Note: Descriptions are shown in the official language in which they were submitted.
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
1
PROCESS FOR TREATMENT OF CRUDE OIL, SLUDGES AND EMULSIONS
Field of the Invention:
The present invention relates to processes for treatment of petroleum/crude
sludge,
emulsions and slop oil. More particularly, the present invention relates to a
process
of removal of bound and unbound water from petroleum/crude sludge, emulsions
and slop oil comprising of hydrocarbons, bound water, unbound water, dissolved
and
un-dissolved solids, into different pure salable streams, particularly but not
restricted
to petroleum industry.
Background of the Invention:
Petroleum crude comes out of oil wells invariably with water, dissolved and un-
dissolved solids and sulfur bearing compounds containing partly both bound
water
and unbound water. This petroleum crude is thereafter treated at group
collection
centers (GCCs, hereinafter) of oil companies wherein firstly the petroleum
crude is
de-sulfurized and then unbound water are removed along with un-dissolved
solids.
However, GCCs do not remove bound water and dissolved solids except in cases
where de-emulsifiers are used. Presumably, because desalting of crude leads to
additional formation of emulsion with bound water, crude with bound water is
sent
to oil wells.
GCC is specifically required to remove sulfur with most of the un-dissolved
solids
from the crude and remove entire water to bring down the crude water content
below
5000 ppm before sending it to refineries. The process of removal of water
mainly
involves allowing the crude to settle in a settling tank wherein the top
layer, middle
layer and bottom layer are formed. The top layer contains pure crude that is
sent to
refineries for further treatment. The middle layer contains water bearing
emulsion
that is sent to tank where it is heated subjected to high voltage oscillating
electric
field and optionally with use of de-emulsifiers where the purpose is to remove
maximum water in least time. The bottom layer normally contains oily water
with
un-dissolved solids which is known as slop oil. Being a pollutant, often the
slop oil is
sent to abandoned oil wells for storage through pipe lines.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
2
In refineries, production, transportation, storage and refining of the crude
oil mostly
create sludge. Sludge is generally a tightly held viscous emulsion of oil,
water and
solids wherein the solid content could vary widely. Whenever oil and water is
mixed
and agitated, sludge gets formed. In refineries, sludge is also formed in the
desalting
unit where crude is washed with fresh water to remove Alkalis that had
ingressed
with seawater. Also, sludge gets produced in hydro-crackers, crude storage
tanks,
slop oil, API separators and the like. Normally 1.6 kgs. of sludge is produced
per
tonne of crude. As per a 1992 US-EPA report, by and large petroleum refineries
unavoidably generate about 30,000 tons of oil sludge waste streams per year
per
refinery. More than 80% of this sludge comes under the EPA hazardous waste
nos. .
F037 and F038. In India, more than 2.62 lac tonnes of sludge is produced in a
year.
Sludge also gets formed, when water in crude is vigorously agitated/ sheared
by
transfer pumps. Being heavier than light oils, it tends to settle at the
bottom of ship ,
load, but gets removed from ship, when crude is pumped out at the refinery.
Apart
from that, we have tank sludge, which is a solid layer that accumulates with
time at
ship bottom, and is removed once in 5 years or so. Typically a 60-M tank
disgorges
1,000 MT of material. About 85 to 90% of it constitutes heavy hydrocarbons
like
paraffin, asphalt, micro-crystalline wax, etc. Often this material is removed
using
high pressure water jets. Sludge also gets generated in post refinery
operations.
When heavy liquid fuels like LSHS or furnace oil are used for power generation
through low speed DG sets 0.5 wt % to 1 wt % sludge gets formed. These DG sets
could either be land based or marine. Sludge also gets produced in waste-oil
re-
conditioning plants. Formation of sludge is a great problem in overall world.
For example, Texaco, (acquired by Chevron in 2001) after oil drilling
operations
from 1964 to 1990, seventy billion litres of toxic petroleum sludge pools were
abandoned in Ecuadorean Amazon rainforest without any remedy. This sludge came
from drilling operations per se and not from production. Chevron has a
patented
technology for treatment of sludge as disclosed in US Pat. No.4,689,155.
However,
still the sludge was dumped into streams and rivers that local people depended
for
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
3
drinking, bathing and fishing. It dug over 900 open Air, unlined waste pits
that still
seep toxins into the ground. This sludge contained chemicals like benzene &
polycyclic aromatic hydrocarbons. What's worse is this dumping was done
intentionally to cut corners and save an estimated US $3 per barrel. The
company
saved US $1.32 billion, but it led to 30,000 Ecuadoreans suffering, with 1,400
of
them dying. This could lead to 10,000 more deaths by 2080.
In case of orient region of Ecuadorean rainforest, which once supported 30,000
people, the land itself has become toxic and water system contaminated. Almost
any
kind of food from this region, whether it's fanned, domesticated, caught in
the wild
or in water is unsafe to eat. Local economies and communities have collapsed.
Eighteen years ago locals filed a class-action law-suit. Damages had been
assessed at
US $27.3 billion. Locals own their case and Chevron was asked to pay US $18
billion. Rather than take responsibility and pay up for this environmental
disaster,
Chevron refused to pay and is waging unprecedented public relations and
lobbying
campaigns to avoid having to clean up the mess.
In case of PdVSA, the Public Sector Oil Company in Venezuela. In August 1999,
it
was slapped with US $1.5 billion in environmental liabilities. Amongst other
things,
it was asked to clean up 15,000 oil pits containing contaminated sludge from
oil
wells. This alone cost the Company US $1 billion. Under pressure from courts,
this
Company which had traditionally ignored the environment has now started
cleaning-
up operations & pledges to rank environmental protection as one of its top
most
goals.
In case of Russia, it generates more than 3 million tonnes of Petroleum Sludge
per
year, more than 33% of that coming from oil wells alone. Russian oil & gas
industry
is the 3rd largest contributor to accumulation of industrial wastes in that
country.
Russia has 7,000 abandoned oil wells. It has 416,000 km of oil pipelines that
often
get damaged due to corrosion. Every year it faces 50,000 to 60,000 pipeline
related
accidents, leading to a leak of 15 million tonnes of oil before automatic flow
blocking mechanisms get activated. About 30% of this ends up in rivers and
lakes,
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
4
i.e. 4 to 5 million tonnes a year. In 1994, at Komi alone 0.1 million tonnes
of oil got
spilled from a single pipeline related accident. In 1993 at Tyagan, in Tyumen
region,
a single pipeline related incident led to a spillage of 0.42 million tonnes of
oil. Russia
has lagoons holding millions of tonnes of sludge. Western Siberia has more
than 3
million tonnes of slop. Tatarstan has over 2.5 million tonnes. Bashkortostan
has 700
lagoons with 2 million tonnes of sludge. Land being cheap, storage of sludge
in
lagoons cost US $20 to $40 per tonne depending on location. They burn away
most
off their sludge causing extensive air pollution. Russia has 27 refineries
with total
capacity of 300 million tonnes. At its Saratov Refinery, lagoons cover more
than 150
ha. The content of oil in its ground water is 7.2 grams per litre. US
companies alone
are currently providing sludge disposal service in Russia worth more than US
$90
million per annum. Russia pays between US $28 to $360 to dispose off a tonne
of
sludge, depending on how far the area is from city & also on the kind of
technology
and equipment used.
The cost of a Russian custom-made sludge processing system built with foreign
components start from US $5 million onwards. These are mostly de-emulsifying
units based on settling tanks, centrifiiges & decanters. Oil skimmed from
therein gets
used in barges & pumps. They also use bio-remediation & incinerators. For de-
emulsification combined with bio-remediation they pay between US $ 160 to 200
per
tonne. For innovative technologies like ultra-sound treatment they pay US $300
per
tonne.
Since sludge is difficult to dispose off, till recently refineries were
dumping it in
tanks, ponds & lagoons. Most refineries in developing and under-developed
countries continue to that even till the present date. Typically, such lagoons
are 4 ha.
in size & contain about 1.2 to 1.6 lakh tonnes of sludge. Several of them
contain
sludge since 1896. Sludges in such old lagoons are known as "weathered sludge"
With age, they tend to get homogenized.
In developed countries like the US, fresh storage of sludge in ponds or
lagoons is
prohibited, unless they are lined with non-permeable materials. Even that is
strongly
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
discouraged. That's because surrounding soil & groundwater get adversely
impacted.
Evaporation of volatiles too causes strong odour & air pollution.
In 1980, US Congress enacted the Comprehensive Environmental Response,
5 Compensation & Liability Act. This created a tax on chemical & petroleum
businesses and the money thus collected went into a large trust known as
Superfund.
That money paid for the cleaning up of all hazardous waste disposal and spill
sites,
like the petroleum sludge lagoons. In 1995 the tax on industries expired. But
the
Superfund Programme continued. Today money is appropriated from the general
tax
revenue, to fund it. US-EPA administers this fund in co-operation with
individual
states.
Recently, cleaning-up of sludge ponds & lagoons has emerged as a lucrative
commercial business. Refineries are keen to recover oil from sludge. When
that's not
possible, they are keen to extract its energy. When even that's not possible,
they try
to convert it into innocuous substances, at the least cost. There are various
efforts
seen in the art for cleaning-up of sludge using various techniques.
= Use of de-emulsifiers/ chemicals is seen in the art for breaking of the
sludge. For
example, Chinese patent document CN101786776 to Norman Kevin, Elk Point
discloses deep treatment process wherein the oil-containing sludge is
introduced into
a regulation pool followed by adding of hot water and subsequent stirring
thereof
such that the fluidity of oil-containing sludge is improved. The sludge is
further
treated in cyclone desalter and sent into a modulation tank where a predefined
quantity of demulsifier is added followed by de-emulsification at an
appropriate
temperature. Also, Czechoslovakian patent document CS8702260-A to Baxa J
entitled "Oil dehydration and desalting- by adding distillation slops and de-
emulsifying vacuum distillation" discloses use of de-emulsifiers.
M/s. Smith & Loveless Inc. treats refinery sludge with chemicals and aeration.
M/s.
Lenntech Petrochemical Company from Netherlands uses chemicals, solvent
extraction, membranes, filtration, floatation, flocculation, reverse osmosis,
etc. to
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
6
recover oil. M/s. Reverse Oil, a Ukrainian-American Joint Venture is
desludging
"Ukrtatnafta" sludge ponds since 1996, with a plethora of chemicals, merely to
minimize its adverse environmental impact. However, sludge breaking with
chemicals/ de-emulsifiers doesn't always affect 100% separation. Also, the use
of de-
emulsifiers is unfit for further use within refineries unless the recovered
oil is
predominantly free from water.
Alternatively, a technique of heating the sludge with solvent, preferably with
Azeotropic solvent mixtures, is also seen in the art. For example, German
patent
document DE 19936474 to Bereznikov Anatoli provides separation of oil-
containing
sludges by heating with a solvent and recycling the solvent is effected using
a
solvent (e.g. toluene) forming a heterogeneous azeotropic mixture with the
aqueous
component. The mixture is steadily mixed to give slurry which is then heated
to its
boiling point. The saturated vapour is condensed and the aqueous component and
the
solid residue removed, this being continued to complete water separation by
controlling the temperature increase. Also, Spanish patent document
ES2047129T3
to Richter Gedeon Vegyeszet discloses dehydration process employing Azeotropic
distillation and more particularly it relates to a process for the vigorous
dehydration
of substances or mixtures, primarily condensation reaction mixtures, (e.g.
direct
esterification, direct acetal formation, direct ketal formation) using
continuous
Azeotropic distillation. Further, US Patent document US 3669847A to Dynamit
Nobel Ag discloses process for separating steam-volatile organic solvents from
industrial process waste waters wherein Steam-volatile organic solvents are
removed
from process waste waters by intimately mixing the process waste waters with
steam
to form an azeotropic steam mixture, withdrawing the Azeotropic steam mixture
from the resultant mixture of steam and water, and condensing said Azeotropic
steam
mixture.
Companies like M/s. CEVA International Inc. & M/s. E & I Technologies, Inc.
recover oil by centrifuging sludge. In collaboration with M/s. Petro-Waste
Services,
Inc. (PWS), CEVA offers equipment in 2 sizes. One processes 200 tonnes of
sludge/day, while the other handles 475 tonnes of sludge a day. Some of these
are
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
7
mobile units. Often when sludge resolution is not possible, refineries
incinerate
them. Due to high water content, here burning is often supported with
supplementary
liquid fuels. M/s. W. N. Best makes incineration systems for processing 0.38
to 26.5
tonnes of petroleum sludge/hour. Many modern refineries dump their sludge in
Coker Plants, where fuel is partially recovered. Hence they don't generate
sludge.
Pollution prevention through non-generation is considered to be most
profitable.
They create what's known as Pet Coke. However, the coke oven plants produce
high
sulfur contents.
Bioremediation is however emerging as major trend. Here sludge is uniformly
mixed
with soil, such that its total hydrocarbon content is limited to ¨3 wt. %.
Naturally
= existing bacteria in soil then degrades hydrocarbons into CO2 & H20 over
a period
of few years. To accelerate this, one increases the supply of air, moisture &
nutrients
into the soil. To increase nutrients, one supplies nitrogen & phosphorus based
fertilizers. A certain density & variety of bacteria also helps. With all
these, one tries
to achieve a significant reduction of hydrocarbons in soil within about a
year. This
process is also known as "land farming", since one works sludge into land with
a
view to achieve its final disposal through the slow process of bacterial
action.
Biopiling is a further improvement in this field where homogenous sludge and
soil
mix are placed over an impermeable base of natural clay, along with wood chips
to
improve permeability. Perforated pipes are connected to a blower or vacuum
pumps
to aerate the soil pile. Leachate collection system is also incorporated for
uniform
addition of water and nutrient.
Globally M/s. Biogenie, M/s. Envirosoil Services Ltd. and M/s. Willacy Oil
Services
Ltd. are active in this field. The LTTD process of Envirosoil treats soil with
sludge in
a plant and once hydrocarbon content in soil is reduced below the acceptable
level of
15 ppm, it is transferred to land. Willacy is very active in Middle East and
Turkey.
In India, M/s. Tata Energy Research Institute (TERI) took 7 years to develop
"Oilzapper". That's an efficient bacterial consortium, developed from 5
bacterial
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
8
isolates, immobilized over powdered corncob. It efficiently degrades oil based
hydrocarbons within about a year. This technology know-how has been
transferred
to M/s. Shriram Biotech Ltd., Hyderabad & M/s. Bharat Petroleum Corporation
Ltd.,
Mumbai. Oilzapper has successfully degraded more than 10,000 tonnes of
petroleum
sludge in India over the last 2 years. Globally, bioremediation costs between
$ 73 to
$ 641 per tonne of sludge.
However, even bioremediation technique has certain limitations. Firstly, the
bioremediation process leads to entire loss of valuable hydrocarbon which is
highly
undesired. Secondly, the bioremediation process is highly expense and consumes
a
lot of time in waste disposal process. Also, the product obtained after
remediation
fails to convert waste into wealth as the product obtained after
bioremediation
treatment is of no use.
Another deadliest pollutant is the slop oil which is normally an oily water
containing
solids and salts. This water is treated at Group Collection Centres (GCCs)
prior
sending it to refineries. Slop oil also gets generated in refineries where the
crude is
added with fresh water for desaltation and removed using same equipments as
that of
GCC thereby adding unnecessary cost. Also, lot of hydrocarbon is lost in such
20, process in addition to generation of polluting slop oil.
This water being a pollutant is normally sent back for storage wherein the
stored
corrosive water may leak out in addition to adding cost of transport for
discharging
the corrosive water in sea water through pipelines. Slop oil also has large
implications on environment where it contaminates sea water thereby effecting
marine life. Further, slop oil is a major source which has always been
neglected
although being a valuable source of oil and water both.
For instance, Russia has more than 4,16,000 km pipeline that often gets
damaged due
to corrosion causing 50-60,000 pipeline related accidents thereby leading to
leak of
millions of tons of oil before automatic flow blocking mechanisms get
activated.
About 30% oils ends in rivers and lakes thereby generating slop oil. In 1993,
Tyagan
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
9
in Tyumen region a single pipeline related incident lead to spillage of 0.42
million of
oil. In 1994 at Komi alone, 0.1 million tons of oil got spilt from a single
pipeline
leakage.
Slop oil even comes from cleaning of oil contaminated equipments including
cleaning of oil carrying ships. Even in industries apart from oil industries,
the
industries where oil is used as coolant or for lubrication slop oil gets
generated.
Conventionally centrifuge technique is used for treatment of slop oil. For
example,
German patent document DE4205885 to Meiken, Bernard entitled "Recovery of
water, gasoline, heavy oils, and solids from slop oils or oil emulsions"
discloses use
of two-phase decanter for centrifuging of slop oil/ emulsions wherein Slop oil
is
heated to 105-135 C in a heating circuit formed by a heater, column, and
pump. The
gases and steam are then drawn from the top of the column, and, from the
bottom of
the column, heated oil slops are taken, cooled, and, in a two-phase decanter
separated
into a centrifuged clean oil-phase and a solid phase. Also, Russian patent
document
RU2217476 teaches processes of the oil-bearing slimes refining and extraction
hydrocarbons from them for refining of the liquid and pasty oily slimes, in
particular
of the bottom sediments, resistant oil-water emulsions, intermediate layers
containing a fair quantity of mechanical impurities. The method provides for
dilution
of the oily slimes with petroleum, its heating and separation in the three-
phase
decanter centrifuge for petroleum, water and a concentrate of mechanical
impurities.
Residual water is separated from petroleum with the light oil fractions in the
distillation column. Further, Chinese patent document CN100582031 to China Nat
Petroleum Corp discloses a process for processing and utilizing for oil field
oil-
containing sewage sludge. The invention relates to the process and utilization
method of the oily sludge wherein the horizontal centrifuge via a secondary
lift pump
is used for dehydration. The dehydrated water enters the coming liquid
pipeline of
the sewage disposal system after centrifuge operation.
However, centrifuge technique is not without limitations. There are generally
two
types of centrifuges that are used in tandem, namely a decanter and disc stack
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
centrifuge. The disc stack centrifuge has advantages of higher G but it is
inefficient
when slop oil contains more amount of solids. The decanters enhance density
difference but they fail in case of handling of heavy crude/ extra heavy crude
contaminated water that has oil density equal to water density. Centrifuge
enhances
5 buoyancy but reduces residence time due to which it is effective only
when the
particle size is more and drag is less. Moreover, surface charge of the oil
particles
tends to prevent oil particles to coalesce and come together. Further, the
centrifuge
can handle ultrafine particles only until population density is very large.
However,
when the population density falls below a particular level mean free path
increases so
10 much that coalescence of droplets fails to occur within the residence
time permitted.
The main fact is that that the centrifuge can make separation only when there
is
coalescence. Hence, centrifuge technique substantially fails to work as
intended
when the slop oil contains either ultrafine oil droplets or highly viscous oil
droplets
containing solids and bound water therein.
Alternatively, use of filtration technique is also seen in the art for sludge
treatment.
For example, Canadian patent document CA1202223 to Amsted Industries
Incorporated discloses a deep bed type filter containing gravity separator.
The bed is
agitated and dislodged oil entrapped in filter bed. Where the oil in the water
is
unusually viscous or has a waxy, tarlike, or sticky consistency, for example,
rejuvenation of the filter bed is enhanced by the addition of a small amount
of a
solvating liquid to the oil-water mixture before filtering. Also, GB1340931 to
Beavon D K teaches a treatment method for oil-water mixture containing also
oily
particulate solids which is treated by passing it through a granular filter
medium to
remove the particulate solids wherein the filtrate obtained is being water or
a mixture
of water and oil. The next is to periodically solvate oil from the granular
filter media
by passing an oil stripping media through the bed in the same direction as the
oil-
water mixture without affecting the integrity of the filter medium followed by
backwashing the filter to remove the now oil-free solids. The oil-water
filtrate
obtained may then be separated by gravity settling.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
11
However, filtration technique substantially fails to produce oil free water
without any
chance of total separation of salable quantity of oil when there is large
population of
ultrafine droplets of sub-micron size. Further, the filtration technique is
highly time
consuming considering the pore size ,of the filtration medium. Moreover,
regeneration of filtration medium is a highly tedious and time consuming task.
Optionally, coagulants or flocculants are also used to overcome above
disclosed
disadvantages of centrifuge and/or filtration. However, these
coagulants/flocculants
deteriorate or contaminate quality of oil. Moreover, the addition of
coagulants and
flocculants is a slow process and time consuming. If the oil droplets are held
by
water then neither filtration nor centrifuge will work unless the emulsifiers
are used.
For example, entire fats cannot be removed from milk by filtration or
centrifuge
because fats are hold by proteins which are emulsifier in this case.
Use of air flotation techniques for removal of emulsified oil particles was
seen in the
art. For example, a research paper entitled "The removal of emulsified oil
particles
from water by floatation" to Christine Angelldou et al., Ind. Eng. Chem.
Process
Des. Dev., 1977, 16 (4), pp 436-441, talks about use of air bubbles by air
flotation
technique for recovery of oil particles wherein the floatation of emulsified
oil
particles suspended in low concentrations in water has been studied. Two oils
were
used wherein the oil concentrations were up to 200 mg/L. To effect the
separation
various cationic surfactants were used in the flotation cell which was
operated batch
wise with an external total recycle. It was found that the rate of floatation
in water
was increased with addition of surfactant up to a limit. The presence of sea
salt
reduced the floatation rate. However, air flotation technique is not without
limitations. Firstly, the air floatation is feasible only for the oil
concentrations up to
200 ppm and it can never go beyond said ppm level. Secondly, these techniques
make use of surfactant that highly contaminates the quality of oil and bound
water.
Further, removal of solid and bound water is impossible in the air floatation
technique.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
12
Accordingly, there exists a need of a process for treatment of a petroleum
sludge
that facilitates recovery of usable oil and usable water from the sludge
considering
enormous volumes of the sludge which is generally found as an untreated waste.
Further, there exists a need of a process that removes bound water from the
petroleum sludge apart from the use of de-emulsifiers which may work in rarest
cases. In addition, there exists a need of a process for treatment of slop oil
that
facilitates recovery of usable water from the slop oil considering enormous
volumes
of the slop oil which is generally found as either physically dispersed in the
water or
bound to water through an emulsifier. Further, there exists a need of a
process that
converts waste slop oil into usable water by a cost effective way in addition
to
recovering usable oil therefrom.
Object of the Invention:
An object of the present invention is to remove bound and unbound water from
petroleum/crude sludge and emulsions, comprising of hydrocarbons, bound water,
unbound water, solids and dissolved salts into different pure salable streams.
-
Another object of the present invention is to provide a process for treatment
of
sludge that is cost effective and which facilitates recovery of pure oil and
water as
complete as possible without deteriorating original composition/
characteristics
thereof.
Further object of the present invention is to provide a process for treatment
of slop
oil to recover usable water from slop oil by an effective and economically
viable
process.
=
Yet another object of the present invention is to recover usable hydrocarbons
from
the slop oil by an effective and economically vial* process in addition to
mitigating
the problems of slop oil pollution.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
13
Summary of the invention
In a preferred embodiment of the present invention, a process for treatment of
a
sludge mixture is disclosed wherein the sludge mixture includes hydrocarbons
with
bound water, unbound water, dissolved and un-dissolved solids therein. The
process
for treatment of the sludge mixture comprises a first step of centrifuging the
sludge
mixture in a first centrifuge provided if the sludge mixture splits into
various
components. The first centrifuge being a batch centrifuge forms a viscous
hydrocarbon layer, a slop oil layer and a free flowing hydrocarbon layer. In
next
step, the viscous hydrocarbon layer is desalted in a first desalter followed
by optional
treatment thereof in a heat based low volatiles stripping vessel for removing
vapors
of low boiling liquid hydrocarbons therefrom. In next step, the vapors of low
boiling
liquid hydrocarbons are condensed in a first condenser for obtaining low
boiling
liquid hydrocarbons along with water for use. Optionally, the crude
hydrocarbons
coming from a group collection center are desalted in a second desalter for
obtaining
desalted product crude thereby removing bound water containing hydrocarbon
layer
that is subsequently mixed with the viscous hydrocarbon layer from the first
-
centrifuge. In next step, the free flowing hydrocarbon layer is desalted in a
third
desalter for entire removal of salts therefrom. In next step, the viscous
hydrocarbon
layer is treated in a homogenizer by adding a first predefined amount of
solvent for
forming a volatiles free non-viscous homogenized stream therefrom. In next
step,
BTX and Ash tests of the non-viscous homogenized stream are performed followed
by treatment thereof in an agitator cum homogenizer thereby adding a second
predefined amount of solvent therein in accordance with the BTX and Ash tests
results. In next step, the non-viscous homogenized stream is centrifuged in a
second
centrifuge for separating a bound water dominant hydrocarbon stream, unbound
water dominant or water free hydrocarbon stream and the slop oil therefrom.
Optionally, the non-viscous homogenized stream is treated in a hot insulated
settling
tank for removal of water free solvent along with hydrocarbons therefrom. In
next
step, the unbound water dominant or water free hydrocarbon stream is heated in
a
first heating vessel thereby optionally adding a predefined amount of free
water. The
first heating vessel operates at a first predefined temperature range thereby
forming a
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
14
first residual phase and a first vapor phase. In next step, the bound water
dominant
hydrocarbon stream is heated in a second heating vessel at a second
temperature
range thereby optionally adding a third predefined amount of additional
solvent. The
second heating vessel forms a second residual phase and a second vapor phase.
In
next step, the first residual phase is centrifuged in a hot centrifuge at a
second
predefined temperature for obtaining volatiles free desalted product
hydrocarbons in
a range of about 96 wt% to 100 wt% along with unbound water having turbidity
at
least below 20 NTU. In next step, the second residual phase is treated in the
first
heating vessel. In next step, the first vapor phase and the second vapor phase
are
condensed through a second condenser for obtaining at least 100% solvent, the
bound water in a range of about 99 wt % to 100 wt % and the free water in a
range of
about 94 wt% to 99 wt%. The solvent is reused in said process.
The first centrifuge reduces quantum of the sludge mixture with bound water
that
requires further processing which reduces cost and time of further processing.
The
free flowing hydrocarbon layer is about 41 wt% typically having 3,864 ppm
water
and 0.88 wt. A) ash with calorific value of 10,635 kcal/kg. The viscous
hydrocarbon
layer is having at least 42.21 wt. % water typically having 8.61 wt. % Ash
with CV
of 5,210 kcal/kg. The first centrifuge enhances separation between the
components
present in the sludge by extending a period of residence time of the sludge
thereby
gradually varying revolutions per minute of the batch centrifuge enabling
collection
of slop oil behind the viscous hydrocarbon layer.
The first desalter, the second desalter and the third desalter retain the
quality of
hydrocarbons coming from different process streams and hence improve
commercial
value thereof. The first desalter, the second desalter and the third desalter
prevent
needless repetition of identical processes done in the group collection center
for
removal of bound and unbound water from crude again into refineries after
desalting
of the crude. The first desalter, the second desalter and the third desalter
prevent
ingression of water into various product hydrocarbon streams in refineries
thereby
preventing accumulation of sludge in downstream of said process and vessels
from
refinery onwards processes. The first desalter, the second desalter and the
third
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
desalter allow the group collection center to dispatch crude without salts and
without
having to worry about either disposal or processing of crude containing bound
water.
The first desalter, the second desalter and the third desalter prevent
corrosion of
pipelines and tankers during transportation. The heat based stripping vessel
separates
5 the low volatiles from the viscous hydrocarbon layer for preventing co-
distillation
thereof along with the solvent during removal of bound water with solvent in
downstream of said process. Removal of the bound water from the viscous
hydrocarbon layer also allows removal of heavy metal, Ash and salts therefrom
which =effectively improves commercial value thereof. The BTX and Ash tests
help
10 assists in determination of amount of solvent to be added in said
process.
The solvent reduces viscosity for removal of bound water from topmost layer of
the
non-viscous homogenized stream on account of viscosity. The solvent help
assists in
homogenization of the sludge that in turn helps sampling and further helps in
15 accurate determination of water and Ash content. The solvent is added in
said
process only for viscous portion of the hydrocarbons which substantially
reduces
overall use of solvent. The solvent is selected from the group of Benzene,
Toluene,
Xylene and similar Azeotropes of water. The solvent helps removal of the bound
water from the top most layer and has least possible thermal damage to the
product
hydrocarbon stream in said top most layer. The solvent stream and the second
centrifuge mutually remove substantial bound water from the viscous
hydrocarbon
layer at an ambient temperature. The solvent depresses the boiling point of
the bound
water. The solvent is added in a range of about 1.8 to 100 times the weight of
water
present in the sludge for removal of entire bound water. The solvent has a
left over
weight ratio of solvent to hydrocarbon in a minimum range of 2.00 to 6.00 for
entire
removal of the bound water at least temperature. The bound water obtained is
high
quality usable water that requires minimal treatment for being used as a
drinking
water. The first predefined temperature of the first heating vessel is in a
range of
about 90 C- 105 C. The second heating vessel is a multi effect evaporator
preferably
with thermal vapor recompression to avoid thermal cracking of the product
hydrocarbon stream. The second heating vessel includes a foam breaker and an
entrainment separator adapted to avoid entrainment of hydrocarbons in
condensate.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
16
The first heating vessel includes a foam breaker and an entrainment separator
adapted to avoid entrainment of hydrocarbons in the condensate. The second
heating
vessel maintains a controlled rate of heating with an optimum ratio of
residual
solvent to water for entire removal of bound water from the hydrocarbon. The
first
and second heating vessels are provided with waste heat for reducing cost of
energy
in said process.
TheY hot centrifuge is a hot settling tank that ensures adequate reduction in
viscosity
of hydrocarbons thereby allowing settling of free water present therein over a
period
of time. The hot centrifuge has a temperature in a range of about 80 C to 94
C. The
hot settling tank may be operated under high pressure so that operating
temperature
can be increased to further reduce the viscosity of hydrocarbon that will
facilitate
faster removal of free water without leading to boiling of water.
In an alternative embodiment of the present invention, a process for pre-
treatment of
slop oil is disclosed where the slop oil contains water, solids, salts and
hydrocarbon
content greater than 10,000 PPM with or without bound water. The process for
pre-
treatment of slop oil comprises an initial step of feeding the slop oil in a
first settling
tank for phase separation thereby forming a substantially unbound water-free
hydrocarbon layer with or without salts, a water dominant hydrocarbon layer,
and a
slop oil layer having hydrocarbon content less than 10,000 PPM. In next step,
the
water dominant layer is treated in a second settling tank by adding a
predefined
amount of alum therein. The second settling tank forms a substantially unbound
water-free hydrocarbon layer, a gelatinous oil bearing layer and alum
containing slop
oil having hydrocarbon content less than 10,000 PPM. Optionally, the
gelatinous oil
bearing layer is centrifuged in a third centrifuge by adding a predefined
amount of
solvent. The third centrifuge forms a solvent layer containing Alum along with
solid
coated with hydrocarbons. The solvent layer contains Alum that is being added
to the
first heating vessel in said process. The third centrifuge helps to quickly
separate
solvent cum hydrocarbon layers and gelatinous oil bearing layer from slop oil.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
17
In yet another alternative embodiment of the present invention, a process for
treatment of slop oil is disclosed wherein the slop oil contains water,
solids, salts and
limited hydrocarbon content less than 10,000 PPM with or without bound water.
The
process comprises an initial step of centrifuging the slop oil through a
fourth
centrifuge for obtaining the slop oil with low turbidity by connecting most
oil present
in a thin top layer. In next step, the above slop oil from is treated in a
high speed
shear mixer by adding a solvent to form a mixture followed by centrifuging
thereof:
in a fifth centrifuge for obtaining a water dominant hydrocarbon layer and a
solvent
dominant hydrocarbon layer therefrom. In next step, BTX and Ash tests of the
solvent dominant hydrocarbon layer are conducted for bound water followed by a
heat treatment thereof in a third heating vessel and a fourth heating vessel.
The third
vessel has a predefined amount of solvent added therein. The fourth vessel is
having
a predefined amount of free water added therein. The third heating vessel and
fourth
heating vessel separate a vapor phase from a liquid phase. The vapor phase is
having
entire remaining solvent and free =water therein. The liquid phase is having
hydrocarbons with limited solids, limited salts and alum therein. In next
step, the =
liquid phase is centrifuged through a sixth centrifuge that is operating at a
predefined
temperature for separating a product hydrocarbon layer from a water layer. The
water layer is having limited salts, limited solids and alum therein. In next
step, the
water layer is treated through a first reverse osmosis plant for obtaining
water for use
and a reject stream. In next step, the vapor phase is condensed through a
third
condenser for obtaining water for use and solvent that can be reused in the
high
speed shear mixer. In next step, the water dominant hydrocarbon layer is
heated in a
fifth heating vessel for separating vapors of solvent therefrom followed by
condensing thereof in the third condenser to obtain solvent for reuse and
water for
use. The fifth heating vessel produces a liquid phase that includes remaining
water,
limited hydrocarbons, salts and solids with a substantially low turbidity. In
next step,
the liquid phase is treated in a settling tank followed by addition of a
predefined
amount of alum therein. The settling tank forms a water dominant alum layer
and a
gelatinous oil bearing layer.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
18
In next step, the water dominant alum layer is filtered in a filtration unit.
The
filtration unit separates the water dominant alum layer into a filtrate stream
and a
residual stream. The filtrate stream includes water, alum and salts therein.
The
residual stream includes wet solids with traces of hydrocarbons, salts and
alum. The
filtrate stream is treated in a second reverse osmosis plant for recovering
usable
water therefrom. The filtration unit in accordance with the present invention
brings
down the turbidity value of the slop oil below 1 NTU. Effectiveness of
filtration
depends on pore size of the filtrate media and nature of hydrocarbons present
in the
slop oil.
In next step, the residual stream is mixed with the gelatinous oil bearing
layer
followed by drying thereof in a first hot dryer for obtaining a viscous liquid
containing hydrocarbons, alum, solids and salts. In next step, the viscous
liquid is
agitated in an agitator cum de-oiling unit by adding a predefined solvent
followed by
treatment thereof through a seventh centrifuge thereby adding water therein.
The
seventh centrifuge provides a water layer, a cake layer and a solvent layer,
the water
layer having alum, salts and limited solvent therein. The cake layer is
preferably a
cake of de-oiled solids with solvent, limited salts and limited alum. The
water is
treated in a sixth heating vessel for obtaining vapors of solvent and water
followed
by treatment thereof through a fourth condenser for obtaining solvent for
reuse and
water either for use or for further treatment in said process. In next step,
the solvent
layer is treated in the fourth heating vessel for recovery of solvent. In next
step, the
= cake layer is treated in a second hot dryer for recovery of solvent
through the
condenser. The second hot dryer produces dried de-oiled solids having traces
of alum
and salts therein.
The third heating vessel is a multiple effect evaporator preferably with
thermal vapor
recompression adapted to avoid thermal cracking of the product hydrocarbon.
The
third heating vessel has a temperature in a range of about 70 C- 150 C. The
fourth
heating vessel has a temperature in a range of about 90 C to 105 C. The
fifth
heating vessel has a temperature in a range of about 90 C to 105 C. The
sixth
centrifuge is a hot centrifuge that has a temperature of about 80 C to 94 C.
The
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
19
sixth centrifuge is a hot settling tank that has a temperature of about 80 C
to 94 C.
The hot settling tank may be operated under high pressure so that operating
temperature can be increased to further reduce the viscosity of hydrocarbon
that will
facilitate faster removal of free water without leading to boiling of water.
The sixth
heating vessel is an evaporator. The sixth heating vessel has a temperature in
a range
of about 90 C to 105 C.
The BTX study and Ash study help assists in determination of amount of solvent
to
be added in said process. The solvent is selected from the group of Benzene,
Toluene, Xylene and other azeotropes of water. The first hot dryer has a
temperature
of about 108 C. The second hot dryer has a temperature of about 200 C. The
first
reverse osmosis plant removes alum, salts and solids to produce water of
usable
quality. Addition of alum in the second settling tank neutralizes surface
charge which
facilitates speedy separation of the hydrocarbons through flocculation and
formation
of the gelatinous oil bearing layer. Addition of alum in third settling tank
when the
slop oil is having turbidity below 90 NTU electrically discharge finest
droplets of the
hydrocarbons and flocculate them thereby reducing turbidity by in a range of
90
wt.%- 99 wt.%. Addition of alum is slow process by itself but it can be
speeded up
by applying heat such that effectiveness of alum treatment is dependent on
temperature and time.
The fourth centrifuge is a multi-pass centrifuge that reduces turbidity value
of slop
oil to a limiting value beyond which centrifuge is unable to produce any
further
-value addition because then size variations of dispersed oil droplets become
narrow
and population density of dispersed oil droplets also falls with increase in
mean free
path, residual droplets are electrically charged and density difference is
very small.
The above lacuna for centrifuge gets magnified when starting turbidity value
of the
slop oil is very high. The solvent is added through the high shear mixer when
centrifuge reaches its limiting value. Addition of solvent enhances the
operating
range of centrifuge by bringing in large variation in droplet size and also by
increasing the population density of droplets along with increasing density
difference
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
between oil and water. The centrifuge again reaches a limiting value at that
point the
residual solvent is boiled out with free water in a temperature range of about
90 C
to 99 C.
5 In further alternative embodiment of the present invention, a process for
treatment of
a sludge mixture comprising of a centrifuge is disclosed. The process for
treatment
using only centrifuge comprises a step of centrifuging the sludge containing
hydrocarbons, bound water, salts and solvents in a centrifuge to break the
binding
between hydrocarbons by increasing residence time of the hydrocarbons in the
10 centrifuge thereby forming three different layers, namely a viscous
hydrocarbon
layer with bound water, salts and solids, a free flowing hydrocarbons layer
with
limited salts and solids and a free water with limited solids and salts. The
centrifuge=
repositions the viscous hydrocarbon layer from a back side to a middle side of
the
centrifuge by slowly increasing revolutions per minute thereof and slowly
decreasing
15 an angle between a vertical axis of centrifuge container and a
horizontal plane
thereof by gradually reducing but not allowing it to become 00. The sludge
mixture
has bound water requiring further processing which reduces further processing
cost
and time. The centrifuge gives a large amount of marketable product
hydrocarbons, =
namely free flowing hydrocarbons.
In yet another embodiment of the present invention, a process for treatment of
sludge
mixture with combined effect of centrifuge and solvent is disclosed wherein
the
sludge mixture contains bound water, salts and solids therein. The process for
treatment comprises an initial step of adding of a predefined amount of
solvent in the
sludge mixture followed by mixing thereof to reduce the viscosity of the
sludge
mixture. In next step, the sludge mixture is centrifuged in the centrifuge to
obtain a
large layer of solvent and hydrocarbon, a layer containing hydrocarbons and
bound
water and a free water layer. The centrifuge has an extended residence time
for
getting less of sludge with bound water therein. The large layer of solvent
and
hydrocarbon is treated for recovery of solvent by boiling through free water
in a
temperature range of 90 C to 99 C at an atmospheric pressure. The sludge
mixture
has bound water requiring further processing reduces thereby saving further
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
21
processing cost and time. The centrifuge gives a large amount of marketable
product
hydrocarbons, namely free flowing hydrocarbons.
Brief Description of Drawings
FIG. 1 is a process flow diagram showing production and collection of crude at
a
group collection center;
FIG. 2 is a process flow diagram showing treatment of a sludge mixture of
FIG.1
prior to removal of bound water therefrom;
FIG. 3 is a process flow diagram showing treatment of the sludge mixture of
FIG. 2
for removal of bound water therefrom;
FIG. 4 is a process flow diagram showing treatment of slop oil with
hydrocarbon
content above 10,000 PPM;
FIG. 5 is a process flow diagram showing treatment of the slop oil with
hydrocarbon
content equal to or less than 10,000 PPM;
FIG. 6 is a continued process flow diagram of FIG.5 showing treatment of the
slop
oil with hydrocarbon content equal or below 10,000 PPM;
FIG. 7 shows a graphical representation of Benzene at a rate of 2500 PPM when
mixed with water using high shear mixer for 1 minute;
FIG. 8 shows a graphical representation of Benzene at a rate of 5000 PPM when
mixed with water using high shear mixer for 1 minute;
FIG. 9 shows a graphical representation of Toluene at a rate of 2500 PPM when
mixed with water using high shear mixer for 1 minute;
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
22
FIG. 10 shows a graphical representation of Toluene at a rate of 5000 PPM when
mixed with water using high shear mixer for 1 minute;
FIG. 11 shows a graphical representation of Xylene at a rate of 2500 PPM when
mixed with water using high shear mixer for 1 minute;
FIG. 12 shows a graphical representation of Xylene at a rate of 5000 PPM when
mixed with water using high shear mixer for 1 minute;
FIG. 13 shows a graphical representation of Coconut Oil at a rate of 2500 PPM
when mixed with Water using high shear mixer for 1 minute;
FIG. 14 shows a graphical representation of Coconut Oil at a rate of 5000 PPM
when mixed with water using high shear mixer for 1 minute;
FIG. 15 shows a graphical representation of Coconut Oil at a rate of 2500 PPM
when mixed with water using high shear mixer for 3 minutes;
FIG. 16 shows a graphical representation of Coconut Oil at a rate of 2500 PPM
when mixed with water using high shear mixer for 5 minutes;
FIG. 17 shows a graphical representation of ONGC Oil at a rate of 2500 PPM
when
mixed with water using high shear mixer for 1 minute;
FIG. 18 shows a graphical representation of ONGC Oil at a rate of 5000 PPM
when
mixed with water using high shear mixer for 5 minutes;
FIG. 19 shows a graphical representation of ONGC Oil at a rate of 2500 PPM
when
mixed with water using high shear mixer for 3 minutes;
FIG. 20 shows a graphical representation of ONGC Oil at a rate of 2500 PPM
when
mixed with water using high shear mixer for 5 minutes; and
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
23
FIG. 21 shows a graphical representation of Diesel at a rate of 2500 PPM when
mixed with water using high shear mixer for 5 minutes.
Detailed Description of the Invention:
The invention described herein is explained using specific exemplary details
or better
understanding. However, the invention disclosed can be worked on by a person
skilled in the art without the use of these specific details.
References in the specification to "one embodiment" or " an embodiment" means
that a particular feature, structure, characteristic, or function described in
connection
with the embodiment is included in at least one embodiment of the invention.
The
appearances of the phrase "in one embodiment" in various places in the
specification
are not necessarily all referring to the same embodiment.
References in the specification to "preferred embodiment" means that a
particular
feature, structure, characteristic, or function described in detail thereby
omitting
known constructions and functions for clear description of the present
invention.
In the description and in the claims, the term "Sludge" is defined broadly as
a
mixture of hydrocarbons, bound and unbound water, dissolved and undissolved
solids and naturally occurring emulsifiers. The sludge in accordance with the
present
invention is a sludge that contains total water content is in a range of 2 wt%
to 95
wt%. However, when total water content is in a range of 2 wt% to 61 wt%, the
entire
water in the hydrocarbons is bound water when emulsifiers are not additionally
added. When the water content is above 61% the water is combination of both
bound
water and unbound water. Sludge is deadly pollutant as it contains heavy
metals and
getting rid of is an expensive affair. It can pollute ground, water and even
air through
low volatiles.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
24
In the description and in the claims, the term "Slop oil" is defined broadly
as a
mixture of hydrocarbons, emulsifiers, un-dissolved solids, hydrocarbon coated
un-
dissolved solids and dissolved solids, bound and unbound water. The slop oil
in
accordance with the present invention is having hydrocarbon content in a range
of 5
ppm- 5 lac ppm. These hydrocarbons are not water soluble. Often when oil
content
extends beyond 10,000 PPM, it will reasonably quickly spilt into 3 layers, a
decantable top layer of pure oil with PPM level of water, a significant water
bearing
oil in the middle where separation rate of pure oil is slow and a residual
bottom layer
which is slop oil containing less than 10,000 PPM.
In the description and in the claims, the term "Bound Water" is defined
broadly as
water that does not come out hydrocarbon inspite centrifuging the sludge at
21893
RCF for at least 10 minutes is bound water.
In the description and in the claims, the term "Unbound Water" is defined
broadly as
any water apart from bound water.
