Note: Descriptions are shown in the official language in which they were submitted.
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
SYSTEMS AND METHODS FOR COLLECTING AND TRANSMITTING ASSAY RESULTS
BACKGROUND OF THE INVENTION
[0001] Existing systems and methods for clinical testing suffer many drawbacks
from the perspectives of
patients, health care professionals, and taxpayers or insurance companies.
Today, consumers can purchase certain
specialized tests from various locations for consumer use. For example, a
consumer can purchase a pregnancy test
at a pharmacy and review the results. However, such results are to be viewed
by the consumer, and are not to be
relied on by the consumer's physician in forming a screening, diagnosis or
treatment plan.
[0002] Additionally, if a test result is to be conducted and to be relied on
by a doctor, physical samples
are transported to a laboratory where the tests on the samples are performed.
For example, blood from a fingerstick
or venous draw is typically collected from a subject at a hospital or
physician's office. The blood sample is shipped
to a Clinical Laboratory Improvement Amendments (CLIA) certified laboratory
which performs the tests and
analysis that is provided to the patient's doctor. Such techniques are
cumbersome and cause significant delay in
providing the result of a test ordered by a physician, especially because the
physical specimens must be transported
to a different site for analysis. Moreover, the sample collection sites often
have limited hours which further causes
inconvenience to patients.
[0003] Conventional techniques are also problematic for certain diagnoses.
Some tests are time
sensitive, and the results of which may take days or weeks to complete. In
such a time, a disease can progress past
the point of treatment. This impairs a medical professional's ability to
provide quality care.
[0004] Traditional systems and methods also affect the integrity and quality
of a clinical test due to
degradation of a sample that often occurs while transporting such sample from
the site of collection to the place
where actual analysis of the sample is performed. For examples, analytes decay
at a certain rate, and the time delay
for analysis can result in loss of the sample integrity. Different
laboratories also work with different qualities which
can result in varying degrees of error. Each laboratory can have its own set
of references that further introduce a
wide range of variability in coefficients of variation. Additionally,
preparation of samples by hand permit upfront
human error to occur from various sample collection sites. These and other
drawbacks inherent in the conventional
setup make it difficult to perform longitudinal analysis with high quality.
[0005] Furthermore, the conventional techniques are typically not very cost
effective. For example,
delays in test results lead to delays in diagnoses and treatments that can
have a deleterious effect on a patient's
health. For example, a disease may progress further, resulting in the patient
needing additional treatment. Payers,
such as health insurance companies and taxpayers contributing to governmental
health programs, end up paying
more to treat problems that could have been averted with more accessible and
faster clinical test results.
UMMARY
[0006] A need exists for improved systems and methods that allow higher
quality of care, more rapid and
accurate screening, diagnosis and/or treatment. Specifically, there is a
considerable need for sample collection,
-1-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
preparation and analysis. A further need exists for easily accessible sample
collection sites, while permitting the
analysis of data that can be relied on by a health care professional.
[0007] Systems and methods are further needed for earlier intervention and
providing high quality of care
with little variability and reduced human error to enable the performance of
longitudinal analysis of data. Systems
and methods disclosed herein meet this need and provide related advantages as
well.
[0008] An aspect of the invention is directed a method of evaluating a
biological sample collected from a
subject, said method comprising: (a) receiving data transmitted from a device
placed in or on the subject or at a
retailer site, wherein the device is configured to process the biological
sample by: (i) receiving the biological
sample; (ii) preparing the biological sample for a subsequent qualitative
and/or quantitative evaluation, to yield data
necessary for the subsequent qualitative and/or quantitative evaluation of
said biological sample; and (iii)
transmitting electronically the data to an authorized analytical facility
and/or an affiliate thereof for performance of
said subsequent qualitative and/or quantitative evaluation; and (b) analyzing
the data transmitted from the device, at
the authorized analytical facility and/or the affiliate thereof, to provide
said qualitative and/or quantitative
evaluation of said biological sample.
[0009] In accordance with another aspect of the invention, a method of
evaluating a biological sample
collected from a subject may comprise: (a) receiving electronic data
representative of an image of said biological
sample and/or an image of a physical process or chemical reaction performed
with said biological sample or a
portion thereof, said data being transmitted from a device placed in or on the
subject or at a designated sample
collection site, wherein the device is configured to process the biological
sample by: (i) receiving the biological
sample; (ii) preparing the biological sample for a subsequent qualitative
and/or quantitative evaluation, wherein said
preparation yields the electronic data representative of the image of said
biological sample and/or the image of the
physical process or the chemical reaction; and (iii) transmitting the
electronic data representative of the image to an
authorized analytical facility and/or an affiliate thereof for performance of
said subsequent qualitative and/or
quantitative evaluation; wherein the processing generates the electronic data
representative of the image necessary
for the subsequent qualitative and/or quantitative evaluation of said
biological sample, and (b) analyzing the
electronic data representative of the image transmitted from the device, at
the authorized analytical facility and/or
the affiliate thereof, to provide said qualitative and/or quantitative
evaluation of said biological sample.
[0010] A method of evaluating a plurality of types of biological samples
collected from a subject may be
provided in accordance with another aspect of the invention. The method may
comprise: (a) receiving data
transmitted from a device placed in or on the subject or at a designated
sample collection site, wherein the device is
configured to process the plurality of types of biological samples by: (i)
receiving the plurality of types of biological
samples; (ii) preparing the biological samples for a subsequent qualitative
and/or quantitative evaluation, to yield
data necessary for the subsequent qualitative and/or quantitative evaluation
of said plurality of types of biological
samples; and (iii) transmitting electronically the data to an authorized
analytical facility and/or an affiliate thereof
for performance of said subsequent qualitative and/or quantitative evaluation;
and (b) analyzing the data transmitted
from the device, at the authorized analytical facility and/or the affiliate
thereof, to provide said qualitative and/or
quantitative evaluation of said plurality of types of biological samples.
[0011] An additional aspect of the invention may be directed to a method of
evaluating a biological
sample collected from a subject at a designated site, said method comprising:
(a) collecting and processing the
-2-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
biological sample at said designated site wherein the sample is collected by a
device that is configured to (i) receive
the biological sample; (ii) prepare the biological sample for a subsequent
qualitative and/or quantitative evaluation,
to yield data necessary for the subsequent qualitative and/or quantitative
evaluation of said biological sample; and
(iii) transmit the data to a health care provider of an authorized analytical
facility and/or an affiliate thereof for
performance of said subsequent qualitative and/or quantitative evaluation; and
(b) transmitting the data to the
authorized analytical facility and/or an affiliate thereof; and (c) analyzing
the data transmitted from the device, at
the authorized analytical facility and/or the affiliate thereof, to provide
said qualitative and/or quantitative
evaluation of said biological sample.
[0012] Also, aspects of the invention may be directed to a method of
evaluating a biological sample
collected from a subject, said method comprising: (a) receiving data
transmitted from a device placed in or on the
subject or at a designated sample collection site, wherein the device is
configured to process the biological sample
by (i) receiving the biological sample; (ii) preparing the biological sample
for a subsequent qualitative and/or
quantitative evaluation, to yield data necessary for the subsequent
qualitative and/or quantitative evaluation of said
biological sample; and (iii) transmitting the data to a health care provider
of an authorized analytical facility and/or
an affiliate thereof for performance of said subsequent qualitative and/or
quantitative; and (b) analyzing the data
transmitted from the device, at the authorized analytical facility and/or the
affiliate thereof, to provide said
qualitative and/or quantitative evaluation of said biological sample; and (c)
verifying (x) whether the subject
received an order from a health care professional to undertake said subsequent
qualitative and/or quantitative
evaluation of said biological sample, or (y) whether the order for the
subsequent qualitative and/or quantitative
evaluation of said biological sample is within the policy restrictions of a
payer or a prescribing physician for said
subsequent qualitative and/or quantitative evaluation, and/or (z) whether the
subject is covered by health insurance
for said qualitative and/or quantitative evaluation of the biological sample;
wherein said verifying step is performed
prior to, concurrently with, or after steps (a) and/or (b).
[0013] A method of performing a pathological study of a biological sample
collected from a subject may
be provided in accordance with another aspect of the invention. The method may
comprise: (a) receiving electronic
data representative of an image of said biological sample, a physical process
and/or chemical reaction performed
with said biological sample or a portion thereof, wherein the data is received
from a device placed in or on the
subject or at a designated sample collection site, wherein the device is
configured to: (i) receive said biological
sample; (ii) prepare the collected biological sample for a subsequent
qualitative and/or quantitative evaluation,
wherein said preparation yields the electronic data representative of the
image of said biological sample and/or the
chemical reaction; and (iii) transmit the electronic data representative of
the image to a pathologist of an authorized
analytical facility and/or its affiliate thereof; (b) analyzing the electronic
data by the pathologist of the authorized
analytical facility and/or the affiliate thereof, to provide said qualitative
and/or quantitative evaluation.
[0014] Additional aspects of the invention may be directed to a method of
performing a pathological
study of a biological sample collected from a subject, said method comprising:
(a) receiving electronic data
representative of an image of said biological sample and/or a chemical
reaction performed with at least one
component from said biological sample from a device placed in or on the
subject or at a designated sample
collection site, wherein the device is configured to: (i) receive said
biological sample; (ii) prepare the collected
biological sample for a subsequent qualitative and/or quantitative evaluation,
wherein said preparation yields the
electronic data representative of the image of said biological sample and/or
the chemical reaction; and (iii) transmit
-3-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
the electronic data representative of the image to a pathologist of an
authorized analytical facility;(b) analyzing the
electronic data by the pathologist of the authorized analytical facility to
provide said subsequent qualitative and/or
quantitative evaluation.
[0015] Furthermore, aspects of the invention may be directed to a method of
evaluating a biological
sample collected from a subject, said method comprising: (a) receiving data
transmitted from a device placed in or
on the subject or at a designated sample collection site, wherein the device
is configured to process the biological
sample by: (i) receiving the biological sample; (ii) preparing the biological
sample for a subsequent qualitative
and/or quantitative evaluation, to yield data necessary for the subsequent
qualitative and/or quantitative evaluation
of said biological sample; and (iii) transmitting electronically the data to
an authorized analytical facility and/or an
affiliate thereof; (b) analyzing the data transmitted from the device, at the
authorized analytical facility and/or the
affiliate thereof, to provide said subsequent qualitative and/or quantitative
evaluation of said biological sample.
[0016] Additional aspects of the invention may be directed to a method of
evaluating a biological sample
collected from a subject, said method comprising: (a) receiving data
transmitted from a device placed in or on the
subject or at a retailer site, wherein the device is configured to process the
biological sample by: (i) receiving the
biological sample; (ii) preparing the biological sample for a subsequent
qualitative and/or quantitative evaluation, to
yield data necessary for the subsequent qualitative and/or quantitative
evaluation of said biological sample; and (iii)
transmitting electronically the data to an authorized analytical facility
and/or an affiliate thereof; and (b) analyzing
the data transmitted from the device, at the authorized analytical facility
and/or the affiliate thereof, to provide said
subsequent qualitative and/or quantitative evaluation of said biological
sample.
[0017] In accordance with additional aspects of the invention, a method of
evaluating a biological sample
may comprise: (a) processing, with the aid of a device, a biological sample
collected from a subject, wherein the
device is placed in or on the subject or at a designated sample collection
site, wherein the processing generates data
necessary for a subsequent qualitative and/or quantitative evaluation of said
biological sample, and wherein the
device is configured to (i) receive the biological sample; (ii) prepare the
biological sample for the subsequent
qualitative and/or quantitative evaluation; and (iii) transmit the data to an
authorized analytical facility and/or an
affiliate thereof; (b) transmitting the data from the device, at the
authorized analytical facility and/or the affiliate
thereof, to provide said subsequent qualitative and/or quantitative evaluation
of said biological sample; and (c)
verifying whether the subject has healthcare coverage, wherein said verifying
step is performed prior to,
concurrently with, or after steps (a) and/or (b).
[0018] A method of evaluating a biological sample collected from a subject may
provided in accordance
with another aspect of the invention. The method may comprise: (a) receiving
electronic data representative of an
image of said biological sample and/or chemical reaction performed with at
least one component from said
biological sample transmitted from a device placed in or on the subject or at
a designated sample collection site,
wherein the device is configured to process the biological sample by: (i)
receiving the biological sample; (ii)
preparing the biological sample for a subsequent qualitative and/or
quantitative evaluation, wherein said preparation
yields the electronic data representative of the image of said biological
sample and/or the chemical reaction; and
(iii) transmitting the electronic data representative of the image to an
authorized analytical facility and/or an affiliate
thereof; wherein the processing generates the electronic data representative
of the image necessary for the
subsequent qualitative and/or quantitative evaluation of said biological
sample, and (b) analyzing the electronic data
-4-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
representative of the image transmitted from the device, at the authorized
analytical facility and/or the affiliate
thereof, to provide said subsequent qualitative and/or quantitative evaluation
of said biological sample.
[0019] Additional aspects may be directed to a method of evaluating a
biological sample collected from a
subject, said method comprising: (a) receiving data transmitted from a device
placed in or on the subject or at a
designated sample collection site, wherein the device is configured to process
the biological sample by (i) receiving
the biological sample; (ii) preparing the biological sample for a subsequent
qualitative and/or quantitative
evaluation, to yield data necessary for the subsequent qualitative and/or
quantitative evaluation of said biological
sample; and (iii) transmitting the data to a health care provider of an
authorized analytical facility and/or an affiliate
thereof; and (b) analyzing the data transmitted from the device, at the
authorized analytical facility and/or the
affiliate thereof, to provide said subsequent qualitative and/or quantitative
evaluation of said biological sample (c)
verifying whether the subject received an order from a health care
professional to undertake said subsequent
qualitative and/or quantitative evaluation of said biological sample, wherein
said verifying step is performed prior
to, concurrently with, or after steps (a) and/or (b).
[0020] Also, aspects of the invention may be directed to a method of
evaluating a biological sample, said
method comprising: (a) processing, with aid of a device, a biological sample
collected from a subject having
received an order for undertaking a subsequent qualitative and/or quantitative
evaluation of the biological sample,
wherein the device is placed in or on the subject or at a designated sample
collection site, wherein the processing
generates data necessary for the subsequent qualitative and/or quantitative
evaluation of said biological sample, and
wherein the device is configured to (i) receive the biological sample; (ii)
prepare the biological sample for a
subsequent qualitative and/or quantitative evaluation; and (iii) transmit the
data to an authorized analytical facility
and/or an affiliate thereof; (b) transmitting the data from the device, for
analysis at the authorized analytical facility
and/or the affiliate thereof, to provide said subsequent qualitative and/or
quantitative evaluation of said biological
sample; and (c) verifying whether the order for the subsequent qualitative
and/or quantitative evaluation of said
biological sample is within the policy restrictions of a payer or a
prescribing physician for said subsequent
qualitative and/or quantitative evaluation, wherein said verifying step is
performed prior to, concurrently with, or
after steps (a) and/or (b).
[0021] Another aspect of the invention provides a method of evaluating a
biological sample collected
from a subject, said method comprising: (a) receiving data transmitted from a
device placed in or on the subject or
at a designated sample collection site, wherein the device is configured to
process the biological sample by: (i)
receiving the biological sample; (ii) preparing the biological sample for a
subsequent qualitative and/or quantitative
evaluation, to yield information necessary for the subsequent qualitative
and/or quantitative evaluation of said
biological sample; and (iii) transmitting electronically the data to an
authorized analytical facility and/or an affiliate
thereof; and (b) analyzing the data transmitted from the device, at the
authorized analytical facility and/or the
affiliate thereof, to provide said subsequent qualitative and/or quantitative
evaluation of said biological sample,
wherein the subsequent qualitative and/or quantitative evaluation of said
biological sample yields a determination of
the presence or concentration of an analyte selected from one or more of the
following: sodium, potassium,
chloride, TCO2, anion Gap, ionized calcium, glucose, urea nitrogen,
creatinine, lactate, hematocrit, hemoglobin, pH,
PCO2, P02, HCO3, base excess, s02, ACT Kaolin, ACT Celite, PT/INR, cTnl, CK-
MB, or BNP.
-5-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[0022] Moreover, aspects of the invention may be directed to a method of
evaluating a plurality of types
of biological samples collected from a subject, said method comprising: (a)
receiving data transmitted from a device
placed in or on the subject or at a designated sample collection site, wherein
the device is configured to process the
plurality of types of biological samples by: (i) receiving the plurality of
types of biological samples; (ii) preparing
the biological samples for a subsequent qualitative and/or quantitative
evaluation, to yield data necessary for the
subsequent qualitative and/or quantitative evaluation of said plurality of
types of biological samples; and (iii)
transmitting electronically the data to an authorized analytical facility
and/or an affiliate thereof; and (b) analyzing
the data transmitted from the device, at the authorized analytical facility
and/or the affiliate thereof, to provide said
subsequent qualitative and/or quantitative evaluation of said plurality of
types of biological samples.
[0023] In practicing any of the methods above or elsewhere herein, alone or in
combination, the
qualitative and/or quantitative evaluation of said biological sample may be
effected without physically transporting
said sample from the site where the sample is collected to an authorized
analytical facility and/or an affiliate
thereof.
[0024] The methods above or elsewhere herein, alone or in combination, may
include methods wherein
the biological sample is selected from the group consisting of blood, serum,
plasma, nasal swab or nasopharyngeal
wash, saliva, urine, tears, gastric fluid, spinal fluid, stool, mucus, sweat,
earwax, oil, glandular secretion, cerebral
spinal fluid, tissue, semen, and vaginal fluid, throat swab, breath, hair,
finger nails, skin, biopsy, placental fluid,
amniotic fluid, cord blood, emphatic fluids, cavity fluids, sputum, mucus,
puss, micropiota, meconium, breast milk
and/or other excretions.
[0025] Any of the methods above or elsewhere herein, alone or in combination,
may be practiced
wherein the biological sample has a volume of 250 microliters (uL) or less.
[0026] In practicing the methods described above or elsewhere herein, alone or
in combination, the
methods may further comprise the step of providing oversight by a health care
professional of the authorized
analytical facility and/or by a software program.
[0027] In some embodiments, the methods above or elsewhere herein, alone or in
combination may
further comprising the step of verifying insurance eligibility of said subject
prior to, concurrent with or subsequent
to said analysis.
[0028] The methods above or elsewhere herein, alone or in combination, may
further comprise
generating a report that comprises the analysis for said subject based on said
qualitative and/or quantitative
evaluation.
[0029] In practicing the methods above or elsewhere herein, alone or in
combination, the analysis may
determine presence or concentration of analyte present in the biological
sample.
[0030] The methods provided above or elsewhere herein, alone or in
combination, may include an
analyte selected from the group consisting of protein, nucleic acid, drug,
drug metabolite, gas, ions, particles, small
molecules and metabolites thereof, elements, toxins, lipids, carbohydrates,
prions, formed elements, and
combination thereof.
[0031] A designated sample collection site may be a retailer location or a
physician's office, in
accordance with the practice of any of the methods described above or
elsewhere herein, alone or in combination.
-6-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
In some embodiments when practicing any of the methods described above or
elsewhere herein, alone or in
combination, the designated sample collection site may be the subject's home.
A designated sample collection site
may be an employer site, provider office, or hospital in methods above or
elsewhere herein, alone or in
combination.
[0032] In practicing the methods above or elsewhere herein, alone or in
combination, a further step may
be provided of aggregating the data to a yield a longitudinal analysis over
time.
[0033] The methods described above or elsewhere herein, alone or in
combination may utilize a
biological sample that is collected from a fingerstick.
[0034] In practicing the methods above or elsewhere herein, alone or in
combination, in some instances,
the processing of the biological sample does not involve a display of the
presence or concentration level of one or
more analyte selected for determination of cardiac markers, chemistries, blood
gases, electrolytes, lactate,
hemoglobin, coagulation or hematology.
[0035] Methods described above or elsewhere herein, alone or in combination,
may include a device that
is configured to verify whether the subject is covered by health insurance for
said qualitative and/or quantitative
evaluation of the biological sample.
[0036] The device may be configured to verify whether the subject received an
order from a health care
professional to undertake said qualitative and/or quantitative evaluation of
the biological sample, in the practice of
any of the methods above or elsewhere herein, alone or in combination.
[0037] In some embodiments, the methods above or elsewhere herein, alone or in
combination, may
include the device that is configured to verify the subject's identity prior
to receiving the biological sample,
transmitting electronically the data, or analyzing the transmitted data. In
some embodiments, the verification of the
subject's identity may comprise receiving a genetic signature of the subject.
In some of the methods described
above or elsewhere herein, alone or in combination, the genetic signature may
be obtained by nucleic acid
amplification of a biological sample from the subject. The verification of the
subject's identity may comprise one
or more biometric measurement of the subject, in the practice of the methods
described above or elsewhere herein,
alone or in combination. The verification of the subject's identity may be
performed by an authorized technician, in
some embodiments of the methods described above or elsewhere herein, alone or
in combination.
[0038] In practicing the methods above or elsewhere herein, alone or in
combination, the identity of the
authorized technician may be verified prior to receiving the biological
sample, transmitting electronically the data,
or analyzing the transmitted data.
[0039] The device may be configured to receive one or more cartridge
configured for the qualitative
and/or quantitative evaluation ordered by a health care professional, in the
practice of one or more of the methods
above or elsewhere herein, alone or in combination.
[0040] In some embodiments, one or more of the methods above or elsewhere
herein, alone or in
combination, may provide cartridge that has one or more identifier that is
readable by the device.
[0041] The methods above or elsewhere herein, alone or in combination, may
further comprise receiving
the identifier information from the device.
-7-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[0042] The performance of methods above or elsewhere herein, alone or in
combination may further
comprise the step of providing one or more protocol to said device based on
the identifier information received,
wherein said protocol effects the preparation of the biological sample.
[0043] In practicing methods above or elsewhere herein, alone or in
combination, the device may be
contained within a housing.
[0044] Methods above or elsewhere herein, alone or in combination, may
comprise a qualitative and/or
quantitative evaluation that involves a determination of clinical relevance of
the biological sample or lack thereof.
[0045] The designated sample collection site may be a retailer location in the
practice of methods above
or elsewhere herein, alone or in combination. In some embodiments of the
invention, including methods above or
elsewhere herein, alone or in combination, the designated sample collection
site is a chain store, pharmacy,
supermarket, or department store. The designated sample collection site may be
the subject's home in methods
above or elsewhere herein, alone or in combination.
[0046] The performance of methods above or elsewhere herein, alone or in
combination may comprise
data that includes electronic bits representative of the sample. The data may
be aggregated and may be useful for
longitudinal analysis over time to facilitate screening, diagnosis, treatment,
and/or disease prevention in the
methods above or elsewhere herein, alone or in combination.
[0047] The biological sample in the methods above or elsewhere herein, alone
or in combination, may
have a volume of 250 microliters ("uL") or less. In some embodiments, the
biological sample may be blood, serum,
saliva, urine, tears, gastric and/or digestive fluid, stool, mucus, sweat,
earwax, oil, glandular secretion, semen, or
vaginal fluid in the methods above or elsewhere herein, alone or in
combination. In the practice of methods above
or elsewhere herein, alone or in combination, the biological sample may be a
tissue sample. The methods above or
elsewhere herein, alone or in combination, may include a biological sample
that is collected from a fingerstick.
[0048] Methods above or elsewhere herein, alone or in combination, may further
comprise generating a
report based on said qualitative and/or quantitative evaluation of said
biological sample. In some embodiments, the
performance of one or more methods above or elsewhere herein, alone or in
combination, may further comprise
transmitting said report to an additional health care professional. The
additional health care professional may have
provided the order to the subject to undertake said qualitative and/or
quantitative evaluation of said biological
sample in methods above or elsewhere herein, alone or in combination. In some
instances, the additional health
care professional is at a different location from the authorized analytical
facility in the performance of methods
above or elsewhere herein, alone or in combination.
[0049] In the practice of methods above or elsewhere herein, alone or in
combination, processing may
include adding one or more reagent or fixatives.
[0050] In some embodiments, the data is transmitted to a cloud computing based
infrastructure in
methods above or elsewhere herein, alone or in combination.
[0051] Methods above or elsewhere herein, alone or in combination, may
comprise an image wherein the
image is a video image. The data may comprise electronic data representative
of an image and/or audio signal in
the practice of methods above or elsewhere herein, alone or in combination.
-8-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[0052] In the practice of methods above or elsewhere herein, alone or in
combination, a payer may
receive an electronic bill from the designated sample collection site.
[0053] A health care professional of the authorized analytical facility may
receive an electronic payment
from the designated sample collection site in the practice of one methods
above or elsewhere herein, alone or in
combination.
[0054] The device utilized in methods above or elsewhere herein, alone or in
combination, may be
configured to additionally prepare the biological sample based on at least one
of: prior preparation of the biological
sample, analysis of the data at the authorized analytical facility and/or the
affiliate thereof.
[0055] In the performance of methods above or elsewhere herein, alone or in
combination the authorized
analytical facility may be separate from the sample collection site.
[0056] A preparation of a biological sample may be automated when practicing
one or more of the
methods above or elsewhere herein, alone or in combination.
[0057] Methods above or elsewhere herein, alone or in combination may further
comprise overseeing
said subsequent qualitative and/or quantitative evaluation. The overseeing
step may be performed by a health care
professional of the authorized analytical facility and/or by a software
program in methods above or elsewhere
herein, alone or in combination. In some embodiments, transmitting the data
from the device may also be for
oversight of said subsequent qualitative and/or quantitative evaluation in
some methods above or elsewhere herein,
alone or in combination. Methods above or elsewhere herein, alone or in
combination, may be provided wherein
the oversight is provided by the health care professional of the authorized
analytical facility and/or by a software
program.
[0058] The data utilized in methods above or elsewhere herein, alone or in
combination, may be
representative of the biological sample and/or any portion thereof. In some
embodiments, the data may be
representative of a preparation of the collected biological sample. The data
may comprise information of one or
more conditions under which a preparation of the collected biological sample
occurs. The one or more conditions
may comprise one or more characteristics listed from the group: amount of the
biological sample, concentration of
the biological sample, quality of the biological sample, temperature, or
humidity.
[0059] In some of the methods above or elsewhere herein, alone or in
combination, the data is
representative of a reaction run by the device. The data may comprise
information of the rate of the reaction. In
some instances, the data may comprise information about a control reaction and
a chemical reaction involving the
biological sample.
[0060] In practicing methods above or elsewhere herein, alone or in
combination, such methods may
further comprise (c) overseeing one or more steps of (i)-(iii) to improve
quality of said evaluation, wherein said
overseeing is performed prior to, concurrently with, or subsequent to any of
steps (i)-(iii).
[0061] Methods above or elsewhere herein, alone or in combination may further
comprise (iv) overseeing
one or more steps of (i)-(iii) to improve quality of said evaluation, wherein
said overseeing is performed prior to,
concurrently with, or subsequent to any of steps (i)-(iii).
-9-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[0062] In some embodiments, methods above or elsewhere herein, alone or in
combination may be
provided wherein the overseeing is of data representative of the biological
sample and/or any portion thereof. The
overseeing may be of data representative of the biological sample and/or any
portion thereof. The overseeing may
be of data representative of a preparation of the collected biological sample.
In some instances, the overseeing is of
data representative of a preparation of the collected biological sample. The
overseeing may be of information of
one or more conditions under which a preparation of the collected biological
sample occurs. In methods above or
elsewhere herein, alone or in combination, overseeing may be of information of
one or more conditions under
which a preparation of the collected biological sample occurs. The overseeing
may be of data that is representative
of a chemical reaction run by the device. In some embodiments, overseeing may
be of data is representative of a
chemical reaction run by the device.
[0063] In the performance of methods above or elsewhere herein, alone or in
combination, the healthcare
coverage may be provided by a health insurance company
[0064] Methods above or elsewhere herein, alone or in combination may comprise
the preparing step that
involves one or more of the types of chemical reactions selected from
immunoassay, nucleic acid assay, receptor-
based assay, cytometric assay, colorimetric assay, enzymatic assay,
electrophoretic assay, electrochemical assay,
spectroscopic assay, chromatographic assay, microscopic assay, topographic
assay, calorimetric assay, turbidmetric
assay, agglutination assay, radioisotope assay, viscometric assay, coagulation
assay, clotting time assay, protein
synthesis assay, histological assay, culture assay, or osmolarity assay.
[0065] The device may be further configured to process the biological sample
by transmitting
electronically data representative of one or more biometric measurement of the
subject, in accordance with methods
above or elsewhere herein, alone or in combination.
[0066] In some methods above or elsewhere herein, alone or in combination, the
processing of the
biological sample does not encompass an analysis of the presence or
concentration level of three or more analytes
belonging to categories of cardiac marker, blood gas, electrolyte, lactate,
hemoglobin, and coagulation factors.
[0067] In some embodiments, the processing of the biological sample does not
encompass an analysis of
the presence or concentration level of three or more analytes belonging to the
following: sodium, potassium,
chloride, TCO2, anion Gap, ionized calcium, glucose, urea nitrogen,
creatinine, lactate, hematocrit, hemoglobin, pH,
PCO2, P02, HCO3, base excess, s02, ACT Kaolin, ACT Celite, PT/INR, cTnl, CK-
MB, and BNP, in the practice of
methods above or elsewhere herein, alone or in combination.
[0068] In the practice of some methods above or elsewhere herein, alone or in
combination, the sample
collection site may be one or more of the following: a hospital, clinic,
military site, or subject's home.
[0069] In some embodiments, data may be displayed on the touch screen after
analysis, for methods
above or elsewhere herein, alone or in combination.
[0070] Methods above or elsewhere herein may include imaging data of body
parts that may be done for
analysis simultaneously with biochemical analyses.
[0071] An aspect of the invention may be directed to a system of evaluating a
biological sample collected
from a subject, said system comprising: (a) a communication unit configured to
receive data from a device placed in
or on the subject or at a designated sample collection site, wherein the
device is configured to process the biological
-10-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
sample, thereby generating data necessary for a subsequent qualitative and/or
quantitative evaluation of said
biological sample, and wherein the device comprises (i) a sample collection
unit configured to receive the biological
sample; (ii) a sample preparation unit configured to prepare the biological
sample for the subsequent qualitative
and/or quantitative evaluation; and (iii) transmission unit configured to
transmit the data to an authorized analytical
facility and/or an affiliate thereof; and (b) a processor that processes said
data for the qualitative and/or quantitative
evaluation of said biological sample at the authorized analytical facility
and/or the affiliate thereof, and wherein said
processor communicates with a record database comprising one or more medical
records and/or insurance
information of the subject.
[0072] Additional aspects of the invention may be directed to a system of
evaluating a biological sample
collected from a subject, said system comprising: (a) a communication unit
configured to receive data from a device
placed in or on the subject or at a designated sample collection site, wherein
the device is configured to process the
biological sample, thereby generating data necessary for a subsequent
qualitative and/or quantitative evaluation of
said biological sample, and wherein the device comprises (i) a sample
collection unit configured to receive the
biological sample; (ii) a sample preparation unit configured to prepare the
biological sample for the subsequent
qualitative and/or quantitative evaluation; and (iii) transmission unit
configured to transmit the data to an authorized
analytical facility and/or an affiliate thereof; (b) a processor that
processes said data for the subsequent qualitative
and/or quantitative evaluation of said biological sample at the authorized
analytical facility and/or the affiliate
thereof, and wherein said processor communicates with a record database
comprising one or more medical records
for the subject, and/or wherein the processor communicates with a payer
database comprising insurance information
for the subject.
[0073] In accordance with another aspect of the invention, a system of
evaluating a blood sample
collected from a subject may be provided. The system may comprise: (a) a
communication unit configured to
receive data from a device placed in or on the subject or at a designated
sample collection site, wherein the device is
configured to process the blood sample, thereby generating data necessary for
a subsequent qualitative and/or
quantitative evaluation of said blood sample, and wherein the device comprises
(i) a sample collection unit
configured to receive the blood sample; (ii) a sample preparation unit
configured to prepare the biological sample
for the subsequent qualitative and/or quantitative evaluation, wherein the
sample preparation unit permits at least
one reagent to be added to the blood sample; and (iii) transmission unit
configured to transmit the data to an
authorized analytical facility and/or an affiliate thereof; and (b) a
processor that processes said data for the
subsequent qualitative and/or quantitative evaluation of said blood sample at
the authorized analytical facility
and/or the affiliate thereof, and wherein said processor accesses a record
database comprising one or more medical
records for the subject, and/or wherein the processor accesses a payer
database comprising insurance information
for the subject.
[0074] A system for rapid evaluation of a biological sample collected from a
subject to aid in screening,
diagnosis, treatment, or prevention of a disease may be provided in accordance
with an additional aspect of the
invention. The system may comprise: a communication unit for receiving from a
device electronic data
representative of an image of said biological sample and/or a chemical
reaction performed with at least one
component from said biological sample; said device being placed in or on the
subject or at a designated sample
collection site, wherein said device is for processing the biological sample
thereby generating the electronic data
representative of the image of said biological sample necessary for a
subsequent qualitative and/or quantitative
-11-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
evaluation of said biological sample, and wherein the device comprises, within
a housing, (i) a sample collection
unit for receiving the biological sample; (ii) a sample preparation unit for
preparing the biological sample for the
subsequent qualitative and/or quantitative evaluation, wherein the preparation
of the biological sample is
automated; (iii) an imaging unit for recording an image of the biological
sample and/or a chemical reaction
performed with at least one component from said biological sample; and (iv) a
transmission unit for transmitting the
electronic data representative of the image and/or the chemical reaction; and
a processor that processes said
electronic data representative of the image for subsequent qualitative and/or
quantitative evaluation of said
biological sample.
[0075] In practicing the systems above or elsewhere herein, alone or in
combination, the process may be
configured to communicate with a payer database comprising the insurance
information for the subject.
[0076] The systems described above or elsewhere herein, alone or in
combination may include a device
that is configured to receive information relating to said qualitative and/or
quantitative evaluation and optionally
display said information on said device.
[0077] The device may comprises a processing unit configured to verify whether
the subject is covered
by health insurance for said qualitative and/or quantitative evaluation of the
biological sample in the practice of
systems above or elsewhere herein, alone or in combination.
[0078] In some embodiments, systems above or elsewhere herein, alone or in
combination may comprise
a device that is configured to verify whether the subject received an order
from a health care professional to
undertake said qualitative and/or quantitative evaluation of the biological
sample.
[0079] The processor provided in systems above or elsewhere herein, alone or
in combination, may
access the records database prior to providing said qualitative and/or
quantitative evaluation. Optionally, the
processor accesses the payer database prior to providing said qualitative
and/or quantitative evaluation in systems
above or elsewhere herein, alone or in combination.
[0080] Prior to providing said qualitative and/or quantitative evaluation,
said systems above or elsewhere
herein, alone or in combination, may determine which records database to
access.
[0081] The device may be configured to receive one or more cartridge
configured for the qualitative
and/or quantitative evaluation ordered by a health care professional, in the
practice of systems above or elsewhere
herein, alone or in combination.
[0082] In some embodiments, the device is contained within a housing in
systems above or elsewhere
herein, alone or in combination.
[0083] In systems above or elsewhere herein, alone or in combination, the
qualitative and/or quantitative
evaluation may involve a determination of clinical relevance of the biological
sample or lack thereof.
[0084] The designated sample collection site may be a chain store, pharmacy,
supermarket, or
department store in systems above or elsewhere herein, alone or in
combination. In some embodiments, the
designated sample collection site is the subject's home.
[0085] The systems above or elsewhere herein, alone or in combination, may
comprise a biological
sample that has a volume of 250 uL or less. The biological sample may be
blood, serum, saliva, urine, tears, gastric
-12-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
and/or digestive fluid, stool, mucus, sweat, earwax, oil, glandular secretion,
semen, or vaginal fluid. In some
instances, the biological sample may be a tissue sample.
[0086] In some systems above or elsewhere herein, alone or in combination, the
biological sample may
be collected from a fingerstick.
[0087] In some embodiments, systems above or elsewhere herein may utilize a
designated sample
collection site that may be a retailer. A designated sample collection site
may be an employer site, provider office,
or hospital in systems above or elsewhere herein, alone or in combination.
[0088] An authorized analytical facility, in some systems above or elsewhere
herein, alone or in
combination, may be separate from the sample collection site.
[0089] A user interface may be accessible by a health care professional for
said subsequent qualitative
and/or quantitative evaluation and/or oversight of said subsequent qualitative
and/or quantitative evaluation in
systems above or elsewhere herein, alone or in combination.
[0090] In systems above or elsewhere herein, alone or in combination, a
processor may further provide
oversight of said subsequent qualitative and/or quantitative evaluation.
[0091] A sample preparation unit may comprise (i) a pipette, and optionally
(ii) one or more of the
following: centrifuge, magnetic separator, filter, vessels, containers, assay
units, reagent units, heater, thermal
controller, cytometer, electromagnetic source, temperature sensor, motion
sensor, or sensor for electrical properties,
in systems above or elsewhere herein, alone or in combination.
[0092] In some embodiments, systems above or elsewhere herein, alone or in
combination may comprise
an image. The image may be static. In some embodiments, the image may be a
video image. Systems above or
elsewhere herein, alone or in combination may include [[a]] the transmission
unit that is configured to transmit the
electronic data representative of the image wirelessly.
[0093] In systems above or elsewhere herein, alone or in combination, data may
comprise electronic data
representative of the image and an audio signal.
[0094] A device in systems above or elsewhere herein, alone or in combination,
may be configured to
receive one or more cartridge configured for the qualitative and/or
quantitative evaluation. In some embodiments,
the cartridge may have one or more identifier that is readable by the device.
[0095] In some systems above or elsewhere herein, alone or in combination, at
least one component may
be a biological analyte made of carbohydrate, lipid, protein or a combination
thereof.
[0096] In utilizing the systems above or elsewhere herein, alone or in
combination, a chemical reaction
may be performed without the biological sample.
[0097] In some embodiments, data may be displayed on the touch screen after
analysis, for systems
above or elsewhere herein, alone or in combination.
[0098] Systems above or elsewhere herein may include imaging data of body
parts that may be done for
analysis simultaneously with biochemical analyses.
-13-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[0099] Some aspects of the invention are directed to a method of performing a
pathological study of a
biological sample collected from a subject, said method comprising (a)
receiving electronic data representative of
an image of said biological sample and/or a chemical reaction performed with
at least one component of the
biological sample from a device placed in or on the subject or at a designated
sample collection site, wherein the
device is configured to: (i) receive said biological sample; (ii) prepare the
collected biological sample for a
subsequent qualitative and/or quantitative evaluation, wherein said
preparation yields the electronic data
representative of the image of said biological sample and/or the chemical
reaction; and (iii) transmit the electronic
data representative of the image to a pathologist of an authorized analytical
facility; and (b) analyzing the electronic
data by the pathologist of the authorized analytical facility to provide said
subsequent qualitative and/or quantitative
evaluation.
[00100] An aspect of the invention is directed to a method of evaluating a
biological sample collected
from a subject. The method comprises (a) receiving data transmitted from a
device placed in or on the subject or at
a designated sample collection site, wherein the device is configured to
process the biological sample by: (i)
receiving the biological sample; (ii) preparing the biological sample for a
subsequent qualitative and/or quantitative
evaluation, to yield data necessary for the subsequent qualitative and/or
quantitative evaluation of said biological
sample; and (iii) transmitting electronically the data to an authorized
analytical facility and/or an affiliate thereof;
and (b) analyzing the data transmitted from the device, at the authorized
analytical facility and/or the affiliate
thereof, to provide said subsequent qualitative and/or quantitative evaluation
of said biological sample. This may
be in contrast to conventional devices which may only transmit results of an
analysis, not data for subsequent
qualitative and/or quantitative evaluation of a sample. Such conventional
devices that only transmit results may not
be relied upon by one or more health care professional in diagnosing, treating
and/or preventing a disease for
subject.
[00101] In some embodiments, the processing of the biological sample does not
encompass an analysis of
the presence or concentration level of three or more analytes belonging to
categories of cardiac marker, blood gas,
electrolyte, lactate, hemoglobin, and coagulation factors. In some instances,
the processing of the biological sample
does not encompass an analysis of the presence or concentration level of three
or more analytes belonging to the
following: sodium, potassium, chloride, TCO2, anion Gap, ionized calcium,
glucose, urea nitrogen, creatinine,
lactate, hematocrit, hemoglobin, pH, PCO2, P02, HCO3, base excess, s02, ACT
Kaolin, ACT Celite, PT/INR, cTnl,
CK-MB, and BNP.
[00102] A method of evaluating a biological sample collected from a subject is
provided in accordance
with another aspect of the invention. The method comprises (a) receiving data
transmitted from a device placed in
or on the subject or at a retailer site, wherein the device is configured to
process the biological sample by: (i)
receiving the biological sample; (ii) preparing the biological sample for a
subsequent qualitative and/or quantitative
evaluation, to yield data necessary for the subsequent qualitative and/or
quantitative evaluation of said biological
sample; and (iii) transmitting electronically the data to an authorized
analytical facility and/or an affiliate thereof;
and (b) analyzing the data transmitted from the device, at the authorized
analytical facility and/or the affiliate
thereof, to provide said subsequent qualitative and/or quantitative evaluation
of said biological sample.
