Note: Descriptions are shown in the official language in which they were submitted.
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME 1 ________________ DE 2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS THEREOF FOR THE
TREATMENT OF INFLAMMATORY DISORDERS
FIELD OF THE INVENTION
[0001] The present invention relates to compounds that are inhibitors of
autotaxin, also known as
ectonucleotide pyrophosphatase/phosphodiesterase 2 (NPP2 or ENPP2), that is
involved in fibrotic
diseases, proliferative diseases, inflammatory diseases, autoimmune diseases,
respiratory diseases,
cardiovascular diseases, neurodegenerative diseases, dermatological disorders,
and/or abnormal
angiogenesis associated diseases. The present invention also provides methods
for the production of a
compound of the invention, pharmaceutical compositions comprising a compound
of the invention,
methods for the prophylaxis and/or treatment of diseases involving fibrotic
diseases, proliferative
diseases, inflammatory diseases, autoimmune diseases, respiratory diseases,
cardiovascular diseases,
neurodegenerative diseases, dermatological disorders, and/or abnormal
angiogenesis associated diseases
by administering a compound of the invention.
BACKGROUND OF THE INVENTION
[0002] Autotaxin (ATX; also known as ENPP2 (ectonucleotide pyrophosphatase
/ phosphodiesterase
2) or lysophospholipase D) is a ¨120 kDa protein that belongs to the ENPP
family of enzymes which is
composed of seven members, out of which ENPP1 and ENPP3 are the closest to
ATX. Whereas ENPP1
and ENPP3 are active in converting ATP into pyrophosphate (a regulator of
mineralization and
calcification processes in bone), ATX is the only ENPP enzyme with
lysophospholipase D (lysoPLD)
activity and is responsible for the hydrolysis of lysophosphatidylcholine
(LPC) to produce the bioactive
lipid lysophosphatidic acid (LPA). Several pieces of evidence have established
ATX as the main source
of LPA in blood. For example, blood LPA and ATX levels have been shown to be
strongly correlated in
humans. In addition, LPA levels are reduced by 50% in mice carrying a
heterozygous null mutation of
ATX (Tanaka, et al., 2006).
[0003] Due to the importance of LPA as a biological mediator, the levels of
bio-activc LPA are
expected to be strictly spatially and temporally controlled. The relatively
short half life of circulating
LPA (¨ 3 min) in mice is in line with this expectation. In the circulation,
where LPC levels are very high
(100-200 04, mainly albumin-bound), ATX is constitutively active but newly
produced LPA is rapidly
degraded by membrane-bound phosphatases and levels of plasma LPA are thereby
kept low (in the low
JIM range). This is confirmed by the fact that in cell-free plasma ex vivo,
LPA levels increase at a steady
rate. In addition, LPA in blood is bound to serum albumin, which might further
reduce the levels of bio-
active LPA. Besides this first level of control of LPA levels, the spatial
control of LPA production is
ensured by the capacity of ATX to bind to cell surface molecules such as
integrins and heparan sulphate
proteoglycans (HSPs) to facilitate LPA release near to its cognate receptors.
Several pieces of evidence
support this hypothesis. First, the structural studies of ATX are supporting
the fact that the ATX structure
1
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
is compatible with such a process (Hausmann, J, 2011). In addition, several
reports indicated how ATX
is involved in lymphocyte homing through the interaction with cell surface
integrins (Kanda, 2008). It
was shown, for example, that ATX can be induced on high endothelial venules
(HEVs) on sites of
inflammation. This ATX expressed by HEVs acts on HEVs in situ to facilitate
lymphocyte binding to
endothelial cells (Nakasaki, et al., 2008). As such, ATX not only drives the
formation of LPA but,
through these cellular interactions, also ensures specificity in LPA
signaling.
[0004] ATX is widely expressed, with highest mRNA levels detected in brain,
lymph nodes, kidney,
and testis. Originally discovered as `autocrine motility factor' in melanoma
cells, ATX has emerged as
the key LPA-producing enzyme in plasma and tissues. Unfortunately, embryonic
lethality has hampered
studies of the importance of ATX in adult life. This embryonic lethality
reflects the key role of LPA in
various developmental processes, vasculogenesis in particular. Knock-out
studies of the LPA receptors
have been more informative in terms of unraveling the physiological role of
LPA. LPA acts through at
least six distinct (G protein)-coupled receptors (LPA1-6) found on the surface
of different cell types,
three of which belong to the edg receptor family and three to the non-edg
receptor family. LPA interacts
with specific G protein-coupled receptors (GPCRs), namely LPA1 (also known as
EDG2), LPA2 (also
known as EDG4), LPA3 (also known as EDG7), LPA4 (also known as GPR23/p2y9),
LPA5 (also known
as GPR92/93), LPA6 (also known as p2y5). LPA has also been described to
interact with three other
GPCRs (GPR87, p2y10, GPR35). In addition, a preference of LPA receptors for
specific LPA species has
been demonstrated (Bandoh, et al., 2000). As such, the specificity of the LPA
activities is controlled by
the expression pattern of the LPA receptors and their downstream signaling
route.
[0005] The main part of the LPA responses arc mediated through trimeric G-
proteins and include but
are not limited to mitogen-activated protein kinase (MAPK) activation,
adenylyl cyclase (AC)
inhibition/activation, phospholipase C (PLC) activationiCa2+ mobilization,
arachidonic acid release,
Akt/PKB activation, and the activation of small GTPases, Rho, ROCK, Rae, and
Ras. Other pathways
that are affected by LPA receptor activation include cell division cycle
42/GTP-binding protein (Cdc42),
proto-oncogene serine/threonine-protein kinase Raf (c-RAF), proto-oncogene
tyrosine-protein kinase Src
(c-src), extracellular signal-regulated kinase (ERK), focal adhesion kinase
(FAK), guanine nucleotide
exchange factor (GEF), glycogen synthase kinase 3b (GSK3b), c-jun amino-
terminal kinase (JNK), MEK,
myosin light chain II (MLC II), nuclear factor kB (NF-kB), N-methyl-D-
aspartate (NMDA) receptor
activation, phosphatidylinosito13-kinase (PI3K), protein kinase A (PKA),
protein kinase C (PKC), and
ras-related C3 botulinum toxin substrate 1 (RAC1). The actual pathway is
influenced by cell type,
expression level of a receptor or signaling protein, receptor usage, and LPA
concentration (Tania, Khan,
Zhang, Li, & Song, 2010). LPA has a broad range of physiological actions and
various cellular effects
(for example blood pressure regulation, platelet activation, smooth muscle
contraction, cell growth, cell
rounding, neurite retraction, actin stress fiber formation and cell
migration),In addition, a preference of
LPA receptors for specific LPA species has been demonstrated (Bandoh, et al.,
2000). The knock-out
studies for these receptors indicated a role in bone development (Gennero, et
al., 2011), and neurogenesis
2
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
(Matas-Rico, et al., 2008), embryo implantation (Ye, et al., 2005) and the
formation of blood and
lymphatic vessels (Sumida, et al., 2010).
[0006] With regard to pathophysiology, a role for LPA and LPA receptors has
been claimed in
various patho-physiological conditions such as proliferative diseases,
neuropathic pain, inflammation,
autoimmune diseases, fibrosis, lymphocyte tracking in lymph nodes, obesity,
diabetes, or embryonic
blood vessel formation.
[0007] The role of LPA in lung fibrosis has been well described in
literature and also an involvement
in asthma has been claimed. The present inventors however are the first to
report a link to chronic
obstructive pulmonary disease (COPD).
[0008] Several lines of evidence suggest a role for ATX in the control of
lung function in disease
through effects on lung epithelial cells, fibroblasts and smooth muscle cells.
In general, inflammatory
conditions in the lung are often described as associated with increased ATX
and LPA levels. Instillation
of LPS in mice, for example, induces increased ATX and LPA levels in the
broncho-alveolar lavage
(BAL) fluid (Zhao, et al., 2011). Also in humans, a segmental LPS challenge
led to increased LPA levels
(Georas, et al., 2007). Overall, the role of LPA in activating lung epithelial
cells, the first line of defense
to inhaled noxious stimuli, towards increased cytokine and chemokine
production and migration have
been extensively described (Zhao & Natarajan, 2013). Exogenous LPA promotes
inflammatory responses
by regulating the expression of chemokines, cytokines, and cytokine receptors
in lung epithelial cells. In
addition to the modulation of inflammatory responses, LPA regulates
cytoskeleton rearrangement and
confers protection against lung injury by enhancing lung epithelial cell
barrier integrity and remodeling.
[0009] In the asthmatic individual, the release of normal repair mediators,
including LPA, is
exaggerated or the actions of the repair mediators are inappropriately
prolonged leading to inappropriate
airway remodeling. Major structural features of the remodeled airway observed
in asthma include a
thickened lamina reticularis (the basement membrane-like structure just
beneath the airway epithelial
cells), increased numbers and activation of myofibroblasts, thickening of the
smooth muscle layer,
increased numbers of mucus glands and mucus secretions, and alterations in the
connective tissue and
capillary bed throughout the airway wall. ATX and/ or LPA may contribute to
these structural changes in
the airway, for example ATX and/ or LPA are involved in acute airway
hyperresponsiveness in asthma.
The lumen of the remodeled asthmatic airway is narrower due to the thickening
of the airway wall, thus
decreasing airflow. Additionally, LPA contributes to the long-term structural
remodeling and the acute
hyperresponsiveness of the asthmatic airway, for example LPA contributes to
the hyper-responsiveness
that is a primary feature of acute exacerbations of asthma. Reports describing
the role of LPA in asthma
generated different conclusions, ranging from a protective role (Zhao, et al.,
2009) to a negative role
(Emo, et al., 2012). The testing of autotaxin inhibitors in models for airway
diseases as described herein
allows for the clarification of the potential of this enzyme as a drug target.
[0010] Fibroblast proliferation and contraction and extracellular matrix
secretion stimulated by LPA
contributes to the fibroproliferative features of other airway diseases, such
as the peribronchiolar fibrosis
present in chronic bronchitis, and interstitial lung diseases and severe
asthma. LPA plays a role in the
3
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
fibrotic interstitial lung diseases and obliterative bronchiolitis, where both
collagen and myofibroblasts
are increased. Studies related to IPF (idiopathic pulmonary fibrosis)
indicated an increase in LPA levels
in the BAL fluid of patients (Tager, et al., 2008). Further LPA1 knock-out and
inhibitor studies revealed
a key role for LPA in fibrotic processes in lung and were complemented by
studies using cell-specific
knock-out mice lacking ATX in bronchial epithelial cells and macrophages.
These mice were shown to be
less sensitive to models of lung fibrosis (Oikonomou, et al., 2012). A role
for LPA in other fibrotic
diseases (kidney and skin) was based on similar types of observations
(Pradere, et al., 2007), (Castelino,
et al., 2011). The role of LPA in lung remodeling relates to the effects of
LPA on both lung fibroblasts
(through LPA1) and epithelial cells (through LPA2) (Xu, et al., 2009) have
demonstrated that LPA2 plays
a key role in the activation of TGFP in epithelial cells under fibrotic
conditions. The role of LPA in
remodeling and fibrosis is relevant to COPD, IPF and asthma, diseases in which
lung remodeling as a
long term outcome will limit lung function. Finally, of interest towards lung
diseases, in mice, ATX is
one of the three main QTLs that appear to be associated with differences in
lung function (Ganguly, et al.,
2007).
[0011] One prominent area of research interest is the role of ATX¨LPA
signaling in cancer
(Braddock, 2010). Although cancer-specific mutations in ATX have not been
identified so far,
overexpression of ATX or individual LPA receptors in xenografted and
transgenic mice promotes tumour
formation, angiogenesis and metastasis. Conversely, ATX knockdown in mammary
carcinoma cells
reduces their metastatic spread to bone. Several human cancers show elevated
ATX and/or aberrant LPA
receptor expression patterns, as revealed by microarray analyses. Autotaxin is
viewed as a pro-metastatic
enzyme. It has initially been isolated from the conditioned medium of human
melanoma cells that
stimulates a myriad of biological activities, including angiogenesis and the
promotion of cell growth,
migration, survival, and differentiation through the production of LPA (Lin M.
E., 2010). LPA
contributes to tumorigenesis by increasing motility and invasiveness of cells.
The initiation, progression
and metastasis of cancer involve several concurrent and sequential processes
including cell proliferation
and growth, survival and anti-apoptosis, migration of cells, penetration of
foreign cells into defined
tissues and/ or organs, and promotion of angiogenesis.
[0012] Therefore, the control of each of these processes by LPA signaling
in physiological and
pathophysiological conditions underscores the potential therapeutic usefulness
of modulating LPA
signaling pathways for the treatment of cancer. In particular, LPA has been
implicated in the initiation or
progression of ovarian cancer, prostate cancer, breast cancer, melanoma, head
and neck cancer, bowel
cancer (colorectal cancer), thyroid cancer, glioblastoma, follicular lymphoma
and other cancers (Gardell,
2006) (Murph, Nguyen, Radhakrishna, & Mills, 2008) (Kishi, 2006).
[0013] Furthermore, autotaxin is implicated in the invasive and metastatic
process of tumor cells,
since ectopic overexpression of autotaxin is frequently observed in malignant
tumor tissues such as
ovarian cancer (Vidot, et al., 2010), breast cancer (Panupinthu, Lee, & Mills,
2010) (Zhang, et al., 2009),
prostate cancer (Nouh, et al., 2009), renal cancer, Hodgkin lymphoma
(Baumforth, 2005), hepatocellular
carcinoma (Wu, et al., 2010), lung cancer (Xu & Prestwich, 2010), and
glioblastoma (Kishi, 2006).
4
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Autotaxin overexpression has also been found in a variety of tumors such as
malignant melanoma,
teratocarcinoma, neuroblastoma, non-small-cell lung cancer, renal cell
carcinoma (Stassar, et al., 2001).
[0014] Furthermore, expression of autotaxin by cancer cells controls
osteolytic bone metastasis
formation. In particular, LPA stimulates directly cancer growth and
metastasis, and osteoclast
differentiation. Therefore, targeting the autotaxin/LPA signaling route has
also been found to be
beneficial in patients with bone metastases (David, 2010). Finally, the
inhibition of autotaxin seems to
provide a beneficial adjuvant to chemotherapy for preventing tumor growth and
metastasis in patients
with high autotaxin expression in their tumors (Gaetano, 2009).
[0015] Upregulation of the autotaxin-LPA signaling pathway has been
observed in a variety of
inflammatory conditions. For example, pro-inflammatory effects of LPA include
degranulation of mast
cells, contraction of smooth-muscle cells and release of cytokines from
dendritic cells.As an indication
for its general role in inflammation, LPA and autotaxin activity have been
shown to be induced by
carageenan injection into the mouse air pouch model, which is used to develop
anti-inflammatory drugs,
including cyclooxygenase inhibitors for arthritis. Furthermore, a reduction in
plasma and air pouch LPA
has been observed in this rat air pouch model using an autotaxin inhibitor,
confirming the role of
autotaxin during inflammation as a major source of LPA (Gierse, 2010). It has
been observed that
autotaxin inhibitors reduce LPA and PGE2 and also reduce inflammatory pain.
[0016] As another general role in inflammatory diseases, LPA has been shown
to induce
chemokinesis in T-cells. Intravenous injection of enzymatically inactive
autotaxin has been shown to
attenuate the homing of T-cells to lymphoid tissues, likely by competing with
endogenous autotaxin and
exerting a dominant-negative effect. In certain instances, autotaxin
facilitates lymphocyte entry into
lymphoid organs (Kanda, 2008). Therefore an autotaxin inhibitor may block
lymphocyte migration into
secondary lymphoid organs and be of benefit in autoimmune diseases.
[0017] Specifically in rheumatoid arthritis, an increased expression of ATX
in synovial fibroblasts
from RA patients was demonstrated and ablation of ATX expression in
mesenchymal cells (including
synovial fibroblasts) resulted in attenuated symptoms in mouse models for
rheumatoid arthritis
(Nikitopoulou, et al., 2012). As such, the role of autotaxin in rheumatoid
arthritis has been strongly
established.
[0018] Several lines of evidence suggest a role for LPA in vascular injury
and atherosclerosis. These
relate to the role of LPA in modulating endothelial barrier function and the
phenotype of vascular smooth
muscle cells and the action of LPA as a weak platelet agonist. Platelets have
been identified as important
participants in LPA production in the circulation in some settings, mainly by
providing sufficient LPC
amounts. Plasma autotaxin associates with platelets during aggregation and
concentrates in arterial
thrombus, and activated but not resting platelets bind recombinant autotaxin
in an integrin-dependent
manner. Experimental induction of thrombocytopenia in rats, using an anti-
platelet antibody, decreases
the production of LPA in serum by almost 50%, which suggests a role for LPA
during clotting. In some
instances, transgenic overexpression of autotaxin elevates circulating LPA
levels and induces a bleeding
diathesis and attenuation of thrombosis in mice. Intravascular administration
of exogenous LPA
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
recapitulates the prolonged bleeding time observed in autotaxin-Tg mice.
Finally, autotaxin +/- mice,
which have ¨50% normal plasma LPA levels, are more prone to thrombosis.
[0019] In addition to a role in blood clotting, LPA has multiple effects on
the endothelial monolayer
permeability increase, and endothelial cells, in particular in critical
aspects of angiogenesis such as cell
migration stimulation and invasion. Furthermore, LPA also exerts migratory and
contractile effects on
vascular smooth muscle cells: autotaxin-mediated LPA production and subsequent
LPA signaling
contributes to vascular development by stimulating endothelial cell migration
and invasion as well as
regulating adhesive interactions with the extracellular matrix and smooth
muscle cells. For example,
similar vascular defects have been observed in autotaxin-deficient mice and in
mice lacking genes
involved in cell migration and adhesion (Van Meeteren, et al., 2006).
Therefore an autotaxin inhibitor
may have benefit in some diseases involving dysregulated angiogenesis.
[0020] LPA induces neuropathic pain as well as demyelination and pain-
related protein expression
changes via LPA1 (Inoue, et al., 2008). ATX heterozygous knockout mice show
about 50% recovery of
nerve injury-induced neuropathic pain compared to wild type mice.
Lysophosphatidylcholine (LPC), also
known as lyso-lecithin, is known to induce neuropathic pain. It has been
observed that LPC-induced
neuropathic pain is partially reduced in ATX heterozygous knockout mice. These
results support the idea
that LPA is produced by autotaxin resulting in neuropathic pain (Lin M. E.,
2010).
[0021] Autotaxin is also implicated in metabolic diseases, in particular
obesity and diabetes
(Federico, et al., 2012). In some instances, autotaxin is responsible for the
lysoPLD activity released by
adipocytes and exerts a paracrine control on preadipocyte growth via an LPA-
dependent mechanism. In
addition, autotaxin is upregulated during adipocyte differentiation and in
genetic obesity. In certain
instances, autotaxin mRNA is upregulated in adipocytes from db/db mice
suggesting that the upregulation
of autotaxin is related to the severe type 2 diabetes phenotype and adipocyte
insuline resistance. In some
instances, upregulation of adipocyte autotaxin is associated with type 2
diabetes in human (Ferry, 2003).
The relationship between adipocyte and autotaxin biology suggests the use of
an autotaxin inhibitor for
the treatment of metabolic diseases.
[0022] Finally, two other conditions clearly related to autotaxin biology
are cholestatic pruritus
(Kremer, et al., 2010) and regulation of ocular pressure (Iyer, et al., 2012).
[0023] The current therapies are not satisfactory and therefore there
remains a need to identify further
compounds that may be of use in the treatment of fibrotic diseases,
proliferative diseases, inflammatory
diseases, autoimmune diseases, respiratory diseases, cardiovascular diseases,
neurodegenerative diseases,
dermatological disorders, and/or abnormal angiogenesis associated diseases.
The present invention
therefore provides compounds, methods for their manufacture and pharmaceutical
compositions
comprising a compound of the invention together with a suitable pharmaceutical
carrier. The present
invention also provides for the use of a compound of the invention in the
preparation of a medicament for
the treatment of fibrotic diseases, proliferative diseases, inflammatory
diseases, autoimmune diseases,
respiratory diseases, cardiovascular diseases, neurodegencrative diseases,
dermatological disorders,
and/or abnormal angiogenesis associated diseases.
6
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
SUMMARY OF THE INVENTION
[0024] The present invention is based on the identification of novel
compounds, and their ability to
act as inhibitors of autotaxin and that they may be useful for the treatment
of fibrotic diseases,
proliferative diseases, inflammatory diseases, autoimmune diseases,
respiratory diseases, cardiovascular
diseases, neurodegenerative diseases, dermatological disorders, and/or
abnormal angiogenesis associated
diseases. The present invention also provides methods for the production of
these compounds,
pharmaceutical compositions comprising these compounds and methods of
treatment for fibrotic diseases,
proliferative diseases, inflammatory diseases, autoimmune diseases,
respiratory diseases, cardiovascular
diseases, neurodegenerative diseases, dermatological disorders, and/or
abnormal angiogenesis associated
diseases by administering the compounds of the invention.
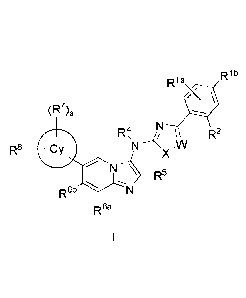
[0025] Accordingly, in a first aspect of the invention, the compounds of
the invention are provided
having a Formula (I):
Rib
Rla
X \
R7)a
R4, R2
R8 4111 NX-W
N R5
R6b
R62
wherein
Ria is H, halo or C1_4 alkyl;
Rib is:
- halo,
- C 1_4 alkyl (which alkyl is optionally substituted with one or more
independently selected
halo), or
- C 1_4 alkoxy (which alkoxy is optionally substituted with one or more
independently selected
halo);
X is ¨S-, -0-, ¨N=CH-, ¨CH=N- or ¨CH=CH-;
W is N, or CR3
when W is N, R2 is:
- H,
- -CN,
- halo,
- Ci_4 alkyl (which alkyl is optionally substituted with one or more
independently selected OH,
or CN),
- -C(=0)CH3,
7
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
- -C(=0)CF3,
- -C(=0)0CH3,
- -C(=0)NH2, or
- -NHC(=0)CH3, or
when W is CR3, one of R2 or R3 is:
- H,
- -CN,
- halo,
- C1-4 alkyl (which alkyl is optionally substituted with one or more
independently selected OH,
or CN),
- -C(=0)CH3,
- -C(=0)CF3,
- -C(=0)0CH3,
- -C(=0)NH2, or
- -NHC(=0)CH3,
and the other is H, or Cm alkyl;
R4 is C1_4 alkyl;
R5 is CIA alkyl optionally substituted with one or more independently selected
CN, OH, halo, or -
C(=0)NH2;
one of R6a or R6b is selected from H, -Cf13, and halo, and the other is H;
Cy is:
- C4_10 cycloalkyl,
- 4-10 membered mono or bicyclic heterocycloalkyl containing one or more
beteroatoms
independently selected from 0, N, and S, or
- 4-7 membered heterocycloalkenyl containing 1 double bond, containing one
or more
heteroatoms independently selected from 0, N, and S;
each R7 is independently selected from:
- OH,
- oxo,
- halo, and
- C1_4 alkyl (which alkyl is optionally substituted with one or more
independently selected OH,
or Ci4 alkoxy);
the subscript a is 0,1 or 2;
R8 is -(Li-Wi).-L2-Gi,
wherein
- L1 is absent, or is -0-, -C(=0)-, -NR', -NRhC(=0)-, or -SO2-;
- WI is C1_4 alkylene;
- the subscript m is 0, or 1;
8
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
- L2 is absent, or is -0-, -C(=0)-, -C(=0)0-, -0C(=0) -C(=0)-C(=0)-, -C(=0)-
C(=0)N1V-,
-NRb-, -C(=0)NRc-, -NRdC(=0)-, -NRiC(=0)0-, -SONRe- or ¨NRfS02-;
- GI is
o H,
o -CN,
o C1_4 alkyl (which alkyl is optionally substituted with one or more
independently
selected ¨CN, OH, halo or phenyl),
o C3_7 cycloalkyl (which cycloalkyl is optionally substituted with ¨NHA
o 5-6 membered heterocycloalkenyl containing 1 double bond containing one
or more
heteroatoms independently selected from 0, N, and S (which heterocycloalkenyl
is
optionally substituted with one or more independently selected R9 groups),
o 4-10 membered mono, bi or spirocyclic heterocycloalkyl containing one or
more
heteroatoms independently selected from 0, N, and S (which hcterocycloalkyl is
optionally substituted with one or more independently selected R9 groups), or
o 5-6 membered heteroaryl containing one or more heteroatoms independently
selected
from 0, N, and S (which heteroaryl is optionally substituted with one or more
independently selected groups),
each R9 is oxo, or R19;
each le is:
- -OH,
- halo,
- -CN,
- CI 4 alkyl (which alkyl is optionally substituted with one or more
independently selected OH,
halo, or phenyl),
- C1_4 alkoxy,
- C3_7 cycloalkyl,
- phenyl,
- -S02CH3,
- -C(=0)C1_4 alkoxy,
- -C(=0)C1_4 alkyl, or
- -NRgC(=0)C1_4 alkyl; and
each Re, Rh, Re, Rd, Re, Rf, Rg, Rh, Ri, and R is independently selected from
H and C14 alkyl.
[0026] In one aspect, the compounds of the invention are inhibitors of
autotaxin. Furthermore, the
compounds of the invention may exhibit low clearance, possibly resulting in
low therapeutic dose levels.
[0027] In a more particular aspect, the compounds of the invention are
active in vivo against IPF
and/or COPD.
[0028] In a particular aspect, the compounds of the invention are provided
for use in the prophylaxis
and/or treatment of fibrotic diseases, proliferative diseases, inflammatory
diseases, autoimmune diseases,
9
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
respiratory diseases, cardiovascular diseases, neurodegenerative diseases,
dermatological disorders,
and/or abnormal angiogenesis associated diseases.
[0029] In a further aspect, the present invention provides pharmaceutical
compositions comprising a
compound of the invention, and a pharmaceutical carrier, excipient or diluent.
In a particular aspect, the
pharmaceutical composition may additionally comprise further therapeutically
active ingredients suitable
for use in combination with the compounds of the invention. In a more
particular aspect, the further
therapeutically active ingredient is an agent for the treatment of fibrotic
diseases, proliferative diseases,
inflammatory diseases, autoimmune diseases, respiratory diseases,
cardiovascular diseases,
neurodegenerative diseases, dermatological disorders, and/or abnormal
angiogenesis associated diseases.
[0030] Moreover, the compounds of the invention, useful in the
pharmaceutical compositions and
treatment methods disclosed herein, are pharmaceutically acceptable as
prepared and used.
[0031] In a further aspect of the invention, this invention provides a
method of treating a mammal, in
particular humans, afflicted with a condition selected from among those listed
herein, and particularly
fibrotic diseases, proliferative diseases, inflammatory diseases, autoimmune
diseases, respiratory
diseases, cardiovascular diseases, neurodegenerative diseases, dermatological
disorders, and/or abnormal
angiogenesis associated diseases, which method comprises administering an
effective amount of the
pharmaceutical composition or compounds of the invention as described herein.
[0032] The present invention also provides pharmaceutical compositions
comprising a compound of
the invention, and a suitable pharmaceutical carrier, excipient or diluent for
use in medicine. In a
particular aspect, the pharmaceutical composition is for use in the
prophylaxis and/or treatment of fibrotic
diseases, proliferative diseases, inflammatory diseases, autoimmunc diseases,
respiratory diseases,
cardiovascular diseases, neurodegenerative diseases, dermatological disorders,
and/or abnormal
angiogenesis associated diseases.
[0033] In additional aspects, this invention provides methods for
synthesizing the compounds of the
invention, with representative synthetic protocols and pathways disclosed
later on herein.
[0034] Other objects and advantages will become apparent to those skilled
in the art from a
consideration of the ensuing detailed description.
[0035] It will be appreciated that compounds of the invention may be
metabolized to yield
biologically active metabolites.
DETAILED DESCRIPTION OF THE INVENTION
Definitions
[0036] The following terms are intended to have the meanings presented
therewith below and are
useful in understanding the description and intended scope of the present
invention.
[0037] When describing the invention, which may include compounds,
pharmaceutical compositions
containing such compounds and methods of using such compounds and
compositions, the following
terms, if present, have the following meanings unless otherwise indicated. It
should also be understood
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
that when described herein any of the moieties defined forth below may be
substituted with a variety of
substituents, and that the respective definitions are intended to include such
substituted moieties within
their scope as set out below. Unless otherwise stated, the term 'substituted'
is to be defined as set out
below. It should be further understood that the terms 'groups' and 'radicals'
can be considered
interchangeable when used herein.
[0038] The articles 'a' and 'an' may be used herein to refer to one or to
more than one (i.e. at least
one) of the grammatical objects of the article. By way of example 'an
analogue' means one analogue or
more than one analogue.
[0039] As used herein the term IPA' relates to lysophosphatidic acid which
is a member of the
membrane-derived bioactive lipid mediators, further comprising sphingosine- 1 -
phosphate (SIP),
lysophosphatidylcholine (LPC), and sphingosylphosphorylcholine (SPC). LPA
interacts with specific G
protein-coupled receptors (GPCRs), namely LPAI, LPA,, LPA3, LPA4, LPA5, LPA6,
LPA7, LPA8, in an
autocrine and paracrinc fashion, to activate intracellular signaling pathways,
and in turn produce a variety
of biological responses.
[0040] 'Alkyl' means straight or branched aliphatic hydrocarbon with the
number of carbon atoms
specified. Particular alkyl groups have 1 to 8 carbon atoms. More particular
is lower alkyl which has 1 to
6 carbon atoms. A further particular group has 1 to 4 carbon atoms. Exemplary
straight chained groups
include methyl, ethyl n-propyl, and n-butyl. Branched means that one or more
lower alkyl groups such as
methyl, ethyl, propyl or butyl is attached to a linear alkyl chain, exemplary
branched chain groups include
isopropyl, iso-butyl, t-butyl and isoamyl.
[0041] `Alkoxy' refers to the group ¨0R26 where R26 is alkyl with the
number of carbon atoms
specified. Particular alkoxy groups are methoxy, ethoxy, n-propoxy,
isopropoxy, n-butoxy, tert-butoxy,
sec-butoxy, n-pentoxy, n-hexoxy, and 1,2-dimethylbutoxy. Particular alkoxy
groups are lower alkoxy,
i.e. with between 1 and 6 carbon atoms. Further particular alkoxy groups have
between 1 and 4 carbon
atoms.
[0042] `Alkylene' refers to divalent alkene radical groups having the
number of carbon atoms
specified, in particular having 1 to 6 carbon atoms and more particularly 1 to
4 carbon atoms which can
be straight-chained or branched. This term is exemplified by groups such as
methylene (-CH2-), ethylene
(-CR2-CF12-), or -CH(CH3)- and the like.
[0043] `Alkenyl' refers to monovalent olefinically (unsaturated)
hydrocarbon groups with the number
of carbon atoms specified. Particular alkenyl has 2 to 8 carbon atoms, and
more particularly, from 2 to 6
carbon atoms, which can be straight-chained or branched and having at least 1
and particularly from 1 to
2 sites of olefinic unsaturation. Particular alkenyl groups include ethenyl (-
CH=CH2), n-propenyl (-
CH2CH=CH2), isopropenyl (-C(CH3)=CH2) and the like.
[0044] 'Amino' refers to the radical -NH2.
[0045] 'Aryl' refers to a monovalent aromatic hydrocarbon group derived by
the removal of one
hydrogen atom from a single carbon atom of a parent aromatic ring system. In
particular aryl refers to an
aromatic ring structure, monocyclic or polycyclic, with the number of ring
atoms specified. Specifically,
Ii
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
the term includes groups that include from 6 to 10 ring members. Where the
aryl group is a monocyclic
ring system it preferentially contains 6 carbon atoms. Particularly aryl
groups include phenyl, naphthyl,
indenyl, and tetrahydronaphthyl.
[0046] `Cycloalkyl'refers to a non-aromatic hydrocarbyl ring structure,
monocyclic or polycyclic,
with the number of ring atoms specified. A cycloalkyl may have from 3 to 10
carbon atoms, and in
particular from 3 to 7 carbon atoms. Such cycloalkyl groups include, by way of
example, single ring
structures such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, and
cycloheptyl.
[0047] `Cyano' refers to the radical -CN.
[0048] 'Halo' or 'halogen' refers to fluoro (F), chloro (Cl), bromo (Br)
and iodo (I). Particular halo
groups are either fluoro or chloro.
[0049] `Hetero' when used to describe a compound or a group present on a
compound means that one
or more carbon atoms in the compound or group have been replaced by a
nitrogen, oxygen, or sulfur
heteroatom. Hetero may be applied to any of the hydrocarbyl groups described
above such as alkyl, e.g.
heteroalkyl, cycloalkyl, e.g. heterocycloalkyl, aryl, e.g. heteroaryl, and the
like having from 1 to 4, and
particularly from 1 to 3 heteroatoms, more typically 1 or 2 heteroatoms, for
example a single heteroatom.
[0050] `Heteroaryl' means an aromatic ring structure, monocyclic or
polycyclic, that includes one or
more heteroatoms independently selected from 0, N and S and the number of ring
atoms specified. In
particular, the aromatic ring structure may have from 5 to 10 ring members.
The heteroaryl group can be,
for example, a five membered or six membered monocyclic ring or a bicyclic
structure formed from fused
five and six membered rings or two fused six membered rings or, by way of a
further example, two fused
five membered rings. Each ring may contain up to four heteroatoms typically
selected from nitrogen,
sulphur and oxygen. Typically the heteroaryl ring will contain up to 4
heteroatoms, more typically up to 3
heteroatoms, more usually up to 2, for example a single heteroatom. in one
embodiment, the heteroaryl
ring contains at least one ring nitrogen atom. The nitrogen atoms in the
heteroaryl rings can be basic, as in
the case of an imidazole or pyridine, or essentially non-basic as in the case
of an indole or pyrrole
nitrogen. In general the number of basic nitrogen atoms present in the
heteroaryl group, including any
amino group substituents of the ring, will be less than five. Examples of five
membered monocyclic
heteroaryl groups include but are not limited to pyrrole, furan, thiophene,
imidazole, furazan, oxazole,
oxadiazolc, oxatriazolc, isoxazole, thiazolc, isothiazolc, thiadiazolc,
pyrazolc, triazolc and tetrazole
groups. Examples of six membered monocyclic heteroaryl groups include but are
not limited to pyridine,
pyrazine, pyridazine, pyrimidine and triazine. Particular examples of bicyclic
heteroaryl groups
containing a five membered ring fused to another five membered ring include
but are not limited to
imidazothiazole and imidazoimidazole. Particular examples of bicyclic
heteroaryl groups containing a six
membered ring fused to a five membered ring include but are not limited to
benzofuran, benzothiophene,
benzoimidazo le, benzoxazole, isob enzoxazo le, b enzis oxazo le,
benzthiazole, b enzisothiazo le,
isobenzofuran, indole, isoindole, isoindolone, indolizine, indoline,
isoindoline, purine (e.g. adenine,
guanine), indazole, imidazopyridincs, imidazopyrimidincs, imidazopyrazincs,
pyrazolopyrimidinc,
triazolopyrimidine, benzodioxole and pyrazolopyridine groups. Particular
examples of bicyclic
12
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
heteroaryl groups containing two fused six membered rings include but are not
limited to quinoline,
isoquinoline, chroman, thiochroman, chromene, isochromene, chroman,
isochroman, benzodioxan,
quinolizine, benzoxazine, benzodiazine, pyridopyridine, quinoxaline,
quinazoline, cinnoline, phthalazine,
naphthyridine and pteridine groups. Particular heteroaryl groups are those
derived from thiophene,
pyrrole, benzothiophene, bcnzofuran, indole, pyridine, quinolinc, imidazolc,
thiazole, oxazole and
pyrazine.
[0051] Examples of representative heteroaryls include the following:
N N
r, N
I )1
N
N
1:111011 = "IN
wherein each Y is selected from >C(=0), NH, 0 and S.
[0052] As used herein, the term cheterocycloalkyr means a stable non-
aromatic ring structure, mono-
cyclic or polycyclic, that includes one or more heteroatoms independently
selected from 0, N and S and
the number of ring atoms specified. The non-aromatic ring structure may have
from 4 to 10 ring
members, and in particular from 4 to 7 ring members. A fused heterocyclic ring
system may include
carbocyclic rings and need only to include one heterocyclic ring. Examples of
heterocyclic rings include,
but are not limited to, morpholine, piperidine (e.g 1-piperidinyl, 2-
piperidinyl, 3-piperidinyl and 4-
piperi dinyl), pyrrol i dine (e.g. 1 -pyrrolidinyl, 2-pyrrolidinyl and 3 -
pyrrol i di nyl), pyrrol i do n e, pyranõ
tetrahydrofuran, tetrahydrothiophene, dioxane, tetrahydropyran (e.g. 4-
tetrahydro pyranyl), imidazoline,
imidazolidinone, oxazoline, thiazoline, 2-pyrazoline, pyrazolidine,
piperazine, and N-alkyl piperazines
such as N-methyl piperazine. Further examples include thiomorpholine and its S-
oxide and S,S-dioxide
(particularly thiomorpholine). Still further examples include azetidine,
piperidone, piperazone, and N-
alkyl piperidines such as N-methyl piperidine. Particular examples of
heterocycloalkyl groups are shown
in the following illustrative examples:
13
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
/¨W
' )
,X
\W \AN
( 1110
wherein each W is selected from CH2, NH, 0 and S; and each Y is selected from
NH, 0, C(=0), SO2, and
S.
[0053] As used herein, the term `heterocycloalkenyl' means a
`heterocycloalkyr, which comprises at
least one double bond. Particular examples of heterocycloalkenyl groups are
shown in the following
illustrative examples:
C ,Z ,W
,z
wherein each W is selected from CH2, NH, 0 and S; each Y is selected from NH,
0, C(=0), SO2, and S;
and each Z is selected from N or CH.
[0054] 'Hydroxyl' refers to the radical -OH.
[0055] `Oxo' refers to the radical =0.
[0056] 'Substituted' refers to a group in which one or more hydrogen atoms
are each independently
replaced with the same or different substituent(s).
[0057] Sulfo' or `sulfonic acid' refers to a radical such as ¨S03H.
[0058] `Thior refers to the group -SH.
[0059] As used herein, term 'substituted with one or more' refers to one to
four substituents. In one
embodiment it refers to one to three substituents. In further embodiments it
refers to one or two
substituents. In a yet further embodiment it refers to one substituent.
[0060] `Thioalkoxy' refers to the group ¨SR26 where R26 is alkyl with the
number of carbon atoms
specified. Particular thioalkoxy groups are thiomethoxy, thioethoxy, n-
thiopropoxy, isothiopropoxy, n-
thiobutoxy, tert-thiobutoxy, sec-thiobutoxy, n-thiopentoxy, n-thiohexoxy, and
1,2-dimethylthiobutoxy.
More particular thioalkoxy groups are lower thioalkoxy, i.e. with between 1
and 6 carbon atoms. Further
particular alkoxy groups have between 1 and 4 carbon atoms.
14
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[0061] One having ordinary skill in the art of organic synthesis will
recognize that the maximum
number of heteroatoms in a stable, chemically feasible heterocyclic ring,
whether it is aromatic or non
aromatic, is determined by the size of the ring, the degree of unsaturation
and the valence of the
heteroatoms. In general, a heterocyclic ring may have one to four heteroatoms
so long as the
heteroaromatic ring is chemically feasible and stable.
[0062] 'Pharmaceutically acceptable' means approved or approvable by a
regulatory agency of the
Federal or a state government or the corresponding agency in countries other
than the United States, or
that is listed in the U.S. Pharmacopoeia or other generally recognized
pharmacopoeia for use in animals,
and more particularly, in humans.
[0063] 'Pharmaceutically acceptable salt' refers to a salt of a compound of
the invention that is
pharmaceutically acceptable and that possesses the desired pharmacological
activity of the parent
compound. In particular, such salts are non-toxic may be inorganic or organic
acid addition salts and base
addition salts. Specifically, such salts include: (1) acid addition salts,
formed with inorganic acids such as
hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric
acid, and the like; or formed
with organic acids such as acetic acid, propionic acid, hexanoic acid,
cyclopentanepropionic acid, glycolic
acid, pyruvic acid, lactic acid, malonic acid, succinic acid, malic acid,
maleic acid, fumaric acid, tartaric
acid, citric acid, benzoic acid, 3-(4-hydroxybenzoyl) benzoic acid, cinnamic
acid, mandelic acid,
methanesulfonic acid, ethanesulfonic acid, 1,2-ethane-disulfonic acid, 2-
hydroxyethanesulfonic acid,
benzenesulfonic acid, 4-chlorobenzenesulfonic acid, 2-naphthalenesulfonic
acid, 4-toluenesulfonic acid,
camphorsulfonic acid, 4-methylbicyclo[2.2.2]-oct-2-ene-l-carboxylic acid,
glucoheptonic acid, 3-
phenylpropionic acid, trimethylacctic acid, tertiary butylacctic acid, lauryl
sulfuric acid, gluconic acid,
glutamic acid, hydroxynaphthoic acid, salicylic acid, stearic acid, muconic
acid, and the like; or (2) salts
formed when an acidic proton present in the parent compound either is replaced
by a metal ion, e.g. an
alkali metal ion, an alkaline earth ion, or an aluminum ion; or coordinates
with an organic base such as
ethanolamine, diethanolamine, triethanolamine, N-methylglucamine and the like.
Salts further include,
by way of example only, sodium, potassium, calcium, magnesium, ammonium,
tetraalkylammonium, and
the like; and when the compound contains a basic functionality, salts of non
toxic organic or inorganic
acids, such as hydrochloride, hydrobromide, tartrate, mesylate, acetate,
maleate, oxalate and the like. The
term 'pharmaceutically acceptable cation' refers to an acceptable cationic
counter-ion of an acidic
functional group. Such cations are exemplified by sodium, potassium, calcium,
magnesium, ammonium,
tetraalkylammonium cations, and the like.
[0064] 'Pharmaceutically acceptable vehicle' refers to a diluent, adjuvant,
excipient or carrier with
which a compound of the invention is administered.
[0065] Trodrugs' refers to compounds, including derivatives of the
compounds of the
invention,which have cleavable groups and become by solvolysis or under
physiological conditions the
compounds of the invention which are pharmaceutically active in vivo. Such
examples include, but are
not limited to, cholinc ester derivatives and the like, N-alkylmorpholinc
esters and the like.
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[0066] 'Solvate' refers to forms of the compound that are associated with a
solvent, usually by a
solvolysis reaction. This physical association includes hydrogen bonding.
Conventional solvents include
water, ethanol, acetic acid and the like. The compounds of the invention may
be prepared e.g. in
crystalline form and may be solvated or hydrated. Suitable solvates include
pharmaceutically acceptable
solvates, such as hydrates, and further include both stoichiometric solvates
and non-stoichiometric
solvates. In certain instances the solvate will be capable of isolation, for
example when one or more
solvent molecules are incorporated in the crystal lattice of the crystalline
solid. 'Solvate' encompasses
both solution-phase and isolable solvates. Representative solvates include
hydrates, ethanolates and
methanolates.
[0067] 'Subject' includes humans. The terms 'human', 'patient' and
'subject' are used
interchangeably herein.
[0068] 'Effective amount' means the amount of a compound of the invention
that, when administered
to a subject for treating a disease, is sufficient to effect such treatment
for the disease. The 'effective
amount' can vary depending on the compound, the disease and its severity, and
the age, weight, etc., of
the subject to be treated.
[0069] 'Preventing' or 'prevention' refers to a reduction in risk of
acquiring or developing a disease
or disorder (i.e. causing at least one of the clinical symptoms of the disease
not to develop in a subject
that may be exposed to a disease-causing agent, or predisposed to the disease
in advance of disease
onset).
[0070] The term 'prophylaxis' is related to 'prevention', and refers to a
measure or procedure the
purpose of which is to prevent, rather than to treat or cure a disease. Non-
limiting examples of
prophylactic measures may include the administration of vaccines; the
administration of low molecular
weight heparin to hospital patients at risk for thrombosis due, for example,
to immobilization; and the
administration of an anti-malarial agent such as chloroquine, in advance of a
visit to a geographical region
where malaria is endemic or the risk of contracting malaria is high.
[0071] 'Treating' or 'treatment' of any disease or disorder refers, in one
embodiment, to ameliorating
the disease or disorder (i.e. arresting the disease or reducing the
manifestation, extent or severity of at
least one of the clinical symptoms thereof). In another embodiment 'treating'
or 'treatment' refers to
ameliorating at least one physical parameter, which may not be discernible by
the subject. In yet another
embodiment, 'treating' or 'treatment' refers to modulating the disease or
disorder, either physically (e.g.
stabilization of a discernible symptom), physiologically (e.g. stabilization
of a physical parameter), or
both. In a further embodiment, 'treating' or 'treatment' relates to slowing
the progression of the disease.
[0072] As used herein the term 'fibrotic diseases' refers to diseases
characterized by excessive
scarring due to excessive production, deposition, and contraction of
extracellular matrix, and are that are
associated with the abnormal accumulation of cells and/or fibronectin and/or
collagen and/or increased
fibroblast recruitment and include but are not limited to fibrosis of
individual organs or tissues such as the
heart, kidney, liver, joints, lung, pleural tissue, peritoneal tissue, skin,
cornea, retina, musculoskeletal and
digestive tract. In particular, the term fibrotic diseases refers to
idiopathic pulmonary fibrosis (IPF);
16
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
cystic fibrosis, other diffuse parenchymal lung diseases of different
etiologies including iatrogenic drug-
induced fibrosis, occupational and/or environmental induced fibrosis,
granulomatous diseases
(sarcoidosis, hypersensitivity pneumonia), collagen vascular disease, alveolar
proteinosis, langerhans cell
granulomatosis, lymphangioleiomyomatosis, inherited diseases (Hermansky-Pudlak
Syndrome, tuberous
sclerosis, neurofibromatosis, metabolic storage disorders, familial
interstitial lung disease); radiation
induced fibrosis; chronic obstructive pulmonary disease (COPD); scleroderma;
bleomycin induced
pulmonary fibrosis; chronic asthma; silicosis; asbestos induced pulmonary
fibrosis; acute respiratory
distress syndrome (ARDS); kidney fibrosis; tubulointerstitium fibrosis;
glomerular nephritis; focal
segmental glomerular sclerosis; IgA nephropathy; hypertension; Alport; gut
fibrosis; liver fibrosis;
cirrhosis; alcohol induced liver fibrosis; toxic/drug induced liver fibrosis;
hemochromatosis; nonalcoholic
steatohepatitis (NASH); biliary duct injury; primary biliary cirrhosis;
infection induced liver fibrosis;
viral induced liver fibrosis; and autoimmune hepatitis; corneal scarring;
hypertrophic scarring; Dupuytren
disease, kcloids, cutaneous fibrosis; cutaneous scleroderma; systemic
sclerosis, spinal cord injury/fibrosis;
myelofibrosis; vascular restenosis; atherosclerosis; arteriosclerosis;
Wegener's granulomatosis; Peyronie's
disease, or chronic lymphocytic. More particularly, the term 'fibrotic
diseases' refers to idiopathic
pulmonary fibrosis (1PF).
[0073] As used herein the term 'proliferative disease(s)' refers to
conditions such as cancer (e.g.
uterine leiomyosarcoma or prostate cancer), myeloproliferafive disorders (e.g.
polycythemia Vera,
essential thrombocytosis and myelofibrosis), leukemia (e.g. acute myeloid
leukaemia, acute and chronic
lymphoblastic leukemia), multiple myeloma, psoriasis, restenosis, scleroderma
or fibrosis. In particular
the term refers to cancer, leukemia, multiple myeloma and psoriasis.
[0074] As used herein, the term 'cancer' refers to a malignant or benign
growth of cells in skin or in
body organs, for example but without limitation, breast, prostate, lung,
kidney, pancreas, stomach or
bowel. A cancer tends to infiltrate into adjacent tissue and spread
(metastasise) to distant organs, for
example to bone, liver, lung or the brain. As used herein the term cancer
includes both metastatic tumour
cell types (such as but not limited to, melanoma, lymphoma, leukaemia,
fibrosarcoma,
rhabdomyosarcoma, and mastocytoma) and types of tissue carcinoma (such as but
not limited to,
colorectal cancer, prostate cancer, small cell lung cancer and non-small cell
lung cancer, breast cancer,
pancreatic cancer, bladder cancer, renal cancer, gastric cancer, glioblastoma,
primary liver cancer, ovarian
cancer, prostate cancer and uterine leiomyosarcoma). In particular, the term
"cancer' refers to acute
lymphoblastic leukemia, acute myeloidleukemia, adrenocortical carcinoma, anal
cancer, appendix cancer,
astrocytomas, atypical teratoid/rhabdoid tumor, basal cell carcinoma, bile
duct cancer, bladder cancer,
bone cancer (osteosarcoma and malignant fibrous histiocytoma), brain stem
glioma, brain tumors, brain
and spinal cord tumors, breast cancer, bronchial tumors, Burkitt lymphoma,
cervical cancer, chronic
lymphocytic leukemia, chronic myelogenous leukemia, colon cancer, colorectal
cancer,
craniopharyngioma, cutaneous T-Cell lymphoma, embryonal tumors, endometrial
cancer,
ependymoblastoma, epcndymoma, esophageal cancer, ewing sarcoma family of
tumors, eye cancer,
retinoblastoma, gallbladder cancer, gastric (stomach) cancer, gastrointestinal
carcinoid tumor,
17
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
gastrointestinal stromal tumor (GIST), gastrointestinal stromal cell tumor,
germ cell tumor, glioma, hairy
cell leukemia, head and neck cancer, hepatocellular (liver) cancer, hodgkin
lymphoma, hypopharyngeal
cancer, intraocular melanoma, islet cell tumors (endocrine pancreas), Kaposi
sarcoma, kidney cancer,
Langerhans cell histiocytosis, laryngeal cancer, leukemia, Acute lymphoblastic
leukemia, acute myeloid
leukemia, chronic lymphocytic leukemia, chronic myclogcnous leukemia, hairy
cell leukemia, liver
cancer, non-small cell lung cancer, small cell lung cancer, Burkitt lymphoma,
cutaneous T-celllymphoma,
Hodgkin lymphoma, non-Hodgkin lymphoma, lymphoma, Waldenstrom
macroglobulinemia,
medulloblastoma, medulloepithelioma, melanoma, mesothelioma, mouth cancer,
chronic myelogenous
leukemia, myeloid leukemia, multiple myeloma, asopharyngeal cancer,
neuroblastoma, non-small cell
lung cancer, oral cancer, oropharyngeal cancer, osteosarcoma, malignant
fibrous histiocytoma of bone,
ovarian cancer, ovarian epithelial cancer, ovarian germ cell tumor, ovarian
low malignant potential tumor,
pancreatic cancer, papillomatosis, parathyroid cancer, penile cancer,
pharyngeal cancer, pineal
parenchymal tumors of intermediate differentiation, pincoblastoma and
supratentorial primitive
neuroectodermal tumors, pituitary tumor, plasma cell neoplasm/multiple
myeloma, pleuropulmonary
blastoma, primary central nervous system lymphoma, prostate cancer, rectal
cancer, renal cell (kidney)
cancer, retinoblastoma, rhabdomyosarcoma, salivary gland cancer, sarcoma,
Ewing sarcoma family of
tumors, sarcoma, kaposi, Sezary syndrome, skin cancer, small cell Lung cancer,
small intestine cancer,
soft tissue sarcoma, squamous cell carcinoma, stomach (gastric) cancer,
supratentorial primitive
neuroectodermal tumors, T -cell lymphoma, testicular cancer, throat cancer,
thymoma and thymic
carcinoma, thyroid cancer, urethral cancer, uterine cancer, uterine sarcoma,
vaginal cancer, vulvar cancer,
Waldenstrom macroglobulincmia, and Wilms tumor
[0075] As used herein the term 'leukemia' refers to neoplastic diseases of
the blood and blood
forming organs. Such diseases can cause bone marrow and immune system
dysfunction, which renders
the host highly susceptible to infection and bleeding. In particular the term
leukemia refers to acute
myeloid leukaemia (AML), and acute lymphoblastic leukemia (ALL) and chronic
lymphoblastic
leukaemia (CLL).
[0076] As used herein the term 'inflammatory diseases' refers to the group
of conditions including,
rheumatoid arthritis, osteoarthritis, juvenile idiopathic arthritis,
psoriasis, psoriatic arthritis, allergic
airway disease (e.g. asthma, rhinitis), chronic obstructive pulmonary disease
(COPD), inflammatory
bowel diseases (e.g. Crohn's disease, ulcerative colitis), endotoxin-driven
disease states (e.g.
complications after bypass surgery or chronic endotoxin states contributing to
e.g. chronic cardiac
failure), and related diseases involving cartilage, such as that of the
joints. Particularly the term refers to
rheumatoid arthritis, osteoarthritis, allergic airway disease (e.g. asthma),
chronic obstructive pulmonary
disease (COPD) and inflammatory bowel diseases (e.g. Crohn's disease and
ulcerative colitis). More
particularly the term refers to rheumatoid arthritis, and chronic obstructive
pulmonary disease (COPD).
[0077] As used herein the term `autoimmune disease(s)' refers to the group
of diseases including
obstructive airways disease, including conditions such as COPD, asthma (e.g
intrinsic asthma, extrinsic
asthma, dust asthma, infantile asthma) particularly chronic or inveterate
asthma (for example late asthma
18
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
and airway hyperreponsiveness), bronchitis, including bronchial asthma,
systemic lupus erythematosus
(SLE), cutaneous lupus erythrematosis, lupus nephritis, dermatomyositis,
Sjogren's syndrome, multiple
sclerosis, psoriasis, dry eye disease, type I diabetes mellitus and
complications associated therewith,
atopic eczema (atopic dermatitis), thyroiditis (Hashimoto's and autoimmune
thyroiditis), contact
dermatitis and further eczematous dermatitis, inflammatory bowel disease (e.g.
Crohn's disease and
ulcerative colitis), atherosclerosis and amyotrophic lateral sclerosis.
Particularly the term refers to
COPD, asthma, systemic lupus erythematosis, type I diabetes mellitus and
inflammatory bowel disease.
[0078] As used herein , the term 'respiratory disease' refers to diseases
affecting the organs that are
involved in breathing, such as the nose, throat, larynx, eustachian tubes,
trachea, bronchi, lungs, related
muscles (e.g., diaphram and intercostals), and nerves. In particular, examples
of respiratory diseases
include asthma, adult respiratory distress syndrome and allergic (extrinsic)
asthma, non-allergic (intrinsic)
asthma, acute severe asthma, chronic asthma, clinical asthma, nocturnal
asthma, allerGen-induced
asthma, aspirin-sensitive asthma, exercise-induced asthma, isocapnic
hyperventilation, child onset
asthma, adult-onset asthma, cough-variant asthma, occupational asthma, steroid-
resistant asthma,
seasonal asthma, seasonal allergic rhinitis, perennial allergic rhinitis,
chronic obstructive pulmonary
disease, including chronic bronchitis or emphysema, pulmonary hypertension,
interstitial lung fibrosis
and/or airway inflammation, cystic fibrosis, and hypoxia.
[0079] As used herein the term 'allergy' refers to the group of conditions
characterized by a
hypersensitivity disorder of the immune system including, allergic airway
disease (e.g. asthma, rhinitis),
sinusitis, eczema and hives, as well as food allergies or allergies to insect
venom.
[0080] As used herein the term 'asthma' as used herein refers to any
disorder of the lungs
characterized by variations in pulmonary gas flow associated with airway
constriction of whatever cause
(intrinsic, extrinsic, or both; allergic or non-allergic). The term asthma may
be used with one or more
adjectives to indicate the cause.
[0081] As used herein the term 'cardiovascular disease' refers to diseases
affecting the heart or blood
vessels or both. In particular, cardiovascular disease includes arrhythmia
(atrial or ventricular or both);
atherosclerosis and its sequelae; angina; cardiac rhythm disturbances;
myocardial ischemia; myocardial
infarction; cardiac or vascular aneurysm; vasculitis, stroke; peripheral
obstructive arteriopathy of a limb,
an organ, or a tissue; reperfusion injury following ischemia of the brain,
heart, kidney or other organ or
tissue; endotoxic, surgical, or traumatic shock; hypertension, valvular heart
disease, heart failure,
abnormal blood pressure, vasoconstriction (including that associated with
migraines); vascular
abnormality, inflammation, insufficiency limited to a single organ or tissue.
[0082] As used herein the term `neurodegenerative diseases' refers to
disorders that are associated
with atrophy of the affected central or peripheral structures of the nervous
system. In particular, the term
`neurodegenerative diseases' refers to diseases such as Alzheimer's disease
and other dementias,
degenerative nerve diseases, encephalitis, epilepsy, genetic brain disorders,
head and brain
malformations, hydrocephalus, stroke, Parkinson's disease, multiple sclerosis,
amyotrophic lateral
sclerosis (ALS or Lou Gehrig's Disease), Huntington's disease, and prion
diseases.
19
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[0083] As used herein the term 'dermatological disorder' refers to a skin
disorder. In particular,
dermatological disorders include proliferative or inflammatory disorders of
the skin such as, atopic
dermatitis, bullous disorders, collagenoses, psoriasis, psoriatic lesions,
dermatitis, contact dermatitis,
eczema, pruritus, urticaria, rosacea, scleroderma, wound healing, scarring,
hypertrophic scarring, keloids,
Kawasaki Disease, rosacca, Sjogren-Larsso Syndrome, or urticaria.
[0084] As used herein the term 'abnormal angiogenesis associated disease'
refers to diseases caused
by the dysregulation of the processes mediating angiogenesis. In particular,
abnormal angiogenesis
associated disease refers to atherosclerosis, hypertension, tumor growth,
inflammation, rheumatoid
arthritis, wet-form macular degeneration, choroidal neovascularization,
retinal neovascularization, and
diabetic retinopathy.
[0085] `Compound(s) of the invention', and equivalent expressions, are
meant to embrace compounds
of the Formula(e) as herein described, which expression includes the
pharmaceutically acceptable salts,
and the solvates, e.g. hydrates, and the solvates of the pharmaceutically
acceptable salts where the context
so permits. Similarly, reference to intermediates, whether or not they
themselves are claimed, is meant to
embrace their salts, and solvates, where the context so permits.
[0086] When ranges are referred to herein, for example but without
limitation, C1-8 alkyl, the citation
of a range should be considered a representation of each member of said range.
[0087] Other derivatives of the compounds of this invention have activity
in both their acid and acid
derivative forms, but in the acid sensitive form often offers advantages of
solubility, tissue compatibility,
or delayed release in the mammalian organism (Bundgard, H, 1985). Prodrugs
include acid derivatives
well know to practitioners of the art, such as, for example, esters prepared
by reaction of the parent acid
with a suitable alcohol, or amides prepared by reaction of the parent acid
compound with a substituted or
unsubstituted amine, or acid anhydrides, or mixed anhydrides. Simple aliphatic
or aromatic esters,
amides and anhydrides derived from acidic groups pendant on the compounds of
this invention are
particularly useful prodrugs. In some cases it is desirable to prepare double
ester type prodrugs such as
(acyloxy)alkyl esters or ((alkoxycarbonyl)oxy)alkylesters. Particular such
prodrugs are the C1-8 alkyl, C2-
8 alkenyl, C6-10 optionally substituted aryl, and (C6-10 aryl)-(C14 alkyl)
esters of the compounds of the
invention.
[0088] As used herein, the term 'isotopic variant' refers to a compound
that contains unnatural
proportions of isotopes at one or more of the atoms that constitute such
compound. For example, an
'isotopic variant' of a compound can contain one or more non-radioactive
isotopes, such as for example,
deuterium (2H or D), carbon-13 (13C), nitroGen-15 (15N), or the like. It will
be understood that, in a
compound where such isotopic substitution is made, the following atoms, where
present, may vary, so
that for example, any hydrogen may be 2H/D, any carbon may be 13C, or any
nitrogen may be 15N, and
that the presence and placement of such atoms may be determined within the
skill of the art. Likewise,
the invention may include the preparation of isotopic variants with
radioisotopes, in the instance for
example, where the resulting compounds may be used for drug and/or substrate
tissue distribution studies.
The radioactive isotopes tritium, i.e. 3H, and carbon-14, i.e. c are
particularly useful for this purpose in
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
view of their ease of incorporation and ready means of detection. Further,
compounds may be prepared
that are substituted with positron emitting isotopes, such as 11C, '8F, 150
and 13N, and would be useful in
Positron Emission Topography (PET) studies for examining substrate receptor
occupancy.
[0089] All isotopic variants of the compounds provided herein, radioactive
or not, are intended to be
encompassed within the scope of the invention.
[0090] It is also to be understood that compounds that have the same
molecular formula but differ in
the nature or sequence of bonding of their atoms or the arrangement of their
atoms in space are termed
'isomers'. Isomers that differ in the arrangement of their atoms in space are
termed `stereoisomers'.
[0091] Stereoisomers that are not mirror images of one another are termed
`diastereomers' and those
that are non-superimposable mirror images of each other are termed
`enantiomers'. When a compound
has an asymmetric center, for example, it is bonded to four different groups,
a pair of enantiomers is
possible. An enantiomer can be characterized by the absolute configuration of
its asymmetric center and
is described by the R- and S-sequencing rules of Cahn and Prelog, or by the
manner in which the
molecule rotates the plane of polarized light and designated as dextrorotatory
or levorotatory (i.e. as (+)
or (-)-isomers respectively). A chiral compound can exist as either individual
enantiomer or as a mixture
thereof A mixture containing equal proportions of the enantiomers is called a
`racemic mixture'.
[0092] `Tautomers' refer to compounds that are interchangeable forms of a
particular compound
structure, and that vary in the displacement of hydrogen atoms and electrons.
Thus, two structures may
be in equilibrium through the movement of 7L electrons and an atom (usually
H). For example, enols and
ketones are tautomers because they are rapidly interconverted by treatment
with either acid or base.
Another example of tautomerism is the aci- and nitro- forms of
phenylnitromethane, that are likewise
formed by treatment with acid or base.
[0093] Tautomeric forms may be relevant to the attainment of the optimal
chemical reactivity and
biological activity of a compound of interest.
[0094] The compounds of the invention may possess one or more asymmetric
centers; such
compounds can therefore be produced as individual (R)- or (S)- stereoisomers
or as mixtures thereof
[0095] Unless indicated otherwise, the description or naming of a
particular compound in the
specification and claims is intended to include both individual enantiomers
and mixtures, racemic or
otherwise, thereof The methods for the determination of stereochemistry and
the separation of
stereoisomers are well-known in the art.
[0096] It will be appreciated that compounds of the invention may be
metabolized to yield
biologically active metabolites.
THE INVENTION
[0097] The present invention is based on the identification of novel
compounds, and their ability to
act as inhibitors of autotaxin and that they may be useful for the treatment
of fibrotic diseases,
proliferative diseases, inflammatory diseases, autoimmune diseases,
respiratory diseases, cardiovascular
21
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
diseases, neurodegenerative diseases, dermatological disorders, and/or
abnormal angiogenesis associated
diseases.
[0098] The present invention also provides methods for the production of
these compounds,
pharmaceutical compositions comprising these compounds and methods of
treatment for fibrotic diseases,
proliferative diseases, inflammatory diseases, autoimmunc diseases,
respiratory diseases, cardiovascular
diseases, neurodegenerative diseases, dermatological disorders, and/or
abnormal angiogenesis associated
diseases by administering the compounds of the invention.
[0099] Accordingly, in a first aspect of the invention, the compounds of
the invention are provided
having a Formula (I):
Rlb
Rla
X \
(R7 )a
R8 N X-W
R6b
R6a
wherein
Rla is H, halo or C1,4 alkyl;
Rib is:
- halo,
- C1_4 alkyl (which alkyl is optionally substituted with one or more
independently selected
halo), or
-CM alkoxy (which alkoxy is optionally substituted with one or more
independently selected
halo);
X is ¨S-, -0-, ¨N=CH-, ¨CH=N- or ¨CH=CH-;
W is N, or CR3
when W is N, R2 is:
- H,
- -CN,
- halo,
- C1.4 alkyl (which alkyl is optionally substituted with one or more
independently selected OH,
or CN),
- -C(=0)CH3,
- -C(=0)CF3,
- -C(=0)0CH3,
- -C(=0)NH2, or
- -NHC(=0)CH3, or
22
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
when W is CR3, one of re or R3 is:
- H,
- -CN,
- halo,
- C1_4 alkyl (which alkyl is optionally substituted with one or more
independently selected OH,
or CN)
- -C(=0)CH3,
- -C(=0)CF3,
- -C(=0)0CH3,
- -C(=0)NH2, or
- -NHC(=0)CH3,
and the other is H, or C1_4 alkyl;
R4 is Ci_4 alkyl;
R5 is C1-4 alkyl optionally substituted with one or more independently
selected CN, OH, halo, or -
C(=0)NH2;
one of R6a or Ra is selected from H, -CH3, and halo, and the other is H;
Cy is:
- C4_10 cycloalkyl,
- 4-10 membered mono or bicyclic heterocycloalkyl containing one or more
heteroatoms
independently selected from 0, N, and S, or
- 4-7 membered hetcrocycloalkenyl containing 1 double bond, containing one
or more
heteroatoms independently selected from 0, N, and S;
each R7 is independently selected from:
- OH,
- oxo,
- halo, and
- C1_4 alkyl (which alkyl is optionally substituted with one or more
independently selected OH,
or C1_4 alkoxy);
the subscript a is 0, 1 or 2;
Rg is -(1-1-W1)m-L2-G1,
wherein
- L1 is absent, or is -0-, -C(=0)-, -NR', -NRhC(=0)-, or -SO2-;
- WI is Ci_4 alkylene;
- the subscript in is 0, or 1;
- L2 is absent, or is -0-, -C(=0)-, -C(=0)0-, -0C(=0) -C(=0)-C(=0)-, -C(=0)-
C(=0)NRa-,
-NRb-, -C(=0)NRe-, -NR6C(=0)-, -NRjC(=0)0-, -S02-, -S02NRe- or ¨NRfS02-;
- Gis
0 H,
23
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
o -CN,
o C1_4 alkyl (which alkyl is optionally substituted with one or more
independently
selected ¨CN, OH, halo or phenyl),
o C3_7 cycloalkyl (which cycloalkyl is optionally substituted with ¨NH,),
o 5-6 membered heterocycloalkenyl containing 1 double bond containing one
or more
heteroatoms independently selected from 0, N, and S (which heterocycloalkenyl
is
optionally substituted with one or more independently selected R9 groups),
o 4-10 membered mono, bi or spirocyclic heterocycloalkyl containing one or
more
heteroatoms independently selected from 0, N, and S (which heterocycloalkyl is
optionally substituted with one or more independently selected R9 groups), or
o 5-6 membered heteroaryl containing one or more heteroatoms independently
selected
from 0, N, and S (which heteroaryl is optionally substituted with one or more
independently selected RI groups),
each R9 is oxo, or Rio;
each Rio is:
- -OH,
- halo,
- -CN,
- C1_4 alkyl (which alkyl is optionally substituted with one or more
independently selected OH,
halo, or phenyl),
- C1-4 alkoxy,
- C3_7 cycloalkyl,
- phenyl,
- -S02CH3,
- -C(=0)C 1_4 alkoxy,
- -C(=0)CI4 alkyl, or
- -NRgC(=0)C1.4 alkyl; and
each Ra, Rh, Re, Rd, Re, Rf, Rg, Rh, R',and 12 is independently selected from
H and C _4 alkyl.
[00100] In one embodiment, a compound of the invention is according to Formula
I, wherein Ria is H.
[00101] In one embodiment, a compound of the invention is according to Formula
I, wherein Ria is
halo. In a particular embodiment, Ria is F, Cl, or Br. In a more particular
embodiment, Ria is F, or Cl. In
a most particular embodiment, Rth is F.
[00102] In one embodiment, a compound of the invention is according to Formula
I, wherein R th is C
4 alkyl. In a particular embodiment, RI' is -CH3, -C1-12-CH3,
¨CH(CH3)2. In a more
particular embodiment, Ria is -CH3, or -CH2-CH3.
[00103] In one embodiment, a compound of the invention is according to Formula
I, wherein Rib is
halo. In a particular embodiment, Rib is F, Cl, or Br. In a more particular
embodiment, Rth is F, or Cl. In
a most particular embodiment, Rib is F.
24
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00104] In one embodiment, a compound of the invention is according to Formula
I, wherein Rib is CI_
4 alkyl. In a particular embodiment, Rib is -CH3, -0-12-CH3, -CF13-0-12-CH3, -
CH(CH3)2. In a more
particular embodiment, Rib is -CH3, or -CH2-CH3
[00105] In one embodiment, a compound of the invention is according to Formula
I, wherein Rib is CI_
4 alkyl substituted with one or more independently selected halo. In a
particular embodiment, Rib is -CF3,
or -CH2-CF3. In a more particular embodiment, Rib is -CF3.
[00106] Tn one embodiment, a compound of the invention is according to Formula
T, wherein Rib is CI
4 alkoxy. In a particular embodiment, Rib is -OCH3, -OCH2-CE3, -OCH2-CH2-CH3, -
OCH(CH3)2. In a
more particular embodiment, Rib is -OCH3, or -0C1-17-CH3 In a most particular
embodiment, Rib is -
OCH3
[00107] In one embodiment, a compound of the invention is according to Formula
I, wherein Rib is CI_
4 alkoxy substituted with one or more independently selected halo. In a more
particular embodiment, Rib
is -0CF3, -OCH?-CHF, or -OCH2-CF3 In a most particular embodiment, Rib is -
0CF3
[00108] In one embodiment, a compound of the invention is according to Formula
I, wherein X is -S-,
-0-, -N=CH-, -CH=N- or -CH=CH-. In a particular embodiment, X is -S-, or -0-.
In another particular
embodiment, X is -N=CH-.
[00109] In one embodiment, a compound of the invention is according to Formula
I, wherein W is N,
and R2 is as previously defined. In a particular embodiment, R2 is H, -CN, -
C(=0)CH3, -C(=0)CF3, -
C(=0)0CH3, -C(=0)NH2, or -NHC(=0)CH3. In a more particular embodiment, R2 is -
CN.
[00110] In one embodiment, a compound of the invention is according to Formula
I, wherein W is N,
and R2 is as previously defined. In a particular embodiment, R2 is halo. In a
more particular
embodiment, R2 is F, Cl, or Br. In a most particular embodiment, R2 is F, or
Cl.
[00111] In one embodiment, a compound of the invention is according to Formula
T, wherein W is N,
and R2 is as previously defined. In a particular embodiment, R2 is C14 alkyl.
In another particular
embodiment, R2 is Ci_4 alkyl substituted with one or more independently
selected OH, and CN. In yet
another particular embodiment, R2 is CI 4 alkyl substituted with one OH, or
CN. In a more particular
embodiment, R2 is -CH3, -CH2-CH3, -CH2-0H, or -CH2-CN. In a most particular
embodiment, R2 is -CI-12-
OH, or -CH2-CN.
[00112] In another embodiment, a compound of the invention is according to
Formula I, wherein W is
CR3, and R2 and R3 are as previously defined. In a particular embodiment, R2
is H, -CN, -C(=0)CH3,
-C(=0)CF3, -C(=0)0CH3, -C(=0)NH2, or -NHC(=0)CH3, and R3 is H, or C1_4 alkyl.
In another
particular embodiment, R2 is H, or C1_4 alkyl, and R3 is H, -CN, -C(0)CH3, -
C(0)CF3, -C(0)OCH3,
-C(=0)NH2, or -NHC(=0)CH3. In a more particular embodiment, R2 is H, -CN, -
C(=0)CH3, -C(=0)CF3,
-C(=0)0CH3, -C(=0)NI-L, or -NHC(=0)CH3, and R3 is H, -CH3, or -CH2-CH3. In
another more
particular embodiment, R2 is H, -CH3, or -CH2-CH3, and R3 is H, -CN, -
C(z0)CH3, -C(r0)CF3,
-C(0)OCH3, -C(=0)NH2, or -NHC(=0)CH3. In a most particular embodiment, R2 is -
CN, and R3 is H,
-CH3, or -CH2-CI-3. In another most particular embodiment, R2 is H, -CH3, or -
CI-19-CH3, and R3 is -CN.
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00113] In another embodiment, a compound of the invention is according to
Formula I, wherein W is
CR3, and R2 and R3 are as previously defined. In a particular embodiment, R2
is halo, and R3 is H, or
C 1_4 alkyl. In another particular embodiment, R2 is H, or C1_4 alkyl, and R3
is halo. In a more particular
embodiment, R2 is F, Cl, or Br, and R3 is H, -CH3, or -CH2-CH3. In another
more particular embodiment,
R2 is H, -CH3, or -CH2-CH3, and R3 is F, Cl, or Br. In a most particular
embodiment, R2 is F, or Cl, and
123 is H, -CH3, or -CH2-CH3. In another most particular embodiment, R2 is H, -
CH3, or -CH2-CH3, and R3
is F, or Cl.
[00114] In another embodiment, a compound of the invention is according to
Formula I, wherein W is
CR3, and R2 and Rg are as previously defined. In a particular embodiment, R2
is C1_4 alkyl, and Rg is H, or
C14 alkyl. In another particular embodiment, R2 is H, or C1_4 alkyl, and R3 is
C14 alkyl. In a more
particular embodiment, R2 is -CH3, or -CH2-CH3, and R3 is H, -CH3, or -CH2-
CH3. In another more
particular embodiment, R2 is H, -CH3, or -CH2-CH3, and R3 is -CH3, or -CH2-
CH3.
[00115] In another embodiment, a compound of the invention is according to
Formula I, wherein W is
CR3, and R2 and R3 are as previously defined. In a particular embodiment, R2
is C1_4 alkyl substituted
with OH, or CN, and R3 is H, or C14 alkyl. In another particular embodiment,
R2 is H, or C14 alkyl, and
R3 is C1_4 alkyl substituted with OH, or CN. In a more particular embodiment,
R2 is -CH2-0H, or
-CH2-CN, and Rg is H, -CH3, or -CH2-CH3. In another more particular
embodiment, R2 is H, -CH3, or
-CY2-CH3, and R3 is -Cfb-OH, or -CW-CN.
[00116] In one embodiment, a compound of the invention is according to Formula
II:
(R7)a
ON
R8
R-
R6b
R6a
I I
wherein the subscript a, R4, R5, ¨6a,
R R6b, R7 and Rg arc as described above.
[00117] In another embodiment, a compound of the invention is according to
Formula III:
(R7)a
R4, /1;1 \
R8 s CN
R-
R6b
R6III
wherein the subscript a, R4, R5, ¨6a,
K R6b, R7 and Rg are as described above.
26
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00118] In one embodiment, a compound of the invention is according to Formula
I, II or III, wherein
R4 is C1_4 alkyl. In a particular embodiment, R4 is -CH3, or -CH2-CH3. In a
more particular embodiment,
R4 is -CH3.
[00119] In one embodiment, a compound of the invention is according to Formula
I, II or III, wherein
R5 is C1_4 alkyl. In a particular embodiment, R5 is -CH3, -CH2-CH3 or -CH2-CH2-
C1-13. In a more
particular embodiment, R5 is -CH3, or -CH2-CH3. In a most particular
embodiment, R5 is -CH2-CH3.
[00120] In one embodiment, a compound of the invention is according to Formula
T, TT or ITT, wherein
R5 is C1_4 alkyl substituted with one or more independently selected CN, OH,
halo, and -C(=0)NH2. In a
particular embodiment, Rs is C1.4 alkyl substituted with one CN, OH, halo, or -
C(=0)NH2. In a more
particular embodiment, R5 is -CH3, -C112-CH3, -CW-CFL-CH3, -CH)-C1-12-CH)-CH3,
-CH(CH3)2, or
-CH2-CH(CH3)2, each of which is substituted with one CN, OH, halo, or -
C(=0)NH2. In another more
particular embodiment, R5 is -CH3, -CH2-CH3, -CI-12-CH2-CH3, -CF2-CH2-CH2-CH3,
or -CH2-CH(CH3)2,
each of which is substituted with one -CN, OH, F, or -C(=0)NH2. In a most
particular embodiment, R5 is
-CH2-CH2-CN, -CH2-CH2-0H, -CH2-CF3, or -CH2-CH2-C(=0)NH2.
[00121] In one embodiment, a compound of the invention is according to Formula
I, IT or TIT, wherein
Cy is C3_10 cyeloalkyl. In a particular embodiment, Cy is cyclobutyl,
cyclopentyl or cyclohexyl. In a
more particular embodiment, Cy is cyclohexyl.
[00122] In one embodiment, a compound of the invention is according to Formula
I, II or III, wherein
Cy is 4-10 membered mono or bicyclic heterocycloalkyl containing one or more
heteroatoms
independently selected from 0, N, and S. In a particular embodiment, Cy is
oxetanyl, azetidinyl,
tetrahydrofuranyl, pyrrolidinyl, tetrahydropyranyl, piperidinyl, piperazinyl,
morpholinyl, or
thiomorpholinyl. In a more particular embodiment, Cy is piperidinyl. In
another more particular
embodiment, Cy is piperazinyl.
[00123] In one embodiment, a compound of the invention is according to Formula
1, II or III, wherein
Cy is 4-7 membered heterocycloalkenyl containing 1 double bond, containing one
or more heteroatoms
independently selected from 0, N, and S. In a particular embodiment, Cy is
dihydrofuranyl,
dihydrothiazolyl, dihydrooxazolyl, dihydropyranyl, tetrahydropyridinyl, or
dihydrothiopyranyl. In a more
particular embodiment, Cy is dihydrooxazolyl.
[00124] In one embodiment, a compound of the invention is according to Formula
I, II or III, wherein
the subscript a is 1 or 2, and R7 is OH, oxo, or halo. In a particular
embodiment, R7 is OH, oxo, F, or Cl.
[00125] In one embodiment, a compound of the invention is according to Formula
T, TT or ITT, wherein
the subscript a is 1 or 2, and R7 is C1_4 alkyl. In a particular embodiment,
R7 is -CH3, -CH2-CH3, or
-CH(CH3)2. In a more particular embodiment, R7 is -CH3.
[00126] In one embodiment, a compound of the invention is according to Formula
I, II or III, wherein
the subscript a is 1 or 2, and R is C1_4 alkyl substituted with OH, or Ci_4
alkoxy. In a particular
embodiment, R7 is -CH3, -CH2-CH3, or -CH(CH3)2, each of which is substituted
with OH, or C1_4 alkoxy.
In a more particular embodiment, R7 is ¨CH2-0H, or ¨CH2-0C1-13.
27
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00127] In one embodiment, a compound of the invention is according to Formula
I, II or III, wherein
the subscript a is 0.
[00128] In one embodiment, a compound of the invention is according to Formula
IVa, IVb, IVc or
IVd:
\
L2 L2
CN N ON
N __
LN
N-4>\
R6b N R6b N
R6a R6a
IVa, IVb,
N
\ CN CN
L2 L2
NI¨ \S N NS
Nri?
R6b N R6b N
R6a R6a
ivc, or iVd
wherein R6a, R6h, LI, Wt, L2, Gt and the subscript m are as previously
described.
[00129] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein the
subscript m is 1, and Li is absent.
[00130] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein the
subscript m is 1, L1 is -NR'-, and R' is as previously described. In a
particular embodiment, le is H. In
another particular embodiment, R' is ¨CI-13, -CH2-CH3, or -CH(CH3)2.
[00131] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein the
subscript m is 1, Li is -NRhC(=0)-, and Rh is as previously described. In a
particular embodiment, Rh is
H. In another particular embodiment, Rh is ¨CH3, -CH2-CH3, or -CH(CH3)2.
[00132] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein the
subscript m is 1, and L1 is -C(=0)-, or -SO2-.
[00133] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein the
subscript m is 1, and WI is CI 4 alkylene. In a particular embodiment, WI is -
CH2-, -CH2-CH2-,
-C(CH3)H-, -CH2-CH2-CH2- or -CH2-C(CH3)H-. In a more particular embodiment, W1
is -CH2-, or
-C(CH3)H-.
[00134] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein the
subscript m is 0.
[00135] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein L2 is
absent.
28
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00136] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein L2 is
-0-.
[00137] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein L2 is
-0-, -C(=0)-, -C(=0)0-, -0C(=0) -C(=0)-C(=0)-, or -SO2-.
[00138] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein L2 is
-C(=0)-C(=0)N1V-, and Ra is as previously described. In a particular
embodiment, Re is H. In another
particular embodiment, Re is ¨CH3, -CH2-CH3, or -CH(CH3)2.
[00139] In one embodiment, a compound of the invention is according to Formula
l-lVd, wherein L2 is
-NRb-, and Rb is as previously described. In a particular embodiment, Rb is H.
In another particular
embodiment, Rb is ¨CH3, -0-12-CH3, or -CH(CH3)2.
[00140] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein L2 is
-C(=0)NRe-, and Re is as previously described. In a particular embodiment, Re
is H. In another particular
embodiment, Re is ¨CH3, -CH2-CH3, or -CH(CH3)2.
[00141] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein L2 is
-NRdC(=0)- , and Rd is as previously described. In a particular embodiment, Rd
is H. in another
particular embodiment, Rd is ¨CH3, -CH2-CH3, or -CH(CH3)2.
[00142] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein L2 is
-NRiC(=0)0-, and Ri is as previously described. In a particular embodiment, Ri
is H. In another
particular embodiment, Ri is ¨CH3, -CH2-CH3, or -CH(CH3)2.
[00143] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein L2 is
-SO2NRe-, and Re is as previously described. In a particular embodiment, Re is
H. In another particular
embodiment, Re is ¨CH3, -CH2-CH3, or -CH(CH3)2.
[00144] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein L2
is¨NRfS02-, and Rf is as previously described. In a particular embodiment, Rf
is H. In another particular
embodiment, le is ¨CH3, -CH2-CH3, or -CH(CH3)2.
[00145] In one embodiment, a compound of the invention is according to Formula
I-lVd, wherein G1 is
H, or CN.
[00146] In one embodiment, a compound of the invention is according to Formula
I-lVd, wherein G1 is
CIA alkyl. In a particular embodiment, G1 is -CH3, or ¨CH2-CH3.
[00147] In one embodiment, a compound of the invention is according to Formula
I-lVd, wherein G1 is
Ci alkyl substituted with ¨CN, OH, halo or phenyl. In a particular embodiment,
G1 is ¨CH3, -CH2-CH3,
or -CH(CH3)2, each of which is substituted with ¨CN, OH, halo or phenyl. In a
more particular
embodiment, G1 is -CF3, -CH2-C1, -CH2-CN, -CH2-0H or ¨CH2-Ph.
[00148] In one embodiment, a compound of the invention is according to Formula
I-lVd, wherein G1 is
C3_7 cycloalkyl. In a particular embodiment, G1 is cyclopropyl, cyclobutyl,
cyclopropyl, or cyclohexyl.
[00149] In one embodiment, a compound of the invention is according to Formula
I-lVd, wherein G1 is
cycloalkyl substituted with ¨NH2. In a particular embodiment, G1 is
cyclopropyl, cyclobutyl,
cyclopropyl, or cyclohexyl, each of which is substituted with ¨NFI2.
29
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00150] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein G1 is
5-6 membered heterocycloalkenyl containing 1 double bond, containing one to
three heteroatoms
independently selected from 0, N, and S. In a particular embodiment, G1 is
dihydrofuranyl,
dihydrothiazolyl, dihydrooxazolyl, dihydropyranyl, or dihydrothiopyranyl.
[00151] In one embodiment, a compound of the invention is according to Formula
I-1Vd, wherein G1 is
5-6 membered heterocycloalkenyl containing 1 double bond, containing one to
three heteroatoms
independently selected from 0, N, and S, substituted with one or more
independently selected R9, and R9
is as previously defined. In another embodiment, G1 is 5-6 membered
heterocycloalkenyl containing 1
double bond, containing one to three heteroatoms independently selected from
0, N, and S, substituted
with one or two independently selected R9, and R9 is as previously defined. In
a particular embodiment,
G1 is dihydrofuranyl, dihydrothiazolyl, dihydrooxazolyl, dihydropyranyl, or
dihydrothiopyranyl, each of
which is substituted with one or two independently selected R9, and R9 is as
previously defined.
[00152] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein G1 is
4-10 membered mono, bi or spirocyclic heterocycloalkyl containing one to three
heteroatoms
independently selected from 0, N, and S. In a particular embodiment, G1 is
oxetanyl, azetidinyl,
tetrahydrofuranyl, pyrolidinyl, tetrahydropyranyl, piperidinyl, piperazinyl,
morpholinyl, thiomorpholinyl,
or 2,6-diaza-spiro[3.3]heptane.
[00153] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein G1 is
4-10 membered mono, bi or spirocyclic heterocycloalkyl containing one to three
heteroatoms
independently selected from 0, N, and S, substituted with one or more
independently selected R9, and R9
is as previously defined. In another embodiment, G1 is 4-10 membered mono, bi
or spirocyclic
heterocycloalkyl containing one to three heteroatoms independently selected
from 0, N, and S,
substituted with one or two independently selected R9, and R9 is as previously
defined. In a particular
embodiment, G1 is oxetanyl, azetidinyl, tetrahydrofuranyl, pyrolidinyl,
tetrahydropyranyl, piperidinyl,
piperazinyl, morpholinyl, thiomorpholinyl, or 2,6-diaza-spiro[3.3]heptanes,
each of which is substituted
with one or two independently selected R9, and R9 is as previously defined.
[00154] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein R9 is
oxo.
[00155] In another embodiment, a compound of the invention is according to
Formula I-IVd, wherein
R9 is RI .
[00156] In one embodiment, a compound of the invention is according to Formula
T-TVd, wherein RI
is selected from OH, F, Cl, and -CN.
[00157] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein R'
is C1..4 alkyl. In a particular embodiment, RI is selected from -CH3, -CH2-
CH3, and -CH(CH3)2. In a
more particular embodiment, RI is selected from -CH3, and -CH2-CH3.
[00158] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein RI
is C1_4 alkyl substituted with one or more independently selected OH, halo,
phenyl. In a further
embodiment, RI is Ci_4 alkyl substituted with one to three independently
selected OH, halo, and phenyl.
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
In a more particular embodiment, Rm is -CH3, -CH2-CH3, and -CH(CH3)2, each of
which is substituted
with one to three independently selected OH, halo, and phenyl. In a most
particular embodiment, RI is -
CF3, -CH2-CH2-0H, and -CH2-phenyl.
[00159] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein le
is C14 alkoxy. In a particular embodiment, le is selected from -OCH3, -OCH2-
CH3, and -0C(CH3)3. In
a particular embodiment, RI is -OCH3.
[00160] Tn one embodiment, a compound of the invention is according to Formula
I-IVd, wherein RI
is selected from -S02CH3, -C(=0)C1.4 alkoxy, and -C(=0)C1_4 alkyl. In a
particular embodiment, RI is
selected from -S02CH3, -C(=0)0CH3, -C(=0)0CH2CH3, -C(=0)0CH(CH3)2, -C(=0)CH3, -
C(=0)CI-2CH3, and -C(=0)OCH(CH3)2. In a most particular embodiment, RI is
selected from -S02a13,
-C(=0)0CH3, and -C(=0)CH3.
[00161] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein le
is ¨NRgC(=0)C1_4 alkyl, and Rg is as described previously. In a particular
embodiment, RI is ¨
NRgC(=0)CH3, or ¨NRgC(=0)CH2CH3, and Rg is as described previously. In a more
particular
embodiment, RI is ¨NRgC(=0)CH3, or ¨NRgC(=0)CH2CH3, and Rg is H, -CH3, or -
CH2CH3. in a most
particular embodiment, RI is ¨NHC(=0)CH3, or ¨NHC(=0)CH2CH3.
[00162] In one embodiment, a compound of the invention is according to Formula
I-lVd, wherein G1 is
4-7 membered heterocycloalkyl containing one or more heteroatoms independently
selected selected from
0, N, and S, each of which is substituted with one or two independently
selected R9 groups, and R9 is
oxo. In a further particular embodiment, G1 is oxetanyl, azetidinyl,
tetrahydrofuranyl, pyrolidinyl,
tetrahydropyranyl, piperidinyl, piperazinyl, morpholinyl, thiomorpholinyl, or
2,6-diaza-spiro[3.3]heptane,
each of which is substituted with one or two independently selected R9 groups,
and R9 is oxo.
[00163] In one embodiment, a compound of the invention is according to Formula
I-1Vd, wherein G1 is
4-7 membered heterocycloalkyl containing one or more heteroatoms independently
selected from 0, N,
and S, each of which is substituted with one or two independently selected R9
groups, R9 is RI , and RI is
as previously described. In a further embodiment, G1 is oxetanyl, azetidinyl,
tetrahydrofuranyl,
pyrolidinyl, tetrahydropyranyl, piperidinyl, piperazinyl, morpholinyl,
thiomorpholinyl, or 2,6-diaza-
spiro[3.3]heptane, each of which is substituted with one or two independently
selected R9 groups, R9 is
R' , and RI is as previously described. In a particular embodiment, RI is
selected from OH, F, Cl, and -
CN.