In the description and in the claims, the term "Dissolved Solids" is defined
broadly
as the solids that are dissolved in the water that comes out with sludge.
In the description and in the claims, the term "Un-dissolved Solids" is
defined
broadly as the heavy metals including radioactive metals that come out from
oil well
along with crude.
Referring to FIG.1, a process flow chart 100 shows a process undergone by a
petroleum crude 102 after being recovered through a plurality of oil wells 101
followed by processing thereof at a group collection center 104 (GCC,
hereinafter) as
illustrated. The crude 102 preferably contains sulfur, bound water, unbound
water,
salts and solids. However, gases, if any, are removed from the crude 102 at
line
101A before being sent to GCC 104. The GCC 104 includes a desulfurization
plant
106 that separates out sulfur from crude 102 via line 108 thereby forming a
sulfur-
free crude stream 110 containing crude with bound water, unbound water, salts
and
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
solids. The sulfur-free crude stream 110 is fed to a gravity based settling
tank 112.
The gravity based settling tank 112 separates crude into three streams namely
an
upper crude stream 114, a middle crude stream 116 and a lower crude stream
118.
The upper crude stream 114 contains crude with salts, limited solids and
traces of
5 water that follows line-A. The lower crude stream 118 contains water with
salts,
solids and limited crude that follows line-B. It is understood here that lower
crude
stream 118 is slop oil stream having less than 10,000 PPM hydrocarbon content
in
this one preferred embodiment. The middle crude stream 116 contains crude with
salts, bound water, unbound water and solids that is fed to a hot insulated
settling
10 tank 120 through line 119.
The hot insulated settling tank 120 operates at an atmospheric pressure and at
a
temperature at about or less than 95 C. A de-emulsifier 122 is optionally
added to
the hot insulating settling tank120 through line124. In addition, a high
voltage
oscillating electric field 125 is given to the hot insulating settling tank
120 in this one
15 embodiment. The hot insulated settling tank 120 treats the middle crude
stream 116
thereby forming three layers therein, namely a top crude layer 126, a middle
crude
layer 128 and bottom crude layer 130. The top crude layer 126 contains crude
with
salts, limited solids and traces of water that follows line-A. The bottom
crude layer
130 contains water with salts, solids and limited crude that follows line-B.
In this one
20 embodiment, the bottom crude layer 130 is slop oil having less than
10,000 PPM
hydrocarbon content. In this one preferred embodiment, the middle crude layer
128
is preferably sludge in accordance with the preferred embodiment which
contains
crude with bound water, salts, limited unbound water and limited solids.
Accordingly, the sludge 128 follows line-C in this one preferred embodiment.
Referring to FIG. 2, a process 200 for treatment of the sludge 128 before
removal of
bound water therefrom is illustrated. The sludge 128 is fed to a first
centrifuge 202
through the line-C. Additionally, a plurality of sludges 204 from all other
sources
with/ without salts is added to the first centrifuge 202 along with the sludge
128. The
first centrifuge 202 is a batch type or multi-pass centrifuge in this one
preferred
embodiment. The first centrifuge 202 forms three layers, namely a top layer
208, a
middle layer 206 and a bottom layer 210. The bottom layer 210 preferably
contains
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
26
water with salts, solids and limited crude. The middle layer 206 is preferably
a
viscous hydrocarbon layer with bound water, limited solids and traces of
unbound
water with/without salts. The top layer 208 preferably contains free flowing
hydrocarbons with or without salts, limited unbound water and limited solids.
In this
one embodiment, the bottom crude layer 210 is slop oil having less than 10,000
PPM
hydrocarbon content.
The middle layer 206 is preferably fed to a first desalter 212 through line
211 if it
contains salts. A predefined amount of free water is added to the first
desalter 212 in
order to obtain an upper stream 213 and a lower stream 214. The lower stream
214
preferably contains water with salts, solids and limited crude which is mixed
with
bottom layer 210 in this one embodiment. The upper stream 213 preferably
contains
desalted viscous hydrocarbons with bound water, limited unbound water and
limited
solids. The upper stream 213 follows line 213-A in this one embodiment.
Alternatively, the middle layer 206 can be directly fed to a homogenizer 216
through
line 215 if the middle layer 206 is without salts and low volatiles. It is
understood
here that the line 215 may be mixed with the line 213-A before being fed to
the
homogenizer 216.
The top layer 208 is preferably fed to a third desalter 218 through line 217
if it
contains salts. A predefined amount of free water is added to the third
desalter 218 in
order to obtain either two or three layers. The third desalter 218 produces an
upper
layer 220, a bottom layer 222 and optionally a middle layer 224 if it has a
fraction
having bound water contained therein. The upper layer 220 is= a free flowing
salt free
hydrocarbon product with limited solids and traces of water. The bottom layer
222
contains water with salts, solids and limited crude that follows line-B. In
this one
embodiment, the bottom crude layer 222 is slop oil having less than 10,000 PPM
hydrocarbon content. The middle layer 224, if formed, is added to the upper
stream
213 in this one embodiment.
The crude stream 114 containing crude with salts, limited solids and traces of
water
following line-A (refer FIG. 1) is fed to a second desalter 228. The second
desalter
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
27
228 preferably forms three layers, namely a top layer 230, a middle layer 232
and a
bottom layer 234. The bottom layer 234 contains water with salts, solids and
limited
crude that follows line-B. In this one embodiment, the bottom crude layer 234
is slop
oil having less than 10,000 PPM hydrocarbon content. The top layer 230 is a
desalted product crude with traces of solids and water which goes back to
refinery as
a product. The middle layer 232 contains desalted viscous hydrocarbons with
bound
water that is added to the stream 213 and fed to the homogenizer 216.
The homogenizer 216 treats desalted viscous hydrocarbon layer with bound
water,
limited unbound water and limited solids thereby adding a limited solvent
stream
236 in case where the hydrocarbons are highly viscous. The homogenizer 216
advantageously facilitates addition of solvent only after reducing volume of
sludge
and specifically for viscous hydrocarbon portion thereby drastically reducing
overall
= use of solvent in the process. The solvent is also added to the
homogenizer 216 in
order to help assist in BTX study being performed during the process. The
solvent
236 also helps assists in reducing viscosity for removing bound water on
account of
viscosity. In this one preferred embodiment, the solvent 236 is selected from
one or
more of the following Benzene, Toluene and Xylene. The homogenizer 216
produces
a non-viscous homogenized stream 238 that follows line-D as illustrated. The
stream
238 preferably contains hydrocarbons that are volatiles free, desalted and non-
viscous. The hydrocarbons in the non-viscous homogenized stream 238 preferably
contain bound water, limited unbound water and limited solids contained
therein.
Optionally, a heat based low volatiles stripping vessel 240 may be employed if
the
desalted viscous hydrocarbons in the stream 213 contain low boiling volatiles
therein. In such case, the stream 213 is sent to a heat based low volatiles
stripping
vessel 240 via line 242 instead of being sent to homogenizer 216 via line 213-
A.
However, the viscous hydrocarbon layer 206 may be directly fed to the heat
based
low volatiles stripping vessel 240 through line 244 if it is free from salts
but contains
only low volatiles therein. The heat based low volatiles stripping vessel 240
is
adapted in the process 200 to prevent the low volatiles to come out with
solvent by
separation thereof which would otherwise contaminate the solvent and removal
of
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
28
these hydrocarbons later on would need fractional distillation which would be
needlessly a costlier affair. Hence, the heat based low volatiles stripping
vessel 240
is adapted in the process to separate the low volatile hydrocarbons. The heat
based
low volatiles stripping vessel 240 is provided with a waste heat to facilitate
heating.
The heat based low volatiles stripping vessel 240 forms a vapor phase 246 and
a
liquid phase 248. The vapor phase 246 preferably contains vapors of low
volatiles,
hydrocarbons and water. The liquid phase 248 preferably contains volatiles
free,
desalted hot hydrocarbons with bound water, limited unbound water and limited
solids.
The vapor phase 246 is sent to a first condenser 250 for removing heat
therefrom
followed by processing through a first condensate/phase separator 252. The
condensate/phase separator 252 preferably forms a first layer 254, a second
layer 256
and a third layer 258. The first layer 254 contains pure water that can be
reused in
the process or packed for sale. The second layer 256 contains low boiling
liquid
hydrocarbons that are mixed with a desalted product crude 230 through line
260. The
third layer 258 contains non condensable vapors of hydrocarbons that are
flared as a
source of heat via line 262 as illustrated.
The liquid phase 248 is fed to a cooling vessel 264 wherein the hot
hydrocarbons are
cooled to a room temperature and added to the homogenizer 216 via line 266 to
subsequently produce the product stream 238 which follows line-D as
illustrated.
It is understood here that, in case of typical sludge from ONGC lagoons, the
first
centrifuge 202 is able to separate sludge wherein one can find a small
fraction of
viscous hydrocarbons floating on the top carrying about 40-44 wt% bound water
and
13% Ash. The free flowing hydrocarbons about 40 wt% are obtained which
contains
0.3 wt% to 0.8 wt% Ash and less than 3000 ppm of water. The water that goes
out is
having turbidity well below 20 NTU. One cannot add this water back to the
hydrocarbons and make sludge thereby preventing reconstitution.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
29
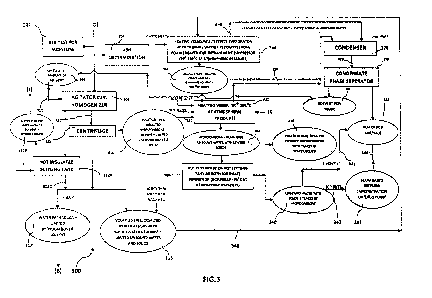
Referring to FIG.3, a process 300 for treatment of the product stream 238 for
removal of bound water is illustrated. The product stream 238 (refer FIG. 2)
is fed to
an agitator cum homogenizer 306 through the line-D after performing a BTX
study
302 and an ash content study 304. The BTX study 302 is performed to detect
moisture content in the product stream 238 and the ash content study 304 is
performed to detect ash content in the product stream 238. A calculated amount
of
solvent 308 is added in the agitator cum homogenizer 306 through line 310. It
is
understood here that the quantum of solvent added has an impact in the
agitator cum
homogenizer 308 in order to bring out the water at least temperature from the
hydrocarbons. In case of Xylene being used as solvent, preferably, ratio of
Xylene to
wt. of hydrocarbon/water (whichever is higher) is 5.5. In case of Toluene
being used
as solvent, preferably, ratio of Toluene to wt. of hydrocarbon/water is 10Ø
In case
of Benzene being used as solvent, preferably, ratio of Benzene to wt. of
hydrocarbon/water is 80Ø
In next step, the contents in the agitator cum homogenizer 306 are fed to a
second
centrifuge 312 through line 311. Optionally, the contents in the agitator cum
= homogenizer 306 are fed to a hot insulating tank 312A that separates out
a water free
top layer 312B containing solvent and hydrocarbons. The water free top layer
312B
follows line-J as shown. The second centrifuge 312 splits the contents in
three layers,
namely a first layer 314, a second layer 316 and a third layer 318. The first
layer 314
is an unbound water dominant hydrocarbon stream that preferably contains
volatiles
free desalted hydrocarbons, solvent, limited unbound water and solids
contained
therein. The second layer 316 is a bound water dominant stream that preferably
contains volatile free desalted hydrocarbons with bound water, solvent,
limited
bound water and solids contained therein. The third layer 318 preferably
contains
water with solids, limited hydrocarbons and solvent that follows line-B. It is
understood here that the contents in the hot insulating tank 312A may be mixed
with
the third layer 318 via line 312C. In this one embodiment, the third layer 318
is slop
oil having less than 10,000 PPM hydrocarbon content.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
It is understood here that in homogenizer 306 one puts hydrocarbons for the
treatment with up to 61% bound water wherein the difference in density between
water and hydrocarbon is in a region of 0.05 gm/cc and containing bound water
which does not come out of the first centrifuge 202 in spite of 21900 RCF for
10
5 minutes. However, after adding solvent 308 followed by reduction in
viscosity in the
second centrifuge 312 the entire bound water comes out in subsequent
processing. It
is understood here that the same hydrocarbon had undergone similar centrifugal
action in first centrifuge 202 where viscosity was reduced still the bound
water that
is recovered here had not come out. This fact of recovery of bound water is a
10 discovery in accordance with the present invention.
The first layer 314 is fed to a first heating vessel 320 through line 322. The
first
heating vessel 320 operates at an atmospheric pressure and a temperature range
of
about 90 C to 105 C, more preferably in a range of about 90 C - 98 C, in
this one
15 preferred embodiment. A predefined amount of free water is added to
the first
heating vessel 320 and waste heat is supplied for heating the first heating
vessel at
the desired temperature in order to produce a first residual phase 324 and a
first
vapor phase 326. In case where hydrocarbons have salt and/or ash or solids
therein,
then free water may perform an additional function of de-salting and de-ashing
apart
20 from boiling out entire pure solvent for re-use or sale at
temperatures below 100 C.
= The first vapor phase 326 preferably contains vapors of entire remaining
solvent and
part of unbound water which is fed to a second condenser 328 where heat is
removed
from the vapors to form a liquid phase that moves to second condensate phase
separator 330 through line 329. The condensate phase separator 330 separates
the
25 liquid phase into a solvent phase 332 and a water phase 334. The
solvent phase 332
is preferably reused in the process. The water phase 334 is pure water having
= turbidity less than 5 NTU which is either recycled in the process or
packed for sale.
The first residual phase 324 preferably contains hydrocarbons and remaining
unbound water with limited solids that is fed to a hot centrifuge/ hot
settling tank
30 336. The hot centrifuge 336 operates at an atmospheric pressure and
preferably at an
inlet temperature less than or equal to 95 C and more preferably at the inlet
temperature of 92 C -93 C. The hot centrifuge 336 preferably separates the
liquid
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
31
stream 324 into two layers, namely a top layer 338 and a bottom layer 340. The
top
layer 338 preferably contains volatiles free desalted hydrocarbon product with
traces
of water/solids having water content less than 5000 ppm. The bottom layer 340
entirely contains unbound water with solids and traces of hydrocarbons. The
bottom
layer 340 is mixed with water phase 334 via line 341 if it has turbidity less
than 5
NTU. Alternatively, the bottom layer 340 is fed to an alum based settling tank
342
via line 343 if the turbidity is greater than 5 NTU. The alum based settling
tank treats
the water to bring the turbidity below 5 NTU followed mixing thereof with
water
phase 334 via line 344. It is understood here that Alum based settling tank
342 may
be a filtration unit or a reverse osmosis plant in other alternative
embodiments of the
present invention.
The second layer 316 is fed to a second heating vessel 346 through line 348
that
operates at an atmospheric pressure and preferably in a temperature ranges of
about
70 C -150 C wherein waste heat is applied for heating purpose. However, it
is
understood that the second heating vessel 346 may be a multi effect evaporator
with
thermal vapor recompression alternative embodiment of the present invention.
Also,
it is understood that the second heating vessel 346 may be a foam breaker and
entrainment suppressor in yet another embodiment of the present invention. A
predefined of solvent may be added to the second layer 316 if required. The
second
heating vessel 346 forms a second vapor phase 350 and a second residual phase
352.
The second vapor phase 350 contains vapors of solvent with entire bound water
and
unbound water which is fed and processed through the second condenser 328 as
per
the treatment process of vapor phase 326 as stated above. The second residual
phase
352 is added the first heating vessel 320 and processed therethrough as
illustrated.
Now referring again to FIGS. 1- 3, in operation, the first centrifuge 202
advantageously allows rapid separation of the sludge into value added layers
at
ambient temperature wherein typical CV of incoming sludge is about 6,044
kcal/kg
with water content about 40 wt. % and ash content about 3.68 wt. %. The first
centrifuge substantially reduces the mass of the sludge to be handled
subsequently by
more than 3 times followed by separating in-coming hydrocarbons into two
fractions
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
32
that commands different market price and in all probability different
subsequent
treatment. Particularly, the first centrifuge 202 operates for 10 minutes at
relative
centrifugal force (RCF, hereinafter) of 4,500 (which requires cycle time of 30
mins.)
to produce about 41 wt.% of the free flowing hydrocarbon layer 220 with 3,864
ppm
water and 0.88 wt.% ash having CV of 10,635 kcal/kg, about 32 wt% of the
viscous
hydrocarbon layer 206 having 42.21 wt.% water and 8.61 wt.% ash with CV of
5,210
kcal/kg, and 26 wt.% of the slop oil after subsequent treatment with Alum
having
less than 20 NTU turbidity.
In operation, the first centrifuge 202 enhances force of buoyancy over
extended time
by gradually increasing the RPM and also by having centrifuge bottles held
onto
rotor through a pivot. The first centrifuge 202 provides an extended residence
time
with enhanced force of buoyancy that allows building up of an adequately large
Kinetic Energy differential between droplets of separating liquids, which
then, on
exceeding a threshold value, provides the energy needed to break the bonds
that were
holding these droplets together. Breaking of bonds was necessary but not
adequate.
Subsequently, these different materials are carried as entirely as possible
through one
another and collect them into distinct, single component layers 206, 208 and
210.
The enhanced or increased residence time or centrifugal force squeezes out
more
water and to a small extent even oil from viscous layer 213 and by doing so
makes it
even further viscous and hence reaching a limiting point beyond which it did
not
make sense to try any further. The Combination of progressively increasing RPM
and of pivots holding centrifuge bottles probably had a couple of additional
impacts.
Initially, a less RPM which is low centrifugal force limits accumulation of
viscous
hydrocarbons that helps in collection of the viscous hydrocarbons as lumps
without
flattening thereof as cakes. Further, a low RPM wherein the force of weight is
larger
than centrifugal force helps collection of the viscous hydrocarbons at the
bottom-
most space within bottles thereby leaving behind ample free space at top. This
helps
initially released weakly bound water to reach the extreme end and then
collect
behind these lumps. The viscous liydrocarbons preferably grow with time as
additional material accreted. Subsequently, more water releases and collects
behind
viscous lumps from top thereby releasing them from the base of the bottle and
then
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
33
slowly moving them towards their final position in the centre. Further, when
RPM
rises, the force of buoyancy increases to swivel out the bottles progressively
by
reducing their angle with horizontal and with that above described process
became
more vivid. Eventually, these bottles become near horizontal but never
completely
horizontal. At the end, there will be some small residual angle with the
horizontal.
Eventually the centrifuge 202 allows the viscous hydrocarbon to get flattened
with
time and high centrifugal force into thick disc shaped layer. But even then
since
these bottles are never truly horizontal this disc has a limited contact with
bottle
surface at its topmost point which provides a relatively easy opening for
water to
penetrate in and collect behind it.
In operation, the three desalters 212, 218, 228 facilitate de-salting of crude
prior to
removing bound water and also prior to dispatching it to refineries. This has
a special
importance in accordance with the present invention. The process 200 includes
placement of desalters 212, 218 and 228 allow crude de-salting at the specific
location within the.proposed process which is different from its current
location. The
desalters 212, 218 and 228 prevent needless, expensive, time-cum-capital
consuming
repetition of crude de-Watering at refineries, after first carrying out
exactly similar
process earlier at GCCs. Besides, the desalters 212, 218 and 228 enhance
product
quality and reduce expense on paid energy, by preventing ingression of water
into
crude stream at refineries. This in turn reduces or eliminates sludge
accumulation in
down-stream product supply chain. The desalters 212, 218 and 228 at our
disclosed
location of the process 200 facilitates mitigation of the problem of bound
water that
gets into Crude while de-salting, without having the advantage of distillation
column.
The desalters 212, 218 and 228 prevent mixing of hydrocarbons having bound
water
with hydrocarbons having unbound water and the product hydrocarbon stream in
comparison to mere de-salting. This also allows preventing mixing of viscous
hydrocarbons with free flowing hydrocarbons. The second desalter 228 has
unique
ability to dispatch de-salted hydrocarbons to refineries without loading them
with
bound water.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
34
In operation, the heat based low volatiles stripping vessel 240 facilitate
stripping in
case of hydrocarbons coming in with bound water and low-boiling volatiles. The
stripping vessel 240 strip these low volatiles and separate them by heating
prior to
removal of bound water using solvents and even prior to addition of solvent
itself in
the process 200 thereby preventing the low boiling volatiles to distill out
with the
solvent during removal of bound water with solvent in downstream of the
process
200 wherein depending on the solvent used the final temperature could rise at
least
as high as 140 C. The stripping vessel 240 also prevents the low volatiles to
enter in
subsequent purification of the solvent which would otherwise become a far more
expensive & elaborate a process. Further, the heat based low volatiles
stripping
vessel 240 prevents low boiling volatiles to distill out with the solvent
during
removal of bound water subsequently with the use of solvent in the homogenizer
306
and the second centrifuge 312 followed by exposure to temperature of at least
as
high as 98 C in the first heating vessel 320. The heat based stripping vessel
facilitates recovery of the low boiling hydrocarbons that can be recycled back
to the
desalted product crude 230 which apart from conservation and economical
advantages help to deliver back the hydrocarbons in as original form as
possible. If a
fraction of low volatiles becomes non-condensable stream 258 due to thermal
cracking then that fraction wduld be either flared or combusted to provide an
additional source of heat.
Moreover, addition of solvent before the second centrifuge 312 is more
important
instead of addition of solvent before the first centrifuge 202. This is partly
because
one would end up consuming more solvent in such case as it will get needlessly
mixed also with the free flowing hydrocarbons. This may lead to an additional
cost
and process for subsequent removal of the solvent from free flowing
hydrocarbons.
Moreover, the removed solvents would get contaminated with low boiling
hydrocarbons in such case. Also, one would end up mixing low valued viscous
hydrocarbons with higher valued free flowing hydrocarbons in such case.
In operation, the second centrifuge 312 treats the solvent bearing sludge
after
removing the clear water with turbidity values from below 20 NTU in certain
cases.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
The second centrifuge 312 removes entire remaining bound water from the sludge
stream fed thereto such as Furnace Oil Sludge, ONGC Viscous Hydrocarbons and
the like. The second centrifuge 312 does not remove the entire bound water
from the
sludge where a part of hydrocarbons holds onto bound water on account of
5 emulsifier. Apart from removing bound water, the second centrifuge also
helps in
reducing ash in hydrocarbons.
However, it is understood here that mere use of the second centrifuge 312
alone and
without solvent could not remove traces of bound water inspite of high
residence
10 time of 10 minutes at an RCF value as high as 21,900 because of high
drag on
account of high viscosity. The use of solvent stream 308 is extremely
important to
reduce viscosity and to make the centrifuge 312 effective when used
subsequently.
On the other hand, solvent by itself is more effective than the centrifuge 312
but still
it fails to remove entire bound water inspite of 72 hours of residence time as
part of
15 the water is tightly held by hydrocarbons. Bound water could not be
separated either
by the use of solvent alone, even when heated to temperatures a little below
their
azeotropic boiling temperature or by the use of centrifuge alone.
Accordingly, combined use of solvent stream 308 with second centrifuge 312 to
separate bound water completely and quickly at ambient temperatures from the
20 viscous sludges is extremely important in accordance with the present
invention. The
process 300 combines the enhanced force of buoyancy due to increased
acceleration
due to gravity and additionally a significant decrease in viscosity of the
sludge by
using the solvent, like Xylene, that is in proportion of two times the weight
of sludge
itself at ambient temperatures and over an extended period of time thereby
affecting
25 complete separation of bound water from viscous hydrocarbons which is
hitherto not
possible either by singly using even 4.87 times more powerful a centrifuge
over
same time alone or by singly using the same solvent in similar proportion even
at
twice the ambient temperature Over 72 hours as against 10 minutes.
Referring to FIG. 4, a process 400 for pretreatment of the slop oil in
accordance with
30 an alternative embodiment of the present invention is shown. The slop
oil stream 402
has hydrocarbon content greater than 10,000 PPM. In this one alternative
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
36
embodiment, the slop oil feed stream 402 preferably contains water with salts,
solids
and limited hydrocarbons with or without bound water. The slop oil feed stream
402
=is sent to a first settling tank or a phase separation column 404 wherein
preferably
three layers are formed, namely a top layer 406, a middle layer 408 and a
bottom
layer 410. The top layer 406 preferably contains free flowing hydrocarbons
with or
without salt along with traces of water and solids. The middle layer 408
preferably
contains hydrocarbons with large amounts of water with or without salts and
solids.
The bottom layer 410 preferably contains water with salts, solids, limited
hydrocarbons which follows line-B. In this one alternative embodiment, the
bottom
layer 410 is slop oil having less than 10,000 PPM hydrocarbon content. The top
layer.
406 is directly stored as a product storage tank 412 through line 411 if it
does not
contain any traces of salts therein. Alternatively, the top layer 406 may be
optionally
fed to third desalter 218 (refer FIG. 2) via line F if it contains salts
therein. The
middle layer 408 is fed to a second settling tank 414 followed by adding a
predefined
amount of alum.
The second settling tank 414 forms a first layer 416, a second layer 418 and a
third
layer 420. The first layer 416 preferably contains free flowing hydrocarbons
with or
without salts and traces of water and solids which is mixed with the top layer
406 in
this one embodiment. The second layer 418 mainly contains alum with water
having
salts, solids, limited hydrocarbons. The second layer 418 is mixed with the
bottom
layer 410 to follow line-B as illustrated. The third layer 420 is a gelatinous
oil
bearing layer containing hydrocarbons, alum, salts, solids and water contained
therein. The third layer 420 follows line-H. It is understood here that
addition of
alum in the second settling tank 414 facilitates speedy separation of the
hydrocarbon
through coagulation and formation of the gelatinous oil bearing layer.
The first settling tank 404 may occasionally produce a fraction 422 which may
contain viscous hydrocarbons with or without salts/ solids/ bound water. The
fraction
422 may be optionally fed to a first desalter 212 via line- I if it contains
salts and
bound water both. The fraction 422 may be optionally fed to second desalter
228 by
mixing with line-A if it contains salts without any bound water therein. The
fraction
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
37
422 may be optionally mixed with an upper stream 213 via line-E if it contains
only
bound water without any salts therein. The fraction 422 may be optionally sent
to a
third centrifuge 424 via line 423 if it contains only solids without any salts
and
bound water therein. A predefined amount of solvent is added to the third
centrifuge
424 in order to separate the fraction 422 into two layers, namely a top layer
426 and
a bottom layer 428. The third centrifuge 424 reduces drag, surface charge on
the
particles of hydrocarbon thereby reducing mean free path and allowing
coalescence
of the particles at ambient temperature. The top layer 426 preferably contains
hydrocarbons and solvent with traces of solids which is sent to the first
heating
vessel 320 via line- J. The bottom layer 428 preferably contains solids that
are coated
with hydrocarbons which follows line-K in this one embodiment. However, the
bottom layer 422 may be directly stored as a product 430 via line 429 if it is
free
from salts, solids and bound water.
Referring to FIGS. 5-6, a process 500 for treatment of the slop oil following
line-B in
accordance with the present invention is shown. The slop oil stream 502
preferably
has a high turbidity and hydrocarbon content less than 10,000 PPM. In this one
embodiment, the slop oil stream 502 preferably contains water with salts,
solids and
limited hydrocarbons with or without bound water. The slop oil feed stream 502
is
fed to a fourth centrifuge 504 to reduce turbidity and obtain a stream 506
having low
turbidity. The fourth centrifuge 504 is a multipass centrifuge that works on
its own as
long as population density of ultrafine particles of hydrocarbons is high
because then
mean free path is low. Because, ultrafine particles can be removed only after
they
coalesce and for coalescing there has to be relative movement between
particles
.This comes only due to relative particle size distribution. This distribution
is very
narrow in the zone of high density of small particles. It is understood here
that the
multipass centrifuge 504 must begin with fresh slop oil. Also, it is
understood that
the gap between slop oil generation and operation of centrifuge 504 should be
as
minimum as possible. Further, it is understood that the fourth centrifuge 504
uses the
relative motion brought by high G till such time that mean free path between
the
hydrocarbon particles is increased beyond maximum capacity thereof.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
38
The stream 506 having low turbidity is fed to a high speed shear mixer 508
wherein
a predefined amount of solvent is added via line 510 thereby forming a mixture
512
that is fed to a fifth centrifuge 514. Addition of solvent followed by high
shear
mixing, in a range of about 8000-10000 RPM, allows formation of the adequate
size
solvent particles, preferably in a range of about 0.5 to 0.8 micron size whose
population density increases by a substantial amount. It is understood that
for
adequate disintegration of solvent there is an optimum mixing time that is
about 1
mm. Further increase in time may result in increase in particle size and fall
in
turbidity. The right particle size of solvent preferably removes almost
similar size of
ultra fine oil particles. Thereafter the coalescence speed increases which
prove to be
a rate controlling step in accordance with the present invention wherein
effect of
coalescence extends in the working range of the fifth centrifuge 514.
Thereafter, the
centrifuge 514 starts working due to high population density and continues
till the
population density falls down to an earlier level. This effectively allows the
oil
particles to completely go out. Moreover, addition of solvent facilitates
coalescence
that enhances the efficiency of the centrifuge 514 by having enhanced sweeping
effect wherein a limiting factor for centrifuge 514 about population density
of ultra
fine droplets is reached with solvent droplets instead of oil droplets.
Addition of solvent in the high speed shear mixer 508 enhances population
density
within the slop oil that makes the fifth centrifuge 514 to efficiently allow
the solvent=
to facilitate coagulation thereby moving the hydrocarbon particles to move
from
bottom and separate with a swiping impact. Addition of solvent in large amount
in
the high speed shear mixer 508 allows replacement of hydrocarbon droplet with
solvent droplet for replacing oil with solvent therein.
The fifth centrifuge 514 preferably forms two layers, namely a top layer 516
and a
bottom layer 518. The top layer 516 is a solvent dominant hydrocarbon layer
that
preferably contains a top layer comprising solvent, hydrocarbons with or
without
bound water, limited free water, limited salts and limited solids. The bottom
layer
518 is a water dominant hydrocarbon layer that preferably contains water,
limited
solvent, limited hydrocarbons, salts, solids with very high turbidity value.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
39
The top layer 516 is subjected to a BTX study 520 to know water and ash
content for
deciding requirement of solvent, if needed. The top layer 516 is added to a
third
heating vessel 522 via line 524 if the top layer 516 contains hydrocarbons
having
bound water contained therein. Alternatively, the top layer 516 is added to a
fourth
heating vessel 526 through line 528 if the top layer 516 contains hydrocarbons
having no bound water contained therein. The third heating vessel 522 may be a
multi effect evaporator with thermal vapor recompression, foam breaker and
entrainment suppressor in other alternative embodiments of the present
invention.
The third heating vessel 522 preferably operates at an atmospheric pressure
and in a
temperature range of about 70 C- 150 C in this one embodiment. A predefined
amount of additional solvent may be added to the third heating vessel 522
based on
the BTX study 520. A predefined amount of waste heat is applied to the third
heating
vessel for increasing the temperature of the third heating vessel 522 and
forming two
phases, namely a vapor phase 530 and a liquid phase 532. The liquid phase 532
preferably contains hydrocarbons, remaining solvent, limited solids and
limited salts
therein. The vapor phase 530 contains vapors having a part of solvent, entire
bound
water and free water therein. The vapor phase 530 is fed to a condenser 536
through
line 538. The condenser 536 removes heat from the vapor phase 530 followed by
processing through a condensate/phase separator 540. The condensate/phase
separator 540 preferably forms a first layer 542 and a second layer 544. The
first
layer 542 contains pure water that can be reused in the process or packed for
sale.
The second layer 544 contains solvent that is reused in the process by mixing
with
the solvent line 510. The liquid phase 532 is free from bound water which is
subsequently added to the fourth heating vessel 526 through line 534. The
fourth
heating vessel 526 operates at an atmospheric pressure and in a temperature
range of
about 90 C to 105 C. A predefined amount of a solvent stream-G (refer FIG. 6)
may
be added to the fourth heating vessel 526 as illustrated. The heating vessel
526
produces a vapor phase 546 and a liquid phase 548. The vapor phase 546
contains
entire remaining solvent and a part of free water. The liquid phase 548
contains
hydrocarbons, remaining free water, limited solids, limited salts and alum.
The vapor
phase 546 is added to the condenser 536 via line 550. The liquid phase 548 is
fed to a
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
sixth centrifuge 552. The sixth centrifuge 552 is a hot centrifuge or hot
settling tank
in this one embodiment that operates at an atmospheric pressure and at a
temperature
equal to or less than 95 C. The sixth centrifuge 552 preferably produces two
layers,
namely a top layer 554 and a bottom layer 556. The top layer 554 is a
hydrocarbon
5 product
having traces of water, salts and solids therein. The top layer 554 is stored
or
packed for sale. The bottom layer 556 contains water, limited salts, limited
solids
and alum. The bottom layer 556 is processed through a RO plant 558 to obtain a
pure
water stream 560 and a reject stream 562. The pure water stream 560 is mixed
with
the first layer 542. The reject strearn 562 follows line-H in this one
embodiment.
The bottom layer 518 is fed to a fifth heating vessel 564 that operates at an
atmospheric pressure and in a temperature range of about 90 C to 105 C. The
fifth
heating vessel 564 is supplied with waste heat to achieve the desired
temperature
range. The fifth heating vessel 564 produces a vapor phase 566 and a liquid
phase
568. The vapor phase 566 preferably contains vapors of solvent and part of
water
that is further processed through the condenser 536 as illustrated. The liquid
phase
568 preferably contains remaining water, limited hydrocarbons, salts and
solids. The
liquid phase 568 has substantially low turbidity which follows line-E as
illustrated.
As shown in FIG. 6, the liquid phase 568 following line-E is fed to a third
settling
tank 602 wherein a predefined amount of alum stream 604 is added. The alum
stream
604 is preferably added to reduce the turbidity of the liquid phase 568 and
bring it
down below 2.0 NTU. The third settling tank 602 may be optionally provided
with
heat to facilitate alum treatment in hot condition. Addition of Alum under
heated
condition at a temperature in a range of about 80 C to 90 C for at least
four hours
may reduce the turbidity of the slop oil by at least 90%. Addition of the alum
stream
604 is more effective under heating that allows wider distribution pattern of
droplets
that firstly allows the oil particles to attach with each other and form a
gel. It is
understood here that efficacy of addition of alum stream 604 is not limited by
availability of ions as is a kinetics related problem. The settling tank 602
preferably
forms two layers, namely a top layer 606 and a bottom layer 608. The top layer
606
is a water dominant alum layer that preferably contains water, alum, solids,
salts and
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
41
traces of hydrocarbons contained therein. The bottom layer 608 is preferably a
gelatinous oil bearing layer containing hydrocarbons, alum, water, solids and
salts
therein. However, it is understood here that the scum may be collected either
at top
or both at the top and bottom depending upon the ppm level in other
alternative
embodiments of the present invention. The top layer 606 is sent to a
filtration unit
610 that splits the top layer 606 into a filtrate stream 612 and a residual
stream 614.
The residual stream 614 preferably contains solids with traces of
hydrocarbons, salts
and alum. The residual stream 614 is mixed with the bottom layer 608 through
line
616. The filtrate stream 612 preferably contains water, alum and salts. The
filtrate
stream 612 is sent to a RO plant 618 through line 617 for obtaining a pure
water
stream 620 if total dissolved solids (TDS, hereinafter) of the filtrate stream
612 is
high else directly stored or packed for sale via stream622 if the TDS is low.
It is '
understood here that addition of alum in third settling tank 602 improves rate
of
filtration in the filtration unit 610 thereby substantially reducing turbidity
below 2
NTU. The bottom layer= 608 is mixed with the third layer 420 (refer FIG. 4)
= following line-H and sent to a first hot dryer 624. The first hot dryer
624 preferably
operates at an atmospheric pressure and .a temperature of about 108 C which
boils
out the water in form of a water vapor stream 626 thereby retaining a viscous
liquid
stream 628 containing hydrocarbons, alum, solids and salts. The viscous liquid
stream 628 is fed to an agitator/ de-oiling unit 630. A predefined amount of
solvent
stream 631 is added to the agitator/de-oiling unit 630 along with the bottom
layer
428 (refer FIG. 4) following line-K and containing solids that are coated with
hydrocarbons. The agitator/ de-oiling unit 630 produces free flowing liquid
stream
632 that preferably contains solvent with hydrocarbons, alum, salts and de-
oiled
solids. The free flowing liquid stream 632 is sent to a seyenth centrifuge 634
through
line 633. It is understood however that the seventh centrifuge 634 may be
phase
separator in other alternative embodiments of the present invention. The
seventh
centrifuge 634 preferably produces three layers, namely a first layer 636, a
second
layer 638 and a third layer 640. The first layer 636 preferably contains
solvent and
hydrocarbons which is added to the heating vessel 526 (as shown in FIG. 5)
through
line-G. The second layer 638 is a water dominant alum layer that preferably
contains
water with alum and salts along with limited solvent. The second layer 638 is
fed to a
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
42
sixth heating vessel 642 which= operates at an atmospheric pressure and in a
temperature range of about 90 C to 105 C. The sixth heating vessel 642
produces
two phases namely, a vapor phase 643 and a liquid phase 644. The liquid phase
644
preferably contains water, alum and salts contained therein. The liquid phase
644 is
recycled to the RO plant 618 via recycle line 646. The vapor phase 643
preferably
contains vapors of solvent and water. The vapor phase 643 is sent to a
condenser 648
for removing heat followed by processing through a condensate/phase separator
650.
The condensate/phase separator 650 preferably forms a first layer 652 and a
second
layer 654. The first layer 652 contains pure water that can be reused in the
process or
packed for sale. The second layer 654 contains solvent that can be reused in
the
process. The third layer 640 preferably contains cake of wet de-oiled solids
with
solvent, limited salts and limited alum. The third layer 640 is sent to a
second hot
drier 656 that operates at an atmospheric pressure and temperature of about
200 C. A
predefined amount of waste heat is applied to the dryer 656 to achieve desired
temperature. The second hot dryer 656 treats the third layer 640 thereby
removing a
vapor stream 658 thereby forming a residual stream 660. The vapor stream 658
preferably contains vapors of solvent and water contained therein. The
residual
stream 660 preferably contains dried de-oiled solids with traces of alum and
salts.
The vapor stream 658 is mixed with the vapor phase 643 and further treated
through
the condenser 648 as illustrated.
Referring now to FIGS. 4-6, in operation, the processes 400 and 500
advantageously
convert pollutants into valuable product streams thereby mitigating problems
of
environmental pollution and damage to environment. In addition, the processes
400
and 500 facilitate best possible recovery of oil and valuable water wherein
the water
can be used as a drinking water for commercial use at a cost less than the
cost of
storage of sludge/ slop oil. The processes 400 and 500 facilitate use of
chemicals for
recovery of oils such that the chemicals used are totally recycled and reused
in the
process. Further, the process of the present invention runs at almost nil
energy cost
by making use of waste heat in overall process.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
43
EXAMPLES
The following examples and comparative examples are provided to demonstrate
particular embodiments of the present invention. It should be appreciated by
those
skill in the art that the methods disclosed in the examples and comparative
examples
that follow merely represent exemplary embodiments of the present invention.
Those
of skill in the art should, in light of the present disclosure, appreciate
that many
changes can be made in the specific embodiments described and still obtain a
like or
similar result without departing from the spirit and scope of the present
invention.
EXAMPLE -1
TABLE 1.1- DESCRIPTION OF OILS USED IN LAB
SI. FURNA
DESCRIPTION
CE OIL DIESEL
No.
1 Wt. % Water in Hydrocarbons as per BTX 0.21 0.01
2 Calorific Value of Oil (kcal/kg) 10,173 11,002
3 Wt. % Ash in Oil 0.23 0.00
TABLE 1.2- PREPARATION OF FURNACE OIL SLUDGE
CV OF
Wt.%
SLUD
Wt.% GE OIL IN
Wt. Wt.% BOUN Wt.%
TURBIDI WT.%
Slud % SLUDG D SLOP
TY OF LOSS
WAT WITH SLOP
SS E WATE OIL
SLOP OF
ge
ER BOUN OIL IN
No. USE FORM R IN FORM OIL MATE
USED PPM
D ED SLUD ED (NTU) RIAL
WATE
GE
1 2.02 0.00 100.00 2.15 9,960 0.00 0.00 0.00 0.00
2 10.01 0.00 100.00 9.91 - 9,148 0.00 0.00
0.00 0.00
3 15.08 0.00 100.00 14.84 8,640 0.00 0.00 0.00 0.00
4 35.00 0.00 100.00 34.65 6,590 0.00 0.00 0.00 0.00
5 47.52 2.51 63.11 19.02 8,134 32.92 464 959 3.97
6 48.53 2.44 57.90 18.92 8,142 38.59 16,740 >10,000 3.50
7 50.01 0.00 100.00 49.94 5,146 0.00 0.00 0.00 0.00
8 60.79 0.00 100.00 59.48 4,109 0.00 0.00 0.00 0.00
9 69.97 0.00 52.90 43.84 5,798 40.31 2,144 2,535 6.79
10 82.60 0.00 31.50 44.77 5,610 67.95 930 1,080 0.55
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
44
11 96.44 1 0.00 5.92 39.78 1 6,130 93.52 1 2,110
2,583 0.57
TABLE 1.3- PREPARATION OF DIESEL SLUDGE
Wt.% CV OF Wt.% PPM
O. T %
F
Wt.% Wt.% Wt.% BOUND SLUDGE SLOP OIL TURBIDITY W
SI.
WATER SLS SLUDGE WATER WITH OIL OF SLOP
LOSS OF
No. IN MATERIA
USED USED FORMED IN BOUND FOR OIL (NTU)
SLOP
SLUDGE WATER MED
OIL
1 50.00 0.00 0.00 5,00,0
100.00 > 10,000 0.00
00
2 48.85 2.43 45.18 6.44 10,160 49 1,43,6
.79 > 10,000 5.02
56
PREPARATION OF SLUDGES /EMULSIONS FOR SUBSEQUENT REMOVAL
OF BOUND AND FREE WATER
A predefined amount of in-house Sludge was prepared with water using
Viscous/Non-Viscous Hydrocarbons in order to understand sludges and also for
subsequent removal of entire Bound Water from therein. Accordingly, weighed
amounts of Water, Hydrocarbons and Sodium Lauryl Sulphate as emulsifier, if
any,
are mixed and then stirred at 10,000 rpm using a high shear Mixer, for 1
minute at a
time and for 5 times, while ensuring that temperature of mixed material never
exceeded 58 C. After every 1 minute of mixing the material was cooled to near
ambient temperature. Subsequently, a representative sample was subjected to 10
minutes of continuous centrifuging at 21,893 relative centrifugal force (RCF),
in a
batch type centrifuge, to find out if any water separated. If yes, then it was
considered as slop oil. The remaining material was considered as sludge with
bound
water. Different types of sludges were prepared as shown in the following
table 1.2
and table 1.3.
It was understood that the sludge with bound water mean from wherein no free
water
visibly emerges out even on batch centrifuging it at RCF of 21,893 with
residence
time of 10 minutes at peak RCF. The sludges were made without using external
emulsifier like Sodium Lauryl Sulphate (SLS) with viscous Furnace Oil but not
with
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
free flowing diesel. It, was observed that, drag on account of viscosity was
an
important reason for hydrocarbons to tightly hold onto fine droplets of water.
It was
observed that for diesel, use of Sodium Lauryl Sulphate was necessary. Even
then, as
can be seen from Table # 3, only 5.96 wt.% of total water present could be
bound to
5 diesel and
82.64 wt.% of diesel could be bound to water, thereby forming 45.18
wt.% Sludge, when using 2.43 wt.% SLS. It was also observed that sludges could
be
prepared using SLS, both with Furnace Oil and Diesel. However, with same
amount
of water, the quantum of diesel sludge with bound water was nearly half of
that one
got with furnace oil. It was further observed that water holding ability of
Furnace Oil
10 in sludge
was deteriorated sharply with the presence of SLS. This was because with
the use of SLS, sludge became far less viscous. This also showed that binding
between hydrocarbons and water on accounts of viscosity and the use of SLS did
not
add up. In other words, their contributions towards binding the two were not
additive. Further, it was observed that with use of SLS, the quantum of sludge
with
15 bound water
dropped down. This was because now only a quarter of water was
present that participated in binding with furnace oil. However, it was
observed that
the strength of binding was a lot stronger than what would have been possible
in the
absence of SLS. It was observed from Table 1.2 that, in Sludge No. 5, SLS was
added to the water prior to production of sludge. While in Sludge No. 6, SLS
was
20 added to a
sludge that had already been prepared with 49.75 wt. % water. From this
Table it was observed that the binding between water and hydrocarbon was
slightly
stronger when SLS was, uniformly dissolved in water prior to production of the
sludge. It was observed that there was an upper limit on how much bound water
can
be held onto by Furnace Oil on account of its viscosity wherein the furnace
oil
25 cannot be
made to hold the entire water as bound water through vigorous mixing
beyond about 1.5 times of its own weight of water as can be clearly seen in
table-2.