[00103] An additional aspect of the invention is a method of evaluating a
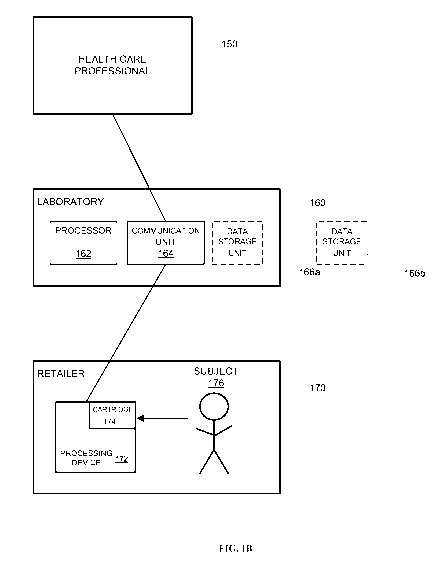
biological sample, said method
comprising: (a) processing, with the aid of a device, a biological sample
collected from a subject, wherein the
-14-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
device is placed in or on the subject or at a designated sample collection
site, wherein the processing generates data
necessary for a subsequent qualitative and/or quantitative evaluation of said
biological sample, and wherein the
device is configured to (i) receive the biological sample; (ii) prepare the
biological sample for the subsequent
qualitative and/or quantitative evaluation; and (iii) transmit the data to an
authorized analytical facility and/or an
affiliate thereof; (b) transmitting the data from the device, at the
authorized analytical facility and/or the affiliate
thereof, to provide said subsequent qualitative and/or quantitative evaluation
of said biological sample; and (c)
verifying whether the subject has healthcare coverage, wherein said verifying
step is performed prior to,
concurrently with, or after steps (a) and/or (b).
[00104] Another aspect of the invention is a method of evaluating a biological
sample collected from a
subject, said method comprising (a) receiving electronic data representative
of an image of said biological sample
and/or chemical reaction performed on a device, wherein the electronic data is
transmitted from a device placed in
or on the subject or at a designated sample collection site, wherein the
device is configured to process the biological
sample by: (i) receiving the biological sample; (ii) preparing the biological
sample for a subsequent qualitative
and/or quantitative evaluation, wherein said preparation yields the electronic
data representative of the image of
said biological sample and/or the chemical reaction; and (iii) transmitting
the electronic data representative of the
image to an authorized analytical facility and/or an affiliate thereof;
wherein the processing generates the electronic
data representative of the image necessary for the subsequent qualitative
and/or quantitative evaluation of said
biological sample; and (b) analyzing the electronic data representative of the
image transmitted from the device, at
the authorized analytical facility and/or the affiliate thereof, to provide
said subsequent qualitative and/or
quantitative evaluation of said biological sample.
[00105] A system of evaluating a biological sample collected from a subject is
provided in accordance
with yet another aspect of the invention. The system comprises (a) a
communication unit configured to receive data
from a device placed in or on the subject or at a designated sample collection
site, wherein the device is configured
to process the biological sample, thereby generating data necessary for a
subsequent qualitative and/or quantitative
evaluation of said biological sample, and wherein the device comprises (i) a
sample collection unit configured to
receive the biological sample; (ii) a sample preparation unit configured to
prepare the biological sample for the
subsequent qualitative and/or quantitative evaluation; and (iii) transmission
unit configured to transmit the data to
an authorized analytical facility and/or an affiliate thereof; (b) a processor
that processes said data for the
subsequent qualitative and/or quantitative evaluation of said biological
sample at the authorized analytical facility
and/or the affiliate thereof, and wherein said processor communicates with a
record database comprising one or
more medical records for the subject, and/or wherein the processor
communicates with a payer database comprising
insurance information for the subject.
[00106] Furthermore, a method of evaluating a biological sample collected from
a subject is provided.
The method comprises (a) receiving data transmitted from a device placed in or
on the subject or at a designated
sample collection site, wherein the device is configured to process the
biological sample by (i) receiving the
biological sample; (ii) preparing the biological sample for a subsequent
qualitative and/or quantitative evaluation, to
yield data necessary for the subsequent qualitative and/or quantitative
evaluation of said biological sample; and (iii)
transmitting the data to a health care provider of an authorized analytical
facility and/or an affiliate thereof; and (b)
analyzing the data transmitted from the device, at the authorized analytical
facility and/or the affiliate thereof, to
provide said subsequent qualitative and/or quantitative evaluation of said
biological sample; and (c) verifying
-15-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
whether the subject received an order from a health care professional to
undertake said subsequent qualitative
and/or quantitative evaluation of said biological sample, wherein said
verifying step is performed prior to,
concurrently with, or after steps (a) and/or (b).
[00107] An additional aspect of the invention is directed to a method of
evaluating a biological sample,
said method comprising (a) processing, with aid of a device, a biological
sample collected from a subject having
received an order for undertaking a subsequent qualitative and/or quantitative
evaluation of the biological sample,
wherein the device is placed in or on the subject or at a designated sample
collection site, wherein the processing
generates data necessary for the subsequent qualitative and/or quantitative
evaluation of said biological sample, and
wherein the device is configured to (i) receive the biological sample; (ii)
prepare the biological sample for a
subsequent qualitative and/or quantitative evaluation; and (iii) transmit the
data to an authorized analytical facility
and/or an affiliate thereof; (b) transmitting the data from the device, for
analysis at the authorized analytical facility
and/or the affiliate thereof, to provide said subsequent qualitative and/or
quantitative evaluation of said biological
sample; and (c) verifying whether the order for the subsequent qualitative
and/or quantitative evaluation of said
biological sample is within the policy restrictions of a payer or a
prescribing physician for said subsequent
qualitative and/or quantitative evaluation, wherein said verifying step is
performed prior to, concurrently with, or
after steps (a) and/or (b).
[00108] A method of evaluating a biological sample collected from a subject is
illustrated in accordance
with an aspect of the invention. The method comprises (a) receiving data
transmitted from a device placed in or on
the subject or at a designated sample collection site, wherein the device is
configured to process the biological
sample by (i) receiving the biological sample; (ii) preparing the biological
sample for a subsequent qualitative
and/or quantitative evaluation, to yield information necessary for the
subsequent qualitative and/or quantitative
evaluation of said biological sample; and (iii) transmitting electronically
the data to an authorized analytical facility
and/or an affiliate thereof; and (b) analyzing the data transmitted from the
device, at the authorized analytical
facility and/or the affiliate thereof, to provide said subsequent qualitative
and/or quantitative evaluation of said
biological sample, wherein the subsequent qualitative and/or quantitative
evaluation of said biological sample yields
a determination of the presence or concentration of an analyte belonging
selected from one or more of the
following: sodium, potassium, chloride, TCO2, anion Gap, ionized calcium,
glucose, urea nitrogen, creatinine,
lactate, hematocrit, hemoglobin, pH, PCO2, P02, HCO3, base excess, s02, ACT
Kaolin, ACT Celite, PT/INR, cTnl,
CK-MB, or BNP.
[00109] In another aspect, the invention provides a system of evaluating a
blood sample collected from a
subject, said system comprising (a) a communication unit configured to receive
data from a device placed in or on
the subject or at a designated sample collection site, wherein the device is
configured to process the blood sample,
thereby generating data necessary for a subsequent qualitative and/or
quantitative evaluation of said blood sample,
and wherein the device comprises (i) a sample collection unit configured to
receive the blood sample; (ii) a sample
preparation unit configured to prepare the biological sample for the
subsequent qualitative and/or quantitative
evaluation, wherein the sample preparation unit permits at least one reagent
to be added to the blood sample; and
(iii) transmission unit configured to transmit the data to an authorized
analytical facility and/or an affiliate thereof;
and (b) a processor that processes said data for the subsequent qualitative
and/or quantitative evaluation of said
blood sample at the authorized analytical facility and/or the affiliate
thereof, and wherein said processor accesses a
-16-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
record database comprising one or more medical records for the subject, and/or
wherein the processor accesses a
payer database comprising insurance information for the subject.
[00110] Another method of evaluating a plurality of types of biological
samples collected from a subject is
provided. The method comprises (a) receiving data transmitted from a device
placed in or on the subject or at a
designated sample collection site, wherein the device is configured to process
the plurality of types of biological
samples by (i) receiving the plurality of types of biological samples; (ii)
preparing the biological samples for a
subsequent qualitative and/or quantitative evaluation, to yield data necessary
for the subsequent qualitative and/or
quantitative evaluation of said plurality of types of biological samples; and
(iii) transmitting electronically the data
to an authorized analytical facility and/or an affiliate thereof; and (b)
analyzing the data transmitted from the device,
at the authorized analytical facility and/or the affiliate thereof, to provide
said subsequent qualitative and/or
quantitative evaluation of said plurality of types of biological samples.
[00111] In some embodiments, the processing of the biological sample does not
involve a display of the
presence or concentration level of one or more analyte selected for
determination of cardiac markers, chemistries,
blood gases, electrolytes, lactate, hemoglobin, coagulation or hematology. In
some embodiments, the processing of
the biological sample does not involve a display of the presence or
concentration level of three or more analytes
belonging to the following: sodium, potassium, chloride, TCO2, anion Gap,
ionized calcium, glucose, urea nitrogen,
creatinine, lactate, hematocrit, hemoglobin, pH, PCO2, P02, HCO3, base excess,
s02, ACT Kaolin, ACT Celite,
PT/INR, cTnl, CK-MB, and BNP. After the subsequent analysis, such information
can be transmitted back to the
device, such as for display, storage, or analysis.
[00112] Furthermore, in some embodiments, the device is configured to verify
whether the subject is
covered by health insurance for said qualitative and/or quantitative
evaluation of the biological sample. The device
can comprise a processing unit configured to verify whether the subject is
covered by health insurance for said
qualitative and/or quantitative evaluation of the biological sample. The
device can be configured to verify whether
the subject received an order from a health care professional to undertake
said qualitative and/or quantitative
evaluation of the biological sample.
[00113] In some instances, the processor accesses the records database prior
to providing said qualitative
and/or quantitative evaluation. The processor may access the payer database
prior to providing said qualitative
and/or quantitative evaluation. In some embodiments, prior to providing said
qualitative and/or quantitative
evaluation, said system determines which records database to access.
[00114] In some embodiments, the device is configured to verify the subject's
identity prior to receiving
the biological sample, transmitting electronically the data, or analyzing the
transmitted data. The verification of the
subject's identity can comprise receiving a genetic signature of the subject.
The genetic signature can be obtained
by nucleic acid amplification of a biological sample from the subject. The
verification of the subject's identity can
comprise one or more biometric measurement of the subject. The verification of
the subject's identity can be
performed by an authorized technician. The identity of the authorized
technician can be verified prior to receiving
the biological sample, transmitting electronically the data, or analyzing the
transmitted data.
[00115] In accordance with some embodiments of the invention, the device can
be configured to receive
one or more cartridge configured for the qualitative and/or quantitative
evaluation ordered by a health care
professional. The device can be configured to receive one or more cartridge
configured for the qualitative and/or
-17-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
quantitative evaluation ordered by a health care professional. The cartridge
can have one or more identifier that is
readable by the device. In some instances, methods are provided further
comprising receiving the identifier
information from the device. Such methods can also further comprise providing
one or more protocol to said device
based on the identifier information received, wherein said protocol effects
the preparation of the biological sample.
A protocol may be provided from a server wirelessly to facilitate preparation
and/or processing of the biological
sample. The protocol may be provided from the cloud or from any external
device.
[00116] In some embodiments, the device is contained within a housing.
[00117] The qualitative and/or quantitative evaluation can involve a
determination of clinical relevance of
the biological sample or lack thereof.
[00118] The designated sample collection site is a retailer location in some
embodiments of the invention.
The designated sample collection site can be a chain store, pharmacy,
supermarket, or department store. The
designated sample collection site can be the subject's home.
[00119] In some embodiments, the data includes electronic bits representative
of the sample. The data can
be aggregated and is useful for longitudinal analysis over time to facilitate
screening, diagnosis, progress treatment,
and/or disease prevention. The data can also be useful and viewable for
longitudinal analysis over time to facilitate
screening, diagnosis, progress treatment, and/or disease prevention, as well
as a better understanding of disease
progression or regression, or efficacy of an intervention, including a
treatment or lifestyle change.
[00120] The biological sample can have a volume of 250 uL or less. The
biological sample is blood,
serum, saliva, urine, tears, gastric and/or digestive fluid, stool, mucus,
sweat, earwax, oil, glandular secretion,
semen, or vaginal fluid. The biological sample can be a tissue sample. The
biological sample can be collected from
a fingerstick.
[00121] In some embodiments, a method can further comprise generating a report
based on said
qualitative and/or quantitative evaluation of said biological sample. The
method can further comprise transmitting
said report to an additional health care professional. In some instances, the
additional health care professional
provided the order to the subject to undertake said qualitative and/or
quantitative evaluation of said biological
sample. The additional health care professional can be at a different location
from the authorized analytical facility.
[00122] In some embodiments, processing includes adding one or more reagent or
fixatives.
[00123] The data can be transmitted to a cloud computing based infrastructure
in accordance with an
embodiment of the invention. The image can be a video image. The data can
comprise electronic data
representative of an image and/or audio signal. The cloud computing based
infrastructure may be self learning.
Data may be provided to a model that may refit and retune based on the data
that is collected. The cloud computing
based infrastructure can perform the analysis.
[00124] In some embodiments, the processor accesses the payer database. A
payer can receive an
electronic bill from the designated sample collection site. A health care
professional of the authorized analytical
facility can receive an electronic payment from the designated sample
collection site.
-18-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00125] The device can be configured to additionally prepare the biological
sample based on at least one
of: prior preparation of the biological sample, analysis of the data at the
authorized analytical facility and/or the
affiliate thereof.
[00126] In some embodiments, the authorized analytical facility is separate
from the sample collection
site.
[00127] The preparation of the biological sample can be automated.
[00128] Methods may be provided further comprising overseeing said subsequent
qualitative and/or
quantitative evaluation. The overseeing step can be performed by a health care
professional of the authorized
analytical facility and/or by a software program. Transmitting the data from
the device can also be for oversight of
said subsequent qualitative and/or quantitative evaluation. The oversight can
be provided by the health care
professional of the authorized analytical facility and/or by a software
program. A user interface can be provided
accessible by a health care professional for said subsequent qualitative
and/or quantitative evaluation and/or
oversight of said subsequent qualitative and/or quantitative evaluation. The
processor can further provide oversight
of said subsequent qualitative and/or quantitative evaluation.
[00129] In some embodiments, the data is representative of the biological
sample and/or any portion
thereof. The data can be representative of a preparation of the collected
biological sample. The data can comprise
information of one or more conditions under which a preparation of the
collected biological sample occurs. One or
more conditions can comprise one or more characteristics listed from the
group: amount of the biological sample,
concentration of the biological sample, quality of the biological sample,
temperature, or humidity. The data can be
representative of a reaction run by the device. The data can comprise
information of the rate, quality, and/or
performance of the reaction. The data can comprise information about a control
reaction and a chemical reaction
involving the biological sample. Collected data can be a photon as a result of
a chemical reaction. Other examples
of data may include electrons, photons, intensities, frequencies, colors,
sounds, or temperatures.
[00130] In some embodiments, methods are provided further comprising (c)
overseeing one or more steps
of (i)-(iii) to improve quality of said evaluation, wherein said overseeing is
performed prior to, concurrently with, or
subsequent to any of steps (i)-(iii). Additionally methods are provided
further comprising (iv) overseeing one or
more steps of (i)-(iii) to improve quality of said evaluation, wherein said
overseeing is performed prior to,
concurrently with, or subsequent to any of steps (i)-(iii). The overseeing can
be of data that is representative of the
biological sample and/or any portion thereof. The overseeing can be of data
that is representative of a preparation
of the collected biological sample. The overseeing can be of information of
one or more conditions under which a
preparation of the collected biological sample occurs. The overseeing can be
of that is data representative of a
reaction run by the device. The overseeing can be of data that is
representative of a reaction run that occurs within
the system.
[00131] In some embodiments, healthcare coverage is provided by a health
insurance company and/or
employer.
[00132] In some embodiments, a preparing step involves one or more of the
types of reactions selected
from immunoassay, nucleic acid assay, receptor-based assay, cytometry,
colorimetric assay, enzymatic assay,
spectroscopic assay (e.g., mass spectrometry, infrared spectroscopy, x-ray
photoelectron spectroscopy),
-19-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
electrophoresis, nucleic acid sequencing, agglutination, chromatography,
coagulation, electrochemical assay,
histology, or cell analysis, including dead and/or live cell analysis,
molecular biology, chemistry, turbidmetric
assay, agglutination assay, radioisotope assay, viscometric assay, coagulation
assay, clotting time assay, protein
synthesis assay, histological assay, culture assay, osmolarity assay,
microscopic assay, topographic assay,
calorimetric assay, and/or other types of assays or combinations thereof.
[00133] The device can be further configured to process the biological sample
by transmitting
electronically data representative of one or more biometric measurements of
the subject.
[00134] In some embodiments, a sample collection site is one or more of the
following: a hospital, clinic,
emergency room, military site, or subject's home.
[00135] An aspect of the invention may be directed to a system for rapid
evaluation of a biological sample
collected from a subject to aid in screening, diagnosis, treatment, or
prevention of a disease, said system
comprising: a communication unit for receiving from a device electronic data
representative of an image of said
biological sample and/or a chemical reaction performed with at least one
component from said biological sample;
said device being placed in or on the subject or at a designated sample
collection site, wherein said device is for
processing the biological sample thereby generating the electronic data
representative of the image of said
biological sample necessary for a subsequent qualitative and/or quantitative
evaluation of said biological sample,
and wherein the device comprises, within a housing, (i) a sample collection
unit for receiving the biological sample;
(ii) a sample preparation unit for preparing the biological sample for the
subsequent qualitative and/or quantitative
evaluation, wherein the preparation of the biological sample is automated;
(iii) an imaging unit for recording an
image of the biological sample and/or a chemical reaction performed with at
least one component from said
biological sample; and (iv) a transmission unit for transmitting the
electronic data representative of the image and/or
the chemical reaction; and a processor that processes said electronic data
representative of the image for subsequent
qualitative and/or quantitative evaluation of said biological sample.
[00136] In some embodiments, the sample preparation unit may comprise (i) a
pipette, and optionally (ii)
one or more of the following: centrifuge, magnetic separator, filter, vessels,
containers, assay units, reagent units,
heater, thermal controller, cytometer, electromagnetic source, temperature
sensor, motion sensor, or sensor for
electrical properties.
[00137] The image may be static and/or a video image. The data may comprise
electronic data
representative of the image and an audio signal.
[00138] The biological sample may be selected from one or more of the
following: blood, serum, saliva,
urine, tears, gastric and/or digestive fluid, stool, mucus, sweat, earwax,
oil, glandular secretion, semen, or vaginal
fluid. In some embodiments, the biological sample has a volume of 250 uL or
less. A component of a biological
sample may be a biological analyte made of carbohydrate, lipid, protein or a
combination thereof. A chemical
reaction may be performed without the biological sample.
[00139] The transmission unit may be configured to transmit the electronic
data representative of the
image wirelessly.
-20-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00140] The device may be configured to receive one or more cartridge
configured for the qualitative
and/or quantitative evaluation. In some embodiments, the cartridge may have
one or more identifier that is readable
by the device.
[00141] In accordance with another aspect of the methods, systems, and devices
disclosed herein,
Applicants disclose a method of evaluating a biological sample collected from
a subject, said method comprising:
[00142] (a) receiving at a laboratory location data transmitted from a device
having a housing, said device
placed in or on the subject or at a designated sample collection site, said
data comprising raw data from said
biological sample, said biological sample comprising a cell, wherein the
device is configured to process the
biological sample within said housing by: (i) receiving the biological sample;
(ii) preparing the biological sample
and yielding raw data within said housing for a subsequent qualitative and/or
quantitative evaluation of said
biological sample, said raw data comprising (1) numerical values
representative of a physical process or chemical
reaction performed by the device and (2) electronic data representative of an
image of a cell in said biological
sample; and (iii) transmitting electronically the raw data from said sample
collection site to an authorized analytical
facility and/or an affiliate thereof for performance of said subsequent
evaluation at said laboratory location;
[00143] (b) analyzing the raw data transmitted from the device at the
authorized analytical facility and/or
the affiliate thereof, to provide said evaluation of said biological sample,
wherein said analysis is performed using a
processor alone or in conjunction with an individual affiliated with the
authorized analytical facility; and
[00144] (c) providing oversight of integrity of said analysis and operation of
said device such that results
generated from said analysis can be utilized by a health care professional for
screening, diagnosis or treatment of
said subject, wherein the oversight is performed at said laboratory location
using a processor alone or in conjunction
with an individual affiliated with the authorized analytical facility. In
embodiments, the evaluation of a biological
sample is effected without physically transporting said sample from the site
where the sample is collected to the
authorized analytical facility or affiliate thereof. The authorized analytical
facility and/or affiliate thereof may be a
Clinical Laboratory Improvement Amendments (CLIA)-compliant laboratory. In
embodiments, a designated sample
collection site may be a retailer site, a military site, the subject's home, a
health assessment location, or a health
treatment location.
[00145] In embodiments of such methods, preparing the biological sample and
yielding raw data within
said housing may comprise transporting a reagent, the biological sample, or a
portion of the biological sample, with
a pipette within the housing; and may comprise centrifuging the biological
sample, or a portion thereof, within the
housing. In embodiments of such methods, electronic data representative of an
image of a cell may include
electronic data derived from an optical assessment of histology of said cell,
morphology of said cell, hematology, or
cell count. In embodiments of such methods, receiving data comprises may
include receiving data from an image of
a physical process or chemical reaction performed by the device with some or
all of a biological sample. In
embodiments of such methods, raw data may be yielded by more than one assay,
including at least two assays
selected from an immunoassay, a nucleic acid assay, a receptor-based assay,
and an enzymatic assay. In
embodiments, raw data may be yielded by at least three assays selected from an
immunoassay, a nucleic acid assay,
a receptor-based assay, and an enzymatic assay.
[00146] A biological sample may be selected from the group consisting of
blood, serum, plasma, nasal
swab, nasopharyngeal wash, saliva, urine, tears, gastric fluid, spinal fluid,
stool, mucus, sweat, earwax, oil,
-21-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
glandular secretion, cerebral spinal fluid, tissue, semen, and vaginal fluid,
throat swab, breath, hair, finger nails,
skin, biopsy, placental fluid, amniotic fluid, cord blood, lymphatic fluids,
cavity fluids, sputum, mucus, pus,
microbiota, meconium, breast milk and other excretions. A biological sample
may be a fluid sample having a
volume of 250 pi., or less.
[00147] In embodiments, the methods include evaluating a plurality of types of
biological samples
collected from a subject, wherein said data transmitted from said device
comprises raw data from said plurality of
types of biological samples, wherein at least one of said biological samples
comprises a cell. In embodiments
including evaluating a plurality of types of samples, yielding raw data may
include yielding raw data from at least
two types of biological samples and at least two assays selected from an
immunoassay, a nucleic acid assay, a
receptor-based assay, and an enzymatic assay. Such evaluation of a plurality
of types of biological samples may be
effected without physically transporting any of the samples from the site
where the sample is collected to the
authorized analytical facility and/or an affiliate thereof. Fluid samples of
such a plurality of types of biological
samples may each have a volume of 250 !AL or less. In embodiments evaluating a
plurality of types of samples,
oversight may include the selection of analysis methodology and procedures for
each of the plurality of types of
biological samples.
[00148] In embodiments of these methods, oversight includes the selection of
analysis methodology and
procedures. In embodiments, these methods include a step of verifying
insurance eligibility of said subject prior to,
concurrent with or subsequent to said analysis. In embodiments, these methods
include generating a report for a
subject based on the evaluation.
[00149] In accordance with aspects of the methods disclosed herein, the device
may be a Clinical
Laboratory Improvement Amendments (CLIA)-waived device. In accordance with
other aspects of the methods
disclosed herein, the device may be a CLIA-compliant device, may be operated
in compliance with CLIA, may be
operated by a CLIA-compliant laboratory, or may be operated in a CLIA-
compliant location; or may be a CLIA-
certified device, may be operated by a CLIA-certified laboratory, or may be
operated in a CLIA-certified location;
or may be a device that has been cleared for use by the U.S. Food and Drug
Administration; or may be a device that
has been classified exempt by the U.S. Food and Drug Administration; or may be
a device that has not been cleared
or approved by any regulatory body. The device may comprise a sample
processing device, or may comprise a
sample processing unit. The device may comprise a device that has been
classified by a regulatory body as a sample
processing device, or as a sample processing unit. The device may be a sample
processing device, or may comprise
a sample processing unit. The device may be a device that has been classified
by a regulatory body as a sample
processing device, or as a sample processing unit.
[00150] In accordance with aspects of the methods disclosed herein, the
authorized analytical facility
and/or affiliate thereof may be a Clinical Laboratory Improvement Amendments
(CLIA)-compliant laboratory, and
may be a CLIA-certified laboratory. In accordance with aspects of the methods
disclosed herein, the device may be
operated under the control or oversight of a CLIA-compliant laboratory, and
may be operated under the control or
oversight of a CLIA-certified laboratory. For example, in accordance with
aspects of the methods disclosed herein,
the device may be a CLIA-waived device operated under the control of a CLIA-
compliant or CLIA-certified
laboratory. In accordance with aspects of the methods disclosed herein, the
device may be a CLIA-waived device
operated under the oversight of a CLIA-compliant laboratory or CLIA-certified
laboratory. In accordance with other
-22-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
aspects of the methods disclosed herein, the device may be operated under the
oversight or control of a CLIA-
compliant laboratory, or a CLIA-certified laboratory, where the device may be
a CLIA-compliant device or a
CLIA-certified device; or may be a device that has been cleared for use by the
U.S. Food and Drug Administration;
or may be a device classified as exempt by the U.S. Food and Drug
Administration; or may be a device that has not
been cleared or approved by any regulatory body.
[00151] In accordance with another aspect of the systems, methods, and devices
disclosed herein,
Applicants disclose a system of evaluating a biological sample collected from
a subject, the system comprising:
[00152] (a) a communication unit placed at a laboratory location configured to
receive data from a device
placed in or on the subject or at a designated sample collection site, wherein
the device comprises a housing and is
configured to process a biological sample within said housing, said biological
sample comprising a cell, said
processing by said device generating raw data for a subsequent qualitative
and/or quantitative evaluation of said
biological sample, and wherein the device comprises: (i) a sample collection
unit within said housing configured to
receive the biological sample; (ii) a sample preparation unit within said
housing configured to prepare the biological
sample and to yield raw data for the evaluation within said housing, wherein
said raw data (1) comprises numerical
values representative of a physical process or chemical reaction performed by
the device and (2) and electronic data
representative of an image of a cell in said biological sample; and a (iii)
transmission unit configured to transmit the
raw data from said sample collection site to an authorized analytical facility
and/or an affiliate thereof at said
laboratory location;
[00153] (b) a processor at said laboratory location that processes said data
alone or in conjunction with an
individual affiliated with the authorized analytical facility for (a) the
evaluation of said biological sample at the
authorized analytical facility and/or the affiliate thereof, and (b) the
oversight of said integrity of evaluation and
operation of said device, such that results generated from said evaluation can
be utilized by a health care
professional for screening, diagnosis or treatment of said subject. An
authorized analytical facility may be a Clinical
Laboratory Improvement Amendments (CLIA)-compliant laboratory. A designated
sample collection site may be a
retailer site, a military site, the subject's home, a health assessment
location, or a health treatment location.
Evaluation of a biological sample may be effected without physically
transporting said sample from the site where
the sample is collected to the authorized analytical facility or an affiliate
thereof.
[00154] In embodiments, sample preparation units of such systems may include a
fluid handling system
comprising a pipette within the housing; and may include a centrifuge within
the housing. Electronic data
representative of an image of a cell in said biological sample may include
electronic data derived from an optical
assessment of histology or morphology of said cells, and raw data may include
raw data from an image of a
physical process or chemical reaction performed by the device with said
biological sample or a portion thereof.
Raw data may include raw data from at least two assays, or from at least three
assays, selected from an
immunoassay, a nucleic acid assay, a receptor-based assay, and an enzymatic
assay.
[00155] In accordance with aspects of the systems disclosed herein, the device
may be a Clinical
Laboratory Improvement Amendments (CLIA)-waived device. In accordance with
other aspects of the systems
disclosed herein, the device may be a CLIA-compliant device, may be operated
in compliance with CLIA, may be
operated by a CLIA-compliant laboratory, or may be operated in a CLIA-
compliant location; or may be a CLIA-
certified device, may be operated by a CLIA-certified laboratory, or may be
operated in a CLIA-certified location;
-23-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
or may be a device that has been cleared for use by the U.S. Food and Drug
Administration; or may be a device that
has been classified exempt by the U.S. Food and Drug Administration; or may be
a device that has not been cleared
or approved by any regulatory body.
[00156] In accordance with aspects of the systems disclosed herein, the
authorized analytical facility
and/or affiliate thereof may be a Clinical Laboratory Improvement Amendments
(CLIA)-compliant laboratory. In
accordance with aspects of the systems disclosed herein, the device may be
operated under the control or oversight
of a CLIA-compliant laboratory, or CLIA-certified laboratory. For example, in
accordance with aspects of the
systems disclosed herein, the device may be a CLIA-waived device operated
under the control of a CLIA-compliant
or CLIA-certified laboratory. In accordance with aspects of the systems
disclosed herein, the device may be a
CLIA-waived device operated under the oversight of a CLIA-compliant of CLIA-
certified laboratory. In accordance
with other aspects of the systems disclosed herein, the device may be operated
under the oversight or control of a
CLIA-compliant or CLIA-certified laboratory, where the device may be a CLIA-
compliant device or CLIA-
certified device; or may be a device that has been cleared for use by the U.S.
Food and Drug Administration; or may
be a device classified as exempt by the U.S. Food and Drug Administration; or
may be a device that has not been
cleared or approved by any regulatory body.
[00157] In embodiments of systems disclosed herein, a processor at a
laboratory location may be
configured to generate a report; may be configured to communicate with a
record database comprising one or more
medical records of, or insurance information for, the subject; and may be
configured to communicate with a payer
database comprising the insurance information for the subject.
[00158] In accordance with another aspect of the methods, systems, and devices
disclosed herein,
Applicants disclose a method of evaluating a biological sample collected from
a subject, said method comprising:
(a) transmitting protocol information from a laboratory to a device placed at
a sample collection site, said device
comprising a housing, wherein said protocol information identifies, updates,
or comprises a protocol that governs
actions performed and data collected by the device; (b) contacting a
biological sample collected from a subject at
said sample collection site with a first plurality of reagents selected from a
second plurality of reagents according to
said protocol, wherein said the number of reagents in said second plurality is
greater than the number of reagents in
said first plurality of reagents; (c) transmitting data from the device to the
laboratory, wherein said data comprises
data obtained from said biological sample within said housing of said device
according to said protocol; (d)
analyzing said transmitted data at said laboratory effective to provide an
evaluation of said biological sample,
wherein said analysis is performed using a processor at said laboratory or by
an individual associated with said
laboratory; and (e) providing oversight, wherein said oversight comprises
oversight of integrity of said analyzing
transmitted data or oversight of the operation of said device at said sample
collection site, effective that results
generated from analyzing transmitted data are suitable for use by a health
care professional for screening, diagnosis
or treatment of the subject, wherein the oversight is performed using a
processor at said laboratory or by an
individual associated with said laboratory. Contacting a biological sample
with a first plurality of reagents may
include transporting a reagent or at least a portion of a biological sample
with a pipette. In embodiments,
transmitted data may be obtained following centrifugation of at least a
portion of a biological sample within the
housing.
-24-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00159] In embodiments, the evaluation of a biological sample is effected
without physically transporting
the sample from the sample collection site to the laboratory location. In
embodiments, a sample collection site may
be selected from the group consisting of a site in said subject, a site on
said subject, a retail site, a military site, the
subject's home, a health assessment location, and a health treatment location.
[00160] In embodiments, transmitting data to a laboratory may include
transmitting raw data to a
laboratory. In embodiments, analyzing transmitted data may be effective to
determine the presence or concentration
of analyte present in the biological sample, or a disease condition associated
with the biological sample. In
embodiments, such methods include transmitting identification information from
the sample collection site to the
laboratory; and include selecting or generating said protocol information
transmitted from the laboratory to the
device based on said identification information. A laboratory may be an
authorized analytical facility, an affiliate of
an authorized analytical facility, or a CLIA-compliant laboratory.
[00161] In embodiments, a device may be configured to accept a cartridge, the
cartridge may include a
second plurality of reagents, and may be configured to allow delivery of said
first plurality of reagents to said
device. A cartridge may be configured to deliver a biological sample to the
device. Identification information may
include subject identifying information, information based on signals
generated related to the sample, information
based on signals generated related to reactions performed with the sample,
information based on signals detected
related to the sample, information based on signals detected related to
reactions performed with the sample, device
identification information, cartridge identification information, component
identifying information, and other
information transmitted from the device.
[00162] In embodiments, a protocol may include instructions regarding one or
more of: preparation of a
sample; preparation of a plurality of samples; performing a chemical reaction;
performing a plurality of chemical
reactions; sequence of performing a plurality of chemical reactions;
performing a clinical test; performing a
plurality of clinical tests; sequence of performing a plurality of clinical
tests; detecting the presence of an analyte;
detecting the presences of a plurality of analytes; sequence of detecting the
presences of a plurality of analytes;
detecting the concentration of an analyte; detecting the concentrations of a
plurality of analytes; sequence of
detecting the concentrations of a plurality of analytes; pre-processing data;
and sequence of steps in processing data.
In embodiments, protocol information may be changed according to transmitted
data obtained from said biological
sample within said housing of said device according to said protocol.
[00163] In embodiments, a biological sample may have a volume of 250 jIL or
less. In embodiments, a
biological sample may include cells, wherein the device is configured to
process the biological sample within said
housing effective to obtain raw data from the sample, raw data may include:
electronic data representative of an
image derived from an optical assessment of histology, morphology, kinematics,
or dynamics of cells in at least a
portion of said biological sample; and (2) data derived from an assay
performed on at least a portion of said
biological sample, wherein said assay is selected from an immunoassay, a
nucleic acid assay, a receptor-based
assay, an enzymatic assay, and a spectroscopic assay. In embodiments, raw data
may be obtained from two or more,
or from three or more, assays selected from immunoassays, nucleic acid assays,
receptor-based assays, enzymatic
assays, and spectroscopic assays. In embodiments, obtaining raw data may
include obtaining electronic data
representative of an image or images derived from two or more optical
assessments selected from optical
-25-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
assessments of histology, morphology, kinematics, and dynamics of cells in at
least a portion of said biological
sample.
[00164] In embodiments, transmitting data may include transmitting data
derived from a plurality of types
of biological samples collected from the subject, at least one of the
biological samples comprising cells.
[00165] Embodiments of the methods disclosed herein include embodiments where
obtaining a sample
comprises obtaining a first sample at a first time, and obtaining a subsequent
sample at a subsequent time.
[00166] In embodiments, providing oversight may include providing oversight of
the operation of the
device by a processor at said laboratory location; and may include selection
of analysis methodology and
procedures used in analyzing the transmitted data. In embodiments, these
methods may include verifying insurance
eligibility of said subject; such verification may be performed at a time
prior to, at a time concurrent with, or at a
time subsequent to, said analysis.
[00167] In embodiments, methods disclosed herein may further comprise
generating a report regarding
said subject based on said evaluation of said biological sample.
[00168] In embodiments of methods, systems, and devices disclosed herein, a
device may comprise a
temperature sensor, wherein the sensor may be configured to detect and measure
temperature within the device or
external to the device. A temperature within the device may include a
temperature of a sample, a reagent, a
component of the device, a region, a surface, or a compartment within the
device. In embodiments of methods,
systems, and devices disclosed herein, a device may comprise a temperature
control component effective to
maintain a temperature in the device at a desired temperature, or within a
desired temperature range. A temperature
may be measured, or may be maintained, on a surface or within a region,
compartment, or component of the device.
In embodiments of the methods, systems, and devices disclosed herein, a device
may be configured to report a
temperature to a laboratory, wherein the temperature may be a temperature of a
surface of the device, a temperature
within the device, a temperature within a region of the device, a temperature
within a compartment of the device, a
temperature within a component of the device, or a temperature external to the
device.
[00169] Other goals and advantages of the invention will be further
appreciated and understood when
considered in conjunction with the following description and accompanying
drawings. While the following
description may contain specific details describing particular embodiments of
the invention, this should not be
construed as limitations to the scope of the invention but rather as an
exemplification of preferable embodiments.
For each aspect of the invention, many variations are possible as suggested
herein that are known to those of
ordinary skill in the art. A variety of changes and modifications can be made
within the scope of the invention
without departing from the spirit thereof.
INCORPORATION BY REFERENCE
[00170] All publications, patents, and patent applications mentioned in this
specification are herein
incorporated by reference to the same extent as if each individual
publication, patent, or patent application was
specifically and individually indicated to be incorporated by reference.
-26-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
BRIEF DESCRIPTION OF THE DRAWINGS
[00171] The novel features of the invention are set forth with particularity
in the appended claims. A
better understanding of the features and advantages of the present invention
will be obtained by reference to the
following detailed description that sets forth illustrative embodiments, in
which the principles of the invention are
utilized, and the accompanying drawings of which:
[00172] FIG. lA shows an operation scheme involving a laboratory, a sample
collection site, and a health
care professional.
[00173] FIG. 1B shows a retailer having a processing device in communication
with a laboratory (e.g. a
CLIA certified laboratory).
[00174] FIG. 2 shows a processing device that can be placed in a designated
sample collection site and is
configured to be in communication over a network with one or more other
devices.
[00175] FIG. 3A illustrates various exemplary components of a processing
device.
[00176] FIG. 3B illustrates another example of a device.
[00177] FIG. 4 shows an example of a sample collection, processing, and
analysis method.
[00178] FIG. 5 shows a laboratory benefit manager in communication with a
payer and a sample
collection site.
[00179] FIG. 6 shows a laboratory benefit system provided in accordance with
an embodiment of the
invention.
[00180] FIG. 7 shows an example of a laboratory benefit manager/wholesaler
model in accordance with
an embodiment of the invention.
[00181] FIG. 8 shows examples of a system providing sample processing,
analysis, and oversight.
[00182] FIG. 9 shows further examples of a system providing sample processing,
analysis, and oversight.
[00183] FIG. 10A shows an example of a laboratory benefit system provided in
accordance with an
embodiment of the invention.
[00184] FIG. 10 B shows an example of a laboratory benefit system provided in
accordance with an
embodiment of the invention.
[00185] FIG. 10 C shows an example of a laboratory benefit system provided in
accordance with an
embodiment of the invention.
[00186] FIG. 10 D shows an example of a laboratory benefit system provided in
accordance with an
embodiment of the invention.
DETAILED DESCRIPTION
[00187] While preferable embodiments of the invention have been shown and
described herein, it will be
obvious to those skilled in the art that such embodiments are provided by way
of example only. Numerous
variations, changes, and substitutions will now occur to those skilled in the
art without departing from the invention.
-27-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
It should be understood that various alternatives to the embodiments of the
invention described herein may be
employed in practicing the invention.
[00188] The invention provides systems and methods for collecting and
transmitting data relating to a
sample, and often representative of the sample so that further analysis of the
sample does not require physical
transportation of the sample. Various aspects of the invention described
herein may be applied to any of the
particular applications set forth below or for any other types of diagnostic
or assay systems. The invention may be
applied as a standalone system or method, or as part of an integrated system,
such as in a system between
laboratories, health care professionals, and sample collection sites. It shall
be understood that different aspects of
the invention can be appreciated individually, collectively, or in combination
with each other.
[00189] It must be noted that, as used in the specification and the appended
claims, the singular forms "a",
"an", and "the" include plural referents unless the context clearly dictates
otherwise. Thus, for example, reference to
"a reagent" refers to a single reagent and to multiple reagents, and reference
to "an assay" refers to a single assay
and to multiple assays.
[00190] As used in the description herein and throughout the claims that
follow, the meaning of "in"
includes "in" and "on" unless the context clearly dictates otherwise.
[00191] As used in the description herein and throughout the claims that
follow, the meaning of "or"
includes both the conjunctive and disjunctive unless the context expressly
dictates otherwise. Thus, the term "or"
includes "and/or" unless the context expressly dictates otherwise.
[00192] As used in the description herein, the term "embodiments" and the
phrase "in embodiments" and
linguistic variants thereof refer to exemplary features, elements,
capabilities, and combinations thereof, of devices,
systems, and methods as disclosed herein. Disclosure of a particular feature,
or element, or capability, or particular
combination of features, elements, or capabilities, is not limiting, but are
illustrative of such, and may include other
combinations and other aspects of such features, elements, and capabilities.
[00193] In this specification and in the claims that follow, reference will be
made to a number of terms,
which shall be defined to have the following meanings:
[00194] The term "analyte" and its plural and other forms, as used herein,
includes without limitation
drugs, prodrugs, pharmaceutical agents, drug metabolites, biomarkers such as
expressed proteins and cell markers,
antibodies, antigens, proteins, hormones, polypeptides, glycoproteins,
polysaccharides, lipids, viruses, cholesterol,
polysaccharides, nucleic acids, genes, nucleic acids, and combinations
thereof.
