Note: Descriptions are shown in the official language in which they were submitted.
CA 02886479 2015-03-27
- SYSTEMS AND METHODS FOR ENHANCING PRODUCTION OF VISCOUS
HYDROCARBONS FROM A SUBTERRANEAN FORMATION
This is a division of co-pending Canadian Patent Application No. 2,824,549
filed
August 22, 2013.
Field
The present disclosure is directed generally to systems and methods for
enhancing
production of viscous hydrocarbons from a subterranean formation, and more
particularly to
systems and methods that utilize a hydrocarbon solvent mixture to reduce a
viscosity of the
viscous hydrocarbons.
Background
Viscous hydrocarbons, which also may be referred to herein as heavy oils
and/or as
bitumen, represent a significant fraction of worldwide hydrocarbon reserves.
These viscous
hydrocarbons may have a relatively high viscosity, precluding their
production, or at least
economic production, by flowing from a subterranean formation. Several methods
have
been utilized to decrease the viscosity of the viscous hydrocarbons, thereby
decreasing a
resistance to flow thereof and/or permitting production of the viscous
hydrocarbons from the
subterranean formation by piping, flowing, and/or pumping the viscous
hydrocarbons from
the subterranean formation. While each of these methods may be effective under
certain
conditions, they each possess inherent limitations.
As an illustrative, non-exclusive example, steam injection may be utilized to
heat the
viscous hydrocarbons and to thereby decrease their viscosity. While water
and/or steam
may represent an effective heat transfer medium, the pressure required to
produce saturated
steam at a desired temperature may be relatively high, limiting the
applicability of steam
recovery processes to high pressure operation and/or requiring a large amount
of energy to
heat the steam and decreasing an overall thermal efficiency of a viscous
hydrocarbon
1
CA 02886479 2015-03-27
- recovery process. In addition, water and/or steam may damage certain
subterranean
formations.
_
As another illustrative, non-exclusive example, cold and/or heated solvents
have
been injected into a subterranean formation to decrease the viscosity of
viscous
hydrocarbons that are present within the subterranean formation. These methods
traditionally inject a pure (i.e., single- component), or at least
substantially pure, volatile
solvent, such as propane, into the subterranean formation and permit the
solvent to dissolve
the viscous hydrocarbons, dilute the viscous hydrocarbons, and/or transfer
thermal energy to
the viscous hydrocarbons. While effective under certain conditions, these
traditional solvent
injection processes suffer from limited injection temperature and/or pressure
operating
ranges, an inability to effectively decrease the viscosity of the viscous
hydrocarbons, and/or
challenges associated with maintaining the traditional solvent in a vaporous
state during
transport to the subterranean formation. Thus, there exists a need for
improved systems and
methods for enhancing production of viscous hydrocarbons from a subterranean
formation.
Summary
A method of selecting a composition of a hydrocarbon solvent mixture for
injection
into a subterranean formation to enhance production of viscous hydrocarbons
therefrom,
wherein the hydrocarbon solvent mixture is injected into the subterranean
formation as a
vapor stream with a vapour pressure may comprise determining a threshold
maximum
pressure of the subterranean formation; determining a stream temperature at
which the vapor
stream is to be injected into the subterranean formation; and selecting the
composition of the
hydrocarbon solvent mixture based, at least in part, on the stream temperature
and the
threshold maximum pressure, wherein the selecting includes: (i) selecting a
first proportion
of the hydrocarbon solvent mixture that comprises a first compound with at
least five
carbon atoms, wherein the first proportion comprises at least 10 mole percent
of the
hydrocarbon solvent mixture; and (ii)selecting a second proportion of the
hydrocarbon
solvent mixture that comprises a second compound with more carbon atoms than
the first
2
CA 02886479 2015-03-27
= compound, wherein the second proportion comprises at least 10 mole
percent of the
hydrocarbon solvent mixture.
The foregoing has broadly outlined the features of the present disclosure so
that the
detailed description that follows may be better understood. Additional
features will also be
described herein.
Brief Description of the Drawings
Fig. 1 is a schematic representation of a hydrocarbon production system.
Fig. 2 is a plot of vapor pressure vs. temperature for a plurality of
hydrocarbons.
Fig. 3 is a histogram depicting a carbon content of compounds that may be
present in
a gas plant condensate.
Fig. 4 is a flowchart depicting disclosure method of enhancing production of
viscous
hydrocarbons from a subterranean formation.
Fig. 5 is a flowchart depicting disclosure method of selecting a composition
of a
hydrocarbon solvent mixture.
It should be noted that the figures are merely examples and no limitations on
the
scope of the present disclosure are intended thereby. Further, the figures are
generally not
drawn to scale, but are drafted for purposes of convenience and clarity in
illustrating various
aspects of the disclosure.
Detailed Description
For the purpose of promoting an understanding of the principles of the
disclosure,
reference will now be made to the features illustrated in the drawings and
specific language
will be used to describe the same. It will nevertheless be understood that no
limitation of the
scope of the disclosure is thereby intended. Any alterations and further
modifications, and
any further applications of the principles of the disclosure as described
herein are
contemplated as would normally occur to one skilled in the art to which the
disclosure
3
CA 02886479 2015-03-27
relates. It will be apparent to those skilled in the relevant art that some
features that are not
relevant to the present disclosure may not be shown in the drawings for the
sake of clarity.
Figs. 1 and 4-5 provide illustrative, non-exclusive examples of hydrocarbon
production systems 10 according to the present disclosure, of methods 100
according to the
present disclosure of enhancing production of viscous hydrocarbons from a
subterranean
formation, and/or of methods 200 according to the present disclosure of
selecting a
composition of a hydrocarbon solvent mixture for injection into the
subterranean formation
as a vapor stream. All elements and/or method steps may not be labeled in each
of Figs. 1
and 4-5, but reference numerals associated therewith may be utilized herein
for consistency.
Elements, components, features, and/or method steps that are discussed herein
with
reference to one or more of Figs. 1 and 4-5 may be included in and/or utilized
with any of
Figs. 1 and 4-5 without departing from the scope of the present disclosure.
