Note: Descriptions are shown in the official language in which they were submitted.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
1
Continuous process for conversion of lignin to useful compounds
PRIORITY AND CROSS REFERENCES
This application claims the priority of United States Provisional Patent
Application No.
61/719,486 filed 28 October 2012, United States Provisional Patent Application
No.
61/751,919 filed 13 January 2013, United States Provisional Patent Application
No.
61/754,611 filed 14 February 2013, United States Provisional Patent
Application No.
61/765,402 filed 15 February 2013, WIPO Application No. PCT/U52013/027393
filed 22
February 2013, WIPO Application No. PCT/EP2013/053625 filed 26 February 2013,
WIPO
Application No. PCT/EP2013/053626 filed 26 February 2013, WIPO Application No.
PCT/EP2013/053628 filed 26 February 2013, WIPO Application No.
PCT/EP2013/053629
filed 26 February 2013, WIPO Application No. PCT/EP2013/053630 filed 26
February 2013,
WIPO Application No. PCT/EP2013/053631 filed 26 February 2013, United States
Patent
Application No. 13/775,229 filed 24 February 2013, United States Patent
Application No.
13/775,230 filed 24 February 2013, United States Patent Application No.
13/775,238 filed 24
February 2013, United States Patent Application No. 13/775,239 filed 24
February 2013,
United States Patent Application No. 13/775,240 filed 24 February 2013, United
States Patent
Application No. 13/775,241 filed 24 February 2013, United States Patent
Application No.
13/775,242 filed 24 February 2013, United States Provisional Patent
Application No.
61/837,262 filed 20 June 2013 and United States Provisional Patent Application
No.
61/866,734 filed 16 August 2013 the teachings of each of which are
incorporated herein by
reference.
BACKGROUND
The conversion of lignin in batch processes using hydrogen and catalysts is
known. For
example, Boocock, D.G.B et al, "The Production of Synthetic Organic Liquids
from Wood
Using a Modified Nickel Catalyst" discloses exposing air dried poplar to
hydrogen and Raney
Nickel in a batch autoclave at 340 C to 350 C for 1 or 2 h to produce "oil
products".
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
2
However, according to Boocock et al, "ltlhe use of Raney nickel has now been
abandoned in
favour of nickel from nickel salts . . ."
The use of catalysts to recover lignin is also known. Zakzeski, Pieter C., et
al; "The Catalytic
Valorization of Lignin for the Production of Renewable Chemicals", 2010 is a
comprehensive
review of catalytic efforts to convert lignin.
While many have proposed theoretical continuous processes, the inventors are
not aware of
any disclosure which is enabling beyond a theoretical basis. For example,
converting solid
lignin presents significant handling problems as documented in PNNL-16079,
September
2006.
"High-pressure feeding systems for biomass slurries have been recognized as a
process development issue at least as long as the modern biomass conversion
systems have been under development since the Arab oil embargo of 1973.
The authors review the state of the art and various slurry pumping systems,
the
vast majority of which include ball check valves. Their conclusion is that
high-pressure feeding remains a problem for small scale production but
believe "the high-pressure feeding of biomass slurries should be more readily
achieved at larger flow rates wherein the fibrous nature of the biomass would
not be expected to bridge and plug the orifices and valves."
There exists therefore the need to provide a pumping and charging scheme for
slurries.
An example of this is in the series of applications US 2011/0312051, US
2011/0312487, US
2011/0312488, US 2011/0313212, US 2011/0313210, US 2011/0313209, US
2011/0313208,
and US 2011/0312050. These applications to common inventors propose a
continuous process
based only upon batch autoclave results demonstrating high catalytic
selectivity to ethylene
glycol. However, the high ethylene glycol yields depend upon the purity of the
cellulose
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
3
feedstock which will intuitively cleave into 3 units of ethylene glycol. Of
the experiments
listed, the experiments using a feedstock closest to a biomass feedstock as
found in the
industrial or natural environment is bleached pulp. However, bleached pulp
only produced a
yield of 37%. When hemi-cellulose is used (xylose), the results are expected
to be shifted
much more away from ethylene glycol to propylene glycol. While the continuous
process is
theoretically described, the application fails to disclose an enabling
continuous process. For
example, the disclosure states that "[m]aterials of a continuous] process must
be capable of
being transported from a low pressure source into the reaction zone, and
products must be
capable of being transported from the reaction zone to the product recovery
zone. Depending
upon the mode of operation, residual solids, if any, must be capable of being
removed from
the reaction zone." This discloses the intuitively obvious requirement to
operate a continuous
process but the statement fails to teach one of ordinary skill how to achieve
those
requirements. Nowhere in the application is this essential problem discussed
or solved. In
fact, during the discussion of Fig. 2 of the publication, the temperature and
pressure conditions
are discussed without any disclosure as to how the slurry can be raised to the
listed pressure of
1800psig, or even 200psig. When considering the transport problem, which, as
of 2006, has
existed since the oil embargo of 1973, a disclosure telling one of ordinary
skill that transport
of materials is critical can hardly be considered enabling.
These series of applications also disclose to keep the water in the reaction
zone in the liquid
phase. In the batch autoclave this occurs due to the sealed nature. However,
it fails to disclose
how this is done, or even if it can be done, in a continuous process.
In order to avoid the problems of pumping and charging as noted, but not
solved, in the above
applications and publications, dissolution of the lignin is proposed. WO
2011/117705 relies
upon dissolving the lignin so that the material can be charged as a liquid
taking full advantage
of the check valve and high pressure liquid charging systems. In fact,
according to WO
2011/117705, "the only limit [is] that the lignin fed to the hydrogenolysis
reaction is well
dissolved, at the feeding temperature, in said solvent."
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
4
Converting the products of a converted lignin feedstream into basic aromatics
has been a long
desire of industry. There have been attempts to convert the products of a
converted lignin
feedstream under low-severity conditions (<190 C). However, these conditions
have proven
unfruitful in yielding selectivity of aromatics in all but a few model
compounds.
There exists therefore the need for a properly enabling disclosure of how to
continuously
convert lignin which includes the handling, charging, and essential conditions
for the process
to be carried out. There also exists the need to provide a process capable of
producing a
substantial proportion of aromatics from a lignin derived feedstream. These
conditions and
steps are believed both novel and inventive and for the first time
experimentally enabled.
SUMMARY
Disclosed herein is a process to convert a converted lignin feedstream to a
converted lignin
product comprised of aromatic compounds. The process disclosed herein
comprises the steps
of exposing the converted lignin feedstream to at least one catalyst in the
presence of a
plurality of hydrogen donor molecules at a reaction temperature in the range
of 190 C to
350 C for a reaction time of at least 30 minutes where the converted lignin
feedstream
comprises phenol oil, and at least some of the plurality of hydrogen donor
molecules are
donated during the exposure of the converted lignin feedstream and the
plurality of hydrogen
donor molecules to the at least one catalyst at the reaction temperature
during the reaction
time.
In one embodiment the at least one catalyst comprises an elemental metal. In
one embodiment
the first catalyst comprises an elemental metal selected from the group
consisting of Platinum,
Palladium, Cesium, Copper, Nickel, Ruthenium, Rhodium, Gold, Iron, Cobalt and
Iridium. In
one embodiment the first catalyst is a bimetallic catalyst comprised of at
least one metal
selected from the group consisting of Platinum, Palladium, Cesium, Copper,
Nickel,
Ruthenium, Rhodium, Gold, Iron, Cobalt and Iridium.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
In one embodiment the ratio of mmol of hydrogen donor molecules to mmol of
catalyst is in a
range selected from the group consisting of between 1.0:1.0 and 5.0:1.0,
between 1.2:1.0 and
4.0:1.0 and between 1.5:1.0 and 3.0:1Ø
In one embodiment at least one of the plurality of hydrogen donor molecules is
selected from
the group consisting of aliphatic polyols, compounds having the formula of
OH
Ri
O
. 2
Where R1 is selected from the group consisting of -OCH2, or -H, or -OH and R2
is selected
from the group consisting of -CH3, -CH2-CH3, -CH2-CH2-CH3, and
-CH2-CH2-CH2-CH3, and compounds having the formula of
Y
Where R is selected from the group consisting of -CH3, -CH2-CH3, -CH2-CH2-CH3,
and -CH2-
CH2-CH2-CH3.
In one embodiment at least one of the plurality of hydrogen donor molecules is
produced from
a previously converted lignin feedstream. In one embodiment at least one of
the plurality of
hydrogen donor molecules is cyclohexanol supplied from a mixture of the
converted lignin
feedstream and a source other than the converted lignin feedstream. In one
embodiment at
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
6
least one of the plurality of hydrogen donor molecules is selected from the
group consisting of
sorbitol, glycerol, xylitol and ethylene glycol.
In one embodiment the mole ratio of hydrogen donor molecules to phenol oil
based upon the
phenol oil having an assigned molecular weight of 150.0 g/mol is in a range
selected from the
group consisting of between 2.0:1.0 and 10.0:1.0, between 3.0:1.0 and 9.0:1.0,
between 4.0:1.0
and 8.0:1.0 and between 5.0:1.0 and 7.0:1Ø
In one embodiment the reaction temperature is in a range having a lower limit
selected from
the group consisting of at least 190 C, at least 200 C, at least 210 C, and
215 C and an
upper limit selected from the group consisting of 250 C, 260 C, 270 C, 310
C, and 320
C.
In one embodiment the process further comprises exposing the converted lignin
feedstream to
H2 gas. In one embodiment the amount of H2 gas is less than 25% of the total
amount of
hydrogen atoms donated from the at least one hydrogen donating compound and
the amount of
H2 gas.
BRIEF DESCRIPTION OF THE DRAWINGS
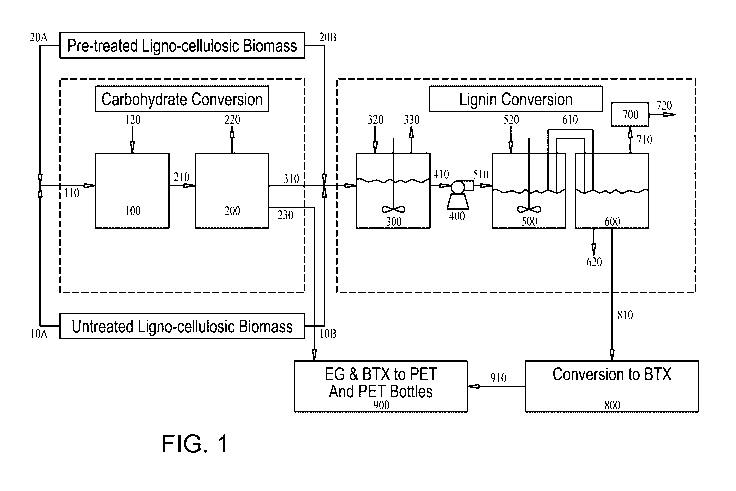
Fig. 1 is a schematic description of the unit operations of a fully integrated
process for
continuously converting ligno-cellulosic biomass feedstock to polyester
bottles.
Fig. 2 shows a further embodiment of the process.
Fig. 3 shows an embodiment with at least a portion of the water from the
lignin conversion
process reused in the pre-treatment or slurry creation step of an integrated
facility.
Fig. 4 shows an embodiment of a continuous stir tank reactor for the lignin
conversion
process.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
7
Fig. 5 shows the effect of mixing type and vacuum upon the final dispersed
concentration
versus time.
Fig. 6 shows the schematic of piston pumps and valves used for charging a
slurry comprised
of lignin to a lignin conversion reactor.
Fig. 7 shows the schematic of piston pumps and valves used for charging a
slurry comprised
of lignin to a lignin conversion reactor.
Fig. 8 shows the schematic of a bubble column.
Fig. 9 shows the ability of a bubble column to convert the slurry comprised of
lignin to lignin
conversion products comparable to those attained from a continuous stir tank
reactor.
DETAILED DESCRIPTION
This specification is an enabling disclosure and an actual reduction to
practice of a continuous
lignin conversion process of high yields, in particular from biomass
feedstock. Approximately
80% of the available lignin in the feedstock is recovered as usable products.
Although not apparent from the numbers, the disclosed process is a very high
yield conversion
process. In approximate terms, 1 kg of biomass feedstock used contained 50%
lignin, 41%
carbohydrates and 9% ash, by weight of the dry feed.
Demonstrated high lignin recovery of the process based upon 1 kg of feedstock
are as follows:
50% by dry weight of the feedstock is not lignin and not used, as it is either
destroyed or, in
the case of ash, simply not available. Of the lignin remaining, 35-40% by
weight of the lignin
is oxygen which is removed from the process (deoxygenated). Thus, while 50% of
the
feedstock is lignin, 40% of that weight is unavailable lignin (oxygen),
leaving only 30% of the
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
8
total weight of the feedstock as the theoretical recoverable amount of lignin.
The experiments
below have recovered up to 24-26% of the feedstock by weight, or approximately
80% of the
theoretically available lignin has been converted to usable oils.
As noted in the background section, many have proposed continuous lignin and
biomass
reactors developed on lignin conversion data from batch autoclaves. These
previous
disclosures have attempted to teach and enable a continuous process. However,
these are non-
enabling disclosures and generally inoperative as the processes fail to
address the problems
facing a continuous process.
As an example, the continuous process produced very little long chain
aliphatic hydrocarbons,
whereas the comparative batch process produced a significant amount of long
chain aliphatic
hydrocarbons. It is believed that the continuous process destroyed the
carbohydrates to very
low molecular weight, low boiling point molecules such as methane and carbon
dioxide and
removed them through the exit gas. In a batch process, these compounds are
kept in the
reactor and are believed to be further converted to long chain aliphatics
(greater than 12
carbons). Therefore, in the continuous process of this disclosure, the amount
of aliphatic
carbons having a number of carbons greater than 11 expressed as a percent of
the total weight
of the conversion products is less than 10% by weight, with less than 8% by
weight more
preferred, with less than 5% by weight even more preferred with less than 2.5%
by weight
most preferred.
The above problem is just one of many encountered by the inventors when trying
to create a
continuous process using industrial ligno-cellulosic feedstocks and not model
compounds.
These problems make it impossible to predict and enably claim a theoretical
continuous
process on the basis of batch data or model compounds.
Not only does this specification fully enable one of ordinary skill to operate
a continuous
process to convert lignin to liquid oils, the specification also discloses the
subsequent use of
the oils to make a polyester bottle or container.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
9
LIGNIN
The claimed process utilizes a feed or feedstock comprising lignin. It can
also utilize a
feedstock consisting of lignin, or a feedstock consisting essentially of
lignin, or a feedstock
comprising at least 95% lignin by weight.
Lignin does not have a single chemical structure. In fact, according to the
Kirk Othmer
Encyclopedia, the exact chemical structure of lignin, as it occurs in wood, is
not known and
because it is hard to extract from wood without changing its structure, the
exact structure may
never be known. While there are many variations of Lignin, the term lignin, as
used in this
specification, refers to any polymer comprising p-hydroxyphenyl units,
syringyl units, and
guaiacyl units.
While pure lignin, such as Organosolv, Acetosolv lignins may be used, the
extraction of lignin
from its natural origins is expensive using organic solvents with the
attendant environmental
issues. The robustness of the claimed process is established by the fact is
the process is
experimentally demonstrated on a continuous basis to convert lignin as lignin
is found in a
lignin-cellulosic biomass feedstock.
LIGNIN CELLULOSIC BIOMASS FEEDSTOCK
The lignin to be converted in this invention can be present as a feed or
feedstock of natural
ligno-cellulosic biomass comprising at least one carbohydrate and lignin.
Depending upon
how the natural ligno-cellulosic biomass is treated another embodiment of the
feedstock may
have the composition and unique decomposition temperatures and surface areas
described
below.
Because the feedstock may use naturally occurring ligno-cellulosic biomass,
the stream will
have relatively young carbon materials. The following, taken from ASTM D 6866
¨ 04
describes the contemporary carbon, which is that found in bio-based
hydrocarbons, as opposed
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
to hydrocarbons derived from oil wells, which was derived from biomass
thousands of years
ago. "[A] direct indication of the relative contribution of fossil carbon and
living biospheric
carbon can be as expressed as the fraction (or percentage) of contemporary
carbon, symbol fc.
This is derived from fm through the use of the observed input function for
atmospheric 14C
over recent decades, representing the combined effects of fossil dilution of
the 14C (minor) and
nuclear testing enhancement (major). The relation between fc and fm is
necessarily a function
of time. By 1985, when the particulate sampling discussed in the cited
reference [of ASTM D
6866 ¨ 04, the teachings of which are incorporated by reference in their
entirety] the fm ratio
had decreased to ca. 1.2."
Fossil carbon is carbon that contains essentially no radiocarbon because its
age is very
much greater than the 5730 year half life of 14C. Modern carbon is explicitly
0.95 times the
specific activity of SRM 4990b (the original oxalic acid radiocarbon
standard), normalized
to 613C = -19%. Functionally, the faction of modern carbon = (1/0.95) where
the unit 1 is
defined as the concentration of 14C contemporaneous with 1950 [A.D.] wood
(that is, pre-
atmospheric nuclear testing) and 0.95 are used to correct for the post 1950
[A.D.] bomb 14C
injection into the atmosphere. As described in the analysis and interpretation
section of the
test method, a 100% 14C indicates an entirely modern carbon source, such as
the products
derived from this process. Therefore, the percent 14C of the product stream
from the process
will be at least 75%, with 85% more preferred, 95% even preferred and at least
99% even
more preferred and at least 100% the most preferred. (The test method notes
that the percent
14C can be slightly greater than 100% for the reasons set forth in the
method). These
percentages can also be equated to the amount of contemporary carbon as well.
Therefore the amount of contemporary carbon relative to the total amount of
carbon is
preferred to be at least 75%, with 85% more preferred, 95% even more preferred
and at least
99% even more preferred and at least 100% the most preferred. Correspondingly,
each carbon
containing compound in the reactor, which includes a plurality of carbon
containing
conversion products will have an amount of contemporary carbon relative to
total amount of
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
11
carbon is preferred to be at least 75%, with 85% more preferred, 95% even
preferred and at
least 99% even more preferred and at least 100% the most preferred.
In general, a natural or naturally occurring ligno-cellulosic biomass can be
one feed stock for
this process. Ligno-cellulosic materials can be described as follows:
Apart from starch, the three major constituents in plant biomass are
cellulose, hemicellulose
and lignin, which are commonly referred to by the generic term lignocellulose.
Polysaccharide-containing biomasses as a generic term include both starch and
ligno-
cellulosic biomasses. Therefore, some types of feedstocks can be plant
biomass,
polysaccharide containing biomass, and ligno-cellulosic biomass.
Polysaccharide-containing biomasses according to the present invention include
any material
containing polymeric sugars e.g. in the form of starch as well as refined
starch, cellulose and
hemicellulose.
Relevant types of naturally occurring biomasses for deriving the claimed
invention may
include biomasses derived from agricultural crops selected from the group
consisting of starch
containing grains, refined starch; corn stover, bagasse, straw e.g. from rice,
wheat, rye, oat,
barley, rape, sorghum; softwood e.g. Pinus sylvestris, Pinus radiate; hardwood
e.g. Salix spp.
Eucalyptus spp.; tubers e.g. beet, potato; cereals from e.g. rice, wheat, rye,
oat, barley, rape,
sorghum and corn; waste paper, fiber fractions from biogas processing, manure,
residues from
oil palm processing, municipal solid waste or the like. Although the
experiments are limited
to a few examples of the enumerated list above, the invention is believed
applicable to all
because the characterization is primarily to the unique characteristics of the
lignin and surface
area.
The ligno-cellulosic biomass feedstock used to derive the composition is
preferably from the
family usually called grasses. The proper name is the family known as Poaceae
or Gramineae
in the Class Liliopsida (the monocots) of the flowering plants. Plants of this
family are
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
12
usually called grasses, or, to distinguish them from other graminoids, true
grasses. Bamboo is
also included. There are about 600 genera and some 9,000-10,000 or more
species of grasses
(Kew Index of World Grass Species).
Poaceae includes the staple food grains and cereal crops grown around the
world, lawn and
forage grasses, and bamboo. Poaceae generally have hollow stems called culms,
which are
plugged (solid) at intervals called nodes, the points along the culm at which
leaves arise. Grass
leaves are usually alternate, distichous (in one plane) or rarely spiral, and
parallel-veined. Each
leaf is differentiated into a lower sheath which hugs the stem for a distance
and a blade with
margins usually entire. The leaf blades of many grasses are hardened with
silica phytoliths,
which helps discourage grazing animals. In some grasses (such as sword grass)
this makes the
edges of the grass blades sharp enough to cut human skin. A membranous
appendage or fringe
of hairs, called the ligule, lies at the junction between sheath and blade,
preventing water or
insects from penetrating into the sheath.
Grass blades grow at the base of the blade and not from elongated stem tips.
This low growth
point evolved in response to grazing animals and allows grasses to be grazed
or mown
regularly without severe damage to the plant.
Flowers of Poaceae are characteristically arranged in spikelets, each spikelet
having one or
more florets (the spikelets are further grouped into panicles or spikes). A
spikelet consists of
two (or sometimes fewer) bracts at the base, called glumes, followed by one or
more florets. A
floret consists of the flower surrounded by two bracts called the lemma (the
external one) and
the palea (the internal). The flowers are usually hermaphroditic (maize,
monoecious, is an
exception) and pollination is almost always anemophilous. The perianth is
reduced to two
scales, called lodicules, that expand and contract to spread the lemma and
palea; these are
generally interpreted to be modified sepals.
The fruit of Poaceae is a caryopsis in which the seed coat is fused to the
fruit wall and thus,
not separable from it (as in a maize kernel).
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
13
There are three general classifications of growth habit present in grasses;
bunch-type (also
called caespitose), stoloniferous and rhizomatous.
The success of the grasses lies in part in their morphology and growth
processes, and in part in
their physiological diversity. Most of the grasses divide into two
physiological groups, using
the C3 and C4 photosynthetic pathways for carbon fixation. The C4 grasses have
a
photosynthetic pathway linked to specialized Kranz leaf anatomy that
particularly adapts them
to hot climates and an atmosphere low in carbon dioxide.
C3 grasses are referred to as "cool season grasses" while C4 plants are
considered "warm
season grasses". Grasses may be either annual or perennial. Examples of annual
cool season
are wheat, rye, annual bluegrass (annual meadowgrass, Poa annua and oat).