Further, it was observed that furnace oil can hold water as bound water only
up to 67
to 82% of its own weight beyond a threshold value. Also, the quantum of sludge
formed with bound water was found to be decreased sharply with increasing
water
30 content. It
was observed that the remaining water stayed as free water with traces of
hydrocarbons in it. That was called slop oil. Though was difficult to produce
fresh
Sludge with more than 60-62 wt. % bound water in it, using furnace oil,
through
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
46
mixing, but then one can always retain the Furnace Oil Sludge with more than
80 wt.
% bound water in it, by removing a part of Furnace oil from an already
prepared
sludge, using a solvent with centrifuge, as can be seen later in Samples 2 to
4 in later
Table 4.3. Finally, it was observed from the calorific values of sludges
deteriorate in
proportion with water content therein.
EXAMPLE-2
PRE-TREATMENT OF INCOMING SLUDGES/EMULSIONS WITH
CENTRIFUGE
It was an aim to determine extent of bound water present in an incoming sludge
thereby splitting it into sludge with bound water and slop oil through
Centrifuge.
Also, it was an aim to establish that with a batch Centrifuge alone it is
possible to
recover value added, marketable hydrocarbons; remove a part of water present
and
also reduce the quantum of Sludge that would require further treatment.
Accordingly,
the Sludges prepared in house and also Lagoon Sludge procured from Oil and
Natural Gas Corporation (ONGC) of India were treated in a Batch Type
Centrifuge
at RCF of 4,500 and 21,893 with varying residence time thereby separating and
weighing the fractions thereafter followed by doing mass balance for the
material
centrifuged. Further, these fractions were evaluated for their moisture
content by a
BTX Process followed by ash content by heating in a muffle furnace and
subsequently for calorific values using bomb calorimeter and turbidity of Free
Water
that came out, with Hach Turbidity Meter. The constituents in the prepared
sludge
and the conditions under which these Sludges were centrifuged are mentioned in
accordance with below mentioned tables 2.1- 2.8.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
47
TABLE 2.1 - CONSTITUENTS IN FURNACE OIL SLUDGE SAMPLES
Sludge Sludge Sludge Sludge
Sludge with
SI. with 47.5 with 50 with 70 with 83
DESCRIPTION 96
Wt. %
No. Wt. % Wt. % Wt. A Wt. %
Water
Water Water Water Water
Wt. of Water taken
1 in High Shear mixer 355.43 500.06 350.10 419.35
1,431.82
in (g)
Wt. of Sodium
Lauryl Sulphate
2 18.75 0.00 0.00 0.00 0.00
added as Emulsifier
in above Water (g)
Wt. of Furnace Oil
3 added to above 374.09 500.13 150.27 88.33 52.91
Water (g)
Total Wt. of Sludge
4 748.27 1,000.19 500.37 507.68 1,484.73
Sample (g)
Wt.% Water in
above Sludge
46.85 49.46 69.55 82.02 95.79
Sample as
determined by BTX
Calorific Value in
6 kcal/kg 5,260 5,148 3,135 1,736 320
TABLE 2.2- CENTRIFUGING DETAILS
Sludge Sludge Sludge Sludge Sludge
SI. DESCRIPTION with 47.5 with 50 with 70 with 83 with 96
No.
Wt. A Wt. % Wt. % Wt. % Wt. %
Water Water Water Water Water
Time taken in mints to Reach
1 Max. Relative Centrifugal 2.60 2.55 2.95 2.80
2.68
Force
Max. Relative Centrifugal
2 Force at the Centrifuge was 21,893 21,893 21,893
21,893 21,893
operated
Holding Time at Max. Relative
3 10 10 10 10 10
____ Centrifugal Force in minutes
Time taken to come back to
4 zero Relative Centrifugal 16.55 16.80 16.50 16.50
16.50
Force
Total Residence Time in mm.
inside centrifuge 29.15 29.35 29.45 29.30
29.18
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
48
TABLE 2.3- RESULTS OF PRE-TREATMENT OF FURNACE OIL SLUDGE SAMPLES IN
CENTRIFUGE
Sludge Sludge Sludge Sludge Sludge
SI. DESCRIPTION
with 47.5 with 50 with 70 with 83 with 96
No. Wt. % Wt. % Wt. % Wt. % Wt. %
Water Water Water Water Water
Wt. % Sludge recovered
1 63.11 97.82 52.90 31.50 5.92 =
having Bound Water therein
Wt.% Bound Water in above
2 Sludge as determined by .19.02 . 49.51 43.84 44.77
39.78
BTX
Calorific Value of Sludge
3 8,134 5,146 5,798 5,610
6,130
____ with Bound Water (kcal/kg)
Wt. % Slop Oil that
4 Separated Out from above 32.92 0.00 40.31 67.95
93.52
Prepared Sludge
Oil Content in Slop Oil
464.00 2,144 930 2,110
(PPM)
6 Turbidity of Slop Oil (NTU) 959 2,535 1,080
2,583
Wt.% Oil IW through
7 0.43 0.83 0.23 0.00 0.00
adhering to Various Surfaces
Wt.% Water lost through
8 Evaporation and Wetting of 3.54 1.35 6.56 0.55 0.56
Surfaces
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
49
Diesel Based Sludges:
TABLE 2.4- CONSTITUENTS IN DIESEL SLUDGE SAMPLES
SI. No. DESCRIPTION Test 1 Test 2 Test 3
Wt. of Water taken in High Shear mixer
302.11 301.84 300.11
in (g)
2
Wt. of Sodium Lauryl Sulphate added
15.00 15.00
as Emulsifier in above Water (g)
3 Wt. of Diesel added to above Water (g) 302.84 _ 301.09 _ 300.21
4 Wt. of Prepared Sludge (g) 619.95 617.93 600.32
Wt.% Water in above Prepared Sludge
48.38 48.31 49.13
as determined by BTX
5,503
6 Calorific Value (kcal/kg) 5,550, 5,518
TABLE 2.5- CENTRIFUGING DETAILS
SI. No. DESCRIPTION Test 1 Test 2 Test 3
Time taken in mints to Reach Max.
1 2.92 2.50 2.50
Relative Centrifugal Force
Max. Relative Centrifugal Force at the
3,502 21,893 21,893
2
Centrifuge was operated
Holding Time at Max. Relative
3 1.00 13.00 10.00
Centrifugal Force in mints _
Time taken to come back to zero
4 2.37 3.50 3.50
Relative Centrifugal Force
Total Residence Time in mints inside
5 6.29 19.00 16.00
, centrifuge
TABLE 2.6- RESULTS OF PRE-TREATMENT OF DIESEL SLUDGE SAMPLES IN
CENTRIFUGE
SI. No. DESCRIPTION Test 1 Test 2 Test 3
Wt. % Sludge recovered having Bound
1 65.87 45.18 0.00
Water therein
2 Wt.% Bound Water in above Sludge as
33.65 6.44
determined by BTX
Calorific Value of Sludge with Bound
3 7,185 10,160
Water (kcal/kg)
Wt. % Slop Oil that Separated Out
4 28.01 49.79 46.12
from above Prepared Sludge
5 Oil Content in Slop Oil (ppm) 2,02,883 1,43,656 37
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
Wt.% Oil lost through adhering to
6 1.36 0.81 2.87
Various Surfaces
Wt.% Water lost through Evaporation
7 4.76 4.21 2.86
and Wetting of Surfaces
Incoming ONGC lagoon sludge:
TABLE 2.7- CENTRIFUGING DETAILS
SI. No. DESCRIPTION TEST 1
TEST 2 TEST 3
1 Wt. of Sludge taken for treatment (g) 700.08 700.91
2,115.91
Wt. % Water in above Sludge as
2 40.28 39.97 40.95
determined by BTX
3 Wt.% Ash Content in above Oil 3.68 3.68 3.70 _
Calorific Value of above Sludge
4 6,018 6,038 5,945
(kcal/kg)
Time taken in mints to Reach Max.
5 2.80 2.75 2.67
_________ Relative Centrifugal Force
Max. Relative Centrifugal Force at the
6 4,500 4,500 21,893
Centrifuge was operated
Holding Time at Max. Relative
7 0.00 10.00 10.00
Centrifugal Force in mints
Time taken to come back to zero
8 16.50 16.65 2.17
Relative Centrifugal Force
Total Residence Time in mints inside
9 19.3 29.45 14.83
centrifuge
TABLE 2.8- RESULTS OF PRE-TREATMENT OF ONGC SLUDGE SAMPLES IN
CENTRIFUGE
SI. No. DESCRIPTION TEST 1
TEST 2 TEST 3
Wt. % Sludge recovered having Bound
1 40.52 31.67 20.85
Water therein
Wt.% Bound Water in above Sludge as
2 52.41 42.21 30.26
determined by BTX
3 Wt.% Ash Content in above Sludge 7.61 8.61 7.23
Calorific Value of Sludge with Bound
4 4,240 5,212 6,635
Water (kcal/kg)
Wt. % Oil that Separated Out from
5 40.39 40.99 42.60
above Sludge
Wt.% Water in above Oil as determined
6 1.02 0.39 0.15
by BTX
7 Calorific Value of Oil (kcal/kg) 10,550 10,633 10,681
8 Wt.% Ash Content in above Oil 1.06 0.88 0.73
9 Wt.% Slop Oil that Separated Out from 18.22 26.46
35.91
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
51
above Sludge
Oil Content in Slop Oil (ppm) 0.00 0.00 0.00
11 Turbidity of Slop Oil (NTU) 122 398 1,132
12 Wt.% Ash Content in above Slop Oil 0.61 = 2.04 5.18
Wt.% Oil + Ash lost through adhering
13 0.35 0.37 0.12
to Various Surfaces
Wt.% Water lost through Evaporation
14 0.52 0.52 0.54
and Wetting of Surfaces
It was observed that there was no impact on furnace oil sludges containing up
to 61
wt. % water while centrifuging the sludges with residence time of 10 minutes
at peak
RCF value of 21,893, provided that the sludge was without emulsifier like SLS.
The
5 entire
sludge was retrieved back expect what might stick to the walls of centrifuge
bottles. This can be seen from Table 2.3, for the sludge with 50 wt. % water
the
calorific value was not rose. However, once wt. % water of the sludge exceeded
certain threshold value, which lies between the values of 61 to 70, then even
without
SLS, if a Furnace Oil Sludge was centrifuged for 10 minutes at a peak RCF of
10 21,893 it
was found to be divided into sludge with bound water and slop oil. It was
observed that if the sludge was having 70 wt.% water and nil SLS, then it was
found
to be divided such that 53 wt.% of the sludge was containing 44 wt.% water and
the
entire water was bound water. The remaining material was found to be the slop
oil.
Alternatively, it was observed that if furnace Oil containing 96 wt. % water
and nil
SLS, then on centrifuging it for 10 minutes at a peak RCF of 21,893 the sludge
was
found to be divided such that only 6 wt. % of it forming the sludge with 40
wt. %
water, the entire water being bound water, and the remaining material mostly
as slop
oil. It was further observed that beyond 70 wt. % water, with increasing water
content, there was less yield of sludge with bound water in it. However, it
was
observed that the quantum of bound water inside the sludge does not vary much.
Moreover, it was observed that for the less yield of sludge more slop oil was
obtained.
Further, it was observed that for furnace oil sludge with 96 wt. % water was
having a
meagre calorific value of 320 kcal/kg. However, on centrifuging the sludge for
10
minutes at a peak RCF of 21,893 it yielded 6 wt. % sludge and that the sludge
was
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
52
having 19 times more energy density in it at 6,130 kcal/kg. Here by
centrifuging it
separated heavier lumps of Sludge, where energy was relatively concentrated
from
the slop oil that has very less energy in it.
Further, it was observed that centrifuging of the furnace oil sludge for 10
minutes at
RCF of 21,893 with emulsifier like SLS in the sludge far lesser quantity of
sludge
was generated with bound water as compared to similar sludge without SLS.
However with SLS, the quality of sludge was far better as it had a lot higher
calorific
value, on account of far less water content. Here again a centrifuge created a
value
by squeezing out water from within the sludge. This was possible only because
of the
presence of SLS, which partially reduced the ability of sludge to hold onto
water on
account of its viscosity. However, it was observed that SLS helped to make a
centrifuge effective and without SLS the centrifuge was having no impact on
the
sludge.
=
Also, it was observed that centrifuging with diesel sludge for 13 minutes at
RCF of
21,893 adds value only when the sludge was having emulsifier like SLS in it.
This
was possible only when the water was bound to the diesel. However, without
SLS,
water was found to be separated from the diesel without any sludge formation.
But
once SLS is present, the centrifuging was able to concentrate 87 wt. % diesel
into the
sludge with just 6.44 wt. % water thereby nearly doubling the energy density
within
the sludge in no time up to a value of 10,160 kcal/kg which was found to be
92% of
energy density of pure diesel.
Also, it was observed that with diesel sludge having SLS, the value added by
centrifuging depends both on the residence time provided and the peak RCF
value
chosen. Here cumulative impact of centrifuging was found to be a helping
factor. It
was observed that with centrifuging of diesel sludge having SLS for 1 minute
at RCF
of 3,502, the squeezing out of water was found to be less. Consequently the
quantum
of sludge generated was found to be increased from 45 wt. % to 66 wt. % while
the
increase in energy density falls from 84% to 29%. This can be seen from Table -
2.6.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
53
It was observed that, without SLS, there was no binding between diesel and
water.
The centrifuge was found to be quickly separating the two thereby giving pure
diesel
at one end. However, with SLS present, the diesel delivered was not entirely
water
free. Hence to that extent, from the perspective of a centrifuge, SLS was
found to aid
in cases of furnace oil based sludges and hinder in case of diesel based
sludges.
Accordingly, with diesel sludge it was established that a batch type
centrifuge can
break emulsifier based bonds between water and diesel. Often centrifuges are
not
expected to remove bound water. However, the bond breaking was more pronounced
with increased residence time at a higher RCF value.
It was observed that the best impact of pre-treatment of sludge with a
centrifuge was
seen with ONGC lagoon sludge which can be seen from Tables 2.7 and 2.8. Here,
it
was observed that one can enhance commercial value of sludge by extracting
from it
41 wt.% of saleable, free flowing hydrocarbons with a calorific value of
10,633
kcal/kg, ash content of 0.88 wt.% and moisture content of 0.39 wt.%. Besides,
it was
also observed that one was able to reduce by weight of sludge requiring
further
treatment by little more than three times with commensurate benefits. Also, it
was
observed that with more residence time or higher RCF value one was able to
squeeze
out more water. It was also observed that pre-treatment of sludge helped in
reducing
salt and ash contents in hydrocarbons. Further, it was confirmed that only
centrifuge
cannot remove entire water from the sludge. It was observed that the
centrifuge
enhances acceleration due to gravity by enormously speeding up the naturally
occurring separation of two different immiscible liquids due to their= density
difference. It was observed that the centrifuge was helping when mean free
path
between tiny droplets of a particular liquid is small followed by
consolidating them
into much larger droplets with reduced drag, which then helped them move even
faster.
Further it was observed that varying acceleration between moving droplets
leads to a
lot larger number of collisions in a given time and hence faster consolidation
followed by setting up a chain effect. Also, with enhanced residence time,
droplets
of two different liquids keep gathering kinetic energy while moving in
opposite
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
54
directions. Once the kinetic energy difference between them exceed a threshold
value, then they were able to break the forces that might be binding these
droplets
together thereby affecting a permanent separation between them, which
otherwise
would have been difficult to achieve with on-line centrifuges capable of
operating at
similar RCF. Accordingly, separation between constituents on lagoon sludge
from
ONGC was ascertained. Further, it was observed that free water can be
collected
from ONGC Sludge, behind the impervious, viscous layer of hydrocarbons with
bound water, inspite of the fact that lumps of viscous layer emerge before the
free
water emerges while centrifuging. Lastly, it was observed that constituents of
lagoon
sludge do not naturally separate out with time, even after decades wherein
quantum
of bonds broken depend on both the operative RCF of centrifuge and the
residence
time of sludge within the centrifuge.
EXAMPLE-3
EFFECT OF USING XYLENE AS SOLVENT ON SLUDGES WITH BOUND
WATER
An impact on release of bound water from sludges with time was studied by
reducing their viscosities through addition of varying amounts of low viscous
solvents like Xylene, at ambient and elevated temperatures. Also, it was
discovered
how water could be held tightly by hydrocarbons. Accordingly, about 58 wt.%
and
200 wt.% xylene was added to viscous Furnace Oil Sludges without SLS prepared
in-house with 50 wt.% bound water therein. Alternatively, about 58 wt.% and
200
wt.% xylene was added to viscous furnace oil sludge with 20.47 wt.% bound
water
and 3.74 wt.% SLS, extracted from in-house Sludge with 47.5 wt.% water and
2.51
wt.% SLS, by centrifuging that for 10 minutes at 21,893 RCF. Further, about 58
wt.% and 200 wt.% xylene was added to viscous ONGC sludge with 42.21 wt.%
bound water and 8.61 wt.% ash that was recovered from batch centrifuging of in-
coming ONGC Lagoon Sludge for 10 minutes at 4,500 RCF. Subsequently, the
mixture was stirred well and kept a part of that low viscous mixtures in the
settling
vessels for 6 and 72 hours at a stretch at ambient temperature of about 28 to
32 C
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
while keeping a yet another set of samples for 6 hours in a water bath at 80-
85 C. At
the end of test period, fractions of material from top, middle and bottom of
the
settling vessels were removed and evaluated for water content using the BTX
process. Subsequently, water present in various levels with the average water
content
5 of the entire mixture was measure. While top-most layer was often nearly
water free,
no free water or slop oil was found to be collected at the bottom-most layer.
The
bottom-most layer was composition wise similar to the middle layer and hence
added
the same in Table Nos. 3-2, 3-4, 3-6 and 3-9.
furnace oil based sludges with 50 wt.% water-
TABLE 3.1- CONSTITUENTS IN FURNACE OIL SLUDGE PLUS XYLENE MIXTURES
Si. DESCRIPTION
Ni
Sample # 1 Sample # 2 Sample # 3
1 Wt. of Sludge with Bound Water (g) 2,319.30 808.29 587.45
21 Wt.% Bound Water in above
50.00 49.75 20.47
Sludge as per BTX
31 Wt. % Sodium Lauryl
0.00 0.00 3.74
Sulphate in Sludge
Wt. of Xylene Added & mixed with above
1,389.40 1604.17
1162.73
7 Sludge (g)
5 Wt.% Xylene in total mixture 37.46 66.50 66.43
6 Wt. % Water in above Sludge with Xylene 31.27 16.67 6.87
Total amount of Sludge +
7 Xylene Mixture prepared for 3,708.70 2412.46 1,750.18
Treatment (g)
TABLE 3.2- TEST RESULTS WITH 37 WT % XYLENE IN FURNACE OIL SLUDGE
WITHOUT EMULSIFIER
Kept in
Kept at Kept at
Water Bath
SI.
N DESCRIPTION 30 C for 6 30 C for at (80-
o.
Hours 72
Hours 85 C) for 6
Hours
Wt. of above Sludge + Xylene Mixture
1 1,005.46 1,002.91 1,002.70
taken for specific Treatment (g)
Wt. of Water-Free Layer with Solvent +
2 65.50 , 66.46 61.34
Furnace Oil Collected (g)
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
56
Wt. of Layer with Solvent + Furnace Oil
3 917.68 898.02 889.61
With Bound Water Collected (g)
4 Wt. of Slop Oil Collected (g) 0.00 0.00 0.00
Wt. of Material Sticking to the Surfaces
(g) 21.60 32.30 27.83
6 Wt. % Evaporation Loss 0.068 0.611 2.386
TABLE 3.3- STUDY OF WT% WATER AT DIFFERENT LAYERS WITHIN MATERIAL
WITH 37 WT% XYLENE IN FURNACE OIL SLUDGE WITHOUT EMULSIFIER
Kept in
Kept at Kept at
Water Bath
SI.
DESCRIPTION 30 C for 6 30 C for at (80-
No.
Hours 72
Hours - 85 C) for 6
Hours
Wt.% Water in top 5.5 Vol. cYck of this layer
1 27.77 0.17 0.07
as determined by BTX
Corrected Wt.% Water in Top 5.5 Vol.%
2 28.51 8.59 28.11
____ after adding back the amount evaporated
Wt.% Water in Middle 89 Vol. % of this
3 31.65 33.00 32.66
layer as determined by BTX
Wt.% Water in Bottom 5.5 Vol. % of this
4 31.75 38.75 41.68
layer as determined by BTX
TABLE 3.4- EFFECT OF 66.5 WT % XYLENE IN FURNACE OIL SLUDGE WITHOUT
EMULSIFIER
Kept in
Kept at Kept at
Water Bath
SI.
DESCRIPTION 30 C for 6 30 C for at (80-
No.
Hours 72
Hours 85 C) for 6
Hours
Wt. of above Sludge + Xylene Mixture
1 493.78 655.1 329.44
taken for specific Treatment (g)
Wt. of Water-Free Layer with Solvent +
2 38.71 46.32 26.54
Furnace Oil Collected (g)
Wt. of Layer with Solvent + Furnace Oil
3 446.98 583.57 266.16
With Bound Water Collected (g)
4 Wt. of Slop Oil Collected (g) 0.00 0.00 0.00
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
57
Wt. of material sticking to surfaces (g) 7.85 20.05
21.50
6 Wt. % Evaporation Loss 0.049 0.788 4.626
TABLE 3.5- STUDY OF WT.% WATER AT DIFFERENT LAYERS WITHIN MATERIAL
WITH 66.5 WT% XYLENE IN FURNACE OIL SLUDGE WITHOUT EMULSIFIER
Kept in
Kept at Kept at Water Bath
SI.
DESCRIPTION 30 C for 6 30 C for at (80-
No.
Hours 72
Hours 85 C) for 6
Hours
Wt.% of total treated material, in the top
1 7.97 7.35 9.07
most layer
Wt.% Water in the top-most layer as
2 0.31 0.56 4.79
___ determined by BTX
Corrected Wt.% Water in Top most layer
3 0.92 10.53 39.52
after adding back the amount evaporated
Wt.% of total treated material, in the
4
middle layer 80.69 79.20 79.89
Wt.% Water in Middle Layer as
5
determined by BTX 15.03 8.82 5.67
Wt.% of total treated material, in the
6 11.34 13.45 11.05
bottom most layer
Wt.% Water in Bottom most layer as
7 determined by BTX 40.24 69.10 80.14
TABLE 3.6- EFFECT OF Wt. 66.5 WT % XYLENE IN FURNACE OIL SLUDGE WITH
EMULSIFIER
Kept in
Kept at Kept at
Water Bath
SI.
DESCRIPTION 30 C for 6 30 C for 72
No. at
(80-85 C)
Hours Hours
for 6 Hours
Wt. of above Sludge + Xylene Mixture
1 328.09 325.53 321.66
taken for specific Treatment (g)
Wt. of Water-Free Layer with Solvent +
2 28.40 17.63 27.91
Furnace Oil Collected (g)
Wt. of Layer with Solvent + Furnace Oil
3 291.71 290.59 279.95
With Bound Water Collected (g)
4 Wt. of Slop Oil Collected (g) 0.00 0.00 0.00
5 Wt. of material sticking to surfaces (g) 7.76 15.11 11.75
6 Wt. % Evaporation Loss 0.07 0.68 0.64
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
58
TABLE 3.7- STUDY OF WT% WATER AT DIFFERENT LAYERS WITHIN MATERIAL WITH
66.5 WT% XYLENE IN FURNACE OIL SLUDGE WITH EMULSIFIER
Kept in
Kept at Kept at
SI.
Water Bath
DESCRIPTION 30 C for 6 30 C for 72
No. at
(80-85 C)
Hours Hours
for 6 Hours
Wt.% of total treated material, in the top
1 8.87 5.72 9.07
most layer
Wt.% Water in the top-most layer as
2 0.21 0.17 0.18
determined by BTX
Corrected Wt.% Water in Top most layer
3 1.01 11.29 7.04
after adding back the amount evaporated
4 Wt.% of total treated material, in the middle
layer 77.52 79.35 78.38
Wt.% Water in Middle Layer as determined
6.78 4.80 4.82
by BTX
Wt.% of total treated material, in the
6 13.60 14.93 12.55
bottom most layer
Wt.% Water in Bottom most layer as
7
1 determined by BTX 2.22 18.14 21.56
TABLE 3.8- CONSTITUENTS IN ONGC SLUDGE PLUS XYLENE MIXTURES
SI. DESCRIPTION Sample #
No. 1
1 Wt. of Sludge with Bound Water (g) 438.66
Wt.% Bound Water in above
2 Sludge as per BTX 42.21
3 Wt. % Ash in Sludge 8.61
Wt. of Xylene Added & mixed with above
4 882.71
Sludge (g)
5 Wt.% Xylene in final mixture 66.80
6 Wt. % Water in above Sludge with Xylene 14.01
Total amount of Sludge + Xylene
7 Mixture prepared for Treatment 1,321.37
(g)
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
59
TABLE 3.9- EFFECT OF 66.5 WT % XYLENE IN ONGC SLUDGE
Kept in
Kept at Kept at
SI.
Water Bath
DESCRIPTION 30 C for 6 30 C for 72
No. at
(80-85 C)
Hours Hours
for 6 Hours
Wt. of above Sludge + Xylene Mixture
1 333.65 333.96 332.14
taken for specific Treatment (g)
Wt. of Water-Free Layer with Solvent +
2 28.92 19.93 24.68
Furnace Oil Collected (g)
Wt. of Layer with Solvent + Furnace Oil
3 295.97 303.91 248.08
, With Bound Water Collected (g)
4 Wt. of Slop Oil Collected (g) 0.00 0.00 0.00
Wt. of material sticking to surfaces (g) 8.55 6.56 46.09
6 Wt. % Evaporation Loss 0.06 1.07 4.00
TABLE 3.10- STUDY OF WT% WATER AT DIFFERENT LAYERS WITHIN MATERIAL WITH
66.5 WT.% XYLENE IN ONGC SLUDGE
Kept in
Kept at Kept at
SI.
Water Bath
DESCRIPTION 30
C for 6 30 C for 72 at (80-85 C)
No.
Hours Hours
for 6 Hours
Wt.% of total treated material, in the top
1 most layer 8.90 6.15 9.05
Wt.% Water in the top-most layer as
2 11.76 2.31 2.71
____ determined by BTX
Corrected Wt.% Water in Top most layer
3 12.36 17.15 36.75
after adding back the amount evaporated
Wt.% of total treated material, in the middle
4 79.59 79.51 78.75
layer
Wt.% Water in Middle Layer as determined
5
by BTX 14.08 13.58 7.92
Wt.% of total treated material, in the
6 bottom most layer 11.51 14.33 12.20
Wt.% Water in Bottom most layer as
'determined by BTX 16.37 16.01 43.81
In earlier Example-1, it was observed that on= addition of external
emulsifiers like
Sodium Lauryl Sulphate to furnace oil based sludges containing up to 61 wt.%
water
with respect to the total water therein, the amount of water held by Furnace
Oil as
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
bound water, dropped down from 100% to a much lower level. For sludges with 48-
49 wt.% water in it, when SLS added was 2.4 to 2.5 wt.%, we found only 25-23
wt.% of total water present in Sludge, remained stuck to furnace oil as bound
water.
But that was measured only after subsequently centrifuging the sludge for 10
5 minutes at 21,893 RCF. However, in accordance with the present Example-3,
the
furnace oil sludge with 47.52 wt. % water and 2.51 wt. % SLS in it,
centrifuging for
10 minutes at 21,893 RCF a viscous sludge was retrieved containing 20.47 wt.%
bound water and 3.74 wt.% SLS therein. The obtained viscous sludge with 20.47
wt.
% bound water alone was taken for the treatment.
In earlier Example-2, it was observed that on batch centrifuging of incoming
ONGC
Lagoon Sludge for 10 minutes at 21,893 RCF or even at 4,500 RCF, the sludge
got
separated into 3 different layers and middle layer was a viscous sludge with
30 wt. %
bound water therein. When RCF was just 4,500, the middle layer had 42.2 1 wt.
%
total water in it, of which only 72 wt. % was bound water. However, in
accordance
with present Example-3, it was evaluated that the middle layer after
centrifuging in-
coming ONGC sludge for 10 minutes at 4,500 RCF produced 42.21 wt. % total
water
in it. In other words, addition of xylene as solvent to viscous sludges alone,
where
except for ONGC Sludge the entire water was bound so tightly to hydrocarbons
that
even on centrifuging for 10 minutes at 21,893 RCF there was no separation of
water.
Also it was observed that with ONGC Sludge, at this high RCF only 28 wt. % of
water was separated.
Accordingly, it was observed that Xylene dramatically reduced viscosity of the
resultant mixture especially when the quantity thereof was added twice that of
the
sludge. Further, the resultant mixture was heated at 80 C -85 C to further
reduce the
viscosity thereof. Apart from reducing viscosity immediately, Xylene was
believed
to enhance the density difference between immiscible hydrocarbons and water.
However, it was observed that no free water or slop oil could separate out
even after
waiting for 6 hours. Hence, it was confirmed that viscosity alone was not
responsible
for hydrocarbons to hold onto such large amounts of water.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
61
As shown in Table 3.1, it was observed that the mixture held even up to 31.27
wt. %
water on an average. It was observed that the dispersed water droplet size was
so tiny
that even with viscosity got immediately reduced and density difference slowly
got
enhanced. However the drag experienced by them remained too high to allow
their
rapid separation. In addition, it might be probably because of dispersed water
droplet
size being just so small that inspite of 10 minutes of stay within a
centrifuge
operating at such large RCF, still immiscible water could not separate from
hydrocarbons even when it was present in quantity as high as 50 wt.%.
In order to evaluate the extent of impact of addition of Xylene to the sludge
on
downward percolation of bound water with time, the table Nos. 3.3, 3.5, 3.7
and 3.10
were read horizontally for the Top-Most, Middle & Bottom-Most layers.
Accordingly, it was observed that addition of xylene beyond a certain
threshold
value is essential to get a large impact on furnace oil based sludges. It was
observed
that for varied the quantum of Xylene added for Furnace Oil based sludges,
Xylene
was effective only when it was present in the final mixture in the levels of
66 wt.%
and the benefit of Xylene was muted when presence thereof was limited to 37
wt.%.
It was observed that even the top-most layer was not anywhere near being water
free
with 37 wt. % Xylene present in the mixture.
Further, it was observed that there was no collection of free water or slop
oil at the
bottom but there was collection of nearly water free hydrocarbons at the top-
most
layer. In addition to the fact that a lot more water got collected in the
bottom most
layer indicated that water droplets were actually very slowly moving down with
=
time. Very slow rate of downwards percolation of water could either be because
of
presence of emulsifiers or due to ultra small size of dispersed water
droplets.
Accordingly, it was identified that the density differential between water and
hydrocarbons diminished and that in turn reduced the force of buoyancy in case
for
water bound to oil due to emulsifiers. Accordingly, it was confirmed that in
case of
emulsifier being present the concentration of water in the bottom most layer
cannot
exceed the average concentration of water present as much as what would be
possible in case there was no emulsifier where other things remaining the
same.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
62
Further, it was observed that using Xylene with additional heating one may get
water
free top-most layer in about 6 hours, even from sludges with high water
content, if
condensed water vapours are prevented from trickling back into that layer or
if one
prevents the condensation of regressing water vapors itself. But the time
required for
this will be much longer if emulsifiers are present in sludge. Also only a
small
fraction of water free hydrocarbons can thus be released from sludges.
Further, it was observed that the temperature had two impacts on the mixture.
Firstly,
apart from further reducing its viscosity, it enhanced the rate of evaporation
of water
from the top-most layer. Again, the latter had two implications. Firstly, it
helped to
reduce water in the top-most layer. Secondly, it enhanced the rate of
condensation.
Part of our settling vessel projected out above the water level in water bath.
Hence,
its top portion was relatively cooler, allowing rapid condensation. With that,
droplets
of condensed water rapidly trickled back into the top-most layer. That in turn
explained why we had more water in the top-most layer as observed from Tables
3.5,
3.7 and 3.10.
Also, it was observed that egressing water vapors could be prevented by not
allowing
the top end of our settling vessel kept in water bath to cool down in addition
to
getting much drier top-most layer. That could also be achieved by preventing
the
condensed water from trickling back into the top-most layer by modifying the
design
of our settling vessel itself.
It was observed that with reduced Xylene and relatively higher viscosity in
the
mixture as seen in Table Nos. 3.5, 3.7 and 3.10 and not repeated in Table 3.3.
It was
observed that it might have an adverse impact on the rate of evaporation. It
was seen
that when xylene present in the mixture was limited to 37 wt. % then only 2.39
wt. %
of the mixture evaporated by way of water vapour. However, when xylene present
in
mixture rose to 66.5 wt. % then about 4.63 wt.% of the mixture evaporated by
way
of water vapour. Accordingly, it was confirmed that with less evaporation
there was
less condensation and hence less harm was done through condensation. This can
be
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
63
ascertained by comparing Table Nos. 3.2 and 3.4. Besides, in Table 3.3, the
top-most
layer consisted of just 6.12 wt. % of total mixture as against 9.07 wt.% in
Table 3.5
where more xylene was used. By considering a thinner layer there was lesser
moisture in top-most layer in as can be seen in Table 3.3.
However, in presence of SLS very low evaporation rate was observed inspite of
equally large reduction is viscosity. It was because when water was bound to
hydrocarbons through emulsifier, the boiling point thereof under a given
pressure
went high and with that evaporation rate at a given temperature came down
along
with condensation rate.
Accordingly it was observed that when water is bound to hydrocarbons through
emulsifier, entire water or hydrocarbons may or may not be bound. Hence the
process of separation between them becomes slow and incomplete without being
completely ceased. Hence when emulsifier is present, deviation from average
water
content in any layer was observed to be less than that without emulsifier.
This too
was borne out from Table Nos. 3.5 and 3.7.
An important observation with ONGC Sludge was that even after reducing its
viscosity immensely by adding twice as much its own weight of xylene there was
little impact on downwards percolation of bound water present therein under
ambient
conditions. As can be seen from Table 3.10, the average water content in
bottom
most layer even after waiting for 72 hours rose to 16 wt.% when overall water
content of mixture itself was 14 wt.%. In contrast, for Furnace Oil Sludge
when
twice its weight of Xylene was added the average water content became 16.67
wt.%.
But after 72 hours of waiting, water in bottommost layer rose to 69 wt.%,
which is
nearly 4 times as much. With ONGC Sludge additional heat however had an
immense impact though not as much as that seen with Furnace Oil Sludge.
It was further observed that solvent, even when added in large amounts, it
does not
quickly and selectively solubilize entire hydrocarbons present in sludge and
then
disgorge out immiscible water due to density difference as commonly assumed.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
64
However, it was only found to weaken the forces that bind water and
hydrocarbons
together and to an extent additionally helped by slowly enhancing density
difference
between them by slowly dissolving hydrocarbons in very small quantities at a
time.
It certainly did not entirely or immediately eliminate the forces that bind
water with
hydrocarbons.
EXAMPLE-4
COMBINED EFFECT OF CENTRIFUGE & SOLVENT ON SLUDGES WITH
BOUND WATER
In order to understand the mechanism and also the impact on the release of
bound
water from hydrocarbons firstly by reducing viscosity of various sludges
followed by
adding solvents, such that the mixture contains 67 wt. % solvents,
subsequently
centrifuging it for 10 minutes at 4,500 RCF and at ambient temperature of
about 28
to 32 C was studied. Specifically, solvents like Xylene and Toluene were added
to =
viscous furnace oil sludge prepared in-house with 50 wt. % water.
Alternatively,
solvents like Xylene and Toluene were added to viscous furnace oil sludge with
20A7 wt.% bound water and 3.74 wt.% SLS, extracted from in-house Sludge with
47.5 wt.% water and 2.51 wt.% SLS, by centrifuging that for 10 minutes at
21,893
RCF. Alternatively, solvents like Xylene and Toluene were added to viscous
ONGC
sludge with 42.21 wt.% bound water recovered after batch centrifuging in-
coming
ONGC Lagoon Sludge for 10 minutes at 4,500 RCF such that the mixture contains
67 wt.% solvent and then after stirring immediately subject it to non-stop
centrifuging at 4,500 RCF for 10 minutes. The process of centrifuging produced
two
or three distinct layers of liquids. The third layer was obtained only in case
of ONGC
Sludge containing clear water. The Top-most layer was invariably water free.
It was
containing bulk of solvent added and also large amounts of hydrocarbons
released
from sludge. The middle layer, in cases where three layers were obtained, was
consisting of hydrocarbons and water. Subsequently, the middle layer was
evaluated.
On centrifuging it for 10 minutes at 21,893 RCF we got sludge with bound
Water,
albeit much smaller in quantity, a free flowing layer of solvent plus some
dissolved
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
hydrocarbons and slightly coloured slop oil. The sludge thus obtained was then
evaluated for bound water using BTX and calorific value using Bomb
Calorimeter.
Furnace Oil Based Sludges with Bound Water-
5
TABLE 4.1 DESCRIPTION OF FURNACE OIL SLUDGE
SI. Sample # Sample # Sample #
DESCRIPTION
No. 1 2 3
1 Wt. of Sludge taken for Treatment (g) 469.69 _
234.47 233.55
2 Wt.% Sodium Lauryl Sulphate '3.74 -
Wt. % Water in above Sludge as
3 20.47 49.91 49.91
determined by BTX
Calorific Value of above Sludge
4 7,970 5,110 5,110
(kcal/kg)
5 Name of Solvent Used Xylene Toluene Xylene
6 Wt. of Solvent Added (g) 940.87 469.75 467.18
7 Final Wt. of Sludge with Solvent (g) 1,410.56 704.22
700.73
TABLE 4.2- CENTRIFUGING DETAILS
SI. Sample # Sample # Sample #
DESCRIPTION
No. 1 2 3
Time taken in minutes to Reach Max.
1
Relative Centrifugal Force 2.65 2.70 2.65
Max. Relative Centrifugal Force on
4,500 4,500 4,500
2 which operated
3 Holding Time at Max. Relative
10 10 10
Centrifugal Force in mutes
Time taken to come back to zero
4
Relative Centrifugal Force 17 16.5 16.5
Total Residence Time in minutes
5
________ inside centrifuge 29.65 29.20 29.15
= TABLE 4.3 COMBINED EFFECT OF CENTRIFUGE WITH SOLVENT ON FURNACE OIL
SLUDGE
SI. Sample # Sample # Sample #
DESCRIPTION
No. 1 2 3
Wt. of Furnace Oil Sludge + Solvent
1 1,408.38 702.94 699.59
Mixture taken for Centrifuge (g)
Wt. of top Most Layer with Furnace
2 703.66 566.16 553.03
Oil + Solvent Recovered (g)
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
66
3 Wt.% of Top Most Layer 49.96 80.54 79.05
4 Wt. of Solvent in Top Most Layer (g) 696.62 466.32 453.54
Wt. of Furnace Oil with 0.23 wt.%
6.83 99.42 99.50
ash in Top Most Layer (g)
Wt. % Water in Top Most Layer as
6 0.03 0.06 0.00
determined by BTX
Calorific Value of Material in Top
7
Most Layer in kcal/kg
Wt. of Middle Layer containing
Furnace Oil + Water + Solvent + Ash
8 + Emulsifier if any, inclusive of 699.00 134.60 144.68
material sticking on surfaces or
evaporated (g)
9 Wt.% of Middle Layer 49.63 19.15 20.68
Wt. of Sludge with Bound Water +
Solvent + Emulsifier + Ash in 663.80 94.46 104.81
_______ Middle_AO- in
Wt. of Solvent + Free Flowing
- 11 Furnace Oil + Ash + Emulsifier in 0.00 1.25 12.92
_______ Middle Layer (g)
Wt. of slightly coloured Free Water
12 in Middle Layer with Emulsifier & 3520 38.89 26.95
Ash (g)
Wt.% of Sludge with Bound Water,
13 Ash and Emulsifier found within the 94.96 70.18 72.44
_______ Middle Layer
Wt.% Water in above Sludge from
14 within the Middle Layer as 9.07 81.66 85.02
determined by BTX
Calorific Value of above Sludge in
9,124 1,860 1,518
kcal/kg
Wt. of Clear Water with Ash &
16 0.00 0.00 0.00
Emulsifier in Bottom Most layer (g)
17 Wt.% of Bottom Most Layer 0.00 0.00 0.00
18 Wt. of loss of material in (g) 5.72 2.18 1.88
19 W% of Loss of Material 0.41 0.31 0.27
ONGC viscous sludge with bound water-
TABLE 4.4- DESCRIPTION OF ONGC VISCOUS SLUDGE
SI. DESCRIPTION Sample # Sample
No. 1 #2
1 Wt. of Sludge taken for Treatment (g) 212.86 233.53
Wt. % Water in above Sludge as
2 42.21 42.21
determined by BTX
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
67
Calorific Value of above Sludge
3 5,213 5,213
(kcal/kg)
4 Name of Solvent Used Toluene Xylene
Wt. of Solvent Added (g) 492.33 468.74
6 Final Wt. of Sludge with Solvent (g) 705.19 702.27
TABLE 4.5- CENTRIFUGING DETAILS
SI.
DESCRIPTION Sample # Sample #
No. 1 2
1 Time taken in minutes to Reach Max.
2.68 2.56
Relative Centrifugal Force
Max. Operative Relative Centrifugal
2 4,500 4,500
Force
Holding Time at Max. Relative
3 10.00 10.00
Centrifugal Force in minutes
Time taken to come back to zero Relative
4 16.60 16.50
= Centrifugal Force in minutes
Total Residence Time in minutes inside
5 29.28 29.06
the centrifuge
TABLE 4.6- COMBINED EFFECT OF CENTRIFUGE WITH SOLVENT
ON ONGC SLUDGE
SI. Sample # Sample #
DESCRIPTION
No. 1 2
1 Wt. of Mixture taken for Centrifuge (g) 705.08 702.14
=
2 Wt. of top Most Layer with Furnace Oil
534.30 511.47
+ Solvent Recovered (g)
3 Wt. % of Top Most Layer 75.78 72.84
4 Wt. of Solvent in Top Most Layer (g) 481.92 455.88
Wt. of ONGC Hydrocarbons in Top Most
5 44.17 47.52
Layer (g)
6 Wt. of Ash in Top Most Layer (g) 8.10 7.98
Wt. % Water in Top Most Layer as
7 0.02 0.02
determined by BTX
Calorific Value of Top Most Layer
8
(kcal/kg)
Wt. of Middle Layer containing ONGC
Hydrocarbons + Water +Solvent + Ash, 108.29 131.13
9
inclusive of material sticking on surfaces
or evaporated (g)
Wt.% of Middle Layer 15.36 18.68
Wt. of Sludge with bound water in
11 73.58 92.90
Middle Layer in (g)
=
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
68
Wt. of Solvent and free Flowing
12 Hydrocarbons with ash in Middle Layer 14.51 16.18
________ (g)
13 Wt. of Unbound, slightly coloured Water
20.20 22.05
with ash in Middle Layer (g)
14 Wt.% Sludge in Middle Layer 67.95 70.85
Wt.% Bound Water in above Sludge in
15 12.52 22.02
Middle Layer as determined by BTX
Calorific Value of Sludge in Middle
8,098 7,149
16 Layer in kcal/kg
Wt. of Bottom Most Layer containing
17 60.00 56.91
clear water (g)
18 Wt.% of Bottom Most Layer 8.51 8.11
19 Turbidity of Bottom Most Layer (NTU) 20.2 19.08
Wt. of un-accountable loss of Material
20 2.48 2.63
(g)
21 Wt.% Loss of Material 0.35 0.37
It was observed that combining solvent with centrifuge does a lot more than
mere
addition of their individual effects. For example, Furnace Oil Sludge without
external emulsifier and with 50 wt. % water could not remove any water or oil
after
centrifuging it for 10 minutes at 21,893 RCF. Subsequently, by adding similar
quantum of same solvent we could get only 8 wt. % of the mixture, in the top
most
layer with 0.31 wt. % water, after waiting for 6 hours. However, even at
reduced
peak RCF of centrifuge to 4,500, i.e. by 4.9 times, thereby keeping the
residence
time at peak RCF the same it was possible to collect 80 wt.% of the mixture in
top-
most layer with nil water in it, in just time of 30 minutes. This was mostly
because
by combining the solvent and centrifuge enhanced the factors that contribute
to
increase force of buoyancy that naturally helps separating two immiscible
liquids.
