Note: Descriptions are shown in the official language in which they were submitted.
CA 02881023 2015-02-04
LEAK-PROOF CONTAINERS, MADE FROM
EXPANDABLE THERMOPLASTIC RESIN BEADS
FIELD OF THE INVENTION
The present invention is directed to a leak proof disposable container;
comprised of a bottom portion composed of expandable thermoplastic beads and a
lidding film. The bottom portion of the container is formed using a
conventional shape
molding process, food or drink is deposited into the bottom portion and a
lidding film is
attached, producing a leak proof container. The container can be used for hot
or cold
liquids, such as noncarbonated beverages, or hot or cold foods such as instant
noodles, soups, fried chicken, yogurt, ice cream and the like.
One object of the invention is to provide a disposable container that reduces
waste; more specifically, replacing the conventional disposable snap-on lid,
with a
thermoplastic lidding film, reduces the weight of the lidding material by 74%.
Another object of the invention is to provide a disposable container that is
100
percent recyclable under the #6 PS (polystyrene) symbol.
Another object of the invention is to provide a leak proof disposable
container
that eliminates leaking, dripping, splashing and spilling of food and/or
drink;
particularly while transporting food or drink in the container, e.g., walking,
bicycling
and driving in a vehicle, etc.
Another object of the invention is to provide a container with excellent heat
insulating properties, such that hot drink and/or food remains hot, and the
consumer
does not experience discomfort while holding the container due to excessive
heat.
The excellent heat insulating properties of the container are also
advantageous in
keeping cold, semi-frozen and frozen food and drink cold.
1
CA 02881023 2015-02-04
In one embodiment of the invention, the leak proof containers are produced
continuously. In another embodiment of the invention, the leak proof
containers are
produced individually, as needed or as purchased in a retail setting.
Another embodiment of the invention includes a hypodermic needle-like
piercing straw. More specifically, when the consumer wishes to consume the
liquid
stored within the leak proof disposable container, the thermoplastic lidding
film is
punctured with the piercing straw and the liquid is consumed through the
straw.
FIELD OF THE INVENTION
The present invention provides a leak proof container for packaging food or
drink comprising: a bottom portion having an upper flange circumferentially
extending
around at least one compartment for supporting a food or drink, wherein said
bottom
portion comprises expanded thermoplastic beads; and a lidding film attached to
said
upper flange, forming a leak proof seal, enclosing said food or drink.
A further embodiment of the present invention provides a leak proof container
wherein said bottom portion is comprised of expandable polystyrene beads.
In a further embodiment, the present invention provides a leak proof container
wherein said bottom portion has a density from 0.5 pounds per cubic foot (8
g/L) to 12
pounds per cubic foot (192 g/L).
In a further embodiment, the present invention provides a leak proof container
wherein said lidding film is a monolayer film.
In a further embodiment, the present invention provides a leak proof container
wherein said monolayer lidding film comprises a styrene butadiene copolymer.
In a further embodiment, the present invention provides a leak proof container
comprising a monolayer lidding film, wherein the monolayer lidding film
comprises a
styrene butadiene copolymer and the Average Plateau Peeling Force required to
peel
2
CA 02881023 2015-02-04
the lidding film from the leak proof container is greater than 0.32 pounds-
force, or 1.4
Newton; wherein said Average Plateau Peeling Force is calculated from an
lnstron
load-displacement curve (extension-mode) as the lidding film is peeled from 1
inch to
3 inches of travel on said upper flange.
In a further embodiment, the present invention provides a leak proof container
comprising a monolayer lidding film, wherein the monolayer lidding film
comprises a
styrene butadiene copolymer and the Average Puncture Force at Straw
Breakthrough
is greater than 2.9 pounds-force, or 13 Newton; wherein said Average Puncture
Force
at Straw Breakthrough is calculated from an Instron load-displacement curve
(compression-mode).
An advantage of the monolayer styrene butadiene copolymer lidding film is a
leak proof container that reduces waste, i.e. replacing the conventional
disposable
snap-on lid with a lighter weight thermoplastic film; thus, reducing the
weight of the
lidding material by 74%.
An additional advantage of the monolayer styrene butadiene copolymer lidding
film is a leak proof container that is 100% recyclable under the #6 PS symbol
(polystyrene).
In a further embodiment, the present invention provides a leak proof container
comprising a monolayer lidding film, wherein the lidding film comprises an
ethylene
vinyl acetate copolymer containing from 3wtcY0 to 16wt% vinyl acetate and has
a melt
index from 0.2 dg/min to 20 dg/min; wherein melt index is determined by ASTM D-
1238 at 190 C and a 2.16kg load.
In a further embodiment the present invention provides a leak proof container
comprising a monolayer lidding film, wherein the lidding film comprises a
polyolefin
3
CA 02881023 2015-02-04
with a DSC melting point from 90 C to 125 C and has a melt index from 0.2
dg/min to
20 dg/min.
Further embodiments of the present invention include leak proof containers
wherein the lidding film is a multilayer film.
In a further embodiment, leak proof containers are lidded with a multilayer
film,
wherein the inner most layer of the multilayer film comprises an ethylene
vinyl acetate
copolymer containing 3wt% to 16wt% vinyl acetate and has a melt index from 0.2
dg/min to 20 dg/min.
In a further embodiment, the leak proof container comprises a multilayer film,
wherein the inner most layer of the multilayer film comprises an ethylene
vinyl acetate
copolymer containing 3wt% to 16wt% vinyl acetate and has a melt index from 0.2
dg/min to 20 dg/min; and the Average Plateau Peeling Force required to peel
this
multilayer lidding film from said leak proof container is greater than 0.24
pounds-force,
or 1.1 Newton.
The present invention provides a leak proof container comprising a multilayer
lidding film, wherein the inner most layer of the multilayer film comprises a
polyolefin
with a DSC melting point from 90 C to 125 C and a melt index from 0.2 dg/min
to 20
dg/min.
The present invention further provides a process for producing a leak proof
container for food or drink comprising the following steps: (A) shape molding
a bottom
portion having an upper flange circumferentially extending around at least one
compartment for supporting a food or drink, wherein said bottom portion
comprises
expanded thermoplastic beads; (B) transporting said bottom portion to a
filling station;
(C) filling said compartment(s) with said food or drink, forming a filled
container; (D)
transporting said filled container to a lidding station; (E) attaching a
lidding film to said
4
CA 02881023 2015-02-04
upper flange on said filled container, forming a leak proof container; (F)
manually
transporting said leak proof container to a point of purchase (single serving
retail
counter), or optionally mechanically transporting said leak proof container to
a fully
integrated bulk packaging line.
The present invention further provides a process wherein the bottom portion
comprises expanded polystyrene beads.
The present invention provides a process wherein said bottom portion has a
density from 0.5 pounds per cubic foot (8 g/L) to 12 pounds per cubic foot
(190 g/L).
The present invention provides a process wherein said lidding film is a
monolayer film.
The present invention provides a process wherein said monolayer lidding film
comprises a styrene butadiene copolymer.
The present invention provides a process that produces a leak proof container
comprising a monolayer lidding film, wherein the monolayer film is comprised
of a
styrene butadiene copolymer; and the Average Plateau Peeling Force required to
peel
this lidding film from said leak proof container is greater than 0.32 pounds-
force, or 1.4
Newton.
The present invention provides a process that produces a leak proof container
comprising a monolayer lidding film, wherein the monolayer film is comprised
of a
styrene butadiene copolymer; and the Average Puncture Force at Straw
Breakthrough
is greater than 2.9 pounds-force, or 13 Newton.
The present invention provides a process that produces a leak proof container
comprising a monolayer lidding film, wherein the monolayer film comprises an
ethylene vinyl acetate copolymer containing from 3wt% to 16wt% vinyl acetate
and
has a melt index from 0.2 dg/min to 20 dg/min.
5
CA 02881023 2015-02-04
The present invention provides a process that produces a leak proof container
comprising a monolayer lidding film, wherein the monolayer film comprises a
polyolefin with a DSC melting point from 90 C to 125 C and a melt index from
0.2
dg/min to 20 dg/min.
Further embodiments of the present invention provide a process that produces
leak proof containers comprising a multilayer lidding film.
The present invention provides a process that produces a leak proof container
comprising a multilayer lidding film, wherein the inner most layer of the
multilayer film
comprises an ethylene vinyl acetate copolymer containing 3wt /0 to 16wt /0
vinyl
acetate and has a melt index from 0.2 dg/min to 20 dg/min.
The present invention provides a process that produces a leak proof container
comprising a multilayer film, wherein the inner most layer of the multilayer
film
comprises an ethylene vinyl acetate copolymer containing 3wt /0 to 16wt /0
vinyl
acetate and has a melt index from 0.2 dg/min to 20 dg/min; and the Average
Plateau
Peeling Force required to peel this multilayer lidding film from said leak
proof
container is greater than 0.24 pounds-force, or 1.1 Newton.
The present invention provides a process that produces a leak proof container
comprising a multilayer lidding film, wherein the inner most layer of the
multilayer film
comprises a polyolefin with a DSC melting point from 90 C to 125 C and a melt
index
from 0.2 dg/min to 20 dg/min.
DEFINITION OF TERMS
Other than in the operating examples or where otherwise indicated, all numbers
or expressions referring to quantities of ingredients, reaction conditions,
etc., used in
the specification and claims are to be understood as modified in all instances
by the
term "about." Accordingly, unless indicated to the contrary, the numerical
parameters
6
CA 02881023 2015-02-04
set forth in the following specification and attached claims are
approximations that can
vary depending upon the desired properties, which the present invention
desires to
obtain. At the very least, and not as an attempt to limit the application of
the doctrine
of equivalents to the scope of the claims, each numerical parameter should at
least be
construed in light of the number of reported significant digits and by
applying ordinary
rounding techniques.
Notwithstanding that the numerical ranges and parameters setting forth the
broad scope of the invention are approximations, the numerical values set
forth in the
specific examples are reported as precisely as possible. Any numerical values,
however, inherently contain certain errors necessarily resulting from the
standard
deviation found in their respective testing measurements.
Also, it should be understood that any numerical range recited herein is
intended to include all sub-ranges subsumed therein. For example, a range of
"1 to
10" is intended to include all sub-ranges between and including the recited
minimum
value of 1 and the recited maximum value of 10; that is, having a minimum
value
equal to or greater than 1 and a maximum value of equal to or less than 10.
Because
the disclosed numerical ranges are continuous, they include every value
between the
minimum and maximum values. Unless expressly indicated otherwise, the various
numerical ranges specified in this application are approximations.
In order to form a more complete understanding of the invention the following
terms are defined and should be used with the accompanying figures and the
description of the various embodiments throughout.
As used herein, the term "monomer" refers to a small molecule that may
chemically react and become chemically bonded with itself; other monomers to
form a
polymer.
7
CA 02881023 2015-02-04
As used herein, the term "polymer" refers to macromolecules composed of one
or more monomers connected together by covalent chemical bonds. The term
polymer is meant to encompass, without limitation, homopolymers, copolymers,
terpolymers, quatropolymers, block polymers, graft copolymers, and blends and
combinations thereof.
The term "homopolymer" refers to a polymer that contains one type of
monomer.
The term "copolymer" refers to a polymer that contains two monomer
molecules that differ in chemical structure randomly bonded together. The term
"terpolymer" refers to a polymer that contains three monomer molecules that
differ in
chemical structure randomly bonded together. The term "quatropolymer" refers
to a
polymer that contains four monomer molecules that differ in chemical structure
randomly bonded together.
As used herein the term "expandable polystyrene beads", or EPS, refers to
spherical polystyrene beads generally prepared by an aqueous suspension
polymerization process. EPS beads are expandable because they contain a
physical
blowing agent, e.g. pentane.
As used herein, the term "styrenic polymer" refers to a polymer derived from
the homopolymerization of styrene or copolymerizing styrene with one or more
monomers, wherein the monomers are covalently linked in a random fashion and
the
resulting polymer contains at least 50 weight percent of one or more monomers
selected from styrene, p-methyl styrene, a-methyl styrene, tertiary butyl
styrene,
dimethyl styrene, nuclear brominated or chlorinated derivatives thereof and
combinations thereof. Non-limiting examples of comonomers include butadiene,
alkyl
acrylates (for example, butyl acrylate, ethyl acrylate and 2-ethylhexyl
acrylate), alkyl
8
CA 02881023 2015-02-04
methacrylates (for example, methyl methacrylate, ethyl methacrylate, butyl
methacrylate and 2-ethylhexyl methacrylate) acrylonitrile, vinyl acetate,
alpha-
methylethylene, divinyl benzene, dimethyl maleate and diethyl maleate.
The term "styrene butadiene copolymer" refers to high impact polystyrenes
(HIPS) and styrene butadiene block copolymers.
The synthesis and morphology of high impact polystyrene is well described by
A. Echte et al. in "Half a Century of Polystyrene ¨ A Survey of the Chemistry
and
Physics of a Pioneering Material", Agnew. Chem. Int. Ed. Engl. 20, 344-361
(1981).
