Note: Descriptions are shown in the official language in which they were submitted.
CA 02888322 2015-04-14
WO 2014/060444 1
PCT/EP2013/071567
Antibodies to Amvioid Beta
Field of the Invention
This invention relates to antibodies that bind to human amyloid beta 1-42
peptide and N-
terminal truncates thereof, collectively referred to as A13n-42 peptides,
wherein n is 1 to 29. It
relates to antibodies that are selective in binding to amyloid beta n-42
peptide over amyloid beta
1-40 peptide. The invention also relates to use of anti-A13n-42 antibodies for
treating conditions
associated with amyloidosis, including Alzheimer's disease.
Background
Alzheimer's disease (AD) is characterised by worsening cognitive impairment,
affecting
memory, that debilitates the patient's social and occupational functioning.
The degenerative
disease causes loss of nerve cells within the brain, which brings about
cognitive difficulties with
language and higher functioning, such as judgement, planning, organisation and
reasoning,
which can lead eventually to personality changes. The end stages of the
disease are
characterised by a complete loss of independent functioning.
Histologically, AD (sporadic and familial) is defined by the presence of
intracellular
neurofibrillary tangles (NFT's) and extracellular plaques. Plaques are
aggregations of amyloid p
peptide (A13) derived from the aberrant cleavage of the amyloid precursor
protein (APP), a
transmembrane protein found in neurons and astrocytes in the brain. A13
deposits are also found
in the blood vessels of AD patients.
Cholinergic neurons are particularly vulnerable in AD, and the consequent
neurotransmitter decline affects other neurotransmitter systems. Other
symptoms of the disease
include oxidative stress, inflammation and neuronal apoptosis (programmed cell
death). In the
AD patient, extensive neuronal cell death leads to cognitive decline and the
eventual death of
the patient. (Younkin, 1995; Borchelt et al., 1996; Selkoe, 1999).
Current treatments are symptomatic only and are seen as minimally effective
with minor
improvements in symptoms for a limited duration of time. However,
overproduction or changes
in A13 levels are believed to be key events in the pathogenesis of sporadic
and early onset AD.
For this reason, A13 has become a major target for the development of drugs
designed to reduce
its formation (Vassar etal., 1999), or to activate mechanisms that accelerate
its clearance from
brain.
The amyloid cascade hypothesis proposes that production of the A13 peptide
adversely
affects neuron function, thereby, leading neuron death and dementia in AD. A13
is produced
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
2
from the amyloid precursor protein (APP) which is cleaved sequentially by
secretases to
generate species of different lengths. The main plaque component is the 42
amino acid isoform
of A131-42 which is involved in the formation of neurotoxic oligomers and
plaque formation in AD
pathogenesis. A number of isoforms of A13 including A131-42, pGluA[33-42, A133-
42 and 4-42
predominate in the AD brain, of which A131-42 and A134-42 are the main forms
in the
hippocampus and cortex of familial and sporadic AD (Portelius etal., 2010).
A13 ending at residue 42 is a minor component of the A13 species produced by
processing
of APP. Other forms include A131-40 and N-terminal truncates A6n-40. However,
A13 ending at
residue 42 is most prone to aggregate and drives the deposition into amyloid
plaques. In
addition to being more prone to aggregate, the A131-42 peptide forms soluble
low-n polymers (or
oligomers) that have been shown to be toxic to neurons in culture. Unlike the
larger
conspicuous fibril deposits, oligomers are not detected in typical pathology
assays. Oligomers
having similar properties have been isolated from AD brains and these are more
closely
associated to disease progression than the plaques (Younkin, 1998; Walsh
etal., 2005a; Walsh
et al., 2005b).
Experimentally generated oligomers applied to brain slices or injected in vivo
cause
failure of hippocampal long-term potentiation (LTP) which is a form of
synaptic information
storage well known as a paradigm for memory mechanisms (Lambert etal., 1998;
Walsh etal.,
2002; Wang et al., 2002). Soluble oligomers have been involved in the physical
degeneration of
synapses (Mucke etal., 2000). Reversal of memory failure by antibodies in
mouse models has
confirmed the emerging concept that oligomers have a major role to play in
synaptic failure.
Genetic evidence suggests that increased amounts of A131-42 and N-terminal
truncates
thereof (A6n-42)are produced in many, if not all, genetic conditions that
cause familial AD
(Borchelt etal., 1996; Duff etal., 1996; Scheuner etal., 1996; Citron etal.,
1998), pointing to
the possibility that amyloid formation may be caused either by increased
generation of A6n-42
or decreased degradation, or both (Glabe, 2000). In particular, familial AD
causing genetic
mutations in the APP gene and/or in the gene encoding the y-secretase complex
component
presenilin increased the production of A131-42 relative to A131-40. It has
also been proposed
that the absolute quantity of peptides produced within the brain might be less
important than the
ratio of A13 peptides (reflected in a changed A131-42 to A131-40 ratio) for
the generation of toxic
A13 species (De Strooper, 2007; Kuperstein etal., 2010). In addition, animal
models of amyloid
deposition, both mice and Drosophila, suggest that A131-42 is required for the
formation of
amyloid deposits (Greeve etal., 2004; lijima etal., 2004; McGowan etal.,
2005).
Results from a vaccination study in 2000 suggested possible new treatment
strategies
for AD. The PDAPP transgenic mouse, which overexpresses mutant human APP (in
which the
amino acid at position 717 is phenylalanine instead of the normal valine),
progressively
develops many of the neuropathological hallmarks of AD in an age- and brain
region-dependent
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
3
manner. Transgenic animals were immunised with A131-42 peptide either before
the onset of
AD-type neuropathologies (at 6 weeks of age) or at an older age (11 months),
when A13
deposition and several of the subsequent neuropathological changes were well
established.
Immunisation of the young animals essentially prevented the development of
plaque formation,
neuritic dystrophy and astrogliosis. Treatment of the older animals also
markedly reduced the
extent and progression of these AD-like neuropathologies. It was shown that
A131-42
immunisation resulted in the generation of anti-A13 antibodies and that A6-
immunoreactive
monocytic/microglial cells appear in the region of remaining plaques (Schenk
etal., 1999;
Schenk etal., 2000). However, the active immunisation approach when applied to
humans
resulted in several cases of meningoencephalitis, most likely due to a T-cell
response, and was
discontinued although the initial results on efficacy were promising (Orgogozo
et al., 2003;
Gilman etal., 2005; Pride etal., 2008).
Following this, several passive vaccination strategies were investigated. The
peripheral
administration of antibodies against A13 was sufficient to reduce amyloid
burden (Bard et al.,
2000). Despite relatively modest antibody serum levels achieved in these
experiments, the
passively administered antibodies were able to cross the blood-brain barrier
and enter the
central nervous system, decorate plaques and induce clearance of pre-existing
amyloid. In a
comparison between an A61-40-specific antibody, an A61-42-specific antibody
and an antibody
directed against residues 1-16 of A13, all antibodies were shown to reduce A13
accumulation in
mouse brain (Levites etal., 2006).
More recently, it has been suggested that CNS penetration is the most likely
route to
effective A13 clearance for passively administered antibodies (Golde et al.,
2009). However, in
addition to the antibodies being able to cross the blood-brain barrier, the
sink hypothesis was
proposed as a possible mechanism of action.
The sink hypothesis states that A13 can be removed from CNS indirectly by
lowering the
concentration of the peptide in the plasma. In the experiments describing
this, an antibody that
binds the A13 in the plasma and thereby sequesters A13 from the CNS was used.
This was
accomplished because the antibody prevents influx of A13 from the plasma to
CNS and/or
changes the equilibrium between the plasma and CNS due to a lowering of the
free A13
concentration in plasma (DeMattos etal., 2001). Amyloid binding agents
unrelated to
antibodies have also been shown to be effective in removing A13 from CNS
through binding in
plasma. Two A13 binding agents, gelsolin and GM1, which sequester plasma A13
were shown to
reduce or prevent brain amyloidosis (Matsuoka et al., 2003).
Regarding safety, one pathogenic feature in AD is cerebral amyloid angiopathy
(CAA)
where there is a replacement of vascular smooth muscle cells with A13, mainly
A131-40, in the
walls of cerebral arteries (Weller et al., 2003). Treating AD patients with
pan-A13 antibodies has
been shown to lead to microhemorrhages reflecting the removal of A13 from the
vessel wall
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
4
(Wilcock et al., 2009) which could be detrimental to patients. One way to
circumvent this has
been to generate de-glycosylated antibodies which may reduce the clearance
mechanisms
contributing to microhemorrhages and/or reduce the rate by which A13 is
cleared from the
vascular deposits, preventing saturation of efflux pathways (Wilcock etal.,
2006).
Targeting the n-4213 peptide species with an A1342 specific antibody would
target the
species which is the key peptide composite in the AD brain and the driver of
plaque formation.
An antibody with a primary specificity for n-42 monomer and low n oligomer
species would not
only deplete these species, but could also prevent the build-up of other
oligomeric species
shown to be toxic to neurons.
Summary of the Invention
This invention relates to fully human antibodies that are specific for A13 1-
42 and N-
terminal truncates thereof and bind to an epitope between amino acids 29-42 of
the A1342
peptide. Antibodies according to this invention may be used for the
preventative and/or
therapeutic treatment of conditions associated with beta amyloid such as AD,
including mild
cognitive impairment (MCI) due to AD, and Down's syndrome.
The invention concerns the use of fully human antibodies to suppress isoforms
of A13
peptide (n-42) in plasma, brain and cerebrospinal fluid (CSF) to prevent
accumulation or
reverse the deposition of A13 n-42 isoforms within the brain and
cerebrovasculature and to
improve cognition.
Described herein is the production of fully human antibodies to the A13 n-42
peptides,
which recognise monomer and low n oligomeric forms (up to and including
pentamer) of A13 n-
42 and are epitope mapped to a region encompassing amino acids 17-42 on the
A1342 peptide,
more specifically to a region encompassing amino acids 29 to 42 on the A1342
peptide.
Antibodies in accordance with the invention are specific for A13 n-42 species
(wherein n
is an integer in the range of from 1 to 29) and thus can be expected to
selectively reduce the
key driver of AD progression. Antibodies in accordance with the invention are
effective in
binding A1342 (not A[340) in human plasma, brain and cerebrospinal fluid (CSF)
leading to
increased clearance of A13 n-42 isoforms from the brain. Antibodies in
accordance with the
invention are also effective in reducing the binding of A1342 soluble
aggregates to neurons and
thus the portion of the antibody that enters the brain will have an effect on
the health of the
neurons.
Described herein are potent, high affinity antibodies, including an antibody
with a KD of
320 pM for monomer. Such high affinity may enable effective suppression of A13
n-42 to levels
enabling AD disease prevention and modification.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
The levels of soluble A1342 and A1340 species can be detected in the brain,
CSF and
blood with standardised assays using antibodies directed against epitopes on
the A13 peptide.
As shown in a rat PK:PD described herein, a dose-dependent suppression of free
A1342 was
observed in the CSF of rats post peripheral administration of antibody. Also
demonstrated is a
5 dose-dependent increase in total A1342 in the brain of rats with
negligible effect on A1340 peptide.
Thus, described herein are antibodies that have the capacity to penetrate the
brain
(0.1% of total peripheral administration in the CSF) and specifically suppress
the key toxic
species A1342 (not A[340) in the CSF.
The specificity and mechanism of action of antibodies according to the
invention may
enable both the prophylactic and therapeutic treatment of a number of diseases
linked to a
build-up of amyloid which accumulates within organs in the body including
different stages of
the AD disease process: prodromal, mild and moderate AD, Down's syndrome as
well as
macular degeneration.
Antibodies according to the invention may have the capacity to reverse
cognitive decline,
treat cognitive decline and prevent cognitive decline in subjects diagnosed
with prodromal, mild
to moderate AD and Down's Syndrome.
Accordingly, a first aspect of the invention relates to binding members for
human A[31-42,
especially antibody molecules.
Binding members, e.g. antibody molecules, according to the invention may have
any or
all of the following properties:
- Binding to soluble monomeric human A131-42 and/or oligomeric A131-
42;
- Selectivity in binding A[31-42 over A[31-40. They may show no
binding to A[31-40, or
binding may be negligible. For example, antibody molecules according to the
invention may bind monomeric A131-42 with a dissociation constant (KD) of 500
pM or
less. They may not bind A131-40, or may bind A131-40 with a KD greater than 1
mM;
- Binding to human A1317-42. Accordingly, the antibody molecule may
recognise an
epitope between amino acids 17-42 of the A[31-42 peptide, more specifically
the
antibody molecule may recognise an epitope between amino acids 29-42 of the
A131-
42 peptide;
- Binding to soluble monomeric human 3pyro-42 (pyroglutamate 3) and 11pyro-42
(pyroglutamate 11)
- Binding to human A131-43; and
- Cross-reactivity with murine A131-42.
A binding member may comprise a set of HCDRs and/or a set of LCDRs of an
antibody
molecule as described herein. Examples of antibody molecules according to the
invention
comprise a VH domain containing a set of HCDRs (HCDR1, HCDR2 and HCDR3) and a
VL
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
6
domain containing a set of LCDRs (LCDR1, LCDR2 and LCDR3), where the HCDRs and
LCDRs are the HCDRs and LCDRs respectively of any of the antibodies Abet0380,
Abet0007,
Abet0144, Abet0319, Abet0321b, Abet0322b, Abet0323b, Abet0328, Abet0329,
Abet0332,
Abet0342, Abet0343, Abet0344, Abet0368, Abet0369, Abet0370, Abet0371,
Abet0372,
Abet0373, Abet0374, Abet0377, Abet0378, Abet0379, Abet0381, Abet0382 and
Abet0383, or a
GL version thereof, whose sequences are shown in the appended sequence
listing.
Correspondence between the antibody molecules and the sequence identifiers in
the sequence
listing is indicated in Table 16.
An antibody molecule for human A[31-42 may comprise
(i) a VH domain comprising a set of HCDRs: HCDR1, HCDR2 and HCDR3,
interspersed
with framework regions, wherein the amino acid sequences of the set of HCDRs
are as shown
in Table 16 for any of antibodies Abet0380, Abet0007, Abet0144, Abet0319,
Abet0321b,
Abet0322b, Abet0323b, Abet0328, Abet0329, Abet0332, Abet0342, Abet0343,
Abet0344,
Abet0368, Abet0369, Abet0370, Abet0371, Abet0372, Abet0373, Abet0374,
Abet0377,
Abet0378, Abet0379, Abet0381, Abet0382 and Abet0383 or a GL version thereof,
or may comprise that set of HCDRs with one or two amino acid mutations; and
(ii) a VL domain comprising a set of LCDRs: LCDR1, LCDR2 and LCDR3,
interspersed
with framework regions, wherein the amino acid sequences of the set of LCDRs
are as shown
in Table 16 for any of antibodies Abet0380, Abet0007, Abet0144, Abet0319,
Abet0321b,
Abet0322b, Abet0323b, Abet0328, Abet0329, Abet0332, Abet0342, Abet0343,
Abet0344,
Abet0368, Abet0369, Abet0370, Abet0371, Abet0372, Abet0373, Abet0374,
Abet0377,
Abet0378, Abet0379, Abet0381, Abet0382 and Abet0383, or a GL version thereof,
or may comprise that set of LCDRs with one or two amino acid mutations.
An antibody molecule according to the invention may comprise
(i) a VH domain comprising the Abet0380 or Abet0380 GL set of HCDRs, wherein
the
amino acid sequences of the Abet0380 HCDRs are
HCDR1 SEQ ID NO: 525,
HCDR2 SEQ ID NO: 526, and
HCDR3 SEQ ID NO: 527,
or may comprise the Abet0380 or Abet0380 GL set of HCDRs with one or two amino
acid mutations, and
(ii) a VL domain comprising the Abet0380 or Abet0380 GL set of LCDRs, wherein
the
amino acid sequences of the Abet0380 LCDRs are
LCDR1 SEQ ID NO: 534
LCDR2 SEQ ID NO: 535, and
LCDR3 SEQ ID NO: 536,
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
7
or may comprise the Abet0380 or Abet0380 GL set of LCDRs with one or two amino
acid mutations.
The antibody molecule may comprise
(i) a VH domain comprising a set of HCDRs: HCDR1, HCDR2 and HCDR3,
interspersed with framework regions, wherein the amino acid sequences of the
HCDRs are
HCDR1 SEQ ID NO: 525,
HCDR2 SEQ ID NO: 526, and
HCDR3 SEQ ID NO: 527,
or may comprise that set of HCDRs with one or more amino acid substitutions,
wherein
the one or more substitutions are selected from those shown in Table 12 or
Table 14;
and
(ii) a VL domain comprising a set of LCDRs: LCDR1, LCDR2 and LCDR3,
interspersed
with framework regions, wherein the amino acid sequences of the LCDRs are
LCDR1 SEQ ID NO: 534
LCDR2 SEQ ID NO: 535, and
LCDR3 SEQ ID NO: 536,
or may comprise that set of LCDRs with one or more amino acid substitutions,
wherein
the one or more substitutions are selected from those shown in Table 13 or
Table 15.
The VH domain of the antibody molecule may comprise a FW1 region in which the
amino acid residues at Kabat positions 26-30 are selected from those shown in
Table 14.
The VH domain of the antibody molecule may comprise heavy chain framework
regions
FW1, FW2, FW3 and FW4, wherein the amino acid sequences of the heavy chain
framework
regions are
FW1 SEQ ID NO: 528
FW2 SEQ ID NO: 529
FW3 SEQ ID NO: 530, and
FW4 SEQ ID NO: 531
or wherein FW1 comprises SEQ ID NO: 528 with one or more amino acid
substitutions,
wherein the one or more substitutions in FW1 are selected from those shown in
Table 12 or
Table 14.
The VL domain of the antibody molecule may comprise light chain framework
regions
FW1, FW2, FW3 and FW4, wherein the amino acid sequences of the light chain
framework
regions are
FW1 SEQ ID NO: 537
FW2 SEQ ID NO: 538
FW3 SEQ ID NO: 539, and
FW4 SEQ ID NO: 540.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
8
An antibody molecule according to the invention may comprise
(i) a VH domain amino acid sequence as shown in Table 16 for any of
Abet0380, Abet0343,
Abet0369, Abet0377 and Abet0382, or a GL version thereof,
or may comprise that amino acid sequence with one or two amino acid mutations;
and
(ii) a VL domain amino acid sequence as shown in Table 16 for any of
Abet0380, Abet0343,
Abet0369, Abet0377 and Abet0382, or a GL version thereof,
or may comprise that amino acid sequence with one or two amino acid mutations.
An antibody molecule according to the invention may comprise a VH domain
having an
amino acid sequence at least 85% identical to SEQ ID NO: 524 and a VL domain
having an
amino acid sequence at least 85 % identical to SEQ ID NO: 533, wherein in the
VH domain:
amino acid 26 is M, G or S;
amino acid 27 is G, F or D;
amino acid 28 is N, T, D or H,
amino acid 29 is F
amino acid 30 is N, S, K, or P;
amino acid 31 is Y, V, R, E, or T;
amino acid 32 is Q, Y, D, S, or E;
amino acid 33 is T, P, I, or V;
amino acid 34 is M;
amino acid 35 is W;
amino acid 50 is V;
amino acid 51 is I;
amino acid 52 is G;
amino acid 52a is K, S, or A;
amino acid 53 is T, S, N, D, G, or Q;
amino acid 54 is N, G, T, or P;
amino acid 55 is E, G, N, K, or T;
amino acid 56 is N, T, R, or K;
amino acid 57 is I, T, K, or V;
amino acid 58 is A, V, or T;
amino acid 59 is Y;
amino acid 60 is A;
amino acid 61 is D;
amino acid 62 is S;
amino acid 63 is V;
amino acid 64 is K;
amino acid 65 is G;
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
9
amino acid 95 is E;
amino acid 96 is W;
amino acid 97 is M
amino acid 98 is D;
amino acid 99 is H;
amino acid 100 is S;
amino acid 100a is R;
amino acid 100b is P;
amino acid 100c is Y;
amino acid 100d is Y;
amino acid 100e is Y;
amino acid 100f is Y;
amino acid 100g is G;
amino acid 100h is M;
amino acid 101 is D;
amino acid 102 is V;
and wherein in the VL domain:
amino acid 24 is S;
amino acid 25 is G;
amino acid 26 is H;
amino acid 27 is N;
amino acid 28 is L, or I;
amino acid 29 is E, or G;
amino acid 30 is D;
amino acid 31 is K;
amino acid 32 is F, or W;
amino acid 33 is A, or V;
amino acid 34 is S;
amino acid 50 is R;
amino acid 51 is D;
amino acid 52 is D;
amino acid 53 is K;
amino acid 54 is R;
amino acid 55 is P;
amino acid 56 is S;
amino acid 89 is S, or Q;
amino acid 90 is S, or A;
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
amino acid 91 is Q;
amino acid 92 is D;
amino acid 93 is T, or S;
amino acid 94 is V, or T;
5 amino acid 95 is T;
amino acid 96 is R;
amino acid 97 is V.
An antibody molecule according to the invention may comprise a VH domain
having an
amino acid sequence at least 85% identical to SEQ ID NO: 524 and a VL domain
having an
10 amino acid sequence at least 85% identical to SEQ ID NO: 533, wherein in
the VH domain:
amino acid 26 is M, G, S, V, A, N, T, or H;
amino acid 27 is G, F, S, Y, E, D, or P;
amino acid 28 is N, Q, H, V, E, T, A, S, D, M, or P;
amino acid 29 is F, I, Y, S, L, or W;
amino acid 30 is N, S, T, Q, K, H, R, G, P, E, K, A, or D;
amino acid 31 is Y, H, K, E, N, T, R, V, P, M, F, I, D, or W;
amino acid 32 is Q, Y, D, N, S, E, or T;
amino acid 33 is T, P, I, or V;
amino acid 34 is M, or L;
amino acid 35 is W;
amino acid 50 is V;
amino acid 51 is I;
amino acid 52 is G;
amino acid 52a is K, S, P, A, N, G, E, D, V, or T;
amino acid 53 is T, S, N, H, Q, D, G, or E;
amino acid 54 is N, G, P, T, Q, E, M, K, or A;
amino acid 55 is E, G, K, N, Q, T, H, D, or A;
amino acid 56 is N, T, A, R, or K;
amino acid 57 is I, T, N, S, K, F, Q, V, or L;
amino acid 58 is A, V, S, T, or N;
amino acid 59 is Y;
amino acid 60 is A;
amino acid 61 is D;
amino acid 62 is S, A, or T;
amino acid 63 is V;
amino acid 64 is K;
amino acid 65 is G;
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
11
amino acid 95 is E;
amino acid 96 is W;
amino acid 97 is M
amino acid 98 is D, or G;
amino acid 99 is H, or R;
amino acid 100 is S;
amino acid 100a is R;
amino acid 100b is P;
amino acid 100c is Y;
amino acid 100d is Y;
amino acid 100e is Y;
amino acid 100f is Y;
amino acid 100g is G;
amino acid 100h is M, or I;
amino acid 101 is D;
amino acid 102 is V, or A;
and wherein in the VL domain:
amino acid 24 is S, or T;
amino acid 25 is G, or T;
amino acid 26 is H, R, or P;
amino acid 27 is N, or H;
amino acid 28 is L, I, V, F, or T;
amino acid 29 is E, M, G, S, or N;
amino acid 30 is D, A, S, G, or H;
amino acid 31 is K, or S;
amino acid 32 is F, or W;
amino acid 33 is A, V, M, T, or I;
amino acid 34 is S, T, or A;
amino acid 50 is R;
amino acid 51 is D;
amino acid 52 is D;
amino acid 53 is K;
amino acid 54 is R;
amino acid 55 is P;
amino acid 56 is S;
amino acid 89 is S, Q, or A;
amino acid 90 is S, A, or T;
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
12
amino acid 91 is Q
amino acid 92 is D, or G;
amino acid 93 is T, Q, S, N, or K;
amino acid 94 is V, T, or F;
amino acid 95 is T;
amino acid 96 is R;
amino acid 97 is V, S, or A.
An antibody molecule according to the invention may comprise:
(i) a VH domain having an amino acid sequence at least 90 % identical to a
VH domain
amino acid sequence shown in Table 16 for any of Abet0380, Abet0343, Abet0369,
Abet0377
and Abet0382, or a GL version thereof; and
(ii) a VL domain having an amino acid sequence at least 90 % identical to a
VL domain
amino acid sequence shown in Table 16 for any of Abet0380, Abet0343, Abet0369,
Abet0377
and Abet0382, or a GL version thereof.
The antibody molecule may comprise a VH domain and a VL domain at least 90 %
identical with the VH domain and VL domain, respectively, of any of Abet0380,
Abet0343,
Abet0369, Abet0377 and Abet0382, or a GL version thereof.
The indicated percentage identity of the VH and/or VL domain may be at least
95 %, at
least 98 % or at least 99 %.
The antibody molecule may comprise the VH domain and VL domain of any of
Abet0380,
Abet0343, Abet0369, Abet0377 and Abet0382 or a GL version thereof.
For example, the antibody molecule may comprise the Abet0380-GL VH domain
amino
acid sequence SEQ ID NO: 524 and the Abet0380-GL VL domain amino acid sequence
SEQ ID
NO: 533.
An antibody molecule according to the invention may be one that competes for
binding
to A131-42 with:
(i) an antibody molecule comprising a VH domain amino acid sequence SEQ ID
NO: 524 and a VL domain amino acid sequence SEQ ID NO: 533,
(ii) an antibody molecule encoded by nucleic acid deposited under accession
number NCIMB 41890, 41891 or 41892.
An antibody molecule may comprise a VH domain and a VL domain encoded by:
(i) the Abet0380-GL nucleic acid sequence deposited under accession number
41890;
(ii) the Abet0144-GL nucleic acid sequence deposited under accession number
41891; or
(ii) the Abet0377-GL nucleic acid sequence deposited under accession number
41892.
The antibody molecule may comprise a VH domain and a VL domain comprising the
HCDRs and LCDRs, respectively, of a deposited antibody mentioned above. The
antibody
molecule may be the antibody encoded by the deposited nucleic acid mentioned
above.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
13
Also described herein are nucleic acid molecules encoding binding members
according
to the invention, host cells containing the nucleic acid, and methods of
producing the binding
members by expressing the nucleic acid and recovering the binding member.
Further aspects of the invention relate to compositions comprising an antibody
molecule
according to any of the preceding claims, and one or more additional
components, such as a
pharmaceutically acceptable excipient, and to such compositions for medical
use.
Compositions comprising binding members according to the present invention may
be provided
for use in a method of treatment of the human or animal body.
Binding members described herein may be used in methods of diagnosis or
treatment in
human or animal subjects, e.g. humans. Binding members of the invention may be
used to
decrease levels of A131-42 in an individual and/or to reduce amyloidosis.
Binding members may
be used to reduce amyloidosis and to treat, reduce or prevent conditions
associated with
amyloidosis. Conditions and diseases that may be treated include Alzheimer's
disease, such as
prodomal, mild or moderate AD. AD treated by the invention may be familial or
sporadic AD.
The invention may be used to prevent, reduce or reverse mild cognitive
impairment (MCI)
associated with AD. Cognition may be improved, and/or cognitive decline may be
lessened, in
AD patients or Down's syndrome patients. The invention may also be used to
treat or prevent
macular degeneration, which is linked with amyloid beta (Ding etal. PNAS
108(28):E279-287
2011).
Accordingly, in a further aspect, the invention provides a method of reducing
amyloidosis,
treating Alzheimer's disease, improving cognition or reducing cognitive
decline in Alzheimer's
disease or Down's syndrome, and/or treating macular degeneration in an
individual, comprising
administering a binding member of the invention to the individual.
These and other aspects of the invention are described in more detail below.
Brief Description of the Drawings
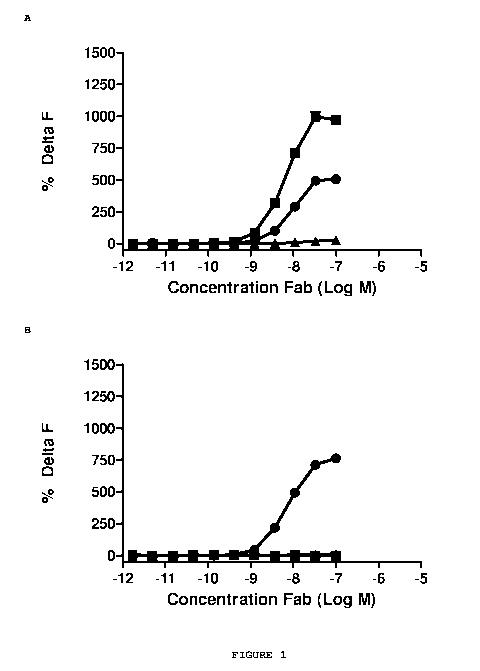
Figure 1 shows the results of the direct binding HTRFTm assay between the
purified
Abet0007 Fab and a series of Amyloid beta peptides. The Abet0007 clone (.)
shows binding to
the human Amyloid beta 1-42 peptide (Figure 1A) and the murine Amyloid beta 1-
42 peptide
(Figure 1C) but shows no binding to the human Amyloid beta 1-40 peptide
(Figure 1B) or the
scrambled human Amyloid beta 1-42 peptide (Figure 1D). The positive control
antibody (.) and
the negative control antibody (=) were used to verify the integrity of the
assay.
Figure 2 shows the inhibition of the formation of the biotinylated human
Amyloid beta 1-
42 peptide and Abet0007 IgG2 complex by increasing concentrations of
competitor peptides.
Complex formation is inhibited by human Amyloid beta 1-42 (.), 11-42 (=), 17-
42(Y) and 1-43
(+) peptides. It is not inhibited by human Amyloid beta 1-40 peptide (.) or by
the negative control
peptide (0).
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
14
Figure 3 shows the Surface Plasmon Resonance (BlAcore) traces for the human
Amyloid beta 1-42 peptide binding to immobilised Abet0007 IgG2 at
concentrations of 100 nM
(top trace), 50 nM, 25 nM, 12.5 nM, 6.2 nM and 3.1 nM (bottom trace) peptide.
Each trace is
fitted to a 1:1 Langmuir model.
Figure 4 shows sample images from the in vitro immunohistochemical staining of
Abet0007 IgG2. (A) A positive control antibody shows strong plaque recognition
(score = 4) on
human AD brain sections (ApoE genotype 3/3; Braak stage 6; 20 pg/ml antibody).
(B) The
Abet0007 IgG2 lead clone shows no plaque recognition (score = 0) on an
adjacent brain section
(20 jig/m1). (C) The same positive control antibody shows strong plaque
recognition (score = 4)
on Tg2576 mouse brain sections (18 month old mice; 20 pg/ml antibody). (D) The
Abet0007
IgG2 lead clone shows no plaque recognition (score = 0) on an adjacent mouse
brain section
(20 jig/m I).
Figure 5 shows the inhibition of the formation of the human Amyloid beta 1-42
and
Abet0042 IgG complex by increasing concentrations of Abet0007 scFv (A) and
Abet0144 scFv
(=). The Abet0144 clone is significantly more potent than the Abet0007 parent
clone in this
assay. A negative control antibody (.) is included for comparison.
Figure 6 shows the Surface Plasmon Resonance (BlAcore) traces for the purified
Abet0144 scFv binding to immobilised human Amyloid beta 1-42 peptide at
concentrations of
400 nM (top trace), 200 nM, 100 nM, 50 nM and 12.5 nM (bottom trace) scFv.
Each trace is
fitted to a 1:1 Langmuir model.
Figure 7 shows the Surface Plasmon Resonance (BlAcore) traces for human
Amyloid
beta 1-42 peptide binding to immobilised Abet0144-GL IgG1-TM antibody at
concentrations of
50 nM (top trace), 25 nM, 12.5 nM, 6.25 nM, 3.13 nM and 1.56 nM (bottom trace)
peptide. Each
trace is fitted to a 1:1 Langmuir model.
Figure 8 shows the Surface Plasmon Resonance (BlAcore) traces for a series of
Amyloid beta peptides at 400 nM binding to immobilised Abet0144-GL IgG1-TM
antibody. There
is clear binding to the biotinylated human Amyloid beta 1-42 peptide (top
trace) and the
unlabelled human Amyloid beta 1-42 peptide (second trace). There is no
discernable binding to
scrambled biotinylated human Amyloid beta 1-42 peptide, biotinylated human
Amyloid beta 1-40
peptide, unlabelled human Amyloid beta 1-40 peptide or biotinylated-insulin
(flat lines).
Figure 9 shows specificity profiling of Abet0144-GL IgG1-TM using a
biochemical
epitope competition assay in which inhibition of the formation of a complex
between biotinylated
human Amyloid beta 1-42 peptide and Abet0144-GL IgG1-TM by increasing
concentrations of
competitor peptides is measured. Complex formation is inhibited by human
Amyloid beta 1-42
(*), pyro 3-42 (V) and pyro 11-42 (o) peptides. No significant inhibition is
observed with human
Amyloid beta 1-40 peptide (=), 1-16 (A) and 12-28(v) peptide truncates or with
the negative
control peptide (0).
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
Figure 10 shows sample images from the in vitro immunohistochemical staining
of
Abet0144-GL IgG1-TM. (A) A positive control antibody shows strong plaque
recognition (score
= 4) on human AD brain sections (ApoE genotype 3/3; 20 pg/ml antibody). (B)
The Abet0144-
GL IgG1-TM lead clone shows some plaque recognition (score = 1.5) on an
adjacent brain
5 section (20 jig/m1). (C) The same positive control antibody shows strong
plaque recognition
(score = 4) on Tg2576 mouse brain sections (18 month old mice; 20 pg/ml
antibody). (D) The
Abet0144-GL IgG1-TM lead clone shows some plaque recognition (score = 1) on an
adjacent
mouse brain section (20 jig/m1).
Figure 11 shows the inhibition of the formation of the human Amyloid beta 1-42
peptide
10 and Abet0144-GL IgG1-TM complex by increasing concentrations of purified
competitor scFv
(.). Four of the most potent scFv clones, Abet0369 (Figure 11A), Abet0377
(Figure 11B),
Abet0380 (Figure 110) and Abet0382 (Figure 11D) all show significant
improvement in potency
over the parent Abet0144-GL scFv sequence (.).
Figure 12 shows the Surface Plasmon Resonance (BlAcore) traces for human
Amyloid
15 beta 1-42 peptide binding to immobilised Abet0380-GL IgG1-TM antibody at
concentrations
from 1024 nM (top trace) to 63 pM (bottom trace) peptide. Each trace is fitted
to a 1:1 Langmuir
model.
Figure 13 shows the Surface Plasmon Resonance (BlAcore) traces for a series of
Amyloid beta peptides binding to immobilised Abet0380-GL IgG1-TM antibody.
There is clear
binding to the biotinylated human Amyloid beta 1-42 peptide (top trace) and
the unlabelled
murine Amyloid beta 1-42 peptide (second trace). There is no discernable
binding to
biotinylated human Amyloid beta 1-40 peptide or unlabelled murine Amyloid beta
1-40 peptide
(flat lines).
Figure 14 shows sample images from the in vitro immunohistochemical staining
of
Abet0380-GL IgG1-TM. (A) A positive control antibody shows strong plaque
recognition (score
= 4) on human AD brain sections (ApoE genotype 3/3, Braak stage 6; 5 pg/ml
antibody). (B)
The Abet0380-GL IgG1-TM lead clone shows strong plaque recognition (score = 3)
on an
adjacent brain section (10 jig/m1). (C) The same positive control antibody
shows strong plaque
recognition (score = 4) on Tg2576 mouse brain sections (22 month old mice; 20
pg/ml antibody).
(D) The Abet0380-GL IgG1-TM lead clone shows strong plaque recognition (score
= 4) on an
adjacent mouse brain section (20 jig/m1).
Figure 15 shows Western Blot analysis of Abeta 42 aggregate preparation and
detection
using the Abet0380-GL IgG1TM. (A) Abet0380-GL IgG1TM detection of non-photo
cross-linked
(non PICUP) A1342 aggregate. (B) Abet0380-GL IgG1TM detection of photo cross-
linked A1342
aggregate (PICUP). Here we demonstrate that Abet0380-GL IgG1TM specifically
recognises
A131-42 monomer and low n oligomer species up to and including pentamer.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
16
Figure 16 shows the dose-dependent reduction of the level of free Amyloid beta
1-42
peptide in the CSF (A), the increase of total Amyloid beta 1-42 peptide in
brain tissue (B) and
the unaffected levels of total Amyloid beta 1-40 peptide in brain tissue (C)
by increasing doses
of Abet0380-GL IgG1-TM antibody in Sprague-Dawley rats receiving repeated
weekly doses
over 14 days.
Figure 17 shows sample images from the immunohistochemical analysis of binding
of
Abet0380-GL IgG1-TM to Amyloid beta plaques in vivo 168 hours after a
peripheral dose to
aged Tg2576 mice. A positive control antibody given at 30 mg/kg shows strong
in vivo plaque
recognition (A), whereas Abet0380-GL IgG1-TM given at 30(B) or 10(0) mg/kg
does not show
any in vivo plaque decoration.
Figure 18 shows the specificity of Abet0380-GL IgG1-TM in competition binding
experiments with a range of different concentrations (10uM down to 0.17nM) of
a panel of full
length, truncate and pyro human Abeta peptides (Abeta 1-42, Abeta 1-43, Abeta
1-16, Abeta
12-28, Abeta 17-42, Abeta pyro-3-42, or Abeta pyro-11-42). Key:
4,- Abeta 1-42
*- Abeta 1-43
/ Abeta 1-16
= Abeta 12-28
4,--- Abeta 17-42
ti- Abeta Pyro-3-42
0--- Abeta Pyro 11-42
O Vehicle 1 (DMS0)
= Vehicle 2 (NH4OH)
The x-axis shows the concentration of Abeta peptide in log M, the y-axis shows
% specific
binding. Inhibition of Abet0380-GL IgG1-TM: N-terminal Biotin Abeta 1-42
binding was
observed with Abeta 1-42, Abeta 1-43, Abeta 17-42, Abeta Pyro-3-42 & Abeta
Pyro-11-42 with
1050 values ranging from 10-8 to 10-9 molar for this group. No inhibition of
Abet0380-GL IgG1-
TM: N-terminal Biotin Abeta 1-42 binding was observed with Abeta 1-16 or Abeta
12-28.
Figure 19 shows the ability of antibody Abet0144-GL to sequester amyloid beta
1-42 in a
normal rat PK-PD study. The x-axis shows vehicle or concentration of Abet0144-
GL (10mg/kg,
or 40 mg/kg), the y-axis shows the concentration of total amyloid beta 1-42 in
CSF in pg/ml.
Free amyloid beta 1-42 in CSF was not significantly altered by either 10 or 40
mg/kg of
Abet0144-GL (5 and 18% increase respectively when compared with vehicle).
Total amyloid
beta 1-42 in CSF was significantly increased by 38% at 10 mg/kg, and by 139%
at 40 mg/kg.
Total amyloid beta 1-42 in brain tissue was also significantly increased, by
16% and 50% at 10
and 40 mg/kg respectively. Data from this study in normal rats, demonstrate
that Abet0144-GL
had no significant effect on free amyloid beta 1-42 levels in CSF, whilst
increasing total amyloid
beta 1-42 levels in both CSF and brain.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
17
Detailed Description
By binding isoforms of A13 peptide 1-42 and N-terminal truncates thereof (n-
42) in
plasma, brain and cerebrospinal fluid (CSF), a binding member according to the
present
invention may prevent accumulation or reverse the deposition of A13 n-42
isoforms within the
brain and cerebrovasculature. Binding members according to the present
invention may bind
and precipitate soluble A131-42 in blood plasma and/or in cerebrospinal fluid
(CSF), thereby
reducing the concentration of A131-42 in the serum and/or CSF, respectively.
This represents a
therapeutic approach for Alzheimer's disease and other conditions associated
with amyloidosis.
Binding members are specific for the target epitope within A1317-42, more
specifically
within A1329-42, and bind this target epitope with high affinity relative to
non-target epitopes, for
example epitopes from A[31-40, thereby targeting the main toxic species linked
with amyloid
plaque formation. For example, a binding member may display a binding affinity
for A131-42
which is at least 10-fold, at least 100-fold, at least 1000-fold or at least
10,000-fold greater than
for A[31-40. Thus, the binding member is selective for binding A[31-42 over
A[31-40. As noted
above, the binding member may bind A131-42 with a dissociation constant (KD)
of 500 pM or
less. Preferably, it shows no significant binding to A131-40. Affinity and
binding can be
determined using surface plasmon resonance using monomeric A13 peptide, as
described in the
Examples.
Binding to A13 can also be measured in a homogenous time resolved fluorescence
(HTRFTm) assay, to determine whether the antibody is able to compete for
binding to A13 with a
reference antibody molecule to the A13 peptide, as described in the Examples.
An HTRFTm assay is a homogeneous assay technology that utilises fluorescence
resonance energy transfer between a donor and acceptor fluorophore that are in
close proximity.
Such assays can be used to measure macromolecular interactions by directly or
indirectly
coupling one of the molecules of interest to a donor fluorophore, europium
(Eu3+) cryptate, and
coupling the other molecule of interest to an acceptor fluorophore XL665, (a
stable cross linked
allophycocyanin). Excitation of the cryptate molecule (at 337 nm) results in
fluorescence
emission at 620nm. The energy from this emission can be transferred to XL665
in close
proximity to the cryptate, resulting in the emission of a specific long-lived
fluorescence (at 665
nm) from the XL665. The specific signals of both the donor (at 620 nm) and the
acceptor (at
665 nm) are measured, allowing the calculation of a 665/620 nm ratio that
compensates for the
presence of coloured compounds in the assay.
A binding member according to the invention may compete for binding to A131-42
and
thus inhibit binding of the reference antibody in an HTFRTm competition assay
with A[31-42, but
not with A131-40. A binding member may show at least 70%, at least 75%, at
least 80%, at
least 85 % or at least 90 % inhibition of Abet0144GL for binding to A[31-42 in
an HTRFTm assay.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
18
Potency of inhibition of binding may be expressed as an 1050 value, in nM
unless
otherwise stated. In functional assays, 1050 is the concentration of an
antibody molecule that
reduces a biological response by 50% of its maximum. In ligand-binding
studies, 1050 is the
concentration that reduces receptor binding by 50% of maximal specific binding
level. 1050 may
be calculated by plotting % of maximal biological response as a function of
the log of the binding
member concentration, and using a software program, such as Prism (Graph Pad)
or Origin
(Origin Labs) to fit a sigmoidal function to the data to generate IC50 values.
Suitable assays for
measuring or determining potency are well known in the art.
A binding member may have an IC50 of 5 nM or less, e.g. 2 nM or less, e.g. 1
nM or less,
in HTRFTm epitope competition assay with Abet0144-GL and A131-42. Abet0144-GL
is an
antibody molecule having VH domain SEQ ID NO: 20 and VL domain SEQ ID NO: 29.
It may
be used in the assay in the same format as the antibody molecule to be tested,
for example in
scFv or IgG, e.g. IgG1 format. Thus, IgG antibody molecules according to the
invention may
compete with Abet0144-GL IgG for binding to human A131-42 in an HTRF epitope
competition
assay. Potency in such an assay may be less than 1 nM.
A binding member according to the invention may show specific binding for A[31-
42 over
A131-40, as determined by an HTRFTm competition assay. In such an assay, A131-
40 may show
no significant inhibition of the binding member binding to the A131-42
peptide, e.g. it may show
less than 20 %, e.g. less than 10 % or less than 5 %, inhibition in such an
assay, and preferably
shows no significant inhibition in such an assay.
Binding members according to the invention recognise an epitope within human
A1317-42,
more specifically within human A1329-42 and may also recognise their target
epitope in A13 from
other species, e.g. mouse or rat. The potency of a binding member as
calculated in an HTRFTm
competition assay using A[31-42 from a first species (e.g. human) may be
compared with
potency of the binding member in the same assay using A131-42 from a second
species (e.g.
mouse A[31-42), in order to assess the extent of cross-reactivity of the
binding member for A131-
42 of the two species. Potency, as determined by IC50 measurements, may be
within 10-fold or
within 100-fold. As noted above, Abet0144GL may be used as reference antibody
in the
HTRFTm competition assay. Binding members described herein may have a greater
potency in
a human A131-42 assay than in a non-human A131-42 assay.
A binding member may comprise an antibody molecule having one or more CDRs,
e.g. a
set of CDRs, within an antibody framework (i.e. an antibody antigen-binding
domain). For
example, an antibody molecule may comprise an antibody VH and/or VL domain. VH
and VL
domains of antibody molecules are also provided as part of the invention. As
is well-known, VH
and VL domains comprise complementarity determining regions, ("CDRs"), and
framework
regions, ("FWs"). A VH domain comprises a set of HCDRs and a VL domain
comprises a set of
LCDRs. An antibody molecule may comprise an antibody VH domain comprising a VH
CDR1,
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
19
CDR2 and CDR3 and/or an antibody VL domain comprising a VL CDR1, CDR2 and
CDR3. VH
or VL domains may further comprise a framework. A VH or VL domain framework
typically
comprises four framework regions, FW1, FW2, FW3 and FW4, which are
interspersed with
CDRs in the following structure: FW1 - CDR1 - FW2 - CDR2 - FW3 - CDR3 - FW4.
Examples of antibody VH and VL domains, FWs and CDRs according to aspects of
the
invention are listed in Tables 5 and 6 and the appended sequence listing that
forms part of the
present disclosure. All VH and VL sequences, CDR sequences, sets of CDRs, sets
of HCDRs
and sets of LCDRs disclosed herein, as well as combinations of these elements,
represent
aspects of the invention. As described herein, a "set of CDRs" comprises CDR1,
CDR2 and
CDR3. Thus, a set of HCDRs refers to HCDR1, HCDR2 and HCDR3, and a set of
LCDRs refers
to LCDR1, LCDR2 and LCDR3. Unless otherwise stated, a "set of CDRs" includes
HCDRs and
LCDRs. Typically antibody molecules of the invention are monoclonal
antibodies.
In other embodiments, a binding member may comprise an antigen-binding site
within a
non-antibody molecule, normally provided by one or more CDRs e.g. a set of
CDRs in a non-
antibody protein scaffold, as discussed further below.
The isolation of a parent antibody molecule designated Abet0007, followed by
directed
mutation of CDR3 and selection of an optimised antibody Abet0144, germlined to
Abet0144-GL
with a set of CDR sequences and framework sequences as shown in Tables 5, 6
and the
sequence listing, is described herein. Through an extensive process of further
optimisation and
recombination of multiple libraries as described in the Examples, a panel of
antibody clones was
generated from Abet0144GL. These further optimised clones are designated
Abet0380,
Abet0319, Abet0321b, Abet0322b, Abet0323b, Abet0328, Abet0329, Abet0332,
Abet0342,
Abet0343, Abet0369, Abet0370, Abet0371, Abet0372, Abet0373, Abet0374,
Abet0377,
Abet0378, Abet0379, Abet0381, Abet0382 and Abet0383. Their CDR sequences and
variable
domain sequences are referenced in Tables 5 and 6 and set out in the sequence
listing.
