Note: Descriptions are shown in the official language in which they were submitted.
PROCESS, METHOD, AND SYSTEM FOR REMOVING HEAVY METALS FROM
FLUIDS
TECHNICAL FIELD
[002] The invention relates generally to a process, method, and system for
removing
heavy metals such as mercury from liquid hydrocarbons.
BACKGROUND
[003] Heavy metals such as mercury can be present in trace amounts in all
types of
hydrocarbon streams such as crude oils. The amount can range from below the
analytical
detection limit to several thousand ppbw (parts per billion by weight)
depending on the
source. It is desirable to remove the trace amounts of these metals from crude
oils.
[004] Various methods to remove trace metal contaminants in liquid hydrocarbon
feed such as mercury have been disclosed.
[005] US Patent Nos. 6537443 and 6685824 disclose processes for removing
mercury, in which the liquid hydrocarbon feed is mixed with sulfur containing
compounds,
and removing the mercury-containing particulates in a pre-coated pressure
filter. A filtering
process is compact, but it may result in loss of hydrocarbons and waste in the
form of oily
solids. In US Patent Publication Nos. US20120067785A1, US20120067784A1,
US20120125816A1, reactive extraction methods are employed, wherein the liquid
hydrocarbon feed stream is brought into contact with additives including but
not limited to an
iodine source, tetrakis(hydroxymethyl)phosphonium sulfate /
tetrakis(hydroxymethyl)
phosphonium chloride, and oxidizing agents, respectively, wherein mercury is
extracted from
the crude oil into a water phase for subsequent removal.
[006] There is a need for improved methods and systems for the removal of
mercury
from liquid hydrocarbon steams, particularly a compact system maximizing oil
recovery and
using lower quantities of chemical reagents than in prior art methods.
Date recu/Date Received 2020-04-14
CA 02898232 2015-07-14
WO 2014/158810
PCT/US2014/020298
SUMMARY
[007] In one aspect, a method for reducing a trace element of mercury in a
crude oil
feedstock is provided. The method comprises the steps: passing the crude oil
feedstock
having a mercury concentration as feed to a filtration device having a filter
element to
generate a filtered crude having a reduced concentration of mercury and a
reject stream
containing crude oil having a concentrated mercury level of at least 10 times
the
concentration of mercury in the crude oil feed; mixing into the reject stream
an effective
amount of an extractive agent to remove a portion of the mercury for a treated
crude oil
having a reduced concentration of mercury.
[008] In one embodiment, the filtration device is a dead-end filter, and the
device is
back-flushed to generate the reject stream. In another embodiment, the device
is a cross-
flow filtration which generates a permeate stream comprising the filtered
crude, and the reject
stream comprising a retentate stream having a mercury concentration of at
least 20 times the
concentration of mercury in the crude oil feedstock.
[009] In another aspect, a method for removing a trace amount of mercury in
liquid
hydrocarbons is disclosed. The process comprises: passing the crude oil feed
through a
filtration device having a filtration element to retain at least 50% of the
mercury on the
filtration media and generate a filtered crude having a reduced concentration
of mercury;
back-flushing the filtration device with a portion of the filtered crude to
generate a reject
stream containing crude oil having a concentrated mercury level of at least 20
times the
concentration of mercury in the filtered crude; mixing into the reject stream
an effective
amount of an extractive agent selected from the group of
tetrakis(hydroxymethyl)
phosphonium sulfate; tetrakis(hydroxymethyl)phosphonium chloride; an oxidizing
agent; an
organic or inorganic sulfidic compound with at least one sulfur atom reactive
with mercury;
and combinations thereof to extract a portion of the mercury into a water
phase; and
separating the water phase containing the mercury from the crude oil for a
treated crude oil
having a reduced concentration of mercury.
[010] In one embodiment, the filtration device is a cross-flow filtration
device. In
another embodiment, the filtration device is a dead-end filtration device
having the filtration
element pre-coated with a filter aid material, e.g., materials including but
not limited to
pearlite, diatomite, cellulose fiber, and combinations thereof
[011] In another aspect, a method for removing a trace amount of mercury in
liquid
hydrocarbons is disclosed. The process comprises the steps of: passing the
crude oil feed
through a dead-end filtration device to retain at least 50% of the mercury on
the filtration
media and generate a filtered crude having a reduced concentration of mercury;
back-flushing
the filtration device with a portion of the filtered crude or other solvents
to generate a reject
stream having a concentrated mercury level of at least 20 times the
concentration of mercury
in the filtered crude; mixing into the reject stream an effective amount of a
reducing agent to
convert a portion of the mercury into a volatile form of mercury; and removing
a portion of
the volatile mercury by at least one of stripping, scrubbing, adsorption, and
combinations
thereof to obtain a treated crude oil having a reduced concentration of
mercury.
[011a] In accordance with another aspect, there is a method for reducing a
trace
element of mercury in a crude oil feedstock, comprising: passing the crude oil
feedstock
having a mercury concentration as feed to a filtration device having at least
a filter element to
generate a filtered crude having a reduced concentration of mercury and a
reject stream
containing crude oil having a concentrated mercury level of at least 10 times
the concentration
of mercury in the crude oil feed; mixing into the reject stream an effective
amount of an
extractive agent to remove at least a portion of the mercury for a treated
crude oil having a
reduced concentration of mercury; wherein the extractive agent is selected
from the group of
oxidizing agents; reducing agents, organic or inorganic sulfidic compounds
with at least one
sulfur atom reactive with mercury; tetrakis(hydroxymethyl)phosphonium sulfate;
tetrakis(hydroxymethyl) phosphonium chloride; and combinations thereof.