[00164] Tn one embodiment, a compound of the invention is according to Formula
I-TVd, wherein G1 is
4-7 membered heterocycloalkyl containing one or more heteroatoms independently
selected from 0, N,
and S, each of which is substituted with one or two independently selected R9
groups, R9 is le, and le is
as previously described. In a further embodiment, G1 is oxetanyl, azetidinyl,
tetrahydrofuranyl,
pyrolidinyl, tetrahydropyranyl, piperidinyl, piperazinyl, morpholinyl,
thiomorpholinyl, or 2,6-diaza-
spiro[3.3]heptane, each of which is substituted with one or two independently
selected R9 groups, R9 is
Rw, and RI is as previously described. In a particular embodiment, RI is
selected C1-4 alkyl. In a more
31
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
particular embodiment, Rm is selected from -CH3, -CH2-CH3, and -CH(CH3)2. In a
most particular
embodiment, RI is selected from -CH3, and -Cf12-CH3.
[00165] In one embodiment, a compound of the invention is according to Formula
I-lVd, wherein G1 is
4-7 membered heterocycloalkyl containing one or more heteroatoms independently
selected from 0, N,
and S, each of which is substituted with one or two independently selected R9
groups, R9 is RI , and RI is
as previously described. In a further embodiment, G1 is oxetanyl, azetidinyl,
tetrahydrofuranyl,
pyrolidinyl, tetrahydropyranyl, piperidinyl, piperazinyl, morpholinyl,
thiomorpholinyl, or 2,6-D iaza-
spiro[3.3]heptane, each of which is substituted with one or two independently
selected R9 groups, R9 is
RI , and RI is as previously described. In a particular embodiment, RI is
C1_4 alkyl substituted with one
or more independently selected OH, halo, or phenyl. In a further embodiment,
RI is CIA alkyl
substituted with one to three independently selected OH, halo, or phenyl. In a
more particular
embodiment, RI is -CH3, -CH2-CH3, or -CH(CH3)2, each of which is substituted
with one to three
independently selected OH, halo, or phenyl. In a most particular embodiment,
RI is -CF3,
or -CH2-phenyl.
[00166] In one embodiment, a compound of the invention is according to Formula
I-lVd, wherein G1 is
4-7 membered heterocycloalkyl containing one or more heteroatoms independently
selected from 0, N,
and S, each of which is substituted with one or two independently selected R9
groups, R9 is R' , and le is
as previously described. In a further embodiment, G1 is oxetanyl, azetidinyl,
tetrahydrofuranyl,
pyrolidinyl, tetrahydropyranyl, piperidinyl, piperazinyl, morpholinyl,
thiomorpholinyl, or 2,6-diaza-
spiro[3.3]heptane, each of which is substituted with one or two independently
selected R9 groups, R9 is
RI , and RI is as previously described. In a particular embodiment, RI is C1-
4 alkoxy. In a more
particular embodiment, re is selected from -OCH3, -OCH2-CH3, and -0C(CH3)3. In
a most particular
embodiment, RI is -OCH3.
[00167] In one embodiment, a compound of the invention is according to Formula
I-IVd, wherein G1 is
4-7 membered heterocycloalkyl containing one or more heteroatoms independently
selected from 0, N,
and S, each of which is substituted with one or two independently selected R9
groups, R9 is RI , and RI is
as previously described. In a further embodiment, G1 is oxetanyl, azetidinyl,
tetrahydrofuranyl,
pyrolidinyl, tetrahydropyranyl, piperidinyl, piperazinyl, morpholinyl,
thiomorpholinyl, or 2,6-Diaza-
spiro[3.3]heptane, each of which is substituted with one or two independently
selected R9 groups, R9 is
RI , and RI is as previously described. In a particular embodiment, RI is
selected from -S02CH3,
-C(=0)C 1_4 alkoxy, and -C(=0)C 1_4 alkyl. In a more particular embodiment, RI
is selected from
-S02CH3, -C(=0)0CH3, -C(=0)0CH2CH3, -C(=0)0CH(CH3)2, -C(=0)CH3, -C(=0)CH2CH3,
and
-C(=0)0CH(CH3)2. In a most particular embodiment, le is selected from -S02CH3,
-C(=0)0CH3, and
-C(=0)CH3.
[00168] In one embodiment, a compound of the invention is according to Formula
I-lVd, wherein G1 is
4-7 membered heterocycloalkyl containing one or more heteroatoms independently
selected from 0, N,
and S, each of which is substituted with one or two independently selected R9
groups, R9 is RI , and RI is
as previously described. In a further embodiment, G1 is oxetanyl, azetidinyl,
tetrahydrofuranyl,
32
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
pyrolidinyl, tetrahydropyranyl, piperidinyl, piperazinyl, morpholinyl,
thiomorpholinyl, or 2,6-Diaza-
spiro[3.3]heptane, each of which is substituted with one or two independently
selected R9 groups, R9 is
RI , and RI is as previously described. In a particular embodiment, RI is
¨NR8C(=0)C1_4 alkyl, and Rg
is as described previously. In a particular embodiment, RI is ¨NRgC(=0)CH3,
or ¨NRgC(=0)CH2CH3,
and Rg is as described previously. In a more particular embodiment, RI is
¨NRgC(=0)CH3, or
¨NRgC(=0)CH2CH3, and Rg is H, -CH3, or -CH2CH3. In a most particular
embodiment, RI is
¨NHC(=0)CH3, or ¨NHC(=0)CH2CH3.
[00169] In one embodiment, a compound of the invention is according to Formula
Va, Vb, Vc, or Vd:
R\ R\
N ,
7¨W1 'N
WI N
N
Gi s CN G s CN
Reb R6b
Rea R6a
Va, Vb,
R\ R\
CN \ CN
Gi
N N
Rsa Rsa
Vc, or Vd
wherein R6a, R6b, W1, and G1 are as described above.
[00170] In one embodiment, a compound of the invention is according to Formula
Va-Vd, wherein Wi
is C1.4 alkylene. In a particular embodiment, W1 is -CH2-, -CH2-CH2-, -C(CH3)H-
, -CH2-CH2-CH2- or
-CH2-C(CH3)H-. In a more particular embodiment, Wi is -CH2-, or -C(CH3)H-. In
a most particular
embodiment, Wt is -CH2-.
33
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00171] In one embodiment, a compound of the invention is according to Formula
VIa, VIb, VIc, or
VId:
0 0
GiN\N....4\ I Grit' N-ThN I
S CN s CN
N ______________________________________________________
R6b N Rsb'Y'''sN
R6a Rsa
Via, Vlb,
CN
Gi N CN N N-N) N
N ______________________________________________________
Feb N R6b N
R6a Rsa
Vic, or Vld
wherein R6a, R6b, and G1 are as described above.
[00172] In one embodiment, a compound of the invention is according to Formula
Va-VId, wherein Gi
is 4-7 membered heterocycloalkyl containing one or more heteroatoms
independently selected selected
from 0, N, and S. In a particular embodiment, G1 is oxetanyl, azetidinyl,
tetrahydrofuranyl, pyrolidinyl,
tetrahydropyranyl, piperidinyl, piperazinyl, morpholinyl, or thiomorpholinyl.
In a more particular
embodiment, G1 is azetidinyl.
[00173] In one embodiment, a compound of the invention is according to Formula
Va-VId, wherein Gi
is 4-7 membered heterocycloalkyl containing one or more heteroatoms
independently selected from 0, N,
and S, which heterocycle is substituted with one or more independently
selected R9 groups. In a further
embodiment, G1 is 4-7 membered heterocycloalkyl containing one or more
heteroatoms independently
selected from 0, N, and S, which heterocycle is substituted with one or two
independently selected R9
groups. In a particular embodiment, G1 is oxetanyl, azetidinyl,
tetrahydrofuranyl, pyrolidinyl,
tetrahydropyranyl, piperidinyl, piperazinyl, morpholinyl, or thiomorpholinyl,
each of which is substituted
with one or two independently selected R9 groups. In a more particular
embodiment, G1 is azetidinyl
substituted with one or two independently selected R9 groups.
[00174] In one embodiment, a compound of the invention is according to Formula
Va-VId, wherein Gi
is 4-7 membered heterocycloalkyl containing one or more heteroatoms
independently selected from 0, N,
and S, each of which is substituted with one or two independently selected R9
groups, and R9 is oxo. In a
further particular embodiment, G1 is oxetanyl, azetidinyl, tetrahydrofuranyl,
pyrolidinyl,
tetrahydropyranyl, piperidinyl, piperazinyl, morpholinyl, or thiomorpholinyl,
each of which is substituted
with one or two independently selected R9 groups, and R9 is oxo.
34
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00175] In one embodiment, a compound of the invention is according to Formula
Va-VId, wherein Gi
is 4-7 membered heterocycloalkyl containing one or more heteroatoms
independently selected selected
from 0, N, and S, each of which is substituted with one or two independently
selected R9 groups, R9 is
RI , and RI is as previously described. In a further embodiment, G1 is
oxetanyl, azetidinyl,
tetrahydrofuranyl, pyrolidinyl, tetrahydropyranyl, piperidinyl, piperazinyl,
morpholinyl, or
thiomorpholinyl, each of which is substituted with one or two independently
selected R9 groups, R9 is RI ,
and RI is as previously described. In a particular embodiment, RI is
selected from OH, F, Cl, and -CN.
In a more particular embodiment, G1 is azetidinyl substituted with one or two
independently selected R9
groups, R9 is RI , and RI is as previously described. In a most particular
embodiment, RI is selected
from OH, F, Cl, and -CN.
[00176] In one embodiment, a compound of the invention is according to Formula
Va-VId, wherein GI
is 4-7 membered heterocycloalkyl containing one or more heteroatoms
independently selected from 0, N,
and S, each of which is substituted with one or two independently selected R9
groups, R9 is RI , and RI is
as previously described. In a further embodiment, G1 is oxetanyl, azetidinyl,
tetrahydrofuranyl,
pyrolidinyl, tetrahydropyranyl, piperidinyl, piperazinyl, morpholinyl, or
thiomorpholinyl, each of which
is substituted with one or two independently selected R9 groups, R9 is RI ,
and RI is as previously
described. In a particular embodiment, le is selected C1_4 alkyl. In a more
particular embodiment, le is
selected from -CH3, -CH2-CH3, and -CH(CH3)2. In a most particular embodiment,
RI is selected from
-CH3, and -CH2-CH3.
[00177] In one embodiment, a compound of the invention is according to Formula
Va-VId, wherein Gi
is 4-7 membered heterocycloalkyl containing one or more heteroatoms
independently selected from 0, N,
and S, each of which is substituted with one or two independently selected R9
groups, R9 is RI , and RI is
as previously described. In a further embodiment, G1 is oxetanyl, azetidinyl,
tetrahydrofuranyl,
pyrolidinyl, tetrahydropyranyl, piperidinyl, piperazinyl, morpholinyl, or
thiomorpholinyl, each of which
is substituted with one or two independently selected R9 groups, R9 is RI ,
and RI is as previously
described. In a particular embodiment, RI is C14 alkyl substituted with one
or more independently
selected OH, halo, phenyl. In a further embodiment, RI is C 1 A alkyl
substituted with one to three
independently selected OH, halo, and phenyl. In a more particular embodiment,
RI is -CH3, -CH2-CH3,
and -CH(CH3)2, each of which is substituted with one to three independently
selected OH, halo, and
phenyl. In a most particular embodiment, RI is -CF3, -CH2-CH2-0H, and -CH2-
phenyl.
[00178] In one embodiment, a compound of the invention is according to Formula
Va-VId, wherein GI
is 4-7 membered heterocycloalkyl containing one or more heteroatoms
independently selected from 0, N,
and S, each of which is substituted with one or two independently selected R9
groups, R9 is le, and le is
as previously described. In a further embodiment, G1 is oxetanyl, azetidinyl,
tetrahydrofuranyl,
pyrolidinyl, tetrahydropyranyl, piperidinyl, piperazinyl, morpholinyl, or
thiomorpholinyl, each of which
is substituted with one or two independently selected R9 groups, R9 is RI ,
and RI is as previously
described. In a particular embodiment, RI is C14 alkoxy. In a more particular
embodiment, RI is
selected from -OCH3, -OCH2-CH3, and -0C(CH3)3. In a most particular
embodiment, RI is -OCH3.
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00179] In one embodiment, a compound of the invention is according to Formula
Va-VId, wherein Gi
is 4-7 membered heterocycloalkyl containing one or more heteroatoms
independently selected from 0, N,
and S, each of which is substituted with one or two independently selected R9
groups, R9 is RI , and RI is
as previously described. In a further embodiment, G1 is oxetanyl, azetidinyl,
tetrahydrofuranyl,
pyrolidinyl, tetrahydropyranyl, piperidinyl, piperazinyl, morpholinyl, or
thiomorpholinyl, each of which
is substituted with one or two independently selected R9 groups, R9 is RI ,
and RI is as previously
described. In a particular embodiment, RI is selected from -S02CH3, -C(=0)C1
alkoxy, and
-C(=0)C1_4 alkyl. In a more particular embodiment, RI is selected from -
S02CH3, -C(=0)0CH3,
-C(=0)0CH2CH3, -C(=0)0CH(CH3)2, -C(=0)CH3, -C(=0)CH2CH3, and -C(=0)0CH(CH3)2.
In a most
particular embodiment, RI is selected from -S02CH3, -C(=0)0CH3, and -
C(=0)CH3.
[00180] In one embodiment, a compound of the invention is according to Formula
Va-VId, wherein GI
is 4-7 membered heterocycloalkyl containing one or more heteroatoms
independently selected from 0, N,
and S, each of which is substituted with one or two independently selected R9
groups, R9 is RI0, and RI is
as previously described. In a further embodiment, Gt is oxetanyl, azetidinyl,
tetrahydrofuranyl,
pyrolidinyl, tetrahydropyranyl, piperidinyl, piperazinyl, morpholinyl, or
thiomorpholinyl, each of which
is substituted with one or two independently selected R9 groups, R9 is RI ,
and RI is as previously
described. In a particular embodiment, le is ¨NRgC(=0)C1_4 alkyl, and Rg is as
described previously. In
a particular embodiment, RI is ¨NRgC(=0)CH3, or -N1VC(=0)CH2CH3, and Rg is as
described
previously. In a more particular embodiment, RI is -NRgC(=0)CH3, or -
NR8C(=0)CH2CH3, and Rg is H,
-CH3, or -CH2CH3. In a most particular embodiment, RI is -NHC(=0)CH3, or
¨NHC(=0)CH2CH3.
[00181] In one embodiment, a compound of the invention is according to Formula
wherein R6a
is H, -CH3 or halo, and R6b is H. In a particular embodiment, R6a is H, -CH3,
F, or Cl, and R6b is H. In a
more particular embodiment, R6a= is H, -CH3, or F, and R6b is H.
[00182] In one embodiment, a compound of the invention is according to Formula
1-Vld, wherein R6a
is H, and Rob is H, -CH3 or halo. In a particular embodiment, R6a is H, and
R6b is H, -CH3, F, or Cl. In a
more particular embodiment, 126 is H, and R6b is H, -CH3, or F.
[00183] In another embodiment, R6a and R6b are both H.
[00184] In one embodiment, a compound of the invention according to Formula I
is selected from:
24(2- ethy1-8 -methy1-6-(piperazin- 1 -yl)imidazo [ 1 ,2- a] pyridin-3 -y1)
(methyl)amino)-4-(4-
fluorophenyl)thiazole-5-carbonitrile,
24(2- ethy1-6-(4-(2-(3 -hydroxyazeti di n- 1 -y1)-2- oxo ethyl)p iperazin- 1 -
y1)-8- methylimidazo[1,2-a]pyridin-
3-y1)(methyflamino)-4-(4-fluorophenyl)thiazole-5-carbonitrile,
24(2- ethy1-6-(4-(2-(3 -hydroxy-3 -methylazetidin- 1 -y1)-2- oxo ethyl)pip
erazin- 1 -y1)-8 -methylimidazo [ 1 ,2-
a] pyridin-3 -y1)(methyl) amino)-4- (4- fluorophenyl)thiazo le-5-carb
nitrite,
(R)-242- ethy1-6- (44243 -hydroxypyrro lidin- 1 -y1)-2-oxoethyl)pip erazin- 1 -
y1)-8 -methylimidazo [ 1 ,2-
a] pyridin-3 -y1)(methyl) amino)-4- (4- fluorophenyl)thiazole-5-carb onitrile,
(S)-24(2- ethyl-644 -(243 -hydroxypyrro lidin- 1 -y1)-2- oxo ethyflpip erazin-
1 -y1)-8-methylimidazo [1 ,2-
a] pyridin-3 -y1)(methyl) amino)-4- (4- fluorophenyl)thiazole-5-carb onitrile,
36
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
2-((2-ethy1-6-(4-(2-(3 -hydroxyazetidin-1 -y1)-2- oxoethyl)-3 ,3 -
dimethylpiperazin-1 -y1)-8-
methylimidazo [1,2-a] pyridin-3 -y1)(methyflamino)-4-(4-fluorophenypthiazole-5-
carbonitrile,
2-(4-(2-ethy1-3 -44-(4-fluorophenyl)thiazol-2-y1)(methyflamino)-8-
methylimidazo [1,2-a] pyridin-6-
yOpiperazin- 1-y1)-1 -(3 -hydroxyazetidin- 1 -y1) ethanone,
(R)-2-(4-(2-ethy1-34(4-(4-fluorophenyl)thiazol-2-y1)(methyflamino)-8-
methylimidazo [1 ,2-a] pyridin-6-
yl)piperazin- 1-y1)-1 -(3 -hydroxypyrro lidin- 1 -y1) ethanone,
(S)-2-(4-(2-ethy1-3 -((4-(4-flu orophenyl)thi azol-2-y1)(methyl)am ino)-8-
methyl imi d azo [1 ,2-a]pyri d i n-6-
yl)piperazin- 1-y1)-1 -(3 -hydroxypyrro lidin- 1 -y1) ethanone,
2-((2-ethy1-6-(1 -(2-(3 -hydroxyazetidin-1 -y1)-2- oxo ethyl)piperidin-4-y1)-8-
methylimidazo [1 ,2-a] pyridin-
3-y1)(methyflamino)-4-(4-fluorophenypthiazole-5-carb onitrile,
2-(ethyl(2-ethyl-8 -methy1-6-( 1 -(methylsulfony1)- 1 ,2,3 ,6-
tetrahydropyridin-4-yl)imidazo [ 1,2-a]pyridin-3-
yl)amino)-4-(4 -fluorophenyl)thiazo le-5-earb onitrile,
24(2- ethy1-8-fluoro-6-(4-(2-(3 -hydroxyazetidin- 1 -y1)-2-oxoethyl)pip erazin-
1 -yl)imidazo[1,2-a]pyridin-3 -
y1)(methyflamino)-4-(4-fluorophenyethiazole-5-carbonitrile,
2-(4-(3-45-eyano-4 -(4-flu orophe nyl)thiazol-2-y1)(m ethyflamin o)-2- ethy1-8-
fluo imid azo [1 ,2-a] pyridin-
6-yl)pip erazin- 1 -y1)-N -methylacetamide,
2-(4-(2-ethy1-8-fluoro-3 4(444 -fluorophenyl)thiazol-2-
y1)(methyl)amino)imidazo [1,2-a]pyridin-6-
yl)piperazin- 1-y1)-1 -(3 -hydroxyazetidin- 1 -y1) ethanone,
(S)-2-(4-(2-ethy1-8-fluoro-3 44-(4-fluorophenyl)thiazol-2-
y1)(methyl)amino)imidazo [1,2-a]pyridin-6-
yOpiperazin- 1-y1)-1 -(3 -hydroxypyrro lidin- 1 -y1) ethanone,
(R)-2-(4-(2-ethy1-8-fluoro-3 4(4-(4-fluorophenyflthiazol-2-
y1)(methypamino)imidazo [ 1 ,2-a] pyridin-6 -
yl)piperazin- 1-y1)-1 -(3 -hydroxypyrro lidin- 1 -y1) ethanone,
2-(4-(2-ethy1-3 -((4-(4-fluoropb enyl)th azol-2-y1)(methyflam ino)-7- m ethyl
imi dazo [1 ,2-a] pyri din-6-
yl)piperidin-1 -y1)-1 -(3 -hydroxyazetidin-l-yl)ethanone,
2-[(2-Ethyl-7-fluoro-6- { 4-[2-(3-hydroxy-azetidin- 1 -y1)-2-oxo- ethyl] -
piperazin- 1-y1} -imidazo [1,2-
a] pyridin-3 -y1)-methyl-amino] -4-(4-fluoro-phenyl)-thiazo le-5-carb
nitrite,
244-(2-Ethy1-7-fluoro-3- {[4-(4-fluoro-phenyl)-thiazol-2-yThmethyl-amino} -
imidazo [ 1,2-a]pyridin-6-y1)-
piperazin- 1 -yl] - 1 -(3-hydroxy-azetidin-1 -y1)- ethanone,
244-(2-Ethy1-7-fluoro-3- {[4-(4-fluoro-pheny1)-thiazol-2-y1]-methyl-amino} -
imidazo [ 1,2-a]pyridin-6-y1)-
piperazin- 1 -yl] - 1 -(3-hydroxy-pyrro lidin- 1 -y1)- ethanone,
2-[4-(2-Ethyl-7-fluoro-3- {[4-(4-fluo ro-ph eny1)-th iazol-2-yl] - methyl-
amino} -imidazo [1 ,2-a]pyri d n-6-y1)-
piperazin- 1 -yl] - 1 -(3-hydroxy-pyffo lidin- 1 -y1)- ethanone,
24443 -((5-cyano-4 -(4-fluorophenyl)thiazol-2-y1)(methyflamino)-2-
ethylimidazo [ 1,2-a] pyridin-6-
yl)piperazin- 1 -y1)-N-methylacetamide,
tert-butyl 4-(2-ethyl-3-44-(4-fluorophenyl)thiazol-2-
y1)(methyl)amino)imidazo [ 1 ,2-al pyridin-6-
yOpiperazine- 1 -earb oxylate,
2-(4-(2-ethy1-3-44-(4-fluorophenyethiazol-2-y1)(methyl)amino)imidazo [1 ,2-a]
pyridin-6 -yl)pip erazin-1 -
y1)- 1 -(3-hydroxyazetidin-1 -y1) ethanone,
37
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
(S)-2-(4-(2-ethy1-3-44-(4-fluorophenyl)thiazol-2-y1)(methypamino)imidazo [1 ,2-
a] pyridin-6 -yl)pip erazin-
1 -y1)- 1 -(3-hydroxypyrro lidin- 1 -yl) ethanone,
(R)-2-(4-(2-ethy1-3-((4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [
1,2-a] pyridin-6 -
yl)piperazin- 1-y1)-1 -(3 -hy clroxypyrro lidin- 1 -y1) ethanone,
N-( 1 -(244 -(2- ethy1-3 -((4-(4- fluorophenyethiazol-2-
y1)(methyDamino)imidazo [1,2-a]pyridin-6-
yl)piperazin- 1 -yl)acetoyl)pyrrolidin-3-yl)acetamide,
2-(4-(2-ethy1-3 -((4-(4-flu orophe nyl)thi azol-2-y1)(m ethyl)amino)i in d azo
[1 ,2-a] pyridi n-6 -yl)p ip erazin-1 -
y1)-1 -(3-fluoroazetidin-1-yl)ethanone,
1 -(3,3-difluoro azetidin-1 -y1)-2-(4-(2- ethy1-3 4(4-(4-fluorophenyethiazol-2-
yl)(methypamino)imidazo [1,2-a]pyridin-6-yl)pip erazin-1 -yl)ethanone,
1 -(azetidin- 1 -y1)-2-(4-(2- ethy1-3-44-(4-fluorophenypthiazol-2-
y1)(methyl)amino)imidazo [ 1,2-a] pyridin-
6-yl)pip erazin- 1 -ypethanone,
2-(4-(2-ethy1-3-44-(4-fluorophenyethiazol-2-y1)(methyl)amino)imidazo [1 ,2-a]
pyridin-6 -yl)pip erazin-1 -
y1)- 1 -(pyn-o lidin- 1 -y1) ethanone,
2-(4-(2-ethy1-3 -((4-(4-fluo roph enyl)th azol-2-y1)(methyl)a mino)i mid azo
[1 ,2-a] pyri di n-6 -yl)p ip e razi n-1 -
y1)-1 -morpholinoethanone,
2-(4-(2-ethy1-3 4(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [1 ,2-
a] pyridin-6 -yl)pip erazin-1 -
yl)acetamide,
2-(4-(2-ethy1-3-((4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [1 ,2-
a]pyridin-6 -yl)pip erazin-1 -
y1)- 1 -(3-(hy droxymethyl)az etidin- 1 -y1) ethanone,
2-(4-(2-ethy1-3-44-(4-fluorophenyl)thiazol-2-y1)(methypamino)imidazo [1 ,2-a]
pyridin-6 -yl)pip erazin-1 -
y1)-N,N-dimethylacetamide,
ethyl 2-(4-(2-ethy1-3 44-(4-fluorophe nyl)th azol-2-y1)(methyl)am
ino)im dazo [ 1 ,2-a] pyridin-6-
yl)piperazin- 1 -yl)acetate,
ethyl 2-(4-
(2-ethyl-3-44-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [ 1 ,2-a]
pyridin-6-
yl)piperazin- 1 -yl)prop ano ate,
2-(4-(2-ethy1-3-44-(4-fluorophenyl)thiazol-2-y1)(methyDamino)imidazo [1 ,2-a]
pyridin-6 -yl)pip erazin- 1 -
yOacetonitrile,
N-(6-(4-(( 1 -cyc lopropyl- 1 H-tetrazol-5-yl)methyl)pip erazin-1 -y1)-2-
ethylimidazo [1 ,2-a] pyridin-3 -y1)-4-(4-
fluoropheny1)-N-methylthiazol-2-amine,
N-(2- ethy1-6-(4-(oxazol-2-ylmethyl)p iperazi n- 1 -y1)1 mi dazo [ 1 ,2-a]pyri
din-3 -y1)-4-(4- flu oroph eny1)-N-
methylthiazol-2-amine,
N-(6-(4-((1 ,2,4-oxadiazol-3-yl)methyl)piperazin- 1 -y1)-2- ethylimidazo [1,2-
a] pyridin-3 -y1)-4-(4-
fluoropheny1)-N-methylthiazol-2-amine,
2-(4-(2-ethy1-3-44-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [1 ,2-a]
pyridin-6 -yl)pip erazin-1 -
yOace tic acid,
2-hydroxyethyl 4-(2-
ethyl-3 4(4-(4-fluorophenyl)thiazol-2-y1)(methyeamino)imidazo [ 1 ,2-a]
pyridin-6-
yl)piperazine- 1 -carb oxylate,
38
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
tert-butyl 2-(4-(2-ethy1-3 4(4-(4-fluorophenyl)thiazol-2-
y1)(methyl)amino)imidazo [ 1 ,2-a] pyridin-6-
yppiperazine- 1 -carb onyl)pyrrolidine- 1 -carb oxylate,
tert-butyl 3-(4-(2-ethyl-3 -44-(4-fluorophenyl)thiazol-2-
y1)(methyl)amino)imidazo [ 1 ,2-al pyridin-6-
yOpiperazine- 1 -carb onyl)pyrrolidine- 1 -carb oxy late,
(4-(2-ethy1-3 -((4 -(4-fluorophenyl)thiazol-2-y1)(methyeamino)imidazo [
pyridin-6-yl)pip erazin- 1 -
yl)(pyrro lidin-2-yemethanone,
(4-(2-ethy1-3 -((4 -(4-flu orophe nypthiazol-2-y1)(m ethyl)ami no) im d azo [
1 ,2-al pyridi n-6-yl)p ip erazi n- 1 -
yl)(pyrro lidin-3 -yemethanone,
1 -(3 -(4-(2-ethy1-3 4(444 -fluorophenyl)thiazol-2-y1)(methypamino)imidazo [ 1
,2-a] pyridin-6-yl)pip erazine-
1 -carb onyl)pyrrolidin- 1 -yl)ethanone,
(4-(2-ethy1-3 -(4-fluorophenypthiazol-2-y1)(methyl)amino)imidazo
[1,2pyridin-6-yl)pip erazin- 1 -
yl)( 1 -(methylsulfonyl)pyrrolidin-3 -yl)methanone,
1 -(4-(2-ethy1-3 -44-(4-fluorophenyethiazol-2-y1)(methyl)amino)imidazo [ 1 ,2-
a] pyridin-6 -yl)pip erazin- 1 -
y1)-2-hydroxyethanone,
1 -(4-(2-ethy1-3 -44-(4-fluoroph enyl)th azol-2-y1)(methyl)am i no) imidazo [1
,2-a] pyri di n-6 -yl)p ip erazi n- 1 -
yl)prop an- 1 -one,
1 -(4-(2-ethy1-3 4(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [ 1 ,2-
a] pyridin-6 -yl)pip erazin- 1 -
y1)-4-hydroxybutan- 1 -one,
4-(dimethylamino)- 1-(4-(2-ethyl-3 4(444 -fluorophenyl)thiazol-2-
y1)(methyl)amino)imidazo [ 1 ,2-
a] pyridin-6-yl)piperazin- 1 -yl)butan- 1 -one,
N-(2- ethy1-6-(4-(methylsulfonyl)pip erazin- 1 -yl)imidazo [ 1,2-a] pyridin-3 -
y1)-4-(4-fluoropheny1)-N-
methylthiazol-2-amine,
N-(6-(4-(3 -chloropropylsulfonyl)piperazin-1 -y1)-2- ethyl i m i dazo [1 ,2-a]
pyridi n-3 -y1)-4-(4-fluoropheny1)-
N -methylthiazol-2-amine,
N-(6-(4-(3 -(dimethylamino)propylsulfonyl)pip erazin- 1 -y1)-2 - ethylimidazo
[ 1 ,2-a] pyridin-3 -y1)-4- (4-
fluoropheny1)-N-methylthiazol-2-amine,
N-(2- ethy1-6-(4-(3 -(pyrrolidin- 1 -yl)propylsulfonyl)piperazin- 1 -
yl)imidazo[ 1 ,2-a] pyridin-3 -y1)-4-(4-
fluoropheny1)-N-methylthiazol-2-amine,
3 -(4-(2-ethy1-3 -44-(4-fluorophenyl)thiazol-2-y1)(methypamino)imidazo [ 1 ,2-
a] pyridin-6 -yl)pip erazin- 1 -
ylsulfonyl)prop an- 1-01,
methyl 2-(4-(2-ethy1-3 -((4-(4-fluorophe nyl)th azol-2-y1)(methyl)am i no)
imi dazo [1 ,2-a]pyridin-6-
yepiperazin-1 -ylsulfonyl)acetate,
2-(4-(2-ethy1-3 4(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [ 1 ,2-
a] pyridin-6 -yl)pip erazin- 1 -
ylsulfonyl)acetic acid,
2-(4-(2-ethy1-3 -44-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [ 1 ,2-
a] pyridin-6 -yl)pip erazin- 1 -
ylsulfonyl)acetamide,
tert-butyl 4-(2-ethyl-3((4(4-fluorophenyl)thiazol-2-y1)(methypamino)imidazo
[ 1 ,2-a] pyridin-6 -y1)-3 -
oxopiperazine- 1 -carb oxylate,
39
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
tert-butyl 4-(3 -
((4- (4-chlorophenyl)thiazol-2-y1)(methyl) amino)-2- ethylimidazo [ 1 ,2-a]
pyridin-6 -y1)-3 -
oxopiperazine- 1 -carb oxylate,
ethyl 2-(4-(2-ethy1-3((4(4-fluorophenyl)thiazol-2-
y1)(methyl)amino)imidazo [ 1 ,2-al pyridin-6 -y1)-3 -
oxopiperazin- 1 -y1) acetate,
1-(2-ethyl-3 (4-fluorophenyl)thiazol-2-y1)(methyflamino)imidazo [1,2-
a]pyridin-6-y1)-4 -
(methylsulfonyl)piperazin-2- one,
N-(2- ethy1-6-(1 -(methylsulfo ny1)- 1 ,2,3 ,6-tetrahydropyri di n-4-yl)imi
dazo [ 1 ,2-a.] pyridi n-3 -y1)-4-(4-
fluoropheny1)-N -methylthiazol-2-amine,
N-(6-( 1 -(chloromethylsulfony1)- 1 ,2,3 ,6-tetrahydropyridin-4-y1)-2-
ethylimidazo [ 1 ,2-a] pyridin-3 -y1)-4-(4-
fluoropheny1)-N-methylthiazol-2-amine,
4-(4-chloropheny1)-N- (2- ethy1-6-( 1 -(methylsulfony1)-2,5-dihydro- 1H-pyrrol-
3 -yflimidazo [ 1 ,2-a] pyridin-
3 -y1)-N-methylthiazol-2-amine,
4-(4-chloropheny1)-N- (2- ethy1-6-(1 -(methylsulfony1)- 1 ,4,5,6-
tetrahydropyridin-3 -yl)imidazo [ 1,2-
a] pyridin-3 -y1)-N-methylthiazol-2-amine,
4-(4-tert-butylphe ny1)-N-(2-ethy1-6-( 1 -(methyl su lfony1)- 1,2,3 ,6-
tetrahydropyridi n-4-yl)i mi d azo [ 1 ,2-
a] pyridin-3 -y1)-N -methylthiazol-2-amine,
N-(2-ethyl-6-(1 -(methylsulfony1)- 1,2,3 ,6-tetrahydropyridin-4-yl)imidazo [
pyridin-3 -y1)-4-(4-
methoxypheny1)-N-methylthiazol-2-amine,
N-(2-ethyl-6-(1 -(methylsulfony1)- 1,2,3 ,6-tetrahydropyridin-4-ypimidazo [
1,2-a] pyridin-3 -y1)-N-methy1-4-
(4-(trifluoromethoxy)phenyl)thiazol-2-amine,
4-(3,4-difluoropheny1)-N-(2 - ethyl-6-(1 -(methylsulfony1)- 1,2,3 ,6-
tetrahydropyridin-4-yl)imidazo [ 1,2-
a] pyridin-3 -y1)-N-methylthiazol-2-amine,
3 -(4-(2-ethy1-3 4(4-(4-fluoroph enyl)th azol-2-y1)(methypam i no) im idazo [1
,2-a.] pyri di n-6 -y1)-5,6-
dihydropyridin- 1 (2H)-ylsulfonyl)propyl acetate,
3 -(4-(2-ethy1-3 4(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [ 1 ,2-
a.] pyridin-6 -y1)-5,6-
dihydropyridin- 1 (2H)-ylsulfonyl)prop an- 1 - ol,
4-(2-ethyl-3((4(4-chlorophenyl)thiazol-2-y1)(methypamino)imidazo [ 1 ,2-a]
pyridin-6-y1)-3 ,6- dihydro-
2H-thiopyran 1,1-dioxide,
N-(2-ethyl-6-(1 -(methylsulfony1)- 1,2,3 ,6-tetrahydropyridin-4-yl)imidazo [
pyridin-3 -y1)-5-fluoro-4-
(4-fluoropheny1)-N-methylthiazol-2-amine,
tert-butyl 4-(2-
ethy1-3 (4-fluorophenyl)th azol- 2-y1)(methyl)am i no) imi dazo [1 ,2-a.]
pyri din-6 -y1)-3 -
hydroxypiperidine- 1 -carboxylate,
4-(2-ethyl-3 (4-fluorophenyl)thiazol-2-
y1)(methypamino)imidazo pyridin-6-y1)- 1 -
(methylsulfonyl)piperidin-3 -ol,
N-(2-ethyl-6-(1 -(methylsulfonyl)pip eridin-4-yl)imidazo [ 1 ,2-al pyridin-3 -
y1)-4- (4-fluoropheny1)-N-
methy lthiazol-2-amine,
4-(4-tert-butylpheny1)-N-(2-ethy1-6-(1 -(methylsulfonyl)piperidin-4-yl)imidazo
[ pyridin-3 -y1)-N-
methylthiazol-2-amine,
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
N-(2- ethy1-6-(1 -(methylsulfonyl)pip eridin-4-yl)imidazo [ 1 ,2-a] pyridin-3 -
y1)-4- (4-methoxypheny1)-N-
methylthiazol-2-amine,
4-(3,4-difluoropheny1)-N-(2 - ethy1-6-(1 -(methylsulfonyl)pip eridin-4-
yl)imidazo [ 1 ,2-a] pyridin-3 -y1)-N-
methylthiazol-2-amine,
N -(2- ethy1-6-(1 -(methylsulfonyl)pip eridin-4-yl)imidazo [ 1 ,2-a] pyridin-3
-y1)-N -methy1-4- (4-
(trifluoromethyl)phenyl)thiazol-2 -amine,
N-(2- ethy1-6-(1 -(methyl sul fo nyi)p ip e ri d i n-4-yl)i mi dazo [ 1 ,2-a]
pyri d i n-3 -y1)-N- methy1-4- (4-
(trifluoromethoxy)phenyl)thiazol-2-amine,
N-(6-( 1 -(3 -chloropropylsulfonyflpip eridin-4-y1)-2-ethylimidazo [1,2-a]
pyridin-3 -y1)-4-(4-fluoropheny1)-
N-methylthiazol-2-amine,
N-(6-( 1-(3 -(dimethylamino)propylsulfonyflpip eridin-4 -y1)-2- ethylimidazo [
1 ,2-a] pyridin-3 -y1)-4-(4-
fluoropheny1)-N-methylthiaz01-2-amine,
N-(2- ethy1-6-(1 -(3 -morpholinopropylsulfonyl)pip eridin-4-yl)imidazo [ 1,2-
a] pyridin-3 -y1)-4-(4-
fluoropheny1)-N-methylthiazol-2-amine,
N-(2-ethyl-6-(1 -(3 -(pyrrol id i n- 1 -yl)p ropylsulfo nyl)piperidin-4-
yl)imidazo[l ,2-a]pyridi n-3 -y1)-4-(4-
fluoropheny1)-N -methylthiazol-2-amine,
N-(6-( 1 -(3 -aminopropylsulfonyl)piperidin-4-y1)-2-ethylimidazo[1,2-a]pyridin-
3-y1)-4-(4-fluoropheny1)-
N-methylthiazol-2-amine,
N-(2-ethyl-6-(1 -(2-morpho lino ethylsulfonyflpiperidin-4-yflimidazo [1,2-a]
pyridin-3 -y1)-4- (4-
fluoropheny1)-N-methy lthiaz01-2-amine,
4-(2-ethyl-3 (4-
fluorophenyl)thiazol-2-y1)(methyflamino)imidazo [1,2-a]pyridin-6-yl)pip
eridine- 1 -
sulfonamide,
3 -(4-(2-ethy1-3 4(4-(4-fluoroph enyflth azol-2-y1)(methypam i no) im idazo [1
,2-a] pyri di n-6 -yl)p ip eri di n- 1 -
ylsulfonyl)propyl acetate,
3 -(4-(2-ethy1-3 4(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [1 ,2-
a] pyridin-6 -yl)pip eridin- 1 -
ylsulfonyl)prop an- 1-01,
3 -(4-(2-ethy1-3 -fluoro-4-(4 -fluorophenyl)thiazol-2-
y1)(methyflamino)imidazo [1,2-a]pyridin-6-
yl)piperidin- 1 -ylsulfony Opropan- 1-01,
2-(24(2-ethy1-64 1 -(methylsulfonyl)piperidin-4-yl)imidazo [1,2-a]pyridin-3 -
y1)(methyl) amino)thiazol-4-
y1)-5 - fluorob enzonitrile,
2-(2-((2-ethy1-6- ( 1 -(m ethyl sulfo nyl)piperidi d azo [1 ,2-a]pyridi n-3
-y1)(methyl)am ino)-5-
methylthiazol-4-y1)-5 -fluoro b enzonitrile,
N-(2-ethyl-6-(1 -(methylsulfonyl)pip eridin-4-yl)imidazo [ 1 ,2-a] pyridin-3 -
y1)-4- (4-fluoro-2-methylpheny1)-
N-methylthiazol-2-amine,
4-(2-chloro-4-fluoropheny1)-N-(2- ethy1-6-( 1 -(methylsulfonyl)piperidin-4-
yl)imidazo [1 ,2-a] pyridin-3 -y1)-
N-methylthiazol-2-amine,
4-(2,4-difluoropheny1)-N-(2 - ethy1-6-(1 -(methylsulfonyl)pip eridin-4-
yl)imidazo [ 1 ,2-a] pyridin-3 -y1)-N-
methylthiazol-2-amine,
41
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
N-(2- ethy1-6-(1 -(methylsulfonyl)pip eridin-4-yl)imidazo [ 1,2-a] pyridin-3 -
y1)-4-(4-fluoropheny1)-N,5-
dimethylthiazol-2-amine,
4-(4-fluoropheny1)-N-(2- ethy1-6-(1 -(methylsulfonyl)pip eridin-4-yl)imidazo [
pyridin-3 -y1)-N-(d3 -
methyl)thiazol-2-amine,
4-(4-fluoropheny1)-N -(2- cthy1-6-(1 -(mc-thylsulfonyl)pip cridin-4-yl)imidazo
[ 1,2-a] pyridin-3 -y1)-N-(d3 -
methyl)-(d-thiazol-2)-amine,
methyl 2-((2-ethyl-6-(1 -(methylsul fo nyl)p ip e ri d n-4 -yl)i mi dazo [ I
,2-a] pyri din-3 -y1)(methyl)amino)-4-(4-
fluorophenyl)thiazole-5-carboxylate,
1 -(2-((2-ethy1-6-(1 -(methylsulfonyl)piperidin-4-yl)imidazo[1,2-a]pyridin-3-
y1)(methyl)amino)-4-(4-
fluorophenyl)thiazol-5-ybethanone,
N-(2-(2-42-ethyl-6-(1-(methylsulfonyl)piperidin-4-yl)imidazo[ 1,2-a] pyridin-3
-y1)(methyDamino)thiazol-
4-y1)-5-fluorophenyl)acetamide,
(2424(2- ethy1-6 -(1 -(methylsulfonyl)pip eridin-4-yeimidazo[ 1,2-a] pyridin-3-
y1)(methypamino)thiazol-4-
y1)-5- fluorophenyl)methanol,
ethyl 2-(4-(2-ethy1-3 4(444- flu orophenyl)thi azol-2-y1)(m ethyl)ami n
o)i m idazo [1 ,2-a] pyri di n-6-y1)-5,6-
dihydropyridin- 1 (2H)-ypacetate,
ethyl 2-(4-
(2-ethyl-34(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [ 1,2-a]
pyridin-6-
yl)piperidin-1 -yeacetate,
2-(4-(2-ethy1-34(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [1,2-a]
pyridin-6-yl)pip eridin- 1 -
y1)- 1 -(3-hydroxy etidin-1 -yOethanone,
(R)-2-(4-(2-cthy1-34(4-(4-fluorophcnyl)thiazol-2-y1)(mcthyl)amino)imidazo [
1,2-a] pyridin-6-yl)pip cridin-
1 -y1)- 1 -(3-hydroxypyrro lidin- 1 -yl)ethanone,
(S)-2-(4-(2-ethy1-34(4-(4-fluorophenyetbiazol-2-y1)(methyDamino)imidazo [1 ,2-
a] pyridin-6-yl)p ip eri di n-
1 -y1)- 1 -(3-hydroxypyrro lidin- 1 -yl)ethanone,
2-(4-(2-ethy1-34(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [1,2-a]
pyridin-6-yl)pip eridin-1 -
y1)- 1 -(3-(hydroxymethyl)azetidin- 1 -yl)ethanone,
2-(4-(2-ethy1-3-44-(4-fluorophenyl)thiazol-2-y1)(methypamino)imidazo [ 1,2-a]
pyridin-6-yl)pip eridin- 1 -
y1)-N,N-dimethy lac etamide,
2-(4-(2-ethy1-3 -44-(4-fluorophcnyl)thiazol-2-y1)(methypamino)imidazo [1,2-a]
pyridin-6-yl)pip eridin- 1 -
y1)- 1 -(pyrm lidin- 1 -yl)ethanone,
(S)- 1 -(2-(4-(2-ethy1-34(4-(4-fluorophenyl)thiazol-2-y1)(methypamino)imidazo
[1 ,2-a] pyri d i n-6-
yl)piperidin-1 -yl)acetoyl)pyrrolidine-3-carbonitrile,
2-(4-(2-ethy1-34(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [1,2-a]
pyridin-6-yl)pip eridin- 1 -
y1)- 1 -(3-(hydroxymethyppyiTo lidin- 1 -yl)ethanone,
4-((4-(2-ethy1-3 - 4444- fluorophenyl)thiazol-2-y1)(methypamino)imidazo [1,2-
a]pyridin-6-yl)pip eridin- 1
yl)methyl)-1,3-dioxo lan-2- one,
2-(4-(2-ethy1-3-44-(4-fluorophenyethiazol-2-y1)(methyl)amino)imidazo [1,2-a]
pyridin-6-yl)pip eridin-1 -
y1)-N-(2-hydroxyethyl)-N-methylacetamide,
42
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
2-(4-(2-ethy1-3 4(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [1 ,2-
a] pyridin-6 -yl)pip eridin- 1 -
y1)-N-methoxy-N-methylac etamide,
N-(cyanomethyl)-2-(4-(2-ethy1-34(4-(4-fluorophenyl)thiazol-2-
y1)(methyl)amino)imidazo [ 1 ,2-al pyridin-
6-yepip eridin- 1 -y1)-N-methylacetamide,
54(4-(2-ethy1-3 -( (4-(4- fluorophenyethiazol-2 -y1)(methyflamino)imidazo [1,2-
a]pyridin-6 -yl)pip cridin- 1 -
yl)methyl)oxazo lidin-2- one,
2-(4-(2-ethy1-34(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [1 ,2-a]
pyridi n-6 -y1)p ip eridi n- 1 -
y1)-N -(3 -hydroxypropyl)acetamide,
1 -(3,3-difluoro azetidin- 1 -y1)-2-(4-(2- ethy1-3 4(4-(4-fluorophenyethiazol-
2-
yl)(methyl) amino)imidazo [1,2-a]pyridin-6-yl)pip eridin- 1 -yflethanone,
2-(4-(2-ethy1-3 -44-(4-fluorophenyethiazol-2-y1)(methyl)amino)imidazo [ 1 ,2-
a] pyridin-6 -yl)pip eridin- 1 -
yl)acetamide,
1 -(4-(2-ethy1-3 -44-(4-fluorophenyethiazol-2-y1)(methyl)amino)imidazo [1 ,2-
a]pyridin-6 -yl)pip cridin- 1 -
y1)-2- (pyiTo lidin- 1 -y1) ethanone,
1 -(4-(2-ethy1-3 -44-(4-fluo roph enyl)th azol-2-y1)(methyl)am i no) imidazo
[1 ,2-a] pyri di n-6 -yl)p ip e ri di n- 1 -
y1)-2- (methylamino)ethanone,
1 -(4-(2-ethy1-3 4(4-(4-fluorophenyl)thiazol-2-y1)(methypamino)imidazo [1 ,2-
a] pyridin-6 -yl)pip eridin- 1 -
y1)-2- (3-hydroxyazetidin- 1 -y1) ethanone,
2-(dimethylamino)- 1-(4-(2-ethyl-3 4(444 -fluorophenyl)thiazol-2-
y1)(methyl)amino)imidazo [ 1 ,2-
a] pyridin-6-yl)piperidin- 1 -y1) ethanone,
3-(dimethylamino)- 1 -(4-(2-ethy1-3 4(4-(4-fluorophenyl)thiazol-2-
y1)(methypamino)imidazo [ 1 ,2-
a] pyridin-6-yl)piperidin- 1 -yl)prop an- 1 -one,
2-(3,3-di fluo ro azetidi n-1 -y1)-1 -(442- etby1-3-44-(4-fluo rophenypth
iazol-2-
yl)(methyflamino)imidazo [1,2-a]pyridin-6-yl)pip eridin- 1 -yl)ethanone,
1 -(4-(2-ethy1-3 4(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [1 ,2-
a] pyridin-6 -yl)pip eridin- 1 -
y1)-3 - (methylamino)prop an- 1 -one,
1 -(4-(2-ethy1-3 -44-(4-fluorophenyl)thiazol-2-y1)(methypamino)imidazo [ 1 ,2-
a] pyridin-6 -yl)pip eridin- 1 -
y1)-2- (3-fluoro azetidin- 1 -ypethanone,
1 -(3-(4-(2-ethy1-3 -((4-(4 -fluorophenyl)thiazol-2-y1)(methyflamino)imidazo
[1 ,2-a] pyridin-6-yl)pip eridin-
1 -yl)azetidin- 1 -yl)ethanone,
5-bro mo-N- (2- ethy1-6-(1 -(methyl sulfonyl)p iperidi n-4-yl)i midazo [1 ,2-
a]pyrid i n-3 -y1)-4-(4-fluoropheny1)-
N-methylthiazol-2-amine,
2-((2-ethyl-6-(I - (methylsulfonyl)pip eridin-4-yl)imidazo [ 1,2-a]pyridin-3-
ye (methyl)amino)-4-(4-
fluorophenyl)thiazo le-5-carb nitrite,
2-((2-ethy1-6-(1 - (methylsulfonyl)pip eridin-4-yl)imidazo [ 1,2-a] pyridin-3-
y1)(methyDamino)-4-(4-
fluorophenyl)thiazole-5-c arb oxamide,
24(2- ethy1-6-(4- (2-(3 -hydroxyazetidin- 1 -y1)-2- oxo cthyl)piperazin- 1 -
yl)imidazo [1 ,2-a] pyridin-3 -
yl)(methyl) amino)-4-(4- fluorophenyethiazole-5-carb onitrile,
43
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
24(2- ethy1-6-(4-(2-(3 -(hydroxymethyl)azetidin-1 -y1)-2-oxo ethyl)piperazin-
1 -yl)imidazo [1,2pyridin-3 -
y1)(methypamino)-4-(4-fluorophenyl)thiazole-5-carbonitrile,
2-(4-(3-45-cyano-4-(4-fluorophenypthiazol-2-y1)(methyDamino)-2-ethylimidazo [
1,2-a] pyridin-6-
yOpiperazin- 1 -y1)-N,N-dimethylacetamide,
24(2- ethy1-6-(1 -(2-(3 -hydroxyazetidin-1 -y1)-2- oxo ethyl)piperidin-4-
yl)imidazo[1,2-a]pyridin-3 -
y1)(methypamino)-4-(4-fluorophenyethiazole-5-carbonitrile,
(R)-2-((2-ethy1-6-(1 -(243 -hydroxypyrrolid in-1 -y1)-2-oxoethyl)piperidin-4-
yl)imidazo [1 ,2-a]pyridin-3-
y1)(methypamino)-4-(4-fluorophenyethiazole-5-carbonitrile,
(S)-24(2-ethy1-6-(1 -(2-(3 -hydroxypyrrolidin- 1 -y1)-2- oxo ethyppip eridin-4-
yl)imidazo pyridin-3 -
y1)(methypamino)-4-(4-fluorophenyl)thiazole-5-carbonitrile,
2-((2-ethyl-6-(1 -(2-(3 -(hydroxymethyl)azetidin- 1 -y1)-2-oxo ethyl)piperidin-
4-yl)imidazo [ 1,2-a] pyridin-3 -
y1)(methyDamino)-4-(4-fluorophenyl)thiazole-5-carbonitrile,
2-(4-(34(5-cyano-4-(4-fluorophenyflthiazol-2-y1)(methypamino)-2-ethylimidazo [
pyridin-6-
yl)piperidin-1 -y1)-N,N-dimethylacetamide,
2-(4-(2-ethy1-3 -44-(4-fluoropheny0-5 -(hyd roxymethyl)th iazol-2-y1)(methyl)
ami no)im idazo [1 ,2-
a] pyridin-6-yl)piperazin- 1-y1)-1 -( 3-( hydroxymethyl)azetidin- 1 -
yl)ethanone,
(2-((2-ethy1-6-(1 -(methylsulfonyl)pip eridin-4-yl)imidazo [ pyridin-3 -
y1)(methyl)amino)-4-(4-
fluorophenyl)thiazol-5-yl)methanol,
(2-((2-ethyl-6-(1 -(methylsulfonyl)pip eridin-4-yl)imidazo [ pyridin-3 -
y1)(methyDamino)-4-(4-
(trifluoromethoxy)phenyl)thiazol-5-y methanol,
(2-((6-(1 -(3-(dimethylamino)propylsulfonyl)piperidin-4-y1)-2-ethylimidazo
[1,2-a] pyridin-3 -
y1)(methypamino)-4-(4-fluorophenyl)thiazol-5-y1),
(2-((2-ethyl-6-(1 -(metbylsulfonyep ip dazo [
pyridin-3 -y1)(methypamino)-4-(4-
(trifluoromethyl)phenyl)thiazol-5 -yemethanol,
2-(4-(2-ethy1-3 -((4-(4-fluoropheny1)-5 -(hydroxymethyl)thiazol-2-
y1)(methyl)amino)imidazo [ 1,2-
a] pyridin-6-yl)piperidin-1 -y1)-1 -(pyrrolidin-1-yl)ethanone,
2-(4-(2-ethy1-3 -44-(4-fluoropheny1)-5 -(hydroxymethyl)thiazol-2-
y1)(methyl)amino)imidazo [ 1,2-
a]pyridin-6-yl)piperidin-1 -y1)-1 -(3 -(hydroxymethyl)azetidin-1 -ypethanone,
2-(dimethylamino)- 1 -(4-(2-ethy1-3 -((4-(4 -fluoropheny1)-5-
(hydroxymethypthiazol-2-
yl)(methypamino)imidazo [1,2-a]pyridin-6-yl)pip eridin- 1 -yl)ethanone,
1-(4-(2-ethyl-3 -((4-(4-flu oropheny1)-5 -(hydroxymethyl)thiazol-2-
y1)(methyl)amino)imidazo [1 ,2-
a] pyridin-6-yl)piperidin-1 -yl)prop an- 1 -one,
2-(4-(2-ethy1-3 4(4-(4-fluorophenyl)-5 -(hydroxymethyl)thiazol-2-
y1)(methyl)amino)imidazo [ 1,2-
a] pyridin-6-yppiperazin- 1 -y1)-N,N-dimethylacetamide,
2-(4-(2-ethy1-3 -44-(4-fluoropheny1)-5 -(hydroxymethyl)thiazol-2-
y1)(methyl)amino)imidazo [ 1,2-
a] pyridin-6-yl)piperidin-1 -y1)-1 -(3 -hydroxyazetidin-1-yl)ethanone,
2-(4-(2-ethy1-3 -44-(4-fluoropheny1)-5 -(hydroxymethyl)thiazol-2-
y1)(methyeamino)imidazo [ 1,2-
a] pyridin-6-yl)piperidin-1 -y1)-N,N-dimethylacetamide,
44
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
2-(4-(2-ethy1-3 4(4-(4-fluorophenyl)-5 -(2,2,2-trifluoro acetoyl)thiazol-2-
y1)(methyeaminotimidazo [ 1,2-
a] pyridin-6-yppiperazin- 1 -y1)-N,N-dimethylacetamide,
1 -(24(2-ethy1-64 1 -(2-(3 -hydroxyazetidin- 1 -y1)-2- oxo ethyl)piperidin-4-
yflimidazo pyridin-3 -
yl)(methyl) amino)-4-(4- fluoropheny Othiazol-5 -y1) -2,2,2-trifluoro
ethanone,
1 -(24(2-ethy1-64 1 -(methylsulfonyl)piperidin-4-yltimidazo[1,2-a]pyridin-3-
y1)(methyl)amino)-4-(4-
fluorophenyl)thiazol-5-y1)-2,2,2-trifluoroethanone,
2-(2-((2-ethy1-6- (p ip erazin- 1 -yl)imi d azo [ 1 ,2-a] pyri di n- 3 -
y1)(methyl) ami n o)-5- methylth iazol-4-y1)-5-
fluor b enzonitrile,
2-(2-((2-ethyl-6-(4-(2-(3 -hydroxyazetidin- 1 -y1)-2- oxo ethyl)piperazin- 1 -
yl)imidazo [ 1 ,2-a] pyridin-3 -
yl)(methyl) amino)-5 -methylthiazol-4-y1)-5 -fluorob enzonitrile,
24443 44-(2-cyano-4-fluoropheny1)-5 -methylthiazol-2-y1)(methyl) amino)-2-
ethylimidazo [ 1 ,2-a] pyridin-
6-yepip erazin- 1 -y1)-N-methy lac etamide,
2-(24(2-ethy1-6- (44243 -fluoro azetidin- 1 -y1)-2-oxoethyflpip erazin- 1 -
yflimidazo[1,2-a]pyridin-3 -
yl)(methyl) amino)thiazol-4-y1)-5-fluorob enzonitrile,
2424(6444243,3 - d i flu o roazetid n- 1 -y1)-2- oxo etbyl)p ip e razi n- 1 -
y1)-2-ethylimid azo [1 ,2-a] pyri di n-3 -
yl)(methyl) amino)thiazol-4-y1)-5-fluoro b enzonitrile,
2-(2-((2-ethyl-6-(4-(2-(3 -hydroxyazetidin- 1 -y1)-2- oxo ethyl)piperazin- 1 -
yl)imidazo [ 1 ,2-a] pyridin-3 -
yl)(methyl) amino)thiazol-4-y1)-5-fluorob enzonitrile,
2-(2-((6-(4-(2-(azetidin- 1 -y1)-2- oxo ethyflpip erazin- 1 -y1)-2-
ethylimidazo [1,2-a]pyridin-3 -
yl)(methyl) amino)thiazol-4-y1)-5-fluorob enzonitrile,
24443 4(4-(2-cyano-4-fluorophenyl)thiazol-2-y1)(methyflamino)-2- ethylimidazo
[ 1,2-a] pyridin-6-
yl)piperazin- 1 -y1)-N,N-dimethylacetamide,
24443 -44-(2-cyano-4-fluorophenyflth iazol-2-y1)(m ethyflam in o)-2- ethyl imi
dazo [ 1 ,2-a] pyridi n-6-
yl)piperazin- 1 -y1)-N -methylacetamide,
2-(24(2-ethy1-64 1 -(2-(3 -hydroxyazetidin- 1 -y1)-2- oxo ethyl)piperidin-4-
yflimidazo pyridin-3 -
yl)(methypamino)thiazol-4-y1)-5-fluorob enzonitrile,
24443 44-(2-cyano-4-fluorophenyl)thiazol-2-y1)(methyflamino)-2- ethylimidazo [
pyridin-6-
yOpiperidin- 1 -y1)-N,N- dimethy lac etamide,
2-(24(2-ethy1-64 1 -(2-(3 -hydroxyazetidin- 1 -y1)-2- oxo ethyflpiperidin-4-
yflimidazo [ 1,2-a]pyridin-3 -
yl)(methyl) amino)-5 -methylthiazol-4-y1)-5 -fluorob enzonitrile,
2-(5-((2-ethy1-6- ( 1 -(2-(3 -hydroxyazeti di n- 1 -y1)-2- oxo ethyl)piperi
din-4-y1) imidazo [1 ,2-a] pyri di n-3 -
ye(methyl) amino)- 1 ,2,4-thiadiazol-3 -y1)-5 -fluor b enzonitrile,
2-(4-(2-(2-cyanoethyl)-34(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo
[ 1 ,2-a] pyridin-6-
yppiperidin- 1 -y1)-N,N-dimethylacetamide,
3 -(3 -((4-(4-fluoropheny1)-5-(hydroxymethyl)thiazol-2-y1)(methyl) amino)-64 1
-(methylsulfonyl)piperidin-
4-yl)imidazo[1,2-a]pyridin-2-yl)propanenitrile,
3-(6-( 1 -(2-(dimethylamino)-2- oxo ethyl)piperidin-4-y1)- 3 4(444-
fluorophenyl)thiazol-2-
yl)(methyflaminotimidazo pyridin-2-yl)propanamide,
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
N-(6-(3 -amino azetidin- 1 -y1)-2-ethylimidazo [1,2-a]pyridin-3-y1)-4-(4-
fluoropheny1)-N-methylthiazol-2-
amine,
2-(1-(2-ethy1-3-44-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [1,2-a]
pyridin-6-yl)azetidin-3-
ylamino)- l-(3 -hydroxyazetidin-1 -yOethanone,
N-( 1 -(2- ethy1-34(4-(4-fluorophcnyl)thiazol-2-y1)(methyl)amino)imidazo [1,2-
a]pyridin-6-yl)azetidin-3 -
y1)-2-(3-hydroxyazetidin-1 -yl)acetamide,
2-(4-(2-ethy1-34(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [1 ,2-
a]pyridin-6-yl)piperazin-1 -
yl) ethanol,
N-(2- ethy1-6-morpho linoimidazo [1,2-a]pyridin-3 -y1)-4-(4-fluoropheny1)-N-
methylthiazol-2-amine,
4-(2-ethyl-3 44-(4-fluorophenyethiazol-2-y1)(methypamino)imidazo [1,2-
a]pyridin-6-y1)-thiomorpho line
1,1-dioxide,
1 -(34(4-(4-chlorophenyl)thiazol-2-y1)(me thyl)amino)-2- ethylimidazo [1,2-a]
pyridin-6-yl)imidazolidin-2-
one,
ethyl 2-(3 -(3 4(4-(4-chlorophenyl)thiazol-2-y1)(methypamino)-2-
ethylimidazo [ 1,2-a] pyridin-6-y1)-2-
oxo imi dazol idin-1 -yl)acetate,
4-(4-chloropheny1)-N-methyl-N-(64 1 -(methylsulfony1)-1,2,3,6-
tetrahydropyridin-4-y1)-2-(2,2,2-
trifluoro ethyl)imidazo [1,2-a] pyridin-3 -yl)thiazol-2-amine,
2-(2-((2-ethy1-6-(1-(methylsulfonyl)piperidin-4-yeimidazo[1,2-a]pyridin-3-
y1)(methyeamino)-4-(4-
fluorophenyl)thiazol-5-yl)acetonitrile,
2- ethyl-N-(4-(4-fluorophenyl)pyrimidin-2-y1)-N-methyl-6-(1 -(methylsulfony1)-
1,2,3,6-tetrahydropyridin-
4-yl)imidazo [ 1,2-a] pyridin-3 -amine,
3-(4-chloropheny1)-N-(2- ethyl-6-(4-(methylsulfonyl)pip erazin- 1 -
yl)imidazo[1,2-a]pyridin-3-y1)-N-
methyl-1,2,4-th iadi azol-5-a mine,
N-(2-ethyl-6-(1 -(methylsulfonyI)-1,2,3,6-tetrahydropyridin-4-yl)imidazo [ 1,2-
a] pyridin-3 -y1)-3-(4-
fluoropheny1)-N-methy1-1,2,4-oxadiazol-5 -amine,
2-(4-(2-ethy1-3 -(4-
fluoropheny1)-1,2,4-thiadiazol-5 -y1)(methyl)amino)imidazo [1,2-a] pyridin-6-
y1)-
5,6-dihydropyridin- 1 (2H)-y1)-N,N-dimethylacetamide,
2-(4-(2-ethyl-34(3-(4-fluoropheny1)-1,2,4-thiadiazol-5-
y1)(methyl)amino)imidazo [1,2-a] pyridin-6-
yl)piperidin-1 -y1)-N,N- dimethylac ctamide,
N-(6-(4-(( 1 H-imidazol-5-yl)methyl)pip erazin- 1 -y1)-2- ethylimidazo [ 1,2-
a] pyridin-3 -y1)-4-(4-
flu oroph eny1)-N-methylthi azol-2-a min e,
N -(2- ethy1-6-(1 -(methylsulfony1)-1,2,3,6-tetrahydropyridin-4-yl)imidazo [
1,2-a] pyridin-3 -y1)-N -methy1-4-
(4-(trifluoromethyl)phenyl)thiazol-2-amine,
N-cyclopropy1-2-(4-(2-ethyl-3-44-(4-fluorophenypthiazol-2-
y1)(methypamino)imidazo [ 1,2-a] pyridin-6-
yl)piperidin-1 -yl)acetamide,
54(4-(2-ethy1-3 - 4444- fluorophenyl)thiazol-2-y1)(methypamino)imidazo [1,2-
a]pyridin-6-y pip eridin- 1 -
yOmethyl)-3-methyloxazolidin-2-one,
46
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
(R)-544-(2-ethy1-34(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [ 1
,2-a] pyridin-6-
yl)piperidin- 1 -yl)methyl)oxazolidin-2-one,
(S)-5-((4-(2-ethy1-3 (4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [ 1
,2-a] pyridin-6-
yOpiperidin- 1 -yl)methyl)oxazolidin-2-one,
44(4-(2-ethy1-3 -( (4-(4- fluorophenyethiazol-2 -y1)(methypamino)imidazo [1,2-
a]pyridin-6 -yl)pip cridin- 1 -
yl)methyl)oxazo lidin-2- one,
N-(2- ethy1-6-(1 -(methylsulfo ny1)- 1 ,2,3 ,6-tetrahydropyri di n-4-yl)imi
dazo [ 1 ,2-a]pyri di n-3 -y1)-3 -(4-
fluoropheny1)-N -methyl- 1 ,2,4-thiadiazol-5 -amine,
1 -(4-(2-ethy1-3 4(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [ 1 ,2-
a] pyridin-6 -yl)pip erazin- 1 -
yl)prop ane- 1 ,2-dione,
5-(4-(2-ethy1-3 -44-(4-fluorophenyethiazol-2-y1)(methypamino)imidazo [ 1 ,2-a]
pyridin-6 -yl)pip erazine- 1 -
carb onyl)pyrro lidin-2- one,
(1 -aminocyclopropyl)(4 -(2- cthy1-3 4(4-(4-fluorophcnyl)thiazol-2-
y1)(methyeamino)imidazo [ 1 ,2-
pyridin-6-yl)piperazin- 1 -yl)methanone,
(S)- 1 -(4-(2-ethy1-3 -((4-(4-fluo rophe nyl)th iazol-2-y1)(methypamino) imid
azo [ 1 ,2-a] pyrid i n-6 -yl)p ip erazin-
1 -y1)-2-hydroxyprop an- 1 -one,
2-(4-(2-ethy1-3 4(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [ 1 ,2-
a] pyridin-6 -yl)pip erazin- 1 -
y1)-2- oxoacetamide,
1 -benzy1-4-(4-(2 - ethy1-3((4(4-fluorophenyl)thiazol-2-
y1)(methyl)amino)imidazo [ 1 ,2-al pyridin-6-
yOpiperazine- 1 -carb ony Opyrrolidin-2-one,
3 -(3 4(4-(4-chlorophcnyl)thiazol-2-y1) (methyl)amino)-2- cthylimidazo [ 1 ,2-
a] pyridin-6 -yl)oxazo lidin-2-
one,
2-(2-ethyl-3((4(4-chl orophe nyl)th iazol-2-y1)(methyl)ami n o)im i dazo [1 ,2-
a] pyri di n-6-y1)-1-
[ 1 ,2]thiazinane- 1, 1 -dioxide,
4-(2-ethyl-3 (4-fluorophenyl)thiazol-2-y1)(methyDamino)imidazo [1,2-
a]pyridin-6-y1)-N-(thiophen-2-
y1)-5,6- dihydropyridine- 1 (2H)-carb oxamide,
4-(4-chloropheny1)-N- (2- ethy1-64 1 -(methylsulfony1)- 1 ,2,3 ,6-
tetrahydropyridin-4-yl)imidazo [ 1,2-
pyridin-3 -y1)-N-methylthiazol-2-amine,
4-(4-chlorophcny1)-N- (2- ethy1-6-(1 -(trifluoromethylsulfony1)- 1,2,3 ,6-
tctrahydropyridin-4 -yl)imidazo [1,2-
pyridin-3 -y1)-N-methylthiazo1-2-amine,
1-(2-ethyl-3 (4-flu o rophenyl)thiazol-2-y1)(m ethypami n o) m idazo [1 ,2-
a]pyri di n-6-yl)p ip eri di n-4-ol,
24443 44-(4-chlorophenyethiazol-2-y1)(methyl) amino)-2 - ethylimidazo [ 1 ,2-
a] pyridin-6-yepiperazin- 1 -
yl) ethanol,
4-(2-ethyl-3((4(4-chlorophenyl)thiazol-2-y1)(methyDamino)imidazo [ 1 ,2-
a]pyridin-6-y1)-
thiomorpholine- 1, 1 -dioxide,
tert-butyl 4-(2-ethy1-3 - 4444- fluorophenyl)thiazol-2-y1)(methypamino)imidazo
[ 1 ,2-a] pyridin-6-y1)-5,6-
dihydropyridinc- 1 (2H)-carb oxylatc,
47
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
1 -(4-(2-ethy1-3 4(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo [ 1 ,2-
a] pyridin-6 -yl)pip eridin- 1 -
yl)prop an- 1 -one,
N-(2- ethy1-6-(pip eridin-4-yl)imidazo [ 1 ,2-a] pyridin-3 -y1)-4- (4-
fluoropheny1)-N-methylthiazol-2-amine,
N-(6-( 1 -benzylpiperidin-4-y1)-2-ethylimidazo [1,2-a]pyridin-3 -y1)-4- (4-
fluoropheny1)-N-methylthiazol-2-
amine,
N-(2-ethyl-6-(1 -isopropylpip eridin-4-yl)imidazo [ 1 ,2-a] pyridin-3 -y1)-4-
(4-fluoropheny1)-N-methylthiazol-
2-am ine,
tert-butyl 4-(2-ethy1-3 -((4-(4-fluorophenyl)thiazol-2-
y1)(methyl)amino)imidazo [ 1 ,2-a] pyridin-6-
yl)piperidine- 1 -carboxylate,
N-(6-(3,6-dihydro-2H-pyran-4-y1)-2-ethylimidazo [ 1 ,2- a] pyridin-3 -y1)-4-(4-
fluoropheny1)-N-
methylthiazol-2-amine,
4-(4-chloropheny1)-N-(6-(3,6-dihydro-2H-pyran-4-y1)-2-ethylimidazo[1,2-a]
pyridin-3 -y1)-N-
methylthiazol-2-amine,
(2-(4-(2-ethy1-3 -((4-(4- fluorophenyethiazol-2 -y1)(methyeamino)imidazo [
pyridin-6 -yepip eridin- 1 -
y1)-4,5 - dihydrooxazol-5 -y1) m ethanol,
2-(4-(2-ethy1-3 -( (4-(4-fluorophenyethiazol-2-y1)(methyl)amino)imidazo [ 1 ,2-
a.] pyridin-6 -yepip eridin- 1 -
y1)- 1 - (3 -hydroxypyrro lidin- 1 -y1) ethanone,
2-(2-((2-ethy1-6- ( 1 -(methylsulfonyppiperidin-4-yeimidazo [
pyridin-3 -y1)(methyl) amino)thiazol-4-
y1)-5 - fluorophenol,
tert-butyl 4-(3-((3 -(4-chloropheny1)- 1,2,4-thiadiazol- 5 -y1)(methypamino)-2-
ethy limidazo [ 1 ,2-µa] pyridin-
6-yl)pip crazinc- 1 -carb oxylatc,
N-(6-(4-(( 1 H-imidazol-2-yl)methyl)pip erazin- 1 -y1)-2- ethylimidazo [ 1 ,2-
a] pyridin-3 -y1)-4- (4-
fluoropheny1)-N-methylth i azol-2-amine,
cyclopropy1(4- (2- ethy1-3 44-(4-fluorophenyflthiazol-2-
y1)(methyflamino)imidazo [ 1,2-a] pyridin-6-
yl)piperazin- 1 -yl)methanone,
ethyl 2-(4-(2-ethy1-3 -44-(4-fluorophenyl)thiazol-2-
y1)(methyeamino)imidazo [ 1 ,2-a] pyridin-6-
yl)piperazin- 1 -y1)-2- oxo acetate,
[6-( 1 , 1 -Dioxo-is othiaz olidin-2-y1)-2-ethyl-imidazo [1,2- a]pyridin-3 -
yl] 4444- fluoro-pheny1)-thiazol-2-y1]-
methyl-amine,
tert-butyl 4-(3-((4-(4-chlorophenyl)thiazol-2-y1)(methyl)amino)-2-ethylimidazo
[ 1 ,2-a] pyridin-6 -y1)-5,6-
dihydropyridi ne-1 (2H)-carboxylate,
4-(4-chloropheny1)-N - (6-(3,6-dihydro-2H-thiopyran-4-y1)-2- ethylimidazo [
pyridin-3 -y1)-N -
methylthiazol-2-amine,
N-(6-(4,4- difluoropip eridin- 1 -y1)-2-ethylimidazo [ 1 ,2-a.] pyridin-3 -y1)-
4-(4-fluoropheny1)-N-
methylthiazol-2-amine,
N-(6-( 1 -(3 -chloropropy ls ulfony1)- 1 ,2,3 ,6-tetrahy dropyridin-4-y1)-2-
ethylimidazo [ 1 ,2-::t] pyridin-3 -y1)-4-
(4-fluoropheny1)-N-methylthiazol-2-aminc,
48
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
tert-butyl 4-(34(4-(4-chlorophenyl)thiazol-2-y1)(methyl)amino)-2-
ethylimidazo[1,2-a]pyridin-6-
yl)piperazine- 1 - carb oxylate,
N-(6-( 1 -(cyclohexylmethyl)piperidin-4-y1)-2- ethylimidazo [ 1 ,2- a] pyridin-
3 -y1)-4-(4-fluoropheny1)-N-
methylthiazol-2-amine,
N-(2-ethy1-6-(5-methy1-4,5-dihydrooxazol-2-yl)imidazo[1,2-a]pyridin-3-y1)-4-(4-
fluoropheny1)-N-
methylthiazol-2-amine,
N-(2- ethy1-6-(4-methy1-4,5 - dihydrooxazol-2 -yl)imi d azo [ 1 ,2-a] pyri d i
n-3 -y1)-4-(4-fluoroph eny1)-N-
methylthiazol-2-amine,
2-(2-ethyl-3 44- (4- fluorophenyl)thiazol-2-y1)(methyl)aminotimidazo [1,2- a]
pyridin-6-y1)-4,5 -
dihydrooxazole-4-carboxylic acid,
(2-(2- ethy1-3 - -(4- fluorophenypthiazol-2-y1)(methyl)amino)imidazo [ 1,2-a]
pyridin-6-y1)-4,5 -
dihydrooxazol-4-yOmethanol, and
4-(4-chloropheny1)-N- (6-(4,5-dihydro oxazol-2 -y1)-2- ethylimidazo [ 1 ,2- a]
pyridin-3 -y1)-N-methylthiazol-2-
amine.
[00185] In one embodiment, a compound of the invention according to Formula I
is 2-((2-ethy1-6-(4-
(2-( 3 -hydroxyazetidin- 1 -y1)-2- oxo ethyl)piperazin- 1 -y1)- 8-
methylimidazo [ 1,2-a]pyridin-3 -
y1)(methypamino)-4-(4-fluorophenyl)thiazole-5-carbonitrile.
[00186] In another embodiment, a compound of the invention according to
Formula I is not 2-42-
ethy1-6-(4-(2-(3 -hydroxyazetidin- 1 -y1)-2-oxoethyl)pip erazin- 1 -y1)-8 -
methylimidazo [ 1 ,2-al pyridin-3 -
yl)(methyl)amino)-4-(4-fluorophenyl)thiazole-5-carbonitrile.
[00187] In one embodiment a compound of the invention is not an isotopic
variant.
[00188] In one aspect a compound of the invention according to any one of the
embodiments herein
described is present as the free base.
[00189] In one aspect a compound of the invention according to any one of the
embodiments herein
described is a pharmaceutically acceptable salt.
[00190] In one aspect a compound of the invention according to any one of the
embodiments herein
described is a solvate of the compound.
[00191] In one aspect a compound of the invention according to any one of the
embodiments herein
described is a solvate of a pharmaceutically acceptable salt of a compound.
[00192] While specified groups for each embodiment have generally been listed
above separately, a
compound of the invention includes one in which several or each embodiment in
the above Formula, as
well as other formulae presented herein, is selected from one or more of
particular members or groups
designated respectively, for each variable. Therefore, this invention is
intended to include all
combinations of such embodiments within its scope.
[00193] While specified groups for each embodiment have generally been listed
above separately, a
compound of the invention may be one for which one or more variables (for
example, R groups) is
selected from one or more embodiments according to any of the Formula(c)
listed above. Therefore, the
49
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
present invention is intended to include all combinations of variables from
any of the disclosed
embodiments within its scope.
[00194] Alternatively, the exclusion of one or more of the specified variables
from a group or an
embodiment, or combinations thereof is also contemplated by the present
invention.
[001951 In certain aspects, the present invention provides prodrugs and
derivatives of the compounds
according to the formulae above. Prodrugs are derivatives of the compounds of
the invention, which have
metabolically cleavable groups and become by solvolysis or under physiological
conditions the
compounds of the invention, which are pharmaceutically active, in vivo. Such
examples include, but are
not limited to, choline ester derivatives and the like, N-alkylmorpholine
esters and the like.
[00196] Other derivatives of the compounds of this invention have activity in
both their acid and acid
derivative forms, but the acid sensitive form often offers advantages of
solubility, tissue compatibility, or
delayed release in the mammalian organism (Bundgard, H, 1985). Prodrugs
include acid derivatives well
know to practitioners of the art, such as, for example, esters prepared by
reaction of the parent acid with a
suitable alcohol, or amides prepared by reaction of the parent acid compound
with a substituted or
unsubstituted amine, or acid anhydrides, or mixed anhydrides. Simple aliphatic
or aromatic esters,
amides and anhydrides derived from acidic groups pendant on the compounds of
this invention are
preferred prodrugs. In some cases it is desirable to prepare double ester type
prodrugs such as
(acyloxy)alkyl esters or ((alkoxycarbonyl)oxy)alkylesters. Particularly useful
are the Ci to Cs alkyl, C2-
C8 alkenyl, aryl, C7-C1 substituted aryl, and C7-C17 arylalkyl esters of the
compounds of the invention.
CLAUSES
1) A compound according to Formula I:
Rib
X \
(R7), Rla
// R2
R8 NX-W
N
R6b
R6a
wherein
Rla is H, halo or C1_4 alkyl;
Rib is:
- halo,
- Ci_4 alkyl (which alkyl is optionally substituted with one or more
independently selected
halo), or
-Ci4 alkoxy (which alkoxy is optionally substituted with one or more
independently selected
halo);
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
X is ¨S-, -0-, ¨N=CH-, ¨CH=N- or ¨CH=CH-;
W is N, or CR3
when W is N, R2 is:
- H,
- -CN,
- halo,
- C1_4 alkyl (which alkyl is optionally substituted with one or more
independently selected OH,
or CN)
- -C(=0)CH3,
- -C(=0)CF3,
- -C(=0)0CH3,
- -C(=0)NH2,
- -NHC(=0)CH3, or
when W is CR3, one of R2 or R3 is:
- H,
- -CN,
- halo,
- C1 alkyl (which alkyl is optionally substituted with one or more
independently selected OH,
or CN)
- -C(=0)CH3,
- -C(=0)CF3,
- -C(=0)0CH3,
- -C(=0)NH2,
- -NHC(=0)CH3,
and the other is H, or C1_4 alkyl;
R4 is C1 4 alkyl;
R5 is C1_4 alkyl optionally substituted with one or more independently
selected CN, OH, halo, or -
C(=0)NH2;
one of R6a or R6b is selected from H, -CH3, and halo, and the other is H;
Cy is:
- C4_10 cycloalkyl,
- 4-10 membered mono or bicyclic heterocycloalkyl containing one or more
heteroatoms
independently selected from 0, N, and S, or
- 4-7 membered heterocycloalkenyl containing 1 double bond, containing one
or more
heteroatoms independently selected from 0, N, and S;
each R7 is independently selected from:
- OH,
- oxo,
51
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
- halo, and
- C14 alkyl (which alkyl is optionally substituted with one or more
independently selected OH,
or C 1_4 alkoxy);
the subscript a is 0, 1 or 2;
R8 is -OLI-Wilin-L2-G1,
wherein
- L1 is absent, or is -0-, -C(=0)-, -NR', -NRhC(=0)-, or -SO2-;
- WI is C1_4 alkylene;
- the subscript m is 0, or 1;
- L2 is absent, or is -0-, -C(=0)-, -C(=0)0-, -0C(=0) -C(=0)-C(=0)-, -C(=0)-
C(=0)NRa-,
-NRb-, -C(=0)NRe-, -NRdC(=0)-, -NRiC(=0)0-, SO2, -SO2NRe- or ¨NRfS02-;
- Gis
o H,
o -CN,
o C14 alkyl (which alkyl is optionally substituted with one or more
independently
selected ¨CN, OH, halo or phenyl),
o C3_7 cycloalkyl (which cycloalkyl is optionally substituted with ¨NH2),
o 5-6 membered heterocycloalkenyl containing 1 double bond containing one
or more
heteroatoms independently selected from 0, N, and S (which heterocycloalkenyl
is
optionally substituted with one or more independently selected R9 groups),
o 4-10 membered mono, bi or spirocyclic heterocycloalkyl containing one or
more
heteroatoms independently selected from 0, N, and S (which heterocycloalkyl is
optionally substituted with one or more independently selected R9 groups), or
o 5-6 membered heteroaryl containing one or more heteroatoms independently
selected
from 0, N, and S (which heteroaryl is optionally substituted with one or more
independently selected le groups),
each R9 is oxo, or R19;
each le is:
- -OH,
- halo,
- -CN,
- C 1_4 alkyl (which alkyl is optionally substituted with one or more
independently selected OH,
halo, or phenyl),
- C14 alkoxy,
- C3_7 cycloalkyl,
- phenyl,
- -S02CH1,
- -C(0)C14 alkoxy,
52
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
- -C(=0)C 1_4 alkyl, or
- -NRgC(=0)C1.4 alkyl; and
each Re, Rb, Re, Rd, Re, Rf, Rg, Rh, R',and Ri is independently selected from
H and Ci_ct alkyl;
or a pharmaceutically acceptable salt, or a solvate, or a pharmaceutically
acceptable salt of a
solvate thereof; or a biologically active metabolite thereof
2) A compound or pharmaceutically acceptable salt thereof, according to
clause 1, wherein Ria is F,
Cl, -CH3 or ¨C2H5.
3) A compound or pharmaceutically acceptable salt thereof, according to
clause 1, wherein Ria is H.
4) A compound or pharmaceutically acceptable salt thereof, according to any
one of clauses 1-3,
wherein Rib is F, Cl, -CH3, ¨C415, -CF3, -OCH3, or -0CF3.
5) A compound or pharmaceutically acceptable salt thereof, according to any
one of clauses 1-3,
wherein Rib is F.
6) A compound or pharmaceutically acceptable salt thereof, according to any
one of clauses 1-5,
wherein X is ¨S- or -0-.
7) A compound or pharmaceutically acceptable salt thereof, according to any
one of clauses 1-5,
wherein X is ¨S-.
8) A compound or pharmaceutically acceptable salt thereof, according to any
one of clauses 1-5,
wherein W is N.
9) A compound or pharmaceutically acceptable salt thereof, according to any
one of clauses 1-5,
wherein W is CR3.
10) A compound or pharmaceutically acceptable salt thereof, according to
clause 9, wherein R3 is H,
CN, F, or Cl.
11) A compound or pharmaceutically acceptable salt thereof, according to
clause 1, wherein the
compound is according to Formula 11:
(R7)a
R4, iljj ON
R8
R6b
Rea
wherein R4, R5, R6a, K6b,
R7, le and the subscript a are according to clause 1.
53
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
12) A compound or pharmaceutically acceptable salt thereof, according to
clause 1, wherein the
compound is according to Formula III:
(R7)a
4
R,
R8 CN
R5
R6b
R6a
wherein R4, R5, R6a, 6b
x, R7, le and the subscript a are according to clause 1.
13) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 1-12,
wherein R4 is -CH3, or ¨C2H5.
14) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 1-12,
wherein R4 is -CH3.
15) A compound or pharmaceutically acceptable salt thereof; according to
any one of clauses 1-14,
wherein R5 is C1_4 alkyl.
16) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 1-14,
wherein fe is -CH3, or ¨C2H5.
17) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 1-14,
wherein R5 is C1,4 alkyl substituted with one CN, OH, halo, or -C(=0)NH2.
18) A compound or pharmaceutically acceptable salt thereof, according to
clause 17, wherein R5 is -
C1-13, ¨C2H5 or ¨C3H2 substituted with one CN, OH, halo, or -C(=0)NH2.
19) A compound or pharmaceutically acceptable salt thereof; according to
clause 17, wherein RD is ¨
CH2-CH2-CN, ¨CH2-CH2-0H, ¨CH2-CF3, or ¨CH2-CH2-C(=0)NH2.
20) A compound or pharmaceutically acceptable salt thereof; according to
any one of clauses 1-19,
wherein Cy is C4_10 cycloalkyl.
21) A compound or pharmaceutically acceptable salt thereof; according to
clause 20, wherein Cy is
cyclobutyl, cyclopentyl or cyclohexyl.
22) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 1-19,
wherein Cy is 4-10 membered mono or bicyclic heterocycloalkyl containing one
or more
heteroatoms independently selected from 0, N, and S.
23) A compound or pharmaceutically acceptable salt thereof, according to
clause 22, wherein Cy is
oxetanyl, azetidinyl, tetrahydrofuranyl, pyrrolidinyl, tetrahydropyranyl,
piperidinyl, piperazinyl,
morpholinyl, or thiomorpholinyl.
54
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
24) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 1-19,
wherein Cy is 4-7 membered heterocycloalkenyl containing 1 double bond,
containing one or more
heteroatoms independently selected from 0, N, and S.
25) A compound or pharmaceutically acceptable salt thereof, according to
clause 24, wherein Cy is
dihydrofuranyl, dihydrothiazolyl, dihydrooxazolyl, dihydropyranyl,
tetrahydropyridinyl, or
dihydrothiopyranyl.
26) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 1-25,
wherein the subscript a is 1 or 2, and R7 is OH, oxo, F, Cl, or ¨CH3.
27) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 1-25,
wherein the subscript a is 0.
28) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 1-27,
wherein R8 is -(L1-W1)m-L2-Gi
29) A compound according to Formula I, II, or III, wherein the compound is
according to Formula IVa,
IVb, IVc or IVd:
L2 / /61\/1-1-1)m. (W1-1-1)m, \ \
L2
N-
S CN CN
N""'S
R6tr.'"--y1N
R6a R6a
IVa, IVb,
N ,
CN L2 L2 N'Th \ N- \ CN
Ns
R6b(N R6bM(LN
Rea Rea
IVC, or IVd
wherein R6a, R6b, LI, W1, L2, G1 and the subscript m are according to clause
1.
30) A compound or pharmaceutically acceptable salt thereof; according to
clause 28, or 29, wherein the
subscript m is 1, L1 is absent.
31) A compound or pharmaceutically acceptable salt thereof, according to
clause 28, or 29, wherein the
subscript m is 1, Li is -C(=0)-, or -SO2-.
32) A compound or pharmaceutically acceptable salt thereof; according to
any one of clauses 28-31,
wherein the subscript m is 1, and WI is C1_4 alkylene.
33) A compound or pharmaceutically acceptable salt thereof, according to
clause 32, wherein the
subscript m is 1, L1 is as defined above and Wi is -CH2-, -CH¨CH2-, -C(CH3)H-,
-CH2-CH2-CH2-
or -CH2-C(CH3)H-.
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
34) A compound or pharmaceutically acceptable salt thereof, according to
clause 28, or 29, wherein the
subscript m is 0.
35) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-34,
wherein L2 is absent.
36) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-34,
wherein L2 is -0-.
37) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-34,
wherein L2 is ¨C(=0)-.
38) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-34,
wherein L2 is ¨C(=0)0- or -0C(=0)-.
39) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-34,
wherein L2 is -C(=0)-C(=0)NRa-.
40) A compound or pharmaceutically acceptable salt thereof, according to
clause 39, wherein Ra is H.
41) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-34,
wherein L2 is -NRb-.
42) A compound or pharmaceutically acceptable salt thereof, according to
clause 41, wherein Rb is H.
43) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-34,
wherein L2 is -C(=0)NR`-.
44) A compound or pharmaceutically acceptable salt thereof, according to
clause 41, wherein Re is H, -
CH3, or ¨CH2-CH3.
45) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-34,
wherein L2 is -NRdC(=0)-.
46) A compound or pharmaceutically acceptable salt thereof, according to
clause 45, wherein Rd is H, -
CH3, or ¨CH2-0-13.
47) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-34,
wherein L2 is - S 02-.
48) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-34,
wherein L2 is - S 0 NRe-.
49) A compound or pharmaceutically acceptable salt thereof, according to
clause 48, wherein Re is H, -
CH3, or ¨CH2-CH3.
50) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-34,
wherein L2 is ¨NRfS02-.
51) A compound or pharmaceutically acceptable salt thereof, according to
clause 50, wherein 12 is H, -
CH3, or ¨CH2-CH3.
52) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-51,
wherein G1 is H, or CN.
53) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-51,
wherein G1 is C 1_4 alkyl.
56
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
54) A compound or pharmaceutically acceptable salt thereof, according to
clause 53, wherein G1 is -
CH3, or ¨CH3-CH3.
55) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-51,
wherein G1 is C1_4 alkyl substituted with ¨CN, OH, halo or phenyl.
56) A compound or pharmaceutically acceptable salt thereof, according to
clause 55, wherein G1 is -
CF3, -CH2-C1, -CH7-CN, -CH2-0H or ¨CH2-Ph.
57) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-51,
wherein GI is C3_7 cycloalkyl.
58) A compound or pharmaceutically acceptable salt thereof, according to
clause 57, wherein G1 is
cyclopropyl, cyclobutyl, cyclopropyl, or cyclohexyl.
59) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-51,
wherein G1 is C3-7 cycloalkyl substituted with ¨NH2.