Germlined VH and VL domain sequences Abet0380GL, Abet0377GL, Abet0343GL,
Abet0369GL and Abet0382GL are shown in Table 8 and Table 9.
For example, Tables 5 and 6 show that Abet0380 has a set of CDRs, in which
HCDR1 is
SEQ ID NO: 525 (Kabat residues 31-35), HCDR2 is SEQ ID NO: 526 (Kabat residues
50-65),
HCDR3 is SEQ ID NO: 527 (Kabat residues 95-102), LCDR1 is SEQ ID NO: 534
(Kabat
residues 24-34), LCDR2 is SEQ ID NO: 535 (Kabat residues 50-56) and LCDR3 is
SEQ ID NO:
536 (Kabat residues 89-97). The other optimised antibody clones are shown in
Tables 5 and 6
in a similar way and are also provided as aspects of the invention.
A binding member for human A[31-42 in accordance with the invention may
comprise
one or more CDRs as described herein, e.g. a set of CDRs. The CDR or set of
CDRs may be
an Abet0380, Abet0319, Abet0321b, Abet0322b, Abet0323b, Abet0328, Abet0329,
Abet0332,
Abet0342, Abet0343, Abet0369, Abet0370, Abet0371, Abet0372, Abet0373,
Abet0374,
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
Abet0377, Abet0378, Abet0379, Abet0381, Abet0382 and Abet0383 set of CDRs, or
a
germlined version thereof, or may be a variant thereof as described herein.
In some embodiments;
HCDR1 is may be 5 amino acids long, consisting of Kabat residues 31-35;
5 HCDR2 may be 17 amino acids long, consisting of Kabat residues 50-65;
HCDR3 may be 16 amino acids long, consisting of Kabat residues 95-102;
LCDR1 may be 11 amino acids long, consisting of Kabat residues 24-34;
LCDR2 may be 7 amino acids long, consisting of Kabat residues 50-56; and/or
LCDR3 may be 9 amino acids long, consisting of Kabat residues 89-97.
10 Binding members may comprise a HCDR1, HCDR2 and/or HCDR3 and/or an
LCDR1,
LCDR2 and/or LCDR3 of any of the antibodies listed in Tables 5 and 6, e.g., a
set of CDRs of
any of the antibodies listed in Table 5 or 6. The binding member may comprise
a set of VH
CDRs of any one of these antibodies. Optionally, it may also comprise a set of
VL CDRs of one
of these antibodies. The VL CDRs may be from the same or a different antibody
as the VH
15 CDRs. A VH domain comprising a set of HCDRs of any of the antibodies
listed in Tables 5,
and/or a VL domain comprising a set of LCDRs of any of the antibodies listed
in Tables 6, are
also provided herein.
A binding member may comprise a set of H and/or L CDRs of any of the
antibodies
listed in Tables 5 and 6 with one or more amino acid mutations, e.g. up to 5,
10 or 15 mutations,
20 within the disclosed set of H and/or L CDRs. A mutation may be an amino
acid substitution,
deletion or insertion. For example, an antibody molecule of the invention may
comprise the set
of H and/or L CDRs from any one of Abet0380, Abet0319, Abet0321b, Abet0322b,
Abet0323b,
Abet0328, Abet0329, Abet0332, Abet0342, Abet0343, Abet0369, Abet0370,
Abet0371,
Abet0372, Abet0373, Abet0374, Abet0377, Abet0378, Abet0379, Abet0381, Abet0382
and
Abet0383, or a germlined version thereof, with one or two amino acid
mutations, e.g.
substitutions.
For example, the binding member may comprise
a VH domain comprising the Abet0380 or Abet0380GL set of HCDRs, wherein the
amino acid sequences of the Abet0380 or Abet0380GL HCDRs are
HCDR1 SEQ ID NO: 525,
HCDR2 SEQ ID NO: 526, and
HCDR3 SEQ ID NO: 527,
or comprising the Abet0380 set of HCDRs with one or two amino acid mutations,
and
(ii) a VL domain comprising the Abet0380 or Abet0380GL set of LCDRs, wherein
the
amino acid sequences of the Abet0380 or Abet0380GL LCDRs are
LCDR1 SEQ ID NO: 534
LCDR2 SEQ ID NO: 535, and
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
21
LCDR3 SEQ ID NO: 536,
or comprising the Abet0380 or Abet0380GL set of LCDRs with one or two amino
acid
mutations.
Mutations may potentially be made at any residue within the set of CDRs. In
some
embodiments, substitutions may be made at the positions substituted in any of
Abet0380,
Abet0319, Abet0321b, Abet0322b, Abet0323b, Abet0328, Abet0329, Abet0332,
Abet0342,
Abet0343, Abet0369, Abet0370, Abet0371, Abet0372, Abet0373, Abet0374,
Abet0377,
Abet0378, Abet0379, Abet0381, Abet0382 and Abet0383 compared with Abet0144GL,
or at the
positions substituted in any of Abet0319, Abet0321b, Abet0322b, Abet0323b,
Abet0328,
Abet0329, Abet0332, Abet0342, Abet0343, Abet0369, Abet0370, Abet0371,
Abet0372,
Abet0373, Abet0374, Abet0377, Abet0378, Abet0379, Abet0381, Abet0382 and
Abet0383
compared with Abet0380, or germlined versions thereof, as shown in Tables 5
and 6.
For example, the one or more substitutions may be at one or more of the
following Kabat
residues:
26, 27, 28, 29 or 30 in VH FW1;
31, 32, 33, 34 or 35 in VH CDR1;
52a, 53, 54, 55, 56, 57, 58 or 62 in VH CDR2;
98, 99, 100h or 102 in VH CDR3;
24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 in VL CDR1;
89, 90, 92, 93, 94 or 97 in VL CDR3.
Examples of possible amino acid substitutions at particular Kabat residue
positions are
shown in Tables 12 and 14 for the VH domain and Tables 13 and 15 for the VL
domain.
As described above, a binding member may comprise an antibody molecule having
one
or more CDRs, e.g. a set of CDRs, within an antibody framework. For example,
one or more
CDRs or a set of CDRs of an antibody may be grafted into a framework (e.g.
human framework)
to provide an antibody molecule. The framework regions may be of human
germline gene
segment sequences. Thus, the framework may be germlined, whereby one or more
residues
within the framework are changed to match the residues at the equivalent
position in the most
similar human germline framework. The skilled person can select a germline
segment that is
closest in sequence to the framework sequence of the antibody before
germlining and test the
affinity or activity of the antibodies to confirm that germlining does not
significantly reduce
antigen binding or potency in assays described herein. Human germline gene
segment
sequences are known to those skilled in the art and can be accessed for
example from the
VBASE compilation (VBASE, MRC Centre of Protein Engineering, UK, 1997,
http//mrc-
cpe.cam.ac.uk).
A binding member as described herein may be an isolated human antibody
molecule
having a VH domain comprising a set of HCDRs in a human germline framework,
e.g. Vh3-23
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
22
DP-47. Thus, the VH domain framework regions FW1, FW2 and/or FW3 may comprise
framework regions of human germline gene segment Vh3-23 DP-47 and/or may be
germlined
by mutating framework residues to match the framework residues of this human
germline gene
segment. FW4 may comprise a framework region of a human germline j segment.
The amino acid sequence of VH FW1 may be SEQ ID NO: 528. VH FW1 contains a
series of residues at Kabat positions 26-30 that are believed to contribute to
antigen binding
and/or to be important for structural conformation of the CDR1 loop.
Substitutions may be
included in SEQ ID NO: 528, for example to synergise with the selected
sequence of HCDR1.
The one or more substitutions may optionally be selected from those shown in
Table 12 or
Table 14.
The amino acid sequence of VH FW2 may be SEQ ID NO: 529. The amino acid
sequence of VH FW3 may be SEQ ID NO: 530. The amino acid sequence of VH FW4
may be
SEQ ID NO: 531.
Normally the binding member also has a VL domain comprising a set of LCDRs,
e.g. in a
human germline framework, e.g. V lambda 23-3 DPL-23. Thus, the VL domain
framework
regions may comprise framework regions FW1, FW2 and/or FW3 of human germline
gene
segment V lambda 23-3 DPL-23 and/or may be germlined by mutating framework
residues to
match the framework residues of this human germline gene segment. FW4 may
comprise a
framework region of a human germline j segment. The amino acid sequence of VL
FW1 may
be SEQ ID NO: 537. The amino acid sequence of VL FW2 may be SEQ ID NO: 538.
The
amino acid sequence of VL FW3 may be SEQ ID NO: 539. The amino acid sequence
of VL
FW4 may be SEQ ID NO: 540.
A germlined VH or VL domain may or may not be germlined at one or more Vernier
residues, but is normally not.
For example, an antibody molecule or a VH domain as described herein may
comprise
the following set of heavy chain framework regions:
FW1 SEQ ID NO: 528;
FW2 SEQ ID NO: 529;
FW3 SEQ ID NO: 530;
FW4 SEQ ID NO: 531;
or may comprise the said set of heavy chain framework regions with 1, 2, 3, 4,
5, 6 or 7
amino acid mutations, e.g. substitutions.
An antibody molecule or a VL domain as described herein may comprise the
following
set of light chain framework regions:
FW1 SEQ ID NO: 537;
FW2 SEQ ID NO: 538;
FW3 SEQ ID NO: 539;
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
23
FW4 SEQ ID NO: 540;
or may comprise the said set of light chain framework regions with 1, 2, 3, 4,
5, or 6
amino acid mutations, e.g. substitutions.
A non-germlined antibody molecule has the same CDRs, but different frameworks,
compared to a germlined antibody molecule. Of the antibody sequences shown
herein in the
appended sequence listing, sequences of Abet0144-GL, Abet0380-GL, Abet0377-GL,
Abet0343-GL, Abet0369-GL, and Abet0382-GL are germlined. Germlined antibodies
of other
antibody molecules whose sequences are disclosed herein may be produced by
germlining
framework regions of their VH and VL domain sequences, optionally to Vh3-23 DP-
47 in the VH
domain and V lambda 23-3 DPL-23 in the VL domain.
Typically, a VH domain is paired with a VL domain to provide an antibody
antigen-
binding site, although as discussed above a VH or VL domain alone may be used
to bind
antigen. For example, the Abet0380-GL VH domain (SEQ ID NO: 524) may be paired
with the
Abet0380-GL VL domain (SEQ ID NO:533), so that an antibody antigen-binding
site is formed
comprising both the Abet0380-GL VH and VL domains. Analogous embodiments are
provided
for the VH and VL domains of the other antibodies disclosed herein. In other
embodiments, the
Abet0380-GL VH is paired with a VL domain other than the Abet0380-GL VL. Light-
chain
promiscuity is well established in the art. Again, analogous embodiments are
provided by the
invention for the other VH and VL domains disclosed herein. Thus, a VH domain
comprising the
VH CDRs or the germlined VH domain sequence of any of Abet0319, Abet0321b,
Abet0322b,
Abet0323b, Abet0328, Abet0329, Abet0332, Abet0342, Abet0343, Abet0369,
Abet0370,
Abet0371, Abet0372, Abet0373, Abet0374, Abet0377, Abet0378, Abet0379,
Abet0380,
Abet0381, Abet0382 and Abet0383 may be paired with a VL domain comprising the
VL CDRs
or germlined VL domain from a different antibody e.g. the VH and VL domains
may be from
different antibodies selected from Abet0319, Abet0321b, Abet0322b, Abet0323b,
Abet0328,
Abet0329, Abet0332, Abet0342, Abet0343, Abet0369, Abet0370, Abet0371,
Abet0372,
Abet0373, Abet0374, Abet0377, Abet0378, Abet0379, Abet0380, Abet0381, Abet0382
and
Abet0383.
A binding member may comprise
(i) a VH domain amino acid sequence as shown in Table 16 or in the appended
sequence
listing for any of Abet0380, Abet0343, Abet0369, Abet0377 and Abet0382, or a
germlined
version thereof,
or comprising that amino acid sequence with one or two amino acid mutations;
and
(ii) a VL domain amino acid sequence as shown in Table 16 or in the
appended sequence
listing for any of Abet0380, Abet0343, Abet0369, Abet0377 and Abet0382, or a
germlined
version thereof,
or comprising that amino acid sequence with one or two amino acid mutations.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
24
An antibody molecule may comprise:
(i) a VH domain having an amino acid sequence at least 90 %, 95 % or 98
% identical to a
VH domain amino acid sequence shown in Table 16 for any of Abet0380, Abet0343,
Abet0369,
Abet0377 and Abet0382, or a germlined version thereof; and
(ii) a VL domain having an amino acid sequence at least 90 %, 95 % or 98 %
identical to a
VL domain amino acid sequence shown in Table 16 for any of Abet0380, Abet0343,
Abet0369,
Abet0377 and Abet0382, or a germlined version thereof.
It may comprise a VH domain and a VL domain at least 90 %, 95 % or 98 %
identical
with the VH domain and VL domain, respectively, of any of Abet0380, Abet0343,
Abet0369,
Abet0377 and Abet0382, or a germlined version thereof.
A binding member may comprise a VH domain and a VL domain in which;
(i) the VH domain amino acid sequence is shown in SEQ ID NO: 524 and the VL
domain
amino acid sequence is shown in SEQ ID NO: 533.
(ii) the VH domain amino acid sequence has 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10
amino acid
substitutions as compared to SEQ ID NO: 524 and the VL domain amino acid
sequence has 1,
2, 3,4, 5, 6, 7, 8,9 or 10 amino acid substitutions as compared to SEQ ID NO:
533; or
(iii) the VH domain amino acid sequence has at least 80%, at least 85%, at
least 90% or
at least 95% sequence identity with SEQ ID NO: 524 and the VL domain amino
acid sequence
has at least 80%, at least 85%, at least 90% or at least 95% sequence identity
with SEQ ID NO:
533.
In some embodiments, an antibody molecule may lack antibody constant regions,
for
example an scFv.
In other embodiments, an antibody molecule may comprise an antibody constant
region.
An antibody molecule may be a whole antibody such as an IgG, i.e. an IgG1,
IgG2, or IgG4, or
may be an antibody fragment or derivative as described below. Antibody
molecules can also
have other formats, e.g. IgG1 with YTE (Dall'Acqua et al. (2002) J.
Immunology, 169: 5171-
5180; Dall'Acqua et al. (2006) J Biol. Chem. 281(33):23514-24) and/or TM
mutations
(Oganesyan et al. (2008) Acta Cryst D64:700-4) in the Fc region.
The invention provides a binding member of the present invention with a
variant Fc
region, wherein the variant comprises a phenylalanine (F) residue at position
234, a
phenylalanine (F) residue or a glutamic acid (E) residue at position 235 and a
serine (S) residue
at position 331, as numbered by the EU index as set forth in Kabat. Such
mutation
combinations are hereinafter referred to as the triple mutant (TM).
A binding member as described herein may comprise a CDR, VH domain, VL domain,
antibody-antigen binding site or antibody molecule which is encoded by the
nucleic acid
sequences and/or the vector of any of:
(i) deposit accession number NCIMB 41889 (Abet0007);
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
(ii) deposit accession number NCIMB 41890 (Abet0380-GL);
(iii) deposit accession number NCIMB 41891 (Abet0144-GL);
(iv) deposit accession number NCIMB 41892 (Abet0377-GL).
A binding member as described herein may be produced or producible from the
nucleic
5 acid, vector or cell line of deposit accession number NCIMB 41889, 41890,
41891 or 41892.
For example, a binding member may be produced by expression of the nucleic
acid or vector of
the cell line of deposit accession number NCIMB 41890. The nucleic acid or
vector may be
expressed any convenient expression system. Alternatively, the binding member
may be
expressed by the cell line of deposit accession number NCIMB 41889, 41890,
41891 or 41892.
10 Aspects of the invention also provide nucleic acid encoding the VH
and/or VL domains,
which is contained in the cell line of accession number 41889, 41890, 41891 or
41892; a vector
comprising said nucleic acid, which is contained in the cell line of accession
number 41889,
41890, 41891 or 41892; and the cells or cell line of accession number 41889,
41890, 41891 or
41892.
15 A binding member according to the present invention may comprise an
antibody antigen
binding site or antibody molecule that competes for binding to human A131-42
with any antibody
molecule encoded by nucleic acid deposited under accession number 41889,
41890, 41891 or
41892, or with an antibody molecule that comprises the VH domain and VL domain
amino acid
sequences of Abet007, Abet0380-GL, Abet0144-GL or Abet0377-GL as set out in
the appended
20 sequence listing.
Binding member
The term binding member describes one member of a pair of molecules that bind
one
another. The members of a binding pair may be naturally derived or wholly or
partially
synthetically produced. One member of the pair of molecules has an area on its
surface, or a
25 cavity, which binds to and is therefore complementary to a particular
spatial and polar
organization of the other member of the pair of molecules. Examples of types
of binding pairs
are antigen-antibody, biotin-avidin, hormone-hormone receptor, receptor-
ligand, enzyme-
substrate. The present invention is concerned with antigen-antibody type
reactions.
A binding member normally comprises a molecule having an antigen-binding site.
For
example, a binding member may be an antibody molecule or a non-antibody
protein that
comprises an antigen-binding site.
An antigen binding site may be provided by means of arrangement of CDRs on non-
antibody protein scaffolds, such as fibronectin or cytochrome B etc. [Haan &
Maggos (2004)
BioCentury, 12(5): A1-A6; Koide et al. (1998) Journal of Molecular Biology,
284: 1141-1151;
Nygren etal. (1997) Current Opinion in Structural Biology, 7:463-4691], or by
randomising or
mutating amino acid residues of a loop within a protein scaffold to confer
binding specificity for a
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
26
desired target. Scaffolds for engineering novel binding sites in proteins have
been reviewed in
detail by Nygren et al. [supra]. Protein scaffolds for antibody mimics are
disclosed in
W000/34784, which is herein incorporated by reference in its entirety, in
which the inventors
describe proteins (antibody mimics) that include a fibronectin type III domain
having at least one
randomised loop. A suitable scaffold into which to graft one or more CDRs,
e.g. a set of
HCDRs or an HCDR3 and/or LCDR3, may be provided by any domain member of the
immunoglobulin gene superfamily. The scaffold may be a human or non-human
protein. An
advantage of a non-antibody protein scaffold is that it may provide an antigen-
binding site in a
scaffold molecule that is smaller and/or easier to manufacture than at least
some antibody
molecules. Small size of a binding member may confer useful physiological
properties, such as
an ability to enter cells, penetrate deep into tissues or reach targets within
other structures, or to
bind within protein cavities of the target antigen. Use of antigen binding
sites in non-antibody
protein scaffolds is reviewed in Wess, 2004 [Wess, L. In: BioCentury, The
Bernstein Report on
BioBusiness, 12(42), A1-A7, 2004]. Typical are proteins having a stable
backbone and one or
more variable loops, in which the amino acid sequence of the loop or loops is
specifically or
randomly mutated to create an antigen-binding site that binds the target
antigen. Such proteins
include the IgG-binding domains of protein A from S. aureus, transferrin,
tetranectin, fibronectin
(e.g. 10th fibronectin type III domain), lipocalins as well as gamma-
crystalline and other Affilin TM
scaffolds (Scil Proteins). Examples of other approaches include synthetic
"Microbodies" based
on cyclotides - small proteins having intra-molecular disulphide bonds,
Microproteins
(VersabodiesTM, Amunix) and ankyrin repeat proteins (DARPins, Molecular
Partners).
In addition to antibody sequences and/or an antigen-binding site, a binding
member may
comprise other amino acids, e.g. forming a peptide or polypeptide, such as a
folded domain, or
to impart to the molecule another functional characteristic in addition to
ability to bind antigen.
Binding members may carry a detectable label, or may be conjugated to a toxin
or a targeting
moiety or enzyme (e.g. via a peptidyl bond or linker). For example, a binding
member may
comprise a catalytic site (e.g. in an enzyme domain) as well as an antigen
binding site, wherein
the antigen binding site binds to the antigen and thus targets the catalytic
site to the antigen.
The catalytic site may inhibit biological function of the antigen, e.g. by
cleavage.
Although, as noted, CDRs can be carried by non-antibody scaffolds, the
structure for
carrying a CDR, e.g. CDR3, or a set of CDRs of the invention will generally be
an antibody
heavy or light chain sequence or substantial portion thereof in which the CDR
or set of CDRs is
located at a location corresponding to the CDR or set of CDRs of naturally
occurring VH and VL
antibody variable domains encoded by rearranged immunoglobulin genes. The
structures and
locations of immunoglobulin variable domains may be determined by reference to
Kabat, et al.,
1987 [Kabat, E.A. et al, Sequences of Proteins of Immunological Interest. 41h
Edition. US
Department of Health and Human Services. 1987] and updates thereof. A number
of academic
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
27
and commercial on-line resources are available to query this database. For
example, see Martin,
A.C.R. Accessing the Kabat Antibody Sequence Database by Computer PROTEINS:
Structure,
Function and Genetics, 25 (1996), 130-133 and the associated on-line resource,
currently at the
web address of http://www.bioinf.org.uk/abs/simkab.html.
By CDR region or CDR, it is intended to indicate the hypervariable regions of
the heavy
and light chains of the immunoglobulin as defined by Kabat etal. 1991 [Kabat,
E.A. et al. (1991)
Sequences of Proteins of Immunological Interest, 5th Edition. US Department of
Health and
Human Services, Public Service, NIH, Washington], and later editions. An
antibody typically
contains 3 heavy chain CDRs and 3 light chain CDRs. The term CDR or CDRs is
used here in
order to indicate, according to the case, one of these regions or several, or
even the whole, of
these regions which contain the majority of the amino acid residues
responsible for the binding
by affinity of the antibody for the antigen or the epitope which it
recognizes.
Among the six short CDR sequences, the third CDR of the heavy chain (HCDR3)
has
greater size variability (greater diversity essentially due to the mechanisms
of arrangement of
the genes which give rise to it). It may be as short as 2 amino acids although
the longest size
known is 26. CDR length may also vary according to the length that can be
accommodated by
the particular underlying framework. Functionally, HCDR3 plays a role in part
in the
determination of the specificity of the antibody (Segal etal., PNAS, 71:4298-
4302, 1974; Amit et
al., Science, 233:747-753, 1986; Chothia etal., J. Mol. Biol., 196:901-917,
1987; Chothia etal.,
Nature, 342:877- 883, 1989; Caton etal., J. Immunol., 144:1965-1968, 199;
Sharon etal.,
PNAS, 87:4814-4817, 1990; Sharon etal., J. Immunol., 144:4863-4869, 1990; and
Kabat etal.,
J. Immunol., 147:1709-1719, 1991).
Antibody Molecule
This describes an immunoglobulin whether natural or partly or wholly
synthetically
produced. The term also covers any polypeptide or protein comprising an
antibody antigen-
binding site. It must be understood here that the invention does not relate to
the antibodies in
natural form, that is to say they are not in their natural environment but
that they have been able
to be isolated or obtained by purification from natural sources, or else
obtained by genetic
recombination, or by chemical synthesis, and that they can then contain
unnatural amino acids
as will be described later. Antibody fragments that comprise an antibody
antigen-binding site
include, but are not limited to, molecules such as Fab, Fab', Fab'-SH, scFv,
Fv, dAb and Fd.
Various other antibody molecules including one or more antibody antigen-
binding sites have
been engineered, including for example Fab2, Fab3, diabodies, triabodies,
tetrabodies and
minibodies. Antibody molecules and methods for their construction and use are
described in
Holliger & Hudson, Nature Biotechnology 23(9):1126-1136 2005.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
28
It is possible to take monoclonal and other antibodies and use techniques of
recombinant DNA technology to produce other antibodies or chimeric molecules
that bind the
target antigen. Such techniques may involve introducing DNA encoding the
immunoglobulin
variable region, or the CDRs, of an antibody to the constant regions, or
constant regions plus
framework regions, of a different immunoglobulin. See, for instance, EP-A-
184187, GB
2188638A or EP-A-239400, and a large body of subsequent literature. A
hybridoma or other
cell producing an antibody may be subject to genetic mutation or other
changes, which may or
may not alter the binding specificity of antibodies produced.
As antibodies can be modified in a number of ways, the term "antibody
molecule" should
be construed as covering any binding member or substance having an antibody
antigen-binding
site with the required specificity and/or binding to antigen. Thus, this term
covers antibody
fragments and derivatives, including any polypeptide comprising an antibody
antigen-binding
site, whether natural or wholly or partially synthetic. Chimeric molecules
comprising an antibody
antigen-binding site, or equivalent, fused to another polypeptide (e.g.
derived from another
species or belonging to another antibody class or subclass) are therefore
included. Cloning and
expression of chimeric antibodies are described in EP-A-0120694 and EP-A-
0125023, and a
large body of subsequent literature.
Further techniques available in the art of antibody engineering have made it
possible to
isolate human and humanised antibodies. For example, human hybridomas can be
made as
described by Kontermann & Dubel [Kontermann, R & Dubel, S, Antibody
Engineering, Springer-
Verlag New York, LLC; 2001, ISBN: 3540413545]. Phage display, another
established
technique for generating binding members has been described in detail in many
publications,
such as Kontermann & Dubel [supra] and W092/01047 (discussed further below),
and US
patents U55969108, U55565332, U55733743, U55858657, US5871907, U55872215,
U55885793, U55962255, U56140471, U56172197, U56225447, U56291650, U56492160,
US6521404.
Transgenic mice in which the mouse antibody genes are inactivated and
functionally
replaced with human antibody genes while leaving intact other components of
the mouse
immune system, can be used for isolating human antibodies [Mendez, M. et al.
(1997) Nature
Genet, 15(2): 146-156]. Humanised antibodies can be produced using techniques
known in the
art such as those disclosed in for example W091/09967, US 5,585,089, EP592106,
US
565,332 and W093/17105. Further, W02004/006955 describes methods for
humanising
antibodies, based on selecting variable region framework sequences from human
antibody
genes by comparing canonical CDR structure types for CDR sequences of the
variable region
of a non-human antibody to canonical CDR structure types for corresponding
CDRs from a
library of human antibody sequences, e.g. germline antibody gene segments.
Human antibody
variable regions having similar canonical CDR structure types to the non-human
CDRs form a
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
29
subset of member human antibody sequences from which to select human framework
sequences. The subset members may be further ranked by amino acid similarity
between the
human and the non-human CDR sequences. In the method of W02004/006955, top
ranking
human sequences are selected to provide the framework sequences for
constructing a chimeric
antibody that functionally replaces human CDR sequences with the non-human CDR
counterparts using the selected subset member human frameworks, thereby
providing a
humanized antibody of high affinity and low immunogenicity without need for
comparing
framework sequences between the non-human and human antibodies. Chimeric
antibodies
made according to the method are also disclosed.
Synthetic antibody molecules may be created by expression from genes generated
by
means of oligonucleotides synthesized and assembled within suitable expression
vectors, for
example as described by Knappik etal. [Knappik etal. J. Mol. Biol. (2000) 296,
57-86] or Krebs
etal. [Krebs etal. Journal of Immunological Methods 254 2001 67-84].
It has been shown that fragments of a whole antibody can perform the function
of
binding antigens. Examples of binding fragments are (i) the Fab fragment
consisting of VL, VH,
CL and CH1 domains; (ii) the Fd fragment consisting of the VH and CH1 domains;
(iii) the Fv
fragment consisting of the VL and VH domains of a single antibody; (iv) the
dAb fragment [Ward,
E.S. etal., Nature 341, 544-546 (1989); McCafferty etal. (1990) Nature, 348,
552-554; Holt etal.
(2003) Trends in Biotechnology 21, 484-490], which consists of a VH or a VL
domain; (v)
isolated CDR regions; (vi) F(ab')2 fragments, a bivalent fragment comprising
two linked Fab
fragments (vii) single chain Fv molecules (scFv), wherein a VH domain and a VL
domain are
linked by a peptide linker which allows the two domains to associate to form
an antigen binding
site [Bird etal., Science, 242, 423-426, 1988; Huston etal., PNAS USA, 85,
5879-5883, 1988];
(viii) bispecific single chain Fv dimers (PCT/U592/09965) and (ix)
"diabodies", multivalent or
multispecific fragments constructed by gene fusion (W094/13804; Holliger, P.
et al., Proc. Natl.
Acad. Sci. USA 90 6444-6448, 1993). Fv, scFv or diabody molecules may be
stabilized by the
incorporation of disulphide bridges linking the VH and VL domains [Reiter, Y.
et al., Nature
Biotech, 14, 1239-1245, 1996]. Minibodies comprising a scFv joined to a CH3
domain may also
be made [Hu, S. etal., Cancer Res., 56, 3055-3061, 1996]. Other examples of
binding
fragments are Fab', which differs from Fab fragments by the addition of a few
residues at the
carboxyl terminus of the heavy chain CH1 domain, including one or more
cysteines from the
antibody hinge region, and Fab'-SH, which is a Fab' fragment in which the
cysteine residue(s) of
the constant domains bear a free thiol group.
Qui etal. [Qui etal., Nat. Biotechnol. 25:921-929 2007] described antibody
molecules
containing just two CDRs linked by a framework region. CDR3 from the VH or VL
domain was
linked to the CDR1 or CDR2 loop of the other domain. Linkage was through the C
terminus of
the selected CDR1 or CDR2 to the N terminus of the CDR3, via a FR region. Qui
etal. selected
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
the FR region having the fewest hydrophobic patches. The best combination for
the antibody
tested was found to be VL CDR1 linked by VH FR2 to VH CDR3 (VHCDR1-VHFR2-
VLCDR3).
At a molecular weight of around 3 kDa, these antibody molecules offer
advantages in terms of
improved tissue penetration as compared with full immunoglobulins (approx. 150
kDa) or scFv
5 (approx. 28 kDa).
Antibody fragments of the invention can be obtained starting from any of the
antibodies
listed herein, by methods such as digestion by enzymes e.g. pepsin or papain
and/or by
cleavage of the disulfide bridges by chemical reduction. In another manner,
the antibody
fragments comprised in the present invention can be obtained by techniques of
genetic
10 recombination likewise well known to the person skilled in the art or
else by peptide synthesis
by means of, for example, automatic peptide synthesizers, such as those
supplied by the
company Applied Biosystems, etc., or by nucleic acid synthesis and expression.
Functional antibody fragments according to the present invention include any
functional
fragment whose half-life is increased by a chemical modification, especially
by PEGylation, or
15 by incorporation in a liposome.
A dAb (domain antibody) is a small monomeric antigen-binding fragment of an
antibody,
namely the variable region of an antibody heavy or light chain. VH dAbs occur
naturally in
camelids (e.g., camel, llama) and may be produced by immunizing a camelid with
a target
antigen, isolating antigen-specific B cells and directly cloning dAb genes
from individual B cells.
20 dAbs are also producible in cell culture. Their small size, good
solubility and temperature
stability makes them particularly physiologically useful and suitable for
selection and affinity
maturation. Camelid VH dAbs are being developed for therapeutic use under the
name
"nanobodiesTm". A binding member of the present invention may be a dAb
comprising a VH or
VL domain substantially as set out herein, or a VH or VL domain comprising a
set of CDRs
25 substantially as set out herein.
Bispecific or bifunctional antibodies form a second generation of monoclonal
antibodies
in which two different variable regions are combined in the same molecule
[Holliger and Bohlen
(1999) Cancer and Metastasis Rev. 18: 411-419]. Their use has been
demonstrated both in the
diagnostic field and in the therapy field from their capacity to recruit new
effector functions or to
30 target several molecules on the surface of tumour cells. Where
bispecific antibodies are to be
used, these may be conventional bispecific antibodies, which can be
manufactured in a variety
of ways [Holliger, P. and Winter G. Current Opinion Biotechnol 4, 446-449.
1993], e.g., prepared
chemically or from hybrid hybridomas, or may be any of the bispecific antibody
fragments
mentioned above. These antibodies can be obtained by chemical methods [Glennie
M J etal.,
1987 J. lmmunol. 139, 2367-2375; Repp R. etal., 1995 J. Hemat. 377-382] or
somatic methods
[Staerz U. D. and Bevan M. J. 1986 PNAS 83; Suresh M. R. etal., 1986 Methods
Enzymol. 121:
210-228] but likewise and preferentially by genetic engineering techniques
which allow the
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
31
heterodimerization to be forced and thus facilitate the process of
purification of the antibody
sought [Merchand etal., 1998 Nature Biotech. 16:677-681]. Examples of
bispecific antibodies
include those of the BiTETm technology in which the binding domains of two
antibodies with
different specificity can be used and directly linked via short flexible
peptides. This combines
two antibodies on a short single polypeptide chain. Diabodies and scFy can be
constructed
without an Fc region, using only variable domains, potentially reducing the
effects of anti-
idiotypic reaction.
Bispecific antibodies can be constructed as entire IgG, as bispecific Fab'2,
as Fab'PEG,
as diabodies or else as bispecific scFv. Further, two bispecific antibodies
can be linked using
routine methods known in the art to form tetravalent antibodies.
Bispecific diabodies, as opposed to bispecific whole antibodies, may also be
particularly
useful because they can be readily constructed and expressed in E. coll.
Diabodies (and many
other polypeptides, such as antibody fragments) of appropriate binding
specificities can be
readily selected using phage display (W094/13804) from libraries. If one arm
of the diabody is
to be kept constant, for instance, with a specificity directed against amyloid
beta as described
herein, then a library can be made where the other arm is varied and an
antibody of appropriate
specificity selected. Bispecific whole antibodies may be made by alternative
engineering
methods as described in Ridgeway etal., 1996 [Ridgeway, J. B. B. etal.,
Protein Eng., 9,616-
621, 1996].
Various methods are available in the art for obtaining antibodies. The
antibodies may be
monoclonal antibodies, especially of human, murine, chimeric or humanized
origin, which can
be obtained according to the standard methods well known to the person skilled
in the art.
In general, for the preparation of monoclonal antibodies or their functional
fragments, especially
of murine origin, it is possible to refer to techniques which are described in
particular in the
manual "Antibodies" [Harlow and Lane, Antibodies: A Laboratory Manual, Cold
Spring Harbor
Laboratory, Cold Spring Harbor N.Y., pp. 726, 1988] or to the technique of
preparation from
hybridomas described by Kohler and Milstein [Kohler and Milstein, Nature,
256:495-497, 1975].
Monoclonal antibodies can be obtained, for example, from an animal cell
immunized with
human A131-42, or one of its fragments containing the epitope recognized by
said monoclonal
antibodies, e.g. A[317-42. Suitable fragments and peptides or polypeptides
comprising them are
described herein, and may be used to immunise animals to generate antibodies
against A131-42.
Said antigen, or one of its fragments, can especially be produced according to
the usual
working methods, by genetic recombination starting with a nucleic acid
sequence contained in
the cDNA sequence coding for A[31-42 or fragment thereof, by peptide synthesis
starting from a
sequence of amino acids comprised in the peptide sequence of the A[31-42
and/or fragment
thereof.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
32
The monoclonal antibodies can, for example, be purified on an affinity column
on which
human A131-42 or one of its fragments containing the epitope recognized by
said monoclonal
antibodies, e.g. A1317-42, has previously been immobilized. More particularly,
the monoclonal
antibodies can be purified by chromatography on protein A and/or G, followed
or not followed by
ion-exchange chromatography aimed at eliminating the residual protein
contaminants as well as
the DNA and the LPS, in itself, followed or not followed by exclusion
chromatography on
Sepharose gel in order to eliminate the potential aggregates due to the
presence of dimers or of
other multimers. In one embodiment, the whole of these techniques can be used
simultaneously or successively.
Antigen-binding site
This describes the part of a molecule that binds to and is complementary to
all or part of
the target antigen. In an antibody molecule, it is referred to as the antibody
antigen-binding site,
and comprises the part of the antibody that binds to and is complementary to
all or part of the
target antigen. Where an antigen is large, an antibody may only bind to a
particular part of the
antigen, which part is termed an epitope. An antibody antigen-binding site may
be provided by
one or more antibody variable domains. An antibody antigen-binding site may
comprise an
antibody light chain variable region (VL) and an antibody heavy chain variable
region (VH).
WO 2006/072620 describes engineering of antigen-binding sites in structural
(non-CDR)
loops extending between beta strands of immunoglobulin domains. An antigen-
binding site may
be engineered in a region of an antibody molecule separate from the natural
location of the
CDRs, e.g. in a framework region of a VH or VL domain, or in an antibody
constant domain,
e.g., CH1 and/or CH3. An antigen-binding site engineered in a structural
region may be
additional to, or instead of, an antigen-binding site formed by sets of CDRs
of a VH and VL
domain. Where multiple antigen-binding sites are present in an antibody
molecule, they may
bind the same antigen (target antigen), thereby increasing valency of the
binding member.
Alternatively, multiple antigen-binding sites may bind different antigens (the
target antigen and
one or more another antigen), and this may be used to add effector functions,
prolong half-life
or improve in vivo delivery of the antibody molecule.
Isolated
This refers to the state in which binding members of the invention, or nucleic
acid
encoding such binding members, will generally be in accordance with the
present invention.
Thus, binding members, VH and/or VL domains, and encoding nucleic acid
molecules and
vectors according to the present invention may be provided isolated and/or
purified, e.g. from
their natural environment, in substantially pure or homogeneous form, or, in
the case of nucleic
acid, free or substantially free of nucleic acid or genes of origin other than
the sequence
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
33
encoding a polypeptide with the required function. Isolated members and
isolated nucleic acid
will be free or substantially free of material with which they are naturally
associated, such as
other polypeptides or nucleic acids with which they are found in their natural
environment, or the
environment in which they are prepared (e.g. cell culture) when such
preparation is by
recombinant DNA technology practised in vitro or in vivo. Members and nucleic
acid may be
formulated with diluents or adjuvants and still for practical purposes be
isolated - for example
the members will normally be mixed with gelatin or other carriers if used to
coat microtitre plates
for use in immunoassays, or will be mixed with pharmaceutically acceptable
carriers or diluents
when used in diagnosis or therapy. Binding members may be glycosylated, either
naturally or
by systems of heterologous eukaryotic cells (e.g. CHO or NSO (ECACC 85110503)
cells, or they
may be (for example if produced by expression in a prokaryotic cell)
unglycosylated.
Heterogeneous preparations comprising antibody molecules also form part of the
invention. For example, such preparations may be mixtures of antibodies with
full-length heavy
chains and heavy chains lacking the C-terminal lysine, with various degrees of
glycosylation
and/or with derivatized amino acids, such as cyclization of an N-terminal
glutamic acid to form a
pyroglutamic acid residue.
As used herein, the phrase "substantially as set out refers to the
characteristic(s) of the
relevant CDRs of the VH or VL domain of binding members described herein will
be either
identical or highly similar to the specified regions of which the sequence is
set out herein. As
described herein, the phrase "highly similar" with respect to specified
region(s) of one or more
variable domains, it is contemplated that 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11,
12, 13, 14 or 15 amino
acid substitutions may be made in the CDR and/or VH or VL domain.
As noted above, a binding member in accordance with the present invention
binds
human A131-42. As described herein, binding members of the present invention
may be
optimised for affinity and/or for potency of inhibition in an HTRFTm
competition assay. Generally,
potency optimisation involves mutating the sequence of a selected binding
member (normally
the variable domain sequence of an antibody) to generate a library of binding
members, which
are then assayed for potency and the more potent binding members are selected.
Thus
selected "potency-optimised" binding members tend to have a higher potency
than the binding
member from which the library was generated. Nevertheless, high potency
binding members
may also be obtained without optimisation, for example a high potency binding
member may be
obtained directly from an initial screen. Assays and potencies are described
in more detail
elsewhere herein. The skilled person can thus generate binding members having
high potency.
In a further aspect, the present invention provides a method of obtaining one
or more
binding members able to bind the antigen, the method including bringing into
contact a library of
binding members according to the invention and said antigen, and selecting one
or more
binding members of the library able to bind said antigen.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
34
The library may be displayed on particles or molecular complexes, e.g.
replicable
genetic packages, such as yeast, bacterial or bacteriophage (e.g. T7)
particles, viruses, cells or
covalent, ribosomal or other in vitro display systems, each particle or
molecular complex
containing nucleic acid encoding the antibody VH variable domain displayed on
it, and
optionally also a displayed VL domain if present. Phage display is described
in W092/01047
and e.g. US patents US5969108, US5565332, US5733743, US5858657, US5871907,
US5872215, US5885793, US5962255, US6140471, US6172197, US6225447, US6291650,
US6492160 and US6521404, each of which is herein incorporated by reference in
their entirety.
Ribosome display is described in Hanes J and Pluckthun A. (1997) Proc Natl
Acad Sci U S A.
1997 May 13;94(10):4937-42; W001/75097 and W02006/072773, each of which is
herein
incorporated by reference in their entirety.
Following selection of binding members able to bind the antigen and displayed
on
bacteriophage or other library particles or molecular complexes, nucleic acid
may be taken from
a bacteriophage or other particle or molecular complex displaying a said
selected binding
member. Such nucleic acid may be used in subsequent production of a binding
member or an
antibody VH or VL variable domain by expression from nucleic acid with the
sequence of
nucleic acid taken from a bacteriophage or other particle or molecular complex
displaying a said
selected binding member.
An antibody VH variable domain with the amino acid sequence of an antibody VH
variable domain of a said selected binding member may be provided in isolated
form, as may a
binding member comprising such a VH domain.
Ability to bind human A[31-42 and A[31-40 may be further tested, also ability
to compete
with, e.g., any of the antibodies as listed herein (e.g. in scFv format and/or
IgG format, e.g. IgG2
or IgG1) for binding to human A131-42. Ability to neutralize A131-42 may be
tested, as discussed
further elsewhere herein.
A binding member may bind human A[31-42 with the affinity of any of the
antibodies
listed in Tables 5 and 6, e.g. scFv, IgG2, IgG1TM or IgG1, or with an affinity
that is better.
Antibody binding affinities are shown in Table 7. Binding affinity and
neutralization potency of
different binding members can be compared under appropriate conditions.
Variants of the VH and VL domains and CDRs described herein, including those
for
which amino acid sequences are set out herein, and which can be employed in
binding
members for A[31-42 can be obtained by means of methods of sequence alteration
or mutation
and screening for antigen binding members with desired characteristics.
Examples of desired
characteristics include but are not limited to: increased binding affinity for
antigen relative to
known antibodies which are specific for the antigen, increased neutralization
of an antigen
activity relative to known antibodies which are specific for the antigen if
the activity is known
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
specified competitive ability with a known antibody or ligand to the antigen
at a specific molar
ratio, ability to immunoprecipitate complex, ability to bind to a specified
epitope: a linear epitope,
e.g., peptide sequence identified using peptide-binding scan as described
herein, e.g., using
peptides screened in linear and/or constrained conformation, or a
conformational epitope,
5 formed by non-continuous residues; and ability to modulate a new
biological activity of human
A131-42. Such methods are also provided herein.
Variants of antibody molecules disclosed herein may be produced and used in
the
present invention. Following the lead of computational chemistry in applying
multivariate data
analysis techniques to the structure/property-activity relationships [see for
example, Wold, etal.
10 Multivariate data analysis in chemistry. Chemometrics¨Mathematics and
Statistics in Chemistry
(Ed.: B. Kowalski); D. Reidel Publishing Company, Dordrecht, Holland, 1984
(ISBN 90-277-
1846-6] quantitative activity-property relationships of antibodies can be
derived using well-
known mathematical techniques, such as statistical regression, pattern
recognition and
classification [see for example Norman et al. Applied Regression Analysis.
Wiley-lnterscience;
15 3rd edition (April 1998) ISBN: 0471170828; Kande!, Abraham etal.
Computer-Assisted
Reasoning in Cluster Analysis. Prentice Hall PTR, (May 11, 1995), ISBN:
0133418847;
Krzanowski, Wojtek. Principles of Multivariate Analysis: A User's Perspective
(Oxford Statistical
Science Series, No 22 (Paper)). Oxford University Press; (December 2000),
ISBN:
0198507089; Witten, Ian H. eta! Data Mining: Practical Machine Learning Tools
and
20 Techniques with Java Implementations. Morgan Kaufmann; (October 11,
1999), ISBN:
1558605525; Denison David G. T. (Editor) eta! Bayesian Methods for Nonlinear
Classification
and Regression (Wiley Series in Probability and Statistics). John Wiley &
Sons; (July 2002),
ISBN: 0471490369; Ghose, Arup K. etal. Combinatorial Library Design and
Evaluation
Principles, Software, Tools, and Applications in Drug Discovery. ISBN: 0-8247-
0487-8]. The
25 properties of antibodies can be derived from empirical and theoretical
models (for example,
analysis of likely contact residues or calculated physicochemical property) of
antibody sequence,
functional and three-dimensional structures and these properties can be
considered individually
and in combination.
An antibody antigen-binding site composed of a VH domain and a VL domain is
typically
30 formed by six loops of polypeptide: three from the light chain variable
domain (VL) and three
from the heavy chain variable domain (VH). Analysis of antibodies of known
atomic structure
has elucidated relationships between the sequence and three-dimensional
structure of antibody
combining sites [Chothia C. etal. Journal Molecular Biology (1992) 227, 799-
817; Al-Lazikani,
et al. Journal Molecular Biology (1997) 273(4), 927-948]. These relationships
imply that,
35 except for the third region (loop) in VH domains, binding site loops
have one of a small number
of main-chain conformations: canonical structures. The canonical structure
formed in a
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
36
particular loop has been shown to be determined by its size and the presence
of certain
residues at key sites in both the loop and in framework regions.
This study of sequence-structure relationship can be used for prediction of
those
residues in an antibody of known sequence, but of an unknown three-dimensional
structure,
which are important in maintaining the three-dimensional structure of its CDR
loops and hence
maintain binding specificity. These predictions can be backed up by comparison
of the
predictions to the output from lead optimization experiments. In a structural
approach, a model
can be created of the antibody molecule [Chothia, etal. Science, 223,755-758
(1986)] using
any freely available or commercial package, such as WAM [Whitelegg, N.R.u. and
Rees, A.R
(2000). Prot. Eng., 12, 815-824]. A protein visualisation and analysis
software package, such
as Insight ll (Accelrys, Inc.) or Deep View [Guex, N. and Peitsch, M.C.
Electrophoresis (1997)
18, 2714-2723] may then be used to evaluate possible substitutions at each
position in the CDR.
This information may then be used to make substitutions likely to have a
minimal or beneficial
effect on activity.
The techniques required to make substitutions within amino acid sequences of
CDRs,
antibody VH or VL domains and binding members generally are available in the
art. Variant
sequences may be made, with substitutions that may or may not be predicted to
have a minimal
or beneficial effect on activity, and tested for ability to bind A[31-42
and/or for any other desired
property.
Variable domain amino acid sequence variants of any of the VH and VL domains
whose
sequences are specifically disclosed herein may be employed in accordance with
the present
invention, as discussed.
As described above, aspects of the invention provide a binding member, such as
an
antibody molecule, comprising a VH domain that has at least 75%, at least 80%,
at least 85%,
at least 90%, at least 93%, at least 94%, at least 95%, at least 96%, at least
97%, at least 98%
or at least 99% amino acid sequence identity with a VH domain of any of the
antibodies listed
herein, for which VH domain sequences are shown in the appended sequence
listing below;
and/or comprising a VL domain that has at least 75%, at least 80%, at least
85%, at least 90%,
at least 93%, at least 94%, at least 95%, at least 96%, at least 97%, at least
98% or at least
99% amino acid sequence identity with a VL domain of any of the antibodies
listed in Table 11,
for which VL domain sequences are shown in the appended sequence listing.
