Note: Descriptions are shown in the official language in which they were submitted.
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
1
TITLE: PROCESSES FOR RECOVERY OF DERIVATIVES OF NATIVE LIGNIN
FIELD
This disclosure relates to processes for recovery of derivatives of native
lignin from
lignocellulosic feedstocks, recovered derivatives of native lignins, and
industrial applications
thereof. More particularly, this disclosure relates to processes for recovery
of derivatives of
native lignin having certain chemical properties as well as uses, processes,
methods, and
compositions thereof.
BACKGROUND
Native lignin is a naturally occurring amorphous complex cross-linked organic
macromolecule that comprises an integral structural component of all plant
biomass. The
chemical structure of lignin is irregular in the sense that different
structural units (e.g.,
phenylpropane units) are not linked to each other in any systematic order. It
is known that native
lignin comprises pluralities of two monolignol monomers that are methoxylated
to various
degrees (trans-coniferyl alcohol and trans-sinapyl alcohol) and a third non-
methoxylated
monolignol (trans-p-coumaryl alcohol). Various combinations of these
monolignols comprise
three building blocks of phenylpropanoid structures i.e. guaiacyl monolignol,
syringyl monolignol
and p-hydroxyphenyl monolignol, respectively, that are polymerized via
specific linkages to form
the native lignin macromolecule.
Extracting native lignin from lignocellulosic biomass during pulping generally
results in
lignin fragmentation into numerous mixtures of irregular components.
Furthermore, the lignin
fragments may react with any chemicals employed in the pulping process.
Consequently, the
generated lignin fractions can be referred to as lignin derivatives and/or
technical lignins. As it is
difficult to elucidate and characterize such complex mixture of molecules,
lignin derivatives are
usually described in terms of the lignocellulosic plant material used, and the
methods by which
they are generated and recovered from lignocellulosic plant material, i.e.
hardwood lignins,
softwood lignins, and annual fiber lignins.
Native lignins are partially depolyrnerized during chemical pulping processes
into lignin
fragments which are soluble in the pulping liquors and subsequently separated
from the
cellulosic pulps. Post-pulping liquors containing lignin and polysaccharide
fragments, and other
extractives, are commonly referred to as "black liquors" or "spent liquors",
depending on the
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
2
chemical pulping process. Such liquors are generally considered a by-product,
and it is common
practice to combust them to recover some energy value in addition to
recovering the cooking
chemicals. However, it is also possible to precipitate and/or recover lignin
derivatives from these
liquors. Each type of chemical pulping process used to separate cellulosic
pulps from other
lignocellulosic components produces lignin derivatives that are very different
in their physico-
chemical, biochemical, and structural properties.
Given that lignin derivatives are available from renewable biomass sources
there is an
interest in using these derivatives in certain industrial processes. For
example, lignin derivatives
obtained via organosolv extraction, such as the Alcell process (Alcell is a
registered trademark
of Lignol Innovations Ltd., Burnaby, BC, CA), have been used in rubber
products, friction
materials, adhesives, resins, plastics, asphalt, cement, casting resins,
agricultural products, and oil-
field products. However, large-scale commercial application of the extracted
lignin derivatives,
particularly those isolated in traditional pulping processes employed in the
manufacture of pulp
and paper, has been limited due to, for example, the inconsistency of their
chemical and
functional properties. This inconsistency may, for example, be due to changes
in feedstock
supplies and the particular extraction/generation/recovery conditions. These
issues are further
complicated by the complexity of the molecular structures of lignin
derivatives produced by the
various extraction methods and the difficulty in performing reliable routine
analyses of the
structural conformity and integrity of recovered lignin derivatives. For
instance, lignin derivatives
are known to have antioxidant properties (e.g. Catignani G.L., Carter M.E.,
Antioxidant
Properties of Lignin, Journal of Food Science, Volume 47, Issue 5, 1982, p.
1745; Pan X. et al. J.
Agric. Food Chem., Vol. 54, No. 16, 2006, pp. 5806-5813) but, to date, these
properties have
been highly variable making the industrial application of lignin derivatives
as an antioxidant
problematic.
Thermoplastics and thermosets are used extensively for a wide variety of
purposes.
Examples of thermoplastics include classes of polyesters, polycarbonates,
polylactates,
polyvinyls, polystyrenes, polyamides, polyacetates, polyacrylates,
polypropylene, and the like.
Polyolefins such as polyethylene and polypropylene represent a large market,
amounting to more
than 100 million metric tons annually worldwide. During manufacturing,
processing and use the
physical and chemical properties of certain thermoplastics can be adversely
affected by various
factors such as exposure to heat, UV radiation, light, oxygen, mechanical
stress or the presence
of impurities. Clearly it is advantageous to mitigate or avoid these problems.
In addition, the
increase in recycling of material has led to an increased need to address
these issues.
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
3
Degradation caused by free radicals, exposure to UV radiation, heat, light,
and
environmental pollutants are frequent causes of the adverse effects. A
stabilizer such as an
antioxidant, anti-ozonant, or UV block is often included in thermoplastic
resins for the purpose
of aiding in the production process and extending the useful life of the
product. Common
examples of stabilizers and antioxidants include amine types, phenolic types,
phenol alkanes,
phosphites, and the like. These additives often have undesirable or even
unacceptable
environmental, health and safety, economic, and/or disposal issues associated
with their use.
Furthermore, certain of these stabilizers/antioxidants can reduce the
biodegradability of the
product.
It has been suggested that lignin may provide a suitable polymeric natural
antioxidant
which has a low level of toxicity toxicity, efficacy, and environmental
profile. See, for example,
A. Gregorova et all, Radical scavenging capacity of lignin and its effect on
processing stabilization
of virgin and recycled polypropylene, Journal of Applied Polymer Science 106-3
(2007) pp. 1626-
1631; C. Pouteau et al. Antioxidant Properties of Lignin in Polypropylene,
Polymer Degradation
and Stability 81(2003) 9-18. For a variety of reasons, despite the advantages,
lignin has not been
adopted for widespread use as an antioxidant. For instance, it is often
problematic to provide
lignins that perform consistently in terms of antioxidant activity. Also, the
processing of the
lignin may introduce substances that are incompatible for use with chemicals
such as polyolefins.
Additionally, the cost of producing and/or purifying the lignin may make it
uneconomic for
certain uses.
SUMMARY
Some embodiments of the present disclosure relate to derivatives of native
lignin having
certain aliphatic hydroxyl contents. Surprisingly, it has been found that
stable and predictable
antioxidant activity is provided by selecting for derivatives of native lignin
having certain
aliphatic hydroxyl contents. Some embodiments of the present disclosure relate
to processes for
organosolv pulping of lignocellulosic biomass feedstocks wherein certain
operating parameters
are selectively manipulated to recover lignin derivatives having certain
aliphatic hydroxyl
contents.
This summary does not necessarily describe all features of the invention.
Other aspects,
features and advantages of the invention will be apparent to those of ordinary
skill in the art
upon review of the following description of specific embodiments of the
invention.
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
4
BRIEF DESCRIPTION OF THE DRAWINGS
The present disclosure will be described in conjunction with reference to the
following
drawings, in which:
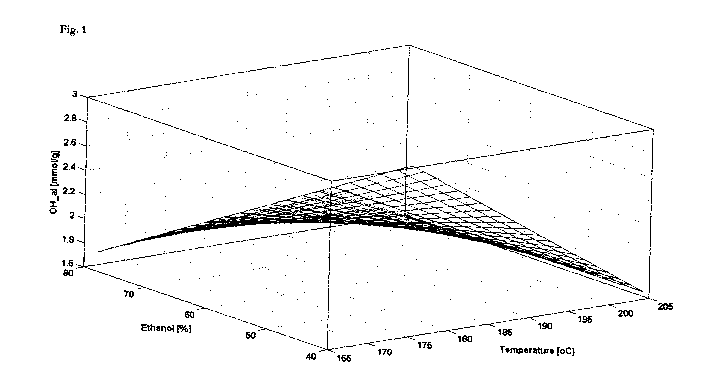
Fig. 1 is a chart showing aliphatic hydroxyl contents of lignin derivatives of
the present
disclosure recovered from aspen as a function of organic solvent concentration
[Ethanol] and
pulping temperature [Temperature] at constant pH of 2.47 and pulping time of
68 min;
Fig. 2 is a chart showing aliphatic hydroxyl contents of lignin derivatives of
the present
disclosure recovered from acacia as a function of pulping time [time] and
acidification of the
organic solvent [pH] at constant organic solvent concentration of 60.0% (w/w)
and pulping
temperature of 185.5 C;
Fig. 3 is a chart showing aliphatic hydroxyl contents of lignin derivatives of
the present
disclosure recovered from eucalyptus as a function of acidification of the
organic solvent [pH]
and pulping temperature [Temperature] at constant organic solvent
concentration of 60.0%
(w/w) and pulping time of 68 min;
Fig. 4 is a chart showing aliphatic hydroxyl contents of lignin derivatives of
the present
disclosure recovered from hybrid spruce as a function of acidification of the
organic solvent
[pH] and pulping time [Time] at constant organic solvent concentration of
60.5% (w/w) and
pulping temperature of 183 C;
Fig. 5 is a chart showing aliphatic hydroxyl contents of lignin derivatives of
the present
disclosure recovered from radiata pine as a function of acidification of the
organic solvent [pH]
and pulping time [Time] at constant organic solvent concentration of 60.5%
(w/w) and pulping
temperature of 183 C;
Fig. 6 is a chart showing aliphatic hydroxyl contents of lignin derivatives of
the present
disclosure recovered from loblolly pine as a function of pulping time [Time]
and pulping
temperature [Temperature, C] at constant pH of the pulping liquor of 2.43 and
organic solvent
concentration of 62% w/w ethanol;
Fig. 7 is a chart showing aliphatic hydroxyl contents of lignin derivatives of
the present
disclosure recovered from wheat straw as a function of organic solvent
concentration [Ethanol]
and pulping time [Time] at constant pulping temperature of 185.5 C and organic
solvent
acidified to a pH of 2.2;
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
Fig. 8 is a chart showing aliphatic hydroxyl contents of lignin derivatives of
the present
disclosure recovered from bagasse as a function of acidification of the
organic solvent [pH] and
pulping time [lime] at constant organic solvent concentration of 55% (w/w) and
pulping
temperature of 179 C; and
Fig. 9 is a chart showing aliphatic hydroxyl contents of lignin derivatives of
the present
disclosure recovered from corn cobs as a function of acidification of the
organic solvent [pH]
and pulping time [Time] at constant organic solvent concentration of 53.5%
(w/w) and pulping
temperature of 177 C.
DETAILED DESCRIPTION
The present disclosure relates to derivatives of native lignin having certain
aliphatic
hydroxyl contents, and to organosolv pulping processes tailored for recovery
of the lignin
derivatives from lignocellulosic biomass feedstocks.
Lignin derivative having lower aliphatic hydroxyl contents have been found to
score
more highly on the Radical Scavenging Index (RSI), a measure of antioxidant
activity. Thus,
selecting for derivatives of native lignin having a lower aliphatic hydroxyl
content results in a
product having a higher and more predictable antioxidant activity. It has been
found that
derivatives of native lignin having an aliphatic hydroxyl content of about
2.35 mmol/g or less
result in a good level of antioxidant activity. For example, about 2.25 mmol/g
or less, about 2.00
mmol/g or less, or about 1.75 mrnol/g or less.
Radical Scavenging Index (RSI) is a measure of radical scavenging capacity.
The assay
uses 2,2-dipheny1-1-picrylhydrazyl (DPPH), a stable free radical which absorbs
light strongly at
515 nm to measure a compound's radical scavenging index (RSI). In its radical
form, DPR1-1.
absorbs strongly at 515 nm and has a deep purple colour. As DPPH gives up its
free electron to
radical scavengers, it loses its purple colour and its absorbance shifts to
520 nm. The greater the
drop in DPPH absorbance at 515 nm after a test compound has been added to the
DPPH
solution, the higher the compound's free RSI and also, its antioxidant
activity. In the present
disclosure, Vitamin E (Vit. E) and butylated hydroxytoluene (BHT) are used as
positive controls.