60) A compound or pharmaceutically acceptable salt thereof, according to
clause 59, wherein Gi is
cyclopropyl, cyclobutyl, cyclopropyl, or cyclohexyl, each of which is
substituted with ¨NH2.
61) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-51,
wherein Gj is 5-6 membered heterocycloalkenyl containing 1 double bond
containing one to three
heteroatoms independently selected from 0, N, and S.
62) A compound or pharmaceutically acceptable salt thereof, according to
clause 61, wherein GI is
dihydrofuranyl, dihydrothiazolyl, dihydrooxazolyl, dihydropyranyl, or
dihydrothiopyranyl.
63) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-51,
wherein G1 is 4-10 membered mono, bi or spirocyclic heterocycloalkyl
containing one to three
heteroatoms independently selected from 0, N, and S.
64) A compound or pharmaceutically acceptable salt thereof, according to
clause 63, wherein G1 is
oxetanyl, azetidinyl, tetrahydrofuranyl, pyrolidinyl, tetrahydropyranyl,
piperidinyl, piperazinyl,
morpholinyl, thiomorpholinyl, or 2,6-Diaza-spiro[3.3]heptane.
65) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-51,
wherein G1 is 4-10 membered mono, bi or spirocyclic heterocycloalkyl
containing one to three
heteroatoms independently selected from 0, N, and S, substituted with one or
two independently
selected R9.
66) A compound or pharmaceutically acceptable salt thereof, according to
clause 65, wherein G1 is
oxetanyl, azetidinyl, tetrahydrofuranyl, pyrolidinyl, tetrahydropyranyl,
piperidinyl, piperazinyl,
morpholinyl, thiomorpholinyl, or 2,6-Diaza-spiro[3.3]heptane, each of which is
substituted with
one or two independently selected R9.
67) A compound or pharmaceutically acceptable salt thereof, according to
clause 65 or 66, wherein R9
is oxo.
68) A compound or pharmaceutically acceptable salt thereof, according to
clause 65 or 66, wherein R9
is RI and RI is selected from OH, F, Cl, and -CN.
57
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
69) A compound or pharmaceutically acceptable salt thereof, according to
clause 65 or 66, wherein R9
is RI and RI is selected from -CH3, -CH2-CH3, -CF3, -CH2-CH2-0H, -CH2-
phenyl,
70) A compound or pharmaceutically acceptable salt thereof; according to
clause 65 or 66, wherein R9
is RI and RI is selected from -OCH3, -OCH2-CH3, and -0C(CH3)3.
71) A compound or pharmaceutically acceptable salt thereof, according to
clause 65 or 66, wherein R9
is RI and RI is selected from -S02CH3, -C(=0)0CH3, and -C(=0)CH3.
72) A compound or pharmaceutically acceptable salt thereof, according to
clause 65 or 66, wherein
each R9 is RI and RI is -NRgC(=0)CH3, or -NRgC(=0)CH2CH3.
73) A compound or pharmaceutically acceptable salt thereof; according to
clause 72, wherein each Rg
is H, -CH3, or -CH2CH3.
74) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 28-51,
wherein G1 is 5-6 membered heteroaryl containing one to three heteroatoms
independently selected
from 0, N, and S.
75) A compound or pharmaceutically acceptable salt thereof, according to
clause 74, wherein G1 is
furanyl, thienyl, pyrrolyl, pyrazolyl, imidazolyl, triazolyl, oxazolyl,
oxadiazolyl, thiazolyl,
thiadiazolyl, pyridyl, pyrazinyl, or pyrimidyl.
76) A compound or pharmaceutically acceptable salt thereof; according to
any one of clauses 28-51,
wherein GI is 5-6 membered heteroaryl containing one to three heteroatoms
independently selected
from 0, N, and S, substituted with one or two independently selected RI .
77) A compound or pharmaceutically acceptable salt thereof, according to
clause 76, wherein G1 is
furanyl, thicnyl, pyrolyl, pyrazolyl, imidazolyl, triazolyl, oxazolyl,
oxadiazolyl, thiazolyl,
thiadiazolyl, pyridyl, pyrazinyl, or pyrimidyl, each of which is substituted
with one or two
independently selected RI .
78) A compound or pharmaceutically acceptable salt thereof, according to
clause 77, wherein RI is
selected from OH, F, Cl, and -CN.
79) A compound or pharmaceutically acceptable salt thereof, according to
clause 77, wherein RI is
selected from -CH3, -CH2-CH3, -CF3, -CF17-CH2-0H, and -CH2-phenyl.
80) A compound or pharmaceutically acceptable salt thereof, according to
clause 77, wherein RI is
selected from -OCH3, -OCH2-CH3, and -0C(CH3)3.
81) A compound or pharmaceutically acceptable salt thereof; according to
clause 77, wherein RI is
selected from -S02CH3, -C(=0)0CH3, and -C(=0)CH3.
82) A compound or pharmaceutically acceptable salt thereof, according to
clause 77, wherein each RI
is -NRgC(=0)CH3, or -NRgC(=0)CH2CH3.
83) A compound or pharmaceutically acceptable salt thereof; according to
clause 82, wherein each Rg
is H, -CH3, or -CH2CH3.
58
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
84) A compound according to clause 1, wherein the compound is according to
Formula Va, Vb, Vc, or
Vd:
\ \
N \N¨<N
G1 s CN G1 s CN
R6trz-N
R6a R6a
Va, Vb,
R\
CN 7¨W1- N \ CN
G1 N S G
S
N ____________________
R6r)./.1-74-N
R6a R6a
VC, or Vd
wherein R6a, Rob, W1, and G1 are according to clause 1.
85) A compound or pharmaceutically acceptable salt thereof, according to
clause 84, wherein W1 is
C1-4 alkylene.
86) A compound or pharmaceutically acceptable salt thereof, according to
clause 85, wherein WI is -
CH2-, -CH2-CH2-, -C(CH3)H-, -CH2-CH2-CH2- or -CH2-C(CH3)H-.
87) A compound according to to clause 1, wherein the compound is according
to Formula VIa, VIb,
Vic, or VId:
0 0
\ GiAN-Th \
S CN CN
N
R6a R6a
Via, VI b,
N
CN
GAN CN
R6a R6a
Vic, or VI d
wherein R6a, R6b, and G1 are according to clause 1.
59
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
88) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 84-87,
wherein G1 is 4-7 membered heterocycloalkyl containing one or more heteroatoms
independently
selected from 0, N, and S.
89) A compound or pharmaceutically acceptable salt thereof, according to
clause 88, wherein G1 is
oxetanyl, azetidinyl, tetrahydrofuranyl, pyrolidinyl, tetrahydropyranyl,
piperidinyl, piperazinyl,
morpholinyl, or thiomorpholinyl.
90) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 84-87,
wherein GI is 4-7 membered heterocycloalkyl containing one or more heteroatoms
independently
selected from 0, N, and S, which heterocycloalkyl is substituted with one or
two independently
selected R9 groups.
91) A compound or pharmaceutically acceptable salt thereof, according to
clause 90, wherein G1 is
oxetanyl, azetidinyl, tetrahydrofuranyl, pyrolidinyl, tetrahydropyranyl,
piperidinyl, piperazinyl,
morpholinyl, or thiomorpholinyl, each of which is substituted with one or two
independently
selected R9 groups.
92) A compound or pharmaceutically acceptable salt thereof, according to
clause 90 or 91, wherein R9
is oxo.
93) A compound or pharmaceutically acceptable salt thereof, according to
clause 90 or 91, wherein R9
is RI and RI is selected from OH, F, Cl, and -CN.
94) A compound or pharmaceutically acceptable salt thereof, according to
clause 90 or 91, wherein R9
is RI and RI is selected from -CH3, -CH2-CH3, -CF3, -CH2-CH2-0H, and -CH2-
phenyl.
95) A compound or pharmaceutically acceptable salt thereof, according to
clause 90 or 91, wherein R9
is RI and RI is selected from -OCH3, -OCH2-CH3, and -0C(CH3)3.
96) A compound or pharmaceutically acceptable salt thereof, according to
clause 90 or 91, wherein R9
is RI and RI is selected from -S02CH3, -C(=0)0CH3, and -C(0)CH.
97) A compound or pharmaceutically acceptable salt thereof, according to
clause 90 or 91, wherein R9
is RI and RI is ¨NRgC(=0)CH3, or ¨NRgC(=0)CH2CH3.
98) A compound or pharmaceutically acceptable salt thereof, according to
clause 90 or 91, wherein
each Rg is H, -CH3, or -CH2CH3.
99) A compound or pharmaceutically acceptable salt thereof, according to
any one of clauses 1-98,
wherein R6a is H, -CH3 or F, and Ra is H.
100) A compound or pharmaceutically acceptable salt thereof, according to any
one of clauses 1-98,
wherein R6a is CH3, and Ra is H.
101) A compound or pharmaceutically acceptable salt thereof, according to any
one of clauses 1-98,
wherein R6a is H, and le is H, -CH3 or F.
102) A compound or pharmaceutically acceptable salt thereof, according to any
one of clauses 1-98,
wherein le and le are H.
103) A compound or pharmaceutically acceptable salt thereof, wherein the
compound is selected from
the compounds of Table III.
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
104) A compound or pharmaceutically acceptable salt thereof, wherein the
compound is 242-ethy1-6-
(44243 -hydroxyazetidin-1 -y1)-2- oxo ethyl)piperazin-1 -y1)-8-methylimidazo
pyridin-3-
yl)(methypamino)-4-(4-fluorophenyl)thiazole-5-carbonitrile.
105) A pharmaceutical composition comprising a pharmaceutically acceptable
carrier and a
pharmaceutically effective amount of a compound according to any one of
clauses 1-104.
106) A pharmaceutical composition according to clause 105, comprising a
further therapeutic agent.
107) A compound or pharmaceutically acceptable salt thereof, according to any
one of clauses 1-104, or
a pharmaceutical composition according to clause 105 or 106, for use in
medicine.
108) A compound or pharmaceutically acceptable salt thereof, according to any
one of clauses 1-104, or
a pharmaceutical composition according to clause 105 or 106, for use in the
treatment of fibrotic
diseases, proliferative diseases, inflammatory diseases, autoimmune diseases,
respiratory diseases,
cardiovascular diseases, neurodegenerative diseases, dermatological disorders,
and/or abnormal
angiogenesis associated diseases.
109) A compound or pharmaceutically acceptable salt thereof, according to any
one of clauses 1-104, or
a pharmaceutical composition according to clause 105 or 106, for use in the
treatment of idiopathic
pulmonary fibrosis.
110) The use of a compound or pharmaceutically acceptable salt thereof or the
pharmaceutical
composition according to clause 108 or 109, wherein said compound or
pharmaceutical
composition is administered in combination with a further therapeutic agent.
111) A method for the treatment, or prevention of diseases or conditions
selected from fibrotic diseases,
proliferative diseases, inflammatory diseases, autoimmune diseases,
respiratory diseases,
cardiovascular diseases, neurodegenerative diseases, dermatological disorders,
and/or abnormal
angiogenesis associated diseases, comprising administering an amount of
compound according to
any one of clauses 1-104, or the pharmaceutical composition according any one
of clauses 105 or
106, sufficient to effect said treatment, or prevention.
112) A method for the treatment, or prevention of diseases or conditions
selected from idiopathic
pulmonary fibrosis, comprising administering an amount of compound according
to any one of
clauses 1-104, or the pharmaceutical composition according any one of clauses
105 or 106,
sufficient to effect said treatment, or prevention.
113) The method according to clause 111 or 112, wherein the compound, or the
pharmaceutical
composition, is administered in combination with a further therapeutic agent.
114) The pharmaceutical composition according to clause 106, or the use
according to clause 110, or the
method according to clause 113, wherein the further therapeutic agent is for
the treatment of
fibrotic diseases, proliferative diseases, inflammatory diseases, autoimmune
diseases, respiratory
diseases, cardiovascular diseases, neurodegenerative diseases, dermatological
disorders, and/or
abnormal angiogenesis associated diseases.
61
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
115) The pharmaceutical composition according to clause 106, or the use
according to clause 110, or the
method according to clause 113, wherein the further therapeutic agent is for
the treatment of
idiopathic pulmonary fibrosis.
PHARNIACEUTICAL COMPOSITIONS
[00197] When employed as a pharmaceutical, a compound of the invention is
typically administered in
the form of a pharmaceutical composition. Such compositions can be prepared in
a manner well known
in the pharmaceutical art and comprise at least one active compound of the
invention according to
Formula 1. Generally, a compound of the invention is administered in a
pharmaceutically effective
amount. The amount of compound of the invention actually administered will
typically be determined by
a physician, in the light of the relevant circumstances, including the
condition to be treated, the chosen
route of administration, the actual compound of the invention administered,
the age, weight, and response
of the individual patient, the severity of the patient's symptoms, and the
like.
[00198] The pharmaceutical compositions of this invention can be administered
by a variety of routes
including oral, rectal, transdermal, subcutaneous, intra-articular,
intravenous, intramuscular, and
intranasal. Depending on the intended route of delivery, a compound of the
invention is preferably
formulated as either injectable or oral compositions or as salves, as lotions
or as patches all for
transdermal administration.
[00199] The compositions for oral administration can take the form of bulk
liquid solutions or
suspensions, or bulk powders. More commonly, however, the compositions are
presented in unit dosage
forms to facilitate accurate dosing. The term 'unit dosage forms' refers to
physically discrete units
suitable as unitary dosages for human subjects and other mammals, each unit
containing a predetermined
quantity of active material calculated to produce the desired therapeutic
effect, in association with a
suitable pharmaceutical excipient, vehicle or carrier. Typical unit dosage
forms include prefilled,
premeasured ampules or syringes of the liquid compositions or pills, tablets,
capsules or the like in the
case of solid compositions. In such compositions, the compound of the
invention according to Formula I
is usually a minor component (from about 0.1 to about 50% by weight or
preferably from about 1 to about
40% by weight) with the remainder being various vehicles or carriers and
processing aids helpful for
forming the desired dosing form.
[00200] Liquid forms suitable for oral administration may include a suitable
aqueous or non-aqueous
vehicle with buffers, suspending and dispensing agents, colorants, flavors and
the like. Solid forms may
include, for example, any of the following ingredients, or compound of the
inventions of a similar nature:
a binder such as microcrystallinc cellulose, gum tragacanth or gelatin; an
cxcipient such as starch or
lactose, a disintegrating agent such as alginic acid, Primogel, or corn
starch; a lubricant such as
magnesium stearate; a glidant such as colloidal silicon dioxide; a sweetening
agent such as sucrose or
saccharin; or a flavoring agent such as peppermint or orange flavoring.
62
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00201] Injectable compositions are typically based upon injectable sterile
saline or phosphate-
buffered saline or other injectable carriers known in the art. As before, the
active compound of the
invention according to Formula I in such compositions is typically a minor
component, often being from
about 0.05 to 10% by weight with the remainder being the injectable carrier
and the like.
[00202] Transdermal compositions are typically formulated as a topical
ointment or cream containing
the active ingredient(s), generally in an amount ranging from about 0.01 to
about 20% by weight,
preferably from about 0.1 to about 20% by weight, preferably from about 0.1 to
about 10% by weight,
and more preferably from about 0.5 to about 15% by weight. When formulated as
an ointment, the active
ingredients will typically be combined with either a paraffinic or a water-
miscible ointment base.
Alternatively, the active ingredients may be formulated in a cream with, for
example an oil-in-water
cream base. Such transdermal formulations are well-known in the art and
generally include additional
ingredients to enhance the dermal penetration of stability of the active
ingredients or the formulation. All
such known transdermal formulations and ingredients arc included within the
scope of this invention.
[00203] A compound of the invention can also be administered by a transdermal
device. Accordingly,
transdermal administration can be accomplished using a patch either of the
reservoir or porous membrane
type, or of a solid matrix variety.
[00204] The above-described components for orally administrable, injectable or
topically
administrable compositions are merely representative. Other materials as well
as processing techniques
and the like are set forth in Part 8 of Remington's Pharmaceutical Sciences,
17th edition, 1985, Mack
Publishing Company, Easton, Pennsylvania, which is incorporated herein by
reference.
[00205] A compound of the invention can also be administered in sustained
release forms or from
sustained release drug delivery systems. A description of representative
sustained release materials can
be found in Remington's Pharmaceutical Sciences.
[00206] The following formulation examples illustrate representative
pharmaceutical compositions that
may be prepared in accordance with this invention. The present invention,
however, is not limited to the
following pharmaceutical compositions.
Formulation 1 - Tablets
[00207] A compound of the invention according to Formula I may be admixed as a
dry powder with a
dry gelatin binder in an approximate 1:2 weight ratio. A minor amount of
magnesium stearate may be
added as a lubricant. The mixture may be formed into 240-270 mg tablets (80-90
mg of active compound
of the invention according to Formula 1 per tablet) in a tablet press.
Formulation 2 - Capsules
[00208] A compound of the invention according to Formula I may be admixed as a
dry powder with a
starch diluent in an approximate 1:1 weight ratio. The mixture may be filled
into 250 mg capsules (125
mg of active compound of the invention according to Formula I per capsule).
63
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Formulation 3 - Liquid
[00209] A compound of the invention according to Formula 1(125 mg), may be
admixed with sucrose
(1.75 g) and xanthan gum (4 mg) and the resultant mixture may be blended,
passed through a No. 10
mesh U.S. sieve, and then mixed with a previously made solution of
microcrystalline cellulose and
sodium carboxymethyl cellulose (11:89, 50 mg) in water. Sodium benzoate (10
mg), flavor, and color
may be diluted with water and added with stirring. Sufficient water may then
be added with stirring.
Further sufficient water may be then added to produce a total volume of 5 mL.
Formulation 4 - Tablets
[00210] A compound of the invention according to Formula I may be admixed as a
dry powder with a
dry gelatin binder in an approximate 1:2 weight ratio. A minor amount of
magnesium stearate may be
added as a lubricant. The mixture may be formed into 450-900 mg tablets (150-
300 mg of active
compound of the invention according to Formula I) in a tablet press.
Formulation 5 - Injection
[00211] A compound of the invention according to Formula I may be dissolved or
suspended in a
buffered sterile saline injectable aqueous medium to a concentration of
approximately 5 mg/mL.
Formulation 6 - Topical
[00212] Stearyl alcohol (250 g) and a white petrolatum (250 g) may be melted
at about 75 C and then a
mixture of A compound of the invention according to Formula I (50 g)
methylparaben (0.25 g),
propylparaben (0.15 g), sodium lauryl sulfate (10 g), and propylene glycol
(120 g) dissolved in water
(about 370 g) may be added and the resulting mixture may be stirred until it
congeals.
METHODS OF TREATMENT
[00213] In one embodiment, the present invention provides compounds of the
invention, or
pharmaceutical compositions comprising a compound of the invention, for use in
medicine. In a
particular embodiment, the present invention provides compounds of the
invention or pharmaceutical
compositions comprising a compound of the invention, for use in the
prophylaxis and/or treatment of
fibrotic diseases, proliferative diseases, inflammatory diseases, autoimmune
diseases, respiratory
diseases, cardiovascular diseases, neurodegenerative diseases, dermatological
disorders, and/or abnormal
angiogenesis associated diseases.
[00214] In another embodiment, the present invention provides compounds of the
invention, or
pharmaceutical compositions comprising a compound of the invention for use in
the manufacture of a
medicament for use in the prophylaxis and/or treatment of fibrotic diseases,
proliferative diseases,
inflammatory diseases, autoimmune diseases, respiratory diseases,
cardiovascular diseases,
neurodegenerative diseases, dermatological disorders, and/or abnormal
angiogenesis associated diseases.
64
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00215] In one embodiment, the present invention provides pharmaceutical
compositions comprising a
compound of the invention, and another therapeutic agent. In a particular
embodiment, the other
therapeutic agent is a fibrotic diseases, proliferative diseases, inflammatory
diseases, autoimmune
diseases, respiratory diseases, cardiovascular diseases, neurodegenerative
diseases, dermatological
disorders, and/or abnormal angiogcnesis associated diseases treatment agent.
[00216] In additional method of treatment aspects, this invention provides
methods of prophylaxis
and/or treatment of a mammal afflicted with fibrotic diseases, proliferative
diseases, inflammatory
diseases, autoimmune diseases, respiratory diseases, cardiovascular diseases,
neurodegenerative diseases,
dermatological disorders, and/or abnormal angiogenesis associated diseases,
which methods comprise the
administration of an effective amount of a compound of the invention or one or
more of the
pharmaceutical compositions herein described for the treatment or prophylaxis
of said condition.
[00217] In one embodiment, the present invention panicles compounds of the
invention or
pharmaceutical compositions comprising a compound of the invention, for use in
the prophylaxis and/or
treatment of fibrotic diseases. In a particular embodiment, the fibrotic
disease is selected from idiopathic
pulmonary fibrosis (IPF), cystic fibrosis, other diffuse parenchymal lung
diseases of different etiologies
including iatrogenic drug-induced fibrosis, occupational and/or environmental
induced fibrosis,
granulomatous diseases (sarcoidosis, hypersensitivity pneumonia), collagen
vascular disease, alveolar
proteinosis, langerhans cell granulomatosis, lymphangioleiomyomatosis,
inherited diseases (Hermansky-
Pudlak Syndrome, tuberous sclerosis, neurofibromatosis, metabolic storage
disorders, familial interstitial
lung disease), radiation induced fibrosis, chronic obstructive pulmonary
disease (COPD), scleroderma,
blcomycin induced pulmonary fibrosis, chronic asthma, silicosis, asbestos
induced pulmonary fibrosis,
acute respiratory distress syndrome (ARDS), kidney fibrosis,
tubulointerstitium fibrosis, glomerular
nephritis, focal segmental glomerular sclerosis, IgA nephropathy,
hypertension, Alport, gut fibrosis, liver
fibrosis, cirrhosis, alcohol induced liver fibrosis, toxic/drug induced liver
fibrosis, hemochromatosis,
nonalcoholic steatohepatitis (NASH), biliary duct injury, primary biliary
cirrhosis, infection induced liver
fibrosis, viral induced liver fibrosis, and autoimmune hepatitis, corneal
scarring, hypertrophic scarring,
Dupuytren disease, keloids, cutaneous fibrosis, cutaneous scleroderma,
systemic sclerosis, spinal cord
injury/fibrosis, myelofibrosis, vascular restenosis, atherosclerosis,
arteriosclerosis, Wegener's
granulomatosis, Pcyronic's disease, or chronic lymphocytic. More particularly,
the fibrotic diseases is
idiopathic pulmonary fibrosis (IPF).
[00218] In another embodiment, the present invention provides compounds of the
invention, or
pharmaceutical compositions comprising a compound of the invention for use in
the manufacture of a
medicament for use in the prophylaxis and/or treatment of fibrotic diseases.
In a particular embodiment,
the fibrotic disease is selected from idiopathic pulmonary fibrosis (IPF),
cystic fibrosis, other diffuse
parenchymal lung diseases of different etiologies including iatrogenic drug-
induced fibrosis, occupational
and/or environmental induced fibrosis, granulomatous diseases (sarcoidosis,
hypersensitivity pneumonia),
collagen vascular disease, alveolar protcinosis, langerhans cell
granulomatosis,
lymphangioleiomyomatosis, inherited diseases (Hermansky-Pudlak Syndrome,
tuberous sclerosis,
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
neurofibromatosis, metabolic storage disorders, familial interstitial lung
disease), radiation induced
fibrosis, chronic obstructive pulmonary disease (COPD), scleroderma, bleomycin
induced pulmonary
fibrosis, chronic asthma, silicosis, asbestos induced pulmonary fibrosis,
acute respiratory distress
syndrome (ARDS), kidney fibrosis, tubulointerstitium fibrosis, glomerular
nephritis, focal segmental
glomerular sclerosis, IgA nephropathy, hypertension, Alport, gut fibrosis,
liver fibrosis, cirrhosis, alcohol
induced liver fibrosis, toxic/drug induced liver fibrosis, hemochromatosis,
nonalcoholic steatohepatitis
(NASH), biliary duct injury, primary biliary cirrhosis, infection induced
liver fibrosis, viral induced liver
fibrosis, and autoimmune hepatitis, corneal scarring, hypertrophic scarring,
Dupuytren disease, keloids,
cutaneous fibrosis, cutaneous scleroderma, systemic sclerosis, spinal cord
injury/fibrosis, myelofibrosis,
vascular restenosis, atherosclerosis, arteriosclerosis, Wegener's
granulomatosis, Peyronie's disease, or
chronic lymphocytic. More particularly, the fibrotic disease is idiopathic
pulmonary fibrosis (IPF).
[00219] In additional method of treatment aspects, this invention provides
methods of prophylaxis
and/or treatment of a mammal afflicted with fibrotic diseases, which methods
comprise the administration
of an effective amount of a compound of the invention or one or more of the
pharmaceutical compositions
herein described for the treatment or prophylaxis of said condition. In a
particular embodiment, the
fibrotic disease is selected from idiopathic pulmonary fibrosis (1PF), cystic
fibrosis, other diffuse
parenchymal lung diseases of different etiologies including iatrogenic drug-
induced fibrosis, occupational
and/or environmental induced fibrosis, granulomatous diseases (sarcoidosis,
hypersensitivity pneumonia),
collagen vascular disease, alveolar proteinosis, langerhans cell
granulomatosis,
lymphangioleiomyomatosis, inherited diseases (Hermansky-Pudlak Syndrome,
tuberous sclerosis,
neurofibromatosis, metabolic storage disorders, familial interstitial lung
disease), radiation induced
fibrosis, chronic obstructive pulmonary disease (COPD), scleroderma, bleomycin
induced pulmonary
fibrosis, chronic asthma, silicosis, asbestos induced pulmonary fibrosis,
acute respiratory distress
syndrome (ARDS), kidney fibrosis, tubulointerstitium fibrosis, glomerular
nephritis, focal segmental
glomerular sclerosis, IgA nephropathy, hypertension, Alport, gut fibrosis,
liver fibrosis, cirrhosis, alcohol
induced liver fibrosis, toxic/drug induced liver fibrosis, hemochromatosis,
nonalcoholic steatohepatitis
(NASH), biliary duct injury, primary biliary cirrhosis, infection induced
liver fibrosis, viral induced liver
fibrosis, and autoimmune hepatitis, corneal scarring, hypertrophic scarring,
Dupuytren disease, keloids,
cutaneous fibrosis, cutaneous scleroderma, systemic sclerosis, spinal cord
injury/fibrosis, myelofibrosis,
vascular restenosis, atherosclerosis, arteriosclerosis, Wegener's
granulomatosis, Peyronie's disease, or
chronic lymphocytic. More particularly, the fibrotic disease is idiopathic
pulmonary fibrosis (IPF).
[00220] A particular regimen of the present method comprises the
administration to a subject suffering
from a fibrotic disease of an effective amount of a compound of the invention
according to Formula I for
a period of time sufficient to reduce the level of fibrosis in the subject,
and preferably terminate the
processes responsible for said fibrosis. A special embodiment of the method
comprises administering of
an effective amount of a compound of the invention according to Formula Ito a
subject patient suffering
from to the development of idiopathic pulmonary fibrosis, for a period of time
sufficient to reduce or
66
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
prevent idiopathic pulmonary fibrosis of said patient, and preferably
terminate, the processes responsible
for said idiopathic pulmonary fibrosis.
[00221] In one embodiment, the present invention provides compounds of the
invention or
pharmaceutical compositions comprising a compound of the invention, for use in
the prophylaxis and/or
treatment of proliferative diseases. In a particular embodiment, the
proliferative disease is selected from
cancer, leukemia, multiple myeloma and psoriasis.
[00222] In another embodiment, the present invention provides compounds of the
invention, or
pharmaceutical compositions comprising a compound of the invention for use in
the manufacture of a
medicament for use in the prophylaxis and/or treatment of proliferative
diseases. In a particular
embodiment, the proliferative disease is selected from cancer, leukemia,
multiple myeloma and psoriasis.
[00223] In additional method of treatment aspects, this invention provides
methods of prophylaxis
and/or treatment of a mammal afflicted with proliferative diseases, which
methods comprise the
administration of an effective amount of a compound of the invention or one or
more of the
pharmaceutical compositions herein described for the treatment or prophylaxis
of said condition. In a
particular embodiment, the proliferative disease is selected from cancer,
leukemia, multiple myeloma and
psoriasis.
[00224] In one embodiment, the present invention provides compounds of the
invention or
pharmaceutical compositions comprising a compound of the invention, for use in
the prophylaxis and/or
treatment of inflammatory diseases. In a particular embodiment, the
inflammatory disease is selected
from rheumatoid arthritis, osteoarthritis, allergic airway disease (e.g.
asthma), chronic obstructive
pulmonary disease (COPD) and inflammatory bowel diseases (e.g. Crohn's disease
and ulcerative colitis).
More particularly, the inflammatory disease is selected from rheumatoid
arthritis, and chronic obstructive
pulmonary disease (COPD).
[00225] In another embodiment, the present invention provides compounds of the
invention, or
pharmaceutical compositions comprising a compound of the invention for use in
the manufacture of a
medicament for use in the prophylaxis and/or treatment of inflammatory
diseases. In a particular
embodiment, the inflammatory disease is selected from rheumatoid arthritis,
osteoarthritis, allergic airway
disease (e.g. asthma), chronic obstructive pulmonary disease (COPD) and
inflammatory bowel diseases
(e.g. Crohn's disease and ulcerative colitis). More particularly, the
inflammatory disease is selected from
rheumatoid arthritis, and chronic obstructive pulmonary disease (COPD).
[00226] In additional method of treatment aspects, this invention provides
methods of prophylaxis
and/or treatment of a mammal afflicted with inflammatory diseases, which
methods comprise the
administration of an effective amount of a compound of the invention or one or
more of the
pharmaceutical compositions herein described for the treatment or prophylaxis
of said condition. In a
particular embodiment, the inflammatory disease is selected from rheumatoid
arthritis, osteoarthritis,
allergic airway disease (e.g. asthma), chronic obstructive pulmonary disease
(COPD) and inflammatory
bowel diseases (e.g. Crohn's disease and ulcerative colitis). More
particularly the inflammatory disease is
selected from rheumatoid arthritis, and chronic obstructive pulmonary disease
(COPD).
67
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00227] In one embodiment, the present invention provides compounds of the
invention or
pharmaceutical compositions comprising a compound of the invention, for use in
the prophylaxis and/or
treatment of autoimmune diseases. In a particular embodiment, the autoimmune
disease is selected from
COPD, asthma (e.g intrinsic asthma, extrinsic asthma, dust asthma, infantily
asthma) particularly chronic
or inveterate asthma (for example late asthma and airway hyperreponsiveness),
bronchitis, including
bronchial asthma, systemic lupus erythematosus (SLE), cutaneous lupus
erythrematosis, lupus nephritis,
dermatomyositis, Sjogren's syndrome, multiple sclerosis, psoriasis, dry eye
disease, type T diabetes
mellitus and complications associated therewith, atopic eczema (atopic
dermatitis), thyroiditis
(Hashimoto's and autoimmune thyroiditis), contact dermatitis and further
eczematous dermatitis,
inflammatory bowel disease (e.g. Crohn's disease and ulcerative colitis),
atherosclerosis and amyotrophic
lateral sclerosis. Particularly, the autoimmune disease is selected from COPD,
asthma, systemic lupus
erythematosis, type I diabetes mellitus and inflammatory bowel disease.
[00228] In another embodiment, the present invention provides compounds of the
invention, or
pharmaceutical compositions comprising a compound of the invention for use in
the manufacture of a
medicament for use in the prophylaxis and/or treatment of autoimmune diseases.
In a particular
embodiment, the autoimmune disease is selected from COPD, asthma (e.g
intrinsic asthma, extrinsic
asthma, dust asthma, infantily asthma) particularly chronic or inveterate
asthma (for example late asthma
and airway hyperreponsiveness), bronchitis, including bronchial asthma,
systemic lupus erythematosus
(SLE), cutaneous lupus erythrematosis, lupus nephritis, dermatomyositis,
Sjogren's syndrome, multiple
sclerosis, psoriasis, dry eye disease, type I diabetes mellitus and
complications associated therewith,
atopic eczema (atopic dermatitis), thyroiditis (Hashimoto's and autoimmune
thyroiditis), contact
dermatitis and further eczematous dermatitis, inflammatory bowel disease (e.g.
Crohn's disease and
ulcerative colitis), atherosclerosis and amyotrophic lateral sclerosis.
Particularly, the autoimmune disease
is selected from COPD, asthma, systemic lupus erythematosis, type I diabetes
mellitus and inflammatory
bowel disease.
[00229] In additional method of treatment aspects, this invention provides
methods of prophylaxis
and/or treatment of a mammal afflicted with autoimmune diseases, which methods
comprise the
administration of an effective amount of a compound of the invention or one or
more of the
pharmaceutical compositions herein described for the treatment or prophylaxis
of said condition. In a
particular embodiment, the autoimmune disease is selected from COPD, asthma
(e.g intrinsic asthma,
extrinsic asthma, dust asthma, infantily asthma) particularly chronic or
inveterate asthma (for example
late asthma and airway hyperreponsiveness), bronchitis, including bronchial
asthma, systemic lupus
erythematosus (SLE), cutaneous lupus erythrematosis, lupus nephritis,
dermatomyositis, Sjogren's
syndrome, multiple sclerosis, psoriasis, dry eye disease, type I diabetes
mellitus and complications
associated therewith, atopic eczema (atopic dermatitis), thyroiditis
(Hashimoto's and autoimmune
thyroiditis), contact dermatitis and further eczematous dermatitis,
inflammatory bowel disease (e.g.
Crohn's disease and ulcerative colitis), atherosclerosis and amyotrophic
lateral sclerosis. Particularly, the
68
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
autoimmune disease is selected from COPD, asthma, systemic lupus
erythematosis, type I diabetes
mellitus and inflammatory bowel disease.
[00230] In one embodiment, the present invention provides compounds of the
invention or
pharmaceutical compositions comprising a compound of the invention, for use in
the prophylaxis and/or
treatment of respiratory diseases. In a particular embodiment, the respiratory
disease is selected from
asthma, adult respiratory distress syndrome and allergic (extrinsic) asthma,
non-allergic (intrinsic)
asthma, acute severe asthma, chronic asthma, clinical asthma, nocturnal
asthma, allergen-induced asthma,
aspirin-sensitive asthma, exercise-induced asthma, isocapnic hyperventilation,
child onset asthma, adult-
onset asthma, cough-variant asthma, occupational asthma, steroid-resistant
asthma, seasonal asthma,
seasonal allergic rhinitis, perennial allergic rhinitis, chronic obstructive
pulmonary disease, including
chronic bronchitis or emphysema, pulmonary hypertension, interstitial lung
fibrosis and/or airway
inflammation and cystic fibrosis, and hypoxia.
[00231] In another embodiment, the present invention provides compounds of the
invention, or
pharmaceutical compositions comprising a compound of the invention for use in
the manufacture of a
medicament for use in the prophylaxis and/or treatment of respiratory
diseases. In a particular
embodiment, the respiratory disease is selected from asthma, adult respiratory
distress syndrome and
allergic (extrinsic) asthma, non-allergic (intrinsic) asthma, acute severe
asthma, chronic asthma, clinical
asthma, nocturnal asthma, allergen-induced asthma, aspirin-sensitive asthma,
exercise-induced asthma,
isocapnic hyperventilation, child onset asthma, adult-onset asthma, cough-
variant asthma, occupational
asthma, steroid-resistant asthma, seasonal asthma, seasonal allergic rhinitis,
perennial allergic rhinitis,
chronic obstructive pulmonary disease, including chronic bronchitis or
emphysema, pulmonary
hypertension, interstitial lung fibrosis and/or airway inflammation and cystic
fibrosis, and hypoxia.
[00232] In additional method of treatment aspects, this invention provides
methods of prophylaxis
and/or treatment of a mammal afflicted with respiratory diseases, which
methods comprise the
administration of an effective amount of a compound of the invention or one or
more of the
pharmaceutical compositions herein described for the treatment or prophylaxis
of said condition. In a
particular embodiment, the respiratory disease is selected from asthma, adult
respiratory distress
syndrome and allergic (extrinsic) asthma, non-allergic (intrinsic) asthma,
acute severe asthma, chronic
asthma, clinical asthma, nocturnal asthma, allergen-induced asthma, aspirin-
sensitive asthma, exercise-
induced asthma, isocapnic hyperventilation, child onset asthma, adult-onset
asthma, cough-variant
asthma, occupational asthma, steroid-resistant asthma, seasonal asthma,
seasonal allergic rhinitis,
perennial allergic rhinitis, chronic obstructive pulmonary disease, including
chronic bronchitis or
emphysema, pulmonary hypertension, interstitial lung fibrosis and/or airway
inflammation and cystic
fibrosis, and hypoxia.
[00233] In one embodiment, the present invention provides compounds of the
invention or
pharmaceutical compositions comprising a compound of the invention, for use in
the prophylaxis and/or
treatment of cardiovascular diseases. In a particular embodiment, the
cardiovascular disease is selected
from arrhythmia (atrial or ventricular or both), atherosclerosis and its
sequelae, angina, cardiac rhythm
69
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
disturbances, myocardial ischemia, myocardial infarction, cardiac or vascular
aneurysm, vasculitis,
stroke, peripheral obstructive arteriopathy of a limb, an organ, or a tissue,
reperfusion injury following
ischemia of the brain, heart, kidney or other organ or tissue, endotoxic,
surgical, or traumatic shock,
hypertension, valvular heart disease, heart failure, abnormal blood pressure,
shock, vasoconstriction
(including that associated with migraines), vascular abnormality,
inflammation, insufficiency limited to a
single organ or tissue.
[00234] In another embodiment, the present invention provides compounds of the
invention, or
pharmaceutical compositions comprising a compound of the invention for use in
the manufacture of a
medicament for use in the prophylaxis and/or treatment of cardiovascular
diseases. In a particular
embodiment, the cardiovascular disease is selected from arrhythmia (atrial or
ventricular or both),
atherosclerosis and its sequelae, angina, cardiac rhythm disturbances,
myocardial ischemia, myocardial
infarction, cardiac or vascular aneurysm, vasculitis, stroke, peripheral
obstructive arteriopathy of a limb,
an organ, or a tissue, reperfusion injury following ischemia of the brain,
heart, kidney or other organ or
tissue, endotoxic, surgical, or traumatic shock, hypertension, valvular heart
disease, heart failure,
abnormal blood pressure, shock, vasoconstriction (including that associated
with migraines), vascular
abnormality, inflammation, insufficiency limited to a single organ or tissue.
[00235] In additional method of treatment aspects, this invention provides
methods of prophylaxis
and/or treatment of a mammal afflicted with cardiovascular diseases, which
methods comprise the
administration of an effective amount of a compound of the invention or one or
more of the
pharmaceutical compositions herein described for the treatment or prophylaxis
of said condition. In a
particular embodiment, the cardiovascular disease is selected from arrhythmia
(atrial or ventricular or
both), atherosclerosis and its sequelae, angina, cardiac rhythm disturbances,
myocardial ischemia,
myocardial infarction, cardiac or vascular aneurysm, vasculitis, stroke,
peripheral obstructive arteriopathy
of a limb, an organ, or a tissue, reperfusion injury following ischemia of the
brain, heart, kidney or other
organ or tissue, endotoxic, surgical, or traumatic shock, hypertension,
valvular heart disease, heart failure,
abnormal blood pressure, shock, vasoconstriction (including that associated
with migraines), vascular
abnormality, inflammation, insufficiency limited to a single organ or tissue.
[00236] In one embodiment, the present invention provides compounds of the
invention or
pharmaceutical compositions comprising a compound of the invention, for use in
the prophylaxis and/or
treatment of neurodegenerative diseases. In a particular embodiment, the
neurodegenerative disease is
selected from Alzheimer's disease and other dementias, brain cancer,
degenerative nerve diseases,
encephalitis, epilepsy, genetic brain disorders, head and brain malformations,
hydrocephalus, stroke,
Parkinson's disease, multiple sclerosis, amyotrophic lateral sclerosis (ALS or
Lou Gehrig's Disease),
Huntington's disease, and prion diseases.
[00237] In another embodiment, the present invention provides compounds of the
invention, or
pharmaceutical compositions comprising a compound of the invention for use in
the manufacture of a
medicament for use in the prophylaxis and/or treatment of neurodegenerative
diseases. In a particular
embodiment, the neurodegenerative disease is selected from Alzheimer's disease
and other dementias,
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
brain cancer, degenerative nerve diseases, encephalitis, epilepsy, genetic
brain disorders, head and brain
malformations, hydrocephalus, stroke, Parkinson's disease, multiple sclerosis,
amyotrophic lateral
sclerosis (ALS or Lou Gehrig's Disease), Huntington's disease, and prion
diseases.
[00238] In additional method of treatment aspects, this invention provides
methods of prophylaxis
and/or treatment of a mammal afflicted with neurodegenerative diseases, which
methods comprise the
administration of an effective amount of a compound of the invention or one or
more of the
pharmaceutical compositions herein described for the treatment or prophylaxis
of said condition. In a
particular embodiment, the neurodegenerative disease is selected from
Alzheimer's disease and other
dementias, brain cancer, degenerative nerve diseases, encephalitis, epilepsy,
genetic brain disorders, head
and brain malformations, hydrocephalus, stroke, Parkinson's disease, multiple
sclerosis, amyotrophic
lateral sclerosis (ALS or Lou Gehrig's Disease), Huntington's disease, and
prion diseases.
[00239] In one embodiment, the present invention provides compounds of the
invention or
pharmaceutical compositions comprising a compound of the invention, for use in
the prophylaxis and/or
treatment of dermatological disorders. In a particular embodiment, the
dermatological disease is selected
from atopic dermatitis, bullous disorders, collagenoses, psoriasis, psoriatic
lesions, dermatitis, contact
dermatitis, eczema, pruritus, urticaria, rosacea, scleroderma, wound healing,
scarring, hypertrophic
scarring, keloids, Kawasaki Disease, rosacea, or Sjogren-Larsso Syndrome.
[00240] In another embodiment, the present invention provides compounds of the
invention, or
pharmaceutical compositions comprising a compound of the invention for use in
the manufacture of a
medicament for use in the prophylaxis and/or treatment of dermatological
disorders. In a particular
embodiment, the dermatological disease is selected from atopic dermatitis,
bullous disorders,
collagenoses, psoriasis, psoriatic lesions, dermatitis, contact dermatitis,
eczema, pruritus, urticaria,
rosacea, scleroderma, wound healing, scarring, hypertrophic scarring, keloids,
Kawasaki Disease,
rosacea, or Sjogren-Larsso Syndrome.
[00241] In additional method of treatment aspects, this invention provides
methods of prophylaxis
and/or treatment of a mammal afflicted with dermatological disorders, which
methods comprise the
administration of an effective amount of a compound of the invention or one or
more of the
pharmaceutical compositions herein described for the treatment or prophylaxis
of said condition. In a
particular embodiment, the dermatological disease is selected from atopic
dermatitis, bullous disorders,
collagenoses, psoriasis, psoriatic lesions, dermatitis, contact dermatitis,
eczema, pruritus, urticaria,
rosacea, scleroderma, wound healing, scarring, hypertrophic scarring, keloids,
Kawasaki Disease,
rosacea, or Sjogren-Larsso Syndrome.
[00242] In one embodiment, the present invention provides compounds of the
invention or
pharmaceutical compositions comprising a compound of the invention, for use in
the prophylaxis and/or
treatment of abnormal angiogenesis associated diseases. In a particular
embodiment, the abnormal
angiogenesis associated disease is selected from atherosclerosis,
hypertension, tumor growth,
inflammation, rheumatoid arthritis, wet-form macular degeneration, choroidal
neovascularization, retinal
neovascularization, diabetic retinopathy, and glioblastoma multiforma.
71
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00243] In another embodiment, the present invention provides compounds of the
invention, or
pharmaceutical compositions comprising a compound of the invention for use in
the manufacture of a
medicament for use in the prophylaxis and/or treatment of abnormal
angiogenesis associated diseases. In
a particular embodiment, the abnormal angiogenesis associated disease is
selected from atherosclerosis,
hypertension, tumor growth, inflammation, rheumatoid arthritis, wet-form
macular degeneration,
choroidal neovascularization, retinal neovascularization, diabetic
retinopathy, and glioblastoma
multiforma.
[00244] In additional method of treatment aspects, this invention provides
methods of prophylaxis
and/or treatment of a mammal afflicted with abnormal angiogenesis associated
diseases, which methods
comprise the administration of an effective amount of a compound of the
invention or one or more of the
pharmaceutical compositions herein described for the treatment or prophylaxis
of said condition. In a
particular embodiment, the abnormal angiogenesis associated disease is
selected from atherosclerosis,
hypertension, tumor growth, inflammation, rheumatoid arthritis, wet-form
macular degeneration,
choroidal neovascularization, retinal neovascularization, diabetic
retinopathy, and glioblastoma
multifonna.
[00245] Injection dose levels range from about 0.1 mg/kg/h to at least 10
mg/kg/h, all for from about 1
to about 120 h and especially 24 to 96 h. A preloading bolus of from about 0.1
mg/kg to about 10 mg/kg
or more may also be administered to achieve adequate steady state levels. The
maximum total dose is not
expected to exceed about 1 g/day for a 40 to 80 kg human patient.
[00246] For the prophylaxis and/or treatment of long-term conditions, such as
degenerative conditions,
the regimen for treatment usually stretches over many months or years so oral
dosing is preferred for
patient convenience and tolerance. With oral dosing, one to four (1-4) regular
doses daily, especially one
to three (1-3) regular doses daily, typically one to two (1-2) regular doses
daily, and most typically one
(1) regular dose daily are representative regimens. Alternatively for long
lasting effect drugs, with oral
dosing, once every other week, once weekly, and once a day are representative
regimens. In particular,
dosage regimen can be every 1-14 days, more particularly 1-10 days, even more
particularly 1-7 days, and
most particularly 1-3 days.
[00247] Using these dosing patterns, each dose provides from about 1 to about
1000 mg of a
compound of the invention, with particular doses each providing from about 10
to about 500 mg and
especially about 30 to about 250 mg.
[00248] Transdermal doses are generally selected to provide similar or lower
blood levels than are
achieved using injection doses.
[00249] When used to prevent the onset of a condition, a compound of the
invention will be
administered to a patient at risk for developing the condition, typically on
the advice and under the
supervision of a physician, at the dosage levels described above. Patients at
risk for developing a
particular condition generally include those that have a family history of the
condition, or those who have
been identified by genetic testing or screening to be particularly susceptible
to developing the condition.
72
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00250] A compound of the invention can be administered as the sole active
agent or it can be
administered in combination with other therapeutic agents, including other
compound of the inventions
that demonstrate the same or a similar therapeutic activity and that are
determined to be safe and
efficacious for such combined administration. In a specific embodiment, co-
administration of two (or
more) agents allows for significantly lower doses of each to be used, thereby
reducing the side effects
seen.
[00251] In one embodiment, a compound of the invention or a pharmaceutical
composition comprising
a compound of the invention is administered as a medicament. In a specific
embodiment, said
pharmaceutical composition additionally comprises a further active ingredient.
[00252] In one embodiment, a compound of the invention is co-administered with
another therapeutic
agent for the treatment and/or prophylaxis of a disease involving
inflammation, particular agents include,
but are not limited to, immunoregulatory agents e.g. azathioprine,
corticosteroids (e.g. prednisolone or
dexamethasone), cyclophosphamide, cyclosporin A, tacrolimus, mycophcnolate,
mofctil, muromonab-
CD3 (OKT3, e.g. Orthocoloneg), ATG, aspirin, acetaminophen, ibuprofen,
naproxen, and piroxicam.
[00253] In one embodiment, a compound of the invention is co-administered with
another therapeutic
agent for the treatment and/or prophylaxis of arthritis (e.g. rheumatoid
arthritis), particular agents include
but are not limited to analgesics, non-steroidal anti-inflammatory drugs
(NSAIDS), steroids, synthetic
DMARDS (for example but without limitation methotrexate, leflunomide,
sulfasalazine, auranofin,
sodium aurothiomalate, penicillamine, chloroquine, hydroxychloroquine,
azathioprine, tofacitinib,
baricitinib, fostamatinib, and cyclosporin), and biological DMARDS (for
example but without limitation
infliximab, ctancrcept, adalimumab, rituximab, and abatacept).
[00254] In one embodiment, a compound of the invention is co-administered with
another therapeutic
agent for the treatment and/or prophylaxis of proliferative disorders,
particular agents include but are not
limited to: methotrexate, leukovorin, adriamycin, prednisone, bleomycin,
cyclophosphamide,
fluorouracil, paclitaxel, docetaxel, vincristine, vinblastine, vinorelbine,
doxorubicin, tamoxifen,
toremifene, megestrol acetate, anastrozole, goserelin, anti-HER2 monoclonal
antibody (e.g. HerceptinTm),
capecitabine, raloxifene hydrochloride, EGFR inhibitors (e.g. lressag,
TarcevaTm, ErbituxTm), VEGF
inhibitors (e.g. AvastinTm), proteasome inhibitors (e.g. VelcadeTm), Glivec
and hsp90 inhibitors (e.g.
17-AAG). Additionally, the compound of the invention according to Formula I
may be administered in
combination with other therapies including, but not limited to, radiotherapy
or surgery. In a specific
embodiment the proliferative disorder is selected from cancer,
myeloproliferative disease or leukaemia.
[00255] In one embodiment, a compound of the invention is co-administered with
another therapeutic
agent for the treatment and/or prophylaxis of autoimmune diseases, particular
agents include but are not
limited to: glucocorticoids, cytostatic agents (e.g. purine analogs),
alkylating agents (e.g nitrogen
mustards (cyclophosphamide), nitrosoureas, platinum compound of the
inventions, and others),
antimetabolites (e.g. methotrexate, azathioprine and mercaptopurine),
cytotoxic antibiotics (e.g.
dactinomycin anthracyclincs, mitomycin C, blcomycin, and mithramycin),
antibodies (e.g. anti-CD20,
anti-CD25 or anti-CD3 (OTK3) monoclonal antibodies, Atgamg and
Thymoglobulineg), cyclosporin,
73
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
tacrolimus, rapamycin (sirolimus), interferons (e.g. IFN-I3), TNF binding
proteins (e.g. infliximab,
etanercept, or adalimumab), mycophenolate, fingolimod and myriocin..
[00256] In one embodiment, a compound of the invention is co-administered with
another therapeutic
agent for the treatment and/or prophylaxis of transplant rejection, particular
agents include but are not
limited to: calcincurin inhibitors (e.g. cyclosporin or tacrolimus (FK506)),
mTOR inhibitors (e.g.
sirolimus, everolimus), anti-proliferatives (e.g. azathioprine, mycophenolic
acid), corticosteroids (e.g.
prednisolone, hydrocortisone), antibodies (e.g. monoclonal anti-IL-2Ra
receptor antibodies, basiliximab,
daclizumab), polyclonal anti-T-cell antibodies (e.g. anti-thymocyte globulin
(ATG), anti-lymphocyte
globulin (ALG)).
[00257] In one embodiment, a compound of the invention is co-administered with
another therapeutic
agent for the treatment and/or prophylaxis of asthma and/or rhinitis and/or
COPD, particular agents
include but are not limited to: beta2-adrenoceptor agonists (e.g. salbutamol,
levalbuterol, terbutaline and
bitolterol), epinephrine (inhaled or tablets), anticholinergics (e.g.
ipratropium bromide), glucocorticoids
(oral or inhaled). Long-acting 132-agonists (e.g. salmeterol, formoterol,
bambuterol, and sustained-release
oral albuterol), combinations of inhaled steroids and long-acting
bronchodilators (e.g.
fluticasone/salmeterol, budesonidelformoterol), leukotriene antagonists and
synthesis inhibitors (e.g.
montelukast, zafirlukast and zileuton), inhibitors of mediator release (e.g.
cromoglycate and ketotifen),
biological regulators of IgE response (e.g. omalizumab), antihistamines (e.g.
ceterizine, cinnarizine,
fexofenadine) and vasoconstrictors (e.g. oxymethazoline, xylomethazoline,
nafazoline and tramazoline).
[00258] Additionally, a compound of the invention may be administered in
combination with
emergency therapies for asthma and/or COPD, such therapies include oxygen or
hcliox administration,
nebulized salbutamol or terbutaline (optionally combined with an
anticholinergic (e.g. ipratropium),
systemic steroids (oral or intravenous, e.g. predni s on e, predni so lo n e,
methylpredn i so lo ne,
dexamethasone, or hydrocortisone), intravenous salbutamol, non-specific beta-
agonists, injected or
inhaled (e.g. epinephrine, isoetharine, isoproterenol, metaproterenol),
anticholinergics (IV or nebulized,
e.g. glycopyrrolate, atropine, ipratropium), methylxanthines (theophylline,
aminophylline, bamiphylline),
inhalation anesthetics that have a bronchodilatory effect (e.g. isoflurane,
halothane, enflurane), ketamine
and intravenous magnesium sulfate.
[00259] In one embodiment, a compound of the invention is co-administered with
another therapeutic
agent for the treatment and/or prophylaxis of inflammatory bowel disease
(IBD), particular agents include
but are not limited to: glucocorticoids (e.g. prednisone, budesonide)
synthetic disease modifying,
immunomodulatory agents (e.g. methotrexate, leflunomide, sulfasalazine,
mesalazine, azathioprine, 6-
mercaptopurine and cyclosporin) and biological disease modifying,
immunomodulatory agents
(infliximab, adalimumab, rituximab, and abatacept).
[00260] In one embodiment, a compound of the invention is co-administered with
another therapeutic
agent for the treatment and/or prophylaxis of SLE, particular agents include
but are not limited to: human
monoclonal antibodies (bclimumab (Bcnlysta)), Disease-modifying antirhcumatic
drugs (DMARDs) such
as antimalarials (e.g. plaquenil, hydroxychloroquine), immunosuppressants
(e.g. methotrexate and
74
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
azathioprine), cyclophosphamide and mycophenolic acid, immunosuppressive drugs
and analgesics, such
as nonsteroidal anti-inflammatory drugs, opiates (e.g. dextropropoxyphene and
co-codamol), opioids (e.g.
hydrocodone, oxycodone, MS Contin, or methadone) and the fentanyl duragesic
transdermal patch.
[00261] In one embodiment, a compound of the invention is co-administered with
another therapeutic
agent for the treatment and/or prophylaxis of psoriasis, particular agents
include but are not limited to:
topical treatments such as bath solutions, moisturizers, medicated creams and
ointments containing coal
tar, dithranol (anthralin), corticosteroids like desoximetasone (TopicortTm),
fluocinonide, vitamin D3
analogues (for example, calcipotriol), argan oil and retinoids (etretinate,
acitretin, tazarotene), systemic
treatments such as methotrexate, cyclosporine, retinoids, tioguanine,
hydroxyurea, sulfasalazine,
mycophenolate mofetil, azathioprine, tacrolimus, fumaric acid esters or
biologics such as AmeviveTM,
EnbrelTm, HumiraTM, RemicadeTM, RaptivaTM and ustekinumab (a IL-12 and IL-23
blocker).
Additionally, a compound of the invention may be administered in combination
with other therapies
including, but not limited to phototherapy, or photochcmotherapy (e.g.
psoralen and ultraviolet A
phototherapy (PUVA)).
[00262] In one embodiment, a compound of the invention is co-administered with
another therapeutic
agent for the treatment and/or prophylaxis of allergic reaction, particular
agents include but are not
limited to: antihistamines (e.g. cetirizine, diphenhydramine, fexofenadine,
levocetirizine), glucocorticoids
(e.g. prednisone, betamethasone, beclomethasone, dexamethasone), epinephrine,
theophylline or anti-
leukotrienes (e.g. montelukast or zafirlukast), anti-cholinergics and
decongestants.
[00263] By co-administration is included any means of delivering two or more
therapeutic agents to
the patient as part of the same treatment regime, as will be apparent to the
skilled person. Whilst the two
or more agents may be administered simultaneously in a single formulation,
i.e. as a single
pharmaceutical composition, this is not essential. The agents may be
administered in different
formulations and at different times.
CHEMICAL SYNTHETIC PROCEDURES
General
[00264] The compound of the invention can be prepared from readily available
starting materials using
the following general methods and procedures. It will be appreciated that
where typical or preferred
process conditions (i.e. reaction temperatures, times, mole ratios of
reactants, solvents, pressures, etc.) are
given, other process conditions can also be used unless otherwise stated.
Optimum reaction conditions
may vary with the particular reactants or solvent used, but such conditions
can be determined by one
skilled in the art by routine optimization procedures.
[00265] Additionally, as will be apparent to those skilled in the art,
conventional protecting groups
may be necessary to prevent certain functional groups from undergoing
undesired reactions. The choice
of a suitable protecting group for a particular functional group as well as
suitable conditions for protection
and deprotection are well known in the art (Greene, T W; Wuts, P GM;, 1991).
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
[00266] The following methods are presented with details as to the preparation
of a compound of the
invention as defined hereinabove and the comparative examples. A compound of
the invention may be
prepared from known or commercially available starting materials and reagents
by one skilled in the art
of organic synthesis.
[00267] All reagents were of commercial grade and were used as received
without further purification,
unless otherwise stated. Commercially available anhydrous solvents were used
for reactions conducted
under inert atmosphere. Reagent grade solvents were used in all other cases,
unless otherwise specified.
Column chromatography was performed on silica gel 60 (35-70 gm). Thin layer
chromatography was
carried out using pre-coated silica gel F-254 plates (thickness 0.25 mm).
IFINMR spectra were recorded
on a Bruker DPX 400 NMR spectrometer (400 MHz or a Bruker Advance 300 NMR
spectrometer (300
MHz). Chemical shifts (6) for 1H NMR spectra are reported in parts per million
(ppm) relative to
tetramethylsilane (6 0.00) or the appropriate residual solvent peak, i.e.
CHC13 (6 7.27), as internal
reference. Multiplicities arc given as singlet (s), doublet (d), triplet (t),
quartet (q), quintuplet (quin),
multiplet (m) and broad (br). Electrospray MS spectra were obtained on a
Waters platform LC/MS
spectrometer or with Waters Acquity UPLC with Waters Acquity PDA detector and
SQD mass
spectrometer. Columns used: UPLC BEH C18 1.7gm 2.1x5 mm VanGuard Pre-column
with Acquity
UPLC BEH C18 1.7um 2.1x30 mm Column or Acquity UPLC BEH C18 1.7um 2.1x50 mm
Column. All
the methods are using MeCN/H20 gradients. MeCN and ff20 contain either 0.1%
Formic Acid or NH3
(10 mM). LC-MS columns used: Waters XBridge Prep OBD C18 5 gm 30 mm ID x 100
mm L
(preparative column) and Waters XBridge BEH C18 5 gm 4.6 mm ID x 100 mm L
(analytical column).
All the methods are using either Me0H/H20 or MeCN/H20 gradients. Me0H, MeCN
and H20 contain
either 0.1% Formic Acid or 0.1% Diethylamine. Microwave heating was performed
with a Biotage
Initiator. Celpure P65 is a filtration aid, commercial product (cas number
61790-53-2).
[00268] List of abbreviations used in the experimental section:
microliter d doublet
APMA 4-aminophenylmercuric acetate 2-
Dicyclohexylphosphino-2'-
DavePhos (N,N- di methyl amino)b iphe
nyl
app t Apparent triplet
Dichloromethane
AUC Area Under the Curve DM
2,3 -Dichloro-5,6-dicyano -1,4-
BAL Broncho-alveolar lavage
DDQ benzoquinone
BALF Broncho-alveolar lavage fluid
DEAD di ethyl azod ic arb oxyl
ate
bd Broad doublet
Described in details
Boc tert-Butyloxy-carbonyl Desc'd
bs Broad singlet DIAD
Diisopropyl azodicarboxylate
BSA Bovine serum albumine DIPE Diisopropylether
bt Broad triplet DIPEA N,N-
diisopropylethylamine
Cat. Catalytic amount DMA Dimethy lac etamide
cDNA copy deoxyribonucleic acid
76
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
N,N-dimethylformamide
DMF MMP Matrix Metall Proteinase
_________________________________ ¨
DMSO Dimethylsulfoxide MS Ms'd Mass
measured by LC-MS
_
1,1'- Bis( diphenylphosphino) MW Molecular weight
dppf ferrocene
N.A. Not
available
_
1-ethyl-3-(3- NBS N-Bromosuccinimide
EDC dimethylaminopropyl)carbodiimid
e) nBuOH n-Butanol
_________________________________ - NMR Nuclear
Magnetic Resonance
N-(3-Dimethylaminopropy1)-N'-
EDC.HCI
ethylcarbodiimide hydrochloride PBF
phosphate buffered fonnalin
eq. Equivalent
PBS Phosphate buffered saline
Et20 Diethyl ether
PCR
Polymerase chain reaction
Et0Ac Ethyl acetate
Tetrakis(triphenylphosphine)palla
_ Pd(PPh3)4
Et0H Ethanol dium(0)
_________________________________ ¨ Pcl/C
Palladium on Carbon 10%
FBS Fetal bovine serum
Tris(dibenzylideneacetone)
FITC Fluorescein Isothiocyanate ¨ Pd2(dba)3
dipalladium(0)
_________________________________ - [1,F-
Fmoc 9-Fluorenylmethoxycarbonyl _ PdC12dppf
Bis(diphenylphosphino)ferrocene]
dichloropalladium(II)
g gram
_
PEG Polyethylene glycol
h hour
_
ppm part-per-million
HOBt Hydroxybenzotriazole
_
HPLC High pressure liquid q quartet
chromatography QrtPCR
quantitative real-time PCR
¨1
HRP horseradish peroxydase
_________________________________ ¨1 QTL quantitative trait loci
Int Intermediate
_ r.t. Room temperature
(2-Biphenyl)di-tert-
JohnPhos
butylphosphine RNA Ribonucleic acid
kg kilogram Rt retention time
L liter s singlet
_
Liquid Chromatography-Mass
LC-MS sept septuplet
Spectrometry
LPC lysophosphatidylcholine t triplet
TEA
Triethylamine
m multiplet
_
MeCN Acetonitrile TFA Tnfluoroacetic acid
_
Me0H Methanol THF Tetrahydrofuran
_________________________________ ¨ (N-ethyl-N-(2-hydroxy-3-
mg milligram
TOOS
sulfopropy1)-3-methylaniline,
_
mm minute sodium salt dihydrate
_
TS Tobacco smoke
mL millilitre
_______________________________________________________________________ ¨1
XantPhos 4,5-Bis(diphenylphosphino)-9,9-
mmol millimoles dimethylxanthene
77
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
SYNTHETIC PREPARATION OF THE COMPOUNDS OF THE INVENTION
Example 1. General Synthetic Methods
1 .1. Synthetic methods overview
...:9,1
R" NH2
R"
i I
RY
Gen-1
Reb ". NH2
R"
ii 10
14F1
RY Gen-2
R" ---1\1
R"
iii
(R7)a
Ra 0
NH2 R4\ f?
RYrr,NL. Rz Hz Gen-8
Ry R8
._..c_RE: Gen-3 X
Gen-6
R" ---N _,,..
R" ---N
R" R".(6---N R"
R"
viii 1
IR'ait 1
(R7)e x
lb i
V R
R 4\
IV Ry,.õ,, R4\ NH
N
Gen-4 xiii Fe NH
/ N*__Rz Gen-9
---- NI- \-->_Rz
R" ---N
1.___ lw R2
X"
R"-----N
R R"
,
R"
Gen-7 - N---
R" ---N1
R" vl 1 xi i 1
Rib
R1.* Rib R
i>\44 (R7)a
R4\ iri\j \ R2
R4\ )21 1 R2
R8
N¨\ W vi
X- Gen-5 --' N----R, Gen-I0
R"-r-' It.
--1\I R"
R"
Where R.3' is halo, NO2, or - C(-0)0alkyl, Rz is R. or an alkyl, alkenyl or
carbonyl group optionally
substituted.
Step 1: method A
Step ii: consists in one of the following methods
B1 (2 steps) : Route using isonitrile reagent then reaction with HCOOH
B2 (2 steps) : Route using KCN then reaction with HCOOH
78
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step iii: consists in one of the following methods
Cl: Alkylation with NaH as base in DMF
C2 : Alkylation with K2CO3 as base in acetone
Step iv: consists in one of the following methods
D1 : Deformylation under acid conditions
D2 : Deformylation under basic conditions
Step v : consists in one of the following methods
El (2 steps) : formation of thiourea then cyclisation to give thiazole
derivative
E2 : Aromatic or heteroaromatic nucleophilic substitution
E3 (3 steps) : formation of thiourea, methylation , then cyclisation to give
oxadiazole
derivative
Cl : NaH, DMF
Step vi: consists in one or several of the following methods
Fl : Buchwald coupling
F2 : Suzuki coupling
F3 : Negishi coupling
F4 : Copper mediated coupling
F5 : Boc deprotection
F6 : Reduction with (F12) in presence of transition metal catalyst
F7 : Boc protection
F8 : Alkylation
F9a and F9b : Amide bond forming reaction
F10 : Reductive amination
Fll : Sulfonylation
Fl2a and Fl2b : Nucleophilic substitution
F13 : Saponification
F14 : Introduction of hydroxymethyl group
F15 : Introduction of trifluoroacetyl group
Fl6a and Fl6b : Halogenation
F17 : Copper mediated cyanation
F18 : Reduction with lithium borohydride
F19 : Synthesis of oxazoline
Step vii: consists in one of methods D
Step viii: consists in one of methods E or method H
Step ix: consists in one of methods C
Step x: consists in one or several methods F
Step xi: consists in one of methods D
Step xii : consists in one or several methods E and F
79
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
El (2 steps) : formation of thiourea then cyclisation to give thiazole
derivative
E4 : Buchwald coupling
E5 (2 steps) : SNAr then Suzuki coupling
Step : consists in one or several methods F
1.2. General methods
1.2.1. General method A: Synthesis of Intermediate Gen-1
B
R NH2 N H2
Rea R6a
Gen-1
[00269] To a solution of amino-pyridine derivative (1 eq.) in MeCN under argon
at 0 C is added NBS
(0.5 eq.). The reaction mixture is stirred at r.t. for 1 h then cooled to 0 C
before introducing additional
NBS (0.5 eq.). The reaction mixture is stirred at r.t. for 1 h then diluted in
Et0Ac. The organic layer is
washed with a saturated NaHCO3 solution, dried over Na2SO4, filtered and
concentrated in vacuo. The
residue is diluted in DCM, washed with a I M NaOH solution. The organic phase
is dried over Na2SO4,
filtered and concentrated in vacuo to give Intermediate Gen-1.
1.2.2. Illustrative synthesis of Intermediate Gen-1 -a : 2-amino-5-hromo-3-
fluoropyridine
Br -N
NH2 N H2
[00270] To a solution of 2-amino-3-fluoro-pyridine (9.4 g, 83.1 mmol, 1 eq.)
in MeCN (470 mL) under
argon at 0 C was added NBS (7.4 g, 41.5 mmol, 0.5 eq.). The reaction mixture
was stirred at r.t. for 1 h
then cooled to 0 C before introducing additional NBS (7.39 g, 41.5 mmol, 0.5
eq.). The reaction mixture
was stirred at Lt. for 1 h then diluted in Et0Ac. The organic layer was washed
with a saturated NaHCO3
solution, dried over Na2SO4, filtered and concentrated in vacuo. The residue
was diluted in DCM, washed
with a 1 N NaOH solution. The organic phase was dried over Na2SO4, filtered
and concentrated in vacuo
to afford Intermediate Gen- 1-a (2-amino-5-bromo-3-fluoropyridine).
[00271] LC-MS: MW (calcd): 190 (79Br), 192 (81Br); MW
(obsd): 191 (79Br M+1), 193 (81Br
M+1)
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
1.2.3. General methods B1 and B2: Synthesis of Intermediate Gen-2
0
RN
Hz
R6b /'\,1/11,NH
R6a
R6a
Gen-1 Gen-2
1.2. 3. 1 . General method B1
0
R z
Re NH
R
R6a 2
R6a
R6a
Gen-1
Gen-2
Step (
[00272] To a solution of amino-pyridine derivative Gen-1 (1 eq.) in nBuOH
under argon are added
successively the aldehyde RzCHO (2.5 eq.), MgCl2 (0.04 eq.) and 1,1,3,3-
tetramethylbutyl isocyanide
(1.15 eq.). The reaction mixture is heated at 130 C for 3.5 h, and then
concentrated in vacuo. The
residue is partitioned between heptane and water, stirred for 15 min and
filtered on Celpure P65. The
resulting solid is then dissolved with DCM, dried over Na2SO4, filtered and
concentrated in vacuo to
afford the expected amine.
Step
[00273] A solution of the above prepared compound (1 eq.) in formic acid is
heated at 80 C for 1 h.
The reaction mixture is concentrated in vacuo. The residue is then triturated
in Et20. The formed
precipitate is filtered, rinsed and dried to afford Intermediate Gen-2.
1.2.3.2. Illustrative synthesis of Intermediate Gen-2-a: N-(6-Bromo-2-ethyl-
8-fluoro-imidazo[1,2-
aJpyridin-3-yl)-formarnide
110
HN HN-4C
N
Br N
i i N
,y11, _______________
NH2
Step i)
[00274] To a solution of 2-amino-3-Fluoro-4-bromo-pyridine (Gen-1 -a) (2 g,
10.5 mmol, 1 eq.) in
nBuOH (12 mL) under argon were added successively propionaldchydc (1.9 mL,
26.2 mmol, 2.5 eq.),
MgCl2 (40 mg, 0.42 mmol, 0.04 eq.) and 1,1,3,3-tetramethylbutyl isocyanide
(2.1 mL, 12 mmol, 1.15
eq.). The reaction mixture was heated at 130 C for 3.5 h, then concentrated
in vacuo.
81
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00275] The residue was partitioned between heptane (10 mL) and water (20 mL),
stirred for 15 min
and filtered on Celpure P65. The resulting solid was then dissolved with DCM,
dried over over Na2SO4,
filtered and concentrated in vacuo to afford the corresponding amine. The
filtrate was further extracted
with DCM, the combined organic layers were washed with water, a 1 M NaOH
solution, and brine dried
over Na2SO4, filtered and concentrated in vacuo to deliver a second batch of
the expected amine.
[00276] 1F1 NMR 6 (ppm) (400 MHz, CDC13): 8.11 (1 H, s), 6.90 (1 H, d),
2.85-2.80 (1 H, m), 2.76 (2
H, q), 1.67(2 H, s), 1.37(3 H, t), 1.16 (6 H, s), 1.11(9 H, s).
[00277] LC-MS: MW (calcd): 369 CHO, 371 (81Br); m/z MW (obsd): 370 (79Br M+1),
372 (81Br
M+1)
Step it)
[00278] A solution of amine (2.9 g, 7.83 mmol, 1 eq.) in formic acid (23 mL)
was heated at 80 C for 1
h. The reaction mixture was then concentrated in vacuo. The residue was
triturated in toluene and
evaporated twice. The resulting solid was taken up in Et20, stirred for 45
min, then filtered, rinsed and
dried to afford Tntermediate Gen-2-a.
[00279] 1H NMR 6 (ppm) (400 MHz, CDC13): 2 rotamers 8.55 (1 H, s), 8.15 (1
H, d), 7.95 (1 H, s),
7.76 (1 H, s), 7.54-7.44 (1 H, m), 7.13-6.96 (3 H, m), 2.80 (2 H, q), 2.74 (2
H, q), 1.33 (3 H, t), 1.31 (3 H,
t).
[00280] LC-MS: MW (calcd): 285 (79Br), 287 (81Br); ink MW (obsd): 286 (79Br
M+1), 288 (81Br
M+1)
1.2.3.3. General method B2
0
NH2H
b/Y\ N
R6 NH2 R6b
R
R6a
R6a
R6a
Gen-1
Gen-2
Step i)
[00281] To a suspension of amino-pyridine derivative Gen-1 (1 eq.) in
toluene are added the aldehyde
RzCHO (1 eq.) and benzotriazole (1 eq.). The mixture is stirred at r.t.
overnight. Additional aldehyde
reagent (0.06 eq.) and benzotriazole (0.06 eq.) are added. After 4 h stirring,
potassium cyanide (1.2 eq.)
is added, followed by Et0H. The reaction mixture is stirred at r.t. for 5
days. The crude product mixture
is then quenched with a 3 M NaOH solution. Solvents are evaporated carefully
in vacuo. The residue is
diluted with water and Et0Ac. The aqueous layer is extracted with Et0Ac. The
combined organic layers
are washed with water and brine, dried over Na2SO4 and concentrated in vacuo.
The crude product
mixture is dissolved in Et0H and carefully added to a solution of acetyl
chloride (2.1 eq.) in Et0H at 0 C.
82
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
The resulting reaction mixture is stirred at r.t. overnight and then
concentrated to dryness to afford the
corresponding imidazo[1,2-a]pyridin-3-ylamine as hydrochloride salt.
Step ii)
[00282] A solution of the above prepared imidazo[1,2-a]pyridin-3-ylamine
hydrochloride salt (1 eq.) in
formic acid is heated at 90 C for 2 h. Solvents are evaporated in vacuo. The
residue is dissolved in water.
The mixture is carefully basified with a saturated NaHCO3 solution until pH 8-
9 is reached. The formed
solid is filtered, washed with water and D1PE and dried to afford Intermediate
Gen-2.
1.2.3.4. Illustrative synthesis of Intermediate Gen-2 -d : N-(6-Broino-2-
ethyl-8-methyl-imidazo[1,2-
alpyridin-3-y1)-formarnide
0
HCI HN
NH2
B
N ____________________________________________________________
N ____________________________________
N
NH 2 N
Step
[00283] To a suspension of 2-amino-5-bromo-3-methylpyridine (420 g, 2.24 mol,
1 eq.) previously
washed with a saturated NaHCO3 solution before use in 1.5 L of toluene under
nitrogen were added
propionaldehyde (248 mL, 3.36 mol, 1.5 eq.) and I H-benzotriazole (281 g, 2.36
mol, 1.05 eq.). The
resulting mixture was stirred 4 h at r.t. before adding 3.5 L of Et0H and
potassium cyanide (175 g, 2.70
mol, 1.2 eq.). The reaction mixture was further stirred overnight at r.t. and
2 h at 78 C. After cooling to
r.t., the mixture was quenched by addition of a 2.5 M NaOH solution (3 L).
[00284] This experiment was performed in four batches with the same quantities
of reagents, the crude
mixture were then pooled together and concentrated in vacuo. The remaining oil
was diluted with Et0Ac
(15 L) and washed with a 2 M NaOH solution (2 x 2 L). The aqueous layer was
extracted twice with
Et0Ac (2 x 1 L). The combined organic layers were then dried over Na2SO4,
filtered and concentrated in
vacuo. The crude mixture was dissolved in Et0H (2 L) and carefully added to a
solution of acetyl
chloride (1 L, 14.0 mol, 1.6 eq.) in Et0H (6 L). The resulting reaction
mixture was stirred at r.t.
overnight and then concentrated to dryness. The residue was triturated in DCM
(7 L) for 3 days, the
precipitate formed was collected, washed with DCM (2 x 500 mL) and dried to
afford 6-Bromo-2-ethyl-
8-methyl-imidazo[1,2-a]pyridin-3-ylamine as a hydrochloride salt.
[00285] 'H NMR 6 (ppm) (400 MHz, DMS0): 8.70 (1 H, s), 7.75 (1 H, s), 4.86
(3 H, bs), 2.81 (2 H,
q), 2.56 (3 H, s), 1.56 (3 H, s).
[00286] LC-MS: MW (calcd): 253 (79Br), 255 (81Br): ink MW (obsd): 254 (79Br
M+1), 256 (81Br
M+1)
83
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step ii,)
[00287] A suspension of the above prepared 6-bromo-2-ethy1-8-methyl-
imidazo[1,2-a]pyridin-3-
ylamine hydrochloride (785 g, 2.70 mol, 1 eq.) in formic acid (713 mL, 18.9
mol, 7 eq.) was heated to
80 C for 2 h. The crude mixture was concentrated in vacuo to low volume (about
400 mL). The residue
was brought up in water (1 L) and a 3 M solution of NaOH (2 L), and further
basified with a saturated
NaHCO3 solution until foaming ceased and pH reached 8-9. After homogenization
for 1 h, the precipitate
was filtered and washed with water (2 x 300 mL). Purification was achieved by
dissolution in a mixture
of toluene and Me0H 3:1 (4 L) followed by concentration in vacuo. Trituration
of the residue in a
mixture of 200 mL of Me0H and 5 L of DIPE, decantation and filtration of the
resulting suspension
afforded N-(6-bromo-2-ethy1-8-methylimidazo[1,2-a]pyridin-3-yl)formamide
(Intermediate Gen-2-d).
[00288] Rotamer A: 1H NMR 6 (ppm) (400 MHz, DMS0): 10.2 (1 H, bs), 8.36 (1 H,
s), 8.11(1 H, s),
7.21 (1 H, s), 2.63-2.60 (2 H, m), 2.56 (3 H, s), 1.24-1.17 (3 H, m)
[00289] Rotamer B: 1H NMR 6 (ppm) (400 MHz, DMS0): 8.51 (1 H, s), 8.23 (1 H,
s), 8.11 (1 H, s),
7.23 (1 H, s), 2.63-2.60 (2 H, m), 2.58 (3 H, s), 1.24-1.17 (3 H, m)
[00290] LC-MS: MW (calcd): 281 (79Br), 283 (81Br); miz MW (obsd): 282 (79Br
M+1), 284 (81Br
M+1)
1.2.4. General methods CI and C2:
Synthesis of Intermediate Gen-3
R4 0
H N 'NA
RY H _____________________ N
Rz Rz
Raa R6a
Gen-2 Gen-3
1.2.4.1. General method Cl
R4, ,
NT"-
õ
N _____________________
Rz
R6bM----LN R6bN Rz
R62 R6a
[00291] To a solution of imidazo[1,2-a]pyridine-3-ylamine derivative (1 eq.)
in DMF is added NaH
(1.5 eq.) portionwise, then alkyl iodide (1.4 eq.). The reaction mixture is
stirred for 1 h then quenched
with water and diluted with Et0Ac. The aqueous layer is extracted with Et0Ac.
The combined organic
layers are washed with water and brine, dried over Na2SO4 and concentrated in
vacuo. The residue is
triturated with DIPE. The solid is filtered, rinsed with DIPE and dried to
give the expected intermediate.
84
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
1.2.4.2. Illustrative synthesis of Intermediate Gen3-b : N-(6-bromo-2-
ethylimidazo[1,2-akyridin-3-
yl)-N-methylfornzamide
0 0
HN A ____________ 'WAN
B N N
-1\1
[00292] NaH (60% suspension in oil, 151 g, 3.76 mol, 1.5 eq.) was added
portionwise at r.t. over a
period of 30 min. to a solution of Intermediate Gen-2-b (673 g, 2.51 mol, 1
eq.) in DMF (6 L). The
internal temperature increased to 35 C during the addition and the reaction
mixture was directly cooled to
15 C. Methyl iodide (502 g, 3.53 mol, 1.4 eq.) was added dropwise over a
period of 1 h. The reaction
mixture was kept below 20 C, stirred for 1 h then quenched with water (220
mL). Solvents were
evaporated in vacuo. The residue was diluted with water (3 L) and Et0Ac (4 L).
The aqueous layer was
extracted with Et0Ac (3 x 1 L). The combined organic layers were washed with
water (2x 3 L) and brine
(1.5 L), dried over Na2SO4 and concentrated in vacuo. The residue was
triturated with DTPE (2 L). The
solids were filtered, rinsed with DIPE (2 x 1 L) and dried to give
Intermediate Gen3-b.
[00293] 'H NMR 6 (ppm) (400 MHz, CDC13): 7.92 (1 H, s), 7.78 (1 H, s), 7.33
(1 H, d), 7.30 (1 H, d),
3.25 (3 H, s), 2.72 (2 H, q), 1.35 (3 H, t).
[00294] LC-MS: MW (calcd): 281 (79Br), 283 (giBr); m/z MW (obsd): 284 (giBr
M+1)
1.2.4.3. General method C2
R4,
NT
R6bN
R6b N
Rea R6a
[00295] To a suspension of imidazo[1,2-a]pyridine-3-ylamine derivative (1 eq.)
in acetone are added
potassium carbonate (3 eq.) and alkyl iodide (1.5 eq. to 1.9 eq.). The
reaction mixture is stirred at a
temperature comprised between r.t. and refluxing temperature. If after
stirring overnight, the reaction is
not complete, additional alkyl iodide (0.07 eq.) is then introduced and
stirring is continued for 1 h. The
reaction mixture is filtered and washed with acetone and DCM. The filtrate is
concentrated in vacuo and
the residue is partitioned between DCM and water. The aqueous layer is further
extracted with DCM.
The combined organic layers are then washed with brine, dried over Na2SO4,
filtered and concentrated in
vacuo. The solid is triturated with Et20 at r.t. for 1 h, filtered off and
dried to afford the expected
Intermediate.
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
1.2.4.4. Illustrative synthesis of Intermediate Gen-3-e: N-(6-bromo-2-ethyl-
8-rnethylirnidazo[1,2-
a]pyridin-3-yl)-N-nzethylformarnide
nO 0
\
H N N H
N
[00296] To a suspension of formamide Gen-2-d (720 g, 2.55 mol, 1 eq.) in 5 L
of acetone were added
potassium carbonate (1 kg, 7.66 mol, 3 eq.) and methyl iodide (700 g, 4.93
mol, 1.9 eq.). The reaction
mixture was heated to 40 C overnight. Additional methyl iodide (25 g, 0.18
mol, 0.07 eq.) was then
introduced and stiffing continued for 1 h at 40 C. The reaction mixture was
filtered and washed with
acetone (2 x 300 mL) and DCM (2 x 300 mL). The filtrate was concentrated in
vacuo and the residue was
partitioned between DCM (3 L) and water (1 L). The aqueous layer was further
extracted with DCM.
The combined organic layers were then washed with brine, dried over Na2SO4,
filtered and concentrated
in vacua. The solid was triturated with Et20 (1 L) at r.t. for 1 h, filtered
off and dried to afford
Intermediate Gen-3-e.
[00297] Rotamer A (Major): 1H NMR 6 (ppm) (400 MHz, CDC13): 8.19 (1 H, s),
7.78 (1 H, s), 7.15 (1
H, s), 3.24 (3 H, s), 2.72 (2 H, q), 2.59 (3 H, s), 1.31 (3 H, t)
[00298] Rotamer B (Minor): 1H NMR ö (ppm) (400 MHz, CDC13): 8.49 (1 H, s),
7.65 (1 H, s), 7.08 (1
H, s), 3.36 (3 H, s), 2.72 (2 H, q), 2.59 (3 H, s), 1.31 (3 H, t)
[00299] LC-MS: MW (calcd): 295 (79BI), 297 (81Br); miz MW (obsd): 296 (79Br
M+1), 298 (81Br
M+1)
1.2.5. General methods D1 and D2:
Synthesis of Intermediate Gen-4
y
Nk NH
RV
RN Rz R6b N R
b
Rea Rea
Gen-3 Gen-4
1.2.5.1. General method D1
0 =
HNH
N N
RN R6bN
R6a R6a
[00300] A 4 M HC1 solution in dioxane or 1.25 M HC1 solution in Me0H (9 eq.)
is added to a solution
of imidazo[1,2-a]pyridine-3-y1 formamide derivative (1 eq.) in Me0H. The
reaction mixture is stirred at
a room temperature or refluxed for 3 h. Additional 4 M HC1 solution (1.5 eq.)
is added and stirring is
86
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
continued until completion of the reaction. The reaction mixture is then
concentrated in vacuo to afford
the expected intermediate.
1.2.5.2. Illustrative synthesis of Intermediate Gen-4-d: (6-Bromo-2-ethyl-8-
methyl-imidazo[1,2-
a]pyridin-3-y0-methyl-amine
0
N N H
Br
N
[00301] Intermediate Gen-3-e (80 g, 270 mmol, 1 eq.) was dissolved in a 1.25 M
HC1 solution in
Me0H (540 mL, 2.5 eq.) and the resulting mixture was refluxed overnight. 270
mL of 1.25 M HCl
solution in Me0H were added and heating continued overnight. After 48 h,
additional 70 mL of the 1.25
M HC1 solution in Me0H were introduced in the reaction mixture. Heating was
maintained overnight
until conversion was complete. The crude mixture was then concentrated in
vacuo and the residue was
partitioned between Et0Ac (300 mL) and water (700 mL). A saturated NaHCO3
solution was added until
pH reached 8-9. The aqueous layer was extracted twice with Et0Ac (2 x 300 mL).
The combined organic
layers were then washed with brine (200 mL), dried over Na2SO4, filtered and
concentrated in vacuo to
give Intermediate Gen-4-d (6-bromo-2-ethyl-8-methyl-imidazo[1,2-a]pyridin-3-
y1)-methyl-amine) as a
free base.
[00302] 1H NMR 6 (ppm) (400 MHz, CDC13): 8.05 (1 H, s), 7.04 (1 H, s), 2.84-
2.78 (5 H, m), 2.60 (3
H, s), 1.35(3 H, t)
[00303] LC-MS: MW (calcd): 267 (79Br), 269 (81Br); m/z MW (obsd): 268 (79Br
M+1), 270 (81Br
M+1)
1.2.5.3. General method D2
0
NH
H
RN ____________________ R'
õõkylz-z. R6b N RzR5a
R62
[00304] A 10 M aqueous KOH solution (15 eq.) is added to a solution of
imidazo[1,2-a]pyridine-3-y1
formamide derivative (1 eq.) in Me0H. The reaction mixture is stirred at r.t.
for 3 h, then quenched with
brine and Me0H is removed in vacua. The remaining aqueous phase is extracted
with DCM three times.
The combined organic layers are washed with brine, dried over MgSO4, filtered
and concentrated in
vacuo to afford the expected intermediate as a free base.
87
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
1 .2. 5.4. Illustrative synthesis of Intermediate Gen-4-a: (6-Bromo-2-ethyl-
8-fluoro-imidazo[1,2-
a]pyridin-3-yl)-rnethyl-amine
0
\
N-4-\ NH
H
[00305] A 10 M aqueous KOH solution (25 mL, 250 mmol, 15 eq.) was added to a
solution of
imidazo-pyridine Intermediate Gen-3-a (5 g, 16.67 mmol, 1 eq.) in 25 mL of
Me0H. The reaction
mixture was stirred at r.t. for 3 h, then quenched with brine and Me0H was
removed in vacuo. The
remaining aqueous phase was extracted with DCM three times. The combined
organic layers were
washed with brine, dried over MgSO4, filtered and concentrated in vacuo to
afford Intermediate Gen-4-a
as a free base.
[00306] LC-MS: MW (calcd): 271 (79Br), 273 (81Br); m/z (obsd): 272 (79Br M+1),
274(81Br M+1)
1.2.6. General Methods El, E2, E3 and C: Synthesis of Intermediate Gen-5
ia Rib Rib
R R1'
R4\
RN NH
R4\ N 2
W R R2
X" X
N R RN_Rz
Rea R6b-/`rL---N
Rea R"
Gen-4 Gen-5 Gen-7
1 .2.6. 1 . General method El
Rib
R2 /
N /IS = R la p
'NH Hs R3
i) N 2
Rz Rz
R6C¨k).--"-LN R6b N
R6a
Rea R62
Step I)
[00307] To a suspension of imidazo[1,2-a]pyridine-3-ylamine derivative (1 eq.)
in DCM is added TEA
(4.5 eq.). The mixture is stirred for 30 min at r.t. then Fmoc-isothiocyanate
(1.3 eq.) is added. The
resulting solution is stirred at r.t. for 3 h. Piperidine (3.2 eq.) is then
introduced and the reaction mixture is
stirred at r.t. overnight. Water is added to the solution and the layers are
separated. The aqueous layer is
extracted with DCM/Me0H. The combined organic layers arc washed with brine,
dried over Na2SO4 and
concentrated in vacuo. The expected product is obtained either by
chromatography on silica gel or
crystallization to afford the corresponding thiourea.
88
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step ii,)
[00308] The above prepared thiourea (1 eq.) is added to a solution of the
corresponding bromo
acetophenone Gen-11 or commercial product (1.3 eq.) in Et0H. The reaction
mixture is stirred at reflux
for 3 h then concentrated in vacuo. The crude product is triturated in hot
Et0Ac and stirred for 30 min,
allowed to cool to r.t., filtered off and rinsed with Et0Ac to afford the
expected Intermediate Gen-5.
1.2.6.2. Illustrative synthesis of Intermediate Gen-5-1) : (6-Broino-2-
ethyl-imidazo[1,2-alpyridin-3-
y1)44-(4-fluoro-phenyl)-thiazol-2-y11-methyl-amine
-ANH2 \N¨s
NH
Step i)
[00309] To a suspension of Intermediate Gen-4-b (170 g, 520 mmol, 1 eq.) in
DCM (3 L), was added
triethylamine (325 mL, 2.34 mol, 4.5 eq.). The mixture was stirred for 30 min
at r.t. then Fmoc-
isothiocyanate (190 g, 676 mmol, 1.3 eq.) was added. The formed solution was
stirred at r.t. for 3 h.
Piperidine (164 mL, 1.66 mol, 3.2 eq.) was added to the solution and the
reaction mixture was stirred at
r.t. overnight. Water (1.5 L) was added to the solution and the layers were
separated. The aqueous layer
was extracted with DCM/Me0H. The combined organic layers were washed with
brine, dried over
Na2SO4 and concentrated in vacua. The residue was triturated with MeCN,
filtered and rinsed with MeCN
and Et20 to afford the corresponding thiourea.