Typically, HIPS is produced by dissolving a rubber, such as polybutadiene, in
styrene
and polymerizing the styrene as well as grafting (covalently linking) a
portion of the
polystyrene macromolecules to the polybutadiene macromolecules. The original
homogeneous solution of polybutadiene in styrene phase separates at relatively
low
styrene conversion. In general, the final morphology of HIPS consists of a
continuous
rigid polystyrene matrix with embedded polybutadiene particles; importantly,
grafted
macromolecules nit the two incompatible phase (polystyrene and butadiene)
together
and significantly influence final properties. Several morphological and
structural
features play an important role in determining the end-use performance of
HIPS, e.g.
rubber content, phase volume ratio, rubber particle size, rubber particle size
distribution, degree of grafting and the degree of cross linking within the
rubber. As a
specific example, it is generally accepted that HIPS impact strength goes
through a
maximum when the rubber particle size is approximately 0.039 mil (1 m) to
0.079 mil
(2 m). Typically, commercial HIPS contains approximately 75wt% polystyrene.
The term "styrene butadiene copolymer" also refers to styrene butadiene block
copolymers. The term "block copolymer" refers to a polymer that contains at
least two
monomer molecules that upon polymerization form at least two chemically
distinct
9
CA 02881023 2015-02-04
regions, segments or blocks. The term block copolymer includes linear block
copolymers, multi-block copolymers and star shaped block copolymers. A block
copolymer can be prepared using a living or anionic polymerization process.
More
specifically, a styrene butadiene block copolymer may be produced using the
following
steps: butadiene monomer is added to a reactor and completely converted to
living
polybutadiene anions using a butyllithium initiator; styrene monomer is then
added to
the reactor and completely polymerized to form poly(butadiene-block-styrene)
anions,
and; the living polymerization system is terminated producing a linear styrene
butadiene block copolymer. Such living polymerization systems allow one to
precisely
control the weight fraction or length of each block. In addition, prior to
termination a
third monomer could be added to form a tri-block copolymer, or multi-block
copolymer,
such as -Ax-By-Cz-, wherein A, B and C represent monomers and x, y, z are
integers
that represent the length of the homopolymer sequence. The term tri-block, or
multi-
block, may also refer to a block copolymer with the following structure: -Ax-
By-Az-,
wherein the two A blocks differ in length. Through the use of a coupling agent
one
can use anionic polymerization to produce star shaped block copolymers such as
-
(AB)-X; wherein X is the coupling agent and n is an integer that represents
the
number of macromolecular arms attached to X, n can be 3, 4, 5 or higher.
Silicon
tetrachloride (SiCI4) is a non-limiting example of a coupling agent which
could be used
to produce a star shaped block copolymer with four arms. It is also possible
to
produce asymmetric star shaped block copolymers wherein the arm lengths differ
in
length, i.e. three short polystyrene arms and one long polystyrene arm
attached to a
polybutadiene core. In general, block copolymers covalently link two (or more)
polymer segments that are immiscible, as a result the homopolymer-like blocks
within
a block copolymer phase separate. For example, in the case of styrene
butadiene
CA 02881023 2015-02-04
block copolymers, the polystyrene and polybutadiene segments microscopically
phase
separate, producing unique morphologies that may be spherical, rod-like or
lamellar in
nature, wherein the phases have dimensions from hundreds to thousands of
angstroms. Such morphological dimensions results in glass-clear transparency,
in
addition the rubbery polybutadiene phases (low Tg) dramatically improve the
impact
resistance of the block copolymer, relative to pure polystyrene.
As used herein, the term "thermoplastic" refers to a broad class of polymers
that soften or become liquid when heated, will flow under pressure and harden
when
cooled. In many cases, thermoplastics are high-molecular-weight polymers that
can
be repeatedly heated and remolded.
As used herein, the term "polyolefin" refers to a broad class of polymers that
includes polyethylene and polypropylene.
As used herein, the term "polyethylene" includes ethylene homopolymers,
ethylene copolymers containing one comonomer, ethylene terpolymers containing
two
comonomers and ethylene quatropolymers containing three comonomers, etc.
Polyethylenes are typically produced using Ziegler/Natta catalysts, chrome
catalysts,
metallocene catalysts or free radical catalysts. Suitable comonomers include
propylene, C4 to C8 a-olefins, vinyl acetate, methyl acrylate, methyl
methacrylate,
acrylic acid and methacrylic acid.
As used herein, the term "polypropylene" includes isotactic, syndiotactic and
atactic polypropylene homopolymers, random propylene copolymers containing one
comonomer, random propylene terpolymers containing two comonomers, random
propylene quatropolymers containing three comonomers, etc., and impact or
heterophasic polypropylenes. Random polypropylenes typically contain less than
20
wt% of comonomer, based on the weight of the random polypropylene; typical
11
CA 02881023 2015-02-04
comonomers include ethylene and C4 to C8 a-olefins. Impact or heterophasic
polypropylenes, typically contain up to 40 wt% of an ethylene/propylene rubber
finely
dispersed in a propylene homopolymer. The ethylene/propylene rubber may also
include one or more of the following monomers; 1,2-propadiene, isoprene, 1,3-
butadiene, 1-5-cyclooctadiene, norbornadiene or dicyclopentadiene.
As used herein, the term "intercalated" refers to the insertion of one or more
polymer molecules within the domain of one or more other polymer molecules
having
a different composition. In the embodiments of this invention, the term
"intercalated
polymer" refers to a styrenic polymer intercalated within polyolefin
particles, produced
by polymerizing a styrenics monomer mixture within a polyolefin particle. U.S.
Patent
7,411,024, U.S. Patent 7,906,589, U.S. Patent 8,101,686 and U.S. Patent
8,168,722,
herein incorporated by reference in their entirety, describe intercalated
polymers
comprised of 20 percent to 60 percent by weight of a polyolefin and from 40
percent to
80 percent by weight of a styrenic polymer, based on the weight to the
interpolymer
resin particles.
As used herein, the term "bottom portion" refers to a container such as a cup,
a
bowl or a tray produced in a shape molding process from expandable
thermoplastic
resin beads. A shape molded bottom portion may contain one or more
"compartments", wherein food or drink can be placed.
As used herein, the term "lidding film" refers to a monolayer or a multilayer
film
that is capable of being attached to said bottom portion, forming a leak-proof
seal.
Sealing may be accomplished by any know method such as heat sealing,
ultrasonic
sealing, pressure sensitive adhesives or hot melt adhesives, etc.
As used herein the term "monolayer film" contains a single layer of one
thermoplastic, or blends of more than one thermoplastic.
12
CA 02881023 2015-02-04
As used herein, a "multilayer film" is comprised of more than one
thermoplastic
layer, or optionally non-thermoplastic layers such as metals or paper
products. A
single layer within a multilayer film may contain a blend of more than one
thermoplastic. Multilayer lidding films are common in food and drink packaging
because one may incorporate additional functionality into the lidding film;
for example,
moisture barrier, oxygen barrier, adhesive layers, toughness, abuse
resistance,
scratch resistance, decorations (print or graphics) and sealability layers.
As used herein, the term "sealant layer" refers to a layer of thermoplastic
film
that is capable of being attached to said bottom portion, forming a leak proof
seal. In
the case of monolayer films, the terms sealant layer and lidding film are
equivalent.
The term "inner most layer" refers to a multilayer film; the inner most layer
of a
multilayer film is the sealant layer that is capable of being attached to said
bottom
portion, forming a leak proof seal. The inner most layer is also in contact
with the
internal environment within the container, the inner most layer may also be in
physical
contact with the food or drink contained within the compartment of the bottom
portion.
The term "outer most layer" refers to a multilayer film. The outer most layer
of
a multilayer film is in contact with the external environment, forming the
outer surface
of the multilayer film.
Herein, the term "barrier layer" refers to a functional layer within a
multilayer
film that protects food or drink from the deleterious effects of moisture
and/or oxygen.
Polyethylene films containing a high density polyethylene (HDPE) barrier layer
provide
reasonable moisture barrier. However, when high moisture and high oxygen
barrier is
required, a wide variety of barrier resins are available. Typical
thermoplastic barrier
resins include, polyvinylalcohol (PVOH), ethylene vinyl alcohol (EVOH),
polyamides
(Nylon), polyesters, polyvinylidene chloride (PVDC), polyacrylonitrile and
acrylonitrile
13
CA 02881023 2015-02-04
copolymers and polyvinylchloride (PVC). Barrier layers may also include a
layer of
thermoplastic film upon which a metal oxide has been applied by vapor
deposition; for
example a thin silicon oxide (SiOx) or aluminum oxide (A10x) layer vapor
deposited on
polypropylene, polyamide or polyethylene terephthalate.
Herein, the term "tie layer" refers to a layer within a multilayer film that
promotes adhesion between adjacent film layers that are dissimilar in chemical
composition. Common tie resins or adhesive resins are functionalized
polyethylenes
containing monomer units derived from C4 to C8 unsaturated anhydrides, or
monoesters of C4 to C8 unsaturated acids having at least two carboxylic acid
groups,
or diesters of C4 to C8 unsaturated acids having at least two carboxylic acid
groups, or
mixtures thereof. Tie layers in multilayer films typically contain less than
20 wt% of a
tie resin blended with a polyolefin, e.g., plastomer, ULDPE, VLDPE, LLDPE,
MDPE,
HDPE or polypropylenes, etc. Depending the multilayer film structure, in some
cases
the following resins may also be effective as tie resins; ethylene/vinyl
acetate
copolymers, ethylene/methyl acrylate copolymers, ethylene/butyl acrylate
copolymers,
very low density polyethylene (VLDPE), ultralow density polyethylene (ULDPE),
as
well as metallocene catalyzed ethylene/a-olefin copolymers.
Herein, polymer density was determined using American Society for Testing
and Materials (ASTM) methods ASTM D1505 or D792.
Herein, polymer melt index was determined using ASTM D1238, Condition I
was measured at 190 C, using a 2.16 kg weight and ,Condition G was measured at
230 C, using a 2.16 kg weight.
Herein, the VICAT softening temperatures of polymers was measured using
ASTM D1525.
Herein, polymer melting temperature was measured using ASTM D3418.
14
CA 02881023 2015-02-04
Herein, the gel content of intercalated polymers was measured using ASTM
D2765 using toluene as solvent.
Herein, water vapor transmission rate (VVVTR) of lidding film, expressed as
grams of water vapor transmitted per 100 square inches of film per day at a
specified
film thickness (mils), org/100 in2/day, was determined using ASTM F 1249-06 at
100 F (37 .8 C) and 100% relative humidity using a MOCON permatron developed
by
Modern Controls Inc.
Herein, oxygen gas transmission rate of lidding film was determined using
ASTM F2622-08.
Herein, lidding film dart impact strength was determined using ASTM D-1709B.
Herein, lidding film machine direction and transverse direction Elmendorf tear
strength and tensile strength was determined using ASTM D-1922 and ASTM D882,
respectively.
Herein, lidding film machine direction and transverse direction tensile
properties
(2% secant modulus, tensile strength at yield, tensile elongation at yield,
tensile
strength at break, tensile elongation at break) were determined using ASTM
D882.
Herein, the optical properties of lidding film were measured as follows: Haze,
ASTM D1003; Clarity ASTM D1746 and; Gloss (20 ) ASTM D2457.
DESCRIPTION OF THE FIGURES
In the accompanying Figures:
Figure 1 illustrates a typical container molded from expandable thermoplastic
beads, which includes a bottom portion 101, an upper flange 102 and a food or
drink
compartment 103. The dimensions r1 and r2 represent the outer and inner radius
of
the upper flange; the difference (ri-r2) defines the width of the upper
flange.
CA 02881023 2015-02-04
Figure 2 illustrates another embodiment, a tray molded from expandable
thermoplastic beads.
Figure 3 illustrates another embodiment, an assembly of four cup-like bottom
portions and four compartments of equal volume molded from expandable
thermoplastic beads.
Figure 4 illustrates another embodiment, an assembly of three tray-like bottom
portions and three compartments that differ in volume molded from expandable
thermoplastic beads.
Figure 5 illustrates various lidding film embodiments; monolayer lidding film
5a,
three layer lidding film 5b, four layer lidding film 5c, five layer lidding
film 5d and seven
layer lidding film 5e.
Figure 6 illustrates an atomic force micrograph (AFM) showing the
heterophasic morphology of an intercalated polymer; styrenic polymer (bright
regions)
intercalated within a polyolefin (dark regions) particle.
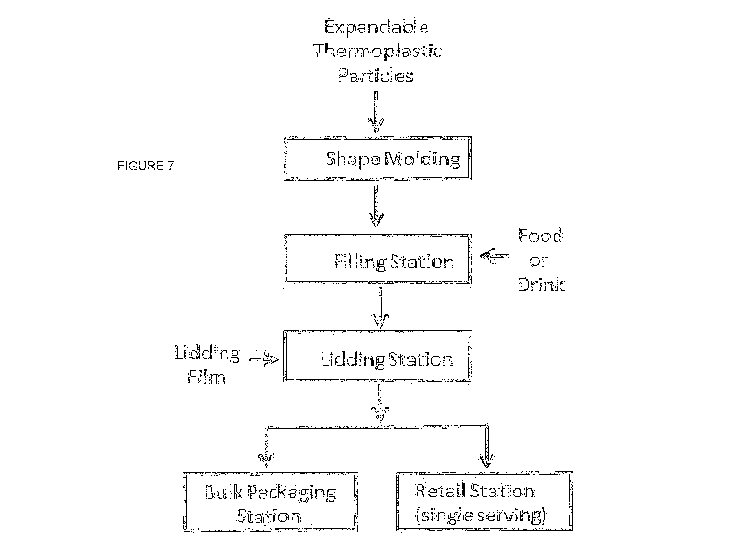
Figure 7 illustrates a process to produce a leak proof container made from
expandable thermoplastic resin beads.
