Note: Descriptions are shown in the official language in which they were submitted.
CA 02884787 2015-03-12
'
=
Processing of Iron Oxide Containing Chloride
The invention concerns a method for processing iron(III) oxide obtained from a
solution
containing iron chloride by means of a hydrolysis step with a reduction of
chloride groups
adhering to the iron(III) oxide.
The invention further concerns the use of the method for processing pickling
liquor.
Pickling is a process step frequently used in the production of steel- or iron-
containing products.
Acids and acid mixtures, especially hydrochloric acid and sulfuric acid, are
used as pickling
medium. The method involving pickling with hydrochloric acid or with mixtures
containing
hydrochloric acid is frequently and readily used due to the quality of final
products that can be
achieved but also due to the capacity of the pickling solution for
regeneration. The acid acts to
dissolve the oxide layers or scale layers that form on the steel or metal
surface during previous
processes such as rolling, annealing, etc. During pickling, scale which
contains iron oxide reacts
with the acid that is used, forming iron chloride and water. During the
pickling process, acid is
consumed until the solution becomes saturated with iron chloride. This
necessitates a
continuous supply of hydrochloric acid to the pickling process. The use of
spray roasting
systems (Ruther or Woodhall-Duckham method) or fluidized bed systems for
regenerating
pickling liquor has therefore become standard practice.
With sufficient quality, the powdered iron oxide that forms as a secondary
product during
regeneration of the pickling liquor is a marketable product. However,
regenerated iron oxide with
even small fractions of residual chloride exhibits highly unfavorable
corrosion behavior in
relation to metallic materials across a broad temperature range. Moreover,
small residual
chloride concentrations interfere with the magnetic and mechanical properties
of ferritic
1
CA 02884787 2015-03-12
materials in which regenerated iron oxide is used as a raw material. In
addition to a low residual
chloride concentration, a high specific surface area of the regenerated iron
chloride is a further
essential quality feature for the industrial (re)usability of regenerated iron
oxide.
WO 2008/070885 A2 discloses a method for reducing the chloride concentration
of iron oxides
containing chloride while at the same time achieving a sufficiently large
specific surface area of
the regenerated iron oxide. In a hydrochloric acid regeneration system that
functions according
to the spray roasting principle, the acid- and iron chloride-containing
pickling liquor to be
regenerated is preconcentrated and then sprayed into a reactor from the top,
after which
circulation is generated in the reactor using one or more burner(s) disposed
on the sides of the
reactor, and the temperature in the reactor is maintained at approximately 550
C, thereby
oxidizing the iron chloride in a roasting process to form powdered iron oxide.
Below the burner
level, superheated steam is then injected via a tangential infeed line in the
cone region of the
reactor and reacts with the chlorides still adhering to the surface of the
iron oxide, forming
gaseous hydrochloric acid, with the chloride concentration of the iron oxide
that is discharged
the reactor having a chloride percentage by weight of less than 0.15 wt%.
Ambient air is then
injected below the burner level to cool the iron oxide in order to prevent
shrinkage of the specific
surface area of the iron oxide.
EP 0 850 881 Al discloses a method for reducing the chloride concentration of
iron oxides
containing chloride while at the same time achieving a sufficiently large
specific surface area of
the regenerated iron oxide. In a spray roaster, the iron chloride-containing
pickling liquor is
sprayed into a reaction chamber which is heated in the area of the burner
level to approximately
650 C and to which fuel gases are supplied, and the solution is thermally
decomposed in said
reaction chamber to form iron oxide granulate and hydrochloric acid gas. Below
the burner level,
the spray roasted iron oxide granulate is cooled in a cooling zone to
temperatures below 450 C
by introducing cooling gases, and is discharged by means of a discharge device
arranged at the
2
CA 02884787 2015-03-12
=
= lower end of the spray roaster, and then conducted across a bed to which
superheated steam is
applied, wherein the iron oxide that is regenerated following the application
of superheated
steam has a residual chloride concentration of less than 500 ppm and a
specific surface area
greater than 3.5 m2/g.