[00310] 1I-1 NMR 6 (ppm) (400 MHz, CDC13): 7.88 (1 H, s), 7.32 (1 H, d),
7.30 (1 H, d), 3.67 (3 H, s),
2.75 (2 H, q), 1.33 (3 H, t).
[00311] LC-MS: MW (calcd): 312 (79Br), 314 (81Br): miz MW (obsd): 313 (79Br
M+1), 315 (81Br
M+1)
Step ii)
[00312] The above prepared thiourea (62.5 g, 180 mmol, 1 eq.) was added to a
solution of 2-bromo-4'-
fluoroacetophenone (50.7 g, 233 mmol, 1.3 eq.) in Et0H (1.5 L). The reaction
mixture was stirred at
reflux for 3 h then concentrated in vacua. The crude product was triturated in
hot Et0Ac and stirred for
30 min, allowed to cool to r.t., filtered off and rinsed with Et0Ac to afford
Intermediate Gen-5-b.
[00313] 1H NMR 6 (ppm) (400 MHz, Me0D): 8.75 (1 H, s), 7.98 (2 H, dd), 7.83-
7.75 (3 H, m), 7.14-
7.03 (3 H, m), 3.63 (3 H, s), 2.86 (2 H, q), 1.36 (3 H, t).
[00314] LC-MS: MW (calcd): 430 (79Br), 432 (81Br): ink MW (obsd): 431 (79Br
M+1), 433 (81Br
M+1)
89
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
1.2.6.3. General method E2
la Rib
R
j/ 1 R2
'NH N\ AA/
X
Rz
R6b N R6b R,¨kyls N
R6a R62
[00315] To a solution of imidazo[1,2-a]pyridine-3-ylamine derivative (1 eq.)
and the halogeno
heteroaryl derivative (1.2 eq.) in THF under argon is added NaH (3 eq.). The
reaction mixture is heated at
90 C overnight. After cooling to r.t. the mixture is slowly quenched by
addition of water and then diluted
with Et0Ac. The organic layer is separated and the aqueous layer extracted
with Et0Ac. The combined
organic layers are washed with water and brine, dried over Na2SO4, filtered
and concentrated in vacuo.
The residue is purified either by chromatography on silica gel or by
crystallization to deliver the expected
intermediate.
1.2.6.4. Illustrative synthesis of Intermediate Gen-5-t: 21(6-Bromo-2-ethyl-
8-methyl-imidazo[1,2-
akyridm-3-y0-methyl-amin61-4-(4-fluoro-phenyl)-thiazole-5-carbonitrtle
N
NH
S CN
[00316] To a solution of amine Gen-4-d (4.4 g, 16.6 mmol, 1 eq.) in THF (44
mL) under argon was
slowly added NaH (60% in oil suspension, 2.0 g, 50.0 mmol, 3 eq.). The
reaction mixture was heated at
90 C for 30 min then cooled to 40 C before adding the chlorothiazole Gen-12-a
(4.74 g, 19.9 mmol, 1.2
eq.). The reaction mixture was stirred at 90 C overnight. After cooling to
r.t. the mixture was slowly
quenched by addition of water and then diluted with Et0Ac. The organic layer
was separated and the
aqueous layer extracted with Et0Ac. The combined organic layers were then
washed with water and
brine, dried over Na2SO4, filtered and concentrated in vacuo. The residue was
triturated in Et20, filtered
and washed with Et20 and MeCN. Recrystallization was performed in MeCN (180
niL) to afford
Intermediate Gen-5-t (2- [(6-Bromo-2-ethy1-8-methyl-imidazo [1,2-a]pyridin-3-
y1)-methyl-amino]-4-(4-
fluoro-pheny1)-thiazole-5-carbonitrile).
[00317] Iff NMR 6 (ppm) (400 MHz, CDC13): 8.15(2 H, dd), 7.80(1 H, s), 7.22-
7.14(3 H, m), 3.62(3
H, s), 2.77 (2 H, q), 2.64 (3 H, s), 1.35 (3 H, t)
[00318] LC-MS: MW (calcd): 469 (79Br), 471 (81Br); m/z MW (obsd): 470 (79Br
M+1), 472 ('1Br
M+1)
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
1 .2. 6.5. General method E3
Rib
, S
NH
i) Nh-12 ii) = NH R6b HI) -\µ'N--
o,\VI/ R2
N z
R
R"R R" Rth
Step I)
[00319] To a suspension of imidazo[1,2-a]pyridine-3-ylamine derivative (1 eq.)
in DCM is added TEA
(4.5 eq.). The mixture is stirred until dissolved at r.t. then Fmoc-
isothiocyanate (2.2 eq.) is added. The
resulting solution is stirred at r.t. overnight. Piperidine (5 eq.) is then
introduced and the reaction mixture
is stirred at r.t. for 4 h. Water is added to the solution and the layers are
separated. The aqueous layer is
extracted with DCM. The combined organic layers are washed with brine, dried
over Na2SO4 and
concentrated in vacuo to give the expected thiourea.
Step ii)
[00320] The above prepared thiourea (1 eq.) is dissolved in acetone and Me0H,
NaHCO3 (1 eq.) and
Met (6 eq.) are added, the reaction mixture is stirred at 60 C for 3 h. Then
the reaction mixture is stirred
at r.t. over 2 d. Then the reaction mixture is concentrated in vacuo, the
residue is dissolved in a mixture of
DCM and Me0H. Solids are filtered off, and the filtrate is concentrated in
vacuo. The residue is purified
by chromatography on silica gel (elution with DCM/MeOH: 100/0 to 90/10) to
afford the expected
methylthiourea.
Step iii)
[00321] TEA (3 eq.) is added to a solution of the above prepared
methylthiourea (1 eq.) in Et0H,
followed by the arylamidoxime derivative (2 eq.), then the reaction mixture is
stirred at 80 C over 2 d.
The reaction mixture is quenched by addition of a saturated NaHCO3 solution
and extracted with DCM
three times. The organic phases are combined, dried over Na2SO4 and
concentrated in vacuo. The residue
is purified by chromatography on silica gel to afford the expected
intermediate.
1 .2. 6.6. Illustrative synthesis of Intermediate Gen-5-ae: (6-Bromo-2-
ethyl-imidazo[1,2-alpyridin-3-
yl)-1-3-(4-fluoro-phenyl)-[1,2,41oxadiazol-5-yll -methyl-amine
411
S N
\NI( \ N 4 .N . NH
N
NH 2 B NH 0
r \
\ N \
91
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step (
[00322] To a suspension of the hydrochloride salt of Gen-4-b (11.6 g, 35.5
mmol, 1 eq.) in 250 mL of
DCM was added TEA (20.49 mL, 147.1 mmol, 4.5 eq.). The mixture was stirred at
r.t. until full
dissolution occurred, then Fmoc-isothiocyanate (21.95 g, 74.5 mmol, 2.2 eq.)
was added. The resulting
solution was stirred at r.t. overnight. Piperidine (17.52 nit, 177.0 mmol, 5
eq.) was then introduced and
the reaction mixture was stirred at r.t. for 4 h. Water was added to the
solution and the layers were
separated. The aqueous layer was extracted with DCM. The combined organic
layers were washed with
brine, dried over Na2SO4 and concentrated in vacuo to give the expected
thiourea.
Step
[00323] The above prepared thiourea (500 mg, 1.59 mmol, I eq.) was dissolved
in 150 mL of acetone
and 30 mL of Me0H, NaHCO3 (134 mg, 1.59 mmol, 1 eq.) and Mel (1.36 g, 9.58
mmol, 6 eq.) were
added, the reaction mixture is stirred at 60 C for 3 h. Then the reaction
mixture was stirred at r.t. over 2 d.
The reaction mixture was then concentrated in vacuo, and the residue was
dissolved in a mixture of DCM
and Me0H (9/1). Solids were filtered off, and the filtrate was concentrated in
vacuo. The residue was
purified by chromatography on silica gel (elution with DCM/MeOH: 100/0 to
90/10) to afford the
expected methylthiourea.
[00324] LC-MS: MW (calcd): 326 (79Br), 328 (81Br); na/z (obsd): 327 (79Br
M+1), 329 (81Br M+1)
Step iii)
[00325] TEA (0.383 mL, 2.75 mmol, 3 eq.) was added to a solution of the above
prepared
methylthiourea (300 mg, 0.917 mmol, 1 eq.) in 10 mL of Et0H, followed by 4-
fluorobenzamidoxime
(283 mg, 1.833 mmol, 2 eq.), then the reaction mixture was stirred at 80 C
over 2 days. The reaction
mixture was quenched by addition of a saturated NaHCO3 solution (100 mL) and
extracted with 20 mL of
DCM three times. The organic phases were combined, dried over Na2SO4 and
concentrated in vacuo.
The residue was purified by chromatography on silica gel (elution with
DCM/MeOH: 100/0 to 95/5) to
afford Intermediate Gen-5-ae.
[00326] LC-MS: MW (calcd): 415 (79Br), 417 (81Br); In/z (obsd): 416 (79Br
M+1), 418 (81Br M+1)
1.2.6.7. General method Cl
b R1 Rlb a
N
R2 R4 p R2
HN ¨ ____________________ N¨N
RY,¨k X
N \i¨Rz
R6b N R6b N
R6a R6a
Gen-7 Gen-5
92
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00327] Intermediates Gen-5 are prepared from intermediates Gen-7 according to
general method Cl
described previously
1.2.7. General methods F, El, E4 and E5: Synthesis of Intermediate Gen-10
Rib Rlb
Rla
X \ (R7)a 4
R,
NH
(IR7) R8
R4, P¨e12 \ R2
-W
x-W Rz
RY. X Rs
Rsb
Rz R82
R8b-N
R6b
R6a
R62
Gen-5 Gen-10 Gen-9
1.2.7.1. General methods Fl
halo
N"¨c_Rz
R6bN Rz
R6a R6a
1.2.7.1.1. General method Fla
[00328] To a
solution of the 6-halo-imidazo[1,2-a]pyridine-3-ylamine derivative (1 eq.) in
toluene
under argon are successively added the corresponding amine (5 eq.), sodium
tert-butoxide (2 eq.), and
then ligand (0.13 eq.) and palladium catalyst (0.1 eq.). The reaction mixture
is heated at 115 C until
completion. After cooling to r.t., the crude product is filtered on Celpure
P65, the residue is washed with
Et0Ac and the filtrate is then concentrated in vacua. The crude product is
purified by chromatography on
silica gel to afford the expected intermediate.
1.2.7.1.2. Illustrative synthesis of Intermediate Gen-] 0-i : 2-((2-ethyl-6-
(piperazin-l-
yl)imidazo[1,2-a]pyridin-3-yl)(methy0amino-4-(4-fluorophenyl)thiazole-5-
carbonitrile
CF CF
N , N
ON H N-Th N_<, ON
N
N __
[00329] To a solution of bromide Gen-5-e (300 mg, 0.66 mmol, 1 eq.) in toluene
(6 mL) under argon
were successively added piperazine (283 mg, 3.28 mmol, 5 eq.), sodium tert-
butoxide (126 mg, 1.31
mmol, 2 eq.), and then JohnPhos (26 mg, 0.085 mmol, 0.13 eq.) and Pd2(dba)3
(60 mg, 0.065 mmol, 0.1
eq.). The reaction mixture was heated at 115 C for 45 min. After cooling to
r.t., the crude product was
93
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
filtered on Celpuret P65, the residue was washed with Et0Ac and the filtrate
was then concentrated in
vacuo. The crude product was purified by chromatography on silica gel (elution
with DCM/Me0H/7 N
NH3 in MeOH: 100/0/0 to 100/8/1) to afford Intermediate Gen-10-i.
[00330] 1H
NMR (ppm) (400 MHz, CDC13): 8.08(1 H, dd), 7.50(1 H, d), 7.46-7.31 (2 H, m),
7.21-
7.11 (3 H, m), 3.61 (3 H, s), 3.06(8 H, bs), 2.73(2 H, q), 1.33(3 H, t).
[00331] LC-MS: MW (calcd): 461; m/z MW (obsd): 462 (M+1)
1 .2. 7.1 . 3. General method Fib
[00332] To a
solution of the 6-halo-imidazo[1,2-a]pyridine-3-ylamine derivative (1 eq.) in
toluene
under argon are successively added the corresponding amine (1.1 to 1.5 eq.),
sodium tert-butoxide (1.18
to 2 eq.), and then JohnPhos, XantPhos or DavePhos (0.06 to 0.1 eq.) and
Pd2(dba)3 (0.02 to 0.05 eq.).
The reaction mixture is heated at 115 C until completion. After cooling to
r.t., the crude product is
filtered on Celpureg P65, the residue is washed with Et0Ac and the filtrate
concentrated in vacuo. The
crude product is purified by chromatography on silica gel to afford the
expected intermediate.
1.2.7.1.4.
Illustrative synthesis of 4-(34[5-Cyano-4-(4-fluoro-phenyl)-thiazol-2-yll-
methyl-amino}-
2-ethyl-8-methyl-imidazo[1,2-a]pyridin-6-y1)-piperazine-1-carboxylic acid tert-
butyl
ester
Soc. N.-)
S CN S ON
[00333] To a solution of Intermediate Gen-5-t (24.2 g, 51.5 mmol, 1 eq.) in
toluene under argon were
successively added N-Boc piperazine (14.4 g, 77.3 mmol, 1.5 eq.), sodium tert-
butoxide (9.9 g, 103
mmol, 2 eq.), JohnPhos (1.54 g, 5.15 mmol, 0.1 eq.) and Pd2(dba)3 (2.36 g,
2.58 mmol, 0.05 eq.). The
reaction mixture was heated at 115 C for 1 h. After cooling to r.t., the crude
product was filtered on
Celpuret P65 and the residue dissolved in Et0Ac and washed with water. The
organic layer was further
washed with brine, dried over Na2SO4, filtered and concentrated in vacuo. The
crude product was
purified by chromatography on silica gel (elution with heptane/Et0Ac: 90/10 to
20/80) to afford the
expected product.
[00334] 11-1
NMR 13 (ppm) (400 MHz, CDC13): 8.16 (2 H, dd), 7.17 (2 H, app t), 6.99 (2 H,
bs), 3.62-
3.53 (4 H, m), 3.60 (3 H, s), 3.04-2.93 (4 H, m), 2.74 (2 H, q), 2.62 (3 H,
s), 1.47 (9 H, s), 1.33 (3 H, t).
[00335] LC-MS: MW (calcd): 575; m/z MW (obsd): 576 (M+1)
94
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
1.2.7.2. General method F2
, I '
R6b
R6a Raa
[00336] To a solution of the bromide Gen-5-b (21.6 g, 45.1 mmol, 1 eq.) in a
mixture dioxane/water
(300 mL/75 mL) under argon were successively added sodium carbonate (14.3 g,
135 mmol, 3 eq.), tert-
butyl 4-(4,4,5,5-tetramethyl- 1,3 ,2-dioxab orol an-2-y1)-5,6-dihydropyridine-
1(2H)-carboxylate (18.1 g,
58.6 mmol, 1.3 eq.), and then Pd(PPh3)4 (3.91 g, 3.38 mmol, 0.075 eq.). The
reaction mixture was heated
at 85 C for 3 h. After cooling to r.t., the crude product is filtered on
Clarcel and the filtrate is
concentrated in vacuo. The residue is purified by chromatography on silica gel
to afford the expected
intermediate.
1.2.7.3. Illustrative synthesis of 4-(2-Ethyl-34[4-(4-fluoro-phenyl)-
thiazol-2-yl]-methyl-amino}-
imidazo[1,2-alpyridin-6-y1)-3,6-dihydro-2H-pyridine-1-carboxylic acid tert-
butyl ester
N¨
'
N
N \
Br,,õ S
¨N
[00337] To a solution of the bromide Gen-5-b (21.6 g, 45.1 mmol, 1 eq.) in a
dioxane/water (300
mL/75 mL) mixture under argon were successively added sodium carbonate (14.3
g, 135 mmol, 3 eq.),
the corresponding boronic ester (18.1 g, 58.6 mmol, 1.3 eq.), and then
Pd(PPh3)4 (3.91 g, 3.38 mmol,
0.075 eq.). The reaction mixture was heated at 85 C for 3 h until completion.
After cooling to r.t., the
reaction mixture was concentrated in vacuo. The crude product was partitioned
between Et0Ac and
water. The aqueous layer was extracted twice with Et0Ac. The combined organic
layers were then
washed with brine, dried over Na2SO4, filtered and concentrated in vacuo. The
residue was then purified
by chromatography on silica gel (elution with DCM/Me0H 100/0 to 97/3) to
afford the expected
compound.
[00338] LC-MS: MW (calcd): 533; m/z MW (obsd): 534 (M+1)
1.2.7.4. General method F3
'
Rz -11.=
R"\-(-1N Rz
R6a R6a
[00339] To a solution of the 6-halo-imidazo[1,2-a]pyridine-3-ylamine
derivative (1 eq.) in DMA under
argon are successively added the copper iodide (0.25 eq.), PdC12dppf (0.1
eq.), and a solution of the
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
corresponding organozinc compound (1.3 eq.) in DMA. The reaction mixture is
heated at 80 C for 3 h
then additional solution of the corresponding organozinc compound (0.6 eq.) in
DMA is added. Stirring
at 80 C is continued overnight. After cooling to r.t., the crude product is
filtered on Celpureg P65, the
residue is washed with Et0Ac and the filtrate is washed with water and brine,
dried over Na2SO4, filtered
and concentrated in vacuo. The crude product is purified by chromatography on
silica gel to afford the
expected intermediate.
1.2.7.5. Illustrative synthesis of 4-(34[5-Cyano-4-(4-fluoro-phenyl)-
thiazol-2-yll -methyl-aniino}-2-
ethyl-8-methyl-imidazo[1,2-akyridin-6-y1)-piperidine-1 -carboxylic acid tert-
butyl ester
N N
Boo .N
S CN S CN
N
[00340] To a solution of the bromide Gen-5-t (600 mg, 1.28 mmol, 1 eq.) in DMA
(4.4 mL) under
argon were successively added the copper iodide (61 mg, 0.32 mmol, 0.25 eq.),
PdC12dppf (93 mg, 0.13
mmol, 0.1 eq.), and a solution of the corresponding organozinc compound
(prepared from 4-iodo-Boc-
piperidine (Corley, et al., 2004)) in DMA (1 M in DMA, 1.66 mL, 1.66 mmol, 1.3
eq.). The reaction
mixture was heated at 80 C for 3 h then additional solution of the
corresponding organozinc compound
(0.5 mL, 0.5 mmol, 0.6 eq.) in DMA was added. Stirring at 80 C was continued
overnight. After cooling
to r.t., the crude product was filtered on Celpureg P65, the residue was
washed with Et0Ac and the
filtrate washed with water and brine, dried over Na2SO4 and concentrated in
vacuo. The crude product
was purified by chromatography on silica gel (elution beptanesiEt0Ac 100/0 to
50/50) to deliver the
expected compound.
[00341] LC-MS: MW (calcd): 574; m/z MW (obsd): 575 (M+1)
1.2.7.6. General method F4
,
z
Rz
R6b R
R6b
R6a
R6a
[00342] To a suspension of 6-halo-imidazo[1,2-a]pyridine-3-ylamine
derivative (1 eq.), potassium
carbonate (2 to 3 eq.), the corresponding amine (1.2 to 2 eq.), and CuI (0.1
to 0.2 eq.) in DMF under
argon, is added the trans-1,2-diaminocyclohexane (0.2 to 0.4 eq.), and then
the reaction mixture is heated
between 85 C and 100 C overnight. After cooling to r.t., the crude product is
filtered on Celite, and the
residue is washed with Et0Ac. The filtrate is washed with a saturated NaHCO3
solution, the two phases
96
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
are separated, and the aqueous phase is washed twice with Et0Ac. The combined
organic layers are
washed with brine, dried over Na2SO4, filtered and concentrated in vacuo. The
crude product is purified
by chromatography on silica gel to afford the expected intermediate.
1.2.7.7. Illustrative synthesis of compound 62. 4-(2-Ethy1-3-1[4-(4-fluoro-
pheny1)-thiazol-2-y1]-
methyl-aminoi-imidazo[1,2-a]pyridin-6-yl)-3-oxo-piperazine-1-carboxylic acid
tert-butyl est
\ ------> Boc.N
N N N
0
[00343] To a suspension of Gen-5-b (600 mg, 1.391 mmol, 1 eq.), potassium
carbonate (577 mg, 4.173
mmol, 3 eq.), 3-oxo-piperazine-1 -carboxylic acid tert-butyl ester (557 mg,
2.78 mmol, 2 eq.), and Cul (53
mg, 0.278 mmol, 0.2 eq.) in DMF (4 mL) under argon, was added the 1,2-
diaminocyclohexane (67 RL,
0.56 mmol, 0.4 eq.), and then the reaction mixture was heated at 100 C
overnight. After cooling to r.t.,
the crude product was filtered on Celite, and the residue was washed with
Et0Ac. The filtrate was washed
with a saturated NaHCO3 solution, the two phases were separated, and the
aqueous phase was washed
twice with Et0Ac. The combined organic layers were washed with brine, dried
over Na2SO4, filtered and
concentrated in vacuo. The crude product was purified by chromatography on
silica gel to afford the
expected compound.
[00344] LC-MS: MW (calcd): 550; m/z MW (obsd): 551 (M+1)
1.2.7.8. General method F5 (Boc removal)
4- 4 -
0 N HN
1.2.7.8.1. General method F5a
[00345] To a solution of the boc protected amine (1 eq.) in DCM is added TFA
(10 eq.). The reaction
mixture is stirred at r.t. until completion. Then the reaction mixture is
partitioned between DCM and
water. The aqueous layer is washed twice with DCM. A saturated Na2CO3 solution
is added to the
aqueous layer until pH reached 8-9 and is extracted with DCM twice. The
combined organic layers are
then washed with brine, dried over Na2SO4, filtered and concentrated in vacuo
to afford the expected
intermediate
97
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
1.2.7.8.2. Illustrative synthesis of Compound 177: [6-(3-Amino-azetidin-l-
y1)-2-ethyl-irnidazo[1,2-
]pyridin-3-yl -[4-(4-fluoro-phenyl)-thiazol-2-yl -methyl-amine
CF CF
HN N
H2NCAN
[00346] [1-(2-Ethyl-3-f[4-(4-fluoro-pheny1)-thiazol-2-yl]-methyl-aminoI -
imidazo[1,2-a]pyridin-6-y1)-
azetidin-3-3/1]-carbamic acid tert-butyl ester was prepared from intermediate
Gen-5-b and 3-N-Boc-
amino-azetidine using method Fib. To a solution of this compound [ (200 mg,
0.383 mmol, 1 eq.) in
DCM (3 mL) was added TFA (291 [it, 3.827 mmol, 10 eq.). The reaction mixture
was stirred at r.t. for
2.5 days, then the reaction mixture was partitioned between DCM and water. The
aqueous layer was
washed twice with DCM. A saturated Na2CO3 solution was added to the aqueous
layer until pH reached
8-9 and was extracted with DCM twice. The combined organic layers were then
washed with brine, dried
over Na2SO4, filtered and concentrated in vacuo to afford the expected
compound.
[00347] LC-MS: MW (calcd): 422; m/z MW (obsd): 423 (M+1)
1.2.7.8.3. General method F5b
[00348] To a solution of the boc protected amine (1 eq.) in Me0H is added a 2
N HC1 solution in Et20
or 4 M HC1 solution in dioxane or 1.25 M HC1 solution in Me0H (6 eq.). The
reaction mixture is stirred
at r.t. until completion then concentrated in vacuo. The residue is
partitioned between Et0Ac and water.
The aqueous layer is extracted twice with Et0Ac. A 2 N NaOH solution is added
to the aqueous layer
until pH reached 8-9 and further extraction with Et0Ac is performed. The
combined organic layers are
then washed with brine, dried over Na2SO4, filtered and concentrated in vacuo
to afford the Intermediate
Gen-10.
1.2.7.8.4. Illustrative synthesis of Compound 1 : 2-[(2-Ethyl-8-methyl-6-
piperazin-l-yl-
imidazo[1,2-alpyridin-3-y1)-methyl-aminoi -4-(4-fluoro-phenyl)-thiazole-5-
carbonitrile
Boc, \N"¨
N 1
S ON S ON
[00349] 4-(3-[[5-Cyano-4-(4-fluoro-pheny1)-thiazol-2-y1]-methyl-aminoI -2-
ethyl-8-methyl-
imidazo[1,2-a]pyridin-6-y1)-piperazine-1 -carboxylic acid tert-butyl ester was
prepared from intermediate
Gen-5-t using Boc-piperazine and method Fib.
98
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00350] To a solution of 4-(3-{[5-Cyano-4-(4-fluoro-pheny1)-thiazol-2-A-methyl-
amino} -2-ethy1-8-
methyl-imidazo[1,2-a]pyridin-6-y1)-piperazine-l-carboxylic acid tert-butyl
ester (24.4 g, 42 mmol, 1 eq.)
in Me0H (100 mL) was added a 2 M HCl solution in Et20 (127 mL, 254 mmol, 6
eq.). The reaction
mixture was stirred at Lt. for 3.5 h then concentrated in vacuo. The residue
was partitioned between
Et0Ac and water. The aqueous layer was extracted twice with Et0Ac. A 2 M NaOH
solution was added
to the aqueous layer until pH reached 8-9 and further extraction with Et0Ac
was performed. The
combined organic layers were then washed with brine, dried over Na2SO4,
filtered and concentrated in
vacuo. The solid was triturated with heptane (100 mL) at r.t. overnight,
filtered off, washed with heptane
and Et20, and dried to afford the expected compound.
[00351] 11-1 NMR 6 (ppm) (400 MHz, CDC13): 8.17 (2 H, dd), 7.18 (2 H, app
t), 6.99 (2 H, bs), 3.61 (3
H, s), 3.09-2.98 (8 H, m), 2.75 (2 H, q), 2.61 (3 H, s), 1.34 (3 H, t).
[00352] LC-MS: MW (caled): 475; m/z MW (obsd): 476 (M+1)
1.2.7.9. General method F6
'N1'
z
z ______________________ IV" R
, R = '1,
R6b R"--" N R6bN
R" 14"
and R6a
[00353] To a solution of the imidazo[1,2-a]pyridine-3-ylamine derivative (1
eq.) in a mixture
THF/Me0H with AcOH (0 to 0.05 eq.) is added Pt02 (15%) or Pd/C (10%). The
flask is evacuated and
backfilled with argon. Then the reaction is evacuated and backfilled with H2
and stirred at r.t. under
atmospheric pressure until completion. The crude product is filtered through a
pad of Clarcel and washed
with Me0H. The filtrate is concentrated under reduced pressure. The residue is
purified to
chromatography on silica gel to afford the expected compound.
1.2.7.10. Illustrative synthesis of 4-(2-Ethyl-3-1[4-(4-fluoro-pheny0-
thiazol-2-y1]-inethyl-amino}-
imidazo[1,2-alpyridin-6-A-piperidine-1-carboxylic acid tert-butyl ester
N N
Boc, N-- oo.
N s-s B
[00354] 4-(2-Ethyl-3- { [4-(4-fluoro-pheny1)-thiazol-2-yl] -methyl-amino } -
imidazo [1,2-a] pyridin-6-y1)-
3,6-dihydro-2H-pyridine-1 -carboxylic acid tert-butyl ester was prepared from
Intermediate Gen-5-b and
tert-butyl 4-(4,4,5,5-tetramethy1-1,3,2-dioxaborol an-2 -y1)-5,6-
dihydropyridine-1(2H)-carb oxylate using
method F2.
[00355] To a solution of 4-(2-Ethyl-3- {[4-(4-fluoro-pheny1)-thiazol-2-y1]-
methyl-amino} -imidazo [1,2-
a]pyridin-6-y1)-3,6-dihydro-2H-pyridine- 1 -carboxylic acid tert-butyl
ester_(60,0 g, 97 mmol, 1 eq.) in a
99
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
mixture of THF (750 mL) and Me0H (750 mL) with AcOH (0.279 mL, 4.83 mmol, 0.05
eq.) was added
Pd/C (10.3 g, 9.67 mmol, 0.1 eq.). The flask was evacuated and backfilled with
argon. Then the reaction
was evacuated and backfilled with H2 and stirred at r.t. under atmospheric
pressure overnight. The crude
product was filtered through a pad of Clarcel and washed with Me0H. The
filtrate was concentrated
under reduced pressure. The residue was purified to chromatography on silica
gel to afford the expected
compound.
[00356] LC-MS: MW (calcd): 535; m/z MW (obsd): 536 (M+1)
1.2.7.11. General method F7
-
- 0 N
HN =
[00357] To a solution of the amino derivative in DCM are added TEA (5 eq.)
then Boc20 (0.9 eq.).The
reaction mixture is stirred at r.t. for 1.5 h then diluted with DCM. The
organic layer is separated and the
aqueous layer extracted with DCM. The combined organic layers are washed with
water and brine, dried
over Na2SO4, filtered and concentrated in vactto to afford the corresponding
intermediate.
1.2.7.12. Illustrative synthesis of 4-(2-Ethyl-3-methylamino-imidazo[1,2-
akyridin-6-yl)-piperidine-
1-carboxylic acid tert-butyl ester
H Bo c,N
N NH NH
- N - N
[00358] (2-Ethyl-6-piperidin-4-yl-imidazo[1,2-a]pyridin-3-y1)-methyl-amine is
prepared from
intermediate Gen-3-b through successive methods F2 (with tert-butyl 4-(4,4,5,5-
tetramethy1-1,3,2-
dioxaborol an-2-y1)-5,6-dihydropyridine-1(2H)-carboxylate), F6 and Dl.
[00359] To a solution of (2-Ethy1-6-piperidin-4-yl-imidazo[1,2-a]pyridin-3-
y1)-methyl-amine (1.67 g,
4.15 mmol, 1 eq.) in DCM (35 mL) were added TEA (2.9 mL, 20.7 mmol, 5 eq.)
then Boc20 (815 mg,
3.74 mmol, 0.9 eq.). The reaction mixture was stirred at r.t. for 1.5 h then
diluted with DCM. The
organic layer was separated and the aqueous layer extracted with DCM. The
combined organic layers
were washed with water and brine, dried over Na2SO4, filtered and concentrated
in vacuo to afford the
expected compound.
[00360] LC-MS: MW (calcd): 358; m/z MW (obsd): 359 (M+1)
1.2.7.13. General method F8
HN
halo
100
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00361] To a solution of amino derivative (1 eq.) in MeCN are added potassium
carbonate (2 eq.) or
TEA (5 eq.) and halogenated derivative Gen-13 (or commercially available
products) (1.5 eq.). The
reaction mixture is heated between 70 C and reflux for 1.5 h to 6 h then
cooled to r.t. The reaction
mixture is quenched with water and diluted with Et0Ac. The aqueous layer is
extracted with Et0Ac.
The combined organic layers arc washed with water and brine, dried over Na2SO4
and concentrated in
vacuo. The residue is purified by chromatography on silica gel to deliver the
expected compound. If the
product is precipitated in the reaction mixture the following work up is used:
after cooling to r.t., the
reaction mixture is filtered. The solid is washed with MeCN, water and dried
in vacuo to afford the
expected product.
1.2.7.14. Illustrative synthesis of Compound 2 : 2-((2-ethyl-6-(4-(2-(3-
hydroxyazetidin-1
oxoethyl)piperazin-1 -y0-8-meihylimidazo[1,2-a]pyridin-3-yl)(methyl) alll ino)-
4-(4-
fluorophenyt9thiazole-5-carbonitrile
HO,,rn
HN N N \N"--4
S CN __________________________________________________ S CN
1\1
[00362] To a solution of amine compound 1 (12.6 g, 27 mmol, 1 eq.) in 100 mL
of MeCN were added
potassium carbonate (7.3 g, 53 mmol, 2 eq.) and Gen13-a (5.2 g, 34 mmol, 1.3
eq.). The reaction mixture
was refluxed for 5.5 h then cooled to r.t. and stirred for 40 h. The crude
product was filtered and washed
with MeCN. The collected precipitate was then suspended in 300 mL of water,
stirred for 1 h, filtered,
and finally washed with water and MeCN. The solid obtained was dried in vacuo
for 48 b to afford
Compound 2.
[00363] NMR (400 MHz, CDC13) 6 ppm 8.20 - 8.12 (2 H, m), 7.22 - 7.13 (2 H,
m), 6.99 (2 H, s),
4.68 (1 H, m), 4.43 (1 H, dd), 4.26 (1 H, dd), 4.14 - 4.05 (1 H, m), 3.88 (1
H, dd), 3.61 (3 H, s), 3.58 -
3.52 (1 H, m), 3.14 - 3.02 (6 H, m), 2.74 (2 H, q), 2.70 - 2.62 (4 H, m), 2.59
(3 H, s), 1.33 (3 H, t)
[00364] LC-MS: MW (calcd): 588; nth MW (obsd): 589 (M+1)
1.2.7.15. General methods F9
1.2.7.15.1. General method F9a
0 0
-
)L. OH HN '
- -
[00365] To a solution of acid (1.1 eq.) in DCM are added HOBT (1.2 eq.) and
EDC.HC1 (1.2 eq.). The
reaction mixture is stirred at r.t. for 45 min then prepared solution of amine
(1 eq.) in DCM with TEA (3
eq.) is added. The reaction mixture is stirred at r.t. until completion, then
water and a solution of HC1 1
M are added, the aqueous layer is extracted with DCM, the organic layer is
washed with a saturated
101
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Na2CO3 solution, and brine, dried over Na2SO4, filtered and concentrated in
vacuo. The residue is
purified by chromatography on silica gel to afford the expected compound.
1.2.7.15.2. Illustrative synthesis of Compound 205: 1-Benzy1-4-[4-(2-ethyl-
3-{[4-(4-fluoro-phenyl)-
thiazol-2-yll-methyl-amino}-imidazo[1,2-akyridin-6-y0-piperazine-1-carbonyl -
pyrrolidin-2-one
111 0
HN1
\N-<
SN N =
[00366] To a solution of 1-benzy1-5-oxo-pyrrolidine-3-carboxylic acid (38
mg, 0.173 mmol, 1.1 eq.) in
DCM (3 mL) were added HOBT (25 mg, 0.188 mmol, 1.2 eq.) and EDC.HC1 (36 mg,
0.188 mmol, 1.2
eq.). The reaction mixture was stirred at r.t. for 45 min then Gen-10-e (80
mg, 0.157 mmol, 1 eq.)
dissolved in DCM (1 mL) with TEA (65 litt, 0.471 mmol, 3 eq.) was added. The
reaction mixture was
stirred at r.t overnight, then water and a solution of HCl 1 M were added, the
aqueous layer was extracted
with DCM, the organic layer was washed with a saturated Na2CO3 solution, and
brine, dried over Na2SO4,
filtered and concentrated in vacuo. The residue was purified by chromatography
on silica gel to afford the
Compound 205
[00367] LC-MS: MW (calcd): 637; m/z MW (obsd): 638 (M+1)
1.2.7.15.3. General method F9b
0 0
ci -
HN
-1-
[00368] To a solution of amine (1 eq.) in DCM are added TEA (4 to5 eq.)
followed by acyl chloride
derivative (1.2 to 2 eq.). The reaction mixture is stirred at r.t. until
completion, then is quenched with
water and the aqueous layer is extracted with DCM twice. The organic layer is
washed with water, and
brine, dried over Na2SO4, filtered and concentrated in vacuo. The residue is
purified by chromatography
on silica gel to afford the expected compound.
1.2.7.15.4. Illustrative synthesis of Compound 48: 1-{314-(2-Ethyl-34[4-(4-
fluoro-phenyl)-thiazol-
2-y1Tmethyl-amino}-imidazon,2-a]pyridin-6-y0-piperazine-1-carbonyl -pyrrolidin-
l-
yll-ethanone
,F
0 0
HN1 N N-
-N
N
102
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00369] To a solution of compound 47 (30 mg, 0.049 mmol, 1 eq.) in DCM (2 mL)
were added TEA
(34 !IL, 0.247 mmol, 5 eq.) followed by the acetyl chloride (7 jiL, 0.099
mmol, 2 eq.). The reaction
mixture was stirred at r.t. for 2 h, then quenched with water and the aqueous
layer was extracted with
DCM twice. The organic layer was washed with water, and brine, dried over
Na2SO4, filtered and
concentrated in vacuo. The residue was purified by chromatography on silica
gel to afford Compound 48
[00370] LC-MS: MW (calcd): 575; m/z MW (obsd): 576 (M+1)
1.2.7.16. General method F10
- 0
H N ' + Th
[00371] To a solution of the appropriate amine (1.0 eq.) in Me0H are added TEA
(0 to 3 eq.), acetic
acid if needed (0 to 3 eq.) and the aldehyde or ketone (1.5 to 2 eq.). The
reaction mixture is stirred at r.t.
for 10 min then NaBH3CN (1.5 to 3 eq.) is added. The reaction mixture is
stirred at r.t. overnight, then
concentrated in vacuo. The residue is dissolved in a mixture of DCM and water,
the two phases are
separated and the aqueous phase is extracted with DCM. The combined organic
layers are washed with a
saturated Na2CO3 solution, and brine, dried over Na2SO4, filtered and
concentrated in vacuo. The residue
was purified by chromatography on silica gel to afford the expected compound.
1.2. 7./7. Illustrative synthesis of Compound 217: [6-(1-Benzyl-piperidin-4-
yl)-2-ethyl-imidazo[1,2-
alpyridin-3-yll-14-(4-fluoro-phenyl)-thiazol-2-yl 1-methyl-amine
HN \N
[00372] To a solution of amine Gen-10-c (40 mg, 0.085 mmol, 1.0 eq.) in MeOH
(2 nit) were added
TEA (35 sL, 0.254 mmol, 3 eq.) and the benzaldehyde (17 sL, 0.169 mmol, 2
eq.). The reaction mixture
was stirred at r.t. for 10 min then NaBH3CN (158 mg, 0.254 mmol, 3 eq.) was
added. The reaction
mixture was stirred at r.t. overnight, then concentrated in vacuo. The residue
was dissolved in a mixture
of DCM and water, the two phases were separated and the aqueous phase is
extracted with DCM. The
combined organic layers were washed with a saturated Na2CO3 solution, and
brine, dried over Na2SO4,
filtered and concentrated in vacuo. The residue was purified by chromatography
on silica gel to afford
Compound 217.
[00373] LC-MS: MW (calcd): 525; m/z MW (obsd): 526 (M+1)
103
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
1.2.7.18. General method F]]
- 0
µ= 0
-CI
,N
= S
0
[00374] To a solution of the appropriate amine (1 eq.) in DCM at 0 C are added
TEA (3 eq.) and
sulfonyl chloride (1.3 to 2 eq.). The reaction mixture is stirred at r.t.
until completion. The crude product
is quenched with water and diluted with DCM, the aqueous layer is extracted
with DCM. The combined
organic layers are washed with water and brine, dried over Na2SO4, filtered
and concentrated in vacuo.
The residue is purified by chromatography on silica gel to afford the expected
compound.
1.2.7.19. Illustrative synthesis of Compound 80 : N-(2-ethyl-6-(1-
(methylsulfonyl)piperidin-4-
yl)im idazo [1,2-a]pyridin-3-yl)-4-(4-fluorophenyl)-AT-inethylth iazol-2-a in
in e
F
0
'
\
, S j
[00375] To a solution of the previously prepared amine Gen-10-c (2.9 g, 6.7
mmol, 1 eq.) in DCM at
0 C were added TEA (2.8 mL, 20.1 mmol, 3 eq.) and mesyl chloride (1.03 mL,
13.3 mmol, 2 eq.). The
reaction mixture was stirred at r.t. for 3 h. The crude product was quenched
with water and diluted with
DCM, the aqueous layer was extracted with DCM. The combined organic layers
were washed with water
and brine, dried over Na2SO4, filtered and concentrated in vacuo. The residue
was purified by
chromatography on silica gel (elution DCM/Me0H 90/10) to afford Compound 80.
[003761 LC-MS: MW (calcd): 513; m/z MW (obsd): 514 (M+1)
1.2.7.20. General methods F12
1.2.7.20.1. General methods Fl 2a
halo
X X= NR, 0, S
[00377] To a solution of the the corresponding nucleophile (2 to 6 eq.) in THF
or DMF are added Nal
or KI (cat) and halogenoalkyl group containing imidazo[1,2-a]pyridine-3-
ylamine derivative Gen-10 (1
eq.). When the nucleophile amine corresponding as a hydrochloride, the amine
is premixed with K2CO3
(5 to 6 eq.) in the solvent for 10 mm before the addition of the catalyst and
the halogenoalkyl group
containing imidazo[1,2-a]pyridine-3-ylamine derivative. The reaction mixture
is heated between 80 C
and 150 C under microwave irradiation or in thermic conditions for 1.5 to 3 h.
After cooling, water and
Et0Ac are added to the reaction mixture, the aqueous layer is extracted with
Et0Ac twice. The combined
organic layers are washed with water and brine, dried over Na2SO4, filtered
and concentrated in vacuo.
The residue is purified by chromatography on silica gel to afford the expected
compound.
104
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
1.2.7.20.2. Illustrative synthesis of Compound 89 : {2-Ethyl-611-(3-
pyrrolidin-1 -yl-propane-1-
sulfony1)-piperidin-4-yll-imidazo[1,2-akyridin-3-yl}-[4-(4-fluoro-phenyl)-
thiazol-2-yll-
methyl-amine
0
---,
N
[NC' _ns) 0 7
,)4
N ' > __
-N
[00378] To a solution of the the pyrrolidine (36 jiL, 0.434 mmol, 5 eq.) in
THF (3 mL) were added NaI
(2 mg, cat) and Compound 86 (50 mg, 0.087 mmol, 1 eq.). The reaction mixture
was heated at 150 C
under microwave irradiation for 2 h. After cooling, water and Et0Ac were added
to the reaction mixture,
the aqueous layer was extracted with Et0Ac twice. The combined organic layers
were washed with water
and brine, dried over Na2SO4, filtered and concentrated in vacuo. The residue
was purified by
chromatography on silica gel to afford Compound 89.
[00379] LC-MS: MW (calcd): 610; in/z MW (obsd): 611 (M+1)
1.2.7.20.3. Illustrative synthesis of Compound 131 : { 2-(3,3-Difluoro-
azetidin-1-A-1-1-4-(2-ethyl-3-
{[4-(4-fluoro-phenyl)-thiazol-2-yll-methyl-amino}-imidazo[1,2-alpyridin-6-yl)-
piperidin-
1-yll-ethanone
0 Fµ
CI ,,J-LN
F)CN,
S
N _____________________________________________________
\
[00380] To a solution of the the 3,3-difluoroazetidine hydrochloride (40 mg,
0.31 mmol, 2 eq.) in DMF
(1.5 mL) was added K2CO3 (111 mg, 0.80 mmol, 5 eq.), the reaction mixture was
stirred at r.t. for 10
min., then KI (4 mg, cat) and the2-Chloro-1-[4-(2-ethy1-3- {[4-(4-fluoro-
phenye-thiazol-2-y1]-methyl-
amino} -imidazo[1,2-a]pyridin-6-y1)-piperidin-l-y1]-ethanone (1 eq.) were
added. The reaction mixture is
heated at 80 C in thermic conditions for 2 h. After cooling, water and Et0Ac
were added to the reaction
mixture, the aqueous layer was extracted with Et0Ac twice. The combined
organic layers were washed
with water and brine, dried over Na2SO4, filtered and concentrated in vacuo.
The residue was purified by
chromatography on silica gel to afford Compound 131.
[00381] LC-MS: MW (calcd): 568; m/z MW (obsd): 569 (M+1)
1.2.7.20.4. General methods Fl 2b
0 0
halo Ao-K'
0
[00382] To a solution of the halogenoalkyl group containing imidazo[1,2-
a]pyridine-3-ylamine
derivative (1 eq.) in DMF is added potassium acetate (3 eq.), the reaction
mixture is heated at 90 C for 4h
105
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
to overnight. After cooling, water and Et0Ac are added to the reaction
mixture, the aqueous layer is
extracted with Et0Ac twice. The combined organic layers are washed with water
and brine, dried over
Na7SO4, filtered and concentrated in vacuo. The residue is purified by
chromatography on silica gel to
afford the expected compound.
1.2.7.20.5.
Illustrative synthesis of Compound 74 : Acetic acid 3-1-4-(2-ethyl-3-{[4-
(411uoro-phenyl)-
thiazol-2-yl -methyl-ainino}-imidazo[1,2-alpyridin-6-y0-3,6-dihydro-21-1-
pyridine-1 -
sulfonyli-propyl ester
0
CI
_// S,
0 Ic Ns N
N _____________________
N
[00383] To a
solution of the the {6-[1 -(3 -Chloro-propane-l-sulfony1)-1,2,3,6-tetrahy dro-
pyridin-4-yl] -
2- ethyl-imidazo
pyridin-3 -yl } - [444 -fluo ro-pheny1)-thiazol-2-yl] -methyl-amine (105 mg,
0.183
mmol, 1 eq.) in DMF (3 mL) was added potassium acetate (54 mg, 0.549 mmol, 3
eq.), the reaction
mixture was heated at 90 C for 4 h. After cooling, water and Et0Ac were added
to the reaction mixture,
the aqueous layer was extracted with Et0Ac twice. The combined organic layers
were washed with water
and brine, dried over Na2SO4, filtered and concentrated in vacuo. The residue
was purified by
chromatography on silica gel to afford Compound 74.
[00384] LC-MS: MW (calcd): 597; MW (obsd): 598 (M+1)
1.2.7.21. General method F13
0 0
A
0 >si.L- 0H HO s
[00385] To a solution of the the corresponding ester (1 eq.) in Et0H or a
mixture of THF/water is
added an excess of a solution of NaOH 1 N or LiOH (5 eq.). The reaction
mixture is stirred at r.t.
overnight, then concentrated in vacuo and the residue is dissolved in a
mixture of DCM and water. The
aqueous layer is extracted with DCM twice, the combined organic layers are
washed with a saturated
NaHCO3 solution, and brine, dried over Na7SO4, filtered and concentrated in
vacuo. The residue is
purified by chromatography on silica gel to afford the expected compound.
106
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
1.2.7.22. Illustrative synthesis of Compound 94: 3-1-4-(2-Ethyl-3-11-4-(4-
fluoro-phenyl)-thiazol-2-yll -
methyl-amino}-imidazo[1, 2-d pyridin-6-A-piperidine-1-sulfony11-propan-1-ol
0
A ,0
0 \ HO
0 0
[00386] To a solution of the the compound 93 (1.14 g, 1.901 mmol, 1 eq.) in
Et0H (15 mL) was added
an excess of a solution of NaOH 1 N (10 mL). The reaction mixture was stirred
at r.t. overnight, then
concentrated in vacuo and the residue was dissolved in a mixture of DCM and
water. The aqueous layer
was extracted with DCM twice, the combined organic layers were washed with a
saturated NaHCO3
solution, and brine, dried over Na2SO4, filtered and concentrated in vacuo.
The residue was purified by
chromatography on silica gel to afford Compound 94.
[00387] LC-MS: MW (calcd): 557; m/z MW (obsd): 558 (M+1)
1.2.7.23. General method F14
Rib Rib
Rla Ria
R2
R2
>('
><N.
Rz OH
RN R6b
R6a
R6a
[00388] To a solution of thiazole derivative (1 eq.) in THF are added
formaldehyde (48 eq.), TEA (5.9
eq.) and water. The reaction mixture is heated to 140 C under microwave
irradiation for 2.5 h. The
crude product mixture is quenched with water and a NH3 aqueous solution. The
aqueous layer is
extracted with Et0Ac. The combined organic layers are washed with water and
brine, dried over Na2SO4,
filtered and concentrated in vacuo. The residue is purified by chromatography
on silica gel to deliver the
expected compound.
1.2.7.24. Illustrative synthesis of compound 147: (2-((2-ethyl-6-(1-
(methylsulfonyOpiperidin-4-
yl)irnidazo[1,2-a]pyridin-3-yl)(methyl)amino)-4-(4-fluorophenyOthiazol-5-
yOmethanol
0
OH
107
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00389] To a solution of compound 80 (500 mg, 0.97 mmol, 1 eq.) in THF (3.5
mL) were added
formaldehyde (37% in water, 3.5 mL, 47 mmol, 48 eq.), TEA (800 [IL, 5.75 mmol,
5.9 eq.) and water
(3.5 mL). The reaction mixture was heated to 140 C under microwave irradiation
for 2.5 h. The crude
mixture was quenched with water and a NH3 aqueous solution. The aqueous layer
was extracted with
Et0Ac. The combined organic layers were washed with water and brine, dried
over Na2SO4, filtered and
concentrated in vacuo. The residue was purified by chromatography on silica
gel (elution with
DCM/MeOH: 100/0 to 97/3) to afford Compound 147.
[00390] LC-MS: MW (calcd): 543; m/z MW (obsd): 544 (M+1)
1.2.7.25. General method F15
Rib
Rib
R2 / R2 /
R1 a
Rla
N s =
'NJ CF3
S Rz
R6b N 0
R6a R6a
[00391] To a solution of thiazolc derivative (1 eq.) in pyridine at 0 C is
slowly added trifluoroacetic
anhydride (6 eq.). The reaction mixture is stirred at 0 C for 1 h then
partitioned between DCM and water.
The organic phase is separated. The aqueous layer is extracted with DCM. The
combined organic layers
are washed with water and brine, dried over MgSO4, filtered and concentrated
in vacuo. The residue is
purified by chromatography on silica gel to afford the expected compound.
1.2.7.26. Illustrative synthesis of compound 160: 1-(242-ethyl-6-(1-
(methylsulfonyl)piperidin-4-
yl)imidazo[1,2-a]pyridin-3-yl)(methyl)amino)-4-(4-fluorophenyl)thiazol-5-y1)-
2,2,2-
trifluoroethanone
0
N
S. 'S. F
N / N \N"¨s
0
[00392] To a solution of compound 80 (80 mg, 0.16 mmol, 1 eq.) in pyridine (5
mL) at 0 C was slowly
added tfifluoroacetic anhydride (150 j.tL, 0.93 mmol, 6 eq.). The reaction
mixture was stirred at 0 C for 1
h then partitioned between DCM and water. The organic phase was separated. The
aqueous layer was
extracted with DCM. The combined organic layers were washed with water and
brine, dried over MgSO4,
filtered and concentrated in vacuo. The residue was purified by chromatography
on silica gel (elution
with DCM/MeOH: 100/0 to 95/5) to afford Compound 160.
[00393] LC-MS: MW (calcd): 609; m/z MW (obsd): 610 (M+1)
108
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
1.2.7.27. General methods F16
Rib lb
R2 / R2 /
\ R,42 Ria
N ¨4\s halo
y
R6b
R6a
R6a
1.2.7.27.1. General method F16a
[00394] To a solution of thiazole derivative (1 eq.) in DCM is added polymer-
supported bromide (1.1
eq.). The mixture is stirred vigorously at r.t. for 4 h. The crude mixture is
filtered, the residue is washed
with DCM and Me0H. The filtrate is concentrated in vacuo, then diluted with
DCM, washed with a
saturated NaHCO3 solution, water and brine, dried over Na2SO4 and concentrated
in vacuo to afford the
expected compound.
1.2.7.27.2. Illustrative synthesis of 2-[4-(3-{[5-Bromo-4-(4-fluoro-pheny1)-
thiazol-2-ylrmethyl-
amino}-2-ethyl-imidazo[1,2-a]pyridin-6-y1)-piperazin-1-y11-1-(3-hydroxyrnethyl-
cyclobutyl)-ethanone
OH OH
t-N,
N
I-, ,N
s Br
0 0
'IC
-N
[00395] To a solution of compound 34 (200 mg, 0.36 mmol, 1 eq.) in DCM (7.5
mL), was added
polymer-supported bromide (1.2-1.8 mmol/g, 244 mg, 0.39 mmol, 1.1 eq.). The
mixture was stirred
vigorously at r.t. for 4 h. The crude mixture was filtered; the residue was
washed with DCM and McOH.
The filtrate was concentrated in vacuo, then diluted with DCM, washed with a
saturated NaHCO3
solution, water and brine, dried over Na2SO4 and concentrated in vacua to
afford the expected compound.
[00396] LC-MS: MW (calcd): 641 (79Br), 643 ("Br); m/z MW (obsd): 642(79Br
M+1), 644(81Br M+1)
1.2.7.27.3. General method Fl6b
[00397] To a solution of thiazole derivative (1 eq.) in MeCN is added
selectfluor (1.2 eq.) portionwise.
The mixture is stirred at r.t. for 20 h to 2 days. The crude mixture is
concentrated in vacua, the residue is
dissolved in mixture of Et0Ac and water. The aqueous layer is extracted with
Et0Ac twice, the
combined organic layers are washed with water, then brine, dried over Na2SO4,
filtered and concentrated
in vacua. The residue is purified by chromatography on silica gel to afford
the expected compound.
109
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
1.2.7.27.4. Illustrative synthesis of compound 95: 3-[4-(2-Ethy1-34[5-
fluoro-4-(4-fluoro-pheny1)-
thiazol-2-yll-rnethyl-amino}-imidazo[1,2-akyridin-6-y1)-piperidine-l-sulfonyll-
propan-
l-ol
,F
0, p Q p
S S F
N7 \
[00398] To a solution of compound 94 (90 mg, 0.161 mmol, 1 eq.) in McCN (5 mL)
was added
selectfluor (69 mg, 0.194 mmol, 1.2 eq.) portionwise. The mixture was stirred
at r.t. for 2 days. The crude
mixture is concentrated in vacuo, the residue was dissolved in mixture of
Et0Ac and water. The aqueous
layer was extracted with Et0Ac twice, the combined organic layers werewashed
with water, and brine,
dried over Na2SO4, filtered and concentrated in vacuo. The residue was
purified by chromatography on
silica gel to afford Compound 95.
[00399] LC-MS: MW (calcd): 575; MW (obsd): 576 (M+1)
1.2.7.28. General method F17
Rib Rib
Ria Rie
R2 R2
s Br s CN
Rz
R6a R6a
[00400] To a solution of the above prepared bromide (1 eq.) in pyridine is
added copper cyanide (5
eq.). The mixture is heated to 160 C under microwave irradiation for 2 h. The
crude mixture is quenched
with water and a NH3 aqueous solution, and diluted in Et0Ac. The organic layer
is separated, the aqueous
layer is extracted with Et0Ac. The combined organic layers are washed with
water and brine, dried over
Na2SO4 and concentrated in vacuo. The residue is purified by chromatography on
silica gel or
preparative LC-MS to afford the expected compound.
1.2.7.29. Illustrative synthesis of compound 139: 2-((2-ethyl-6-(4-(2-(3-
(hydroxymethyl)azetidin-1 -y1)-
2-oxoethyl)piperazin-l-y0imidazo[1,2-alpyridin-3-y0(inethyl)ainino)-4-(4-
fluorophenyl)thiazole-5-carbonitrile
OH OH
\ NP
0 N S Br ______________ 0 L. N S CN
N
110
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
[00401] The bromo derivative (128 mg, 0.20 mmol, 1 eq.), obtained by
bromination of compound 34
by the general method F16a, was dissolved in pyridine (3 mL), then copper
cyanide (89 mg, 1 mmol, 5
eq.) was added. The mixture was heated to 160 C under microwave irradiation
for 2 h. The crude
mixture was quenched with water and a NH3 aqueous solution, and diluted in
Et0Ac. The organic layer
was separated; the aqueous layer was extracted with Et0Ac. The combined
organic layers were washed
with water and brine, dried over Na2SO4 and concentrated in vacua. The residue
was purified by
preparative LC-MS to afford Compound 139.
[00402] LC-MS: MW (calcd): 588; mh MW (obsd): 589 (M+1)
1.2.7.30. General method F18
Rib Rib
R1 a
2 Ria
R2
4 N 4 N
R \ R \
0
S S
\ ie R. OH
R6bN
R" R"
[00403] To a solution of ester derivative (1 eq.) in anhydrous THE at 0 C is
added LiBH4 (2 M in
THF, 5 eq.). The reaction mixture is allowed to warm to r.t. then stirred
overnight at 80 C. Solid sodium
sulfate hydrate is added and the mixture is kept stirring for 10 min. The
reaction mixture is then filtered
and the solid is rinsed with THF. The filtrate is concentrated to give the
hydroxymethyl derivative that
can be used as such in the next step or purified by chromatography.
1.2.7.31. Illustrative synthesis of 4-(2-Ethyl-34[4-(4-fluoro-pheny0-5-
hydroxyrnethyl-thiazol-2-yli-
tnethyl-amino}-inndazo[1,2-alpyridin-6-yl)-piperazine-1-carboxylic acid tert-
butyl ester
0 0
N OAN
OH
[00404] To a solution of Intermediate Gen-10-ag (723 mg, 1.22 mmol, 1 eq.) in
anhydrous THF (12
mL) at 0 C was added LiBH4 (2 M in THF, 3 mL, 6.1 mmol, 5 eq.). The reaction
mixture was allowed to
warm to r.t. then stirred overnight at 80 C. Solid sodium sulfate hydrate was
added and the mixture was
kept stirring for 10 min. The reaction mixture was then filtered and the solid
rinsed with THF. The filtrate
was concentrated to give the expected compound used as such in the next step.
LC-MS: MW (calcd): 566; m/z (obsd): 567 (M+1)
111
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
1.2.7.32. General Method F19 :General synthesis of oxazoline derivative
0 ' N. _
HO N
_Rz 0 Rz
R
R6a R6a
[00405] 3-Amino-imidazo[1,2-a]pyridine-6-carboxylic acid (2-hydroxy-ethyl)-
amide derivative is
prepared from Intermediate Gen-5 (with RY=CO2R) and 2-ethanolamine derivative
via successive general
synthetic methods F13 and F9a. To the 3-amino-imidazo[1,2-a]pyridine-6-
carboxylic acid (2-hydroxy-
ethyl)-amidederivative prepared from Intermediate Gen-5-aa with the desired
amine via successive
general synthetic methods F13 and F9a (1 eq.) in anhydrous DCM arc added
triphenylphosphine (1.5 eq.)
and DDQ (1.5 eq.). The reaction mixture is stirred at r.t. for 0.5 h then the
solvent is evaporated. The
crude product is purified by chromatography to give the expected compound.
1.2.7.33. Illustrative synthesis of compound 240 : [6-(4,5-Dihydro-oxazol-2-
yl)-2-ethyl-imidazo[1,2-
a]pyridin-3-yli-14-(4-fluoro-phenyl)-thiazol-2-ylrmethyl-amine
AO.
N N
\ \ \
0
"--1\1
[00406] To a solution of
3 4(4-(4-chlorophenyl)thiazol-2-y1) (me thyl)amino)-2- ethyl-N-(2-
hydroxyethypimidazo[1,2-a]pyridine-6-carboxamide (40 mg, 0.09 mmol, 1 eq.),
prepared from
Intermediate Gen-5-aa and ethanolamine via successive general synthetic
methods F13 and F9a) in DCM
(1.5 mL), were added triphenylphosphine (35 mg, 0.13 mmol, 1.5 eq.) and DDQ
(30 mg, 0.13 mmol, 1.5
eq.). The reaction mixture was stirred at room temperature for 0.5 h and then
concentrated. The crude
product was purified by chromatography on silica gel (eluent DCM/Me0H 100/0 to
97/3) to give
Compound 240.
[00407] LC-MS: MW (calcd): 437; m/z (obsd): 438(M+1)
1.2.7.34. General Method El
Rib
R2 /
R1a
N H N H 2 N¨\\
S R3
i) N ii) ====,'
Rz
Rz
R6b--kyl" N R6bN R6bN
R6a
R6a R6a
112
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00408] Intermediates Gen-10 are prepared from intermediates Gen-9 according
to general method El
described previously.
1.2.7.35. General method E4
Rib
R2 /
R1 a
' NH N N X -W
Rz
R6N R6ID= N
R6a R6a
[00409] To a
previously degassed solution of halogeno lieteroaryl derivative (1 eq.), the
imidazo[1,2-
a]pyridine-3-ylamine derivative (1.2 eq.), cesium carbonate (3 eq.), and
Xantphos (0.15 eq.) in dioxane
under argon is added Pd(OAc)2 (0.2 eq.). The reaction mixture is heated to
reflux until completion. After
cooling to r.t., the reaction mixture is partitioned between water and Et0Ac
and the layers separated. The
aqueous layer is extracted with Et0Ac. The combined organic layers are washed
with water and brine,
dried Na2SO4, filtered and concentrated in vacuo. The crude product is
purified by chromatography on
silica gel to afford the expected intermediate.
1.2.7.36.
Illustrative synthesis of 4-(2-Ethyl-34[3-(4-flitoro-phenAl 1 ,2,4Jthiadiazol-
5-ylrinethyl-
amino}-intidazo[1,2-a]pyridin-6-y1)-3,6-dihydro-2H-pyridine-1-carboxylic acid
tert-butyl
ester
,F
\/\ 0
_
_)1
0 N I N
S-
I¨ ______________________ \
[00410] To a
previously degassed solution of 5-chloro-3-(4-fluoropheny1)-1,2,4-thiadiazole
(800 mg,
3.73 mmol, 1 eq.), amine Gen-9-c (1.57 g, 4.41 mmol, 1.2 eq.), cesium
carbonate (3.64 g, 11.2 mmol, 3
eq.), and Xantphos (323 mg, 0.56 mmol, 0.15 eq.) in dioxane (20 mL) under
argon was added Pd(0A02
(167 mg, 0.74 mmol, 0.2 eq.). The reaction mixture was heated to reflux for 3
h. After cooling to r.t., the
reaction mixture was partitioned between water and Et0Ac and the layers
separated. The aqueous layer
was extracted twice with Et0Ac. The combined organic layers were washed with
water and brine, dried
Na7SO4, filtered and concentrated in vacuo. The crude product was purified by
chromatography on silica
gel (elution with heptane/Et0Ac: 80/20 to 40/60) to afford the expected
compound.
[00411] LC-MS: MW (calcd): 534; m/z MW (obsd): 535 (M+1)
113
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
1.2.7.37. General method E5
R1b
Rla
Br
R2
N--7(
)(N. \N ' NH ' N S-N
NK,ii)
Rz
Rz
i)
R"
R6a R6a R6a
Step i
[00412] A solution of imidazo[1,2-a]pyridine-3-ylamine derivative (1 eq.) and
3-Bromo-5-chloro-
1,2,4-thiadiazole (3.3 eq.) in McCN (2.5 mL) under argon in a sealed tube is
heated at 90 C overnight.
After cooling to r.t. the mixture is concentrated in vacuo. The residue is
purified by chromatography on
silica gel.
Step ii
[00413] To a solution of the above prepared bromo derivative (1 eq.) in a
mixture dioxane/water under
argon are added cesium fluoride (2.1 eq.), the corresponding aryl boronic acid
derivative (1.2 eq.), and
then PdC12(P -tBu2(p-NMe2Ph))2 (0.1 eq.). The reaction mixture is heated at 80
C for 48 h. After cooling
to r.t., the reaction mixture is partitioned between water and Et0Ac and the
layers separated. The
aqueous layer is extracted twice with Et0Ac. The combined organic layers are
washed with water and
brine, dried Na2SO4, filtered and concentrated in vacuo. The crude product is
purified by chromatography
on silica gel to afford the expected intermediate.
1.2.7.38. Illustrative synthesis of 4-(3-0-(2-Cyano-4-fluoro-phenyl)-
[1,2,4]thiadiazol-5-yll -methyl-
arninol-2-ethyl-imidazo[1,2-a]pyridin-6-y1)-piperidine-l-carboxylic acid tert-
butyl ester
4. JCL J,
N :0 \ Br 0 P- _/\,N
S ii) 0 N
NH 0 , \s-N CN
N ___________________________________________________________
Step i
[00414] A solution of Intermediate Gen-9-d (500 mg, 1.40 mmol, 1 eq.) and 3-
Bromo-5-chloro-1,2,4-
thiadiazole (917 mg, 4.61 mmol, 3.3 eq.) in MeCN (2.5 mL) under argon in a
sealed tube was heated at
90 C overnight. After cooling to r.t. the mixture was concentrated in vacuo.
The residue was purified by
chromatography on silica gel (elution with DCM/MeOH: 100/0 to 96/4) to afford
the expected compound.
[00415] LC-MS: MW (calcd): 520 (79Br), 522 (81Br); m/z MW (obsd): 521 (79Br
M+1), 523 (81Br
M+1)
114
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step ii
[00416] To a solution of bromide obtained previously in step i) (120 mg, 0.23
mmol, 1 eq.) in a
mixture dioxane/water (1.1 / 0.68 mL) under argon were added cesium fluoride
(73 mg, 0.48 mmol, 2.1
eq.), 2-Cyano-4-fluorobenzeneboronic acid, pinacol ester (69 mg, 0.28 mmol,
1.2 eq.), and then Pd(P-
tBu2(p-NMe2Ph))2 (16 mg, 0.022 mmol, 0.1 eq.). The reaction mixture was heated
at 80 C for 48 h.
After cooling to r.t., the reaction mixture was partitioned between water and
Et0Ac and the layers
separated. The aqueous layer was extracted twice with Et0Ac. The combined
organic layers were
washed with water and brine, dried over Na2SO4, filtered and concentrated in
vacuo. The crude product
was purified by chromatography on silica gel (elution with DCM/MeOH: 100/0 to
96/4) to afford the
expected compound.
[00417] LC-MS: MW (calcd): 561; m/z MW (obsd): 562 (M+1)
1.2.8. General method D1. Synthesis of Intermediate Gen-6
0
NH2
H N R N
RYNH _______________ Rz
Rz R6b- N
Rea
Rea
Gen-2 Gen-6
[00418] Intermediates Gen-6 are prepared from intermediates Gen-2 according to
general method D1
described previously.
1.2.9. General methods El or H1 :
Synthesis of Intermediate Gen-7
Ria Rlb
NH 2 N
R2
N -W
R6b N
Rz RY N_-( X
R7
R62 õ,-)) =
R6b N
R6a
Gen-6 Gen-7
1.2.9.1. General method El
[00419] Intermediates Gen-7 are prepared from intermediates Gen-6 according to
general method El
described previously
115
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
1.2.9.2. General method HI
R1 b
R1a
NH2 N
ii) H N R2
RZ R6b N
RN N N
R6a
R6a N
R62
Gen-6 Gen-7
Step i
[00420] To a solution of the corresponding intermediate (1 eq.) in DCM at 0 C
are added calcium
carbonate (3 eq.) and a few min later thiophosgene (1.2 eq.). The reaction
mixture is stirred at 0 C for 3.5
h then quenched with water. The layers are separated. The aqueous phase is
extracted with DCM. The
combined organic layers are washed with water and brine, dried over Na2SO4 and
concentrated in vacuo
to give the expected isothiocyanate.
Step ii
[00421] To a solution of the above prepared isothiocyanate (1 eq.) in DMF are
added DIPEA (, 1.1 eq.)
and then the corresponding benzamidine hydrochloride (1 eq.). The reaction
mixture is stirred at r.t.
overnight. DIAD (1.1 eq.) is added and the resulting mixture is heated at 80 C
for 45 min. Stirring is
continued at r.t. for 3 h then water and Et0Ac were added. The layers are
separated. The aqueous phase is
extracted with Et0Ac. The combined organic layers are washed with water and
brine, dried over Na2SO4
and concentrated in vacuo. The residue is purified by chromatography on silica
gel.and give Intermediate
Gen-7
1.2.9.3. .. Illustrative synthesis of Intermediate Gen-7-a: (6-Bromo-2-ethyl-
imidazo[1,2-d]pyridin-3-
y1)43-(4-chloro-phenylH1 ,2,41thiadiazol-5-yll -amine
CI
411
HN---1 NH2
Br N
Step i
[00422] To a suspension of hydrochloride salt of Intermediate Gen-6-a_(530
mg,1.92 mmol, 1 eq.) in
DCM (7 mL) at 0 C were added calcium carbonate (799 mg, 5.75 mmol, 3 eq.) and
a few min later
thiophosgene (176 uL, 2.30 mmol, 1.2 eq.). The reaction mixture was stirred at
0 C for 3.5 h then
quenched with water. The layers were separated. The aqueous phase was
extracted with DCM. The
116
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
combined organic layers were washed with water and brine, dried over Na2SO4
and concentrated in vacuo
to afford 6-Bromo-2-ethyl-3-isothiocyanato-imidazo[1,2-a]pyridine
isothiocyanate.
[00423] LC-MS: MW (calcd): 281 (79Br), 283 (81Br); MW (obsd): 282 (79Br
M+1), 284 (81Br
M+1)
Step ii
[00424] To a solution of the above prepared isothiocyanate (305 mg,1.08 mmol,
1 eq.) in DMF (6 mL)
were added DIPEA (207 j.tL, 1.19 mmol, 1.1 eq.) and then 4-chloro-benzamidine
hydrochloride (207 mg,
1.08 mmol, 1 eq.). The reaction mixture was stirred at r.t. overnight. DIAD
(236 IA., 1.19 mmol, 1.1 eq.)
was added and the resulting mixture was heated at 80 C for 45 min. Stirring
was continued at r.t. for 3 h
then water and Et0Ac were added. The layers were separated. The aqueous phase
was extracted with
Et0Ac. The combined organic layers were washed with water and brine, dried
over Na2SO4 and
concentrated in vacuo. The residue was purified by chromatography on silica
gel (elution with
heptane/Et0Ac: 100/0 to 50/50 then DCM/Me0H 98/2) to afford Intermediate Gen-7-
a.
[00425] LC-MS: MW (calcd): 433 (79Br), 435 (81Br); m/z MW (obsd): 434 (79Br),
436 (81Br M+1)
1.2.10. General methods F: Synthesis of Intermediate Gen-8
(R7). R40
0 \
\ N
R8
H N
\ ¨Rz
R6b
R6a
R6a
Gen-3 Gen-8
[00426] Intermediates Gen-8 are prepared from Intermediates Gen-3 according to
one or several
general methods F described previously
1.2.11. General methods D and F : Synthesis of Intermediate Gen-9
4 r-,4 (R7). R (R7)0
\ R4 0\ II
NH NH
Rs
____________________________________________________ R8
______________________ 3P- 1\142\ z
Rz
R6b
R6b
R6b
R6a
R6a
R6a
Gen-4 Gen-9 Gen-8
1.2.11.1. General method D and F
[00427] intermediates Gen-9 are prepared from Intermediates Gen-8 according to
general method D1
and one or several general methods F described previously.
117
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
1.2.11.2. General method F
[00428] Intermediates Gen-9 are prepared from Intermediate Gen-4 according to
one or several general
methods F described previously
1.2.12. General method G1: General Synthesis of a¨halogenoketones Gen-11
o la lb R12
R Br
R11,c/v.,,rx
R3 R3
R2 0 R2 0
Gen-1 1
[00429] To a solution of ketone (1 eq.) in MeCN is added
phenyltrimethylammonium tribromide (1
eq.). The resulting mixture is stirred at r.t. for 3 h, and then is
concentrated in vacuo. The organic residue
is dissolved in Et0Ac and the organic layer is washed with water, with brine,
dried over Na2SO4, filtered
and concentrated in vacuo to give the Intermediate Gen-11. The crude product
is used directly for the
next step without purification
1.2.13. Illustrative synthesis of Intermediate Gen-11-a : 2-(2-Bromo-acetyl)-5-
fluoro-benzonitrile
Br
CN 0
Br 0 CN 0
Step i
[00430] To a solution of 1-(2-Bromo-4-fluoro-phenyl)-ethanone (3.0 g, 13.82
mmol, 1 eq.) in DMA
(150 mL) under argon was added Zn(CN)2 (1.6 g, 13.82 mmol, 1 eq.), Pd2(dba)3
(1.26 g, 1.38 mmol, 0.1
eq.), dppf (1.53 g, 2.76 mmol, 0.2 eq.) and Zn dust (107.8 mg, 1.65 mmol, 0.12
eq.). The reaction
mixture was heated at 100 C for 1.4 h, after cooling to r.t. the mixture was
slowly quenched by addition
of water and then diluted with Et0Ac. The organic layer was separated and the
aqueous layer extracted
with Et0Ac twice. The combined organic layers were washed with water, dried
over Na2SO4, filtered and
concentrated in vacuo. The residue was purified either by chromatography on
silica gel to deliver
Intermediate 2-acetyl-5-fluoro-benzonitrile
[00431] LC-MS: MW (calcd): 163; m/z MW (obsd): 164 (M+1)
Step ii
[00432] To a solution of 2-acetyl-5-fluoro-benzonitrile (1.52 g, 9.33 mmol, 1
eq.) in MeCN (40 mL)
was added phenyltrimethylammonium tribromide (3.51 g, 1 eq.). The resulting
mixture was stirred at r.t.
for 3 h, and then is concentrated in vacuo. The organic residue was dissolved
in Et0Ac and the organic
layer was washed with water, with brine, dried over Na2SO4, filtered and
concentrated in vacuo to give
the Intermediate Gen-11-a. The crude was used directly for the next step
without purification
118
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00433] LC-MS: MW (calcd): 241 (79Br), 243 (81Br); in/z MW (obsd): 242 (79Br
M+1), 244 ('Br
M+1)
1.2.14. General method G2: General Synthesis of ehlorothiazoles Gen-12:
Rib R1a
R1a Rla Rlb
X \
R2
0
R2 Cl¨
Rib
CN H2N R2
S CN s
Gen-12
Step i
[00434] To a solution of benzoylacetonitrile derivative (1 eq.) in Et0H is
added pyridine (1 eq.). The
resulting mixture is stirred at 70 C for 15 min then cooled at r.t. A
previously stirred suspension of
thiourea (2 eq.) and iodine (1 eq.) in Et0H is then slowly added. After 1 h at
r.t. a cold 1 M Na2S203
solution is added under stirring. The resulting precipitate is filtered,
washed with water, and finally dried
under vacuo to afford the amino-4-phenyl-thiazole-5-carbonitrile derivative.
Step ii
[00435] To a solution of copper (II) chloride (1.2 eq.) in MeCN is added
dropwise tert-butyl nitrite (1.5
eq.). After stirring at r.t. for 30 min, the amino-4-phenyl-thiazole-5-
carbonitrile (1 eq.) is introduced
portionwise and stirring is continued for 1 h. The reaction mixture is then
carefully quenched by addition
of a 1 N HC1 solution. After 15 min stirring, the organic phase is separated;
the aqueous phase is further
extracted with Et0Ac. The combined organic layers are washed with brine, dried
over Na2SO4, filtered
and concentrated in vacuo. The crude product product is filtered on a silica
plug and eluted with DCM.
Solvents arc evaporated and the residue is finally triturated in heptanc,
filtered and dried to give
Intermediate Gen-12.
1.2.15. Illustrative synthesis of Intermediate Gen-12-a 2-Chloro-4-(4-fluoro-
phenyl)-thiazole-5-
carbonitrile:
0
H2N CI
CN S CN S CN
Step i
[00436] To a solution of 4-fluorobenzoylacetonitrile (50 g, 306 mmol, 1 eq.)
in Et0H (600 mL) was
added pyridine (24.7 mL, 306 mmol, 1 eq.). The resulting mixture was stirred
at 70 C for 15 min then
cooled to r.t. A previously stirred suspension of thiourea (46.7 g, 613 mmol,
2 eq.) and iodine (77.8 g, 306
119
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
mmol, 1 eq.) in Et0H (300 mL) was then slowly added. After 1 h at r.t. a cold
1 M Na2S203 solution
(360 mL) was added under stirring. The resulting precipitate was filtered,
washed with water, and finally
dried under vacuo to afford 2-Amino-4-(4-fluoro-pheny1)-thiazole-5-
carbonitrile.
[00437] tH NMR 6 (ppm) (400 MHz, DMS0): 8.26 (2 H, s), 7.97 (2 H, dd), 7.36 (2
H, t)
Step ii
[00438] To a solution of copper (TT) chloride (36.8 g, 273 mmol, 1.2 eq.) in
MeCN (500 mL) was
added dropwise tert-butyl nitrite (40.7 mL, 342 mmol, 1.5 eq.). After stirring
at r.t. for 30 min, amine
previously obtained in step i (50 g, 228 mmol, 1 eq.) was introduced
portionwise and stirring was
continued for 1 h. The reaction mixture was then carefully quenched by
addition of a 1 N HC1 solution
(750 mL). After 15 min stirring, the organic phase was separated; the aqueous
phase was further extracted
with Et0Ac. The combined organic layers were washed with brine, dried over
Na2SO4, filtered and
concentrated in vacuo. The crude product product was filtered on a silica plug
(250 g) and eluted with
DCM. Solvents were evaporated and the residue was finally triturated in
heptane, filtered and dried to
afford 2-Chlo ro-4- (4-flu o ro-phenyl)-tbiazo I e-5-carb o nitril e
(Intermediate Gen- 12-a).
[00439] tH NMR 6 (ppm) (400 MHz, DMS0): 8.06 (2 H, dd), 7.46 (2 H, dd)
1.2.16. General method G3: General Synthesis of intermediaire Gen-13
0
0
)1)
N ss Cl")
-1-
H CI
CI
1.2.16.1. General method G3a
[00440] To a suspension of potassium carbonate (2.2 eq.) in water is added the
amine derivative (1
eq.). The reaction mixture is stirred at r.t. until complete dissolution, then
diluted with DCM and cooled
to 0 C prior to the dropwise introduction of chloroacetyl chloride (1.2 eq.)
over 30 min. After 2 h stirring
at r.t., the reaction mixture is filtered, the organic layer and the aqueous
phase are separated, and the
aqueous phase is extracted either with DCM or with a mixture of Et0Ac/nBu0H
1:1. The combined
organic layers are dried over Na9SO4, filtered and concentrated in vacuo. The
residue is suspended in
acetone and stirred vigorously for 20 mm, filtered and the filtrate was
concentrated in vacuo to afford
Intermediate Gen-13
1.2.16.2. Illustrative synthesis of Intermediate Gen-13-a : 2-Chloro-1-(3-
hydroxy-azetidin- 1 -y1)-
ethanone:
0 HO HO
CI )1) _____________________________________
.C\I\JH CI
HCI 'CI
120
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
[00441] To a suspension of potassium carbonate (13.9 g, 100 mmol, 2.2 eq.) in
water (33.5 mL) was
added hydroxyazetidine hydrochloride (5 g, 45.6 mmol, 1 eq.). The reaction
mixture was stirred at r.t.
until complete dissolution, then diluted with 33.5 mL of DCM and cooled to 0 C
prior to the dropwise
introduction of chloroacetyl chloride (4.4 mL, 54.8 mmol, 1.2 eq.) over 30 min
After 2 h stirring at r.t.,
the reaction mixture was filtered, the organic layer was separated, and the
aqueous phase was extracted
with a mixture Et0Ac/nBuOH 1:1 (6 x 16 mL). The combined organic layers were
dried over Na2SO4,
filtered and concentrated in vacuo. The residue was suspended in acetone (48
mL) and stirred vigorously
for 20 min, filtered and the filtrate was concentrated in vacuo to afford
Intermediate Gen-13-a.
[00442] '1-1 NMR 6 (ppm) (400 MHz, CDC13): 4.77-4.68 (1 H, m), 4.50 (1 H,
dd), 4.50 (1 H, dd), 4.32
(1 H, dd), 4.16 (1 H, dd), 3.89 (2 H, s), 2.55 (1 H, d).
1.2.17. General method G3b
[00443] To a solution of chloroacetyl chloride (1 eq.) and TEA (1.5 eq.) in
DCM at 0 C is added the
amine derivative (1.1 eq.). The reaction mixture is stirred overnight at r.t.,
then concentrated in vacuo.
The residue is suspended in acetone and stirred vigorously for 20 min,
filtered and the filtrate is
concentrated in vacuo to afford Intermediate Gen-13 which is used directly
without further purification.
1.2.17.1.
Illustrative synthesis of Intermediate Gen-13-o : 2-Chloro-N-methoxy-N-
inethylacetamide
0
-NH CI)) __________
0
0 CI
CI
[00444] To a solution of chloroacetyl chloride (0.195 mL, 1.21 mmol, 1 eq.)
and TEA (0.253 mL, 1.81
mmol, 1.5 eq.) in 3 mL of DCM at 0 C was added the N,0-dimethylhydroxylamine
(0.081 g, 1.33 mmol,
1.1 eq.). The reaction mixture was stirred overnight at r.t., then
concentrated in vacuo. The residue was
suspended in acetone and stirred vigorously for 20 min, filtered and the
filtrate was concentrated in vacuo
to afford Intermediate Gen-13-o which was used directly without further
purification.
[00445] LC-MS: MW (calcd): 137 (35C1) 139 (37C1); m/z MW (obsd): 138 (35C1
M+1), 140 (37C1 M+1)
Example 2. Preparation of the compounds of the invention.
2.1. Compound 1: 242-ethyl-8-methyl-6-(piperazin-l-yl)imidazo[1,2-a]pyridin-3-
yl)(methyl)amino)-4-
(4-fluorophenyl)thiazole-5-carbonitrile
HN
N
S CN
N
1\1-
-1\1
121
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step i: 6-Bromo-2-ethyl-8-methyl-imidazo[1,2-c]pyridin-3-ylamine
NH2
Br
N
NH2
[00446] To a suspension of 2-amino-5-bromo-3-methylpyridine (420 g, 2.24 mol,
1 eq.) previously
washed before use with a saturated NaHCO3 solution in 1.5 L of toluene under
nitrogen were added
propionaldehyde (248 mL, 3.36 mol, 1.5 eq.) and 1H-benzotriazole (281 g, 2.36
mol, 1.05 eq.). The
resulting mixture was stirred 4 11 at r.t. before adding 3.5 L of Et0H and
potassium cyanide (175 g, 2.70
mol, 1.2 eq.). The reaction mixture was further stirred overnight at r.t. and
2 h at 78 C. After cooling to
r.t., the mixture was quenched by addition of a 2.5 M NaOH solution (3 L).
[00447] This experiment was performed in four batches with the same quantities
of reagents, the crude
mixture were then pooled together and concentrated in vacuo to low volume. The
remaining oil was
diluted with Et0Ac (15 L) and washed with a 2 M NaOH solution (2 x 2 L). The
aqueous layer was
extracted twice with Et0Ac (2 x 1 L). The combined organic layers were then
dried over Na2SO4, filtered
and concentrated in vacuo. The crude mixture was dissolved in Et0H (2 L) and
carefully added to a
solution of acetyl chloride (1 L, 14.0 mol, 1.6 eq.) in Et0H (6 L). The
resulting reaction mixture was
stirred at r.t. overnight and then concentrated to dryness. The residue was
triturated in DCM (7 L) for 3
days, the precipitate formed was collected, washed with DCM (2 x 500 mL) and
dried to afford 6-bromo-
2-ethy1-8-methylimidazo[1,2-a]pyridin-3-amine as a hydrochloride salt.
[00448] 1F1
NMR 6 (ppm) (400 MHz, DMS0): 8.70 (1 H, s), 7.75 (1 H, s), 4.86 (3 H, bs),
2.81 (2 H,
q), 2.56 (3 H, s), 1.56 (3 H, t).
[00449] LC-MS: MW (calcd): 253 (79Br) and 255 (81Br);
(obsd): 254 (79Br M+1) and 256 (81Br
M+1)
Step ii: N-(6-Brorno-2-ethyl-8-inethyl-imidazo[1,2-alpyridin-3-y1)-fonnarnide
(Gen-2-d)
0
NH2 H
1\1Brc".
[00450] A suspension of the latter compound (785 g, 2.70 mol, 1 eq.) in formic
acid (713 mL, 18.9
mol, 7 eq.) was heated to 80 C for 2 h. The crude mixture was concentrated in
vacuo to low volume
(about 400 mL). The residue was brought up in water (1 L) and a 3 M solution
of NaOH (2 L), and
further basified with a saturated NaHCO3 solution until foaming ceased and pH
reached 8-9. After
homogenization for 1 h, the precipitate was filtered and washed with water (2
x 300 mL). Purification
was achieved by dissolution in a mixture of toluene and McOH 3:1 (4 L)
followed by concentration in
vacuo. Trituration of the residue in a mixture of 200 mL of Me0H and 5 L of
DIPE, decantation and
122
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
filtration of the resulting suspension afforded Intermediate Gen-2-d N-(6-
bromo-2-ethy1-8-
methylimidazo [1,2-a] pyridin-3 -y1) formamide.
[00451] 1F1 NMR 6 (ppm) (400 MHz, DMS0): presence of 2 rotamers 10.2 (1 H, bs,
one rotamer),
8.51 (1 H, s, one rotamer), 8.36 (1 H, s, one rotamer), 8.23 (1 H, s, one
rotamer), 8.11 (1 H, s, both
rotamers), 7.23 (1 H, s, one rotamer), 7.21 (1 H, s, one rotamer), 2.63-2.60
(2 H, m, both rotamers), 2.58
(3 H, s, one rotamer), 2.56 (3 H, s, one rotamer), 1.24-1.17 (3 H, m, both
rotamers)
[00452] LC-MS: MW (calcd): 281 (79Br) and 283 (8113r); m/z (obsd; miz (obsd):
282 (79Br M+1) and
284 (81Br M+1)
Step iii: N-(6-Brorno-2-ethyl-8-nzethyl-iinidazo[1,2-a]pyridin-3-y1)-N-methyl-
fornzainide (Gen-3-e)
0 0
N
Br
N Br
N
[00453] To a suspension of formamide Gen-2-d (720 g, 2.55 mol, 1 eq.) in 5 L
of acetone were added
potassium carbonate (1 kg, 7.66 mol, 3 eq.) and methyl iodide (700 g, 4.93
mol, 1.9 eq.). The reaction
mixture was heated to 40 C overnight. Additional methyl iodide (25 g, 0.18
mol, 0.07 eq.) was then
introduced and stirring continued for 1 h at 40 C. The reaction mixture was
filtered and washed with
acetone (2 x 300 mL) and DCM (2 x 300 mL). The filtrate was concentrated in
vacuo and the residue was
partitioned between DCM (3 L) and water (1 L). The aqueous layer was further
extracted with DCM. The
combined organic layers were then washed with brine, dried over Na2SO4,
filtered and concentrated in
vacuo. The solid was triturated with Et20 (1 L) at r.t. for 1 h, filtered off
and dried to afford the
Intermediate Gen-3-e.
[00454] 1H NMR ii (ppm) (400 MHz, CDC13), presence of 2 rotamers, major
rotamer: 8.19 (1 H, s),
7.78 (1 H, s), 7.15 (1 H, s), 3.24 (3 H, s), 2.72 (2 H, q), 2.59 (3 H, s),
1.31 (3 H, t)
[00455] Iff NMR (ppm) (400 MHz, CDC13), minor rotamer: 8.49 (1 H, s), 7.65
(1 H, s), 7.08 (1 H, s),
3.36 (3 H, s), 2.72 (2 H, q), 2.59 (3 H, s), 1.31 (3 H, t)
[00456] LC-MS: MW (calcd): 295 (79Br) and 297 (8IBr); miz (obsd): 296 (79Br
M+1) and 298 ('Br
M+1)
Step iv: (6-Bromo-2-ethyl-8-methyl-imidazo[1,2-alpyridin-3-y1)-inethyl-atnine
0
N-1( NH
N
123
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00457] The above prepared formamide Gen-3-e (80 g, 270 mmol, 1 eq.) was
dissolved in a 1.25 M
HCl solution in Me0H (540 mL, 2.5 eq.) and the resulting mixture was heated at
110 C overnight. 270
mL of the 1.25 M HCl solution in Me0H were added and heating continued
overnight. After 48 h,
additional 70 mL of the 1.25 M HC1 solution in Me0H were introduced in the
reaction mixture. Heating
was maintained overnight until conversion was complete. The crude mixture was
then concentrated in
vacuo and the residue was partitioned between Et0Ac (300 mL) and water (700
mL). A saturated
NaHCO3 solution was added until pH reached 8-9. The aqueous layer was
extracted twice with Et0Ac (2
x 300 mL). The combined organic layers were then washed with brine (200 mL),
dried over Na2SO4,
filtered and concentrated in vacuo to give (6-bromo-2-ethy1-8-methyl-
imidazo[1,2-a]pyridin-3-y1)-
methyl-amine Gen-4-d as a free base.
[00458] 1F1 NMR 6 (ppm) (400 MHz, CDC13): 8.05 (1 H, s), 7.04 (1 H, s),
2.84-2.78 (5 H, m), 2.60 (3
H, s), 1.35 (3 H, t)
[00459] LC-MS: MW (calcd): 267 (79Br) and 269 (81Br); m/z (obsd): 268 (79Br
M+1) and 270 (81Br
M+1)
Step v: 24(6-Bromo-2-ethyl-8-methyl-imidazo[1,2-cdpyridin-3-y1)-methyl-amino]-
4-(4-fluoro-phenyl)-
thiazole-5-carbonitrite
CF
NH CI
S CN
Br S CN
[00460] To a solution of amine Gen-4-d (4.4 g, 16.6 mmol, 1 eq.) in THE (44
mL) under argon was
slowly added NaH (60% in oil suspension, 2.0 g, 50.0 mmol, 3 eq.). The
reaction mixture was heated at
90 C for 30 min then cooled to 40 C before adding the chlorothiazolc Gen-12-a
(4.74 g, 19.9 mmol, 1.2
eq.). The reaction mixture was stirred at 90 C overnight. After cooling to
r.t. the mixture was slowly
quenched by addition of water and then diluted with Et0Ac. The organic layer
was separated and the
aqueous layer extracted with Et0Ac. The combined organic layers were then
washed with water and
brine, dried over Na2SO4, filtered and concentrated in vacuo. The residue was
triturated in Et20, filtered
and washed with Et20 and MeCN. Recrystallization was performed in MeCN (180
mL) to afford 2-[(6-
Bromo-2-ethy1-8-methyl-imidazo [1,2-al pyridin-3 -y1)-methyl-amino] -4-(4-fluo
ro-pheny1)-thiazo le-5-
carbonitrile (Intermediate Gen-5-t).