Figure 8 illustrates one embodiment of a sealing station, with a sealing
device
61, a heat sealing disk 63, a cutting disk 64, a continuous roll of lidding
film 62, a
lower platform 66 and a bottom portion 60.
Figure 9 illustrates a top view of a leak proof container, wherein a leak
proof
seal 71, attaches the bottom portion 70 to the lidding film 72. The dimensions
r11 and
r12 represent the outer and inner radius of the upper flange; the dimension
r13 is the
lidding film radius, the difference (ri3-rii) defines the lidding film
overhang.
Figure 10 illustrates the DSC melting points of EVA copolymers as a function
of
vinyl acetate content (wt%).
16
CA 02881023 2015-02-04
Figure 11 illustrates the DSC of a poly(ethylene-co-ethylene vinyl acetate)
copolymer, Elvax 3135X, 12% VA, 0.3512 (190 C), 0.93g/cc, available from
DuPont.
Peak melting point of 94.78 C.
Figure 12 illustrates the DSC thermogram of a poly(ethylene-co-1-octene)
plastomer, Affinity PL1881G, 0.904g/cc, 1.0 melt index (190 C), available from
Dow
Chemical. Peak melting point of 97.48 C.
Figure 13 illustrates the DSC thermogram of a poly(propylene-co-ethylene)
copolymer, Adsyl 5C 37 F, available from LyondellBasell, 0.90g/cc and 5.512
(230 C).
DSC melting peaks at 104.69 C, 134.80 C and 145.65 C.
Figure 14 plots the compressive load (lb-f), or the lidding film puncture
strength,
of lidding film Example 3 (five specimens) as a function of compressive
extension
(inches).
Figure 15 plots the extensional load (lb-f), or the lidding film peel
strength, of
lidding film Example 3 (five specimens) as a function of extension (inches).
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
The manufacture of molded articles, such as cups, bowls and the like from
expanded thermoplastic beads is known. Any suitable expandable resin beads can
be used, in a shape molding process to form a cup or bowl as shown in Figure
1,
comprising a bottom portion 101, an upper flange 102 and a compartment 103 for
holding food or drink. Herein, the term "bottom portion" refers to any molded
shape, a
cup, a bowl, a tray, etc. Suitable expandable resin beads include those with
dimensions that allow the expandable and/or pre-expanded beads to be fed to a
two-
part mold without clogging or obstructing the feed channels in the mold and
are able
to expand and fuse together to form the molded bottom portion 101 shown in
Figure 1.
Suitable expandable resin beads, include but are not limited to, those that
contain
17
CA 02881023 2015-02-04
homopolymers of vinyl aromatic monomers. Suitable vinyl aromatic monomers
include, but are not limited to, styrene, isopropylstyrene, alpha-
methylstyrene, nuclear
methylstyrenes, chlorostyrene, tert-butylstyrene. In an embodiment of the
invention,
the vinyl aromatic monomers can be copolymerized with one or more other
monomers, non-limiting examples being divinylbenzene, conjugated dienes (non-
limiting examples being butadiene, isoprene, 1, 3- and 2,4- hexadiene), alkyl
methacrylates, alkyl acrylates, acrylonitrile, and maleic anhydride, where the
vinyl
aromatic monomer is present in at least 50% by weight of the copolymer. In
many
embodiments of the invention, styrenic polymers are used, particularly
polystyrene,
however, other suitable polymers can be used, such as polyolefins (e.g.
polyethylene,
polypropylene), polycarbonates, polyphenylene oxides, and mixtures thereof.
In one embodiment of the invention, the resin beads comprise expandable
polystyrene (EPS) beads. EPS is generally prepared by an aqueous suspension
polymerization process, which produces spherical polystyrene beads, which
become
expandable after impregnation with a blowing agent. When heated the
impregnated
polystyrene beads soften, the blowing agent vaporizes and the beads expand.
Expandable polystyrene beads can be screened to relatively precise bead sizes.
Typically, bead diameters are within the range of from 0.008 inch to 0.02 inch
(0.2 mm
to 0.5 mm). Occasionally, molded articles (cups and bowls) are made from beads
having bead diameters as high as 0.03 inches (about 0.8 mm).
In the present invention, the resin beads are formed via polymerization in a
suspension process, from which essentially spherical resin beads are produced.
These beads are useful for making the bottom portion shown in Figure 1.
However,
polymers derived any polymerization process can be pelletized to from
cylindrical or
spherical resin beads of appropriate dimensions, impregnated with a blowing
agent
18
CA 02881023 2015-02-04
and shape molded to produce a bottom portion; non-limiting examples of
polymerization processes include bulk, solution or gas phase processes.
In an embodiment of the invention, resin beads containing any of the above-
mentioned polymers have a minimum average particle size of at least 0.4 mil
(10 pm),
in some situations at least 1 mil (25 pm), in some cases at least 2 mil (50
pm), in other
cases at least 3 mil (75 pm), in some instances at least 4 mil (100 pm) and in
other
instances at least 6 mil (150 pm). Also, the resin beads can have a maximum
average particle size of up to 24 mil (600 pm), in some instances up to 22 mil
(550
pm), in other instances up to 20 mil (500 pm), in some cases up to 18 mil (450
pm), in
other cases up to 16 mil (400 pm), and in some situations up to 14 mil (350
pm). The
maximum average size of the resin beads will be limited by the dimensions of
the two-
part mold to allow for feeding of the expandable and/or pre-expanded resin
beads into
the mold that forms the bottom portion. The size of resin beads used in this
embodiment can be any value or can range between any of the values recited
above.
The number average particle size and size distribution of the resin beads can
be determined using low angle light scattering, which can provide a weight
average
value. A non-limiting example of such a device is Model LA-910 Laser
Diffraction
Particle Size Analyzer available from Horiba Ltd., Kyoto, Japan.
In an embodiment of the invention, the polymers in the resin bead have a
weight average molecular weight (Mw) of at least 25,000, in some cases at
least
50,000, and in other cases at least 75,000 and the Mw can be up to 1,000,000,
in
some cases up to 750,000 and in other cases up to 500,000. The weight average
molecular weight of the polymers in the resin bead can be any value or can
range
between any of the values recited above. Unless stated otherwise, all
molecular
weight values are determined using gel permeation chromatography (GPC) using
19
CA 02881023 2015-02-04
appropriate polystyrene standards. Unless otherwise indicated, the molecular
weight
values indicated herein are weight average molecular weights (Mw).
In an embodiment of the invention, after polymerization, the resin beads are
isolated and dried and then suspended in an aqueouS system. As used herein,
"aqueous system" means a solution or mixture containing at least 50 weight%
water
as the solution medium and/or continuous phase. Dispersing aids, nonionic
surfactants and/or waxes can also be added to the aqueous system. When the
resin
beads are dispersed in the aqueous system, one or more blowing agents can be
added.
The expandable thermoplastic beads or resin beads can optionally be
impregnated using any conventional method with a suitable blowing agent. As a
non-
limiting example, the impregnation can be achieved by adding the blowing agent
to
the aqueous suspension during the polymerization of the polymer, or
alternatively by
re-suspending the beads or resin beads in an aqueous medium and then
incorporating the blowing agent as taught in U.S. Pat. No. 2,983,692. Any
gaseous
material or material which will produce gases on heating can be used as the
blowing
agent. Conventional blowing agents include aliphatic hydrocarbons containing 4
to 6
carbon atoms in the molecule, such as butanes, pentanes, hexanes, and the
halogenated hydrocarbons, e.g. CFC's and HCFC's, which boil at a temperature
below the softening point of the polymer chosen. Mixtures of these aliphatic
hydrocarbon blowing agents can also be used.
Alternatively, water can be blended with these aliphatic hydrocarbons blowing
agents or water can be used as the sole blowing agent as taught in U.S. Pat.
Nos.
6,127,439; 6,160,027; and 6,242,540 in these patents, water-retaining agents
are
used. The weight percentage of water for use as the blowing agent can range
from 1
I
CA 02881023 2015-02-04
wt% to 20 wt%. The texts of U.S. Pat. No. 6,127,439 U.S. Pat. No 6,160,027 and
U.S. Pat. No. 6,242,540 are incorporated herein by r Iference.
In an embodiment of the invention, the blowing agent can include one or more
selected from nitrogen, sulfur hexafluoride (SF6), argon, carbon dioxide,
1,1,1,2-
tetrafluoroethane (HFC-134a), 1,1,2,2-tetrafluoroethane (HFC-134), 1,1,1,3,3-
pentafluoropropane, difluoromethane (HFC-32), 1,1-difluoroethane (HFC-152a),
pentafluoroethane (HFC-125), fluoroethane (HFC-161) and 1,1,1-trifluoroethane
(HFC-143a), methane, ethane, propane, n-butane, isobutane, n-pentane,
isopentane,
cyclopentane, neopentane, hexane, azodicarbonamide, azodiisobutyro-nitrile,
benzenesulfonylhydrazide, 4,4-oxybenzene sulfonyl-semicarbazide, p-toluene
sulfonyl
semi-carbazide, barium azodicarboxylate, N,N'-dimethyl-N,N'-
dinitrosoterephthalamide, trihydrazino triazine, mixtures of citric acid and
sodium
bicarbonate, and combinations thereof.
In an embodiment of the invention, the blowing agent can be present in the
resin beads at a level of less than 14 wt%, in some situations less than 6
wt%, in
some cases ranging from about 2 wt% to about 5 wt%, and in other cases ranging
from about 2.5 wt% to about 3.5 wt% based on the weight of the resin bead.
Any suitable dispersing aid can be used in the present invention. Suitable
dispersing aids prevent the resin beads from sticking together when dispersed
in the
aqueous system. Examples of suitable dispersing aids include, but are not
limited to
finely divided water-insoluble inorganic substances such as tricalcium
phosphate, zinc
oxide, bentonite, talc, kaolin, magnesium carbonate, aluminum oxide and the
like as
well as water-soluble polymers such as polyvinyl alcohol, alkyl aryl
sulfonates,
hydroxyethyl cellulose, polyacrylic acid, methyl cellulose, polyvinyl
pyrrolidone, and
the like, sodium linear alkyl benzene sulfonates, such as sodium
dodecylbenzene-
21
CA 02881023 2015-02-04
sulfonate, and combinations thereof. In an embodiment of the invention, the
dispersing aid includes tricalcium phosphate together with a sodium linear
alkylbenzene sulfonate. The amount of the dispersing aid necessary will vary
depending on a number of factors but will generally be at least about 0.01
parts, in
some cases at least about 0.05 parts, and in other cases at least about 0.1
parts and
can be up to about 2 parts, in some cases up to about 1 parts, and in other
cases up
to about 0.75 parts by weight per 100 parts by weight of resin beads. The
amount of
the dispersing aid can be any value or can range between any of the values
recited
above.
One or more non-ionic surfactants can be included such as polyoxyalkylene
derivatives of sorbitan fatty acid esters, such as C8 to C32 linear or
branched with up to
five units of unsaturation, non-limiting examples being oleates, stearates,
monolaurates and monostearates, an ethylene oxide/propylene oxide block
copolymer, or other non-ionic or anionic surface active agent can be added to
the
aqueous suspension if desired. In an embodiment of the invention, the amount
of
surfactant is at least 0.01 parts, in some cases at least 0.05 parts, and in
other cases
at least 0.1 parts and can be up to 2 parts, in some cases up to 1 parts, and
in other
cases up to 0.75 parts by weight per 100 parts by weight of resin beads. The
amount
of surfactant can be any value or can range between any of the values recited
above.
In an embodiment of the invention, the HLB (Hydrophilic/Lipophilic/-Balance)
of the
above-mentioned polyoxyalkylene containing surfactants is at least 8, in some
cases
at least 10 and in other cases at least 12 and can be up to 22, in some cases
up to 20
and in other cases at least 18. HLB is applicable to non-ionic surfactants and
it
predicts water solubility, the higher the HLB the more hydrophilic or water
soluble the
non-ionic surfactant. The HLB of the polyoxyalkylene containing surfactants
can be
22
CA 02881023 2015-02-04
any value or can range between any of the values recited above. The non-ionic
surfactants can aid in the formation of fine cell structure in the expanded
resin beads.
The waxes are used in the present invention were selected to promote the
formation of fine cell structure in the expanded resin beads. At atmospheric
pressure,
waxes are typically solid at 20 C and below, in some cases 25 C and below, and
in
other cases 30 C and below, and are liquid at 125 C and above, in some cases
150 C and above, and in other cases 200 C and above.
In an embodiment of the invention, the waxes are selected from natural and/or
synthetic waxes. As such, the waxes used in the present invention can be one
or
more materials selected from C10 to C32, in some instances C12 to C32, in some
cases
C14 to C32, and in other cases C16 to C32 linear, branched or cyclic alkyl,
alkenyl, aryl,
alkaryl, or aralkyl alcohols; C10 to C32, in some instances C12 to C32, in
some cases C14
to C32, and in other cases C16 to C32 linear, branched or cyclic alkyl,
alkenyl, aryl,
alkaryl, or aralkyl carboxylic acids and/or their corresponding ammonium and
metal
salts or C1 to C32, in some instances C12 to C32, in some cases C14 to C32,
and in other
cases C16 to C32 linear, branched or cyclic alkyl, alkenyl, aryl, alkaryl, or
aralkyl esters;
C10 to C32, in some instances C12 to C32, in some cases C14 to C32, and in
other cases
C16 to C32 linear, branched or cyclic alkyl, alkenyl, aryl, alkaryl, or
aralkyl
hydrocarbons; polyethylene; polypropylene; polyester; polyether; and
combinations
thereof, so long as they meet a combination of liquid and solid temperatures
as
defined above. The polyethylene, polypropylene, polyester, and polyether waxes
can
have a molecular weight (Mw) of from about 1,000 to about 100,000 so long as
they
meet a combination of liquid and solid temperatures as defined above
In an embodiment of the invention, the amount of wax is at least 0.01 parts,
in
some cases at least 0.05 parts, and in other cases at least 0.1 parts and can
be up to
23
CA 02881023 2015-02-04
2 parts, in some cases up to 1 parts, and in other cases up to 0.75 parts by
weight per
100 parts by weight of resin beads. The amount of wax can be any value or can
range between any of the values recited above.
