Note: Descriptions are shown in the official language in which they were submitted.
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
DEVICES, SYSTEMS, AND METHODS ASSOCIATED WITH ANALYTE
MONITORING DEVICES AND DEVICES INCORPORATING THE SAME
PRIORITY
[0001] The present application claims priority under 35 U.S.C. 119(e) to U.S.
Provisional
Application No. 61/801,518 filed March 15, 2013, entitled "Devices, Systems,
and
Methods Associated with Analyte Monitoring Devices and Devices Incorporating
the
Same," the disclosure of which is incorporated herein by reference for all
purposes.
BACKGROUND
[0002] Analyte monitoring devices have been used as medical diagnostic devices
to
determine a level of analyte from a sample. One common application is glucose
measurements. For example, an analyte monitoring device is used with a remote
sensor to
perform an analyte reading. The sensor may be configured for implantation
(e.g.,
subcutaneous, venous, or arterial implantation) into a patient. The analyte
monitoring
device processes signals from the remote sensor to determine the concentration
or level of
analyte in the subcutaneous tissue and may display the current level of the
analyte.
BRIEF DESCRIPTION OF THE DRAWINGS
[0003] The embodiments of the present disclosure are best understood from the
following
detailed description when read in conjunction with the accompanying drawings.
It is
emphasized that, according to common practice, the various features of the
drawings are
not to-scale. On the contrary, the dimensions of the various features are
arbitrarily
expanded or reduced for clarity. Included are the following:
[0004] FIG. 1 illustrates an in vivo analyte monitoring system in accordance
with certain
embodiments of the present disclosure;
[0005] FIG. 2 illustrates a block diagram of a system including an analyte
monitoring
device and remote data processing device in accordance with certain
embodiments of the
present disclosure;
[0006] FIG. 3 illustrates an analyte monitoring device used with a remote
sensor in
accordance with certain embodiments of the present disclosure;
-1-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0007] FIG. 4 illustrates a method of powering on an analyte monitoring
device, such as a
glucose monitoring device in accordance with certain embodiments of the
present
disclosure;
[0008] FIG. 5A illustrates a method of navigating to a home screen from other
screens on
an analyte monitoring device in accordance with certain embodiments of the
present
disclosure;
[0009] FIG. 5B illustrates a method of powering an analyte monitoring device
off in
accordance with certain embodiments of the present disclosure;
[0010] FIG. 5C illustrates a method of powering an analyte monitoring device
off in
accordance with certain embodiments of the present disclosure;
[0011] FIG. 5D illustrates a method of powering off an analyte monitoring
device in
accordance with certain embodiments of the present disclosure;
[0012] FIG. 5E illustrates a battery low screen for an analyte monitoring
device that
indicates that the battery of the device is low in accordance with certain
embodiments of
the present disclosure;
[0013] FIG. 5F illustrates a method for linking an analyte monitoring device
to a PC and
transferring data between the two in accordance with certain embodiments of
the present
disclosure;
[0014] FIG. 5G illustrates a method for charging a battery for an analyte
monitoring device
in accordance with certain embodiments of the present disclosure;
[0015] FIG. 6A illustrates a method for performing a sensor scan or system
check in
accordance with certain embodiments of the present disclosure;
[0016] FIG. 6B illustrates a method for activating a sensor in accordance with
certain
embodiments of the present disclosure;
[0017] FIG. 6C illustrates a method for activating a sensor in accordance with
certain
embodiments of the present disclosure;
[0018] FIG. 6D illustrates a method for scanning a sensor if the sensor is
nearing expiration
in accordance with certain embodiments of the present disclosure;
[0019] FIG. 6E illustrates exemplary graphical user interface displaying
sensor life in
varying increments in accordance with certain embodiments of the present
disclosure;
[0020] FIG. 7 illustrates a method for activating a sensor after the device is
powered on in
accordance with certain embodiments of the present disclosure;
-2-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0021] FIG. 8 illustrates a method for scanning a sensor with an analyte
monitoring device
in accordance with certain embodiments of the present disclosure;
[0022] FIG. 9A illustrates a method for performing two scans within a
predetermined
period of time with the analyte reader in accordance with certain embodiments
of the
present disclosure;
[0023] FIG. 9B illustrates a method of logging a dose calculation in
accordance with certain
embodiments of the present disclosure;
[0024] FIG. 9C illustrates a method for enabling two consecutive scans in
accordance with
certain embodiments of the present disclosure;
[0025] FIGS. 10A and 10L illustrate example Sensor Results screens in
accordance with
certain embodiments of the present disclosure;
[0026] FIG. 10B and 10M illustrate example Results Non-Actionable screens in
accordance
with certain embodiments of the present disclosure;
[0027] FIG. 10C illustrates an example Results - Masked screen in accordance
with certain
embodiments of the present disclosure;
[0028] FIG. 10D illustrates an interface for indicating that the sensor
temperature is too
high in accordance with certain embodiments of the present disclosure;
[0029] FIG. 10E illustrates an interface for indicating that the sensor
temperature is too low
in accordance with certain embodiments of the present disclosure;
[0030] FIG. 1OF illustrates an interface for indicating that the sensor
reading is out of range
in accordance with certain embodiments of the present disclosure;
[0031] FIG. 10G illustrates an interface for indicating that the sensor
reading is out of range
in accordance with certain embodiments of the present disclosure;
[0032] FIG. 10H illustrates an interface for indicating that the sensor
reading is high in
accordance with certain embodiments of the present disclosure;
[0033] FIG. 101 illustrates an interface for indicating that the sensor
reading is low in
accordance with certain embodiments of the present disclosure;
[0034] FIG. 10J illustrates an interface for indicating that a high analyte
level is projected in
accordance with certain embodiments of the present disclosure;
[0035] FIG. 10K illustrates an interface for indicating that a low analyte
level is projected
in accordance with certain embodiments of the present disclosure;
[0036] FIG. 11 illustrates an example method for performing a blood glucose
test with a
test strip in accordance with certain embodiments of the present disclosure;
-3-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0037] FIG. 12A illustrates an example Blood Glucose Results screen in
accordance with
certain embodiments of the present disclosure;
[0038] FIG. 12B illustrates an example interface for indicating the results of
a blood
glucose control solution test in accordance with certain embodiments of the
present
disclosure;
[0039] FIG. 12C illustrates example interfaces for indicating that the analyte
monitoring
device's temperature is too high or too low in accordance with certain
embodiments of the
present disclosure;
[0040] FIG. 12D illustrates an interface for indicating that the measurement
reading is out
of range in accordance with certain embodiments of the present disclosure;
[0041] FIG. 12E illustrates an interface for indicating that the measurement
reading is out
of range in accordance with certain embodiments of the present disclosure;
[0042] FIG. 12F illustrates an interface for indicating that the measurement
reading is high
in accordance with certain embodiments of the present disclosure;
[0043] FIG. 12G illustrates an interface for indicating that the measurement
reading is low
in accordance with certain embodiments of the present disclosure;
[0044] FIGS. 12H and 121 illustrate display screens for masked results of a
blood glucose
control solution test and blood glucose test strip results, respectively in
accordance with
certain embodiments of the present disclosure;
[0045] FIG. 13 illustrates an example method for performing a ketone test with
a ketone
test strip in accordance with certain embodiments of the present disclosure;
[0046] FIG. 14A illustrates an example Ketone Results screen in accordance
with certain
embodiments of the present disclosure;
[0047] FIG. 14B illustrates an example interface for indicating the results of
a ketone
control solution test in accordance with certain embodiments of the present
disclosure;
[0048] FIG. 14C illustrates example interfaces for indicating that the analyte
monitoring
device's temperature is too high or too low in accordance with certain
embodiments of the
present disclosure;
[0049] FIG. 14D illustrates an interface for indicating that the measurement
reading is out
of range in accordance with certain embodiments of the present disclosure;
[0050] FIG. 14E illustrates an interface for indicating that the ketone
measurement reading
is high in accordance with certain embodiments of the present disclosure;
-4-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0051] FIGS. 14F and 14G illustrate display screens for masked results of a
Ketone control
solution test and Ketone test strip results, respectively in accordance with
certain
embodiments of the present disclosure;
[0052] FIGS. 15A-15E illustrate a Notes Interface for entering notes on a
Reader in
accordance with certain embodiments of the present disclosure;
[0053] FIGS. 16A-16C illustrate an Insulin on Board Interface for entering
insulin on board
information on a Reader in accordance with certain embodiments of the present
disclosure;
[0054] FIGS. 17A-17E illustrate an Insulin Calculator Interface for using an
insulin
calculator on a Reader in accordance with certain embodiments of the present
disclosure;
[0055] FIGS. 18A and 18B illustrate a Dose Detail Interface for displaying
insulin dose
details on a Reader in accordance with certain embodiments of the present
disclosure;
[0056] FIGS. 19A-19D illustrate a Reminders Interface for setting reminders on
a Reader in
accordance with certain embodiments of the present disclosure;
[0057] FIGS. 20A and 20B illustrate a Receive Reminder Interface for receiving
reminders
on a Reader in accordance with certain embodiments of the present disclosure;
[0058] FIG. 21A illustrates an exemplary interface for providing a summaries
menu on the
analyte monitoring device in accordance with certain embodiments of the
present
disclosure;
[0059] FIG. 21B illustrates a Daily Graph screen for showing a daily graph of
sensor
readings obtained over a single day or 24 hour time period in accordance with
certain
embodiments of the present disclosure;
[0060] FIG. 21C illustrates an exemplary Average Glucose Summary interface for
providing a graph of sensor readings obtained over a time period that is
summarized with
respect to a predetermined time period in accordance with certain embodiments
of the
present disclosure;
[0061] FIG. 21D illustrates an exemplary screen for showing the percentage of
time the
sensor readings were within a target zone in accordance with certain
embodiments of the
present disclosure;
[0062] FIG. 21E illustrates an exemplary screen for showing a graph of the
number of
events associated with sensor readings obtained over a time period, wherein
the events are
summarized with respect to a predetermined time period in accordance with
certain
embodiments of the present disclosure;
-5-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0063] FIG. 21F illustrates an exemplary screen for indicating information
associated with
the use of the sensor over a time period in accordance with certain
embodiments of the
present disclosure;
[0064] FIG. 21G illustrates an example interface for providing a summaries
menu on the
analyte monitoring device, when the device is in masked mode in accordance
with certain
embodiments of the present disclosure;
[0065] FIGS. 22A-22F illustrate a Logbook Interface for displaying, adding
and/or editing
logbook entries on a Reader in accordance with certain embodiments of the
present
disclosure;
[0066] FIGS 23A-23C illustrate a Strip/Hardware Errors Interface for
displaying error
messages on a Reader and steps for recovering date and time following time
loss in
accordance with certain embodiments of the present disclosure;
[0067] FIG. 24 illustrates methods for first starting an analyte monitoring
device in
accordance with certain embodiments of the present disclosure;
[0068] FIG. 25 illustrates a method of entering settings on an analyte
monitoring device in
accordance with certain embodiments of the present disclosure;
[0069] FIG. 26 illustrates methods of entering settings on an analyte
monitoring device in
accordance with certain embodiments of the present disclosure;
[0070] FIG. 27 illustrates methods of checking the system status of an analyte
monitoring
device in accordance with certain embodiments of the present disclosure;
[0071] FIG. 28 illustrates a method of setting a masked mode for an analyte
monitoring
device in accordance with certain embodiments of the present disclosure;
[0072] FIG. 29 illustrates a method of activating a calculator feature on an
analyte
monitoring device in accordance with certain embodiments of the present
disclosure;
[0073] FIG. 30 illustrates a method of setting up a calculator on an analyte
monitoring
device in accordance with certain embodiments of the present disclosure;
[0074] FIG. 31 illustrates a method of setting up a calculator on an analyte
monitoring
device in accordance with certain embodiments of the present disclosure;
[0075] FIG. 32 illustrates a method for a correction setup in accordance with
certain
embodiments of the present disclosure;
[0076] FIG. 33 illustrates a method for an insulin on board setup and a method
for saving
settings in accordance with certain embodiments of the present disclosure;
-6-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0077] FIG. 34 illustrates example animation interfaces in accordance with
certain
embodiments of the present disclosure;
[0078] FIG. 35 illustrates example animation interfaces in accordance with
certain
embodiments of the present disclosure;
[0079] FIG. 36 illustrates a method when starting up Remote Device (RD)
software on a
remote data processing device in accordance with certain embodiments of the
present
disclosure;
[0080] FIG. 37 illustrates a flowchart for the data management software
startup process in
accordance with certain embodiments of the present disclosure;
[0081] FIG. 38 illustrates an example Welcome screen in accordance with
certain
embodiments of the present disclosure;
[0082] FIG. 39 illustrates a flowchart for a method of navigating through the
Reader mode
for accessing setting and functions that are used to setup and control the
Reader device in
accordance with certain embodiments of the present disclosure;
[0083] FIG. 40 illustrates an example Reader Landing screen in accordance with
certain
embodiments of the present disclosure;
[0084] FIG. 41 illustrates a screen indicating that the Reader is out of sync
in accordance
with certain embodiments of the present disclosure;
[0085] FIG. 42 illustrates a Profile screen in accordance with certain
embodiments of the
present disclosure;
[0086] FIG. 43 illustrates an Insulin Calculator Setup Interface for health
management
software configured for an easy insulin calculator in accordance with certain
embodiments
of the present disclosure;
[0087] FIG. 44 illustrates an Insulin Calculator Setup Interface for health
management
software configured for an advanced insulin calculator set to count carbs by
grams of
carbs in accordance with certain embodiments of the present disclosure;
[0088] FIGS. 45A and 45B illustrate an Insulin Calculator Setup Interface for
health
management software configured for an advanced insulin calculator set to count
carbs by
grams of carbs and by time of day in accordance with certain embodiments of
the present
disclosure;
[0089] FIGS. 46A and 46B illustrate an Insulin Calculator Setup Interface for
health
management software configured for an advanced insulin calculator set to count
carbs by
servings of carbs and by time of day, according to embodiments of the present
disclosure;
-7-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0090] FIG. 47 illustrates a Masked Mode setup screen in accordance with
certain
embodiments of the present disclosure;
[0091] FIG. 48 illustrates a method for syncing a Reader device with a remote
device in
accordance with certain embodiments of the present disclosure;
[0092] FIG. 49 illustrates a method for a Guided Reader Setup interface in
accordance with
certain embodiments of the present disclosure;
[0093] FIG. 50 illustrates a single screen of a Guided Reader setup interface
in accordance
with certain embodiments of the present disclosure;
[0094] FIG. 51 illustrates a flowchart for navigating through the Reports mode
of the RD
software in accordance with certain embodiments of the present disclosure;
[0095] FIG. 52 illustrates an example Reports landing screen in accordance
with certain
embodiments of the present disclosure;
[0096] FIG. 53 illustrates an example Generate Reports screen in accordance
with certain
embodiments of the present disclosure;
[0097] FIG. 54 illustrates an example Logbook Report screen when viewed from
the
Reports Mode in accordance with certain embodiments of the present disclosure;
[0098] FIG. 55 illustrates a flowchart for a Guided Reports Setup interface in
accordance
with certain embodiments of the present disclosure;
[0099] FIG. 56 illustrates a method for exporting Reader data in accordance
with certain
embodiments of the present disclosure;
[0100] FIG. 57 illustrates an exemplary Snapshot report for a specific time
frame in
accordance with certain embodiments of the present disclosure;
[0101] FIG. 58 illustrates a Calendar report in accordance with certain
embodiments of the
present disclosure;
[0102] FIG. 59 illustrates a Daily Patterns report in accordance with certain
embodiments
of the present disclosure;
[0103] FIG. 60 illustrates a Meal Patterns report in accordance with certain
embodiments of
the present disclosure;
[0104] FIG. 61 illustrates the first page of a Daily Statistics report for a
given time period in
accordance with certain embodiments of the present disclosure;
[0105] FIG. 62 illustrates an exemplary Logbook report in accordance with
certain
embodiments of the present disclosure;
-8-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0106] FIG. 63 illustrates an exemplary Logbook report in accordance with
certain
embodiments of the present disclosure;
[0107] FIG. 64 illustrates an example Reader Settings Report in accordance
with certain
embodiments of the present disclosure t;
[0108] FIG. 65 illustrates a Reader Settings Report in accordance with certain
embodiments
of the present disclosure;
[0109] FIG. 66 illustrates a home screen for the data management software when
the
Reader device is not connected to the PC on which the data management software
resides
in accordance with certain embodiments of the present disclosure;
[0110] FIGS. 67A and 67B illustrate a home screen for the data management
software when
an unconfigured Reader device is connected to the PC on which the data
management
software resides in accordance with certain embodiments of the present
disclosure;
[0111] FIG. 68 illustrates a home screen for the data management software when
the
Reader device is connected to the PC on which the data management software
resides in
accordance with certain embodiments of the present disclosure;
[0112] FIGS. 69A and 68B illustrate a home screen for the data management
software when
the time and date of connected Reader device isn't synchronized with the time
of the
computer on which the software is loaded in accordance with certain
embodiments of the
present disclosure;
[0113] FIGS. 70A-70D illustrate a home screen for the data management software
when an
update to the software is available, in accordance with certain embodiments of
the present
disclosure;
[0114] FIG. 71 illustrates a generate reports screen for the data management
software in
accordance with certain embodiments of the present disclosure;
[0115] FIG. 72 illustrates an exemplary snapshot report in accordance with
certain
embodiments of the present disclosure;
[0116] FIGS. 73A and 73B illustrate exemplary daily patterns reports in
accordance with
certain embodiments of the present disclosure;
[0117] FIGS. 74A and 74B illustrate exemplary advanced daily pattern reports
in
accordance with certain embodiments of the present disclosure;
[0118] FIG. 75 illustrates an exemplary mealtime pattern report in accordance
with certain
embodiments of the present disclosure;
-9-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0119] FIG. 76 illustrates an exemplary monthly summery report in accordance
with certain
embodiments of the present disclosure;
[0120] FIG. 77 illustrates an exemplary weekly summary report in accordance
with certain
embodiments of the present disclosure;
[0121] FIG. 78 illustrates an exemplary daily log report in accordance with
certain
embodiments of the present disclosure;
[0122] FIGS. 79A and 79B illustrate an exemplary reader details report in
accordance with
certain embodiments of the present disclosure ;
[0123] FIG. 80 illustrates an exemplary frame of the generate reports menu for
setting
report parameters in accordance with certain embodiments of the present
disclosure;
[0124] FIGS. 81A and 81B illustrate an advanced daily pattern report settings
screen in
accordance with certain embodiments of the present disclosure sure;
[0125] FIGS. 82-86 illustrate exemplary screens for adjusting settings of the
Reader device
in accordance with certain embodiments of the present disclosure;
[0126] FIGS. 87A-87E illustrate screens associated with professional options
of the Reader
device in accordance with certain embodiments of the present disclosure;
[0127] FIG. 88 illustrates options available in main systems menus of the data
management
software in accordance with certain embodiments of the present disclosure; and
[0128] FIG. 89 illustrates an auto-save option screen in accordance with
certain
embodiments of the present disclosure.
DETAILED DESCRIPTION
[0129] Before the embodiments of the present disclosure are described, it is
to be
understood that the present disclosure is not limited to particular aspects
described, as such
may, of course, vary. It is also to be understood that the terminology used
herein is for the
purpose of describing particular aspects only, and is not intended to be
limiting, since the
scope of the present disclosure will be limited only by the appended claims.
[0130] Where a range of values is provided, it is understood that each
intervening value, to
the tenth of the unit of the lower limit unless the context clearly dictates
otherwise,
between the upper and lower limits of that range is also specifically
disclosed. Each
smaller range between any stated value or intervening value in a stated range
and any
other stated or intervening value in that stated range is encompassed within
the present
disclosure. The upper and lower limits of these smaller ranges may
independently be
-10-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
included or excluded in the range, and each range where either, neither or
both limits are
included in the smaller ranges is also encompassed within the present
disclosure, subject
to any specifically excluded limit in the stated range. Where the stated range
includes one
or both of the limits, ranges excluding either or both of those included
limits are also
included in the present disclosure.
[0131] Unless defined otherwise, all technical and scientific terms used
herein have the
same meaning as commonly understood by one of ordinary skill in the art to
which this
present disclosure belongs. Although any methods and materials similar or
equivalent to
those described herein can be used in the practice or testing of the present
disclosure, some
potential and preferred methods and materials are now described. All
publications
mentioned herein are incorporated herein by reference to disclose and describe
the
methods and/or materials in connection with which the publications are cited.
It is
understood that the present disclosure supersedes any disclosure of an
incorporated
publication to the extent there is a contradiction.
[0132] It must be noted that as used herein and in the appended claims, the
singular forms
"a", "an", and "the" include plural referents unless the context clearly
dictates otherwise.