Aspects of the invention provide a binding member, such as an antibody
molecule,
comprising a VH domain having a set of VH CDRs that have at least 75%, at
least 80%, at least
85%, at least 90%, at least 93%, at least 94%, at least 95%, at least 96%, at
least 97%, at least
98% or at least 99% amino acid sequence identity with the set of VH CDRs of
any of the
antibodies listed herein, for which VH CDR sequences are shown herein; and/or
comprising a
VL domain having a set of VL CDRs that have at that has at least 75%, at least
80%, at least
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
37
85%, at least 90%, at least 93%, at least 94%, at least 95%, at least 96%, at
least 97%, at least
98% or at least 99% amino acid sequence identity with the set of VL CDRs of
any of the
antibodies listed herein, for which the VL CDR sequences are shown in herein.
Algorithms that can be used to calculate % identity of two amino acid
sequences include
e.g. BLAST [Altschul etal. (1990) J. Mol. Biol. 215: 405-410], FASTA [Pearson
and
Lipman (1988) PNAS USA 85: 2444-2448], or the Smith-Waterman algorithm [Smith
and
Waterman (1981) J. Mol Biol. 147: 195-197] e.g., employing default parameters.
Particular variable domains may include one or more amino acid sequence
mutations
(substitution, deletion, and/or insertion of an amino acid residue), and less
than about 15 14, 13,
12, 11, 10, 9, 8, 7, 6, 5, 4, 3 or 2.
Mutations may be made in one or more framework regions and/or one or more
CDRs.
The mutations normally do not result in loss of function, so a binding member
comprising a
thus-altered amino acid sequence may retain an ability to bind human A[31-42.
It may retain the
same quantitative binding and/or neutralizing ability as a binding member in
which the alteration
is not made, e.g., as measured in an assay described herein. The binding
member comprising a
thus-altered amino acid sequence may have an improved ability to bind human
A[31-42.
Mutation may comprise replacing one or more amino acid residues with a non-
naturally
occurring or non-standard amino acid, modifying one or more amino acid residue
into a non-
naturally occurring or non-standard form, or inserting one or more non-
naturally occurring or
non-standard amino acid into the sequence. Examples of numbers and locations
of alterations
in sequences of the invention are described elsewhere herein. Naturally
occurring amino acids
include the 20 "standard" L-amino acids identified as G, A, V, L, I, M, P, F,
W, S, T, N, Q, Y, C,
K, R, H, D, E by their standard single-letter codes. Non-standard amino acids
include any other
residue that may be incorporated into a polypeptide backbone or result from
modification of an
existing amino acid residue. Non-standard amino acids may be naturally
occurring or non-
naturally occurring. Several naturally occurring non-standard amino acids are
known in the art,
such as 4-hydroxyproline, 5-hydroxylysine, 3-methylhistidine, N-acetylserine,
etc. [Voet & Voet,
Biochemistry, 2nd Edition, (Wiley) 1995]. Those amino acid residues that are
derivatised at
their N-alpha position will only be located at the N-terminus of an amino-acid
sequence.
Normally in the present invention an amino acid is an L-amino acid, but it may
be a D-amino acid.
Alteration may therefore comprise modifying an L-amino acid into, or replacing
it with, a D-amino
acid. Methylated, acetylated and/or phosphorylated forms of amino acids are
also known, and
amino acids in the present invention may be subject to such modification.
Amino acid sequences in antibody domains and binding members of the invention
may
comprise non-natural or non-standard amino acids described above. Non-standard
amino acids
(e.g. D-amino acids) may be incorporated into an amino acid sequence during
synthesis, or by
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
38
modification or replacement of the "original" standard amino acids after
synthesis of the amino
acid sequence.
Use of non-standard and/or non-naturally occurring amino acids increases
structural and
functional diversity, and can thus increase the potential for achieving
desired binding and
neutralising properties in a binding member of the invention. Additionally, D-
amino acids and
analogues have been shown to have different pharmacokinetic profiles compared
with standard
L-amino acids, owing to in vivo degradation of polypeptides having L-amino
acids after
administration to an animal, e.g., a human, meaning that D-amino acids are
advantageous for
some in vivo applications.
Novel VH or VL regions carrying CDR-derived sequences of the invention may be
generated using random mutagenesis of one or more selected VH and/or VL genes
to generate
mutations within the entire variable domain. Such a technique is described by
Gram etal.
[Gram etal., 1992, Proc. Natl. Acad. Sci., USA, 89:3576-3580], who used error-
prone PCR. In
some embodiments one or two amino acid substitutions are made within an entire
variable
domain or set of CDRs.
Another method that may be used is to direct mutagenesis to CDR regions of VH
or VL
genes. Such techniques are disclosed by Barbas etal. [Barbas etal., 1994,
Proc. Natl. Acad.
Sci., USA, 91:3809-3813] and Schier et al. [Schier et al., 1996, J. MoL Biol.
263:551-567].
All the above-described techniques are known as such in the art and the
skilled person
will be able to use such techniques to provide binding members of the
invention using routine
methodology in the art.
A further aspect of the invention provides a method for obtaining an antibody
antigen-
binding site for human A131-42, the method comprising providing by way of
substitution, deletion,
or insertion of one or more amino acids in the amino acid sequence of a VH
domain set out
herein a VH domain which is an amino acid sequence variant of the VH domain,
optionally
combining the VH domain thus provided with one or more VL domains, and testing
the VH
domain or VH/VL combination or combinations to identify a binding member or an
antibody
antigen-binding site for A[31-42 and optionally with one or more desired
properties. Said VL
domain may have an amino acid sequence which is substantially as set out
herein. An
analogous method may be employed in which one or more sequence variants of a
VL domain
disclosed herein are combined with one or more VH domains.
As noted above, a CDR amino acid sequence substantially as set out herein may
be
incorporated as a CDR in a human antibody variable domain or a substantial
portion thereof.
The HCDR3 sequences substantially as set out herein represent embodiments of
the present
invention and each of these may be incorporated as a HCDR3 in a human heavy
chain variable
domain or a substantial portion thereof.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
39
Variable domains employed in the invention may be obtained or derived from any
germline or rearranged human variable domain, or may be a synthetic variable
domain based
on consensus or actual sequences of known human variable domains. A variable
domain can
be derived from a non-human antibody. A CDR sequence of the invention (e.g.
CDR3) may be
introduced into a repertoire of variable domains lacking a CDR (e.g. CDR3),
using recombinant
DNA technology. For example, Marks et al. [Marks eta! Bio/Technology, 1992,
10:779-783]
describe methods of producing repertoires of antibody variable domains in
which consensus
primers directed at or adjacent to the 5 end of the variable domain area are
used in conjunction
with consensus primers to the third framework region of human VH genes to
provide a
repertoire of VH variable domains lacking a CDR3. Marks etal. further describe
how this
repertoire may be combined with a CDR3 of a particular antibody. Using
analogous techniques,
the CDR3-derived sequences of the present invention may be shuffled with
repertoires of VH or
VL domains lacking a CDR3, and the shuffled complete VH or VL domains combined
with a
cognate VL or VH domain to provide binding members of the invention. The
repertoire may
then be displayed in a suitable host system, such as the phage display system
of W092/01047,
which is herein incorporated by reference in its entirety, or any of a
subsequent large body of
literature, including Kay, Winter & McCafferty [Kay, B.K., Winter, J., and
McCafferty, J. (1996)
Phage Display of Peptides and Proteins: A Laboratory Manual, San Diego:
Academic Press], so
that suitable binding members may be selected. A repertoire may consist of
from anything from
104 individual members upwards, for example at least 105, at least 106, at
least 107, at least 108,
at least 109 or at least 10" members or more. Other suitable host systems
include, but are not
limited to yeast display, bacterial display, T7 display, viral display, cell
display, ribosome display
and covalent display.
A method of preparing a binding member for human A[31-42 is provided, which
method
comprises:
(a) providing a starting repertoire of nucleic acids encoding a VH domain
which
either include a CDR3 to be replaced or lack a CDR3 encoding region;
(b) combining said repertoire with a donor nucleic acid encoding an amino
acid
sequence substantially as set out herein for a VH CDR3, for example a VH CDR3
shown in
Table 11, such that said donor nucleic acid is inserted into the CDR3 region
in the repertoire, so
as to provide a product repertoire of nucleic acids encoding a VH domain;
(c) expressing the nucleic acids of said product repertoire;
(d) selecting a binding member for human A131-42; and
(e) recovering said binding member or nucleic acid encoding it.
Again, an analogous method may be employed in which a VL CDR3 of the invention
is
combined with a repertoire of nucleic acids encoding a VL domain that either
include a CDR3 to
be replaced or lack a CDR3 encoding region.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
Similarly, one or more, or all three CDRs may be grafted into a repertoire of
VH or VL
domains that are then screened for a binding member or binding members for
human A131-42.
For example, an HCDR1, HCDR2 and/or HCDR3, e.g., a set of HCDRs, from one or
more of the antibodies listed in Table 5 or Table 6 may be employed, and/or an
LCDR1, LCDR2
5 and/or LCDR3, e.g., set of LCDRs, from one or more of the antibodies
listed herein may be
employed.
Similarly, other VH and VL domains, sets of CDRs and sets of HCDRs and/or sets
of
LCDRs disclosed herein may be employed.
A substantial portion of an immunoglobulin variable domain may comprise at
least the
10 three CDR regions, together with their intervening framework regions.
The portion may also
include at least about 50 % of either or both of the first and fourth
framework regions, the 50 %
being the C-terminal 50 % of the first framework region and the N-terminal 50
% of the fourth
framework region. Additional residues at the N-terminal or C-terminal end of
the substantial
part of the variable domain may be those not normally associated with
naturally-occurring
15 variable domain regions. For example, construction of binding members of
the present
invention made by recombinant DNA techniques may result in the introduction of
N- or C-
terminal residues encoded by linkers introduced to facilitate cloning or other
manipulation steps.
Other manipulation steps include the introduction of linkers to join variable
domains of the
invention to further protein sequences including antibody constant regions,
other variable
20 domains (for example in the production of diabodies) or
detectable/functional labels as
discussed in more detail elsewhere herein.
Although in some aspects of the invention, binding members comprise a pair of
VH and
VL domains, single binding domains based on either VH or VL domain sequences
form further
aspects of the invention. It is known that single immunoglobulin domains,
especially VH
25 domains, are capable of binding target antigens in a specific manner.
For example, see the
discussion of dAbs above.
In the case of either of the single binding domains, these domains may be used
to
screen for complementary domains capable of forming a two-domain binding
member able to
bind A[31-42. This may be achieved by phage display screening methods using
the so-called
30 hierarchical dual combinatorial approach as disclosed in W092/01047,
herein incorporated by
reference in its entirety, in which an individual colony containing either an
H or L chain clone is
used to infect a complete library of clones encoding the other chain (L or H)
and the resulting
two-chain binding member is selected in accordance with phage display
techniques, such as
those described in that reference. This technique is also disclosed in Marks
etal.,
35 Bio/Technology, 1992, 10:779-783.
Binding members of the present invention may further comprise antibody
constant
regions or parts thereof, e.g., human antibody constant regions or parts
thereof. For example, a
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
41
VL domain may be attached at its C-terminal end to antibody light chain
constant domains
including human CK or a chains. Similarly, a binding member based on a VH
domain may be
attached at its C-terminal end to all or part (e.g., a CH1 domain) of an
immunoglobulin heavy
chain derived from any antibody isotype, e.g. IgG, IgA, IgE and IgM and any of
the isotype sub-
classes, particularly IgG2, IgG1 and IgG4. IgG2 may be advantageous in some
embodiments
owing to its lack of effector functions. In other embodiments, IgG1 may be
advantageous due to
its effector function and ease of manufacture. Any synthetic or other constant
region variant that
has these properties and stabilizes variable regions may also be useful in the
present invention.
Binding members may be labelled with a detectable or functional label. Thus, a
binding
member or antibody molecule can be present in the form of an immunoconjugate
so as to
obtain a detectable and/or quantifiable signal. An immunoconjugate may
comprise an antibody
molecule of the invention conjugated with detectable or functional label. A
detectable label as
referred to herein may be any label which produces or can be induced to
produce a signal,
including but not limited to fluorescers, chemiluminescers (e.g., horseradish
peroxidase),
coloured labels (e.g. latex [blue] or colloidal gold [red]), radiolabels,
enzymes, photosensitisers
and magnetic labels. The amount of label bound at a surface, e.g., a surface
of a capillary bore,
may therefore be detected and/or measured by detecting fluorescence or
luminescence, colour,
radioactivity, enzyme activity, light absorbance or changes in magnetic field.
Detectable labels
may be attached to binding members using conventional chemistry. Preferably, a
detectable
label is a label detectable by optical interrogation, e.g., with a digital
camera or flatbed scanner.
Labels that can be detected by optical interrogation include fluorescers,
chemiluminescers and
coloured labels. The mechanism by which a signal can be generated for optical
detection
includes (but is not necessarily limited to): light absorption, light
scattering, light diffraction, light
reflection, fluorescence or luminescence.
Suitable labels include, by way of illustration and not limitation,
- enzymes, such as alkaline phosphatase, glucose-6-phosphate
dehydrogenase
("G6PDH"), alpha-D-galactosidase, glucose oxydase, glucose amylase, carbonic
anhydrase,
acetylcholinesterase, lysozyme, malate dehydrogenase and peroxidase, e.g.,
horseradish
peroxidase;
- dyes;
- fluorescent labels or fluorescers, such as fluorescein and its
derivatives, fluorochrome,
rhodamine compounds and derivatives, GFP (GFP for "Green Fluorescent
Protein"), dansyl,
umbelliferone, phycoerythrin, phycocyanin, allophycocyanin, o-phthaldehyde,
and
fluorescamine; fluorophores such as lanthanide cryptates and chelates, e.g.,
Europium etc
(Perkin Elmer and Cis Biointernational),
- chemoluminescent labels or chemiluminescers, such as isoluminol,
luminol and the
dioxetanes;
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
42
- bio-luminescent labels, such as lucif erase and luciferin;
- sensitizers;
- coenzymes;
- enzyme substrates;
- radiolabels, including but not limited to, bromine'', carbon14, cobalt57,
fluorine8, gallium87,
gallium88, hydrogen3 (tritium), indium111, indiumi13m, iodinei23m, iodine125,
iodin 126
e
, iodine131,
iodine133, mercury", mercury203, phosphorous32, rhenium"m, rhenium101,
rhenium',
ruthenium95, ruthenium97, ruthenium" , ruthenium", scandium47, selenium75,
sulphur35,
technetium99, technetium99m, tellurium121rn, tellurium122m, tellurium125m,
thUliUM165, thUliUM167,
thulium", yttrium199 and other radiolabels mentioned herein;
- particles, such as latex or carbon particles; metal sol;
crystallite; liposomes; cells, etc.,
which may be further labelled with a dye, catalyst or other detectable group;
- molecules such as biotin, digoxygenin or 5-bromodeoxyuridine;
- toxin moieties, such as for example a toxin moiety selected from a
group of
Pseudomonas exotoxin (PE or a cytotoxic fragment or mutant thereof), Diptheria
toxin or a
cytotoxic fragment or mutant thereof, a Botulinum toxin A, B, C, D, E or F,
ricin or a cytotoxic
fragment thereof e.g. ricin A, abrin or a cytotoxic fragment thereof, saporin
or a cytotoxic
fragment thereof, pokeweed antiviral toxin or a cytotoxic fragment thereof and
bryodin 1 or a
cytotoxic fragment thereof.
Examples of suitable enzymes and coenzymes are disclosed in US4275149, and
US4318980. Suitable fluorescers and chemiluminescers are also disclosed in
U54275149.
Labels further include chemical moieties, such as biotin that may be detected
via binding to a
specific cognate detectable moiety, e.g., labelled avidin or streptavidin.
Detectable labels may
be attached to antibodies of the invention using conventional chemistry known
in the art.
lmmunoconjugates or their functional fragments can be prepared by methods
known to
the person skilled in the art. They can be coupled to enzymes or to
fluorescent labels directly or
by the intermediary of a spacer group or of a linking group, such as a
polyaldehyde, like
glutaraldehyde, ethylenediaminetetraacetic acid (EDTA), diethylene-
triaminepentaacetic acid
(DPTA), or in the presence of coupling agents, such as those mentioned above
for the
therapeutic conjugates. Conjugates containing labels of fluorescein type can
be prepared by
reaction with an isothiocyanate.
The methods known in the art for coupling the therapeutic radioisotopes to the
antibodies either directly or via a chelating agent, such as EDTA, DTPA
mentioned above may
also be used for the radioelements which can be used in diagnosis. It is
likewise possible to
perform labelling with sodium125 by the chloramine T method [Hunter W. M. and
Greenwood F.
C. (1962) Nature 194:495] or else with technetium99m by the technique of
U54424200 or
attached via DTPA as described in U54479930.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
43
There are numerous methods by which the label can produce a signal detectable
by
external means, for example, by visual examination, electromagnetic radiation,
heat, and
chemical reagents. The label can also be bound to another binding member that
binds the
antibody of the invention, or to a support.
The label can directly produce a signal, and therefore, additional components
are not
required to produce a signal. Numerous organic molecules, for example
fluorescers, are able to
absorb ultraviolet and visible light, where the light absorption transfers
energy to these
molecules and elevates them to an excited energy state. This absorbed energy
is then
dissipated by emission of light at a second wavelength. This second wavelength
emission may
also transfer energy to a labelled acceptor molecule, and the resultant energy
dissipated from
the acceptor molecule by emission of light for example fluorescence resonance
energy transfer
(FRET). Other labels that directly produce a signal include radioactive
isotopes and dyes.
Alternatively, the label may need other components to produce a signal, and
the signal
producing system would then include all the components required to produce a
measurable
signal, which may include substrates, coenzymes, enhancers, additional
enzymes, substances
that react with enzymic products, catalysts, activators, cofactors,
inhibitors, scavengers, metal
ions, and a specific binding substance required for binding of signal
generating substances. A
detailed discussion of suitable signal producing systems can be found in
US5185243.
An aspect of the invention provides a method comprising causing or allowing
binding of
a binding member as provided herein to human A131-42. As noted, such binding
may take place
in vivo, e.g. following administration of a binding member, or nucleic acid
encoding a binding
member, or it may take place in vitro, for example in ELISA, Western blotting,
immunocytochemistry, immunoprecipitation, affinity chromatography, and
biochemical or cell-
based assays.
The present invention also provides for measuring levels of antigen directly,
e.g., in
plasma or CSF, by employing a binding member according to the invention for
example in a
biosensor system. For instance, a method of detecting and/or measuring binding
to human
A131-42 may comprise, (i) exposing said binding member to A131-42 and (ii)
detecting binding of
said binding member to A131-42, wherein binding is detected using any method
or detectable
label described herein. The A131-42 may be monomeric or oligomeric A131-42,
preferably
monomeric A131-42. This, and any other binding detection method described
herein, may be
interpreted directly by the person performing the method, for instance, by
visually observing a
detectable label. Alternatively, this method, or any other binding detection
method described
herein, may produce a report in the form of an autoradiograph, a photograph, a
computer
printout, a flow cytometry report, a graph, a chart, a test tube or container
or well containing the
result, or any other visual or physical representation of a result of the
method.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
44
The amount of binding of the binding member to A[31-42 may be determined.
Quantitation may be related to the amount of the antigen in a test sample,
which may be of
diagnostic interest. Screening for A131-42 binding and/or the quantitation
thereof may be useful,
for instance, in screening patients for diseases or disorders referred to
herein and/or any other
disease or disorder involving aberrant A[31-42 levels and/or activity.
A diagnostic method may comprise (i) obtaining a tissue or fluid sample from a
subject,
e.g., a patient suspected or believed to have a condition or disease mentioned
herein, (ii)
exposing said tissue or fluid sample to one or more binding members of the
present invention;
and (iii) detecting bound A[31-42 as compared with a control sample, wherein
an increase in the
amount of A131-42 binding as compared with the control may indicate an
aberrant level of A131-
42. Tissue or fluid samples to be tested include blood, serum, plasma, CSF,
urine, biopsy
material, tumours, or any tissue suspected of containing aberrant A131-42
levels. Subjects
testing positive for aberrant A[31-42 levels or activity may also benefit from
the treatment
methods disclosed later herein.
Those skilled in the art are able to choose a suitable mode of determining
binding of the
binding member to an antigen according to their preference and general
knowledge, in light of
the methods disclosed herein.
The reactivities of binding members in a sample may be determined by any
appropriate
means. Radioimmunoassay (RIA) is one possibility. Radioactive labelled antigen
is mixed with
unlabelled antigen (the test sample) and allowed to bind to the binding
member. Bound antigen
is physically separated from unbound antigen and the amount of radioactive
antigen bound to
the binding member determined. The more antigen there is in the test sample
the less
radioactive antigen will bind to the binding member. A competitive binding
assay may also be
used with non-radioactive antigen, using antigen or an analogue linked to a
reporter molecule.
The reporter molecule may be a fluorochrome, phosphor or laser dye with
spectrally isolated
absorption or emission characteristics. Suitable fluorochromes include
fluorescein, rhodamine,
phycoerythrin and Texas Red, and lanthanide chelates or cryptates. Suitable
chromogenic
dyes include diaminobenzidine.
Other reporters include macromolecular colloidal particles or particulate
material, such
as latex beads that are colored, magnetic or paramagnetic, and biologically or
chemically active
agents that can directly or indirectly cause detectable signals to be visually
observed,
electronically detected or otherwise recorded. These molecules may be enzymes,
which
catalyze reactions that develop, or change colours or cause changes in
electrical properties, for
example. They may be molecularly excitable, such that electronic transitions
between energy
states result in characteristic spectral absorptions or emissions. They may
include chemical
entities used in conjunction with biosensors. Biotin / avidin or biotin /
streptavidin and alkaline
phosphatase detection systems may be employed.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
The signals generated by individual binding member-reporter conjugates may be
used to
derive quantifiable absolute or relative data of the relevant binding member
binding in samples
(normal and test).
A kit comprising a binding member as described herein is also provided as an
aspect of
5 the present invention. In the kit, the binding member may be labelled to
allow its reactivity in a
sample to be determined, e.g., as described further below. Further, the
binding member may or
may not be attached to a solid support. Components of a kit are generally
sterile and in sealed
vials or other containers. Kits may be employed in diagnostic analysis or
other methods for
which binding members are useful. A kit may be for use in a method described
above. A kit
10 may contain instructions for use of the components in a method, e.g., a
method in accordance
with the present invention. Ancillary materials to assist in or to enable
performing such a
method may be included within a kit of the invention. The ancillary materials
include a second,
different binding member which binds to the first binding member and is
conjugated to a
detectable label (e.g., a fluorescent label, radioactive isotope or enzyme).
Antibody-based kits
15 may also comprise beads for conducting an immunoprecipitation. Each
component of the kits is
generally in its own suitable container. Thus, these kits generally comprise
distinct containers
suitable for each binding member. Further, the kits may comprise instructions
for performing
the assay and methods for interpreting and analyzing the data resulting from
the performance of
the assay.
20 The present invention also provides the use of a binding member as above
for
measuring antigen levels in a competition assay, that is to say a method of
measuring the level
of antigen in a sample by employing a binding member as provided by the
present invention in a
competition assay. This may be where the physical separation of bound from
unbound antigen
is not required. Linking a reporter molecule to the binding member so that a
physical or optical
25 change occurs on binding is one possibility. The reporter molecule may
directly or indirectly
generate detectable signals, which may be quantifiable. The linkage of
reporter molecules may
be directly or indirectly, covalently, e.g., via a peptide bond or non-
covalently. Linkage via a
peptide bond may be as a result of recombinant expression of a gene fusion
encoding antibody
and reporter molecule.
30 Competition between binding members may be assayed easily in vitro, for
example
using ELISA and/or by a biochemical competition assay such as one tagging a
specific reporter
molecule to one binding member which can be detected in the presence of one or
more other
untagged binding members, to enable identification of binding members which
bind the same
epitope or an overlapping epitope. Such methods are readily known to one of
ordinary skill in
35 the art, and are described in more detail herein.
The present invention extends to a binding member that competes for binding to
human
A131-42 with any binding member defined herein, e.g., any of the antibodies
listed in Tables 5
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
46
and 6, e.g., in IgG2, IgG1 or IgG1 triple mutation ("TM"; Oganesyan etal.
(2008) Acta
Crystallogr D Biol Crystallogr. 64(Pt 6):700-4) format. Competition between
binding members
may be assayed easily in vitro, for example by tagging a specific reporter
molecule to one
binding member which can be detected in the presence of other untagged binding
member(s),
to enable identification of binding members which bind the same epitope or an
overlapping
epitope. Competition may be determined for example using ELISA in which A131-
42 is
immobilized to a plate and a first tagged or labelled binding member along
with one or more
other untagged or unlabelled binding members is added to the plate. Presence
of an untagged
binding member that competes with the tagged binding member is observed by a
decrease in
the signal emitted by the tagged binding member.
Competition assays can also be used in epitope mapping. In one instance
epitope
mapping may be used to identify the epitope bound by a binding member which
optionally may
have optimized neutralizing and/or modulating characteristics. Such an epitope
can be linear or
conformational. A conformational epitope can comprise at least two different
fragments of A13,
wherein said fragments are positioned in proximity to each other when the A13
peptide is folded
in its tertiary or quaternary structure to form a conformational epitope which
is recognized by an
inhibitor of A13, such as a A13 - binding member. In testing for competition a
peptide fragment of
the antigen may be employed, especially a peptide including or consisting
essentially of an
epitope of interest. A peptide having the epitope sequence plus one or more
amino acids at
either end may be used. Binding members according to the present invention may
be such that
their binding for antigen is inhibited by a peptide with or including the
sequence given.
In further aspects, the invention provides an isolated nucleic acid which
comprises a
sequence encoding a binding member, VH domain and/or VL domain according to
the present
invention, and methods of preparing a binding member, a VH domain and/or a VL
domain of the
invention, which comprise expressing said nucleic acid under conditions to
bring about
production of said binding member, VH domain and/or VL domain, and recovering
it. Examples
of encoding nucleic acid sequences are set out in the Tables and the appended
sequence
listing. Nucleic acid sequences according to the present invention may
comprise DNA or RNA
and may be wholly or partially synthetic. Reference to a nucleotide sequence
as set out herein
encompasses a DNA molecule with the specified sequence, and encompasses a RNA
molecule
with the specified sequence in which U is substituted for T, unless context
requires otherwise
The present invention also provides constructs in the form of plasmids,
vectors, such as
a plasmid or phage vector, transcription or expression cassettes which
comprise at least one
polynucleotide as above, for example operably linked to a regulatory element.
A further aspect provides a host cell containing or transformed with the
nucleic acids
and/or vectors of the invention. The present invention also provides a
recombinant host cell line
that comprises one or more constructs as above. A nucleic acid sequence
encoding any CDR
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
47
or set of CDRs or VH domain or VL domain or antibody antigen-binding site or
antibody
molecule, e.g. scFv or IgG (e.g. IgG2, IgG1 or IgG1TM) as provided, forms an
aspect of the
present invention, along with a method of production of the encoded product,
which method
comprises expression from encoding nucleic acid sequences thereof. Expression
may
conveniently be achieved by culturing recombinant host cells containing the
nucleic acid under
appropriate conditions. Following production by expression a VH or VL domain,
or binding
member may be isolated and/or purified using any suitable technique, then used
as appropriate.
Accordingly, another aspect of the invention is a method of production of an
antibody VH
variable domain, the method including causing expression from encoding nucleic
acid
sequences. Such a method may comprise culturing host cells under conditions
for production of
said antibody VH variable domain.
Analogous methods for production of VL variable domains and binding members
comprising a VH and/or VL domain are provided as further aspects of the
present invention.
A method of production may comprise a step of isolation and/or purification of
the
product. A method of production may comprise formulating the product into a
composition
including at least one additional component, such as a pharmaceutically
acceptable excipient.
Systems for cloning and expression of a polypeptide in a variety of different
host cells
are well known. Suitable host cells include bacteria, mammalian cells, plant
cells, filamentous
fungi, yeast and baculovirus systems and transgenic plants and animals. The
expression of
antibodies and antibody fragments in prokaryotic cells is well established in
the art. For a
review, see for example PlOckthun [PlOckthun, A. Bio/Technology 9: 545-551
(1991)]. A
common bacterial host is E. coil.
Expression in eukaryotic cells in culture is also available to those skilled
in the art as an
option for production of a binding member [Chadd HE and Chamow SM (2001)
Current Opinion
in Biotechnology 12: 188-194; Andersen DC and Krummen L (2002) Current Opinion
in
Biotechnology 13: 117; Larrick JW and Thomas DW (2001) Current Opinion in
Biotechnology
12:411-418].
Mammalian cell lines available in the art for expression of a heterologous
polypeptide
include Chinese hamster ovary (CHO) cells, HeLa cells, baby hamster kidney
cells, NSO mouse
melanoma cells, YB2/0 rat myeloma cells, human embryonic kidney cells, human
embryonic
retina cells and many others.
Suitable vectors can be chosen or constructed, containing appropriate
regulatory
sequences, including promoter sequences, terminator sequences, polyadenylation
sequences,
enhancer sequences, marker genes and other sequences as appropriate. Vectors
may be
plasmids e.g. phagemid, or viral, e.g. 'phage, as appropriate [Sambrook and
Russell, Molecular
Cloning: a Laboratory Manual: 3rd edition, 2001, Cold Spring Harbor Laboratory
Press]. Many
known techniques and protocols for manipulation of nucleic acid, for example
in preparation of
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
48
nucleic acid constructs, mutagenesis, sequencing, introduction of DNA into
cells and gene
expression, and analysis of proteins, are described in detail in Ausubel etal.
[Ausubel etal. eds.,
Short Protocols in Molecular Biology: A Compendium of Methods from Current
Protocols in
Molecular Biology, John Wiley & Sons, 41h edition 1999].
A further aspect of the present invention provides a host cell containing
nucleic acid as
disclosed herein. Such a host cell may be in vitro and may be in culture. Such
a host cell may
be in vivo. In vivo presence of the host cell may allow intra-cellular
expression of the binding
members of the present invention as "intrabodies" or intra-cellular
antibodies. Intrabodies may
be used for gene therapy.
Another aspect provides a method comprising introducing nucleic acid of the
invention
into a host cell. The introduction may employ any available technique. For
eukaryotic cells,
suitable techniques may include calcium phosphate transfection, DEAE-Dextran,
electroporation, liposome-mediated transfection and transduction using
retrovirus or other virus,
e.g., Vaccinia, or for insect cells, Baculovirus. Introducing nucleic acid in
the host cell, in,
particular a eukaryotic cell may use a viral or a plasmid based system. The
plasmid system may
be maintained episomally or may be incorporated into the host cell or into an
artificial
chromosome. Incorporation may be either by random or targeted integration of
one or more
copies at single or multiple loci. For bacterial cells, suitable techniques
may include calcium
chloride transformation, electroporation and transfection using bacteriophage.
The introduction may be followed by causing or allowing expression from the
nucleic
acid, e.g., by culturing host cells under conditions for expression of the
gene. The purification of
the expressed product may be achieved by methods known to one of skill in the
art.
Nucleic acid of the invention may be integrated into the genome (e.g.,
chromosome) of
the host cell. Integration may be promoted by inclusion of sequences that
promote
recombination with the genome, in accordance with standard techniques.
The present invention also provides a method that comprises using a construct
as stated
above in an expression system in order to express a binding member or
polypeptide as above.
Binding members according to the invention may be used in a treatment (which
may
include prophylactic treatment) of a disease or disorder in the human or
animal body (e.g., in a
human patient), which comprises administering the binding member to the
patient. Conditions
treatable according to the invention are described elsewhere herein, including
preventative
treatment and reduction of severity of the condition or one or more of its
symptoms, or delaying
or reducing risk of onset.
Accordingly, the invention provides a method of treating or reducing the
severity of at
least one symptom of any of the conditions mentioned herein, comprising
administering to a
patient in need thereof an effective amount of one or more binding members of
the present
invention alone or in a combined therapeutic regimen with another appropriate
medicament
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
49
known in the art or described herein such that the severity of at least one
symptom of any of the
above disorders is reduced.
The term "effective dose" is defined as an amount sufficient to cure or at
least partially
arrest the disease and its complications in a patient already suffering from
the disease.
The present invention is directed inter alia to treatment of Alzheimer's
disease and other
amyloidogenic diseases by administration of therapeutic antibody of the
invention to a patient
under conditions that generate a beneficial therapeutic response in a patient
(e.g., a reduction
of A131-42 in CSF, a reduction of plaque burden, inhibition of plaque
formation, reduction of
neuritic dystrophy, improvement in cognitive function, and/or reversal,
reduction or prevention of
cognitive decline) in the patient, for example, for the prevention or
treatment of an
amyloidogenic disease.
As used herein, "treatment" is defined as the application or administration of
a
therapeutic agent to a patient, who has a disease or condition associated with
amyloidosis; or a
symptom of, or a predisposition towards such disease or condition associated
with amyloidosis,
with the purpose to cure, heal, alleviate, relieve, alter, remedy, ameliorate,
improve or affect the
disease, condition, symptoms thereof or the predisposition thereto.
The invention provides methods of preventing or treating a disease associated
with
amyloid deposits of A13 in the brain of a patient. Such diseases include
Alzheimer's disease,
Down's syndrome, and cognitive impairment. Cognitive impairment can occur with
or without
other characteristics of an amyloidogenic disease. The invention provides
methods of treatment
of macular degeneration, a condition which is linked with Aft Methods of the
invention may
involve administering an effective dose to a patient of an antibody that
specifically binds to 1 ¨
42 A13 and N-terminal truncates thereof. Such methods are particularly useful
for preventing or
treating Alzheimer's disease in human patients.
Antibodies of the invention may be used in therapeutic regimes for preventing
or
ameliorating the neuropathology and, in some patients, the cognitive
impairment associated
with Alzheimer's disease.
Patients amenable to treatment include patients showing symptoms and also
individuals
at risk of disease but not showing symptoms. For Alzheimer's disease,
potentially anyone is at
risk if he or she lives for a sufficiently long time. Antibodies of the
invention can be administered
prophylactically to a subject without any assessment of the risk of the
subject patient. Patients
amenable to treatment include individuals who have a known genetic risk of
Alzheimer's
disease, for example individuals who have blood relatives with this disease
and those whose
risk is determined by analysis of genetic or biochemical markers. Genetic
markers of
predisposition towards Alzheimer's disease include mutations in the APP gene,
particularly
mutations at position 717 and positions 670 and 671 referred to as the Hardy
and Swedish
mutations respectively. Other markers of risk are mutations in the presenilin
genes, PS1 and
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
PS2, and ApoE4, a family history of AD, hypercholesterolemia or
atherosclerosis. Individuals
suffering from Alzheimer's disease can be diagnosed by the characteristic
dementia associated
with the disease, as well as by the presence of risk factors described above.
A number of
diagnostic tests are available to assist in identification Alzheimer's disease
in an individual.
5 These include measurement of CSF tau and A131-42 levels. Elevated tau and
decreased A131-42
levels may signify the presence of AD. Individuals suffering from Alzheimer's
disease can also
be diagnosed by NINCDS-ADRDA or DSM-IV-TR criteria.
In asymptomatic patients, treatment can begin at any age (e.g., 10, 20, 30).
Generally,
treatment is commenced in later life, for example when a patient reaches his
or her 40's, 50's,
10 60's or 70's. Treatment may involve multiple doses over a period of
time, which may be for the
duration of the remaining life of the patient. The need for administration of
repeat doses can be
monitored by measuring antibody levels over time.
For prophylaxis, pharmaceutical compositions or medicaments are administered
to a
patient susceptible to, or otherwise at risk of, Alzheimer's disease in an
amount sufficient to
15 eliminate or reduce the risk, lessen the severity, or delay the outset
of the disease, including
biochemical, histologic, cognitive impairment and/or behavioral symptoms of
the disease, its
complications and intermediate pathological phenotypes presenting during
development of the
disease. For therapeutic applications, compositions or medicaments are
administered to a
patient suspected of, or already suffering from such a disease in an amount
sufficient to cure, or
20 at least partially arrest, the symptoms of the disease (biochemical,
histologic, cognitive
impairment and/or behavioural), including its complications and intermediate
pathological
phenotypes in development of the disease.
The invention provides a method of treating an individual comprising
administering to an
individual in need thereof an effective amount of one or more binding members
of the present
25 invention alone or in a combined therapeutic regimen with another
appropriate medicament
known in the art or described herein. A method of treatment may comprise
administering an
effective amount of a binding member described herein to a patient in need
thereof, wherein
levels of A131-42 are decreased in blood plasma and / or CSF.
A method of treatment may comprise (i) identifying a patient having a
condition
30 associated with amyloidosis as mentioned herein, and (ii) administering
an effective amount of a
binding member described herein to the patient, wherein levels of A131-42 are
decreased in
blood plasma and/or CSF, and amyloidosis is reduced. An effective amount is an
amount that
decreases the level of A[31-42 so as to decrease or lessen the severity of at
least one symptom
of the particular disease or disorder being treated, but not necessarily cure
the disease or
35 disorder.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
51
The invention also provides a method of antagonising at least one effect of
A131-42
comprising contacting with or administering an effective amount of one or more
binding
members of the present invention such that said at least one effect of A[31-42
is antagonised.
Accordingly, further aspects of the invention provide methods of treatment
comprising
administration of a binding member as provided, pharmaceutical compositions
comprising such
a binding member, and use of such a binding member in the manufacture of a
medicament for
administration, for example in a method of making a medicament or
pharmaceutical
composition comprising formulating the binding member with a pharmaceutically
acceptable
excipient. A pharmaceutically acceptable excipient may be a compound or a
combination of
compounds entering into a pharmaceutical composition not provoking secondary
reactions and
which allows, for example, facilitation of the administration of the binding
member, an increase
in its lifespan and/or in its efficacy in the body, an increase in its
solubility in solution or else an
improvement in its conservation. These pharmaceutically acceptable vehicles
are well known
and will be adapted by the person skilled in the art as a function of the
nature and of the mode
of administration of the active compound(s) chosen.
Binding members as described herein will usually be administered in the form
of a
pharmaceutical composition, which may comprise at least one component in
addition to the
binding member. Thus pharmaceutical compositions according to the present
invention, and for
use in accordance with the present invention, may comprise, in addition to a
binding member, a
pharmaceutically acceptable excipient, carrier, buffer, stabilizer or other
materials well known to
those skilled in the art. Such materials should be non-toxic and should not
interfere with the
efficacy of the active ingredient. The precise nature of the carrier or other
material will depend
on the route of administration.
For injectable formulations, e.g., for intra-venous or subcutaneous injection,
the active
ingredient will be in the form of a parenterally acceptable aqueous solution
which is pyrogen-
free and has suitable pH, isotonicity and stability. Binding members as
described herein may
be formulated in liquid, semi-solid or solid forms depending on the
physicochemical properties
of the molecule and the route of delivery. Formulations may include
excipients, or combinations
of excipients, for example: sugars, amino acids and surfactants. Liquid
formulations may include
a wide range of antibody concentrations and pH. Solid formulations may be
produced by
lyophilisation, spray drying, or drying by supercritical fluid technology, for
example. Treatment
may be given by injection (for example, subcutaneously, or intra-venously. The
treatment may
be administered by pulse infusion, particularly with declining doses of the
binding member. The
route of administration can be determined by the physicochemical
characteristics of the
treatment, by special considerations for the disease or by the requirement to
optimize efficacy
or to minimize side-effects. One particular route of administration is intra-
venous. Another route
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
52
of administering pharmaceutical compositions of the present invention is
subcutaneously.
Subcutaneous injection using a needle-free device is also advantageous.
A composition may be administered alone or in combination with other
treatments, either
simultaneously or sequentially dependent upon the condition to be treated.
A binding member may be used as part of a combination therapy in conjunction
with an
additional medicinal component. Combination treatments may be used to provide
significant
synergistic effects, particularly the combination of a binding member with one
or more other
drugs. A binding member may be administered concurrently or sequentially or as
a combined
preparation with another therapeutic agent or agents, for the treatment of one
or more of the
conditions listed herein.
A binding member and one or more of the above additional medicinal components
may
be used in the manufacture of a medicament. The medicament may be for separate
or
combined administration to an individual, and accordingly may comprise the
binding member
and the additional component as a combined preparation or as separate
preparations.
Separate preparations may be used to facilitate separate and sequential or
simultaneous
administration, and allow administration of the components by different routes
e.g. oral and
parenteral administration.
Compositions provided may be administered to mammals. Administration is
normally in
a "therapeutically effective amount", this being sufficient to show benefit to
a patient. Such
benefit may be at least amelioration of at least one symptom. The actual
amount administered,
and rate and time-course of administration, will depend on the nature and
severity of what is
being treated, the particular mammal being treated, the clinical condition of
the individual patient,
the cause of the disorder, the site of delivery of the composition, the type
of binding member,
the method of administration, the scheduling of administration and other
factors known to
medical practitioners. Prescription of treatment, e.g. decisions on dosage
etc, is within the
responsibility of general practitioners and other medical doctors and may
depend on the
severity of the symptoms and/or progression of a disease being treated. A
therapeutically
effective amount or suitable dose of a binding member of the invention can be
determined by
comparing its in vitro activity and in vivo activity in an animal model.
Methods for extrapolation
of effective dosages in test animals to humans are known. The precise dose
will depend upon
a number of factors, including whether the antibody is for diagnosis,
prevention or for treatment,
the size and location of the area to be treated, the precise nature of the
antibody (e.g., whole
antibody, fragment or diabody) and the nature of any detectable label or other
molecule
attached to the antibody. A typical antibody dose will be in the range 100 pg
to 1 g for systemic
applications. An initial higher loading dose, followed by one or more lower
doses, may be
administered. Typically, the antibody will be a whole antibody, e.g., the IgG1
or IgG1-TM isotype.
Treatments may be repeated at daily, twice-weekly, weekly or monthly
intervals, at the
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
53
discretion of the physician. Treatments may be every two to four weeks for
subcutaneous
administration and every four to eight weeks for intra-venous administration.
Treatment may be
periodic, and the period between administrations is about two weeks or more,
e.g., about three
weeks or more, about four weeks or more, or about once a month.
Various further aspects and embodiments of the present invention will be
apparent to
those skilled in the art in view of the present disclosure.
All documents, including database references and accession numbers, patents,
patent
applications and publications, mentioned in this specification are
incorporated herein by
reference in their entirety for all purposes.
"and/or" where used herein is to be taken as specific disclosure of each of
the two
specified features or components with or without the other. For example "A
and/or B" is to be
taken as specific disclosure of each of (i) A, (ii) B and (iii) A and B, just
as if each is set out
individually herein.
Unless context dictates otherwise, the descriptions and definitions of the
features set out
above are not limited to any particular aspect or embodiment of the invention
and apply equally
to all aspects and embodiments which are described.
Certain aspects and embodiments of the invention will now be illustrated by
way of
example and with reference to the accompanying figures and tables.
Examples
The following sequences have been deposited with NCIMB, Ferguson Building,
Craibstone
Estate, Bucksburn, Aberdeen, AB21 9YA. Scotland, UK:
E. coli TOP10 cells Abet0007 = NCIMB 41889
E. coli T0P10 cells Abet0380-GL = NCIMB 41890
E. coli T0P10 cells Abet0144-GL = NCIMB 41891
E. coli T0P10 cells Abet0377-GL = NCIMB 41892
Date of deposit = 02 November 2011
Example 1. Anti-Amyloid beta 1-42 specific antibody generation and lead
selection
1.1 Formulation of Amyloid beta peptides
Biotinylated human Amyloid beta 1-42 peptide (rPeptide, USA; cat: A1117 or
Bachem
AG, Switzerland; cat: H-5642) was resuspended to 1 mg/ml in 1% ammonium
hydroxide
solution (v/v) and stored in aliquots at -80 C until required. An identical
procedure was followed
for unlabelled human Amyloid beta 1-42 peptide (Anaspec, USA; cat: 64129),
unlabelled human
Amyloid beta 1-40 peptide (rPeptide, USA; cat: A1155), biotinylated human
Amyloid beta 1-40
peptide (rPeptide, USA; cat: A111 or Bachem AG, Switzerland; cat: H-5914),
biotinylated
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
54
murine Amyloid beta 1-42 peptide (Anaspec, USA; cat: 61718-01) and
biotinylated murine
Amyloid beta 1-40 peptide (Anaspec, USA; cat: 61717).
1.2 Selections
The Fab310-Lambda phage display library (Dyax, USA) cloned into a phagemid
vector
based on the filamentous phage M13 was used for selections (Hoet et al.,
2005). Anti-Amyloid
beta 1-42 specific Fab antibodies were isolated from the phage display
libraries using a series
of selection cycles on synthetic human biotinylated Amyloid beta 1-42
(rPeptide, USA)
essentially as previously described (Hawkins et al., 1992; Vaughan et al.,
1996). In brief, for the
first round of solution-phase selections, biotinylated amyloid-beta 1-42 in
Dulbecco's phosphate
buffered saline (DPBS, pH 7.4) was added to purified phage particles that had
been pre-
incubated for 1 hour in Marvel-PBS (3% w/v) containing a 100-fold excess of
unlabelled human
Amyloid beta 1-40 peptide (Anaspec, USA). Phage particles that bound to the
biotinylated
Amyloid beta 1-42 peptide were captured using Streptavidin-coupled
paramagnetic beads
(lnvitrogen Life Technologies, UK) and weakly-bound phage were removed by a
series of wash
cycles using PBS-Tween (0.1% v/v). Bound phage particles were eluted from the
beads,
infected into E. coliTG1 bacteria and rescued for the next round of selection
(Vaughan et al.,
1996). Two subsequent rounds of selection were carried out as previously
described but with a
reduced concentration of biotinylated Amyloid beta 1-42 antigen.
1.3 Identification of Amyloid beta 1-42 specific clones using a direct-binding
assay on unpurified
Fab fragments
To produce soluble single chain Fab fragments (sFab) the genelll tether was
removed
from the Fab310-Lambda display cassette using standard cleavage and ligation
techniques.
Briefly, the phagemid vectors were isolated from the round 3 output using
standard DNA
purification kits (QIAgen, UK) and the genelll tether sequence was removed
from the vector
using a M/ul restriction digest (Hoet et al., 2005). Religated vectors were
transformed back into
TG1 cells and individual colonies were picked for analysis.
Unpurified sFab from periplasmic preparations were screened in a homogeneous
time-
resolved fluorescence (HTRFTm, CisBio International, France) binding assay
using an EnVision
plate reader (PerkinElmer, USA). In this assay, binding of unpurified sFab to
human Amyloid
beta 1-42 peptide was assessed by measuring the fluorescence resonance energy
transfer
(FRET) between the histidine tagged sFab and the biotinylated peptide using
streptavidin
cryptate and anti-6his-XL665 detection reagents (CisBio International, France;
cat: 610SAKLB
and 61H ISXLB respectively). Selection outputs were screened as unpurified
bacterial
periplasmic extracts containing sFab, prepared in 50 mM MOPS buffer pH 7.4,
0.5 mM EDTA
and 0.5 M sucrose. Ten microlitres of unpurified sFab samples were added to a
Costar 384
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
well assay plate (Corning, USA; cat: 3676). This was followed by the addition
of 5 pl of 20 nM
synthetic human Amyloid beta 1-42 and 5 pl of a combined solution of 6 nM
streptavidin
cryptate and 20 nM anti-his-XL665. Non-specific binding wells (negative
controls) were defined
for each plate by using a negative control unpurified sFab in place of the
test sFab sample.