The resin beads used in the invention are advantageously solid particles in
the
form of thermoplastic resin beads produced from suspension polymerization as
indicated above. The polymer is formed as a slurry of finely divided beads in
the
aqueous suspension. The beads are recovered by washing and drying.
In an embodiment of the invention, the resulting resin beads can be screened
to remove any resin beads with particle sizes that are too large. In many
cases, resin
beads having a particle size greater than 24 mil (600 pm), in some cases
greater than
20mil (500 pm) and in other cases greater than 16 mil (400 pm) are removed by
screening.
The production of shape molded articles from impregnated polystyrene beads
is generally done in two steps. First, the blowing agent impregnated
polystyrene
beads are pre-expanded to a density from about 0.5 to 12 pounds per cubic
foot,
hereafter pcf, (8 to 192 g/L); an example of this first step shown Table 2.
Second, the
pre-expanded beads are heated in a closed mold to further expand the pre-
expanded
beads to form a fused or molded article having the shape of the mold, i.e., a
cup or
bowl. An example of this second step is shown in Table 3. Such EPS cups and
bowls
are lightweight, have adequate structural properties and have excellent
insulating
properties.
For a better understanding of the present invention, Figures 1 through 5 are
presented; however, these figures are intended purely as examples and are not
to be
construed as limiting.
24
CA 02881023 2015-02-04
Figure 1, shows EPS beads molded into a cup or bowl shape, with a bottom
portion 101 and an upper flange 102. The bottom portion forms a hollow
compartment
103 within which food or drink is placed. The bottom portion can be any number
of
shapes, e.g. the tray shapes shown in Figure 2. Figure 2 shows an upper flange
202,
a tray-like bottom portion 201 and a tray-like compartment 203. Figure 3 shows
an
upper flange 302 with four cup-like bottom portions, bottom portion 301a, 301c
and
301d can be seen in Figure 3, while bottom portion 301b is obscured. Figure 3
shows
four cup-like compartments 303a, 303b, 303c and 303d of equal volume. Figure 4
shows an upper flange 402 and three tray-like bottom portions; bottom potion
401a
and 401c can be seen in Figure 4, while bottom portion 401b is obscured.
Figure 4
shows three tray-like compartments 403a, 403b and 403c that differ in volume.
The
dimensions of the bottom portions and the dimensions of the multiple
compartments
are not critical; rather, these dimensions are governed by practical
requirements such
as the standard sizes for food and drink containers; for example, cups from 8
ounces
to 32 ounces are common (from about 237 mL to about 947 mL) and food
compartments typically vary from 1 ounce to 40 ounces (from about 30 mL to
about
1183 mL).
In Figure 1, the width of the upper flange (wf) is the difference bptween the
upper flange's outer radius, r1, and the upper flange's inner radius, r2; wf =
r1 ¨ r2. The
width of the upper flange, wf, must be large enough such that a leak proof
seal is
formed between the upper flange and the lidding film. In some instances, wf is
from
0.039 inches to 0.39 inches (1 mm to 10 mm), in some cases from 0.079 inches
to
0.31 inches (2 mm to 8 mm) and in other cases 0.1 inch to 0.2 inch (2.5 mm to
5 mm).
In the case of a bottom portion containing multiple compartments, perforation
lines, or
easy-failure lines may be incorporated into the bottom portion by a variety of
methods
CA 02881023 2015-02-04
know to those skilled in the art, such as cutting, punching, nicking with
blades, heat
treatment, laser radiation, electron beam radiation, el4ctrostatic erosion,
dissolving
with solvents or etching by chemical reaction. Perforation lines or easy-
failure lines
facilitate the separation of one multiple compartment from the remaining
multiple
compartments, while maintaining a leak proof seal on all compartments.
Figure 5 shows several non-limiting examples of lidding film. At the top of
figure 5, film 5a illustrates a monolayer 10 of lidding film comprised of at
least one
thermoplastic. In the case of monolayer film 5a, such a film must be capable
of
sealing to the bottom portion forming a leak proof seal; thus one could also
call film 5a
the sealant film and/or the lidding film. Non-limiting examples of a
thermoplastic
monolayer include: styrene butadiene copolymers, blends of styrene butadiene
copolymer with other styrenic polymers, blends of styrene butadiene copolymers
with
intercalated polymers, a polyolefin, polyolefin blends and polyolefins blended
with
intercalated polymers. Particularly suitable polyolefins and/or polyolefin
blend
components include metallocene catalyzed ethylene copolymers commonly referred
to as elastomers, metallocene catalyzed ethylene copolymers commonly referred
to
as plastomers, ultralow density polyethylene (ULDPE), very low density
polyethylene
(VLDPE), linear low density polyethylene (LLDPE), medium density polyethylene
(MDPE), high density polyethylene (HDPE), low density polyethylene (LDPE),
ethylene vinyl acetate copolymers (EVA), ethylene alkyl acrylate copolymers,
ethylene acrylic acid copolymers, metal salts of ethylene acrylic acid
(commonly
referred to as ionomers), random propylene copolymers and impact or
heterophasic
propylene copolymers. An example of a 4.09 mil thick (104 m) monolayer
styrene
butadiene copolymer film is shown in Table 11. Typical densities of film grade
styrene
butadiene copolymers range from 1.00 g/cm to 1.05 g/cm, as determined by ASTM
26
CA 02881023 2015-02-04
D792. The melt indexes of film grade styrene butadiene copolymers range from 3
to
20 g/10 min, as determined by ASTM D1238 measured at 200 C using a 5kg load.
Embodiments of this invention also include multilayer lidding films, i.e.
films
containing more than one chemically distinct layer of thermoplastic, or
optionally
layers of non-thermoplastic materials such as aluminum foil or paper products,
etc.
Film 5b, shown in Figure 5, illustrates a three layer lidding film with three
chemically distinct layers: layer 20 is an outer most layer; layer 21 is a
core layer; and
layer 22 is an inner most layer. In film 5b the core layer is thicker than the
outer most
and inner most layers. In this non-limiting example, the core layer could be a
lower
cost thermoplastic, i.e. lower cost relative to monolayer film 5a because the
3-layer
film 5b uses less of a higher cost sealant thermoplastic. Not wishing to be
bound to
any particular three layer lidding film composition, an example of a three
layer lidding
film is LLDPE/LDPE/EVA where the EVA (poly(ethylene-co-vinyl acetate)) is the
inner
most layer, or sealant layer. Three layer film embodiments also include
thermoplastic
blends; more specifically, any one of the three layers in film 5b, any two of
the three
layers in film 5b, or all three layers in film 5b may be comprised of blends
of more than
one thermoplastic.
Film 5c, shown in Figure 5, illustrates a four layer lidding film with four
chemically distinct layer: layer 30 is an outer most layer; layer 31is an
outer-most-
intermediate layer; layer 32 is an inner-most-intermediate layer and; layer 33
is an
inner most layer. As shown, the outer most intermediate layer of film 5c is
the
thickest. A non-limiting examples of four layer lidding films are
polyamide/adhesive/LLDPE/plastomer or PET/adhesive/LLDPE/plastomer where the
polyolefin plastomer is the inner most layer, or sealant layer; in these 4-
layer
structures it would be desirable that the adhesive layer is relatively thin,
for example
27
CA 02881023 2015-02-04
about 1 to 5 percent of the total film thickness. In the case of lidding film
5c, shown in
Figure 5, embodiments also include thermoplastic bl-nds; more specifically,
any one
of the four layers in film 5c, any two of the four layers in film 5c, any
three of the four
layers in film 5c or all four of the layers in film 5c may be comprised of
blends of more
than one thermoplastic. Examples of four layer films :re shown in: Table 5,
film
Example 1 is a 3.071 mil (77.9 pm) thick film with PE /adhesive/LDPE/EVA (3.2%
VA)
layers, and; Table 9, film Example 2 is a 2.043 mil (5 .9 pm) thick film with
PET/adhesive/polypropylene/poly(propylene-co-ethyl,-ne) layers.
Film 5d, shown in Figure 5, illustrates a five la er lidding film with five
chemically distinct layers of approximately equal thic ness: layer 40 is an
outer most
layer; layer 41 is a outer-most-intermediate layer; layrr 42 is a core layer;
layer 43 is
an inner-most-intermediate layer; and layer 44 is an i ner most layer, or
sealant layer.
Not wishing to be bound to any particular five layer lioding film composition,
an
example of a five layer lidding film is LLDPE/tie-layer EVOH/tie-
layer/plastomer, where
the polyolefin plastomer is the inner most, or sealant ayer. The core layer in
this five
layer example is EVOH (ethylene vinyl alcohol) whic is a barrier resin (oxygen
barrier). The intermediate layers of tie resin layers a - required to prevent
delamination between the EVOH and polyolefin layer.. Five layer embodiments
also
include thermoplastic blends, e.g. any one, any two, .: ny three, any four or
all five of
the layers of film 5d shown in Figure 5, may be comp ised of blends of more
than one
thermoplastic. In multilayer food packaging films the number of individual
layers
typically increases when moisture barrier layers and/or oxygen barrier layers
are
introduced; which generally requires additional tie re-in layers. Barrier
layers
containing EVOH or polyamides are frequently sand iched between polyolefin
layers,
28
CA 02881023 2015-02-04
where the polyolefin layers provide functionality such as sealability,
toughness,
puncture resistance and abuse and/or scratch resistance.
Film 5e, shown in Figure 5, illustrates a seven layer lidding film with seven
chemically distinct layers: layer 50 is an outer most layer; layer 51 is an
outer-most-
intermediate layer; layer 52 is an outer-most-core intermediate layer; layer
53 is the
core layer; layer 54 is an inner-most-core intermediate layer; layer 55 is an
inner-
most-intermediate layer, and; layer 56 is the inner most layer, or sealant
layer. Not
wishing to be bound to any particular seven layer lidding film composition, an
example
of a seven layer lidding film is LLDPE/tie layer/polyamide/EVOH/polyamide/tie
layerNLDPE, where the VLPE is the inner most layer, or sealant layer. Such a
seven
layer structure is a high barrier film, will excellent barrier properties with
respect to
both oxygen and moisture. Seven layer embodiments also include thermoplastic
blends; wherein any one, any two, any three, any four, any five, any six or
all seven
layers of film 5e shown in Figure 5 may be comprised of blends of more than
one
thermoplastic.
Not wishing to be bound by any particular lidding film thickness, the total
thickness of the monolayer or multilayer lidding films shown in Figure 5 may
vary from
0.5 mil to 16 mil (13 pm to 406 pm), in some instances from 1.0 mil to 8 mil
(25 pm to
203 pm) and in other cases from 2.0 mil to 4 mil (51 pm to102 pm). It will be
appreciated by those skilled in the art that one can optimize lidding film
performance
through: the selection of thermoplastic(s); the blending of thermoplastics;
adjusting the
thickness of individual layers (layer ratios) and; adjusting the overall, or
total,
thickness. A non-limiting example of layer ratios for a three layer
LLDPE/LDPE/EVA
film is 20/60/20. By adjusting layer ratios, an experienced artisan can
optimize lidding
29
CA 02881023 2015-02-04
film performance attributes; non-limiting examples of performance attributes
lidding
film sealability, peelability, puncture resistance, toughness and optical
properties, etc.
A non-limiting example of layer ratios for a four layer film
PET/adhesive/LDPE/EVA are 20/2/43/35, as shown in Table 5 for film Example 1.
A non-limiting example of layer ratios for a five layer LLDPE/tie
layer/EVOH/tie-
layer/plastomer film are 60/10/10/10/10.
A non-limiting example of layer ratios for a seven layer LLDPE/tie
layer/polyamide/EVOH/polyamide/tie layerNLDPE film is 25/10/10/10/10/10/25.
Additional embodiments of this invention includes films containing more than 7
layers; such embodiments also include so call "microlayered" films containing
greater
than 100 layers.
Another embodiment includes multilayer films containing non-thermoplastic
materials such as paper products or metals such as aluminum, or a
thermoplastic
layer having a vapor deposited metal on its surface, for example a thin
silicon oxide
(SiOx) or aluminum oxide (A10) layer vapor deposited on polypropylene.
Another lidding film embodiment includes at least one layer of intercalated
polymer, or intercalated polymer blended with other thermoplastics. As used
herein,
the term "intercalated" refers to the insertion of one or more polymer
molecules within
the domain of one or more other polymer molecules having a different
composition.
More specifically, styrenic polymers are inserted into polyolefin particles by
polymerizing a styrenic monomer mixture within the polyolefin particles, as
described
in U.S. Pat. No. 7,411,024; the disclosure of which is incorporated herein by
reference
in its entirety. The intercalated polymer typically contains 20% to 60% by
weight of an
uncross-linked polyolefin, e.g. polyethylene, polypropylene, and from 40% to
80% by
CA 02881023 2015-02-04
weight of a vinyl aromatic monomer, e.g., styrene; baed on the weight of the
intercalated polymer.