Thus, for example, reference to "a program update" includes a plurality of
such program
updates and reference to "the program update" includes reference to one or
more program
updates and equivalents thereof known to those skilled in the art, and so
forth.
[0133] Generally, embodiments of the present disclosure relate to in vivo
methods and
devices for detecting at least one analyte such as glucose in body fluid.
Accordingly,
embodiments include in vivo analyte sensors configured so that at least a
portion of the
sensor is positioned in the body of a user (e.g., within the ISF), to obtain
information about
at least one analyte of the body, e.g., transcutaneously positioned in user's
body. In certain
embodiments, an in vivo analyte sensor is coupled to an electronics unit that
is maintained
on the body of the user such as on a skin surface, where such coupling
provides on body,
in vivo analyte sensor electronics assemblies.
[0134] In certain embodiments, analyte information is communicated from a
first device
such as an on body electronics unit to a second analyte monitoring device
which may
include user interface features, including a display, and/or the like.
[0135] In many embodiments of the system, analyte information derived by the
sensor/on
body electronics (for example, on body electronics assembly) is made available
in a user-
usable or viewable form only when queried by the user such that the timing of
data
-11-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
communication is selected by the user. The on-body electronics may take
periodic
measurement and record such data until an on-demand reading is taken by the
user (e.g.,
the display device brought in close vicinity of the on-body electronics and
sensor). Upon
communication, the on-body electronics may communicate the recorded data for a
set time
period. For example, the on-body electronics may have 8 hours of memory in
which it
stores periodic measurements taken every 15 minutes. When an on-demand reading
is
taken, the entire 8 hours is transferred to the device. It should be
appreciated that if the
user does not take an on-demand reading for longer than 8 hours, some of the
data may be
lost.
[0136] Accordingly, in certain embodiments once a sensor electronics assembly
is placed
on the body so that at least a portion of the in vivo sensor is in contact
with bodily fluid
such as ISF and the sensor is electrically coupled to the electronics unit,
sensor derived
analyte information may be communicated from the on body electronics to a
display
device on-demand by powering on the display device, and executing a software
algorithm
stored in and accessed from a memory of the display device, to generate one or
more
request commands, control signal or data packet to send to the on body
electronics. The
software algorithm executed under, for example, the control of the
microprocessor or
application specific integrated circuit (ASIC) of the display device may
include routines to
detect the position of the on body electronics relative to the display device
to initiate the
transmission of the generated request command, control signal and/or data
packet.
[0137] Display devices may also include programming stored in memory for
execution by
one or more microprocessors and/or ASICs to generate and transmit the one or
more
request command, control signal or data packet to send to the on body
electronics in
response to a user activation of an input mechanism on the display device such
as
depressing a button on the display device, triggering a soft button associated
with the data
communication function, and so on. The input mechanism may be alternatively or
additionally provided on or in the on body electronics which may be configured
for user
activation. In certain embodiments, voice commands or audible signals may be
used to
prompt or instruct the microprocessor or ASIC to execute the software
routine(s) stored in
the memory to generate and transmit the one or more request command, control
signal or
data packet to the on body device. In the embodiments that are voice activated
or
responsive to voice commands or audible signals, on body electronics and/or
display
device includes a microphone, a speaker, and processing routines stored in the
respective
-12-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
memories of the on body electronics and/or the display device to process the
voice
commands and/or audible signals. In certain embodiments, positioning the on
body device
and the display device within a predetermined distance (e.g., close proximity)
relative to
each other initiates one or more software routines stored in the memory of the
display
device to generate and transmit a request command, control signal or data
packet.
[0138] Different types and/or forms and/or amounts of information may be sent
for each on
demand reading, including but not limited to one or more of current analyte
level
information (i.e., real time or the most recently obtained analyte level
information
temporally corresponding to the time the reading is initiated), rate of change
of an analyte
over a predetermined time period, rate of the rate of change of an analyte
(acceleration in
the rate of change), historical analyte information corresponding to analyte
information
obtained prior to a given reading and stored in memory of the assembly. Some
or all of
real time, historical, rate of change, rate of rate of change (such as
acceleration or
deceleration) information may be sent to a display device for a given reading.
In certain
embodiments, the type and/or form and/or amount of information sent to a
display device
may be preprogrammed and/or unchangeable (e.g., preset at manufacturing), or
may not
be preprogrammed and/or unchangeable so that it may be selectable and/or
changeable in
the field one or more times (e.g., by activating a switch of the system, etc).
Accordingly,
in certain embodiments, for each on demand reading, a display device will
output a current
(real time) sensor-derived analyte value (e.g., in numerical format), a
current rate of
analyte change (e.g., in the form of an analyte rate indicator such as a arrow
pointing in a
direction to indicate the current rate), and analyte trend history data based
on sensor
readings acquired by and stored in memory of on body electronics (e.g., in the
form of a
graphical trace). Additionally, the on skin or sensor temperature reading or
measurement
associated with each on demand reading may be communicated from the on body
electronics to the display device. The temperature reading or measurement,
however, may
not be output or displayed on the display device, but rather, used in
conjunction with a
software routine executed by the display device to correct or compensate the
analyte
measurement output to the user on the display device.
[0139] As described, embodiments include in vivo analyte sensors and on body
electronics
that together provide body wearable sensor electronics assemblies (also
referred to herein
as a "patch"). In certain embodiments, in vivo analyte sensors are fully
integrated with on
body electronics (fixedly connected during manufacture), while in other
embodiments they
-13-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
are separate but connectable post manufacture (e.g., before, during or after
sensor insertion
into a body). On body electronics may include an in vivo glucose sensor,
electronics,
battery, and antenna encased (except for the sensor portion that is for in
vivo positioning)
in a waterproof housing that includes or is attachable to an adhesive pad.
[0140] Embodiments include sensor insertion devices, which also may be
referred to herein
as sensor delivery units, or the like. Insertion devices may retain on body
electronics
assemblies completely in an interior compartment, i.e., an insertion device
may be "pre-
loaded" with on body electronics assemblies during the manufacturing process
(e.g., on
body electronics may be packaged in a sterile interior compartment of an
insertion device).
In such embodiments, insertion devices may form sensor assembly packages
(including
sterile packages) for pre-use or new on body electronics assemblies, and
insertion devices
configured to apply on body electronics assemblies to recipient bodies.
[0141] Embodiments include portable handheld display devices, as separate
devices and
spaced apart from an on body electronics assembly, that collects information
from the
assemblies and provide sensor derived analyte readings to users. Such devices
may also be
referred to as meters, readers, monitors, receivers, human interface devices,
companions,
or the like. Certain embodiments may include an integrated in vitro analyte
meter. In
certain embodiments, display devices include one or more wired or wireless
communications ports such as USB, serial, parallel, or the like, configured to
establish
communication between a display device and another unit (e.g., on body
electronics,
power unit to recharge a battery, a PC, etc).
[0142] Compatible informatics software in certain embodiments include, for
example, but
not limited to stand alone or network connection enabled data management
software
program, resident or running on a display device, personal computer, a server
terminal, for
example, to perform data analysis, charting, data storage, data archiving and
data
communication as well as data synchronization. Informatics software in certain
embodiments may also include software for executing field upgradable functions
to
upgrade firmware of a display device and/or on body electronics unit to
upgrade the
resident software on the display device and/or the on body electronics unit,
e.g., with
versions of firmware that include additional features and/or include software
bugs or
errors fixed, etc.
-14-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0143] Embodiments may include a haptic feedback feature such as a vibration
motor or the
like, configured so that corresponding notifications (e.g., a successful on-
demand reading
received at a display device), may be delivered in the form of haptic
feedback.
[0144] Embodiments include programming embedded on a computer readable medium,
i.e.,
computer-based application software (may also be referred to herein as
informatics
software or programming or the like) that processes analyte information
obtained from the
system and/or user self-reported data. Application software may be installed
on a host
computer such as a mobile telephone, PC, an Internet-enabled human interface
device
such as an Internet-enabled phone, personal digital assistant, or the like, by
a display
device or an on body electronics unit. Informatics programming may transform
data
acquired and stored on a display device or on body unit for use by a user.
[0145] Embodiments of the subject disclosure are described primarily with
respect to
glucose monitoring devices and systems, and methods of glucose monitoring, for
convenience only and such description is in no way intended to limit the scope
of the
disclosure. It is to be understood that the analyte monitoring system may be
configured to
monitor a variety of analytes at the same time or at different times.
[0146] For example, analytes that may be monitored include, but are not
limited to, acetyl
choline, amylase, bilirubin, cholesterol, chorionic gonadotropin, creatine
kinase (e.g., CK-
MB), creatine, DNA, fructosamine, glucose, glutamine, growth hormones,
hormones,
ketones, lactate, oxygen, peroxide, prostate-specific antigen, prothrombin,
RNA, thyroid
stimulating hormone, and troponin. The concentration of drugs, such as, for
example,
antibiotics (e.g., gentamicin, vancomycin, and the like), digitoxin, digoxin,
drugs of abuse,
theophylline, and warfarin, may also be monitored. In those embodiments that
monitor
more than one analyte, the analytes may be monitored at the same or different
times, with
a single sensor or with a plurality of sensors which may use the same on body
electronics
(e.g., simultaneously) or with different on body electronics.
[0147] As described in detail below, embodiments include devices, systems,
kits and/or
methods to monitor one or more physiological parameters such as, for example,
but not
limited to, analyte levels, temperature levels, heart rate, user activity
level, over a
predetermined monitoring time period. Also provided are methods of
manufacturing.
Predetermined monitoring time periods may be less than about 1 hour, or may
include
about 1 hour or more, e.g., about a few hours or more, e.g., about a few days
of more, e.g.,
about 3 or more days, e.g., about 5 days or more, e.g., about 7 days or more,
e.g., about 10
-15-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
days or more, e.g., about 14 days or more, e.g., about several weeks, e.g.,
about 1 month or
more. In certain embodiments, after the expiration of the predetermined
monitoring time
period, one or more features of the system may be automatically deactivated or
disabled at
the on body electronics assembly and/or display device.
[0148] For example, a predetermined monitoring time period may begin with
positioning
the sensor in vivo and in contact with a body fluid such as ISF, and/or with
the initiation
(or powering on to full operational mode) of the on body electronics.
Initialization of on
body electronics may be implemented with a command generated and transmitted
by a
display device in response to the activation of a switch and/or by placing the
display
device within a predetermined distance (e.g., close proximity) to the on body
electronics,
or by user manual activation of a switch on the on body electronics unit,
e.g., depressing a
button, or such activation may be caused by the insertion device, e.g., as
described in U.S.
Patent Application No. 12/698,129 filed on February 1,2010 and U.S.
Provisional
Application Nos. 61/238,646, 61/246,825, 61/247,516, 61/249,535, 61/317,243,
61/345,562, and 61/361,374, the disclosures of each of which are incorporated
herein by
reference for all purposes.
[0149] When initialized in response to a received command from a display
device, the on
body electronics retrieves and executes from its memory software routine to
fully power
on the components of the on body electronics, effectively placing the on body
electronics
in full operational mode in response to receiving the activation command from
the display
device. For example, prior to the receipt of the command from the display
device, a
portion of the components in the on body electronics may be powered by its
internal
power supply such as a battery while another portion of the components in the
on body
electronics may be in powered down or low power including no power, inactive
mode, or
all components may be in an inactive mode, powered down mode. Upon receipt of
the
command, the remaining portion (or all) of the components of the on body
electronics is
switched to active, fully operational mode.
[0150] Embodiments include transcutaneous sensors and also wholly implantable
sensors
and wholly implantable assemblies in which a single assembly including the
analyte
sensor and electronics are provided in a sealed housing (e.g., hermetically
sealed
biocompatible housing) for implantation in a user's body for monitoring one or
more
physiological parameters.
-16-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0151] Exemplary analyte monitoring systems that relate to the present
disclosure and that
may be utilized in connection with the disclosed analyte measurement system
include
those described in U.S. Patent No. 7,041,468; U.S. Pat. No. 5,356,786; U.S.
Pat. No.
6,175,752; U.S. Pat. No. 6,560,471; U.S. Pat. No. 5,262,035; U.S. Pat. No.
6,881,551;
U.S. Pat. No. 6,121,009; U.S. Pat. No. 7,167,818; U.S. Pat. No. 6,270,455;
U.S. Pat. No.
6,161,095; U.S. Pat. No. 5,918,603; U.S. Pat. No. 6,144,837; U.S. Pat. No.
5,601,435;
U.S. Pat. No. 5,822,715; U.S. Pat. No. 5,899,855; U.S. Pat. No. 6,071,391;
U.S. Pat. No.
6,120,676; U.S. Pat. No. 6,143,164; U.S. Pat. No. 6,299,757; U.S. Pat. No.
6,338,790;
U.S. Pat. No. 6,377,894; U.S. Pat. No. 6,600,997; U.S. Pat. No. 6,773,671;
U.S. Pat. No.
6,514,460; U.S. Pat. No. 6,592,745; U.S. Pat. No. 5,628,890; U.S. Pat. No.
5,820,551;
U.S. Pat. No. 6,736,957; U.S. Pat. No. 4,545,382; U.S. Pat. No. 4,711,245;
U.S. Pat. No.
5,509,410; U.S. Pat. No. 6,540,891; U.S. Pat. No. 6,730,200; U.S. Pat. No.
6,764,581;
U.S. Pat. No. 6,299,757; U.S. Pat. No. 6,461,496; U.S. Pat. No. 6,503,381;
U.S. Pat. No.
6,591,125; U.S. Pat. No. 6,616,819; U.S. Pat. No. 6,618,934; U.S. Pat. No.
6,676,816;
U.S. Pat. No. 6,749,740; U.S. Pat. No. 6,893,545; U.S. Pat. No. 6,942,518;
U.S. Pat. No.
6,514,718; U.S. Pat. No. 5,264,014; U.S. Pat. No. 5,262,305; U.S. Pat. No.
5,320,715;
U.S. Pat. No. 5,593,852; U.S. Pat. No. 6,746,582; U.S. Pat. No. 6,284,478;
U.S. Pat. No.
7,299,082; U.S. Patent Application No. 61/149,639, entitled "Compact On-Body
Physiological Monitoring Device and Methods Thereof', U.S. Patent Application
No.
11/461,725, filed August 1, 2006, entitled "Analyte Sensors and Methods"; U.S.
Patent
Application No. 12/495,709, filed June 30, 2009, entitled "Extruded Electrode
Structures
and Methods of Using Same"; U.S. Patent Application Publication No.
US2004/0186365;
U.S. Patent Application Publication No. 2007/0095661; U.S. Patent Application
Publication No. 2006/0091006; U.S. Patent Application Publication No.
2006/0025662;
U.S. Patent Application Publication No. 2008/0267823; U.S. Patent Application
Publication No. 2007/0108048; U.S. Patent Application Publication No.
2008/0102441;
U.S. Patent Application Publication No. 2008/0066305; U.S. Patent Application
Publication No. 2007/0199818; U.S. Patent Application Publication No.
2008/0148873;
U.S. Patent Application Publication No. 2007/0068807; US patent Application
Publication
No. 2010/0198034; and US provisional application no. 61/149,639 titled
"Compact On-
Body Physiological Monitoring Device and Methods Thereof', the disclosures of
each of
which are incorporated herein by reference in their entirety.
-17-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0152] Additional relevant subject matter is provided in the following
disclosures: U.S.
Provisional Application Nos. 61/498,142 filed June 17, 2011; U.S. Application
Nos.
13/071,461, 13/071,487, and 13/071,497, which were all filed on March 24,
2011, and
13/091,557 which was filed on April 21, 2011; US Patent Application
Publication No. US
2010/0081905, 2011/0021889, 2010/0230285, and 2011/0021889; and U.S. Patent
Nos.
6,736,957, 7,501,053 and 7,754,093; the disclosures of which are each
incorporated by
reference herein in their entirety and for all purposes.
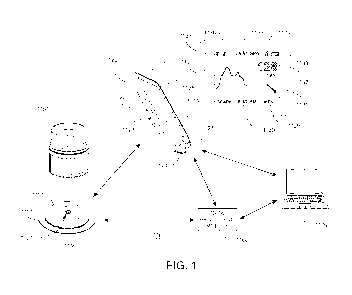
[0153] FIG. 1 illustrates an example embodiment of In-Vivo Analyte Monitoring
System
and is described below. FIG. 1 shows an exemplary in vivo-based analyte
monitoring
system 1100 in accordance with embodiments of the present disclosure. As
shown, in
certain embodiments, analyte monitoring system 1100 includes on body
electronics 1110
electrically coupled to in vivo analyte sensor 1101 (a proximal portion of
which is shown
in FIG. 1) and attached to adhesive layer 1140 for attachment on a skin
surface on the
body of a user. On body electronics 1110 includes on body housing 1119 that
defines an
interior compartment. Also shown in FIG. 1 is insertion device 1150 that, when
operated,
transcutaneously positions a portion of analyte sensor 1101 through a skin
surface and in
fluid contact with ISF , and positions on body electronics 1110 and adhesive
layer 1140 on
a skin surface In certain embodiments, on body electronics 1110, analyte
sensor 1101 and
adhesive layer 1140 are sealed within the housing of insertion device 150
before use, and
in certain embodiments, adhesive layer 1140 is also sealed within the housing
or itself
provides a terminal seal of the insertion device 1150. Devices, systems and
methods that
maybe used with embodiments herein are described, e.g., in U.S. Patent
Application No.
12/698,129 and U.S. Provisional Application Nos. 61/238,646, 61/246,825,
61/247,516,
61/249,535, 61/317,243, 61/345,562, and 61/361,374, the disclosures of each of
which are
incorporated herein by reference for all purposes.
[0154] Referring back to the FIG. 1, analyte monitoring system 100 includes
display device
1120 which includes a display 1122 to output information to the user, an input
component
1121 such as a button, actuator, a touch sensitive switch, a capacitive
switch, pressure
sensitive switch, jog wheel or the like, to input data or command to display
device 1120 or
otherwise control the operation of display device 1120. It is noted that some
embodiments
may include display-less devices or devices without any user interface
components. These
devices may be functionalized to store data as a data logger and/or provide a
conduit to
transfer data from on body electronics and/or a display-less device to another
device
-18-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
and/or location. Embodiments will be described herein as display devices for
exemplary
purposes which are in no way intended to limit the embodiments of the present
disclosure.
It will be apparent that display-less devices may also be used in certain
embodiments.
[0155] In certain embodiments, display 1122 and input component 1121 may be
integrated
into a single component, for example a display that can detect the presence
and location of
a physical contact touch upon the display such as a touch screen user
interface. In such
embodiments, the user may control the operation of display device 1120 by
utilizing a set
of pre-programmed motion commands, including, but not limited to, single or
double
tapping the display, dragging a finger or instrument across the display,
motioning multiple
fingers or instruments toward one another, motioning multiple fingers or
instruments away
from one another, etc. In certain embodiments, a display includes a touch
screen having
areas of pixels with single or dual function capacitive elements that serve as
LCD
elements and touch sensors.
[0156] Display device 1120 may be a dynamic color LCD display. In certain
embodiments,
display device 1120 may have preset and customizable options, including
display
resolution, quality and backlight options. Display device, in one embodiment,
may have a
dynamic color palette of up to 65,000 colors and include graphical displays
from 256 color
subsets of the 65,000 color dynamic display. In other embodiments, the LCD
display may
include a backlight, which may be an LED backlight. The LCD backlight may be
preprogrammed to dim or shut off after certain periods of time of non-activity
elapse. For
example, in certain embodiments, the default time before the display shuts off
may be 1
minute for most screens, including a dimming feature of the backlight after 15
seconds. In
some embodiments, the time until display shut off or display dim may vary
based on the
current screen or mode of the device, e.g., an apply blood to test strip
screen, as described
herein below in more detail, may have a longer time out than the default 1
minute, e.g., 2
minutes. In other embodiments, the display does not dim or turn off unless a
user manually
commands the device to turn off the display.
[0157] Display device 1120 also includes data communication port 1123 for
wired data
communication with external devices such as remote terminal (personal
computer) 1170,
for example. Example embodiments of the data communication port 1123 include
USB
port, mini USB port, RS-232 port, Ethernet port, Firewire port, or other
similar data
communication ports configured to connect to the compatible data cables.
Display device
1120 may also include an integrated in vitro glucose meter, including in vitro
test strip
-19-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
port 1124 to receive an in vitro glucose test strip for performing in vitro
blood glucose
measurements.
[0158] Referring still to FIG. 1, display 1122 in certain embodiments is
configured to
display a variety of information - some or all of which may be displayed at
the same or
different time on display 1122. Display 1122 may include but is not limited to
graphical
display 1138, for example, providing a graphical output of glucose values over
a
monitored time period (which may show important markers such as meals,
exercise, sleep,
heart rate, blood pressure, etc, numerical display 1132, for example,
providing monitored
glucose values (acquired or received in response to the request for the
information), and
trend or directional arrow display 1131 that indicates a rate of analyte
change and/or a rate
of the rate of analyte change, e.g., by moving locations on display 1122.