In general, elements and/or method steps that are likely to be included are
illustrated
in solid lines, while elements and/or method steps that may be optional are
illustrated in
dashed lines. However, elements and/or method steps that are shown in solid
lines are not
necessarily essential, and an element and/or method step shown in solid lines
may be
omitted without departing from the scope of the present disclosure.
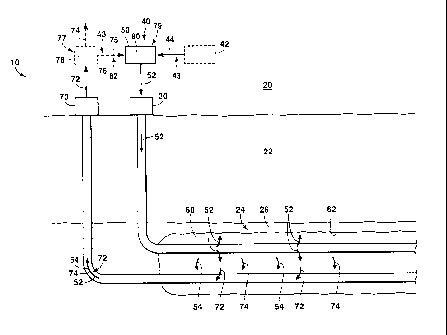
Fig. 1 is a schematic representation of a hydrocarbon production system 10
that may
be utilized with, may be included in, and/or may include the systems and
methods according
to the present disclosure. Hydrocarbon production system 10 may include an
injection well
and a production well 70 that extend between a surface region 20 and a
subterranean
formation 24 that is present within a subsurface region 22.
Injection well 30 may be in fluid communication with an injectant supply
system 40.
Injection well 30 may be configured to receive a hydrocarbon solvent mixture
44 from any
25 suitable source (e.g., a storage structure 42). The hydrocarbon solvent
mixture 44 may be
provided to a vaporization assembly 50 to generate a vapor stream 52. The
vapor stream 52
may be provided to subterranean formation 24 via injection well 30.
Once provided to the subterranean formation, the vapor stream 52 may condense
within a vapor chamber 60. When the vapor stream 52 condenses, the vapor
stream 52 may
30 release latent heat (or latent heat of condensation), transfer thermal
energy to the
4
CA 02886479 2015-03-27
,
- subterranean formation, and/or generate a condensate 54.
Condensation of the vapor stream
52 may heat viscous hydrocarbons 26 that may be present within the
subterranean
formation, thereby decreasing a viscosity of the viscous hydrocarbons. Vapor
stream 52
and/or condensate 54 may combine with, mix with, be dissolved in, dissolve,
and/or dilute
viscous hydrocarbons 26, thereby further decreasing the viscosity of the
viscous
hydrocarbons.
The energy transfer between vapor stream 52 and viscous hydrocarbons 26 and/or
the mixing of vapor stream 52 with viscous hydrocarbons 26 may generate
reduced-
viscosity hydrocarbons 74, which may flow to production well 70. After flowing
to the
production well 70, the reduced-viscosity hydrocarbons 74 may be produced from
the
subterranean formation as a reduced-viscosity hydrocarbon mixture 72. The
reduced-
viscosity hydrocarbon mixture may comprise reduced-viscosity hydrocarbons 74,
vapor
stream 52, and/or condensate 54 in any suitable ratio and/or relative
proportion.
Hydrocarbon production system 10 may include a condensate recovery system 77.
The condensate recovery system 77 may include and/or be a separation assembly
78.
Condensate recovery system 77 may receive reduced-viscosity hydrocarbon
mixture 72.
Condensate recovery system 77 may separate the reduced-viscosity hydrocarbon
mixture
into reduced-viscosity hydrocarbons 74, light hydrocarbon gasses 75, and/or
recovered
hydrocarbon solvent 76.
Reduced-viscosity hydrocarbons 74 may be removed from the hydrocarbon
production system, utilized in another downstream process of the hydrocarbon
production
system, and/or pipelined or otherwise transported to a suitable processing
site, such as a
hydrocarbon refinery, for further processing.
Recovered hydrocarbon solvent 76 may be utilized as a feed stream 43 that may
be
combined with (or may be) hydrocarbon solvent mixture 44 to generate vapor
stream 52.
Light hydrocarbon gasses 75 may include hydrocarbons and/or carbon compounds
with four or fewer carbon atoms, such as methane, ethane, propane, and/or
butane. Light
hydrocarbon gasses 75 may be provided to vaporization assembly 50 as a fuel
stream that
may be combusted to heat hydrocarbon solvent mixture 44.
5
CA 02886479 2015-03-27
Hydrocarbon production system 10 may include a solvent purification system 79.
Solvent purification system 79 may include a purification assembly 80. Solvent
purification
system 79 may be configured to receive a feed stream 43 from any suitable
source. For
example, feed stream 43 may be provided by storage structure 42 and/or may be
separated
from reduced- viscosity hydrocarbons 72 and recovered hydrocarbon solvent 76.
Regardless
of the source of feed stream 43, the solvent purification system 79 may be
configured to
remove one or more components from the feed stream 43 to generate hydrocarbon
solvent
mixture 44 with a target, or desired, composition. The hydrocarbon solvent
mixture then
may be provided to vaporization assembly 50 to generate vapor stream 52.
Injectant supply system 40 may receive hydrocarbon solvent mixture 44, such as
from storage structure 42. Injectant supply system 40 may vaporize the
hydrocarbon solvent
mixture within vaporization assembly 50 to generate vapor stream 52. Injectant
supply
system 40 may receive recovered hydrocarbon solvent 76 from condensate
recovery system
77. Injectant supply system 40 may vaporize the recovered hydrocarbon solvent
within
vaporization assembly 50 to generate vapor stream 52. Injectant supply system
40 may
receive feed stream 43, such as from storage structure 42 and/or from
condensate recovery
system 77. Injectant supply system may purify the feed stream within
purification assembly
80 to generate hydrocarbon solvent mixture 44, with the hydrocarbon solvent
mixture then
being vaporized within vaporization assembly 50 to generate vapor stream 52.
