Note: Descriptions are shown in the official language in which they were submitted.
CA 02888907 2015-04-23
=
STABLE ANALOGS OF PEPTIDE AND POLYPEPTIDE THERAPEUTICS
This application is a divisional application of Canadian Patent Application
2,525,574 filed on May 17, 2004.
Bacicround of the Invention
Polypeptide and peptide therapeutics are widely used in medical practice.
Their
ease of production, either by recombinant DNA technology or peptide
synthesizers,
ensures their continued use in a variety of circumstances in the years to
come.
Accordingly, polypeptide therapeutics, such as hormones, cytokines and growth
factors, represent an important class of therapeutic agents. Certain native
polypeptides,
however, can be inactivated rapidly in vivo via proteolysis or isomerization.
Such
inactivation can be inconvenient in cases where it is desired to maintain a
consistent or
sustained blood level of the therapeutic over a period of time, as repeated
administrations are then necessary. In certain instances, one or more of the
proteolytic
products of the polypeptide can be antagonistic to the activity of the intact
polypeptide.
/5 In these cases, administration of additional therapeutic alone may be
insufficient to
overcome the antagonist effect of the proteolytic products.
- To further illustrate, one class of peptide hormones whose prolonged
presence
in the blood may be beneficial include glucagon-like peptides 1 and 2 (GLP-1
and
GLP-2 respectively), ,glucose-dependent insulinotropic peptide (GIP),
neuropeptide Y
(NPY), pancreatic polypeptide (PP), and peptide YY (PYY)._ GLP-1 is an
important
polypeptide hormone with regulatory function in glucose metabolism and
gastrointestinal secretion and metabolism. Current efforts show that GLP-1 is
a growth
factor for beta cells in the pancreas and perhaps is involved in cell
differentiation in
other organs as well. GLP-2 is a 33-amino acid peptide having therapeutic
application
in the treatment ofdiseases of the gastrointestinal tract. In particular, it
has been
determined that GLP-2 acts as a trophic agent to enhance and maintain proper
gastrointestinal function, as well as to promote growth of intestinal tissues
(See, e.g.,
U.S. Patent Serial Nos. 5,834,428; 5,789,379; and 5,990,077; and International
Publication No. WO 98/52600). GM is a 42-amino acid peptide synthesized and
secreted from endocrine cells in the small intestine (See R. A. Pederson, et
al.,
Endocrinology 99, 780- 785 (1976) and T. B. Usdin, et al., Endocrinology 133,
2861-
2870 (1993)). GIP infusions have been shown to inhibit the effects of glucagon
on the
liver while enhancing those of insulin. Additionally, GIP has dual effects on
hepatic
blood flow, increasing flow through the portal vein and inhibiting flow
through the
- 1 -
CA 02888907 2015-04-23
hepatic artery. Neuropeptide Y is a 36-amino acid member of the pancreatic
polypeptide family. It is highly concentrated in both the central and
peripheral
mammalian nervous system, is the most potent substance known to cause an
increase in
feeding, and may play a role in the genetic basis of Type II Diabetes Mellitus
(See U.S.
Patents 6410701, 6075009, 5026685, 5328899, and K. Tatemoto, Proc. Natl. Acad.
Sci.
USA 79, 5485-5489 (1982)). Peptide YY (PYY) and pancreatic polypeptide (PP)
are
structurally related peptide hormones involved in memory loss, depression,
anxiety,
epilepsy, pain, hypertension, and sleep and eating disorders.
These polypeptide hormones, and other polypeptide factors, are believed to be
degraded by members of the post-proline cleaving class of serine proteinase
enzymes,
such as dipeptidyl peptidase IV (DPP IV). DPP IV is a membrane associated
serine
peptidase which cleaves N-tezminal dipeptides from a peptide chain containing
in the
penultimate (P1) position, preferably, a proline residue, or an alanirte
residue if the N-
terminal residue (P2) is histidine or a large aromatic such as tyrosine,
tryptophan or
phenylalanine. The smino terminus sequences of GLP-1, GIP, and GLP-2 are His-
Ala-
Glu, Tyr-Ala-Glu, and His-Ala-Asp respectively. The amino terminal sequences
of
NPY, PP, and PYY are Tyr-Pro-Ser, Ala-Pro-Leu and Tyr-Pro-Ile respectively.
Hence,
DPP IV has been implicated in the regulation of the activity of each of these
polypeptide hormones, as well as other polypeptides, in vivo.
DPP IV-mediated removal of Xaa-Ala or Xaa-Pro dipeptides, wherein Xaa is an
amino acid residue, from the N-terminus of the bioactive peptide hormones
mentioned
above renders them inactive, or even antagonistic. Accordingly, cleavage and
inactivation of peptide hormones by serine proteinases such as DPP IV is just
one
example that illustrates the significant limitation imposed by proteolysis for
the use of
therapeutic polypeptides. The discovery of analogs that exhibit stability
towards
proteolysis, such as DPP IV-mediated inactivation, is therefore of substantial
interest.
Accordingly, there is a need in the art for proteolysis-resistant peptide
hormones.
Summary of the Invention
The present invention generally provides compositions of peptide or
polypeptide analogs (herein "P'i analogs") that are resistant to cleavage by
proteinases
(e.g., analogs that are resistant to proteolysis).
- 2 -
CA 02888907 2015-04-23
One aspect of the invention relates to the discovery that modification of
substrates for post-proline cleaving proteinases at the P'i position (the
residue to the
carboxy terminal side of the amide cleavage site) can produce substrate
analogs with
greatly reduce susceptibility to enzyme-mediated cleavage relative to the
native
substrate, yet retain the biological activity of the native substrate. For
example,
modification of substrates of the post-proline cleaving serine proteinase DPP
IV with
an amino acid analog at the P'i residue (of the DPP IV cleavage site) results
in a
substrate analog with reduce susceptibility to cleavage by DPP IV, yet retains
the
biological activity of the underlying substrate.
Another aspect of the invention relates to the more general observation that
modification of proteinase substrates at the P'1 residue (of the cleavage
site) with an
amino acid analog having a tetrasubstituted Cf3 carbon can markedly increase
the in
vivo half-life of the resulting analog, e.g., which may have a longer duration
of
biological action and/or reduced clearance relative ot the wild-type
polypeptide. Based
on this discovery, and its applicability to substrates cleaved by a diverse
range of
proteinases, the present invention provides a method for producing P'i analogs
of
' substrates for such proteinases as serine proteinases, metalloproteinases,
aspartic
proteinases, and cysteine proteinases.
The present invention also provides pharmaceutical compositions comprising
one or more of the subject "P'i analogs". Exemplary pharmaceutical
compositions
comprise one or more P'1 analogs formulated with pharmaceutically acceptable
carriers
or excipients.
Another aspect of the present invention is a method of treating a disease in a
subject comprising administering a therapeutically effective amount of one or
more of
said P'i analogs. The subject P'1 analogs can be administered alone, or can be
administered as part of a therapeutic regimen including other therapies
appropriate to
the specific disease indication. By way of example, administration of a P'i
analog for
the treatment of diabetes may be used alone, or may be used in combination
with
modulation of diet and exercise, and/or with administration of insulin.
Further
exemplary combinatorial methods of treatment comprise administration of a P'1
analog
and administration of an inhibitor of the particular enzyme that cleaves the
native
polypeptide. Such an inhibitor may be specific to the particular enzyme (e.g.,
a DPP IV
- 3 -
CA 02888907 2015-04-23
specific inhibitor) or may be more generic to the enzyme class (e.g., a serine
protease
inhibitor).
Another aspect of the present invention is use of the subject P'i analogs for
diagnostic purposes.
Another aspect of the present invention is use of the subject P'i analogs for
the
manufacture of a medicament for providing proteinase resistant peptides.
Another aspect of the present invention is use of a 13,1 analog in the
manufacture
of a therapeutic medicament.
Yet another aspect of the present invention is a method of conducting a
business
comprising, identifying, manufacturing, marketing, distributing, and licensing
a P'1
analog, pharmaceutical compositions thereof, and/or kits including the P'1
analog.
In any of the foregoing aspects, the present invention contemplates
compositions and methods wherein the P'1 analog is an analog of a polypeptide
hormone such as glucagon-like peptide, NPY, PPY, secretin, GL-1, GLP-2, and
GIP.
However, the present invention recognizes that any polypeptide or peptide
hormone
that is cleaved by a proteinase may be modified at the cleavage site as
described herein
to provide P'i analogs that are resistant to proteolysis. Furthermore, the
present
invention recognizes that P'i analogs resistant to any of a number of classes
of
proteinases can be readily designed based on our knowledge of the cleavage
site of
those enzymes and based on the teachings of this application. Exemplary
classes of
proteinases include metalloproteinases, aspartic proteinases, cysteine
proteinases, and
seine proteinases.
Brief Description of the Figures
Figure 1 shows a schematic of the degradation of a native GLP-1 by DPP IV.
Figure 2 summarizes HPLC/MS results demonstrating that two different peptide
analogs of GLP-1 (7-37) are resistant to cleavage by DPP IV.
Figure 3 shows that the 3-dimethyl-aspartate substituted GLP-1 analog
maintains functional activities of native GLP-1. The graph at the left shows
that GLP-1
and GLP-1 (3-DMA) bind receptor with similar, although not identical,
affinities. The
graph at the right shows that GLP-1 and GLP-1 (3-DMA) have substantially
identical
- 4 -
CA 02888907 2015-04-23
signaling potential as measured by cAMP production following exposure to GLP-1
or
GLP-1 analog.
Figure 4 shows that the 3-butyl-methyl-glycine substituted GLP-1 (GLP-1
(BM)) analog maintains functional activity of native GLP-1. The graph shows
that
GLP-1 and GLP-1 (BM) have substantially identical signaling potential as
measured by
cAMP production following exposure to GLP-1 or GLP-1 analog.
Figure 5 shows GLP-1 (7-37) amide treated with human DPP-IV for two hours
(bottom) compared to untreated peptide (top) by HPLC/MS. Note that treatment
of
GLP-1 (7-37) with DPP IV resulted in a time dependent loss of peptide
10(
Figure 6 shows the results of treating a GLP-1 analog containing a tertiary-
leucine (TLE) residue in place of the P'l glutamic acid with human DPP-IV for
two
hours (bottom) compared to untreated peptide (top) by HPLC/MS. Note that the
TLE-
GLP1 analog was resistant to degradation by DPP IV.
Figure 7 shows that substitution of a tertiary-leucine (TLE) at the P'l
position
of a model peptide substrate for the serine protease thrombin results in the
production
of a peptide analog resistant to cleavage by thrombin.
. Figure 8 shows the percent change in blood glucose in diabetic mice
for
Exendin-4 over time for three different doses (40 jig, 4p,g, and 0.4 g) as
compared to a
saline control solution.
Figure 9 shows the percent change in blood glucose in diabetic mice for a GLP-
1(TPA1B4) analog at a dose of 40 jig over time compared to the percent change
in
blood glucose for a saline or GLP-1 control.
Figure 10 shows the percent change in blood glucose in diabetic mice for a
GLP-1(TPA1B4) analog for three different doses (800 jig, 80 g, and 8 jig)
over time
compared to a saline control.
Figure 11 shows the percent change in blood glucose in diabetic mice for a
GLP-1 analog (TPA1B4) at a dose of 20 mg/kg over time compared to the percent
change in blood glucose for a saline or GLP-1 control.
- 5 -
CA 02888907 2015-04-23
Figure 12 shows the blood glucose level in diabetic mice for a GLP-1 analog
(TPA1B4) at a dose of 20 mg/kg over time compared to the blood glucose level
for a
saline or GLP-1 control.
Figure 13 shows the percent change in blood glucose for Exendin-4 over time
for three different doses (8 jig, 0.8 jig, and 0.08 jig) compared to a saline
control.
Figure 14 shows the persent change in blood glucose for GLP-1 over time for a
dose of 800 jig compared to a saline control.
Figure 15 shows the percent change in blood glucose for a GLP-1 analog
(P1732) for two different doses (8 jig and 0.8 jig) as compared to a saline
control.
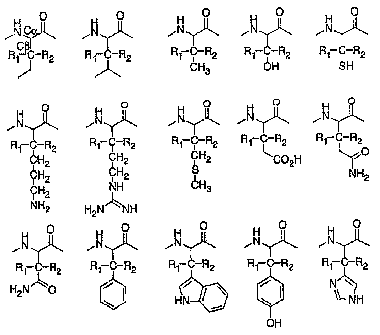
Figure 16 shows exemplary embodiments of Formula (IA wherein naturally
occurring amino acids have been modified at the f3-position (3-position) with
R1 and
R2.
Detailed Description of the Invention
I. Overview
The present invention generally relates to peptide and P'1 analogs that have
increased in vivo half-lives, e.g., resulting from reduced susceptibility to
cleavage by
proteolytic enzymes, yet retain the desired acivity of the original substrate.
The P'1
analogs of the present invention include analogs of growth factors, cytoldnes,
peptide
hormones and other polypeptides and peptides whose activity and/or half-life
in VIVO
are ordinarily regulated by proteolytic cleavage.
One aspect of the invention relates to the discovery that modification of
substrates for post-proline cleaving proteinases at the P'i position (the
residue to the
carboxy terminal side d the amide cleavage site) can produce substrate analogs
with
greatly reduce susceptibility to enzyme-mediated cleavage relative to the
native
substrate, yet retain the biological activitrof the native substrate. For
example,
modification of substrates of the post-proline cleaving serine proteinase DPP
IV with
an amino acid analog at the P'1 residue (of the DPP IV cleavage site) results
in a
substrate analog with reduce susceptibility to cleavage by DPP IV, yet retains
the
biological activity of the underlying substrate.
- 6 -
CA 02888907 2015-04-23
While replacing the P'i residue with another naturally occurring amino acid is
contemplated, in preferred embodiments, the P'1 residue is replaced with a non-
naturally occurring amino acid analog, and even more preferably, with one
which is a
structural analog, e.g., retaining similar attributes with respect to steric
and/or
electronic nature. To illustrate, in certain embodiments the present invention
provides a
- modified polypeptide which is rendered less susceptible
to proteolysis by a post-proline
cleaving proteinases, such as dipeptidylpeptidase IV (DPP-IV), wherein the
= polypeptide has been modified at the P'i position with an amino acid or
amino acid
analog of Formula I:
0
R2
R3
wherein,
R1 and R2 are independentl}rselected from lower alkyl, heteroalkyl,
cycloalkyl,
heterocycloalkyl, aryl, heteroaryl, alkoxyl, carboxyl, carboxarnide, carbonyl,
halogen, hydroxyl, amine, or cyano, or R1 and R2 taken together form a ring of
4-7 atoms;
R3 is selected from lower alkyl, heteroalkyl, cycloalkyl, heterocycloalkyl,
aryl,
heteroaryl, amino, alkoxyl, halogen, carboxyl, carboxsmide, carbonyl, cyano,
thioalkyl,
acylamino, amido, cyano, nitro, azido, sulfate, sulfonate, sulfonamido, -
(CH2)mR4, -
(C112)m0H, -(CHAnCOOH, -(CH2)m0-lower alkyl, -(CH2)m0-1ower alkenyl, -
=(CH2),10(CH2)mR4, -(CH2)mSH, -(C112)mS-lower alkyl, -(CH2)mS-lower alkenyl,
-(CH2)nS(CH2)m-R4, (CH2).NH2, -(CH2)mNC(=NH)NH2, -(CH2).C(=0)N112, or -
(CH2)mNH2,;
R4 represents, independently for each occurrence, aryl, aralkyl, cycloalkyl,
cycloalkenyl, or heterocycle;
m is 0, 1, or 2;
and n is 0, 1, or 2.
- 7 -
CA 02888907 2015-04-23
In certain preferred embodiments, R1 and R2 each independently represent a
small hydrophobic group, such as a lower alkyl (preferably methyl, ethyl, or
propyl,
and even more preferably a methyl), a halogen, or a halogenated lower alkyl.
In certain preferred embodiments, R3 represents a lower alkyl, more preferably
methyl, ethyl or propyl, and even more preferably a methyl. In other preferred
embodiments, R3 represents an aryl, such as phenyl or hydroxyphenyl
(preferably para-
hydroxy). In yet other preferred embodiments, R3 represents a hydroxyl group.
In still
other preferred embodiments, R3 represents -(CH2)mCOOH, and preferably where m
is
preferably 0 or 1.
In certain preferred embodiments, n is 0.