[00461] 1H NMR 6 (ppm) (400 MHz, CDC13): 8.15 (2 H, dd), 7.80 (1 H, s),
7.22-7.14 (3 H, m), 3.62 (3
H, s), 2.77 (2 H, q), 2.64 (3 H, s), 1.35 (3 H, t)
[00462] LC-MS: MW (calcd): 469 (79Br), 471 (81Br); ni/z (obsd): 470 (79Br
M+1), 472 (81Br M+1)
124
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step vi: 4-63-{115-Cyano-4-(4-fluoro-pheny1)-thiazol-2-ylrmethyl-amino}-2-
ethyl-8-rnethyl-iinidazo[1,2-
akyridin-6-y1)-piperazine-1-carboxylic acid tert-butyl ester
S CN L. S CN
N
[00463] To a solution of the above prepared bromide Gen-5-t (24.2 g, 51.5
mmol, 1 eq.) in toluene
(345 mL) under argon were successively added N-Boc piperazine (14.4 g, 77.3
mmol, 1.5 eq.), sodium
tert-butoxide (9.9 g, 103 mmol, 2 eq.) and then JohnPhos (1.54 g, 5.15 mmol,
0.1 eq.) and Pd2(dba)3 (2.36
g, 2.58 mmol, 0.05 eq.). The reaction mixture was heated at 115 C for 1 h.
After cooling to r.t., the crude
product was filtered on Celpure P65 and the residue washed with water and
Et0Ac. The organic layer of
the filtrate was washed with brine, dried over Na2SO4, filtered and
concentrated in vacuo. The crude
product was purified by chromatography on silica gel (elution with
heptane/Et0Ac: 90/10 to 20/80) to
afford the expected compound.
[00464] tH NMR 6 (ppm) (400 MHz, CDC13): 8.16 (2 H, dd), 7.17 (2 H, app t),
6.99 (2 H, bs), 3.62-
3.53 (4 H, m) 3.60 (3 H, s), 3.04-2.93 (4 H, m), 2.74 (2 H, q), 2.62 (3 H, s),
1.47 (9 H, s), 1.33 (3 H, t)
[00465] LC-MS: MW (calcd): 575; m/z (obsd): 576 (M+1)
Step vii: 2-[(2-Ethyl-8-methyl-6-piperazin-l-yl-imidazo[1,2-a]pyridin-3-y1)-
inethyl-ainino]-4-(4-fluoro-
pheny1)-thiazole-5-carbonitrile
S CN S CN
1\1 jõi
LI\J)
[00466] To a solution of 4-(3-{[5-Cyano-4-(4-fluoro-phenyl)-thiazol-2-A-methyl-
aminol -2-ethy1-8-
methyl-imidazo[1,2-alpyridin-6-y1)-piperazine-1-carboxylic acid tert-butyl
ester (24.4 g, 42 mmol, 1 eq.)
in Me0H (100 mL) was added a 2 M HC1 solution in Et20 (127 mL, 254 mmol, 6
eq.). The reaction
mixture was stirred at r.t. for 3.5 h then concentrated in vacuo. The residue
was partitioned between
Et0Ac and water. The aqueous layer was extracted twice with Et0Ac. A 2 M NaOH
solution was added
to the aqueous layer until pH reached 8-9 and further extraction with Et0Ac
was performed. The
combined organic layers were then washed with brine, dried over Na2SO4,
filtered and concentrated in
vacuo. The solid was triturated with heptane (100 mL) at r.t. overnight,
filtered off, washed with heptane
and Et20, and dried to afford Compound 1.
[00467] tH NMR 6 (ppm) (400 MHz, CDC13): 8.17 (2 H, dd), 7.18 (2 H, app t),
6.99 (2 H, bs), 3.61 (3
H, s), 3.09-2.98 (8 H, m), 2.75 (2 H, q), 2.61 (3 H, s), 1.34 (3 H, t)
125
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00468] LC-MS: MW (calcd): 475; m/z (obsd): 476 (M+1)
2.2. Compound 2: 242-ethyl-6-(4-(2-(3-hydroxyazetidin-l-y1)-2-
oxoethyl)piperazin-l-y1)-8-
methylimidazo[1,2-akyridin-3-y1)(methyl)alllino)-4-(4-fluorophenyOthiazole-5-
carbonitrile
\-41 0
HN \N--<N
S CN S CN
[00469] To a solution of amine compound 1(12.6 g, 27 mmol, 1 eq.) in 100 mL of
MeCN were added
potassium carbonate (7.3 g, 53 mmol, 2 eq.) and Gen13-a (5.2 g, 34 mmol, 1.3
eq.). The reaction mixture
was refluxed for 5.5 h then cooled to r.t. and stirred for 40 h. The crude
product was filtered and washed
with MeCN. The collected precipitate was then suspended in 300 mL of water,
stirred for 1 h, filtered,
and finally washed with water and MeCN. The solid obtained was dried in vacuo
for 48 h to afford
Compound 2.
[00470]
IFINMR (400 MHz, CDC13) 6 ppm 8.20 - 8.12 (2 H, m), 7.22 - 7.13 (2 H, m), 6.99
(2 H, s),
4.68 (1 H, m), 4.43 (1 H, dd), 4.26 (1 H, dd), 4.14 - 4.05 (1 H, m), 3.88 (1
H, dd), 3.61 (3 H, s), 3.58 -
3.52 (1 H, m), 3.14 - 3.02 (6 H, m), 2.74 (2 H, q), 2.70 - 2.62 (4 H, m), 2.59
(3 H, s), 1.33 (3 H, t)
[00471] LC-MS: MW (calcd): 588; m/z (obsd): 589 (M+1)
2.3. Compounds 3-5
[00472] Compounds 3-5 listed in the table of compounds were prepared similarly
as compound 2
following general synthetic method F8 using Compound 1 and Intermediates Gen-
13-b, Gen-13-g, Gen-
1 3-h listed in the table of synthetic intermediates. Analytical details for
these compounds and the
following compounds are provided in the table of analytical details.
Analytical details for synthetic
intermediates are provided in the table of synthetic intermediates.
2.4. Compound 6: 2-((2-ethy1-6-(4-(2-(3-hydroxyazetidin-1-y1)-2-oxoethyl)-3,3-
dimethylpiperazin-1-y1)-
8-rnethylirn idazo ,2-akyridin-3-y1)(rn ethyl) a m ino)-4-(4-fluo rophenyl)th
iazo e- 5 -carbon itrile
Step i : 2-0-(3,3-Dimethyl-piperazin-l-y1)-2-ethyl-8-methyl-imidazo[1,2-
a]pyridin-3-y1J-rnethyl-amino}-
4-(4-fluoro-phenA-thiazole-5-carbonitrile.
\N HN \N
s CN s CN
126
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00473] Intermediate Gen-10-x 2- { [6-(3 ,3 -Dimethyl-piperazin-1 -y1)-2-
ethyl-8-methyl-imidazo [1,2-
a]pyridin-3-y1]-methyl-aminoI -4-(4-fluoro-phenyl)-thiazole-5-carbonitrile is
prepared from Intermediate
Gen-5-t and 2,2-dimethylpiperazine following general synthetic method Fla.
Step ii: 2-[(2-Ethy1-6q4-1-2-(3-hydroxy-azetidin-1-y1)-2-oxo-ethy1]-3,3-
dimethyl-piperazin-1-3;1}-8-
methyl-imidazo[1,2-cdpyridin-3-y0-methyl-aminol-4-(47fluoro-phenyl)-thiazole-5-
carbonitrile
HO
H N-41
S CN S CN
N N
[00474] Compound 6 is obtained from Intermediate Gen-10-x by alkylation with
Intermediate Gen-13-
a following general synthetic method F8.
2.5. Compound 7: 244-(2-ethy1-3-((4-(4-fluorophenyOthiazol-2-y1)(methy0amino)-
8-
methylimidazo[1,2-akyridin-6-Apiperazin-l-y1)-1-(3-hydroxyazetidin-1-
y0ethanone
HO
\ N
N I
Step i: (6-bromo-2-ethyl-8-methyl-imidazo[1,2-cdpyridin-3-y1)44-(4-fluoro-
phenyl)-thiazol-2-y1Pmethyl-
amine
NH \ N
I
Br*, S
N \ _______________________________________________
[00475] Intermediate Gen-5-d (6-bromo-2-ethy1-8-methyl-imidazo[1,2-a]pyridin-3-
y1)-[4-(4-fluoro-
pheny1)-thiazol-2-y1]-methyl-amine is prepared from Intermediate Gen-4-d and 2-
bromo-4'-
fluoroacetophenone following general synthetic method El.
127
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step ii: (2-ethy1-8-methy1-6-piperazin-l-yl-imidazo[1,2-cdpyridin-3-y1)-[4-(4-
fluoro-pheny0-thiazol-2-y1J-
methyl-amine
N N
I H N N
S
N \ N __ S
[00476] intermediate Gen-10-n (2- ethy1-8-methyl-6-piperazin- 1 -yl-imi
dazo pyridin-3 -y1)- [4 -(4-
fluoro-pheny1)-thiazol-2-yl]-methyl-amine is prepared from Intermediate Gen-5-
d and piperazine
following general synthetic method F1a.
Step iii: 2-(4-(2-ethy1-34(4-(411uorophenyl)thiazol-2-y1)(methyl)amino)-8-
methylimidazo[1,2-a]pyridin-
6-Aptperazin - 1 -y1)- 1 -(3-hyd roxyazetid in- 1 -yl)etha n one
0
\ N
HN-Th I
S
N \ S
N \
[00477] Compound 7 is obtained by alkylation of Intermediate Gen-10-n with
Intermediate Gen-13-a
following general synthetic method F8.
2.6. Compounds 8 and 9
[00478] Compounds 8-9 listed in the table of compounds arc prepared similarly
as compound 7
following general synthetic method F8 using Intermediates Gen-10-n, Gen-13-g,
Gen-13-h listed in the
table of synthetic intermediates.
2.7. Compound 10: 242-ethyl-6-(1-(2-(3-hydroxyazetidin-l-y1)-2-
oxoethyl)piperidin-4-y1)-8-
rnethylimidazo[1,2-4pyridin-3-y0Onethyl)amino)-4-(4-fluorophenyOthiazole-5-
carbonitrile
N
-N
cN N N
0
HO
128
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step 0 2-[(2-ethyl-8-methyl-6-piperidin-4-yl-imidazo[1,2-a]pyridin-3-y0-methyl-
amino]-4-(4-fluoro-
pheny0-thiazole-5-carbonitrile
\ HN----.N."
NK
S CN S CN
N
[00479] Synthetic Intermediate Gen-10-o 2-[(2-ethyl-8-methyl-6-piperidin-4-
yl-imidazo pyridin-
3-y1)-methyl-amino]-4-(4-fluoro-pheny1)-thiazole-5-carbonitrile is obtained
from Intermediate Gen-5-t
using general synthetic methods F3 followed by F5b.
Step 2-((2-
ethy1-6-(1-(2-(3-hydroxyazetidin-1-y1)-2-oxoethyl)pipericlin-4-y0-8-
methylimidazo[1,2-
a]oyridin-3-y00nethy0amino)-4-(4-fluoropheny0thiazole-5-carbonitrile
[00480] Compound 10 is then obtained by alkylation of Intermediate Gen-10-o
with Intermediate Gen-
13-a using general synthetic method F8.
2.8. Compound 11: 2-(ethyl(2-ethyl-8-methyl-6-(1-(methylsulfony0-1,2, 3,6-
tetrahydropyridin-4-
y0 imidazo[1,2-d 1pyridin-3-y0amino)-4-(4-fluoropheny0thiazole-5-carbonitrile
0
0 s\ CN
N _____________________________________
Step 0 N-(6-brorno-2-ethyl-8-methylimidazo[1,2-4 pyridin-3-y0-N-
ethylformarnide
//0 0
HN¨jk N1(
BrJ H _______________________________
[00481] Intermediate Gen-3 -h N-(6-
bromo-2- ethyl- 8-methylimidazo[l,2pyridin-3 -y1)-N-
ethylformamide is prepared from Inteimediate Gen-2-d with iodoethane according
to general synthetic
method C2.
129
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step ii)
0
11
NH
N
[00482] Reaction of Intermediate Gen-3-h following general synthetic method D1
affords Intermediate
Gen-4-h.
Step iii)
NH
BrN
CN
N
[00483] Reaction of Intermediate Gen-4-h with Intermediate Gen-12-a following
general synthetic
method E2 affords Intermediate Gen-5-h.
Step iv)
0
N
CN N CN
N \
[00484] Suzuki coupling of Intermediate Gen-5 -h with 1-(methylsulfony1)-4-
(4,4,5,5-tetramethyl-
1,3,2-dioxaborolan-2-y1)-1,2,3,6-tetrahydropyridine following general
synthetic method F2 afford
compound 11.
130
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
2.9. Compound 12: 2-(12-ethy1-8-fluoro-6-(4-(2-(3-hydroxyazetidin-1-y1)-2-
oxoethyl)piperazin-1-
y1)Unidazo[1,2-u]pyridin-3-y1)(nzethyl)arnino)-4-(4-fluorophenyl)thiazole-5-
carbonitrile
HO N
j/
N C N
0 N
N
Step I)
Br.N
yNH 2
NH 2
[004851 Intermediate Gen- 1-a 2-amino-5-bromo-3 -fluoropyridine is prepared
from 2-amino-3 -
fluoropyridine using general synthetic method A.
Step ii)
0
N
ii)
Br
N H2
[004861 Intermediate Gen-2-a N-(6-bromo-2-ethy1-8-fluoro-imidazo[1,2-a]pyridin-
3-y1)-formamide is
prepared from Intermediate Gen-1 -a and propionaldehyde using general
synthetic method Bl.
Step iii)
0
0 NH
k11-1 Br H
[004871 Intermediate Gen-2-a is methylated with iodomethane following general
method C2 to give
Intermediate Gen-3-a N-(6-bromo-2-ethy1-8-fluoro-imidazo[1,2-a]pyridin-3-y1)-N-
methyl-formamide.
131
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step iv)
0
\
NH
Br,
[00488] Formyl group of Intermediate Gen-3-a is removed under conditions of
general synthetic
method D1 to furnish Intermediate Gen-4-a (6-bromo-2-ethy1-8-fluoro-
imidazo[1,2-a]pyridin-3-y1)-
methyl-amine.
Step v)
NH
S C N
sy-LN)
[00489] Reaction of Intermediate Gen-4-a with Intermediate Gen-12-a following
general synthetic
method E2 afforded Intermediate Gen-5-r 2-[(6-bromo-2-ethy1-8-fluoro-
imidazo[1,2-a]pyridin-3-y1)-
methyl-amino]-4-(4-fluoro-pheny1)-thiazole-5-carbonitrile.
Step vi)
S
H-Th CN N
S CN
BrNJ
[00490] Reaction of Intermediate Gen-5-r with piperazine following general
synthetic method Fla
afforded Intermediate Gen-10-m 2-[(2-ethy1-8-fluoro-6-piperazin-1-yl-
imidazo[1,2-a]pyridin-3-y1)-
methyl-amino]-4-(4-fluoro-pheny1)-thiazole-5-carbonitrile.
132
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step vii)
HO
\ N
S CN S CN
Y-1\1)
[00491] Alkylation of Intermediate Gen-10-m with Intermediate Gen-13-a
following general synthetic
method FS gave compound 12.
2.10. Compound 13: 2-(4-(3-((5-cyano-4-(4-fluorophenyl)thiazol-2-
y1)(methyl)amino)-2-ethyl-8-
fluoroimiduzo[1,2-c]pyridin-6-Aptperazin-1-y0-N-methylacetamide
S CN
Y-1\12
[00492] Compounds 13 listed in the table of compounds is prepared similarly as
compound 12
following general synthetic method FS using Intermediates Gen-10-m and 2-
chloro-N-methylacetamide.
2.11. Compound 14: 2-(4-(2-ethyl-8-flitoro-3-((4-(4-fluorophenyl)thiazol-2-
y1)(methyl)amino)imidazo[1,2-cdpyridin-6-y1)piperazin-1-321)-1-(3-
hydroxyazetidin-l-Aethanone
HO
\¨N 0
S
Step i)
BrN
NH
\N¨s
133
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00493] Intermediate Gen-5-a (6-bromo-2-ethy1-8-fluoro-imidazo[1,2-a]pyridin-3-
y1)-[4-(4-fluoro-
pheny1)-thiazol-2-y1]-methyl-amine is prepared from Intermediate Gen-4-a and 2-
bromo-4'-
fluoroacetophenone following general synthetic method El.
Step ii)
µF
HN
N µµc
S
\
N
[00494] Intermediate Gen-10-1 (2 - ethy1-8-fluoro-6-piperazin-1 -yl-imidazo
[1,2-a]pyridin-3 -y1)- [4 -(4-
fluoro-pheny1)-thiazol-2-y1]-methyl-amine is prepared from Intermediate Gen-5-
a and piperazine
following general synthetic method Fla.
Step iii)
,F
z/
;.0
N--(/
HN N N"] p
S ,N
N
\
[00495] Compound 14 is obtained by alkylation of Intermediate Gen-10-1 with
Intermediate Gen-13-a
following general synthetic method F8.
2.12. Compounds 15-16
[00496] Compounds 15-16 listed in the table of compounds arre prepared
similarly as compound 7
following general synthetic method F8 using Intermediates Gen-10-1, Gen-13-g,
Gen-13-h listed in the
table of synthetic Intermediates.
2.13. Compound 17: 2-(4-(2-ethy1-34(4-(4-fluorophenyOthiazol-2-
y1)(ntethyl)amino)-7-
methylimidazo[1,2-afryridin-6-Apiperidin-1-y1)-1-(3-hydroxyazetidin-1-
Aethanone
N
N N
0
HO
134
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step i) N-(6-Bromo-2-ethyl-7-rnethyl-imidazo[1,2-alpyridin-3-yl)-forrnamide
0
BrN N'
)1, B r
N"¨c
[00497] Intermediate Gen-2-h N-(6-Bromo-2-ethy1-7-methyl-imidazo[1,2-a]pyridin-
3-y1)-forrnamide
is prepared from 2-amino-4-bromo-5-methylpyridine and propionaldehyde
following general synthetic
method Bl.
Step ii) N-(6-Brorno-2-ethyl-7-inethyl-imidazo[1,2-alpyridin-3-yl)-N-
methylTformamide
0
0
Br H
Br, N ___
iN
[00498] Intermediate Gen-2-h is methylated with iodomethane following general
method C2 to give
Intermediate Gen-3-i.
Step 4-1-2-Ethyl-3-(formyl-inethyl-arnino)-7-inethyl-iniidazo[],2-
alpyridin-6-yl -piperidine-1-
carboxylic acid tert-butyl ester
0
0 0
H
N-4>,
[00499] Negishi coupling of Gen-3-i with N-boc-4-iodopiperidine derived
organozinc reagent
following general synthetic method F3 afforded Intermediate Gen-8-f.
Step iv) 442-Ethyl-7-methyl-3-rnethylainino-imidazo[1,2-alpyridin-6-A-
piperidine-1-carboxylic acid
tert-butyl ester
o
HN NH N- NH
___________________ H
\
-N
[00500] Removal of formyl and boc groups of Intermediate Gen-8-f and
protection of piperidine with
Boc group is achieved under conditions of general synthetic methods D1 and F7
to furnish Intermediate
Gen-9-g.
135
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step v) (2-Ethy1-7-methy1-6-pperidin-4-yl-imidazo[1,2-cdpyridin-3-y1)-1-4-(4-
fluoro-pheny1)-thiazol-2-y1J-
methyl-amine
F
1 01 0
..\-'0'-''N''''''= \ F:/
NH 4, 1,0
--')--'---N--\ - 0 N
\ P-5
N----', '
Ns- \F
\
_________________________________________________ H N-4
NC;,
)-- \
\
[00501] Intermediate Gen-10-s is prepared from Intermediate Gen-9-g and 2-
bromo-4'-
fluoroacetophenone under conditions of general synthetic method El followed by
boc removal with
general synthetic method F5b.
Step vi) 2-[4-(2-Ethyl-3-{ [4-(4-fluoro-pheny1)-thiazol-2-yll -rnethyl-
amino}-7-methyl-imidazo[1,2-
cdpyridin-6-y1)-piperidin-1-yll-1-(3-hydroxy-azetidin-1-y1)-ethanone
,F F
HO,
(i z---- \---\
0
,-----__/-- -N"-e
N----( N-
HN--- ____________________________
Isl''-- \ NI--1
N-----\S-)
7-'\Ki-N---- '' s 'N"--µ(
. N ,
[00502] Alkylation of Intermediate Gen-1 0-s with Intermediate Gen-1 3-a
following general synthetic
method F8 gave compound 17.
2.14. Compound 18: 2-[(2-Ethy1-7-fluoro-6-{412-(3-hydroxy-azetidin-l-y1)-2-oxo-
ethyl] -piperazin-1 -
yl,Limidazo [1 ,2-al pyridin-3-yI)-methyl-amino] -4-(411uoro-pheny1)-thiazole-
5-carbonitrile
F
HO,r___.\
N
N--- \
s CN
FN \
Step I)
II ________________________________ ._ Br1,1
1--
F" NH, F-' 'NH,
[00503] Intermediate Gen- 1-b is prepared from 2-amino-4-fluoropyridine
following general synthetic
method A.
Step ii)
o
___________________________________ (---< - " H //
N
Br ,N
----`,
Br. ---, 'N
Br. ____ H
"- - - ___.(
ii
F,= ¨ A 'NH2
F" --: )----1q. ' ' F
136
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00504] Intermediate Gen-2-i is prepared from Intermediate Gen- 1-b and
propionaldehyde following
general synthetic method Bl.
Step iii)
0 0
NI-14
FN
[00505] Intermediate Gen-2-i is methylated with iodomethane following general
method Cl to give
Intermediate Gen-3-j.
Step iv)
0
NH
,
[00506] Formyl group of Intermediate Gen-3-j is removed under conditions of
general synthetic
method D2 to furnish Intermediate Gen-4-g.
Step v)
NH
N-(
Br.
N
S \ C
F N N
\
N
[00507] Reaction of Intermediate Gen-4-g with Intermediate Gen-12-a following
general synthetic
method E2 afforded Intermediate Gen-5-u.
Step vi)
_____________________________________ HN
CN S CN
Brj 'N-
N'
[00508] Reaction of Intermediate Gen-5-u with piperazine following general
synthetic method F la
afforded Intermediate Gen-10-r.
137
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step vii)
HOõ
0
\
HN N
N _4 \) \
S- CN N'Th
CN
, N
\
-N FN
[00509] Alkylation of Intermediate Gen-10-r with Intermediate Gen-13-a
following general synthetic
method FS gave compound 18.
2.15. Compound 24. 2-(4-(2-ethyl-3-((4-(47fluorophenyOthiazol-2-
y1)(methyl)aminofinfidazo[1,2-
ulpyridin-6-Apiperuzin-l-y1)-1-(3-hydroxyazetidin-1-Aethunone
N
si
N
0
HO
Step I)
NH
Br N
\\
\
N
[00510] Intermediate Gen-5-b (6-Bromo-2-ethyl-imidazo[1,2-a]pyridin-3-y1)-[4-
(4-fluoro-pheny1)-
thiazol-2-yThmethyl-amine is prepared from Gen-4-b (6-Bromo-2-ethyl-imidazo
[1,2-a]pyridin-3-y1)-
methyl-amine and 2-Bromo-4'-fluoroacetophenone following general synthetic
method El.
Step it)
,F ,F
N
N--(µ
HN
N S
N \
[00511] Reaction of Intermediate Gen-5-b with piperazine following general
synthetic method Fla
afforded Intermediate Gen-10-e 2-Ethy1-6-piperazin-l-yl-imidazo[1,2-a]pyridin-
3-y1)-[4-(4-fluoro-
phenyl)-thiazol-2-y1]-methyl-amine.
138
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step in)
HO_
S
\)¨
N N
[00512] Alkylation of Intermediate Gen-10-e with Intermediate Gen-13-a
following general synthetic
method F8 give compound 24.
2.16. Compounds 25 to 41
[00513] These compounds listed in Table II are prepared similarly as compound
24 following general
synthetic method F8 using Intermediates Gen-10-e and respectively
Intermediates Gen-13-g, Gen-13-h,
Gen-13-j, Gen-13-d, Gen-13- e, Gen-13-f, 1 -(Chloroac etyl)pyrrolidine, Gen-13-
1 , 2-Chloroacetamide,
Gen-13-c, 2-Chloro-N,N-dimethyl-acetamide, ethyl 2-chloroacetate, Ethyl 2-
Chloropropionate,
Chloroacetonitrile, 5-(chloromethyl)-1-
cyclopropy1-1H-tetrazole, 2-Chloromethyl-oxazole, 3-
(Chloromethyl)-1,2,4-oxadiazole.
2.17. Compound 43: 2-hydroxyethyl 4-(2-ethy1-34(4-(4-fluorophenyOthiazo1-2-
y1)(methyl)amino)imidazo[1,2-alpyridin-6-yOpiperazine-1-carboxylate
0
N ,
HO
0 N-Th N
[00514] The compound 43 is prepared similarly as compound 24 until the
Intermediate Gen-10-e
,F
0
FIN \NI-4;1
Is1"¨N---
/ N
\
[00515] TEA (89 L, 0.64 mmol, 3 eq.) and potassium carbonate (88 mg, 0.64
mmol, 3 eq.) were
added to a solution of hydrochloride salt of Gen-10-e (100 mg, 0.21 mmol, 1
eq.) in DCM (3 mL)
followed by ethylene carbonate (28 mg, 0.32 mmol, 1.5 eq.). The reaction
mixture was stirred at r.t. for
18 h. Then DMF (1 mL) was added and the reaction was stirred at 80 C for 15 h.
The reaction was
quenched by addition of water. The mixture was extracted with DCM, combined
organic layers were
dried over sodium sulfate, filtered and evaporated. The crude product was
purified by chromatography on
silica gel (elution with DCM/Me0H 100/0 to 98/2) to afford Compound 43.
139
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00516] 1H NMR (400 MHz, Me0D-d4) 6 ppm 7.89 (2 H, dd), 7.50 - 7.34 (3 H, m),
7.16 - 7.05 (2 H,
m), 6.94 (1 H, s), 4.17 - 4.10 (2 H, m), 3.74 - 3.67 (2 H, m), 3.68 - 3.57 (7
H, m), 3.14 - 2.98 (4 H, m),
2.69 (2 H, q), 1.30 (3 H, t)
[00517] LC-MS: MW (caled): 524; m/z MW (obsd): 525 (M+1)
2.18. Compound 61: 2-(4-(2-ethy1-34(4-(411uorophenyl)thiazol-2-
y1)(methyl)aminofimidazo11,2-
alpyridin-6-Apiperazin-1-ylculfonyl)acetamide :
N
-N
001 0 N
H2N:
0
Step 1)
O.. _CI 0.01
S,
'0
CI 0 0_0
1
[00518] To a solution of chlorosulfonylacetyl chloride (419 uL, 3.955 mmol, 1
eq.) in Et20 (4 mL), at
0 C was added Me0H (160 uL, 3.955 mmol, 1 eq.). The reaction mixture is
strirred at 0 C for 3 h, then
concentrated in vacuo to give chlorosulfonyl-acetic acid methyl ester.
Step it)
N
S
HN) 0 0
HU-
0
[00519] Compound 60 was obtained by reaction of intermediate Gen-10-e with
chlorosulfonyl-acetic
acid methyl ester following general synthetic methods Fl 1 and F13.
140
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step
F
N F
N
S m
0 N
HO
H2N
0
[00520] To a solution of compound 60 (38 mg, 0.068 mmol, 1 eq.) in DCM (3 mL)
and THF (2 nit)
were added HOBT (11 mg, 0.082 mmol, 1.2 eq.) and EDC.HC1 (14 mg, 0.075 mmol,
1.1 eq.). The
reaction mixture was stirred at r.t. for 1.25 h then a solution of ammoniac 7
M in MeON (2 drops) was
added. The reaction mixture was stirred at r.t. for 30 min., then filtered on
celite and the filtrate was
concentrated in vacuo. The residue was purified by preparative HPLC to afford
the compound 61.
[00521] 11-1 NMR (400 MHz, CDC13) 6 ppm 7.88 - 7.85 (2 H, m), 7.62 (1 H,
d), 7.23 (1 H, d), 7.17 -
7.07 (3 H, m), 6.70 (1 H, s), 6.51 (1 H, bs)NH, 5.68 (1 H, bs)NH, 3.90 (2 H,
s), 3.61 (3 H, s), 3.56 - 3.51
(4 H, m), 3.18 - 3.06 (4 H, m), 2.75 (2 H, q), 1.34 (3 H, t)
[00522] LC-MS: MW (calcd): 557; m/z MW (obsd): 558 (M+1)
2.19. Compound 64: ethyl 2-(4-(2-ethyl-3-(14-(4-fluorophenyl)thiazol-2-
y1)(inethyl)aniinofimidazo[1,2-
akyridin-6-y1)-3-oxopiperazin-1-y0acetate
N
N /1\I
0 rN
0
[00523] Compound 64 is prepared from Intermediate Gen-5-b and 3-0xo-piperazine-
1 -carboxylic acid
tert-butyl ester using the method F4, following by the method F5b to give 1-(2-
Ethy1-3-1[4-(4-fluoro-
phenyl)-thiazol-2-yl] -methyl-amino} -imi dazo [1,2-a] pyri din-6-y1)-
piperazin-2- on e (Gen-10-d).
N N
NN _________________________________________________ NN
rN 0
0
141
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00524] To a solution of Gen-10-d (60 mg, 0.133 mmol, 1 eq.) in 4 mL of DMF at
r.t. was added NaH
60% in oil (8 mg, 0.199 mmol, 1.5 eq.). The reaction mixture was stirred for 1
h at r.t. then ethyl bromo
acetate (0.018 mL, 0.159 mmol, 1.2 eq.) was added. The reaction mixture was
stirred at r.t. for 2 h. The
reaction mixture was quenched with a saturated solution of ammonium chloride.
The aqueous phase was
extracted with AcOEt. The combined organic layers were washed with a saturated
solution of sodium
carbonate, with brine, dried over Na2SO4, filtered and concentrated in vacuo.
The crude product was
purified by chromatography on silica gel to afford Compound 64.
[00525] LC-MS: MW (calcd): 536; m/z MW (obsd): 537 (M+1)
2.20. Compound 76: 4-(2-ethy1-3-((4-(4-chloropheny0thiazol-2-
y1)(methyl)arnino)imidazo[1,2-
cdpyridin-6-y1)-3,6-dihydro-2H-thiopyran 1,1-dioxide
CI
0µ
0'2S1 \N¨s
[00526] Compound 76 is prepared from Intermediate Gen-5-i and 2-(3,6-Dihydro-
2H-thiopyran-4-y1)-
4,4,5,5-tetramethy141,3,21dioxaborolane using method F2 to give 4-(2-ethy1-344-
(4-
chlorophenyl)thiazol-2-y1)(methyl)amino)imidazo[1,2pyridin-6-y1)-3 ,6-dihydro-
2H-thiopyran (Gen-
10-g).
N-( 9 õ
S1\ \,
[00527] Oxone tetrabutylammonium salt (489 mg, 0.3 mmol, 1.5 eq.) was added to
a solution of 4-(2-
ethy1-34(4-(4-chlorophenyl)thiazol-2-y1)(methyl)amino)imidazo[1,2-a]pyridin-6-
y1)-3,6-dihydro-2H-
thiopyran Gen-10-g (93.5 mg, 0.2 mmol, 1 eq.) in DCM (1.1 mL). The reaction
mixture was stirred at r.t.
for 4 h, then brine was added and layers were separated. The aqueous layer was
extracted with
dichloromethane, then combined organic layers were dried over sodium sulfate,
filtered and evaporated.
The crude product was purified by chromatography on silica gel (elution with
DCM/McOH 100/0 to
98/2) to afford Compound 76.
[00528] Iff
NMR (400 MHz, CDC13) 6 ppm 7.81 (2 H, d), 7.77 (1 H, bs), 7.61 (1 H, d), 7.37
(2 H, d),
7.29 (1 H, d), 6.76 (1 H, s), 5.90 (1 H, t), 3.79 (2 H, bs), 3.63 (3 H, s),
3.26 - 3.20 (2 H, m), 3.15 - 3.07 (2
H, m), 2.77 (2 H, q), 1.35 (3 H, t)
[00529] LC-MS: MW (calcd): 498 (35C1), 500 (37C1); m/z MW (obsd): 499 (Cl
M+1), 501 (37C1 M+1)
142
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
2.21. Compound 78: tert-butyl 442-ethyl-3-(14-(4-fluorophenyl)thiazol-2-
A(methyOarninofirnidazo[1,2-
alpyridin-6-y1)-3-hydroxypiperidine-1-carhoxylate
0
\
OANoH
[00530] Compound 78 is prepared from Intermediate Gen-5-c and 4-(4,4,5,5-
tetramethyl-
[1,3,2]dioxaborolan-2-y1)-piperidine-1 -carboxylic acid tert-butyl ester using
method F2 to give 4-(2-
Ethyl-3- { [4-(4-fluoro-pheny1)-thiazol-2-yl] -methyl-amino } -imidazo
pyridin-6-y1)-3,6- dihydro-2H-
pyridine-1 -carboxylic acid tert-butyl ester.
0 0 411
N
I
[00531] To a solution of 4-(2-Ethy1-3-{[4-(4-fluoro-pheny1)-thiazol-2-y1]-
methyl-amino}-imidazo[1,2-
a]pyridin-6-y1)-3,6-dihydro-2H-pyridine-1-carboxylic acid tert-butyl ester
(0.15 g, 0.28 mmol, 1 eq.) in 6
mL of THF at 0 C under nitrogen was added BH3.THF 1 M in THF (2.81 mL, 2.81
mmol, 10 eq.). The
reaction mixture was stirred at 0 C for 4 h, then at r.t. for 14 h. The
reaction mixture was cooled to 0 C,
then NaOH 2 M (6 mL) and H202 30% aqueous (6 mL) were added. The reaction
mixture was refluxed
for 3 h then cooled to r.t. and THF was removed in vacuo. The remaining
aqueous phase was extracted
with DCM three times. The combined organic layers were washed with brine,
dried over Na2SO4, filtered
and concentrated in vacuo. The crude product was purified by chromatography on
silica gel (elution with
heptane/Et0Ac: 100/0 to 40/60) to afford Compound 78.
[00532] NMR (400 MHz, Me0D-d4) 6 ppm 8.04 (1 H, d), 7.96 - 7.92 (2 H, m),
7.65 - 7.61 (1 H,
m), 7.57 - 7.54 (1 H, m), 7.10 (2 H, t), 7.03 (1 H, s), 4.37 - 4.33 (1 H, m),
4.19 - 4.16 (1 H, m), 3.75 - 3.61
(4 H, m), 2.91 - 2.78 (3 H, m), 2.73 -2.60 (2 H, m), 1.91 - 1.66 (2 H, m),
1.52 (9 H, d), 1.39 (3H, t)
[00533] LC-MS: MW (calcd): 551; m/z MW (obsd): 552 (M+1)
143
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
2.22. Compound 90: N-(6-(143-aminopropylsulfonyl)piperidin-4-y1)-2-
ethylimidazo[1,2-a]pyridin-3-y0-
4-(4-fluoropheny0-N-methylthiazol-2-amine
N
N
0
H2NSc)
Step it
[00534]
Reaction of Intermediate Gen-10-c and 3 -chlo ro-prop ane-1 -sulfonyl chloride
following
method F11 then general synthetic method Fl2a with phtalimide afforded
Intermediate Gen-10-p.
Step ii) {611-(3-Amino-propane-1-sulfonyl)-piperidin-4-y11-2-ethyl-imidazo[1,2-
a]pyridin-3-y1)-
0-(4-fluoro-pheny0-thiazol-2-ylrmethyl-amine
F
F
N N \
S N \
N S
0 0
0
H2NSN
0
[00535] To a solution of Intermediate Gen-10-p (0.053 g, 0.077 mmol, 1 eq.) in
4 mL of Et0H at r.t.
was added hydrazine hydrate (0.013 mL, 0.270 mmol, 3.5 eq.). The reaction
mixture was stirred 2 h at
90 C and at r.t. overnight. The reaction mixture was concentrated in vacuo.
The residue was taken up
with DCM and a saturated Na2CO3 solution. The aqueous phase was extracted with
DCM. The combined
organic layers were washed with brine, dried over Na2SO4, filtered and
concentrated in vacuo. The crude
product was purified by chromatography on silica gel (elution with DCM/Me0H)
to afford Compound
90.
[00536] 11-1
NMR (300 MHz, CDC13) 6 ppm 7.85(2 H, dd), 7.61 (1 H, s), 7.57(1 H, d), 7.19 -
7.02 (3
H, m), 6.67 (1 H, s), 3.92 (2 H, bd), 3.61 (3 H, s), 3.16 - 3.02 (1 H, m),
3.01 - 2.69 (7 H, m), 2.68 - 2.51 (1
H, m), 2.11 - 1.97 (2 H, m), 1.96 - 1.87(2 H, m), 1.86 - 1.66(2 H, m), 1.34 (3
H, t)
[00537] LC-MS: MW (calcd): 556; m/z MW (obsd): 557 (M+1)
144
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
2.23. Compound 91 : N-(2-ethy1-6-(1-(2-morpholinoethylsulfonyl)piperidin-4-
yOirnidazo[1,2-a]pyridin-
3-y1)-4-(4-fluoropheny0-N-methylthiazol-2-amine :
N N
-N
-N
\
N =
0
N
[00538] To a solution of Gen-10-y (0.026 g, 0.049 mmol, 1 eq.) in 2 mL of MeCN
at r.t. was added
morpholine (0.013 mL, 0.148 mmol, 3 eq.). The reaction mixture was stirred for
45 min at r.t. then ethyl
bromo acetate (0.009 mL, 0.079 mmol, 1.2 eq.) was added. The reaction mixture
was stirred at 90 C for
4.5 h and at r.t. overnight. The reaction mixture was concentrated in vacuo.
The crude product was
purified by chromatography on silica gel (elution with DCM/MeOH: 100/0 to
98/2) to afford Compound
91.
[00539] '1-1 NMR (400 MHz, Me0D-d4) 6 ppm 7.99 - 7.81 (3 H, m), 7.51 (2 H,
dd), 7.10 (2 H, t), 6.96
(1 H, s), 3.84 (2 H, d), 3.75 - 3.58 (7 H, m), 3.24 (2 H, t), 2.95 (2 H, t),
2.89 - 2.66 (5 H, m), 2.55 (4 H,
bs), 1.92 (2 H, d), 1.85 - 1.67 (2 H, m), 1.32 (3 H, t)
[00540] LC-MS: MW (caled): 612; iin/z MW (obsd): 613 (M+1)
2.24. Compound 92: 4-(2-ethyl-344-(47fluorophenyl)thiazol-2-
y0(methyParnino)irnidazo[1,2-
alpyridin-6-Apiperidine-1-sulfonamide
N N
o
N
NCW
FI,N
[00541] To a solution of Gen-10-c (0.05 g, 0.115 mmol, 1 eq.) in dioxane (2
mL) was added sulfamide
(0.039 g, 0.402 mmol, 3.5 eq.). The reaction mixture was heated at 110 C under
microwave irradiation
for 45 min. After cooling, water and Et0Ac were added to the reaction mixture,
the aqueous layer was
extracted with Et0Ac twice. The combined organic layers were washed with water
and brine, dried over
Na2SO4, filtered and concentrated in vacuo. The residue was purified by
chromatography on silica gel to
afford Compound 92.
[00542] 11-1 NMR (400 MHz, Me0D-d4) 6 ppm 7.93 - 7.84 (3 H, m), 7.53 (1 H,
dd), 7.40 (1 H, dd),
7.11 (2 H, t), 6.94 (1 H, s), 3.75 (2 H, d), 3.62 (3 H, s), 2.76 - 2.66 (5 H,
m), 1.90 (2 H, bd), 1.83 (2 H,
qd), 1.32(3 H, t)
[00543] LC-MS: MW (calcd): 514; m/z MW (obsd): 515 (M+1)
145
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
2.25. Compound 105: 1-(2-(12-ethyl-6-(1-(methylsulfonyl)piperidin-4-
Aimidazo[1,2-alpyridin-3-
y1)(nzethyl)arnino)-4-(4-fluorophenyOthiazol-5-Aethanone
,O
[00544] To a solution of Compound 104 (0.06 g, 0.11 mmol, 1 eq.) in 2 mL of
THF at -78 C under
argon was added MeLi 1.6 M in Et20 (0.1 mL, 0.16 mmol, 1.45 eq.) dropwise. The
reaction mixture was
stirred at -78 C for 1 h, then warmed up to r.t. and stirred for 4 d. The
reaction mixture was quenched
with a saturated solution of ammonium chloride, the aqueous phase was
extracted with AcOEt. The
combined organic layers were washed with brine, dried over Na2SO4, filtered
and concentrated in vacuo.
The crude product was purified by preparative HPLC to afford Compound 105.
[00545] Iff NMR (400 MHz, Me0D-d4) 6 ppm 7.96 (1 H, s), 7.69 - 7.60 (2 H,
m), 7.55 (1 H, d), 7.44
(1 H, dd), 7.23 (2 H, t), 3.85 (2 H, d), 3.59 (3 H, s), 2.91 - 2.81 (5 H, m),
2.80 - 2.68 (3 H, m), 2.00 (3 H,
s), 1.99 - 1.92 (2 H, m), 1.91 - 1.77 (2 H, m), 1.33 (3 H, t)
[00546] LC-MS: MW (calcd): 555; m/z MW (obsd): 556 (M+1)
2.26. Compound 106: N-(2-(2-(12-ethyl-6-(1-(rnethylsulfonyl)piperidin-4-
yl)imidazo[1,2-alpyridin-3-
y1)(methyl)amino)thiazol-4-y1)-5-fluorophenyl)acetainide
F
N N
Br S 0 NH S
"1/.
-s-N
\µ
0 0
[00547] To Intermediate Gen-10-al (0.07 g, 0.118 mmol, 1 eq.), acetamide
(0.009 g, 0.141 mmol, 1.2
eq.), cesium carbonate (0.054 g, 0.165 mmol, 1.4 eq.), Xantphos (0.010 g,
0.017 mmol, 0.15 eq.) and
Pd2(dba)3 (0.006 g, 0.006 mmol, 0.05 eq.) in a sealed tube under argon was
added degased dioxane. The
reaction mixture was heated at 100 C for 6 h. After cooling to r.t., the
reaction mixture was filtered on
Celpure0 P65 and the residue is washed with Et0Ac. The filtrate was
concentrated in vacuo and the
crude product was purified by chromatography on silica gel (elution with
DCM/Me0H) to afford
Compound 106.
[00548] 11-1 NMR (400 MHz, CDC13) 6 ppm 11.56 (1 H, bs), 8.43 (1 H, d),
7.66 (1 H, d), 7.61 (1 H, s),
7.55 (1 H, dd), 7.22 (1 H, d), 6.84 - 6.73 (2 H, m), 3.96 (2 H, d), 3.63 (3 H,
s), 2.87 - 2.71 (7 H, m), 2.71 -
2.54 (1 H, m), 2.09(3 H, s), 2.02 - 1.92 (2 H, m), 1.91 - 1.77(2 H, m), 1.37(3
H, t)
[00549] LC-MS: MW (calcd): 570; m/z MW (obsd): 571 (M+1)
146
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
2.27. Compound 107: (2-(2-((2-ethy1-6-(1-(methylsulfonyl)piperidin-4-
y0imidazo[1,2-alpyridin-3-
y1)(nzethyl)amino)thiazol-4-y0-5-fluorophenyOmethanol
N N
\)--N
Br S
HO N
N. ,.
õ
0 0
[00550] To a solution of Intermediate Gen-10-al (0.050 g, 0.084 mmol, I eq.)
in 0.84 mL of THF at -
78 C under argon was added iPrMgCl.LiC1 1.3 M in THF (0.32 mL, 0.421 mmol, 5
eq.) dropwise. The
reaction mixture was warmed up from -78 C to 0 C over 1 h, then was added
iPrMgCl.LiC1 1.3 M in
THF (0.32 mL, 0.421 mmol, 5 eq.). The reaction mixture was warmed up to r.t.
and stirred for 1.5 h. At
this point, paraformaldehyde (0.025 g, 0.843 mmol, 10 eq.) was added and the
reaction mixture was left
under stirring overnight. The reaction mixture was quenched with a saturated
solution of ammonium
chloride and then filtered on celite. The two phases of the filtrate were
separated. The aqueous phase was
extracted with Et0Ac. The combined organic layers were washed with brine,
dried over MgSO4, filtered
and concentrated in vacuo. The crude product was purified by preparative HPLC
to afford Compound
107.
[00551] 1H
NMR (300 MHz, CDC13) 6 ppm 7.61 (2 H, dd), 7.53 (1 H, dd), 7.17 (2 H, td),
7.05 (1 H,
td), 6.64 (1 H, s), 4.60 (2 H, d), 3.97 (2 H, d), 3.58 (3 H, s), 2.87 - 2.59
(8 H, m), 2.05 - 1.76 (4 H, m),
1.36 (3 H, t)
[00552] LC-MS: MW (calcd): 543; m/z MW (obsd): 544 (M+1)
2.28. Compound 137: 2-((2-ethy1-6-(1-(methylsulfonyl)piperidin-4-Aimidazo[1,2-
alpyridin-3-
y1)(methyl)amino)-4-(4-fluorophenyOthiazole-5-carboxamide
/F
\
0
/9
CN 6/ NH,
0
\'>
-N
[00553] A solution of compound 136 (0.050 g, 0.093 mmol, 1 eq.) in H2SO4 (208
[tL, 3.90 mmol, 42
eq.) was stirred at r.t. overnight. Then water was added to the reaction
mixture water, and the reaction was
neutralized with a saturated NaHCO3 solution. The crude product was filtered
and washed with water
twice. The solid obtained was dried in vacuo for 48 b to afford Compound 137.
[00554] NMR
(400 MHz, DMSO-d6) 6 ppm 8.10 (1 H, s), 7.75 (2 H, t), 7.54 (1 H, d), 7.36 (1
H, d),
7.25 (2 H, t), 3.68 (2 H, d), 3.50 (3 H, s), 3.30 (2 H, s)NH2, 2.89 (3 H, s),
2.82 - 2.71 (3 H, m), 2.63 (2 H,
q), 1.92 - 1.83 (2 H, m), 1.81 - 1.71 (2 H, m), 1.25 (3 H, t)
LC-MS: MW (calcd): 556; m/z MW (obsd): 557 (M+1)
147
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
2.29. Compound 174: 2-(4-(2-(2-cyanoethyl)-344-
(47fluorophenyl)thiazol-2-
y1)(rnethyl)amino)imidazo[1,2-akyridin-6-Apiperidin-1-y1)-N,N-
dimethylacetamide
/ N
N¨
O
=N
Step i)
0
0 N H
NH,
[00555] Reaction of 2-amino-5-bromo-pyridine with Z-cinnamaldehyde according
to general method
B1 afforded Intermediate Gen-2-g (E)-N-(6-bromo-2-styrylimidazo[1,2-a]pyridin-
3-y1)-formamide.
Step ii)
0 0
IN114 \
N-1(H
Br=N H Br,
N
[00556] Methylation of Intermediate Gen-2-g following general method Cl led to
Intermediate Gen-3-
g (E)-N-(6-bromo-2-styrylimidazo[1,2-a]pyridin-3-y1)-N-methylformamide.
[00557] LC-MS: MW (calcd): 355 (79Br), 357 ("Br); m/z (obsd): 356 (79Br M+1),
358 ("Br M+1)
Step iii)
0
\ BrN
NH
H Br
[00558] Deformylation of Intermediate Gen-3-g is performed according to method
D1 to give
Intermediate Gen-4-f (E)-6-bromo-N-methyl-2-styrylimidazo[1,2-a]pyridin-3-
amine.
[00559] LC-MS: MW (calcd): 327 (79Br), 329 ("Br); m/z (obsd): 328 (79Br M+1),
330 ("Br M+1)
148
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step iv)
\N¨\ NH c
[00560] Intermediate Gen-4-f is reacted with 2-bromo-1-(4-fluorophenyeethanone
according to
general method El to lead to Intermediate Gen-5-p (E)-N-(6-bromo-2-
styrylimidazo[1,2-a]pyridin-3-y1)-
4-(4-fluoropheny1)-N-methylthiazol-2-amine.
[00561] LC-MS: MW (calcd): 504 (79Br), 506 (81Br); m/z (obsd): 505 (79Br M+1),
507 (81Br M+1)
Step v)
\N i)¨s \Ns ii)
\N¨
Br
N \ OH B
HO
Gen-5-p Gen-5-ab Gen-5-p
Step i)
[00562] To a solution of Intermediate Gen-5-p (16.2 g, 32.1 mmol, 1 eq.) in
DCM (500 mL) cooled at
3 C was added Osmium tetroxide (in t-BuOH, 14.6 g, 1.44 mmol, 0.045 eq.). N-
methylmorpholine-4-
oxide (8.6 g, 63.6 mmol, 2 eq.) was added and the reaction kept stirring.
After 20 min an additional
portion of N-methylmorpholine-4-oxide (4.3 g, 31.8 mmol, 1 eq.) was added,
this operation was
performed 7 times (until complete conversion of starting material was
observed). The reaction was
quenched by addition of water (500 mL). Layers were separated and the aqueous
layer was extracted with
DCM (2 x 100 mL). The combined organic layers were dried over sodium sulfate,
filtered and
concentrated. The crude product was purified by chromatography on silica gel
(elution with DCM/Me0H
99.5/0.5 to 95/5) to give Intermediate Gen-5-q (6-bromo-344-(4-
fluorophenyl)thiazol-2-
y1)(methypamino)imidazo[1,2-a]pyridine-2-carbaldehyde) and Gen-5-ab 1-(6-bromo-
3-((4-(4-
fluorophenyethiazol-2-y1)(methypamino)imidazo [1,2- a] pyridin-2-y1)-2-
phenylethane-1,2- diol.
[00563] Gen-5-q LC-MS: MW (calcd): 430 (79Br), 432 (81Br); m/z (obsd): 431
(79Br M+1), 433 (81Br
M+1)
[00564] Gen-5-ab LC-MS: MW (calcd): 538 (79Br), 540 (81Br); m/z (obsd): 539
(79Br M+1), 541 (81Br
M+1)
149
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step ii,)
[00565] A solution of 1-(6-bromo-3-44-(4-fluorophenyl)thiazol-2-
y1)(methypamino)imidazo[1,2-
alpyridin-2-y1)-2-phenylethane-1,2-diol (Gen-5-ab) (10.8 g, 20 mmol, 1 eq.) in
DCM (500 mL) was
cooled to -4 C. Lead tetraacetate (dried before use, 13.3 g, 30 mmol, 1.5 eq.)
was added and stirred at -
4 C for 0.5 h. The reaction was quenched by addition of water (500 mL). The
mixture was filtered and
the layers in the filtrate were separated. The aqueous layer was extracted
with DCM (2 x 200 mL). The
combined organic layers were dried over sodium sulfate, filtered and
concentrated. The crude product
was purified by chromatography on silica gel (elution with heptane/Et0Ac 100/0
to 50/50) to give an
additional amount of Intermediate Gen-5-q 6-bromo-344-(4-fluorophenyl)thiazol-
2-
yl)(methyl)amino)imidazo pyridine-2-carb aldehyde.
[00566] LC-MS: MW (calcd): 430 (79Br), 432 (giBr); m/z (obsd): 431 (79Br M+1),
433 (giBr M+1)
Step iii)
/ N
N I0 iii)
0, // N
=N
Gen-5-q Gen-5-ac
[00567] Sodium hydride (60% in oil suspension, 55.2 mg, 1.38 mmol, 1.15 eq.)
was added to a
solution of diethyl cyanomethylphosphonate (234 mg, 1.32 mmol, 1.1 eq.) in
anhydrous THF (5 mL). The
resulting white suspension was stirred for 10 min at room temperature and then
cooled to -78 C.
Intermediate Gen-5-q (518 mg, 1.2 mmol, 1 eq.) dissolved in anhydrous THF (10
mL) was added with a
syringe pump at a rate of 60 mL/h. The mixture was stirred for 0.5 h at -78 C,
then allowed to warm to
room temperature and stirred for additional 20 min. The reaction mixture was
quenched by addition of
water (100 mL) and extracted with Et0Ac (3 x 30 mL). The organic layers were
combined, dried over
sodium sulfate, filtered and concentrated. The crude product was purified by
chromatography on silica gel
(elution with heptanes/Et0Ac 70/30 to 65/35) to give 3-(6-bromo-34(4-(4-
fluorophenyl)thiazol-2-
y1)(methyl)amino)imidazo[1,2-a]pyridine-2-yl)acrylonitrile (Gen-5-ac).
[00568] LC-MS: MW (calcd): 453 (Br), 455 (8113r); m/z (obsd): 454 (7913r M+1),
456 (8113r M+1)
150
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step vii)
N
\ 0 -1µ1
S-
L
BrN-0 >c, I õL(
-N" =-=
- N
_________________ N
¨N
[00569] Suzuki coupling of 3-(6-
bromo-34(4-(4-fluorophenypthiazol-2-
y1)(methyl)amino)imidazo[1,2-a]pyridine-2-yl)acrylonitrile (Gen-5-ac) with
tert-butyl 444,4,5,5-
tetramethy1-1,3,2-dioxaborol an-2-y1)-5,6-dihydropyridine-1(2H)-carboxylate
according to general
method F2 afforded tert-butyl 4-(2-
(2-cyanoviny1)-3-((4-(4-fluorophenyl)thiazol-2-
y1)(methypamino)imidazo[1,2-a]pyridine-6-y1)-5,6-dihydropyridine-1(2H)-
carboxylate.
[00570] LC-MS: MW (calcd): 556; MW (obsd): 557 (M+1)
Step viii)
11101
Si(N¨ S
\-1), ___________________
[00571] Reduction of the latter compound is performed according to general
method F6 in presence of
a catalytic amount of acetic acid to give tert-butyl 4-(2-(2-cyanoethyl)-34(4-
(4-fluorophenypthiazol-2-
y1)(methypamino)imidazo[1,2-a]pyridine-6-yepiperidine-1-carboxylate.
[00572] LC-MS: MW (calcd): 560; m/z (obsd): 561 (M+1)
Step ix)
0 /N /N
A
sAN¨ 1-11\1-'s S
N4>
=N =N
[00573] Boc deprotection of the latter compound is performed according to
general method F5b to
afford Intermediate Gen-10-aa.
[00574] LC-MS: MW (calcd): 460; m/z (obsd): 461 (M+1)
151
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step x)
110
/N I /
H N
õNli/a ______________________________
0 0
11-4>
=N =N
[00575] Intermediate Gen-10-aa is alkylated with 2-chloro-N,N-
dimethylacetamide using general
method F8 to give the expected compound 174.
2.30. Compound 175: 3-(3-(14-(4-fluoropheny1)-5-(hydroxymethyl)thiazol-2-
y1)(methyl)amino)-6-(1-
(methylsu1fony0piperidin-4-y0imidazo[1,2-a]pyridin-2-y1)propanenitrile
Step I)
=
/1N
/ N
HN S
N S
N
/ =N
[00576] Intermediate Gen-10-aa is sulfonylated with methanesulfonyl chloride
using general method
Fll to give 3 -(3 -((4-(4- fluorophenyethiazol-2-y1)(methyl) amino)-6- (1 -
(methylsulfonyl)piperidin-4 -
yl)imidaz o [1,2-a]pyridin-2-yepropanenitrile.
[00577] LC-MS: MW (calcd): 538; m/z (obsd): 539 (M+1)
Step ii:
HO
0 / N 0 / N
0, 0
'S,N 0- S
/ N / N N
/ ___________________________ =N / __ =N
[00578] The latter compound is reacted under conditions of general method F14
to give the expected
compound 175.
152
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
2.31. Compound 176: 3-(6-(1-(2-(dimethylamino)-2-oxoethyl)piperidin-4-
y1)-344-(4-
fluorophenyl)thiazol-2-y1)(methyl)amino)irnidazo[1,2-akyridin-2-Apropanamide
Step i)
/ N
N
N 0 0
0,
0 BrNJ
KC)
0
[00579] Sodium hydride (60% in oil suspension, 92 mg, 2.3 mmol, 1.15 eq.) was
added to a solution of
methyl 2-(dimethoxyphosphoryl)acetate (401 mg, 2.2 mmol, 1.1 eq.) in anhydrous
THF (20 mL). The
resulting white suspension was stirred for 20 min at room temperature and then
cooled to -78 C.
Intermediate Gen-5-q (863 mg, 2.0 mmol, 1 eq.) dissolved in anhydrous THF (15
mL) was added with a
syringe pump at a rate of 45 mL/h. The mixture was stirred for 50 min at -78
C, then allowed to warm to
room temperature and stirred for additional 40 min. The reaction mixture was
evaporated. Brine (50 mL)
was added to the crude product and the mixture was extracted with Et0Ac (3 x
50 mL). The organic
layers were combined, dried over sodium sulfate, filtered and concentrated.
The crude product was
purified by chromatography on silica gel (elution with heptanes/Et0Ac 100/0 to
50/50) to give the
Intermediate Gen-5-ad.
[00580] LC-MS: MW (calcd): 486 (79Br), 488 (giBr); m/z (obsd): 487 (79Br M+1),
489 (giBr M+1)
Step ii)
0
- 0 N 0 ,
-0\
S-
N-
13N 07\
N __
0 0
[005811 Suzuki coupling of methyl 3-(6-bromo-34(4-(4-fluorophenyfithiazol-2-
y1)(methyl)amino)imidazo[1,2-a]pyridin-2-yl)acrylate with tert-butyl 4-
(4,4,5,5-tetramethy1-1,3,2-
dioxaborol an-2-y1)-5,6-dihydropyridine-1(2H)-carboxylate according to general
method F2 afforded tert-
butyl 4-(3 - ((4-(4 -fluorophenyl)thiazol-2-y1) (methyl)amino)-2- ( 3 -methoxy-
3-oxoprop-1 -enyeimidazo [1,2-
a] pyridin-6-y1)-5,6- dihydropyridine-1(2H)-carb oxylate.
[00582] LC-MS: MW (calcd): 589; MW (obsd): 590 (M+1)
153
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Step iii)
>, 0 1 /s-P( N
N- 0 N N-
N e
0 0
[00583] Reduction of the latter compound is performed according to general
method F6 in presence of
a catalytic amount of acetic acid to give tert-butyl 4-(34(4-(4-
fluorophenyl)thiazol-2-y1)(methyDamino)-
2-(3-methoxy-3-oxopropypimidazo[1,2-a]pyridin-6-yOpiperidine-1-carboxylate.
[00584] LC-MS: MW (calcd): 593; (obsd): 594 (M+1)
Step iv)
11110 110
/S-1ZN-- /S-11(N--
0 OH
[00585] The latter compound is saponified according to general method F13 to
afford 3-(6-(1-(tert-
butoxycarbonyl)piperidin-4-y1)-344-(4-fluorophenyl)thiazol-2-
y1)(methyl)amino)imidazo[1,2-a]pyridin-
2-yl)propanoic acid.
[00586] LC-MS: MW (calcd): 579; (obsd): 580 (M+1)
Step v)
110
0 / /
0-Jt`N S0)1- N
e
OH NH2
[00587] A solution of 3-(6-(1-(tert-butoxycarbonyl)piperidin-4-y1)-344-(4-
fluorophenypthiazol-2-
y1)(methypamino)imidazo[1,2-a]pyridin-2-yl)propanoic acid (220 mg, 0.38 mmol,
1 eq.) and TEA (211
[iL, 1.52 mmol, 4 eq.) in DMF (3 mL) was stirred for 10 mm at r.t.. Propane
phosphonic acid anhydride
154
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
(226 nt, 0.76 mmol, 2 eq.) followed by ammonium chloride (40.7 mg, 0.76 mmol,
2 eq.) were added and
the resulting mixture was stirred at r.t. for 4 days. TEA (211 !IL, 1.52 mmol,
4 eq.), propane phosphonic
acid anhydride (226 L, 0.76 mmol, 2 eq.) and ammonium chloride (40.7 mg, 0.76
mmol, 2 eq.) were
added and the reaction mixture kept stirring for 2 h. Brine was added (100
niL) and the mixture was
extracted with DCM (3 x 20 mt.). The organic layers were combined, dried over
sodium sulfate, filtered
and concentrated. The crude product was purified by chromatography on silica
gel (elution with
DCM/Me0H 100/0 to 80/20) to give tert-butyl 44243 -a mi no-3 - oxop ropy1)-3
4(444 -
fluorophenyethiazol-2-y1)(methypaminolimidazo [1,2- a] pyridin-6-yl)pip
eridine-1 -carb oxylate (Gen-10-
am).
[00588] LC-MS: MW (calcd): 578; m/z (obsd): 579 (M+1)
Step vi)
,
0
HN S-
0 Nil I
N
N
\ NH2 NH2
[00589] Boc deprotection of the latter compound is performed according to
general method F5b to
afford 3-(34(4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)-6-(piperidin-4-
yl)imidazo[1,2-a]pyridin-2-
yl)propanamide.
[00590] LC-MS: MW (calcd): 478; m/z (obsd): 479 (M+1)
Step vii)
I.
/N NO /N
HN s
N
N,2 ______________________________________________________ N,2
[00591] The latter compound was alkylated with 2-chloro-N,N-dimethylacetamide
using general
method FS to give the expected compound 176.
155
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
2.32. Compound 184: ethyl 2-(3-(34(4-(4-chlorophenyOthiazol-2-
y1)(methyl)amino)-2-
ethylirnidazo[1,2-alpyridin-6-yl)-2-oxonnidazolidin-1-yOacetate
CI
N
N N
\-0 N4
0
0
[00592] To a solution of Gen-10-a (0.30 g, 0.066 mmol, 1 eq.) in 3 mL of DMF
at r.t. was added NaH
60% in oil (0.004 g, 0.099 mmol, 1.5 eq.). The reaction mixture was stirred 45
min at r.t. then ethyl
bromo acetate (0.009 mL, 0.079 mmol, 1.2 eq.) was added. The reaction mixture
was stirred at r.t. for 1 h.
The reaction mixture was quenched with a saturated solution of ammonium
chloride. The aqueous phase
was extracted with AcOEt. The combined organic layers were washed with a
saturated solution of sodium
carbonate, with brine, dried over Na2SO4, filtered and concentrated in vacuo.
The crude product was
purified by chromatography on silica gel (elution with Heptane/AcOEt: 100/0 to
50/50) to afford
Compound 184.
[00593] tH NMR (400 MHz, Me0D) 6 ppm 8.39 - 8.28 (1 H, m), 7.84 (2 H, d), 7.71
(1 H, dd), 7.57 (1
H, dd), 7.40 - 7.33 (2 H, m), 7.00 (1 H, s), 4.18 (2 H, q), 4.01 (2 H, s),
3.93 - 3.86 (2 H, m), 3.67 - 3.57 (5
H, m), 2.72 (2 H, q), 1.31 (3 H, t), 1.25 (3 H, t)
[00594] LC-MS: MW (calcd): 538 (35C1), 540 (37C1); m/z MW (obsd): 539 (3'C1
M+1), 541 (37C1 M+1)
2.33. Compound 186: 2-(24(2-ethyl-6-(1-(inethylvulfonyl)piperidin-4-
Aimidazo[1,2-alpyridin-3-
yl)(methyl)amino)-4-(4-fluorophenyl)thiazol-5-yl)acetonitrile
0
S,
0 CN
S
N
/F
0 0
-S \ ,01-1 0' 'N
- L
\ \
[00595] To a solution of compound 147 (50 mg, 0.092 mmol, 1 eq.) with
triphenyl phosphine (60 mg,
0.23 mmol, 2.5 eq.) in THE (1.5 mL) at 0 C were added acetone cyanohydrine (23
mg, 0.27 mmol, 3 eq.)
156
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
and DEAD (40 mg, 0.23 mmol, 2.5 eq.). The reaction mixture was stirred at 0 C
for 3 h, then quenched
by addition of a saturated NaHCO3 solution and Et0Ac. The organic layer was
washed with a saturated
NaHCO3 soltution, water, and brine, dried over Na2SO4, filtered and
concentrated in vacuo. The crude
product was purified by preparative HPLC to afford Compound 186.
[00596] ILI NMR (400 MHz, CDC13) 6 ppm 7.64 - 7.57 (4 H, m), 7.22 - 7.14 (3 H,
m), 3.97 (2 H, d),
3.72 (2 H, d), 3.56 (3 H, s), 2.85 - 2.72 (7 H, m), 2.71 - 2.60 (1 H, m), 2.06
- 1.95 (2 H, m), 1.86 (2 H,
qd), 1.37(3 H, t)
[00597] LC-MS: MW (calcd): 552; m/z (obsd): 553 (M+1)
2.34. Compound 187: 2-ethyl-N-(4-(4-fluorophenyl)pyrimidin-2-y1)-N-methyl-6-(1-
(methylsulfony1)-
1,2,3,6-tetrahydropyridin-4-Aimidazo[1,2-alpyridin-3-amine
0 0
. N-
S j
`N
0, PO
,,
S N NH S \
N '
[00598] To Intermediate Gen-9-a (90 mg, 0.27 mmol, 1 eq.), 2-chloro-4-(4-
fluoropheny1)-pyrimidine
(63 mg, 0.30 mmol, 1.1 eq.), cesium carbonate (0.177 g, 0.54 mmol, 2 eq.),
Xantphos (0.008 g, 0.014
mmol, 0.05 eq.) and palladium acetate (0.003 g, 0.009 mmol, 0.03 eq.) in a
sealed tube under nitrogen
was added degazed dioxane. The reaction mixture was heated at 130 C overnight.
After cooling to r.t.,
the reaction mixture was quenched with water. The aqueous phase was extracted
with AcOEt three times.
The combined organic layers were washed with brine, dried over Na2SO4,
filtered and concentrated in
vacuo. The crude product was purified by chromatography on silica gel (elution
with DCM/MeOH: 100/0
to 95/5) to afford Compound 187.
[00599] 11-1 NMR (300 MHz, CDC13) 6 ppm 8.41 (1 H, d), 8.05 - 7.90 (2 H,
m), 7.65 - 7.42 (2 H, m),
7.28 (1 H, dd), 7.13 (3 H, d), 6.03 (1 H, t), 4.00 - 3.87 (2 H, m), 3.62 (3 H,
s), 3.47 (2 H, t), 2.82 (3 H, s),
2.73 (2 H, q), 2.64 - 2.43 (2 H, m), 1.28 (3 H, t)
[00600] LC-MS: MW (calcd): 506; m/z MW (obsd): 507 (M+1)
157
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
2.35. Compound 202 : (1-aminocyclopropyl)(4-(2-ethyl-3-((4-
(47fluorophenyl)thiazol-2-
y1)(nzethyl)amino)ililidazo[1,2-cdpyridin-6-Apiperazin-l-yOmethanone
N N
______________________________________ 3.-
HN.,) Fnnoc.N Kir N
0
Step (
[00601] Intermediate Gen-10-e was reacted with Fmoc-1-amino-l-cyclopropane
carboxylic acid
according to general synthetic method F9a to give Fmoc-protected derivative.
Step ii)
N N
________________________________________ 3.-
Fmoc N N
H2N
0 0
[00602] To a solution of Fmoc-protected derivative obtained in step i) above
(66 mg, 0.089 mmol, 1
eq.) in a mixture of DCM/DMF (2/4 mL) were added pyridine (100 pi, excess.)
then morpholine (78 1AL,
0.89 mmol, 10 eq.). The reaction mixture was stirred at r.t. for 20 h, then
diluted with water and Et0Ac.
The aqueous layer was extracted with Et0Ac, then the organic layer was washed
with water, and brine,
dried over Na2SO4, filtered and concentrated in vacuo. The crude product was
purified by
chromatography on silica gel (elution with DCM/MeOH: 100/0 to 98/2) to afford
Compound 202.
[00603] 1H NMR (300 MHz, CDC13) ppm 7.86 (2 H, dd), 7.54 (1 H, d), 7.24 - 7.04
(4 H, m), 6.68 (1
H, s), 3.84 (4 H, bt), 3.61 (3 H, s), 3.04 (4 H, bt), 2.73 (2 H, q), 1.33 (3
H, t), 1.07 - 0.99 (2 H, m), 0.86 -
0.77 (2 H, m)
[00604] LC-MS: MW (calcd): 519; m/z (obsd): 520 (M+1)
158
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
2.36. Compound 204: 2-(4-
(2-ethyl-3-((4-(4-fluorophenyl)thiazol-2-y1)(methyl)amino)imidazo[1,2-
alpyridin-6-Apiperazin-1-y1)-2-oxoacetamide
Step i)
F, F-,r;
N
r- 0 r N
N N)
HO' H2N-
0 0
[00605]
Intermediate Gen-10-e was reacted with chloro-oxo-acetic acid ethyl ester
following successive general synthetic methods F9b and F13 to give [4-(2-ethyl-
3-{[4-(4-fluoro-
phenyl)-thiazol-2-y1]-methyl-amino -imidazo [1 ,2-a]pyridin-6-y1)-piperazin-1 -
y1]-oxo-acetic acid
(Intermediate Gen-10-an).
FAN, F
N
-N
-N
\
N - N
====-/
I
0 r-N
H 0 -iLyN
H2N)YNJ
0 0
[00606] To a solution of Intermediate Gen-10-an (24 mg, 0.047 mmol, 1 eq.) in
DCM (3 mL) and THF
(2 mL) were added HOBT (8 mg, 0.057 mmol, 1.2 eq.) and EDC.HC1 (10 mg, 0.075
mmol, 1.1 eq.). The
reaction mixture was stirred at r.t. for 1 h then a solution of ammoniac 7 N
in Me0H (3 drops) was added.
The reaction mixture was stirred at r.t. for 3 h, then filtered on celite and
the filtrate was concentrated in
vacuo. The residue was purified by preparative HPLC to afford Compound 204.
[00607] 11-1
NMR (400 MHz, CDC13) 6 ppm 7.87 - 7.81 (2 H, m), 7.53 (1 H, dd), 7.20 (1 H,
d), 7.14 -
7.05 (3 H, m), 7.04 (1 H, bs) NH, 6.66 (1 H, s), 5.59 (1 H, bs) NH, 4.24 (2 H,
t), 3.80 (2 H, t), 3.59 (3 H,
s), 2.72 (2 H, q), 3.11 - 3.05 (4 H, m), 1.33 (3 H, t)
[00608] LC-MS: MW (calcd): 507; m/z MW (obsd): 508 (M+1)
2.37. Compound 211: 1-(2-ethyl-3-((4-(4fluorophenyl)thiazol-2-
y1)(methyl)amino)imidazo[1,2-
akyridin-6-Apiperidin-4-ol
Step (
CO
0_0
N-"""c
.1\1
159
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00609] Intermediate Gen-5-c is reacted under conditions of general method Fib
with 1,4-Dioxa-8-
aza-spiro [4.5] decane to give the [6-(1,4-Dioxa-8-aza-spiro [4.5] dec-8 -y1)-
2- ethyl- imidazo pyridin-3 -
y11- [4-(4-fluoro-phenyl)-thiazol-2-yll -methyl-amine (Intermediate Gen-10-ap)
Step U.)
CO 0
0
¨3. UN,"
N
[00610] To as solution of Intermediate Gen-10-ap (162 mg, 0.33 mmol, 1 eq.) in
a THF/water (1/1)
mixture (1 mL) was added HC1 (6 N in water, 1 mL). The reaction mixture was
stirred at 60 C overnight.
Solvents were evaporated, then aqueous sodium carbonate was added and the
mixture was extracted with
ethyl acetate. Combined organic layers were washed with water, dried over
Na2SO4, filtered and
concentrated. The crude product was purified by chromatography on silica gel
(elution with
heptane/Et0Ac: 100/0 to 0/100) to afford Intermediate Gen-10-aq
[00611] LC-MS: MW (calcd): 449; m/z (obsd): 450 (M+1)
Step id)
F
0 HO
Th N1 ¨1¨\\
N .s,
S
\>
=
'N \
[00612] To a
solution of Intermediate Gen-10-aq (70 mg, 0.16 mmol, 1 eq.) in ethanol (0.5
mL)
stirred at 0 C was added sodium borohydride (5 mg, 0.12 mmol, 0.8 eq.). The
reaction was stirred
overnight allowing the temperature to rise to room temperature. Solvent was
evaporated, aqueous
ammonium chloride was added and the mixture was extracted with ethyl acetate.
The combined organic
layers were dried over sodium sulfate, filtered and evaporated. Compound 211
was then obtained by
purification by preparative LC-MS.
[00613] LC-MS: MW (calcd): 451; m/z (obsd): 452 (M+1)
2.38. Compound 222: {2-[4-(2-Ethyl-3-{14-(4-fluoro-phenyl)-thiazol-2-yli-
methyl-aminol-imtdazo[1,2-
alpyridin-6-y1)-ptperazin-1-y1J-4,5-dihydro-oxazol-5-y1}-methanol
c":7
HO /¨N
\ /11
HN
//
I
1¨
N N
160
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
[00614] A solution of Intermediate Gen-10-c (300 mg, 0.69 mmol, 1 eq.), 4-
(chloromethyl)-1,3-
oxazolidin-2-one (103 mg, 0.76 n-nnol, 1.1 eq.), Cs2CO3 (450 mg, 1.38 mmol, 2
eq.) and NaI (103 mg,
0.69 mmol, 1 eq.) in DMF (3 mL) was stirred at 90 C overnight. Water was added
and the reaction
mixture was extracted with Et0Ac. The combined organic layers were dried over
sodium sulfate, filtered
and evaporated. The crude product was purified by chromatography on silica gel
(elution with
DCM/MeOH: 100/0 to 90/10) and preparative HPLC to give Compound 222.
[00615] LC-MS: MW (calcd): 534; m/z (obsd): 535 (M+1)
2.39. Compound 227: Cyclopropyl-[4-(2-ethy1-3-1[4-(4-fluoro-pheny1)-thiazol-2-
y11-methyl-amino}-
inzidazo[],2-cdpyridin-6-y1)-piperazin-1-y11-methanone
F,
-N N,
s s'
HN
N
r
N A
0
[00616] To a solution of Gen-10-e (200 mg, 0.423 mmol, 1 eq.) in DCM (3 mL)
were added TEA (294
mg, 2.114 mmol, 5 eq.) followed by the 4-Bromo-butyryl chloride (73 pt, 0.634
n-nnol, 1.5 eq.). The
reaction mixture was stirred at r.t. overnight, then was quenched with water
and the aqueous layer was
extracted with DCM twice. The organic layer was washed with water, and brine,
dried over Na2SO4,
filtered and concentrated in vacuo. The residue was purified by chromatography
on silica gel (elution
with DCM/MeOH: 100/0 to 97/3) to afford the Compound 227.
[00617] LC-MS: MW (calcd): 504; m/z MW (obsd): 505 (M+1)
2.40. Compound 229: [6-(1,1-Dioxo-isothiazolidin-2-y1)-2-ethyl-imidazo[1,2-
a]pyridin-3-y11-[4-(4-
fluoro-pheny1)-thiazol-2-y1]-rnethyl-amine
Step i)
N N
S N
I-12N
[00618] To a a solution of ammonium chloride (440 mg, 8.23 mmol, 4 eq.) in
water (10 mL) were
added a solution of Gen-5-z (818 mg, 2.058 mmol, 1 eq.) in Me0H (5 mL) and THF
(5 mL), followed by
iron (460 mg, 8.23 mmol, 4 eq.). The reaction mixture was stirred at 70 C for
3 h. The solvents were
evaporated. The residue was resuspended/dissolved in Et0Ac/water, this mixture
was filtered over Celite
prior to separation. The separated aqueous phase was extracted once more with
Et0Ac, The combined
161
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
organic phases were dried over Na2SO4, filtered and concentrated in vacuo. The
residue was purified by
chromatography on silica gel (elution with DCM/MeOH: 100/0 to 95/5) to afford
Intermediate Gen-5-af.
[00619] LC-MS: MW (calcd): 367; m/z MW (obsd): 368 (M+1)
Step
F-
7N / n,/
\
¨S
.N :N
0, ,9
sK
1-1,N1
[00620] Intermediate Gen-5-af was reacted with 3-chloro-propane- 1 -
sulfonyl chloride using
general method Fll to give Intermediate 3-Chloro-propane-1-sulfonic acid (2-
ethy1-3-1[4-(4-fluoro-
pheny1)-thiazol-2-y1]-methyl-amino}-imidazo[1,2-a]pyridin-6-y1)-amide
(Intermediate Gen-10-ao).
[00621] LC-MS: MW (calcd): 507 (35C1), 509 (37C1); m/z MW (obsd): 508 (3'Cl
M+1), 510 (37C1 M+1)
Step iii)
F. F
N
/
N N
1
p
j
N
[00622] To a solution of the the chlorine derivative Gen-10-ao (55 mg,
0.108 mmol, 1 eq.) in
DMF (2 mL) was added potassium acetate (32 mg, 0.325 mmol, 3 eq.), the
reaction mixture was heated at
90 C for 1 h, then at 60 C overnight. After cooling, water and Et0Ac were
added to the reaction mixture,
the aqueous layer was extracted with Et0Ac twice. The combined organic layers
were washed with water
and brine, dried over Na2SO4, filtered and concentrated in vacua. The residue
was purified by
chromatography on silica gel (elution with DCM/MeOH: 100/0 to 95/5) to afford
Compound 229.
[00623] LC-MS: MW (calcd): 471; m/z MW (obsd): 472 (M+1)
2.41. Intermediate Gen-13-j : N-11-(2-Chloro-acety1)-pyrrolidin-3-yll-
acetamide
0\\
HN
HO-1) ______________________________________
NH CI
'CI
[00624] To a solution of chloroacetic acid (0.715 g, 7.56 mmol, 1 eq.) in DCM
(15 mL) and THF (12
mL) at r.t. was added EDC.HC1 (1.89 g, 9.83 mmol, 1.3 eq.) and HOBt (1.33 g,
9.83 mmol, 1.3 eq.). The
reaction mixture was stirred at r.t. for 30 mm and then 3-acetamidopyrrolidine
(1.26 g, 9.83 mmol, 1.3
162
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
eq.)was added. After stirring two days at r.t., the reaction mixture was
diluted with water, HC1 1 M, and
DCM. The aqueous phase was extracted with DCM five times. The combined organic
layers were washed
with a saturated NaHCO3 solution, with brine, dried over Na2SO4, filtered and
concentrated in vacuo.
The resulting crude oil was used as such in the next step.
[00625] 11-1 NMR (400 MHz, CDCL-d) i ppm 5.33 - 5.01 (2 H, m), 4.60 - 4.41
(1 H, m), 3.79 - 3.65 (2
H, m), 3.63 - 3.44 (2 H, m), 1.97 (3 H, d), 1.90 - 1.80 (2 H, m)
[00626]
2.42. Intermediate Gen-11-d: 2-Brorno-1-(4-fluoro-pheny1)-d2 ethanone
Br
[00627] A mixture of starting material (Kolonko & Reich, 2008) (1.04 g, 7.37
mmol, 1 eq.) and Br2
immobilized on resin used in large excess in deuterated chloroform was shaken
overnight at r.t.. The resin
was filtered off, washed with deuterated chloroform. The filtrate was
concentrated in vacua and the crude
product was used directly in the next step.
Table I. Intermediates used
towards the compounds of the invention.
Int - MS
Int Structures Name MW
Mtd Ms'd
191
BrN 190 (79Br
Gen-
2-amino-5-Bromo-3-
A (79Br), M+1),
1-a fluoropyridine 192 193
(81131") (81Br
M+1)
190
N
Gen- 5-Bromo-4-fluoro- (79
I A Br)192 ,
N.A.
1-b NH2 pyridin-2-ylamine
(9 'Br)
o 286
N-(6-Bromo-2-ethyl-8- 285 (79Br
Gen- Br, H fluoro-
imidazo[1,2- Gen-1-a (79Br), M+1),
2-a
N a]pyridin-3-y1)- B1 287 288
formamidc (81Br)
(81Br
M+1)
268
0 267 C9Br
N-(6-Bromo-2-ethyl-
Gen- (79Br), M+1),
2-b H imidazo[1,2-a]pyridin- B1
269 270
3-y1)-formamide
("Br) ("Br
M+1)
163
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
0
1111--k N-(2-Ethy1-6-iodo-
Gen- 316
I ,...__,....--.., imi dazo [-a] pyr 1,2idin- BI 315
2-c --- N"--4) H (M+1)
-"--N \ 3 -y1)- form ami de
S'42-
0
H // N-(6-Bromo-2- ethyl-8 - 281
N-----\ 284
H
Gen- Br..N_-- methyl-imidazo [1,2- (79Br),
(81Br
B1 or B2
2-d )--,- \ a] pyridin-3 -y1)- 283
'y N (81B0 1\4+1)
formamide
280
o N-(6-Bromo-2- 279
(79Br
r.11(
Gen- --1 cyclopropyl- (79Br), M+1),
Br,ct,.., ..ii_<II B2
2-e imidazo [1,2-a] pyridin- 281 282
''' ----N 3 -y1)- formamide (81Br)
(81Br
M+1)
0
N-[6-Bromo-2-(2,2,2- 321
Gen- H trifluoro-ethyl)- (79Br),
Bro..j, .......,c B1 N.A.
2-f imidazo [1,2-a] pyridin- 323
N'' N CF, 3 -yll - formamide (81Br)
0
V( (E)-N-(6-bromo-2- 341
Gen- Br...,N \ H styrylimidazo [1,2-
B1 (79Br),
N.A.
2-g a] pyridin-3 -y1)- 343
N \ formamide (81Br)
282
0
IV( H N-(6-Bromo-2-ethyl-7- 281 (79Br
Gen- methyl-imidazo [1,2- (79Br),
M+1),
2-h 13r,,,,-,N,
a] pyridin-3 -y1)- B1
283 284
=S'',)-.:'-"--N \ formamide (81Br)
(81Br
M+1)
286
0
r\ILAH N-(6-Bromo-2- ethyl-7- 285 (79Br
Gen- flu oro-imi dazo [1,2- Gen- 1-b
(79Br), M+1)
2-i
Br - . . . N 6
formamide a] pyridin-3 -y1)- B1 287 288
Fr--- \ (81B0 (81Br
M+1)
0
kill( N-(2-Ethyl-6-nitro-
Gen- H 235
2-j 02NN, imidazo [1,2-al pyridin- B1 234
(M+1)
-'N \ 3 -y1)- formamide
'S-,---
0 H 2-Ethyl-3-formylamino- No
0
Gen- Jt !\I ¨ H imidazo [1,2-a] pyridine-
B1 247 LC-
2-k o --% NI- 6-carboxylic acid MSN
-N methyl ester MR
o 300
\ N --I( N-(6-Bromo-2-ethyl-8- 299 (79Br
Gen- Br.µ,,..,-,N...i H fluoro-imi dazo [1,2- Gen-2-a
(79Br), M+1),
3-a a]pyridin-3-y1)-N- C2 301 302
Y-1\11 \
methyl-formamide (81Br) (81Br
F M+1)
164
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
282
0
\ N
N -(6-Bromo-2- ethyl- 281 ("Br
--14
Gen- imidazo [1,2-a] pyridin- Gen-2-6 ("Br),
M+1),
3-b Br. N___. H
3-y1)-N-methyl- Cl 283 284
formamide (81Br) (81Br
M+1)
o N-(6-Bromo-2-
ethyl- 284
CD; //
N----\
Gen- imidazo [1,2-a] pyridin- Gen-2-6 ("Br),
N.A.
3-c Br .,,,N___%' H
3 -y1)-N-(c/3-methyl)- Cl 286
formamide ("Br)
o N-(2-Ethy1-6-iodo-
N - \
Gen- 1, ___( H i mi dazo [1 ,2-a]pyridi n- Gen-
2-c 329 330
3-d -N ) 3-y1)-N-methyl- Cl (M+1)
formamide
296
o
1 -41 N-(6-Bromo-2-ethyl-8- 295 ("Br
N Gen- Br ,..,-.N_- - methyl-imidazo [1 ,2- Gen-2-d
("Br), M+1),
3-c
"1-''''Ns \ a] pyridin-3 -y1)-N- C2 297 298
methyl-formamide (81Br) ("Br
M+1)
294
O N-(6-Bromo-2-
293 (79Br
\ N---k cyclopropyl-
Gen- Gen-2-e (79Br ), M+1),
3-f N_ H imidazo [1 ,2-a] pyridin-
C 1 295 296
3-y1)-N-methyl-
s,-)z-----""N (81B1") (81Br
formamide
M+ 1)
356
o
µ // (E)-N-(6-bromo-2- 355 (79Br
N- \
Gen- Br ,,, I H
styrylimidazo [1,2- Gen-2-g ("Br), M+1),
3-g \\ ¨ a]pyridin-3-y1)-N- Cl 357 358
% methylformamide (81Br) (81Br
M+1)
0
310
N --k N-(6-Bromo-2- ethyl-8- 309 (79Br
Gen- H methyl-imidazo [1,2- Gen-2-d (79B
r),3 M+1),
Br...,Nr....
3-h a] pyridin-3 -y1)-N-ethyl- C2 11 312
formamidc (81Br) (81Br
M+1)
296
0
NH N-(6-Bromo-2-ethyl-7- 295 (79Br
Gen- H methyl-imidaz o [1 ,2- Gen-2-h (79B
r),2 M+1),
3-i Br,.,--,N,c
a] pyridin-3 -y1)-N- Cl 97 298
--)-"N \ methyl-formamide (Thr) ("Br
M+1)
0 300
\ p
N----\ N-(6-Bromo-2-ethyl-7- 299 (79Br
Gen- H fluoro-imidazo [1,2- Gen-2-i (79B
r),3 M+1),
3-j
a] pyridin-3 -y1)-N- Cl 01 302
methyl-formamide (81Br) (81Br
M+1 )
0 N-(2-Ethy1-6-nitro-
\ N--k
Gen- H imidazo [1 ,2-a.] pyrid in- Gen-2-j 249
3-k 02N. ,,.,..,N_..._
3-y1)-N-methyl- C2 248
(M+1)
formamide
165
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
o 2-Ethy1-3-(formyl-
o
N-----' methyl-amino)-
Gen- 11 H imidazo [1,2-al pyridine- Gen-2-k
261 262
3-1 'o ' '¨' 'Isl- C1 (M+1
,i- 7---\ 6-carboxylic acid
methyl ester
272
\
NH (6-Bromo-2-ethyl-8- 271 (79Br
Gen- Br-,,N...._ fluoro-imidazo [1,2- Gen-3-a (79B
r),2 M+1),
4-a yl---11 \ a] pyridin-3 -y1)-methyl- D1
73 274
amine (81Br) (81Br
F
M+1)
254
\ 253 (79Br
Gen NH (6-Bromo-2-ethyl-
-
Br,_--N_... imidazo [1,2-a] pyridin-
G en-3 -b (79B r),2 M+1),
4-b Dl 55 256
s-.)--:-'N \ 3-y1)-methyl-amine
(81Br) (81Br
M+1)
,
NH (2-Ethy1-6-io do-
Gen- Gen-3-d 302
l'Il-N----( imidazo [1,2-a] pyridin- 301
4-cD1 (M+1)
¨11 \ 3-y1)-methyl-amine
268
NH (6-Bromo-2-ethyl-8- 267 (79Br
Gen- 13r----N---1' methyl-imidazo [1,2- Gen-3-e
(79B r),2 M+1),
4-d a] pyridin-3 -y1)-methyl- D1 69 270
amine (81Br) (81Br
M+1)
266
\ (6-Bromo-2- 265 (79Br
Gen Br Nr... NH
- cyclopropyl- Gen-3-f (79Br),
M+1),
4-e
imidazo [1,2-a] pyridin- D1 267 268
<1
'\'/1------N 3-y1)-methyl-amine (Thr) ("Br
M+1)
328
\
327 (79Br
NH (E)-6-bromo-N-methyl-
Gen- Br .._ Gen-3-g (79B r),
M+1),
2-styrylimidazo [12-
D1 329(81B 330
,
4-f
' 1%1"L'N \µ ( \) a] pyridin-3 -amine
µ1\ r) (81Br
M+1 )
\ (6-Bromo-2-ethyl-7- 271
NH
Gen- fluoro-imidazo [1,2- Gen-3-j (79Br),
N.A.