The lignin derivative samples (1.0 ¨ 2.0 mg), Vit. E control samples (1.0-2.0
mg), and BHT
control samples (6.0 ¨ 8.0 mg) are prepared for testing by being placed into
microcentrifuge
tubes after which, each was diluted with 1.0 mL of 90% (v/v) aqueous dioxane,
vortexed,
transferred to new microcentrifuge tubes and further diluted 50/50 with 90%
aqueous dioxane
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
6
to give stock concentrations of 0.5-1.0 mg/mL for the samples and Vitamin E
and 3.0-4.0
mg/mL for BHT. An indicating (purple) DPPH stable free radical solution is
made by dissolving
3.78 mg DPPH in 100 mL 90% dioxane (95.9 1.1M). Samples and standards are
serially diluted to
fill columns of a quartz 96-well plate (8 dilutions). The assays were
performed by placing aliquots
of the sample stock solutions into two rows of wells in a 96-well plate. The
first row served as
the reference row while the second row received DPPH aliquots. 165 'IL of 90%
dioxane was
added to each well and mixed. Aliquots of the mixed samples in each row are
transferred to the
adjacent row which is further diluted with 165 !IL of 90% dioxane in each
well. The mixing,
transferring and dilution are repeated until the last row of wells is
prepared. The same volume of
aliquots is removed from the last row. The 96-well plate also contains a row
of wells that
received only the 90% dioxane. In the final step of the preparation procedure,
165 L of the
DPPH solution is added to all the control and analytical columns by using an 8-
channel auto-
pipette and an Eppendorf reagent reservoir as quickly as possible. As soon as
all reagents are
added, the plate is placed into a plate-reading spectrophotometer (Molecular
Devices, Sunnyvale,
CA, USA, Spectra Max Plus), and absorbance measurements are carried out. The
program for
the spectrophotometer (SOFTmax software) consists of a timing sequence of 16
min and a
reading of the entire plate at 515 nm. RSI is defined as the inverse of the
concentration which
produces 50% inhibition in DPPH absorbance at 515 nm. The results are then
'normalized' by
dividing sample RSI by the RSI value for the BHT control. The normalized RSI
is represented
by this acronym "NRSI".
The present disclosure provides processes for recovery of derivatives of
native lignin
during or after organosolv pulping of lignocellulosic feedstocks. The pulp may
be from any
suitable lignocellulosic feedstock including hardwoods, softwoods, annual
fibres, and
combinations thereof.
Hardwood feedstocks include Acacia; Afzelia; Synsepalum duloificum; Albizia;
Alder
(e.g. Alnus glutinosa, Alnus rubra); Applewood; Arbutus; Ash (e.g. F. nigra,
F. quadrangulata, F.
excelsior, F. pennylvanica lanceolata, F. latifolia, F. profunda, F.
ameficana); Aspen (e.g. P. grandidentata,
P. tremula, P. tremuloides); Australian Red Cedar (Toona ciliata); Ayna
(Dz1rtemonanthus benthamianus);
Balsa (Ochroma pyramidale); Basswood (e.g. T. ameficana, T. beterophylla);
Beech (e.g. F. glvatica, F.
grandifolia); Bitch; (e.g. Betula populifolia, B. nigra, B. papyrifira, B.
lenta, B. alleghaniensis/ B. lutea, B.
pendula, B. pubescens); Blackbean; Blackwood; Bocote; Boxelder; Boxwood;
Brazilwood; Bubinga;
Buckeye (e.g. Aesculus hippocastanum, Aesculus glabra, Aesculus flava/
Aesculus octandra); Butternut;
Catalpa; Cherry (e.g. Prunus serotina, Prunus pennylvanica, Prunus avium);
Crabwood; Chestnut;
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
7
Coachwood; Cocobolo; Corkwood; Cottonwood (e.g. Populus balsam/era, Populus
deltoides, Populus
sargentii, Populus heterophylla); Cucumbertree; Dogwood (e.g. Cormisfiorida,
Cornus nuttallu); Ebony
(e.g. Dio.ipyros kur#i, Diooros melanida, Dioipyros crass#7ora); Elm (e.g.
Ulmus americana, Ulmus
procera, Ulmus thomasii, Ulmus rubra, Ulmus glabra); Eucalyptus; Greenheart;
Grenadilla; Gum (e.g.
Nyssa sylvatica, Eucalyptus globulus, Liquidambar styractflua, Nyssa
aquatica); Hickory (e.g. Caga alba,
Caga glabra, Caga ovata, Caga laciniosa); Hornbeam; Hophornbeam; Ipe; Iroko;
Ironwood (e.g.
Bangkirai, Cenpinus caroliniana, Casuarina equisetifolia, Choricbangaipia
subaigentea, Copaffera spp.,
Eusideroglon .z2vageti, Guajacum officinale, Guajacum sanctum, Hopea odorata,
Ipe, Krugiodendron
ferreum, Lyonothamnus lyonzi (L. flofibundus), Mesua ferrea, Olea spp., Olnga
tesota, Ostga virginiana,
Parrotia persica, Tabebuia serratifolia); Jacaranda; Jotoba; Lacewood; Laurel;
Limba; Ligrium vitae;
Locust (e.g. Robinia pseudacacia, Gleditsia triacanthos); Mahogany; Maple
(e.g. Acer saccharum, Acer
nigrum, Acer negundo, Acer rubrum, Acer sacchminum, Acer pseudoplatanus);
Meranti; Mpingo; Oak (e.g.
Quercus macrocaipa, Quercus alba, Quercus stellata, Quercus bicolor, Quercus
virginiana, Quercus michauxii,
Quercus prinus, Quercus muhlenbeigii, Quercus chgsolepis, Quercus lyrata,
Quercus robur, Quercus petraea,
Quercus rubra, Quercus velutina, Quercus laurifolia, Quercus falcata, Quercus
nigra, Quercus phellos, Quercus
texana); Obeche; Okoume; Oregon Myrtle; California Bay Laurel; Pear; Poplar
(e.g. P.
balsamifera, P. nigra, Hybrid Poplar (Populus x canadensis)); Ramin; Red
cedar; Rosewood; Sal;
Sandalwood; Sassafras; Satinwood; Silky Oak; Silver Wattle; Snakewood;
Sourwood; Spanish-
cedar; American sycamore; Teak; Walnut (e.g. Juglans nigra, Juglans regia);
Willow (e.g. Salix nigra,
Salix alba); Yellow-poplar (Litiodendron tuligera); Bamboo; Palmwood; and
combinations/hybrids
thereof.
For example, hardwood feedstocks for the present disclosure may be selected
from
Acacia, Aspen, Beech, Eucalyptus, Maple, Birch, Gum, Oak, Poplar, and
combinations/hybrids
thereof. The hardwood feedstocks for the present disclosure may be selected
from Populus spp.
(e.g. P. grandidentata, P. tremula, P. tremuloides, P. balsam/era, P.
deltoides, P. saigentii, P. heterophylla, P.
balsanifera, P. nigra, Populus X canadensis), Eucalyptus iv. (e.g. E.
astrigens, e. clivicola, E. dielsii, E.
forrestiana, E. gardneri, E. globulus, E. nitans, E. occidentalis, E.ornata,
E. salubnir, E. ipathulata), Acacia
ipp. (e.g. A. albida, A. cavenia, A. dealbata, A. decurrens, A. famesiana, A.
meamsii, A. melanoxylon, A.
nilotica, A. penninervis, A. pycnatha, A. saligna, and combinations thereof.
It has been found that derivatives of native lignin from hardwood feedstocks
having an
aliphatic hydroxyl content of about 2.35 rnmol/g or less have a good level of
antioxidant activity.
For example, about 2.25 mrnol/g or less, about 2mmol/g or less, or about 1.75
rnmol/g or less.
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
8
In the present disclosure, "aliphatic hydroxyl content" refers to the quantity
of aliphatic
hydroxyl groups in the lignin derivative and is the arithmetic sum of the
quantity of Primary and
Secondary Hydroxyl Groups (al-OH = pr-OH + sec-OH). The aliphatic hydroxyl
content can be
measured using quantitative 13C high resolution NMR spectroscopy of acetylated
lignin (using
1,3,5-trioxane as internal reference).
For the data analysis "BASEOPT" (DIGMOD set to baseopt) routine in TopSpin
2.1.4
was used to predict the first FID data point back at the mid-point of "C r.f.
pulse in the digitally
filtered data was used. For the NMR spectra recording a Bruker AVANCE II
digital NMR
spectrometer running TopSpin 2.1 was used. The spectrometer used a Bruker 54
mm bore
Ultrashield magnet operating at 14.1 Tesla (600.13 MHz for 1H, 150.90 MHz for
13C). The
spectrometer was coupled with a Bruker QNP cryoprobe (5 mm NMR samples, 13C
direct
observe on inner coil, 1H outer coil) that had both coils cooled by helium gas
to 20K and all
preamplifiers cooled to 77K for maximum sensitivity. Sample temperature was
maintained at 300
K 0.1 K using a Bruker BVT 3000 temperature unit and a Bruker BCUO5 cooler
with ca. 95%
nitrogen gas flowing over the sample tube at a rate of 800 L/h.
Derivatives of native lignin according to the present disclosure, coming from
hardwood
feedstocks tend to have a normalized RSI of 30 or greater, 40 or greater, 50
or greater, 60 or
greater, 70 or greater.
Softwood feedstocks include Araucaria (e.g. A. cunninghamii, A. angustifolia,
A. araucana);
softwood Cedar (e.g. Juniperus virginiana, Tbuja plicata, Tbuja occidentalis,
Cbamaeoptis thyoides
Callitropsis nootkatensis); Cypress (e.g. Cbamaegparis, Cup ressus Taxodium,
Cup ressus arkonica,
Taxodium distichum, Chamaegparis obtusa, Chamaegpatis lawsoniana, Cup ressus
semperviren); Rocky
Mountain Douglas-fir; European Yew; Fir (e.g. Abies balsamea, Abies alba,
Abies procera, Abies
arnabilis); Hemlock (e.g. Tsuga canadensis, Tsuga mertensiana, Tsuga
heterophylla); Kauri; Kaya; Larch
(e.g. Larix decidua, Larix kaempferi, Larix laricina, Larix occidentalis);
Pine (e.g. Pinus nigra, Pinus
banksiana, Pinus contorta, Pinus radiata, Pinus ponderosa, Pinus resinosa,
Pinta glvestris, Pinus strobus,
Pinus monticola, Pinus lambertiana, Pinus taeda, Pinus palustfis, Pinus
figida, Pinus echinata); Redwood;
Rimu; Spruce (e.g. Picea abies, Picea mariana, Picea rubens, Picea sitchensis,
Picea glauca); Sugi; and
combinations/hybrids thereof.
For example, softwood feedstocks which may be used herein include cedar; fir;
pine;
spruce; and combinations thereof. The softwood feedstocks for the present
disclosure may be
selected from loblolly pine (P. taeda), radiata pine, jack pine, spruce (e.g.,
white, interior, black),
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
9
Douglas fir, black spruce, and combinations/hybrids thereof. The softwood
feedstocks for the
present disclosure may be selected from pine (e.g. Pinus radiata, Pinus
taeda); spruce; and
combinations/hybrids thereof.
It has been found that derivatives of native lignin from softwood feedstocks
having an
aliphatic hydroxyl content of about 2.35 mmol/g or less have a good level of
antioxidant activity.
For example, about 2.25 rnmol/g or less, about 2mmol/g or less, or about 1.75
mmol/g or less.
Derivatives of native lignin according to the present disclosure, coming from
softwood
feedstocks tend to have a normalized RSI of 15 or greater, 25 or greater, 30
or greater, 35 or
greater, 40 or greater.
Annual fibre feedstocks include, for example, flax; cereal straw (wheat,
barley, oats, rye);
bagasse; corn; hemp, fruit pulp, alfa grass, switchgrass, miscanthus, kenaf,
and
combinations/hybrids thereof. For example, the annual fibre feedstock may be
selected from
wheat straw, corn stover, corn cobs, sugar cane bagasse, and
combinations/hybrids thereof.
Derivatives of native lignin according to the present disclosure, coming from
annual fibre
feedstocks tend to have a normalized RSI of 15 or greater, 20 or greater, 25
or greater, 30 or
greater, 35 or greater.
In an embodiment of the present disclosure, derivatives of native lignin from
annual fibre
feedstocks have an aliphatic hydroxyl content of about 3.75 mmol/g or less;
3.5 mmol/g or less;
3.25 mmol/g or less; 3 mmol/g or less; 2.75 mmol/g or less; 2.5 mmol/g or
less; 2.35 mmol/g
or less; 2.25 mmol/g or less.
The derivatives of native lignin will vary with the type of process used to
separate native
lignins from cellulose and other biomass constituents. Examples of extractive
technologies
include (1) solvent extraction of finely ground wood; (2) acidic dioxane
extraction (acidolysis) of
wood; (3) steam explosion; or (4) acid hydrolysis methods. Furthermore,
derivatives of native
lignin can be recovered after pulping of lignocellulosic biomass including
industrially operated
kraft and soda pulping (and their modifications) and sulphite pulping. It
should be noted that
kraft pulping, sulphite pulping, and ASAM organosolv pulping will generate
derivatives of native
lignin containing significant amounts of organically-bound sulphur which may
make them
unsuitable for certain uses.
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
Four major "organosolv" pulping methods have been proposed. Organosolv
extraction
tends to produce highly-purified lignin mixtures. The first organosolv method
uses
ethanol/solvent pulping (aka the Alcell process); the second organosolv
method uses alkaline
sulphite anthraquinone methanol pulping (aka the "ASAM" process); the third
organosolv
process uses methanol pulping followed by methanol, NaOH, and anthraquinone
pulping (aka
the "Organocell" process); the fourth organosolv process uses acetic
acid/hydrochloric acid
pulping (aka the "Acetosolv" process).