In certain preferred embodiments of such substrate analogs, the P'i is an
amino
acid analog having a tetrasubstituted Cf3 carbon, such as represented in
Formula II:
0
Cos===õ,),7s\sõ.
R2 __________________________________
R3
wherein R1 and R2 each independently represent a lower alkyl or a halogen; R3
represents a lower alkyl, an aryl, a hydroxyl group, -(CH2)mCOOH, -(CH2)mNf12,
-
(CH2)mNC(=NH)NH2, -(CH2)mC(=0)NH2, -SH, or -(CH2)mSCH3; and m is 0, 1, or 2.
In certain preferred embodiments, RI and R2 are independently selected from
methyl, ethyl, or propyl, and even more preferably a methyl.
In certain preferred embodiments, R3 represents a lower alkyl, more preferably
methyl, ethyl, or propyl, and even more preferably a methyl. In other
preferred
embodiments, R3 represents an aryl, such as a phenyl, hydroxyphenyl
(preferably para-
hydroxy), indole or imidazole. In yet other preferred embodiments, R3
represents a
hydroxyl group. In certain preferred embodiments, R3 represents -COOH or -
CH2COOH. In still other preferred embodiments, R3 represents -
CH2CH2NC(=NH)NH2, -CH2C(=0)NH2, -CH2CH2C(=0)NH2, -SH, or -CH2SCH3.
- 8 -
CA 02888907 2015-04-23
Another aspect of the invention relates to the more general observation that
modification of proteinase substrates at the P'1 residue (of the cleavage
site) with an
amino acid analog having a tetra-substituted CI3 carbon can markedly increase
the in
vivo half-life of the resulting analog, e.g., which may have a longer duration
of
biological action and/or reduced clearance relative ot the wild-type
polypeptide. Based
on this discovery, and its applicability to substrates cleaved by a diverse
range of
proteinases, the present invention provides a method for producing P'1 analogs
of
substrates for such proteinases as serine proteinases, metalloproteinases,
aspartic
proteinases, and cysteine proteinases.
In certain preferred embodiments, the P'1 is an amino acid analog having a
tetrasubstituted CI3 carbon, such as represented in Formula II:
0
N
R2 __________________________________
\R3
wherein R1 and R2 each independently represent a lower alkyl or a halogen; R3
represents lower alkyl, aryl, hydroxyl group, -(CH2)õ,COOH, -(CH2).NC(=NH)NH2,
-
(CH2).C(=0)NH2, -(CH2)mNH2, -SH, -(CH2)mSCH3; and m is 0, 1, or 2.
In certain preferred embodiments, R1 and R2 each independently represent
methyl, ethyl or propyl, and even more preferably methyl.
In certain preferred embodiments, R3 represents lower alkyl, more preferably
methyl, ethyl or propyl, and even more preferably methyl. In other preferred
embodiments, R3 represents an aryl group, such as a phenyl, hydroxyphenyl
(preferably
p-hydroxy), indole, or imidazole. In yet other preferred embodiments, R3
represents a
hydroxyl group. In certain preferred embodiments, R3 represents -COOH or -
CH2COOH. In still other preferred embodiments, R3 represents -
CH2CH2NC(----NH)N1-12, -CH2C(----0)NH2, -CH2CH2C(=0)NH2, -SH, or -CH2SCH3. For
examples of preferred embodiments of modified naturally occurring amino acids,
see
Figure 16.
- 9 -
CA 02888907 2015-04-23
=
II. Definitions
The term "substrate" refers to a substrate of an enzyme which is catalytically
acted on and chemically converted by the enzyme to product(s).
The binding site for a peptide substrate consists of a series of "specificity
subsites" across the surface of the enzyme. The term "specificity subsite"
refers to a
pocket or other site on the enzyme capable of interacting with a portion of a
substrate
for the enzyme.
In discussing the interactions of peptides and protein substrates with
proteinases, e.g., serine and cysteine proteinases and the like, the present
application
utilins the nomenclature of Schechter and Berger [(1967) Biochem. Biophys.
Res.
Commun. 27:157-162)]. The individual amino acid residues of a substrate or
inhibitor
are designated -P2-P1-P'1-P'2-, etc. and the corresponding subsites of the
enzyme are
designated S2, Sl, S'1, S12, etc. The scissile bond of the substrate is the
amide bond
linking the P1 and P'1 residues.
A "P'i residue" refers to the amino acid residue of a substrate polypeptide
that
becomes the new amino terminus of product polypeptide resulting from
proteinase-
mediated cleavage of the amide backbone of the substrate polypeptide. To
further
illustrate, a substrate polypeptide includes an amide backbone bond that is
subject to a
proteolytic reaction represented by the general scheme:
P 1
0 R` 0 R'
=
0 R 0
P1
By the term "amino acid residue" is meant an amino acid. In general the
abbreviations used herein for designating the naturally occurring amino acids
are based
on recommendations of the IUPAC-TUB Commission on Biochemical Nomenclature
(see Biochemistry (1972) 11:1726-1732). For instance Met, Ile, Leu, Ala and
Gly
-10-
CA 02888907 2015-04-23
represent "residues" of methionine, isoleucme, leucine, alanine and glycine,
respectively. By the residue is meant a radical derived from the corresponding
a-
.
amino acid by eliminating the OH portion of the carboxyl group and the H
portion of
the a-amino group.
5 The term "amino acid side chain" is that part of an amino acid residue
exclusive
of the backbone, as defined by K. D. Kopple, "Peptides and Amino Acids", W. A.
Benjamin Inc., New York and Amsterdam, 1966, pages 2 and 33; examples of such
side chains of the common amino acids are -CH2CH2SCH3 (the side chain of
methionine), -CH2(CH3)-CH2CH3 (the side chain of isoleucine), -CH2CH(CH3)2
(the
10 side chain of leucine) or H-(the side chain of glycine). These
sidechains are pendant
from the backbone Ca carbon.
The term "tetra-substituted Cf3 carbon" refers to a carbon atom which is (i)
directly pendant from the Ca carbon of the amino acid backbone, and (ii)
includes four
pendant substituents (including the Ca carbon), none of which is hydrogen.
15 As used herein, "protein" is a polymer consisting essentially of any
of the 20
amino acids. Although "polypeptide" is often used in reference to relatively
large
proteins, and "peptide" is often used in reference to small protein, usage of
these terms
in the art overlaps and is varied. Unless evident from the Context, the terms
"peptide(s)", "protein(s)" and "polypeptide(s)" are used interchangeably
herein.
20 As used herein, the term "nucleic acid" refers to polynucleotides such
as
deoxyribonucleic acid (DNA), and, where appropriate, ribonucleic acid (RNA).
The
term should also be understood to include, as equivalents, analogs of either
RNA or
DNA made from nucleotide analogs, and, as applicable to the embodiment being
described, single (sense or antisense) and double-stranded polynucleotides.
25 The International Union of Biochemistry and Molecular
Biology (1984) has
recommended the use of the term "peptidase" for the subset of peptide bond
hydrolases
(Subclass E.0 3.4.). The widely used term "protease" is synonymous with
"peptidase",
and they are used interchangeably herein. Peptidases comprise two groups of
enzymes:
the endopeptidases and the exopeptidases. Endopeptidases cleave peptide bonds
at
30 points within a protein, and exopeptidases remove amino
acids sequentially from either
the N- or C-terminus.
- 11 -
CA 02888907 2015-04-23
The term "proteinase" is also used as a synonym for endopeptidase. Proteinase
= are classified according to their catalytic mechanisms. Four mechanistic
classes have
been recogni7ed by the International Union of Biochemistry and Molecular
Biology:
serine proteinases, cysteine proteinases, aspartic proteinases, and
metalloproteinases.
The class "serine proteinases" comprises two distinct families: the
chymotrypsin family which includes the mammalian enzymes such as chymotrypsin,
trypsin, elastase or kallilcrein, and the substilisin family which includes
the bacterial
enzymes such as subtilisin. The general three-dimensional structure is
different in the
two families but they have the same active site geometry and catalysis
proceeds via the
same mechanism. The serine proteinases exhibit different substrate
specificities which
are related to amino acid substitutions in the various enzyme subsites (see
the
nomenclature of Schechter and Berger) interacting with the substrate residues.
Three
residues which form the catalytic triad are essential in the catalytic
process: His-57,
Asp-102 and Ser-195 (chymotrypsinagen numbering).
The family of "cysteine proteinases" includes the plant peptidases such as
f
papain, actinidin or bromelain, several mammalian lysosomal cathepsins, the
cytosolic
calpains (calcium-activated), and several parasitic peptidases (e.g.,
Trypanosoma,
Schistosoma). Papain is the archetype and the best studied member of the
family.
Most of the "aspartic proteinases" belong to the pepsin family. The pepsin
,family includes digestive enzymes such as pepsin and chymosin as well as
lysosomal
cathepsins D, processing enzymes such as renin, and certain fungal peptidases
(penicillopepsin, rhizopuspepsin, endothiapepsin). A second family comprises
viral
proteinases such as the peptidase from the AlDS virus (HIV) also called
retropepsin.
The "metalloproteinases" are found in bacteria, fungi as well as in higher
organisms. They differ widely in their sequences and their structures but the
great
majority of enzymes contain a zinc atom which is catalytically active. Many
enzymes
contain the sequence ITEXXEI, which provides two histidine ligands for the
zinc
whereas the third ligand is either a glutamic acid (thermolysin, neprilysin,
alanyl
aminopeptidase) or a histidine (astacin).
-12-
CA 02888907 2015-04-23
The term "agonist", as used herein, is meant to refer to a peptide or 13'1
analog
that retains the bioactivity of the native substrate of interest so as to
produce a similar
biological effect when administered to an animal.
The term "antagonist" refers to a peptide or P'i analog that does not retain
the
bioactivity of the native substrate of interest, or at least at a reduced
level of activity
relative to the native substrate, and inhibits the biological action of the
native substrate.
The term "analog" refers to a.molecule substantially similar in function to
either
the entire receptor molecule or to a fragment thereof.
The term "derivative with minor modifications" with respect to a parent
chemical compound, for example an amino acid analog, is used to refer to
compounds
which are chemically similar to the parent chemical compound. Preferably, a
derivative with minor modifications will have minor structural modifications
and hence
may be considered as "structural analogs" of the original compound.
"Heart-related ailments" includes any chronic or acute pathological event
involving the heart and/or associated tissue (e.g., the pericardium, aorta and
other
associated blood vessels), including ischemia-reperf-usion injury; congestive
heart
failure; cardiac arrest; myocardial infarction; cardiotoxicity caused by
compounds such
as drugs (e.g., doxorubicin, herceptin, thioridazine and cisapride); cardiac
damage due
to parasitic infection (bacteria, fungi, rickettsiae, and viruses, e.g.,
syphilis, chronic
Trypanosoma cruzi infection); fulminant cardiac amyloidosis; heart surgery;
heart
trsnsplantation; traumatic cardiac injury (eg., penetrating or blunt cardiac
injury, and
aortic valve rapture), surgical repair of a thoracic aortic aneurysm.; a
suprarenal aortic
aneurysm; cardiogenic shock due to myocardial infarction or cardiac failure;
neurogenic shock and anaphylaxis.
"Instruction(s)" as used herein means a product label and/or documents
describing relevant materials or methodologies pertaining to use of a kit or
packaged
pharmaceutical. These materials may include any combination of the following:
background information, list of components, proposed dosages, warnings
regarding
possible sideeffects, instructions for administering the drug, technical
support, and any
other related documents.
- 13 -
CA 02888907 2015-04-23
The phrase "pharmaceutically acceptable" is employed herein to refer to those
ligands, materials, compositions, and/or dosage forms which are, within the
scope of
sound medical judgment, suitable for use in contact with the tissues of human
beings
and animals, substantially non-pyrogenic, without excessive toxicity,
irritation, allergic
5 response, or other problem or complication, commensurate with a
reasonable
benefit/risk ratio.
The phrase "pharmaceutically acceptable carrier" as used herein means a
pharmaceutically acceptable material, composition or vehicle, such as a liquid
or solid
filler, diluent, excipient, solvent or encapsulating material, involved in
carrying or
10 transporting the subject chemical from one organ or portion of the body,
to another
organ or portion of the body. Each carrier must be "acceptable" in the sense
of being
compatible with the other ingredients of the formulation, not injurious to the
patient,
and substantially non-pyrogenic. Some examples of materials which can serve as
.
pharmaceutically acceptable carriers include: (1) sugars, such as lactose,
glucose, and
15 sucrose; (2) starches, such as corn starch and potato starch; (3)
cellulose, and its
derivatives, such as sodium carboxymethyl cellulose, ethyl cellulose, and
cellulose
acetate; (4) powdered tragacanth; (5) malt; (6) gelatin; (7) talc; (8)
excipients, such as
= cocoa butter and suppository waxes; (9) oils, such as peanut oil,
cottonseed oil,
safflower oil, sesame oil, olive oil, corn oil, and soybean oil; (10) glycols,
such as
20 propylene glycol; (11) polyols, such as glycerin, sorbitol, marmitol,
and polyethylene
glycol; (12) esters, such as ethyl oleate and ethyl laurate; (13) agar; (14)
buffering
agents, such as magnesium hydroxide and aluminum hydroxide; (15) alginic acid;
(16)
pyrogen-free water; (17) isotonic saline; (18) Ringer's solution; (19) ethyl
alcohol; (20)
phosphate buffer solutions; and (21) other non-toxic compatible substances
employed
25 in pharmaceutical formulations. In certain embodiments,
pharmaceutical compositions
of the present invention are non-pyrogenic, i.e., do not induce significant
temperature
elevations when administered to a patient. '
The term "pharmaceutically acceptable salts" refers to the relatively non-
toxic,
inorganic and organic acid addition salts of the inhibitor(s). These salts can
be
30 prepared in situ during the final isolation and purification
of the inhibitor(s), or by
separately reacting a purified inhibitor(s) in its free base form with a
suitable organic or
inorganic acid, and isolating the salt thus formed. Representative salts
include the
- 14-
CA 02888907 2015-04-23
hydrobromide, hydrochloride, sulfate, bisulfate, phosphate, nitrate, acetate,
valerate,
oleate, palmitate, stearate, laurate, benzoate, lactate, phosphate, tosylate,
citrate,
maleate, fumarate, succinate, tartrate, naphthylate, mesylate, glucoheptonate,
lactobionate, and laurylsulphonate salts, and the like. (See, for example,
Berge et al.
(1977) "Pharmaceutical Salts", J. Phann. Sci. 66:1-19)
In other cases, the inhibitors useful in the methods of the present invention
may
contain one or more acidic functional groups and, thus, are capable of forming
pharmaceutically acceptable salts with pharmaceutically acceptable bases. The
term
"pharmaceutically acceptable salts" in these instances refers to the
relatively non-toxic
inorganic and organic base addition salts of an inhibitor(s). These salts can
likewise be
prepared in situ during the final isolation and purification of the
inhibitor(s), or by
separately reacting the purified inhibitor(s) in its free acid form with a
suitable base,
such as the hydroxide, carbonate, or bicarbonate of a pharmaceutically
acceptable metal
cation, with ammonia, or with a pharmaceutically acceptable organic primary,
secondary, or tertiary amine. Representative alkali or alkaline earth salts
include the
lithium, sodium, potassium, calcium, magnesium, and aluminum salts, and the
like.
Representative organic amines useful for the formation of base addition salts
include
ethylamine, diethylamine, ethylenediamine, ethanolamine, diethanolamine,
piperazine,
and the like (see, for example, Berge et al., supra).
= 20 The term "preventing" is art-recognized, and when used in relation
to a
condition, such as a local recurrence (e.g., pain), a disease such as cancer,
a
syndrome complex such as heart failure or any other medical condition, is well
understood in the art, and includes administration of a composition which
reduces the frequency of, or delays the onset of, symptoms of a medical
condition in a subject relative to a subject which does not receive the
composition. Thus, prevention of cancer includes, for example, reducing the
number of detectable cancerous growths in a population of patients receiving a
prophylactic treatment relative to an untreated control population, and/or
delaying the appearance of detectable cancerous growths in a treated
population
versus an untreated control population, e.g., by a statistically and/or
clinically
significant amount. Prevention of an infection includes, for example, reducing
the number of diagnoses of the infection in a treated population versus an
-15-
CA 02888907 2015-04-23
untreated control population, and/or delaying the onset of symptoms of the
= infection in a treated population versus an untreated control population.