Examples of
perennial cool season are orchard grass (cocksfoot, Dactylis glomerata),
fescue (Festuca spp),
Kentucky Bluegrass and perennial ryegrass (Lolium perenne). Examples of annual
warm
season are corn, sudangrass and pearl millet. Examples of Perennial Warm
Season are big
bluestem, indian grass, bermuda grass and switch grass.
One classification of the grass family recognizes twelve subfamilies: These
are 1)
anomochlooideae, a small lineage of broad-leaved grasses that includes two
genera
(Anomochloa, Streptochaeta); 2) Pharoideae, a small lineage of grasses that
includes three
genera, including Pharus and Leptaspis; 3) Puelioideae a small lineage that
includes the
African genus Puelia; 4) Pooideae which includes wheat, barley, oats, brome-
grass (Bronnus)
and reed-grasses (Calamagrostis); 5) Bambusoideae which includes bamboo; 6)
Ehrhartoideae, which includes rice, and wild rice; 7) Arundinoideae, which
includes the giant
reed and common reed; 8) Centothecoideae, a small subfamily of 11 genera that
is sometimes
included in Panicoideae; 9) Chloridoideae including the lovegrasses
(Eragrostis, ca. 350
species, including teff), dropseeds (Sporobolus, some 160 species), finger
millet (Eleusine
coracana (L.) Gaertn.), and the muhly grasses (Muhlenbergia, ca. 175 species);
10)
Panicoideae including panic grass, maize, sorghum, sugar cane, most millets,
fonio and
bluestem grasses; 11) Micrairoideae and 12) Danthoniodieae including pampas
grass; with
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
14
Poa which is a genus of about 500 species of grasses, native to the temperate
regions of both
hemispheres.
Agricultural grasses grown for their edible seeds are called cereals. Three
common cereals
are rice, wheat and maize (corn). Of all crops, 70% are grasses.
Sugarcane is the major source of sugar production. Grasses are used for
construction.
Scaffolding made from bamboo is able to withstand typhoon force winds that
would break
steel scaffolding. Larger bamboos and Arundo donax have stout culms that can
be used in a
manner similar to timber, and grass roots stabilize the sod of sod houses.
Arundo is used to
make reeds for woodwind instruments, and bamboo is used for innumerable
implements.
Another naturally occurring ligno-cellulosic biomass feedstock may be woody
plants or
woods. A woody plant is a plant that uses wood as its structural tissue. These
are typically
perennial plants whose stems and larger roots are reinforced with wood
produced adjacent to
the vascular tissues. The main stem, larger branches, and roots of these
plants are usually
covered by a layer of thickened bark. Woody plants are usually either trees,
shrubs, or lianas.
Wood is a structural cellular adaptation that allows woody plants to grow from
above ground
stems year after year, thus making some woody plants the largest and tallest
plants.
These plants need a vascular system to move water and nutrients from the roots
to the leaves
(xylem) and to move sugars from the leaves to the rest of the plant (phloem).
There are two
kinds of xylem: primary that is formed during primary growth from procambium
and
secondary xylem that is formed during secondary growth from vascular cambium.
What is usually called "wood" is the secondary xylem of such plants.
The two main groups in which secondary xylem can be found are:
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
1) conifers (Coniferae): there are some six hundred species of conifers. All
species have
secondary xylem, which is relatively uniform in structure throughout this
group. Many
conifers become tall trees: the secondary xylem of such trees is marketed as
softwood.
2) angiosperms (Angiospermae): there are some quarter of a million to four
hundred
thousand species of angiosperms. Within this group secondary xylem has not
been found
in the monocots (e.g. Poaceae). Many non-monocot angiosperms become trees, and
the
secondary xylem of these is marketed as hardwood.
The term softwood useful in this process is used to describe wood from trees
that belong to
gymnosperms. The gymnosperms are plants with naked seeds not enclosed in an
ovary. These
seed "fruits" are considered more primitive than hardwoods. Softwood trees are
usually
evergreen, bear cones, and have needles or scale like leaves. They include
conifer species e.g.
pine, spruces, firs, and cedars. Wood hardness varies among the conifer
species.
The term hardwood useful for this process is used to describe wood from trees
that belong to
the angiosperm family. Angiosperms are plants with ovules enclosed for
protection in an
ovary. When fertilized, these ovules develop into seeds. The hardwood trees
are usually
broad-leaved; in temperate and boreal latitudes they are mostly deciduous, but
in tropics and
subtropics mostly evergreen. These leaves can be either simple (single blades)
or they can be
compound with leaflets attached to a leaf stem. Although variable in shape all
hardwood
leaves have a distinct network of fine veins. The hardwood plants include e.g.
Aspen, Birch,
Cherry, Maple, Oak and Teak.
Therefore a preferred naturally occurring ligno-cellulosic biomass may be
selected from the
group consisting of the grasses and woods. Another preferred naturally
occurring ligno-
cellulosic biomass can be selected from the group consisting of the plants
belonging to the
conifers, angiosperms, Poaceae and families. Another preferred naturally
occurring ligno-
cellulosic biomass may be that biomass having at least 10% by weight of it dry
matter as
cellulose, or more preferably at least 5% by weight of its dry matter as
cellulose.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
16
The carbohydrate(s) comprising the invention is selected from the group of
carbohydrates
based upon the glucose, xylose, and mannose monomers and mixtures thereof.
The feedstock comprising lignin can be naturally occurring ligno-cellulosic
biomass that has
been ground to small particles, or one which has been further processed. One
process for
creating the feedstock comprising lignin, comprises the following steps.
PREFERABLE PRETREATMENT
It has been theorized that pretreatment of the feedstock is a solution to the
challenge of
processing an insoluble solid feedstock comprising lignin or polysaccharides
in a pressurized
environment. According to US 2011/0312051, sizing, grinding, drying, hot
catalytic treatment
and combinations thereof are suitable pretreatment of the feedstock to
facilitate the continuous
transporting of the feedstock. While not presenting any experimental evidence,
US
2011/0312051 claims that mild acid hydrolysis of polysaccharides, catalytic
hydrogenation of
polysaccharides, or enzymatic hydrolysis of polysaccharides are all suitable
to create a
transportable feedstock. US 2011/0312051 also claims that hot water treatment,
steam
treatment, thermal treatment, chemical treatment, biological treatment, or
catalytic treatment
may result in lower molecular weight polysaccharides and depolymerized lignins
that are more
easily transported as compared to the untreated ones. While this may help
transport, there is
no disclosure or solution to how to pressurize the solid/liquid slurry
resulting from the pre-
treatment. In fact, as the inventors have learned the conventional wisdom and
conventional
systems used for pressuring slurries failed when pre-treated ligno-cellulosic
biomass feedstock
is used.
In the integrated second generation industrial operations, pre-treatment is
often used to ensure
that the structure of the ligno-cellulosic content is rendered more accessible
to the catalysts,
such as enzymes, and at the same time the concentrations of harmful inhibitory
by-products
such as acetic acid, furfural and hydroxymethyl furfural remain substantially
low. There are
several strategies to achieve increased accessibility, many of which may yet
be invented.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
17
The current pre-treatment strategies imply subjecting the ligno-cellulosic
biomass material to
temperatures between 110-250 C for 1-60 mm e.g.:
Hot water extraction
Multistage dilute acid hydrolysis, which removes dissolved material before
inhibitory
substances are formed
Dilute acid hydrolyses at relatively low severity conditions
Alkaline wet oxidation
Steam explosion.
A preferred pretreatment of a naturally occurring ligno-cellulosic biomass
includes a soaking
of the naturally occurring ligno-cellulosic biomass feedstock and a steam
explosion of at least
a part of the soaked naturally occurring ligno-cellulosic biomass feedstock.
The soaking occurs in a substance such as water in either vapor form, steam,
or liquid form or
liquid and steam together, to produce a product. The product is a soaked
biomass containing a
first liquid, with the first liquid usually being water in its liquid or vapor
form or some
mixture.
This soaking can be done by any number of techniques that expose a substance
to water,
which could be steam or liquid or mixture of steam and water, or, more in
general, to water at
high temperature and high pressure. The temperature should be in one of the
following
ranges: 145 to 165 C, 120 to 210 C, 140 to 210 C, 150 to 200 C, 155 to 185 C,
160 to
180 C. Although the time could be lengthy, such as up to but less than 24
hours, or less than
16 hours, or less than 12 hours, or less than 9 hours, or less than 6 hours;
the time of exposure
is preferably quite short, ranging from 1 minute to 6 hours, from 1 minute to
4 hours, from 1
minute to 3 hours, from 1 minute to 2.5 hours, more preferably 5 minutes to
1.5 hours, 5
minutes to 1 hour, 15 minutes to 1 hour.
If steam is used, it is preferably saturated, but could be superheated. The
soaking step can be
batch or continuous, with or without stirring. A low temperature soak prior to
the high
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
18
temperature soak can be used. The temperature of the low temperature soak is
in the range of
25 to 90 C. Although the time could be lengthy, such as up to but less than 24
hours, or less
than 16 hours, or less than 12 hours, or less than 9 hours or less than 6
hours; the time of
exposure is preferably quite short, ranging from 1 minute to 6 hours, from 1
minute to 4 hours,
from 1 minute to 3 hours, from 1 minute to 2.5 hours, more preferably 5
minutes to 1.5 hours,
minutes to 1 hour, 15 minutes to 1 hour.
Either soaking step could also include the addition of other compounds, e.g.
H2SO4, NH3, in
order to achieve higher performance later on in the process. However, it is
preferred that acid,
base or halogens not be used anywhere in the process or pre-treatment. The
feedstock is
preferably void of added sulfur, halogens, or nitrogen. The amount of sulfur,
if present, in the
composition is in the range of 0 to 1% by dry weight of the total composition.
Additionally,
the amount of total halogens, if present, are in the range of 0 to 1% by dry
weight of the total
composition. By keeping halogens from the feedstock, there are no halogens in
the lignin
conversion products.
The product comprising the first liquid is then passed to a separation step
where the first liquid
is separated from the soaked biomass. The liquid will not completely separate
so that at least a
portion of the liquid is separated, with preferably as much liquid as possible
in an economic
time frame. The liquid from this separation step is known as the first liquid
stream comprising
the first liquid. The first liquid will be the liquid used in the soaking,
generally water and the
soluble species of the feedstock. These water soluble species are glucan,
xylan, galactan,
arabinan, glucolygomers, xyloolygomers, galactolygomers and arabinolygomers.
The solid
biomass is called the first solid stream as it contains most, if not all, of
the solids.
The separation of the liquid can again be done by known techniques and likely
some which
have yet to be invented. A preferred piece of equipment is a press, as a press
will generate a
liquid under high pressure.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
19
The first solid stream is then steam exploded to create a steam exploded
stream, comprising
solids and a second liquid. Steam explosion is a well known technique in the
biomass field
and any of the systems available today and in the future are believed suitable
for this step.
The severity of the steam explosion is known in the literature as Ro, and is a
function of time
and temperature and is expressed as
Ro = texpl(T-100)/14.751
with temperature, T expressed in Celsius and time, t, expressed in common
units.
The formula is also expressed as Log(Ro), namely
Log(Ro) = Ln(t) + KT-100)/14.751.
Log(Ro) is preferably in the ranges of 2.8 to 5.3, 3 to 5.3, 3 to 5.0 and 3 to
4.3.
The steam exploded stream may be optionally washed at least with water and
there may be
other additives used as well. It is conceivable that another liquid may be
used in the future, so
water is not believed to be absolutely essential. At this point, water is the
preferred liquid and
if water is used, it is considered the third liquid. The liquid effluent from
the optional wash is
the third liquid stream. This wash step is not considered essential and is
optional.
The washed exploded stream is then processed to remove at least a portion of
the liquid in the
washed exploded material. This separation step is also optional. The term at
least a portion is
removed, is to remind one that while removal of as much liquid as possible is
desirable
(pressing), it is unlikely that 100% removal is possible. In any event, 100%
removal of the
water is not desirable since water is needed for the subsequent hydrolysis
reaction. The
preferred process for this step is again a press, but other known techniques
and those not
invented yet are believed to be suitable. The products separated from this
process are solids in
the second solid stream and liquids in the second liquid stream.
The steam exploded stream is then subjected to hydrolysis to create a
hydrolyzed stream.
Optionally at least a part of the liquid of the first liquid stream is added
to the steam exploded
stream. Also, water is optionally added. Hydrolysis of the steam exploded
stream is realized
by contacting the steam exploded stream with a catalyst. Enzymes and enzyme
composition is
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
the preferred catalyst. While laccase, an enzyme known to alter lignin, may be
used, the
composition is preferably void of at least one enzyme which converts lignin. A
preferred
hydrolysis of the steam exploded stream comprises the step of:
A) Contacting the steam exploded stream with at least a portion of a solvent,
the
solvent comprised of water soluble hydrolyzed species; wherein at least some
of the
water soluble hydrolyzed species are the same as the water soluble hydrolyzed
species
obtainable from the hydrolysis of the steam exploded stream;
B) maintaining the contact between the steam exploded stream and the solvent
at a
temperature in the range of 20 C to 200 C for a time in the range of 5 minutes
to 72
hours to create a hydrolyzed stream from the steam exploded stream.
The hydrolyzed stream is comprised of carbohydrate monomers selected from the
group
consisting of glucose, xylose, and mannose.
The hydrolyzed stream is subjected to fermentation to create a fermented
stream comprised of
the composition and water. The fermentation is performed by means of addition
of yeast or
yeast composition to the hydrolyzed stream.
Eventually hydrolysis and fermentation can be performed simultaneously,
according to the
well known technique of simultaneous saccharification and fermentation (SSF).
The composition derived from naturally occurring ligno-cellulosic biomass is
separated from
the water in the fermented stream. The separation of the liquid can be done by
known
techniques and likely some which have yet to be invented. A preferred piece of
equipment is a
press.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
21
The composition is different from naturally occurring ligno-cellulosic biomass
in that it has a
large surface area as calculated according to the standard Brunauer, Emmett
and Teller (BET)
method.
The BET surface area of the dry composition is at least 4 m2/gm more
preferably in the range
of 4 to 80 m2/gm, with 4 to 50 m2/gm being more preferable, 4 to 25 m2/gm
being even more
preferred, and 4 to 15 m2/gm being even more preferred and 4 to 12 m2/gm being
the most
preferred.
The composition is further characterized by the peaks generated during a
thermal gravimetric
analysis, known as TGA.
In thermogravimetric analysis, the plot of the weight with respect to
temperature and the plot
of the first derivative of weight with respect to temperature are commonly
used.
If the decomposition of the material or of a component of the material occurs
in a specific
range of temperature, the plot of the first derivative of weight with respect
to temperature
presents a maximum in the specific range of temperature, defined also as first
derivative peak.
The value of temperature corresponding to the first derivative peak is
considered the
decomposition temperature of the material or of that component of the
material.
The material is a composition of many components, which decompose in different
specific
temperature ranges, the plot of the first derivative of weight with respect to
temperature
presents first derivative peaks associated to the decomposition of each
component in each
specific temperature range. The temperature values corresponding to the first
derivative peaks
are considered the decomposition temperatures of each component of the
material.
As a general rule, a maximum is located between two minima. The values of
temperature
corresponding to the minima are considered as the initial decomposition
temperature and the
final decomposition temperature of the decomposition temperature range of the
component
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
22
whose decomposition temperature corresponds to the first derivative peak
comprised between
the two minima. In this way, a derivative peak corresponds to decomposition
temperature
range. The weight loss of the material in the range between the initial
decomposition
temperature and the final decomposition temperature is associated to the
decomposition of that
component of the material and to the first derivative peak.
Should the naturally occurring ligno-cellulosic biomass used to derive the
lignin composition
be a mixture of different species of grasses or plants or other materials,
then the mixture of the
naturally occurring ligno-cellulosic biomass is what should be used for the
comparison with
the material from which the composition was derived.
The composition created has the characteristics that temperature corresponding
to the
maximum value of the first lignin decomposition peak is less than the
temperature
corresponding to the maximum value of the first lignin decomposition peak of
the naturally
occurring ligno-cellulosic biomass. This difference is marked with the maximum
value of the
first lignin decomposition peak being less than the temperature corresponding
to the maximum
value of the first lignin decomposition peak of the naturally occurring ligno-
cellulosic biomass
by a value selected from the group consisting of at least 10 C, at least 15
C, at least 20 C,
and at least 25 C.
This reduction in the maximum value of the first lignin decomposition
temperature can be
compared to the maximum value of the first lignin decomposition temperature
after pre-
treatment.
Additionally, the absolute mass on a dry basis associated with the first
lignin decomposition
peak of the claimed lignin composition is greater than the absolute mass on a
dry basis of the
second lignin decomposition peak. While for Arundo donax, the absolute mass of
the first
decomposition temperature of the naturally occurring ligno-cellulosic biomass
is greater than
the absolute mass of the second decomposition temperature of the naturally
occurring ligno-
cellulosic biomass, this is not true for many ligno-cellulosic biomasses such
as corn stover and
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
23
wheat straw. However, after conversion, the lignin composition derived from
these biomasses
has a mass on a dry basis associated with the first lignin decomposition
temperature that is
greater than the mass on a dry basis associated with the second lignin
decomposition
temperature.
The feedstock can be further characterized by comparing the temperature
associated with the
maximum value of the first lignin decomposition range with the temperature
associated with
the maximum value of the first lignin decomposition range of the ligno-
cellulosic biomass
used to derive the feedstock.
The feedstock can also be further characterized by the relative amount of
carbohydrates, which
include glucans and xylans, present on a dry basis. The composition may have
the amount of
total carbohydrates present in the composition in the range of 10 to 60% of
the dry weight of
the composition, with 10 to 40% more preferred with 5 to 35% even most
preferred.
Provided, of course, that the amount of total lignin present in the
composition is in the range
of 30 to 80% of the dry weight of the composition and the weight percent of
the carbohydrates
plus the weight percent of the lignin is less than 100% of the dry weight of
the feedstock.
Because the composition of the feedstock comprising lignin may vary with the
starting
material from which it is derived, the naturally occurring ligno-cellulosic
biomass from which
the feedstock is derived can be selected from the group consisting of the
grasses and food
crops.
SLURRY CREATION
Lignin may be charged to a lignin conversion reactor (500) as a solid slurried
in a liquid. In a
preferred embodiment the liquid may comprise water. In another embodiment, the
liquid may
comprise a hydrogen donor. The use of hydrogen donors is well known and
described in
Wang, X, and Rinaldi, R.; "Exploiting H-Transfer reactions with RANEY Ni for
upgrade of
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
24
phenolic and aromatic biorefinery feeds under unusual, low severity
conditions:", Energy
Environ. Sci., 2012, 5, 8244
It has been discovered that a slurry comprised of lignin has several unique
characteristics
making it difficult to create, maintain and handle, and in many instances a
slurry comprised of
lignin behaves in the opposite manner of traditional slurries.
The solid content of a slurry comprised of lignin should be in the range of
about 1 to 70% by
weight with 5 to 35% by weight solids content more preferred. Traditionally,
slurries are
easier to maintain when the solids content is low. Surprisingly, a slurry
comprised of lignin is
easier to maintain when the solids content is high (greater than 20% by weight
solids).
The particle size of the slurry comprised of lignin should be such that the
number average size
is in the range of less than 200 micron with less than 150 micron being
preferred and less than
100 micron being most preferred. Particle size reduction is not necessary when
the feedstock
comprising lignin has been steam exploded. However, particle size reduction is
considered
necessary if the practitioner is starting with naturally occurring lignin,
such as wood chips.
No surfactants or emulsifying agents are needed, but they can be used.
There are several strategies for creating a slurry comprised of lignin
depending upon the
manufacturing location of the claimed process. If the lignin conversion is co-
sited with the
pre-treatment or carbohydrate conversion of the ligno-cellulosic biomass (10),
then the lignin
may already be present in a slurry form, often called the stillage or stillage
lignin, with little or
no water soluble sugars, or void of water soluble sugars. When the ligno-
cellulosic biomass
(10) is passed through the pre-treatment or carbohydrate conversion process
first, the water
soluble sugars are converted to species other than sugars. The water soluble
sugars will have
been washed off, extracted or converted by the enzymes or catalysts to species
other than
sugars, leaving the bottoms which are comprised of lignin and unconverted,
insoluble
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
carbohydrates, many of which are still bound with the lignin. These bottoms
are void of or
substantially void of free water soluble sugars.
In this iterated embodiment, the bottoms, (or stillage or stillage lignin as
it is often called), of
the sugar or carbohydrate conversion process, (e.g. fermentation), are passed
directly to a next
process which could further remove more carbohydrates; or the bottoms are
passed directly to
the lignin conversion process described herein. In this manner, the water from
the
carbohydrate conversion process which would otherwise have to be treated via
expensive
waste water treatment plant(s) is used as a slurry liquid to maintain or
create the slurry
comprised of lignin to feed the lignin conversion process. The stillage
lignin, which is the
slurry liquid removed from the carbohydrate conversion process comprising the
lignin, is then
cleaned in situ by the hydrogen of the lignin conversion process while at the
same time,
converting the lignin. As described later, the slurry liquid coming from the
lignin conversion
process will have significantly less total biochemical oxygen demand, also
known as BOD's,
and/or chemical oxygen demand, also known as COD's, relative to the amounts of
BOD's and
COD's in the incoming slurry liquid from the stillage lignin, thus reducing
the amount of, and
cost of waste water treatment needed before releasing the slurry liquid to the
environment.
The BOD's and COD's have been chemically destroyed by the conditions of the
lignin
conversion process.
In a further refinement, at least a portion of the slurry liquid from the
lignin conversion
process can be used as make up water or steam in a pre-treatment process, thus
significantly
reducing the amount and cost of water treatment. (See Fig. 3)
This schematic is demonstrated in Fig. 3, wherein the ligno-cellulosic biomass
(10) enters the
pre-treatment process and the pre-treated ligno-cellulosic biomass is passed
to the
carbohydrate conversion process, in this instance fermentation. In the
carbohydrate
conversion process, the sugars are converted to the final product or products.
