Note: Descriptions are shown in the official language in which they were submitted.
CA 02888140 2015-04-10
1
NOVEL PYRAZOLE DERIVATIVE
Technical Field
The present invention relates to a novel compound; a
tau protein (hereinafter also referred to as tau)
aggregation inhibitor, a P-secretase inhibitor, and a p-
amyloid protein (hereinafter also referred to as AP)
aggregation inhibitor using the same; and a novel pyrazole
derivative useful for the prevention or treatment of
diseases such as dementia and Alzheimer's disease and a
pharmaceutical composition containing the same.
Background Art
Senile dementia has become a serious medical and social
problem along with the rapid aging of society in recent
years and the development of effective anti-dementia drugs
has been greatly desired. There are already very many
studies on Alzheimer's disease but the cause of the disease
has not been clearly defined. Drugs such as
acetylcholinesterase inhibitors including donepezil
(Aricept (registered trademark)), galantamine (Reminyl) and
rivastigmine (Exelon/Rivastach), and NMDA receptor
antagonists including memantine hydrochloride (Memary) have
been used as Alzheimer's therapeutic drugs. These drugs
are very useful as symptomatic therapy but are not drugs
for fundamental treatment.
Alzheimer's disease is considered to be caused by
aggregation of AP, aggregation of tau, and the like. Hence,
a substance that inhibits aggregation of these proteins
CA 02888140 2015-04-10
2
could be used as a fundamental therapeutic drug for
Alzheimer's disease.
Yang et al. have reported that curcumin has an AP
aggregation inhibitory activity, a disaggregation activity
on AP aggregate, and the like (Non Patent Literature 1).
The inventors of the present invention have revealed that
curcumin and its derivatives have an inhibitory activity
against secretase, which is involved in the generation of
AP (Patent Literature 1 and 2). Narlawar et al. have
synthesized curcumin derivatives by replacing the 1,3-
dicarbonyl moiety with a pyrazole ring and reported that
these compounds have a tau aggregation inhibitory activity
(Non Patent Literature 2).
Citation List
Patent Literature
Patent Literature 1: WO 2008/066151
Patent Literature 2: WO 2009/145219
Non Patent Literature
Non Patent Literature 1: Fusheng Yang et al., J. Biol.
Chem. 2005, Feb 18; 280 (7) 5892-5901
Non Patent Literature 2: Rajeshwar Narlawar et al.,
ChemMedChem 2008, 3, 165-172
Summary of Invention
Technical Problem
As described in the above literature, a curcumin
derivative can be a promising candidate for a fundamental
CA 02888140 2015-04-10
3
therapeutic drug for Alzheimer's disease. Under this
technical background, an object of the present invention is
thus to provide a novel therapeutic means for Alzheimer's
disease.
Solution to Problem
The inventors succeeded in creating a novel compound
based on technical ideas that are distinct from those
forming the basis of known compounds and found that the
compound has an excellent pharmacological activity. The
inventors further conducted extensive studies to complete
the present invention.
That is, as a result of the extensive investigations to
solve the above problems, the inventors succeeded in
synthesizing a curcumin derivative by replacing the 1,3-
dicarbonyl moiety of curcumin with a pyrazole ring and
replacing at least one of the 4-hydroxy-3-methoxyphenyl
groups at both ends with a substituent, and found that this
novel compound has a potent tau aggregation inhibitory
activity. The inventors also found that this derivative
has a high brain penetration and also possesses a p-
secretase inhibitory activity and an AP aggregation
inhibitory activity.
A curcumin derivative in which the 1,3-dicarbonyl
moiety of curcumin is replaced with a pyrazole ring is
described in Non Patent Literature 2 etc. A curcumin
derivative in which one of the 4-hydroxy-3-methoxyphenyl
groups at both ends of curcumin is replaced with a
substituent is described in Patent Literature 1 and 2, etc.
CA 02888140 2015-04-10
4
However, the derivative of the present invention which has
a pyrazole ring and in which at least one of the 4-hydroxy-
3-methoxyphenyl groups at both ends is replaced with a
substituent is a novel compound having a chemical structure
that is unique to the present invention and distinct from
those of compounds disclosed in known literature.
The tau aggregation inhibitory activity of a curcumin
derivative is described in Non Patent Literature 2. In
this literature, the nitrogen atom at position 1 of the
pyrazole ring is replaced with various groups, and the tau
aggregation inhibitory activities of the derivatives
significantly vary with the groups introduced into the ring.
However, no modification was made to the benzene rings at
both ends and thus all the synthesized derivatives have the
4-hydroxy-3-methoxyphenyl groups as curcumin does.
Therefore, the person skilled in the art who has read Non
Patent Literature 2 is expected to consider that the group
introduced into the pyrazole ring plays an important role
for the tau aggregation inhibitory activity and that the 4-
hydroxy-3-methoxyphenyl groups at both ends are irrelevant
to the tau aggregation inhibitory activity. Thus the
person skilled in the art would not attempt to replace the
4-hydroxy-3-methoxyphenyl groups with a substituent.
That is, the present invention relates to the following.
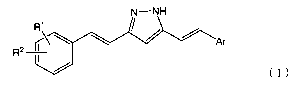
[1] A compound represented by the following general formula
(I):
CA 02888140 2015-04-10
N--NH
R1
A z
Ar
R2 II
(I)
[wherein
Rl represents a hydrogen atom, a halogen atom, a
hydroxy group, a nitro group, an amino group, a cyano group,
5 a C1-6 alkyl group optionally having one or more
substituents, a C1-6 alkoxy group optionally having one or
more substituents, a mono- or di(C1_6 alkyl)amino group
optionally having one or more substituents, a C1-6 alkylthio
group optionally having one or more substituents, a C1-6
alkylsulfonyl group optionally having one or more
substituents, a C1-6 acyl group optionally having one or
more substituents, a C1-6 acylamino group optionally having
one or more substituents, a C2-6 alkenyl group optionally
having one or more substituents, a C2-6 alkenyloxy group
optionally having one or more substituents, a mono- or
di(C2_6 alkenyl)amino group optionally having one or more
substituents, a 02-6 alkenylthio group optionally having one
or more substituents, or a carbamoyl group optionally
having one or more substituents;
R2 represents a group represented by the following
general formula (II):
R3--eY)n
( I I)
(wherein m and n each represent an integer of 0 or 1,
A represents -0-, -NH-, -S-, -SO- or -SO2-,
CA 02888140 2015-04-10
6
Y represents a C1-6 alkylene group, a C2-6 alkenylene
group or a C2-6 alkynylene group, and
R3 represents a nitrogen-containing heterocyclic group
optionally having one or more substituents, a C1-6 alkoxy
group optionally having one or more substituents, a mono-
or di(C1_6 alkyl)amino group optionally having one or more
substituents, a mono- or di(C2..6 alkenyl)amino group
optionally having one or more substituents, or a carbamoyl
group optionally having one or more substituents);
Rl and R2 may be joined to form a ring together with the
benzene ring; and
Ar represents a homocyclic or heterocyclic group
optionally having one or more substituents],
or a salt thereof.
[2] The compound according to the above [1] or a salt
thereof, wherein m is 1 and A is -0-.
[3] The compound according to the above [2] or a salt
thereof, wherein R2 is a morpholinomethoxy group, a
morpholinoethoxy group, a pyridylmethoxy group, a
pyridylethoxy group, a 2-pyrrolidinoethoxy group, a 2-
piperidinoethoxy group, a 2-(4-
(substituted)piperazino)ethoxy group, or a 2-(1,1-dioxo-
1,4-thiazinan-4-yl)ethoxy group.
[4] The compound according to the above [3] or a salt
thereof, wherein R2 is a morpholinoethoxy group.
[5] The compound according to the above [1] or a salt
thereof, wherein R2 is a morpholinomethyl group, a (4-
(substituted)piperazino)methyl group, a (1,1-dioxo-1,4-
thiazinan-4-yl)methyl group, a piperidinomethyl group, a
CA 02888140 2015-04-10
7
pyrrolidinomethyl group, a 2-morpholinoethyl group, a 2-(4-
(substituted)piperazino)ethyl group, a 2-(1,1-dioxo-1,4-
thiazinan-4-yl)ethyl group, a 2-piperidinoethyl group, a 2-
pyrrolidinoethyl group, or a 2-morpholinoethanesulfonyl
group.
[6] The compound according to the above [1] or a salt
thereof, wherein R2 is a 4-(substituted)piperazino group or
a 4-(substituted)-1,4-diazepano group, with the exception
of the case where Ar is a homocyclic group optionally
having a substituent.
[7] The compound according to any one of the above [1] to
[6] or a salt thereof, wherein Ar is a bicyclic group
having a benzene skeleton and optionally having one or more
substituents.
[8] The compound according to the above [7] or a salt
thereof, wherein the bicyclic group having a benzene
skeleton is a 1,3-benzodioxole group, a 1,4-benzodioxan-5-
yl group, a 1,4-benzodioxan-6-y1 group, a 1,4-benzodioxin-
2-y1 group, a quinolino group, or an indolyl group.
[9] The compound according to any one of the above [1] to
[5] or a salt thereof, wherein Ar is a phenyl group
optionally having one or more substituents, a pyrrolyl
group optionally having one or more substituents, a pyridyl
group optionally having one or more substituents, a pyrazyl
group optionally having one or more substituents, an
imidazolyl group optionally having one or more substituents,
or a furyl group optionally having one or more substituents.
[10] The compound according to the above [6] or a salt
thereof, wherein Ar is a pyrrolyl group optionally having
CA 02888140 2015-04-10
8
one or more substituents, a pyridyl group optionally having
one or more substituents, a pyrazyl group optionally having
one or more substituents, an imidazolyl group optionally
having one or more substituents, or a furyl group
optionally having one or more substituents.
[11] The compound according to the above [1] or a salt
thereof, wherein Ar is represented by the following general
formula (III):
R1'
I / 1
( I )
(wherein
RI' represents a hydrogen atom, a halogen atom, a
hydroxy group, a nitro group, an amino group, a cyano group,
a C1-6 alkyl group optionally having one or more
substituents, a C1-6 alkoxy group optionally having one or
more substituents, a mono- or di(C1_6 alkyl)amino group
optionally having one or more substituents, a C1-6 alkylthio
group optionally having one or more substituents, a C1-6
alkylsulfonyl group optionally having one or more
substituents, a C1-6 acyl group optionally having one or
more substituents, a C1-6 acylamino group optionally having
one or more substituents, a C2-6 alkenyl group optionally
having one or more substituents, a C2-6 alkenyloxy group
optionally having one or more substituents, a mono- or
di(C2._6 alkenyl)amino group optionally having one or more
substituents, a C2-6 alkenylthio group optionally having one
CA 02888140 2015-04-10
9
or more substituents, or a carbamoyl group optionally
having one or more substituents;
R2' represents a group represented by the following
general formula (IV):
(Iv)
(wherein m' and n' each represent an integer of 0 or 1,
A' represents -0-, -NH-, -S-, -SO- or -502-,
Y' represents a C1-6 alkylene group, a C2-6 alkenylene
group or a C2-6 alkynylene group, and
R3' represents a nitrogen-containing heterocyclic group
optionally having one or more substituents, a C1-6 alkoxy
group optionally having one or more substituents, a mono-
or di(C1_6 alkyl)amino group optionally having one or more
substituents, a mono- or di(C2_6 alkenyl)amino group
optionally having one or more substituents, or a carbamoyl
group optionally having one or more substituents), and
R1' and R2' may form a ring together with the benzene
ring).
[12] A tau aggregation inhibitor comprising the compound
according to any one of the above [1] to [11] or a salt
thereof as an active ingredient.
[13] A P-secretase inhibitor comprising the compound
according to any one of the above [1] to [11] or a salt
thereof as an active ingredient.
[14] An AP aggregation inhibitor comprising the compound
according to any one of the above [1] to [11] or a salt
thereof as an active ingredient.
CA 02888140 2015-04-10
[15] A pharmaceutical composition comprising the compound
according to any one of the above [1] to [11] or a salt
thereof as an active ingredient.
[16] The pharmaceutical composition according to the above
5 [15] for use in the prevention or treatment of a disease in
which tau, P-secretase or AP is involved.
[17] Use of the compound according to the above [1] or a
salt thereof in the production of a prophylactic or
therapeutic preparation for a disease in which tau, p¨
lc) secretase or AP is involved.
[18] The compound according to any one of the above [1] to
[11] or a salt thereof for use in the prevention or
treatment of a disease in which tau, P-secretase or AP is
involved.
[19] A method for preventing or treating a disease in which
tau, P-secretase or AP is involved, the method comprising
the step of administering the compound according to the
above [1] to a patient.
[20] The pharmaceutical composition according to the above
[15] for use in the prevention or treatment of Alzheimer's
disease.
[21] Use of the compound according to the above [1] or a
salt thereof in the production of a prophylactic or
therapeutic preparation for Alzheimer's disease.
[22] The compound according to any one of the above [1] to
[11] or a salt thereof for use in the prevention or
treatment of Alzheimer's disease.
CA 02888140 2015-04-10
II
[23] A method for preventing or treating Alzheimer's
disease, the method comprising the step of administering
the compound according to the above [1] to a patient.
[24] An oral or parenteral preparation comprising the
compound according to any one of the above [1] to [11] or a
salt thereof and one or more pharmacologically acceptable
carriers.
Advantageous Effects of Invention
The compound of the present invention is remarkably
excellent in a tau aggregation inhibitory activity, a p-
secretase inhibitory activity, an AP aggregation inhibitory
activity, and/or the like, and is thus useful as a
therapeutic drug for Alzheimer's disease and the like. The
compound of the present invention also has a high brain
penetration, and thus a highly efficient therapeutic drug
can be provided.
Description of Embodiments
The present invention will be described in detail below.
In the present invention, the term "halogen atom" means
a fluorine atom, a chlorine atom, a bromine atom, and an
iodine atom. The term "01_6 alkyl group" means a linear or
branched alkyl group of 1 to 6 carbon atoms, and examples
thereof include methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec-butyl, tert-butyl, n-pentyl, neopentyl, and
n-hexyl groups. The term "halo C1-6 alkyl group" means a
linear or branched 1- to 6-carbon alkyl group substituted
with one or more halogen atoms that may be the same or
CA 02888140 2015-04-10
12
different, and examples thereof include trifluoromethyl,
difluoromethyl, perfluoroethyl,
hexafluoroisopropyl,
perfluoroisopropyl, chloromethyl, bromomethyl, 1-bromoethyl,
and 2,3-dibromopropyl groups.
The term "C1-6 alkoxy group" means a linear or branched
alkoxy group of 1 to 6 carbon atoms, and examples thereof
include methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy,
sec-butoxy, tert-butoxy, n-pentyloxy,
isopentyloxy,
neopentyloxy, and n-hexyloxy groups. The term "halo C1-6
alkoxy group" means a linear or branched 1- to 6-carbon
alkoxy group substituted with one or more halogen atoms
that may be the same or different, and examples thereof
include trifluoromethoxy, difluoromethoxy, perfluoroethoxy,
perfluoroisopropoxy, chloromethoxy, bromomethoxy,
1-
bromoethoxy, and 2,3-dibromopropoxy groups.
The term "01_6 acyl group" means a linear or branched
acyl group of 1 to 6 carbon atoms, and examples thereof
include formyl, acetyl, propionyl, butyryl,
2-
methylpropionyl, pivaloyl, pentanoyl, 3-methylbutyryl, and
hexanoyl groups. The term "halo C1-6 acyl group" means a
linear or branched 1- to 6-carbon acyl group substituted
with one or more halogen atoms that may be the same or
different, and examples thereof include chloroformyl,
bromoformyl, dichloroacetyl, dibromoacetyl, and
trifluoroacetyl groups.
The term "C1-6 alkylsulfonyl group" means a linear or
branched alkylsulfonyl group of 1 to 6 carbon atoms, and
examples thereof include methylsulfonyl, ethylsulfonyl, n-
propylsulfonyl, isopropylsulfonyl, n-butylsulfonyl, sec-
CA 02888140 2015-04-10
13
butylsulfonyl, tert-butylsulfonyl, n-
pentylsulfonyl,
isopentylsulfonyl, and n-hexylsulfonyl groups. The term
"halo C1-6 alkylsulfonyl group" means a linear or branched
1- to 6-carbon alkylsulfonyl group substituted with one or
more halogen atoms that may be the same or different, and
examples thereof include
trifluoromethylsulfonyl,
difluoromethylsulfonyl,
perfluoroethylsulfonyl,
perfluoroisopropylsulfonyl,
chloromethylsulfonyl,
bromomethylsulfonyl, 1-bromoethylsulfonyl, and 2,3-
dibromopropylsulfonyl groups.
The term "mono- or di(C1_6 alkyl)amino group" means an
amino group mono- or di-substituted with the above 01-6
alkyl group, and examples thereof include methylamino,
ethylamino, propylamino, isopropylamino, butylamino, sec-
butylamino, tert-butylamino, dimethylamino, diethylamino,
ethylmethylamino, dipropylamino, methylpropylamino, and
diisopropylamino groups. The term "C1-6 alkylthio group"
means a linear or branched alkylthio group of 1 to 6 carbon
atoms, and examples thereof include methylthio, ethylthio,
n-propylthio, isopropylthio, n-butylthio, sec-butylthio,
tert-butylthio, n-pentylthio, isopentylthio, and n-
hexylthio groups. The term "01_6 acylamino group" means an
amino group substituted with the above 01-6 acyl group, and
examples thereof include acetylamino and propionylamino
groups.
The term "02-6 alkenyl group" means a linear or branched
alkenyl group of 2 to 6 carbon atoms, and examples thereof
include vinyl, propenyl, and butenyl groups. The term "C2-6
alkenyloxy group" means a linear or branched alkenyloxy
CA 02888140 2015-04-10
14
group of 2 to 6 carbon atoms, and examples thereof include
propenyloxy, butenyloxy, and pentenyloxy groups. The term
"mono- or di(C2_6 alkenyl)amino group" means an alkenyl
amino group mono- or di-substituted with the above C2-6
alkenyl group, and examples thereof include vinylamino,
propenylamino, butenylamino, and divinylamino groups.
The term "C2-6 alkenylthio group" means a linear or
branched alkenylthio group of 2 to 6 carbon atoms, and
examples thereof include vinylthio, 1-propenylthio,
isopropenylthio, 1-butenylthio, 2-butenylthio, and 2-
methylallylthio groups. The term "nitrogen-containing
heterocyclic group" means a saturated or unsaturated
heterocyclic group containing one or more nitrogen atoms
and optionally containing one or more oxygen and/or sulfur
atoms, and examples thereof include pyridyl, pyrimidyl,
pyrazyl, morpholino, 4-(substituted)piperazino (e.g., 4-
methylpiperazino etc.), 1,1-
dioxo-1,4-thiazinan-4-yl,
piperidino, pyrrolidino, thiazolyl, azepan-l-yl, and 4-
(substituted)-1,4-diazepano (e.g., 4-methyl-1,4-diazepano)
groups.
The present invention relates to a compound represented
by the following general formula (I):
N--NH
R1
Z
Ar
R2
(I)
, and
a salt thereof.
In the above general formula (I), RI represents a
hydrogen atom, a halogen atom, a hydroxy group, a nitro
CA 02888140 2015-04-10
group, an amino group, a cyano group, a C1-6 alkyl group
optionally having one or more substituents, a Ci_6 alkoxy
group optionally having one or more substituents, a mono-
or di(C1_6 alkyl)amino group optionally having one or more
5 substituents, a 01-6 alkylthio group optionally having one
or more substituents, a 01-6 alkylsulfonyl group optionally
having one or more substituents, a C1_6 acyl group
optionally having one or more substituents, a C1-6 acylamino
group optionally having one or more substituents, a C2-6
10 alkenyl group optionally having one or more substituents, a
C2-6 alkenyloxy group optionally having one or more
substituents, a mono- or di(C2_6 alkenyl)amino group
optionally having one or more substituents, a C2-6
alkenylthio group optionally having one or more
15 substituents, a carbamoyl group optionally having one or
more substituents, or the like.
The substituents in R1 are not particularly limited as
long as the effects of the present invention are not
impaired, and examples thereof include a halogen atom, a
hydroxy group, a nitro group, an amino group, a morpholino
group, and a pyridyl group. R1 is preferably a hydroxy
group, a C1-6 alkyl group optionally having one or more
substituents, a C1-6 alkoxy group optionally having one or
more substituents, or the like, and is more preferably
hydroxy, methoxy, ethoxy,
morpholinomethoxy,
morpholinoethoxy, pyridylmethoxy, or pyridylethoxy group,
or the like. The position of R1 on the benzene ring is not
particularly limited as long as the effects of the present
invention are not impaired, but when the position of the
CA 02888140 2015-04-10
16
carbon atom bound to the pyrazole ring via the vinyl group
is assigned position 1, the position of 170 on the benzene
ring is preferably position 2 or 3, more preferably
position 2.
In the above general formula (I), R2 represents a group
represented by the following general formula (II):
R3¨eY)n (Ar
( I I )
=
In the above general formula (II), m and n each
represent an integer of 0 or 1. A represents -0-, -NH-, -
S-, -SO-, -SO2-, or the like, and preferably represents -0-,
-502-, or the like. Y represents a C1-6 alkylene group, a
02-6 alkenylene group, a 02-6 alkynylene group, or the like,
and preferably represents a methylene group, an ethylene
group, or the like. R3 represents a nitrogen-containing
heterocyclic group optionally having one or more
substituents, a C1-6 alkoxy group optionally having one or
more substituents, a mono- or di(C1_6 alkyl)amino group
optionally having one or more substituents, a mono- or
di(C2-6 alkenyl)amino group optionally having one or more
substituents, or a carbamoyl group optionally having one or
more substituents.
The substituents in R3 in the above general formula
(II) are not particularly limited as long as the effects of
the present invention are not impaired. In cases where R3
is a nitrogen-containing heterocyclic group, the
substituents are preferably a halogen atom, a hydroxy group,
a nitro group, an amino group, a C1-6 alkyl group, a halo
CA 02888140 2015-04-10
17
C1-6 alkyl group, a 01_6 alkoxy group, a halo C1_6 alkoxy
group, a 01-6 acyl group, a halo C1-6 acyl group, a 01-6
alkylsulfonyl group, a halo 01-6 alkylsulfonyl group, or the
like. In cases where R3 is a C1-6 alkoxy group, the
substituents are preferably a methoxy group, an ethoxy
group, a 2-methoxyethoxy group, or the like. In cases
where R3 is a mono- or di(C1_6 alkyl)amino group or a mono-
or di(alkenyl)amino group, the substituents are preferably
a 01_6 alkoxy group, a halo 01_6 alkoxy group, a 01-6
alkylsulfonyl group, a halo 01-6 alkylsulfonyl group, or the
like. In cases where R3 is a carbamoyl group, the
substituents are preferably a 01-6 alkyl group, a 01-6 alkoxy
group, or the like.
Preferred specific examples of the group represented by
R3 include pyridyl, pyrimidyl, pyrazyl, morpholino, 4-
(substituted)piperazino (e.g., 4-methylpiperazino), 4-
(substituted)-1,4-diazepano (e.g., 4-methyl-1,4-diazepano),
1,1-dioxo-1,4-thiazinan-4-yl, piperidino, pyrrolidino,
thiazolyl, methoxy, ethoxy, propoxy, dimethylamino,
diethylamino, isopropylamino, N,N-bis(2-methoxyethyl)amino,
N-2-methoxyethyl-N-methylamino, N,N-bis(2-
methylsulfonylethyl)amino, N-methyl-N-(2-
methylsulfonylethyl)amino, and dimethylcarbamoyl groups.
Particularly preferred specific examples of the group
represented by R2 include morpholinoethoxy,
morpholinomethoxy, pyridylmethoxy, pyridylethoxy, 2-
pyrrolidinoethoxy, 2-piperidinoethoxy, 2-(4-
(substituted)piperazino)ethoxy, 2-(1,1-dioxo-1,4-thiazinan-
4-yl)ethoxy, morpholinomethyl, (4-
CA 02888140 2015-04-10
18
(substituted)piperazino)methyl, (1,1-dioxo-1,4-thiazinan-4-
yl)methyl, piperidinomethyl, pyrrolidinomethyl, 2-
morpholinoethyl, 2-(4-(substituted)piperazino)ethyl, 2-
(1,1-dioxo-1,4-thiazinan-4-yl)ethyl, 2-piperidinoethyl, 2-
pyrrolidinoethyl, 2-morpholinoethanesulfonyl,
methoxymethoxy, methoxyethoxy, methoxymethyl, methoxyethyl,
dimethylamino, diethylamino, isopropylamino, N,N-bis(2-
methoxyethyl)amino, N-2-methoxyethyl-N-methylamino, N,N-
bis(2-methylsulfonylethyl)amino, N-methyl-N-(2-
methylsulfonylethyl)amino, dimethylaminomethyl,
diethylaminomethyl, N,N-bis(2-methoxyethyl)aminomethyl,
dimethylaminoethoxy, 4-(substituted)piperazino, 4-
(substituted)-1,4-diazepano, and dimethylcarbamoylethoxy
groups. The substituents represented by the term
"substituted" enclosed in the parentheses are not
particularly limited as long as the present invention is
not impaired, but are preferably a hydrogen atom, a C1-6
alkyl group, or the like, and are particularly preferably a
hydrogen atom, a methyl group, an ethyl group, an isopropyl
group, or the like. The position of R2 on the benzene ring
is not particularly limited as long as the effects of the
present invention are not impaired, but when the position
of the carbon atom bound to the pyrazole ring via the vinyl
group is assigned position 1, the position of R2 on the
benzene ring is preferably position 3 or 4, more preferably
position 4.