Prevention of pain includes, for example, reducing the magnitude of, or
alternatively delaying, pain sensations experienced by subjects in a treated
5 population versus an untreated control population.
A "therapeutically effective amount" of a compound, e.g., such as a
polypeptide
or peptide analog of the present invention, with respect to use in treatment,
refers to an
amount of the polypeptide or peptide in a preparation which, when administered
as part
of a desired dosage regimen (to a mammal, preferably a human) alleviates a
symptom,
10 ameliorates a condition, or slows the onset of disease conditions
according to clinically
acceptable standards for the disorder or condition to be treated or the
cosmetic purpose,
e.g., at a reasonable benefit/risk ratio applicable to any medical treatment.
The term "alkyl" refers to a fully saturated branched or unbranched carbon
chain radical having the number of carbon atoms specified, or up to 30 carbon
atoms if
15 no specification is made. For example, a "lower alkyl" refers to an
alkyl having from 1
to 10 carbon atoms, such as methyl, ethyl, propyl, butyl, pentyl, hexyl,
heptyl, and
octyl, and those which are positional isomers of these alkyls. Alkyl of 10 to
30 carbon
atoms includes decyl, undecyl, dodecyl, tridecyl, tetradecyl, pentadecyl,
hexadecyl,
heptadecyl, octadecyl, nonadecyl, eicosyl, heneicosyl, docosyl, tricosyl. and
tetracosyl.
20 In preferred embodiments, a straight chain or branched chain alkyl has
30 or fewer
carbon atoms in its backbone (e.g., C1-C30 for straight chains, c3-c30 for
branched
chains), and more preferably 20 or fewer. Likewise, preferred cycloalkyls have
from 3-
carbon atoms in their ring structure, and more preferably have 5, 6, or 7
carbons in
the ring structure.
25 Moreover, the term "alkyl" (or "lower alkyl") as used
throughout the
specification, examples, and claims is intended to include both unsubstituted
and
substituted alky chains, the latter of which refers to alkyl moieties having
substituents
replacing a hydrogen on one or more carbons of the hydrocarbon backbone. Such
substituents can include, for example, a halogen, a hydroxyl, a carbonyl (such
as a
30 carboxyl, an alkoxycarbonyl, a formyl, or an acyl), a
thiocarbonyl (such as a thioester, a
thioacetate, or a thioformate), an alkoxyl, a phosphoryl, a phosphate, a
phosphonate, a
phosphinate, an amino, an amido, an amidine, a cyano, a nitro, a sulfhydryl,
an
-16-
CA 02888907 2015-04-23
alkylthio, a sulfate, a sulfonate, a sulfamoyl, a sulfonamido, a sulfonyl, a
heterocyclyl,
an aralkyl, or an aromatic or heteroaromatic moiety. It will be understood by
those
sldlled in the art that the moieties substituted on the hydrocarbon chain can
themselves
be substituted, if appropriate. For instance, the substituents of a
substituted alkyl may
include substituted and unsubstituted forms of amino, azido, imino, amido,
phosphoryl
(including phosphonate and phosphinate), sulfonyl (including sulfate,
sulfonamido,
sulfamoyl and sulfonate), and silyl groups, as well as ethers, alkylthios,
carbonyls
(including ketones, aldehydes, carboxylates, and esters), -CF3, -CN and the
like.
Exemplary substituted alkyls are described below. Cycloalkyls can be further
substituted with alkyls, alkenyls, alkoxyls, alkylthios, aminoalkyls, carbonyl-
substituted
alkyls, -CF3, -CN, and the like.
Unless the number of carbons is otherwise specified, "lower alkyl", as used
herein, means an alkyl group, as defmed above, but having from one to ten
carbons,
more preferably from one to six carbon atoms in its backbone structure such as
methyl,
ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, and tert-butyl.
Likewise, "lower
alkenyl" and "lower alkynyl" have similar chain lengths. Throughout the
application,
preferred alkyl groups are lower alkyls. In preferred embodiments, a
substituent
designated herein as alkyl is a lower alkyl.
The term "carbocycle", as used herein, refers to an aromatic or non-aromatic
ring in which each atom of the ring is carbon.
The term "aryl" as used herein includes 5-, 6- and 7-membered single-ring
aromatic groups that may include from zero to four heteroatoms, for example,
benzene,
pyrrole, furan, thiophene, imidazole, oxazole, thiazole, ttiazole, pyrazole,
pyridine,
pyrazine, pyridazine and pyrimidine, and the like. Those aryl groups having
heteroatoms in the ring structure may also be referred to as "aryl
heterocycles" or
"heteroaromatics". The aromatic ring can be substituted at one or more ring
positions
with such substituents as described above, for example, halogen, azide, alkyl,
aralkyl,
alkenyl, alkynyl, cycloalkyl, hydroxyl, alkoxyl, amino, nitro, sulfhydryl,
imino, amido,
phosphonate, phosphinate, carbonyl, carboxyl, silyl, ether, alkylthio,
sulfonyl,
sulfonamido, ketone, aldehyde, ester, heterocyclyl, aromatic or heteroaromatic
moieties, -CF3, -CN, or the like. The term "aryl" also includes polycyclic
ring systems
having two or more cyclic rings in which two or more carbons are common to two
-17-
CA 02888907 2015-04-23
adjoining rings (the rings are "fused rings") wherein at least one of the
rings is
aromatic, e.g., the other cyclic rings can be cycloalkyls, cycloalkenyls,
cycloalkynyls,
aryls and/or heterocyclyls.
"Alkenyl" refers to any branched or unbranched unsaturated* carbon chain
radical having the number of carbon atoms specified, or up to 26 carbon atoms
if no
limitation on the number of carbon atoms is specified; and having 1 or more
double
bonds in the radical. Alkenyl of 6 to 26 carbon atoms is exemplified by
hexenyl,
heptenyl, octenyl, nonenyl, decenyl, undecenyl, dodenyl, tridecenyl,
tetradecenyl,
pentadecenyl, hexadecenyl, heptadecenyl, octadecenyl, nonadecenyl, eicosenyl,
heneicosoenyl, docosenyl, tricosenyl and tetracosenyl, in their various
isomeric forms,
where the unsaturated bond(s) can be located anywhere in the radical and can
have
either the (Z) or the (E) configuration about the double bond(s).
The term "alkynyi" refers to hydrocarbyl radicals of the scope of alkenyl, but
having one or more triple bonds in the radical.
The terms "alkoxyl" or "alkoxy" as used herein refers to an allcyl group, as
defined below, having an oxygen radical attached thereto. Representative
alkoxy
= groups include methoxy, ethoxy, propoxy, tert-butoxy and the like. An
"ether" is two
hydrocarbons covalently linked by an oxygen. Accordingly, the substituent of
an alkyl
that renders that alkyl an ether is or resembles an alkoxyl, such as can be
represented by
one of -0-alkyl, -0-allcenyl, -0-alkynyl, -0-(CH2).-R1, where m and R1 are
described
below.
The terms "heterocycly1" or "heterocyclic group" refer to 3- to 10-membered
ring structures, more preferably 3- to 7-membered rings, whose ring structures
include
one to four heteroatoms. Heterocycles can also be polycycles. Heterocyclyl
groups
include, for example, thiophene, thianthrene, furan, pyran, isobenzofuran,
chromene,
xanthene, phenoxathiin, pyrrole, imidazole, pyrazole, isothiazole, isoxazole,
pyridine,
pyrazine, pyrimidine, pyridazine, indolizine, isoindole, indole, indazole,
purine,
quinolizine, isoquinoline, quinoline, phthalazine, naphthyridine, quinoxaline,
quinazoline, cinnoline, pteridine, carbazole, carboline, phenanthridine,
acridine,
pyrimidine, phenanthroline, phenazine, phenarsazine, phenothiazine, furazan,
phenoxazine, pyrrolidine, oxolane, thiolane, oxazole, piperidine, piperazine,
morpholine, lactones, lactams such as azetidinones and pyrrolidinones,
sultams,
- 18-
CA 02888907 2015-04-23
sultones, and the like. The heterocyclic ring can be substituted at one or
more positions
with such substituents as described above, as for example, halogen, alkyl,
aralkyl,
alkenyl, alkynyl, cycloalkyl, hydroxyl, amino, nitro, sulfhydryl, imino,
smido,
phosphate, phosphonate, phosphinate, carbonyl, carboxyl, silyl, sulfamoyl,
sulfinyl,
ether, alkylthio, sulfonyl, ketone, aldehyde, ester, a heterocyclyl, an
aromatic or
heteroaromatic moiety, -CF3, -CN, or the like.
The term "alkylthio" refers to an alkyl group, as defined above, having a
sulfur
radical attached thereto. In preferred embodiments, the "alkylthio" moiety is
represented by one of-(S)-alkyl, -(S)-alkenyl, -(S)-alicynyl, and -(S)-(CH2)m-
Ri,
wherein m and R1 are defmed below. Representative alkylthio groups include
methylthio, ethylthio, and the like.
As used herein, the term "nitro" means -NO2; the term "halogen" designates F,
Cl, Br or I; the term "sulthydryl" means -SH; the term "hydroxyl" means -OH;
and the
term "sulfonyl" means -SOT-.
The terms "amine" and "amino" are art-recogni7ed and refer to both
unsubstituted and substituted amines, e.g., a moiety that can be represented
by the
general formulae:
R6
,R6
FR or ii¨N¨R5
R3 R3
wherein R3, R5 and R6 each independently represent a hydrogen, an alkyl, an
alkenyl,
-(CH2)m-RI, or R3 and R5 taken together with the N atom to which they are
attached
complete a heterocycle having from 4 to 8 atoms in the ring structure; R1
represents an
alkenyl, aryl, cycloalkyl, a cycloalkenyl, a heterocyclyl or a polycyclyl; and
m is zero
or an integer in the range of 1 to 8. In preferred embodiments, only one of R3
or R5 can
be a carbonyl, e.g., R3, R5 and the nitrogentogether do not forin an imide. In
even
more preferred embodiments, R3 and R5 (and optionally IQ each independently
represent a hydrogen, an alkyl, an alkenyl, or -(C112)m-Ri. Thus, the term
"alkylamine"
as used herein means an amine group, as defined above, having a substituted or
unsubstituted alkyl attached thereto, i.e., at least one of R3 and R5 is an
alkyl group. In
certain embodiments, an amino group or an alkylamine is basic, meaning it has
a Plc?
- 19 -
CA 02888907 2015-04-23
7.00. The protonated forms of these functional groups have pKas relative to
water
above 7.00.
The term "carbonyl" is art-recognized and includes such moieties as can be
represented by the general formula:
,R
tzt, x 7 or
XR8
wherein X is a bond or represents an oxygen or a sulfur, and R7 represents a
hydrogen,
an alkyl, an allcenyl, -(CH2)arRi or a pharmaceutically acceptable salt, R8
represents a
hydrogen, an alkyl, an alkenyl or -(CH2)m-Ri, where m and R1 are as defined
above.
Where X is an oxygen and R7 or Rg is not hydrogen, the formula represents an
"ester".
Where X is an oxygen, and R7 is as defined above, the moiety is referred to
herein as a
carboxyl group, and particularly when R7 is a hydrogen, the formula represents
a
"carboxylic acid". Where X is an oxygen, and Rg is hydrogen, the formula
represents a
"formate". In general, where the oxygen atom of the above formula is replaced
by
sulfur, the formula represents a "thiocarbonyl" group. Where X is a sulfur and
R7 or Rg
is not hydrogen, the formula represents a "thioester" group. Where X is a
sulfur and R7
is hydrogen, the formula represents a"thiocarboxylic acid" group. Where X is a
sulfur
and R8 is hydrogen, the formula represents a "thioformate" group. On the other
hand,
where X is a bond, and R7 is not hydrogen, the above formula represents a
"ketone"
group. Where X is a bond, and R7 is hydrogen, the above formula represents an
"aldehyde" group.
As used herein, the term "substituted" is contemplated to include all
permissible
substituents of organic compounds. In a broad aspect, the permissible
substituents
include acyclic and cyclic, branched and unbranched, carbocyclic and
heterocyclic,
aromatic and nonaromatic substituents of organic compounds. Mustrative
substituents
include, for example, those described hereih above. The permissible
substituents can
be one or more and the same or different for appropriate organic compounds.
For
purposes of this invention, the heteroatoms such as nitrogen may have hydrogen
substituents and/or any permissible substituents of organic compounds
described herein
which satisfy the valences of the heteroatoms. This invention is not intended
to be
limited in any manner by the permissible substituents of organic compounds. It
will be
- 20 -
CA 02888907 2015-04-23
understood that "substitution" or "substituted with" includes the implicit
proviso that
such substitution is in accordance with permitted valence of the substituted
atom and
the substituent, and that the substitution results in a stable compound, e.g.,
which does
not spontaneously undergo transformation such as by rearrangement,
cyclization,
elimination, etc.
The term "sulfamoyl" is art-recognized and includes a moiety that can be
represented by the general formula:
0
¨S--N
11
in which R3 and R5 are as defined above.
The term "sulfate" is art recognized and includes a moiety that can be
represented by the general fornmla:
0
" R
0 7
in which R7 is as defined above.
The term "sulfamido" is art recognized and includes a moiety that can be
represented by the general formula:
0
ti
1¨N¨S¨R8
I I
R30
in which R2 and R4 are as defined above.
The term "sulfonate" is art-recognized and includes a moiety that can be
represented by the general formula:
0
11
"
0 R7
in which R7 is an electron pair, hydrogen, alkyl, cycloalkyl, or aryl.
-21
CA 02888907 2015-04-23
The terms "sulfoxido" or "sulfinyl", as used herein, refers to a moiety that
can
be represented by the general formula:
0
a II
t¨S¨Ri2
in which R12 is selected from the group consisting of hydrogen, alkyl,
alkenyl, alkynyl,
cycloalkyl, heterocyclyl, aralkyl, or aryl.
Analogous substitutions can be made to alkenyl and alkynyl groups to produce,
for example, aminoalkenyls, aminoalkynyls, amidoalkenyls, amidoalkynyls,
iminoalkenyls, iminoalkynyls, thioalkenyls, thioalkynyls, carbonyl-substituted
alkenyls
or alkynyls.
As used herein, the definition of each expression, e.g., alkyl, m, n, etc.,
when it
occurs more than once in any structure, is intended to be independent of its
definition
elsewhere in the same structure.
For purposes of this invention, the chemical elements are identified in
accordance with the Periodic Table of the Elements, CAS version, Handbook of
Chemistry and Physics, 67th Ed., 1986-87, inside cover. Also for purposes of
this
invention, the term "hydrocarbon" is contemplated to include all permissible
compounds having at least one hydrogen and one carbon atom. In a broad aspect,
the
permissible hydrocarbons include acyclic and cyclic, branched and unbranched,
carbocyclic and heterocyclic, aromatic and nonaromatic organic compounds which
can
be substituted or unsubstituted.
III Exemplag Embodiments
(a) P analogs
The present invention provides for the manufacture and use of peptide and P'i
analogs resistant to proteinase-mediated cleavage. Given a native polypeptide
typically
cleaved by a particular proteinase (e.g., a metalloproteinase, a cysteine
proteinase, an
aspartic proteinase, or a serine proteinase), one can readily determine the
site within the
native polypeptide at which the proteinase cleaves (the cleavage site). Once
the
cleavage site is identified, P'i analogs can be readily made according to the
methods of
the present invention. Given the depth of understanding in the art of
enzymology, the
- 22 -
CA 02888907 2015-04-23
preferred cleavage sites of a large number of proteinases are known, and the
identification of the consensus cleavage site in a given native polypeptide
can be
rapidly and easily accomplished by simply examining the amino acid sequence.
In the event that the cleavage site within a particular polypeptide is not
known
or can not be rapidly determined by simply examining the amino acid sequence,
the
cleavage site can be determined by simply incubating native polypeptide and
proteinase
to allow cleavage, separating the cleaved polypeptide species (e.g., by
electrophoresis),
and sequencing the cleaved peptide fragments. By determining the sequence of
the
ends of the cleaved peptide fragment, and comparing this sequence to that of
the full-
length polypeptide sequence, one can rapidly and easily identify or verify the
cleavage
site within a native polypeptide at which a proteinase acts.
Another exemplary method for rapidly deternaining the substrate specificity of
a
proteinase is provided, for example, by PCT Publication W00061789.
