an embodiment of an electrolytic cell 10 for production of rare earth
metals.
The cell 10 includes a housing 12 having a floor 14, a sump 16, one or more
cathodes
18, and one or more pairs of anodes 20.
The housing 12 is formed from anti-corrosive materials which are inert in view
of the
electrolyte composition and operating conditions, as has been described in the
preceding paragraphs. In particular, the anti-corrosive materials used to
internally line
the housing 12 should be resistant to forming an alloy with the rare earth
metals
produced therein. In one embodiment the housing 12 may be lined internally
with
refractory materials. Suitable refractory materials include, but are not
limited to,
carbon, silicon carbide, silicon nitride, boron nitride, or certain stainless
steels such as
will be well known to those skilled in the art.
The inclined floor 14 has one or more inclined channels 22 disposed therein
along
which molten rare earth metals produced in the electrolytic cell 10 can drain.
In one
embodiment, the one or more inclined channels 22 are inclined from the
horizontal at
an angle a of up to about 10 .
In the embodiment shown in Figure 2, the channel 22 has a rectangular cross-
section.
It will be appreciated, however, that in alternative embodiments, the cross-
section of
the channel 26 may take other forms, such as a V-shape or a U-shape.
In some forms of the invention the floor 14 may be provided with more than one
inclined channel 22, as shown in Figure 2. In these particular forms the
channels 22
are configured in adjacent lateral parallel alignment with one another. In
general, the
channel(s) 22 may be aligned along or spaced equidistantly from a central
longitudinal
axis of the floor 14 in the housing 12. In this arrangement, the channel(s) 22
in the
floor 14 may be located proximal to an underside 24 of the one or more
cathodes 18 to
receive molten rare earth metals produced on the one or more cathodes 18.
The floor 14, or an upper surface of the floor 14, may be formed from anti-
corrosive
materials similar to or the same as those materials selected for the lining of
the cell
housing 12. All surfaces having direct contact with the rare earth metal
product,
including the channel(s) 22 and the sump 16 should be resistant to forming
alloys with
the rare earth metals produced in the electrolytic bath. Suitable lining
materials for the
channel(s) 22 and the sump 16 include, but are not limited to, metals such as
tungsten,
molybdenum, or tantalum.
CA 02879712 2015-01-20
WO 2013/170299
PCT/AU2013/000500
The sump 16 is configured to receive, in use, molten rare earth metal produced
on the
one or more cathodes 18 which collects in the channel and drains towards the
lower
end 26 of the channel 22. The sump 16 is spaced apart and isolated from the
one or
5 more cathodes 18 and the one or more anodes 20.
The sump 16 may be provided with a heater to maintain a temperature above the
liquidus of the molten rare earth metal. The sump 16 may also be provided with
a port
(not shown) from which molten rare earth metal may be tapped as required. The
sump
10 16 may be formed from inert metals similar to those used for the housing
12.
The arrangement allows for continuous removal of molten rare earth metal
product
from the floor 14 of the cell 10 which prevents pooling of the molten rare
earth metal
product and consequently provides several advantages. In prior art
electrolytic cells
where a pool of molten rare earth metal product is allowed to form,
particularly on the
floor of the cell or at a cathodic surface, it is common for the molten rare
earth metal
product to become contaminated with 'sludge' which comprises undissolved and
partially molten rare earth feed material, reaction intermediates, and
byproducts. In the
electrolytic cell 10 disclosed herein, in the absence of molten rare earth
metal product,
the sludge remains in contact with the molten electrolyte and is thereby
provided with
an opportunity for re-dissolution in the molten electrolyte.
The molten rare earth metal product collected in the sump 16 is spaced apart
from and
isolated from the one or more cathodes 18 and the one or more anodes 20.
Consequently, the molten rare earth metal is protected from reaction and/or
contamination with fugitive carbon arising from the one or more anodes 20, and
back
reactions with off gases from the one or more anodes 20.
The one or more cathodes 18 are suspended in the electrolyte bath 11 contained
within the cell housing 12 above the channel 22 in substantially vertical
alignment
therewith. In the form as illustrated, the cathodes 18 comprise plates of
cathodic
material having an upper surface 28 and opposing elongate surfaces 30, with
the
underside 24 being disposed above the channel 22 in so that molten rare earth
metal
produced on the opposing surfaces 30 may fall under gravity directly into the
underlying channel 22. The opposing surfaces 30 of the cathodes 18 are
supported by
an inert refractory filler material 32 which further avoids the formation of
an inactive
electrolyte zone in the cell 10.