Common to the prior art methods described is that the roasting process used
for regenerating or
regeneration of the iron oxide is energy intensive, and the energy required to
power the thermal
decomposition is supplied by fossil fuels, so that a corresponding quantity of
greenhouse gases
is produced. Moreover, the specific surface area and the chloride
concentration of the iron oxide
and/or the amount of chloride adhering to the iron oxide are substantially
dependent on the
temperature of the roasting reaction in the reactor. At routine roasting
temperatures of less than
800 C, only an improvable, insufficient reduction of the chloride
concentration of the iron oxide
and/or the chloride adherent to the iron oxide is obtained. Sufficient
reduction of the chloride
concentration and/or of the chloride adherent to the iron oxide first occurs
at temperatures of
between 800 and 1000 C. However, thermal treatment at such high temperatures
results in a
decrease in the specific surface area of the iron oxide which is negative for
the subsequent
industrial use of the iron oxides. Moreover, systems in which iron oxide can
be annealed at such
high temperatures of up to 1000 C and then cooled are technically complex.
The object of the invention is therefore to devise a solution that will make
it possible to remove
adherent chloride from iron(III) oxide which is obtained by means of a
hydrothermal method in
the processing of a solution containing iron chloride, and at the same time to
maintain a high
specific surface area of the iron oxide.
In a method of the type described in detail in the introductory part, this
object is attained
according to the invention in that the iron oxide (Fe203) is treated in a
leaching stage with a
basic solution having a pH value of > 7, preferably > 8.
3
CA 02884787 2015-03-12
= This object is likewise attained according to the invention by the use of
a method according to
claim 14.
By treating the obtained iron oxide according to the invention in a leaching
stage with a basic
solution having a pH value of greater than 7, preferably greater than 8, the
chlorides adherent to
the iron oxide are bound by at least one component of the basic solution, so
that an effective
reduction in the chloride concentration of the iron oxide (Fe203) or in the
chloride still adherent
to the respective iron oxide particles or the adherent chlorine ions is
achieved. However,
treatment with the basic solution has no effect on the specific surface area
of the iron oxide
produced by means of a hydrothermal process, so that the specific surface area
of the
regenerated iron oxide that is present at the start of the leaching step in
the leaching stage is
maintained and preserved. The method according to the invention has
substantially lower
energy requirements as compared with spray roasting methods known in the art
which achieve
similar chloride reductions at temperatures of between 800 and 1000 C, and
therefore the
greenhouse gas emissions produced during the regeneration process as a whole
can be
reduced significantly. In general, with the solutions according to the
invention, adherent chloride
is detached from iron(III) oxide obtained by means of a hydrothermal process
during the
processing of a solution containing iron chloride, and at the same time, a
high specific surface
area of the iron oxide is obtained or maintained. With the solutions according
to the invention,
methods are provided by which the chloride concentration (Cl) in iron oxide
(Fe203) produced
by means of a hydrothermal process is reduced or diminished in an
environmentally safe and
energy saving manner by mixing or treating the iron oxide with a solution that
increases the pH
value.
In an advantageous manner, the solution containing iron chloride, for example
a solution
containing hydrochloric acid, which forms during pickling of an object made of
a material that
contains iron, in particular steel, with hydrochloric acid as the pickling
liquor, is subjected for its
regeneration to a hydrolysis step during treatment in a thermal treatment
stage, resulting in
4
CA 02884787 2015-03-12
the iron(III) oxide to be further processed. The invention embodiment is
therefore further
characterized in that the iron oxide (Fe203) to be treated with the basic
solution is produced
hydrothermally, preferably by means of an acid regeneration process carried
out within a
temperature range of 130-190 C, in particular by means of a hydrothermal
process. In
particular, this is carried out by means of a hydrothermal process with/in a
corresponding
system. The thermal treatment stage is thus a component of an acid
regeneration process,
preferably a spray roasting process or a fluidized bed process having a
corresponding spray
roasting system or fluidized bed system.