[00195] As used herein, the term "cell count" refers to a qualitative or
quantitative assessment of the
number of cells in a sample, or in a volume, or in a field of view, or on a
surface. An estimate of the number of
cells in a sample, e.g., by absorbance of light passing through, or scattered
or otherwise altered by cells in a sample;
by light or other radiation emitted by cells expressing fluorescent or
otherwise detectable proteins, or labeled by
dyes, radionuclides, or other markers; an enumeration of the number of cells
in an image (e.g., an image of a blood
or urine sample, a tissue slice, or other biological sample, acquired by a
camera, a microscope, or other optical
imaging device), and a quantitative measurement of the number of cells
adherent to a surface are all examples of a
cell count.
-28-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00196] As used herein, the term "cytometry" refers to observations, analysis,
methods, and results
regarding cells of a biological sample, where the cells are substantially at
rest in a fluid or on a substrate. Cells
detected and analysed by cytometry may be detected and measured by any
optical, electrical or acoustic detector.
Cytometry may include preparing and analyzing images of cells in or from a
biological sample (e.g., two-
dimensional images). The cells may be labeled (e.g., with fluorescent,
chemiluminescent, enzymatic, or other
labels) and plated (e.g., allowed to settle on a substrate) and detected.
Cells may be, for example, imaged by a
camera. A microscope may be used for cell imaging in cytometry; for example,
cells may be imaged by a camera
and a microscope, e.g., by a camera forming an image using a microscope. An
image formed by, and used for,
cytometry typically includes more than one cell. As used herein, the term
"cytometry" refers to measurements made
without substantial cell motion with respect to the device (e.g., the cells
are observed and imaged while at rest with
respect to a surface of the device). In this regard, the term "cytometry" as
used herein is different from, and refers to
different observations, analysis, methods, and results than are referred to by
the more limited term "flow
cytometry."
[00197] As used herein, the term "flow cytometry" refers to observations and
analysis of cells, where the
cells are in motion within a device, or with respect to a surface of a device.
Flow cytometry typically uses a mobile
liquid medium that sequentially carries individual cells to an optical,
electrical or acoustic detector; e.g., in a typical
flow cytometer, cells are carried by a moving fluid past a stationary
detector.
[00198] As used herein, the term "microscopy" refers to the use of magnifying
lenses and other optical
methods to provide an image of a target, such as a cell or a plurality of
cells. Microscopy typically uses optical or
acoustic means to detect stationary cells, generally by recording at least one
magnified image. An image formed by
microscopy typically provides greater resolution than is possible when viewing
the target by eye alone; for example,
the image may be enlarged. Optical methods and techniques used in microscopy,
in addition to the use of lenses for
refraction, include the use of mirrors, prisms, filters, polarizers, gratings,
grids, light sources, scanning methods and
techniques (e.g., as used in confocal microscopy), special apertures (e.g., a
pin-hole), and other methods.
[00199] As used herein, "spectroscopy" refers to assays and measurements using
light intensity, including
light intensity as a function of light wavelength to detect and assess a
sample. Spectoscopy includes measurements
of the reflection or transmission of electromagnetic waves, including visible,
UV, and infrared light. Spectroscopy
includes any and all assays that produce luminescence or change light (e.g.,
color chemistry). These may include
one or more of the following: spectrophotometry, fluorimetry, luminometry,
turbidimetry, nephelometry,
refractometry, polarimetry, and measurement of agglutination.
[00200] The term "fluorimetry" as used herein refers to measuring the light
emitted by a fluorescent
molecule coupled to a subject upon exciting the fluorescent molecule with
incident light.
[00201] As used herein, "luminometry" refers to measurements and observations
that use no external
illumination method, but instead detects electromagnetic radiation emitted by
or from the object, chemical reaction,
or area of interest. The emitted light may be weak, and thus luminometry may
require the detection of low light or
other radiation levels; such signals can be detected using an extremely
sensitive sensor such as a photomultiplier
tube (PMT). Luminometry includes assays that produce chemiluminescence, such
as those using luciferases or
some assays using peroxidases.
-29-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00202] As used herein, "turbidimetry" refers to detection, measurement, or
observation of a sample or
reaction in a sample by backlighting the sample and components within the
sample with white light, with the result
being sensed by an imaging sensor. The reduction of the intensity of the
transmitted light is measured (the intensity
of the incident light being known) . Turbidimetry may be used, for example, to
determine a concentration of cells in
solution. In some embodiments, turbimetry is measured by nephelometry.
[00203] As used herein, the term "nephelometry" refers to measurements of
light that is transmitted or
scattered after passing through a suspension, e.g., a suspension of target
analytes in a solution. For example, the
amount of a substrate bound to an immunoglobin such as IgM, IgG, and IgA may
be measured by nephelometry.
[00204] As used herein, the term "polarimetry" refers to measurements of the
polarization of light or other
electromagnetic radiation following reflection, refraction, or other contact
with subject object or field. Polarimetry
assays include circular dichroism, which may provide structural information
and light scattering assays, which may
provide information about the size and/or shape of the subject. One
nonlimiting example of light scattering assays
uses dynamic light scattering (DLS).
[00205] As used herein, the terms "colorant" and "chromogen" refer to a
compound which produces or
provides a detectable change in color, absorbance, turbidity, or other optical
property of a medium. Chromagens
may be used to signal the occurrence, progress, or results of a chemical
reaction, which may be detected and
measured by colorimetric or other means (e.g., by luminometer,
spectrophotometer, or other light detector).
[00206] As used herein, the terms "product formation," "colored product,"
"colored product formation,"
and the like are used to refer to the act of, and the products that result
from, addition of a colorant to a solution. For
example, addition of a colorant to a solution may result in a reaction
effective to alter an optical property of the
solution. Such a reaction may result in the formation of molecules originally
not present in the solution, or may
result in the aggregation of molecules or compounds previously in the
solution, or may result in the degradation or
other alteration of molecules or compounds previously in the solution,
effective to alter the color, absorbance,
and/or other optical properties of a solution to which a colorant is added.
[00207] As used herein, the terms "reflex" and "reflex testing" refer to the
initiation, modification or
repetition of a protocol, measurement, assay method, or analysis based on
information or results obtained following
an initial measurement, assay, or analysis. A reflex test may be performed
where information is obtained by an
initial measurement that can be supplemented by further testing, suggests that
a more precise or specific test should
be performed, or that an additional test should be performed for an analyte or
condition related to or suggested by
the initial measurement. Typically, a reflex test is performed based on the
results of an initial test; the initial test is
typically less sensitive, cheaper or faster than the reflex test. For example,
reflex testing may occur when, after
detecting a possible abnormality with an initial PAP smear, a more specific or
precise test, such as one using nucleic
acid analysis, may be performed to more accurately assess the subject's
condition. Another example of reflex
testing might include measurement of the folate level in the blood of a
subject based on finding a low hematocrit
level in an initial blood test.
[00208] As used herein, the terms "sample" and "biological sample" include the
entire sample and include
a portion, or portions, of the entire sample, unless the context clearly
dictates otherwise. A "sample" may include,
but is not limited to, a sample selected from the group consisting of blood,
serum, plasma, a nasal swab, a
nasopharyngeal wash, saliva, urine, tears, a gastric fluid, spinal fluid,
stool, mucus, sweat, earwax, oil, a glandular
-30-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
secretion, cerebral spinal fluid, tissue, semen, vaginal fluid, a throat swab,
breath, hair, finger nails, skin, biopsy
tissue or fluid, placental fluid, amniotic fluid, cord blood, lymphatic
fluids, cavity fluids (e.g, a fluid collected or
drained from a body cavity of a subject), sputum, mucus, pus, microbiota,
meconium, breast milk and other
secretions and excretions.
[00209] As used herein, "processing" a sample refers to actions taken to
receive, modify, test, represent,
and characterize a sample, and may include diluting a sample, subjecting a
sample to treatment (e.g., centrifugation,
filtration, or other treatment), fractionating or separating sample
constituents, contacting a sample with a reagent,
staining or dying a sample, observing a sample, testing a sample, or other
action, e.g., for determination of
characteristics or properties of the sample, Processing a sample may include
obtaining an image or representation of
a sample or sample constituents,
[00210] As used herein, the term "sample collection site" refers to a location
at which a sample may be
obtained from a subject. A sample collection site may be, for example, a
retailer location (e.g., a chain store,
pharmacy, supermarket, or department store), a provider office, a physician's
office, a hospital, the subject's home,
a military site, an employer site, or other site or combination of sites. As
used herein, the term "sample collection
site" may also refer to a proprietor or representative of a business, service,
or institution located at, or affiliated
with, the site. Thus, for example, the phrases "an electronic bill from the
designated sample collection site" and "an
electronic payment from the designated sample collection site" refer to a bill
or payment from the proprietor, or the
representative of the proprietor, located at, or affiliated with, the sample
collection site.
[00211] As used herein, the terms "retailer", "retailer site, "retail site",
and the like, refer to a location at
which a retail operations (sales and other commercial transactions) occur. As
used herein, these terms may also
refer to a proprietor or representative of a business, service, or institution
located at, or affiliated with, the retail site.
[00212] As used herein, the phrase "no processing of the sample by the subject
or by an operator" and
linguistic equivalents means that a sample, such as a blood sample, urine
sample, stool sample, or other sample,
may be placed directly in a container for further processing, and that no
action other than those required to effect the
placement of the sample in the container is required.
[00213] As used herein, "numerical values", such as, e.g., numerical values
representative of a physical
process or chemical reaction performed by the device, refer to output of a
sensor, detector, or other component or
device for measuring a physical parameter such as light intensity, absorbance
of light, temperature, pH, or other
parameter useful for the measurement of a physical process or chemical
reaction. Such numerical values may be
analog values (i.e., continuous variables) or digital values (i.e., discrete
variables), and may be summed, averaged,
normalized, binned, or otherwise manipulated as needed or convenient for the
measurement.
[00214] As used herein, "electronic data representative of an image" refers to
electronic output of a
camera, microscope, charge-coupled device, or other sensor capable of
providing data that can be used to form an
image or a representation of an image. Where configured to form or provide an
image (e.g., in arrays), sensors and
components including photodiodes, photomultiplier tubes, photoelectric cells,
and other light-sensitive electronic
components may be used to provide, in whole or in part, electronic data
representative of an image.
-31-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00215] As used herein, the term "data" includes all information, in any form,
obtained from or related to
a test, measurement, or observation of a sample (including a portion of a
sample) or a reaction in which a sample
participates. Data includes raw data, pre-processed data, and processed data.
[00216] As used herein, "raw data" includes signals and direct read-outs from
sensors, cameras, and other
components and instruments which detect or measure properties or
characteristics of a sample. For example, raw
data includes voltage or current output from a sensor, detector, counter,
camera, or other component or device; raw
data includes digital or analog numerical output from a sensor, detector,
counter, camera, or other component or
device; and raw data may include digitized or filtered output from a sensor,
detector, counter, camera, or other
component or device. For example, raw data includes the output of a
luminometer, which may include output in
"relative light units" which are related to the number of photons detected by
the luminometer. Raw data may
include a JPEG, bitmap, or other image file produced by a camera. Raw data may
include cell counts; light intensity
(at a particular wavelength, or at or within a range of wavelengths); a rate
of change of the output of a detector; a
difference between similar measurements made at two times; a number of events
detected; the number of events
detected within a pre-set range or that meet a pre-set criterion; the minimum
value measured within a time period,
or within a field of view; the maximum value measured within a time period, or
within a field of view; and other
data. Where sufficient, raw data may be used without further processing or
analysis; typically, raw data is further
processed or used for further analysis related to the sample, the subject, or
for other purposes.
[00217] As used herein, "pre-processed" data includes data derived from raw
data that has been baseline
corrected, filtered, summed, averaged, normalized, scaled, or otherwise
manipulated. Pre-processed data may
include binned data, or transformed data (e.g., time domain data transformed
by Fourier Transform to frequency
domain), or may be combined with other data. The pre-processing may put the
data into a desired form, and may
involve modifying the format of data; however, data pre-processing does not
perform actual data analysis or
comparison with any threshold values, and does not alter the content of the
data.
[00218] As used herein, "processed data" includes data and analyses resulting
from combination,
manipulation or analysis of raw data, pre-processed data, or other processed
data. Processing may include
comparison (e.g., with a baseline, threshold, standard curve, historical data,
or data from other sensors),
combination, mathematical manipulation or correction, curve-fitting, use of
data as the basis of mathematical or
other analytical reasoning (including deductive, inductive, Bayesian, or other
reasoning), and other forms of
processing known to those of skill in the art.
[00219] As used herein, the term "immunoassay" refers to tests which utilize
antibodies (including
antibody fragments) and their binding to target molecules to label, identify,
quantify, or otherwise provide
information regarding the presence, amounts, and properties of target
molecules and samples containing them. One
useful immunoassay that can be run on a device disclosed herein is an ELISA
(Enzyme-Linked ImmunoSorbent
Assay),Immunoassays also include, for example, competitive binding assays,
sandwich assays, Western blots, and
other assays utilizing antibodies and antibody fragments.
[00220] As used herein, the term "nucleic acid" refers to molecules formed of
chains of nuclecotides, such
as deoxyribonucleic acid molecules (DNA), ribonucleic acid molecules (RNA),
and including locked nucleic acids,
"peptide nucleic acids" (PNA) and other nucleic acid analogs similar to, or
which mimic, DNA or RNA.
-32-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00221] As used herein, the term "nucleic acid assay" refers to any and all
assays which utilize nucleic
acids or which detect nucleic acids in a sample. Nucleic acids hybridize to
complementary nucleic acids, a property
which is useful for identifying target nucleic acids, and for identifying
samples, whether fluid, tissue, or other
samples, containing target nucleic acids. Nucleic acid assays use, and
include, techniques for amplifying target
nucleic acids (e.g., by producing copies of target nucleic acids, or copies of
nucleic acids that are complementary to
target nucleic acids). Nucleic acid assays include, for example, assays using
polymerase chain reaction (PCR),
Southern blots, Northern blots, and other assays which may identify and allow
detection of nucleic acids in a
sample.
[00222] As used herein, the term "receptor-based assay" refers to an assay
which utilizes, or detects, the
binding of a receptor to its ligand, or the dissociation of a ligand from its
receptor. Such assays may use the binding
directly to detect or quantify the presence or amount of a receptor or ligand,
may use competitive binding
techniques to detect or quantify the presence or amount of a target molecule
in a sample, or may detect or quantify
the presence or amount of a target by use of other methods based on binding
between a receptor and ligand or
ligands.
[00223] As used herein, the term "enzymatic assay" refers to an assay which
utilizes, or detects, the
presence or action of an enzyme. For example, an assay which provides a
substrate for a target enzyme, and detects
the presence of that enzyme, or quantifies the activity of that enzyme in a
sample following addition of the substrate
is an enzymatic assay. An assay which utilizes the enzymatic production of a
detectable substance is another
example of an enzymatic assay; for example, colorimetric assays (e.g., in
which a detectable product is produced by
an enzyme, which may be an endogenous enzyme or which may be supplied with the
assay reagents) such as assays
in which horseradish peroxidase or alkaline phosphatase is used to produce a
colored product as an indicator of the
progress of the reaction or presence of a target, are enzymatic assays.
[00224] As used herein, "Clinical Laboratory Improvement Amendments" and
"CLIA" refer to sections of
42 U.S.C. Part F, e.g., subpart 2, sections 263a through 263a7, Federal
Regulations 42 CFR Chapter W (sections
493.1 to 493.2001), and related laws, regulations, and as amended. Regulations
pursuant to CLIA are administered
by the Centers for Medicare and Medicaid Services (CMS) of the United States
Department of Health and Human
Services.
[00225] As used herein, the term "CLIA-compliant" means that a device, a
procedure, an operation, a
laboratory, or other facility, complies with CLIA, as amended.
[00226] As used herein, the term "CLIA-certified" means a device, a procedure,
an operation, a
laboratory, or other facility, that has been certified, by an appropriate
regulatory body empowered to do so, as
compliant with CLIA, as amended.
[00227] As used herein, the term "CLIA-compliant laboratory" means a
laboratory, or other facility, that
complies with CLIA, as amended.
[00228] As used herein, the term "CLIA-certified laboratory" means a
laboratory, or other facility, that
has been certified, by an appropriate regulatory body empowered to do so, as
being compliant with CLIA, as
amended. A CLIA-certified laboratory is a CLIA-compliant laboratory.
-33-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00229] As used herein, a device that is a "CLIA-compliant device" is a device
that complies with CLIA,
as amended, or whose use complies with CLIA, as amended.
[00230] As used herein, a "CLIA-waived device" is a device for which a
certificate of waiver, under
CLIA, has been issued by an appropriate regulatory body empowered to do so, as
compliant with CLIA, as
amended, or whose use complies with a certificate of waiver, under CLIA,
issued by an appropriate regulatory body
empowered to do so. A CLIA-waived device is a CLIA-compliant device.
[00231] As used herein, a "CLIA-certified device" is a device that has been
certified, by an appropriate
regulatory body empowered to do so, as compliant with CLIA, as amended, or
whose use complies with a
certification issued under CLIA by an appropriate regulatory body empowered to
do so. A CLIA-certified device is
a CLIA-compliant device.
[00232] As used herein, a device that has been "cleared under section 510(k)
of the U.S. Food, Drug and
Cosmetic Act" means a device that has been cleared by the U.S. Food and Drug
Administration, or its successor, for
sale or use in the United States under section 510(k) of the U.S. Food, Drug
and Cosmetic Act.
[00233] As used herein, a device for which there is "no substantial equivalent
under section 510(k) of the
U.S. Food, Drug and Cosmetic Act" means a device for which no substantial
equivalent device has been approved
for sale in the United States under section 510(k) of the U.S. Food, Drug and
Cosmetic Act.
[00234] As used herein, a device that has "not been cleared or approved by any
regulatory body" means a
device that has not received certification under CLIA, and has not been
cleared by the U.S. Food and Drug
Administration, or its successor, for sale or use in the United States under
section 510(k) of the U.S. Food, Drug and
Cosmetic Act.
[00235] As used herein, the phrase "operated under the control of a CLIA-
compliant laboratory" means
that the operation of a device, method, or system is being controlled by a
CLIA-compliant laboratory.
[00236] As used herein, the phrase "operated under the oversight of a CLIA-
compliant laboratory" means
that the operation of a device, method, or system is under the oversight of a
CLIA-compliant laboratory.
[00237] As used herein, the phrase "operated under the control or oversight of
a CLIA-compliant
laboratory" means: that the operation of a device, method, or system is being
controlled by a CLIA-compliant
laboratory; that the operation of a device, method, or system is under the
oversight of a CLIA-compliant laboratory;
or that the operation of a device, method, or system is being controlled by,
and is under the oversight of, a CLIA-
compliant laboratory.
[00238] FIG. lA shows a system comprising a laboratory 110, a designated
sample collection site 120,
and a health care professional 100. A device 130 may be provided at the
designated sample collection site. A
sample collection site may be a first location, and a laboratory may be
provided at a second location. The first
location and the second location may be different locations. The first and
second locations may be located so that
they are not proximate to one another. A health care professional may be
provided at a third location, although
he/she may be affiliated with, employed by, or contracted by the laboratory.
The third location may be a different
location from the first and second locations. The third location may be
located so that it is not proximate to the first
or second locations. A laboratory, health care professional, and sample
collection site may all be at different
-34-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
locations from one another. In one example, a laboratory, health care
professional, and/or sample collection site
may be at separate facilities. Alternatively, one or more of them may be at
the same location.
[00239] A laboratory can be an entity or facility or system or device capable
of performing a clinical test
or analyzing collected data. A laboratory can provide controlled conditions in
which scientific research,
experiments, and measurement can be performed. The laboratory can be a medical
laboratory or clinical laboratory
where tests can be done on clinical specimens, or analysis can occur on data
collected from clinical specimens, in
order to get information about the health of a patient as pertaining to the
screening, diagnosis, prognosis, treatment,
and/or prevention of disease. A clinical specimen may be a sample collected
from a subject. Preferably, a clinical
specimen may be collected from the subject at a sample collection site that is
at a separate facility from the
laboratory, as described in further detail elsewhere herein. The clinical
specimen may be collected from the subject
using a device, which is placed at a designated sample collection site or in
or on the subject.
In some embodiments, a laboratory may be a certified laboratory. The certified
laboratory may be an
authorized analytical facility. In some embodiments, authorized analytical
facilities may include contracted
analytical facilities. For example, a certified laboratory or other laboratory
may send images to experts at another
laboratory (which may be a certified laboratory) for analysis.
[00240] Any description herein of a laboratory may apply to an authorized
analytical facility and vice
versa. In some instances, the laboratory may be certified by a governmental
agency or professional association. A
laboratory may receive certification or oversight by a regulatory body. In one
example, the laboratory may be
certified by an entity, such as Centers for Medicare & Medicaid Services
(CMS), College of American Pathologists,
ISO standards 15189 or 17025 or equivalents thereof. For instance, an
authorized analytical facility may be a
Clinical Laboratory Improvement Amendments (CLIA) certified laboratory in the
United States or its equivalent in
a foreign jurisdiction.
[00241] An authorized analytical facility is typically subject to oversight or
regulation. For example, a
laboratory may have oversight by a board-certified entity (which may include
one or more board-certified
personnel). In some embodiments, oversight can include validating one or more
clinical test. Oversight may also
include assessing the performance of, correcting, calibrating, running
controls, replicates, adjusting, or analyzing
one or more clinical test. Oversight can include evaluation of one or more
sets of data to provide a quality control
for a clinical test. The authorized analytical facility can have one or more
qualified person providing the oversight.
For example, one or more pathologist or other health care professional may
review data and/or analysis that is
processed by the facility. At an authorized analytical facility, a trained
pathologist or other certified health care
professional may provide oversight. In some instances, the certified health
care professional providing oversight
may be one or more of the following: a doctor certified in pathology, a doctor
with laboratory training or experience
in the specialty areas of service for which the health care professional is
responsible, or an individual with
experience or laboratory training in the specialty.
[00242] The oversight may further include the certified health care
professional who may establish the
procedures and rules in the laboratory, deal with problems that arise, and/or
train/evaluate the lab personnel.
Oversight may also include selecting test methodology, verifying test
procedures and establishment of laboratory's
test performance characteristics, enrollment in participation in an HHS
approved proficiency testing program,
establishing a quality control program appropriate for the testing performed,
establishing the parameters for
-35-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
acceptable levels of analytic performance, ensuring that those levels are
maintained throughout the entire testing
process, resolving technical problems and ensuring that remedial actions are
taken when test systems deviate from
the established performance specifications, ensuring patient test results are
not reported until all corrective actions
have been taken, identifying training needs and assuring that each individual
performing tests receives regular in-
service training and education, evaluating the competency of all testing
personnel and assuring that the staff
maintain their competency to perform test procedures (e.g., also procedures
for evaluation of the staff: direct
observation of routine test performance, monitoring the recording/reporting of
results, review of intermediate test
results, records, etc, observation of performance of instrument maintenance,
assessment of test performance,
assessment of problem solving skills), and/or evaluating and documenting the
performance of individuals
responsible for moderate complexity testing (e.g., semiannually during the
first year; thereafter, at least annually
unless test methodology or instrumentation changes). Oversight may include
reviewing and/or verifying
functionality of laboratory procedures or devices, and/or validity of data
collected and/or generated. The oversight
may assure the quality of the rest and/or put the data into a condition upon
which a health care professional can rely
upon it to provide a screening, diagnosis, treatment, including but not
limited to prophylactic treatment. Oversight
may include reviewing a test empirically. Oversight may include one or more,
two or more, or any of the number
of items described elsewhere herein.
[00243] In some instances, the oversight may be provided by an oversight
software program rather than
the certified health care professional. In some instances, one, two or more of
the types of oversight provided may
be implemented by an oversight software program. A combination of an oversight
software program and health
care professional may be employed to provide oversight. In some instances,
one, two or more of the types of
oversight may be implemented by a health care professional over a software
program. For example, the health care
professional may determine the procedures and rules associated with the
software program. In some instances, the
software program may be self-learning. The software program may access an
increasing pool of data and/or
evolving rules or procedures.
[00244] In some embodiments, the oversight software program may be provided on
a device. The
oversight software program may be provided at a sample collection site, on or
off the device. The software program
may be provided a laboratory, such as an authorized analytical facility. In
some instances, the device may receive
updates to the oversight software program. The updates may or may not be
provided by the laboratory. The
oversight software may be stored in a memory, and may include computer
readable media comprising code,
instructions, or logics that may be capable of executing a step.
[00245] In some instances, the oversight software may include one or more
algorithm that may review a
qualitative and/or quantitative evaluation of the sample that may be
performed. The oversight software program
may look for outliers, may determine whether the qualitative and/or
quantitative evaluation was properly
performed, may perform one or more comparison with records or data points, may
perform statistical analysis of the
evaluation, or any other oversight action as described elsewhere herein. The
oversight software may be able to
perform one or more calibrations and/or diagnostics.
[00246] A health care professional of an authorized analytical facility may
receive and/or view data. A
health care professional of an authorized analytical facility may be
affiliated with or associated with the authorized
analytical facility. In some instances, the health care professional may be
employed by or under contract with the
-36-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
authorized analytical facility. The health care professional may be located at
the authorized analytical facility, may
be located remotely from the authorized analytical facility, or in another
analytical facility (e.g., hospital, center of
excellence, specialized leading path/group). In some instances, the health
care professional is not required to be on-
site at all times while testing is performed, or when data is received at an
authorized analytical facility, but may be
available on an as needed basis to provide consultation. The health care
professional may be accessible to provide
on-site, telephone and/or electronic consultation.
[00247] The health care professional providing oversight may be a different
individual from or the same
individual as the health care professional that may receive a report from the
authorized analytical facility for
diagnosing, treating, monitoring, or preventing a disease for the subject. For
example, a pathologist of an
authorized analytical facility may be a different individual from a
prescribing physician of the subject. A health
care professional of authorized analytical facility may be a reviewing health
care professional or an overseeing
health care professional. The health care professional who may receive the
report may be the health care
professional who has ordered the test that the subject has undertaken. A
different health care professional may
provide analysis, and a different health care professional may provide
oversight. Alternatively, the same health care
professional may provide both analysis and oversight.
[00248] A designated sample collection site may be a point of service (POS)
location. Any disclosure
herein of a sample collection site may also apply to a point of service
location and vice versa. A point of service
location where a sample may be collected from a subject or provided by a
subject may be a location remote to the
laboratory. The sample collection site may have a separate facility from a
laboratory. The sample may or may not
be collected fresh from the subject at the sample collection site.
Alternatively, the sample may be collected from
the subject elsewhere and brought to the sample collection site. A sample
collection site at a point of service
location may be a blood collection center, or any other bodily fluid
collection center. The sample collection site
may be a biological sample collection center. In some embodiments, a sample
collection site may be a retailer.
Examples of retailers are provided in further detail elsewhere herein. Other
examples of sample collection sites
may include hospitals, clinics, health care professionals' offices, schools,
day-care centers, health centers, assisted
living residences, government offices, traveling medical care units, mobile
units, emergency vehicles (e.g., air, boat,
ambulance), or the home. For example, a sample collection site may be a
subject's home. A sample collection site
may be at a sample acquisition site and/or health assessment and/or treatment
locations (which may include any of
the sample collection sites described elsewhere herein including but not
limited to emergency rooms, doctors'
offices, urgent care, tents for screening (which may be in remote locations),
a health care professional walking into
someone's house to provide home care). A sample collection site may be any
location where a sample from the
subject is received by the device. Any location may be designated as a sample
collection site. The designation may
be made by any party, including but not limited to the laboratory, entity
associated with the laboratory,
governmental agency, or regulatory body. Any description herein relating to
sample collection site or point of
service may relate to or be applied to retailers, hospitals, clinics, or any
other examples provided herein and vice
versa.
[00249] A device may be provided at the sample collection site. The device may
be configured to accept a
sample. The device may be referred to as a sample collection device. The
device may also be referred to as a
sample processing device. The device may also be referred to as a reader
device. Any description of a reader
device may apply to any device that may be capable of receiving a sample
and/or processing the sample. The
-37-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
device may accept a sample collected from a subject at the sample collection
site, or that the subject or subject's
proxy brings to the service location. The device may directly collect the
sample from the subject, or an intermediate
device or technique may be used to collect the sample from the subject.
Examples of collection techniques and
mechanisms are described in greater detail elsewhere herein.
[00250] In some instances, the device may be placed in or on a subject. For
example, a device may be
ingested by a subject (see e.g. U.S. Patent Publication No. 2006/0182738, U.S.
Patent Publication No.
2006/0062852, U.S. Patent Publication No. 2005/0147559, U.S. Patent
Publication No. 2010/0081894, which are
hereby incorporated by reference in their entirety). The device may be a pill
or have another format that may pass
through the digestive tract of a subject. The device may be implanted within
the subject. For example, the device
may be subcutaneously implanted within the subject. In another example, the
device may be worn by the subject.
The device may be attached to the subject via strap, adhesive, integrated into
clothing, or any other technique. The
device may comprise one or more needle or microneedle that may penetrate the
skin of the subject. The device may
be a patch that may be worn by the patient. The device may include an
automated lancing cartridge. The cartridge
may be disposable. One or more disposable component may be used to collect a
sample from a subject. The
disposable component may provide the sample to a non-disposable device.
Alternatively, the disposable component
may be the sample processing device.
[00251] The device may receive a sample from the subject at one time.
Alternatively, the device may
periodically receive a sample from the subject. This may be at regularly
scheduled intervals or in response to one or
more detected conditions. The device may optionally administer therapy to the
subject. The device may administer
one or more therapeutic agent to the subject. The therapeutic agent may be
administered at scheduled intervals or in
response to one or more detected conditions. The therapeutic agent may be
administered in response to one or more
detected conditions from the sample.
[00252] In some instances, the device may be provided to a subject at a
designated sample collection site.
Alternatively, the subject may obtain or come into contact with the device at
any other location.
[00253] Examples of samples may include various fluid or solid samples. In
some instances, the sample
can be a bodily fluid sample from the subject. The sample can be an aqueous or
gaseous sample. In some
instances, solid or semi-solid samples can be provided. The sample can include
tissues and/or cells collected from
the subject. The sample can be a biological sample. Examples of biological
samples can include but are not limited
to, blood, serum, plasma, a nasal swab, a nasopharyngeal wash, saliva, urine,
gastric fluid, spinal fluid, tears, stool,
mucus, sweat, earwax, oil, a glandular secretion, cerebral spinal fluid,
tissue, semen, vaginal fluid, interstitial fluids
derived from tumorous tissue, ocular fluids, spinal fluid, a throat swab,
breath, hair, finger nails, skin, biopsy,
placental fluid, amniotic fluid, cord blood, lymphatic fluids, cavity fluids,
sputum, pus, microbiota, meconium,
breast milk and/or other excretions. The samples may include nasopharyngeal
wash. Nasal swabs, throat swabs,
stool samples, hair, finger nail, ear wax, breath, and other solid, semi-
solid, or gaseous samples may be processed in
an extraction buffer, e.g., for a fixed or variable amount of time, prior to
their analysis. The extraction buffer or an
aliquot thereof may then be processed similarly to other fluid samples if
desired. Examples of tissue samples of the
subject may include but are not limited to, connective tissue, muscle tissue,
nervous tissue, epithelial tissue,
cartilage, cancerous sample, or bone. The sample may be provided from a human
or animal. The sample may be
provided from a mammal, vertebrate, such as mtrines, simians, humans, farm
animals, sport animals, or pets. The
-38-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
sample may be collected from a living or dead subject. The sample may be
collected fresh from a subject or may
have undergone some form of pre-processing, storage, or transport.
[00254] One or more, two or more, three or more, four or more, five or more,
six or more, seven or more,
eight or more, ten or more, twelve or more, fifteen or more, or twenty or more
different types of samples may be
collected from a subject. A single type of sample or a plurality of types of
samples may be collected from the
subject simultaneously or at different times. A single type of sample or a
plurality of types of samples may be
received or capable of being received by the device simultaneously or at
different times. A plurality of types of
samples may be processed by the device in parallel and/or in sequence. For
example, a device may be capable of
receiving both a bodily fluid and a tissue, or a stool sample and a bodily
fluid. In another example, a device may be
capable of receiving a plurality of types of bodily fluids, such as blood and
urine. For example, the device may be
capable of receiving one or more type, two or more type, three or more types,
four or more types, five or more
types, six or more types, seven or more types, eight or more types, ten or
more types, or twenty or more types of
bodily fluid.
[00255] Different collection mechanisms or the same collection mechanism of a
device may be used to
collect a plurality of types of samples.
[00256] A subject may provide a sample, and/or the sample may be collected
from a subject. A subject
may be a human or animal. The subject may be a mammal, vertebrate, such as
murines, simians, humans, farm
animals, sport animals, or pets. The subject may be living or dead. The
subject may be a patient, clinical subject,
or pre-clinical subject. A subject may be undergoing screening, diagnosis,
treatment, monitoring and/or disease
prevention. The subject may or may not be under the care of a health care
professional. The subject may be a
person of any age, an infant, a toddler, an adult or an elderly.
[00257] Any volume of sample may be provided from the subject. Examples of
volumes may include, but
are not limited to, about 10 mL or less, 5 mL or less, 3 mL or less, 1
microliter (nL, also "uL" herein) or less, 500
pi., or less, 300 pi., or less, 250 pi., or less, 200 pi., or less, 170 pi.,
or less, 150 pi., or less, 125 pi., or less, 100 pi., or
less, 75 pi., or less, 50 pi., or less, 25 pi., or less, 20 pi., or less, 15
pi., or less, 10 pi., or less, 5 pi., or less, 3 pi., or
less, 1 pi., or less, 500 nL or less, 250 nL or less, 100 nL or less, 50 nL or
less, 20 nL or less, 10 nL or less, 5 nL or
less, 1 nL or less, 500 pL or less, 100 pL or less, 50 pL or less, or 1 pL or
less. The amount of sample may be about
a drop of a sample. The amount of sample may be the amount collected from a
pricked finger or fingerstick. The
amount of sample may be the amount collected from a microneedle or a venous
draw. Any volume, including those
described herein, may be provided to the device.
[00258] A health care professional may include a person or entity that is
associated with the health care
system. A health care professional may be a medical health care provider. A
health care professional may be a
doctor. A health care professional may be an individual or an institution that
provides preventive, curative,
promotional or rehabilitative health care services in a systematic way to
individuals, families and/or communities.
Examples of health care professionals may include physicians (including
general practitioners and specialists),
dentists, audiologists, speech pathologists, physician assistants, nurses,
midwives, pharmaconomists/pharmacists,
dietitians, therapists, psychologists, chiropractors, clinical officers,
physical therapists, phlebotomists, occupational
therapists, optometrists, emergency medical technicians, paramedics, medical
laboratory technicians, medical
prosthetic technicians, radiographers, social workers, and a wide variety of
other human resources trained to
-39-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
provide some type of health care service. A health care professional may or
may not be certified to write
prescriptions. A health care professional may work in or be affiliated with
hospitals, health care centers and other
service delivery points, or also in academic training, research and
administration. Some health care professionals
may provide care and treatment services for patients in private homes.
Community health workers may work
outside of formal health care institutions. Managers of health care services,
medical records and health information
technicians and other support workers may also be health care professionals or
affiliated with a health care provider.
[00259] In some embodiments, the health care professional may already be
familiar with the subject or
have communicated with the subject. The subject may be a patient of the health
care professional. In some
instances, the health care professional may have prescribed the subject to
undergo a clinical test. The health care
professional may have instructed or suggested to the subject to undergo a
clinical test conducted at the sample
collection site or by the laboratory. In one example, the health care
professional may be the subject's primary care
physician. The health care professional may be any type of physician for the
subject (including general
practitioners, and specialists).
[00260] A health care professional may receive a report from an authorized
analytical facility. The health
care professional that receives a report may be an ordering health care
professional or health care professional in the
analytical facility and/or sample collection site.
[00261] A laboratory 110 may be in communication with a sample collection site
120 and a health care
professional 100. The laboratory may be in communication with any number of
sample collection sites and health
care professionals. For example, the laboratory may be in communication with
one or more, two or more, three or
more, five or more, ten or more, fifteen or more, twenty or more, 30 or more,
50 or more, 100 or more, 200 or more,
500 or more, 1000 or more, 5000 or more, 10,000 or more, 100,000 or more, or
1,000,000 or more sample
collection sites and/or health care professionals. In some systems, one, two,
three, four, or more laboratories may
be provided that may communicate with any number of sample collection sites
and/or health care professionals.
The laboratories may or may not communicate with one another. The sample
collection sites, laboratories, and/or
health care professionals may be scattered geographically at any location. In
some embodiments, the sample
collection sites and/or health care professionals in communication with a
laboratory may be in the same geographic
region (e.g., town, city, state, region, country). Alternatively, the sample
collection sites and/or health care
professionals in communication with a laboratory may be scattered anywhere
globally.
[00262] The laboratory may communicate with the health care professional and
the sample collection site
in any manner known in the art. In some embodiments, the laboratory may
communicate directly with a device
located at the sample collection site or in or on a subject. Such
communications may be via electronic signals,
radiofrequency signals, optical signals, cellular signals, or any other type
of signals that may be transmitted via a
wired or wireless connection. Any transmission of data or description of
electronic data or transmission described
elsewhere herein may occur via electronic signals, radiofrequency signals,
optical signals, cellular signals, or any
other type of signals that may be transmitted via a wired or wireless
connection. For example, data may be
transmitted electronically from a sample collection site to a laboratory and
vice versa. Data may be transmitted
from a device which may be at the sample collection site or in or on a subject
to the laboratory and vice versa.
Similarly, data may be transmitted electronically from a laboratory to a
health care professional and vice versa. The
communications may be over a network such as a local area network (LAN), wide
area network (WAN) such as the
-40-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
Internet, personal area network, a telecommunications network such as a
telephone network, cell phone network,
mobile network, a wireless network, a data-providing network, or any other
type of network. The communications
may utilize wireless technology, such as Bluetooth or RTM technology.
Alternatively, various communication
methods may be utilized, such as a dial-up wired connection with a modem, a
direct link such as TI, ISDN, or cable
line. In some embodiments, a wireless connection may be using exemplary
wireless networks such as cellular,
satellite, or pager networks, GPRS, or a local data transport system such as
Ethernet or token ring over a LAN. In
some embodiments, the device may communicate wirelessly using infrared
communication components. A device
130, personal computer, server, laptop computer, tablet, mobile phone, cell
phone, satellite phone, smartphone (e.g.,
iPhone, Android, Blackberry, Palm, Symbian, Windows), personal digital
assistant, Bluetooth device, pager, land-
line phone, or other network device may be used in order to provide
communications. Such devices may be
communication-enabled devices.
[00263] The laboratory may communicate with a device at a sample collection
site, or in or on a subject.
The device from the sample collection site may communicate with any
communication-enabled device of the
laboratory. The device may provide data to a cloud computing infrastructure
that may be accessed by any
communication-enabled device of the laboratory. The device may transmit data
to the laboratory.
[00264] The data provided by the device may include data relating to a sample
from a subject. The data
may be information necessary and/or sufficient for a qualitative and/or
quantitative evaluation of the sample. The
data may include information for oversight. The data may include information
for analysis. The data may be an
electronic representation of a sample. An electronic representation of a
sample may include an electronic
representation of the entire sample and/or any portion thereof. The data may
be electronic data. In some instances,
the data may be electronic bits representative of the sample or reaction or
reagents. The data may be digital and/or
analog. The data may be representative of one or more measurable parameter
relating to, based on, or of the
sample.
[00265] The data may be representative of a sample and/or any portion thereof.
In some embodiments, the
data is representative of a preparation of the collected biological sample.
The data may be collected prior to,
during, and/or after the preparation of the sample. The data may be collected
over time. The data may comprise
information of one or more conditions under which a preparation of the
collected biological sample occurs.
Examples of such conditions may comprise one or more characteristics listed
from the group: amount of the
biological sample, concentration of the biological sample, quality of the
biological sample, temperature, or
humidity. Such conditions may include environmental conditions. Environmental
conditions may refer to
conditions of the sample, and/or the surroundings of the sample. The
environmental conditions may be provided
prior to, during, and/or after the sample is received by the device, prepared
by the device, and/or data is transmitted
by the device.
[00266] The data may include amounts, concentrations, proportions, purity, or
other information of
sample, reagents, diluents, wash, dyes or any other material that may be
involved in the preparation of a sample,
reactions, and/or controls/calibrations on the device. Physical and/or
chemical properties of a sample and/or other
materials, and/or a chemical reaction may be measured at one or more points in
time, and may be aggregated as
data. In some embodiments, the data may determine whether a sample, reagent,
diluents, wash, dye, or any other
material is suitable for use in the device for said sample preparation and/or
to permit subsequent qualitative and/or
-41-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
quantitative evaluation. For example, the data may be indicative of any error
conditions that may indicate a sample
and/or any of the other materials have gone bad, or are otherwise unsuitable.
In some instances, data is collected
during any processes the device is performing.
[00267] In some embodiments, the data may be representative of a chemical
reaction which may be run by
the device. The chemical reaction may include a chemical reaction with the
sample, or without the sample. The
chemical reaction may include one or more reagents that may react with the
sample. The chemical reaction may
include a control or calibration reaction. The data representative of the
reaction may include one or more
measurement of the chemical reaction. The data may also include the rate or
speed of the chemical reaction, and/or
the acceleration of the chemical reaction. The data may include how complete a
chemical reaction is (e.g., whether
the chemical reaction has started, whether the chemical reaction is taking
place, whether the chemical reaction is
complete, how far along the chemical reaction is -- e.g., 10%, 50%, etc.). The
data may comprise information about
a control reaction and a chemical reaction involving the biological sample.