Organosolv extraction processes, particularly the Alcell process, tend to be
less
aggressive and can be used to separate highly purified lignin and other useful
materials from
biomass without excessively altering or damaging the lignin. Such processes
can therefore be
used to maximize the value from all the components making up the biomass.
Organosolv
extraction processes however typically involve extraction at higher
temperatures and pressures
with a flammable solvent than other industrial methods and thus are generally
more complex and
expensive.
A description of the Alcell process can be found in US Patent 4,764,596
(herein
incorporated by reference). The process generally comprises pulping a fibrous
biomass feedstock
with primarily an ethanol/water solvent solution under conditions that
included: (a) 60%
ethanol/40% water, (b) temperature of about 180 C to about 210 C, (c)
pressure of about 20
atm to about 35 atm, and (d) a processing time ranging from 30 to 120 minutes.
Derivatives of
native lignin are fractionated from the native lignins into the pulping liquor
which also receives
solubilised hemicelluloses, other saccharides and other extractive such as
resins, organic acids,
phenols, and tannins. Organosolv pulping liquors comprising the fractionated
derivatives of
native lignin and other extractives from the fibrous biomass feedstocks, are
often called "black
liquors". The organic acid extractives released by organosolv pulping
significantly acidify of the
black liquors to pH levels of about 5.5 and lower. After separation from the
cellulosic pulps
produced during the pulping process, the derivatives of native lignin are
recovered from the
black liquors by depressurization/flashing followed by dilution with cold
water which will cause
the fractionated derivatives of native lignin to precipitate thereby enabling
their recovery by
standard solids/liquids separation processes. Various disclosures exemplified
by US Patent No.
7,465,791 and PCT Patent Application Publication No. WO 2007/129921, describe
modifications to the Alcell organosolv process for the purposes of increasing
the yields of
fractionated derivatives of native lignin recovered from fibrous biomass
feedstocks during
biorefming. Modifications to the Alcell organosolv process conditions
included adjusting: (a)
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
11
ethanol concentration in the pulping solution to a value selected from a range
of 60% - 80%
ethanol, (b) temperature to a value selected from a range of 120 C to 350 C,
(c) pressure to a
value selected from a range of 15 atm to 35 atm, and (d) processing time to a
duration from a
range of 20 minutes to about 2 hours. Some modifications to the Alcell
organosolv process also
include the addition of an acid catalyst to the pulping solution to lower its
pH to a value from
the range of about 1.5-5.5.
The present disclosure provides a process for producing derivatives of native
lignin from
lignocellulosic biomass feedstocks wherein the lignin derivatives have certain
aliphatic hydroxyl
contents selected before pulping is commenced, said process comprising:
(a) pulping a fibrous biomass feedstock with an organic solvent/water solvent
solution
using a combination of the following operating conditions or parameters: (1) a
selected organic
solvent concentration, (2) a selected degree of acidification of the organic
solvent, (3) a selected
temperature at which the pulping is conducted, and (4) a selected time period
during which
pulping is conducted,
(b) separating the cellulosic pulps from the black liquor produced during
pulping, and
(c) recovering derivatives of native lignin from the black liquor.
The organic solvent may be selected from short-chain aliphatic alcohols such
as
methanol, ethanol, propanol, and combinations thereof. For example, the
solvent may be
ethanol. The liquor solution may comprise about 20%, by weight, or greater,
about 30% or
greater, about 50% or greater, about 60% or greater, about 70% or greater, of
ethanol.
The pH of the organic solvent may be adjusted to, for example, from about 1 to
about 6,
or from about 1.5 to about 5.5.
Step (a) of the process may be carried out at a temperature of from about 100
C and
greater, or about 120 C and greater, or about 140 C and greater, or about 160
C and greater, or
about 170 C and greater, or about 180 C and greater. The process may be
carried out at a
temperature of from to about 300 C and less, or about 280 C and less, or about
260 C and less,
or about 240 C and less, or about 220 C and less, or about 210 C and less, or
about 205 C and
less, or about 200 C and less.
Step (a) of the process may be carried out at a pressure of about 5 atm and
greater, or
about 10 atm and greater, or about 15 atm and greater, or about 20 atm and
greater, or about 25
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
12
atm and greater, or about 30 atm and greater. The process may be carried out
at a pressure of
about 150 atm and less, or about 125 atm and less, or about 115 atm and less,
or about 100 atm
and less, or about 90 atm and less, or about 80 atm and less.
The fibrous biomass may be treated with the solvent solution of step (a) for
about 1
minute or more, about 5 minutes or more, about 10 minutes or more, about 15
minutes or more,
about 30 minutes or more. The fibrous biomass may be treated with the solvent
solution of step
(a) for about 360 minutes or less, about 300 minutes or less, about 240
minutes or less, about
180 minutes or less, about 120 minutes or less.
The present disclosure provides a process for producing a lignin derivative
having an
aliphatic hydroxyl content of about 2.35 mmol/g or less, about 2.25 mmol/g or
less, about
2tnmol/g or less, or about 1.75 mmol/g or less. Said process comprises:
a) commingling a fibrous biomass feedstock in a vessel with a selected organic
solvent/water solvent solution having a selectively adjusted pH, wherein:
i. the solution comprises about 30% or greater, by weight, of organic
solvent; and
the pH of the organic solvent is adjusted from about 1 to about 5.5;
b) heating the commingled fibrous biomass and pH-adjusted organic solvent to a
temperature selected from the range of about 100 C to about 300 C;
c) raising the pressure in the vessel to about 10 atm or greater;
d) maintaining the elevated temperature and pressure for a period of time
selected from the
range of about 1 minute to about 360 minutes while continuously commingling
fibrous
biomass and pH-adjusted organic solvent thereby producing cellulosic pulps and
a black
liquor, and;
e) separating the cellulosic pulps from the pulp liquor
f) recovering derivatives of native lignin.
g) Liquor-to-biomass ratios can be varied from 2:1 to 15:1 wt:wt
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
13
The present disclosure provides a process for producing a hardwood lignin
derivative
having an aliphatic hydroxyl content of about 2.35 mmol/g or less, about 2.25
mmol/g or less,
about 2 mmol/g or less, or about 1.75 mmol/g or less, said process comprises:
a) pulping a fibrous biomass feedstock in a vessel with a pH-adjusted organic
solvent/water
solvent solution to form a liquor, wherein:
i. the solution comprises a selected concentration of organic solvent of
about 30% or greater; and
the pH of the organic solvent is selectively adjusted from about 1 to
about 5.5;
b) heating the liquor to a selected temperature of about 100 C or greater;
c) raising the pressure in the vessel to about 10 atm or greater;
d) maintaining the elevated temperature and pressure for a selected period of
time of 1
minute or longer;
e) separating the cellulosic pulps from the pulp liquor
f) recovering derivatives of native lignin.
The present disclosure provides a process for producing a softwood lignin
derivative
having an aliphatic hydroxyl content of about 2.35 mmol/g or less, about 2.25
mmol/g or less,
about 2 mmol/g or less, or about 1.75 mmol/g or less, said process comprises:
a) commingling a fibrous biomass feedstock in a vessel with a selected organic
solvent/water solvent solution having a selectively adjusted pH, wherein:
i. the solution comprises about 30% or greater, by weight, of organic
solvent; and
the pH of the organic solvent is adjusted from about 1 to about 5.5;
b) heating the commingled fibrous biomass and pH-adjusted organic solvent to a
temperature selected from the range of about 100 C to about 300 C;
c) raising the pressure in the vessel to about 10 atrn or greater;
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
14
d) maintaining the elevated temperature and pressure for a period of time
selected from the
range of about 1 minute to about 360 minutes while continuously commingling
fibrous
biomass and pH-adjusted organic solvent thereby producing cellulosic pulps and
a black
liquor, and;
e) separating the cellulosic pulps from the pulp liquor
The present disclosure provides a process for producing an annual fibre lignin
derivative
having an aliphatic hydroxyl content of about 3.75 mmol/g or less; 3.5 mmol/g
or less; 3.25
mmol/g or less; 3 mmol/g or less; 2.75 mmol/g or less; 2.5 mmol/g or less;
2.35 mmol/g or
less; 2.25 mmol/g or less, said process comprises:
a) commingling a fibrous biomass feedstock in a vessel with a selected organic
solvent/water solvent solution having a selectively adjusted pH, wherein:
i. the solution comprises about 30% or greater, by weight, of organic
solvent; and
the pH of the organic solvent is adjusted from about 1 to about 5.5;
b) heating the commingled fibrous biomass and pH-adjusted organic solvent to a
temperature selected from the range of about 100 C to about 300 C;
c) raising the pressure in the vessel to about 10 atm or greater;
d) maintaining the elevated temperature and pressure for a period of time
selected from the
range of about 1 minute to about 360 minutes while continuously commingling
fibrous
biomass and pH-adjusted organic solvent thereby producing cellulosic pulps and
a black
liquor, and;
e) separating the cellulosic pulps from the pulp liquor
The present disclosure relates to methods for determining suitable operating
conditions
for organosolv pulping of lignocellulosic biomass feedstocks for production of
derivatives of
native lignin having certain desirable aliphatic hydroxyl contents. Such
operating conditions may
be determined, for example, by selecting a target operating value for each of
at least two process
parameters while keeping other process parameters constant. Suitable process
parameters that
can be manipulated by selection of target operating values include: (a)
concentration of organic
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
solvent in the pulping liquor, (b) degree of acidification of the organic
solvent prior to
commencing pulping, (c) temperature at which pulping is conducted, (d)
duration of the pulping
period, and (e) liquor-to-biomass ratios among others. Suitable target
operating values can be
determined by empirically modelling the performance results collected from a
series of
preliminary organosolv pulping runs with subsamples of a selected
lignocellulosic feedstock
wherein at least one process parameter has been adjusted between each of the
runs. Exemplary
performance results are the aliphatic hydroxyl contents of lignin derivatives
recovered from each
preliminary organosolv pulping run. A suitable number of preliminary pulping
runs is about 10,
or about 15, or about 20 about 25 or about 30. The performance results in
combination with the
manipulated process parameters can be used for equations for identification of
suitable target
operating values for one or more organosolv processing conditions for
lignocellulosic biomass
feedstocks from which lignin derivatives have desirable chemical or structural
or functional
attributes can be recovered. Such equations can be derived from performance
results by
mathematical tools and software exemplified by Matlab Version 7.7Ø471
R2008b (Matlab is a
registered trademark of The Mathworks Inc., Natick, MA, USA) with a Model-
Based Calibration
Toolbox Version 3.5 supplied by The Mathworks Inc.
In reference to use of the Matlab software tools for generating the predictive
equations
for selected organosolv process conditions, suitable model characteristics
include:
Model Class: Linear Models
Linear Model Subclass: polynomial
Interaction order: 2
Suitable model terms include:
Constant terms: 1
Linear terms: 4
Second Order Terms: 10
Total Number Terms: 15
Stepwise: Minimize PRESS with 50 maximum iterations
Suitable experimental designs include:
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
16
Experimental Design Type: Sobol Sequence
Number of Points: all available points in Tables 1, 3, and 4
Input factors: 4 (the process parameters Cooking time [Time], cooking
temperature
[Temperature], cooking pH [pH], solvent concentration [SOLVENT]
The maximum and minimum values used in each model should be those maximum and
minimum values observed in the actual performance data points collected for
both the input and
output variables ("responses").
