Note: Descriptions are shown in the official language in which they were submitted.
SOLID TABLET UNIT DOSE OVEN CLEANER
FIELD OF THE INVENTION
[0001] The present invention relates generally to the field of
solidification and solidification
matrices. The present invention relates to solidification of a phosphate-free
alkaline detergent
composition. In particular, the present invention relates to a phosphate-free
alkaline detergent
composition including sodium hydroxide, sodium carbonate, anhydrous sodium
metasilicate and
preferably a polycarboxylic acid polymer as part of the solidification matrix.
BACKGROUND
[0002] The use of solidification technology and solid block detergents in
institutional and
industrial operations was pioneered in the SOLID POWER brand technology
claimed for example in
Fernholz et al., U.S. Reissue Pat, Nos. 32,762 and 32,818. Additionally,
sodium carbonate hydrate cast
solid products using substantially hydrated sodium carbonate materials was
disclosed in Heile et al.,
U.S. Pat. Nos. 4,595,520 and 4,680,134.
[0003] In more recent years, attention has been directed to producing
highly effective
detergent materials from less caustic materials such as soda ash, also known
as sodium carbonate.
Early work in developing the sodium carbonate based detergents found that
sodium carbonate hydrate-
based materials often swelled, (i.e., were dimensionally unstable) after
solidification. Such swelling
can interfere with packaging, dispensing, and use. The dimensional instability
of the solid materials
relates to the unstable nature of various hydrate forms prepared in
manufacturing the sodium carbonate
solid materials. Early products made with hydrated sodium carbonate typically
comprised of
anhydrous, a one mole hydrate, a seven mole hydrate, a ten mole hydrate or
more mixtures thereof.
However, after the product had been manufactured and stored at ambient
temperatures, the hydration
state of the initial product was found to shift between hydrate forms, e.g.,
one, seven, and ten mole
hydrates, resulting in dimensional instability of the block chemicals. In
these conventional solid form
compositions, changes in water content and temperature lead to structural and
dimensional change,
which may lead to a failure of the solid form, resulting in problems such as
the inability of the solid
form to fit into dispensers for use.
1
CA 2895835 2017-09-13
[0004] Additionally, conventional solid alkaline detergents,
particularly those
intended for institutional and commercial use, generally require phosphates in
their
compositions. The phosphates typically serve multiple purposes in the
compositions, for
example, to control the rate of solidification, to remove and suspend soils,
and as an
effective hardness sequestrant. It was found, disclosed, and claimed in U.S.
Pat. Nos.
6,258,765, 6,156,715, 6,150,324, and 6,177,392, that a solid block functional
material
could be made using a binding agent that includes a carbonate salt, an organic
acetate,
such as an aminocarboxylate, or phosphonate component and water. Due to
ecological
concerns, further work has recently been directed to replacing phosphorous-
containing
compounds in detergents. In addition, nitrilotriacetic acid (NTA)-containing
aminocarboxylate components used in place of phosphorous-containing compounds
in
some instances as a binding agents and hardness sequestrants, are believed to
be
carcinogenic. As such, their use has also been curtailed.
[0005] The need for solidification matrices for solid, alkaline
detergents has
required numerous modifications, including removal of phosphate and/or NTA.
Additional modifications include the formulation of solidification matrices
incorporatine,
caustic material (sodium hydroxide) in combination with the less caustic
materials, such as
soda ash (e.g. sodium carbonate), continue to present difficulty in
establishing solid,
physically stable tablet compositions. It has been shown that highly caustic
powders for
solidification fail to consistently form stable compositions, such as tablets.
Therefore,
there is a need for using lower levels of sodium hydroxide in combination with
other less
caustic materials in order to formulate dimensionally-stable solid
compositions. These and
other aspects of forming physically stable detergent compositions provide the
background
against which the present invention is provided.
[0006] In an aspect of the present invention, a physically stable
phosphate-free
alkaline detergent tablet composition for combination ovens is provided.
[0007] In an aspect of the present invention, methods for employing
ash- and/or
hydroxide-hydration to form a physically stable, phosphate-free alkaline
detergent tablet
containing sodium carbonate, sodium hydroxide and sodium metasilicate are
provided.
[0008] In a further aspect of the invention, the compositions and
methods of the
invention provide physically stable compositions having durable cleaning
performance,
including for example in cleaning combination ovens.
2
CA 2895835 2019-03-13
SUMMARY
[0009] One embodiment of the present invention is a solid detergent
composition
that comprises an alkali metal hydroxide, a polycarboxylie acid polymer,
sodium
carbonate, water and at least one functional ingredient. According to an
embodiment, if
the solid detergent composition heated at a temperature of 120 degrees
Fahrenheit, the
composition remains dimensionally stable and has a growth exponent of less
than 3%.
The composition is preferably free of phosphate.
[0010] Another embodiment is a solid detergent composition comprising
between
about between about 5% and about 70% sodium hydroxide by weight of the solid
detergent composition; between about 20% and about 90% sodium carbonate by
weight of
the solid detergent composition; between about 0.1% and about 15%
polycarboxylic acid
polymer by weight of the solid detergent composition; between about 0.1% and
about 10%
water by weight of the solid detergent composition; between about 1% and about
50%
secondary alkalinity source by weight of the solid detergent composition;
between about
1% and about 50% chelant by weight of the solid detergent composition; wherein
the
detergent composition is free of phosphate. If heated at a temperature of
120 degrees
Fahrenheit, the solid detergent composition is dimensionally stable and has a
growth
exponent of less than 3%.
[0011] Yet a further embodiment is a method of forming a solid a
detergent
composition by combining sodium carbonate, an anhydrous silicate secondary
alkalinity
source and at least one additional functional component to form a powder pre-
mix; and
mixing the powder pre-mix with a water source to form a solid hydrate; and
combining the
solid hydrate with a source of sodium hydroxide and optionally a
polycarboxylic acid
polymer. In an aspect of the invention the produced solid detergent
compositions are
phosphorous free, and if heated at a temperature of 120 degrees Fahrenheit,
the solid
detergent compositions are dimensionally stable and have a growth exponent of
less than
3%.
[0012] While multiple embodiments are disclosed, still other
embodiments of the
present invention will become apparent to those skilled in the art from the
following
detailed description, which shows and describes illustrative embodiments of
the invention.
Accordingly, the drawings and detailed description are to be regarded as
illustrative in
nature and not restrictive.
3
CA 2895835 2019-03-13
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
BRIEF DESCRIPTION OF THE DRAWINGS
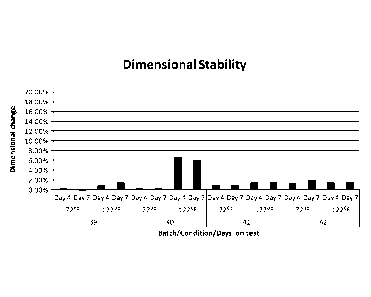
[0013] Figure 1 is a graph showing the dimensional stability of various
exemplary
formulations according to embodiments of the methods and compositions of the
invention.
[0014] Figure 2 is a graph showing compression strength of various
exemplary
formulations according to embodiments of the methods and compositions of the
invention.
[0015] Figure 3 is a graph showing the dimensional stability of various
exemplary
formulations according to embodiments of the methods and compositions of the
invention.
[0016] Figure 4 is a graph showing compression strength of various
exemplary
formulations according to embodiments of the methods and compositions of the
invention.
[0017] Various embodiments of the present invention will be described in
detail
with reference to the drawings, wherein like reference numerals represent like
parts
throughout the several views. Reference to various embodiments does not limit
the scope
of the invention. Figures represented herein are not limitations to the
various
embodiments according to the invention and are presented for exemplary
illustration of the
invention.
DETAILED DESCRIPTION
[0018] The embodiments of this invention are not limited to particular
solid
detergent compositions as they may vary as understood by skilled artisans. It
is further to
be understood that all terminology used herein is for the purpose of
describing particular
embodiments only, and is not intended to be limiting in any manner or scope.
For
example, as used in this specification and the appended claims, the singular
forms "a."
"an" and "the" can include plural referents unless the content clearly
indicates otherwise.
Further, all units, prefixes, and symbols may be denoted in its SI accepted
form. Numeric
ranges recited within the specification are inclusive of the numbers defining
the range and
include each integer within the defined range.
[0019] Unless defined otherwise, all technical and scientific terms used
herein
have the same meaning as commonly understood by one of ordinary skill in the
art to
which embodiments of the invention pertain. Many methods and materials
similar,
modified, or equivalent to those described herein can be used in the practice
of the
embodiments of the present invention without undue experimentation, the
preferred
materials and methods are described herein. In describing and claiming the
embodiments
4
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
of the present invention, the following terminology will be used in accordance
with the
definitions set out below.
[0020] The term "about," as used herein, refers to variation in the
numerical
quantity that can occur, for example, through typical measuring and liquid
handling
procedures used for making concentrates or use solutions in the real world;
through
inadvertent error in these procedures; through differences in the manufacture,
source, or
purity of the ingredients used to make the compositions or carry out the
methods; and the
like. Whether or not modified by the term "about", the claims include
equivalents to the
quantities and refers to variation in the numerical quantity that can occur.
[0021] The terms "dimensional stability" and "dimensionally stable" as used
herein, refer to a solid product having a growth exponent of less than about
3%, preferably
less than about 2%.
[0022] The term "weight percent," "wt-%," "percent by weight," "% by
weight,"
and variations thereof, as used herein, refer to the concentration of a
substance as the
weight of that substance divided by the total weight of the composition and
multiplied by
100. It is understood that, as used here, "percent," "%," and the like are
intended to be
synonymous with "weight percent," "wt-%," etc.
[0023] According to embodiments of the invention, the solid compositions
overcome a need in the prior art by providing a dimensionally stable solid
composition for
use in any pressed, extruded or cast solid composition containing a hydratable
salt, an
alkalinity active (e.g. alkali metal hydroxide) and water. In preferred
aspects, the
dimensionally stable solid compositions are not used in cast solid
compositions. In
particular, the composition would be useful for preparing a solid detergent
composition
that may be employed in any of a wide variety of situations where a
dimensionally-stable,
caustic-containing alkaline detergent that is substantially phosphorous-free
and
nitrilotiiacetic acid (NTA)-free solid product is desired. Substantially
phosphorus-free
means a solidification matrix having less than approximately 0.5 wt-%, more
particularly,
less than approximately 0.1 wt-%, and even more particularly less than
approximately 0.01
wt-% phosphorous based on the total weight of the solidification matrix. NTA-
free means
a solidification matrix having less than approximately 0.5 wt-%, less than
approximately
0.1 wt-%, and often less than approximately 0.01 wt-% NTA based on the total
weight of
the solidification matrix. Accordingly, the embodiments of the present
invention are
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
particularly useful in cleaning applications where it is desired to use an
environmentally
friendly solid detergent.
[0024] The solidification matrix of the present invention may be employed
in any
of a wide variety of situations in which a dimensionally stable solid product
is desired.
The solidification matrix is dimensionally stable and has an appropriate rate
of
solidification. In addition, the solidification matrix may be free of
phosphorous and NTA,
making the solidification matrix particularly useful in cleaning applications
where it is
desired to use an environmentally friendly, solid alkaline detergent. Such
applications
include, but are not limited to: phosphate-free alkaline detergent use in
combination ovens,
such as those used in various food service industries. Additional applications
may include,
for example, machine and manual warewashing employing a ware wash detergent,
presoaks, fryer boil outs, power soak sinks and related applications, soak
tanks, instrument
reprocessing, laundry and textile cleaning and destaining, carpet cleaning and
destaining,
vehicle cleaning and care applications, surface cleaning and destaining,
kitchen and bath
cleaning and destaining, floor cleaning and destaining, cleaning in place
operations,
general purpose cleaning and destaining, and/or industrial or household
cleaners. Methods
suitable for preparing a solid detergent composition using the solidification
matrix are also
provided.