It is preferable
to introduce the slurry liquid from the lignin conversion process (620), prior
to or
simultaneously with the steam explosion step of the pretreatment process.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
26
The bottoms, or stillage, comprising the lignin, slurry liquid, and possibly
carbohydrates, is
passed to the slurry creation step, (300). If the stillage lignin is a
sufficiently stable slurry and
of desired concentrations, (e.g. solids, buffers, pH), it can be passed
directly to (400), the
slurry pump, without any further treatment, e.g. water dilution or water
reduction, agitation,
vacuum.
If adjustments are needed, the slurry comprised of lignin is brought to the
optimum slurry
conditions by adjusting the solids concentration under agitation and
optionally vacuum.
Usually this is under high shear agitation of the slurry comprised of lignin.
In some embodiments, the bottoms of the carbohydrate conversion process will
be shipped to
a different location for the lignin conversion. While it is possible to ship
the already slurried
stillage, the cost of shipping water may make shipping cost prohibitive. In
this instance, it is
anticipated that the feedstock comprised of lignin will be shipped as a solid
and often dry with
as much water having been removed as possible; usually by a filter press,
drying, or both.
Oftentimes, the solid feedstock comprising lignin will be chilled or even
frozen to prevent
microbial growth during shipment or storage. The slurry liquid from the
dewatering process is
often sent to waste water treatment where it is cleaned to remove BOD' s and
COD' s, and then
released to the environment or reused in parts of the pre-treatment process.
It is this external
treatment step which can be minimized or reduced by re-using or recycling at
least a portion of
the slurry liquid from the lignin conversion process.
It has been directly observed that the feedstock comprising lignin is
excessively intractable
and the particles are very difficult to separate. This is particularly the
case when the feedstock
comprising lignin has been subjected to dewatering pressure to dewater, as in
a filter press.
Visible light microscopic examination shows the feedstock comprising lignin to
have tendrils
with tentacles and hooks, much like Velcro .
As stated earlier, if the feedstock after the carbohydrate conversion step is
already a slurry, it
may be possible to add the slurry directly to the process without further
treatment. However,
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
27
generally this is not expected. After carbohydrate conversion, there is likely
to be trapped
gasses in the stillage lignin which should be removed.
If the lignin conversion is not co-sited with the pre-treatment or
fermentation of the ligno-
cellulosic biomass (10), then one strategy for creating the slurry comprised
of lignin is to use a
machine capable of applying high shear forces and apply high shear forces to
the unslurried
solid feedstock comprising lignin. High shear forces may be achieved by
feeding the solid
feedstock comprising lignin through a compounder. Preferred compounder
embodiments
include a twin screw co-rotating screws compounder, a twin screw counter-
rotating screws
compounder, an extruder, a banbury, or another device known for imparting
mechanical forces
to the material processed through it.
The amount of mechanical forces required is related to the amount of energy
required to make
the solid feedstock comprising lignin readily dispersible. The more mechanical
forces applied
to the solid feedstock comprising lignin, the easier the dispersion. The
amount of mechanical
forces required can be determined iteratively by comparing the energy consumed
with the
energy required to disperse the resulting solid into the slurry liquid of the
slurry. Techniques
to vary the amount and type of mechanical forces applied to the solid
feedstock comprising
lignin depend upon the equipment and are well known in the art to those
familiar with the
particular machine being used.
A slurry liquid can be added to the solid feedstock comprising lignin to
produce a slurry
comprised of lignin. It is preferred that the slurry liquid be added to the
solid feedstock
comprising lignin after exiting the compounder. In this regard, the solid
feedstock comprising
lignin is void of free liquid meaning that free liquid comprises less than 5%
of the weight of
the composition with no free liquid being preferred. In another embodiment,
the slurry liquid
may be added to the solid feedstock comprising lignin in the compounder. In a
preferred
embodiment the slurry liquid comprises water. In another embodiment, the
slurry liquid may
comprise a hydrogen donor. It should be noted that for the purposes of this
specification, the
slurry liquid is also known as a carrier liquid as well.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
28
The amount of energy consumed by the compounder necessary to create a solid
feedstock
comprising lignin that is readily dispersible into a slurry liquid and/or has
a low viscosity
when dispersed into a slurry liquid can be determined by measuring the torque.
The solid
feedstock comprising lignin is readily dispersed into a slurry liquid when the
amount of torque
required to disperse the solid feedstock comprising lignin into the slurry
liquid in the absence
of a hydrolysis catalyst is less than 50% of the amount of torque required to
disperse the solid
feedstock comprising lignin into the slurry liquid under the same conditions,
prior to the
application of the mechanical forces.
The amount of torque is the total amount of energy applied to the solid-slurry
liquid mixture to
disperse the solid into the slurry liquid. The amount of torque can be
determined by the area
under the curve of the line of the torque applied at a given point in time, t,
corresponding to
the point at which the solid is considered dispersed into the slurry liquid. A
solid is considered
dispersed into the slurry liquid when the numeral average of the percent of
dry matter content
of a statistically valid number of aliquots of the slurry liquid is within
2.5% of the percent of
the total dry matter content in the slurry liquid.
The viscosity of the slurry comprised of lignin, measured at 25 C, a shear
rate of 10s-1, of the
mechanically dispersed solid feedstock comprising lignin dispersed in the
slurry liquid content
should be less than the viscosity of a slurry of the solid feedstock comprised
of lignin
dispersed in the slurry liquid prior to mechanical treatment; when measured
under the same
conditions (e.g. dry matter content).
After producing the slurry comprised of lignin, the slurry comprised of lignin
may be
maintained by way of mechanical agitation.
Another strategy for creating the slurry comprised of lignin where the lignin
conversion is not
co-sited with the pre-treatment or fermentation of the ligno-cellulosic
biomass (10) is to
expose the solid feedstock comprising lignin in a slurry liquid, preferably
water, to a vacuum
or pressure less than atmospheric pressure, with less than 0.8 bar being
preferred, with less
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
29
than 0.7 bar being more preferred, less than 0.4 bar being even more preferred
with less than
0.2 bar being the most preferred. The feedstock comprising lignin will rapidly
expand into
small particles, disassociate, and disperse. In this way, high shear mixing
and/or high shear
forces are avoided with higher concentrations possible. It is preferred to
have at least some
mechanical agitation occurring simultaneously with the vacuum step so as to
more rapidly
disperse the particles. The Slurry Creation Experimental Section and Figure 5
quantitatively
show the advantage of using vacuum on the solid feedstock comprising lignin
prior to
increasing the pressure on the slurry. The vacuum may be applied
simultaneously with shear
and agitation, through a conveying screw. The minimum time for the vacuum to
remain
applied is the time sufficient to disperse the particles to greater than 50%
of the theoretical
dispersion at 25 C, with greater than 75% dispersion at 25 C more preferred
and greater than
90% dispersion at 25 C the most preferred. It is preferred that the solid
feedstock comprising
lignin be surrounded or encompassed by a slurry liquid for full effectiveness
of the vacuum.
In a preferred embodiment this slurry liquid is water. In another embodiment,
this slurry
liquid comprises a hydrogen donor. 100% dispersion at 25 C is the theoretical
dispersion.
The amount of dispersion is determined by measuring the amount of solids in a
sample after 2
minutes of settling. If there were 16 gms of solid in 84 gms of liquid, the
dry matter content at
100% dispersion would be 16 %. At 50% of the theoretical dispersion, the dry
matter content
of the sample after 2 minutes of settling would be 8%.
A final strategy for creating the slurry comprised of lignin where the lignin
conversion is not
co-sited with the pre-treatment or fermentation of the ligno-cellulosic
biomass (10) is to
expose the solid feedstock comprising lignin in a slurry liquid, preferably
water, to high shear
such as that found in a blender, which over time will also disperse the
particles of the
feedstock comprising lignin throughout the slurry. In another embodiment, the
slurry liquid is
a hydrogen donor.
In most instances the slurry liquid will be water or water in combination with
at least one
hydrogen donor. The ratio amount of the weight of the water of the slurry
liquid to the dry
weight of the lignin feedstock is preferably in the range of 0.3 to 9, with
0.5 to 9 more
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
preferred, with 1 to 9 even more preferred with 2 to 9 another preferred ratio
and 3 to 5 an
even more preferred ratio.
SLURRY CREATION EXPERIMENTS
Experiments were conducted for evaluating slurry preparation under vacuum
treatment in
comparison with slurry preparation under standard mechanical agitation.
Slurry Creation Experiment 1
An amount of 450g of lignin-rich composition, having a dry matter of 53%, was
inserted into a
3 liter round bottom flask with 1050g of water, to reach a theoretical
concentration of 16% of
dry matter of lignin-rich composition in the mixture. No mechanical mixing was
applied.
The flask had a dimension of approximately 16cm and was equipped with a
stirrer with a
dimension of approximately 6cm.
The flask was sealed and vacuum of 29.8mmHg was applied for 5 minutes and
removed. After
2 minutes of sedimentation time, a first sampling of the slurry comprised of
lignin was
extracted.
Mechanical agitation was applied to the slurry comprised of lignin at
atmospheric pressure for
1 minute, then mechanical agitation was stopped and after 2 minutes of
sedimentation time a
sampling was extracted. The mechanical agitation procedure was repeated
further for 5, 10,
30, and 60 minutes of agitation time and samplings were extracted after a
sedimentation time
of 2 minutes each time.
No chunks were present at the bottom of the flask and the slurry comprised of
lignin appeared
to be homogeneously mixed.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
31
Slurry Creation Experiment 2
A control experiment was realized by inserting an amount of 450g of lignin-
rich composition,
having a dry matter of 53%, into a 3 liter round bottom flask with 1050g of
water, to reach a
theoretical concentration of 16% of dry matter of lignin-rich composition in
the mixture.
The flask and mechanical stirrer were the same as in the experiment conducted
with vacuum.
The slurry comprised of lignin was subjected only to mechanical agitation, and
samplings
were extracted after 5, 1, 5, 10, 30, 60 minutes of agitation. Before each
sampling, the
mechanical agitation was stopped for 2 minutes of sedimentation time.
A relevant amount of chunks were present at the bottom of the flask and the
slurry comprised
of lignin appeared to be inhomogeneous.
The mechanical agitation was obtained by stirring the slurry comprised of
lignin at 250rpm in
both the experiments.
Concentration of dry matter of the lignin-rich composition was determined by
drying samples
in an oven at 105 C for 15 hours.
Figure 5 reports the graph of percent complete dispersion of the lignin-rich
composition in the
slurry comprised of lignin. The percent complete dispersion is the
concentration of dry matter
of lignin-rich composition in the slurry comprised of lignin normalized with
respect to the
theoretical concentration.
The experiment demonstrates that by applying a vacuum the time needed to
obtain a full
dispersion of the lignin-rich composition in the slurry comprised of lignin is
strongly reduced,
thereby enabling mixing energy savings, time savings and slurry tank volume
reduction.
SLURRY PRESSURIZING AND TRANSPORT
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
32
After the slurry comprised of lignin is created it must be brought to a
pressure slightly greater
than the lignin conversion reactor pressure plus the pressure from the slurry
pump exit to the
lignin conversion reactor (500), so that the slurry can be charged into the
lignin conversion
reactor (500).
The slurry comprised of lignin can be pressurized using a slurry pump (400).
For the purposes
of this specification the term slurry pump (400) is meant to refer to any pump
which can reach
the desired pressures, such as a piston pump and/or a syringe pump. A multi-
stage centrifugal
pump may also reach the required pressures. The slurry pump (400), which is
depicted as a
piston pump used in the experiments will have an inlet valve (350). The inlet
valve position
can span the range from fully open to fully closed. Therefore, the inlet valve
position can be
selected from the group consisting of open, closed and at least partially
open, wherein open
means fully open (the restrictions across the valve as measured by pressure
drop are the
minimum possible), closed means fully closed so that no liquid or gas can pass
through the
valve, and at least partially open means the valve is not fully closed and not
fully open, but
somewhere in between fully closed and fully open. The slurry pump (400) will
have an outlet
valve (450). The outlet valve can be present in an outlet valve position
selected from the
group consisting of open, closed and at least partially open, with open,
closed and at least
partially open having the same meanings as for the inlet valve position.
The slurry pump (400) will further comprise a piston (420) and a piston
chamber (425). The
piston (420) forms a seal inside and against the piston chamber (425) to form
a pump cavity.
The size of the cavity depends upon where the piston (420) is within the
piston chamber (425).
The slurry comprised of lignin is passed through the inlet valve (350) which
is in the inlet
valve position of at least partially open or open (430A) into the pump cavity
formed by
withdrawing at least a portion of the piston (420) from the piston chamber
(425). During this
inlet step, the outlet valve (450) is in the closed outlet valve position
(440B). The pump cavity
will be at an inlet pump cavity pressure. After an amount of slurry comprised
of lignin enters
the pump cavity, the inlet valve position is changed to closed (430B), or in
other words, the
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
33
inlet valve is closed. A force is then placed on or applied to the piston
(420) in the piston
chamber (425) until the pressure of the slurry comprised of lignin reaches the
discharge
pressure which is greater than the reactor operating pressure, also known as
the lignin
conversion reactor pressure or deoxygenation pressure. The reactor operates in
the ranges of
80 to 245 bar, 80 to 210 bar, 90 to 210 bar and 90 to 175 bar. Therefore the
discharge pressure
of the pump should also be in the above ranges of 80 to 245 bar, 80 to 210
bar, 90 to 210 bar
and 90 to 175 bar, but greater than the lignin conversion pressure. It should
also be noted for
the purposes of this specification that the terms lignin conversion vessel and
lignin conversion
reactor are interchangeable.
At least a portion of the slurry comprised of lignin is discharged from the
pump cavity by
opening the outlet valve (450), also known as changing the outlet valve
position to a position
selected from the group consisting of at least partially open and open. The
piston (420) is
further forced into the pump body to reduce the volume of the pump cavity and
push at least a
portion of the slurry comprised of lignin through the outlet valve (450). The
outlet valve (450)
is connected to the lignin conversion reactor (500) by tubing, piping or other
connection. By
connected to the lignin conversion reactor it is meant that material from the
pump cavity can
flow through the outlet valve and into the lignin conversion reactor (500)
generally through a
pipe, a tube or through a series of connected pipes or tubes. In one
embodiment there may be
a plurality of additional valves between the outlet valve and the lignin
conversion reactor
(500), such as a valve for isolating the lignin conversion reactor (500).
In order for the process to run in a continuous manner it is not necessary
that the slurry
comprised of lignin is continuously introduced to the lignin conversion
reactor (500). For
example, when only one piston pump is used, the slurry comprised of lignin is
introduced into
the lignin conversion reactor (500) in steady aliquots or pulses. Thus there
are moments when
there is no product entering the lignin conversion reactor. But, over time,
the mass introduced
into the lignin conversion reactor equals the mass removed from the lignin
conversion reactor.
One distinguishing feature between a continuous and a batch process is that,
in a continuous
process, the reaction is occurring or progressing at the same time that either
the slurry
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
34
comprised of lignin is introduced into the lignin conversion reactor (500)
and/or the lignin
conversion products are removed from the lignin conversion reactor. Another
way to state this
is that the conversion (e.g. deoxygenating, or hydrogenating) in the lignin
conversion reactor
occurs while simultaneously, or at the same time, removing at least a portion
of the lignin
conversion reactor contents from the lignin conversion reactor (500). Such
removal is done in
a continuous manner which includes an aliquot or pulse removal.
The previous art proposes the use of piston pumps or syringe pumps for high
pressure reactor
charging. However, the consensus of the art is to use check valves. This
simple elegant
approach has been used for years. However, as discovered by the inventors,
check valves and
other valve configurations will not work with a slurry comprised of lignin.
The inventors
consulted multiple pump and valve experts and evaluated the myriad of
solutions proposed by
the experts, none of which allowed the slurry comprised of lignin to be
continuously charged
to the lignin conversion reactor. A pressure could not be maintained or could
not be
maintained for long. The observations indicated that the tough, fibrous nature
of lignin allows
the lignin from the slurry comprised of lignin to get stuck in the valve seats
and build up in
areas of low flow or high impaction causing the valves to plug.
What was discovered is that a more complicated valving system worked. It was
discovered
that the industry standard and use of a simple check valve had to be replaced
with a valve
having a position that could be controlled and that the valve should provide
unrestricted and
unobstructed flow of the slurry comprising lignin through the valve or its
flow path. By
unrestricted flow it is meant that the flow of the slurry comprising lignin
through the valve
(flow path) does not change directions, such as in a bend, and does not
increase in linear
velocity, such as in a narrowing of the flow path. By unobstructed flow it is
meant that the
flow path does not contain any additional elements, such as the insert body of
a butterfly
valve, in the path of the slurry flow such that the slurry will have to flow
around or strike the
additional element when the valve is in the fully open position. Further, the
flow path does
not contain additional dead zones, such as the seat groove of a gate valve.
Dead zones, such
as the seat groove of a gate valve will fill with slurry when the valve is
open and, when the
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
valve is closed, the gate will compress the slurry into the groove which will
allow for
accumulation and compression of the slurry comprised of lignin in the groove.
In this
instance, over time the valve will not seat or seal, and will fail to hold
pressure.
By way of example, but not limitation, a valve that provides for unrestricted
and unobstructed
flow of the slurry comprising lignin may include a ball valve, a full port
ball valve or a full
port fixed ball valve. In contrast, traditional valves such as most globe
valves, most angle
valves, most diaphragm valves, most butterfly valves and most check valves
restrict and/or
obstruct the flow of the slurry comprised of lignin and will cause the lignin
from the slurry
comprised of lignin to build up in areas of low flow or high impaction causing
the valves to
eventually plug or not seat or seal, and fail to hold pressure. (Examples of
such valves are
described in Chemical Engineers' Handbook, Fifth Edition, Perry & Chilton, p 6-
54 through
6-57, 1973). In practice, this build up of lignin from the slurry comprised of
lignin may occur
quite rapidly, in some cases so rapidly that no amount of the slurry comprised
of lignin will be
charged through the inlet valve and into the pump cavity. (See Slurry Pumping
Experiment 1).
By removing the check valve, the system was no longer automatic within the
valve but needed
special additional controls to turn each valve on and off in a synchronized
manner. Therefore,
in direct opposite of the prior art, and what the pump and valve experts
proposed to the
inventors on many occasions, the process only functioned when the inlet valve
(350) and the
outlet valve (450) were not check valves, but valves that provide for
unrestricted and
unobstructed flow. (A check valve being a valve which prevents the reversal of
flow). It is
preferable that the pressurization process, discharge and ultimate charge into
the reactor be
void of any check valves in the path of slurry flow. Alternatively, the slurry
does not flow
through a check valve into the slurry pump (400) to enter the reactor.
Different embodiments are available. For example there could be a plurality of
slurry pumps
comprising at least two piston pumps. Where there are two piston pumps each
piston pump
may have its own inlet valve and its own outlet valve (e.g. the first piston
pump has a first
inlet valve (350A) and a first outlet valve (450A) while the second piston
pump has a second
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
36
inlet valve (350B) and a second outlet valve (450B)). The plurality of slurry
pumps can be in
a parallel configuration. It is possible for two piston pumps in a parallel
configuration to share
the same inlet valve (350) and/or outlet valve (450). Another configuration is
where the inlet
valve (350) and outlet valve (450) are the same valve.
Eventually at least a portion of the slurry comprising lignin, a portion of
which is in a solid
form, is introduced into the lignin conversion reactor (500). The lignin
conversion reactor will
have a lignin conversion pressure and lignin conversion temperature. The
lignin conversion
pressure will be at least slightly less than the slurry pump discharge
pressure which is at least
the amount of pressure drop from the slurry pump (400) to the lignin
conversion reactor inlet.
Generally, the slurry pump discharge pressure will be greater than the lignin
conversion
pressure, with the slurry pump discharge pressure being greater than the
lignin conversion
reactor pressure plus the absolute amount of pressure drop in the process from
the slurry pump
discharge to the lignin conversion reactor (500).
SLURRY PUMPING EXPERIMENTS
Experiments were conducted for charging a slurry comprised of lignin to a
pressurized lignin
conversion reactor. The following procedures were applied to all the
experiments, unless
differently specified.
De-ionized water was added to a lignin-rich composition obtained from the
pretreatement of
ligno-cellulosic biomass to obtain a slurry comprised of lignin having a dry
matter content of
20 weight percent of the mass of the slurry. The mixture was inserted into a
blender (Waring
Blender, model HGBSSSS6) and thoroughly mixed intermittently for one to two
minutes to
reach a homogeneous slurry. The homogeneity of the slurry was evaluated by
eye. The slurry
was inserted into a mix tank (340) with constant agitation. The mix tank (340)
was a stainless
steel, dish bottom tank with a volume of approximately 1 L containing a
standard laboratory
paddle mixer and a bottom discharge port connected to a Chandler Quizix QX
dual syringe
pump having two pump cavities. Inlet valves (350) were inserted between the
mix tank (340)
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
37
and the two pump cavities of the Chandler Quizix QX dual syringe pump. The
Chandler
Quizix QX dual syringe pump was connected by tubing to a Parr 4575 reactor
equipped with a
dual 450 pitched turbine blade, cooling coil, separate gas and slurry feed
ports and a discharge
dip tube (610). Outlet valves (450) were inserted between the two pump
cavities of the
Chandler Quizix QX dual syringe pump and the Parr reactor. Between 200 and 400
scfh of
hydrogen at a temperature of 20 C was inserted into the Parr reactor to reach
a pressure of
48.3 bar. The Parr reactor was heated to a temperature corresponding to 90% of
the reaction
temperature and a continuous flow of Hydrogen was started into the Parr
reactor. Final
temperature and pressure in the Parr reactor varied between 275-325 C and 100
and 175 bar.
The pressure was measured by means of a pressure transducer (Ashcroft Type 62)
connected
to the Parr reactor.
The slurry comprised of lignin was passed from the mix tank (340) into the
first of the two
pump cavities of the Chandler Quizix QX dual syringe pump by changing the
inlet valve
position of the first inlet valve (350A) corresponding to the first pump
cavity to the open
position (430A) by means of an actuator. After the slurry comprised of lignin
reached the first
pump cavity, the first inlet valve (350A) corresponding to the first pump
cavity was changed
to the closed inlet valve position (430B) by means of an actuator. After the
first inlet valve
(350A) corresponding to the first pump cavity was closed, the slurry comprised
of lignin was
passed from the mix tank (340) into the second of the two pump cavities of the
Chandler
Quizix QX dual syringe pump by changing the inlet valve position of the second
inlet valve
(350B) corresponding to the second pump cavity to the open position (430A) by
means of an
actuator.