Not wishing to be bound by any particular monolayer lidding film composition,
but one or more intercalated polymers may comprise 100`)/0 of the lidding film
or 100%
of one or more layers of the lidding film. In addition, intercalated polymers
may be
blended with one or more thermoplastics, such as polyolefins or styrene
butadiene
copolymers. The amount of intercalated polymer in any layer may range from 0.5
wt%
to 100 wt%, based on the total weight of the intercalated polymer plus the
other
thermoplastics, in some cases from 5 wt% to 80 wt% and in other cases from 10
wt%
to 50 wt% of intercalated polymer. Suitable thermoplastics for blending
include
styrene butadiene copolymers, styrenic polymers or polyolefins.
Not wishing to be bound by any particular theory, the heterophasic morphology
of intercalated polymers in the sealant layer of the lidding film is
advantageous when
sealing to a bottom portion composed of expandable polystyrene. The
heterophasic
morphology of intercalated polymers is shown in Figure 6, the brighter regions
correspond to styrenic polymer and the darker regions correspond to
poly(ethylene-
co-vinyl acetate) containing 4 wt% vinyl acetate. In a multilayer lidding
film, the
presence of intercalated polymer in intermediate layers, or the outer layers,
is
advantageous, for example, in producing a lidding film that is easy to
puncture with a
piercing straw. The amount and types of intercalated polymer in the lidding
film is
determined based on the desired end use and physical properties, particularly
the
strength of the seal between the lidding film and the bottom portion, and
puncture
strength of the lidding film.
Monolayer thermoplastic lidding films are typically produced using a blown
film
or cast film process. In the blown film process, thermoplastic pellets are
melted in an
31
CA 02881023 2015-02-04
extruder and passed through an annular or tubular die. A molten thermoplastic
tube is
extruded, inflated with air, solidified, collapsed into a Sheet and the sheet
collected on
a product roll. In the cast film process, thermoplastic pellets are melted in
an extruder,
passed through a slot die, solidified on a chill roll and collected on a
product roll.
Similarly, multilayer thermoplastic films can be produced using blown or cast
film processes by adding additional extruders and using multilayer extrusion
dies.
Monolayer or multilayer lidding film may also be printed or decorated. Lidding
film
may also be produced by extrusion coating or extrusion lamination, these
processes
allow one to adhesively bond incompatible thermoplastic polymer layers and/or
combine dissimilar materials such as aluminum foil or paper products with
thermoplastics, as well as protect a high quality print or decoration layer
with a
protective thermoplastic layer.
The lidding film can optionally include, depending on its intended use,
additives
and adjuvants, which can include, without limitation, anti-blocking agents,
antioxidants, anti-static additives, colorants, dyes, filler materials, heat
stabilizers,
impact modifiers, light stabilizers, light absorbers, lubricants, pigments,
plasticizers,
slip agents, softening agents, and combinations thereof.
Suitable anti-blocking agents, slip agents and lubricants include without
limitation silicone oils, liquid paraffin, synthetic paraffin, mineral oils,
petrolatum,
petroleum wax, polyethylene wax, hydrogenated polybutene, higher fatty acids
and
the metal salts thereof, linear fatty alcohols, glycerine, sorbitol, propylene
glycol, fatty
acid esters of monohydroxy or polyhydroxy alcohols, phthalates, hydrogenated
castor
oil, beeswax, acetylated monoglyceride, hydrogenated sperm oil, ethylenebis
fatty
acid esters, and higher fatty amides. Suitable lubricants include, but are not
limited to,
ester waxes such as the glycerol types, the polymeric complex esters, the
oxidized
32
CA 02881023 2015-02-04
polyethylene type ester waxes and the like, metallic stearates such as barium,
calcium, magnesium, zinc and aluminum stearate, salts of 12-hydroxystearic
acid,
amides of 12-hydroxystearic acid, stearic acid esters of polyethylene glycols,
castor
oil, ethylene-bis-stearamide, ethylene- bis-cocamide, ethylene-bis-lauramide,
pentaerythritol adipate stearate and combinations thereof in an amount of from
0.1
wt% to 2 wt% of the lidding film composition.
Suitable antioxidants include without limitation Vitamin E, citric acid,
ascorbic
acid, ascorbyl palmitrate, butylated phenolic antioxidants, tert-
butylhydroquinone
(TBHQ) and propyl gallate (PG), butylated hydroxyanisole (BHA), butylated
hydroxytoluene (BHT), and hindered phenolics such as IRGANOX 1010 and
IRGANOX 1076 available from Ciba Specialty Chemicals Corp., Tarrytown, NY.
Suitable heat stabilizers include, without limitation, phosphite or
phosphonite
stabilizers and hindered phenols, non-limiting examples being the IRGANOX
stabilizers and antioxidants available from Ciba Specialty Chemicals. When
used, the
heat stabilizers are included in an amount of 0.1 wt% to 2 wt% of the lidding
film
compositions.
Suitable anti-static agents include, without limitation, glycerine fatty acid,
esters, sorbitan fatty acid esters, propylene glycol fatty acid esters,
stearyl citrate,
pentaerythritol fatty acid esters, polyglycerine fatty acid esters, and
polyoxethylene
glycerine fatty acid esters in an amount of from 0.01 wt% to 2 wt% of the
lidding film
compositions.
Suitable colorants, dyes and pigments are those that do not adversely impact
the desirable physical properties of the lidding film include, without
limitation, white or
any colored pigment. In embodiments of the invention, suitable white pigments
contain
titanium oxide, zinc oxide, magnesium oxide, cadmium oxide, zinc chloride,
calcium
33
CA 02881023 2015-02-04
carbonate, magnesium carbonate, kaolin clay and combinations thereof in an
amount
of 0.1 wt% to 20 wt% of the lidding film. In embodiments of the invention, the
colored
pigment can include carbon black, phthalocyanine blue, Congo red, titanium
yellow or
any other colored pigment typically used in the printing industry in an amount
of 0.1
wt% to 20 wt% of the lidding film. In embodiments of the invention, the
colorants,
dyes and pigments include inorganic pigments including, without limitation,
titanium
dioxide, iron oxide, zinc chromate, cadmium sulfides, chromium oxides and
sodium
aluminum silicate complexes. In embodiments of the invention, the colorants,
dyes
and pigments include organic type pigments, which include without limitation,
azo and
diazo pigments, carbon black, phthalocyanines, quinacridone pigments, perylene
pigments, isoindolinone, anthraquinones, thioindigo and solvent dyes.
Suitable fillers are those that do not adversely impact, and in some cases
enhance, the desirable physical properties of the lidding film. Suitable
fillers, include,
without limitation, talc, silica, alumina, calcium carbonate in ground and
precipitated
form, barium sulfate, talc, metallic powder, glass spheres, barium stearate,
calcium
stearate, aluminum oxide, aluminum hydroxide, glass, clays such as kaolin and
montmorolites, mica, silica, alumina, metallic powder, glass spheres, titanium
dioxide,
diatomaceous earth, calcium stearate, aluminum oxide, aluminum hydroxide, and
fiberglass, and combinations thereof can be incorporated into the polymer
composition
in order to reduce cost or to add desired properties to the lidding film. The
amount of
filler is desirably less than 20% of the total weight of the lidding film as
long as this
amount does not alter the properties of the lidding film.
Suitable impact modifiers include, without limitation, styrene butadiene
copolymers (e.g. HIPS or styrene butadiene block copolymers), acrylonitrile
butadiene
styrene terpolymers (ABS), copolymers of C1-C12 linear, branched or cyclic
olefins,
34
CA 02881023 2015-02-04
copolymers of C1-C12 linear, branched or cyclic alkyl esters of (meth)acrylic
acid and
styrenic monomers, ethylene propylene diene monomer (EPDM) rubbers and
styrene/ethylene copolymers. The amount of impact modifier used is typically
in the
range of 0.5 wt% to 25 wt% of the lidding film.
Suitable softening agents and plasticizers include, without limitation,
cumarone-
indene resin, terpene resins, and oils in an amount of about 2 parts by weight
or less
based on 100 parts by weight of the lidding film.
Figure 7 is a sketch of a process to make a leak proof container comprising a
bottom portion composed of expanded thermoplastic beads, a compartment
containing food or drink and a lidding film. The first process step in Figure
7 is
referred to as "shape molding". Typically shape molding is a two-step process.
First,
blowing agent impregnated expandable beads are pre-expanded to a density from
2
pet to 12 pet (32 g/L to 192 g/L) forming a "pre-puff. This first step, which
is typically
called the pre-expansion step, can be accomplished by heating the blowing
agent
impregnated beads using any conventional heating medium such as steam, hot
air,
hot water, or radiant heat. In the second step, typically referred to as shape
molding,
the bottom portion is formed. Expandable and/or pre-expanded resin beads are
injected into the mold cavity. When the mold cavity is full, steam is applied
thereby
heating the mold and further expanding the pre-puff to form the bottom
portion. At the
end of the heating cycle, the mold is water cooled, the male portion and
female portion
of the mold are separated and compressed air is supplied in order to
facilitate the
ejection of the bottom portion from the mold. Typically, molded EPS bottom
portions
have a density from about 2 pcf to 8 pcf (32 g/L to 128 g/L). As shown in
Figure 1
through 4, the bottom portion, or portions, may be a cup, bowl or tray;
comprising one
or more compartments. Table 1 summarizes the EPS that was used to produce the
CA 02881023 2015-02-04
bottom portions, i.e. DYLITE F271T, expandable polystyrene beads, available
from
NOVA Chemicals Inc.
It will be appreciated by those skilled in the art that processes and
equipment
are available for forming, heating, cooling, holding and delivering foods or
liquids into
containers, or bottom portions. In addition, a non-limiting embodiment,
includes filling
the bottom portion manually, and then manually transporting the filled bottom
portion
to the sealing station; such an embodiment would be common in a retail or
convenience store setting. The filling station step shown in Figure 7 is
intended to
include all processes that are used to deliver liquids and/or solid foods to a
container,
including manual processes.
Referring to Figure 7, after the food or drink has been deposited into the
compartment (or compartments), the bottom portion (or bottom portions) is
transported to the lidding station. Figure 8 illustrates one embodiment of a
lidding
station, where the lidding station consists of a sealing device 61 device
adapted to
move in the vertical direction between an open position and a closed position,
as
shown by position a (top of Figure 8) and position b (middle of Figure 8),
respectively.
A continuous roll of lidding film 62 is shown in Figure 8. The bottom portion,
60, rests
on a lower platform 66. In transitioning from the opened position a, to the
closed
position b, the sealing device 61 moves downward. In the closed position b,
the
sealing device produces intimate contacts between: i) a heat sealing disk 63
and the
outer most layer of the lidding film, and ii) the inner most layer of the
lidding film and
the bottom portion 60. Heat from the sealing disk 63, is transferred to the
interlace
between the inner most layer and the bottom portion 60, whereupon the inner
most
layer and the bottom portion fuse together and upon cooling a leak proof seal
is
formed. In the case of a continuous roll of lidding film the sealing device
also includes
36
CA 02881023 2015-02-04
a cutting disk 64. More specifically, when in closed position b, the sealing
disk 63
forms the leak proof heat seal and simultaneously the cutting disk 64 cuts the
lidding
film from the continuous roll. As shown in the lower portion of Figure 8,
after sealing
and cutting are complete, the sealing device 61 returns to the open position
and the
leak proof disposable container 65 can be transferred manually or mechanically
from
the lower plafform 66.
The cutting disk and heat sealing disk are non-overlapping and concentric,
with
the cutting disk having the larger diameter. Referring to Figure 9, when the
lidding film
is simultaneously sealed and cut, a disk of lidding film with radius r13 is
produced.
Radius r13 is larger than the outer radius of the upper flange, r11, producing
a lidding
film overhang with a radius (ri3-rii). The length of the lidding film overhang
is not
critical. In some cases (r13-rii) was from 0 mm to 1 mm, in some situations
from 1 mm
to 5 mm, in other cases from 5 mm to 10 mm and in still other cases from 10 mm
to 20
mm. The width of the upper flange, wf =(r13-ri2), must be wide enough such
that a
leak proof heat seal is formed between the upper flange and the inner most
layer of
the lidding film. In some cases wf is at least 0.039 inches (1 mm), in some
instances
from 0.039 inches to 0.39 inches (1 mm to 10 mm), in other cases from 0.079
inches
to 0.31 inches (2 mm to 8 mm) and in still other cases 0.1 inch to 0.2 inch
(2.5 mm to
5 mm).
In the embodiment shown in Figure 8 the continuous web of lidding film may
move in an intermittent fashion. More specifically, the lidding film 62
advances
horizontally, then stops while the sealing device 61 closes to lid the bottom
portion 60
and then opens to release the leak proof container 65 and accept the next
bottom
portion 60. In another embodiment, the continuous web of lidding film advances
horizontally with constant velocity, and the sealing device is adapted to move
back
37
CA 02881023 2015-02-04
and forth horizontally. Table 6 summarizes the operating conditions of the
sealing
device used in this work, the sealing device used was an intermittent
Automatic
Sealing Machine (Model ET-999S), available from Boba Tea Direct.