The present invention provides generoli7able methods for constructing
proteinase resistant P'i analogs. The present invention contemplates the
design and use
of P' analogs resistant to metalloproteinases, cysteine proteinases, aspartic
proteinases,
and serine proteinases. For instant, the subject analogs can be rendered
resistant to
cleavage by proteinases selected from: an aminopeptidase (EC 3.4.11.-), a
dipeptidase
= (EC 3.4.13.-), a dipeptidyl-peptidase or tripeptidyl peptidase (EC
3.4.14.-), a peptidyl-
dipeptidase (EC 3.4.15.-), a serine-type carboxypeptidase (EC 3.4.16.-), a
metallocarboxypeptidase (EC 3.4.17.-), a cysteine-type carboxypeptidase (EC
3.4.18.-),
an omegapeptidase (EC 3.4.19.-), a serine proteinase (EC 3.4.21.-), a cysteine
proteinase (EC 3.4.22.-), an aspartic proteinase (EC 3.4.23.-), a metallo
proteinase (EC
3.4.24.-), or a proteinase of unknown mechanism (EC 3.4.99.-). The EC
designation
following each class of proteinase is that used in the recommendation of the
International Union of Biochemistry and Molecular Biology (1984), and these
subclass
headings are provided here for reference.
To further illustrate the exemplary proteinases for which proteinase-resistant
P'1
analogs are contemplated, an non-exhaustive list of proteinases include:
leucyl
aminopeptidase, membrane alanine aminopeptidase, cystinyl aminopeptidase,
tripeptide
aminopeptidase, prolyl aminopeptidase, aminopeptidase B, glutamyl
aminopeptidase,
Xaa-Pro aminopeptidase, bacterial leucyl aminopeptidase, clostridial
aminopeptidase,
- 23 -
CA 02888907 2015-04-23
cytosol alanyl aminopeptidase, lysyl aminopeptidase, Xaa-Trp aminopeptidase,
= tryptophanyl aminopeptidase, methionyl aminopeptidase, D-stereospecific
aminopeptidase, aminopeptidase Ey, vacuolar aminopeptidase I, Xaa-His
dipeptidase,
Xaa-Arg dipeptidase, Xaa-methyl-His dipeptidase, Cys-Gly dipeptidase, Glu-Glu
5 dipeptidase, Pro-Xaa dipeptidase, Xaa-Pro dipeptidase, Met-Xaa
dipeptidase, non-
stereospecific dipeptidase, cytosol non-specific dipeptidase, membrane
dipeptidase,
Beta-Ala-His dipeptidase, Dipeptidyl-peptidase I (DPP 1), Dipeptidyl-peptidase
II (DPP
ID, Dipeptidyl-peptidase III (DPP 111), Dipeptidyl-peptidase IV(DPP IV),
Dipeptidyl-
dipeptidase, Tripeptidyl-peptidase 1, Tripeptidyl-peptidase 11, Xaa-Pro
dipeptidyl-
10 peptidase, peptidyl-dipeptidase A, peptidyl-dipeptidase B, peptidyl-
dipeptidase Dcp,
lysosomal Pro-X carboxypeptidase, Serine-type D-Ala-D-Ala carboxypeptidase,
carboxypeptidase C, carboxypeptidase D, carboxypeptidase A, carboxypeptidase
B,
lysine(arginine) carboxypeptidase, Gly-X carboxypeptidase, alanine
carboxypeptidase,
muramoylpentapeptide carboxypeptidase, carboxypeptidase H, glutamate
15 carboxypeptidase, carboxypeptidase M, murarnoyltetrapeptide
carboxypeptidase, zinc
D-Ala-D-Ala carboxypeptidase, carboxypeptidase A2, membrane Pro-X
carboxypeptidase, tubulinyl-Tyr carboxypeptidase, carboxypeptidase T,
thermostable
carboxypeptidase 1, carboxypeptidase U, glutamate carboxypeptidase 11,
metallocarboxypeptidase D, cysteine-type carboxypeptidase, acylaminoacyl-
peptidase,
20 peptidyl-glycinamidase, pyroglutamyl-pepticlase I, beta-aspartyl-
peptidase,
pyroglutarnyl-peptidase 11, N-formylmethionyl-peptidase, pteroylpoly-gamma-
glutamate carboxypeptidase, gamma-glutamyl hydrolase, gamma-D-glutamyl-meso-
diaminopimelate peptidase I, chymotrypsin, chymotrypsin C, metridin, trypsin,
thrombin, coagulation factor Xa, plasmin, enteropeptidase, acrosin, alpha-
lytic
25 endopeptidase, glutamyl endopeptidase, cathepsin G,
coagulation factor VI1a,
coagulation factor IXa, cucumisin, prolyl oligopeptidase, coagulation factor
X1a,
brachyurin, plasma kallikrein, tissue kallikrein, pancreatic elastase,
leukocyte elastase,
coagulation factor XI1a, chymase, complement component Clr, complement
component Cls, classical-complement pathway C3/C5 convertase, complement
factor
30 I, complement factor D, alternative-complement pathway
C3/C5 convertase, cerevisin,
hypodermin C, lysyl endopeptidase, endopeptidase La, gamma-renin, venombin AB,
leucyl endopeptidase, tryptase, scutelarin, kexin, subtilisin, oryzin,
proteinase K,
thermomycolin, thermitase, endopeptidase So, T-plasminogen activator, protein
C
- 24 -
CA 02888907 2015-04-23
(activated), pancreatic endopeptidase E, pancreatic elastase 11, IgA-specific
serine
endopeptidase, U-plasminogen activator, venombin A, fain, myeloblastin,
semenogelase, granzyme A, granzyme B, streptogrisin A, streptogrisin B,
glutamyl
endopeptidase 11, oligopeptidase B, limulus clotting factor C, limulus
clotting factor B,
limulus clotting enzyme, omptin, repressor lexA, signal peptidase I,
togavirin, flavirin,
endopeptidase Clp, proprotein convertase 1, proprotein convertase 2, snake
venom
factor V activator, lactocepin, cathepsin B, papain, ficain, chymopapain,
asclepain,
clostripain, streptopain, actinidain, cathepsin L, cathepsin H, calpain,
cathepsin T,
glycyl endopeptidase, cancer procoagulant, cathepsin S, picomain 3C, picomain
2A,
caricain, ananain, stem bromelain, fruit bromelain, legumain, histolysain,
caspase-1,
gingipain R, cathepsin K, pepsin A, pepsin B, gastricsin, chymosin, cathepsin
D,
neopenthesin, renin, retropepsin, pro-opiomelanocortin converting enzyme,
aspergillopepsin I, aspergillopepsin II, penicillopepsin, rhizopuspepsin,
endothiapepsin,
mucoropepsin, candidapepsin, saccharopepsin, rhodotorulapepsin, physaropepsin,
acrocylindropepsin, polyporopepsin, pycnoporopepsin, scytalidopepsin A,
scytalidopepsin B, xanthomonapepsin, cathepsin E, barrierpepsin, signal
peptidase 11,
pseudomonapepsin, plasmepsin I, plasmepsinII, phytepsin, atrolysin A,
microbial
collagenase, leucolysin, interstitial collagenase, neprilysin, envelysin, IgA-
specific
metalloendopeptidase, procollagen N-endopeptidase, thimet oligopeptidase,
neurolysin,
stromelysin 1, meprin A, procollagen C-endopeptidase, peptidyl-Lys
metalloendopeptidase, astacin, stromelysin 2, matrilysin, gelatinase A,
aeromonolysin,
pseudolysin, thermolysin, bacillolysin, aureolysin, coccolysin, mycolysin,
beta-lytic
metalloendopeptidase, peptidyl-Asp metalloendopeptidase, neutrophil
collagenase,
gelatinase B, leishmanolysin, saccharolysin, autolysin, deuterolysin,
serralysin,
atrolysin B, atrolysin C, atroxase, atrolysin E, atrolysin F, adamalysin,
horrilysin,
ruberlysin, bothropasin, bothrolysin, ophiolysin, trimerelysin I, trimerelysin
11,
mucrolysin, pitrilysin, insulysin, 0-sialoglycoprotein endopeptidase,
russellysin,
mitochondrial intermediate peptidase, dactylysin, nardilysin, magnolysin,
meprin B,
mitochondrial processing peptidase, macrophage elastase, choriolysin L,
choriolysin H,
tentoxilysin, bontoxilysin, oligopeptidase A, endothelin-converting enzyme 1,
fibrolase,
jararhagin, fragilysin, and multicatalytic endopeptidase complex.
One aspect of the present invention is a =polypeptide sequence encoding for a
proteinase-resistant analog of a polypeptide hormone that has an N-terminal
sequence
- 25 - =
CA 02888907 2015-04-23
selected from NH2-Xaa-Ala-Yaa- and NH2-Xaa-Pro-Yaa-, where Xaa and Yaa each
independently represent an amino acid residue. In certain embodiments, Xaa is
an
amino acid with aromatic side chain. In certain embodiments, Xaa is selected
from
histidine, tyrosine, tryptophan, and phenylalanine. In certain embodiments,
Yaa is an
amino acid residue with an acidic side chain. In certain embodiments, Yaa, is
selected
from aspartic acid and glutamic acid.
By way of example, in certain embodiments, the proteinase is a serine
proteinase. In some embodiment the proteinase is a dipeptidyl peptidase. An
exemplary dipeptidyl peptidase is dipeptidyl peptidase IV (DPP IV). DPP IV
activity
alters the biological activity of a large number of bioactive proteins and
polypeptides.
In addition to the potential DPP IV substrates disclosed in U.S. Patent
6,090,786, the
present invention is also directed to analogs of GLP-1, GLP-2, and GIP. In
certain
embodiments, the peptide hormone is a naturally occurring variety found in
mammals.
In certain embodiments, the peptide hormone is a naturally, or artificially
mutated
variety of a naturally occurring (wild type) peptide hormone. Thus, natural
and
synthetic peptide hormones are within the scope of peptide hormones
contemplated for
the modifications. Thus in certain embodiments, the present invention provides
DPP
IV proteolysis-resistant analogs of the aforementioned peptide hormones.
To provide further illustration of proteinase-resistant P'i analogs, Table I
provides a list of several human hormones that are substrates of DPP IV The
13,1 amino
acid in each peptide hormone is labeled with an asterisk. Exemplary analogs
are
shown, wherein X is an amino acid analog having a sidechain represented in,
for
example, Formula II above. One can readily construct a similar table
comprising
substrates for other serine proteinases and readily identify the P'i amino
acid.
Similarly, one can readily construct a table comprising substrates for a given
aspartic
proteinase, cysteine proteinase, or metalloproteinase and identify the P'1
amino acid.
Table 1: Exemplary analogs of DPP IV substrates
Native sequence Exemplary Analog
Human HAE*GTFTSDVSSITEGQ
HAXGTFTSDVSSYLEGQA
glucagon-like peptide AA10EFIAWLVKGR AKEFIAWLVKGRG
GLP-1(7-37)
Human HAE*GTFTSDVSSYLEGQ
HAXGTFTSDVSSYLEGQA
glucagon-like peptide AAICEFIAWLVKGR-NH2 AKEFIAWLVKGR-NH2
1: GLP-1 (7-36)N112
- 26 -
CA 02888907 2015-04-23
Human HAD*GSFSDEMNTELDNL
HAXGSFSDEMNTILDNLA
glucagon-like peptide AARDF1NWLIQTKITD ARDFINWLIQTKITD
2, GLP-2
Human YAE*GTFISDYSIAMDKI
YAXGTFISDYSIAMDKIHQ
glucose-dependent
HQQDFVNWLLAQICGICKNDWICH QDFVNWLLAQKGICKNDWKHNIT
insulinotropic NITQ
_ polypeptide, GIP
Human YPS*KPDNPGEDAPAED
YPXKPDNPGEDAPAEDM
neuropeptide Y, NPY MARYYSALRHYINLITRQRY ARYYSALRHYINLITRQRY
Human APL*EPVYPGDNATPEQ
APXEPVYPGDNATPEQIVIA
pancreatic polypeptide MAQYAADLRRY QYAADLRRY
PP
Human YPI*KPEAPGEDASPEEL
YPXKPEAPGEDASPEELN
peptide YY NRYYASLRHYLNLVTRQRY RITYASLRHYLNLVIRQRY
exendin-4 HGE*GTFTSDLSKEMEEE
HGXGTFTSDLSICEMEEEA
(GLP-1 analog) AVRLFIEWLKNGGPSSGAPPPS- VRLFIEWLICNGGPSSGAPPPS-NH2
NH2
exendin-3 1-1SD*G11.1 SDLSKQMEEE
HSXGTFTSDLSKQMEEEA
(GLP-1 analog) AVRLFIEWLKNGGPSSGAPPPS VRLFIEWLICNGGPSSGAPPPS
In certain embodiments of the GLP-1(7-37), GLP-1(7-36)NH2, GLP-1 (7-36)-
Exendin tail-NH2, GLP-2, GIP and exendin-3 analogs, X is an amino acid analog
of
Formula (II). In preferred embodiments, X is an amino acid analog of Formula
(II)
wherein R1 and R2 each independently represent methyl, ethyl, or propyl. In
the most
preferred embodiment, X is an amino acid analog of Formula (11), wherein both
R1 and
R2 are methyl, and R3 is selected from -COOH and -CH2-COOH.
In certain preferred embodiments of the NPY analogs, X is an amino acid
analog of Formula (11). In preferred embodiments, X is an amino acid analog of
Formula (II) wherein R1 and R2 each independently represent methyl, ethyl, or
propyl.
In the most preferred embodiment, X is an amino acid analog of Formula (11),
wherein
both R1 and R2 are methyl, and R3 represents -OH.
In certain preferred embodiments of the pancreatic polypeptide PP and peptide
YY (PYY) analogs, X is an amino acid analog of Formula (II). In preferred
embodiments, X is an amino acid analog of Formula (II) wherein R1, R2, and R3
each
independently represent methyl, ethyl, or propyl. In the most preferred
embodiment, X
is an amino acid analog of Formula (11), wherein both R1 and R2 are methyl,
and R3
represents --CH(CH3)2 or ¨CH2-CH3.
In certain preferred embodiments of the exendin-4 analogs, X is an amino acid
analog of Formula (II). In preferred embodiments, X is an amino acid analog of
Formula OD wherein R1 and R2 each independently represent methyl, ethyl, or
propyl,
and R3 represents -(CH2).,-C(=0)NH2 (wherein m is 0, 1, or 2). In the most
preferred
-27-
CA 02888907 2015-04-23
embodiment, X is an amino acid analog of Formula (11), wherein both R1 and R2
are
methyl, and R3 represents -CH2-C(=0)NH2.
More generally, the present invention specifically contemplates the generation
of analogs for peptide and polypeptide factors that have an amino acid
sequence
Xaa-Ala-Yaa-R or Xaa-Pro-Yaa-R'
wherein Xaa and Yaa represent amino acid residues, and R and R',
independently for each occurrence, represent polypeptide chains comprising 1
to about
100 amino acid residues and wherein in the analog sequence Yaa is replaced by
an
amino acid residue represented by Formula I or Formula H. The invention
further
contemplates the modification of variant polypeptides that differ in sequence
from the
wildtype polypeptide inorder to produce variant P'i analogs. Such variants are
at least
80%, 85%, 90%, 95%, 97%, 99%, or greater than 99% identical to the wildtype
polypeptide.
In certain embodiments, R is a polypeptide having an amino acid sequence
selected
from the group consisting of
GTFTSDVSSYLEGQAAKEFIAWLVKGR,
GTFTSDVSSYLEGQAAKEFIAWLVKGRPSSGAPPPS-N1-12,
GTFTSDVSSYLEGQAAKEFIAWLVKGR-NH2,
GSFSDEMNTILDNLAARDFINWLIQTKITD, and
=
GTFISDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ,
or a sequence that differs by 5 or fewer amino acid residues thereto, 'even
more
preferably differs by no more than 4, 3, or even 2 amino acid residues.
In certain embodiments, R is a polypeptide having an amino acid sequence
selected
from the group consisting of
ICPDNPGEDAPAEDMARYYSALRHYINLITRQRY,
EPVYPGDNATPEQMAQYAADLRRY, and
KPEAPGEDASPEELNRVYASLRHYLNLVTRQRY,
or a sequence that differs by 5 or fewer amino acid residues thereto, even
more
preferably differs by no more than 4, 3, or even 2 amino acid residues.
Proteinase-resistant GHRH analogs provide still further illustration of the
generalizable methods and compositions of the present invention. Regulated
expression of the growth hormone (GH) pathway is essential for optimal linear
growth,
- 28 -
CA 02888907 2015-04-23
as well as for homeostasis of carbohydrate, protein, and fat metabolism.