5 Cross-reactive sFab clones were identified using a concurrent assay with
human Amyloid beta
1-40 peptide. All dilutions were performed in 50 mM MOPS pH 7.4 (Sigma, UK;
cat: M9381)
containing 0.4 M KF (BDH Chemicals, USA; cat: 103444T), 0.1% fatty acid free
bovine serum
albumin (Sigma, UK; cat: A6003) and 0.1% Tween 20 (v/v) (Sigma, UK; cat:
P2287) (assay
buffer). Assay plates were incubated for 4 hours at room temperature prior to
reading time
10 resolved fluorescence on an En Vision plate reader (PerkinElmer, USA)
using an excitation
wavelength of 320 nm and measuring the emission at 620 nm and 665 nm (100
flashes).
Data were analysed by calculating % Delta F values for each sample. % Delta F
was
determined according to equation 1.
15 Equation 1:
% Delta F = (sample 665 nm / 620 nm ratio) ¨ (negative control 665 nm / 620 nm
ratio) x 100
(negative control 665 nm / 620 nm ratio)
1.4 Direct binding assay of purified sFab fragments
Unpurified sFab periplasm extracts that showed specific binding to human
Amyloid beta
1-42 peptide by HTRFTm assay were subjected to DNA sequencing (Osbourn et al.,
1996;
20 Vaughan etal., 1996). The sFab with unique protein sequences were
expressed in E. coli and
purified by affinity chromatography (essentially as described (Bannister
etal., 2006)). The
Amyloid beta binding profile of each purified sFab was determined by testing a
dilution series of
the purified sFab in the HTRFTm assay described in section 1.3, substituting
the unpurified sFab
periplasmic preparation with the purified sFab. The purified sFab were tested
concurrently for
25 binding to biotinylated human Amyloid beta 1-42 peptide, biotinylated
murine Amyloid beta 1-42
peptide and biotinylated human Amyloid beta 1-40 peptide. In addition, sFab
were tested for
binding to scrambled human Amyloid beta 1-42 peptide (Anaspec, custom
synthesis) in a
separate HTRFTm experiment in order to control for any non-specific peptide
binding. Data were
analysed by calculating the % Delta F values as described in section 1.3.
30 Example results for purified Abet0007 sFab are shown in Figure 1. These
results
demonstrate that Abet0007 specifically binds to human Amyloid beta 1-42
peptide over human
Amyloid beta 1-40 peptide and scrambled human Amyloid beta 1-42 peptide. In
addition,
Abet0007 sFab is cross-reactive with murine Amyloid beta 1-42 peptide.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
56
1.5 Reformatting of Amyloid beta 1-42 specific antibody Fabs to IgG2 format
Thirteen Amyloid beta 1-42 specific clones were converted from Fab to IgG2 by
sub-
cloning the variable heavy chain (VH) and variable light chain (VI) domains
into vectors
expressing whole human antibody heavy and light chains respectively. The VH
domains were
codon optimised in-house before sub-cloning since the Dyax FAB310 library VH
domains are
semi-synthetic and failed to express in sufficient quantities within the
mammalian cells
described herein. An in-house germline matched VH, which is known to express
well as a full-
length human IgG in mammalian cells, was used as a template to alter the DNA
codon usage of
the Dyax VH domains whilst retaining the original amino acid sequence. The
codon optimised
variable heavy chains were cloned into a mammalian expression vector (pEU 9.2)
containing
the human heavy chain constant domains and regulatory elements to express
whole IgG2
heavy chain in mammalian cells. Similarly, the variable light chain domain was
cloned into a
mammalian expression vector (pEU4.4) for the expression of the human lambda
light chain
constant domains and regulatory elements to express whole IgG light chain in
mammalian cells.
Vectors for the expression of heavy chains and light chains were originally
described in Persic
et al. (Persic et aL, 1997). To obtain clones as IgG2, the heavy and light
chain IgG expression
vectors were transiently transfected into HEK293-EBNA (lnvitrogen, UK; cat:
R620-07) or
CEP6-CHO (produced in-house) mammalian cells where the antibody was expressed
and
secreted into the medium. Harvested media was filtered prior to purification.
The IgGs were
purified using Protein A chromatography (BiosepraTM, Pall, USA or MabSelect
SuRe, GE
Healthcare, UK). Culture supernatants were loaded onto an appropriate Protein
A column pre
equilibrated in 50 mM Tris pH 8.0, 250 mM NaCI. Bound IgG was eluted from the
column using
0.1 M Sodium citrate pH 3.0 and the eluate was neutralised by the addition of
1 M Tris buffer
(pH 10.0). The IgGs were buffer exchanged into Dulbecco's PBS using NAP-10
buffer
exchange columns (GE Healthcare, UK; cat: 17-0854-02). The purified IgGs were
passed
through a 0.2 micrometer filter and the concentration of IgG was determined by
absorbance at
280 nm using an extinction coefficient based on the amino acid sequence of the
IgG. The
purified IgGs were analysed for aggregation or degradation using SEC-HPLC and
SDS-PAGE
techniques.
1.6 Specificity determination of lead antibodies in an Amyloid beta peptide
competition HTRFTm
assay
As described above, the purified sFab fragments that bound specifically to the
Amyloid
beta 1-42 peptide were converted to recombinant IgG. To test the specificity
of these IgGs for
binding to other human Amyloid beta peptides, a competition assay was
developed. In this
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
57
assay unlabelled human Amyloid beta peptides 1-42 (rPeptide, USA; cat: A1165),
1-40
(rPeptide, USA; cat: A1155), 11-42 (rPeptide, USA; cat: A1063), 17-42
(rPeptide, USA; cat:
A1058) and 1-43 (Anaspec, USA; cat: 25356) were incubated with the lead IgG
and biotinylated
human Amyloid beta peptide 1-42 (rPeptide, USA; cat: A1117). Briefly, a
dilution series of each
test peptide was combined with 0.3 nM test IgG and 5 nM biotinylated human
Amyloid beta 1-42
peptide. The competition of each peptide was assessed by detecting the loss of
binding of the
lead IgG to the biotinylated 1-42 peptide by measuring the fluorescence
resonance energy
transfer (FRET) between the IgG and biotinylated 1-42 Amyloid beta using
streptavidin cryptate
(CisBio International, France; cat: 610SAKLB) and anti-human Fc IgG XL665
(CisBio
International, France; cat: 61HFCXLB) detection reagents.
Streptavidin cryptate and anti-human Fe IgG XL665 were combined at 7 nM and 5
nM
respectively in assay buffer containing 50 mM MOPS pH 7.4 (Sigma, UK; cat:
M9381), 0.4 M KF
(BDH Chemicals, USA; cat: 103444T), 0.1% fatty acid free bovine serum albumin
(Sigma, UK;
cat: A6003) and 0.1% Tween 20 (v/v) (Sigma, UK; cat: P2287). 5 pl of this
solution were added
to the assay plate (Costar 384 well black shallow well, Corning Life
Sciences; cat: 3676).
Amyloid beta peptides were serially diluted in assay buffer using a Greiner 96
well U bottom
plate (Greiner BioOne, Germany; cat: 650201). 5 pl of each peptide dilution
were transferred in
duplicate to the assay plate using a MiniTrakTm (PerkinElmer, USA) liquid
handling robot. The
test IgG was diluted to 1.2 nM in assay buffer and 5 pl were added to the
assay plate. A 20 nM
solution of biotinylated human Amyloid beta 1-42 peptide was prepared in assay
buffer and 5 pl
of this solution were added to the assay plates. Non-specific binding wells
(negative controls)
were defined for each plate by replacing the test IgG with 5 pl of assay
buffer. Assay plates
were incubated for 4 hours at room temperature prior to reading time resolved
fluorescence at
620 nm and 665 nm emission wavelengths using an EnVision plate reader
(PerkinElmer, USA).
Data were analysed by calculating % Delta F values for each sample. % Delta F
was
determined according to equation 1. % Delta F values were subsequently used to
calculate %
specific binding as described in equation 2.
Equation 2:
% specific binding = % Delta F of sample x 100
% Delta F of total binding control
Example results for Abet0007 IgG are shown in Figure 2. These results
demonstrate
Abet0007 IgG binds to human Amyloid beta 1-42 peptide, but not to human
Amyloid beta 1-40
peptide. This antibody also binds to the truncated peptides 11-42 and 17-42
and to the human
Amyloid beta 1-43 peptide.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
58
1.7 Determination of binding affinities of lead antibodies to human amyloid
beta 1-42 using
Surface Plasmon Resonance
The BlAcore T-100 (GE Healthcare, UK) biosensor instrument was used to assess
the
kinetic parameters of the interaction between each lead antibody and
synthetically produced
human Amyloid beta 1-42 peptide. These experiments were performed essentially
as described
by Karlsson et al. (Karlsson et al., 1991).
The biosensor uses the optical effects of surface plasmon resonance (SPR) to
study
changes in surface concentration resulting from the interaction of an analyte
molecule that is
flowed over a ligand molecule that is immobilised on the dextran layer of a
biosensor chip.
Typically, a defined concentration of the analyte species is passed over the
coupled ligand and
any binding is detected as an increase in local SPR signal (association
phase). This is followed
by a period of buffer flow, during which dissociation of the analyte species
from the surface
immobilised ligand can be observed as a decrease in signal (dissociation
phase). The
remaining analyte can then be stripped from the chip-bound ligand and the
procedure repeated
at several different analyte concentrations. The experiment is designed such
that neither the
absolute binding capacity nor kinetic profile of the coupled ligand change
significantly during the
entire experiment and can be monitored using a series of controls employed
throughout the
experiment. A proprietary HEPES buffered saline containing EDTA (HBS-EP+, GE
Healthcare,
UK) is typically used as the diluent buffer for the analyte samples and as the
flow buffer during
the dissociation phase. The experimental data is recorded over time as
'Resonance Units'
(RUs), which are arbitrary units that directly correspond to the SPR signal.
The RUs are directly
proportional to changes in the refractive index on the chip surface, which in
turn is an
approximate measure of the mass of analyte bound. The proprietary
BlAevaluation software
package can then be used to process data and fit binding models to the data
sets. Returned
association (ka, M-1 s-1) and dissociation (kd, s-1) rate constants allow
calculation of dissociation
(KD, M) affinity constants.
The affinity of binding between each test IgG and human Amyloid beta 1-42
peptide was
estimated using assays in which the antibody was covalently coupled by amine-
linkage to a
proprietary CM5 chip surface to a final surface density of approximately 2,000
RU. The chip
surface was regenerated between cycles by a single 40 second injection of 10
mM Glycine pH
2.0 to remove ligand bound to the antibody. The regeneration did not result in
a significant loss
of peptide binding activity.
A series of dilutions of synthetic human Amyloid beta 1-42 peptide (1.6 ¨ 100
nM) were
sequentially passed over the antibody surface for a sufficient amount of time
to observe
sensorgrams that could be fitted to an appropriate binding model with
confidence. Blank
reference flow-cell data were subtracted from each IgG dataset and a zero-
concentration buffer
blank was double-reference subtracted from the main data set to reduce the
impact of any
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
59
buffer artefacts or non-specific binding effects. An appropriate binding model
was then fitted
simultaneously to the data from each analyte titration using the BlAevaluation
software.
The validity of the data was assessed using the calculated Chi2 value, with an
acceptable value being under 2 RU2. The overall success of the fit was
estimated using the
residuals, with a deviation of under 2 RUs being acceptable.
Example results for Abet0007 IgG2 are shown in Figure 3. The mean association
rate
constant (ka), dissociation rate constant (kd) and dissociation constant (KD)
are 1.6 x 105 vls-i,
7.4 x 10-2 s-1 and 473 nM respectively. These parameters were derived from a
1:1 Langmuir fit
to the data.
1.8 Functional characterisation of lead antibodies by depletion of Amyloid
beta peptides from
human plasma
The lead antibodies were tested in a plasma depletion assay to investigate
their ability to
immunoprecipitate human Amyloid beta 1-42 peptide from human blood plasma.
This assay
was used to provide evidence of functional efficacy for each antibody.
Briefly, human plasma
samples were incubated with each test IgG for 3 hours after which the
antibodies and any
bound ligands were removed. The Amyloid beta 1-42 peptide content of the human
plasma
samples was assayed using standard techniques before and after
immunoprecipitation, and
these values were used to determine the efficacy of the antibody. The plasma
samples were
also assayed for any depletion in Amyloid beta 1-40 peptide to assess the
specificity of the lead
antibodies
Each test antibody was separately covalently linked to Dynabeads M-270
Carboxylic
Acid magnetic beads (Invitrogen Life Technologies, UK; cat: 143-05D) according
to the
manufacturer's instructions. For each test IgG 100 pl of Dynabeads were washed
twice with 100
pl of 25 mM MES, pH 5 (Sigma, UK; cat: M5287) using a magnet to separate the
beads from
the suspension. Immediately before use, EDC (Pierce, Thermo Scientific, USA;
cat: 22981) was
dissolved in cold 25 mM MES, pH 5 to a final concentration of 50 mg/ml. A 50
mg/ml solution of
NHS (Pierce, Thermo Scientific; cat: 24500) was similarly prepared in 25 mM
MES, pH 5. 50 pl
of NHS solution and 50 .I EDC solution were added to the washed Dynabeads,
which were
mixed well and incubated with slow-tilt rotation at room temperature for 30
minutes. A magnet
was used to pellet the beads and the supernatant was removed. The beads were
washed twice
with 100 pl of 25 mM MES, pH 5. Each test IgG was diluted to 0.6 mg/ml in a
total volume of
100 pl of 25 mM MES, pH 5. The washed beads were resuspended in the ligand
solution and
were incubated for at least 30 minutes at room temperature with slow tilt
rotation. The beads
were subsequently pelleted using a magnet and washed once with 100 pl of 50 mM
Tris buffer,
pH 7.4 (Sigma, UK). The beads were then resuspended in 100 .I of Marvel-PBS
(3% w/v) and
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
incubated overnight at 4 C. The beads were washed twice with Dulbecco's PBS
(100 pl) and
resuspended in PBS (100 III).
EDTA-plasma samples were isolated from anonymous donors at the AstraZeneca
Health Clinic in Sodertalje, Sweden. Approximately 100 ml of blood were
collected from each
5 donor, and this was pooled into two 50 ml tubes. EDTA was added to a
final concentration of 5
mM to prevent clotting, and the tubes were spun at 4,000 x g for 10 minutes at
4 C. The
supernatant (plasma) was collected, aliquotted into 1 ml tubes, and stored at -
80 C until
required. The samples were assayed for both Amyloid beta 1-42 peptide and
Amyloid beta 1-40
peptide content as described below.
10 Each set of antibody coated beads was separately incubated with a 1 ml
aliquot of
EDTA-plasma for 3 hours at 4 C with slow tilt rotation, and the beads were
then pelleted using a
magnet. The supernatant was carefully removed from the beads and was assayed
for both
Amyloid beta 1-42 peptide and Amyloid beta 1-40 peptide as described below.
Analysis of the Amyloid beta 1-40 peptide content of the plasma samples was
performed
15 using the A13 40 Human ELISA Kit from lnvitrogen (UK; cat: KHB3482)
according to the
manufacturer's instructions. Briefly, a human Amyloid beta 1-40 standard was
used to create a
dilution series from 1 ng/ml down to 7.81 pg/ml. The dilutions were made using
Standard
Dilution Buffer containing 1 protease inhibitor tablet (Roche, UK; cat:
11697498001) per 50 ml
diluent. 50 pl of each dilution was then added to a different microtitre well
pre-coated with a
20 proprietary monoclonal antibody specific for the N-terminus of the
Amyloid beta peptide. EDTA-
plasma samples were centrifuged for 10 minutes at 4 C and 2,000 x g and 50 jil
of each sample
supernatant were added to a separate well in the same microtitre plate as the
standards. 50 pl
of proprietary Hu Al3 Detection Antibody, which specifically recognises the C-
terminus of
Amyloid beta 1-40 peptide, were then added to each microtitre well. The plate
was covered with
25 a plate seal and incubated for 3 hours at room temperature with shaking.
The plate was washed
four times with 400 pl of wash buffer, allowing the plate to soak for 15-30
seconds during each
wash. The plate was then inverted and tapped dry. 100 pl of proprietary Anti-
Rabbit IgG HRP
conjugate were added to each well, the plate was covered with a plate seal,
and the samples
were incubated for 30 minutes at room temperature. Again, the plate was washed
four times
30 with 400 pl of wash buffer, allowing the plate to soak for 15-30 seconds
during each wash. The
plate was then inverted and tapped dry. 100 pl of proprietary Stabilised
Chromogen were added
to each well, and the plate was incubated in the dark for 20 minutes at room
temperature. This
was followed by the addition of 100 pl of proprietary Stop Solution to each
well. The absorbance
of each well was read at 450 nm within 2 hours of adding the Stop Solution. A
standard curve
35 was produced from the human Amyloid beta 1-40 dilution series, and this
was used to
determine the concentration of human Amyloid beta 1-40 in the test EDTA-plasma
samples.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
61
Analysis of the Amyloid beta 1-42 peptide content of the plasma samples was
performed
using the INNOTEST 8-Amyloid (1 -42) kit from Innogenetics (Belgium; cat:
80177) essentially
according to the manufacturer's instructions. Briefly, a human Amyloid beta 1-
42 standard was
used to create a dilution series from 1 ng/ml down to 7.81 pg/ml. 100 pl of
each dilution was
then added to a different microtitre well pre-coated with a proprietary
monoclonal antibody
specific for the C-terminus of the human Amyloid beta 1-42 peptide. EDTA-
plasma samples
were centrifuged for 10 minutes at 4 C and 2,000 x g and 100 jil of each
sample supernatant
were added to a separate well in the same microtitre plate as the standards.
The plate was
covered with a plate seal and incubated for 3 hours at room temperature with
shaking. The plate
was washed five times with 400 pl of wash buffer and was then inverted and
tapped dry. 100 pl
of proprietary conjugate 1 (Cl HS), which recognises the N-terminus of the
Amyloid beta peptide,
were added to each well. The plate was covered with a plate seal, and the
samples were
incubated for 1 hour at room temperature. Again, the plate was washed five
times with 400 pl of
wash buffer, and was then inverted and tapped dry. 100 pl of conjugate 2 (C2),
a streptavidin-
HRP conjugate that binds to the biotinylated Cl HS antibody, were then added
to each well. The
plate was covered with a plate seal and incubated for 30 minutes at room
temperature. The
plate was washed five times with 400 pl of wash buffer, and was then inverted
and tapped dry.
100 pl of proprietary substrate solution were added to each well, the plate
was incubated for 30
minutes at room temperature, and then 100 pl of stop solution were added to
each well. The
absorbance of each well was read at 450 nm within 15 minutes of adding the
stop solution. A
standard curve was produced from the human Amyloid beta 1-42 dilution series,
and this was
used to determine the concentration of human Amyloid beta 1-42 in the test
EDTA-plasma
samples.
In one experiment, the Abet0007 IgG2 antibody reduced the levels of human
Amyloid
beta 1-42 peptide in plasma from 80.47 pg m1-1 to 60.56 pg m1-1 (a 25%
reduction). In a second
experiment, the levels were reduced from 197.43 pg m1-1 to 154.45 pg m1-1 (a
22% reduction).
1.9 Identification of lead clones with low affinity for native Amyloid beta
using in vitro
immunohisto chemistry
The lead antibodies were tested for their ability to bind to Amyloid beta,
with the aim of
identifying lead clones with low affinity for native forms of the Amyloid beta
peptide. Briefly, the
lead antibodies were screened on human Alzheimer's Disease brain sections and
Tg2576
mouse brain sections to identify anti Amyloid beta 1-42 antibodies that bound
to native Amyloid
in vitro.
Tg2576 mice are transgenic mice that over-express the gene for human Amyloid
Precursor Protein (APP). This gene carries two point mutations in the gamma-
secretase
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
62
cleavage site (Lys670Asn and Met671Leu) that leads to formation of Amyloid
beta plaques in
the cortex and hippocampus of the mice, starting at an age of approximately 9
months.
Human brain tissue was isolated from the frontal cortex (inferior frontal
gyrus) of two
individuals with severe Alzheimer's Disease (ApoE genotype 3/3, Braak stage 6
and ApoE
genotype 4/3, Braak stage 5 respectively). As a control, equivalent tissue was
isolated from one
non-dementia individual (ApoE genotype 3/3, Braak stage 1). All three tissues
were supplied by
the Netherlands Brain Bank (NBB). The quality of the sections was verified by
haematoxylin/eosin staining before use. Mouse brain tissue was isolated from
Tg2576 mice at
an age of 15 months (2 mice), 18 months (6 mice), and 22 months (2 mice).
Paraffin embedded brain sections, 4-6 pm in width, were prepared for
immunohistochemistry by first removing the paraffin support matrix. Sections
were washed with
Xylene (5 minutes x2), absolute ethanol (3 minutes x2), 95% ethanol (3 minutes
x2), 70%
ethanol (3 minutes x2), 90% formic acid (Sigma Aldrich, UK; cat: 06440; 10
minutes), tap water
(20 minutes x3) and PBS (5 minutes x2). The sections were then boiled in Diva
Decloaker
solution (Biocare Medical, USA; cat: DV2004 G1) in a microwave oven for 20
minutes at 100 C.
The samples were subsequently cooled to 40 C in a waterbath and then washed
with distilled
water (5 minutes) and PBS (5 minutes x3).
The lead IgG2 antibodies were tested at concentrations of 2, 5 and 20 pg m1-1.
Binding of
these IgGs was detected using a rabbit anti-human secondary antibody (Dako,
Denmark; cat:
A0482) at a 1 in 400 dilution, followed by the OmniMap anti-rabbit HRP
conjugate antibody
(Ventana Medical Systems, USA; cat: 760-4311). The signal was detected using
the
ChromoMap DAB kit (Ventana Medical Systems, USA; cat: 760-159). These staining
steps
were performed using a BenchMark automated slide preparation system (Ventana
Medical
Systems, USA) according to standard protocols.
Scoring of the staining was carried out in a blinded fashion by at least two
different
people under 20-40x optical magnification. In vitro plaque binding was
designated using a scale
from 0 (no staining of plaques) up to 4 (intense staining of plaques).
Abet0007 IgG2 showed no staining of plaques in either the human Alzheimer's
Disease
brains or the mouse Tg2576 brains (score = 0). In contrast, a positive control
antibody produced
a score of 4 on adjacent sections under the same conditions. Representative
images are
presented in Figure 4.
Example 2. Antibody optimisation of Abet0007 through directed mutation of the
Complementarity Determining Regions 3 (CDR3)
2.1 Conversion of Abet0007 parent clone to scFv format
The parent clone was converted from IgG2 format to single chain variable
fragment
(scFv) format in preparation for affinity optimisation. The codon-optimised
variable heavy (VH)
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
63
and variable light (VL) domains were amplified separately from their
respective IgG vectors with
the addition of specific cloning sites and a flexible linker region.
Recombinatorial PCR was then
performed to generate a complete scFv construct, which was cloned into the
pCantab10.5
phagemid vector, essentially as described in Vaughan etal. (Vaughan et al.,
1996). The
pCantab10.5 vector is a modified version of the pCantab6 vector that contains
additional
restriction sites to facilitate the addition of tags other than the standard
His and myc tags.
2.2 Optimisation of Abet0007 by targeted mutagenesis
The lead antibody (Abet0007) was optimised for improved affinity to human
Amyloid
beta 1-42 peptide using a targeted mutagenesis approach with affinity-based
phage display
selections. Large scFv-phage libraries derived from Abet0007 were created by
oligonucleotide-
directed mutagenesis of the variable heavy (VH) and variable light (VL) chain
complementarity
determining regions 3 (CDR3) using standard molecular biology techniques as
described by
Clackson and Lowman ((2004) A Practical Approach, Oxford University Press).
The libraries were subjected to affinity-based phage display selections in
order to enrich
for variants with higher affinity for human Amyloid beta 1-42 peptide. The
selections were
performed essentially as described previously (Hawkins et aL, 1992; Schier
etal., 1996;
Thompson et aL, 1996). In brief, the scFv phage particles were incubated with
biotinylated
human Amyloid beta 1-42 peptide (rPeptide, USA; cat: A1117) in solution. ScFv-
phage that
bound to the antigen were then captured on streptavidin-coated paramagnetic
beads
(Dynabeads M280, lnvitrogen Life Sciences, UK) following the manufacturer's
recommendations. The selected scFv-phage particles were then rescued as
described
previously (Osbourn et al., 1996), and the selection process was repeated in
the presence of
decreasing concentrations of biotinylated human Amyloid beta 1-42 antigen (200
nM to 2 nM
over 3 rounds).
2.3 Identification of improved clones using a direct binding assay
One thousand seven hundred and sixty scFv were randomly selected from
selection
rounds 2 and 3 from the targeted mutagenesis approach described in section
2.2. These clones
were screened using a direct binding assay essentially as described in section
1.3. Briefly,
unpurified scFv from periplasmic preparations were tested for increased
binding to biotinylated
human Amyloid beta 1-42 peptide and detected using HTRFTm technology with
streptavidin
cryptate and anti-6his-XL665 detection reagents.
Unpurified scFv from periplasmic preparations were prepared in 50 mM MOPS
buffer pH
7.4 including 0.5 mM EDTA and 0.5 M sucrose and were subsequently diluted to
1% in assay
buffer containing 50 mM MOPS pH 7.4 (Sigma, UK; cat: M9381), 0.4 M potassium
fluoride
(BDH Chemicals, USA; cat: 103444T), 0.1% fatty acid free bovine serum albumin
(Sigma, UK;
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
64
cat: A6003) and 0.1% Tween 20 (v/v) (Sigma, UK; cat: P2287) using a Greiner
384 well V
bottom plate (Greiner BioOne, Germany; cat: 781280). 5 I of diluted scFv
sample were
transferred to the assay plate (Costar 384 well black shallow well, Corning
Life Sciences; cat:
3676) using a MiniTrakTm (PerkinElmer, USA) liquid handling robot. 5 I of
assay buffer was
then added to each well. A 20 nM solution of biotinylated human Amyloid beta 1-
42 peptide
(rPeptide, USA; cat: A1117) was prepared in assay buffer and 5 I this
solution were added to
the assay plates. Streptavidin cryptate (Cisbio International, France; cat:
610SAKLB) and anti-
His6-XL665 (Cisbio International, France; cat: 61H ISXLB) detection reagents
were combined in
assay buffer to give concentrations of 7 nM streptavidin cryptate and 60 nM
anti-His6-XL665
and then 5 I of this detection cocktail were added to all wells of the assay
plates. Non-specific
binding wells (negative controls) were defined for each plate by replacing
scFv sample with 5 I
assay buffer. Assay plates were sealed and incubated for 2.5 hours at room
temperature prior
to reading time resolved fluorescence at 620 nm and 665 nm emission
wavelengths using an
EnVision plate reader (PerkinElmer, USA). Data analysis was performed as
described
previously (Section 1.3) and % Delta F values were used to compare assay
signals in adjacent
wells. A "hit" was defined as a scFv sample that generated a % Delta F greater
than the signal
observed for Abet0007 scFv.
2.4 Confirmation of improved clones using an epitope competition assay
Clones that displayed a higher binding to human Amyloid beta 1-42 peptide than
the
Abet0007 parent clone were subjected to DNA sequencing (Osbourn et al., 1996;
Vaughan et
al., 1996). The scFv with unique protein sequences were expressed in E. coli
and purified by
affinity chromatography followed by buffer exchange. The binding affinities of
these scFv were
then tested in an epitope competition assay against a benchmark antibody
called Abet0042,
which has a similar affinity for human Amyloid beta 1-42 peptide as Abet0007.
In this
competition assay, the binding of Abet0042 IgG to biotinylated human Amyloid
beta 1-42
peptide is competed against the test scFv samples. The binding of Abet0042 IgG
to biotinylated
human Amyloid beta 1-42 peptide is detected using the HTRFTm technology as
described
previously (section 1.6). Briefly, a dilution series of purified scFv are
added to a mixture of
Abet0042 IgG, biotinylated Amyloid beta 1-42 peptide, streptavidin cryptate
and anti-human Fc
IgG XL665. The time resolved fluorescence was read after two hours incubation
at room
temperature.
Purified scFv were serially diluted in assay buffer containing 50 mM MOPS
buffer pH 7.4
(Sigma, UK; cat: M9381), 0.4 M potassium fluoride (BDH Chemicals, USA; cat:
103444T), 0.1%
fatty acid free bovine serum albumin (Sigma, UK; cat: A6003) and 0.1% Tween 20
(Sigma, UK;
cat: P2287) using a Greiner 96 well U bottom plate (Greiner BioOne, Germany;
cat: 650201). 5
I of each dilution of scFv was transferred in duplicate to the assay plate
(Costar 384 well
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
black shallow well, Corning Life Sciences; cat: 3676) using a MiniTrakTm
(PerkinElmer, USA)
liquid handling robot. A 20 nM solution of biotinylated Amyloid beta 1-42
peptide (rPeptide,
USA; cat: A1117) was prepared in assay buffer and 5 I of the biotinylated
peptide solution was
added to the assay plates, to give a final concentration of 5 nM peptide in
the final assay
5 volume of 20 pl. The assay plates were sealed and incubated at room
temperature for 1 hour.
Streptavidin cryptate and anti-human Fe IgG XL665 were combined at 7 nM and 20
nM
respectively in assay buffer and 5 I of this solution were added to the assay
plate. Abet0042
IgG was diluted to 2.4 nM in assay buffer and 5 I were added to the assay
plate to generate a
final IgG concentration of 0.6 nM. Non-specific binding wells (negative
controls) were defined for
10 each plate by replacing Abet0042 IgG with 5 I assay buffer. Assay
plates were sealed and
incubated for 2 hours at room temperature prior to reading time resolved
fluorescence at 620
nm and 665 nm emission wavelengths using an En Vision plate reader
(PerkinElmer, USA).
Data were analysed by calculating the % Delta F value and the % specific
binding for
each sample. % Delta F was determined according to Equation 1 and % specific
binding was
15 calculated using Equation 2. IC50 values were determined using Prism
(Graphpad Software,
USA) by curve fitting using a four parameter logistic equation , as described
in equation 3.
Equation 3:
Y = Bottom + Top ¨ Bottom
1 + 10"((L0gEC50¨ X)* HillSlope)
Where Y is specific binding and X is the logarithm of concentration.
Example results are shown in Figure 5. The original lead, Abet0007, has an
IC50 of 159
nM while the most improved clone, Abet0144, has an IC50 value of 5.5 nM.
2.5 Kinetic profiling of affinity improved clones in purified scFv format by
Surface Plasmon
Resonance
Surface Plasmon Resonance was used to analyse the purified seFv clones that
had
shown significant improvement in binding affinity for human Amyloid beta 1-42
peptide over the
parent sequence, Abet0007, in the HTRFTm epitope competition assay (section
2.4). Briefly, the
BlAcore T-100 (GE Healthcare, UK) biosensor instrument was used to assess the
kinetic
parameters of the interaction between each purified seFv and synthetically-
produced human
Amyloid beta 1-42 peptide. These experiments were performed essentially as
described by
Karlsson et al. (Karlsson et al., 1991). For further details see section 1.7.
The affinity of binding between each test seFv and human Amyloid beta 1-42 was
estimated using assays in which biotinylated synthetic human Amyloid beta 1-42
peptide
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
66
(rPeptide, USA; cat: A1117) was non-covalently bound via a biotin/streptavidin
interaction to a
proprietary SA sensor chip to a final surface density of approximately 700 RU.
The chip surface
was regenerated between cycles by a single 20 second injection of 10 mM
Glycine pH 2.0 to
remove scFv bound to the peptide. The regeneration did not result in a
significant loss of
peptide binding capacity.
A series of dilutions of each purified scFv (12.5 ¨ 400 nM) were sequentially
passed over
the peptide surface for a sufficient amount of time to observe sensorgrams
that could be fitted to
an appropriate binding model with confidence. A zero-concentration buffer
blank was subtracted
from the main dataset to reduce the impact of any buffer artefacts or non-
specific binding effects.
An appropriate binding model was then fitted simultaneously to the data from
each analyte
titration using the BlAevaluation software.
The validity of the data was assessed using the calculated Chi2 value, with an
acceptable value being under 4 RU2. The overall success of the fit was
estimated using the
residuals, with a deviation of under 20 RUs being acceptable.
Example results for Abet0144 scFv are shown in Figure 6. The association rate
constant (ka),
dissociation rate constant (kd) and dissociation constant (KD) are 2.01 x 105
Nes-1, 6.66 x 10-3
s-1 and 33.2 nM respectively. These parameters were derived from a 1:1
Langmuir fit to the data.
2.6 Reformatting of affinity improved scFv to human IgG1-TM
The IgG1-TM antibody format is a human IgG1 isotype containing three single
amino
acid substitutions (Triple Mutant: TM) within the lower hinge and CH2 constant
domain
(Oganesyan et al., 2008). When introduced into the lower hinge and CH2 domain
of human
IgG1 molecules, the triple mutation L234F/L235E/P331S (TM') causes a profound
decrease in
their binding to human CD64, CD32A, CD16 and C1q. These TM mutations are used
to create
a human isotype with very low effector function. ScFv were reformatted to IgG1-
TM by
subcloning the variable heavy chain (VH) and variable light chain (VL) domains
into vectors
expressing whole human antibody heavy and light chains respectively. The
variable heavy
chain was cloned into a mammalian expression vector (pEU 1.4) containing the
human heavy
chain constant domains and regulatory elements to express whole IgG1-TM heavy
chain in
mammalian cells. Similarly, the variable light chain domain was cloned into a
mammalian
expression vector (pEU 4.4) for the expression of the human lambda light chain
constant
domains and regulatory elements to express whole IgG light chain in mammalian
cells. IgG
antibodies were expressed and purified essentially as described in Section
1.5.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
67
2.7 Germ/Thing
The amino acid sequences of the VH and VL domains of the affinity optimised
Amyloid
beta 1-42 peptide specific antibodies were aligned to the known human germline
sequences in
the VBASE database (Tomlinson etal., 1992), and the closest germline was
identified by
sequence similarity. For the VH domains of the optimised antibody lineage this
was Vh3-23
(DP-47) and for the VL domains it was VA3-3r (DPL-23).
The germlining process consisted of reverting framework residues in the VH and
VL
domains to the closest germline sequence to identically match human
antibodies. For Abet0144,
no residues required changing in the VH domain (Table 1) but a total of 5
changes were made in
the framework of the VL domain. These changes occurred at Kabat positions 1,
2, 3, 40 and 81
(Table 2). The Vernier residues (Foote et al., 1992), were not germlined,
apart from residue 2 in
the light chain sequence which was germlined at the same time as the flanking
residues 1 and 3.
Germlining of these amino acid residues was carried out using standard site
directed
mutagenesis techniques with the appropriate mutagenic primers as described by
Clackson and
Lowman (Clackson etal., 2004).
Kabat FW1 CDR1
Numbering VH co Lc) ap r. co cs) (c) cr:i cc 'C3= I
c7) A' 2 2
Vernier =
VH3-23 EVQLLESGGGLVQPGGSLRLSCAASGFTFSSYAMS
Abet0144 EVQL LESGGGLVQPGGSLRLSCAASGFTFSVYTMW
Abet0144-GLEVQLLESGGGLVQPGGSLRLSCAASGFTFSVYTMW
Kabat FW2 CDR2
Numbering V I 1 cr 'e op 2 =Ti 4 (4-) `,`? '4 41; 2 D
Vernier = = =
VH3-23 WVRQAPGKGLEWVSA I SGSGGSTYYADSVKG
Abet0144 WVRQAPGKGLEWVSV I GSSGGTT VYADSVKG
Abet0144-GLWVRQAPGKGLEWVSV I GSSGG;T TVYADSVKG
Kabat FW3
Numbering VH D D D `,Y)). 1`,µ! P. Lr(). (r). P `0=0)
c7D a g3 a 2 2 cr'D 2 2 2 2 2 0)
Vernier = = e =
VH3-23 RFT I SRDNSKNTLYLQMNSLRAEDTAVYYCAK
Abet0144 RFT I SRDNSKNTLYLQMNSLRAEDTAVYYCAR
Abet0144-GL RFT I SRDNSKNTLYLQMNSLRAEDTAVYYCA
CDR3 FW4
Kabat
LO CO 6) 0 C=1
Numbering VH g; 8 8 8 8 8 0, 8 8 0 0 c,
Vernier
VH3-23
Abet0144 EWMDHSRPYYYYGMDVWGQGT LVTVSS
Abet0144-GLEWMDHSRPYYYYGMDVWGQGT L VT VSS
Table 1: A sequence alignment of the Abet0144 and Abet0144-GL clones to the
VH3-23
(DP47) germline. Residues that are different from germline are highlighted.
Vernier residues
are indicated by circles (.). No changes were made in the VH domain to
germline the Abet0144
clone.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
68
Kabat FW 1 CDR 1
Numbering ¨ CO LO N CO 0) C (C) C (7)
Vernier a a
VL3-3R SYELTQPPS - VSVSPGQTAS I TCSGDKLGDKYAC
Abet0144 '-_,SVLTQPPS - VSVSPGQTAS I TCSGHNLEDKFAS
Abet0144-GL SYELTQPPS - VSVSPGQTAS I TCSGHNLEDKFATh
Kabat FW 2 CDR 2
Numbering L P, c%) c:4 3 "41)- '4? L4 '`,'? 2 7, FA
LC?) 7, 2 2
Vernier a aaaa
VL3-3R WYQQKPGQSPVLV I YQDSKRPS
Abet0144 WYQQK GQSPVLV I YRDDKRPS
Abet0144-GL WYQQKHGQSPVLV I YRDDKRPS
Kabat FW3
Numbering `A g 2 A L,c2 ,S) gLi2 `,&) 8 c'TD 2 2 A 2
2
Vernier ea e
VL3-3R GI PERFSGSNSGNTAT LT I SGTQAMDEADYYC
Abet0144 GI PERFSASNSGHTAT LT I SGTQATDEADYYC
Abet0144-GL GI PERFSASNSGHTATLT I SGTQA-MDEADYYC
Kabat CDR3 FW4
Numbering V 8 E) 8 8 3 8 8 g
I_
Vernier
VL3-3R ............................ Q A WD
Abet0144 QAQDSTTRVFGGGTKLTVL
Abet0144-GL QAQDSTTRVFGGGTKLTVL
Table 2: A sequence alignment of the Abet0144 and Abet0144-GL clones to the
VA3-3R (DPL-
23) germline. Residues that are different from germline are highlighted.
Vernier residues are
indicated by circles (.). Five changes were made in the VL domain to germline
the Abet0144
clone. The Vernier 2 residue was reverted to germline at the same time as
residues 1 and 3.
Reverting this residue did not impact on antibody potency.
2.8 Determination of the binding kinetics of affinity-optimised IgGs using
Surface Plasmon
Resonance
Surface Plasmon Resonance was used to analyse the binding kinetics of the
affinity-
optimised IgGs (section 2.6) and their germ lined counterparts (section 2.7).
Briefly, the BlAcore
T-100 (GE Healthcare, UK) biosensor instrument was used to assess the kinetic
parameters of
the interaction between each test IgG and synthetically produced human Amyloid
beta 1-42
peptide. These experiments were performed essentially as described by Karlsson
et al.
(Karlsson et al., 1991). For further details see section 1.7.
The affinity of binding between each test IgG and human Amyloid beta 1-42 was
estimated using assays in which the antibody was covalently coupled by amine
linkage to a
proprietary CM3 chip surface to a final surface density of approximately 2,000
RU. The chip
surface was regenerated between cycles by a single 40 second injection of 10
mM Glycine pH
2.0 to remove ligand bound to the antibody. The regeneration did not result in
a significant loss
of peptide binding capacity.
A series of dilutions of synthetic human Amyloid beta 1-42 peptide (1.6 ¨ 50
nM) were
sequentially passed over the antibody surface for a sufficient amount of time
to observe
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
69
sensorgrams that could be fitted to an appropriate binding model with
confidence. Blank
reference flow-cell data were subtracted from each IgG dataset and a zero-
concentration buffer
blank was double-reference subtracted from the main dataset to reduce the
impact of any buffer
artefacts or non-specific binding effects. An appropriate binding model was
then fitted
simultaneously to the data from each analyte titration using the BlAevaluation
software.
The validity of the data was assessed using the calculated Chi2 value, with an
acceptable value being under 2 RU2. The overall success of the fit was
estimated using the
residuals, with a deviation of under 2 RUs being acceptable.
Example results for Abet0144-GL (germlined) IgG1-TM are shown in Figure 7. The
association rate constant (ka), dissociation rate constant (kd) and
dissociation constant (KD) are
2.08 x 105 M-1 s-1, 1.97 x 10-3 s-1 and 9.50 nM respectively. These parameters
were derived from
a 1:1 Langmuir fit to the data.
2.9 Specificity profiling of affinity optimised IgGs using Surface Plasmon
Resonance
Surface Plasmon Resonance was used to verify the specificity of the affinity-
optimised
IgGs for the human Amyloid beta 1-42 peptide. Briefly, the BlAcore T-100 (GE
Healthcare, UK)
biosensor instrument was used to assess the kinetic parameters of the
interaction between
each test IgG and a range of small peptides including synthetically-produced
human Amyloid
beta 1-42 and human Amyloid beta 1-40. These experiments were performed
essentially as
described by Karlsson et al. (Karlsson et al., 1991). For further details see
section 1.7.
The affinity of binding between each test IgG and each peptide was estimated
using
assays in which the antibody was covalently coupled by amine-linkage to a
proprietary CM3
chip surface to a final surface density of approximately 2,000 RU. The chip
surface was
regenerated between cycles by a single 40 second injection of 10 mM Glycine pH
2.0 to remove
ligand bound to the antibody. The regeneration did not result in a significant
loss of peptide
binding capacity.
Each test peptide at 400 nM was sequentially passed over the antibody surface
for a
sufficient amount of time to observe sensorgrams that either showed no binding
or that could be
fitted to an appropriate binding model with confidence. Blank reference flow-
cell data were
subtracted from each IgG dataset and a zero-concentration buffer blank was
double-reference
subtracted from the main dataset to reduce the impact of any buffer artefacts
or non-specific
binding effects.
Example results for Abet0144-GL (germlined) IgG1-TM are shown in Figure 8. Two
peptides (biotinylated human Amyloid beta 1-42, (rPeptide, USA; cat: A1117)
and unlabelled
human Amyloid beta 1-42 (rPeptide, USA; cat: A1165)) showed strong binding to
the antibody,
whilst three peptides (biotinylated scrambled human Amyloid beta 1-42
(Anaspec, USA; custom
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
synthesis), biotinylated human Amyloid beta 1-40 (rPeptide, USA; cat: A1111)
and unlabelled
human Amyloid beta 1-40 (Anaspec, USA; cat: 24236)) showed no binding to the
antibody.
5 2.10 Specificity profiling of Abet0144-GL IgG1-TM in biochemical epitope
competition assay
format
To test the specificity of Abet0144-GL IgG1-TM for binding to other human
Amyloid beta
peptides, a competition assay was developed. In this assay a fixed
concentration (1.5nM) of
biotinylated human Amyloid beta 1-42 peptide (rPeptide, USA; cat: A1117) was
incubated with a
10 range of different concentrations of unlabelled human Amyloid beta
peptides including 1-42, 1-
40, 1-16, 12-28, pyro 3-42, pyro 11-42 and scrambled 1-42 (Anaspec cat: 20276,
24236, 24226,
24230, 29907, 29903 and 25383, respectively) in the presence of a fixed
concentration
(0.28nM) of Abet0144-GL IgG1-TM. Peptide competition was assessed by detecting
the
inhibition of binding of Abet0144-GL IgG1-TM to the biotinylated human Amyloid
beta 1-42
15 peptide using time resolved fluorescence resonance energy transfer (TR-
FRET). This involved
the use of Europium cryptate labelled anti-human Fc IgG (CisBio International,
France; cat:
61HFCKLB) and XL665 labelled streptavidin (CisBio International, France; cat:
611SAXLB) TR-
FRET detection reagents.
Experiments were set up in Costar 384 well shallow bottom microtitre plates.
5u1/ well of
20 biotinylated human Amyloid beta 1-42 (6nM) was added to all wells of the
assay plate (except
for negative binding assay control wells) in order to give a final assay
concentration of 1.5nM.
5u1 of assay buffer only was added to the negative binding assay control
wells. A duplicate 11
point 1:3 serial titration was prepared or each test peptide starting at a top
concentration of
40uM in order to give a top final assay peptide concentration of 10uM. 5u1 per
well of each test
25 peptide serial dilution was then transferred onto the 384 well assay
plate. 5u1 of assay buffer
only was added to the total and negative binding assay control wells. Abet0144-
GL IgG1-TM
was diluted to give a working solution at 1.12nM. 5u1 per well of this
solution was added to all
wells of the 384 well assay plate in order to result in a final assay Abet0144-
GL-IgG1-TM
concentration of 0.28nM. Finally, a combined solution was prepared containing
Europium
30 cryptate labelled anti-human Fc (2.4nM) and XL665 labelled streptavidin
(60nM). 5u1 per well of
this solution was added to all wells of the 384 well assay plate such that the
final concentrations
of Europium cryptate labelled anti-human Fc and XL665 labelled streptavidin
were 0.6nM and
15nM, respectively. Note that the final assay volume was 20u1 and that each
individual assay
component was added as a 5u1 addition at four times the required final assay
concentration. All
35 reagents were diluted in an assay buffer containing 50 mM MOPS pH 7.4
(Sigma, UK; cat:
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
71
M9381), 0.4 M KF (BDH Chemicals, USA; cat: 103444T), 0.1% fatty acid free
bovine serum
albumin (Sigma, UK; cat: A6003) and 0.1% Tween 20 (v/v) (Sigma, UK; cat:
P2287).
Assay plates were incubated for 2 hours at room temperature prior to reading
time
resolved fluorescence at 620 nm and 665 nm emission wavelengths using an En
Vision plate
reader (Perkin Elmer, USA). Data was analysed by calculating % Delta F values
for each
sample. % Delta F was determined according to equation 1.
Equation 1:
% Delta F = (sample 665 nm / 620 nm ratio) ¨ (negative control 665 nm / 620 nm
ratio) x 100
(negative control 665 nm / 620 nm ratio)
% Delta F values were used to calculate % specific binding as described in
equation 2.
Equation 2:
% specific binding = % Delta F of sample x 100
% Delta F of total binding control
Example results for Abet0144-GL IgG1-TM are shown in Figure 9. These results
demonstrate that Abet0144-GL IgG1-TM binds to human Amyloid beta 1-42, pyro 3-
42 and pyro
11-42 peptides but does not bind to human Amyloid beta 1-40 peptide or to the
peptide
truncates 1-16 and 12-28.
