Note: Descriptions are shown in the official language in which they were submitted.
CA 02882522 2015-02-19
WO 2014/030030
PCT/IB2012/054210
1
METHOD OF PRODUCING A SUGAR PRODUCT FROM FRUIT
TECHNICAL FIELD
The present invention relates to a method of
producing a liquid or solid sugar product, in particular
glucose and fructose, from fruit.
BACKGROUND ART
Producing sugar, such as glucose and fructose, from
fruit juice, in particular grape juice, is known.
The juice, obtained for example by pressing the
fruit, is normally processed first to eliminate non-
sugar components, and then by chromatography to separate
the sugars, mainly glucose from fructose. The resulting
products, in liquid form or later crystallized, are
normally used in the food industry or consumed as they
are, for example, as sweeteners.
EP1734108 and EP2425723 describe a method of
producing sugar products from grapes, in which a
concentrated rectified must solution is chromatography
processed to obtain a glucose solution and a fructose
solution, which are then crystallized.
Prior to the chromatography stage, the juice
obtained from crushing the grapes is clarified and
demineralized, and then concentrated to the right
concentration for chromatography processing. The
EP1734108 and EP2425723 method, however, has the
CA 02882522 2015-02-19
WO 2014/030030
PCT/IB2012/054210
2
drawback of including no treatment to reduce the colour
of the sugar solutions, and no control of the
hydroxymethylfurfurol (HMF) content, which has
crystallization inhibiting properties. Also, the
chromatography process fails to provide for complete,
satisfactory separation, and more specifically for
correct, uniform extraction, of the glucose and
fructose. Demand therefore exists for a method of
separating sugars, particularly glucose and fructose,
from fruit juices, designed to eliminate the above
drawbacks, and which, in particular, provides for
controlling hydroxymethylfurfurol levels;
correct,
uniform extraction of sugars from fruit, while at the
same time safeguarding the integrity of the sugars; and
low energy consumption.
DESCRIPTION OF THE INVENTION
It is an object of the present invention to provide
a method of producing a sugar product from fruit,
designed to permit hydroxymethylfurfurol level control;
correct uniform sugar extraction from fruit, while at
the same time safeguarding the integrity of the sugars;
lower energy consumption than known methods; and product
purity comparable with that of known methods.
According to the present invention, there is
provided a method as claimed in Claim 1.
BRIEF DESCRIPTION OF THE DRAWINGS
CA 02882522 2015-02-19
2014-06-20 15:48 Studio Torts 5p +39 011 5622102 >> 923994465
P 10/11
PCT/IB 2012/054 210¨ 20-06-2014
3
A non-limiting embodiment of the invention will be
described by way of example, with particular reference
to grapes and to the attached drawing, in which :
Figure 1 shows a chromatography system for
separating a glucose solution and fructose solution in
accordance with the present invention.
BEST WAY OF IMPLEMENTING THE INVENTION
According to one non-limiting embodiment, the term
'juice' refers to grape juice, and in particular to
juice obtained from crushing grapes.
After being filtered to remove any remaining
solids, the juice so formed is clarified, e.g. by adding
gelatine, bentonite and carbon, and demineralized, e.g.
on ion resins. Alternatively, clarification and
demineralization may be performed as described in
EP2425723.
This stage produces a clarified, demineralized
fruit juice with a sugar, in particular glucose and
fructose, percentage of over 935t, e.g. 98.5t, with
reference to the dry substance.
Next, the clarified, demineralized fruit juice is
concentrated to reduce its water content, but without
altering the composition of its solid portion. The
obtained concentrated juice, for example rectified
concentrated must, is a colourless solution
containing over 98%, with reference to the dry substance, of
Duration: 20.06.2014 16:53:41 - 20.06.2014 16:56:49. This page 10 of AMENDED
SHEET2o14 16:56:35
Received at the EPO on Jun 20, 2014 16:56:49. Page 10 of 11
CA 02882522 2015-02-19
WO 2014/030030
PCT/IB2012/054210
4
fructose and glucose, and the rest of which is made up
of components such as alcohol, flavonoids and other
polyphenols.