Growth
hormone synthesis and its pulsatile secretion from the anterior pituitary is
stimulated by
growth hormone¨releasing hormone (GBRH) and inhibited by somatostatin, both
hypothalamic hormones. Gro*th hormone increases production of insnlin-like
growth
5 factor-] (IGF-I) primarily in the liver, as well as other target organs.
Linear growth velocity and body composition respond to GH or GBRH
replacement therapies in a broad spectrum of conditions, both in humans and in
farm
animals. The etiology of these conditions can vary significantly. In 50% of
human GH
deficiencies the GBRII¨GH¨IGF-I axis is functionally intact but does not
elicit the
10 appropriate biological responses in its target tissues. Similar
phenotypes are produced
by genetic defects at different points in the GH axis, as well as in non¨GH-
deficient
short stature. In several conditions characterized by growth retardation in
which the
GHRH¨GH¨IGF¨I axis is functional, such as Turner's syndrome,
hypochondroplasia,
Crohn's disease, intrauterine growth retardation, or chronic renal
insufficiency,
15 therapeutic administration of GBRH or GH has been shown to be effective
in
promoting growth.
In the elderly, there is considerable decrement in the activity of the GHRH¨
.
GH¨IGF-I axis that results in reduced GH secretion and IGF-I production. These
changes are associated with a loss of skeletal muscle mass (sarcopenia),
osteoporosis,
20 increased fat deposition, and decreased lean body mass. It has been
demonstrated that
the development of these changes can be offset by recombinant GH therapy.
Current GH therapy has several shortcomings, however, including frequent
subcutaneous or intravenous injections, insulin resistance, and impaired
glucose
tolerance. Children treated with GH are vulnerable also to premature
epiphyseal closure
25 and slippage of the capital femoral epiphysis. In domestic
livestock, GHRH and GH
stimulate milk production, increase feed-to-milk conversion, and sustain
growth,
primarily by increasing lean body mass, and increase overall feed efficiency.
Hot and
chilled carcass weights are increased, and carcass lipid (percentage of soft-
tissue mass)
is decreased by GHRH.
30 Although GIIRH protein therapy entrains and stimulates
normal cyclical GH
secretion with virtually no side effects, the short half-life of the molecule
in vivo
requires frequent (one to three times per day) intravenous, subcutaneous, or
intranasal
- 29 -
'
CA 02888907 2015-04-23
(at a 300-fold higher dose) administrations. Thus, recombinant GHRH
administration
is not practical as a chronic therapy.
GHRH has a primary sequence as indicated below. The P'1 amino acid (in this
case as aspartic acid) is marked-by an asterisk and bolded.
5 YAD*AIFTNSYRKVLGQLSARKLLQDIMSRQQGESNQERGARARL
GBRH is cleaved by the post-proline cleaving enzyme prolyl endopeptidase
(PEP). PEP is a cytosolic endopeptidase which cleaves a variety of substrates
in
addition to GBRH including neuroactive peptides, such as arginine vasopressin,
luteinizing hormone-releasing hormone, thyrotropin releasing hormone, alpha-
10 melanocyte secreting hormone, substance P, oxytocin, bradykinin,
neurotensin and
angiotensin (Ag) I and II.
Accordingly, in certain embodiment, the invention contemplates GIFRH analogs
having an amino acid sequence represented in the general formula:
Tyr-Ala-Yaa-R
15 wherein Yaa represent amino acid having a sidechain represented in
Formula I
or Fonnula Et above, and R represents a polypeptide chain having the sequence
= AlFTNSYRKVLGQLSARKLLQDIMSRQQGESNQERGARARL, or a sequence that
differs by 5 or fewer amino acid residues thereto, even more preferably
differs by no
more than 4, 3, or even 2 amino acid residues. In certain preferred
embodiments, Ri
20 and 122 each independently represent a methyl, ethyl or propyl, and even
more
preferably a methyl, and R3 represents -COOH or -CH2COOH.
To provide an additional example still, two of the primary sites of actions
for
angiotensin (ANG)-(1---7) are the vasculature and the kidney. ANG-(1---7) is
hydrolyzed primarily to ANG-(1---5) by pulmonary membranes. The ANG-converting
25 enzyme (ACE) inhibitor lisinopril abolished the generation
of ANG-(1---5), as well as
that of smaller metabolites. Accordingly, a class of (ANG)-(1 --- 7) peptide
analogs
resistant to cleavage could have the same or similar effect as ACE inhibitor.
In other
words, such peptide analogs would increase the effective concentration and/or
half-life
of (ANG)-(1 --- 7).
30 Yet another example with important applications to the
generation of
therapeutic agents for the treatment of disease is IGFBP-3. IGEBP-3 in serum
and
-30-
CA 02888907 2015-04-23
other body fluids is cleaved by proteinases, and the proteolytic products have
greatly
reduced or no affinity for IGF's. Increased proteolysis of IGFBP-3 has been
observed
in various clinical and physiological conditions including both pregnancy and
certain
cancers. Accordingly, the design of proteinase resistant IGFBP-3 analogs may
be
particularly useful in maintaining appropriate IGFBP-3 levels, for example in
cancers
associated with increased proteolysis of IGFBP-3.
The above cited examples are meant solely for illustration. The present
invention provides a generalizable method by which virtually any polypeptide
that is a
substrate for a proteinase can be manipulated with a tetra-substitution at the
cleavage
site to produce a proteinase resistant P'i analog. Exemplary polypeptides that
are
substrates for proteinases and accordingly which can be manipulated at the
cleavage
site to produce a proteinase resistant P'1 analog include, without limitation,
enkephalin,
Leu-enkephalin, Met-enkephalin, angiotensin I, an.giotensin 11, vasopressin,
endothelin,
vasoactive intestinal peptide, neurotensin, endorphins, insulin, grarnicidin,
paracelsin,
delta-sleep inducing peptide, gonadotropin-releasing hormone, human
parathyroid
hormone (1-34), truncated erythropoietin analogues described in Wrighton et
al., 1996,
Science 273:458-463), specifically EMP-1, Atrial natriuretic peptide (ANP,
ANF),
human brain natriuretic peptide (hBNP), cecropin, kinetensin, neurophysins,
elafin,
guamerin, atriopeptin I, atriopeptin 11, atriopeptin deltorphin I,
deltorphin II,
vasotocin, bradykinin, dynorphin, dynorphin A, dynorphin B, growth hormone
release
factor, growth hormone, growth hormone releasing peptide, oxytocin,
calcitonin,
calcitonin gene-related peptide, calcitonin gene-related peptide 1J growth
hormone
releasing peptide, tachykinin, adrenocorticotropic hormone (ACTH), brain
natriuretic
polypeptide, cholecystokinin, corticotropin releasing factor, diazeparn
binding inhibitor
fragment, FMRF-amide, galanin, gastric releasing polypeptide, gastric
inhibitory
polypeptide, gastrin, gastrin releasing peptide, glucagon, glucagon-like
peptide-1,
glucagon-like peptide-2, LHRH, melanin concentrating hormone, melanocyte
stimulating hormone (MSH), alpha-MSH, morphine modulating peptides, motilin,
neurokinin A, neurolcinin B, neuromedin 13, neuromedin C, neuromedin K,
neuromedin N, neuromedin U, neuropeptide K, neuropeptide Y, pituitary
adenylate
cyclase activating polypeptide (PACAP), pancreatic polypeptide, peptide YY,
peptide
histidine-methionine amide (PHN/1), secretin, somatostatin, substance K,
thyrotropin-
releasing hormone (TRH), kyotorphin, melanostatin (MIF-1), thrombopoeitin
analogs,
-31 -
CA 02888907 2015-04-23
in particular AF 12505, insulin-like growth factor I (57-70), insulin-like
growth factor I
(30-41), insulin-like growth factor I (24-41), insulin-like growth factor II
(33-40),
insulin-like growth factor II (33-40), insulin-like growth factor II (69-84),
growth
hormone (GH)-releasing peptide6 (GHRP-6), f3-Interleulcin 1 (163-171), 13-
Interleulcin
II (44-56), Interleukin II (60-70), epidermal growth factor, bivalirudin
(Hirulog),
hiralog-I, C-type natriuretic peptide, ornipressin (also known as 8-omithine-
vasopressin), octreotide, eptifibatide, calcitonin gene-related peptide
(CGRP),
endomorphin-1; endomorphin-2, nociceptin, angiotensinogen, adrenomodullin,
antiarrhytmic peptide (AA-P); Antagonist G, indolicidin, osteocalcin,
cortistatin 29,
cortistatin 14, PD-145065, PD-142893, fibrinogen binding inhibitor peptide,
leptin, GR
83074, parathyroid hormone related peptide, angiotensinogen, leupeptin, and
any
modified or truricated analog thereof.
In many embodiments, the analog will be selected to retain one or more of the
in vitro or in vivo activity of the native substrate. The in vitro and in
vivo activities
may be measured using any protocol available to one of ordinary skill that are
appropriate for the particular polypeptide. Exemplary functional activities
that can be
measured to ascertain whether a 13'1 analog maintains the same or similar
functional
activity include ability of the polypeptide to bind its receptor(s) in a cell
based or cell
free assay, ability of the polypeptide to induce a change (e.g.,
proliferation,
differentiation, survival, growth, migration, etc) in a cell responsive to the
polypeptide,
. ability of the polypeptide to modulate the expression of one or more
other genes or
proteins in a cell responsive to the polypeptide.
In certain embodiments, the analog has substantially similar activitiy as the
native polypeptide (e.g., about 80%, 90%, 100%, 110%, or 120% as active as the
native
polypeptide). In some embodiment, the analog is less active than the native
polypeptide (e.g., about 50%, 60%, 70%, or 75% as active as the native
polypeptide).
We note that an analog that is somewhat less active may be useful, such as in
vivo or in
cell culture, if the decrease in activity still provides the ability to
provide a sufficient
local concemtration of analog for a sufficient period of dine. Thus, an
increase in half-
life obtained by proteinase resistance may off-set the decrease in activity
caused by the
construction of the analog. In still other embodiment, the analog is more
active that the
native polypeptide (e.g., about 130%, 150%, 175%, 200%, 300%, 500%, 800%, or
even
1000% as active as the native polypeptide). In any of the foregoing, by
"activitiy" is
- 32 -
CA 02888907 2015-04-23
meant one or more functions of the native polypeptide. For example, an
activity (e.g., a
biological function) of a polypeptide may be receptor binding, cofactor
interaction,
ability to bind to DNA, ability to act as a transcriptional activator or
repressor, the
ability to participate in a particular signal transduction pathway, and the
ability to
influence cell behavior (e.g., proliferation, differentiation, survival, or
migration).
Such activites may be expressed, for example, as relative binding constants
(such as for receptor binding), effective concentrations (EC50) and/or
effective doses
(EDso).
Exemplary P'/ analogs have an increased half life incomparison to the native
polypeptide (in vitro and/or in vivo) due to the resistance of the P'1 analogs
to a
proteinase which typically cleaves the native polypeptide. However, it will be
generally appreciated that various P'1 analogs will have different half-lives
(as well as a
different change in half-life in comparison to the native polypeptide). The in
vitro
and/or in vivo half-life can be readily measured by one of skill in the art
using standard
methods. In certain embodiments, the analog has an in vitro or in vivo half
life that is
about a factor of 0.5, 0.6, 0.7, 0.8., 0.9, 1.0, 1.3, 1.5, 2, 3, 5, 10, 25,
30, 50, 75, 100, or
even greater than 100 times the in vitro and/or in vivo half-life of the
native
polypeptide under similar half-life measurement assay conditions.
(b) Synthesis of peptide hormone analogs
The peptides of the invention can be prepared by standard solid phase
synthesis.
See, e.g., Stewart, J. M., et al., Solid Phase Synthesis (Pierce Chemical Co.,
2d ed.
1984).
The analogs of the invention can be prepared using standard solid-phase
techniques for the synthesis of peptides. As is generally known, peptides of
the
requisite length can be prepared using commercially available equipment and
reagents
following the manufacturers instructions fcir blocking interfering groups,
protecting the
amino acid to be reacted, coupling, deprotection, and capping of unreacted
residues.
Suitable equipment can be obtained, for example, from Applied BioSystems in
Foster
City, Calif, or Biosearch Corporation in San Raphael, Calif.
In a preferred method, the peptides are synthesized using standard automated
solid-phase synthesis protocols employing t-butoxycarbonyl-alpha-amino acids
with
- 33 -
CA 02888907 2015-04-23
appropriate side-chain protection. Completed peptide is removed from the solid
phase
support with simultaneous side-chain deprotection using the standard hydrogen
fluoride
method. Crude peptides are further purified by semi-preparative reverse phase-
HPLC
(Vydac C18) using acetonitrile gradients in 0.1% trifluoroacetic acid (TFA).
The
peptides are vacuum dried to remove acetonitrile and lyophilized from a
solution of
0.1% TFA in water. Purity is verified by analytical RP-HPLC. The peptides can
be
lyophilized and then Solubilized in either water or 0.01M acetic acid at
concentrations
of 1-2 mg/mL by weight.
The use of the aforementioned synthetic methods is needed if nonencoded
amino acids or the D-forms of amino acids occur in the peptides. However, for
peptides
which are gene-encoded, recourse can also be had to recombinant techniques
using
readily synthesized DNA sequences in commercially available expression
systems.
Accordingly, one aspect of the present invention is a method of preparing an
analog of a polypeptide, wherein said peptide is resistant to a proteinase
selected from
the group consisting of a metalloproteinase, a serine proteinase, an aspartic
proteinase,
and a cysteine proteinase. In one embodiment, the analog is resistant to a
serine
proteinase. In another embodiment, the serine proteinase is a dipeptidyl
peptidase such
as a post-proline cleaving dipeptidyl peptidase. In yet another embodiment,
the post-
proline cleaving dipeptidyl peptidase is DPP IV. In any of the foregoing,
preparation of
the proteinase resistant peptide analog may comprise substituting one or more
amino
acid residues in the peptide hormone with an amino acid residue represented by
Formula I or Formula II shown above.
Another aspect of the present invention is a method for preparing an analog of
a
peptide hormone, wherein the peptide hormone has an N-terminal amino acid
sequence
Xaa-Ala-Yaa-R, or Xaa-Pro-Yaa-R', wherein Xaa and Yaa represent amino acid
residues and R and R', independently for each occurrence, represent
polypeptide chains
comprising 1 to about 100 amino acid residues (preferably about ( 90, < 80, <
70, < 60,
< 50, < 40, < 30, < 20, or even < 10 amino acid residues) arid wherein in the
analog
sequence Yaa is replaced by an amino acid residue represented by Formula I or
Formula II shown above.
(c) Functional Assays
- 34 -
CA 02888907 2015-04-23
As outlined in detail herein, the present invention provides a generalizable
way
to make proteinase resistant P'i analogs. Based .on knowledge of the cleavage
site for a
particular enzyme in a given substrate, and based on the guidance provided
herein for
constructing proteinase resistart analogs, a number of P'i analogs resistant
to cleavage
5 by, for example, serine proteinases, metalloproteinases, aspartic
proteinases, and
cysteine proteinases, can be readily constructed. Once candidate P'i analogs
have been
made, the activity of the P'i analog (e.g., the suitability of the candidate
analog as a
proteinase substrate) can be readily measured and compared to that of the
native
polypeptide.
10 A variety of methods for assessing whether a candidate analog is
resistant to
proteolysis are available in the art. For example, the ability of a particular
proteinase to
cleave a P'i analog can be measured in a cell free system in vitro. In one
such
embodiment of a cell free assay system, candidate substrate (e.g., P'i analog
and/or
native polypeptide) is end labeled with a detectable label such as
radioactivity.
15 Labelled substrate is incubated in the presence of proteinase. Over
time, samples of the
reaction mixture can be stopped and run on a gel. A shift in the size of the
radioactive
band indicates that the polypeptide is cleaved by the proteinase, and the rate
at which
this shift occurs indicates the rate at which the polypeptide is cleaved by
the proteinase.
=
This rate can be compared to that observed with the native polypeptide.
20 To further illustrate, an exemplary experiment to test a particular
P'i analog
might involve the following. The native polypeptide and the putative P'i
analog are
each radioactively labelled (note: for the purposes of labeling, all that is
necessary is
that cleavage of the polypeptide produces a radioactive fragment which differs
in size
from the full length labeled polypeptide). The labeled native polypeptide and
P'1
25 analog are incubated with the particular proteinase.