[011b] In accordance with a further aspect, there is a method for reducing a
trace
element of mercury in a crude oil feed, comprising: passing the crude oil feed
having a
mercury concentration through a filtration device having a filter element to
retain at least
50% of the mercury on the filter element and generate a filtered crude having
a reduced
concentration of mercury; back-flushing the filtration element with a portion
of the filtered
crude to generate a reject stream containing crude oil having a concentrated
mercury level of
at least 20 times the concentration of mercury in the crude oil feed; mixing
into the reject
stream an effective amount of a reducing agent to convert a portion of the
mercury into a
volatile mercury; removing a portion of the volatile mercury by one of
stripping, scrubbing,
3
Date Recue/Date Received 2022-04-05
adsorption, and combinations thereof to obtain a treated crude oil having a
reduced
concentration of mercury.
[011c] In accordance with a further aspect, there is a method for reducing a
trace
element of mercury in a crude oil feedstock, comprising: passing the crude oil
feedstock
.. having a mercury concentration as feed to a filtration device having at
least a filter element to
generate a filtered crude having a reduced concentration of mercury and a
reject stream
containing crude oil having a concentrated mercury level of at least 10 times
the concentration
of mercury in the crude oil feed; mixing into the reject stream an effective
amount of an
extractive agent to remove at least a portion of the mercury for a treated
crude oil having a
reduced concentration of mercury; and mixing a complexing agent into the
mixture of the
reject stream and the extractive agent, wherein the filtration device is
selected from: a dead-
end filtration device, a dynamic filtration device, a vibratory shear enhanced
processing filter,
and a cross-flow filter device, wherein the extractive agent is selected from
the group of
oxidizing agents; reducing agents, organic or inorganic sulfidic compounds
with at least one
.. sulfur atom reactive with mercury; tetrakis(hydroxymethyl)phosphonium
sulfate;
tetrakis(hydroxymethyl) phosphonium chloride; and combinations thereof, and
wherein the
complexing agent is selected from the group of thiol groups, thiophene groups,
thioether
groups, thiazole groups, thalocyanine groups, thiourenium groups, amino
groups,
polyethylene imine groups, hydrazido groups, N-thiocarbamoyl-polyalkylene
polyamino
.. groups, sulfides, ammonium thiosulfate, alkali metal thiosulfates, alkaline
earth metal
thiosulfates, iron thiosulfates, alkali metal dithionites, alkaline earth
metal dithionites,
polyamines, and mixtures thereof.
DRAWINGS
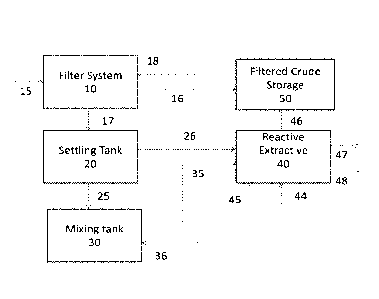
[012] Figure 1 is a block diagram of embodiments of a system and a process to
remove mercury from oily solids.
DETAILED DESCRIPTION
[013] The following terms will be used throughout the specification and will
have
the following meanings unless otherwise indicated.
3a
Date Recue/Date Received 2022-04-05
[014] "Crude oil" refers to both crude oil and condensate. Crude, crude oil,
crudes
and liquid hydrocarbons are used interchangeably and each is intended to
include both a
single crude and blends of crudes.
[015] "Trace amount" refers to the amount of mercury in the crude oil, which
varies
depending on the source, e.g., from a few ppb to up to 30,000 ppb.
[016] "Dead-end filtration" (conventional or normal filtration) refers to a
filter
system where substantially the entire liquid portion of the slurry, rather
than just a fraction, is
forced through the filter element, with most or all of the solids retained on
the filter element
as filter cake.
[017] "Cross-flow" filtration (or crossflow filtration or tangential flow
filtration
(TFF)) refers to a filtration technique in which the feed stream flows
parallel or tangentially
along the surface of the filter element (membrane) and the filtrate flows
across the filter
element, and typically only a portion of the liquid in the solids-containing
stream passes
through the filter element. In cross-flow filtration, solid material which is
smaller than the
3b
Date Recue/Date Received 2022-04-05
CA 02898232 2015-07-14
WO 2014/158810
PCT/US2014/020298
filter element pore size passes through (across) the element as permeate or
filtrate, and
everything else is retained on the feed side of the element as retentate or
concentrate.
[018] "Diafiltration" (DF) refers to a cross-flow filtration process wherein a
buffer
material, e.g., a solvent, is added into the feed stream and / or the
filtering process while
filtrate is removed continuously from the process.
[019] "Dynamic filtration" is an extension of cross-flow filtration, wherein
the filter
medium is kept essentially free from plugging or fouling by using rotary,
oscillating, or
vibratory motion of the filtration membrane relative to the feed slurry to
disrupt the formation
of cake layers adjacent to the filter medium. These results are accomplished
by moving the
material being filtered fast enough relative to the filtration medium to
produce high shear
rates as well as high lift forces on the particles.
[020] As used herein, the term cross-flow filtration (or filter) includes
diafiltration
and dynamic filtration techniques / apparatuses.
[021] Crudes may contain small amounts of mercury, which may be present as
elemental mercury Hg , ionic mercury, inorganic mercury compounds, and / or
organic
mercury compounds. Examples include but are not limited to: mercuric halides
(e.g., HgXY,
X and Y could be halides, oxygen, or halogen-oxides), mercurous halides (e.g.,
HgAY, X
and Y could be halides, oxygen, or halogen-oxides), mercuric oxides (e.g.,
Hg0), mercuric
sulfide (e.g., HgS, meta-cinnabar and/or cinnabar), mercuric sulfate (HgSO4),
mercurous
sulfate (Hg2SO4), mercury selenide (e.g., HgSe2, HgSe8, HgSe), mercury
hydroxides, and
organo-mercury compounds (e.g., alkyl mercury compounds) and mixtures of
thereof.
[022] The invention relates to the removal of trace mercury in crude oil in a
mercury
removal process comprising a filtration step and a reactive extraction step,
for a compact
system requiring less chemical reagents than in the prior art.