Solidification Matrices and Solid Detergent Compositions
[0025] The solidification matrix generally includes an alkali metal
hydroxide
alkalinity source, a hydratable salt, such as sodium carbonate (soda ash), a
polycarboxylic
acid polymer and a water charge for forming solid compositions. The
solidification
matrices may further include chelants, corrosion inhibitors, additional water
conditioning
agents and/or additional alkalinity sources. The solidification matrices may
comprise,
consist of and/or consist essentially of an alkali metal hydroxide, a
hydratable salt, a
polycarboxylic acid polymer, a chelant, additional alkalinity and/or corrosion
inhibitor
source and/or a water charge.
[0026] Suitable component concentrations for the solidification matrix
range from
between approximately 1% and 90% by weight alkali metal hydroxide alkalinity,
0.1%
and approximately 15% by weight polycarboxylic acid polymer, between
approximately
0.1% and approximately 25% by weight water, and between approximately 20% and
approximately 90% by weight sodium carbonate. Particularly suitable component
6
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
concentrations for the solidification matrix range from between approximately
5% and
70% by weight alkali metal hydroxide alkalinity, 1% and approximately 10% by
weight
polycarboxylic acid polymer, between approximately 0.1% and approximately 10%
by
weight water, and between approximately 25% and approximately 90% by weight
sodium
carbonate. More particularly suitable component concentrations for the
solidification
matrix range from between approximately 10% and 50% by weight alkali metal
hydroxide
alkalinity, 2.5% and approximately 10% by weight polycarboxylic acid polymer,
between
approximately 1% and approximately 5% by weight water, and between
approximately
30% and approximately 70% by weight sodium carbonate. Unexpectedly, according
to
the invention the dimensionally stable solid compositions have alkali metal
hydroxide (e.g.
sodium hydroxide) content up to at least 30% or greater, preferably up to at
least 40% or
greater, or up to at least 50% or greater, overcoming a significant limitation
in the art.
[0027] In additional aspects of the invention the component concentrations
for the
solidification matrix further include the following ranges from between
approximately
0.1% and 50% by weight chelant, such as sodium gluconate, and 0.1% and 50% by
weight
secondary alkalinity source and/or corrosion inhibitor. Particularly suitable
component
concentrations for the solidification matrix range from between approximately
1% and
50% by weight chelant, and 1% and 50% by weight secondary alkalinity source
and/or
corrosion inhibitor. More particularly suitable component concentrations for
the
solidification matrix range from between approximately 5% and 25% by weight
chelant,
and 1% and 20% by weight secondary alkalinity source and/or corrosion
inhibitor. Those
skilled in the art will appreciate other suitable component concentration
ranges for
obtaining comparable properties of the solidification matrix. Without being
limited to the
scope of the invention, all numeric ranges recited herein are inclusive of the
numbers
defining the range and include each integer within the defined range.
Alkalinity Source
[0028] The solid detergent composition includes an effective amount of one
or
more alkalinity sources to provide effective cleaning of a substrate and
improve soil
removal performance of the solid detergent compositions. Preferably, the
alkalinity source
is an alkali metal hydroxide and is provided in an effective amount to improve
substrate
cleaning and soil removal. The compositions of the invention include the
alkalinity source
in an amount of at least about 1% by weight, at least about 5% by weight, or
at least about
7
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
10% by weight. In preferred aspects, the alkalinity source constitutes between
about 1%
and about 90% by weight, between about 5% and about 70% by weight, between
about
10% and about 50% by weight, and most preferably between about 20% and about
40%
by weight of the total weight of the solid detergent composition.
[0029] An effective amount of the alkalinity sources should be considered
as an
amount that provides a use composition having a pH of at least about 8,
preferably at least
about 10, and more preferably at least about 12. When the use composition has
a pII of
between about 8 and about 10, it can be considered mildly alkaline, and when
the pH is
greater than about 12, the use composition can be considered caustic.
[0030] Examples of suitable alkaline sources of the solid detergent
composition
include, but are not limited to an alkali metal hydroxide. Exemplary alkali
metal
hydroxides that can be used include, but are not limited to sodium, lithium,
or potassium
hydroxide. The alkali metal hydroxide may be added to the composition in any
form
known in the art, including as solid beads, dissolved in an aqueous solution,
or a
combination thereof. Alkali metal hydroxides are commercially available as a
solid in the
form of prilled solids or beads having a mix of particle sizes ranging from
about 12-100
U.S. mesh, or as an aqueous solution, as for example, as a 45% and a 50% by
weight
solution. It is preferred that the alkali metal hydroxide according to the
invention is added
in the form of prilled solids or beads.
Hydratable Salt
[0031] The solid detergent compositions according to the invention comprise
at
least one hydratable salt. In one embodiment the hydratable salt is sodium
carbonate (aka
soda ash or ash) and/or potassium carbonate (aka potash). In a preferred
aspect, the
hydratable salt is sodium carbonate and excludes potassium carbonate. The
hydratable salt
is provided in the ranges from between approximately 20% and approximately 90%
by
weight, preferably between approximately 25% and approximately 90% by weight,
and
more preferably between approximately 30% and approximately 70% by weight
hydratable salt, such as sodium carbonate. Those skilled in the art will
appreciate other
suitable component concentration ranges for obtaining comparable properties of
the
solidification matrix.
[0032] In other embodiments, the hydratable salt may be combined with other
solidification agents. For example, the hydratable salt may be used with
additional
8
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
solidification agents that are inorganic in nature and may also act optionally
as a source of
alkalinity. In certain embodiments, the secondary solidification agent may
include, but are
not limited to: additional alkali metal hydroxides, anhydrous sodium
carbonate, anhydrous
sodium sulfate, anhydrous sodium acetate, and other known hydratable compounds
or
combinations thereof. According to a preferred embodiment, the secondary
hydratable
salt comprises sodium metasilicate and/or anhydrous sodium metasilicate. The
amount of
secondary solidifying agent necessary to achieve solidification depends upon
several
factors, including the exact solidifying agent employed, the amount of water
in the
composition, and the hydration capacity of the other detergent components. In
certain
embodiments, the secondary solidifying agent may also serve as an additional
alkaline
source.
Polycarboxylic Acid Polymers
[0033] The solid alkaline detergent compositions according to the invention
include a polycarboxylic acid polymer or salt or derivative thereof. As
referred to herein,
the reference to any polycarboxylic acid polymer shall further encompass the
salt or
derivative thereof as also being a suitable polymer for use in the solid
alkaline detergent
compositions according to the invention. Examples of particularly suitable
polycarboxylic
acid polymers include, but are not limited to: polyacrylic acid polymers,
polyacrylic acid
polymers modified by a fatty acid end group ("modified polyacrylic acid
polymers"),
polymaleic acid polymers and combinations of these polymer materials. Salts of
each of
the polycarboxylic acid polymers may further be employed for the solid
alkaline detergent
compositions.
[0034] Non-limiting examples of polycarboxylic acid polymer salts include
polyacrylic acid salts and derivatives, such as water soluble acrylic
polymers. Such
polymers include, but are not limited to, polyacrylic acid, polymethacrylic
acid, acrylic
acid, acrylic acid-methacrylic acid copolymers, polymaleic acid, hydrolyzed
polyacrylamide, hydrolyzed methacrylamide, hydrolyzed acrylamide-
methacrylamide
copolymers, hydrolyzed polyacrylonitrile, hydrolyzed polymethacrylonitrile,
hydrolyzed
acrylonitrile methacrylonitrile copolymers, and the like, or combinations
thereof or
copolymers thereof. Water soluble salts or partial salts of these polymers
such as their
respective alkali metal (e.g., sodium, potassium, or combinations thereof) or
ammonium
salts can also be used according to the invention.
9
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
[0035] Examples of particularly suitable polyacrylic acid polymers and
modified
polyacrylic acid polymers and salts and derivatives thereof, include those
having a
molecular weight of between about 1,000 and about 100,000. Examples of more
particularly suitable polymaleic acid polymers and salts and derivatives
thereof include
those having a molecular weight of between about 500 and about 5,000. An
example of
particularly suitable commercially available polyacrylic acid polymer and
salts and
derivatives thereof includes, but is not limited to, Acusol 445ND, available
from Rohm &
Haas EEC, Philadelphia, PA. An example of particularly suitable commercially
available
modified polyacrylic acid polymer includes, but is not limited to, Alcosperse
325,
available from Alco Chemical, Chattanooga, TN. Examples of particularly
suitable
commercially available polymaleic acid polymers include, but are not limited
to: Belclene
200, available from Houghton Chemical Corporation, Boston, MA and Aquatreat AR-
801,
available from Alco Chemical, Chattanooga, TN.
[0036] In one embodiment, the solidification matrix of the present
invention
includes at least one polyacrylic acid polymer or salt or derivative thereof.
For example,
the solidification matrix may include between about 0.1% and 15% by weight,
more
particularly, between about 0.5% and 15% by weight polyacrylic acid polymer,
between
about 0.1% and 10% by weight, between about 1% and 10% by weight, more
particularly,
between about 2.5% and 10% by weight. Without being limited to the scope of
the
invention, all numeric ranges recited herein are inclusive of the numbers
defining the
range and include each integer within the defined range.
[0037] In alternative embodiments, the solidification matrix may include a
polymaleic acid polymer and at least two polyacrylic acid polymers having
different
molecular weights. In a further embodiment, the solidification matrix includes
at least one
carboxylic acid salt in addition to the at least one polycarboxylic acid
polymer. Suitable
carboxylic acid salts include straight chain saturated carboxylic acid salts
such as acetic
acid, gluconic acid, malic acid, succinic acid, glutaric acid, adipic acid,
tartaric acid, citric
acid or combinations thereof. In one example the solidification includes
between about
0.1% and 10% by weight carboxylic acid salt, for example citric acid salt.
Water
[0038] According to aspects of the invention, water may be both
independently
added to the solidification matrix and/or may be provided in the
solidification matrix as a
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
result of its presence in an aqueous material that is added to the detergent
composition.
Preferably, the secondary alkalinity source (e.g. silicate or metasilicate) is
provided as an
anhydrous silicate and therefore does not introduce water into the
solidification matrix.
However, the remaining components added to the detergent composition may
include
water or may be prepared in an aqueous premix available for reaction with the
solidification matrix component(s). Water is introduced into the
solidification matrix to
provide the solidification matrix with desired cohesive strength or
compressibility and to
provide a desired rate of solidification (e.g. hydroxide and/or ash hydration
according to
the aspects of the invention). In addition, it is expected that the aqueous
medium may help
in the solidification process when is desired to form the concentrate as a
solid. The water
may also be provided as deionized water or as softened water.
[0039] The amount of water in the resulting solid detergent composition
will
depend on the methods of forming employed for the solid detergent composition
(e.g.
processing forming techniques). As the methods and compositions of the present
invention are not preferred for use in casting (solidification occurring
within a container),
a lower amount of water is employed. The use of forming techniques includes a
relatively
smaller amount of water for solidification compared with the casting
techniques. When
preparing the solid detergent composition by forming techniques, water may be
present in
ranges of between about 1% and about 25% by weight, particularly between about
1% and
about 20% by weight, and more particularly between about 2% and about 10% by
weight.
Without being limited to the scope of the invention, all numeric ranges
recited herein are
inclusive of the numbers defining the range and include each integer within
the defined
range.
[0040] The solidification matrix may be phosphorus-free and/or
nitrilotriacetic
acid (NTA)-free to make the solid detergent composition more environmentally
beneficial.
Phosphorus-free means a solidification matrix having less than approximately
0.5 wt-%,
more particularly, less than approximately 0.1 wt-%, and even more
particularly less than
approximately 0.01 wt-% phosphorous based on the total weight of the
solidification
matrix. NTA-free means a solidification matrix having less than approximately
0.5 wt-%,
less than approximately 0.1 wt-%, and often less than approximately 0.01 wt-%
NTA
based on the total weight of the solidification matrix. When the
solidification matrix is
NTA-free, the solidification matrix and resulting solid detergent composition
is also
compatible with chlorine, which functions as an anti-redeposition and stain-
removal agent.
