Note: Descriptions are shown in the official language in which they were submitted.
CA 2,886,957
Blakes Ref: 72571/00022
1 USE OF OX0 COMPOUNDS FOR LOWERING APO C3
2 [001] The present disclosure relates to a method of reducing
apolipoprotein C-
3 III (apoC-III) mRNA or protein in a subject in need thereof, comprising
administering to the
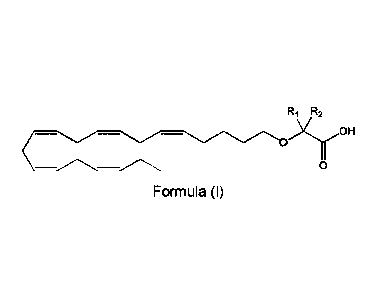
4 subject a pharmaceutically effective amount of a compound of Formula (I):
R1 R2
6 Formula (I)
7 or a pharmaceutically acceptable salt or ester thereof,
8 wherein R1 and R2 are independently chosen from a hydrogen atom or
linear, branched,
9 and/or cyclic Ci-C6 alkyl groups, with the proviso that R1 and R2 are not
both hydrogen. Such
methods, compounds, and compositions are useful to treat conditions caused by,
associated
11 with, or aggravated by, elevated hepatic and /or plasma apoC-III such as
hypertriglyceridemia
12 (HTG), hyperchylomicronemia, dyslipidemia, pancreatitis and in the
prevention and/or treatment
13 of one or more of cardiovascular disease or metabolic disorder, or a
symptom thereof.
14 [002] Dietary polyunsaturated fatty acids (PUFAs), including omega-3
fatty acids,
have effects on diverse physiological processes impacting normal health and
chronic diseases,
16 such as the regulation of plasma lipid levels, cardiovascular and immune
functions, insulin
17 action, neuronal development, and visual function.
18 [003] Omega-3 fatty acids, e.g., (5Z,8Z,11Z,14Z,17Z)-icosa-
5,8,11,14,17-pentaenoic
19 acid (EPA) and (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoic
acid (DHA),
regulate plasma lipid levels, cardiovascular and immune functions, insulin
action, and neuronal
21 development, and visual function. Omega-3 fatty acids have been shown to
have beneficial
22 effects on the risk factors for cardiovascular diseases, for example
hypertension and
23 hypertriglyceridemia (HTG), and on the coagulation factor VII
phospholipid complex activity.
24 [004] WO 2010/128401 discloses that 2-((5Z,8Z,11Z,14Z,17Z)-icosa-
5,8,11,14,17-
pentaenyloxy)butanoic acid favorably influences lipid profiles and inhibits
i.a. development of
26 atherosclerosis, decreases total cholesterol and increases HDL
cholesterol as compared to a
27 control. Those results demonstrate that 2-((5Z,8Z,11Z,14Z,17Z)-icosa-
5,8,11,14,17-
28 pentaenyloxy)butanoic acid and its derivatives may be useful in the
prevention or treatement of
29 various conditions, such as inflammation, hyperlipidemic conditions,
obesity, fatty liver disease,
atherosclerosis, peripheral insulin resistance, and/or diabetic conditions.
Further use of 2-
1
22713041.2
Date Recue/Date Received 2021-08-30
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 ((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaenyloxy)butanoic acid and
its derivatives for
2 treating different diseases or conditions is disclosed in WO 2012/059818.
3 [005] More particularly W02012/059818 describes a method of treating or
preventing
4 at least one disease or condition selected from elevated Apo B, primary
hypercholesterolemia
(heterozygous familial and nonfamilial), and primary dysbetalipoproteinemia
(Fredrickson Type
6 III) in a subject in need thereof, comprising administering to the
subject a pharmaceutically
7 effective amount of a compound of Formula (I). However although it is
already established that
8 Apo B and Apo E (dysbetalipoproteinemia) related pathways are positively
affected by
9 compounds of Formula (I), data from 2 clinical studies in distinct
patient populations surprisingly
revealed that an additional apolipoprotein, apoC-Ill, is also potently reduced
by compounds of
11 Formula (I).
12 [006] ApoC-Ill is a glycoprotein produced primarily by the liver
whose function is
13 believed to involve promoting the assembly and secretion of triglyceride-
rich VLDL particles
14 from hepatic cells under lipid-rich conditions (Sundaram Met al., J
Lipid Research, vol. 51,
2010). In plasma it is largely associated with very low-density lipoprotein
(VLDL), high-density
16 lipoprotein (HDL) and chylomicrons. An increase in apoC-III levels
induces the development of
17 hypertriglyceridemia. The mechanisms by which apoC-III expression
increase plasma
18 triglycerides are partially mediated via inhibition of lipoprotein
lipase and hepatic lipase; it
19 thereby delays the catabolism of triglyceride-rich particles. ApoC-III
is also thought to inhibit
hepatic uptake of triglyceride rich particles. The clinical importance of apoC-
III has been
21 established by studies demonstrating that carriers of rare mutations
that disrupt apoC-III
22 function have both lower TG levels and a reduced risk of
coronary/ischemic heart disease (N
23 Engl J Med. 2014 Jul 3;371(1):22-31, Loss-of-function mutations in
APOC3, triglycerides, and
24 coronary disease).
[007] The long-chain omega-3 fatty acids, EPA and DHA, are well established in
the
26 treatment of HTG. Given the recent identification of apoC-III as both a
pivotal regulator in
27 triglyceride levels and as a genetically validated target for the
prevention of coronary heart
28 disease, the effects of omega-3 fatty acids in various forms and
compositions upon plasma
29 apoC-III levels have been investigated. By way of example US2014/0221486
claims a method
for reducing an apoC-Ill level of a subject either on statin therapy and
having baseline fasting
31 triglycerides of about 200 mg/di to about 499 mg/di, or a subject having
fasting baseline
32 triglycerides of at least about 500 mg/di, by administering a
pharmaceutical composition
33 comprising about 1 g to about 4 g of ethyl eicosapentaenoate per day to
the subject. US
2
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 2013/0177643 claims a method of lowering serum or plasma apoC-III levels,
comprising
2 administering a pharmaceutical composition comprising: EPA, substantially
in free acid form, in
3 an amount of at least about 50% (ala); DHA, substantially in free acid
form, in an amount of at
4 least about 15% (a/a); DPA, substantially in free acid form, in an amount
of at least about 1%
(a/a); in an amount and for a duration sufficient to reduce serum or plasma
apoC-III from pre-
6 treatment levels. Yet another example can be found in US2014/0094520
claiming a method of
7 reducing a lipid parameter level in a subject from a baseline lipid
parameter level, wherein the
8 lipid parameter is selected from a group consisting of inter alia apoC-
Ill, comprising
9 administering to the subject a composition comprising fatty acids,
wherein at least 50 percent by
weight of the fatty acids comprise omega-3 fatty acids, salts, esters, or
derivatives thereof,
11 wherein the omega-3 fatty acids comprise eicosapentaenoic acid (EPA),
docosapentaenoic acid
12 (DPA) and wherein the ratio of docosahexaenoic acid to DHA to EPA
(DHA:EPA) is less than
13 1:10, and wherein the ratio of DHA to DPA (DHA:DPA) is less than 2:1.
14 [008] Effective lowering of hepatic/plasma apoC-III with an orally
delivered omega-
3/omega-3 derivative offers an attractive treatment option for selected
patient populations if
16 clinically relevant reductions can be achieved. Although it is yet to be
determined what degree
17 of reduction in apoC-III is 'clinically relevant', studies in subjects
with loss-of-function apoC-III
18 mutations show that apoC-III levels 46% lower than non-carriers are
associated with a 40%
19 lower risk of coronary heart disease (CHD) (N Engl J Med. 2014 Jul
3;371(1):22-31, Loss-of-
function mutations in APOC3, triglycerides, and coronary disease). In addition
to the reduced
21 apoC-III concentrations, carriers also had 39% lower TG concentrations
than non-carriers.
22 Given that loss-of-function represents life-long exposure, it is
therefore conceivable that
23 therapies aimed at reducing apoC-III over a shorter time frame should
aim for apoC-III
24 reductions as close to (or higher) than those associated with loss-of-
function mutations if
beneficial effects upon CHD are to be achieved. As the apoC-III results
achieved with naturally
26 occurring omega-3 lipids are relatively modest (see Example 26),
compounds that more potently
27 reduce apoC-Ill may offer not only superior triglyceride lowering but
also superior
28 cardioprotective effects.
29 [009] The present disclosure relates to a method of reducing
apolipoprotein C-
III (apoC-III) mRNA or protein in a subject in need thereof, comprising
adrhinistering to the
31 subject a pharmaceutically effective amount of a compound of Formula
(I):
3
22713041.1
CA 02886957 2015-04-01
CA Application
Makes Ref: 72571/00022
R1 R2
o,,===\cõ,,OH
1
2 Formula (I)
3 or a pharmaceutically acceptable salt or ester thereof,
4 wherein R1 and R2 are independently chosen from a hydrogen atom or
linear, branched,
and/or cyclic 01-C2 alkyl groups, with the proviso that RI and R2 are not both
hydrogen.
6 [010] A number of metabolic diseases or conditions are closely
associated with
7 increased risk of cardiovascular events. Such diseases or conditions
include, but are not limited
8 to, diabetes mellitus type I and type II, metabolic syndrome,
dyslipidemic conditions such as
9 hypercholesterolemia, hyperlipidemia, mixed dyslipidemia,
hypertriglyceridemia,
hyperchyolomicronemia, and various familial dyslipidemias.
11 [011] In at least one embodiment the disease or condition is chosen
from any of
12 hypertriglyceridemia (HTG), hyperchylomicronemia, dyslipidemia, and
pancreatitis and in the
13 prevention and/or treatment of one or more of cardiovascular disease or
metabolic disorder, or a
14 symptom thereof.
[012] The present disclosure also includes a method of reducing apoC-III in
a subject
16 in need thereof, the method comprising administering to the subject a
pharmaceutically effective
17 amount of 2-((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-
yloxy)butanoic acid:
0
18 0
19 or a pharmaceutically acceptable salt or ester thereof.
21 Brief description of the drawings
22 [013] Figure 1 discloses the relative hepatic apoC-III gene expression
for a compound
23 of Formula (I), a control, and a reference compound.
24 Description
[014] Particular aspects of the disclosure are described in greater detail
below. The
26 terms and definitions as used in the present application and as
clarified herein are intended to
27 represent the meaning within the present disclosure.
28 [015] The singular forms ''a," "an," and "the" include plural reference
unless the context
29 dictates otherwise.
4
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 [016] The terms "approximately" and "about mean to be nearly the same as
a
2 referenced number or value. As used herein, the terms "approximately" and
"about"
3 should be generally understood to encompass 5% of a specified amount,
frequency, or
4 value.
[017] The terms "treat," "treating," and "treatment" include any therapeutic
6 application that can benefit a human or non-human mammal. Both human and
veterinary
7 treatments are within the scope of the present disclosure. Treatment may
be responsive
8 to an existing condition or it may be prophylactic, i.e., preventative.
9 [018] The terms "administer," "administration,'' and "administering" as
used herein
refer to (1) providing, giving, dosing and/or prescribing by either a health
practitioner or his
11 authorized agent or under his direction a compound or composition
according to the
12 present disclosure, and (2) putting into, taking or consuming by the
human patient or
13 person himself or herself, or non-human mammal a compound or composition
according
14 to the present disclosure.
[019] The term "pharmaceutically effective amount" means an amount sufficient
to
16 achieve the desired pharmacological and/or therapeutic effects, i.e., an
amount of the
17 disclosed compound that is effective for its intended purpose. While
individual
18 subject/patient needs may vary, the determination of optimal ranges for
effective amounts
19 of the disclosed compound is within the skill of the art. Generally, the
dosage regimen for
treating a disease and/or condition with the compounds presently disclosed may
be
21 determined according to a variety of factors such as the type, age,
weight, sex, diet, and/or
22 medical condition of the subject/patient.
23 [020] The term "pharmaceutical composition" means a compound according
to
24 the present disclosure in any form suitable for medical use.