Following incubation, both native
polypeptide and P'1 analog are separated by gel electrophoresis, and the
migration of
the labeled species is examined. Since the particular proteinase is known to
cleave the
native polypeptide, one would expect to see a shift in the size of the labeled
fragment of
the native polypeptide (before and after incubation with enzyme) with the
smaller
30 fragment corresponding to a cleavage product. However, if
the P'i analog is resistant
to proteolysis, this shift in mobility following incubation with proteinase
will either not
-35-
CA 02888907 2015-04-23
occur, or will occur much more slowly than occurs for the proteolysis of the
native
= protein.
The relative ability of a proteinase to cleave a P'l analog in comparison to a
native polypeptide can also be assessed in a cell based in vitro system. In
one such cell
5 based assay, a cell which expresses a given proteinase is contacted with
a native
polypeptide or a P'1 analog such that the native polypeptide or P'i analog is
expressed
in the cell. Much like in the cell free assay described above, the native
polypeptide and
1 analog are detectably labelled. Cleavage of the native polypeptide and the
P'1
analog can be measured and compared by extracting protein from the cells and
10 measuring the migration of labeled protein.
In a further example of a cell based assay, a cell which does not express a
given
proteinase is contacted with a detectably labeled native polypeptide or P'1
analog such
'that the native polypeptide or P'1 analog is expressed in the cell. The cell
is further
contacted with the particular proteinase such that the proteinase is expressed
in the cell.
15 Cleavage of the native polypeptide and the P'1 analog can be measured
and compared
by extracting protein from the cells and measuring the migration of labeled
protein.
In any of the aforementioned cell based assays, the invention contemplates the
use of any of a number of primary cells or cell lines. In some instances, it
may be
advantageous to select a particular cell or cell line in which to conduct in
vitro analysis..
20 For example, it may be advantageous in some instances to select a cell
line that is more
closely related to the cell type in which one eventually wishes to use the P'i
analog.
However, in other instances, it may be most useful to perform initial
screening and
testing of candidate P'1 analogs in a possibly unrelated cell type or cell
line selected
primarily based on convenience, and perform later safety and efficacy testing
in more
25 specific cell lines or in animal models as needed.
In addition to cell free and cell-based assays, the proteinase resistance of a
particular P'i analog can be measured in vivo using any of a number of animal
models.
Initial testing of the proteolysis of a given P'i analog can be assessed in
wildtype
animals. During such initial testing, the potential positive or negative
effects of the 1'1
30 analog are not the question, but rather the question is
whether a particular P'1 analog is
resistant to proteolysis. Once a particular P'1 analog is shown to be
resistant to
proteolysis using any of the cell free, cell based, or in vivo assays
described above,
-36-
CA 02888907 2015-04-23
further in vitro and in vivo testing of the P'1 analog can be conducted to
ascertain the
therapeutic effectiveness of the P'i analog.
Additional assays can be used to evaluate the specific functional activity of
a
proteinase resistant P'i analog. Such assays can be selected based on the
particular P't
analog. For example, when the polypeptide is a growth factor, the functional
activity of
the growth factor analog can be assessed by measuring the ability of the
growth factor
to bind its growth factor receptor in a cell free or cell based assay, and
comparing this
to the ability of the native growth factor. When the polypeptide is a peptide
hormone,
the functional activity of the peptide hormone analog can be assessed by
measuring the
ability of the peptide hormone analog to bind its receptor in a cell free or
cell based
assay, and comparing this to the ability of the native peptide hormone. When
the
polypeptide is a transcription factor, the functional activity of the
transcription factor
= analog can be assessed by measuring the ability to bind to an appropriate
DNA
consensus sequence or the ability to activate a reporter construct containing
an
appropriate consensus sequence, and comparing this ability to that of the
native
transcription factor. In any of these examples, functional activity can also
be measured
in animal models.
The following illustrative example provides potential methods of assessing a
functional activity of analogs of a particular polypeptide.
1. Assays of Insulinatropic Activity
In certain embodiments, the P'1 analogs of the present invention are peptide
hormone analogs. Active GLP-1 peptides, 7-34, 7-35, 7-36, and 7-37, have
insulinotorpic activity, and the invention provides methods for making peptide
analogs
of these active GLP-1 peptides. The resistance of GLP-1 peptide analogs to
proteolysis
can be readily measured. Additionally, the functional activity of the GLP-1
peptide
analogs can be demonstrated by examining,the insulinotropic properties of the
peptide
hormone analogs. Insulinotrophic activity may be determined, for example, by
providing a given peptide analog to animal cells, or injecting that analog
into animals
and monitoring the release of immunoreactive insulin (IRI) into the media or
circulatory system of the animal, respectively. The presence of }RI can be
detected
through the use of a radioimmunoassay which can specifically detect insulin.
-37-
CA 02888907 2015-04-23
The db/db mouse is a genetically obese and diabetic strain of mouse. The db/db
mouse develops hyperglycemia and hyperinsulinemia concomitant with its
=
development of obesity and thus serves as a model of obese type 2 diabetes
(NIDDM).
The db/db mice can be purchased from, for example, The Jackson Laboratories
(Bar
5 Harbor, Me.). In an exemplary embodiment, for treatment of the mice with
a regimen
including a peptide hormone analog or control, sub-orbital sinus blood samples
are
taken before and at some time (e.g., 60 minutes) after dosing of each animal.
Blood
glucose measurements can be made by any of several conventional techniques,
such as
using a glucose meter. The blood glucose levels of the control and peptide
hormone
10 analog dosed animals are compared
The metabolic fate of exogenous GLP-1 analog can also be followed in either
nondiabetic and type If diabetic subjects, and the effect of a candidate
analog
determined. For instance, a combination of high-pressure liquid chromatography
(IIPLC), specific radioimmunoassays (RIAs), and an enzyme-linked immunosorbent
15 assay (ELISA), can be used, whereby intact biologically active GLP-1 and
its
metabolites can be detected. See, for example, Deacon et al. (1995) Diabetes
44:1126-
1131. To illustrate, after GLP-1 analog administration, the intact peptide can
be
measured using an NH2-terminally directed RIA or ELISA, while the difference
in
concentration between these assays and a CO2H-terminal-specific RIA allowed
20 determination of NH2-terminally truncated metabolites.
Without the analog,
subcutaneous GLP-1 is rapidly degraded in a time-dependent manner, forming a
metabolite which co-elutes on BPLC with GLP-I(9-36) amide and has the same
immunoreactive profile. For instance, thirty minutes after subcutaneous GLP-1
administration to diabetic patients (n is 8), the metabolite accounted for
88.5 4- 1.9% of
25 the increase in plasma immunoreactivity determined by the
CO2H-terminal RIA, which
was higher than the levels measured in healthy subjects (78.4 + 3.2%; n = 8;
P.( 0.05).
See Deacon et al., supra. Intravenously infused GLP-I was also extensively
degraded.
Other methods of measuring insulinotropic activities of GLP-1 analogs are
disclosed in U.S. Patent 5,545,618.
30 (d) Pharmaceutical Preparations
For therapeutic use, the chosen P'1 analog is formulated with a carrier that
is
pharmaceutically acceptable and is appropriate for administering a
therapeutically
-38-
CA 02888907 2015-04-23
effective amount of the P'i analog to a subject using a dosage adapted for a
chosen
route of administration, i.e., oral, intravenous, or parenteral, so as to
deliver the peptide
to the desired tissue. In certain embodiments, the analogs are non-pyrogenic,
i.e., do
not trigger elevation of a patient's body temperature by more than a
clinically
5 acceptable amount. Suitable pharmaceutically acceptable carriers are
those used
conventionally with peptide-based drugs, such as diluents, excipients and the
like.
Reference may be made to "Remington's Pharmaceutical Sciences", 17th Ed., Mack
Publishing Company, Easton, Pa., 1985, for guidance on drug formulations
generally.
In one embodiment of the invention, the compounds are formulated for
administration
10 by infusion, e.g., when used as liquid nutritional supplements for
patients on total
parenteral nutrition therapy, or by injection, e.g., sub-cutaneously,
intramuscularly or
intravenously, and are accordingly utilized as aqueous solutions in sterile
and pyrogen-
free form and optionally buffered to physiologically tolerable pH, e.g., a
slightly acidic
or physiological pH. Thus, the compounds may be administered in a vehicle such
as
15 distilled water or, more desirably, in saline, phosphate buffered saline
or 5% dextrose
solution. Water solubility/of the 13'1 analog may be enhanced, if desired, by
incorporating a solubility enhancer, such as acetic acid or sodium hydroxide.
The P'1 analogs of this invention can be provided in the form of
=
pharmaceutically acceptable salts. Examples of such salts include, but are not
limited
20 to, those formed with organic acids (e.g., acetic, lactic, maleic,
citric, malic, ascorbic,
succinic, benzoic, methanesulfonic, or toluenesulfonic acid), inorganic acids
(e.g.,
hydrochloric acid, sulfuric acid, or phosphoric acid), and polymeric acids
(e.g., tannic
acid, carboxymethyl cellulose, polylactic, polyglycolic, or copolymers of
polylactic-
glycolic acids).
25 A therapeutically effective amount of a P'1 analog of this invention
and a
pharmaceutically acceptable carrier substance (e.g., magnesium carbonate,
lactose, or a
phospholipid with which the therapeutic analog can form a micelle) together
form a
therapeutic composition (e.g., a pill, tablet, capsule, or liquid) for
administration (e.g.,
orally, intravenously, transdermally, pulmonarily, vaginally, subcutaneously,
nasally,
30 iontophoretically, intratracheally, intracranially,
intramyocardially, intraperidardially,
intramuscularly) to a subject. The pill, tablet, or capsule that is to be
administered
orally can be coated with a substance for protecting the active composition
from the
- 39 -
CA 02888907 2015-04-23
gastric acid or intestinal enzymes in the stomach for a period of time
sufficient to allow
it to pass undigested into the small intestine. The therapeutic composition
can also be
in the form of a biodegradable or nonbiodegradable sustained release
formulation for
subcutaneous or intramuscular administration. See, e.g., U.S. Patent Serial
Nos.
3,773,919 and 4, 767,628 and PCT Application No. WO 94/15587. Continuous
administration can also be achieved using an implantable or external pump
(e.g.,
1NFUSA1DTm pump). The administration can also be conducted intermittently,
e.g.,
single daily injection, or continuously at a low dose, e.g., sustained release
formulation.
Therapeutic or diagnostic compositions of the invention are administered to an
individual in amounts sufficient to treat or diagnos disorders. The effective
dose of a
peptide of the present invention for treating the above-mentioned diseases or
disorders
varies depending upon the manner of administration, the age and the body
weight of the
subject, and the condition of the subject to be treated, and ultimately will
be decided by
the attending physician or veterinarian.
Also contemplated within the scope of this invention is a peptide covered by
the
above generic formula for use in treating diseases or disorders associated
with aberrant
glucose metabolism, lipid metabolism or eating disorder.
Other features and advantages of the present invention will be apparent from
the
detailed description and from the claims.
(v) Methods of Use
(1) Diagnostic uses
The peptide hormone analogs of the invention may be used in radiolabeled or
unlabeled form to diagnose or treat a variety of disease states including but
not limited
to those associated with glucose metabolism, lipid metabolism, food intake,
and
hypertension.
Preferably, radiolabeled complexes of the compounds of the invention are used
for such diagnoses and treatments. Radiolabeled embodiments, of the compounds
of
the invention may be used in radioisotope guided surgery, as described in WO
93/18797 and in Woltering, et al. (1994) Surgery 116, 1139-1147. In a
preferred
embodiment, a complex of a .gamma.-emitting radionuclide such as 99Tc and a
compound of the invention is used to diagnose an SSTR-expressing tumor, and
- 40 -
CA 02888907 2015-04-23
subsequently, a complex of Ý-emitting radionuclide such as 188Re or 186Re with
the
compound is used te treat the tumor.
For diagnostic purposes, an effective diagnostic amount of the diagnostic or
radiodiagnostic agent of the invention is administered, preferably
intravenously. An
effective diagnostic amount is defmed as the amount of diagnostic or
radiodiagnostic
agent necessary to effect localization and detection of the label in vivo
using
conventional methodologies such as magnetic resonance, computerized
tomography,
gamma scintigraphy, SPECT, PET, and the like.
For diagnosis using scintigraphic imaging, preferably, 99Tc-labeled compounds
of the invention are administered in a single unit injectable dose. The 99Tc-
labeled
compounds provided by the invention may be administered intravenously in any
conventional medium for intravenous injection such as an aqueous saline
medium, or in
blood plasma medium. Generally, the unit dose to be administered has a
radioactivity
of about 0.01 mCi to about 100 mCi, preferably 1 mCi to 50 mCi. The solution
to be
injected at unit dosage is from about 0.01 mL to about 10 mL. After
intravenous
administration, imaging in vivo can take place in a matter of a few minutes.
However,
imaging can take place, if desired, hours or even longer after the
radiolabeled
compound is injected into a patient. In most instances, a sufficient amount of
the
administered dose will accumulate in the area to be imaged within about 0.1 of
an hour
to permit the taking of scintiphotos. Any conventional method of scintigraphic
imaging
for diagnostic purposes can be utilized in accordance with this invention.
(2) Methods of Treatment
P'1 analogs provide improved methods of treating any disease or condition that
can be treated with a given polypeptide therapeutic composition, wherein the
polypeptide is normally cleaved in vivo by a proteinase. Given that
proteolysis
decreases or eliminates the availability of the therapeutic, and in some
instances leads
to the production of functionally antagonistic products, the safety and
efficacy of many
polypeptide therapeutics which can be used to treat particular diseases and
conditions is
greatly compromised. Accordingly, the methods and compositions of proteinase
resistant 13'1 analogs provides improved methods of treating any of a number
of diverse
diseases and conditions.
-41 -
CA 02888907 2015-04-23
To more explicitly illustrate the applicability of P'1 analogs in improved
= methods of treating a variety of diseases and conditions, we provide the
following non-
limiting examples. In certain embodiments, the P'i analogs of the present
invention are
peptide hormone analogs. These peptide hormones possess, in certain
embodiments,
5 the ability to
lower blood glucose levels, to relieve obesity, to alleviate impaired
glucose tolerance, to inhibit hepatic glucose neogenesis, and to lower blood
lipid levels
and to inhibit aldose reductase. They are thus useful for the prevention
and/or therapy
of congestive heart failure, hyperglycemia, obesity, hyperlipidemia, diabetic
complications (including retinopathy, nephropathy, neuropathy, cataracts,
coronary
10 artery disease and arteriosclerosis) and furthermore for obesity-related
hypertension
and osteoporosis. Thus one aspect of the present invention is a method for
treating a
disease in a patient or subject comprising administering a therapeutically
effective
amount of one or more peptide hormone analogs, such as the peptide hormone
analogs
disclosed herein.
15 In certain embodiments, the proteolysis-resistant analogs for use in a
method of
treatment comprise P'i analogs of active GLP-1 peptides. GLP-1 peptides of
various
lengths are known to be biologically active including: GLP-1(7-34), GLP-1(7-
35);
= GLP-1(7-36), and GLP-1(7-37) the sequences of which are listed below:
GLP-1(7-37): HAE GTFTSDVSSY LEGQAAKEFI AWLVKGRG;
20 GLP-1 (7-36):
HAE GTFTSDVSSY LEGQAAKEFI AWLVKGR(-NH2);
GLP-1 (7-35): HAE GTFTSDVSSY LEGQAAKEFI AWLVK; and
GLP-1 (7-34): HAE GTFTSDVSSY LEGQAAKEFI AWLV.
In certain embodiments, the present invention relates to a method for
modifying
glucose metabolism. P'i analogs of GLP-1 peptides may be administered to
patient
25 suffering from diabetes mellitus. Diabetes mellitus is a
disease characterized by
hyperglycemia occurring from a relative or absolute decrease in insulin
secretion,
decreased insulin sensitivity, or insulin resistance. The morbidity and
mortality of this
disease result from vascular, renal, and neurological complications. An oral
glucose
tolerance test is a clinical test used to diagnose diabetes. In an oral
glucose tolerance
30 test, a patient's physiological response to a glucose load or
challenge is evaluated.
After ingesting the glucose, the patient's physiological response to the
glucose
- 42 -
CA 02888907 2015-04-23
challenge is evaluated. Generally, this is accomplished by determining the
patient's
blood glucose levels (the concentration of glucose in the patient's plasma,
serum, or
whole blood) for several predetermined points in time.