11
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
Additional Functional Materials
[0041] The components of the solidification matrix can be combined with
various
functional components used to form a solid detergent composition. In some
embodiments,
the solidification matrix including the alkali metal hydroxide, secondary
alkalinity source,
chelant, polycarboxylic acid polymer, water, and sodium carbonate make up a
large
amount, or even substantially all of the total weight of the detergent
composition, for
example, in embodiments having few or no additional functional materials
disposed
therein. In these embodiments, the component concentrations ranges provided
above for
the solidification matrix are representative of the ranges of those same
components in the
detergent composition. For example, such compositions may include between
about 5%
and 70% alkali metal hydroxide, 0.1% and 15% by weight polycarboxylic acid
polymer,
between about 0.1% and about 10% by weight water, between about 20% and 90% by
weight sodium carbonate, between about 1% and 50% by weight secondary
alkalinity
source, and between about 1% and 50% by weight chelant, with the balance of
the
composition comprising the additional functional components. Without being
limited to
the scope of the invention, all numeric ranges recited herein are inclusive of
the numbers
defining the range and include each integer within the defined range.
[0042] The functional materials provide desired properties and
functionalities to
the solid detergent composition. For the purpose of this application, the term
"functional
materials" includes a material that when dispersed or dissolved in a use
and/or concentrate
solution, such as an aqueous solution, provides a beneficial property in a
particular use.
Some particular examples of functional materials are discussed in more detail
below,
although the particular materials discussed are given by way of example only,
and that a
broad variety of other functional materials may be used. For example, many of
the
functional materials discussed below relate to materials used in cleaning
and/or destaining
applications. However, other embodiments may include functional materials for
use in
other applications.
Secondary Alkaline Source
[0043] The solid detergent composition can include an effective amount of
one or
more secondary alkaline sources to provide alkalinity and/or enhance cleaning
of a
substrate and/or improve soil removal performance of the solid detergent
composition. As
12
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
with the alkali metal hydroxide alkalinity source, the secondary alkaline
source may be
provided in concentrate form.
[0044] Examples of suitable secondary alkaline sources of the solid
detergent
composition include, but are not limited to an alkali metal carbonates, such
as sodium or
potassium carbonate, bicarbonate, sesquicarbonate, and mixtures thereof; metal
silicates,
such as sodium or potassium silicate or metasilicate, and mixtures thereof;
metal borates,
such as sodium or potassium borate, and mixtures thereof; and ethanolamines
and amines,
and mixtures thereof. Such secondary alkalinity agents are commonly available
in either
aqueous or powdered form, either of which is useful in formulating the present
solid
detergent compositions. According to preferred embodiments of the invention,
the
secondary alkalinity agent is provided in a solid form.
[0045] In an aspect, silicates are preferred for use as secondary
alkalinity sources.
Silicates are known for conventional benefits of corrosion inhibition and/or
anti-
redeposition efficacy in addition to providing alkalinity. In an aspect of the
present
invention, the silicate secondary alkalinity source is not provided in amounts
sufficient for
metal protection (i.e. corrosion inhibition) as a result of the solidification
composition
containing the alkali metal hydroxide component. Exemplary silicates include,
but are not
limited to: sodium silicate and potassium silicate. As referred to herein,
silicates may
further include metasilicates (e.g potassium or sodium metasilicates).
Silicates and/or
metasilicates can be provided as powdered, particulate or granular silicates
and/or
metasilicates. In addition, the silicates and/or metasilicates can be either
anhydrous or
contain water of hydration. In a preferred aspect of the invention, the
silicates and/or
metasilicates are anhydrous.
[0046] In some aspects, the secondary alkaline source is provided in an
amount of
between about 0.1% and about 50% by weight, between about 0.5% and about 50%
by
weight, between about 1% and about 50% by weight, between about 1% and about
25%
by weight, and between about 5% and about 15% by weight of the total weight of
the solid
detergent composition. Without being limited to the scope of the invention,
all numeric
ranges recited herein are inclusive of the numbers defining the range and
include each
integer within the defined range.
Surfactants
13
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
[0047] The solid detergent composition can include at least one cleaning
agent
comprising a surfactant or surfactant system. A variety of surfactants can be
used in a
solid detergent composition, including, but not limited to: anionic, nonionic,
cationic, and
zwitterionic surfactants. Surfactants are an optional component of the solid
detergent
composition and can be excluded from the concentrate. Exemplary surfactants
that can be
used are commercially available from a number of sources. For a discussion of
surfactants, see Kirk-Othmer, Encyclopedia of Chemical Technology, Third
Edition,
volume 8, pages 900-912, which is herein incorporated by reference in its
entirety. When
the solid detergent composition includes a cleaning agent, the cleaning agent
is provided
in an amount effective to provide a desired level of cleaning. The solid
detergent
composition, when provided as a concentrate, can include the cleaning agent in
a range of
about 0.05% to about 20% by weight, about 0.5% to about 15% by weight, about
1% to
about 15% by weight, about 1.5% to about 10% by weight, and about 2% to about
8% by
weight. Additional exemplary ranges of surfactant in a concentrate include
about 0.5% to
about 8% by weight, and about 1% to about 5% by weight. Without being limited
to the
scope of the invention, all numeric ranges recited herein are inclusive of the
numbers
defining the range and include each integer within the defined range.
[0048] Examples of anionic surfactants useful in the solid detergent
composition
include, but are not limited to: carboxylates such as alkylcarboxylates and
polyalkoxycarboxylates, alcohol ethoxylate carboxylates, nonylphenol
ethoxylate
carboxylates; sulfonates such as alkylsulfonates, alkylbenzenesulfonates,
alkylarylsulfonates, sulfonated fatty acid esters; sulfates such as sulfated
alcohols, sulfated
alcohol ethoxylates, sulfated alkylphenols, alkylsulfates, sulfosuccinates,
and alkylether
sulfates. Exemplary anionic surfactants include, but are not limited to:
sodium
alkylarylsulfonate, alpha-olefinsulfonate, and fatty alcohol sulfates.
[0049] Examples of nonionic surfactants useful in the solid detergent
composition
include, but are not limited to, those having a polyalkylene oxide polymer as
a portion of
the surfactant molecule. Such nonionic surfactants include, but are not
limited to:
chlorine-, benzyl-, methyl-, ethyl-, propyl-, butyl- and other like alkyl-
capped
polyethylene glycol ethers of fatty alcohols; polyalkylene oxide free
nonionics such as
alkyl polyglycosides; sorbitan and sucrose esters and their ethoxylates;
alkoxylated amines
such as alkoxylated ethylene diamine; alcohol alkoxylates such as alcohol
ethoxylate
propoxylates, alcohol propoxylates, alcohol propoxylate ethoxylate
propoxylates, alcohol
14
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
ethoxylate butoxylates; nonylphenol ethoxylate, polyoxyethylene glycol ether;
carboxylic
acid esters such as glycerol esters, polyoxyethylene esters, ethoxylated and
glycol esters of
fatty acids; carboxylic amides such as diethanolamine condensates,
monoalkanolamine
condensates, polyoxyethylene fatty acid amides; and polyalkylene oxide block
copolymers. An example of a commercially available ethylene oxide/propylene
oxide
block copolymer includes, but is not limited to, PLURONIC , available from
BASF
Corporation, Florham Park, NJ. An example of a commercially available silicone
surfactant includes, but is not limited to, ABIL B8852, available from
Goldschmidt
Chemical Corporation, Hopewell, VA.
[0050] Examples of cationic surfactants that can be used in the solid
detergent
composition include, but are not limited to: amines such as primary, secondary
and tertiary
monoamines with C18 alkyl or alkenyl chains, ethoxylated alkylamines,
alkoxylates of
ethylenediamine, imidazoles such as a 1-(2-hydroxyethyl)-2-imidazoline, a 2-
alky1-1-(2-
hydroxyethyl)-2-imidazoline, and the like; and quaternary ammonium salts, as
for
example, alkylquaternary ammonium chloride surfactants such as
n-alkyl(C12-C18)dimethylbenzyl ammonium chloride,
n-tetradecyldimethylbenzylammonium chloride monohydrate, and a naphthylene-
substituted quaternary ammonium chloride such as dimethyl-l-
naphthylmethylammonium
chloride. The cationic surfactant can be used to provide sanitizing
properties.
[0051] Examples of zwitterionic surfactants that can be used in the solid
detergent
composition include, but are not limited to: betaines, imidazolines, and
propionates.
[0052] Because the solid detergent composition is intended to be used in an
automatic dishwashing or warewashing machine or a combination oven, the
surfactants
selected, if any surfactant is used, can be those that provide an acceptable
level of foaming
when used inside a dishwashing or warewashing machine or a combination oven.
Solid
detergent compositions for use in automatic dishwashing or warewashing
machines are
generally considered to be low-foaming compositions. Low foaming surfactants
that
provide the desired level of detersive activity are advantageous in an
environment such as
a dishwashing machine where the presence of large amounts of foaming can be
problematic. In addition to selecting low foaming surfactants, defoaming
agents can also
be utilized to reduce the generation of foam. Accordingly, surfactants that
are considered
low foaming surfactants can be used. In addition, other surfactants can be
used in
conjunction with a defoaming agent to control the level of foaming.
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
Chelants, Builders and/or Water Conditioners
[0053] The solid detergent composition can include one or more building
agents,
also called chelating or sequestering agents (e.g., builders), including, but
not limited to:
an aminocarboxylic acid, or a polyacrylate. In general, a chelating agent is a
molecule
capable of coordinating (i.e., binding) the metal ions commonly found in
natural water to
prevent the metal ions from interfering with the action of the other detersive
ingredients of
a cleaning composition. Preferable levels of addition for builders that can
also be
chelating or sequestering agents are between about 0.1% to about 70% by
weight, about
1% to about 60% by weight, or about 1.5% to about 50% by weight. If the solid
detergent is provided as a concentrate, the concentrate can include between
approximately
1% to approximately 60% by weight, between approximately 3% to approximately
50%
by weight, and between approximately 6% to approximately 45% by weight of the
builders. Additional ranges of the builders include between approximately 3%
to
approximately 20% by weight, between approximately 6% to approximately 15% by
weight, between approximately 25% to approximately 50% by weight, and between
approximately 35% to approximately 45% by weight. Without being limited to the
scope
of the invention, all numeric ranges recited herein are inclusive of the
numbers defining
the range and include each integer within the defined range.
[0054] In a preferred aspect, a chelant (e.g. sodium gluconate) is provided
in an
amount of between about 0.1% and about 50% by weight, between about 0.5% and
about
50% by weight, between about 1% and about 50% by weight, between about 1% and
about 25% by weight, and between about 5% and about 25% by weight of the total
weight
of the solid detergent composition. Without being limited to the scope of the
invention, all
numeric ranges recited herein are inclusive of the numbers defining the range
and include
each integer within the defined range.
[0055] Examples of preferred chelants for use in the non-phosphate alkaline
detergent compositions include carboxylates such as citrate, tartrate or
gluconate are
suitable. In a preferred aspect, sodium gluconate is employed as a chelant for
the solid
alkaline detergent compositions.
[0056] The solid detergent compositions can contain a non-phosphorus based
builder. Although various components may include trace amounts of phosphorous,
a
composition that is considered free of phosphorous generally does not include
phosphate
16
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
or phosphonate builder or chelating components as an intentionally added
component.
Carboxylates such as citrate, tartrate or gluconate are suitable. Useful
aminocarboxylic
acid materials containing little or no NTA include, but are not limited to: N-
hydroxyethylaminodiacetic acid, ethylenediaminetetraacetic acid (EDTA),
hydroxyethylenediaminetetraacetic acid, diethylenetriaminepentaacetic acid,
N-hydroxyethyl-ethylenediaminetriacetic acid (HEDTA),
diethylenetriaminepentaacetic
acid (DTPA), and other similar acids having an amino group with a carboxylic
acid
substituent.