In the above general formula (I), RI- and R2 may be
joined together to form a ring. In this case, RI- and R2
form a fused ring or the like together with the benzene
CA 02888140 2015-04-10
19
ring to which RI- and R2 are attached. Examples of the fused
ring include a 1,3-benzodioxole group optionally having one
or more substituents, a 1,4-benzodioxan-5-y1 group
optionally having one or more substituents, a 1,4-
benzodioxan-6-y1 group optionally having one or more
substituents, a 1,4-benzodioxin-6-y1 group optionally
having one or more substituents, a 1,4-benzodioxin-2-y1
group optionally having one or more substituents, a
quinolino group optionally having one or more substituents,
an isoquinolino group optionally having one or more
substituents, a quinoxalino group optionally having one or
more substituents, and an indolyl group optionally having
one or more substituents.
The substituents in the above fused rings are not
particularly limited and examples thereof include those
exemplified above for RI, R2 and R3.
In the above general formula (I), Ar represents a
homocyclic or heterocyclic group optionally having one or
more substituents. The substituents are not particularly
limited as long as the effects of the present invention are
not impaired. Preferred examples thereof include a halogen
atom, a hydroxy group, a nitro group, an amino group, a C1-6
alkyl group, a halo C1_6 alkyl group, a C1-6 alkoxy group, a
halo C1-6 alkoxy group, a C1-6 acyl group, a halo C1-6 acyl
group, a C1-6 alkylsulfonyl group, and a halo C1-6
alkylsulfonyl group, and particularly preferred examples
thereof include amino, hydroxy, methyl, ethyl, methoxy, and
ethoxy groups. Preferred examples of the homocyclic or
heterocyclic group include a monocyclic group such as
CA 02888140 2015-04-10
phenyl, pyrrolyl, imidazolyl, furyl, pyridyl, and pyrazyl
groups, and a bicyclic group having a benzene skeleton.
Preferred examples of the bicyclic group having a
benzene skeleton include 1,3-benzodioxole, 1,4-benzodioxan-
5 5-yl, 1,4-benzodioxan-6-yl, 1,4-benzodioxin-6-yl, 1,4-
benzodioxin-2-yl, quinolino, isoquinolino, quinoxalino, and
indolyl groups. Particularly preferred examples of the
indolyl group optionally having one or more substituents
include a 1-methylindoly1 group.
10 In cases where Ar is a phenyl group optionally having
one or more substituents, Ar is preferably a substituent
represented by, for example, the following general formula
(III):
/
;;FI=1
(1 1).
15 In the above general formula (I), R1' represents a
hydrogen atom, a halogen atom, a hydroxy group, a nitro
group, an amino group, a cyano group, a 01-6 alkyl group
optionally having one or more substituents, a C1-6 alkoxy
group optionally having one or more substituents, a mono-
20 or di(C1_6 alkyl)amino group optionally having one or more
substituents, a C1-6 alkylthio group optionally having one
or more substituents, a C1-6 alkylsulfonyl group optionally
having one or more substituents, a C1-6 acyl group
optionally having one or more substituents, a C1-6 acylamino
group optionally having one or more substituents, a 02-6
alkenyl group optionally having one or more substituents, a
CA 02888140 2015-04-10
21
C2-6 alkenyloxy group optionally having one or more
substituents, a mono- or di(C2_6 alkenyl)amino group
optionally having one or more substituents, a C2-6
alkenylthio group optionally having one or more
substituents, a carbamoyl group optionally having one or
more substituents, or the like.
The substituents in R1' are not particularly limited as
long as the effects of the present invention are not
impaired, and examples thereof include a halogen atom, a
hydroxy group, a nitro group, an amino group, a morpholino
group, and a pyridyl group. R1' is preferably a hydroxy
group, a C1-6 alkyl group optionally having one or more
substituents, a C1-6 alkoxy group optionally having one or
more substituents, or the like, and is more preferably
hydroxy, methoxy, ethoxy,
morpholinomethoxy,
morpholinoethoxy, pyridylmethoxy, or pyridylethoxy group,
or the like. The position of R1' on the benzene ring is
not particularly limited as long as the effects of the
present invention are not impaired, but when the position
of the carbon atom bound to the pyrazole ring via the vinyl
group is assigned position 1, the position of R1' on the
benzene ring is preferably position 2 or 3, more preferably
position 2.
In the above general formula (III), R2' represents a
group represented by the following general formula (IV):
R3'¨EYI)' A'rrn
n
( v )
CA 02888140 2015-04-10
22
In the above general formula (IV), m' and n' each
represent an integer of 0 or 1. A' represents -0-, -NH-, -
S-, -SO-, -502-, or the like, and preferably represents -0-,
-SO2-, or the like. Y' represents a 01-6 alkylene group, a
C2-6 alkenylene group, a C2-6 alkynylene group, or the like,
and preferably represents a methylene group, an ethylene
group, or the like. R3' represents a nitrogen-containing
heterocyclic group optionally having one or more
substituents, a C1-6 alkoxy group optionally having one or
more substituents, a mono- or di(C1-6 alkyl)amino group
optionally having one or more substituents, a mono- or
di(C2_6 alkenyl)amino group optionally having one or more
substituents, or a carbamoyl group optionally having one or
more substituents.
The substituents in R3' in the above general formula
(IV) are not particularly limited as long as the effects of
the present invention are not impaired. In cases where R3'
is a nitrogen-containing heterocyclic group, the
substituents are preferably a halogen atom, a hydroxy group,
a nitro group, an amino group, a C1-6 alkyl group, a halo
C1-6 alkyl group, a C1-6 alkoxy group, a halo C1-6 alkoxy
group, a CI-6 acyl group, a halo C1-6 acyl group, a C1-6
alkylsulfonyl group, a halo C1-6 alkylsulfonyl group, or the
like. In cases where R3' is a C1-6 alkoxy group, the
substituents are preferably a methoxy group, an ethoxy
group, a 2-methoxyethoxy group, or the like. In cases
where R3' is a mono- or di(C1_6 alkyl)amino group or a mono-
or di(alkenyl)amino group, the substituents are preferably
a C1-6 alkoxy group, a halo C1-6 alkoxy group, a 01-6
CA 02888140 2015-04-10
23
alkylsulfonyl group, a halo C1_6 alkylsulfonyl group, or the
like. In cases where R3' is a carbamoyl group, the
substituents are preferably a C1-6 alkyl group, a C1_6 alkoxy
group, or the like.
Preferred specific examples of the group represented by
R3' include pyridyl, pyrimidyl, pyrazyl, morpholino, 4-
(substituted)piperazino (e.g., 4-methylpiperazino), 1,1-
dioxo-1,4-thiazinan-4-yl, piperidino, pyrrolidino,
thiazolyl, methoxy, ethoxy, propoxy, dimethylamino,
diethylamino, isopropylamino, N,N-bis(2-methoxyethyl)amino,
N-2-methoxyethyl-N-methylamino, N,N-bis(2-
methylsulfonylethyl)amino, N-methyl-N-(2-
methylsulfonylethyl)amino, and dimethylcarbamoyl groups.
Particularly preferred specific examples of the group
represented by R2' include morpholinoethoxy,
morpholinomethoxy, pyridylmethoxy, pyridylethoxy, 2-
pyrrolidinoethoxy, 2-piperidinoethoxy, 2-(4-
(substituted)piperazino)ethoxy, 2-(1,1-dioxo-1,4-thiazinan-
4-yl)ethoxy, morpholinomethyl, (4-
(substituted)piperazino)methyl, (1,1-dioxo-1,4-thiazinan-4-
yl)methyl, piperidinomethyl, pyrrolidinomethyl, 2-
morpholinoethyl, 2-(4-(substituted)piperazino)ethyl, 2-
(1,1-dioxo-1,4-thiazinan-4-yl)ethyl, 2-piperidinoethyl, 2-
pyrrolidinoethyl, 2-morpholinoethanesulfonyl,
methoxymethoxy, methoxyethoxy, methoxymethyl, methoxyethyl,
dimethylamino, diethylamino, isopropylamino, N,N-bis(2-
methoxyethyl)amino, N-2-methoxyethyl-N-methylamino, N,N-
bis(2-methylsulfonylethyl)amino, N-methyl-N-(2-
methylsulfonylethyl)amino, dimethylaminomethyl,
CA 02888140 2015-04-10
24
diethylaminomethyl, N,N-bis(2-methoxyethyl)aminomethyl,
dimethylaminoethoxy, and dimethylcarbamoylethoxy groups.
The substituents represented by the term "substituted"
enclosed in the parentheses are not particularly limited as
long as the present invention is not impaired, but are
preferably a hydrogen atom, a C1-6 alkyl group, or the like,
and are particularly preferably a hydrogen atom, a methyl
group, an ethyl group, an isopropyl group, or the like.
The position of R2' on the benzene ring is not particularly
limited as long as the effects of the present invention are
not impaired, but when the position of the carbon atom
bound to the pyrazole ring via the vinyl group is assigned
position 1, the position of R2' on the benzene ring is
preferably position 3 or 4, more preferably position 4.
In the above general formula (III), RI' and R2' may be
joined together to form a ring. In this case, RI' and R2'
form a fused ring or the like together with the benzene
ring to which RI' and R2' are attached. Examples of the
fused ring include those exemplified above for the bicyclic
group having a benzene skeleton.
The substituent represented by the general formula
(III) may be the same as or different from the phenyl group
substituted with RI and R2 in the general formula (I). When
they are the same, the compound or a salt thereof is easier
to synthesize and is thus industrially preferable.
In the general formula (I), in cases where R2 is a
morpholinomethoxy, morpholinoethoxy or pyridylmethoxy group,
Ar is particularly preferably, for example, a phenyl group
optionally having one or more substituents (e.g., a
CA 02888140 2015-04-10
morpholinomethoxy phenyl group, a dimethylcarbamoylmethoxy
phenyl group, a dimethoxyphenyl group, or the like), a
pyrrolyl group optionally having one or more substituents,
or a bicyclic group having a benzene skeleton and
5 optionally having one or more substituents (e.g., a 1,3-
benzodioxole group, a 1,4-benzodioxan-6-y1 group, an
indolyl group, a 1-methylindoly1 group, or the like). In
cases where R2 is a pyridylmethoxy group, Ar is
particularly preferably, for example, a pyrrolyl group
10 optionally having one or more substituents, or a bicyclic
group having a benzene skeleton (e.g., a 1-methylindoly1
group or the like). Preferably, RI, R2 and/or Ar are basic
groups so that the compound or a salt thereof is excellent
in water solubility and therefore can readily undergo oral
15 absorption and the like.
A salt of the compound represented by the general
formula (I) is also encompassed in the present invention.
The salt is preferably a pharmacologically acceptable salt
and examples thereof include hydrohalic acid salts such as
20 hydrofluoride, hydrochloride, hydrobromide, and
hydroiodide; inorganic acid salts such as sulfate, nitrate,
perchlorate, phosphate, carbonate, and bicarbonate; organic
carboxylic acid salts such as acetate, oxalate, maleate,
tartrate, and fumarate; organic sulfonic acid salts such as
25 methanesulfonate, trifluoromethanesulfonate,
ethanesulfonate, benzenesulfonate, toluenesulfonate, and
camphorsulfonate; amino acid salts such as aspartate and
glutamate; amine salts such as trimethylamine salt,
triethylamine salt, procaine salt, pyridine salt, and
CA 02888140 2015-04-10
26
phenethylbenzylamine salt; alkali metal salts such as
sodium salt and potassium salt; alkaline earth metal salts
such as magnesium salt and calcium salt; etc. In view of
the water solubility, oral absorbability, efficacy, and the
like, preferred are hydrochloride and oxalate.
The compound represented by the general formula (I) can
be produced by known methods described in, for example,
Rajeshwar Narlawar et al., ChemMedChem 2008, 3, 165-172, WO
2008/066151, WO 2009/145219, or the like, or by any
combination of the methods described in the literature, or
by a method known per se or an equivalent method thereto.
Specifically, the compound can be produced by, for example,
the following Steps 1 and 2, but the production method is
not limited thereto.
o o
Ar
(B)
0
0 0
Ar' 1
2
Ar2
P (I)
Arl in the above formula is either the phenyl ring
substituted with R1 and R2 or a ring represented by Ar in
the compound represented by the general formula (I) of the
present invention, and Ar2 is the other one. In Step 1, an
aldehyde represented by the general formula (A) is allowed
to react with a compound represented by the general formula
(B) in the presence of a solvent and a catalyst to give a
diketone represented by the general formula (C).
The solvent used in the reaction is not particularly
limited as long as it does not inhibit the reaction, and
CA 02888140 2015-04-10
27
examples thereof include ethyl acetate, N,N-
dimethylacetamide, N,N-dimethylformamide, 1-methy1-2-
pyrrolidone, dimethylsulfoxide, tetrahydrofuran,
acetonitrile, etc. These solvents may be used alone or in
combination of two or more kinds thereof at a given mixing
ratio.
The catalyst used in the reaction is also not
particularly limited. Examples thereof include bases such
as a primary amine and a secondary amine, and specific
examples thereof include n-butylamine, ethanolamine,
piperidine, morpholine, etc.
In Step 1, a water scavenger may be added in order to
capture the water produced by the reaction. Examples of
the water scavenger include an alkyl borate, an alkyl
phosphate, an orthoester, etc., and specific examples
thereof include trimethyl orthoformate and tri-n-butyl
borate.
In Step 1, the quantitative ratio of the aldehyde
represented by the general formula (A) and the compound
represented by the general formula (B) is not particularly
limited as long as the reaction proceeds. However, in view
of the reaction efficiency and the like, the amount of the
compound is preferably 0.5 to 10 mol, more preferably 0.5
to 2 mol, relative to 1 mol of the aldehyde.
In Step 1, the reaction temperature is not particularly
limited as long as the reaction proceeds. However, in view
of the reaction efficiency and the like, the reaction
temperature is preferably 0 to 200 C, more preferably 50 to
100 C.
CA 02888140 2015-04-10
28
In Step 1, the reaction duration is not particularly
limited as long as the reaction proceeds. However, in view
of the production efficiency and the like, the reaction
duration is preferably 0.5 to 48 hours, more preferably 1
to 24 hours.
The aldehyde represented by the general formula (A) and
the compound represented by the general formula (B) that
are used in Step 1 may be commercially available products
or those synthesized by a known method (for example, the
method described in WO 2008/066151 or WO 2009/145219) or
other methods.
In Step 2, a diketone represented by the general
formula (C) is allowed to react with a hydrazine in the
presence of a solvent to give a compound represented by the
general formula (I).
The hydrazine used in the reaction is not particularly
limited and examples thereof include hydrazine monohydrate,
hydrazine aqueous solution, anhydrous hydrazine, hydrazine
acetate, hydrazine monohydrochloride, hydrazine
dihydrochloride, a derivative thereof, etc.
The solvent used in the reaction is not particularly
limited as long as it does not inhibit the reaction and
examples thereof include protic solvents such as acetic
acid, methanol, ethanol, and water; non-protic solvents
such as ethyl acetate, toluene, tetrahydrofuran, methylene
chloride, chloroform, etc. These solvents may be used
alone or, if desired, in combination of two or more kinds
thereof at a given mixing ratio.
CA 02888140 2015-04-10
29
In Step 2, the quantitative ratio of the diketone
represented by the general formula (C) and the hydrazine is
not particularly limited as long as the reaction proceeds.
However, in view of the reaction efficiency and the like,
the amount of the hydrazine is preferably 1 to 50 mol, more
preferably 2 to 10 mol, relative to 1 mol of the diketone.
In Step 2, the reaction temperature is not particularly
limited as long as the reaction proceeds. However, in view
of the reaction efficiency and the like, the reaction
temperature is preferably 20 to 120 C, more preferably 50
to 80 C.
In Step 2, the reaction duration is not particularly
limited as long as the reaction proceeds. However, in view
of the production efficiency and the like, the reaction
duration is preferably 0.2 to 24 hours, more preferably 0.5
to 6 hours.
The compound of the present invention may be
administered to a subject, alone or in combination with one
or more of other compounds of the present invention, or
with one or more compounds other than the compounds of the
present invention. The compound of the present invention
may be administered as a preparation or pharmaceutical
composition comprising one or more pharmacologically
acceptable carriers. The effective dosage of the compound
of the present invention as an active ingredient and
frequency of administration may vary with the dosage form,
the age, body weight, symptoms, etc. of the patient, and
the like, but the daily dosage is usually about 0.01 to 100
mg/kg, more preferably about 1 to 50 mg/kg.
CA 02888140 2015-04-10
The subject is not particularly limited and examples
thereof include mammals such as a human, a monkey, a
hamadryas baboon, a chimpanzee, a mouse, a rat, a guinea
pig, a hamster, a rabbit, a cat, a dog, a sheep, a goat, a
5 pig and a cattle.
The route of administration of the preparation or
pharmaceutical composition comprising the compound of the
present invention as an active ingredient is not
particularly limited, and the preparation or pharmaceutical
10 composition may be administered orally or parenterally by a
usual method. Examples of the parenteral administration
include rectal administration, nasal administration,
transpulmonary administration, dermal administration, and
injection administration (e.g., intravenous administration,
15 intraspinal administration, epidural administration,
intramuscular administration, subcutaneous administration,
intraperitoneal administration,
intraarterial
administration, intraarticular administration, intracardiac
administration, intracystic administration, intracutaneous
20 administration, intralesional administration, intraocular
administration, intrathoracic administration, subarachnoid
administration, intrauterine
administration,
intraventricular administration, etc.).
The form of the preparation or pharmaceutical
25 composition comprising the compound of the present
invention as an active ingredient is not particularly
limited, and examples thereof include oral or parenteral
preparations such as tablets, powders, granules, capsules,
oral solutions, emulsions, elixirs, lemonades, suspensions,
CA 02888140 2015-04-10
31
syrups, oromucosal tablets, oral jellies, inhalations,
suppositories, injections, ointments, ophthalmic ointments,
ophthalmic preparations, nasal preparations, ear
preparations, patches, solutions for external application.
The dosage of the preparation or pharmaceutical
composition of the present invention can be determined as
appropriate depending on the severity of the symptom, the
age, sex, and body weight of the patient, the route of
administration, the type of the salt, the specific disease,
etc.
Since the compound of the present invention has a tau
aggregation inhibitory activity, a P-secretase inhibitory
activity, and/or an AP aggregation inhibitory activity, the
compound of the present invention is effective in
preventing and treating diseases in which tau, P-secretase
or AP is involved, such as Alzheimer's disease (familial
Alzheimer's disease and sporadic Alzheimer's disease),
senile dementia, Down syndrome, Parkinson's disease,
Creutzfeldt-Jakob disease, amyotrophic lateral sclerosis,
diabetic neuropathy, Huntington's chorea, multiple
sclerosis, etc. Among these nervous diseases, the compound
of the present invention is especially effective in
preventing and treating Alzheimer's disease.
The compound of the present invention can be formulated
by a commonly used method into dosage forms such as tablets,
powders, granules, capsules, oral solutions, emulsions,
elixirs, lemonades, suspensions, syrups, oromucosal tablets,
oral jellies, inhalations, suppositories, injections,
ointments, ophthalmic ointments, ophthalmic preparations,
CA 02888140 2015-04-10
32
nasal preparations, ear preparations, patches, solutions
for external application, etc. For the formulation into
such dosage forms, excipients, binders, lubricants,
colorants and flavor modifiers that are usually used for
the formulation of medicines can be used, and as needed
stabilizers, emulsifiers, absorption enhancers, surfactants,
pH adjusters, preservatives, antioxidants, and/or the like
can also be used. Thus, ingredients that are usually used
as raw materials of a pharmaceutical preparation may be
mixed with the compound and formulated into a dosage form
by a conventional method.
For example, in the production of oral preparations, a
crystalline or amorphous compound of the present invention
is mixed with excipients, and as needed with additives such
as binders, disintegrators, lubricants, colorants, flavor
modifiers, etc., and formed into powders, fine granules,
granules, tablets, coated tablets, capsules, etc. by a
conventional method. Examples of the additives include
animal and vegetable fats and oils such as soybean oil,
beef tallow, and synthetic glyceride; hydrocarbons such as
liquid paraffin, squalane, and hard paraffin; ester oils
such as octyldodecyl myristate and isopropyl myristate;
higher alcohols such as cetostearyl alcohol and behenyl
alcohol; silicone resins; silicone oils; surfactants such
as polyoxyethylene fatty acid ester, sorbitan fatty acid
ester, glycerin fatty acid ester, polyoxyethylene sorbitan
fatty acid ester, polyoxyethylene hardened castor oil, and
polyoxyethylene-polyoxypropylene block copolymer; water
soluble polymers such as hydroxyethyl cellulose,
CA 02888140 2015-04-10
33
hydroxypropyl methylcellulose,
hydroxypropyl
methylcellulose phthalate, polyacrylate, carboxy vinyl
polymer, polyethylene glycol, polyvinylpyrrolidone, and
methylcellulose; lower alcohols such as ethanol and
isopropanol; polyalcohols such as glycerin, propylene
glycol, dipropylene glycol, and sorbitol; sugars such as
glucose and sucrose; inorganic powders such as anhydrous
silicic acid, magnesium aluminum silicate, and aluminium
silicate; purified water; etc.
Examples of the excipients include lactose, corn starch,
saccharose, glucose, mannitol, sorbitol, crystalline
cellulose, silicon dioxide, etc. Examples of the binders
include polyvinyl alcohol, polyvinyl ether, methylcellulose,
ethylcellulose, gum arabic, tragacanth, gelatin, shellac,
hydroxypropyl methylcellulose, hydroxypropyl cellulose,
polyvinylpyrrolidone, polypropylene glycol-polyoxyethylene
block polymer, meglumine, etc. Examples of the
disintegrators include starch, agar, gelatin powder,
crystalline cellulose, calcium carbonate, sodium
bicarbonate, calcium citrate, dextrin, pectin,
carboxymethyl cellulose calcium, etc. Examples of the
lubricants include magnesium stearate, talc, polyethylene
glycol, silica, hydrogenated vegetable oil, etc. Examples
of the colorants include a colorant that is approved as
additives to a medicine, etc. Examples of the flavor
modifiers include cocoa powder, menthol, aromatic powder,
mentha oil, borneol, cinnamon powder, etc.
Needless to say, in the production of the tablets or
granules, coating of the tablets or granules with a sugar
CA 02888140 2015-04-10
34
or the like may be performed as needed. In the production
of solutions such as syrups, emulsions, elixirs, lemonades,
suspensions, and injections, the compound of the present
invention can be mixed as needed with further additives
such as pH adjusters, solubilizers, emulsifiers,
disintegrators, isotonic agents, solubilization assisting
agents, stabilizers, and the like, and formed into such
solutions by a conventional method.
In the production of external medicines, the production
method is not limited and the production can be carried out
by a conventional method. That is, for the formulation of
external medicines, various types of raw materials that are
usually used for medicines, quasi drugs, cosmetics, or the
like can be used as a base ingredient. Specific examples
of the base ingredients to be used include animal and
vegetable oils, mineral oils, ester oils, waxes, higher
alcohols, fatty acids, silicone oils, surfactants,
phospholipids, alcohols, polyalcohols, water soluble
polymers, clay minerals, water-insoluble natural or
synthetic polymers such as resins, plastics and rubbers,
purified water, etc. Further, pH adjusters, antioxidants,
chelating agents, antibacterial and antifungal agents,
colorants, flavors, and/or the like can be added as needed.
The base ingredients of the external medicines of the
present invention are not limited to the above ingredients.
As needed, other ingredients can be added and examples
thereof include ingredients having a differentiation
inducing activity, blood flow increasing agents,
bactericides, anti-inflammatories, cell
activators,
CA 02888140 2015-04-10
vitamins, amino acids, moisturizers, keratolytic agents,
etc. The amount of the base ingredients to be added is
determined so that the concentration will be a usual base
ingredient concentration in the production of external
5 medicines.
The compound of the present invention may be provided
as a food or drink, a feed, or a food additive.
The food or drink of the present invention may contain
one or more types of food additives commonly used in food
10 or drink, and examples of the food additives include
sweeteners, colorants, preservatives,
thickening
stabilizers, antioxidants, color fixatives, bleaching
agents, antifungal agents, gum bases, bittering agents,
enzymes, brighteners, acidulants, seasonings, emulsifiers,
15 fortifiers, processing aids, flavors, and spice extracts.
The food or drink of the present invention includes health
foods, functional foods, foods for specified health use,
foods for babies, foods for small children, foods for
pregnant women and nursing mothers, foods for elderly
20 people, and foods for sick people.
The form of the food or drink of the present invention
is not particularly limited. Specific examples thereof
include so-called dietary supplements in forms of tablets,
granules, powders, energy drinks, or the like. Other
25 examples thereof include drinks, such as tea drink,
refreshing drink, carbonated drink, nutritional drink,
fruit juice, and lactic drink; noodles, such as buckwheat
noodle, wheat noodle, Chinese noodle, and instant noodle;
sweets and bakery products, such as drop, candy, gum,
CA 02888140 2015-04-10
36
chocolate, snack, biscuit, jelly, jam, cream, pastry, and
bread; fishery or livestock products, such as fish sausage,
ham, and sausage; dairy products, such as processed milk
and fermented milk; fats, oils and processed foods thereof,
such as vegetable oil, oil for deep frying, margarine,
mayonnaise, shortening, whipped cream, and dressing;
seasonings, such as sauce and dipping sauce; retort pouch
foods, such as curry, stew, rice-bowl cuisine, porridge,
and rice soup; and frozen desserts, such as ice cream,
sherbet, and shaved ice.
The amount of intake of the food or drink of the
present invention is not particularly limited, and may be
determined depending on the form of the food or drink, the
age, sex, condition, and the like of the subject who is to
take the food or drink, and other conditions.