In an alternative embodiment, the lidding film sealing device 61 is stationary
and the lower platform 66 is adapted to move in the vertical direction between
an
opened position and a closed position. In transitioning from the opened
position to the
closed position, the lower platform moves upward. In the closed position the
bottom
portion 60 produces intimate contacts between: i) the bottom portion and the
inner
most layer of the lidding film, and ii) the outer most layer of the lidding
film and the
heat sealing disk 63. When the bottom portion and the inner most lidding film
layer
are in intimate contact, heat from the sealing disk is transferred to the
interface
between the inner most layer and the bottom portion whereupon the inner most
layer
and the bottom portion fuse together and upon cooling a leak proof seal is
formed. In
the case of a continuous roll of lidding film the sealing device 64
simultaneously cuts
the lidding film as the leak proof seal is formed. In the open position, the
leak proof
container is not in contact with the lidding film, and the lidding film is not
in contact
with sealing device, such that the leak proof container can be transferred
manually or
mechanically from the sealing station.
In the case of heat sealing, the optimal temperature and time required to form
a
leak proof seal depends on the thermal properties of both the lidding film and
the
bottom portion. In the case of 2 mil thick (51 micron) monolayer films
comprised of
polyethylene sealants, sealing disk temperatures between 90 C and 150 C are
typical,
with sealing disk dwell times between 0.25 seconds and 1.5 seconds. In the
case of
styrene butadiene copolymers and sealant grade polypropylenes, higher sealing
disk
temperature between 130 C and 190 C are more typical. Given a specific lidding
film
38
CA 02881023 2015-02-04
thickness and lidding film structure, one skilled in the art can optimize
sealing
conditions to produce a leak proof seal. Relevant data to the skill artisan is
shown in
Tables 7, 8, 10, 13 and 14 and Figures 10, 11, 12 and 13. More specifically:
the
physical properties of a poly(ethylene-co-vinyl acetate) sealant, or EVA,
Elvax 3135X
are summarized in Table 7 and melting point information is shown in Figures 10
and
11; the physical properties of a polyolefin plastomer sealant Affinity PL 188G
are
summarized in Table 8 and a DSC melting curve is shown in Figure 12; the
physical
properties of a poly(propylene-co-ethylene sealant Adsyl 5C 37F are summarized
in
Table 10 and the DSC melting curve is shown in Figure 13; the physical
properties of
a high impact polystyrene (HIPS), lnnova RC600, are summarized in Table 13,
and;
the physical properties of a styrene butadiene block copolymer are summarized
in
Table 14. Styrene butadiene copolymers with melt indexes that range from 3 to
20
g/10 min are suitable for lidding film applications, wherein the melt index is
determined
using ASTM D1238 and measured at 200 C using a 5kg load. Film grade styrene
butadiene copolymers typically have densities that range from 1.00 to 1.04
g/cc, as
measured by ASTM D792.
Not wishing to be bound by any theory, it is believed that to achieve a leak-
proof seal by heat sealing the most critical material parameters are the
softening/melting behavior of the sealant layer, the softening/glass
transition behavior
of the EPS bottom portion (cup or bowl) and the settings or parameters on the
heat
sealing device, i.e., the heat sealing parameters shown in Table 6. As
demonstrated
by lidding film Example 1, a leak proof seal was produced when the inner most
layer
was composed of an EVA containing 3.2 wt% of vinyl acetate. Figure 10 suggests
that a leak proof seals can be obtained with EVA copolymers up to about 12 wt%
vinyl
acetate. More specifically, the DSC melting point of an EVA containing 12 wt%
vinyl
39
CA 02881023 2015-02-04
acetate is similar to the 95 C glass transition of EPS (relative to pure
polystyrene, the
glass transition of EPS is depressed due to the presence of the pentane
blowing
agent which acts as a plasticizer). The DSC thermogram of a 12 wt% vinyl
acetate
EVA copolymer is shown in Figure 11, specifically, Elvax 3135X (available from
DuPont Packaging & Industrial Polymers). As shown in Figure 11, the peak DSC
melting point of Elvax 3135X is 94.78 C, which is close to the glass
transition
temperature of EPS. Additional physical properties of Elvax 3135X are
summarized in
Table 7. Suitable lidding films, where the inner most layer contains an EVA,
with a
DSC melting point between about 90 C and about 125 C, are suitable to form a
leak
proof seal with an EPS bottom portion (cup, bowl, tray, etc.). In addition,
one would
expect lidding films, wherein the inner most layer contains a blend of more
than one
thermoplastic, wherein at least 30 wt% is an EVA copolymer with a DSC melting
point
between about 90 C and about 125 C, to form a leak proof seal with an EPS
bottom
portion.
Similar to EVA's, poly(ethylene-co-a-olefin) plastomers also have well defined
melting points. Figure 12 shows the DSC thermogram of a poly(ethylene-co-1-
octene)
plastomer, Affinity PL1881G, 0.904g/cm3 and 1.0 melt index (available from Dow
Chemical). The peak DSC melting point of Affinity PL1881G is 97.48 C, which is
close to the glass transition temperature of EPS. Additional physical
properties of
Affinity PL1881G are summarized in Table 8. Suitable lidding films, where the
inner
most layer contains a polyolefin plastomer, with a DSC melting point between
about
90 C and about 125 C, should form a leak proof seal with an EPS bottom portion
(cup, bowl, tray, etc.). In addition, lidding films, wherein the inner most
layer contains
a blend of more than one thermoplastic, wherein at least 30 wt% is a
polyolefin
CA 02881023 2015-02-04
plastomer with a DSC melting point between about 90 C and about 125 C, should
form a leak proof seal with an EPS bottom portion.
Specific polypropylene sealants are also suitable sealants. For example, Adsyl
5C37F, commercially available from LyondellBasell Industries, has a seal
initiation
temperature of 105 C, as shown in Table 10. This seal initiation temperature
is
supported by the DSC thermogram of Adsyl 5C37F, as indicated by the broad
melting
peak at 104.69 C shown in Figure 13. Portions of Adsyl 5C37F also melt at
134.80 C
and 145.65 C. Additional physical properties of Adsyl 5C37F are summarized in
Table 10.
The lidding film need not be supplied to the sealing station from a continuous
roll. Rather, pre-cut pieces of lidding film of circular, ellipsoid, square,
rectangle or
polygon shapes may fed to the sealing station mechanically or manually. In the
case
of bottom portions with multiple compartments the sealing and cutting device
may be
adapted to individually seal each compartment, such that each compartment is
isolated from all other compartments. In another embodiment, sealing device
may be
adapted to include a perforation template, such that peroration lines, or easy-
failure
lines may be incorporated into the assembly of leak proof containers. Easy-
failure
lines facilitate the separation of one compartment from the multiple
compartment
assembly, while maintaining a leak proof seal on all compartments. A variety
of
methods know to those skilled in the art, can be used to incorporate easy-
failure lines;
such as cutting, punching, nicking with blades, heat treatment, laser
radiation, electron
beam radiation, electrostatic erosion, dissolving with solvents or etching by
chemical
reaction.
Recall Figure 7 illustrating a process to produce a leak proof container made
from expandable thermoplastic resin beads. After lidding is complete, the
process
41
CA 02881023 2015-02-04
bifurcates into: i) a bulk packaging station, or; ii) a retail station. These
two processes
differ significantly in the degree of automation and output rates, i.e., leak
proof
containers produced per unit time.
High throughput, fully integrated bulk packaging lines are well known per se
and will not be described in detail. In brief, integrated food packaging
plants may
include: shape molding equipment and filling machines followed by at least one
downstream packaging train which may including food package accumulators,
stacking, cardboard box packers, pallet systems and stretch film wrappers.
Such high
throughput lines are typically computer controlled and monitored with the aim
of
optimizing the interaction between the filling machines and downstream
packaging
operations to maximize packaging line output.
In contrast with bulk packaging stations, the Retail Station (single serving)
shown in Figure 7 represents embodiments such as retail stores, convenience
stores
or take-out restaurants. In such settings, manual filling, manual lidding and
manually
transportation from station to station may be more cost effective than high
speed
automated packaging equipment.
An additional embodiment of the present invention includes an optional
disposable drinking straw. The drinking straw may be composed of any suitable
material. For example, a straw produced from one or more thermoplastics by an
extrusion process where a straw-like tube is extruded and cut to length.
Optionally,
one end of the straw is cut in a hypodermic needle-like fashion, hereafter
referred to
as a "piercing straw". The consumer can use the piercing straw to puncture the
lidding film of the leak proof container, inserting the piercing straw into a
liquid and
consume the liquid through the piercing straw. Although not leak proof, an
adequate
seal remains between the piercing straw and the pierced lidding film, due to
the elastic
42
CA 02881023 2015-02-04
nature of the lidding film. The leak proof seal remains intact between the
lidding film
and upper flange of the bottom portion. As a result, once pierced with the
piercing
straw, the leak proof container prevents splashing or loss of the liquid
during transport,
for example walking, riding a bicycle or sudden stops in a motor vehicle.
Optionally,
one could completely remove the lidding film from the bottom portion; insert a
straw or
piercing straw into the liquid and drink.
In a retail store, convenience store or take-out restaurant setting, a straw
or
piercing straw could be a separate item where the consumer selects the straw
or
piercing straw from a dispenser. Optionally, one could produce a leak proof
container
and straw or piercing straw that is 100 percent recyclable under the #6 PS
symbol.
In a high throughput, fully integrated bulk packaging line the optional straw
or
piercing straw could be added in an automated straw attachment step and
packaged
along with the leak proof container. Optionally, one could produce a leak
proof
container and straw or piercing straw combination that is 100 % recyclable
under the
#6 PS symbol.
The puncture performance and peel performance of the leak proof containers
were evaluated by developing the following the in-house tests.
Lidding Film Puncture Test
An Instron Model 4400R equipped with Instron Bluehill 2 software and a 100
pound load cell was used to generate a displacement-load curve in compression
mode to measure the puncture strength of the lidding film attached to the EPS
bottom
portion. The bottom portions tested were 16 ounce (0.47 liters) noodle bowls
fabricated from the expandable polystyrene beads (EPS) described in Table 1.
The
EPS beads were expanded and the noodle bowls were molded (forming the bottom
43
CA 02881023 2015-02-04
portion) as described in Example 1. Noodle bowl r1 and r2 dimensions were
1.860
inches (4.724 cm) and 1.716 inches (4.359 cm), respectively, thus the upper
flange
dimension was 0.144 inch (0.365 cm); see Figure 1.
To prepare the noodle bowls for puncture testing a galvanized steel washer
weighing 0.2 pounds (91 grams) and with dimensions of 0.18 inch (0.46 cm)
thick, 2.5
inch (6.4 cm) diameter with a 1 inch (2.5 cm) hole in the center was placed in
the
bottom of each noodle bowl. The noodle bowl containing the washer was lidded
with
lidding film (Example 1 and Example 3) using the Automatic Sealing Machine
(Model
ET-999S) as described in Example 1 above. The washer containing leak proof
container was mounted into the lnstron as described in the following
paragraph.
Four clear Plexiglass acrylic sheets, 4 inches (10 cm) square and 0.177 mil
(0.45 cm) thick, were glued together using GE Silicone II glue forming a
testing
platform. A puck-shaped rare earth magnet, 1.57 cm diameter (4 cm) and 0.47
inch
thick (1.2 cm), was glued (GE Silicone II) to the bottom of the testing
plafform, wherein
the magnet was placed precisely in the center of the testing platform, forming
a
magnetic fixture. The bottom of the magnetic fixture (magnet side) was
centered over
and magnetically attached to an lnstron compression platen, thus securely
attaching
the magnetic fixture to the lnstron. The lnstron compression platen was a
stainless
steel plate mounted into a tool steel support column that was pinned directly
into the
lnstron base. A circular rubber spacer, 2.33 inch (5.92 cm) in diameter and
0.1 inch
(0.25 cm) thick was centered on the top of the magnetic fixture and the washer
containing leak proof container was placed on top of the rubber spacer,
forming a
puncture test specimen. The rubber spacer fit snuggly into the empty space
defined
by the bottom rim on the noodle bowl and the top of the magnetic fixture. If
the rubber
spacer was not employed a cylindrical air gap of about 0.1 inch (0.25 cm) in
height
44
CA 02881023 2015-02-04
and 2.35 inches (5.97 cm) in radius would exist between the bottom of the
noodle
bowl and the magnetic fixture; given such an air gap, magnetic forces would
distort
the shape of the washer containing leak proof container, or alternatively pull
the
washer completely through the bottom of the noodle bowl. The rubber spacer was
reusable and was used for all puncture and peel tests. The acrylic sheets used
to
fabricate the magnetic fixture provide a flat and rigid testing platform as
well as allow
one to control (strengthen or weaken) the magnetic force on the washer in the
leak
proof container, i.e. four acrylic sheets were used in puncture testing and
two acrylic
sheets were used in peel testing.
In the puncture test the lnstron's upper test fixture was adapted to hold a
piercing straw. The piercing straw as fabricated as described in the rest of
this
paragraph. Drinking straws were purchased from a convenience store with the
following dimensions: length 7 inch (18 cm), outside diameter 0.45 inch (1.1
cm) and
wall thickness 0.014 inch (0.036 cm). With the long dimension of the straw
oriented in
a vertical fashion, the lower end of the straw was cut at a 45 angle from
horizontal
(forming a hypodermic needle-like lower end) and 4 inches of straw was cut
(horizontally) from the top end of the straw and discarded. These two cuts
produced a
hypodermic needle like piercing straw that was about 3 inches (7.6 cm) in
length. The
reduced straw length increased the effective stiffness of the straw and made
it easier
to mount the straw in the Instron such that the straw was oriented
perpendicular to the
lidding film surface.