[023] Filtration Process Step: In one embodiment, the liquid hydrocarbon is
first
treated in a filtration process step, wherein a portion of mercury particulate
mercury and
solids containing adsorbed mercury are removed.
[024] In one embodiment, the system comprises a dead-end filtration device
selected
from the group of sand filter, multimedia filter, cartridge filter, bag
filter, employing a filter
element (membrane), employed in a form known in the art, e.g., cartridges,
screens, bags,
pleated filter, spiral wound filters, etc. As the crude is forced through the
filter element by
pressure drop, e.g., between 5 to 50 psig, solids as well as mercury
containing particulates
deposit on the filter element(s), resulting in a crude with a reduced
concentration of mercury.
4
CA 02898232 2015-07-14
WO 2014/158810
PCT/US2014/020298
[025] In one embodiment, the filter element is a stainless steel sintered
metal filter
with no pre-coating, having pore size ranges from 0.5 to 5 microns. In another
embodiment,
the filter element is pre-coated with a filter aid material known in the art,
e.g., pearlite,
diatomite (diatomaceous earth or "DE"), cellulose fiber, or combinations
thereof. The filter
aid material has a median particle size of 0.1 to 100 um and at a thickness of
at least 1 mm in
one embodiment; a median particle size ranging from 1 to 50 um in a second
embodiment;
and from 3 to 20 um in a third embodiment. In one embodiment, the filter aid
layer has a
thickness of 2-10 mm. In yet another embodiment, the filter aid layer has a
thickness of less
than 1" (2.54 cm). The filter aid material has a median particle size ranging
from Ito 50 p.m
in one embodiment; and from 3 to 20 p.m in a second embodiment.
[026] In another embodiment, the filter system comprises a cross-flow filter
device.
The cross-flow device is of the dynamic filtration type in one embodiment. In
a second
embodiment, the cross-flow filter device is of a vibratory shear enhanced
processing (VSEP)
filter type from New Logic Research, Inc. of Emeryville, CA and similar
devices from other
manufacturers. The cross-flow filter device separates a mercury containing
crude feed into
two streams, a first stream which passes through the filter membrane
containing crude with a
reduced mercury concentration ("permeate stream"), and a second stream
("retentate stream")
with the remainder of the crude feed, solids, and particulates, which does not
pass through the
filter membrane, having mercury concentration of at least 10 - 50 times the
mercury
concentration in the first stream.
[027] In one embodiment of a cross-flow filtration operation, a portion of the
retentate stream is recycled and combined with the liquid hydrocarbon feed to
the cross-flow
filter. The amount of the recycle stream in the recirculation loop can be
varied to allow
further concentration of the mercury in the reject (retentate) stream, provide
buffer from
process upsets, and control of the concentration in the reject stream for
further Hg removal
treatment. A portion of the retentate stream ranging from 1 to 25% of the
total stream can be
continuously or periodically purged from the cross-flow filtration process as
a reject stream,
allowing control of the amount of mercury and other matters from the system.
In one
embodiment, a portion the retentate stream equivalent to about 1-10% of the
feed to the
filtration system is purged for further treatment in the reactive extraction
process step.
[028] Any suitable filtration element (membrane) can be utilized in the
crossflow or
dead-end filtration assembly. In one embodiment, the filter element comprises
a porous
material which permits crude oil and solids below a certain size to flow
through as the
5
CA 02898232 2015-07-14
WO 2014/158810
PCT/US2014/020298
filtrate (or permeate) while retaining particles, including mercury-containing
particles, in the
retentate. The filter membrane is of sufficient nominal pore size for at least
50% of the
crude to pass through in one embodiment; at least 60% in a second embodiment;
at least 70%
in a third embodiment; and at least 80% in a fourth embodiment. The filter
membrane has a
pore size of 0.1 -50 um in one embodiment; of 0.5 -20 um in a second
embodiment; and at
least 1 um in a third embodiment.
[029] Polymers, organic materials, inorganic ceramic materials, and metals are
suitable for use as construction materials for the membrane in the cross-flow
filtration device,
or the filter element in the dead-end filtration device, as long as it does
not undergo
significant chemical changes to substantially impair the desired properties of
the filtered
crude. In one embodiment, the material is an inorganic material such as a
ceramic (silicon
carbide, zirconium oxide, titanium oxide, etc.) having the ability to
withstand harsh
environments. In another embodiment, the material is a metal such as stainless
steel,
titanium, or nickel-copper alloy.
[030] Over time, filtration becomes more difficult as pressure builds up
across the
filter apparatus with the filter element being clogged up with particulates.
The filter is
periodically (or whenever needed as clogged) back-flushed to remove oily
solids, which
comprise filtered particulates and pre-coated filter aid material (if any was
applied). In one
embodiment, the back-flushing is carried out by reversing the flow direction
of the filtrate
stream to force oily solids off the membrane / screen, generating a reject
stream. In another
embodiment, the trans-membrane pressure is periodically inverted by the use of
a secondary
pump. In one embodiment, the filter device is back-flushed with a fluid to
force the filtered
particulates and filter aid materials (if any was applied) off the filter
element and out of the
filter system. This back flushing also forces a portion of the hyrocarbon
liquids out of the
filter system with the solids as a reject stream.
[031] In one embodiment, a gas, e.g., methane, nitrogen, carbon dioxide, etc.,
is used
for the back-flushing. In another embodiment, in addition to or in place of
using a gas, the
filtered crude or a solvent (or a mixture thereof) is used to extract the oily
solids. The
extraction solvent is a light specific gravity solvent or solvent mixtures,
such as, for example,
xylene, benzene, toluene, kerosene, reformate (light aromatics), light
naphtha, heavy naphtha,
light cycle oil (LCO), medium cycle oil (MCO), propane, diesel boiling range
material,
which is used to "wash" the filter membrane / screen / filter aid and remove
the oily solids,
generating a reject stream.