[0159] As further shown in FIG. 1, display 1122 may also include date display
1135
providing for example, date information for the user, time of day information
display 1139
providing time of day information to the user, battery level indicator display
1133 which
graphically shows the condition of the battery (rechargeable or disposable) of
the display
device 1120, sensor calibration status icon display 1134 for example, in
monitoring
systems that require periodic, routine or a predetermined number of user
calibration
events, notifying the user that the analyte sensor calibration is necessary,
audio/vibratory
settings icon display 1136 for displaying the status of the audio/vibratory
output or alarm
state, and wireless connectivity status icon display 1137 that provides
indication of
wireless communication connection with other devices such as on body
electronics, data
processing module1160, and/or remote terminal 1170. As additionally shown in
FIG. 1,
display 1122 may further include simulated touch screen buttons 1125,1126 for
accessing
menus, changing display graph output configurations or otherwise for
controlling the
operation of display device 1120.
[0160] Referring back to FIG. 1, in certain embodiments, display 1122 of
display device
1120 may be additionally, or instead of visual display, configured to output
alarms
notifications such as alarm and/or alert notifications, glucose values etc,
which may be
audible, tactile, or any combination thereof In one aspect, the display device
1120 may
include other output components such as a speaker, vibratory output component
and the
like to provide audible and/or vibratory output indication to the user in
addition to the
visual output indication provided on display 1122. Further details and other
display
embodiments can be found in, e.g., U.S. Patent Application No. 12/871,901,
U.S.
-20-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
provisional application nos. 61/238,672, 61/247,541, 61/297,625, the
disclosures of each
of which are incorporated herein by reference for all purposes.
[0161] After the positioning of on body electronics 1110 on the skin surface
and analyte
sensor 1101 in vivo to establish fluid contact with ISF (or other appropriate
body fluid), on
body electronics 1110 in certain embodiments is configured to wirelessly
communicate
analyte related data (such as, for example, data corresponding to monitored
analyte level
and/or monitored temperature data, and/or stored historical analyte related
data) when on
body electronics 1110 receives a command or request signal from display device
120. In
certain embodiments, on body electronics 1110 may be configured to at least
periodically
broadcast real time data associated with monitored analyte level which is
received by
display device 1120 when display device 1120 is within communication range of
the data
broadcast from on body electronics 1110, i.e., it does not need a command or
request from
a display device to send information.
[0162] For example, display device 1120 may be configured to transmit one or
more
commands to on body electronics 1110 to initiate data transfer, and in
response, on body
electronics 1110 may be configured to wirelessly transmit stored analyte
related data
collected during the monitoring time period to display device 1120. Display
device 1120
may in turn be connected to a remote terminal 1170 such as a personal computer
and
functions as a data conduit to transfer the stored analyte level information
from the on
body electronics 1110 to remote terminal 1170. In certain embodiments, the
received data
from the on body electronics 1110 may be stored (permanently or temporarily)
in one or
more memory of the display device 1120. In certain other embodiments, display
device
1120 is configured as a data conduit to pass the data received from on body
electronics
1110 to remote terminal 1170 that is connected to display device 1120.
[0163] Referring still to FIG. 1, also shown in analyte monitoring system 1100
are data
processing module 1160 and remote terminal 1170. Remote terminal 1170 may
include a
personal computer, a server terminal a laptop computer or other suitable data
processing
devices including software for data management and analysis and communication
with the
components in the analyte monitoring system 1100. For example, remote terminal
1170
may be connected to a local area network (LAN), a wide area network (WAN), or
other
data network for uni-directional or bi-directional data communication between
remote
terminal 1170 and display device 1120 and/or data processing module 1160.
-21-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0164] Remote terminal 1170 in certain embodiments may include one or more
computer
terminals located at a physician's office or a hospital. For example, remote
terminal 1170
may be located at a location other than the location of display device 1120.
Remote
terminal 1170 and display device 1120 could be in different rooms or different
buildings.
Remote terminal 1170 and display device 1120 could be at least about one mile
apart, e.g.,
at least about 110 miles apart, e.g., at least about 1100 miles apart. For
example, remote
terminal 1170 could be in the same city as display device 1120, remote
terminal 1170
could be in a different city than display device 1120, remote terminal 1170
could be in the
same state as display device 1120, remote terminal 1170 could be in a
different state than
display device 1120, remote terminal 1170 could be in the same country as
display device
1120, or remote terminal 1170 could be in a different country than display
device 1120,
for example. In certain embodiments, a separate, optional data
communication/processing
device such as data processing module 1160 may be provided in analyte
monitoring
system 1100. Data processing module 160 may include components to communicate
using
one or more wireless communication protocols such as, for example, but not
limited to,
infrared (IR) protocol, Bluetooth0 protocol, Zigbee0 protocol, and 802.11
wireless LAN
protocol. Additional description of communication protocols including those
based on
Bluetooth0 protocol and/or Zigbee0 protocol can be found in U.S. Patent
Publication No.
2006/0193375 incorporated herein by reference for all purposes. Data
processing module
1160 may further include communication ports, drivers or connectors to
establish wired
communication with one or more of display device1120, on body electronics
1110, or
remote terminal 1170 including, for example, but not limited to USB connector
and/or
USB port, Ethernet connector and/or port, FireWire connector and/or port, or
RS-232 port
and/or connector.
[0165] In certain embodiments, control logic or microprocessors of on body
electronics
1110 include software programs to determine future or anticipated analyte
levels based on
information obtained from analyte sensor 1101, e.g., the current analyte
level, the rate of
change of the analyte level, the acceleration of the analyte level change,
and/or analyte
trend information determined based on stored monitored analyte data providing
a
historical trend or direction of analyte level fluctuation as function time
during monitored
time period. Predictive alarm parameters may be programmed or programmable in
display
device 1120, or the on body electronics 1110, or both, and output to the user
in advance of
-22-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
anticipating the user's analyte level reaching the future level. This provides
the user an
opportunity to take timely corrective action.
[0166] Information, such as variation or fluctuation of the monitored analyte
level as a
function of time over the monitored time period providing analyte trend
information, for
example, may be determined by one or more control logic or microprocessors of
display
device 1120, data processing module 160, and/or remote terminal 1170, and/or
on body
electronics 1110. Such information may be displayed as, for example, a graph
(such as a
line graph) to indicate to the user the current and/or historical and/or and
predicted future
analyte levels as measured and predicted by the analyte monitoring system
1100. Such
information may also be displayed as directional arrows (for example, see
trend or
directional arrow display 1131) or other icon(s), e.g., the position of which
on the screen
relative to a reference point indicated whether the analyte level is
increasing or decreasing
as well as the acceleration or deceleration of the increase or decrease in
analyte level. This
information may be utilized by the user to determine any necessary corrective
actions to
ensure the analyte level remains within an acceptable and/or clinically safe
range. Other
visual indicators, including colors, flashing, fading, etc., as well as audio
indicators
including a change in pitch, volume, or tone of an audio output and/or
vibratory or other
tactile indicators may also be incorporated into the display of trend data as
means of
notifying the user of the current level and/or direction and/or rate of change
of the
monitored analyte level. For example, based on a determined rate of glucose
change,
programmed clinically significant glucose threshold levels (e.g.,
hyperglycemic and/or
hypoglycemic levels), and current analyte level derived by an in vivo analyte
sensor, the
system 1100 may include an algorithm stored on computer readable medium to
determine
the time it will take to reach a clinically significant level and will output
notification in
advance of reaching the clinically significant level, e.g., 30 minutes before
a clinically
significant level is anticipated, and/or 20 minutes, and/or 10 minutes, and/or
5 minutes,
and/or 3 minutes, and/or 1 minute, and so on, with outputs increasing in
intensity or the
like.
[0167] Referring again back to FIG. 1, in certain embodiments, software
algorithm(s) for
execution by data processing module 1160 may be stored in an external memory
device
such as an SD card, microSD card, compact flash card, XD card, Memory Stick
card,
Memory Stick Duo card, or USB memory stick/device including executable
programs
stored in such devices for execution upon connection to the respective one or
more of the
-23-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
on body electronics 1110, remote terminal 1170 or display device 1120. In a
further
aspect, software algorithms for execution by data processing module 160 may be
provided
to a communication device such as a mobile telephone including, for example,
WiFi or
Internet enabled smart phones or personal digital assistants (PDAs) as a
downloadable
application for execution by the downloading communication device.
[0168] Examples of smart phones include Windows , AndroidTM, iPhone0 operating
system, Palm WebOSTM, Blackberry operating system, or Symbian0 operating
system based mobile telephones with data network connectivity functionality
for data
communication over an internet connection and/or a local area network (LAN).
PDAs as
described above include, for example, portable electronic devices including
one or more
microprocessors and data communication capability with a user interface (e.g.,
display/output unit and/or input unit, and configured for performing data
processing, data
upload/download over the internet, for example. In such embodiments, remote
terminal
170 may be configured to provide the executable application software to the
one or more
of the communication devices described above when communication between the
remote
terminal 1170 and the devices are established.
[0169] In still further embodiments, executable software applications may be
provided
over-the-air (OTA) as an OTA download such that wired connection to remote
terminal
1170 is not necessary. For example, executable applications may be
automatically
downloaded as software download to the communication device, and depending
upon the
configuration of the communication device, installed on the device for use
automatically,
or based on user confirmation or acknowledgement on the communication device
to
execute the installation of the application. The OTA download and installation
of software
may include software applications and/or routines that are updates or upgrades
to the
existing functions or features of data processing module 1160 and/or display
device 1120.
[0170] Referring back to remote terminal 1170 of FIG. 1, in certain
embodiments, new
software and/or software updates such as software patches or fixes, firmware
updates or
software driver upgrades, among others, for display device 1120 and/or on body
electronics 1110 and/or data processing module 1160 may be provided by remote
terminal
1170 when communication between the remote terminal 1170 and display device
1120
and/or data processing module 1160 is established. For example, software
upgrades,
executable programming changes or modification for on body electronics 1110
may be
received from remote terminal 1170 by one or more of display device 1120 or
data
-24-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
processing module 1160, and thereafter, provided to on body electronics 1110
to update its
software or programmable functions. For example, in certain embodiments,
software
received and installed in on body electronics 1110 may include software bug
fixes,
modification to the previously stalled software parameters (modification to
analyte related
data storage time interval, resetting or adjusting time base or information of
on body
electronics 1110, modification to the transmitted data type, data transmission
sequence, or
data storage time period, among others). Additional details describing field
upgradability
of software of portable electronic devices, and data processing are provided
in U.S.
Application Nos. 12/698,124, 12/794,721, 12/699,653, and 12/699,844, and U.S.
Provisional Application Nos. 61,359,265, and 61/325,155 the disclosure of
which is
incorporated by reference herein for all purposes.
[0171] In some aspects, the display device (also referred to herein as
"analyte monitoring
device" or simply "device") is configured to receive a signal from a remote
sensor using
radio-frequency identification (RFID) technology.
[0172] This configuration may be used to provide glucose on demand
capabilities, for
example, in which case when a measurement reading is desired, the analyte
monitoring
device is brought within close vicinity of the implantable sensor. It should
be appreciated
that in other embodiments the wireless communication unit may communicate with
the
sensor using a different wireless communication technology than RFID. When
within
range, the device may be configured to verify that the sensor is the
appropriate sensor that
it has been configured to operate with. If not, the device ignores the sensor
and does not
initiate operation with the sensor. If so, the device initiates operation with
the sensor.
[0173] The analyte monitoring device may perform a variety of functions,
including for
example: modifying the signals from the sensor using calibration data and/or
measurements from a temperature probe (not shown); determining a level of an
analyte in
the interstitial fluid; determining a level of an analyte in the bloodstream
based on the
sensor measurements in the interstitial fluid; determining if the level, rate
of change,
and/or acceleration in the rate of change of the analyte exceeds or meets one
or more
threshold values; activating an alarm system if a threshold value is met or
exceeded;
evaluating trends in the level of an analyte based on a series of sensor
signals; therapy
management (e.g., determine a dose of a medication, etc.); and reduce noise or
error
contributions (e.g., through signal averaging or comparing readings from
multiple
electrodes); etc. The analyte monitoring device may be simple and perform only
one or a
-25-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
small number of these functions or the analyte monitoring device may perform
all or most
of these functions.
Software for the Remote Device
[0174] In some aspects, the analyte monitoring device may be communicatively
coupled to
a remote data processing device for management purposes. Remote device may
include,
for example, a personal computer, laptop, PDA, cellular phone, smartphone, set-
top box,
etc. The remote device may include, for example, a control unit including any
variety of
processor, microprocessor, microcontroller, etc. The remote device may also
include a
memory unit comprising non-volatile memory and volatile memory.
[0175] The term "remote device" is used herein to represent any device that is
external to
the analyte monitoring device. The remote device may require software to fully
communicate with the analyte monitoring device, manage data from the analyte
monitoring device, modify settings on the analyte monitoring device, or
otherwise operate
with analyte monitoring device. This auto-assisting user interface software is
referred to
herein as "remote device software" or "RD software" or "data management
software" to
distinguish it from the user interface software running on the analyte
monitoring device.
The RD software may be obtained from one or more methods such as downloading
from
the web, CD-ROM, memory stick, etc. The RD software is generally discussed
here and
additional details regarding various flows and screens are provided later.
[0176] In some embodiments, the analyte monitoring device includes the RD
software
programs and/or applications to be run on the remote device. In some
instances, the RD
software may be configured to automatically launch when the analyte monitoring
device is
coupled to the computer. For example, the analyte monitoring device may
include an
installer program that is stored in non-volatile memory and executed when the
analyte
monitoring device is coupled to the remote device. The installer program may
be executed
when the user couples the analyte monitoring device to the remote device. The
installer
program may then initiate the launch of the RD software on the remote device.
[0177] In some embodiments, the RD software is not stored in non-volatile
memory on the
remote device. The RD software is stored on the analyte monitoring device and
used to
launch the RD software on the remote device is coupled to the analyte
monitoring device.
[0178] In some embodiments, the RD software may be downloaded and stored in
non-
volatile memory on the remote device. For example, the RD software may be
downloaded
-26-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
via a network connection (e.g., via an intern& connection), by storage device
(e.g., CD-
ROM, memory stick, etc.), and/or downloaded from the analyte monitoring
device. In
some instances, the RD software is capable of being run even when the device
is not
coupled to the computer.
[0179] It should be understood that the RD software may be compatible with
various
hardware systems (e.g., PC, MAC) and various operating systems (e.g., Windows,
MAC
OS, Linux).
[0180] The analyte monitoring device may be communicatively coupled to the
remote
device via wired technologies. Example wired technologies may include, but are
not
limited to, the following technologies, or family of technologies: USB,
FireWire, SPI,
SDIO, RS-232 port, etc.
[0181] The analyte monitoring device may include, for example, a communication
connector unit to permit wired communication and coupling to the remote
device. The
communication connector unit provides the capability to communicate with a
remote
device having an appropriate interface to operatively couple with the
communication
connector. In some embodiments, the communication connector is configured to
communicate with a smartphone such as an iPhone or Blackberry.
[0182] The communication connector unit may be any variety of connection
interfaces -
e.g., male or female connection interfaces. Using USB as an example, the
communication
connector may be any of the variety of USB plugs or USB receptacles/ports. As
USB
receptacles are typically located on computer and other devices, a
corresponding USB
plug used as a communication connector unit will enable the analyte monitoring
device to
be plugged directly into the USB receptacle, avoiding the use of cables. In
other instances,
the appropriate USB receptacle may be used on the analyte monitoring device to
enable
communication using a USB cable (similar to many other devices such as digital
cameras,
cellular phones, smartphones, etc.).
[0183] It should be appreciated that the in some embodiments the analyte
monitoring device
may be communicably coupled to the remote device via wireless technology. In
such
instances, the analyte monitoring device may include corresponding
transmitters,
receivers, and/or transceivers. The analyte monitoring device may be
configured to
wirelessly communicate using a technology including, but not limited to, radio
frequency
(RF) communication, Zigbee0 communication protocols, WiFi, infrared, wireless
Universal Serial Bus (USB), Ultra Wide Band (UWB), Bluetooth0 communication
-27-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
protocols, and cellular communication, such as code division multiple access
(CDMA) or
Global System for Mobile communications (GSM), etc.
[0184] The functionality of the RD software launched on the remote device may
include a
variety of functions relating to, for example, data acquisition; data
management;
management of features, settings, configurations, etc., of the analyte
monitoring device;
generation, saving, transmitting, and/or printing of reports, management of
updates (e.g.,
field updates to device firmware and RD software); access to training content,
web-based
content, web-based marketing; etc.
[0185] The RD software may be launched on a remote device and used by the user
(e.g., the
patient) and/or a health care provider (HCP) (e.g., physician, hospital staff,
etc.). For
example, the HCP and/or patient may use the RD software on a remote device to
analyze
the patient data, view and print reports, view and change device settings,
update device
firmware and application software, etc.
[0186] In some instances, the RD software may initiate a comparison between
the time date
on the analyte monitoring device and that on the remote device and/or remote
time server
accessed via an intern& connection from the remote device. The RD software may
account
for discrepancies and take action accordingly. For example, thresholds may be
set (e.g., 5
minute difference) and if the threshold is reached, the analyte monitoring
device prompts
the user with a warning, question, indicator, etc., to acknowledge the
discrepancy and/or
remedy the discrepancy (e.g., adjust the time on one of the devices). In some
instances, a
similar comparison may be performed by the RD software to account for other
discrepancies between the analyte monitoring device and remote device - e.g.,
discrepancies between data logs, data values, stored files, device and/or user
interface
configurations and settings, etc. The appropriate action can then be taken or
requested.
[0187] Various defaults and customized configurations and settings may be
established for
generating, printing, saving, exporting, etc., reports. For example, the
various formats for
the report may be established (e.g., layout, style, themes, color, etc.);
various file types to
save the report as (e.g., PDF, Word document, Excel spreadsheet, etc. In some
instances,
for example, the RD software may provide the user with the ability to export
tab-delimited
text files or XML exports of the meter data (e.g., including blood glucose,
ketones, carbs,
insulin, and event tags, etc.). In some instances, the RD software may enable
the user to
save, print, and/or export preferences, including favorite reports, target
blood glucose
-28-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
ranges, auto save, auto print, color/black and white printing, device/software
update
settings for multiple devices, etc.
[0188] In some aspects, the RD software is used to control the configuration
of the device
and data from the device. This control may be utilized by the user and/or HCP.
In some
instances, the RD software shall provide access to one or more informative
documents,
trainings, tutorials, etc. For example, the RD software application may
provide links or to
manufacturer sponsored websites intended for any variety of purposes such as
marketing
and training content.
[0189] In some aspects, the RD software may include an update management
function to
help facilitate the detection, download, and installation of updates (e.g.,
firmware,
informatics application updates, etc.) for the analyte meter device and/or the
RD software.
The updates may be detected and downloaded automatically in some instances
(e.g., when
an intern& connection is active) and/or detected and downloaded upon user
confirmation
or request. In some instances, updates to the software shall also update its
installation files
stored on the device. Moreover, in some instances, when the device firmware is
updated,
required labeling/user documentation is also updated on the device. In some
instances,
when device firmware is updated, the existing device settings and testing
history (e.g.,
blood glucose, insulin, carb data, etc.) is preserved.
[0190] In some aspects of the present disclosure, data management software may
be loaded
and launched on a remote data processing device to operate with a coupled
analyte
monitoring device. The data management software may include one or more GUI's
for
communicating with the analyte monitoring device. It should be appreciated a
GUI may be
used to represent one or more of graphical elements displayed on the display
of the remote
device for interfacing with the user. Thus, "graphical user interface" or
"GUI" may
encompass the entire display, an application window, pop-up windows, menus,
progress
and status bars, buttons, etc.
[0191] In some aspects of the present disclosure, the data management software
provides a
meter mode to provide access to settings and functions that are used to setup
and control
the analyte monitoring device. The data management software may also provide a
meter
setup mode to guide the user through the initial setup of the analyte
monitoring device.
The data management software may provide a reports mode to provide access to
settings
and function for creating, viewing, saving, and/or printing various reports.
In addition, the
data management software may provide a reports setup mode to guide a user
through the
-29-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
initial reports setup and creation process. The data management software may
also provide
the function for users to export data from the analyte monitoring device -
e.g., as a tab-
delimited file or other spreadsheet-compatible format. In some instances, the
data
management software may provide functions for providing help documents,
tutorials, etc.
to the user. The data management software may provide functions for checking
for
software update and for acquiring updates. For example, checks may be
automatically
initiated and/or initiated by the user. In some instances, the software
updates may be
checked for and acquired via a network connection on the remote device.