The compound of the present invention or a salt thereof
can be used for a therapeutic method for a disease in which
tau, P-secretase or AP is involved. Specific examples of
the method include the following (a) to (c):
(a) a therapeutic method for a disease in which tau is
involved, the method comprising the step of administering a
compound represented by the general formula (I) or a salt
thereof to a patient with a disease in which tau is
involved;
(b) a therapeutic method for a disease in which P-secretase
is involved, the method comprising the step of
administering a compound represented by the general formula
(I) or a salt thereof to a patient with a disease in which
P-secretase is involved; and
CA 02888140 2015-04-10
37
(c) a therapeutic method for a disease in which AP is
involved, the method comprising the step of administering a
compound represented by the general formula (I) or a salt
thereof to a patient with a disease in which AP is involved.
The diseases in which tau, P-secretase or AP is
involved preferably include diseases of which the onset
mechanism involves tau, P-secretase or AP. Specific
examples of such diseases include Alzheimer's disease
(familial Alzheimer's disease and sporadic Alzheimer's
disease), senile dementia, Down syndrome, Parkinson's
disease, Creutzfeldt-Jakob disease, amyotrophic lateral
sclerosis, diabetic neuropathy, Huntington's chorea,
multiple sclerosis, etc. Among these, preferred is
Alzheimer's disease.
The route of administration, dosage form, and dosage of
the compound of the present invention, and the subject to
which the compound is to be administered may be the same as
those described above for the preparation comprising the
compound of the present invention.
The compound of the present invention can also be used
for a method for inhibiting tau aggregation, a method for
inhibiting P-secretase, and a method for inhibiting Ap
aggregation. Specific examples of the method include the
following (d) to (i):
(d) a method for inhibiting tau aggregation, the method
comprising the step of administering a compound represented
by the general formula (I) or a salt thereof to a mammal
including human, thereby inhibiting tau aggregation in the
body of the mammal including human;
CA 02888140 2015-04-10
38
(e) a method for inhibiting tau aggregation, the method
comprising the step of bringing a compound represented by
the general formula (I) or a salt thereof in contact with
tau;
(f) a method for inhibiting P-secretase, the method
comprising the step of administering a compound represented
by the general formula (I) or a salt thereof to a mammal
including human, thereby inhibiting P-secretase in the
living human body;
(g) a method for inhibiting P-secretase, the method
comprising the step of bringing a compound represented by
the general formula (I) or a salt thereof in contact with
P-secretase;
(h) a method for inhibiting AP aggregation, the method
comprising the step of administering a compound represented
by the general formula (I) or a salt thereof to a mammal
including human, thereby inhibiting AP aggregation in the
body of the mammal including human; and
(i) a method for inhibiting AP aggregation, the method
comprising the step of bringing a compound represented by
the general formula (I) or a salt thereof in contact with
A.
The salt in the above methods may be a
pharmacologically acceptable salt.
The present invention also includes, as one embodiment,
use of the compound of the present invention or a salt
thereof in the production of a prophylactic or therapeutic
preparation for a disease in which tau, P-secretase or AP
is involved. The disease in which tau, P-secretase or AP
CA 02888140 2015-04-10
39
is involved may be the same as those described above, and
is preferably Alzheimer's disease.
The present invention also includes, as another
embodiment, the compound of the present invention or a salt
thereof for use in the prevention or treatment of a disease
in which tau, P-secretase or AP is involved. The disease
in which tau, P-secretase or AP is involved may be the same
as those described above, and is preferably Alzheimer's
disease.
Examples
The present invention will be described in further
detail with reference to Examples etc., but the present
invention is not limited thereto. Various modifications of
the present invention can be made by a person who has
common knowledge in the art, without departing from the
scope of the technical idea of the present invention.
In Examples, the compound of the general formula (I) is
detected as a mixture of two tautomers represented by the
general formulas (I) and (I') shown below, depending on the
measurement conditions of 114-NMR. The two tautomers are
isomers of the same substance. Therefore, the synthetic
compounds in Examples can be named based on either the
general formula (I) or the general formula (I').
N-NH HN-N
RI RI
Z
r.\
Ar
R2 41-111'. R2 II
(I) )
CA 02888140 2015-04-10
A compound represented by the above general formula (C)
is an important intermediate in the present invention. The
compound of the general formula (C) can exist in tautomeric
forms including a keto form and an enol form, and these
5 tautomeric forms are the same substance. Therefore, such
an intermediate can be named based on any of the general
formula (C), the general formula (C'), and the general
formula (C").
0 OH 0 0 OH 0
Arl Ar2 Arl Ar2 Arl
Ar2
(C') (C) (C'')
10 The following abbreviations are also used herein.
Table 1
Abbreviation Reagent, solvent, etc.
AcOEt Ethyl acetate
AcOH Acetic acid
CHCI3 Chloroform
IBCF lsobutyl chloroformate
K2003 Potassium carbonate
LAH Lithium aluminium hydride
MgSO4 Magnesium sulfate
Na2SO4 Sodium sulfate
NaHCO3 Sodium hydrogen carbonate
NaOH Sodium hydroxide
PTLC Preparative thin-layer chromatography
THF Tetrahydrofuran
Example 1: Synthesis of 4-[2-[4-[(E)-2-[5-[(E)-2-(1H-indo1-
6-yl)viny11-1H-pyrazol-3-yl]vinyl]phenoxy]ethylimorpholine
15 Example 1-1: Synthesis of (1E,6E)-1-(1H-indo1-6-y1)-7-[4-
(2-morpholinoethoxy)phenyl]hepta-1,6-diene-3,5-dione
To 2.5 g of (E)-6-(1H-indo1-6-yl)hex-5-ene-2,4-dione
were added 44 mL of AcOEt and 1.07 g of boron oxide, and
CA 02888140 2015-04-10
41
the mixture was stirred at 70 C for 40 minutes. To this,
2.27 g of 4-(2-morpholinoethoxy)benzaldehyde and 7.07 mL of
tributyl borate were successively added, and the mixture
was stirred at the same temperature for 40 minutes. Then,
0.11 mL of piperidine was added, and the mixture was
stirred at 70 C for 1.5 hours and was allowed to cool back
to room temperature. To the reaction mixture, 22 mL of a
20% K2CO3 aqueous solution and 20 mL of THF were added and
the mixture was stirred at room temperature for 10 minutes.
The organic layer was separated, washed with saturated
brine, dried over MgSO4, and concentrated. The residue was
purified by silica gel column chromatography (CHC13/acetone
or CHC13/methanol system) to give 1.62 g of the title
compound (33% yield).
Example 1-2: Synthesis of 4-[2-[4-[(E)-2-[5-[(E)-2-(1H-
indo1-6-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
To 1.5 g of (1E,6E)-1-(1H-indo1-6-y1)-7-[4-(2-
morpholinoethoxy)phenyl]hepta-1,6-diene-3,5-dione were
added 13.5 mL of AcOH and 1.31 mL of hydrazine monohydrate,
and the mixture was stirred at 60 C for 3 hours. The
reaction mixture was allowed to cool back to room
temperature and poured into a stirring mixture of 18.7 g of
K2CO3, 95 mL of cold water, 30 mL of AcOEt and 20 mL of THF.
The organic layer was separated, washed with saturated
brine, dried over MgSO4, and concentrated. The residue was
purified by silica gel column chromatography (CHC13/acetone
CA 02888140 2015-04-10
42
or CHC13/methanol system) to give 0.62 g of the title
compound (42% yield).
Example 2: Synthesis of 4-[2-[4-[(E)-2-[5-[(E)-2-(1,3-
benzodioxo1-5-yl)vinyl]-1H-pyrazol-3-yl]viny1]-3-
methoxyphenoxy]ethyl]morpholine
Example 2-1: Synthesis of (1E,6E)-1-(1,3-benzodioxo1-5-y1)-
7-[2-methoxy-4-(2-morpholinoethoxy)phenyl]hepta-1,6-diene-
3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 2-2: Synthesis of 4-[2-[4-[(E)-2-[5-[(E)-2-(1,3-
benzodioxo1-5-yl)vinyl]-1H-pyrazol-3-yl]viny1]-3-
methoxyphenoxy]ethyl]morpholine
The title compound was obtained in the same manner as
in Example 1-2.
Example 3: Synthesis of 4-[2-[4-[(E)-2-[5-[(E)-2-(1,3-
benzodioxo1-5-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
Example 3-1: Synthesis of (1E,6E)-1-(1,3-benzodioxo1-5-y1)-
7-[4-(2-morpholinoethoxy)phenyl]hepta-1,6-diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
CA 02888140 2015-04-10
43
Example 3-2: Synthesis of 4-[2-[4-[(E)-2-[5-[(E)-2-(1,3-
benzodioxo1-5-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
The title compound was obtained in the same manner as
in Example 1-2.
Example 4: Synthesis of 6-[(E)-2-[3-[(E)-2-[2-methoxy-4-
[(4-methylpiperazin-1-yl)methyl]phenyl]viny11-1H-pyrazol-5-
yl]viny1]-1H-indole
Example 4-1: Synthesis of 2-methoxy-4-[(4-methylpiperazin-
1-yl)methyl]benzoic acid methyl ester
To 100 mL of ethanol were added 10.0 g of 1-
methylpiperazine and 20.2 g of triethylamine. To the
mixture cooled with ice, 25 mL of a solution of 24.6 g of
4-(bromomethyl)-2-methoxy-benzoic acid methyl ester in
ethanol was added dropwise. The mixture was stirred at
room temperature for 18 hours. The reaction mixture was
concentrated. To the residue, 75 mL of water and 250 mL of
methylene chloride were added, and the organic layer was
separated. The obtained organic layer was dried over Na2SO4
and concentrated. The residue was purified by amino-silica
gel column chromatography (heptane/AcOEt system) to give
22.4 g of the title compound (85% yield).
Example 4-2: Synthesis of [2-methoxy-4-[(4-methylpiperazin-
l-yl)methyl]phenyl]methanol
To 200 mL of a suspension of 5.21 g of LAH in THF
cooled with ice was added dropwise, under argon flow, 50 mL
CA 02888140 2015-04-10
44
of a solution of 25.5 g of 2-methoxy-4-[(4-methylpiperazin-
1-yl)methyl]benzoic acid methyl ester in THF. The mixture
was stirred under ice cooling for 1 hour and further
stirred at room temperature for 22 hours. To the reaction
mixture cooled with ice, 25 mL of a 10% NaOH water was
added dropwise. After 30-minute stirring, the mixture was
filtered through Celite. The filtrate was washed with 150
mL of THF and then was concentrated. To the residue, 50 mL
of saturated brine and 300 mL of dichloromethane were added,
and the organic layer was separated. The organic layer was
concentrated. The residue was purified by silica gel
column chromatography (CHC13/methanol system) to give 22.4
g of the title compound (98% yield).
Example 4-3: Synthesis of 2-methoxy-4-[(4-methylpiperazin-
l-yl)methyl]benzaldehyde
To 220 mL of a solution of 22.2 g of [2-methoxy-4-[(4-
methylpiperazin-1-yl)methyl]phenyllmethanol in CHC13 was
added 61.8 g of manganese dioxide, and the mixture was
stirred at 55 C for 13 hours. The reaction mixture was
allowed to stand to cool. After insoluble matter was
filtered off, the mother liquor was concentrated to give
21.8 g of the title compound (99% yield).
Example 4-4: Synthesis of (1E,6E)-1-(1H-indo1-6-y1)-7-[2-
methoxy-4-[(4-methylpiperazin-l-yl)methyl]phenyl]hepta-1,6-
diene-3,5-dione
To 200 mL of a solution of 7.56 g of (E)-6-(1H-indo1-6-
yl)hex-5-ene-2,4-dione in AcOEt was added 3.24 g of boron
CA 02888140 2015-04-10
oxide, and the mixture was stirred at 70 C for 1 hour. To
this, 15 mL of a solution of 8.68 g of 2-methoxy-4-[(4-
methylpiperazin-l-yl)methyl]benzaldehyde in AcOEt and 18.3
mL of triisopropyl borate were successively added, and the
5 mixture was stirred at the same temperature for 40 minutes.
Then, 10 mL of a solution of 0.35 mL of piperidine in AcOEt
was added. The mixture was stirred at 70 C for 3 hours and
was allowed to cool back to room temperature. To the
reaction mixture, 100 mL of a 10% K2CO3 aqueous solution
10 was added, and the mixture was stirred for 1 hour. The
organic layer was separated and concentrated. The residue
was purified by amino-silica gel column chromatography
(heptane/AcOEt system) to give 7.14 g of the title compound
(47% yield).
Example 4-5: Synthesis of 6-[(E)-2-[3-[(E)-2-[2-methoxy-4-
[(4-methylpiperazin-l-yl)methyl]phenyl]viny11-1H-pyrazol-5-
yl]viny1]-1H-indole
The title compound was obtained in the same manner as
in Example 1-2.
Example 5: Synthesis of 3,5-bis[(E)-2-[4-(2-
morpholinoethoxy)phenyl]vinyl]-1H-pyrazole
Example 5-1: Synthesis of (1E,6E)-1,7-bis[4-(2-
morpholinoethoxy)phenyl]hepta-1,6-diene-3,5-dione
To 2.0 g of pentane-2,4-dione were added 10.0 mL of
AcOEt and 0.70 g of boron oxide, and the mixture was
stirred at 70 C for 1 hour. To the reaction mixture, 30 mL
CA 02888140 2015-04-10
46
of a solution of 9.4 g of 4-(2-
morpholinoethoxy)benzaldehyde in AcOEt and 10.7 mL of
tributyl borate were successively added, and the mixture
was stirred at the same temperature for 1 hour. To this,
0.4 mL of 1-butylamine was added, and the mixture was
stirred at 70 C for 2.5 hours and was allowed to cool back
to room temperature. To the reaction mixture, 50 mL of a
10% K2CO3 aqueous solution and 30 mL of THF were added, and
the mixture was stirred at room temperature for 10 minutes.
The organic layer was separated, washed with saturated
brine, dried over MgSO4, and concentrated. The residue was
treated with 100 mL of diethyl ether to give 2.51 g of the
title compound as a powder (24% yield).
Example 5-2: Synthesis of 3,5-bis[(E)-2-[4-(2-
morpholinoethoxy)phenyl]viny1]-1H-pyrazole
The title compound was obtained in the same manner as
in Example 1-2.
Example 6: Synthesis of N,N-dimethy1-2-[4-[(E)-2-[3-[(E)-2-
[4-(2-morpholinoethoxy)phenyl]viny1]-1H-pyrazol-5-
yl]vinyl]phenoxy]acetamide
Example 6-1: Synthesis of (E)-6-[4-(2-
morpholinoethoxy)phenyl]hex-5-ene-2,4-dione
To 30.0 g of (E)-3-[4-(2-morpholinoethoxy)pheny1]-2-
propenoic acid was added 600 mL of THF under argon flow.
To the mixture cooled with ice, 12.1 g of triethylamine and
15.5 g of IBCF were successively added dropwise. The
CA 02888140 2015-04-10
47
mixture was stirred at room temperature for 2 hours to
prepare an acid anhydride. In a separate container, 200 mL
of THF and 16.3 g of acetylacetone were added to 15.5 g of
magnesium chloride under argon flow. To the mixture cooled
with ice, 17.5 mL of pyridine was added dropwise over 25
minutes. The mixture was stirred at room temperature for 2
hours. To the reaction mixture cooled with ice, the above-
prepared acid anhydride cooled with ice was added dropwise
over 45 minutes. The mixture was stirred at room
temperature overnight. The reaction mixture was added to 2
L of a saturated aqueous ammonium chloride solution and 2 L
of CHC13, and the organic layer was separated. The
obtained organic layer was dried over Na2SO4 and
concentrated.
The residue was dissolved in 100 mL of THF. While the
mixture was stirred under ice cooling, 40 mL of a 28%
ammonia water was added dropwise. The mixture was stirred
at the same temperature for 1 hour. The reaction mixture
was added to 1 L of CHC13 and 1 L of saturated brine, and
the organic layer was separated. The obtained organic
layer was dried over Na2SO4 and concentrated. The residue
was purified by silica gel column chromatography
(CHC13/methanol system) to give 26.5 g of the title
compound (77% yield).
Example 6-2: Synthesis of N,N-dimethy1-2-[4-[(1E,6E)-7-[4-
(2-morpholinoethoxy)pheny1]-3,5-dioxo-hepta-1,6-
dienyllphenoxylacetamide
CA 02888140 2015-04-10
48
The title compound was obtained in the same manner as
in Example 4-4.
Example 6-3: Synthesis of N,N-dimethy1-2-[4-[(E)-2-[3-[(E)-
2-[4-(2-morpholinoethoxy)phenyl]viny1]-1H-pyrazol-5-
yl]vinyl]phenoxy]acetamide
To 2.70 g of N,N-dimethy1-2-[4-[(1E,6E)-7-[4-(2-
morpholinoethoxy)pheny1]-3,5-dioxo-hepta-1,6-
dienyl]phenoxy]acetamide were added 2.80 g of hydrazine
dihydrochloride and 54 mL of methanol, and the mixture was
stirred at 40 C overnight. The reaction mixture was
allowed to stand to cool, and poured into 200 mL of a
saturated aqueous NaHCO3 solution. To this, 600 mL of
AcOEt was added, and the organic layer was separated. The
obtained organic layer was washed with saturated brine,
dried over Na2SO4, and concentrated. The residue was
purified by silica gel column chromatography
(CHC13/methanol system) to give 1.76 g of the title
compound (66% yield).
Example 7: Synthesis of 4-[2-[4-[(E)-2-[5-[(E)-2-(3,4-
dimethoxyphenyl)viny1]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
Example 7-1: Synthesis of (1E,6E)-1-(3,4-dimethoxypheny1)-
7-[4-(2-morpholinoethoxy)phenyl]hepta-1,6-diene-3,5-dione
The title compound was obtained in the same manner as
in Example 4-4.
CA 02888140 2015-04-10
49
Example 7-2: Synthesis of 4-[2-[4-[(E)-2-[5-[(E)-2-(3,4-
dimethoxyphenyl)viny1]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
The title compound was obtained in the same manner as
in Example 6-3.
Example 8: Synthesis of 4-[2-[4-[(E)-2-[5-[(E)-2-(3H-
benzimidazol-5-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
Example 8-1: Synthesis of (1E,6E)-1-(3H-benzimidazol-5-y1)-
7-[4-(2-morpholinoethoxy)phenyl]hepta-1,6-diene-3,5-dione
The title compound was obtained in the same manner as
in Example 4-4.
Example 8-2: Synthesis of 4-[2-[4-[(E)-2-[5-[(E)-2-(3H-
benzimidazol-5-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
The title compound was obtained in the same manner as
in Example 6-3.
Example 9: Synthesis of 2-[[3-methoxy-4-[(E)-2-[5-[(E)-2-
(1H-pyrrol-2-y1)yinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
Example 9-1: Synthesis of (1E,6E)-1-[2-methoxy-4-(2-
pyridylmethoxy)pheny1]-7-(1H-pyrrol-2-yl)hepta-1,6-diene-
3,5-dione
CA 02888140 2015-04-10
The title compound was obtained in the same manner as
in Example 1-1.
Example 9-2: Synthesis of 2-[[3-methoxy-4-[(E)-2-[5-[(E)-2-
5 (1H-pyrrol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
To 30 mg of (1E,6E)-1-[2-methoxy-4-(2-
pyridylmethoxy)pheny1]-7-(1H-pyrrol-2-yl)hepta-1,6-diene-
3,5-dione were added 1 mL of THE, 3 L of AcOH and 4 L of
10 hydrazine monohydrate, and the mixture was stirred at 60 C
for 4 hours. To this, 500 L of AcOH was added and the
mixture was stirred at 80 C for 1 hour. To this, 4 L of
hydrazine monohydrate was added and the mixture was stirred
at 80 C for 1 hour. The reaction mixture was allowed to
15 cool back to room temperature, and a saturated aqueous
NaHCO3 solution was added. After extraction with AcOEt,
the organic layer was separated. The obtained organic
layer was washed with saturated brine, dried over MgS01,
and concentrated. The residue was purified by PTLC
20 (CHC13/methanol or AcOEt system) to give 8 mg of the title
compound (27% yield).
Example 10: Synthesis of 3-benzyloxy-N,N-diethy1-4-[(E)-2-
[5-[(E)-2-(1-methylindo1-6-y1)viny11-1H-pyrazol-3-
25 yl]vinyl]aniline
Example 10-1: Synthesis of (E)-6-(1-methylindo1-6-yl)hex-5-
ene-2,4-dione
CA 02888140 2015-04-10
51
The title compound was obtained in the same manner as
in Example 6-1.
Example 10-2: Synthesis of (1E,6E)-1-[2-benzyloxy-4-
(diethylamino)pheny1]-7-(1-methylindo1-6-y1)hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 10-3: Synthesis of 3-benzyloxy-N,N-diethy1-4-[(E)-
2-[5-[(E)-2-(1-methylindo1-6-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]aniline
The title compound was obtained in the same manner as
in Example 9-2.
Example 11: Synthesis of N,N-diethy1-4-[(E)-2-[5-[(E)-2-(1-
methylindo1-6-y1)vinyl]-1H-pyrazol-3-yl]viny1]-3-(2-
morpholinoethoxy)aniline
Example 11-1: Synthesis of (1E,6E)-1-[4-(diethylamino)-2-
(2-morpholinoethoxy)pheny11-7-(1-methylindo1-6-y1)hepta-
1,6-diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 11-2: Synthesis of N,N-diethy1-4-[(E)-2-[5-[(E)-2-
(1-methylindo1-6-y1)vinyl]-1H-pyrazol-3-yl]viny1]-3-(2-
morpholinoethoxy)aniline
CA 02888140 2015-04-10
52
The title compound was obtained in the same manner as
in Example 9-2.
Example 12: Synthesis of 2-[(E)-2-[5-[(E)-2-(1-methylindol-
6-yl)viny1]-1H-pyrazol-3-yl]viny1]-5-(2-
pyridylmethoxy)phenol
Example 12-1: Synthesis of (1E,6E)-1-[2-hydroxy-4-(2-
pyridylmethoxy)pheny1]-7-(1-methylindol-6-yl)hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 12-2: Synthesis of 2-[(E)-2-[5-[(E)-2-(1-
methylindo1-6-yl)vinyl]-1H-pyrazol-3-yl]viny1]-5-(2-
pyridylmethoxy)phenol
The title compound was obtained in the same manner as
in Example 9-2.
Example 13: Synthesis of 4-[2-[2-[(E)-2-[5-[(E)-2-(1H-
indo1-6-yl)vinyl]-1H-pyrazol-3-yl]viny1]-5-(2-
pyridylmethoxy)phenoxy]ethyl]morpholine
Example 13-1: Synthesis of 2-(2-morpholinoethoxy)-4-(2-
pyridylmethoxy)benzaldehyde
To 1.0 g of 2-hydroxy-4-(2-pyridylmethoxy)benzaldehyde
were successively added 30 mL of acetonitrile, 4.26 g of
cesium carbonate and 0.85 g of 4-(2-chloroethyl)morpholine
hydrochloride. The mixture was stirred at 90 C for 2 hours.
CA 02888140 2015-04-10
53
To the reaction mixture, 50 mL of water and 150 mL of AcOEt
were added, and the organic layer was separated. The
obtained organic layer was dried over Na2SO4 and
concentrated to give 1.56 g of the title compound in
quantitative yield.
Example 13-2: (1E,6E)-1-(1H-indo1-6-y1)-7-[2-(2-
morpholinoethoxy)-4-(2-pyridylmethoxy)phenyl]hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 13-3: Synthesis of 4-[2-[2-[(E)-2-[5-[(E)-2-(1H-
indo1-6-yl)vinyl]-1H-pyrazol-3-yl]viny1]-5-(2-
pyridylmethoxy)phenoxy]ethyl]morpholine
The title compound was obtained in the same manner as
in Example 9-2.
Example 14: Synthesis of 4-[2-[3-methoxy-4-[(E)-2-[5-[(E)-
2-(1-methylindo1-6-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
Example 14-1: Synthesis of (1E,6E)-1-[2-methoxy-4-(2-
morpholinoethoxy)pheny1]-7-(1-methylindol-6-yl)hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
CA 02888140 2015-04-10
54
Example 14-2: Synthesis of 4-[2-[3-methoxy-4-[(E)-2-[5-
[(E)-2-(1-methylindo1-6-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
The title compound was obtained in the same manner as
in Example 1-2.
Example 15: Synthesis of 4-[2-[4-[(E)-2-[5-[(E)-2-(1-
methylindo1-6-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
Example 15-1: Synthesis of (1E,6E)-1-(1-methylindo1-6-y1)-
7-[4-(2-morpholinoethoxy)phenyl]hepta-1,6-diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 15-2: Synthesis of 4-[2-[4-[(E)-2-[5-[(E)-2-(1-
methylindo1-6-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
The title compound was obtained in the same manner as
in Example 1-2.
Example 16: Synthesis of 6-[(E)-2-[3-[(E)-2-[2-fluoro-4-(2-
pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-yl]viny1]-1-
methylindole
Example 16-1: Synthesis of (1E,6E)-1-[2-fluoro-4-(2-
pyridylmethoxy)pheny1]-7-(1-methylindol-6-yl)hepta-1,6-
diene-3,5-dione
CA 02888140 2015-04-10
The title compound was obtained in the same manner as
in Example 1-1.
Example 16-2: Synthesis of 6-[(E)-2-[3-[(E)-2-[2-fluoro-4-
5 (2-pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-yl]viny1]-1-
methylindole
The title compound was obtained in the same manner as
in Example 1-2.