The reduction in the chloride concentration of the iron oxide (Fe203)
containing chloride or in the
chloride still adherent to the respective iron oxide particles is expediently
carried out at
pressures of 0 to 100 bar. The invention therefore further provides that the
iron oxide (Fe203) is
treated in a leaching step of the leaching stage at a pressure of between 0
and 100 bar.
It is further expedient according to the embodiment of the invention that the
iron oxide (Fe203) is
treated in a leaching step of the leaching stage at a temperature of between 0
and 300 C. This
temperature range can be achieved with an acceptable level of energy
consumption, and
ensures that the specific surface area of the iron oxide does not change.
Although in principle it is sufficient for the success of the method according
to the invention to
establish a pH value of greater than 7 in the leaching stage, the method can
be carried out
particularly effectively if the iron oxide (Fe203) is treated in the leaching
stage with a basic
solution having a pH value of between 11 and 14, which likewise characterizes
the embodiment
of the invention.
CA 02884787 2015-03-12
It is likewise particularly advantageous if, according to a further embodiment
of the invention,
the iron oxide (Fe203) is treated in the leaching stage at a temperature of
between 10 and 80 C
and/or at ambient pressure.
In the execution of the method, it can be provided that, after the basic
solution is added to the
chloride-containing iron oxide (Fe203) and after the chloride concentration of
the or on the iron
oxide (Fe203) has been reduced/diminished, an oxide/leaching solution mixture
results, which
contains both "dechlorinated" iron oxide (Fe203) and a basic solution. This is
then fed to a solid-
liquid separation unit in which the "dechlorinated" iron oxide (Fe203) and the
basic solution are
separated from the oxide-leaching solution mixture. The separated
"dechlorinated" iron oxide
(Fe203) can be rinsed with rinsing water and the separated basic solution can
be returned to the
leaching stage.
The invention therefore further provides that the iron oxide (Fe203)/solution
mixture is
separated, in particular mechanically, in a solid-liquid separation stage that
follows the leaching
stage, into an iron oxide-containing solid portion and a solution-containing
liquid portion.
It is also expedient for the recovery of the chloride if the solid-liquid
separation stage comprises
a rinsing treatment of the solid portion using a rinsing medium, which is
likewise provided by the
invention. Additional residues are also rinsed out of the iron oxide.
To use the basic solution efficiently, the invention also provides that the
solution-containing
liquid portion is returned at least partially to the leaching stage.
The invention is further characterized in that surplus basic solution and/or a
portion of the iron
6
CA 02884787 2015-03-12
oxide (Fe203)/solution mixture that forms in the leaching stage is removed
from the leaching
stage as discharge water by means of an overflow device.
In particular, a further development of the invention is also characterized in
that the iron oxide
(Fe203) of the iron oxide-containing solid portion has a chloride adherence
(Cl) of 0.18 wt%,
in particular 15 wt%. The residual chloride concentration of the regenerated
iron oxide is
therefore low enough for industrial applications.
It is further advantageous for the invention for both the chlorinated iron
oxide (Fe203) supplied to
the leaching stage and the "dechlorinated" iron oxide (Fe203) to have a BET
surface area of
more than 15 m2/g. An iron chloride thus regenerated not only has a
sufficiently low residual
chloride fraction, but also has a sufficiently high specific surface area for
industrial applications,
so that it is a marketable product that meets industrial requirements. In a
further embodiment,
the invention is therefore characterized in that the iron oxide (Fe203) of the
iron oxide-containing
solid portion has a specific BET surface area of > 15 m2/g, in particular of
20-30 m2/g, preferably
of 22-26 m2/g, and also in that the specific BET surface area of the iron
oxide (Fe203) passes
through the leaching stage unchanged.
Finally, in a further advantageous embodiment, the invention provides that a
leaching solution
having an alkaline or alkaline-earth base, in particular sodium hydroxide
solution (NaOH) is
used as the basic solution. Sodium hydroxide solution is one of the most
widely used laboratory
and industrial chemicals, and due to its industrial mass production is
available as a cost-
effective basic solution.