These reactions may occur
simultaneously and/or sequentially. The data may pertain to one or more
chemical reactions that may or may not
occur simultaneously. The data may pertain to one or more sample preparation
step that may or may not occur
simultaneously. The data may also include physical processing, such as
centrifugation, pulveration, or any other
actions described herein, which may be represented through bits of data. The
data can be utilized for oversight
functionally performed on-board, remotely by a health care professional,
and/or an external device configured to
render such oversight.
[00268] The occurrence, progress, or results of a chemical reaction may be
detected and measure by
colorimetric or other means. For example, chromogens (also termed herein,
e.g., colorants, colored products, and
other terms) that may be used with systems, devices, and methods provided
herein may include, for example, i)
substrates which may be oxidized (e.g. molecules that change color upon
oxidization, such as by peroxidase and
hydrogen peroxide), for example: aniline derivatives [e.g. 2-amino-4-
hydroxybenzenesulfonic acid (AHBS)(forms a
yellow dye upon oxidation which may be monitored at 415 nm); N-(2-hydroxy-3-
sulfopropy1)-3,5-
dimethyoxyaniline (forms a dye upon oxidation that may be monitored at 610
nm)], o-dianisidine (forms a yellow-
orange dye upon oxidation that may be monitored at 405 nm), 10-acetyl-3,7-
dihydroxyphenoxazine (ADHP) (forms
a dye upon oxidation that may be monitored, for example, colorimetrically at
570 nm or fluorescently at EX/EM =
535/587 nm), ii) substrates of kinases (e.g. molecules that change color upon
phosphorylation), for example: iii)
substrates of phosphatases (e.g. molecules that change color upon
dephosphorylation), for example: p-nitrophenyl
phosphate (pNPP) (forms p-nitrophenol upon dephosphorylation, which may be
measured by absorbance at 405
nm); iv) substrates of hydrolases (e.g. molecules that change color upon
hydrolysis), for example: ortho-
nitrophenyl-beta-galactoside (ONPG) (may be hydrolyzed by beta-galactosidase
to galactose and ortho-nitrophenol;
ortho-nitrophenol may be measured by absorbance at 420 nm); v) substrates
which may change color upon complex
formation, for example: o-cresolphthalein (forms a complex with calcium, which
may be measured by absorbance
at 575 nm).
[00269] Hydrogen peroxide, in the presence of horseradish peroxidase and
colorants such as an amino-
antipyrene (e.g., 4-aminoantipyrene) and an aniline-containing compound (e.g.,
N-Ethyl-N-(2-hydroxy-3-
sulfopropy1)-3,5-dimethoxyaniline) may react to form a colored product (e.g.,
a Trinder (e.g., quinoneimine) dye as
indicated in the figure).
-42-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00270] For example, HRP may react with an aniline-containing compound such as
N-Ethyl-N-(2-
hydroxy-3-sulfopropy1)-3,5-dimethoxyaniline (ALPS), or with an aminoantipyrene
compound such as 4-
aminoantipyrene or with phenolic compounds. Thus, for example, a peroxidase
(e.g., HRP, myeloperoxidase, or
other peroxidase), an aniline-containing compound, and an aminoantipyrene may
all be termed "colorants" or
"chromagens." In further examples, HRP may react with a benzidine-containing
compound (e.g., with
diaminobenzidine (DAB); tetramethylbenzidine(TMB); 2,2'-azino-bis(3-
ethylbenzothiazoline-6-sulphonic acid)
(DABS); 3-dimethylaminobenzoic acid (DMAB); hydroquinone; o-tolidine; o-
phenylenediamine; o-chlorophenol;
p-hydroxy-benzenesulfonate; p-anisidine; a Trinder reagent (such as 4-
aminoantipyrene,
methyiben7othia7otinonehydrazone (MBT11), or other compound for producing a
Trinder dye); and derivatives and
related compounds) to form a colored product. HRP or other peroxidase may also
react with other compounds to
form a chemiluminescent product; for example, HRP or other peroxidase may
react with luminol to form a
chemiluminescent product (other molecules may be present, and may enhance such
reactions; for example, HRP-
mediated production of luminescent products from luminol is enhanced in the
presence of 4-iodophenol).
[00271] Further chromagens include, for example, alkaline phosphatase;
resazurin (7-Hydroxy-3H-
phenoxazin-3-one 10-oxide); 10-acetyl-3,7-dihydroxyphenoxazine (Amplex Red)
and similar compounds (e.g.,
Amplex UltraRed (A36006 from Life Technologies, Carlsbad, CA 92008); resorufin
compounds (e.g., 7-
ethoxyresorufin); dyes such as e.g., fluorescein, calcein, rhodamine, and
ethidium dyes; N-methy1-4-hydrazino-7-
nitrobenzofurazan; acridinium (acridine-9-carboxylic acid) esters and
compounds which react with these
compounds to alter an optical property of a solution; phenols and phenol
derivatives (e.g., p-iodophenol and p-
phenylphenol); luminescent amines, including amine adducts (e.g., as may be
derived from copper cyanide), and
other molecules. It will be understood that other enzymes and reactants may be
used to form colored products, or to
detect an analyte in a biological sample, such as a blood sample.
[00272] In some examples, the data may be one or more image, and/or audio data
representative of the
sample. An image may be a digital image or an analog image. The audio data may
be digital and/or analog. The
data may include a video representative of the sample. An image may include a
video image. The data may include
electronic data representative of a digital image and/or audio data of the
sample. In one example, the data may
include video imaging that may capture changes over time. For example, a video
may be provided to provide
evaluation on dynamic actions, such as lysing, agglutination, mixing, movement
of cells or other molecules in a
sample or matrix, or assays.
[00273] The data may be collected at one time, or at a plurality of times. The
data may be collected at
discrete points in time, or may be continuously collected over time. Data
collected over time may be aggregated
and/or analyzed. In some instances, data may be aggregated and may be useful
for longitudinal analysis over time
to facilitate screening, diagnosis, treatment, and/or disease prevention.
[00274] Data may be collected from a device over time. The aggregated data
from a single device for a
given sample may be useful to facilitate the qualitative and/or quantitative
evaluation of the sample. For example, it
may be useful to determine how a sample reacts and/or changes over time in
order to provide a screening, diagnosis,
treatment, and/or disease prevention.
[00275] In some embodiments, data may be displayed in a lab report, medical
record, or any other type of
display. The display may show patient health, provider's level of care,
disease regression, progression, and/or onset
-43-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
through longitudinal analysis of high integrity data that is may be obtainable
more frequently or obtained frequently
through the described infrastructure over time.
[00276] Data may be collected from multiple devices. The aggregated data from
multiple devices may be
useful to facilitate the qualitative and/or quantitative evaluation of the
sample. The aggregated data may include
data relating to samples collected from a single subject, received at the
multiple devices. Alternatively, the
aggregated data may include data relating to samples collected from other
subjects, received at the multiple devices.
The aggregated data may be collected and/or stored in a database. The database
may be accessed to provide data to
perform a longitudinal analysis that takes past collected data into account.
Trends, and changes over time may be
monitored. The multiple devices may be standardized and/or may provide data
that is of sufficient quality,
precision, and/or accuracy in order to aggregate the data and perform a
longitudinal analysis therefrom. Very little
or no variation may be provided between devices. The devices may also create
standardized environments in which
the sample preparation may occur. The standardized environments may also be
provided during a chemical
reaction. The devices may also provide standardized pre-analytic steps. The
multiple devices may be distributed
globally. This may provide a global evaluation infrastructure, which may
better permit the monitoring of disease
progression and/or regression. By standardizing a device, data may be
longitudinally analyzed looking at velocity
of markers in one or more subject over time. The data may be analyzed and/or
displayed in a form of lab report or
electronic medical record or decision support system for consumers, providers,
and/or payers (e.g., health plans,
employers, governmental payers, etc.). Such display may include displays of
data over time, which may include
trending analysis or other analysis relating to changes in values, rates of
changes, or rates of rates of change.
[00277] The data may be of a quality suitable for a longitudinal analysis over
time. The suitable quality of
data may be useful for lab reports and/or electronic medical records that may
incorporate data collected over time.
This may include data collected over long periods of time (e.g., multiple
visits, or based off multiple samples), or
shorter periods of time (within a single visit, or based on single received
sample). The data may have a sufficient
quality, precision, and/or accuracy for longitudinal analysis. For example,
the sample may be collected from a
subject a plurality of times. The sample may be collected from the subject at
different times. The samples may be
collected at predetermined intervals or according to a predetermined schedule.
Alternatively, samples may be
collected from the subject when one or more condition or event triggers the
collection. Multiple collections of
samples may permit the sample to be analyzed over a period of time, thereby
permitting longitudinal analysis. In
some embodiments, in order to permit longitudinal analysis, the data may have
a high degree of precision and/or
accuracy. In one example, the data may have a coefficient of variation of 20%
or less, 15% or less, 10% or less, 9
% or less, 8% or less, 7% or less, 6% or less, 5% or less, 4% or less, 3% or
less, 2% or less, 1% or less, 0.5% or
less, or 0.1% or less over time. In some instances, the multiple devices may
provide data having a coefficient of
variation of 20% or less, 15% or less, 10% or less, 9 % or less, 8% or less,
7% or less, 6% or less, 5% or less, 4% or
less, 3% or less, 2% or less, 1% or less, 0.5% or less, or 0.1% or less over
time.
[00278] The data over time may be analyzed longitudinally. This may include
the change in data over
time, the rate of change of data over time, the rate of change of the rate of
change of data over time, or any
derivative thereof. For example, velocity and/or acceleration of data change
may be collected and/or analyzed. The
increase and/or decrease in the data values and/or the various rates of change
may be beneficial in determining a
screening, diagnosis, treatment, and/or disease prevention.
-44-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00279] The device is capable of processing a sample collected from a subject
to yield data for subsequent
analysis. The device may be configured to facilitate collection of the sample
from the subject. The device may be
configured to receive the sample from the subject. The device may be
configured to prepare the sample for a
clinical test to detect and/or quantitate an analyte of interest. The device
may comprise one or more reagents useful
for the clinical test. The preparation or the clinical test may include a
chemical reaction with the reagents. The
device may include one or more detector that may be capable of detecting
signals generated from processing the
sample. The device may transmit data relating to the sample. The data relating
to the sample may include the raw
data from the detected signals, such signals relating to unreacted sample, a
sample that has undergone a reaction,
and/or device configurations. In some instances, the device may pre-process
some of the raw data to get it into a
desired format, and transmit the pre-processed data. In some instances, the
device may perform one or more
analysis step, and transmit analyzed data. Alternatively, the device does not
perform any pre-processing and/or
analysis. The pre-processing and/or analysis may occur at the laboratory. In
some instances, pre-processing and/or
analysis may occur at both the device and the laboratory. The laboratory may
also include a hospital who may be
leveraging its pathologists so data can be transmitted to centers of
excellence for the analysis of different types of
specific conditions.
[00280] In embodiments, a device may monitor its environment, including its
internal and external
environment. In embodiments, a device may provide device environmental
information to a laboratory. Device
environmental information includes, e.g., internal temperature, external
temperature, internal humidity, external
humidity, time, status of components, error codes, images from an internal
camera, images from an external camera,
air pressure (barometric pressure), and other information. In embodiments, an
internal camera may be fixed at an
internal location. In embodiments, an internal camera may be fixed at an
internal location and may be configured to
rotate, scan, or otherwise provide views of multiple areas or regions within
the device. In embodiments, an internal
camera may be movable within the device; for example, an internal camera may
be mounted on a movable element,
such as a pipette, within the device. In embodiments, an internal camera may
be movable within the device and may
be configured to rotate, scan, or otherwise provide multiple views of areas
within the device from multiple locations
within the device. In embodiments, an external camera may be fixed at an
external location. In embodiments, an
external camera may be fixed at an external location and may be configured to
rotate, scan, or otherwise provide
multiple views of areas outside the device. In embodiments, an external camera
may be movable on or around the
outside of the device. In embodiments, an external camera may be movable and
may be configured to rotate, scan,
or otherwise provide multiple views of areas outside the device from multiple
locations on or around the outside of
the device.
[00281] Thus, in embodiments, monitoring, and reporting the results of such
monitoring, of the device
environment may be part of the oversight of the operation of the device and of
the integrity of results and analysis.
Such monitoring provides information, oversight, and control of quality
regarding the device and its output (of the
condition, operation, results generated, and data transmitted). Such
monitoring may include: measurement and
control of air temperature; measurement and control of liquid (volume,
temperature, mixing, etc.); monitoring of
sample collection; imaging of a cartridge to determine the position of a
cartridge in the device; imaging of a
cartridge to correct its position, if necessary, per such imaging of the
cartridge in the device; imaging of tips to
confirm proper engagement with pipette nozzles; imaging of liquid volumes in
tips; imaging of bubbles in liquids, if
any; imaging of samples to assess sample volume, sample quality and presence
or absence of conditions which may
-45-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
interfere with proper processing or analysis (such as hemolysis, lipemic,
icteric conditions of a sample); feedback
control and error detection of status and condition of motors on a pipette for
control and oversight concerning
accurate aspiration and dispensing of liquids; feedback control and error
detection of a centrifuge for precise
centrifugal force control, determination of position, and other information
regarding status and condition of a
centrifuge; feedback control and error detection for gantry, robots and
shuttle positions, including or oversight and
control of the positioning of pipettes, cartridges, and tips/cuvettes for
processing and analysis of a sample; control
of reactions (between a sample and a reagent) to confirm proper device
operation (e.g., using sensors, pipette,
gantry, thermal sensors and control, etc.); monitoring and control of the
proper state of reagents; the running of
control reactions at the same time as the sample analysis, or beforehand or
subsequently; performance of replicate
analysis of the sample to enhance precision; performing blank reads to control
for possible changes in background
signals in samples and for possible small fluctuations in sensor performance
and in output of light sources.
[00282] In embodiments, monitoring, and reporting the results of such
monitoring, may include
calibration. Calibration of the device, reagents, disposables, and of their
manufacture and assembly may be part of
the oversight of the operation of the device and of the integrity of results
and analysis. During manufacturing, each
device is calibrated to a set of controlled standards. During manufacturing,
reagents and disposables (e.g., tips,
cuvettes, and other elements) is calibrated to a set of controlled standards,
and identification information related to
each cartridge containing these reagents and disposables includes information
about these calibrations. Such
calibration may include: each pipette nozzle of each pipette is calibrated¨
for example, for each pipette nozzle,
information regarding how a given displacement of the motor translates to a
given amount of liquid volume is
measured and provided in the information recorded for each motor for use in
protocols used with the device in
which that motor is a component; each sensor and illumination source in a
device is calibrated against a set of
controlled standards, and provided in the information for each device, so that
the resulting signals from all sensors
across all devices results in the same measurement; each motor control
algorithm is calibrated during manufacturing
such that speeds and position of the motors can be controlled similarly across
each device; each camera and each
illumination source is characterized, including a flat field correction;
during manufacturing, each of lot reagents is
calibrated such that any change in potency of the reagents still results in
the same analytic result. Thus, since each
sample is analyzed by processing on the device (for which such component and
device specific calibration
information is known) the raw data resulting from sample processing can be
calibrated and corrected according to
the information and calibrations for each reagent, and each device and its
components. Such oversight and
calibration insures the integrity of the results obtained, and thus also
provides for the integrity of analysis of such
results. The raw data from a device is analyzed by utilizing the device-
specific calibration and the reagent lot-
specific calibration to arrive at the result. Each result arrived at in this
way is thus accurate, precise, and reliable,
and may be compared with analogous results obtained from other samples in the
same instrument, and with other
instruments and samples, reducing variance and errors and allowing for better
analysis and better confidence in
diagnoses and inferences drawn from the analysis of samples.
[00283] Quality control runs may be performed on a device and reagents on a
periodic basis, e.g., as
overseen by CLIA, to ensure that the reagents and devices are still performing
within specifications. If
discrepancies are found, reagents and/or devices may be determined to need
recalibration. Devices can be
recalibrated in the field by running defined protocols, which may or may not
require inserting a calibration cartridge
into the device. Reagents may be recalibrated by generating a standard curve
using the same lot of reagents and
-46-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
deriving a calibration function. Such reagent recalibration can performed on
any one of the devices and can be
applied to all devices.
[00284] Transmission of device environmental information to a laboratory is
useful for the oversight and
control of the device, including being useful for the oversight and control of
the operation of the device.
Transmission of device environmental information to a laboratory is useful for
maintaining the integrity of the
operation and control of the device, quality control of the operation and
control of the device, and for reducing
variation or error in the data collection and sample processing performed by
the device. Device environmental
information may be used, for example, by a laboratory to modify, correct, or
update a protocol or other instruction
or command to a device. Device environmental information may be used, for
example, by a laboratory to modify,
correct, or update an analysis of data received from a device. For example,
transmission of temperature information
to a laboratory is useful for the oversight and control of the device, and is
useful in the analysis by the laboratory of
data provided by the device to the laboratory.
[00285] In embodiments, a device may be configured to control the temperature
within the device, or
within a portion of the device. Such control improves the reproducibility of
measurements made within the device,
may unify or provide regularity of conditions for all samples, and reduce the
variability of measurements and data,
e.g., as measured by the coefficient of variance of multiple measurements or
replicate measurements. Temperature
information may be useful for quality control. In embodiments, a device may
monitor temperature and control its
internal temperature. Temperature control may be useful for quality control. A
device that monitors and controls its
temperature may transmit temperature information to a laboratory; a laboratory
may use such temperature
information in the control of the operation of the instrument, in the
oversight of the instrument, and in the analysis
of data transmitted from the instrument.
[00286] In embodiments, a device may be configured to acquire images from
within the device, or within
a portion of the device. Such images may provide information about the
position, condition, availability, or other
information regarding components, reagents, supplies, or samples within the
device, and may provide information
used in control of the operation of the device. Such images may be useful for
quality control. A device that acquires
images from within the device may transmit image information to a laboratory;
a laboratory may use such image
information in the control of the operation of the instrument, in the
oversight of the instrument, and in the analysis
of data transmitted from the instrument.
[00287] In one scenario, a device may perform a sample preparation step
without performing any analysis
or receiving any oversight. The data from the sample preparation step may be
sent to the laboratory, which may
perform the analysis, and which may be an authorized analytical facility that
includes oversight. In another
scenario, the device may perform one or more sample preparation step and may
perform analysis on board. Data
from the analysis may be sent to an authorized analytical facility, which may
provide oversight. Alternatively,
oversight may occur on board the device.
[00288] In some embodiments, oversight may include a review of the data in raw
form, pre-processed
form, or after analysis. Oversight may occur of a qualitative and/or
quantitative evaluation of the sample.
Examples of a qualitative evaluation of the sample may include but are not
limited to review of an image, video, or
audio file. Examples of a quantitative evaluation of the sample may include a
numerical value indicating a presence
or concentration level of a signal, series of signals, or an analyte.
Oversight may include one or more, or two or
-47-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
more of the examples provided elsewhere herein. Oversight may be provided by a
health care professional of an
authorized analytical facility. In some other instances, oversight may be
provided by a software program or
automated review system. The software program and/or automated review system
may or may not be under the
review or care of a qualified person, such as a health care professional (such
as a laboratory director).
[00289] The device may duplicate manual analytical procedures. In some
instances, the device may
perform automatically various steps, such as pipetting, preparing filtrates,
heating, and/or measuring color intensity.
The device may be used in conjunction with materials to measure one or more
analytes. The device may measure
the presence or concentration of one or more analytes. The device may include
reagent-containing components that
may serve as reaction units. Examples of device components and steps that may
be taken by the device can be
described in greater detail elsewhere herein.
[00290] The laboratory may communicate with a health care professional. The
laboratory may generate a
report based on analyzed data. In some instances, the laboratory may analyze
raw data or pre-processed data
provided from the device. Alternatively, the laboratory may receive analyzed
data from the device. The laboratory
may or may not perform further analysis and/or oversight from analyzed data
received from the device.
[00291] The laboratory and/or device may generate a report that may present
the analyzed data in a
meaningful or desired manner. The report may have a format that may enable a
viewer of the report to rely on the
report to make a medical determination. The laboratory and/or device may
transmit the report to a health care
professional (or laboratory director). In some embodiments, a pathologist,
other health care professional, or other
qualified person may review the report prior to transmitting the report to the
health care professional. A reviewing
health care professional may review the report or qualitative and/or
quantitative evaluation useful for generating the
report prior to transmission to an ordering health care professional. Review
or oversight may occur of the analyzed
data and/or report at the laboratory. Alternatively, review or oversight may
occur on-board the device. The health
care professional who receives the report may or may not rely on the report
for screening, diagnosis, treatment
and/or disease prevention of the subject.
[00292] The laboratory and/or device may also provide a report to the subject.
The report provided to the
subject may be the same as or different from the report provided to the health
care professional. The report
provided to the health care professional may have more detail or vice versa.
The formats between the reports
provided to the subject and the health care professional may or may not vary.
Alternatively, the laboratory and/or
device does not provide a report to the subject. The subject may receive
information based on the report from the
health care professional. A device or laboratory can directly provide a lab
report automatically to a consumer upon
a test being performed and/or analysis being done, or being sent to a
physician for review and/or after the
physician's review.
[00293] Any transmission of data and/or reports may incorporate the use of a
cloud computing
infrastructure. The sending party may provide the data to or have the data on
a cloud computing infrastructure. The
receiving party and/or parties (e.g., health care professional or patient) may
access the cloud computing
infrastructure. The cloud computing infrastructure may be provided on the
sending party side and/or the receiving
party side. Alternatively, traditional fixed data storage techniques may be
employed.
[00294] FIG. 1B shows a retailer 170 having a processing device 172 in
communication with a laboratory
160. The laboratory or reader device may be in communication with a health
care professional 150. As previously
-48-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
described, any discussion herein of retailers or other examples of sample
collection sites may apply to any type of
sample collection site, and vice versa. A retailer may be provided at a first
location and a health care professional
may be provided at a second location. The first location and the second
location may be different locations. In
some embodiments, the first and second locations are not proximate to one
another. A laboratory may be provided
at a third location. The third location may be a different location from the
first and/or second location. For
example, the first, second, and third locations need not be proximate to one
another. The first, second, and/or third
locations may be located in different facilities. Alternatively, the first,
second, and/or third could all be the same
location (point of service).
[00295] A retailer may be an entity that sells a product or service. In some
embodiments, the product or
service may relate to health or medical care. For example, the retailer may
sell medicine or health care supplies
and/or insurance. In some embodiments, a retailer may be a pharmacy (e.g.,
retail pharmacy, clinical pharmacy,
hospital pharmacy), drugstore, chain store, supermarket, or grocer. Examples
of retailers may include but are not
limited to Walgreens, CVS Pharmacy, Duane Reade, Walmart, Target, Rite Aid,
Kroger, Costco, Kaiser
Permanente, or Sears.
[00296] A retailer may be provided at a retailer location. In some
embodiments, the retailer may be at a
different geographic location than a health care professional and/or
laboratory location. Alternatively, the health
care professional may be provided at the retailer location.
[00297] A retailer 170 may have a sample processing device 172 at the
retailer's location. In some
embodiments, the retailer may have one or more, two or more, three or more,
four or more, five or more, six or
more, or ten or more sample processing devices at the retailer's location. The
sample processing device may be a
point of service device. The sample processing devices may be capable of
communication with communication-
enabled devices. For example, the sample processing devices at a retailer
location may communicate with one
another. Alternatively, sample processing devices may communicate with other
reader devices at different
locations, such as other sample collection sites, or in or on a subject.
Sample processing devices may communicate
with other types of communication-enabled devices, such as a computer at a
laboratory and/or biometric devices.
Such communications may be wired or wireless.
[00298] The sample processing device 172 may be configured to accept a sample.
The sample processing
device may be configured to collect the sample directly from a subject. The
sample processing device may be
configured to perform one or more sample preparation step on the subject. The
sample processing device may be
configured to run an assay. In some embodiments, the sample processing device
may be configured to run one or
more assay. The sample processing device may be capable of performing
multiplexed assays on a single sample.
Where desired, the device is configured to perform at least 2, 3, 4, 5, 6, 7,
8, 9, 10, 20, 30, 40, 50, 100, 200, 500,
1000 or more assays. The plurality of assays may be run simultaneously in
parallel. One or more control assays
and/or calibrators (e.g., including a configuration with a control of a
calibrator for the assay/tests) can also be
incorporated into the device to be performed in parallel if desired. In some
instances, assays may be run in
sequence, or any combination of in sequence and in parallel, based on the
sample. The reader device may be
effecting one, two, or more chemical reactions or other processing tests
(e.g., pulverizing). The sample processing
device may be configured to detect one or more signal relating to the sample.
The sample may be a sample of
bodily fluid, a biological sample, or any other example as provided elsewhere
herein.
-49-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00299] In some embodiments, the sample processing device 172 may comprise a
cartridge 174. The
cartridge may be removable from the sample processing device. In some
embodiments, a sample may be provided
to the cartridge of the sample processing device. Alternatively, the sample
may be provided to another portion of
the sample processing device. The cartridge and/or device may comprise a
sample collection unit that may be
configured to accept a sample. The sample processing device is described in
further detail elsewhere herein. The
cartridge and device may be integrated into a single device or may be
separable devices. A device may include a
pill or patch that may link to a mobile device or other network device for
processing.
[00300] A subject 176 may be provided at the retailer 170. The subject may
provide a sample of bodily
fluid to the sample processing device 172 and/or cartridge 174 of the device.
A bodily fluid may be drawn from a
subject and provided to a device in a variety of ways, including but not
limited to, fingerstick, lancing, injection,
and/or pipetting. The bodily fluid may be collected using venous, or non-
venous methods. The bodily fluid may be
provided using a bodily fluid collector. A bodily fluid collector may include
a lancet, microneedle, porous
membrane (e.g., for a pill), capillary, tube, pipette, syringe, venous draw,
or any other collector described elsewhere
herein. In one embodiment, a lancet punctures the skin and withdraws a sample
using, for example, gravity,
capillary action, aspiration, or vacuum force. The lancet may be part of the
sample processing device, part of the
cartridge of the device, part of a system, or a standalone component. Where
needed, the lancet may be activated by
a variety of mechanical, electrical, electromechanical, or any other known
activation mechanism or any
combination of such methods. In one example, a subject's finger (or other
portion of the subject's body) may be
punctured to yield a bodily fluid. The bodily fluid may be collected using a
capillary tube, pipette, or any other
mechanism known in the art. The capillary tube or pipette may be separate from
the device and/or cartridge, or may
be a part of a device and/or cartridge. A transfer device may require no
additional processing steps, and may be
pre-coated with anti-coagulants or other pre-treatments in a single step. In
another embodiment where no active
mechanism is required, a subject can simply provide a bodily fluid to the
device and/or cartridge, as for example,
could occur with a saliva sample, or touching a pierced body part to a surface
directly. The collected fluid can be
placed within the device. A bodily fluid collector may be attached to the
device, removably attachable to the
device, or may be provided separately from the device.
[00301] A cartridge 174 may be inserted into the sample processing device 172
or otherwise interfaced
with the sample processing device. The cartridge may be removed from the
sample processing device. In one
example, a sample may be provided to a sample collection unit of the
cartridge. The sample may be provided
directly to the cartridge. The sample may or may not be provided to the sample
collection unit via a bodily fluid
collector. A bodily fluid collector may be attached to the cartridge,
removably attachable to the cartridge, or may
be provided separately from the cartridge. The bodily fluid collector may or
may not be integral to the sample
collection unit. The cartridge may then be inserted into the sample processing
device. Alternatively, the sample
may be provided directly to the sample processing device, which may or may not
utilize the cartridge. The
cartridge may comprise one or more reagents, which may be used in the
operation of the sample processing device.
Alternatively, one or more reagents may already be provided onboard the sample
processing device.
[00302] The cartridge may or may not be disposable. Cartridges may be
specially configured for one or
more types of clinical tests. For example, a first cartridge may have a first
configuration to enable a first set of tests,
and a second cartridge may have a second configuration to enable a second set
of tests. Alternatively, universal
-50-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
cartridges that may be configured for the same selection of tests may be
provided. In some instances, universal
cartridges may be dynamically programmed for certain tests through remote or
on-board protocols.
[00303] When a cartridge is inserted into the sample processing device, one or
more components of the
cartridge may be brought into fluid communication with other components of the
sample processing device. For
example, if a sample is collected at a cartridge, the sample may be
transferred to other portions of the sample
processing device. Similarly, if one or more reagents are provided on a
cartridge, the reagents may be transferred to
other portions of the sample processing device, or other components of the
sample processing device may be
brought to the reagents. One or more components of the cartridge may be
transferred in an automated fashion to
other portions of the sample processing device, and vice versa. In some
embodiments, the reagents or components
of a cartridge may remain on-board the cartridge. In some embodiments, no
fluidics are included that require
tubing or maintenance (e.g., manual or automated maintenance).
[00304] The sample processing device may be configured to be placed in or on a
subject. The sample
processing device may receive a sample from the subject through a housing of
the device. For example, if the
sample processing device is ingestible or implanted within a subject, it may
include a housing or a biocompatible
coating. The biocompatible coating may be permeable to the desired sample. The
sample may penetrate the
coating or housing of the sample processing device, thereby being received by
the sample processing device. If the
sample processing device is on the subject, the sample may be received through
the housing and/or coating of the
device. Alternatively, the sample may be received using one or more needle or
microneedle that may be provided
on the device (which may or may not be provided on the cartridge portion of
the device).
[00305] The sample processing device may be configured to facilitate sample
collection, prepare the
sample for a clinical test, and/or may comprise one or more reagents useful
for a clinical test. In some
embodiments, the sample processing device may be configured to run one or more
test from the sample. A
chemical reaction or other processing step may be performed, with or without
the sample. In some embodiments,
assays, such as immunoassays or nucleic acid assays may be run. Examples of
steps and/or tests that may be
prepared or run by the device may include, but are not limited to immunoassay,
nucleic acid assay, receptor-based
assay, cytometric assay, colorimetric assay, enzymatic assay, electrophoretic
assay, electrochemical assay,
spectroscopic assay, chromatographic assay, microscopic assay, topographic
assay, calorimetric assay, turbidmetric
assay, agglutination assay, radioisotope assay, viscometric assay, coagulation
assay, clotting time assay, protein
synthesis assay, histological assay, culture assay, osmolarity assay, and/or
other types of assays, centrifugation,
separation, filtration, dilution, enriching, purification, precipitation,
pulverization, incubation, pipetting, transport,
cell lysis, or other sample preparation steps, or combinations thereof. Sample
processing may include chemical
reactions and/or physical processing. Sample processing may include the
assessment of histology, morphology,
kinematics, dynamics, and/or state of a sample, which may include such
assessment for cells. The device may
perform one or more, two or more, three or more, or four or more of these
steps/tests.
[00306] Processing of a biological sample may include preprocessing (e.g.,
preparation of a sample for a
subsequent processing or measurement), processing (e.g., alteration of a
sample so that it differs from its original, or
previous, state), and post-processing (e.g., fixing a sample, or disposing of
all or a portion of a sample following its
measurement or use). A biological sample may be divided into portions, such as
aliquots of a blood or urine sample,
or such as slicing, mincing, or dividing a tissue sample into two or more
pieces. Processing of a biological sample,
-51-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
such as blood sample, may include mixing, stirring, sonication,
homogenization, or other processing of a sample or
of a portion of the sample. Processing of a biological sample, such as blood
sample, may include centrifugation of a
sample or a portion thereof. Processing of a biological sample, such as blood
sample, may include providing time
for components of the sample to separate or settle, and may include filtration
(e.g., passing the sample or a portion
thereof through a filter). Processing of a biological sample, such as blood
sample, may include allowing or causing
a blood sample to coagulate. Processing of a biological sample, such as blood
sample, may include concentration of
the sample, or of a portion of the sample (e.g., by sedimentation or
centrifugation of a blood sample, or of a solution
containing a homogenate of tissue from a tissue sample) to provide a pellet
and a supernatant. Processing of a
biological sample, such as blood sample, may include dilution of a portion of
the sample. Dilution may be of an
entire sample, or of a portion of a sample, including dilution of a pellet or
of a supernatant from sample. A
biological sample may be diluted with water, or with a saline solution, such
as a buffered saline solution. A
biological sample may be diluted with a solution which may or may not include
a fixative (e.g., formaldehyde,
paraformaldehyde, or other agent which cross-links proteins). A biological
sample may be diluted with a solution
effective that an osmotic gradient is produced between the surrounding
solution and the interior, or an interior
compartment, of such cells, effective that the cell volume is altered. For
example, where the resulting solution
concentration following dilution is less than the effective concentration of
the interior of a cell, or of an interior cell
compartment, the volume of such a cell will increase (i.e., the cell will
swell). A biological sample may be diluted
with a solution which may or may not include an osmoticant (such as, for
example, glucose, sucrose, or other sugar;
salts such as sodium, potassium, ammonium, or other salt; or other osmotically
active compound or ingredient). In
embodiments, an osmoticant may be effective to maintain the integrity of cells
in the sample, by, for example,
stabilizing or reducing possible osmotic gradients between the surrounding
solution and the interior, or an interior
compartment, of such cells. In embodiments, an osmoticant may be effective to
provide or to increase osmotic
gradients between the surrounding solution and the interior, or an interior
compartment, of such cells, effective that
the cells at least partially collapse (where the cellular interior or an
interior compartment is less concentrated than
the surrounding solution), or effective that the cells swell (where the
cellular interior or an interior compartment is
more concentrated than the surrounding solution).
[00307] A biological sample may be dyed, or markers may be added to the
sample, or the sample may be
otherwise prepared for detection, visualization, or quantification of the
sample, a portion of a sample, a component
part of a sample, or a portion of a cell or structure within a sample. For
example, a biological sample may be
contacted with a solution containing a dye. A dye may stain or otherwise make
visible a cell, or a portion of a cell,
or a material or molecule associated with a cell in a sample. A dye may bind
to or be altered by an element,
compound, or other component of a sample; for example a dye may change color,
or otherwise alter one of more of
its properties, including its optical properties, in response to a change or
differential in the pH of a solution in which
it is present; a dye may change color, or otherwise alter one of more of its
properties, including its optical
properties, in response to a change or differential in the concentration of an
element or compound (e.g., sodium,
calcium, CO2, glucose, or other ion, element, or compound) present in a
solution in which the dye is present. For
example, a biological sample may be contacted with a solution containing an
antibody or an antibody fragment. For
example, a biological sample may be contacted with a solution that includes
particles. Particles added to a
biological sample may serve as standards (e.g., may serve as size standards,
where the size or size distribution of the
particles is known, or as concentration standards, where the number, amount,
or concentration of the particles is
-52-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
known), or may serve as markers (e.g., where the particles bind or adhere to
particular cells or types of cells, to
particular cell markers or cellular compartments, or where the particles bind
to all cells in a sample).
[00308] The sample processing device may be configured to perform one, two or
more assays on a small
sample of bodily fluid. One or more chemical reaction may take place on a
sample having a volume, as described
elsewhere herein. For example one or more chemical reaction may take place in
a pill having less than femtoliter
volumes. In an instance, the sample collection unit is configured to receive a
volume of the bodily fluid sample
equivalent to a single drop or less of blood or interstitial fluid. The sample
collection unit may be able to collect a
volume of bodily fluid sample without piercing a subject's skin. In one
example, light may be shined to optically
measure a sample. In additional examples, ultrasound, MRI, or a scan may be
used to perform analysis non-
invasively.
[00309] The device may be capable of performing all on-board steps in a short
amount of time. For
example, from sample collection from a subject to transmitting data and/or to
analysis may take about 3 hours or
less, 2 hours or less, 1 hour or less, 50 minutes or less, 45 minutes or less,
40 minutes or less, 30 minutes or less, 20
minutes or less, 15 minutes or less, 10 minutes or less, 5 minutes or less, 4
minutes or less, 3 minutes or less, 2
minutes or less, 1 minute or less, 50 seconds or less, 40 seconds or less, 30
seconds or less, 20 seconds or less, 10
seconds or less, 5 seconds or less, 3 seconds or less, 1 second or less, 500
ms or less, 200 ms or less, or 100 ms or
less. The amount of time from accepting a sample within the device to
transmitting data and/or to analysis from the
device may take about 3 hours or less, 2 hours or less, 1 hour or less, 50
minutes or less, 45 minutes or less, 40
minutes or less, 30 minutes or less, 20 minutes or less, 15 minutes or less,
10 minutes or less, 5 minutes or less, 4
minutes or less, 3 minutes or less, 2 minutes or less, 1 minute or less, 50
seconds or less, 40 seconds or less, 30
seconds or less, 20 seconds or less, 10 seconds or less, 5 seconds or less, 3
seconds or less, 1 second or less, 500 ms
or less, 200 ms or less, or 100 ms or less.
[00310] A laboratory, device, or other entity or software may perform analysis
on the data in real-time.
Analysis may include qualitative and/or quantitative evaluation of a sample. A
laboratory, device, or other entity
may analyze the data within 48 hours or less, 36 hours or less, 24 hours or
less, 12 hours or less, 8 hours or less, 6
hours or less, 4 hours or less, 3 hours or less, 2 hours or less, 1 hour or
less, 45 minutes or less, 30 minutes or less,
20 minutes or less, 15 minutes or less, 10 minutes or less, 5 minutes or less,
3 minutes or less, 1 minute or less, 30
seconds or less, 15 seconds or less, 10 seconds or less, 5 seconds or less, or
1 second or less. The analysis may
include the comparison of the data with one or more threshold value. The
analysis may or may not include review
by a pathologist or other qualified person. The time included for analysis may
or may not include time to generate a
report based on the data. The time included for analysis may or may not
include the time it takes to transmit a
report to a health care professional.
[00311] A device 172 may be provided to a sample collection site 170 by a
laboratory 160. The device
may be sold to the sample collection site, leased/rented by the sample
collection site, or the sample collection site
may be used as a location at which the laboratory may conduct sample
collection and/or other steps.
[00312] Similarly, one or more cartridge 174 may be provided to the sample
collection site 170 by the
laboratory 160. Alternatively, the cartridge may be provided by another
source. The cartridge may be sold to the
sample collection site, leased/rented by the sample collection site, or may be
utilized as part of the location where
-53-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
the laboratory may collect samples and/or perform other steps. The cartridge
may be from a same or different
source as the device.
[00313] A laboratory 160 may have a processor 162 and a communication unit
164. A laboratory may be
provided within a facility. The processor and communication unit may be
provided within the facility. The
laboratory may have one or a plurality of processors and one or a plurality of
communication units.
[00314] A processor 162 may be configured to generate a report for a health
care professional 150. The
processor may be on a server side with a software performing the processing.
The processor may generate the
report based on data received from the sample processing device 172 or may
provide oversight or analysis. The
processor may perform qualitative and/or quantitative evaluation of the
sample. In some embodiments, the
processor may compare data received from the sample processing device with a
threshold value. The threshold
value may be for one or more analyte. Said comparison may include a comparison
of whether a data value is
greater than, equal to, or less than a threshold value. The comparison may
include whether the data value is
qualitatively and/or quantitatively the same as the threshold value. The
comparison may include one or more forms
of statistical or physiological analysis of the data in relation to one or
more stored values. Examples may include
best-fit analysis, and/or analysis such as curve fitting, extrapolation,
interpolation, regression analysis, least squares,
mean calculations, multivariate, simulation analysis, or variation
calculations. The processor may analyze the data
received from the sample processing device. The processor may be configured to
perform one or more steps for
statistical analysis of the data.
[00315] In some embodiments, a threshold value may refer to a single value.
The threshold value may be
a numerical value or an alphanumeric value. The threshold value may be a
string or any other form of data. The
threshold value may refer to a range of values and/or set of values. A
threshold value may refer to a single value or
a plurality of values. A plurality of values may fall within one or more
continuous spectrum. Alternatively, the
plurality of values may be discrete. Examples of threshold ranges may include
1-100 units, or 5-10 units, and
examples of threshold sets may include values falling within a list selected
from 1 unit, 3 units, 5 units, 8 units, 13
units, 20 units, or 50 units. A unit may refer to any dimension or measureable
quantity. Such values are provided
by way of example only. In some instances, the processor may compare one or
more image, video, or audio file or
other data. The processor may make such comparisons against one or more
reference image, video, or audio file or
other data. An algorithm may be capable of evaluating one or more feature of
the files or other data. In some
instances, the processor may automatically sort the files for viewing by a
health care professional.
[00316] The processor may be able to access one or more data storage unit
166a, 166b which may contain
stored information. The stored information may include the threshold value for
one or more analyte. The threshold
value may be useful for determining the presence or concentration of the one
or more analyte. The threshold value
may be useful for detecting situations where an alert may be useful. The data
storage unit may include any other
information relating to sample preparation or clinical tests that may be run
on a sample. The data storage unit may
include records or other information that may be useful for generating a
report for a health care professional. The
data storage units may also be capable of storing computer readable media
which may include code, logic, or
instructions for the processor to perform one or more step.