[0057] Water conditioning polymers can be used as non-phosphorus containing
builders. Exemplary water conditioning polymers include, but are not limited
to:
polycarboxylates. Exemplary polycarboxylates that can be used as builders
and/or water
conditioning polymers include, but are not limited to: those having pendant
carboxylate (-
0O2-) groups such as polyacrylic acid, maleic acid, maleic/olefin copolymer,
sulfonated
copolymer or terpolymer, acrylic/maleic copolymer, polymethacrylic acid,
acrylic
acid-methacrylic acid copolymers, hydrolyzed polyacrylamide, hydrolyzed
polymethacrylamide, hydrolyzed polyamide-methacrylamide copolymers, hydrolyzed
polyacrylonitrile, hydrolyzed polymethacrylonitrile, and hydrolyzed
acrylonitrile-
methacrylonitrile copolymers. For a further discussion of chelating
agents/sequestrants,
see Kirk-Othmer, Encyclopedia of Chemical Technology, Third Edition. volume 5,
pages
339-366 and volume 23, pages 319-320, the disclosure of which is incorporated
by
reference herein. These materials may also be used at substoichiometric levels
to function
as crystal modifiers
Hardening Agents
[0058] The solid detergent compositions can also include a hardening agent
in
addition to, or in the form of, the builder. A hardening agent is a compound
or system of
compounds, organic or inorganic, which significantly contributes to the
uniform
solidification of the composition. Preferably, the hardening agents are
compatible with the
cleaning agents, including the sodium hydroxide active alkalinity, and other
active
ingredients of the composition and are capable of providing an effective
amount of
hardness and/or aqueous solubility to the processed composition. The hardening
agents
should also be capable of forming a homogeneous matrix with the cleaning agent
and
17
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
other ingredients when mixed and solidified to provide a uniform dissolution
of the
cleaning agent from the solid detergent composition during use.
[0059] The amount of hardening agent included in the solid detergent
composition
will vary according to factors including, but not limited to: the type of
solid detergent
composition being prepared, the ingredients of the solid detergent
composition, the
intended use of the composition, the quantity of dispensing solution applied
to the solid
composition over time during use, the temperature of the dispensing solution,
the hardness
of the dispensing solution, the physical size of the solid detergent
composition, the
concentration of the other ingredients, and the concentration of the cleaning
agent in the
composition. It is prefen-ed that the amount of the hardening agent included
in the solid
detergent composition is effective to combine with the cleaning agent and
other
ingredients of the composition to form a homogeneous mixture under continuous
mixing
conditions and a temperature at or below the melting temperature of the
hardening agent.
[0060] It is also preferred that the hardening agent form a matrix with the
cleaning
agent and other ingredients which will harden to a solid form under ambient
temperatures
of approximately 30 C to approximately 50 C, particularly approximately 35
C to
approximately 45 C. after mixing ceases and the mixture is dispensed from the
mixing
system, within approximately less than 1 minute, or from about 1 minute to
approximately
3 hours, particularly approximately less than 2 minutes to approximately 2
hours, and
particularly approximately less than 5 minutes to approximately 1 hour. A
minimal
amount of heat from an external source may be applied to the mixture to
facilitate
processing of the mixture. It is preferred that the amount of the hardening
agent included
in the solid detergent composition is effective to provide a desired hardness
and desired
rate of controlled solubility of the processed composition when placed in an
aqueous
medium to achieve a desired rate of dispensing the cleaning agent from the
solidified
composition during use.
[0061] The hardening agent may be an organic or an inorganic hardening
agent.
According to an aspect of the invention, it is preferred that the organic
hardening agent is
not a polyethylene glycol (PEG) compound, such as shown in Examples 10-23
according
to the invention. Examples of polyethylene glycols include, but are not
limited to: solid
polyethylene glycols of the general formula H(OCH2CH2)110H, where n is greater
than 15,
particularly approximately 30 to approximately 1700 having a variety of
molecular
weights. It is further preferred that the hardening agent is not urea and/or
urea particles.
18
[0062] Preferred inorganic hardening agents are hydratable inorganic
salts, including, but not
limited to: sulfates and bicarbonates. The inorganic hardening agents are
present at concentrations of
up to approximately 50% by weight, particularly approximately 1% to
approximately 25% by weight,
and more particularly approximately 5% to approximately 15% by weight. Without
being limited to
the scope of the invention, all numeric ranges recited herein are inclusive of
the numbers defining the
range and include each integer within the defined range.
Bleaching Agents
[0063] Bleaching agents suitable for use in the solid detergent
composition for lightening or
whitening a substrate include bleaching compounds capable of liberating an
active halogen species,
such as C12, Br2, -OC I" and/or -0Br", under conditions typically encountered
during the cleansing
process. Suitable bleaching agents for use in the solid detergent compositions
include, but are not
limited to: chlorine-containing compounds such as chlorines, hypochlorites, or
chloramines.
Exemplary halogen-releasing compounds include, but are not limited to: the
alkali metal
dichloroisocyanurates, chlorinated trisodium phosphate, the alkali metal
hypochlorites,
monochloramine, and dichloramine. Encapsulated chlorine sources may also be
used to enhance the
stability of the chlorine source in the composition (see, for example, U.S.
Patent Nos. 4,618,914 and
4,830,773). A bleaching agent may also be a peroxygen or active oxygen source
such as hydrogen
peroxide, perborates, sodium carbonate peroxyhydrate, potassium
permonosulfate, and sodium
perborate mono and tetrahydrate, with and without activators such as
tetraacetylethylene diamine.
[0064] When the concentrate includes a bleaching agent, it can be included
in an amount of
between approximately 0.1% and approximately 60% by weight, between
approximately 1% and
approximately 20% by weight, between approximately 3% and approximately 8% by
weight, and
between approximately 3% and approximately 6% by weight. Without being limited
to the scope of
the invention, all numeric ranges recited herein are inclusive of the numbers
defining the range and
include each integer within the defined range.
Fillers
19
CA 2895835 2017-09-13
[0065] The solid detergent composition can include an effective amount of
detergent fillers
which do not perform as a cleaning agent per se, but cooperates with the
cleaning agent to enhance the
overall cleaning capacity of the composition. Examples of detergent fillers
suitable for use in the
present cleaning compositions include, but are not limited to: sodium sulfate
and sodium chloride.
When the concentrate includes detergent filler, it can be included in an
amount up to approximately
50% by weight, between approximately 1% and approximately 30% by weight, or
between
approximately 1.5% and approximately 25% by weight. Without being limited to
the scope of the
invention, all numeric ranges recited herein are inclusive of the numbers
defining the range and
include each integer within the defined range.
Defoaming Agents
[0066] A defoaming agent for reducing the stability of foam may also be
included in the
warewashing composition. Examples of defoaming agents include, but are not
limited to: ethylene
oxide/propylene block copolymers such as those available under the name
Pluronic N-3; silicone
compounds such as silica dispersed in polydimethylsiloxane,
polydimethylsiloxane, and functionalized
polydimethylsiloxane such as those available under the name Abil B9952; fatty
amides, hydrocarbon
waxes, fatty acids, fatty esters, fatty alcohols, fatty acid soaps,
ethoxylates, mineral oils, polyethylene
glycol esters, and alkyl phosphate esters such as monostearyl phosphate.
[0067] A discussion of defoaming agents may be found, for example, in U.S.
Patent No.
3,048,548 to Martin et al., U.S. Patent No. 3,334,147 to Brunelle et al., and
U.S. Patent No. 3,442,242
to Rue et al. When the concentrate includes a defoaming agent, the defoaming
agent can be provided
in an amount of between approximately 0.0001% and approximately 10% by weight,
between
approximately 0.001% and approximately 5% by weight, or between approximately
0.01% and
approximately 1.0% by weight. Without being limited to the scope of the
invention, all numeric ranges
recited herein are inclusive of the numbers defining the range and include
each integer within the
defined range.
Anti-Redeposition Agents
[0068] The solid detergent composition can include an anti-redeposition
agent for facilitating
sustained suspension of soils in a cleaning solution and preventing the
removed
CA 2895835 2017-09-13
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
soils from being redeposited onto the substrate being cleaned. Examples of
suitable
anti-redeposition agents include, but are not limited to: polyacrylates,
styrene maleic
anhydride copolymers, cellulosic derivatives such as hydroxyethyl cellulose,
hydroxypropyl cellulose and carboxymethyl cellulose. When the concentrate
includes an
anti-redeposition agent, the anti-redeposition agent can be included in an
amount of
between approximately 0.5% and approximately 10% by weight, and between
approximately 1% and approximately 5% by weight. Without being limited to the
scope of
the invention, all numeric ranges recited herein are inclusive of the numbers
defining the
range and include each integer within the defined range.
Stabilizing Agents
[0069] The solid detergent composition may also include stabilizing agents.
Examples of suitable stabilizing agents include, but are not limited to:
borate,
calcium/magnesium ions, propylene glycol, and mixtures thereof. The
concentrate need
not include a stabilizing agent, but when the concentrate includes a
stabilizing agent, it can
be included in an amount that provides the desired level of stability of the
concentrate.
Exemplary ranges of the stabilizing agent include up to approximately 20% by
weight,
between approximately 0.5% and approximately 15% by weight, and between
approximately 2% and approximately 10% by weight. Without being limited to the
scope
of the invention, all numeric ranges recited herein are inclusive of the
numbers defining
the range and include each integer within the defined range.
Dispersants
[0070] The solid detergent composition may also include dispersants.
Examples of
suitable dispersants that can be used in the solid detergent composition
include, but are not
limited to: maleic acid/olefin copolymers, polyacrylic acid, and mixtures
thereof. The
concentrate need not include a dispersant, but when a dispersant is included
it can be
included in an amount that provides the desired dispersant properties.
Exemplary ranges
of the dispersant in the concentrate can be up to approximately 20% by weight,
between
approximately 0.5% and approximately 15% by weight, and between approximately
2%
and approximately 9% by weight. Without being limited to the scope of the
invention, all
numeric ranges recited herein are inclusive of the numbers defining the range
and include
each integer within the defined range.
21
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
Enzymes
[0071] Enzymes that can be included in the solid detergent composition
include
those enzymes that aid in the removal of starch and/or protein stains.
Exemplary types of
enzymes include, but are not limited to: proteases, alpha-amylases, and
mixtures thereof.
Exemplary proteases that can be used include, but are not limited to: those
derived from
Bacillus licheniformix, Bacillus lenus, Bacillus alcalophilus, and Bacillus
amyloliquefacins. Exemplary alpha-amylases include Bacillus subtilis, Bacillus
amyloliquefaceins and Bacillus licheniformis. The concentrate need not include
an
enzyme, but when the concentrate includes an enzyme, it can be included in an
amount
that provides the desired enzymatic activity when the solid detergent
composition is
provided as a use composition. Exemplary ranges of the enzyme in the
concentrate
include up to approximately 15% by weight, between approximately 0.5% to
approximately 10% by weight, and between approximately 1% to approximately 5%
by
weight. Without being limited to the scope of the invention, all numeric
ranges recited
herein are inclusive of the numbers defining the range and include each
integer within the
defined range.
Glass and Metal Corrosion Inhibitors
[0072] The solid detergent composition can include a metal corrosion
inhibitor in
an amount up to approximately 50% by weight, between approximately 1% and
approximately 40% by weight, or between approximately 3% and approximately 30%
by
weight. Without being limited to the scope of the invention, all numeric
ranges recited
herein are inclusive of the numbers defining the range and include each
integer within the
defined range.
[0073] The corrosion inhibitor is included in the solid detergent
composition in an
amount sufficient to provide a use solution that exhibits a rate of corrosion
and/or etching
of glass that is less than the rate of corrosion and/or etching of glass for
an otherwise
identical use solution except for the absence of the corrosion inhibitor. It
is expected that
the use solution will include at least approximately 6 parts per million (ppm)
of the
corrosion inhibitor to provide desired corrosion inhibition properties. It is
expected that
larger amounts of corrosion inhibitor can be used in the use solution without
deleterious
effects. It is expected that at a certain point, the additive effect of
increased corrosion
22
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
and/or etching resistance with increasing corrosion inhibitor concentration
will be lost, and
additional corrosion inhibitor will simply increase the cost of using the
solid detergent
composition. The use solution can include between approximately 6 ppm and
approximately 300 ppm of the corrosion inhibitor, and between approximately 20
ppm and
approximately 200 ppm of the corrosion inhibitor. Examples of suitable
corrosion
inhibitors include, but are not limited to: a combination of a source of
aluminum ion and a
source of zinc ion, as well as an alkaline metal silicate or hydrate thereof.