[021] The compounds of Formula (I) may exist in various stereoisomeric forms,
26 including enantiomers, diastereomers, or mixtures thereof. It will be
understood that the
27 invention encompasses all optical isomers of the compounds of Formula
(I) and mixtures
28 thereof. Hence, compounds of Formula (I) that exist as diastereomers,
racemates, and/or
29 enantiomers are within the scope of the present disclosure.
[022] The present disclosure relates to a method of reducing apolipoprotein
C-III
31 (apoC-III) mRNA or protein in a subject in need thereof, comprising
administering to the subject
32 a pharmaceutically effective amount of a compound of Formula (I):
5
22713041.1
CA 02886957 2015-04-01
CA Application
Blokes Ref: 72571/00022
R1 R2
OH
O)C
1 0
2 Formula (I)
3 or a pharmaceutically acceptable salt or ester thereof,
4 wherein RI and R2 are independently chosen from a hydrogen atom or
linear, branched,
and/or cyclic C1-C6 alkyl groups, with the proviso that R1 and R2 are not both
hydrogen.
6 [023] In at least one embodiment, the present disclosure relates to
use of a
7 pharmaceutically effective amount of a compound of Formula (I):
R1 R2
8 6
9 Formula (I)
or a pharmaceutically acceptable sailor ester thereof, for reducing
apolipoprotein
11 C-III (apoC-III) nnRNA or protein in a subject in need thereof,
12 wherein RI and R2 are independently chosen from a hydrogen atom or
linear, branched,
13 and/or cyclic C1-06 alkyl groups, with the proviso that Aland R2 are not
both hydrogen.
14 [024] In at least one embodiment, R1 and R2 are chosen from a
hydrogen atom, a
methyl group, an ethyl group, a n-propyl group, and an isopropyl group.
16 [025] In at least one embodiment, R1 and R2are chosen from a hydrogen
atom, a
17 methyl group, and an ethyl group.
18 [026] In at least one embodiment, one of R1 and R2 is a hydrogen atom
and the other
19 one of R, and R2 is chosen from a C1-03 alkyl group. In one embodiment
one of R1 and R2 is a
hydrogen atom and the other one of RI and R2 is chosen from a methyl group or
an ethyl group.
21 [027] In at least one embodiment, the compound is present in its
various
22 stereoisonneric forms, such as an enantiomer (R or S), diastereomer, or
mixtures thereof.
23 [028] In at least one embodiment, the compound is present in racennic
form.
24 [029] In cases, where the compound according to Formula (I) is a salt
of a
counter-ion with at least one stereogenic center, or ester of an alcohol with
at least one
26 stereogenic center, the compound may have multiple stereocenters. In
those situations,
27 the compounds of the present disclosure may exist as diastereomers.
Thus, in at least
28 one embodiment, the compounds of the present disclosure are present as
at least one
29 diastereomer.
6
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 [030] In at least one embodiment, the compound of the present
disclosure is 2-
2 ((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yloxy)butanoic acid:
3 0
4 [031] In at least one embodiments the compound of the present
disclosure is present
in its S and/or R form represented by the formulas:
6 0 and - o
7 [032] In at least one embodiment the disease or condition is chosen
from any of
8 hypertriglyceridemia (HTG), hyperchylomicronemia, dyslipidemia, and
pancreatitis and in the
9 prevention and/or treatment of one or more of cardiovascular disease or
metabolic disorder, or a
symptom thereof. In one embodiment the disease or condition is chosen from any
of
11 hyperchylomicronemia, pancreatitis and in the prevention and/or
treatment of one or more of
12 cardiovascular disease or metabolic disorder, or a symptom thereof. In
one embodiment the
13 disease or condition is chosen from any of hyperchylomicronemia and
pancreatitis.
14 [033] Compounds of Formula (I) can be prepared as described, for
example, in
PCT Application WO 2010/128401 filed May 7,2010, and according to Examples 1-
23
16 below.
17 [034] Examples 1-23 are exemplary and one skilled in the art would
understand
18 how to apply these general methods to arrive at other compounds within
the scope of
19 Formula (I). Compounds of the present disclosure may be in the form of a
pharmaceutically
acceptable salt or ester. For example, the compounds of Formula (I) may be in
the form of
21 esters, such as a phospholipid, a glyceride or a C1-C6-alkyl ester. In
at least one
22 embodiment, the ester is chosen from a glyceride or a 01-C6-alkyl ester.
In at least one
23 embodiment, the ester is chosen from a triglyceride, a 1,2-diglyceride,
a 1,3-diglyceride, a
24 1-monoglyceride, a 2-monoglyceride, a methyl ester, an ethyl ester, a
propyl ester, a
isopropyl ester, a n-butyl ester and a tert-butyl ester. In at least one
embodiment, the
26 compound of Formula (I) is present as a methyl ester, an ethyl ester, an
isopropyl ester, a
27 n-butyl ester or a tert-butyl ester, for example as a methyl ester or an
ethyl ester. It has
28 been proven by in-vitro digestion studies in a bio relevant media that
esters represented by
7
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 Formula (I) (i.e., the ethyl ester and the butyl ester) will be rapidly
hydrolyzed in the
2 gastrointestinal tract.
3 [035] Salts suitable for the present disclosure include, but are not
limited to, salts
4 of NH4'; metal ions such as Li, Na, K., Mg2 , or Ca2.; a protonated
primary amine such as
tert-butyl ammonium, (3S,5S,7S)-adamantan-1-ammonium, 1,3-dihydroxy-2-
6 (hydroxymethyl)propan-2-ammonium, a protonated aminopyridine (e.g.,
pyridine-2-
7 ammonium); a protonated secondary amine such as diethylammonium,
2,3,4,5,6-
8 pentahydroxy-N-methylhexan-1-ammonium, N-ethylnaphthalen-1-ammonium, a
protonated
9 tertiary amine such as 4-methylmorpi iolin-4-ium, a protonated quaternary
amine such as 2-
hydroxy-N,N,N-trimethylethan-1-aminium and a protonated guanidine such as
amino((4-
11 amino-4-carboxybutyl)amino)methaniminium or a protonated heterocycle
such as 1 H-
12 imidazol-3-ium. Additional examples of suitable salts include salts of a
diprotonated
13 diamine such as ethane-1,2-diammonium or piperazine-1,4-diium. Other
salts according
14 to the present disclosure may comprise protonated Chitosan:
OH \ OH
\ 0
\ HO 0 HO
NH3* NH2 /
m
15=
16 [036] In at least embodiment the salts are chosen from a sodium salt,
a calcium
17 salt, and a choline salt.
18 [037] The present disclosure provides for a method of reducing apoC-
III in a
19 subject in need thereof, comprising administering to the subject a
pharmaceutically
effective amount of a compound of Formula (I). The subject may be a human or a
non-
21 human mammal. The compounds presently disclosed may be administered as a
22 medicament, such as in a pharmaceutical composition.
23 [038] In at least one embodiment, the present disclosure relates to a
method for
24 reducing an apoC-III level of a subject on statin therapy and having
baseline fasting
triglycerides of about 200 mg/di to about 499 mg/dl by administering to the
subject a
26 pharmaceutical effective amount of a compound of Formula (I). In another
embodiment the
27 present disclosure relates to use of a pharmaceutical effective amount
of a compound of
28 Formula (I), in the manufacture of a medicament for reducing an apoC-III
level of a subject
29 on statin therapy and having baseline fasting triglycerides of about 200
mg/dlto about 499
8
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 mg/c11. The apoC-111 level can be reduced by at least about 20%, by at
least about 25%, by
2 at least about 30% or by at least about 35%.
3 [039] In at least one embodiment, the disclosure relates to a method
for reducing
4 an apoC-III level of a subject having baseline fasting triglycerides of
about 200 mg/ell to
about 499 mg/di by administering to the subject a pharmaceutical effective
amount of a
6 compound of Formula (1). In another embodiment the present disclosure
relates to use of a
7 pharmaceutical effective amount of a compound of Formula (1), in the
manufacture of a
8 medicament for reducing an apoC-III level of a subject having baseline
fasting triglycerides
9 of about 200 mg/di to about 499 mg/ell. The apoC-III level can be reduced
by at least about
20%, by at least about 25%, by at least about 30% or by at least about 35%.
11 [040] In at least one embodiment, the present disclosure relates to a
method for
12 reducing an apoC-III level of a subject on statin therapy and having
baseline fasting
13 triglycerides of above 500 mg/dl by administering to the subject a
pharmaceutical effective
14 amount of a compound of Formula (I). In another embodiment the present
disclosure
relates to use of a pharmaceutical effective amount of a compound of Formula
(1), in the
16 manufacture of a medicament for reducing an apoC-III level of a subject
on statin therapy
17 and having baseline fasting triglycerides of above 500 mg/d1. The apoC-
III level can be
18 reduced by at least about 25%, by at least about 30%, by at least about
35% or by at least
19 about 40%.
[041] In at least one embodiment, the disclosure relates to a method for
reducing
21 an apoC-111 level of a subject having baseline fasting triglycerides of
above 500 mg/di by
22 administering to the subject a pharmaceutical effective amount of a
compound of Formula
23 (I). In another embodiment the present disclosure relates to use of a
pharmaceutical
24 effective amount of a compound of Formula (I), in the manufacture of a
medicament for
reducing an apoC-III level of a subject having baseline fasting triglycerides
of above 500
26 mg/c11. The apoC-111 level can be reduced by at least about 25%, by at
least about 30%, by
27 at least about 35% or by at least about 40%.
28 [042] The present disclosure also relates to a method for reducing an
apoC-III
29 level of a subject having baseline fasting LDL-cholesterol of at least
2.5 nnmol/L (-97mg/dI)
by administering to the subject a pharmaceutical effective amount of a
compound of
31 Formula (1). In another embodiment the present disclosure relates to use
of a
32 pharmaceutical effective amount of a compound of Formula (I), in the
manufacture of a
33 medicament for reducing an apoC-III level of a subject having baseline
fasting LDL-
9
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 cholesterol of at least 2.5 mmol/L (-97mg/dI). The apoC-III level can be
reduced by at least
2 about 25%, by at least about 30%, by at least about 35% or by at least
about 40%.
3 [043] In at least one embodiment the present disclosure relates to a
method for
4 reducing apoC-III in a subject in need thereof, comprising administering
to the subject a
pharmaceutically effective amount of a dyslipidemic agent such as for example
a statin and a
6 compound of Formula (I).
7 [044] The composition presently disclosed may comprise at least one
compound
8 of Formula (I) and optionally at least one non-active pharmaceutical
ingredient, i.e.,
9 excipient. Non-active ingredients may solubilize, suspend, thicken,
dilute, emulsify.
stabilize, preserve, protect, color, flavor, and/or fashion active ingredients
into an
11 applicable and efficacious preparation, such that it may be safe,
convenient, and/or
12 otherwise acceptable for use. Examples of excipients include, but are
not limited to,
13 solvents, carriers, diluents, binders, fillers, sweeteners, aromas, pH
modifiers, viscosity
14 modifiers, antioxidants, extenders, humectants, disintegrating agents,
solution-retarding
agents, absorption accelerators, wetting agents, absorbents, lubricants,
coloring agents,
16 dispersing agents, and preservatives. Excipients may have more than one
role or
17 function, or may be classified in more than one group; classifications
are descriptive only
18 and are not intended to be limiting. ;-1 some embodiments, for example,
the at least one
19 excipient may be chosen from corn starch, lactose, glucose,
microcrystalline cellulose,
magnesium stearate, polyvinylpyrrolidone, citric acid, tartaric acid, water,
ethanol, glycerol,
21 sorbitol, polyethylene glycol, propylene glycol, cetylstearyl alcohol,
carboxymethylcellulose,
22 and fatty substances such as hard fat or suitable mixtures thereof. In
some embodiments,
23 the compositions presently disclosed comprise at least one compound of
Formula (I) and
24 at least one pharmaceutically acceptable antioxidant, e.g., tocopherol
such as alpha-
tocopherol, beta-tocopherol, gamma-tocopherol, and de/ta-tocopherol, or
mixtures thereof,
26 BHA such as 2-tert-butyl-4-hydroxyanisole and 3-tert-butyl-4-
hydroxyanisole, or mixtures
27 thereof and BHT (3,5-di-tert-butyl-4-hydroxytoluene), or mixtures
thereof.