Br..7.,N___
4-g a] pyridin-3 -y1)-methyl- D2 273
amine (81Br)
/ 282
(6-Bromo-2-cthy1-8- 281 (79Br
NH
Gen- Br / methyl-imidazo [1,2- Gen-3-h (79Br),
M+1),
,N_\ \
4-h I õLs \ \ a] pyridin-3 -y1)- ethyl- D1 283 284
T \N
amine (81Br) (81Br
M+1)
\
NH (2-Ethyl-6-nitro-
Gen- Gen-3-k 221
0,N. _.õ2,1\r..., imidazo [1 ,2-a]pyridin- 220
4-i D1 (M+1)
L-z---NI \ 3-y1)-methyl-amine
166
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
2-Ethy1-3-methylamino-
o
NH
Gen- imidazo[1,2-a]pyridine- Gen-3-1 233 234
- --1-1------, -----
4-j o - N:Liõ \,,) \ 6-carboxylic acid D1 (M+1)
methyl ester
F
/¨
(\Y 449
(6-Bromo-2-ethyl-8-
/ 448 (79Br
N--,K
\ __4, \ fluoro-imidazo[1,2-
a]pyridin-3-y1)-[4-(4- Gen-4-a (79Br), M+1),
Gen-
5-a N sv El 450 451
fluoro-pheny1)-thiazol-
(81Br) (81Br
zi,1,N\/ \ 2-A-methyl-amine
M+1)
F
F 431
0 (6-Bromo-2-ethyl-
430 (79Br
imidazo[1,2-a]pyridin-
Gen- Gen-4-b (79Br),
M+1),
5-b '14----1 3-y1)-[4-(4-fluoro-
El 431 433
Br,, ,,,,,,N- - phe methyl-amine ny1)-thiazol-2-y1]-
(8 'Br) (8 'Br
N \ M+1)
-
F
/1 (2-Ethy1-6-iodo-
imidazo[1,2-a]pyridin-
Gen- N----)----/ Gen-4-c 479
\ N---- 3-y1)-[4-(4-fluoro- 478
5-c El (M+1)
1-. / s phenyl)-thiazol-2-y1}-
'r N---
\ methyl-amine
F
-------/ 445
/ \\ (6-Bromo-2-ethy1-8-
)--------/ 444 (79Br
4'1S-3/ methyl-imidazo[1,2-
Gen-4-d (79Br), M+1),
Gen- '14¨ alpyridin-3-y1)-[4-(4-
5d El 446 447
fluoro-pheny1)-thiazol-
(81Br) (81Br
2-A-methyl-amine
M+1)
/F 456
/2-----
\ 2- }2-[(6-Bromo-2-ethyl-
455 79Br
imidazo[1,2-a]pyridin-
Gen- \ ¶C/1,1 3-y1)-methyl-
amino]- Gen-4-b (79Br), M+1),
5-c N s El 457 458
Br. ,-,-, _ thiazol-4-y1}-5-fluoro-
'isLN \ benzonitrile (81Br) (81Br
, M+1)
OMe
(2-Ethy1-6-iodo-
Gen- N--/ imidazo[1,2-a]pyridin-
Gen-4-c 491
5-f \N-- 3-y1)-[4-(4-methoxy-
El 490
(M+1)
, s pheny1)-thiazol-2-y1]-
11-1-
methyl-amine
, - [4-(4-tert-Buty1-pheny1)-
--.-1-
thiazol-2-y1]-(2-ethyl-6-
\
Gen- ,--___/ Gen-4-c 517
5-g s p---cc iodo-imidazo[1,2- El 516
(M+1)
N-1''s-) a]pyridin-3-y1)-methyl-
l'---%'N--- amine
167
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
F
(2-[(6-Bromo-2-ethyl-8- 484
1----\
f-----,/- methyl-imidazo [1,2- 483 (79Br
,/ N- (
Gen- a] pyridin-3 -y1)- ethyl- Gen-4-h
(79Br), M+ 1),
5-h Br, 'N- l'S -/1CN amino]-4-(4-fluoro- E2 485
486
-N-
phenyl)-thiazo le-5- (81Br) (81Br
1 carbonitrile M+1)
CI 495
4-(4-Chloro-pheny1)-
494 (35C1
,, thiazol-2-yl] - (2- ethy1-6-
Gen- Gen-4-c (35C1), M+ 1 )
\ --4) iodo-imidazo [1,2-
5-i N
/ s El 496 497
a] pyridin-3 -y1)-methyl-
(37C1) (370
'L---N \ amine
M+1)
OCF3 (2-Ethy1-6-io do-
imidazo [1,2-a] pyridin-
Gen- \ /1/4- 3-y1)-methyl- [4- (4- Gen-4-c
544 545
5-j N-`s ' trifluoromethoxy- El (M+ 1)
pheny1)-thiazo 1-2-yl] -
amine
CF3
(2-Ethy1-6-io do-
-_---,_-_,/ imidazo [1,2-a] pyridin-
Gen- N--(/ Gen-4-c 529
5-k 1j14---s 3-y1)-methyl- [4- (4-
El 528
(M+ 1)
I- trifluoromethyl-phenyl)-
`('-'N---
-NI \ thiazol-2-y1]-amine
F
[4-(3,4-Difluoro-
-4µ1
___)õ,____/--F pheny1)-thiazol-2-yl] -(2-
Gen- Gen-4-c 497
5-1 1N5) ethyl-6-io do-
E 1 496
(M+1)
'Y 'N--4, imidazo [1,2-a] pyridin-
1 i,N, \ 3-y1)-methyl-amine
F
, 470
( 1 2- 2- [(6-Bromo-2- ethyl-
469 (79Br
imidazo ---( [1,2-a] pyridin-
Gen- N 2-" Gen-4-b (79Br),
M+1),
s _I/ i CN 3-y1)-methyl-amino] -5-
5-m N- \ El 471 472
Br, / S - methyl-thiazol-4-yll -5-
- (
NI- \ "BO ("Br
1 -L---N/ \ fluoro-benzon itri 1 e
M+ 1)
_ F (6-Bromo-2- 443
cyclopropyl- 442 (79Br
Gen-
'-18-1 imidazo [1,2-a] pyridin- Gen-4-
e (79Br), M+1),
N--
-n 3-y1)- [4-(4-fluoro- El 444
445
phenyl)-thiazol-2-yl] - (81Br) (81Br
<I
methyl-amine M+ 1)
449
(6-Bromo-2-ethy1-7-
448 (79Br
fluoro-imidazo [ 1,2-
Gen- Gen-4-g (79Br),
M+1),
N----17---( - a] pyridin-3 -y1)- [4-(4-
5-0 ' El 450 451
Br-",õ--õN__2- s fluoro-pheny1)-thiazol-
(8IBr) (81Br
)----- \
F ------ N ' 2-y1]-methyl-amine
M+1)
F
505
(E)-N -(6 - bromo-2 -
504 (79Br
¨
styrylimidazo [1,2-
Gen- lq - --\' Gen-4-f (79Br), M+1),
\ /
a]pyri din-3-y1)-4-(4-
5-p Br, ,N_j4 El 506 507
'r _,_ ---, _
- -ni / \ fluoropheny1)-N-
I methylthiazol-2-amine (81Br)
(81Br
- M+1)
168
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
F 431
:--,-----/-1 6-bromo-3-((4-(4-
fluorophenyl)thiazol-2- Gen-5-ab 74930 ("Br
Gen- 'N-)I-1 yl)(methyl)amino)imida See cpd ( Br), M+1),
5-q . _ 432 433
13r- -11k1-4 8 ZO[1,2-a]pyridine-2- 174
(81Br) (81Br
N) o carbaldehyde
M+1)
F
2-[(6-Bromo-2-ethyl-8- 474
7----\c'
fluoro-imidazo [1,2- 473 ("Br
Gen- 'N-44 \ a]pyridin-3-y1)-methyl- Gen-4-a (79Br),
M+1),
5-r Br.,,,. j S CN amino]-4-(4-fluoro- E2
475 476
'INI"'\
I I, y---\ phenyl)-thiazole-5- (81Br)
(81Br
N ,
F carbonitrile M+1)
F 457
(I-1 2-[(6-Bromo-2-ethyl-
456 (79Br
/ imidazo [1 ,2-a]pyridin-
Gen- µ14-7 3-y1)-methyl-amino]-4- Gen-4-b (79Br), M+1),
5-s E2 457 459
/ s¨CN
Br (4-fluoro-phenyl)- le
(81Br) (81Br
thiazole-5-carbonitrile
- --- N \ M+1)
F
--\( 2-[(6-Bromo-2-ethyl-8- 470
methyl-imidazo [1,2- 469 (79Br
Gen- \ N--j- a]pyridin-3-y1)-methyl- Gen-4-d (79Br),
M+1),
5-t S CN amino]-4-(4-fluoro- E2 471 472
phenyl)-thiazole-5-
carbonitrile (81Br) (81Br
M+1)
F 2-[(6-Bromo-2-ethyl-7- 474
------ fluoro-imidazo [1,2- 473
(79Br
Gen- N ----/ a]pyridin-3-y1)-methyl- Gen-4-g (79Br), M+1),
N s CN
'-- ------
5-u amino]-4-(4-fluoro- E2 475 476
Br,õ.õ,_, Nr4 "
phenyl)-thiazole-5- (81Br) (81Br
F N \ carbonitrile M+1)
500
(79Br
(47 99B9r 35CI
35C1)
ci
[6-Bromo-2-(2,2,2-
501 Al ( Br
' M+1),
502
___ / trifluoro-ethyl)-
(Br 35
imidazo[1,2-a]pyridin- Gen-7-b 3i r,1 Cl,
Gen- N-
- 79Db
sNI
Br -- s \ 3 ,
r
5-v -y1]- [4-(4-chloro- C 1 "Br . N__ --
T ',Lõ \>- \ phenyl)-thiazol-2-y1]- 37C1), 37C1
N CF, methyl-amine 503 M+1),
(81Br r5P,,.4
7C1) 387cL)11.
M+1)
169
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
448
447 (79Br
35c1
("Br
CI
(6-Brom o-2-etbyl-
35C1)' M+1),
450
,---\
-,-...: imidazo[1,2-a]pyridin-
(4814B9r (3851Br
Gen- \ ril-1-( 3-y1)- [3 -(4-chloro- Gen-7-a 35ci,
9C1,
5-w N¨Cs-N phenyl)- Cl
Br.,..N_-(
[1,2,4]thiadiazol-5-y1]- "Br 7
37BCr1
\ 370),
methyl-amine 451 M+1),
452
(81Br
(81Br
37C1) 37C1
M+1)
(6-Bromo-2-ethyl- 432
(IF
)---,----- / imidazo[1,2-a]pyridin- 431 (79Br
Gen- N---( 3-y1)- [3 -(4-fluoro- Gen-7-c (79Br),
M+1),
\ // \
5-x N- \ N phenyl)- Cl 433 434
Br _._. S
=ri-- \> \ [1,2,4]thiadiazol-
5-y1]- (81Br) (81Br
methyl-amine M+1)
-N \
447
446 (3759Br
, 79 Cl
31 Br M+1),
a 5C1)' 449
4111 (6-Bromo-2-ethyl- 448
imidazo [1,2-a] pyridin-
(81Br (81Br
Gen- N Gen-4-b - Cl,
\ N---- \ 3-y1)- [4-(4-cbloro-
5-y El 3'Cl, 79Br
S phenyl)-thiazol-2-y1]-
Brr\i_. "Br... 37C1
methyl-amine 37C1),
\ 450
451
(Br (81Br
37c1) ,,,, ( Br
7l._,1) 37Cl
M+1)
F
di(2-Ethyl-6-nitro-
Gemimidazo[1,2-a] pyridin-
N Gen-4-i 398
\ \ 3-y1)- [4-(4-fluoro- 397
5-z N--- El (M+1)
02N 1\1_.
S phenyl)-thiazol-2-yl] -
)N \ ..,,,..,
methyl-amine
.'--
F
2-Etby1-3- {[4-(4-fluoro-
N
phenyl)-thiazol-2-y1]-
Gen- Ni \ methyl-amino{ - Gen-4-j 410 411
o \
5-aa s imidazo[1,2-a]pyridine- El (M+1)
o N \ 6-carboxylic acid
.. ---N \ methyl ester
170
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
Gen- F
:-. 1-(6-Bromo-3-{[4-(4- 539
fluoro-phenyl)-thiazol- 538 5-ab (79Br
(N-7( 2-y11-methyl-amino} - Gen-5-p (79Br), M+1),
µN---s see Cpd
imidazo[1,2-a]pyridin- 540 541
Br N>
, OH 174
_ 2-y1)-2-phenyl-ethane- (81Br) (81Br
..-"'-----:õ- -----"'N --(,
1,2-diol M+1)
HO
Gen- F 454
5-ac (-I 3-(6-Bromo-3-{[4-(4-
453 (79Br
N
fluoro-phenyl)-thiazol- Gen-5-q (79Br), M+1),
-2' 2-y1]-methyl-amino}- see Cpd 455
456
Br _. ''' imidazo[1,2-a]pyridin- 174
("Br) ("Br
1 1 \\ 2-y1)-aerylonitrile
N \ CN M+1)
Gen- f(F._Th.v_,
5-ad 3-(6-Bromo-3-{[4-(4- 487
), fluoro-pheny1)-thiazol-
Gen-5-q 4 86 (79Br
2-y1]-methyl-amino}- (79Br),
M+1),
\ 0 S---' N¨ imidazo[1,2-a]pyridin- See Cpd488
489
176
2-y1)-acrylic acid methyl ("Br (81Br
--, J/ \--0
-- ester M+1)
o
Gen- F
ill 415 (
(6-Bromo-2-ethyl- 416
imidazo[1,2-alpyridin-
5-ae 79Br
N \ 3-y1)-[3-(4-fluoro- Gen-4-b (79Br), M+1),
-N phenyl)- E3 417 418
Br 0 . N___ [1,2,4]oxadiazol-5-y1]- (81Br) ("Br
')-"N \ methyl-amine M+1)
Gen- F
5-af N / 2-Ethyl-N-[4-(4-fluoro-
\
S i õ, phenyl)-thiazol-2-y1]-N- Gen-5-z
See Cpd 367 368
N / IN methyl-imidazo[1,2- (M+1)
..- ../ 229
a]pyridine-3,6-diamine
H2N,---1,--
CI
. 2-Ethyl-3-{[4-(4-chloro- 427
N phenyl)-thiazol-2-y1]- 426 (35C1
Gen- methyl-amino}-
Gen-4-j (35C1), M+1),
5-ag
imidazo[1,2-a]pyridine- El 428 429(3
'0A.---/-" N---- - s 6-carboxylic acid (37C1)
Cl
methyl ester M+1)
240
NH2 239 (79Br
6-Bromo-2-ethyl-
Gen- BrN....- imidazo[1,2-
a]pyridin- Gen-2-b (79Br), M+1),
6-a D1 241 242
N \ 3-ylamine
(81Br) (81Br
M+1)
171
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
294
N H2 6-Bromo-2-(2,2,2- 293 (79Br
Gen- Br,,,,. ,N._...c trifluoro-ethyl)- Gen-2-f
(79Br), M+1),
6-b N \C F3 imidazo [1,2-a] pyridin- D1 295 295
3-ylamine (81Br) (81Br
M+1)
434
4 3 3 (3795 B r
(79Br Cl
M+1),
, a 35C1)' 436
/ ¨ (6-Bromo-2-ethyl-
(/ imidazo [1,2-a] pyridin- 435 (siBr
(Br 35i,
Gen- N 3-y1)- [3 -(4-chloro- Gen-6-a ,sc 1,
CI,
H -79 --
7-a NJ- o_N phenyl)- HI 79B r 79Br
Br.1 ,-,,,,___< '
[1,2,4]thiadiazol-5-y1]- 37c1
\ > 370),
amine M+1 ),
437
438
81Br
(81 Br
7CI) 37CI
M+1)
487
(79Br
486 35n1
CI 3(5C1), lvi79BY ,/,41),
[6-Bromo-2-(2,2,2-
488 489
tri fluoro-ethyl)- (81131. (81Br
N 35Cl, Gen- H/ --3 imi dazo [1 ,2-a]pyridi n- Gen-
6-b
N \-'
7b Br ,Nr.- ' 3 -y I] - [4-(4-chloro- El
79Br 79Br
phenyl)-thiazo I-2-y I] - )-- Cl 37C1),
amine M+1),
F 490
491
c81Br
(81Br
7C1) 37C1
1\4+1 )
F
(6-Bromo-2-ethyl- 418
imidazo [1,2-a] pyridin- 417 (79Br
Gen- N---( 3-y1)- [3 -(4-fluoro- Gen-6-a (79Br),
M+1),
H // 1
7-c N ----\ N phenyl)- H1 419 420
Br. N..-, )- S [1,2,4]thiadiazol-5-yl] - (81Br) (81Br
T N \ amine M+1)
N-[2-Ethyl-6-(1-
H methanesulfonyl-
Gen-
1 ,2,3 ,6-tetrahydro-
L_,I,,,_,,,,., N----0 pyridin-4-yI)- Gen-3 -b
362 363
8-a / N1-- imidazo [1,2-a] pyridin- F2 (M+1)
\ 3-y1]-N-methyl-
formamide
N-[2-Ethyl-6-(1 -
o \ o methanesulfonyl-
Gen-
11,--,
'N4 piperidin-4-yI)- Gen-8-a 365
, , 0 364
8-b ., '11---s imidazo [1,2-a] pyridin- F6
(M+1)
N ' 3-yI]-N-methyl-
formamide
172
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
442-Ethy1-3-(formyl-
0
,>,,, -It _----, o methyl-amino)-
Gen- o N ' \ N 1/ imidazo[1,2-a]pyridin- Gen-3 -b
387(M
8-c 1-.õ--, .---. _j\ H
- N" ' \\ 6-y1]-piperidine-1- F2-F6 386
+1)
\ carboxylic acid tert-
butyl ester
N-[2-Ethy1-6-(1-
o, o methanesulfonyl-
.= ,./ PD H
Gem sN___(
1,2,3,6-tetrahydro-
Gen-3-c 366
8-d
pyridin-4-y1)- 365
- y 'N" \\
K F2 (M+1)
--'=N1 \ imidazo[1,2-a]pyridin-
3-y1]-N-(di-methyl)-
formamide
N-[2-Ethyl-6-(1-
0 0 D
\ / /.-D H methanesulfonyl-
s, - D------\ /
Gen-
N" ' µ14-,\ piperidin-4-y1)- Gen-8-d
368
L / o 367
8-e --''---1, 'N-A imidazo[1,2-a]pyridin- F6 (M+1)
N \ 3-y1]-N-(d3-methyl)-
formamide
o
4-[2-Ethy1-3-(formyl-
XOJN, \ icr methyl-amino)-7-
Gen- methyl-imidazo[1,2- Gen-3-i 401
400
8-f I- _.----, ,,,,.<-----.N_--4, H a]pyridin-6-y1]-
F3 (M+1)
)'-----N> N piperidine-l-carboxylic
acid tert-butyl ester
442-Ethy1-3-(formyl-
0
-, ji o methyl-amino)-
Gen- 0' 'N i 'N---1( imidazo[1,2-a]pyridin- Gen-3-b
385
i H 384
8-g '---\'-'--';-'N¨ 6-y1]-3,6-dihydro-2H- F2
(M+1)
N/ \ pyridine-1-carboxylic
acid tert-butyl ester
o 442-Ethy1-3-(formyl-
.> .. \ /H methyl-amino)-
Gen- " /N-----/o imidazo[1,2-a]pyridin- Gen-3 -b
388
387
8-h --- ----/----N--- 6-3/11-piperazine-1- Fib (M+1)
-)------N>- \ carboxylic acid ter--
butyl ester
2-Ethy1-6-(1-
,\ methanesulfonyl-
Gen- NH 1,2,3,6-tetrahydro- Gen-4-b
334 335
9-a 7
pyridin-4-y1)- F2 (M+1)
I 1 \
'-----N \ imidazo[1,2-a]pyridin-
3-3/1]-methyl-amine
o o [2-Ethyl-6-(1-
\ /
t methanesulfonyl-
Gen- N NH Gen-8-b 337
9-b ', '14"-- piperidin-4-y1)-
D1 336
- 7 \ imidazo[1,2-a]pyridin-
(M+1)
LN 3-yThmethyl-amine
4-(2-Ethy1-3-
o Gen-4-b
0 trt N------ \ methylamino-
F2
Gen- NH imidazo[1,2-a]pyridin-
or 356 357
9-c -r--' N---- 6-y1)-3,6-dihydro-2H- (M+1)
)N2
\ pyridine-1-carboxylic Gen-8-g
D2
acid tert-butylester
173
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
4-(2-Ethyl-3 -
0
methylamino-
Gen- 0 N ' NH imidazo [1,2-a] pyridin- Gen-8-c 359
9-d L-..._,.---. \.....
''',- N 6-y1)-pip eridine-1 - Dl-F7 358
(M+1)
-'i\( \ carboxylic acid ter-
butyl ester
4-(2-Ethy1-3-
j0: _ methylamino-
\
Gen- -0' NH imidazo [1,2-a] pyridin- Gen-8-h 360
359
9-e --...---"-.1.,-.2---N--,,, 6-y1)-pip erazine-1 - D1-F7
(M+1)
,¨,-,-.-N \ carboxylic acid tert-
butyl ester
o\ 0 D [2-Ethyl-6-(1-
Gen-
.D- -1--\ D methanesulfonyl-
NH
piperidin-4-y1)- Gen-8-e
339 340
9-f ,-------..,õ - ---N¨, Dl (M+1)
imidazo [1,2-a]pyridin-
3 -yl] -(d3-methyl)-amine
4-(2-Ethyl-7-methyl-3 -
0
U (d3-methyl)amino-
Gen- o' 'N1 NH [ imidazo [1,2-a] pyridin- Gen-8-f 373 , J
372
9-g \. _{
- N \> 6-yI)-pip eridine-1 - D1-F7
(M+1)
carboxylic acid tert-
butyl ester
CI
/
453
Cpd 1-(3- {[4-(4-Chloro-
452 (35C1
183 )-- phenyl)-thiazo1-2-y1]-
N¨( Gen-5-i (35C1), M+1)
or , Gen- ___4 methyl-amino}-2-ethyl-
F4 454 455
/ ¨1 N S' imidazo[1,2-a]pyridin-
HN \ õ.N. i (37C1) (37C1
10-a 8-- ---; N , ,> 6-y1)-imidazolidin-2-one
o
M+1)
;----,.-N \
F
/--( [2-Ethyl-6-(1,2,3,6-
( )
tetrahydro-pyridin-4-y1)-
Gen- N- K imidazo[1,2-a]pyridin- Gen-5-b 434
433
10-b HN--- N
--- 3 -yl] - [4-(4-fluoro- F2-F5b (M+1)
" N"---- pheny1)-thiazol-2-y1]-
)':¨\\ methyl-amine
F Gen-5-b
Cpd /--(
K )
j (2-Ethyl-6-piperidin-4- F2-F6-
216 yl-imidazo [1,2- F5b
N 436
or ' " ---,K\ alpyridin-3 -y1)- [4-(4- or 435
...--.. (M+1)
Gen HN --- N's fluoro-pheny1)-thiazol- Gen-9-c
10c L_c. ,
I N,,,i \ 2-yThmethyl-amine El -F6-
F5b
F
7---( 1-(2-Ethyl-3-{[4-(4-
/ fluoro-phenyl)-th iazol-
Gen- Gen-5-b 451
2-yl] -methyl-amino { - 450
10-d HN1'-' \N--1;ISI imidazo[1,2-a]pyridin-
F4-F5b (M+1)
II 6-y1)-piperazin-2-one
0 ---1----N \
174
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
F
-K
(2-Ethy1-6-piperazin-1-
Gen-5-b
7- ¨ yl-imidazo [1,2-
Gen- 1`,1--C a] pyridin-3 -y1)- [4-(4- Flb-F5b
436 437
10-e Hu'I
N or (M+1)
1 S fluoro-pheny1)-thiazol-
Fla
N
2-34]-methyl-amine
¨ ) \
-N '
F
/
2- {2- [(2-Ethy1-6-
piperidin-4-yl-
Gen- N--<_, \cN imidazo[1,2-a]pyridin- Gen-9-d
474 475
10-f HN '-'--- \ NI--j' \ 3-y1)-methyl-amino] -5- E1-F5b
(M+l)
methyl-thiazol-4-yll -5-
. ;. fluoro-benzonitrile
-s---N \
CI
Cpd
N__,(,-------" [4-(4-Chloro-phenyl)- 467
thiazol-2-y1]- [6-(3,6- 466 (35C1
231
dihydro-2H-thiopyran- Gen-5-i (35C1), M+1)
or s - ---, \ 1)
Gen- 1 N \,s., 4-y1)-2-ethyl- F2 468
469
' 7-'---;)'N -)-- imidazo [1,2-a] pyridin- (37C1) ("Cl
10-g
3-yThmethyl-amine M+1)
F
/¨( 2-{2-[(2-Ethyl-6-
\ ) piperidin-4-yl-
Gen- ---? KCN imidazo[1,2-a] pyridin- Gen-9-d 461
10-h H -'
N- S 3-y1)-methyl-amino]- El-F5b 460
HI(
(M+1)
thiazol-4-y11-5-fluoro-
--N \ benzonitrile
F
/ 2-{2-[(2-Ethyl-6-
piperazin-l-yl-
- ,
Gen- N-_-\(1- eN imidazo [1,2-a] pyridin- Gen-5-e 462
461
10-i HN ' 3-y1)-methyl-amino]- Fla
(M+1)
[ 1 N- s
thiazol-4-y11-5-fluoro-
,¨ benzonitrile
---",--, ) --------Nz \
CI
(
/¨ \ [4-(4-Chloro-phenyl)- 436
thiazol-2-y1]- [6-(2,5- 435 (35C1
Gen- N--/ dihydro-1H-pyrrol-3- Gen-5-i (35C1), M+1),
10-j /_____ \ _I y1)-2- ethyl-imidazo [1,2- F2-
F5a 437 438
HN N N1 =s-
a] pyridin-3 -yl] -methyl- (37C1) (37C
7-"--
amine M+1)
\
F
2- [(2-Ethy1-6-pip erazin-
1-yl-imidazo [1,2-
G en- )µ a] pyridin-3 -y1)-methyl- Gen-5-s 462
10- HN k M \ _ j(--
N s CN amino]-4-(4-fluoro- Fla 461
(M+1)
pheny1)-thiazole-5-
--L'N\ carbonitrile
175
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
F
,-- (2-Ethyl-8-fluo ro-6-
N
4, piperazin-l-yl-
Gen- \ II \ imidazo [1,2-a] pyrid in- Gen-5-a
454 N.A.
10-1 HN-----1 Jr `s' 3-y1)- [4-(4-fluoro- Fla
pheny1)-thiazol-2-y1]-
\ methyl-amine
F
F
) /---K\ 2-[(2-Ethy1-8-fluoro-6-
//
/ piperazin-l-yl-
Gen- \ jj-S, imidazo [1,2-a] pyridin- Gen-5-r 47, 480
10-m HN 1
N s CN 3-y1)-methyl-amino] -4- Fla (M+1)
L.
(4-fluoro-phenye-
-L- Ltsi \
1 z thiazole-5-carbonitrile
F
F
,-- --\ (2-Ethyl-8-methyl-6-
)-__- piperazin-l-yl-
HN-- N----(
Gen- imidazo [1,2-a] pyridin- Gen-5-d 450 451
-1 \N----'\ \)
10-n L-N, .. _,- S- 3-y1)- [4-(4-fluoro- Fla
(M+1)
nil \ s
pheny1)-thiazol-2-y1]-
--- -"k-----N \ methyl-amine
/F
/>------c
2j 2- [(2-Ethy1-8 -methy1-6-
N- 1-11\1 \ piperidin-4-yl-
4 1_,
'-
474
Gen- N---- \ ' CN imidazo [1,2-a] pyridin- Gen-5-t 475
/ s"-
10-0 L7'''-.,--'N---- 3-y1)-methyl-amino] -4- F3-F5b
(M+1)
L ) (4-fluoro-phenyl)-
.---z- , \
-.-- N thiazole-5-carbonitrile
2- {3 - [4-(2-Ethy1-3 - {[4-
(4-fluoro-phenyl)-
l thiazol-2-yl] -methy-
N ,,., Gen-10-c
Gen- _ J amino} -imidazo [1,2-
F11- 686 687
10-p ( a] pyridin-6-
y1)- (M+1)
F 12a
,N.S-14-- piperi dine-l-sul folly]] -
i3
o propyl} - is o indo le-1,3 -
d lone
F
_C
(2-Ethy1-7-fluoro-6-
)=- piperazin-l-yl-
Gen- N- 4 imidazo [1,2-a] pyridin- Gen-5-o
454 455
10-CI FIN'-' \ __., ,
N" s" 3-y1)- [4-(4-fluoro- Fla
(M+1)
pheny1)-thiazol-2-y1]-
\
methyl-amine
F
F
== 2-[(2-Ethy1-7-fluoro-6-
)- - piperazin-l-yl-
Gen- N--' imidazo [1,2-a] pyridin- Gen-5-u 479 480
10-1. HN_ - ' \N-- \\) 3-y1)-methyl-amino] -4- Fla
(M+1)
S CN
Ni
(4-fluoro-pheny1)-
thiazole-5-carbonitrile
F7 7 'NJ \
176
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
,F
/ - (2-Ethyl-7-methyl-6-
// piperidin-4-yl-
Gcn- N--(/ imidazo [1,2-a] pyridin- Gcn-9-g
449 450
10-s HN'` \ I 171 --4 3-y1)- [4-(4-fluoro- E1-
F5b (M+1)
s
¨ pheny1)-thiazol-2-y1]-
t¨ ) \ methyl-amine
,F
i¨ 2-{5-[(2-Ethyl-6-
.
piperidin-4-yl-
)
Gen- N---\c imidazo [1,2-a] pyridin- Gen-9-d 462
104 HN --
" 'N4,1s,N 'N 3-y1)-methyl-amino]- E5-F5b 461 ow 1
)
l
,,. ,-----------N*
-1--, \
N \ [1,2,4]thiadiazol-3 -y1} -
5-fluoro-benzonitrile
CI
/4 3-(4-Chloro-phenyl)- 454
[1,2,4]thiadiazol-5-y1]- 453 (35C1
Gen- N---\( (2-ethyl-6-piperazin-1- Gen-5-w (35C1), M+1),
10-u HN' N i \ N "---s- yl-imidazo [1,2-
F1b-F5b 455 456
a] pyridin-3 -y1)-methyl- (37C1) (37C
--.¨'L'--- NI/ \ amine M+1)
F [2-Ethy1-6-(1,2,3,6-
/ ¨ tetrahydro-pyridin-4-y1)-
Gen-9-c
l- - imidazo [1,2-a] pyridin- E4-F5b
N__-- N Gen- N-
10-v MI 7( 3-y11- [3 -(4-fluoro- or
434 04+1 )
435
----'' \- s
1 phenyl)- Gen-5-x
,-, _,-c
[1,2,4]thiadiazol-5-y1]- F2-F5b
methyl-amine
F
¨K (2-Ethyl-6-piperidin-4-
K/\ /> yl-imidazo [1,2-
> / Gen-10-
Gen- N--\K a] pyridin-3 -y1)- [3 -(4- 437
10-w HN---"- \ N¨ N fluoro-phenyl)- v 436
(M+1)
14-' F6
I--õ_, ---, [1,2,4]thiadiazol-5-y1]-
Isj-'--ni" \ s methyl-amine
F
/ 2-1[643,3 -Dimcthyl-
-..\
piperazin-l-y1)-2- ethyl-
8-methyl-im idazo [1 ,2-
Gen- \ i --7 Gen-5-t 504
a] pyrid i n-3 -yl] -methyl- 503
10-x I-IN-C N- `s-- CN Fla (M+1)
amino} -4-(4-fluoro-
T
pheny1)-thiazole-5-
I carbonitrile
F iisN / [6-(1-Ethenesulfonyl-
\ --- 1 \I r
piperi din-4-y1)-2- ethyl-
s
Gen- N / N imidazo [1,2-a] pyridin- Gen-10-c 525 526
---- --
10-y I 3 -yl] - [4-(4-fluoro- F 1 1 (M+1)
pheny1)-thiazol-2-yl] -
methyl-amine
so
177
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
F
<--
(2-Cyclopropy1-6-
piperidin-4-yl-
Gen- !,i--
imidazo[1,2-a]pyridin- Gen-5-n
448
10-Z HN ----' \ 3-y1)-[4-(4-fluoro- F2-F6-
447
(M+1)
[ 1 ,N- s-
pheny1)-thiazol-2-y1]- F5b
j------N methyl-amine
F
110 3-(3- { [4-(4-Fluoro-
pheny1)-thiazol-2-y1]- Gen-5-ac
F2-F6-
Gen- methyl-amino} -6- 461(M
/N F5b 460
10-aa sAN¨ piperidin-4-yl- +1)
HN See Cpd
imidazo[1,2-a]pyridin-
2-y1)-propionitrile 174
''''= --N \ =N
F
/-4 4-(2-Ethyl-3- { [4-(4-
K\ //
Gen- / fluoro-pheny1)-thiazol-
10- ni--\} 2-y1]-methyl-amino} - Cpd 78
451 N.A.
HN-^ \ NS ) F5b
ab L -k imidazo[1,2-alpyridin-
y ---' --y-; N \ \ 6-y1)-piperidin-3-ol
OH
zo¨
< [2-Ethy1-6-(1,2,3,6-
N > tetrahydro-pyridin-4-y1)-
Gen- imidazo[1,2-alpyridin- Gen-5-f 446
N __C 445
10-ac HN-----'' N S ' 3-y1]-[4-(4-methoxy- F2-F5b
(M+1)
pheny1)-thiazol-2-y1]-
methyl-amine
ci.,._
(I ;) [4-(4-Chloro-phenyl)-
504
I thiazol-2-y1]-methy146- 503 (35C1
Gen- (1,2,3,6-tetrahydro-
Gen-5-v (35C1), M+1),
10- \ pyridin-4-y1)-2-(2,2,2-
zs;AN F2-F5a 505 506
ad HI\1 4 ¨ trifluoro-ethyl)- (37c 0 C7ci
- ------,--/-N- \\ imidazo[1,2-a]pyridin-
)--.-- >- \ 3-yThamine M+1).
N 4-F
cI
)----
;) [4-(4-Chloro-phenyl)-
'. thiazol-2-y1]-[2-ethy1-6- 449
Gen-
(1,4,5,6-tetrahydro- Gen-5-i (35C1),4
-N N.A.
10-ac 11 pyridin-3-y1)- F2-F5a 51
s ----\
1 N¨ imidazo[1,2-a]pyridin- (37C1)
HN ), - 3-34]-methyl-amine
Y - --,
\
N '
178
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
F
)---.
(2-Cyclopropy1-6-
Gen- ),. piperazin-l-yl-
imidazo [1,2-a] pyridin- Gen-5-n- 449
' 1s1 448
10-af 2 3-y1)- [4-(4-fluoro- F1b-F5 a (M+1)
HN ---1.--- S- ,
N ¨ phenyl)-thiazol-2-yl] -
methyl-amine
+, -- ---- ../-.------,..,
4-(2-Ethyl-3- {[4-(4-
F
fluoro-phenyl)-5 -
methoxycarb onyl-
Gen- A_Y.., \ rl thiazol-2-yl] -methyl-
Gen-9-e 595
10- ' u Z.
,N _--1,1,-(N-----'S--f amino} -imidazo [1,2- El
594
(M+1)
ag L'N\)--\ 'C) a] pyridin-6-y1)-
piperazine-1-carboxylic
acid tert-butyl ester
2CF,
[2-Ethy1-6-(1,2,3,6-
Y-- tetrahydro-pyridin-4-y1)-
Gen- N--,K imidazo [1,2-a] pyridin-
10- HN 1/ '-, \
N-- 3-yThmethyl- [444- Gen-5- j-
499 500
ah JL, - i - trifluoromethoxy-
F2-F5b (M+1)
--- ----% 'N- ,
pheny1)-thiazol-2-y1]-
-- N
amine
cF3
1-/- [2-Ethy1-6-(1,2,3,6-
tetrahydro-pyridin-4-y1)-
Gen- N-7( imidazo [1,2-a] pyridin- Gen-5-k
483
N.A.
10-ai HN--- '_4l 3-y1}-methyl-[4-(4- F2-F5b
'S'
- ---, ---N- trifluoromethyl-pheny1)-
\ thiazol-2-y1]-amine
N
F
/¨ [4-(3,4-Difluoro-
')---F phenyl)-thiazol-2-yl] - [2-
>--
Gen-
Nill, ethy1-6-(1,2,3,6- Gen-5-1
451 452
10-aj i-IN''- tetrahydro-pyridin-4-y1)-
F2-F5b (M+1)
,------, ----- 4 imidazo [1,2-a] pyridin-
L---- 3-3/1}-methyl-amine
N
CI
/
/ [4-(4-Chloro-phenyl)- 450
thiazol-2-yl] - [2-ethyl-6- 449 (35C1
Gen-
(1,2,3,6-tetrahydro- Gen-5-i (35C1), M+1),
10- N--,
\ _._, ) pyridin-4-y1)- F2-F5b 451 452
[
ak HN' . - N '
i S
imidazo[1,2-a]pyridin- (37C1) (37C1
L__, > \ 3-yThmethyl-amine M+1)
N
179
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
F
N mi [4-(2-Bromo-4-fluoro-
592
\ -----).___-- phenyl)-thiazol-2-y1]-[2-
Br S ethy1-6-(1- 591 (79Br
Gen- ,,N ,,N Gen-9-b (79Br) M+1),
10-al I methanesulfonyl-
El 593 594
piperidin-4-y1)-
(81Br) (Br
imidazo[1,2-a]pyridin-
0, N ..,,- M+1)
'S'
3-yThmethyl-amine
--- ,µ
0
F(____
4-(2-(2-Carbamoyl-
Q ethyl)-3-{[4-(4-fluoro-
\ phenyl)-thiazol-2-y1]- Gen-5-ad
Gen- ---N
-,L ()) '_1( methyl-amino}- F2-F6- 579
10- 0 -N--------,, S NI_
imidazo[1,2-a]pyridin- F13 see 578
(M+1)
am ' ---r, 'N----
6-y1)-piperidine-1- Cpd 176
N \-4 carboxylic acid ten-
NH2
butyl ester
F, 7
/ [4-(2-Ethyl-3 {[4-(4-
si \F.-sr( \ fluoro-pheny1)-thiazol-
Gen- N z,N 2-y1]-methyl-amino}- Gen-10-e 509
10-
{ imidazo[1,2-a]pyridin- F9b-F13 508
(M+1)
an
o r- N' 6-y1)-pip erazin-l-yl] -
1] I 1
r\L oxo-acetic acid
0
F
3-Chloro-propane-1- 508
N /
Gen- k -,).---N sulfonic acid (2-ethyl-
3- 507 ("Cl
\ s -----=-- {[4-(4-fluoro-pheny1)- Gen-5-af ("CD, M+1),
10- thiazol-2-y1]-methyl- Fl 1 509 510
ao 0, p 1 amino}-imidazo[1,2-
(37C1); (37C1
01S,N,---7,,-
alpyridin-6-y1)-amide M+1)
H
F
0 [6-(1,4-Dioxa-8-aza-
/- -0 spiro[4.5]dec-8-y1)-2-
Gen- N
10- .
b c ) NN----1 ethyl-imidazo[1,2- Gen-5-c
493 494
I S a]pyridin-3-y1]-[4-(4- Fib (M+1)
ap
I I)_ fluoro-pheny1)-thiazol-
,,,,N. µ
2-yid-methyl-amine
F
/-- 1-(2-Ethyl-3- {[4-(4-
_ \) G en-10-
Gen- 0 N-----/ fluoro-pheny1)-thiazol-
-\
! \N =- =\) 2-yid-methyl-amino} - aP 450
10-
449
aq \¨N s imidazo[1,2-a]pyridin-
See Cpd (M+1)
6-y1)-piperidin-4-one 211
--)'--N \
180
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
4-(2-Ethyl-3- { [4-(4-
F
fluoro-pheny1)-5 -
methoxycarb onyl-
G en- 40 i(t-N---, `N_ /N ---\ /0
thiazol-2-yl] -methyl- Gen-9-d 594
I 593
I 0-ar ''LC,'N-S amino} -imidazo [1,2- El (M+1)
/0
a]pyridin-6-y1)-
piperidine-1-carboxylic
acid tert-butyl ester
_ ,F
//----\C 2- [(2-Ethy1-8 -methy1-6-
piperazin-l-yl-
Cpd FiN-^
\N----/N-1 imidazo [1,2-a] pyridin- Gen-5-t
475 476
1 s-- -CN 3-y1)-methyl-amino] -4- F1b-
F5b (M+1)
---------- -----N
(4-fluoro-phenyl)-
- 'LlsI--\µ thiazole-5-carbonitrile
F 4-(2-Ethy1-3- { [4-(4-
' fluoro-pheny1)-thiazol-
y---% 2-yl] -methyl-amino } - Gen-5-c
Cpd 552
78 -'<-o1N-- \ _41--sS imidazo [1,2-a] pyridin- F2-
see 551
(M+1)
6-y1)-3 -hydroxy- Cpd 78
I_ = \ piperidine-l-carboxylic
OH L---, ----N
acid tert- butyl ester
F
/- -< 2- {2- [(2-Ethy1-6-
i---\ piperazin-l-yl-
Cpd N N1-- C imidazo [1,2-a]
pyridin- Gen-5-m 476
475
161 HN''', 'Ts1--c 3-y0-methyl-amino] -5- Fla (M+1)
õ,, ,.......,...; --..w4, methyl-thiazol-4-y1} -5-
N fluoro-benzonitrile
/F
C) [6-(3 -Amino-azetidin-1-
y1)-2- ethyl- imidazo [1,2-
Cpd Gen-5-b 423
s ---- a] pyridin-3 -yl] -[4-(4- 422
177 H2N._-\ F lb-F5a (M+1)
N- 8 fluoro-pheny1)-thiazol-
\---N.
2-yThmethyl- amine
242
CN o 241 (79Br
Gen- J. jt 2-(2-Bromo-acetyl)-5- G1 (79Br), ..
M+1),
11-a )
, Br fluoro-benzonitrile 243 244
F "
("BO ("Br
M+1)
294
(79Br,
9Br)
Br 0
296
Gen- 2-Bromo-1-(2-bromo-4-
G1 (79Br' N.A.
11-b 11101 Br fluoro-phenyl)-ethanone
s
F I Br)
298
(81Br'
g I Br)
CN 0 255
Gen- 2-(2-Bromo-propiony1)- (79B r),
- r G1 N.A.
11-c
F)- Br 5-fluoro-benzonitrile 257
(81Br)
181
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
see 218
o
,.--..õ--,i,11,, Br 2-Bromo-1-(4-fluoro- example (79Br),
N.A.
Gen-
11-d D/\D pheny1)-(d2- ethanone) Gen-11- 220
F ---"
d (81Br)
F 239
238 (35C1
2-Chloro-4-(4-fluoro-
Gen- ("CD, M+1),
phenyl)-thiazole-5- G2
12-a N
carbonitrile 240 241
ji \ _ (37C1) ("Cl
CI- M --- N M+1)
149
Gen- a._ ..11..._ 2-Chloro-1-(3-hydroxy-
G3 a (35C1),
N.A
13-a Is1A.OH azetidin-l-y1)-ethanone 151
(37C1)
164
163 ("Cl
o 2-Chloro-1-(3 -hydroxy-
1
a., -,N3c_ (3'c 1), M+1)
Gen-
3 -methyl-azetidin-1-y1)- G3 a
13-b 165 166
OH ethanone (37C1) ("Cl
M+1)
164
163 (35C1
'
2-Chloro-1-(3-
Gen- ci j.1, ("C1), M+1)
13-c Na hydroxymethyl-azetidin- G3b
165 166
1-y1)- ethanone (37C1) (37C1
M+1)
151
Gen- a 1(N G3 a
2-Chloro-1-(3-fluoro- ("CD,
'''-' -----\
153
N. A
13-d - -11F azetidin-l-ye-ethanone
(37c 0
170
169 (35C1
o 2-Chloro-1-(3,3 -
Gen- ci,ANac_F
difluoro-azetidin-l-y1)- G3 a (35C1), M+1)
13-e 171 172
F ethanone (37C1) (37C1
M+1)
133
Gen- ajN3 1-Azetidin-l-y1-2- (35C1),
13-f chloro-ethanone G3 a N.A.
135
(37C1)
164
163 (35C1
,õ? (S)-2-Chloro-1-(3 -
Gen- aõI, -, (35C1), M+1)
NL J-01-1 hydroxy-pyrrolidin-1- G3 a
13-g 165 166
y1)- ethanone (37C1) (37C1
M+1)
164
163 (35C1
(R)-2-Chloro-1-(3 -
Gen- a- 3,,, OH , hydroxy-pyrrolidin-1- G3b (35CD, M+1)
13-h 7_ j 165 166
y1)- ethanone (37C1) (37C1
M+1)
182
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
Int - MS
Int Structures Name MW
Mtd Ms'd
173
172 (35C1
(S)-1-(2-Chloro-acety1)-
Gen- (35C1), M+1),
cijm_ \,.....cN pyrrolidine-3- G3b
13-i \ , 174 175
carbonitrile (37C1) (37C1
M+1)
o
N-[1-(2-Chloro-acety1)- 204
';.s
Gen- see Gen- ( CD
pyrrolidin-3-y1]-
206 ' N.A.
13-j 1-___ / \/,__-- 13-j
o acetamide
(37C1)
2-Chloro-1-(3- 177
Gen- a -V. hydroxymethyl- (35C1),
G3b N.A.
13-k r' -1-1-\oFi pyrrolidin-1-y1)- 179
ethanone (37C1)
163
Gen- ck._,ZN, 2-Chloro-1-morpholin-
G3b (35C1),
N.A.
13-1 4-yl-ethanone 165
(37C1)
134
133 (35C1
Gen- 2-Chloro-N- (35C1), M+1),
ck_ 5.) N_A G3b
13-m H cyclopropyl-acetamide 135 136
(37C1) (37C1
M+1)
151
Gen-
CL2-Chloro-N-(2-hydroxy-
o (35C1),
ethyl)-N-methyl- G3b N.A.
13-n y- \-OH 153
acetamide (37C1)
138
137 (35C1
o
Gen-
ci U N-o G3b 2-Chloro-N-methoxy-N-
(35C1), M+1)
, ..-- ----,
13-o I ' methyl-acetamide 139 140
(37C1) (37C1
M+1)
146
o 2-Chloro-N-
Gen- ci, H (35C1),
cyanomethyl-N-methyl- G3b N.A.
13-p I 148
N acetamide (37C1)
151
Gen- 2-Chloro-N-(3 -hydroxy- (35CD,
CI., 5.',N ____ \ G3b N.A.
13-q 11 OH propy1)-acetamide 153
(37C1)
Table II. NMR data of the Intermediates used towards the compounds of the
invention.
Int NMR data (6)
1H NMR (400 MHz, CDC13) 6 ppm, presence of 2 rotamers 8.71 (0.5 H, bs), 8.56
(0.5 H,
Gen- s), 8.52 (0.5 H, bs), 8.16 (0.5 H, d), 8.04 (0.5 H, bs), 7.77 (0.5 H,
dd), 7.71 (0.5 H, dd),
2-k 7.67 (0.5 H, bs), 7.53 (0.5 H, d), 7.49 (0.5 H, d), 3.95 (1.5 H,
s), 3.92 (1.5 H, s), 2.79 (1 H,
q), 2.72(1 H, q), 1.36 - 1.26(3 H, m)
183
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Int NMR data (6)
Gen- 1H NMR (400 MHz, CDC13) 6 ppm 8.22 (1 H, s), 7.94 (1 H, s), 7.50 (1 H,
d), 7.34 (1 H,
3-c d), 2.75 (2H, q), 1.34 (3 H, t)
Gen- 1H NMR (300 MHz, CDC13) 6 ppm 7.56 (1 H, dd), 7.41 (1 H, dd), 7.20 - 7.08
(1 H, m),
11-b 4.48 (2 H, s)
Gen- 1H NMR (400 MHz, CDC13) 6 ppm 8.03 (1 H, dd), 7.55 (1 H, dd), 7.46 - 7.38
(1 H, m),
11-c 5.26(1 H, q), 1.96(3 H, d)
Gen-
11d NMR (400 MHz, CDC13) 6 ppm 8.01 - 7.96 (2 H, m), 7.17 - 7.08 (2
H, m)
Gen- 1H NMR (400 MHz, CDC13) 6 ppm 4.78 - 4.68 (1 H, m), 4.56 - 4.47 (1 H, m),
4.38 - 4.29
13-a (1 H, m), 4.16 (1 H, ddd), 3.97 (1 H, dd), 3.91 (2 H, s), 2.56 (1 H, d)
Gen- 1H NMR (300 MHz, CDC13) 6 ppm 5.49 - 5.40 (0.5 H, m), 5.32 - 5.20 (0.5 H,
m), 4.69 -
13-d 4.50 (1 H, m), 4.49 - 4.29 (2 H, m), 4.28 - 4.10 (1 H, m), 3.93 (2 H, s)
Gen- 1H NMR (300 MHz, CDC13) 6 ppm 4.18 (2 H, t), 3.96 (2 H, t), 3.73 (2 H,
s), 2.27 - 2.14 (2
13-f H, m)
Gen- 1H NMR (400 MHz, CDC13) 6 ppm 5.33 - 5.01 (2 H, m), 4.60 - 4.41 (1 H, m),
3.79 - 3.65
13-j (2 H, m), 3.63 - 3.44 (2 H, m), 1.97(3 H, d), 1.90 - 1.80 (2 H, m)
Table III. Illustrative compounds of the invention.
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
2-((2-ethyl-8-methyl-6-
(piperazin-1
yl)imidazo[1,2-
HN F 1 b-F5b 476
1 I I s- CN a]pyridin-3-
(Gen-5-t) 475
(M+1)
'N = N - - yl)(methyl)amino)-4-(4-
N\> fluorophenyl)thiazole-5-
carbonitrile
2-42-ethy1-6-(4-(2-(3 -
HO _/ F hydroxyazetidin-1 -
y1)-2 -
\ -N ) \ o o ethyl)pip erazin- 1 -
y1)-8-
F8 589
2
N L-CN methylimidazo[1,2- 588
(C pd 1)
(M+1)
alpyridin-3-
y1)(methypamino)-4-(4-
fluorophenypthiazole-5-
carbonitrile
184
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
2-( (2- ethy1-6-(442-(3 -
hydroxy-3 ¨
F
HO /7- --(:µ methylazetidin-l-y1)-2-
-\No
oxoethyl)piperazin-1-
3 602 \ ni-- \ y1)-8- F8 603
rt s--- ---CN methyl imi dazo[1,2- (Cpd 1)
(M+1)
a]pyridin-3-
yl)(methyl)amino)-4-(4-
fluorophenyl)thiazo le-5-
carbonitrile
(R)-2-((2-ethyl-6-(4-(2-
(3 -hydroxypyrro lidin-1-
H. -1 ---\c'F
y1)-2-
HO N0 =¨... oxo ethyl)pip erazin-1 -
4 ,,,-----1
'N---r).--
¨ y1)-8- F8
methylimi dazo [1,2- (Cpd 1) 602 603
S CN
(M+1)
'--= -r- -N- ,
--i)--Nr-- \ alpyrid in-3-
I yl)(methyl)amino)-4-(4-
fluorophenyl)thiazo le-5-
carbonitrile
(S)-2-((2-ethyl-6-(4-(2-
(3 -hydroxypyrro lidin-1 ¨
F
HO¨ci\ ¨ ti
¨' '-iC) /7---<
c' y1)-2-
_-,-- / B 1/B2-
oxoethyl)piperazin-1-
N¨(/ Cl¨D1¨
, ------, y1)-8- 603
"1 1
"---4s-\)---CN E2-Flb- 602
S c methyl imi dazo [1,2- (M+1)
F5b-F8
¨\ a]pyridin-3-
(Cpd 1)
yl)(methyl)amino)-4-(4-
fluoropheny Othiazo le-5-
carbonitrile
F 2-((2- ethy1-6-(4-(243 -
Ho \ /7---) hydroxyazetidin-l-y1)-2-
¨N
oxoethyl)-3,3-
,0
N---(
)---/ dimethylpiperazin-1-y1)- F8
617
6
i>.- \ IL )----cN 8-methylimidazo [1,2- (Gen-
10- 616
1,,I
pl- -S alpyridin-3- x) (M+1)
,,,,r__&,>_
yl)(methyl)amino)-4-(4-
-, fluorophenyl)thiazo le-5-
carbonitrile
2-(4-(2-ethy1-344-(4-
HO
F fluorophenyl)thiazol-2-
N
I yl)(methyl)amino)-8-
I' methylimidazo [1,2- F8 (Gen-
563 564
7 ''¨'
Ll \ N¨ )'
alpyridin-6- 10n) (M+1)
L )N1> \ yl)piperazin-1-y1)-1-(3-
-,y
hydroxyazetidin-l-
ypethanone
185
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
OH F
(R)-2-(4-(2- ethyl-34(4-
K/ (4-fluorophenyl)thiazol-
ro 2' 2-y1)(methyl)amino)-8-
N¨ methylimidazo [1,2- F8 (Gen- 578
8
'N'-''
I I \ ii i
N s a]pyridin-6- 10n) 577
(M+1)
yl)piperazin-l-y1)-1-(3-
----- ''N"
'N ---- \
hydroxypyrrolidin-l-
r-
I yl)ethanone
OH F
(S)-2-(4-(2-ethyl-3-((4-
, f-----j
(4-fluorophenyl)thiazol-
\--N
r 2-y1)(methyl) amino)-8-
ro
N ¨ \ methylimidazo [1,2- F8 (Gen- 578
577
N . a]pyridin-6- 10n) (M+1)
- '-ri'------N----% yl)pip erazin-l-y1)-1-(3 -
N
1 \ hydroxypyrro lidin-1-
'
yl)ethanone
24(2- ethy1-6-(1-(2-(3 -
Fõ,------
hydroxyazetidin-l-y1)-2-
oxoethyl)piperidin-4-
)------N y1)-8- F8
CN N ,.., 588
-- --..,
I methylimidazo [1,2- (Gen-10- 587
(M+1)
0 r .--, , a]pyridin-3- o)
"Ty yl)(methyl) amino)-4-(4-
HO
fluorophenyl)thiazo le-5-
carbonitrile
2-(ethyl(2-ethy1-8-
F methyl-641-
1
AI' (methylsulfony1)-
o
---\ n \
6 N 1 N---`= tetrahydropyridin-4- F2 564 565
yl)imidazo [1,2- (Gen-5-W S ON
(M+1)
a]pyridin-3-yDamino)-4 -
---N \ (4-
fluorophcnyl)thiazo le-5-
carbonitrile
F
24(2- ethy1-8-fluo ro-6-
/¨c (4-(2-(3-
hydroxyazetidin-l-y1)-2-
H0,_\
12 oxo ethyl)pip erazin-1 - F8
\---.N ,õ
'''N' , fi---µ, yl)imidazo [1,2- (Gen-10- 592 593
's CN (M+1)
o c N a]pyridin-3- m)
¨ ----1,-- ,(.1)____Ni, \
yl)(methyl)amino)-4-(4-
fluorophenyl)thiazo le-5-
carbonitrile
F 24443 4(5-cyano-4-(4-
H r---- \S fluorophenyl)thi azol-2-
,Np
yl)(methyl)amino)-2-
N- F8
13
' N----
N'1 ethyl-8- 551
'-`, il--. 1;1 / s CN fluoroimidazo [1,2- (Gcn-10-
550(M+1)
m)
,- J'--N> \ a]pyridin-6-
1 yl)piperazin-l-y1)-N-
F methylacetamide
186
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F 2-(4-(2-ethy1-8-fluoro-3 -
HO. i---- ((444-
--,---- fluorophenyl)thiazol-2-
N- -/ F8
14 'N' i µN--, yl)(methyl)amino)imida
(Gen-10- 567 568
s- zo[1,2-a] pyri din-6-
1) (M+1)
yl)piperazin-l-y1)-1-(3-
',, N ,
hydroxyazetidin-l-
yl)ethanone
HO F (S)-2-(4-(2-ethy1-8-
r---, A- --/ fluoro-3-44-(4-
i t/
\ -t=1,_.,,0 /)--=-:___--/ fluorophenyl)thiazol-2-
F8
N--1 yl)(methyl)amino)imida 582
15 -.N.- 1
\ N---- \) (Gen-10- 581
LN,, 11 s.' ZO [1,2-a] pyridin-6-
1) (M+1)
yl)piperazin-l-y1)-1-(3-
' N hydroxypyrrolidin-1-
-)-- .¨.\
F ypetlianone
HOt/F (R)-2-(4-(2-ethyl-8-
(.1 e fluoro-3-((4-(4-
fluorophenyl)thiazol-2-
/ F8
N--. t 16 N
yl)(methyl)amino)imida 582
'N'-'`I \ ---- \)
' ' ' ZO [1,2-a] pyridin-6- (Gen-10- 581
s (M+1)
'--- iq"--t
yl)piperazin-1-y1)-1-(3-
1)
hydroxypyrrolidin-1-
F yl)ethanone
F 4
/ 2-(4-(2-ethy1-3-44-(4-
\ N-- N )____C fluorophenyl)thiazol-2-
S ¨ yl)(methyl)amino)-7-
N / N
--- --.../ methylimidazo [1,2- F8 563
17 I a] pyridin-6-yl)pip eridin- (Gen-10- 562
(M+1)
0 1-y1)-1-(3-
(---.- s)
H 0
C iN N ' ' 7 ' hydroxyazetidin-l-
yOethanone
F 2- [(2-Ethy1-7-fluoro-6-
HO,rn
4111 {4- [2-(3 -hydroxy-
I. azetidin-1-y1)-2-oxo-
F8
18 NrTh \1\11 \ ethyl] -pip erazin-l-y1{ - 593
1 S CN imidazo [1,2-a]
pyridin-3- (Gcn-10- 592 (4+1)
---- N \ r)
\ y1)-methy1-amino]-4-(4-
fluoro-pheny1)-thiazole-
5-carbonitrile
F 2- [4-(2-Ethy1-7-fluoro-
H0,,_.n
4111 3- {[4-(4-fluoro-pheny1)-
\---h,e0
thiazol-2-yl] -methyl-
F8
19 Le") \ N¨ \ amino{ -imidazo [1,2-
(Gen-10- 567 568
s alpyridi n-6-y1)- (M+1)
cl)
p iperazin-l-yl] -1-(3 -
F
hydroxy-azetidin-l-y1)-
ethanone
187
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
HO F (R)-2-[4-(2-Ethy1-7-
bN,,c0 ii, flpuhoe rnoy-13) - tli [1 i 4 a-z(40 -1 fl2u yo IT -
A-Bl-C 1-
D2-E1- 594
20 Ni \ N-4,1 \ methyl-amino}-
F 1 a-F8 581 (M+N
imi dazo [1,2-a] pyridin-6-
(Gen-10- a)
y1)-piperazin-1-y1]-1 -(3-
CO
hydroxy-pyrro lidin-1-
y1)- ethanone
Ho_ F (S)-244-(2-Ethy1-7-
411 C fluoro-3- { [444- fluoro-
1N ,e,0 pheny1)-thiazol-2-y1]-
F8 594
21 '1\INI \ IN ---j.s \ methyl-amino)-
(Gen-10- 581 (M N
imidazo [1,2-a] pyridin-6-
cl)
y1)-pip erazin-1-y1]-1- (3 -
a)
F1."-N \ hydroxy-pyrro lidin-1-
y1)- ethanone
F
0 24443 -((5-cyano-4-(4-
fluorophenyl)thiazol-2-
\
HN-.10 yl)(methyl)amino)-2- F8
588
22 L-N 7Th \I N¨Es sN \ CN ethylimidazo [1,2-
(Gen-10- 587
(M+1)
L..../N,õ N.,..... alpyridin-6- k)
/1-'sN \ yl)piperazin-l-y1)-N-
methylacetamide
F-
-' N_ / tert-butyl 4-(2-ethy1-3-
y' ((444-
I s/ _-_-z__--(\
fluorophenyl)thiazol-2-
N z N Fib 537
23 5 </' yl)(methyl)amino)imida 536
(Gen-5-c) (M+1)
i---N- - zo[1,2-a]pyridin-6-
N J yl)piperazine-l-
,,õo
carb oxyl ate
o
F \ (- ' 7-
2-(4-(2-ethyl-3-((4-(4-
=,--N \ / fluorophenyl)thiazol-2-
F8
s r---- \ yl)(methyl)amino)imida (Gen-10- 550
N ,N
24 I ' zo[1,2-a]pyridin-6- 549
c) (M+1)
yl)piperazin-l-y1)-1-(3-
ri J hydroxyazetidin-1-
HO" yl)ethanone
F- (S)-2-(4-(2-ethyl-3-((4-
(4-fluorophenyethiazol-
F8
\ \---S7¨ ; '( 2-
N N yl)(methyl)amino)imida (Gen-10- 564
25 ,- ---/-
I 1 zo [1,2-a] pyridin-6- c) 563
(M+1)
H yl)piperazin-l-y1)-1-(3-
,,
HO. / N - hydroxypyrrolidin-1-
\_ I ypethanone
188
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
(R)-2-(4-(2- ethyl-34(4-
1 (4-fluorophenyethiazol-
--
2- F8
-s
yl)(methyl)amino)imida (Gen-10- 564
26 I zo[1,2-a] pyri din-6- e) 563
(M+1)
0 r,,,,,_,
yl)piperazin-l-y1)-1-(3 -
H0--(--1,1'-''N'¨
-- , hydroxypyrro lidin-1-
yl)ethanone
z
N-(142-(4-(2-ethy1-3-
-- ((4-(4-
\ ---s/ --z----K\N fluorophenyl)thiazol-2-
, ,I1, F8
yl)(methyl)amino)imida 605
27 (Gen-10- 604
? , -----N---:---- zo[1,2-a]pyridin-6- (M+1)
e)
-----\ _J-K N yl)piperazin-l-
H
yl)acetoyl)pyrro lidi n-3-
\,
yl)acetamide
_ /F 2-(4-(2-ethy1-344-(4-
F,
"\-- \ fluorophenyl)thiazol-2-
/\=------/ yl)(methyl)amino)imida F8
28 ' -t-\( zo[1,2-alpyridin-6- (Gen-10- 551
552
'N/----\ 'N, - ''s ) (M+1)
i
,_____<,/-õ, _ yl)pip crazin-l-y1)-1-(3 - c)
1 \
fluoroazetidin-1-
---").--N
ypethanone
F 1-(3,3-difluoroazetidin-
F /
(/7-----\\' 1-y1)-2-(4-(2-ethy1-3-
((4-(4-
F8
29 '-fs
\ Ni_r_t3 fluorophenyl)thiazol-2- (Gen-10 569 570
l/---- -
yl)(methyl)amino)imida (M+1)
------' ----- N----, )
zo [1,2-a] pyridin-6-
e)'N \ yl)piperazin-l-
yl)ethanone
F 1-(azetidin-l-y1)-2-(4-
(2- ethy1-3-((4-(4-
)----- /- fluo rophenyl)th iazol-2- F8
30 ,\,,N* N
ii ---\c yl)(methyl)amino)imida (Gen-10- 533
534
-N'Th (M+1)
s zo [1,2-a] pyridin-6- e)
yl)piperazin-l-
L----j'N \
yl)ethanone
F
^ / 2-(4-(2-ethy1-344-(4-
/:
fluorophenyl)thiazol-2-
F8
31 'N'/------) \NI -( yl)(methyl)amino)imida
zo [1,2-a] pyridin-6- (Gen-10-
547 548
\
yi\l----- - -1 's-'
yl)piperazin-l-y1)-1- e) (M+1)
:ii \
(pyrrolidin-l-
yl)ethanone
F2 / 2-(4-(2-ethy1-344-(4-
-
s 't-----\ fluorophcnyl)thiazol-2-
F8
rsi 7,N yl)(methyl)amino)imida 564
32 (Gen-10- 563
- zo [1,2-a] pyridin-6- (M+1)
o r N e)
- r N ,N j yl)piperazin-l-y1)-1 _ --
O J morpholinoethanone
189
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F
¨ 2-(4-(2-ethy1-3-44-(4-
H2N0 K7 1/
____/ fluorophenyl)thiazol-2- F8
,
N--7( yl)(methyl)amino)imida (Gen-10- 494
33 \ // I\ 493
''N'----A N-----\ ZO[1,2-a]pyridin-6- e) (M+1)
s yl)p ip erazin-1-
1
"-k-----N/ \ yl)acetamide
OH 2-(4-(2-ethy1-344-(4-
I /F
fluorophenyl)thiazol-2-
F8
'.% )_--, yl)(methyl)amino)imida
(Gen-10- 564
34 \N--!,-,.)C zo [1,2-a] pyridin-6-
e) 563
(M+1)
L.1 yl)piperazin-1-y1)-1-(3-
N,,---õ. .\
(hydroxymethyl)azetidin
-1-ypethan one
F
I %-----( 2-(4-(2-ethy1-3-((4-(4-
0N ) \) fluorophenyl)thiazol-2-
F8
N yl)(methyl)amino)imida 522
--=/ --( (Gen-10- 521
V" ZO[1,2-a] pyri din-6- (M+1)
e)
yl)piperazin-l-y1)-N,N-
\ dimethylacetamide
F
e---- ethyl 2-(4-(2-ethyl-3-
*-----1/ ((4-(4-
36 '' --ir--N'--' -j1
\N- .-'' fluorophenyl)thiazol-2- F8
(Gen-10- 522 523
I___ fj / s yl)(methyl)amino)imida (M+1)
e)
zo [1,2-a] pyridin-6-
--'-----,._..- -j---'-'N \ yl)piperazin-l-yl)acetate
, ethyl 24442- ethy1-3-
1 ((4-(4-
c)--.
fluorophenyl)thiazol-2- F8
537
37 'N' ) \ N4, ,,,
yl)(methyl)amino)imida (Gen-10- 536
7-. õ, (M+1)
" i --,j, F zo [1,2-a] pyridin-6- e)
yl)pip erazin-1-
yl)propano ate
F -----
----,(z
l... N / 244-(2-ethy1-344-(4-
-- - - ---- - ¨ - - - r\ -----N / fluorophenyl)thiazol-2-
L s' --)-------A F8
y)(mey)amno)ma
38 N l thl iiid 476
N (Gen-10- 475
zo[1,2-a]pyridin-6- (M+1)
e)
yl)piperazin-1 -
NI; r' 'NI
yl)acetonitrile
F ---- N-(6-(4-0-
N /
------z '7\ ---1µ1, cyclopropy1-1H-
'¨s tetrazol-5-
,NN F8
yl)methyl)piperazin-1- 559
39 (Gen-10- 558
/!\I 'N r-' N ''-- y1)-2-ethylimidazo [1,2-
e) (M+1)
N 14 a] pyri din-3 -y1)-4- (4-
fluoropheny1)-7V-
methylthiazol-2-amine
190
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F --f L- N-(2-ethy1-6-(4-(oxazol-
N / 2-ylmethyl)piperazin-1-
yl)imidazo [1,2- FR
40 ,N,N (Gen-10- 517 518
' I a] pyridin-3-y1)-4-(4-
e) (M+1)
/;---N
fluoropheny1)-N-
, : N ---,
methylthiazol-2-amine
0--
F z--- N-(6-(4-((1,2,4-
7
1 N / oxadiazol-3-
---7 .-- )------N \ /
yl)methyl)piperazin-1- F8
---s -)------\- ,, 519
41 ,rsi.õIN y1)-2-ethylimidazo [1,2-
(Gen-10- 518
(M+1)
alpyridin-3-y1)-4-(4- e)
p¨N r--N----
1 fluoropheny1)-N-
i\i'---N1-- methylthiazo1-2-amine
/F
/F------( 2-(4-(2-ethy1-3-44-(4-
v \)
HOO fluorophenyl)thiazol-2-
42 'N-'1 N---7(
-/s yl)(methyl)amino)imida F13
494 495
zo [1,2-a] pyridin-6- (Cpd 36) \N=- (M+1)
N -
7 ---- ,- ---...-- -------- yl)piperazin-l-yl)acetic
\) s
N \ acid
2-hydroxyethyl 4-(2-
--, ----ci --=NI, / .. ethyl-3-((4-(4-
'¨s 1----\., fluorophenyl)thiazol-2-
see Cpd
43 525
43 ErN/.'.
¨ -. yl)(methyl)amino)imida
(Gen-10- 524
(M+1)
r Isr ZO[1,2-a]pyridin-6-
., Ø N, J yl)piperazine-1-
e)
HO - 1(1' ¨
carboxylate
F, tert-butyl 2-(4-(2-ethyl-
-t N r¨
N / 3-((4-(4-
,,...-- \
fluorophenyl)thiazol-2-
F9a
yl)(methyl)amino)imida 634
44 -I (Gen-10- 633
r r¨N --- zo[1,2-a]pyridin-6-
e) (M+1)
<
= N yl)piperazine-1-
' i" TI carb onyl)pyrroli dine-1-
---- 0 - ;(:) 0
carboxylate
tert-butyl 3-(4-(2-ethyl-
-('./
\ '---%____/¨ fluorophenyl)thiazol-2-
\ /
N /
,- --,/ F9a
yl)(methyl)amino)imida 634
45 o--K7 I zo[1,2-a]pyridin-6- (Gen-10- 633
(M+1)
e)
yl)piperazine-1-
\
carb onyl)pyrrolidinc-1 -
11
o carboxylate
(4-(2-ethy1-344-(4-
-,--7 -0--N, /¨ fluorophenyl)thiazol-2-
'---s ------ \N yl)(methyl)amino)imida F5b
534
46 I - zo [1,2-a] pyridin-6- (Cpd 44) 533
(M+1)
7-7------- yl)piperazin-1-
yl)(pyrrolidin-2-
H -r ¨ yl)methanone
o
191
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F-------.
T N / (4-(2- ethy1-3 4(444-
------, -c_. __----N / fluorophenyl)thiazol-2-
' yl)(methyl)amino)imida
N N F5b 534
47 -, zo [1,2-a] pyridin-6- 533
H (Cpd 45) (M+1)
N----,1 !''''-isr'' yl)pip erazin-1-
yl)(pyrrolidin-3-
'11 - yl)methanone
o
F. -,i 1 -(3 -(4-(2-etby1-3 44-
,
(4- flu orophenyl)thiazo1-
LS )==\ 2-
F9b
,o A,j, /,N yl)(methyl)amino)imida 576
48
-----' 1 zo [1,2-a] pyridin-6- (Cpd 47) 575
(M+1)
ii-__I i 4.1
yl)piperazine-1-
\
I I carbonyl)pyrrolidin-l-
o yl)ethanone
' \
N / (4-(2- ethy1-3 4(444-
------- ---cr -,----N ,' fluorophenyl)thiazol-2-
\
0 r,N. :Isi yl)(methyl)amino)imida Fll
612
49 11
---S-- '' zo [1,2-a]pyridin-6- (Cpd 47) 611
(M+1)
\N yl)piperazin- I -y1)(1-
- N ¨
/
N i (methylsulfonyppyrrolid
---_,-- ---,,_ -
I in-3 -yl)methanone
0
F--C)¨ N / 1 -(4-(2-ethy1-3 4(444-
----- ---- =)---N /
L-6 [-----\ fluorophenyl)thiazol-2-
F9a
N / N yl)(methyl)amino)imida 495
I zo[1,2-c]pyridin-6- (Gen-10- 494
(M+1)
.--- -,..N.-----,_-.% 1 1 yl)piperazin-1-y1)-2-
e)
HO 11 ¨ hydroxyethanone
0
N /
1 -(4-(2-ethy1-3 4(444-
---_----)-- ' .
'-s
fluorophenyethiazol-2-
F9b
7N,,N yl)(methyl)amino)imida 493
51
I zo[1,2-c]pyridin-6- (Gen-10- 492
(M+1)
e)
yl)piperazin-1-
,----,,i -, ,-- yl)prop an-1 - one
j-
F¨ - ---
N 1 1-(4-(2-ethy1-344-(4-
---41\ __-(--
S ---- fluorophenyl)thiazol-2-
F9b
7k,,N yl)(methyl)amino)imida 523
52
I zo [1,2-a] pyridin-6- (Gen-10- 522
(M+1)
yl)piperazin-1-y1)-4-
e)
hydroxybutan-1- one
o
192
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F ,
y , 4-(dimethylamino)-1 -(4-
/
1 % ' t_-___-(
(2- ethy1-3 4(444-
S
fluorophenyl)thiazol-2- F9b-F12a
550
53 ,- ---
I yl)(methyl)amino)imida (Gen-10- 549
(M+1)
r Ni zo[1,2-a] pyri din-6- e)
yl)piperazi n-l-yl)butan-
'14--1-c1N-'
1-one
F
/7----- N -(2- ethy1-6-(4-
\/ (methylsulfonyl)piperazi
N_T----'-----/ n-l-yl)imidazo [1 Fll
,2- 515
54 ,-s- N'I \ -N- a] pyridin-3 -y1)-4-(4- (Gen-10-
514(M+1)
\ fluoropheny1)-N- e)
- > \ methylthiazol-2-amine
F z"-----
----c
N / N-(6-(4-(3-
-,----_,_õ, _ = . n; ,
chloropropylsulfonyl)pi
S N/,4 perazin-1-y1)-2- Fll 577
,
55 ethylimidazo [1,2- (Gen-10- 576
(M+1)
r'N-"---% a] pyridin-3 -y1)-4-(4- c)
,N,_,J fluoropheny1)-N-
CI--õSµ methylthiazol-2-amine
NO
F,Z"- 11 /
N-(6-(4-(3-
_
/¨ (dimethylamino)propyls
s -r---- \ ulfonyl)pip erazin-1 -y1)-
,,, N N
56 2-ethylimidazo [1,2- F12a
586585
(Cpd 55) (M+1)
r¨N---r- a] pyridin-3 -y1)-4-(4-
ii Rµs,N.j fluoropheny1)-N-
,--
c, ------ -- - methylthiazol-2-amine
,.õ
1 , N-(2-ethyl-6-(4-(3-
F._(--).N /
----- ----(\ (pyrrolidi n-1-
\-----s l¨ \NI yl)propyls ulfony pip era
,Nõ¨/. F12a 612
57 f zin-1-yl)imidazo[1,2-
(Cpd 55) 611
(M+1)
N a] pyridin-3 -y1)-4-(4-
- r ... rIv i fluoropheny1)-N-
s
o methylthiazol-2-amine
F- (7,.,, NJ, /
3 -(4-(2-ethyl-3 4(444-
-----z --c,- ,¨N \ / fluorophenyl)thiazol-2-
; N yl)(methyl)amino)imida F12b-F13 559
58 zo [1,2-a]pyridin-6- (Cpd 55) 558
(M+1)
N-----.
0 Nr j yl)piperazin-1 -
HO µ'µS -`-
- , ylsulfonyl)propan-l-ol
'o
F ,_(5-,-----
methyl 24442- ethyl-3-
../ /¨ ((4-(4-
õ r...,õ z
L s )------\ fluorophenyl)thi azol-2- Fll
,N.õ,,,õN 573
59 yl)(methyl)amino)imida (Gen-10- 572
I r N - ZO[1,2-a]pyridin-6- e) (M+1)
9.1 (:),\ ,N, yl)piperazin-1-
'-o -- c, ylsulfonyl)acetate
193
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F 2-(4-(2-ethy1-3 4(444-
----- -----. ¨Ns\ /---
4 [----\ fluorophenyl)thiazol-2-
,õN,,,),N yl)(methyl)amino)imida F13 559
60 558
I zo [1,2-a] pyridin-6- (Cpd 59) (M+1)
yl)piperazin-1-
(i)t \\s,N,,) ylsulfonyl)acctic acid
HO' '---" \
0
F7
2-(4-(2-ethyl-3-((4-(4-
---
fluorophenyl)thiazol-2
-s )------, -I\ see Cpd
N N yl)(methyl)amino)imida 558
61 ,-
I 61 557
zo [1,2-a] pyridin-6- (M+1)
o 0 r " yl)pip erazin-1-
(Cpd 60)
I _.\\s,Nõ,_ ,, ylsulfonyl)acetamide
Hp(
F - ----
Ti y , tert-butyl 4-(2-ethyl-3-
--' \ =';7.¨N /--- ((4-(4-
fluorophenyl)thiazol-2-
,N = N F4 551
62 ---
, yl)(methypamino)imida
(Gen-5-b) 550
(M+1)
zo[1,2-a]pyridin-6-y1)-
-,, õ0õN, _ .,===0 3 -oxopiperazine-1 -
/II 'f
o carboxylate
a - ¨ tert-butyl 4434(444-
566
chlorophenyl)thiazol-2-
-- - ---- :,=.=-=-N '¨
yl)(methyl)amino)-2- (35C1
567
F4
,
63 I ethylimidazo [1,2-
(Gen-5-i) 5)68 (35C1
'-1-- f---N- a]pyridin-6-y1)-3-
(37C1 M+1)
-fl
0õN -0 , ,=-k-, oxopiperazine-1-
¨
)
O carboxylate
F , ethyl 2-(4-(2-ethyl-3-
-1
N I ((444-
').---N /
fluorophcnyl)thiazol-2- see Cpd64 537
-s
v
64 ,N ,,,,N yl)(methyl)amino)imida 536
I zo [1,2-cdpyridi n-6-y1)- (Gen-10-
(M+1)
o r-- 'N''''''-- d)
it ,L 3-oxop iperazi n-1-
'0- -)1 =0 yl)acetate
1-(2- ethy1-3 -((4-(4-
fluorophenyl)thiazol-2-
A
yl)(methypamino)imida F11
N 529
65 ,- --, /
J zo[1,2-a]pyridin-6-y1)- (Gen-10- 528
(M+1)
_ ----N. ----õ7 4- d)
o I [
(m ethyl sul fo nyl)p ip erazi
s ¨ o
7 , n-2-one
o
N -(2- ethy1-6-( 1-
(methylsulfony1)-
Vi--N)----(/ 1,2,3,6-
s , F11
66
N N tetrahydropyridin-4- 512
1 yl)imidazo [1,2-
(M+1) (Gen-10- 511
0 i a]pyridin-3-y1)-4-(4-
b)
,\
fluoropheny1)-N-
0 methylthiazol-2-amine
194
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F N-(6-(1-
T N / (chloromethylsulfonye-
, VrN \r_i_
1,2,3,6-
s Fll
tetrahydropyridin-4-y1)- 546
67 . ---,
I 2-etbylimidazo [1,2- (Gen-10-
545
(M+1)
b)
a] pyridi n-3 -y1)-4-(4-
_ rU
ci b , -- '' fluoropheny1)-N-
\\o methylthiazol-2-amine
CI
,.( 4-(4-chloropheny1)-N-
(2- ethy1-6-(1- 513 514
, (35C1 (35C1
(methylsulfony1)-2,5- Fl 1
68 ,i-N dihydro-1H-pyrrol-3- (Gen-10-
), M+1)
\ il 515 516
s-----\ yl)imidazo [1,2- .1)
o /------,, N¨ CO
(37c1
a] pyridin-3 -y1)-N-
) M+1)
, 1-, \> \ methylthiazo1-2-amine
---- --N
CI
/--._
\----- 4-(4-chloropheny1)-N-
(2- ethy1-6-(1- 527 528
,
I (methyls ulfony1)-
,,5,6- Eli
14 ("C1
(35C1
69 (Gcn-10-
), M+1)
tetrahydropyridin-3- 529 530
r-----,, s ,N______
yl)imidazo [1,2- ae) (37C1 (37C1
,N,,--7-,, ., a] pyridin-3 -y1)-N- )
M+1)
O o ri___ ) \
methylthiazol-2-amine
N ,
4-(4-tert-butylpbeny1)-
z .-r
1,1 / N-(2-ethyl-6-(1-
_ /
\ r (methylsulfony1)-
F2
s N 1,2,3,6- 550
70 N ,
(Gen-5- 549
tetrahydropyridin-4- (M+1)
,%----'' yl)imidazo [1,2- g)-
0 I
a] pyridin-3 -y1)-N-
\o methylthiazol-2-amine
I o N-(2- ethy1-6-(1-
__y/,------
(methylsulfony1)-
/-- 1,2,3,6-
Fll
tetrahydropyridin-4- 524
71 1,1,N (Gen-10- 523
I yl)imidazo [1,2-
ac) (M+1)
a] pyridin-3 -y1)-4-(4-
r
methoxypheny1)-N-
s'
, metbylthiazol-2-amine
o
F F F N-(2- ethy1-6-(1 -
(methylsulfony1)-
0,7,
1,2,3,6-
tetrahydropyridin-4- Fll
578
72 1---s
N N yl)imidazo [1,2- (Gen-10-
577
---../ (M+1)
I a] pyridin-3 -y1)-N- ah)
,- --.- ---
1 methy1-4-(4-
9 N (trifluoromethoxy)pheny
'o 1)thiazol-2-amine
195
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F
F, 4-(3,4-difluoropheny1)-
,N / N-(2-ethyl-6-(1-
s - (methylsulfony1)-
1,2,3,6- Fll
530
73 NN 'I (Gen-10- 529 tetrahydropyridin-
4- (M+1)
aj)
yl)imi dazo [1,2-
c)\ N - a]pyridin-3-y1)-N-
* .`--- methylthiazol-2-amine
0
3 -(4-(2-ethy1-3 4(444-
fluorophenyethiazol-2-
2'----.-----N)-=(r--
NI, N yl)(methyl)amino)imida F11-F12b
598
74 -I' J zo[1,2-a]pyridin-6-y1)- (Gcn-10- 597
(M+1)
. r" 5,6-dihydropyridin- b)-
1(2H)-ylsulfonyl)propyl
o acetate
F, .,-.:% - ,
3 -(4-(2-ethyl-3 4(444-
N /
, N _
fluorophenyl)thiazol-2-
\ I--
yl)(methyl)amino)imida
N / N F13 556
75 zo[1,2-a]pyridin-6-y1)- 555
I (Cpd 74) (M+1)
r----,,,--, 5,6-dihydropyridin-
9\_ N ,- 1(2H)-
(10- ----
O ylsulfonyl)propan-1-ol
CI
)-----, 4-(2- ethy1-3 4(444- 498
499
) chlorophenyl)thiazol-2- see (3C1 (35C1
76 yl)(methyl)amino)imida Cpd76
(Gen-10-g ), M+1)
zo [1,2-a] pyrid in-6-y1)- 500 501
,µ
3,6-dihy dro-2H- or cpd (37CI (37c1
1 \) thiopyran 1,1-dioxide ) M+1)
F N-(2-ethyl-6-(1-
N / (methylsulfony1)-
----
1,2,3,6-
F ,õd ---'\
N , N tetrahydropyridin-4- F16b
530
77 -= /
1 yl)imidazo [1,2- (Cpd 66) 529
(M+1)
1 a] pyridin-3-y1)-5-fluo ro-
4-(4-fluoropheny1)-N-
' O methylthiazo1-2-amine
.N / tert-butyl 442- ethy1-3 -
((4-(4-
\----s --)--'\ fluorophenyl)thiazol-2- F2-see
N N 552
78 OH yl)(methyl)amino)imida Cpd 78 551
----, -7,- zo [1,2-a] pyridin-6-y1)-3- (Gen-5-c) (M+1)
0 N , hydroxypip eridine-1-
7' If --- carboxylate
o
196
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F %------
4-(2-ethy1-34(4-(4-
fluorophenyl)thiazo1-2-
---S ------ A Fll
,N yl)(methyl)amino)imida 530
79 r r,j zo[1,2-a]pyridin-6-y1)-1- (Gen-10- 529
(M+1)
(methylsulfonyl)piperidi ab)
o, isl. 7 n-3 - ol
-3
' \\ -
0
F,Q ,
N-(2-ethyl-6-(1-
NI _N,/, z
\\
(methylsulfonyl)piperidi --s Ts---\ F11-F6
NN n-4-yl)imidazo[1,2- 514
80 I a] pyridin-3 -y1)-4-(4- (Gen-10- 513
(M+1)
7 r fluoropheny1)-N-
b)
\ N
-- , methylthiazol-2-amine
o
,-_
4-(4-tert-butylpheny1)-
-s N-(2-ethyl-6-(1-
(methylsulfonyl)pip eridi F2-F6
552
81 7NN
I n-4-yl)imidazo[1,2- (G en-5- 551
(M+1)
g)-
1--- '-{-----'--- a] pyridin-3 -y1)-N-
9, N. methylthiazo1-2-amine
s' -
,6
O ,,,
N / N-(2- ethy1-6-(1-
/
I-S -)-----= (methylsulfonyl)piperidi
n-4-yl)imidazo[1,2- F11-F6
526
82 N (Gen-10- 525
'I a] pyridin-3 -y1)-4-(4-
(M+1)
methoxypheny1)-N-
ac)
R .,11
, s ----- methylthiazo1-2-amine
'o
F
Fõ \
f 4-(3,4-difluoropheny1)-
N-(2- ethy1-6-(1-
(m ethyl sul fonyl)pip eri di F11-F6
532
83
--- --../ (Gen-10- 531
I n-4-yl)imidazo[1,2- (M+1)
aj)
a] pyridin-3 -y1)-N-
2s, methylthiazol-2-amine
o
F õ4--_. N, / N-(2- ethy1-6-(1 -
S (methylsulfonyl)piperidi
n-4-yl)imidazo[1,2- F11-F6
564
84 N--:i-N a] pyridin-3 -y1)-N - (Gen-10- 563
(M+1)
r%----L- methyl-4-(4- ai)
o I (trifluoromethyl)phenyl)
s --,, 7- thiazol-2-amine
,- \ o
F F F
-To N-(2- ethy1-6-(1-
V N / (methylsulfonyl)piperidi
n-4-yl)imidazo[1,2- F6-F11
----C:?---N, /
580
85 ' s )----
N ,.,,N a] pyridin-3 -y1)-N- (Gen-10- 579
methyl-4-(4- au) (M+1)
Ali (trifluoromethoxy)pheny
s,
O 1)thiazol-2-amine
197
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F
N / N-(6-(1-(3-
575 576
- )------ chloropropylsulfonyl)pi
peridin-4-y1)-2- Fl 1 (35c1 (35c1
,N,,,,,,s, ), M+1)
86
I ethyl imi d azo [1,2- (Gen-10-
577 578
a]pyridin-3-y1)-4-(4- c) (37c1 (37c1
fluoropheny1)-N-
) M+1)
methylthiazol-2-amine
0
F c---- _
N / N-(6-(1-(3-
--z N '
(dimethylamino)propyls
s / N ulfonyl)piperidin-4 -y1)-
F12a 585
87 2-ethylimidazo [1,2- 584
(Cpd 86) (M+1)
a] pyridin-3 -y1)-4-(4-
I 0 fluoropheny1)-N-
,\ õA., _,
,1\1. , S\ methylthiazol-2-amine
0
__(,;----
N-(2-ethyl-6-(1-(3-
F
), N /
----z =( )--%_____,(¨ morpholinopropylsulfon
L--s yl)piperidin-4-
F12a 627
88 ---
1 yl)imidazo [1,2-
(Cpd 86) 626
(M+1)
z-- --- -77 a] pyridin-3 -y1)-4-(4-
o I fluoropheny1)-N -
,N ,, \\s'N'--7- methylthiazol-2-amine
0
i N-(2-ethyl-6-(1-(3-
(pyrrolidin-l-
CIN*K.- yl)propylsulfonyl)piperi
N F12a 611
89 I din-4-yl)imidazo [1,2-
(Cpd 86) 610
(M+1)
a] pyridin-3 -y1)-4-(4-
ri o (
fluoropheny1)-N-
rsi \, ,-- ,s --
C methylthiazol-2-amine
FT
N / N-(6-(1-(3 -
aminopropylsulfonyl)pi
see Cpd
peridin-4-y1)-2-
N ,,N 90 557
90 ,
I ethylimidazo [1,2-
(Gen-10- 556
(M+1)
a] pyridin-3 -y1)-4-(4-
o I fluoropheny1)-N- 11)
\\ __N., ,
H2N -, S, ¨ methylthiazol-2-amine
O
--( N-(2-ethyl-6-(1-(2-
/
-N __---, morpholinoethylsulfonyl
)piperidin-4-
SCC
Cpd91 613
91 yl)imidazo [1,2- 612
(Gen-10- (M+1)
o I a] pyridin-3 -y1)-4-(4-
Y)
- 14 s = fluoropheny1)-N-
\
o i 0 methylthiazol-2-amine
198
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
1 /---N / 4-(2- ethy1-3 4(444-
---- - ---
[ \
fluorophenyl)thiazol-2- see
s
,NA, " yl)(methyl)amino)imida Cpd92) 515
92
I zo[1,2-a]pyridin-6- (Gen-10- 514 (M+1)
r----------"-. yl)pip eridine-1- c)
µ ti
['s' sulfonamide
H2N Ne,
FT
N /
3 -(4-(2-ethy1-3 4(444-
_.., , /
--- L:7----N,H fluorophenyl)thiazol-2-
yl)(methyl)amino)imida
Fl2b 600
93 'I , zo[1,2-a] pyridin-6- 599
.----õ,,,-----._--- (Cpd 86) (M+1)
0 r yl)piperidin-1-
0 N, ylsulfonyl)propyl
0 acetate
o
F-
3 -(4-(2-ethy1-3 4(444-
/ fluorophenyl)thiazol-2-
S N ji
yl)(methyl)amino)imida F13 558
94 .--- ---.7
I zo[1,2-a]pyridin-6- (Cpd 93) 557
(M+1)
yl)piperidin-1-
ylsulfonyl)propan-l-ol
o
F N J 3 -(442- ethyl-34(5-
--
fluoro-4-(4-
)----s fluorophenyl)thiazol-2-
F ,, F 1 6b 576
'I yl)(methyl)amino)imida 575
(Cpd 94) (M+1)
zo [1,2-a] pyridin-6-
Ho
..µ N yl)piperidin-l-
õ,_ ,s-" -----[
O ylsulfonyl)propan-l-ol
,F 2-(2-((2-ethy1-6-(1-
/7---
(methylsulfonyl)piperidi
o n- 4- = = yennidazo[1,2-
El 539
96 s,, _-- \, _ )) CN a]pyridin-3-
538
o ' N ' (Gen-9-
b) (M+1)
j s' yl)(methyl)amino)thiazo
1-4-y1)-5 -
------------1-1--- N\ \ fluorobenzonitrile
)
2-(2-((2-ethy1-6-(1-
õ1:rõ:
/ _ C (m ethyl sul fo nyl)p ip eri di n-4-yl)imid azo [1,2-
/ _NI ,N El 553
97 _ -,--
I a]pyridin-3- 552
(Gen-9-b) (M+1)
r ' yl)(methyl)amino)-5-
o. methylthiazol-4-y1)-5-
--s ¨
o fluorobenzonitrile
/F N-(2-ethyl-6-(l-
(7 1 (m ethyl sul fonyl)pip eri di
o i\---- n-4-yl)imidazo[1,2-
1/
0 N
N N- ,( E 1 528
98 -s \ ___--<? \\ \ a] pyridin-3 -
y1)-4-(4- 527
' -- (Gen-9-b) (M+1)
L. / S fluoro-2-methylpheny1)-
-,-- --_--- -N-
N-methylthiazol-2-
-` [-NI \ amine
199
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F
4-(2-chloro-4-
\/ \ 547
fluoropheny1)-N-(2-
("Cl
o o
N---- ethyl-6-(1- 548
S , ¨ a El )
99 / N.- I' \ \I
(methylsulfonyl)piperidi ("C 1
s (Gen-9-b) 549
2 1 id 4 n--yl)imazo[,- M+1)
(37C 1
a] pyridin-3 -y1)-N-
"---- ---> -N N )
methylthiazol-2-amine
F
/\ >
;----(/ 4-(2,4-difluoropheny1)-
cil / N-(2-ethyl-6-(1-
N ¨( (methylsulfonyl)piperidi E 1
532
100 ? N.,- \ / \I F 531
n-4-yl)imidazo[1,2- (Gen-9-b) (M+1)
a] pyridin-3 -y1)-N-
methylthiazol-2-amine
F
N -(2- ethy1-6-(1-
o
,4/'---
_______ 2 (methylsulfonyl)piperidi
1 N---/. n-4-yl)imidazo[1,2- El 528
101 s --- 'N---/ 527
o 'N '- a]
pyridin-3 -y1)-4-(4- (Gen-9b) (M+1)
I S
-N- fluoropheny1)-N,5-
., dimethylthiazol-2-amine
F
4-(4-fluoropheny1)-N-
)--, (2- ethy1-6- (1-
102
(methylsulfonyl)pip eridi El 517
\\ IP 7 N
\ iP D n-4-yl)imidazo[1,2- (Gen-9-
f) 516
(M+1)
s
-- ,N --------. S"--\ it
N-- D \ a] pyridin-3 -y1)-N-(d3-
c methy1)thiazo1-2 -amine
--`- -1----- \
F
2--õ 4-(4- fluoropheny1)-N-
k ,) (2- ethy1-6- (1-
BI-C1-
1-,, (methylsulfonyl)piperidi
F2-F6- 518
103 c) p D--__( 1.ri D n-4-yl)imidazo[1,2-
DI-El 517
(M+1)
N,^ S--I*, _____4-D a] pyridin-3 -y1)-N-(d3-
N
D I õ methyl)-(d-thiazol-2)- (G en-9-f)
amine
' 'L-N/ \
/ F methyl 24(2- ethy1-6-(1-
/;------\ \
,/ (methylsulfonyl)piperidi
o ,)---__--/ n-4-yeimidazo[1,2-
11, El 572
104 0 -N-^ \ , P1----\, 0 a]pyridin-3-
571
(Gen-9-b) (M+1)
N¨\s, 1,--,
yl)(methyl)amino)-4-(4-
J-- > \ fluorophenyl)thiazole-5-
'¨ N carboxylate
F 1-(2-((2- ethyl-6- (1-
(7 -\ (methylsulfonyl)piperidi
o n-4-yl)imidazo [1,2- see
Cpd
---, , 556
105 s ----õ rij---//
\\N--, .2---,,,,\ n a]pyridin-3- 105 555
(M+1)
s - ' yl)(methyl)amino)-4-(4- (Cpd 104)
------- ------ N-- \
L ')--- fluorophenyl)thi azol-5-
-.,/-- N \ ypethanone
200
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F'--f. N-(2-(2-((2-ethy1-6-(1-
. (methylsulfonyl)piperidi
see Cpd
N
/H S ,.I,L N n-4-yl)imidazo[1,2-
106 c
' ,;:,
a]pyridin-3- 106
570 571
(Gen-10- (M+1)
_, --. --: ..---.-- yl)(methyl)amino)thiazo
I ¨ ¨
al)
ch, lq. 7
' s -
fluorophenyl)acetamide
o
(2424(2- ethy1-6 -(1-
(methylsulfonyl)piperidi
see cpd
\ n-4-yl)imidazo[1,2-
HO' ,N, ,N 107 544
alpyridin-3-
(Gen-10- 543
(M+1)
107
I yl)(methyl)amino)thiazo
al)
o,õs A, _ ,,-- 1-4-y1)-5-
- 8 fluorophenyl)methanol
F -,,_(.2- ethyl 24442- ethy1-3-
N /
-7----1).- --- N \ " ((4-(4-
fluorophenyl)thi azol-2- F8
520
108 r\I , N
rr yl)(methyl)amino)imida (Gen-10- 519
(M+1)
1 zo[1,2-a]pyridin-6-y1)- .. b)-
o r- --,,--------
5,6-dihydropyridin-
II '
1(2H)-yl)acetate
F', ----
-N\ / ethyl 2-(4-(2-ethyl-3-
---\-; -;r___ /
((4-(4-
------\ fluorophenyl)thiazol-2- F8
109 N.. ,N (Gen-10- 521 522
yl)(methyl)amino)imida _, (M+1)
c)
0 ,--------- ----, --;----2 .. zo [1,2-a] pyridin-6-
yl)pip eridin-1-yl)ac etate
F--õ,,,,-õ- -
\ \)-.. ,N / 2-(4-(2-ethy1-344-(4-
------- --c- ¨NI /--- L fluorophenyl)thiazol-2-
N / N yl)(methyl)amino)imida F8
,. ,.õ, 549
110 I zo [1,2-a] pyridin-6- (Gen-10- 548
(M+1)
0 r --..---õ-- yl)piperidin-l-y1)-1-(3- c)
-11- [NI 71s1 hydroxyazetidin-1-
HO-
¨-
-----/ yl)ethanone
F, ,1õ;:, ----,
(R)-2-(4-(2- ethyl-34(4-
(4-fluorophenyl)thiazol-
2- F8
111 I yl)(methyl)amino)imida (Gen-10- 562 563
o '---'-'- zo[1,2-a]pyridin-6- c)
(M+1)
yl)piperidin-l-y1)-1-(3-
<_ hydroxypyrro lidin-1 -
HO yl)ethanone
201
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
(S)-2-(4-(2-ethy1-3-((4-
(4- fluorophenyethiazol-
L--s r-----\- , 2- F8
Ni , N
yl)(methyl)amino)imida (Gen-10- 563
112 562
? r------ zo[1,2-a]pyridin-6- c) (M+1)
,) N yl)piperidin-1-y1)-1-(3-
,/--N --' --'
)---J hydroxypyrro lidin-1 -
HO yl)ethanone
F ----
2-(4-(2-ethy1-34(4-(4-
/
,__ LN:7_ /,
fluorophenyethiazol-2-
y1)(methyl)amino)imida F8 563
õ/N
113 i zo[1,2-a]pyridin-6- (Gen-10- 562
(M+1)
yl)piperidin-1-y1)-1-(3- c)
:it iv
, -_-
(hydroxymethypazetidin
HO, -1--- / -1 -yl)ethanone
F,_
N / 2-(4-(2-ethy1-34(4-(4-
_, \ ,'7---N\ /
fluorophenyl)thiazol-2- F8
S
i r-----\
N /N yl)(methyl)aminonmida (Gen-10- 521
I
114 ,- -,,-
zo[1,2-a]pyridin-6- c) 520
(M+1)
o r--,--_=-
yl)pip eridin-1 -y1)-N,N-
I dimethylacetamide
F--Cz
N i 2-(4-(2-ethy1-344-(4-
-- fluorophenypthiazol-2-
-
, yl)(methyl)amino)imida F8
N / 547
115 -- --,,,i
I zo[1,2-a] pyrid in-6- (Gen-10- 546
(M+1)
O '-`1% yl)piperidin-l-y1)-1- c)
H 1
- (pyrrolidin-1-7--,,,, - ,NI--, ---
yl)ethanone
(S)-1-(2-(4-(2-ethy1-3-
F,_ õ.õ-: , N
/ ((4-(4-
--- /
fluorophenyl)thi azol-2- F8
N, ,N 116 571
yl)(methyl)amino)imida (Gen-10- 572
I zo[1,2-a]pyridin-6- c) (M+1)
o..---,, --- ---
r
yl)piperidin-1-
Nc. / //"N"--"'-'. yl)acetoyl)pyrrolidine-3-
1 carbonitrile
Ho 2-(4-(2-ethy1-344-(4-
c, fluorophenyl)thiazol-2-
r yl)(methyl)amino)imida F8
0N-_; /L, 577
117 ¨ / N ZO[1,2-a]pyridin-6- (Gen-10- 576
S.-1( yl)p ip eridi n-1 -y1)-1-(3 - c) (M+1)
(hydroxymethyl)pyrroli
J,- )-- din-l-yl)ethanone
,o ,F 4-((4-(2-ethy1-34(4-(4-
9-- (''-----, fluorophenyl)thiazol-2-
, ,o
' )------/ yl)(methyl)amino)imida F8
118 k_ -
N- N--(
\ Nj/µ ) ZO [1,2¨a] pyridin-6- (Gen-10- 535 536
4 S yl)piperidin-1- c) (M+1)
Is1 ,t, yl)methyl)-1,3-dioxolan-
----N \ 2-one
202
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
OH
F 2-(4-(2-ethy1-344-(4-
r fluorophenyl)thiazol-2-
,, N0
yl)(methyl)amino)imida F8
551
119 'Isl'-' , // \ ZO[1,2-a]pyridin-6- (Gen-10- 550
N- \ ) (M+1)
i s- yl)piperidin-1-y1)-N-(2- c)
-,_
1 .> ` hydroxyethyl)-N-
N \ methylacetamide
(
2-(4-(2-ethy1-344-(4-
--
o-- fluorophenyethiazol-2-
1 \ yl)(methyl)amino)imida F8
0 rsi., 537
120 -N ZO [1,2-a] pyridin-6- (Gen-10- 536
¨ \s--k
yl)piperidin-1-y1)-N- c) (M+1)
methoxy-N-
------ -------- 'NJ
methylacetamide
-
-( / N-(cyanomethyl)-2-(4-
F
----r( ',)--N / (2- ethyl-34(444-
fluorophenyl)thiazol-2- F8
NI ,N 546
121 .--- - .-,
I yl)(methyl)amino)imida (Gen-10- 545
(M+1)
0 ,,_ ,._,..--- zo[1,2-a]pyridin-6- c)
II i
- )-L. N NI" yl)pip eridin-l-y1)-N-
'----'. '-'--- '
N methylacetamide
F
5-((4-(2-ethy1-3-((4-(4-
fluorophenypthiazol-2-
yl)(methyl)amino)imida F8
536
122 'N zo[1,2-a]pyridin-6- (Gen-10- 534
0 (M+1)
N¨
yl)piperidin-1- c)
NC-,..------,..,---;--N¨
H yl)methyl)oxazolidin-2-
\ one
------ N \
HO
F 2-(4-(2-ethy1-344-(4-
-
1 \%------\( fluorophenyl)thiazol-2-
HN
}--------) yl)(methyl)amino)imida F8
N 551
123 zo[1,2-a]pyridin-6- (Gen-10- 550
yl)piperidin-1-y1)-N-(3- c) (M+1)
hydroxypropyl)acetamid
e
F-72 1-(3,3-difluoroazetidin-
,(- N /
----",_,Z-------r -----N, / 1-y1)-2-(4-(2-ethy1-3-
\
\---s ----C- ((4-(4-
124
I, fluorophenyl)thiazol-2-
F8
y1)(methyl)amino)imida (Gen-10- 568 569
(M+1)
C'-- zo [1,2-a] pyridin-6- c)
F-i-j - yl)piperidin-1-
F yl)ethanone
203
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F
/---r--< 2-(4-(2-ethy1-3-((4-(4-
o NH ,,
----- 2 ).\ /% nuorophenyethiazol-2-
--- F8
125 N N-7(
yl)(methyl)amino)imida
(Gen-10- 492 493
N 2 ZO [1,2-al pyridin-6- (M+1)
_.__ s c)
- -4:- -N \\ yl)piperidin-1-
si \ yl)acetami de
-N \
F
/;----- 1 -(4-(2-ethy1-3 -((4-(4-
__/ fluorophenyl)thiazol-2-
( :.)