[00317] In some embodiments, a data storage unit 166a may be provided at the
laboratory 160. The
processor may be able to access the local data storage unit. In another
embodiment, the data storage unit 166b may
-54-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
be provided remote to the laboratory. For example, the data storage unit may
be provided at a sample collection site
170 or with a health care professional 150. The data storage unit may be
provided on the device. Alternatively, the
data storage unit may be provided at any other location. Any combination of
data storage unit locations may be
utilized by the processor. For example, the processor may access data storage
units that may be provided at the
laboratory and external to the laboratory.
[00318] In some embodiments, the data storage units may be electronic medical
records (EMR) or EMR
databases. The data storage units may contain information associated with a
subject. The information associated
with the subject may include medical records of the subject, health history of
the subject, identifying information
associated with the subject, payment information associated with the subject,
or any other information associated
with the subject. The data storage units may be payer databases. The data
storage units may include information
associated with a payer, such as a health insurance company or governmental
payer. Such information may include
treatment records, insurance records, or financial information associated with
the subject.
[00319] One or more communication unit 164 may be provided at the laboratory
160. The laboratory may
be at the same location as or different location from, or may actually be the
same as the sample collection or
processing center or provider or hospital office/location. Any description
herein of the laboratory may apply to any
other locations provided herein and vice versa. The communication unit may be
configured to receive data from a
device 172. The communication unit may receive data relating to a sample of a
subject from the device at a sample
collection site 170. The communication unit may receive information about the
subject from the device and/or the
sample collection site. The communication unit may receive identifying
information about the subject. The
communication unit may receive information from the device and/or any other
machine (e.g., biometric devices,
mobile devices) or entity associated with the sample collection site.
[00320] The communication unit 164 may be configured to transmit data to a
device 172 and/or any other
machine or entity associated with the sample collection site 170. In some
embodiments, the communication unit
may provide one or more protocol to the device. The communication may provide
the protocol in addition to
receiving data. The protocol may effect the collection of a sample, prepare
the sample for a clinical test, or permit a
chemical reaction with one or more reagents on the device. The protocol may
effect the running of the clinical test
on the device. The protocol may effect the detection of the presence and/or
concentration of an analyte at the
device. Any description of detection and/or analysis relating to the presence
and/or concentration of an analyte may
include and/or be applied to assessing a disease condition. The protocol may
effect the pre-processing of raw data
and/or analysis of data at the device.
[00321] The communication unit may permit two-way communication unit between
the sample collection
site and the laboratory. The communication unit may permit two-way
communication between a sample processing
device at a sample collection site or in or on a subject, and a processor at
the laboratory. In some embodiments, one
or more protocol may be sent to a device based on data sent by the device. The
data sent by the device may include
subject identifying information, information based on signals generated and/or
detected relating to the sample or
reactions, device identification information, cartridge identification
information, or any other information sent from
the device. Data may be collected from the device depending on protocols
provided to the device. The protocols
may govern the type of data that is collected and the actions performed by the
device. In some embodiments, one,
two, or more subsequent sets of protocols may be sent to a device based on
data collected from the device. The data
-55-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
from the device may provide feedback which may govern further actions to be
taken by the device, dictated by the
protocols.
[00322] In alternate embodiments of the invention, the laboratory need not
send protocols to the device.
The protocols may be stored locally on the device. Alternatively, the system
may provide protocols to the device.
The protocols may be provided from an entity external to the device. The
protocols may be on a cartridge.
[00323] The laboratory may have an output unit which may display or transmit
the report to the health
care professional. The output unit may be a video display. Alternatively, the
output unit may be a communication
unit. In one example, the output unit may be a touchscreen. The touchscreen
may have an intrinsic imaging
capability through built-in sensors, which may include LEDs or other light
sources.
[00324] The device may have one or more identifier. The device may be capable
of transmitting the
device identifier to the laboratory. One or more components of the device may
have an identifier. For example, a
cartridge may have one or more identifier. The cartridge identifier may be
readable by the device. For example,
when a cartridge is provided to the device, the device may automatically read
the cartridge identifier. The device
may transmit the cartridge identifier or other component identifiers to the
laboratory. The device, cartridge, or other
component identifiers may provide information about the configuration and/or
capabilities of the device, cartridge,
or other components respectively. For example, an identifier may indicate
which reagents or device components
are available. A protocol may be transmitted to the device from the laboratory
based on the identification
information received or from a device to a laboratory for review. A protocol
may be run on the device based on the
identification information.
[00325] An identifier may be a physical object formed on the device,
cartridge, or other component. For
example, the identifier may be read by an optical scanner. In some
embodiments, a camera may capture an image
of the identifier and the image may be analyzed to identify the device,
cartridge, or other component. In one
example, the identifier may be a barcode. A barcode may be a 1D or 2D barcode.
In some embodiments, the
identifier may emit one or more signal that may identify the device,
cartridge, or component. For example, the
identifier may provide an infrared, ultrasonic, optical, audio, electrical, or
other signal that may indicate the identity
of the device, cartridge, or component. The identifier may utilize a
radiofrequency identification (RFID) tag. The
identifier may be stored on a memory of the device, cartridge, or other
component. In one example, the identifier
may be a computer readable medium.
[00326] The communication unit 164 may be configured to transmit data to a
health care professional 150.
In some embodiments, the communication unit may transmit a report or
thefanalysis generated based on data
relating to the sample. The communication unit may be in communication with a
network device used by the health
care professional. For example, the communication unit may be capable of
communicating with a computer, tablet,
or mobile device of the health care professional.
[00327] Alternatively, another entity or source may generate a report, and/or
transmit a report to the health
care professional. For example, a laboratory may analyze data provided by the
device at a sample collection site or
in or on a subject or by a laboratory, hospital, sample collection center, or
any other location described herein. The
laboratory, device or another entity may generate a report or thefanalysis
based on the analyzed data. The report
may include longitudinal data over time, which may include concentration or
presence of one or more analytes or
changes in disease states over time. The report and/or analysis may make use
of clinical outcome assessments, such
-56-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
as those described in U.S. Patent Publication No. 2009/0318775, which is
hereby incorporated by reference in its
entirety. The laboratory, device, the other entity, or an additional entity
may transmit the report to the health care
professional. Various rounds of analysis or data processing may occur by one
or more entity. The various entities
may be provided at different facilities. Alternatively, some of the various
entities may be provided at the same
facility.
[00328] In some embodiments, the processor, communication unit, and data
storage unit may be provided
on the same machine. Alternatively, two or more of the processor,
communication unit, and data storage unit may
be provided on the same machine. The machine may be a computer, or any other
network device as described
elsewhere herein. Two or more of the processor, communication unit, and data
storage may be located on a
laboratory-located computer. Alternatively, the processor, communication unit,
and data storage may all be located
on different machines. In some instances, multiple processors, communication
units, and data storage units may be
provided that may be distributed over one or a plurality of machines.
[00329] FIG. 2 shows a sample processing device 200 in communication over a
network 202 with one or
more other devices 204a, 204b.
[00330] A sample processing device may be described further elsewhere herein.
The sample processing
device may be configured to accept one or more cartridge. The sample
processing device may be configured to
accept a sample from a subject. The sample processing device may be configured
to facilitate collection of the
sample, prepare the sample for a clinical test, and/or effect a chemical
reaction with one or more reagents or other
chemical or physical processing. The sample processing device may be
configured to detect one or more signals
relating to the sample. The sample processing device may be configured to run
a test. The test may include running
one or more chemical reactions. The sample processing device may be configured
to identify one or more
properties of the sample. In some embodiments, the device may not be
configured to perform a qualitative and/or
quantitative evaluation of the sample on board the device. Alternatively, the
device may perform such a qualitative
and/or quantitative evaluation. For instance, the sample processing device may
be configured to detect the presence
or concentration of one analyte or a plurality of analytes or a disease
condition in the sample (e.g., in or through a
bodily fluid, secretion, tissue, or other sample). Alternatively, the sample
processing device may be configured to
detect signals that may be analyzed to detect the presence or concentration of
one or more analytes (which may be
indicative of a disease condition) or a disease condition in the sample. The
signals may be analyzed on board the
device, or at another location. Running a clinical test may or may not include
any analysis or comparison of data
collected.
[00331] A sample processing device 200 may be configured to communicate over a
network 202. The
sample processing device may include a communication module that may interface
with the network. The sample
processing device may be connected to the network via a wired connection or
wirelessly. The network may be a
local area network (LAN) or a wide area network (WAN) such as the Internet. In
some embodiments, the network
may be a personal area network. The network may include the cloud. The sample
processing device may be
connected to the network without requiring an intermediary device. Any other
description of networks provided
herein may be applied.
[00332] In some embodiments, the sample processing device 200 may communicate
over the network 202
with another device 204a, 204b. The other device may be a communication-
enabled device. For example, the
-57-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
other device may be a client computer or a mobile device comprising a video
display with at least one display page
comprising data. The other device may be any type of networked device,
including but not limited to a personal
computer, server computer, or laptop computer; personal digital assistants
(PDAs) such as a Palm-based device or
Windows CE device; phones such as cellular phones, smartphones (e.g., iPhone,
Android, Blackberry, etc.), or
location-aware portable phones (such as GPS); a roaming device, such as a
network-connected roaming device; a
wireless device such as a wireless email device or other device capable of
communicating wireless with a computer
network; or any other type of network device that may communicate possibly
over a network and handle electronic
transactions. Any discussion of any device mentioned may also apply to other
devices, including those described
elsewhere herein. The sample processing device may communicate with one or
more, two or more, three or more,
or any number of other devices. Such communication may or may not be
simultaneous. Such communication may
include providing data to a cloud computing infrastructure or any other type
of data storage infrastructure which
may be accessed by other devices.
[00333] The other device 204a, 204b that may communicate with the sample
processing device 200 may
have a video display. Video displays may include components upon which
information may be displayed in a
manner perceptible to a user, such as, for example, a computer monitor,
cathode ray tube, liquid crystal display,
light emitting diode display, touchpad or touchscreen display, and/or other
means known in the art for emitting a
visually perceptible output. Video displays may be electronically connected to
a client computer according to
hardware and software known in the art.
[00334] In one implementation of the invention, a display page may include a
computer file residing in
memory which may be transmitted from a server over a network to a client
computer or other device, which can
store it in memory. A client computer may receive tangible computer readable
media, which may contain
instructions, logic, data, or code that may be stored in persistent or
temporary memory of the client computer, or
may somehow affect or initiate action by a client computer. Similarly, one or
more devices may communicate with
one or more client computers across a network, and may transmit computer files
residing in memory. One or more
devices may communicate computer files or links that may provide access to
other computer files.
[00335] At a client computer 204a, mobile device 204b, or any other network
device as described
elsewhere herein, the display page may be interpreted by software residing in
memory of the client computer,
mobile device, or network device, causing the computer file to be displayed on
a video display in a manner
perceivable by a user. The display pages described herein may be created using
a software language known in the
art such as, for example, the hypertext mark up language ("HTML"), the dynamic
hypertext mark up language
("DHTML"), the extensible hypertext mark up language ("XHTML"), the extensible
mark up language ("XML"), or
another software language that may be used to create a computer file
displayable on a video or other display in a
manner perceivable by a user. Any computer readable media with logic, code,
data, instructions, may be used to
implement any software or steps or methodology. Where a network comprises the
Internet, a display page may
comprise a webpage of a type known in the art.
[00336] A display page according to the invention may include embedded
functions comprising software
programs stored on a memory device, such as, for example, VBScript routines,
JScript routines, JavaScript routines,
Java applets, ActiveX components, ASP.NET, AJAX, Flash applets, Silverlight
applets, or AIR routines.
-58-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00337] A display page may comprise well known features of graphical user
interface technology, such as,
for example, frames, windows, scroll bars, buttons, icons, and hyperlinks, and
well known features such as a "point
and click" interface or a touchscreen interface. Pointing to and clicking on a
graphical user interface button, icon,
menu option, or hyperlink also is known as "selecting" the button, option, or
hyperlink. A display page according to
the invention also may incorporate multimedia features, multi-touch, pixel
sense, IR LED based surfaces, vision-
based interactions with or without cameras.
[00338] A user interface may be displayed on a video display and/or display
page. The user interface may
display a report generated based on analyzed data relating to the sample. The
report may include information about
the presence or concentration of one or more analyte. The user interface may
display raw or analyzed data relating
to the sample. The data may include information about the presence or
concentration of one or more analyte. The
user interface may display an alert. One example of an alert may be if an
error is detected on the device, or if an
analyte concentration exceeds a predetermined threshold.
[00339] In some embodiments, one or more network devices 204a, 204b may be
provided at a laboratory
facility. The network devices at the laboratory may receive or access data
provided by the sample processing
device 200. In some other embodiments, one or more network devices may be
provided at a health care
professional location. In some embodiments, both laboratory devices and health
care professional devices may be
able to receive or access data provided by the sample processing device. In an
additional example, the one or more
network devices may belong to the subject. One or more of the laboratory,
health care professional, or subject may
have a network device able to receive or access data provided by the sample
processing device. The one or more
laboratory health care professional and/or subject, or the network device of
the laboratory, health care professional,
and/or subject may be authenticated prior to being granted access to the data.
For example, the laboratory
personnel, health care professional, and/or subject may have a login ID and/or
password in order to access the data.
In some embodiments, the data can be sent to the email of the laboratory
personnel, health care professional, and/or
subject.
[00340] In some embodiments, the sample processing device may provide data to
a cloud computing
infrastructure. The network device (e.g., of a laboratory, health care
professional, or other entity) may access the
cloud computing infrastructure. In some embodiments, on-demand provision of
computational resources (data,
software) may occur via a computer network, rather than from a local computer.
The network device may contain
very little software or data (perhaps a minimal operating system and web
browser only), serving as a basic display
terminal connected to the Internet. Since the cloud may be the underlying
delivery mechanism, cloud-based
applications and services may support any type of software application or
service. Information provided by the
sample processing device and/or accessed by the network devices may be
distributed over various computational
resources. Alternatively, they may be stored in one or more fixed data storage
unit or database.
[00341] FIG. 3A illustrates a high level example of a sample processing device
300. A sample processing
device may be provided at any location, including a sample collection site.
The sample processing device may be in
or on a subject, or may be carried by the subject. The sample processing
device may be easily mobile or
transportable. The sample processing device may travel with the subject. The
sample processing device may be a
benchtop device or a handheld device. The sample processing device may be
located remote to a laboratory. Any
-59-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
number of sample processing devices may be distributed geographically in any
manner. For example, one or more
sample collection sites may have one or more devices.
[00342] The sample processing device 300 may be configured to accept a
removable cartridge 350. The
removable cartridge and/or device may have any other characteristics or
components as described elsewhere herein.
The removable cartridge may be configured to accept a sample and/or deliver
the sample to the device. The
removable cartridge may have one or more reagents provided thereon. For
example FIG. 3B provides an
illustration of one or more reagents provided on the removable cartridge.
Alternatively, one or more reagents 370
may be provided on board the device, such as shown in FIG. 3A. The device may
comprise one or more reagent
units that may contain and/or confine one or more reagents. The reagents may
originally be provided on the device,
the reagents may be provided to the reagent units from or on the cartridge, or
both on-board the device and within
the cartridge.
[00343] In other embodiments, the sample processing device need not have a
removable cartridge. One or
more functions as described for the cartridge may be provided by the device
itself.
[00344] The sample processing device and/or a cartridge may comprise all
reagents, liquid- and solid-
phase reagents, required to perform one or more of the chemical reactions
and/or other processing steps, including
physical processing, as described elsewhere herein. For example, for a
luminogenic ELISA assay the reagents
within the device may include a sample diluent, a detector conjugate (for
example, three enzyme-labeled
antibodies), a surface labeled with antibodies binders, a wash solution, and
an enzyme substrate.
[00345] Enzyme-linked ImmunoSorbent assays ("ELISA") are assays using
antibodies to bind a target
analyte in a solution or on a substrate. One useful immunoassay that can be
run on a device disclosed herein is
ELISA. For example, tips having adherent antibodies or target antigens may be
used in ELISAs performed by
devices and according to methods disclosed herein.
[00346] Performing an ELISA generally involves at least one antibody capable
of binding an antigen of
interest (i.e., an analyte that is indicative of influenza viral infection). A
sample containing or suspected to contain
the antigen of interest is immobilized on a support (e.g., a tip or other
support having a surface for immobilization)
either non-specifically (e.g., via adsorption to the surface) or specifically
(e.g., via capture by another antibody
specific to the same antigen, in a "sandwich" ELISA). After the antigen is
immobilized the detection antibody is
added, forming a complex with the antigen. The detection antibody can be
conjugated to an enzyme, or can itself be
detected by a secondary antibody which is in turn conjugated to an enzyme.
Upon addition of a substrate for the
conjugated enzyme, a detectable signal is generated which indicates the
presence and/or quantity of the antigen in
the sample. The choice of substrates will depend on the enzyme conjugated.
Suitable substrates include fluorogenic
and chromogenic substrates. Those of skill in the art would be understand and
be able to determine which
parameters of such assays that can be modified to increase the signal detected
as well as other variations of ELISAs
known in the art.
[00347] In some ELISAs, a solid phase capture surface can include an attached
first antibody to which a
sample (e.g., diluted blood, plasma, or biological specimen) can be added. If
present, an analyte in the sample can
bind to the first antibody and become immobilized. An enzyme reagent can be
added that includes, for example, an
antibody coupled or conjugated to an enzyme (e.g., alkaline phosphatase or
horseradish peroxidase) that produces a
detectable product, or can be otherwise detected. If the antibody portion of
the enzyme reagent can bind the analyte,
-60-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
then the enzyme reagent also becomes immobilized at the capture surface.
Addition of a substrate for the enzyme
can result in a product producing an effect, for example, light that can be
measured and plotted as shown. In this
manner the amount of analyte present in a sample can be measured.
[00348] Thus, for example, an exemplary ELISA which may be performed using a
device, system, or
method as disclosed herein includes a solid phase capture surface (e.g., a
tip) on which a first antibody is
immobilized. The first antibody is specific for a test antigen (e.g., antibody
specific for a target blood analyte, such
as cholesterol, or for e.g., neuraminidase on the coat of a virus of interest,
or other antigen). If the test antigen is
present in a test sample (e.g., whole blood, plasma, or serum) that is exposed
to the antibody immobilized on the
surface, then the test antigen can become immobilized (captured) at the
capture surface. Addition of a second,
labeled antibody that binds to the first antibody (e.g., where the first
antibody has a biotin label, and the second
antibody has an avidin label and a detectable label; or where the first
antibody is a sheep antibody including an Fc
portion, the second antibody may be an antibody targeting sheep Fc and labeled
with alkaline phosphatase (AP)
which can be detected following addition of AP substrate) allows the detection
and quantification of the amount of
antigen in the sample. The first antibody, which is bound to the substrate, is
not washed out by the addition of the
second antibody. Such detection and quantification may be accomplished by
providing substrate for alkaline
phosphatase, leading to the production of colored, fluorescent, luminescent
(e.g., chemiluminescent), or otherwise
detectable compounds which may be detected and measured.
[00349] Alternatively, after the blood sample is placed in contact with the
surface having the immobilized
first antibody (labeled with an enzyme which catalyzes a reaction that
produces a first detectable compound) that
targets a first antigen, a second antibody, targeting a second antigen and
labeled with a second enzyme which can
produce a second detectable compound may be added. The first antibody, which
is bound to the substrate, is not
washed out by the addition of the second antibody, and may be detected by
providing the substrate and proper
reaction conditions for the production of a first detectable product by enzyme
linked to the first antibody. Binding
and subsequent detection of the second, labeled antibody at the capture
surface indicates the presence of both the
first and the second test antigens in the test sample. Both the first and
second detectable compounds produced by
the enzymes linked to the antibodies may be detected by any means desired,
including by detection of fluorescence,
luminescence, chemiluminescence, absorbance, or other means for detecting the
products of the enzymatic reactions
due to the attached enzymes.
[00350] For example, photomultipliers tubes, charge-coupled devices,
photodiodes, cameras,
spectrophotometers, and other components and devices may be used to measure
light emitted or affected during the
performance of an ELISA. For example, the amount of light detected (e.g., in
relative light units, or other
measurements of luminosity) during the performance of an ELISA on a sample may
be compared to a standard
curve (e.g., a calibration curve prepared for a particular assay, device,
cartridge, or reagent) to calculate the
concentration of the target analyte in the sample. Analytes that have been
detected and their levels measured in
blood samples using ELISAs performed on devices and systems as disclosed
herein include: vitamin B-12, folic
acid, thyroxine, testosterone, estradiol, cotinine, vancomycin, hemoglobin
Ale, prostate specific antigen, human
chorionic gonadotropin, luteinizing hormone, parathyroid hormone, alpha-
fetoprotein, prealbumin, cardiac troponin
T, C-reactive protein, hepatitis B surface antigen (HbsAg), immunoglobulin E
(IgE), immunoglobulin G (IgG),
Dengue virus IgG, rheumatoid factor IgM, West Nile Virus IgM, anti-HIV 1
antibodies, anti-HIV 2 antibodies, anti-
nuclear antibodies, influenza A, influenza B, and streptococcus.
-61-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00351] ELISAs are also used, for example, in competitive binding experiments,
in which the
concentration of an analyte in a solution may be measured by adding a known
amount of labeled analyte, and
measuring the binding of the analyte. Increased concentrations of the sample
analyte (which does not include the
label) interfere ("compete") with the binding of the labeled analyte, allowing
calculation of the amount of analyte in
the sample.
[00352] Additional reagents can be provided as needed. In some embodiments,
reagents can be
incorporated into a device to provide for sample pretreatment. Examples of
pretreatment reagents include, without
limitation, white cell lysis reagents, reagents for liberating analytes from
binding factors in the sample, enzymes,
and detergents. The pretreatment reagents can also be added to a diluent
contained within the device.
[00353] Reagents according to the present invention include without limitation
wash buffers, enzyme
substrates, dilution buffers, conjugates, enzyme-labeled conjugates, DNA
amplifiers, sample diluents, wash
solutions, sample pre-treatment reagents including additives such as
detergents, polymers, chelating agents,
albumin-binding reagents, enzyme inhibitors, enzymes, anticoagulants, red-cell
agglutinating agents, antibodies, or
other materials necessary to run an assay on a device. An enzyme-labeled
conjugate can be either a polyclonal
antibody or monoclonal antibody labeled with an enzyme that can yield a
detectable signal upon reaction with an
appropriate substrate. Non-limiting examples of such enzymes are alkaline
phosphatase and horseradish peroxidase.
In some embodiments, the reagents comprise immunoassay reagents. Reagents
defining assay specificity may be
provided, which may optionally include, for example, monoclonal antibodies,
polyclonal antibodies, proteins,
nucleic acid probes or other polymers such as affinity matrices, carbohydrates
or lipids. In general, reagents,
especially those that are relatively unstable when mixed with liquid, are
confined separately in a defined region (for
example, a reagent unit) within the device and/or cartridge.
[00354] In some embodiments, a reagent unit may contain a small volume of
reagent. For example, a
reagent unit may contain approximately about 5 microliters or less to about 1
milliliter of liquid. In some
embodiments, the unit may contain about 20-200 microliters of liquid. In a
further embodiment, the reagent unit
contains 100 microliters of fluid. In an embodiment, a reagent unit contains
about 40 microliters of fluid. A reagent
unit may include any volume described elsewhere herein, which may include
volumes of sample. The volume of
liquid in a reagent unit may vary depending on the type of assay being run or
the sample of bodily fluid provided. In
an embodiment, the volumes of the reagents do not have to be predetermined,
but must be more than a known
minimum. In some embodiments, the reagents are initially stored dry and
dissolved upon initiation of the assay
being run on the device.
[00355] The sample processing device may comprise a display 310. The display
may be a video display
or other type of user interface. The display may function as a user interface.
The display may permit a user to
operate the sample processing device. The display may be configured to accept
an input from the user relating to a
subject identity, other information about the subject, information about the
sample, information about one or more
clinical test, information about sample preparation steps, information about a
laboratory, and/or information about a
medical care provider.
[00356] The display may output information to an operator of the device. The
display may prompt the
operator to perform one or more steps in the operation of the device. The
display may display information about the
sample collected, the subject, and/or data relating to one or more preparation
step performed or chemical reaction
-62-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
run. The display may output information about one or more automated process
that may be implemented by the
device. The display may provide one or more alert for an error detected, or
when one or more parameters are met
(e.g., certain detected signals exceed a predetermined threshold). A display
may display results on the device.
[00357] The sample processing device 300 may comprise one or more components
useful for collecting
the sample, preparing the sample for a clinical test, and/or running a
chemical reaction, or other test or analysis.
The sample processing device may also comprise one or more components useful
for detecting one or more signal
relating to the sample or components of the device. For example, the sample
processing device may include, but is
not limited to, a sample collection unit, centrifuge, magnetic separator,
filter, pipette or other fluid handling system,
vessels, containers, assay units, reagent units, heater, thermal block,
cytometer, spectrophotometer, imaging
systems, microscopy station, light source, optical detector, photometer,
temperature sensor, motion sensor, or sensor
for electrical properties. Fluid may be transferred from one component to
another via a fluid handling system, such
as a pipette, channels, or pumps.
[00358] In some embodiments, the fluid handling system may be a pipettor. The
pipettor may be a multi-
head pipettor. In some instances, each of the pipette heads may be of the same
type or may be of different types.
For example, the pipette heads may be air displacement pipettes and/or
positive displacement pipettes. In some
instances, the fluid handling system may be capable of picking up and/or
removing one or more pipette tip. The
pipette tips may be individually added or removed from the pipette head. The
pipette head may transfer the pipette
tip from a first location to a second location. A pipette tip may be capable
of connecting to and forming a fluid-
tight seal with a pipette head or screwing into it or attaching in other ways.
A sample or other fluid may be
aspirated and/or dispensed by the pipette tip.
[00359] The pipette tip may have an interior surface and an exterior surface.
The pipette tip may have a
first end and an opposing second end. In some embodiments, both the first and
second ends may be open. In some
embodiments, the first end may have a diameter that is greater than the
diameter of the second end. The pipette tip
may or may not be coated with reagents and/or capturing binders such as
antibodies. In some instances, an interior
surface of the pipette tip may be coated with a reagent and/or capturing
binders. A chemical reaction may take
place within the pipette tip. The chemical reaction may take place within the
pipette tip while the tip is attached to a
pipette head, or when the tip is separated from the pipette head.
Alternatively, chemical reactions may take place
within one or more vessel. The pipette may deliver a sample or other fluid to,
or aspirate a sample or other fluid
from, a vessel. The pipette tip may be capable of being at least partially
inserted into a vessel.
[00360] The pipettor may be utilized to transfer a sample or other fluid
within the device. The pipettor
may assist with the preparation of a sample. The pipettor may assist with the
running of a chemical reaction.
[00361] The sample processing device may be capable of performing at least one
sample preparation step
and/or running one or more, two or more, three or more, four or more, five or
more, six or more, seven or more,
eight or more, nine or more, ten or more, twenty or more, thirty or more, or
fifty or more chemical reactions. The
device may be capable of performing one or more, two or more, three or more,
four or more, five or more, six or
more, seven or more, eight or more, nine or more, ten or more, twenty or more,
thirty or more, or fifty or more
different types of assays. These may occur simultaneously and/or in sequence.
The sample preparation and/or
chemical reactions that may occur may be governed by protocols that may be
individualized to a subject's needs
and/or sent back and forth from a server and/or stored or inputted locally.
The subject's needs may be based on a
-63-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
prescription or instructions that the subject has received from a health care
professional. The device may be
configured to accommodate a wide range of sample preparation and/or chemical
reactions.
[00362] The sample processing device 300 may include one or more detector 360
which may be capable
of detecting one or more signal relating to the sample. The detector may be
able to detect all emissions from the
electromagnetic spectrum. Alternatively the detector may be able to detect a
selected range of emission from the
electromagnetic spectrum. For example, an optical detector may detect an
optical signal relating to a chemical
reaction that had taken place on the device. An electrical property sensor or
other sensor may detect the voltage,
current, impedance, resistance, or any other electrical property of a sample.
A temperature sensor may determine
the temperature of a thermal block, upon which a sample may rest. A sensor may
determine the speed of a
centrifuge. A sensor may determine the position, velocity, and/or acceleration
of a pipette and/or the successful
execution of a protocol.
[00363] One or more detectable signal may be detected by a detector 360. The
detectable signal can be a
luminescent signal, including but not limited to photoluminescence,
electroluminescence, chemiluminescence,
fluorescence, phosphorescence or any emission from the electromagnetic
spectrum. In some embodiments, one or
more label may be employed during a chemical reaction. The label may permit
the generation of a detectable
signal. Methods of detecting labels are well known to those of skill in the
art. Thus, for example, where the label is
a radioactive label, means for detection may include a scintillation counter
or photographic film as in
autoradiography. Where the label is a fluorescent label, it may be detected by
exciting the fluorochrome with the
appropriate wavelength of light and detecting the resulting fluorescence by,
for example, microscopy, visual
inspection, via photographic film, by the use of electronic detectors such as
digital cameras, charge coupled devices
(CCDs) or photomultipliers and phototubes, or other detection device. In some
instances, cameras may utilize
CCDs, CMOS, may be lensless cameras (e.g., Frankencamera), open-source
cameras, or may utilize or any other
visual detection technology known or later developed in the art. In some
embodiments, imaging devices may
employ 2-d imaging, 3-d imaging, and/or 4-d imaging (incorporating changes
over time). Similarly, enzymatic
labels are detected by providing appropriate substrates for the enzyme and
detecting the resulting reaction product.
Finally, simple colorimetric labels are often detected simply by observing the
color associated with the label. For
example, conjugated gold often appears pink, while various conjugated beads
appear the color of the bead.
[00364] In some embodiments, an imaging unit may be provided. Examples of
imaging units may include
any of the detectors and/or optical detection devices as described elsewhere
herein. For example, imaging units
may be cameras which may utilize CCDs, CMOS, may be lensless cameras (e.g.,
Frankencamera), open-source
cameras, or may utilize or any other visual detection technology known or
later developed in the art. An imaging
unit may capture static images and/or may capture moving images. For example,
the imaging unit may capture a
series of digital images. An imaging unit may capture video images. An imaging
device may be a camera or a
sensor that detects and/or records electromagnetic radiation and associated
spatial and/or temporal dimensions.
[00365] In one example, the imaging unit may capture one or more digital image
of a sample. For
example, the imaging unit may capture an image of a tissue sample. The picture
of the tissue sample may be
transmitted to a pathologist or other health care professional. Analysis
and/or oversight may occur for the image of
the tissue sample. Analysis and/or oversight may occur on-board or remotely,
by a health care professional or a
software program. In other examples, the imaging unit may capture images of a
sample, and/or any form of
-64-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
preparation of the sample such as chemical reactions or physical processing
steps occurring with the sample. For
example, a video may be taken of a chemical reaction. Any description herein
of data may also apply to data
representative of images, and vice versa.
[00366] Imaging may be used to, e.g., detect, examine, identify, characterize,
and quantify cells in a
sample, such as in, e.g., a blood sample, a urine sample, biopsy tissue, or
other sample. Such use of imaging and
other techniques may be termed "cytometry." Cytometry includes observations
and measurements of cells, such as
red blood cells, platelets, white blood cells, including qualitative and
quantitative observations and measurements of
cell numbers, cell types, cell surface markers, internal cellular markers, and
other characteristics of cells of interest.
Where a biological sample includes or is a blood sample, the sample may be
divided into portions, and may be
diluted (e.g., to provide greater volume for ease of handling, to alter the
density or concentration of cellular
components in the sample to provide a desired diluted density, concentration,
or cell number or range of these, etc.).
The sample may be treated with agents which affect coagulation, or may be
treated or handled so as to concentrate
or precipitate sample components (e.g., ethylene diamine tetraacetic acid
(EDTA) or heparin may be added to the
sample, or the sample may be centrifuged or cells allowed to settle). A sample
may be treated by adding dyes or
other reagents which may react with and mark particular cells or particular
cellular components. For example, dyes
which mark cell nuclei (e.g., hematoxylin dyes, cyanine dyes, drag dyes such
as Draq5, and others); dyes which
mark cell cytoplasm (e.g., eosin dyes, including fluorescein dyes, and others)
may be used separately or together to
aid in visualization, identification, and quantification of cells. More
specific markers, including antibodies and
antibody fragments specific for cellular targets, such as cell surface
proteins, intracellular proteins and
compartments, and other targets, are also useful in cytometry.
[00367] Biological samples may be measured and analyzed by cytometry using
optical means, including,
for example, photodiode detectors, photomultipliers, charge-coupled devices,
laser diodes, spectrophotometers,
cameras, microscopes, or other devices which measure light intensity (of a
single wavelength, of multiple
wavelengths, or of a range, or ranges, of wavelengths of light), form an
image, or both. A field of view including a
sample may be imaged, or may be scanned, or both, using such detectors. A
biological sample may be measured
and analyzed by cytometry prior to processing, dilution, separation,
centrifugation, coagulation, or other alteration.
A biological sample may be measured and analyzed by cytometry during or
following processing, dilution,
separation, centrifugation, coagulation, or other alteration of the sample.
For example, a biological sample may be
measured and analyzed by cytometry directly following receipt of the sample.
In other examples, a biological
sample may be measured and analyzed by cytometry during or after processing,
dilution, separation, centrifugation,
coagulation, or other alteration of the sample.
[00368] For example, a blood sample may be prepared for cytometry by
sedimentation or centrifugation.
A sedimented or pellet portion of such a sample may be resuspended in a buffer
of choice prior to cytometric
analysis (e.g., by aspiration, stirring, sonication, or other processing). A
biological sample may be diluted or
resuspended with water, or with a saline solution, such as a buffered saline
solution prior to cytometric analysis. A
solution used for such dilution or resuspension may or may not include a
fixative (e.g., formaldehyde,
paraformaldehyde, or other agent which cross-links proteins). A solution used
for such dilution or resuspension may
provide an osmotic gradient between the surrounding solution and the interior,
or an interior compartment, of cells
in the sample, effective that the cell volume of some or all cells in the
sample is altered. For example, where the
resulting solution concentration following dilution is less than the effective
concentration of the interior of a cell, or
-65-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
of an interior cell compartment, the volume of such a cell will increase
(i.e., the cell will swell). A biological sample
may be diluted with a solution which may or may not include an osmoticant
(such as, for example, glucose, sucrose,
or other sugar; salts such as sodium, potassium, ammonium, or other salt; or
other osmotically active compound or
ingredient). In embodiments, an osmoticant may be effective to maintain the
integrity of cells in the sample, by, for
example, stabilizing or reducing possible osmotic gradients between the
surrounding solution and the interior, or an
interior compartment, of such cells. In embodiments, an osmoticant may be
effective to provide or to increase
osmotic gradients between the surrounding solution and the interior, or an
interior compartment, of such cells,
effective that the cells at least partially collapse (where the cellular
interior or an interior compartment is less
concentrated than the surrounding solution), or effective that the cells swell
(where the cellular interior or an
interior compartment is more concentrated than the surrounding solution).
[00369] For example, a biological sample may be measured or analyzed following
dilution of the sample
with a solution including dyes. For example, a biological sample may be
measured or analyzed following dilution
of a portion of the sample with a solution including antibodies or antibody
fragments. For example, a biological
sample may be measured or analyzed following dilution of the sample with a
solution including particles. Particles
added to a biological sample may serve as standards (e.g., may serve as size
standards, where the size or size
distribution of the particles is known, or as concentration standards, where
the number, amount, or concentration of
the particles is known), or may serve as markers (e.g., where the particles
bind or adhere to particular cells or types
of cells, to particular cell markers or cellular compartments, or where the
particles bind to all cells in a sample).
[00370] For example, a biological sample may be measured or analyzed following
processing which may
separate one or more types of cells from another cell type or types. Such
separation may be accomplished by gravity
(e.g., sedimentation); centrifugation; filtration; contact with a substrate
(e.g., a surface, such as a wall or a bead,
containing antibodies, lectins, or other components which may bind or adhere
to one cell type in preference to
another cell type); or other means. Separation may be aided or accomplished by
alteration of a cell type or types.
For example, a solution may be added to a biological sample, such as a blood
sample, which causes some or all
cells in the sample to swell. Where one type of cell swells faster than
another type or types of cell, cell types may be
differentiated by observing or measuring the sample following addition of the
solution. Such observations and
measurements may be made at a time, or at multiple times, selected so as to
accentuate the differences in response
(e.g., size, volume, internal concentration, or other property affected by
such swelling) and so to increase the
sensitivity and accuracy of the observations and measurements. In some
instances, a type or types of cells may burst
in response to such swelling, allowing for improved observations and
measurements of the remaining cell type or
types in the sample.
[00371] Observation, measurement and analysis of a biological sample by
cytometry may include
photometric measurements, for example, using a photodiode, a photomultiplier,
a laser diode, a spectrophotometer,
a charge-coupled device, a camera, a microscope, or other means or device.
Cytometry may include preparing and
analyzing images of cells in a biological sample (e.g., two-dimensional
images), where the cells are labeled (e.g.,
with fluorescent, chemiluminescent, enzymatic, or other labels) and plated
(e.g., allowed to settle on a substrate)
and imaged by a camera. The camera may include a lens, and may be attached to
or used in conjunction with a
microscope. Cells may be identified in the two-dimensional images by their
attached labels (e.g., from light emitted
by the labels).
-66-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00372] An image of cells prepared and analyzed by a cytometer as disclosed
herein may include no cells,
one cell, or multiple cells. A cell or cell in an image of a cytometer, as
disclosed herein, may be labeled, as
disclosed above. A cell or cell in an image of a cytometer, as disclosed
herein, may be labeled, as disclosed above,
effective to identify the image, and the subject from whom the sample was
taken.
[00373] Cytometric measurements of cells from samples of blood have been made
using devices, systems,
and methods embodying features disclosed herein. Cytometric images have been
used, for example, to count the
numbers of cells (e.g., providing the number of cells per volume of blood), to
determine the sizes and size
distribution of cells in the sample (including mean, standard deviation, and
other size measures), and to identify cell
types based on cell surface markers. Typically, the total concentration of red
blood cells and platelets is in the
range of about 1 -3 x 106 cells per microliter (e.g., about 2 x 106 cells per
microliter). Cells were allowed to settle on
the floor of a channel within the cuvette, allowing an image to be taken of a
single focal plane that was sufficient to
detect all the cells in the volume within the image area; thus a count of the
cells in a single image provided a count
of the cells in the volume of blood represented by that image. Since the
dimensions of the channel were known, the
volume of blood above the area shown in the image allowed accurate calculation
of the density of cells within that
volume. Volume is also measured by inclusion of known concentrations of beads
(the images of which are
distinguishable from blood cell images) as indicators; since the concentration
of the beads was known, counting the
numbers of beads in a field allowed a precise calculation of the volume of
sample from which an image was taken.
[00374] Optical images included fluorescence images for detecting red, blue
and green wavelengths
(useful, for example, for detecting and measuring fluorescence from dyes
attached to specific cell-surface markers),
and darkfield images (useful for detecting cell shape and outlines, and for
measuring cell size and volume).
Fluorescence images may be used, for example, to identify and quantitate
different cell types in the sample.
Darkfield images are based on light scattered from the objects in the cuvette,
and they provide information
regarding the size, shape, and number of objects in the cuvette. Forward
scatter measurements were used to
quantify cell size. Side scatter measurements were used to determine,
identify, and categorize cell morphology.
[00375] Data from the images was processed by a controller associated with the
sample processing device.
The following measurements may be calculated: 1) number of red blood cells in
the cuvette; 2) average volume of
red blood cells in the cuvette; 3) red blood cell distribution width (RDW) of
red blood cells in the cuvette; 4)
number of platelets in the cuvette; 5) average volume of platelets in the
cuvette; and 6) platelet distribution width of
platelets in the cuvette.
[00376] Microscopy methods that may be used with devices and systems disclosed
herein include but are
not limited to bright field, oblique illumination, dark field, dispersion
staining, phase contrast, differential
interference contrast (DIC), polarized light, epifluorescence, interference
reflection, fluorescence, confocal
(including CLASS), confocal laser scanning microscopy (CLSM), structured
illumination, stimulated emission
depletion, electron, scanning probe, infrared, laser, widefield, light field
microscopy, lensless on-chip holographic
microscopy, digital and conventional holographic microscopy, extended depth-of-
field microscopy, optical scatter
imaging microscopy, deconvolution microscopy, defocusing microscopy,
quantitative phase microscopy, diffraction
phase microscopy, confocal Raman microscopy, scanning acoustic microscopy and
X-ray microscopy.
Magnification levels used by microscopy may include, as nonlimiting examples,
up to 2x, 5x, 10x, 20x, 40x, 60x,
100x, 100x, 1000x, or higher magnifications. Feasible magnification levels
will vary with the type of microscopy
-67-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
used. For example, images produced by some forms of electron microscopy may
involve magnification of up to
hundreds of thousands of times. Multiple microscopy images may be recorded for
the same sample to generate
time-resolved data, including videos. Individual or multiple cells may be
imaged simultaneously, by parallel
imaging or by recording one image that encompasses multiple cells. A
microscopic objective may be immersed in
media to change its optical properties, such as through oil immersion. A
microscopic objective may be moved
relative to the sample by means of a rotating CAM to change the focus.
Cytometry data may be processed
automatically or manually, and may further include analyses of cell or tissue
morphology, such as by a pathologist
for diagnostic purposes.