2.11 Functional characterisation of lead antibodies by depletion of Amyloid
beta peptides from
human plasma
The lead antibodies were tested in a plasma depletion assay to investigate
their ability to
immunoprecipitate human Amyloid beta 1-42 peptide from human blood plasma.
This assay
was used to provide evidence of functional efficacy for each antibody. The
assays were
performed exactly as described in Section 1.8.
In one experiment, the Abet0144-GL IgG1-TM antibody reduced the levels of
human
Amyloid beta 1-42 peptide in plasma from 13.54 pg m1-1 to 9.86 pg m1-1 (a 27%
reduction). In a
second experiment, the levels were reduced from 40.06 pg m1-1 to 34.65 pg m1-1
(a 14%
reduction).
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
72
2.12 Identification of improved clones with native binding for Amyloid beta
using in vitro
immunohisto chemistry
The affinity-optimised IgGs were tested for their ability to bind to Amyloid
beta, with the
aim of identifying lead clones which recognise native Amyloid beta peptide.
Briefly, the lead
antibodies were screened on human Alzheimer's Disease brain sections and
Tg2576 mouse
brain sections to identify anti-Amyloid beta 1-42 antibodies that bound to
native Amyloid in vitro.
The experiments were performed essentially as described in section 1.9.
In these experiments, human brain tissue was isolated from the frontal cortex
and
hippocampus of two individuals with severe Alzheimer's Disease (female, ApoE
4/3, 86 years;
female, ApoE 3/3, 67 years). Mouse brain tissue was isolated from Tg2576 mice
at an age of 18
months (6 mice). Antibodies were tested at concentrations of 2, 5 and 20 ug m1-
1.
In one experiment, the Abet0144-GL IgG1-TM antibody did not stain diffuse
plaques
(DP) or cerebral amyloid angiopathy (CAA) plaques (score = 0). It did,
however, stain core
plaques (CP) with a score of 1 on Tg2576 brain sections, and a score of 1.5 on
human AD brain
sections. In contrast, a positive control antibody produced a score of 3-4 on
all plaques (CP, DP,
CAA) on adjacent sections under the same conditions. Representative images are
shown in
Figure 10.
Example 3. Antibody optimisation of Abet0144-GL through mutation of all six
CDRs including
flanking Vernier residues
3.1 Conversion of Abet0144-GL parent clone to scFv format compatible with
Ribosome Display
The parent clone was converted from IgG1-TM format to single chain variable
fragment
(scFv) format in preparation for affinity optimisation. The codon-optimised
variable heavy (VH)
and variable light (VI) domains were amplified separately from their
respective IgG vectors with
the addition of specific cloning sites and a flexible linker region.
Recombinatorial PCR was then
performed to generate a complete scFv construct, which was cloned into a
modified pUC vector
(pUC-RD) containing the structural features necessary for ribosome display.
These features
include a 5' and 3' stem loop to prevent degradation of the mRNA transcript by
exonucleases, a
Shine-Dalgarno sequence to promote ribosome binding to the mRNA transcript,
and a genelll
spacer that allows the translated scFv molecule to fold while still remaining
attached to the
ribosome (Groves et al., 2005).
3.2 Optimisation of Abet0144-GL by targeted mutagenesis
The lead antibody (Abet0144-GL) was further optimised for improved affinity to
human
Amyloid beta 1-42 peptide using a targeted mutagenesis approach with affinity-
based ribosome
display selections. Large scFv-ribosome libraries derived from Abet0144-GL
were created by
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
73
oligonucleotide-directed mutagenesis of all six variable heavy (VH) and
variable light (VI) chain
complementarity determining regions (CDRs) using standard molecular biology
techniques as
described by Clackson and Lowman (Clackson etal., 2004). The mutated sequences
from each
CDR were affinity optimised as a separate library. The five Vernier residues
preceding the
VHCDR1 (Kabat residues 26-30) were also randomised using targeted mutagenesis
and these
sequences were combined and matured with the remaining VHCDR1 library. All
libraries were
subjected to affinity-based ribosome display selections in order to enrich for
variants with higher
affinity for human Amyloid beta 1-42 peptide. The selections were performed
essentially as
described previously (Hanes etal., 2000).
In brief, the six targeted mutagenesis libraries of the Abet0144-GL lead
clone, one
covering each CDR, were separately transcribed into mRNA. Using a process of
stalled
translation, mRNA-ribosome-scFv tertiary complexes were formed (Hanes etal.,
1997). These
complexes were then subjected to four rounds of selection incubated in the
presence of
decreasing concentrations of synthetic biotinylated human Amyloid beta 1-42
peptide (Bachem,
Germany; cat: H-5642) (100 nM to 10 nM) to select for variants with higher
affinity for human
Amyloid beta 1-42 peptide. Those complexes that bound to the antigen were then
captured on
streptavidin-coated paramagnetic beads (DynabeadsTM, lnvitrogen, UK; cat: 112-
05D) and non-
specific ribosome complexes were washed away. mRNA was subsequently isolated
from the
bound ribosomal complexes, reverse transcribed to cDNA and them amplified by
PCR. This
DNA was used for the next round of selection.
After four rounds of affinity maturation, each selection output was cloned out
for
screening purposes. ScFv isolated by ribosome display were cloned into the
phagemid vector
pCANTAB6 by NotliNcol restriction endonuclease digestion of the ribosome
display construct
(New England BioLabs, USA; cat: R0189L, R0193L) followed by ligation into
Notll Alcol digested
pCANTAB6 using T4 DNA ligase (New England BioLabs, USA; cat: M0202L)
essentially as
described by McCafferty et al. (McCafferty etal., 1994).
3.3 Identification of improved clones using an epitope competition assay
Two thousand and twenty four scFv chosen at random from selection rounds 3 and
4 of
the targeted mutagenesis approach described in section 3.2 were expressed in
bacteria to
produce unpurified periplasmic scFv. Those scFv capable of binding synthetic
human amyloid
beta 1-42 peptide via the same epitope as Abet0144-GL IgG1-TM were elucidated
in a
competition format assay, using the HTRFTm platform. Specifically,
fluorescence resonance
energy transfer (FRET) was measured between streptavidin cryptate (associated
with
biotinylated amyloid beta 1-42 peptide) and anti-human Fc XL665 (associated
with Abet0144-
GL IgG1-TM) in the presence of a single concentration of each unpurified
periplasmic test scFv.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
74
Successful occupation of the Abet0144-GL IgG1-TM epitope on the peptide by
scFv resulted in
a reduction in FRET, as measured on a fluorescence plate reader.
A 'Total' binding signal was determined by analysing the binding of Abet0144-
GL IgG1-
TM to synthetic human Amyloid beta 1-42 peptide in the absence of competitor
peptide. The
'Sample' signals were derived from analysing the binding of Abet0144-GL IgG1-
TM to synthetic
human Amyloid beta 1-42 peptide in the presence of a test scFv sample.
Finally, a `Cryptate
Blank' signal was determined by analysing the fluorescence mediated by the
detection reagent
cocktail alone.
Unpurified periplasmic scFv were supplied in sample buffer consisting of 50 mM
MOPS,
pH 7.4, 0.5 mM EDTA, and 0.5 M sucrose. For profiling, scFv samples were
diluted in a 384-
well V-bottom plate to 50% of the original stock concentration in assay
buffer, consisting of 50
mM MOPS, pH 7.4, 0.4 M potassium fluoride, 0.1% fatty-acid-free bovine serum
albumin and
0.1% Tween 20 (v/v). 5 I of each newly-diluted scFv was transferred to the
'Sample' wells of a
black, shallow, solid bottom, non-binding 384-well assay plate using a liquid
handling robot. The
remaining reagents (prepared in assay buffer) were added to the assay plate by
multichannel
pipette in the following order: 5 I sample buffer (to 'Total' and `Cryptate
Blank' wells), 10 I
assay buffer (to `Cryptate Blank' wells), 5 I 2 nM Abet0144-GL IgG1-TM (to
'Sample' and
'Total' wells), 5 I 5 nM biotinylated human Amyloid beta 1-42 peptide (to
'Sample' and 'Total'
wells), and 5 I detection cocktail, consisting of 6 nM streptavidin cryptate
and 60 nM anti-His6-
2 0 XL665 (to all wells). Assay plates were sealed and then incubated for 3
hours at room
temperature in the dark, prior to measuring time-resolved fluorescence at 620
and 665 nm
emission wavelengths on a fluorescence plate reader.
Data were analysed by calculating % Delta F values for each sample. % Delta F
was
determined according to equation 4.
Equation 4:
% Delta F = (Sample 665 nm / 620 nm ratio) ¨ (Cryptate Blank 665 nm / 620 nm
ratio) x 100
(Cryptate Blank 665 nm / 620 nm ratio)
Delta F values were subsequently used to calculate normalised binding values
as
described in equation 5.
Equation 5:
Normalised data (% Total) = % Delta F of sample x 100
% Delta F of Total binding control
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
Unpurified periplasmic scFv demonstrating significant inhibition of Abet0144-
GL IgG1-
TM binding to Amyloid beta 1-42 peptide were subjected to DNA sequencing
(Osbourn et al.,
1996; Vaughan etal., 1996). The scFv found to have unique protein sequences
were
5 expressed in E. coli and purified by affinity chromatography followed by
buffer exchange.
The potency of each purified scFv was determined by testing a dilution series
of the
scFv (typically 4 pM ¨ 1200 nM) in the epitope competition assay described
above. Data were
again analysed by calculating the % Delta F and % Total binding values for
each sample. In
addition, a % Inhibition value for each concentration of purified scFv was
also calculated as
10 described in Equation 6:
Equation 6:
% Inhibition = 100 - % Total Binding
ScFv sample concentration was plotted against % Inhibition using scientific
graphing
software, and any concentration-dependant responses were fitted with non-
linear regression
curves. 1050 values were obtained from these analyses with Hill-slopes
constrained to a value of
-1. The most potent clone from this round of selections, Abet0286, had an 1050
of 1.8 nM and
came from the VLCDR1 targeted mutagenesis library.
Reagent/Equipment sources: MOPS (Sigma, UK; cat: M9381), potassium fluoride
(BDH
chemicals, USA; cat: A6003), fatty-acid-free bovine serum albumin (Sigma, UK;
cat: A6003),
Tween 20 (Sigma, UK; cat: P2287), Abet0144-GL IgG1-TM (produced in-house),
biotinylated
human Amyloid beta 1-42 peptide (rpeptide, USA; cat: A1117), Streptavidin
cryptate (Cisbio,
France; cat: 610SAKLB), anti-His6-XL665 (Cisbio, France; cat: 61HISXLB), 384-
well assay
plates (Corning, Costar Life Sciences; cat: 3676), 384-well dilution plates
(Greiner BioOne,
Germany; cat: 781280), liquid handling robot (MiniTrakTm, Perkin Elmer, USA),
fluorescence
plate reader (EnvisionTM, Perkin Elmer, USA), HTRF technology (Cisbio
International, France),
graphing/statistical software (Prism, Graphpad USA).
3.4 Recombination of successful selection outputs to produce "binary"
libraries, and their
subsequent affinity optimisation
The epitope competition assay described in Section 3.3 was used to judge
whether a
particular scFv-ribosome library had been affinity matured over the first four
rounds of selection.
Two of the libraries, the VHCDR3 and the VLCDR2 targeted mutagenesis
libraries, had shown
no improvement over the parent Abet0144-GL clone and were not progressed
further.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
76
The remaining four targeted mutagenesis libraries, (covering the VHCDR1,
VHCDR2,
VLCDR1 and VLCDR3), had shown affinity improvements and were recombined in a
pair-wise
fashion to produce six "binary" recombination libraries in which two of the
six CDRs were
mutated. For example, the affinity matured library covering the VHCDR1 was
randomly
recombined with the affinity matured VHCDR2 library to generate a VH1 :VH2
library. The
remaining libraries were produced as: VH1:VL1, VH1:VL3, VH2:VL1, VH2:VL3 and
VL1:VL3. A
subset of each recombination library was cloned out as previously described
(Section 3.2) and
was sent for sequencing to verify the integrity of each library.
Selections were then continued as previously described (section 3.2) in the
presence of
decreasing concentrations of biotinylated synthetic human Amyloid beta 1-42
peptide (5 nM and
2 nM for rounds 5 and 6 respectively). As before, each selection output was
cloned out for
screening purposes (section 3.2).
One thousand nine hundred and thirty-six scFv, randomly selected from
selection rounds
5 and 6, were screened in an epitope competition assay as described in section
3.3. Due to the
increase in potency of these clones, the unpurified scFy were first diluted to
25% before addition
to the assay plates. As previously, clones that showed significant inhibitory
properties were sent
for DNA sequencing, and unique clones were produced and analysed as purified
scFy (section
3.3). The most potent clone from these selections, Abet0303, had a potency of
0.84 nM and
came from the VH1:VH2 recombination library.
3.5 Recombination of binary selection outputs to produce "ternary" libraries,
and their
subsequent affinity optimisation
The epitope competition assay described in Section 3.3 was used to judge
whether each
binary library had been affinity matured over the previous two rounds of
selection (5 and 6). All
libraries had shown affinity improvements, and were therefore considered for
further affinity
maturation.
The six binary libraries (section 3.4) were recombined with the successful
round 4
outputs (section 3.2) in a pair-wise fashion to form four "ternary"
recombination libraries in which
three of the six CDRs were mutated. For example, the VH2:VL3 binary library
(round 6 output)
was recombined with the VHCDR1 targeted mutagenesis library (round 4 output)
to generate a
VH1 :VH2:VL3 library. Similar constructs were also created by combining the
VH1:VH2 binary
library (round 6 output) with the VLCDR3 targeted mutagenesis library (round 4
output). These
two individual libraries were pooled to create the VH1 :VH2:VL3 ternary
library.
Care was taken not to destroy the synergy between CDRs that had been co-
optimised.
For example, the VH1 :VL3 binary library was not recombined with the VHCDR2
targeted
mutagenesis library since this manipulation would have destroyed the synergy
between the co-
optimised VHCDR1 and VLCDR3 sequences. A complete list of all ternary
libraries and their
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
77
derivations is given in Table 3. A subset of each recombination library was
cloned out as
previously described (Section 3.2) and was sent for sequencing to verify the
integrity of each
library.
Formed From
Ternary Constituent
Round 6 output Round 4 output
Library Libraries
VH1 : VH2 : VH1 a Vlll : VH2 VLCDR1
VH1 =: VH2 =: VL1
VH1 : VH2 : VH1 b V112 : VL1 V11CDR1
VH1 : VH2 : VH3 a V111: V112 VLCDR3
VII 1 : V112 : VL3
VH1 : VH2 : VH3 b VH2 : VL3 V11CDR1
VH1 : VL1 : VH3 a VH1: VL1 VLCDR3
VII 1 : VI, 1 : =VI, 3
VII 1 : VI, 1 : VI, 3 b VL1 : VL3 V11CDR1
V112 : VL1 : VH3 a V112 : VL1 VLCDR3
V112 :VL1 :VL3
V112 : VL1 : VH3 b VL1 : VL3 VBCDR2
Table 3: A description of the four ternary libraries that were matured during
rounds 7 and 8 of
the second Lead Optimisation campaign. Each library comprised two constituent
libraries,
generated from a random pairwise recombination of a round 6 output binary
library and a round
4 output targeted mutagenesis library.
Selections were then continued as previously described (section 3.2) in the
presence of
decreasing concentrations of biotinylated synthetic human Amyloid beta 1-42
peptide (500 pM
and 200 pM for rounds 7 and 8 respectively). As before, each selection output
was cloned out
for screening purposes (section 3.2).
One thousand four hundred and eight scFv, randomly selected from selection
rounds 7
and 8, were screened in an epitope competition assay as described in section
3.3. As with the
"binary" screen, the unpurified scFv were first diluted to 25% before addition
to the assay plates.
As previously, clones that showed significant inhibitory properties were sent
for DNA
sequencing, and unique clones were produced and analysed as purified scFv
(section 3.3). The
most potent clone from these selections, Abet0343, had a potency of 0.48 nM
and came from
the VH1:VH2:VL3 recombination library.
3.6 Recombination of ternary selection outputs to produce "quaternary"
libraries, and their
subsequent affinity optimisation
The epitope competition assay described in Section 3.3 was used to judge
whether each
ternary library had been affinity matured over the previous two rounds of
selection (7 and 8). All
libraries had shown affinity improvements, and were therefore considered for
further affinity
maturation.
The VH1:VH2:VL1 ternary library (round 8 output) was recombined with the
VLCDR3
targeted mutagenesis library (round 4 output) and the VH2:VL1:VL3 ternary
library (round 8
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
78
output) was recombined with the VHCDR1 targeted mutagenesis library (round 4
output).
Separately, the VH1 :VH2 binary library (round 6 output) was recombined with
the VL1:VL3 binary
library (round 6 output). These three individual libraries were then pooled to
create a single
"quaternary" library, VH1 :VH2:VL1 :VL3, in which four of the six CDRs were
mutated.
Care was taken not to destroy the synergy between CDRs that had been co-
optimised.
For example, the VH1 :VL2:VL3 ternary library was not recombined with the
VLCDR1 targeted
mutagenesis library since this manipulation would have destroyed the synergy
between the co-
optimised VHCDR1/VHCDR2 and VLCDR3 sequences. A subset of each recombination
library
was cloned out as previously described (Section 3.2) and was sent for
sequencing to verify the
integrity of each library.
Selections were then continued as previously described (section 3.2) in the
presence of
decreasing concentrations of biotinylated synthetic human Amyloid beta 1-42
peptide (50 pM to
10 pM for rounds 9 to 11). As before, each selection output was cloned out for
screening
purposes (section 3.2).
One thousand six hundred and seventy two scFv, randomly selected from
selection
rounds 9 to 11, were screened in an epitope competition assay as described in
section 3.3. Due
to the increase in potency of these clones, the unpurified scFv were first
diluted to 3.13% before
addition to the assay plates. As previously, clones that showed significant
inhibitory properties
were sent for DNA sequencing, and unique clones were produced and analysed as
purified
scFv (section 3.3). The most potent clone from these selections, Abet0377, had
a potency of
0.32 nM (n=2 data). Sample inhibition curves are shown in Figure 11, and data
for 24 of the
highest potency clones are shown in Table 4. The corresponding protein
sequences are listed in
Tables 5 and 6.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
79
Selection Number
of
Clone IC50 (nM) Range
round
repeats
Abet0144-GL 14 8.1 - 18 7
Abet0319 7 0.68 0.52 - 0.76
3
Abet0321b 7 0.73 0.69 - 0.76
2
Abet0322b 7 0.71 0.43 - 0.98
2
Abet0323b 8 0.67 0.57 - 0.76
2
Abet0328 8 0.55 1
Abet0329 8 0.63 1
Abet0332 8 0.91 1
Abet0342 8 0.59 1
Abet0343 8 0.48 1
Abet0344 7 0.77 1
Abet0368 11 0.55 1
Abet0369 10 0.36 0.30 - 0.41
3
Abet0370 10 0.76 1
Abet0371 11 0.50 0.46 - 0.53
2
Abet0372 10 0.38 0.26 - 0.49
2
Abet0373 10 0.84 1
Abet0374 10 0.42 0.41 - 0.43
2
Abet0377 10 0.32 0.29 - 0.35
2
Abet0378 9 0.97 1
Abet0379 9 0.69 1
Abet0380 10 0.43 0.38 - 0.47
2
Abet0381 10 0.47 1
Abet0382 10 0.66 1
Abet0383 11 0.75 1
Table 4: Example potency data for optimised scFy clones when evaluated in the
Abet0144-GL
HTRFTm epitope competition assay. Where the assay was performed more than
once, the
absolute range of 1050 values is provided.
Table 5 (see below): Sequence alignment of the VH domains of the optimised non-
germlined
clones described herein. Changes from the parent sequence (Abet0144-GL) are
highlighted.
Residues are designated according to the Kabat numbering system.
Table 6 (see below): Sequence alignment of the VL domains of the optimised non-
germlined
clones described herein. Changes from the parent sequence (Abet0144-GL) are
highlighted.
Residues are designated according to the Kabat numbering system. Note that
Abet0378 has an
amber stop codon "B" present in the VL sequence at position 91, which was
introduced as a
change from glutamine during optimisation. The antibody was produced as an
scFy fragment in
the E. coli strain TG1 used for expression in which the amber stop codon is
read as glutamine.
80
FW 1 CDR 1 FW
2 CDR 2
Kabat
Numbering VH ¨ cv co .t ,r) (ID N. op (3) 0 ¨ (`i c") 't u) (ip r" c cv) 8
0)0)10). .(1-, &) R (c). (8 8 8 7, FA 8 8 8 8 rc,) 8 8 0 ..,- (.4 .c;?- 4
v-t) 4' 4. (4 (--,(? ..9 8 F.',4 69, E,',' 8 8 8 N. P, 8 8 OW 8 8 8
Abet0144-GLEVQL LESGGGLVQPGGSLRLSCAASGFT FSVYTMWWVRQAPGKGLEWVSV
IGSSGGTTVYADSVKG 0
Abet0319 V TV 1 NKD
t=J
Abet0321b Al H TNHDP
A 0
Abet0322b NEE
,T,YI,IP A A
4-
Abet0323b JS QED
PNPKNNA 0
Abet0328 :DA H.TD R
AHTNNSA 0
Abet0329 T NTKRE
HQER S 0
4-
Abet0332 E D WHTD,I
R N NKK I A 4-
Abet0342 D FRSV
AQTQNKA 4-
Abet0343 N NHQV
KTNEN I A
Abet0344 ,S
GNETRKA
Abet0368 n , 3 P S P
KDTQNST
Abet0369 , I KN R
KDETR FN
Abet0370 I FMSA
ETPERQA
Abet0371 DA FFD
Abet0372 C1\1
NI E KGMNNVS
Abet0373 V D ERSV
fR GKTN I T
Abet0374 0 K DTP
DQNHKKA
Abet0377 N NEQ L
VGTKN I A T
Abet0378 F ETD I
TNTDNVA
Abet0379 D A ETP L
ER NQNK A
Abet0380 M ,3 N
NYQ R KTNEN I A P
Abet0381 EF'R
F RE TQPNR LT
Abet0382 H TNS I
EAHR VT 2
Abet0383 K DW P
R I ADNAK I A 002
Iv
FW 3 CDR 3
FW 4 Iv
Kabat
NurnberingVry 8 .',3 8 8 , p, F, pi, re : ), =g- r, p.,.,, rr:-, g 2 (r -, 2 8
(7, 8 (I -a ('-µ1 co 71- Lo op r- co CD 0 ,- Cµi CO 71" L0 CO t=== CO CC 0 0 0
0 0 0 0 0 0 0C'J 0 0 0 0 0 0 0
0, 0, 0, CO CO CO CO CO CO CO 0) 0) 0) 0) 0) CC a) a) a) a) , o o o o o 0 o o
ul
1
Abet0144-GLRFT I SRDNSKNT L YLQMNSLRAEDTAVYYCAREWMDHSRPYYYYGMDVWGQGT LVTVSS
2.
,
Abet0319
0.1-
Abet0321b
Abet0322b --1.-
Abet0323b
Abet0328 R
Abet0329
Abet0332
Abet0342
Abet0343
Abet0344 R i
Abet0368 D K
Abet0369
Abet0370 ,,
.0
Abet0371
Abet0372
Abet0373
M
Abet0374
U .0
t=J
Abet0377
0
Abet0378
--
Abet0379 D)
Cn)
0
Abet0380
Abet0381
I P
Abet0382
!A
0
Abet0383 G
P
Table 5
81
FW 1 CDR 1
FW 2 CDR 2
Kabat
0
Numbering V, .- CV CO 71- LO OD N. CO CY) '- Co CO 't L CD N' CD CD ca Fj
gji 2 g 8 8 [=,-,cc4 g g 8 8 1 c=o- '4.14- ,u7, 2 8 w
o
Abet0144-GL SYEL TQPPS - VSVSPGQT AS I TCSGHNLEDKF ASWYQQK Pq.c.,.SP V L V I
YRDDKRPS
Abet0319 I M WV
Abet0321b
I ,
Abet0322b G
0
Abet0323b
Abet0328
4-,
Abet0329 V S WMT
Abet0332 I G A W V
I
Abet0342 __
Abet0343 ] 1 ,,, ! `I,. V
Abet0344 Q :_, V
Abet0368 T
Abet0369 ,_' R I G SWV A
Abet0370 TT PHFNS
Abet0371 L SSWV
Abet0372
Abet0373
Abet0374 , T G G ___
Abet0377 T H WI
Abet0378
P
Abet0379 1,:l r'-_ ,./
Abet0380
2
Abet0381
Abet0382 I
oo
Abet0383
Iv
o"
Kabat FW 3 CDR
3 FW 4 cni
1
Numbering V[
1
Abet0144-GL G I PERFSASNSGHT AT L T I SGTQAMDEADYYCQAQDSTTRVFGGGTK L TV L
Abet0319 T S S T V
Abet0321b T 59 T V
Abet0322b E T S S T V
Abet0323b 1 V T SS T V
I
Abet0328 . T 99 T V
Abet0329 T S S T V
Abet0332 T G Q
V S,
Abet0342 lr
Abet0343 T SS T V
Abet0344 T A T N F
Abet0368 Ad T S S T V
Abet0369 T SS
Abet0370
R n
Abet0371 T SS T V
Abet0372 T S S T V
M
Abet0373 E ' 1- _
Abet0374 , F T S S T V
l=J
Abet0377 T T 99 T V
0
Abet0378 T SSB T V
Abet0379 T 1 A T N F
Abet0380 T S S T V
Abet0381 T N S S T V
A
Abet0382 T T S S K V
Abet0383 l- S S T V
Table 6
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
82
3.7 Kinetic profiling of affinity improved clones in purified scFv format by
Surface Plasmon
Resonance
Surface Plasmon Resonance was used to analyse the purified scFv clones that
had
shown significant improvement in binding affinity for human Amyloid beta 1-42
peptide over the
parent sequence, Abet0144-GL, in the HTRFTm epitope competition assay
(sections 3.3-3.6).
Briefly, the ProteOn Protein Interaction Array System (BioRad, USA) was used
to assess the
kinetic parameters of the interaction between each purified scFv and
synthetically produced
human Amyloid beta 1-42 peptide. These experiments were performed essentially
as described
by Karlsson et al. (Karlsson et al., 1991). For further details see section
1.7.
The affinity of binding between each test scFv and human Amyloid beta 1-42 was
estimated using assays in which biotinylated synthetic human Amyloid beta 1-42
peptide
(rPeptide, USA; cat: A1117) was non-covalently bound via a biotin/streptavidin
interaction to a
proprietary streptavidin chip (NTA 176-5021) at five different surface
densities. The chip
surface was regenerated between cycles by a single 60 second injection of 10
mM Glycine pH
2.0 to remove scFv bound to the peptide. The regeneration did not result in a
significant loss of
scFv binding capacity.
Each scFv at 100 ¨ 200 nM was sequentially passed over the peptide surface for
a
sufficient amount of time to observe sensorgrams that could be fitted to an
appropriate binding
model with confidence. An irrelevant scFv blank was subtracted from the main
dataset to
reduce the impact of any buffer artefacts or non-specific binding effects. An
appropriate binding
model was then fitted to the data.
For Abet0380 scFv, the association rate constant (ka), dissociation rate
constant (kd)
and dissociation constant (KD) are 1.93 x 105 M-1 s-1, 2.85 x 10-5 s-1 and 148
pM respectively.
These parameters were derived from a 1:1 Langmuir fit to the data.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
83
Clone ka (4-4 s-1) kd (s-1) KD 00
Abet0144-GL 1.16E+05 6.60E-03 5.87E-08
Abet0319 3.29E+05 1.29E-04 3.91E-10
Abet0321b 1.50E+05 3.33E-05 2.22E-10
Abet0322b 2.03E+05 1.65E-04 8.12E-10
Abet0323b 2.10E+05 1.88E-04 8.94E-10
Abet0328 1.41E+05 1.03E-04 7.29E-10
Abet0329 1.97E+05 1.38E-04 7.01E-10
Abet0332 3.29E+05 1.29E-04 3.91E-10
Abet0342 1.36E+05 5.73E-05 4.21E-10
Abet0343 1.20E+05 2.25E-05 1.88E-10
Abet0344 7.75E+04 5.73E-05 7.39E-10
Abet0368 1.87E+05 9.00E-05 4.82E-10
Abet0369 3.27E+05 4.34E-05 1.33E-10
Abet0370 1.19E+05 7.76E-05 6.51E-10
Abet0371 3.57E+05 2.72E-04 7.62E-10
Abet0372 2.43E+05 1.76E-04 7.24E-10
Abet0373 1.85E+05 8.92E-05 4.83E-10
Abet0374 2.56E+05 6.04E-05 2.36E-10
Abet0377 1.96E+05 3.02E-05 1.54E-10
Abet0378 1.36E+05 6.41E-05 4.72E-10
Abet0379 1.34E+05 4.39E-05 3.27E-10
Abet0380 1.93E+05 2.85E-05 1.48E-10
Abet0381 2.13E+05 5.14E-05 2.41E-10
Abet0382 2.25E+05 7.97E-05 3.54E-10
Abet0383 1.81E+05 3.94E-05 2.17E-10
Table 7: Example kinetic data for optimised scFv clones binding to synthetic
biotinylated human
Amyloid beta 1-42 peptide, as determined by Surface Plasmon Resonance.
3.8 Reformatting of affinity improved scFv to human IgG1-TM
The IgG1-TM antibody format is discussed in section 2.6. ScFv were reformatted
to
IgG1-TM by subcloning the variable heavy chain (VH) and variable light chain
(VL) domains into
vectors expressing whole human antibody heavy and light chains respectively.
The variable
heavy chain was cloned into a mammalian expression vector (pEU 1.4) containing
the human
heavy chain constant domains and regulatory elements to express whole IgG1-TM
heavy chain
in mammalian cells. Similarly, the variable light chain domain was cloned into
a mammalian
expression vector (pEU 4.4) for the expression of the human lambda light chain
constant
domains and regulatory elements to express whole IgG light chain in mammalian
cells.
To obtain antibodies as IgG, the heavy and light chain IgG expression vectors
were
transiently transfected into HEK293-EBNA mammalian cells (Invitrogen, UK; cat:
R620-07)
where the IgGs were expressed and secreted into the medium. Harvests were
pooled and
filtered prior to purification. The IgG was purified using Protein A
chromatography. Culture
supernatants were loaded onto an appropriate ceramic Protein A column
(BioSepra - Pall,
USA) and washed with 50 mM Tris-HCI pH 8.0, 250 mM NaCI. Bound IgG was eluted
from the
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
84
column using 0.1 M Sodium Citrate (pH 3.0) and neutralised by the addition of
Tris-HCI (pH 9.0).
The eluted material was buffer exchanged into PBS using NAP-10 buffer exchange
columns
(GE Healthcare, UK; cat: 17-0854-02) and the purified IgGs were passed through
a 0.2 pm filter.
The concentration of IgG was determined spectrophotometrically using an
extinction coefficient
based on the amino acid sequence of the IgG. The purified IgGs were analysed
for aggregation
or degradation using SEC-HPLC and by SDS-PAGE.
3.9 Germlining
Five of the most potent IgGs were selected for germlining, based on an
experimental
characterisation of their corresponding scFv. Purified scFy of clones
Abet0343, Abet0369,
Abet0377, Abet0380 and Abet0382 all exhibited IC50 values of less than 750 pM,
as determined
by epitope competition assay (Table 4), and all had an experimental
dissociation constant of
less than 250 pM, as determined by Surface Plasmon Resonance, Table 7.
The germlining process consisted of reverting framework residues in the VH and
VL
domains to the closest germline sequence to identically match human
antibodies. For the VH
domains of the optimised antibody lineage this was Vh3-23 (DP-47) and for the
VL domains it
was VA3-3r (DPL-23). For Abet0380, 1 residue required changing in the VH
domain at Kabat
position 43 (Table 8) and 1 residue required changing in the VL domain at
Kabat position 81
(Table 9). The remaining four sequences required between two and five changes
(Tables 8 and
9). The Vernier residues (Foote etal., 1992), were not germlined, apart from
residue 2 in the
light chain sequence of Abet0343, which was germlined for at the same time as
the flanking
residues 1 and 3. Germlining of these amino acid residues was carried out
using standard site-
directed mutagenesis techniques with the appropriate mutagenic primers as
described by
Clackson and Lowman (Clackson etal., 2004).
85
FW1 CDR1
FW2 CDR2
Kabat
0
Numbering VH Cµi co 1.0 co (3) " (0 C a) 2 2 2 -A-
Lcr,) 2 2 2 (.7., 2 2 7r (cyg; 2 2 (.77_ :7T_ v_ L. 7 , (4! 1.;
4_ ,(2 ;74 CO 2 ;,; ,c2, (T,
Vernier eeee
-C;
Abet0144-GLEVQL L ESGGGLVQPGGS LR LSCAASGF T F SVYTMWWVRQAPGKGL EWVSV I
GSSGGTTVYADSVKG
Abet0343 N NHQV
KTNEN I A
Abet0369 S Q I KN
KDETRFN
Abet0377 N NEQ L
VGTKN I A
Abet0380 MGN NYQ
KTNEN I A
Abet0382 H TNS I
EAHRVT
FW3
CDR3 FW4
Kabat
4) r CO =71- LO CO N. op cp) (N
Numbering Vu 2 (NE; 2 2 P. 'I `1! ! co µ01 ("0101 -
(.0(`'01 g 01 2 0( = 0NO o go) 2 '(;) `,31 "(;) T,rc-,;`:)61C) CC.'2
2', -
Vernier 1 =
Abet0144-GLRFT I SRDNSKNT L Y LQMNS LRAEDTAVYYCAREWMDHSRPYYYYGMDVWGQGT LVTVSS
Abet0343
Abet0369
Abet0377
Abet0380
Abet0382
Table 8: Sequence alignment of the VH domains of the five clones selected for
germlining. The two residues that were reverted to germline are
indicated by shaded boxes. The positions of the Vernier residues are indicated
by circles (.).
86
FW 1 CDR 1
FW 2 CDR 2 0
Kabat
Numbering VI_ c\i co 71- in co i=-= co o) C Cr) 71- L (13
C (7) Lc.n.,, rc-4- g,) c9, c7; icro) (%- Ff;
'.77 s7,\1. F70,. 3 f,s4 is?,
Vernier = =
= = = = = =
Abet0144-GL SYELTQPPS - VSVSPGQT AS I TCSGHNLEDKFASWYQQKPGQSPVLV I YRDDKRPS
Abet0343 L S V
Abet0369 G R I G
SWV A
Abet0377 T H
W I
Abet0380
Abet0382
Kabat FW 3
CDR 3 FW 4
Numbering VI- lic) E.?) PO) 3 7) \ 10 2 (7 -0 3 3 C(8 (73 fz.) 'rz e. I
cr . R.) C0
07)
`03 CO 09 SS CO. CO CO) 8 `,1 Col g E g.)) 8 E) c8 8 3 L8 8 8
Vernier = = = = =
= I
Abet0144-GL G I PER FSASNSGHT AT LT I SGTQAMDEADYYCQAQDSTIRVFGGGTK L TV L
Abet0343 I S S
T V 0
1.)
Abet0369 I
S S T V
Abet0377 I I
S S T V
1.)
Abet0380 I
S S T V 1.)
Abet0382 I
S S K V
0
0
Table 9: Sequence alignment of the VL domains of the five clones selected for
germlining. The thirteen residues that were reverted to germline are
indicated by shaded boxes. The positions of the Vernier residues are indicated
by circles (.).The Vernier 2 residue in Abet0343 was reverted to
germ-line at the same time as residues 1 and 3. Reverting this residue did not
impact on antibody potency.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
87
3.10 Determination of the binding kinetics of affinity-optimised IgGs using
Surface Plasmon
Resonance
Surface Plasmon Resonance was used to analyse the binding kinetics of the
affinity-
optimised IgGs (section 3.8) and their germlined counterparts (section 3.9).
Briefly, the BlAcore
T-100 (GE Healthcare, UK) biosensor instrument was used to assess the kinetic
parameters of
the interaction between each test IgG and synthetically-produced human Amyloid
beta 1-42
peptide. These experiments were performed essentially as described by Karlsson
et al.
(Karlsson et aL, 1991). For further details see section 1.7.
The affinity of binding between each test IgG and human Amyloid beta 1-42 was
estimated using assays in which each antibody was non-covalently captured by a
protein G
surface that was itself amine linked to a proprietary CM5 chip. The chip
surface was
regenerated between cycles by paired 40 second injections of 10 mM Glycine pH
2.0 to remove
ligand and bound antibody. The test antibody was then reapplied for each
peptide injection.
A series of dilutions of synthetic human Amyloid beta 1-42 peptide (0.063 ¨
1024 nM)
were sequentially passed over the antibody surface for a sufficient amount of
time to observe
sensorgrams that could be fitted to an appropriate binding model with
confidence. Blank
reference flow-cell data were subtracted from each IgG dataset and a zero-
concentration
antibody-only buffer blank was double-reference subtracted from the main
dataset. An
appropriate binding model was then fitted simultaneously to the data from each
analyte titration
using the BlAevaluation software.
The validity of the data was assessed using the calculated Chi2 value, with an
acceptable value being under 2 RU2. The overall success of the fit was
estimated using the
residuals, with a deviation of under 2 RUs being acceptable.
Example results for Abet0380-GL (germlined) IgG1-TM are shown in Figure 12.
The
association rate constant (ka), dissociation rate constant (kd) and
dissociation constant (KD) are
9.52 x 105 M-1 s-1, 3.07 x 10-4 s-1 and 322 pM respectively. These parameters
were derived from
a 1:1 Langmuir fit to the data.
3.11 Specificity profiling of affinity-optimised IgGs using Surface Plasmon
Resonance
Surface Plasmon Resonance was used to verify the specificity of the affinity-
optimised
IgGs for the human Amyloid beta 1-42 peptide. Briefly, the BlAcore2000 (GE
Healthcare, UK)
biosensor instrument was used to assess the kinetic parameters of the
interaction between
each test IgG and a range of small peptides including synthetically-produced
human Amyloid
beta 1-42 and human Amyloid beta 1-40. These experiments were performed
essentially as
described by Karlsson et al. (Karlsson et al., 1991). For further details see
section 1.7.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
88
The interaction between each test IgG and each peptide was estimated using
assays in
which the antibody was non-covalently captured by a protein G surface that was
itself amine
linked to a proprietary CM5 chip. The interaction between antibody and peptide
was observed
using a 5 application single cycle approach. The chip surface was regenerated
between cycles
by paired 40 second injections of 10 mM Glycine pH 2.0 to remove ligand and
bound antibody.
The test antibody was then reapplied for each peptide injection cycle.
Each test peptide (between 64 and 1024 nM) was sequentially passed over the
antibody
surface for a sufficient amount of time to observe sensorgrams that either
showed no binding or
that could be fitted to an appropriate binding model with confidence. Blank
reference flow-cell
data were subtracted from each IgG dataset and a zero-concentration antibody-
only buffer
blank was double-reference subtracted from the main dataset.
Example results for Abet0380-GL (germlined) IgG1-TM are shown in Figure 13.
Two
peptides (biotinylated human Amyloid beta 1-42, (rPeptide, USA; cat: A1117)
and unlabelled
murine Amyloid beta 1-42 (rPeptide, USA; cat: A1008) showed strong binding to
the antibody,
whilst two peptides biotinylated human Amyloid beta 1-40 (rPeptide, USA; cat:
A1111) and
unlabelled murine Amyloid beta 1-40 (rPeptide, USA; cat: A1007) showed no
binding to the
antibody.
3.12 Affinity of the most potent IgGs for native Amyloid beta using in vitro
immunohistochemistry
The most potent IgGs were tested for their ability to bind to Amyloid beta,
with the aim of
estimating the affinity of these clones for native forms of the Amyloid beta
peptide. Briefly, the
lead antibodies were screened on human Alzheimer's Disease brain sections and
Tg2576
mouse brain sections to identify anti-Amyloid beta 1-42 antibodies that bound
to Amyloid
plaques in vitro. The experiments were performed essentially as described in
section 1.9.
In these experiments, human brain tissue was isolated from the frontal cortex
of two
individuals with severe Alzheimer's Disease (ApoE genotype 3/3, Braak stage 6
and ApoE
genotype 4/3, Braak stage 5). As a control, equivalent tissue was isolated
from one non-
dementia individual (ApoE genotype 3/3, Braak stage 1). Mouse brain tissue was
isolated from
Tg2576 mice at an age of 15 months (2 mice) and 22 months (2 mice). Antibodies
were tested
at concentrations of 2, 5, 10 and 20 ug m1-1.
In one experiment, the Abet0380-GL IgG1-TM antibody stained core plaques (CP)
with a
score of 4 on Tg2576 brain sections, and a score of 3 on human AD brain
sections. It also
stained diffuse plaques (DP) and cerebral amyloid angiopathy (CAA) plaques,
but to a lesser
extent. In contrast, a positive control antibody produced a score of 3-4 on
all plaques (CP, DP,
CAA) on adjacent sections under the same conditions. Representative images are
shown in
Figure 14.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
89
3.13 Demonstrating Abet0380-GL IgGl-TM Abeta42 recognition profile by western
blot
To cross-link the A1342 oligomers before SDS-PAGE, PICUP (photo-induced cross-
linking of
peptides) was carried out as follows. A 1 mM solution of Ru(Bpy) was created
by adding 2 jil of
stock (at 10 mM) to 18 jil of 1xPBS. In addition, a 20 mM solution of ammonium
persulphate
(APS) was created by adding 2 jil of stock (at 200 mM) to 18 jil of 1xPBS.
Unused stock was
immediately snap-frozen on dry ice and returned to the -80 C freezer. In the
dark room, 5 jil of
Ru(Bpy) was added to 80u1 of aggregate (neat 10uM sample), followed by 5 jil
of APS.
Samples were irradiated with a lamp in the dark room for 10secs. 30uIs of (4x)
LDS Sample
buffer was added immediately.
SDS-PAGE was then performed on cross-linked (PICUP) and non-cross-linked A131-
42
aggregate. The solutions were incubated in a hot block at 70 C for 10 minutes.
Meanwhile, a
marker was created by combining 5 jil of Magic Mark XP Western Protein
Standard, 5 jil of
Novex Sharp Pre-stained Protein Standard. After the ten-minute incubation, the
samples plus
marker were loaded onto a NuPAGE Novex 4-12% Bis-Tris Gels (1.0 mm, 15 well,
15 jil per
well) with MES running buffer. The gels were run at 200 V for 35 minutes.
The gel was then blotted onto a PVDF membrane using an iBlot machine from
lnvitrogen, for 7
minutes at 20V (program P3).
Once blotting was complete, the gel stack was disassembled and the PVDF
membrane was
then blocked in 50 ml of 4% MPBST (4% Marvel in PBST) for one hour at room
temperature
with gentle rotation. The blots were then cut with a scalpel for probing with
individual antibodies.
This was a 1 hour incubation with the primary antibody solution (2ug/m1 in 10
ml of 3% MPBST).
Next, the membrane was washed 5x with PBST, 5 minutes each, and was then
incubated in
secondary antibody solution (1 jil anti-human Fc specific - HRP conjugate in
10 ml of PBST) for
1 hour at room temperature. The membrane was washed 3x with PBST and 2x with
PBS, 5
minutes each.
During the final washes, the chemi-luminescence SuperSignal West Dura
substrate (Thermo
Scientific; 34075) were allowed to warm to room temperature. 600u1 of each of
the 2 solutions
were combined. The PBS was decanted from the PVDF membrane, and then a pipette
was
used to cover the membrane with the mixed Dura reagents. The reaction was
allowed to
proceed for -5 minutes (during which time the VerscDoc Imaging System was set
up) and then
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
an image was taken with 30sec exposure (with enhancement using the transform
filter). A
representative image is shown in Figure 15.
Example 4. Studies demonstrating a specific functional response of Abet0380-GL
laG1 -TM
5 antibody in vivo
4.1 Functional characterisation of Abet0380-GL IgGl-TM by reduction of free
Amyloid beta 1-42
peptide in vivo
Eight-week old male albino Harlan Sprague-Dawley rats (n = 8-12) received a
single
dose of Abet0380-GL IgG1-TM antibody by intravenous injection with a dosing
vehicle of 25 mM
10 Histidine, 7% Sucrose, 0.02% p80 surfactant, pH 6.0 at 5 ml/kg. Dosing
solutions were made
just before dosing. Animals were anaesthetised at the time indicated and
cerebrospinal fluid
(CSF) was aspirated from the cisterna magna. CSF samples were centrifuged for
10 minutes at
approximately 3000 x g at 4 C within 20 minutes of sampling to remove cells or
debris. Samples
were then frozen on dry ice and stored at -70 C for subsequent analysis.
15 Animals were sacrificed by decapitation, brain tissue was dissected and
Amyloid beta
peptides were extracted from brain tissue in diethylamine (DEA; Fluka, Sigma,
UK; cat: 31729).
Briefly, frozen brain tissue was homogenised in 0.2% DEA and 50 mM NaCI
(Merck, USA; cat:
1.06404.1000). Brain homogenates were ultracentrifuged at 133,000 x g, for 1
hour. Recovered
supernatants were neutralised to pH 8.0 with 2 M Tris-HCI (TRIZMAe-
hydrochloride; Sigma, UK;
20 cat: 93363) and stored at -70 C until analysis. Animal experimentations
were performed in
accordance with relevant guidelines and regulations provided by the Swedish
Board of
Agriculture. The ethical permission was provided by an ethical board
specialised in animal
experimentations: the Stockholm &Ara Animal Research Ethical Board.
Measurement of free Amyloid beta 1-42 peptide in rat CSF was conducted using
25 immunoprecipitation to remove Abet0380-GL bound Amyloid beta 1-42
peptide, followed by
analysis by a commercial ELISA kit obtained from lnvitrogen. Briefly, a
solution of protein A
beads (Dynabeads Protein A; lnvitrogen, UK; cat: 100-02D) was added to a 96
well non-skirted
plate (polypropylene 0.2 ml; VWR International, UK; cat: 10732-4828) and
washed twice with
TBST (50 mM TBS; Sigma, UK; cat: T6664 plus 0.1% Tween20) using a magnet
(DynaMag TM
30 96 side; lnvitrogen, UK; cat: 123.31D) to separate the beads from the
solution. Thawed rat CSF
samples (40 pl) were added to each well and incubated at 40 C with tilt
rotation for 1 hour. The
beads were then pelleted using the magnet and 30 pl of immunoprecipitated CSF
samples were
transferred to a 96 well plate from the ELISA kit (mouse Amyloid beta (1-42)
colorimetric ELISA
kit; lnvitrogen, UK; cat: KMB3441) with 70 pl of the Standard Diluent Buffer
already added
35 (supplemented with protease inhibitor; Roche, UK; cat: 11836153001).