Exemplary Devices & Systems
[0192] FIG. 2 illustrates a block diagram of a system including an analyte
monitoring
device and remote data processing device, according to some embodiments.
System 1500
is shown to comprising analyte monitoring device 1501 communicably coupled to
remote
device 1505. In some instances, as shown, remote device 1505 may have network
access
to a network 1510 in which a second remote device 1515 is shown coupled to. It
should be
understood that network 1510 may include one or more networks, including LANs,
WANs, and/or the internet.
[0193] Analyte monitoring device 1501 is shown removably coupled to remote
device 1505
via communication connector unit 1422. Communication connector unit, for
example,
includes a USB plug which couples with a USB receptacle 1507 in remote device
1505.
Remote device 1505 may include peripheral devices, such as printer, keyboard,
monitor,
CD drive, etc. Remote device 1505 may also include, as shown, a network
interface 1530
which connects it to network 510. Remote device 1515 is also connected to
network 1510
and may communicate with remote devicel 505 via network 1510.
[0194] The following paragraphs describe system 1500 during operation,
according to some
embodiments. In some instances, the analyte monitoring device described is a
glucose
monitoring device which measures the glucose concentration level of a blood
sample. It
should be understood that the description applies equally to other analytes
and to other
forms of samples.
[0195] In use, analyte monitoring device 1501 receives a test strip 1525 for
measuring an
analyte level of a sample applied to test strip 1525. Test strip 1525 is
received at strip port
unit 1520. Analyte monitoring device 1501 performs a measurement computation
on the
sample and the user can view the measurement reading on, for example, a
touchscreen
-30-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
display (not shown). The user may also be presented with a menu on the
touchscreen
display to view and select - e.g., menus for storing data, downloading data,
performing
bolus calculations based on the measurement, etc.
[0196] The user may couple the analyte monitoring device 1501 to remote device
505 (e.g.,
a personal computer) via a communication connector unit. For example, the user
may
decide to store the measurement data and then choose to download stored test
data
(including stored measurement readings) to a remote device 1505.
[0197] Analyte monitoring devicel 501 may then be coupled to remote device
1505 via
communication connector unit 1422. Communication connector unit 1422 may, for
example, include a USB plug which couples to a USB receptacle 1507 on remote
device
1505.
[0198] In some instances, the analyte monitoring device 1501 may be powered by
the
remote device 1505 when coupled via the communication connector unit 1422. In
such
case, the user would couple the analyte monitoring device 1501 to the remote
device 1505
and then insert test strip 1525 into the strip port 1520 to take a measurement
reading. In
some instances, the analyte monitoring device includes its own power source,
such as
button or AAA-size batteries, for example, and is not powered by the remote
device 1505.
[0199] In some instances, the analyte monitoring device may be "locked" or
prevented from
performing a test while coupled to the remote device 1505. For example,
medical device
regulations such as high voltage isolation testing may be required if the
analyte monitoring
device is configured to perform tests while coupled to a remote device. Thus,
"locking" or
preventing the analyte monitoring device from performing a test while coupled
to the
remote device allows the analyte monitoring device to not be subjected to the
additional
testing, if so desired.
[0200] In some aspects, the analyte monitoring device 1501 may initiate a user
interface
application (e.g., RD software) to execute on the analyte monitoring device,
and/or the
remote device 1505 when coupled to the remote device 1505. The user interface
application may be stored in a memory unit on the analyte monitoring device
1501, for
example. In some aspects, the user is not required to have previously loaded
software on
the remote device 1505 to operate with the analyte monitoring device 1501. In
some
aspects, the analyte monitoring device may be configured to initiate the user
interface
application automatically upon coupling to the remote device. It should be
understood that
the user interface application may be configured to be compatible with various
hardware
-31-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
systems (e.g., PC, MAC) and various operating systems (e.g., Windows, MAC OS,
Linux).
[0201] The user interface application may include, for example, diabetes
management
related applications. The user interface application may provide a variety of
menus,
selections, charts, alarms, reminders, visual indicators, etc. For example,
the user may be
presented with menus and options, such as whether to take a measurement
reading, to
view stored measurement readings, to store data, to download data, to perform
bolus
calculation based on the measurement, etc.
[0202] The user interface program may, for example, allow the user to perform
the
following steps: (1) generate a replica of the test data stored on the analyte
monitoring
device 1501, on the remote device 1505; and (2) synchronize test data from the
analyte
monitoring device 1501 to the database on the remote device 1505. Meter
settings and/or
user settings/preferences from the analyte monitoring device may also be
included in the
test data and synchronized with the remote device. Date and time for the
remote device
1505 and analyte monitoring device 1501 may also be synched.
[0203] To read test data from the analyte monitoring device 1501 and write it
to the remote
device 1505, it is recognized herein that data in the remote device may be
organized into
tables, which may be organized into records, which may be broken down into
predefined
fields. Similarly, at some level data will be organized into records with a
consistent field
structure on the analyte monitoring device 1501. The user interface
application may read
test data from the analyte monitoring device and write it out to tables on the
remote device
1505. The user interface application may also read data from table in the
remote device
1505 and write them out to the analyte monitoring device 1501. Various types
of data
conversion may be used. For example, data residing in fields in the analyte
monitoring
device may be converted from the format it exists in the analyte monitoring
device to a
format compatible with the remote device, and vice versa. The logical
structure of the
records in the two systems may be different.
[0204] Remote device 1505 may include peripheral devices, such as printer,
keyboard,
monitor, CD drive, etc. Remote device 1505 includes a network interface which
connects
it to network 1510 (e.g., the internet). The user interface application may
provide the user
with the option to view test data on the monitor, to store test data on
storage media (e.g.,
CD-ROM, memory card, etc.), further analyze and/or manipulate test data,
transmit data to
another device), and/or print out test data such as charts, reports, etc., on
the printer.
-32-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0205] As shown, remote device 1505 may also include a network interface 1530
(e.g.,
network interface card (NIC), modem, router, RF front end, etc.) used to
connect the
remote device 1505 to network 1510. For example, in some aspects, analyte
monitoring
device 1501 may couple via a USB connection to the remote device which may be
a
personal computer or laptop connected to the internet using a wireless modem
and/or
router. In some aspects, analyte monitoring device 1501 may couple via a micro
USB
connection to a remote device 1505 which is a smartphone having an RF front
end to
access a mobile network. The user interface application may provide a user
interface for
using the network connection of the remote device 1505 - e.g., to forward test
data to a
physician, hospital, health provider, and/or other third party located at a
second remote
device 1515 on network 1510. Appropriate action may then be taken by the
receiving
party at the second remote device 1515.
[0206] Referring back to FIG. 2, the analyte monitoring device may include a
wireless
communication unit, for example, which may include, for example, a receiver
and/or
transmitter for communicating with another device, e.g., remote device 1505, a
medication
delivery device, and/or a patient monitoring device (e.g., a continuous
glucose monitoring
device or a health management system, such as the CoPilotTM system available
from
Abbott Diabetes Care Inc., Alameda, CA), etc. The wireless communication unit
may be
configured to wirelessly communicate using a technology including, but not
limited to,
radio frequency (RF) communication, Zigbee0 communication protocols, WiFi,
infrared,
wireless Universal Serial Bus (USB), Ultra Wide Band (UWB), Bluetooth0
communication protocols, and cellular communication, such as code division
multiple
access (CDMA) or Global System for Mobile communications (GSM), etc. In some
aspects, the wireless communication unit is configured for bi-directional
radio frequency
(RF) communication with another device to transmit and/or receive data to and
from the
analyte monitoring device 1501.
[0207] In some aspects, the wireless communication unit may be used to
communicate with
a remote device as described above for the communication connector unit. In
some aspects
where the analyte monitoring device includes a communication connector unit,
the
wireless communication unit may replace or provide an optional channel of
communication for the functions provided by the communication connector unit
discussed
above. Referring back to FIG. 2, analyte monitoring device 1501 may be coupled
to
remote device 1505 via a wireless communication unit and provide an optional
alternative
-33-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
communication channel with remote device 1505. In some aspects, analyte
monitoring
device 1501 may not include a communication connector unit 1422, and instead
only
communicate with the remote device 1505 via a wireless communication unit
present on
analyte monitoring device 1501. In some aspects, the analyte monitoring device
is
configured to receive a program update from a remote device via the wireless
communication unit.
[0208] In some aspects, the wireless communication module may be configured to
communicate with a smartphone (e.g., iPhone, Blackberry, etc). It is typical
for
smartphones to include various wireless technologies such as Wi-Fi, infrared,
Bluetooth0,
etc.
[0209] In some aspects, the analyte monitoring device may be configured to
wirelessly
communicate via the wireless communication unit with a server device, e.g.,
using a
common standard such as 802.11 or Bluetooth0 RF protocol, or an IrDA infrared
protocol. The server device could be another portable device, such as a
Personal Digital
Assistant (PDA) or notebook computer, or a larger device such as a desktop
computer,
appliance, etc. In some aspects, the server device has a display, such as a
liquid crystal
display (LCD), as well as an input device, such as buttons, a keyboard, mouse
or
touchscreen. With such an arrangement, the user can control the meter
indirectly by
interacting with the user interface(s) of the server device, which in turn
interacts with the
meter across a wireless link.
[0210] In some aspects, the wireless communication module is used to
communicate with a
remote sensor - e.g., a sensor configured for implantation into a patient or
user. Examples
of sensors for use in the analyte monitoring systems of the present disclosure
are described
in U.S. Patent No. 6,175,752; and U.S. Patent Application, Ser. No.
09/034,372,
incorporated herein by reference. Additional information regarding sensors and
continuous
analyte monitoring systems and devices are described in U.S. Patent Nos.
5,356,786; U.S.
Pat. No. 6,175,752; U.S. Pat. No. 6,560,471; U.S. Pat. No. 5,262,035; U.S.
Pat. No.
6,881,551; U.S. Pat. No. 6,121,009; U.S. Pat. No. 7,167,818; U.S. Pat. No.
6,270,455;
U.S. Pat. No. 6,161,095; U.S. Pat. No. 5,918,603; U.S. Pat. No. 6,144,837;
U.S. Pat. No.
5,601,435; U.S. Pat. No. 5,822,715; U.S. Pat. No. 5,899,855; U.S. Pat. No.
6,071,391;
U.S. Pat. No. 6,120,676; U.S. Pat. No. 6,143,164; U.S. Pat. No. 6,299,757;
U.S. Pat. No.
6,338,790; U.S. Pat. No. 6,377,894; U.S. Pat. No. 6,600,997; U.S. Pat. No.
6,773,671;
U.S. Pat. No. 6,514,460; U.S. Pat. No. 6,592,745; U.S. Pat. No. 5,628,890;
U.S. Pat. No.
-34-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
5,820,551; U.S. Pat. No. 6,736,957; U.S. Pat. No. 4,545,382; U.S. Pat. No.
4,711,245;
U.S. Pat. No. 5,509,410; U.S. Pat. No. 6,540,891; U.S. Pat. No. 6,730,100;
U.S. Pat. No.
6,764,581; U.S. Pat. No. 6,299,757; U.S. Pat. No. 6,461,496; U.S. Pat. No.
6,503,381;
U.S. Pat. No. 6,591,125; U.S. Pat. No. 6,616,819; U.S. Pat. No. 6,618,934;
U.S. Pat. No.
6,676,816; U.S. Pat. No. 6,749,740; U.S. Pat. No. 6,893,545; U.S. Pat. No.
6,942,518;
U.S. Pat. No. 6,514,718; U.S. Pat. No. 5,264,014; U.S. Pat. No. 5,262,305;
U.S. Pat. No.
5,320,715; U.S. Pat. No. 5,593,852; U.S. Pat. No. 6,746,582; U.S. Pat. No.
6,284,478;
U.S. Pat. No. 7,299,082; U.S. patent application Ser. No. 10/745,878 filed
Dec. 26, 1003
entitled "Continuous Glucose Monitoring System and Methods of Use"; and U.S.
Application No. 61/149,639 entitled "Compact On-Body Physiological Monitoring
Device
and Methods Thereof', the disclosures of each which are incorporated by
reference herein.
[0211] FIG. 3 illustrates an analyte monitoring device used with a remote
sensor, according
to some embodiments. Sensor 1605 may be configured for implantation (e.g.,
subcutaneous, venous, or arterial implantation) into a patient. The sensor
1605 is coupled
to sensor control unit 1610 which is typically attached to the skin of a
patient. The sensor
control unit 1610 operates the sensor 1605, including, for example, providing
a voltage
across the electrodes of the sensor 1605 and collecting signals from the
sensor 1605. The
sensor control unit 1610 may evaluate the signals from the sensor 605 and/or
transmit the
signals to wireless communication unit 1423 on analyte monitoring device 1501
for
evaluation.
[0212] In some aspects, the wireless communication unit 1423 is configured to
receive a
signal from a remote sensor using radio-frequency identification (RFID)
technology. This
configuration may be used to provide glucose on demand capabilities, in which
case when
a measurement reading is desired, the analyte monitoring device is brought
within close
vicinity of the implantable sensor. In some instances, RFID technology may be
used in
continuous glucose monitoring (CGM) applications.
[0213] The analyte monitoring device 1501 processes the signals from the on-
skin sensor
control unit 1610 to determine the concentration or level of analyte in the
subcutaneous
tissue and may display the current level of the analyte via display unit 1421.
Furthermore,
the sensor control unit 1610 and/or the analyte monitoring device 1501 may
indicate to the
patient, via, for example, an audible, visual, or other sensory-stimulating
alarm, when the
level of the analyte is at or near a threshold level. For example, if glucose
is monitored
-35-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
then an alarm may be used to alert the patient to a hypoglycemic or
hyperglycemic glucose
level and/or to impending hypoglycemia or hyperglycemia.
[0214] The analyte monitoring device 1501 may perform a variety of functions,
including
for example: modifying the signals from the sensor 605 using calibration data
and/or
measurements from a temperature probe (not shown); determining a level of an
analyte in
the interstitial fluid; determining a level of an analyte in the bloodstream
based on the
sensor measurements in the interstitial fluid; determining if the level, rate
of change,
and/or acceleration in the rate of change of the analyte exceeds or meets one
or more
threshold values; activating an alarm system if a threshold value is met or
exceeded;
evaluating trends in the level of an analyte based on a series of sensor
signals; therapy
management (e.g., determine a dose of a medication, etc.); and reduce noise or
error
contributions (e.g., through signal averaging or comparing readings from
multiple
electrodes); etc. The analyte monitoring device may be simple and perform only
one or a
small number of these functions or the analyte monitoring device may perform
all or most
of these functions.
[0215] Analyte monitoring device 1501 may communicate with a remote device 505
via
communication connector unit 1422, and/or wireless communication unit 1423,
and/or a
second wireless communication unit (not shown), as described earlier. It
should also be
understood that the analyte monitoring device may be configured with one or
more
wireless communication units.
User Interface for the Analyte Monitoring Device
[0216] In some aspects, the analyte monitoring device includes software used
to perform
various operation and functions with the device, such as, but not limited to,
the functions
described above. The device may include, for example, software instructions
that are
stored within a machine-readable storage medium (e.g., flash memory or other
non-
volatile memory) and executed by one or more general-purpose or special-
purpose
programmable microprocessors and/or microcontrollers, or other type of
processing
device. It should be appreciated that machine-readable storage medium may
include any
variety of non-volatile memory (e.g., Flash memory) or volatile memory (e.g.,
random
access memory (RAM)), and may include one or more memory components.
[0217] In some aspects, the analyte monitoring device may include software
that is used to
provide the overall user interface for operation of the device and general
user-experience
-36-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
with the device. The user interface may encompass graphical user interfaces
(GUIs) that
are displayed for a variety of features that may be provided by the device -
e.g., Home
screen, Glucose Reading screen, Logbook screen, Reader Summary screens, Reader
Usage
screens, etc. The user interface also encompasses screen navigation/flows for
various
operations that may be performed by the device - e.g., on-demand readings;
activating a
patch; replacing a patch; providing the status of a patch; notification of
patch expiration;
activating various information screens such as logbooks, summary screens,
usage reports,
etc.; creation of reports for display, communication, printing, etc.; etc.
[0218] It should be noted that the term "sensor" and "patch" are used herein
to refer
generally to the implanted sensor and on-body electronics together.
[0219] In some aspects of the present disclosure, the analyte monitoring
device provides
various graphical user interfaces (GUIs) or screens that are displayed on a
display of the
analyte monitoring device to assist the user with operation of the device or
provide
information to the user. It should be understood that the terms "graphical
user interface",
"GUI", "interface" and "screen" are used broadly herein to represent any
graphical
interface element displayed on the display, and are used interchangeably. For
example, the
graphical user interface may comprise a graphical icon, element, picture,
video, text box,
pop-up window, application window, home screen, etc.
[0220] Furthermore, it must be noted that the terms "graphical user
interface", "GUI",
"interface", and "screen" are used broadly herein and may include plural
referents unless
the context clearly dictates otherwise. Therefore, for example, reference to a
"Setup
screen" may include one or more screens in the setup process, and reference to
"the Setup
screen" may include reference to one or more program updates and equivalents
thereof
known to those skilled in the art, and so forth.
[0221] Furthermore, it should be understood that one or more GUIs may be
implemented
for various features, functions and/or settings. Further, different GUIs may
be combined in
some instances without compromising the underlying principles of the
disclosure. Still
further, the term
[0222] The user may navigate through branches of various screens via trigger
elements on
the device. The trigger elements may be any variety of trigger elements -
e.g., buttons,
keys, toggle switches, wheels, arrows, etc. The trigger elements may be
physical and
tangible trigger elements located on the device (e.g., hardware buttons or
keys on the
housing or keyboard, etc.) and/or may be nontangible trigger elements (e.g.,
graphical user
-37-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
interface elements) displayed on the device. It should also be understood that
the branches
of navigation may be displayed on the home screen (e.g., as icons on the
display) and
triggered by corresponding physical and tangible trigger elements on the
housing of
keyboard.
[0223] In some embodiments, a touchscreen display is implemented and the
trigger
elements are icons displayed on the touchscreen. The trigger element is
activated by the
user touching the corresponding trigger element (e.g., icon). It should be
understood that
icons is used broadly herein to represent any text, image, video, graphic,
etc. For example,
the trigger element may be suggestive of its function or feature - e.g., an
image of a gear
representing a trigger element for accessing the setup menu, an arrow keys,
check boxes,
toggle switches, buttons (e.g., with identifying text or image inside), etc.
Home Screen:
[0224] In some aspects of the present disclosure a Home screen is provided.
The home
screen or landing screen is displayed on the display of the analyte monitoring
device and
functions as a reference point or relative reference point to perform various
functions or
features on the device. From the home screen the user can navigate to any of
the various
GUI's to perform or access various functions and features of the device. For
instance, the
user can navigate to a screen enabling the user to access a logbook, setup
menu,
reminders, etc. From that point, the user may access additional features and
functions
related to the selected item.
Active Screens:
[0225] In some aspects of the present disclosure an Active screen is provided.
The Active
Screen (e.g., Scan Prompt screen) awaits the user to perform an on-demand
reading or
otherwise "ping", "scan", or "swipe" the sensor. It should be appreciated that
the term
"ping", "scan", and "swipe" are used interchangeably herein and refer broadly
to bringing
the analyte monitoring device in sufficiently close distance of the sensor to
perform a
communication (e.g., on-demand reading, sensor activation, etc.).
On-Demand Reader Screens:
[0226] In some aspects of the present disclosure, On-Demand Reader screens are
provided
to convey information pertaining to analyte readings (e.g., glucose levels).
While
embodiments are described in relation to on-demand glucose readings, it should
be
appreciated that other analytes may be implemented in other embodiments.
-38-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0227] In some instances, the On-Demand Reader screens may include one or more
of the
following: a reading, trend symbol, trigger element for calculating insulin,
trigger element
for food intake, trigger element for adding notes, trigger element for
switching between
screens, patch status information, trail information, the current time,
battery status, etc.
The reading provides glucose levels for a current reading. The trend symbol
provides
trending information related to increasing or decreasing patterns of glucose
levels. When
activated, the trigger element for calculating insulin displays a screen for
providing an
insulin calculation for the user - e.g., based on the current glucose reading.
When
activated, the trigger element for food intake displays a screen for entering
food intake
and/or displaying food intake. When activated, the trigger element for notes
displays a
screen for entering notes and/or displaying previously entered notes. The
trail information
displays readings leading up to the current reading.
[0228] Some of the embodiments have a single screen layout in which all the
information
for the on-demand glucose reading is displayed on a single screen. Other
embodiments
include a dual screen layout which provides the information for the on-demand
glucose
reading over two screens. The user may switch between the two-screens with a
trigger
element such as an icon or box on the screen. In some instances, the user may
be able to
switch between screens by sliding a finger across the display of the device.