[00377] The microscopic objective can be finely positioned to focus the image
via an actuator, such as by
a cam connected to a motor. The objective may be focused on one or more planes
of the sample. Focusing may be
automated by image analysis procedures by computing the image sharpness of
digital images among other methods.
[00378] Cell counting can be performed using cytometry, e.g., imaging with or
without microscopy. In
situations where the subjects may be bright-field illuminated, the preferred
embodiment is to illuminate the subjects
from the front with a white light and to sense the cells with an imaging
sensor. Subsequent digital processing will
count the cells. Where the cells are infrequent or are small, the preferred
embodiment is to attach a specific or non-
specific fluorescent marker, and then illuminate the subject field with a
laser or other suitable light source. Confocal
scanning imaging may be used. In embodiments, up to 500 or 1000 cells of any
given type may be counted. In
other embodiments, various numbers of cells of any given type may be counted,
including but not limited to more
than or equal to about 1 cell, 5 cells, 10 cells, 30 cells, 50 cells, 100
cells, 150 cells, 200 cells, 300 cells, 500 cells,
700 cells, 1000 cells, 1500 cells, 2000 cells, 3000 cells, 5000 cells. Cells
may be counted using available counting
algorithms. Cells can be recognized by their characteristic fluorescence, size
and shape.
[00379] In some microscopy embodiments, brightfield illumination may be
achieved by the use of a white
light source along with a stage-condenser to create Koehler illumination.
Brightfield images of cells, which may
detect properties similar to that of forward scattering in flow cytometry, can
reveal cell size, phase-dense material
within the cells and colored features in the cell if the cells have been
previously stained. In one example
embodiment, the Wright-Giemsa staining method can be used to stain human whole
blood smear. Brightfield
imaging shows characteristic patterns of staining of human leukocytes. The
characteristically shaped red cells can
also be identified in these images.
[00380] In some microscopy embodiments, darkfield imaging may be achieved by
the use of a ringlight
based illumination scheme, or other epi- or trans-darkfield illumination
schemes available. Darkfield imaging may,
for example, be used to determine light scattering properties of cells,
equivalent to side scatter in flow cytometry,
such as when imaging human leukocytes. The internal and external features of
the cell which scatter more light
appear brighter and the features which scatter lesser amounts of light appear
darker in a darkfield image. Cells such
as granulocytes have internal granules of size range (100-500nm) which can
scatter significant amount of light and
generally appear brighter in darkfield images. Furthermore, the outer boundary
of any cell may scatter light and
may appear as a ring of bright light. The diameter of this ring may directly
give the size of the cell. Microscopy
methods may additionally be used to measure cell volume. For example, red
blood cell volume may be measured.
To increase accuracy, red blood cells may be transformed into spheres through
the use of anionic or zwitterionic
-68-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
surfactants, and dark field imaging used to measure the size of each sphere,
from which cell volumes may be
calculated.
[00381] In some microscopy embodiments, small cells or formed elements which
may be below the
diffraction-limited resolution limit of the microscope, may be labeled with
fluorescent markers; the sample may be
excited with light of appropriate wavelength and an image may be captured. The
diffraction pattern of the
fluorescent light emitted by the labeled cell may be quantified using computer
analysis and correlated with the size
of the cell. The computer programs used for these embodiments is described
elsewhere herein. To improve the
accuracy of this method, the cells may be transformed into spheres by the use
of anionic and zwitterionic
surfactants.
[00382] Cell imaging may be used to extract one or more of the following kinds
of information for each
cell (but is not limited to the following): Cell size; Quantitative measure of
cell granularity or light scattering (also
popularly called side scatter, based on flow cytometry parlance); Quantitative
measure of fluorescence in each
spectral channel of imaging, after compensating for cross-talk between
spectral channels, or intracellular
distribution pattern of fluorescent or other staining; Shape of the cell, as
quantified by standard and custom shape
attributes such as aspect ratio, Feret diameters, Kurtosis, moment of inertia,
circularity, solidity etc.; Color, color
distribution and shape of the cell, in cases where the cells have been stained
with dyes (not attached to antibodies or
other types of receptor);Intracellular patterns of staining or scattering,
color or fluorescence that are defined as
quantitative metrics of a biological feature such as morphology, for example
density of granules within cells in a
darkfield image, or the number and size of nucleolar lobes in a Giemsa-Wright
stained image of polymorphonuclear
neutrophils etc.; Co-localization of features of the cell revealed in images
acquired in different channels;Spatial
location of individual cells, cellular structures, populations of cells,
intracellular proteins, ions, carbohydrates and
lipids or secretions (such as to determine the source of secreted proteins).
[00383] A wide range of cell-based assays can be designed to use the
information gathered by cytometry.
For example, an assay for performing a 5-part leukocyte differential may be
provided. The reportables in this case
may, for example, be number of cells per microliter of blood for the following
types of leukocytes: monocytes,
lymphocytes, neutrophils, basophils and eosinophils. Reportables may also be
used to classify leukocyte
differentiation, or identify T and B-cell populations.
[00384] Fluorescence microscopy generally involves labeling of cells or other
samples with fluorescent
labels, described in more detail below. Microscopic imaging of fluorescently
labeled samples may gather
information regarding the presence, amounts, and locations of the target that
is labeled at a given moment in time or
over a period of time. Fluorescence may also be used to enhance sensitivity
for detecting cells, cellular structures,
or cellular function. In fluorescence microscopy, a beam of light is used to
excite the fluorescent molecules, which
then emit light of a different wavelength for detection. Sources of light for
exciting fluorophores are well known in
the art, including but not limited to xenon lamps, lasers, LEDs, and
photodiodes. Detectors include but are not
limited to PMTs, CCDs, and cameras.
[00385] Spectroscopy includes any and all assays that produce luminescence or
change light (e.g., color
chemistry). These may include one or more of the following: spectrophotometry,
fluorimetry, luminometry,
turbidimetry, nephelometry, refractometry, polarimetry, and measurement of
agglutination.
-69-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00386] Spectrophotometry refers to measuring a subject's reflection or
transmission of electromagnetic
waves, including visible, UV, and infrared light. Spectrophotometry may, for
example, be used to determine
nucleic acid concentrations in a sample, such as by measuring absorbance at a
wavelength of about 260 nm, to
determine protein concentration by measuring absorbance at a wavelength of
about 280 nm, and/or to determine salt
concentration by measuring absorbance at a wavelength of about 230 nm.
[00387] Other examples of spectrophotometry may include infrared (IR)
spectroscopy. Examples of
infrared spectroscopy include near-infrared spectroscopy, far-infrared
spectroscopy laser-Raman spectroscopy,
Raman confocal laser spectroscopy, Fourier Transform infrared spectroscopy,
and any other infrared spectroscopy
technique. Frequencies of less than about 650 cm-1 are typically used for far-
infrared spectroscopy, frequencies
greater than about 4000 cm-1 are typically used for near-infrared
spectroscopy, while frequencies between about
biomedical applications, including in screening and diagnosis of cancer,
arthritis, and other diseases, determining
chemical compositions of biological fluids, determining septic state, and
others. IR spectroscopy may be used on
solid samples, such as tissue biopsies, cell cultures, or Pap smears; or on
liquid samples, such as blood, urine,
synovial fluid, mucus, and others. IR spectroscopy may be used to
differentiate between normal and cancerous cells
as described in U.S. Pat. No. 5,186,162, herein incorporated by reference. IR
spectroscopy may also be used on
blood samples to detect markers for cancers of various solid organs. IR
spectroscopy may also be used to determine
cellular immunity in patients, such as to diagnose immunodeficiencies,
autoimmune disorders, infectious diseases,
allergies, hypersensitivity, and tissue transplant compatibility.
[00388] IR spectroscopy may be used to determine glucose levels in blood,
which is of use for diabetic
patients, such as for monitoring insulin response. IR spectroscopy may further
be used to measure other substances
in blood samples, such as alcohol levels, fatty acid content, cholesterol
levels, hemoglobin concentration. IR
spectroscopy can also distinguish between synovial fluid from healthy and
arthritic patients.
[00389] Fluorimetry refers to measuring the light emitted by a fluorescent
molecule coupled to a subject
upon exciting the fluorescent molecule with incident light. Fluorimetry may
use any of the fluorescent molecules,
labels, and targets as described for cytometric assays above. In some
embodiments, fluorimetry uses substrate
molecules that change in fluorescence based on an enzymatic activity, such as
converting NAD+ to NADH or vice
versa or producing beta-galactosidase from a precursor molecule. Fluorimetry
may be used with a polarized
excitation source to measure fluorescence polarization or anisotropy of a
subject, which may provide information
about the size and/or binding state.
[00390] Colorimetry refers to measuring the transmissive color absorption of a
subject, preferably by
backlighting the subject with white light with the result sensed by an imaging
sensor. Examples include some
assays that use oxidases or peroxidases combined with a dye that becomes
colored in the presence of hydrogen
peroxide. One method that measures peroxidase activity in whole cell
suspensions of human white blood cells is
disclosed in Menegazzi, et al., J. Leukocyte Biol 52: 619-624 (1992), which is
herein incorporated by reference in
its entirety. Such assays may be used to detect analytes that include but are
not limited to alcohols, cholesterols,
lactate, uric acid, glycerol, triglycerides, glutamate, glucose, choline,
NADH. Some of the enzymes that may be
used include horseradish peroxidase, lactoperoxidase, microperoxidase, alcohol
oxidase, cholesterol oxidase,
NADH oxidase. Other nonlimiting examples of colorimetric assays include dye-
based assays to determine protein
concentration, such as Bradford, Lowry, biureat, and Nano-orange methods. The
pH of a sample may also be
-70-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
determined by colorimetric assays with indicator dyes, including but not
limited to phenolphtalein, thymolphtalein,
alizarin Yellow R, indigo carmine, m-cresol purple, cresol red, thymol blue,
xylenol blue, 2,2',2",4,4'-
pentamethoxytriphenyl carbinol, benzopurpurin 4B, metanil yellow, 4-
phenylazodiphenylamine, malachite green,
quinaldine red, orange IV, thymol blue, xylenol blue, and combinations
thereof.
[00391] Luminometry uses no illumination method as the subject emits its own
photons. The emitted light
can be weak and can be detecting using an extremely sensitive sensor such as a
photomultiplier tube (PMT).
Luminometry includes assays that produce chemiluminescence, such as those
using luciferases or some assays
using peroxidases.
[00392] For turbidimetry, the preferred embodiment for sensing is backlighting
the subject with white
light with the result sensed by an imaging sensor. For turbidimetry, the
reduction of the intensity of the transmitted
light is measured. Turbidimetry may be used, for example, to determine a
concentration of cells in solution. In some
embodiments, turbimetry is measured by nephelometry.
[00393] Nephelometry measures the light that is transmitted or scattered after
passing through a subject in
a suspension, typically a substrate bound to an immunoglobin such as IgM, IgG,
and IgA.
[00394] Polarimetry measures the polarization of, typically, electromagnetic
waves by a subject.
Polarimetry assays include circular dichroism, which may provide structural
information and light scattering assays,
which may provide information about the size and/or shape of the subject. One
nonlimiting example of light
scattering assays uses dynamic light scattering (DLS). Subjects for these
assays do not require labeling.
[00395] The sample processing device 300 may have a processor 330 that may
provide instructions to one
or more components of the device. The processor may act as a controller that
may instruct one or more component
of the device. For example, the processor may provide an instruction to a
pipette to aspirate or dispense a fluid.
The processor may provide an instruction that controls the temperature of a
heater (which may optionally heat
and/or cool the device). The processor may provide an instruction to an
optical detector to detect one or more
signal. The processor may also receive instructions and/or collected data. For
example, a processor may act in
accordance with one or more protocol. The protocol may be provided on board
the device or may be provided from
a source external to the device. The processor may also receive data regarding
signals detected by the device. The
processor may or may not analyze signals that have been detected by the
device. The processor may or may not
compare one or more detected signal with a threshold value.
[00396] A communication module 340 may be provided on the device 300. A
communication unit may
be part of a laboratory or set-up which includes the device. The communication
module may permit the device to
communicate with an external machine. For example, the communication module
may receive one or more
protocol or set of instructions from an external source. In some embodiments,
the external source may be a
laboratory. The communication module may also permit the device to transmit
data to an external machine. Data
may be transmitted via a transmission unit. For example, the device may
transmit data to a laboratory or to a health
care professional. The device may transmit data to a cloud computing
infrastructure, which may be accessed by a
laboratory, health care professional, or other entity. The communication
module may permit wireless and/or wired
communication.
-71-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00397] The sample processing device 300 may also comprise a power module 320.
The power module
may connect the device to an external power source, or may be provided as an
internal local power source. For
example, the power module may connect the device to a grid or utility. The
device may include a plug that may be
connected to an electric socket. The device may be connected to any other
external power source, which may
include an electricity generation device, such as a generator, or any
renewable energy source (e.g., solar, wind,
water, geothermal), or energy storage source (e.g., battery, ultracapacitor).
The power module may be a local
power source. For example, the power module may be an energy storage device,
such as a battery or ultracapacitor.
Any battery chemistry known or later developed in the art may be used.
Alternatively, a local power source may
include a local energy generation device, such as a device that utilizes
renewable energy. The power module may
provide electricity to run the rest of the sample processing device.
[00398] One or more component of the device may be contained within a housing.
The housing may
partially or completely surround components of the device. A display may be
provided on the housing so that the
display may be visible.
[00399] The device may be a benchtop device. The device may be portable or
worn. A plurality of
devices may fit within a room. The device may have a total volume of less
than, greater than, or equal to about 4
m3, 3 m3, 2.5 m3, 2 m3, 1.5 m3, 1 m3, 0.75 m3, 0.5 m3, 0.3 m3, 0.2 m3, 0.1 m3,
0.08 m3, 0.05 m3, 0.03 m3, 0.01 m3,
0.005 m3, 0.001 m3, 500 cm3, 100 cm3, 50 cm3, 10 cm3, 5 cm3, 1 cm3, 0.5 cm3,
0.1 cm3, 0.05 cm3, or 0.01 cm3. The
device may have a footprint covering a lateral area of the device. In some
embodiments, the device footprint may
be less than, greater than, or equal to about 4 m2, 3 m2, 2.5 m2, 2 m2, 1.5
m2, 1 m2, 0.75 m2, 0.5 m2, 0.3 m2, 0.2 m2,
0.1 m2, 0.08 m2, 0.05 m2, 0.03 m2, 100 cm2, 80 cm2, 70 cm2, 60 cm2, 50 cm2, 40
cm2, 30 cm2, 20 cm2, 15 cm2, 10
cm2, 7 cm2, 5 cm2, 1 cm2, 0.5 cm2, 0.1 cm2, 0.05 cm2, or 0.01 cm2. The device
may have a lateral dimension (e.g.,
width, length, or diameter) or a height less than, greater than, or equal to
about 4 m, 3 m, 2.5 m, 2 m, 1.5 m, 1.2 m,
1 m, 80 cm, 70 cm, 60 cm, 50 cm, 40 cm, 30 cm, 25 cm, 20 cm, 15 cm, 12 cm, 10
cm, 8 cm, 5 cm, 3 cm, 1 cm, 0.5
cm, 0.1 cm, 0.05 cm, or 0.01 cm. The lateral dimensions and/or height may vary
from one another. Alternatively,
they may be the same. In some instances, the device may be a tall and thin
device, or may be a short and squat
device. The height to lateral dimension ratio may be greater than or equal to
100:1,50:1, 30:1, 20:1, 10:1, 9:1, 8:1,
7:1, 6:1, 5:1,4:1, 3:1,2:1, 1:1, 1:2, 1:3, 1:4, 1:5, 1:6, 1:7, 1:8, 1:9, 1:10,
1:20, 1:30, 1:50, or 1:100.
[00400] The device may have any weight. The device may be capable of being
lifted manually by a
human. The device may be capable of being on or in a human. The device may be
molted or mounted to a ground,
wall, ceiling, and/or wall. The device may be sized and/or shaped to be
ingestible by a human. Examples of device
weights may include but are not limited to less than, greater than, or equal
to about 20 kg, 15 kg, 10 kg, 8 kg, 6 kg,
kg, 4 kg, 3 kg, 2 kg, 1 kg, 0.7 kg, 0.5 kg, 0.3 kg, 0.1 kg, 0.05 kg, 0.01 kg,
5 g, 1 g, 0.5 g, 0.1 g, 0.05 g, or 0.01 g.
[00401] In some embodiments, methods above, alone or in combination, are
implemented with the aid of
one or more systems and devices provided in Patent Cooperation Treaty
Application No. PCT/US2011/53188;
Patent Cooperation Treaty Application No. PCT/US2011/53189; Patent Cooperation
Treaty Application No.
PCT/US2012/57155; U.S. Patent Application 13/244,946; U.S. Patent Application
13/244,947; U.S. Patent
Application 13/244,949; U.S. Patent Application 13/244,950; U.S. Patent
Application 13/244,951; U.S. Patent
Application 13/244,952; U.S. Patent Application 13/244,953; U.S. Patent
Application 13/244,954; U.S. Patent
Application 13/244,956; and U.S. Patent Application 13/769,779 , entitled
"Systems and Methods for Multi-
-72-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
Analysis," filed February 18, 2013 (which also claims priority to
PCT/US2011/53188), the contents of all of which
applications are hereby incorporated by reference herein in their entireties.
[00402] FIG. 4 shows an example of a sample collection, processing, and
analysis method. One or more
of the following steps may occur in such a method. The order of the steps may
be modified, or one or more step
may be optional or may be substituted by another step. Steps illustrated in
Fig. 4 by boxes numbered from 400 to
440 represent steps which may be considered pre-analytic steps. Pre-analytic
steps include sample collection,
verification, preparation, including oversight, calibration, running of
controls, acquisition of raw data, and other
steps as shown in the figure and as disclosed herein. In embodiments,
oversight of pre-analytic steps may be
performed at one or more times before, during, and after sample collection and
testing, or may be continuous
throughout a period or periods before, during, and after sample collection and
testing. Steps illustrated in Fig. 4 by
boxes numbered from 450 to 460 represent steps which may be considered
analytic steps. Analytic steps include
analysis of data received from a device at a sample collection site, as
indicated in the figure and as disclosed herein.
In embodiments, oversight of analytic steps may be performed at one or more
times before, during, and after
analysis, or may be continuous throughout a period or periods before, during,
and after analysis. Steps illustrated in
Fig. 4 by boxes numbered from 470 to 480 represent steps which may be
considered post-analytic steps. Post-
analytic steps include review of the analysis of data, comparison with
controls, calibrations, device and sample
identification and information, review of report generation and of the report
generated for a particular test, and other
steps as indicated in the figure and as disclosed herein. In embodiments,
oversight of post-analytic steps may be
performed at one or more times before, during, and after post-analysis, or may
be continuous throughout a period or
periods before, during, and after post-analysis. Methods disclosed herein, and
devices and systems useful in the
practice of such methods, may be, or may be used with, or may be part of, a
laboratory automation system (LAS),
or a laboratory information system (LIS), or an electronic medical record
system (EMR). In embodiments of the
methods, systems, and devices disclosed herein, a method, system or device as
disclosed herein may be integrated
with a LAS, a LIS, or an EMR. In embodiments, an EMR may be integrated with an
LAS, a LIS, and methods,
systems, or devices as disclosed herein.
[00403] The method may include collecting a sample from a subject 400,
preparing the sample for running
a chemical reaction 410, permitting a chemical reaction with one or more
reagent 420, detecting a signal relating to
the sample, chemical reaction, and/or component of the device 430, pre-
processing the detected signals without
performing analysis, analyzing the data 450, generating a report based on the
data 460, transmitting a report 470,
providing the report to a health care professional 480, and/or displaying a
report on the device and/or screen or
other display device.
[00404] One or more of these steps may be provided by any device or entity.
The demarcations illustrated
in the figures are provided by way of example only, and are in no way
limiting. For example, a sample may be
collected 400 external to a device 490. Alternatively, the sample may be
collected directly at the device, or may be
collected by the device. This may occur at a sample collection site. The
sample prep 410, chemical reaction 420, or
signal detection steps 430, may be performed by the device 490.
[00405] In some embodiments, a sample may be prepared for a subsequent
qualitative and/or quantitative
evaluation. Such a sample preparation for evaluation step may include one or
more of the sample prep 410,
chemical reaction 420, and/or signal detection 430 steps. In some embodiments,
a sample may be processed by
-73-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
receiving the sample 400, and/or preparing the sample for a subsequent
qualitative and/or quantitative evaluation, to
yield data necessary for the subsequent qualitative and/or quantitative
evaluation. Sample processing may also
include transmitting the data from the device. In some instances, the data may
be transmitted to a health care
professional of an authorized analytical facility.
[00406] One, two or all these of these steps may take place, and one, two, or
all of the steps that take place
may occur at the device at a sample collection site. Alternatively, they may
take place at another entity, such as a
laboratory. The point of service site near or on the body (such as the home)
of the subject may be a laboratory or
sample collection site.
[00407] Data collected by the device may be in a raw state. This may include
signals detected at the
device. The data may optionally undergo pre-processing 440. Data pre-
processing does not perform actual data
analysis or comparison with any threshold values. Data pre-processing may
involve modifying the format of data.
In some instances, data pre-processing may occur at a device 490 at a sample
collection site. Then the pre-
processed data may be transmitted to a laboratory. Alternatively, data pre-
processing 440 may occur at a laboratory
492. Raw data may be sent from a device to the laboratory where pre-processing
may occur. Alternatively, no pre-
processing occurs within the method.
[00408] Examples of raw data include, but are not limited to, the following:
light data, including light
intensity, wavelength, polarization, and other data regarding light, e.g.,
output from optical detectors such as
photomultiplier tubes, photodiodes, charge-coupled devices, luminometers,
spectrophotometers, cameras, and other
light sensing components and devices, including absorbance data, transmittance
data, turbidity data, luminosity
data, wavelength data (including intensity at one, two, or more wavelengths or
across a range of wavelengths),
reflectance data, refractance data, birefringence data, polarization, and
other light data. Light data may provide
information about the presence, number, or other characteristic of an analyte,
cell, particle, or other subject of
interest in a sample, tissue, container, or field of view. Light data may
provide information about the presence,
progress, or completion of a chemical reaction or physical process.
[00409] Raw data includes image data, e.g., data from digital or analog
cameras, including images of
tissues, including tissue slices and biopsy samples; images of cells,
particles, crystals, or other elements which may
be present in a sample; images may include two-dimensional images of cells
particles, or crystals, such as
fluorescently labeled or chemiluminescent cells; two-dimensional images of
light scattered by cells, particles, or
crystals; two-dimensional images of cells, particles or crystals taken using
phase contrast, bright-field, dark-field,
interference contrast, or other technique; two-dimensional images of cells in
a tissue slice or other histological
specimen, where the cells may, or may not be stained. Images may include three-
dimensional images of cells
particles, or crystals, such as fluorescently labeled or chemiluminescent
cells; three-dimensional images of light
scattered by cells, particles, or crystals; three-dimensional images of cells,
particles or crystals taken using phase
contrast, bright-field, dark-field, interference contrast, or other technique;
three-dimensional images of cells in a
tissue slice or other histological specimen, where the cells may, or may not
be stained. Raw data includes cell
counts, cell shape, numbers of stained or labeled cells, and intensity of
staining or labeling.
[00410] Raw data includes temperature measurements, duration measurements, pH
measurements, ion and
other analyte measurements, particle counts, hematocrit measurements, and
other measurements of chemical and
physical characteristics of a sample, or of sample constituents. Raw data
includes data measuring or indicating the
-74-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
progress of a chemical reaction or physical change, including color changes
due to enzymatic action on the product
of a chemical reaction. Such raw data provides data from chemical assays,
including measurements of light emitted
or absorbed during or following chemical reactions, binding reactions,
competition reactions, and other reactions
and assays. Such raw data may provide a direct measure of the chemical
reaction or assay, or further processing or
analysis based on the raw data may be required to provide a desired
measurement.
[00411] Raw data may be pre-processed by a device prior to transmission to a
laboratory location. In
embodiments, raw data is transmitted from a sample collection location to a
laboratory location without pre-
processing.
[00412] Data analysis may occur 450 in accordance with an embodiment of the
invention. Data analysis
may include a subsequent qualitative and/or quantitative evaluation of a
sample. The quantitative and/or qualitative
analysis may involve a determination of clinical relevance of the biological
sample or lack thereof. Data analysis
may include one or more comparison of the data with a threshold value. Said
comparison may be used to determine
the presence or concentration of one or more analyte, or may be useful for
analytical methods and/or pathological
analysis described elsewhere herein. Data analysis may occur at a laboratory
492. In some embodiments, the
laboratory may be a certified laboratory. The data that may be analyzed may be
raw data or pre-processed data. A
device may process a sample without analyzing the sample. Data analysis does
not occur on the device in this
scenario. In some embodiments, processing the sample on the device does not
yield a determination of the presence
or concentration level of one or more analytes, two or more analytes, three or
more analytes, four or more analytes,
five or more analytes, six or more analytes, seven or more analytes, eight or
more analytes, nine or more analytes,
ten or more analytes, twelve or more analytes, fifteen or more analytes, or
twenty or more analytes. In some
instances, processing the sample on the device does not yield a determination
of the presence or concentration of
one or more, or any number of analytes (including those described elsewhere
herein), belonging to the categories of
cardiac marker, blood gas, electrolyte, lactate, hemoglobin, or coagulation
factors. In some embodiments,
processing the sample on the device does not yield a determination of the
presence or concentration of one or more,
two or more, three or more, or any number of analytes (including those
described elsewhere herein), belonging to
the following: sodium, potassium, chloride, TCO2, anion Gap, ionized calcium,
glucose, urea nitrogen, creatinine,
lactate, hematocrit, hemoglobin, pH, PCO2, P02, HCO3, base excess, s02, ACT
Kaolin, ACT Celite, PT/INR, cTnl,
CK-MB, and BNP. In some instances, processing the sample does not include a
display of the presence or
concentration of one or more, or any number of analytes (including those
described elsewhere herein), belonging to
the categories of cardiac marker, blood gas, electrolyte, lactate, hemoglobin,
or coagulation factors. Similarly, in
some instances, processing the sample does not include a display of the
presence or concentration of one or more, or
any number of analytes (including those described elsewhere herein), belonging
to the following: sodium,
potassium, chloride, TCO2, anion Gap, ionized calcium, glucose, urea nitrogen,
creatinine, lactate, hematocrit,
hemoglobin, pH, PCO2, P02, HCO3, base excess, s02, ACT Kaolin, ACT Celite,
PT/INR, cTnl, CK-MB, and BNP.
[00413] Data analysis may include a qualitative and/or quantitative evaluation
of the sample. Said
qualitative and/or quantitative evaluation of the sample may yield a
determination of the presence or concentration
of one or more, two or more, three or more, four or more, five or more, six or
more, ten or more, fifteen or more, or
twenty or more analytes. In some examples, analytes may belong to categories
involved in one or more of the
following types of research and/or analyses: immunoassay, nucleic acid assay,
receptor-based assay, cytometric
assay, colorimetric assay, enzymatic assay, electrophoretic assay,
electrochemical assay, spectroscopic assay,
-75-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
chromatographic assay, microscopic assay, topographic assay, calorimetric
assay, turbidmetric assay, agglutination
assay, radioisotope assay, viscometric assay, coagulation assay, clotting time
assay, protein synthesis assay,
histological assay, culture assay, osmolarity assay, and/or other types of
assays or combinations thereof. Analytes
being tested may be involved in one or more types of reactions selected from
the following: Chemistry - Routine
Chemistry, Hematology (includes cell-based assays, coagulation and andrology),
Microbiology ¨ Bacteriology
(includes "Molecular Biology"), Chemistry ¨ Endocrinology, Microbiology ¨
Virology, Diagnostic Immunology -
General Immunology, Chemistry ¨ Urinalysis, Immunohematology - ABO Group & Rh
type, Diagnostic
Immunology - Syphilis Serology, Chemistry ¨ Toxicology, Immunohematology -
Antibody Detection (transfusion),
Immunohematology - Antibody Detection (non-transfusion), Histocompatibility,
Microbiology ¨
Mycobacteriology, Microbiology ¨ Mycology, Microbiology ¨ Parasitology,
Immunohematology - Antibody
Identification, Immunohematology - Compatibility Testing, Pathology ¨
Histopathology, Pathology - Oral
Pathology, Pathology ¨ Cytology, Radiobioassay, and/or Clinical Cytogenetics.
One or more measurement may
include: proteins, nucleic acids (DNA, RNA, hybrids thereof, microRNA, RNAi,
EGS, Antisense), metabolites,
gasses, ions, particles (including crystals), small molecules and metabolites
thereof, elements, toxins, enzymes,
lipids, carbohydrates, prion, formed elements (e.g., cellular entities (e.g.,
whole cell, cell debris, cell surface
markers)). In some embodiments, one or more analytes belonging to categories
of cardiac marker, blood gas,
electrolyte, lactate, hemoglobin, or coagulation factors. In some embodiments,
one or more analytes may include
sodium, potassium, chloride, TCO2, anion Gap, ionized calcium, glucose, urea
nitrogen, creatinine, lactate,
hematocrit, hemoglobin, pH, PCO2, P02, HCO3, base excess, s02, ACT Kaolin, ACT
Celite, PT/INR, cTnl, CK-
MB, and/or BNP.
[00414] The data that may be analyzed may be provided from a device 490 or may
be modified at the
laboratory 492 or other entity prior to being analyzed. In another embodiment
of the invention, the data analysis
450 may occur on the device without occurring at a laboratory. Alternatively,
data analysis may occur on both the
device and at the laboratory or the device may be the laboratory. The analysis
may occur at a point of service
location, such as a home, office, doctor's office/hospital, retailer site, or
other point of service location. Any
description herein of a laboratory location or other location, may apply to
any other point of service location
described elsewhere herein.
[00415] A report may be generated 460 based on the data. A report may be based
on analyzed data 450 or
may be based on data in its raw or pre-processed form. The report may be
generated based on a qualitative and/or
quantitative evaluation of the sample. The report may be generated at a
laboratory 492, such as an authorized
analytical facility. Alternatively, the report can be generated at the device,
or by any other entity. The report may
be transmitted 470. The report may be transmitted by the same entity that
generated the report. Alternatively, a
different entity can transmit the report. The report may be transmitted by a
laboratory 492, such as an authorized
analytical facility, a device 490, cartridge, or any other entity.
[00416] The report may be received by a health care professional 480. The
health care professional may
be provided at a location separate from the device 490 and/or the laboratory
492. The health care professional may
be capable of relying on the report in order to diagnose, treat, and/or
provide disease prevention for the subject.
[00417] Thus, as previously described, any one or more of these steps may be
optional. Any one or more
of these steps may be performed at a sample collection site or in or on a
subject by a device 490 or may be
-76-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
performed at a laboratory 492, or at any other entity. In some embodiments,
the location where a data analysis 450
step may be performed may be certified, or may undergo review or oversight.
[00418] A device may be configured to process a sample. Sample processing may
include receiving a
sample 400 and/or preparing a sample for subsequent qualitative and/or
quantitative evaluation, to yield necessary
for the subsequent qualitative and/or quantitative evaluation. Preparing the
sample for subsequent qualitative and/or
quantitative evaluation may include one or more sample preparation step 410,
chemical reaction step or physical
processing step 420, and/or detection step 430. Processing the sample may
include adding one or more reagent or
fixatives. Sample processing may optionally also include transmitting data
electronically. The data may be
transmitted to a health care professional of an authorized analytical facility
and/or displayed on the screen. The data
may be transmitted and/or displayed simultaneously.
[00419] The sample may be collected from a subject 400 in any manner described
elsewhere herein. For
example, a fingerstick may collect the sample from the subject. In other
examples, feces, urine, or tissue may be
collected in an operating and/or emergency room, or any other sample
collection mechanism described elsewhere
herein may be utilized. The collected sample may be provided to a device 490.
The sample collection may occur at
a sample collection site, or elsewhere. The sample may be provided to the
device at a sample collection site.
[00420] Optionally, the sample may be prepared for a chemical reaction and/or
physical processing step
410. The sample preparation step may include one or more of the following:
centrifugation, separation, filtration,
dilution, enriching, purification, precipitation, incubation, pipetting,
transport, chromatography, cell lysis,
cytometry, pulverization, grinding, activation, ultrasonication, micro column
processing, processing with magnetic
beads or nanoparticles, or other sample preparation steps. The sample may be
transferred within a device. Sample
preparation may include one or more step to separate blood into serum and/or
particulate fractions, or to separate
any other sample into various components. Sample preparation may include one
or more step to dilute and/or
concentrate blood, or other biological samples. Sample preparation may include
adding an anti-coagulant or other
ingredients to a sample. Sample preparation may also include purification of a
sample. Sample preparation may
involve altering the density of a sample, and/or creating a density profile of
a sample. In some instances, denser
portions of a sample may be separated from less dense portions of a sample.
Sample preparation may include
separating solid components of a sample from aqueous components of a sample.
In some examples, sample
preparation may involve centrifugation, incubation and/or cell lysis. Sample
preparation may include causing the
sample to flow, such as a laminar flow. Sample preparation may include
transporting a sample from one portion of
a device to another. Sample preparation may include incubating a sample. The
sample preparation may include a
process to render a biological sample applicable prior to undergoing a
chemical reaction and/or running an assay.
The sample preparation step may render a biological sample ready for running
one or more clinical test, which may
include adding a series of reagents, running a protocol and/or running an
assay.
[00421] Optionally, the sample may undergo a chemical reaction with a reagent
420. The chemical
reaction may occur following a sample preparation step. Alternatively, the
chemical reaction need not follow a
sample preparation step. Sample preparation steps may occur prior to,
concurrently with, and/or after a chemical
reaction. In some embodiments, preparing a sample for qualitative and/or
quantitative evaluation may include
permitting a chemical reaction. One or more type of assay, as described
elsewhere herein may occur. For example,
a sample preparation step (or e.g., a chemical reaction that may occur while
preparing a sample for qualitative
-77-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
and/or quantitative evaluation) may include one or more of the types of
chemical reactions selected from
immunoassay, nucleic acid assay, receptor-based assay, cytometric assay,
colorimetric assay, enzymatic assay,
electrophoretic assay, electrochemical assay, spectroscopic assay,
chromatographic assay, microscopic assay,
topographic assay, calorimetric assay, turbidmetric assay, agglutination
assay, radioisotope assay, viscometric
assay, coagulation assay, clotting time assay, protein synthesis assay,
histological assay, culture assay, osmolarity
assay, and/or other types of assays or combinations thereof. In some
embodiments, a heater and/or thermal block
may be employed. The chemical reaction may include providing the sample at a
desired temperature. The
chemical reaction may also include maintaining and/or varying the temperature
of the sample before, during, and/or
after the chemical reaction. Any description herein of chemical reaction may
include any type of reaction that may
occur in the device. For instance, chemical reactions may include physical
interactions, chemical interactions,
and/or other physical interactions or transformations. In some embodiments, a
display (such as a screen) or sensors
in a device may conduct imaging externally. For example, the device may be
capable of conducting MRI,
ultrasound, or other scans.
[00422] The sample preparation and/or chemical reaction may occur in response
to one or more
instructions. The instructions may be stored locally on the device or may be
provided from an external source. In
some embodiments, the external source is a laboratory. In some embodiments,
the sample preparation and/or
chemical reaction procedures may be self-educated. For example, they may be
capable of picking up different ways
of preparing a sample and/or making it ready for analysis. In some
embodiments, the sample preparation
procedures may be able to self adjust to utilize various sample preparation
techniques given a set of parameters.
The sample preparation adjustment or maintenance may or may not rely on
signals detected relating to a sample,
and/or to parameters and/or instructions provided by an operator. The sample
preparation procedures may be self-
learning. One or more controller that may provide instructions to conduct a
sample preparation and/or chemical
reaction may be capable of self-learning.
[00423] The adjustments may be made in response to new instructions that may
be generated locally on
the device or that may be provided from the external source. For example, new
instructions may be updated and/or
pushed down from the external source. There may be a dynamic process in which
the sample preparation and/or
chemical reaction and/or physical processing steps are performed in accordance
with changeable instructions. Any
description herein relating to a sample preparation and/or chemical reaction
may also include any physical
processing steps.
[00424] One or more signal may be detected 430 from the device. The signal may
be detected after a
sample preparation step has been done and/or after a chemical reaction and/or
physical processing step has taken
place. In some embodiments, one or more signal may be detected even if no
sample preparation and/or chemical
reaction has taken place on the sample. The signals may be based on a reading
of a sample that may or may not
have undergone an assay. The signals may be based on a measurement relating to
the device.
[00425] In some instances, one or more additional sample preparation steps may
occur. For instance, an
additional sample preparation for qualitative and/or quantitative evaluation
may occur. Such preparation may be
made based on at least one of: prior preparation of the biological sample
and/or analysis of the data by the health
care professional. Reflex testing may occur based on earlier results. The
reflex testing may occur in an automatic
-78-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
and dynamic manner before, during, or after the test/analyses. Earlier
evaluation may yield further testing, which
may be automated.
[00426] Optionally, data may undergo pre-processing 440. Raw data of detected
signals may or may not
undergo pre-processing. Pre-processing may affect the format of the raw data.
For example, the pre-processing
may normalize a format of the data. The pre-processing may put the data into a
desired form. Pre-processing may
occur without performing any analysis of the data. In some embodiments, the
pre-processing may alter the form of
the data without altering the content of the data. In some instances, pre-
processing does not compare the data with
any threshold values or perform any valuation judgments.
[00427] The data may be analyzed 450, as described elsewhere herein. Data
analysis may include a
subsequent qualitative and/or quantitative evaluation of a sample. Optionally,
a report may be generated based on
the raw data, pre-processed data, or the analyzed data. The report and/or the
data may be transmitted to a health
care professional. A software system may perform chemical analysis and/or
pathological analysis, or these could be
distributed amongst combinations of lab, clinical, and referenced/contracted
specialty personnel (e.g., lab and
John's Hopkins laboratory for specialty experts of some diseases or to engage
them as part of/in a certified
laboratory).
[00428] In some embodiments, the report may be reviewed before being
transmitted to the health care
professional. In some instances, the data may be reviewed before or after the
report is generated. The review may
occur by one or more pathologist or other qualified person. The pathologist
may be associated with a laboratory
492. The pathologist may or may not be physically located at the laboratory
facility. The pathologist may be
employed by the laboratory. For an authorized analytical facility, oversight
may be provided via a regulatory body.
In some embodiments, the laboratory may be a CLIA certified laboratory. A
board certified entity (which may
include board-certified personnel) may review the data/reports and provide a
measure of quality control and
verification. In some embodiments, the board certified entity may include one
or more pathologist.
[00429] In some embodiments, a device may be a certified device. The device
may be under the oversight
of a regulatory body. A board certified entity may review the data/reports of
the device and provide a measure of
quality control, performance of calibrators, of a test, and verification. A
health care professional may review and/or
provide oversight of the data/reports from the device. Alternatively, a
software program may be provided that may
review data generated by the device. The software program may be created by or
under the review of a health care
professional. The software program may be maintained by an authorized person,
such as a health care professional.
[00430] FIG. 8 shows examples of a system providing sample processing,
analysis, and oversight.
[00431] FIG. 8(i) shows an example of a device 800 which may be capable of
performing a sample
processing 802 step. The device may be capable of communicating with a
laboratory 810. The laboratory may be
capable of performing a subsequent analysis 812 step and may provide oversight
814. Oversight and/or analysis
may be provided by a health care professional and/or software program. The
device may communicate with the
laboratory across a network 850, including any of those described elsewhere
herein. A cloud computing
infrastructure may be provided. The device may be provided in or on a subject,
or at a sample collection site. The
laboratory may be an authorized analytical facility, such as a CLIA certified
facility which could be the device or
cartridge.
-79-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00432] FIG. 8(11) shows an example of a device 820 which may be capable of
performing a sample
processing 822 step and an analysis step 824. The device may be capable of
communicating with a laboratory 830.
The laboratory may be capable of providing oversight 832. Oversight may be
provided by a health care
professional and/or a software program. The device may communicate with the
laboratory across a network 860,
including any of those described elsewhere herein. A cloud computing
infrastructure may be provided. The cloud
computing infrastructure may be part of the system/infrastructure/device. The
device may be provided in or on a
subject, or at a sample collection site. The laboratory may be an authorized
analytical facility, such as a CLIA
certified facility.
[00433] FIG. 8(iii) shows an example of a device 840 which may be capable of
performing a sample
processing 842 step, analysis step 844, and providing oversight 846. In some
embodiments, the oversight may be
provided by an oversight software program on the device. The device may
communicate with a network 870,
including any of those described elsewhere herein. A cloud computing
infrastructure may be provided. The device
may be provided in or on a subject, or at a sample collection site. In some
embodiments, the device may be
certified by a regulatory body. In some instances, the device may be CLIA
certified.
[00434] In embodiments of the devices, systems, and methods disclosed herein,
the handling of a sample
may comprise one of three stages: a first stage which may be termed a "pre-
analysis" stage; a second stage which
may be termed an "analysis" stage; and a third stage which may be termed a
"post-analysis" stage. In embodiments,
a pre-analysis stage may include such actions and steps as, e.g., sample
collection, sample preparation, sample
testing, detection of a signal from a sample or observation or imaging of a
sample; data pre-processing (if any), and
other actions and steps prior to analysis of data obtained regarding a sample.