10 Example 17: Synthesis of 1-methy1-6-[(E)-2-[3-[(E)-2-[4-(2-
pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-yl]vinyl]indole
Example 17-1: Synthesis of (1E,6E)-1-(1-methylindo1-6-y1)-
7-[4-(2-pyridylmethoxy)phenyl]hepta-1,6-diene-3,5-dione
15 The title compound was obtained in the same manner as
in Example 1-1.
Example 17-2: Synthesis of 1-methy1-6-[(E)-2-[3-[(E)-2-[4-
(2-pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-
20 yl]vinyl]indole
The title compound was obtained in the same manner as
in Example 1-2.
Example 18: Synthesis of 1-methy1-6-[(E)-2-[3-[(E)-2-[4-(4-
25 methylpiperazin-1-yl)phenyl]viny1]-1H-pyrazol-5-
yl]vinyl]indole
CA 02888140 2015-04-10
56
Example 18-1: Synthesis of (1E,6E)-1-(1-methylindo1-6-y1)-
7-[4-(4-methylpiperazin-1-yl)phenyl]hepta-1,6-diene-3,5-
dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 18-2: Synthesis of 1-methy1-6-[(E)-2-[3-[(E)-2-[4-
(4-methylpiperazin-1-yl)phenyl]viny1]-1H-pyrazol-5-
yl]vinyl]indole
The title compound was obtained in the same manner as
in Example 1-2.
Example 19: Synthesis of 2-[[3-fluoro-4-[(E)-2-[5-[(E)-2-
(1-methylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
ylivinyl]phenoxy]methyl]pyridine
Example 19-1: Synthesis of (E)-3-[2-fluoro-4-(2-
pyridylmethoxy)pheny1]-2-propenoic acid
To 1.8 g of malonic acid were added 18 mL of pyridine,
2.0 g of 2-fluoro-4-(2-pyridylmethoxy)benzaldehyde and 0.21
mL of piperidine. The mixture was stirred at 75 C for 1.5
hours and further stirred at 95 C for 1 hour. The reaction
mixture was cooled with ice, water was added thereto, and
10% hydrochloric acid was added to adjust the pH to 4. The
resulting precipitate was separated by filtration to give
2.0 g of the title compound (85% yield).
Example 19-2: Synthesis of (E)-6-[2-fluoro-4-(2-
pyridylmethoxy)phenyl]hex-5-ene-2,4-dione
CA 02888140 2015-04-10
57
The title compound was obtained in the same manner as
in Example 6-1.
Example 19-3: Synthesis of (1E,6E)-1-[2-fluoro-4-(2-
pyridylmethoxy)pheny1]-7-(1-methylpyrrol-2-y1)hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 19-4: Synthesis of 2-[[3-fluoro-4-[(E)-2-[5-[(E)-2-
(1-methylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
The title compound was obtained in the same manner as
in Example 1-2.
Example 20: Synthesis of 2-[[4-[(E)-2-[5-[(E)-2-(1-
methylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
Example 20-1: Synthesis of (E)-6-[4-(2-
pyridylmethoxy)phenyl]hex-5-ene-2,4-dione
The title compound was obtained in the same manner as
in Example 6-1.
Example 20-2: Synthesis of (1E,6E)-1-(1-methylpyrrol-2-y1)-
7-[4-(2-pyridylmethoxy)phenyl]hepta-1,6-diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
CA 02888140 2015-04-10
58
Example 20-3: Synthesis of 2-[[4-[(E)-2-[5-[(E)-2-(1-
methylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
The title compound was obtained in the same manner as
in Example 1-2.
Example 21: Synthesis of 4-[2-[3-methoxy-4-[(E)-2-[5-[(E)-
2-(1-methylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
Example 21-1: Synthesis of (1E,6E)-1-[2-methoxy-4-(2-
morpholinoethoxy)pheny1]-7-(1-methylpyrrol-2-yl)hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 21-2: Synthesis of 4-[2-[3-methoxy-4-[(E)-2-[5-
[(E)-2-(1-methylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
The title compound was obtained in the same manner as
in Example 1-2.
Example 22: Synthesis of 2-[[3-methoxy-4-[(E)-2-[5-[(E)-2-
(1-methylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
Example 22-1: Synthesis of (1E,6E)-1-[2-methoxy-4-(2-
pyridylmethoxy)pheny1]-7-(1-methylpyrrol-2-yl)hepta-1,6-
diene-3,5-dione
CA 02888140 2015-04-10
59
The title compound was obtained in the same manner as
in Example 1-1.
Example 22-2: Synthesis of 2-[[3-methoxy-4-[(E)-2-[5-[(E)-
2-(1-methylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
The title compound was obtained in the same manner as
in Example 1-2.
Example 23: Synthesis of 2-[[4-[(E)-2-[5-[(E)-2-(1,5-
dimethylpyrrol-2-yl)vinyl]-1H-pyrazol-3-yl]viny1]-3-
methoxyphenoxy]methyl]pyridine
Example 23-1: Synthesis of (1E,6E)-1-(1,5-dimethylpyrrol-2-
y1)-7-[2-methoxy-4-(2-pyridylmethoxy)phenyl]hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 23-2: Synthesis of 2-[[4-[(E)-2-[5-[(E)-2-(1,5-
dimethylpyrrol-2-yl)vinyl]-1H-pyrazol-3-yl]viny1]-3-
methoxyphenoxy]methyl]pyridine
The title compound was obtained in the same manner as
in Example 1-2.
Example 24: Synthesis of 2-[[3-methoxy-4-[(E)-2-[5-[(E)-2-
(1,3,5-trimethylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
CA 02888140 2015-04-10
Example 24-1: Synthesis of (1E,6E)-1-[2-methoxy-4-(2-
pyridylmethoxy)pheny1]-7-(1,3,5-trimethylpyrrol-2-yl)hepta-
1,6-diene-3,5-dione
The title compound was obtained in the same manner as
5 in Example 1-1.
Example 24-2: Synthesis of 2-[[3-methoxy-4-[(E)-2-[5-[(E)-
2-(1,3,5-trimethylpyrrol-2-yl)viny11-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
10 The title compound was obtained in the same manner as
in Example 1-2.
Example 25: Synthesis of 2-[(E)-2-[3-[(E)-2-[2-methoxy-4-
(2-pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-yl]viny1]-6-
15 methylpyridine
Example 25-1: Synthesis of (1E,6E)-1-[2-methoxy-4-(2-
pyridylmethoxy)pheny1]-7-(6-methy1-2-pyridyl)hepta-1,6-
diene-3,5-dione
20 The title compound was obtained in the same manner as
in Example 1-1.
Example 25-2: Synthesis of 2-[(E)-2-[3-[(E)-2-[2-methoxy-4-
(2-pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-yl]viny1]-6-
25 methylpyridine
The title compound was obtained in the same manner as
in Example 1-2.
CA 02888140 2015-04-10
61
Example 26: Synthesis of 3-[(E)-2-[3-[(E)-2-[2-methoxy-4-
(2-pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-yl]viny1]-2-
methylpyridine
Example 26-1: Synthesis of (1E,6E)-1-[2-methoxy-4-(2-
pyridylmethoxy)pheny1]-7-(2-methyl-3-pyridyl)hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 26-2: Synthesis of 3-[(E)-2-[3-[(E)-2-[2-methoxy-4-
(2-pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-yl]viny1]-2-
methylpyridine
The title compound was obtained in the same manner as
in Example 1-2.
Example 27: Synthesis of 1-methy1-4-[4-[(E)-2-[5-[(E)-2-(1-
methylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenyl]piperazine
Example 27-1: Synthesis of (E)-6-(1-methylpyrrol-2-yl)hex-
5-ene-2,4-dione
The title compound was obtained in the same manner as
in Example 6-1.
Example 27-2: Synthesis of (1E,6E)-1-[4-(4-methylpiperazin-
l-yl)pheny1]-7-(1-methylpyrrol-2-yl)hepta-1,6-diene-3,5-
dione
CA 02888140 2015-04-10
62
The title compound was obtained in the same manner as
in Example 1-1.
Example 27-3: Synthesis of 1-methy1-4-[4-[(E)-2-[5-[(E)-2-
(1-methylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
ylivinyl]phenyl]piperazine
The title compound was obtained in the same manner as
in Example 1-2.
Example 28: Synthesis of 1-methy1-4-[4-[(E)-2-[5-[(E)-2-(1-
methylpyrrol-2-y1)vinyl]-1H-pyrazol-3-yl]vinyl]pheny11-1,4-
diazepane
Example 28-1: Synthesis of (1E,6E)-1-[4-(4-methy1-1,4-
diazepan-l-yl)phenyl]-7-(1-methylpyrrol-2-yl)hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 28-2: Synthesis of 1-methy1-4-[4-[(E)-2-[5-[(E)-2-
(1-methylpyrrol-2-y1)vinyl]-1H-pyrazol-3-yl]vinyl]pheny1]-
1,4-diazepane
The title compound was obtained in the same manner as
in Example 1-2.
Example 29: Synthesis of 3-[(E)-2-(4-isopropoxy-2-
methoxyphenyl)viny11-5-[(E)-2-(1-methylpyrrol-2-yl)viny1]-
1H-pyrazole
CA 02888140 2015-04-10
63
Example 29-1: Synthesis of (1E,6E)-1-(4-isopropoxy-2-
methoxypheny1)-7-(1-methylpyrrol-2-yl)hepta-1,6-diene-3,5-
dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 29-2: Synthesis of 3-[(E)-2-(4-isopropoxy-2-
methoxyphenyl)viny1]-5-[(E)-2-(1-methylpyrrol-2-yl)viny11-
1H-pyrazole
The title compound was obtained in the same manner as
in Example 1-2.
Example 30: Synthesis of 1-methy1-4-[2-[4-[(E)-2-[5-[(E)-2-
(1-methylpyrrol-2-y1)vinyl]-1H-pyrazol-3-
yl]vinyl]phenyl]ethyl]piperazine
Example 30-1: Synthesis of 4-[2-(4-methylpiperazin-1-
yl)ethyl]benzaldehyde
To 87 mL of a solution of 8.7 g of 1-[2-(4-
bromophenyl)ethy1]-4-methylpiperazine in THE' cooled
to -70 C was added dropwise 21.1 mL of a solution of n-
butyllithium (1.6 mol/L) in hexane, and the mixture was
stirred at the same temperature for 30 minutes. While the
reaction mixture was stirred at the same temperature, 3.6
mL of N,N-dimethylformamide was added dropwise. The
mixture was stirred at the same temperature for 30 minutes.
The cooling bath was removed, and the mixture was allowed
to warm to -10 C, poured into a mixture of 12 mL of 1 N
hydrochloric acid and 100 mL of cold water, and subjected
CA 02888140 2015-04-10
64
to extraction with CHC13. The obtained extract was dried
over MgSO4 and concentrated to give 7.04 g of the title
compound (98%).
Example 30-2: Synthesis of (1E,6E)-1-[4-[2-(4-
methylpiperazin-l-yl)ethyl]phenyl]-7-(1-methylpyrrol-2-
yl)hepta-1,6-diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 30-3: Synthesis of 1-methy1-4-[2-[4-[(E)-2-[5-[(E)-
2-(1-methylpyrrol-2-y1)vinyll-1H-pyrazol-3-
yl]vinyl]phenyllethyllpiperazine
The title compound was obtained in the same manner as
in Example 1-2.
Example 31: Synthesis of 2-[2-[3-methoxy-4-[(E)-2-[5-[(E)-
2-(1-methylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]pyridine
Example 31-1: Synthesis of 2-methoxy-4-[2-(2-
pyridyl)ethoxy]benzaldehyde
To a stirring ice-cooled mixture of 5.0 g of 4-hydroxy-
2-methoxybenzaldehyde, 4.5 g of 2-(2-pyridyl)ethanol and
10.3 g of triphenylphosphine was added dropwise 15.8 g of a
40% solution of diethyl azodicarboxylate in toluene. The
mixture was stirred at room temperature for 5 hours. The
reaction mixture was concentrated, purified by silica gel
column chromatography (hexane/acetone system) and further
CA 02888140 2015-04-10
purified by silica gel column chromatography (hexane/AcOEt
system) to give 5.84 g of the title compound (69% yield).
Example 31-2: Synthesis of (1E,6E)-1-[2-methoxy-4-[2-(2-
5 pyridyl)ethoxy]pheny1]-7-(1-methylpyrrol-2-yl)hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
10 Example 31-3: Synthesis of 2-[2-[3-methoxy-4-[(E)-2-[5-
[(E)-2-(1-methylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]pyridine
The title compound was obtained in the same manner as
in Example 1-2.
Example 32: Synthesis of 2-[[3-methy1-4-[(E)-2-[5-[(E)-2-
(1-methylpyrrol-2-y1)vinyll-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
Example 32-1: Synthesis of 2-methy1-4-(2-
pyridylmethoxy)benzoic acid methyl ester
To 0.85 g of 4-hydroxy-2-methyl benzoic acid methyl
ester were added 12 mL of acetonitrile, 2.12 g of K2CO3 and
0.84 g of 2-(chloromethyl)pyridine hydrochloride, and the
mixture was stirred at 90 C for 4 hours. The reaction
mixture was allowed to cool back to room temperature and
neutralized by the addition of 5% hydrochloric acid. To
this, 150 mL of AcOEt was added, and the organic layer was
separated. The obtained organic layer was washed with
CA 02888140 2015-04-10
66
saturated brine, dried over MgSO4, and concentrated. The
residue was purified by silica gel column chromatography
(hexane/AcOEt system) to give 1.18 g of the title compound
(89% yield).
Example 32-2: Synthesis of [2-methy1-4-(2-
pyridylmethoxy)phenyl]methanol
To 1.0 g of 2-methyl-4-(2-pyridylmethoxy)benzoic acid
methyl ester was added 8 mL of toluene under argon
atmosphere. To the mixture cooled to -78 C, 8.7 mL of a
solution of diisobutylaluminium hydride (1.0 mol/L) in
toluene was added dropwise. The mixture was stirred at the
same temperature for 6 hours and further stirred at -35 C
for 1 hour. To the reaction mixture, a saturated aqueous
Rochelle salt solution was added, and the mixture was
stirred at room temperature for 30 minutes. To this, 150
mL of diethyl ether was added, and the organic layer was
separated. The obtained organic layer was dried over Na2SO4
and concentrated to give 0.83 g of the title compound (93%
yield).
Example 32-3: Synthesis of 2-methy1-4-(2-
pyridylmethoxy)benzaldehyde
To 0.72 g of [2-methyl-4--(2-
pyridylmethoxy)phenylimethanol were added 30 mL of CHC13
and 7.2 g of manganese dioxide, and the mixture was stirred
at room temperature for 2 hours. Insoluble matter was
filtered off from the reaction mixture, and the mother
CA 02888140 2015-04-10
67
liquor was concentrated to give 0.70 g of the title
compound (99% yield).
Example 32-4: Synthesis of (1E,6E)-1-[2-methy1-4-(2-
pyridylmethoxy)pheny1]-7-(1-methylpyrrol-2-y1)hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 32-5: Synthesis of 2-[[3-methy1-4-[(E)-2-[5-[(E)-2-
(1-methylpyrrol-2-y1)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
The title compound was obtained in the same manner as
in Example 1-2.
Example 33: Synthesis of 2-[2-[3-[(E)-2-[5-[(E)-2-(1-
methylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]pyridine
Example 33-1: Synthesis of (1E,6E)-1-(1-methylpyrrol-2-y1)-
7-[3-[2-(2-pyridyflethoxy]phenyl]hepta-1,6-diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 33-2: Synthesis of 2-[2-[3-[(E)-2-[5-[(E)-2-(1-
methylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]pyridine
The title compound was obtained in the same manner as
in Example 1-2.
CA 02888140 2015-04-10
68
Example 34: Synthesis of 2-[[3,5-dimethoxy-4-[(E)-2-[5-
[(E)-2-(1-methylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
Example 34-1: Synthesis of 2,6-dimethoxy-4-(2-
pyridylmethoxy)benzaldehyde
The title compound was obtained in the same manner as
in Example 32-1.
Example 34-2: Synthesis of (1E,6E)-1-[2,6-dimethoxy-4-(2-
pyridylmethoxy)pheny1]-7-(1-methylpyrrol-2-yl)hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 34-3: Synthesis of 2-[[3,5-dimethoxy-4-[(E)-2-[5-
[(E)-2-(1-methylpyrrol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
The title compound was obtained in the same manner as
in Example 1-2.
Example 35: Synthesis of 2-[[3-methoxy-4-[(E)-2-[5-[(E)-2-
(1-methylimidazol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
Example 35-1: Synthesis of (1E,6E)-1-[2-methoxy-4-(2-
pyridylmethoxy)pheny1]-7-(1-methylimidazol-2-yl)hepta-1,6-
diene-3,5-dione
CA 02888140 2015-04-10
69
The title compound was obtained in the same manner as
in Example 1-1.
Example 35-2: Synthesis of 2-[[3-methoxy-4-[(E)-2-[5-[(E)-
2-(1-methylimidazol-2-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
The title compound was obtained in the same manner as
in Example 1-2.
Example 36: Synthesis of 2-[[3-methoxy-4-[(E)-2-[5-[(E)-2-
(3-methylimidazol-4-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
Example 36-1: Synthesis of (1E,6E)-1-[2-methoxy-4-(2-
pyridylmethoxy)pheny1]-7-(3-methylimidazol-4-y1)hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 36-2: Synthesis of 2-[[3-methoxy-4-[(E)-2-[5-[(E)-
2-(3-methylimidazol-4-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
The title compound was obtained in the same manner as
in Example 1-2.
Example 37: Synthesis of 2-[(E)-2-[3-[(E)-2-[2-methoxy-4-
(2-pyridylmethoxy)phenyl]viny11-1H-pyrazol-5-
yl]vinyl]pyridine
CA 02888140 2015-04-10
Example 37-1: Synthesis of (1E,6E)-1-[2-methoxy-4-(2-
pyridylmethoxy)pheny1]-7-(2-pyridyl)hepta-1,6-diene-3,5-
dione
The title compound was obtained in the same manner as
5 in Example 1-1.
Example 37-2: Synthesis of 2-[(E)-2-[3-[(E)-2-[2-methoxy-4-
(2-pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-
yl]vinyl]pyridine
10 The title compound was obtained in the same manner as
in Example 1-2.
Example 38: Synthesis of 7-[(E)-2-[3-[(E)-2-[2-methoxy-4-
(2-pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-
15 yl]vinyl]quinoline
Example 38-1: Synthesis of (1E,6E)-1-[2-methoxy-4-(2-
pyridylmethoxy)pheny1]-7-(7-quinolyl)hepta-1,6-diene-3,5-
dione
20 The title compound was obtained in the same manner as
in Example 1-1.
Example 38-2: Synthesis of 7-[(E)-2-[3-[(E)-2-[2-methoxy-4-
(2-pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-
25 yl]vinyl]quinoline
The title compound was obtained in the same manner as
in Example 1-2.
CA 02888140 2015-04-10
71
Example 39: Synthesis of 2-[[4-[(E)-2-[5-[(E)-2-(2-
furyl)viny1]-1H-pyrazol-3-yl]viny1]-3-
methoxyphenoxy]methyl]pyridine
Example 39-1: Synthesis of (1E,6E)-1-(2-fury1)-7-[2-
methoxy-4-(2-pyridylmethoxy)phenyl]hepta-1,6-diene-3,5-
dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 39-2: Synthesis of 2-[[4-[(E)-2-[5-[(E)-2-(2-
furyl)viny1]-1H-pyrazol-3-yl]viny1]-3-
methoxyphenoxy]methyl]pyridine
The title compound was obtained in the same manner as
in Example 1-2.
Example 40: Synthesis of 2-[[3-methoxy-4-[(E)-2-[5-[(E)-2-
(5-methy1-2-furyl)viny1]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
Example 40-1: Synthesis of (1E,6E)-1-[2-methoxy-4-(2-
pyridylmethoxy)pheny1]-7-(5-methyl-2-furyl)hepta-1,6-diene-
3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 40-2: Synthesis of 2-[[3-methoxy-4-[(E)-2-[5-[(E)-
2-(5-methyl-2-furyl)viny1]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
CA 02888140 2015-04-10
72
The title compound was obtained in the same manner as
in Example 1-2.
Example 41: Synthesis of 2-[[4-[(E)-2-[5-[(E)-2-(4,5-
dimethy1-2-furyl)viny1]-1H-pyrazol-3-yl]viny1]-3-
methoxyphenoxy]methyl]pyridine
Example 41-1: Synthesis of (1E,6E)-1-(4,5-dimethy1-2-
fury1)-7-[2-methoxy-4-(2-pyridylmethoxy)phenyl]hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 41-2: Synthesis of 2-[[4-[(E)-2-[5-[(E)-2-(4,5-
dimethy1-2-furyl)viny1]-1H-pyrazol-3-yl]viny1]-3-
methoxyphenoxy]methyl]pyridine
The title compound was obtained in the same manner as
in Example 1-2.
Example 42: Synthesis of 2-[[3-methoxy-4-[(E)-2-[5-[(E)-2-
(2-methylpyrazol-3-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
Example 42-1: Synthesis of (1E,6E)-1-[2-methoxy-4-(2-
pyridylmethoxy)phenyl]-7-(2-methylpyrazol-3-y1)hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
CA 02888140 2015-04-10
73
Example 42-2: Synthesis of 2-[[3-methoxy-4-[(E)-2-[5-[(E)-
2-(2-methylpyrazol-3-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]methyl]pyridine
The title compound was obtained in the same manner as
in Example 1-2.
Example 43: Synthesis of 1-methy1-6-[(E)-2-[3-[(E)-2-[4-(4-
methy1-1,4-diazepan-1-y1)phenyl]vinyl]-1H-pyrazol-5-
yl]vinyl]indole
Example 43-1: Synthesis of (1E,6E)-1-[4-(4-methy1-1,4-
diazepan-l-y1)phenyl]-7-(1-methylindol-6-y1)hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 43-2: Synthesis of 1-methy1-6-[(E)-2-[3-[(E)-2-[4-
(4-methy1-1,4-diazepan-1-yl)phenyl]vinyl]-1H-pyrazol-5-
yl]vinyl]indole
The title compound was obtained in the same manner as
in Example 1-2.
Example 44: Synthesis of 6-[(E)-2-[3-[(E)-2-E2-methoxy-4-
[2-(2-pyridyl)ethoxy]phenyliviny1]-1H-pyrazol-5-yl]viny1]-
1-methylindole
Example 44-1: Synthesis of (1E,6E)-1-[2-methoxy-4-[2-(2-
pyridyl)ethoxy]phenyl]-7-(1-methylindol-6-yl)hepta-1,6-
diene-3,5-dione
CA 02888140 2015-04-10
74
The title compound was obtained in the same manner as
in Example 1-1.
Example 44-2: Synthesis of 6-[(E)-2-[3-[(E)-2-[2-methoxy-4-
[2-(2-pyridyl)ethoxy]phenyl]viny1]-1H-pyrazol-5-yl]viny1]-
1-methylindole
The title compound was obtained in the same manner as
in Example 1-2.
Example 45: Synthesis of 6-[(E)-2-[3-[(E)-2-[2,6-dimethoxy-
4-(2-pyridylmethoxy)phenyllvinyl]-1H-pyrazol-5-yl]viny1]-1-
methylindole
Example 45-1: Synthesis of (1E,6E)-1-[2,6-dimethoxy-4-(2-
pyridylmethoxy)pheny1]-7-(1-methylindo1-6-y1)hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 45-2: Synthesis of 6-[(E)-2-[3-[(E)-2-[2,6-
dimethoxy-4-(2-pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-
yl]viny1]-1-methylindole
The title compound was obtained in the same manner as
in Example 1-2.
Example 46: Synthesis of 6-[(E)-2-[3-[(E)-2-[3-fluoro-4-(2-
pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-ylivinyl]-1-
methylindole
CA 02888140 2015-04-10
Example 46-1: Synthesis of (1E,6E)-1-[3-fluoro-4-(2-
pyridylmethoxy)pheny1]-7-(1-methylindo1-6-yl)hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
5 in Example 1-1.
Example 46-2: Synthesis of 6-[(E)-2-[3-[(E)-2-[3-fluoro-4-
(2-pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-yl]viny1]-1-
methylindole
10 The title compound was obtained in the same manner as
in Example 1-2.
Example 47: Synthesis of 4-[[4-[(E)-2-[5-[(E)-2-(1H-indol-
6-yl)viny1]-1H-pyrazol-3-yl]vinyl]phenyl]methyl]morpholine
Example 47-1: Synthesis of (1E,6E)-1-(1H-indo1-6-y1)-7-[4-
(morpholinomethyl)phenyl]hepta-1,6-diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 47-2: Synthesis of 4-[[4-[(E)-2-[5-[(E)-2-(1H-
indo1-6-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenyl]methyl]morpholine
The title compound was obtained in the same manner as
in Example 1-2.
Example 48: Synthesis of 4-[2-[4-[(E)-2-[5-[(E)-2-(1H-
indo1-6-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenyl]ethyl]morpholine
CA 02888140 2015-04-10
76
Example 48-1: Synthesis of (1E,6E)-1-(1H-indo1-6-y1)-7-[4-
(2-morpholinoethyl)phenyl]hepta-1,6-diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 48-2: Synthesis of 4-[2-[4-[(E)-2-[5-[(E)-2-(1H-
indo1-6-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenyl]ethyl]morpholine
The title compound was obtained in the same manner as
in Example 1-2.