Calibration standard
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
91
samples were added to the plate in duplicate and the plate was incubated for 2
hours at room
temperature with shaking. The plate was washed 4 times with 400 pl of wash
buffer, 100 pl of
the detection antibody solution was added to each well and the plate was
incubated for 1 hour
at room temperature with shaking. Again, the plate was washed 4 times with 400
pl of wash
buffer, 100 pl of the secondary antibody working solution was added to each
well and the plate
was incubated for 30 minutes at room temperature with shaking. Finally, the
plate was washed
4 times with 400 pl of wash buffer, 100 pl of stabilised Chromogen was added
to each well and
the plate was incubated for 30 minutes at room temperature in the dark. To
stop the reaction,
100 pl of Stop Solution was added to each well and the plate was read within 2
hours at an
absorbance of 450 nm. Single CSF samples were analyzed and data analysis was
performed
using Prism 4 (Graph Pad, USA) with one-way ANOVA on log transformed data
without
adjustment for multiple comparisons.
Measurement of total (free and Abet0380-GL bound) Amyloid beta 1-42 peptide in
rat
brain homogenates was performed using modifications of the mouse Amyloid beta
(1-42)
colorimetric ELISA kit (Invitrogen, UK; cat: KMB3441). The kit detection
antibody was replaced
by an excess of Abet0380-GL IgG1-TM antibody and the secondary antibody by an
anti-human
IgG HRP-conjugate antibody (Jackson ImmunoResearch, UK; cat: 109-035-098).
Briefly,
thawed brain homogenates of 50 pl diluted 1:2 in Sample Diluent (supplemented
with protease
inhibitor; Roche, UK; cat: 11836153001) and standard samples were added in
duplicate to the
96 well ELISA plate. An excess of Abet0380-GL IgG1-TM antibody (50 p1,4 pg/ml)
was added
to each well and the plate was then incubated for 3 hours at room temperature.
The plate was
washed 4 times with 400 pl of wash buffer, 100 pl of the secondary antibody
working solution
was added to each well and the plate was incubated for 30 minutes at room
temperature.
Finally, the plate was washed 4 times with 400 pl of wash buffer, 100 pl of
stabilised
Chromogen was added to each well and the plate was incubated for 15 minutes at
room
temperature in the dark. To stop the reaction, 100 pl of Stop Solution was
added to each well
and the plate was read within 2 hours at an absorbance of 450 nm. Data
analysis was
performed using Prism 4 (GraphPad, USA) with one-way ANOVA on log transformed
data
without adjustment for multiple comparisons.
Measurement of total Amyloid beta 1-40 peptide in rat brain homogenates was
performed using the mouse Amyloid beta (1-40) colorimetric ELISA kit
(lnvitrogen, UK; cat:
KMB3481). Briefly, thawed brain homogenates of 50 pl and standard samples,
diluted in
Sample Diluent (supplemented with protease inhibitor; Roche, UK; cat:
11836153001), were
added in duplicate to the 96 well ELISA plate. 50 pl of the detection antibody
solution were
added to each well and the plate was incubated for 3 hours at room
temperature. The plate was
washed 4 times with 400 pl of wash buffer, 100 pl of the secondary antibody
working solution
was added to each well and the plate was incubated for 30 minutes at room
temperature.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
92
Finally, the plate was washed 4 times with 400 pl of wash buffer, 100 pl of
stabilised
Chromogen was added to each well and the plate was incubated for 30 minutes at
room
temperature in the dark. To stop the reaction, 100 pl of Stop Solution was
added to each well
and the plate was read within 2 hours at an absorbance of 450 nm. Data
analysis was
performed using Prism 4 (GraphPad, USA) with one-way ANOVA on log transformed
data
without adjustment for multiple comparisons.
4.2 Functional characterisation of Abet0380-GL IgGl-TM by reduction of free
Amyloid beta 1-42
peptide in vivo
A single dose of the Abet0380-GL IgG1-TM antibody at 20 mg/kg reduced the CSF
level
of free Amyloid beta 1-42 peptide in rats to the limit of quantification at 72
or 168 hours after
dose in the assay described in Section 4.1 (data not shown). To further
investigate the effect of
the Abet0380-GL IgG1-TM antibody in vivo, rats were administered weekly doses
of 0.25, 0.5, 1,
5 or 10 mg/kg over 14 days. Animals were euthanized 168 hours after the second
dose to
measure levels of free Amyloid beta 1-42 peptide in CSF as well as total
Amyloid beta 1-42 or
1-40 peptides in brain tissue.
A dose-dependent decrease of free Amyloid beta 1-42 was demonstrated in CSF
(Figure
16A). The two highest doses of 5 and 10 mg/kg reduced Amyloid beta 1-42
peptide to the limit
of quantification in the assay used, whereas doses of 0.5 and 1 mg/kg
significantly reduced
Amyloid beta 1-42 peptide by 47% and 61% respectively when compared to the
vehicle control.
The lowest dose, 0.25 mg/kg, gave a 14% reduction of free Amyloid beta 1-42
peptide in CSF,
but failed to reach statistical significance. Due to sequestration of Amyloid
beta 1-42 peptide by
Abet0380-GL IgG1-TM antibody, a dose-dependent increase of total Amyloid beta
1-42 peptide
was demonstrated in brain tissue (Figure 16B). However, the level of total
Amyloid beta 1-40
peptide in brain tissue was unaffected (Figure 160), thus demonstrating the
specificity of
Abet0380-GL IgG1-TM for Amyloid beta 1-42 peptide. In summary, the above
results from rat
studies showed that the Abet0380-GL IgG1-TM antibody reduced the level of free
Amyloid beta
1-42 peptide in CSF with an ED50 between 0.5 and 1 mg/kg.
4.3 Functional characterisation of Abet0380-GL IgG1TM ¨ demonstration of non
plaque binding
in vivo - no binding of Abet0380-GL IgGl-TM to Amyloid beta plaques in vivo
168 hours after a
peripheral dose to aged Tg2576 mice
Abet0380-GL IgG1-TM was tested for its ability to bind to Amyloid beta plaques
in aged Tg2576
mice after a single peripheral dose. Animal experimentations were performed in
accordance
with relevant guidelines and regulations provided by the Swedish Board of
Agriculture. The
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
93
ethical permission was provided by an ethical board specialised in animal
experimentations: the
Stockholm Sodra Animal Research Ethical Board.
Seventeen-month old female Tg2576 mice (n=5) received a single dose of
vehicle, a positive
control antibody at 30 mg/kg or the Abet0380-GL IgG1-TM antibody at 10 or 30
mg/kg by
intravenous injection with a dosing vehicle of 25 mM Histidine, 7% Sucrose,
0.02% p80
surfactant, pH 6.0 at 5 mUkg. At 168 hours after dose, animals were deeply
anaesthetised and
perfused with room temperature PBS followed by cold (4 C) phosphate buffered
4%
paraformaldehyde (PFA). Animals were then sacrificed by decapitation and
brains were
dissected and immersions fix in PFA at 4 C for 72 hours. The fixative was
exchanged to PBS
containing 0.1% sodium azide and tissues were stored at 4 C until further
processed.
lmmunohistochemistry was performed on brain sections to evaluate the degree of
binding of
Abet0380-GL IgG1-TM to Amyloid beta plaques in vivo. Briefly, paraffin
embedded brain
sections were prepared for immunohistochemistry as described in section 1.9.
Detection of
Abet0380-GL IgG1-TM or the positive control antibody deposited within brain
parenchyma was
conducted using a rabbit-anti-mouse IgG1 and IgG2-specific secondary antibody
from
Epitomics. The staining was performed on the Ventana robot, using the OmniMap
detection
system (Ventana Medical Systems, USA). For spiking ex vivo, consecutive tissue
sections were
stained in vitro with the injected Abet0380-GL IgG1-TM or positive control
antibody in excess.
Secondary antibodies and chromogenes were the same as above.
Scoring of the staining was carried out in a blinded fashion under 10x optical
magnification. The
distribution of decorated plaques was noted. The intensity of plaque labelling
was scored
according to a relative intensity scale from 0 (no staining of plaques) up to
4 (intense decoration
of plaques).
Abet0380-GL IgG1-TM did not decorate Amyloid beta plaques or cerebral amyloid
angiopathy
(CAA) in vivo at 168 hours after a peripheral dose of 10 or 30 mg/kg. The
positive control
antibody demonstrated intense to low in vivo plaque decoration. A partial and
focal distribution
pattern was apparent, with core plaques, diffuse plaques and CAA in all
animals.
Representative images are shown in Figure 17. Spiking ex vivo of brain tissue
from the same
animals with Abet0380-GL IgG1-TM and the positive control antibody confirmed
the previously
demonstrated ex vivo plaque binding capacity of the injected antibodies (not
shown).
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
94
Example 5. Anti-A/31-42 Sequences
Examples of sequences of antibody molecules are listed in the appended
sequence
listing, including example antibody VH domains, VL domains, individual CDR
sequences, sets
of HCDRs, sets of LCDRs, and framework regions.
Sequences of the 24 optimised clones listed in Table 7 were compared. Tables
10 and
11 show % sequence identity between the VH and VL domains respectively.
0
Homology of the VH Percent
Identity t.)
domain 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
24 25 26 27 28 29 30
1-,
Abet0144-GL 1
94.4 92.8 92.0 90.4 88.0 91.2 87.2
90.4 90.4 92.8 88.8 89.6 89.6 93.6 89.6 89.6 89.6 89.6 90.4 88.8 88.8 88.0
91.2 88.0 90.4 90.4 89.6 89.6 91.2 .6.
Abet0319
2 5.8 94.4 91.2 90.4 88.0 90.4 88.0 88.0
88.8 87.2 86.4 88.8 87.2 93.6 89.6 87.2 87.2 88.0 88.8 86.4 88.8 87.2 88.8
84.0 88.8 89.6 88.0 89.6 88.8 -1
cA
Abet0321b
3 7.6 5.8 91.2 89.6 88.0 89.6 88.8 88.8
90.4 87.2 87.2 87.2 88.8 92.0 88.8 87.2 88.8 88.0 89.6 88.0 88.8 86.4 89.6
85.6 90.4 88.0 88.0 89.6 89.6 =
.6.
Abet0322b
4 8.5 9.4 9.4 88.0 85.6 88.0 85.6 87.2
87.2 85.6 86.4 86.4 86.4 90.4 87.2 86.4 87.2 86.4 87.2 86.4 87.2 85.6 88.0
84.0 87.2 87.2 86.4 88.0 88.0 .6.
.6.
Abet0323b
5 10.3 10.3 11.2 13.1 904 89.6 88.8
90.4 90.4 90.4 88.0 88.0 89.6 88.8 90.4 88.0 88.8 91.2 92.0 88.0 90.4 88.8
88.8 86.4 90.4 88.8 91.2 91.2 , 88.8
Abet0328
6 13.1 13.1 13.1 16.0 10.3 88.8 88.8 90.4
88.8 87.2 88.0 87.2 87.2 88.8 91.2 86.4 88.0 88.8 91.2 85.6 88.8 88.0 87.2
84.8 88.8 88.0 88.8 89.6 87.2
Abet0329
7 9.4 10.3 11.2 13.1 11.2 12.2 86.4 89.6
90.4 88.8 87.2 89.6 89.6 88.8 90.4 88.8 88.8 88.8 88.8 88.0 89.6 89.6 90.4
84.8 90.4 90.4 88.8 90.4 90.4
Abet0332
8 14.1 13.1 12.2 16.0 12.2 12.2 15.0
87.2 88.8 85.6 84.8 86.4 86.4 87.2 88.0 87.2 88.0 87.2 89.6 86.4 88.0 86.4
87.2 87.2 88.8 87.2 87.2 88.8 , 87.2
Abet0342
9 10.3 13.1 12.2 14.1 10.3 10.3 11.2 14.1
928 90.4 92.8 88.0 91.2 87.2 89.6 92.8 92.0 91.2 92.8 88.8 89.6 88.8 91.2
88.0 92.8 88.8 91.2 90.4 91.2
Abet0343
10 10.3 12.2 10.3 14.1 10.3 12.2 10.3 12.2
7.6 89.6 90.4 88.8 92.0 87.2 91.2 91.2 91.2 93.6 92.0 88.0 96.0 87.2 90.4
88.8 100.0 89.6 93.6 96.8 90.4
Abet0344
11 7.6 14.1 14.1 16.0 10.3 14.1 12.2 16.0 10.3
11.2 87.2 90.4 89.6 86.4 88.0 88.0 89.6 88.8 90.4 87.2 88.0 87.2 89.6
88.0 89.6 91.2 88.8 88.8 89.6
Abet0368
12 12.2 15.0 14.1 15.0 13.1 13.1 14.1 17.0 7.6
10.3 14.1 88.0 89.6 85.6 88.8 90.4 88.8 88.8 90.4 88.8 88.0 86.4 90.4 86.4
90.4 88.8 88.8 88.8 90.4
Abet0369
13 11.2 12.2 14.1 15.0 13.1 14.1 11.2 15.0
13.1 12.2 10.3 13.1 880 86.4 89.6 87.2 88.0 87.2 88.0 84.8 88.0 88.0 88.8
84.8 88.8 99.2 87.2 88.8 88.8
Abet0370
14 11.2 14.1 12.2 15.0 11.2 14.1 11.2 15.0 9.4
8.5 11.2 11.2 13.1 87.2 88.0 88.8 89.6 88.8 90.4 88.0 89.6 88.8 92.0 86.4
92.0 88.8 88.8 90.4 92.0
Abet0371
15 6.7 6.7 8.5 10.3 12.2 12.2 12.2 14.1 14.1
14.1 15.0 16.0 15.0 14.1 88.8 86.4 86.4 86.4 88.0 85.6 87.2 87.2 88.0
83.2 87.2 87.2 86.4 88.0 88.0
Abet0372
16 11.2 11.2 12.2 14.1 10.3 9.4 10.3 13.1
11.2 9.4 13.1 12.2 11.2 13.1 ' 12.2 88.8 88.0 90.4 90.4 86.4 91.2 90.4
89.6 84.8 91.2 90.4 90.4 92.0 , 89.6 õ, P
Abet0373
17 11.2 14.1 14.1 15.0 13.1 15.0 12.2 14.1 7.6
9.4 13.1 10.3 14.1 12.2 15.0 12.2 88.0 89.6 90.4 88.8 88.0 87.2 91.2 87.2
91.2 88.0 89.6 88.8 91.2
Abet0374
18 11.2 14.1 12.2 14.1 12.2 13.1 12.2 13.1 8.5
9.4 11.2 12.2 13.1 11.2 15.0 13.1 13.1 88.8 90.4 89.6 88.8 87.2 90.4 88.8
91.2 88.8 88.8 89.6 90.4
Abet0377 19 11.2 13.1 13.1 15.0 9.4 12.2 12.2
14.1 9.4 6.7 12.2 12.2 14.1 12.2 15.0 10.3 11.2 122
91.2 88.8 92.0 86.4 88.8 86.4 93.6 88.0 100.0 92.8 88.8
n,
Abet0378 20 10.3 12.2 11.2 14.1
8.5 9.4 12.2 11.2 7.6 8.5 10.3 10.3 13.1
10.3 13.1 10.3 10.3 10.3 ' 9.4 888 89.6 88.0 92.0 87.2 92.0 88.8 91.2
90.4 , 92.0 n,
n,
Abet0379
21 12.2 15.0 13.1 15.0 13.1 16.0 13.1 15.0
12.2 13.1 14.1 12.2 17.0 13.1 16.0 15.0 12.2 11.2 12.2 12.2 86.4 84.8 88.0
87.2 88.0 85.6 88.8 86.4 88.0 0
1-
Abet0380 22 12.2 12.2 12.2 14.1 10.3 12.2 11.2
13.1 11.2 _____________________ 4.1 13.1 13.1 13.1 11.2 14.1 9.4 13.1
12.2 8.5 11.2 15.0 87.2 88.0 86.4 96.0 88.8 92.0 99.2 , 88.0 0,
-
38.8 83.2 87.2 88.8 86.4 88.0 88.8
i
Abet0381 23 13.1 14.1 15.0 16.0 12.2 13.1 11.2
15.0 12.2 14.1 14.1 15.0 13.1 12.2 14.1 10.3 14.1
14.1 15.0 13.1 17.0 14.1 i , ip
Ø
I
Abet0382
24 9.4 12.2 11.2 13.1 12.2 14.1 10.3 14.1 9.4
10.3 11.2 10.3 12.2 8.5 13.1 11.2 9.4 10.3 12.2 8.5 13.1 13.1 1221 864
90.4 89.6 88.8 88.8 100.0 1-
Oh
Abet0383
25 13.1 18.0 16.0 18.0 15.0 17.0 17.0 14.1
13.1 12.2 13.1 15.0 17.0 15.0 19.1 17.0 14.1 12.2 15.0 14.1 14.1 15.0 19.1
15.0 88.8 85.6 86.4 87.2 86.4
Abet0343-GL 26 10.3 12.2 10.3 14.1 10.3 12.2 10.3 12.2 7.6 0.0 11.2 10.3 12.2
8.5 14.1 9.4 9.4 9.4 6.7 8.5 13.1 4.1 14.1 10.3 12.2 89.6 93.6 96.8 90.4
Abet0369-GL 27 10.3 11.2 13.1 14.1 12.2 13.1 10.3 14.1 12.2 11.2
9.4 12.2 0.8 12.2 14.1 10.3 13.1
12.2 13.1 12.2 16.0 12.2 12.2 11.2 16.0- 11 2 f88.0 89.6 89.6
Abet0377-GL 28 11.2 13.1 13.1 15.0 9.4 12.2 12.2 14.1
9.4 6.7 12.2 12.2 14.1 12.2 15.0 10.3 11.2 12.2 0.0
9.4 12.2 8.5 15.0 12.2 15.0 6.7 13.1 , 1, 92.8 88.8
Abet0380-GL 29 11.2 11.2 11.2 . 13.1 , 9.4 11.2 , 10.3 12.2 10.3 3.3
12.2 12.2 12.2 10.3 13.1 8.5 12.2 11.2 7.6 10.3 15.0 0.8
13.1 12.2 14.1 3.3 11.2 7.6 : -.4 88.8
Abet0382-GL 30 9.4 12.2 11.2 13.1 12.2 14.1 10.3 14.1 9.4 10.3 11.2 10.3
12.2 8.5 13.1 11.2 9.4 10.3 12.2 8.5 13.1 13.1 12.2 0.0 15.0 10.3 11.2 12.2
12.2 =
Percent Divergence
IV
Table 10: Sequence identity across the entire VH sequence (Kabat residues
14113) of the twenty four non-germlined and the five germlined n
antibodies described herein. All sequences are within 86.4% of the Abet0380-GL
lead clone (highlighted values). m
,-o
t..,
1-,
c.,.)
--.1
1-,
un
cA
--.1
Homology of the VI_ Percent
Identity
domain 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24
25 26 27 28 29 30
Abet0144-GL 1
89.6 94.3 93.4 93.4 92.5 90.6 89.6
99.1 91.5 91.5 92.5 87.7 92.5 89.6 95.3 94.3 91.5 90.6 94.3 90.6 95.3 91.5
94.3 94.3 96.2 89.6 92.5 962 96.2
Abet0319
2 11.2 93.4 93.4 92.5 91.5 94.3 91.5
88.7 90.6 86.8 92.5 93.4 84.0 95.3 94.3 93.4 91.5 93.4 93.4 85.8 94.3 92.5
91.5 94.3 93.4 93.4 93.4 93 4 93.4
tµ.)
Abet0321b
3 5.9 6.9 97.2 97.2 96.2 94.3 88.7 93.4
95.3 91.5 96.2 91.5 86.8 93.4 99.1 98.1 95.3 94.3 98.1 90.6 99.1 95.3 96.2
98.1 98.1 91.5 94.3 98.1 98.1
Abet0322b
4 6.9 6.9 2.9 96.2 95.3 94.3 89.6 92.5
94.3 90.6 95.3 92.5 86.8 93.4 98.1 99.1 96.2 93.4 97.2 89.6 98.1 94.3 95.3
99.1 97.2 92.5 93.4 97.2 97.2 r,
Abet0323b
5 6.9 8.0 2.9 3.9 97.2 93.4 87.7 92.5
94.3 90.6 95.3 90.6 85.8 92.5 98.1 97.2 94.3 93.4 97.2 89.6 98.1 94.3 95.3
97.2 97.2 90.6 93.4 97.2 97.2
Abet0328
6 8.0 9.0 3.9 4.9 2.9 92.5 86.8 91.5
93.4 89.6 94.3 91.5 85.8 91.5 97.2 96.2 93.4 92.5 96.2 88.7 97.2 93.4 94.3
96.2 96.2 91.5 92.5 96.2 96.2 g;
Abet0329
7 10.1 5.9 5.9 5.9 6.9 8.0 89.6 89.6
91.5 87.7 93.4 93.4 84.9 95.3 95.3 94.3 92.5 94.3 94.3 86.8 95.3 92.5 92.5
95.3 94.3 93.4 94.3 94.3 94.3
Abet0332
8 11.2 9.0 12.3 11.2 13.4 14.6 11.2 88.7
85.8 84.9 87.7 89.6 84.9 91.5 89.6 88.7 88.7 89.6 88.7 84.0 89.6 88.7 87.7
90.6 88.7 89.6 89.6 88.7 88.7 .6'
Abet0342 9 0.9 12.3 6.9
8.0 8.0 9.0 11.2 12.3 90.6 , 90.6
91.5 86.8 91.5 88.7 94.3 93.4 90.6 89.6 93.4 89.6 94.3 90.6 93.4 93.4 95.3
88.7 91.5 95.3 95.3
Abet0343
10 9.0 10.1 4.9 5.9 5.9 6.9 9.0 15.7 10.1
96.2 95.3 89.6 84.0 90.6 96.2 95.3 92.5 91.5 95.3 95.3 96.2 92.5 93.4 95.3
95.3 88.7 91.5 95.3 95.3
Abet0344 11
9.0 14.6 9.0 10.1 10.1 11.2 13.4 16.9 10.1
3.9 91 5 85.8 84.0 86.8 92.5 91.5 88.7 87.7 91.5 99.1 92.5 88.7 90.6
91.5 91.5 84.9 87.7 91.5 91.5
Abet0368
12 8.0 8.0 3.9 4.9 4.9 5.9 6.9 13.4 9.0 4.9
9.0 90.6 84.9 92.5 97.2 96.2 93.4 93.4 96.2 90.6 97.2 94.3 94.3 96.2 96.2
90.6 93.4 96.2 96.2
Abet0369
13 13.4 6.9 9.0 8.0 10.1 9.0 6.9 11.2 14.6
11.2 15.7 10.1 83.0 95.3 92.5 91.5 90.6 91.5 91.5 84.9 92.5 90.6 89.6
93.4 91.5 98.1 91.5 91.5 91.5
Abet0370
14 8.0 18.1 14.6 14.6 15.7 15.7 16.9 16.9
9.0 18.1 18.1 16.9 19.3 85.8 87.7 86.8 85.8 84.9 86.8 83.0 87.7 84.0 86.8
87.7 88.7 84.9 86.8 88.7 88.7
Abet0371
15 11.2 4.9 6.9 6.9 8.0 9.0 4.9 9.0 12.3
10.1 14.6 8.0 4.9 15.7 94.3 93.4 92.5 94.3 93.4 85.8 94.3 92.5 91.5 94.3
93.4 95.3 94.3 93.4 93.4
Abet0372 16 4.9 5.9 0.9 1.9 1.9 2.9
4.9 11.2 5.9 3.9 8.0 2.9 8.0
13.4 5.9 i 991 96.2 95.3 99.1 91.5 100.0 96.2 97.2 99.1 99.1 92.5 95.3
99.1 99.1
Abet0373
17 5.9 6.9 1.9 0.9 2.9 3.9 5.9 12.3 6.9 4.9
9.0 3.9 9.0 14.6 6.9 0.9 95.3 94.3 98.1 90.6 99.1 95.3 96.2 98.1 98.1 91.5
94.3 98.1 98.1
Abet0374
18 9.0 9.0 4.9 3.9 5.9 6.9 8.0 12.3 10.1 8.0
12.3 6.9 10.1 15.7 8.0 3.9 4.9 92.5 95.3 87.7 96.2 92.5 93.4 97.2 95.3
90.6 92.5 95.3 95.3
Abet0377
19 10.1 6.9 5.9 6.9 6.9 8.0 5.9 11.2 11.2
9.0 13.4 6.9 9.0 16.9 5.9 4.9 5.9 8.0 94.3 86.8 95.3 92.5 94.3 94.3 94.3
91.5 98.1 94.3 94.3
Abet0378
20 5.9 6.9 1.9 2.9 2.9 3.9 5.9 12.3 6.9 4.9
9.0 3.9 9.0 14.6 6.9 0.9 1.9 4.9 5.9 _90.6 99.1 95.3 96.2 98.1 98.1 91.5
94.3 98.1 98.1 0
Abet0379 21 10.1 15.7 10.1 11.2 11.2 12.3
14.6 18.1 11.2 4.9 0.9 10.1 16.9 19.3 15.7 9.0 10.1
13.4 14.6 10.1 91 5 87.7 89.6 90.6 90.6 84.0 86.8 90.6 90.6
Abet0380 22 4.9 5.9 0.9 1.9 1.9 2.9 4.9 11.2 5.9
3.9 8.0 2.9 8.0 13.4 5.9 0.0 0.9
3.9 4.9 0.9 9.0 96.2 97.2 99.1 99.1 92.5 95.3 99.1 , 99.1
Abet0381 23 9.0 8.0 4.9 5.9 5.9 6.9
8.0 12.3 10.1 8.0 12.3 5.9 10.1 18.1 8.0 3.9 4.9
8.0 8.0 4.9 13.4 3.9 1 93.4 95.3 95.3 90.6 92.5 95.3 95.3
Abet0382
24 5.9 9.0 3.9 4.9 4.9 5.9 8.0 13.4 6.9 6.9
10.1 5.9 11.2 14.6 9.0 2.9 3.9 6.9 5.9 3.9 11.2 2.9 6.9 96.2 96.2 89.6
92.5 96.2 96.2
Abet0383 25 5.9 5.9 1.9 0.9 2.9 3.9 4.9 10.1 6.9 4.9 9.0 3.9 6.9 13.4 5.9 0.9
1.9 2.9 5.9 1.9 10.1 0.9 4.9 3.9 981 93.4 94.3 98.1 98.1
Abet0343-GL 26 3.9 6.9 1.9 2.9 2.9 3.9 5.9 12.3
4.9 4.9 9.0 3.9 9.0 12.3 6.9 0.9 1.9 4.9 5.9 1.9
10.1 0.9 4.9 3.9 1 9 93.4 96.2 100.-8- 100.0 0
Abet0369-GL 27 11.2 6.9 9.0 8.0 10.1 9.0 6.9 11.2 12.3 12.3 16.9 10.1 1.9 16.9
4.9 8.0 9.0 10.1 9.0 9.0 18.1 8.0 10.1 11.2 69 69 93.4 934 93.4
Abet0377-GL 28 8.0 6.9 5.9 6.9 6.9 8.0 5.9 11.2 9.0 9.0 13.4 6.9 9.0 14.6 5.9
4.9 5.9 8.0 1.9 5.9 14.6 4.9 8.0 8.0 5.9 3.9 6.9 962 96.2
Abet0380-GL 29 3.9 6.9 1.9 2.9 2.9 3.9 5.9 12.3
4.9 4.9 9.0 3.9 9.0 12.3 6.9 0.9 1.9 4.9 5.9 1.9
10.1 0.9 , 4.9 3.9 1.9 0.0 6.9 39 100.0
Abet0382-GL 30 3.9 6.9 1.9 2.9 2.9 3.9 5.9 12.3 4.9 4.9 9.0 3.9 9.0 12.3 6.9
0.9 1.9 4.9 5.9 1.9 10.1 0.9 4.9 3.9 1.9 0.0 6.9 3.9 0.0
Percent Divergence
Table 11: Sequence identity across the entire VL sequence (Kabat residues
14107) of the twenty four non-germlined and the five germlined
antibodies described herein. All sequences are within 88.7% of the Abet0380-GL
lead clone (highlighted values).
CA 02888322 2015-04-14
WO 2014/060444 97
PCT/EP2013/071567
Kabat Abet0380-
number GL Other example residues
26 M G S
27 G F D
,.¨ 28 N T D H
s
Li- 29 F
i
> 30 N S K P
31 Y V R E T
_ 32 Q Y D S E
cc 33 T P I V
a
0 34 M
i
> 35 W
50 V
51 I
52 G
52a K S A
53 T S N D G Q
54 N G T P
55 E G N K T
56 N T R K
57 I T K V
58 A V T
59 Y
60 A
61 D
62 S
c\J
cc 63 V
a
0 64 K
I
> 65 G
95 E
96 W
97 M
98 D
99 H
100 S
100a R
100b P
100c Y
100d Y
100e Y
100f Y
100g G
co
cc 100h M
a
0 101 D
I
> 102 V
Table 12: Examples of residues at each position within the VH CDRs and Vernier
Residues.
CA 02888322 2015-04-14
WO 2014/060444 98
PCT/EP2013/071567
Kabat Abet0380-
number GL Other example residues
24 S
25 G
26 H
27 N
28 L I
29 E G
30 D
31 K
E 32 F W
a
0 33 A V
¨I 34
> S
50 R
51 D
52 D
53 K
c\J
CC 54 R
a
_1 56 S
>
89 S Q
90 S A
91 Q
92 D
93 T S
94 V T
co
cc 95 T
a
_1 97 V
>
Table 13: Examples of residues at each position within the VL CDRs.
CA 02888322 2015-04-14
WO 2014/060444 99
PCT/EP2013/071567
Kabat
0380-GL Substitutions in other optimised clones
number
26 M G, S, V, A, N, T, H
27 G F, S, Y, E, D, P
¨ 28 N Q, H, V, E, T, A, S, D, M, P
u- 29 F I, Y, S, L, W
i
> 30 N S, T, Q, K, H, R, G, P, E, K, A, D
31 Y H, K, E, N, T, R, V, P, M, F, I, D, W
_ 32 Q Y, D, N, S, E, T
cc 33 T P, I, V, A, I
a
0 34 M L
I
> 35 W
50 V
51 I
52 G
52a K S, P, A, N, G, E, D, V, T
53 T S, N, H, Q, D, G, E
54 N G, P, T, Q, E, M, K, A
55 E G, K, N, Q, T, H, D, A
56 N T, A, R, K
57 I T, N, S, K, F, Q, V, L
58 A V, S, T, N
59 Y
60 A
61 D
62 S A, T
c\J
cc 63 V
a
0 64 K
I
> 65 G
95 E
96 W
97 M
98 D G
99 H R
100 S
100a R
100b P
100c Y
100d Y
100e Y
100f Y
100g G
0,
cc 100h M I
a
0 101 D
i
> 102 V A
Table 14: Substitutions observed in VH CDRs and FW1 in 24 optimised clones
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
100
Kabat
number 0380-GL Substitutions in other optimised clones
24 S T
25 G T
26 H R, P
27 N H
28 L I, V, F, T
29 E M, G, S, N
30 D A, S, G, H
31 K S
E 32 F W
a
0 33 A V, M, T, I
¨ 34 I
> S T, A
50 R
51 D
52 D
53 K
c\J
cc 54 R
a
0 55 P
_i 56 S
>
89 S Q, A
90 S A, T
91 Q
92 D G
93 T 0, S, N, K
94 V T, F
co
cc 95 T
a
0 96 R
_i 97 V S, A
>
Table 15: Substitutions observed in VL CDRs in 24 optimised clones
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
101
Table 16: Correspondence between the antibody sequences mentioned herein and
the
sequences in the Sequence Listing at the end of this document.
1 Abet0007 VH DNA
2 Abet0007 VH PRT
3 Abet0007 CDR1 PRT
4 Abet0007 CDR2 PRT
Abet0007 CDR3 PRT
6 Abet0007 FW1 PRT
7 Abet0007 FW2 PRT
8 Abet0007 FW3 PRT
9 Abet0007 FW4 PRT
Abet0007 VL DNA
11 Abet0007 VL PRT
12 Abet0007 CDR1 PRT
13 Abet0007 CDR2 PRT
14 Abet0007 CDR3 PRT
Abet0007 FW1 PRT
16 Abet0007 FW2 PRT
17 Abet0007 FW3 PRT
18 Abet0007 FW4 PRT
19 Abet0144-GL VH DNA
Abet0144-GL VH PRT
21 Abet0144-GL CDR1 PRT
22 Abet0144-GL CDR2 PRT
23 Abet0144-GL CDR3 PRT
24 Abet0144-GL FW1 PRT
Abet0144-GL FW2 PRT
26 Abet0144-GL FW3 PRT
27 Abet0144-GL FW4 PRT
28 Abet0144-GL VL DNA
29 Abet0144-GL VL PRT
Abet0144-GL CDR1 PRT
31 Abet0144-GL CDR2 PRT
32 Abet0144-GL CDR3 PRT
33 Abet0144-GL FW1 PRT
34 Abet0144-GL FW2 PRT
Abet0144-GL FW3 PRT
36 Abet0144-GL FW4 PRT
37 Abet0319 VH DNA
38 Abet0319 VH PRT
39 Abet0319 CDR1 PRT
Abet0319 CDR2 PRT
41 Abet0319 CDR3 PRT
42 Abet0319 FW1 PRT
CA 02888322 2015-04-19
WO 2014/060444
PCT/EP2013/071567
102
43 Abet0319 FW2 PRT
44 Abet0319 FW3 PRT
45 Abet0319 FW4 PRT
46 Abet0319 VL DNA
47 Abet0319 VL PRT
48 Abet0319 CDR1 PRT
49 Abet0319 CDR2 PRT
50 Abet0319 CDR3 PRT
51 Abet0319 FW1 PRT
52 Abet0319 FW2 PRT
53 Abet0319 FW3 PRT
54 Abet0319 FW4 PRT
55 Abet0321b VH DNA
56 Abet0321b VH PRT
57 Abet0321b CDR1 PRT
58 Abet0321b CDR2 PRT
59 Abet0321b CDR3 PRT
60 Abet0321b FW1 PRT
61 Abet0321b FW2 PRT
62 Abet0321b FW3 PRT
63 Abet0321b FW4 PRT
64 Abet0321b VL DNA
65 Abet0321b VL PRT
66 Abet0321b CDR1 PRT
67 Abet0321b CDR2 PRT
68 Abet0321b CDR3 PRT
69 Abet0321b FW1 PRT
70 Abet0321b FW2 PRT
71 Abet0321b FW3 PRT
72 Abet0321b FW4 PRT
73 Abet0322b VH DNA
74 Abet0322b VH PRT
75 Abet0322b CDR1 PRT
76 Abet0322b CDR2 PRT
77 Abet0322b CDR3 PRT
78 Abet0322b FW1 PRT
79 Abet0322b FW2 PRT
80 Abet0322b FW3 PRT
81 Abet0322b FW4 PRT
82 Abet0322b VL DNA
83 Abet0322b VL PRT
84 Abet0322b CDR1 PRT
85 Abet0322b CDR2 PRT
86 Abet0322b CDR3 PRT
87 Abet0322b FW1 PRT
CA 02888322 2015-04-19
WO 2014/060444
PCT/EP2013/071567
103
88 Abet0322b FW2 PRT
89 Abet0322b FW3 PRT
90 Abet0322b FW4 PRT
91 Abet0323b VH DNA
92 Abet0323b VH PRT
93 Abet0323b CDR1 PRT
94 Abet0323b CDR2 PRT
95 Abet0323b CDR3 PRT
96 Abet0323b FW1 PRT
97 Abet0323b FW2 PRT
98 Abet0323b FW3 PRT
99 Abet0323b FW4 PRT
100 Abet0323b VL DNA
101 Abet0323b VL PRT
102 Abet0323b CDR1 PRT
103 Abet0323b CDR2 PRT
104 Abet0323b CDR3 PRT
105 Abet0323b FW1 PRT
106 Abet0323b FW2 PRT
107 Abet0323b FW3 PRT
108 Abet0323b FW4 PRT
109 Abet0328 VH DNA
110 Abet0328 VH PRT
111 Abet0328 CDR1 PRT
112 Abet0328 CDR2 PRT
113 Abet0328 CDR3 PRT
114 Abet0328 FW1 PRT
115 Abet0328 FW2 PRT
116 Abet0328 FW3 PRT
117 Abet0328 FW4 PRT
118 Abet0328 VL DNA
119 Abet0328 VL PRT
120 Abet0328 CDR1 PRT
121 Abet0328 CDR2 PRT
122 Abet0328 CDR3 PRT
123 Abet0328 FW1 PRT
124 Abet0328 FW2 PRT
125 Abet0328 FW3 PRT
126 Abet0328 FW4 PRT
127 Abet0329 VH DNA
128 Abet0329 VH PRT
129 Abet0329 CDR1 PRT
130 Abet0329 CDR2 PRT
131 Abet0329 CDR3 PRT
132 Abet0329 FW1 PRT
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
104
133 Abet0329 FW2 PRT
134 Abet0329 FW3 PRT
135 Abet0329 FW4 PRT
136 Abet0329 VL DNA
137 Abet0329 VL PRT
138 Abet0329 CDR1 PRT
139 Abet0329 CDR2 PRT
140 Abet0329 CDR3 PRT
141 Abet0329 FW1 PRT
142 Abet0329 FW2 PRT
143 Abet0329 FW3 PRT
144 Abet0329 FW4 PRT
145 Abet0332 VH DNA
146 Abet0332 VH PRT
147 Abet0332 CDR1 PRT
148 Abet0332 CDR2 PRT
149 Abet0332 CDR3 PRT
150 Abet0332 FW1 PRT
151 Abet0332 FW2 PRT
152 Abet0332 FW3 PRT
153 Abet0332 FW4 PRT
154 Abet0332 VL DNA
155 Abet0332 VL PRT
156 Abet0332 CDR1 PRT
157 Abet0332 CDR2 PRT
158 Abet0332 CDR3 PRT
159 Abet0332 FW1 PRT
160 Abet0332 FW2 PRT
161 Abet0332 FW3 PRT
162 Abet0332 FW4 PRT
163 Abet0342 VH DNA
164 Abet0342 VH PRT
165 Abet0342 CDR1 PRT
166 Abet0342 CDR2 PRT
167 Abet0342 CDR3 PRT
168 Abet0342 FW1 PRT
169 Abet0342 FW2 PRT
170 Abet0342 FW3 PRT
171 Abet0342 FW4 PRT
172 Abet0342 VL DNA
173 Abet0342 VL PRT
174 Abet0342 CDR1 PRT
175 Abet0342 CDR2 PRT
176 Abet0342 CDR3 PRT
177 Abet0342 FW1 PRT
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
105
178 Abet0342 FW2 PRT
179 Abet0342 FW3 PRT
180 Abet0342 FW4 PRT
181 Abet0343 VH DNA
182 Abet0343 VH PRT
183 Abet0343 CDR1 PRT
184 Abet0343 CDR2 PRT
185 Abet0343 CDR3 PRT
186 Abet0343 FW1 PRT
187 Abet0343 FW2 PRT
188 Abet0343 FW3 PRT
189 Abet0343 FW4 PRT
190 Abet0343 VL DNA
191 Abet0343 VL PRT
192 Abet0343 CDR1 PRT
193 Abet0343 CDR2 PRT
194 Abet0343 CDR3 PRT
195 Abet0343 FW1 PRT
196 Abet0343 FW2 PRT
197 Abet0343 FW3 PRT
198 Abet0343 FW4 PRT
199 Abet0344 VH DNA
200 Abet0344 VH PRT
201 Abet0344 CDR1 PRT
202 Abet0344 CDR2 PRT
203 Abet0344 CDR3 PRT
204 Abet0344 FW1 PRT
205 Abet0344 FW2 PRT
206 Abet0344 FW3 PRT
207 Abet0344 FW4 PRT
208 Abet0344 VL DNA
209 Abet0344 VL PRT
210 Abet0344 CDR1 PRT
211 Abet0344 CDR2 PRT
212 Abet0344 CDR3 PRT
213 Abet0344 FW1 PRT
214 Abet0344 FW2 PRT
215 Abet0344 FW3 PRT
216 Abet0344 FW4 PRT
217 Abet0368 VH DNA
218 Abet0368 VH PRT
219 Abet0368 CDR1 PRT
220 Abet0368 CDR2 PRT
221 Abet0368 CDR3 PRT
222 Abet0368 FW1 PRT
CA 02888322 2015-04-19
WO 2014/060444
PCT/EP2013/071567
106
223 Abet0368 FW2 PRT
224 Abet0368 FW3 PRT
225 Abet0368 FW4 PRT
226 Abet0368 VL DNA
227 Abet0368 VL PRT
228 Abet0368 CDR1 PRT
229 Abet0368 CDR2 PRT
230 Abet0368 CDR3 PRT
231 Abet0368 FW1 PRT
232 Abet0368 FW2 PRT
233 Abet0368 FW3 PRT
234 Abet0368 FW4 PRT
235 Abet0369 VH DNA
236 Abet0369 VH PRT
237 Abet0369 CDR1 PRT
238 Abet0369 CDR2 PRT
239 Abet0369 CDR3 PRT
240 Abet0369 FW1 PRT
241 Abet0369 FW2 PRT
242 Abet0369 FW3 PRT
243 Abet0369 FW4 PRT
244 Abet0369 VL DNA
245 Abet0369 VL PRT
246 Abet0369 CDR1 PRT
247 Abet0369 CDR2 PRT
248 Abet0369 CDR3 PRT
249 Abet0369 FW1 PRT
250 Abet0369 FW2 PRT
251 Abet0369 FW3 PRT
252 Abet0369 FW4 PRT
253 Abet0370 VH DNA
254 Abet0370 VH PRT
255 Abet0370 CDR1 PRT
256 Abet0370 CDR2 PRT
257 Abet0370 CDR3 PRT
258 Abet0370 FW1 PRT
259 Abet0370 FW2 PRT
260 Abet0370 FW3 PRT
261 Abet0370 FW4 PRT
262 Abet0370 VL DNA
263 Abet0370 VL PRT
264 Abet0370 CDR1 PRT
265 Abet0370 CDR2 PRT
266 Abet0370 CDR3 PRT
267 Abet0370 FW1 PRT
CA 02888322 2015-04-19
WO 2014/060444
PCT/EP2013/071567
107
268 Abet0370 FW2 PRT
269 Abet0370 FW3 PRT
270 Abet0370 FW4 PRT
271 Abet0371 VH DNA
272 Abet0371 VH PRT
273 Abet0371 CDR1 PRT
274 Abet0371 CDR2 PRT
275 Abet0371 CDR3 PRT
276 Abet0371 FW1 PRT
277 Abet0371 FW2 PRT
278 Abet0371 FW3 PRT
279 Abet0371 FW4 PRT
280 Abet0371 VL DNA
281 Abet0371 VL PRT
282 Abet0371 CDR1 PRT
283 Abet0371 CDR2 PRT
284 Abet0371 CDR3 PRT
285 Abet0371 FW1 PRT
286 Abet0371 FW2 PRT
287 Abet0371 FW3 PRT
288 Abet0371 FW4 PRT
289 Abet0372 VH DNA
290 Abet0372 VH PRT
291 Abet0372 CDR1 PRT
292 Abet0372 CDR2 PRT
293 Abet0372 CDR3 PRT
294 Abet0372 FW1 PRT
295 Abet0372 FW2 PRT
296 Abet0372 FW3 PRT
297 Abet0372 FW4 PRT
298 Abet0372 VL DNA
299 Abet0372 VL PRT
300 Abet0372 CDR1 PRT
301 Abet0372 CDR2 PRT
302 Abet0372 CDR3 PRT
303 Abet0372 FW1 PRT
304 Abet0372 FW2 PRT
305 Abet0372 FW3 PRT
306 Abet0372 FW4 PRT
307 Abet0373 VH DNA
308 Abet0373 VH PRT
309 Abet0373 CDR1 PRT
310 Abet0373 CDR2 PRT
311 Abet0373 CDR3 PRT
312 Abet0373 FW1 PRT
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
108
313 Abet0373 FW2 PRT
314 Abet0373 FW3 PRT
315 Abet0373 FW4 PRT
316 Abet0373 VL DNA
317 Abet0373 VL PRT
318 Abet0373 CDR1 PRT
319 Abet0373 CDR2 PRT
320 Abet0373 CDR3 PRT
321 Abet0373 FW1 PRT
322 Abet0373 FW2 PRT
323 Abet0373 FW3 PRT
324 Abet0373 FW4 PRT
325 Abet0374 VH DNA
326 Abet0374 VH PRT
327 Abet0374 CDR1 PRT
328 Abet0374 CDR2 PRT
329 Abet0374 CDR3 PRT
330 Abet0374 FW1 PRT
331 Abet0374 FW2 PRT
332 Abet0374 FW3 PRT
333 Abet0374 FW4 PRT
334 Abet0374 VL DNA
335 Abet0374 VL PRT
336 Abet0374 CDR1 PRT
337 Abet0374 CDR2 PRT
338 Abet0374 CDR3 PRT
339 Abet0374 FW1 PRT
340 Abet0374 FW2 PRT
341 Abet0374 FW3 PRT
342 Abet0374 FW4 PRT
343 Abet0377 VH DNA
344 Abet0377 VH PRT
345 Abet0377 CDR1 PRT
346 Abet0377 CDR2 PRT
347 Abet0377 CDR3 PRT
348 Abet0377 FW1 PRT
349 Abet0377 FW2 PRT
350 Abet0377 FW3 PRT
351 Abet0377 FW4 PRT
352 Abet0377 VL DNA
353 Abet0377 VL PRT
354 Abet0377 CDR1 PRT
355 Abet0377 CDR2 PRT
356 Abet0377 CDR3 PRT
357 Abet0377 FW1 PRT
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
109
358 Abet0377 FW2 PRT
359 Abet0377 FW3 PRT
360 Abet0377 FW4 PRT
361 Abet0378 VH DNA
362 Abet0378 VH PRT
363 Abet0378 CDR1 PRT
364 Abet0378 CDR2 PRT
365 Abet0378 CDR3 PRT
366 Abet0378 FW1 PRT
367 Abet0378 FW2 PRT
368 Abet0378 FW3 PRT
369 Abet0378 FW4 PRT
370 Abet0378 VL DNA
371 Abet0378 VL PRT
372 Abet0378 CDR1 PRT
373 Abet0378 CDR2 PRT
374 Abet0378 CDR3 PRT
375 Abet0378 FW1 PRT
376 Abet0378 FW2 PRT
377 Abet0378 FW3 PRT
378 Abet0378 FW4 PRT
379 Abet0379 VH DNA
380 Abet0379 VH PRT
381 Abet0379 CDR1 PRT
382 Abet0379 CDR2 PRT
383 Abet0379 CDR3 PRT
384 Abet0379 FW1 PRT
385 Abet0379 FW2 PRT
386 Abet0379 FW3 PRT
387 Abet0379 FW4 PRT
388 Abet0379 VL DNA
389 Abet0379 VL PRT
390 Abet0379 CDR1 PRT
391 Abet0379 CDR2 PRT
392 Abet0379 CDR3 PRT
393 Abet0379 FW1 PRT
394 Abet0379 FW2 PRT
395 Abet0379 FW3 PRT
396 Abet0379 FW4 PRT
397 Abet0380 VH DNA
398 Abet0380 VH PRT
399 Abet0380 CDR1 PRT
400 Abet0380 CDR2 PRT
401 Abet0380 CDR3 PRT
402 Abet0380 FW1 PRT
CA 02888322 2015-04-19
WO 2014/060444
PCT/EP2013/071567
110
403 Abet0380 FW2 PRT
404 Abet0380 FW3 PRT
405 Abet0380 FW4 PRT
406 Abet0380 VL DNA
407 Abet0380 VL PRT
408 Abet0380 CDR1 PRT
409 Abet0380 CDR2 PRT
410 Abet0380 CDR3 PRT
411 Abet0380 FW1 PRT
412 Abet0380 FW2 PRT
413 Abet0380 FW3 PRT
414 Abet0380 FW4 PRT
415 Abet0381 VH DNA
416 Abet0381 VH PRT
417 Abet0381 CDR1 PRT
418 Abet0381 CDR2 PRT
419 Abet0381 CDR3 PRT
420 Abet0381 FW1 PRT
421 Abet0381 FW2 PRT
422 Abet0381 FW3 PRT
423 Abet0381 FW4 PRT
424 Abet0381 VL DNA
425 Abet0381 VL PRT
426 Abet0381 CDR1 PRT
427 Abet0381 CDR2 PRT
428 Abet0381 CDR3 PRT
429 Abet0381 FW1 PRT
430 Abet0381 FW2 PRT
431 Abet0381 FW3 PRT
432 Abet0381 FW4 PRT
433 Abet0382 VH DNA
434 Abet0382 VH PRT
435 Abet0382 CDR1 PRT
436 Abet0382 CDR2 PRT
437 Abet0382 CDR3 PRT
438 Abet0382 FW1 PRT
439 Abet0382 FW2 PRT
440 Abet0382 FW3 PRT
441 Abet0382 FW4 PRT
442 Abet0382 VL DNA
443 Abet0382 VL PRT
444 Abet0382 CDR1 PRT
445 Abet0382 CDR2 PRT
446 Abet0382 CDR3 PRT
447 Abet0382 FW1 PRT
CA 02888322 2015-04-19
WO 2014/060444
PCT/EP2013/071567
111
448 Abet0382 FW2 PRT
449 Abet0382 FW3 PRT
450 Abet0382 FW4 PRT
451 Abet0383 VH DNA
452 Abet0383 VH PRT
453 Abet0383 CDR1 PRT
454 Abet0383 CDR2 PRT
455 Abet0383 CDR3 PRT
456 Abet0383 FW1 PRT
457 Abet0383 FW2 PRT
458 Abet0383 FW3 PRT
459 Abet0383 FW4 PRT
460 Abet0383 VL DNA
461 Abet0383 VL PRT
462 Abet0383 CDR1 PRT
463 Abet0383 CDR2 PRT
464 Abet0383 CDR3 PRT
465 Abet0383 FW1 PRT
466 Abet0383 FW2 PRT
467 Abet0383 FW3 PRT
468 Abet0383 FW4 PRT
469 Abet0343-GL VH DNA
470 Abet0343-GL VH PRT
471 Abet0343-GL CDR1 PRT
472 Abet0343-GL CDR2 PRT
473 Abet0343-GL CDR3 PRT
474 Abet0343-GL FW1 PRT
475 Abet0343-GL FW2 PRT
476 Abet0343-GL FW3 PRT
477 Abet0343-GL FW4 PRT
478 Abet0343-GL VL DNA
479 Abet0343-GL VL PRT
480 Abet0343-GL CDR1 PRT
481 Abet0343-GL CDR2 PRT
482 Abet0343-GL CDR3 PRT
483 Abet0343-GL FW1 PRT
484 Abet0343-GL FW2 PRT
485 Abet0343-GL FW3 PRT
486 Abet0343-GL FW4 PRT
487 Abet0369-GL VH DNA
488 Abet0369-GL VH PRT
489 Abet0369-GL CDR1 PRT
490 Abet0369-GL CDR2 PRT
491 Abet0369-GL CDR3 PRT
492 Abet0369-GL FW1 PRT
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
112
493 Abet0369-GL FW2 PRT
494 Abet0369-GL FW3 PRT
495 Abet0369-GL FW4 PRT
496 Abet0369-GL VL DNA
497 Abet0369-GL VL PRT
498 Abet0369-GL CDR1 PRT
499 Abet0369-GL CDR2 PRT
500 Abet0369-GL CDR3 PRT
501 Abet0369-GL FW1 PRT
502 Abet0369-GL FW2 PRT
503 Abet0369-GL FW3 PRT
504 Abet0369-GL FW4 PRT
505 Abet0377-GL VH DNA
506 Abet0377-GL VH PRT
507 Abet0377-GL CDR1 PRT
508 Abet0377-GL CDR2 PRT
509 Abet0377-GL CDR3 PRT
510 Abet0377-GL FW1 PRT
511 Abet0377-GL FW2 PRT
512 Abet0377-GL FW3 PRT
513 Abet0377-GL FW4 PRT
514 Abet0377-GL VL DNA
515 Abet0377-GL VL PRT
516 Abet0377-GL CDR1 PRT
517 Abet0377-GL CDR2 PRT
518 Abet0377-GL CDR3 PRT
519 Abet0377-GL FW1 PRT
520 Abet0377-GL FW2 PRT
521 Abet0377-GL FW3 PRT
522 Abet0377-GL FW4 PRT
523 Abet0380-GL VH DNA
524 Abet0380-GL VH PRT
525 Abet0380-GL CDR1 PRT
526 Abet0380-GL CDR2 PRT
527 Abet0380-GL CDR3 PRT
528 Abet0380-GL FW1 PRT
529 Abet0380-GL FW2 PRT
530 Abet0380-GL FW3 PRT
531 Abet0380-GL FW4 PRT
532 Abet0380-GL VL DNA
533 Abet0380-GL VL PRT
534 Abet0380-GL CDR1 PRT
535 Abet0380-GL CDR2 PRT
536 Abet0380-GL CDR3 PRT
537 Abet0380-GL FW1 PRT
CA 02888322 2015-04-14
WO 2014/060444 PCT/EP2013/071567
113
538 Abet0380-GL FW2 PRT
539 Abet0380-GL FW3 PRT
540 Abet0380-GL FW4 PRT
541 Abet0382-GL VH DNA
542 Abet0382-GL VH PRT
543 Abet0382-GL CDR1 PRT
544 Abet0382-GL CDR2 PRT
545 Abet0382-GL CDR3 PRT
546 Abet0382-GL FW1 PRT
547 Abet0382-GL FW2 PRT
548 Abet0382-GL FW3 PRT
549 Abet0382-GL FW4 PRT
550 Abet0382-GL VL DNA
551 Abet0382-GL VL PRT
552 Abet0382-GL CDR1 PRT
553 Abet0382-GL CDR2 PRT
554 Abet0382-GL CDR3 PRT
555 Abet0382-GL FW1 PRT
556 Abet0382-GL FW2 PRT
557 Abet0382-GL FW3 PRT
558 Abet0382-GL FW4 PRT
Example 6: Specificity of Abet0380-GL IgGl-TM in competition binding
experiments
The specificity of Abet0380-GL IgG1-TM was examined in competition binding
experiments. In
brief Abet0380-GL IgG1-TM (0.5nM) was incubated (1hr at room temperature) with
a range of
different concentrations (10uM down to 0.17nM) of a panel of full length,
truncate and pyro
human Abeta peptides (Abeta 1-42, Abeta 1-43, Abeta 1-16, Abeta 12-28, Abeta
17-42, Abeta
pyro-3-42, or Abeta pyro-11-42).
