Note: Descriptions are shown in the official language in which they were submitted.
CA 02883078 2016-07-20
SYSTEM FOR INCREASING SWELLING EFFICIENCY BY ION REMOVAL
BACKGROUND
[0001/2] Isolation of downhole environments depends on the deployment of a
downhole tool that effectively seals the entirety of the borehole or a portion
thereof, for
example, an annulus between a casing wall and production tube. Swellable
packers, for
example, are particularly useful in that they automatically expand to fill the
cross-sectional
area of a borehole in response to one or more downhole fluids. Consequently,
swellable
packers can be placed in borehole locations that have a smaller inner diameter
than the cross-
sectional area of the fully expanded swellable packer. However, certain
downhole
conditions, such as the presence of monovalent and polyvalent cations (e.g.,
Ca2+, Zn2F, etc.)
in the aqueous downhole fluids contacting the swellable packer, tend to
decrease both the
amount of swelling and the rate at which the packer swells, and may also
accelerate
degradation of the packer. In order to overcome these issues and to
continually improve upon
swelling efficiency under a variety of conditions, the industry is always
desirous of new and
alternate swelling systems.
SUMMARY
[0003] A swellable system reactive to a flow of fluid, including an article
including a
swellable material operatively arranged to swell upon exposure to a flow of
fluid, the flow of
fluid containing ions therein; and a filter material disposed with the
swellable material and
operatively arranged to remove the ions from the flow of fluid before exposure
to the swellable
material.
[0004] A method of operating a swellable system including filtering ions from
a flow
of fluid with a filter material; and swelling a swellable material responsive
to the flow of fluid
upon exposure to the fluid.
1
CA 02883078 2015-02-24
WO 2014/055891 PCT/US2013/063501
BRIEF DESCRIPTION OF THE DRAWINGS
[0005] The following descriptions should not be considered limiting in any
way.
With reference to the accompanying drawings, like elements are numbered alike:
[0006] Figure 1 is a cross-sectional view of a swellable article in an initial
configuration;
[0007] Figure 2 is a cross-sectional view of the swellable article of Figure 1
in a
swelled configuration;
[0008] Figure 3 is a swellable system according to an embodiment disclosed
herein
where a swellable article is disposed with a filter material in a shell
covering a swellable core;
and
[0009] Figure 4 is a swellable system according to another embodiment
disclosed
herein where a filter material is separately disposed from a swellable
article.
DETAILED DESCRIPTION
[0010] A detailed description of one or more embodiments of the disclosed
apparatus
and method are presented herein by way of exemplification and not limitation
with reference
to the Figures.
[0011] Referring now to Figure 1, a system 10 including a tubular or string 12
and a
downhole article 14, e.g., a packer or sealing element, disposed thereon is
illustrated. The
downhole article 14 includes, for example, a base composition and a filter
component,
discussed in more detail below. The base composition comprises an elastomeric
material
and/or an absorbent material. Due to fluid absorption by the absorbent
material, e.g.
absorption of water, brine, hydrocarbons, etc., the article 14 expands or
swells to a second
configuration shown in Figure 2. Various absorbent materials are known and
used in the art.
For example, with respect to water swellable embodiments any so-called Super
Absorbent
Polymer could be used, or those marketed by Nippon Shokubai Co., Ltd. under
the name
AQUALICO CS-65. The elastomeric material is included, for example, to provide
a seal
against a downhole structure 16, e.g., a borehole in a subterranean formation
18, shown in
Figure 2. Of course, the structure 16 could be any other tubing, casing,
liner, etc. located
downhole and engagable by the article 14. The elastomeric material could be
any swellable
or non-swellable material. In some embodiments, the elastomeric material is
absorbent with
respect to one or more downhole fluids thus also encompassing the absorbent
material. In
this way, for example, the article 14 can be run-in having an initially
radially compressed
configuration, exposed to fluids once located downhole, and expanded to engage
between the
2
CA 02883078 2015-02-24
WO 2014/055891 PCT/US2013/063501
tubular 12 and the structure 16. In one embodiment, the structure 16 is
isolated by expansion
of the article 14 such that fluids (e.g., from the formation 18) are
substantially prevented from
flowing past the article 14 once the article 14 is expanded.