As discussed, conventional hydrocarbon production systems that utilize an
injected
vapor stream to decrease the viscosity of high viscosity hydrocarbons
traditionally utilize a
pure (i.e., single-component), or at least substantially pure, injected vapor
stream that
comprises a light hydrocarbon, such as propane. In contrast, the systems and
methods
according to the present disclosure may utilize hydrocarbon solvent mixture 44
to generate
vapor stream 52. Hydrocarbon solvent mixture 44 may include a heavy
hydrocarbon
fraction that comprises, consists of, or consists essentially of hydrocarbons
with five or more
carbon atoms. The heavy hydrocarbon fraction may comprise greater than or
equal to 10
mole percent, greater than or equal to 20 mole percent, greater than or equal
to 30 mole
percent greater than or equal to 40 mole percent, greater than or equal to 50
mole percent,
greater than or equal to 60 mole percent, greater than or equal to 70 mole
percent, or greater
6
CA 02886479 2015-03-27
than or equal to 80 mole percent of the hydrocarbon solvent mixture.
Additionally or
alternatively, the heavy hydrocarbon fraction also may comprise less than or
equal to 99
mole percent, less than or equal to 95 mole percent, less than or equal to 90
mole percent,
less than or equal to 80 mole percent, less than or equal to 70 mole percent,
less than or
equal to 60 mole percent, or less than or equal to 50 mole percent of the
hydrocarbon solvent
mixture. Suitable ranges may include combinations of any upper and lower
amount of mole
percentage listed above. Additional examples of suitable mole percentages may
include any
of the illustrative threshold amounts listed above.
The heavy hydrocarbon fraction may include at least a first compound that has
five
or more carbon atoms and a second compound that has more carbon atoms than the
first
compound. The first compound and the second compound each may comprise at
least 10
mole percent of hydrocarbon solvent mixture 44. For example, the first and/or
second
compounds may comprise at least 20 mole percent, at least 30 mole percent, at
least 40 mole
percent, at least 50 mole percent, at least 60 mole percent, at least 70 mole
percent, or at
least 80 mole percent of the hydrocarbon solvent mixture. Suitable ranges may
include
combinations of any upper and lower amount of mole percentage listed above.
The heavy hydrocarbon fraction may comprise any suitable hydrocarbon
molecules,
materials, and/or compounds. For example, the heavy hydrocarbon fraction may
comprise
one or more of alkanes, n-alkanes, branched alkanes, alkenes, n-alkenes,
branched alkenes,
alkynes, n-alkynes, branched alkynes, aromatic hydrocarbons, and/or cyclic
hydrocarbons.
As used herein, a "compound that has five or more carbon atoms" may include
any
suitable single chemical species that includes five or more carbon atoms. A
"compound that
has five or more carbon atoms" also may include any suitable mixture of
chemical species.
Each of the chemical species in the mixture of chemical species may include
five or more
carbon atoms and each of the chemical species in the mixture of chemical
species also may
include the same number of carbon atoms as the other chemical species in the
mixture of
chemical species.
For example, a compound that has five carbon atoms may include a pentane, n-
pentane, a branched pentane, cyclopentane, a pentene, n-pentene, a branched
pentene,
cyclopentene, a pentyne, n-pentyne, a branched pentyne, cyclopentyne,
methylbutane,
7
CA 02886479 2015-03-27
dimethylpropane, ethylpropane, and/or any other hydrocarbon with five carbon
atoms. A
compound with six carbon atoms, seven carbon atoms, or eight carbon atoms may
include a
single chemical species with six carbon atoms, seven carbon atoms, or eight
carbon atoms,
respectively, and/or may include a mixture of chemical species that each
include six carbon
atoms, seven carbon atoms, or eight carbon atoms, respectively.
Generating vapor stream 52 from hydrocarbon solvent mixture 44 may provide
advantages over more traditional hydrocarbon production systems that utilize
an injected
vapor stream that is formed from a substantially pure light hydrocarbon. For
example, and
as illustrated in Fig. 2 (which is a plot of vapor pressure vs. temperature
for a number of
hydrocarbons with varying carbon content), compounds with a larger number of
carbon
atoms generally exhibit a lower vapor pressure at a given temperature when
compared to
compounds with a smaller number of carbon atoms. Thus, injecting vapor stream
52 that is
formed from hydrocarbon solvent mixture 44, a majority of which comprises
compounds
with five or more carbon atoms, may permit injecting the vapor stream at a
lower pressure
for a given temperature when compared to propane injection and/or may permit
tailoring
(i.e., selecting, regulating, and/or controlling) a temperature-pressure
behavior of the vapor
stream to a given subterranean formation.
Vapor stream 52 may be injected into subterranean formation 24 at a stream
temperature. A composition of hydrocarbon solvent mixture 44 may be selected
such that
the vapor pressure of the hydrocarbon solvent mixture at the stream
temperature is less than
a threshold maximum pressure of the subterranean formation. This may prevent
damage to
the subterranean formation and/or escape of vapor stream 52 from the
subterranean
formation. Threshold maximum pressures may include, for example, a
characteristic
pressure of the subterranean formation, such as a fracture pressure of the
subterranean
formation, a hydrostatic pressure within the subterranean formation, a
lithostatic pressure
within the subterranean formation, a gas cap pressure for a gas cap that is
present within the
subterranean formation, and/or an aquifer pressure for an aquifer that is
located above and/or
under the subterranean formation. The above-mentioned pressures may be
measured and/or
determined in any suitable manner. For example, this may include measuring a
selected
pressure with a downhole pressure sensor, calculating the pressure from any
suitable
8
CA 02886479 2015-03-27
property and/or characteristic of the subterranean formation, and/or
estimating the pressure,
such as via modeling the subterranean formation. The threshold pressures
disclosed herein
may be selected to correspond in any suitable or desired manner to one or more
of these
measured or calculated pressures. For example, the threshold pressures
disclosed herein
may be selected to be, to be greater than, to be less than, to be within a
selected range of, to
be a selected percentage of, to be within a selected constant of, etc. one or
more of these
selected or measured pressures. A threshold pressure may be a user-selected,
or operator-
selected, value that does not directly correspond to a measured or calculated
pressure.
The threshold maximum pressure also may be related to and/or based upon the
characteristic pressure of the subterranean formation. This may include
threshold maximum
pressures that are less than or equal to 95%, less than or equal to 90%, less
than or equal to
85%, less than or equal to 80%, less than or equal to 75%, less than or equal
to 70%, less
than or equal to 65%, less than or equal to 60%, less than or equal to 55%, or
less than or
equal to 50% of the characteristic pressure for the subterranean formation
and/or threshold
maximum pressures that are at least 20%, at least 25%, at least 30%, at least
35%, at least
40%, at least 45%, at least 50%, at least 55%, at least 60%, at least 65%, at
least 70%, at
least 75%, or at least 80% of the characteristic pressure for the subterranean
formation.