Thus, in one aspect, the present invention relates to therapeutic and related
uses
of proteolysis-resistant GLP-1 analogs for treating heart-related ailments,
hyperglycemia, obesity, hyperlipidemia, diabetic complications (including
retinopathy,
nephropathy, neuropathy, cataracts, coronary artery disease and
arteriosclerosis) and
furthermore for obesity-related hypertension and osteoporosis.
In certain embodiments, the subject GLP-1 analogs can be used as part of
treatment regimens for various heart-related ailments. Exemplary heart related
ailments include myocardial infarction, ischemia-reperfusion injury,
congestive heart
failure, and cardiac arrest. The subject GLP-1 analogs can also be used in the
= prevention of heart related ailments.
In certain embodiments, the subject analogs can be used to induce arousal for
the treatment or amelioration of depression, schizoaffective disorders, sleep
apnea,
attention deficit syndromes with poor concentration, memory loss,
forgetfulness, and
narcolepsy.
.In certain embodiments, therapeutically effective amounts of proteolysis-
v
resistant GLP-2 analogs may be administered to patients suffering from
gastrointestinal
diseases. It has been determined that GLP-2 acts as a trophic agent, to
promote growth
of gastrointestinal tissue. The effect of GLP-2 is marked particularly by
increased
growth of the small bowel, and is therefore herein referred to as an
"intestinotrophic"
effect.
Thus, in one aspect, the present invention relates to therapeutic and related
uses
of GLP-2 analogs for promoting the growth and proliferation of
gastrointestinal tissue,
most particularly small bowel tissue. For histance, the subject method can be
used as
part of a regimen for treating injury, inflammation or resection of intestinal
tissue, e.g.,
where enhanced growth and repair of the intestinal mucosal epithelial is
desired.
With respect to small bowel tissue, such growth is measured conveniently as an
increase in small bowel mass and length, relative to an untreated control. The
effect of
subject GLP-2 analogs on small bowel also manifests as an increase in the
height of the
- 43 -
CA 02888907 2015-04-23
crypt plus villus axis. Such activity is referred to herein as an
"intestinotrophic"
= activity. The efficacy of the subject method may also be detectable as an
increase in
crypt cell proliferation and/or a decrease in small bowel epithelium
apoptosis. These
cellular effects may be noted most significantly in relation to the jejunum,
including the
5 distal jejunum and particularly the proximal jejunum, and also in the
distal ileum. A
compound is considered to have "intestinotrophic effect" if a test animal
exhibits
significantly increased small bowel weight, increased height of the crypt plus
villus
axis, or increased crypt cell proliferation or decreased small bowel
epithelium apoptosis
when treated with the compound (or genetically engineered to express it
themselves).
10 A model suitable for determining such'gastrointestinal growth is
described by US
Patent 5,834,428.
In general, patients who would benefit from either increased small intestinal
mass and consequent increased small bowel mucosal function are candidates for
treatment by the subject method. Particular conditions that may be treated
include the
15 various forms of sprue including celiac sprue which results from a toxic
reaction to a-
gliadin from wheat, and is marked by a tremendous loss of villae of the bowel;
tropical
sprue which results from infection and is marked by partial flattening of the
villae;
= hypogarnmaglobulinemic sprue which is observed commonly in patients with
common
variable immunodeficiency or hypogammaglobulinemia and is marked by
significant
20 decrease in villus height. The therapeutic efficacy of the treatment may
be monitored
by enteric biopsy to examine the villus morphology, by biochemical assessment
of
nutrient absorption, by patient weight gain, or by amelioration of the
symptoms
associated with these conditions. Other conditions that may be treated by the
subject
method, or for which the subject method may be useful prophylactically,
include
25 radiation enteritis, infectious or post-infectious
enteritis, regional enteritis (Crohn's
disease), small intestinal damage due to toxic or other chemotherapeutic
agents, and
patients with short bowel syndrome.
More generally, the present invention provides a therapeutic method for
treating
digestive tract diseases. The term "digestive tract" as used herein means a
tube through
30 which food passes, including stomach and intestine. The
term "digestive tract diseases"
as used herein means diseases accompanied by a qualitative or quantitative
abnormality
in the digestive tract mucosa, which include, e.g., ulceric or inflammatory
bowel
- 44 -
CA 02888907 2015-04-23
disease; congenital or acquired digestion and absorption disorder including
malabsorption syndrome; disease caused by loss of a mucosal barrier function
of the
=
gut; and protein-losing gastroenteropathy. The ulceric disease includes, e.g.,
gastric
ulcer, duodenal ulcer, small intestinal ulcer, colonic ulcer and rectal ulcer.
The
5 inflammatory bowel disease includes, e.g., esophagitis, gastritis,
duodenitis, enteritis,
colitis, Crohn's disease, proctitis, gastrointestinal Behcet, radiation
enteritis, radiation
colitis, radiation proctitis, enteritis and medicamentosa. The malabsorption
syndromes
includes the essential malabsorption syndrome such as disaccharide-decomposing
enzyme deficiency, glucose-galactose malabsorption, fractose malabsorption;
10 secondary malabsorption syndromes, e.g., the disorder caused by a
mucosal atrophy in
the digestive tract through the intravenous or parenteral nutrition or
elemental diet, the
disease caused by the resection and shunt of the small intestine such as short
gut
syndrome, cul-de-sac syndrome; and indigestible malabsorption syndrome such as
the
disease caused by resection of the stomach, e.g., dumping syndrome.
15 The term "therapeutic agent for digestive tract diseases" as used
herein means
the agents for the prevention and treatment of the digestive tract diseases,
which
include, e.g., the therapeutic agent for digestive tract ulcer, the
therapeutic agent for
inflammatory digestive tract disease, the therapeutic agent for mucosal
atrophy in the
=
digestive tract and the therapeutic agent for digestive tract wound, the
amelioration
20 agent for the function of the digestive tract including the agent for
recovery of the
mucosal barrier function and the amelioration agent for digestive and
absorptive
function. The ulcers include digestive ulcers and erosions, acute ulcers,
namely, acute
mucosal lesions.
The subject method, because of promoting proliferation of intestinal mucosa,
25 can be used in the treatment and prevention of pathologic
conditions of insufficiency in
digestion and absorption, that is, treatment and prevention of mucosal atrophy
or
=
treatment of hypoplasia of the digestive tract tissues and decrease in these
tissues by
surgical removal as well as improvement of digestion and absorption. Further,
the
subject method can be used in the treatment of pathologic mucosal conditions
due to
30 inflammatory diseases such as enteritis, Crohn's disease
and ulceric colitis and also in
the treatment of reduction in function of the digestive tract after operation,
for example,
in dumping syndrome as well as in the treatment of duodenal ulcer in
conjunction with
- 45 -
CA 02888907 2015-04-23
the inhibition of peristalsis of the stomach and rapid migration of food from
the
stomach to the jejunum. Furthermore, glicentin can effectively be used in
promoting
cure of surgical invasion as well as in improving functions of the digestive
tract. Thus,
the present invention also provides a therapeutic agent for atrophy of the
digestive tract
mucosa, a therapeutic agent for wounds in the digestive tract and a drug for
improving
functions of the digestive tract which comprise glicentin as active
ingredients.
Additionally, the subject method can be used to alter the pharmacokinetics of
pancreatic peptide, Peptide YY and neuropeptide Y, all of which are members of
the
pancreatic polypeptide family. Specifically, DPP IV has been implicated in the
processing of those peptides in a manner which alters receptor selectivity,
and thus DPP
IV resistant analogs of each of these peptides can be readily designed.
Neuropeptide Y (NPY) is believed to act in the regulation of vascular smooth
muscle tone, as well as regulation of blood pressure. NPY also decreases
cardiac
contractility. NPY is also the most powerful appetite stimulant known (Wilding
et al.,
(1992) J Endocrinology 132:299-302). The centrally evoked food intake
(appetite
stimulation) effect is predominantly mediated by NPY Yl receptors and causes
increase in body fat stores (Stanley et al., (1989) Physiology and Behavior
46:173-177).
By way of example, one possible use of NPY analogs is in the manufacture of
therapeutics that increase appetite. Although much of the world strives to
lose weight,
in a munber of contexts, the goal is to gain weight. The incidence of eating
disorders is
on the rise around the world. Over time, individuals with eating disorders
suffer from a
pathological lose of appetite, and this lose of appetite makes re-feeding
extremely
difficult. Such difficulty often persists even when the individual's weight
has reached a
life-threateningly low level. Accordingly, the use of agents which stimulate
the
appetite would greatly enhance the ability of health care providers to
encourage and
support re-feeding of severely malnourished eating disorder patients.
The difficulty encountered by individuals attempting to re-feed following
prolonged periods of malnutrition is not limited to individuals with eating
disorders.
Malnutrition due to any cause can result in a serious suppression of appetite
and this
can be a barrier to quickly and easily facilitating proper nutrition in these
individuals.
Therapeutics that stimulate appetite would have great utility in the treatment
of
malnourished individuals.
- 46 - =
CA 02888907 2015-04-23
LOSS of appetite and wasting syndromes are often associated with other
diseases
and conditions. For example, patients with various forms of cancer and AIDS
often
experience wasting. This significant weight loss, as well as loss of muscle
mass, can
lead to a variety of other complications including loss of energy and further
suppression
of the immune system. Accordingly, therapeutics which help to counter the loss
of
appetite and wasting associated with other diseases and treatments would
greatly
improve the quality of life of patients battling any of a number of diseases.
A final example pertains to the administration of therapeutics that stimulate
appeptite and stimulate weight gain in the agricultural arena. Such agents
could be
used to help raise animals, such as commercial livestock, with a higher
average weight
and/or a higher average fat content. By way of example, such therapeutics
could be
administrated, for example in animal feed or water, to cows, pigs, chickens,
sheep,
turkeys, goat, buffalo, ostrich, and the like to poduce larger animals for
sale in the food
industry.
Peptide YY (PYY) and pancreatic polypeptide (PP) are involved in eating
disorders, gastrointestinal disorders, and p an cr e ati c tumors. (See U.S.
Patent
5,574,010)
DPP IV has also been implicated in the metabolism and inactivation of growth
hormone-releasing factor (GHRF). GHRF is a member of the family of homologous
peptides that includes glucagon, secretin, vasoactive intestinal peptide
(VIP), peptide
histidine isoleucine (PHI), pituitary adenylate cyclase activating peptide
(PACAP),
gastric inhibitory peptide (GIP) and helodermin (Kubiak et al. (1994) Peptide
Res
7:153). GHRF is secreted by the hypothalamus, and stimulates the release of
growth
hormone (GH) from the anterior pituitary. Thus, the subject method can be used
to
improve clinical therapy for certain growth hormone deficient children, and in
clinical
therapy of adults to improve nutrition and to alter body composition (muscle
vs. fat).
The subject method can also be used in veterinary practice, for example, to
develop
higher yield milk production and higher yield, leaner livestock.
The invention contemplates the use ofri analogs in methods of treatment
wherein the P'1 analog alone constitutes the therapeutic regimen, as well as
methods of
treatment that utilize administration of one or more P'i analogs as part of a
more
complex multi-factorial therapeutic regimen. For example, in the case of
methods of
- 47 -
CA 02888907 2015-04-23
treating diabetes and/or complications of diabetes, the present invention
contemplates
methods of treating diabetes by administering a P,1 analog such as a GLP-1
analog.
The present invention further contemplates that, in some circumstances, it may
be
preferably to administer more than one P'i analog. For example, the method of
treatment may comprise administration of two or more 13'1 analogs. Such P'i
analogs
may be analogs of the same polypeptide (e.g., two different GLP-1 analogs), or
may be
analogs of distinct polypeptides. Furthermore the invention contemplates that
administratin of one or more P'i analogs may be used as part of a complex
therapeutic
regimen. In the case of a method of treating diabetes or complications of
diabetes, an
exemplary therapeutic regimen may include administration of one or more 13'1
analog,
administration of insulin, modulation of diet, and modulation of exercise.
In still a further example of a multi-faceted therapeutic regimen, the
invention
contemplates the administration of one or more P'i analogs and one or more
agents that
inhibit the enzymatic activity of the particular enzyme that endogenouely
cleaves the
native protein. In the case of GLP-1, an exemplary method would comprise
administration of one or more peptide analogs with one or more inhibitors of
DPP IV.
inhibitors of a particular enzyme may be specific (e.g., an inhibitor that
modulates only
the activity of DPP IV) or the inhibitor may be more promiscuous (e.g., an
inhibitor
that modulates the activity of multiple serine proteases). Additionally, the
invention
contemplates the administration of one or more P'1 analogs and one or more
enzymes
that degrade the particular enzyme that endogenouely cleaves the native
protein. In the
case of GLP-1, an exemplary method would comprise administration of one or
more
peptide analogs with one or more enzymes that degrade DPP IV. Such enzymes may
be specific (e.g., an enzyme that only degrades DPP IV) or the enzyme may
degrade
multiple other protein (e.g., an enzyme that degrades several serine
proteases).
Business Methods
Other aspects of the invention provide for certain methods of doing business.
In
particular, practicing the methods of the invention may identify certain
peptidase
resistant P'1 analogs, such as peptide hormone analogs. This technical step,
when
combined with one of more additional steps, provides for novel approaches to
conduct
a pharmaceutical, agrochemical, biotechnological, or preferably a life-science
business.
For example, P'i analogs according to the present invention can be tested for
efficacy
- 48 -
CA 02888907 2015-04-23
as therapeutics in a variety of disease models, and the potential therapeutic
= compositions can then be tested for toxicity and other safety-profiling
before
formulating, packaging and subsequently marketing the resulting formulation
for the
treatment of disease. Alternatively, the rights to develop and market such
formulations
5 or to conduct such steps may be licensed to a third party for
consideration. In certain
other aspects of the invention, the P'i analogs thus identified may have
utility in the
form of information that can be provided to a third party for consideration
such that an
improved understanding of the fimction or side effects of said P'i analogs in
a
biological or therapeutic context is obtained.
10 In certain embodiments, the initially identified P'i analog can be
subjected to
further optimization, e.g., to further refine the structure of a lead analog.
Such
optimization may lead to the development of analogs that combine maximal
resistance
to proteolysis with other diserable pharmacological characteristics including:
solubility,
permeability, bioavailability, toxicity, mutagenicity, and pharmacolcinetics.
15 Structural modifications are made to a lead analog to address issues
with the
parameters listed above. These modifications however, must take into account
possible
effects on the analog's potency and activity. For example, if the toxicity of
a lead
analog is high when tested in an animal model, modifications can bemade to the
analog
in an effort to decrease toxicity while maintaining the desired characteristic
of
20 proteinase resistance.
Candidate analogs (whether or not said analogs are modified to alter to
improve
in vivo characteristics) or combinations thereof, must be tested for efficacy
and toxicity
in animal models. Such therapeutic profiling is commonly employed in the
pharmaceutical arts. Before testing an experimental therapeutic in humans,
extensive
25 therapeutic profiling (preclinical testing) must be
completed to establish initial
parameters for safety arid efficacy. Preclinical testing establishes a
mechanism of
action for the therapeutic, its bioavailability, absorption, distribution,
metabolism, and
elimination through studies performed in vitro (that is, in test tubes,
beakers, petri
dishes, etc.) and in animals. Animal studies are used to assess whether the
therapeutic
30 will provide the desired results. Varying doses of the
experimental therapeutic are
administered to test the therapeutic's efficacy, identify harmful side-effects
that may
occur, and evaluate toxicity.
- 49 -
CA 02888907 2015-04-23
Briefly, one of skill in the art will recognize that the identification of a
candidate, proteinaSe resistant analog is a first step in developing a
pharmaceutical
=
preparation useful for administration. Administration of an amount of a
pharmaceutical
preparation comprising said P'1 analog effective to treat a condition or
disease must be
5 both safe and effective. Early stage drug trials, routinely used in the
art, help to address
concerns of the safety and efficacy of a potential pharmaceutical. In the
specific case
of a P'i analog, efficacy of the pharmaceutical preparation could be readily
evaluated
first in cell culture, and then in a mouse or rat model. Cell culture systems
and animal
models appropriate for the particular disease indication for which a given P'i
analog
10 will be used can be readily selected by one of skill in the art.