[0074] The corrosion inhibitor can refer to the combination of a source of
aluminum ion and a source of zinc ion. The source of aluminum ion and the
source of
zinc ion provide aluminum ion and zinc ion, respectively, when the solid
detergent
composition is provided in the form of a use solution. The amount of the
corrosion
inhibitor is calculated based upon the combined amount of the source of
aluminum ion and
the source of zinc ion. Anything that provides an aluminum ion in a use
solution can be
referred to as a source of aluminum ion, and anything that provides a zinc ion
when
provided in a use solution can be referred to as a source of zinc ion. It is
not necessary for
the source of aluminum ion and/or the source of zinc ion to react to form the
aluminum ion
and/or the zinc ion. Aluminum ions can be considered a source of aluminum ion,
and zinc
ions can be considered a source of zinc ion. The source of aluminum ion and
the source of
zinc ion can be provided as organic salts, inorganic salts, and mixtures
thereof. Exemplary
sources of aluminum ion include, but are not limited to: aluminum salts such
as sodium
aluminate, aluminum bromide, aluminum chlorate, aluminum chloride, aluminum
iodide,
aluminum nitrate, aluminum sulfate, aluminum acetate, aluminum formate.
aluminum
tartrate, aluminum lactate, aluminum oleate, aluminum bromate, aluminum
borate,
aluminum potassium sulfate, aluminum zinc sulfate, and aluminum phosphate.
Exemplary
sources of zinc ion include, but are not limited to: zinc salts such as zinc
chloride, zinc
sulfate, zinc nitrate, zinc iodide, zinc thiocyanate, zinc fluorosilicate,
zinc dichromate, zinc
chlorate, sodium zincate, zinc gluconate, zinc acetate, zinc benzoate, zinc
citrate, zinc
lactate, zinc formate, zinc bromate, zinc bromide, zinc fluoride, zinc
fluorosilicate, and
zinc salicylate.
[0075] The applicants discovered that by controlling the ratio of the
aluminum ion
to the zinc ion in the use solution, it is possible to provide reduced
corrosion and/or
etching of glassware and ceramics compared with the use of either component
alone. That
is, the combination of the aluminum ion and the zinc ion can provide a synergy
in the
23
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
reduction of corrosion and/or etching. The ratio of the source of aluminum ion
to the
source of zinc ion can be controlled to provide a synergistic effect. In
general, the weight
ratio of aluminum ion to zinc ion in the use solution can be between at least
approximately
6:1, can be less than approximately 1:20, and can be between approximately 2:1
and
approximately 1:15.
[0076] An effective amount of an alkaline metal silicate or hydrate thereof
can be
employed in the compositions and processes of the invention to form a stable
solid
detergent composition having metal protecting capacity. The silicates employed
in the
compositions of the invention are those that have conventionally been used in
solid
detergent formulations. For example, typical alkali metal silicates are those
powdered,
particulate or granular silicates which are either anhydrous or preferably
which contain
water of hydration (approximately 5% to approximately 25% by weight,
particularly
approximately 15% to approximately 20% by weight water of hydration). These
silicates
are preferably sodium silicates and have a Na20:Si02 ratio of approximately
1:1 to
approximately 1:5, respectively, and typically contain available water in the
amount of
from approximately 5% to approximately 25% by weight. In general, the
silicates have a
Na20:Si02 ratio of approximately 1:1 to approximately 1:3.75, particularly
approximately
1:1.5 to approximately 1:3.75 and most particularly approximately 1:1.5 to
approximately
1:2.5. A silicate with a Na20:Si02 ratio of approximately 1:2 and
approximately 16% to
approximately 22% by weight water of hydration, is most preferred. For
example, such
silicates are available in powder form as GD Silicate and in granular form as
Britesil H-20,
available from PQ Corporation, Valley Forge, PA. These ratios may be obtained
with
single silicate compositions or combinations of silicates which upon
combination result in
the preferred ratio. The hydrated silicates at preferred ratios, a Na.,,O:Sia)
ratio of
approximately 1:1.5 to approximately 1:2.5, have been found to provide the
optimum
metal protection and rapidly form a solid detergent. Hydrated silicates are
preferred.
[0077] Silicates can be included in the solid detergent composition to
provide for
metal protection but are additionally known to provide alkalinity and
additionally function
as anti-redeposition agents. Exemplary silicates include, but are not limited
to: sodium
silicate and potassium silicate. The solid detergent composition can be
provided without
silicates, but when silicates are included, they can be included in amounts
that provide for
desired metal protection. The concentrate can include silicates in amounts of
at least
approximately 1% by weight, at least approximately 5% by weight, at least
approximately
24
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
10% by weight, and at least approximately 15% by weight. In addition, in order
to
provide sufficient room for other components in the concentrate, the silicate
component
can be provided at a level of less than approximately 35% by weight, less than
approximately 25% by weight, less than approximately 20% by weight, and less
than
approximately 15% by weight. Without being limited to the scope of the
invention, all
numeric ranges recited herein are inclusive of the numbers defining the range
and include
each integer within the defined range.
Fragrances and Dyes
[0078] Various dyes, odorants including perfumes, and other aesthetic
enhancing
agents can also be included in the composition. Suitable dyes that may be
included to alter
the appearance of the composition, include, but are not limited to: Direct
Blue 86,
available from Mac Dye-Chem Industries, Ahmedabad, India; Fastusol Blue,
available
from Mobay Chemical Corporation, Pittsburgh, PA; Acid Orange 7, available from
American Cyanamid Company, Wayne, NJ; Basic Violet 10 and Sandolan Blue/Acid
Blue
182, available from Sandoz, Princeton, NJ; Acid Yellow 23, available from
Chemos
GmbH, Regenstauf, Germany; Acid Yellow 17, available from Sigma Chemical, St.
Louis,
MO; Sap Green and Metanil Yellow, available from Keyston Analine and Chemical,
Chicago, IL; Acid Blue 9, available from Emerald Hilton Davis, LLC,
Cincinnati. OH;
Hisol Fast Red and Fluorescein, available from Capitol Color and Chemical
Company,
Newark, NJ; and Acid Green 25, Ciba Specialty Chemicals Corporation,
Greenboro. NC.
[0079] Fragrances or perfumes that may be included in the compositions
include,
but are not limited to: terpenoids such as citronellol, aldehydes such as amyl
cinnamaldehyde, a jasmine such as CIS-jasmine or jasmal, and vanillin.
Flow Aids
[0080] Various flow aids can also be included in the composition. Flow aids
may
further be referred to as carriers and/or glidants and are generally known for
improving the
processing of compositions, such as the solid detergent compositions according
to the
invention. Suitable components for improving the flowability of the homogenous
powder
components according to the invention, may include for example, inorganic or
organic
agents. According to an aspect, inorganic agents are preferred, including for
example
silicas, borates, acetate salts, sulfate salts and the like. Silicas,
including for example the
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
precipitated or fumed forms (e.g., Sipernat , Aerosil , CAB-0-SIID) can be
employed
and are commercially available, for example from Evonik Industries.
Methods of Making and Use
[0081] Without being limited to a particular theory of the invention, the
actual
solidification mechanism may occur through hydroxide hydration, e.g. the
interaction of
the sodium hydroxide (or other alkali metal hydroxide) with water. It is
believed that the
combination of the sodium hydroxide and secondary alkalinity source (e.g.
sodium
metasilicate) along with the polycarboxylic acid polymer functions to control
the kinetics
and thermodynamics of the solidification process and provides a solidification
matrix in
which additional functional materials may be bound to form a functional solid
composition. For example, the polycarboxylic acid polymer and other functional
ingredients may have efficacy in stabilizing the hydroxide by acting as donor
and/or
acceptor of free water.
[0082] In other aspects of the invention, there may be aspects of
solidification as a
result of ash hydration, e.g. the interaction of the hydratable salt with
water. For example,
according to such embodiments, the carbonate hydrates by acting as a donor
and/or
acceptor of free water.
[0083] According to aspects of the invention, by controlling the rate of
water
migration for hydration of the ash and/or hydroxide alkalinity source, the
rate of
solidification of the detergent compositions may be controlled to provide
process and
dimensional stability to the resulting solid detergent composition product.
The rate of
solidification is significant because if the solidification matrix solidifies
too quickly, the
composition may solidify during mixing and stop processing. If the
solidification matrix
solidifies too slowly, valuable process time is lost.
[0084] Without being limited to a particular theory of the invention, in an
aspect
the polycarboxylic acid polymer may assist in providing dimensional stability
to the end
product by ensuring that the solid product containing the sodium hydroxide and
secondary
alkalinity source (e.g. sodium metasilicate) does not swell. If the solid
product swells after
solidification, various problems may occur, including but not limited to:
decreased
density, integrity, and appearance; and inability to dispense or package the
solid product.
Generally, a solid product is considered to have dimensional stability if the
solid product
has a growth exponent of less than about 3% and particularly less than about
2%. Growth
26
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
exponent refers to the percent growth or swelling of a product over a period
of time after
solidification under normal transport/storage conditions. Because normal
transport/storage
conditions for detergent products often results in the detergent composition
being
subjected to an elevated temperature, the growth exponent of a solid detergent
product
may be determined by measuring one or more dimensions of the product prior to
and after
heating at between 100 F and 120 F. The measured dimension or dimensions
depends
on the shape of the solid product and the manner in which it swells. For
tablets, the
change in both diameter and height is generally measured and added together to
determine
the growth exponent. For capsules, just the diameter is normally measured.
[0085] In general, a solid detergent composition using the solidification
matrix of
the present invention can be created by combining the alkali metal hydroxide
alkalinity
source (e.g. sodium hydroxide), secondary alkalinity source (e.g. anhydrous
sodium
metasilicate), polycarboxylic acid polymer, sodium carbonate, water, and any
additional
functional components and allowing the components to interact and solidify.
[0086] For example, in a first embodiment, the solid detergent composition
may
include sodium hydroxide, anhydrous sodium metasilicate, polycarboxylic acid
polymer, a
water charge, sodium carbonate, a chelant and optional functional ingredients.
In an
exemplary embodiment, the solid detergent composition includes between
approximately
1% and 90% by weight alkali metal hydroxide alkalinity, 0.1% and approximately
15% by
weight polycarboxylic acid polymer, between approximately 0.1% and
approximately
25% by weight water, between approximately 20% and approximately 90% by weight
sodium carbonate, between approximately 0.1% and 50% by weight chelant, such
as
sodium gluconate, and 0.1% and 50% by weight secondary alkalinity source
and/or
corrosion inhibitor. In another exemplary embodiment, the solid detergent
composition
includes between approximately 5% and 70% by weight alkali metal hydroxide
alkalinity,
1% and approximately 10% by weight polycarboxylic acid polymer, between
approximately 0.1% and approximately 10% by weight water, between
approximately
25% and approximately 90% by weight sodium carbonate, between approximately 1%
and
50% by weight chelant, and 1% and 50% by weight secondary alkalinity source
and/or
corrosion inhibitor. In yet another exemplary embodiment the solid detergent
composition
includes between approximately 10% and 50% by weight alkali metal hydroxide
alkalinity, 2.5% and approximately 10% by weight polycarboxylic acid polymer,
between
approximately 1% and approximately 5% by weight water, between approximately
30%
27
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
and approximately 70% by weight sodium carbonate, between approximately 1% and
50%
by weight chelant, and 1% and 50% by weight secondary alkalinity source and/or
corrosion inhibitor. Without being limited to the scope of the invention, all
numeric ranges
recited herein are inclusive of the numbers defining the range and include
each integer
within the defined range.
[0087] In some embodiments, the relative amounts of sodium hydroxide, water
and polycarboxylic acid polymer are controlled within a composition. The
solidification
matrix and additional functional components harden into solid form due to the
chemical
reaction of the sodium hydroxide and water (hydroxide hydration), and/or the
sodium
carbonate (ash hydration) with the water. As the solidification matrix
solidifies, a binder
composition can form to bind and solidify the components. At least a portion
of the
ingredients associate to form the binder while the balance of the ingredients
forms the
remainder of the solid composition. The solidification process may last from a
few
minutes to about six hours, depending on factors including, but not limited
to: the size of
the formed composition, the ingredients of the composition, and the
temperature of the
composition.