6
[032] In one embodiment of a cross-flow filtration operation, instead of or in
addition to periodic back-flushing with a gas, the filtered crude, or an
extracting solvent, a
small amount of the solvent is optionally added to the feed stream to be
filtered, with the
weight ratio of the solvent being slowing increasing overtime to facilitate
the filtration
operation or decreasing the frequency of back-flushing. The solvent feed is
added in a
weight ratio of solvent to feed of 0 at the start of the filtering operation,
to 10:1 toward the
end of the operation as the pressure begins to build up as the membrane
becomes clogged.
[033] In one embodiment, the filter device comprises a plurality of filter
elements
with means within the assembly for back-flushing at least one of the filter
screens /
membranes without interrupting the operation while the device is on-stream,
with the back-
flushed device being isolated from the crude feed. In yet another embodiment,
the filter
device is of a clean-in-place (CIP) type known in the art, with accessory
pumps, holding
tanks, and the like supplying solvents and / or reactive agents such as sodium
hypochlorite
and sulfidic compounds to alleviate fouling and pressure build-up in the
filtration system.
[034] Descriptions and operations of filter devices that can be used in the
filtration
process step include and are not limited to US patent publications
US20120132597A1 titled
"Cross-flow filtration with turbulence and back-flushing action for use with
online chemical
monitors," US8128829 titled "Cross flow filter device," US3994810 titled
"Onstream back-
flush filter," and US5587074 titled "Fluid filter with enhanced back-flush
flow," US6322698
titled "Vibratory separation systems and membrane separation units".
[035] In one embodiment and in addition to filtration, the liquid hydrocarbon
is
optionally treated with an organic or inorganic sulfidic compound with at
least one sulfur
atom reactive with mercury as disclosed in US Patent Nos. 6537443 and 6685824.
In one
embodiment, the sulfidic compound when dissolved in water yields S2-, SH-, Sx2-
, or Sx11-
anions, and a solution with a pH greater than 7. Examplary sulfidic compounds
include but
are not limited to potassium or sodium sulfide (Na2S), sodium hydrosulfide
(NaSH),
potassium or sodium polysulfide (Na2Sx), ammonium sulfide [(NH4)2S], ammonium
hydrosulfide (NH4HS), ammonium polysulfide [(NH4)2Sx], Group 1 and Group 2
counterparts of these materials, and combinations thereof. The treating
sulfidic compound is
added for a concentration of 1.0 and about 10000 ppbw in one embodiment; and
about 5.0
ppbw and about 1000 ppbw in a second embodiment.
7
Date recu/Date Received 2020-04-14
CA 02898232 2015-07-14
WO 2014/158810
PCT/US2014/020298
[036] In one embodiment, the sulfidic treatment is in-situ in the filtering
operation
with the use of filter aid materials pretreated or coated with the organic or
inorganic sulfidic
compound. In another embodiment, the crude feed is mixed with the sulfidic
compound
prior to the filter operation, in an in-line static mixer or a mixing tank
with a residence time
of at least 1 minute, wherein any mercury precipitate formed is removed in the
filtration step.
In another embodiment, the mixing time is at least 15 minutes.
[037] Depending on the initial concentration of mercury in the liquid
hydrocarbon
feed, the filtration step results in two streams, a first stream for further
mercury removal
("reject stream") containing optional extract solvent, oily solids, and less
than 10 vol. % of
the original crude feed with a mercury concentration of much higher than in
the original
crude feed; and a second stream with filtered crude containing at least 90
vol. % of the
original crude feed, for further processing or sale.
[038] The reject stream has a mercury concentration of at least 20 times the
concentration of mercury in the filtered crude in one embodiment; at least 50
times in a
second embodiment; at least 100 times in a third embodiment; and at least 1000
times in a
fourth embodiment. The first stream has a mercury concentration of at least 5
times the
mercury concentration in the original crude feed in one embodiment; at least
10 times in a
second embodiment; and at least 100 times in a third embodiment.
[039] The filtered crude stream has a reduced mercury concentration of less
than
1000 ppbw in one embodiment; less than 500 ppbw in a second embodiment; less
than 300
ppbw n a third embodiment; less than 100 ppbw in a third embodiment; and less
than 50
ppbw in a fourth embodiment. With optional treatment with a sulfidic compound,
the
mercury in the filtered crude is reduced to less than 100 ppbw in one
embodiment; less than
75 ppbw in a second embodiment; and less than 50 ppbw in a third embodiment.
[040] Reactive Extraction Process Step: The reject stream, i.e., the crude
with a
concentrated mercury level is further treated with chemical reagents to lower
its mercury
level. In the reactive extraction process, the reject stream is brought into
contact with one or
more extractive agents selected from the group of
tetrakis(hydroxymethyl)phosphonium
sulfate; tetrakis(hydroxymethyl)phosphonium chloride; an oxidizing agent; an
organic or
inorganic sulfidic compound with at least one sulfur atom reactive with
mercury; and
combinations thereof. In one embodiment, a solvent such as water may also be
added along
with the extractive agent. The extractive agent extracts a portion of mercury
into the water
phase for subsequent removal in a phase separation process step. At least 50%
of the
8
CA 02898232 2015-07-14
WO 2014/158810
PCT/US2014/020298
mercury is extracted from the crude oil into the water phase in one
embodiment; at least 75%
extraction in a second embodiment; at least 90% extraction in a third
embodiment.
[041] In another embodiment, the crude is treated with a reducing agent
("reductant") as an extractive agent, wherein the reductant coverts at least
25% of the non-
volatile mercury portion of the mercury to a volatile (strippable) form. The
mercury is then
removed from the crude via stripping with a stripping gas known in the art,
e.g., natural gas,
methane, nitrogen, or combinations thereof
[042] The extractive agent can be employed in any form of a liquid, a powder,
slurry, aqueous form, a gas, a material on a support, or combinations thereof.