[0012] Downhole fluids typically comprise an aqueous component, which more
accurately is a brine containing various ions, e.g., metal cations from
dissolved salts. As
noted above, monovalent and polyvalent cations can interact with the absorbent
material, and
decrease the overall rate and ratio of expansion of the absorbent material,
thereby hindering
the sealing efficacy of the article. It has been generally found that
polyvalent cations such as
Ca 2+, Zn2+5 etc. have a more profound effect on the performance of swellable
materials,
particularly in water swellable articles, than monovalent cations and are thus
usually more
desirable to be removed. It is to be appreciated that while water-swellable
materials are
discussed as an exemplary embodiment that is adversely affected by the
presence of cations,
other materials may be swellable in response to different fluids and/or
adversely affected by
anions. For example, in one embodiment the swellable material is adversely
affected (e.g.,
reduced swelling, shorter life span, slower swelling rate, etc.) by the
presence of anions. For
this reason, the term "ions" as used herein will refer to any cation or anion
that has a negative
effect on the performance of a corresponding swellable material.
[0013] To mitigate the deleterious effect of such ions on the absorbent
material, the
filter material acts to remove or filter ions from the downhole fluids before
they interact with
the swellable material. By remove or filter, it is meant that the filter
material captures or
holds the ions in, at, or proximate a capture site or location proximate to
the filter material, or
otherwise neutralizes the ions such that the flow of fluid is at least
partially relatively devoid
of ions downstream of the filter material. Thus, while the ions are still
technically in the
fluid, they are prevented from adversely affecting the swelling of the
swellable material and
therefore considered to be removed or filtered. The removal, filtering, or
capture may be
done by chemical or physical bonding between the filter material and the ions,
physisorption
or chemisorption at or by the filter material or a surface thereof,
electrostatic and/or van der
Waals attraction between the filter material or an atomic structure thereof
(e.g.,
functionalized group) and the ions, etc., examples of which are discussed in
more detail
below.
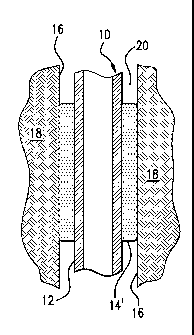
[0014] In the embodiment of Figures 1 and 2, the filter material, the
elastomeric
material, and/or the absorbent material can all be mixed together, e.g.,
homogeneously, then
formed into the article 14. An alternate embodiment for a system 22 is shown
in Figure 3, the
system 22 including an article 24 on a tubular or string 26. The article 24 is
formed from a
3
CA 02883078 2015-02-24
WO 2014/055891 PCT/US2013/063501
core 28 and a shell 30. In this embodiment, the core 28 includes the
aforementioned
swellable material, while the shell 30 includes the filter material. The core
28 and the shell
30 may both, for example, include suitable elastomeric and/or filler materials
to provide
sealing for the article 24 and to impart chemical and physical properties to
the article 24. In
this way, the flow of fluid to which the swellable material in the core 28 is
reactive will first
be filtered of ions by the filter material in the shell 30.
[0015] A system 32 according to another embodiment is shown in Figure 4 in
which a
swellable article 34 is disposed with a tubular or string 36. In this
embodiment, a formation
38 is separated from the article 34 by a radially disposed tubular or string
40, e.g., a casing,
liner, tubing, etc. The tubular/string 40 includes at least one port or
opening 42 for enabling a
flow of fluid, generally designated by an arrow 44, to encounter the article
34. The filter
material can be arranged in a plug 46 positioned in the opening 42, in a
membrane or film 48
positioned over the opening 42, etc. The plug 46 can be formed as any suitable
fluid
permeable member for creating a passageway for communicating fluid to the
swellable
material. In this way, the flow of fluid is filtered by the filter material
before it reaches the
article 34. The plug 46 and/or the membrane 48 could be formed from any
suitable
permeable material, e.g., a porous foam, fibers, with the filter material
disposed in or with the
permeable material, e.g., in pores of the permeable material.
[0016] In another embodiment, essentially a combination of the above, the
shell 30
could be a protective or elastomeric shell impermeable to downhole fluids and
resistant to
corrosion and degradation. A permeable plug, such as discussed with respect to
the plug 46
could be included in the shell 30 as opposed the an outer tubular 40. In this
way, the
swellable article will benefit from an outer shell made of an elastomeric or
other material that
can be selected to provide beneficial properties such as corrosion resistance,
fluid
impermeability, etc., while also maintaining the advantageous ion filtering
properties
provided by the current invention as discussed herein.