In the puncture test, the downward movement of the lnstron's upper test
fixture,
travelling at a crosshead speed of 20 inches per minute (50.8 cm/min),
generated a
displacement-load curve in compressive mode as the straw descended, contacted
and pierced the lidding film. A typical displacement-load curve is shown in
Figure 14.
CA 02881023 2015-02-04
In Figure 14, five leak proof containers (specimen 1 to 5) were tested,
wherein the
lidding film was the same, i.e. lidding film Example 3 which was the monolayer
styrene
butadiene film described in Table 11. In Figure 14, each successive
displacement-
load curve was arbitrarily shifted to the right, this shift made it easier for
the viewer to
inspect the shape of each curve. The average puncture test results (average of
five
specimens) are summarized in Table 15. The "Average Puncture Force at Straw
Breakthrough" was the average compressive force in pounds force (lb-f)
required to
pierce the lidding film.
Lidding Film Peel Test
An Instron Model 4400R equipped with Instron Bluehill 2 Software and a 100
pound load cell was used to generate a displacement-load curve in extension
mode to
measure the peel strength of the lidding film attached to the EPS bottom
portion. The
washer containing leak proof containers tested were fabricated as described
above in
the Lidding Film Puncture Test, hereafter the puncture test. A peel test
magnetic
fixture was fabricated as described in the puncture test, with the exception
that two
acrylic sheets were used rather than four. In peel testing a higher magnetic
force was
required to hold the washer containing leak proof container in place, thus the
number
of acrylic sheets were reduced from four to two. The peel test magnetic
fixture was
attached to the Instron as described in the puncture test above. A peel test
specimen
was formed by centering the rubber spacer on the top of the magnetic fixture
and the
washer containing leak proof container was placed on top of the rubber spacer.
In the peel test, the lnstron's upper test fixture was adapted to hold a
stainless
steel fishing leader (wire). The top loop on the leader was directly attached
to the
Instron load cell by sliding the load cell pin through the loop in the leader.
The bottom
loop on the leader was attached to a strong metal clip and the jaws of the
metal clip
46
CA 02881023 2015-02-04
were attached to the lidding film overhang. Figure 9 defines a lidding film
overhang of
length (r13-ri 1).
With the washer containing leak proof container securely attached to the
magnetic fixture and the strong metal clip attached to the lidding film
overhang, the
upward movement of the lnstron's upper text fixture, travelling at a crosshead
speed
of 20 inches per minute (50.8 cm/min), generated a displacement-load curve, or
a
peeling force curve. Atypical peeling force curve is shown in Figure 15. In
Figure 15
five leak proof containers (specimen 1 to 5) were tested, wherein the lidding
film was
the same, i.e. lidding film Example 3, the monolayer styrene butadiene film
described
in Table 11. In Figure 15, each successive displacement-load curve was
arbitrarily
shifted to the right, this shift made it easier for the viewer to inspect the
shape of each
curve.
As shown in Figure 15 there was an initial spike (the left-most peak in Figure
15) in the peeling force at the point where the leak proof seal was broken;
this initial
spike was called the "Average Peel Force at Start of Lidding Film Peel Off (lb-
f)" in
Table 16. As shown in Figure 15, a plateau in the peeling force was observed
starting
at an extension of about 1 inch (2.54 cm) and ending at an extension of about
3
inches (7.6 cm). This plateau in peeling force was called the "Average Plateau
Peeling Force" in Table 16. The Average Plateau Peeling Force is the average
of five
specimens, and it is the peeling force measure as the lidding film is peeled
from the
upper flange between 1 inch and 3 inches of travel on the upper flange. As
shown in
Figure 15 at the end of the peel test, at the moment the lidding film was
completely
detached from the upper flange, there was a second spike in peeling force (the
right-
most peak in Figure 15); this second spike was called the "Average Peel Force
at End
47
CA 02881023 2015-02-04
of Lidding Film Peel Off (lb-f)" in Table 16. The average peel test results
(average of
five specimens) are summarized in Table 16.
The present invention will further be described by reference to the following
examples. The following examples are merely illustrative of the invention and
are not
intended to limit the scope of the invention.
EXAMPLES
Example 1
The specifications of expandable polystyrene beads, DYLITE F271T available
from NOVA Chemicals are summarized in Table 1.
F271T beads were pre-expanded to produce pre-puff using the continuous pre-
expansion conditions shown in Table 2. Pre-expansion was carried out in a
Thermoware VA-40 continuous type pre-expander, available from Thermoware EPS
Machinery bv in Barneveld, The Netherlands. Pre-expansion conditions were
adjusted to produce two samples of pre-puff, a high density pre-puff or pre-
foam from
3.95 pcf to 4.15 pcf (63.3 g/L to 66.5 g/L) and a low density pre-puff or pre-
foam from
2.70 pcf to 2.85 pcf (43.2 g/L to 45.7 g/L).
After pre-expansion, the pre-puff was air dried for 5 minutes to remove
moisture and aged from 2 hours to 4 hours prior to molding.
Expandable polystyrene containers, cups or bowls, can be fabricated by any
conventional molding machine that has an inner shell and an outer shell, for
example,
Cup Production Model 6-VLC-125 machine made by Autonational B.V., or Model M10
cup machine made by Master Machine and Tool, LLC. 12 ounce (0.35 L) and 16
ounce (0.47 L) cups, and a 16 ounce (0.47 L) noodle bowls were molded using
the
conditions shown in Table 3, using the Model 6-VLC-125 machine made by
Autonational B.V. Once molded, the containers were aged at least 24 hours
prior to
48
CA 02881023 2015-02-04
attaching the lidding film. The width of the upper flange, wf, of the
containers, cups
and bowl, are shown in Table 4, wf is an important parameter; the wider the
upper
flange the greater the contact area between the bottom portion (EPS cup or
bowl) and
the lidding film.
Lidding film Example 1 was purchased from Boba Tea Direct, 9674 E.
Arapahoe Road #155, Greenwood Village, Colorado 80112. Table 5 shows the
structure and composition of lidding film Example 1. The data in Table 5 was
generated by FTIR-microscopy; films samples were cut using a Leitz 1400
Microtome
and the chemical composition of each layer of the multilayer film, or
monolayer film,
was determined using a Nicolet Avatar 360 FT-IR spectrometer. As shown in
Table 5,
lidding film Example 1 had to a total thickness of 3.071 mil (77.9 pm) and
consisted of
four chemically distinct layers. The inner most layer, or sealant layer, of
film Example
1 was composed of poly(ethylene-co-vinyl acetate) or EVA. The EVA in film
Example
1 contained 3.2wt /0 vinyl acetate. An EVA containing 3.2wt% of vinyl acetate
has a
DSC melting point of 108.5 C, based on the linear regression line shown in
Figure 10.
As shown in Table 5, the outer most layer of Example 1 was composed of
polyethylene terephthalate (PET). The PET layer was adhesively laminated to an
intermediate layer of high pressure low density polyethylene (LDPE), as shown
in
Table 5.
Lidding film Example 1 was sealed to the bottom portion (EPS cups and bowls)
using Y-Fang Sealing Machine LTD, Automatic Sealing Machine (Model ET-999S),
available from Boba Tea Direct. The Model ET-999S was equipped with a 3.74
inch
(95 mm) diameter heat sealing ring and a universal cutter base that fits 3.62
inch (92
mm), 3.74 inch (95 mm) and 3.86 inch (98 mm) diameter cups. The experimental
conditions used to attach lidding film Example 1 to EPS cups and bowls are
shown in
49
CA 02881023 2015-02-04
Table 6. The successful attachment of lidding film Example 1 to EPS cups and
bowls
was a surprising result. The manufacturer, Boba Tea Direct, stated that they
do not
have a sealing machine that will attached lidding film to EPS cups and their
machines
can only attach lidding film to polypropylene or polyethylene terephthalate
cups.
Example 2
Expandable polystyrene beads, DYLITE F271T available from NOVA
Chemicals, was pre-expanded and molded into cups and bowls, as described in
Example 1.
In Example 2, lidding film Example 2 was used. Film Example 2 was a 2.043
mil thick (51.9 pm) four layer film, as shown in Table 9. The data in Table 9
was
generated by FTIR-microscopy, as described in Example 1. The inner most layer,
or
sealant layer, was composed of a random poly(propylene-co-ethylene) sealant.
As
shown in Table 9, the outer most layer of Example 2 was composed of
polyethylene
terephthalate (PET). The PET layer was adhesively laminated to an intermediate
layer of polypropylene. Lidding film Example 2 was purchased from Boba Tea
Direct,
9674 E. Arapahoe Road #155, Greenwood Village, Colorado 80112.
Regardless of the setting used on the Automatic Sealing Machine (Model ET-
999S), lidding film Example 2 could not be attached to the EPS cups or bowl.
Not wishing to be bound by any theory, it is believed that in the case of
lidding
film Sample #2, the DSC melting point of the poly(propylene-co-ethylene) inner
most
layer was too high. In other words, a leak-proof seal between the inner most
layer of
the lidding film and EPS bottom portion could not be achieved due to a
mismatch in
thermal properties. However, lower temperature poly(propylene-co-ethylene)
sealants
are available. For example, Adsyl 5C37F, commercially available from
LyondellBasell
Industries, has a seal initiation temperature of 105 C, as shown in Table 10.
This seal
CA 02881023 2015-02-04
initiation temperature is supported by the DSC thermogram of Adsyl 5C37F, as
indicated by the broad melting peak at 104.69 C shown in Figure 13. Portions
of
Adsyl 5C37F also melt at 134.80 C and 145.65 C. Additional physical properties
of
Adsyl 5C37F are summarized in Table 10.
Example 3
Expandable polystyrene beads, DYLITE F271T available from NOVA
Chemicals, was pre-expanded and molded into cups and bowls, as described in
Example 1.
In Example 3, lidding film Example 3 was used. As shown in Table 11, film
Example 3 was a 4.09 mil thick (104 pm) monolayer film containing a styrene
butadiene copolymer. The data in Table 11 was generated by FTIR-microscopy, as
described in Example 1. Film Example 3 was manufactured by Multiplastics, Inc.
7770 North Central Drive, Lewis Center, Ohio 43035, USA. Table 12 summarizes
additional Multiplastic Inc. technical data on film Example 3, referred to as
"370W
White Lidding Film". Styrene butadiene copolymers are available from a variety
of
suppliers. For example, Table 13 summarizes a suitable high impact polystyrene
lnnova RC600 available from lnnova SA, Higienopolis, Porto Alegre, RS Brazil;
and
Table 14 summarizes a suitable styrene butadiene block copolymer, K-Resin SBC
KRO1BR, available from Chevron Phillips Chemical Company LLC, The Woodlands,
TX USA.
Lidding film Example 3 was attached to the bottom portion (EPS cups and
bowls) using Y-Fang Sealing Machine LTD, Automatic Sealing Machine (Model ET-
999S), available from Boba Tea Direct. The attachment and operation of the
Automatic Sealing Machine is described in Example 1. The successful attachment
of
lidding film Example 3 to EPS cups and bowls produces a leak proof container.
Such
51
CA 02881023 2015-02-04
a leak proof container has the advantage of being 100 percent recyclable under
the #6
PS symbol (polystyrene); in addition, the use of a thermoplastic lidding film
reduces
the mass of lidding material by 74% relative to the commonly used snap-on
polystyrene lid.
Lidding Film Puncture Test Results
The average puncture test results (average of five specimens) are summarized
in Table 15, as well as the standard deviations, for the two lidding films
tested, i.e. film
Examples 1 and 3. Film Example 2 was not tested because this film could not be
attached to the EPS noodle bowl, or bottom portion. The "average puncture
force at
straw breakthrough" was the average compressive force in pounds force (lb-f)
required to pierce the lidding film and the "average extension at straw
breakthrough"
was distance the straw travelled from the point of contacting the lidding film
to the
point of piercing the lidding film.
Lidding Film Peel Test Results
The average peel test results (average of five specimens) are summarized in
Table 16, as well as the standard deviations, for the two lidding films
tested, i.e. film
Examples 1 and 3. Film Example 2 was not tested because this film could not be
attached to the EPS noodle bowl, or bottom portion.
52
CA 02881023 2015-02-04
TABLE 1
Specification of DYLITE F271T, expandable polystyrene beads, available from
NOVA Chemicals Inc.