Reader Summary/Reports Screens:
[0229] In some aspects of the present disclosure, the analyte monitoring
device displays
summary screens that convey a collection of information associated with
readings that
have been performed.
Event Summary Views:
[0230] The Event Summary screen displays summarized information regarding the
history
of the user's readings.
Event Detail View:
[0231] The Event Detail screen displays a detailed view of the user's readings
associated
with an event - e.g., a hypoglycemic event.
Logbook screen:
[0232] The Logbook screen displays recorded readings and associated data
regarding the
user's readings.
-39-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Usage Report:
[0233] The Usage Report screen displays the meter utilization to indicate user
engagement.
Any gaps in time (e.g., extended durations where the user did not take any
readings) are
recorded and shown in the Usage Report.
Personalization Picture:
[0234] In some embodiments, the analyte monitoring device includes a
personalization
screen that displays a personalized image - e.g., selected or uploaded by the
user. The
personalized screen may be displayed at specific times. For example, the
personalized
screen may be displayed after the device is powered on. Once the power up
process is
complete, the analyte monitoring device displays another screen, such as an
active screen.
In another embodiment, the home screen is displayed after the power up
process.
Screen Qualities:
[0235] It should be appreciated that the analyte monitoring device may be
implemented
with different screen qualities - e.g., gray-scale and higher-resolution. In
some
embodiments, the analyte monitoring device may be capable of being operated in
different
screen qualities. For example, the analyte monitoring device may include a
color
touchscreen and be capable of being run in color mode or gray-scale mode.
[0236] The following paragraphs describe various navigation flows between user
screens
for performing various functions and features of the device.
Navigation Flows
[0237] The following paragraphs describe various navigation flows between user
screens
for performing various functions and features of the analyte monitoring
device. For
example, software or firmware implementing flows introduced herein may be
stored on a
machine-readable storage medium and may be executed by one or more general-
purpose
or special-purpose programmable microprocessors.
Hardware
[0238] An exemplary embodiment of a graphical user interface which may be
utilized in
connection with an analyte monitoring device (e.g., analyte reader device) as
described
herein and which functions to perform various hardware-related actions is
provided.
-40-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Hardware - Power On Interface
[0239] FIG. 4 illustrates a method 100 of powering on an analyte monitoring
device, such
as a glucose monitoring device, according to one embodiment. The analyte
monitoring
device may also be referred to herein as a "reader" which takes measurement
readings
from the analyte sensor. From the off state, as represented by block 102, the
device can be
powered on by depressing a power button - e.g., by a "short press" or a "long
press", as
represented by block 104. At block 106, a test is performed to determine if
the battery is
depleted. If so, the device is powered off, as shown by block 107. In some
instances, when
the device recovers from a power loss, it can remember "sensor" status
information and
resume use of the same sensor if the sensor has not expired - e.g., a 14-day
period, or other
predetermined period, has not elapsed. If the battery is determined to not be
depleted, then
the device is powered on, as shown at block 108.
[0240] At block 110, the device processor determines the presence of any
errors in the
hardware functionality of the device. Upon being powered on, if the device
processor
determines a hardware failure, such as the device is not working properly, the
device will
display a hardware error message as shown at block 111, such as those
described herein
below in the present application. If no hardware errors are detected, at block
112, it is
determined if this is the first time the device is to be set up. If it is the
first time, then a
First Start procedure is initiated to begin the setup of the device, as shown
at block 114. If
it is determined that this is not a first time setup, then it is determined if
the sensor has
already been activated. If not activated, then the home screen is displayed,
as shown at
block 118. If already activated, then it is determined whether the sensor is
expired, as
shown at block 120. If the sensor is expired, it is determined if the sensor
expiration
message has been previously displayed, as shown at block 130. If so, then the
home screen
is displayed, as shown at block 134. If not, then a sensor expiration screen
is displayed, as
shown at block 132. In some instances, an audible notification may also be
provided. The
sensor expiration screen may also inform the use that a new sensor must be
started to take
a glucose reading. Once confirmed by the user, for example by pressing "ok",
the home
screen is displayed on the device.
[0241] Referring back to block 120, if the sensor is not expired, then it is
determined if the
sensor is warming up, as shown by block 122. If not, then a prompt is
displayed for a
sensor scan. If the sensor is warming up, then a warm-up message screen is
displayed that
informs the user that the sensor is warming up, as shown at block 126. In some
instances,
-41-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
such as shown, the warm-up message screen indicates the time remaining before
the
sensor can be used. An audible reminder may also be present, as shown. Once
confirmed
by the user, for example by pressing "ok", the home screen is displayed on the
device.
Go Home / Off Interface
[0242] FIG. 5A illustrates a method 140 of navigating to a home screen from
other screens
for an analyte monitoring device, according to one embodiment. At block 141,
the device
is displaying a screen other than the home screen. At block 142 the home
trigger button is
pressed (e.g., by a short press). When the home trigger button is pressed, the
device
processor checks whether the sensor is expired, e.g., after 14 days, as shown
at block 143.
If the sensor is expired, then a sensor expiration screen is displayed, as
shown at block
144. In some instances, an audible notification may also be provided. The
sensor
expiration screen may also inform the user that a new sensor must be started
to take a
glucose reading. Once confirmed by the user, for example by pressing "ok", the
home
screen is displayed on the device, as shown at block 145. If the sensor is not
expired, then
the home screen is displayed, as shown at block 146. An alternative home
screen 148 is
shown that does not include a reminder. In some instances, a short press of
the trigger
button for the home screen is not activated when a test strip is inserted
within the device a
prior to receiving a result. It should be appreciated that there may be some
screens in
which the home trigger button does not lead to a display of the home screen.
In some
embodiments, from some screens, pressing the home trigger button does not
navigate to
the home screen. Such exceptions are described herein below.
[0243] FIG. 5B illustrates a method 150 of powering an analyte monitoring
device off,
according to one embodiment. At block 152, a current screen is displayed on
the device.
When a screen has been displayed for a predetermined period of time - e.g., 45
seconds or
some other period of time, the screen brightness may dim to say power, as
shown at block
154. If the user touches the dimmed screen the display returns to full
brightness and the
timeout timer resets. After the screen is dimmed, if another predetermined
period of time
has passed without any activity - e.g., 15 seconds or other time period - then
the device
will power off It should be appreciated that there may be certain screens in
which such
method does not apply.
[0244] FIG. 5C illustrates a method 160 of powering an analyte monitoring
device off,
according to one embodiment. From the home screen, shown at block 162, the
pressing
(e.g., short or long) of the home trigger button, as shown at block 164, will
trigger the
-42-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
display of a screen indicating the device is to powering off The user may be
provided with
an option to cancel the power down process. If no selection is detected, as
shown at block
168, then the device may power down after a predetermined period of time -
e.g., 5
seconds or some other time period, as represented by block 172. If at block
166 the trigger
button is pressed, as shown at block 170, then the device is powered off, as
represented by
block 172.
[0245] FIG. 5D illustrates a method 180 of powering off an analyte monitoring
device,
according to one embodiment. From a current screen, shown at block 182, the
depression
of the trigger button (e.g., a long depression) triggers the display of a
power down screen
shown at block 186. The user may be provided with an option to cancel the
power down
process. If no selection is detected, as shown at block 188, then the device
may power
down after a predetermined period of time - e.g., 5 seconds or some other time
period, as
represented by block 192. If at block 186 the trigger button is pressed, as
shown at block
190, then the device is powered off, as represented by block 192.
Low Battery Interface
[0246] FIG. 5E illustrates a battery low screen 196 for an analyte monitoring
device that
indicates that the battery of the device is low, according to one embodiment.
In one
embodiment, for example, this screen may appear after scanning of the sensor
or a strip
test, but before the results are shown. In some instance, this screen will
start appearing
when the battery capacity reaches a level equivalent to 1 day of typical use.
This may be a
predetermined value or based on a user history. In some instances, when the
screen is
display, a reminder auditory/vibratory signal is the only signal broadcast.
The standard
confirmation signal is omitted. A user confirmation element may be displayed
that directs
the user to scan results when the user confirms. In some instances, if the
charger is
plugged in from the battery low screen, the device will transition to the
results screen.
PC link / Data Transfer Interface
[0247] FIG. 5F illustrates a method 200 for linking an analyte monitoring
device to a PC
and transferring data between the two, according to one embodiment. At block
202, the
device is connected to the PC via a wired or wireless connection, and data is
transferred
between the device and the PC. When data is being transferred, a data transfer
screen is
displayed and indicates that the data transfer is in process. The example
shown also
instructs the user not to unplug the device from the computer. If the data
transfer
completes or the connection between the device and PC (e.g., USB connection)
is
-43-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
disconnected, then the home screen is displayed. In one embodiment, if the
initial Reader
settings have not been saved the Reader will go to the First Start procedure
after
attempting to transfer data. It should be appreciated that engineering
implementation may
dictate changes to these screens in other embodiments. In some instances, if
the device is
plugged into a computer before initial setup, the language will default to
English until it is
set by PC App.
Charging Battery Interface
[0248] FIG. 5G illustrates a method 210 for charging a battery for an analyte
monitoring
device, according to one embodiment. The device may be charged by connecting
the
device to a PC or other device (e.g., via a USB connection, etc.) or by
connecting the
device to an AC adapter that is plugged into an AC outlet, for example. When
the charger
is plugged in from the off state, the home screen is displayed and indicates
the device is
charging - e.g., via a charging icon or symbol, such as a battery symbol -
such as shown at
block 212. In one embodiment, while charging every screen is subject to a
standard time-
out period, such as a 60 second time-out period for instance.
[0249] If the device is disconnected from the charger (e.g., from the USB
connection port),
shown at 216, then the home screen continues to be displayed without the
charging symbol
or icon. This may be replaced by an icon or symbol indicating the current
state of the
battery life. In one embodiment, if the device is displaying any screen,
including the home
screen, it will remain on that screen when the charger is disconnected, and
the
predetermined timeout period will reset when the charger is disconnected.
Home Screen
[0250] An exemplary embodiment of a graphical user interface which may be
utilized in
connection with a reader as described herein and which functions to navigate
the device in
relation to the Home screen is provided.
Sensor Scan or System Check Interface
[0251] FIG. 6A illustrates a method 220 for performing a sensor scan or system
check,
according to one embodiment. From the home screen at block 222, if a glucose
check is
initiated, it is determined if a system check is required, as shown at block
230. If so, then a
system check is performed, as shown at block 232. If a system check is not
required, then
a sensor scan prompt is displayed, as shown at block 234. The home screen may
enable
other features to be initiated as well. For example, a reader's summaries menu
screen may
-44-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
be displayed when the corresponding icon is triggered at the home screen, as
shown by
block 224. Also, a settings screen may be displayed when the corresponding
icon is
triggered at the home screen, as shown by block 226.
[0252] In addition, an insulin on board screen may be displayed when the
corresponding
icon is triggered at the home screen, as shown by block 236. The insulin on
board screen
provides information related to the estimated active insulin remaining in a
user's body
according to insulin data previously entered. Additional details may be
provided when a
corresponding icon is initiated from the insulin on board screen. In one
embodiment, a
timer counts down from time of dose for insulin duration as set in a
calculator setup. In
one embodiment, the insulin on board screen with active countdown appears if
the insulin
on board is enabled in the calculator setup and the insulin on board is
calculated to be
present. The icon shown provides a visual indication that reflects the amount
of rapid-
acting insulin estimated to be still in body - e.g., a percentage of the body-
shaped icon
filled corresponding to a percentage of the active insulin remaining in the
body.
[0253] Also, a reminder screen may be displayed when the corresponding icon is
triggered
at the home screen, as shown by block 228. In one embodiment, when a reminder
is set,
the time at which the alarm will sound appears next to a reminders icon. If,
for example,
multiple reminders are active, the time shown is the time of the reminder that
will sound
soonest.
Expired/No Sensor Interface
[0254] FIG. 6B illustrates a method for activating a sensor, according to one
embodiment.
In the embodiment shown, the home screen 242 indicates that no sensor is
currently paired
to the analyte monitoring device is displayed on the device. For example, a
sensor may
have never been paired to the device, or a previously paired sensor may have
expired.
From home screen 242, the user may trigger the activation of a sensor by
pressing or
otherwise selecting a corresponding icon or other trigger element displayed on
the display
of the device.
Sensor Warming Up Interface
[0255] FIG. 6C illustrates a method 246 for activating a sensor, according to
one
embodiment. After a user initiates the activation of a sensor, e.g., as
represented by block
250, the device may indicate if the sensor is warming up. As illustrated,
during the warm
up period, the home screen 248 indicates that the sensor is warming up. For
example,
-45-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
home screen 248 indicates the time remaining before the sensor is ready, as
shown in
screen 248 - e.g., "Ready in 60 min".
Sensor Near Expiration Interface
[0256] FIG. 6D illustrates a method 252 for scanning a sensor if the sensor is
nearing
expiration, according to one embodiment. In the embodiment shown, a screen 254
indicates the time remaining before the currently paired sensor is expired -
e.g., in 16
hours. From home screen 254, the user may still trigger the activation of a
sensor by
pressing or otherwise selecting a corresponding icon or other trigger element
displayed on
the display of the device, since the sensor is not yet expired.
Sensor Life Display in Title Bar
[0257] FIG. 6E illustrates exemplary embodiments of a graphical user interface
displaying
sensor life in varying increments. In the embodiment shown, sensor life is
displayed in
three ranges: days, hours, and minutes as shown in screens 257, 258 and 259,
respectively.
In certain embodiments, the sensor life is rounded up - e.g., 2 days 1 hour is
displayed as 3
days, 1 day 20 hours is displayed as 2 days, 1 hour 10 minutes is displayed as
2 hours. In
embodiments, the sensor life display is independent of a user adjustable date
and time.
Sensor Activation
[0258] An exemplary embodiment of a graphical user interface which may be
utilized in
connection with a reader as described herein and which functions activate the
sensor is
provided.
[0259] FIG. 7 illustrates a method 260 for activating a sensor after the
device is powered
on, according to one embodiment. At block 261, the device is powered on. Once
powered,
a home screen 262 is displayed. The home screen 262 indicates that there is no
active
sensor, and further includes an icon or other trigger element that enables the
start of a new
sensor. Once icon is selected by the user, as represented by block 263, a
"Start New
Sensor" screen 264 for starting the sensor is displayed. The start new sensor
screen 264
enables the user to start a new sensor - e.g., by informing the user to scan
the sensor to
start it. Upon the start of the scan, the device waits (e.g., 15 seconds) for
the scan to
complete, as shown by blocks 265 and 266. If the wait period elapses and the
scan is not
yet complete, the device may display a screen informing the user that the scan
has timed
out 267.
-46-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0260] Upon completion of the scan, to start a pairing of the device and
sensor, the device
may check the sensor for errors, including an integrity check of the scan 268,
a health
check of the sensor 270 and an expiration of the sensor check 272. If the
device
determines that the scan failed, the device may display a screen indicating
that a scan error
occurred, as shown at block 269. If the device determines that the sensor in
not
functioning properly, the device may display a screen indicating to replace
the sensor with
a new sensor 271. If the scan succeeds and the sensor is found to be
functioning, the
device may check the expiration information of the sensor, and if expired,
e.g., a user tried
to continue use of an expired sensor, the device may display a sensor expired
screen 273.
From any of the scan error, replace sensor, and sensor expired screens,
selecting "ok" may
navigate the device back to the home screen 262 to start a new sensor again.
[0261] Referring to block 275, if the sensor has already been activated by
another device,
then a screen 276 indicating that the sensor is already paired with another
device is
displayed. Upon user confirmation, the home screen is displayed, as shown at
block 277.
In certain embodiments, when a sensor is already paired with another reader
device, use of
that sensor with the new reader device is not allowed.
[0262] In some instances, the device may include a masked mode that enables
the user to
take readings of a paired sensor, but does not display the resulting readings
to the user, or
otherwise limits the resulting data to the user. After a non-expired sensor
has been
successfully scanned, the device may display a masked mode screen to indicate
a masked
mode is currently enabled. For example, masked mode screen 279 indicates that
the device
will operate in the masked mode, unless otherwise changed. In some instances,
the initial
configuration may be set up by the doctor or other health care profession for
the patient. In
certain configurations, the masked mode may only be deactivated by a health
care
professional. If the device is not operating in a masked mode, or after the
user confirms
the masked mode, then a warm up message screen 280 is displayed that indicates
that the
sensor is warming up. The remaining time for warm up may also be displayed. If
the user
confirms the message, for example by selecting the "ok" icon, then the device
may
navigate back to the home screen 281 and the home screen is displayed and
indicates the
sensor is warming up - e.g., by showing the remaining time until the sensor is
ready. If the
user attempts to perform a sensor scan as shown by block 282 - e.g., by
selecting the check
glucose icon, then the warm up message screen 281 is displayed again. In some
instances,
the home screen may be displayed after a predetermined time showing the warm
up
-47-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
message or the device will automatically navigate to the home screen 283 after
the sensor
is ready. In certain embodiments, the device will not display a message screen
showing "0
minutes" remaining.
Sensor Scan and Results
[0263] Exemplary embodiment of a graphical user interfaces which may be
utilized in
connection with a reader as described herein and which function to scan and to
provide
results are provided.
Sensor Scan Interface
[0264] FIG. 8 illustrates a method 300 for scanning a sensor with an analyte
monitoring
device, according to one embodiment. At block 301, the device is powered on. A
scan
prompt screen 302 is displayed and indicates to the user to scan the sensor to
check an
analyte level (e.g., glucose level).
[0265] In the embodiment shown, an icon or trigger element is provided on
screen 302 for
going to the home screen 303. If selected by the user, the home screen 303 is
displayed.
From the home screen 303, the user may initiate a sensor scan thereafter and
return to the
scan prompt screen 302.
[0266] Upon the start of the scan, the device waits (e.g., 15 seconds) for the
scan to
complete, as shown by blocks 304 and 305. If the wait period elapses and the
scan is not
yet complete, the device may display a screen informing the user that the scan
has timed
out 306. Upon completion of the scan, the device may perform an integrity
check of the
scan 307. If the device determines that the scan failed, the device may
display a screen
indicating that a scan error occurred, as shown at block 308 and instruct the
user to scan
the sensor again.
[0267] If the sensor is a new inactive sensor, as shown at block 309, then a
new sensor
detected screen 310 is shown and indicates that the sensor is a new sensor.
Screen 310
provides the user with an option to start the new sensor or not. If the user
elects to start the
new sensor, then the device initiates the sensor activation process, as shown
by block 312.
If the user elects to not start the new sensor, then the device displays the
home screen, as
shown by block 311.
[0268] If the device determines that the current sensor is inactive, as shown
at block 313,
then a check sensor screen 314 is displayed and indicates that there may be a
problem with
the sensor. The check sensor screen 314, in some embodiments, may suggest to
the user to
check if the sensor is loose or has fallen out. If the sensor is loose, the
loose sensor should
-48-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
be removed and a new sensor shall be paired. If the sensor is correctly
applied, then the
sensor is scanned again.
[0269] Referring to block 315, if the sensor has already been activated by
another device,
then a screen 316 indicating that the sensor is already paired with another
device is
displayed. Upon user confirmation, the home screen is displayed. In certain
embodiments,
when a sensor is already paired with another reader device, use of that sensor
with the new
reader device is not allowed.
[0270] The device may check the expiration information of the sensor as shown
at block
317, and if expired, e.g., a user tried to reuse an expired sensor, the device
may display a
sensor expired screen 318. From any of the scan error, replace sensor, and
sensor expired
screens, selecting "ok" may navigate the device back to the home screen to
start a new
sensor again.
[0271] If the device determines that the sensor in not functioning properly,
the device may
display a screen indicating to replace the sensor with a new sensor 271. If
the scan
succeeds and the sensor is found to be functioning, the device determines
whether the
sensor is to expire within a predetermined amount of time, such as within the
next 3 days,
as shown at block 321. It should be appreciated that the amount of time may
vary in other
embodiments, and in one embodiment check to see if the sensor is expired. It
should also
be appreciated that the predetermined amount of time may be preprogrammed in
manufacturing, and/or set within the settings, etc. If at block 321, it is
determined that the
sensor is expiring within the predetermined amount of time, then a sensor near
expiration
screen 322 is displayed to indicate the sensor is close to expiring. The
remaining time until
the sensor expires may be displayed, for example. In certain embodiments, the
remaining
time message is displayed after the first scan of the day for the last three
days of the sensor
life and the screen is shown after every scan for the last 8 hours before
sensor expiration.
The screen showing the remaining time until sensor expiration may be displayed
as days
remaining, when the length of time is 2 days or more, hours remaining, when
the length of
time is between 2 hours and 1 day, and minutes remaining, when the length of
time is less
than an hour, wherein the time remaining is rounded up as described above in
conjunction
with FIG. 6E. If the time remaining screen is still active when the time
remaining reaches
zero, the device may automatically navigate to the Sensor Results with no
active sensor
screen, as described herein below.