[0017] In one embodiment, the filter material comprises one or more graphene-
based
compounds. By graphene-based it is meant a compound that includes or is
derived from
graphene, such as graphene itself, graphite, graphite oxide, graphene oxide,
etc. The
compounds could take any form used with such graphene-based compounds, such as
sheets
or nanosheets, particles, flakes, nanotubes, etc. Advantageously, the unique
properties of
graphene enable effective donor¨acceptor interactions between both the anions
and the
cations and the graphene flakes or particles. The graphene-based materials,
associated oxides,
or other derivatives or functionalized compounds thereof may contain a
corresponding
4
CA 02883078 2015-02-24
WO 2014/055891 PCT/US2013/063501
relatively large number of capture sites for attracting and binding ions via
van der Waals
and/or Coulombic interactions. Of course, other materials with electron-rich
surfaces can be
used for similarly filtering cations, while highly electron deficient
materials may be utilized
with respect to anions.
[0018] To further increase the ability of graphene-based filter materials to
capture the
aforementioned polyvalent cations, the filter materials can be functionalized
to include one or
more functional groups. The process of forming graphite or graphene oxide, for
example,
results in the inclusion of various functional groups that are relatively
negatively charged
(e.g., carboxylic acid groups) or polar (e.g., carbonyl groups). Polyvalent
cations will be
attracted to and captured by these groups. In one embodiment the filter
material is covalently
modified with thiol groups according to known diazonium chemistry procedures.
Thiol
groups are naturally excellent at capturing positively charged ions, notably
doubly charged
mercury cations, although other metallic cations ions such as the
aforementioned Ca2 ', Zn2 ',
etc., contained in downhole brines will also be readily captured by thiol
groups. Other
functional groups such as disulfide groups, carboxylic acid, sulfonic acid
groups may also be
used for their ability to capture polyvalent cations, particularly doubly
charged cations. Other
functional groups include chelating ligand groups, such as iminodiacetic acid,
iminodiacetic
acid group,N45-amino-1-carboxy-(t-butyppentyl]iminodi-t-butylacetate) group, N-
(5-amino-
1-carboxypentyl)imino-diacetic acid group, N-(5-amino-1-
carboxypentyl)iminodiacetic acid
tri-t-butyl ester group, aminocaproic nitrilotriacetic acid group,
aminocaproic nitrilotriacetic
acid tri-tert-butylester group, 2-aminooxyethyliminodiacetic acid group, and
others that
would be recognized by those of ordinary skill in the art in view of the
disclosure herein.
[0019] The graphene-based materials could also be functionalized to filter
anions,
e.g., with quaternary ammonium, quaternary phosphonium, ternary sulfonium,
cyclopropenylium cations, or primary, secondary, ternary amino, or other
groups. These
groups are either positively charged or become protonated in acidic
environments and thus
require anions to compensate for the charge. In some situations, the anion can
be exchanged
with another anion while preserving charge. For example, in one embodiment,
the graphene-
based material is functionalized with a quaternary ammonium group, the
positive charge of
which is balanced by hydroxide anions. In this example, in brine containing
S042- anions,
one S042- anion will be captured and two hydroxide anions (OH-) will be
released. In an
embodiment, a mixture of graphene-based material functionalized with sulfonic
acid groups
and graphene-based material functionalized with quarternary ammonium groups
balanced by
hydroxide anions is used to neutralize a CaC12 brine. In the cation-exchange
process, Ca2 '
CA 02883078 2015-02-24
WO 2014/055891 PCT/US2013/063501
cations are captured with a simultaneous release of two H ' ions for each Ca2'
cation. In the
anion-exchange process, Cl- ions are captured by the quaternary ammonium group
with a
simultaneous release of OH- anion for each a ion. Recombination of released H
' and Off
ions results in the formation of water molecules, which may contribute to the
swelling
process of water-swellable materials.
[0020] While the invention has been described with reference to an exemplary
embodiment or embodiments, it will be understood by those skilled in the art
that various
changes may be made and equivalents may be substituted for elements thereof
without
departing from the scope of the invention. In addition, many modifications may
be made to
adapt a particular situation or material to the teachings of the invention
without departing
from the essential scope thereof. Therefore, it is intended that the invention
not be limited to
the particular embodiment disclosed as the best mode contemplated for carrying
out this
invention, but that the invention will include all embodiments falling within
the scope of the
claims. Also, in the drawings and the description, there have been disclosed
exemplary
embodiments of the invention and, although specific terms may have been
employed, they
are unless otherwise stated used in a generic and descriptive sense only and
not for purposes
of limitation, the scope of the invention therefore not being so limited.
Moreover, the use of
the terms first, second, etc. do not denote any order or importance, but
rather the terms first,
second, etc. are used to distinguish one element from another. Furthermore,
the use of the
terms a, an, etc. do not denote a limitation of quantity, but rather denote
the presence of at
least one of the referenced item.
6