Suitable ranges may include combinations of any upper and lower amount of
characteristic
pressure listed above. Additional examples of suitable threshold maximum
pressures may
include any of the illustrative threshold amounts listed above.
Non-exclusive examples of vapor pressures for hydrocarbon solvent mixtures
that
may be utilized with and/or included in the systems and methods according to
the present
disclosure include vapor pressures that are greater than a lower threshold
pressure of at least
0.2 megapascals (MPa), at least 0.3 MPa, at least 0.4 MPa, at least 0.5 MPa,
at least 0.6
MPa, at least 0.7 MPa, at least 0.8 MPa, at least 0.9 MPa, at least 1 MPa, at
least 1.1 MPa, at
least 1.2 MPa, at least 1.3 MPa, at least 1.4 MPa, at least 1.5 MPa, at least
1.6 MPa, at least
1.7 MPa, at least 1.8 MPa, at least 1.9 MPa, at least 2 MPa, at least 2.1 MPa,
at least 2.2
MPa, at least 2.3 MPa, at least 2.4 MPa, and/or at least 2.5 MPa. Additionally
or
alternatively, the vapor pressure for the hydrocarbon solvent mixture may be
less than an
upper threshold pressure that is less than or equal to 3 MPa, less than or
equal to 2.9 MPa,
9
CA 02886479 2015-03-27
less than or equal to 2.8 MPa, less than or equal to 2.7 MPa, less than or
equal to 2.6 MPa,
less than or equal to 2.5 MPa, less than or equal to 2.4 MPa, less than or
equal to 2.3 MPa,
less than or equal to 2.2 MPa, less than or equal to 2.1 MPa, less than or
equal to 2 MPa, less
than or equal to 1.9 MPa, less than or equal to 1.8 MPa, less than or equal to
1.7 MPa, less
than or equal to 1.6 MPa, less than or equal to 1.5 MPa, less than or equal to
1.4 MPa, less
than or equal to 1.3 MPa, less than or equal to 1.2 MPa, less than or equal to
1.1 MPa, less
than or equal to 1 MPa, less than or equal to 0.9 MPa, less than or equal to
0.8 MPa, less
than or equal to 0.7 MPa, less than or equal to 0.6 MPa, less than or equal to
0.5 MPa, less
than or equal to 0.4 MPa, and/or less than or equal to 0.3 MPa. Suitable
ranges may include
combinations of any upper and lower amount of pressure listed above.
Additional examples
of suitable pressures may include any of the illustrative threshold amounts
listed above.
Non-exclusive examples of stream temperatures of vapor stream 52 when it is
injected into injection well 30 include stream temperatures of at least 30 C,
at least 35 C, at
least 40 C, at least 4 C, at least 50 C, at least 55 C, at least 60 C, at
least 65 C, at least
70 C, at least 75 C, at least 80 C, at least 85 C, at least 90 C, at least 95
C, at least 100 C, at
least 105 C, at least 110 C, at least 115 C, at least 120 C, at least 125 C,
at least 130 C, at
least 135 C, at least 140 C, at least 145 C, at least 150 C, at least 155 C,
at least 160 C, at
least 165 C, at least 170 C, at least 175 C, at least 180 C, at least 185 C,
at least 190 C, at
least 195 C, at least 200 C, at least 205 C, and/or at least 210 C.
Additionally or
alternatively, the stream temperature also may be less than or equal to 250 C,
less than or
equal to 240 C, less than or equal to 230 C, less than or equal to 220 C, less
than or equal to
210 C, less than or equal to 200 C, less than or equal to 190 C, less than or
equal to 180 C,
less than or equal to 170 C, less than or equal to 160 C, less than or equal
to 150 C, less than
or equal to 140 C, less than or equal to 130 C, less than or equal to 120 C,
less than or equal
to 110 C, less than or equal to 100 C, less than or equal to 90 C, less than
or equal to 80 C,
less than or equal to 70 C, less than or equal to 60 C, less than or equal to
50 C, and/or less
than or equal to 40 C. Suitable ranges may include combinations of any upper
and lower
amount of stream temperatures listed above. Additional examples of suitable
stream
temperatures may include any of the illustrative threshold amounts listed
above.
CA 02886479 2015-03-27
The composition of hydrocarbon solvent mixture 44 may be selected such that a
dew
point temperature of vapor stream 52 and a bubble point temperature of the
hydrocarbon
solvent mixture differ by at least a threshold temperature difference.
Illustrative, non-
exclusive examples of the threshold temperature difference include threshold
temperature
differences of at least 10 C, at least 15 C, at least 20 C, at least 25 C, at
least 30 C, at least
35 C, at least 40 C, at least 45 C, at least 50 C, at least 55 C, at least 60
C, at least 65 C, at
least 70 C, at least 75 C, at least 80 C, at least 85 C, at least 90 C, at
least 95 C, or at least
100 C. Additional examples and/or ranges of temperature differences may be
based upon
the difference between any include combinations of any upper and lower stream
temperatures listed above.
When vapor stream 52 is injected into subterranean formation 24 via injection
well
30 (as illustrated in Fig. 1), the vapor stream may decrease in temperature
(or lose thermal
energy) while being conveyed through the injection well to the subterranean
formation
and/or while being conveyed through the subterranean formation from injection
well 30 to
an interface 62 between vapor chamber 60 and viscous hydrocarbons 26 that are
not within
the vapor chamber. Thus, and for traditional single-component vapor streams,
the vapor
stream must be superheated significantly prior to being injected into the
subterranean
formation and/or a significant portion of the vapor stream will condense prior
to reaching
interface 62.