The concentrated concentrated rectified must is
then heated to a temperature of 50-70 C, in particular
57-65 C, e.g. 60 C. Advantageously, at these
temperatures, the concentrated concentrated rectified
must has a viscosity (<10 mPa*s) allowing an uniform
leading front during chromatography process.
At these temperatures, the glucose and fructose in
the juice can be separated effectively with no need for
a high-pressure eluent, and above all without altering
the stability of the sugars and/or deteriorating the
chromatography resins.
So, despite operating at higher than the ambient
temperature of known methods, improved sugar separation
allows a considerable reduction in total energy
consumption of the method according to the invention as
a whole.
The concentrated concentrated rectified must is
then subjected to chromatographic separation, in
particular on ion-exchange resins, more specifically on
cation resins, and preferably on styrene-divinylbenzene
resins derivatized with sulphone groups. The resins used
may have the characteristics described in EP2425723.
Chromatographic separation is performed in a
CA 02882522 2015-02-19
WO 2014/030030
PCT/1B2012/054210
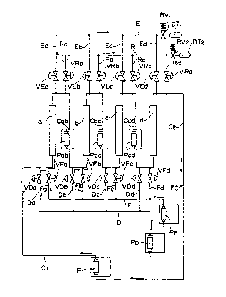
simulated fluid-bed system as shown in Figure 1.
The Figure 1 system comprises four columns : a, b,
c and d, each with a pump (P, Pab, Pbc, Pcd) for pumping
the circulating fluid, i.e. the eluent, through the
5 columns.
The four columns are filled with a cation resin,
preferably styrene-divinylbenzene resin derivatized with
sulphone groups.
The outlet of column a is connected to the inlet of
column b by a line Cab fitted with pump Pab; the outlet
of column b is connected to the inlet of column c by a
line Cbc fitted with pump Pbc; the outlet of column C is
connected to the inlet of column d by a line Ccd fitted
with pump Pcd; the outlet of column d is connected to
the inlet of pump P by a line C2; and the outlet of pump
P is connected to the inlet of column a by a line CI.
Columns a-d, lines Cl, C2, Cab, Cbc, Ccd and pumps P,
Pab, Pbc, Pcd thus form a separation circuit FC, through
which the circulating fluid, i.e. the eluent, e.g.
deionized water, circulates in the direction of the
arrows in Figure 1.
Eluent feed lines Da-Dd and concentrated rectified
must feed lines Fa-Fd into separation circuit FC are
connected to separation circuit FC close to the
respective inlets of columns a-d.
Glucose extraction lines Ea-Ed, for extracting a
CA 02882522 2015-02-19
WO 2014/030030
PCT/1B2012/054210
6
glucose-enriched fraction from separation circuit FC,
and fructose extraction lines Ra-Rd, for extracting a
fructose-enriched fraction from separation circuit FC,
are connected to separation circuit FC close to the
respective outlets of columns a-d.
Eluent feed lines Da-Dd branch off from an eluent
feed pipe D connected at one end to an eluent pump PD;
and concentrated rectified must feed lines Fa-Fd branch
off from a concentrated rectified must feed pipe F
connected at one end to a concentrated rectified must
pump PF.
Open-close valves VDa-VDd are installed along
respective eluent feed lines Da-Dd; and open-close
valves VFa-VFd are installed along respective
concentrated rectified must feed lines Fa-Fd.
Open-close valves VEa-VEd are installed along
respective glucose extraction lines Ea-Ed; and open-
close valves VRa-VRd are installed along respective
fructose extraction lines Ra-Rd.
Glucose extraction lines Ea-Ed are connected to a
glucose extraction pipe E.
Fructose extraction lines Ra-Rd are connected to a
fructose extraction pipe R.
The Figure 1 system operates as follows.