Different
.. extractive agents can be added, e.g., in one embodiment after the addition
of an oxidant, a
reducing agent is added. In another embodiment, the crude is brought into
contact directly
with a reducing agent without any oxidant addition.
[043] The amount of extractive agent needed for mercury removal is at least
equal to
the amount of mercury to be removed on a molar basis (1:1), if not in an
excess amount. In
.. one embodiment, the molar ratio ranges from 2:1 to 5,000:1. In another
embodiment, from
10:1 to 2,500:1. In yet another embodiment, the molar ratio ranges from 5:1 to
10,000:1.
[044] The contact with the extractive agent can be at any temperature that is
sufficiently high enough for the crude to be liquid. The contact is at room
temperature in one
embodiment; at a sufficiently elevated temperature, e.g., at least 50 C, in
another
embodiment; for at least a minute in one embodiment; at least 1 hr in another
embodiment;
and at least 2 hrs. in yet another embodiment.
[045] The contact between the reject stream with concentrated mercury level
and the
extractive agent can be either via a non-dispersive or dispersive method. The
dispersive
contacting method can be via mixing valves, static mixers or mixing tanks or
vessels, or other
methods known in the art. The non-dispersive method can be any of packed inert
particle
beds, fiber film contactors, or other method known in the art.
[046] In one embodiment, the extractive agent is an organic or inorganic
sulfidic
compound, which converts or extracts non-volatile mercury from the crude oil
to a water-
soluble form. The reactive extractive agent can be the same or different
sulfur compound
used in the filtration process (if any was used). Examples include but are not
limited to alkali
metal sulfides, alkaline earth metal sulfides, alkali metal polysulfides,
alkaline earth metal
polysulfides, alkali metal trithiocarbonates, dithiocarbamates, either in the
monomeric or
polymeric form, sulfurized olefins, mercaptans, thiophenes, thiophenols, mono
and dithio
9
CA 02898232 2015-07-14
WO 2014/158810
PCT/US2014/020298
organic acids, and mono and dithioesters, and mixtures thereof. In one
embodiment, the
sulfidic compound is water-soluble monatomic sulfur compound, e.g., any of
sodium
hydrosulfide, potassium hydrosulfide, ammonium hydrosulfide, sodium sulfide,
potassium
sulfide, calcium sulfide, magnesium sulfide, and ammonium sulfide.
[047] In another embodiment, the extractive agent is an oxidizing agent
("oxidant")
to extract mercury from the crude oil forming a soluble mercury compound. The
oxidant in
one embodiment is selected from the group of iodine sources, oxyhalites,
hydroperoxides,
organic peroxides, inorganic peracids and salts thereof, organic peracids and
salts thereof,
ozone, and combinations thereof. In one embodiment, the oxidant is selected
from the group
of elemental halogens or halogen containing compounds, e.g., chlorine, iodine,
fluorine or
bromine, alkali metal salts of halogens, e.g., halides, chlorine dioxide, etc.
In another
embodiment, the oxidant is an iodide of a heavy metal cation. In yet another
embodiment,
the oxidant is selected from ammonium iodide, an alkaline metal iodide, and
etheylenediamine dihydroiodide. In one embodiment, the oxidant is selected
from the group
.. of hypochlorite ions pa such as Na0C1, Na0C12, Na0C13, Na0C14, Ca(0C1)2,
NaC103,
NaC102, etc.), vanadium oxytrichloride, Fenton's reagent, hypobromite ions,
chlorine
dioxine, iodate 103- (such as potassium iodate KI03 and sodium iodate NaI03),
and mixtures
thereof. In one embodiment, the oxidant is selected from KMn04, K2S208,
K2Cr07, and C12.
[048] In one embodiment, the extractive agent is a reducing agent
("reductant"),
which can be added as the only extracting agent. In another embodiment, the
reducing agent
is added in addition to the oxidizing agent (and other optional reagents such
as demulsifiers)
for a portion of the mercury to be converted from a non-volatile to a volatile
form. The
oxidant / reductant can be introduced continuously, e.g., in a water stream
being brought into
contact continuously with a crude oil stream, or intermittently, e.g.,
injection of a water
.. stream batch-wise.
[049] Examples of reducing agents include but are not limited to reduced
sulfur
compounds contain at least one sulfur atom in an oxidation state less than +6.
(e.g., sodium
thiosulfate, sodium or potassium bisulfite, metabisulfite, or sulfite);
ferrous and ferric
compounds include inorganic and organic ferrous compounds; stannous compounds
which
include inorganic stannous compounds and organic stannous compounds; oxalates
which
include oxalic acid, inorganic oxalates and organic oxalates; cuprous
compounds include
inorganic and organic cuprous compounds; organic acids decompose to form CO2
upon
heating and act as reducing agents; nitrogen compounds include hydroxylamine
compounds
CA 02898232 2015-07-14
WO 2014/158810
PCT/US2014/020298
and hydrazine; sodium borohydride; diisobutylaluminium hydride (DIBAL-H);
thiourea; a
transition metal halide such as ferric chloride, zinc chloride, NiC12; SO2 in
N2 or other inert
gases, hydrogen; hydrogen sulfide; and hydrocarbons such as CO2 and carbon
monoxide.
[050] After the addition of an extractive agent that converts some of the
mercury in
the concentrated crude to a soluble form, e.g., iodine source or an oxidant,
the treated crude
having a reduced concentration of mercury can be separated from the aqueous
phase
containing the extracted mercury by methods known in the art, e.g., gravity
settling,
coalescing, etc., using separation devices such as centrifuges, hydrocyclones,
separators,
mesh coalescer etc.
[051] In one embodiment, the removal of mercury from the treated crude can be
enhanced with the addition of a complexing agent to the oil-water emulsion
mixture, added in
a sufficient amount to effectively stabilize (forming complexes with) the
soluble mercury.