CA 02879712 2015-01-20
WO 2013/170299
PCT/AU2013/000500
11
The cathodes 18 are configured in adjacent alignment with one another whereby
opposing elongate surfaces 30 of adjacent cathodes 18 are respectively
longitudinally
aligned with one another and respective opposing end surfaces of adjacent
cathodes
18 face one another. It will be appreciated by persons skilled in the art that
spacing
between facing opposing end surfaces of adjacent cathodes 18 is as narrow as
possible.
The plates of cathodic material are correspondingly sized so that, in the
arrangement
as described above, an effective length of the adjacently disposed cathodes 18
is
substantially the same as or marginally shorter than the length of the channel
22.
Alternatively, a single cathode 18 having a similar length as the channel 22
may be
employed in the electrolytic cell 10 as disclosed herein.
The opposing elongate surfaces 30 of the cathodes 18 are downwardly and
outwardly
inclined at an angle from the vertical, whereby a cross-sectional shape of the
cathode
18 is substantially triangular. The opposing elongate surfaces 30 may be
inclined from
the vertical by angle 13 of up to about 45 , and preferably from 2 to 100
.
The angle of inclination is selected on the basis of optimised bubble-driven
flow of
electrolyte to achieve good mixing with feed material, and maintenance of high
Faraday yield. The desired angle 13 may be determined by computational
modelling for
the specific cell geometry.
In embodiments where a single rare earth metal or an alloy of rare earth
metals is the
desired electrolytic product, the cathodes 18 may be formed from an
electrically
conductive material with sufficient resistive heat properties to ensure free
flow of the
molten rare earth metals at temperatures marginally greater than their melting
points.
Such materials should be resistant to forming alloys with the rare earth
metals
produced in the electrolytic bath. Suitable materials include, but are not
limited to,
metals such as tungsten, molybdenum, or tantalum.
In alternative embodiments where an alloy of iron with one or more rare earth
metals is
desired, the cathode 18 may be formed from iron. It will be appreciated by
persons
skilled in the art that in these particular embodiments, the cathode 18 will
be consumed
during the electrolytic process for production of the iron-rare earth metal
alloy.
CA 02879712 2015-01-20
WO 2013/170299
PCT/AU2013/000500
12
In the embodiment shown in Figures 1 and 2, a plurality of pairs of anodes 20
are
suspended within the cell housing 12. Each anode 20 in the pair is spaced
apart from
respective opposing elongate surfaces 30 of the cathodes 18. In the form as
illustrated, the anodes 20 comprise plates of consumable anodic material
having an
upper surface 32, a lower surface 34, opposing distal and proximal elongate
surfaces
36a, 36b and opposing ends 38. Distal elongate surface 36a of each anode 20
may be
substantially vertical or may be inclined from the vertical. The proximal
elongate
surface 36b is inclined from the vertical. The proximal elongate side 36b may
be
inclined from the vertical by angle 13' of up to about 45 , and preferably
from 2 to 100
,
tapering toward the lower surface 34 of the anode 20.
The proximal elongate surfaces 36b of the anodes 18 face respective opposing
elongate surfaces 30 of the cathodes 18. Both surfaces 36b and 30 are inclined
from
the vertical by corresponding angle 13' such that the said surfaces 36b and 30
are
spaced apart in parallel alignment with one another so as to define a
substantially
constant anode-cathode distance therebetween.
The anodes 20 are configured in adjacent alignment with one another whereby
opposing elongate surfaces 36a, 36b of adjacent anodes 20 are respectively
longitudinally aligned with one another and respective opposing ends 38 of
adjacent
anodes 20 face one another. It will be appreciated by persons skilled in the
art that
spacing between facing opposing ends 38 of adjacent anodes 20 is as narrow as
possible.
The plates of anodic material are correspondingly sized so that, in the
arrangement as
described above, an effective length of the adjacently disposed anodes 20 is
substantially the same as or marginally shorter than the length of the channel
22.
Alternatively, a single pair of anodes 20 having a similar length as the
channel 22 may
be employed in the electrolytic cell 10 as disclosed herein.
Suitable examples of consumable anodic material include, but are not limited
to,
carbon-based materials in particular high purity carbon, electrode grade
graphite,
calcined petroleum coke-coal tar pitch formulations. Such formulations will be
well
known to those skilled in electrolytic production of rare earth metals and
other metals
such as aluminium.
CA 02879712 2015-01-20
WO 2013/170299
PCT/AU2013/000500
13
The anodes are consumed as the electrolysis process progresses and the angle
of
inclination 13 of proximal elongate side 36b remains substantially constant.
Gas
bubbles released from the anode 20 are therefore retained close to the
proximal
elongate surface 36b as the gas bubbles rise to the electrolyte surface, by
virtue of the
inclined profile of proximal elongate surface 36b, as illustrated in Figure 2.