After initiating the system by filling it with
eluent - preferably deionized water or at any rate water
GA 02882522 2015-02-19
WO 2014/030030
PCT/1B2012/054210
7
with a low calcium content, which acts as the
circulating fluid - one of eluent feed lines Da-Dd, one
of glucose extraction lines Ea-Ed, one of concentrated
rectified must feed lines Fa-Fd and one of fructose
extraction lines Ra-Rd are connected so as to be
positioned in the following order: eluent feed line,
glucose extraction line, concentrated rectified must
feed line, fructose extraction line along the direction
of the circulation fluid in the separation circuit FC,
through opening and closing of valves VDa-VDd, VEa-VEd,
VFa-VFd and VRa-VRd.
For example, eluent feed line Da, glucose
extraction line Ea, concentrated rectified must feed
line Fc and fructose extraction line Rc are connected to
separation circuit FC by opening valves VDa, VEa, VFc,
VRc and closing all the other valves.
This results in the formation of an elution zone IV
in column a, between eluent feed line Da and glucose
extraction line Ea; a concentration zone III in column
b, between glucose extraction line Ea and concentrated
rectified must feed line Fc; a refining zone II in
column c, between concentrated rectified must feed line
Fc and fructose extraction line Rc; and an adsorption
zone I in column d, between fructose extraction line Rc
and eluent feed line Da.
In column a containing the elution zone, the resin-
CA 02882522 2015-02-19
WO 2014/030030
PCT/IB2012/054210
8
retained glucose is eluted by the eluent from line Da,
and is transferred to the circulating fluid in
separation circuit FC. At the inlet of column a, the
circulating fluid therefore contains roughly no glucose.
This concentration increases as the circulating fluid
flows through column a, and, at the outlet of column a,
the circulating fluid contains a large amount of glucose
(glucose-enriched fraction), part of which is extracted
from the separation circuit by glucose extraction line
Ea and collected in a glucose tank, and the rest of
which is fed into column b.
In column b containing concentration zone III, the
glucose in the circulating fluid from column a is
retained by the resin as the circulating fluid flows
through column b. In the meantime, the fructose in
column b, retained by the resin to a lesser degree than
the glucose, is transferred to the circulating fluid, so
the glucose concentration in the circulating fluid
flowing through column b decreases, while the fructose
concentration increases. The circulating fluid from
column b is then fed into column c together with a
portion of concentrated rectified must from line Pc.
In column c containing refining zone II, the
glucose in the concentrated rectified must from line Pc
and in the circulating fluid is retained by the resin,
and the fructose previously retained by the resin in
CA 02882522 2015-02-19
WO 2014/030030
PCT/IB2012/054210
9
column c is transferred to the circulating fluid. At the
inlet of column c, the circulating fluid therefore
contains roughly no glucose, whereas the fructose
concentration increases as the circulating fluid flows
through column c. The circulating fluid from column c
and containing a large amount of fructose (fructose-
enriched fraction) is partly extracted by fructose
extraction line Rc and collected in a fructose tank, and
partly fed into column d.
In column d containing adsorption zone I, the large
amount of fructose in the circulating fluid from column
c is retained by the resin, so, at the outlet of column
d, the circulating fluid contains practically no glucose
and no fructose, and is fed back into column a along
lines C1 and C2.
After a given time interval (6.5 minutes), valves
VDa, VEa, VFc, VRc are closed and valves VDb, VEb, VFd,
VRd are opened. Opening and closing the valves above
determines a transfer of the elution zone IV from column
a to column b, of the concentration zone III from column
b to column c, of the refining zone II from column c to
column -d, and of the adsorption zone I from column d to
column a.
In column b, the resin-retained glucose is
therefore eluted by the eluent from line Db; and the
circulating fluid from column b (glucose-enriched
CA 02882522 2015-02-19
WO 2014/030030
PCT/IB2012/054210
fraction) is partly extracted by glucose extraction line
Eb and collected in a glucose tank, and the rest fed
into column c along line Cbc.