This amount as expressed as molar ratio of complexing agent to soluble mercury
ranges from
1:1 to 5,000:1 in one embodiment; from 5:1 to 1000:1 in a second embodiment;
and 10:10 to
500:1 in a third embodiment. Mercury forms coordination complexes with
compounds
including but not limited to oxygen, sulfur, phosphorous and nitrogen
containing compound,
e.g., thiol groups, thiophene groups, thioether groups, thiazole groups,
thalocyanine groups,
thiourenium groups, amino groups, polyethylene imine groups, hydrazido groups,
N-
thiocarbamoyl-polyalkylene polyamino groups, derivatives thereof, and mixtures
thereof. Tn
another embodiment, the complexing agent is an inorganic sulfur compound
selected from
sulfides, ammonium thiosulfate, alkali metal thiosulfates, alkaline earth
metal thiosulfates,
iron thiosulfates, alkali metal dithionites, and alkaline earth metal
dithionites, and mixtures
thereof. In yet another embodiment, the complexing agent is a polyamine for
forming stable
cationic complexes with mercury ions.
[052] In one embodiment with the use of a reductant as a extractive agent, the
volatile mercury is stripped from the treated crude oil using methods and
equipment known in
the art, e.g., a stripping unit, an adsorption bed, etc. In one embodiment,
the crude oil is sent
to a stripping unit with the addition of a stripping (carrier) gas for the
removal of the volatile
mercury from the crude into the stripping gas. The crude removed from the
bottom of the
unit contains less than 50% of the mercury originally in the crude (both
volatile and non-
volatile forms) in one embodiment.
[053] The treated crude oil can be combined with the filtered crude oil to
form a
combined crude oil product stream having a reduced concentration of mercury,
e.g., less than
11
100 ppbw in one embodiment. The combined crude oil product stream in one
embodiment is
at least 95% volume of the crude oil feedstock to the filtration unit; and at
least 98 vol. % in a
second embodiment.
[054] Stripping of Volatile Mercury: In one embodiment, with the conversion of
a
portion the mercury from a non-volatile to a volatile form, the volatile
mercury is stripped
from the reject stream while it is in contact with the extracting agents,
e.g., oxidant and / or
reductant, with a stripping (carrier) gas. In another embodiment, the volatile
mercury is
removed from the treated crude using methods and equipment known in the art,
e.g., a
stripping unit, an adsorption bed, etc.
[055] After treatment with the extractive agents, the concentration of mercury
in the
treated crude oil is reduced to 100 ppbw or less in one embodiment; 50 ppbw or
less in a
second embodiment; 20 ppbw or less in a third embodiment; and less than 10
ppbw in a fourth
embodiment. In yet another embodiment, at least 75% of the mercury is
extracted from the
crude oil in the reject stream. In another embodiment, the removal or the
reduction is at least
90%
[056] Examples of extractive agents and methods for mercury removal using
extractive agents are disclosed in US Patent Publication Nos. US20120125816A1,
US20120125817A1, US20120125818A1, US20120067784A1, US20120067785A1,
U520120067786A1, and U520120067779A1.
[057] Figure Illustrating Embodiments: Reference will be made to Figure 1 for
a
diagram schematically illustrating various embodiments of a system for
removing mercury
from oily solids.
[058] In Figure 1, a crude oil stream containing mercury 15 is sent to
filtration
system 10, which in one embodiment is a bank of filter elements in the form of
dead-end
filtration or cross- flow filtration. In one embodiment, a gas stream 18 is
used for the back-
flushing of the filter element. In another embodiment, an extraction solvent
stream is used
for the back-flushing instead of or in addition to the gas stream 18. Although
not shown, in
one embodiment, the filtration system includes a recirculation loop with one
or more
recirculation pumps for the recycling of the retentate stream, with a portion
of the retentate
stream being purged from the recycled retentate stream continuously or
periodically to form
the reject stream for further treatment. The filtered crude 16 with a reduced
concentration of
mercury is sent to storage tank 50 for sale or further treatment. The reject
stream 17
12
Date recu/Date Received 2020-04-14
CA 02898232 2015-07-14
WO 2014/158810
PCT/US2014/020298
containing the back-flushed crude and /or the purged portion of the retentate
stream is sent to
settling tank 20. The reject stream 17 has a mercury concentration of 2-50
times the
concentration of mercury in the feed stream 15.
[059] In one embodiment of an oxidation-complexation process for the removal
of
mercury (as shown in dotted lines), at least an oxidizing agent 36 is added to
the reject stream
25 in a mixing tank 30, and the mixture of oxidizing agent and crude oil 35 is
directed to the
reactive extraction process step 40, with the addition of an aqueous stream
containing
reducing / complexing reagent 45. Waste water 47 containing mercury is sent to
disposal or
re-injected into a reservoir, and crude 46 with reduced mercury content is
sent to storage 50.
[060] In another embodiment with the use of direct reduction for the removal
of
mercury (solid lines), from the settling tank 20, stream 26 containing back-
flushed crude and
/ or purged retentate stream is directed to the reactive extraction process
step 40, wherein at
least an aqueous stream containing a reducing agent 45 is added for the
conversion wherein a
portion of non-volatile mercury is converted to volatile strippable mercury.
In one
embodiment, a stripping gas 44, e.g., N2, CO2, H2, methane, argon, helium,
steam, natural gas,
and combinations thereof is employed to remove the volatile mercury. From this
process
step, gas stream 48 containing mercury is sent to disposal, re-injected into a
reservoir or
treated with an adsorbent material by methods known in the art for mercury
removal from gas
streams. Crude 46 with reduced mercury content is sent to storage 50.
[061] In a third embodiment of a sulfidic extraction process for the removal
of
mercury (as shown in dotted lines), an aqueous stream 45' containing an
inorganic sulfidic
compound is added to the extraction step 40 for the conversion of or
extraction of non-
volatile mercury from the crude oil stream 26 to a water-soluble form. Waste
water 47
containing water-soluble mercury is sent to disposal or re-injected into a
reservoir, and crude
46 with reduced mercury content is sent to storage 50.