Following the incubation between Abet0380-GL IgG1-TM and the Abeta peptides N-
terminal
biotin Abeta 1-42 (1.5nM) was added followed by a europium cryptate labelled
anti-human Fe
antibody (0.8nM) (CisBio Cat. No. 61HFCKLB) and streptavidin-XLe" (5nM)
(CisBio Cat. No.
611SAXLB). The assay was then incubated for a further 2hrs at room temperature
before
reading on an Envision plate reader (PerkinElmer) using a standard homogeneous
time
resolved fluorescence (HTRF) read protocol. In the absence of competition, the
interaction of N-
terminal biotin Abeta 1-42 with Abet0380-GL IgG1-TM (in complex with
streptavidin-XLe" and
and europium cryptate labelled anti-human Fc antibody, respectively) could
then be measured
via time resolved fluorescence resonance energy transfer (TR-FRET) due to the
proximity of the
europium cryptate donor and XL665 acceptor fluorophores. Competition of the
Abet0380-GL
CA 02888322 2015-04-14
WO 2014/060444 PCT/EP2013/071567
114
IgG1-TM: N-terminal biotin Abeta 1-42 interaction by test peptides therefore
resulted in a
reduction in assay signal. Results were expressed as % specific binding where
100% specific
binding was derived from wells containing streptavidin-XLenu (5nM), N-terminal
biotin Abeta 1-42
(1.5nM), Abet0380-GL IgG1-TM (0.5nM) & europium crptate labelled anti-human Fc
antibody
(0.8nM). 0% specific binding was derived from wells in which Abet0380-GL IgG1-
TM had been
omitted.
The final assay volume was 20 1 and all reagents were prepared in an assay
buffer comprising
MOPS pH7.4 (50mM), potassium fluoride (0.4M), tween 20 (0.1%) & fatty acid
free BSA (0.1%).
The assay was performed in low volume 384 well black assay plates (Costar
3676).
In summary, inhibition of Abet0380-GL IgG1-TM: N-terminal Biotin Abeta 1-42
binding was
observed with Abeta 1-42, Abeta 1-43, Abeta 17-42, Abeta Pyro-3-42 & Abeta
Pyro-11-42 with
IC50 values ranging from 10-8 to 10-9 molar for this group. No inhibition of
Abet0380-GL IgG1-
TM: N-terminal Biotin Abeta 1-42 binding was observed with Abeta 1-16 or Abeta
12-28 (Figure
18).
Example 7: Ability of antibody Abet0144-GL to sequester amyloid beta 1-42 in a
normal rat PK-
PD study
The ability of antibody Abet0144-GL to sequester amyloid beta 1-42 was
investigated in a PK-
PD study in normal rats. Rats were intravenously administered Abet0144-GL (10
or 40 mg/kg)
or vehicle weekly for 2 weeks (on days 0 and 7), and sacrificed a week after
the 2nd dose. CSF
was sampled for free and total amyloid beta 1-42, and brain was sampled for
total amyloid beta
1-42 measurement. Free and total amyloid beta 1-42 levels were measured using
assays
described above.
As shown in Figure 19, free amyloid beta 1-42 in CSF was not significantly
altered by either 10
or 40 mg/kg of Abet0144-GL (5 and 18% increase, respectively when compared
with vehicle;
Figure 19). Total amyloid beta 1-42 in CSF was significantly increased by 38%
at 10 mg/kg,
and by 139% at 40 mg/kg. Total amyloid beta 1-42 in brain tissue was also
significantly
increased, by 16% and 50% at 10 and 40 mg/kg, respectively. In summary, data
from this study
in normal rats, demonstrated that Abet0144-GL had no significant effect on
free amyloid beta 1-
42 levels in CSF, whilst increasing total amyloid beta 1-42 levels in both CSF
and brain. This
was the profile that would be expected from an antibody with an affinity for
target in the tens of
nM range.
CA 02888322 2015-04-14
WO 2014/060444 PCT/EP2013/071567
115
References
Bannister, D., Wilson, A., Prowse, L., Walsh, M., Ho!gate, R., Jermutus, L.
and Wilkinson, T.
(2006). Parallel, high-throughput purification of recombinant antibodies for
in vivo cell
assays. BiotechnoL Bioeng. 94, 931-937.
Bard, F., Cannon, C., Barbour, R., Burke, R. L., Games, D., Grajeda, H.,
Guido, T., Hu, K.,
Huang, J., Johnson-Wood, K., Khan, K., Kholodenko, D., Lee, M., Lieberburg,
I., Motter,
R., Nguyen, M., Soriano, F., Vasquez, N., Weiss, K., Welch, B., Seubert, P.,
Schenk, D.
and Yednock, T. (2000). Peripherally administered antibodies against amyloid
beta-
peptide enter the central nervous system and reduce pathology in a mouse model
of
Alzheimer disease. Nat. Med. 6,916-919.
Borchelt, D. R., Thinakaran, G., Eckman, C. B., Lee, M. K., Davenport, F.,
Ratovitsky, T., Prada,
C. M., Kim, G., Seekins, S., Yager, D., Slunt, H. H., Wang, R., Seeger, M.,
Levey, A. I.,
Gandy, S. E., Copeland, N. G., Jenkins, N. A., Price, D. L., Younkin, S. G.
and Sisodia,
S. S. (1996). Familial Alzheimer's disease-linked presenilin 1 variants
elevate Abeta1-
42/1-40 ratio in vitro and in vivo. Neuron 17, 1005-1013.
Citron, M., Eckman, C. B., Diehl, T. S., Corcoran, C., Ostaszewski, B. L.,
Xia, W., Levesque, G.,
St George Hyslop, P., Younkin, S. G. and Selkoe, D. J. (1998). Additive
effects of PS1
and APP mutations on secretion of the 42-residue amyloid beta-protein.
NeurobioL Dis.
5, 107-116.
Clackson, T. and Lowman, H. B. (2004). Phage display: a practical approach,
Oxford University
Press.
De Strooper, B. (2007). Loss-of-function presenilin mutations in Alzheimer
disease. Talking
Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 8,
141-146.
DeMattos, R. B., Bales, K. R., Cummins, D. J., Dodart, J. C., Paul, S. M. and
Holtzman, D. M.
(2001). Peripheral anti-A beta antibody alters CNS and plasma A beta clearance
and
decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc.
Natl.
Acad. ScL USA 98, 8850-8855.
Duff, K., Eckman, C., Zehr, C., Yu, X., Prada, C. M., Perez-tur, J., Hutton,
M., Buee, L.,
Harigaya, Y., Yager, D., Morgan, D., Gordon, M. N., Holcomb, L., Refolo, L.,
Zenk, B.,
Hardy, J. and Younkin, S. (1996). Increased amyloid-beta42(43) in brains of
mice
expressing mutant presenilin 1. Nature 383, 710-713.
Foote, J. and Winter, G. (1992). Antibody framework residues affecting the
conformation of the
hypervariable loops. J. MoL Biol. 224, 487-499.
Gilman, S., Koller, M., Black, R. S., Jenkins, L., Griffith, S. G., Fox, N.
C., Eisner, L., Kirby, L.,
Rovira, M. B., Forette, F. and Orgogozo, J. M. (2005). Clinical effects of
Abeta
CA 02888322 2015-04-14
WO 2014/060444 PCT/EP2013/071567
116
immunization (AN1792) in patients with AD in an interrupted trial. Neurology
64, 1553-
1562.
Glabe, C. (2000). Does Alzheimer disease tilt the scales of amyloid
degradation versus
accumulation? Nat. Med. 6, 133-134.
GoIde, T. E., Das, P. and Levites, Y. (2009). Quantitativeand Mechanistic
Studies of Ab
lmmunotherapy. CNS & Neuro. Dis. - Drug Targets 8, 31-49
Greeve, I., Kretzschmar, D., Tschape, J. A., Beyn, A., Bre!linger, C.,
Schweizer, M., Nitsch, R.
M. and Reifegerste, R. (2004). Age-dependent neurodegeneration and Alzheimer-
amyloid plaque formation in transgenic Drosophila. J. NeuroscL 24, 3899-3906.
Groves, M. A. and Osbourn, J. K. (2005). Applications of ribosome display to
antibody drug
discovery. Expert Opin. Biol. Ther. 5, 125-135.
Hanes, J., Jermutus, L. and Pluckthun, A. (2000). Selecting and evolving
functional proteins in
vitro by ribosome display. Methods EnzymoL 328, 404-430.
Hanes, J. and Pluckthun, A. (1997). In vitro selection and evolution of
functional proteins by
using ribosome display. Proc. NatL Acad. ScL USA 94, 4937-4942.
Hawkins, R. E., Russell, S. J. and Winter, G. (1992). Selection of phage
antibodies by binding
affinity. Mimicking affinity maturation. J. MoL Biol. 226, 889-896.
Hoet, R. M., Cohen, E. H., Kent, R. B., Rookey, K., Schoonbroodt, S., Hogan,
S., Rem, L.,
Frans, N., Daukandt, M., Pieters, H., van Hegelsom, R., Neer, N. C., Nastri,
H. G.,
Rondon, I. J., Leeds, J. A., Hufton, S. E., Huang, L., Kashin, I., Devlin, M.,
Kuang, G.,
Steukers, M., Viswanathan, M., Nixon, A. E., Sexton, D. J., Hoogenboom, H. R.
and
Ladner, R. C. (2005). Generation of high-affinity human antibodies by
combining donor-
derived and synthetic complementarity-determining-region diversity. Nat.
BiotechnoL 23,
344-348.
lijima, K., Liu, H. P., Chiang, A. S., Hearn, S. A., Konsolaki, M. and Zhong,
Y. (2004). Dissecting
the pathological effects of human Abeta40 and Abeta42 in Drosophila: a
potential model
for Alzheimer's disease. Proc. NatL Acad. ScL USA 101, 6623-6628.
Karlsson, R., Michaelsson, A. and Mattsson, L. (1991). Kinetic analysis of
monoclonal antibody-
antigen interactions with a new biosensor based analytical system. J. Immunol
Methods
145, 229-240.
Kuperstein, I., Broersen, K., Benilova, I., Rozenski, J., Jonckheere, W.,
Debulpaep, M.,
Vandersteen, A., Segers-Nolten, I., Van Der Werf, K., Subramaniam, V.,
Braeken, D.,
Callewaert, G., Bartic, C., D'Hooge, R., Martins, I. C., Rousseau, F.,
Schymkowitz, J.
and De Strooper, B. (2010). Neurotoxicity of Alzheimer's disease Abeta
peptides is
induced by small changes in the Abeta42 to Abeta40 ratio. EMBO J. 29, 3408-
3420.
Lambert, M. P., Barlow, A. K., Chromy, B. A., Edwards, C., Freed, R.,
Liosatos, M., Morgan, T.
E., Rozovsky, I., Trommer, B., Viola, K. L., Wals, P., Zhang, C., Finch, C.
E., Krafft, G. A.
CA 02888322 2015-04-14
WO 2014/060444 PCT/EP2013/071567
117
and Klein, W. L. (1998). Diffusible, nonfibrillar ligands derived from Abeta1-
42 are potent
central nervous system neurotoxins. Proc. Natl. Acad. ScL USA 95, 6448-6453.
Levites, Y., Das, P., Price, R. W., Rochette, M. J., Kostura, L. A., McGowan,
E. M., Murphy, M.
P. and GoIde, T. E. (2006). Anti-Abeta42- and anti-Abeta40-specific mAbs
attenuate
amyloid deposition in an Alzheimer disease mouse model. J. Clin. Invest. 116,
193-201.
Matsuoka, Y., Saito, M., LaFrancois, J., Saito, M., Gaynor, K., Olm, V., Wang,
L., Casey, E., Lu,
Y., Shiratori, C., Lemere, C. and Duff, K. (2003). Novel therapeutic approach
for the
treatment of Alzheimer's disease by peripheral administration of agents with
an affinity to
beta-amyloid. J. NeuroscL 23, 29-33.
McCafferty, J., Fitzgerald, K. J., Earnshaw, J., Chiswell, D. J., Link, J.,
Smith, R. and Kenten, J.
(1994). Selection and rapid purification of murine antibody fragments that
bind a
transition-state analog by phage display. App!. Biochem. BiotechnoL 47, 157-
171;
discussion 171-153.
McGowan, E., Pickford, F., Kim, J., Onstead, L., Eriksen, J., Yu, C., Skipper,
L., Murphy, M. P.,
Beard, J., Das, P., Jansen, K., Delucia, M., Lin, W. L., Dolios, G., Wang, R.,
Eckman, C.
B., Dickson, D. W., Hutton, M., Hardy, J. and Golde, T. (2005). Abeta42 is
essential for
parenchymal and vascular amyloid deposition in mice. Neuron 47, 191-199.
Mucke, L., Masliah, E., Yu, G. Q., Mallory, M., Rockenstein, E. M., Tatsuno,
G., Hu, K.,
Kholodenko, D., Johnson-Wood, K. and McConlogue, L. (2000). High-level
neuronal
expression of abeta 1-42 in wild-type human amyloid protein precursor
transgenic mice:
synaptotoxicity without plaque formation. J. NeuroscL 20, 4050-4058.
Oganesyan, V., Gao, C., Shirinian, L., Wu, H. and Dall'Acqua, W. F. (2008).
Structural
characterization of a human Fc fragment engineered for lack of effector
functions. Acta
Crystallogr. D BioL Crystallogr. 64, 700-704.
Orgogozo, J. M., Gilman, S., Dartigues, J. F., Laurent, B., Puel, M., Kirby,
L. C., Jouanny, P.,
Dubois, B., Eisner, L., Flitman, S., Michel, B. F., Boada, M., Frank, A. and
Hock, C.
(2003). Subacute meningoencephalitis in a subset of patients with AD after
Abeta42
immunization. Neurology 61, 46-54.
Osbourn, J. K., Field, A., Wilton, J., Derbyshire, E., Earnshaw, J. C., Jones,
P. T., Allen, D. and
McCafferty, J. (1996). Generation of a panel of related human scFv antibodies
with high
affinities for human CEA. Immunotechnology 2, 181-196.
Persic, L., Roberts, A., Wilton, J., Cattaneo, A., Bradbury, A. and
Hoogenboom, H. R. (1997).
An integrated vector system for the eukaryotic expression of antibodies or
their
fragments after selection from phage display libraries. Gene 187, 9-18.
Portelius, E., Bogdanovic, N., Gustavsson, M. K., Volkmann, I., Brinkmalm, G.,
Zetterberg, H.,
Winblad, B. and Blennow, K. (2010). Mass spectrometric characterization of
brain
CA 02888322 2015-04-14
WO 2014/060444 PCT/EP2013/071567
118
amyloid beta isoform signatures in familial and sporadic Alzheimer's disease.
Acta
NeuropathoL 120, 185-193.
Pride, M., Seubert, P., Grundman, M., Hagen, M., Eldridge, J. and Black, R. S.
(2008). Progress
in the active immunotherapeutic approach to Alzheimer's disease: clinical
investigations
into AN1792-associated meningoencephalitis. Neurodegener. Dis. 5, 194-196.
Schenk, D., Barbour, R., Dunn, W., Gordon, G., Grajeda, H., Guido, T., Hu, K.,
Huang, J.,
Johnson-Wood, K., Khan, K., Kholodenko, D., Lee, M., Liao, Z., Lieberburg, I.,
Motter, R.,
Mutter, L., Soriano, F., Shopp, G., Vasquez, N., Vandevert, C., Walker, S.,
Wogulis, M.,
Yednock, T., Games, D. and Seubert, P. (1999). Immunization with amyloid-beta
attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400,
173-177.
Schenk, D. B., Seubert, P., Lieberburg, I. and Wallace, J. (2000). beta-
peptide immunization: a
possible new treatment for Alzheimer disease. Arch. NeuroL 57, 934-936.
Scheuner, D., Eckman, C., Jensen, M., Song, X., Citron, M., Suzuki, N., Bird,
T. D., Hardy, J.,
Hutton, M., Kukull, W., Larson, E., Levy-Lahad, E., Viitanen, M., Peskind, E.,
Poorkaj, P.,
Schellenberg, G., Tanzi, R., Wasco, W., Lannfelt, L., Selkoe, D. and Younkin,
S. (1996).
Secreted amyloid beta-protein similar to that in the senile plaques of
Alzheimer's disease
is increased in vivo by the presenilin 1 and 2 and APP mutations linked to
familial
Alzheimer's disease. Nat. Med. 2, 864-870.
Schier, R., Bye, J., ApeII, G., McCall, A., Adams, G. P., Malmqvist, M.,
Weiner, L. M. and Marks,
J. D. (1996). Isolation of high-affinity monomeric human anti-c-erbB-2 single
chain Fv
using affinity-driven selection. J. MoL Biol. 255, 28-43.
Selkoe, D. J. (1999). Translating cell biology into therapeutic advances in
Alzheimer's disease.
Nature 399, A23-31.
Thompson, J., Pope, T., Tung, J. S., Chan, C., Hollis, G., Mark, G. and
Johnson, K. S. (1996).
Affinity maturation of a high-affinity human monoclonal antibody against the
third
hypervariable loop of human immunodeficiency virus: use of phage display to
improve
affinity and broaden strain reactivity. J. MoL BioL 256, 77-88.
Tomlinson, I. M., Walter, G., Marks, J. D., Llewelyn, M. B. and Winter, G.
(1992). The repertoire
of human germline VH sequences reveals about fifty groups of VH segments with
different hypervariable loops. J. MoL BioL 227, 776-798.
Vassar, R., Bennett, B. D., Babu-Khan, S., Kahn, S., Mendiaz, E. A., Denis,
P., Teplow, D. B.,
Ross, S., Amarante, P., Loeloff, R., Luo, Y., Fisher, S., Fuller, J., Edenson,
S., Lile, J.,
Jarosinski, M. A., Biere, A. L., Curran, E., Burgess, T., Louis, J. C.,
Collins, F., Treanor,
J., Rogers, G. and Citron, M. (1999). Beta-secretase cleavage of Alzheimer's
amyloid
precursor protein by the transmembrane aspartic protease BACE. Science 286,
735-741.
Vaughan, T. J., Williams, A. J., Pritchard, K., Osbourn, J. K., Pope, A. R.,
Earnshaw, J. C.,
McCafferty, J., Hodits, R. A., Wilton, J. and Johnson, K. S. (1996). Human
antibodies
CA 02888322 2015-04-14
WO 2014/060444 PCT/EP2013/071567
119
with sub-nanomolar affinities isolated from a large non-immunized phage
display library.
Nat. Biotechnol. 14, 309-314.
Walsh, D. M., Klyubin, I., Fadeeva, J. V., Cullen, W. K., Anwyl, R., Wolfe, M.
S., Rowan, M. J.
and Selkoe, D. J. (2002). Naturally secreted oligomers of amyloid beta protein
potently
inhibit hippocampal long-term potentiation in vivo. Nature 416, 535-539.
Walsh, D. M., Klyubin, I., Shankar, G. M., Townsend, M., Fadeeva, J. V.,
Betts, V., Podlisny, M.
B., Cleary, J. P., Ashe, K. H., Rowan, M. J. and Selkoe, D. J. (2005a). The
role of cell-
derived oligomers of Abeta in Alzheimer's disease and avenues for therapeutic
intervention. Biochem. Soc. Trans. 33, 1087-1090.
Walsh, D. M., Townsend, M., Podlisny, M. B., Shankar, G. M., Fadeeva, J. V.,
El Agnaf, 0.,
Hartley, D. M. and Selkoe, D. J. (2005b). Certain inhibitors of synthetic
amyloid beta-
peptide (Abeta) fibrillogenesis block oligomerization of natural Abeta and
thereby rescue
long-term potentiation. J. NeuroscL 25, 2455-2462.
Wang, H. W., Pasternak, J. F., Kuo, H., Ristic, H., Lambert, M. P., Chromy,
B., Viola, K. L., Klein,
W. L., Stine, W. B., Krafft, G. A. and Trommer, B. L. (2002). Soluble
oligomers of beta
amyloid (1-42) inhibit long-term potentiation but not long-term depression in
rat dentate
gyrus. Brain Res. 924, 133-140.
Weller, R. 0. and Nicoll, J. A. (2003). Cerebral amyloid angiopathy:
pathogenesis and effects on
the ageing and Alzheimer brain. NeuroL Res. 25, 611-616.
Wilcock, D. M., Alamed, J., Gottschall, P. E., Grimm, J., Rosenthal, A., Pons,
J., Ronan, V.,
Symmonds, K., Gordon, M. N. and Morgan, D. (2006). Deglycosylated anti-amyloid-
beta
antibodies eliminate cognitive deficits and reduce parenchymal amyloid with
minimal
vascular consequences in aged amyloid precursor protein transgenic mice. J.
NeuroscL
26, 5340-5346.
Wilcock, D. M. and Colton, C. A. (2009). lmmunotherapy, vascular pathology,
and
microhemorrhages in transgenic mice. CNS NeuroL Disord. Drug. Targets. 8, 50-
64.
Younkin, S. G. (1995). Evidence that A beta 42 is the real culprit in
Alzheimer's disease. Ann.
NeuroL 37, 287-288.
Younkin, S. G. (1998). The role of A beta 42 in Alzheimer's disease. J.
PhysioL Paris 92, 289-
292.
Other references are included in the text.
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
120
<110> MedImmune Limited
<120> Antibodies to Amyloid Beta
<160> 558
<170> Medimmune Ltd patent software March 2010 release. Output verified by
USPTO Checker
version 4.4Ø
<210> 1
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0007
<400> 1
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctggatt cacctttagc gtttatacta tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggttcta gtggtggtac gacagtttac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagaaggg 300
cagcagctgg tacgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 2
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0007
<400> 2
Glu Val Gln Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Val Tyr
20 25 30
Thr Met Trp Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Ser Ser Gly Gly Thr Thr Val Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Gly Gln Gln Leu Val Arg Pro Tyr Tyr Tyr Tyr Gly Net
100 105 110
Asp Val Trp Gly Gln Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 3
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0007
<400> 3
Val Tyr Thr Met Trp
5
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
121
<210> 4
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0007
<400> 4
Val Ile Gly Ser Ser Gly Gly Thr Thr Val Tyr Ala Asp Ser Val Lys
10 15
Gly
<210> 5
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0007
<400> 5
Glu Gly Gin Gin Leu Val Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 6
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0007
<400> 6
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser
20 25 30
<210> 7
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0007
<400> 7
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 8
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0007
<400> 8
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 9
<211> 11
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
122
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0007
<400> 9
Trp Gly Gln Gly Thr Leu Val Thr Val Ser Ser
10
<210> 10
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0007
<400> 10
cagagcgtct tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt ggaagataaa tttgcttcct ggtatcaaca gaagtcaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg tcaggcgcag gacagtacca ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 11
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0007
<400> 11
Gln Ser Val Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
5 10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gln Gln Lys Ser Gly Gln Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Gln Ala Gln Asp Ser Thr Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 12
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0007
<400> 12
Ser Gly His Asn Leu Glu Asp Lys Phe Ala Ser
5 10
<210> 13
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
123
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0007
<400> 13
Arg Asp Asp Lys Arg Pro Ser
<210> 14
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0007
<400> 14
Gin Ala Gin Asp Ser Thr Thr Arg Val
5
<210> 15
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0007
<400> 15
Gin Ser Val Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 16
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0007
<400> 16
Trp Tyr Gin Gin Lys Ser Gly Gin Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 17
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0007
<400> 17
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gin Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 18
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0007
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
124
<400> 18
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
10
<210> 19
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0144-GL
<400> 19
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctggatt cacctttagc gtttatacta tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggttcta gtggtggtac gacagtttac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 20
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0144-GL
<400> 20
Glu Val Gln Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Val Tyr
20 25 30
Thr Met Trp Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Ser Ser Gly Gly Thr Thr Val Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gln Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 21
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0144-GL
<400> 21
Val Tyr Thr Met Trp
5
<210> 22
<211> 17
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0144-GL
<400> 22
Val Ile Gly Ser Ser Gly Gly Thr Thr Val Tyr Ala Asp Ser Val Lys
10 15
Gly
<210> 23
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0144-GL
<400> 23
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 24
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0144-GL
<400> 24
Glu Val Gln Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser
20 25 30
<210> 25
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0144-GL
<400> 25
Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 26
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0144-GL
<400> 26
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gln
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 27
<211> 11
<212> PRT
<213> Homo sapiens
<220>
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
126
<223> Abet0144-GL
<400> 27
Trp Gly Gln Gly Thr Leu Val Thr Val Ser Ser
10
<210> 28
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0144-GL
<400> 28
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt ggaagataaa tttgcttcct ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctatg 240
gatgaggctg actattactg tcaggcgcag gacagtacca ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 29
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0144-GL
<400> 29
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
5 10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Met
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Gln Ala Gln Asp Ser Thr Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 30
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0144-GL
<400> 30
Ser Gly His Asn Leu Glu Asp Lys Phe Ala Ser
5 10
<210> 31
<211> 7
<212> PRT
<213> Homo sapiens
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
127
<220>
<223> Abet0144-GL
<400> 31
Arg Asp Asp Lys Arg Pro Ser
<210> 32
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0144-GL
<400> 32
Gln Ala Gln Asp Ser Thr Thr Arg Val
5
<210> 33
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0144-GL
<400> 33
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 34
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0144-GL
<400> 34
Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 35
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0144-GL
<400> 35
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gln Ala Met Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 36
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0144-GL
<400> 36
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
128
<210> 37
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0319
<400> 37
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcgg cctctgtctc cgtgtacaac aaggacacta tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggttcta gtggtggcac gacagtctac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 38
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0319
<400> 38
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Val Ser Val Tyr Asn Lys Asp
20 25 30
Thr Met Trp Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Ser Ser Gly Gly Thr Thr Val Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 39
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0319
<400> 39
Lys Asp Thr Met Trp
5
<210> 40
<211> 17
<212> PRT
<213> Homo sapiens
<220>
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
129
<223> Abet0319
<400> 40
Val Ile Gly Ser Ser Gly Gly Thr Thr Val Tyr Ala Asp Ser Val Lys
10 15
Gly
<210> 41
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0319
<400> 41
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 42
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0319
<400> 42
Glu Val Gln Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Val Ser Val Tyr Asn
20 25 30
<210> 43
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0319
<400> 43
Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 44
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0319
<400> 44
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gln
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 45
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0319
<400> 45
Trp Gly Gln Gly Thr Leu Val Thr Val Ser Ser
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
130
<210> 46
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0319
<400> 46
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataacat catggacaag tgggtctctt ggtatcaaca gaagccaggc 120
cggtcccctg ccctggtaat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 47
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0319
<400> 47
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Ile Net Asp Lys Trp Val
25 30
Ser Trp Tyr Gin Gin Lys Pro Gly Arg Ser Pro Ala Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gin Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gin Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 48
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0319
<400> 48
Ser Gly His Asn Ile Met Asp Lys Trp Val Ser
5 10
<210> 49
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0319
<400> 49
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
131
Arg Asp Asp Lys Arg Pro Ser
<210> 50
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0319
<400> 50
Ser Ser Gin Asp Thr Val Thr Arg Val
5
<210> 51
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0319
<400> 51
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 52
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0319
<400> 52
Trp Tyr Gin Gin Lys Pro Gly Arg Ser Pro Ala Leu Val Ile Tyr
5 10 15
<210> 53
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0319
<400> 53
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gln Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 54
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0319
<400> 54
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 55
<211> 375
<212> DNA
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
132
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 55
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctgcgta ccactcgaac cacgacccta tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggttcta gtggtggtac gacagcttac 180
gcagactccg tgaagggccg gttcaccatc tccagagata attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 56
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 56
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Ala Tyr His Ser Asn His Asp
20 25 30
Pro Met Trp Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Ser Ser Gly Gly Thr Thr Ala Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 57
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 57
His Asp Pro Met Trp
5
<210> 58
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 58
Val Ile Gly Ser Ser Gly Gly Thr Thr Ala Tyr Ala Asp Ser Val Lys
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
133
15
Gly
<210> 59
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 59
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 60
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 60
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Ala Tyr His Ser Asn
25 30
<210> 61
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 61
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 62
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 62
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 63
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 63
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 64
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
134
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 64
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt ggaagataaa tttgcttcct ggtatcaaca gaagccaggc 120
cagtcccctg tcctgatcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 65
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 65
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Ile Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gin Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gin Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 66
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 66
Ser Gly His Asn Leu Glu Asp Lys Phe Ala Ser
5 10
<210> 67
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 67
Arg Asp Asp Lys Arg Pro Ser
5
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
135
<210> 68
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 68
Ser Ser Gin Asp Thr Val Thr Arg Val
<210> 69
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 69
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 70
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 70
Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Ile Ile Tyr
5 10 15
<210> 71
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 71
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gin Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 72
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0321b
<400> 72
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 73
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0322b
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
136
<400> 73
gaggtgcagc tgttggagtc tggaggaggc ctggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctaacga agagttccag tacaacccta tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggttcta gtggtggtgc gacagtttac 180
gcagacgccg tgaagggccg gttcaccatc tccagagaca attccgagaa cacgctgtat 240
ctgcaaatga acagcctaag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 74
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0322b
<400> 74
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Asn Glu Glu Phe Gin Tyr Asn
20 25 30
Pro Met Trp Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Ser Ser Gly Gly Ala Thr Val Tyr Ala Asp Ala Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Glu Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 75
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0322b
<400> 75
Tyr Asn Pro Met Trp
5
<210> 76
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0322b
<400> 76
Val Ile Gly Ser Ser Gly Gly Ala Thr Val Tyr Ala Asp Ala Val Lys
5 10 15
Gly
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
137
<210> 77
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0322b
<400> 77
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
10 15
<210> 78
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0322b
<400> 78
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Asn Glu Glu Phe Gin
20 25 30
<210> 79
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0322b
<400> 79
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 80
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0322b
<400> 80
Arg Phe Thr Ile Ser Arg Asp Asn Ser Glu Asn Thr Leu Tyr Leu Gin
5 10 15
Net Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 81
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0322b
<400> 81
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 82
<211> 318
<212> DNA
<213> Homo sapiens
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
138
<220>
<223> Abet0322b
<400> 82
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt gggagataaa tttgcttcct ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagagat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtcctg 318
<210> 83
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0322b
<400> 83
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Gly Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Glu Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gln Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 84
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0322b
<400> 84
Ser Gly His Asn Leu Gly Asp Lys Phe Ala Ser
5 10
<210> 85
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0322b
<400> 85
Arg Asp Asp Lys Arg Pro Ser
5
<210> 86
<211> 9
<212> PRT
<213> Homo sapiens
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
139
<220>
<223> Abet0322b
<400> 86
Ser Ser Gln Asp Thr Val Thr Arg Val
<210> 87
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0322b
<400> 87
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 88
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0322b
<400> 88
Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 89
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0322b
<400> 89
Glu Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gln Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 90
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0322b
<400> 90
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 91
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 91
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
140
tcctgtgcag cctctaccag cacgttccag gaagacacta tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggtccca acccgaagaa caacgcctac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 92
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 92
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Thr Ser Thr Phe Gin Glu Asp
20 25 30
Thr Met Trp Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Pro Asn Pro Lys Asn Asn Ala Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 93
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 93
Glu Asp Thr Met Trp
5
<210> 94
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 94
Val Ile Gly Pro Asn Pro Lys Asn Asn Ala Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 95
<211> 16
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
141
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 95
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
10 15
<210> 96
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 96
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Thr Ser Thr Phe Gin
20 25 30
<210> 97
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 97
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 98
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 98
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 99
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 99
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 100
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 100
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
142
tcgtacgagt tgactcagcc accctcagta tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt ggaagataaa tttgcttcct ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctctggggt ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga tcgtccta 318
<210> 101
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 101
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Val Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gin Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Ile Val Leu
100 105
<210> 102
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 102
Ser Gly His Asn Leu Glu Asp Lys Phe Ala Ser
5 10
<210> 103
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 103
Arg Asp Asp Lys Arg Pro Ser
5
<210> 104
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0323b
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
143
<400> 104
Ser Ser Gin Asp Thr Val Thr Arg Val
<210> 105
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 105
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 106
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 106
Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 107
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 107
Gly Val Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gin Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 108
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0323b
<400> 108
Phe Gly Gly Gly Thr Lys Leu Ile Val Leu
5 10
<210> 109
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0328
<400> 109
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctccagaga ccccttcaag gcggacacta tgtggtgggt ccgccaggct 120
ccaaggaaga ggctggagtg ggtctcagtt attggtgccc acaccaccaa cagcgcgtac 180
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
144
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccgct cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 110
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0328
<400> 110
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Arg Asp Pro Phe Lys Ala Asp
20 25 30
Thr Met Trp Trp Val Arg Gin Ala Pro Arg Lys Arg Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Ala His Thr Thr Asn Ser Ala Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp Arg Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 111
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0328
<400> 111
Ala Asp Thr Met Trp
5
<210> 112
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0328
<400> 112
Val Ile Gly Ala His Thr Thr Asn Ser Ala Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 113
<211> 16
<212> PRT
<213> Homo sapiens
<220>
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
145
<223> Abet0328
<400> 113
Glu Trp Met Asp Arg Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
10 15
<210> 114
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0328
<400> 114
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Arg Asp Pro Phe Lys
20 25 30
<210> 115
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0328
<400> 115
Trp Val Arg Gin Ala Pro Arg Lys Arg Leu Glu Trp Val Ser
5 10
<210> 116
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0328
<400> 116
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 117
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0328
<400> 117
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 118
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0328
<400> 118
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggtcagcatc 60
acctgctctg gacgtaactt ggaagataaa tttgcttcct ggtatcaaca gaagccaggc 120
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
146
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcaggggt ccctgagcga 180
ttctctgcct ccaactccgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 119
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0328
<400> 119
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
10 15
Thr Val Ser Ile Thr Cys Ser Gly Arg Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Val Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gin Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gin Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 120
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0328
<400> 120
Ser Gly Arg Asn Leu Glu Asp Lys Phe Ala Ser
5 10
<210> 121
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0328
<400> 121
Arg Asp Asp Lys Arg Pro Ser
5
<210> 122
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0328
<400> 122
Ser Ser Gin Asp Thr Val Thr Arg Val
5
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
147
<210> 123
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0328
<400> 123
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
10 15
Thr Val Ser Ile Thr Cys
<210> 124
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0328
<400> 124
Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 125
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0328
<400> 125
Gly Val Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gin Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 126
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0328
<400> 126
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 127
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0329
<400> 127
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctacgtt taacctcaag cgcgagacta tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctccgtt attggttccc accaggagcg cacgagctac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
148
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 128
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0329
<400> 128
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Thr Phe Asn Leu Lys Arg Glu
20 25 30
Thr Met Trp Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Ser His Gin Glu Arg Thr Ser Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 129
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0329
<400> 129
Arg Glu Thr Met Trp
5
<210> 130
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0329
<400> 130
Val Ile Gly Ser His Gin Glu Arg Thr Ser Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 131
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0329
<400> 131
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
149
15
<210> 132
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0329
<400> 132
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Thr Phe Asn Leu Lys
25 30
<210> 133
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0329
<400> 133
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 134
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0329
<400> 134
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gln
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 135
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0329
<400> 135
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 136
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0329
<400> 136
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataacgt gagcgacaag tggatgacgt ggtatcagca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaagctacg 240
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
150
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 137
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0329
<400> 137
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Val Ser Asp Lys Trp Met
20 25 30
Thr Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gln Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 138
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0329
<400> 138
Ser Gly His Asn Val Ser Asp Lys Trp Met Thr
5 10
<210> 139
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0329
<400> 139
Arg Asp Asp Lys Arg Pro Ser
5
<210> 140
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0329
<400> 140
Ser Ser Gln Asp Thr Val Thr Arg Val
5
<210> 141
<211> 22
<212> PRT
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
151
<213> Homo sapiens
<220>
<223> Abet0329
<400> 141
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
10 15
Thr Ala Ser Ile Thr Cys
<210> 142
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0329
<400> 142
Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 143
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0329
<400> 143
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gln Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 144
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0329
<400> 144
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 145
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0332
<400> 145
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggagtc cctgagactc 60
tcctgtgcag cctcttccga ctcctggcac accgacatta tgtggtgggt ccgccaggct 120
ccagggaaga ggctggagtg ggtctcagtt attggtaact cgaacaagaa gatcgcctac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct catca 375
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
152
<210> 146
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0332
<400> 146
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Glu
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Ser Asp Ser Trp His Thr Asp
20 25 30
Ile Met Trp Trp Val Arg Gin Ala Pro Gly Lys Arg Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Asn Ser Asn Lys Lys Ile Ala Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 147
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0332
<400> 147
Thr Asp Ile Met Trp
5
<210> 148
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0332
<400> 148
Val Ile Gly Asn Ser Asn Lys Lys Ile Ala Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 149
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0332
<400> 149
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 150
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
153
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0332
<400> 150
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Glu
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Ser Asp Ser Trp His
20 25 30
<210> 151
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0332
<400> 151
Trp Val Arg Gin Ala Pro Gly Lys Arg Leu Glu Trp Val Ser
5 10
<210> 152
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0332
<400> 152
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 153
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0332
<400> 153
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 154
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0332
<400> 154
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc cagggcagac ggccagcatc 60
acctgctctg gacataacat cggcgcgaag tgggtgagct ggtatcaaca gaagccaggc 120
cagtcaccta tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg tcaggcgcag ggccaggtga ccaggtcgtt cggcggaggg 300
accaagctga ccgtccta 318
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
154
<210> 155
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0332
<400> 155
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Ile Gly Ala Lys Trp Val
20 25 30
Ser Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Ile Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Gin Ala Gin Gly Gin Val Thr Arg Ser
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 156
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0332
<400> 156
Ser Gly His Asn Ile Gly Ala Lys Trp Val Ser
5 10
<210> 157
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0332
<400> 157
Arg Asp Asp Lys Arg Pro Ser
5
<210> 158
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0332
<400> 158
Gin Ala Gin Gly Gin Val Thr Arg Ser
5
<210> 159
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0332
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
155
<400> 159
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
10 15
Thr Ala Ser Ile Thr Cys
<210> 160
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0332
<400> 160
Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Ile Leu Val Ile Tyr
5 10 15
<210> 161
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0332
<400> 161
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gin Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 162
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0332
<400> 162
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 163
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0342
<400> 163
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctggatt cgactttcgc aggtccgtca tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggtgccc agacccagaa caaggcgtac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 164
<211> 125
<212> PRT
CA 02888322 200-04-14
WO 2014/060444
PCT/EP2013/071567
156
<213> Homo sapiens
<220>
<223> Abet0342
<400> 164
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asp Phe Arg Arg Ser
20 25 30
Val Met Trp Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Ala Gin Thr Gin Asn Lys Ala Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 165
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0342
<400> 165
Arg Ser Val Met Trp
5
<210> 166
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0342
<400> 166
Val Ile Gly Ala Gin Thr Gin Asn Lys Ala Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 167
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0342
<400> 167
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 168
<211> 30
<212> PRT
<213> Homo sapiens
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
157
<220>
<223> Abet0342
<400> 168
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asp Phe Arg
20 25 30
<210> 169
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0342
<400> 169
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 170
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0342
<400> 170
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 171
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0342
<400> 171
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 172
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0342
<400> 172
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt ggaagataaa tttgcttcct ggtatcaaca gaagccaggc 120
cagtcccccg tcctggtcat ctatcgggat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg ggacactgcc actctgacca tcagcgggac ccaggctatg 240
gatgaggctg actattactg tcaggcgcag gacagtacca ctcgagtgtt cggcggaggg 300
actaagctga ccgtccta 318
<210> 173
<211> 106
<212> PRT
CA 02888322 200-04-14
WO 2014/060444
PCT/EP2013/071567
158
<213> Homo sapiens
<220>
<223> Abet0342
<400> 173
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly Asp Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Met
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Gln Ala Gln Asp Ser Thr Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 174
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0342
<400> 174
Ser Gly His Asn Leu Glu Asp Lys Phe Ala Ser
5 10
<210> 175
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0342
<400> 175
Arg Asp Asp Lys Arg Pro Ser
5
<210> 176
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0342
<400> 176
Gln Ala Gln Asp Ser Thr Thr Arg Val
5
<210> 177
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0342
<400> 177
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
5 10 15
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
159
Thr Ala Ser Ile Thr Cys
<210> 178
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0342
<400> 178
Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 179
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0342
<400> 179
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly Asp Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gln Ala Met Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 180
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0342
<400> 180
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 181
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0343
<400> 181
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctggatt caactttaac caccaggtga tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggtaaga ccaacgagaa catcgcctac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact ctcgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 182
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
160
<400> 182
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Phe Asn His Gin
20 25 30
Val Met Trp Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Lys Thr Asn Glu Asn Ile Ala Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 183
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343
<400> 183
His Gin Val Met Trp
5
<210> 184
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343
<400> 184
Val Ile Gly Lys Thr Asn Glu Asn Ile Ala Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 185
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343
<400> 185
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 186
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343
<400> 186
ak 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
161
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Phe Asn
20 25 30
<210> 187
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343
<400> 187
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 188
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343
<400> 188
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 189
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343
<400> 189
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 190
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0343
<400> 190
cagagcgtct tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt ggaagataaa tttgcttcct ggtatcaaca gaagtcaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 191
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
162
<400> 191
Gin Ser Val Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gin Gin Lys Ser Gly Gin Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gin Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gin Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 192
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343
<400> 192
Ser Gly His Asn Leu Glu Asp Lys Phe Ala Ser
5 10
<210> 193
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343
<400> 193
Arg Asp Asp Lys Arg Pro Ser
5
<210> 194
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343
<400> 194
Ser Ser Gin Asp Thr Val Thr Arg Val
5
<210> 195
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343
<400> 195
Gin Ser Val Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
163
<210> 196
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343
<400> 196
Trp Tyr Gin Gin Lys Ser Gly Gin Ser Pro Val Leu Val Ile Tyr
10 15
<210> 197
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343
<400> 197
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gin Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 198
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343
<400> 198
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 199
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0344
<400> 199
gaggtgcagc tattggagtc tgggggaggc ttggtacagc ctggggggtc cctgagtctc 60
tcctgtgcag cctctggatt cacctttagc gtttatacta tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggtggga acgagacccg gaaggcctac 180
gcagactccg tgaagggccg gttcaccatc tccagggaca attccaagaa caggctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 200
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0344
<400> 200
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
164
Ser Leu Ser Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser Val Tyr
20 25 30
Thr Met Trp Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Gly Asn Glu Thr Arg Lys Ala Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Arg Leu Tyr
65 70 75 80
Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gln Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 201
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0344
<400> 201
Val Tyr Thr Met Trp
<210> 202
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0344
<400> 202
Val Ile Gly Gly Asn Glu Thr Arg Lys Ala Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 203
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0344
<400> 203
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Net Asp Val
5 10 15
<210> 204
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0344
<400> 204
Glu Val Gln Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly
5 10 15
Ser Leu Ser Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Ser
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
165
20 25 30
<210> 205
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0344
<400> 205
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
10
<210> 206
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0344
<400> 206
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Arg Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 207
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0344
<400> 207
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 208
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0344
<400> 208
cagagcgtct tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt ggaagataaa tttgcttcct ggtatcaaca gaagtcaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg tgcgacccag gacaacttca ctcgagtgtt cggcggaggc 300
accaagctga ccgtccta 318
<210> 209
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0344
<400> 209
Gln Ser Val Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
166
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gln Gln Lys Ser Gly Gln Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ala Thr Gln Asp Asn Phe Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 210
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0344
<400> 210
Ser Gly His Asn Leu Glu Asp Lys Phe Ala Ser
10
<210> 211
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0344
<400> 211
Arg Asp Asp Lys Arg Pro Ser
5
<210> 212
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0344
<400> 212
Ala Thr Gln Asp Asn Phe Thr Arg Val
5
<210> 213
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0344
<400> 213
Gln Ser Val Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 214
<211> 15
<212> PRT
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
167
<213> Homo sapiens
<220>
<223> Abet0344
<400> 214
Trp Tyr Gin Gin Lys Ser Gly Gin Ser Pro Val Leu Val Ile Tyr
10 15
<210> 215
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0344
<400> 215
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gin Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 216
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0344
<400> 216
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 217
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0368
<400> 217
gaggtgcagc tgttggagtc tgggggaggc ttagtacagc cgggggggtc cctgagactc 60
tcctgtgcag cctctggatt cgactttggg ccgagcccta tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggtaagg acacccagaa cagcacgtac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagga cacgctgtat 240
ctgcaaatga acagcctgaa agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 218
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0368
<400> 218
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asp Phe Gly Pro Ser
20 25 30
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
168
Pro Met Trp Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Lys Asp Thr Gln Asn Ser Thr Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asp Thr Leu Tyr
65 70 75 80
Leu Gln Met Asn Ser Leu Lys Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gln Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 219
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0368
<400> 219
Pro Ser Pro Met Trp
<210> 220
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0368
<400> 220
Val Ile Gly Lys Asp Thr Gln Asn Ser Thr Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 221
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0368
<400> 221
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 222
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0368
<400> 222
Glu Val Gln Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asp Phe Gly
20 25 30
<210> 223
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
169
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0368
<400> 223
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
10
<210> 224
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0368
<400> 224
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asp Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Lys Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 225
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0368
<400> 225
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 226
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0368
<400> 226
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt ggaagataaa tttacttcct ggtatcaaca gaagtcaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcggggc ccaggctacg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 227
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0368
<400> 227
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Thr
20 25 30
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
170
Ser Trp Tyr Gin Gin Lys Ser Gly Gin Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Ala Gin Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gin Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 228
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0368
<400> 228
Ser Gly His Asn Leu Glu Asp Lys Phe Thr Ser
10
<210> 229
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0368
<400> 229
Arg Asp Asp Lys Arg Pro Ser
5
<210> 230
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0368
<400> 230
Ser Ser Gin Asp Thr Val Thr Arg Val
5
<210> 231
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0368
<400> 231
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 232
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0368
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
171
<400> 232
Trp Tyr Gln Gln Lys Ser Gly Gln Ser Pro Val Leu Val Ile Tyr
10 15
<210> 233
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0368
<400> 233
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Ala Gln Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 234
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0368
<400> 234
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 235
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0369
<400> 235
gaggtgcagc tgttggagtc tgggggaggc ctggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctcttcgtt ccagatctcg aagaacacta tgtggtgggt ccgccgggct 120
ccagggaagg ggctggagtg ggtctcagtt attggtaagg acgagacccg cttcaactac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa caccctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 236
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369
<400> 236
Glu Val Gln Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Ser Phe Gln Ile Ser Lys Asn
20 25 30
Thr Met Trp Trp Val Arg Arg Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Lys Asp Glu Thr Arg Phe Asn Tyr Ala Asp Ser Val
CA 02888322 200-04-14
WO 2014/060444
PCT/EP2013/071567
172
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 237
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369
<400> 237
Lys Asn Thr Met Trp
<210> 238
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369
<400> 238
Val Ile Gly Lys Asp Glu Thr Arg Phe Asn Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 239
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369
<400> 239
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 240
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369
<400> 240
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Ser Phe Gin Ile Ser
20 25 30
<210> 241
<211> 14
<212> PRT
<213> Homo sapiens
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