[0088] According to an aspect of the invention, the hydratable salt (e.g.
sodium
carbonate), secondary alkalinity source (e.g. anhydrous sodium metasilicate)
and at least
one additional functional ingredient are combined into a homogenous powder
mixture. A
water source is added to the homogenous powder mixture prior to incorporation
into the
detergent composition, and can be provided as a solid hydrate. According to an
aspect of
the invention, the addition of water to the homogenous powder mixture is
referred to
herein as a water "charge." A water charge is included in the solidification
matrix for
subsequent combination with the sodium hydroxide (and optionally the
polycarboxylic
acid polymer). In an aspect, a water charge of less than about 10% is
preferred, from about
1% to about 10%, or from about 2% to about 10%.
[0089] Thereafter, the solid hydrate is then combined with sodium hydroxide
(and
optionally the polycarboxylic acid polymer). The sodium hydroxide is combined
with the
water and sodium carbonate matrix when added to the detergent composition for
the
detergent composition to effectively solidify according to the methods of the
invention. In
general, an effective amount of the sodium hydroxide and sodium carbonate
refer to
amounts that effectively control the kinetics and thermodynamics of the
solidification
28
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
system by controlling the rate and movement of water into the hydroxide
hydration
process and/or ash hydration process.
[0090] Solid detergent compositions formed using the solidification matrix
are
produced using a batch or continuous mixing system. In an exemplary
embodiment, a
processing method of a tablet press is used to form tablets from the
homogeneous mixtures
according to the methods of the invention. In some embodiments, the processing
temperature is at or below the melting temperature of the components. The
processed
mixture may be dispensed from the mixer by forming, or other suitable means,
whereupon
the detergent composition hardens to a solid form. The structure of the matrix
may be
characterized according to its hardness, melting point, material distribution,
crystal
structure, and other like properties according to known methods in the art.
Generally, a
solid detergent composition processed according to the method of the invention
is
substantially homogeneous with regard to the distribution of ingredients
throughout its
mass and is dimensionally stable.
[0091] Specifically, in a forming process, the liquid and solid components
are
introduced into the final mixing system and are continuously mixed until the
components
form a substantially homogeneous semi-solid mixture in which the components
are
distributed throughout its mass. In an exemplary embodiment, the components
are mixed
in the mixing system for at least approximately 5 seconds. The mixture is then
discharged
from the mixing system into, or through, a die or other shaping means. The
product is
then packaged. In an exemplary embodiment, the formed composition begins to
harden to
a solid form in less than 1 minute, or between approximately 1 minute and
approximately
3 hours. Particularly, the formed composition begins to harden to a solid form
in between
a few seconds to about 1 minute. More particularly, the formed composition
begins to
harden to a solid form in between approximately a few seconds to about 2
minutes.
[0092] By the term "solid form", it is meant that the hardened composition
will not
flow and will substantially retain its shape under moderate stress or pressure
or mere
gravity. The degree of hardness of the solid composition may range from that
of a fused
solid product which is relatively dense and hard, for example, like concrete,
to a
consistency characterized as being a hardened paste. In addition, the term
"solid" refers to
the state of the detergent composition under the expected conditions of
storage and use of
the solid detergent composition. In general, it is expected that the detergent
composition
29
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
will remain in solid form when exposed to temperatures of up to approximately
100 F and
particularly greater than approximately 120 F.
[0093] The resulting solid detergent composition may take forms including,
but
not limited to: an extruded, molded or formed solid pellet, block, tablet,
powder, granule,
flake; or the formed solid can thereafter be ground or formed into a powder,
granule, or
flake. In an embodiment of the invention, the solid detergent compositions are
not cast
solid products. In an exemplary embodiment, extruded pellet materials formed
by the
solidification matrix have a weight of between approximately 50 grams and
approximately
250 grams, extruded solids formed by the solidification matrix have a weight
of
approximately 100 grams or greater, and solid block detergents formed by the
solidification matrix have a mass of between approximately 0.25 and
approximately 10
kilograms. The solid compositions provide for a stabilized source of
functional materials.
In some embodiments, the solid composition may be dissolved, for example, in
an
aqueous or other medium, to create a concentrated and/or use solution. The
solution may
be directed to a storage reservoir for later use and/or dilution, or may be
applied directly to
a point of use.
[0094] In certain embodiments, the solid detergent composition is provided
in the
form of a unit dose. A unit dose refers to a solid detergent composition unit
sized so that
the entire unit is used during a single washing cycle. According to aspects of
the
invention, when the solid detergent composition is provided as a unit dose, it
is typically
provided as an extruded pellet, or a tablet having a size of between
approximately 1 gram
and approximately 250 grams.
[0095] In other embodiments, the solid detergent composition is provided in
the
form of a multiple-use solid, such as a block or a plurality of pellets, and
can be repeatedly
used to generate aqueous detergent compositions for multiple washing cycles.
In certain
embodiments, the solid detergent composition is provided as an extruded block,
or a tablet
having a mass of between approximately 5 grams and approximately 10 kilograms.
In
certain embodiments, a multiple-use form of the solid detergent composition
has a mass
between approximately 1 kilogram and approximately 10 kilograms. In further
embodiments, a multiple-use form of the solid detergent composition has a mass
of
between approximately 5 kilograms and about approximately 8 kilograms. In
other
embodiments, a multiple-use form of the solid detergent composition has a mass
of
between about approximately 5 grams and approximately 1 kilogram, or between
approximately 5 grams
and approximately 500 grams.
[0096] Although the detergent composition is discussed as being formed
into a solid product, the
detergent composition may also be provided in the form of a paste. When the
concentrate is provided in the
form of a paste, enough water is added to the detergent composition such that
complete solidification of the
detergent composition is precluded. In addition, dispersants and other
components may be incorporated
into the detergent composition in order to maintain a desired distribution of
components.
[0097] The various solidification matrices of the present invention may be
employed in a wide
variety of cleaning applications. In some aspects, the solid detergent
compositions of the invention are
suitable for use in any applications requiring an environmentally friendly,
solid alkaline detergent. Such
applications include, but are not limited to: phosphate-free alkaline
detergent use in combination ovens,
such as those used in various food service industries. Additional applications
may include, for example,
machine warewashing employing a ware wash detergent, presoaks, fryer boil
outs, power soak sinks and
related applications, soak tanks, instrument reprocessing, laundry and textile
cleaning and destaining, carpet
cleaning and destaining, vehicle cleaning and care applications, surface
cleaning and destaining, kitchen
and bath cleaning and destaining, floor cleaning and destaining, cleaning in
place operations, general
purpose cleaning and destaining, and/or industrial or household cleaners.
[0098] In preferred aspects, the solid detergent compositions are
particularly suited for cleaning
combination ovens. Various descriptions of combination ovens are disclosed,
for example, in U.S. Pat.
Nos. 5,368,008, 5,640,946, and 6,410,890, EP 0652405 and DE 2842771. For
example, combination ovens
may refer to apparatuses having a double oven- steamer, a double oven-boiler,
or having at least one oven
chamber and a steam generator and/or boiler. The solid detergent compositions
according to the invention
can be provided for cleaning of the combination oven apparatuses known in the
art.
[0099] In some aspects, the solid detergent compositions may be added
directly to a combination
oven apparatus, for example through a funnel or other member, as described and
depicted for example in
U.S. Pat. No. 5,640,946, instead of having to access the steamer and/or boiler
components of the
combination ovens. These and other inlet points for supplying a solid
detergent composition according to
the invention will be
31
CA 2895835 2017-09-13
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
readily ascertainable by those skilled in the art. In some aspects, the solid
detergent
compositions according to the invention may be initially used to generate an
aqueous
solution or suspension for delivery to a combination over for cleaning
according to the
invention. Thereafter, the liquid compositions are applied to the internal
surfaces of the
apparatus, such as for example, through the use of spray nozzles and/or spray
jets or the
like.
[00100] The methods of cleaning using the solid detergent compositions
according
to the invention may further include one or more rinse steps, decalcification
steps, a
prewash step, and/or a soak step.
EXAMPLES
[00101] The present invention is more particularly described in the
following
examples that are intended as illustrations only, since numerous modifications
and
variations within the scope of the present invention will be apparent to those
skilled in the
art. Unless otherwise noted, all parts, percentages, and ratios reported in
the following
examples are on a weight basis, and all reagents used in the examples were
obtained, or
are available, from the chemical suppliers described below, or may be
synthesized by
conventional techniques.
[00102] The following test method was used to characterize the compositions
produced in Examples 1, 2, and 3 and Comparative Examples A and B:
Dimensional Stability Test for Formed Products
[00103] Approximately 50 grams batch of the product using a polycarboxylic
acid
polymer as part of the solidification matrix was first pressed in a die at
approximately
1000 pounds per square inch (psi) for approximately 20 seconds to form
tablets. The
diameter and height of the tablets were measured and recorded. The tablets
were
maintained at room temperature for one day and then placed in an oven at a
temperature of
approximately 120 F. After the tablets were removed from the oven, the
diameters and
heights of the tablets were again measured and recorded. The growth exponent
was
determined for the tablets by measuring growth based on the cumulative change
in the
diameter and height of the tablet after heating.
Examples 1, 2, and 3 and Comparative Examples A and B
32
CA 02895835 2015-06-18
WO 2014/107460 PCT/US2013/078513
[00104] Examples 1, 2, and 3 are compositions of the present invention
using a
polycarboxylic acid polymer as part of a solidification matrix. In particular,
the
compositions of Examples 1, 2, and 3 used a polyacrylic acid polymer, a
modified
polyacrylic acid polymer, and a polymaleic acid polymer, respectively, as part
of the
solidification matrix. In addition, the compositions of Examples 1, 2, and 3
also included
component concentrations (in weight percent) of sodium carbonate (soda ash or
dense
ash), sodium bicarbonate, sodium metasilicate, a builder, surfactant,
defoamers, sodium
hydroxide, and water as provided in Table 1. The sodium carbonate, sodium
bicarbonate,
sodium metasilicate, builder, surfactant, and defoamers were premixed to form
a powder
premix and the polycarboxylic acid polymer, sodium hydroxide, and water were
premixed
to form a liquid premix. The powder premix and the liquid premix were then
mixed
together to form the composition. Approximately 50 grams of the composition
were
pressed into a tablet at approximately 1000 psi for approximately 20 seconds.
[00105] The composition of Comparative Example A was prepared as in
Examples
1. 2, and 3, except that the composition of Comparative Example A did not
include a
polycarboxylic acid polymer.
[00106] The composition of Comparative Example B was prepared as in Example
1
except for the addition of Trilon M Powder, which is a methylglycinediacetic
acid
(MGDA) powder. Table 1 provides the component concentrations for the
compositions of
Example 1, 2, and 3 and Comparative Example A. Table 2 provides the component
concentrations of Comparative Example B.
[00107] Table 1
Component Example 1 Example 2 Example 3 Comp.
Example A
Sodium carbonate, wt.% 55.76 56.76 57.33 58.19
Sodium bicarbonate, wt.% 2.88 2.88 2.88 2.88
Sodium metasilicate, wt.% 3 3 3 3
Builder, wt.% 20 20 20 20
Nonionic surfactant, wt.% 3.53 3.53 3.53 3.53
Defoamer, wt.% 1.06 1.06 1.06 1.06
Polyacrylic acid, wt.% 7.34 0 0 0
Modified polyacrylic acid, wt.% 0 9 0 0
Polymaleic acid, wt.% 0 0 7.05 0
Sodium hydroxide (50%), wt.% 2.66 0 2.37 0
Water, wt.% 3.77 3.77 2.78 11.34
33
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
[00108] Table 2
Component Comp.
Example B
Sodium carbonate, wt.% 43.52
Trilon M Powder, wt.% 13.24
Sodium bicarbonate, wt.% 2.88
Anhydrous metasilicate, wt.% 3.00
Builder, wt.% 20.00
Nonionic surfactant, wt.% 3.53
Defoamer, wt.% 1.06
Modified polyacrylic acid, wt.% 9.00
Water, wt.% 3.77
[00109] The compositions of Examples 1, 2, and 3 and Comparative Example A
were then subjected to the dimensional stability test for formed products, as
discussed
above, to observe the dimensional stability of the compositions after heating.
The results
are tabulated below in Table 3.