Example 49: Synthesis of 4-[3-[4-[(E)-2-[5-[(E)-2-(1H-
indo1-6-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenyl]propyl]morpholine
Example 49-1: Synthesis of (1E,6E)-1-(1H-indo1-6-y1)-7-[4-
(3-morpholinopropyl)phenyl]hepta-1,6-diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 49-2: Synthesis of 4-[3-[4-[(E)-2-[5-[(E)-2-(1H-
ind01-6-yl)viny11-1H-pyrazol-3-
yl]vinyl]phenyl]propyl]morpholine
The title compound was obtained in the same manner as
in Example 1-2.
Example 50: Synthesis of 6-[(E)-2-[3-[(E)-2-[4-[(4-
methylpiperazin-1-yl)methyl]phenyl]viny1]-1H-pyrazol-5-
yl]viny1]-1H-indole
CA 02888140 2015-04-10
77
Example 50-1: Synthesis of (1E,6E)-1-(1H-indo1-6-y1)-7-[4-
[(4-methylpiperazin-l-yl)methyl]phenyl]hepta-1,6-diene-3,5-
dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 50-2: Synthesis of 6-[(E)-2-[3-[(E)-2-[4-[(4-
methylpiperazin-1-yl)methyl]phenyl]viny1]-1H-pyrazol-5-
yl]viny1]-1H-indole
The title compound was obtained in the same manner as
in Example 1-2.
Example 51: Synthesis of 6-[(E)-2-0-[(E)-2-[4-[2-(4-
methylpiperazin-l-yl)ethyl]phenyl]vinyl]-1H-pyrazol-5-
yl]viny1]-1H-indole
Example 51-1: Synthesis of (1E,6E)-1-(1H-indo1-6-y1)-7-[4-
[2-(4-methylpiperazin-1-yl)ethyl]phenyl]hepta-1,6-diene-
3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 51-2: Synthesis of 6-[(E)-2-[3-[(E)-2-[4-[2-(4-
methylpiperazin-l-yl)ethyl]phenyl]vinyl]-1H-pyrazol-5-
yl]viny1]-1H-indole
The title compound was obtained in the same manner as
in Example 1-2.
CA 02888140 2015-04-10
78
Example 52: Synthesis of 4-[2-[2-[(E)-2-[5-[(E)-2-(1H-
indo1-6-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
Example 52-1: Synthesis of (1E,6E)-1-(1H-indo1-6-y1)-7-[2-
(2-morpholinoethoxy)phenyl]hepta-1,6-diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 52-2: Synthesis of 4-[2-[2-[(E)-2-[5-[(E)-2-(1H-
indo1-6-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
The title compound was obtained in the same manner as
in Example 1-2.
Example 53: Synthesis of 4-[2-[3-[(E)-2-[5-[(E)-2-(1H-
indo1-6-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
Example 53-1: Synthesis of (1E,6E)-1-(1H-indo1-6-y1)-7-[3-
(2-morpholinoethoxy)phenyl]hepta-1,6-diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 53-2: Synthesis of 4-[2-[3-[(E)-2-[5-[(E)-2-(1H-
indo1-6-yl)vinyl]-1H-pyrazol-3-
yl]vinyl]phenoxy]ethyl]morpholine
The title compound was obtained in the same manner as
in Example 1-2.
CA 02888140 2015-04-10
79
Example 54: Synthesis of 6-[(E)-2-[3-[(E)-2-[2-methoxy-4-
[2-(2-pyridyl)ethoxy]phenyl]viny1]-1H-pyrazol-5-yl]viny1]-
1H-indole
Example 54-1: Synthesis of (1E,6E)-1-(1H-indo1-6-y1)-7-[2-
methoxy-4-[2-(2-pyridyl)ethoxy]phenyl]hepta-1,6-diene-3,5-
dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 54-2: Synthesis of 6-[(E)-2-[3-[(E)-2-[2-methoxy-4-
[2-(2-pyridyl)ethoxy]phenyl]viny1]-1H-pyrazol-5-yl]viny1]-
1H-indole
The title compound was obtained in the same manner as
in Example 1-2.
Example 55: Synthesis of 4-[2-[4-[(E)-2-[5-[(E)-2-(2,3-
dihydro-1,4-benzodioxin-6-yl)viny1]-1H-pyrazol-3-yl]viny1]-
3-methoxyphenoxy]ethyl]morpholine
Example 55-1: Synthesis of (E)-6-(2,3-dihydro-1,4-
benzodioxin-6-yl)hex-5-ene-2,4-dione
The title compound was obtained in the same manner as
in Example 6-1.
Example 55-2: Synthesis of (1E,6E)-1-(2,3-dihydro-1,4-
benzodioxin-6-y1)-7-[2-methoxy-4-(2-
morpholinoethoxy)phenyl]hepta-1,6-diene-3,5-dione
CA 02888140 2015-04-10
The title compound was obtained in the same manner as
in Example 1-1.
Example 55-3: Synthesis of 4-[2-[4-[(E)-2-[5-[(E)-2-(2,3-
5 dihydro-1,4-benzodioxin-6-yl)viny1]-1H-pyrazol-3-yl]viny1]-
3-methoxyphenoxy]ethyl]morpholine
The title compound was obtained in the same manner as
in Example 1-2.
10 Example 56: Synthesis of 4-[2-[3-methoxy-4-[(E)-2-[3-[(E)-
2-[2-methoxy-4-(2-morpholinoethoxy)phenyl]viny1]-1H-
pyrazol-5-yl]vinyl]phenoxy]ethyl]morpholine
Example 56-1: Synthesis of (1E,6E)-1,7-bis[2-methoxy-4-(2-
15 morpholinoethoxy)phenyl]hepta-1,6-diene-3,5-dione
The title compound was obtained in the same manner as
in Example 5-1.
Example 56-2: Synthesis of 4-[2-[3-methoxy-4-[(E)-2-[3-
20 [(E)-2-[2-methoxy-4-(2-morpholinoethoxy)phenyl]viny1]-1H-
pyrazol-5-yl]vinyl]phenoxy]ethyl]morpholine
The title compound was obtained in the same manner as
in Example 1-2.
25 Example 57: Synthesis of 1-methyl-2-[(E)-2-[3-[(E)-2-[4-(2-
pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-yl]vinyl]indole
Example 57-1: Synthesis of (1E,6E)-1-(1-methylindo1-2-y1)-
7-[4-(2-pyridylmethoxy)phenyl]hepta-1,6-diene-3,5-dione
CA 02888140 2015-04-10
81
The title compound was obtained in the same manner as
in Example 1-1.
Example 57-2: Synthesis of 1-methy1-2-[(E)-2-[3-[(E)-2-[4-
(2-pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-
yl]vinyl]indole
The title compound was obtained in the same manner as
in Example 1-2.
Example 58: Synthesis of 5-[(E)-2-[3-[(E)-2-[2-methoxy-4-
(2-pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-yl]viny1]-1-
methylindole
Example 58-1: Synthesis of (1E,6E)-1-[2-methoxy-4-(2-
pyridylmethoxy)pheny1]-7-(1-methylindo1-5-y1)hepta-1,6-
diene-3,5-dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 58-2: Synthesis of 5-[(E)-2-[3-[(E)-2-[2-methoxy-4-
(2-pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-yl]viny1]-1-
methylindole
The title compound was obtained in the same manner as
in Example 1-2.
Example 59: Synthesis of 1-methy1-6-[(E)-2-[3-[(E)-2-[2-
methy1-4-(2-pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-
yl]vinyl]indole
CA 02888140 2015-04-10
82
Example 59-1: Synthesis of (1E,6E)-1-(1-methylindo1-6-y1)-
7-[2-methy1-4-(2-pyridylmethoxy)phenyl]hepta-1,6-diene-3,5-
dione
The title compound was obtained in the same manner as
in Example 1-1.
Example 59-2: Synthesis of 1-methy1-6-[(E)-2-[3-[(E)-2-[2-
methy1-4-(2-pyridylmethoxy)phenyl]viny1]-1H-pyrazol-5-
yl]vinyl]indole
The title compound was obtained in the same manner as
in Example 1-2.
The structural formulas and analytical data of the
compounds obtained in the above Examples are shown in the
tables below.
Ex.: example; St.: chemical structure; Dat.: analytical
data
CA 02888140 2015-04-10
83
Table 2
St.
Ex.
Dat.
1-1 0 0
CD N
,
'H NMR CDCI3): 2.56-2.61 (m, 4H), 2.82 (t, 2H, J=5.7 Hz), 3.72-3.76
(m, 4H), 4.15 (t,
2H, J=5.7 Hz), 5.81 (s, 1H), 6.51 (d, 1H, J=16.0 Hz), 6.56-6.58 (m, 1H), 6.64
(d, 1H,
J=16.0 Hz), 6.92(d, 2H, J=8.8 Hz), 7.28-7.30(m, 1H), 7.40 (dd, 1H, J=1.3Hz,
8.2 Hz), 7.50
(d, 2H, J=8.8 Hz), 7.56 (s, 1H), 7.62 (d, 1H, J=16.0 Hz), 7.63 (d, 1H, J=8.2
Hz), 7.79 (d, 1H,
J=16.0 Hz), 8.30 (s, 1H), 16.07 (brs, 1H)
MS (El) m/z 444 (Mt).
1-2 N NH
N
'H NMR (c CDCI3): 2.55-2.60 (m, 4H), 2.80 (t, 2H, J=5.7 Hz), 3.72-3.75 (m,
4H), 4.11 (t,
2H, J=5.7 Hz), 6.52-6.54 (m, 1H), 6.60 (s, 1H), 6.87 (d, 2H, J=8.7 Hz), 6.92
(d, 1H, J=16.4
Hz), 7.01-7.06 (m, 2H), 7.18-7.20 (m, 1H), 7.19 (d, 1H, J=16.4 Hz), 7.31 (dd,
1H, J=1.4Hz,
8.3 Hz), 7.40 (d, 2H, J=8.7 Hz), 7.43-7.45 (m, 1H), 7.60 (d, 1H, J=8.3 Hz),
8.13 (s, 1H),
9.32-11.42 (br, 1H)
MS (El) m/z 440 (Mt).
2-1 OCH3 0 0
0
>
MS (E0 m/z 479 (M)
2-2 OCH3 N NH
() 0
'H NMR ( CDCI3): 2.61-2.74 (m, 4H), 2.84-2.93 (m, 2H), 3.76-3.83 (m, 4H), 3.87
(s, 3H),
4.16-4.22 (m, 2H), 5.97 (s, 2H) ,6.48-6.52 (m, 2H), 6.58 (s, 1H), 6.79 (d, 1H
J=7.8 Hz),
6.88 (d, 1H, J=16.5 Hz), 6.91-6.97 (m, 2H), 7.00 (d, 1H, J=16.5 Hz), 7.03 (d,
1H, J=1.4
Hz), 7.29 (d, 1H, J=16.5 Hz), 7.43 (d, 1H, J=8.2 Hz)
MS (El) m/z 475 (M.).
CA 02888140 2015-04-10
84
Table 3
3-1
0 0
0
MS (El) m/z 449 (W)
3-2 N¨NH
0
I , >
'H NMR (d CDCI3): 2.56-2.60 (m, 4H), 2.81 (t, 2H, J=5.7 Hz), 3.74 (m, 4H),
4.14 (t, 2H,
J=5.7 Hz), 5.98 (s, 2H) ,6.57 (s, 1H), 6.79 (d, 1H, J=7.8 Hz), 6.84-6.94 (m,
5H), 6.97-7.04
(m, 3H), 7.40-7.43 (m, 2H), 9.48-11.04 (br, 1H)
MS (El) m/z 445 (M+).
CA 02888140 2015-04-10
Table 4
4-1 OCH3
H3C CO2CH3
1H NMR ( CDCI3): 2.29 (s, 3H), 2.47 (brs, 8H), 3.52 (s, 2H), 3.88 (s, 3H),
3.91 (s, 3H),
6.94 (d, 1H, J=7.9 Hz), 7.00 (s, 1H), 7.74 (d, 1H, J=7.9 Hz)
4-2 OCH3
40 OH
'H NMR ( a CDCI3): 2.28 (s, 3H), 2.47 (brs, 8H), 3.49 (s, 2H), 3.87 (s, 3H),
4.66 (s, 2H),
6.86-6.92 (m, 2H), 7.20 (d, 1H, J=7.7 Hz)
4-3 OCH3
si CHO
'H NMR ( 4 CDCI3): 2.30 (s, 3H), 2.48 (brs, 8H), 3.53 (s, 2H), 3.94 (s, 3H),
6.97-7.02 (m,
2H), 7.77 (d, 1H, J=7.7 Hz), 10.42 (s, 1H)
4-4 OCH3 0 0
H3C,N N
/
'H NMR ( DMSO-d6): 2.16 (s, 3H), 2.37 (brs, 8H), 3.48 (s, 2H), 3.89 (s, 3H),
6.14 (s, 1H),
6.47-6.51 (m, 1H), 6.84-6.99 Cm, 3H), 7.02 (s, 1H), 7.43 (d, 1H, J=8.3 Hz),
7.48-7.51 (m,
1H), 7.60 (d, 1H, J=8.3 Hz), 7.66-7.74 (m, 2H), 7.79 (d, 1H, J=15.8 Hz), 7.85
(d, 1H,
J=15.9 Hz), 11.41 (brs, 1H)
MS (ES1) m/z 459 (W2H)., 915 (2M+Hr.
4-5 OCH3 N NH
N
'H NMR (c CDCI3): 2.31 (s, 3H), 2.50 (brs, 8H), 3.51 (s, 2H), 3.91 (s, 3H),
6.54-6.56 (m,
1H), 6.67 (s, 1H), 6.91-6.94 (m, 2H), 7.05 (d, 1H, J=16.6 Hz), 7.07 (d, 1H,
J=16.6 Hz),
7.18-7.28 (m, 2H), 7.34 (dd, 1H, J=1.5Hz, 8.5 Hz), 7.38 (d, 1H, J=16.6 Hz),
7.47-7.50 (m,
2H), 7.62 (d, 1H, J=8.0 Hz), 8.21 (brs, 1H)
CA 02888140 2015-04-10
86
Table 5
5-1 0 0
o
r0
0
'H NMR (15 CDCI3): 2.56-2.61 (m, 8H), 2.81 a, 4H, J=5.7 Hz), 3.72-3.76 (m,
8H), 4.15 (t,
4H, J=5.7 Hz), 5.77 (s, 1H), 6.49 (d, 2H, J=16.0 Hz), 6.92 (d, 4H, J=8.9 Hz),
7.50 (d, 4H,
J=8.9 Hz), 7.61 (d, 2H, J=16.0 Hz), 16.00 (brs, 1H)
MS (El) m/z 534 (M).
5-2
N NH
,
'H NMR CDCI3): 2.56-2.61 (m, 8H), 2.81 (t, 4H, J=5.7 Hz), 3.72-3.76
(m, 8H), 4.13 (t,
4H, J=5.7 Hz), 6.57 (s, 11-1), 6.89 (d, 2H, J=16.4 Hz), 6.89 (d, 4H, J=8.8
Hz), 7.02 (d, 2H,
J=16.4 Hz), 7.41 (d, 4H, J=8.8 Hz)
MS (El) m/z 530 (M.).
6-1 0 0
C)
CH3
'H NMR ( CDCI3): 2.16 (s, 3H), 2.56-2.60 (m, 4H), 2.81 (t, 2H, J=5.7 Hz), 3.72-
3.76 (m,
4H), 4.14 (t, 2H, J=5.7 Hz), 5.62 (s, 1H), 6.34 (d, 1H, J=15.7 Hz), 6.89-6.93
(m, 2H),
7.45-7.49 (m, 2H), 7.55 (d, 1H, J=15.8 Hz)
MS (ESI) m/z 318(M+H).
6-2 0 0
I
I cH3
O(N
t,H3
0
'H NMR CDCI3): 2.57-2.61 (m, 4H), 2.82 (t, 2H, J=5.7 Hz), 2.99 (s,
3H), 3,10 (s, 3H),
3.72-3.77 (m, 4H), 4.15 (t, 2H, J=5.7 Hz), 4.74 (s, 2H), 5.79 (s, 1H), 6.50
(d, 2H, J=15.7
Hz), 6.90-6.99 (m, 4H), 7.48-7.54 (m, 4H), 7.61 (d, 1H, J=15.8 Hz), 7.62 (d,
1H, J=15.8
Hz)
MS (ESI) m/z 508 (M+2H).
6-3 N NH
I \
I ?H3
N,CH3
0
1H NMR (<5 DMSO-d6): 2.45-2.52 (m, 4H), 2.69 (t, 2H, J=5.8 Hz), 2.85 (s, 3H),
3.00 (s,
3H), 3.56-3.60 (m, 4H), 4.10 (t, 2H, J=5.7 Hz), 4.83 (s, 2H), 6.67 (s, 1H),
6.87-7.17 (m,
8H), 7.43-7.52 (m, 4H), 12.89 (s, 1H)
CA 02888140 2015-04-10
87
Table 6
7-1 0 0
OCH3
0
1FINMR CDCI3): 2.56-2.62 (m, 4H), 2.82 (t, 2H, J=5.7 Hz), 3.72-3.77
(m, 4H), 3.927 (s,
3H), 3.940 (s, 3H), 4.15 (t, 2H, J=5.6 Hz), 5.80 (s, 1H), 6.50 (d, 2H, J=15.8
Hz), 6.86-6.95
(m, 3H), 7.07-7.10 (m, 1H), 7.15 (dd, 1H, J=1.9, 8.3 Hz), 7.48-7.53 (m, 2H),
7.61 (d, 1H,
J=15.8 Hz), 7.62 (d, 1H, J=15.9 Hz)
MS (ESO m/z 466 (M+H)+.
7-2 N NH
OCH3
N
IH NMR ( CDCI3): 2.57-2.61 (m, 4H), 2.81 (t, 2H, J=5.8 Hz), 3.72-3.77 (m, 4H),
3.876 (s,
3H), 3.884 (s, 3H), 4.11 (t, 2H, J=5.7 Hz), 6.60 (s, 1H), 6.78-7.08 (m, 9H),
7.35-7.40 (m,
2H)
8-1 0 0
\ N
MS (ESI) m/z 446 (M+H).
8-2 N NH
o
\ I
I
N
IH NMR ( DMSO-d6): 2.45-2.53 (m, 4H), 2.69 (t, 2H, J=5.6 Hz), 3.56-3.61 (m,
4H),
4.08-4.13 (m, 2H), 6.72 (s, 1H), 6.90-7.35 (m, 6H), 7.42-7.85 (m, 5H), 8.19-
8.26 (m, 1H),
12.49 (brs, 1H), 12.92 (brs, 1H)
CA 02888140 2015-04-10
88
Table 7
9-1
OCH3 0 0
N
/
'MS (El) m/z 402 (M+).
9-2 OCH3 N-NH
N
/
1FINMR ( acetone-d6): 3.90 (s, 3H), 5.23 (s, 2H), 6.10-6.13 (m, 1H), 6.26-6.28
(m, 1H),
6.56 (s, 1H), 6.65 (dd, 1H, J=2.6 Hz, 8.4 Hz), 6.74 (d, 1H, J=2.6 Hz), 6.77
(d, 1H, J=16.8
Hz), 6.82-6.84 (m, 1H), 7.03 (d, 1H, J=16.8 Hz), 7.06 (d, 1H, J=16.8 Hz), 7.29-
7.33 (m,
1H), 7.35 (d, 1H, J=16.8 Hz), 7.51 (d, 1H, J=8.4 Hz), 7.56 (br d, 1H, J=7.7),
7.82 (dt, 1H,
J=1.8 Hz, 7.7 Hz), 8.57-8.60 (m, 1H), 10.33 (brs, 1H)
MS (El) m/z 398 (M).
10-1
0 0 k ,CH3
IN
I
MS (El) m/z 241 (M').
10-2
0 0 0C H3
/
N
MS (El) m/z 506 (Ml.
10-3
0 N-NH
N/CH3
1H NMR ( cpci3): 1.13 (t, 6H, J=7.2 Hz), 3.33 (q, 4H, J=7.2 Hz), 3.80 (s, 3H),
5.16 (s,
2H), 6.21 (d, 1H, J=2.0 Hz), 6.31 (dd, 1H, J=2.0 Hz, 8.7 Hz), 6.46 (d, 1H,
J=3.1 Hz), 6.55
(s, 1H), 6.88 (d, 1H, J=16.4 Hz), 7.05 (d, 1H, J=3.1 Hz), 7.09 (d, 1H, J=16.4
Hz), 7.21-7.49
(m, 10H), 7.58 (1H, d, J=8.2 Hz)
MS (El) m/z 502 (M+).
CA 02888140 2015-04-10
89
Table 8
11-1 CY]
c,, N
0 0 CH3
, N
N
MS (El) m/z 529 (M+).
11-2
01
N¨NH CH3
N
1H NMR(CDCI3): 1.19(t, 6H, J=7.2 Hz), 2.66-2.70(m, 4H), 2.93(t, 2H, J=5.9 Hz),
3.38
(q, 4H, J=7.2 Hz), 3.75-3.81 (m, 7H), 4.20 (t, 2H, J=5.9 Hz), 6.19 (d, 1H,
J=2.5 Hz), 6.32
(dd, 1H, J=2.5 Hz, 8.7 Hz), 6.46 (d, 1H, J=3.1 Hz), 6.55 (s, 1H), 6.88 (d, 1H,
J=16.4 Hz),
7.05 (d, 1H, J=3.1 Hz), 7.10 (d, 1H, J=16.4 Hz), 7.24 (d, 1H, J=16.4 Hz), 7.30
(d, 1H,
J=16.4 Hz), 7.33 (dd, 1H, J=1.5 Hz, 8.2 Hz), 7.39 (d, 1H, J=8.7 Hz), 7.43
(brs, 1H), 7.58 (d,
1H, J=8.2 Hz)
MS (El) m/z 525 (M+).
12-1 OH 0 0 /CH3
, N
I
MS (El) m/z 452 (ht).
12-2
OH N¨NHC H3
LJ
N
1H NMR (c acetone-d6): 3.87 (s, 3H), 5.17 (s, 2H), 6.41 (d, 1H, J=3.1 Hz),
6.57-6.61 (m,
2H), 6.69 (s, 1H), 7.09 (d, 1H, J=16.9 Hz), 7.15 (d, 1H, J=16.4 Hz), 7.23 (d,
1H, J=3.1 Hz),
7.29-7.33 (m, 2H), 7.34 (d, 1H, J=16.4 Hz), 7.41 (d, 1H, J=16.9 Hz), 7.48 (d,
1H, J=8.2
Hz), 7.52 (br d, 1H, J=7.7 Hz), 7.54 (d, 1H, J=8.7 Hz), 7.56 (brs, 1H), 7.81
(dt, 1H, J=2.1
Hz, 7.7 Hz), 8.56-8.59 (m, 1H), 8.13-9.43 (br, 1H), 11.20-12.53 (br, 1H)
MS (El) m/z 448 (M').
CA 02888140 2015-04-10
Table 9
13-1 01
0
110
1H NMR ( CDCI3): 2.56-2.61 (m, 4H), 2.85 (t, 2H, J=5.6 Hz), 3.71-3.74 (m, 4H),
4.18 (t,
2H, J=5.6 Hz), 5.26 (s, 2H), 6.57-6.59 (m, 1H), 6.62-6.65 (m, 1H), 7.24-7.28
(m, 1H),
7.47-7.50 (m, 1H), 7.72-7.76 (m, 1H), 7.81 (d, 1H, J=8.7 Hz), 8.61-8.63 (m,
1H), 10.31 (s,
1H)
13-2
N
0 0
\ N
MS (El) m/z 551 (se)
13-3
N¨NH
I
N
1
1H NMR (1:$ DMSO-d6): 2.49-2.56 (m, 4H), 2.77-2.82 (m, 2H), 3.59-3.63 (m, 4H),
4.17 (t,
2H, J=5.7 Hz), 5.22 (s, 2H), 6.40-6.44 (m, 1H), 6.61-6.69 (m, 2H), 6.75-6.79
(m, 1H),
6.98-7.10 (m, 2H), 7.20-7.31 (m, 3H), 7.34-7.40 (m, 2H), 7.46-7.57 (m, 4H),
7.84-7.88 (m,
1H), 8.58-8.61 (m, 1H), 11.18 (brs, 1H), 12.87 (brs, 1H)
MS (El) m/z 547 (Mt).
CA 02888140 2015-04-10
91
Table 10
St.
Ex.
Dat.
14-1 OCH3 0 0 CH3
0-Th
I
N
1H NMR (ô, CDC13) : 2. 56-2. 60 (n, 4H), 2. 82 (t, 2H, J=5. 8 Hz) , 3. 72-3.
76
(m, 4H), 3.83 (s, 3H) , 3.88 (s, 3H), 4. 15 (t, 2H, J=5. 8 Hz) , 5. 83 (s, 1H)
,
6.48-6. 53 (m, 3H), 6. 64 (d, 1H, J=16. 0 Hz), 6. 66 (d, 1H, J=16. 0 Hz), 7.
12
(d, 1H, J=3. 2 Hz), 7.38 (dd, 1H, J=1. 3, 8.3 Hz), 7. 47-7. 49 (n, 2H), 7.60
(d, 111, J=8. 3 Hz) , 7. 81 (d, 1H, J=16. 0 Hz) , 7.90 (d, 1H, J=16. 0 Hz)
MS (El) m/z 488 (C.
14-2 OCH3 N -NH CH3
1
1H NMR (8, CDCI3) : 2. 59-2. 63 (in, 4H), 2. 83 (t, 2H, J=5. 7 Hz). 3. 75-3.
78
(m, 411), 3. 79 (s, 3H), 3. 86 (s, 3H), 4. 14 (t, 2H, J=5. 7 Hz), 6. 45-6. 50
(m, 3H), 6. 64 (s, 1H), 6.99 (d, 1H, J=16. 6 Hz) , 7.06 (d, 1H, J=3. 1 Hz) ,
7.09 (d, 1H, J=16. 5 Hz), 7.24 (d, 1H, J=16. 6 Hz) , 7. 30-7. 35 (m, 2H) ,
7. 40-7. 42 (in, 1H), 7.45 (d, 111, J=9. 2 Hz), 1.58 (d, 1H, J=8. 2 Hz),
MS (El) m/z 484 (M').