After the first inlet valve (350A) corresponding to the first pump cavity was
closed (430B), the
Chandler Quizix QX dual syringe pump pressurized the slurry comprised of
lignin in the first
pump cavity to a pressure greater than that of the Parr reactor. While the
slurry comprised of
lignin in the first pump cavity was being pressurized both the first inlet
valve (350A) and the
first outlet valve (450A) were closed. After the slurry comprised of lignin in
the first pump
cavity was pressurized to a pressure greater than that of the Parr reactor,
the first outlet valve
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
38
(450A) corresponding to the first pump cavity was changed to the open position
(440A) by
means of an actuator, allowing the pressurized slurry comprised of lignin in
the first pump
cavity to be charged to the Parr reactor.
After the first outlet valve (450A) corresponding to the first pump cavity was
opened, the
second inlet valve (350B) corresponding to the second pump cavity was changed
to the closed
position (430B) by means of an actuator. After the second inlet valve (350B)
corresponding to
the second pump cavity was closed (430B), the Chandler Quizix QX dual syringe
pump
pressurized the slurry comprised of lignin in the second pump cavity to a
pressure greater than
that of the Parr reactor. While the slurry comprised of lignin in the second
pump cavity was
being pressurized both the second inlet valve (350B) and the second outlet
valve (450B) were
closed. The pressure of the Parr reactor is the deoxygenation pressure and can
range from 90
to 175 bar. After the slurry comprised of lignin in the second pump cavity was
pressurized to
a pressure greater than that of the Parr reactor, the first outlet valve
(450A) corresponding to
the first pump cavity was changed to the closed position (440B) by means of an
actuator.
After the first outlet valve (450A) corresponding to the first pump cavity was
closed, the
second outlet valve (450B) corresponding to the second pump cavity was changed
to the open
(440A) position by means of an actuator, allowing the pressurized slurry
comprised of lignin
in the second pump cavity to be charged to the Parr reactor.
After the second outlet valve (450B) corresponding to the second pump cavity
was opened,
the first inlet valve (350A) corresponding to the first pump cavity was
changed to the open
position (430A) by means of an actuator, allowing additional slurry comprised
of lignin from
the mix tank (340) into the first pump cavity to be pressurized and
subsequently charged to the
Parr reactor.
Slurry Pumping Experiments 1 and 2
For Slurry Pumping Experiments 1 and 2, the inlet valves and outlet valves
were small orifice,
rising stem valves from Vindum Engineering, Model No. CV-505-SS. These valves
were
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
39
recommended by an expert in the field of slurry pumping, and were represented
as sufficient
for charging a slurry comprised of lignin to a pressurized reactor.
For Experiment 1, when the inlet valve corresponding to the first pump cavity
was changed to
the open position, it immediately plugged with solid lignin from the slurry
comprised of
lignin. No amount of the slurry comprised of lignin reached the first pump
cavity, the outlet
valve corresponding to the first pump cavity, or the Parr reactor.
For Experiment 2, an expert in the field of slurry pumping recommended
pressurizing the mix
tank (340) to between 2.5 to 3 bar to assist with charging the slurry
comprised of lignin
through the inlet valves into the pump cavities. The expert represented that
pressurizing the
mix tank (340) would allow the slurry comprised of lignin to pass through the
inlet valves into
the pump cavities without plugging the inlet valves. When the inlet valve
corresponding to the
first pump cavity was changed to the open position, it immediately plugged
with solid lignin
from the slurry comprised of lignin without any amount of the slurry comprised
of lignin
reaching the first pump cavity, the outlet valves, or the Parr reactor.
Slurry Pumping Experiments 3 and 4
For Experiments 3 and 4, an expert in the field of slurry pumping recommended
that the inlet
valves and outlet valves be replaced with Swagelock Bellows Seal Valves, Model
No. SS-
HBS6-C. The inlet valves and outlet valves of Experiments 3 and 4 had a larger
orifice than
those of Experiments 1 and 2, and the expert represented that these larger
orifices would allow
the slurry comprised of lignin to pass through the inlet valves into the pump
cavities without
plugging the inlet valves.
For Experiment 3, when the inlet valve corresponding to the first pump cavity
was changed to
the open position, it allowed a portion of the slurry comprised of lignin into
the first pump
cavity to be charged to the Parr reactor. However, after a time of between 15
and 20 minutes
the inlet valves again plugged with solid lignin from the slurry comprised of
lignin.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
For Experiment 4, an expert in the field of slurry pumping recommended
pressurizing the mix
tank (340) to between 2.5 and 3 bar to assist with charging the slurry
comprised of lignin
through the inlet valves into the pump cavities. The expert again represented
that pressurizing
the mix tank (340) would allow the slurry comprised of lignin to pass through
the inlet valves
into the pump cavities without plugging the inlet valves. When the inlet valve
corresponding
to the first pump cavity was changed to the open position, it allowed a
portion of the slurry
comprised of lignin into the first pump cavity to be charged to the Parr 4575
reactor.
However, after a time of between 25 and 30 minutes the inlet valves again
plugged with solid
lignin from the slurry comprised of lignin.
Slurry Pumping Experiments 5 and 6
For Experiment 5, the inventors decided to replace the inlet valves with
Swagelok 60 Series 3
piece Ball Valves, Model No. SS-62T56. The outlet valves were the same
Swagelock
Bellows Seal Valves used in Experiments 3 and 4. When the inlet valve
corresponding to the
first pump cavity was changed to the open position, it allowed a portion of
the slurry
comprised of lignin into the first pump cavity, which was subsequently passed
through the
outlet valve corresponding to the first pump cavity and charged to the Parr
reactor. The
process was run for a period of approximately two days, at which time the
outlet valves
became plugged with solid lignin from the slurry comprised of lignin.
For Experiment 6, the inlet valves were the same Swagelok 60 Series 3 piece
Ball Valves as
those used in Experiment 5, however, the inventors decided to replace the
outlet valves with
Swagelok 60 Series 3 piece Ball Valves, Model No. SS-62T56. When the inlet
valve
corresponding to the first pump cavity was changed to the open position, it
allowed a portion
of the slurry comprised of lignin into the first pump cavity, which was
subsequently passed
through the outlet valve corresponding to the first pump cavity and charged to
the Parr reactor.
The pump was then able to continuously charge the slurry comprised of lignin
into the Parr
reactor without plugging the inlet valves or outlet valves. It was not
necessary to pressurize
the mix tank (340) in order to charge the reactor.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
41
CHAR PREVENTION
One of the difficulties in any continuous lignin conversion process is
avoiding the formation
of char. Char formation results in decreased yields of lignin conversion
products, and disrupts
the continuous nature of the lignin conversion process, as the lignin
conversion process must
be shut down and the char removed from the lignin conversion reactor before
continuing the
process.
The Inventors discovered that, to avoid char, the deoxygenation, which is the
exposure of the
lignin to hydrogen as either H2 gas or via a hydrogen donor, occurs at a
lignin conversion
temperature and a lignin conversion pressure, wherein the lignin conversion
temperature is in
the range of greater than the boiling point of the liquid composition in the
reactor at
atmospheric pressure, and less than the critical temperature of the liquid
composition, with the
lignin conversion pressure being greater than the bubble pressure of the
liquid composition in
the reactor at the lignin conversion temperature, subject to the condition
that the lignin
conversion pressure is selected so as to avoid the formation of char.
The liquid composition of the reactor is the composition of the liquid
components that are
added to the vessel. For example, in one embodiment, the liquid composition is
almost pure
water with dissolved species. In the case of pure water the hydrogen would
come from added
hydrogen gas. In the case of pure water or substantially pure water, the
bubble pressure is the
vapor pressure of the water at the lignin conversion temperature. In another
embodiment, the
liquid composition could comprise water and a hydrogen donor. This liquid
composition has
its own bubble pressure and critical temperature forming the lower and upper
boundary of the
temperature range, subject to the additional condition that the lignin
conversion pressure be
selected so as to avoid char formation after two residence cycles, which can
be visually
verified by opening the reactor after two residence cycles and observing the
presence or
absence of char ¨ a dark residue coating the reactor. The reactor will also be
void of any
liquid.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
42
What has been discovered is that the lignin conversion pressure is also a
function of the
amount of gas exiting the reactor. The higher the amount of gas used, such as
in hydrogen gas
or nitrogen, the greater the pressure required. In the instance of a hydrogen
donor, less gas is
used and thus a lower lignin conversion pressure is needed to prevent char.
The proper lower lignin conversion pressure can be easily empirically
established as follows.
One can determine the liquid composition charged to the reactor. In most cases
it will be
water from the slurry and whatever hydrogen donor compounds, if any, are used.
The design
will include a flow rate for the gas exiting the reactor. While the
calculations can be done
manually, a commercial simulation package can be used to determine the vapor
liquid
equilibrium conditions (bubble pressure) of the liquid mixture. This is
demonstrated in Table
2 which is the "calculated reactor pressure for liquid water" using water as
the liquid. As can
be seen by the table, the theoretical calculations are a close approximation,
but in the case of
water, the actual pressure was still greater than the calculated amount based
upon the pure
components. Once the approximation is determined, the reaction can be
conducted for two
residence cycles, the vessel opened and examined for char. If there is char,
the reaction
pressure is increased until there is no char and thus subject to the condition
that no char is
formed after two residence cycles.
A residence cycle is the amount of time to turn over the reactor contents. If
the residence
volume is 4 L in the vessel and the vessel is being charged at a volumetric
flow rate at
operating conditions of 1 Uhr, the residence cycle is 4 hours and 2 residence
cycles is 8 hours.
At 2 Uhr, the residence cycle is 2 hrs and 2 residence cycles is 4 hours.
As demonstrated above the lignin conversion process should occur at a lignin
conversion
temperature, where the lignin conversion temperature is in the range of
greater than the boiling
point of the slurry liquid at atmospheric pressure, and less than the critical
temperature of the
slurry liquid, subject to the condition that the lignin conversion pressure is
greater than the
bubble pressure of the slurry liquid at the lignin conversion temperature and
the lignin
conversion pressure is selected so as to avoid the formation of char.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
43
To avoid char formation, the lignin conversion pressure should be selected so
that the lignin
conversion pressure is greater than the bubble pressure of the slurry liquid
at the lignin
conversion temperature. Bubble pressure is the sum of the partial vapor
pressures of all
components in the lignin conversion reactor.
When the slurry liquid is comprised of water, the lignin conversion process
should occur at a
lignin conversion temperature below the critical temperature of water.
Generally, the lignin conversion process will occur at a lignin conversion
temperature in the
range of 190 C to 370 C. The lignin conversion temperature range is preferably
selected from
the group consisting of 190 C to 370 C, 210 C to 370 C, 220 C to 360 C, 240 C
to 360 C,
250 C to 360 C, 280 C to 360 C, 290 C to 350 C, and 300 C to 330 C.
Where the slurry liquid is comprised of a hydrogen donor, the lignin
conversion process may
occur at a lignin conversion temperature in the range of 190 C to 350 C with
200 C to 310 C
being more preferred, 210 C to 300 C being even more preferred, and 210 C to
280 C being
most preferred.
The hydrogen donor may also be introduced into the lignin conversion reactor
separately from
the liquid slurry. The hydrogen donor may also come from the carbohydrate
conversion step,
thus the ligno-cellulosic biomass is generating its own hydrogen for use in
the process. In
such a process, the hydrogen donor, such as ethylene glycol, could be
manufactured in the
carbohydrate conversion step of Figure 3 and passed to the liquid slurry and
introduced into
the lignin conversion reactor via stream 325.
In order to avoid char it is also important to control the lignin conversion
pressure as described
above. . The lignin conversion pressure is in a range preferably selected from
the group
consisting of 70 bar to 300 bar, 80 bar to 245 bar, 82 bar to 242 bar, 82 bar
to 210 bar, 90 bar
to 207 bar and 90 bar to 172 bar.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
44
The continuous lignin conversion in the presence of carbohydrates should occur
at a lignin
conversion pressure higher than the theoretical equilibrium vapor pressure of
water at the
lignin conversion temperature. It was directly observed that char was formed
when the lignin
conversion pressure was even greater than the calculated water vapor pressure
at the lignin
conversion temperature accounting for the exiting gas sweeping across the top
of the liquid.
No char was observed when the lignin conversion pressure was substantially
higher than the
calculated water vapor pressure at the lignin conversion temperature. What was
discovered is
that to avoid char formation in a continuous process it was necessary to
maintain at least a
portion of the reactor contents as a liquid, but to do so, required pressures
much higher than
expected or would have been predicted.
Char formation is not seen in batch reactor conditions because batch reactor
conditions are
always at theoretical equilibrium. When the exit sweeping gas is introduced in
the continuous
process, the equilibrium conditions no longer exist and the pressure required
to keep at least
some of the reactor contents as a liquid in the lignin conversion reactor is
substantially higher
than conventional wisdom or innovation would teach. While process simulations
can be made
to initially approximate the lignin conversion pressure at given conditions,
the actual
minimum lignin conversion pressure can be easily empirically established by
increasing the
pressure until no char is observed. Those practicing the invention are
cautioned that the
increase in pressure can be large depending upon the flow rates from the
reactor.
CHAR PREVENTION EXPERIMENTS
The following procedures were applied to all the experiments, unless
differently specified.
De-ionized water was added to a lignin-rich composition obtained from the
pretreatement of
ligno-cellulosic biomass to obtain a slurry comprised of lignin having a dry
matter content of
20 weight percent of the mass of the slurry. The mixture was inserted into a
blender (Waring
Blender, model HGBSSSS6) and thoroughly mixed intermittently for 10 min. to
reach a
homogenous slurry. The homogeneity of the slurry was evaluated by eye. The
slurry was
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
inserted into a mix tank with constant agitation. The mix tank was a stainless
steel, dish
bottom tank with a bottom discharge port connected to a Chandler Quizix QX
dual syringe
pump having two pump cavities. Inlet valves were inserted between the mix tank
and the two
pump cavities of the Chandler Quizix QX dual syringe pump. The Chandler Quizix
QX dual
syringe pump was connected by tubing to a Parr 4575 reactor equipped with a
dual 450 pitched
turbine blade, cooling coil, separate gas and slurry feed ports and a
discharge dip tube. Outlet
valves were inserted between the two pump cavities of the Chandler Quizix QX
dual syringe
pump and the Parr reactor.
Hydrogen at a temperature of 20 C was inserted into the Parr reactor to reach
a pressure of
48.3 bar. The Parr reactor was heated to a temperature corresponding to 90% of
the reaction
temperature and continuous flow of Hydrogen was started into the Parr reactor.
The pressure
was measured by means of a pressure transducer (Ashcroft Type 62) connected to
the Parr
reactor.
The slurry comprised of lignin was passed from the mix tank through the
Chandler Quizix QX
dual syringe pump and into the Parr reactor by opening and closing the inlet
and outlet valves
in a manner that allowed the slurry comprised of lignin to pass continuously
into the Parr
reactor.
Experiments were conducted according to the described procedure. Experimental
parameters
are reported in Table 1.
CA 02889702 2015-04-27
WO 2014/063852
PCT/EP2013/067734
46
TABLE 1 EXPERIMENTAL PARAMETERS
Exp. Temp H2 Press. Flow Rate Lignin-rich Residen
Catalyst to Unreacted %
No. ( C) Flow (bar) Slurry Solids
composition cc time Lignin-rich Lignin
Catalyst
(sccm) (mL/ (g/ Concentratio (min) composition
(% of Loss
n (wt%) ratio
Theoretical)
mm) min)
1 340 150 156.1 2.8 0.42 15 53 0.50
2 340 500 173.4 5.6 0.84 15 26 2.60
3 340 500 173.4 2.8 0.42 15 51 1.25
4 305 100 122.4 3.8 0.19 5 45 0.25 3.1
13.3
325 100 166.5 3.8 0.19 5 42 0.25 0.2 1.7
6 305 800 122.4 3.8 0.19 5 45 2.00 0.6
1.3
7 325 100 166.5 2.3 0.12 5 70 0.25 0.3
1.1
8 305 100 122.4 3.8 0.57 15 45 2.00 20.8
18.4
Large amounts of char without liquid water was observed in the reaction
products of
experiments 1-3. No char and liquid water was observed in Experiments 4 - 8.
It is believed that it is necessary to have liquid present, such as water in
the liquid phase, for
the reaction to progress as opposed to decomposition.
What was discovered was that even though the reactor was operated at a total
system (reactor)
pressure well above the vapor pressure of water at the 340 C (146.1 bar) vs.
the gas pressure,
there was still no water or solvent present.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
47
TABLE 2 COMPARISON OF REACTOR CONDITIONS VS CHAR FORMATION
Exp. Temp. Vapor Minimum calculated Reactor Char
No. pressure of Reactor pressure for Pressure
pure water Liquid Water (bar)
1 340 146.1 165.3 156.1 Yes
2 340 146.1 172.9 173.4 Yes
3 340 146.1 196.3 173.4 Yes
4 305 92.1 95.6 122.4 No
325 120.7 125.8 166.5 No
6 305 92.1 116.3 122.4 No
7 325 120.7 128.6 166.5 No
8 305 92.1 98.2 122.4 No
CATALYST RETENTION AND SEPARATION
Because the lignin conversion catalyst is present as free particles (625), and
not a fixed bed,
the lignin conversion catalyst needs separated from the lignin conversion
products. The
catalyst particles (625) can be separated from the liquid lignin conversion
products after the
liquid lignin conversion products are removed from the lignin conversion
reactor (500) by
filtering, settling, centrifuging, solid bowl centrifuging, cycloning or other
processes known in
the art. The separated catalyst is then either re-introduced into the lignin
conversion reactor
for further reactions, treated for replenishment and then reused, or
discarded. These traditional
methods are known.
It has been discovered that the free catalyst particles (625) can be separated
from the lignin
conversion products in situ, that is within the lignin conversion reactor
(500) while the
continuous catalytic conversion of the lignin feedstock to lignin conversion
products is
occurring. Thus, the lignin conversion products can be separated from the
catalyst particles
(625) during the continuous catalytic conversion of a lignin feedstock to
lignin conversion
products.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
48
This separation is done by gravity settling, wherein the fluid linear velocity
(meters/min) of
the lignin conversion products (liquid and gas) leaving the lignin conversion
reactor is less
than the gravitational linear settling velocity of the catalyst particles
(625) in the liquid/gas
lignin conversion product stream exiting the reactor. Therefore, as long as
the lignin
conversion products being removed from the lignin conversion reactor are
removed from the
lignin conversion reactor at a linear velocity less than the settling velocity
of the catalyst
particles (625) and from a point higher (relative to gravity) than the liquid
level in the reactor,
catalyst particles will stay in the lignin conversion reactor.
The liquid level of the lignin conversion reactor is at the physical interface
of the bulk liquid
phase and bulk gas phase in the lignin conversion reactor (500). The bulk gas
phase is a
continuous gas phase which has a specific gravity which is less than the
specific gravity of the
bulk liquid phase. The bulk gas phase may have droplets of liquid in the bulk
gas phase.
Likewise, the bulk liquid phase is a continuous liquid phase and will have
dissolved gases and
gas bubbles.
The height relative to the liquid level at which the lignin conversion
products are removed
from the lignin conversion reactor is called the disengagement height. The
disengagement
height is greater than the catalysts particles travel height which is the
height the catalyst
particles (625) will reach when carried along with the lignin conversion
products. Because the
settling velocity of the catalyst particles is greater than the lignin
conversion products removal
velocity, the catalyst particles (625) will eventually drop back into the
lignin conversion
reactor (500) so long as the disengagement height in the settling zone as
discussed below is
large enough relative to the travel height so that at least a majority of the
catalyst particles
(625) do not reach the point at which the lignin conversion products are
removed from the
lignin conversion reactor.
In practice, so long as the settling velocity of the catalyst particles is
substantially greater than
the liquid lignin conversion products removal velocity, the disengagement
height should be
large enough so that at least a majority of the catalyst particles (625) never
reach the point at
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
49
which the liquid lignin conversion products are removed from the lignin
conversion reactor.
For example, where the liquid lignin conversion products are removed through
an "L" shaped
dip tube having a dip tube major length (612) and a dip tube minor length
(614) as shown in
Figure 4, the disengagement point must be less than the dip tube minor length
(614). If the dip
tube minor length (614) is one meter, the settling velocity of the catalyst
particles is 1.2 meters
per second, and the liquid lignin conversion products removal velocity is 1
meter per second
the liquid lignin conversion products will reach the disengagement height
(which is also the
dip tube minor length (614)) in one second. Because the catalyst particles
(625) have a
settling velocity which is 0.2 meters per second greater than the liquid
lignin conversion
products velocity, the catalyst particles (625) will travel up the dip tube
(610) at a velocity
which is 0.2 meters per second less (0.8 meters per second in this example)
than the liquid
lignin conversion products travel up the dip tube. As a result, when the
liquid lignin
conversion products reach the disengagement height (which is also the dip tube
minor length
(614)) of one meter after one second, the catalyst particles (625) will have
only travelled 0.8
meters. In this manner, the catalyst particles never reach the disengagement
height and will
"settle" back into the lignin conversion reactor (500).
Conversely, if the settling velocity of the catalyst particles is less than
the liquid lignin
conversion products removal velocity, the catalyst particles (625) will reach
or exceed the
disengagement height and will be removed from the reactor. For instance, if
the settling
velocity of the catalyst particles is 0.8 meters per second and the liquid
lignin conversion
products removal velocity is 1 meter per second, the catalyst particles (625)
will be travelling
at a velocity at least equal to the liquid lignin conversion products. In this
manner the catalyst
particles will reach the disengagement height at least at the same time as the
liquid lignin
conversion products, and will thereby be removed from the lignin conversion
reactor (500)
through the dip tube (610).