However, and since vapor stream 52 according to the present disclosure is
formed
from hydrocarbon solvent mixture 44, only a portion, such as a minority
portion, of the
vapor stream (such as a lower vapor pressure portion, a higher molecular
weight portion,
and/or a portion that is formed from hydrocarbon compounds with a greater
number of
carbon atoms) may condense during transport between surface region 20 and
subterranean
formation 24 and/or during transport between injection well 30 and interface
62. Thus, this
portion of vapor stream 52 may act as a "thermal buffer" for a remainder of
vapor stream 52,
decreasing a potential for undesired condensation of the remainder of the
vapor stream. This
may increase an overall efficiency of hydrocarbon production system 10, may
permit the
hydrocarbon production system to operate with less energy, and/or may permit
vapor stream
52 to extend farther into subterranean formation 24 prior to condensing within
the
11
CA 02886479 2015-03-27
= subterranean formation, when compared to traditional vapor injection
processes that do not
utilize hydrocarbon solvent mixture 44.
Hydrocarbon solvent mixture 44 may be obtained from any suitable source. As
illustrative, non-exclusive examples, hydrocarbon solvent mixture 44 may
include, be
obtained from, and/or be a gas plant condensate and/or a crude oil refinery
condensate. Fig.
3 is a histogram depicting a mole fraction of hydrocarbons that may be present
in a given
gas plant condensate as a function of the carbon content of the hydrocarbons.
As may be
seen in Fig. 3, the gas plant condensate may include a significant fraction of
compounds
with five or more carbon atoms and thus may be suitable for use as hydrocarbon
solvent
mixture 44, either directly or after further purification and/or separation
(such as via solvent
purification system 79).
Thus, and when hydrocarbon solvent mixture 44 includes gas plant condensate
(such
as the gas plant condensate of Fig. 3), solvent purification system 79 may be
utilized to
remove one or more components from the gas plant condensate to generate a
desired
composition for the hydrocarbon solvent mixture. For example, solvent
purification system
79 may remove at least a portion of the compounds with four or fewer carbon
atoms from
the gas plant condensate. As another example, solvent purification system 79
may remove
at least a portion of one or more of the compounds with five or more carbon
atoms from the
gas plant condensate.
Hydrocarbon solvent mixture 44 may define any suitable composition. As
illustrative, non-exclusive examples, a majority fraction, at least 50 mole
percent, at least 60
mole percent, at least 70 mole percent, at least 80 mole percent, at least 90
mole percent, or
at least 95 mole percent of hydrocarbon solvent mixture 44 may comprise a
compound with
five carbon atoms, a compound with six carbon atoms, a compound with seven
carbon
atoms, and/or a compound with eight carbon atoms. As additional illustrative,
non-
exclusive examples, the first compound may be pentane and/or the second
compound may
be hexane.
Hydrocarbon solvent mixture 44 may comprise any suitable number of compounds
and/or chemical species. The hydrocarbon solvent mixture may include a third
compound
that includes more carbon atoms than the second compound. When the hydrocarbon
solvent
12
CA 02886479 2015-03-27
= mixture includes the third compound, the third compound may comprise any
suitable
portion, or fraction, of the hydrocarbon solvent mixture. The third compound
may comprise
at least 20 mole percent, at least 30 mole percent, at least 40 mole percent,
at least 50 mole
percent, at least 60 mole percent, or at least 70 mole percent of the
hydrocarbon solvent
mixture.
The hydrocarbon solvent mixture 44 may include a light hydrocarbon fraction
that
includes hydrocarbons with fewer than five carbon atoms, such as hydrocarbons
with one
carbon atom, two carbon atoms, three carbon atoms, and/or four carbon atoms;
however, this
light hydrocarbon fraction (when present) may comprise a minority portion of
the
hydrocarbon solvent mixture. The light hydrocarbon fraction may comprise at
least 5 mole
percent, at least 10 mole percent, at least 15 mole percent, at least 20 mole
percent, at least
30 mole percent, at least 40 mole percent, at least 50 mole percent, or at
least 60 mole
percent of the hydrocarbon solvent mixture. The light hydrocarbon fraction may
comprise
less than or equal to 70 mole percent, less than 60 or equal to mole percent,
less than or
equal to 50 mole percent, less than or equal to 40 mole percent, less than or
equal to 30 mole
percent, less than or equal to 20 mole percent, less than or equal to 15 mole
percent, or less
than or equal to 10 mole percent of the hydrocarbon solvent mixture. Suitable
ranges may
include combinations of any upper and lower amount of hydrocarbon fractions
listed above.
Additional examples of suitable mole percentages of light hydrocarbons may
include any of
the illustrative threshold amounts listed above.
Condensate recovery system 77 may include any suitable structure, such as at
least
one separation assembly 78, that is configured to separate at least a portion
of condensate 54
from reduced-viscosity hydrocarbon mixture 72 and/or from reduced-viscosity
hydrocarbons
74 that are present within the reduced-viscosity hydrocarbon mixture and to
generate
recovered hydrocarbon solvent 76. This may include any suitable (single stage)
separation
vessel, (multistage) distillation assembly, liquid-liquid separation, or
extraction, assembly
and/or any suitable gas-liquid separation, or extraction, assembly. Condensate
recovery
system 77 may include a recycle conduit 82 that is configured to convey the
recovered
hydrocarbon solvent stream, which also may be referred to herein as condensate
54 and/or as
a portion of the condensate stream, to vaporization assembly 50.
13
CA 02886479 2015-03-27
Solvent purification system 79 may include any suitable structure, such as at
least
one purification assembly 80, that may be configured to receive any suitable
feed stream 43,
such as a hydrocarbon feedstock stream and/or recovered hydrocarbon solvent
76, and to
purify the feed stream to generate hydrocarbon solvent mixture 44. This may
include any
suitable liquid-liquid separation, or extraction, assembly, any suitable gas-
liquid separation,
or extraction, assembly, any suitable gas-gas separation, or extraction,
assembly, single
stage separation vessel, and/or any suitable (multistage) distillation
assembly. In addition,
solvent purification system 79 may be configured to produce hydrocarbon
solvent mixture
44 with any suitable composition, such as those that are discussed herein.
This may include
removing compounds with fewer than five carbon atoms from the feed stream to
generate
the hydrocarbon solvent mixture.