15-1 0 0 Th pH,
--- N
MS (El) m/z 458 (C.
15-2 N NH
CH,
N
N
1H NMR ( 6 , CDC13) : 2. 57-2. 61 (in, 4H), 2. 82 (t, 2H, J=5. 7 Hz) , 3. 73-
3. 76
(m, 411), 3. 80 (s, 3H) , 4. 14 (t, 2H, J=5. 7 Hz) , 6. 46-6. 47 (in, 1H), 6.
61
(s, 1H), 6. 88-6. 97 (in, 311) , 7. 02-7. 08 (m, 3H), 7. 23 (d, 1H, J=16. 5
Hz),
7.31 (dd, 1H, J=1. 4, 8.3 Hz), 7. 40-7. 44 (n, 2H), 7.59 (d, 1H, J=8. 3 Hz) ,
7. 77 (d, 1H, J=8. 7 Hz)
MS (El) m/z 454 (W).
CA 02888140 2015-04-10
92
Table 11
16-1 F 0 0
?H3
N
1
MS (El) m/z 454 (M').
16-2 F N-NH
1 OH
3
N
1H NMR ( , CDC 13) : 3.80 (s, 3H) , 5. 21 (s, 2H), 6.47 (dd, 1H, J=0. 7 Hz,
3. 2 Hz), 6. 65 (s, 1H), 6. 73 (dd, 1H, J=2. 5 Hz, 12.3 Hz), 6. 79 (dd, 1H,
J=2. 5 Hz, 8.7 Hz), 7.02 (d, 1H, J=16. 7 Hz), 7.05 (d, 1H, J=16. 9 Hz), 7.06
(d, 1H, J=3. 0 Hz), 7. 16 (d, 1H, J=16. 7 Hz), 7. 21-7. 26 (m, 2H), 7. 32 (dd,
1H, J=1. 35 Hz, 8.3 Hz), 7.40 (s, 1H), 7. 45-7. 51 (in, 2H), 7.59 (d, 1H, J=8.
3
Hz) , 7.70-7. 75 (in, 1H), 8.62 (d, 1H, J=4. 3 Hz)
MS (El) m/z 450 (14.).
17-1 0 0 OH3
7 N
I /
1H NMR (& CDC 13) : 3. 84 (s, 3H) , 5. 25 (s, 2H), 5.82 (s, 1H), 6. 49-6. 53
(in, 2H) , 6. 67 (d, 1H, J=15. 7 Hz) , 7. 00-7. 02 (m, 2H) , 7. 13 (d, 1H,
J=3. 0
Hz) , 7_ 23-7. 27 (n, 1H), 7_ 39 (dd, 1H, J=1. 2, 8.4 Hz), 7. 48-7. 53 (in,
4H) ,
7. 60-7. 64 (in, 2H) , 7. 71-7. 75 (m, 1H) , 7. 83 (d, 1H, J=15. 7 Hz), 8. 60-
8. 63
(in, 1H), 16.11 (brs, 1H) ,
MS (El) m/z 436 (M).
17-2 N-NH
OH
3
N
0
1
1H NMR (a, CDC 13) : 3. 77 (s. 3H) , 5. 22 (s, 2H), 6. 46 (d, 1H, J=3. 0 Hz) ,
6.62 (s, 1H) , 6_ 91-6. 97 (m, 3H), 7.04-7. 10 (in, 3H) , 7. 22-7. 27 (n, 2H)
,
7.31 (dd, 1H, J=1. 0, 8.3 Hz), 7. 40-7. 43 (m, 3H) , 7. 51-7. 53 (n, 1H) ,
7.58
(d, 1H, J=8. 3 Hz), 7. 70-7. 74 (in, 1H) , 8. 60-8. 62 (n, 1H) ,
MS (El) m/z 432 (W).
CA 02888140 2015-04-10
93
Table 12
18-1 0 0
CH,
N
H3C,.Nõ)
1H NMR ( , CDC 13) 2. 35 (s. 3H), 2. 56 (t, 411, J=4. 9 Hz), 3_ 32
(t, 411,
J=4. 9 Hz), 3. 83 (s, 311), 5. 81 (s, 1H), 6. 44-6. 51 (m, 2H) , 6. 65 (d,
111,
J=15. 7 Hz) , 6.89 (d, 2H, J=8. 6 Hz), 7.12 (d, 1H, J=2. 9 Hz), 7.38 (d, 1H,
J=8. 3 Hz), 7. 44-7. 50 (m, 311), 7. 58-7. 64 (m, 2H) , 7. 81 (d, 1H, J=15.]
Hz) ,
16.15 (brs, 1H)
MS (El) m/z 427 NO.
18-2 N-NH
1 pH 3
N
H3C
1H NMR (6, CDC13) 2. 38 (s, 3H), 2_ 57-2. 64 (m, 4H), 3. 24-3. 31 (m, 411),
3.80 (s, 3H) , 6. 45-6. 48 (m, 111), 6.60-6. 62 (in, 111) , 6. 87-6. 92 (rn,
311) ,
6. 99-7_ 10 (m, 3H), 7. 20-7. 27 (m, 111) , 7. 32 (d, 1H, J=8. 5 Hz), 7. 39-7.
43
(m, 311), 7. 57-7. 61 (in, 1H)
MS (El) m/z 423 (M).
CA 02888140 2015-04-10
94
Table 13
19-1 F 0
OH
1H NMR 6 , DMSO-de) : 5.25 (s, 2H). 6.46 (d, 1H, J=16. 1 Hz), 6. 92-6. 96
(in, 1H), 7. 02-7.07 (n, 1H), 7. 35-7. 39 (n, 1H) , 7. 52-7. 54 (in, 1H), 7.
59
(d, 1H, J=16. 1 Hz), 7.76-7. 80 (in, 1H), 7. 83-7. 88 (m, 1H), 8. 58-8. 60 (n,
1H), 12.41 (brs, 1H)
MS (El) m/z 273 (M*).
19-2 F 0 0
CH3
1H NMR (8, CDCI3) : 2. 16 (s, 3H) , 5.23 (s, 2H), 5. 63-5. 64 (in, 1H). 6. 44-
6. 48
(m, 1H), 6. 72-6. 76 (in, 1H), 6. 79-6. 82 (m, 1H), 7. 25-7. 29 (in, 1H), 7.
42-7. 47
(in, 1H), 7. 48-7. 50 (m, 1H), 7. 63 (d, 1H, J=16. 1 Hz), 7. 72-7. 77 (n, 1H)
,
8. 60-8. 63 (in, 1H)
MS (El) m/z 313 (M).
19-3 0 0 CH3
/
0
1H NMR (8, CDC 13) : 3. 74 (s, 3H), 5. 23 (s, 2H), 5. 72 (s, 1H), 6. 19-6. 22
(m, 1H), 6.37 (d, 1H, J=15. 4 Hz), 6.59 (d, 1H, J=16. 1 Hz), 6. 71-6. 77 (n,
2H) , 6. 77-6. 79 (in, 1H), 6.81 (dd, 1H, J=2. 5Hz, 8.8 Hz), 7. 24-7. 28 (m,
1H) ,
7.45-7. 51 (m, 2H), 7. 61 (d, 1H, J=15. 4 Hz), 7. 67 (d, 1H, J=16. 1 Hz).
7. 72-7. 76 (in, 1H), 8. 61-8.63 (in, 1H) , 16. 10 (brs, 1H)
MS (El) m/z 404 (M*).
19-4 F N -NH CH3
/
1H NMR (8, CDC13) : 3. 70 (s, 3H), 5. 21 (s, 2H), 6. 14-6. 16 (in, 1H), 6. 50
(dd, 1H, J=1. 4Hz, 3.8 Hz), 6.58 (s, 1H), 6.65 (t, 1H, J=2. 1 Hz) , 6. 71-6.
81
(in, 3H), 6. 94-7. 02 (in, 2H) , 7. 14 (d, 1H, J=16. 7 Hz), 7. 24-7. 27 (in,
1H) .
7.47 (t, 1H, J=8. 7 Hz) , 7.50 (d, 1H, J=8. 0 Hz), 7. 71-7.75 (in, 1H) , 8.62
(d, 1H, J=4. 9 Hz)
MS (E1) m/z 400 (Mt)
CA 02888140 2015-04-10
Table 14
20-1 0 0
1401 CH3
1
1H NMR (ó, CDC13) : 2. 15 (s, 3H) , 5. 24 (s, 2H) , 5. 61 (s, 1H) , 6. 34 (d,
1H, J=16. 0 Hz) , 6.99 (d, 2H, J=8. 7 Hz) , 7.23 (dd, 1H, J=5. 0Hz, 7.6 Hz) ,
7.47 (d, 2H, J=8. 7 Hz) , 7. 48-7. 51 (m, 1H) , 7.55 (d, 1H, J=16. 0 Hz) ,
7.69-7. 73 (m, 1H) , 8. 60-8.62 (in, 1H) , 15.43 (brs, 1H)
20-2 0 0 CH3
N
,
/
1
1H NMR (a, CDC13) : 3. 74 (s, 3H), 5. 25 (s, 2H), 5. 71 (s, 1H). 6. 19-6.21
(m, 1H), 6.37 (d, 1H, J=15. 4 Hz), 6.48 (d, 1H, J=15. 8 Hz), 6. 71-6. 73 (m,
1H). 6. 77-6. 78 (m, 1H), 7. 00 (d, 2H, J=8. 8 Hz) , 7. 23-7. 27 (m, 1H),
7.48-7. 52 (m, 1H) , 7. 50 (d, 2H, J=8. 8 Hz), 7. 59 (d, 1H, J=15. 8 Hz) , 7.
60
(d. 1H, J=15. 4 Hz) , 7. 71-7. 75 (m, 1H), 8. 61 (d, 1H, J=4. 2 Hz)
MS (El) m/z 386 (W).
20-3 N-NH CH3
N
,
/
1H NMR ( , CDC13) : 3. 69 (s, 3H), 5. 23 (s, 2H), 6. 15 (t, 1H. J=3. 2 Hz) ,
6.49 (dd, 1H, J=1. 5Hz, 3. 7 Hz), 6. 54 (s, 1H) , 6. 64-6. 66 (m, 1H) , 6. 76
(d,
1H, J=16. 2 Hz), 6. 90 (d, 1H, J=16. 5 Hz) , 6. 96 (d, 1H, J=16. 2 Hz), 6. 98
(d, 2H, J=8. 8 Hz) , 7.02 (d, 1H, J=16. 5 Hz), 7. 22-7. 26 (m, 1H) , 7. 42 (d,
2H, J=8. 8 Hz) , 7. 52 (d, 1H, J=7. 9 Hz), 7. 70-7. 74 (m, 1H) , 8. 60-8. 62
(m,
1H)
MS (El) m/z 382 (M).
CA 02888140 2015-04-10
96
Table 15
21-1 OCH3 0 0 CH3
I
1 /
N
MS (El) m/z 438 (M).
21-2 OCH3 N-NH
CH
3
I
111 NMR (a, CDC13) : 2. 57-2. 60 (in, 4H), 2. 81 (t, 2H, J=5. 7 Hz) , 3. 69
(s,
3H), 3. 73-3. 76 (m, 4[1), 3. 86 (s, 311), 4. 13 (t, 2H, J=5. 7 Hz), 6. 13-6.
15
(m, 111), 6. 47-6.51 (in, 311), 6. 54 (s, 1H), 6. 63-6.64 (m, 111) , 6. 77 (d,
111,
J=16. 0 Hz) , 6. 95 (d, 111, J=16. 5 Hz) , 6. 96 (d, 1H, J=16. 0 Hz) , 7. 29
(d,
111, J=16. 5 Hz) , 7.43 (d, 1H, J=8. 3 Hz)
MS (El) m/z 434 (W).
22-1 OCH3 0 0 OH3
/
I
MS (E rniz 416 (Mi.
22-2 OCH3 N -NH CH3
,
/
I
1H NMR (a, CDC13) : 3. 68 (s, 311), 3. 84 (s, 311), 5. 23 (s, 211) , 6. 13-6.
15
(m, 1H), 6.47 (dd, 1H, J=1. 4, 3.4 Hz) , 6. 54 (s, 1H) , 6. 55-6. 59 (m, 2H) ,
6.62-6. 63 (m, 1H), 6. 77 (d, 111, J=16. 0 Hz) , 6. 95 (d, 111, J=16. 5 Hz) ,
6. 96
(d, 1H, J=16. 0 Hz) , 7. 22-7. 25 (in, 111) , 7. 29 (d, 111, J=16. 5 Hz) , 7.
42 (d,
111, J=8. 7 Hz), 7. 51-7. 53 (m, 111), 7. 70-7. 74 (m, 111) , 8. 60-8. 62 (m,
1H)
MS (El) m/z 412 (W).
CA 02888140 2015-04-10
97
Table 16
23-1 OCH3 0 0 CH3
r\'1
/ CH3
1H NMR ( 6 , CDC 13) .2.28 (s, 3H) , 3.59 (s, 3H) , 3.86 (s, 3H) , 5.25 (s.
211) , 5. 69 (s, 1H) , 5.99 (d, 111, J=3. 9 Hz) , 6. 32 (d, Ili, J=15. 5 Hz),
6.56-6. 61 (m, 3H) , 6. 66 (d, 1H, J=3. 9 Hz) , 7.24-7. 28 (in, 111) , 7.46
(d,
1H, J=9. 3 Hz), 7. 50-7. 53 (In, 1H) , 7.60 (d, 1H, J=15. 5 Hz) 7. 71-7. 76
(In,
1H) , 7.85 (d, 1H, J=16. 2 Hz) , 8. 60-8. 63 (m, 111)
MS (El) m/z 430 (M').
23-2 OCH3 N-NH CH3
cH3
111 NMR 6 , CDC 13) : 2. 25 (s, 311) , 3. 56 (s, 3H) , 3. 85 (s, 3H) 5.
23 (s,
2H) , 5. 90-5.95 (in. 1H) , 6. 40-6. 45 (m, 1H), 6.52-6. 61 (n, 3H) 6. 71
(d,
111, J=16. 5 Hz), 6.97 (d, 1H, J=17. 0 Hz) , 7.03 (d, 1H, J=16. 5 Hz) , 7. 21-
7. 35
(m, 2H), 7. 42 (d, 1H, J=8. 5 Hz) , 7. 50-7. 54 (n, 1H) , 7. 69-7. 76 (in,
1H),
8. 68-8. 64 (in, 1H)
MS (El) m/z 426 (M*)
24-1 OCH3 0 0 CH3
iS
11
/ CH3
H3C
1H NMR (5, CDC 13) 2. 24 (s, 3H) , 2.27 (s,
311) , 3. 58 (s, 3H), 3.86 (s,
311) , 5. 24 (s, 2H), 5.70 (s, 1H) , 5. 86 (s, 1H) , 6. 18 (d, 1H, J=15. 5 Hz)
,
6.56-6. 60 (n, 311) , 7. 23-7. 27 (m, 1H) , 7.46 (d, 1H, J=9. 2 Hz) 7. 49-
7.53
(in, 111). 7. 69-7.75 (m, 211), 7. 84 (d, 1H, J=16. 1 Hz) , 8.60-8. 63 (n. 1H)
MS (El) m/z 444 (M*).
24-2 OCH3 N-NH 91-13
/ CH3
H3C
1H NMR (5, CDC13) 2. 22 (s, 3H) , 2.23 (s.
311) , 3. 54 (s. 3H) , 3.86 (s,
3H) , 5.24 (s, 211) , 5.80 (s. 1H) 6. 52-6. 60 (m, 4H) , 6.97
(d, 1H, J=16. 7
Hz) , 7. 01 (d, 111, J=16. 7 Hz) , 7. 23-7. 27 (in, 111), 7.30 (d, 1H, J=16. 7
Hz) ,
7.44 (d, 1H, J=8. 3 Hz) , 7. 51-7. 54 (in, 1H) . 7. 71-7. 75 (in, 1H) , 8. 61-
8. 63
(m. 1H)
MS (El) m/z 440 (M*).
CA 02888140 2015-04-10
98
Table 17
25-1 OCH3 0 0
NCH3
1
1H NMR ( a , CDC 13) : 2.60 (s, 3H). 3.88 (s, 3H) , 5.25 (s, 2H) , 5.92 (s,
1H) , 6. 58-6. 61 (m, 2H), 6. 66 (d, 1H, J=16. 0 Hz) , 7. 09-7. 12 (m, 1H) ,
7. 17
(d, 1H, J=15. 5 Hz), 7. 20-7.23 (n, 1H), 7. 24-7. 27 (in, 1H) , 7.48-7. 53 (n,
2H) , 7. 56-7. 61 (n, 2H) , 7. 71-7. 76 (n, 1H) , 7. 93 (d, 1H, J=16. 0 Hz) ,
8. 61-8. 63 (in, 1H), 15.85 (brs, 1H)
MS (El) m/z 428 (M).
25-2 OCH3 N-NH
N CH
1H NMR ( a , CDC 13):2. 59 (s, 3H), 3. 86 (s, 3H); 5. 24 (s, 2H) , 6. 56-6. 60
(m. 2H) , 6.68 (s, 1H) , 6.97 (d, 1H, J=16. 6 Hz) , 7. 02-7. 04 (m, 1H) , 7.
16
(d, 1H, J=16. 2 Hz) , 7. 23-7. 27 (in, 2H) , 7.30 (d, 1H, J=16. 6 Hz) , 7.44
(d,
1H, J=8. 3 Hz), 7. 51-7. 58 (m, 3H) , 7. 71-7. 75 (in, 1H) , 8. 61-8. 63 (in,
1H)
MS (El) m/z 424 00 .
26-1 OCH3 0 0 CH3
, , N
I
MS (El) mh 428 (Mb).
26-2 OCH3 N-NH CH3
`s, N
1H NMR (5, CDC 13) 2.67 (s, 3H), 3.86 (s, 3M), 5. 24 (s, 2H) , 6. 56-6. 61
(n, 2H) , 6. 65 (s, 1H), 6.95 (d. 1H, J=16. 4 Hz), 7. 00 (d, 1H, J=16. 4 Hz),
7. 15 (dd, 1H, J=4. 7, 7.9 Hz), 7. 23-7. 28 (in, 2H). 7.31 (d, 1H, J=16. 4
Hz),
1.43 (d, 1H, J=8.6 Hz), 7.53 (d, 1H. J=7. 9 Hz) . 1.14 (dt, 1H. J=1. 9, 7.6
Hz) , 1.83 (dd, 1H, J=1. 6, 7. 9 Hz) , 8.40 (dd. 1H, J=1. 6, 4. 7 Hz), 8. 61-
8. 63
(m, 1H)
MS (El) m/z 424 (M`).
CA 02888140 2015-04-10
99
Table 18
27-1 0 0 CH3
H3C
/
1H NMR (& CDC 13) : 2. 12 (s, 3H) , 3.71 (s, 3H) , 5.55 (s, 1H) , 6. 17-6. 19
(m, 1H), 6.20 (d, 1H, J=15. 3 Hz) , 6. 65-6.66 (m, 1H), 6. 74-6. 75 (m. 1H) ,
752 (d, 1H, J=15.3 Hz), 15.63 (brs, IH)
27-2 0 0 CH3
/
1H NMR ( , CDC 13) 2. 85 (s, 3H). 3.06 (t, 4H, J=5. 0 Hz), 3.82 (t, 4H,
J=5. 0 Hz) , 4.22 (s, 3H) , 6. 19 (s, 1H) , 6. 68-6. 70 (m, 1H) , 6.85 (d, 1H,
J=15. 4 Hz), 6. 94 (d, 1H, J=16. 0 Hz) , 7. 19 (dd. 1H, J=1. 3 Hz, 3.9 Hz) ,
7. 24-7. 26 (m, III), 7. 38 (d, 2H, J=8. 9 Hz) , 7. 95 (d, 2H, J=8. 9 Hz) , 8.
07
(d, 111, J=16. 0 Hz), 8.08 (d, 1H, J=15. 4 Hz). 16. 68 (brs, 1H)
MS CE!) m/z 377 (M+).
27-3 N-NH CH3
/
,1\1)
H3C
1H NMR(5, CDCI3) : 2. 38 (s, 3H), 2. 58-2. 64 (m, 4H) , 3. 28 (t, 4H, J=4. 8
Hz) , 3. 69 (s, 3H), 6. 15 (dd. IH, J=2. 8 Hz, 3. 6 Hz), 6.48 (dd, 1H, J=1. 5
Hz, 3. 6 Hz), 6. 52 (s, 1H) , 6. 63-6. 65 (in, 1H) , 6. 76 (d, 1H, J=16. 2 Hz)
,
6.86 (d, 1H, J=16. 4 Hz) , 6.90 (d, 2H, J=8. 8 Hz) , 6.95 (d, 1H, J=16. 2 Hz)
,
6. 99 (d, 1H, J=16. 4 Hz), 7. 39 (d. 2H, J=8. 8 Hz)
MS (El) m/z 373 (M1.
CA 02888140 2015-04-10
100
Table 19
28-1 0 0 CH3
/
H3C-N\ )
1H NMR ( 6 , CDC13) : 2.02 (quint, 2H, J=5. 9 Hz), 2.38 (s, 3H), 2. 54-2. 58
(in, 211), 2. 69-2. 73 (in, 2H) , 3. 52-3. 56 (n, 2H), 3. 60-3. 64 (m, 2H), 3.
73
(s, 311) , 5.68 (s, 1H), 6. 18-6.21 (in, 1H) , 6.36 (d, 1H, J=15. 5 Hz), 6.40
(d, 1F1, J=15. 7 Hz), 6. 66-6. 70 (in, 3H) 6. 75-6. 77 (in, 1H) ,
7.42-7. 45 (m,
211), 7.57 (d, 1H, J=15. 5 Hz) , 7. 59 (d, 111, J=15. 6 Hz)
MS (El) m/z 391 (Mi.
28-2 N-NH CH3
/
H3C-N\
1H NMR ( 6 , CDC13) : 2. 00-2. 06 (in, 2H). 2.39 (s, 3H), 2. 55-2. 60 (m,
211),
2. 70-2. 74 (in, 2H), 3. 50-3. 54 (m, 2H) , 3. 59-3. 62 (n, 2H), 3. 69 (s,
311),
6. 14-6. 16 (in, 111), 6. 47-6.53 (m, 211), 6. 63-6. 69 (n, 3H) , 6. 78 (d,
1H,
J=16. 1 Hz), 6. 79 (d, 1H, J=16. 4 Hz) , 6. 96 (d, 1H, J=16. 1 Hz), 6. 98 (d,
111, J=16. 4 Hz) , 7.34-7. 38 (n, 2H)
MS (E I ) m/z 387 (M.) .
29-1 OCH3 0 0 CH3
CH3
H3C-1.0 /
1H NMR ( 6 , CDC13) : 1.36 (d, 6H, J=5. 8 Hz) , 3. 73 (s, 3H), 3.86 (s, 3H),
4.57-4. 63 (m, 111) , 5. 71 (s, 1H), 6. 18-6.20 (in, 1H), 6. 36 (d, 1H, J=15.
4
Hz), 6. 44 (d, 1H, J=2. 4 Hz), 6.49 (dd, 1H, J=2. 4 Hz, 8. 5 Hz), 6. 59 (d,
1H, J=15. 9 Hz) , 6.68-6. 70 (in, 1H) 6. 74-6. 76 (n, 1H), 7.45 (d, 1H,
J=8. 5
Hz) , 7.58 (d, 1H, J=15. 4 Hz) , 7.87 (d, 1H, J=15. 9 Hz)
MS (El) m/z 367 (111')
29-2 OCH3 N-NH CH3
CH3
/
H3C 0 -
1H NMR (15, CDC13) : 1. 35 (d, 6H. J=6. 1 Hz) , 3. 69 (s, 311). 3. 85 (s, 3H)
4. 53-4. 61 (in, 1H), 6. 13-6. 15 (m, 1H) , 6.45 (d, 1H, J=2. 3 Hz), 6.46-6.
49
(n, 211) , 6. 54 (s. 111), 6. 62-6. 64 (m, 1H) , 6. 77 (d. 1H, J=16. 2 Hz) ,
6. 95
(d, 1H, J=16. 7 Hz) , 7.01 (d, 1H, J=16. 2 Hz) , 7.30 (d, 1H, J=16. 7 Hz) ,
7.41
(d, 1H, J=8. 5 Hz)
MS (El) m/i 363 (Mi.
CA 02888140 2015-04-10
101
Table 20
30-1 ip CHO
rN
H3C,N,)
1H NMR (5, CDC I3) : 2. 30 (s, 3H), 2. 40-2. 66 (m, 10H) , 2. 86-2. 90 (m,
210.
7. 37 (d, 2H, J=8. 0 Hz) , 7. 80 (d, 2H, J=8. 0 Hz), 9. 98 (s, 1H)
30-2 0 0 CH3
/
MS (El) m/z 405 (M.).
30-3 N-NH CH3
/
H3C,N,,)
1H NMR (5, CDC13) : 2.30 (s, 3H) , 2.43-2. 66 (m, 10H) , 2. 79-2. 83 (m, 2H).
3. 70 (s, 3H) , 6. 14-6. 16 (m, 1H), 6. 49 (dd, 1H, J=1. 4 Hz, 3. 7 Hz), 6.55
(s, 1H) , 6.64-6. 65 (m, 1H), 6. 75 (d, 1H, J=16. 3 Hz), 6.95 (d, 1H, J=16. 5
Hz) , 6.98 (d, 1H, J=16. 3 Hz), 7.05 (d, 1H, J=16. 5 Hz), 7.20 (d, 2H, J=8. 1
Hz) , 7.40 (d, 2H, J=8. 1 Hz)
MS (El) m/z 401 (M.).