This modelling approach can be used to select and manipulate organosolv
process
conditions to recover lignin derivatives that have certain targeted ranges of
chemical and/or
structural attributes, for example, one or more of:
- non-conjugated carbonyl groups/g lignin derivative in the range of about
0.09 to
about 1.62 CO-nc mmol/g;
- conjugated carbonyl groups/g lignin derivative in the range of about 0.31
to about
1.36 CO-conj mmol/g;
- total carbonyl groups/g lignin derivative in the range of about 0.51 to
about 2.72 CO
tot mmol/g;
- primary hydroxyl groups/g lignin derivative in the range of about 0.48 to
about 3.62
pr-OH mmol/g;
- secondary hydroxyl groups/g lignin derivative in the range of about 0 to
about 3.19
sec-OH sec mmol/g;
- aliphatic hydroxyl groups/g lignin derivative in the range of about 0.53
to about 6.62
al-OH mmol/g;
- phenolic hydroxyl groups/g of lignin derivative in the range of about
2.00 mmol to
about 7.12 ph-OH mmol/g;
- total hydroxyl groups/g lignin derivative in the range of about 4.73 to
about 10.28
tot-OH mmol/g;
- 0 to about 2.46 mmol/g of aliphatic carboxylic and/or aliphatic ester
groups (COOR
al mmol/g);
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
17
- conjugated carboxylic and/or conjugated ester groups/g lignin derivative
in the range
of about 0 to about 2.20 COOR con mmol/g;
- carboxylic or ester group/g lignin derivative in the range of about 0 to
about 4.46
COOR tot mmol/g;
- methoxyl groups/g lignin derivative in the range of about 3.61 to about
8.46 0-me
mmol/g;
- ethoxyl or other alkoxy groups/g lignin derivative in the range of about
0.28 to about
1.34 0-et mmol/g;
- syringyl groups/g lignin derivative in the range of about 0 to about 3.60
S mmol/g;
- guaiacyl groups /g lignin derivative in the range of about 1.33 to about
7.78 G
mmol/g;
- S/G ratio in the range of about 0.41 to about 41.87;
- 0 to about 1.91 p-hydroxyphenyl units (or H-units)/g lignin derivative
mmol/g;
- p-5 structural moitie/g lignin derivative in the range of about 0 to
about 0.68
mmol/g;
- P-P structural moitiey/g lignin derivative in the range of about 0 to
about 0.46 p-i3
mmol/g;
- 3-0-4 structural moitiey/g lignin derivative in the range of about 0 to
about 2.66 f3-
0-4 mmol/g;
- degree of condensation (DC) in the range of about 0.78 to about 85.0 %;
- number-average molecular weight (Mn, g/mol) in the range of about 536.50
Daltons
to about 1464.00 Daltons;
- weight-average molecular weight (Mw, g/mol) in the range of about 965.00
Daltons
to about 3366.50 Daltons;
- Z average molecular weight (Mz, g/mol) in the range of about 1378.50
Daltons to
about 5625.00 Daltons;
- polydispersity (D) in the range of about 1.46 to about 3.04 (Mw/Mn);
- carbon content (% dry weight) in the range of about 60.54% to about
72.50%;
- hydrogen content ( /0 dry weight) in the range of about 4.52% to about
7.24%; and
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
18
- oxygen content (% dry weight) in the range of about 21.90% to about
35.38%;
- nitrogen content (')/0 dry weight) in the range of about 0.08% to about
2.82%;
- sulphur content ( /0 dry weight) in the range of about 0.50% to about
1.25%.
This modelling approach can be used to select and manipulate organosolv
process
conditions to recover lignin derivatives that have certain targeted ranges of
functional attributes,
for example, one or more of:
- radical scavaging index (RSI) in the range of about 5.44 to about 53.36;
- glass transition temperature (Tg) in the range of about 51 C to about
127 C;
- melt flow index (MFI) in the range of about 0 g/10 min to about 878.00
g/10 sec;
- viscosity (V) of a phenol-formaldehyde resin containing these lignin
derivatives at
40% phenol replacement level in the range of about 50 cP to about 20,000 cP;
and
- normalized shear strength as measured by the automated bonding evaluation
system
(ABES) of a phenol-formaldehyde resin where 40% of the phenol has been
replaced
by the lignin derivative about 2,034 MPa*cm2/g to about 3796 MPa*cm2/g.
The derivatives of native lignin recovered with the processes described herein
may be
incorporated into polymer compositions. The compositions herein may comprise a
lignin
derivative according to the present disclosure and a polymer-forming
component. As used
herein, the term 'polymer-forming component' means a component that is capable
of being
polymerized into a polymer as well as a polymer that has already been formed.
For example, in
certain embodiments the polymer-forming component may comprise monomer units
which are
capable of being polymerized. In certain embodiments the polymer component may
comprise
oligomer units that are capable of being polymerized. In certain embodiments
the polymer
component may comprise a polymer that is already substantially polymerized.
Polymers forming components for use herein may result in thermoplastic
polymers such
as epoxy resins, urea-formaldehyde resins, polyimides and the like, and
thermosets such as
phenol-formaldehyde resins, and the like. For example, polyalkenes such as
polyethylene or
polypropylene.
Typically, the lignin derivative will comprise from about 0.1%, by weight, or
greater,
about 0.5% or greater, about 1% or greater, of the composition. Typically, the
lignin derivative
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
19
will comprise from about 80%, by weight, or less, about 60% or less, about 40%
or less, about
20% or less, about 10% or less, about 5% or less, of the composition.
The compositions comprise lignin derivative and polymer-forming component but
may
comprise a variety of other optional ingredients such as adhesion promoters;
biocides
(antibacterials, fungicides, and moldicides), anti-fogging agents; anti-static
agents; bonding,
blowing and foaming agents; dispersants; fillers and extenders; fire and flame
retardants and
smoke suppressants; impact modifiers; initiators; lubricants; micas; pigments,
colorants and dyes;
plasticizers; processing aids; release agents; silanes, titanates and
zirconates; slip and anti-blocking
agents; stabilizers; stearates; ultraviolet light absorbers; foaming agents;
defoamers; hardeners;
odorants; deodorants; antifouling agents; viscosity regulators; waxes; and
combinations thereof
The present disclosure provides the use of the present derivatives of native
lignin as an
antioxidant. For example, the present use may be as an antioxidant additive
for use with
thermoplastic polymers such as polyethylene, polypropylene, polyamides, and
combinations
thereof.
The present disclosure provides methods of producing derivatives of native
lignin having
an aliphatic hydroxyl content of about 2.35 mmol/g or less, about 2.25 mmol/g
or less, about 2
mmol/g or less, or about 1.75 mmol/g or less.
The present disclosure provides methods of producing derivatives of native
hardwood
lignin having an aliphatic hydroxyl content of about 2.35 mmol/g or less
result, about 2.25
mmol/g or less, about 2mmol/g or less, or about 1.75 mmol/g or less.
The present disclosure provides methods of producing derivatives of native
softwood
lignin having an aliphatic hydroxyl content of about 2.35 mmol/g or less,
about 2.25 mmol/g or
less, about 2mmol/g or less, or about 1.75 mmol/g or less.
The present disclosure provides methods of producing derivatives of native
annual fibre
lignin having an aliphatic hydroxyl content of about 3.75 mmol/g or less; 3.5
mmol/g or less;
3.25 mmol/g or less; 3 mmol/g or less; 2.75 mmol/g or less; 2.5 mmol/g or
less; 2.35 mmol/g
or less; 2.25 mmol/g or less.
The present disclosure provides methods of producing derivatives of native
lignin having
a normalized RSI of 15 or greater, 20 or greater, 25 or greater, 30 or
greater, 35 or greater, 40 or
greater, 50 or greater, 60 or greater, 70 or greater.
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
The present disclosure provides methods of producing derivatives of native
hardwood
lignin having a normalized RSI of 15 or greater, 20 or greater, 25 or greater,
30 or greater, 35 or
greater, 40 or greater, 50 or greater, 60 or greater, 70 or greater.
The present disclosure provides methods of producing derivatives of native
softwood
lignin having a normalized RSI of 15 or greater, 20 or greater, 25 or greater,
30 or greater, 35 or
greater, 40 or greater.
The present disclosure provides methods of producing derivatives of native
annual fibre
lignin having a normalized RSI of 15 or greater, 20 or greater, 25 or greater,
30 or greater, 35 or
greater.
All citations are herein incorporated by reference, as if each individual
publication was
specifically and individually indicated to be incorporated by reference herein
and as though it
were fully set forth herein. Citation of references herein is not to be
construed nor considered as
an admission that such references are prior art to the present invention.
One or more currently preferred embodiments of the invention have been
described by
way of example. The invention includes all embodiments, modifications and
variations
substantially as hereinbefore described and with reference to the examples and
figures. It will be
apparent to persons skilled in the art that a number of variations and
modifications can be made
without departing from the scope of the invention as defined in the claims.
Examples of such
modifications include the substitution of known equivalents for any aspect of
the invention in
order to achieve the same result in substantially the same way.
The following examples are intended to be exemplary of the invention and are
not
intended to be limiting.
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
21
EXAMPLES
EXAMPLE 1: Recovery of derivatives of native lignin from hardwood feedstocks,
softwood feedstocks, and annual fibre feedstocks.
The three hardwood feedstocks chips were prepared from: (1) aspen trees grown
in
British Columbia, Canada, (2) acacia grown in Chile, and (3) eucalyptus grown
in Chile.
Subsamples of the three hardwood plant species were individually pulped using
an autocatalysed
ethanol pulping process organosolv process wherein a different set of pulping
conditions was
used for each subsample. The individual sets of pulping conditions applied to
hardwood species
are listed in Tables 1(a)-1(c). Twenty seven different combinations of pulping
conditions were
tested with each of BC aspen (Table 1(a)), Chilean Acacia dealbata (Table
1(b)), and Chilean
Eucalyptus nitens (Table 1(c)).
For each subsample, the ethanol pulping solvent was prepared as listed in its
respective
table. First, the ethanol was partially diluted with water after which, a
suitable amount of
sulphuric acid was added to achieve the target final acidity after which, the
ethanol solution was
further diluted with water to achieve the target ethanol concentration.
The raw lignin content of each fibrous biomass subsample was determined using
the
Mason lignin determination method. Then, after adding the fibrous biomass
subsample to a
pressure vessel (100-700g odw chips), the tailored ethanol-based pulping
solvent was added to
the vessel (6:1 liquor:wood ratio), after which it was pressurized and brought
up to the target
temperature listed in the table. The biomass subsample was then "cooked" for
the specified
period of time after which, the pulping process was stopped. After pulping,
the derivatives of
native lignin were recovered by transferring the contents of the pressure
vessel to a press. The
solids were then squeezed in a press and filtered through a coarse silk screen
which separated the
chip residues from the fine particles and the liquids. Next, the fine
particles were separated from
the liquids by filtering the suspension separated from the chip residues,
through fine filter paper.
The fine particles represent derivatives of native lignin that were extracted
and which
precipitated from solution after cooling and is herein referred to as self-
precipitated derivatives
of native lignin designated in the tables as "SPL". Finally, the derivatives
of native lignin still
remaining in the filtered liquid were precipitated from solution by dilution
with cold water. The
derivatives of native lignin precipitated by cold-water dilution are referred
to herein as
precipitated lignin or "PL". After determination of the dry weights of SPL and
PL derivatives of
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
22
native lignin, the relative yield of each lignin derivative was determined in
reference to the total
lignin value determined for the biomass sample before pulping. The original
lignin and
carbohydrates content of each fibrous biomass subsample was determined using
the methods
described in National Renewable Energy Laboratory (NREL) Technical Report
entitled
"Determination of Structural Carbohydrates and Lignin in Biomass" - Laboratory
Analytical
Procedure (1P-510-42618 (25 April 2008)). Ash and extractives content were
evaluated
according to the standard TAPPI procedures.The yields of SPL and PL
derivatives of native
lignin for each subsample are expressed on a weight A basis relative to the
total lignin value in
raw biomass, and listed in Tables 1(a)-1(c) for the hardwood feedstocks,
Tables 3(a)-3(c) for the
softwood feedstocks, and Tables 4(a)-4(c) for the annual fibre feedstocks in
columns next to the
processing conditions used for each subsamples. Table 2 shows the chemical
composition of the
raw lignocellulosic biomass samples used in this disclosure. The chip residues
remaining after the
first filleting step were pressed, dried and weighed. The yield of de-
lignified residues, "pulp",
(referred to in Tables 1, 3, 4 as "PBY") is expressed on a % basis relative to
the dry weight of the
pre-pulping biomass subsample.
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
23
Table 1 (a): Organosolv processing conditions for hardwood feedstocks.