[00110] Table 3
Example Dimension Initial Post-heating % Growth
Example 1 Diameter. mm 44.69 44.96 0.6
Height, mm 20.64 20.87 1.1
Example 2 Diameter. mm 44.69 44.71 0
Height, mm 19.76 19.64 -0.6
Example 3 Diameter. mm 45.03 45.44 0.9
Height, mm 19.66 19.89 1.2
Comparative Diameter. mm 44.77 46 2.7
Example A Height, mm 19.38 20.96 8.2
[00111] As illustrated in Table 3, the formed products of the compositions
of
Examples 1, 2, and 3 exhibited considerably less swelling than the formed
product of the
composition of Comparative Example A. In particular, the product of the
composition of
Example 1 had only a 0.6% growth in diameter and a 1.1% growth in height
resulting in a
growth exponent of 1.7%. The product of the composition of Example 2 had a 0%
growth
in diameter and a -0.6% growth in height resulting in no positive growth
exponent. The
product of the composition of Example 3 only had a 0.9% growth in diameter and
a 1.2%
growth in height resulting in a growth exponent of 2.1%. By comparison, the
product of
the composition of Comparative Example A had a 2.7% growth in diameter and an
8.2%
growth in height resulting in a growth exponent of 10.9%.
34
CA 02895835 2015-06-18
WO 2014/107460 PCT/US2013/078513
[001 1 2] The only difference in the compositions of Examples 1, 2, and 3
and
Comparative Example A was the presence of a polycarboxylic acid polymer. It is
thus
believed that the polycarboxylic acid polymer aided in the dimensional
stability of the
products of the compositions of Example 1, Example 2, and Example 3. Because
the
composition of Comparative Example A did not contain a polycarboxylic acid
polymer,
the composition did not include a mechanism for controlling the movement of
water
within the solid product.
[00113] Six tablet samples of the composition of Comparative Example B were
also
tested for swelling. The diameter and height of each such tablet were measured
and
recorded. The tablets were maintained at room temperature for one day and then
placed in
an oven heated to a temperature of approximately 120 F. When the first tablet
was
removed from the oven, the tablet crumbled, indicating a lack of a
dimensionally stable
product. The remaining samples were successfully removed from the oven and the
diameter and height of each tablet were measured and recorded as set forth in
Table 4
below.
[00114] Table 4
Comparative Initial Post- % Total
Example C heating Growth Growth
Sample 2 Diameter 44.35 45.25 2.029 4.65
(mm)
Height (mm) 19.49 20.00 2.617
Sample 3 Diameter 44.23 45.20 2.193 5.29
(mm)
Height (mm) 19.04 19.63 3.099
Sample 4 Diameter 44.52 45.23 1.595 4.69
(mm)
Height (mm) 19.38 19.98 3.096
Sample 5 Diameter 44.38 45.17 1.780 4.65
(mm)
Height (mm) 19.22 19.75 2.758
Sample 6 Diameter 44.23 45.11 1.990 5.02
(mm)
Height (mm) 19.12 19.70 3.033
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
[00115] As can be seen by the results in Table 4, when subjected to a
temperature of
120 degrees Fahrenheit, each of Samples 1, 2, 3, 4, 5 and 6 including MGDA
were not
stable and/or exhibited growth of over 4.5%.
Dimensional Stability Test for Cast Products
[00116] Approximately 4000 grams batch of the product using a
polycarboxylic
acid polymer as part of the solidification matrix was first poured into a
capsule. The
diameter of the capsule was measured and recorded. The capsule was maintained
at room
temperature for one day, held in an oven at a temperature of approximately 104
F for two
days, and then returned to room temperature. After the capsule returned to
room
temperature, the diameter of the capsule was again measured and recorded. The
growth
exponent was determined for the capsules by measuring growth based on the
change in the
diameter after heating.
Examples 4, 5, and 6 and Comparative Example C
[00117] Examples 4, 5, and 6 are compositions of the present invention
using a
polycarboxylic acid polymer as a part of the solidification matrix. In
particular, the
composition of Example 4 used a polyacrylic acid polymer as part of the
solidification
matrix, the composition of Example 5 used a modified polyacrylic acid polymer
as part of
the solidification matrix, and the composition of Example 6 used polymaleic
acid polymer
as part of the solidification matrix. Each of the compositions of Examples 4.
5. and 6 also
included component concentrations (in weight percent) of softened water,
builder, water
conditioner, sodium hydroxide 50%, sodium carbonate (dense ash), anionic
surfactant, and
nonionic surfactant, as provided in Table 3. The liquids (softened water,
builder, water
conditioner, polycarboxylic acid polymer, and sodium hydroxide 50%) were
premixed in
order to form a liquid premix and the powders (sodium carbonate, anionic
surfactant, and
nonionic surfactant) were premixed in order to form a powder premix. The
liquid premix
and the powder premix were then mixed to form the composition, which was
subsequently
poured into capsules.
[00118] The composition of Comparative Example C was prepared as in
Examples
4. 5, and 6 except that the composition of Comparative Example C did not
contain a
polycarboxylic acid polymer but did contain the same quantity of available
water.
36
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
[00119] Table 5 provides the component concentrations for the compositions
of
Examples 4-6 and Comparative Example C.
[00120] Table 5
Component Example 4 Example 5 Example 6 Comp.
Example C
Water, softened, wt.% 22.49 22.5 20.49 24
Builder, wt.% 4 4 0 4
Water conditioner wt.% 3 3 3 3
Polyacrylic acid, wt.% 0 10 0 0
Modified polyacrylic acid, 10 0 0 0
wt.%
Polymaleic Acid, wt.% 0 0 10 0
NaOH, 50%, wt.% 0 0 3.4 0
Sodium carbonate, wt.% 55.51 55.5 58.12 63.64
Anionic surfactant, wt.% 1 1 1 1
Nonionic surfactant, wt.% 4 4 4 4
[00121] After the compositions of Examples 4, 5, and 6 and Comparative
Example
C were formed, they were subjected to the dimensional stability test for cast
products, as
discussed above, to observe the dimensional stability of the compositions
after heating.
The results are tabulated below in Table 6.
[00122] Table 6
Initial Post- % Growth
heating
Example 4 Diameter, mm 161 162 0.6
Example 5 Diameter, mm 159 161 1.3
Example 6 Diameter, mm 159 162 1.9
Comp. Example C Diameter, mm 162 170 4.9
[00123] As illustrated in Table 4, the cast products of the compositions of
Examples
4. 5, and 6 exhibited considerably less swelling than the cast product of the
composition of
Comparative Example C. In particular, the product of the composition of
Example 4
experienced only a 0.6% growth in diameter resulting in a 0.6% growth
exponent, the
product of Example 5 experienced only a 1.3% growth in diameter resulting in a
1.3%
growth exponent, and the product of the composition of Example 6 experienced
only a
1.9% growth in diameter resulting in a 1.9% growth exponent. By comparison,
the
37
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
product of the composition of Comparative Example C had a 4.9% growth in
diameter
resulting in a 4.9% growth exponent.
[00124] The only difference in the compositions of Examples 4, 5, and 6 and
Comparative Example C was the presence of a polycarboxylic acid polymer. It is
thus
believed that the polycarboxylic acid polymer aided in the dimensional
stability of the
products of the compositions of Examples 4, 5, and 6. By contrast, because the
composition of Comparative Example C did not contain a polycarboxylic acid
polymer,
the composition did not contain a mechanism for controlling the movement of
water
within the solid product.
Examples 7, 8 and 9
[00125] Examples 7, 8 and 9 compare cleaning performance when various
combinations of polymaleic acid and polyacrylic acid were utilized. The
composition of
each Example is set forth in Table 7. To form the compositions, the sodium
carbonate,
builder, surfactant, and disaccharide were premixed to form a powder premix
and the
polycarboxylic acid polymer, potassium hydroxide, phosphonate and water were
premixed
to form a liquid premix. The powder premix and the liquid premix were then
mixed
together to form the composition. Approximately 1000 grams of the composition
was
pressed into a tablet at approximately 1000 psi for approximately 20 seconds
and allowed
to solidify.
[00126] The resulting tablets were employed in an AM-14 automatic
dishwasher
machine dispensing 17 grain water. Glassware was then subjected to 100 wash
and rinse
cycles and tested for cleanliness. Cleanliness was measured in two ways.
First, a
luminosity value was determined by acquiring a digital optical image of the
glassware, and
then analyzing a luminosity value via computer analysis. The luminosity test
indicates the
degree of film present on the glass surface, with a lower value indicating
less film and a
cleaner glass. Second, a visual rating was measured on a 1 to 5 rating scale,
with a lower
visual rating indicating a cleaner glass. The results of these tests are set
forth in Table 8.
[00127] Table 7
Raw Material Example 7 Example 8 Example 9
Sodium carbonate 71.80 71.80 71.80
38
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
Builder 7.50 7.50 7.50
Nonionic surfactant 3.68 3.68 3.68
Nonionic surfactant 2.02 2.02 2.02
Water 9.39 7.61 8.39
Disaccharide 2.00 2.00 2.00
Polymaleic Acid (Belclene 200) 1.00 1.00 0.00
Polyacrylic acid 4500 0.90 0.00 0.90
Polyacrylic acid 11,000 0.96 0.00 0.96
Phosphonate 0.30 0.30 0.30
Potassium Hydroxide 0.45 0.45 0.45
100.00 96.36 98.00
1000 PPM 946 ppm 980 ppm
[00128] Table 8
Example 7 Example 8 Example 9
visual luminosity visual luminosity visual luminosity
Glass rating value rating value rating value
1 2.50 15610 3.00 17720 3.00 19653
2 2.00 14250 3.00 16752 3.00 19539
3 2.00 14664 3.00 16955 3.50 24913
4 2.00 15005 3.50 21742 3.50 20485
2.50 14949 3.00 16615 3.00 18191
6 2.50 15389 3.50 18392 3.00 18759
Plastic 2.5 N/A 2.5 N/A 4 N/A
6 Glass
Average: 2.29 14978 3.07 18029 3.29 20257
6 Glass Std.
Dev.: 0.27 490 0.35 1939 0.39 2413
4 Glass
Average: 2.13 14717 3.13 18016 3.25 20782
4 Glass Std.
Dev.: 0.25 345 0.25 2488 0.29 2910
39
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
[00129] The results
set forth in Table 8 indicate that the combination of polymaleic
acid and polyacrylic acid provides improved cleaning performance versus
polymaleic acid
or polyacrylic acid alone.
Examples 10-23
[00130] A series of experiments using different concentrations and sources
of water,
chelants, and binders were employed to evaluate the use of sodium hydroxide
alkalinity to
generate a solid alkaline detergent composition according to the objectives of
the
invention. Various formulas and mix instructions for making solid, phosphate-
free alkaline
detergent tablets were evaluated to provide methods for making the physically
stability
and durable cleaning compositions according to the invention. Table 9 shows
the
components evaluated including: potassium carbonate (alkaline builder); sodium
sulfate
(filler); sodium gluconate (chelant); disodium metasilicate (alkalinity
source, corrosion
inhibitor); sodium hydroxide (active cleaner, caustic); and PEG (binder).
[00131] Table 9
Component Examples
11 12 13 14 15 16
potassium carbonate. wt.% 28.33 30 35 33.33 30 35 35
sodium sulfate, wt.% 13.34 15 15 13.34 15 5 5
sodium gluconate, wt.% 10 10 10 10 10 10 10
disodium metasilicate, wt.% 15 15 15 15 15 15 15
Sodium hydroxide, wt.% 25 25 25 25 25 25 25
PEG 8000, wt.% 8.33 5 0 3.33 5 10 10
Total 100 100 100 100 100 100 100
Component Examples
17 18 19 20 21 22 23
potassium carbonate. wt.% 33.33 30 35 31.66 25 25 35
sodium sulfate, wt.% 8.34 10 15 11.67 15 15 10
sodium gluconate, wt.% 10 10 10 10 10 10 10
disodium metasilicate, wt.% 15 15 15 15 15 15 15
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
Sodium hydroxide, wt.% 25 25 25 25 25 25 25
PEG 8000, wt.% 8.33 10 0 6.67 10 10 5
Total 100 100 100 100 100 100 100
[00132] Good batch mixing and powder transfer was observed for Examples 10-
23.