\ N)
yl)(methyl)amino)imida F9b-F12a
547
126 ---- '-- Ny' ' --c ` zo[1,2-a] pyrid in-6-
(Gen-10- 546
t N s
(M+1)
yl)piperidin-1-y0-2- c)
(pyrrolidin-l-
yl)ethanone
F
F 1 -(4-(2-ethy1-344-(4-
fluorophenyl)thiazol-2-
\ F9b-F12a
127 11-1 yl)(methyl)amino)imida 507
N 1k
H 'N'''' 'N ---C ,) ZO [1,2-a] pyridin-6- (Gen-
10- 506(M+1)
s yl)piperidin-1-y1)-2- c)
(methylamino)ethanone
/ F 1 -(4-(2-ethy1-3 4(444-
( \\., fluorophenyl)thiazol-2-
HOõ _ 0 2,-----__/
yl)(methyl)amino)imida
128 ZO[1,2-a]pyridin-6- F9b-F12a
549
548
- ----
N \ yl)pip eridin-1 -y1)-2-(3 - (Gen-
10c) (M+1)
hydroxyazetidin-l-
yl)ethanone
F
/7---- 2-(dimethylamino)-1 -(4-
(2- ethy1-3 4(444-
--
\ ' N- -\ )7( fluorophenyl)thiazol-
2- F9b-F12a
129 ' NjCL'Isii-''
yl)(methyl)amino)imida (Gen-10- 520 521
,^,-.- - ---- µs zo [1,2-a] pyridin-6- c)
(M+1)
yl)piperidin-l-
yl)ethanone
F 3 -(dimethylamino)-1 -(4-
,
\ \ 1 (2- ethy1-3 4(444-
-------/- fluorophenyl)thiazol-2- F9b-F12a
535
130 ,r,j, INI. \ 7,,N)
_1 yl)(methyl)amino)imida (Gen-10- 534
S ZO[1,2-a]pyridin-6- c) (M+1)
i \\ yl)piperidin-1-
-Nr \ yl)prop an-1 - onc
F 2-(3,3-di fluoroazeti din-
0, 1-y1)-1 -(442- ethy1-3-
F ((4-(4-
F9b-F12a
0N
fluorophenyl)thiazol-2- 569
131 - ''- 'Isr¨N µN"---s ) (Gen-10- 568
yl)(methyl)amino)imida (M+1)
c)
zo [1,2-a] pyridin-6-
yl)piperidin-1-
yl)ethanone
204
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F 144-(2-ethy1-344-(4-
¨ -\ fluorophenyethiazol-2-
,
yl)(methyl)amino)imida F9b-F12a
zo [1,2-a] pyridin-6- (Gen-10- 520
521
132 --N-"---1-N-"- \ \ (M+1)
H
I s yepiperidin-1-y1)-3- c)
(methyl ami no)propan-1 -
one
F
//------<, 1 -(4-(2-ethy1-3 4(444-
fluorophenyl)thiazol-2-
F, _ 0
\N, Jt
yl)(methyl)amino)imida F9b-F12a
551
133 --- 'NNN---11,) zo [1,2-a] pyridin-6-
(Gen-10- 550
(M+1)
yl)piperidin-l-y1)-2-(3- c)
fluoroazetidin-1-
yl)ethanone
F- ./----.
1-(3 -(4-(2-ethyl-3 -44-
(4-fluorophenyl)thiazol-
2-
F10-F5b-
134 1 yl)(methyl)amino)imida
F9 (Gen- 532 533
zo [1,2-a] pyridin-6- (M+1)
10-c)
1U. yl)piperidi n-1-
yl)azetidi n-1 - 1r.N
yl)ethanone
0
F 5-bromo-N-(2-ethyl-6-
( \ (1-
(: N1)-:------./ (methylsulfonyl)piperidi
Fl6a 593
135 o'''s'N' `1,1--/ \ n-4-yl)imidazo [1,2-
(Cpd 80) 592
(M+1)
S Br Br a] pyridi n-3 -y1)-4-(4-
---' NI¨ fluoropheny1)-N-
,
methylthiazol-2-amine
F 24(2- ethy1-6-(1-
--- \
(methylsulfonyl)piperidi
, )=----- / n-4-yl)imidazo[1,2-
F17 539
136 (:)S- N \' 7
N-1
a]pyridin-3- 538
L, _ 11 `s-)'-- cl \I yl)(methyl)amino)-4-(4-
(Cpd 135) (M+1)
fluorophenyl)thiazo le-5-
' j-----N \ carbonitrile
F 24(2- ethy1-6-(1-
(/---1 (methylsulfonyl)piperidi
o N 557 -- / n-4-
yl)imidazo [1,2- see Cpd
137 SN''-- \ ---/ a]pyridin-3- 137 556
_ s. _ _ 2 (M+1)
o L N
,,, , ,
s yl)(methyl)amino)-4-(4- (Cpd 136)
¨ ---- 'N"---,
o fluorophenypthiazo le-5-
'N \ carboxamide
2-42- ethy1-6-(4-(2-(3 -
, ,--____c \/ F
HO hydroxyazetidin-l-y1)-2-
`r¨ o x
\
0 C oethyl)piperazin-1-
" )'---)
yl)imidazo [1,2- F 1 6a-F17 575
138 -N-') \N--t3 (M+1) 574
alpyridin-3- (Cpd 24)
s ---CN
-4- N yl)(m ethyl) amino)-4-(4-
fluorophenyl)thiazo le-5-
carbonitrile
205
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
OH 24(2-ethy1-6-(4-(2-(3-
F (hydroxymethyl)azetidin
-1----\ //z---1/
-1-y1)-2-
1 oxoethyDpiperazin-1-
139 -14---1 \1,1¨.-1,-2 yl)imidazo[1,2- F16a-F17
588 589
L 11 / s -CN (Cpd 34) (M+1)
---. , ---,- 'lc - \ alpyridin-3-
,-1---r,)2 \ yl)(methyl)amino)-4-(4-
fluorophenyl)thiazole-5-
carbonitrile
,F 2-(4-(34(5-cyano-4-(4-
fluorophenyl)thiazol-2-
,-NO
)=---/-- yl)(methyl)amino)-2-
140 ''N' -'N N----c" Fl6a-F17
547
' I 14---( \-,-. ethylimidazo[1,2-
546
-CN a]pyridin-6-
(Cpd 35) (M+1)
yl)piperazin-l-y1)-N,N-
/L'N\/ \ dimethylacetamide
2-42-ethy1-6-(1-(2-(3-
/ E hydroxyazetidin-l-y1)-2-
HO'Y ----\
\--N0 oxoethyl)piperidin-4-
yl)imidazo[1,2- F16a-F17 574
141 rkl"' ) \ N---- \, alpyridin-3- 573
(M+1)
-CN (Cpd 110)
yl)(methyl)amino)-4-(4-
- \
--.-----N \ fluorophenyl)thiazole-5-
carbonitrile
F_,------, (R)-2-((2-ethy1-6-(1-(2-
(3-hydroxypyrrolidin-1-
---_ ------ '':\,--N /¨
)\---S/ )------- y1)-2-
NC N ,,,- oxoethyppiperidin-4-
, ---../ Fl6a-F17 588
142 I yl)imidazo[1,2- 587
O ---------------- (Cpd 111) (M+1)
alpyridin-3-
yl)(methyl)amino)-4-(4-
fluorophenyl)thiazole-5-
HO carbonitrile
(S)-242-ethy1-6-(1-(2-
(3-hydroxypyrrolidin-1-
y1)-2-
NC NNoxoethyppiperidin-4-
)----s
F16a-F17 588
143 X yl)imidazo[1,2-
o
(Cpd 112) 587
(M+1)
alpyridin-3-
yl)(methyl)amino)-4-(4-
\ i
\i- fluorophenyl)thiazole-5-
HO
carbonitrile
24(2-ethy1-6-(1-(2-(3 -
F (hydroxymethyl)azetidin
OH 0 -1-y1)-2-
711 oxoethyl)piperidin-4-
F16a-F17 588
144
A
CN yl)imidazo[1,2- 587
- --._.----õ-,--I,r-\
a]pyridin-3-
(Cpd 113) (M+1)
) \ yl)(methyl)amino)-4-(4-
fluorophenyl)thiazole-5-
carbonitrile
206
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F
/7--- 2-(4-(34(5-cyano-4-(4-
...,õ0 // fluorophenyl)thiazol-2-
_) --<\ , yl)(methyl)amino)-2-
Fl6a-F17 546
145µ14 . ,--_ ethylimidazo[1,2- 545
/ S CN (Cp N'---
' (M+1)
N --- alpyridin-6-yl)piperidin- d 114)
1-y1)-N,N-
dimethylacetamide
2-(4-(2-ethy1-344-(4-
OH fluoropheny1)-5-
l F
----\ --- (hydroxymethyl)thiazol-
\\
- - ),--_/-- 2-
F14 594
146 \ N--'1 yl)(methyl)amino)imida 593
1%1Th (Cpd 34) (M+1)
ZO[1,2-a]pyridin-6-
-..,-
\ OH yl)piperazin-1-y1)-1-(3-
'
(hydroxymethyl)azetidin
-1-yl)ethanone
/F (2-((2-ethy1-6-(1-
(1 (methylsulfonyl)piperidi
o n-4-yl)imidazo[1,2-
11/ NI F14 544
147 -s . ..
\ µINI---/ ----\ a]pyridin-3- 543
o ri - (Cpd 80)
(M+1)
¨ i s- yl)(methyl)amino)-4-(4-
--- ----- =N-
OH fluorophenyl)thiazol-5-
------ ------ ¨NI \ yl)methanol
F, F
F'I (242-ethy1-6-(1-
0, ,,,--
- (methylsulfonyl)piperidi
Y-s ' n-4-yl)imidazo[1,2-
F14 610
148 HO¨ , N N1 a]pyridin-3- 609
(Cpd 85) (M+1)
yl)(methyl)amino)-4-(4-
O r' T (trifluoromethoxy)pheny
-s 1)thiazol-5-yOmethanol
,
0
--V si / (2-((6-(1-(3-
- A
% '1' ¨N \ / (dimethylamino)propyls
)\---s/ --(\ ulfonyflpiperidin-4-y1)-
Ho---/ ri N 2-ethylimidazo[1,2- F14
615
149 // 614
(Cpd 87) (M+1)
o
yl)(meat]hPyYrpaidminin-3o-)-4-(4-
I 1 -
\\ ,1µ1,,, ,,-- fluorophenyl)thiazol-5-
.N. -S
yp
0
F/ F (24(2-ethy1-6-(1-
F-' -----i-J_
,...e,/ / (methylsulfonyl)piperidi
.-s' ---- \ n-4-yl)imidazo[1,2-
F14 594
150 HO- / N N
-, ----:/ a]pyridin-3- 593
yl)(methyl)amino)-4-(4- (Cpd 84) (M+1)
r
0. ,N., ,.(trifluoromethyl)phenyl)
-
\\ thiazol-5-yemethanol
o
207
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F 2-(4-(2-ethy1-3 -((4-(4-
I
,
2 ----\ fluoropheny1)-5-
(hydroxymethypthiazol-
HO \ 2-
F14 577
151 1----- \ \_____,; N yl)(methyl)amino)imida 576
' (Cpd 115) (M+1)
s---1-( zo[1,2-alpyridin-6-
0
H l'i yl)piperidin-l-y1)-1-
,õ,-, ¨
(4\' (pyrrolidin-l-
yl)ethanone
F
)-- 2-(4-(2-ethy1-3 4(444-
fluoropheny1)-5-
(hydroxymethyl)thiazol-
OH
HO 1 2-
F14 593
152 -'\--- \
\ Iri
s- \ yl)(methyl)amino)imida
zo [1,2-a] pyridin-6- (Cpd 113) 592
(M+1)
II ' N ---- yl)piperidin-1-y1)-1-(3-
o J- N> 4
(bydroxymethypazeti din \
-1 -ypethan one
2-(dimethylamino)- 1 -(4-
r (2- ethy1-34(4-(4-
e----7 fluoropheny1)-5-
)=------z (hydroxymethyl)thiazol-
153 N N¨(' F14
µN--' .\ 2-
(Cpd 129) 550 551
(M+1)
isil, 1 , s \ yl)(methyl)amino)imida
OH
- N ZO[1,2-a]pyridin-6-
----- -N' yl)piperidin-l-
yl)cthanone
'F 1 -(4-(2-ethy1-3 -((4-(4-
/-----
fluoropheny1)-5-
j)I _(___,,C' (hydroxymethyl)thiazol-
\ N F9b-F14
154 '- NI.' 2- (Gen-10- 521 522
s- O yl)(methyl)amino)imida (M+1)
c)
ri
zo [1,2-al pyridin-6-
'N yl)piperidin-1-
yl)prop an-1 -one
,F 2-(4-(2-ethy1-344-(4- Bl-C1-
\/,
fluoropheny1)-5- F1b-D1-
nic) ,
-----' )-------- z (hydroxymethypthiazol- F7-E1-
155 'N -----"-
I i P1----\(
\ N/----Cs 551 , OH 2- F18-F5b- 552
yl)(methyl)amino)imida F8 (M+1)
N_ J\
,)---- H
'----- N \ ZO[1,2-a] pyrid in-6- (Gen-10-
yl)piperazin-l-y1)-N,N- ag)
dimethylacetamide
F-_ C.--\1 2-(4-(2-ethyl-3-((4-(4-
/ fluoropheny1)-5-
-
/
)\--- ' --s-(-, (hydroxymethyl)thiazol- F18-F5b-
HO¨y ,NN 2- F8
579
156 yl)(methyl)amino)imida (Gcn- 10- 578
(M+1)
zo [1,2-a] pyridin-6- ar)
o r-- --- õ
1-õ, ,,..N., ,.-- yl)piperidin-l-y1)-1-(3-
L - - hydroxyazetidi n-1 -
HO' ypethanone
208
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F 'Y 2-(4-(2-ethy1-344-(4-
1, )-_, ,N / fluoropheny1)-5-
y -r\ --N / F18-F5b-
1----s r-----\ (hydroxymethyl)thiazol-
F8
HO --z N , N 2-
157 .--- -...,/
I yl)(methyl)amino)imida (Gen-10- 550 N.M
o i - - - - ' zo[1,2-a]pyridin-6-
ar)
yl)piperidin-l-y1)-N,N-
I dimethylacetamide
F
2-(4-(2-ethy1-344-(4-
I ,/ ------, fluoropheny1)-5-(2,2,2-
\ \
INI...,,,0 trifluoroacetoyl)thiazol-
_¨_y
/
158 N----( 2- F15 618
'1`1:-:N. K, \0F
yl)(methyl)amino)imida (Cpd 35)
' I 617 (4+1)
7 \\ F ZO[1,2-a]pyridin-6-
--'----11/ N F yl)piperazin-l-y1)-N,N-
dimethylacetamide
1-(24(2-ethy1-6-(1-(2-
F (3-hydroxyazetidin-1 -
HO /i-----
''c"---\ ( \ ) y1)-2-
-N ,0 )'------/- I oxoethyl)piperidin-4-
yl)imidazo[1,2- F15 644 645
159
F 0
a]pyridin-3- (Cpd 110) (M+1)
,...õ._
F F yl)(methyl)amino)-4-(4-
fluorophenyl)thiazol-5-
y1)-2,2,2-
trifluoroethanone
F 1-(2-((2-ethy1-6-(1-
/1 (methylsulfonyl)piperidi
o -------/ n-4-yl)imidazo[1,2-
N-- F a]pyridin-3- F15 610
N¨ \ 2--õ./- F yl)(methyl)amino)-4-(4- (Cpd 80) 609
T
(M+1)
, _ -- -----,-,------N4 S' '1\ \F fluorophenyl)thiazol-5-
0
y1)-2,2,2-
trifluoroethanone
zF 2-(2-((2-ethy1-6-
7 (piperazin-l-
yl)imidazo[1,2- Fla
161 Hl' \ N ------:
, ---1 ,CN a]pyridin-3- (Gen-5- 475 476
N----- 4\s- - (M+1)
yl)(methyl)amino)-5- m)
, -----K,
methylthiazol-4-y1)-5-
` --=-----N \ fluorobenzonitrile
2-(24(2-ethy1-6-(4-(2-
F (3-hydroxyazetidin-1 -
HO
)-
''C¨\N /2----- c y1)-2-
v-- oxoethyl)piperazin-1-
F8 589
162 'N------i \N ,r1 CN yl)imidazo[1,2-
(Cpd 161) 588
(M+1)
a]pyridin-3-
C--
--------I-N' \ yl)(methyl)amino)-5-
methylthiazol-4-y1)-5-
fluorobenzonitrile
209
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F 24443 -((4-(2-cyano-4-
fluoropheny1)-5-
FINI
7----( methylthiazol-2-
163
N--, ' ,õ yl)(methyl)amino)-2- F8 547
tsi'M \N--/ 1 r = et -iy unidazo[1,2-
(Cpd 161) 546
(M+1)
8 a]pyridin-6-
\>_
J,,,, \ yl)pip erazin-1-y1)-N-
methylacetamide
F
N / 2-(2-((2-ethyl-6-(4-(2-
\ --N (3-fluoroazetidin-1-y1)-
CN S )---------C,, 2-oxoethyl)pip erazin-1-
.- yl)imidazo [1,2- F8 577
164
I a]pyridin-3- (Gen-10- 576 (M+1)
0 IV"'N yl)(methyl)amino)thiazo i)
N,) 1-4-y1)-5 -
,CiN
fluorobenzonitrile
F
2-(2-((6-(4-(2-(3,3-
1'
F lc, /
difluoroazetidin-1-y1)-2-
------1yN
oxoethyl)piperazin-1 -
CN -S / F8
N N
y1)-2-ethylimidazo [1,2- 595
165
(M+1) I a]pyrid in-3- (Gen-10- 594
5r--N- i)
t,N,J yl)(methyl)amino)thiazo
F )t--.JN 1-4-y1)-5-
F' fluorobenzonitrile
2-(2-((2-ethyl-6-(4-(2-
F (3-hydroxyazetidin-1-
HO____
--( y1)-2-
N --- ' oxoethyl)piperazin-1- F8
575
166 'Isr-) ,y \ CN yl)imidazo [1,2- (Gen-10- 574
(M+1)
= s- a]pyridin-3- i)
N N
yl)(m ethyl)arnino)thiazo
L' -------N \
1-4-y1)-5 -
fluorob enzonitrile
F , ,-- 7 2-(2-((6-(4-(2-(azetidin-
\ ,N / 1-y1)-2-
\ ------N /
oxoethyl)piperazin-1-
CN -S / k. F8
N/ ; . y1)-2-
ethylimidazo [1,2- 559
167 .-- --,
I ,I a]pyridin-3- (Gen-10- 558
(M+1)
O i)
yl)(methyl)amino)thiazo
1-4-y1)-5 -
fluorob enzonitrile
/ k
F--,_ j"- 2-(4-(344-(2-cyano-4-
------ NN /__ fluorophenyl)thiazol-2-
N ti )---K, yl)(methyl)amino)-2- F8
,N,,N 547
168 1 ethylimidazo [1,2- (Gen-10-
546
(M+1)
o ri ri a]pyridin-6- i)
yl)piperazin-1-y1)-N,N-
- N-)1"----' ''---
/ dimethylacetamide
210
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F /-----
--V \\ 2-(4-(344-(2-cyano-4-
¨ z -c'N-`----N/ / fluorophenyl)thiazol-2-
oN ---S / ¨ = yl)(methyl)amino)-2- F8
N N 533
169 --- ----, ethylimidazo [1,2- (Gen-10- 532
I (M+1)
alpyridin-6- i)
yl)piperazin-1-y1)-N-
_
'N
H methylacetamide
2424(2- ethy1-6-(1-(2-
F (3-hydroxyazetidin-1-
H0¨<", y1)-2-
\7N ,.(:) z ---,-( ox oethyl)p ip eri d i n-4-
F8
574
170 " N¨ c yl)imidazo [1,2- (Gen-10- 573
'N---4''s- (M+1)
a]pyridin-3- h)
---. ,---, ,,---N¨k
1--=N\2 \ yl)(methyl)amino)thiazo
1-4-y1)-5 -
fluorob enzonitrile
F
//---1 2-(4-(3-((4-(2-cyano-4-
fluorophenyl)thiazol-2-
171 Isl--1
yl)(methyl)amino)-2- F8
Isl- -'
\ µ) CN ethylimidazo [1,2- (Gen-10- 545
s' 546
/ (M+1)
a] pyridin-6-yl)pip eridin- h)
-----Isiz \ 1-y1)-N,N-
dimethylacetamide
2-(2-((2-ethyl-6-(1-(2-
7_ __.(F (3-hydroxyazetidin-1 -
HO y1)-2-
F8
N , oxoethyl)pip eridin-4-
587 588
172 \ N((, \,..õ, CN
yl)imidazo [1,2- (Gen-10-
s' - f) (M+1)
j
alpyridin-3-
yl)(methypamino)-5-
methylthiazol-4-y1)-5-
fluorobenzonitrile
2-(5-((2-ethyl-6-(1-(2-
F
HO //----K r (3-hydroxyazeti din-1 -
-----c
N--(/ `cN oxoethyl)piperidin-4- F8
173 isl"'' µ
N--'s-N yl)imidazo [1,2- (Gen-10- 574 575
I (M+1)
alpyridin-3- t)
yl)(methyl)amino)-
1,2,4-thiadiazol-3 -y1)-5-
fluorob enzonitrile
F,
2-(4-(2-(2-cyanoethyl)-
0 3-((4-(4-
, fluorophenyethiazol-2- see Cpd
174 I N
A yl)(methyl)amino)imida 174-F8
(Gen-10- 545 546
(M+1)
N s ZO [1,2-a] pyridin-6-
- ir =N '
_rj - yl)piperidin-1-3/1)-N,N- aa)
di methyl acetami d e
---2---N \ ¨N
211
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
3-(3-((4-(4-
F,..,..
fluoropheny1)-5-
(3 (hydroxymethyl)thiazol-
HO 2-y1)(methyl)amino)-6- F11-F14
569
175 0 OH (Gen-10- 568
_ SA (M+1)
S.
/ N ' N - (m ethyl sul fo nyl)p ip eri di aa)
..-- n-4-yl)imidazo[1,2-
a]pyridin-2-
yl)propanenitrile
F 3-(6-(1-(2-
(I)- (dimethylamino)-2-
see Cpd
-,. oxoethyl)piperidin-4-
176- F5b-
y1)-3-((4-(4- 564
176 N --- \s--q F8 563
µN-- fluorophenyl)thiazol-2-
(Gen-10- (M+1)
o , N4 yl)(methyl)amino)imida
zo [1,2-a] pyriclin-2- am)
''',---- -----N ¨c:)
yl)propanamide
\ , N-(6-(3-amin o azetidin-
------- \ )-- N \ / 1-y1)-2-
s s---- \ ethylimidazo [1,2- F1b-F5a
423
177 ,r,,j N
ri = .7 422
J! a] pyridin-3 -y1)-4-(4- (Gen-5-b) (M+1)
fluoropheny1)-N-
methylthiazol-2-amine
H2N
F,
T N / 2-(1 -(2-ethyl-3-((4-(4-
fluorophenyl)thiazol-2-
\ -s \F-----\õ, yl)(methyl)amino)imida
N /,P, F8 536
178
'l zo[1,2-a]pyridin-6-
(Cpd 177) 535
(M+1)
HO yl)azetidin-3-ylamino)-
,N,-/----/ 1-(3-hydroxyazetidin-1-
1 I H yl)ethanone
0
I
F, N-(1-(2-ethy1-3 4(444-
fluorophenyl)thi azol-2-
\ si )_--- yl)(methyl)amino)imida
179 ,N.õi`i zo[1,2-a]pyridin-6- F9b-F8 536535
I j yl)azetidin-3-y1)-2-(3- (Cpd 177)
(M+1)
HO , -1 0 n_N.---", __-
''-N it L__./ hydroxyazetidin-1-
-1\1'
H yl)acetamide
), ,N, / 2-(4-(2-ethy1-3 4(444-
,
---- r
. -N, / fluorophenyl)thiazol-2-
L--4 F.---\- yl)(methyl)amino)imida Fib 481
180 N /N
---- --,../ 480
I zo[1,2-a]pyridin-6- (Gen-5b) (M+1)
y 1)pip erazin-1 -
HO
I i
y ethanol
,-,, ,.,, ,
-
N-(2-ethyl-6-
morpholinoimidazo [1,2-
d --- - Fib 438
181 N. N a] pyridin-3 -y1)-4-(4- 437
i T fl (Gen-5-b) (M+1)
uoropheny1)-N-
r III
o methylthiazol-2-amine
212
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F
----(
N / 4-(2-ethyl-3 4(444-
r; )----N /
fluorophenyl)tbiazol-2-
S V- ,--'-'\
N N yl)(methyl)amino)imida Fib 182 485 486
I zo[1,2-a]pyridin-6-y1)- (Gen-5-c) (M+1)
thiomorpho line 1,1-
-S. dioxide
11 -
o
CL -
- k ,N, / 1-(3-((4-(4- 452 453
183 z ---\'. \-.>-N \ /
chlorophenyl)thiazol-2- (C1 (C1
¨s --2-7\
(or N . N yl)(methyl)amino)-2- F4 ), M+1)
- ---../-
Gen- I ethylimidazo [1,2- (Gen-5-i)
454 455
10-a) /--N- a]pyridin-6- ("Cl ("Cl
(
\ , N yl)imidazo lidin-2- one ) M+1)
-----`
H 0
CI,u__,_
7
ethyl 243434(444-
, N/ 538 539
- \ )---- chlorophenyethiazol-2- (C1 (C1
iiõN ,õ, N yl)(methyl)amino)-2- sec Cpd
184
_4_,..:)- ethylimidazo [1,2- 184-F8 ), M+1)
540 541
, a]pyridin-6-y1)-2- (Cpd 183) (37c1 ("c1
oxoimidazolidin-1-
) M+1)
li¨ o yl)acetate
CI 4-(4-ehloropheny1)-N-
)
/
-, methyl-N-(6-(1-
z1 581
(methylsulfony1)- (35C1
/N 1,2,3,6- Fll
185 o o
V 'q-i tetrahydropyridin-4-
y1)- (Gen-10- )' NA
ki- 583
'N"'- ' - ' 2-(2,2,2- ad) L t (37C1
trifluoroethypimidazo [1,
,L > \ /
---- N A¨F 2-a]pyridin-3-yl)thiazol-
, ,
F F 2-amine
F
r 1 c 2-(2-((2-ethyl-6-(i-
0 __) (methylsulfonyl)piperidi
1 N---/ K n-4-yl)imidazo[1,2- see cpd
553
186 o / N N----C
, $ zCN a]pyridin-3- 186 552
(M+1)
yl)(methyl)amino)-4-(4- (Cpd 147)
-- N-
\ fluorophenyethiazol-5-
)------'N' \
yl)acetonitrilc
2-ethyl-N-(4-(4-
0, , o N7------ fluorophenyl)pyrimidin-
s , ,, ,
\ ___-/ \ 2-y1)-N-methy1-6-(1- See Cpd
506
L ,,. .rsi
(methylsulfony1)- 187 507
_- -N \
187 , -;----- --, .---",---'"N-- 7---- \
/ 1,2,3,6- (G en-9-a) (M+1)
` \
-------- \\ F tetrahydropyridin-4-
yl)imidazo [1,2-
a] pyridin-3 -amine
ct-..,,,,
/
3 -(4-chloropheny1)-N-
N _ 531 532
-----rz --c\ - ____14 i (2-ethyl-6-(4- ("Cl
(35C1
N-s ----\- .. (methylsulfonyl)piperazi
Fll
,N, /,N ), M+1)
188
1 n-l-yl)imidazo [1,2- (Gen-10-
533 534
r---N '- a] pyridin-3 -y1)-N- u) ("Cl (37C1
R ri methy1-1,2,4-thiadiazol-
\s- -- , 5-amine ) M+1)
o
213
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
,F N -(2- ethy1-6-(1-
( z;--- (methylsulfony1)-
1,2,3,6-
1 N----' tetrahydropyridin-4- F2
// \\ 497
189 o - N '-- N---\. ,N yl)imi dazo [1,2- (G en-5-
496
/ o (M+1)
a] pyridi n-3 -y1)-3 -(4- ae)
----,-,- -õõ,....-,õ ----,N---\
=-.---.. ..)---z------N \ fluoropheny1)-N-
methy1-1,2,4-oxadiazol-
5-amine
F_,
2-(442-ethyl-3 -43 -(4-
7 ) fluoropheny1)-1,2,4-
thiadiazol-5-
F8
yl)(methyl)amino)imida 520
190 I N '14
' 2 ZO r 1,2-al pyridin-6-y1)- (Gen-10- 519
(M+1)
s- \ v)II 14- 5,6-dihydropyridin-
'- 7-' '14----- 1(2H)-y1)-N,N -
1'--N\/ \ dimethylacetamide
F,
2-(442-ethyl-3 -((3 -(4-
C--) flu oroph eny1)-1,2,4-
I ='' thiadiazol-5- F8
yl)(methyl)amino)imida (gen-10- 521 522
191 1 N N yl)(m
(M+1)
11 1
,..,,--,. g -I( ZO r 1,2-al pyridin-6- w)
11 /
N-
o yl)pip eridin-1 -y1)-N,N-
-------------2-- ,N--
j------.N dimethylacetamide
F ,'-----
L N -(6-(4-((1H-imidazol-
-- 5-yl)methyl)piperazin-1 -
F10
6 N 7 N y1)-2-ethylimidazo [1,2- 517
192 ...-- ---.7. (Gen-10- 516
I a] pyridin-3 -y1)-4-(4- (M+1)
e)
, yv -Th ,------N------,/,=- fluoropheny1)-N-
/ 'tJ, 1 j
'N' -%tµi'-/ methylthiazol-2-amine
H
F N-(2- ethy1-6-(1 -
(methyl sul fony1)-
\ N / 1,2,3,6-
tetrahydropyridin-4- Fll
'---s 562
193 N N
----' ---,./' y 1)imidaz o [1,2- (Gen-10- 561
(M+1)
õ---,,, ---, ---,-- a] pyridin-3 -y1)-N- ai)
c) r T methyl-4-(4-
(trifluoromethyl)phenyl)
O thiazol-2-amine
\ ,N / N-cyc lopropy1-2 -(442-
/- ethy1-3-((4-(4-
\ \ __4 ,) \
fluorophenyl)thiazol-2- F8
533
194 ,,N, N
yl)(methyl)amino)imida (Gen-10- 532
(M+1)
o ---- .2,1 ,----,- zo [1,2-a] pyridin-6-
c)
/ \`
yl)piperidi n-1-
1\l' '------' '----- yl)acetamide
H
214
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
Fl_ 5-((4-(2-ethy1-3-((4-(4-
\ ,;\ fluorophenyl)thiazol-2-
,L yl)(methyl)amino)imida FR
549
195 zo[1,2-a]pyridin-6- (Gen-10- 548
(M+1)
'-T r' N--- yl)piperidin-l- c)
N
yOmethyl)-3-
--- ---r-N-4),
)-----N \ methyloxazolidin-2- one
F
), (R)-54(4-(2-ethy1-34(4-
----1,
\
(4-fluorophenyl)thiazol-
), 2-
535
yl)(methyl)amino)imida F8
196 - 'N (Gen-10- 534
_I/
0 --õ-- 's zo [1,2-a]
pyridin-6- (M+1)
c)
o__---K ..'r
! yl)piperidin-1-
N----j '-Y.7'Isl---- yl)methyl)oxazolidin-2-
H
one
c ; - (S)-54(4-(2-ethy1-34(4-
(4-fluorophenyl)thiazol-
2-
F8
''N yl)(methyl)amino)imida 535
197 (Gen-10- 534
k zo [1,2-a] pyridin-6- (M+1)
o---/ ' ('-`1,1"-- N
S _____ c)
_i yl)piperidin-1-
\N z-- ----,,,,--(
H 7 \\ yemethypoxazolidin-2-
- N one
F,
o
4-((4-(2-ethy1-3-((4-(4-
(\' 1
---o L----(' fluorophenypthiazol-2-
y1)(methyl)amino)imida F8
HN i , 535
198 N
_IL zo[1,2-a]pyridin-6- (Gen-10- 534
(M+1)
'1,1--- s- `N- yl)piperidin-1- c)
l I yl)methypoxazolidin-2-
--- ---- -N---.
1 \>¨ one
F N -(2- ethy1-6-(1 -
-.N /
----r , (methylsulfony1)-
I, I)õ.
7 \\ ----1µ1 / 1,2,3,6-
N--- 7--------K,. tetrahydropyridin-4- Fll
,,,, ,N 513
199 yl)imidazo[1,2- (Gen-10- 512
I (M+1)
- a] pyridin-3 -y1)-3 -(4- v)
(:::\ .N fluoropheny1)-N-
s.- -' methy1-1,2,4-thiadiazol-
\\0
5-amine
F --
--1- \I
'. ----N / 1-(4-(2-ethyl-3-((4-(4-
-f,
fluorophenyl)thiazol-2-
F9a
N / N
200 ---- -,/ yl)(methyl)amino)imida 507
(Gen-10- 506
zo [1,2-a] pyridin-6- (M+1)
riv lij -
yl)pip crazin-1-
e)
---- -- --, - yl)propane-1,2-dione
II
o
215
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
._j N / 5-(4-(2-ethy1-34(4-(4-
- A )----N \ /¨ fluorophenyl)thiazol-2-
s r-----c yl)(methyl)amino)imida F9a
,,N _N 548
201
I zo [1,2-a] pyridin-6- (Gen-10- 547
(M+1)
.---s, .---,-- yl)pip erazine-1- e)
0=1=1 N. [1 i carbonyl)pyrrolidin-2-
1 ¨'
one
o
F,
1 N / (1-aminocyclopropyl)(4-
- - 7
(2- ethy1-34(4-(4-
Ls' )------ see Cpd
fluorophenyl)thiazol-2-
N N 202 520
202
I yl)(methyl)amino)imida 519
(Gen-10- (M+1)
zo [1,2-a] pyridin-6-
' , j yl)piperazin-1- e)
H2N- 1-11'l "--. yl)methanone
0
F ---
' N j (S)-1-(4-(2- ethyl-34(4-
(4-fluorophenyl)thiazol-
' -g -----A 2- F9a
,r,i, ,,7N 509
203
I
yl)(methyl)amino)imida (Gen-10- 508
(M+1)
pH 1 N- ZO [1,2-a] pyridin-6- e)
N J yl)pip erazin-l-y1)-2-
I I hydroxyprop an-1- one
0
2-(4-(2-ethy1-344-(4-
-c-\
2---S )--'-'(,n, fluorophenyl)thiazol-
2- see Cpd
,,,,),,ri yl)(methyl)amino)imida 204 508
204 507
zo [1,2-a] pyridin-6- (Gen-10- (M+1)
,.- ----. _.-----, .-..----
0 i N ¨ yl)piperazin-1-y1)-2- an)
it H2N'--1 N oxoacetamide
'
0
F--y7-----
\ -... N , 1-b enzy1-4-(4-(2- ethyl-
34(444-
<-----,---,- --12-'s--N)õ('¨
fluorophenyl)thiazol-2-
F9a
yl)(methyl)amino)imida 638
205 / ---- , 9\ zo [1,2-a] pyridin-6- (Gen-10- 637
(M+1)
- K e)
rN. ,
yl)pip erazine-1-
\--N--1 '-''
\rõ N -I
) carbony1)pyrro1idin-2-
0 one
CI
N / 3-(3-((4-(4- 453 454
--N
chlorophenyl)thiazol-2- (3C1
(35C1
S
206 N z N
/ '../ yl)(methyl)amino)-2- F4 ), M+1)
ethyl imidazo[1,2- (Gen-5-i) 455
456
N,....." a]pyridin-6- (37C1 (37C1
C---k yl)oxazolidin-2- one ) M+1)
0
216
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
ci, --------.
2-(2-ethy1-34(4-(4- 501 502
L---=-/./,,, -"-- ----,---N- N /¨
chloroph enyl)thi azol-2- (35C1 ("Cl
--s i-
207 ikJ ,,,N yl)(methyl)amino)imida F4
), M+1)
I zo[1,2-
a]pyridin-6-y1)-1- (Gen-5-i) 503 504
[1,2]thiazinane-1,1- (37C1 (37C1
dioxide ) M+1)
o
F --
L N / -( 4-(2-ethy1-34(4-(4-
/ fluorophenyl)thiazol-2-
yl)(methyl)amino)imida
,,,,,/' F2 559
208 'I zo[1,2-a]pyridin-6-y1)-
(Gen-5-b) 558
(M+1)
H r--- .--' N-(thiophen-2-y1)-5,6-
sõ ,N, , N, , dihydropyridine-1(2H)-
carboxamide
o
ci- , -,,--- -, 4-(4-chloropheny1)-N-
11 / (2- ethy1-6-(1- 527 528
(methylsulfony1)- (35C1
("Cl
'1---s Fll
rN, 4N 1,2,3,6- ), M+1),
209 --
tetrahydropyrid in-4- (Gen 10 529 530
., ak)
0 I yl)imidazo [1,2- (37C1 (37C1
., ,N, ,,
--- a] pyridin-3 -y1)-N- )
M+1)
o methylthiazol-2-amine
cr, --- -
4-(4-chloropheny1)-N-
- ----- ---\-: '------N /
\--- '----- (2-ethyl-6-(1- 581 582
S/ ) N
(trifluoromethylsulfonyl
Fll ("Cl ("Cl
)-1,2,3,6- ), M+1),
210 I (Gen-10-
----,, ----, .------- tetrahydropyridin-4- 583 584
0 r ak)
---\ N yl)imidazo [1,2- (37C1 (37C1
F µ\0 a] pyridin-3 -y1)-N - )
M+1)
F methylthiazol-2-amine
F
r-----µ 1-(2-ethyl-3-((4-(4-
HO )-------/- ' fluorophenyl)thiazol-2-
see Cpd
211 1-----\
( / N----(
\ N----(/ yl)(methyl)amino)imida 211
(Gen-10- 451 452
/ S-' ZO [1,2-a] pyridin-6-
(M+1)
Nr,
_/1--==N' \ yl)piperidin-4-ol acl)
a - 2-(4-(3-((4-(4-
chlorophenyl)thiazol-2-
yl)(methyl)amino)-2-
s ..)-----N F 1 b 497
212 --"--./ ethylimidazo [1,2- 496
(Gen-5-y) (M+1)
a]pyridin-6-
1 I yl)piperazin-1 -
HO --' yl)ethanol
CL. /
- -
1 \ N / 4-(2-ethy1-34(4-(4-
----S )-----
chlorophenyl)thiazol-2-
yl)(methyl)amino)imida Fib 502
- 501
N
213 ---'N
zo [1,2-a]pyridin-6-y1)- (Gen-5-y) (M+1)
-s 2 thiomorpholine-1,1-
dioxide
1`---
o
217
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F, -----..
Lts_.1 / tert-butyl 4-(2-ethy1-3-
--N /
\ ;r- ______ ((4-(4-
s i ,,,
N Pi
<- ----.-7 fluorophenyl)thiazol-2-
214 yl)(methyl)amino)imida F2
533 534
zo[1,2-a]pyridin-6-y1)- (Gen-5-b) (M+1)
0,, .N.,,
1 5,6-dihydropyridinc-
__ yo 1(2H)-carboxylatc
\\_ N / 1-(4-(2-ethy1-344-(4-
--,-
---Si )'---r(= fluorophenyl)thiazol-2-
F9b-F6
N, N yl)(methyl)amino)imida
215 -r (Gen-10- 491 492
zo [1,2-a] pyridin-6- (M+1)
r yl)piperidin-1-
b)
,--õ,õ
yl)prop an-1- one
0
F _1,7 ,
216 N-(2- ethy1-6-(pip eridin-
\ s' s)-----A, 4-yl)imidazo[1,2- El-F6-
(Gen 436
N .., a] pyridin-3 -y1)-4-(4- F5a 435
-10- . ---,-
fluoropheny1)-N- (Gen-9-c) (M+1)
c) r--,...- õ._.---
methylthiazol-2-amine
HN ,
-,---
F , ---2'
¨L N, / N-(6-(1-
\ / \ ' benzylpiperidin-4-y1)-2-
¨s T----- azo F10
\ ethylimid [1,2- 526
217 N,,;,N (Gen-10- 525
i a] pyridin-3 -y1)-4-(4-
c) (M+1)
fluoropheny1)-N -
methylthiazo1-2-amine
-
, ¨
F ¨
1 N / N-(2-ethyl-6-(l-
N/
s¨r____
is opropylpip eridin-4-
F10
,N, /N yl)imidazo [1,2- 478
218 J a] pyridin-3 -y1)-4-(4- (Gen-10- 477
(M+1)
,-- ----,_. c)
fluorophcny1)-N -
-----",_ methylthiazol-2-amine
F. N / tert-butyl 4-(2-ethyl-3 - ((4-(4-
/ -----(
-s .. ,Ni fluorophenyl)thiazol-2-
F2-F6 536
219 - N--,-,
yl)(methyl)amino)imida
(Gen-5-c) 535
(M+1)
r--,, ,- zo[1,2-a]pyridin-6-
,.0 yl)pip eridine-1-
1 101 ¨ carboxylate
F.
L N / N-(6-(3,6-dihydro-2H-
----,--, ')---N, /
- -s pyran-4-y1)-2-
ethylimidazo [1,2- F2 435
220 N .k, .
--,./- a] pyridin-3 -y1)-4-(4- (Gen-5-b) 434
(M+1)
I
flu oropheny1)-N-
I methylthiazol-2-amine
o ,
218
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
N i
4-(4-chloropheny1)-N- 450 451
s 1
221 N (6-(3,6-dihydro-2H-
pyran-4-y1)-2- F2 ( C1
"Cl (
), M+1)
õN
I ethylimidazo [1,2- (Gen-5-i) 452
453
f,.., a] pyridin-3 -y1)-N- (37C1 ("Cl
methylthiazol-2-amine ) M+1)
OH F
;------\ (2-(-(- ethyl-3-((4-(4-
2----o ) fluorophcnyethiazol-2-
2--,
N--(
'' -- yl)(methyl)amino)imida see Cpd
222 - N N- --- zo [1,2-a] pyridin-6-
222 535
(Gen-10- 534
S
(------------,-õ------N-----N y1)piperidin-1-y1)-4,5-
(M+1)
- > \ dihydrooxazol-5- c)
\
yl)methanol
F,_
/
-("\ N,,--N r- 2-(4-(2-ethy1-344-(4-
LL
fluorophenyl)thiazol-2-
N N yl)(methyl)amino)imida F8
563
223 zo[1,2-a]pyridin-6-
(Gen-10- 562
,- (M+1)
O r -
yl)pip eridin-l-y1)-1-(3- c)
Ho
hydroxypyrro lidin-1- --(.µz---N ''-- -7
yl)cthanone
/F
jz---- ---(
2-(2-((2-ethy1-6-(1-
O )---- (m ethyl sul fonyl)pip eri di
- \ _// \\ OH n-4-yl)imidazo[1,2- El 529 530
' N'
224 0 ' 1 N ----N ,
r s a]pyridin-3- (Gen-9-b) (M+1)
yl)(methyl)amino)thiazo
1¨ \i' \
---N 1-4-y1)-5 -fluorophenol
tert-butyl 4-(34(3-(4-
/ chlorophcny1)-1,2,4- 553 554
N¨s ------- \ thiadiazol-5-
Fib ("Cl ("Cl
225
,õ/N yl)(methyD (Gen
amino)-2- ), M+1),
-5-
ethyl imidazo [ w)
1,2- 555 556
a]pyridin-6- ("Cl ("Cl
0,1.4 ) yl)pip erazine-1- ) M+1)
o carboxylate
N / N-(6-(4-((1H-imidazol-
, - -( - N r-
\\____
S - \ 2-yl)methyl)piperazin-1 -
F10
226 , N,, , N y1)-2-ethyl im i d azo [1,2-
(Gen-10- 516 517
a] pyridin-3 -y1)-4-(4- (M+1)
fluoropheny1)-N-
e)
k N methylthiazol-2-amine
H
219
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
N / )..__ cyclopropy1(4-(2-ethyl-
N /¨ 3-((4-(4-
- I --'- see Cpd
$ fluorophenyl)thiazol-2-
N, ,z,N 227 505
227
I yl)(methyl)amino)imida
(Gen-10- 504
(M+1)
N --' ZO [1,2¨a] pyridin-6-
/\ N yl)pip erazin-1- e)
11 ¨ yl)methanone
0
F,,,r,,, .c1
ethyl 2-(4-(2-ethyl-3-
N, /
((4-(4-
fluorophenyl)thi azol-2- F9b
;14 537
228 I r yl)(methyl)amino)imida (Gen-10- 536
(M+1)
o r N''--- ZO [1,2¨a] pyridin-6-
e)
N 01 yl)piperazin-1-y1)-2-
-'o
II '' oxo acetate
o
F¨, --
N / [6-(1,1-Dioxo-
L¨ rs------( isothiazolidin-2-y1)-2- see Cpd
N ,N ethyl-imidazo [1,2- 229
229 , , // 471 472
a]pyridin-3-yl] -[4-(4- (Gen-10- (M+1)
fluoro-phenyl)-thiazol- ao)
\
2-y1]-methyl-amine
11
o
CI-
---( 0
/ tert-butyl 4-(3-((4-(4-
chlorophenyl)thiazol-2- 549 550
-s/ NN fl ----- \ (35C1 (C1
yl)(methyl)amino)-2-
F2 ), 1\4+1),
230
'1 ethylimidazo [1,2-
(Gen-5-i) 551 552
-00, a]pyri d i n-6-y1)-5,6-
I - dihydropyridine-1(2H)- (37C1 (37C1
r - carboxylate ) M+1)
/ 0
CI
I
/--------)
z, 4-(4-chloropheny1)-N- 466
231 y'' (6-(3,6-dihydro-2H- ("Cl
(Gen thiopyran-4-y1)-2- F2 ),
2 N N.M.
-10- 1,
, \s----3 \ ethylimidazo [1,2- (Gen-5-i) 468
g) s ;
I '
N---- a] pyridin-3 -y1)-N- (37C1
methylthiazol-2-amine )
---NI \\
F
/
----c N-(6-(4,4-
F )----,__- ,/ difluoropiperidin-1 -y1)-
F ¨7L--- \) N¨
\N--- 2-ethylimidazo [1,2- F 1 b
232
, a] pyridin-3 -y1)-4-(4- (Gen-5-c) 471
472
,rI s-
N ¨ = fluoropheny1)-N -
I J. _ ')---\ methylthiazol-2-amine
N \
220
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int) MW
Ms'd
F _,.., N-(6-(1-(3-
Lõ / chloropropylsulfony1)- 573 574
-Ls'r"\y,-(' 1,2,3,6- ("Cl
(35C1
Fll
N, ,N tetrahydropyridin-4-y1)- ), M+1),
233 -.-/- (Gen-10-
2-ethy limidazo [1,2- 575 576
\--,."- b)
0 I ' a] pyridin-3 -y1)-4-(4-
(37C1 (37C1
-\\ fluoropheny1)-N- ) M+1)
6 methylthiazol-2-amine
CI -
,N / tert-butyl 4-(3-((4-(4-
552 553
7\ "--- )----( chlorophenyl)thiazol-2- ("Cl (35C1
yl)(methyl)amino)-2-
Fib ), M+1),
234
I ethylimidazo [1,2-
2 \ 2 (Gen-5-y) 554 555
alpyridin-6-
1-- r ; (37C1 (37C1
0
yl)pip erazine-1-
, IsL ) m+i)
r carboxylate
o
N-(6-(1-
F -(,7), , ¨N N / (cyclohexylmethyl)piper
- - ----c-\ /
idin-4-y1)-2- F10
I--
\s 532
235 N N
-.% ethylimidazo [1,2- (Gen-10-
531
(M+1)
a] pyridin-3 -y1)-4-(4- c)
------. r-------------------
fluoropheny1)-N-
- ,----- N, ,---- methylthiazol-2-amine
F
/7-- N-(2- ethy1-6-(5-methyl-
4,5-dihydro oxazol-2- F13-F9a-
)¨
N¨/ yl)imidazo [1,2- F19
436
236
)--o
\ , 1
, ._' __----'
N -- a] pyridin-3-y1)-4-(4- (Gen-5- (M+1)
fluoropheny1)-N- aa) 435
S
N' 'N- \,
7 \) \ methylthiazol-2-amine
F
/
µ \ N-(2- ethy1-6-(4-methyl-
4,5-dihydro oxazol-2- F13-F9a-
-
237 N¨
// yl)imi dazo [1,2- F19
a]pyridin-3-y1)-4-(4- (Gen-5- 435
436
(M+1)
fluoropheny1)-N- aa)
'NI¨
J methylthiazol-2-amine
N \µ
/F
2-(2-ethy1-34(4-(4-
\ 2 fluorophenyl)thiazol-2- F13-F9a-
N-4 yl)(methyl)amino)imida F19
466
238 465
o r----0 \N-- ZO[1,2-a]pyridin-6-
y1)- (Gen-5- (M+1)
----K 's' HO Is r 'f \\ 4,5-dihydrooxazole-4-
aa)
r '
----- / \ carboxylic acid
'N \
F
i (2-(2-ethyl-3-((4-(4-
\ ___/) fluorophenyl)thiazol-2- F13-F9a-
yl)(methyl)amino)imida F19 452
239 i--- 451
HO\ 7-9 N , ZO[1,2-a]pyridin-6-y1)- (Gen-5- (M+1)
-;-)- -,.. __= '''' 4,5-dihydro oxazol-4- aa)
yOmethanol
221
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
MS
Cpd Structures Name Mtd (Int)
MW
Ms'd
4-(4-chloropheny1)-N- 437 438
(6-(4,5-dihydrooxazol- F13-F9a- (35C1 (35C1
240 !7\
2-y1)-2- F19 ),
M+1),
)1
ethylimidazo[1,2- (Gen-5- 439 440
S a] pyridin-3 -y1)-N - ag)
(37C1 (37C1
N- methylthiazol-2-amine ) M+1)
I
N
Table IV. NMR data of the compounds of the invention.