Vaporization assembly 50 may include any suitable structure that is configured
to
vaporize hydrocarbon solvent mixture 44 to generate vapor stream 52.
Vaporization
assembly 50 may include a heating assembly that is configured to heat and
vaporize the
hydrocarbon solvent mixture. Vaporization assembly 50 may include a steam co-
injection
assembly that is configured to co-inject steam into injection well 30 with
hydrocarbon
solvent mixture 44. The steam may heat and vaporize the hydrocarbon solvent
mixture to
generate vapor stream 52. This may include heating and vaporizing the
hydrocarbon solvent
mixture prior to the hydrocarbon solvent mixture being supplied to the
injection well (as
illustrated in Fig. 1). Additionally or alternatively, this also may include
heating and
vaporizing the hydrocarbon solvent mixture within the injection well (or
subsequent to
supply to the injection well).
Injection well 30 may include any suitable structure that may form a fluid
conduit to
convey vapor stream 52 to, or into, subterranean formation 24. Similarly,
production well
70 may include any suitable structure that may form a fluid conduit to convey
reduced-
viscosity hydrocarbon mixture 72 from subterranean formation 24 to, toward,
and/or
proximal, surface region 20. As illustrated, for example, in Fig. 1, injection
well 30 may be
spaced apart from production well 70. Production well 70 may extend at least
partially
below injection well 30, may extend at least partially vertically below
injection well 30,
and/or may define a greater distance (or average distance) from surface region
20 when
14
CA 02886479 2015-03-27
compared to injection well 30. At least a portion of production well 70 may be
parallel to,
or at least substantially parallel to, a corresponding portion of injection
well 30. At least a
portion of injection well 30, and/or of production well 70, may include a
horizontal, or at
least substantially horizontal, portion.
Fig. 4 is a flowchart depicting methods 100 according to the present
disclosure of
enhancing production of viscous hydrocarbons from a subterranean formation.
Methods 100
may include preheating at least a portion of the subterranean formation at
105, selecting a
composition of a hydrocarbon solvent mixture at 110, and/or regulating the
composition of
the hydrocarbon solvent mixture at 115. Methods 100 may include heating the
hydrocarbon
solvent mixture to generate a vapor stream at a stream temperature at 120 and
injecting the
vapor stream into the subterranean formation at 125. Methods 100 also may
include
condensing the vapor stream within the subterranean formation at 130 to
generate a
condensate and/or generating reduced-viscosity hydrocarbons at 135. Methods
100 further
may include producing the reduced-viscosity hydrocarbons at 140 and may
include
producing the condensate at 145 and/or recycling the condensate at 150.
Preheating a portion of the subterranean formation at 105 may include
preheating, or
providing thermal energy to, the subterranean formation in any suitable manner
and may be
performed prior to the injecting at 125. The preheating at 105 may include
electrically
preheating the subterranean formation, chemically preheating the subterranean
formation,
and/or injecting a preheating steam stream into the subterranean formation.
The preheating
at 105 may include preheating any suitable portion of the subterranean
formation, such as a
portion of the subterranean formation that is proximal to the injection well,
a portion of the
subterranean formation that is proximal to the production well, and/or a
portion of the
subterranean formation that defines a vapor chamber that receives the vapor
stream.
Selecting the composition of a hydrocarbon solvent mixture at 110 may include
selecting the composition of the hydrocarbon solvent mixture such that a vapor
pressure of
the hydrocarbon solvent mixture is less than a threshold maximum pressure of
the
subterranean formation, such that the vapor pressure of the hydrocarbon
solvent mixture is
at least a lower threshold pressure, and/or such that the vapor pressure of
the hydrocarbon
solvent mixture is less than an upper threshold pressure. Illustrative, non-
exclusive
CA 02886479 2015-03-27
= examples of the threshold maximum pressure, the lower threshold pressure,
and the upper
threshold pressure are discussed herein. Additionally or alternatively, the
selecting at 110
also may include selecting using any of the subsequently described methods
200.
Regulating the composition of the hydrocarbon solvent mixture at 115 may
include
regulating the composition, or chemical composition, of the hydrocarbon
solvent mixture in
any suitable manner. The regulating at 115 may include receiving a hydrocarbon
feedstock,
or a feed stream, that comprises a desired composition for the hydrocarbon
solvent mixture,
and the regulating further may include utilizing the hydrocarbon feedstock as
the
hydrocarbon solvent mixture. The regulating at 115 may include receiving the
hydrocarbon
feedstock and altering a composition of the hydrocarbon feedstock to generate
the
hydrocarbon solvent mixture. The altering may include diluting the hydrocarbon
feedstock,
distilling the hydrocarbon feedstock, removing a portion of the hydrocarbon
feedstock,
and/or decreasing a proportion of the hydrocarbon feedstock that comprises
compounds with
fewer than five carbon atoms to generate the hydrocarbon solvent mixture.
Illustrative, non-
exclusive examples of the composition, or the desired composition, of the
hydrocarbon
solvent mixture are discussed in more detail herein.
Heating the hydrocarbon solvent mixture to generate a vapor stream at 120 may
include heating the hydrocarbon solvent mixture in any suitable manner to
generate the
vapor stream at a suitable stream temperature. Illustrative, non-exclusive
examples of the
stream temperature are disclosed herein.
The heating at 120 may include directly heating the hydrocarbon solvent
mixture in a
surface region to generate the vapor stream. The heating at 120 may include co-
injecting the
hydrocarbon solvent mixture and a steam stream to vaporize the hydrocarbon
solvent
mixture. When the heating at 120 includes co-injecting the steam stream, the
steam stream
may be a saturated steam stream. Additionally or alternatively, the co-
injecting may include
co-injecting at least 5, at least 6, at least 7, at least 8, at least 9 at
least 10, at least 20, at least
25, at least 50, at least 75, or at least 100 moles of the hydrocarbon solvent
mixture for each
mole of steam.