Briefly, mice or rats
could be administered varying doses of said pharmaceutical preparations over
various
time schedules. The route of administration would be appropriately selected
based on
the particular characteristics of the agent and on the cell type to which
delivery of the
P'1 analog is desired. Control mice can be administered a placebo (e.g.,
carrier or
15 excipient alone).
In one embodiment, the step of therapeutic profiling includes toxicity testing
of
analogs in cell cultures and in animals; analysis of pharmacokinetics and
metabolism of
= the candidate analog; and determination of efficacy in animal models of
diseases. In
certain instances, the method can include analyzing structure-activity
relationship and
20 optimi7ing lead analogs based on efficacy, safety and pharmacokinetic
profiles. The
goal of such steps is the selection of analog candidates for pre-clinical
studies to lead to
filing of Investigational New Drug applications ("IND") with the FDA prior to
human
clinical trials.
Between lead optimization and therapeutic profiling, one goal is to develop a
25 P'1 analog that is resistant to a particular protease and
can be administered with
minimal side-effects. In the case of analogs for in vitro use, exemplary
analogs should
not be exceptionally toxic to cells in culture, should not be mutagenic to
cells in
culture, and should not be carcinogenic to cells in culture. In the case of
analogs for in
vivo use, exemplary analogs should not be exceptionally toxic (e.g., should
have only
30 tolerable side-effects when administered to patients),
should not be mutagenic, and
should not be carcinogenic.
-50-
=
CA 02888907 2015-04-23
By toxicity profiling is meant the evaluation of potentially harmful side-
effects
which may occur when an effective amount of a pharmaceutical preparation is
administered. A side-effect may or may not be harmful, and the determination
of
whether a side effect associated with a pharmaceutical preparation is an
acceptable side
effect is made by the Food and Drug Administration during the regulatory
approval
process. This determination does not follow hard and fast rules, and that
which is
considered an acceptable side effect varies due to factors including: (a) the
severity of
the condition being treated, and (b) the availability of other treatments and
the side-
effects currently associated with these available treatments. For example, the
term
cancer encompasses a complex family of disease states related to mis-regulated
cell
growth, proliferation, and differentiation. Many forms of cancer are
particularly
devastating diseases which cause severe pain, loss of function of the effected
tissue,
and death. Chemotheraputic drugs are an important part of the standard therapy
for
many forms of cancer. Although chemotherapeutics themselves can have serious
side-
effects including hair-loss, severe nausea, weight-loss, and sterility, such
side-effects
are considered acceptable given the severity of the disease they aim to treat.
In the
context of the present invention, whether a side-effect is considered
significant will
depend on the condition to be treated and the availability of other methods to
treat that
condition.
Toxicity tests can be conducted in tandem with efficacy tests, and mice
administered effective doses of the pharmaceutical preparation can be
monitored for
adverse reactions to the preparation.
One or More proteinase resistant P'i analogs, which are proven safe and
effective in animal studies, can be formulated into a pharmaceutical
preparation. Such
pharmaceutical preparations can then be marketed, distributed, and sold.
Exemplary
P'1 analogs and pharmaceutical preparation of such analogs may be marketed and
sold
alone, or may be sold as a pharmaceutical Package and/or kit. Furthermore, in
any of
the foregoing aspects, a method of conducting a business based on the design
of one or
more P'i analogs may optionally include a system for billing a patient and/or
the
patient's insurance provider, as well as a system for collecting appropriate
reimbursement from the patient and/or the patient's insurance provider.
-51 -
CA 02888907 2015-04-23
Examples
The following examples are shown by way of illustration and not by way of
limitation.
Example 1: Proteinase Resistant GLP-1 Analogs
5 Administration of GLP-1 is a candidate therapeutic for diabetes.
However, one
of the barriers to the efficacy of a treatment based on GLP-1 adminstration is
the rapid
in vivo degradation of GLP-1 by DPP IV. DPP IV cleaves GLP-1 near the N-
terminus
between alanine and glutamic acid, and previous studies have indicated that
this
cleaveage occurs extremely rapidly following administration of exogenous GLP-1
10 (Figure 1).
To generate peptide analogs resistant to proteolysis, we constructed analogs
containing tetra-substitutions at the P'l position of GLP-1. In the following
examples,
GLP1(7-37) was used. Briefly, we made substitutions at the P'l glutamic acid
of GLP-
1. Two specific substitutions that were made and tested were 3-dimethyl-
aspartate and
15 3-butyl-methyl-glycine. The resulting GLP-1 analogs were referred to as
GLP-1
(3DMA) (wherein the P'l substitution was 3-dimethyl-aspartate) and GLP-1 (BM)
= (wherein the P'l substitution was 3-butyl-methyl-glycine.
Figure 2 summarizes experiments which demonstrated that both GLP-1
= (3DMA) and GLP-1 (BM) were resistant to cleavage by DPP IV in comparison
to
20 native GLP-1. However, it is most desirable to produce peptide analogs
that are not
only resistant to proteolysis, but also retain all or much of the biological
activity of the
native peptide. Accordingly, we conducted a series of experiments to ascertain
whether
these GLP-1 analogs which display robust resistance to degradation by DPP IV
also
retain biological activities of native GLP-1
25 Example 2: Proteinase Resistant GLP-1 Analogs Retain Functional Activity
of Native
GLP-1
We conducted a series of experiments to assess the functional activity of both
GLP-1 (3DMA) and GLP-1 (BM) in comparison to native GLP-1 peptide. Figures 3-4
summarize the results of these experiements. Briefly, we examined two
functional
30 properties of GLP-1: binding of GLP-1 to its receptor and
signal transduction as
assayed by production of cAMP. Figure 3 summarizes experiments which examined
- 52 -
CA 02888907 2015-04-23
the activity of GLP-1 (3DMA). The left panel compares the kinetics of receptor
binding. We note that GLP-1 (3-DMA) retained the ability to bind the GLP-1
receptor.
Additionally, we note that the binding were similar, although not identical,
to that of
the native peptide.
Further analysis is provided in the right hand panel which summarizes an assay
to ascertain whether GLP-1 (3DMA) potentiates GLP-1 signaling in a manner
similar
to the native peptide. COS-7 cells (approx 106/10 cm plate) were transiently
transfected with cDNA encoding the human GLP-1 receptor. One day after
transfection, the cells were trypsinind and seeded in 24-well plates (density
of approx
105/well). Two days following transfection, the cells were incubated for one
hour at
room temperature either with native GLP-1 (0.3 iM), GLP-1 (3DMA) (10 iiM), or
in
the absence of either peptide. cAMP content, which correlates with receptor-
mediated
signaling, was measured in the cell lysate by proximity scintillation
radioimmunoassay.
As shown in Figure 3, GLP-1 (3DMA) potentiated signaling via the GLP-1
receptor to
an extent indistinguishable from native GLP-1.
Figure 4 summarizes similar experiments in which the activity of GLP-1 (BM)
was measured. Briefly, COS-7 cells (approx 106/10 cm plate) were transiently
transfected with cDNA encoding the htunan GLP-1 receptor. One day after
transfection, the cells were trypsinized and seeded in 24-well plates (density
of approx
105/well). Two days following transfection, the cells were incubated for one
hour at
room temperature either with native GLP-1 (0.3 !AM), GLP-1 (BM) (10 p,M), or
in the
absence of either peptide. cAMP content, which correlates with receptor-
mediated
signaling, was measured in the cell lysate by proximity scintillation
radioimmunoassay.
As shown in Figure 4, GLP-1 (BM) potentiated signaling via the GLP-1 receptor
to an
extent indistinguishable from native GLP-1.
Example 3: Tert-leucine Substituted GLP-1 Analogs are Resistant to DPP IV
Degradation
The data provided in examples 1 and 2 demonstrated that two distinct
substitutions at the P'l position of GLP-1 yielded proteinase resisitant
peptide analogs.
We have additionally demonstrated that a third substitution at the P'l
positions also
yields a proteinase resistant peptide analog. Briefly, the P'l glutamic acid
of GLP-1 (7-
. - 53 -
CA 02888907 2015-04-23
37) was substituted with tertiary leucine (TLE), and the ability of DPP IV to
cleave this
peptide analog was assessed.
Figure 5 shows HPLC/MS analysis of GLP-1 (7-37) following two hours of
treatment with human DPP IV (bottom chromatogram) in comparison to GLP-1 (7-
37)
in the absence of proteinase (top chromatogram). As expected, treatment with
DPP IV
resulted in a time dependent degradation of GLP-1.
Figure 6 shows IIPLC/MS analysis of a TLE-modified GLP-1 (7-37) analog.
TLE-modified GLP-1 analog was treated with human DPP IV for two hours, and
degradation of the analog over time was compared to that of analog in the
absence of
DPP IV. Comparison of the chromatograms (note: the top panel corresponds to
the
untreated peptide analog and the bottom panel corresponds to the treated
peptide
analog) demonstrated that TLE-modified GLP-1 is resistant to degradation by
DPP IV.
Example 4: Substitution at the P'l Position Confers Resistance to Other
Proteinases
The foregoing examples provide extensive evidence demonstrating that a
variety of substitutions at the P'l position confer resistance to degradation
by the serine
protease DPP IV. However, this method of tetra-substitution at the P'l
position to
confer proteinase resistance is not specific to substrates cleaved by DPP IV.
We have
=
also demonstrated that tetra-substitution at the P'l position of a model
substrate confers
resistance to cleavage by thrombin. Although thrombin is a serine proteinase,
it
recognizes a cleavage site distinct from that of DPP IV, and the results
summarized
=
herein indicate the broad applicability of the methods of the present
invention for
constructing P'1 analogs resistant to degradation by any of a number of
proteinases.
Figure 7 summarizes experiments which demonstrated that substitution of a
tertiary leucine (TLE) at the P'l position of a model thrombin substrate
conferred
resistance to proteolysis. Briefly, the peptide WALAPRSFA is a model substrate
for
thrombin. Thrombin cleaves after the arginine residue. Accordingly, the serine
residue
of this model peptide is the P'l positions.
WALAPR SFA
In the above schematic, the P'l position serine residue is indicated in bold
type
and an arrow denotes the site of cleavage by thrombin after the arginine
residue.
=
- 54 -
CA 02888907 2015-04-23
To test the ability of tetra-substitution at the P' 1 position to confer
resistance to
thrombin proteolysis, we prepared model peptide in which the P'l position
contained a
tertiary leucine (TLE). The model peptide analog is represented below, wherein
X is
used to indicate the TLE substitution.
WALAPRXF'A
To compare digestion of the model peptide analog by thrombin with that of the
native model peptide, peptides were digested for 4 hours at 23 C with 10 nM
thrombin
in 0.1 M HEPES pH 8, 0.14 M NaC1, 5 mM CaC12, 0.5% PEG6000. Following
digestion, C18 reverse phase HPLC of the digests was compared to the
undigested
peptides, and the mass spectra of the major peaks are shown for each
chromatogram in
Figure 7. As shown in Figure 7, unmodified peptide was efficiently cleaved by
thrombin to yield the cleavage product WALAPR. In contrast, the TLE.
substituted
peptide analog was not cleaved by thrombin under these conditions.
Example 5: In-vivo results for stable dimethylaspartate GLP-1 Analogs
. Figure 8 shows the percent change in blood glucose in diabetic mice for
Exendin-4 over time for three different doses (40 jig, 4pg, and 0.4 pg) as
compared to a
saline control solution.
Figure 9 shows the percent change in blood glucose in diabetic mice for a GLP-
1(TPA1B4) analog at a dose 0f40 pg over time compared to the percent change in
blood glucose for a saline or GLP-1 control.
Figure 10 shows the percent change in blood glucose in diabetic mice for a
GLP-1(TPA1B4) analog for three different doses (800 pg, 80 pg, and 8 pg) over
time
compared to a saline control.
The GLP-1 analog TPA1B4 is an analog of GLP-1 residues 7-36 with a C-
terminal amide and a fl-dimethyl aspartate residue at position 9. The sequence
for
TPA1B4 is:
HAXGTFTSDVSSYLEGQAAKEFIAWLVKGR-N112
In-vivo experiments were performed using female BKS.Cg-m+/-LePr(db)/J
mice that were purchased at 5-7 weeks of age and allowed to adjust to vivarium
conditions for two weeks prior to the start of the experiments. The mice were
housed
- 55 -
CA 02888907 2015-04-23
in pressurized, individually ventilated cages. A standard rodent diet was used
with
food and water provided ad libitum. Blood glucose was measured with a
ThereaSense
Freestyle blood glucose monitor. The tail vein was nicked with a needle to
obtain a
small drop of blood (about 10 AL) for each measurement. The GLP-1 analog
(TPA1B4) and exendin-4 were dissolved in phosphate buffered saline (PBS)
administered by intraperitoneal injection of the indicated dose in 0.2 mL. The
saline
control for this experiment was a 0.2 mL injection of PBS. Blood glucose
measurements were taken at t = 0, 30 min, 1 h, 2 h, 3 h, 4 h, 5 h (and 6 h).
The values
in Figures 8 and 9 are the average of five mice.
Figure 11 shows the percent change in blood glucose in diabetic mice for a
GLP-1 analog (TPA1B4) at a dose of 20 mg/kg over time compared to the percent
change in blood glucose for a saline or GLP-1 control.
Figure 12 shows the blood glucose level in diabetic mice for a GLP-1 analog
(TPA1B4) at a dose of 20 mg/kg over time compared to the blood glucose level
for a
saline or GLP-1 control.
Female BKS.Cg-m+/ LePr(db)/J mice were purchased at 5-7 weeks of age and
= allowed to adjust to vivarium conditions for two weeks prior to the start
of the
experiments. The mice were housed in pressurized, individually ventilated
cages. A
standard rodent diet was used with food and water provided ad libitum. Blood
glucose
was measured with a ThereaSense Freestyle blood glucose monitor. The tail vein
was
nicked with a needle to obtain a small drop of blood (about 10 AL) for each
measurement. The mice were fasted for two hours prior to administration of the
dose
and throughout the experiment. The GLP-1 analog (TPA1B4) and GLP-1 were
dissolved in phosphate buffered saline (PBS) and administered by
intraperitoneal
injection of the indicated dose in 0.4 mL. The saline control for this
experiment was a
0.4 mL injection of PBS. Blood glucose measurements were taken t = 0, 30 min,
1 h,
and 4 h. Values plotted are the average of ten mice.
Figure 13 shows the percent change in blood glucose for Exendin-4 over time
for three different doses (8 pg, 0.8 pg, and 0.08 pg) as compared to a saline
control.
Figure 14 shows the percent change in blood glucose for GLP-1 over time for a
dose of 800 pg compared to a saline control.
-56-
CA 02888907 2015-04-23
Figure 15 shows the percent change in blood glucose for a GLP-1 analog
= (P1732) for two different doses (8 ptg and 0.8 AO as compared to a saline
control.
The GLP-1 analog P1732 is an analog of GLP-1 residues 7-36 that incorporates
a portion of the Exendin-4 tail with a C-terminal amide and a fl-dimethyl
aspartate
5 residue at position 9. The sequence for P1732 is:
HAXGTFTSDVSSYLEGQAAKEFIAWLVKGRPSSGAPPPS-NH2
In-vivo experiments were performed using female BKS.Cg-m+/ LePr(db)/J
mice that were purchased at 5-7 weeks of age. and allowed to adjust to
vivarium
conditions for two weeks prior to the start of experiments. The mice were
house in
10 pressurized, individually ventilated cages. A standard rodent diet was
used with food
and water provided ad libitum. Blood glucose was measured with a ThereaSense
Freestyle blood glucose monintor. The tail vein was nicked with a needle to
obtain a
small drop of blood (-10 pl) for each measurement. The mice were fasted for 2
hours
prior to administration of the dose and throughout the experiment. The GLP-1
analog
15 (P1732) was dissolved in phosphite buffered saline (PBS) and
administered by
intraperitoneal injection of the indicated dose in 0.4 ml. The saline control
for this
experiment was a 0.4 ml injection of PBS. Blood glucose measurements were made
prior to the injection and at 30, 60 and 240 minutes post injection. Values
plotted are
the average of 5 mice for the P1732 data and 10 mice for the saline control.
20 Figure 16 shows exemplary embodiments of Formula (11), wherein
naturally
occurring amino acids have been modified at the fl-position (3-position) with
R1 and R2
where R1 and R2 are independently lower alkyl or halogen. In preferred
embodiments,
R1 and R2 are both lower alkyl. In a more preferred embodiment, R1 and R2 are
independently methyl, ethyl, or propyl. In the most preferred embodiment, both
R1 and
25 R2 are methyl.
-57-