In column c, the glucose in the circulating fluid
5 from column b is retained by the resin, and the
fructose, retained by the resin to a lesser degree, is
transferred to the circulating fluid, so the glucose
concentration in the circulating fluid decreases, while
the fructose concentration increases as the circulating
10 fluid flows through column c.
The circulating fluid from column c is fed into
column d together with the concentrated rectified must
from concentrated rectified must feed line Fd. In column
d, the glucose in the concentrated rectified must and in
the circulating fluid is retained by the resin, and the
fructose is transferred to the circulating fluid. And,
at the outlet of column d, part of the circulating fluid
(fructose-enriched fraction) is extracted by fructose
extraction line Rd and collected in a fructose tank, and
the rest is fed into column a.
In column a, the glucose and fructose in the
circulating fluid are retained by the resin, so the
circulating fluid from column a has no glucose and no
fructose, and is fed back into column b.
After a given time interval (6.5 minutes), valves
VDb, VEb, VFd, VRd are closed and valves VDc, VEc, VFa,
CA 02882522 2015-02-19
WO 2014/030030
PCT/IB2012/054210
11
VRa are opened. Opening and closing the valves above
determines a transfer of the elution zone IV from column
b to column c, of the concentration zone III from column
c to column d, of the refining zone II from column d to
column a, and of the adsorption zone I from column a to
column b; and the process is repeated as described
above.
Chromatographic separation is performed at a
temperature of 50-70 C, and more specifically of 57-
65 C, e.g. 60 C.
At these temperatures, the glucose and fructose in
the juice can be separated effectively with no need for
a high-pressure eluent, and above all without altering
the stability of the sugars and/or deteriorating the
chromatography resins.
On the other hand, these temperatures greatly
increase the amount of hydroxymethylfurfurol (HMF)
derived from thermal decomposition of the sugars.
In the production method according to the
invention, the concentrated rectified must and the
sugars in it are kept several days in the system. Before
it is crystallized, the sugar molecule remains in the
process solutions in the system for a period of 5-10
days, and at temperatures of 70-28 C, so a certain
amount of HMF, however small, is inevitably produced.
excessive amounts of hydroxymethylfurfurol may
CA 02882522 2015-02-19
WO 2014/030030 PCT/1B2012/054210
12
render the crystallization stage ineffective and greatly
reduce crystallization efficiency. In fact,
hydroxymethylfurfurol is a known
fructose
crystallization inhibitor.
Excessive amounts of hydroxymethylfurfurol have
the following effect on fructose crystallization :
20 ppm of HMF in the fructose syrup - 6096
crystallization efficiency
50 ppm of HMF in the fructose syrup - 5596
crystallization efficiency
200 ppm of HMF in the fructose syrup - 4596
crystallization efficiency
The chromatographic separation stage is therefore
followed by a filtration stage using carbon filters
(e.g. active-carbon - 3 micron filtration BECODISC
Filters ()) which, in addition to reducing the colour of
the chromatography eluate, also retains the HMF
contained in it, to achieve a high degree of
crystallization efficiency.
Temperature is preferably maintained constant at
both the chromatographic and filtration stages.
The post-filtration fructose- and glucose-enriched
fractions are then concentrated, e.g. in steam
concentrators, to the Brix required to produce liquid
sugar products for consumption or crystallization -
roughly 88 Brix for fructose and 75 Brix for glucose.
13
After chromatography and filtration, the glucose-
enriched fraction is over 86% pure, and the fructose-
enriched fraction over 96% pure.
Chromatographic separation, filtration and
concentration are performed at a preferably constant
temperature of 50-70 C, and more specifically 57-65 C,
e.g. 60 C.
The liquid sugar products obtained may then be
crystallized by cooling - and possibly inseminating
W fructose or glucose crystals - from a temperature of 50-
70 C to a temperature of 25-30 C. These crystallization
temperatures, as opposed to the 12-13 C temperatures
used in known methods, have the advantage of improving
sugar crystallization efficiency and greatly reducing
energy consumption.
The crystallized end products obtained are over 99%
pure.
CA 2882522 2018-10-31