Advantageously, this reduces the opportunity for contact of the evolved gas
with metal
forming on the cathode 18, hence improving Faraday efficiency and avoiding
insoluble
sludges formed by back reaction therewith.
Under most operating conditions the ACD in the electrolytic cell, as disclosed
herein,
may be between about 30 mm to about 200 mm, although an ACD of between about
50 mm to about 100 mm is preferred. The person skilled in the art may readily
determine an appropriate ACD depending on the desired heat generation in the
electrolyte zone, electrolyte flows for optimum solubility of the feed
material, and
optimisation of the process yield (Faraday efficiency).
The anode is consumed during electrolysis and consequently the ACD may
increase
as electrolysis progresses. The electrolysis cell 10 disclosed herein may be
provided
with a device 40 operatively associated with the one or more anodes 20 to
control the
ACD, in particular to maintain a substantially constant ACD. Said device 40
may
comprise a horizontal positioning apparatus in operative communication with
the one
or more anodes 20. In use, the horizontal positioning apparatus may laterally
translate
the one or more anodes 20 toward the cathode 18 in response to a rate at which
the
anode 20 is consumed so that the ACD may remain substantially constant. The
rate of
anode consumption may be determined by reference to current flow.
Alternatively, the
horizontal positioning apparatus may translate the one or more anodes 20 in
response
to variation in cell resistance from a predetermined value.
Consequent to anode consumption, the volume occupied by the anodes 20 in the
electrolytic cell 10 decreases thereby lowering the height of the electrolyte
bath in the
housing 12. Similarly, the intermittent cell operations such as the
replacement of spent
anodes with new anodes, and the removal of rare earth metal product from the
cell, will
result in substantial and undesirable variation in the height of the
electrolyte bath and
the electrode immersion depth. The electrolysis cell 10 disclosed herein may
be
provided with a displacement device 42 to control the height of the
electrolyte bath in
the housing 12, in particular to maintain a substantially constant height of
the
CA 02879712 2015-01-20
WO 2013/170299
PCT/AU2013/000500
14
electrolyte bath in the housing 12 . The displacement device 42 may comprise
an inert
body which is suspended in the housing 12 and positionable in a vertical
direction. In
use, the inert body may be downwardly or upwardly translated in response to
specific
cell operation so that the height of the electrolyte bath may remain
substantially
constant. The inert body may take any suitable form, for example a bar as
illustrated in
the Figures.
The displacement device 42 may formed from similar refractory materials as the
inner
linings of the housing 12 as described previously.
In use, the electrolysis process may be performed by charging the molten
electrolyte to
the electrolytic cell 10 as described herein. An alternating current may be
supplied
between the cathodes 18 and the anodes 20 and the resistance of the electrodes
18,
raises the operating temperature of the electrolytic cell 10 to a
predetermined
15 temperature. The feed material is then charged to the electrolytic cell
10 and dissolves
in the molten electrolyte. A direct current in a range of 5-100 kiloannperes
is supplied
to the anodes 20, whereupon electrolysis of the dissolved feed material
commences.
In the electrolytic reaction the feed material is reduced to molten rare earth
metal(s) on
the opposing elongate surfaces 30 of the cathode 18. The molten rare earth
metal(s)
20 subsequently fall into the channel 22 and drain along the channel 22
into the sump 16,
which is tapped as required. Feed material may be regularly charged to the
electrolytic
cell 10 into areas of high electrolyte flow, at a rate corresponding more or
less to the
consumption rate. It will be appreciated by those familiar with the art that
the feed rate
may be finely controlled to achieve a target cell resistance corresponding to
the
desired concentration of feed in the electrolyte.
The electrolysis process may be performed under an inert or low oxygen
atmosphere
within the electrolytic cell 10. The inert atmosphere may be established and
maintained by supplying an inert gas or gas mixtures to the electrolytic cell
10 to
exclude air therefrom and thereby prevent undesirable reactions with the
molten
electrolyte and/or the electrodes 18, 20. Suitable examples of inert gases
include, but
are not limited to, helium, argon, and nitrogen.
Numerous variations and modifications will suggest themselves to persons
skilled in
the relevant art, in addition to those already described, without departing
from the basic
inventive concepts. All such variations and modifications are to be considered
within
the scope of the present invention, the nature of which is to be determined
from the
CA 02879712 2015-01-20
WO 2013/170299
PCT/AU2013/000500
preceding description.
In the claims which follow, and in the preceding description, except where the
context
requires otherwise due to express language or necessary implication, the word
5 "comprise" and variations such as "comprises" or "comprising" are
used in an inclusive
sense, i.e. to specify the presence of the stated features but not to preclude
the
presence or addition of further features in various embodiments of the
apparatus and
method as disclosed herein.