Injecting the vapor stream into the subterranean formation at 125 may include
injecting the vapor stream via an injection well that extends within the
subterranean
16
CA 02886479 2015-03-27
= formation and/or injecting the vapor stream to decrease a viscosity of
viscous hydrocarbons
that may be present within the subterranean formation. This may include
injecting to
facilitate and/or produce the generating at 135.
The injecting at 125 may include flowing the vapor stream through, or through
at
least a portion of, the injection well and into the subterranean formation.
The injecting at
125 also may include contacting the vapor stream with the viscous hydrocarbons
within the
subterranean formation.
Condensing the vapor stream within the subterranean formation at 130 may
include
condensing any suitable portion of the vapor stream to release a latent heat
of condensation
of the vapor stream, heat the subterranean formation, heat the viscous
hydrocarbons, and/or
generate the reduced-viscosity hydrocarbons within the subterranean formation.
The
condensing at 130 may include condensing a majority, at least 50%, at least
60%, at least
70%, at least 80%, at least 90%, at least 95%, at least 99%, or substantially
all of the vapor
stream within the subterranean formation. The condensing at 130 may include
generating a
condensate, which also may be referred to herein as a condensate stream, from
the vapor
stream and/or within the subterranean formation. The condensing at 130 may
include
regulating a temperature within the subterranean formation to facilitate, or
permit, the
condensing at 130.
Generating reduced-viscosity hydrocarbons at 135 may include generating the
reduced- viscosity hydrocarbons in any suitable manner. The generating at 135
may be
facilitated by, produced by, and/or a result of the injecting at 125 and/or
the condensing at
130. The generating at 135 also may include dissolving the condensate in the
viscous
hydrocarbons, dissolving the viscous hydrocarbons in the condensate, and/or
diluting the
viscous hydrocarbons with the condensate to generate the reduced-viscosity
hydrocarbons.
Producing the reduced-viscosity hydrocarbons at 140 may include producing the
reduced- viscosity hydrocarbons via any suitable production well, which may
extend within
the subterranean formation and/or may be spaced apart from the injection well.
This may
include flowing the reduced-viscosity hydrocarbons from the subterranean
formation,
through the production well, and to, proximal to, and/or toward the surface
region.
17
CA 02886479 2015-03-27
The producing at 140 may include producing asphaltenes. The asphaltenes may be
present within the subterranean formation and/or within the viscous
hydrocarbons. The
asphaltenes may be produced as a portion of the reduced-viscosity hydrocarbons
(and/or the
reduced-viscosity hydrocarbons may include, or comprise, asphaltenes). The
injecting at
125 may include injecting into a stimulated region of the subterranean
formation that
includes asphaltenes, and the producing at 140 may include producing at least
a threshold
fraction of the asphaltenes from the stimulated region. This may include
producing at least
wt%, at least 20 wt%, at least 30 wt%, at least 40 wt%, at least 50 wt%, at
least 60 wt%,
at least 70 wt%, at least 80 wt%, or at least 90 wt% of the asphaltenes that
are, or were,
10 present within the stimulated region prior to the injecting at 125.
Producing the condensate at 145 may include producing the condensate, or
condensate stream, that is generated during the condensing at 130. The
producing at 145
may include producing the condensate with the reduced-viscosity hydrocarbons
and/or
producing a reduced- viscosity hydrocarbon mixture that includes the reduced-
viscosity
hydrocarbons and the condensate.
Recycling the condensate at 150 may include recycling the condensate in any
suitable manner. The recycling at 150 may include separating at least a
separated portion of
the condensate from the reduced-viscosity hydrocarbon mixture and/or from the
reduced-
viscosity hydrocarbons. The recycling at 150 also may include utilizing at
least a recycled
portion of the condensate, which also may be referred to herein as a recovered
hydrocarbon
solvent, as, or as a portion of, the hydrocarbon solvent mixture and/or
returning the recycled
portion of the condensate to the subterranean formation via the injection
well. The recycling
at 150 further may include purifying the recycled portion of the condensate
prior to utilizing
the recycled portion of the condensate and/or prior to returning the recycled
portion of the
condensate to the subterranean formation.
Fig. 5 is a flowchart depicting illustrative, non-exclusive examples of
methods 200
according to the present disclosure of selecting a composition of a
hydrocarbon solvent
mixture for injection into a subterranean formation as a vapor stream to
enhance production
of viscous hydrocarbons from the subterranean formation. Methods 200 may
include
determining a threshold maximum pressure for the subterranean formation at
210,
18
CA 02886479 2015-03-27
= determining a stream temperature at which the vapor stream is injected
into the subterranean
formation at 220, and selecting a composition of the hydrocarbon solvent
mixture at 230.
Methods 200 may include injecting the vapor stream into the subterranean
formation at 240
and/or producing reduced- viscosity hydrocarbons from the subterranean
formation at 250.
Determining the threshold maximum pressure for the subterranean formation at
210
may include determining any suitable threshold maximum pressure for the
subterranean
formation. Illustrative, non-exclusive examples of the threshold maximum
pressure are
discussed in more detail herein.
Determining the stream temperature at which the vapor stream is injected into
the
subterranean formation at 220 may include determining the stream temperature
in any
suitable manner. The determining at 220 may include determining a thermally
efficient
stream temperature. The determining at 220 may include determining a stream
temperature
at which a viscosity, or average viscosity, of the viscous hydrocarbons is yet
another
illustrative, non-exclusive example, the determining at 220 may include
determining a
stream temperature at which a production rate of the viscous hydrocarbons from
the
subterranean formation is at least a threshold production rate. Illustrative,
non-exclusive
examples of the stream temperature are disclosed herein.
Selecting the composition of the hydrocarbon solvent mixture at 230 may
include
selecting the composition of the hydrocarbon solvent mixture based, at least
in part, on the
stream temperature and/or on the threshold maximum pressure.