28 [045] The compositions presently disclosed may be formulated in oral
29 administration forms, e.g., tablets or gelatin soft or hard capsules.
The dosage form can be
of any shape suitable for oral administration, such as spherical, oval,
ellipsoidal, cube-
31 shaped, regular, and/or irregular shaped. Conventional formulation
techniques known in
32 the art, may be used to formulate the compounds according to the present
disclosure. In
33 some embodiments, the composition may be in the form of a gelatin
capsule or a tablet.
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 [046] A suitable daily dosage of a compound of Formula (I) may range
from
2 about 5 mg to about 2 g. For example, in some embodiments, the daily dose
ranges from
3 about 50 mg to about 1 g, from about 100 mg to about 1 g, from about 50
mg to about 800
4 .. mg, from about 100 mg to about 800 mg, from about 100 mg to about 600 mg.
In at least
one embodiment, the daily dose ranges from about 200 mg to about 600 mg. The
6 compounds may be administered, for example, once, twice, or three times
per day. In at
7 .. least one embodiment, the compound of Formula (I) is administered in an
amount ranging
8 .. from about 200 mg to about 800 mg per dose. In at least one embodiment,
the compound
9 of Formula (I) is administered once per day.
[047] The present inventors have found that compounds of Formula (I), such
as
11 2-((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yloxy)butanoic acid,
have
12 remarkably good pharmaceutical activity. Surprisingly, the compounds of
Formula (I)
13 .. presently disclosed exhibit improved biological activity compared to
naturally occurring
14 .. omega-3 fatty acids, such as EPA and DHA for reducing apoC-Ill.
[048] In some embodiments, for example, compounds of Formula (I) may reduce
16 the median levels of apoC-III in plasma or in the liver by at least 25-
30% versus
17 baseline, i.e., a superior decrease to that achieved with available
EPA/DHA/DPA
18 .. combinations. As compounds of Formula (I) have been shown to decrease
hepatic
19 .. apoC-III mRNA in pre-clinical models (and thus presumably also hepatic
production/secretion), the addition of lipid-lowering drugs that reduce apoC-
III via
21 increased hepatic uptake of apo B particles, e.g., statins or PCSK-9
inhibitors, could
22 .. be expected to exert additional plasma apoC-III lowering effects.
23 Examples
24 [049] The present disclosure may be further described by the
following non-
limiting examples, in which standard techniques known to the skilled chemist
and
26 techniques analogous to those described in these examples may be used
where
27 .. appropriate. It is understood that the skilled artisan will envision
additional embodiments
28 .. consistent with the disclosure provided herein.
29 [050] Unless otherwise stated, reactions were carried out at room
temperature,
.. typically in the range between 18-25 C with solvents of HPLC grade under
anhydrous
31 conditions. Evaporations were carried out by rotary evaporation in
vacuo. Column
32 .. chromatography was performed by the flash procedure on silica gel.
Nuclear magnetic
33 .. resonance (NMR) shift values were recorded on a Bruker Avance DPX 200 or
300, or on
11
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 an AVII 400 instrument with peak multiplicities described as follows: s,
singlet; d, doublet;
2 dd, double doublet; t, triplet; q, quartet; p, pentet; m, multiplett; br,
broad. Mass spectra
3 were recorded with a GI956A mass spectrometer (electrospray, 3000 V)
switching positive
4 and negative ionization mode. Reported yields are illustrative and do not
necessarily
represent the maximum yield attainable.
6 [051] Example 1: Preparation of fert-butyl 2-((5Z,8Z,11Z,14Z,17Z)-
icosa-
7 5,8,11,14,17-pentaen-1-yloxy)butanoate:
o
-----õ---,-----......-----.0 -.....--
)y
- 8 _,-,.. 6
9 [052] Tetrabutylammonium chloride (0.55 g, 1.98 mmol) was added to a
solution
of (5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-ol, (3.50 g, 12.1 mmol) in
toluene
11 (35 mL) at room temperature under nitrogen. An aqueous solution of
sodium hydroxide
12 (50% (w/w), 11.7 mL) was added under vigorous stirring at room
temperature, followed by
13 t-butyl 2-bromobutyrate (5.41 g, 24.3 mmol). The resulting mixture was
heated to 50 C and
14 additional t-butyl 2-bromobutyrate was added after 1.5 hours (2.70 g,
12.1 mmol), 3.5
hours (2.709, 12.1 mmol) and 4.5 hours (2.709, 12.1 mmol) and stirred for 12
hours in
16 total. After cooling to room temperature, ice water (25 mL) was added
and the resulting
17 two phases were separated. The organic phase was washed with a mixture
of NaOH (5%)
18 and brine, dried (MgSO4), filtered and concentrated. The residue was
purified by flash
19 chromatography on silica gel using increasingly polar mixtures of
heptane and ethyl acetate
(100:0 -> 95:5) as eluent. Concentration of the appropriate fractions afforded
1.87 g (36%
21 yield) of the title compound as an oil. 1H NMR (300 MHz, CDCI3): 5 0.85-
1.10 (m, 6H),
22 1.35-1.54 (m, 11H), 1.53-1.87 (m, 4H), 1.96-2.26 (m, 4H), 2.70-3.02 (m,
8H), 3.31 (dt, 1H),
23 3.51-3.67 (m, 2H), 5.10-5.58 (m, 10H).
24 [053] Example 2: Preparation of 2-((5Z,8Z,11Z,14Z,17Z)-icosa-
5,8,11,14,17-
pentaenyloxy)butanoic acid (Compound A):
26 roil
27 [054] tert-Butyl 2-((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-
28 yloxy)butanoate (19.6 g, 45.5 mmol) was dissolved in dichloromethane
(200 mL) and
29 placed under nitrogen. Trifluoroacetic acid (50 mL) was added and the
reaction mixture was
stirred at room temperature for one hour. Water was added and the aqueous
phase was
12
22713041.1
CA 02886957 2015-04-01
CA Application
Blakos Ref: 72571/00022
1 extracted twice with dichloromethane. The combined organic extract was
washed with
2 brine, dried (Na2SO4), filtered and concentrated. The residue was
subjected to flash
3 chromatography on silica gel using increasingly polar mixtures of
heptane, ethyl acetate
4 and formic acid (90: 10:1 ->80:20:1) as eluent. Concentration of the
appropriate fractions
afforded 12.1 g (71% yield) of the title compound as an oi1.11-1-NMR (300 MHz,
C0CI3): 6
6 0.90-1.00 (m, 6H), 1.50 (m, 2H), 1.70 (m, 2H), 1.80 (m, 2H), 2.10 (m,
4H), 2.80-2.90 (m,
7 8H), 3.50 (m, 1H), 3.60 (m, 1H), 3.75 (t, 1H), 5.30-5.50 (m, 10H); MS
(electrospray): 373.2
8 [M-H].
9 [055] Example 3: Preparation of (4S,5R)-3-((S)-2-((5Z,8Z,11Z,14Z,17Z)-
icosa-
5,8,11,14,17-pentaenyloxy)butanoy1)-4-methy1-5-phenyloxazolidin-2-one and
(4S,5R)-
11 3-((R)-2-((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaenyloxy)butanoy1)-
4-methy1-5-
12 phenyloxazolidin-2-one:
/-
13 o 6 a
14 [056] DMAP (1.10 g, 8.90 mmol) and DCC (1.90 g, 9.30 mmol) were added
to a
mixture of 2-((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaenyloxy)butanoic
acid (3.20 g,
16 8.50 mmol) in dry dichloromethane (100 nnL) held at 0 C under nitrogen.
The resulting
17 mixture was stirred at 0 C for 20 minutes. (4S,5R)-4-methyl-5-
phenyloxazolidin-2-one (1.50
18 g, 8.50 mmol) was added and the resulting turbid mixture was stirred at
ambient
19 temperature for five days. The mixture was filtrated and concentrated
under reduced
pressure to give a crude product containing the desired product as a mixture
of two
21 diastereomers. The residue was purified by flash chromatography on
silica gel using 15%
22 ethyl acetate in heptane as eluent. The two diastereomers were separated
and the
23 appropriate fractions were concentrated. (4S,5R)-3-((S)-2-
((5Z,8Z,11Z,14Z,17Z)-icosa-
24 5,8,11,14,17-pentaenyloxy)butanoy1)-4-methy1-5- phenyloxazolidin-2-one
eluted first and
was obtained in 1.1 9(40% yield) as an oil. (4S,5R)-3-((R)-2-
((57,87,11Z,147,17Z)-icosa-
26 5,8,11,14,17-pentaenyloxy)butanoy1)-4-methy1-5-phenyloxazolidin-2-one
was obtained in
27 0.95 g (34% yield) as an oil.
28 [057] (4S,5R)-3-((S)-2-((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-
29 pentaenyloxy)butanoy1)-4-methy1-5-phenyloxazolidin-2-one (El ):1H-NMR
(300 MHz,
CDCI3): b 0.90 (d, 3H), 1.00 (t, 3H), 1.07 (t, 3H), 1.45-1.57 (m, 2H), 1.62-
1.76 (m, 3H), 1.85-
13
22713041.1
CA 2,886,957
Blakes Ref: 72571/00022
1 1.95 (m, 1H), 2.05-2.15 (m, 4H), 2.87 (m, 8H), 3.39 (m, 1H), 3.57 (m,
1H), 4.85-4.92 (m,
2 2H), 5.30-5.45 (m, 10H), 5.75 (d, 1H), 7.32 (m, 2H), 7.43 (m, 3H).
3 [058] (4S,5R)-3-((R)-2-((5Z,8Z,11Z,14Z,17Z)-icosa-5, 8,11,14,17-
4 pentaenyloxy)butanoyI)-4-methyl-5-phenyloxazolidin-2-one (E2): 1H-NMR
(300 MHz,
CDCI3): 6 0.98 (d, 3H), 0.99 (t, 3H), 1.08 (t, 3H), 1.40-1.52 (m, 2H), 1.55-
1.75 (m, 3H), 1.80-
6 1.90 (m, 1H), 2.05-2.15 (m, 4H), 2.84 (m, 8H), 3.39 (m, 1H), 3.56 (m,
1H), 4.79 (pent, 1H),
7 4.97 (dd, 1H), 5.30-5.45 (m, 10H), 5.71 (d, 1H), 7.33 (m, 2H), 7.43 (m,
3H).
8 [059] Example 4: Preparation of (S)-24(52,8Z,11Z,14Z,17Z)-icosa-
9 5,8,11,14,17-pentaenyloxy)butanoic acid:
OH
11 [060] Hydrogen peroxide (35% in water, 0.75 mL, 8.54 mmol) and
lithium
12 hydroxide monohydrate (0.18 g, 4.27 mmol) was added to a solution of
(45,5R)-34(S)-2-
13 ((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaenyloxy)butanoyI)-4-methyl-
5-
14 phenyloxazolidin-2-one (1.10 g, 2.13 mmol) in tetrahydrofuran (12 mL)
and water (4 mL)
held at 0 C under nitrogen. The reaction mixture was stirred at 0 C for 30
minutes. 10%
16 Na2S03 (aq) (30 mL) was added, the pH was adjusted to -2 with 2M HCI and
the mixture
17 was extracted twice with heptane (30 mL). The combined organic extract
was dried
18 (Na2SO4), filtered and concentrated. The residue was subjected to flash
chromatography
19 on silica gel using increasingly polar mixtures of heptane and ethyl
acetate (98:8 -> 1:1) as
eluent. Concentration of the appropriate fractions afforded 0.48 g (60 %
yield) of the title
21 compound as an oil. 1H-NMR (300 MHz, CDCI3): 6 0.90-1.00 (m, 6H), 1.48
(m, 2H), 1.65
22 (m, 2H), 1.85 (m, 2H), 2.10(m, 4H), 2.80-2.90 (m, 8H), 3.55 (m, 1H),
3.60 (m, 1H), 3.88 (t,
23 1H), 5.35-5.45 (m, 10H); MS (electrospray): 373.3 [M-H]; Rik -37
(c=0.104, ethanol).