For example, a pre-analytic action may
include initiation of a test, and may include contacting a sample with a
reagent, detection of the amount of light
emitted from, or the amount of light absorbed by, a sample following contact
with a reagent, or other such action or
step. In embodiments, an analysis stage may include such actions and steps as,
e.g., analysis of data obtained
regarding a sample, generation of a report regarding the sample and its
analysis, and other actions and steps
regarding the analysis of data obtained regarding a sample. For example, an
analytic action or step may include
correcting raw data based on device environmental data and/or calibrations
specific for the device or reagents used
to examine the sample; calculation of a value, e.g., a concentration value, a
hematocrit value, a volume of a cell, or
other value; determination of a type or types of cells, particles, or other
subject in a sample; or other step or action.
In embodiments, a post-analysis stage may include such actions and steps as,
e.g., transmission of a report regarding
a sample, contacting or receiving communications from a health care
professional or other provider regarding such
a report, and other actions and steps subsequent to the analysis of data
obtained regarding a sample. For example, a
post-analytic action or step may comprise a determination of whether or not a
test is accurate, e.g., by comparing
outliers, controls, and replicates to the results of an analysis. For example,
a post-analytic action or step may
comprise highlighting values or results that are outliers or may be a cause
for concern (e.g., above or below a
normal or acceptable range, or indicative of an abnormal condition), or
combinations of results which, together,
may indicate the presence of an abnormal condition. Such post-analytic action,
communicated to a physician or
other health-care provider may better insure that the physician or other
health-care provider is made aware of, and
cognizant of, possible concerns and may thus be more likely to take
appropriate action.
[00435] In embodiments of devices, systems and methods disclosed herein, a
test of a sample may be
performed within a device.
-80-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00436] In embodiments of devices, systems and methods disclosed herein, a
test of a sample may be
initiated within a device, and raw data derived from the sample may be
transmitted to a different location, e.g., a
laboratory location. Transmission of raw data may be via the cloud or other
network. In embodiments where a test
of a sample may be initiated within a device, and raw data is transmitted to
another location, the test may be
completed at a location other than within the device; for example, a test may
be completed at a laboratory location
using raw data transmitted to the laboratory location via the cloud, or via
another network. In such embodiments,
the test of the sample is started at the sample collection site (within the
device) and is completed at the laboratory
location. In embodiments, a test of the sample may be started and completed
within the device at the sample
collection site. In embodiments, testing of a sample may be started and
completed within a device, and raw data
derived from the sample may be transmitted to a different location, e.g., a
laboratory location.
[00437] In embodiments of the devices, systems, and methods disclosed herein,
the device may be placed
at a sample collection site that is physically distant from the laboratory,
and processes a sample without physical
transport of the sample from the device to the laboratory. In embodiments of
the devices, systems, and methods
disclosed herein, the device, although placed at a sample collection site that
is physically distant from the
laboratory, operates under the control of the laboratory, and operates under
the oversight of the laboratory. In
embodiments of the devices, systems, and methods disclosed herein, control and
oversight of the device at a sample
collection site may be effected by a processor at the laboratory location or
by an individual affiliated with the
laboratory. The laboratory may be an authorized analytical facility, and may
be a CLIA-compliant laboratory,
CLIA-certified laboratory, or CLIA-waived laboratory.
[00438] A device as disclosed herein may be, or comprise, a sample processing
device. A device as
disclosed herein may be, or comprise, a sample processing unit.
[00439] In embodiments, a device for performing a sample processing step as
disclosed herein may be a
CLIA-certified device; may be a device operated in a CLIA-certified laboratory
or location; may be a CLIA-
compliant device; may be a device operated in a CLIA-compliant laboratory or
location; may be a device operated
by a CLIA-certified operator; may be a device operated by a CLIA-compliant
operator; may be a device operated in
a CLIA-compliant manner; may be a CLIA-waived device; may be a device cleared
for use by a regulatory body
empowered to do so; may be a device cleared for use by the U.S. Food and Drug
Administration; may be a device
classified as exempt by the U.S. Food and Drug Administration; may be a device
cleared for use, e.g., under section
510(k) of the U.S. Food, Drug and Cosmetic Act; may be a device with no
substantial equivalent under section
510(k) of the U.S. Food, Drug and Cosmetic Act; or the device may have no
governmental certification.
[00440] In embodiments, a device as disclosed herein may be or comprise a CLIA-
waived device, and
may perform a CLIA-compliant or CLIA-certified test on or with a sample. In
embodiments, a device as disclosed
herein may be or comprise a CLIA-waived device, and may perform a CLIA-waived
test on or with a sample. In
embodiments, a device as disclosed herein may be or comprise a CLIA-compliant
or CLIA-certified device, and
may perform a CLIA-compliant or CLIA-certified test on or with a sample. In
embodiments, a device as disclosed
herein may be or comprise a CLIA-compliant or CLIA-certified device, and may
perform a CLIA-waived test on or
with a sample. In embodiments, a device as disclosed herein may be in or at a
CLIA-compliant or CLIA-certified
laboratory or location, and may perform a CLIA-compliant or CLIA-certified
test on or with a sample. In
-81-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
embodiments, a device as disclosed herein may be in or at a CLIA-compliant or
CLIA-certified laboratory or
location, and may perform a CLIA-waived test on or with a sample.
[00441] In embodiments, a device as disclosed herein may operate under the
oversight or control of a
laboratory. For example, a device as disclosed herein may process a sample at
a sample collection site under the
oversight of a laboratory, where the laboratory is placed at a laboratory
location that is physically distant from the
sample collection site. Thus, in embodiments, the device, while physically
distant from the laboratory, operates as
part of the laboratory due to its operation being controlled by the
laboratory. In embodiments, the device, while
physically distant from the laboratory, operates as part of the laboratory due
to its operation being under the
oversight of the laboratory. In embodiments, testing may be performed on a
device located at a sample collection
site, and raw data and/or results may be transmitted to a laboratory at a
laboratory location. Such raw data and/or
results may be analyzed at a laboratory at a laboratory location. The
laboratory may be an authorized analytical
facility, and may be a CLIA certified laboratory. Thus, laboratory certified
results may be obtained from testing
performed at a sample collection site.
[00442] In embodiments, testing may be initiated on a device located at a
sample collection site, and may
be continued and may be completed in a laboratory at a laboratory location
(e.g., using raw data transmitted from
the device). In embodiments, testing may thus be completed at a laboratory
location, and analysis may be
performed at a laboratory location from results obtained from testing
initiated at a sample collection site and
completed in a laboratory at a laboratory location. The laboratory may be an
authorized analytical facility, and may
be a CLIA certified laboratory. Thus, laboratory certified results may be
obtained from testing initiated at a sample
collection site and completed in a laboratory at a laboratory location.
[00443] In embodiments, oversight of the operation of a device may be on-
going, e.g., may comprise
oversight at multiple times during the operation of a device, and may comprise
continuous oversight of the
operation of a device during the operation of the device. For example,
oversight may comprise oversight of the
operation of the device at multiple times during the testing of a sample, and
may comprise continuous oversight of
the operation of the device during the testing of a sample. Oversight of a
protocol for the operation of a device
during the testing of a sample may include updating the protocol with new
process steps, corrected process steps, or
new commands regarding process steps. Oversight may be provided or performed
using a processor. Oversight may
be provided or performed using a processor at a sample collection site (e.g.,
on a device), using a processor at a
laboratory location, using a processor that is part of the cloud, or of a
network (which cloud or network may or may
not include a processor on a device or at a laboratory location). Oversight
may be performed or provided by an
individual (e.g., an individual affiliated with an authorized analytical
facility). Oversight may include using any one
or more of such processors in conjunction with an individual (e.g., an
individual affiliated with an authorized
analytical facility).
[00444] Protocols may receive and utilize identifying data and information
identifying the device in use,
the cartridge, the sample, the patient, and other information and data.
Identifying information and data may identify
a particular device, its past history, present status, present condition,
environmental information regarding the
device and its environs, and other data and information. For example, in
embodiments, patient identification may be
retained with each sample from beginning to end of sample collection, testing,
analysis, and reporting of results. In
embodiments, patient identification may be retained with each sample from
beginning to end of sample collection,
-82-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
testing, analysis, reporting of results, and billing. Identifying information
and data may identify a particular
cartridge, its contents (including reagents, disposables, and sample
information), its past history, present status,
present condition, environmental information regarding the cartridge and its
environs, and other data and
information. Identifying information and data may identify a particular
patient, including a patient's identity, age,
sex, medications, medical history, test history, present status, present
condition, and other data and information.
Identifying information and data may identify a particular sample, its mode of
collection, patient derived from,
condition (sample volume, whether contacted with heparin, EDTA, filtered,
coagulated, whether or not there is an
air bubble present, whether a blood sample may have undergone hemolysis, or is
lipemic), and other data and
information. Identifying information and data may specify an analysis to be
performed, or that has been performed,
on data from a sample. Identifying information and data may specify post-
analytic actions to be performed, such as
regarding a report, comparison with other test data or analysis, or other
action or step.
[00445] Protocols may be updated and may be tailored to specific devices, or
environmental conditions
within or around the device, e.g., taking into account the specific properties
and characteristics of sensors, motors,
physical dimensions, and other properties and characteristics of an individual
device; taking into account the
temperature, humidity, air pressure, position and movement of internal modules
or components within the device;
condition and status of motors, sensors, or other components; taking into
account calibrations of motors, sensors,
and other components; and other properties and characteristics. Protocols may
be updated and may be tailored to
specific reagents and disposables, e.g., taking into account control reagents,
calibrations of reagents, the specific
properties and characteristics of reagents in a cartridge, of tips in a
cartridge, of the temperature of a cartridge, or
other characteristic or property. Oversight may comprise oversight during or
following analysis of data, and may
provide for further testing, or re-testing of a sample or of additional
samples from a patient.
[00446] Use of protocols that include device-specific information, cartridge-
specific information, or other
information and data specific for sensors, devices, reagents, and other
components and elements of testing of a
sample, including calibration and control information, provides for correction
and scaling of raw data to insure that
values and observations obtained from such raw data is correct, consistent,
and reproducible. Such values and
observations obtained are reproducible across devices, and over time (e.g.,
from subsequent samples) allowing for
comparison of patient results with group and historical values for better
screening, diagnosis and treatment based on
test results and analysis. Oversight as disclosed herein provides oversight of
the integrity of performance of tests,
and of results of tests, including oversight of each sample, oversight of the
testing of each sample, oversight of the
scaling and calibration of raw data obtained during testing, oversight of test
integrity, oversight of use and analysis
of data in view of controls, calibrators, replicates, and outliers, oversight
of the analysis of data in view of device
and reagent environmental and calibration information, oversight of the
analysis of test results, oversight of the
reporting of test results and analysis, and oversight of post-analytic
activity and communications. Device and
cartridge information may be retained in the cloud or other network; may be
retained on or with each device or
cartridge; or may be retained, partially or completely, in both locations.
[00447] Devices, systems, methods, and protocols as disclosed herein provide
multifunctional capabilities,
allowing a single device at a sample collection site to perform multiple
tests, including multiple types of tests, on a
sample; such test may be run rapidly, requiring only small amounts of sample
(e.g., requiring only the amount of
blood available from a finger-stick), and may be run reliably and accurately,
allowing for reproducibility and
reliable comparison across devices and samples. Thus, the devices disclosed
herein are multifunctional; the assays
-83-
CA 02901016 2015-08-11
WO 2014/127285
PCT/US2014/016593
they perform are multifunctional; the controls are multifunctional; and the
oversight is multifunctional.
Multifunctional includes the ability to perform multiple types of assays,
including nucleic acid assays, general
chemistry assays, cytometry (e.g., cell imaging, flow cytometry) assays, ELISA
assays, and others (e.g., as
disclosed in U.S. Patent Application U.S. Patent Application 13/244,947 and in
U.S. Patent Application
____________________________________________________________________ ,
entitled "Systems and Methods for Multi-Purpose Analysis," filed February 18,
2013), on a single
device and from a single sample.
[00448] Devices, systems, methods, and protocols as disclosed herein provide
rapid results from testing a
small volume sample; such testing and results can be automated, and the rapid
provision of such results, e.g. by a
laboratory to a health care provider, provides rapid and reliable information
for better patient care. Laboratories,
such as CLIA-compliant and CLIA-certified laboratories, may have pathologists
in place, or readily available, for
review and interpretation of results, for further analysis, and for possible
recommendations regarding further testing
and/or treatment. As disclosed herein, embodiments of devices, systems,
methods, including protocols and
oversight as disclosed herein may include some or all of the features,
elements, and capabilities disclosed, and may
include these features, elements, and capabilities in any combination of some
or all of such features, elements, and
capabilities.
[00449] In embodiments, oversight may include at least the following
processes: 1) sample collection; 2)
receipt of data (e.g., test data, raw data, device data, cartridge data,
sample data, patient data, etc.); 3) recognition of
a status or problem related to the data); analysis of the status or problem in
view of the sample, the test being
performed, the device, reagents, etc. performing the test, etc.; and 4)
sending instructions to the device, operator, or
physician (e.g., general practitioner, pathologist, or other healthcare
provider) based on the data and analysis, for
further action or modification or cessation of present actions. Such oversight
may be intermittent (e.g., occur once
or at a few times before, during, or after testing), or may be continuous (on-
going during testing, or on-going from a
time before testing, and may continue through or beyond the period of
testing). In embodiments, oversight may be
dynamic oversight, allowing updating of protocols as events occur or data is
obtained, to insure proper procedures,
proper quality, proper integrity, and proper results from such testing. Such
oversight and testing may be "patient-
aware", i.e. may take into account the identity, characteristics (e.g., age,
sex, medical history, medication, condition,
physician, insurance coverage, or other factor related to the patient for whom
the test is being conducted. In this
way, the sample and sample analysis may always be linked to the patient
throughout the testing process ¨ at the pre-
analytical stage, at the analytical stage, and at the post-analytical stage.
As disclosed herein, all these tests and
analysis may be provided without physically transporting a sample to a
laboratory location. Sample collection and
processing by the device at a sample collection site, followed by transmission
of raw data to the laboratory location
allows testing, analysis, and reporting of result to be performed without
physically transporting a sample to a
laboratory location. The transmission of raw data to the laboratory location
provides "digital" transport of a sample
or effectively a "virtual sample" obviating a need for physically transporting
a sample to a laboratory location.
Oversight and control of the operation of the device at the sample collection
site by a protocol, provided by the
cloud or other network, or directly from a laboratory location, allows for the
control and operation of the device by
the laboratory. Such control and operation may be by a CLIA-compliant
laboratory, which may be a CLIA-certified
laboratory.
[00450] For example, protocols used by the device in processing the sample may
be provided by the
laboratory, may be updated by the laboratory, and their proper application may
be overseen by the laboratory.
-84-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
Protocols may include instructions and procedures directing the forms of
processing and testing to apply to a
sample; the order of performing such processing and testing of a sample; the
timing of such processing and testing
of a sample; the pre-processing, if any, of data obtained by the processing
and testing of a sample; the compilation
of data, including raw data (and pre-processed data, if any), obtained by the
processing and testing of a sample; the
transmission of data, including raw data (and pre-processed data, if any),
obtained by the processing and testing of a
sample, to a laboratory; the receipt of further instructions from a laboratory
in response to the data transmitted from
the device to the laboratory; further processing or testing of a sample
pursuant to any further instructions from a
laboratory in response to the data transmitted from the device to the
laboratory; and the disposal of sample and other
wastes following such processing and testing of a sample.
[00451] In embodiments, oversight by the laboratory may include oversight of
the operation of the device;
oversight of sensing operations within the device; oversight of the analysis
of data transmitted by the device to the
laboratory; oversight of a report generated pursuant to the analysis of such
data; oversight of billing for services
provided; and other oversight. In embodiments, oversight by a laboratory may
include oversight of the collection of
a sample at the sample collection site, of the placement of the sample in a
cartridge at the sample collection site, of
the placement of a cartridge in a device at a sample collection site, or other
oversight. Such oversight may include
confirmation that proper instructions for such procedures had been provided,
and that proper procedures had been
followed. In embodiments, oversight may include oversight of the confirmation
of insurance coverage of a subject
prior to, concurrent with, or subsequent to the processing a sample. Control
or oversight of the device may be
implemented, for example, via electronic communication such as via a cloud
computing infrastructure, other
telephonic communication (which may include microwave or radio components,
e.g., via cell-phone links), radio
communication, infrared linkages, and communication utilizing other forms of
electromagnetic radiation. Electronic
communication between a device at a sample collection site and a laboratory at
a laboratory location may include
downloading of protocols; transfer or updating of protocols; communication of
device information (e.g., device
identification; device status; temperature or other environmental information;
date, time or sequence information;
supply status (e.g., supply of reagents, supply of cartridges, or other
materials); and other device information);
communication of patient or subject information; communication of sample
information (e.g., identification
information related to a sample; data obtained from a sample; information
regarding processing applied to a sample;
information regarding equipment and procedures used to obtain data from the
sample; and other data regarding or
derived from a sample); communication of insurance information; communication
of payment information; and
communication of other information. Such oversight may be provided or
performed using a processor, e.g., a
processor at a sample collection site, at a laboratory location, that is part
of the cloud, or of a network; may be
provided or performed by an individual (e.g., an individual affiliated with an
authorized analytical facility); and
may include using a processor in conjunction with an individual (e.g., an
individual affiliated with an authorized
analytical facility).
[00452] In embodiments, oversight by the laboratory may include oversight of
the operation of the device.
In embodiments, oversight of operation of the device may include checking the
status of the device. In
embodiments, oversight of operation of the device may include performing
calibration of the device. In
embodiments, oversight of operation of the device may include oversight of the
identification of a subject prior to
processing a sample. Oversight of operation of the device may include
oversight of the identification of an operator
(e.g., regarding proper certification, eligibility, etc.) prior to processing
a sample. In embodiments, oversight of
-85-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
operation of the device may include oversight of the identification of a
cartridge for use in processing and testing a
sample. In embodiments, oversight of operation of the device may include
providing a protocol, oversight of receipt
of a protocol, or updating of a protocol for use in processing and testing a
sample. In embodiments, oversight of
operation of the device may include oversight of the processing and testing of
a sample by the device, including
oversight of selection and use of reagents, transport of reagents and sample
within the device, performance of
sample processing and testing within the device, collection of data from the
sample, collection of data from control
or calibration reagents and tests; and related sample processing and testing
operations. In embodiments, oversight of
operation of the device may include oversight of any pre-processing of data
obtained by testing of a sample. In
embodiments, oversight of the operation of the device may include instructions
to re-test a sample, or to test
controls, replicates, or other materials. Oversight of the operation of the
device may include collection of device
information, including device identification, device status, temperature, and
other information. In embodiments,
oversight of the operation of the device may include collection of images
transmitted by the device, e.g., from a
camera within the device. In embodiments, oversight of the operation of the
device may include analysis of device
information or of images transmitted by the device. In embodiments, oversight
of the operation of the device may
include transmission, receipt, or analysis of quality control information,
device status or condition information,
assay information, control information or data, calibration information or
data, or other information. Such oversight
of operations may be provided or performed using a processor, e.g., a
processor at a sample collection site, at a
laboratory location, that is part of the cloud, or of a network; may be
provided or performed by an individual (e.g.,
an individual affiliated with an authorized analytical facility); and may
include using a processor in conjunction
with an individual (e.g., an individual affiliated with an authorized
analytical facility).
[00453] In embodiments, oversight of the operation of the device may include
transmission of instructions
to the device. Instructions may include protocols, instructions to begin
operations according to a protocol,
instructions to interrupt or pause operations according to a protocol,
instructions to stop or end operations according
to a protocol, instructions to dynamically adjust or modify operation or a
protocol, and other instructions. In
embodiments, oversight of the operation of the device may include transmission
of instructions from the laboratory
location to the device at the sample collection site. In embodiments,
oversight of the operation of the device may
include transmission of instructions from the laboratory location to the
device at the sample collection site
accordance with procedures and requirements of a regulatory body, an
authorized analytical facility, or a CLIA-
certified laboratory. In embodiments, oversight may include oversight by
personnel of a laboratory, such as CLIA-
compliant laboratory or a CLIA-certified laboratory. In embodiments, oversight
may include remote oversight by
personnel of a laboratory, such as CLIA-compliant laboratory or a CLIA-
certified laboratory. In embodiments,
oversight by personnel of a laboratory may include oversight to insure that
appropriate sample collection, sample
processing, and other steps are taken. In embodiments, oversight of the
operation of the device may include
transmission of instructions to the device prior to processing a sample. In
embodiments, oversight of the operation
of the device may include transmission of instructions to the device prior to
testing a sample. In embodiments,
oversight of the operation of the device may include transmission of
instructions to the device for collecting data
from a sample. In embodiments, oversight of operation of the device may
include oversight of transmission of the
data, including raw data and pre-processed data (if any) to a laboratory. In
embodiments, oversight of the operation
of the device may include instructions to collect data from controls,
replicates, device information, or other
information. In embodiments, oversight of operation of the device may include
oversight of receipt of instructions
-86-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
from the laboratory pursuant to the transmission of data following processing
and testing of a sample. In
embodiments, oversight of operation of the device may include transmission of
instructions from the laboratory to
the device effecting retesting of a sample pursuant to data or information
transmitted from the device to the
laboratory. In embodiments, oversight of operation of the device may include
oversight of the disposal of sample
and any waste following processing and testing of the sample. In embodiments,
oversight of operation of the device
may include other device operations. Such oversight may be provided or
performed using a processor, e.g., a
processor at a sample collection site, at a laboratory location, that is part
of the cloud, or of a network; may be
provided or performed by an individual (e.g., an individual affiliated with an
authorized analytical facility); and
may include using a processor in conjunction with an individual (e.g., an
individual affiliated with an authorized
analytical facility).
[00454] Analysis of the sample may be performed at a location physically
distant from the sample
collection site at the laboratory (which may be an authorized analytical
facility, and may be a CLIA certified
laboratory). Analysis of the sample may be performed at a location physically
distant from the sample collection
site according to the requirements of a regulatory body. A regulatory body may
be a CLIA regulatory body, may be
the U.S. Food and Drug Administration, or other regulatory body. A regulatory
body may be a U.S. regulatory
body, may be an international regulatory body, or may be a regulatory body of
a nation other than the United States.
[00455] In embodiments, oversight by the laboratory may include oversight of
the analysis of data
transmitted by the device to the laboratory. In embodiments, oversight of
analysis of data transmitted by the device
may include pre-analytic oversight, analytic oversight, and post-analytic
oversight. In embodiments, oversight of
analysis of data transmitted by the device may include oversight of the
transmission of raw data to the laboratory. In
embodiments, oversight of analysis of data transmitted by the device may
include oversight of the transmission of
pre-processed data to the laboratory. In embodiments, oversight of analysis of
data transmitted by the device may
include oversight of the analysis of data, including raw data, pre-processed
data, and other data, that is performed at
the laboratory. In embodiments, oversight of data transmission may include
oversight of encryption of data,
oversight of mode of transmission of data, oversight of timing or sequence of
transmission of data, and confirmation
of complete transmission or receipt of data. In embodiments, oversight of
analysis of data transmitted by the device
may include oversight of the analysis of data, including raw data, pre-
processed data, and other data, by a processor
at the laboratory. In embodiments, oversight of analysis of data transmitted
by the device may include oversight of
the analysis of data, including raw data, pre-processed data, and other data,
that is performed in conjunction with an
individual at the laboratory, or affiliated with the laboratory. In
embodiments, oversight of analysis of data
transmitted by the device may include oversight of the analysis of data,
including raw data, pre-processed data, in
accordance with procedures and requirements of a regulatory body. In
embodiments, oversight of analysis of data
transmitted by the device may include oversight of the analysis of data,
including raw data, pre-processed data, in
accordance with procedures and requirements of an authorized analytical
facility. In embodiments, oversight of
analysis of data transmitted by the device may include oversight of the
analysis of data, including raw data, pre-
processed data, in accordance with procedures and requirements of a CLIA-
certified laboratory. Such oversight of
analysis may be provided or performed using a processor, e.g., a processor at
a sample collection site, at a
laboratory location, that is part of the cloud, or of a network; may be
provided or performed by an individual (e.g.,
an individual affiliated with an authorized analytical facility); and may
include using a processor in conjunction
with an individual (e.g., an individual affiliated with an authorized
analytical facility).
-87-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00456] In embodiments, oversight of analysis of data transmitted by the
device may include performance
of the analysis at the laboratory location. In embodiments, oversight of
analysis of data may be performed by a
processor, and may include software oversight, oversight by or in conjunction
with an individual (e.g., an individual
affiliated with an authorized analytical facility), oversight by or in
conjunction with a laboratory automation system
(LAS), oversight by or in conjunction with a laboratory information system
(LIS), or oversight by or in conjunction
with an electronic medical records system (EMR). In embodiments, oversight of
analysis of data transmitted by the
device may include transmission of instructions from the laboratory location
to the device at the sample collection
site; such transmission of instructions from the laboratory location to the
device at the sample collection site may be
in accordance with procedures and requirements of a regulatory body, an
authorized analytical facility, or a CLIA-
certified laboratory. In embodiments, oversight of analysis of data
transmitted by the device may include receipt at
the laboratory of device information, cartridge information, patient
identification information, calibration
information, and other information from the device. Such oversight may be
provided or performed using a
processor; may be provided or performed by an individual (e.g., an individual
affiliated with an authorized
analytical facility); and may include using a processor in conjunction with an
individual (e.g., an individual
affiliated with an authorized analytical facility).
[00457] In embodiments, oversight of a report generated pursuant to the
analysis of data transmitted by a
device to a laboratory may include compilation of data and analysis to be
reported; oversight including review and
oversight of the integrity of the process, the operation, and of the assay
that generated the data to be reported,
including review and oversight of data integrity, testing integrity, and
analysis integrity; preparation of a report;
review of the report for accuracy and completeness; review of the report via a
processor at the laboratory location;
review of the report by an individual affiliated with the laboratory;
oversight of the transmission of the report to
recipients, including oversight of the transmission of the report to
recipients, including confirmation of proper
confidentiality and confirmation of its receipt; and other oversight. In
embodiments, oversight of a report generated
pursuant to the analysis of data transmitted by a device to a laboratory may
be performed in accordance with
procedures and requirements of a regulatory body, an authorized analytical
facility, or a CLIA-certified laboratory.
Such oversight of report generation may utilize automation of technical and
operational steps and so be effective to
minimize possible human error. Such reporting oversight may be provided or
performed using a processor; may be
provided or performed by an individual (e.g., an individual affiliated with an
authorized analytical facility); and
may include using a processor in conjunction with an individual (e.g., an
individual affiliated with an authorized
analytical facility).
[00458] In embodiments, oversight of billing for services provided may be
performed using a processor at
the laboratory location. In embodiments, oversight of billing for services
provided may be performed by an
individual affiliated with the laboratory. In embodiments, oversight of
billing for services provided may be
performed in accordance with procedures and requirements of a regulatory body,
an authorized analytical facility,
or a CLIA-certified laboratory. Such billing oversight may be provided or
performed using a processor; may be
provided or performed by an individual (e.g., an individual affiliated with an
authorized analytical facility); and
may include using a processor in conjunction with an individual (e.g., an
individual affiliated with an authorized
analytical facility).
[00459] For example, in embodiments of the devices, systems, and methods
disclosed herein, e.g., as
illustrated in Fig. 8, a device at a sample collection site may receive a
protocol from a laboratory at a laboratory
-88-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
location. In embodiments, the protocol may be updated by further instructions
from the laboratory. A protocol may
include instructions regarding a cartridge, or cartridges, which may be used
in accordance with the protocol. A
subject may wish to provide a sample for testing. In embodiments of the
devices, systems, and methods disclosed
herein, the subject may provide identification information, or testing
information (e.g., an order from a physician or
other health provider regarding a test or tests to be performed;
identification information regarding an operator, etc.)
to the device or to an operator of the device at the sample collection site.
The device may provide such
identification or testing information to the laboratory. The laboratory may
use such identification or testing
information to determine the eligibility or appropriateness of the test or of
the subject for the test (e.g., by
determination of insurance status and coverage, billing information, sex, age,
or health status of the subject, or other
means). In view of the identification or testing information, the laboratory
may provide instructions to the subject or
operator (.e.g., via a device user interface, a device audio link, telephone,
or other means) regarding collection of a
sample, the proper cartridge to be used, or other information. Collection of
the sample may require no processing of
the sample by the subject or by an operator. For example, sample collection
may be automated, sample processing
may be automated, and other functions may be automated, providing better
control and integrity of sample
collection, processing and analysis; such control may aid in compliance with
CLIA or other regulatory standards,
e.g., by reducing the possibility of operator variance or error. In view of
the identification or testing information,
the laboratory may provide instructions, including but not limited to, a
protocol, to the device. Such instructions
may cause the device (e.g., via a user interface, audio output, or other
means) to request confirmation or further
information from the subject or operator at the sample collection site. A
sample may be obtained from the subject at
the sample collection site. The sample may be placed in the device, or the
sample may be placed in a cartridge and
the cartridge with the sample may be placed in the device. The device may
transmit status, test, or identification
information to the laboratory. In view of the cartridge, status, test, or
identification information, the laboratory may
transmit instructions, including but not limited to a protocol, to the device.
For example, the laboratory may
transmit instructions to the device effecting the rejection of the sample, or
of the cartridge with the sample, if the
sample, cartridge, protocol, identification information, or other information
do not match or are otherwise
incompatible with proper operation, processing, or testing of the sample by
the device, or if the subject lacks
insurance coverage or if the test is otherwise inappropriate for the subject,
the cartridge, or the device. In view of
the cartridge, status, test, or identification information, the laboratory may
transmit a protocol, or update a protocol,
to the device. The laboratory may transmit instructions to the device
effecting the processing and testing of the
sample. The transmission of instructions from the laboratory to the device may
be via the cloud, telephone, radio,
network, LAN, other electronic or electromagnetic means, or any other
communications link. Instructions effecting
the processing and testing of a sample may include instructions effecting
transport of the sample, of reagents, or of
device components within or to the device; may include instructions effecting
mixing of the sample and reagents;
may include instructions effecting processing and/or testing of the sample;
may include instructions effecting
observation or measurement of the sample, including instructions effecting
acquisition of data from the sample;
may include instructions effecting the transmission of data (which may include
raw data and pre-processed data)
from the device to the laboratory; and may include instructions effecting the
disposal of the sample and waste
resulting from the processing and testing of the sample. These instructions
enable the device to process a sample
without physical transport of the sample from the device to the laboratory.
The laboratory may analyze the data
received from the device. Laboratory analysis of data received from the device
may be dynamic analysis (e.g.,
analysis may be confirmed, altered or updated in view of information or data
provided with or about the sample or
-89-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
test). The laboratory may provide further instructions to the device pursuant
to the analysis of the data received
from the device. Such further instructions may effect further processing or
testing of the sample in the device at the
sample collection site. The laboratory may prepare a report based on the data
from the sample and its analysis. The
laboratory may notify a subject, a health provider, an insurance company, or a
payer regarding the processing and
testing of the sample. The laboratory may send a report to a subject, a health
provider, an insurance company, or a
payer regarding the processing and testing of the sample. The laboratory may
prepare bill information, or may
prepare a bill, regarding the processing and testing of the sample. The
laboratory may send a bill regarding the
processing and testing of the sample to the subject, an insurance company, or
a payer. Thus, control and oversight
of the device at a sample collection site may be effected by a processor at
the laboratory location, by an individual
affiliated with the laboratory, or both. The laboratory may be an authorized
analytical facility, and may be a CLIA
certified or CLIA-compliant laboratory.
[00460] In embodiments, oversight of the operation of a device, oversight of
the analysis of data from a
sample, or other oversight may comprise oversight by software. Such oversight
software may be software cleared
under section 510(k) of the U.S. Food, Drug and Cosmetic Act, or cleared or
approved under other statutes or by
another regulatory body, and such software may be run by, or running in a CLIA-
compliant or CLIA-certified
laboratory or location. Such oversight software may be software cleared under
section 510(k) of the U.S. Food,
Drug and Cosmetic Act, or cleared or approved under other statutes or by
another regulatory body, and such
software may be run by, or running in a location other than a CLIA-compliant
or CLIA-certified laboratory or
location. Such oversight software may be software cleared under section 510(k)
of the U.S. Food, Drug and
Cosmetic Act, or cleared or approved under other statutes or by another
regulatory body, and such software may be
run by, or running in the cloud or other network; such software running in the
cloud or other network may be run
by, or running under the oversight of a CLIA-compliant or CLIA-certified
laboratory or location, or may not be run
by, or running under the oversight of a CLIA-compliant or CLIA-certified
laboratory or location.
[00461] In some embodiments, a method for evaluating a biological sample may
be provided. The
method may include receiving and/or preparing a sample on board a device. The
method may include performing
analysis on-board the device. Alternatively, the method may include performing
analysis external and/or remote to
the device. For example, the analysis may occur at a laboratory or by an
affiliate of the laboratory. In some
embodiments, the analysis may occur both on-board the device and external to
the device.
[00462] The analysis may be performed by a health care professional of a
laboratory, or any other affiliate
of the laboratory. The analysis may be performed by a software program. A
processor may perform one or more
steps of the software program, thereby effecting such analysis. In some
embodiments, one, two or more types of
analysis may be provided by the analysis software program. In some
embodiments, the analysis may be performed
by both the health care professional and the software program. In some
examples, the analysis may be performed
by a software program on-board the device, by a health care professional
external to the device, and/or by a
software program external to the device.
[00463] The method may further include providing oversight of the analysis.
The method may include
performing oversight on-board the device. Alternatively, the method may
include performing oversight external
and/or remote to the device. For example, the oversight may occur at a
laboratory or by an affiliate of the
-90-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
laboratory. The laboratory may be an authorized analytical facility, and may
be CLIA-certified laboratory. In some
embodiments, the oversight may occur both on-board the device and external to
the device.
[00464] In some embodiments, analysis may be conducted by a health care
professional and oversight
may be conducted by a health care professional, analysis may be conducted by a
health care professional and
oversight may be conducted by a software program, analysis may be conducted by
a software program and
oversight may be conducted by a health care professional, or analysis may be
conducted by a software program and
oversight may be conducted by a software program. The same health care
professional or different health care
professionals may be used for analysis and/or oversight. The same software
program or different software
programs may be used for analysis and/or oversight. Any description of
laboratories, health care professionals,
software, and/or infrastructure that may perform oversight may also apply to
analysis, or vice versa.
[00465] The oversight may be performed by a health care professional of a
laboratory, or any other
affiliate of the laboratory. The oversight may be performed by a software
program. A processor may perform one
or more steps of the software program, thereby effecting such oversight. In
some embodiments, the oversight may
be performed by both the health care professional and the software program. In
some examples, the oversight may
be performed by a software program on-board the device, by a health care
professional external to the device,
and/or by a software program external to the device. Any combination of
analysis and oversight may be provided.
[00466] Oversight may include pre-analytical oversight, may include analytical
oversight, and may
include post-analytical oversight. Pre-analytical oversight may include
oversight of the acquisition and processing
of a sample by a device at a sample collection site. Such oversight may be
performed at a laboratory location by a
processor or by an individual affiliated with a laboratory. The laboratory may
be a sample collection site, and
sample collection may be manual or may be automated. The laboratory may be an
authorized analytical facility, and
may be a CLIA-certified laboratory.
[00467] Analytical oversight may include oversight of the acquisition of data
from a sample by the device.
Such oversight may be performed at a laboratory location by a processor or by
an individual affiliated with a
laboratory, which may be may be an authorized analytical facility, and may be
a CLIA-certified laboratory.
Analytical oversight may include oversight of the transmission of data from
the device to the laboratory. Oversight
of data transmission may include oversight of encryption of data, oversight of
mode of transmission of data,
oversight of timing or sequence of transmission of data, and confirmation of
complete transmission or receipt of
data. Such oversight may be performed at a laboratory location by a processor
or by an individual affiliated with a
laboratory, which may be may be an authorized analytical facility, and may be
a CLIA-certified laboratory.
Analytical oversight may include oversight of the analysis of data transmitted
from the device to the laboratory.
Oversight of the analysis of data transmitted from the device to the
laboratory may include instructions to collect
data from controls, replicates, device information, or other information.
Oversight of the analysis of data transmitted
from the device to the laboratory may include use of controls for multiple
assay methodologies available or in use
on the same device, available or in use at the same time, or at substantially
the same time, for analysis, calibration,
or control. Oversight of the analysis of data transmitted from the device to
the laboratory may include comparisons
with, or use of, data from controls, replicates, device information, or other
information. Such oversight, including
oversight of multiple assay methodologies, may be performed at the same time,
or at substantially the same time.
-91-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
Such oversight may be performed at a laboratory location by a processor or by
an individual affiliated with a
laboratory, which may be may be an authorized analytical facility, and may be
a CLIA-certified laboratory.
[00468] Post-analytical oversight may include preparation of a report
regarding the data and the analysis
of data obtained from a sample. Post-analytical oversight may include
identification of outliers or other data or
information requiring further review. Post-analytical oversight may include
use of a processor to provide further
analysis regarding clinically abnormal values or other data or information
requiring further review. Post-analytical
oversight may include notification of an individual affiliated with the
laboratory regarding outliers or other data or
information requiring further review. Post-analytical oversight may include
providing an individual affiliated with
the laboratory with data, analysis, or information regarding outliers or other
data or information requiring further
review. Post-analytical oversight may be performed at a laboratory location by
a processor or by an individual
affiliated with a laboratory, which may be may be an authorized analytical
facility, and may be a CLIA-certified
laboratory.
[00469] FIG. 5 shows a laboratory benefit management (LBM) entity 510 in
communication with a payer
500 and sample collection site 520. The LBM may be in communication with a
payer at a payer location and the
sample collection site at a point of service location. The LBM may be provided
at a facility at the LBM location.
The LBM may be at a different location than the payer and the sample
collection site. In some embodiments, the
sample collection site may be a retailer, insurance company, entity, or any
sample collection site as described
elsewhere herein. For example, the payer, LBM, and point of service may be
provided in different facilities.
[00470] The LBM 510 may be an entity. For example, the LBM may be a company,
corporation,
organization, partnership, business, or one or more individuals that form an
entity. The LBM may be configured to
communicate with one or more other entity regarding financial transactions and
services. The LBM may provide
instructions regarding financial transactions and services and manage
financial processes.
[00471] The payer 500 may be an entity that may pay or partially pay for one
or more health or medical
related services for a subject. The payer may have a contract or agreement
with the subject or a sponsor of the
subject to provide some form of medical coverage. The payer may be a public
payer or private payer. In some
instances, the payer may be a government payer or a health insurance company.
Examples of government payers
may include, but are not limited to Medicare, Medicaid, Federal Employees
Health Benefits Program, Veterans
Health Administration, State Children's Health Insurance Program, Military
Health System/TRICARE, Indian
Health Service, or other publicly funded health insurance programs. Examples
of types of private payers may
include, but are not limited to, health maintenance organizations (HMO),
preferred provider organization (PPO),
independent practice association (IPA), point of service (POS) plans, or
managed care or indemnity insurance plans.
Examples of health insurance companies may include but are not limited to
Aetna, Blue Cross Blue Shield
Association, CIGNA, Kaiser Permanente, Humana, Health Net, UnitedHealth Group,
or Wellpoint.
[00472] The sample collection site 520 may be a point of service location. A
sample collection site may
be provided at a point of service location. Any discussion of a point of
service may also apply to a sample
collection site at a point of service location. A point of service location
may be a location remote to the LBM where
a sample may be collected from a subject or provided by a subject. In some
embodiments, a sample collection site
may be a retailer. Examples of point of service locations and retailers are
provided in further detail elsewhere
-92-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
herein. In some embodiments, the sample collection site may comprise a device,
as described in further detail
elsewhere herein.
[00473] The LBM may receive information from a sample collection site, and/or
may receive information
from a payer. The LBM may provide information to a sample collection site,
and/or may provide information to a
payer. The LBM may communicate with the payer and sample collection site in
any manner known or later
developed in the art, including, but not limited to using a sample processing
device, network device, mobile device,
telephone, postage, courier, delivery, or any other communication techniques
described elsewhere herein. The
communication may occur over a network, including any form of network as
described elsewhere herein. One-way
or two-way communication may be provided between the LBM and the payer, and
between the LBM and the
sample collection site. The LBM, payer, and sample collection site may have
one or more communication unit.
The communication unit may be configured to provide communication between the
LBM, payer, and sample
collection site. The communication unit may be configured to provide wireless
or wired communication.
[00474] The LBM may also perform financial transactions with the payer and
with the sample collection
site. In some instances, the financial transactions may be two-way financial
transactions, or may be one-way
financial transactions. In one example, the payer may pay the LBM. The LBM may
pay the sample collection site.
The payment the LBM provides the sample collection site may be derived from
the payment the LBM receives
from the payer.
[00475] The LBM, payer, and sample collection site may have a processor and
memory that may keep
track of the communications and/or payments. The LBM, payer, and/or sample
collection site may interact with
one or more third party that may keep track of the communications and/or
payments. The one or more third parties
may be financial institutions. A processor may have access to one or more
memory that may contain information
about payments received or disbursed. For example, an LBM may have a processor
that accesses one or more
memory or data storage unit containing information about a payment received
from the payer and a payment
provided to a sample collection site.
[00476] The payments may be provided based on use of a device provided at the
sample collection site.