In a preferred embodiment, the lignin conversion reactor will have an
agitation zone and a
settling zone, also known as a decantation zone. In the settling zone, the
liquid phase of the
reactor is exposed to less agitation than in the agitation zone. The settling
zone can be created
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
by use of a dip tube as discussed below. The internal of the dip tube sees
very little agitation
and is thus the settling zone in that embodiment. The settling zone can also
be created by
placing baffles above the agitator but below the liquid level to create a
still spot. Another way
is to have a separate reactor or vessel which does not have agitation. This
configuration is
described in the bubble column section. The lignin conversion products are
removed from the
settling zone at a lignin conversion products removal velocity. In order for
more efficient
removal of the catalyst, the lignin conversion products removal is subject to
the condition that
to reach the point in the lignin conversion reactor which is higher relative
to gravity than the
liquid level of the lignin conversion reactor, the lignin conversion products
must leave the
agitation zone and pass through a portion of the settling zone
Figure 4 demonstrates an embodiment of the principles. In this embodiment, the
product is
removed via a dip tube (610), where the lignin conversion products must exit
up and out the
dip tube. As the lignin conversion products travel up the tube, the first
catalyst particles (625)
travel with it. However, the first catalyst particle will have a terminal or
settling velocity ¨
that is the speed at which the particle drops through the liquid lignin
conversion products of
the reactor. If one observes catalyst particles (625) coming out the dip tube
(610), it is a
simple matter to enlarge the diameter of the dip tube to reduce the lignin
conversion products
velocity relative to gravity (slow down the speed) so that the conversion
products travel up the
tube relative to gravity at a speed less than the speed at which the first
catalyst particles are
dropping down the tube, thus keeping the catalyst in the reactor. If one
wished to purge the
catalyst, or add new catalyst so that the old catalyst could be removed, one
would reduce the
diameter of the tube (increasing the flow rate) and have catalyst particles
(625) flow out of the
lignin conversion reactor (500). The catalyst removal and replenishment can be
done
continuously so that a predetermined percentage of catalyst is removed and
replenished on a
continuous basis.
In practice, the catalyst particles (625) will vary in size and shape, each
having a different
settling velocity. Therefore, the preferred lignin conversion products removal
velocity is less
than the settling velocity of at least 75% by weight of the catalyst
particles, with a lignin
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
51
conversion products removal velocity less than the settling velocity of at
least 85% by weight
of the catalyst particles being more preferred, with a lignin conversion
products removal
velocity less than the settling velocity of at least 90% by weight of the
catalyst particles being
even more preferred, with a lignin conversion products removal velocity less
than the settling
velocity of at least 95% by weight of the catalyst particles being yet even
more preferred, with
a lignin conversion products removal velocity less than the settling velocity
of 100% by
weight of the catalyst particles being most preferred.
The "75% by weight of the catalyst particles" means that 75% by weight of the
total amount
of catalyst in the reactor remains in the reactor and 25% by weight of the
total amount of the
catalyst in the reactor is removed. Alternatively, the percent equals
100 * R 1[R +X]
Where R is the weight of the catalyst remaining, X is the weight of the
catalyst exited or
removed from the reactor. The 100 is to make the number a percent.
One of ordinary skill can now easily see how a properly designed system could
continually
replenish catalyst ¨ say add 5% by weight of new catalyst while removing 5% by
weight.
Thus, the catalyst is constantly being turned over.
CATALYST RETENTION EXPERIMENTS
Experiments were conducted for retaining catalyst in the reactor. The
following procedures
were applied to all the experiments, unless differently specified.
De-ionized water was added to a lignin-rich composition obtained from the
pretreatment of
ligno-cellulosic biomass to obtain a slurry comprised of lignin having a dry
matter content of
20 weight percent of the mass of the slurry. The mixture was inserted into a
blender (Waring
Blender, model HGBSSSS6) and thoroughly mixed intermittently for 10 min. to
reach a
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
52
homogenous slurry. The homogeneity of the slurry was evaluated by eye. The
slurry was
inserted into a mix tank (340) with constant agitation. The mix tank (340) was
a stainless
steel, dish bottom tank with a bottom discharge port connected to a Chandler
Quizix QX dual
syringe pump having two pump cavities. Inlet valves (350) were inserted
between the mix
tank (340) and the two pump cavities of the Chandler Quizix QX dual syringe
pump. The
Chandler Quizix QX dual syringe pump was connected by tubing to a Parr 4575
reactor
equipped with a dual 450 pitched turbine blade, cooling coil, separate gas and
slurry feed ports
and a stainless steel discharge dip tube (610) having an outside diameter of
0.25 inches and an
inside diameter of 0.152 inches. Outlet valves were inserted between the two
pump cavities of
the Chandler Quizix QX dual syringe pump and the Parr reactor.
The lignin conversion reactor pressure was controlled by a Mity Mite Model 91
Back Pressure
Regulator (BPR) positioned in the lignin conversion reactor discharge line
between the Parr
reactor and the products receiver. The lignin conversion pressure was measured
by means of a
pressure transducer (Ashcroft Type 62) connected to the Parr reactor.
The Parr reactor was charged with 150 mL of de-ionized water prior to
beginning the
experiments. The lignin conversion reactor pressure was increased to 48.3 bar
by way of 20
C hydrogen. The lignin conversion reactor was heated to 90% of the lignin
conversion
temperature prior to charging the slurry comprised of lignin to the lignin
conversion reactor.
After increasing the temperature to 90% of the lignin conversion temperature,
additional de-
ionized water was passed from the mix tank (340) through the Chandler Quizix
QX dual
syringe pump into the lignin conversion reactor (500) at a rate of 2.8 mL/min.
Hydrogen flow
was added to the lignin conversion reactor at a rate of 150 sccm. At this
point, the temperature
in the lignin conversion reactor was increased to 100% of the lignin
conversion temperature,
and the lignin conversion reactor pressure was adjusted via the BPR to the
desired operating
pressure as reflected in the experiments.
Slurry comprised of lignin was then charged to the reactor through the
Chandler Quizix QX
dual syringe pump at a rate of 2.8 mL/min. The slurry comprised of lignin was
passed from
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
53
the mix tank (340) through the Chandler Quizix QX dual syringe pump and into
the Parr
reactor by opening and closing the inlet valves (350) and outlet valves (450)
in a manner that
allowed the lignin slurry to pass continuously into the Parr reactor. The
lignin conversion
products were continuously removed from the lignin conversion reactor (500)
via the dip tube
(610) and cooled to approximately 35 C before passing through the BPR. After
passing
through the BPR, the lignin conversion products were collected in a stainless
steel products
receiver fitted with a vent line to allow non-condensable gases from the
lignin conversion
reactor to separate from the liquid lignin conversion products.
The lignin conversion reactor was allowed to reach steady state conditions,
and after four
reactor residence cycles, the lignin conversion products were collected in the
products receiver
for approximately one additional reactor residence cycle. At this time, all
feed streams to the
lignin conversion reactor were stopped, and the lignin conversion reactor was
isolated from
the products receiver by way of an isolation valve. The lignin conversion
reactor was cooled
to approximately 30 C and the pressure was reduced to atmospheric pressure by
opening a
vent valve.
The liquid lignin conversion products were mixed with an equal amount of
methyl tertiary
butyl ether (MTBE). This mixture was filtered through a Buchner funnel fitted
with a
Whatman #1 filter paper.
Catalyst Retention Experiment 1
For Experiment 1, sponge nickel catalyst was added directly to the slurry
comprised of lignin
resulting in a slurry comprised of 13.5 weight percent lignin on a dry basis
and 7.0 weight
percent sponge nickel catalyst on a dry basis. The sponge nickel catalyst had
a particle size
range of between 10 and 40 pm. The lignin conversion reactor was operated at
340 C and
156.4 bar, which is approximately 10 bar above the vapor pressure of water at
340 C. At
operating conditions, the average residence time of the slurry comprised of
lignin was 53
minutes.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
54
Surprisingly, after the experiment was stopped and the liquid lignin
conversion products were
filtered, very little catalyst was observed on the filter paper, and in one
instance, no catalyst
was observed at all. Where catalyst was observed on the filter paper, it was
observed as fine
particles of catalyst. When the Parr reactor was shut down and opened, it was
surprisingly
observed that nearly all of the catalyst remained in the lignin conversion
reactor.
Catalyst Retention Experiment 2
For Experiment 2, 28 g on a dry basis of the sponge nickel catalyst was
charged directly to the
Parr reactor, along with the initial 150 mL of de-ionized water, prior to
beginning the
experiment. No amount of catalyst was added to the slurry comprised of lignin
prior to
charging the slurry comprised of lignin to the lignin conversion reactor. As a
result, the slurry
comprised of lignin contained 15 weight percent lignin on a dry basis. The
lignin conversion
reactor was operated at 340 C and 173.4 bar, which is approximately 17 bar
above the vapor
pressure of water at 340 C. Hydrogen flow rate was increased to 500 sccm.
Slurry feed rate
and average residence time remained the same as in Experiment 1.
Surprisingly, after the experiment was stopped and the liquid lignin
conversion products were
filtered, it was observed that the majority of the catalyst remained in the
lignin conversion
reactor (500). Finer particles of catalyst were observed on the filter paper.
It was also
surprisingly observed that, where higher rates of lignin conversion were
attained, less catalyst
was removed from the lignin conversion reactor as evidenced by less catalyst
present on the
filter paper.
It is believed that the settling velocity of the catalyst particles is greater
than the velocity of the
removal of lignin conversion products from the lignin conversion reactor (500)
through the dip
tube (610). This results in the surprising and advantageous retention of
catalyst in the lignin
conversion reactor. It is further believed that the fibrous, Velcro -like
nature of the lignin-
rich composition in the slurry comprised of lignin will attach itself to the
catalyst particles
(625) and remove them from the lignin conversion reactor where lower levels of
lignin
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
conversion are achieved. It is further believed that, where removal of all or
a portion of the
catalyst from the lignin conversion reactor is desired, all or a portion of
the catalyst can be
removed from the Parr reactor by decreasing the diameter and length of the dip
tube, thereby
increasing the velocity of the removal of lignin conversion products from the
Parr reactor to a
level greater than that of the settling velocity of the catalyst.
BUBBLE COLUMN REACTOR
Although the process can be operated where the lignin conversion reactor is a
continuous stir
tank reactor (CSTR), the CSTR requires a high amount of energy input, and the
high pressure
required to convert lignin on a continuous basis results in an unreasonably
large reactor when
utilizing a CSTR. It has been discovered that a bubble column reactor requires
less energy
input and allows for a smaller reactor for a continuous lignin conversion
process.
One alternative to the CSTR is the ebullating bed reactor, as described in US
Patent 4,240,644.
One version of ebullated bed is a bubble column reactor. A bubble column
reactor consists of
at least one vertical cylinder at least partially filled with liquid. Gas is
fed to the bottom of the
cylinder through a gas feed tube causing a turbulent upward stream of bubbles.
In a preferred
embodiment the gas may be hydrogen or nitrogen. In a preferred embodiment the
liquid may
comprise water. In a further embodiment the liquid may comprise a hydrogen
donor. The gas
flow could be nitrogen or hydrogen gas, at a sufficient rate to keep the
catalyst particles
fluidized within the liquid components of the reactor.
In a preferred embodiment, the bubble column reactor will also comprise a gas
distributor at
the bottom of the vertical cylinder to allow for even distribution of gas
bubbles. A preferred
gas distributer is comprised of a material which is not corroded by the
reactants, such as a
stainless steel mesh.
A slurry comprised of lignin can be fed to the bottom of the vertical cylinder
through a slurry
feed tube. The amount of slurry comprised of lignin fed to the bubble column
reactor can be
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
56
varied to achieve increased rates of lignin conversion as described in the
experimental section
below based on temperature, pressure, hydrogen flow, amount of catalyst and
residence time.
In one embodiment a plurality of catalysts may be charged to the bubble column
reactor
through the slurry feed tube. In another embodiment a plurality of catalysts
may be charged
directly to the bubble column reactor prior to charging the hydrogen and/or
slurry comprised
of lignin to the bubble column reactor.
The reactor scheme for the bubble column may also include a second column for
the
disengagment of the solid unreacted lignin and catalyst to flow by gravity
into the bottom of
the bubble column or ebullating reactor and be recontacted with fresh gas.
The bubble column reactor may also comprise a heating element which allows for
regulation
of the bubble column reactor temperature. Preferably this heating element
comprises a
plurality of heating coils wrapped around the vertical cylinder. In a
preferred embodiment the
bubble column reactor temperature is between 220 C and 350 C. The reactor
conditions of
pressure and temperature should be selected so as to prevent char formation as
discussed
earlier.
Bubble column reactor pressure may be varied based upon the bubble column
reactor
temperature and gas flow rate as described in the experimental section below.
In a preferred
embodiment the bubble column reactor pressure is between 150 bar and 230 bar.
A dip tube may be inserted at the top of the vertical cylinder for removing a
plurality of the
lignin conversion products to a products receiver.
In one embodiment the bubble column reactor may consist of a plurality of
vertical cylinders,
each having a separate gas feed tube, a separate slurry feed tube and a
separate dip tube.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
57
What was found is that, by utilizing a bubble column reactor instead of a
CSTR, significant
amounts of energy savings can be attained due to the lack of a separate
stirring element.
Additionally, the bubble column results in higher rates of conversion than a
CSTR while
converting the slurry comprised of lignin to similar products.
BUBBLE COLUMN REACTOR EXPERIMENTS
The following procedures were applied to all the experiments, unless
differently specified.
De-ionized water was added to a lignin-rich composition obtained from the
pretreatment of
ligno-cellulosic biomass to obtain a slurry comprised of lignin having a dry
matter solids
content of 5 weight percent of the mass of the slurry comprised of lignin. The
mixture was
inserted into a blender (Waring Blender, model HGBSS6) and thoroughly mixed
intermittently
at thirty second intervals (thirty seconds of mixing followed by thirty
seconds without mixing)
for 10 mm. to reach a visually homogenous slurry. (See Experimental
establishing the ability of
the Waring HGBSS6 Blender to homogenously disperse on a quantitative basis).
The homogeneity
of the slurry comprised of lignin was evaluated by eye.
The slurry comprised of lignin was inserted into a mix tank with constant
agitation. The mix
tank was a stainless steel, dish bottom tank with a bottom discharge port
connected to a
Chandler Quizix QX dual syringe pump having two pump cavities. Inlet ball
valves were
inserted between the mix tank and the two pump cavities of the Chandler Quizix
QX dual
syringe pump. The Chandler Quizix QX dual syringe pump was connected by stream
(1510)
to a bubble column reactor having an inside diameter (1540) of one inch, a
height (1545) of
thirty inches, a heating element (1550), a gas distributor (1570) comprised of
stainless steel
mesh having a length of two inches, a slurry feed tube (1560) at the bottom of
the column
having a length of six inches for feeding the lignin slurry to the bubble
column reactor, and a
dip tube (1565) having a length of eight inches connected to a transfer line
(1580) at the top of
the bubble column reactor for removal of reaction products to a products
receiver. The
products receiver was maintained at the same pressure as the bubble column
reactor. The
bubble column reactor further contained a vent (1520) connected to a rupture
disk (1521) and
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
58
a pressure transducer (1522). The bubble column reactor further contained a
thermal well
(1590) for measuring temperature inside the bubble column reactor during the
experiment.
The slurry comprised of lignin was passed from the mix tank through the
Chandler Quizix QX
dual syringe pump and into the bubble column reactor by opening and closing
the inlet and
outlet valves in a manner that allowed the lignin slurry to pass continuously
into the bubble
column reactor.
The inventors conducted a set of seven experiments. The results of these
experiments are
summarized below in Table 3 and Table 4.
Bubble Column Experiment 1
For Experiment 1, 43 g of Raney Nickel catalyst (1500) was charged directly to
the bubble
column reactor, along with 150 g of liquid water, prior to beginning the
experiment.
Hydrogen was swept through the system continuously at a gas flow rate of 300
scc/m through
the gas feed tube (1530) and into the gas distributor (1570). The bubble
column reactor was
heated to a bubble column reactor temperature of 310 C to achieve a target
bubble column
reactor pressure of 165.5 bar. Slurry comprised of lignin was fed to the
bubble column reactor
at a rate of 3 mL/min. The slurry comprised of lignin was continuously fed to
and removed
from the bubble column reactor for a period of five hours or a total of 4.1
residence cycles of
slurry comprised of lignin through the reactor. The total amount of slurry
comprised of lignin
passed through the system was 45 g. When the inventors concluded the
experiment, 11.1293 g
of un-reacted slurry comprised of lignin remained in the bubble column
reactor, however, in
removing the un-reacted slurry comprised of lignin from the bubble column an
unknown
quantity was spilled.
What was observed was that the lignin conversion products were phenol oils
that were nearly
identical in composition as measured by G.C. Mass Spectrometer to the phenol
oils produced
during a lignin conversion process in a continuous stir tank reactor (CSTR)
(See Figure 9).
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
59
Conversion rate of the slurry comprised of lignin was 75.27%, not taking into
account the
unknown quantity of un-reacted slurry comprised of lignin which was spilled.
Bubble Column Experiment 2
For Experiment 2, the inventors increased the bubble column reactor
temperature from 310 C
to 318 C. The constant amount of slurry comprised of lignin present in the
bubble column
reactor after reaching assumed steady state during the experiment was 15.2587
g. All other
conditions remained the same as in Experiment 1. When the inventors concluded
the
experiment, 15.2587 g of un-reacted slurry comprised of lignin remained in the
bubble column
reactor.
What was observed was that the increased bubble column reactor temperature
resulted in a
rate of conversion of the slurry comprised of lignin of 66.09%.
Bubble Column Experiment 3
For Experiment 3, the inventors reduced the amount of catalyst charged to the
bubble column
reactor from 43 g to 21.5 g. The constant amount of slurry comprised of lignin
present in the
bubble column reactor after reaching assumed steady state during the
experiment was 16.5924
g. All other conditions remained the same as in Experiment 2. When the
inventors concluded
the experiment, 16.5924 g of un-reacted slurry comprised of lignin remained in
the bubble
column reactor.
What was observed was that the reduced catalyst in the bubble column reactor
resulted in a
reduced rate of conversion of the slurry comprised of lignin of 63.13%.
Bubble Column Experiment 4
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
For Experiment 4, the inventors increased the bubble column reactor pressure
from 166.49 bar
to 172.4 bar and reduced the rate of slurry flow from 3 mL/min to 2 mL/min.
Total run time
was increased to six hours and forty minutes, and total input of the slurry
comprised of lignin
was decreased to 40 g. The number of turns of slurry comprised of lignin
through the bubble
column reactor decreased to 3.62. The total amount of slurry comprised of
lignin present in
the bubble column reactor after reaching assumed steady state during the
experiment was
18.4116 g. All other conditions remained the same as in Experiment 2. When the
inventors
concluded the experiment, 18.4116 g of un-reacted slurry comprised of lignin
remained in the
bubble column reactor.
What was observed was that the reduced slurry flow resulted in a lower rate of
conversion of
the slurry comprised of lignin of 53.97%.
Bubble Column Experiment 5
For Experiment 5, the inventors further reduced the rate of slurry flow from 2
mL/min to 1.2
mL/min. Total run time was increased to ten hours, and total input of the
slurry comprised of
lignin was decreased to 36 g. The number of residence cycles of slurry
comprised of lignin
through the reactor decreased to 3.26. The total amount of slurry comprised of
lignin present
in the bubble column reactor after reaching assumed steady state during the
experiment was
14.2125 g. All other conditions remained the same as in Experiment 4. When the
inventors
concluded the experiment, 14.2125 g of un-reacted slurry comprised of lignin
remained in the
bubble column reactor.
At times of four hours, eight hours, and ten hours, the products receiver was
de-pressurized
and discharged. After four hours, the products receiver contained 0.89 g of
phenol oils. After
eight hours the products receiver contained 3.25 g of phenol oils. After ten
hours the products
receiver contained 0.97 g of phenol oils. Upon completion of the experiment,
it was further
observed that 2.4 g of phenol oils remained present in the transfer line. When
the residual
solids were drained from the bubble column reactor, filtered, washed with
acetone and
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
61
Rotovapped, it was further observed that 1 g of phenol oils was present in the
residual solids.
Total, 8.51 g of phenol oils were collected resulting in a phenol oils yield %
based on the
amount of converted slurry comprised of lignin of 39.06%. The phenol oils
yield % based on
the amount of slurry comprised of lignin charged to the bubble column reactor
was 23.64%.
What was observed was that, despite the reduced slurry flow, the increased
total run time
resulted in a higher rate of conversion of the slurry comprised of lignin of
60.52%
Bubble Column Experiment 6
For Experiment 6, the inventors increased the gas flow through the reactor
from 300 scc/m to
600 scc/m resulting in a bubble column reactor pressure increase from 172.4
bar to 187.2 bar.
Total run time was also increased to twelve hours. This resulted in an
increased total input of
slurry comprised of lignin of 72 g. The number of residence cycles of slurry
comprised of
lignin through the reactor increased to 7. The total amount of slurry
comprised of lignin
present in the bubble column reactor at any one time during the experiment was
23.5214 g.
All other conditions remained the same as in Experiment 4. When the inventors
concluded the
experiment, 23.5214 g of slurry comprised of lignin remained in the bubble
column reactor.
At times of two hours forty minutes, five hours twenty minutes, eight hours,
ten hours forty
minutes and twelve hours the products receiver was de-pressurized and
discharged. After two
hours forty minutes the products receiver contained 1.43 g of phenol oils.
After five hours
twenty minutes the products receiver contained 3.27 g of phenol oils. After
eight hours the
products receiver contained 2.64 g of phenol oils. After ten hours forty
minutes the products
receiver contained 4.7 g of phenol oils. After twelve hours the products
receiver contained
3.57 g of phenol oils. Upon completion of the experiment, it was further
observed that 9.29 g
of phenol oils remained present in the transfer line. When the residual solids
were drained
from the bubble column reactor, filtered, washed with acetone and Rotovapped,
it was further
observed that 1.05 g of phenol oils was present in the residual solids. Total,
25.95 g of phenol
oils were collected resulting in a phenol oils yield percentage based on the
amount of
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
62
converted slurry comprised of lignin of 53.53%. The phenol oils yield % based
on the amount
of slurry comprised of lignin charged to the bubble column reactor was 36.04%.
What was observed was that the increased gas flow rate resulted in a higher
rate of conversion
of the slurry comprised of lignin of 67.33%. It was further observed that
increasing the gas
flow rate increased the phenol oils yield percentage both based upon the
amount of converted
slurry comprised of lignin and on the amount of slurry comprised of lignin
charged to the
bubble column reactor.