24 [061] Example 5: Preparation of (R)-2-((5Z,8Z,11Z,14Z,17Z)-icosa-
5,8,11,14,17-pentaenyloxy)butanoic acid:
26
27 [062] Hydrogen peroxide (35% in water, 0.65 mL, 7.37 mmol) and
lithium
28 hydroxide monohydrate (0.15 g, 3.69 mmol) was added to a solution of
(4S,5R)-3-((R)-2-
29 ((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaenyloxy)butanoyI)-4-methyl-
5-
14
23872079.1
CA 2 88 6957 2020-03-31
CA 2,886,957
Blakes Ref: 72571/00022
1 phenyloxazolidin-2-one (0.95 g, 1.84 mmol) in tetrahydrofuran (12 mL) and
water (4 mL)
2 held at 0 C under nitrogen. The reaction mixture was stirred at 0 C for
30 minutes. 10%
3 Na2S03 (aq) (30 mL) was added, the pH was adjusted to 2 with 2M HCI and
the mixture
4 was extracted twice with heptane (30 mL). The combined organic extract
was dried
(Na2SO4), filtered and concentrated. The residue was subjected to flash
chromatography on
6 silica gel using increasingly polar mixtures of heptane and ethyl acetate
(98:8 -> 50:50) as
7 eluent. Concentration of the appropriate fractions afforded 0.19 g (29%
yield) of the title
8 compound as an oil. 1H-NMR (300 MHz, CDC13); 50.90-1.00 (m, 6H), 1.48 (m,
2H), 1.65
9 (m, 2H), 1.85 (m, 2H), 2.10 (m, 4H), 2.80-2.90 (m, 8H), 3.55 (m, 1H),
3.60 (m, 1H), 3.88 (t,
1H), 5.35-5.45 (m, 10H); MS (electrospray): 373.3 EM-H]-; [a]D -31 (c=0.088,
ethanol).
11 [063] Example 6: Preparation of fert-butyl 2-((5Z,8Z,11Z,142,172)-icosa-
12 6,8,11,14,17-pentaenyloxy)propanoate:
13 0 I
14 [064] A mixture of (5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-ol,
(1.00 g,
3.47 mmol), tetrabutylammonium chloride (0.24 g, 0.87 mmol) and t-butyl 2-
bromo-
16 propionate (3.62 g, 17.3 mmol) was dissolved in toluene (36 mL) and
placed under
17 nitrogen. An aqueous solution of sodium hydroxide (50%, 8 mL) was added
slowly under
18 vigorous stirring and the resulting mixture was stirred at ambient
temperature for twenty
19 hours. Water was added and the mixture was extracted three times with
ether. The
combined organic extract was washed with brine, dried (Na2SO4), filtered and
21 concentrated. The residue was purified by flash chromatography on silica
gel using 2%
22 ethyl acetate in heptane as eluent. Concentration of the appropriate
fractions afforded
23 1.40 g (90% yield) of the title compound as an oil. 1H-NMR (300 MHz,
CDCI3): 50.95 (t,
24 3H), 1.41 (d, 3H), 1.48 (s, 9H), 1.48-1.66 (m, 4H), 2.05 (m, 4H), 2.83
(m, 8H), 3.35 (m,
1H), 3.55 (m, 1H), 3.79 (q, 1H), 5.32-5.44 (m, 10H).
26 [065] Example 7: Preparation of 2-((5Z,8Z,11Z,14Z,17Z)-icosa-
6,8,11,14,17-
27 pentaenyloxy)propanoic acid:
28 0
23872079.1
CA 2886957 2020-03-31
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 [066] Trifluoroacetic acid (2 mL) was added to a solution of 2-
2 ((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaenyloxy)propanoate (1.40 g,
3.36 mmol) in
3 dichloromethane (10 mL) held under nitrogen and the reaction mixture was
stirred at room
4 temperature for three hours. Diethyl ether (50 mL) was added and the
organic phase was
washed with water (30 mL), dried (Na2SO4) and concentrated. The residue was
subjected
6 to flash chromatography on silica gel using increasingly polar mixtures
of heptane, ethyl
7 acetate and formic acid (95:5:0.25 ->80:20:1) as eluent. Concentration of
the appropriate
8 fractions afforded 0.67 g of slightly impure product. This material was
dissolved in heptane
9 (15 mL), washed three times with water (5 mL). dried (Na2SO4), filtered
and concentrated
to afford 0.50 g (41% yield) of the title compound as an oil. 1H-NMR (300 MHz,
CDCI3):
11 0.99 (t, 3H), 1.40-1.48 (m, 5H), 1.67 (m, 2H), 2.09 (m, 4H), 2.80-2.60
(m, 8H), 3.53 (m,
12 2H), 4.01 (q, 1H), 5.31-5.47 (m, 10H); MS (electrospray): 359.2 [M-H].
13 [067] Example 8: Preparation of tert-butyl 2-((5Z,8Z,11Z,14Z,17Z)-icosa-
14 5,8,11,14,17-pentaenyloxy)-2-methylpropanoate:
-
16 [068] A mixture of (5Z,87,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-
ol, (0.83 g,
17 3.14 mmol), tetrabutylammonium chloride (0.24 g, 0.85 mmol) and t-butyl
2-bromo
18 isobutyrate (3.509, 15.7 mmol) was dissolved in toluene (15 mL) and
placed under
19 nitrogen. An aqueous solution of sodium hydroxide (50%, 5 mL) was added
slowly under
vigorous stirring at room temperature. The resulting mixture was heated to 60
C and stirred
21 for six hours. The mixture was cooled, added water and extracted three
times with ether.
22 The combined organic extract was washed with brine, dried (Na2SO4),
filtered and
23 concentrated. The residue was purified by flash chromatography on silica
gel using a
24 gradient of 5-10% ethyl acetate in heptane as eluent. Concentration of
the appropriate
fractions afforded 0.60 g (44% yield) of the title compound as an oil. MS
(electrospray):
26 453.3 [M+Na].
27 [069] Example 9: Preparation of 2-((5Z,BZ,11Z,14Z,17Z)-icosa-
5,8,11,14,17-
28 pentaenyloxy)-2-methylpropanoic acid:
Ar-011
- -
29
16
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 [070] Trifluoroacetic acid (5 mL) was added to a solution of tert-
butyl 2-
2 ((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaenyloxy)-2-methylpropanoate
(600 mg, 1.39
3 mmol) in dichloromethane (20 mL) under nitrogen and the reaction mixture
was stirred at
4 room temperature for two hours. Water was added and the aqueous phase was
extracted
twice with dichloromethane. The combined organic extract was washed with
brine, dried
6 (Na2SO4), filtered and concentrated. The residue was purified by flash
chromatography on
7 silica gel using a mixture of heptane, ethyl acetate and formic acid
(80:20:1) as eluent. The
8 appropriate fractions were concentrated and the residue (135 mg) was
purified further by
9 flash chromatography on silica gel using a gradient of 5-10% of a mixture
of ethyl acetate
and formic acid (95:5) in heptane as eluent. Concentration of the appropriate
fractions
11 afforded 80 mg slightly impure product. This material was dissolved in
heptane (5 mL),
12 washed twice with water (5 mL), dried (Na2SO4), filtered and
concentrated to afford 40 mg
13 (8% yield) of the title compound as an oil. 1H-NMR (300 MHz, 0DCI3): 5
0.99 (t, 3H), 1.47
14 (s, 6H), 1.64 (m, 2H), 2.07 (m, 4H), 2.81-2.88 (m, 8H), 3.46 (t, 2H),
5.29-5.44 (m, 10H); MS
(electrospray): 373.3 [M-H].
16 [071] Example 10: Preparation of tert-butyl 2-ethy1-2-
((5Z,8Z,11Z,14Z,17Z)-
17 icosa-5,8,11,14,17-pentaen-1-yloxy)butanoate:
18
19 [072] tert-Butyl 2-((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-
yloxy)butanoate (480 mg, 1.11 mmol) was added dropwise over 30 minutes to a
solution of
21 lithium diisopropylamine (LDA) (2.0 M, 750 L, 1.50 mmol) in dry
tetrahydrofuran (10 mL)
22 held at -70 C under nitrogen. The reaction mixture was stirred for 30
minutes. Ethyl iodide
23 (312 mg, 2.00 mmol) was added in one portion and the resulting mixture
was warmed to
24 ambient temperature during 1 hour. The reaction mixture was stirred at
ambient
temperature for 17 hours. The mixture was poured into saturated NH4CI (aq.)
(50 mL) and
26 extracted with heptane (2 x 50 mL). The combined organic phases was
washed
27 successively with brine (50 mL), 0.25 M HCI (50 mL) and brine (50 mL),
dried (MgSO4),
28 filtered and concentrated. The residue was purified by flash
chromatography on silica gel
29 using increasingly polar mixtures of heptane and ethyl acetate (100:0 ->
95:5) as eluent.
Concentration of the appropriate fractions afforded 343 mg (67% yield) of the
title
31 compound as an oil. 11-I NMR (300 MHz, CDCI3): 5 0.84 (t, 6H), 0.99 (td,
3H), 1.35-1.55 (m,
17
22713041.1
= CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 11 H), 1.54-1.69 (m, 2H), 1.68-1.87 (m, 4H), 1.99-2.24 (m, 4H), 2.74-2.99
(m, 8H), 3.31 (t,
2 2H), 5.23-5.52 (m, 10H); MS (electrospray): 401.3 [M-1]-.
3 [073] Example 11: Preparation of 2-ethy1-2-((5Z,8Z,11Z,14Z,17Z)-icosa-
4 5,8,11,14,17-pentaen-1-yloxy)butanoic acid:
_ 1
6 [074] A mixture of formic acid (5 ml) and tert-butyl 2-ethyl-2-
7 ((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yloxy)butanoate (250
mg, 0.55 mmol)
8 was stirred vigorously under nitrogen at room temperature for 4.5 hours.
The formic acid
9 was removed in vacuo. The residue was purified by flash chromatography on
silica gel
using increasingly polar mixtures of heptane and ethyl acetate (100:0 ->
80:20) as eluent.
11 Concentration of the appropriate fractions afforded 163 mg (74% yield)
of the title
12 compound as an oil. 1H NMR (300 MHz, CDCI3): 5 0.86 (t, 6H), 0.99 (t,
3H), 1.36-1.57 (m,
13 2H), 1.68 (dd, 2H), 1.73-1.98 (m, 4H), 2.11 (tt, 4H), 2.70-3.01 (m, 8H),
3.39 (t, 2H), 5.20-
14 5.56 (m, 10H). MS (electrospray): 481.4 [M+Na]*.
[075] Example 12: Preparation of ethyl 2-(((5Z,8Z,11Z,14Z,17Z)-icosa-
16 5,8,11,14,17-pentaen-1-yl)oxy)butanoate:
17 oio
18 [076] Dicyclohexylmethanediimine (DCC) (304 mg, 1.47 mmol) and N,N-
19 dimethylpyridin-4-amine (DMAP) (10 mg, 0.067 mmol) were added to a
stirred solution of 2-
(((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yl)oxy)butaroic acid (501.3
mg, 1.335
21 mrrol) in dichloromethane (DCM) (4 mL) at 0 C under N2-atmosphere. The
mixture was stirred
22 for 5 minutes, before ethanol (Et0H) (0.16 mL, 2.67 mmol) was added. The
resulting mixture
23 was stirred at room temperature for 20 hours. The reaction mixture was
purified by flash
24 chromatography on silica gel using increasingly mixtures of heptane and
ethyl acetate (100:0
99:1) as eluent. Concentration of the appropriate fractions afforded 473 mg
(88% yield) of the
26 title compound as an oil. 1H NMR (300 MHz, chloroform-d) 5 0.95 (2 x t,
6H), 1.37-1.48 (m, 2H),
27 1.54-1.79 (m, 4H), 2.01-2.10 (m, 4H), 2.77-2.84 (m, 8H), 3.27-3.34 (m,
1H), 3.53-3.60 (m, 1H),
28 3.69-3.73 (dd, 1H), 4.13-4.24 (m, 2H), 5.25-5.33 (m, 10H), MS
(electrospray): 425.3 [M+Na];
29 HRMS (electrospray): Found 425.3021 [M Na], calcd. for [C26H4203+Nalt
425.3031.