In the following, the invention will be described by way of example in
reference to a drawing.
The drawing shows
7
CA 02884787 2015-03-12
=
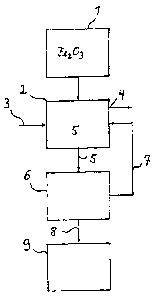
Fig 1 a schematic representation of a block diagram of a method
according to the
invention.
In the embodiment example shown, in a first process stage 1, iron oxide
(Fe203) is produced by
means of one or more process step(s) comprising a hydrolysis step from a
solution containing
iron chloride, said solution being obtained, for example, from the pickling of
steel using
hydrochloric acid, wherein the iron oxide contains chloride groups (Cl) and
therefore chloride
ions on an order of magnitude greater than 0.15 wt%, generally on an order of
magnitude of
approximately 0.55 wt%, and a specific BET surface area of more than 15 m2/g.
In a subsequent leaching stage 2, a basic solution 3 or leaching solution
having a pH value
greater than 8 is added to the iron oxide (Fe203). Said solution is preferably
sodium hydroxide
solution (NaOH). The sodium ions (Nat) react with the chloride ions (Cr),
which become
detached from the iron oxide (Fe203), resulting in a reduction in the chloride
concentration of the
iron oxide (Fe203) containing chloride or in the chloride still adherent to
the respective iron oxide
particles or in the adherent chloride ions. This is achieved without any
significant change, in
particular without a reduction in the specific (BET) surface area of the iron
oxide (Fe203).
Surplus basic solution or leaching solution and/or a portion of the iron oxide
(Fe203)/solution
mixture 5 that forms in leaching stage 2 is discharged via an overflow device
4 as discharge
water. The iron oxide (Fe203)/solution mixture 5 obtained in leaching stage 2,
which contains
both "dechlorinated" iron oxide (Fe203) and sodium chloride (NaCl)-containing
solution or
leaching solution that has formed, for example, is fed to a subsequent solid-
liquid separation
stage 6. The "dechlorinated" iron oxide (Fe203) that is discharged from
leaching stage 2 still has
a BET surface area of more than 15 m2/g.
In solid-liquid separation stage 6, the "dechlorinated" iron oxide (Fe203) is
separated from iron
oxide (Fe203)/solution mixture 5 as an iron oxide-containing solid portion
(8), and basic solution
or leaching solution is separated out as a solution- or leaching solution-
containing liquid portion
8
CA 02884787 2015-03-12
=(7). The separated "dechlorinated" iron oxide (Fe203) or the iron oxide-
containing solid portion
can be rinsed in solid-liquid separation stage 6 with a rinsing medium, for
example water. The
separated solution- or leaching solution-containing liquid portion is returned
as basic solution or
leaching solution to the leaching stage, as indicated by reference sign 7. In
the iron oxide-
containing solid portion 8 which is discharged from solid-liquid separation
stage 6, the
separated, "dechlorinated" and optionally rinsed iron oxide (Fe203) has a
chloride concentration
of less than 0.18 wt%, even of less than 0.15 wt%, and still has a specific
(BET) surface area of
more than 15 m2/g.
The iron oxide-containing solid portion 8 is fed for final processing to one
or more additional
process stage(s), denoted together by reference sign 9. The further processing
steps of process
stage(s) 9 comprise drying and/or packaging and/or pelletizing the obtained
iron oxide (Fe203).
The iron oxide (Fe203) recovered or obtained according to the embodiment has a
concentration
of chloride ions (Cl) of 0.13 to 0.17 wt% and a specific BET surface area of
22-26 m2/g. The
chlorine or chlorine ion concentration (CV) is determined in the dry sample by
means of
MatrixPro x-ray fluorescence analysis (model number 2.699) as a bed sample in
a helium
atmosphere. The Q+ method specific to the device is used for analysis. The
specific surface
area is measured using the BET method by means of gas adsorption.
9