However, on other hand combining the solvent and centrifuge by reducing
viscosity
drag was substantially reduced that inhibited such separation.
As can be clearly seen from Table Nos. 4.1 to 4.3, in case of furnace oil
sludge with
emulsifier like SLS, the size of topmost water-free layer shrinks sharply from
80 to
50 wt.%. This clearly established that even a combination of solvent and
centrifuge
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
69
is less effective when water is additionally bound to hydrocarbons through an
emulsifier. With shrinking of the top most layer, middle layer expanded from
20
wt.% to 50 wt.% and while doing so the presence of sludge with bound water
therein
went up from 72 to 95 wt.%. Even more important act was that bound water in
within higher fraction of sludge fallen from 85 wt.% to 9 wt.% thereby
enhancing
the calorific value of that sludge from 1,520 to 9124 kcal/kg.
With the presence of the emulsifier like SLS, the hydrocarbon component in
Sludge
went up slightly from 76 to 87 wt.% while its water content came down from 20
to 9
wt.% on account of the combined treatment with Solvent and Centrifuge. But
when
SLS was not present, with same treatment furnace oil component in the sludge
fell
down dramatically from 50 to 15 wt.% while water content therein went up from
50
to 85 wt.%. When SLS was present, the mass of sludge required further
treatment
actually increased by 41 wt. % as compared to reduction by 55 to 57 wt. % in
the
absence of SLS. This basically implied that with presence of SLS, the increase
in
hydrocarbon content in sludge was not so much on account of some water moving
out from it, but because of a lot more solvent coming into it with very little
amounts
of furnace oil leaving the sludge. When SLS was present only 2 wt. % of
furnace oil
present in sludge left and moved into top most layer while 25 wt.% of xylene
added
moved into the sludge and then got very tightly bounded to water. Here the
topmost
layer was consisting mostly of Xylene alone and hence it was only slightly
coloured.
On the contrary, in the absence of SLS, about 86 wt.% of furnace oil present
in
sludge moved out into the water-free topmost layer while nil xylene moved in.
Hence it was only in the absence of SLS that combination of xylene and
centrifuge
was able to remove 87 wt. % of furnace oil from within the sludge.
Accordingly, the
combined effect of solvent with centrifuge was ascertained.
It was observed that with furnace oil sludge, use of Toluene as Solvent was
found to
be equally good as that of Xylene except for the fact that with Toluene the
mass of
sludge was found to be shrinked by about 4.6 wt. % more time and reduced its
water
content by about 3.3 wt.% more as compared to xylene thereby raising its
calorific
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
value from 1,520 to 1,860 kcal/kg. Also with Toluene the middle layer was
found to
contain less amount of solvent with dissolved hydrocarbon therein.
Toluene was observed to remove 85 wt% hydrocarbons present in sludge along
with
5 34 wt. % of water as against Xylene removing 86 wt.% hydrocarbons along
with 87
wt. % hydrocarbons and 26 wt.% water present in sludge. Toluene was found to
extract water from the sludge in a relatively better manner while Xylene seems
to
extract hydrocarbons a little better manner in comparison to Toluene.
10 It was observed with ONGC sludge that the combined use of solvent and
centrifuge
can remove free water with turbidity values in the range of 20 NTU. In case of
ONGC Sludge, Toluene was preferred over Xylene as it reduced mass of sludge
with
bound water by a factor of 2.89 against with that of 2.51 with Xylene.
Similarly, the
factors for furnace oil sludge without external emulsifier were respectively
observed
15 to be 2.48 and 2.30. Hence the combination of solvent cum centrifuge was
found to
work better with ONGC sludge. In case of ONGC sludge, Toluene was found to
reduce hydrocarbon content in sludge by 48 wt. % while Xylene reduces it by 46
wt.
%. Similar figures for furnace oil sludge without external emulsifier are 85
wt. %
and 87 wt. % respectively.
In case of ONGC Sludge, Toluene was found to reduce water content in sludge by
90
wt. % while Xylene was found to reduce the water content by 79 wt.%.
Similarly, for
furnace oil based sludge without external emulsifier, Toluene was found to
reduce
water content in sludge by 34 % and Xylene was found to reduce water content
in
the sludge by 26 wt. %.
Hence, it was confirmed that water content is far more easily extractable in
case of
ONGC sludge as compared to furnace oil based sludge without external
emulsifier.
This was also seen by free water collecting at the bottom. Toluene was found
to be
particularly far better when removal of water from the sludge. Accordingly,
use of
Toluene was preferred for ONGC sludge.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
71
Further, it was observed that removal of bound water from the sludge preferred
rather than extraction of hydrocarbons from sludge because removal of bound
water
from the sludge was found to increase calorific value of sludge without
loading too
much of hydrocarbons in solvent. However, the solvent can be reused as such
prior
to separating hydrocarbons from solvent when solvent has fewer hydrocarbons
therein.
In case of ONGC Sludge, it was possible to extract close to 50 wt. %
hydrocarbons
from the sludge containing bound water therein without loading solvent with
hydrocarbons in topmost layer beyond 8.5 or 9.5 wt. % when using Toluene and
Xylene respectively. This was because a lot of released hydrocarbons were not
solubilised by solvent collected in topmost water-free layer.
EXAMPLE-5:
STUDY OF PURE AZEOTROPIC BOILING WITH WATER
A study was conducted in order to understand and evaluate pure azeotropic
boiling
of solvents like Benzene, Toluene and Xylene with water at an atmospheric
pressure
933 mbar followed by comparison of results with similar values from the
literature.
A predefine weighed amounts of solvents and de-ionized water in certain
proportions
were taken in Round Bottom (RB) Flask of Dean and Stark Apparatus followed by
heating in a mantle furnace. A condenser having chilled water at 6 C is
attached to
the RB flask where vapours of solvent and water are condensed. A stop cork at
bottom the receiver was positioned which was periodically opened to
periodically
collect the condensate and weigh the immiscible constituents individually
after
separating them in the separating flask. The temperature of material at near
bottom in
the RB Flask was continuously recorded using a digital thermometer.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
72
TABLE 5.1- DESCRIPTION OF SOLVENTS USED
SI.
DESCRIPTION
Benzene Toluene Xylene
No.
1 Wt.% Water in Solvent through BTX 0.001994 0.003996 0.003998
2 Calorific Value of Solvent (kcal/kg) 9,995 10,074 10,205
Boiling Point of pure Solvent in C at Sea Level
3 80.20 110.80 138.40
as per Literature
Minimum Azeotropic Boiling Point in C at Sea
4 69.30 84.10 90.00
Level with Water as per Literature,
Minimum Azeotropic Boiling Wt. Ratio with
10.24 3.95 150
, Water per unit Wt. of Water as per Literature .
TABLE 5.2- STUDY OF AZEO TROPIC BOILING OF SOLVENTS WITH WATER AT 933 mbar
SI.
DESCRIPTION
Benzene Toluene Xylene
No.
1 Wt. of Water Taken in RB Flask (g) 100.35 150.40 306.94
_ 2 Wt. of Solvent Taken in RB Flask (g) 1003.42 601.33
538.17
3 Initial Wt. Ratio of Solvent to Water 10.00 4.00 1.75
Observed temperature range of Azeotropic
4 74.67-
76.03 93.4-93.9 95.55-95.85
Boiling ( C)
5 Initial Wt. Ratio of Solvent to Water Collected 33.45 5.65
2.20
6 Final Wt. Ratio of Solvent to Water Collected 102.15 10.14
2.23
7 Average Wt. Ratio of Solvent to Water Collected 61.97 9.34
2.08
Wt. Ratio of Solvent to Water Left over in RB
8 6.48 2.56 1.52
Flask at the End of Experiment
Wt. of Water left in RB Flask at the end of
9 93.99 116.41 180.06
Experiment (g)
Wt. % Loss due to Evaporation, etc. 0.38 0.67 0.36
It was observed that minimum azeotropic boiling point with water ought to be a
fixed point. Yet a small range for boiling point was observed. It was observed
that
5 inspite of lower atmospheric pressure at our place, on an average we get
6.05 C
higher minimum azeotropic boiling point with Benzene; 9.55 C with Toluene and
5.7 C with Xylene than what was reported in the literature. Part of this could
be
because there was no stirrer inside the RB Flask.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
73
In case of minimum azeotropic boiling weight ratio, higher values were
observed
than what was reported in the literature. It was observed that for Benzene, it
was
found to be higher by 3.3 times. For toluene, initially it was higher by 1.43
times. For
Xylene, initially it .was higher by 1.46 times. In case of benzene, quantity
of solvent
floating over water was very high and therefore entrainment was also expected
to be
the maximum.
It was observed that weight ratio of solvent emerging with water vapor
increased
with time. This happened significantly only for Benzene and Toluene. Moreover,
the
weight ratio of solvent to water in residual material in RB Flask
progressively
became far lower than the minimum azeotropic boiling ratio itself for Benzene
and
Toluene. Presumably, it was found that if one boils these solvents with water,
with
less solvent to water ratio by weight than the minimum azeotropic boiling
ratio, then
a lot more solvent was found to be gone out per unit of water removed.
EXAMPLE- 6
STUDY OF ABOVE AZEOTROPIC BOILING IN PRESENCE OF FURNACE
OIL, WITH AND WITHOUT BOUND AND FREE WATER
In order to better understand behavior of solvents at boiling in presence of
furnace
oil and modification thereof in presence of bound water and free water
thereafter,
predefined weighed amounts of Toluene and Furnace Oil in certain proportions
were
taken in the RB Flask in first instance. Subsequently, Benzene, Toluene and
Xylene
were taken at a time with Furnace Oil based sludge containing bound water.
Thereafter, each of the above solvents was taken at a time with furnace oil
with
drinking water in a specific proportion in the RB Flask. This RB Flask was a
part of
Dean and Stark Apparatus that was heated in mantle furnace. A condenser with
chilled water supply was attached to the RB flask with supply of chilled water
at 6
C wherein the vapours of solvent and water were condensed. A Stop Cork at
bottom
of the receiver was attached to periodically collect condensate and
individually
weigh the immiscible constituents after first separating them in a separating
flask.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
74
The temperature of material at near bottom in the RB Flask was continuously
recorded using a digital thermometer.
TABLE 6.1- STUDY OF VARIATION IN BOILING POINT OF SOLVENTS IN
PRESENCE OF FURNACE OIL AT 933 mbar
SI.
DESCRIPTION Benzene
Toluene Xylene
No.
Wt. of Furnace Oil Taken in RB Flask
1
(g) 300.41
2 Wt. of Solvent Taken in RB Flask (g) 300.20
Initial Wt. Ratio of Solvent to Furnace
3
Oil 1.00
Observed Boiling Temperature range at 110.93-
4
933 mbar ( C) 356.15
Wt. of Solvent Collected by the end (g) - 305.96
6 Wt. % Loss due to Evaporation, etc. 0.21
TABLE 6.2- STUDY OF VARIATION IN BOILING POINT OF SOLVENTS IN
PRESENCE OF FURNACE OIL & BOUND WATER AT 933 mbar
SI.
DESCRIPTION Benzene
Toluene Xylene
No.
1 Wt. of Sludge taken in RB Flask (g) 25.68 152.45 150.37
Wt. of Bound Water Present in Sludge
2 12.79 /5.94 74.90
(g)
Wt. of Furnace Oil Present in Sludge
3 12.89 76.51 75.47
(g)
4 Wt of Solvent added in RB Flask (g) 1024.04 759.46 415.39
Initial Wt. Ratio of Solvent to Water
5 80.06 10.00 5.55
present
Initial Wt. Ratio of Solvent to Furnace
6 79.44 9.92 5.50
Oil Present
Observed Boiling Temperature Range 72.11- 91.2- 96.33-
at 933 mbar ( C) 79.54 108.86 136.28
Initial Wt. Ratio of Solvent to Water
8 19.61 4.82 2.06
Collected
Final Wt. Ratio of Solvent to Water
9 122.66 104.67 10.35
Collected
Average Wt. Ratio of Solvent to Water
82.34 6.93 1.92
Collected
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
11 Wt. of Total Water collected (g) 11.48 74.50 74.32
12 Wt. of Total Solvent collected (g) 945.32 516.07 142.68
13 Rate of Water Collection (g/min) 0.30 0.33
Wt. Ratio of Solvent to Furnace Oil
14 Left over in RB Flask at the End of 5.67 _ 3.44 3.59
Experiment
Wt. of Solvent left over in RB Flask at
the end of Experiment (g) 73.11 236.52 270.72
Wt. of Furnace Oil left over in RB
16 12.89 76.51 75.47
Flask at the end of Experiment (g)
Residual Water present in left over
17 Solvent cum Furnace Oil in ppm as 1,551.59 2,614.04 530.01
determined by BTX Test
18 Wt. % Loss due to Evaporation, etc. 0.69 0.89 0.45
TABLE 6.3-STUDY OF VARIATION IN BOILING POINT OF SOLVENTS IN
PRESENCE OF FURNACE OIL & FREE WATER AT 933 mbar
SI.
DESCRIPTION Benzene Toluene Xylene
No.
. Wt. of Furnace Oil Taken in RB Flask
1 151.88 150.00 153.31
(g)
2 Wt of Solvent Taken in RB Flask (g) 304.40 600.01 460.26
Wt. of Free Water added in RB Flask
3 456.77 600.36 921.76
(g) ____
Initial Wt. Ratio of Water to Solvent
4 1.50 1.00 2.00
present
Initial Wt. Ratio of Solvent to Furnace
5 2.00 4.00 3.00
Oil
6
Observed Boiling Temperature Range 86.7- 97.28- 96.89-
at 933 mbar ( C) 98.31 98.5 97.58
Initial Wt. Ratio of Solvent to Water
7 558.63 4.85 2.07
Collected
Final Wt. Ratio of Solvent to Water
0.03 0.08 0.05
8 Collected
9 Average Wt. Ratio of Solvent to Water
2.26 2.38 1.05
Collected
10 Total Wt. of Water Collected (g) 136.28 252.31 489.69
11 Total Wt. of Solvent Collected (g) 307.89 602.66 464.66
12 Rate of Solvent Collection (g/min) 2.81 2.96 1.76
Wt. of Furnace Oil Left behind in RB
13 148.39 147.35 148.91
Flask at the End of Experiment (g)
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
76
Wt. of Free Water left behind in RB
14 315.54 343.86 424.34
Flask at the end of Experiment(g)
15 Wt. % Loss due to Evaporation, etc. 0.54 0.31 0.50
It was observed that when Toluene and furnace Oil were taken in proportion of
50:
50 by weight, they both being non-polar form a solution. It was observed that
the
boiling point of Toluene went up from 110.8 C since the boiling point of
Furnace
Oil is in the region of 350 C. It was observed that when boiling began,
Toluene
preferentially boiled out as its boiling point was lot lower than that of
Furnace Oil.
The boiling point continuously rose as Toluene kept getting depleted as can be
clearly seen from Table 6.1. =
As shown in Table 6.1, the boiling began at 110.93 C at atmospheric pressure
of
933 mbar. Boiling Point of pure Toluene is 110.80 C at sea level. Boiling
ended at
350.15 that being the initial boiling point of pure Furnace Oil. We actually
collected
back slightly more solvent than what we added. At the end some Furnace Oil too
came out.
It was observed that when solvent was added to any sludge for boiling out the
bound
water from hydrocarbons, there was yet another phenomenon working apart from
solvent helping to depress the boiling point of water and vice versa which was
vast
reduction in viscosity of furnace oil that helped in weakening of the forces
that bind
furnace oil to the bound water. It was observed that the boiling point of
bound water
reduced by reducing viscosity itself.
As can be seen in Table 6.2, instead of pure furnace oil in case of furnace
oil based
sludge with 50 wt. % bound water, it was observed that water was held onto
furnace
oil so tightly that even on centrifuging it for 10 minutes at 21,893 RCF, nil
water was
found to be separated from the furnace oil. Under such a circumstance, entire
bound
water got removed in temperature range of 91.2 C to 108.86 C through boiling
along with 69 wt. % of Toluene that was originally present. Average weight
ratio at
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
77
which Toluene and Water came out was 6.93. As compared to a mixture of pure
water and toluene, here the boiling point rose towards the end once ratio of
Toluene
to furnace oil dropped down below a certain point. It was because with Toluene
being in solution with furnace oil, the latter raised the boiling point of
former as seen
in Table 6.1. Also wt. ratio of regressing solvent to water fell down from
9.34 to 6.93
(as shown in Table 5.2) as entrainment became less since here the solvent was
in
solution with furnace oil.
Similarly, with Xylene, entire bound water got removed with 35 wt. % Xylene.
It
was observed that even the rate of water removal was better at 0.33 g/minute
as
compared to 0.30 g/minute for Toluene.
=
Further, the temperature range was observed to be higher. It was in a range of
about
96.33 C to 136.28 C. This was because boiling point of pure xylene was higher-
than
that of toluene as can be seen from Table 5.1. Interestingly, it was observed
that the
end boiling point rose close to that of the boiling point of pure solvent.
This was
presumably because towards the end, with depletion of bound water, the left
over
material in the Rl3 flask became similar to the starting material as per Table
6.1.
Here too the weight ratio of egressing solvent to water was found to be 1.92
which
was lower than that 2.08 as indicated in Table 5.2.
It was observed that rise in temperature occurred towards the end when bound
water
was getting depleted. However, the use of multi-effect evaporator for boiling
out
water and solvent from hydrocarbons was preferred. The multi-effect evaporator
allowed material to be in sections/vessels where ambient pressure and hence
absolute
temperature was low by the time boiling point rose. This successfully
prevented
thermal cracking.
It was observed that with benzene, entire bound water got removed with 93 wt.
% of
Benzene. Benzene was found to be weakest in removing bound water as per unit
of
bound water removed 82 units of benzene by weight were required. Benzene was
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
78
found to be the slowest amongst the solvents however it was found to have an
advantage that the entire process got over by 80 C.
Here the boiling point range was found to vary from 72.11 C to 79.54 C. It
was
observed that weight ratio of egressing benzene to water for boiling out pure
water
was 82.34 on an average, which was however more than 61.97 as seen in Table
5.2.
This showed here that the water was indeed bound and not free. Therefore, a
lot
more benzene was required as having weakest ability to remove free water.
When solvent boiled out bound water, towards the end the quantum of solvent
required to drive out a unit mass of water rose very sharply along rising
boiling
point. Also, it was observed that quantum of required solvent went up partly
because
statistically it was then difficult for egressing solvent vapours to encounter
residual
water before emerging from furnace oil. It could also be partly because the
last bit of
water could be most tightly bound to furnace oil. Boiling point also rose
towards the
end as there was no further depression of boiling point of solvent present in
solution
with higher boiling furnace oil. The depression in the boiling point of the
solvent was
available earlier because of water being present was now no longer available.
A behavioral study of bound water in furnace oil based sludge after being
replaced
with free water can be seen from Table 6.3. Here drinking water was added in
the
RB Flask in specific proportions with pure furnace oil and solvent wherein
water
added was in excess. It was observed here that instead the entire solvent went
out
and that too with far lower boiling point range, leaving behind excess free
water with
furnace oil. Free water was not found to be mixed with furnace oil and that
was
removed by gravity based separation. It was observed that all three solvents
were
entirely boiled out within a temperature range of 97 C to 99 C except for
benzene
where boiling started from 86.7 C and went up to 98.31 C. Interestingly, it
was
observed that the free water drove out entire solvent from Furnace Oil and the
final
boiling point approached that of free water temperature and not that of
solvent
temperature. When free water drove out solvent, the weight ratio of egressing
solvent
to water was a lot lower than minimum azeotropic boiling ratio. This was
totally
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
79
reverse of what happened when solvent was driving out entire bound water.
While
entire or slightly excess solvent was removed only 30 wt.% of original free
water left
while boiling out benzene, 42 wt.% free water left for boiling out toluene and
53
wt.% water left for boiling out Xylene. As can be seen from Table 6.3 that the
weight
ratio of egressing solvent to water was almost same i.e. 2.26 and 2.38 for
Benzene
and Toluene respectively. A fraction of water left when removing Toluene due
to
addition of less water for removing toluene. Xylene was found to be more
effective
in removing bound water and Toluene was found to be more effective in removing
free water.
It was observed that the rate of solvent removal from the solution with higher
boiling
hydrocarbons like furnace oil was a lot faster with free water than the rate
of bound
water removal from same furnace oil in sludge with same solvents. For example,
Xylene removed bound water from furnace oil at the rate of 0.33 g/min.
However,
free water removed Xylene from furnace oil at the rate of 1.76 g/min. This was
because of slow heating when removing bound water from furnace oil using
xylene.
This happened because water was held to furnace oil in sludge a lot more
tightly than
the binding strength between Xylene and furnace oil on account of their
solubility.
Further, it was observed that when only traces of solvent were present in
Furnace
Oil, a lot more water was required to remove a given mass of solvent towards
the
end. This was because with minuscule solvent left in furnace oil, the boiling
point
approached that of furnace oil or 350 C although the solvent was being boiled
out at
less than 99 C. Statistically it was found lot more difficult for egressing
water
vapour to encounter solvent present within a far larger volume of furnace oil
when
only traces of solvent were present.
Accordingly, it was confirmed that the boiling point of solvents like Benzene,
Toluene and Xylene can be substantially depressed by adding free water to the
solution of hydrocarbons when these solvents were present in the solution with
other
hydrocarbons having substantially higher boiling points. It was also confirmed
that
=
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
one can choose to remove either the entire water or entire solvent by varying
their
initial ratio in the mixture in case where furnace oil was present.
EXAMPLE-7
5
REMOVAL OF BOUND WATER FROM FURNACE OIL SLUDGES WITH 50
WT.% BOUND WATER IN IT, BY BOILING IT WITH AZEOTROPIC
SOLVENTS
10 In order to evaluate implications of using different quantity of various
solvents on
removal of bound water from Furnace Oil Sludges with 50 wt. % Bound Water in
it,
predefined proportions of sludge and solvent by weight were taken in the RB
flask of
Dean and Stark Apparatus and followed by continuous heating thereof in the
mantle
heater while continuously monitoring the temperature of material in RB Flask
with
15 digital thermometer. The vapours of bound water and solvent were
collected in the
receiver after condensing them with circulating cold water 5 C to 6 C in the
insulated condenser. The condensates were out and collected in separating
flask
using the stop cork at the bottom of the receiver. After phase separation,
water and
solvent collected were individually weighed each time.
Removal of bound water with Xylene-
TABLE 7.1A- REMOVAL OF BOUND WATER FROM FURNACE OIL SLUDGES
BY VARYING PROPORTIONS OF XYLENE AT 933 mbar
SI. TEST
TEST TEST TEST
DESCRIPTION
No. 1 2 3 4
1 Wt. of Sludge taken in RB Flask (g) 150.22
150.15 152.45 150.90
2 Wt.% Water Present in Sludge 49.81 49.81 49.81
49.81
3 Wt. of Furnace Oil Present in Sludge (g) 75.39 75.37 76.51
75.73
4 Wt. of Solvent added in RB Flask (g) 123.52
138.69 155.87 171.07
5 Initial Ratio of Solvent to Water by Wt. 1.65 1.85 2.05
2.28
97.03- 96.25- 98.49- 95.76-
6 Observed Boiling Temperature Range ( C)
151.26 180.1 176.66 162.68
7 Initial Wt. Ratio of Solvent to Water Collected 2.05 2.04
2.01 2.10
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
81
8 Final Wt. Ratio of Solvent to Water Collected 4.11 1.44 4.24
8.52
Average Wt. Ratio of Solvent to Water
9 1.79 1.86 1.92 2.07
Collected
Wt.% Bound Water collected during
97.38 98.90 99.93 99.92
Experiment inclusive of losses
Wt.% of Solvent collected during Experiment
100.00 99.50 92.09 90.92
inclusive of losses
Wt.% of Furnace Oil collected during
12 2.67 0.00 0.00 0.00
Experiment
13 Average Rate of Water Collection (g/min) 0.52 0.34 0.34
0.33
Wt. Ratio of Solvent to Furnace Oil Left
14
over in RB Flask at the End of Experiment 0.00 0.01 0.16 0.46
Residual Water present in left over Solvent
cum Furnace Oil in ppm as determined by 25,998 10,880 653 792
BTX Test
16 Wt. % Loss due to Evaporation, etc. 2.87 2.39 1.04 0.91
TABLE 7.1B- REMOVAL OF BOUND WATER FROM FURNACE OIL SLUDGES BY
VARYING PROPORTIONS OF XYLENE AT 933 mbar
SI. No. DESCRIPTION TEST TESTTEST 3
1 2
1 Wt. of Sludge taken in RB Flask (g) 153.66 151.67
153.05
2 Wt.% Water Present in Sludge 49.81 49.81 49.81
4 Wt. of Furnace Oil Present in Sludge (g) 77.12 76.12 76.81
5 Wt. of Solvent added in RB Flask (g) 191.45 226.71
268.04
6 Initial Ratio of Solvent to Water by Wt. 2.50 3.00 3.52
98.71- 97.91- 99.76-
7 Observed Boiling Temperature Range ( C)
146.04 142.66 139.41
8 Initial Wt. Ratio of Solvent to Water Collected 2.04 2.04
2.16
9 Final Wt. Ratio of Solvent to Water Collected 1.71 2.90
8.50
10 Average Wt. Ratio of Solvent to Water Collected 1.83 2.07
2.48
Wt.% Bound Water collected during Experiment
11 99.87 99.87 99.86
inclusive of losses
Wt.% Solvent collected during Experiment
12 73.60 68.81 71.49
inclusive of losses
13 Wt.% of Furnace Oil collected during Experiment 0.00 0.00
0.00
14 Average Rate of Water Collection (g/min) 0.35 0.47 0.35
Wt. Ratio of Solvent to Furnace Oil Left over
15 in RB Flask at the End of Experiment 0.66 0.93 0.99
Residual Water present in left over Solvent
16 cum Furnace Oil in ppm as determined by 1,297 1,314 1,432
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
82
BTX Test
17 Wt. % Loss due to Evaporation, etc. 1.01 1.00 0.95
TABLE 7.1C- REMOVAL OF BOUND WATER FROM FURNACE OIL SLUDGES BY
= VARYING PROPORTIONS OF XYLENE AT 933 mbar
SI. No. DESCRIPTION TEST
TEST TEST
1 2 3
1 Wt. of Sludge taken in RB Flask (g) 150.50
150.45 150.37
2 Wt.% Water Present in Sludge 49.81 49.81 49.81
= 3 Wt. of Furnace Oil
Present in Sludge (g) 75.53 75.51 75.47
4 Wt. of Solvent added in RB Flask (g) 300.00
338.51 415.39
Initial Ratio of Solvent to Water by Wt. 4.00 4.52 5.54
98.29- 96.64- 96.33-
6 Observed Boiling Temperature Range ( C)
137.94 137.68 136.28
7 Initial Wt. Ratio of Solvent to Water Collected 2.14 2.02
2.06
8 Final Wt. Ratio of Solvent to Water Collected 31.12 9.74
10.35
9 Average Wt. Ratio of Solvent to Water Collected 2.69 1.86
1.92
Wt.% Bound Water collected during Experiment
99.97 99.92 99.95
inclusive of losses
Wt.% Solvent collected during Experiment
11 67.57 41.12 34.83
inclusive of losses
= 12 Wt.% of Furnace Oil
collected during Experiment 0.00 0.00 0.00
13 Average Rate of Water Collection (g/min) 0.35 0.33
0.33
Wt. Ratio of Solvent to Furnace Oil Left over in
14 RB Flask at the End of Experiment 1.29 2.64 3.59
= Residual Water present in left over Solvent cum
Furnace Oil in ppm as determined by BTX Test 265 795 530
16 Wt. % Loss due to Evaporation, etc. 0.63 0.46 0.45
=
Removal of Bound Water With Toluene-
TABLE 7.2- REMOVAL OF BOUND WATER FROM FURNACE OIL SLUDGES
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
83
BY VARYING PROPORTIONS OF TOLUENE AT 933 mbar
SI. TEST TEST
DESCRIPTION TEST 1
No. 2 3
1 Wt. of Sludge taken in RB Flask (g) 150.71 151.06 152.45
2 Wt.% Water Present in Sludge 49.81 49.81 49.81
3 Wt. of Furnace Oil Present in Sludge (g) 75.64 75.82 76.51
4 Wt. of Solvent added in RB Flask (g) 225.61 451.67
759.46
Initial Ratio of Solvent to Water by Wt. 3.00 6.00 10.00
98.00- .06- .
6 Observed Boiling Temperature Range ( C) 89 8928-
126.10 146.44 108.87
= 7 Initial Wt. Ratio of Solvent
to Water Collected 5.45 5.29 4.82
8 Final Wt. Ratio of Solvent to Water Collected 0.93 10.24
14.87
9 Average Wt. Ratio of Solvent to Water Collected 3.63 5.97
6.93
Wt.% Bound Water collected during Experiment
86A0 98.23 99.74
inclusive of losses
Wt.% Solvent collected during Experiment
11 100.00 96.50 68.86
inclusive of losses
12 Wt.% of Furnace Oil collected
during Experiment 4.10 0.00 0.00
13 Average Rate of Water Collection
(g/min) 0.36 0.33 0.30
Wt. Ratio of Solvent to Furnace Oil Left over in
14 RB Flask at the End of Experiment
0.00 0.21 3.09
Residual Water present in left over Solvent cum
Furnace Oil in ppm as determined by BTX Test 1,34,981 17,542 2,614
16 Wt.% Loss due to Evaporation, etc.
1.61 0.52 0.89
Removal of bound water with Benzene-
TABLE 7.3- REMOVAL OF BOUND WATER FROM FURNACE OIL SLUDGES BY
I VARYING PROPORTIONS OF BENZENE AT 933 mbar
DESCRIPTION TEST 1 TEST 2
No.
1 Wt. of Sludge taken in RB Flask (g) 50.52 25.68
2 Wt.% Water Present in Sludge 49.80 49.80
3 Wt. of Furnace Oil Present in Sludge (g) 25.36 12.89
4 Wt. of Solvent added in RB Flask (g) 604.11 1024.04
5 Initial Ratio of Solvent to Water by Wt. 24.01 80.06
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
84
6 Observed Boiling Temperature Range ( C) 76.26-94.67 72.11-
81.05
7 Initial Wt. Ratio of Solvent to Water Collected 26.93
19.61
8 Final Wt. Ratio of Solvent to Water Collected 116.33 122.66
9 Average Wt. Ratio of Solvent to Water Collected 48.36
82.34
Wt.% Bound Water collected during Experiment
' 57.79 98.84
inclusive of losses
Wt.% Solvent collected during Experiment inclusive
11 98.28 96.25
of losses
12 Wt.% of Furnace Oil collected during Experiment 0.00
0.00
13 Average Rate of Water Collection (g/min) 0.04 0.02
Wt. Ratio of Solvent to Furnace Oil Left over in
14 RB Flask at the End of Experiment 0.41 2.98
Residual Water present in left over Solvent cum
Furnace Oil in ppm as determined by BTX Test 4,18,770 1,552
16 Wt. % Loss due to Evaporation, etc. 0.92 0.69
It was observed that in all above tables, entire water present in the sludge
was bound
water. It was observed that addition of 5.5 times the weight of water present
in the
sludge was must in case where Xylene was used as solvent. However, it might be
5 reduced to
3.5- 4.5 times, without much rise in maximum temperature at the end of
the experiment. It was observed that if Xylene was taken up to 3 times the
weight of
water present then temperature at the end was not only high but also there was
residual moisture in left over material. It was observed that addition of 10
times the
weight of water present in sludge was must in case where Toluene was used as
10 solvent.
It was observed that moisture in residual Furnace Oil cum Solvent was low.
It was observed that addition of 80 times the weight of water present in
sludge was
must in case where Benzene was used as a solvent. With this one can remove
almost
the entire bound water present in sludge.
15 As can be
seen in Table 7-2, temperature rise was observed to be maximum when
average weight ratio of Toluene to Water boiling out was approximately equal
to
original weight ratio that was present at the start of the process inspite of
which
entire water cannot be removed from sludge. This was because under such a
condition neither water was able to boil out entire solvent nor the solvent
was able to
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
boil out entire water. However, then water may keep accumulating over time and
may boil out entire solvent from the mixture if average weight ratio of
solvent to
water boiling out was more than what was originally present as in Test 1. On
the
contrary as seen in Test 3, it was seen that if average weight ratio of
solvent to water
5 boiling out was far lower than what was originally present, then solvent
kept
accumulating over time thereby ensuring that entire water got removed from
sludge
through boiling. For Xylene, the worst weight ratio to have for solvent to
water was
1.85. This was because the average weight ratio of solvent to water boiling
out was
the same as what was present right in the beginning.
It was observed that final temperature was very high when one began the
process by
having the same weight ratio of solvent and water in mixture as one would find
on an
=
average in the vapour phase. It was also observed that it was very difficult
to remove ,
entire water or solvent from sludge under that condition. It was observed that
at least
1 wt. % to 1.5 wt. % water stayed back along with similar amounts of solvent.
Removal of this residual amount of water and solvent was difficult inspite of
massive
rise in temperature.
When Xylene was added either 1.65 or 1.85 times the weight of water present in
furnace oil sludge with 50 wt. % water, at the end we found that our purpose
was
defeated. Instead of solvent removing entire bound water, the bound water
actually
ended up removing entire solvent through boiling. This was because the average
egressing ratio of solvent to water by weight itself was 1.86 when xylene was
1.85
times the weight of water present. In the end it was observed that 0.54 wt. %
Xylene
was left behind with 1.09 wt.% water inspite of final temperature rising to
180.1 C.
Similarly, it was observed that for Toluene and Benzene too there may be a cut
off
point for the amount of solvent added. If solvent added is less than that cut-
off value
then instead of solvent removing entire bound water one may find the bound
water
has boiled out the entire solvent instead. For Toluene, that amount was
observed to
be between 5 - 6 times the weight of water for furnace oil sludge with 50 wt.%
water.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
86
For Benzene, that amount was about 80 times the weight of water in furnace oil
sludge with 50 wt.% water.
It was observed that rate of water removal with Xylene was 0.33 g/min, with
Toluene 0.30 g/min but with benzene it was only 0.02 g/min. Hence it was
established that one may require a lot more benzene to take out a unit mass of
water
due to which the rate of water removal is so low. However, it was observed
that only
advantage with benzene was operative temperature range which was found 72 C
to
80 C as against 96 C to 138 C for Xylene and 89 C to 109 C for Toluene.
Here
above rates of water removal with Xylene, Toluene and Benzene were found to be
smaller as compared to the rates of removal of free water with same solvents
(refer
Table 5.2). It was observed that, the quantity of Xylene egressing with unit
weight of
water was rising when initial ratio of solvent to water was in a range of
about 1.65 to
4. Even for Toluene and Benzene, with more solvent added, more solvent found
egressed per unit mass of water removed through boiling.
As can be seen from Test 3 in Table 7.1C that it was possible for average
weight
ratio of solvent to water boiling out to be lower than both the initial and
final weight
ratio in which they boil out. That was because the minimum ratio in which they
boil
out was not at the very start but somewhere soon after that. Also it can be
seen from
Table 7.1C that a very steep rise in the weight ratio in which they boil out
towards
the end was observed when initial weight ratio of solvent to water was very
high.
Once that happened the end temperature still increased up, but then only up to
the
boiling point of pure solvent under ambient pressure and not well above it.
Inspite of
the weight ratio of egressing solvent to water increased up steeply towards
the end. It
was observed that average weight ratio was not differ much than their initial
weight
ratio. This was because such a sharp rise in their weight ratio and also in
their boiling
point was seen over a very short period of time towards the very end of the
process.
On the contrary, as can be seen from Table 7.1A and 7.1B, the final
temperature
reached exceeded the boiling point of pure xylene under similar ambient
pressure
when weight of xylene added is the range of 1.85 to 3.0 times the weight of
water
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
87
present in sludge. Accordingly, it was concluded that lower the ratio higher
will be
the final temperature rise. This may be probably because when xylene was
relatively
less, it cannot adequately depress the boiling point of last bits of bound
water in
furnace oil. Hence, it was confirmed that first the quantity of solvent added
at the
start has to be such that at the end of the process, for a given heating rate
enough
residual solvent remains back in furnace oil to adequately reduces its
viscosity and
also to adequately depress the boiling point of last bits of bound water in
furnace oil.
Further it was observed that one must remove entire bound water with large
amounts
of solvent still remaining behind in residual furnace oil in order to get low
operative
temperatures that probably might have helped in three ways. Firstly, furnace
oil
remained free flowing with very small viscosity till the end. Secondly, the
boiling
point was not significantly raised till the end by presence of soluble furnace
oil with
large fraction of residual solvent. Thirdly, it was easier for it to drive out
traces of
water towards the end with large presence of residual solvent.
It was observed that the left over weight ratio of solvent to oil was observed
to be
Minimum of 3.59, 3.09 and 2.98 for Xylene, Toluene and Benzene respectively
for
removing entire bound water from less viscous Oil. However, to get above
mentioned left over ratios one has to take initial wt. ratio of solvent to
water/oil are
5.5, 10.0 and 80.0 for Xylene, Toluene and Benzene respectively and follow
optimally controlled rate of heating.
EXAMPLE- 8
ESTABLISHMENT OF THE BASIS OF EVALUATING QUANTITY OF
ADDITION OF SOLVENT
In order to establish the basis of evaluating how much solvent one should add
was
based on amount of water present in Sludge or the amount of hydrocarbons
present
therein. Accordingly, predefined proportions of sludge and solvent by weight
were
taken in the RB flask of Dean and Stark Apparatus and followed by continuous
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
88
heating thereof in the mantle heater while continuously monitoring the
temperature
of material in RB Flask with digital thermometer. The vapours of bound water
and
solvent were collected in the receiver after condensing them with circulating
cold
water at 5 C to 6 C in the insulated condenser. The condensates were out and
collected in separating flask using the stop cork at the bottom of the
receiver. After
phase separation, water and solvent collected were individually weighed each
time.
TABLE 8.1- DETERMINING BASIS FOR ADDING XYLENE FOR REMOVAL OF
BOUND WATER FROM FURNACE OIL SLUDGES AT 933 mbar
SI. DESCRIPTION TEST TEST TEST
TEST
No. 1 2 3 4
1 Wt. of Sludge taken in RB Flask (g) 150.76 151.08 150.62
151.42
2 Wt.% Water Present in Sludge 14.84 14.84 59.48 59.48
3 Wt. of Furnace Oil Present in Sludge (g) 128.38 128.66
61.03 61.36
4 Wt. of Solvent added in RB Flask (g) 414.65
707.70 492.74 339.34
5 Initial Ratio of Solvent to Water by Wt. 18.53 31.57 5.50
3.77
Initial Ratio of Solvent to Furnace Oil by
6 3.23 5.50 8.07 5.53
Wt.
Observed Boiling Temperature Range 96.34-
93.02- 96.25- 97.30-
( C) 139.53
140.12 135.32 137.48
Initial Wt. Ratio of Solvent to Water
8 2.17 2.12 2.06 2.00
_______ Collected
9 Final Wt. Ratio of Solvent to Water
17.78 82.63 459.75 3.93
Collected
Average Wt. Ratio of Solvent to Water
2.38 3.30 2.08 1.96
Collected
11 Wt.% Water collected during Experiment 99.75 99.91 99.99
99.93
inclusive of losses
Wt.% Solvent collected during Experiment
12 12.86 10.61 37.24 50.97
inclusive of losses
Wt.% of Furnace Oil collected during
13 0.00 0.00 0.00 0.00
_______ Experiment
14 Average Rate of Water Collection (g/min) 0.14 0.16 0.27
0.30
Wt. Ratio of Solvent to Furnace Oil Left
over in RB Flask at the End of Experiment 2.81 4.92 5.07 2.71
Residual Water present in left over
16 Solvent cum Furnace Oil in ppm as 500 159 85 1,037
_______ determined by BTX Test ____________________________________
17 Wt. % Loss due to Evaporation, etc. 0.31 0.30 0.50 0.65
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
89
It was observed that the best results were obtained when residual solvent to
furnace
oil ratio, by weight, at the end of the experiment was high. In Test-3, where
the
above residual ratio was 5.07, least temperature rise was observed with
maximum
bound water removed with residual moisture level being just 85 ppm. It was
established that it would not matter whether one considers the weight of water
or
weight of furnace oil in sludge for evaluating the quantity of solvent to be
added.
As can be seen from Test Nos. 1 and 2, rate of water collection was observed
to be
significantly low when there was less water in Furnace Oil Sludges. This was
inspite
the fact that per unit mass of egressing solvent more water was removed at
that time.
Apparently, water in Furnace Oil based Sludge was limited to the extent to
which it
can depress the boiling point of solvent.
EXAMPLE-9
EVALUATION OF EFFICACY OF PROCESS FOR REMOVAL OF BOUND
WATER FROM DIFFERENT FURNACE OIL SLUDGES
It was an aim of the experiment to evaluate efficacy of our Process for
removal of
bound water from different Furnace Oil Sludges with varying water content with
entire water present being only bound water. Accordingly, predefined
proportions of
sludge and solvent by weight were taken in the RB flask of Dean and Stark
Apparatus and followed by continuous heating thereof in the mantle heater
while
continuously monitoring the temperature of material in RB Flask with digital
thermometer. The vapours of bound water and solvent were collected in the
receiver
after condensing them with circulating cold water at 5 C to 6 C in the
insulated
condenser. The condensates were out and collected in separating flask using
the stop
cork at the bottom of the receiver. After phase separation, water and solvent
collected were individually weighed each time. Here bound water content in
furnace
oil sludges was varied from 2.15 % to 84.94 wt. %.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
TABLE 9.1A- REMOVAL OF BOUND WATER FROM FURNACE
OIL SLUDGES WITH VARYING WT% WATER BUT FIXED
PROPORTION OF XYLENE AT 933 mbar
SI.
No DESCRIPTION TEST 1 TEST 2 TEST
3
Wt. of Sludge taken in RB
1 150.29 150.21 151.08
Flask (g)
Wt.% Bound Water Present
2.15 9.91 14.84
2. in Sludge
Wt. of Furnace Oil Present
3 147.05 135.32 128.66
in Sludge (g)
Wt. of Solvent added in RB
4 809.39 745.87 707.70
Flask (g)
Initial Ratio of Solvent to
5 249.81 50.09 / 31.56
Water by Wt.
Initial Ratio of Solvent to
6 5.50 5.51 5.50
Furnace Oil by Wt.