18
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 [077] Example 13: Preparation of isopropyl 2-(((5Z,8Z,11Z,14Z,17Z)-
icosa-
2 5,8,11,14,17-pentaen-1-yl)oxy)butanoate
3 o
4 [078] DOC (310 mg, 1.47 mmol) and DMAP (9 mg, 0.067 mmol) were added
to a
stirred solution of 2-(((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-
yl)oxy)butanoic acid
6 (501 mg, 1.335 mmol) in DCM (4 mL) at 0 C under N2-atmosphere. The
mixture was stirred for
7 5 minutes, before isopropanol (rPrOH) (0.16 mL, 2.67 mmol) was added. The
resulting mixture
8 was stirred at room temperature for 20 hours. The mixture was filtered
and concentrated in
9 vacuo. The residue was added heptane (50 mL), filtered and concentrated
in vacuo. The
residue was purified by flash chromatography on silica gel using 1 % ethyl
acetate in heptane as
11 eluent, Concentration of the appropriate fractions afforded 496 mg (89%
yield) of the title
12 compound as an oil. 1E1 NMR (300 MHz, CDCI3): 6 0.97(2 x t, 6H), 1.25
(m, 6H), 1.42-1.50 (m,
13 2H), 1.61-1.70 (m, 2H), 1.70-1.77 (m, 2H), 2.05-2.12 (m, 4H), 2.79-2.86
(m, 8H), 3.29-3.34 (m,
14 1H), 3.54-3.59 (m, 1H), 3.67-3.71 (m, 1H), 5.06-5.10 (m, 1H), 5.31-5.42
(m, 10 H); MS
(electrospray): 439.3 [M+Na].
16 [079] Example 14: Preparation of methyl 2-(((5Z,8Z,11Z,14Z,17Z)-icosa-
17 5,8,11,14,17-pentaen-1-yl)oxy)butanoate:
18
19 [080] Sulphuric acid (0.049 :711, 0.918 mmol) was added to a solution
of 2-
(((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yl)oxy)butanoic acid ((385
mg, 1,028
21 mmol) in methanol (20 ml) at room temperature under N2-athmosphere and
the resulting mixture
22 was stirred at room temperature overnight. MS (electrospray): 389.3
[M+1]+,
23 [081] Example 15: Preparation of butyl 2-(((5Z,8Z,11Z,14Z,17Z)-icosa-
24 5,8,11,14,17-pentaen-1-yl)oxy)butanoate:
26 [082] Sulphuric acid (0.049 ml, 0.918 mmol) was added to a solution
of 2-
27 (((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yl)oxy)butanoic acid
((33 g, 88 mmol) in
19
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 butan-1-ol (400 mL, 4.37 mol) at room temperature under N2-atmosphere and
the reaction
2 mixture was stirred for 120 hours. Heptane (400 mL) and ethyl acetate
(400 mL) was added,
3 and the solution was washed with saturated aq. NaHCO3 (3x300 mL) and
water (2x300 mL).
4 The combined aqueous phase was extracted with heptane/ether (1:1) (2x300
mL). The
combined organic phase was dried (Na2SO4), filtered and concentrated in vacua.
The residue
6 was purified by flash chromatography using increasingly mixtures of
heptane and ethyl acetate
7 (99:1 4 96:4) as eluent. Concentration of the appropriate fractions
afforded 26.3 g (67% yield)
8 of title compound as an oil. 1H NMR (400 MHz, CD0I3) 5 0.93-1.02 (m, 9H),
1.36-1.51 (m, 4H),
9 1.60-1.70 (m, 4H), 1.72-1,84 (m, 2H), 2.05-2.16 (m,4H), 2.78-2.92 (m,
8H), 3.28-3.39 (m, 1H),
3.54-3.65 (m, 1H), 3.70-3.82 (m, 1I-1), 4.08-4.24 (m, 2H), 5.27-5.48 (m, 10H),
MS (electrospray):
11 453.2 [M+Na].
12 [083] Example 16: Preparation of 2,3-dihydroxypropyl 2-
(((5Z,8Z,11Z,14Z,17Z)-
13 icosa-5,8,11,14,17-pentaen-1-yl)oxy)butanoate:
14 [084] Step a) Preparation (2,2-dimethy1-1,3-dioxolan-4-yl)methyl 2-
(((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yl)oxy)butanoate
oX/r i=/
16
17 [085] 2-(((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-
yl)oxy)butanoic acid (25
18 g, 66,7 mmol) and DMAP (8.15 g, 66.7 mmol) were added to a solution of
2,2-dimethy1-1,3-
19 dioxolane-4-methanol (7.54 mL, 60.7 mmol) in chloroform (150 mL) under
nitrogen atmosphere.
A solution of DCC (13.77 g, 66,7 mmol) in chloroform (65 mL) was then added
drop wise at
21 ambient temperature. The mixture was stirred overnight and concentrated
in vacua The
22 residue was purified by flash chromatography on silica gel using
increasingly polar mixtures of
23 10 % ethyl acetate in heptane as eluent. Concentration of the
appropriate fractions afforded
24 19.6 g (66% yield) of the title product as an oil. 1H NMR (300 MHz,
CDCI3) 6 0.99 (t, 6H), 1.37-
1.40 (m, 3H), 1.41-1.53 (m, 5H), 1.59-1.71 (m, 2H), 1.72-1.85 (m, 2H), 2.05-
2.14 (m, 4H), 2.74-
26 2.95 (m, 8H), 3.31-3.38 (m, 1H), 3.57-3.65(m, 1H), 372-3.86(m, 2H), 4.07-
4.12 (m, 1H), 4.15-
27 4.27 (m, 2H), 4.29-4.40 (m, 1H), 5.23-5.50 (m, 10H). MS (electrospray):
511.3 [M+Na].
28 [086] Step b) Preparation of 2,3-dihydroxypropyl 2-
(((5Z,8Z,11Z,14Z,17Z)-icosa-
29 5,8,11,14,17-pentaen-1-yl)oxy)butanoate
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
¨ ¨ 0
1 0
2 [087] To a solution of (2,2-dimethy1-1,3-dioxolan-4-yl)methyl 2-
3 (((5Z,8Z,117,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yl)oxy)butanoate (27.5
g, 56.3 mmol) in
4 dioxane (280 mL) at room temperature under nitrogen was added aq. HCI
(37% (w/w), 28 mL,
341 mmol) and the mixture was stirred for 60 minutes. The mixture was then
carefully poured
6 into sat. aq. NaHCO3 (500 mL) and extracted with Et0Ac (2x300 mL). The
organic phase was
7 washed with 1M HG] (200 mL), brine (200 mL), dried (Na2SO4), filtered and
concentrated in
8 vacuo. The residue was purified by flash chromatography on silica gel
using heptane and ethyl
9 acetate (50:50) as eluent. Concentration of the appropriate fractions
afforded 19 g of the title
product as an oil, contaminated with -10% of the isomer 1,3-dihydroxypropan-2-
y12-
11 (((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yl)oxy)butanoate. The
material was mixed
12 with 1.35 gram of another batch, before further purified by preparative
HPLC. An isocratic 17:83
13 mixture of water/acetonitrile (9:1) to acetonitrile (100%) was used as
eluent. Concentration of
14 the appropriate fractions afforded 11.3 g (38% yield) of the title
product as an oil. 'H NMR (300
MHz, CDCI3) 5 0.97-1.03 (m, 6H), 1.41-1.51 (m, 2H), 1.59-1.69 (m, 2H), 1.72-
1.87 (m, 2H),
16 2.05-2.14 (m, 5H), 2.56 (s, 1H), 2.73-2.94 (m, 8H), 3.33-3.40 (m, 1H),
3.55-3.68 (m, 2H), 3.69-
17 3.77 (m, 1H), 3.79-3.85 (m, 1H), 3.93-4.03 (m, 1H), 4.15-4.37 (m, 2H),
5.25-5.51 (m, 10H). MS
18 (electrospray): 471.3 [M+Na].
19 [088] Example 17: Preparation of 1,3-dihydroxypropan-2-yi 2-
(((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yl)oxy)butanoate
21 [089] Step a) Preparation of oxiran-2-ylmethyl 2-
(((5Z,8Z,11Z,14Z,17Z)-icosa-
22 5,8,11 ,14,17-pentaen-1-yl)oxy)butanoate
/0\
offo
23 0
24 [090] A mixture of 2-(((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-
1-
yl)oxy)butanoic acid (800 mg, 2.14 mmol), glycidol (0.17 mL, 2.56 mmol), 1-(3-
26 dimethylaminopropy1)-3-ethylcarbodiimide hydrochloride (EDC*HCI) (491
mg, 2.56 mmol) and 4-
27 dimethylaminopyridine (DMAP) (313 mg, 2.56 mmol) in dry DCM (7 mL) was
stirred at room
28 temperature under N2-atmosphere. The reaction mixture was concentrated
in vacua The
29 residue was purified by flash chromatography on silica gel using
increasingly polar mixtures of
21
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 heptane and ethyl acetate (99:1 --> 95:5) as eluent. Concentration of the
appropriate fractions
2 afforded 527 mg (57% yield) of the title product as an oil. 1H NMR (400
MHz, 000I3) 5 0.94-
3 0.98 (m, 6H), 1.40-1.44(m, 2H), 1.57-1.64(m, 2H), i.70-1.82(m, 2H), 2.02-
2.12 (m, 4H), 2.63
4 (bs, 1H), 2.78-2.84 (m, 9H), 3.20 (bs, 1H), 3.30-3.35 (m, 1H), 3.55-3.61
(m, 1H), 3.77-3.80 (m,
1H), 3.94-4.01 (m, 1H), 4.42-4.48 (m, 1H), 5.36-5.26 (m, 10H). MS
(electrospray): 453.3
6 [M+Na].
7 [091] Step b) Preparation of 2-((2-(((5Z,8Z,11Z,14Z,17Z)-icosa-
5,8,11,14,17-pentaen-
8 1-yl)oxy) butanoyl)oxy)propane-1 ,3-diy1 bis(2,2,2-trifluoroacetate)
9 OIC F3
[092] Trifluoroacetic anhydride (TFAA) (0.55 mL, 3.96 mmol) in dry DCM (3
mL) was
11 added portion wise to a precooled solution of oxiran-2-ylmethyl 2-
(((5Z,8Z,11Z,14Z,17Z)-icosa-
12 5,8,11,14,17-pentaen-1-yl)oxy)butanoate (286 mg, 0.66 mmol) in dry DCM
(3 mL) at -20 C
13 under N2-atmosphere. The cooling bath was removed and the mixture was
stirred for 19 hours
14 at ambient temperature, before reaction mixture was concentrated in
vacuo pressure. The
residue was dissolved in toluene (6 mL) and passed through a pad of silica
(6.5 g) eluting with
16 toluene (150 mL). Concentration in vacuo to afforded 357 mg (84% yield)
of the title compound
17 as an oil. 1H NMR (400 MHz, CDCI3) 50.95 (2xt, 6H), 1.38-1.45 (m, 2H),
1.57-1.63 (m, 2H),
18 1.66-1.78 (m, 2H), 2.09-2.02 (m, 4H), 2.78-2.84 (m, 8H), 3.27-3.33 (m,
1H), 3.51-3.56(m, 1H),
19 3.77 (dd, 1H), 4.30-4.53 (m, 2H), 4.60-4.69 (m, 2H), 5.17-5.43 (m, 10H),
5.43-5.55 (m, 1H). MS
(electrospray): 661.1 [M+Na].
21 [093] Step c) Preparation of 1,3-dihydroxypropan-2-y12-
(((5Z,8Z,11Z,14Z,17Z)-icosa-
22 5,8,11,14,17-pentaen-1-yl)oxy)butanoate
oco.,1))H
n
23 - OH
24 [094] A solution of pyridine (0.4 m1_, 4.95 mmol) and methanol (0.3
mL, 7.41 mmol) in
pentane/DCM (2:1) (4.5 mL) was added drop wise to a solution of 2-((2-
(((5Z,8Z,11Z,14Z,177)-
26 icosa-5,8,11,14,17-pentaen-1-yl)oxy)butanoyl)oxy)propane-1,3-diy1
bis(2,2,2-trifluoroacetate)
27 (354 mg, 0.552 mmol) in pentane/DCM (2:1) (5 mL) cooled to -20 C under
N2-atmosphere.