-49-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0272] If at block 321, it is determined that the sensor is not expiring
within the
predetermined amount of time, then it is determined whether the sensor battery
is low, as
represented at block 325. If the battery is low, then at block 326, the device
indicates that
the battery is low - e.g., via a low battery screen, or a low battery icon,
etc. In some
instances, a reading is not taken and no results are shown for the scan.
[0273] If the battery is not low, then at block 327, it is determined if the
skin temperature is
too hot or cold - e.g., using a "safe" range of temperatures. The sensor may
provide the
temperature data to the device. If the temperature is determined to be too hot
or cold, then
the device displays the resulting reading and indicates that the sensor
temperature was too
hot or too cold, respectively, as shown at block 328. If the skin temperature
is not too hot
or cold, then it is determined whether the quality of the data is acceptable,
as shown at
block 329. If the data quality checks fail, the device may display a sensor
error screen 330,
informing the user that the glucose reading is unavailable. In certain
embodiments, the
device may suggest to the user to rescan after a predetermined waiting period,
such as 10
minutes. Upon selection by the user of the "ok" confirmation element, the
device may
navigate back to the home screen 331.
[0274] As shown at block 332, it is determined whether the resulting reading
is out of
range. If the sensor is out of range, then it is determined if a high or low
glucose condition
has occurred. For example, a target range or acceptable range may be preset by
the
manufacturer or customizable by the user. If a high or low condition is
present, then the
user is taken to the Sensor Results screen, as shown at block 333, and the
high or low
condition may be indicated on the Sensor Results screen (e.g., via a message,
icon, etc.)
along with the sensor results.
[0275] Referring back to block 332, if it is determined that the sensor is not
out of range,
then it is determined if the glucose level was a high or low glucose level
334. For example,
a high or low glucose level may be preset by the manufacturer or customizable
by the user.
If a high or low glucose level is present, then the user is taken to the
Sensor Results
screen, as shown at block 335, and the sensor results may be displayed. If a
high or low
condition is not present, then it is determined whether a high or low
condition is projected,
as shown at block 336. A high or low condition may be projected, for example,
by trends
in the measurement readings. If a high or low condition is projected, then the
user is taken
to the Sensor Results screen and the projected high or low condition is
indicated on the
Sensor Results screen along with the sensor results, as shown at block 337.
-50-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0276] In some instances, the device is programmed such that the skin
temperature test
takes priority over other test conditions (e.g., out-of-range test, high/low
glucose test,
projected high/low glucose test, etc.) that may occur simultaneously.
Consecutive Scans Interface
[0277] An exemplary embodiment of a graphical user interface which may be
utilized in
connection with a reader as described herein and which functions to provide
navigation
when consecutive scans are present is provided.
Two Scans within Predetermined Period of Time
[0278] In some aspects, the analyte reader device is programmed to perform
specific
navigations when the device is scanned multiple times within a predetermined
period of
time.
[0279] FIG. 9A illustrates a method 350 for performing two scans within a
predetermined
period of time with the analyte reader, according to one embodiment. After the
first scan is
performed, a Sensor Results screen is displayed, as shown at block 352. If the
power is
turned off or the Sensor Results screen is left before a predetermined period
of time (e.g.,
3 minutes in the example embodiment shown), the results from the first scan
are saved, as
represented by block 354. If the device is powered back on, or another scan is
attempted,
within the predetermined period of time, the device will prevent the user from
performing
a new scan and display a No New Scan screen indicating that the a new sensor
reading is
not available at this time., as shown by blocks 356 and 358. The No New Scan
screen may
also indicate the time remaining until another scan, as well as, enable the
user to view the
last Sensor Results screen if desired, as represented by Sensor Results screen
362.
Device Timeout at the Suggest Dose Screen from BG Result
[0280] In some aspects, the analyte reader device is programmed to return to a
saved
suggested dose screen if the reader is powered off or leaves a
"Calculator/Suggested
Dose" screen when a dose has been calculated but not logged, and the device is
powered
back on or another scan is attempted within a predetermined period of time.
[0281] FIG. 9B illustrates a method 364 of logging a dose calculation,
according to one
embodiment. After a glucose reading is taken, a "Calculator/Suggested Dose"
(CALC
SGTD) screen 366 may be displayed to calculate and log a suggested dose of
insulin.
From screen 366, the user can adjust the suggested does if desired and log the
dose.
However, if within a predetermined period of time (e.g., 3 minutes in the
embodiment
-51-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
shown), the device is powered off, or screen 366 is left, after a suggested
dose has been
calculated but not logged, then the suggested dose data is saved, as shown at
block 368. If
the device is powered back on, or another glucose scan is attempted, within
the
predetermined period of time, as shown by block 370, then the user is returned
to the
CALC SGTD screen 372 having the saved suggested dose data. From here, the user
may
continue with the dose calculation and log the calculated dose. If a dose is
logged at this
point, the dose is stored within the Logbook along with the test results. In
one
embodiment, the dose is logged as having been taken at the time the test was
taken rather
than at the time the dose was actually logged. The insulin in body countdown,
however,
starts from the time at which the does is actually logged.
Suggested Dose from BG Result Logged
[0282] In some aspects of the present disclosure, after a first scan and
calculated insulin
dose logged, the device is programmed to prevent a second scan within a
predetermined
period of time from a first scan. The user will not be able to perform the
second scan, but
will be able to view the Logbook.
[0283] FIG. 9C illustrates a method 374 for enabling two consecutive scans,
according to
one embodiment. At block 376, the reader is powered off, or the Sensor Results
screen is
left, within a predetermined period of time after a first scan was taken
(e.g., 3 minutes in
the embodiment shown). If the device is powered back on, or another scan is
attempted,
within the predetermined period of time from the first scan, as shown at block
378, then
the second scan is prevented from occurring until the predetermined period of
time has
lapsed. A No New Scan screen 380 is displayed indicating that another scan is
not
currently permitted. In some instances, as shown, screen 380 provides the time
remaining
until another scan can be taken. In the embodiment shown, user confirmation
form screen
380 takes the user to the Home screen as shown at block 382. Screen 380 may
also
provide a selection to allow the user to view the last reading in the Logbook,
as shown at
block 384.
Sensor Results Interface
[0284] In some aspects of the present disclosure, a graphical user interface
is provided that
may be utilized in connection with a reader as described herein and which
functions
generally to provide sensor results.
-52-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Results
[0285] FIG. 10A illustrates an example Sensor Results screen 388, according to
one
embodiment. Screen 388 may be provided, for example, after a scan has been
successfully
performed. The example sensor results screen 388 is shown to include a sensor
reading
389a, a graph 389b of glucose readings taken, and various reader/sensor
related
information, such as a trending arrow, current time, battery life, sensor
expiration, etc. In
certain embodiments, time may be displayed in 24-hours format or 12-hour
(am/pm)
format. In certain embodiments, numerical glucose results may be displayed in
selectable
units, e.g. mg/dL as shown in FIG. 10A, or mmol/L as shown in FIG. 10L, or
others. A
trigger element 389c for navigating to the Notes interface is also provided to
take the user
to the Notes screen as shown by block 390. It should be appreciated that other
combinations of these, and other, features and trigger elements may be
included in other
embodiments.
[0286] In one embodiment, graph 389b does not display if the current glucose
value is
unavailable. Instead, a screen indicating that the glucose results are
unavailable is
displayed.
[0287] In one embodiment, graph 389b displays the past resulting readings for
a given time
period. The total time period may be sectionalized into a first predetermined
period of time
389d, and a second predetermined period of time 389f. The second predetermined
period
389f is subsequent to the first predetermined period of time 389d and includes
more recent
readings than the first predetermined period of time389d. For example, in the
embodiment
shown, at time 10:23pm, graph 389b displays readings in a first predetermined
time period
389d of 8 hours (e.g., 2pm to lOpm), and also displays more recent readings
within the
second predetermined time period 389f of 1 hour (e.g., lOpm to llpm). As graph
389b is
displayed at 10:23pm, readings for the last 23 minutes (e.g., lOpm to 10:23pm)
are shown
in the second predetermined time period 389f. As subsequent readings are
taken, graph
389b will track the readings within the second predetermined time period 389f.
Once
subsequent readings are taken for the entire second predetermined period of
time 389f, the
entire plot of readings are shifted in time by the second predetermined period
of time 389f
(e.g., 1 hour). In other words, once subsequent readings are obtained up to 1
lpm, the plot
of readings from 3pm to 1 lpm will shift to the first predetermined period of
time 389d,
and the second predetermined time period 389f will begin without any readings
and start
to track subsequent readings between 1 lpm and 12pm. Once subsequent readings
are
-53-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
obtained up to 12pm, then entire plot of readings are again shifted by the
second
predetermined period of time 389f (e.g., 1 hour), and the process repeats.
[0288] In one embodiment, when scanning is first started and no data has been
obtained for
the graph 389b, the graph 389b is not displayed until the device has obtained
sensor
readings for at least the first predetermined period of time 389d (e.g., 8
hours).
[0289] Thus, graph 389b begins with readings for at least the entire first
predetermined
period of time 389d. For example, in one embodiment, this threshold time
period is equal
to the first predetermined period of time 389d. In another embodiment, the
threshold time
period is longer than the first predetermined period of time such that sensor
readings have
been obtained for part or all of the second predetermined period of time.
[0290] It is appreciated that in other embodiments, a template of the graph
may be
displayed at first, but no sensor readings are shown on the graph until the
device has
obtained sensor readings for at least the first predetermined period of time
389d.
Non-Actionable Reading
[0291] FIG. 10B illustrates an example Results Non-Actionable screen 394,
according to
one embodiment. When a scan is performed and the resulting reading is non-
actionable, a
Results Non-Actionable screen 394 indicates to the user that the result is non-
actionable
and that the result should be confirmed. For example, a non-actionable result
is a reading
that is determined to be too high or too low for a therapy treatment,
decision, or
recommendation to be made - e.g., an insulin calculation. Screen 394 includes
a trigger
element for providing more information to the user regarding the non-
actionable reading.
When selected by the user, a Non-Actionable Message screen 396 is provided
with
additional details, recommendations, etc., such as recommending that the user
take a test
strip measurement before making any treatment decisions. User confirmation
(e.g., by
touching the "OK" button 396a) will return the user to the previously
displayed Results
screen. In certain embodiments, a Results Non-Actionable screen may also
include
additional icons as described below, such as a high glucose icon as
illustrated in FIG.
10M. FIG. 10M illustrates a display screen 394a including both a Results Non-
Actionable
icon and a High Glucose icon.
Results - Masked Mode
[0292] FIG. 10C illustrates an example Results - Masked screen 398, according
to one
embodiment. When a scan is performed while the device is set for the Masked
Mode, a
Results - Masked screen 398 is displayed and indicates to the user that the
reading was
-54-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
successful. Screen 398 does not provide the resulting reading to the user. The
resulting
reading is stored along with resulting readings from other scans in the
device. These stored
readings can later be accessed by a physician or other health care
professional at a later
time. For example, the physician or HCP may download the data during the next
patient
visit (e.g., via a wired or wireless connection between the reader and the
physician's or
HCP's computer,); or the data may be transferred via the intern& from the
reader (or
patient's PC) to the physician's or HCP's computer or server; etc.
Sensor Temperature (Too Hot / Too Cold)
[0293] FIG. 10D illustrates an interface for indicating that the sensor
temperature is too
high, according to one embodiment. The Results Sensor Temp High screen 402 is
displayed to indicate to the user that the sensor temperature is too high,
such as with
respect to a predetermined "safe" range of temperatures. For example, screen
402 may
have a warning message and/or icon indicating a high sensor temperature and
may further
indicate that a glucose reading is not available, as shown. Screen 402
provides a trigger
element 402a for providing additional information regarding the high sensor
temperature.
For example, when the user touches the touch-sensitive button 402a, another
screen 404 is
displayed to provide additional details or information, such as that the
sensor temperature
is too high and that the user should try again later.
[0294] FIG. 10E illustrates an interface for indicating that the sensor
temperature is too
low, according to one embodiment. The Results Sensor Temp Low screen 406 is
displayed
to indicate to the user that the sensor temperature is too low, such as with
respect to a
predetermined "safe" range of temperatures. For example, screen 406 may have a
warning
message and/or icon indicating a low sensor temperature and may further
indicate that a
glucose reading is not available, as shown. Screen 406 provides a trigger
element 406a for
providing additional information regarding the high sensor temperature. For
example,
when the user touches the touch-sensitive button 406a, another screen 408 is
displayed to
provide additional details or information, such as that the sensor temperature
is too high
and that the user should try again later.
Out of Range High/Low Readings
[0295] FIG. 1OF illustrates an interface for indicating that the sensor
reading is out of
range, according to one embodiment. The Results Sensor HI screen 412 is
displayed to
indicate to the user that the sensor reading is too high and out of range of
readings. Instead
of a sensor reading being displayed, screen 412 displays an indicator element
412a, such
-55-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
as a message, icon, symbol, etc. For example, in the embodiment shown, the
term "HI"
412a is shown in place of a sensor reading to indicate that the reading is out
of range and
too high.
[0296] The predetermined upper threshold reading value may be determined based
on a
predetermined number (e.g., 500 mg/dL), or may be determined relative to a
predetermined "acceptable" range of readings to display (e.g., 40 mg/dL to 500
mg/dL), or
with respect to a target range (e.g., 350 mg/dL over the target range), etc.
[0297] Screen 412 may also include a graph 412b of sensor readings that also
indicates a
target range 412D. Screen 412 provides a trigger element 412c for providing
additional
information, warning, instructions, etc., regarding the out of range and high
sensor
reading. For example, when the user touches the touch-sensitive button 412c,
another
screen 414 is displayed to provide additional details or information, such as
that the
glucose level is out of range high, that a high glucose level may be
dangerous, and that the
user should check again later and treat as recommended by their health care
professional.
Upon user confirmation (e.g., via selection of the "OK" button) the user is
taken back to
screen 412.
[0298] FIG. 10G illustrates an interface for indicating that the sensor
reading is out of
range, according to one embodiment. The Results Sensor LO screen 416 is
displayed to
indicate to the user that the sensor reading is out of range and too low.
Instead of a sensor
reading being displayed, screen 416 displays an indicator element 416a, such
as a
message, icon, symbol, etc. For example, in the embodiment shown, the term
"LO" 416a
is shown in place of a sensor reading to indicate that the reading is out of
range and too
low.
[0299] The predetermined lower threshold reading value may be determined based
on a
predetermined number (e.g., 40 mg/dL), or may be determined relative to a
determined
"acceptable" range of readings to display (e.g., 40 mg/dL to 500 mg/dL), or
with respect to
a target range (e.g., 20 mg/dL below the target range), etc.
[0300] Screen 416 may also include a graph 416b of sensor readings that also
indicates a
target range 416D. Screen 416 provides a trigger element 416c for providing
additional
information, warning, instructions, etc., regarding the out of range and low
sensor reading.
For example, when the user touches the touch-sensitive button 416c, another
screen 418 is
displayed to provide additional details or information, such as that the
glucose level is out
of range low, that a low glucose level may be dangerous, and that the user
should check
-56-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
again later and treat as recommended by their health care professional. Upon
user
confirmation (e.g., via selection of the "OK" button) the user is taken back
to screen 416.
High/Low Sensor Readings
[0301] FIG. 10H illustrates an interface for indicating that the sensor
reading is high,
according to one embodiment. The Results Sensor High screen 422 is displayed
to indicate
to the user that the sensor reading is a high reading, such as via a message,
icon, symbol,
etc., 422c. For example, in the embodiment shown, after a high reading is
obtained, screen
422 is displayed and the sensor reading 422a is shown. In addition to showing
the sensor
reading 422a, screen 422 includes a trigger element 422c for indicating the
high reading
and for accessing additional information, warning, instructions, etc.,
regarding the high
sensor reading. For example, when the user touches the touch-sensitive button
422c,
another screen 424 is displayed to provide additional details or information
about the high
reading, such as that the glucose level is high, that a high glucose level may
be dangerous,
and that the user should check again later and treat as recommended by their
health care
professional. In certain embodiments, the high reading screen may include
reminder
options. When a reminder on the high reading screen is selected, the reminder
will appear
on the reminder list as described below. The device will check if the reminder
list is full
prior to adding the new reminder. As described below, the reminders are only
saved upon
navigation back to the home screen from the results screen or when the device
times out
on the results screen. Upon user confirmation (e.g., via selection of the "OK"
button) the
user is taken back to screen 422.
[0302] The predetermined upper threshold reading value may be determined based
on a
predetermined number (e.g., 240 mg/dL), or may be determined relative to a
predetermined range of readings (e.g., 70mg/dL to 240 mg/dL), or with respect
to a target
range (e.g., 120 mg/dL over the target range), etc.
[0303] Screen 422 may also include a graph 422b of sensor readings that also
indicates a
target range 422d. In addition, screen 422 may include a distinguishing
element 422f for
identifying the high reading on the graph 422b - e.g., in the embodiment
shown, an
encircled dot 422f. Screen 422 may also include other trigger elements, such
as a trigger
element 422e for initiating the Notes interface.
[0304] FIG. 101 illustrates an interface for indicating that the sensor
reading is low,
according to one embodiment. The Results Sensor Low screen 426 is displayed to
indicate
to the user that the sensor reading is a low reading (e.g., with respect to a
predetermined
-57-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
"acceptable" reading range), such as via a message, icon, symbol, etc., 426c.
For example,
in the embodiment shown, after a low reading is obtained, screen 426 is
displayed and the
sensor reading 426a is shown. In addition to showing the sensor reading 426a,
screen 426
includes a trigger element 426c for indicating the low reading and for
accessing additional
information, warning, instructions, etc., regarding the low sensor reading.
For example,
when the user touches the touch-sensitive button 426c, another screen 428 is
displayed to
provide additional details or information about the low reading, such as that
the glucose
level is low, that a low glucose level may be dangerous, and that the user
should check
again later and treat as recommended by their health care professional. In
certain
embodiments, the low reading screen may include reminder options. When a
reminder on
the low reading screen is selected, the reminder will appear on the reminder
list as
described below. The device will check if the reminder list is full prior to
adding the new
reminder. As described below, the reminders are only saved upon navigation
back to the
home screen from the results screen or when the device times out on the
results screen.
Upon user confirmation (e.g., via selection of the "OK" button) the user is
taken back to
screen 426. In certain embodiments, the reminder times may be different for
low and high
glucose levels, for example, 20 minutes for low glucose and 2 hours for high
glucose.
Certainly, within the scope of the present disclosure, the low glucose
reminder time may
include other suitable times such as 15 minutes, 10 minutes, or 30 minutes,
while the high
glucose reminder time may include other suitable times such as one hour, 1.5
hours, 30
minutes and the like.
[0305] The predetermined lower threshold reading value may be determined based
on a
predetermined number (e.g., 70 mg/dL), or may be determined relative to a
predetermined
range of readings (e.g., 70mg/dL to 240 mg/dL), or with respect to a target
range (e.g., 10
mg/dL below the target range), etc.
[0306] Screen 426 may also include a graph 426b of sensor readings that also
indicates a
target range 426d. In addition, screen 426 may include a distinguishing
element 426f for
identifying the low reading on the graph 426b - e.g., in the embodiment shown,
an
encircled dot 426f. Screen 426 may also include other trigger elements, such
as a trigger
element 426e for initiating the Notes interface.
Projected High/Low Sensor Readings
[0307] FIG. 10J illustrates an interface for indicating that a high analyte
level is projected,
according to one embodiment. The Results Sensor Projected High screen 432 is
displayed
-58-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
to indicate to the user that a high analyte level is projected based on the
current sensor
reading and trend, such as via a message, icon, symbol, etc., 432c. For
example, in the
embodiment shown, after a sensor reading is obtained, screen 432 is displayed
and the
sensor reading 432a is shown. In addition to showing the sensor reading 432a,
screen 432
includes a trigger element 432c for indicating a projected high analyte level
and for
accessing additional information, warning, instructions, etc., regarding the
projected high
analyte level. For example, when the user touches the touch-sensitive button
432c, another
screen 434 is displayed to provide additional details or information about the
projected
high analyte level, such as that the glucose level is high, that a high
glucose level may be
dangerous, and that the user should check again later and treat as recommended
by their
health care professional. In certain embodiments, the projected high reading
screen may
include reminder options. When a reminder on the projected high reading screen
is
selected, the reminder will appear on the reminder list as described below.
The device will
check if the reminder list is full prior to adding the new reminder. As
described below, the
reminders are only saved upon navigation back to the home screen from the
results screen
or when the device times out on the results screen. Upon user confirmation
(e.g., via
selection of the "OK" button) the user is taken back to screen 432.
[0308] Projected high glucose readings may be based on the projected glucose
level of the
user within a predetermined upcoming period of time - e.g., within the next 15
minutes.