Cpd NMR data (6)
1 1H NMR (400 MHz, CDC13) 6 ppm 8.23 - 8.13 (2 H, m), 7.24 - 7.14 (2
H, in), 7.00 (2 H,
d), 3.62 (3 H, s), 3.09 (8 H, bs), 2.77 (2 H, q), 2.62 (3 H, s), 1.35 (3 H, t)
'H NMR (400 MHz, CDC13) 6 ppm 8.20 - 8.12 (2 H, m), 7.22 - 7.13 (2 H, m), 6.99
(2 H,
2 s), 4.68 (1 H, m), 4.43 (1 H, dd), 4.26 (1 H, dd), 4.14 - 4.05 (1
H, m), 3.88 (1 H, dd), 3.61
(3 H, s), 3.58 - 3.52 (1 H, m), 3.14 - 3.02 (6 H, m), 2.74 (2 H, q), 2.70 -
2.62 (4 H, m),
2.59 (3 H, s), 1.33 (3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 8.22 - 8.08 (2 H, m), 7.17 (2 H, t), 6.99 (2 H,
s), 4.24 -
3 4.03 (2 H, m), 3.94 (2 H, s), 3.61 (3 H, s), 3.09 (6 H, bs.), 2.82
- 2.64 (6 H, m), 2.60 (3 H,
s), 1.53 (3 H, s), 1.33 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.21 - 8.12 (2 H, m), 7.17 (2 H, t), 6.99 (2 H,
s), 4.58 -
4 4.45 (1 H, m), 3.71 - 3.48 (7 H, m), 3.23 - 3.15 (2 H, m), 3.09 (4
H, bs), 2.78 - 2.69 (6 H,
m), 2.60 (3 H, s), 2.07 -2.00 (1 H, m), 2.00 - 1.90 (1 H, m), 1.33 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.21 - 8.12 (2 H, m), 7.24 - 7.12 (2 H, m), 6.99
(2 H,
s), 4.57 - 4.46 (1 H, m), 3.70 - 3.53 (7 H, m), 3.28 - 3.04 (6 H, m), 2.83 -
2.67 (6 H, m),
2.62 (3 H, s), 2.10 - 2.00 (1 H, m), 1.99 - 1.89 (1 H, m), 1.34 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 8.25 - 7.99 (2 H, m), 7.33 - 7.06 (4 H, m),
4.62 -
6 4.49 (2 H, m), 4.19 (1 H, dd), 4.15 - 4.08 (1 H, m), 3.75 (1 H,
dd), 3.64 (3 H, s), 3.21 -
3.02 (4 H, m), 2.88 (2 H, s), 2.79 - 2.66 (4 H, m), 2.55 (3 H, s), 1.32 (3 H,
t), 1.14 (6 H, s)
1H NMR (300 MHz, Me0D-d4) 6 ppm 7.95 - 7.84 (2 H, m), 7.22 - 7.16 (2 H, m),
7.11 (2
H, t), 6.94 (1 H, s), 4.61 - 4.51 (1 H, m), 4.50 - 4.41 (1 H, m), 4.20 (1 H,
dd), 4.04 (1 H,
7
dd), 3.75 (1 H, dd), 3.60 (3 H, s), 3.17 - 3.01 (6 H, m), 2.72 (2 H, q), 2.65
(4 H, bt), 2.55 (3
H, s), 1.31 (3 H, t)
1H NMR (400 MHz, Me0D -d4) 6 ppm 7.89 (2 H, dd), 7.19 (2 H, d), 7.11 (2 H, t),
6.93 (1
8 H, s), 4.46 -4.35 (1 H, m), 3.69 - 3.43 (7 H, m), 3.27 -3.18 (2 H,
m), 3.17 - 3.03 (4 H, m),
2.76 - 2.65 (6 H, m), 2.55 (3 H, s), 2.10 - 1.83 (2 H, m), 1.31 (3 H, t)
222
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (300 MHz, Me0D-d4) 6 ppm 7.97 - 7.89 (2 H, m), 7.20 (2 H, bs), 7.13 (2
H, t),
9 6.95 (1 H, s), 4.48 - 4.39 (1 H, m), 3.71 - 3.42 (7 H, m), 3.25 (2 H,
d), 3.17 - 3.04 (4 H,
m), 2.76 - 2.65 (6 H, m), 2.56(3 H, s), 2.11 - 1.88(2 H, m), 1.31 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 8.27 - 8.04 (2 H, m), 7.78 (1 H, s), 7.33 -
7.16 (3 H,
m), 4.61 - 4.54 (1 H, m), 4.50 (1 H, dd), 4.25 - 4.18 (1 H, m), 4.06 (1 H,
dd), 3.77 (1 H,
dd), 3.67 (3 H, s), 3.08 (2 H, d), 3.05 - 2.96 (2 H, m), 2.76 (2 H, qd), 2.59
(4 H, bs), 2.27 -
2.16(2 H, m), 1.88 - 1.78 (4 H, m), 1.34(3 H, t)
1H NMR (400 MHz, Mc0D- c/4) 6 ppm 8.21 - 8.18 (2 H, m), 7.96 (1 H, s), 7.52 (1
H, s),
7.30 (2 H, t), 6.31 (1 H, bs), 4.37 -4.28 (1 H, m), 4.14 -4.05 (1 H, m), 4.01 -
3.99 (2 H,
11
m), 3.57 - 3.50 (2 H, in), 2.94 (3 H, s), 2.86 - 2.76 (2 H, m), 2.68 (5 H,
bs), 1.43 (3 H, t),
1.38 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.21 - 8.13 (2 H, m), 7.22 - 7.15 (2 H, m), 6.97
- 6.89
12 (2 H, m), 4.74 - 4.65 (1 H, m), 4.48 - 4.40 (1 H, m), 4.33 - 4.24 (1 H,
m), 4.14 - 4.07 (1 H,
m), 3.90 (1 H, dd), 3.63 (3 H, bs), 3.17 - 3.05 (6 H, m), 2.79 - 2.66 (6 H,
m), 1.36 (3 H, t)
13 1H NMR (300 MHz, CDC13) 6 ppm 8.22 - 8.11 (2 H, m), 7.25 - 7.13 (2 H,
m), 7.02 - 6.86
(2 H, m), 3.63 (3 H, s), 3.13 (6 H, bs), 2.85 (3 H, d), 2.81 -2.68 (6 H, m),
1.36 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm) 7.89 - 7.83 (2 H, m), 7.09 (2 H, t), 7.00 (1 H,
s), 6.86
14 (1 H, dd), 6.69 (1 H, s), 4.68 (1 H, bs), 4.47 - 4.40 (1 H, m), 4.57 -
4.25 (1 H, m), 4.08 (1
H, dd), 3.89(1 H, dd), 3.60(3 H, s), 3.11 - 3.02 (6 H, m), 2.74(2 H, q), 2.68 -
2.62 (4 H,
m),1.33 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.89 - 7.83 (2 H, m), 7.09 (2 H, t), 7.00 (1 H,
s), 6.87
(1 H, d), 6.69 (1 H, s), 4.58 - 4.48 (1 H, m), 3.71 - 3.51 (7 H, m),3.23 -
3.14 (2 H, in), 3.00
- 3.12 (4 H, m), 2.78 - 2.64 (6 H, m), 2.12 - 1.89 (2 H, m), 1.33 (3 H, t)
1H NMR (300 MHz, Me0D-d4) 6 ppm 7.97 - 7.82 (2 H, m), 7.28 - 7.17 (2 H, m),
7.11 (2
16 H, t), 6.98 (1 H, s), 4.47 - 4.33 (1 H, in), 3.73 - 3.40 (7 H, m), 3.24
(2 H, d), 3.18 - 3.02 (4
H, m), 2.77 -2.61 (6 H, m), 2.14 - 1.84 (2 H, m), 1.31 (3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 7.84 - 7.79 (2 H, m), 7.52 (1 H, s), 7.34 (1 H,
s), 7.04
17 (2 H, t), 6.62 (1 H, s), 4.65 - 4.58 (1 H, m), 4.41 - 4.36 (1 H, in),
4.24 - 4.18 (1 H, in), 4.05
- 4.01 (1 H, m), 3.86 - 3.81 (1 H, m), 3.55 (3 H, s), 3.01 - 2.93 (4 H, m),
2.71 - 2.54 (3 H,
m), 2.36 (3 H, s), 2.25 - 2.13 (2 H, m), 2.04 - 1.57 (4 H, m), 1.28 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.18 - 8.14(2 H, in), 7.26 - 7.16 (4 H, m), 4.70
- 4.68
18 (1 H, m), 4.47 - 4.43 (1 H, m), 4.30 - 4.26 (1 H, m), 4.12 - 4.08 (1 H,
m), 3.91 - 3.87 (1 H,
m), 3.62 (3 H, s), 3.14 - 3.06 (6 H, m), 2.73 - 2.68 (6 H, m), 1.34 (3 H, t)
1H NMR (400 MHz, DMSO) 6 ppm 8.02 - 7.95 (2 H, in), 7.68 (1 H, d), 7.49 (1 H,
d), 7.32
19 - 7.23 (3 H, m), 4.50 - 4.32 (2 H, m), 4.09 - 4.01 (1 H, m), 3.98 -
3.90 (1 H, m), 3.67 - 3.52
(4 H, m), 3.10 - 2.90 (6 H, m), 2.68 -2.42 (6 H, m), 1.23 (3 H, t)
223
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, CDC13) 6 ppm 8.17(1 H, s), 7.87 - 7.83 (2 H, m), 7.37(1 H,
d), 7.10
20 (2 H, t), 6.69 (1 H, s), 4.55 - 4.50 (1 H, m), 3.69 - 3.51 (7 H, m),
3.47 - 3.12 (2 H, m), 3.06
(4 H, bs), 2.80 - 2.68 (6 H, m), 1.99 - 2.07 (2 H, m), 1.31 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.87- 7.84(2 H, m), 7.36 - 7.23 (2 H, m), 7.10(2
H,
21 t), 6.89 (1 H, s), 4.55 - 4.50 (1 H, m), 3.69 - 3.52 (7 H, m), 3.22 -
3.18 (2 H, m), 3.05 (4 H,
bs), 2.75- 2.67 (6 H, m), 2.10 - 1.96 (2 H, m), 1.31 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 8.19 - 8.11 (2 H, m), 7.51 - 7.36 (3 H, m),
7.24 (2
22 H, t), 3.65 (3 H, s), 3.20 - 3.14 (4 H, m), 3.05 (2 H, s), 2.75 (3 H,
s), 2.74 - 2.64 (6 H, m),
1.32 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.92 - 7.88 (2 H, m), 7.48 - 7.45 (1 H, m),
7.40 -
23 7.37 (2 H, m), 7.14 - 7.09 (2 H, m), 6.95 (1 H, s), 3.61 (3 H, s), 3.55
(4 H, t), 3.09 - 2.96 (4
H, m), 2.69 (2 H, q), 1.45 (9 H, s), 1.31 (3H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.94 - 7.84 (2 H, m), 7.44 (1 H, d), 7.40 -
7.29 (2
24 H, m), 7.11(2 H, m), 6.94 (1 H, s), 4.58 - 4.51 (1 H, m), 4.50 - 4.42
(1 H, m), 4.23 - 4.16
(1 H, m), 4.04 (1 H, dd), 3.75 (1 H, dd), 3.60 (3 H, s), 3.19 - 3.00 (6 H, m),
2.73 - 2.61 (6
H, m), 1.30(3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.94 - 7.82 (2 H, m), 7.59 - 7.53 (1 H, m), 7.24
- 7.04
25 (4 H, m), 6.70 (1 H, s), 4.60 - 4.50 (1 H, m), 3.74 - 3.56 (7 H, m),
3.23 (2 H, d), 3.12 (4 H,
bs), 2.76(6 H, m), 2.11 - 1.92 (2 H, m), 1.37(3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 7.96 - 7.77 (2 H, m), 7.52 (1 H, d), 7.19 - 7.04
(4 H,
26 m), 6.67 (1 H, s), 4.57 - 4.45 (1 H, m), 3.78 - 3.52 (7 H, m), 3.19 (2
H, d), 3.14 - 3.00 (4
H, m), 2.79 -2.63 (6 H, m), 2.07 - 1.98 (1 H, m), 1.98 - 1.89 (1 H, in),
1.32(3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.86 (2 H, dd), 7.49 (1 H, d), 7.22 - 7.04 (4 H,
m),
27 6.67 (1 H, s), 5.89 - 5.75 (1 H, m)NH, 4.53 - 4.40 (1 H, m), 3.86 -
3.68 (1 H, m), 3.67 -
3.51 (5 H, m), 3.47 - 3.35 (1 H, in), 3.23 - 3.13 (2 H, m), 3.07 (4 H, bs),
2.78 - 2.65 (6 H,
m), 2.34 -2.10 (1 H, m), 2.00 - 1.95 (3 H, m), 1.93 - 1.77 (1 H, m), 1.33 (3
H, t)
NMR (400 MHz, Me0D-d4) 6 ppm 7.90 (2 H, dd), 7.45 (1 H, d), 7.40 - 7.29 (2 H,
m),
28 7.12 (2 H, t), 6.95 (1 H, d), 5.44 - 5.36 (0.5 H, m), 5.31 - 5.22 (0.5
H, m), 4.66 - 4.51 (1 H,
m), 4.42 - 4.22 (2 H, m), 4.10 - 3.96 (1 H, m), 3.61 (3 H, s), 3.20 - 3.03 (6
H, m), 2.77 -
2.61 (6 H, m), 1.31 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.97 - 7.84 (2 H, in), 7.44 (1 H, d), 7.39 -
7.30 (2
30 H, m), 7.15 - 7.07 (2 H, m), 6.93 (1 H, s), 4.27 (2 H, t), 4.00 (2 H,
s), 3.60 (3 H, s), 3.16 -
3.03 (6 H, m), 2.74 - 2.60 (6 H, m), 2.28 (2 H, q), 1.30 (3 H, t)
224
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, Me0D-d4) 6 ppm 8.01 - 7.80 (2 H, m), 7.44 (1 H, d), 7.39 -
7.30 (2
31 H, m), 7.14 - 7.07 (2 H, m), 6.96 - 6.91 (1 H, m), 3.60 (3 H, s), 3.52
(2 H, t), 3.41 (2 H, t),
3.23 (2 H, bs), 3.17 - 3.05 (4 H, m), 2.74 - 2.67 (6 H, m), 1.95 (2 H, quin),
1.85 (2 H,
quin), 1.30(3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.86 (2 H, dd), 7.54 (1 H, d), 7.20 - 7.03 (4 H,
m),
32 6.68 (1 H, s), 3.71 - 3.57(11 H, m), 3.24 (2 H, s), 3.07 (4 H, d), 2.78
-2.64 (6 H, m), 1.34
(3 H, t)
1H NMR (300 MHz, Me0D-d4) 6 ppm 7.96 - 7.83 (2 H, m), 7.51 - 7.28 (3 H, m),
7.19 -
33 7.05 (2 H, m), 6.95 (1 H, s), 3.61 (3 H, s), 3.13 (4 H, d), 3.05 (2 H,
s), 2.76 - 2.64 (6 H, m),
1.31 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.92 - 7.79 (2 H, m), 7.56 (1 H, d), 7.22 - 7.15
(2 H,
34 m), 7.10 (2 H, t), 6.69 (1 H, s), 4.29 (1 H, t), 4.13 - 4.01 (2 H, m),
3.85 - 3.72 (3 H, m),
3.61 (3 H, s), 3.08 (6 H, s), 2.85 - 2.63 (7 H, m), 1.34 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.87 (2 H, dd), 7.49 (1 H, d), 7.20 - 7.06 (4 H,
m),
35 6.67 (1 H, s), 3.61 (3 H, s), 3.23 (2 H, s), 3.13 - 3.02 (7 H, m), 2.95
(3 H, s), 2.77 - 2.66 (6
H, m), 1.33(3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.86 (2 H, dd), 7.50 (1 H, d), 7.20 - 7.04 (4 H,
m),
36 6.67 (1 H, s), 4.19 (2 H, d), 3.61 (3 H, s), 3.27 (2 H, s), 3.10 (4 H,
m), 2.79 -2.68 (6 H, m),
1.33 (3 H, t), 1.28 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.86 (2 H, dd), 7.49 (1 H, d), 7.19 - 7.05 (4 H,
m),
37 6.67 (1 H, s), 4.18 (2 H, dd), 3.60 (3 H, s), 3.33 (1 H, q), 3.12 -
2.96 (4 H, m), 2.83 - 2.66
(6 H, in), 1.37 - 1.24 (9 H, in)
1H NMR (400 MHz, Me0D-c/4) 6 ppm 7.90 - 7.85 (2 H, m), 7.45 (1 H, d), 7.35 (1
H, dd),
38 7.32 (1 H, d), 7.09 (2 H, t), 6.90 (1 H, s), 3.67 (2 H, s), 3.59 (3 H,
s), 3.04-2.15 (4 H, m),
2.70 (4 H, bt), 2.67 (2 H, q), 1.29 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.89 - 7.84 (2 H, m), 7.44 (1 H, d), 7.36 (1
H, dd),
39 7.31 (1 H, d), 7.13 - 7.09 (2 H, m), 6.91 (1 H, s), 3.97 (2 H, s), 2.85
(1 H, sept), 3.59 (3 H,
s), 3.33 - 3.29 (4 H, in), 3.14 - 3.03 (4 H, m), 2.70 - 2.65 (6 H, m), 1.29 (3
H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.92 - 7.85 (3 H, m), 7.44 (1 H, dd), 7.38 -
7.29 (2
40 H, m), 7.15(1 H, d), 7.11 (2 H, t), 6.93(1 H, s), 3.77(2 H, s), 3.59(3
H, s), 3.16 - 3.02 (4
H, m), 2.73 - 2.63 (6 H, m), 1.30 (3 H, t)
1H NMR (400 MHz, CDCL) 6 ppm 8.70 (1 H, bs), 7.86 - 7.81 (2 H, m), 7.67 (1 H,
d),
41 7.23 (1 H, dd), 7.17 (1 H, d), 7.11 - 7.06 (2 H, m), 6.69 (1 H, s),
3.83 (2 H, s), 3.59 (3 H,
s), 3.15 -3.04 (4 H, m), 2.79 -2.71 (6 H, 111), 1.35 (3 H, t)
225
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.93 - 7.86 (2 H, m), 7.45 (1 H, dd), 7.41 -
7.35 (2
42 H, m), 7.11 (2 H, t), 6.95 (1 H, s), 3.61 (3 H, s), 3.25 - 3.13 (6 H,
m), 2.96 - 2.89 (4 H, m),
2.70 (2 H, q), 1.31 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.89 (2 H, dd), 7.50 - 7.34 (3 H, m), 7.16 -
7.05 (2
43 H, m), 6.94 (1 H, s), 4.17 - 4.10 (2 H, m), 3.74 - 3.67 (2 H, m), 3.68 -
3.57 (7 H, m), 3.14 -
2.98 (4 H, m), 2.69 (2 H, q), 1.30 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm (2 conformers) 7.86 (2 H, dd), 7.57 - 7.47 (1 H,
m),
7.19 (1 H, s), 7.16 - 7.02 (3 H, m), 6.67 (1 H, s), 4.67 (0.5 H, dd), 4.54
(0.5 H, d), 3.77 (3
44
H, bs), 3.70 - 3.33 (6 H, m), 3.18 -2.89 (4 H, m), 2.72 (2 H, q), 2.28 - 1.93
(2 H, m), 1.92
- 1.77 (2 H, m), 1.49 - 1.29 (12 H, m)
1H NMR (400 MHz, CDC13) 6 ppm 7.84 (2 H, dd), 7.51 (1 H, d), 7.19 (1 H, s),
7.15 - 7.02
(3 H, m), 6.66 (1 H, s), 3.88 - 3.62 (4 H, m), 3.59 (3 H, s), 3.51 (3 H, bs),
3.32 (1 H, bs),
3.18(1 H, bs), 3.02(4 H, bs), 2.71 (2 H, q), 2.39 - 2.53 (0.5 H, m), 2.18 -
2.33 (0.5 H, m),
2.14 - 1.94 (1 H, m), 1.43 (9 H, s), 1.32 (3 H, t)
1H NMR (400 MHz, Me0D- c/.4) 6 ppm 8.08 (1 H, dd), 7.92 - 7.85 (3 H, m), 7.78
(1 H, s),
46 7.21 (1 H, s), 7.15 (2 H, t), 4.81 - 4.76 (1 H, m), 3.91 - 3.75 (4 H,
in), 3.72 (3 H, s), 3.50 -
3.29 (6 H, m), 2.94 (2 H, q), 2.64 -2.53 (1 H, m), 2.21 - 1.93 (3 H, m), 1.44
(3H, t)
1H NMR (400 MHz, Me0D- c/4) 6 ppm 8.09 - 8.05 (1 H, m), 7.94 - 7.85 (3 H, m),
7.75 (1
47 H, sb), 7.21 (1 H, s), 7.15 (2 H, t), 3.86 - 3.52 (9 H, m), 3.49 - 3.26
(7 H, m), 2.94 (2 H, q),
2.48 - 2.28 (1 H, m), 2.18 - 2.07 (1 H, m), 1.44 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.90 - 7.86 (2 H, m), 7.73 - 7.66 (1 H, m), 7.25
- 7.25
48 (2 H, m), 7.16 - 7.11 (2 H, m), 6.73 (1 H, s), 3.88 - 3.76 (2 H, m),
3.76 - 3.61 (6 H, m),
3.60 - 3.18 (4 H, m), 3.16 - 3.02 (4 H, m), 2.80 (2 H, q), 2.50 - 2.40 (1 H,
m), 2.20 - 2.04
(4 H, m), 1.39 (3H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.90 - 7.82 (2 H, m), 7.59(1 H, d), 7.22(1 H,
d), 7.16
(1 H, dd), 7.10(2 H, t), 6.69 (1 H, s), 3.83 - 3.75 (2 H, m), 3.71 - 3.65 (2
H, m), 3.61 (3 H,
49
s), 3.60 - 3.55 (2 H, m), 3.55 - 3.46 (1 H, m), 3.38 - 3.29 (2 H, m), 3.11 -
3.00 (4 H, m),
2.91 (3 H, s), 2.75 (2 H, q), 2.29 - 2.09 (2 H, m), 1.35 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.88 (2 H, dd), 7.47 (1 H, dd), 7.42 - 7.34 (2
H, m),
7.11(2 H, t), 6.93 (1 H, s), 4.23 (2 H, s), 3.73 (2 H, bt), 3.60 (3 H, s),
3.54 (2 H, bt), 3.14 -
2.99 (4 H, m), 2.69 (2 H, q), 1.30 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.88 (2 H, dd), 7.50 - 7.44 (1 H, m), 7.42 -
7.32 (2
51 H, m), 7.10 (2 H, t), 6.92 (1 H, s), 3.71 (2 H, s), 3.65 (2 H, t), 3.60
(3 H, s), 3.12 - 2.96 (4
H, m), 2.68 (2 H, q), 2.40 (2 H, q), 1.29(3 H, t), 1.10 (3 H, t)
226
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, CDC13) 6 ppm 7.86 - 7.82 (2 H, m), 7.51 (1 H, d), 7.18(1 H,
d), 7.13
52 - 7.05 (3 H, m), 6.66 (1 H, s), 3.76 (2 H, t), 3.67 (2 H, t), 3.62 (2
H, t), 3.58 (3 H, s), 3.04 -
2.95 (4 H, m), 2.70 (2 H, q), 2.50 (2 H, t), 1.90 (2 H, quint), 1.31 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.92 - 7.82 (2 H, m), 7.52 (1 H, d), 7.20 (1 H,
d), 7.15
53 - 7.04 (3 H, m), 6.67 (1 H, s), 3.77 (2 H, t), 3.67 - 3.58 (5 H, m),
3.06 - 2.94 (4 H, m), 2.73
(2 H, q), 2.40 (2 H, t), 2.33 (2 H, t), 2.22 (6 H, s), 1.82 (2 H, quin), 1.33
(3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.85(2 H, dd), 7.63 (1 H, d), 7.25(1 H, s),
7.18(1 H,
54 bd), 7.09 (2 H, t), 6.70 (1 H, s), 3.62 (3 H, s), 3.44 - 3.34 (4 H, m),
3.21 - 3.10 (4 H, m),
2.83 (3 H, s), 2.76 (2 H, q), 1.35 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.91 - 7.87 (2 H, m), 7.47 (1 H, d), 7.40 -
7.36 (2
55 H, m), 7.14 - 7.08 (2 H, m), 6.94 (1 H, s), 3.69 (2 H, t), 3.60 (3 H,
s), 3.42 (4 H, bt), 3.21 -
3.11(6 H, m), 2.69(2 H, q), 2.26 -2.19 (2 H, m), 1.30 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.91 - 7.83 (2 H, m), 7.53 (1 H, d), 7.23 (1 H,
d), 7.14
56 - 7.06 (3 H, m), 6.68 (1 H, s), 3.62 (3 H, s), 3.46 (4 H, t), 3.16 -
3.05 (6 H, m), 2.74 (2 H,
d), 2.70 -2.57 (2H, m), 2.37 (6 H, bs), 2.19 - 2.08 (2H, m), 1.34 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.87 (2 H, dd), 7.52 (1 H, d), 7.23 (1 H, d),
7.15 - 7.06
57 (3 H, m), 6.68 (1 H, s), 3.61 (3 H, s), 3.45 (4 H, bt), 3.17 - 3.03 (6
H, m), 2.73 (2 H, q),
2.69 - 2.53 (6 H, m), 2.12 - 2.00 (2 H, m), 1.82 (4 H, bs), 1.34 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.88 (2 H, dd), 7.47 (1 H, dd), 7.42 - 7.31 (2
H, m),
58 7.10 (2 H, t), 6.92 (1 H, s), 3.64 (2 H, t), 3.60 (3 H, s), 3.40 (4 H,
bt), 3.22 - 3.06 (6 H, m),
2.69 (2 H, q), 2.03 - 1.90 (2 H, m), 1.30 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.90 (2 H, dd), 7.48 (1 H, dd), 7.44 - 7.34 (2
H, m),
59 7.12 (2 H, t), 6.95 (1 H, s), 3.76 (3 H, s), 3.61 (3 H, s), 3.48 (4 H,
bt), 3.23 - 3.08 (4 H, m),
2.70 (2 H, q), 1.31 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.95 (1 H, d), 7.81 - 7.78 (2 H, m), 7.28 (1 H,
d), 7.21
60 - 7.22 (1 H, m), 7.09 - 7.04 (2 H, m), 6.71 (1 H, s), 4.01 (2 H, s),
3.58 (7 H, s), 3.58 (4 H,
m), 2.77 (2 H, q), 1.32 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.88 - 7.85 (2 H, m), 7.62 (1 H, d), 7.23 (1 H,
d), 7.17
61 - 7.07 (3 H, m), 6.70 (1 H, s), 6.51 (1 H, bs)NH, 5.68 (1 H, bs)NH,
2.90 (2 H, s), 3.61 (3
H, s), 3.56 - 3.51 (4 H, m), 3.18 - 3.06 (4 H, m), 2.75 (2 H, q), 1.34 (3 H,
t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 8.27 (1 H, d), 7.91 - 7.81 (2 H, m), 7.59 (1
H, dd),
62 7.39 (1 H, dd), 7.09 (2 H, t), 6.92 (1 H, s), 4.18 (2 H, bs), 3.81 -
3.67 (4 H, m), 3.60 (3 H,
s), 2.73 (2 H, q), 1.47 (9 H, s), 1.32 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 8.29 (1 H, dd), 7.84 (2 H, d), 7.60 (1 H, dd),
7.45 -
63 7.33 (3 H, m), 7.03 (1 H, s), 4.19 (2 H, s), 3.83 - 3.70 (4 H, m), 3.61
(3 H, s), 2.74 (2 H, q),
1.48 (9 H, s), 1.33 (3 H, t)
227
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, CDC13) 6 ppm 7.89 - 7.86 (3 H, m), 7.66 (2 H, d), 7.25 - 7.22
(1 H,
64 m), 7.14 - 7.10 (2 H, m), 4.27 - 4.21 (2 H, m), 3.82 - 3.66 (2 H, m),
3.64 (3 H, s), 3.54 (2
H, s), 3.39 (2 H, s), 3.07 - 3.04 (2 H, m), 2.79 (2 H, q), 1.37 (3 H, t), 1.32
(3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 8.36 (1 H, dd), 7.94 (2 H, dd), 7.67 (1 H,
dd), 7.46
65 (1 H, dd), 7.16 (2 H, t), 7.05 - 6.90 (1 H, m), 4.11 (2 H, s), 3.89(2
H, dd), 3.75 -3.61 (5 H,
m), 3.03 (3 H, s), 2.81 (2 H, q), 1.39 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.86 (2 H, dd), 7.73 (1 H, s), 7.61 (1 H, d),
7.37 (1 H,
66 dd), 7.10 (2 H, t), 6.69 (1 H, s), 6.10 (1 H, bs), 3.97 (2 H, bs), 3.63
(3 H, s), 3.57 - 3.45 (2
H, m), 2.86 (3 H, s), 2.77 (2 H, q), 2.61 (2 H, bs), 1.36 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.95 (1 H, s), 7.91 - 7.86 (2 H, m), 7.61 (1
H, dd),
67 7.54 (1 H, dd), 7.14 - 7.08 (2 H, m), 6.96 (1 H, s), 6.24 - 6.22 (1 H,
m), 4.83 (2 H, s), 4.13
- 4.09 (2 H, m), 3.66 (2 H, td), 3.63 (3 H, s), 2.73 (2 H, q), 2.66 - 2.53 (2
H, m), 1.32 (3 H,
t)
1H NMR (300 MHz, CDC13) 6 ppm 7.82 (2 H, d), 7.73 (1 H, d), 7.62 (1 H, s),
7.47 (1 H,
68 d), 7.38 (2 H, d), 6.77 (1 H, s), 6.22 (1 H, bs), 4.55 - 4.31 (4 H, m),
3.63 (3 H, s), 2.90 (3
H, s), 2.79 (2 H, q), 1.37 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.81 (2 H, d), 7.77 - 7.72 (2 H, m), 7.45 - 7.34
(3 H,
69 m), 6.79 (1 H, s), 6.30 - 6.20 (1 H, m), 4.12 - 4.04 (2 H, m), 3.64 (3
H, s), 3.50 - 3.36 (2 H,
m), 2.87 (3 H, s), 2.80 (2 H, q), 2.53 - 2.45 (2 H, m), 1.39 (3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 7.84 - 7.77 (2 H, m), 7.73 (1 H, bs), 7.58 (1 H,
dd),
70 7.43 (2 H, d), 7.34 (1 H, dd), 6.70 (1 H, s), 6.16 - 6.05 (1 H, m),
3.96 (2 H, bd), 3.63 (3 H,
s), 3.55 - 3.45 (2 H, m), 2.85 (3 H, s), 2.76 (2 H, q), 2.64 - 2.55 (2 H, m),
1.39 - 1.32 (12
H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.20 (1 H, bd), 7.87 (1 H, s), 7.78 (1 H, bd),
7.73 (2
71 H, d), 6.92 (2 H, d), 6.72 (1 H, s), 6.20 (1 H, bs), 3.97 (2 H, bs),
3.83 (3 H, s), 3.64 (3 H,
bs), 3.54-3.46 (2 H, m), 2.92 (2 H, q), 2.84 (3 H, s), 2.60 (2 H, bs), 1.46 (3
H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.94 - 7.87 (2 H, m), 7.72 (1 H, bs), 7.59 (1 H,
d), 7.37
72 (1 H, dd), 7.26 (2 H, bd), 6.76 (1 H, s), 6.10 (1 H, m), 3.97 (2 H,
bs), 3.63 (3 H, s), 3.55 -
3.44 (2 H, m), 2.85 (3 H, s), 2.76 (2 H, q), 2.60 (2 H, bs), 1.36 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.72 (2 H, s), 7.62 - 7.54 (2 H, m), 7.35 (1 H,
dd), 7.19
73 (1 H, q), 6.71 (1 H, s), 6.13 - 6.06 (1 H, in), 3.96 (2 H, d), 3.62 (3
H, s), 3.58 - 3.43 (2 H,
m), 2.85 (3 H, s), 2.75 (2 H, q), 2.61 (2 H, bs), 1.35 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.93 (1 H, s), 7.91 - 7.82 (2 H, m), 7.63 -
7.48 (2 H,
m), 7.09 (2 H, t), 6.93 (1 H, s), 6.21 (1 H, t), 4.13 (2 H, t), 4.00 - 3.93 (2
H, m), 3.62 (3 H,
74
s), 3.50 (2 H, bt), 3.17 - 3.06 (2 H, m), 2.71 (2 H, q), 2.64 - 2.47 (2 H, m),
2.13 - 2.03 (2
H, m), 1.99 (3 H, s), 1.31 (3 H, t)
228
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.92 (1 H, s), 7.87 (2 H, dd), 7.61 - 7.47 (2
H, m),
7.09 (2 H, t), 6.92 (1 H, s), 6.25 - 6.17 (1 H, m), 3.98 - 3.93 (2 H, m), 3.66
- 3.58 (5 H, m),
3.49 (2 H, t), 3.18 - 3.04 (2 H, m), 2.71 (2 H, q), 2.64 -2.47 (2 H, m), 2.03 -
1.90 (2 H, m),
1.31 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.81 (2 H, d), 7.77 (1 H, bs), 7.61 (1 H, d),
7.37 (2 H,
76 d), 7.29 (1 H, d), 6.76 (1 H, s), 5.90 (1 H, t), 3.79 (2 H, bs), 3.63
(3 H, s), 3.26 - 3.20 (2 H,
m), 3.15 - 3.07 (2 H, m), 2.77(2 H, q), 1.35 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.93 - 7.84 (2 H, m), 7.74 (1 H, s), 7.67 (1 H,
dd), 7.40
77 (1 H, dd), 7.17 - 7.09 (2 H, m), 6.17 - 6.08 (1 H, m), 3.98 (2 H, bs),
3.46 - 3.59 (5 H, m),
2.86 (3 H, s), 2.77 (2 H, q), 2.58 - 2.68 (2 H, m), 1.36 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 8.04 (1 H, d), 7.96 - 7.92 (2 H, m), 7.65 -
7.61 (1
78 H, m), 7.57 - 7.54 (1 H, m), 7.10 (2 H, t), 7.03 (1 H, s), 4.37 - 4.33
(1 H, m), 4.19 - 4.16 (1
H, m), 3.75 - 3.61 (4 H, in), 2.91 -2.78 (3 H, in), 2.73 - 2.60 (2 H, m), 1.91
- 1.66(2 H,
m), 1.52 (9 H, d), 1.39 (3H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 8.00 - 7.79 (3 H, m), 7.55 (1 H, d), 7.48 -
7.41 (1
H, m), 7.12 (2 H, t), 6.95 (1 H, d), 3.96 - 3.87 (1 H, in), 3.85 - 3.72 (2 H,
in), 3.63 (3 H, d),
79
2.87 (3 H, s), 2.85 - 2.77 (1 H, m), 2.73 (2 H, q), 2.63 - 2.52 (2 H, m), 1.97
- 1.89 (2 H,
m), 1.32(3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 7.87 (2 H, dd), 7.67 - 7.53 (2 H, in), 7.21 -
7.05 (3 H,
m), 6.69 (1 H, s), 3.95 (2 H, d), 3.63 (3 H, s), 2.82 - 2.70 (7 H, m), 2.68 -
2.55 (1 H, m),
2.04 - 1.74 (4 H, m), 1.35 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.80 (2 H, d), 7.63 (1 H, bs), 7.58 (1 H, d),
7.43 (2 H,
d), 7.15 (1 H, dd), 6.70 (1 H, s), 3.99 - 3.90 (2 H, m), 3.62 (3 H, s), 2.81
(3 H, s), 2.79 -
81
2.69 (4 H, m), 2.66 - 2.55 (1 H, m), 1.96 (2 H, d), 1.90 - 1.75 (2 H, m), 1.35
(9 H, s), 1.26
(3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 7.82 (2 H, d), 7.70 - 7.52 (2 H, m), 7.16 (1 H,
dd),
82 6.95 (2 H, d), 6.62 (1 H, s), 3.94 (2 H, d), 3.86 (3 H, s), 3.63 (3 H,
s), 2.84 - 2.69 (7 H, m),
2.68 - 2.58 (1 H, m), 2.02 - 1.92 (2 H, m), 1.92 - 1.74 (2 H, m), 1.35 (3 H,
t)
1H NMR (400 MHz, CDC13) 6 ppm 7.73(1 H, ddd), 7.63 - 7.53 (3 H, m), 7.24 -7.11
(2 H,
83 m), 6.70 (1 H, s), 3.95 (2 H, d), 3.62 (3 H, s), 2.81 (3 H, s), 2.79 -
2.69 (4 H, m), 2.68 -
2.56 (1 H, m), 2.01 - 1.92 (2 H, m), 1.82 (2 H, qd), 1.35 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.00 (2 H, d), 7.71 - 7.56 (4 H, m), 7.17 (1 H,
dd),
84 6.88 (1 H, s), 3.95 (2 H, d), 3.64 (3 H, s), 2.81 (3 H, s), 2.80 - 2.71
(4 H, m), 2.68 - 2.57 (1
H, m), 1.97(2 H, d), 1.85 (2 H, qd), 1.36(3 H, t)
229
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, CDC13-d) 6 ppm 7.91 (2 H, d), 7.62 (1 H, s), 7.59 (1 H, d),
7.26 (2 H,
85 d), 7.16 (1 H, dd), 6.75 (1 H, s), 3.95 (2 H, d), 3.63 (3 H, s), 2.81
(3 H, s), 2.79 -2.70 (4 H,
m), 2.68 - 2.57 (1 H, m), 2.01 - 1.92 (2 H, m), 1.90 - 1.74 (2 H, m), 1.35 (3
H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.95 - 7.83 (3 H, m), 7.52 (1 H, dd), 7.39 (1
H, dd),
86 7.19 - 7.04 (2 H, m), 6.94 (1 H, s), 3.85 (2 H, bd), 3.70 (2 H, t),
3.62 (3 H, s), 3.23 - 3.14
(2 H, m), 2.93 (2 H, bt), 2.83 - 2.66 (3 H, m), 2.27 - 2.14 (2 H, m), 1.90 (2
H, bd), 1.77 (2
H, qd), 1.32 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.91 - 7.82 (2 H, m), 7.61 (1 H, s), 7.57 (1 H,
d), 7.19
87 - 7.06 (3 H, m), 6.68 (1 H, s), 3.94 (2 H, d), 3.62 (3 H, s), 3.05 -
2.96 (2 H, m), 2.85 (2 H,
t), 2.75 (2 H, q), 2.66 - 2.55 (1 H, m), 2.41 (2 H, t), 2.24 (6 H, s), 2.05 -
1.88 (4 H, m),
1.86 - 1.71 (2 H, m), 1.34 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.92 - 7.84 (3 H, m), 7.53 (1 H, d), 7.39 (1
H, dd),
88 7.11 (2 H, t), 6.93 (1 H, s), 3.84 (2 H, d), 3.68 (4 H, t), 3.62 (3 H,
s), 3.08 (2 H, t), 2.91 (2
H, t), 2.81 -2.66 (3 H, m), 2.52 -2.38 (6 H, m), 2.01 - 1.85 (4 H, m), 1.75 (2
H, q), 1.31 (3
H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.94 - 7.82 (3 H, m), 7.53 (1 H, d), 7.40 (1
H, dd),
89 7.11(2 H, t), 6.94 (1 H, s), 3.85 (2 H, d), 3.62 (3 H, s), 3.08 (2 H,
t), 2.92 (2 H, t), 2.82 -
2.68 (3 H, m), 2.67 -2.55 (6 H, m), 2.05 - 1.88 (4 H, m), 1.86 - 1.71 (6 H,
m), 1.32 (3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 7.85 (2 H, dd), 7.61 (1 H, s), 7.57 (1 H, d),
7.19 - 7.02
90 (3 H, m), 6.67 (1 H, s), 3.92 (2 H, bd), 3.61 (3 H, s), 3.16 - 3.02 (1
H, m), 3.01 -2.69 (7 H,
m), 2.68 - 2.51 (1 H, m), 2.11 - 1.97 (2 H, m), 1.96 - 1.87 (2 H, m), 1.86 -
1.66 (2 H, m),
1.34 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.99 -7.81 (3 H, m), 7.51 (2 H, dd), 7.10(2 H,
t),
91 6.96 (1 H, s), 3.84 (2 H, d), 3.75 - 3.58 (7 H, m), 3.24 (2 H, t), 2.95
(2 H, t), 2.89 - 2.66 (5
H, m), 2.55 (4 H, bs), 1.92 (2 H, d), 1.85 - 1.67 (2 H, m), 1.32 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.93 - 7.84 (3 H, m), 7.53 (1 H, dd), 7.40 (1
H, dd),
92 7.11 (2 H, t), 6.94 (1 H, s), 3.75 (2 H, d), 3.62 (3 H, s), 2.76 - 2.66
(5 H, m), 1.90 (2 H,
bd), 1.83 (2 H, qd), 1.32 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.91 - 7.82 (3 H, m), 7.52 (1 H, d), 7.38 (1
H, dd),
7.10 (2 H, t), 6.92 (1 H, s), 4.15 (2 H, t), 3.83 (2 H, d), 3.61 (3 H, s),
3.14 - 3.05 (2 H, m),
93
2.96 - 2.86 (2 H, 111), 2.77 - 2.64 (3 H, m), 2.15 - 2.04 (2 H, m), 2.02 (3 H,
s), 1.95 - 1.85
(2 H, m), 1.75 (2 H, dd), 1.31 (3 H, t)
'I-INMR (400 MHz, Me0D-d4) 6 ppm 7.96 - 7.81 (3 H, m), 7.53(1 H, d), 7.41(1 H,
dd),
94 7.11(2 H, t), 6.95 (1 H, s), 3.85 (2 H, d), 3.65 (2 H, t), 3.62 (3 H,
s), 3.13 - 3.07 (2 H, m),
2.93 (2 H, bt), 2.82 - 2.68 (3 H, m), 2.03 - 1.87 (4 H, m), 1.85 - 1.70 (2 H,
m), 1.32 (3 H, t)
230
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, CDC13) 6 ppm 7.89(2 H, dd), 7.63 (1 H, s), 7.59(1 H, d),
7.18(1 H,
dd), 7.13 (2 H, t), 4.00 - 3.92 (2 H, m), 3.80 (2 H, t), 3.52 (3 H, s), 3.12 -
3.06 (2 H,
m),2.93 - 2.84 (2 H, m), 2.75 (2 H, q), 2.71 - 2.60 (1 H, m), 2.14 - 2.03 (2
H, m), 2.00 -
1.89 (2 H, m), 1.88 - 1.74 (2 H, m), 1.36(3 H, t)
1H NMR (400 MHz, Mc0D-c/4) 6 ppm 8.05 (1 H, dd), 7.92 (1 H, s), 7.61 (1 H,
dd), 7.56 -
96 7.47 (2 H, m), 7.43 (1 H, dd), 7.27 (1 H, s), 3.84 (2 H, d), 3.64 (3 H,
s), 2.91 - 2.68 (8 H,
m), 2.00 - 1.93 (2 H, m), 1.89 - 1.76 (2 H, m), 1.33 (3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 7.69 - 7.58 (3 H, m), 7.47 (1 H, dd), 7.38 (1 H,
td),
97 7.20 (1 H, d), 3.97 (2 H, d), 3.53 (3 H, s), 2.89 - 2.61 (8 H, m), 2.24
(3 H, s), 2.07 - 1.94 (2
H, m), 1.93 - 1.77 (2 H, m), 1.38(3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.71 - 7.62 (2 H, m), 7.58 (1 H, d), 7.15 (1 H,
dd),
98 7.01 - 6.91 (2 H, m), 6.48 (1 H, s), 3.97 (2 H, d), 3.58 (3 H, s), 2.83
(3 H, s), 2.81 - 2.72 (4
H, 2.72 - 2.61 (1 H, m), 2.53 (3 H, s), 2.04 - 1.94 (2 H, in), 1.91 -
1.82 (2 H, in), 1.36
(3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.08 (1 H, dd), 7.63 (1 H, bs), 7.60 (1 H, d),
7.19 (2 H,
99 ddd), 7.11(1 H, s), 7.10 - 7.05 (I H, m), 3.96 (2 H, d), 3.60 (3 H, s),
2.82 (3 H, s), 2.81 -
2.70 (4 H, m), 2.68 -2.58 (1 H, m), 2.03 - 1.93 (2 H, m), 1.93 - 1.76 (2 H,
m), 1.36(3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.29 - 8.18 (1 H, m), 7.66 - 7.56 (2 H, m), 7.16
(1 H,
100 dd), 7.03 - 6.95 (2 H, m), 6.89 (1 H, ddd), 3.95 (2 H, d), 3.62 (3 H,
s), 2.82 (3 H, s), 2.82 -
2.71 (4 H, m), 2.66 - 2.57 (1 H, m), 2.01 - 1.94 (2 H, m), 1.88 - 1.81 (2 H,
m), 1.35 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.71 - 7.61 (3 H, m), 7.57 (1 H, d), 7.18 - 7.05
(3 H,
101 in), 3.96 (2 H, d), 3.54 (3 H, s), 2.88 - 2.71 (7 H, m), 2.69 - 2.56 (1
H, m), 2.32 (3 H, s),
2.04 - 1.93 (2 H, m), 1.92 - 1.77 (2 H, m), 1.36 (3 H, t)
NMR (400 MHz, CDC13) 6 ppm 7.87(2 H, dd), 7.62(1 H, s), 7.59(1 H, d), 7.15(1
H,
102 dd), 7.11 (2 H, t), 6.68(1 H, s), 3.95 (2 H, d), 2.82(3 H, s), 2.80 -
2.70 (4 H, in), 2.68 -
2.55 (1 H, m), 2.03 - 1.91 (2 H, m), 1.91 - 1.76 (2 H, m), 1.35 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.89 - 7.76 (3 H, m), 7.66 (1 H, s), 7.28 (1 H,
dd), 7.10
103 (2 H, t), 3.95 (2 H, d), 2.83 -2.71 (7 H, in), 2.69 - 2.60 (1 H, in),
2.00 - 1.93 (2 H, m), 1.90
- 1.76 (2 H, m), 1.34 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.93 - 7.83 (2 H, m), 7.63 - 7.53 (2 H, m), 7.22
- 7.05
104 (3 H, in), 3.97 (2 H, bd), 3.67 (3 H, s), 3.60 (3 H, s), 2.83 (3 H, s),
2.82 - 2.72 (4 H,
2.70 - 2.59 (1 H, m), 2.04 - 1.93 (2 H, m), 1.92 - 1.78 (2 H, m), 1.37 (3 H,
t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.96 (1 H, s), 7.69 - 7.60 (2 H, m), 7.55 (1
H, d),
105 7.44 (1 H, dd), 7.23 (2 H, t), 3.85 (2 H, d), 3.59 (3 H, s), 2.91 -
2.81 (5 H, m), 2.80 - 2.68
(3 H, m), 2.00 (3 H, s), 1.99 - 1.92 (2 H, m), 1.91 - 1.77 (2 H, m), 1.33 (3
H, t)
231
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, CDC13) 6 ppm 11.56 (1 H, bs), 8.43 (1 H, d), 7.66 (1 H, d),
7.61 (1 H,
106 s), 7.55 (1 H, dd), 7.22 (1 H, d), 6.84 - 6.73 (2 H, m), 3.96 (2 H, d),
3.63 (3 H, s), 2.87 -
2.71 (7 H, m), 2.71 - 2.54 (1 H, m), 2.09 (3 H, s), 2.02 - 1.92 (2 H, m), 1.91
- 1.77 (2 H,
m), 1.37 (3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 7.61 (2 H, dd), 7.53 (1 H, dd), 7.17 (2 H, td),
7.05 (1
107 H, td), 6.64 (1 H, s), 4.60 (2 H, d), 3.97 (2 H, d), 3.58 (3 H, s),
2.87 - 2.59 (8 H, m), 2.05 -
1.76 (4 H, m), 1.36 (3 H, t)
1H NMR (400 MHz, Mc0D-d4) 6 ppm 7.92 - 7.82 (3 H, m), 7.61 (1 H, dd), 7.51 (1
H, d),
108 7.10 (2 H, t), 6.94 (1 H, s), 6.20 (1 H, bs), 4.18 (2 H, q), 3.62 (3 H,
s), 3.39 (2 H, s), 3.36 -
3.32 (2 H, m), 2.86(2 H, bt), 2.72(2 H, q), 2.61 - 2.49 (2 H, m), 1.31 (3 H,
t), 1.26 (3 H, t)
1H NMR (400 MHz, Mc0D-d4) 6 ppm 7.88 (2 H, dd), 7.83 (1 H, s), 7.50 (1 H, dd),
7.39 (1
109 H, dd), 7.14 - 7.07 (2 H, m), 6.93 (1 H, s), 4.17 (2 H, q), 3.61 (3 H,
s), 3.24 (2 H, s), 3.07 -
3.00 (2 H, m), 2.71 (2 H, q), 2.66 - 2.54 (1 H, m), 2.38 -2.22 (2 H, m), 1.87 -
1.74 (4 H,
m), 1.31 (3 H, t), 1.26 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.90 - 7.81 (2 H, m), 7.61 (1 H, bs), 7.55 (1 H,
d), 7.20
110 (1 H, dd), 7.13 - 7.06 (2 H, m), 6.67 (1 H, s), 4.72 - 4.65 (1 H, m),
4.49 - 4.42 (1 H, m),
4.31 - 4.24 (1 H, m), 4.11 (1 H, dd), 3.90 (1 H, dd), 3.61 (3 H, s), 3.10 -
2.99 (4 H, m),
2.73 (2 H, q), 2.56 - 2.45 (1 H, m), 2.28 - 2.17 (2 H, m), 1.94 - 1.76 (4 H,
m), 1.34 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.93 - 7.83 (2 H, m), 7.61 (1 H, s), 7.54 (1 H,
d), 7.18
(1 H, d), 7.10 (2 H, t), 6.67 (1 H, s), 4.59 - 4.45 (1 H, m), 3.72 - 3.50 (7
H, m), 3.18 (1 H,
111
d), 3.14 (1 H, s), 3.01 - 3.11 (2 H, m), 2.74 (2 H, q), 2.57 - 2.47 (1 H, m),
2.27 -2.17 (2 H,
m), 1.86 - 1.70 (6 H, m), 1.34 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.87(2 H, dd), 7.61 (1 H, s), 7.54(1 H, d),
7.18(1 H,
112 dt), 7.10(2 H, t), 6.67(1 H, s), 4.59 - 4.48 (1 H, m), 3.72 - 3.50 (7
H, m), 3.21 -3.13 (2 H,
m), 3.13 - 3.01 (2 H, m), 2.75 (2 H, q), 2.56 - 2.44 (1 H, m), 2.28 - 2.18 (2
H, m), 2.09 -
1.93 (2 H, m), 1.82(4 H, m), 1.34 (3 H, t)
'FINMR (300 MHz, CDC13) 6 ppm 7.87(2 H, dd), 7.61 (1 H, s), 7.55(1 H, d),
7.18(1 H,
113 dd), 7.10 (2 H, t), 6.68 (1 H, s), 4.31 (1 H, t), 4.14 - 4.00 (2 H, m),
3.86 - 3.74 (3 H, m),
3.62 (3 H, s), 3.09 - 2.98 (4 H, m), 2.78 (3 H, m), 2.60 - 2.44 (1 H, m), 2.27
- 2.14 (2 H,
m), 1.89 - 1.78 (4 H, m), 1.35 (3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 7.87 (2 H, dd), 7.61 (1 H, s), 7.54 (1 H, dd),
7.18 (1 H,
114 dd), 7.10 (2 H, t), 6.67 (1 H, s), 3.61 (3 H, s), 3.19 (2 H, s), 3.12-
2.99 (5 H, m), 2.95 (3 H,
s), 2.74 (2 H, q), 2.57 - 2.42 (1 H, m), 2.29 - 2.11 (2 H, m), 1.87 - 1.74 (4
H, m), 1.34 (3
H, t)
232
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, CDC13) 6 ppm 7.91 - 7.83 (2 H, m), 7.61 (1 H, bs), 7.54 (1 H,
d), 7.18
115 (1 H, dd), 7.10 (2 H, t), 6.67(1 H, s), 3.61 (3 H, s), 3.49 (4 H, q),
3.15 (2 H, s), 3.07 (2 H,
bd), 2.75 (2 H, q), 2.55 - 2.44 (1 H, m), 2.28 - 2.17 (2 H, m), 1.94 (2 H, q),
1.89 - 1.77 (6
H, m), 1.34(3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.87 (2 H, dd), 7.61 (1 H, s), 7.55 (1 H, d),
7.22 - 7.15
116 (1 H, m), 7.11 (2 H, t), 6.68 (1 H, s), 3.93 (1 H, d), 3.84 - 3.66 (3
H, m), 3.62 (3 H, s), 3.22
- 3.08 (3 H, m), 3.08 - 2.94 (2 H, m), 2.75 (2 H, q), 2.57 - 2.44 (1 H, m),
2.34 (1 H, q),
2.31 -2.12 (3 H, m), 1.89 - 1.76 (4 H, m), 1.35 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.91 - 7.88 (2 H, m), 7.64 (1 H, bs), 7.57 (1 H,
d), 7.23
117 - 7.20(1 H, m), 7.13 (2 H, t), 6.70(1 H, s), 3.73 - 3.61 (7 H, m), 3.57
- 3.40(2 H, m), 3.31
- 3.08 (4 H, m), 2.78 (2 H, q), 2.57 - 2.34 (2 H, m), 2.29 - 2.17 (2 H, m),
1.91 -2.04 (1 H,
m), 1.90 - 1.80 (4 H, m), 1.72 - 1.62 (1 H, m), 1.37 (3H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.86(2 H, dd), 7.60(1 H, s), 7.54(1 H, d),
7.17(1 H,
118 dd), 7.09(2 H, t), 6.67(1 H, s), 4.85 - 4.77 (1 H, m), 4.52(1 H, t),
4.23 (1 H, dd), 3.61 (3
H, s), 3.00 (2 H, t), 2.78 - 2.67 (4 H, m), 2.53 - 2.43 (1 H, m), 2.36 - 2.19
(2 H, m), 1.87 -
1.79 (2 H, m), 1.79 - 1.66 (2 H, m), 1.33 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.87(2 H, dd), 7.62(1 H, s), 7.55(1 H, d),
7.19(1 H,
119 d), 7.10 (2 H, t), 6.67 (1 H, s), 3.82 - 3.73 (2 H, m), 3.61 (3 H, s),
3.58 - 3.51 (2 H, m),
3.25 (2 H, s), 3.18 - 3.05 (2 H, m), 2.98 (3 H, s), 2.74 (2 H, q), 2.60 - 2.49
(1 H, m), 2.34 -
2.21 (2 H, m) , 1.87 - 1.79 (2 H, m), 1.79 - 1.66 (2 H, m), 1.34 (3 H, t)
'I-INMR (400 MHz, CDC13) 6 ppm 7.87(2 H, dd), 7.61 (1 H, s), 7.54(1 H, d),
7.19(1 H,
120 dd), 7.10 (2 H, t), 6.67 (1 H, s), 3.71 (3 H, s), 3.61 (3 H, s), 3.35
(2 H, s), 3.19 (3 H, s),
3.11(2 H, bd), 2.75 (2 H, q), 2.56 - 2.44 (1 H, m), 2.32 - 2.19 (2 H, m), 1.80
- 1.72 (4 H,
m), 1.34(3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm (2 conformers) 7.87 (2 H, dd), 7.62 (1 H, s),
7.55 (1 H,
121 d), 7.23 - 7.15 (1 H, m), 7.11 (2 H, t), 6.68 (1 H, s), 4.62 (0.7 H,
s), 4.35 (1.3 H, s), 3.62 (3
H, s), 3.31 - 3.20 (4 H, m), 3.06 (1 H, s), 3.04 - 2.87 (2 H, m), 2.75 (2 H,
q), 2.57 - 2.45 (1
H, m), 2.30 - 2.14 (2 H, m), 1.93 - 1.74 (4 H, m), 1.35 (3 H, t)
1H NMR (300 MHz, CDC13) 8 ppm 7.85 (2 H, dd), 7.66 - 7.48 (2 H, m), 7.22 -
6.99 (3 H,
122 m), 6.66(1 H, s), 6.11 (1 H, s)NH, 4.84 - 4.69 (1 H, m), 3.73 - 3.52 (4 H,
m), 3.42 - 3.26
(1 H, m), 3.09 (1 H, d), 2.96 (1 H, d), 2.80 - 2.66 (3 H, m), 2.65 - 2.57 (1
H, m), 2.56 -
2.40 (1 H, m), 2.34 - 2.08 (2 H, m), 1.93 - 1.64 (4 H, m), 1.32 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.90 - 7.82 (2 H, m), 7.62 (1 H, s), 7.57 (1 H,
d), 7.18
123 (1 H, dd), 7.15 - 7.06 (2 H, m), 6.68 (1 H, s), 3.66 - 3.57 (5 H, m),
3.47 (2 H, q), 3.07 (2 H,
s), 2.99 - 2.90 (3 H, m),2.81 - 2.68 (3 H, m), 2.57 - 2.48 (1 H, m), 2.30 (2
H, t), 1.93 - 1.82
(2 H, in), 1.78 - 1.66 (2 H, in), 1.39 - 1.29 (5 H, m)
233
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, CDC13) 6 ppm 7.87 - 7.84 (2 H, m), 7.60 (1 H, bs), 7.54 (1 H,
d),
124 7.16 (1 H, dd), 7.09 (2 H, t), 6.66 (1 H, s), 4.58 (2 H, t), 4.33 (2 H,
t), 3.60 (3 H, s), 3.12 (2
H, s), 2.97 (2 H, bd), 2.74 (2 H, q), 2.53-2.45 (1 H, m), 2.17 (2 H, bt), 1.85-
1.69 (4 H, m),
1.32(3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.90 - 7.84 (2 H, m), 7.62 (1 H, s), 7.56 (1 H,
d), 7.17
125 (1 H, dd), 7.14 - 7.07 (2 H, m), 7.06 - 7.02 (1 H, bs)NH, 6.68 (1 H,
s), 5.44 - 5.34 (1 H,
bs)NH, 3.63 (3 H, s), 3.06 - 2.96 (4 H, m), 2.76 (2 H, q), 2.57 - 2.47 (1 H,
m), 2.28 (2 H,
bt), 1.92 - 1.84 (2 H, m), 1.81 - 1.69 (2 H, m), 1.35 (3 H, t)
NMR (400 MHz, CDC13) 6 ppm 7.87(2 H, dd), 7.64 - 7.52 (2 H, m), 7.17 - 7.05 (3
H,
m), 6.68 (1 H, s), 4.76 (1 H, d), 4.21 (1 H, d), 3.62 (3 H, s), 3.50 - 3.25 (2
H, m), 3.08 (1
126
H, t), 2.82 - 2.69 (3 H, m), 2.69 - 2.51 (5 H, m), 1.90 (2 H, d), 1.85 - 1.73
(4 H, m), 1.60 (2
H, quin), 1.35 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.90 - 7.82 (2 H, m), 7.60 (1 H, s), 7.56 (1 H,
d), 7.18
127 - 7.05 (3 H, m), 6.67 (1 H, s), 4.78 (1 H, d), 3.88 (1 H, d), 3.62 (3
H, s), 3.43 (2 H, d), 3.09
(1 H, bt), 2.82 - 2.71 (3 H, m), 2.66 (1 H, bt), 2.46 (3 H, s), 1.91 (2 H, d),
1.71 - 1.54 (2 H,
m), 1.34(3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.93 - 7.78 (2 H, m), 7.67 - 7.51 (2 H, m), 7.20
- 7.03
128 (3 H, m), 6.68 (1 H, s), 4.72 (1 H, d), 4.55 - 4.36 (1 H, m), 3.94 (1
H, d), 3.85 - 3.70 (2 H,
m), 3.62 (3 H, s), 3.49 - 3.28 (2 H, m), 3.22 - 3.00 (2 H, m), 2.85 - 2.68 (3
H, m), 2.67 -
2.25 (2 H, m), 1.89(2 H, d), 1.76 - 1.46 (2 H, m), 1.34 (3 H, t)
'I-INMR (300 MHz, CDC13) 6 ppm 7.85(2 H, dd), 7.64 - 7.50 (2 H, m), 7.17 -
7.02 (3 H,
129 m), 6.67 (1 H, s), 4.74 (1 H, d), 4.23 (1 H, d), 3.61 (3 H, s), 3.22 -
2.98 (3 H, m), 2.82 -
2.69 (3 H, m), 2.60 (1 H, t), 2.29 (6 H, s), 1.89 (2 H, d), 1.73 - 1.45 (2 H,
m), 1.33 (3 H, t)
'I-INMR (400 MHz, CDC13) 6 ppm 7.86 (2 H, dd), 7.60 (1 H, s), 7.55 (1 H, d),
7.17 - 7.04
130 (3 H, m), 6.67 (1 H, s), 4.82 - 4.72 (1 H, m), 3.99 (1 H, d), 3.61 (3
H, s), 3.12 (1 H, t), 2.84
- 2.68 (5 H, m), 2.67 - 2.56 (3 H, m), 2.36 (6 H, s), 1.90 (2 H, t), 1.68 -
1.51 (2 H, m), 1.33
(3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.86(2 H, dd), 7.62 - 7.55 (2 H, m), 7.15 - 7.05
(3 H,
131 m), 6.68 (1 H, s), 4.72 (1 H, d), 3.92 (1 H, d), 3.74 (4 H, t), 3.62 (3
H, s), 3.47 (2 H, q),
3.10 (1 H, bt), 2.75 (3 H, q), 2.62 (1 H, bt), 1.91 (2 H, d), 1.70 - 1.51 (2
H, m), 1.34 (3 H,
t)
1H NMR (400 MHz, CDC13) 6 ppm 7.85 (2 H, dd), 7.60 (1 H, s), 7.55 (1 H, d),
7.17 - 7.02
132 (3 H, m), 6.67 (1 H, s), 4.76 (1 H, d), 3.96 (1 H, d), 3.61 (3 H, s),
3.24 (1 H, bs)NH, 3.11
(1 H, t), 2.92 (2 H, t), 2.80 - 2.71 (3 H, m), 2.69 - 2.61 (3 H, m), 2.49 (3
H, s), 1.96 - 1.82
(2 H, m), 1.69 - 1.50 (2 H, m), 1.33 (3 H, t)
234
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, CDC13) 6 ppm 7.90 - 7.82 (2 H, m), 7.62 - 7.56 (2 H, m), 7.17
- 7.06
133 (3 H, m), 6.68 (1 H, s), 5.24 (0.5 H, quin), 5.09 (0.5 H, quin), 4.73
(1 H, d), 4.01 - 3.76 (3
H, m), 3.62 (3 H, s), 3.42 (2 H, q), 3.33 (1 H, dd), 3.27 (1 H, dd), 3.10 (1
H, t), 2.82 - 2.69
(3 H, m), 2.61 (1 H, t), 1.90 (2 H, d), 1.74 - 1.50 (2 H, m), 1.35 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.85 (2 H, dd), 7.65 - 7.58 (2 H, m), 7.23 (1 H,
d),
134 7.10 (2 H, t), 6.68 (1 H, s), 4.13 (1 H, t), 4.07 - 3.96 (2 H, m), 3.87
(1 H, dd), 3.61 (3 H, s),
3.20 - 3.10 (1 H, m), 3.01 - 2.88 (2 H, m), 2.76(2 H, q), 2.63 -2.51 (1 H, m),
2.04- 1.84
(7 H, m), 1.77 (2 H, t), 1.35 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.50 - 8.43 (1 H, m), 7.93 - 7.87 (2 H, m), 7.85
(1 H,
135 s), 7.83 - 7.76 (1 H, m), 7.12 (2 H, t), 4.00 (2 H, bd), 3.61 (3 H, s),
2.99 (2 H, q), 2.88 -
2.78 (6 H, m), 2.09 - 1.99 (2 H, m), 1.93 - 1.79 (2 H, m), 1.54 (3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 8.23 - 8.12 (2 H, m), 7.68 - 7.53 (2 H, m), 7.25
- 7.13
136 (3 H, m), 3.98 (2 H, d), 3.65 (3 H, s), 2.89 - 2.70 (8 H, m), 2.10 -
1.94 (2 H, m), 1.94 -
1.77(2 H, m), 1.38(3 H, t)
1H NMR (400 MHz, DMSO-d6) 8 ppm 8.10 (1 H, 7.75 (2
H, t), 7.54 (1 H, d), 7.36 (1
137 H, d), 7.25 (2 H, t), 3.68 (2 H, d), 3.50 (3 H, s), 3.30 (2 H, s)NH2,
2.89 (3 H, s), 2.82 -
2.71 (3 H, m), 2.63 (2 H, q), 1.92 - 1.83 (2 H, m), 1.81 - 1.71 (2 H, m), 1.25
(3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 8.16 (2 H, dd), 7.58 - 7.36 (3 H, m), 7.26 (2
H, t),
138 4.61 - 4.53 (1 H, m), 4.54 - 4.42 (1 H, m), 4.21 (1 H, dd), 4.05 (1 H,
dd), 3.77 (1 H, dd),
3.66 (3 H, s), 3.20 - 3.09 (6 H, m), 2.76 - 2.62 (6 H, m), 1.33 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 8.16 (2 H, dd), 7.51 - 7.38 (3 H, m), 7.26 (2
H, t),
139 4.37 - 4.28 (1 H, m), 4.09 - 3.99 (2 H, m), 3.81 - 3.72 (1 H, m), 3.70 -
3.63 (5 H, m), 3.15
(4 H, d), 3.11(2 H, s), 2.83 -2.64 (7 H, m), 1.33 (3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 8.22 - 8.10 (2 H, m), 7.52 (1 H, d), 7.23 - 7.14
(3 H,
140 m), 7.10 (1 H, d), 3.62 (3 H, s), 3.24 (2 H, d), 3.16 - 3.06 (7 H, m),
2.95 (3 H, s), 2.79 -
2.65 (6 H, m), 1.35 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.22 - 8.12 (2 H, m), 7.63 - 7.54 (2 H, m), 7.24
(1 H,
141 dd), 7.19 (2 H, t), 4.74 - 4.66 (1 H, m), 4.49 - 4.42 (1 H, m), 4.29 (1
H, dd), 4.11(1 H, dd),
3.90 (1 H, dd), 3.64 (3 H, s), 3.08 - 2.99 (4 H, m), 2.75 (2 H, q), 2.57 -
2.47 (1 H, m), 2.23
- 2.14 (2 H, m), 1.89 - 1.77 (4 H, m), 1.36 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.19 - 8.11 (2 H, m), 7.59 - 7.52 (2 H, m),
7.24(1 H,
142 d), 7.21 - 7.13 (2 H, m), 4.51 (1 H, dd), 3.71 - 3.57 (5 H, m), 3.56 -
3.46 (1 H, m), 3.19 -
3.00 (4 H, m), 2.73 (2 H, q), 2.57 - 2.46 (1 H, m), 2.28 - 2.08 (4 H, m), 2.09
- 1.94 (1 H,
m), 1.87 - 1.76 (4 H, m), 1.34 (3 H, t)
235
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.89 (2 H, dd), 7.48 - 7.42 (1 H, m), 7.40 -
7.28 (2
142 H, m), 7.11 (2 H, t), 6.94(1 H, s), 4.66(2 H, t), 4.33 (2 H, t), 3.61
(3 H, s), 3.19(2 H, s),
3.17 -3.03 (4 H, m), 2.74 - 2.61 (6 H, m), 1.30 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 8.15 (2 H, dd), 7.95 (1 H, s), 7.52 (2 H, dd),
7.25 (2
143 H, t), 4.39(1 H, d), 3.67 - 3.58 (4 H, m), 3.57 - 3.41 (3 H, m), 3.23 -
3.14 (2 H, m), 3.11 -
3.00 (2 H, m), 2.74 (2 H, q), 2.67 - 2.57 (1 H, m), 2.29 - 2.17 (2 H, m), 2.06
- 1.91 (2 H,
m), 1.89 - 1.76 (4 H, m), 1.34 (3 H, t)
1H NMR (400 MHz, CDC1,) 6 ppm 8.22 - 8.12 (2 H, m), 7.64 - 7.53 (2 H, m), 7.27
- 7.23
144 (1 H, m), 7.19 (2 H, t), 4.33 - 4.26 (1 H, m), 4.13 - 4.04 (2 H, m),
3.86 - 3.77 (3 H, m),
3.64 (3 H, s), 3.16 - 3.07 (4 H, in), 2.87 - 2.80 (1 H, in), 2.75 (2 H, q),
2.64 - 2.55 (1 H,
m), 2.47 - 2.34 (2 H, m), 1.90 - 1.84 (4 H, m), 1.37 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.21 - 8.12 (2 H, m), 7.63 - 7.50 (2 H, m), 7.24
(1 H,
145 dd), 7.18 (2 H, t), 3.63 (3 H, s), 3.23 (2 H, s), 3.11 - 3.03 (5 H,
in), 2.95 (3 H, s), 2.75 (2 H,
q), 2.60 -2.49 (1 H, m), 2.34 -2.22 (2 H, m), 1.90 - 1.80 (4 H, m), 1.36 (3 H,
t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.70 (2 H, dd), 7.47 - 7.41 (1 H, m), 7.40 -
7.30 (2
146 H, m), 7.17 (2 H, t), 4.56 (2 H, s), 4.32 (1 H, t), 4.09 - 3.98 (2 H,
m), 3.76 (1 H, dd), 3.67
(2 H, d), 3.55 (3 H, s), 3.18 - 3.05 (6 H, m), 2.83 - 2.74 (1 H, m), 2.74 -
2.62 (6 H, m),
1.32 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.78 - 7.71 (2 H, m), 7.63 (1 H, s), 7.59 (1 H,
d), 7.21
147 - 7.10 (3 H, m), 4.69 (2 H, s), 3.96 (2 H, d), 3.57 (3 H, s), 2.83 (3
H, s), 2.81 - 2.70 (4 H,
m), 2.69 - 2.59 (1 H, m), 2.03 - 1.94 (2 H, m), 1.91 - 1.77 (2 H, m), 1.34 (3
H, t)
1H NMR (300 MHz, CDC13) 6 ppm 7.85 - 7.75 (2 H, iii), 7.68 - 7.58 (2 H, in),
7.30 (2 H,
148 dd), 7.20 (1 H, dd), 4.71 (2 H, s), 3.97 (2 H, d), 3.57 (3 H, s), 2.83
(3 H, s), 2.76 (4 H, m),
2.71 -2.57 (1 H, m), 1.99 (2 H, d), 1.85 (2 H, bq), 1.35 (3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 7.80 - 7.71 (2 H, m), 7.61 (1 H, bs), 7.54 (1 H,
d), 7.19
149 - 7.09 (3 H, m), 4.68 (2 H, s), 3.93 (2 H, d), 3.56 (3 H, s), 3.06 -
2.97 (2 H, m), 2.94 - 2.81
(2 H, m), 2.77 - 2.58 (3 H, m), 2.44 (2 H, t), 2.25 (6 H, s), 2.08 - 1.98 (4
H, m), 1.89 - 1.71
(2 H, m), 1.32 (3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 7.91 (2 H, d), 7.71 (2 H, d), 7.63 (1 H, s),
7.55 (1 H,
150 d), 7.16 (1 H, dd), 4.72 (2 H, s), 3.95 (2 H, d), 3.57 (3 H, s), 2.88 -
2.56 (8 H, m), 2.04 -
1.74 (4 H, m), 1.30(3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.76 (2 H, dd), 7.60 (1 H, s), 7.47 (1 H, d),
7.19 - 7.08
151 (3 H, m), 4.66 (2 H, s), 3.53 (3 H, s), 3.46 (4 H, q), 3.13 (2 H, s),
3.04 (2 H, bd), 2.71 -
2.60 (2 H, m), 2.54 - 2.44 (1 H, m), 2.27 - 2.14 (2 H, m), 1.99 - 1.89 (2 H,
m), 1.88 - 1.75
(6 H, m), 1.28 (3 H, t)
236
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, CDC13) 6 ppm 7.74 (2 H, dd), 7.60 (1 H, s), 7.48 (1 H, d),
7.19 - 7.08
152 (3 H, m), 4.66 (2 H, s), 4.29 - 4.21 (1 H, m), 4.03 (2 H, bt), 3.81 -
3.72 (3 H, m), 3.54 (3
H, s), 3.07 - 2.96 (4 H, m), 2.84 - 2.73 (1 H, m), 2.68 (2 H, q), 2.56 - 2.43
(1 H, m), 2.28 -
2.13 (2 H, m), 1.80(4 H, bs.), 1.29(3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.79 - 7.72 (2 H, m), 7.60 (1 H, s), 7.53 (1 H,
d), 7.19
153 - 7.08 (3 H, m), 4.75 (1 H, d), 4.68 (2 H, s), 4.25 (1 H, d), 3.56 (3
H, s), 3.24 - 3.03 (3 H,
m), 2.81 - 2.58 (4 H, m), 2.32(6 H, s), 1.91 (2 H, d), 1.71 - 1.52(2 H, m),
1.33(3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 7.76 (2 H, dd), 7.60 (1 H, s), 7.53 (1 H, d),
7.18 - 7.10
154 (3 H, m), 4.81 (1 H, d), 4.68 (2 H, s), 3.99 (1 H, d), 3.56 (3 H, s),
3.12 (1 H, t), 2.82 - 2.54
(4 H, m), 2.38 (2 H, q), 1.99 - 1.83 (2 H, m), 1.80 - 1.50 (2 H, m), 1.32(3 H,
t), 1.17 (3 H,
t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.78 - 7.64 (2 H, m), 7.43 (1 H, d), 7.40 -
7.32 (2
155 H, m), 7.17 (2 H, t), 4.56(2 H, s), 3.55 (3 H, s), 3.28 (2 H, s), 3.18 -
3.10 (4 H, m), 3.09 (3
H, s), 2.93 (3 H, s), 2.74 -2.65 (6 H, m), 1.31 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.74 (2 H, dd), 7.60 (1 H, s), 7.52 (1 H, d),
7.21 - 7.09
(3 H, m), 4.73 -4.65 (3 H, m), 4.50 -4.43 (1 H, m), 4.27(1 H, dd), 4.10 (1 H,
dd), 3.89 (1
156
H, dd), 3.56 (3 H, s), 3.05 (2 H, s), 3.01 (2 H, d), 2.74 (2 H, q), 2.55 -
2.46 (1 H, m), 2.25 -
2.14 (2 H, m), 1.89 - 1.80 (2 H, m), 1.74 - 1.56 (2 H, m), 1.34 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.75 (2 H, dd), 7.61 (1 H, s), 7.53 (1 H, d),
7.22 - 7.11
157 (3 H, m), 4.67 (2 H, s), 3.56 (3 H, s), 3.21 (2 H, s), 3.09 (3 H, s),
3.08 - 3.00 (2 H, m), 2.96
(3 H, s), 2.74 (2 H, q), 2.58 - 2.46 (1 H, m), 2.27 - 2.17 (2 H, m), 1.89 -
1.79 (4 H, m),
1.35 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.79 (2 H, dd), 7.58 (1 H, d), 7.22 (1 H, dd),
7.17 -
158 7.11 (3 H, m), 3.63(3 H, s), 3.30(2 H, d), 3.19 - 3.11 (4 H, m), 3.07(3
H, s), 2.95(3 H, s),
2.85 -2.71 (6 H, m), 1.36 (3 H, t)
1H NMR (400 MHz, CDCL) 6 ppm 7.86 - 7.73 (2 H, m), 7.67 - 7.53 (2 H, m), 7.26 -
7.24
159 (1 H, m), 7.15 (2 H, t), 4.73 - 4.65 (1 H, m), 4.49 - 4.40 (1 H, m),
4.29 (1 H, dd), 4.11 (1
H, dd), 3.92 (1 H, dd), 3.64 (3 H, s), 3.13 (4 H, bs), 2.82 - 2.71 (2 H, m),
2.62 - 2.51 (1 H,
m), 2.39 - 2.24 (2 H, m), 1.88 (4 H, bs), 1.38 (3 H, t)
'I-INMR (300 MHz, CDC13) 6 ppm 7.84 - 7.75 (2 H, m), 7.67 - 7.57 (2 H, m),
7.23 (1 H,
160 dd), 7.15 (2 H, t), 4.03 - 3.93 (2 H, m), 3.65 (3 H, s), 2.85 - 2.72 (7
H, m), 2.73 - 2.59 (1
H, m), 2.07 - 1.95 (2 H, m), 1.88 (2 H, qd), 1.38 (3 H, t)
'I-INMR (400 MHz, CDC13) 6 ppm 7.63 - 7.59 (1 H, m), 7.51 - 7.44 (2 H, m),
7.40 - 7.33
161 (1 H, m), 7.21 (1 H, d), 7.11 (1 H, dd), 3.51 (3 H, s), 3.13 (8 H, bs),
2.75(2 H, q), 2.22(3
H, s), 1.35 (3 H, t)
237
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (300 MHz, CDC13) 8 ppm 7.62 (1 H, dd), 7.54 (1 H, d), 7.46 (1 H, dd),
7.42 -
162 7.33 (1 H, m), 7.19 (2 H, s), 4.68 (1 H, bs), 4.52 - 4.41 (1 H, m),
4.29 (1 H, dd), 4.13 (1 H,
dd), 3.91 (1 H, dd), 3.52 (3 H, s), 3.17 - 3.02 (6 H, m), 2.82 - 2.64 (6 H,
m), 2.24 (3 H, s),
1.37(3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 7.61 (2 H, dd), 7.47 (1 H, dd), 7.33 - 7.43 (1
H, m),
163 7.05 - 7.24 (2 H, m), 3.53 (3 H, s), 3.04 - 3.18 (6 H, m), 2.86 (3 H,
d), 2.64 - 2.83 (6 H,
m), 2.24 (3 H, s), 1.39 (3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 8.05 (1 H, dd), 7.62 (1 H, d), 7.45 (1 H, dd),
7.38 (1
164 H, m), 7.16 - 7.25 (3 H, m), 5.36 - 5.45 (0.5 H, m), 5.18 - 5.25 (0.5
H, m), 4.45 - 4.61 (1
H, m), 4.24 - 4.44 (2 H, m), 4.05 - 4.23 (1 H, m), 3.62 (3 H, s), 3.05 - 3.17
(6 H, m), 2.64 -
2.82 (6 H, m), 1.37 (3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 8.04 (1 H, dd), 7.65 (1 H, d), 7.45 (1 H, dd),
7.37 (1
165 H, ddd), 7.26 - 7.16 (3 H, m), 4.58 (2 H, t), 4.36 (2 H, t), 3.62 (3 H,
s), 3.19 (2 H, s), 3.16 -
3.07 (4 H, m), 2.78 (2 H, q), 2.72 - 2.63 (4 H, m), 1.38 (3 H, t)
1H NMR (300 MHz, CDC13) 8 ppm 8.07 (1 H, dd), 7.57 - 7.32 (3 H, m), 7.22 -
7.09 (3 H,
166 m), 4.77 - 4.62 (1 H, m), 4.46 (1 H, t), 4.28 (1 H, dd), 4.11 (1 H,
dd), 3.90 (1 H, dd), 3.61
(3 H, s), 3.18 - 3.03 (6 H, m), 2.82 -2.61 (6 H, m), 1.34 (3 H, t)
1H NMR (400 MHz, CDC13) 8 ppm 8.06 (1 H, dd), 7.62 (1 H, d), 7.45 (1 H, dd),
7.41 -
167 7.34 (1 H, m), 7.25 - 7.16 (3 H, m), 4.24 (2 H, t), 4.06 (2 H, t), 3.61
(3 H, s), 3.18 - 3.05 (6
H, m), 2.81 - 2.67 (6 H, m), 2.29 (2 H, quin), 1.37 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.07 (1 H, dd), 7.59 (1 H, d), 7.44 (1 H, dd),
7.37 (1
168 H, dd), 7.24 - 7.15 (3 H, m), 3.61 (3 H, s), 3.26 (2 H, s), 3.18 -3.03
(7 H, m), 2.95 (3 H, s),
2.81 -2.68 (6 H, m), 1.35 (3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 8.03 (1 H, dd), 7.68 (1 H, d), 7.45 (1 H, dd),
7.38 (1
169 H, dd), 7.22 - 7.17 (2 H, m), 7.11 - 7.01 (1 H, m), 3.62 (3 H, s), 3.17
- 3.07 (6 H, m), 2.85
(3 H, d), 2.79 (2 H, q), 2.76 -2. 68 (4 H, m), 1.38 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.09 (1 H, dd), 7.61 (1 H, s), 7.54 (1 H, d),
7.45 (1 H,
dd), 7.41 - 7.34 (1 H, m), 7.23 - 7.16 (2 H, m), 4.74 - 4.65 (1 H, m), 4.51 -
4.41 (1 H,
170 4.36 - 4.23 (1 H, m), 4.19 - 4.07 (1 H, m), 3.98 - 3.83 (1 H, m), 3.62
(3 H, s), 3.10 - 2.96
(4 H, m), 2.76 (2 H, q), 2.60 - 2.44 (1 H, m), 2.27 - 2.15 (2 H, m), 1.84 (4
H, bs), 1.35 (3
H, t)
1H NMR (300 MHz, Me0D-d4) 8 ppm 8.10- 8.01 (1 H, m), 7.90(1 H, s), 7.63 (1 H,
dd),
171 7.56 - 7.46 (2 H, m), 7.42 (1 H, dd), 7.30 (1 H, s), 3.64 (3 H, s),
3.26 (2 H, s), 3.10 (3 H,
s), 3.09 - 2.99 (2 H, m), 2.94 (3 H, s), 2.74 (2 H, q), 2.68 - 2.57 (1 H, m),
2.30 - 2.18 (2 H,
m), 1.90 - 1.79 (4 H, m), 1.33 (3 H, t)
238
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.86 (1 H, s), 7.70 - 7.64 (2 H, m), 7.56 -
7.48 (2 H,
172 m), 7.43 - 7.38 (1 H, m), 4.62 - 4.55 (1 H, m), 4.54 - 4.47 (1 H, m),
4.22 (1 H, dd), 4.07 (1
H, ddd), 3.78 (1 H, dd), 3.53 (3 H, s), 3.10 (2 H, s), 3.02 (2 H, bd), 2.74 (2
H, q), 2.69 -
2.58 (1 H, in), 2.28 -2.19 (5 H, m), 1.90 - 1.79 (4 H, m), 1.34 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.36 (1 H, dd), 7.62 - 7.55 (2 H, m), 7.52 (1 H,
dd),
7.43 173 - 7.35 (1 H, m), 7.23 (1 H, dd), 4.73 - 4.64 (1 H, m), 4.51 -
4.42 (1 H, m), 4.29 (1 H,
dd), 4.12 (1 H, dd), 3.90 (1 H, dd), 3.71 (3 H, s), 3.11 - 3.01 (4 H, m), 2.75
(2 H, q), 2.58 -
2.48 (1 H, m), 2.29 - 2.18 (2 H, m), 1.92 - 1.78 (4 H, m), 1.35 (3 H, t)
1H NMR (400 MHz, DMS0) 6 ppm 8.04 (1 H, s), 7.96 - 7.90 (2 H, m), 7.61 - 7.55
(1 H,
174 m), 7.37 (1 H, dd), 7.28 - 7.20 (3 H, m), 3.58 (3 H, s), 3.14 (2 H, s),
3.01 (3 H, s), 2.91 (6
H, bs), 2.79 (3 H, s), 2.60 - 2.54 (1 H, m), 2.12 - 2.05 (2 H, m), 1.73 - 1.62
(4 H, m)
1H NMR (400 MHz, CDC13) 6 ppm 7.71 (2 H, dd), 7.64 (1 H, s), 7.56 (1 H, d),
7.21 (1 H,
1 dd), 7.14 (2 H, t), 4.68 (2 H, bd), 3.96 (2 H, bd), 3.09 - 3.05 (2 H,
m), 2.98 - 2.91 (1 H, m),
2.89 - 2.73 (6H, m), 2.68 - 2.61 (1 H, m), 2.28 - 2.16 (1 H, bs), 2.01 - 1.95
(2 H, m), 1.91 -
1.78 (2 H, m)
1H NMR (400 MHz, DMSO-d) 6 ppm 7.99 (1 H, s), 7.96 - 7.91 (2 H, m), 7.50 (1 H,
d),
176 7.36 (1 H, bs)NH, 7.32 (1 H, d), 7.27 - 7.21 (3 H, m), 6.78 (1 H,bs)NH,
3.55 (3 H, s), 3.10
(2 H, s), 3.01 (3 H, s), 2.90 (2 H, d), 2.87 - 2.65 (5 H, m), 2.60 - 2.42 (3
H, m), 2.12 - 2.04
(2 H, m), 1.75 - 1.60 (4H, m)
1H NMR (300 MHz, CDC13) 6 ppm 7.92 - 7.83 (2 H, m), 7.48 (1 H, d), 7.15 - 7.04
(2 H,
177 m), 6.86 (1 H, d), 6.73 (1 H, dd), 6.68 (1 H, s), 4.12 (2 H, t), 4.01 -
3.90 (1 H, m), 3.60 (3
H, s), 3.41 (2 H, q), 2.72 (2 H, q), 1.78 (2 H, bs)NH2, 1.34 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.86 (2 H, dd), 7.45 (1 H, d), 7.10 (2 H, t),
6.85 (1 H,
178 d), 6.73 (1 H, dd), 6.67 (1 H, s), 4.76 - 4.64 (1 H, m), 4.33 - 4.21 (2
H, m), 4.03 (2 H, bt),
3.98 (1 H, dd), 3.90 (1 H, dd), 3.80 - 3.69 (1 H, m), 3.59 (3 H, s), 3.56 -
3.45 (2 H, m),
3.20 (2 H, s), 2.71 (2 H, q), 1.32 (3 H, t)
'I-INMR (400 MHz, CDC13) 6 ppm 7.87 (2 H, dd), 7.49 (1 H, d), 7.42 (1 H, d)NH,
7.10 (2
179 H, t), 6.88 (1 H, d), 6.72 (1 H, dd), 6.68 (1 H, s), 4.86 (1 H, d),
4.46 (1 H, t), 4.19 (2 H, td),
3.71 (2 H, dd), 3.66 - 3.54 (5 H, m), 3.17 (2 H, s), 3.14 - 3.04 (2 H, m),
2.73 (2 H, q), 1.34
(3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.86 (2 H, dd), 7.50 (1 H, d), 7.18 (1 H, s),
7.17 - 7.06
180 (3 H, m), 6.67 (1 H, s), 3.67 - 3.62 (2 H, m), 3.61 (3 H, s), 3.09 -
3.04 (4 H, m), 2.77 - 2.57
(8 H, m), 1.33 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.90 (2 H, dd), 7.47 (1 H, d), 7.42 - 7.29 (2
H, m),
181 7.12 (2 H, t), 6.95 (1 H, s), 3.81 (4 H, bt), 3.61 (3 H, s), 3.12 -2.94
(4 H, m), 2.70 (2 H, q),
1.31 (3 H, t)
239
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.94 - 7.85 (2 H, m), 7.56 (1 H, dd), 7.49 (1
H, dd),
182 7.40(1 H, dd), 7.11 (2 H, t), 6.95(1 H, s), 3.71 - 3.64 (4 H, m),
3.62(3 H, s), 3.22 - 3.16
(4 H, m), 2.71 (2 H, q), 1.31 (3 H, t)
183 ,
'HNMR (400 MHz, Me0D-d4) 6 ppm 8.31 - 8.28(1 H, m), 7.88 - 7.84 (2 H, m),
7.72(1
(Gen
H, dd), 7.54 (1 H, dd), 7.41 - 7.35 (2 H, m), 7.02 (1 H, s), 3.98 - 3.93 (2 H,
m), 3.62 (3 H,
--
s), 3.56 - 3.52 (2 H, m), 2.72 (2 H, q), 1.32 (3H, t)
a)
1H NMR (400 MHz, Me0D-d4) 6 ppm 8.39 - 8.28 (1 H, m), 7.84 (2 H, d), 7.71 (1
H, dd),
184 7.57 (1 H, dd), 7.40 - 7.33 (2 H, m), 7.00 (1 H, s), 4.18 (2 H, q),
4.01 (2 H, s), 3.93 - 3.86
(2 H, m), 3.67 - 3.57 (5 H, m), 2.72 (2 H, q), 1.31 (3 H, t), 1.25 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.83 - 7.72 (4 H, m), 7.51 (1 H, d), 7.38 (2 H,
d), 6.81
185 (1 H, s), 6.19 - 6.09 (1 H, m), 3.98 (2 H, bd), 3.71 - 3.56 (5 H, m),
3.56 - 3.46 (2 H, m),
2.86 (3 H, s), 2.66 - 2.57 (2 Hon)
1H NMR (400 MHz, CDC13) 6 ppm 7.64 - 7.57 (4 H, m), 7.22 - 7.14 (3 H, m), 3.97
(2 H,
186 d), 3.72 (2 H, d), 3.56 (3 H, s), 2.85 - 2.72 (7 H, m), 2.71 - 2.60 (1
H, m), 2.06 - 1.95 (2 H,
m), 1.86 (2 H, qd), 1.37 (3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 8.41 (1 H, d), 8.05 - 7.90 (2 H, m), 7.65 - 7.42
(2 H,
187 m), 7.28 (1 H, dd), 7.13 (3 H, d), 6.03 (1 H, t), 4.00 - 3.87 (2 H, m),
3.62 (3 H, s), 3.47 (2
H, t), 2.82 (3 H, s), 2.73 (2 H, q), 2.64 - 2.43 (2 H, in), 1.28 (3 H, t)
1H NMR (400 MHz, CDCL) 6 ppm 8.18 (2 H, d), 7.58 (1 H, d), 7.43 (2 H, d), 7.23
- 7.13
188 (2 H, m), 3.67 (3 H, s), 3.44 - 3.36 (4 H, m), 3.22 - 3.09 (4 H, m),
2.83 (3 H, s), 2.74 (2 H,
q), 1.35(3 H, t)
1H NMR (300 MHz, CDC13) 6 ppm 8.01 - 7.97 (2 H, m), 7.67 (1 H, s), 7.59 (1 H,
d), 7.34
189 (1 H, dd), 7.14 (2 H, t), 6.10 (1 H, s), 3.98 (2 H, bs), 3.63 (3 H, s),
3.52 (2 H, q), 2.86 (3 H,
s), 2.76 (2 H, d), 2.62 (2 H, bs), 1.35 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.24 (2 H, dd), 7.67 (1 H, s), 7.56 (1 H, d),
7.41 (1 H,
190 dd), 7.14 (2 H, t), 6.08 (1 H, bs), 3.67 (3 H, s), 3.31 (2 H, s), 3.27
(2 H, bs), 3.08 (3 H, s),
2.95 (3 H, s), 2.83 - 2.49 (4 H, m), 2.54 - 2.45 (2 H, m), 1.35 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 8.28 - 8.20 (2 H, m), 7.61 - 7.53 (2 H, m), 7.21
(1 H,
191 dd), 7.14 (2 H, t), 3.67 (3 H, s), 3.19 (2 H, s), 3.08 (3 H, s), 3.03
(2 H, bd), 2.95 (3 H, s),
2.74 (2 H, q), 2.56 - 2.42 (1 H, m), 2.24 -2.15 (2 H, m), 1.92 - 1.68 (4 H,
m), 1.35 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.92 - 7.80 (2 H, m), 7.64 (1 H, s), 7.47 (1 H,
d), 7.17
192 (1 H, s), 7.13 - 7.05 (3 H, m), 7.01 (1 H, s), 6.66 (1 H, s), 3.66 (2
H, s), 3.59 (3 H, s), 3.08
(4 H, d), 2.77 - 2.65 (6 H, m), 1.32 (3 H, t)
240
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, CDC13) 6 ppm 7.99 (2 H, d), 7.73 (1 H, s), 7.66 (2 H, d),
7.59 (1 H,
193 dd), 7.35 (1 H, dd), 6.88 (1 H, s), 6.13 - 6.08 (1 H, m), 3.96 (2 H,
d), 3.64 (3 H, s), 3.56 -
3.43 (2 H, m), 2.85 (3 H, s), 2.76 (2 H, q), 2.64 - 2.56 (2 H, m), 1.36 (3 H,
t)
1H NMR (400 MHz, CDC13) 6 ppm 7.87(2 H, dd), 7.62(1 H, s), 7.56(1 H, d),
7.18(1 H,
194 dd), 7.11 (2 H, t), 6.69 (1 H, s), 3.63 (3 H, s), 2.99 (2 H, s), 2.91
(2 H, d), 2.82 - 2.68 (3 H,
m), 2.57 - 2.45 (1 H, m), 2.24 (2 H, bt), 1.86 (2 H, d), 1.77 - 1.66 (2 H, m),
1.35 (3 H, t),
0.85 - 0.74 (2 H, m), 0.57 - 0.48 (2 H, m)
1H NMR (400 MHz, CDC13) 6 ppm 7.90 - 7.84 (2 H, m), 7.61 (1 H, s), 7.57 (1 H,
d), 7.18
195 (1 H, dd), 7.10 (2 H, t), 6.67 (1 H, s), 4.71 - 4.61 (1 H, m), 3.65 -
3.58 (4 H, m), 3.31 -
3.24 (1 H, t), 3.13 (1 H, bd), 3.00 (1 H, bd), 2.88 (3 H, s), 2.75 (2 H, q),
2.68 (2 H, dd),
2.55 - 2.46 (1 H, m), 2.36 - 2.18 (2 H, m), 1.86 - 1.70 (4 H, m), 1.34 (3 H,
t)
1H NMR (400 MHz, CDC13) 6 ppm 7.87 (2 H, dd), 7.60 (1 H, s), 7.54 (1 H, d),
7.17 (1 H,
196 dd), 7.10 (2 H, t), 6.67 (1 H, s), 5.23 - 5.08 (1 H, m)NH, 4.79 (1 H,
quin), 3.67 (1 H, t),
3.61 (3 H, s), 3.37 (1 H, t), 3.09 (1 H, bd), 2.99 (1 H, bd), 2.79 - 2.69 (3
H, m), 2.68 - 2.60
(1 H, m), 2.54 - 2.43 (1 H, m), 2.34 -2.17 (2 H, m), 1.86 - 1.65 (4 H, m),
1.34 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.86(2 H, dd), 7.60(1 H, s), 7.54(1 H, d),
7.16(1 H,
197 dd), 7.10 (2 H, t), 6.67 (1 H, s), 5.10 - 4.86 (1 H, m)NH, 4.79 (1 H,
quin), 3.67 (1 H, t),
3.61 (3 H, s), 3.37 (1 H, t), 3.09 (1 H, bs), 2.99 (1 H, bs), 2.79 - 2.60 (4
H, m), 2.55 - 2.42
(1 H, m), 2.34 -2.17 (2 H, m), 1.86 - 1.67 (4 H, m), 1.34(3 H, t)
1H NMR (400 MHz, DMSO-d6) 6 ppm7.95 - 7.90 (3 H, m), 7.66 (1 H, s), 7.51 (1 H,
d),
198 7.31 - 7.20 (4 H, m), 4.34 (1 H, t), 4.01 - 3.97 (1 H, m), 3.96 - 3.88
(1 H, m), 3.54 (3 H, s),
2.96 -2.88 (2 H, m), 2.63 - 2.38 (3 H, m), 2.38 - 2.35 (2 H, m), 2.09 - 1.93
(2 H, m), 1.72 -
1.56(4 H, m), 1.21 (3 H, t)
NMR (400 MHz, CDC13) 6 ppm 8.28 - 8.19 (2 H, m), 7.77 - 7.68 (2 H, m), 7.46 (1
H,
199 dd), 7.14 (2 H, t), 6.15 - 6.08 (1 H, m), 4.01 - 3.95 (2 H, m), 3.69 (3
H, s), 3.58 - 3.44 (2
H, m), 2.86 (3 H, s), 2.79 (2 H, q), 2.68 - 2.57 (2 H, m), 1.35 (3 H, t)
NMR (400 MHz, CDC13) 6 ppm 7.87 - 7.83 (2 H, m), 7.54 (1 H, d), 7.21 (1 H, d),
7.13
200 - 7.06 (3 H, m), 6.67 (1 H, s), 3.78 (2 H, t), 3.65 (2 H, t), 3.60 (3
H, s), 3.09 - 3.03 (4 H,
m), 2.73 (2 H, q), 2.43 (3 H, s), 1.33 (3 H, t)
NMR (400 MHz, CDC13) 6 ppm 7.85 (2 H, dd), 7.53 (1 H, d), 7.22 (1 H, d), 7.15 -
7.01
201 (3 H, m), 6.67 (1 H, s), 6.49 (1 H, d)NH, 4.48 (1 H, dd), 3.84 - 3.74
(2 H, m), 3.60 (5 H,
bs), 3.12 - 2.96 (4 H, m), 2.73 (2 H, q), 2.50 - 2.28 (3 H, m), 2.16 - 2.06 (1
H, m), 1.33 (3
H, t)
1H NMR (300 MHz, CDC13) 6 ppm 7.86 (2 H, dd), 7.54 (1 H, d), 7.24 - 7.04 (4 H,
m),
202 6.68 (1 H, s), 3.84 (4 H, bt), 3.61 (3 H, s), 3.04 (4 H, bt), 2.73 (2
H, q), 1.33 (3 H, t), 1.07 -
0.99 (2 H, m), 0.86 - 0.77 (2 H, m)
241
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, CDC13) 6 ppm 7.85 (2 H, dd), 7.56 (1 H, d), 7.22 (1 H, d),
7.17 - 7.03
203 (3 H, m), 6.68 (1 H, s), 4.52 - 4.43 (1 H, m), 3.93 - 3.70 (2 H, m),
3.66 - 3.51 (5 H, m),
3.13 -2.97 (4 H, m), 2.74(2 H, q), 1.40 - 1.28 (6 H, m)
1H NMR (400 MHz, CDC13) 6 ppm 7.87 - 7.81 (2 H, m), 7.53 (1 H, dd), 7.20 (1 H,
d),
204 7.14 - 7.05 (3 H, m), 7.04 (1 H, bs) NH, 6.66 (1 H, s), 5.59 (1 H, bs)
NH, 4.24 (2 H, t),
3.80 (2 H, t), 3.59(3 H, s), 2.72 (2 H, q), 3.11 -3.05 (4 H, m), 1.33 (3 H, t)
1H NMR (400 MHz, CDC13) 6 ppm 7.85 (2 H, dd), 7.54 (1 H, d), 7.35 - 7.18 (6 H,
m),
205 7.14 - 7.02 (3 H, m), 6.68 (1 H, s), 4.56 (1 H, d), 4.35 (1 H, dd),
3.88 - 3.69 (2 H, m), 3.67
- 3.50 (6 H, m), 3.46 - 3.29 (2 H, m), 3.10 - 2.91 (4 H, m), 2.85 - 2.60 (4 H,
m), 1.33 (3 H,
t)
1H NMR (400 MHz, Mc0D-d4) 6 ppm 8.33 (1 H, dd), 7.84 - 7.81 (2 H, m), 7.70 (1
H, dd),
206 7.58 (1 H, dd), 7.36 - 7.34 (2 H, m), 7.00 (1 H, s), 4.49 - 4.45 (2 H,
m), 4.09 - 4.04 (2 H,
m), 3.60 (3H, s), 2.72 (2 H, q), 1.32 (3 H, s)
1H NMR (400 MHz, Mc0D-d4) 6 ppm 8.03 (1 H, dd), 7.85 (2 H, d), 7.56 (1 H, dd),
7.46
207 (1 H, dd), 7.37 (2 H, d), 7.03 (1 H, s), 3.71 - 3.65 (2 H, m), 3.61 (3
H, s), 3.29 - 3.23 (2 H,
m), 2.73 (2 H, q), 2.30 - 2.21 (2 H, m), 1.92 - 1.83 (2 H, m), 1.32 (3 H, t)
1H NMR (400 MHz, Mc0D-d4) 6 ppm 7.92 - 7.84 (3 H, m), 7.61 (1 H, dd), 7.52 (1
H, d),
208 7.14 - 7.07 (2 H, m), 6.93 (1 H, s), 6.81 - 6.75 (2 H, m), 6.64 - 6.62
(1 H, m), 6.28 - 6.18
(1 H, m), 4.16 - 4.14 (2 H, m), 3.70 (2 H, t), 3.62 (3 H, s), 2.72 (2 H, q),
2.64 - 2.44 (2 H,
m), 1.29(3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.95 (1 H, s), 7.91 - 7.81 (2 H, m), 7.62 (1
H, dd),
209 7.54 (1 H, dd), 7.42 - 7.33 (2 H, m), 7.04 (1 H, s), 6.25 (1 H, t),
3.95 - 3.91 (2 H, m), 3.63
(3 H, s), 3.47 (2 H, td), 2.87 (3 H, s), 2.73 (2 H, q), 2.67 - 2.50 (2 H, m),
1.32 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.95 (1 H, s), 7.90 - 7.80 (2 H, m), 7.62 (1
H, dd),
210 7.54 (1 H, dd), 7.43 - 7.33 (2 H, m), 7.04 (1 H, s), 6.28 - 6.20 (1 H,
m), 4.24 - 4.15 (2 H,
m), 3.80 - 3.67 (2 H, m), 3.63 (3 H, s), 2.73 (2 H, q), 2.69 - 2.55 (2 H, m),
1.32 (3 H, t)
NMR (400 MHz, Me0D-d4) 6 ppm 7.91 (2 H, dd), 7.48 -7.31 (3 H, m), 7.12(2 H,
t),
211 6.95 (1 H, s), 3.77 - 3.68 (1 H, m), 3.61 (3 H, s), 3.51 - 3.40 (2 H,
m), 2.87 - 2.74 (2 H, m),
2.69 (2 H, q), 2.02 - 1.90 (2 H, m), 1.73 - 1.59 (2 H, m), 1.31 (3 H, t)
1H NMR (300 MHz, Me0D-d4) 6 ppm 7.92 - 7.84 (2 H, m), 7.50 - 7.31 (5 H, m),
7.04 (1
212 H, s), 3.71 (2 H, t), 3.62 (3 H, s), 3.18 - 3.05 (4 H, m), 2.76 - 2.66
(6 H, m), 2.60 (2 H, t),
1.32 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.87 (2 H, d), 7.57 (1 H, bd), 7.50 (1 H, d),
7.43 -
213 7.36 (3 H, m), 7.04 (1 H, s), 3.71 - 3.64 (4 H, m), 3.62 (3 H, s), 3.24
- 3.17 (4 H, m), 2.70
(2 H, q), 1.31 (3 H, t)
242
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd NMR data (6)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.90 - 7.86 (3 H, m), 7.60(1 H, dd), 7.51 (1
H, d),
214 7.12 - 7.07 (2 H, m), 6.93 (1 H, s), 6.18 (1 H, bs), 4.04 (2 H,
bs), 3.61 (3 H, s), 3.59 (2 H,
t), 2.71 (2 H, q), 2.55 - 2.38 (2 H, m), 1.45 (9 H, t), 1.31 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.94 - 7.82 (3 H, m), 7.52 (1 H, d), 7.38 (1
H, dd),
215 7.11 (2 H, t), 6.94(1 H, s), 4.66(1 H, d), 4.07(1 H, d), 3.62(3 H,
s), 3.16(1 H, t), 2.89(1
H, t), 2.79 - 2.57 (3 H, m), 2.42 (2 H, q), 1.88 (2 H, t), 1.77 - 1.51 (2 H,
m), 1.31 (3 H, t),
1.11 (3 H, t)
216 1H NMR (400 MHz, CDC13) 6 ppm7.90 - 7.83 (2 H, m), 7.61 (1 H, s),
7.55 (1 H, d), 7.18
(Gen
-10-
(1 H, dd), 7.10 (2 H, t), 6.67 (1 H, s), 3.61 (3 H, s), 3.21 - 3.14 (2 H, m),
3.82 - 3.67 (4 H,
m), 2.65 - 2.55 (1 H, m), 2.88 - 2.78 (2 H, m), 2.70 - 2.51 (2 H, m), 1.34 (3
H,
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.90 - 7.80 (2 H, m), 7.80 (1 H, bs), 7.51 -
7.49 (1
217 H, m), 7.37 (1 H, dd), 7.33 (5 H, m), 7.12 - 7.08 (2 H, m), 6.92
(1 H, s), 3.60 (3H, s), 3.54
(2 H, s), 2.99 (2 H, d), 2.70 (2H, q), 2.65 - 2.56 (1 H, m), 2.22 - 2.06 (2 H,
m), 1.87 - 1.67
(4 H, m), 1.31 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.89 - 7.86 (2 H, m), 7.81 (1 H, s), 7.51 (1
H, d),
218 7.38 (1 H, dd), 7.13 - 7.08 (2 H, m), 6.93 (1 H, s), 3.61 (3 H,
s), 3.02 - 2.97 (2 H, m), 2.81
- 2.57 (4 H, m), 2.32(2 H, t), 1.90- 1.84(2 H, m), 1.79- 1.62(2 H, m), 1.31
(3 H, t), 1.11
- 1.05 (6 H, m)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.95 - 7.82 (3 H, m), 7.51 (1 H, dd), 7.37 (1
H, dd),
219 7.11(2 H, t), 6.93 (1 H, s), 4.18 (2 H, bd), 3.61 (3 H, s), 2.87 -
2.76 (1 H, m), 2.75 - 2.62
(4 H, m), 1.81 (2 H, d), 1.59(2 H, qd), 1.44 (9 H, s), 1.31 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.86 - 7.91 (3 H, m), 7.60 (1 H, dd), 7.52 (1
H, dd),
220 7.07 - 7.14 (2 H, m), 6.94 (1 H, s), 6.31 - 6.27 (1 H, m), 4.31 -
4.26 (2 H, m), 3.87 (2 H, t),
3.62 (3 H, s), 2.71 (2 H, q), 2.38 - 2.52 (2 H, m), 1.31 (3 H, t)
1H NMR (400 MHz, Me0D-d4) 6 ppm 7.90 - 7.80 (3 H, m), 7.60 (1 H, dd), 7.52 (1
H, dd),
221 7.39 - 7.31 (2 H, m), 7.01 (1 H, s), 6.30 - 6.24 (1 H, m), 4.29 -
4.23 (2 H, m), 3.86 (2 H, t),
3.61 (3 H, s), 2.70 (2 H, q), 2.53 - 2.35 (2 H, m), 1.31 (3 H, t)
BIOLOGICAL EXAMPLES
Example 3. In vitro assays
3. /. Principle
[00628] The principle of the assay consists in quantifying the released
choline with an enzymatic
method using choline oxidase and peroxidase. Choline oxydation by choline
oxydase releases betaine and
peroxide. The latter is quantified in presence of HRP that converts the
peroxide detection agent TOOS
and 4-aminoantipyrine into quinoneimine dye. The appearance of quinoneimine
dye is measured
243
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
spectrophotometrically at 555 nm and is proportional to the amount of choline
released by ENPP2.
Inhibition of ENPP2 will result in a decrease of the signal.
3.2. Human ENPP2 (hENPP2) assay
3.2.1. LPC as substrate
[00629] Compound IC50 values are determined in a hENPP2 (UniProtKB/SwissProt
Sequence ref
Q13822) biochemical assay using LPC as substrate.
[00630] 5 j.tL of a dilution series of compound, starting from 20 j.iM
highest concentration, 1/5
dilution, is added to the wells. hENPP2 is used at a final concentration of 1
ug/mL or 3 tig/mL (it will be
appreciated by the skilled person that the potency read out is independent of
the enzyme concentration).
The enzyme is diluted in 50 mM Tris-HC1 pH 8.5, 500 mM NaCl, 5 mM KC1, 10 mM
CaCl2, 0.1% fatty
acid free BSA in a total volume of 10 pL. the reaction is started by the
addition of 10 tiL of 150 jiM LPC
(palmitoyl 16:0) diluted in the same buffer as described above and the mixture
is incubated at 37 C for 30
mM. The reaction is terminated and choline quantified by the addition of a 25
tiL of a mixture containing
0.6 U/mL of choline oxidase, 0.6 U/mL of peroxydase, 1.8 mM TOOS, 1.2 mM amino-
antipyrine, 20 mM
EGTA (stop-developer solution) diluted in the buffer described above.
Luminescence is read on the
Envision after an incubation of 30 min at room temperature (Excitation 555 nm,
excitation light = 70%).
Table V. LPC hENPP2 assay IGO of the compounds of the invention.
> 1000 nM
** > 500 - 1000 I'M
*** > 100 - 500 nM
**** 0.01 - 100 nM
Cpd # LPC ¨ IC50 Cpd # LPC ¨ ICso
2 *** 37
4 *** 38
*** 39
12 **** 40
22 **** 41
23 42
24 *** 43
25 *** 44
26 *** 45
27 *** 46
28 *** 47
29 *** 48
30 **** 49
31 **** 50 **
32 *** 51
33 ** 52
34 *** 53
35 *** 54 **
36 *** 55
244
CA 02902103 2015-08-21
WO 2014/139882
PCT/EP2014/054440
Cpd # LPC - IC50 Cpd # LPC - IC50
56 ** 111 ***
57 * 112 ***
58 * 113 ***
59 * 114 ***
60 * 115 ****
61 * 116 ***
62 * 117 ***
63 * 118 ***
64 * 119 **
65 * 121 **
66 * 122 **
67 * 123 *
68 * 124 *
69 * 125 *
70 * 126 ***
71 * 127 **
72 *** 128 ***
73 * 129 ***
74 * 130 ***
75 ** 131 ***
76 * 132 *
77 * 133 ***
78 * 134 **
79 * 135 *
80 ** 136 ***
81 * 137 *
82 * 138 ****
83 * 139 ****
84 * 140 ****
85 * 141 ****
86 * 142 ****
87 *** 143 ****
88 *** 144 ****
89 *** 145 ****
90 ** 146 **
91 ** 147 ***
92 * 148 *
93 *** 149 ***
94 ** 150 **
95 * 151 **
96 *** 152 **
97 *** 153 **
98 ** 154 ***
99 * 155 **
100 * 156 **
101 * 157 *
102 * 158 ***
103 *** 159 ****
104 * 160 ***
105 *** 161 *
106 * 162 ***
107 ** 163 **
108 * 164 ****
109 *** 165 ***
110 *** 166 ****
245
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd # LPC ¨ IC50 Cpd # LPC ¨ IC50
167 **** 201 *
168 **** 202 *
169 **** 203 *
170 **** 204 *
171 **** 205 **
172 *** 206 *
173 **** 207 *
174 **** 208 *
175 ** 209 *
176 * 210 *
177 ** 211 *
178 *** 212 *
179 * 213 *
180 * 214 *
182 * 215 ***
183 * 216 *
184 * 217 *
185 * 218 *
186 * 219 *
187 * 220 *
188 * 221 *
189 * 222 **
190 * 223 ***
191 * 224 *
192 ** 225 *
193 * 226 *
194 * 227 *
195 * 228 *
196 * 229 *
197 * 233 not active
198 * 239 *
199 *
200 *
3.2.2. FS-3 as substrate
[00631] Compound IC50 values are determined in a fluorescent hENPP2
(UniProtKB/SwissProt
Sequence ref Q13822) biochemical assay using the fluorogenic autotaxin
substrate FS-3 as substrate. FS-
3 is a doubly labeled analog of LPC wherein the fluorophore is quenched
through intramolecular energy
transfer. Without hENPP2, the emission of the probe is quenched. If the
substrate is hydrolyzed by
hENPP2, the emission of the probe is not quenched anymore resulting in a
fluorescence increase.
Inhibition of hENPP2 by compounds will result in a decrease of the signal.
[00632] 10 L, of a dilution series of compound, starting from 20 M
highest concentration, 1/5
dilution, is added to the wells. hENPP2 is used at a final concentration of
0.4 g/mL or 0.64 ug/mL (it
will be appreciated by the skilled person that the potency read out is
independent of the enzyme
concentration). The enzyme is diluted in 50 inM Tris-HC1 pH 8.0, 250 inM NaCl,
5 mM KC1, 1 niM
MgCl2, 1 mM CaCl2, 0.1% fatty acid free BSA in a total volume of 20 L. Enzyme
mixture is added to
compounds and the resulting mixture is incubated for 30 min at room
temperature under shaking. The
reaction is started by the addition of 20 L of 0.75 M FS-3 diluted in the
same buffer as described above
246
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
and the mixture is incubated at 30 C for 30 mm. Fluorescence is read on the
Envision (Excitation 485
nm, emission 520 nM).
Table VI. FS3 hENPP2 assay IC50 of
the compounds of the invention.
* > 1000 nM
** > 500 - 1000 nNI
*** > 100 - 500 nM
**** 0.01 -100 nM
Cpd # FS3 ¨ IC50 Cpd # FS3 ¨ IC50
2 **** 75 ****
4 **** 76 ****
**** 77 ****
12 **** 78 ****
22 **** 79 ****
23 **** 80 ****
24 **** 81 ****
25 **** 82 ****
27 **** 86 ****
35 **** 87 ****
36 **** 88 ****
38 **** 89 ****
39 **** 90 ****
40 **** 91 ****
41 **** 92 ****
42 **** 93 ****
43 **** 94 ****
46 **** 95 ****
47 **** 96 ****
49 **** 98 ****
50 **** 99 ****
51 **** 100 ****
52 **** 101 ****
53 **** 103 ****
54 **** 105 ****
55 **** 106 ****
56 **** 108 ****
57 **** 109 ****
58 **** 110 ****
59 **** 111 ****
60 **** 112 ****
61 **** 113 ****
62 **** 114 ****
63 **** 115 ****
64 **** 116 ****
65 **** 118 ****
66 **** 124 ****
67 **** 125 ****
68 **** 126 ****
69 **** 128 ****
74 **** 133 ****
247
CA 02902103 2015-08-21
WO 2014/139882 PCT/EP2014/054440
Cpd # FS3 ¨ IC50 Cpd # FS3 ¨ IC50
134 **** 200 ****
135 **** 201 ****
136 **** 203 ****
137 **** 204 ****
138 **** 206 ****
141 **** 207 ****
143 **** 208 ****
145 **** 209 ****
147 **** 210 ****
155 **** 211 ****
156 **** 212 ****
157 **** 213 ****
158 **** 214 ****
159 **** 215 ****
160 **** 216 ****
162 **** 217 ****
166 **** 218 ****
167 **** 219 ****
168 **** 220 ****
170 **** 221 ****
171 **** 224 ****
172 **** 225 ****
173 **** 226 ****
174 **** 227 ****
179 **** 229 ***
180 **** 230 ****
182 **** 231 ****
183 **** 232 ****
184 **** 233 ****
185 **** 234 ****
186 **** 235 ****
187 **** 236 ***
188 **** 237 ****
189 **** 238 ***
191 **** 239 ****
192 **** Gen-10-e ****
194 ****
199 ***
3.3. House ENPP2 (ntENPP2)
3.3.1. LPC as substrate
[00633] Compound IC50 values are determined in a mENPP2 (UniProtKB/SwissProt
Sequence ref
Q9R1E6) biochemical assay using LPC as substrate.
[00634] Five L of a dilution series of compound, starting from 20 ttM highest
concentration, 1/5
dilution, is added to the wells. mENPP2 is used at a final concentration of 1
g/mL. The enzyme is
diluted in 50 mM Tris-HC1 pH 8.5, 500 mM NaC1, 5 mM KC1, 10 mM CaCl2, 0.1%
fatty acid free BSA
in a total volume of 10 L. the reaction is started by the addition of 10 L
of 150 M LPC (palmitoyl
16:0) diluted in the same buffer as described above and the mixture is
incubated at 37 C for 30 min. The
reaction is terminated and choline quantified by the addition of a 25 L of a
mixture containing 0.6 U/mL
of eholine oxidase, 0.6 U/mL of peroxydase, 1.8 mM TOOS, 1.2 mM amino-
antipyrine, 20 mM EGTA
248
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME 1 ________________ DE 2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.