28 The cooling bath was removed and the mixture was stirred for 3 hours at
room temperature,
29 before concentrated in vacuo. The residue was purified by flash
chromatography on silica gel
22
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 using
increasingly polar mixtures of heptane and ethyl acetate (95:5 -> 90:10
80:20 -> 50:50)
2 as eluent. Concentration of the appropriate fractions afforded 223 mg of
the title product as
3 crude oil. Purification by preparative HPLC afforded 58 mg (22% yield) of
the title compound as
4 an oil. 1H NMR (400 MHz, CDCI3) O 0.95 (t, 3H), 0.96 (t, 3H), 1.38-1.45
(m, 2H), 1.54-1.64 (m,
2H), 1.67-1.84 (m, 2H), 2.01-2.09 (m, 4H), 2.45 (bs, 2H), 2.83-2.77 (m, 8H),
3.36-3.30 (m, 1H),
6 3.60-3.55 (m, 1H), 3.84-3.78 (m, 5H), 4.98-4.93 (m, 1H), 5.65-5.09 (m,
10H). MS (electrospray):
7 471.1 [M+Nar.
8 [095] Example 18: Preparation of 3-hydroxypropane-1,2-diy1 bis(2-
9 (((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yl)oxy)butanoate)
[096] Step a) Preparation of tert-butyl((2,2-dimethy1-1,3-dioxolan-4-
11 yl) methoxy)dimethylsi lane
0 0
12 /
\--OTBDMS
13 [097] fert-Butyl-chlorodimethylsilane (14.41 g, 91 mmol) was added to
a solution of
14 (2,2-dimethy1-1,3-dioxolan-4-yl)methanol (10 g, 76 mmol) and imidazole
(7.73g, 114 mmol) in
THF (100 mL) at ambient temperature under nitrogen atmosphere. The mixture was
stirred for
16 1.5 hours, poured into water (200 mL) and extracted with tert-butyl
methyl ether (2x150 mL).
17 The phases were separated and the organic layer was washed with water
(100 mL), brine (100
18 mL), dried (Na2SO4), filtered and concentrated in vacuo. The residue was
purified by flash
19 chromatography on silica gel using 3% ethyl acetate in heptane as
eluent. Concentration of the
appropriate fractions afforded 18 g (97% yield) of the title compound as an
oil. 1H NMR (300
21 MHz, CDCI3) 6 0.02-0.05 (m, 6H), 0.85-0.89 (m, 9H), 1.31-1.35 (m, 3H),
1.35-1.40 (m, 3H),
22 3.50-3.60 (m, 1H), 3.63-3.72 (m, 1H), 3.75-3.85 (m, 1H), 3.96-4.05 (m,
1H), 4.07-4.18 (m, 1H).
23 MS (electrospray): 229.2 [M+Na].
24 [098] Step b) Preparation of 3-((tert-butyldimethylsilyl)oxy)propane-
1,2-diol
HO OH
OTBDMS
26 [099] To a solution of tert-butyl((2,2-dimethy1-1,3-dioxolan-4-
yl)methoxy)-
27 dimethylsilane in chloroform (60 mL) was added FeCI3x6H20 absorbed on
silica gel (30 g, 11.9
28 mmol) and the mixture was stirred overnight. The mixture was filtered
and concentrated in
29 vacuo. The residue was purified by flash chromatography on silica gel
using increasingly polar
23
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 mixtures of heptane and ethyl acetate (50:50 ¨> 25:75) as eluent.
Concentration of the
2 appropriate fractions afforded 760 mg (9% yield) of the title compound as
an oil. 1H NMR (300
3 MHz, CDCI3) 5 0.09-0.12 (m, 6H). 0.91- 0.95 (m, 9H), 2.11-2.17 (m, 1H),
2.60 (d, 1H), 3.57-
4 3.85 (m, 5H). MS (electrospray): 229.2 [M+Na].
[0100] Step c) Preparation of 3-((tert-butyldimethylsilyl)oxy)propane-1,2-diy1
bis(2-
6 (((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yl)oxy)butanoate)
_
oOTBDMS
7
8 [0101] To a solution of 3-((tert-butyldimethylsilyl)oxy)propane-1,2-diol
(0.91 g, 4.41
9 mmol) in DMF (20 ml) under N2-atmosphere at ambient temperature were
added 2-
(((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yl)oxy)butanoic acid (3.47
g, 9.3 mmol),
11 DMAP (1.13 g, 9.3 mmol), 1-(3-dimethylaminopropy1)-3-ethylcarbodiimide
hydrochloride (DCI)
12 (1.776 g, 9.26 mmol) and dry DCM (60 m1). The mixture was stirred
overnight, before the
13 reaction mixture was diluted with diethyl ether (200 mL). The mixture
was washed with 1M HCI
14 (100 mL) and brine (100 mL), dried (Na2SO4), filtered and concentrated
in vacuo. The residue
was purified by flash chromatography on silica gel using 3% ethyl acetate in
heptane as eluent.
16 Concentration of the appropriate fractions afforded 2.26 g (56% yield)
of the title compound as
17 an oil. 1H NMR (300 MHz, CDCI3) 6 0.08 (s, 6H), 0.90 (d, 9H), 0.95-1.03
(m, 12H), 1.40-1.52
18 (m, 4H), 1.58-1.69(m, 4H), 1.70-1.83(m, 4H), 2.04-2.15(m, 8H), 2.77-2.92
(m, 16H), 3.27-3.37
19 (m, 2H), 3.57-3.67 (m, 2H), 3.72-3.80 (in, 4H), 4.14-4.32 (m, 1H), 4.41-
4.56 (m, 1H), 5.09-5.22
(m, 1H), 5.23-5.49 (m, 20H). MS (electrospray): 941.6 [M+Na].
21 [0102] Step d) Preparation of 3-hydroxypropane-1.2-diy1 bis(2-
(((5Z,8Z,11Z,14Z,17Z)-
22 icosa-5,8,11,14,17-pentaen-1-yl)oxy)butanoate)
o o
23
24 [0103] To a solution of 3-((tert-butyldimethylsilypoxy)propane-1,2-diy1
bis(2-
(((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yl)oxy)butanoate) (2.26 g,
2.46 mmol) in
26 dioxane (100 mL) was added aq. HC1 (37% (w/w, 2 mL) and the mixture was
stirred for 3 hours
27 under nitrogen atmosphere at ambient temperature, before concentrated in
vacuo. The residue
24
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 was purified by flash chromatography on silica gel using 15 `)/0 ethyl
acetate in heptane as
2 eluent. Concentration of the appropriate fractions afforded 0.83 g (42%
yield) of the title
3 compound as an oil. 1H NMR (300 MHz, 00CI3) 5 0.96-1.03 (m, 12H), 1.40-
1.53 (m, 4H), 1.58-
4 1.68(m, 4H), 1.70-1.85 (m, 4H), 1.87-2.01 (m, 1H), 2.05-2.15(m, 8H), 2.75-
2.95 (m, 16H), 3.28-
3.41 (m, 2H), 3.56-3.65(m, 2H), 3.73-3.85(m, 4H), 4.24-4.37(m, 1H), 4.42-
4.53(m, 1H), 5.14-
6 5.23 (m. 1H), 5.26-5.51 (m, 20H). MS (electrospray); 827.5 [M+Na].
7 [0104] Example 19: Preparation of 2-hydroxypropane-1,3-diy1 bis(2-
8 (((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yl)oxy)butanoate):
9 [0105] Step a) 2-oxopropane-1,3-diy1 bis(2-(((5Z,8Z,11Z,14Z,17Z)-icosa-
5,8,11,14.17-
pentaen-1-yl)oxy)butanoate)
0
11 0 0
12 [0106] 2-(((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-
yl)oxy)butanoic acid
13 (5.0 g, 13.4 mmol) and DMAP (1,63 g, 13.4 mmol) were added to a solution
of 1,3-
14 dihydroxyacetone dimer (1.1459, 6.36 mmol) in chloroform (25 mL) under
nitrogen atmosphere.
A solution of DCC (2.75 g, 13.35 mmol) in chloroform (10 mL) was then added
drop wise at
16 ambient temperature. The mixture was stirred overnight at room
temperature, before
17 concentrated in vacuo. The residue was purified by flash chromatography
on silica gel using
18 increasingly polar mixtures of
heptane and ethyl acetate (90:10 88:12) as eluent.
19 Concentration of the appropriate fractions afforded 2.4 g (47% yield) of
the title compound as an
oil. 'H NMR (300 MHz, CD0I3) 50.97-1.06 (m, 12H). 1.38-1.53 (m, 4H), 1.57-1.73
(m, 4H),
21 1.73-1.96 (m, 4H), 2.03-2.17 (m, 8H), 2.76-2.92 (m, 16H), 3.35-3.42 (m,
2H), 3.63-3.70 (m, 2H),
22 3.89 (dd, 2H), 4.75-4.93 (m, 4H), 5.27-5.49 (m, 20H). MS (electrospray):
827.5 [M-FNar.
23 [0107] Step b) 2-hydroxypropane-1,3-diy1 bis(2-(((5Z,8Z,11Z,14Z,17Z)-
icosa-
24 5,8,11,14,17-pentaen-1-yl)oxy)butanoate)
ecOH
.r y0
26 [0108] NaBH4 (0.336 g, 8.87 mmol) was added carefully to a solution of 2-
oxopropane-
27 1,3-diy1 bis(2-(((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1 -
yl)oxy)butanoate) (3.24 g,
28 4.03 mmol) in THF (55 mL) and water (4 mL) at 0 C. The mixture was
stirred for 15 minutes at
29 0 CC. Acetic acid (1 mL) was then added carefully followed by ethyl
acetate (100 mL). The
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 mixture was washed with water (100 mL), saturated aq. NaHCO3 (100 mL) and
brine, before
2 dried (Na2SO4), filtered and concentrated in vacua The residue was
combined with another
3 batch of the material before purified by flash chromatography on silica
gel using 15% ethyl
4 acetate in heptane as eluent. Concentration of the appropriate fractions
afforded 1.62 g (50%
yield) of the title compound as an oil. 1H NMR (300 MHz, CDCI3) 5 0.97-1.03
(m, 12H), 1.41-
6 1.52 (m, 4H), 1.58-1.69 (m, 6H), 1.71-1.87 (m, 4H), 2.05-2.14 (m, 8H),
2.38-2.42 (m, 1H). 2.78-
7 2.92 (m, 16H), 3.32-3.39 (m, 2H), 3.57-3.64 (m, 2H), 3.80-3.84 (m, 2H),
4.05-4.34 (m, 5H), 5.26-
8 5.49 (m, 20H). MS (electrospray): 827.5 [M+Na].
9 [0109] Example 20: Preparation of propane-1,2,3-triy1 tris(2-
(((5Z,8Z,11Z,14Z,17Z)-
icosa-5,8,11,14,17-pentaen-1-yl)oxy)hutanoate)
0 o
¨
11
12 [0110] 2-(((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-
yl)oxy)butanoic acid
13 (4.0 g, 10.7 mmol), 4-dimethylaminopyridine (1.305 g, 10.7 mmol), 1-(3-
dimethylaminopropyI)-3-
14 ethylcarbodiimide hydrochloride (2.047 g, 10.7 mmol) and dry DCM (30 ml)
was added to a
solution of glycerol (0,173 ml, 2,373 mmol) in DMF (10 ml) under N2-atmosphere
at room
16 temperature. The mixture was stirred overnight, before the reaction
mixture was diluted with
17 diethyl ether (250 mL). The mixture was washed with aq. 1M HCI (100 mL)
and brine (100 mL),
18 before dried (Na2SO4), filtered and evaporated in vacuo. The residue was
purified by flash
19 chromatography on silica gel using 5% ethyl acetate in heptane as
eluent. Concentration of the
appropriate fractions afforded 2.1 g (73 % yield) of the title compound as an
oil. 1H NMR (300
21 MHz, CDCI3) 0.91-1.05 (m, 18H), 1.40-1.52 (m, 6H), 1.57-1.69 (m, 6H),
1.69-1.86 (m, 6H),
22 2.01-2.17 (m, 12H), 2.69-2.96 (m, 24H), 3.27-3.38 (m, 3H), 3.53-3.67 (m,
3H), 3.73-3.81 (m,
23 3H), 4.17-4.27 (m, 2H), 4.37-4.54 (m, 2H), 5.28-5.47 (m, 30H). MS
(electrospray): 1183.8
24 [M+Na].