CA 02888140 2015-04-10
102
Table 21
31-1 OCH3
ari CHO
MS (El) m/z 257 (M1 .
31-2 OCH3 0 0 9H3
/
1H NMR (ci, CDC13) 3. 28 (t, 2H, J=6. 6 Hz), 3. 73 (s, 3H), 3.86 (s, 3H) ,
4.42 (t, 2H, J=6_ 6 Hz) , 5. 71 (s, 1H) , 6. 17-6. 21 (m, 1H), 6.36 (d, 1H,
J=15. 4
Hz) , 6.45 (d, 1H, J=2. 0 Hz) , 6. 53 (dd, 1H, J=2. 0Hz, 8. 6 Hz), 6. 59 (d,
1H,
J=16. 0 Hz) , 6. 90 (d, 1H, J=3. 68 Hz), 6. 75 (s, 1H), 7. 13-7. 18 (m, 1H),
7. 24-7. 28 (m, 1H) , 7. 45 (d, 1H, J=8. 6 Hz), 7.58 (d, 1H, J=15. 4 Hz) ,
7. 60-7. 66 (m, 1H), 7. 87 (d, 1H, J=16. 0 Hz) , 8. 57 (d, 1H, J=4. 7 Hz) ,
16. 18
(brs, 1H)
MS (El) m/z 430 (M').
31-3 OCH3 N -NH 9H3
/
1H NMR (8, CDCI3): 3. 28 (t, 2H, J=6. 7 Hz), 3. 70 (s, 3H), 3.84 (s, 3H),
4.40 (t, 2H, J=6. 7 Hz) , 6. 15 (t, 1H, J=3. 3 Hz) , 6.45 (d, 1H, J=2. 2 Hz),
6.48-6. 52 (m, 2H) , 6. 55 (s, 1H), 6. 63-6. 65 (m, 1H), 6. 77 (d, 1H, J=16. 5
Hz), 6.94 (d, 1H, J=16. 9 Hz) , 6.99 (d, 1H, J=16. 5 Hz), 7.17 (dd, 1H,
J=5. 1Hz, 7. 45 Hz) , 7. 25-7. 32 (m, 2H) , 7. 41 (d, 1H, J=8. 6 Hz) , 7. 62-
7. 66
(m, 1H) , 8. 56-8. 58 Cm, 1H)
MS (El) m/z 426 (M*).
CA 02888140 2015-04-10
103
Table 22
32-1 CH3 0
(00 OCH3
1H NMR ((5, CDC13) : 2. 59 (s, 3H) , 3. 85 (s, 3 H), 5. 24 (s, 2H), 6. 82 (dd,
1H, J=2. 5Hz, 8. 8 Hz), 6. 85 (d, 1H, J=2. 5 Hz), 7. 22-7. 26 (in, 1H), 7. 48-
7. 51
(in, 1H) , 7. 70-7. 74 (m, 1H), 7.93 (d, 1H, J=8. 7 Hz) , 8. 60-8. 62 (n, 1H)
MS (El) m/z 257 (M).
32-2 CH3
OH
1H NMR ( (5 , CDCI3) : 2. 36 (s, 3H), 4.64 (s, 2H). 5. 19 (s, 2K), 6_ 78 (dd,
1H, J=2. 7Hz, 8.4 Hz), 6.85 (d, 1H, J=2. 6 Hz), 7.21-7. 25 (m, 2K), 7. 50-7.53
(in, 1K), 7. 69-7. 73 (n, 1H), 8.58-8. 61 (in, 1H)
MS (El) m/z 229 (M+)
32-3 CH3
401 CHO
1H NMR ((5, CDC13) : 2. 65 (s, 3K), 5. 27 (s, 2K), 6. 86 (d, 1H, J=2. 4 Hz),
6. 93 (dd. 1H, J=2. 6Hz, 8.5 Hz) , 7. 24-7.27 (m, 1H) , 7. 48-7. 50 (n, 1H),
7. 71-7_ 77 (m, 2H), 8_ 61-8.63 (m, 1K), 10. 12 (s, 111)
MS (El) m/z 227 (M+).
32-4 CH3 0 0 CH
1 /
1H NMR ((5,CDC13) : 2. 44 (s. 311) 3. 74 (s, 3H), 5.23
(s, 2K), 5. 70 (s,
1K), 6. 19-6. 21 (m, 1K), 6.37 (d, 1H, J=15. 5 Hz), 6.43 (d, 1H, J=15. 5 Hz),
6. 71-6. 73 (in, 1K). 6. 77-6.78 (m, 1K), 6. 83-6.86 (in, 2K), 7. 22-7.27 (in,
1K), 7. 49-7. 52 (in, 1K), 7. 55-7. 58 (in, 1K), 7. 60 (d, 1H, J=15. 5 Hz) ,
7. 70-7. 74 (in, 1H), 7. 86 (d, 1H, J=15. 6 Hz) , 8. 60-8. 62 (n, 111)
MS (El) m/z 400 (111')
CA 02888140 2015-04-10
104
Table 23
32-5 CH3 N-NH CH3
,-"
/
1H NMR (a, CDC13) : 2. 40 (s, 3H), 3. 70 (s, 3H), 5. 22 (s, 2H) , 6. 15-6. 16
(m, 1H) , 6.49 (dd, 1H, J=1. 6Hz, 3.8 Hz), 6.55 (s, 1H). 6. 64-6. 66 (in, 1H).
6. 77 (d, 1H, J=16. 3 Hz), 6. 80-6. 85 (m, 3H) , 6.96 (d, 1H, J=16. 3 Hz),
7. 21-7. 25 On, 2H), 7. 49-7. 53 (m, 2W, 7. 70-7. 74 (n, 1H) , 8. 60-8. 62 (m,
1H)
MS (El) m/z 396 (M*).
Table 24
33-1 o o
CH,
N 0
1H NMR , CDC 13) 3. 30 (t, 2H, J=6. 6 Hz) , 3. 74 (s, 3H), 4. 42
(t, 2H,
J=6. 6 Hz), 5. 73 (s, 1H), 6. 20-6.22 (m, 1H), 6.38 (d, 1H, J=15. 3 Hz). 6.56
(d, 1H, J=15. 9 Hz), 6. 72-6. 74 (in, 1H), 6. 78-6. 79 (m, 1H), 6. 90-6. 93
(in,
1H) , 7. 06-7. 08 (n, 1H) , 7. 11-7. 13 (n, 1H), 7. 16-7.20 (m, 1H) , 7. 25-7.
31
(in, 2H), 7.56 (d, 1H, J=15. 8 Hz), 7.62 (d, 1H, J=15. 6 Hz), 7. 63-7. 67 (rn,
1H) , 8. 56-8. 59 (in, 1W, 16.07 (brs, 1H) ,
MS (El) m/z 400 (M.).
33-2 _NH CH,
1H NMR , CDC13) 3.31 (t, 2H, J=6. 6 Hz) , 3.70 (s, 3H) , 4.40 (t,
2H,
J=6. 6 Hz), 6. 14-6. 17 (in, 1H). 6. 49-6_ 52 (n, 1H) , 6. 57 (s, 1H), 6. 64-
6. 67
(in, 1H), 6. 73-6. 78 (m, 1H) , 6. 81-6. 85 (m, 1H), 6. 98 (d, 1H, J=16. 2 Hz)
,
7. 01-7. 08 (m. 4H) , 7. 17-7. 21 (n, 1H), 7. 22-7. 28 (n, 1H), 7. 30-7. 34
(in,
1H), 7. 64-7. 69 (in, 1H), 8. 56-8. 59 (in, 1H)
MS (El) m/z 396 (C.
CA 02888140 2015-04-10
105
Table 25
34-1 OCH3
dit CHO
rf,Nr0 4111111 OCH3
1H NMR ((5, CDC 13) : 3. 85 (s, 6H), 50028 (s, 2H). 6.20 (s, 2H), 7. 25-7. 28
(m, 1H), 7. 51 (d, 1H, J=7. 8 Hz), 7. 73-7. 77 (in, 1H), 8. 60-8. 62 (m, 111)
,
10.35 (s, 1H)
MS (El) m/z 273 (M.).
34-2 ocH, 0 0 CH3
G.: 3
1H NMR ((5, CDC13) : 3. 72 (s, 3H) , 3. 85 (s, 6H) , 5. 25 (s, 2H) , 5. 71 (s,
1H) , 6. 17-6. 19 (m, 1H), 6. 23 (s, 2H), 6. 36 (d, 1H, J=15. 4 Hz), 6. 66-6.
68
(m, 1H), 6. 73-6. 75 (m, 1H) , 6.97 (d, 1H, J=16. 0 Hz) , 7. 23-7. 26 (in, 1H)
,
7. 51 (d, 1H, J=7. 7 Hz) , 7.56 (d, 1H, J=15. 4 Hz), 7. 71-7. 75 (m, 1H), 8.03
(d, 1H, J=16. 0 Hz), 8.61 (d, 1H, J=4. 5 Hz) , 16. 26 (brs, 1H)
MS (El) m/z 446 (M).
34-3 OCH3 N-NH CH3
OCH
1H NMR ( (5 , CDC 13) 3.69 (s, 3H), 3.84 (s, 6H), 5.24 (s, 2H), 6. 13-6. 15
(m, 1H) 6. 26 (s, 2H), 6. 47 (dd. 1F1, J=1. 6Hz, 3. 7 Hz), 6. 54 (s,
1H) ,
6. 61-6. 63 (m, 1H), 6. 80 (d, 1H, J=16. 2 Hz) , 6. 97 (d, 1H, J=16. 2 Hz) ,
7.22-7. 26 (In, 1H) , 7. 34 (s, 2H) , 7.53 (d, 11-1, J=7. 9 Hz) , 7. 71-7. 75
(m,
1H) , 8. 61 (d, 1H, J=5. 0 Hz)
MS (El) m/z 442 (M).
CA 02888140 2015-04-10
106
Table 26
35-1 ocH, o o
1H NMR (iS, CDCI3) : 3. 76 (s, 3H), 3_ 87 (s, 3H) , 5.25 (s, 2H), 5. 81 (s,
1H) , 6. 58-6. 61 (in, 2H) , 6. 64 (d, 1H, J=16. 0 Hz), 6. 95-6. 96 (m, 1H) ,
7. 04
(d, 1H, J=15. 1 Hz), 7. 16-7. 17 (m, 1H), 7. 22-7. 26 (m, 1H) , 7.44 (d, 1H,
J=15. 1 Hz), 7. 47-7. 52 (m, 2H) , 7. 70-7. 74 (in, 1H), 7. 93 (d, 1H, J=16. 0
Hz),
8. 60-8. 62 (in, 1H), 15.93 (brs, 1H)
MS (El) m/z 417 (M*).
35-2 OCH3 N-NH cry3
,N
1H NMR (6 , CDC13) : 3. 71 (s, 3H), 3. 85 (s, 311), 5. 23 (s, 2H), 6. 56-6. 60
(m, 3K). 6.86-6. 88 (in, 1H) , 6.96 (d, 1H, J=16. 8 Hz), 6.97 (d, III, J=16. 1
Hz), 7_ 09-7. 11 (m, 1H) , 7. 22-7. 25 (in, 1H), 7. 29 (d, 1H, J=16. 8 Hz), 7_
42
(d, 1H, J=8. 3 Hz), 7.46 (d, 1H, J=16. 1 Hz), 7. 51-7. 53 (in, 1H), 7. 70-7.
74
(m, 1H), 8. 59-8. 62 (in, 111)
MS (El) m/z 413 (W)
36-1 ocH3 0 0 CH3
1.4
-N
MS (El) m/z 417 (M`).
36-2 ocH3 N NH CH3
=- 0
1H NMR ('5, CDC13) :3. 70 (s, 3H), 3.86 (s, 3K), 5. 24 (s, 2H), 6. 56-6.60
(m, 3H), 6.89-6. 90 (m, 2K), 6.93 (d, 1H, J=16. 5 Hz) , 7.23-7. 31 (m, 3K),
7.42 (d, 1H, J=8. 2 Hz) , 7. 43-7. 44 (m, 1K), 7.51-7. 54 (m, 1H), 7. 71-7. 75
(m, 1K). 8. 61-8. 63 (m, 1H)
MS (El) m/z 413 (M1
CA 02888140 2015-04-10
107
Table 27
37-1 ocH,
O0
ob-
MS (El) m/z 414 (M).
37-2 OCH3 N NH
I
I
N
1H NMR (5, CDCI3) : 3.85 (s, 3H), 5. 24 (s, 2H), 6.56-6. 59 (m, 2K), 6. 66
(s, 1H), 6. 97 (d, 1H, J=16. 5 Hz), 7. 13-7. 18 (in, 2H), 7. 22-7. 25 (m, 1H)
,
7. 30 (d, 1H, J=16. 5 Hz) , 7. 37-7.40 (m, 1H), 7. 43 (d, 1H, J=8. 3 Hz) ,
7.51-7. 53 (in, 1K), 7.56 (d, 1H, J=16. 1 Hz) , 7.63-7. 67 (n, 1H), 7.70-7. 74
(n, 1H), 8. 59-8. 62 (m, 2H)
MS (El) m/z 410 (M*).
38-1 OCH3 0 0
101 N
V
(.-VJ
MS (El) m/z 464 (C.
38-2 OCH3 N-NH
so N
1H NMR (a, Acetone-d,) : 3. 92 (s, 3K), 5. 24 (s, 2K), 6. 67 (dd, 1H, J=2. 6,
8. 4 Hz) , 6. 76 (d, 1H, J=2. 6 Hz), 6. 80 (s, 1K), 7.09 (d, 1H, J=16. 7 Hz),
7. 30-7_ 33 (m, 1H), 7. 37-7. 49 (m, 411) , 7. 54 (d, 1H, J=9. 0 Hz) , 7. 66-
7. 68
(d, 1H, J=8. 4 Hz), 7. 80-7. 84 (in, 1H), 7. 90-7. 95 (m, 2K), 8. 09-8. 11 (m,
1K), 8. 26-8.29 (m, 1K), 8. 58-8. 60 (m, 1K), 8.89 (dd, 1H, J=1. 9, 3. 9 Hz)
MS (El) m/z 460 (W).
CA 02888140 2015-04-10
108
Table 28
39-1 OCH, 0 0
,N =
(1 :Co
1H NMR (8, CDC13) : 3.87 (s, 3H) , 5. 25 (s, 2H) , 5. 78 (s, 1H), 6. 46-6. 48
(in, 1H), 6. 51 (d, 1H, J=15. 4 Hz) , 6. 57-6. 61 (m, 3H) , 6. 62 (d, 1H,
J=15. 4
Hz) , 7. 22-7. 26 (n, 1H) , 7. 39 (d, 1H, J=15. 4 Hz) , 7. 45-7. 49 (n, 2H) ,
7.50
(d, 1H, J=7. 7 Hz) , 7. 70-7. 74 (in, 1H) , 7. 90 (d, 1H, J=15. 4 Hz) , 8. 61
(d,
1H, J=4. 5 Hz)
MS (El) m/z 403 (M+).
39-2 OCH3 N-NH
I
0
1H NMR ( 6 , CDCI3) : 3. 85 (s, 3H) , 5. 24 (s, 2H) , 6. 37-6. 39 (n, 1H), 6.
41-6.42
(m, 1H) , 6. 55-6. 59 (m, 3H) 6. 93-6.98 (n, 3H) , 7_ 23-7. 26 (m, 1H),
7.32
(d, 1H, J=16. 6 Hz) , 7.39 (d, 1H, J=1. 6 Hz) , 7.42 (d, 1H, J=8.4 Hz) , 7.53
(d, 1H, J=7. 9 Hz) , 7. 71-7. 75 (in, 1H) , 8.60-8. 62 (in, 1H)
MS (El) m/z 399 (M.).
40-1 OCH3 0 0
0
-CH,
1H NMR (8, CDC13) : 2. 36 (s, 3H) , 3. 86 (s, 3H) , 5. 24 (s, 2H), 5. 75 (s,
1H) , 6. 07-6.09 (in, 1H), 6.43 (d, 1H, J=15. 4 Hz) , 6.49-6. 50 (in, 1H) ,
6.57-6. 62 (n, 3H) , 7. 22-7. 26 (n, 1H), 7. 33 (d, 1H, J=15. 4 Hz) , 7. 45-7.
48
(n, 1H) , 7. 50 (d, 1H, J=7. 7 Hz), 7. 70-7. 74 (n, 1H) , 7. 88 (d, 1H, J=16.
0
Hz) , 8. 60-8. 62 (n, 1H) , 16.02 (brs, 1H)
MS (El) m/z 417 (M).
40-2 0(213 N- NH
0
1H NMR (8, CDC13) : 2.33 (s, 3H) , 3. 85 (s, 3H) , 5. 23 (s, 2H) , 5. 99-6.01
(m, 1H) , 6.24 (d, 1H, J=3. 15 Hz) , 6.53 (s. 1H), 6. 56-6. 59 (n, 2H) , 6.79
(d, 1H, J=16. 2 Hz) , 6.87 (d, 1H, J=16. 2 Hz) , 6.95 (d, 1H, J=16. 6
Hz) ,
7. 21-7. 25 (m, 1H) , 7. 28 (d, 1H, J=16. 6 Hz). 7.43 (d, 1H, J=8. 3 Hz) , 7.
52
(d, 1H, J=7. 9 Hz) , 7. 70-7. 74 (n, 1H) , 8. 60-8. 62 (in, 1H)
MS (El) m/z 413 (M').
CA 02888140 2015-04-10
109
Table 29
41-1 cad, 0 0
--CH3
Cr =
0
CH3
1H NMR ( 6 , CDCI3) : 1. 95 (s, 311) , 2. 27 (s, 3H), 3. 86 (s, 3H). 5. 25 (s,
211) , 5. 74 (s, 1H), 6. 38-6. 42 (m, 211) , 6. 57-6. 62 (m, 3H) , 7. 23-7. 31
(m,
211) , 7. 47 (d, 1H, J=9. 3 Hz) , 7. 49-7. 53 (m, 1H), 7. 71-7. 75 (m, 111) ,
7. 88
(d, 1H, J=16. 3 Hz) , 8. 59-8. 63 (m, 111)
MS (El) m/z 431 (M).
41-2 0CH3 N-NH
I
CH3
1H NMR ( a , CDC13) : 1. 94 (s, 3H) , 2. 24 (s, 3H), 3.85 (s, 311), 5.23 (s,
2H) , 6. 16 (s, 111), 6. 53-6. 59 (m, 3H), 6. 78 (d, 111, J=16. 4 Hz), 6. 83
(d,
1H, J=16. 4 Hz), 6. 96 (d, 1H, J=16. 8 Hz) , 7. 22-7.26 (m, 1H) , 7. 30 (d,
111,
J=16. 8 Hz) , 7.43 (d, 1H, J=8. 5 Hz), 7. 51-7. 53 (m, 111) , 7. 70-7. 74 (m,
111) ,
8.60-8. 62 (m, 1H)
MS (El) m/z 427 CM).
42-1 ocH3 o o cH,
,N.
,
0
MS (El) m/z 417 CM).
42-2 OCH3 N NH CH
101 I rj N
-J
111 NMR C6 , CDC 13) : 3. 85 (s, 3H) , 3. 95 (s, 3H), 5. 24 (s, 2H) , 6.47 (d,
1H, J=2. 0 Hz), 6.57 (dd, 1H, J=2. 4, 8.6 Hz) , 6.59 (d, 111, J=2. 4 Hz), 6.60
(s, 111), 6. 93 (d, 1H, J=16. 7 Hz), 6. 97 (d, 1H, J=16. 3 Hz), 7.03 (d, 1H,
J=16. 3 Hz) , 7. 23-7. 27 (m, 1H) , 7.31 (d, 1H, J=16. 7 Hz) , 7.41 (d, 1H,
J=8. 3
Hz) , 7. 44 (d, 111, J=2. 0 Hz) , 7. 51-7. 54 (m, 1H), 7. 71-7. 75 (in, 1H),
8. 61-8. 63 (m, 1H)
MS (El) m/z 413 (M+).
CA 02888140 2015-04-10
110
Table 30
43-1 o 0 CH3
, N
I /
11 A
H3C-N.õ
1H NMR (8 CDC13) : 2. 00-2. 06 (in, 2H), 2. 39 (s, 3H), 2. 55-2. 59 (n,
211),
2. 71-2. 74 (in, 2H), 3. 53-3. 56 (m, 2K), 3. 61-3. 64 (m, 2H) , 3. 83 (s,
311),
5.79 (s, 1H), 6.43 (d, 1H, J=15. 5 Hz) , 6. 48-6. 50 (m, 1K), 6. 65 (d, 1H,
J=15. 8 Hz) , 6.67-6. 70 (in, 2K). 7. 12 (d, 1H, J=3. 1 Hz) , 7. 37-7. 40 (n,
111)
7. 43-7. 49 (m, 311), 7. 59-7. 64 (m, 2H), 7. 80 (d, 1H, J=15. 7 Hz)
MS (El) m/z 441 (M*).
43-2 N-NH
CH3
N
.I /
H3C- Nxi
1H NMR (8, CDC13) : 2. 01-2. 07 (m, 2H), 2.39 (s, 3H), 2. 56-2. 61 (in, 2K),
2.71-2. 75 (m, 2H), 3. 51-3. 54 (in, 2K), 3. 60-3. 63 (in, 2H), 3.81 (s, 3H),
6. 46-6. 48 (m, 1H), 6. 59 (s, 1H), 6. 66-6. 69 (in, 2K). 6. 82 (d, 1H, J=16.
5
Hz) , 7.00 (d, 1H, J=16. 4 Hz), 7. 05-7. 10 (in, 2H), 7. 23 (d, 1H, J=16. 4
Hz),
7. 31-7. 34 (m, 1H), 7. 36-7_ 39 (n, 2H), 7. 41-7.43 (m, 111), 7. 59 (d, 1H,
J=8. 1
Hz)
MS (El) m/z 437 (M*).
44-1 ocH3 0 0 CH3
MS (E ifin 480 (M*) .
44-2 OCH3 N-NH CH3
(1
1H-NMR (CDC13) : 6 3.28 (t, 2H, J=6. 7 Hz), 3. 79 (s, 3K), 3.84 (s, 3K), 4.40
(t, 2H, J=6. 7 Hz) , 6.45-6. 47 (rn, 2H) 6. 51 (dd. 1H, J=2. 3Hz, 8. 3 Hz) ,
6. 62
(s, ill), 6.96 (d, 1H, J=16.5 Hz), 7.05 (d, 111, J=3.0 Hz) , 7.08 (d, 1H,
J=16. 5 Hz), 7. 14-7. 18 (m, 1K), 7.23 (d, 111, J=16. 5 Hz), 7. 26-7. 29 (n,
III),
7. 29-7. 33 (m, 2K), 7. 40 (brs. 1K), 7.42 (d, 111, J=8. 6 Hz), 7.58 (d, 1H,
J=8. 3 Hz), 7. 61-7. 65 (m, 1K). 8. 56-8. 58 (m. 111)
MS (El) m/z 476 (M+).
CA 02888140 2015-04-10
111
Table 31
45-1 OCH3 0 0 CH,
l'1,)
OCH3
1H NMR (a, CDC I3) 3. 82 (s, 3H) , 3. 86 (s, 6H) , 5. 25 (s, 2H), 5. 82 (s,
1H) , 6. 24 (s, 2H) , 6. 48 (d, 1H, J=3. 2 Hz) , 6. 66 (d, 1H, J=15. 4 Hz) ,
7. 01
(d, 1H, J=16. 0 Hz) , 7. 11 (d, 1H, J=3. 2 Hz) , 7. 23-7. 26 (m, 1H) , 7. 37
(d,
1H, J=1. 3 Hz, 8.4 Hz), 7.47 (s, 1H), 7. 52 (d, 1H, J=7. 7 Hz) , 7. 60 (d, 1H,
J=8. 4 Hz), 7. 71-7_ 75 (m, 1H), T 79 (d, 1H, J15.4 Hz), 8.07 (d, 1H, J=16. 0
Hz) , 8. 60-8. 62 Cm, 1H) , 16.23 (brs, 1H)
MS (El) m/z 496 (Mi.
45-2 OCH3 N -NH CH3
igh .0 N
Cr OCH3
1H NMR (a, CDCI3) : 3. 79 (s, 3H) , 3. 85 (s, 6H). 5. 24 (s, 2H), 6. 26 (s,
2H) , 6. 45 (d, 1H, J=3. 2 Hz), 6. 63 (s, 1U), 7. 04 (d, 1H, J=2. 75 Hz) , 7.
11
(d, 1H, J=16. 1 Hz), 7. 22-7.27 (m, 2H) , 7. 31 (dd, 1H, J=1. 4 Hz, 8.3 Hz),
7. 37 (s, 2H) , 7.41 (s, 1H) , 7. 53 (d, 1H, J=7. 75 Hz), 7. 57 (d, 1H, J=8. 3
Hz) , 7. 71-7. 75 (m, 1W, 8.60-8. 62 (m, 1H)
MS (El) m/z 492 (U.
46-1 0 0 c1-13
N 40--N
MS (El) m/z 454 (41')
46-2 N NH pH3
F
..-N\
111"
MS (El) m/z 450 (M.).