BC aspen
Time Temp. Ethanol PL OH-pr OH-sec OH-al
Run # pHNRSI
in
m C % % mmol/g mmol/g mmol/g
1 2.09 65 196 60 82.7 0.48 0.05 0.53
102.63
2 2.03 104 197 68 61.4 0.58 0.00 0.58
94.12
3 2.02 114 195 43 43.6 0.66 0.10 0.76
90.90
4 1.97 89 172 79 62.2 0.78 0.05 0.83
79.83
2.17 83 189 64 71.6 0.78 0.11 0.89 81.02
6 1.78 30 170 59 65.8 0.84 0.11 0.95
62.80
7 1.96 42 176 51 68.7 0.87 0.11 0.98
89.63
8 2.12 101 180 48 61.6 0.89 0.16 1.05
71.83
9 2.06 27 193 51 65.7 1.03 0.06 1.09
83.85
2.52 90 171 77 53.8 1.15 0.00 1.15 67.17
11 2.18 50 205 45 58.2 0.96 0.27 1.22
74.68
12 1.64 60 167 43 49.7 1.08 0.18 1.27
87.80
13 2.29 115 201 73 60.1 1.00 0.38 1.38
67.68
14 2.00 33 181 44 55.9 1.13 0.30 1.43
85.54
2.22 94 177 47 58.3 1.41 0.35 1.76 57.41
16 2.34 44 174 68 51.3 1.24 0.54 1.78
56.63
17 2.30 87 183 54 63.8 1.32 0.52 1.84
64.00
18 2.29 46 169 73 52.4 1.54 0.43 1.97
60.54
19 2.26 63 190 47 46.8 1.39 0.75 2.13
60.51
2.10 21 166 46 38.5 1.44 0.78 2.21 61.98
21 2.70 82 191 41 49.5 1.41 0.97 2.38
62.85
22 2.81 113 180 67 48.2 1.94 1.34 3.28
44.02
23 3.30 107 170 61 35.5 2.00 2.00 3.99
27.27
24 3.27 100 166 65 27.6 2.16 1.95 4.11
34.71
2.94 56 176 60 42.0 2.21 2.04 4.25 36.85
26 1.87 67 194 58 106.0 0.60 0.05 4.37
97.39
27 1.68 79 173 49 62.1 0.79 0.11 4.46
84.41
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
24
Table 1 (b): Chilean Acacia dealbata
Acid Time Temp. Ethanol PL OH-pr OH-sec OH-al
Run # pH.NRSI
n
% m C % % mmol/g mmol/g mmol/g
1 2.01 1.61 104 197 68 67.88 0.76 0.00 0.76 121.76
2 2.11 1.00 114 195 43 46.56 1.24 0.00 1.24 130.15
3 2.20 1.28 65 196 60 65.67 0.69 0.75 1.44 93.15
4 2.00 1.51 67 194 58 66.21 1.08 0.38 1.46 94.90
1.90 2.47 42 176 51 63.39 1.25 0.30 1.55
86.39
6 2.03 1.31 89 172 79 47.77 0.91 0.73 1.64 90.37
7 1.96 1.40 33 181 44 51.05 1.32 0.75 2.07 75.59
8 2.35 0.60 50 205 45 51.25 1.48 0.83 2.31
80.44
9 2.22 0.90 83 189 64 54.92 1.20 1.33 2.53
85.22
2.16 1.01 27 193 51 54.00 1.44 1.11 2.54 72.39
11 2.40 0.81 90 171 77 36.50 1.82 0.79 2.60
71.61
12 2.04 1.60 79 173 49 61.53 1.20
1.58 2.78 63.46
13 1.79 2.41 30 170 59 59.10 1.29 1.53 2.82
74.95
14 1.82 2.20 60 167 43 53.70 1.45 1.57 3.02
68.76
2.58 0.51 115 201 73 49.61 2.03 1.33 3.36
92.49
16 2.27 0.81 101 180 48 56.26 1.77 2.01 3.78 57.24
17 2.44 0.61 94 177 47 50.46 2.24 1.64 3.88
82.07
18 2.34 0.90 46 169 73 38.40 1.85 2.08 3.93
54.83
19 2.42 0.61 87 183 54 54.44 2.34 1.65 3.99 73.34
2.38 0.70 44 174 68 35.32 2.59 1.45 4.04
69.02
21 2.75 0.20 82 191 41 38.49 2.42 1.76 4.18
59.07
22 2.18 1.09 21 166 46 42.54 2.43 1.78 4.21 61.29
23 2.40 0.51 63 190 47 50.98 2.38 1.88 4.26
61.15
24 3.19 0.11 100 166 65 14.07 3.21 2.45 5.66
54.54
2.80 0.30 113 180 67 36.52 3.13 2.55 5.68
49.80
26 2.93 0.20 56 176 60 27.88 2.98 2.98 5.95 47.02
27 3.20 0.10 107 170 61 25.97 3.43 3.19 6.62
57.85
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
Table 1 (c): Chilean Eucalyptus nitens
Time Temp. Ethanol PL OH-pr OH-sec OH-al
Run # pH.NRSI
mn C % % mmol/g mmol/g mmol/g
1 1.88 104 197 68 81.7 0.57 0.13 0.70
109.66
2 1.96 67 194 58 77.4 0.58 0.19 0.78
108.27
3 2.01 65 196 60 81.8 0.62 0.25 0.86
119.66
4 1.93 114 195 43 43.9 0.88 0.20 1.08
112.88
5 2.53 115 201 73 61.8 0.72 0.50 1.22
101.95
6 2.10 83 189 64 69.4 0.76 0.69 1.46
91.17
7 2.05 89 172 79 53.0 0.91 0.56 1.47
92.24
8 1.87 79 173 49 63.6 0.88 0.61 1.49
67.29
9 2.17 27 193 51 66.8 0.86 0.73 1.60
87.59
10 2.17 101 180 48 63.4 0.91 0.70 1.61
85.93
11 2.25 90 171 77 48.9 1.01 0.68 1.69
94.66
12 1.90 33 181 44 59.5 1.09 0.61 1.70
87.78
13 1.74 42 176 51 66.9 1.17 0.55 1.73
81.93
14 1.77 30 170 59 61.6 1.11 0.72 1.83
77.44
15 1.65 60 167 43 57.3 1.13 0.71 1.84
83.29
16 2.26 46 169 73 48.5 1.07 1.19 2.27
75.61
17 2.30 87 183 54 65.6 1.20 1.07 2.27
75.59
18 2.30 44 174 68 49.0 1.37 1.18 2.55
69.83
19 2.30 50 205 45 66.7 1.87 0.73 2.60
83.34
20 2.34 63 190 47 61.9 2.07 0.55 2.63
78.71
21 2.66 82 191 41 43.4 1.58 1.23 2.81
68.29
22 2.49 113 180 67 55.1 2.04 1.22 3.26
60.61
23 2.00 21 166 46 50.5 1.47 1.96 3.43
69.66
24 2.33 94 177 47 63.3 1.20 2.41 3.61
75.05
25 2.82 56 176 60 42.7 2.74 2.02 4.76
45.98
26 3.22 107 170 61 43.6 2.70 2.70 5.40
55.27
27 3.13 100 166 65 25.6 3.07 2.45 5.52
36.93
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
26
Table 2: Chemical composition of lignocellulosic biomass samples (`)/0 dry
weight)
Biomass Arabinan Galactan Glucan
Xylan Mannan Lignin Ash Extractives
Sample
Alberta 2.39 0.67 41.10 22.41 0 20.94 4.96
1.40
Wheat Straw
Chilean 0.55 0.64 44.65 19.78 1.35 22.85 0.54
1.63
Acacia dealbata
Brazilian 1.87 0.51 38.15 21.79 0.21 24.10 5.39
1.36
Sugarcane
Bagasse
British 1.00 2.00 43.75 4.97 11.70 22.85 1.00
0.86
Columbia
Hybrid
Spruce (Picea
engelmannii x
Picea glauca)
Chilean Pinus 1.52 2.73 46.04 6.12 11.36 24.15 0.67
0.37
radiata
European 3.07 1.02 35.79 30.85 0 18.41 1.43
1.63
Corn Cobs
Southeastern 1.40 2.61 41.64 6.87 10.10 30.84 0.54
2.06
US Pinus taeda
British 0.44 0.43 48.76 16.44 1.48 22.84 0.25
2.63
Columbia
Aspen
(Populus
tremuloides)
Chilean 0.96 0.61 47.70 17.52 1.12 27.57 0.01
0.99
Euca(yptus
nitens
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
27
Characterization of the aliphatic hydroxyl content of derivatives of native
lignin
recovered from three species of hardwoods.
Derivatives of native lignin recovered from hardwood feedstocks were analyzed
to
determine mmol of primary hydroxyl groups/g sample (OH-pr mmol/g) and mmol of
secondary
hydroxyl groups/g sample (OH-sec mmol/g). These data were then used to
calculate mmol
aliphatic hydroxyl groups/g sample (OH-al mmol/g).
The hydroxyl contents were determined by analyses of NMR spectra recorded on a
Bruker 700 MHz spectrometer equipped with Cryoprobe at 300 K using ca 30%
solutions of
sample in DMSO-d6. Chemical shifts were referenced to TMS (0.0 ppm). To ensure
more
accurate baseline, especially in the carbonyl region (215-185 ppm), the
spectra were recorded
over the interval 2404-40) ppm. The following conditions were provided for the
quantitative
13C-NMR:
1. Inverse gate detection;
2. a 90 pulse;
3. Complete relaxation of all nuclei was achieved by addition of chromium
(III)
acetylacetonate (0.01 M) with a 1.2 s acquisition time and 1.7 s relaxation
delay.
The NMR spectra were Fourier-transformed, phased, calibrated using TMS signals
as a
reference (0 ppm), and the baseline was corrected by using a polynomial
function. The
correction of baseline was done using the following interval references for
adjustment to zero:
(220-215ppm)-(185-182ppm)-(97-92ppm)-(5-(-20)ppm). No other regions were
forced to 0. The
signals in the quantitative 13c NMR spectra were assigned on the base of 2D
HSQC NMR and a
known database. After the baseline correction the spectra were integrated
using the area of
internal standard (IS), trioxane, as the reference. Each spectrum was
processed (as described) at
least twice to ensure good reproducibility of the quantification. The
calculation of the quantity of
specific moieties was done as follows:
For non-acetylated lignins: X (mmol/g lignin)= (30mug*I,$)*1000
For acetylated lignins: X (mmol/g lignin) = Ix*rnis/(30mug*Its ¨
42*I0Htotal * m,$)*1000
Where X was the amount of the specific moiety; Ix, 'is and I0,_õota, were the
resonance values of
the specific moiety (Table 3), the internal standard and total OH groups,
correspondingly; mt,g
and mls are the masses of the lignin and internal standard.
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
28
Table 3:
Symbol Ix in Calculation Equation Analytical Method
Resonance at 171.5-169.7 ppm in the
OH-pr
quantitative 13C NMR spectra of acetylated Quantitative 13C High Resolution
NMR of mmol/g lignins minus resonance at 171.5-169.7 acetylated lignin
using 1,3,5-trioxane as internal
i
ppm n the quantitative 13C NMR spectra reference
of non-acetylated lignins
Resonance at 169.7-169.2 ppm in the
OH secquantitative 13C NMR spectra of acetylated Quantitative 13C High
Resolution NMR of
mmol/ lignins minus resonance at 169.7-169.2 acetylated lignin using
1,3,5-trioxane as internal
g
ppm in the quantitative 13C NMR spectra reference
of non-acetylated lignins
OH-al
OH-al = OH-pr + OH-sec
mmol/g
The aliphatic hydroxyl content of the PL lignin derivatives from each of the
twenty seven
samples of aspen chips are shown in Table 1(a). The contents ranged from 0.70
mmol/g in run 1
to 5.52 mmol/g in run 27.
The aliphatic hydroxyl contents of the PL lignin derivatives from each of the
twenty
seven samples of acacia chips are shown in Table 1(b). The contents ranged
from 0.76 mmol/g
in run 1 to 6.62 mmol/g in run 27.
The aliphatic hydroxyl contents of the PL lignin derivatives from each of the
twenty
seven samples of eucalyptus chips are shown in Table 1(c). The contents ranged
from 0.70
mmol/g in run 1 to 5.52 mmol/g in run 27.
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
29
Characterization of the NRSI of derivatives of native lignin recovered from
three species
of hardwoods.
Each of the lignin derivative subsamples produced above was assessed for its
radical
scavenging index (RSI). The potential antioxidant activity of each PL lignin
derivative was
determined by measuring its radical savaging capacity. The assay used 2,2-
dipheny1-1-
picrylhydrazyl (DPPH), a stabile free radical which absorbs light strongly at
515 nm to measure a
compound's radical scavenging index (RSI). In its radical form, DPPI-1.
absorbs strongly at 515
nm and has a deep purple colour. As DPPH gives up its free electron to radical
scavengers, it
loses its purple colour and its absorbance shifts to 520 nm. The greater the
drop in DPPH
absorbance at 515 nm after a test compound has been added to the DPPH
solution, the higher
the compound's free RSI and also, its antioxidant activity. In the present
study, Vit. E and BHT
were used as positive controls. PL lignin derivative subsamples (1.0 ¨ 2.0
mg), Vit. E control
samples (1.0 ¨ 2.0 mg), and BHT control samples (6.0 ¨ 8.0 mg) were prepared
for testing by
being placed into epitubes after which, each was diluted with 1.0 mL of 90%
(v/v) aqueous
dioxane, vortexed, transferred to new epitubes and then further diluted 50/50
with 90% aqueous
dioxane to give stock concentrations of 0.5-1.0 mg/mL for samples and Vitamin
E and 3.0-4.0
mg/mL for BHT. An indicating (purple) DPPH stable free radical solution is
made by dissolving
3.78 mg DPPH in 100 mL 90% dioxane (95.9 1.1.A4). Samples and standards are
serial diluted to
fill columns of a quartz 96-well plate (8 dilutions). The assays were
performed by placing aliquots
of the sample stock solutions into two rows of wells in a 96-well plate. The
first row served as
the reference row while the second row received DPPH aliquots. 165 [stI, of
90% dioxane was
added to each well and mixed. Aliquots of the mixed samples in each row are
transferred to the
adjacent row with is further diluted with 165 pL of 90% dioxane in each well.