The LBM may request a payment from the payer based on use of the device. The
LBM may provide a payment to
the sample collection site based on use of the device. Alternatively, the LBM
may request a payment from the
sample collection site based on use of the device.
[00477] The LBM may comprise one or more data storage unit comprising
information of the subject, or
may have the ability to access information of the subject, said informing
comprising insurance status of said subject,
copayment status of prior and pending clinical test(s), medical records
relating to the subject, payment information
relating to the subject, identification information of the subject, or other
information associate with the subject or
financial transactions associated with the subject.
[00478] In some alternate embodiments, a payer may receive an electronic bill
from a sample collections
site and/or an LBM. In some instances, a health care professional may receive
an electronic payment from the
sample collection site and/or the LBM.
[00479] FIG. 6 shows a laboratory benefit system provided in accordance with
an embodiment of the
invention. A point of service 620 may be in communication with a laboratory
630. The point of service may be a
-93-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
sample collection site and any description herein of a point of service may
also apply to a sample collection site and
vice versa. The point of service may also be in communication with an LBM 610
who may also be in
communication with a payer 600. The LBM and the laboratory may be in
communication with a health care
professional 640. A subject 650 may provide a sample to a point of service.
[00480] A point of service 620 may be a sample collection center that may have
a device that may be
configured to facilitate collection of a biological sample from a subject 650.
As previously described, the sample
may be collected from the subject at the point of service, or may be provided
to the device at the point of service.
[00481] The sample collection center may be capable of communicating with a
laboratory 630. The
laboratory may be a certified laboratory. The sample collection center may
communicate with the laboratory via a
sample processing device located at the sample collection center. The sample
collection center may communicate
with the laboratory in additional ways. Data collected by the device may be
transmitted from the point of service
620 to the laboratory. Such data may be related to the sample collected from
the subject. Any type of data
described previously herein, including raw data, pre-processed data, or
analyzed data may be provided to the
laboratory.
[00482] The laboratory may provide the device to the point of service
location. In one example, the
laboratory may either sell or lease/rent the device to the sample collection
center. The laboratory may request a
payment from the sample collection center for the sales and/or leasing of the
device to the sample collection center.
The sample collection center may provide a payment to the laboratory for the
ownership or use of the device. The
device may be operated by a device operator. The operator may be affiliated
with the point of service location. The
operator may be an employee or otherwise affiliated with the sample collection
center. The operator may or may
not be trained in the use of the device. The sample collection center may be
another entity separate from the
laboratory. The sample collection center may be affiliated with the point of
service location or may be operated by
a separate entity. The sample collection center may be any of the point of
service locations described elsewhere
herein, including but not limited to retailers (e.g., Blue Cross, Blue Shield,
Health Net, Aetna, Cigna), hospitals,
medical facilities, and any other point of service. In one example, the device
may be operated by a technician or
other individual associated with a retailer or other point of service. The
laboratory may be functioning as a
wholesaler of the device. Alternatively, one or more intermediary entities may
be provided that may purchase
devices from the laboratory, and in turn provide/sell devices to point of
service locations.
[00483] In an alternate example, the laboratory may pay the point of service
location for providing the
device at the sample collection center, which may be located at the point of
service location. The laboratory may
pay the point of service location for permitting use of the device at the
point of service location and for permitting
the setup of the sample collection center at the point of service. For
example, the laboratory may be permitted to
rent out space at a retailer, where the laboratory may setup a sample
collection center having one or more devices.
The device may be operated by personnel who is or is not trained in the use of
the device. The device operator may
be affiliated with the laboratory. The device operator may or may not be an
employee of the laboratory. The device
and device operator may be using the point of service location as a sample
collection site that is remote to the
laboratory.
[00484] The laboratory may provide a cartridge to a point of service location.
The cartridge may be
configured to be inserted into, or otherwise interface with the device. The
cartridge may or may not be disposable.
-94-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
The laboratory may or may not provide disposables to the service location for
use with the device. Any description
herein of cartridges may also apply to the disposables and vice versa. In one
example, the laboratory may either
sell the cartridge to the sample collection center. The sample collection
center may be affiliated with the point of
service location and/or with a separate entity. The sample collection center
may be run by the point of service
location and/or a separate entity. The laboratory may request a payment from
the sample collection center for the
sales of the cartridge to the sample collection center. The sample collection
center may provide a payment to the
laboratory for the cartridges. The operator of the device may be affiliated
with the point of service location. The
laboratory may be functioning as a wholesaler of the cartridge. Alternatively,
one or more intermediary entities
may be provided that may purchase cartridges from the laboratory, and in turn
provide/sell cartridges to point of
service locations.
[00485] In an alternate example, the laboratory need not request payment from
the for providing the
cartridge at the sample collection center. The device may be operated by
personnel who is or is not trained in the
use of the device. The device operator may be affiliated with the laboratory.
The device operator may or may not
be an employee of the laboratory. The device and device operator may be using
the point of service location as a
sample collection site that is remote to the laboratory. The cartridge may be
used as part of the sample collection
service at the point of service location, for a device that may be operated by
a laboratory-affiliated individual.
[00486] The laboratory 630 may be capable of communicating with a health care
professional 640. The
health care professional may be at a location separate from the laboratory and
the point of service. The health care
professional may or may not have an existing relationship with the subject
650. The health care professional may
have issued a prescription for the subject to go to the point of service
location and perform one or more test. The
health care professional may or may not have a relationship with point of
service or with the laboratory. In some
embodiments, the laboratory may send a report to the health care professional.
The medical report may be based on
data collected from a device at the point of service. The medical report may
be based on an analysis of the data
collected from the device. In some embodiments, analysis of data may include
the comparison of collected data
with one or more threshold value to determine the presence or concentration of
at least one analyte. In some
embodiments, the laboratory may have a processor that may be configured to
access a data storage unit that may
have information relating to the one or more threshold value. The analysis may
occur at the laboratory 630 and the
report may be generated at the laboratory. Alternatively, the analysis may
occur at the device and the report may be
generated by the device or at the laboratory.
[00487] In some embodiments, a report may be provided to a subject 650. The
report transmitted to the
subject may or may not be the same as the report provided to the health care
professional 640. The reports may be
sent simultaneously, or the health care professional may receive the report
first, or vice versa.
[00488] An LBM 610 may be provided that may communicate with a payer 600 and a
point of service
620. The LBM may or may not communicate with a health care professional 640
and/or a laboratory 630.
[00489] The laboratory 630 and LBM 610 may be separate entities. The
laboratory and LBM may be
separate corporations, companies, organizations, institutions, partnerships,
one or more individuals, or any other
type of entity as described elsewhere herein. The laboratory and LBM may be
incorporated as separate legal
entities. The LBM may be a laboratory benefits manager, and the laboratory may
be a wholesaler. The laboratory
and LBM may be housed in separate facilities. Alternatively, they may share
facilities.
-95-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
[00490] The LBM 610 may charge a payer 600 based on use of the device at the
point of service 620. For
example, per use of the device, the LBM may charge the payer a fee. The size
of the fee may depend on one or
more factors, such as the type of use of the device (e.g., number of analytes
whose presence or concentration were
detected, the number of chemical reactions, the amount of sample preparation,
the types of reactions that take place,
the number of device components that are used), the analysis conducted in
relationship to the data collected from
the device (e.g., more complex analysis may result in a different fee from
more straightforward analysis), the payer
relationship with the subject, the payer relationship with the point of
service if any. The LBM and payer may have
an agreement in place that may determine the payment plan between the payer
and the LBM.
[00491] The LBM 610 may provide a payment to a point of service 620 based on
use of the device at the
point of service. For example, per use of the device, the LBM may provide a
payment to the point of service. In
another example, for the amount of time that the device is located at the
point of service, the LBM may provide a
payment to the point of service. The size of the fee may depend on one or more
factors, such as the type of use of
the device (e.g., number of analytes whose presence or concentration were
detected, the number of chemical
reactions, the amount of sample preparation, the types of reactions that take
place, the number of device
components that are used), the analysis conducted in relationship to the data
collected from the device (e.g., more
complex analysis may result in a different fee from more straightforward
analysis). The LBM and point of service
may have an agreement in place that may determine the payment plan between the
point of service and the LBM
and the LBM. In alternate embodiments, the LBM may provide a payment to a
laboratory 630. Any description
herein of providing payment to a point of service may also apply to a
laboratory. The LBM may provide a payment
to the laboratory instead of providing a payment to the point of service, or
in addition to providing a payment to the
point of service.
[00492] In some embodiments, the LBM 610 may divide a payment received from
the payer 600 into a
technical fee and a professional fee. In one example, the LBM may provide a
payment to a health care professional
640 based on the professional fee. The LBM may provide a payment to the sample
collection center 620 based on
the technical fee. In some embodiments, the sample collection center may be
operated by a point of service, such as
a retailer, hospital, or any other point of service. In some embodiments, the
sample collection center may be
operated by a laboratory. The payment may be provided to the entity for the
point of service location, or to a
laboratory who may be operating a sample collection center at a point of
service location.
[00493] The LBM may make the determination of how to divide the payment from
the payer. The
technical fee and/or professional fees may be based on agreements that the LBM
may have with the health care
professional, point of service, and/or laboratory. The professional fee may
also or alternatively be based on
agreements that the health care professional may have with the payer and/or
laboratory.
[00494] The LBM may further divide the payment from the payer into a
transaction fee. The transaction
fee may be an amount that goes to the LBM. The LBM may be able to keep a
fraction of the payment made by the
payer.
[00495] FIG. 7 shows an example of a lab benefits manager/wholesaler model in
accordance with an
embodiment of the invention. A retailer 700 (or other point of service), such
as a pharmacy, may have one or more
sample processing device located at the retailer site. A retailer technician
may operate the sample processing
-96-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
device, and may place a cartridge into the device 710. The cartridge may or
may not contain a sample from a
subject collected at the retailer site.
[00496] A laboratory benefit manager 720 may be an LBM as described elsewhere
herein. The laboratory
benefit manager may be an entity.
[00497] A laboratory benefit manager 720 and a wholesaler 730 may be provided
within the model. The
laboratory benefit manager and the wholesaler may be separate entities. The
laboratory benefit manager and the
wholesaler may be separate legal entities, corporate entities, corporations,
partnerships, organizations, and/or groups
of one or more individuals. The laboratory benefit manager and the wholesaler
may be housed in different facilities
or in the same facility.
[00498] A laboratory benefit manager 720 may be in communication with one or
more payers 740. The
laboratory benefit manager may issue an invoice for a service to the payers.
The payer may pay the laboratory
benefit manager. For example, the laboratory benefit manager may request a $a
(e.g., $28 to provide a numerical
example) fee from the payer, who pays the laboratory benefit manager, the $a.
The laboratory benefit manager may
retain a LBM fee. For example, a $b (e.g., $1 to provide a numerical example)
fee may be retained by the
laboratory benefit manager.
[00499] The laboratory benefit manager 720 may reimburse the retailer 700 for
the balance of the amount.
For example, the laboratory benefit manager may pay the retailer the remaining
Sc, (e.g., $27). Sc may equal $a
minus $b.
[00500] The retailer may also have fees associated with the laboratory benefit
manager and/or the
wholesaler. For example, the retailer may have an agent fee that the retailer
may pay the laboratory benefit
manager. In one example, the agent fee is $d (e.g., $8 to provide a numerical
example). The retailer may also issue
a purchase order or pay for a product. For example, the retailer may pay for
the purchase or use of the device at the
retailer site and/or cartridges. The retailer may pay the laboratory benefit
manager. Alternatively, the retailer may
pay the wholesaler for the purchase or use of the device and/or cartridges. In
one example, the payment for the
product may be $e (e.g., $9 to provide a numerical example).
[00501] From a laboratory benefit manager perspective, there may be a
financial benefit to following the
model. For example, the laboratory benefit manager may he receive an LBM fee
based on the device use. For
example, the LBM fee may be $b per transaction. The laboratory benefit manager
may also receive an agent fee
from the retailer. For example, the laboratory benefit manager may receive an
$d admin fee. In some instances, the
laboratory benefit manager may also receive a product fee from the retailer.
For example, the laboratory benefit
manager may receive a $e product fee.
[00502] From a retailer perspective, there may be financial benefit to
following the model. For example,
the retailer may receive a service income of Sc. The service income may be
provided through the laboratory benefit
manager. The laboratory benefit manager may provide the service income based
on a payment received from a
payer. The laboratory benefit manager may subtract an LBM fee from the amount
received from the payer, and
may pass the rest on to the retailer as a service income. In additional
embodiments, the laboratory benefit manager
may also subtract a professional fee, which may be provided to a health care
professional or other entity, with the
remainder of the balance going to the retailer as a service income. Thus, as
shown in FIG. 7, the total revenue may
-97-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
be provided from a Sc service income. Costs to the retailer may include an
administration fee (e.g., the $d fee
shown), and/or a product fee (e.g., the $e fee shown). The costs may be about
$f (e.g., $17 to provide a numerical
example). $f may equal $d plus $e. The costs to the retailer may be lower than
the service income. For example, a
$g (e.g., $10 to provide a numerical example) gross margin is illustrated for
the retailer. In some instances, $g = Sc
minus $f.
[00503] The table below illustrates examples of the model.
[00506] Service Income
[00508] Total Revenue [00509] $c
[00510]
[00512] $f ($d admin fee + $e
[00511] COGS product cost)
[00513]
[00514] Gross Margin [00515] $g
[00516] Any of the dollar amounts are provided by way of example only and
shall not be construed as
limiting. Any numerical value may be inserted for the various dollar values.
[00517] In some embodiments, a subject may be associated with a payer. For
example, a payer, such as a
health insurance company, government payer, or any other payer as described
herein, may provide coverage for the
subject. A payer may pay some or all of the subject's medical bills. In some
embodiments, when a subject arrives
at a point of service, the identification of the subject may be verified. The
identification of the subject may be
verified using the device, and/or verified by personnel at the point of
service. For example, the personnel at the
point of service may view the subject's identification and/or insurance card.
The device may or may not capture an
image of the subject and/or collect one or more biometric parameter from the
subject. Verification may occur on-
board the device. Alternatively, the identification of the subject may be
collected at the point of service and may be
further verified at another entity or location. For example, a laboratory,
health care professional, or payer may
verify the subject identity. The device, laboratory, health care professional,
and/or payer may be capable of
accessing subject information, such as electronic health records. Verification
may occur rapidly and/or in real-time.
For example, verification may occur within 10 minutes or less, 5 minutes or
less, 3 minutes or less, 1 minute or less,
45 seconds or less, 30 seconds or less, 20 seconds or less, 15 seconds or
less, 10 seconds or less, 5 seconds or less, 3
seconds or less, 1 second or less, 0.5 seconds or less, or 0.1 seconds or
less. The verification may be automated
without requiring any human intervention.
[00518] The system may verify the identity of the subject for the system's
records, insurance coverage, to
prevent fraud, or any other purpose. The verification may be performed by the
device. The verification may occur
at any time. In one example, the subject's identity may be verified prior to
preparing the subject's sample for the
test. The subject's identity may be verified prior to providing a sample to
the device and/or cartridge. The
-98-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
verification of the subject's identity may be provided prior to, currently
with, or after verifying the subject's
insurance coverage. The verification of the subject's identity may be provided
prior to, currently with, or after
verifying the subject has received a prescription to undergo said qualitative
and/or quantitative evaluation. The
verification may take place through communications with the medical care
provider, laboratory, payer, laboratory
benefits manager, or any other entity. Verification may occur by accessing one
or more data storage units. The
data storage units may include an electronic medical records database and/or a
payer database. Verification may
occur rapidly and/or in real-time. For example, verification may occur within
10 minutes or less, 5 minutes or less,
3 minutes or less, 1 minute or less, 45 seconds or less, 30 seconds or less,
20 seconds or less, 15 seconds or less, 10
seconds or less, 5 seconds or less, 3 seconds or less, 1 second or less, 0.5
seconds or less, or 0.1 seconds or less.
The verification may be automated without requiring any human intervention.
[00519] The verification may include information provided by the subject. For
example, the verification
may include scanning an identification card and/or insurance card of the
subject. The verification may include
taking a picture of the subject and/or the subject's face. For example, the
verification may include taking a two-
dimensional or three-dimensional snapshot of the subject. Cameras may be used
which may provide a two-
dimensional digital image of the subject and/or that may be capable of
formulating a three-dimensional or four-
dimensional image of the subject. A four-dimensional image of the subject may
incorporate changes over time.
The verification may include taking a picture of the subject's face for
identification. The verification may include
taking a picture of another portion of the subject's face for identification,
including but not limited to the patient's
whole body, arm, hand, leg, torso, foot, or any other portion of the body. The
verification may employ a video
camera and/or a microphone that may capture additional visual and/or audio
information. The verification may
include comparing the subject's movements (e.g., gait), or voice.
[00520] The verification may include entering personal information related to
the subject, such as the
subject's name, insurance policy number, answers to key questions, and/or any
other information. The verification
may include collecting one or more biometric read-out of the subject. For
example, the verification may include a
fingerprint, handprint, footprint, retinal scan, temperature readout, weight,
height, audio information, electrical
readouts, or any other information. The biometric information may be collected
by the device. For example, the
device may have a touchscreen upon which the subject may put the subject's
palm to be read by the device. The
touchscreen may be capable of scanning one or more body part of the subject,
and/or receiving a temperature,
electrical, and/or pressure readout from the subject. Alternatively, the
device may receive the biometric information
from other devices. For example, the device may receive the subject's weight
from a scale that may be separate
from the device. The information may be sent directly from the other devices
(e.g., over wired or wireless
connection) or may be entered manually.
[00521] The verification may also include information based on a sample
collected from the subject. For
example, the verification may include a genetic signature of the subject. When
the sample is provided to the device,
the device may use at least part of the sample to determine the genetic
signature of the subject. For example, the
device may perform one or more nucleic acid amplification step and may
determine key genetic markers for the
subject. This may form the subject's genetic signature. The subject's genetic
signature may be obtained prior to,
concurrently with, or after processing the sample on the device. The subject's
genetic signature may be stored on
one or more data storage unit. For example, the subject's genetic signature
may be stored in the subject's electronic
medical records. The subject's collected genetic signature may be compared
with the subject's genetic signature
-99-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
already stored in the records, if it exists. Any other unique identifying
characteristic of the subject may be used to
verify the subject's identity.
[00522] Methods for the amplification of nucleic acids, including DNA and/or
RNA, are known in the art.
Amplification methods may involve changes in temperature, such as a heat
denaturation step, or may be isothermal
processes that do not require heat denaturation. The polymerase chain reaction
(PCR) uses multiple cycles of
denaturation, annealing of primer pairs to opposite strands, and primer
extension to exponentially increase copy
numbers of the target sequence. Denaturation of annealed nucleic acid strands
may be achieved by the application
of heat, increasing local metal ion concentrations (e.g. US6277605),
ultrasound radiation (e.g. WO/2000/049176),
application of voltage (e.g. US5527670, US6033850, US5939291, and US6333157),
and application of an
electromagnetic field in combination with primers bound to a magnetically-
responsive material (e.g. US5545540),
which are hereby incorporated by reference in their entirety. In a variation
called RT-PCR, reverse transcriptase
(RT) is used to make a complementary DNA (cDNA) from RNA, and the cDNA is then
amplified by PCR to
produce multiple copies of DNA (e.g. US5322770 and US5310652, which are hereby
incorporated by reference in
their entirety).
[00523] One example of an isothermal amplification method is strand
displacement amplification,
commonly referred to as SDA, which uses cycles of annealing pairs of primer
sequences to opposite strands of a
target sequence, primer extension in the presence of a dNTP to produce a
duplex hemiphosphorothioated primer
extension product, endonuclease-mediated nicking of a hemimodified restriction
endonuclease recognition site, and
polymerase-mediated primer extension from the 3' end of the nick to displace
an existing strand and produce a
strand for the next round of primer annealing, nicking and strand
displacement, resulting in geometric amplification
of product (e.g. US5270184 and US5455166, which are hereby incorporated by
reference in their entirety).
Thermophilic SDA (tSDA) uses thermophilic endonucleases and polymerases at
higher temperatures in essentially
the same method (European Pat. No. 0 684 315, which is hereby incorporated by
reference in its entirety).
[00524] Other amplification methods include rolling circle amplification (RCA)
(e.g., Lizardi, "Rolling
Circle Replication Reporter Systems," U.S. Pat. No. 5,854,033); helicase
dependent amplification (HDA) (e.g.,
Kong et al., "Helicase Dependent Amplification Nucleic Acids," U.S. Pat.
Appin. Pub. No. US 2004-0058378 Al);
and loop-mediated isothermal amplification (LAMP) (e.g., Notomi et al.,
"Process for Synthesizing Nucleic Acid,"
U.S. Pat. No. 6,410,278), which are hereby incorporated by reference in their
entirety. In some cases, isothermal
amplification utilizes transcription by an RNA polymerase from a promoter
sequence, such as may be incorporated
into an oligonucleotide primer. Transcription-based amplification methods
commonly used in the art include
nucleic acid sequence based amplification, also referred to as NASBA (e.g.
US5130238); methods which rely on
the use of an RNA replicase to amplify the probe molecule itself, commonly
referred to as Q13 replicase (e.g.,
Lizardi, P. et al. (1988) BioTechnol. 6, 1197-1202); self-sustained sequence
replication (e.g., Guatelli, J. et al.
(1990) Proc. Natl. Acad. Sci. USA 87, 1874-1878; Landgren (1993) Trends in
Genetics 9, 199-202; and HELEN H.
LEE et al., NUCLEIC ACID AMPLIFICATION T ECHNOLOGIES (1997)); and methods for
generating
additional transcription templates (e.g. U55480784 and US5399491), which are
hereby incorporated by reference in
their entirety. Further methods of isothermal nucleic acid amplification
include the use of primers containing non-
canonical nucleotides (e.g. uracil or RNA nucleotides) in combination with an
enzyme that cleaves nucleic acids at
the non-canonical nucleotides (e.g. DNA glycosylase or RNaseH) to expose
binding sites for additional primers
-100-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
(e.g. US6251639, US6946251, and US7824890), which are hereby incorporated by
reference in their entirety.
Isothermal amplification processes can be linear or exponential.
[00525] Nucleic acid amplification for subject identification may comprise
sequential, parallel, or
simultaneous amplification of a plurality of nucleic acid sequences, such as
about, less than about, or more than
about 10, 11, 12, 13, 14, 15, 20, 25, 30, 35 ,40, 50, 100, or more target
sequences. In some embodiments, a subjects
entire genome or entire transcriptome is non-specifically amplified, the
products of which are probed for one or
more identifying sequence characteristics. An identifying sequence
characteristic includes any feature of a nucleic
acid sequence that can serve as a basis of differentiation between
individuals. In some embodiments, an individual
is uniquely identified to a selected statistical significance using about,
less than about, or more than about 10, 11,
12, 13, 14, 15, 20, 25, 30, 35 ,40, 50, 100, or more identifying sequences. In
some embodiments, the statistical
significance is about, or smaller than about 102, 10-3, 10, 10-5, 10-6, 10, 10-
8, 10-9, 10-10, 1041, 10-12, 10-13, 10-14,
10-15, or smaller. Examples of identifying sequences include Restriction
Fragment Length Polymorphisms (RFLP;
Botstein, et al., Am. J. Hum. Genet. 32: 314-331, 1980; WO 90/13668), Single
Nucleotide Polymorphisms (SNPs;
Kwok, et al., Genomics 31: 123-126, 1996), Randomly Amplified Polymorphic DNA
(RAPD; Williams, et al.,
Nucl. Acids Res. 18: 6531-6535, 1990), Simple Sequence Repeats (SSRs; Zhao &
Kochert, Plant Mol. Biol. 21:
607-614, 1993; Zietkiewicz, et al. Genomics 20: 176-183, 1989), Amplified
Fragment Length Polymorphisms
(AFLP; Vos, et al., Nucl. Acids Res. 21: 4407-4414, 1995), Short Tandem
Repeats ( STRs), Variable Number of
Tandem Repeats (VNTR), microsatellites (Tautz, Nucl. Acids. Res. 17: 6463-
6471, 1989; Weber and May, Am. J.
Hum. Genet. 44: 388-396, 1989), Inter-Retrotransposon Amplified Polymorphism
(IRAP), Long Interspersed
Elements (LINE), Long Tandem Repeats (LTR), Mobile Elements (ME),
Retrotransposon Microsatellite Amplified
Polymorphisms (REMAP), Retrotransposon-Based Insertion Polymorphisms (RBIP),
Short Interspersed Elements
(SINE), and Sequence Specific Amplified Polymorphism (SSAP). Additional
examples of identifying sequences
are known in the art, for example in US20030170705, which is incorporated
herein by reference. A genetic
signature may consist of multiple identifying sequences of a single type (e.g.
SNPs), or may comprise a
combination of two or more different types of identifying sequences in any
number or combination.
[00526] Genetic signatures can be used in any process requiring the
identification of one or more subjects,
such as in paternity or maternity testing, in immigration and inheritance
disputes, in breeding tests in animals, in
zygosity testing in twins, in tests for inbreeding in humans and animals; in
evaluation of transplant suitability such
as with bone marrow transplants; in identification of human and animal
remains; in quality control of cultured cells;
in forensic testing such as forensic analysis of semen samples, blood stains,
and other biological materials; in
characterization of the genetic makeup of a tumor by testing for loss of
heterozygosity; and in determining the
allelic frequency of a particular identifying sequence. Samples useful in the
generation of a genetics signature
include evidence from a crime scene, blood, blood stains, semen, semen stains,
bone, teeth, hair, saliva, urine, feces,
fingernails, muscle or other soft tissue, cigarettes, stamps, envelopes,
dandruff, fingerprints, items containing any of
these, and combinations thereof. In some embodiments, two or more genetic
signatures are generated and
compared. In some embodiments, one or more genetics signatures are compared to
one or more known genetic
signatures, such as genetic signatures contained in a database.
[00527] A system may also verify whether the subject has received instruction
to undergo a clinical test
from a health care professional. The system may thus verify whether a subject
has received an order from a health
care professional to undertake a qualitative and/or quantitative evaluation of
a biological sample. For example, the
-101-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
system may verify whether the subject has received a prescription from the
health care professional to take the test.
The system may verify whether the subject has received instructions from the
health care professional to provide a
sample to the device. The system may also verify whether the subject was
authorized to go to a particular point of
service to undergo the test. The verification may occur with aid of the
device. The verification may occur at any
time. In one example, the subject's authorization to take the test may be
verified prior to preparing the subject's
sample for the test. The subject's authorization to take the test may be
verified prior to providing a sample to the
device and/or cartridge. The verification of the subject's authorization may
be provided after verifying the subject's
identification. The verification of the subject's authorization may be
provided before or after verifying the subject
has insurance coverage for the clinical test. The system may verify whether
the subject is covered by health
insurance for a qualitative and/or quantitative evaluation of a sample, within
the verifying step is performed prior to,
concurrently with, or after processing a biological sample with the aid of a
device, or transmitting the data from the
device. The verification may take place through communications with the
medical care provider, laboratory, payer,
laboratory benefits manager, or any other entity. Verification may occur
rapidly and/or in real-time. For example,
verification may occur within 10 minutes or less, 5 minutes or less, 3 minutes
or less, 1 minute or less, 45 seconds
or less, 30 seconds or less, 20 seconds or less, 15 seconds or less, 10
seconds or less, 5 seconds or less, 3 seconds or
less, 1 second or less, 0.5 seconds or less, or 0.1 seconds or less. The
verification may be automated without
requiring any human intervention.
[00528] The system may also verify whether the subject has insurance coverage
for the clinical test. The
system may verify whether the subject has insurance coverage to provide a
sample to the device. The system may
also verify whether the subject has insurance coverage for going to the point
of service and undergoing the test. The
verification may occur at any time. In one example, the subject's insurance
coverage may be verified prior to
preparing the subject's sample for the test. The subject's insurance coverage
may be verified prior to providing a
sample to the device and/or cartridge. The verification of the subject's
insurance coverage may be provided after
verifying the subject's identification. The verification of the subject's
insurance coverage may be provided before
or after verifying the subject has received a prescription to take the
clinical test. The verification may take place
through communications with the medical care provider, laboratory, payer,
laboratory benefits manager, or any
other entity. The verification may occur with the aid of the device.
Verification may occur rapidly and/or in real-
time. For example, verification may occur within 10 minutes or less, 5 minutes
or less, 3 minutes or less, 1 minute
or less, 45 seconds or less, 30 seconds or less, 20 seconds or less, 15
seconds or less, 10 seconds or less, 5 seconds
or less, 3 seconds or less, 1 second or less, 0.5 seconds or less, or 0.1
seconds or less. The verification may be
automated without requiring any human intervention.
[00529] The system may also verify whether the clinical test is appropriate
for the subject. The system
may verify whether an order for a qualitative and/or quantitative evaluation
is within a set of policy restrictions.
Such policy restrictions may form guidelines. Such policy restrictions may be
policy restriction of a payer,
prescribing physician or other ordering health care professional, laboratory,
governmental or regulatory body, or
any other entity. Such verification may depend on one or more known
characteristic of the subject including but
not limited to gender, age, or past medical history. A clinical decision
support system may be provided. The
system may be capable of accessing one or more medical records, or information
associated with the subject. The
system may be able to access records relating to the identity of the subject,
insurance coverage of the subject, past
and present medical treatments of the subject, biological features of the
subject, and/or prescriptions provided to the
-102-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
subject. The system may be able to access electronic health records and/or
pull up patient records and history. The
system may also be able to pull up payer records, such as insurance and
financial information relating to the subject.
The verification may occur with the aid of the device.
[00530] In some embodiments, prior to providing a qualitative and/or
quantitative evaluation, the system
may be capable of accessing one or more records database and/or payer
database. In some instances, the system
may be capable of determining which records database and/or payer database to
access prior to providing said
qualitative and/or quantitative evaluation, and/or prior to accessing said
databases. The system may make such
determination based on the subject's identity, the subject's payer
information, information collected about the
sample, the proposed qualitative and/or quantitative evaluation, and/or any
other information.
[00531] In one example, an inappropriate test may be a pregnancy test for a
male subject or a PSA level
(prostrate-specific antigen) for a female subject. Such tests may fall outside
the policy restrictions of a payer or
prescribing physician. Such ordering errors may be detectable by reviewing the
test ordered and information
associated with the subject. Such information associated with the subject may
include medical records for the
subject or identifying information about the subject. In one example, the
appropriateness of the test is verified prior
to preparing the subject's sample for the test. The subject's test
appropriateness may be verified prior to,
concurrently with, or subsequent to providing a sample to the device and/or
cartridge. The verification of the
subject's test appropriateness may be provided after or prior to verifying the
subject's identification and/or
insurance coverage. The verification may take place through communications
with the medical care provider,
laboratory, payer, laboratory benefits manager, or any other entity. A
clinical decision support system may operate
rapidly and/or in real-time. For example, verification may occur within 10
minutes or less, 5 minutes or less, 3
minutes or less, 1 minute or less, 45 seconds or less, 30 seconds or less, 20
seconds or less, 15 seconds or less, 10
seconds or less, 5 seconds or less, 3 seconds or less, 1 second or less, 0.5
seconds or less, or 0.1 seconds or less.
The clinical decision support system may be automated without requiring any
human intervention.
[00532] In some embodiments, qualified personnel may assist with collecting
the subject's identity and/or
providing a sample from the subject to the device. The qualified personnel may
be an authorized technician that has
been trained to use the device. The qualified personnel may be a designated
operator of the device. The qualified
personnel may or may not be a health care professional. In some embodiments,
the identity of the qualified
personnel may be verified. The qualified personnel's identity may be verified
prior to, currently with, or after
receiving the biological sample, transmitting the data from the device
electronically and/or analyzing the
transmitted data. The qualified personnel's identity may be verified prior to,
currently with, or after verifying the
identity of the subject. The qualified person's identity may be verified using
one or more of the techniques
described elsewhere herein.
[00533] FIG. 9 shows further examples of a system providing sample processing,
analysis, and oversight.
The numbers in the boxes in Fig. 9 have the same meanings as the corresponding
numbers in Fig. 8. As shown in
Fig. 9, arrows from the oversight box indicate that oversight may be oversight
of analysis, oversight of
communication over a network (such as the cloud, as exemplified by the cloud
cartoon in the figure), and oversight
of processing, e.g., oversight of the operation of a device processing a
sample. As discussed above, oversight of
operation of a device may be continuous oversight, e.g., during processing of
a sample, and may include oversight
in view of device information (including device identification, device status,
device condition, and other device
-103-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
information), cartridge information, sample information, patient information,
environmental information regarding
a device, cartridge, sample, or other environmental information, and other
information and data about or transmitted
from a device. Such oversight may be oversight of oversight of analysis,
oversight of communication, and oversight
of processing, for each example in which oversight may be located at a
laboratory location, or at a sample collection
site. In embodiments, oversight may be located in the cloud or other network.
In further embodiments, oversight
includes oversight of post-analytic actions or steps.
[00534] FIG. 10A, 10B, 10C, and Fig. 10D show examples of a laboratory benefit
management system
provided in accordance with an embodiment of the invention. Advantages of
scenarios as illustrated in Figs. 10A,
10B, 10C, and 10D, and as discussed herein, include providing a retailer with
the ability to recognize revenue, e.g.,
upon receipt of payment. Such payment may be received from a customer, as
shown in Figs. 10A, 10B, 10C, and
10D; may be received from a laboratory, as shown in Fig. 10A and 10B; or from
a LBM, as shown in Fig. 10C and
10D. As illustrated in the figure, a laboratory benefits manager (LBM) may
communicate with, or be part of, a
laboratory. (The dashed box around the boxes representing Laboratory and LBM,
respectively, indicate that a
laboratory and a LBM may be the same entity, or may be separate entities.) A
test may be offered at a sample
collection site, such as a retail site. The boxes labeled "Retailer" represent
a sample collection site, which may be,
for example, a store, another commercial location, a pharmacy, a health care
facility, or other sample collection site.
A customer, indicated by the boxes labeled "Customer", may desire a service,
such as a blood test, a urine test, or
other test; the customer may pay a retailer for such a test; alternatively, a
customer may pay only a portion of the
amount owed for such a test (e.g., a copay). In embodiments, a customer does
not pay the retailer, and the retailer
receives payment from another party (e.g., a laboratory, a LBM, an insurance
company, a health plan, a
governmental agency, or other payer). A laboratory may provide services (e.g.,
may perform tests of a biological
sample), may provide equipment, may provide disposables, and may do other acts
for which payment may be
expected. A laboratory or a LBM may send an invoice (e.g., a bill) to a payer
requesting payment, as indicated by
the arrow marked "Bill" in the figures. Dollar signs indicate payments. The
head of the arrows indicates the
directionality of the indicated action; for example, the upward-pointing arrow
in figure 10A, labeled "Bill",
indicates that an LBM may bill a payer; and the downward-pointing arrow near
the arrow labeled "Bill" indicates
that a payer may provide a payment to the LBM. As shown, a laboratory may
receive payments from a payer. A
laboratory may provide payment or other moneys to a LBM. A laboratory may
share payments or other moneys
with a LBM. A LBM may receive payments from a payer. A LBM may provide payment
or other moneys to a
laboratory. A laboratory may share payments or other moneys with a LBM. In
embodiments, a laboratory may
provide information along with, separate from, or in addition to the payment
or moneys, e.g., identification
information, test information, insurance information, or other information. A
LBM may manage payer relationships
and contacts. A LBM may pay a retailer. For example, a LBM may reimburse a
retailer an amount of money, e.g.,
according to a test that was performed. In embodiments, a laboratory may
reimburse a retailer. A retailer may pay a
fee or provide other payment to a LBM (e.g., may pay a service fee, an agent
fee, or other fee). In embodiments, a
laboratory or LBM may retain a fee from moneys paid by a retailer. In
embodiments, a laboratory or LBM may
retain a fee from moneys paid by a payer; in embodiments, a LBM may pay a fee
to a laboratory. A laboratory may
be an authorized laboratory, and may be a CLIA-compliant or CLIA-certified
laboratory.
[00535] As indicated by the dashed boxes, a laboratory and a LBM may be the
same entity, or may be
separate entities. In addition, a laboratory may be a wholesaler, i.e., may
provide equipment, supplies, etc. (e.g.,
-104-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
devices, cartridges, and other materials useful for the practice of the
methods disclosed herein, and for obtaining the
devices and systems as disclosed herein). In embodiments, such items may be
provided by third parties that need
not be a laboratory.
[00536] As indicated in Fig. 10A, a customer may deal directly with a
retailer, and may provide payment
to a retailer. A retailer may deal with a laboratory, and pay, or pass payment
to a laboratory (e.g., for services,
equipment, materials, or other payments); the laboratory may pay a retailer
(e.g., a fee). A laboratory may deal with
a LBM, and payments (including fees, reimbursements, or other payments) may
pass in either or both directions
between a LBM and a laboratory. A LBM may deal with a payer (e.g., a health
plan, insurance company,
governmental agency, or other payer) by, for example, billing the payer for
services (e.g., for the service provided
to the customer) or for other costs or billable actions. A payer may pay a LBM
per such a bill. In embodiments, a
LBM and a laboratory may be the same entity, in which case the payer and the
retailer deal with that entity.
[00537] As indicated in Fig. 10B, a customer may deal directly with a
retailer, and may provide payment
to a retailer. A retailer may deal with a laboratory, and pay, or pass payment
to a laboratory (e.g., for services,
equipment, materials, or other payments); the laboratory may pay a retailer
(e.g., a fee). A laboratory may deal with
a LBM, and payments (including fees, reimbursements, or other payments) may
pass in either or both directions
between a LBM and a laboratory. A laboratory may deal with a payer (e.g., a
health plan, insurance company,
governmental agency, or other payer) by, for example, billing the payer for
services (e.g., for the service provided
to the customer) or for other costs or billable actions. A payer may pay a
laboratory per such a bill. In the scenario
illustrated in Fig. 10B, the LBM does not deal directly with the payer, and
the retailer does not deal directly with the
LBM. In embodiments, a LBM and a laboratory may be the same entity, in which
case the payer and the retailer
deal with that entity.
[00538] As indicated in Fig. 10C, a customer may deal directly with a
retailer, and may provide payment
to a retailer. A retailer may deal with a LBM, and pay, or pass payment to a
LBM (e.g., for services, equipment,
materials, or other payments); the LBM may pay a retailer (e.g., a fee). A
laboratory may deal with a LBM, and
payments (including fees, reimbursements, or other payments) may pass in
either or both directions between a LBM
and a laboratory. A laboratory may deal with a payer (e.g., a health plan,
insurance company, governmental
agency, or other payer) by, for example, billing the payer for services (e.g.,
for the service provided to the customer)
or for other costs or billable actions. A payer may pay a laboratory per such
a bill. In the scenario illustrated in Fig.
10C, the retailer deals with the LBM, and does not deal directly with the
laboratory; and the payer deals with the
laboratory, and does not deal directly with the LBM. In embodiments, a LBM and
a laboratory may be the same
entity, in which case the payer and the retailer deal with that entity.
[00539] As indicated in Fig. 10D, a customer may deal directly with a
retailer, and may provide payment
to a retailer. A retailer may deal with a LBM, and pay, or pass payment to a
LBM (e.g., for services, equipment,
materials, or other payments); the LBM may pay a retailer (e.g., a fee). A
laboratory may deal with a LBM, and
payments (including fees, reimbursements, or other payments) may pass from the
laboratory to the LBM. In the
scenario illustrated in Fig. 10D, the laboratory pays the LBM, but the LBM
does not pay the laboratory (the
laboratory receives payment from the payer. A laboratory may deal with a payer
(e.g., a health plan, insurance
company, governmental agency, or other payer) by, for example, billing the
payer for services (e.g., for the service
provided to the customer) or for other costs or billable actions. A payer may
pay a laboratory per such a bill. In the
-105-
CA 02901016 2015-08-11
WO 2014/127285 PCT/US2014/016593
scenario illustrated in Fig. 10D, the LBM does not deal directly with the
payer; and the laboratory does not deal
directly with the retailer. In embodiments, a LBM and a laboratory may be the
same entity, in which case the payer
and the retailer deal with that entity.
[00540] The publications discussed or cited herein are provided solely for
their disclosure prior to the
filing date of the present application. Nothing herein is to be construed as
an admission that the present invention is
not entitled to antedate such publication by virtue of prior invention.
Further, the dates of publication provided may
be different from the actual publication dates which may need to be
independently confirmed. All publications
mentioned herein are incorporated herein by reference to disclose and describe
the structures and/or methods in
connection with which the publications are cited. The following applications
are also incorporated herein by
reference for all purposes: US Applications Ser. Nos. 61/766,076 and
13/769,779.
[00541] It should be understood from the foregoing that, while particular
implementations have been
illustrated and described, various modifications can be made thereto and are
contemplated herein. It is also not
intended that the invention be limited by the specific examples provided
within the specification. While the
invention has been described with reference to the aforementioned
specification, the descriptions and illustrations of
the preferable embodiments herein are not meant to be construed in a limiting
sense. Furthermore, it shall be
understood that all aspects of the invention are not limited to the specific
depictions, configurations or relative
proportions set forth herein which depend upon a variety of conditions and
variables. Various modifications in
form and detail of the embodiments of the invention will be apparent to a
person skilled in the art. It is therefore
contemplated that the invention shall also cover any such modifications,
variations and equivalents.
-106-