Observed Boiling 12 93.02-
7 1.34-137.19 95.16-139.75
Temperature Range ( C) 140.12
Initial Wt. Ratio of Solvent
8 3.36 2.24 2.12
to Water Collected
Final Wt. Ratio of Solvent to
9 23.95 136.40 82.63
Water Collected
Average Wt. Ratio of
10 14.79 2.90 3.30
Solvent to Water Collected
Wt.% Water collected
11 during Experiment inclusive 107.52 98.70 99.91
of losses
Wt.% Solvent collected
12 during Experiment inclusive 6.50 5.92 10.61
of losses
Wt.% of Furnace Oil
13 0.00 0.00 0.00
collected during Experiment
Average Rate of Water
14 0.13 0.17 0.16
Collection (g/min)
Wt. Ratio of Solvent to
Furnace Oil Left over in RB
15 5.15 5.18 4.92
Flask at the End of
Experiment
Residual Water present in
left over Solvent cum
16 484 1,428 159
Furnace Oil in ppm as
determined by BTX Test
Wt. % Loss due to
17 0.12 0.17 0.30
Evaporation, etc.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
91
TABLE 9.1B- REMOVAL OF BOUND WATER FROM FURNACE OIL SLUDGES
WITH VARYING WT% WATER BUT FIXED PROPORTION OF XYLENE AT
933 mbar
DESCRIPTION TEST 1 TEST 2 TEST 3 TEST 4
No.
Wt. of Sludge taken in RB Flask
1 153.07 150.37 150.62 150.12
(g)
Wt.% Bound Water Present in
= 2 29.89 49.81 59.48 84.94
Sludge
Wt. of Furnace Oil/Hydrocarbons
3 107.31 75.47 61.03 22.61
Present in Sludge (g)
Wt. of Solvent added in RB Flask
4 590.67 415.39 492.74 701.31
(g)
Initial Ratio of Solvent to Water
12.91 5.54 5.50 5.50
by Wt.
Initial Ratio of Solvent to
6 5.50 5.50 8.07 31.02
Furnace Oil by Wt.
Observed Boiling Temperature 96.82- 96.33- 96.25- 97.89-
7
Range ( C) 135.98 136.28 135.32 137.91
Initial Wt. Ratio of Solvent to
8 2.17 2.06 2.06 2.01
Water Collected
Final Wt. Ratio of Solvent to
9 41.90 10.35 459.75 57.89
Water Collected
Average Wt. Ratio of Solvent to
3.06 1.92 2.08 2.40
Water Collected
Wt.% Water collected during
11 99.97 99.93 99.99 99.98
Experiment inclusive of losses
12 Wt.% Solvent collected during
33.84 34.69 37.24 43.56
Experiment inclusive of losses
Wt.% of Furnace Oil collected
13 0.00 0.00 0.00 0.00
during Experiment
Average Rate of Water
14 0.27 0.33 0.27 0.31
Collection (g/min)
Wt. Ratio of Solvent to Furnace
Oil Left over in RB Flask at the 4.19 3.59 5.07 17.51
End of Experiment
Residual Water present in left
over Solvent cum Furnace Oil
1,239 530 85 885
16 in ppm as determined by BTX
Test
Wt. % Loss due to Evaporation,
17 0.30 0.45 0.50 0.49
etc.
It was observed that entire water in these sludges was bound water. It was
understood that it was not possible to prepare sludge by mixing water with
furnace
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
92
oil, with more than 60 wt. % or 61 wt. % water in it with entire water being
bound
water. As explained earlier in Example- 1, if one tries to mix 85 wt. % water
with 15
wt. % furnace oil it forms a mixture of sludge with bound water and slop oil.
But still
it was indirectly possible to get furnace oil sludge with 85 wt.% bound water
in it.
For that furnace oil sludge with 50 wt.% bound water was added with twice if
its
weight of solvent like xylene and then centrifuged for 10 minutes at 21,893
RCF.
Most furnace oil in sludge was moved out with solvent leaving behind 14.5 wt.%
of
initial sludge cum solvent as a stable viscous sludge containing 15 wt.%
furnace oil
with 85 wt.% bound water in that. This sludge was taken for Test 4 in Table
9.1B
and then removed bound water from therein.
In the range of 2 wt. % to 85 wt. % bound water, entire bound water was
removed
from the sludges without temperature exceeding 140.12 C using Xylene as
Solvent.
Throughout Xylene added was either 5.5 times the weight of furnace oil or
water
present in sludge, whichever allowed adding more solvent thereto. In case
where
final temperature rose to 140.12 C, the least water was obtained at 159 ppm
left
behind in residual material at the end of the experiment.
It was also observed that the rate of water removal was distinctly low when
water
content in sludge is 15 wt. % or lower. Rate of bound water removal was
inversely
proportional to binding strength of water to furnace oil. This strength was
higher
when total water content was lower.
Further, it was found that the maximum rate of water removal was 0.33 g/min.
Yet
this maximum rate of water collection was 4.15 times lower than 1.37 g/min,
which
was the rate of free water collection with Xylene (as shown in Table 5.2).
Accordingly, it was proved that the water removed here was bound water and not
free water.
It was also found that boiling point itself starts from 121 C when water
present in
furnace oil sludge was only 2.15 wt. % whereas for all other cases is starts
from 95
C or 96 C. Also, on an average 14.79 times of xylene egresses out when water
present was 2.15 wt.% per unit mass of water removed. This high value might
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
93
possibly be due to enormous quantity of Xylene being present. Normally, this
weight
ratio was observed to be varying from 3.3 to 1.92.
It was observed that more than 100 wt.% water got collected when water present
in
furnace oil sludge is 2.15 wt.%. This may have happened because error in BTX
result was high especially with low moisture. Also, even otherwise BTX
indicated a
slightly lower value for water present than what was actually present.
EXAMPLE- 10
REMOVAL OF BOUND WATER FROM FURNACE OIL SLUDGES HAVING 50
WT. % BOUND WATER IN IT, BY VARYING THE RATE OF HEATING,
AFTER ADDING VARYING PROPORTIONS OF SOLVENTS
It was an aim of the experiment to evaluate the impact of varying rate of
heating,
with different proportions of solvent added, on removal of entire bound water
from
furnace oil sludges with 50 wt.% water in it. Accordingly, predefined
proportions of
sludge and solvent by weight were taken in the RB flask of Dean and Stark
Apparatus and followed by continuous heating thereof in the mantle heater
while
continuously monitoring the temperature of material in RB Flask with digital
thermometer. The vapours of bound water and solvent were Collected in the
receiver
after condensing them with circulating cold water at 5 C to 6 C in the
insulated
condenser. The condensates were out and collected in separating flask using
the stop
cork at the bottom of the receiver. After phase separation, water and solvent
collected were individually weighed each time. However, except for the above
mentioned facts, the rate of heating was varied with the input voltage to
mantle
heater. This variation in heating rate meant that in most cases 25 ml of
condensates
in approximately 30, 20 or 10 minutes was collected.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
94
TABLE 10.1-REMOVAL OF BOUND WATER FROM SLUDGES WITH 1.85 WTRATIO
OF XYLENE TO WATER BUT WITH VARYING RATE OF HEATING AT 933 mbar
=No DESCRIPTION TEST 1
TEST 2 TEST
3
1 Wt. of Sludge taken in RB Flask (g) 153.90 151.63 150.15
2 Wt.% Water Present in Sludge 49.81 49.81 49.81
3 Wt. of Furnace Oil Present in Sludge (g) 77.24 76.10 75.37
4 Wt. of Solvent added in RB Flask (g) 141.98 140.86 138.69
Initial Ratio of Solvent to Water by Wt. 1.85 1.86 1.85
95.46- 97.70- 96.25-
6 Observed Boiling Temperature Range ( C)
179.31 173.19 180.1
7 Initial Wt. Ratio of Solvent to Water Collected 2.01 2.06
2.04
8 Final Wt. Ratio of Solvent to Water Collected 1.21 0.52
1.44
9 Average Wt. Ratio of Solvent to Water Collected 1.86 1.83
1.86
Wt.% Water collected during Experiment inclusive of
98.71 99.50 98.90
losses
Wt.% Solvent collected during Experiment inclusive -
11 98.96 98.53 99.50
of losses
12 Wt.% of Furnace Oil collected during Experiment 0.00 0.00
0.00
13 Average Rate of Water Collection (g/min) 0.83 0.48 0.34
14 Average Rate of Condensate Collection (g/min) 1.87 1.35
0.97
Wt. Ratio of Solvent to Furnace Oil Left over in
RB Flask at the End of Experiment 0.02 0.03 0.01
Residual Water present in left over Solvent cum
16 Furnace Oil in ppm as determined by BTX Test 12,817 4,993
10,880
17 Wt.% Loss due to Evaporation, etc. 0.64 1.46 2.39
TABLE 10.2-REMOVAL OF BOUND WATER FROM SLUDGES WITH 2.25 INITIAL
WT.RATIO OF XYLENE TO WATER BUT WITH VARYING RATE OF HEATING AT 933
mbar
SI.
No DESCRIPTION TEST 1 TEST 2 TEST 3
1 Wt. of Sludge taken in RB Flask (g) 150.07 155.07 153.66
2 Wt.% Water Present in Sludge 49.82 49.82 49.80
- 3 Wt. of Furnace Oil Present in Sludge (g) 75.32 77.82 77.12
- 4 Wt. of Solvent added in RB Flask (g) 187.31 193.30 191.45
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
5 Initial Ratio of Solvent to Water by Wt. 2.50 2.50
2.50
.
______________________________________________________________________________
-
96.34- 95.17- 98.71-
6 Observed Boiling Temperature Range ( C)
147.74 147.35 146.04
7 Initial Wt. Ratio of Solvent to Water Collected 1.98
1.95 2.04
8 Final Wt. Ratio of Solvent to Water Collected 1.28 6.25
1.71
9 Average Wt. Ratio of Solvent to Water Collected -
2.02 1.81 1.83
Wt.% Water collected during Experiment inclusive of 99.97
99.96 =
99.87
losses
Wt.% Solvent collected during Experiment inclusive
11 80.71 72.65 73.60
of losses
12 Wt.% of Furnace Oil collected during Experiment 0.00'
0.00 0.00 1
-
______________________________________________________________________________
13 Average Rate of Water Collection (g/min) 1.01 0.47
0.35
14 Average Rate of Condensate Collection (g/min) 3.02 1.53
0.95
Wt. Ratio of Solvent to Furnace Oil Left over in
RB Flask at the End of Experiment 0.48 0.68 , 0.66
= Residual Water present in left over Solvent cum
16 Furnace Oil in ppm as determined by BTX Test 266 386
1,297 =
17 Wt.% Loss due to Evaporation, etc. 0.96 0.95
1.01
TABLE 10.3-REMOVAL OF BOUND WATER FROM SLUDGES WITH 3.5 INITIAL
WT.RATIO OF XYLENE TO WATER BUT WITH VARYING RATE OF HEATING AT 933
mbar
SI. ,
DESCRIPTION = TEST 1 TEST 2 TEST 3
No.
1 Wt. of Sludge taken in RB Flask (g) 150.86 150.71
153.05
2 Wt. /0 Water Present in Sludge 49.81 49.81
49.81
3 Wt. of Furnace Oil Present in Sludge (g) 75.71 75.64
76.81
4 Wt. of Solvent added in RB Flask (g) 263.55 263.18
268.04
5 Initial Ratio of Solvent to Water by Wt. 3.51 3.51
3.52
98.29- 97.12- 99.76-
6 Observed Boiling Temperature Range ( C)
143.2 134.91 139.41
7 Initial Wt. Ratio of Solvent to Water Collected 2.00
2.15 2.16
8 Final Wt. Ratio of Solvent to Water Collected 11.02
1.68. 8.50
9 Average Wt. Ratio of Solvent to Water Collected 2.14
1.86 2.48
Wt.% Water collected during Experiment inclusive of
10 99.84 99.53
99.86
losses
Wt.% Solvent collected during Experiment inclusive
11 61.44 53.00 71.49
of losses
12 Wt.% of Furnace Oil collected during Experiment 0.00
0.00 0.00
13 Average Rate of Water Collection (g/min) 0.93 0.32
0.35
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
96
14 Average Rate of Condensate Collection (g/min) 2.86 1.12
1.00
Wt. Ratio of Solvent to Furnace Oil Left over in
15 RB Flask at the End of Experiment .1.34 1.64 0.99
Residual Water present in left over Solvent cum
16 Furnace Oil in ppm as determined by BTX Test 1,585 4,627
1,432
17 Wt.% Loss due to Evaporation, etc. 0.72 0.68 0.95
TABLE 10.4- REMOVAL OF BOUND WATER FROM SLUDGES WITH 5.5 INITIAL
WT.RATIO OF XYLENE TO WATER WITH VARYING RATE OF HEATING AT 933 mbar
SI.
No DESCRIPTION
TEST 1 TEST 2 TEST 3
=
1 Wt. of Sludge taken in RB Flask (g) 153.09 155.51 150.37
2 Wt.% Water Present in Sludge 49.81 49.81 49.81
3 Wt. of Furnace Oil Present in Sludge (g) 76.83 78.05 75.47
=
4 Wt. of Solvent added in RB Flask (g) 419.48 426.63 415.39
Initial Ratio of Solvent to Water by Wt. 5.50 5.51 5.54
95.74- 95.16-
96.33-
6 Observed Boiling Temperature Range ( C)
137.26 137.45
136.28
7 Initial Wt. Ratio of Solvent to Water Collected 2.03 2.04
2.06
8 Final Wt. Ratio of Solvent to Water Collected 7.93 23.35
10.35
9 Average Wt. Ratio of Solvent to Water Collected 2.20 2.34
1.92
Wt.% Water collected during Experiment inclusive of
99.97 99.97 99.93
losses
Wt.% Solvent collected during Experiment inclusive
11 39.65 42.69 34.69
of losses
12 Wt.% of Furnace Oil collected during Experiment 0.00 0.00
0.00
13 Average Rate of Water Collection (g/min) 0.95 0.42 0.33
14 Average Rate of Condensate Collection (g/min) 2.69 1.18
0.93
Wt. Ratio of Solvent to Furnace Oil Left over in
RB Flask at the End of Experiment 3.30 3.13 3.59
Residual Water present in left over Solvent cum
16 Furnace Oil in ppm as determined by BTX Test 272 267 530
17 Wt.% Loss due to Evaporation, etc. 0.27 0.49 0.45
Xylene was added to furnace oil sludges with 50 wt.% Bound Water, in 4 weight
ratios, i.e. 1.85, 2.50, 3.50 and 5.50 with respect to water present in
Sludge. And then
for each ratio the heating rate was varied. It was observed that impact of
varying
5
heating rate was marginal except for weight ratios 1.85, 3.50 and 5.50. It was
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
97
observed that medium rate of heating was necessary for weight ratio of 1.85
where
water removal rate was 0.48 g/min or condensate removal rate was 1.35 g/min.
It
was observed that best results in terms of low residual moisture were obtained
in left
over material with least rise in temperature. This was found to be a very
sensitive
ratio since residual solvent staying back in left over furnace oil was
extremely small
and hence slight variation therein mattered a lot for removal of last bit of
bound
water with minimal temperature rise. At this rate of heating a little more
water got
boiled out as compared to solvent, thereby allowing little more solvent to
accumulate
and subsequently residual water rapidly boiled out at that elevated
temperature.
Accordingly, it was established that for any amount of solvent added one must
always try to leave behind maximum amount of solvent in residual furnace oil
by
allowing on an average least amount of solvent to boil out per unit mass of
water
removed through boiling if one wants to remove entire water from sludge at
least
temperature. However, slight increase in mass of residual solvent staying
behind till
the end might have played a huge role in ensuring complete removal of water
from
sludges at minimal temperature. It was observed that slowest rate of heating
was
suitable for weight ratios of 3.50 and 5.5 although for 3.50 there was not
much
difference between the medium and slow rates. However, for 5.5 the issue was
clear.
For ratio of 3.50, medium rate of heating was not found to be feasible inspite
of more
solvent being left behind as more water was also left behind. Here the extra
water
left behind was significant to remove one has to increase the temperature
substantially and also consume large amounts of residual solvent.
However, it was observed that Xylene failed to boil out entire water when
weight of
Xylene added was 1.65 times the weight of water in sludge. Instead, water
present in
sludge boiled out entire Xylene. This happened because average weight ratio in
which solvent and water boil out was 1.79 (as can be seen from Test-1 in Table
7.1)
which was a lot higher than initial weight ratio in which they were present
prior to
boiling. Here, it was established that the rate of heating might have to be
fast instead.
Referring to Table Nos. 7.1A and 7.1C it was seen that when Xylene added was
1.65
times the weight of water, water was boiled out at an average rate of 0.52
g/min as
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
98
against a value of 0.33 g/min when weight xylene added was 5.5 times the
weight of
water present. Accordingly, it was established that the heating rate must be
slow
when entire water has to be boiled out with preferred weight ratio of Xylene
and the
heating rate must be fast when solvent has to be driven out.
EXAMPLE- 11
IMPACT OF SEVERLY CONTROLLED RATE OF HEATING ON REMOVAL
OF BOUND WATER FROM FURNACE OIL SLUDGES USING SOLVENTS
A role of additional and severe controlled heating rate on removal of last
fractions of
bound water present in sludges was studied. Accordingly, predefined
proportions of
sludge and solvent by weight were taken in the RB flask of Dean and Stark
= Apparatus and followed by continuous heating thereof in the mantle heater
while
continuously monitoring the temperature of material in RB Flask with digital
= thermometer. The vapours of bound water and solvent were collected in the
receiver
after condensing them with circulating cold water at 5 C to 6 C in the
insulated
condenser. The condensates were out and collected in separating flask using
the stop
cork at the bottom of the receiver. After phase separation, water and solvent
-20 collected were individually weighed each time. This was except of the
fact that
towards the end of the process, when about 6 wt.% of water was left in sludge
the
rate of heating was substantially reduced, even periodically allowing the
temperature
of mixture to fall by 3 C to 10 C. The idea was to hold the mixture within a
fixed
temperature range for much longer time, by firstly reducing and then by
increasing
temperature of residual material within that range in small steps. Also this
meant
entirely stopping and re-starting the boiling process a large number of times.
This
allowed final traces of water to emerge from sludge with lots of solvent in
sharp and
short bursts. However, care was exerted to ensure that condensates did not
overflow
from condenser top.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
99
TABLE 11-A- IMPACT OF SEVERELY CONTROLLING RATE OF HEATING AT END
OF THE PROCESS WHEN USING XYLENE TO REMOVE BOUND WATER
,
SI. Successful Failed
Successful
Failed
No DESCRIPTION Experiment # 1 Experiment
Experiment Experiment
#1 #2
#2
=
1 Wt. of Sludge taken in RB Flask (g) 300.25
150.22 301.18 . 150.90
Wt.% Bound Water Present in
2 49.91 49.81 49.91 49.81
Sludge
3 Wt. of Furnace Oil Present in
150.39 75.39 150.85 75.73
Sludge (g)
Wt. of Solvent added in RB Flask
4 247.38 123.52 338.41 171.07
(g)
Initial Ratio of Solvent to Water
5 1.65 1.65 2.25 2.28
by Wt.
,
_______________________________________________________________________________
_
Observed Boiling Temperature
97.18-232.8 97.03- 97.41- 95.76-
6 Range ( C) 151.26 307.31 162.68
Initial Wt. Ratio of Solvent to
7 2.15 2.05 2.04 2.10
Water Collected
,
Final Wt. Ratio of Solvent to Water
8 1.46 = 4.11 29.53 8.52
=
Collected
Average Wt. Ratio of Solvent to
9 1.69 1.79 2.25 2.07 '
Water Collected
Wt.% Water collected during
10 98.50 97.38 98.81 99.92
-
Experiment inclusive of losses
Wt.% Solvent collected during
11 ' 100.00 100.00 98.97 90.92
Experiment inclusive of losses
Wt.% of Furnace Oil collected
12 1.30 2.67 0.00 0.00
during Experiment
Average Rate of Water Collection
13 0.78 0.52 0.31 0.33
(g/min)
Average Rate of Condensates
14 1.20 - 1.83 2.30 0.50
Collection (g/min)
Wt. Ratio of Solvent to Furnace Oil
15 Left over in RB Flask at the End of 0.02 0.46
Experiment
Residual Water present in left
16 over Solvent cum Furnace Oil in_ 25,998.14 11,692 792.29
ppm as determined by BTX Test
17 Wt.% Loss due to Evaporation, etc. 0.57 2.87 0.48 0.91
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
100
TABLE 11-B- RESIDUAL WEIGHT RATIO OF XYLENE TO WATER IN RB FLASK
AS THE PROCESS PROGRESSES WITH TIME
SI Failed Successful Failed Successful
DESCRIPTION Experiment Experiment
Experiment Experiment
N. o.
#1 #1 #2 #2
Wt. Ratio of Solvent to
1 Water Left over in RB 1.61 1.59 2.28
2.30
Flask After 1st Collection
Wt. Ratio of Solvent to
Water Left over in RB
2 1.58 1.54 2.36 2.39 ,
Flask After 2nd
Collection
Wt. Ratio of Solvent to
3 Water Left over in RB 1.55 1.48 2.50
2.54
Flask After 3rd Collection
Wt. Ratio of Solvent to
4 Water Left over in RB :1.53 1.39 2.68
2.81
Flask After 4th Collection
Wt. Ratio of Solvent to
Water Left over in RB 1.49 1.24 3.07 3.37
Flask After 5th Collection
Wt. Ratio of Solvent to
6 Water Left over in RB 1.44 0.42 3.99
5.08
Flask After 6th Collection
Wt. Ratio of Solvent to
7 Water Left over in RB 1.39, 0.32 6.72
5.89
Flask After 7th Collection
Wt. Ratio of Solvent to
8 Water Left over in RB 1.31 0.24 8.32
7.20
Flask After 8th Collection
Wt. Ratio of Solvent to
9 Water Left over in RB 1.23 0.19 17.02
8.85
Flask After 9th Collection
Wt. Ratio of Solvent to
Water Left over in RB
1.12 2.40 9.71
Flask After 10th
Collection
Wt. Ratio of Solvent to
Water Left over in RB
11 1.03 10.19
Flask After 11th
Collection
Wt. Ratio of Solvent to
Water Left over in RB
12 1.03 9.47
Flask After 12th
Collection
Wt. Ratio of Solvent to
Water Left over in R13
13 1.10
Flask After 13th
Collection
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
101
Wt. Ratio of Solvent to =
IWater Left over in RB 14 - 1.04
Flask After 14th
Collection
Wt. Ratio of Solvent to
Water Left over in RB
15 1.16
Flask After 15th
Collection
Wt. Ratio of Solvent to
16
Water Left over in RB
1.11
Flask After 16th
Collection
Wt. Ratio of Solvent to
17 Water Left over in RB 1.04
Flask After 17th
WI. Ratio of Solvent to
Water Left over in RB
18 0.81
Flask After 18th
Collection
Wt. Ratio of Solvent to
Water Left over in RB
19 0.49
Flask After 19th
Collection
As can be seen from Table 11-A, average rate of condensate collection was only
1.20
g/min as against a value of 1.83 g/min for successful experiment. Consequently
higher amounts of xylene did not boil out, per unit mass of water removed
through
boiling. Hence as can be seen from Table 11-B that the left over weight ratio
of
solvent to water was slightly higher for failed experiment till 5th collection
and at the
top of that we did not severely slow down the heating rate towards the end of
the
process as explained above in procedure. Consequently, as can be seen from
Tablel 1-B that due to high rate of heating we ended up boiling out solvent
and water
almost in equal ratio from 6th till 19th collection.
It was observed that at least egressing solvent was slightly more than the
water up to
10th collection and therefore the residual weight ratio of solvent to water at
least
kept falling slightly. But from 11th till 17th collection they almost boiled
out in equal
proportion. Water cannot stay back with temperature of mixture approaching 232
C.
More amount of heat was fed at a rate faster than what could be consumed
through
boiling. However, more solvent got removed through boiling than water because
of
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
102
substantially slowed down rate of heating towards the end due to which enough
latent heat was not supplied for boiling out water.
In case where weight of Xylene added was 2.28 times the weight of water
present.
Under this situation, weight ratio in which xylene and water boil out was 2.07
when
heated slowly such that the rate of condensate collection .was only 0.50
g/min. This
allowed xylene to accumulate instead by preferentially removing water from
sludge
through boiling. Here, firstly the overall rate of heating of the failed
sample was high
=with condensate collection rate being 2.30 g/min due to which it started with
boiling
out little more solvent per unit mass of water removed as compared to slower
rate of
heating. Fortunately not much harm was done till 3rd collection. In 4th and
5th
collection more solvent left with fast heating. Additionally, towards the end
the rate
of heating was not slowed down for the failed sample. It was observed that the
temperature of residual furnace oil kept rising as the rate of heat supply far
exceeded
the requirement. With that then firstly water started going out rapidly since
had the
ability to soak up heat on account of its high latent heat. Therefore, from
7th
collection onwards, residual weight ratio of solvent to water that remained
behind
kept rising and it rose up dramatically after 9th collection. After most water
left then
with high temperature rate of solvent boiling went up so dramatically, at the
cost 'of
water removal. Consequently, after 10th collection inspite of the fact that
some water
was still left behind, there was hardly any solvent present to remove it. As
can be
seen in Table 11-A that at the end weight ratio of residual solvent to furnace
oil fell
down to 0.02. Taking out residual water from furnace oil was desirable for
this ratio
of initial xylene added by depressing its boiling point with the help from
large
amounts of residual solvent. This was how inspite of having exceeded 300 C the
entire water from furnace oil was removed.
Accordingly, it was ascertained that by opting for slow rate of heating
allowed less
solvent to go out by end of 6th collection and consequently a higher weight
ratio of
solvent to water was left behind in the RB Flask. Subsequently, heat closer to
the
required rate to boil out small amounts of water and solvent from furnace oil
was
supplied by drastically slowing the heating rate. From beginning of 7th till
the end of
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
103
1 1 th collection water boiled out only in slight preference to solvent and
not huge
preference as earlier. Consequently, in the end lot of solvent remained behind
in the
RB Flask to drive out the last bit of water without furnace oil temperature
rising only
up to 163 C and not 307 C.
Finally, it was observed that with fast heating rate water preferentially
boiled over
solvent by a relatively large margin as it required a lot more latent heat for
phase
= transformation. Also, it was observed that with ultra slow heating rate
once again
= water preferentially boiled over solvent provided that the residual
weight ratio of left
e.10 over solvent to water was high but by a relatively narrow margin as
reason being that
the rate of heat supply was not the driving factor. Lastly, it was observed
that the
water preferentially boiled out with very low rate of heating if solvent was
present in
higher quantity than water.
EXAMPLE -12
REMOVAL OF BOUND WATER FROM DIESEL SLUDGES CONTAINING
EMULSIFIER BY BOILING IT WITH AZEOTROPIC SOLVENTS
It was an aim of the experiment to evaluate the process of boiling out bound
and
unbound water with azeotropic solvents from diesel sludges containing
emulsifier(s).
Accordingly, predefined weight proportions of sludge containing emulsifier and
azeotropic solvent mixture were taken in the RB flask of Dean and Stark
Apparatus
and followed by continuous heating thereof in the mantle heater while
continuously
monitoring the temperature of material in RB Flask with digital thermometer.
The
vapours of bound water and solvent were collected in the receiver after
condensing
them with circulating cold water at 5 C to 6 C in the insulated condenser.
The
condensates were out and collected in separating flask using the stop cork at
the
bottom of the receiver. After phase separation, water and solvent collected
were
= individually weighed each time.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
104
TABLE 12- REMOVAL OF BOUND AND UNBOUND WATER FROM DIESEL
SLUDGES USING XYLENE & TOLUENE AT 933 mbar
SI.
DESCRIPTION TEST 1 TEST
2
No.
1 Wt. of Sludge taken in RB Flask (g) 150.43 151.60
2 Wt.% of Total Water Present in Sludge 48.00 48.20
3 Wt.% of Bound Water present in Sludge 2.91 2.90
4 Wt.% of Un-Bound Water present in Sludge 45.09 45.30
Wt. of Sodium Lauryl Sulphate Present in Sludge (g) 3.64 3.67
6 Wt. of Diesel Present in Sludge (g) 74.58 74.86
7 Name of Solvent Used Toluene Xylene
8 Wt of Solvent added in RB Flask (g) 722.30 402.36
9 Initial Ratio of Solvent to Water by Wt. 10.00 5.51
Observed Boiling Temperature Range ( C) 85.69-110.61 93.51-139.24
11 Initial Wt. Ratio of Solvent to Water Collected 6.00
2.15
12 Final Wt. Ratio of Solvent to Water. Collected 73.02 76.50
13 Average Wt. Ratio of Solvent to Water Collected 6.69
2.62
Wt.% Water collected during Experiment inclusive of
14 100.00 99.91
losses
Wt.% Solvent collected during Experiment inclusive
67.04 48.05
of losses
16 Wt.% of Diesel collected during Experiment 0.00 0.00
17 Rate of Water Collection (g/min) 0.47 0.74
Wt. Ratio of Solvent to Diesel Left over in RB Flask
18 at the End of Experiment 3.19 2.79
Residual Water present in left over Solvent cum
19 Diesel in ppm as determined by BTX Test 0 838
Wt. % Loss due to Evaporation, etc. 0.63 0.45
. It
was observed that only a tiny fraction of bound water was present in the
diesel
sludge when an emulsifier like sodium lauryl sulphate was added thereto. In
other
words, only about 6 wt.% of total water present got so tightly bound to diesel
that
5 even on centrifuging it for 10 minutes at 21,893 RCF none of that
water was
separated from diesel. The rate of water removal was about 2.2 times faster
when
using Xylene and about 1.6 times faster when using Toluene in comparison to
furnace oil sludge wherein the bound water was present in an amount of 49.81
wt. %,
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
105
as Clearly seen in 7.1C and 7.2. This was because only a small fraction of
water
present was bound water.
It was observed that water collection rate was however significantly lower
than that
observed in Table 5.2 where Xylene and Toluene removed the free water
respectively at the rate of 1.37 g/min and 0.67 g/min. It was observed here
that
Xylene ended up removing entire water from sludge, i.e. both bound and free
water,
as the weight of Xylene added was 5.51 times the weight of total water
present.
It was observed that Toluene and Xylene both remove the bound and unbound
water
present in sludge inspite of the fact that most of the water present was
unbound
water, still the final temperature shot up to 110.61 C and 139.24 C
respectively.
This was because boiling points of these solvents cannot be depressed any
further
once entire water was removed. Once entire water was removed what was left was
a
solution of solvents and diesel, where diesel was having a slightly higher
boiling
point. It was observed that at that stage solvents began to boil out at their
respective
boiling points under given ambient pressure with further application of heat.
Inspite
of the fact that final weight ratio of solvent to water collected was very
high, the
average ratio was still very small and only slightly higher than the initial
weight ratio
implies that there was a brief and sharp rise in weight ratio of solvent to
water only
towards the end of the process.
EXAMPLE-13
REMOVAL OF BOUND WATER FROM ONGC SLUDGES, WITH 42 WT. %
BOUND WATER THEREIN, BY BOILING WITH AZEOTROPIC SOLVENTS
It was an aim to evaluate implications of using different quantities of
various =
Solvents on removal of bound water from ONGC Viscous Sludges with 42.21 wt. %
bound water in it. Accordingly, predefined weight proportions of ONGC sludge
and
azeotropic solvent mixture were taken in the RB flask of Dean and Stark
Apparatus
and followed by continuous heating thereof in the mantle heater while
continuously
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
106
monitoring the temperature of material in RB Flask with digital thermometer.
The
vapours of bound water and solvent were collected in the receiver after
condensing
them with circulating cold water at 5 C to 6 C in the insulated condenser.
The
condensates were out and collected in separating flask using the stop cork at
the
bottom of the receiver. After phase separation, water and solvent collected
were
individually weighed each time.
ONGC Sludges with Bound Water-
TABLE 13.1- REMOVAL OF BOUND WATER FROM ONGC SLUDGES BY
VARYING PROPORTIONS OF SOLVENTS AT 933 mbar
SI
DESCRIPTION TEST
TEST TEST TEST
1 2 3 4
o.
1 Wt. of Sludge taken in RB Flask (g) 150.58 154.75 150.1
157.6
7 7
2 Wt.% Water Present in Sludge 42.21 42.21
42.21 42.21
3 Wt. of Hydrocarbons Present in Sludge (g) 87.02 89.43
86.78 91.12
Toluen Toluen Xylen Xylen
4 Name of Solvent Used
5 Wt. of Solvent added in RB Flask (g) 874.45 653.48
479.7 367.8
0 7
6 Initial Ratio of Solvent to Water by Wt. 13.76 10.00 7.57
5.53
7 Initial Ratio of Solvent to Hydrocarbons by Wt. 10.05 7.31
5.53 4.04
0 101.3
97.60-
93.1- 90.10- 2-
8 Observed Boiling Temperature Range ( C) 137.0
108.38 108.93 136.6
6
7
9 Initial Wt. Ratio of Solvent to Water Collected 4.98 4.92 2.05
2.04
10 Final Wt. Ratio of Solvent to Water Collected 50.13 127.17
54.36 17.75
11 Average Wt. Ratio of Solvent to Water Collected 6.06 6.03 2.53
2.27
Wt.% Water collected during Experiment
12 99.94 99.85 99.97 99.78
inclusive of losses
Wt.% Solvent collected during Experiment
13 44.38 59.94
33.84 41.75
inclusive of losses
Wt.% Hydrocarbons collected during the
14 0.00 0.00 0.00 0.00
Experiment
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
107
15 Average Rate of Water Collection (g/min) 0.26 0.45 0.34
0.90
Wt. Ratio of Solvent to Hydrocarbons Left over
16 5.59 2. 93 3.66 2.35
in RB Flask at the End of Experiment
Residual Water present in left over Solvent
17 cum ONGC Sludge in ppm as determined by 473 1,118 239
1,614
BTX Test
18 Wt. % Loss due to Evaporation, etc. 0.48 0.53 0.44 0.59
It was observed that when Toluene and Xylene were respectively aVed in weight
ratio of 10 and 5.5 with respect to either the weight of water or hydrocarbons
present
in sludge thereby facilitating addition of maximum quantity of solvent. It was
observed that slow rate of heating was preferred for both Toluene and Xylene
that
left behind more solvent over hydrocarbons in the end. It was established that
it was
possible to remove almost entire bound water present in viscous ONGC sludge
without allowing the temperature to rise above boiling points of these pure
solvents
by adding optimal quantum of solvent and with slow rate of heating and under
atmospheric pressure.
EXAMPLE- 14
REMOVAL OF BOUND WATER FROM DIFFERENT SLUDGES BY
COMBINED USE OF AZEOTROPIC SOLVENTS
It was an aim to evaluate implications of combining the use of Xylene and
Toluene
on removal of Bound Water from ONGC and Furnace Oil sludges with respectively
42.21 wt.% and 49.81 wt.% bound water therein. Accordingly, predefined weight
proportions of sludge and azeotropic solvent mixture were taken in the RB
flask of
Dean and Stark Apparatus and followed by continuous heating thereof in the
mantle
heater while continuously monitoring the temperature of material in RB Flask
with
digital thermometer. The vapourS, of bound water and solvent were collected in
the
receiver after condensing them with circulating cold water at 5 C to 6 C in
the
insulated condenser. The condensates were out and collected in separating
flask
using the stop cork at the bottom of the receiver. After phase separation,
water and
solvent collected were individually weighed each time.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
108
TABLE 14.1-REMOVAL OF BOUND WATER FROM SLUDGES WITH
COMBINED USE OF XYLENE and TOLUENE AT 933 mbar
SI ONGC Furnace
.
DESCRIPTION Viscous Oil
No.
Sludge Sludge
1 Wt. of Sludge taken in RB Flask (g) 150.48 151.16
2 Wt.% Water Present in Sludge 42.21 49.81
3 Wt. of Hydrocarbons Present in Sludge (g) 86.96 75.86
4 Wt of Xylene added in RB Flask (g) 239.28 207.34
Initial Ratio of Xylene to Water by Wt. 3.77 2.75
6 Initial Ratio of Xylene to Hydrocarbons by Wt. 2.75 2.73
7 Wt of Toluene added in RB Flask (g) 435.75 376.53
8 Initial Ratio of Toluene to Water by Wt. 6.86 5.00
9 Initial Ratio of Toluene to Hydrocarbons by Wt. 5.01 4.96
91.74- 87.68-
Observed Boiling Temperature Range ( C)
118.62 124.69
11 Initial Wt. Ratio of Solvent to Water Collected 4.18 4.45
12 Final Wt. Ratio of Solvent to Water Collected = 145.62 67.63
13 Average Wt. Ratio of Solvent to Water Collected 4.03 3.86
14 Wt.% Water collected during Experiment inclusive of losses 99.74
99.89
Wt.% Solvent collected during Experiment inclusive of losses 38.06 49.69
16 Wt.% Hydrocarbons collected during Experiment 0.00 0.00
17 Average Rate of Water Collection (g/min) 0.30 0.28
Wt. Ratio of Solvent to Hydrocarbons Left over in RB Flask
184.81 3.87
at the End of Experiment
Residual Water present in left over Solvent cum
191,893 1,097
Hydrocarbons in ppm as determined by BTX Test
Wt. % Loss due to Evaporation, etc. 0.64 0.65
5
These tests established that entire bound water can be removed from both ONGC
as
well as furnace oil sludges with combined use of Xylene and Toluene as
azeotropic
solvents. Also, it was observed that the observed maximum boiling temperature
was
almost mid way between maximum boiling temperatures when using these solvents
10 individually for the ONGC and furnace oil sludges.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
109
EXAMPLE-15
COMPLETE REMOVAL OF SOLVENTS FROM HYDROCARBONS BY
HEATING WITH FREE WATER
It was an aim of the experiment to establish and evaluate the process of
boiling out
entire solvents like Xylene, Toluene and Benzene from furnace oil at below 100
C
under atmospheric pressure of 933 mbar by adding free water therein.
Accordingly,
weighed amounts of furnace oil, solvent and water were added in specific
proportions in the RB Flask of Dean and Stark Apparatus followed by heating
them
in a mantle heater while periodically noting down the temperature of material
in the
RB Flask with digital thermometer. It was ensured that initial weight ratio of
solvent
to furnace oil was more than what was left behind in the RB Flask after
removing
entire bound water from furnace oil sludges. The vapors of water and solvent
that
boiled out were condensed in an insulated condenser where water was circulated
at 5
oC to 6 C. The condensates were collected in a receiver thereby using a stop
cork at
bottom of the receiver to periodically drain out condensate in separating
flask while
noting the time elapsed. After immediate phase separation the solvent and
water
collected were individually weighed. At the end material left in RB Flask was
weighed and mass balance thereof was performed.
TABLE 15.1A- REMOVAL OF XYLENE FROM FURNACE OIL WITH
VARYING PROPORTIONS OF FREE WATER AT 933 mbar
SI.
DESCRIPTION TEST TEST TEST
No. 1 2 3
1 Wt. of Furnace Oil Taken in RB Flask (g) 151.60 153.86 152.46
2 Wt of Solvent Taken in RB Flask (g) 910.51 923.44 915.03
3 Wt. of Free Water added in R13 Flask (g) 682.98 923.82 1,374.4
4
4 Initial Wt. Ratio of Water to Solvent 0.75 1.00 1.50
5 Initial Wt. Ratio of Solvent to Furnace Oil 6.00 6.00 6.00
6
Observed Boiling Temperature Range 97.03- 96.24- 96.74-
( C) 102.28 97.90 97.71
7 Initial Wt. Ratio of Solvent to Water 2.18 2.21 2.04
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
110
Collected
Final Wt. Ratio of Solvent to Water
0.52 0.08 0.03
8 Collected
Average Wt. Ratio of Solvent to Water
9 1.55 1.39 1.10
Collected
Total Wt. of Water Collected (g) 588.19 669.49 837.39
11 Total Wt. of Solvent Collected (g) 913.99 928.82
919.35
Average Rate of Solvent Collection
12 2.64 3.23 2.68
(g/min)
Wt. of Furnace Oil Left behind in RB
13 148.12 148.48 148.14
Flask at the End of Experiment (g)
Wt. of Free Water left behind in RB Flask
14 87.03 246.24 529.96
at the end of Experiment(g)
Wt. % Loss due to Evaporation, etc. 0.33 0.40 0.29
TABLE 15.1B- REMOVAL OF XYLENE FROM FURNACE OIL WITH VARYING
PROPORTIONS OF FREE WATER AT 933 mbar
SI.
TEST 1 TEST TEST TEST 4
DESCRIPTION
No. 2 3
Wt. of Furnace Oil Taken in RB Flask
1 151.18 150.93 153.31 151.32
(g)
2 Wt of Solvent Taken in RB Flask (g) 453.55
452.94 460.26 454.23
Wt. of Free Water added in RB Flask
3 454.02 679.55 921.76 1,135.85
(g)
4 Initial Wt. Ratio of Water to Solvent 1.00 1.50 2.00
2.50
Initial Wt. Ratio of Solvent to Furnace
5 3.00 3.00 3.00 3.00
Oil
Observed Boiling Temperature Range 97.85- 96.93- 96.89- 96.53-
6
( C) 114.7 109.05 97.58 97.51
Initial Wt. Ratio of Solvent to Water
7 2.14 2.17 2.07 1.93
Collected
Final Wt. Ratio of Solvent to Water
8 0.11 0.09 0.05 0.05
Collected
Average Wt. Ratio of Solvent to
9 1.26 1.17 1.05 0.86
Water Collected
10 Total Wt. of Water Collected (g) 364.39 392.49 489.69 534.10
11 Total Wt. of Solvent Collected (g) 458.72
458.02 464.66 458.47
Average Rate of Solvent Collection
12 2.16 2.82 1.76 2.16
(g/min)
Wt. of Furnace Oil Left behind in RB
13 146.01 145.85 148.91 147.08
Flask at the End of Experiment (g)
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
111
Wt. of Free Water left behind in RB
14
84.09 283.23 424.34 596.02
Flask at the end of Experiment(g)
15 Wt. % Loss due to Evaporation, etc. 0.52 0.23
0.50 0.33
TABLE 15.2A- REMOVAL OF TOLUENE FROM FURNACE OIL WITH VARYING
PROPORTIONS OF FREE WATER AT 933 mbar
SI. TEST TEST TEST TEST
DESCRIPTION
No. 1 2 3 4
1 Wt. of Furnace Oil Taken in RB Flask (g) 151.90 153.81 150.95
150.32
2 Wt of Solvent Taken in RB Flask (g) 456.13 461.66 453.01
450.42
3 Wt. of Free Water added in RB Flask (g) 228.62 346.72 453.70
675.64
4 Initial Wt. Ratio of Water to Solvent 0.50 0.75 1.00
1.50
Initial Wt. Ratio of Solvent to Furnace Oil 3.00 3.00 3.00 3.00
Observed Boiling Temperature Range 96.15- 96.89- 96.40- 95.90-
. 6
( C) 121.44 103.86 98.30 97.6
Initial Wt. Ratio of Solvent to Water
7 6.00 672 5.11 4.73
Collected
Final Wt. Ratio of Solvent to Water
8 0.21 0.18 4.15 1.98
Collected
Average Wt. Ratio of Solvent to Water
9 2.77 2.89 2.35 1.87
Collected
Total Wt. of Water Collected (g) 166.29 160.54 194.22 241.15
11 Total Wt. of Solvent Collected (g) 461.03 464.68 456.24 452.12
Average Rate of Solvent Collection
12 3.48 3.16 3.72 2.29
(g/min)
Wt. of Furnace Oil Left behind in RB
13 147.00 150.79 147.72 148.62
Flask at the End of Experiment (g)
Wt. of Free Water left behind in RB Flask
14 58.65 182.21 252.54 417.00
at the end of Experiment(g)
Wt. % Loss due to Evaporation, etc. 0.44 0.41 0.66 1.37
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
112
TABLE 15.2B-REMOVAL OF TOLUENE FROM FURNACE OIL WITH
VARYING PROPORTIONS OF FREE WATER AT 933 mbar
SI. TEST TEST TEST
DESCRIPTION
No. 1 2 3
1 Wt. of Furnace Oil Taken in RB Flask (g) 150.59 150.20
150.00
= 2 Wt of Solvent Taken in
RB Flask (g) 602.89 600.95 600.01
3 Wt. of Free Water added in RB Flask (g) 301.66 450.90
600.36
4 Initial Wt. Ratio of Water to Solvent 0.50 0.75
1.00
= 5 Initial Wt. Ratio of
Solvent to Furnace Oil 4.00 4.00 4.00
Observed Boiling Temperature Range 95.17- 94.78- 97.28-
6
( C) 111.78 111.98 98.5
Initial Wt. Ratio of Solvent to Water
7 6.61 4.97 4.85
Collected
Final Wt. Ratio of Solvent to Water
8 0.74 0.11 0.08
Collected
Average Wt. Ratio of Solvent to Water
9 5.06 2.48 2.38
Collected
Total Wt. of Water Collected (g) 119.60 244.24 252.31
11 Total Wt. of Solvent Collected (g) 605.84 606.01 602.66
Average Rate of Solvent Collection
12 6.42 4.04 2.96
(g/min)
Wt. of Furnace Oil Left behind in RB
13 147.64 145.14 147.35
Flask at the End of Experiment (g)
Wt. of Free Water left behind in RB Flask
14 177.53 199.47 343.86
at the end of Experiment(g)
Wt. % Loss due to Evaporation, etc. 0.43 0.60 0.31
TABLE 15.3A- REMOVAL OF BENZENE FROM FURNACE OIL WITH VARYING
PROPORTIONS OF FREE WATER AT 933 mbar
SI.