The mixing,
transferring and dilution are repeated until the last row of wells was
prepared. The same volume
of aliquots was removed from the last row. The 96-well plate also contains a
row of wells that
received only the 90% dioxane. In the final step of the preparation procedure,
165 pL of the
DPPH solution is added to all the control and analytical columns by using an 8-
channel auto-
pipette and an Eppendore reagent reservoir as quickly as possible. As soon as
all reagents were
added, the plate was placed into a plate-reading spectrophotometer, and
absorbance
measurements were performed. The program for the spectrophotometer (SOFTmax
software)
consists of a timing sequence of 16 min and a reading of the entire plate at
515 nm. RSI (radical
scavenging index) is defined as the inverse of the concentration which
produces 50% inhibition
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
in DPPH absorbance at 515 nm. The results are then 'normalized' by dividing
sample RSI by the
RSI value for the BHT control.
The NRSI values for lignin derivatives recovered from BC aspen are shown in
Table 1(a).
The NRSI values for lignin derivatives recovered from Chilean acacia biomass
are shown in
Table 1(b). The NRSI values for lignin derivatives recovered from Chilean
eucalyptus biomass
are shown in Table 1(c).
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
31
EXAMPLE 2: Recovery of derivatives of native lignin from softwood feedstocks.
Three softwood feedstocks chips were prepared from: (1) hybrid spruce (Picea
engelmannii
x Picea glauca) trees grown in British Columbia, (1) radiata pine grown in
Chile, and (2) loblolly
pine (Pinus taeda) grown in the southeast USA. Subsamples of the three plant
species were
individually pulped using an autocatalysed ethanol pulping process wherein a
different set of
pulping conditions was used for each subsample.
The individual sets of pulping conditions applied to softwood species are
listed in Tables
3(a)-3(c). Twenty nine different combinations of pulping conditions were
tested with each of BC
hybrid spruce (Table 3(a)) and Chilean radiata pine (Table 3(b)), while 30
combinations of
pulping combinations were tested with southeastern US loblolly pine (Table
3(c)).
For each subsample, the ethanol pulping solvent was prepared as listed in its
respective
table. First, the ethanol was partially diluted with water after which, a
suitable amount of
sulphuric acid was added to achieve the target final acidity after which, the
ethanol solution was
further diluted with water to achieve the target ethanol concentration.
The raw lignin content of each fibrous biomass subsample was determined using
the
methods described in National Renewable Energy Laboratory (NREL) Technical
Report entitled
"Determination of Structural Carbohydrates and Lignin in Biomass" - Laboratory
Analytical
Procedure (1P-510-42618 (25 April 2008)). Then, after adding the fibrous
biomass subsample to
a pressure vessel (100-700 g odw chips), the tailored ethanol-based pulping
solvent was added to
the vessel (6:1 liquor:wood ratio) after which it was brought up to the target
temperature and
pressure listed in the table. The biomass subsample was then "cooked" for the
specified period
of time, after which, the pulping process was stopped. After pulping, the
derivatives of native
lignin were recovered by transferring the contents of pressure vessel to a
press. The solids were
then squeezed in a press and filtered through a coarse silk screen which
separated the chip
residues from the fine particles and the liquids. Next, the fine particles
were separated from the
liquids by filtering the suspension separated from the chip residues, through
fine filter paper. The
fine particles represent derivatives of native lignin that were extracted and
which precipitated
from solution after cooling and is herein referred to as self-precipitated
derivatives of native
lignin designated in the tables as "SPL". Finally, the derivatives of native
lignin still remaining in
the filtered liquid were precipitated from solution by dilution with cold
water. The derivatives of
native lignin precipitated by cold-water dilution are referred to herein as
precipitated lignin or
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
32
"PL". After determination of the dry weights of SPL and PL derivatives of
native lignin, the
relative yield of each lignin derivative was determined in reference to the
total lignin value
determined for the biomass sample before pulping. The yields of SPL and PL
derivatives of
native lignin for each subsample are expressed on a weight A basis relative
to its total lignin
value, and listed in Tables 4(a)-4(c) for the softwood feedstocks in columns
next to the
processing conditions used for each subsamples. The chip residues remaining
after the first
filtering step were pressed, dried and weighed. The yield of de-lignified
residues, "pulp" referred
to in Tables 4(a)-4(c) as "PBY", are expressed on a % yield basis relative to
the dry weight of the
pre-pulping biomass subsample.
Characterization of the aliphatic hydroxyl content of derivatives of native
lignin
recovered from three species of softwoods.
Derivatives of native lignin recovered from softwood feedstocks were analyzed
as
discussed above to determine rnmol primary hydroxyl groups/g sample (OH-pr
mmol/g) and
mmol secondary hydroxyl groups/g sample (OH-sec mmol/g). These data were then
used to
calculate mmol aliphatic hydroxyl groups/g sample (OH-al mmol/g).
The aliphatic hydroxyl contents of the PL lignin derivatives from each of the
twenty nine
samples of spruce woodchips are shown in Table 4(a). The contents ranged from
1.72 mmol/g
in run 1 to 4.75 mmol/g in run 29.
The aliphatic hydroxyl contents of the PL lignin derivatives from each of the
twenty nine
samples of radiata pine woodchips are shown in Table 4(b). The contents ranged
from 2.18
mmol/g in run 1 to 5.09 mmol/g in run 29.
The aliphatic hydroxyl contents of the PL lignin derivatives from each of the
thirty
samples of loblolly pine chips are shown in Table 4(c). The contents ranged
from 1.35 mmol/g
in run 1 to 4.39 mmol/g in run 30.
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
33
Table 4(a): Organosolv processing conditions for softwood feedstocks.
BC hybrid spruce
Acid Time Temp. Ethanol PL OH-pr OH-sec OH-al
Run # pH
NRSI
% min C % % mmol/g mmol/g mmol/g
1 2.02 1.20 58 191 46 44.84 1.57 0.14 1.72
61.61
2 1.96 1.80 46 187 49 43.57 1.47 0.32 1.79
58.80
3 2.08 1.40 43 189 61 67.77 1.67 0.29 1.96
46.83
4 2.09 1.60 50 183 77 72.10 1.74 0.28 2.02
40.66
1.80 2.60 32 182 50 44.49 1.82 0.42 2.24 35.37
6 2.26 1.10 54 185 76 75.89 1.94 0.55 2.49
31.90
7 1.95 1.90 33 179 57 63.89 2.05 0.55 2.60
27.44
8 2.18 0.90 55 184 47 40.42 2.19 0.48 2.66
28.15
9 1.81 2.50 36 175 78 71.84 2.18 0.56 2.75
26.22
2.49 0.35 79 198 42 26.78 2.23 0.58 2.81 22.29
11 1.72 2.90 34 168 43 40.04 2.44 0.49 2.93
23.98
12 2.16 0.98 38 178 44 26.24 2.51 0.43 2.94
22.22
13 2.12 1.20 41 181 68 70.59 2.35 0.69 3.04
30.63
14 2.14 1.30 46 175 68 66.06 2.44 0.61 3.05
23.39
2.11 1.30 34 172 79 56.41 1.94 1.20 3.14 15.76
16 1.82 2.10 39 170 46 33.37 2.61 0.58 3.19
17.63
17 2.69 0.23 110 191 44 30.37 2.39 0.91 3.30
24.17
18 2.52 0.47 57 194 61 59.02 2.88 0.60 3.48
21.88
19 2.08 1.00 59 171 42 24.55 2.94 0.59 3.53
18.93
2.65 0.38 73 189 54 52.60 2.89 0.67 3.55 18.78
21 2.60 0.31 84 184 76 36.43 2.95 0.69 3.64
16.71
22 1.89 2.10 31 167 52 44.49 2.18 1.58 3.76
15.88
23 2.37 0.52 77 178 46 35.16 2.24 1.56 3.79
14.90
24 2.43 0.43 49 179 45 28.34 3.07 0.84 3.91
13.98
2.66 0.36 61 188 67 54.42 3.23 0.70 3.93 18.54
26 2.42 0.64 51 176 65 64.03 2.38 1.66 4.04
15.15
27 3.15 0.13 53 199 73 24.55 2.85 1.19 4.04
16.90
28 3.02 0.12 86 186 47 30.28 3.14 1.39 4.53
22.32
29 2.88 0.17 60 182 62 29.21 3.34 1.40 4.75
13.27
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
34
Table 3 (b): Chilean radiata pine
Acid Time Temp. Ethanol PL OH-pr OH-sec OH-al
Run # pH
NRSI
% min C % % mmol/g mmol/g mmol/g
1 2.04 1.20 58 191 46 34.21 1.74 0.44 2.18 64.24
2 2.06 1.60 50 183 77 65.00 1.77 0.42 2.19 36.10
3 1.72 2.60 32 182 50 46.46 1.79 0.41 2.20 42.49
4 2.12 1.40 43 189 61 65.40 1.79 0.43 2.22 35.27
1.86 2.50 36 175 78 59.66 1.87 0.36 2.23 33.71
6 1.92 1.80 46 187 49 42.72 1.90 0.35 2.26 37.44
7 1.92 1.90 33 179 57 48.76 2.31 0.49 2.80 36.98
8 2.28 1.10 54 185 76 79.49 2.17 0.72 2.90 49.41
9 2.50 0.35 79 198 42 31.87 2.36 0.71 3.07 35.93
1.73 2.90 34 168 43 29.72 2.45 0.65 3.10 22.44
11 2.08 0.98 38 178 44 28.17 2.65 0.63 3.27 20.83
12 2.23 0.90 55 184 47 38.00 2.62 0.69 3.31 28.27
13 2.12 1.30 46 175 68 82.49 2.87 0.72 3.59 23.29
14 2.15 L20 41 181 68 60.93 2.75 0.85 3.60 30.02
2.50 0.47 57 194 61 48.55 2.94 0.72 3.66 27.20
16 1.80 2.10 39 170 46 31.81 3.01 0.70 3.71 18.71
17 2.06 1.00 59 171 42 22.85 3.00 0.71 3.72 20.93
18 2.70 0.31 84 184 76 33.09 3.02 0.72 3.73 20.78
19 2.38 0.52 77 178 46 29.70 3.05 0.71 3.77 18.00
2.63 0.23 110 191 44 27.01 2.72 1.06 3.78 29.76
21 2.52 0.38 73 189 54 31.35 3.18 0.72 3.90 16.80
22 1.79 2.10 31 167 52 37.58 3.25 0.68 3.92 16.15
23 2.73 0.36 61 188 67 46.40 3.23 0.72 3.95 13.90
24 2.04 1.30 34 172 79 50.87 3.32 0.70 4.02 17.61
2.30 0.64 51 176 65 57.35 3.62 0.72 4.35 14.63
26 3.08 0.13 53 199 73 22.04 3.12 1.35 4.47 18.15
27 2.50 0.43 49 179 45 25.33 3.09 1.54 4.63 14.24
28 2.95 0.12 86 186 47 28.54 3.23 1.54 4.77 19.76
29 3.01 0.17 60 182 62 22.12 3.55 1.53 5.09 13.68
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
Table 3 (c): Southeastern US loblolly pine
Acid Time Temp. Ethanol PL OH-pr OH-sec OH-al
Run # pH
NRSI
% min C % % mmol/g mmol/g mmol/g
1 2.05 1.20 33 192 82 65.1 1.16 0.19 1.35 48.73
2 2.12 1.20 58 191 46 36.9 1.42 0.03 1.42 55.39
3 2.00 1.60 50 183 77 69.1 1.42 0.03 1.43 44.54
4 2.01 1.40 43 189 61 63.3 1.55 0.03 1.58 46.95
5 1.65 2.60 32 182 50 41.6 1.74 0.00 1.74 47.49
6 2.13 1.10 54 185 76 69.9 1.29 0.58 1.87 31.66
7 1.80 1.80 46 187 49 42.3 1.74 0.13 1.87 53.44
8 2.02 1.20 41 181 68 58.0 1.68 0.26 1.94 32.17
9 1.90 2.50 36 175 78 66.8 1.68 0.26 1.94 32.73
10 2.33 0.90 55 184 47 36.0 1.87 0.26 2.13 34.41
11 2.07 1.70 43 176 81 62.1 1.94 0.39 2.32 31.29
12 1.90 1.90 33 179 57 53.1 2.06 0.45 2.52 24.37
13 1.83 2.10 39 170 46 29.8 2.19 0.45 2.65 27.78
14 2.10 1.30 34 172 79 50.2 2.32 0.45 2.77 20.05
15 1.80 2.90 34 168 43 25.3 2.26 0.52 2.77 33.44
16 2.17 1.30 46 175 68 58.9 2.26 0.58 2.84 27.85
17 2.52 0.35 79 198 42 16.4 2.19 0.71 2.90 27.95
18 2.58 0.64 51 176 65 48.7 2.13 0.77 2.90 13.78
19 2.15 1.00 59 171 42 20.5 2.32 0.58 2.90 23.61
20 2.25 0.98 38 178 44 38.5 2.45 0.52 2.97 23.34
21 1.87 2.10 31 167 52 39.0 2.58 0.52 3.10 19.27
22 2.65 0.31 84 184 76 34.1 2.52 0.65 3.16 19.24
23 2.47 0.47 57 194 61 46.9 2.39 0.77 3.16 26.27
24 2.92 0.17 60 182 62 38.5 2.65 0.65 3.29 15.93
25 2.50 0.38 73 189 54 39.3 2.58 0.84 3.42 27.78
26 2.39 0.43 49 179 45 30.7 2.65 0.84 3.48 18.02
27 2.77 0.23 110 191 44 12.5 2.52 1.03 3.55 22.80
28 3.20 0.13 53 199 73 23.3 2.65 1.16 3.81 24.56
29 2.80 0.36 61 188 67 24.8 2.97 1.29 4.26 17.00
30 2.99 0.12 86 186 47 27.9 2.97 1.42 4.39 18.12
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
36
Characterization of the NRSI of derivatives of native lignin recovered from
three species
of softwoods.