The predetermined upper threshold reading value may be determined based on a
predetermined number (e.g., 240 mg/dL), or may be determined relative to a
predetermined range of readings (e.g., 70mg/dL to 240 mg/dL), or with respect
to a target
range (e.g., 120 mg/dL over the target range), etc.
[0309] Screen 432 may also include a graph 432b of sensor readings that also
indicates a
target range 432d. In addition, screen 432 may include a distinguishing
element 432f for
identifying the projected high reading on the graph 432b - e.g., in the
embodiment shown,
an encircled dot 432f. Screen 432 may also include other trigger elements,
such as a
trigger element 432e for initiating the Notes interface.
[0310] FIG. 10K illustrates an interface for indicating that a low analyte
level is projected,
according to one embodiment. The Results Sensor Projected Low screen 436 is
displayed
to indicate to the user that a low analyte level is projected based on the
current sensor
reading and trend, such as via a message, icon, symbol, etc., 436c. For
example, in the
embodiment shown, after a sensor reading is obtained, screen 426 is displayed
and the
-59-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
sensor reading 436a is shown. In addition to showing the sensor reading 436a,
screen 436
includes a trigger element 436c for indicating the projected low analyte level
and for
accessing additional information, warning, instructions, etc., regarding the
projected low
analyte level. For example, when the user touches the touch-sensitive button
436c, another
screen 438 is displayed to provide additional details or information about the
projected
low analyte level, such as that the glucose level is low, that a low glucose
level may be
dangerous, and that the user should check again later and treat as recommended
by their
health care professional. In certain embodiments, the projected low reading
screen may
include reminder options. When a reminder on the projected low reading screen
is
selected, the reminder will appear on the reminder list as described below.
The device will
check if the reminder list is full prior to adding the new reminder. As
described below, the
reminders are only saved upon navigation back to the home screen from the
results screen
or when the device times out on the results screen. Upon user confirmation
(e.g., via
selection of the "OK" button) the user is taken back to screen 436.
[0311] Projected low glucose readings may be based on the projected glucose
level of the
user within a predetermined upcoming period of time - e.g., within the next 15
minutes.
The predetermined lower threshold reading value may be determined based on a
predetermined number (e.g., 70 mg/dL), or may be determined relative to a
predetermined
range of readings (e.g., 70mg/dL to 240 mg/dL), or with respect to a target
range (e.g., 10
mg/dL below the target range), etc.
[0312] Screen 436 may also include a graph 436b of sensor readings that also
indicates a
target range 436d. In addition, screen 436 may include a distinguishing
element 436f for
identifying the projected low reading on the graph 436b¨e.g., in the
embodiment shown,
an encircled dot 436f. Screen 436 may also include other trigger elements,
such as a
trigger element 436e for initiating the Notes interface.
Blood Glucose Test & Results
[0313] In some aspects of the present disclosure, a graphical user interface
is provided that
may be utilized in connection with a reader as described herein and which
functions
generally to provide perform an analyte test and provide test results.
Blood Glucose Strip Test Interface
[0314] FIG. 11 illustrates an example method 440 for performing a blood
glucose test with
a test strip, according to one embodiment. At block 442, a test strip is
inserted within an
-60-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
analyte monitoring device that is powered off. Once inserted into the device,
the device
powers on, as shown at block 444. The strip port light, such as a light
emitting diode for
example, turns on and provides light to the strip port, as shown at block 446.
At block 448,
it is determined whether a universal serial bus (USB) connection is
established with the
device. It should be appreciated that other communication technologies may be
implemented in other embodiments¨e.g., micro-USB, mini USB, RS-232, Ethernet,
Firewire, or other data communication connections. If a USB connection is
established,
then the strip test is prevented and a Strip Test Disabled screen 450 is
displayed to indicate
that the strip test is not permitted and that the user should unplug the
device to perform a
glucose test. If the test strip or the USB connection is removed, then the
Reader will
display the Home screen.
[0315] If at block 448, it is determined that a USB connection is not
established, then it is
determined if the inserted test strip is a blood glucose test strip or a
ketone test strip, as
shown at block 452. The test strip may include an identifying element, such as
a specific
contact configuration, to enable the device to identify what type of test
strip it is. If it is
determined that the test strip is a ketone test strip, then the device
initiates the Ketone Test
interface to perform a ketone test measurement, as shown by block 454. If, on
the other
hand, the test strip is determined to be a blood glucose test strip, then it
is determined if
the device temperature is outside of the device's operating range, as
represented by block
456 and reference path F.
[0316] If it is determined that the temperature is above or below the device's
operating
range, then the in certain embodiments, the port light is turned off and a
Strip/Hardware
Error screen 458 indicating that the operating temperature is too hot or too
cold is
displayed.
[0317] If at block 456, it is determined that the temperature is within the
device's operating
range, then the strip is checked for errors, including checking the strip for
damage or
incompatibility, as shown at block 460, or a check to see if blood was applied
to the strip
too soon or the strip was used, as shown at block 464. If the device
determines the strip is
damaged, incompatible or already used, the display may navigate to a
Strip/Hardware
Error screen 462, 466.
[0318] If there are no determined strip errors, an Add Blood interface 468 is
displayed to
indicate that blood may be applied to the test strip. If the test strip is
removed at this point,
as shown by block 470, then the port light turns off and the Home screen is
displayed, as
-61-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
represented by block 471. If at screen 468, blood is applied to the test
strip, as shown by
block 472, then it is determined if there was sufficient blood applied to
accurately perform
a test measurement, as shown by block 474. It should be appreciated that the
timing of this
determination may vary. If not enough blood is present, then the
Strip/Hardware Errors
interface is displayed to indicate that not enough blood was applied or that
there was an
error, as shown by block 476.
[0319] If it is determined that sufficient blood has been applied to the test
strip, then the
port light turns off, as shown by block 478, and a test measurement is
performed. A
Waiting interface 480 may be displayed while the test measurement is being
performed.
At block 482, it is determined if the results are ready. If ready, then the
Strips Test Results
screen 486 is displayed to indicate the resulting reading. If not ready (e.g.,
after a
predetermined timeout period), then the Strip/Hardware Errors screen 484 is
displayed to
indicate that there was an error in the measurement. In certain embodiments,
the device
may also perform a low batter test prior to displaying the results as
described above in
conjunction with FIG. 8.
[0320] Looking ahead to FIG. 34, an example Waiting interface according to one
embodiment is illustrated. Waiting interface 2400 is animated. For example,
the a first
screen 2402 includes a butterfly 2401 at the bottom left corner. As the next
screen 2404 is
displayed, the butterfly 2401 is located in a different position. Similarly,
at the next screen
2406, the butterfly 2401 is again moved to a different position. When
displayed
consecutively, an animated sequence results.
[0321] FIG. 34 also illustrates an example animation interface 2408 to
instruct the user to
add blood, according to one embodiment. Screens 2410, 2412, and 2414 display
an image
of a finger with blood on it and an analyte monitoring device with test strip
inserted into
the strip port. The finger and test strip move closer as the sequence of
screens 2410, 2412,
and 2414 progress to animate the application of blood process. Animation
interface 2408
may be displayed, for example, when the analyte monitoring device is ready to
receive
blood
[0322] FIG. 34 also illustrates an example animation interface 2415 to
instruct the user to
add blood during a ketone test, according to one embodiment. Similarly to
animation
interface 2408, screens 2416, 2418, and 2420 display an image of a finger with
blood on it
and an analyte monitoring device with test strip inserted into the strip port.
The finger and
test strip move closer as the sequence of screens 2416, 2418, and 2420
progress to animate
-62-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
the application of blood process. Animation interface 2408, however includes
an
indication that a Ketone test is being performed - e.g., displaying the words,
"Ketone
Test".
Blood Glucose Strip Test Results Interface
[0323] In some aspects of the present disclosure, a graphical user interface
is provided that
may be utilized in connection with a reader as described herein and which
functions
generally to provide blood glucose strip test results.
Results Screen
[0324] FIG. 12A illustrates an example blood glucose results (Results BG)
screen 490,
according to one embodiment. Screen 490 may be provided, for example, after a
test strip
measurement has been successfully performed. The example Results BG screen 490
is
shown to include a test strip measurement 490a and various analyte and device
related
information, such as indicator elements for a blood glucose strip test 490b,
current time
490d, battery life 490e, etc. A trigger element 490c for navigating to the
Notes interface is
also provided to take the user to the Notes screen as shown by block 494. It
should be
appreciated that other combinations of these, and other, features and trigger
elements may
be included in other embodiments.
[0325] For analyte monitoring devices with an active insulin calculator,
Screen 492 may be
provided, for example, after a test strip measurement has been successfully
performed.
The example Results BG screen 492 is shown to include a test strip measurement
492a and
various analyte and device related information, such as indicator elements for
a blood
glucose strip test 492b, current time 492d, battery life 492e, etc. A trigger
element 492c
for navigating to the Notes interface is also provided to take the user to the
Notes screen as
shown by block 494. A trigger element 492f for navigating to an Insulin
Calculation
interface is also provided as shown by block 496 to provide a calculated
insulin dose based
on the test measurement 492a. In certain embodiments, the Insulin Calculation
interface
may be an Advanced Calculator interface or an Easy Calculator interface, based
upon the
current settings of the device, as described in further detail below. It
should be appreciated
that other combinations of these, and other, features and trigger elements may
be included
in other embodiments.
-63-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Control Solution Screen
[0326] FIG. 12B illustrates an example interface for indicating the results of
a blood
glucose control solution test, according to one embodiment. The Results
Control Solution
(CTRL SLTN) screen 498 includes the results 498a of a blood glucose control
solution
test and various analyte and device related information, such as indicator
elements for
indicating the blood glucose control solution test 498b, indicating the
current time 498d,
indicating the battery life 498e, etc.
Reader Temperature Warning (Too Hot / Too Cold) Screen
[0327] FIG. 12C illustrates example interfaces for indicating that the analyte
monitoring
device's temperature is too high or too low, according to one embodiment.
Results BG
Temp High2/Low2 screen 502 is displayed to indicate to the user that the
device
temperature is either too high or too low to provide a reading, such as with
respect to a
predetermined "safe" range of temperatures to perform an accurate test
measurement. For
example, screen 502 may have a warning message and/or icon 502a indicating too
high or
too low of a temperature and may further indicate that a glucose reading is
not available,
as shown. Screen 502 provides a trigger element 502b for providing additional
information
regarding the too high or too low device temperature. For example, when the
user touches
the touch-sensitive button 502b, another screen 504 or 506 is displayed to
provide
additional details or information, such as that the device temperature is too
low or too
high, respectively, and that the user should try again later. The user can
confirm by
pressing the "OK" button and return to the previously displayed Results
screen, for
instance.
[0328] Results BG Temp Highl/Lowl screen 508 is an example interface that is
displayed
when a test measurement is available, but also indicates to the user that the
device
temperature is either high or low, such as with respect to a predetermined
"safe" range of
temperatures. For example, screen 508 displays the resulting test measurement
508a and
also displays a warning message and/or icon 502b indicating a high or low
device
temperature. Icon 502b also serves as a trigger element for providing
additional
information regarding the high or low device temperature. For example, when
the user
touches the touch-sensitive button 502b, another screen 512 or 510 is
displayed to provide
additional details or information, such as that the device temperature is too
low or too
high, respectively, and that the user should try again later. In one
embodiment, the device
-64-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
is programmed such that the temperature error takes precedence over other
notifications
that may be implemented.
Out of Range High/Low Screen
[0329] FIG. 12D illustrates an interface for indicating that the measurement
reading is out
of range, according to one embodiment. The Results BG HI screen 516 is
displayed to
indicate to the user that the sensor reading is too high and out of range of
readings. Instead
of a measurement reading being displayed, screen 516 displays an indicator
element 516a,
such as a message, icon, symbol, etc. For example, in the embodiment shown,
the term
"HI" 516a is shown in place of a measurement reading to indicate that the
reading is out of
range and too high.
[0330] The predetermined upper threshold reading value may be determined based
on a
predetermined number (e.g., 500 mg/dL), or may be determined relative to a
predetermined "acceptable" range of readings to display (e.g., 20 mg/dL to 500
mg/dL), or
with respect to a target range (e.g., 350 mg/dL over the target range), etc.
[0331] Screen 516 provides a trigger element 516 for providing additional
information,
warning, instructions, etc., regarding the out of range and high measurement
reading. For
example, when the user touches the touch-sensitive button 516b, another screen
518 is
displayed to provide additional details or information, such as that the
glucose level is out
of range high, that a high glucose level may be dangerous, and that the user
should check
again later and treat as recommended by their health care professional. Upon
user
confirmation (e.g., via selection of the "OK" button) the user is taken back
to screen 516.
[0332] FIG. 12E illustrates an interface for indicating that the measurement
reading is out
of range, according to one embodiment. The Results BG LO screen 520 is
displayed to
indicate to the user that the measurement reading is out of range and too low.
Instead of a
sensor reading being displayed, screen 520 displays an indicator element 520q,
such as a
message, icon, symbol, etc. For example, in the embodiment shown, the term
"LO" 520a
is shown in place of a measurement reading to indicate that the reading is out
of range and
too low.
[0333] The predetermined lower threshold reading value may be determined based
on a
predetermined number (e.g., 20 mg/dL), or may be determined relative to a
determined
"acceptable" range of readings to display (e.g., 20 mg/dL to 500 mg/dL), or
with respect to
a target range (e.g., 40 mg/dL below the target range), etc.
-65-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0334] Screen 520 provides a trigger element 520a for providing additional
information,
warning, instructions, etc., regarding the out of range and low measurement
reading. For
example, when the user touches the touch-sensitive button 520b, another screen
522 is
displayed to provide additional details or information, such as that the
glucose level is out
of range low, that a low glucose level may be dangerous, and that the user
should check
again later and treat as recommended by their health care professional. Upon
user
confirmation (e.g., via selection of the "OK" button) the user is taken back
to screen 520.
High/Low Glucose Screen
[0335] FIG. 12F illustrates an interface for indicating that the measurement
reading is high,
according to one embodiment. The Results BG High screen 526 is displayed to
indicate to
the user that the measurement reading is a high reading, such as via a
message, icon,
symbol, trigger element, etc., 526c. For example, in the embodiment shown,
after a high
reading is obtained, screen 526 is displayed and the measurement reading 526a
is shown.
In addition to showing the sensor reading 526a, screen 526 includes a trigger
element 526c
for indicating the high reading and for accessing additional information,
warning,
instructions, recommendations such as recommendation to consider checking
ketones, etc.,
regarding the high measurement reading. For example, when the user touches the
touch-
sensitive button 422c, another screen 528 is displayed to provide additional
details or
information about the high reading, such as that the glucose level is high,
that a high
glucose level may be dangerous, and that the user should check again later and
treat as
recommended by their health care professional. In certain embodiments, the
high glucose
level screen may include reminder options. When a reminder on the high glucose
level
screen is selected, the reminder will appear on the reminder list as described
below. The
device will check if the reminder list is full prior to adding the new
reminder. As described
below, the reminders are only saved upon navigation back to the home screen
from the
results screen or when the device times out on the results screen. Upon user
confirmation
(e.g., via selection of the "OK" button) the user is taken back to screen 526.
[0336] The predetermined upper threshold reading value may be determined based
on a
predetermined number (e.g., 240 mg/dL), or may be determined relative to a
predetermined range of readings (e.g., 70mg/dL to 240 mg/dL), or with respect
to a target
range (e.g., 120 mg/dL over the target range), etc.
[0337] Screen 526 may also include an indicator element (e.g., icon, symbol,
etc.) 526b that
indicates that the measurement reading pertains to a blood glucose strip test.
In the
-66-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
embodiment shown, a symbol of a blood drop 526b is used. Screen 526 may also
include
other trigger elements, such as a trigger element 526e for initiating the
Notes interface.
[0338] FIG. 12G illustrates an interface for indicating that the measurement
reading is low,
according to one embodiment. The Results BG Low screen 530 is displayed to
indicate to
the user that the measurement reading is a low reading (e.g., with respect to
a
predetermined "acceptable" reading range), such as via a message, icon,
symbol, trigger
element, etc., 530c. For example, in the embodiment shown, after a low reading
is
obtained, screen 530 is displayed and the measurement reading 530a is shown.
In addition
to showing the measurement reading 530a, screen 530 includes a trigger element
530c for
indicating the low reading and for accessing additional information, warning,
instructions,
etc., regarding the low sensor reading. For example, when the user touches the
touch-
sensitive button 530c, another screen 532 is displayed to provide additional
details or
information about the low reading, such as that the glucose level is low, that
a low glucose
level may be dangerous, and that the user should check again later and treat
as
recommended by their health care professional. In certain embodiments, the low
glucose
level screen may include reminder options. When a reminder on low high glucose
level
screen is selected, the reminder will appear on the reminder list as described
below. As
discussed above, in certain embodiments, the reminder times may be configured
to be
different for high and low glucose results ¨ for example, 15 minutes for low
glucose and 1
or 2 hours for high glucose. The device will check if the reminder list is
full prior to
adding the new reminder. As described below, the reminders are only saved upon
navigation back to the home screen from the results screen or when the device
times out
on the results screen. Upon user confirmation (e.g., via selection of the "OK"
button) the
user is taken back to screen 530.
[0339] The predetermined lower threshold reading value may be determined based
on a
predetermined number (e.g., 70 mg/dL), or may be determined relative to a
predetermined
range of readings (e.g., 70mg/dL to 240 mg/dL), or with respect to a target
range (e.g., 10
mg/dL below the target range), etc.
[0340] Screen 530 may also include an indicator element (e.g., icon, symbol,
etc.) 530b that
indicates that the measurement reading pertains to a blood glucose strip test.
In the
embodiment shown, a symbol of a blood drop 530b is used. Screen 530 may also
include
other trigger elements, such as a trigger element 530e for initiating the
Notes interface.
-67-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Masked Mode
[0341] When the device is in masked mode, the results of a blood glucose
control solution
test or blood glucose test strip results may be masked on the display. FIGS.
12H and 121
illustrate display screens for masked results of a blood glucose control
solution test and
blood glucose test strip results, respectively.
Ketone Test & Results
[0342] In some aspects of the present disclosure, a graphical user interface
is provided that
may be utilized in connection with a reader as described herein and which
functions
generally to provide perform an ketone strip test measurement and provide test
results.
Ketone Strip Test Interface
[0343] FIG. 13 illustrates an example method 534 for performing a ketone test
with a
ketone test strip, according to one embodiment. At block 536, a ketone test
strip is inserted
within an analyte monitoring device that is powered off Once inserted into the
device, the
device powers on, as shown at block 538. The strip port light, such as a light
emitting
diode for example, turns on and provides light to the strip port, as shown at
block 540. At
block 542, it is determined whether a universal serial bus (USB) connection is
established
with the device. Again, it should be appreciated that other communication
technologies
may be implemented in other embodiments - e.g., micro-USB, mini USB, RS-232,
Ethernet, Firewire, or other data communication connections. If a USB
connection is
established, then the strip test is prevented and a Strip Test Disabled screen
544 is
displayed to indicate that the strip test is not permitted and that the user
should unplug the
device to perform a ketone or blood glucose test. If the test strip or the USB
connection is
removed, then the Reader will display the Home screen.
[0344] If at block 542, it is determined that a USB connection is not
established, then it is
determined if the inserted test strip is a blood glucose test strip or a
ketone test strip, as
shown at block 546. The test strip may include an identifying element, such as
a specific
contact configuration, to enable the device to identify what type of test
strip it is. If it is
determined that the test strip is a blood glucose test strip, then the device
initiates the
Blood Glucose Strip Test interface to perform a blood glucose test
measurement, as shown
by block 548. If, on the other hand, the test strip is determined to be a
ketone test strip,
then it is determined if the device temperature is outside of the device's
operating range,
as represented by block 550 and reference path H. If it is determined that the
temperature
-68-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
is above or below the device's operating range, then the port light is turned
off, and a
Strip/Hardware Error screen 551 indicating that the operating temperature is
too hot or too
cold is displayed.
[0345] If at block 550, it is determined that the temperature is within the
device's operating
range, then the strip is checked for errors, including checking the strip for
damage or
incompatibility, as shown at block 552, or a check to see if blood was applied
to the strip
too soon or the strip was used, as shown at block 554. If the device
determines the strip is
damaged, incompatible or already used, the display may navigate to a
Strip/Hardware
Error screen 553, 555.
[0346] If there are no determined strip errors, a P K Add Blood interface 556
is displayed to
indicate that blood may be applied to the test strip. If the test strip is
removed at this point,
as shown by block 558, then the port light turns off and the Home screen is
displayed, as
represented by block 560. If at screen 556, blood is applied to the test
strip, as shown by
block 564, then it is determined if there was sufficient blood applied to
accurately perform
a test measurement, as shown by block 566 and reference path I. It should be
appreciated
that the timing of this determination may vary. If not enough blood is
present, then the
Strip/Hardware Errors interface is displayed to indicate that not enough blood
was applied
or that there was an error, as shown by block 568.