[0111] Example 21: Preparation of calcium 2-(((5Z,8Z,11Z,14Z,17Z)-icosa-
26 5,8,11,14,17-pentaen-1-yl)oxy)butanoate
26
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
¨ ¨
cw-
- ¨ ¨ ¨ ¨
1
2 [0112] 2-(((5Z,8Z,11Z,14Z,17Z)-lcosa-5,8,11,14,17-pentaen-1-
yl)oxy)butanoic acid
3 (1.87 g, 4.99 mmol, 93%) was mixed with CaCO3 (0.25 g, 2.50 mmol). Water
(1 ml) was added
4 and the mixture was stirred with mechanical stirring at RT for 1 hour.
CO2 develops. Dense and
homogeneous pasta was formed. With stirring, acetone (7 ml) was added. A solid
materiel
6 separates. The solid materiel was filtered of and dried over nitrogen
sealed and stored in the
7 fridge at 4 'C. Yield: 1.86 grams (95 %). The solid was not further
characterized by analytical or
8 spectroscopic methods, but a few experiments indicating that the calcium
salt has formed was
9 performed:
= The solid materiel melts on a hot plate below 100 C. No sharp melting point
was
11 determined
12 = The material do not liberate CO2 on addition of acid, but
"dissolves" and
13 precipitates as an oil
14 [0113] Example 22: Preparation of sodium 2-(((5Z,8Z,11Z,14Z,17Z)-icosa-
5,8,11,14,17-pentaen-1-yl)oxy)butanoate
16 [0114] 2-(((5Z,8Z,11Z,14Z,17Z)-lcosa-5,8,11 ,14,17-pentaen-1-
yl)oxy)butanoic acid
17 (1.87g. 4.99 mmol, 93%) was mixed with NaHCO3 (0.420 g, 5.00 mmol).
Water (1 ml) was
18 added and the mixture was stirred with mechanical stirring at RT for 1
hour. CO2 develops, and
19 a thick homogeneous pasta was formed. With stirring, ethanol (7 ml) was
added to the reaction
flask. The sodium salt formed from 2-(((5Z,8Z,11Z,14Z,17Z)-lcosa-5,8,11,14,17-
pentaen-1-
21 yl)oxy)butanoic acid goes into solution upon addition of ethanol (7 m1).
Small amounts of
22 unreacted NaHCO3 was filtered of and the solution was evaporated to
dryness. The crude
23 slightly viscous oil was evaporated two times with 96 % ethanol to
remove traces of water.
24 [0115] Example 23: Preparation of 2-hydroxy-N,N,N-trimethylethan-1-
aminium 2-
(((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yl)oxy)butanoate
26 [0116] Choline hydroxide (327.7 pL) in water was pipetted into a
scintillation vial with
27 ca. 2.5mL MTBE and 7.5 mL of n-Heptane. Within a nitrogen chamber, 2-
28 (((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-yl)oxy)butanoic acid
(500 mg, 95.8%) was
29 transferred into the vial. Within a nitrogen chamber ca.1.0 mL of water
was added to the vial
27
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 slowly and under stirring. The vial was then sealed. The reaction mixture
was stirred for ca. 30
2 minutes. The formed 2-(((5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaen-1-
yl)oxy)butanoic
3 acid choline salt was a rigid, gel-like material which was filtered on a
Buchner funnel. The wet
4 material on the filter was washed 3 times using 1mL of MTBE. The washed
material appeared
as a rigid gel-like solid.
6 [0117] Example 24 Pre-clinical Study
7 [0118] Evaluation of apoC-Ill regulation in a dyslipidennic mouse model
8 (APOE*3Leiden transgenic mice)
9 [0119] The APOE*3Leiden transgenic mouse is expressing a variant of the
human
apolipoprotein E3 (APOE3), the APOE*3Leiden, in addition to the human
apolipoprotein Cl
11 (APOC1). The APOE*3Leiden transgenic mice exhibit elevated plasma
cholesterol and
12 triglyceride levels, mainly confined to the VLDL/LDL sized lipoprotein
fraction (Van den
13 Maagdenberg AMJM et al, Transgenic mice carrying the apolipoprotein E3-
Leiden gene exhibit
14 hyperlipoproteinemia, J Biol Chem 1993; 268:10540-10545). In contrast to
normal wild-type
mice, the APOE*3Leiden transgenic mice are highly responsive to diet and
hypolipidemic drugs
16 affecting plasma VLDL and chylomicron levels (Van Vlijmen B et al, Diet-
induced
17 hyperlipoproteinemia and atherosclerosis in apolipoprotein E3-Leiden
transgenic mice, J Clin
18 Invest 1994; 93:1403-1410; Groot PHE, at al, Quantitative assessment of
aortic atherosclerosis
19 in apoE3Leiden transgenic mice and its relationship to serum cholesterol
exposure, Arterioscler
Thromb Vasc Biol 1996; 16: 926-933). Consequently, this model is appropriate
to evaluate
21 effects of lipid lowering drugs.
22 [0120] In this study, female APOE*3Leiden transgenic mice were put on a
semi-
23 synthetic Western-type diet (WTD; 15% cocoa butter, 40% sucrose and
0.25% cholesterol; all
24 wiw). After 4 weeks with this diet the plasma cholesterol level reached
mildly elevated levels of
about 12-15 mmo1/1. The mice were then sub-divided into groups of 10 mice
each, matched for
26 plasma cholesterol, triglycerides and body weight (t=0). The test
substances were tested at 0.3
27 mmol/kg bw/day and were administered orally as admix to the WTD. After 4
weeks, all animals
28 were sacrificed and serum and tissues were collected.
29 [0121] Liver tissues were stored in RNA later (Qiagen) at -80 C. Tissue
was
homogenized in RLT buffer with dithiothreitol (Qiagen) and RNA was isolated
using the RNeasy
31 kit (Qiagen), following the manufacturer's procedure. The quality of the
isolated RNA was tested
32 on a Bioanalyser (Agilent) showing RIN (RNA integrity number) values
between 8.1 and 9.5
33 which indicates good quality. cDNA was synthesized by the "RNA to cDNA"
kit (Applied
28
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 Biosystems). Gene expression was measure using Low Density Arrays (LDA,
specific for
2 mouse RNA (Applied Biosystems)). Each sample was measured in 3 parallels,
and the results
3 are presented as the mean value relative to control (WTD without
addition). The fold change in
4 gene expression was calculated by the AACI method, using Rp1p0 as
housekeeping gene and
the mean of the control samples as calibrator.
6 [0122] The results shown in Figure 1 establish that mice fed Compound A
(Example 2)
7 have significantly lower apoC-III expression than mice fed a standard WTD
(P< 0.05, Student T-
8 test). The effect of Compound A is more potent than the effect of
reference Compound 12, an
9 EPA derivative prepared according to Example 20 of W02010/008299 having
the following
structure:
¨ sQ(OH
0
11
12 Reference Compound 12
13 [0123] In addition, the ability of both compounds to reduce plasma TG
was measured.
14 Both compounds reduced TG levels with 69% compared to control. This
confirms that there is
no direct correlation between the observed apoC-III reduction and TG lowering-
effect.
16 [0124] Example 25 Clinical Studies
17 [0125] The apoC-III reducing properties of Compound A have been
demonstrated in two
18 12-week studies and one 4-week study in patients with dyslipidemia. All
three studies
19 demonstrated clinically and statistically significant reductions in apoC-
III with Compound A
treatment.
21 [0126] Example 25A Population having sever hypertriglyceridemia
22 [0127] This study investigated patients with fasting plasma triglyceride
levels above
23 500 mg/dL. The primary objective of this study was to evaluate the
efficacy of Compound A
24 (Example 2) 600 mg once daily (QD) orally by assessment of the
percentage change in
triglycerides (TG) from baseline after 12 weeks of treatment. One of the
secondary objectives
26 was to evaluate the impact of Compound A on plasma levels of apoC-Ill.
27 [0128] This Phase II, multicenter, proof of concept study consisted of a
6-to 8-week
28 screening period (which included a 4- or 6-week diet and lifestyle
stabilization/washout period
29 and a 2-week TG qualifying period), and a 12-week, double-blind,
randomized, parallel group,
placebo-controlled treatment period.
29
22713041,1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 [0129] After confirmation of qualifying fasting TG values, eligible
subjects entered the
2 12-week, randomized, double-blind treatment period. At Visit 4 (Week 0),
subjects were
3 randomly assigned in a 1:1 ratio to 1 of the following treatment groups:
Compound A 600 mg
4 QD or placebo QD.
[0130] Approximately 43 subjects per treatment group (approximately 86
subjects
6 total) were to be randomized in this study. Stratification was by
baseline TG level (700 mg/dL
7 or >700 mg/dL), statin use at randomization, and gender.
8 [0131] The population for this study was men and women (women of
childbearing
9 potential were required to use adequate methods to avoid pregnancy)
between the ages of 18
to 79 years of age, inclusive. Subjects on stable lipid-lowering statin
therapy and subjects not on
11 non-statin lipid-lowering therapy were eligible to enroll in the study.
Subjects were required to
12 have an average fasting TG level .500 mg/dL and _1500 mg/dL from Visit 2
and Visit 3 values
13 or Visit 3 and Visit 3.1 values prior to randomization.
14 [0132] The Intent-to-Treat (ITT) Population consisted of all randomized
subjects who
took at least 1 dose of investigational product, had a baseline efficacy
measurement, and had at
16 least 1 post-randomization efficacy measurement. The ITT Population was
the primary analysis
17 population. All efficacy analyses were performed on the ITT Population.
18 [0133] Summary statistics (n, mean, standard deviation [SD], median,
minimum, and
19 maximum) for the baseline and post-baseline measurements, the percent
changes, or changes
from baseline were presented by treatment group and by visit for all efficacy
variables analyzed.
21 [0134] The primary efficacy analysis was performed using an analysis of
covariance
22 (ANCOVA) model with treatment, gender, and the use of statin therapy at
randomization as
23 factors and baseline TG value as a covariate. The least-squares means,
standard errors, and 2-
24 tailed 95% confidence intervals (Cis) for each treatment group and for
the comparison between
Compound A and placebo were provided.
26 [0135] An ANCOVA model was used for the analysis of secondary efficacy
variables
27 with treatment, gender, and the use of statin therapy at randomization
as factors and the
28 baseline value of the respective efficacy variable as a covariate.
29 [0136] The population recruited for the current study included men
(69.0%) and
women (31.0%) with a mean age of 52.5 years. Approximately 21% of subjects in
both
31 treatment groups received statin therapy through the study. All other
non-statin lipid-altering
32 medications were discontinued at screening. Mean compliance to study
medication during the
33 study was 96.5% for the placebo group and 99.9% for the Compound A 600
mg group.
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 [0137] In the ITT Population, the least-squares (LS) mean percent change
in apoC-III
2 was -38.0 % (-47.5, -28.5) vs baseline and -34.7% (-46.5, -22.8) versus
placebo.
3 [0138] Example 25B Population having mixed dyslipidemia
4 [0139] This study investigated patients with fasting plasma TO levels
between 200 and
499 mg/dL and non-high density lipoprotein cholesterol (non-HDL-C) above 130
mg/dL already
6 receiving treatment with statins. The primary objective of this study was
to evaluate the efficacy
7 of Compound A (Example 2) 600 mg QD orally by assessment of the
percentage change in
8 triglycerides non-HDL-C from baseline after 12 weeks of treatment. One of
the secondary
9 objectives was to evaluate the impact of Compound A on plasma levels of
apoC-Ill.
[0140] This Phase II, multicenter, proof of concept study consisted of a 6-to
8-week
11 screening period (which included a 4- or 6-week diet and lifestyle
stabilization/washout period
12 and a 2-week TG and non-HDL-C qualifying period), and a 12-week, double-
blind, randomized,
13 parallel group, placebo-controlled treatment period.