DESCRIPTION TEST 1 TEST 2 TEST 3
TEST 4
No.
Wt. of Furnace Oil Taken in
1 151.64 150.45 151.88 154.18
RB Flask (g)
Wt of Solvent Taken in RB
2 303.29 301.05 304.40
308.36
________________ Flask (g)
Wt. of Free Water added in
3 151.70 301.53 456.77 616.83
RB Flask (g)
= Initial Wt. Ratio of Water to
4 0.50 1.00 1.50 2.00
Solvent
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
113
Initial Wt. Ratio of Solvent to
2.00 2.00 2.00 2.00
Furnace Oil
Observed Boiling 77.39- 80.85- 82.27-
6 86.70-98.31
Temperature Range ( C) 135.43 105.00 97.82
Initial Wt. Ratio of Solvent to
7 54.92 67.90 558.63 64.73
Water Collected
Final Wt. Ratio of Solvent to
0.16 0.08 0.03 0.03
8 Water Collected
Average Wt. Ratio of Solvent
9 5.19 - 2.94 2.26 1.82
to Water Collected
Total Wt. of Water Collected
59.23 103.51 136.28 170.95
(g)
Total Wt. of Solvent Collected
307.61 304.68 307.89 312.14
11 _____ (g)
Average Rate of Solvent
12 3.55 2.77 2.81 2.28
Collection (g/min)
Wt. of Furnace Oil Left
13 behind in RB Flask at the End 147.32 146.82 148.39
150.40
of Experiment (g)
Wt. of Free Water left behind
14 in RB Flask at the end of 87.66 192.59 315.54 438.83
Experiment(g)
Wt. % Loss due to
0.79 0.72 0.54 0.77
Evaporation, etc.
TABLE 15.3B- REMOVAL OF BENZENE FROM FURNACE OIL WITH
VARYING PROPORTIONS OF FREE WATER AT 933 mbar
SI.
TEST TES
TEST 1
DESCRIPTION TEST 4
No. 2 T3
Wt. of Furnace Oil Taken in RB Flask 151.16 150.71 158.58
160.
1
(g) 10
Wt of Solvent Taken in RB Flask (g) 480.
475.74
48
Wt. of Free Water added in RB Flask 912.29 453.21 951.49
720.
3
(g) 95
4 Initial Wt. Ratio of Water to Solvent 3.02 1.00 1.50
2.00
Initial Wt. Ratio of Solvent to Furnace
5 2.00 3.01 3.00 3.00
Oil
80.7
Observed Boiling Temperature Range 86.50- 77.78- 1- 80.12 -
6
( C) 97.63
111.98 102. 98.59
5
7 Initial Wt. Ratio of Solvent to Water 114.52 56.28 106.
47.31
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
114
Collected 53
Final Wt. Ratio of Solvent to Water
8 0.05 0.03 0.04 0.05
Collected
9
Average Wt. Ratio of Solvent to Water
2.09 2.98 3.04 3.34
Collected
Total Wt. of Water Collected (g) 159.
145.64 152.88 3 143.37
8
Total Wt. of Solvent Collected (g) 485.
11 304.68 455.37 479.55
09
Average Rate of Solvent Collection
12 2.76 3.06 3.46 3.79
(g/min)
Wt. of Furnace Oil Left behind in RB 155.
13 148.88 147.58 154.77
Flask at the End of Experiment (g) 49
14 Wt. of Free Water left behind in RB 556.
761.77 290.45 801.55
Flask at the end of Experiment(g) 51
Wt. % Loss due to Evaporation, etc. 0.36 0.94 0.34 0.41
TABLE -15.4- COMPLETE REMOVAL OF 50:50
XYLENE AND TOLUENE FROM FURNACE OIL WITH
FREE WATER AT 933 mbar
SI. TEST
DESCRIPTION
No. 1
1 Wt. of Furnace Oil Taken in RB Flask (g) 151.21
2 Wt of Toluene Taken in RB Flask (g) 226.65
3 Wt of Xylene Taken in RB Flask (g) 227.16
4 Wt. of Free Water added in RB Flask (g) 682.02
5 Initial Wt. Ratio of Water to Xylene 3.00
6 Initial Wt. Ratio of Xylene to Furnace Oil 1.50
7 Initial Wt. Ratio of Water to Toluene 3.00
8 Initial Wt. Ratio of Toluene to Furnace Oil 1.50
Observed Boiling Temperature Range 96.20-
( C) 97.18
Initial Wt. Ratio of Solvent to Water
10 3.65
Collected
Final Wt. Ratio of Solvent to Water
11 0.06
Collected
=
Average Wt. Ratio of Solvent to Water
12 1.35
Collected
13 Total Wt. of Water Collected (g) 337.21
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
115
14 Total Wt. of Solvent Collected (g) 456.61
15 Average Rate of Solvent Collection
1.58
(gimin)
Wt. of Furnace Oil Left behind in RB
148A1
16 Flask at the End of Experiment (g)
Wt. of Free Water left behind in RB Flask
17 339.40
at the end of Experiment(g)
18 Wt. % Loss due to Evaporation, etc. 0.42
TABLE 15.5-COMPLETE REMOVAL OF SOLVENT FROM
DIESEL WITH FREE WATER AT 933 rnbar
SI. TEST TEST
DESCRIPTION
No. 1 2
1 Wt. of Diesel Taken in RB Flask (g) 75.35 75.29
Name of Solvent taken Toluen
2 Xylene
3 Wt of Solvent Taken in RB Flask (g) 301.15 226.07
4 Wt. of Free Water added in RB Flask (g) 303.20 454.56
Initial Wt. Ratio of Water to Solvent 1.01 2.01
6 Initial Wt. Ratio of Solvent to Diesel 4.00 3.00
Observed Boiling Temperature Range 92.54- 96.50-
( C) 97.24 97.27
Initial Wt. Ratio of Solvent to Water
8 5.17 1.98
Collected
9 Final Wt. Ratio of Solvent to Water
0.82 0.61
Collected
Average Wt. Ratio of Solvent to Water
a.57 1.15
Collected
ii Total Wt. of Water Collected (g) 86.33 200.23 -
12 Total Wt. of Solvent Collected (g) 308.36 232.99
Average Rate of Solvent Collection
13 3.30 2.04
(g/min)
Wt. of Diesel Left behind in RB Flask at
14 68.14 68.37
the End of Experiment (g)
Wt. of Free Water left behind in RB Flask
211.77 250.15
at the end of Experiment(g)
16 Wt. % Loss due to Evaporation, etc. 0.75 0.55
TABLE 15.6- COMPLETE REMOVAL OF SOLVENT FROM ONGC
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
116
VISCOUS DEWATERED HYDROCARBONS WITH FREE WATER
AT 933 mbar
SI. TEST TEST
DESCRIPTION
No. 1 2
Wt. of ONGC Hydrocarbons Taken in RB
1 86.07 86.09
Flask (g)
Toluen
2 Name of Solvent taken Xylene
3 Wt. of Solvent Taken in RB Flask (g) 378.73 322.13
4 Wt. of Free Water added in RB Flask (g) 379.16 644.34
Initial Wt. Ratio of Water to Solvent 1.00 2.00
Initial Wt. Ratio of Solvent to ONGC
6 4.40 3.74
Hydrocarbons
Observed Boiling Temperature Range 88.21- 93.30-
( C) 99.40 96.33
Initial Wt. Ratio of Solvent to Water
4.89 1.98
8 Collected
9 Final Wt. Ratio of Solvent to Water
0.08 0.03
Collected
Average Wt. Ratio of Solvent to Water
2.16 1.07
Collected
11 Total Wt. of Water Collected (g) 177.25 304.88
12 Total Wt. of Solvent Collected (g) 382.89 326.77
Average Rate of Solvent Collection
13 1.35 1.17
(g/min)
Wt. of ONGC Hydrocarbons Left behind
14 81.91 81.45
in RB Flask at the End of Experiment (g)
Wt. of Free Water left behind in RB Flask 196.82 335.34
at the end of Experiment(g)
16 Wt.% Loss due to Evaporation, etc. 0.60 0.39
It was observed that the solvent cannot be entirely boiled out from the
furnace oil in
absence of free water without the boiling point of solvent eventually rising
up to 350
C which was the initial boiling point for pure furnace oil. It was observed
that in
5 case where initial weight ratio of solvent to furnace oil as 1 or more,
the solvent
might invariably begin to boil at boiling point of pure solvent under similar
pressure.
But eventually with last bits of solvents boiling out, its boiling point may
approach
that of pure furnace oil, that being 350 C under atmospheric pressure of 933
mbar.
For Toluene, this was clearly seen in Table 6.1. However, it was seen that
entire
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
117
solvent can be boiled out from same furnace oil at less than 100 C under a
pressure
of 933 mbar when free water was present in appropriate quantity. For Toluene,
the
boiling temperature range in present case was observed to be 95.90 to 97.60 C
as
seen from Table 15.2A. However, said range was 110.93 to 350.15 without
presence
of free water as can be seen from Table 6.1.
Further, it was observed that by adding Xylene 5.5 times the weight of water
was
present in the sludge when removing almost entire bound water from the furnace
oil
sludge with 50 wt. % bound water therein. It was seen that at the end of the
process =
weight ratio of Xylene to furnace oil that was left behind was 3.59.
Therefore,
Xylene was added= 6 times the weight of furnace oil and water added was 3
times the
weight of furnace oil present. It was found that the boiling temperature was
in a
range of 96.24 to 97.90 C in case where Xylene was added 6 times the weight of
furnace oil and when water added was 1 times the initial weight of Xylene. It
was
found that the boiling temperature was in a range of 96.89 C to 97.58 C in
case
where Xylene was added 3 times the weight of furnace oil and water added was
2.00
times the initial weight of Xylene. Accordingly, it was ascertained that with
less
proportion of solvent present more free water was needed to retain boiling
point
range of solvent below 100 C.
Further, as seen earlier at the end of bound water removal from above furnace
oil
sludge, the weight ratio of Toluene to furnace oil left behind was 3.09 as per
table
7.2 and the weight ratio of benzene to furnace oil left behind was 2.98 as per
table
7.3. Hence, the process was started by adding toluene 4 times the weight of
furnace
oil present and then 3 times the weight of furnace oil present. A preferred
initial
weight ratio of free water to solvent was 1 in both cases. With preferred
amount of
free water, boiling point range for Toluene was 97.28 C to 98.50 C and 96.40
C to
98.30 C respectively.
For benzene, the weight ratio Benzene was 3 times the weight of furnace oil
present
and then 2 times the weight of furnace oil present. It was seen that for 3
times
benzene, preferred initial weight ratio of free water to solvent was 2.
However with 2
times benzene, preferred initial weight ratio of free water to solvent was
1.50 times
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
118
instead. It was seen that for both these preferred quantum of free water, the
boiling
temperature range was 80.12 - 98.59 C and 86.70 - 98.31 C respectively.
Accordingly, it was ascertained that apparently there was no limitation on how
much
solvent could initially be present in furnace oil or type of solvent that
could be
present. However, entire solvent, whether benzene, toluene or xylene, could be
removed through boiling at temperatures below 100 C by adding appropriate
quantity of free water prior to heating. In fact, more the initial solvent
present often
less was the weight ratio of free water to solvent to be added. Further, it
was seen
that increasing the quantum of free water beyond a certain limit, not only the
boiling
point range for solvent fell down but also the quantity of solvent removed by
unit
mass of water boiling out also fell down.
In all cases more than 100 wt.% solvent was collected inspite of not
considering
some solvent that would have evaporated. That was because towards the end
boiling
was terminated after collecting some furnace oil too. Yet, it was seen that
the final
boiling point for solvent always remained below 100 C under 933 mbar. Along
with
solvent some furnace oil was also collected only to ensure 100% removal of
solvent.
Therefore, furnace oil got slightly depleted. But once this solvent was re-
used there
could be no further depletion of furnace oil.
It was seen that average weight ratio of solvent to free water collected was
almost
always less than average weight ratio of solvent to bound water collected. The
average collection temperature was also observed to be less with preferred
initial
weight ratio of free water to solvent.
As seen in Table 15.4, when mixed solvents like xylene and toluene were
present in
furnace oil for instance in 50:50 ratio by weight, even they can be entirely
removed
through boiling, at temperatures below 100 C by ensuring that the weight of
free
water added was 1.50 times the combined weight of initial solvents present.
However, as indicated in Table 15.4, when using mixed solvents to boil out
entire
bound water from sludges, the weight ratio of solvent to furnace oil left
behind at the
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
119
end of the process was higher. Apparently there was no upper limit on how much
solvent can be present in furnace oil, as long as appropriate amount of free
water was
added prior to boiling.
As can be seen in Table nos. 15.5 and 15.6 that entire solvent present can be
boiled
out below the predefined temperature in case of hydrocarbons such as free
flowing
Diesel or highly viscous dewatered ONGC hydrocarbons and the like. In case
where
hydrocarbons have salt and/or ash or solids therein, then free water may
perform an
additional function of de-salting and de-ashing apart from boiling out entire
pure
-10 solvent for re-use or sale at temperatures below 100 C.
EXAMPLE -16
SEPARATION OF FREE WATER AND FURNACE OIL
It was an aim to establish that free water can be separated from even viscous
= hydrocarbons with time through gravity based settling or centrifuge.
Accordingly,
weighed amounts of viscous furnace oil and free water were taken in the RB
flask
and vigorously boiled for 15 minutes. Thereafter, in hot condition the
contents were
transferred in a pre-heated and insulated separating flask. It was seen that
bulk of
free water separated from immiscible furnace oil due to density difference and
gravity. Accumulated free water was removed after about 30 minutes from bottom
of
separating flask. The remaining material within the separating flask, after
removing
its insulation, inside hot air oven for 48 hours while maintaining its
temperature at 90
C. Periodically, the collected water was removed from bottom of separating
flask.
After 48 hours, the remaining material was taken out, homogenized and then
tested
for residual moisture using the BTX process. Subsequently, the boiling was
repeated
and the hot material was transferred into un-insulated separating flask. The
material
was removed soon after removal of bulk of free water therefrom. The remaining
furnace oil with 17.33 wt.% moisture was then taken out. Part of it was again
heated
and rest was allowed to cool to a room temperature. Both these hot and cold
fractions
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
120
were centrifuged for 5 minutes at 4,500 RCF. After centrifuge, 150 g of
furnace oil
was removed from top and tested for moisture through BTX Process.
TABLE 16.1- SEPARATION OF FREE WATER FROM FURNACE OIL THROUGH
GRAVITY BASED SETTLING
SI.
TEST 1 TEST TEST
DESCRIPTION
No. 2 3
1,000.2 1,001.6
1 Wt. of Furnace Oil taken For Treatment (g) 1,000.18 8
6
1,001.6 1,000.2
2 Wt. of Water taken for boiling (g) 1,000.58
1 3
3 Loss in weight during boiling (g) 10.22 9.90 9.54
4 Pouring temperature of boiled mixture in
98.27 97.93 98.95
insulated separating flask ( C)
Loss in weight during pouring in Separating
38.43 50.98 33.49
Flask (g)
6 Holding Time (min) 29.52 30.12 32.84
Temperature of Material at the time of
7 92.50 93.20 93.80
removing Free Water from bottom ( C)
Wt. of Water collected from insulated
8 927.35 931.27 934.23
separating flask (g) =
9 Turbidity of above Water (NTU) 6.86 6.79 7.02
Remaining Wt. of Furnace Oil + Free Water
1 024.76 1,009.7'
1,024.6
in Separating Flask (g) 4 3
11 Holding Time in Oven (hrs) 48.00 48.00 48.00
12 Set Temperature of Oven ( C) 90.00 90.00 90.00
13 Wt. of Total Water Collected Periodically
47.62 40.24 41.84
from Separating Flask in 48 Hours
14 Temperature of material Immediately after
83.40 84.1 83.50
taking out from Oven ( C)
Wt. of Furnace oil Collected at the end of
957.57 943.70 962.72
Experiment
Water Present in recovered Furnace Oil
16 3,509 2,590 3,197
through BTX Process in ppm
Wt. of Material adhering to various surfaces
11.28 13.03 12.22
17
(g)
18 Wt. of Material lost due to Evaporation, etc. 8.29 10.33
7.85
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
121
TABLE 16.2- SEPARATION OF FREE WATER FROM FURNACE OIL
BY HOT CENTRIFUGE
SI. No. DESCRIPTION TEST TEST
1 2
1 Wt. of Furnace Oil + Free water taken For 1,043.4 1,141.1
Centrifuge (g) 9 4
W. t= % Water present in above Material
2 16.17 16.48
before Centrifuge
3 Temperature of material before Centrifuge
87.88 90.01
( C)
Time taken in minutes to Reach Max. 2.80 2.75
4
Relative Centrifugal Force
Max. Relative Centrifugal Force at which
4,500 4,500
Centrifuge operated
Holding Time at Max. Relative Centrifugal
6 5 5 00 00
. .
Force in minutes
Time taken in minutes to reduce RPM to
7 17.15 16.50
Zero
Total Residence Time in minutes inside
8 Centrifuge 24.95 24.25
Temperature of material after Centrifuge
9 59.90 60.01
( C)
Residual Water present in Furnace Oil after
Centrifuge as determined by BTX Process 5,313 2,987
(1)Pm)
=
TABLE 16.3- SEPARATION OF FREE WATER FROM FURNACE OIL
BY COLD CENTRIFUGE
SI. No. DESCRIPTION TEST TEST
1 2
1 Wt. of Furnace Oil + Free water taken For 1,135.8 1,128.8
Centrifuge (g) 3 3
Wt. % Water present in above Material
2 17.33 17.28
before Centrifuge
3 Temperature of material before Centrifuge 32.10 30.90
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
122
( C)
Time taken in minutes to Reach Max.
2.90 2.65
Relative Centrifugal Force
Max. Relative Centrifugal Force at which
6 4500 4500
Centrifuge operated
Holding Time at Max. Relative Centrifugal
7 5.00 5.00
Force in minutes
8 Time taken to de-accelerate to zero RPM 16.35 16.25
Total Residence Time in minutes inside
9 24.25 23.90
centrifuge
Temperature of material after Centrifuge
33.88 32.98
__________ 1 C)
Residual Water in Furnace Oil after
1,17,89 1,12,68
11 Centrifuge as determined by BTX Process
5 8
(ppm)
It was observed that, it was difficult to remove entire free water from the
furnace oil
being relatively viscous. It has to be heated to about 99 C to reduce its
viscosity and
then transferred hot with least fall in temperature, into a pre-heated and
well.
5 insulated
separating flask. On retaining there for about 30 minutes with less than 6
oC fall in temperature, about 94 wt. % to 95 wt. % of water drained out and
collected
at the bottom of the separating flask. Further, the entire remaining material
was
heated at about 85-90 C to obtain remaining 5 wt.% to 6 wt.% water in next 48
hours. Finally it was observed that less than 3,500 ppm residual water was
left in
10 Furnace oil
after periodical removal of free water collected from bottom of the
separating flask. Accordingly, the parameters like the settling time required,
maximum temperature needed for heating and residual water content in viscous
hydrocarbon were established. It was seen that about 83 wt. % water was
removed
by gravity settling under hot condition followed by centrifuging it for 5
minutes at
4,500 RCF. But still residual moisture in Furnace Oil fell down from 17.3 wt.
% to
11.8 wt. %. However, hot centrifuge with inlet temperature of 90 C however
worked wherein residual moisture in Furnace Oil was reduced from 16.48 wt.% to
2,900 ppm after centrifuging for 5 minutes at 4,500 RCF. It was evident that
the
recovered water was perfect for industrial use with turbidity values of 6 NTU
to 7
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
123
NTU which was almost oil free that could be further processed for production
of
drinking water.
EXAMPLE - 17
RECOVERY OF PURE HYDROCARBONS, BOUND WATER, SOLVENT AND
THEN FREE WATER FROM PETROLEUM SLUDGES
It was an aim to quantitatively and qualitatively retrieve back pure
hydrocarbons and
entire water, inclusive of entire bound water, present in various sludges and
also
retrieve back the entire solvent and free water in accordance with process of
the
present invention.
Accordingly, weight fraction of bound and unbound water present in sludges
were
firstly determined and then calculated amount of solvent was added therein
followed
by heating in a Dean and Stark Apparatus using Mantle Heater. Accordingly,
entire
bound and free water present in sludge was removed with combined effect of
solvent
cum heat. Subsequently, entire water was condensed and collected along with
part of .
solvent used. Further, a calculated amount of free water was added to residual
matter
in RB Flask and once again heated using the same apparatus. Subsequently,
entire
remaining solvent was removed and collected along with some free water.
Thereafter, the entire amount of remaining free water from residual
hydrocarbons
was collected through gravity separation after heating the hydrocarbons and
retaining
them in a hot condition for a predefined time period in case where
hydrocarbons
were found viscous. Finally, both the waters and hydrocarbons were evaluated
for
their quality/purity and in addition the quantities retrieved were evaluated
by doing a
mass balance study.
Furnace Oil Sludges-
TABLE 17.1-REMOVAL OF ENTIRE BOUND WATER FROM FURNACE OIL
SLUDGES WITH BOUND WATER ALONE, BY BOILING WITH AZEOTROPIC
SOLVENTS
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
124
SI.
DESCRIPTION TEST 1 TEST 2
No.
1 Wt. of Sludge taken (Kg) 1.002 1.000
2 Wt.% of Bound Water Present in Sludge 49.81 49.81
3 Wt. % of Furnace Oil Present in Sludge 50.19 50.19
4 Name of Solvent added Xylene Toluene
Wt. of Solvent added (Kg) ,2.742 4.981
6 Initial Wt. Ratio of Solvent to Water Present 5.50
10.00
Initial Wt. Ratio of Solvent to Furnace Oil
7 5.46 9.92
Present
Observed Boiling Temperature Range
8 96.5-136.9 89.4-108.3
( C)
Initial Wt. Ratio of Solvent to Water
9 2.06 4.82
Collected
Final Wt. Ratio of Solvent to Water
10.36 14.87
Collected
11 Average Wt. Ratio of Solvent to Water
1.92 6.93
Collected
Wt.% Water collected during Experiment
12 99.96 98.13
inclusive of losses
Wt.% Solvent collected during Experiment
13 34.81 67.95
inclusive of losses
Wt. Ratio of Solvent to Furnace Oil Left over
14 3.55 3.11
in RB Flask at the End of Experiment
Residual Water present in left over
Solvent cum Furnace Oil in PPM as 410 1,119
determined by BTX Test
5 TABLE
17.2- REMOVAL OF ENTIRE SOLVENTS= FROM DE-WATERED
FURNACE OIL BY USING FREE WATER
SI.
DESCRIPTION TEST 1 TEST 2
No.
Wt. of De-Watered Furnace Oil present in
1 0.503 0.502
RB Flask (kg)
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
125
2 Wt. of Solvent Present in RB Flask (kg) 1.7835 1.561
3 Wt. of Free Water added in RB Flask (kg) 3.569 1.561
4 Initial Wt. Ratio of Water to Solvent 2.00 1.00
Observed Boiling Temperature Range
96.89-97.58 96.40-98.30
( C)
Initial Wt. Ratio of Solvent to Water
6 2.07 5.12
Collected
Final Wt. Ratio of Solvent to Water
7 0.05 4.14
Collected
Average Wt. Ratio of Solvent to Water
8 1.05 2.35
Collected
9 Total Wt. of Free Water Collected (kg) 1.896 0.67
Total Wt. of Solvent Collected (kg) 1.788 1.565
Wt. of Furnace Oil left behind in RB Flask at
11 0.499 0.498
the end of Experiment (kg)
Wt. of Free Water left behind in RB Flask at
1.634 0.86
12 the end of Experiment(kg)
13 Wt. % Loss due to Evaporation, etc. 0.51 0.66
TABLE 17.3- REMOVAL OF ENTIRE FREE WATER FROM SOLVENT AND
BOUND WATER FREE FURNACE OIL
SI.
DESCRIPTION TEST 1 TEST 2
No.
Total Wt. of Furnace Oil & Free Water taken
1 2.142 1.367
for further processing (kg)
Total Wt. of Water collected by Gravity
2 Separation in 48 hours when retained at 85 to 1.587 0.844
90uC (kg)
3 Wt. of Furnace Oil recovered (kg) 0.493 0.492
4 Moisture in Furnace Oil as per BTX
3,320 4,291
(PP1n)
I 5 Turbidity of recovered Free Water (NTU) 4.8 3.9
Wt.% Material lost due to Evaporation,
6 2.86 2.23
adhering to various surfaces, etc.
5 TABLE 17.4- TEST RESULTS
SI.
Description TEST 1 TEST 2
No
1 Wt. % Furnace Oil recovered 98.90 98.81
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
126
Calorific value of recovered Furnace Oil
2 10,182 10,169
(kcal/kg)
Calorific value of original Furnace Oil
3 10,173 10.173
(kcal/kg)
Wt. % Solvent recovered for Re-use
4 inclusive of that lost by adhering to 99.54 99.13
glasswares
Wt. % Bound Water recovered for Re-use 99.95 98.13
6 Wt. % Free Water recovered for Re-use 96.60 97.18
TABLE 17.5- QUALITY OF RECOVERED BOUND WATER FROM FURNACE OIL
SLUDGE
UNIT
OF PERMISS
SL. TEST
METHOD MEASU IBLE RESULTS
NO. PARAMETERS
REMEN LIMITS
= Hazen
1 Max 15 1.00
Colourhazen IS:3025 P4 Units
IS:3025 (Part
2 Agreeable Agreeable
Odour 5) - 1983
IS:3025 (Part
3 0 6.5-8.5 7.5
_____ pH at 25 C 11) - 1983
IS:3025 (Part 7
4 Agreeable Agreeable
Taste & 8) - 1984
IS:3025 (Part
NTU Max 5 1.4
5 Turbidity 10) - 1984
Dissolved solids at IS:3025 (Part
6 mg/L Max 2000 46.00
180 C 16) - 1984
7 Aluminium as Al APHA 3125 mg/L Max 0.2 <0.005
Ammonia (as Total
8 mg/L Max 0.5 <0.05
Ammonia-N) IS:3025 P34
Anionic surface
9 active agents (as Annex K of IS: mg/L Max 1.0
<0.02
MBAS) 13428
Barium as Ba APHA 3125 mg/L Max 0.7 <0.005
11 Boron as B EPA 200.8 mg/L Max 1.0 <0.005
IS:3025 (Part
12 mg/L Max 200 8.80
Calcium as Ca 40) - 1991
Chloramines (as
mg/L = Max 4.0 <0.05
13 C12) IS:3025 P26
14 Chloride as Cl EPA 300.1 mg/L = Max 1000 2.77
Copper as Cu APHA 3125 mg/L Max 1.5 <0.005
16 Fluoride as F EPA 300.1 mg/L Max 1.5 <0.1
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
127
Free residual IS:3025 (Part
17 mg/L MM 1.0 <0.1
Chlorine 26) - 1986
18 Iron as Fe APHA 3125 mg/L Max 0.3 <0.005
IS:3025 (Part
19 mg/L Max 100 2.43
Magnesium as Mg 46) - 1994
20 Manganese as Mn APHA 3125 mg/L Max 0.3 <0.005
clause 6 of
21 IS:3025 Part39 mg/L Max 0.5 Absent
Mineral Oil -1991
22 Nitrate as NO3 EPA 300.1 mg/L Max 45 10.12
Phenolics as IS:3025 Part 43
mg/L Max 0.002 <0.001
23 C6H5OH - 1992
24 Selenium as Se APHA 3125 mg/L Max 0.01 <0.001
25 Silver as Ag APHA 3125 mg/L Max 0.1 <0.005
26 Sulphate as SO4 EPA 300.1 mg/L Max 400 2.60
IS:3025 (Part
27 mg/L Max 9.05 <0.02
Sulphide as H2S 29) - 1986
Total Alkalinity as
28 mg/L Max 600 40.00
CaCo3 IS:3025 P-23
Total Hardness as
29 mg/L Max 600 32.00
CaCo3 IS:3025 P21
30 Zinc as Zn APHA 3125 mg/L Max 15 <0.005
31 Cadmium as Cd APHA 3125 mg/L Max 0.003 <0.001
32 Cyanide as CN IS:3025 P-34 mg/L Max 0.05 <0.02
33 Lead Pb APHA 3125 mg/L Max 0.01 <0.005
34 Mercury as Hg EPA 200.8 mg/L Max 0.001 <0.0005
Molybdenum as
35 ing/L Max 0.07 0.002
Mo APHA 3125
36 Nickel as Ni APHA 3125 mg/L Max 0.02 <0.005
37 Total Arsenic as APHA 3125 mg/L Max 0.05 <0.001
Total Chromium as
38 mg/L Max 0.05 <0.005
Cr APHA 3125
Chemical Oxygen
mg/L 15.00
39 Demand SM:5002-B
Total Organic IS:3025 (part
40 mg/L 17.65
Carbon NO. 44)
Biochemical
41 Oxygen Demand - APHA-5310-B mg/L 4.00
3 days at 27 C
MPN/100
42 <10 <2
Coliforms IS 1622:1981 ml
MPN/100
43 Absent Absent
Escherichia Coli IS 1622:1981 ml
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
128
TABLE 17.6- REMOVAL OF ENTIRE BOUND WATER FROM ONGC VISCOUS
SLUDGES WITH BOUND WATER ALONE, BY BOILING WITH AZEOTROPIC
SOLVENTS
SI. No. DESCRIPTION TEST 1 TEST 2
1 Wt. of Sludge taken (kg) 1.000 1.001
2 Wt.% Bound Water Present in Sludge 42.21 42.21
3 Wt.% Hydrocarbon Present in Sludge 57.79 57.79
4 Name of Solvents added Xylene Toluene
Wt. of Solvent added (kg) 3.195 5.815
Initial Wt. Ratio of Solvent to Water
6 , 7.57 13.76
added
Initial Wt. Ratio of Solvent to
7 5.53 10.05
Hydrocarbon added
Observed Boiling Temperature Range
8 101.21-136.61 93.08-108.32
( C)
Initial Wt. Ratio of Solvent to Water
9 2.09 5.04
Collected
Final Wt. Ratio of Solvent to Water
54.31 50.13
Collected
Average Wt. Ratio of Solvent to Water
11 2.54 6.08
Collected
Wt.% Water collected during
12 99.64 99.30
Experiment inclusive of losses
Wt.% Solvent collected during
33.31 43.89
13 Experiment inclusive of losses
Wt. Ratio of Solvent to Hydrocarbons
14 Left over in RB Flask at the End of 3.65 5.52
Experiment
Residual Water present in left over
Solvent cum Hydrocarbons in ppm as 329 290
determined by BTX Process
5
TABLE 17-7- REMOVAL OF ENTIRE SOLVENTS FROM DE-WATERED
HYDROCARBONS BY USING FREE WATER
SI. No. DESCRIPTION TEST 1 TEST 2
Wt. of De-Watered Hydrocarbons present
1 0.578 0.579
in RB Flask (kg)
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
129
2 Wt of Solvent Present in RB Flask (kg) 2.107 3.193
Wt. of Free Water added in RB Flask
3 4.215 3.197
(kg)
4 Initial Wt. Ratio of Water to Solvent 2.00 1.00
Observed Boiling Temperature Range
93.25-96.30 88.18-99.26
( C)
Initial Wt. Ratio of Solvent to Water
6 2.01 4.93
Collected
Final Wt. Ratio of Solvent to Water
7 0.03 0.07
Collected
Average Wt. Ratio of Solvent to Water
8 1.05 2.14
' Collected
9 Total Wt. of Free Water Collected (kg) 1.994 1.49
Total Wt. of Solvent Collected (kg) 2.114 3.198
Wt. of Hydrocarbons Left behind in RB
11 0.572 0.574
Flask at the End of Experiment (kg)
12 Wt. of Free Water left behind in RB
2.194 1.659
Flask at the end of Experiment (kg)
13 Wt. % Loss due to Evaporation, etc. 0.40 0.62
TABLE 17.8- SEPARATION OF ENTIRE FREE WATER FROM HYDROCARBONS
SI. No. DESCRIPTION TEST 1 TEST 2
Total Wt. of Hydrocarbons & Free water
1 2.765 2.233
(kg)
Wt. of Free Water collected by Gravity
2 separation while material was kept in 2.144 1.608
oven at 90 C for 48 lirs.(kg)
3 Wt. of Hydrocarbons recovered (kg) 0.559 0.564
Moisture in Hydrocarbons as per BTX
2,579 3,421
4 (1)Pm)
5 Turbidity of recovered Free Water (NTU) 4.6 4.0
6 Wt.% Material loss due to Evaporation,
adhering various surfaces, etc. 2.27 2.71
TABLE 17.9- TEST RESULTS
SI. No. DESCRIPTION TEST 1 TEST 2
1 Wt.% Hydrocarbons recovered 97.72 98.32
Calorific value of recovered
2 10,629 10,641
Hydrocarbons Lkcal/kg)
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
130
Wt. % Solvent recovered for Re-use
3 inclusive of materials adhering on 99.27 98.8
glasswares
4 Wt.% Bound Water recovered for re-use 99.64 99.30
Wt.% Free Water recovered for re-use 97.73 96.93
TABLE 17.10-REMOVAL OF ENTIRE WATER FROM FREE FLOWING DIESEL
SLUDGES WITH BOUND AND FREE WATER BOTH, BY BOILING WITH
AZEO TROPIC SOLVENTS
5
SI. No. DESCRIPTION TEST 1 TEST 2
1 Wt. of Diesel Sludge taken (kg) 1.003 1.005
2 Wt.% Water Present in Sludge 48.20 48.00
Wt.% Sodium Lauryl Sulphate Present in
3 2A2 2.45
Sludge
4 Wt.% Diesel Present in Sludge 49.38 49.55
5 Name of Solvents added Xylene Toluene
6 Wt. of Solvent added (kg) 2.660 4.824 _
7 Initial Wt. Ratio of Solvent to Water Present 5.50 10.00
8 Initial Wt. Ratio of Solvent to Diesel Present 5.37 9.69
93.45- 85.60-
9 Observed Boiling Temperature Range ( C)
139.19 110.53
Initial Wt. Ratio of Solvent to Water
2.18 6.09
Collected
Final Wt. Ratio of Solvent to Water
76.50 73.02
11 Collected
Average Wt. Ratio of Solvent to Water
12 2.64 6.66
Collected
13 Wt.% Water collected during Experiment 99.99 100.00
14 Wt.% Solvent collected during Experiment 48.05
67.04
Wt. Ratio of Solvent to Diesel Left over in
2.78 3.17
RB Flask at the End of Experiment
Residual Water present in left over Solvent
16 cum Diesel in ppm as determined by BTX 96 0.00
Test
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
131
TABLE 17.11- REMOVAL OF ENTIRE SOLVENTS FROM DE-WATERED DIESEL
BY USING FREE WATER
SI. No. DESCRIPTION TEST 1 TEST 2
Wt. of De-Watered Diesel Present in RB
1 0.495 0.498
Flask (kg)
2 Wt. of Solvent Present in RB Flask (kg) 1.377 1.578
3 Wt. of Free Water added in RB Flask (kg) 2.767 1.594
4 Initial Wt. Ratio of Water to Solvent 2.01 1.01
Observed Boiling Temperature Range ( C) 96.42-97.21 92.42-97.18
Initial Wt. Ratio of Solvent to Water
6 2.01 5.19
Collected
Final Wt. Ratio of Solvent to Water
0.60 0.82
7 Collected
Q Average Wt. Ratio of Solvent to Water
1.13 3.49
Collected
9 Total Wt. of Free Water Collected (kg) 1.223 0.453
Total Wt. of Solvent Collected (kg) 1.383 1.585
Wt. of Diesel Left behind in RB Flask at the
11 0.489 0.491
End of Experiment (kg)
Wt. of Free Water left behind in RB Flask at
12 1.522 1.112
the end of Experiment (kg)
13 Wt. % Loss due to Evaporation, etc. 0.48 0.78
TABLE 17.12- SEPARATION OF FREE WATER FROM DIESEL
SI. No. DESCRIPTION TEST 1 TEST 2
1 Total Wt. of Diesel & Free water (kg) 2.011 1.603
Total Wt. of Water collected by Gravity
2 1.495 1.101
separation (kg)
3 Wt. of Diesel recovered (kg) 0.470 0.471
I 4 Moisture in Diesel as per BTX (PPM) 21 72
5 Turbidity of recovered Free Water (NTU) 2.9 2.1
Wt.% Material loss due to Evaporation,
6 2.31 1.94
adhering to various surfaces, etc.
5
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
132
TABLE 17.13- TEST RESULTS
SI. No Description TEST 1 TEST 2
1 Wt. % of Diesel recovered 96.12 95.92
Calorific value of Recovered Diesel
2 11,023 11,020
, (kcal/kg)
3 Calorific value of Original Diesel (kcal/kg) 11,002
11,002
Wt. % Solvent recovered for Re-use
4 inclusive of materials adhering on 98.10 99.75
Glasswares
Wt. % Water recovered from Sludge for re-
99.99 99.99
use
6 Wt. %Tree Water recovered for reuse 98.2 99.01
Referring to tables 17.1- 17.13, the total furnace oil that was present in
sludges
retrieved was about 99 wt. %. This was inspite of the fact that a tiny
fraction thereof
5 got removed along with solvent collected. It was seen that the furnace
oil retrieved
was having about 3,806 ppm of residual moisture on an average as against
original
water content of 2,100 ppm therein. Inspite of slightly higher water content
the
recovered furnace oil was observed to have a calorific value of 10, 176
kcal/kg on an
average as against the value of 10,172 kcal/kg for original furnace oil.
Further, it was observed that about 98 wt. % of the hydrocarbons present in
Sludges
were retrieved for ONGC hydrocarbons with 3,000 ppm of residual water on an
average thereby having a calorific value which was observed to be 10,635
kcal/kg on
an average. Further, it was observed that about 96 wt % diesel was retrieved
on an
average from Diesel sludges with average moisture level of 47 ppm and with an
average calorific value of 11,021 kcal/kg as against to that of original
diesel having
calorific value 11,002 kcal/kg.
Further, it was observed that more than 99 wt. % bound water was retrieved
from
furnace oil sludges with an excellent quality as can be clearly seen in table
17-5. The
bound water recovered from ONGC sludge recovery was observed to be 99.5 wt. %.
The bound water recovered from diesel sludges was observed to be 100 wt. %.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
133
Further, it was observed that more than 99 wt. % solvent was retrieved from
furnace
oil sludges. The solvent recovery for ONCG sludges was observed to be 99 wt.
%.
The solvent recovery for Diesel Sludges was found to be 98.9 wt. %.
Further, it was observed that free water retrieved from furnace oil sludges
was about
96.5 wt.% on an average. The free water recovery for ONGC sludges was 97.3 wt.
%. The free water recovery for diesel based sludges was found to be 98.6 wt.
%. The
free water obtained was in large in quantity and was under process for more
than 48
hours with multiple steps.
EXAMPLE-18
PREPARATION OF OIL COATED SAND AND DEOILING OF SAND USING
XYLENE FOLLOWED BY RECOVERY OF PURE SAND, OIL AND XYLENE
In order to study removal of hydrocarbons from solids Furnace Oil and ONGC
free
flowing Oil coated sand samples were prepared. These sand samples were treated
using solvent like Xylene and thereafter quantitatively and qualitatively the
recovery
of sand, oils, Xylene and water was evaluated. Firstly, weighed amounts of
oils into
weighed sand which was water washed, completely dried and very clean. After
mixing oils into sand, the oil coated sand samples were washed in separate
batches of
Xylene. Progressively, oil was moved from sand into Xylene. The washing was
stopped once turbidity value and colour of pure Xylene did not change much
from its
original state after last washing cycle of the sand. At this stage, sand was
believed to
be coated with Xylene while entire oil on the sand was believed to be moved
into
spent Xylene. The Xylene coated sand was slowly heated beyond the boiling
point of
Xylene in Buchi Rotary Evaporator. The vapors of Xylene were condensed and
collected. Subsequently, Xylene oil mixture was heated with free water to boil
out
entire Xylene with some free water in Dean and Stark apparatus thereby leaving
behind oil with a fraction of free water. Subsequently, free water was removed
through gravity separation while keeping entire material at 85 C-90 C for 48
hours.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
134
Finally recovered sand, oil and free water were evaluated for quality followed
by
doing mass balance thereof.
TABLE 18.1- PRODUCTION OF OILY SAND
= SL
Description Expt-1 Expt-2
No.
1 Wt. of sand taken (kg) 1.00 1.00
Final Turbidity of water after
= 2 washing of sand with water 1.21
1.15
(NTU)
Wt.% loss of pure sand on
3 0.16 0.16
heating it at 815 C for 1 hr.
ONGC FREE
4 Type of Oil added FLOWING FURNACE OIL
HYDROCARBONS
Wt. of Oil added to Sand (kg) 0.10 0.15
6 Total Mass of Oily Sand (kg) 1.10 1.15
7 Wt.% oil present in Oily Sand 9.21 13.16
5 '
TABLE 18.2- REMOVAL OF OIL FROM OILY SAND BY WASHING WITH
XYLENE
SI.= Description Expt-1 Expt-2
No
Wt. Ratio of Xylene to Oily sand in
1 1.5 2.0
each washing
Wt. of Xylene added in each washing
2 1.65 2.31
(kg) =
Minimum number of washings
3 4 7
required to wash out Oil
4 Total amount of Xylene used (kg) 7.300 15.240
Turbidity of xylene after final washing
0.463 0.501
5 of sand (NTU)
6 Turbidity of pure Xylene (NTU) 0.421 0.421
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
135
TABLE 18.3- RECOVERY OF SAND, OIL AND XYLENE
SI.
Description Expt-1 Expt-2
No
Step 1: Recovery of Xylene sticking
from sand after washings
1 Total Wt. of sand & Xylene (kg) 1.27 1.98
= ____________________________________________________________________
2 Wt. of sand after Dried at 1609C (kg) 1.00 1.001
Wt. of Solvent collected as condensate
3 0.26
(kg) 0.96
Step 2: Separation of Xylene from
Oil
Step 2a: Addition of Water & Boiling
4 Wt. of Solvent & Oil taken (kg) 6.76 13.72
Wt. of Free Water added (kg) 13.51 27.44
6 Max. Temp in C while Boiling 97.5 97.8
Wt. of Solvent collected as condensate
7 6.75 13.72
(kg)
Wt. of Water collected as condensate
' 8 7.18 14.58
(kg)
Wt. of Free Water & Oil remaining
9 6.34 12.87
(kg)
Step 2b: Separation of Oil and Water
by gravity separation & Periodic
removal of separated water by
keeping material in oven at 90 C for
48 hours
Wt. of Oil recovered inclusive of
0.10 0.15
sticking on glassware (kg)
Moisture in Oil after Step 2b as per
11 3,420 3,123
BTX (ppm)
Turbidity of recovered Free Water after
12 4.2 3.6
Step 2b (NTU)
5
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
136
TABLE 18.4- TEST RESULTS
SI.