173
<220>
<223> Abet0369
<400> 241
Trp Val Arg Arg Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
10
<210> 242
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369
<400> 242
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gln
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 243
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369
<400> 243
Trp Gly Gln Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 244
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0369
<400> 244
tcgtacgggt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacgtaacat cggggacagc tgggtcgcgt ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 245
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369
<400> 245
Ser Tyr Gly Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
5 10 15
Thr Ala Ser Ile Thr Cys Ser Gly Arg Asn Ile Gly Asp Ser Trp Val
20 25 30
Ala Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
174
0 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gln Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 246
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369
<400> 246
Ser Gly Arg Asn Ile Gly Asp Ser Trp Val Ala
10
<210> 247
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369
<400> 247
Arg Asp Asp Lys Arg Pro Ser
5
<210> 248
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369
<400> 248
Ser Ser Gln Asp Thr Val Thr Arg Val
5
<210> 249
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369
<400> 249
Ser Tyr Gly Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 250
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369
<400> 250
Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
5 10 15
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
175
<210> 251
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369
<400> 251
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
10 15
Leu Thr Ile Ser Gly Thr Gln Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 252
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369
<400> 252
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 253
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0370
<400> 253
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctggatt ccactttccc atgagcgcca tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtc attggtgaga ccccggagag gcaggcctac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagag cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 254
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0370
<400> 254
Glu Val Gln Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe His Phe Pro Met Ser
20 25 30
Ala Met Trp Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Glu Thr Pro Glu Arg Gln Ala Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Ser Thr Leu Tyr
65 70 75 80
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
176
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 255
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0370
<400> 255
Met Ser Ala Met Trp
<210> 256
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0370
<400> 256
Val Ile Gly Glu Thr Pro Glu Arg Gin Ala Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 257
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0370
<400> 257
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 258
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0370
<400> 258
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe His Phe Pro
20 25 30
<210> 259
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0370
<400> 259
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
177
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
10
<210> 260
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0370
<400> 260
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Ser Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 261
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0370
<400> 261
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 262
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0370
<400> 262
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgcacga ccccgcactt caacagcaaa tttgcttcct ggtatcaaca gaagccgggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctatg 240
gatgaggctg actattactg tcaggcgcag gatagtacca ctcgagtgtt cggcggaggg 300
accaggctga ccgtccta 318
<210> 263
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0370
<400> 263
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys Thr Thr Pro His Phe Asn Ser Lys Phe Ala
20 25 30
Ser Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gin Ala Met
65 70 75 80
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
178
Asp Glu Ala Asp Tyr Tyr Cys Gln Ala Gln Asp Ser Thr Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Arg Leu Thr Val Leu
100 105
<210> 264
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0370
<400> 264
Thr Thr Pro His Phe Asn Ser Lys Phe Ala Ser
10
<210> 265
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0370
<400> 265
Arg Asp Asp Lys Arg Pro Ser
5
<210> 266
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0370
<400> 266
Gln Ala Gln Asp Ser Thr Thr Arg Val
5
<210> 267
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0370
<400> 267
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 268
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0370
<400> 268
Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 269
<211> 32
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
179
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0370
<400> 269
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
10 15
Leu Thr Ile Ser Gly Thr Gln Ala Met Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 270
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0370
<400> 270
Phe Gly Gly Gly Thr Arg Leu Thr Val Leu
5 10
<210> 271
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0371
<400> 271
gaggtgcagc tgtcggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctcacga cgccttcccc ttcgacacta tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggttcta gtggtggtac gacagtttac 180
gcagactccg tgaagggccg gttcaccgtt tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 272
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0371
<400> 272
Glu Val Gln Leu Ser Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser His Asp Ala Phe Pro Phe Asp
20 25 30
Thr Met Trp Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Ser Ser Gly Gly Thr Thr Val Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Val Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
180
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gln Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 273
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0371
<400> 273
Phe Asp Thr Met Trp
<210> 274
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0371
<400> 274
Val Ile Gly Ser Ser Gly Gly Thr Thr Val Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 275
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0371
<400> 275
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 276
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0371
<400> 276
Glu Val Gln Leu Ser Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser His Asp Ala Phe Pro
20 25 30
<210> 277
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0371
<400> 277
Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
181
<210> 278
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0371
<400> 278
Arg Phe Thr Val Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 279
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0371
<400> 279
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 280
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0371
<400> 280
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctccg gacataacat ctcgtcgagc tgggtctcct ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtctt cggcggaggg 300
accaagctga ccgtccta 318
<210> 281
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0371
<400> 281
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Ile Ser Ser Ser Trp Val
20 25 30
Ser Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gin Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gin Asp Thr Val Thr Arg Val
85 90 95
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
182
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 282
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0371
<400> 282
Ser Gly His Asn Ile Ser Ser Ser Trp Val Ser
10
<210> 283
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0371
<400> 283
Arg Asp Asp Lys Arg Pro Ser
5
<210> 284
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0371
<400> 284
Ser Ser Gin Asp Thr Val Thr Arg Val
5
<210> 285
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0371
<400> 285
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 286
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0371
<400> 286
Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 287
<211> 32
<212> PRT
<213> Homo sapiens
<220>
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
183
<223> Abet0371
<400> 287
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
10 15
Leu Thr Ile Ser Gly Thr Gln Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 288
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0371
<400> 288
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 289
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0372
<400> 289
gaggtgcagc tgttggagtc tggggggggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctagcga catgttcaac atcgagacca tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggtaagg ggatgaacaa cgtctcgtac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 290
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0372
<400> 290
Glu Val Gln Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Ser Asp Met Phe Asn Ile Glu
20 25 30
Thr Met Trp Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Lys Gly Met Asn Asn Val Ser Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gln Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gln Gly Thr Leu Val Thr Val Ser Ser
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
184
<210> 291
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0372
<400> 291
Ile Glu Thr Met Trp
<210> 292
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0372
<400> 292
Val Ile Gly Lys Gly Met Asn Asn Val Ser Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 293
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0372
<400> 293
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 294
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0372
<400> 294
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Ser Asp Met Phe Asn
20 25 30
<210> 295
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0372
<400> 295
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 296
<211> 32
<212> PRT
<213> Homo sapiens
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
185
<220>
<223> Abet0372
<400> 296
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 297
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0372
<400> 297
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 298
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0372
<400> 298
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt ggaagataaa tttgcttcct ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca ttagcgggac ccaggctacg 240
gatgaggctg attattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 299
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0372
<400> 299
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gin Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gin Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
186
<210> 300
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0372
<400> 300
Ser Gly His Asn Leu Glu Asp Lys Phe Ala Ser
10
<210> 301
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0372
<400> 301
Arg Asp Asp Lys Arg Pro Ser
5
<210> 302
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0372
<400> 302
Ser Ser Gln Asp Thr Val Thr Arg Val
5
<210> 303
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0372
<400> 303
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 304
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0372
<400> 304
Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 305
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0372
<400> 305
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
187
10 15
Leu Thr Ile Ser Gly Thr Gin Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 306
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0372
<400> 306
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 307
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0373
<400> 307
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgtag cctccggatt cgactttgag cggtccgtca tgtggtgggt ccgccaggct 120
ccagggaaga ggctggagtg ggtctcagtt attggtagcg ggaagaccaa catcacctac 180
gcagactccg tgaagggccg gtttaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 308
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0373
<400> 308
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Val Ala Ser Gly Phe Asp Phe Glu Arg Ser
20 25 30
Val Met Trp Trp Val Arg Gin Ala Pro Gly Lys Arg Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Ser Gly Lys Thr Asn Ile Thr Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 309
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
188
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0373
<400> 309
Arg Ser Val Met Trp
<210> 310
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0373
<400> 310
Val Ile Gly Ser Gly Lys Thr Asn Ile Thr Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 311
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0373
<400> 311
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 312
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0373
<400> 312
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Val Ala Ser Gly Phe Asp Phe Glu
20 25 30
<210> 313
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0373
<400> 313
Trp Val Arg Gin Ala Pro Gly Lys Arg Leu Glu Trp Val Ser
5 10
<210> 314
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0373
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
189
<400> 314
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 315
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0373
<400> 315
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 316
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0373
<400> 316
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc cagggcagac ggccagcatc 60
acctgctctg gtcataactt ggaggataaa tttgcttcct ggtatcaaca gaagccaggc 120
cagtcccccg tcctggtcat ctatcgagat gacaagcggc cctcagagat ccctgagcga 180
ttctctgcct ccaactctgg gcacaccgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 317
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0373
<400> 317
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Glu Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gin Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gin Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 318
<211> 11
<212> PRT
<213> Homo sapiens
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
190
<220>
<223> Abet0373
<400> 318
Ser Gly His Asn Leu Glu Asp Lys Phe Ala Ser
10
<210> 319
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0373
<400> 319
Arg Asp Asp Lys Arg Pro Ser
5
<210> 320
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0373
<400> 320
Ser Ser Gln Asp Thr Val Thr Arg Val
5
<210> 321
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0373
<400> 321
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 322
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0373
<400> 322
Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 323
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0373
<400> 323
Glu Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gln Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
191
<210> 324
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0373
<400> 324
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
10
<210> 325
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0374
<400> 325
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctggatt ccagtttaag gacacgccca tgtggtgggt ccgccaggct 120
ccagggaagg ggctagagtg ggtctcagtt attggtgacc agaaccacaa gaaggcctac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg cctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 326
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0374
<400> 326
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Gin Phe Lys Asp Thr
20 25 30
Pro Met Trp Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Asp Gin Asn His Lys Lys Ala Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Ala Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 327
<211> 5
<212> PRT
<213> Homo sapiens
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
192
<220>
<223> Abet0374
<400> 327
Asp Thr Pro Met Trp
<210> 328
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0374
<400> 328
Val Ile Gly Asp Gin Asn His Lys Lys Ala Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 329
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0374
<400> 329
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Ala
5 10 15
<210> 330
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0374
<400> 330
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Gin Phe Lys
20 25 30
<210> 331
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0374
<400> 331
Trp Val Arg Gln Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 332
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0374
<400> 332
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
193
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 333
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0374
<400> 333
Trp Gly Gln Gly Thr Leu Val Thr Val Ser Ser
10
<210> 334
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0374
<400> 334
tcgtacgagt tgactcagcc accctcagtg tccgtgaccc caggacagac ggccagcatc 60
acctgctctg gacataactt gggaggtaaa tttgcttcct ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactttgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 335
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0374
<400> 335
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Thr Pro Gly Gln
5 10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Gly Gly Lys Phe Ala
20 25 30
Ser Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Phe Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gln Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 336
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0374
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
194
<400> 336
Ser Gly His Asn Leu Gly Gly Lys Phe Ala Ser
10
<210> 337
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0374
<400> 337
Arg Asp Asp Lys Arg Pro Ser
5
<210> 338
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0374
<400> 338
Ser Ser Gln Asp Thr Val Thr Arg Val
5
<210> 339
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0374
<400> 339
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Thr Pro Gly Gln
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 340
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0374
<400> 340
Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 341
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0374
<400> 341
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Phe Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gln Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 342
<211> 10
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
195
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0374
<400> 342
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
10
<210> 343
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0377
<400> 343
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctggatt caactttaac gagcagaccc tctggtgggt ccgccaagcc 120
ccagggaaag ggctggagtg ggtctcagtt attggtgtgg ggaccaagaa catcgcctac 180
gcagacaccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctggggaca ggggaccctg 360
gtcaccgtct cctca 375
<210> 344
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377
<400> 344
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Phe Asn Glu Gin
20 25 30
Thr Leu Trp Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Val Gly Thr Lys Asn Ile Ala Tyr Ala Asp Thr Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gln Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 345
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377
<400> 345
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
196
Glu Gin Thr Leu Trp
<210> 346
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377
<400> 346
Val Ile Gly Val Gly Thr Lys Asn Ile Ala Tyr Ala Asp Thr Val Lys
5 10 15
Gly
<210> 347
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377
<400> 347
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 348
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377
<400> 348
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Phe Asn
20 25 30
<210> 349
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377
<400> 349
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 350
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377
<400> 350
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
197
<210> 351
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377
<400> 351
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
10
<210> 352
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0377
<400> 352
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataacac cgagcacaag tggatctcgt ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcca ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 353
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377
<400> 353
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Thr Glu His Lys Trp Ile
20 25 30
Ser Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Thr
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gin Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 354
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377
<400> 354
Ser Gly His Asn Thr Glu His Lys Trp Ile Ser
5 10
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
198
<210> 355
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377
<400> 355
Arg Asp Asp Lys Arg Pro Ser
<210> 356
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377
<400> 356
Ser Ser Gin Asp Thr Val Thr Arg Val
5
<210> 357
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377
<400> 357
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 358
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377
<400> 358
Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 359
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377
<400> 359
Gly Ile Pro Glu Arg Phe Ser Ala Thr Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gin Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 360
<211> 10
<212> PRT
<213> Homo sapiens
<220>
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
199
<223> Abet0377
<400> 360
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
10
<210> 361
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0378
<400> 361
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctggatt cccctttgag accgacatca tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggtacca acaccgacaa cgtcgcctac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 362
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0378
<400> 362
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Pro Phe Glu Thr Asp
20 25 30
Ile Met Trp Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Thr Asn Thr Asp Asn Val Ala Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 363
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0378
<400> 363
Thr Asp Ile Met Trp
5
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
200
<210> 364
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0378
<400> 364
Val Ile Gly Thr Asn Thr Asp Asn Val Ala Tyr Ala Asp Ser Val Lys
10 15
Gly
<210> 365
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0378
<400> 365
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 366
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0378
<400> 366
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Pro Phe Glu
20 25 30
<210> 367
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0378
<400> 367
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 368
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0378
<400> 368
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 369
<211> 11
<212> PRT
<213> Homo sapiens
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
201
<220>
<223> Abet0378
<400> 369
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
10
<210> 370
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0378
<400> 370
tcgtacgagt tgacccagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt ggaagataaa tttgcttcct ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg ctcgtcctag gacacggtga ctcgggtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 371
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0378
<400> 371
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gin Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Ter Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 372
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0378
<400> 372
Ser Gly His Asn Leu Glu Asp Lys Phe Ala Ser
5 10
<210> 373
<211> 7
<212> PRT
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
202
<213> Homo sapiens
<220>
<223> Abet0378
<400> 373
Arg Asp Asp Lys Arg Pro Ser
<210> 374
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0378
<400> 374
Ser Ser Ter Asp Thr Val Thr Arg Val
5
<210> 375
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0378
<400> 375
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 376
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0378
<400> 376
Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 377
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0378
<400> 377
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gln Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 378
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0378
<400> 378
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
203
<210> 379
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0379
<400> 379
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctggatt cgactttgcc gagacgcctt tgtggtgggt ccgccaggct 120
ccaggggaga ggctggagtg ggtctcagtt attggtagca accagaacaa gaccgcctac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagga cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 380
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0379
<400> 380
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asp Phe Ala Glu Thr
25 30
Pro Leu Trp Trp Val Arg Gin Ala Pro Gly Glu Arg Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Ser Asn Gin Asn Lys Thr Ala Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asp Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 381
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0379
<400> 381
Glu Thr Pro Leu Trp
5
<210> 382
<211> 17
<212> PRT
<213> Homo sapiens
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
204
<220>
<223> Abet0379
<400> 382
Val Ile Gly Ser Asn Gin Asn Lys Thr Ala Tyr Ala Asp Ser Val Lys
10 15
Gly
<210> 383
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0379
<400> 383
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 384
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0379
<400> 384
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asp Phe Ala
20 25 30
<210> 385
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0379
<400> 385
Trp Val Arg Gin Ala Pro Gly Glu Arg Leu Glu Trp Val Ser
5 10
<210> 386
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0379
<400> 386
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asp Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 387
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0379
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
205
<400> 387
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
10
<210> 388
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0379
<400> 388
cagagcgtct tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt ggaagataaa tttgcttcct ggtatcaaca gaagtcaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactccgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatggggctg actattactg tgcgacccag gacaacttca ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 389
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0379
<400> 389
Gin Ser Val Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gin Gin Lys Ser Gly Gin Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gin Ala Thr
65 70 75 80
Asp Gly Ala Asp Tyr Tyr Cys Ala Thr Gin Asp Asn Phe Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 390
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0379
<400> 390
Ser Gly His Asn Leu Glu Asp Lys Phe Ala Ser
5 10
<210> 391
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0379
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
206
<400> 391
Arg Asp Asp Lys Arg Pro Ser
<210> 392
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0379
<400> 392
Ala Thr Gln Asp Asn Phe Thr Arg Val
5
<210> 393
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0379
<400> 393
Gln Ser Val Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 394
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0379
<400> 394
Trp Tyr Gln Gln Lys Ser Gly Gln Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 395
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0379
<400> 395
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gln Ala Thr Asp Gly Ala Asp Tyr Tyr Cys
20 25 30
<210> 396
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0379
<400> 396
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 397
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
207
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0380
<400> 397
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctatggg caacttcaac taccagacta tgtggtgggt ccgccaggct 120
ccagggaggg ggctggagtg ggtctcagtt attggtaaga ccaacgagaa catcgcctac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 398
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380
<400> 398
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Met Gly Asn Phe Asn Tyr Gin
20 25 30
Thr Met Trp Trp Val Arg Gin Ala Pro Gly Arg Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Lys Thr Asn Glu Asn Ile Ala Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 399
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380
<400> 399
Tyr Gin Thr Met Trp
5
<210> 400
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
208
<400> 400
Val Ile Gly Lys Thr Asn Glu Asn Ile Ala Tyr Ala Asp Ser Val Lys
10 15
Gly
<210> 401
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380
<400> 401
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 402
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380
<400> 402
Glu Val Gln Leu Leu Glu Ser Gly Gly Gly Leu Val Gln Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Met Gly Asn Phe Asn
20 25 30
<210> 403
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380
<400> 403
Trp Val Arg Gln Ala Pro Gly Arg Gly Leu Glu Trp Val Ser
5 10
<210> 404
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380
<400> 404
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gln
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 405
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380
<400> 405
Trp Gly Gln Gly Thr Leu Val Thr Val Ser Ser
5 10
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
209
<210> 406
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0380
<400> 406
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt ggaagataaa tttgcttcct ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 407
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380
<400> 407
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gin Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 408
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380
<400> 408
Ser Gly His Asn Leu Glu Asp Lys Phe Ala Ser
5 10
<210> 409
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380
<400> 409
Arg Asp Asp Lys Arg Pro Ser
5
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
210
<210> 410
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380
<400> 410
Ser Ser Gin Asp Thr Val Thr Arg Val
<210> 411
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380
<400> 411
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 412
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380
<400> 412
Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 413
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380
<400> 413
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gin Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 414
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380
<400> 414
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 415
<211> 375
<212> DNA
<213> Homo sapiens
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
211
<220>
<223> Abet0381
<400> 415
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cttgagactc 60
tcctgtgcag cctcttcccc gtcgttcccg cgggagacca tgtggtgggt ccgccaggct 120
ccagggaagg ggcttgagtg ggtctcagtt attggtaccc agccgaaccg cttgacgtac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatagacg tctgggggca ggggaccctg 360
gtcaccgtct cccca 375
<210> 416
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0381
<400> 416
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Ser Pro Ser Phe Pro Arg Glu
20 25 30
Thr Met Trp Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Thr Gin Pro Asn Arg Leu Thr Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Ile
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Pro
115 120 125
<210> 417
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0381
<400> 417
Arg Glu Thr Met Trp
5
<210> 418
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0381
<400> 418
Val Ile Gly Thr Gin Pro Asn Arg Leu Thr Tyr Ala Asp Ser Val Lys
5 10 15
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
212
Gly
<210> 419
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0381
<400> 419
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Ile Asp Val
10 15
<210> 420
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0381
<400> 420
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Ser Pro Ser Phe Pro
20 25 30
<210> 421
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0381
<400> 421
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 422
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0381
<400> 422
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 423
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0381
<400> 423
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Pro
5 10
<210> 424
<211> 318
<212> DNA
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
213
<213> Homo sapiens
<220>
<223> Abet0381
<400> 424
tcgtacgagt tgactcagcc accctcagtg tccgcgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt ggaagataaa tttgtttcct ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcgac cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggcta actattactg ttcgtcccag gacacggtga ctcgagcgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 425
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0381
<400> 425
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Ala Ser Pro Gly Gln
10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Val
20 25 30
Ser Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Thr
65 70 75 80
Asp Glu Ala Asn Tyr Tyr Cys Ser Ser Gln Asp Thr Val Thr Arg Ala
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 426
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0381
<400> 426
Ser Gly His Asn Leu Glu Asp Lys Phe Val Ser
5 10
<210> 427
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0381
<400> 427
Arg Asp Asp Lys Arg Pro Ser
5
<210> 428
<211> 9
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
214
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0381
<400> 428
Ser Ser Gin Asp Thr Val Thr Arg Ala
<210> 429
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0381
<400> 429
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Ala Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 430
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0381
<400> 430
Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 431
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0381
<400> 431
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gin Ala Thr Asp Glu Ala Asn Tyr Tyr Cys
20 25 30
<210> 432
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0381
<400> 432
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 433
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0382
<400> 433
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
215
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgaggctc 60
tcctgtgcag cctctggatt ccactttacc aactccatca tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggtagcg aggcgcaccg cgtcacgtac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 434
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382
<400> 434
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe His Phe Thr Asn Ser
20 25 30
Ile Met Trp Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Ser Glu Ala His Arg Val Thr Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 435
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382
<400> 435
Asn Ser Ile Met Trp
5
<210> 436
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382
<400> 436
Val Ile Gly Ser Glu Ala His Arg Val Thr Tyr Ala Asp Ser Val Lys
5 10 15
Gly
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
216
<210> 437
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382
<400> 437
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
10 15
<210> 438
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382
<400> 438
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe His Phe Thr
20 25 30
<210> 439
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382
<400> 439
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 440
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382
<400> 440
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 441
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382
<400> 441
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 442
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0382
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
217
<400> 442
tcgtacgagt tgattcagcc accctcagtg tccgtgtccc caggacagac agccagcatc 60
acctgctctg gacataactt ggaagataaa tttgcttcct ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcca ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctacg 240
gatgaggctg actattactg ttcgtcccag gactcggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 443
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382
<400> 443
Ser Tyr Glu Leu Ile Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Thr
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gin Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gin Asp Ser Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 444
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382
<400> 444
Ser Gly His Asn Leu Glu Asp Lys Phe Ala Ser
5 10
<210> 445
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382
<400> 445
Arg Asp Asp Lys Arg Pro Ser
5
<210> 446
<211> 9
<212> PRT
<213> Homo sapiens
<220>
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
218
<223> Abet0382
<400> 446
Ser Ser Gin Asp Ser Val Thr Arg Val
<210> 447
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382
<400> 447
Ser Tyr Glu Leu Ile Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 448
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382
<400> 448
Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 449
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382
<400> 449
Gly Ile Pro Glu Arg Phe Ser Ala Thr Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gin Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 450
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382
<400> 450
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 451
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0383
<400> 451
gaggtgcagc tgttggagtc cgggggaggc ttggtacagc ctggggggtc cctgaaactc 60
tcctgtgcag cctctggatt cacgtttgac tggtacccga tgtggtgggt ccgccaggct 120
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
219
ccagggaaga ggctggagtg gatctcagtt attggtgcgg acaacgccaa gatcgcctac 180
gcagactccg tgaagggccg gtttaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atgggccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccccg 360
gtcaccgtct cctca 375
<210> 452
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0383
<400> 452
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Lys Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Asp Trp Tyr
20 25 30
Pro Met Trp Trp Val Arg Gin Ala Pro Gly Lys Arg Leu Glu Trp Ile
35 40 45
Ser Val Ile Gly Ala Asp Asn Ala Lys Ile Ala Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Gly His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Pro Val Thr Val Ser Ser
115 120 125
<210> 453
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0383
<400> 453
Trp Tyr Pro Met Trp
5
<210> 454
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0383
<400> 454
Val Ile Gly Ala Asp Asn Ala Lys Ile Ala Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 455
<211> 16
<212> PRT
<213> Homo sapiens
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
220
<220>
<223> Abet0383
<400> 455
Glu Trp Met Gly His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
10 15
<210> 456
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0383
<400> 456
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Lys Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe Asp
20 25 30
<210> 457
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0383
<400> 457
Trp Val Arg Gin Ala Pro Gly Lys Arg Leu Glu Trp Ile Ser
5 10
<210> 458
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0383
<400> 458
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 459
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0383
<400> 459
Trp Gly Gin Gly Thr Pro Val Thr Val Ser Ser
5 10
<210> 460
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0383
<400> 460
tcgtacgagt tgactcagcc accctcagta tccgtgtccc caggacagac ggccagcatc 60
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
221
acctgctctg gacataactt gggagataaa tttgcttcct ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca ttagcgggac ccaggctacg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtcctg 318
<210> 461
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0383
<400> 461
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Gly Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Thr
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gln Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 462
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0383
<400> 462
Ser Gly His Asn Leu Gly Asp Lys Phe Ala Ser
5 10
<210> 463
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0383
<400> 463
Arg Asp Asp Lys Arg Pro Ser
5
<210> 464
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0383
<400> 464
Ser Ser Gln Asp Thr Val Thr Arg Val
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
222
<210> 465
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0383
<400> 465
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
10 15
Thr Ala Ser Ile Thr Cys
<210> 466
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0383
<400> 466
Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 467
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0383
<400> 467
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gin Ala Thr Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 468
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0383
<400> 468
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 469
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 469
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctggatt caactttaac caccaggtga tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggtaaga ccaacgagaa catcgcctac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
223
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact ctcgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 470
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 470
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Phe Asn His Gin
20 25 30
Val Met Trp Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Lys Thr Asn Glu Asn Ile Ala Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 471
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 471
His Gin Val Met Trp
5
<210> 472
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 472
Val Ile Gly Lys Thr Asn Glu Asn Ile Ala Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 473
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343-GL
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
224
<400> 473
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
10 15
<210> 474
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 474
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Phe Asn
20 25 30
<210> 475
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 475
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 476
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 476
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 477
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 477
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 478
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 478
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt ggaagataaa tttgcttcct ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
225
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctatg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 479
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 479
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gin Ala Met
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gin Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 480
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 480
Ser Gly His Asn Leu Glu Asp Lys Phe Ala Ser
5 10
<210> 481
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 481
Arg Asp Asp Lys Arg Pro Ser
5
<210> 482
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 482
Ser Ser Gin Asp Thr Val Thr Arg Val
5
<210> 483
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
226
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 483
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
10 15
Thr Ala Ser Ile Thr Cys
<210> 484
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 484
Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 485
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 485
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gln Ala Met Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 486
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0343-GL
<400> 486
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 487
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 487
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctcttcgtt ccagatctcg aagaacacta tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggtaagg acgagacccg cttcaactac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
227
gtcaccgtct cctca 375
<210> 488
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 488
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Ser Phe Gin Ile Ser Lys Asn
20 25 30
Thr Met Trp Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Lys Asp Glu Thr Arg Phe Asn Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 489
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 489
Lys Asn Thr Met Trp
5
<210> 490
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 490
Val Ile Gly Lys Asp Glu Thr Arg Phe Asn Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 491
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 491
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
228
<210> 492
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 492
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Ser Phe Gin Ile Ser
20 25 30
<210> 493
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 493
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 494
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 494
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 495
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 495
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 496
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 496
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacgtaacat cggggacagc tgggtcgcgt ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctatg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
229
accaagctga ccgtccta 318
<210> 497
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 497
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
10 15
Thr Ala Ser Ile Thr Cys Ser Gly Arg Asn Ile Gly Asp Ser Trp Val
20 25 30
Ala Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Met
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gln Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 498
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 498
Ser Gly Arg Asn Ile Gly Asp Ser Trp Val Ala
5 10
<210> 499
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 499
Arg Asp Asp Lys Arg Pro Ser
5
<210> 500
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 500
Ser Ser Gln Asp Thr Val Thr Arg Val
5
<210> 501
<211> 22
<212> PRT
<213> Homo sapiens
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
230
<220>
<223> Abet0369-GL
<400> 501
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
10 15
Thr Ala Ser Ile Thr Cys
<210> 502
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 502
Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 503
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 503
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gin Ala Met Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 504
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0369-GL
<400> 504
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 505
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 505
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctggatt caactttaac gagcagaccc tctggtgggt ccgccaagcc 120
ccagggaaag ggctggagtg ggtctcagtt attggtgtgg ggaccaagaa catcgcctac 180
gcagacaccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctggggaca ggggaccctg 360
gtcaccgtct cctca 375
<210> 506
CA 02888322 200-04-14
WO 2014/060444
PCT/EP2013/071567
231
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 506
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Phe Asn Glu Gin
20 25 30
Thr Leu Trp Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Val Gly Thr Lys Asn Ile Ala Tyr Ala Asp Thr Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 507
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 507
Glu Gin Thr Leu Trp
5
<210> 508
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 508
Val Ile Gly Val Gly Thr Lys Asn Ile Ala Tyr Ala Asp Thr Val Lys
5 10 15
Gly
<210> 509
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 509
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 510
<211> 30
<212> PRT
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
232
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 510
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe Asn Phe Asn
20 25 30
<210> 511
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 511
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 512
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 512
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 513
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 513
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 514
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 514
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataacac cgagcacaag tggatctcgt ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctatg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 515
CA 02888322 200-04-14
WO 2014/060444
PCT/EP2013/071567
233
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 515
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Thr Glu His Lys Trp Ile
20 25 30
Ser Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Met
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gln Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 516
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 516
Ser Gly His Asn Thr Glu His Lys Trp Ile Ser
5 10
<210> 517
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 517
Arg Asp Asp Lys Arg Pro Ser
5
<210> 518
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 518
Ser Ser Gln Asp Thr Val Thr Arg Val
5
<210> 519
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 519
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
234
Ser Tyr Glu Leu Thr Gin Pro Pro Ser Val Ser Val Ser Pro Gly Gin
10 15
Thr Ala Ser Ile Thr Cys
<210> 520
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 520
Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 521
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 521
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gin Ala Met Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 522
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0377-GL
<400> 522
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 523
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0380-GL
<400> 523
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgagactc 60
tcctgtgcag cctctatggg caacttcaac taccagacta tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggtaaga ccaacgagaa catcgcctac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 524
<211> 125
<212> PRT
<213> Homo sapiens
CA 02888322 200-04-14
WO 2014/060444
PCT/EP2013/071567
235
<220>
<223> Abet0380-GL
<400> 524
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Met Gly Asn Phe Asn Tyr Gin
20 25 30
Thr Met Trp Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Lys Thr Asn Glu Asn Ile Ala Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Net
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 525
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380-GL
<400> 525
Tyr Gin Thr Met Trp
5
<210> 526
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380-GL
<400> 526
Val Ile Gly Lys Thr Asn Glu Asn Ile Ala Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 527
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380-GL
<400> 527
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 528
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380-GL
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
236
<400> 528
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Met Gly Asn Phe Asn
20 25 30
<210> 529
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380-GL
<400> 529
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
5 10
<210> 530
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380-GL
<400> 530
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 531
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380-GL
<400> 531
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 532
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0380-GL
<400> 532
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt ggaagataaa tttgcttcct ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctatg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 533
<211> 106
<212> PRT
<213> Homo sapiens
CA 02888322 200-04-14
WO 2014/060444
PCT/EP2013/071567
237
<220>
<223> Abet0380-GL
<400> 533
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Met
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gln Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 534
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380-GL
<400> 534
Ser Gly His Asn Leu Glu Asp Lys Phe Ala Ser
5 10
<210> 535
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380-GL
<400> 535
Arg Asp Asp Lys Arg Pro Ser
5
<210> 536
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380-GL
<400> 536
Ser Ser Gln Asp Thr Val Thr Arg Val
5
<210> 537
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380-GL
<400> 537
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
5 10 15
Thr Ala Ser Ile Thr Cys
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
238
<210> 538
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380-GL
<400> 538
Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
5 10 15
<210> 539
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380-GL
<400> 539
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gln Ala Met Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 540
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0380-GL
<400> 540
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
<210> 541
<211> 375
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0382-GL
<400> 541
gaggtgcagc tgttggagtc tgggggaggc ttggtacagc ctggggggtc cctgaggctc 60
tcctgtgcag cctctggatt ccactttacc aactccatca tgtggtgggt ccgccaggct 120
ccagggaagg ggctggagtg ggtctcagtt attggtagcg aggcgcaccg cgtcacgtac 180
gcagactccg tgaagggccg gttcaccatc tccagagaca attccaagaa cacgctgtat 240
ctgcaaatga acagcctgag agccgaggac acggccgtgt attactgtgc gagagagtgg 300
atggaccact cccgccccta ctactactac ggtatggacg tctgggggca ggggaccctg 360
gtcaccgtct cctca 375
<210> 542
<211> 125
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382-GL
<400> 542
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
239
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
10 15
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe His Phe Thr Asn Ser
20 25 30
Ile Met Trp Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val
35 40 45
Ser Val Ile Gly Ser Glu Ala His Arg Val Thr Tyr Ala Asp Ser Val
50 55 60
Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr
65 70 75 80
Leu Gin Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys
85 90 95
Ala Arg Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met
100 105 110
Asp Val Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
115 120 125
<210> 543
<211> 5
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382-GL
<400> 543
Asn Ser Ile Met Trp
5
<210> 544
<211> 17
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382-GL
<400> 544
Val Ile Gly Ser Glu Ala His Arg Val Thr Tyr Ala Asp Ser Val Lys
5 10 15
Gly
<210> 545
<211> 16
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382-GL
<400> 545
Glu Trp Met Asp His Ser Arg Pro Tyr Tyr Tyr Tyr Gly Met Asp Val
5 10 15
<210> 546
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382-GL
<400> 546
Glu Val Gin Leu Leu Glu Ser Gly Gly Gly Leu Val Gin Pro Gly Gly
5 10 15
ak 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
240
Ser Leu Arg Leu Ser Cys Ala Ala Ser Gly Phe His Phe Thr
20 25 30
<210> 547
<211> 14
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382-GL
<400> 547
Trp Val Arg Gin Ala Pro Gly Lys Gly Leu Glu Trp Val Ser
10
<210> 548
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382-GL
<400> 548
Arg Phe Thr Ile Ser Arg Asp Asn Ser Lys Asn Thr Leu Tyr Leu Gin
5 10 15
Met Asn Ser Leu Arg Ala Glu Asp Thr Ala Val Tyr Tyr Cys Ala Arg
20 25 30
<210> 549
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382-GL
<400> 549
Trp Gly Gin Gly Thr Leu Val Thr Val Ser Ser
5 10
<210> 550
<211> 318
<212> DNA
<213> Homo sapiens
<220>
<223> Abet0382-GL
<400> 550
tcgtacgagt tgactcagcc accctcagtg tccgtgtccc caggacagac ggccagcatc 60
acctgctctg gacataactt ggaagataaa tttgcttcct ggtatcaaca gaagccaggc 120
cagtcccctg tcctggtcat ctatcgagat gacaagcggc cctcagggat ccctgagcga 180
ttctctgcct ccaactctgg gcacactgcc actctgacca tcagcgggac ccaggctatg 240
gatgaggctg actattactg ttcgtcccag gacacggtga ctcgagtgtt cggcggaggg 300
accaagctga ccgtccta 318
<210> 551
<211> 106
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382-GL
<400> 551
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
241
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
10 15
Thr Ala Ser Ile Thr Cys Ser Gly His Asn Leu Glu Asp Lys Phe Ala
20 25 30
Ser Trp Tyr Gln Gln Lys Pro Gly Gln Ser Pro Val Leu Val Ile Tyr
35 40 45
Arg Asp Asp Lys Arg Pro Ser Gly Ile Pro Glu Arg Phe Ser Ala Ser
50 55 60
Asn Ser Gly His Thr Ala Thr Leu Thr Ile Ser Gly Thr Gln Ala Met
65 70 75 80
Asp Glu Ala Asp Tyr Tyr Cys Ser Ser Gln Asp Thr Val Thr Arg Val
85 90 95
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
100 105
<210> 552
<211> 11
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382-GL
<400> 552
Ser Gly His Asn Leu Glu Asp Lys Phe Ala Ser
5 10
<210> 553
<211> 7
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382-GL
<400> 553
Arg Asp Asp Lys Arg Pro Ser
5
<210> 554
<211> 9
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382-GL
<400> 554
Ser Ser Gln Asp Thr Val Thr Arg Val
5
<210> 555
<211> 22
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382-GL
<400> 555
Ser Tyr Glu Leu Thr Gln Pro Pro Ser Val Ser Val Ser Pro Gly Gln
5 10 15
Thr Ala Ser Ile Thr Cys
<210> 556
CA 02888322 2015-04-14
WO 2014/060444 PCT/EP2013/071567
242
<211> 15
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382¨GL
<400> 556
Trp Tyr Gin Gin Lys Pro Gly Gin Ser Pro Val Leu Val Ile Tyr
10 15
<210> 557
<211> 32
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382¨GL
<400> 557
Gly Ile Pro Glu Arg Phe Ser Ala Ser Asn Ser Gly His Thr Ala Thr
5 10 15
Leu Thr Ile Ser Gly Thr Gin Ala Met Asp Glu Ala Asp Tyr Tyr Cys
20 25 30
<210> 558
<211> 10
<212> PRT
<213> Homo sapiens
<220>
<223> Abet0382¨GL
<400> 558
Phe Gly Gly Gly Thr Lys Leu Thr Val Leu
5 10
Sequences for Human A131-42, human A131-40, human A1317-42, human A131-43,
murine A131-42 and
truncates
Biotinylated Human Amyloid Beta 1-42 peptide:
Biotin-DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA (SEQ ID NO: 559)
Human Amyloid Beta 1-42 peptide:
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA (SEQ ID NO: 560)
Biotinylated Human Amyloid Beta 1-40 peptide:
Biotin-DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV (SEQ ID NO: 561)
Human Amyloid Beta 1-40 peptide:
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV (SEQ ID NO: 562)
Murine Amyloid Beta 1-42 peptide:
DAEFGHDSGFEVRHQKLVFFAEDVGSNKGAIIGLMVGGVVIA (SEQ ID NO: 563)
Biotinylated Murine Amyloid Beta 1-42 peptide:
Biotin-DAEFGHDSGFEVRHQKLVFFAEDVGSNKGAIIGLMVGGVVIA (SEQ ID NO: 564)
Biotinylated-LC-Murine Amyloid Beta 1-42 peptide:
Biotin-(Linker Chain)-DAEFGHDSGFEVRHQKLVFFAEDVGSNKGAIIGLMVGGVVIA (SEQ ID NO:
565)
Murine Amyloid Beta 1-40 peptide:
DAEFGHDSGFEVRHQKLVFFAEDVGSNKGAIIGLMVGGVV (SEQ ID NO: 566)
Biotinylated-LC-Murine Amyloid Beta 1-40 peptide:
CA 02888322 2015-04-14
WO 2014/060444
PCT/EP2013/071567
243
Biotin-(Linker Chain)-DAEFGHDSGFEVRHQKLVFFAEDVGSNKGAIIGLMVGGVV (SEQ ID NO:
567)
Biotinylated Scrambled Amyloid Beta 1-42 peptide:
Biotin-AIAEGDSHVLKEGAYMEIFDVQGHVFGGLIFRVVDLGSHNVA (SEQ ID NO: 568)
Human Amyloid Beta 1-43 peptide:
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIAT (SEQ ID NO: 569)
Human Amyloid Beta 29-42 truncated peptide:
KKKGAIIGLMVGGVVIA (SEQ ID NO: 570)
Human Amyloid Beta 29-40 truncated peptide:
KKKGAIIGLMVGGVV (SEQ ID NO: 571)
Human Amyloid Beta 1-16 truncated peptide:
DAEFRHDSGYEVHHQK (SEQ ID NO: 572)
Human Amyloid Beta 11-22 truncated peptide:
EVRHQKLVFFAE (SEQ ID NO: 573)
Human Amyloid Beta 12-28 truncated peptide:
VRHQKLVFFAEDVGSNK (SEQ ID NO: 574)
Human Amyloid Beta 17-42 truncated peptide:
LVFFAEDVGSNKGAIIGLMVGGVVIA (SEQ ID NO: 575)
Human Amyloid Beta 11-42 truncated peptide:
EVRHQKLVFFAEDVGSNKGAIIGLMVGGVVIA (SEQ ID NO: 576)
Human Amyloid Beta 3-42 truncated peptide:
EFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA (SEQ ID NO: 577)