Bubble Column Experiment 7
For Experiment 7, the inventors increased the bubble column reactor
temperature to 335 C
resulting in an increased bubble column reactor pressure of 207.9 bar. The
inventors also
increased the amount of catalyst charged to the bubble column reactor to 85 g
and the rate of
slurry flow from 2 mL/min to 3 mL/min. Total run time was decreased to five
hours. This
resulted in a decreased total input of slurry comprised of lignin of 45 g. The
number of
residence cycles of slurry comprised of lignin through the reactor decreased
to 4.3. The total
amount of slurry comprised of lignin present in the bubble column reactor at
any one time
during the experiment was 12.082 g. All other conditions remained the same as
in Experiment
6. When the inventors concluded the experiment, 12.082 g of slurry comprised
of lignin
remained in the bubble column reactor.
At times of two hours, four hours, and five hours, the products receiver was
de-pressurized
and discharged. After two hours the products receiver contained 2.69 g of
phenol oils. After
four hours the products receiver contained 1.34 g of phenol oils. After five
hours the products
receiver contained 0.36 g of phenol oils. Upon completion of the experiment,
it was further
observed that 11.92 g of phenol oils remained present in the transfer line.
When the residual
solids were drained from the bubble column reactor, filtered, washed with
acetone and
Rotovapped, it was further observed that 1.25 g of phenol oils was present in
the residual
solids. Total, 17.56 g of phenol oils were collected resulting in a phenol
oils yield % based on
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
63
the amount of converted lignin of 53.34%. The phenol oils yield % based on the
amount of
slurry comprised of lignin charged to the bubble column reactor was 39.02%.
What was observed was that increasing the bubble column reactor temperature,
amount of
catalyst and gas flow resulted in a higher rate of conversion than any of the
previous six
experiments. Further, it was observed that the higher rate of conversion
resulted in an
increased phenol oils yield % based on the amount of slurry comprised of
lignin charged to the
bubble column reactor, despite not resulting in an increased phenol oils yield
% based on the
amount of converted lignin.
Table 3
Exp. Temp. Pressure H20 Catalyst (g) Slurry Slurry H2 Total
Residence
No. ( C) (bar) (g) Flow (wt%) Flow Lignin in
Cycles
(mL/min) (scc/m) B.C.
BC1 310 165.5 150 43 3 5 300 * 4.1
BC2 318 165.5 150 43 3 5 300 15.2587 4.1
BC3 318 165.5 150 21.5 3 5 300 16.5924 4.1
BC4 318 172.4 150 43 2 5 300 18.4116 3.62
BC5 318 172.4 150 43 1.2 5 300 14.2125 3.26
BC6 318 187.2 150 43 2 5 600 23.5214 7
BC7 335 207.9 150 85 3 5 600 12.082 4.3
* Total slurry comprised of lignin in the bubble column reactor is equivalent
to the amount of unconverted lignin
slurry remaining in the bubble column reactor upon shutdown. In BC1, 11.1293 g
of unconverted lignin
remained in the bubble column reactor, however an unknown quantity of un-
reacted lignin was spilled upon
removal from the bubble column reactor at the end of the Experiment resulting
in inaccurate measurements.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
64
Table 4
Exp. Rate of Total Phenol Oils Phenol Catalyst
Lignin Catalyst/Lign
No. Conversion Phenol Yield % Oils Yield
Remaining in Remaining in in Remaining
(%) Oils (g) (converted) % Reactor (g)
Reactor (g) in Reactor (g)
(charged)
BC1 * N/A N/A N/A 24.03 *
2.16/1
BC2 66.09 N/A N/A N/A 19.71 15.2587
1.29/1
BC3 63.13 N/A N/A N/A 15.83 16.5924
0.95/1
BC4 53.97 N/A N/A N/A 29.71 18.4116
2.16/1
BC5 60.52 8.51 39.06 23.64 27.62 14.2125
1.94/1
BC6 67.33 25.95 53.53 36.04 30.81 23.5214
1.31/1
BC7 73.15 17.56 53.34 39.02 56.79 12.082 4.7/1
* 11.1293 g of unconverted lignin remained in the reactor resulting in a rate
of conversion in Experiment BC1 of
75.27%, however an unknown quantity of un-reacted lignin was spilled upon
removal from the bubble column
reactor at the end of the Experiment resulting in inaccurate measurements.
The lignin conversion process is considered a continuous process because the
lignin
conversion products are removed from the lignin conversion reactor (500) in a
continuous
manner. The reactants, such as the component of the slurry comprised of lignin
are generally
introduced into the lignin conversion reactor in a continuous manner as well.
"A continuous
manner" does not mean that that feedstock or products are continuously
introduced or
removed at the same rate. For example, when only one piston pump is used, the
slurry
comprised of lignin is introduced into the lignin conversion reactor (500) in
steady aliquots or
pulses. Thus there are moments, when there is no product entering the lignin
conversion
reactor. But over time, the mass introduced into the lignin conversion reactor
equals the mass
removed from the lignin conversion reactor. One distinguishing feature between
a continuous
and a batch process is that, in the continuous process, the reaction is
occurring or progressing
at the same time that either the reactant feeds are introduced into the lignin
conversion reactor
and/or the lignin conversion products are removed from the lignin conversion
reactor.
Another way to state this that the conversion (e.g. deoxygenating, or
hydrogenating) in the
lignin conversion reactor occurs while simultaneously, or at the same time,
removing at least a
portion of the lignin conversion products from the lignin conversion reactor.
Such removal is
done in a continuous manner which includes a pulse removal.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
The invented process converts the lignin in the feedstock to several different
product types.
As described later, the process conditions can be set to produce one class of
compounds at the
expense of another class of compounds.
The lignin conversion can be considered as a deoxygenation of lignin. The
lignin will not
convert to a single product, but to a plurality of lignin conversion products.
The feedstock
comprising lignin is exposed to additional hydrogen (H2) gas which can be
added in the
conventional manner according to the temperature and pressure of the lignin
conversion
reactor. The plurality of lignin conversion products may be void of ethylene
glycol or
propylene glycol.
There will also be a first catalyst present in the lignin conversion reactor
(500). The reason it
is called a first catalyst is that there may be a second catalyst added to the
lignin conversion
reactor or a second catalyst may be used to further react the lignin
conversion products in a
different step. While there may be a second catalyst, it is possible in one
embodiment that
there is only one catalyst, the first catalyst. The lignin conversion reactor
may be void a
second catalyst.
The lignin conversion products may comprise compounds which are found in jet
fuel, or the
lignin conversion products may be further converted to compounds comprising
jet fuel.
The first catalyst can be any one of the catalysts known to catalyze the
reaction of hydrogen
with lignin. The first catalyst used in the conversion process is preferably a
sponge elemental
metal catalyst comprising at least one sponge elemental metal created by the
Raney process as
described and claimed in US 1,628,190, the teachings of which are incorporated
in their
entirety. The process as claimed creates an alloy of at least a first metal
and a second metal
dissolves the second metal out of the first metal, leaving behind a finely
divided elemental first
metal with high surface area. This high surface area is often described as a
sponge structure.
The preferred first catalyst of the lignin conversion process is known as
Raney Nickel, or
where the finely divided elemental metal is nickel. Another preferred metal is
a metal selected
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
66
from the group consisting of palladium, platinum, nickel, ruthenium, rhodium,
molybdenum,
cobalt, and iron. Because water is a feature of the reaction, the catalyst
structure, particularly
its support must be hydrothermally stable. Due to the heterogeneous nature, at
least a portion
of the first catalyst is present as a plurality of particles, or in particle
form. At a least a portion
of the first catalyst, if not all of the first catalyst, is not present as a
fixed bed.
The first catalyst may or may not be supported or unsupported, but is
generally not present as
a fixed bed. If a fixed bed catalyst is used, the feedstock should be present
as a liquid as
opposed to a slurry so that solids do not plug the pores of the fixed bed. The
contemplation of
a fixed bed is part of the conception because it is believed that many of the
enabling principles
of this process are applicable to both a slurry feedstock and a liquid
feedstock without solids,
or at least less than 1% solids by weight, of a slurry where the solids are
present in a size less
than the pores of the fixed bed.
The amount of the first catalyst can be expressed by the weight of the
elemental nickel to the
dry weight of the lignin feedstock, where the weight of the elemental nickel
to the dry weight
of the lignin in the feedstock should be in the range of about 0.25 to about
2.0, with the range
of about 0.3 to about 1.5 being more preferred with at least about 0.5 to 1.0
being the most
preferred. In one embodiment, the process is void of a catalytic amount of a
second catalyst.
The second catalyst, if used, can be any of the standard hydrogenation
catalysts known, with
the preferred second catalyst being the same as the first catalyst. When the
second catalyst is
the same as the first catalyst, the amount of the second catalyst is the same
as the amount of
the first catalyst. When deoxygenation and dehydrogenation are conducted
simultaneously in
the same vessel, there is no additional second catalyst added as the first
catalyst and its amount
becomes the second catalyst for the purposes of the dehydrogenation reaction.
There is also a preferred introduction of a third catalyst, which is different
from the first and
second catalysts. The preferred third catalyst is a Zeolite creating
heterogeneous cites for the
reactions to progress in an acidic environment.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
67
CRYSTALLINE METAL OXIDE CATALYSTS
Although sponge elemental metal catalysts created by the Raney process can be
used in this
process, they have many disadvantages. Sponge elemental metal catalysts
created by the
Raney process, such as Raney Nickel, require extreme precautions before,
during and after the
reaction. Raney Nickel in particular is a pyrophoric catalyst, and must be
maintained in an
aqueous environment in order to avoid spontaneous combustion.
In an alternative embodiment, the catalyst comprises a crystalline metal oxide
catalyst.
Crystalline metal oxide catalysts are not pyrophoric catalysts, and can be
handled in ambient
conditions unlike Raney Nickel which requires special handling conditions and
storage in an
aqueous environment.
The crystalline metal oxide catalyst may be a crystalline mono-metallic oxide
catalyst or a
crystalline bi-metallic oxide catalyst. In a preferred embodiment, the
crystalline metal oxide
catalyst is in nanoparticle form having an average crystallite particle size
of less than 250 nm,
with an average crystallite particle size of less than 150 nm being more
preferred, an average
crystallite particle size of less than 100 nm being even more preferred and an
average
crystallite particle size of less than 50 nm being most preferred.
Where the catalyst is a crystalline mono-metallic oxide catalyst, the metal
may be selected
from the group consisting of Cesium, Copper, Nickel, Iron, Zinc and Cobalt.
One preferred
crystalline mono-metallic oxide catalyst is nickel oxide.
In one embodiment, the crystalline metallic oxide catalyst is a crystalline bi-
metallic oxide
catalyst. Crystalline bi-metallic oxide catalyst can be obtained from any of
the known
processes, and those yet to be discovered. In general, a crystalline mono-
metallic oxide
catalyst, such as nickel oxide, is doped with atoms of a second metal, such as
zinc, iron or
cobalt. In this process, some of the metal species of the crystalline mono-
metallic oxide
catalyst are replaced with a different metallic species, resulting in a
crystalline bi-metallic
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
68
oxide catalyst. The crystalline mono-metallic oxide catalyst may be doped with
one or more
than one metal. For instance, the crystalline mono-metallic oxide catalyst may
be doped with
zinc and iron metal oxides.
Where the catalyst is a crystalline bi-metallic oxide catalyst, the catalyst
will be comprised of
at least two metals, wherein at least one of the metals is selected from the
group consisting of
Platinum, Palladium, Cesium, Copper, Nickel, Ruthenium, Rhodium, Gold, Iron,
Cobalt and
Iridium. Preferred bi-metallic oxide catalysts include bi-metallic catalysts
comprising nickel
oxide doped with at least one element selected from the group consisting of
zinc, iron and
cobalt.
In a preferred embodiment, the crystalline metal oxide catalyst is present as
free particles. In
another embodiment, at least a portion of the crystalline metal oxide catalyst
may be present in
a fixed bed catalytic process.
Preferably the crystalline metal oxide catalyst will convert lignin to useful
compounds in a
liquid solvent. In a preferred embodiment the liquid solvent is water. In an
alternative
embodiment the liquid solvent is an organic solvent such as methanol.
Crystalline metal oxide catalysts also demonstrate high yield conversion of
lignin to phenolic
compounds, and are highly selective towards functionalized phenols.
Experiments were run
demonstrating the ability of crystalline metal oxide catalysts to convert
lignin to phenolic
compounds.
CRYSTALLINE METAL OXIDE CATALYST EXPERIMENTS
The pre-treated lignin feedstream and sufficient water from a source other
than the pre-treated
lignin feedstream to reach a dry matter concentration of 5 weight percent were
inserted along
with a catalyst in a 50 mL parr mini reactor. After the materials were
inserted into the reactor,
the reactor was pressurized to about 15 bar with nitrogen, stirred for five
minutes, and vented.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
69
The purging cycle was repeated two more times and then two times with
hydrogen. Finally,
the reactor was pressurized at 25 C to a hydrogen pressure of 200 psi and then
heated with an
electric to the reaction temperature. Once the internal temperature of the
reactor was
stabilized, the reactor was stirred for the reaction time of 60 minutes. Once
the reaction time
was completed, the heating element was removed and the reactor was allowed to
cool using an
ice bath. Once the reactor was cooled to a temperature of 24 C, the gas
sample was collected
for further analysis and the reactor was vented until the pressure in the
reactor was reduced to
0 psi.
Nanoparticles of nickel oxide were used as a catalyst. Average particle size
of the nickel
oxide catalyst particles was reported from the manufacturer Sigma-Aldrich Co.,
LLC from St.
Louis, Missouri, USA. In certain experiments the nanoparticles of nickel oxide
were reduced
to metallic nickel in hydrogen at 400 C for two hours prior to charging to
the reactor. In
other experiments the nanoparticles of nickel oxide were not reduced in
hydrogen prior to
charging to the reactor.
Upon completion of the reaction time and cooling and venting of the reactor,
the reaction
products were removed and analyzed to determine the amount of lignin that was
converted and
the yield of phenols in the conversion products. The conversion rate was
determined by
filtering the reaction mixture, and the filtered solution was extracted using
dichloromethane.
The remaining organic layer was rotovapped, and the remaining solids were
ashed to
determine the conversion percentage of the process. The remaining conversion
products were
sent for GC/MS analysis to determine the yield of phenols and the type of
phenols produced.
Crystalline Metal Oxide Experiment 1
For Experiment 1, the Inventors used reduced nanoparticles of nickel oxide as
a catalyst. 0.8 g
of catalyst was charged to the reactor along with 1.5 g of lignin in the form
of a lignin slurry.
The solvent used to create the slurry was deionized water. The reactor was
heated to a
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
reaction temperature of 305 C and time zero was started. The reactor was
further pressurized
to a reaction pressure of 200 psi with hydrogen gas.
Upon ending the Experiment, the profile of the reaction products showed that
83.0% of the
lignin charged to the reactor was converted. This demonstrated that
nanoparticles of nickel
oxide could be utilized as a catalyst for the conversion of lignin.
Crystalline Metal Oxide Experiment 2
For Experiment 2, the Inventors maintained all of the conditions of Experiment
1, except that
0.918 g of catalyst was charged to the reactor along with 2.5 g of lignin in
the form of a lignin
slurry.
Upon ending the Experiment, the profile of the reaction products showed that
79.4% of the
lignin charged to the reactor was converted. However, the yield of phenols
based upon the
79.4% of the lignin which was converted was only 16.6%. This demonstrated that
reduced
nanoparticles of nickel oxide may produce phenol oils, but not in a high yield
relative to the
amount of conversion products. It should be noted here that only 55% of the
pre-treated lignin
feedstream charged to the reactor comprises lignin.
GC/MS of the reaction products indicates that the nanoparticles of nickel
oxide demonstrate
high selectivity towards "light" phenols as opposed to "heavies", heavies
being defined as
molecules having long and short chain hydrocarbons as side products.
Crystalline Metal Oxide Experiment 3
For Experiment 3, the Inventors used an unreduced nanoparticle of nickel
oxide. All other
conditions remained the same as in Experiment 1.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
71
Upon ending the Experiment, the reaction products showed that 77.0% of the
lignin charged to
the reactor was converted. This demonstrated that the unreduced nanoparticles
of nickel oxide
will convert lignin, but that they will not do so as efficiently as reduced
nanoparticles of nickel
oxide.
Crystalline Metal Oxide Experiment 4
For Experiment 4, the Inventors used an unreduced nanoparticle of nickel
oxide. All other
conditions remained the same as in Experiment 2.
Upon ending the Experiment, the reaction products showed that 68.8% of the
lignin charged to
the reactor was converted. Also, the reaction products showed that 25.0% of
the 68.8% of the
lignin that was converted was converted to phenols. Again, it is important to
note here that
only 55% of the pre-treated lignin feedstream comprises lignin. This is an
increase of 8.4%
yield over the reduced nanoparticles of nickel oxide. This demonstrates that,
while the
unreduced nanoparticles of nickel oxide may not provide similar conversion
rates to the
reduced nanoparticles of nickel oxide, the unreduced nanoparticles of nickel
oxide are yielding
a higher percentage of phenols relative to the amount of lignin converted.
GC/MS of the reaction products further indicates that the unreduced
nanoparticles of nickel
oxide demonstrate similar selectivity away from heavies and towards "light"
phenols as the
reduced nanoparticles of nickel oxide.
Crystalline Metal Oxide Experiment 5
For Experiment 5, the Inventors decreased the amount of unreduced
nanoparticles of nickel
oxide to 0.45 g. All other conditions remained the same as Experiment 4.
Upon ending the experiment, the reaction products showed that 61.8 % of the
lignin charged
to the reactor was converted, but that, of that 61.8 %, 23.3% had been
converted to phenols.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
72
This demonstrates that, while decreasing the amount of unreduced nanoparticles
of nickel
oxide may decrease the amount of lignin converted, it does not significantly
reduce the yield
of phenols found in the converted lignin.
Crystalline Metal Oxide Experiment 6
For Experiment 6, the Inventors increased the reaction temperature from 305 C
to 315 C, and
increased the amount of unreduced nanop articles of nickel oxide to 0.918 g.
All other
conditions remained the same as Experiment 4.
Upon ending the experiment, the reaction products showed that 72.0 % of the
lignin charged
to the reactor was converted, but that, of that 72.0 %, only 19.4 % had been
converted to
phenols. This demonstrates that, while increasing the reaction temperature may
increase the
amount of lignin converted, it has a negative impact on the yield of phenols
found in the
converted lignin.
Crystalline Metal Oxide Experiment 7
For Experiment 7, the Inventors used methanol (Me0H) as the solvent for
creating the lignin
slurry as opposed to distilled water. Also, the Inventors lowered the reaction
temperature from
305 C to 290 C. All other conditions remained the same as Experiment 4.
Upon ending the experiment, the reaction products showed that 85.0 % of the
lignin charged
to the reactor was converted. However, the final pressure in the reactor prior
to cooling was
significantly higher than in Experiment 4 (1508 psi vs. 251 psi). Also, GC/MS
of the
conversion products indicated that 13.0 % of the conversion products were
methane as
opposed to only 1.3 % for Experiment 4.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
73
This demonstrates that, while methanol may be used as a solvent in this
reaction, and that it
may increase the amount of lignin converted, it also has the detrimental
effect of producing
more methane than when distilled water is used as the solvent.
Crystalline Metal Oxide Experiment 8
For Experiment 8, the Inventors used an unreduced macro size crystalline
particle of nickel
oxide as the catalyst. All other conditions remained the same as Experiment 4.
Upon ending the experiment, the reaction products showed that 75.7 % of the
lignin charged
to the reactor was converted, but that only 10.6 % of the converted lignin was
phenols. This
demonstrates the need for nanoparticles of nickel oxide as opposed to
macroparticles of nickel
oxide when seeking to convert lignin to phenols.
Operating conditions and conversion data are reported below in Table 5.
TABLE 5
0
t4
Run Catalyst Catalyst Amount of Amount of Solvent Temp. Starting
% % Yield Ending =
-,
4.
No. Particle Catalyst Lignin (g) ( C) Pressure
Conversion of Phenol Pressure -6-
c,
Size (urn) (g) (dry) (psi)
Oils (psi) c...
oo
1 Reduced <50 0.8 1.5 H20 305 200
83.0 N/A 255 t4
(NiO)
2 Reduced <50 0.918 2.5 H20 305 200
79.4 16.6 235
(NiO)
3 Unreduced <50 0.8 1.5 H20 . 305 200
77.0 N/A 235
NiO)
4 Unreduced <50 0.918 2.5 H20 305 200
68.8 25.0 251
NiO)
0
Unreduced <50 0.45 2.5 H20 305 200
61.8 23.3 244 2
(NiO)
0
0
-4
-.
6 Unreduced <50 0.918 2.5 H20 315 200
72.0 19.4 277 .
(NiO)
.
P.
7 Unreduced <50 0.918 2.5 Me0H 290 200
85.0 N/A 1508 1'
2
(NiO)
8 Unreduced 2510 0.92 2.5 H20 305 200
75.7 10.6 250
Macro (NiO)
v
n
,-3
C11
V
e4
=
t.,
a
C'
-
-
44
A
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
By way of further experimentation, the Inventors obtained nanoparticles of
nickel oxide which
had been doped with other metal oxides (crystalline bi-metallic oxide
catalysts). Average
particle size was reported by the manufacturer Sigma-Aldrich Co., LLC from St.
Louis,
Missouri, USA. Operating conditions and conversion data for nanoparticles of
nickel oxides
doped with other metals are reported below in Table 6. Operating conditions
and conversion
data for Experiment 4 are included for comparison of the nanoparticles of
nickel oxides doped
with other metals to those that have not been doped with other metals.
Crystalline Metal Oxide Experiment 9
For Experiment 9, the Inventors obtained nickel cobalt oxide nanopowder (Ni-
Co0) number
634360-25G from Sigma-Aldrich. This catalyst had an average particle size of
less than 150 nm.
All other operating conditions remained the same as Experiment 4.
Upon ending the experiment, the reaction products showed that 68.7% of the
lignin had been
converted, and that 23.2% of the converted lignin were phenols. GC/MS of the
reaction products
further indicates the selectivity towards "light" phenols as seen in
Experiment 4. This
demonstrates that there is no significant difference in the conversion
percentage, yield of phenols
or type of phenols produced between nanoparticles of nickel oxide and
nanoparticles of nickel
oxide doped with cobalt oxide.
Crystalline Metal Oxide Experiment 10
For Experiment 10, the Inventors obtained iron nickel oxide nanopowder (Fe-
NiO) number
637149-25G from Sigma-Aldrich. This catalyst had an average particle size of
less than 50 nm.