14 [0141] After confirmation of qualifying fasting TO and non-HDL-C values,
eligible
subjects entered the 12-week, randomized, double-blind treatment period. At
Visit 4 (Week 0),
16 subjects were randomly assigned in a 1:1 ratio to 1 of the following
treatment groups:
17 Compound A 600 mg QD or placebo QD.
18 [0142] The population for this study was men and women (women of
childbearing
19 potential were required to use adequate methods to avoid pregnancy)
between the ages of 18
to 79 years of age, inclusive. Subjects on stable lipid-lowering statin
therapy and subjects not on
21 non-statin lipid-lowering therapy were eligible to enroll in the study.
Subjects were required to
22 have an average fasting TG level between 200 and 499 mg/dL and non-HDL-C
values above
23 130 mg/dL from Visit 2 and Visit 3 values or Visit 3 and Visit 3.1
values prior to randomization.
24 [0143] The Intent-to-Treat (ITT) Population consisted of all randomized
subjects who
took at least 1 dose of investigational product, had a baseline efficacy
measurement, and had at
26 least 1 post-randomization efficacy measurement. The ITT Population was
the primary analysis
27 population. All efficacy analyses were performed on the ITT Population.
28 [0144] Summary statistics (n, mean, standard deviation [SD], median,
minimum, and
29 maximum) for the baseline and post-baseline measurements, the percent
changes, or changes
from baseline were presented by treatment group and by visit for all efficacy
variables analyzed.
31 [0145] The primary efficacy analysis was performed using an ANCOVA model
with
32 randomization as factor and baseline non-HDL-C value as a covariate. The
least-squares
31
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 means, standard errors, and 2-tailed 95% Cls for each treatment group and
for the comparison
2 between Compound A and placebo were provided.
3 [0146] The primary efficacy analysis was based on the 12-week completer
population.
4 [0147] The population recruited for the current study included men
(58.4%) and
women (46.1%) with a mean age of 58.3 years. All subjects were required to be
on statin
6 therapy (with or without ezetimibe) during the study. All other non-
statin lipid-altering
7 medications were discontinued at screening. Mean compliance to study
medication during the
8 study was 97.2% for the placebo group and 95.3% for the Compound A group.
9 [0148] The baseline mean non-HDL-C level for the study population was
165.9 mg/cIL;
the baseline median TG level was 262.0 mg/dL.
11 [0149] In the 12-week completer population, the LS mean percent change
in ApoC-III
12 was -32.5 % (-38.4, -26.6) vs baseline and - 20.8 cYc, (-28.8, -12.7) vs
placebo.
13 [0150] Example 25A refers to studies in patients with very high
triglycerides (TG 500-
14 2000 mg/di). Example 25B refers to studies in statin stable patients
with mixed dyslipidemia and
persistent hypertriglyceridernia (TG 200-499 mg/di). The studies included in
each section are
16 similar in design, with comparable patient populations.
17 [0151] Example 25C Population having hypercholesterolemia
18 [0152] This study investigated subjects with fasting LDL-C of at least
2.5 mmol (-97
19 mg/di). The objective of the study was to determine the pharmacodynamics
and lipid lowering
effects of Compound A (Example 2) following 4 weeks of treatment in male,
21 hypercholesterolemic subjects withdrawn from stable statin therapy.
22 [0153] The population for this study consisted of men between 18 and 65
years of any
23 ethnic origin and with a BM' between 18.0 and 35.0 kg/m2.
24 [0154] This Phase lb study consisted of a 4-5 week screening period, and
a 4 week
double-blind, randomized, placebo-controlled treatment period.
26 [0155] All subjects had to be on lipid-lowering statin therapy for at
least 3 months prior
27 to the first screening visit, and at stable statin dose for at least 4
weeks prior to the first
28 screening vist.
29 [0156] Statin treatment was withdrawn at the first screening visit, and
remained
withdrawn for the entire screening period. Following withdrawal of statin
medication for at least
31 21 days subject had to have an LDL-C of at least 2.5 mmo1/1 (-97 mmo1/1)
at the secondary
32 screening visit and an increase in LDL-C of at least 20% between the
first screening visit and
33 the secondary screening visit prior to randomization.
32
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 [0157] After confirmation of qualifying fasting LDL-C, eligible subjects
entered a 4-
2 week double blind, randomized, placebo-controlled treatment period.
Subjects were randomly
3 assigned in a 3:1 ratio to one of the following treatment groups:
Compound A 600 mg OD
4 (N=18) or placebo QD (N=6).
[0158] Blood lipids were measured at the end of the screening period and after
4
6 weeks of treatment. Exploratory pharmacodynarnic measurements included
LDL-C, VLDL-C,
7 TC, TG, HDL-C, Non-HDL-C, and Apo B. The impact of Compound A on Apo C-
III was also
8 measured.
9 [0159] Summary statistics for baseline is given as mean with coefficient
of variance.
The mean changes from baseline with 95% confidence intervals were presented by
treatment
11 group for efficacy variables analyzed.
12 [0160] Analyses were performed using analysis of covariance (ANCOVA)
model on
13 changes from baseline with baseline included as covariate
14 [0161] The population recruited for the current study included white
males (100%) with
a mean age of 55 years, mean weight of 85 kg, and mean BMI of 27.9 kg/m2.
16 [0162] The mean percent change in Apo C-III after treatment with
Compound A was -
17 42% vs baseline. This change was statistically significant.
18 [0163] Example 26 Comparative reductions in apoC-111 achieved by EPA/DHA
19 versus Compound A
[0164] (a) Effects of EPA/DHA formulations versus Compound A on plasma apoC-
III
21 and other lipid parameters in sublects with severe HTG
22 [0165] The MARINE trial:
23 [0166] In a double blind, randomized, placebo controlled study the
effect of
24 eicosapentaenoic acid ethyl ester (>96% by weight of the concentrate)
(Vascepa) on apoC-III
was investigated in 229 patients with fasting plasma TG of 500-2000 mg/d1.
Vascepa 4 g/day for
26 12 weeks reduced median apoC-III levels from 25.6 mg/dl to 19.7 mg/di,
corresponding tea
27 median change from baseline of -10.1% [Journal of Clinical Lipidology
2014;8(3): 313-314,
28 lcosapent Ethyl (eicosapentaenoic acid ethyl ester): Effects on
Apolipoprotein C-III in patients
29 from the MARINE and ANCHOR studies.] (Table 1).
[0167] The EVOLVE trial:
31 [0168] In a double blind, randomized, placebo controlled study the
effect of a
32 combination of EPA and DHA as free fatty acids (55% by weight of EPA and
20 cY. by weight of
33 DHA) (Epanova) on apoC-III was investigated in 399 patients with fasting
plasma TG of 500-
33
22713041.1
CA 02886957 2015-04-01
CA Application
Bakes Ref: 72571/00022
=
1 2000 mg/d1. Epanova 4 g/day for 12 weeks resulted in a median apoC-III
change from baseline
2 of -15% [Circulation 2012;126: A19030, Abstract 19030: Apolipoprotein C-
III is Significantly
3 Reduced by Prescription Omega-3 Free Fatty Acids (Epanova) in Patients
with Severe
4 Hypertriglyceridemia and Changes Correlate with Increases in LDL-C: A Sub-
analysis of the
EVOLVE trial] (Table 1).
6
7 Table 1. Effect of treatment with omega-3 prescription pharmaceuticals
and Compound A in
8 subjects with TG > 500 mg/di. Values are median % changes from baseline.
Non-
TG ApoC-III VLDL-C LDL-C HDL-C
HDL-C
Compound A -51 -41 -8 -51 43 24
Vascepa (omega-3) -26.6 -10.1 -7.7 -25.2 -4.5 -3.5
Epanova (omega-3) -30.9 -15.0 -9.6 -33.0 19.4 5.8
9
[0169] (b) Effects of EPA/DHA formulations versus Compound A on plasma ApoC-
III
11 and other lipid parameters in statin stable subjects with mixed
dyslipidemia and persistent
12 hypertriolyceridemia
13 [0170] The ANCHOR trial:
14 [0171] In a double blind, randomized, placebo controlled study the
effect of
eicosapentaenoic acid ethyl ester (Vascepa) on apoC-III was investigated in
702 statin stable
16 patients with mixed dyslipidemia and persistent hypertriglyceridemia
with fasting plasma TG of
17 200-499 mg/c11. Vascepa 4 g/day for 12 weeks reduced median apoC-III
levels from 15.2 mg/di
18 to 13.7 mg/d1, corresponding to a median change from baseline of -9.4%
[Journal of Clinical
19 Lipidology 2015, in press, http://dx.doi.orq/10.1016/i.jacl.2014.11.009,
Effects of icosapent ethyl
on lipoprotein particle concentration and size in statin-treated patients with
persistent high
21 triglycerides (the ANCHOR study)] (Table 2).
22 [0172] The ESPRIT trial:
23 [0173] In a double blind, randomized, placebo controlled study the
effect of a
24 combination of EPA and DHA as free fatty acids (Epanova) on apoC-III was
investigated in 647
statin stable patients with mixed dyslipidemia and persistent
hypertriglyceridemia with fasting
26 plasma TG of 200-499 mg/d1. Epanova 4 g/day for 12 weeks resulted in a
mean apcC-111
27 change from baseline of -13.1% [JACC 2013;61: E1468, A highly
bioavailable omega-3 fatty
34
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 acid reduces non-high density lipoprotein cholesterol in high-risk
patients treated with a statin
2 and residual hypertriglyceridemia (ESPRIT trial)] (Table 2).
3 [0174] The COMBOS trial:
4 [0175] In a double blind randomized study the effect of a combination of
EPA and DHA
ethyl esters (46.5 % by weight of EPA EE and 37.5 % by weight of DHA EE)
(Lovaza) on apoC-
6 III was investigated in 256 statin stable patients with mixed
dyslipidemia and persistent
7 hypertriglyceridemia with fasting plasma TG of 200-400 mg/d1. Lovaza 4
g/day for weeks
8 resulted in a median apoC-III change from baseline of -7.8% [Clinical
Therapeutics 2007;29(7):
9 1354-1367, Efficacy and tolerability of adding prescription Omega-3 fatty
acids 4 g/cIto
simvastatin 40 mg/d in hypertriglyceridemic patients: An 8-week, randomized,
double-blind,
11 placebo-controlled study] (Table 2).
12
13 Table 2. Effect of treatment with omega-3 prescription pharmaceuticals
and Compound A in
14 subjects with mixed dyslipidemia with persistent hypertriglyceridemia
(TG = 200-499 mg/di).
Values are median % changes from baseline *.
Non-
TG ApoC-Ill Apo B VLDL-C LDL-C
HDL-C
Compound A -43 -35 -10 -6 -39 0
Vascepa (omega-3) -17.5 -9.4 -5.0 -2.2 -12.1 1.5
Epanova (omega-3) -20.6 -13.1* -6.9 -2.1 -21.5
1.3
Lovaza (omega-3) -29.5 -7.8 -9.0 -4.2 -27.5 0.7
16 * ApoC-111 value for Epanova is mean % change from baseline
17
18 [0176] Summary of comparative reductions in plasma apoC-III with EPA/DHA
19 versus Compound A
[0177] Although head-to-head trials have not been completed, the comparable
patient
21 populations and study designs provide a reasonable benchmark from which
to compare the
22 efficacy of Compound A versus EPA/DHA in lowering plasma apoC-Ill. There
are two notable
23 differentiating factors between the naturally occurring omega-3 fatty
acids and Compound A.
24 [0178] The first is the superior potency of Compound A, which achieved a
median
reductions in apoC-III of 35 and 41% in the mixed dyslipidemic and severe HTG
patient
26 populations respectively. This compares with apoC-III reductions of only
7.8-15% in the
27 EPA/DHA studies.
22713041.1
CA 02886957 2015-04-01
CA Application
Blakes Ref: 72571/00022
1 [0179] The second differentiating factor is the low-dose of Compound A
needed
2 (600mg QD) versus the 4g dose in the EPA/DHA studies. On a gram for gram
basis, this
3 difference is even greater for Compound A and clearly demonstrates the
potency of this
4 molecule in reducing plasma apoC-III versus EPA/DHA. As previously
mentioned, pre-clinical
models suggest that the apoC-III lowering is independent of TG lowering
(Figure 1).
36
22713041.1