Description . Expt-1 Expt-2
No
Wt. % sand recovered inclusive of
1 99.94 99.93
materials sticking on glasswares
Turbidity of water after washing of
0.542 0.589
2 de-oiled sand (NTU)
Wt.% loss of dried sand after heating
3 0.11 0.11
it at 815 C for 1 hour
Wt. % Solvent recovered for Re-use
4 inclusive of materials sticking on 96.25 96.41
glasswares
Moisture in recovered Solvent as per
144 163 ,
BTX (ppm)
Wt. % Oil recovered inclusive of
6 99.07 98.95
_______ materials sticking on glasswares
7 Moisture in recovered Oils as per
3,420 3,123
BTX (ppm)
Calorific value of recovered Oil
10,580 10,164
8 (kcal/kg)
'9 Calorific value of original Oil
10,652 10,173
(kcal/kg)
Wt. % Free Water recovered for re-
use inclusive of water sticking on 98.08 98.37
glasswares
Referring to tables 18.1- 18.4, it was observed that recovery of sand was
about 100
wt. %. Further, it was seen that recovery of oils was about 99 wt. % and
recovery of
5 solvent was 96 wt. % inclusive of all weighable materials sticking on
various
surfaces. Even free water employed to boil out solvent from oils was retrieved
up to
98 wt. %. The sand recovered was oil free and whose wt. % loss on heating at
815 C
for 1 hour was 0.11 wt. % which was less than 0.16 wt. % for oil free, fresh
sand.
The turbidity was observed to be only 0.56 NTU as against the value of 1.2 NTU
for
10 water that was used for washing fines free fresh sand. The recovered
ONGC free
flowing oil was having only 3,420 ppm of residual moisture with a calorific
value of
10,580 kcal/kg as against residual moisture of 3,900 ppm and calorific value
of
10,652 kcal/kg for original ONGC Oil. It was seen that the recovered furnace
oil was
having residual moisture of about 3,123 ppm with calorific value of 10,164
kcal/kg
as against original furnace oil having residual moisture of 2100 ppm and
calorific
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
137
value of 10,173 kcal/kg. It was seen that the recovered Solvent had barely 153
ppm
moisture in it on an average as against 40 ppm moisture in original Xylene
used.
Further, it was observed that amount of Xylene required to wash unit mass of
oil
coated sand depends on both the type of oil that coats the sand and the amount
of oil
coating the sand. The weight of Xylene required was about 7 times the weight
of oily
sand for removing 9.2 wt. % ONGC free flowing oil. The weight of Xylene
required
was instead about 13 times for completely de-oiling the sand that contain
13.12 wt.
% Furnace oil.
EXAMPLE-19
EFFECT OF TIME RELATED CHANGE IN TURBIDITY VALUES OF SLOP
OILS
It was an aim to evaluate change in turbidity values of slop oils with time.
Further, it
was an aim to observe which hydro-carbons fragment easily to produce stable
slop
oils. Also, it was an aim to study why solvents behave differently from oils.
Accordingly, slop oils were prepared with different oils and solvents. These
oils/
hydrocarbons were added to water in varying parts per million and then
vigorously
fragmented in high shear mixer at 10,000 RPM over varying time. Subsequently,
a
representative sample was subjected to Turbidity test at wavelength of 455nm
with
Hach Turbidity Meter. The turbidity readings were measured in NTU (Normal
Turbidity Unit) thereby taking turbidity values of these slop oils at regular
intervals
of time till they reached near constant values.
=
TABLE 19.1- DESCRIPTION OF OILS & SOLVENTS USED FOR PREPARATION
OF SLOP OILS
SI. DESCRIPTION OF Wt.% WATER Wt.% ASH CALORIFIC
NO. HYDROCARBONS PRESENT CONTENT VALUE
(kcal/kg)
0.04 Wt.% Free
1 Coconut Oil 0.01 8,972
Water
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
138
0.39 Wt.% Free
2 ONGC Oil 0.88 10,633
Water
ONGC Viscous 42.21 Wt.% Bound
3 8.60 5,213
Hydrocarbons Water
0.01 Wt.% Free
4 Diesel 0.00 11,002
Water
0.21 Wt.% Bound
Furnace Oil 0.23 10,173
Water
0.004 Wt.% Free
6 Xylene . 0.00 10,205
Water
0.004 Wt.% Free
7 Toluene 0.00 10,074
Water
0.002 Wt.% Free
8 Benzene 0.00 9,995
Water
It was observed that unlike Xylene and Toluene, Benzene failed to easily
fragment or
remain fragmented into fine droplets even over short periods of time with
vigorous
stirring in water and therefore Benzene was believed to be not as suitable as
Xylene
5 and Toluene
for mopping up ultra-fine oil droplets from slop oils. This was indicated
by its turbidity values of 8 to 12 NTU as shown in FIG.7 and FIG.8. The
turbidity
values of transparent liquids were indicative of population density of
droplets having
diameters of order of 455 nm per unit volume of liquid.
Slop Oils contain all sizes of oil droplets. Amongst them, ultra-fines were
found to
be most difficult to mop up. Benzene was found little less effective than
Xylene and
Toluene when removal of ultra-fine droplets was an object. It was found that
very
large droplets of solvents were better suited for removing all oil droplets,
other than a
fraction of those which were ultra-fine in size. Large droplets of solvents
work faster
in removing bulk of the oil present. As these were the ones that swept away
and then
carried with them large numbers of smaller oil droplets while rising up due to
buoyancy.
It was seen that only a tiny fraction of total oil present resided in
difficult-to-remove
ultra-fine droplets. However, the ultra fine droplets were found contributing
towards
turbidity to a certain extent. Hence, relatively very large droplets of
solvents like
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
139
those of Benzene or even Xylene and Toluene cannot lower turbidity of slop
oils
beyond a point when they were hand mixed into slop oils, with mild mixing in
particular.
Accordingly, it was established that one must use solvents that immediately
fragment
into ultra-fine droplets and then quickly coalesce into very large sized
droplets to
derive advantages of all sizes of solvent droplets. Although both Xylene and
Toluene
were found good for processing Slop Oils, however, Xylene was found to be
better
than Toluene since it initially fragmented into a lot smaller sized droplets.
FIGS. 9 -12 as against FIGS. 13-20, bear testimony to a statement that good
solvents
coalesce very rapidly unlike hydrocarbons present in the slop oils. Most
dispersed
hydrocarbons, other than few like Diesel, took days to coalesce and reduce
their
turbidity. However, the turbidity values of Toluene and Xylene fell down in
hours.
Also, it was seen that the turbidity values of Xylene and Toluene fell down
sharply
with increasing concentration, unlike those of most hydrocarbons. This can be
clearly seen by comparing FIG. 9 with FIG. 14. It was seen that, with
solvents,
higher concentration did not lead to higher population density of ultra-fine
droplets.
Instead it triggered instant coalescence.
Further, it was seen that turbidity values of Toluene and Xylene dropped down
steeply by increasing mixing time at 10,000 RPM. This was contrary to what
happened with most hydrocarbons, including Diesel. For hydrocarbons like
Diesel
and ONGC Oil, increased time of mixing caused further fragmentation with
increased population density of ultra-fine droplets. But for coconut oil it
initially
narrowed variations in droplet size. The slop oil may be made lot more stable
by
making droplet size more uniform with which turbidity values do not change
with
time.
For solvents, however, more mixing resulted in unstable rise in surface energy
which
then triggered immediate coalescence. It was seen that by extending mixing
time
from 1 to 5 minutes at 10,000 RPM and for 2500 ppm, the turbidity value of
Toluene
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
140
fell down from 1,570 and 4,682 NTU to 54 and 874 NTU. It was established that
solvent coalesced rapidly that helped them to grow into large droplets quickly
that
reduced drag which then helped them to rise rapidly due to buoyancy. Here,
Toluene
was observed to surpasses Xylene.
In case of Diesel, it was seen that Diesel too got fragmented initially but
not as much
as Toluene and Xylene. It was also seen that Diesel was extremely fast as
compared
to other hydrocarbons with regard to coalescence, but still not found as fast
as that of
Toluene and Xylene. After 13 mins of mixing at 10,000 RPM turbidity values for
2,500 ppm Diesel Slop Oil was 3,852 NTU, while for same ppm & RPM, turbidity
values of Toluene and Xylene after 5 mins of mixing alone were 54 and 874 NTU
respectively. However, as clearly seen in FIG. 21, Diesel based Slop Oils were
found
easiest to be processed for recovery of oil and clean water due to rapid
coalescing
nature.
In case with 2,500 ppm coconut oil in water having 3 mins of mixing at 10,000
RPM
gave very stable slop oil. But this stability vanished with further increase
in mixing
time. Accordingly, it was established that for very stable coconut oil based
slop oils,
one must begin with vigorous mixing before beginning to process them for
recovery
of pure oil and water.
It appeared that highly stable ONGC free flowing oil based slop oils could be
formed
either by extending their time of mixing or by increasing their hydrocarbon
concentration. However, it was found uncertain that to what extent that value
depends on color of slop oil and to what extent on oil droplet size.
EXAMPLE-20:
EFFECT OF HEAT ON TURBIDITY OF SLOP OILS
It was an aim to understand the effect of heat on turbidity of slop oils when
heated in
an oven at 85 to 95 C for few hours or subjected to vigorous boiling for five
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
141
minutes. Accordingly, low and medium turbidity slop oils were prepared with
Coconut Oil and Free Flowing ONGC Oil as explained below in Table Nos. 20.1
and
20.3. Only the turbidity values were measured for low turbidity slop oils
immediately before and after heating. Additionally, Coconut Oil based slop
oils with
medium turbidity were subjected to our five-step process meant for reduction
in
turbidity, with and without initial heating as explained in Table 20.4. ,
TABLE 20.1- PRODUCTION OF SLOP OILS USING HIGH SHEAR MIXING
MACHINE
SI. TEST TEST
DESCRIPTION TEST 3
TEST 4
No. 1 2
Free Free
Flowing Flowing Coconut Coconut
1 Name of Oil Used
ONGC ONGC Oil Oil
Oil Oil
2 Total Mass of Slop Oil (kg) 0.64 0.58 0.54 0.43
3 Mixing Time (min) 5 5 5 5
4 RPM of Mixing 10,000 10,000 10,000 10,000
5 Oil Content in Slop Oil (ppm) 30 85 60 99
Time elapsed before checking of
6 2.03 3.06 1.36 0.7
Turbidity (min)
7 Average Turbidity of Slop Oil (NTU) 39 579 53 72
TABLE 20.2- RESULTS OF VIGOROUS BOILING OF ABOVE SLOP OILS
SI. TEST TEST
DESCRIPTION TEST 3
TEST 4
No. 1 2
1 Time for Boiling (min) 7 7 7 7
2 Temperature Range ( C) 95-98 95-98 95-98 95-98
Average Turbidity of Slop Oil
3 after heating (NTU) 38.2 435 52.1 68.9
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
142
TABLE 20.3- PRODUCTION OF COCONUT OIL BASED SLOP OILS USING
HIGH SHEAR MIXING MACHINE
SI.
Treatment Treatment Treatment
DESCRIPTION with with without
No. Heating Boiling Heat
1 = Total Mass of Slop Oil (Kg) 0.50 0.50 0.49
2 Mixing Time (min) 5.00 5.00 5.00
3 RPM of Mixing 10,000 10,000 10,000
4 Oil content in Slop Oil (ppm) 2,499 2,499 =2,499
Time elapsed before checking of Turbidity
4.00 3.70 4.05
(mm)
6 Turbidity of Slop Oil (NTU) 4,701 4,814 4,763
TABLE 20.4- STEP WISE RESULTS ON PROCESSING OF ABOVE SLOP OILS
5 WITHOUT REMOVING THE EFFECT OF TIME
Treatmen Treatme Treatmen
SI.
N DESCRIPTION t with nt with t without
o.
Heating Boiling Heat
Step 1: Heating of Slop Oil
1 Time Span in minutes 126 5
2 Temperature range ( C) 80 -90 95-98
3 Turbidity of Slop Oil after heating (NTU) 4,277 4,099
Step 2: Centrifuging of Slop Oil at 4,500 RCF
twice with NIL residence time at peak RCF
value
4 Turbidity of Slop Oil after 2nd Centrifuge (NTU) 553 386 797
Step 3: Addition of Solvent, Centrifuging it
once under same conditions as above and then
removal of entire top layer of Solvent + Oil
Toluene Toluene Toluene
5 Name & Wt.% of Solvent added (7%) (7%) (7%)
Vigorous Vigorou Vigorous
6 Mode of Solvent Addition hand s hand hand
mixed mixed mixed
Turbidity of Slop Oil after removal of Top Layer
7 109 120 200
(NTU)
Step 4: Removal of Solvent from Slop Oil
through Boiling with Free Water present
therein
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
143
Turbidity of Slop Oil in NTU on cooling after
8 addition of make-up water that was lost through 14.4 14.6
12.0
boiling
Step 5: Addition of Alum with Residence Time
in Settling Vessel
9 Wt. `)/0 Alum added to Slop Oil 0.05 0.05 0.05
Time permitted for Flocculation of Oil and Solids
24.48 23.86 16.20
in Settling Vessel in Hours
11 Turbidity after Flocculation (NTU) 9.64 9.80 9.00
=
Step 6: Filtration
Turbidity of Filtrate after using 41 Grade
12 3.42
Whatman filter paper (NTU) ¨
Turbidity of filtrate after using 40 Grade
13 0.37 0.30 0.78
Whatman filter paper (NTU)
Total time elapsed in hrs since preparation of
14 28 26 23.36
Slop Oil
Turbidity of Control Sample of same Slop Oil
3099 3184 3148
after same time since its preparation (NTU) , , ,
The study on effect of vigorous boiling on turbidity of slop oils as shown in
tables
20.1 and 20.2 was found to be important because that was limited to low
turbidity,
less ppm slop oils alone. This kind of slop oils were obtained after 2nd step
of our 5-
5 step process, i.e. after centrifuging for third time with solvent and
then removing
entire topmost layer of solvent cum hydrocarbons.
Thereafter entire dispersed solvent from slop oils was boiled out with help
from free
water present therein. 'While so doing, we wanted to separately evaluate the
impact
of boiling alone, apart from that of solvent removed, on residual turbidity of
slop
10 oils.
The study here showed that impact of boiling was small unless residual value
of slop
oils after boiling out solvent was high. The impact of boiling was further
reduced for
residual values ranging from 38 to 69 NTU and turbidity of slop oils by 2.09
to 4.5%
15 of residual values. However, for high residual values like 435 NTU,
reduction in
turbidity on account of boiling alone was by 33% of residual turbidity value.
Boiling
related reduction in value was additional to that due to removal of solvent.
The part
of these reductions in turbidity values were on account of passage of time
too.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
144
Accordingly it was concluded that impact of boiling can be neglected for low
residual turbidity values.
As seen in table nos. 20.3 and 20.4, about 9 to 14 % immediate reduction in
turbidity
values was observed with medium turbidity coconut oil based slop oils due to
heating without removing impact of passage of time. This impact was observed
to be
more with boiling. Effect of heating or boiling on turbidity values of coconut
oil
=based slop oils progressively reduced with subsequent processing of slop
oils. It was
seen that the impact of both heating and boiling was completely vanished after
boiling out solvents or prior to adding of alum. Hence; it was ascertained
that prior
heating or boiling of slop oils is not required.
EXAMPLE- 21
TIME ADJUSTED EFFECT OF SOLVENT ALONE ON REDUCING
TURBIDITY OF SLOP OILS
An impact of using low viscous solvent, like Toluene, alone on reduction in
turbidity
of slop oils after removing the impact of time from reported turbidity results
was
studied.
Accordingly, 5 Wt. % Toluene was added to the prepared slop oils. Toluene was
mixed in them using high shear mixer at 10,000 RPM for 1 minute. Before
addition
of Solvent, Slop Oils were tested for turbidity and time was noted. Solvent
added
samples were allowed to stand for 20 hours for most Oil and Solvent to collect
at the
top. Later, top layer containing solvent and oil was separated from each Slop
Oil
Sample and remaining material was tested for turbidity after homogenization
and
again the time was noted. Subsequently, entire residual solvent was boiled
out, in
temperature range of 95 C to 98 C from remaining material with help from
free
water present in sop oil. After cooling, make up water was added to replace
the water
lost through boiling. Thereafter, slop oils were again tested for turbidity
and time
was noted. Here each turbidity value of test sample was time-adjusted. This
was
done with control samples where turbidity values continuously changed, often
only
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
145
reducing, with passage of time. After identical elapsed time, turbidity values
of
control samples were added to those of test samples that removed the impact of
time
from reported values, thus reflecting the impact of solvent alone.
TABLE 21.1- PRODUCTION OF ONGC BASED SLOP OILS USING HIGH SHEAR
MACHINE
SI.
DESCRIPTION ONGC Free Flowing Oil
NO.
1 Total Mass of Slop Oils (kg) 0.51 0.60 0.49 0.56 "
2 Time for mixing (min) 1 5 1 5
RPM used for producing Slop
3 10,000 10,000 10,000 10,000
Oils
4 Oil content in Slop Oils (ppm) 2,499 2,501 4,988 5,003
Time elapsed before checking
5 3.88 2.03 2.40 2.80
Turbidity (min)
6
Average Turbidity of Slop Oils
5,626 9,894 >10,000 >10,000
(NTU)
TABLE 21.2- TIME ADJUSTED RESULTS ON ADDITION OF SOLVENT TO
ABOVE SLOP OILS
SI.
DESCRIPTION ONGC Free Flowing Oil
NO.
Toluene Toluene Toluene Toluene
1 Name & Wt. /0 Solvent added
(5%) (5%) (5%) (5%)
Turbidity of Slop Oils before
2 5,626 9,894 >10,000 >10,000
addition of Solvent (NTU)
Turbidity of Slop Oils after.
3 >10,000 >10,000 >10,000 >10,000
addition of Solvent (NTU)
Turbidity of Slop Oils after
4 removing residual Solvent 9,052 >10,000 >10,000 >10,000
through Boiling (NTU)
TABLE 21.3 -PRODUCTION OF COCONUT OIL BASED SLOP OILS USING
HIGH SHEAR MACHINE
SI.
DESCRIPTION Coconut Oil
NO.
1 Total Mass of Slop Oils (kg) 0.53 0.56 0.51 0.54
I 2 Time of mixing (min) 1 5 1 5
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
146
RPM used for producing Slop
3 10,000 10,000 10,000 10,000
Oils
4 Oil content in Slop Oils (ppm) 2,498 2,501 5,003 4,951
Time elapsed before checking
3.12 2.70 1.96 1.72
Turbidity (min)
Average Turbidity of Slop Oils
6 4,354 4,856 7,816 8,886
(NTU)
TABLE 21.4 -PROCESSING OF ABOVE SLOP OIL BY USE OF SOLVENT
SI.
DESCRIPTION Coconut Oil
NO. =
Toluene Toluene Toluene Toluene
1 Name & Wt. Solvent Solvent added
(5%) (5%) (5%) (5%)
Turbidity of Slop Oils before
2 4,354 4,856 , 7,816 8,886
addition of Solvent (NTU)
Turbidity of Slop Oils after
3 >10,000 >10,000 >10,000 >10,000
addition of Solvent (NTU)
Turbidity of Slop Oils after
4 removal of Solvent through 8,167 6,823 >10,000 >10,000
Azeotropic Boiling (NTU)
For slop oils produced from ONGC Free Flowing Oil, impact of mixing time
5 required for their production was large compared to that for Coconut Oil
based slop
oils. It was observed that the turbidity value went up from 5,626 to 9,894 NTU
for
2,500 ppm ONGC slop oil with increase in mixing time from 1 to 5 minutes.
Under
same conditions, for Coconut Oil based Slop Oils it rose from 4,354 to 4,856
NTU.
For Slop Oils produced from ONGC Free Flowing Oil impact of increase in
concentration of hydrocarbons on turbidity value was also slightly larger than
that
for Coconut Oil based Slop Oils.
It was seen that addition of Toluene never helped, either with Coconut or with
ONGC slop oils. It could not reduce turbidity of these slop oils inspite of
removing
large amounts of hydrocarbons from slop oil and retaining them in the topmost
layer
along with it. On the contrary, after addition of solvent turbidity values
infact went
up inspite of boiling entire solvent that was added. For 2,500 Slop Oils rise
in value
was lot more in case of 1 minutes mixed Slop Oils as compared to 5 minutes
mixed
Slop Oils.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
147
Solvent was invariably added into slop oils by mixing it for 1 minute at
10,000 RPM.
Probably this mixing might have further fragmented existing droplets of
Coconut Oil
and ONGC Oil and that could have raised turbidity values by increasing the
population density of ultra-fine droplets. Impact of further fragmentation was
expected to be higher in case of slop oils produced through 1 minute of mixing
as
compared to those that had been generated after 5 minutes of mixing. Hence
turbidity values of slop oils after 1 minute mixing we found to be increasing
a lot
more. Probably use of solvent might have failed also because most of the
solvent
got consumed removing large oil droplets. Consequently, ultra fine droplets
might
have been remained intact with additional need of solvents in batches.
It was seen that with 5,000 ppm slop oils, the-differential rise for 1 and 5
minutes
slop oils could not be established as final values exceeded our test equipment
range.
However, with 5,000 ppm Coconut Oil based Slop Oils, at least their turbidity
values
went up with use of solvent even after boiling out the entire solvent that was
added.
EXAMPLE-22
TIME ADJUSTED EFFECT OF CENTRIFUGE ALONE ON REDUCING
TURBIDITY OF SLOP OILS
Effect of centrifuge alone on reducing turbidity of slop oils after removing
the
impact of time from reported turbidity results was studied. Accordingly, the
slop oils
were prepared using Free Flowing ONGC Oil and also Coconut Oil under
parameters as explained in table nos. 22.1 and 22.3. Table Nos. 22.2 and 22.4
showed time adjusted results of three rounds of centrifuge as well as the
conditions
under which samples were centrifuged. After each round of centrifuge turbidity
values were tested and time was noted only after carefully removing the entire
top
layer of accumulated oil. It was understood that meaning of time adjusted
results
have been explained under procedure explained in Example-21.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
148
TABLE 22.1- PRODUCTION OF ONGC OIL BASED SLOP OIL USING HIGH
SHEAR MIXING MACHINE
SI. TEST TEST TEST TEST
DESCRIPTION
NO. 1 2 3 4
1 Total Mass of Slop Oil (kg) 0.49 0.50 0.51 0.50
2 Mixing Time (mm) 1 5 1 5
3 RPM of Mixing 10,000 10,000 10,000
10,000
4 Oil content in Slop Oil (ppm) 2,496 2,486 5,001
4,993
Time elapsed before checking of Turbidity
3.02 3.35 4.10 4.10 -
(mm)
6 Average Turbidity of Slop Oil (NTU) 5,911 >10,000
>10,000 >10,000
5 TABLE
22.2- RESULTS OF CENTRIFUGING ABOVE ONGC OIL BASED SLOP
OILS
SI. TEST TEST TEST TEST
DESCRIPTION
No. 1 2 3 4
Step:1 Centrifuging of Slop Oil at 4,500
RCF with NIL residence time at peak
= RCF value
Turbidity of Slop Oil before Centrifuge
1 5,911 >10,000 >10,000 >10,000
(NTU)
Turbidity of Slop Oil after 1st Centrifuge
2 1,302 2,382 2,307 4,682
(NTU)
Turbidity of Slop Oil after 2nd Centrifuge
3 891 1,746 1,518 3,976
(NTU)
Turbidity of Slop Oil after 3rd Centrifuge
4 811 1,639 1,260 3,551
(NTU)
TABLE 22.3- PRODUCTION OF COCONUT OIL BASED SLOP OIL USING
HIGH SHEAR MIXING MACHINE
SI. TEST TEST TEST TEST
DESCRIPTION
NO. 1 2 3 4
1 Total Mass of Slop Oil (kg) 0.49 0.49 0.50 0.49
2 Mixing Time (min) 1 5 1 5
3 RPM of Mixing 10,000 10,000 10,000
10,000
4 Oil content in Slop Oil (ppm) 2,496 2,499 4,975
4,990
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
149
Time elapsed before checking of Turbidity
4.10 4.30 1.09 11.96
(min)
6 Average Turbidity of Slop Oil (NTU) 4,408 4,829 7,962
8,823
TABLE 22.4 - RESULTS OF CENTRIFUGING ABOVE COCONUT OIL BASED
SLOP OILS
SI. TEST
TEST TEST TEST
DESCRIPTION
NO. s 1 2 3 4
Step:! Centrifuging of Slop Oil at 4,500
=
RCF with NIL residence time at peak
RCF value
Turbidity of Slop Oil before Centrifuge
1 4408 4829 7,962 8,823
(NTU) , ,
Turbidity of Slop Oil after 1st Centrifuge
2 856 1,080 2,109 3,347
(NTU)
Turbidity of Slop Oil after 2nd Centrifuge
3 433 625 1,933 2,694
(NTU)
Turbidity of Slop Oil after 3rd Centrifuge
4 354 387 1,679 2,627
(NTU)
5
It was seen that the turbidity values of slop oils prepared from Free Flowing
ONGC
Oil was always higher than that of Coconut Oil based slop oils prepared under
similar conditions. The turbidity of Coconut Oil based Slop Oils increased
both with
mixing time employed for their preparation and also with concentration of
hydrocarbons present wherein the concentration of hydrocarbons present was
having
greater impact than mixing time.
Time adjusted impact of centrifuge was found to be substantially dependent on
starting turbidity values. It progressively reduced turbidity with every
successive
operation. It was seen that impact of first round of centrifuge was large. In
successive rounds, the impact kept diminishing. It was observed that the
centrifuge
was lot more effective in removing large sized oil droplets since the impact
of drag
was less on the droplets. It was seen that force of buoyancy worked better
with large
sized droplets with substantial reduction in turbidity in the first round. It
was seen
that the centrifuge became ineffective due to one or more of the following
reasons.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
150
Firstly, centrifuge could have become ineffective once size variations of
dispersed
oil droplets became narrow. Secondly, centrifuge could hay
e become ineffective once population density of dispersed droplets falls with
increasing mean free path. Thirdly, centrifuge could have become ineffective
as
initial turbidity values were too large. Fourthly, centrifuge could have
become
ineffective due to dispersed oil droplets that might have electrically
charged. Lastly,
centrifuge could have become ineffective due to small density difference
between oil
and water.
It was established that narrowing of variations in droplet size could have
resulted in
movement of all droplets with same velocity and acceleration. This could have
resulted in fewer collisions and slower rate of coalescence. Also, efficacy of
centrifuge could have dropped down with absence of sweeping effect of large
sized
oil droplets. Further, uniform size , of dispersed droplets could have
impaired the
centrifuge lot more than their small population density with large mean free
path. It
was seen that with too high initial turbidity value or initial population
density of
ultra-fine oil droplets, the centrifuge slowed down and that then impacted its
efficacy. It was seen that, residual turbidity after 31d attempt at centrifuge
was
invariably large when initial turbidity values were high.
It was concluded that the centrifuge cannot reduce turbidity of slop oils to
the
required value of 1 to 4 NTU. In fact, the limiting turbidity values of the
centrifuge
were lot higher. This was more so in cases of colored slop oils. It was
further
concluded that the density difference between oil and water and also the RCF
and
residence time inside the centrifuge play significant role in this regard.
EXAMPLE- 23
TIME-SOLVENT-CENTRIFUGE ADJUSTED COMBINED EFFECT OF
CENTRIFUGE AND SOLVENT ALONE, ON REDUCING TURBIDITY OF
SLOP OILS
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
151
Combined use of Solvent and Centrifuge on reducing Turbidity of Slop Oils was
studied after removing individual effects of time, centrifuge and also that of
solvent,
from all reported turbidity results. Accordingly, slop oils were prepared
under
conditions given in table nos. 23.1 and 23.3. Subsequently, the turbidity
values of
slop oils were measured. Thereafter, these slop oils were centrifuged twice
with nil
residence time at maximum RCF of 4,500. Further, the solvents were added by
mixing them into slop oils for 1 minute at 10,000 RPM. Thereafter, the
contents were
centrifuged once again with nil residence time at maximum RCF of 4,500.
Subsequently, residual solvent from slop oil was boiled out in temperature
range of
95 C to 98 C with help from free water present in slop oils after entirely
removing
the top layer of solvent cum oil. After cooling, make up water was added that
was
lost through vigorous boiling. Then, the remaining material was tested for
turbidity
values and also the time was noted. To remove impact of time, we added to
above
results the amount by which turbidity values would have reduced if we had
retained '
them in vessels for same periods of time since their production. Next to
remove the
impact of solvent alone, the time adjusted amount by which turbidity values
went up
on addition of solvents into slop oils by mixing them in for 1 minute at
10,000 RPM
was subtracted. Finally, to remove the impact of centrifuge alone, we added to
above
results the time adjusted amount by which turbidity values of slop oils had
got
reduced after centrifuging them thrice with nil residence at maximum RCF of
4,500.
TABLE 23.1 PRODUCTION OF ONGC OIL BASED SLOP OILS USING HIGH
SHEAR MIXING MACHINE
SI. TEST
TEST TEST TEST
DESCRIPTION=NO. 1 2 3 4
1 Total Mass of Slop Oil (kg) 0.51 0.47 0.49 0.51
2 Mixing Time (mm) 1 5 1 5
3 RPM of Mixing 10,000 10,000 10,000 10,000
4 Oil content in Slop Oil (ppm) 2,498 2,498 4,999 5,006
Time elapsed before checking of
5 1.85 2.12 3.10 3.56
Turbidity (mm)
Average Turbidity of Slop Oil
6 6,080 >10,000 >10,000 >10,000
(NTU)
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
152
TABLE 23.2 PROCESSING OF ABOVE SLOP OILS BY COMBINING THE USE OF
SOLVENT AND CENTRIFUGE
SI. TEST TEST TEST
TEST
DESCRIPTION.NO.1 2 3 4
Turbidity of Slop Oils before
1 adding Solvent & doing 6,080 >10,000 >10,000 >10,000
Centrifuge (NTU)
Toluene Toluene Toluene Toluene
2 Name & Wt.% of Solvent added
(5%) (5%) (5%) (7%)
Turbidity of Slop Oil after adding
Solvent; doing centrifuge and
3 3,572
then removing Solvent through
Azeotropic Boiling (NTU)
TABLE 23.3- PRODUCTION OF COCONUT OIL BASED SLOP OILS USING
HIGH SHEAR MIXING MACHINE
SI. TEST TEST TEST
TEST
DESCRIPTION
NO. 1 2 3 4
1 Total Mass of Slop Oil (kg) 0.52 0.50 0.50 0.51
2 Mixing Time (min) 1 5 1 5
3 RPM of Mixing 10,000 10,000 10,000
10,000
4 Oil content in Slop Oil (ppm) 2,501 2,499 4,994
4,999
Time elapsed before checking of
5 1.88 2.85 2.88 4.30
Turbidity (min)
Average Turbidity of Slop Oil
6 4,672 4,653 7,332 8,685
(NTU)
TABLE 23A- PROCESSING OF ABOVE SLOP OILS BY COMBINING THE USE
OF SOLVENT AND CENTRIFUGE
SI. TEST TEST TEST
TEST
DESCRIPTION
NO. 1 2 3 4
Turbidity of Slop Oils before
1 4,672 4,653 7,332 8,685
adding Solvent & doing
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
153
Centrifuge (NTU)
Toluene Toluene Toluene Toluene
2 Name & Wt.0/0 of Solvent added
(5%) (5%) (5%) (7%)
Turbidity of Slop Oil after adding
Solvent; doing centrifuge and
3 1,118 3,213
then removing Solvent through
Azeotropic Boiling (NTU)
It was seen from test Nos. 2,3 and 4 in table 23.2 and test Nos. 3 and 4 in
Table 23.4
that we could not get values because we could quantify the impact of adding
solvents
to slop oils for these tests as can seen from table Nos. 21.2 & 21.4. However,
without
quantifying the impact of using solvents, we cannot remove the impact of
solvent for
these tests.
However, from test no. 1 for 2,500 ppm ONGC Oil based Slop Oil and from test
no.
1 and 2 for 1 and 5 minutes mixed, 2,500 ppm Coconut Oil based Slop Oils, it
was
found that these two unit operations reduced turbidity of slop oils only when
combined. The table nos. 23.2 and 23.4 show that there was a synergetic effect
in
combining the use of Solvent with that of centrifuge. Use of Solvent alone
actually
increased the turbidity of Slop Oils by a large margin. Use of centrifuge by
itself
succeeded well, only when initial turbidity values were not large. But when
solvent =
was combined with centrifuge, it not only wiped out the entire negative impact
of
using solvent alone, but it additionally benefited in cases where initial
turbidity
values where large like ONGC slop oils.
It was ascertained that the centrifuge must preferentially be used for
removing large
oil droplets while solvents must be used for removing ultra fine droplets.
Solvents
must be added only after centrifuge has ceased to be effective for want of
wide
droplet size distribution or low population density of fine droplets or small
density
difference between oils and water. This combination was found must when
initially
turbidity of slop oils was large.
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
154
EXAMPLE-24
EFFECT OF USING ALUM ON REDUCING TURBIDITY OF SLOP OILS
Impact of alum addition on reduction of turbidity of slop oils was studied.
Accordingly, slop oil samples were prepared with both low and high turbidity
values
as per conditions mentioned in below mentioned tables 24.1A and 24.2A. Alum
was
added and settling time was provided as per figures mentioned in tables 24.1B
and
24.2B. Alum was added in 3 different proportions for high turbidity samples
and
turbidity values were evaluated over 4 days with and without adjusting the
effect of
time.
Effect of Alum on low ppm slop oil- ,
TABLE 24.1A- PRODUCTION OF SLOP OILS USING HIGH SHEAR MIXING
MACHINE
SI. DESCRIPTION
TEST TEST TEST TEST TEST
NO. 1 2 3 4
Name of Oil Used ONGC
Free Flowing Oil Coconut Oil
1 Total Mass of Slop Oil (kg) 0.45 0.46 0.59 0.57 0.48
2 Mixing Time (min) 5 5 5 5 5
0000 10,
3 RPM of Mixing 10,000 10,000 10, 10,000
0 0
4 Oil content in Slop Oil (ppm) 9 20 45 7 60
=Time elapsed before checking of
5 5.4 4.2 4.2 6.6 3.6
= Turbidity (min)
6 Average Turbidity of Slop Oil (NTU) 7 19 60 8 56
TABLE 24.1B- IMPACT OF ALUM ON ABOVE SLOP OILS
1
bESCRIPTION TEST TEST TEST TEST TEST
NO. 1 2 3 4 5
Turbidity of Slop Oil before adding Alum
1 6.6 19 60 8 55
(NTU)
2 Wt.% Alum added to Slop Oil 0.05 0.05 0.05
0.05 0.05
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
155
3 Time permitted for Settling (Hrs) 19.03 28.61 26.86
27.46 24
Turbidity Values after above Settling Time
4
4.4 2.0 4.5 1.6 10.1
(NTU)
) Wt.% reduction in Turbidity of Slop Oil by
33.33 89.47 92.50 80.00 81.64
adding Alum
Effect of Mum on high ppm slop oil-
TABLE 24.2A- PRODUCTION OF SLOP OILS USING HIGH SHEAR MIXING
MACHINE
SI.
DESCRIPTION TEST 1 TEST 2
TEST 3
NO.
Coconut
Name of Oil Used ONGC Oil Coconut
Oil
Oil
1 Total Mass of Slop Oil (kg) 0.50 0.52
0.60
2 Mixing Time (min) 3 5
5
3 RPM of Mixing 10,000 10,000
10,000
4 Oil content in Slop Oil (ppm) 2,495 2,438
499
= Time elapsed before checking of
5 3.6 3.6
5.4
= Turbidity (min)
6 Average Turbidity of Slop Oil (NTU) 5,643 4,308
484
5
TABLE 24.2B- IMPACT OF ALUM ON ABOVE SLOP OILS
SI.
DESCRIPTIONS TEST 1
TEST 2 TEST 3
NO.
Turbidity of Slop Oil before adding Alum
1 4,755 4,675 484
(NTU)
2 Wt. % of Alum added to Slop Oil 0.05 0.05
0.05
3 Time permitted for Settling (Hrs) 23.08 24.00
25.08
Turbidity Values including the Impact of Time
4 45 18.7 8.6
(NTU)
Turbidity Values excluding the Impact of
5 1702 1923
Time (NTU)
Wt.% reduction in Turbidity of Slop Oil by
6 99.05 99.60 98.22 =
adding Alum
Effect of Mum with different compositions on slop oil-
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
156
TABLE 24.3A- PRODUCTION OF SLOP OILS USING HIGH SHEAR MIXING
MACHINE
SI.
DESCRIPTIONS TEST 1 TEST 2
NO.
ONGC Free
Name of Oil Used Coconut Oil
= Flowing Oil
1 Total Mass of Slop Oil (kg) 0.49 0.50
2 Mixing Time (min) 5 5
3 RPM of Mixing 10,000 10,000
4 Oil content in Slop Oil (ppm) 2,498 2,496
Time elapsed before checking Turbidity
1.98 2.18
(min)
6 Average Turbidity of Slop Oil (NTU) >10,000 4,740
TABLE 24.3B- ADDITION OF ALUM WITH DIFFERENT PROPORTIONS TO
5 SLOP OIL
SI.
DESCRIPTIONS TEST 1 TEST 2
NO.
Step-1: Addition of Alum with Residence
Time in Settling Vessel
Turbidity of Slop Oil before adding Alum
1 >10,000 4,740
(NTU)
0.0 0.1 0.1 0.0 0.1
0.1
2 Wt. % Alum added to Slop Oil
5 0 5 5 0 5
Day-1
24. 24. 24. 24. 24. 24.
3 Time permitted for Settling (hrs)
05 14 13 02 55 50
Turbidity Values including the impact of 4,1 5.4 5.3 9.5 3.5
3.5
4
time (NTU) 65 4 8 2 9 4
Turbidity Values excluding the impact of 4,3
204 204 194 188 188
5 time (NTU) 64
Day-2
48. 47. 47. 47. 47. 47.
6 Time permitted for Settling (Hrs)
06 96 95 95 96 97
Turbidity Values including the impact of 2,4 5.3 2.5 6.2 2.6
7 3.9
time (NTU) 68 6 3 7 7
Turbidity Values excluding the impact of 2,6
8 171 170
105 108 105
time (NTU) 34
Day-3
71. 71. 71. 71. 71.
71.
9 Time permitted for Settling (Hrs)
52 36 34 46 33 28
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
157
Turbidity Values including the impact of 366 2.6 4.7
4.7 3.3
5
time (NTU) 1 2 9 1
Turbidity Values excluding the impact of
11 691 330 328 16 16 15
time (NTU)
Day-4
96. 96. 96. 96. 95. 96.
12 Time for Settling (Hrs)
17 14 11 49 87 34
2
13 3
Turbidity Values after including impact of 10. 1.1 3.4 2.1 2.2
.
time (NTU) 6 4 9 9 6
Turbidity Values after excluding the impact
14 171 162 161 35 34 34
of time (NTU)
It was observed that impact of Alum on reducing turbidity of slop oils in 24
hours
was more when initial turbidity values were large. As seen in table 24.2B, on
removing the impact of time, one can see that Impact of Alum alone on
reduction of
5 turbidity values of slop oils was about 1.5 times more than that of time
itself.
As seen from table 24.3B, addition of 0.05 wt. % alum is not adequate when
initial
turbidity values are more than 10,000 NTU. Addition of Alum must be increased
to
0.1 wt. %. However, beyond addition of 0.1 wt. % alum there was no further
10 improvement
seen. Hence, it was ascertained that amount of Alum added was
important only when one was interested to get quick results in a day or two.
Combined impact of alum and time was more than adequate to reduce turbidity
values from greater than 10,000 NTU to about 5.5 NTU if given 24 hours.
However,
then removal of the oil layer contaminated with Alum from water was found
rather
difficult. Besides, calorific value of oil was reduced by 2% as shown below in
Example-29. Also, it was observed that alkali content of oil went up with
contamination from Alum. It was also observed that viscosity of oil
dramatically
changes per wt. % of Alum present therein.
EXAMPLE-25
EFFECT OF COMBINED USE OF ALUM, HEAT AND TIME ON REDUCING
TURBIDITY OF SLOP OILS
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
158
It was an aim to evaluate the impact of combined use of Alum, heat and time on
reducing turbidity of slop oils and comparing that with just the use of alum
with time
alone. Accordingly, low turbidity slop oil samples were prepared as per
conditions
mentioned in table-25.1. Thereafter, Alum was added and kept part of samples
at
ambient conditions and their initial and final turbidity values were tested
over
varying time from 3 hours to 5.8 hours. The remaining part of samples were
heated
in oven at 80 C over varying time from 1 to 4 hours and even these were
tested for
initial and final turbidity values. Subsequently, make up water was added for
heated
samples to replenish evaporated water.
TABLE 25.1- PRODUCTION OF SLOP OILS USING HIGH SHEAR MIXING
MACHINE
TEST TEST TEST TEST
DESCRIPTION
SI. No. _ 1 2 3 4
ONGC Free
Name of Oil UsedCoconut Oil
Flowing Oil
= 1 Total Mass of Slop Oil (kg)
0.57 0.54 0.63 0.56
2 Mixing Time (mm) 5 5 5 5
3 RPM of Mixing 10,000 10,000 10,000 10,000
_
4 Oil content in Slop Oil (ppm) 30 60 60 99
_
5 Time elapsed before checking Turbidity
4.00 4.12 2.36 1.82
(min)
6 Average Turbidity of Slop Oil (NTU) 42.1 90.6 56.1
66.5
TABLE 25.2- ADDING ALUM AND HEATING OF ABOVE SLOP OILS
SI.
DESCRIPTION
TEST 1 TEST 2 TEST 3 TEST 4
NO.
Step-1: Addition of Alum with Residence
time in Settling Vessel
Turbidity of Slop Oil before adding Alum
1 42.1 90.6 56.1 66.5
(NTU)
2 Wt % of Alum added to Slop Oil 0.05 0.05 0.05
0.05
Time permitted for Flocculation of Oil 8z
3 5.04 5.84 3.44 4.56
Solids in Settling Vessel (Hrs)
4 Turbidity after Flocculation (NTU) 11.4 92.7 75.9
72.9
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
159
Step-2: Heating of Alum added Slop Oil
1 Time kept for heating (Hrs) 4 4 - 1
3
2 Set Temperature of instrument ( C) 80 80 80
80
Average Turbidity of Alum added Slop Oil
3 2.92 19.1 18.5 10.8
after heating (NTU)
Time taken for the process of heating alum
4 5.11 5.72 3.22 4.43
added Slop oil (Hrs)
As can be seen from table 25.2, the combined impact of alum, heat and time was
found to be far better than that of just alum and time alone, on reduction of
turbidity
values of low turbidity ONGC and Coconut Oil based Slop Oils. In Test-1, it
was
observed that turbidity value fell down by 73% in 5.04 hours in case on non-
heating
of Alum added slop oil. However, Alum added slop oil when heated at 80 C for
4
hours, the turbidity of the slop oil fell down by 93% in 5.11 hours. In tests
2, 3 and 4
when not heated turbidity values were in fact found to be increased by 2.3%,
35.3%
and 9.6% in 5.84, 3.44 and 4.56 hours respectively. The turbidity values of
slop oils
actually went up even more than their initial values with time when Alum added
slop
oil was not heated with lower initial turbidity and lesser settling time.
However, the turbidity value fell down by 67% instead in case where Alum added
slop oil sample when heated at 80 C even with low initial turbidity value of
56.1
NTU and even with less time of 1 hour. Also, with initial turbidity value of
42.1
NTU and with 4 hours of heating at 80 C the fall was 93% in 5.11 hours. It
ascertained that more the length of time over which samples were heated faster
was
the fall in turbidity values. This experiment established the fact that
treatment with
Alum could be speeded up to reduce our overall processing time if needed by
applying low intensity heat.
EXAMPLE-26-
EFFECT OF FILTRATION ON REDUCING TURBIDITY OF SLOP OILS
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
160
In order to evaluate impacts of fast and slow filtration rates on reduction of
turbidity
values of high and low initial turbidity slop oils, the slop oil samples were
prepared
as per conditions mentioned in table 26.1. These samples were filtered
repeatedly
four times using 40 and 41 Grade Whatman cellulose Filter Papers. In one set
of
readings the same filter paper was repeatedly used while in the other set of
readings
new filter papers were used each time. The turbidity values were noted before
and
after each filtration. The time taken for filtration of a given weight of slop
oil was
also noted each time to arrive at the rate of filtration.