Powder appearance varied from batch to batch and particle size uniformity was
dependent
upon content of polyethylene glycol (PEG). A visual evaluation showed that the
powders
not including PEG in its composition were most uniform, but agglomerate
formation
increases as the amount of PEG in the formula increases. Agglomerates are
formed when
the molten PEG was added to the batch during mixing.
[00133] Dimensional Stability Test for Extruded and Press Solids. Screening
was
done at 2500, 3000, and 4000 lbs. It was found that pressing at 4000 lbs.
produced the best
tablets. Tablets made at this load had good crisp edges, smooth surfaces and
high
compression strength. Tablets made at the other two loads had a more porous
surface and
weak edges. Tablet release from the pressing mold for all 14 runs was poor.
The pressing
head had to be washed free of residue after every 2-3 tablets pressed. For
example, if the
residue was not removed after the third tablet, then the fourth tablet would
not pull free of
the pressing head and could only be removed either by blunt force or
dissolving the tablet
with water.
[00134] Using PEG 8000 as the potential binder in the high alkaline formula
caused
the tablets to brown over time. The color stability was dependent on how much
PEG was
included in the formula. Examples 12 and 19 contained no PEG and showed no
discoloration over time. For all Examples that contained PEG 8000, browning
was
observed as early as 1 day in the 122 F chamber.
[00135] Dimensional Stability. Initial height, diameter, and visual
appearance were
recorded. Next the tablets were sealed in a plastic bag and placed inside two
different
environmental chambers for a week. The two environmental chambers used were
ambient
(72 F, 50% Relative Humidity) and 122 F (relative humidity NA). After one week
the
tablets were re-measured and the visual appearance recorded.
[00136] Procedure for Determining Compression Resistance of Extruded and
Pressed Products. One tablet from each batch was tested within three hours of
being
made. After the one week dimensional stability tests were complete the 122 F
tablets were
cooled to ambient conditions, and then compression strength was measured for
both set of
41
CA 02895835 2015-06-18
WO 2014/107460 PCT/US2013/078513
tablets. The formulations tested were not physically stable at 122 F. Tablets
swelled
between 4 % and 10%, and tablets containing both PEG 8000 and sodium hydroxide
turned brown. The results show that the compositions according to the
invention do not
include PEG as a preferred binder for the stable, phosphate-free alkaline oven
cleaner
tablet compositions.
Examples 24-29
[00137] Based on the results of Examples 10-23, alternative solidification
mechanisms, namely carbonate hydration, were further evaluated. Hydrate
potassium and
sodium carbonates were evaluated to determine effect on tablet stability using
different
chelants.
[00138] Table 10 shows the components evaluated including: potassium
carbonate /
potash (alkaline builder); sodium carbonate / ash (alkaline builder); sodium
citrate
(chelant, binder); sodium hydroxide (active cleaner, caustic, hydration
(50%)); water
(hydration); disodium metasilicate (alkalinity source, corrosion inhibitor);
and sodium
sulfate (filler).
[00139] Table 10
Component Examples
24 35 26 27 28 29
potassium carbonate. wt.% 25 25 0 0 25 25
Sodium carbonate, wt.% 0 0 25 25 0 0
sodium citrate, wt.% 10 10 10 10 10 10
Sodium hydroxide (50%), wt.% 5 7 5 7 0 0
water, wt.% 0 0 0 0 2.5 4
disodium metasilicate, wt.% 15 15 15 15 15 15
sodium sulfate, wt.% 20 18 20 18 22.5 21
Sodium hydroxide, wt.% 25 25 25 25 25 25
Total 100 100 100 100 100 100
[00140] The potassium carbonate, sodium carbonate and sodium citrate were
added
to the ribbon blender and mixed. The sodium hydroxide (50%) and water were
added and
42
CA 02895835 2015-06-18
WO 2014/107460 PCT/US2013/078513
mixed until the powder appeared dry. The disodium metasilicate, sodium sulfate
and
sodium hydroxide were added and mixed until the powder appeared uniform.
Examples
24-29 did not provide adequate mixing; powders were wet, clumpy, and hard to
get out of
the ribbon blender. Because of poor mixing the powders were not pressed into
tablets and
the experiment was terminated.
Examples 30-38
[00141] Based on the results of Examples 24-29, the use of sodium carbonate
in
place of potassium carbonate was evaluated. The use of sodium carbonate (dense
ash) was
evaluated in compositions to obtain a more physically stable tablet
formulation.
[00142] Table 11 shows the components evaluated including: sodium carbonate
(alkaline builder); sodium citrate (chelant, binder); citrate solution (33%);
sodium
hydroxide (active cleaner, caustic, hydration (50%)); water (hydration);
disodium
metasilicate (alkalinity source, corrosion inhibitor); and sodium sulfate
(filler).
[00143] Table 11
Component Examples
30 31 32 33 34 35 36 37 38
sodium carbonate, wt.% 25 45.5 47.5 46.5 45.5 47.5 44 25 25
sodium citrate, wt.% 10 10 10 10 7.75 8.75 10 10 10
Citrate/water sol (33%), 0 0 0 0 6.8 3.78 0 0 0
wt.%
water, wt.% 2.5 4.5 2.5 3.5 0 0 6 4.5 2.5
disodium metasilicate, 15 15 15 15 15 15 15 15 15
wt.%
sodium sulfate, wt.% 22.5 0 0 0 0 0 0 20.5 22.5
Sodium hydroxide, wt.% 25 25 25 25 25 25 25 25 25
Total 100 100 100 100 100 100 100 100 100
[00144] For examples 30-36, the sodium carbonate was added to the ribbon
blender
and mixed. The sodium citrate was slowly added. Then the water/citrate
solutions and
disodium metasilicate were mixed until the powder was uniform. Thereafter, 60
gram
43
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
tablets were pressed at 4000 pounds (approximately 1600 psi) and at 2500
pounds, holding
for 3 seconds using the 1.75" diameter tablet die and the Carver press. Then
the force
required to break a tablet was measured (higher force/pressure required
translates to a
more durable tablet). The tablets were then placed on dimensional stability
testing for one
week at room temperature and 122 F.
[00145] For examples 37-38, the sodium carbonate and sodium citrate was
added to
the ribbon blender and mixed. The water and disodium metasilicate were added
and mixed
until the powder was uniform. Then the 60 gram tablets were pressed at 4000
pounds
(approximately 1600 psi) and at 2500 pounds, holding for 3 seconds using the
1.75"
diameter tablet die and the Carver press. The force required to break a tablet
was measured
(higher force/pressure required translates to a more durable tablet). Then the
tablets were
then placed on dimensional stability testing for one week at room temperature
and 122 F.
[00146] Although the formulations tested were physically stable at 72 F,
the
formulations were not physically stable at 122 F. Tablets swelled between 9%
and 18%
and compression strengths were weak in all Examples 30-38. Example 33 showed
improved compression strength and dimensional stability compared to the other
examples,
however the formulation required additional improvements to the solidification
and
dimensional stability.
Examples 39- 42
[00147] Based on the results of Examples 30-38, the use of a different
grade of
sodium metasilicate was evaluated. Anhydrous sodium metasilicate was used in
formulations with potassium carbonate at a lower water level to assess
improvement of
solidification and dimensional stability. Table 12 shows the components
evaluated in the
Example compositions.
[00148] Table 12
Component Examples
39 40
potassium carbonate. wt.% 25% 150 a 48% 288 g
sodium citrate, wt.% 10% 60 g 10% 60 g
water, wt.% 1% 6 a 2% 12 g
44
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
disodium metasilicate, wt.% 15% 90 g 15% 90 g
sodium sulfate, wt.% 24% 144 a
Sodium hydroxide, wt.% 25% 150 a 25% 150 g
-
Total 100 600 g 100 600 g
Component Examples
41 42
sodium carbonate, wt.% 25% 150 a 46.5% 279 g
sodium citrate, wt.% 10% 60g 10% 60g
anhydrous metasilicate, wt.% 35.5% 213 0 15% 90 g
water, wt.% 405% 27 g 3.5% 21 g
sodium hydroxide, wt.% 25% 150g 25% 150g
Total 100 600 g 100 600 g
[00149] For examples 39-40, the potassium carbonate and sodium citrate were
added to the ribbon blender and mixed. The water was slowly added. Then the
disodium
metasilicate, sodium sulfate and sodium hydroxide were mixed until the powder
was
uniform. Thereafter, 25 gram tablets were pressed at 2000 pounds, holding for
3 seconds
using the 1.25" diameter tablet die and the Carver press. Then the force
required to break a
tablet was measured (higher force/pressure required translates to a more
durable tablet).
The tablets were then placed on dimensional stability testing for one week at
room
temperature and 122 F.
[00150] For examples 41-42, the sodium carbonate, sodium citrate and
anhydrous
metasilicate were added to the ribbon blender and mixed. The water was slowly
added.
Then the sodium hydroxide was mixed until the powder was uniform. Thereafter,
25 gram
tablets were pressed at 2000 pounds, holding for 3 seconds using the 1.25"
diameter tablet
die and the Carver press. Then the force required to break a tablet was
measured (higher
force/pressure required translates to a more durable tablet). The tablets were
then placed
on dimensional stability testing for one week at room temperature and 122 F.
[00151] As shown in FIGS. 1-2, the formulation of the table compositions
with
anhydrous sodium metasilicate generated stronger tablets with less swelling
than the prior
Examples using disodium metasilicate penta-hydrate. In addition, the Example
shows that
the use of potassium carbonate is capable of yielding dimensionally-stable
solid
CA 02895835 2015-06-18
WO 2014/107460
PCT/US2013/078513
compositions when the water charge employed during mixing is less than about
1%.
However, the additional benefits of using sodium carbonate, namely improved
cleaning
performance, result in its preferred use over the potassium carbonate.
Examples 43-46
[00152] Based on the results of Examples 39-42, the use of varying
concentrations
and sources of water to make a durable and physically stable tablet
composition were
evaluated. Table 13 shows the components evaluated in the Example
compositions.
[00153] Table 13
Component Examples
43 44 45 46
sodium carbonate, wt.% 40 37 37 42
d-gluconic acid, monosodium salt, wt.% 15 15 15 0
sodium citrate, wt.% 0 0 0 10
disodium metasilicate penta-hydrate, wt.% 10 10 0 0
anhydrous metasilicate, wt.% 0 0 10 10
water, wt.% 0 3 3 3
sodium polyacrylate, wt.% 5 5 5 5
sodium hydroxide, wt.% 30 30 30 30
Total 100 100 100 100
Total Water 4.2 7.2 3 4.2
[00154] For examples 43-46, the sodium carbonate, d-gluconic acid, sodium
citrate,
sodium metasilicate and/or anhydrous metasilicate were added to the ribbon
blender and
mixed. The water was slowly added. Then the sodium polyacrylate polymer and
sodium
hydroxide were mixed until the powder was uniform. Thereafter, 60 gram tablets
were
pressed at 4000 pounds (approximately 1600 psi) and at 2500 pounds, holding
for 3
seconds using the 1.75" diameter tablet die and the Carver press. Then the
force required
to break a tablet was measured (higher force/pressure required translates to a
more durable
tablet). The tablets were then placed on dimensional stability testing for one
week at room
temperature and 122 F.
46
[00155] As shown in FIGS. 3-4 the Example formulations that were mixed with
a liquid water
charge had higher compression strengths than the Example formulations that did
not have a free water
charge. Example 45 yielded the hardest tablets with the least amount of
swelling (indicating greatest
dimensional stability).
[00156] Although the present invention has been described with reference to
preferred
embodiments, workers skilled in the art will recognize that changes may be
made in form and detail
without departing from the spirit and scope of the invention.
[00157] Various modifications and additions can be made to the exemplary
embodiments
discussed without departing from the scope of the present invention. For
example, while the
embodiments described above refer to particular features, the scope of this
invention also includes
embodiments having different combinations of features and embodiments that do
not include all of the
above described features.
[00158] All publications and patent applications in this specification are
indicative of the level
of ordinary skill in the art to which this invention pertains.
47
CA 2895835 2017-09-13