[062] The system as illustrated can be any of a mobile unit, located on-shore
such as
in a refinery, or off-shore on a facility such as an FPSO or other offshore
facility for the
production of oil and/or gas.
[063] EXAMPLES: The illustrative examples are intended to be non-limiting.
[064] Examples 1 - 2: Different 50 API crude and 55 API natural gas
condensate
samples with starting Hg concentration ranging from 588 to 2200 ppbw are
processed using
cross-flow filtration conducted at 175 C and 75 psig, employing a Teflon on
Woven
Fiberglass membrane having a pore size of 1 i.tm. The retentate is recycled
back to the filter
13
CA 02898232 2015-07-14
WO 2014/158810
PCT/US2014/020298
system in a recirculation loop with the use of a recirculation pump to combine
with the feed
to the system. The recirculation pump also maintains a sufficient velocity
through the tubes
of the filter housing (greater than 10 feet/second) to avoid membrane fouling.
A portion of
the retentate in an amount of about 2-10% the feed to filtration system is
continuously
purged from the system. The filtered products are expected to have a mercury
concentration
of less than 100 ppbw. The purged retentate is expected to have a
concentration of 10-50
times the mercury concentration of the feed to the filter system.
[065] Example 3: The filtration in Examples 1-2 continues until there is a
substantial pressure build-up, e.g., going from 10-15 psi at the beginning to
25-30psi. The
filter element is back-flushed with nitrogen, along with a small amount of the
filtered oil.
The back-flushed oil samples are placed into centrifuge tubes, shaken by hand
vigorously for
about 2 minutes. The back-flushed oil samples are expected to have a
concentrated mercury
level of at least 10,000 ppwb, if not at least 50,000 ppbw.
[066] Example 4: Various samples of 50 mL of the back-flushed oil with
concentrated mercury level in Example 3 are combined with the purged retentate
streams, and
added to a number of 10mL Teflon-capped centrifuge tubes. Different oxidants
are as shown
in Table 2. The tubes are shaken vigorously for about 2 minutes. 5 mL of
distilled water is
added to tube. A pre-determined volume of TETREN as complexing agent is added
for a
final concentration of 300M. Tubes are again shaken by hand vigorously for
about 2
minutes, then centrifuged for 1 minute to separate oil from water. Aliquots of
both oil and
water from each are analyzed for mercury with resulting concentrations as
listed in Table 2.
It is expected that the mercury removal efficiency is as previously obtained
in US Patent
Publication No. 20120125817.
[067] Table 2
No. Oxidant Dosage Hg in oil Hg in water Hg
ppbw ppb ppb removal %
1 None ¨ control >10,000 <1000 3.7
2 Iodine 1000 <100 > 1000 >90
3 Sodium polysulfide 29,000 <100 > 1000 > 90
4 OxoneTM 7260 <150 > 1000 > 80
5 Iodine 7260 <150 >1000 >80
[068] For the avoidance of doubt, the present application includes the subject-
matter
defined in the following numbered paragraphs:
14
CA 02898232 2015-07-14
WO 2014/158810
PCT/US2014/020298
[069] Paragraph 1. A method for reducing a trace element of mercury in a crude
oil
feedstock, comprising: passing the crude oil feedstock having a mercury
concentration as
feed to a filtration device having at least a filter element to generate a
filtered crude having a
reduced concentration of mercury and a reject stream containing crude oil
having a
concentrated mercury level of at least 10 times the concentration of mercury
in the crude oil
feed; mixing into the reject stream an effective amount of an extractive agent
to remove at
least a portion of the mercury for a treated crude oil having a reduced
concentration of
mercury.
[070] Paragraph 2. The method of Paragraph 1, where the treated crude oil is
combined with the filtered crude oil to form a combined product stream having
a mercury
concentration of less than 100 ppbw.
[071] Paragraph 3. The method of Paragraph 2, wherein the combined product
stream is at least 98 vol. % of the crude oil feedstock.
[072] Paragraph 4. The method of Paragraph 1, wherein the extractive agent is
selected from the group of oxidizing agents; reducing agents, organic or
inorganic sulfidic
compounds with at least one sulfur atom reactive with mercury;
tetrakis(hydroxymethyl)
phosphonium sulfate; tetrakis(hydroxymetbyl)phosphonium chloride; and
combinations
thereof.
[073] Paragraph 5. The method of Paragraph 1, wherein the extractive agent
extracts a portion of the mercury into a water phase, and wherein the method
further
comprises: separating the water phase containing the mercury from the crude
oil for the
treated crude oil to have a concentration of mercury of less than 100 ppbw.
[074] Paragraph 6. The method of Paragraph 1, wherein the filtration device is
periodically back-flushed to generate the reject stream.
[075] Paragraph 7. The method of Paragraph 6, wherein the filtration device is
back-flushed with any of: an extraction solvent; a portion of the filtered
crude; a gas selected
from methane, nitrogen, carbon dioxide; and combinations thereof to generate
the reject
stream.
[076] Paragraph 8. The method of Paragraph 1, wherein the filtration device is
a
dead-end filtration device and wherein at least 50% of the mercury is retained
on the filter
element.
[077] Paragraph 9. The method of Paragraph 8, wherein the filter element is
pre-
coated with a filter aid material.
CA 02898232 2015-07-14
WO 2014/158810
PCT/US2014/020298
[078] Paragraph 10. The method of Paragraph 9, wherein the filter aid material
has a
median particle size of 0.1 to 100 gm and the filter aid pre-coat is at least
1 mm thick.
[079] Paragraph 11. The method of Paragraph 9, wherein the filter aid material
has a
median particle size of 3 to 20 gm and the filter aid pre-coat has a thickness
of 2-10 mm.