Each of the lignin derivative subsamples produced above were assessed for its
radical
scavenging index (RSI). The potential antioxidant activity of each PL lignin
derivative was
determined as described above. The NRSI values for lignin derivatives
recovered from hybrid
spruce biomass are shown in Table 4(a). The NRSI values for lignin derivatives
recovered from
radiate pine biomass are shown in Table 4(b). The NRSI values for lignin
derivatives recovered
from loblolly pine biomass are shown in Table 4(c).
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
37
EXAMPLE 3: Recovery of derivatives of native lignin from three annual fibre
feedstocks.
Three annual fibre feedstocks were prepared from : (1) wheat straw from
Alberta
Canada, (2) sugarcane bagasse from Brazil, and (3) corn cobs from crops
produced in Europe.
Subsamples of the three plant species were individually pulped using an
autocatalysed ethanol
pulping process based on the Alcell organosolv process wherein a different
set of pulping
conditions was used for each subsample.
The individual sets of pulping conditions applied to annual fiber feedstocks
are listed in
Tables 5(a)-5(c). Twenty seven different combinations of pulping conditions
were tested with
each of Alberta wheat straw (Table 5(a)) and European corn cobs (Table 5(c)),
and twenty six
combinations were tested with Brazilian sugarcane bagasse (Table 5(b)).
For each subsample, the ethanol pulping solvent was prepared as listed in its
respective
table. First, the ethanol was partially diluted with water after which, a
suitable amount of
sulphuric acid was added to achieve the target final acidity after which, the
ethanol solution was
further diluted with water to achieve the target ethanol concentration.
The raw lignin content of each fibrous biomass subsample was determined using
the
Klason lignin determination method. Then, after adding the fibrous biomass
subsample to a
pressure vessel (100-700 g odw chips), the tailored ethanol-based pulping
solvent was added to
the vessel (6:1 liquor:wood ratio) after which it was brought up to the target
temperature and
pressure listed in the table. The biomass subsample was then "cooked" for the
specified period
of time after which, the pulping process was stopped. After pulping, the
derivatives of native
lignin were recovered by transferring the contents of pressure vessel to a
press. The solids were
then squeezed in a press and filtered through a coarse silk screen which
separated the chip
residues from the fine particles and the liquids. Next, the fine particles
were separated from the
liquids by filtering the suspension separated from the chip residues, through
fine filter paper. The
fine particles represent derivatives of native lignin that were extracted and
which precipitated
from solution after cooling and is herein referred to as self-precipitated
derivatives of native
lignin designated in the tables as "SPL". Finally, the derivatives of native
lignin still remaining in
the filtered liquid were precipitated from solution by dilution with cold
water. The derivatives of
native lignin precipitated by cold-water dilution are referred to herein as
precipitated lignin or
"PL". After determination of the dry weights of SPL and PL derivatives of
native lignin, the
relative yield of each lignin derivative was determined in reference to the
Mason lignin value
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
38
determined for the biomass sample before pulping. The yields of SPL and PL
derivatives of
native lignin for each subsample are expressed on a weight % basis relative to
its total lignin
value, and listed in Tables 5(a)-5(c) for the annual fibre feedstocks in
columns next to the
processing conditions used for each subsamples. The chip residues remaining
after the first
filtering step were pressed, dried and weighed. The yield of de-lignified
residues, referred to in
Tables 5(a)-5(c) as "PBY", are expressed on a % yield basis relative to the
dry weight of the pre-
pulping biomass subsample.
Characterization of the aliphatic hydroxyl content of derivatives of native
lignin
recovered from three species of annual fibre feedstocks.
Derivatives of native lignin recovered from annual fiber feedstocks were
analyzed as
described above to determine mmol primary hydroxyl groups/g sample (OH-pr
mmol/g) and
mmol secondary hydroxyl groups/g sample (OH-sec mmol/g). These data were then
used to
calculate mmol aliphatic hydroxyl groups/g sample (OH-al mmol/g)
The aliphatic hydroxyl contents of the PL lignin derivatives from each of the
twenty
seven samples of wheat straw biomass are shown in Table 5(a). The contents
ranged from 2.03
mmol/g in 2.03 run 1 to 3.59 mmol/g in run 27.
The aliphatic hydroxyl contents of the PL lignin derivatives from each of the
twenty six
samples of sugarcane bagasse biomass are shown in Table 5(b). The contents
ranged from 1.72
mmol/g in run 1 to 3.70 mmol/g in run 26.
The aliphatic hydroxyl contents of the PL lignin derivatives from each of the
twenty
seven samples of corn cob biomass are shown in Table 5(c). The contents ranged
from 1.58
mmol/g in run 1 to 4.59 mmol/g in run 27.
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
39
Table 5(a): Organosolv processing conditions for annual fibre feedstocks.
Alberta wheat straw
Time Temp. Ethanol PL OH-pr OH-sec OH-al
Run # pH NRSI
min C % mmol/g mmol/g mmol/g
1 2.86 90 195 41 38.17 1.20 0.82 2.03 54.02
2 2.15 39 189 50 47.53 1.31 1.15 2.46 44.71
3 2.26 49 192 37 37.01 1.36 1.10 2.47 55.32
4 2.23 100 190 67 56.88 1.53 0.96 2.49 55.76
1.80 42 179 51 52.09 1.66 0.89 2.55 34.17
6 2.09 32 187 69 49.66 1.59 0.97 2.56 37.02
7 2.07 67 189 51 54.08 1.52 1.05 2.58 52.10
8 1.85 70 185 47 47.92 1.59 0.99 2.58 43.22
9 1.96 56 175 68 53.59 1.74 0.87 2.60 35.85
2.21 87 181 66 46.06 1.62 1.19 2.81 36.46
11 2.24 48 184 65 43.48 1.67 1.15 2.82 36.63
12 1.76 37 180 36 24.73 1.75 1.10 2.84 41.10
13 2.03 66 166 71 46.36 1.77 1.08 2.85 27.90
14 2.10 106 176 38 35.07 1.61 1.25 2.86 45.20
2.34 99 183 54 53.25 1.71 1.19 2.90 40.66
16 2.49 53 185 72 30.91 1.69 1.34 3.03 35.02
17 2.59 27 163 63 19.82 1.29 1.75 3.05 25.41
18 2.40 94 178 61 36.39 1.64 1.42 3.06 39.29
19 2.03 77 176 42 40.81 1.66 1.42 3.08 42.17
2.20 64 165 65 23.88 1.69 1.47 3.16 30.85
21 1.97 93 165 40 31.81 1.69 1.47 3.16 40.17
22 2.65 59 182 45 25.31 1.67 1.52 3.19 29.68
23 2.61 72 162 70 19.08 1.55 1.64 3.19 24.37
24 2.67 74 175 53 9.24 1.72 1.48 3.19 29.51
2.21 48 174 57 9.82 1.65 1.65 3.30 27.51
26 2.45 79 178 49 39.27 1.80 1.56 3.36 36.83
27 2.12 62 172 35 20.65 1.66 1.93 3.59 24.59
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
Table 5(b): Brazilian sugarcane bagasse
Time Temp. Ethanol PL OH-pr OH-sec OH-al
Run # pH NRSI
min C % % mmol/g mmol/g mmol/g
1 2.08 48 184 65 45.13 0.93 0.79 1.72 35.80
2 2.19 61 178 66 49.76 1.02 0.73 1.74 52.34
3 2.36 34 180 45 44.27 0.99 0.99 1.99 49.32
4 2.01 23 170 66 39.56 1.19 0.89 2.09 41.80
5 2.43 79 178 49 40.84 1.57 0.60 2.17 40.56
6 2.44 50 192 43 37.36 1.02 1.17 2.20 46.90
7 2.50 26 183 71 45.82 1.66 0.58 2.24 32.49
8 2.06 47 176 38 34.11 1.15 1.15 2.30 50.07
9 2.19 54 164 58 44.95 1.31 1.02 2.34 38.73
10 2.51 78 166 62 44.94 1.28 1.05 2.34 42.49
11 2.10 28 171 46 43.75 1.36 1.13 2.49 48.63
12 2.08 44 161 52 44.20 1.43 1.07 2.50 38.93
13 2.93 69 184 42 30.26 1.47 1.05 2.51 37.80
14 2.77 95 168 53 39.46 1.64 1.00 2.64 34.10
15 2.68 57 188 63 47.51 1.74 1.02 2.76 29.49
16 2.37 52 172 50 42.06 1.91 0.85 2.76 33.41
17 2.38 42 173 60 43.37 1.70 1.06 2.76 30.85
18 2.41 68 162 47 35.99 1.79 1.00 2.79 34.71
19 2.84 98 174 37 24.45 1.31 1.52 2.83 41.37
20 2.91 59 182 39 28.67 1.59 1.28 2.87 42.05
21 3.26 32 197 51 42.14 1.53 1.39 2.92 39.05
22 2.92 88 171 73 8.67 1.51 1.51 3.02 24.49
23 3.19 81 181 57 31.83 1.50 1.69 3.19 25.37
24 2.63 72 162 70 32.48 1.79 1.48 3.26 22.37
25 2.55 27 163 63 43.46 2.05 1.28 3.33 22.85
26 2.75 37 167 40 21.56 1.31 2.39 3.70 24.20
CA 02895215 2015-06-16
WO 2014/094104
PCT/CA2012/001172
41
Table 5(c): European corn cobs
Time Temp. Ethanol PL OH-pr OH-sec OH-al
Run # pHNRSI
min C % % mmol/g mmol/g mmol/g
1 2.18 100 190 67 56.58 0.95 0.63 1.58 45.15
2 1.76 37 180 36 33.32 0.66 0.98 1.64 45.32
3 1.85 42 179 51 52.34 0.71 1.03 1.73 52.54
4 1.93 70 185 47 49.02 0.65 1.16 1.81 43.90
2.10 67 189 51 52.01 0.64 1.22 1.86 45.98
6 1.89 56 175 68 49.73 0.56 1.31 1.87 39.29
7 2.21 48 184 65 49.62 0.50 1.62 2.12 40.34
8 2.33 49 192 37 29.35 1.49 0.65 2.14 53.29
9 1.98 66 166 71 47.93 0.68 1.48 2.15 39.88
2.04 32 187 69 45.27 0.74 1.42 2.16 38.37
11 2.14 87 181 66 52.93 0.69 1.50 2.19 43.54
12 1.93 93 165 40 35.49 1.58 0.76 2.34 32.56
13 2.17 99 183 54 50.71 1.24 1.11 2.35 49.00
14 2.11 106 176 38 32.28 0.82 1.58 2.39 42.20
2.18 53 185 72 42.72 0.60 1.81 2.41 31.73
16 2.00 77 176 42 38.75 0.81 1.61 2.42 40.61
17 2.54 72 162 70 20.05 0.75 1.74 2.49 26.80
18 2.81 61 178 66 31.52 0.89 1.66 2.55 36.15
19 2.53 27 163 63 18.86 0.83 1.77 2.61 24.98
2.09 48 174 57 47.17 0.61 2.01 2.63 35.41
21 2.36 79 178 49 45.43 1.29 1.41 2.69 44.17
22 2.28 64 165 65 41.20 0.69 2.01 2.69 29.68
23 2.31 94 178 61 47.72 0.80 1.97 2.77 38.29
24 2.42 79 169 59 27.61 1.21 1.59 2.80 26.24
2.19 39 189 50 47.01 1.85 0.99 2.84 47.24
26 2.50 59 182 45 37.77 1.37 1.79 3.16 51.41
27 1.90 62 172 35 23.86 2.29 2.29 4.59 43.98
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
42
Characterization of the RSI of derivatives of native lignin recovered from
three species of
annual fibre feedstocks.
Each of the lignin derivative subsamples produced above was assessed for its
radical
scavenging index (RSI). The potential antioxidant activity of each PL lignin
derivative was
determined by measuring its radical savaging capacity as described above.