All other operating conditions remained the same as Experiment 4.
Upon ending the experiment, the reaction products showed that 67.8 % of the
lignin had been
converted, but that only 17.3 % of the converted lignin was phenols. GC/MS of
the reaction
products further shows selectivity towards "light" phenols as seen in
Experiment 4. This
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
76
demonstrates that nanoparticles of nickel oxide doped with iron oxide do not
function as well for
converting lignin to phenols as nanoparticles of nickel oxide.
Crystalline Metal Oxide Experiment 11
For Experiment 11, the Inventors obtained nickel zinc iron oxide nanopowder
(Ni-Zn-FeO)
number 641669-1OG from Sigma Aldrich. This catalyst had an average particle
size of less than
100 nm. All other operating conditions remained the same as Experiment 4.
Upon ending the experiment, the reaction products showed that 67.8% of the
lignin had been
converted, and that, surprisingly 37.2 % of the converted lignin was phenols.
GC/MS of the
reaction products further indicates that the selectivity towards "light"
phenols as seen in
Experiment 4. The demonstrates that nanoparticles of nickel oxide doped with
zinc and iron are
highly desirable when seeking to convert lignin to phenols.
TABLE 6
0
Run Catalyst Catalyst Amount of Amount of Temp. Starting
AI A Yield of Ending
No. Particle Catalyst (g) Lignin (g) ( C) Pressure
Conversion Phenol Oils Pressure
Size (urn) (dry) (psi)
I (Psi)
4 Unreduced NiO <50 0.918 2.5 305 200
68.6 25.0 251
9 Unreduced Ni-Co <150 0.92 2.5 305 200
68.7 23.2 251
Unreduced Ni-Fe <50 0.92 2.5 305 200 67.8
17.3 248
11 Unreduced Ni-Zn- <100 0.92 2.5 305 200
67.8 37.2 258
Fe
ps,0
- 4
r
N)
r
131
r
N)
r21
t.)
C4
C.N
4.=
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
78
HYDROGEN DONOR SOLVENTS
Once the lignin feedstream has been converted to a converted lignin
feedstream, the converted
lignin feedstream may be further converted to an aromatic converted lignin
product. The
converted lignin feedstream suitable for this process will comprise products
derived from the
lignin of ligno-cellulosic biomass. Typically the product derived from the
lignin of a lingo-
cellulosic biomass is a phenol oil which is the term used to describe the
composition consisting
of all of the phenols in the converted lignin feedstream.
The converted lignin feedstream is combined with one species or multiple
species of molecules.
These hydrogen donor molecules, considered reactants, may be selected from the
group
consisting of hydrogen donor molecules produced from a previously converted
lignin
feedstream, hydrogen donor molecules derived from a source other than a
product stream from a
previously converted lignin feedstream and mixtures thereof.
A hydrogen donor molecule donates at least one hydrogen atom, both of which
are consumed
during the process. Examples of hydrogen donor molecules are those compounds
selected from
the group consisting of aliphatic polyols having a formula of H-11-1-C-OHln-H,
where n is an
integer from 2 to 10, included in this group are sorbitol (n=6), glycerol
(n=3), xylitol (n=5) and
ethylene glycol (n=2). Thus, the hydrogen donor molecule can be selected from
the group
consisting of sorbitol, glycerol, xylitol and ethylene glycol.
Another group of hydrogen donor molecules are those molecules having the
formula of:
OH
Ri
O
. 2
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
79
Where R1 is selected from the group consisting of -OCH2, -H, and -OH and R2 is
selected from
the group consisting of -CH3, -CH2-CH3, -CH2-CH2-CH3, and -CH2-CH2-CH2-CH3.
Another group of hydrogen donor molecules are those molecules having the
formula of:
Y
Where R is selected from the group consisting of -CH3, -CH2-CH3, -CH2-CH2-CH3,
and -CH2-
CH2-CH2-CH3.
The hydrogen donor molecules are preferably not molecules that produce an
aldehyde as one of
the final conversion products of the donation process. Terminal alcohols like
methanol and
propanol molecules produce an aldehyde as one of the final conversion products
of the donation
process. It is preferred that the hydrogen donor molecules do not produce an
aldehyde as one of
the final conversion products of the donation process because the aldehyde
creates side products
in later processing.
The hydrogen donor molecules can also be supplied from a product stream from a
previously
converted lignin feedstream wherein the product stream includes cyclohexanol
and substituted
cyclohexanols. Hydrogen donor molecules selected from a source other than the
products from a
previously converted lignin feedstream include isopropanol, ethylene glycol,
glycerol,
cyclohexanol and substituted cyclohexanols. In a more preferred embodiment the
hydrogen
donor molecule is isopropanol. In an even more preferred embodiment the
plurality of hydrogen
donor molecules comprise a mixture of cyclohexanol and substituted
cyclohexanols from the
product of a previously converted lignin feedstream and cyclohexanol and
substituted
cyclohexanols from a source other than the product of a previously converted
lignin feedstream.
In a most preferred embodiment the hydrogen donor molecule is cyclohexanol and
substituted
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
cyclohexanols derived from and separated from the converted lignin feedstream
during an earlier
process. In one embodiment the hydrogen donor molecule is present with water
as well.
The required amount of hydrogen donor molecules or mixture thereof can be
determined by the
mole ratio of moles of hydrogen donor molecule(s) to moles of phenol oil where
the phenol oil is
assigned an average molecular weight of 150 g/mol. The mole ratio of moles of
hydrogen donor
molecule(s) to moles of phenol oil should preferably be in the range of
between 2.0:1.0 and
10.0:1.0 with a range of between 3.0:1.0 and 9.0:1.0 being more preferred, a
range of between
4.0:1.0 and 8.0:1.0 being even more preferred and a range of between 5.0:1.0
and 7.0:1.0 being
most preferred.
The role of H2 gas has been found to act as a poison to the conversion to
aromatics. Thus, the
amount of H2 gas, if added to the reaction, should be kept at less than 25% of
the total amount of
hydrogen atoms 1H1 and H2 molecules used in the process representing the
following formula:
2 x [H2]
([H] + (2 x [H2])) x 100 < 25%
The converted liquid feedstream and hydrogen donor molecules are exposed to a
metal catalyst,
preferably a Nickel containing catalyst. Examples of nickel containing
catalysts are described
herein and include the heterogeneous Raney Nickel catalysts and heterogeneous
and
homogeneous Nickel Oxide catalysts.
The ratio of mmol of hydrogen donor molecules to mmol of catalyst metals is
preferred to be in
the range of between 1.0:1.0 and 5.0:1.0 with a range of between 1.2:1.0 and
4.0:1.0 being more
preferred and a range of between 1.5:1.0 and 3.0:1.0 being most preferred.
Only the mmol of
metals in the catalyst are used to calculate the mmol of catalyst.
The materials are exposed to each other at a reaction temperature in the range
of 190 C to 350
C, with 200 C to 310 C being more preferred, with 210 C to 300 C being
even more
preferred and 210 C to 280 C being the most preferred. The reaction time
depends upon the
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
81
amount of catalyst by weight, the reaction temperature and the moles of
hydrogen donor
molecules (not H2 gas). Generally this is in the range of 15 minutes to 6
hours, but times of 10
minutes to 15 hours are conceivable.
What has been discovered and demonstrated in the experimental section is that
when the reaction
temperatures are severe (>190 C or >200 C), the amount of aromatic reaction
products
unexpectedly shifts from less than 5% of the reaction products to greater than
20% of the
reaction products with greater than 30% of the reaction products being more
preferred, greater
than 40% of the reaction products being even more preferred and a majority of
the reaction
products (greater than 50% of the reaction products) being most preferred.
The process can be run in both batch and continuous mode. In continuous mode
the product is
being removed from the reaction vessel while the reaction is occurring. Where
indicated, the
examples were produced on a continuous stirred thermal reactor, a CSTR,
although any reactor
capable of removing product from the reaction vessel while the reaction is
occurring can be used
for a continuous process.
Since the lignin often comes with intractable carbohydrates, it may be
preferable to treat the
feedstock first with a carbohydrate conversion step. Fermentation is one such
carbohydrate
conversion step. Another carbohydrate conversion step and embodied in Figure 1
is to create a
slurry lignin feedstock comprised of carbohydrates and lignin, feed it to a
carbohydrate
conversion reactor as described in United States Patent Publication Numbers
U52011/312487,
U52011/312488 and U52011/0313212 by pressuring the slurry feedstock as
described in this
specification and feeding into a first reaction zone and
a) contacting, the lignin slurry feedstock in a continuous manner, in a first
reaction zone,
hydrogen, water, with a catalyst to generate an effluent stream comprising at
least one
polyol, hydrogen, water and at least one co-product, wherein the hydrogen,
water, and
feedstock comprising cellulose are flowing in a continuous manner, and wherein
the
catalyst in the consists essentially of at least two active metal components
selected
from the group consisting of:
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
82
(i) Mo, W, V, Ni, Co, Fe, Ta, Nb, Ti, o, Zr and combinations thereof wherein
the
metal is in the elemental state or the metal is a carbide compound, a nitride
compound, or a phosphide compound;
(ii) Pt, Pd, Ru, and combinations thereof wherein the metal is in the
elemental
state; and
(iii) any combination of (i) and (ii);
b). separating hydrogen from the effluent stream and recycling at least a
portion of the
separated hydrogen to the reaction zone;
c). separating water from the effluent stream and recycling at least a portion
of the
separated water to the reaction zone; and
d). recovering the polyol from the effluent stream or passing the polyol along
as the
plurality of hydrogen donor molecules.
Depending upon catalyst selection and operations this will produce a mixture
of polyols such as
ethylene glycol and propylene glycol which could be used together as the
plurality of hydrogen
donor molecules.
HYDROGEN DONOR EXPERIMENTS
The below experiments establish the ability for hydrogen donor molecules to
convert a converted
lignin feedstream to a product comprising a majority of the conversion
compounds as aromatic
compounds which are referred to as reformate.
CA 02889702 2015-04-27
WO 2014/063852
PCT/EP2013/067734
83
Table 7: Phenol to Reformate (aromatic compounds) without external hydrogen
(Hz)
Run Experiment Temperature Cold Mole ratio of H Ratio of
( C), Residence Pressure Donating
Phenol Oil
Time (h) and Nitrogen Molecules* to
(mmol) to
Stir (bar) Phenol Oil
Catalyst**
(mmol)
1 Ethylene 225 C , 5h and 1 6.4:1.0 2.2:1.0
Glycol as 900 rpm
Hydrogen
Donor entity
2 Isopropyl 230 C, 5h and 1 6.4:1.0 2.2:1.0
Alcohol as 900 rpm
Hydrogen
Donor entity
3 Cyclohexanol 240 C, 5h and 1 6.4:1.0 2.2:1.0
as Hydrogen 900 rpm
Donor entity
4 Glycerol as 250 C, 5h and 1 5.2:1.0
2.6:1.0
Hydrogen 900 rpm
Donor entity
* The moles of H Donating Molecules are the moles of the entire hydrogen donor
entity
(ethylene glycol, isopropyl alcohol, etc.)
** Catalyst in all Runs was Wet Grace Raney Ni 2800, available from W.R.
Grace. Mmol of
catalyst includes only the metal content of the catalyst.
The mmol of Phenol Oil is calculated as follows: The amount of phenol oil
consists of all of
the phenols (typically 5 different types of phenol units are present but with
similar backbone
alkyl phenol unit). The phenol oil has an assigned average molecular weight of
150.0 g/mol
which is used as the repetitive unit when calculating the amount of mmol of
phenol oil in the
crude mixture, so 5.0g phenol oil has 33.3 mmol of phenols.
The following data sets experimentally establish the ability of the hydrogen
transfer and or
hydrogen donor process to produce an aromatic rich stream of high selectivity
relative to the
prior art.
The experiments are sorted into three tables. Table 7a is the gross
characterization of the batch
process operated on a feedstream derived as described above. The reaction
conditions in the
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
84
batch process was to use 2.0mmol of phenol oil for every 1.0g wet Raney Ni
2800 having a 1:1
ratio by weight of nickel to H20.
Table 7a ¨ Working Examples - BATCH
Run H Donor Feed Temp, Extent of Reaction
Selectivity
Time % Phenols % of Phenols % of % of
% of Converted
=Ji
Converted Left Converted Converted
Products which are
during Unconverted Products Products Hydrogenated
reaction after reaction which are which are
Products
aromatic not
Cyclo - Cyclo -
compounds aromatic alcohols alkanes
(mol %) compounds
(mol%)
I Ethylene Crude 240 C, 86.5 13.3 49.0 51.0
0.0 0.0
Glycol Phenol 5h
(EG) Oil
CO
2 Isopropyl Crude 230 C, 76.1 23.9 46.7 53.3
1.7 4.0 GA to
Alcohol Phenol 5h
(IPA) Oil
2
=
3 Cyclo- Crude 225 C, 92.5 7.5 25.4 74.6
30.4 28.0
hexanol Phenol 5h
(CH) Oil
4 Glycerol Crude 250 C, 62.5 28.6 72.1 27.9
0.63 0.56
(GY) Phenol 5h
Oil
C21
C'
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
86
Table 7b is the gross characterization of the feeds and prior art lower
temperatures as indicated in
the Table 7b. The reaction conditions according to the prior art was 0.2 g of
a Model Phenol
Compound Feed and 1.0 g wet Raney Ni 2800 haying a 1:1 ratio of grams of
nickel to grams of
H20.
Table 7b ¨ PRIOR ART COMPARATIVE EXAMPLES - BATCH
0
H Donor Model Temp, Extent of Reaction
Selectivity
Phenol Time % Phenols % of % of % of Converted
Products which 00
Compound Converted Conversion Converted are
Hydrogenated Products
Feed during Products Products Cyclo - alcohols
Cyclo - alkanes
reaction remaining which are
as Phenols aromatic
compounds
(mol %)
Isopropyl .õ.0 OH = 120 C, 100.0 13.0 3.0
83.0 1.0
Alcohol * 3h
(Rinaldi
et a]. )
Oe
Isopropyl 0_ 120 C, 100.0 NA 1.0 97.0
2.0
ro
Alcohol U 3h
(Rinaldi
et al!)
Isopropyl Pretreated 160 C, NA NA 0.0 NA
NA
Alcohol Bio-oil 3h
(Rinaldi
et al!)
aReference: Rinaldi et al. Energy Environ. Sci., 2012, 5, 8244-8260; NA: Not
available; Pressure; Atmospheric
C21
a
C'
C=4
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
88
In Table 8, the distribution and high yield of the aromatics is demonstrated.
For instance, of the
total amount of products of the reaction, aromatics comprised 48.97% of the
products when
ethylene glycol was the hydrogen donor. Notably, benzene is 15% of the
aromatics when
cyclohexanol is the donor.
Table 8: Product Distribution of Batch Reaction Products
H Temp Cony. % of converted products which are Hydro-
Heavies
Donor ( C), (%)* aromatic compounds based on total area genated
(%)***
Time under a GC/MS curve Products
(h) (%)**
B T EB PB X Other CA CK
(%) (%) (%) (%) (%) (%) (%) (%)
EG 225,5 86.5 4.67 8.34 4.23 4.11 3.07 24.55 0.0 0.0 36.6
IPA 230, 5 76.1 5.24 5.68 6.0 4.9 1.0 23.8 1.7
4.0 23.6
CH 240, 5 92.5 15.1 1.0 1.5 1.2 0.0 6.7 30.8
27.9 8.4
GY 250, 5 62.5 0.17 1.89 2.38 1.29 1.01 31.54 0.56
0.63 28.3
EG:ethylene glycol; IPA:Isopropyl alcohol; CH:cyclohexanol; GY:glycerol;
B:benzene; T:toluene;
EB:ethylbenzene; PB:propylbenzene; X: xylenes; CA: Cycloalcohols; CK:
Cycloalkanes
* Cony. (%) is the percent of phenols converted during the reaction.
** Hydrogenated Products (%) is the percent of the converted products which
are hydrogenated.
*** Heavies are defined as molecules having long and short chain hydrocarbons
as side products.
The process was scaled to a continuous reaction under the following
conditions.
Conversion of Phenol Oil with H-Donor Solvent in CSTR
Experiment CSTR 1
H-Donor = lsopropanol
Total Reactor Volume = 500 ml
Reactor Volume Used = 250 ml
Feed Composition = 15w1% Phenol Oil in lsopropanol (14.0:1.0 mol ratio H-
Donor to Phenol Oil,
MW= 148)
Reactor Temperature = 230 C
Reactor Pressure = 68.95 bar
Nitrogen Flow Rate = 50 sccm
Agitator Speed = 600 rpm
Feed Flow Rate = 1.10 ml/min at 20 C = 2.10 ml/min at 230 C (density @
20 C = 0.787 g/ml,
density at 230 C = 0.412 g/ml)
Average Residence Time = 119 min
Catalyst Amt = 85 g wet Grace 2800 Raney Ni
Experiment CSTR 2
H-Donor = lsopropanol
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
89
Total Reactor Volume = 500 ml
Reactor Volume Used = 250 ml
Feed Composition = 15w1% Phenol Oil in lsopropanol (14.0:1.0 mol ratio H-
Donor to Phenol Oil,
MW= 148)
Reactor Temperature = 250 C
Reactor Pressure = 89.63 bar
Nitrogen Flow Rate = 50 sccm
Agitator Speed = 600 rpm
Feed Flow Rate = 0.76 ml/min at 20 C = 2.10 ml/min at 250 C (density @
20 C = 0.787 g/ml,
density at 250 C = 0.284 g/ml)
Average Residence Time = 119 min
Catalyst Amt = 85 g wet Grace 2800 Raney Ni
Experiment CSTR 3
H-Donor = Cyclohexanol
Total Reactor Volume = 500 ml
Reactor Volume Used = 250 ml
Feed Composition = 10 wt% Phenol Oil in Cyclohexanol (13.3:1.0 mol ratio H-
Donor to Phenol Oil,
MW= 148)
Reactor Temperature = 250 C
Reactor Pressure = 68.95 bar
Nitrogen Flow Rate = 100 sccm
Agitator Speed = 600 rpm
Feed Flow Rate = 2.00 ml/min at 20 C = 2.66 ml/min at 250 C (density @
20 C = 0.951 g/ml,
density at 250 C = 0.715 g/ml)
Average Residence Time = 94 minutes
Catalyst Amt = 50 g wet Grace 2800 Raney Ni
Experiment CSTR 4
H-Donor = Cyclohexanol
Total Reactor Volume = 500 ml
Reactor Volume Used = 250 ml
Feed Composition = 10 wt% Phenol Oil in Cyclohexanol (13.3:1.0 mol ratio H-
Donor to Phenol Oil,
MW= 148)
Reactor Temperature = 280 C
Reactor Pressure = 68.95 bar
Nitrogen Flow Rate = 100 sccm
Agitator Speed = 600 rpm
Feed Flow Rate = 1.80 ml/min at 20 C = 2.54 ml/min at 280 C (density @
20 C = 0.951 g/ml,
density at 280 C = 0.673 g/ml)
Average Residence Time = 98 minutes
Catalyst Amt = 50 g wet Grace 2800 Raney Ni
Experiment CSTR 5
H-Donor = 4-Methylcyclohexanol
Total Reactor Volume = 500 ml
Reactor Volume Used = 250 ml
Feed Composition = 9 wt% Phenol Oil in 4-Methylcyclohexanol (13.1:1.0 mol
ratio H-Donor to
Phenol Oil, MW= 148)
Reactor Temperature = 250 C
Reactor Pressure = 68.95 bar
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
Nitrogen Flow Rate = 400 sccm
Agitator Speed = 600 rpm
Feed Flow Rate = 1.90 ml/min at 20 C = 2.50 ml/min at 250 C (density @
20 C = 0.913 g/ml,
density at 250 C = 0.693 g/ml)
Average Residence Time = 100 minutes
Catalyst Amt = 50 g wet Grace 2800 Raney Ni
Experiment CSTR 6
H-Donor = 4-Methylcyclohexanol
Total Reactor Volume = 500 ml
Reactor Volume Used = 250 ml
Feed Composition = 9 wt% Phenol Oil in 4-Methylcyclohexanol (13.1:1.0 mol
ratio H-Donor to
Phenol Oil, MW= 148)
Reactor Temperature = 280 C
Reactor Pressure = 68.95 bar
Nitrogen Flow Rate = 100 sccm
Agitator Speed = 600 rpm
Feed Flow Rate = 1.70 ml/min at 20 C = 2.37 ml/min at 280 C (density @
20 C = 0.913 g/ml,
density at 280 C = 0.654 g/ml)
Average Residence Time = 105 minutes
Catalyst Amt = 50 g wet Grace 2800 Raney Ni
Table 9 shows the difference between the batch and CSTR reaction processes.
0
Table 9: Batch Vs CSTR
t4
=
-,
4.
-6-
H Temp Extent of Selectivity
w
Donor ( C), Reaction
00
..A
% of converted products which are aromatic compounds
Hydro-genated Heavies
Time (Cony. %*)
based on total area under a GC/MS curve
Products (%)**
(h)
B (%) T (%) LB (%) PB (%) X (%) Other CA (%) CK (%)
(%)
IPA 250 C, 87.9 183 6.6 12.7 8.2 0.0 14.1
4.2 7.1 16.5
(CSTR) 2h
IPA 230 C, 76.1 5.24 5.68 6.0 4.9 1.0 23.8
1.7 4.0 23.6
(Batch) 5h
0
IPA 200 C, 76.7 1.3 1.1 3.8 2.2 0.0 7.8
19.1 24.7 16.6 .
0
(Batch) 5h
.
4...
4
e
EG:ethylene glycol; IPA; Isopropyl alcohol; CH:cyclohexanol; B:benzene;
T:toluene; EB:ethylbenzene; PB:propylbenzene; X: .
.,
xylenes; CA: Cycloalcohols; CK: Cycloalkanes
6-
,
.,
* Cony. % is the percent of phenols converted during the reaction.
.
,
** Hydrogenated Products (%) is the percent of the converted products which
are hydrogenated. ..,
*** Heavies are defined as molecules having long and short chain hydrocarbons
as side products.
v
n
,-3
C21
V
e4
=
t.,
a
C'
-
-
44
A
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
92
As discussed herein, the above process can be preceeded by a carbohydrate
conversion process
which is fed by ligno-cellulosic biomass.