TABLE 26.1- PRODUCTION OF SLOP OILS USING HIGH SHEAR MIXING
MACHINE
SI.
DESCRIPTION TEST 1 TEST 2
No.
ONGC Free
Name of Oil UsedCoconut Oil
Flowing Oil
1 Total Mass of Slop Oil (kg) 0.49 0.51
2 Mixing Time (min) 5 5
3 RPM of Mixing 10,000 10,000
4 Oil content in Slop Oil (ppm) 2,480 2,497
5 Time elapsed before checking
4.70 3.72
Turbidity (min)
Average Turbidity of Slop Oil
6 9356 4,434
(NTU) ,
TABLE 26.2- FILTRATION PROCESS USING WHATMAN FILTER PAPERS FOR
ABOVE SLOP OILS
= SI.
N DESCRIPTION TEST 1 TEST 2
a
Step-4: Filtration using
Whatman Filter paper
Same Filter Each Time Same
Filter Each Time
1 Mode of using Filter Paper Paper used New
Filter Paper used New Filter
each time Paper used each
time Paper used
2 Grade of Whatman Filter
Paper used 40 41 40 41 40 41 40 41
3 Turbidity .After 1st
Filtration (NTU) 1,971 6,007 2,063 6,543 3,888 4,596 3,918 4,689
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
161
Flow Rate of Slop Oil
4 collected for 1st Filtration 2.07 13.95 2.4 7.78 7.48
45.43 6.73 91.31
(g/min)
Turbidity : After 2nd
1,096 3,033 1,147 4,370 3,646 4,650 3,373 4,666
Filtration (NTU)
Flow Rate of Slop Oil
6 collected for 2nd Filtration 0.62 2.9 6.95 46.33 7.46
54.13 6.4 82.1
(g/min)
Turbidity : After 3rd
7 730 1,960 764 4,486 3,292 4,629 2,877 4,489
Filtration (NTU)
Flow Rate of Slop Oil
8 collected for 3rd Filtration 0.73 1.83 5.31 27.66 5.38
33.29 3.18 72.44
(g/min)
Turbidity : After 4th
9 539 1,021 564 4,224 2,906 4,670 2,476 4,428
Filtration (NTU)
Flow Rate of Slop Oil
collected for 4th Filtration 0.66 1.53 5.46 64.19 3.81
22.65 8.68 89.06
(g/min)
Total time elapsed since
11 3.26 2.15
preparation of Slop Oil (hrs)
Turbidity of Control Sample
of same Slop Oil after same
12 9,857 4,438
time since its preparation
(NTU)
It was seen that 40 Grade Whatman filter paper having 8 micron pore size gave
much
lower turbidity values after each filtration however found to be slow while
filtering.
It was even slower when the same paper was repeatedly used each time. It was
also
5 found to be far slower in case of high turbidity slop oils. It was
observed that the
process slowed down but the quality of filtrate was improved with repeated use
of
same filter paper in order to get lower turbidity values. It was seen that
filtration
failed to give consistent results each time. The rate of filtration changed
each time
and reduction in turbidity values also changed accordingly. It was seen that
efficacy
10 of filtration was dependent on the nature of hydrocarbon in the slop
oil. For instance,
as can be seen from Table 26.2 that filtration was lot less effective for
Coconut Oil
based slop oils than ONGC Oil based slop oils.
However, it was seen that other than as finishing step for reduction of last
bits of
turbidity values it was found to be a desirable industrial process because of
one or
more of the following reasons. Firstly, the filtration process was found to be
very
slow process. Secondly, the filtration process was found to be an inconsistent
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
162
process. Thirdly, the filtration medium was found to be blocked fast when pore
size
was small thereby making further process even slower. Fourthly, the
hydrocarbon
present in slop oil cannot be recovered easily or in saleable form. Lastly,
presence of
solids in slop oils further impaired this process in terms of its efficacy and
flow rates.
EXAMPLE-27
EFFECT OF COMBINING USES OF ALUM AND FILTRATION ON
REDUCTION OF TURBIDITY OF SLOP OILS
In order to evaluate impact of combined use of both Alum and filtration on
reduction
of turbidity of slop oils having both low and medium turbidity values, slop
oil
samples were prepared under conditions mentioned in below tables 27.1A and
27.2A. Alum was added to these samples after testing for initial turbidity
values 0.05
wt. %. The turbidity values of these samples were tested again after close to
24
= hours. Further, the samples were successively filtered with Grade 41 and
then with
Grade 40 Whatman Cellulose Filter Papers and after each filtration reduction
in
turbidity values were recorded.
Effect of Alum and filtration on Turbidity of Low ppm Slop Oils-
TABLE 27.1A- PRODUCTION OF SLOP OILS USING HIGH SHEAR MIXING
MACHINE
SI.
TEST TEST TEST TEST TEST
DESCRIPTION
NO 1 2 3 4 5
Name of Oil Used ONGC Free Flowing Oil CoConut
Oil
1 Total Mass of Slop Oil (kg) 0.45 0.46 0.59 0.57 0.48
2 RPM of Mixing 10,000
10,000 10,000 10,000 10,000
3 Mixing Time (min) 5 5 5 5 5
4 Oil content in Slop Oil (ppm) 9 20 45 7 60
Time elapsed before checking
5 0.09 0.07 0.07 0.11 0.06
Turbidity (min)
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
163
6 Average Turbidity of Slop Oil
(NTU) 7 19 60 8 56
TABLE 27.1B- PROCESSING OF ABOVE SLOP OILS BY ADDITION OF ALUM
AND THEN FILTRATION WITH WHATMAN CELLULOSE FILTER PAPERS.
SI.
NO DESCRIPTION ONGC Free Coconut
Oil
Flowing Oil
1 Turbidity of Slop Oils before adding Alum
6.6 19 60 8 55
(NTU)
2 Wt. % of Alum added to Slop Oils 0.05 0.05 0.05 0.05
0.05
3 Time permitted for Settling (Hrs) 19.03
28.61 26.86 27.46 24.00
4 Turbidity after above Settling Time (NTU) 4.4 2.0 4.5
1.6 10.1
% Reduction in Turbidity of Slop Oils by
33.33 89.47 92.50 80.00 81.64
adding Alum
Turbidity of Filtrate after using 41 Grade
6 1.8 1.3 5.2 0.94 3.8
Whatman Filter Paper (NTU)
Turbidity of Filtrate after using 40 Grade
7 0.68 0.75 1.9 0.53 0.19
Whatman Filter Paper (NTU)
5
Effect of Alum and filtration on turbidity of high ppm slop oils-
TABLE 27.2A- PRODUCTION OF SLOP OILS USING HIGH SHEAR MIXING
MACHINE
SI.
DESCRIPTION TEST 1
TEST TEST3
NO. 2
ONGC
Coconu Coconut
Name of Oil Used Free
t Oil Oil
Flowing Oil
1 Total Mass of Slop Oil (kg) 0.50 0.52 0.60
2 Mixing Time (mm) 10,000 19,000
10,000
3 RPM of Mixing 3 5 5
4 Oil content in Slop Oil (ppm) 2,495 2,438 499
5 Time elapsed before checking Turbidity (min) 0.06 0.06
0.09
6 Average Turbidity of Slop Oil (NTU) 5,643 4,508
484
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
164
TABLE 27.2B- PROCESSING OF ABOVE SLOP OILS BY ADDITION OF ALUM
AND THEN FILTRATION WITH WHATMAN CELLULOSE FILTER PAPERS
SI.
DESCRIPTION TEST
TEST TEST
NO. 1 2 3
1 Turbidity of Slop Oils before adding Alum (NTU) 4,755 4,675
484
2 Wt.% Alum added to Slop Oils 0.05 0.05 0.05
3 Time permitted for Settling (Hrs) 23.08 24.00 25.08
4 Turbidity Values without adjusting impact of Time
45 18.7 8.6
(NTU)
Turbidity Values after adjusting impact of Time
1702 1923
(NTU) , ,
% Reduction in Turbidity of Slop Oils by adding
6 99.05 99.60 98.22
Alum
Turbidity of Filtrate after using 41 Grade
7 16.7 9.4 2.33
Whatman Filter Paper (NTU)
Turbidity of Filtrate after using 40 Grade
0.58 0.77 1.05
Whatman Filter Paper (NTU)
It was observed that percentage impact of Alum was lot more, in same time span
and
5 with same dosage, for slop oils with large initial turbidity values as
can be seen by
comparing table nos. 27.1B and 27.2B. It was further seen that Alum could make
use
of centrifuge and solvent redundant. But it was hold untrue. This was because
Alum
took the same time and dosage to reduce turbidity of slop oil from 4,755 to 45
NTU
as much as it took to reduce it from 45 to 4.5 NTU. Alum was needed to reduce
turbidity up to 2-5 NTU. Therefore, it was ascertained that starting turbidity
for
Alum must be below 60 to 70 NTU. Once turbidity values were brought down from
2-5 NTU then even fast filtration was observed to be very effective for
delivering
water with around 1 NTU and also the load on or blocking of filtering media
was
observed to be small. Secondly, Alum was found to be adversely affecting the
quality of oil collected from slop oils. Accordingly, it was ascertained that
if quality
of oil collected is not important and if time taken for filtration and
saturation of
filtering media can be ignored, then alum cum filtration can make the use of
centrifuge and solvent redundantly as far as processing of slop oils is
concerned.
EXAMPLE- 28
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
165
OVERALL EFFECT OF COMBINING THE USE OF CENTRIFUGE, SOLVENT,
ALUM & FILTRATION WITH VARYING SOLVENTS ON REDUCING
TURBIDITY OF VARIOUS SLOP OILS
It was an aim to evaluate combined effect of centrifuge, solvent, alum and
filtration
and also the effect of various solvents on reducing turbidity of slop oils
prepared
from various oils/hydrocarbons. Accordingly, slop oils were prepared as per
conditions mentioned in table nos. 28.1A, 28.2A, 28.3A, 28.4A, 28.5A, 28.6A
and
28-7A. Procedures of preparation were also mentioned in table nos. 28.1B,
28.2B,
28.3B, 28.4B, 28.5B, 28.6B and 28.7B. Subsequently, solvents like Toluene and
Xylene were used mixed in different proportions. The solvents were mixed with
the
slop oils using high shear mixer at 8,090 RPM for 1 minute. The oil content in
slop
oils was varied from 5 PPM to 4, 99,052 PPM. The various oils used were
selected
from one or more of the following Coconut Oil, Furnace Oil, Diesel, ONGC Free
Flowing Oil and ONGC viscous hydrocarbons. Subsequently, all four processing
steps involving the use of Centrifuge, Solvent, Alum and Filtration were
employed in
sequential manner. Accordingly, following observations were made.
Coconut oil based slop oils-
TABLE 28.1A- PRODUCTION OF COCONUT OIL BASED SLOP OILS USING
HIGH SHEAR MIXING MACHINE
SI.
DESCRIPTION TEST 1 TEST 2 TEST 3 TEST 4
No.
Total Mass of Slop Oils
1 0.52 0.66 0.51 0.51
(kg)
2 Mixing Time (min) 5 5 5 5
3 RPM of Mixing 10,000 10,000 10,000 10,000
Oil content in Slop Oil
4 5 80 4,996 4,999
(13Pm)
Time elapsed before
5 checking of Turbidity 4.10 12.76 5.55 4.30
(min)
Average Turbidity of
6 22 65 9 070 8,685
Slop Oils (NTU) ,
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
166
TABLE 28.1B-PROCESSING OF ABOVE SLOP OILS BY COMBINING THE USE
OF CENTRIFUGE, SOLVENT ALUM AND FILTRATION
SI.
DESCRIPTION TEST 1 TEST 2 TEST 3 TEST 4
No.
Step 1: Centrifuging of
Slop Oils at 4,500 RCF
twice with NIL Residence
time at peak RCF value
Average Turbidity of Slop
1 Oils after 2nd Centrifuge 16 24 2,354 2,358
(NTU)
Step 2: Addition of
Solvent & then
Centrifuging it once
again under same
conditions as above and
then removal of entire
top layer of Solvent + Oil
2 Name & Wt.% of Solvent Xylene Xylene Xylene Toluene
added (5%) (5%) (7.2%) (7%)
3 Mode of Solvent Addition High shear High shear High shear High
shear
mixing mixing mixing mixing
4 Time of Mixing (min) 1 minute 1 minute 1 minute 1
minute
RPM used for mixing
5 8,090 8,090 8,090 8,090
Solvent
Average Turbidity of Slop
6 Oils after removal of top 29 62 421 395
layer (NTU)
Step 3: Removal of
Solvent from Slop Oils
through Boiling with
Free Water present
therein
Average Turbidity of Slop
Oils in NTU on cooling
7 after addition of make-up 6 12 42 54
water that was lost through
boiling
Step 4: Addition of Alum
with Residence Time in
Settling Vessel
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
167
Wt. % of Alum added to
8 0.05 0.05 0.05 0.05
Slop Oil
Time permitted for
9 25.31 25.48 23.15 23.26
Settling (hrs)
= Average Turbidity after
above Settling Time 0.743 0.602 1.38 5.01
(NTU)
=
Step 6: Filtration
Average Turbidity of
Filtrate after using 41
11 0.647 0.601 1.27 3.26
Grade Whatman Filter
Paper (NTU)
Average Turbidity of
Filtrate after using 40
12 0.452 0.554 0.34 1.04
Grade Whatman Filter
Paper (NTU)
Total time elapsed since
13 preparation of Slop Oils 27.31 27.48 25.15 25.26
(hrs)
TABLE 28.2A- PRODUCTION OF COCONUT OIL BASED SLOP OILS USING
HIGH SHEAR MIXING MACHINE
SI
= DESCRIPTION TEST 1 TEST 2 TEST 3 TEST 4 TEST 5
o.
Total Mass of Slop
1 0.51 0.51 0.50 0.50 0.50
Oils (kg)
2 Mixing Time (min) 5 5 5 5 5
3 RPM of Mixing 10,000 10,000 10,000 10,000 10,000
Oil content in Slop
4 9,976 9,967 100,069 250,079 499,052
Oil (ppm)
Time elapsed before
5 checking of 3.03 4.66 4.80 6.18 5.30
Turbidity (min)
Average Turbidity of
6 >10000 >10000 7,977 8,498 >10,000
Slop Oils (NTU)
5
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
168
TABLE 28.2B- PROCESSING OF ABOVE SLOP OIL BY COMBINING THE USE
OF CENTRIFUGE WITH SOLVENT, ALUM AND THEN FILTRATION
SI. TEST
DESCRIPTION TEST 1 TEST 2 TEST 3 TEST 4
No. 5
Step 1: Centrifuging of
Slop Oil at 4,500 RCF
twice with NIL
Residence time at peak
RCF value
Average Turbidity of
1 Slop Oil after 2nd 3,494 3,499 >10,000 >10,000
1,554
Centrifuge (NW)
Step 2: Addition of
Solvent & then
Centrifuging it once =
under same conditions
as above and then
removal of entire top
layer of Solvent + Oil
2 Name & Wt.% Solvent Toluene Xylene Xylene Xylene
Xylene
added (7%) (7%) (22%) (17%) (20%)
Mode of Solvent High High High High High
3 shear shear shear shear shear
addition
mixing mixing mixing mixing mixing
4 Time of mixing (mm) 1 minute 1
minute 1 minute 1 minute 1 minute
Maximum RPM used for
8,090 8,090 8,090 8,090 8,090
mixing Solvent
Average Turbidity of
6 Slop Oil after removal of 450 2,216 556 245 566
top layer (NTU)
Step 3: Removal of
Solvent from Slop Oil
through boiling with
free water present
therein
Average Turbidity of
Slop Oil in NTU on
I 7 cooling after addition of 39 103 48 15 56
make-up water that was
lost through boiling
Step 5: Addition of
Alum with Residence
Time in Settling Vessel
Wt.% Alum added to
8 0.05 0.05 0.05 0.05 0.05
Slop Oil
Time permitted for
9 22.14 22.30 22.86 22.50 19.18
Settling (brs)
Average Turbidity after
1.59 9.84 14.4 2.02 52.2
above Settling Time
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
169
(NTU)
=
Step 6: Filtration
Average Turbidity of
Filtrate after using 41
1.34 5.68 10.6 1.36 43.7
11 Grade Whatman Filter
paper (NTU)
Average Turbidity of
Filtrate after using 40
12 0.83 1.26 2.21 1.32 9.61
Grade Whatman Filter
paper (NTU)
Total time elapsed since
13 preparation of Slop Oil 24.14 24.30 27.86 27.93 24.54
(hrs)
Furnace Oil based Slop Oils-
TABLE 28.3A- PRODUCTION OF FURNACE OIL BASED SLOP OILS USING
HIGH SHEAR MIXING MACHINE
SI.
DESCRIPTION TEST 1 TEST 2 TEST 3
_ No.
1 Total Mass of Slop Oils (kg) 0.49 0.49 0.49
2 Mixing Time (min) 5 5 5
3 RPM of Mixing 10,000 10,000 10,000
4 Oil content in Slop Oil (ppm) 1,346 '1,132 2,498
Time elapsed before checking of
5 4.20 6.52 5.35
Turbidity (mm)
Average Turbidity of Slop Oils
6 3,370 2,959 6,742
(NTU)
TABLE 28.3B- PROCESSING OF ABOVE SLOP OIL BY COMBINING THE USE
OF CENTRIFUGE WITH SOLVENT, ALUM AND THEN FILTRATION
SL
DESCRIPTION TEST 1 TEST 2 TEST 3
No.
Step 1: Centrifuging of Slop Oil at
4,500 RCF twice with NIL Residence
time at peak RCF value
Average Turbidity of Slop Oil after 2nd
1 2 225 1,065 1,961
Centrifuge (NTU) ,
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
170
Step 2: Addition of Solvent & then
Centrifuging it once under same
conditions as above and then removal
_ of entire top layer of Solvent + Oil
2 Name & Wt. % Solvent added Xylene Xylene Xylene
High High High
3 Mode of Solvent addition shear shear shear
mixing mixing mixing
4 Time of mixing (min) 1 minute 1 minute 1 minute
RPM used for mixing Solvent 8,090 8,090 8,090
Average Turbidity of Slop Oil after
6 161 20 69
removal of top layer (NTU)
Step 3: Removal of Solvent from Slop
Oil through Boiling with free water
present therein
Average Turbidity of Slop Oil on
7 cooling after addition of make-up water 38 12 39
that was lost through boiling (NTU)
Step 4: Addition of Alum with
Residence Time in Settling vessel
8 Wt.% Alum added to Slop Oil 0.05 0.05 0.05
9 Time permitted for Settling Vessel (hrs) 18.60 18.30
18.23
Average Turbidity after above Settling
1.81 0.672 1.34
Time (NTU)
Step 5: Filtration
11 Average Turbidity of Filtrate after using
1.28 0.417 1.15
41 Grade Whatman Filter paper (NTU)
Average Turbidity of Filtrate after using
0.938 0.254 1.11
12 40 Grade Whatman Filter paper (NTU)
Total time elapsed since preparation of
22.58 22.36 23.40
13 Slop Oil (hrs)
Diesel based Slop Oils-
5 TABLE
28.4A- PRODUCTION OF DIESEL BASED SLOP OILS USING HIGH
SHEAR MIXING MACHINE
SI.
DESCRIPTION TEST 1 TEST 2 TEST 3
No.
1 Total Mass of Slop Oils (kg) 0.51 0.49 . 0.52
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
171
2 Mixing Time (min) 13.53 13.42 13.48
3 RPM of Mixing 8,090 8,030 8,130
4 Oil content in Slop Oil (ppm) 2,492 2,499 7,442
Time elapsed before checking
5.47 3.45 3.57
of Turbidity (min)
Average Turbidity of Slop Oils
6 3,442 3,852 9,702
(NTU)
TABLE 28.4B- PROCESSING OF SLOP OIL FROM TABLE 28.4A BY
COMBINING THE USE OF CENTRIFUGE WITH SOLVENT, ALUM AND THEN
5 FILTRATION
SI.
DESCRIPTION TEST 1 TEST 2 TEST 3
No.
Step 1: Centrifuging of Slop Oil at
4,500 RCF twice with NIL
Residence time at peak RCF value
Average Turbidity of Slop Oil after
1 37 28 67
2nd Centrifuge (NTU)
Step 2: Addition of Solvent & then
Centrifuging it once under same
conditions as above and then
removal of entire top layer of
Solvent + Oil
Toluene Xylene Toluene
2 Name & Wt. % Solvent added
(2%) (2%) (2%)
Vigorous Vigorous Vigorous
3 Mode of Solvent addition
hand mix hand mix hand mix
4 Time of mixing (min)
Maximum RPM used for mixing
5
______ Solvent
6 Average Turbidity of Slop Oil after
removal of top layer(NTU) 35
46 77
Step 3: Removal of top layer
Solvent + Oil
7 Wt. % of Diesel + Solvent recovered 97.78 97.34 97.12
8 Moisture in above as per BTX (ppm) 312 284 322
Step 4: Removal of Solvent from
Slop Oil through boiling with free
water present therein
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
172
Average Turbidity of Slop Oil in NTU
9 on cooling after addition of make-up 14 10 31
water that was lost through boiling
Step 5: Addition of Alum with
Residence Time in Settling vessel
Wt. % Alum added to Slop Oil 0.05 0.05 0.05
11 Time permitted for Settling (hrs) 18.12 17.54 18.31
Average Turbidity after above
12 1.18 0.356 1.39
Settling Time (NTU)
Step 6: Filtration
Average Turbidity of Filtrate after
13 using 41 Grade Whatman Filter paper 1.07 0.352 0.79
(NTU)
Average Turbidity of Filtrate after
14 using 40 Grade Whatman Filter paper 0.49 0.249 0.68
(NTU)
Total time elapsed since preparation
25.48 24.3 25.40
of Slop Oil (hrs)
Average Turbidity of Control Sample
16 of same Slop Oil after same time since 277 330
its preparation (NTU)
ONGC Free Flowing Oil based Slop Oils-
TABLE 28.5A- PRODUCTION OF ONGC OIL BASED SLOP OILS USING HIGH
5 SHEAR MIXING MACHINE
TEST TEST TEST TEST
SI.No. DESCRIPTION TEST 5
TEST 6
1 2 3 4
Total Mass of
1 0.52 0.48 0.51 0.49 0.50 0.51
Slop Oils (kg)
Mixing Time
2 5 5 5 5 5 5
(min)
I 3 RPM of Mixing 10,000 10,000 10,000
10,000 10,000 10,000
Oil content in Slop
4 500 500 1,482 1,489 4,999 5,006
Oil (ppm)
Time elapsed
5 before checking of 4.3 3.26 . 4.11 3.19 4.17 3.34
Turbidity (min)
Average Turbidity
6 of Slop Oils 1,511 1,567 4,859 5,149
>10,000 >10,000
(NTU)
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
173
TABLE 28.5B- PROCESSING OF ABOVE SLOP OIL BY COMBINING THE USE
OF CENTRIFUGE WITH SOLVENT, ALUM AND THEN FILTRATION
SI. TEST TEST TEST TEST TEST TEST
DESCRIPTION
No. 1 2 3 4 5 6
Step 1:
Centrifuging of
Slop Oil at 4,500
RCF twice with
NIL Residence
time at peak RCF
value
Average Turbidity
of Slop Oil after
1 686 645 1,783 1,792 4,364 4,427
2nd Centrifuge
(NTU)
Step 2: Addition
of Solvent & then
Centrifuging it
once under same
conditions as
above and then
removal of entire
top layer of
Solvent + Oil
2 Name & Wt.% Toluene
Xylene Toluene Xylene Xylene Toluene
Solvent added (5%) (5%) (7%) (7%) (7%) (7%)
Mode of Solvent High High High High High High
3shear shear shear shear shear shear
addition
mixing mixing mixing mixing mixing mixing
Time of mixing 1 1 1 1 1 1
4
(min) minute minute minute minute minute minute
Maximum RPM
used for mixing 8,090 8,090 8,090 8,090 8,090 8,090
Solvent
Average Turbidity
6 of Slop Oil after 2,512 824 615 1,564 2,115 1,930
removal of top
layer (NTU)
Step 3: Removal
of Solvent from
Slop Oil through
boiling with free
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
174
water present
therein
Average Turbidity
of Slop Oil on
cooling 7 after
59 33 34 41 72 89
addition of make-
up water that was
lost through boiling
Step 4: Addition
of Alum with
Residence Time in
Settling Vessel
Wt. `)/0 Alum added
8 0.05 0.05 0.05 0.05 0.05 0.05
to Slop Oil
Time permitted for
9 24.10 24.20
23.40 24.60 24.40 23.48
Settling (hrs)
Average Turbidity
after above Settling 20.9 11 2.63 7.89 5.83 2.84
Time (NTU)
Step 5: Filtration
Average Turbidity
of Filtrate after
11 using 41 Grade 3.83 2.35 3.55 3.07 1.64
Whatman Filter
paper (NTU)
Average Turbidity
of Filtrate after
12 using 40 Grade 1.2 1.71 0.81 1.26 1.04 1.14
Whatman Filter
paper (NTU)
Total time elapsed
13 since preparation 26.10 26.2 25.45 26.6 26.4 25.48
of Slop Oil (hrs)
Average Turbidity
of Control Sample
of same Slop Oil
14 - - 3,683 3,701
after same time
since its
preparation (NTU)
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
175
TABLE 28.6A- PRODUCTION OF ONGC OIL BASED SLOP OILS USING HIGH
SHEAR MIXING MACHINE
SI.
DESCRIPTION TEST 1 TEST 2 TEST 3 TEST 4
No.
Total Mass of Slop
1 0.49 0.49 0.51 0.5
Oils (kg)
2 Mixing Time (mm) 1 5 5 5
3 RPM of Mixing 10,000 10,000 10,000 10,000
Oil content in Slop
2,498 2,498 9,997 47,618
4 Oil (ppm)
Time elapsed before
checking of 3.54 4.10 4.20 5.43 =
Turbidity (min)
Average Turbidity
6 4,630 6,119 >10,000 >10,000
of Slop Oils (NTU)
5 TABLE 28.6B- PROCESSING OF SLOP OIL FROM TABLE 28.6A BY
COMBINING THE USE OF CENTRIFUGE WITH SOLVENT, ALUM AND THEN
FILTRATION
SI.
TEST
NO DESCRIPTION 1 TEST 2 TEST 3 TEST 4
Step 1: Centrifuging
of Slop Oil at 4,500
RCF multiple times
with NIL Residence
time at peak RCF
. value
Tolue
Name & Wt.% Solvent Toluene Toluene Toluene
1 ne
added (18%) (18%) (85%) (310%)
Vigor Mild Mild
Vigor Vigor
Mode of Solvent ous Vigorous
ous ous
hand
hand
2
addition hand hand mix. hand hand
mix mix
mix mix mix
Average Turbidity of
3 Slop Oil after removal 1,570 2,170 108 139 2,300
710
of top layer (NTU)
Step 2: Removal of
top layer Solvent + =
Oil
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
176
Wt. % ONGC Oil+ 95.64
4 92.95% 93.79% 95.68%
Solvent recovered
Moisture in above as
9,246 3,894 802 2,154
per BTX (ppm)
Step 3: Removal of
Solvent from Slop Oil
through Boiling with
Free Water present
therein
Average Turbidity of
Slop Oil in NTU on
cooling after addition
31
6 42 35 37 39 40
of make-up water that
was lost through
boiling
Step 4: Addition of
Alum with Residence
Time in Settling
Vessel
Wt. % of Alum added
7 0.05 0.05 0.05 0.05 0.05 0.05
to Slop Oil
Time permitted for
8 18.32 19.36 24.45 24.56 20.40 20.50
Settling (hrs)
Average Turbidity
9 after above Settling 14.9 24 11 10 4.44 4.7
Time (NTU)
Step 5: Filtration
Average Turbidity of
Filtrate after using 41
3.03 7.32 3.69 2.32 2.59 3.20
Grade Whatman Filter
paper (NTU)
Average Turbidity of
Filtrate after using 40
0.834 1.32 1.02 0.948 1.75 1.78
Grade Whatman Filter
_ paper (NTU)
Total time elapsed
12 since preparation of 20.32 21.36 26.45 26.56 25.4
25.50
Slop Oil (hrs)
Average Turbidity of
Control Sample of
13 same Slop Oil after 3,866 3,827
same time since its
preparation (NTU)
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
177
Viscous ONGC Hydrocarbons-
TABLE 28.7A-PRODUCTION OF VISCOUS ONGC HYDROCARBONS BASED
SLOP OILS USING HIGH SHEAR MIXING MACHINE
SI.
DESCRIPTION TEST! TEST 2 TEST 3
No.
1 Total Mass of Slop Oils (kg) 0.49 0.49 0.51
2 Mixing Time (min) 5.00 5.00 5.00
3 RPM of Mixing 10,000 10,000 10,000
Viscous hydrocarbon content in slop
2,488 4,995 9,999
4 ______ oil (ppm)
Oil content in slop oil (ppm) 1,232 2,474 4,952
6 Ash content in slop oil (ppm) 206 413 827
7 Bound water in slop oil (ppm) 1,050 2,108 4,220
Time elapsed before checking of
8 3.90 7.19 4.30
Turbidity (min)
Average Turbidity of Slop Oils
1,637 3,272 8,633
9 (NTU)
5
TABLE 28.7B- PROCESSING OF ABOVE SLOP OIL BY COMBINING THE USE
OF CENTRIFUGE WITH SOLVENT, ALUM AND THEN FILTRATION
SI.
DESCRIPTION TEST 1 TEST 2
TEST 3
No.
Step 1: Centrifuging of Slop Oil at 4,500
RCF twice with NIL Residence time at
peak RCF value
Average Turbidity of Slop Oil after 2nd
1 876 1174 1540
Centrifuge (NTU)
Step 2: Addition of Solvent & then
Centrifuging it once under same
conditions as above and then removal of
entire top layer of Solvent + Oil
Xylene Xylene
Toluene
2 Name & Wt. % Solvent added
(5%) (5%) (5%)
Vigorous Vigorous
High shear
3 Mode of Solvent addition
hand mix hand mix
mixing
4 Time of mixing (min) 1
minute
5 RPM used for mixing Solvent
8,090
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
178
Average Turbidity of Slop Oil after
6 4,731 3,615 4,176
removal of top layer (NTU)
Step 3: Removal of top layer Solvent +
Oil
Wt. % ONGC Viscous hydrocarbons +
7 97.37 98.03 96.48
Solvent recovered
Wt. % Moisture in above as per BTX
8 24,743 29,884
26,112
(PM)
Step 4: Removal of Solvent from Slop
Oil through boiling with Free Water
present therein
Average Turbidity of Slop Oil in NTU on
9 cooling after addition of make-up water 236 220
238
that was lost through boiling
Step 5: Addition of Alum with Residence
Time in Settling Vessel
Wt. % of Alum added to Slop Oil 0.05 0.05 0.05
11 Time permitted for Settling (hrs) 38.12 38.3
40.09
Average Turbidity after above Settling
12 8.91 6.07 5.49
Time (NTU)
Step 6: Filtration
Average Turbidity of Filtrate after using 41
13 3.85 2.35 1.99
Grade Whatman Filter paper (NTU)
Average Turbidity of Filtrate after using 40
0.69
14 1.26
0.65
Grade Whatman Filter paper (NTU)
Total time elapsed since preparation of
40.12 41.53 41.36
Slop Oil (hrs)
It was observed that when water insoluble solids are present in slop oils,
like those
containing ONGC viscous hydrocarbons, it was necessary to incorporate
filtration
after addition of alum to get low turbidity values as filtration was found to
be more
5 effective in removing very fine ash particles. This could be seen from
Table 28.7B. It
was further ascertained that the diesel slop oils were the easiest to process.
Further, it was seen that incorporation of all four unit operations like
centrifuge,
addition of solvent, addition of alum followed by filtration in process made
10 processing of the slop oil faster with least operative problems and with
excellent
results. Further, it was seen that the pollution problem was found to be
entirely
mitigated. Besides, it was found that said operations entirely recover almost
excellent
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
179
quality hydrocarbons and water for use or sale. Energy consumption was found
to be
very small. Also, the solvent employed was fully recovered in its original
form for
re-use.
It was seen that the quantity of solvent required and mode of solvent addition
were
dependent on quantity of hydrocarbons present in slop oils. The quantity of
solvent
required was more when hydrocarbons-were lot more and least agitation was
needed
while adding solvent. It was seen that mere hand shaking was preferred mode of
adding solvent when hydrocarbon content in slop oils was high as can be
clearly seen
from test 4 in table 28.6B. It was also seen that even mild hand shaking was
equally
effective. It was also seen that with collection of hydrocarbons became very
easy
with the use of solvent. The weight percent collected also went up. Finally,
it was
seen that often solvent cum oil layer was having very little water therein.
EXAMPLE-29
EFFICACY OF THE PROCESS OF PRESENT INVENTION WITH SLOP OILS
CONTAINING VERY HIGH HYDROCARBON CONTENT
It was an aim to study efficacy of the combined process with slop oils having
very
high hydro-carbon content inclusive of recovery of hydrocarbons and solvent.
Accordingly, the slop oils were prepared with Coconut Oil under conditions as
mentioned below in table 29.1. These slop oil samples were retained in a
separating
flask for 48 hours that lead to formation of three layers. The top layer
obtained was
containing pure oil. The middle layer obtained was containing oil and water
both
while the bottom layer was containing mostly water with little Oil therein.
The
bottom layer was removed and treated as slop oil along with slop oil coming
from
middle layer as explained below.
The middle Layer was treated with Alum and retained in Separating Flask for
another 48 hours that lead to further formation of three layers, i.e. top
layer
containing pure oil, middle layer containing oil and alum with water and
bottom
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498 PCT/1N2013/000764
180
layer of slop oil. The layer containing alum was dried and tested for
Calorific Value.
The weight percent recovery of pure coconut oil from top and middle layers and
calorific value of dried alum layer can be seen in table 29.2 while results of
treatment
of slop oil along with weight percent recovery of coconut oil cum solvent can
be
seen in table 29.3.
TABLE 29.1- PRODUCTION OF COCONUT OIL BASED SLOP OILS USING
HIGH SHEAR MIXING MACHINE
SI.
DESCRIPTION TEST 1 TEST 2
No.
1 Total Mass of Slop Oils (kg) 0.50 0.50
2 Mixing Time (min) 5 5
3 RPM of Mixing 10,000
10,000
4 Oil content in Slop Oil (ppm) 95,921
240,150
5 Time elapsed before checking of Turbidity (min) 5.30 4.82
6 Average Turbidity of Slop Oils (NTU) >10,000 >10,000
TABLE 29.2- FRACTION OF OIL COLLECTED FROM DIFFERENT SECTIONS
SI.
DESCRIPTION TEST 1 TEST 2
No.
1 Wt % of Oil collected from top most layer 16.38 33.68
2 Wt % of Oil collected from middle layer 63.60 54.16
Calorific value of Scum obtained from middle
3 8,682
_______ layer (kcal/kg)
4 Calorific value of Original Coconut Oil (kcal/kg) 8,972
8,972
5 Wt 'Yo of Oil lost adhering to various surfaces 7.19 8.82
TABLE 29.3- PROCESSING OF BOTTOM LAYER SLOP OIL BY COMBINING
THE USE OF CENTRIFUGE WITH SOLVENT, ALUM AND THEN FILTRATION
SI.
DESCRIPTION TEST 1 TEST 2
No.
Step 1: Centrifuging of Slop Oil at 4,500,RCF
twice with NIL Residence time at peak RCF
value
Average Turbidity of Slop Oil after 2nd
1 >10,000 >10,000
Centrifuge (NTU)
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
181
Step 2: Addition of Solvent & Centrifuging it
once under same conditions as above and then
removal of entire top layer of Solvent + Oil
2 Name & Wt. % of Solvent added Xylene (25%) Xylene
(25%)
High shear High shear
3 Mode of Solvent addition
mixing mixing
4 Time of mixing (min) 1 minute 1 minute
Maximum RPM used for mixing Solvent 8,090 8,090
Average Turbidity of Slop Oil after removal of top
6 ,
1726 1,415
layer (NTU)
Step 3: Removal of top layer Solvent + Oil
7 Wt. % of Coconut oil + Solvent recovered 96.70 94.3
8 Moisture in above as per BTX (ppm) 18,155 16,550
Step 4: Removal of Solvent from Slop Oil
through Boiling with Free water present
therein
Average Turbidity of Slop Oil in NTU on cooling
9 after addition of make-up water that was lost 60 53
through boiling
Step 5: Addition of Alum with Residence Time
in Settling Vessel
Wt. % of Alum added to Slop Oil 0.05 0.05
11 Time permitted for Settling (hrs) 17.48 29.96
Average Turbidity after above Settling Time
12 3.15 4.53
(NTU)
Step 6: Filtration
Average Turbidity of Filtrate after using 41 Grade
13 2.33 3.24
Whatman Filter paper (NTU)
Average Turbidity of Filtrate after using 40 Grade
14 0.748 1.72
Whatman Filter paper (NTU)
Total time elapsed since preparation of Slop Oil
25.78 38.11
(hrs)
It was observed that, for a given hydrocarbon, higher the quantum of
hydrocarbons
in Slop Oils; easier it was for pure hydrocarbons to separate out with
settling. Also, it
was observed that presence of Alum in coconut oil reduced its calorific value
by 3.2
5 wt.% apart from making it viscous and increasing its alkali and ash
contents.
EXAMPLE-30
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
182
QUALITATIVE AND QUANTITATIVE RECOVERY OF HYDROCARBONS,
SOLVENT AND WATER FROM LARGE SAMPLES OF SLOP OILS USING
ENTIRE PROCESS OF THE PRESENT INVENTION
It was an aim to study efficacy of the process of present invention with large
scale
slop oils by evaluating the quantity and quality of hydrocarbons, solvent and
water
recovered. Accordingly, the slop oil samples were prepared as per conditions
mentioned in table 30.1. The slop oils were then treated as per procedure
mentioned
in table 30.2. Subsequently, hydrocarbons and solvent layer removed from step-
3 in
table 30.2 was next treated as per procedure mentioned in Example-15 and
Example-
16. The results obtained can be clearly seen in table 30.3.
TABLE 30.1- PRODUCTION OF VISCOUS ONGC HYDROCARBONS BASED
SLOP OILS USING HIGH SHEAR MIXING MACHINE
SI.
DESCRIPTION TEST 1 TEST 2
No.
ONGC
Name of Oil Used,.Diesel
Sods
1 Total Mass of Slop Oils (kg) 20.02 20.13
I 2 Mixing Time (min) 5 13.33
I 3 RPM of Mixing 10,000 8,130
Viscous Hydrocarbon containing bound water and 9 974
4
ash in Slop Oil (ppm)
5 Hydrocarbons content in slop oil (ppm) 4939 7,446
6 Ash content in Slop oil (ppm) 825
7 Bound water in Slop oil (ppm) 4,210
8 Time elapsed before checking of Turbidity (min) 4.17
4.68
9 Average Turbidity of Slop Oils in (NTU) 7,742 9,264
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
183
TABLE 30.2- PROCESSING OF ABOVE SLOP OIL BY COMBINING THE USE OF
CENTRIFUGE WITH SOLVENT, ALUM AND FILTRATION ALONG WITH THE
RECOVERY OF OIL
SI.
DESCRIPTION TEST 1 TEST 2
No.
Step 1: Centrifuging of Slop Oil at 4,500 RCF
twice with NIL Residence time at peak RCF
value
Average Turbidity of Slop Oil after 2nd
1 1,436 64
Centrifuge (NTU)
Step 2: Addition of Solvent & then
Centrifuging it once under same conditions as
above and then removal of entire top layer of
Solvent + Oil
2 Name & Wt. % of Solvent added Xylene Xylene
High shear Vigorous
3 Mode of Solvent addition
mixing hand mix
4 Time of mixing (min) 1 minute
RPM used for mixing Solvent 8090
Average Turbidity of Slop Oil after removal of top
6 2,733 78
layer (NTU) =
Step 3: Removal of top layer Solvent + Oil
Wt.% ONGC Viscous hydrocarbons + Solvent
7 97.11 97.88
recovered
8 Wt. % Moisture in above as per BTX (ppm) 25,534 541
Step 4: Removal of Solvent from Slop Oil
through Boiling with Free Water present
therein
Average Turbidity of Slop Oil in NTU after
9 addition of make-up water that was lost through 140 33
boiling (NTU)
Step 5: Addition of Alum with Residence time
in Settling Vessel
Wt. % of Alum added to Slop Oil 0.05 0.05
11 Time petinitted for Settling (hrs) 38.69 18.12
12 Average Turbidity after above Settling Time
4.96 4.64
(NTU)
Step 6: Filtration
Average Turbidity of Filtrate after using 41 Grade
13 2.13 2.54
Whatman Filter paper (NTU)
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
184
Average Turbidity of Filtrate after using 40 Grade
14 0.47 0.95
Whatman Filter paper (NTU)
Total time elapsed since preparation of Slop Oil
15 40.7 25.3
(hrs)
TABLE 30.3-SEPARATION OF SOLVENT FROM OIL
SI. ONGC
DESCRIPTION Diesel
No. Solids
Step 1: Addition of Water & Boiling
1 Wt of Solvent & Oil taken (kg) 1.0989 0.5469
2 Wt of Free Water added (kg) 2.0088 0.8052
3 Max. Temp in C while Boiling 97.2 97.4
4 Wt of Solvent collected as condensate (kg) 1.0135
0.4092
Wt of Water collected as condensate (kg) 0.896 0.3543
Wt of Free Water & Oil remaining in RB flask in
1.181
6 0.5792
(kg)
Step 2: Hot Centrifuging at 4,500 RCF once
with residence time of 10 minutes
Temp. in C of Free Water & Oil at the start of
7 92.3 92
Centrifuging
8 Moisture in Oil after Step 2 as per BTX (ppm) 25,700 342
Average Turbidity of recovered Free Water after
9 13.6 4.2
Step 2 (NTU)
=
TABLE 30.4- TEST RESULTS
SI. ONGC
DESCRIPTION Diesel
No. Solids
Wt. % Solvent recovered for Reuse inclusive of
1 99.25 99.65
material adhering on glasswares
2 Moisture in recovered Solvent as per BTX (ppm) 56 48
Wt. % Hydrocarbons recovered inclusive of
3 94.89 95.31
material adhering on glasswares
4 Moisture in recovered Oil as per BTX (ppm) 25,700 342
5 Calorific value of recovered Oil (kcal/kg) 10,428
11,027
6 Calorific value of original Oil (kcal/kg) 10,652 11,002
7
Wt. % of Water recovered from Slop Oil inclusive 98.15 98.86
of materials adhering on glasswares
SUBSTITUTE SHEET (RULE 26)
CA 02894026 2015-06-05
WO 2014/091498
PCT/1N2013/000764
185
8 Final Turbidity of above Water (NTU) 0.47 0.95
Wt. % of Free Water that was used for Solvent/Oil
9 separation recovered for reuse inclusive of 98.08 98.38
materials adhering on glasswares
Average Turbidity of Free Water recovered for
13.6 4.2
reuse (NTU)
It was observed that the qualitative cum quantitative recoveries of solvent
and water
from above slop oils were extremely good. It was further seen that small
fractions of
hydrocarbons were boiled out with solvent thus depressing its weight percent
5 recovery. Finally, it was seen that moisture in ONGC hydrocarbons could
have been
lower if said process had opted for hot settling over 48 hours. Finally, it
was
observed that it was lot more difficult to remove free water from viscous
hydrocarbons.
10 The present invention has been described in an illustrative manner, and
it is to be
understood that the terminology used is intended to be in the nature of
description
rather than of limitation. It is not intended to be exhaustive or to limit the
invention
,to the precise form disclosed. Many modifications and verifications are
possible in
light of the above teaching. It is intended that the scope of the invention be
limited
not by this detailed description, but rather by the claims appended hereto. It
is also to
be understood that the following claims are intended to cover all of the
generic and
specific features of the invention described herein.
SUBSTITUTE SHEET (RULE 26)