CA 02888140 2015-04-10
112
Table 32
47-1
111 NMR ( , CDCI3) : 2. 44-2.
48 (in, 411), 3.52 (s, 2H), 3. 70-3. 74 (m, 4H),
5.84 (s, 1H) , 6. 57-6.59 (m, 1H) , 6.62 (d, 1H, J=15. 9 Hz) , 6.66 (d, 1H,
J=15. 8 Hz), 7. 29-7. 31 (in, 1H), 7. 35-7. 38 (in, 2H), 7. 39-7. 42 (m, 1H),
7. 50-7. 53 (m, 211), 7. 57-7. 58 (m, 1H) , 7. 63-7. 67 (m, 2H), 7.81 (d, 111,
J=15.7 Hz) , 8.31 (brs, 1H), 16.03 (brs, 111)
MS (El) m/z 414 (W).
47-2
CC1N, 40,14/
111 NMR ( , CDCI3) : 2. 44-2.
48 (m, 411), 3.51 (s, 2H), 3. 70-3. 74 (m, 4H),
6. 54-6. 55 (m, 111), 6.63 (s, 111) , 7.03 (d, 1H, J=16. 3 Hz) , 7.04 (d, 1H,
J=16. 5 Hz), 7.09 (d, 1H, J=16. 5 Hz) , 7.20 (d, 1H, J=16. 2 Hz), 7. 21-7. 23
(m, 111), 7. 30-7. 34 (m, 3H), 7. 44-7. 47 (m, 311), 7.61 (d, 1H, J=8. 3 Hz),
8.16 (brs, 1H)
MS (El) m/z 410 (W).
48-1 o 0
SO N
I /
0õ)
111 NMR (a, CDCI3) : 2. 49-2.56 (m, 4H), 2. 59-2. 64 (m, 2H) , 2.81-2. 86 (m,
211) , 3. 72-3. 76 (in, 411) , 5. 83 (s, 111) , 6. 56-6. 58 (m, 111) , 6. 59
(d, 1H, J=16. 0
Hz) , 6.65 (d, 1H, J=16. 0 Hz) , 7.24 (d, 2H, J=8. 2 Hz), 7. 28-7. 30 (m, 1H),
7. 39-7. 41 (m, 111), 7. 48 (d, 2H, J=8. 2 Hz) , 7. 57 (s, 1H) , 7. 61-7. 65
(m,
2H) , 7.80 (d, 1H, J=16.0 Hz) , 8.30 (brs, 111) , 16.00 (brs, 111)
MS (El) m/z 428 (W).
48-2 N NH11:41
1H NMR (a. DMSO-d6) : 2. 41-2.44 (m, 4H) , 2. 49-2.55 (m, 2H). 2. 74 (t, 2H,
J=7. 7 Hz), 3. 56-3. 60 (m, 411) 6. 42 (brs, 1H) ,
6. 73 (s, 111), 6. 96-7. 39 (m,
8H) , 7. 43-7. 56 (in, 4H) , 11. 04-11. 17 (in, 111), 12. 88 (s, 111)
MS (El) m/z 424 (W)
CA 02888140 2015-04-10
113
Table 33
49-1 o o
gel
111 NMR (8, CDC13) : 1. 81-1. 88 (m, 211) , 2. 35-2. 51 (m, 6H), 2. 68 (t, 2H,
J=7. 7 Hz), 3. 70-3. 77 (in, 411), 5.83 (s, 1H) , 6. 57-6. 62 (in, 211), 6.66
(d,
111, J=15. 9 Hz) , 7. 20-7. 23 (n, 2H) , 7.30-7. 31 (in, 1H) , 7.41 (dd, 111,
J=1. 4,
8. 2 Hz), 7. 47-7. 50 (m, 211) , 7. 57-7, 58 (in, 111), 7. 62-7. 66 (m, 211),
7. 81
(d, 111, J=15.9 Hz) , 8.32 (brs, 111)
MS (El) m/z 442 (C.
49-2 N-NH
'K.>J
ill
I
NMR (8, CDC13) : 1. 81-1. 88 (n, 2H), 2. 39 (t, 2H, J=7. 6 Hz) , 2. 44-2. 49
(n, 4H) , 2. 65 (t, 2H, J=7. 7 Hz), 3. 72-3. 76 (m, 4H), 6. 54-6. 56 (in,
111),
6.64 (s, 1H), 7. 00-7.06 (m, 211) , 7.08 (d, 111, J=16. 6 Hz), 7. 16-7. 23 (m,
411) , 7. 33 (dd, 1H, J=1. 2, 8. 5 Hz), 7. 40-7. 43 (in, 211), 7. 47-7. 48 (m,
1H),
7.61 (d, 1H, J=8.3 Hz), 8.19 (brs, 111)
MS (El) m/z 438 (M.).
00
50-1
N
IJJ
I _;
1H NMR (8, CDCI3) : 2. 29 (s, 311), 2. 35-2. 61 (m, 811) , 3. 53 (s, 211), 5.
84
(s, 1H), 6. 57-6. 59 (n, 1H) , 6. 61 (d, 1H, J=15. 9 Hz) , 6.66 (d, 1H, J=15.
8
Hz) , 7. 29-7. 31 (n, 111) , 7. 34-7. 37 (in, 211), 7. 40-7.42 (n, 1H), 7. 49-
7. 52
(m, 2H), 7. 56-7. 58 (m, 1H) , 7. 64 (d, 111, J=8_ 3 Hz) , 7. 65 (d, 111,
J=15. 9
Hz) , 7.81 (d, 1H, J=15.7 Hz) , 8.33 (brs, 1H) , 16.03 (brs, 111)
MS (El) m/z 427 (C.
50-2 N NH
111 NMR (8. CDC13) r 2. 29 (s, 311), 2. 40-2.57 (n, 811). 3. 51 (s, 2H). 6. 54-
6.56
(m, 111), 6. 63 (s, 111). 7. 02 (d, 111, J=16. 4 Hz), 7. 03 (d, 111,
J=16. 5 Hz) ,
7.08 (d, 1H, J=16. 5 Hz) , 7. 19 (d, 1H, J=16. 5 Hz), 7. 20-7.23 (rn, 1H)
7. 29-7. 34 (rn, 311), 7. 42-7. 48 (n, 311) , 7. 61 (d, 1H, J=8. 3 Hz). 8. 23
(brs,
111)
MS (El) m/z 423 (M').
CA 02888140 2015-04-10
114
Table 34
51-1 9 o
1H NMR ( (5 , CDC13) : 2. 30 (s, 3H), 2. 40-2. 65 (n, 10H) , 2.80-2. 85 (in,
2H),
5.83 (s, 1H) , 6. 56-6. 61 (m, 2H), 6.65 (d, 1H, J=15. 9 Hz), 7.23 (d, 2H,
J=8. 1 Hz) , 7. 28-7.30 (m, 1H), 7.40 (dd, 1H, J=1. 5 Hz, 8.05 Hz), 7.47 (d,
2H, J=8. 1 Hz) , 7.56 (s, 1H) 7. 61-7. 65 (n, 2H) , 7.80 (d, 1H, J=15. 9
Hz) ,
8.34 (brs, 1H) , 15.96 (brs, 1H)
MS (El) m/z 441 (M).
51-2 N-NH
I ,
N
/
H3C-
1H NMR ( (5 , CDC13) : 2.32 (s, 3H), 2. 41-2. 69 (in, 10H), 2. 78-2. 85 (n,
2H),
6. 54-6. 56 (in, 1H), 6.63 (s, 1H), 7.01 (d, 1H, J=16. 5 Hz), 7.03 (d, 1H,
J=16.4 Hz), 7.07 (d, 1H, J=16. 5 Hz), 7. 16-7. 22 (in, 3H) , 7. 22-7. 24 (m,
1H),
7.33 (dd, 1H, J=1. 3 Hz, 8.2 Hz), 7.42 (d, 2H, J=8. 1 Hz) , 7.48 (brs, 1H),
7.62 (d, 1H, J=8. 2 Hz), 8.19 (brs, 1H)
MS (El) m/z 437 (M.).
52-1
o o
N
1H NMR ( (5 , CDC13) : 2. 63 (t, 4H, J=4. 8 Hz), 2.89 (t, 2H, J=5. 8 Hz), 3.
76
(t, 4H, J=4. 8 Hz), 4. 20 (t, 2H, J=5. 8 Hz), 5. 83 (s, 1H), 6. 56-6. 58 (n,
1H) , 6.65 (d, 1H, J=16.0 Hz), 6.76 (d, 1H, J=16.0 Hz) , 6.93 (d, 1H, J=8.0
Hz). 6. 96-7. 00 (in, 1H), 7. 28-7. 34 (m, 2H), 7.40 (dd, 1H, J=1. 5 Hz, 8.0
Hz) , T 54-7.58 (n, 2H) , 7.64 (d. 1H, J=8. 25 Hz), 7.80 (d. 1H, J=16. 0 Hz),
7.97 (d, 1H, J=16. 0 Hz) , 8.31 (brs, 1H) , 16.01 (brs, 1H)
MS (El) m/z 444 (W).
52-2 o'
0 N -NFI
-44
1H NMR ((5, CDC13) : 2. 62 (t, 4H, J=4. 6 Hz), 2. 88 (t, 2H, J=5. 8 Hz), 3. 75
(t, 4H, J=4. 6 Hz), 4. 18 (t, 2H, J=5. 8 Hz) , 6.52-6. 54 (in, 1H), 6.61 (s,
1H) , 6.90 (d, 1H, J=7. 7 Hz), 6. 94-6.98 (n, 1H), 7.06 (d, 1H, J=16. 3 Hz)
7. 13 (d, 1H, J=16. 7 Hz) , 7. 18-7. 25 (in, 3H), 7. 31-7. 34 (in, 1H) , 7.42-
7. 47
(in, 2H) , 7. 55 (dd, 1H, J=1. 6 Hz, 7. 7 Hz) , 7. 60 (d, 1H, J=8. 2 Hz), 8.
15
(brs, 1H)
MS (El) miz 440 (M).
CA 02888140 2015-04-10
115
Table 35
7 0
53-1
N
1H NMR (& CDC13) : 2. 56-2_ 62 (m, 4H), 2. 81-2. 84 (m, 2H) , 3. 72-3. 76 (m,
4H) , 4. 13-4. 17 (m, 2H), 5.84 (s, 1K), 6. 56-6. 59 (m, 1H), 6.60 (d, 1H,
J=15. 9
Hz) 6. 65 (d, 1H, J=15. 9 Hz) , 6.92 (dd, 1H, J=2. 45 Hz, 8. 1 Hz), 7.08-7. 10
(m, 1H), 7. 15 (d, 1H, J=7. 85 Hz), 7. 27-7.32 (m, 2K), 7. 39-7.42 (m, 1H),
7. 57 (s, 1K), 7.60 (d, 1H, J=15. 9 Hz) , 7.64 (d, 1H, J=8. 1 Hz). 7. 81 (d,
1H, J=15. 9 Hz). 8.32 (brs, 1H), 15.97 (brs, 1H)
MS (El) m/z 444 (M.) .
53-2 N NH
(2) )
1H NMR C5, CDC 13) 2.68 (brs, 4H) ,
2.90 (brs, 2H) , 3.79 (brs, 4H), 4.21
(brs, 2H) 6. 54-6. 56 (m,
1H), 6. 63 (s, 1K), 6. 82-6. 85 (m, 1H), 7. 02 (d,
1H, J=16. 3 Hz), 7. 04-7. 06 (m, 3K), 7. 10 (d, 1H, J=7. 7 Hz), 7. 19 (d, 1H,
J=16. 3 Hz) , 7. 22-7. 24 (m, 1H), 7. 25-7. 29 (m, 1H), 7.33 (dd, 1H, J=1. 4
Hz,
8.3 Hz) , 7.48 (s, 1K), 7.62 (d, 1H, J=8. 3 Hz), 8. 19 (brs, 1H)
MS (El) m/z 440 (M+)
OCH3 0 0
54-1 _11
I
N 0
MS (El) m/z 466 (M).
OCH3 N-NH
54-2
1H NMR C6 , CDC 13) : 3. 29 (t, 2H, J=6. 7 Hz) , 3. 86 (s, 3H) , 4.41 (t, 2H,
J=6. 7 Hz) , 6. 47 (d, 1H, J=2. 3 Hz) , 6. 52-6. 56 (m, 2K), 6. 62 (s, 1H), 6.
96
(d, 1H, J=16. 5 Hz), 7. 05 (d, 1H, J=16. 5 Hz) , 7. 15-7. 23 (m, 3H), 7. 24-7.
28
(m, 1H), 7. 30 (d, 1H, J=16. 5 Hz) , 7.33 (dd, 1H, J=1. 4Hz, 8. 3 Hz) , 7. 43
(d, 1H, J=8. 3 Hz) , 7.49 (s, 1H) 7. 61 (d, 1H, J=8.
7 Hz) , 7. 61-7. 66 (m,
1H) 8. 17 (brs, 1K), 8. 56-8. 59 (m, 1H)
MS (El) m/z 462 (Mi.
CA 02888140 2015-04-10
116
Table 36
55-1111 j0t, 9
.)
NMR ((5, CDC13) : 2. 15 (s, 3H), 4. 25-4. 30 (m, 4H) , 5. 61 (s, 1H) , 6. 31
(d, 1H, J=15. 7 Hz), 6.86 (d, 1H, J=8. 3 Hz) , 7.02 (dd, 1H, J=2. 0Hz, 8.3
Hz) , 7.05 (d, 111, J=2. 0 Hz) , 7_ 48 (d, 1H, J=15. 7 Hz), 15.41 (brs, 1H)
55-2 ocH, o o
o
-o-
MS (El) m/z 493 (W).
55-3 OCH3 N NH
07') .7 40 0
CY-
1H NMR ( (5 , CDC]) 2. 58-2. 61 (m, 411). 2. 81 (t, 2H, J=5. 7 Hz) , 3. 73-3.
76
(m, 4H) , 3. 86 (s, 3H) , 4. 14 (t, 2H, J=5. 7 Hz), 4. 26-4. 27 (m, 4H) , 6.
48-6. 51
(in, 2H) , 6.58 (s, 1H), 6.84 (d, 1H, J=8. 3 Hz) , 6. 89 (d, 1H, J=16. 5 Hz),
6. 93-6. 99 (m, 3H) , 7. 01 (d, 1H, J=1. 8 Hz), 7. 29 (d, 1H, J=16. 5 Hz), 7.
43
(d, 1H, J=8. 7 Hz)
MS (El) m/z 489 (W).
OCH3 0 0 OCH3
56-1
I
1H NMR ( , CDC13) : 2. 56-2. 60 (m, 811), 2. 81 (t, 4H, J=5. 7 Hz), 3. 72-3.
75
(m, 8H), 3.88 (s, 6H), 4.15 (t, 4H, J=5. 7 Hz), 5.79 (s, 1H), 6.48 (d, 2H,
J=2. 5 Hz) , 6. 51 (dd, 2H, J=2_ 5Hz, 8.5 Hz), 6. 62 (d, 211, J=16. 0 Hz) , 7.
47
(d, 2H, J=8. 5 Hz) , 7. 77 (d, 2H, J=16. 0 Hz), 16. 13 (brs, 1FI) ,
MS (E1) m/z 594 (W).
OCH3 N NH OCH3
56-2
o
0 )
1H NMR ( (5 , CDCI3) : 2. 57-2. 61 (m, 811), 2. 81 (t, 4H, J=5. 7 Hz) , 3. 73-
3. 76
(m. 8H), 3. 86 (s, 611) 4. 14 (t, 411, J=5. 7 Hz) , 6. 47-6. 52 (in, 4H) ,
6. 61
(s, 1H) , 6. 97 (d, 211, J=16. 5 Hz) , 7.30 (d, 2H, J=16. 5 Hz), 7.44 (d, 2H,
J=8. 3 Hz)
MS (El) m/z 590 (W).
(A) Z9V z/w (13) SW
(HI 'sJel) 66 IF (H1 'w) 09 "8-89 "8 (HI 'w) V8 "L-08 "L 'th)
ZL L-OL L (HI 'Ill) 89 "L-99 "L (z1-1 E "8=1' 'Hl `P) 25 "L
(41 L 8 'V "1=1' 111
'PP) 9V - L (HI 'w) FV L-9E L (HZ 'Iu) VE -L-6Z L (zH
z"C=1" 'HI 'P) 1Z -L
(HZ 'al) 60 'L-P0 'L (zH C Z=1" 'HI 'P) VL 9 (HZ 'w) 89 '9-99 '9
([11 wl)
9V "9-1717 9 (HZ '9) CZ 9 (HE '9) 166 (HE '9) 178 "C : (9p-auo4aov ' HI
61-13,
CI
Mr.
HN -N Z-89
" (JD 99t z/111 (I3)
eHo,
1101
0 0 EHOO 1-85
(411) ZEV ziw 01) SW
(zH V -V=I"' 'HI 'P) 19 (HI 'w) VUL-OL-L (zH 8 1=1' 'HI 'P)
85 L (41 6 -L=r 'HI 'P) Z9 (zH L -8=1' 'HZ 'P) (HE
'w) 02 "L-81 "L
(zH E -91=1' 'HI 'P) LI (41 E '91=1' 'H1 'P) 01 "L (HI '1u) Z I
'L-L0 L (zH
"91=r 'HI 'P) VU 'L '(zH L '8=1' 'HZ 'P) L6 =9 '(zH V "91=1' 'HI 'P) 689
'9) 08 '9 (HI '9) Z9 '9 (HZ '9) CZ '9 (HE '9) 18 (c
pap ) èWJN H1
0-11 I
oj
HO HN N Z-LS
(A) 92V z/w (13) SW
r
N
4111147
'HO 0 o I-L5
LeIqI
LIT
OT-VO-STOZ 0VT888Z0 VD
CA 02888140 2015-04-10
118
Table 38
59-1 CHs 0 0 pH,
00 N
1.1 /
111 NMR (ô, CDC13) : 2. 45 (s, 3H) , 3. 83 (s, 3H) , 5. 23 (s, 2H) , 5. 82 (s,
111) , 6. 45-6.50 (m, 211) , 6.67 (d, 1H, J=15. 8 Hz) , 6. 84-6. 87 (m, 2H) ,
7. 13
(d, IH, J=3. 0 Hz) , 7. 22-7. 27 (m, IH) , 7. 37-7. 40 On, IH) ,
7. 48-7. 52 (m,
211) , 7. 56-7. 63 (m, 211) , 7. 70-7. 74 (m, 1H), 7. 83 (d, IH, J=15. 8 Hz) ,
7. 89
(d, 111, J=15. 8 Hz) , 8. 60-8. 62 (m, 111)
MS (El) m/z 450 (kr).
59-2 CH3 N-NH pH3
N
j
111 NMR Co. CDC13) : 2.41 (s, 311) , 3. 80 (s, 311) , 5.22 (s, 211) , 6. 46-6.
48
(m, 1H), 6. 63 (s, 1H) , 6. 82-6.87 (m, 311) , 7.06 (d, 111, J=3.
1 Hz) , 7.07
(d, 1H, J=16. 5 Hz) , 7. 21-7. 28 (m, 3H) , 7. 31-7. 33 (m, 1H), 7. 40-7. 42
(m,
111) , 7. 50-7. 54 (m, 2H) , 7. 59 (d, 1H, J=8. 2 Hz) , 7. 70-7. 74 (m, 1H) ,
8. 60-8. 62 (m, 111)
MS (El) m/z 446 (M').
Pharmacological test example 1: Determination of tau
aggregation inhibitory activity
Recombinant three-repeat microtubule-binding domain
(3R-MBD) of tau protein was expressed in E. coli and was
purified and used for the experiment. The purified tau
solution was diluted with a 50 mM Tris-HC1 buffer (pH 7.6)
to a final concentration of 10 M. The test compounds were
prepared using dimethylsulfoxide (hereinafter also referred
to as DMSO) at 20-fold of their final concentrations and
added to the tau solution so that the DMSO concentration
would be 5%. To this solution, heparin was added so that
the final concentration would be 10 M and the plate was
left to stand at 37 C for 16 hours. Thioflavin T was added
CA 02888140 2015-04-10
119
to the plate so that the concentration would be 10 M and
the fluorescence intensity was measured with a fluorescence
plate reader (PerkinElmer, Inc.)(excitation wavelength: 440
nm; fluorescence wavelength: 486 nm).
The final concentration of each compound at the time of
measurement was set at 0.1, 0.3, 1, 3, and 10 M. The
sample to which only DMSO was added was used as a negative
control and its fluorescence intensity was taken as 0%
inhibitory activity to determine the 50% inhibitory
concentration (IC50) of each compound. The results are
shown in Table 39. Table 39 also shows the calculated
maximum inhibition (%) of the compounds that exhibited
particularly high maximum inhibition () at the
concentrations used for the determination of the tau
aggregation inhibitory activity.
Table 39
Maximum
Compound ICso (j1M)
inhibition (%)
Example 16-2 0.6
Example 17-2 0.65
Example 19-4 0.51
Example 20-3 0.55 89
Example 21-2 89
Example 22-2 0.66 90
Example 23-2 0.59 92
Example 25-2 0.84
Example 27-3 91
Example 28-2 93
Example 32-5 0.64 90
Example 42-2 0.83
Example 43-2 92
Example 53-2 0.84
As is apparent from Table 39, some of the compounds of
the present invention exhibited a particularly high tau
CA 02888140 2015-04-10
120
aggregation inhibitory activity. The compounds that
exhibited high maximum inhibition are expected to have
improved therapeutic effects and are thus more preferred.
Pharmacological test example 2: Determination of p-
secretase inhibitory activity
The P-secretase inhibitory activity was measured with
BACE-1 FRET assay Kit (Invitrogen). The test compounds
were prepared using DMSO at 30-fold of their final
concentrations and added to an assay buffer so that the
DMSO concentration would be 10%. To each of the solutions,
equal volumes of a human recombinant P-secretase (1 U/mL)
and a fluorescent substrate peptide (750 nM), each
dissolved in an assay buffer, were added and the solutions
were left to stand for 1 hour. The fluorescence intensity
was measured with a fluorescence plate reader (excitation
wavelength: 545 nm; fluorescence wavelength: 590 nm).
The final concentration of each compound at the time of
measurement was set at 0.1, 0.3, 1, 3, and 10 M, at 0.3, 1,
3, 10, and 30 M, or at 1, 3, 10, 30, and 100 M. The
sample to which only DMSO was added was used as a negative
control and its fluorescence intensity was taken as 0%
inhibitory activity to determine the 50% inhibitory
concentration (ICH) of each compound for the evaluation of
P-secretase inhibitory activity of each compound. The
fluorescent substrate peptide had the amino acid sequence
of Ser-Glu-Val-Asn-Leu-Asp-Ala-Glu-Lys-Arg, in which the
Ser residue at position 1 was labeled with a fluorescent
CA 02888140 2015-04-10
121
donor (Cy3) and the Lys at position 9 was labeled with a
fluorescence quencher (Cy5Q).
The compounds of the present invention exhibited a high
P-secretase inhibitory activity.
Pharmacological test example 3: Determination of AP
aggregation inhibitory activity
AP 1-42 (Peptide Institute, Inc.) was dissolved in 0.1%
ammonia water so that the concentration would be 0.5 mM,
and then diluted with PBS to 20 M. The test compounds
were prepared using DMSO at 100-fold of their final
concentrations and then diluted with PBS so that the DMSO
concentration would be 2%. The AP solution and each of the
test compound solutions were mixed at an equivalent ratio
and the mixtures were left to stand at 37 C for 24 hours.
To each of the mixtures, an equal volume of a thioflavin T
solution adjusted with a 100 mM Tris-glycine buffer (pH
8.5) to 6 M was added. The fluorescence intensity was
measured with a fluorescence plate reader (excitation
wavelength: 440 nm; fluorescence wavelength: 486 nm).
The final concentration of each compound at the time of
measurement was set at 0.1, 0.3, 1, 3, and 10 m or at 2, 4,
8, and 16 M. The sample to which only DMSO was added was
used as a negative control and its fluorescence intensity
was taken as 0% inhibitory activity. With the use of the
fluorescence intensity of the control taken as 0%
inhibitory activity, the 50% inhibitory concentration
(IC50) of each compound was calculated to evaluate the AP
aggregation inhibitory activity of each compound.
CA 02888140 2015-04-10
122
The compounds of the present invention exhibited a high
AP aggregation inhibitory activity.
Pharmacological test example 4: Concentration measurement
in brain after oral administration
Each of the compounds of the present invention shown in
Tables 40 and 41 and the compound of Example 2 of JP 2012-
229208 A was orally administered to ICR mice (male, 7 weeks
old) in a single dose of 5 mg/kg. At 3, 6 and 9 hours
after the administration, 1, 3, 6 and 9 hours after the
administration, or 1, 3, 6, 9 and 24 hours after the
administration, the mice were subjected to perfusion by
infusing physiological saline into the heart under
inhalational anesthesia with isoflurane, and the whole
brain was harvested. Physiological saline was added to the
harvested brain in an amount equal to twice the wet weight
of the brain and the brain was homogenized. Then, an equal
volume of methanol was added thereto, and the mixture was
deproteinized. The concentration of each compound in the
sample was measured by LC/MS/MS. The test results are
shown in Tables 40 and 41.
Table 40
AUCo-sh in brain
Compound
(ng=hr/g-tissue)
Example 22-2 458
Example 44-2 869
JP 2012-229208 A, Example 2 224
Table 41
AUCo-24h in brain
Compound
(ng.hrig-tissue)
Example 14-2 24639
Example 18-2 9781
CA 02888140 2015-04-10
123
Example 19-4 3499
Example 20-3 5306
Example 27-3 1302
JP 2012-229208 A, Example 2 645 to 677
Industrial Applicability
The compound of the present invention is useful as a
prophylactic drug, a therapeutic drug, and the like for
Alzheimer's disease and therefore the present invention can
be applied to industrial fields such as pharmaceutical
industry.