Additionally or
alternatively, the selecting at 230 also may include selecting, at 232, a
first proportion of the
hydrocarbon solvent mixture that comprises a first compound with at least five
carbon
atoms, selecting, at 234, a second proportion of the hydrocarbon solvent
mixture that
comprises a second compound with more carbon atoms than the first compound,
and/or
(optionally) selecting, at 236, a third (or additional) proportion of the
hydrocarbon solvent
mixture that comprises a third (or additional) compound with more carbon atoms
than the
second (or a prior) compound. The selecting at 230 further may include
selecting such that
the first proportion, the second proportion, and/or the third proportion (when
present)
individually comprise at least 10, at least 20, at least 30, at least 40, at
least 50, or at least 60
mole percent of the hydrocarbon solvent mixture. Additionally or
alternatively, the
19
CA 02886479 2015-03-27
= selecting at 230 also may include selecting such that the first compound,
the second
compound, and/or the third compound (when present) together comprise at least
10, at least
20, at least 30, at least 40, at least 50, at least 60, at least 70, at least
80, at least 90, at least
95, or at least 99 mole percent of the hydrocarbon solvent mixture and/or such
that the
hydrocarbon solvent mixture comprises at least 50, at least 60, at least 70,
at least 80, at least
90, at least 95, or at least 99 mole percent hydrocarbons.
The selecting at 230 also may include selecting such that a vapor pressure of
the
hydrocarbon solvent mixture at a stream temperature of the vapor stream is
less than the
maximum threshold pressure of the subterranean formation. Illustrative, non-
exclusive
examples of the stream temperature are disclosed herein.
This selecting may include increasing the first proportion of the hydrocarbon
solvent
mixture and/or decreasing the second proportion of the hydrocarbon solvent
mixture to
increase the vapor pressure of the hydrocarbon solvent mixture. Additionally
or
alternatively, this may include decreasing the first proportion of the
hydrocarbon solvent
mixture and/or increasing the second proportion of the hydrocarbon solvent
mixture to
decrease the vapor pressure of the hydrocarbon solvent mixture.
The selecting at 230 also may include selecting such that the vapor pressure
of the
hydrocarbon solvent mixture is less than an upper threshold pressure and/or
greater than a
lower threshold pressure. Illustrative, non-exclusive examples of the upper
threshold
pressure and/or of the lower threshold pressure are disclosed herein.
When the viscous hydrocarbons include asphaltenes, the selecting at 230
further may
include selecting such that at least a threshold fraction of the asphaltenes
within the sample
are soluble within the hydrocarbon solvent mixture at the temperature and
pressure at which
the hydrocarbon solvent mixture contacts the viscous hydrocarbons within the
subterranean
formation. This may include measuring the solubility of the asphaltenes within
the
hydrocarbon solvent mixture. This is in direct contrast to traditional solvent
injection
processes, which typically are unable to remove asphaltenes, or at least a
significant fraction
of the asphaltenes, from the subterranean formation.
Illustrative, non-exclusive examples of the threshold fraction include
threshold
fractions of at least 20 weight % (wt%), at least 30 wt%, at least 40 wt%, at
least 50 wt%, at
CA 02886479 2015-03-27
= least 60 wt%, at least 70 wt%, at least 80 wt%, at least 90 wt%, at least
95 wt%, or at least
99 wt%. Additionally or alternatively, the selecting at 230 also may include
selecting such
that a solubility of the asphaltenes within the hydrocarbon solvent mixture is
greater than a
solubility of the asphaltenes in propane and/or butane.
Injecting the vapor stream into the subterranean formation at 240 may include
injecting the vapor stream into the subterranean formation in any suitable
manner to
generate reduced- viscosity hydrocarbons within the subterranean formation. As
an
illustrative, non-exclusive example, the injecting at 240 may be at least
substantially similar
to the injecting at 125, which is discussed in more detail herein with
reference to Fig. 4.
Producing reduced-viscosity hydrocarbons from the subterranean formation at
250
may include producing the reduced-viscosity hydrocarbons in any suitable
manner. As an
illustrative, non-exclusive example, the producing at 250 may be at least
substantially
similar to the producing at 140, which is discussed in more detail herein with
reference to
Fig. 4.
In the present disclosure, several of the illustrative, non-exclusive examples
have
been discussed and/or presented in the context of flow diagrams, or flow
charts, in which the
methods are shown and described as a series of blocks, or steps. Unless
specifically set
forth in the accompanying description, the order of the blocks may vary from
the illustrated
order in the flow diagram, including with two or more of the blocks (or steps)
occurring in a
different order and/or concurrently.
As used herein, the term "and/or" placed between a first entity and a second
entity
means one of (1) the first entity, (2) the second entity, and (3) the first
entity and the second
entity. Multiple entities listed with "and/or" should be construed in the same
manner, i.e.,
"one or more" of the entities so conjoined. Other entities may optionally be
present other
than the entities specifically identified by the "and/or" clause, whether
related or unrelated
to those entities specifically identified.
As used herein, the phrase "at least one," in reference to a list of one or
more entities
should be understood to mean at least one entity selected from any one or more
of the entity
in the list of entities, but not necessarily including at least one of each
and every entity
specifically listed within the list of entities and not excluding any
combinations of entities in
21
= = CA 02886479 2016-03-07
the list of entities. This definition also allows that entities may optionally
be present other
than the entities specifically identified within the list of entities to which
the phrase "at least
one" refers, whether related or unrelated to those entities specifically
identified.
As used herein the terms "adapted" and "configured" mean that the element,
component, or other subject matter is designed and/or intended to perform a
given function.
Thus, the use of the terms "adapted" and "configured" should not be construed
to mean that
a given element, component, or other subject matter is simply "capable of"
performing a
given function but that the element, component, and/or other subject matter is
specifically
selected, created, implemented, utilized, programmed, and/or designed for the
purpose of
performing the function. It is also within the scope of the present disclosure
that elements,
components, and/or other recited subject matter that is recited as being
adapted to perform a
particular function may additionally or alternatively be described as being
configured to
perform that function, and vice versa.
Industrial Applicability
The systems and methods disclosed herein are applicable to the oil and gas
industry.
The subject matter of the disclosure includes all novel and non-obvious
combinations
and subcombinations of the various elements, features, functions and/or
properties disclosed
herein. Similarly, where the claims recite "a" or "a first" element or the
equivalent thereof,
such claims should be understood to include incorporation of one or more such
elements,
neither requiring nor excluding two or more such elements.
22