The above process can use a feedstock from a commercial lignocellulosic
ethanol plant, but at
the same time is flexible enough to use lignin-containing raw materials from
other processes.
The current raw material is derived from a naturally occurring lignocellulosic
biomass, after the
majority of the carbohydrate fraction has been biologically converted to
ethanol. The sulfur
content of the feedstock is near to zero, and consequently no desulfurization
is required to obtain
jet fuels (in contrast to a fossil feedstock).
In most second-generation biofuels processes, the lignin co-product is
collected after distillation
and used as boiler fuel to generate steam and power. This is not necessarily
the best use of these
lignin rich residues (LRR).
The envisioned process is one in which the biorefinery produces ethanol (or
some other product)
from the carbohydrate fraction of the ligno-cellulosic biomass while the LRR
is utilized as a
feedstock for fuels and chemicals produced using at least the above process if
not others for
lignin conversion.
For example, ethylene glycol used in the hydrogen donor solvent process would
come from the
conversion of the carbohydrates to ethylene glycol as described in the art.
Other alcohols are
well known as well. The carbohydrate conversion could be catalytic or
enzymatic. Because the
lignin conversion process does not use pure hydrogen donors, the need to
purify the carbohydrate
conversion products, such as ethylene glycol is not necessary.
The plurality of conversion products preferably comprise at least one product
selected from the
group consisting of carbon dioxide, methane, ethane, phenols, benzene,
toluene, and xylenes.
It should be evident from Figure 4 how the reaction process can be operated as
a CSTR ¨
continuous stirred tank reactor.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
93
The invention taught by the in situ separation using a dip tube is applicable
to almost any solid -
liquid where the solids are present as finely dispersed particles. This aspect
of the invention is
not limited to a lignin conversion process.
Another embodiment of the process is that the plurality of lignin conversion
products are cooled
after leaving the reactor separating the vapor from the liquid and solids,
with the back pressure
regulator (700) located after the liquid solids separator (600), the pressure
of the lignin
conversion process can now be controlled.
The temperature of the lignin conversion products generated by the lignin
conversion process are
substantially greater than the temperature of the steam, soaking and
fermentation processes of the
pre-treatment and carbohydrate conversion processes that would precede the
lignin conversion
process. The inventors clearly contemplate that in the integrated or co-sited
operation that the
heat from the lignin conversion products would be transferred to soaking,
steam pretreatment,
hydroylsis, and/or fermentation processes of the pre-treatment process.
Once these liquid lignin conversion products are obtained, they can then be
subsequently
converted to a number of different chemical feedstocks and intermediates. One
preferred
intermediate is at least one polyester intermediate selected from the group
consisting of ethylene
glycol, terephthalic acid, and isophthalic acid. Once the intermediate is
made, the conversion of
the intermediate to polyester and subsequent articles such as soft drink
bottles, beer bottles, and
other packaging articles can be accomplished using the conventional techniques
known today
and those yet to be invented.
Since the lignin often comes with intractable carbohydrates, it may be
preferable to treat the
feedstock first with a carbohydrate conversion step to obtain carbohydrate
conversion products.
In a preferred embodiment, the carbohydrate conversion products are selected
from the group
consisting of alcohols, polyols, glucans, gluco-lignins and cellulose.
Fermentation is one such carbohydrate conversion step. Another carbohydrate
conversion step
and embodied in Figure 1 is to create a slurry feedstock comprised of
carbohydrates and lignin,
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
94
feed it to a carbohydrate conversion reactor as described in US2011/312487 and
US2011/312488
and US2011/0313212 by pressuring the slurry feedstock as described in this
specification and
feeding it into a first reaction zone and
a) contacting, the lignin slurry feedstock in a continuous manner, in a first
reaction zone, with
hydrogen, water, and a catalyst to generate an effluent stream comprising at
least one polyol,
hydrogen, water and at least one co-product, wherein the hydrogen, water, and
feedstock
comprising cellulose are flowing in a continuous manner, and wherein the
catalyst in the first
reaction zone consists essentially of at least two active metal components
selected from the
group consisting of:
(i) Mo, W, V, Ni, Co, Fe, Ta, Nb, Ti, o, Zr and combinations thereof wherein
the metal is
in the elemental state or the metal is a carbide compound, a nitride compound,
or a
phosphide compound;
(ii) Pt, Pd, Ru, and combinations thereof wherein the metal is in the
elemental state; and
(iii) any combination of (i) and (ii);
b) separating hydrogen from the effluent stream and recycling at least a
portion of the separated
hydrogen to the reaction zone;
c) separating water from the effluent stream and recycling at least a portion
of the separated
water to the reaction zone; and
d) recovering the polyols from the effluent stream.
After recovering the converted carbohydrates, such as the polyols from the
effluent stream, to
create a secondary feedstock stream comprising lignin, the secondary feedstock
stream
comprising lignin can be again optionally pressurized and fed into the lignin
conversion reactor
(500) to convert lignin into the phenols and other component in the plurality
of lignin conversion
products.
In a preferred embodiment, the polyols, such as ethylene glycol and propylene
glycol may be
used as a hydrogen donor to convert the lignin to lignin conversion products.
In another
embodiment, the hydrogen from the effluent stream may be used as a source of
hydrogen to
convert the lignin to lignin conversion products. Also, the water from the
effluent stream may be
recycled or reused as treatment water for pretreating the ligno-cellulosic
biomass feedstock.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
Now that the fundamental operations have been explained, one can turn to
Figure 1 to describe
one embodiment and its variations. As depicted in Figure 1, the conversion of
the ligno-
cellulosic biomass can begin with either pre-treated ligno-cellulosic biomass
(20A or 20B) or
untreated ligno-cellulosic biomass (10A or 10B). The A stream is fed into an
optional
carbohydrate conversion process to convert the carbohydrates to useful
products prior to
converting the lignin. The chosen feedstock enters the carbohydrate conversion
reactor (100) via
stream (110). Additional reactants, such as hydrogen are added into (120). If
the ligno-
cellulosic biomass is added as a slurry and a catalyst is used, the handling
principles described
creating the continuous process apply and reduce this process to practice as
well. After
conversion, the carbohydrate conversion products are passed from the
carbohydrate conversion
reactor (100) to carbohydrate conversion product recovery (200) via stream
(210). There can be
two types of carbohydrate conversion products, one being gas exiting via
(220). This gas could
be methane which can be converted to hydrogen by known technologies such as
steam
reforming. The hydrogen would be used either to convert more carbohydrates or
lignin by
introducing the hydrogen into lignin conversion reactor (500) via stream
(520). Should the
embodiment produce ethylene glycol, that ethylene glycol would be transferred
via stream (230)
to a polyester manufacturing facility which would convert the ethylene glycol
into polyester
resin which is later converted to finished polyester articles such as preforms
and polyester
bottles.
The lignin from the carbohydrate conversion process enters the lignin slurry
creation step (300)
via stream (310). The embodiment without the first carbohydrate conversion
step is depicted by
streams (20B) and (10B) respectively. As contemplated by the inventors, these
could directly
feed, and have been proven to be continuously converted when fed directly into
the slurry
creation step (300). Makeup water or other solvent is added via stream (320)
with the optional
vacuum being applied through stream (330).
If the ligno-cellulosic feedstocks of either (20B) or (10B) are already in a
slurry form, step (300)
can be skipped and the streams (10B) or (20B) fed directly into the slurry
pump or slurry pumps
(400) via stream (410). The pumping system as described above increases the
pressure of the
slurry to greater than the reactor conversion pressure of the lignin
conversion reactor (500).
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
96
After pressurizing the slurry to greater than the reactor conversion pressure
of the lignin
conversion reactor, the slurry pump will discharge the slurry comprised of
lignin through an
outlet valve (450) to the lignin conversion reactor (500) through stream
(510). Lignin
conversion reactor (500) will contain the lignin slurry and at least the first
catalyst. Hydrogen
will enter the lignin conversion reactor (500) at pressure through stream
(520). As a CSTR, the
lignin conversion products are passed up through dip tube (610), with the
catalyst settling back
down into the lignin conversion reactor (500). Vessel (600) is the liquid
solids separator, with
the gas by-products exiting the separation vessel (600) via stream (710) and
passing into the back
pressure regulator (700) which controls the pressure of the whole system.
After reducing the
pressure, the gasses are passed through stream (720). If carbohydrates were
introduced into the
lignin conversion reactor, then stream (720) will contain methane, a
conversion product of the
carbohydrates, thus the carbohydrate conversion process has been done in situ
with the lignin
conversion. The methane can be further converted to hydrogen through steam
reforming for
example and re-used in the process, thus making the process at least partially
self-sufficient in
hydrogen.
The solids from the lignin conversion process are separated from the liquids
in step (600) with
the solid passing in stream (620) and the liquids passing to the BTX
conversion step (800) via
stream (810). Stream (650) of Figure 3 shows the separation of water from the
lignin conversion
process. While the water will be present in the liquid phase, there may be
some water vapor
present in (720) as well. As depicted in Figure 1, in this embodiment, at
least a portion of the
water is re-used to create or supplement the slurry comprised of lignin. As
the lignin conversion
process is a net water producer, some water will be purged in stream (620).
The conversion of phenols to BTX is a well known chemistry with several routes
being
available. As the lignin conversion process produces predominantly phenols,
the conversion of
phenols by the known routes is considered well within the scope of one of
ordinary skill. Once
the BTX (benzene, toluene, xylenes) is formed it can be passed to a conversion
step to convert
the BTX to terephthalic acid, react the terephthalic acid with ethylene glycol
and make polyester
resin and subsequently articles from polyester resin (900) via stream (910),It
is again well within
the scope of one of ordinary skill to convert these products to terephthalic
acid, react the
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
97
terephthalic acid with ethylene glycol to make polyester resin and
subsequently articles from the
polyester resin such as films, trays, preforms, bottles and jars.
INTEGRATED PROCESS EXPERIMENTS
Material Preparation
The experiments used a composition obtained from wheat straw as a starting raw
material.
The raw material was subjected to a soaking treatment in water at a
temperature of 155 C for 65
minutes then steam exploded at a temperature of 190 C for 4 minutes.
The steam exploded material and the liquids from soaking material were mixed
together and
subjected to enzymatic hydrolysis, fermentation to ethanol and distillation.
Detailed parameters used are considered not relevant for the experiments,
provided that the
percentage content of the composition is preserved.
The mixture of liquid and solids after distillation was pressed at 15 bar and
at a temperature of
80 C to obtain a dense and compact solid, having a dry matter content of 55%
and characterized
by the following composition on a dry matter basis.
TABLE 10 LIGNIN FEEDSTOCK ANALYSIS
ELEMENT Percentage content
Ash 13.04
Lignin 49.71
Glucan 21.77
Xylan 6.81
Other compounds 8.67
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
98
The lignin-rich composition was subjected to a temperature lower than 0 C and
kept frozen until
experiments execution.
Lignin conversion procedure
The following procedures were applied to all the experiments not using the
bubble column,
unless differently specified.
Frozen lignin-rich composition was naturally unfrozen until reaching a
temperature of 20 C.
De-ionized water was added to the lignin-rich composition to reach the final
lignin-rich
composition concentration in the slurry planned in each experiment. The
mixture was inserted
into a blender (Waring Blender, model HGBSS6) and thoroughly mixed
intermittently (e.g.
pulsed on for 30 sec, left off for 30 sec) for 10 mm to reach a homogeneous
slurry. The
homogeneity of the slurry was evaluated by eye.
The slurry was inserted into a mix tank with constant agitation. The mix tank
was a stainless
steel, dish bottom tank with a bottom discharge port connected to a Chandler
Quizix QX dual
syringe pump equipped with full port ball valves, connected to the lignin
conversion reactor.
The pump discharge was connected to the reactor with tubing.
The lignin conversion reactor was a Parr 4575 reactor equipped with a dual 45
pitched turbine
blade, cooling coil, separate gas and slurry feed ports and a discharge dip
tube. The reactor was
charged with water (-220 mL) and catalyst (Johnson Matthey A-5000 sponge
catalyst) according
to the experimental conditions of each experiment and sealed. The weight of
catalyst introduced
is indicated as the ratio between the weight of the catalyst and the weight of
dry matter of the
lignin-rich composition added to the lignin conversion reactor in one
residence time. Hydrogen
at a temperature of 20 C was inserted into the lignin conversion reactor to
reach a pressure of
48.3 bar. The lignin conversion reactor was heated to a temperature
corresponding to 90% of the
reaction temperature and continuous flow of Hydrogen was started into the
lignin conversion
reactor. The lignin conversion reactor was connected to a products receiver,
maintained at 25 C.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
99
The pressure was measured by means of a pressure transducer (Ashcroft Type 62)
connected to
the lignin conversion reactor and controlled by means of a back-pressure
regulator (Dresser Mity
Mite 5000, model 91) placed downstream of the products receiver. Temperature
was increased
to the reaction temperature and the flow of slurry comprised of lignin was
introduced into the
lignin conversion reactor. The slurry flow rate was calculated for obtaining
the residence time of
the lignin feed in the reactor in each experiment at the operating conditions.
After a time
corresponding to 3 residence times steady conditions were considered to be
reached and solid
and liquid reaction products were collected into the receiver for a time
corresponding to 1
residence time. The receiver was depressurized to atmospheric pressure, the
non-gaseous
reaction products were extracted with methyl tert-butyl ether organic solvent,
filtered, and the
liquid phases were separated by a separatory funnel.
This system was continuously operated many times without shutting down for up
to 2 shifts
(approximately 16 hours).
Experiments were conducted according to the described procedure. Experimental
parameters are
reported in Table 11.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
100
TABLE 11 EXPERIMENTAL PARAMETERS
Exp. Temp H2 Press. Flow Rate Lignin-rich
Residence Catalyst Unreacted %
No. ( C) Flow (bar) Slurry Solids
composition time (min) to Lignin- Lignin
Catalyst
(sccm) (mL/ (g/ Concentratio rich (% of
Loss
n (wt%) compositi
Theoretical)
mm) min)
on ratio
1 340 150 156.1 2.8 0.42 15 53 0.50
2 340 500 173.4 5.6 0.84 15 26 2.60
3 340 500 173.4 2.8 0.42 15 51 1.25
4 305 100 122.4 3.8 0.19 5 45 0.25
3.1 13.3
325 100 166.5 3.8 0.19 5 42 0.25 0.2
1.7
6 305 800 122.4 3.8 0.19 5 45 2.00 0.6
1.3
7 325 100 166.5 2.3 0.12 5 70 0.25 0.3
1.1
8 305 100 122.4 3.8 0.57 15 45 2.00
20.8 18.4
The experiments produced the following main products:
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
101
TABLE 12 Lignin Conversion Products for Table 11, Experiment 4
Product ID Relative Amount (Area % of G.C.)
Exp 4 Exp 5 Exp 6 Exp 7 Exp
8
2-Methoxyphenol 10.908 13.87 6.337 11.641
6.578
2,6 Dimethoxyphenol 8.673 9.69 5.918 7.229
5.315
4-Ethyl-2-methoxy-phenol 8.139 9.728 8.729 9.994
8.802
2,6-Dimethoxy-4-propylphenol 5.764 3.063 8.458 5.261
7.637
2-Methoxy-4-propyl-phenol 5.118 2.322 5.417 4.042
5.798
4-Ethylphenol 4.563 5.335 5.265 6.228
5.081/ 1.38
1-(4-Hydroxy-3,5-dimethoxypheny1)-
4.288 2.943 1.868
1.635
ethanone
2,6-Dimethoxy-4-ethylphenol 3.859 3.529 6.363 2.634
3.02
Cyclopentanone 2.57 1.667 1.764 1.087
2-Methyl-2-Cyclopenten-1-one 2.233 2.525 2.431
1.244 3 methyl?
2-Methoxy-4-methylphenol 2.153 2.576 2.12 2.18
1.377
2-methyl-Cyclopentanone 2.142 1.772 2.194
1.208
Phenol 1.932 2.808 2.753/ 2.054
2,6-Dimethoxy-methylphenol 1.858 2.504 2.107 1.975
1.365
2,6-Dimethoxy-4-(2-propeny1)-phenol 1.239 1.184 2.987
1.192 1.179
2-Methyl-Cyclopentanone 1.324
1.208
>C20 Aliphatic 2.114
>C20 Aliphatic 1.902
Formic Acid, 1,1, dimethlyehtyl ester 1.406
Cyclohexanol 1.263
>C10 Aliphatic 1.146
>C20 Aliphatic 1.131
2,3-Dimethy1-2-Cyclopenten-1-one 1.329
Eugenol 1.086
1.435
Cyclohexanone, 3-ethenyl 1.074
1.559
Flopropione
1.471
>C20 Aliphatic
1.228
1 Those unidentified general compounds had a 20% match in the library with the
listed
compound so they are noted only by the number of carbons.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
102
RECYCLE OF WASTE WATER TREATMENT
What has also been discovered is that the lignin conversion process of
catalytic hydrogenation
removes much of the contaminants from the water of the stillage entering the
process.
This was easily demonstrated by analyzing the chemical oxygen demand, also
known as CODs
of the stillage from the fermentation (carbohydrate conversion process) prior
to the lignin
conversion process and then analyzing the CODs from the water phase after the
lignin
conversion process.
Observationally, the untreated stillage in a glass sample container appeared
as a dark brown
homogeneous solution. Prior to being processed in the lignin conversion
process the liquid
fraction was dark brown to black, indicating a large amount of soluble
contaminants. After
passing the water through the lignin conversion process (as part of the ligno-
cellulosic biomass
feedstock) the water was separated from the organic products. The water was no
longer dark, but
an amber straw gold.
When measured for CODs, the untreated stillage was 54,000 mg/L of COD. The
CODs of the
water after processing in the lignin conversion process was 17,000 mg/L, a 69%
reduction of
CODs.
Thus, one embodiment of the process will produce an aqueous phase having a COD
concentration preferably less than 50% of the COD concentration of the aqueous
phase of the
lignin feedstock of the lignin conversion process. With less than 40% being
more preferred and
less than 32% being most preferred.
The aqueous phase can be recycled or reused, with or without further COD
removal or reduction
of COD concentration, in the carbohydrate conversion step as the soaking
water, the water of the
steam explosion or other wash water or fermentation streams; or it can be re-
used or recycled in
the lignin conversion step as part of the slurry creation or make up water.
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
103
The re-use or recycle of just 10% of the aqueous phase has massive
implications for the waste
water treatment, which is a significant part of the expense of operating a
carbohydrate
conversion process, a lignin conversion process, or an integrated process.
The water from lignin-cellulosic feedstock was removed and visual and
analytically evaluated
prior to being processed in the lignin conversion process.
This reuse of the water is depicted in Figure 3, where at least a portion of
the water from the
reaction is separated from the lignin conversion products and re-used in the
process. The water
depicted as stream (650) could be used for the slurry at stream (320) or as
part of the hydrolysis
step at (120) of the carbohydrate conversion or used in the soaking or steam
explosion steps of
the pre-treatment. If not reused, the water is generally sent to waste water
treatment for further
purification and re-introduction into the environment.
Analytical measurements
1. Composition of lignin-rich composition
The composition of lignin-rich composition was determined according to the
following standard
procedures:
Determination of Structural Carbohydrates and Lignin in Biomass
Laboratory Analytical Procedure (LAP) Issue Date: 4/25/2008
Technical Report NREL/TP-510-42618 Revised April 2008
Determination of Extractives in Biomass
Laboratory Analytical Procedure (LAP) Issue Date: 7/17/2005
Technical Report NREL/TP-510-42619 January 2008
Preparation of Samples for Compositional Analysis
Laboratory Analytical Procedure (LAP) Issue Date: 9/28/2005
Technical Report NREL/TP-510-42620 January 2008
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
104
Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid
Process
Samples
Laboratory Analytical Procedure (LAP) Issue Date: 3/31/2008
Technical Report NREL/TP-510-42621 Revised March 2008
Determination of Ash in Biomass
Laboratory Analytical Procedure (LAP) Issue Date: 7/17/2005
Technical Report NREL/TP-510-42622 January 2008
Determination of Sugars, By Products, and Degradation Products in Liquid
Fraction
Process Samples
Laboratory Analytical Procedure (LAP) Issue Date: 12/08/2006
Technical Report NREL/TP-510-42623 January 2008
Determination of Insoluble Solids in Pretreated Biomass Material
Laboratory Analytical Procedure (LAP) Issue Date: 03/21/2008
NREL/TP-510-42627 March 2008
2. Composition of liquid products
The composition of liquid products were determined by means of Agilent 7890
Gas
chromatogram and Agilent 5975C Mass Detector, according to the following
procedure and
parameters.
Injector parameters in the Gas chromatogram:
Injection volume: 2 ul
Pulsed spilt injection
Injection pulsed pressure: 50 psi for 0.5 min
Temperature: 220 C
Pressure: 20.386 psi
Septum purge: 3 ml/min
Split ratio: 10:1
CA 02889702 2015-04-27
WO 2014/063852
PCT/EP2013/067734
105
Split flow 13 ml/min
Analytical Column:
Column: Restek RXI-5Sil MS, 30 meter, 0.25 mm ID, 0.5um df
Flow (He): 1.3 ml/min
MSD transfer line: (mass detector)
Temperature profile: 280 C for entire run
Column transfer line: HP-101 methyl siloxane-101 methyl siloxane: 12m x 200um
x 0.25um
Oven Parameters: (connected to the column)
40 C for 1 min
12 C/min to 220 C for 0 mins
30 C/min to 300 C for 17 mins
Detector parameters:
Temperature: 310 C
H2 flow: 45m1/min
Air flow: 450 ml/min
Makeup flow: 26.730 ml/min
MS acquisition parameters:
EM voltage: 1871
Low mass: 10
High mass: 350.00
Threshold: 25
# samples: 3
MS source : 230 C
MS quad: 150 C
CA 02889702 2015-04-27
WO 2014/063852 PCT/EP2013/067734
106
Products and related percentage content relative to the weight of liquid
products were identified
by means of NIST 2008 peak identification software. Only products
corresponding to an area
greater than 1% of the whole spectrum area are reported.
3. Composition of solid products
The filtered solids were dried and then ashed. The burnt portion were
considered unreacted
lignin. The ash portion was considered nickel catalyst.
4. Composition of gas products
The non-condensed gases were identified by gas chromatography.