Typical Values Typical Values
Parameter (English Units) (S.I. Units)
Bead Size 0.012 to 0.02 0.3 to 0.5 mm
inches
Pentane Content 5.3 to 5.9 %
based on the weight of the EPS beads
Bulk Density 38 ¨ 40 lbs/ft3 608 ¨ 640 g/L
Thermal Resistance 4.2 per inch
(R-value)
Thermal 0.235 33.9
Conductivity Btu in/(hr ft2 F) milliWatts/(m K)
(K-factor, Lambda)
Coefficient of Linear 3.5x105 in/in/ F 6.3 cm/cm/ C
Expansion
Maximum 175 F 80 C
Continuous Service
Temperature
53
CA 02881023 2015-02-04
TABLE 2
Pre-expansion experimental conditions to produced EPS pre-puff at two
densities
Container Density (Target) 2.75 pet 4.05 pcf
(44 g/1) (65 g/1)
Feeder Screw Speed 6 rpm 6 rpm
Inlet Steam Temperature 94.0 to 95.5 C 91.0 to 93.0 C
Steam Pressure 0.50 to 0.60 bar 0.50
to 0.60 bar
(0.05 to 0.06 MPa) (0.05 to 0.06 MPa)
Air Pressure 0.40 to 0.60 bar 0.75
to 1.00 bar
(0.04 to 0.06 MPa) (0.075 to 0.1 MPa)
Fluidized Bed Dryer Air 175 to 185 F 175 to185 F
Temperature (79.4 to 85 C) (79.4 to 85 C)
Pre-puff Density Range 2.70 to 2.85 pet 3.95
to 4.15 pcf
(43.2 to 45.7 g/L) (63.3 to 66.5 g/L)
TABLE 3
Container molding conditions using the M-10 cup molding machine made by
Master Machine and Tool, LLC. Containers were produced at two densities
Container Density (Target) 2.75 pet 4.05 pet
(44 g/L) (65 g/L)
Mold Used 12 or 16 ounce cup 16 ounce noodle bowl
(0.35 L or 0.47 L) (0.47 L)
Pre-puff Aging Time 2 to 4 hours 2 to 4 hours
54
CA 02881023 2015-02-04
(after Pre-Expansion)
Steam Pressure 22 to 28 psig 22 to 28 psig
(0.15 to 0.19 MPa) (0.15
to 0.19 MPa)
Air Pressure 90 to 100 psig 90 to 100 psig
(0.62 to 0.69 MPa) (0.62
to 0.69 MPa)
Cooling Water Temperature 100 to 115 F 100 to 115 F
(38 to 46 C) (38 to 46 C)
Fill Time 1.0 to 1.5 seconds 2.0
to 3.0 seconds
Pre-heat Time 1.5 to 2.5 seconds 2.0
to 3.0 seconds
Cook Time 2.5 to 3.5 seconds 4.0
to 6.0 seconds
Cool Time 3.0 to 4.0 seconds 4.5
to 6.0 seconds
Total Cycle Time 12.0 to 13.5 seconds
18.5 to 20.0 seconds
TABLE 4
Upper Flange dimensions of 12, 16 and 32 ounce cups and 16 ounce noodle
bowl
Cups 16 ounce
12 ounce 16 ounce 32 ounce step noodle bowl
Dimension (0.35 L) (0.47 L) cup (0.47
L)
(0.94 L)
Upper Flange 0.126 inch 0.116 inch
0.122 inch 0.144 inch
Width (3.21 mm) (2.96 mm)
(3.09 mm) (3.77 mm)
Wf = (ri-r2)
55
CA 02881023 2015-02-04
TABLE 5
Multilayer structure of lidding film Example 1 (12-10952)
Film Layer Film Layer
Film Layer Thickness Thickness Material
(mils) (micron)
1 1.08 27.4 EVA (3.2% VA)
Inner most, or Sealant Layer
2 1.33 33.7 LDPE
3 0.051 1.3 Adhesive
4 0.61 15.5 Polyethylene
terephthalate
Total: 3.071 Total: 77.9
TABLE 6
Liddinq film attachment (sealing) conditions used: Automatic Sealing Machine
(Model ET-999S), available from Boba Tea Direct
Sealing Conditions
Container 12 or 16 ounce cup
16 ounce noodle bowl
(0.35 L or 0.47 L) =
(0.47L)
Sealing Temperature Setting 120-140 C 120-140 C
Sealing Time 1.5-2.5 seconds 1.5-2.5 seconds
Settling/Rest Time 1.5-2.5 seconds 1.5-2.5 seconds
56
CA 02881023 2015-02-04
TABLE 7
Poly(ethylene-co-ethylene vinyl acetate). Elvax 3135X Ethylene Vinyl Acetate
Copolymer, available from DuPont Packaging and Industrial Polymers. Data
extracted from Elvax 3135X Technical Datasheet
Property Nominal Value Test Method
Unit
Specific Gravity 0.930 g/cm3 ASTM
D792, ISO 1183
Melt Mass-Flow Rate (MFR) 0.35 g/10min ASTM
D1238, ISO 1133
(190 C/2.16kg)
Vinyl Acetate Content 12.0 wt%
Vicat Softening 82.0 C ASTM
D1525, ISO 306
Temperature
Melting Temperature (DSC) 95 C ASTM
D3418, ISO 3146
Freezing Point (DSC) 78 C ASTM
D3418, ISO 3146
Extrusion Melt Temperature < 230 C
Fabrication Conditions for blown film: screw size 2.5 in. (63.5mm); extruder
barrel
24:1 L/D; screw type DSB II; die gap 70 mil (1.8 mm); melt temperature 430 F
(221 C); output 6 lb/hr/in of die circumference; die diameter 6 inch; blow-up
ratio
2.5:1; screw speed 4Orpm.
57
CA 02881023 2015-02-04
TABLE 8
Poly(ethylene-co-1-octene) plastomer, Affinity PL 1881G, available from Dow
Chemical Data extracted from PL 1881G Technical Datasheet
Property Nominal Value Test Method
Unit
Specific Gravity 0.904 g/cm3 ASTM D792
Melt Mass-Flow Rate (MFR) 1.0 g/10min ASTM D1238
(190 C/2.16kg)
Seal initiation Temperature 85 C Dow Internal Method
Melting Temperature (DSC) 100 C Dow Internal Method
Vicat Softening Temperature 86.0 C ASTM D1525
Coefficient of Friction 0.15 ASTM D1894
(vs Itself-Dynamic)
Film Thickness ¨ Tested 51 pm
Film Puncture Energy 8.09 J Dow Internal Method
Film Puncture Force 52.3 N Dow Internal Method
Film Puncture Resistance 21.9 J/cm3 Dow Internal
Method
Secant Modulus 2% Secant, MD 97.4 MPa ASTM D882
Secant Modulus 2% Secant, TD 96.9 MPa ASTM D882
Tensile Strength MD Yield 8.07 MPa ASTM D882
Tensile Strength TD Yield 7.17 MPa ASTM D882
Tensile Strength MD Break 45.4 MPa ASTM D882
Tensile Strength TD Break 42.5 MPa ASTM D882
Tensile Elongation, MD Break 590 % ASTM D882
58
CA 02881023 2015-02-04
Tensile Elongation, TD Break 630 `)/0 ASTM D882
Dart Drop Impact > 830 g ASTM
D1709B
Elmendorf Tear Strength, MD 560 g ASTM D1922
Elmendorf Tear Strength, TD 730 g ASTM D1922
Block Force 70 g ASTM D3354-89
Gloss (20 ) 112 ASTM D2457
Clarity 83.0 ASTM D1746
Haze 3.2% ASTM D1003
Extrusion Melt Temperature 221 C
Fabrication Conditions for blown film: screw size 2.5 in. (63.5mm); extruder
barrel
24:1 LID; screw type DSB II; die gap 70 mil (1.8 mm); melt temperature 430 F
(221 C); output 6 lb/hr/in of die circumference; die diameter 6 inch; blow-up
ratio
2.5:1; screw speed 40rpm.
TABLE 9
Multilayer structure of lidding film Example 2 (12-10953)
Film Layer Film Layer
Film Layer Thickness Thickness Material
(mils) (micron)
1 0.25 6.3
Poly(propylene-co-ethylene)
Inner most, or Sealant Layer
2 1.23 31.3 Polypropylene
3 0.067 1.7 Adhesive
4 0.496 12.6
Polyethylene terephthalate
Total: 2.043 Total: 51.9
59
CA 02881023 2015-02-04
TABLE 10
Poly(propylene-co-ethylene) sealant Adsyl 5 C 37 F, available from
LyondellBasell Industries. Data extracted from Adsyl 5 C 37 F Technical
Datasheet
Nominal
Property Value Unit Test Method
Specific Gravity 0.900
g/cm3 ASTM D792, ISO 1183/A
Melt Mass-Flow Rate (MFR) 5.5 g/10min ASTM D1238, ISO 1133
(230 C/2. 16kg)
Seal initiation Temperature 105 C
Melting Temperature 132 C ISO 11357-3
Vicat Softening Temperature 107 C ISO 306/A50
Tensile Strength (Yield) 21.4 MPa ASTM D638
Tensile Elongation (Yield) 13 % ASTM D638
Flexural Modulus 1% Secant 648 MPa ASTM D790A
Film Thickness ¨ Tested 50 pm
Tensile Modulus MD, Cast Film 280 MPa ISO 527-3/25
Secant Modulus TD, Cast Film 280 MPa ISO 527-3/25
Tensile Stress MD Yield, Cast Film 14.0 MPa ISO 527-3/500
Tensile Stress TD Yield, Cast Film 14.0 MPa ISO 527-3/500
Tensile Stress MD Break, Cast Film 45.0 MPa ISO 527-3/500
Tensile Stress TD Break, Cast Film 35.0 MPa ISO 527-3/500
Tensile Elongation MD Yield, Cast 17 % ISO 527-3/500
Film
CA 02881023 2015-02-04
Tensile Elongation TD Yield, Cast Film 15 % ISO 527-
3/500
Tensile Elongation MD Break, Cast 900 % ISO 527-
3/500
Film
Tensile Elongation TD Break, Cast 800 % ISO 527-
3/500
Film
Notched Izod Impact (23 C) 85 J/m ASTM
D256A
Deflection Temperature Under Load, 62.8 C ASTM
D648
0.45 MPa unannealed
Deflection Temperature Under Load, 62.0 C ISO 75-
2/B
0.45 MPa unannealed
Gloss (45 , 50pm, Cast Film) 87 ASTM
D2457
Haze 1.0% ASTM
D1003
TABLE 11
Monolayer structure of lidding film Example 3 (13-09907)
Film Layer Film Layer
Film Layer Thickness Thickness Material
(mils) (micron)
Styrene Butadiene
1 4.09 104
Copolymer
Total: 4.09 Total: 104
61
CA 02881023 2015-02-04
TABLE 12
Example 3, data extracted from Multiplastics, Inc. product data sheet on 370W
White Lidding Film
Units
Property Typical Values
Vicat Softening
F ( C) 194(90)
Point
psi (MD (Machine Direction)) 4,500
Tensile Strength psi (TD (Transverse
4,700
Direction))
% (MD) 55
Elongation at Break
% (TD) 45
COF 0.44 to 0.55
Gloss (45 ) 16 to 25
Dynes (treated side) ?_ 45
Treatment Level
Dynes (untreated side) 38
VVVTR (water vapor
g/100in2/24hr 1.14
transmission rate)
OTR (oxygen
cm3/100in2/24hr 93.6
transmission rate)
CO2 transmission
cm3/100in2/24hr 645
rate
62
CA 02881023 2015-02-04
TABLE 13
High Impact Polystyrene (HIPS), Innova RC600, data extracted from Innova SA
technical datasheet
Nominal Value
Property Unit Test Method
Specific Gravity 1.04 g/cm3 ASTM D792, ISO 1183
Melt Mass Flow Rate (MFR)
6.0 g/10min ASTM D1238, ISO 1133
(200 C/5kg)
Vicat Softening Point 196 F (91 C) ASTM D1525, ISO 306/A50
Tensile Strength 3,916 psi
ASTM D638
(Breakõ 23 C) (27 MPa)
Tensile Elongation
40 A ATM D638, ISO 527-2
(Break, 23 C)
Rockwell Hardness (L-scale) 80 ASTM D785, ISO 2039-2
Notched Izod Impact
70 J/m ASTM D256
(23 C, 3.2 mm)
63
CA 02881023 2015-02-04
TABLE 14
Styrene butadiene block copolymer (K-Resin SBC KRO1BR); data extracted from
Chevron Phillip Chemical Company LLC technical datasheet
Nominal Value
Property Unit Test Method
Specific Gravity 1.01 g/cm3 ASTM D792
Melt Mass-Flow Rate (MFR) 8.0 g/10min ASTM D1238
(230 C/2.16kg)
Vicat Softening Point 194 F (90 C) ASTM D1525
Tensile Strength (Yield) 4,844 psi ASTM D638
Tensile Elongation (Break) 30 A ASTM D638
Durometer Hardness (Shore 69 ASTM D2240
D)
TABLE 15
Leak proof container lidding film puncture test results (average of five
puncture
tests)
Film Example Film Example Film Example
1 2 3
Average puncture force
film failed
at straw breakthrough 4.68 0.73 5.31 0.76
to seal
(lb-f)
Average extension at
film failed
straw breakthough 0.348 0.049 0.269 0.020
to seal
(inches)
64
CA 02881023 2015-02-04
TABLE 16
Leak proof container lidding film peel test results (average of five peel
tests)
Measurement Film Example 1 Film
Example Film Example 3
2
Average Peel Force at
Start of Lidding Film film failed
1.69 0.13 1.26 0.092
Peel Off to seal
(lb-f)
Average Peel Extension
film failed
at Start of Lidding Film 0.196 0.013 0.197 0.020
to seal
Peel Off (inches)
"Average Plateau
Peeling Force (lb-f)"
film failed
peeling force between 1 0.480 0.078 0.384 0.019
to seal
inch and 3 inch of travel
on the upper flange
Average Peel Force at
End of Lidding Film Peel film failed
2.02 0.17 1.28 0.22
Off to seal
(lb-f)
Average Peel Extension
film failed
at End of Lidding Film 3.65 0.02 3.56 0.019
to seal
Peel Off (inches)
CA 02881023 2015-02-04
While the present invention has been particularly set forth in terms of
specific
embodiments thereof, it will be understood in view of the instant disclosure
that
numerous variations upon the invention are now enabled yet reside within the
scope
of the invention. Accordingly, the invention is to be broadly construed and
limited only
by the scope and spirit of the claims now appended hereto.
66