The NRSI values for lignin derivatives recovered from wheat straw biomass are
shown in
Table 5(a). The NRSI values for lignin derivatives recovered from sugarcane
bagasse biomass are
shown in Table 5(b). The NRSI values for lignin derivatives recovered from
corn cob biomass
are shown in Table 5(c).
EXAMPLE 4: Predictive equations for selective recovery of lignin derivatives
having
targeted aliphatic hydroxyl contents, from organosolv pulping of
hardwood biomass feedstocks
BC Aspen:
In reference to the operating conditions for the twenty seven preliminary
organosolv
pulping runs with subsamples of aspen shown in Table 1(a), the intervals used
for model
generation were: (a) pH = [1.64, 3.30]; (b) Ethanol concentration in the
organic solvent (/0 w/w)
= [41, 79]; (c) pulping time duration (min) = [21, 1151; and (d) pulping
temperature ( C) = [166,
205].
The equation derived from the aliphatic hydroxyl data shown in Table 1(a) for
selection
of two or more operating conditions for production of lignin derivatives
having an aliphatic
hydroxyl content from the range of about 0.48 mmol/g to about 4.94 mmol/g, is:
13.9417-0.0764507*Temperature-0.20763*Ethano1+0.566778*pH*pH-
0.00303132*pH*Time+0.00106268*Temperature*Ethanol
EQ 1
Fig. 1 shows aliphatic hydroxyl contents of lignin derivatives recovered from
aspen as a
function of organic solvent concentration [Ethanol] and pulping temperature
[Temperature] at
constant pH of 2.47 and pulping time of 68 min., and shows process conditions
suitable for
producing lignin derivatives of the present disclosures have either decreased
or increased
aliphatic hydroxyl contents.
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
43
Chilean acacia:
In reference to the operating conditions for the twenty seven preliminary
organosolv
pulping runs with subsamples of acacia shown in Table 1(b), the intervals used
for model
generation were: (a) pH = [1.79, 3.20]; (b) Ethanol concentration in the
organic solvent (/0 w/w)
= [41, 79]; (c) pulping time duration (min) = [21, 115]; and (d) pulping
temperature ( C) = [166,
205].
The equation derived from the aliphatic hydroxyl data shown in Table 1(a) for
selection
of two or more operating conditions for production of lignin derivatives
having an aliphatic
hydroxyl content from the range of about 0.68 mmol/g to about 7.28 mmol/g, is:
44.4758-17.3944*pH-0.342106*Temperature+0.373582*Ethanol-
0.0133583*pH*Time + 0.124198*p H*Temperature +0.000205204*Time*Time-
0.00333743*Ethanol*Ethanol
EQ 2
Fig. 2 shows aliphatic hydroxyl contents of lignin derivatives recovered from
acacia as a
function of pulping time [time] and acidification of the organic solvent [pH]
at constant organic
solvent concentration of 60.0 /0 (w/w) and pulping temperature of 185.5 C, and
shows process
conditions suitable for producing lignin derivatives of the present
disclosures having either
decreased or increased aliphatic hydroxyl contents.
Chilean eucalyptus:
In reference to the operating conditions for the twenty seven preliminary
organosolv
pulping runs with subsamples of eucalyptus shown in Table 1(c), the intervals
used for model
generation were: (a) pH = [1.65, 3.22]; (b) Ethanol concentration in the
organic solvent ( /0 w/w)
= [41, 79]; (c) pulping time duration (min) = [21, 115]; and (d) pulping
temperature ( C) = [166,
205].
The equation derived from the aliphatic hydroxyl data shown in Table 1(a) for
selection
of two or more operating conditions for production of lignin derivatives
having an aliphatic
hydroxyl content from the range of about 0.63 mmol/g to about 6.07 mmol/g, is:
42.1508+ 4.21822*pH-0.5579848Temperature+0.352034*Ethanol-
0.0197431*pH*Time+ 0.000758397*Time*Ethano1+0.00148659*Temperature*Temperat
ure-0.000837671*Temperature*Ethano1-0.00251297*Ethanol*Ethanol
EQ 3
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
44
Fig. 3 shows aliphatic hydroxyl contents of lignin derivatives recovered from
eucalyptus
as a function of acidification of the organic solvent [pH] and pulping
temperature [Temperature]
at constant organic solvent concentration of 60.0 /0 (w/w) and pulping time of
68 min, and
shows process conditions suitable for producing lignin derivatives of the
present disclosures
having either decreased or increased aliphatic hydroxyl contents.
EXAMPLE 5: Predictive equations for selective recovery of lignin derivatives
having
targeted aliphatic hydroxyl contents, from organosolv pulping of
softwood biomass feedstocks
BC hybrid spruce:
In reference to the operating conditions for the twenty seven preliminary
organosolv
pulping runs with subsamples of hybrid spruce shown in Table 4(a), the
intervals used for model
generation were: (a) pH = [1.72,3.15]; (b) Ethanol concentration in the
organic solvent ( /0 w/w)
= [42, 79]; (c) pulping time duration (min) = [31, 110]; and (d) pulping
temperature ( C) = [167,
199].
The equation derived from the aliphatic hydroxyl data shown in Table 4(a) for
selection
of two or more operating conditions for production of lignin derivatives
having an aliphatic
hydroxyl content of about 5.23 rnmol/g or less, is:
65.2341 -0.689028*Temp erature + 0.170969*Ethano1+0.0217104*pH*Temperature-
0.0267202*pH*Ethanol-
0.000116382*Time*Temperature+0.000382542*Time*Ethano1+0.00156337*Temperatur
e*Temperature-0.00113549+Ethanol*Ethanol
EQ 4
Fig. 4 shows aliphatic hydroxyl contents of lignin derivatives recovered from
hybrid
spruce as a function of acidification of the organic solvent [pH] and pulping
time [Time] at
constant organic solvent concentration of 60.5% (w/w) and pulping temperature
of 183 C, and
shows process conditions suitable for producing lignin derivatives of the
present disclosures
having either decreased or increased aliphatic hydroxyl contents.
Chilean radiata pine:
In reference to the operating conditions for the twenty seven preliminary
organosolv
pulping runs with subsamples of radiata pine shown in Table 4(b), the
intervals used for model
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
generation were: (a) pH = [1.72,3.08]; (b) Ethanol concentration in the
organic solvent (/0 w/w)
= [42, 79]; (c) pulping time duration (min) = [31, 110]; and (d) pulping
temperature ( C) = [167,
199].
The equation derived from the aliphatic hydroxyl data shown in Table 4(b) for
selection
of two or more operating conditions for production of lignin derivatives
having an aliphatic
hydroxyl content of about 1.96 mmol/g to about 5.60 mmol/g, is:
13.9072-0.103563*Temperature+0.124245*Ethanol-
1 .31731*pH*pH+ 0.0386197*pH*Temperature+ 0.040326*pH*Ethano1+0.000100503*Ti
me*Time-0.000526035*Time*Ethano1-0.00112536*Temperature*Ethanol
EQ 5
Fig. 5 shows aliphatic hydroxyl contents of lignin derivatives recovered from
radiata pine
as a function of acidification of the organic solvent [pH] and pulping time
[Time] at constant
organic solvent concentration of 60.5% (w/w) and pulping temperature of 183 C,
and shows
process conditions suitable for producing lignin derivatives of the present
disclosures having
either decreased or increased aliphatic hydroxyl contents.
Southeastern USA loblolly pine:
In reference to the operating conditions for the twenty seven preliminary
organosolv
pulping runs with subsamples of loblolly pine shown in Table 4(c), the
intervals used for model
generation were: (a) pH = [1.49, 3.52]; (b) Ethanol concentration in the
organic solvent (% w/w)
= [37.8, 90.2]; (c) pulping time duration (min) = [27.9, 121]; and (d) pulping
temperature ( C) =
[150.3, 218.9].
The equation derived from the aliphatic hydroxyl data shown in Table 4(c) for
selection
of two or more operating conditions for production of lignin derivatives
having an aliphatic
hydroxyl content of about 1.22 mmol/g to about 4.83 mmol/g, is:
19.7852-11.2196*pH-0.153691*Time-
2.39789*pH*pH+0.0880747*pH*Time+0.102069*pH*Temperature+0.0203294*pH*Et
hano1-0.000537328*Time*Time-0.000706365*Temperature*Temperature-
0.000470555*Ethanol*Ethanol
EQ 6
Fig. 6 is a chart showing aliphatic hydroxyl contents of lignin derivatives of
the present
disclosure recovered from loblolly pine as a function of pulping time [Time]
and pulping
temperature [Temperature, C] at constant pH of the pulping liquor of 2.43 and
organic solvent
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
46
concentration of 62% w/w ethanol, and process conditions suitable for
producing lignin
derivatives of the present disclosures having either decreased or increased
aliphatic hydroxyl
contents;
EXAMPLE 6: Predictive equations for selective recovery of lignin derivatives
having
targeted aliphatic hydroxyl contents, from organosolv pulping of annual
fibre biomass feedstocks
Alberta wheat straw:
In reference to the operating conditions for the twenty seven preliminary
organosolv
pulping runs with subsamples of wheat straw shown in Table 5(a), the intervals
used for model
generation were: (a) pH = [1.76, 2.86]; (b) Ethanol concentration in the
organic solvent (/0 w/w)
= [36, 72]; (c) pulping time duration (min) = [27, 106]; and (d) pulping
temperature ( C) = [162,
195].
The equation derived from the aliphatic hydroxyl data shown in Table 5(a) for
selection
of two or more operating conditions for production of lignin derivatives
having an aliphatic
hydroxyl content of about 1.83 mmol/g to about 3.95 mmol/g, is:
-20.3795 + 5.44647*pH + 0.286802*Temperature-0.218004*Ethanol-
1.35259*pH*pH+ 0.00661225*pH*Time+ 0.0170796*pH*Ethanol-
0.000166011*Time*Tirne+0.0000958888*Tirne*Ethanol-
0.00103049*Temperature*Temperature + 0.000921376*Temperature*Ethanol
EQ 7
Fig. 7 shows aliphatic hydroxyl contents of lignin derivatives recovered from
wheat straw
as a function of organic solvent concentration [Ethanol] and pulping time
[Time] at constant
pulping temperature of 185.5 C and organic solvent acidified to a pH of 2.2,
and shows process
conditions suitable for producing lignin derivatives of the present
disclosures that have either
decreased or increased aliphatic hydroxyl contents.
Brazilian sugarcane bagasse:
In reference to the operating conditions for the twenty six preliminary
otganosolv
pulping runs with subsamples of sugarcane bagasse shown in Table 5(b), the
intervals used for
model generation were: (a) pH = [2.01, 3.26]; (b) Ethanol concentration in the
organic solvent
CA 02895215 2015-06-16
WO 2014/094104 PCT/CA2012/001172
47
(/o w/w) = [37, 73]; (c) pulping time duration (min) = [23, 98]; and (d)
pulping temperature ( C)
= [161, 197].
The equation derived from the aliphatic hydroxyl data shown in Table 5(b) for
selection
of two or more operating conditions for production of lignin derivatives
having an aliphatic
hydroxyl content of about 1.55 mmol/g to about 3.84 mmol/g, is:
37.6682-0.119057*Time-0.309507*Temperature-
0.126539*Ethano1+0.0255398*pH*Ethanol+ 0.000640605*Time*Temperature + 0.000691
701*Temperature*Temperature+ 0.000531287*Ethanol*Ethanol
EQ 8
Fig. 8 shows aliphatic hydroxyl contents of lignin derivatives recovered from
bagasse as a
function of acidification of the organic solvent [pH] and pulping time [Time]
at constant organic
solvent concentration of 55% (w/w) and pulping temperature of 179 C, and shows
process
conditions suitable for producing lignin derivatives of the present
disclosures having either
decreased or increased aliphatic hydroxyl contents.
European corn cobs:
In reference to the operating conditions for the twenty seven preliminary
organosolv
pulping runs with subsamples of corn cob biomass shown in Table 5(c), the
intervals used for
model generation were: (a) pH = [1.76, 2.81]; (b) Ethanol concentration in the
organic solvent
(`)/o w/w) = [35, 72]; (c) pulping time duration (min) = [27, 106]; and (d)
pulping temperature
( C) = [162, 192].
The equation derived from the aliphatic hydroxyl data shown in Table 5(c) for
selection
of two or more operating conditions for production of lignin derivatives
having an aliphatic
hydroxyl content of about 1.42 mmol/g to about 5.05 mmol/g, is:
-44.7775 + 0.544455*Temperature-2.22722*pH*pH+ 0.0637232*pH*Temp erature-
0.000080298*Time*Ethano1-0.00200084*Temperature*Temperature
EQ 9
Fig. 9 shows aliphatic hydroxyl contents of lignin derivatives recovered from
corn cobs
as a function of acidification of the organic solvent [pH] and pulping time
[Time] at constant
organic solvent concentration of 53.5% (w/w) and pulping temperature of 177 C,
and shows
process conditions suitable for producing lignin derivatives of the present
disclosures having
either decreased or increased aliphatic hydroxyl contents.