[080] Paragraph 12. The method of Paragraph 9, wherein the filter aid material
is
selected from pearlite, diatomite, cellulose fiber, and combinations thereof.
[081] Paragraph 13. The method of Paragraph 9, wherein the filter aid material
is
diatomite pretreated with an organic or inorganic sulfidic compound with at
least one sulfur
atom reactive with mercury.
[082] Paragraph 14. The method of Paragraph 1, wherein the filtration device
is a
cross-flow filter device.
[083] Paragraph 15. The method of Paragraph 14, wherein at least a portion of
the
retentate stream is purged to generate the reject stream.
[084] Paragraph 16. The method of Paragraph 14, wherein the cross-flow
filtration
device generates a permeate stream comprising the filtered crude having a
reduced
concentration of mercury, and a retentate stream having a mercury
concentration of at least
10 times the first concentration of mercury.
[085] Paragraph 17. The method of Paragraph 14, wherein a portion of the
retentate
stream is recycled in a recirculation loop and combined with the crude oil
feedstock as feed to
the filtration device.
[086] Paragraph 18. The method of Paragraph 14, wherein the permeate stream
has
a reduced concentration of mercury of less than 100 ppbw.
[087] Paragraph 19. The method of Paragraph 14, wherein the cross-flow
filtration
device is periodically back-flushed with an extraction solvent to generate a
back-flushed
stream, and wherein the back-flushed stream is added to the retentate stream
to generate the
reject stream.
[088] Paragraph 20. The method of Paragraph 1, wherein the filtration device
is a
dynamic filtration device.
[089] Paragraph 21. The method of Paragraph 20, wherein the filtration device
is a
vibratory shear enhanced processing filter.
[090] Paragraph 22. The method of Paragraph 1, wherein the extractive agent is
an
organic or inorganic sulfidic compound selected from the group of alkali metal
sulfides,
alkaline earth metal sulfides, alkali metal polysulfides, alkaline earth metal
polysulfides,
16
CA 02898232 2015-07-14
WO 2014/158810
PCT/US2014/020298
alkali metal trithiocarbonates, dithiocarbamates, either in the monomeric or
polymeric form,
sulfurized olefins, mercaptans, thiophenes, thiophenols, mono and dithio
organic acids, and
mono and dithioesters, and mixtures thereof.
[091] Paragraph 23. The method of Paragraph 1, wherein the extractive agent is
a
water-soluble monatomic sulfur compound selected from the group of sodium
hydrosulfide,
potassium hydrosulfide, ammonium hydrosulfide, sodium sulfide, potassium
sulfide, calcium
sulfide, magnesium sulfide, ammonium sulfide, and mixtures thereof.
[092] Paragraph 24. The method of Paragraph 1, wherein the extractive agent is
an
oxidant selected from the group of iodine sources, oxyhalites, hydroperoxides,
organic
peroxides, inorganic peracids and salts thereof, organic peracids and salts
thereof, ozone,
hypochlorite ions, vanadium oxytrichloride, Fenton's reagent, hypobromite
ions, chlorine
dioxine, iodate 103, , and mixtures thereof.
[093] Paragraph 25. The method of Paragraph 2, further comprising: mixing a
complexing agent into the mixture of the reject stream and the extractive
agent, wherein the
complexing agent is selected from the group of thiol groups, thiophene groups,
thioether
groups, thiazole groups, thalocyanine groups, thiourenium groups, amino
groups,
polyethylene imine groups, hydrazido groups, N-thiocarbamoyl-polyalkylene
polyamino
groups, sulfides, ammonium thiosulfate, alkali metal thiosulfates, alkaline
earth metal
thiosulfates, iron thiosulfates, alkali metal dithionites, and alkaline earth
metal dithionites,
polyamines, and mixtures thereof.
[094] Paragraph 26. The method of Paragraph 6, wherein the filtration device
is
back-flushed with a portion of the filtered crude in an amount of less than 10
vol. % of the
crude oil feed.
[095] Paragraph 27. The method of Paragraph 1, wherein the reject stream has a
mercury level of at least 50 times the concentration of mercury in the crude
oil feed.
[096] Paragraph 28. The method of Paragraph 26, wherein the filtered crude
contains less than 100 ppbw mercury.
[097] Paragraph 29. The method of Paragraph 27, wherein the filtered crude
contains less than 50 ppbw mercury.
[098] Paragraph 30. The method of Paragraph 1, wherein the treated crude
contains
less than 100 ppbw mercury.
[099] Paragraph 31. A method for reducing a trace element of mercury in a
crude
oil feed, comprising: passing the crude oil feed having a mercury
concentration through a
17
CA 02898232 2015-07-14
WO 2014/158810
PCT/US2014/020298
filtration device having a filter element to retain at least 50% of the
mercury on the filter
element and generate a filtered crude having a reduced concentration of
mercury; back-
flushing the filtration element with a portion of the filtered crude to
generate a reject stream
containing crude oil having a concentrated mercury level of at least 20 times
the
concentration of mercury in the crude oil feed; mixing into the reject stream
an effective
amount of a reducing agent to convert a portion of the mercury into a volatile
mercury;
removing a portion of the volatile mercury by one of stripping, scrubbing,
adsorption, and
combinations thereof to obtain a treated crude oil having a reduced
concentration of
mercury.
101001 The method of Paragraph 31, wherein the reducing agent is selected from
sulfur compounds containing at least one sulfur atom having an oxidation state
less than +6;
ferrous compounds; stannous compounds; oxalates; cuprous compounds; organic
acids
which decompose to form CO2 upon heating; hydroxylamine compounds; hydrazine
compounds; sodium borohydride; diisobutylaluminium hydride; thiourea;
transition metal
halides; sulfites, bisulfites and metabisulfites; oxalic acid, cuprous
chloride, stannous
chloride, sodium borohydride, and mixtures thereof
18