[0347] If it is determined that sufficient blood has been applied to the test
strip, then a
Waiting interface 570 is displayed while a test measurement is being
performed. At block
572, it is determined if the results are ready. If ready, then the Strips Test
Results screen
576 is displayed to indicate the resulting reading. If not ready (e.g., after
a predetermined
timeout period), then the Strip/Hardware Errors screen 574 is displayed to
indicate that
there was an error in calculating a measurement. In certain embodiments, the
device may
also perform a low battery test prior to displaying the results as described
above in
conjunction with FIG. 8.
Ketone Strip Test Results Interface
[0348] In some aspects of the present disclosure, a graphical user interface
is provided that
may be utilized in connection with a reader as described herein and which
functions
generally to provide ketone strip test results.
Results Screen
[0349] FIG. 14A illustrates an example Ketone Results screen 580, according to
one
embodiment. Screen 580 may be provided, for example, after a ketone test strip
-69-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
measurement has been successfully performed. The example Ketone Results screen
580 is
shown to include a ketone test strip measurement 580a and various analyte and
device
related information, such as indicator elements for indicating the ketone
strip test 580b,
indicating the current time 580d, indicating the battery life 580e, etc. In
one embodiment,
the indicator element 580b for identifying the ketone test strip measurement
is the same
symbol as the indicator element for a blood glucose test strip measurement. In
such case,
the indicator element 580b is generally indicating a test strip measurement
result versus a
sensor reading result. It should be appreciated that in other embodiments,
different
indicator elements may be used for the ketone strip test and the blood glucose
strip test to
distinguish between the two tests.
[0350] A trigger element 580c for navigating to the Notes interface is also
provided to take
the user to the Notes screen as shown by block 582. It should be appreciated
that other
combinations of these, and other, features and trigger elements may be
included in other
embodiments.
[0351] In one embodiment, the insulin calculator is enabled if the setting is
enabled and if
both of the following are met: 1) the ketone test has been performed within a
predetermined period of time (e.g., 15 minutes) of successful strip test or
another
predetermined period of time (e.g., 3 minutes) of a successful sensor test;
and 2) insulin
was not logged with that recent glucose test. Unless both conditions are met,
the insulin
calculator button is not provided.
Control Solution Screen
[0352] FIG. 14B illustrates an example interface for indicating the results of
a ketone
control solution test, according to one embodiment. The Ketone Control
Solution Results
screen 584 includes the results 584a of a ketone control solution test and
various analyte
and device related information, such as indicator elements for indicating a
control solution
test 584b, indicating the current time 584d, indicating the battery life 584e,
etc. In one
embodiment, the indicator element 584b for identifying the ketone control
solution test is
the same symbol as the indicator element for a blood glucose control solution
test. In such
case, the indicator element 584b is generally indicating a control solution
test. It should be
appreciated that in other embodiments, different indicator elements may be
used for the
ketone control solution test and the blood glucose control solution test to
distinguish
between the two tests.
-70-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Reader Temperature Warning (Too Hot / Too Cold) Screen
[0353] FIG. 14C illustrates example interfaces for indicating that the analyte
monitoring
device's temperature is too high or too low, according to one embodiment.
Results Ketone
Temp High2/Low2 screen 588 is displayed to indicate to the user that the
device
temperature is either too high or too low to provide a ketone reading, such as
with respect
to a predetermined "safe" range of temperatures to perform an accurate ketone
test
measurement. For example, screen 588 may have a warning message and/or icon
588a
indicating too high or too low of a temperature and may further indicate that
a ketone
reading is not available, as shown. Screen 588 provides a trigger element 588b
for
providing additional information regarding the too high or too low device
temperature. For
example, when the user touches the touch-sensitive button 588b, another screen
590 or
592 is displayed to provide additional details or information, such as that
the device
temperature is too low or too high, respectively, and that the user should try
again later.
[0354] Results Ketone Temp Highl/Lowl screen 594 is an example interface that
is
displayed when a ketone test measurement is available, but also indicates to
the user that
the device temperature is either high or low, such as with respect to a
predetermined
"safe" range of temperatures. For example, screen 594 displays the resulting
test
measurement 594a and also displays a warning message and/or icon 594b
indicating a
high or low device temperature. Icon 502b also serves as a trigger element for
providing
additional information regarding the high or low device temperature. For
example, when
the user touches the touch-sensitive button 502b, another screen 598 or 596 is
displayed to
provide additional details or information, such as that the device temperature
is too low or
too high, respectively, and that the user should try again later.
Out of Range High Screen
[0355] FIG. 14D illustrates an interface for indicating that the measurement
reading is out
of range, according to one embodiment. The Ketone Results Out of Range High
screen
602 is displayed to indicate to the user that the ketone measurement reading
is too high
and out of range of readings. Instead of a ketone measurement reading being
displayed,
screen 602 displays an indicator element 602a, such as a message, icon,
symbol, etc. For
example, in the embodiment shown, the term "HI" 602a is shown in place of a
measurement reading to indicate that the reading is out of range and too high.
-71-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0356] The predetermined upper threshold reading value may be determined based
on a
predetermined number, or may be determined relative to a predetermined
"acceptable"
range of readings to display, or with respect to a target range, etc.
[0357] Screen 602 provides a trigger element 602b for providing additional
information,
warning, instructions, etc., regarding the out of range and high ketone
measurement
reading. For example, when the user touches the touch-sensitive button 602b,
another
screen 604 is displayed to provide additional details or information, such as
that the ketone
level is out of range high, that a high ketone level may be dangerous, and
that the user
should check again later and treat as recommended by their health care
professional. Upon
user confirmation (e.g., via selection of the "OK" button) the user is taken
back to screen
602.
High Ketones Screen
[0358] FIG. 14E illustrates an interface for indicating that the ketone
measurement reading
is high, according to one embodiment. The Ketone Result - High Ketones screen
608 is
displayed to indicate to the user that the ketone measurement reading is a
high reading,
such as via a message, icon, symbol, trigger element, etc., 608c.
[0359] The predetermined upper threshold reading value may be determined based
on a
predetermined number, or may be determined relative to a predetermined range
of
readings, or with respect to a target range, etc.
[0360] In the embodiment shown, after a high ketone reading is obtained,
screen 608 is
displayed and the measurement reading 608a is shown. In addition to showing
the sensor
reading 608a, screen 608 includes a trigger element 608c for indicating the
high ketone
reading and for accessing additional information, warning, instructions, etc.,
regarding the
high measurement reading. For example, when the user touches the touch-
sensitive button
608c, another screen 610 or 612 is displayed to provide additional details or
information
about the high reading, such as that the ketone level is high, that a high
ketone level may
be dangerous, and that the user should check again later and treat as
recommended by their
health care professional. In the embodiment shown, screen 610 is displayed if
the ketone
reading is between a predetermined range (e.g., .6 to 1.5) and the second
screen 612 is
displayed if the ketone reading is between a high predetermined range (e.g.,
1.6 to 8).
[0361] Screen 608 may also include an indicator element (e.g., icon, symbol,
etc.) 608b that
indicates that the measurement reading pertains to a strip test measurement
(e.g., ketone
test strip measurement). In the embodiment shown, a symbol of a blood drop
608b is used.
-72-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Screen 608 may also include other trigger elements, such as a trigger element
608e for
initiating the Notes interface.
Masked Mode
[0362] When the device is in masked mode, the results of a Ketone control
solution test or
Ketone test strip results may be masked on the display. FIGS. 14F and 14G
illustrate
display screens for masked results of a Ketone control solution test and
Ketone test strip
results, respectively.
Notes Interface
[0363] An exemplary embodiment of a graphical user interface which may be
utilized in
connection with a Reader as described herein and which facilitates a Notes
procedure 3000
for entering notes into the logbook is provided. This graphical user interface
is now
described in greater detail with reference to FIG. 15.
[0364] A graphical user interface which facilitates the Notes procedure 3000
of the reader
may include two different notes interfaces; a first notes interface for
embodiments where
the insulin calculator is disabled 3002, and a second notes interface for
embodiments
where the insulin calculator is enabled 3004. The Note (Insulin Calculator
Disabled)
Interface 3002, shown in FIG. 15A, begins with the display of an "Add to
Logbook"
screen 3006. This "Add to Logbook" screen 3006 includes a list of several
different notes
that may be entered into the Logbook. The list of user selectable notes may
include default
(e.g., preset notes) and, in some instances, custom (e.g., user-defined)
notes. For example,
the default notes may include one or more of the following: rapid-acting
insulin 3008,
long-acting insulin 3010, food 3012, exercise 3014, and medication 3016. The
custom
notes may include any notes generated by the user through the associated
informatics
software. In some cases, 12 notes may be included in the list of user-
selectable notes. For
example, 5 default notes and 7 custom notes may be included. In certain
embodiments, 4
user-selectable notes are displayed on the touchscreen at once. If there are
more than 4
user-selectable notes, the list of notes may be scrolled as necessary to view
the list using
scroll touchscreen buttons, such as down arrow (e.g., down triangle)
touchscreen button
3018 and up arrow (e.g., up triangle) touchscreen button 3020. One or more
user-
selectable notes may be selected by touching the touchscreen checkbox adjacent
the note
desired to be selected. Touching a touchscreen checkbox will toggle the
checkbox from a
checked to unchecked state indicating whether the associated not is selected
or not
-73-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
selected, respectively. For instance, the rapid-acting insulin note may be
selected by
touching the touchscreen checkbox 3022, which then displays a check mark in
the
touchscreen checkbox to indicate that the rapid-acting insulin note has been
selected. The
other user-selectable notes may be selected or unselected (e.g., checked or
unchecked) as
desired in an analogous manner.
[0365] The selection of notes may be saved by touching the "OK" touchscreen
button 3024,
which saves the selection of user-selectable notes and returns the graphical
user interface
to the previous screen. If the "OK" touchscreen button 3024 is pressed without
changing
the selection of user-selectable notes, then the previous selection of user-
selectable notes is
retained and saved.
[0366] In some embodiments, the amount of rapid-acting insulin, long-acting
insulin, food,
exercise, and medication may be entered by touching the numerical input
touchscreen
button (e.g., the "1 2 3" touchscreen button) associated with the desired
selection. In some
cases, the numerical input touchscreen button may not be displayed if the
corresponding
touchscreen checkbox is not checked, and may only be displayed if the
corresponding
touchscreen checkbox is checked. For example, when rapid-acting insulin
checkbox 3008
is unchecked, the numerical input touchscreen button 3026 for rapid-acting
insulin is not
displayed. When the rapid-acting insulin checkbox is checked 3022, the
numerical input
touchscreen button 3026 for rapid-acting insulin is displayed and may be
selected.
[0367] The amount of rapid-acting insulin may be entered by pressing the
numerical input
touchscreen button 3026 associated with the rapid-acting insulin note
selection. Pressing
the numerical input touchscreen button 3026 for rapid-acting insulin will
cause a
numerical input screen 3028 for rapid acting insulin (e.g., the "Enter Rapid-
Acting
Insulin" screen) to be displayed, as shown by reference path (J) (see FIG.
15B). In the
numerical input screen 3028 for rapid-acting insulin, the amount (e.g., units)
of rapid-
acting insulin may be entered. The numerical input screen initially displays
no value (e.g.,
"- 2 is displayed in place of any numbers), however, the amount of rapid-
acting insulin
may be adjusted by pressing the up arrow 3030 (e.g., "+") touchscreen button
or the down
arrow 3032 (e.g., "-") touchscreen button to increase or decrease,
respectively, the amount
of rapid-acting insulin as desired. For example, the amount of rapid-acting
insulin 3034
may be adjusted by 1 unit increments by tapping the up arrow 3030 or the down
arrow
3032. In some embodiments, the amount increases or decreases as appropriate at
a slow-
rate for the first 2 seconds and at a faster rate after 2 seconds when the up
or down arrow
-74-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
is pressed and held. Once the desired amount of rapid-acting insulin is
entered, the amount
may be saved by pressing the "OK" touchscreen button 3036, which saves the
entered
amount of rapid-acting insulin and then returns the graphical user interface
to the "Add to
Logbook" screen 3006, as shown by reference path (J). Numerical values for
long-acting
insulin may be entered in an analogous manner by pressing the numerical input
touchscreen button 3038 (e.g., the "1 2 3" touchscreen button) associated with
the long-
acting insulin selection on the "Add to Logbook" screen 3006.
[0368] Numerical values for food may be entered by pressing the numerical
input
touchscreen button 3040 (e.g., the "1 2 3" touchscreen button) associated with
the food
selection on the "Add to Logbook" screen 3006. Pressing the numerical input
touchscreen
button 3040 for food will cause a numerical input screen 3042 for
carbohydrates to be
displayed (e.g., the "Enter Carbs" screen), as shown by reference path (J)
(see FIG. 15C).
In the numerical input screen 3042 for food, the amount (e.g., grams) of carbs
may be
entered. The numerical input screen initially displays no value (e.g., "- 2 is
displayed in
place of any numbers), however, the amount of carbs may be adjusted by
pressing the up
arrow 3044 (e.g., "+") touchscreen button or the down arrow 3046 (e.g., "-")
touchscreen
button to increase or decrease, respectively, the amount of carbs as desired.
For example,
the amount of carbs 3048 may be adjusted by 1 gram increments by tapping the
up arrow
3044 or the down arrow 3046. In some embodiments, the amount increases or
decreases as
appropriate at a slow-rate for the first 2 seconds and at a faster rate after
2 seconds when
the up or down arrow is pressed and held. Once the desired amount of carbs is
entered, the
amount may be saved by pressing the "OK" touchscreen button 3050, which saves
the
entered amount of carbs and then returns the graphical user interface to the
"Add to
Logbook" screen 3006, as shown by reference path (J).
[0369] In certain embodiments, instead of entering the amount of food as grams
of carbs,
the amount of food may be entered as servings of carbs, as shown in FIG. 15E.
In the
numerical input screen 3068 for servings of carbs, the screen initially
displays no value
(e.g., "- 2 is displayed in place of any numbers), however, the servings of
carbs may be
adjusted by pressing the up arrow 3070 (e.g., "+") touchscreen button or the
down arrow
3072 (e.g., "-") touchscreen button to increase or decrease, respectively, the
servings of
carbs as desired. For example, the servings of carbs 3074 may be adjusted by
0.5 serving
increments by tapping the up arrow 3070 or the down arrow 3072. In some
embodiments,
the amount increases or decreases as appropriate at a slow-rate for the first
2 seconds and
-75-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
at a faster rate after 2 seconds when the up or down arrow is pressed and
held. In some
cases, the numerical input screen 3068 for servings of carbs may include a
display of the
grams of carbs 3076 that corresponds to the number of servings entered. In
some cases, the
numerical input screen 3068 for servings of carbs may include a display of the
energy
(e.g., kcal) of carbs 3078 that corresponds to the number of servings entered.
Once the
desired servings of carbs is entered, the value may be saved by pressing the
"OK"
touchscreen button 3080, which saves the entered servings of carbs and then
returns the
graphical user interface to the "Add to Logbook" screen 3006.
[0370] Referring back to the numerical input screen 3042 for the amount (e.g.,
grams) of
carbs (see FIG. 15C), the numerical input screen 3042 also includes two
touchscreen
checkboxes configured to allow the selection of reminders to check a user's
glucose. For
example, the numerical input screen 3042 includes a "1 hr" touchscreen
checkbox 3052
and a "2 hr" touchscreen checkbox 3054. None, one or both of the touchscreen
checkboxes may be selected as desired. Pressing the "1 hr" touchscreen
checkbox 3052
will display a check mark in the "1 hr" touchscreen checkbox 3052 indicating
that the "1
hr" touchscreen checkbox 3052 has been selected and will set the Reader to
alert the user
to check the user's glucose level after 1 hour. Pressing the "2 hr"
touchscreen checkbox
3054 will display a check mark in the "2 hr" touchscreen checkbox 3054
indicating that
the "2 hr" touchscreen checkbox 3054 has been selected and will set the Reader
to alert
the user to check the user's glucose level after 2 hours. The check glucose
reminder
checkboxes may also be displayed on the numerical input screen 3068 for
entering the
servings of carbs as described above (see FIG. 15E).
[0371] As indicated above, a second notes interface may be provided for
embodiments
where the insulin calculator is enabled 3004. The Note (Insulin Calculator
Enabled)
Interface 3004, shown in FIG. 15D, begins with the display of an "Add to
Logbook"
screen 3056. This "Add to Logbook" screen 3056 includes a list of several
different notes
that may be entered into the Logbook, similar to the "Add to Logbook" screen
3006 in the
Note (Insulin Calculator Disabled) Interface 3002 described above. In the "Add
to
Logbook" screen 3056 of the Note (Insulin Calculator Enabled) Interface 3004,
the user-
selectable notes (e.g., default and custom notes) may be selected (e.g.,
checked) or
unselected (e.g., unchecked) as desired in an analogous manner as described
above in
relation to the Note (Insulin Calculator Disabled) Interface 3002. In
addition, the
numerical input touchscreen buttons (e.g., the "1 2 3" touchscreen buttons) of
the Note
-76-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
(Insulin Calculator Enabled) Interface 3004 function in an analogous manner as
described
above in relation to the Note (Insulin Calculator Disabled) Interface 3002.
[0372] In the Note (Insulin Calculator Enabled) Interface 3004, the insulin on
board and
rapid-acting insulin calculator features of the graphical user interface are
enabled. For
instance, in the Note (Insulin Calculator Enabled) Interface 3004, rather than
displaying a
numerical input touchscreen button for rapid-acting insulin, a calculator
touchscreen
button 3058 is displayed with the rapid-acting insulin selection. Pressing the
calculator
touchscreen button 3058 will cause the graphical user interface to display the
Insulin on
Board Interface 3060 (see FIG.16) and the Insulin Calculator Interface 3062
(see FIG. 17).
In some embodiments, if food information (e.g., amount of carbs) is entered as
described
above, then when the calculator touchscreen button 3058 is pressed, the amount
of carbs
that was previously entered will automatically be displayed in the
corresponding insulin
on board and calculator interface screens.
[0373] In certain embodiments, the Notes - From Logbook Entry Interface 3064
may be
displayed from the Logbook Entry screen if the "add or edit notes" touchscreen
button is
pressed. The Notes - From Logbook Entry Interface 3064 begins with the display
of an
"Add to Logbook" screen 3066. This "Add to Logbook" screen 3066 includes a
list of
several different user-selectable notes that may be entered into the Logbook,
similar to
user-selectable notes described above. In certain embodiments, the "Add to
Logbook"
screen 3066 of the Notes - From Logbook Entry Interface 3064 does not include
a user-
selectable note for rapid-acting insulin or food. The other default notes
(e.g., long-acting
insulin, exercise, medication) and custom notes may still be available for
selection by the
user as desired.
Insulin on Board Interface
[0374] As described above, pressing the calculator touchscreen button 3058
from the "Add
to Logbook" screen 3056 of the Notes (Insulin Calculator Enabled) Interface
3004, will
cause the graphical user interface to display the Insulin on Board Interface
3060 (see
FIG.16). Regarding FIG. 16A, in certain embodiments, a user's insulin on board
(I0B)
information is used in the calculation of a recommended rapid-acting insulin
dosage
amount. For example, if an insulin dosage has been logged (e.g., logged as a
calculated
dose, or entered into the logbook as a rapid-acting dose), then the insulin
dosage
-77-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
information may be used in the calculation of a recommended rapid-acting
insulin dosage
amount 3082.
[0375] In certain embodiments, a user's insulin on board information is used
in the
calculation of a recommended rapid-acting insulin dosage amount if the user's
most recent
insulin dose was administered within a certain time period. In some instances,
the insulin
calculator may be partially locked out if the difference between the current
time and the
time the most recent rapid-acting insulin was administered is less than a lock
out time
period (e.g., the most recent insulin dose was administered within a preceding
lockout
time period, such as within the past 2 hours). During the lockout time period,
the insulin
calculator may be programmed to only calculate a meal bolus and may not
calculate an
additional correction bolus 3084. During the lockout time period, the insulin
calculator
may not include insulin on board into the calculation of a meal bolus.
[0376] If the difference between the current time and the time the most recent
insulin bolus
was administered is greater than a threshold time period (e.g., the lockout
time period) and
less than the duration of insulin action, then the insulin calculator may be
programmed to
include the user's IOB into the calculation of the recommended rapid-acting
insulin
dosage amount 3086. In the time period between the end of the lockout time
period and
the end of the user's duration of insulin action, the insulin calculator may
be programmed
to determine the recommended rapid-acting insulin dosage amount based on the
determined analyte concentration and the insulin on board information. For
instance, in the
time period between the end of the lockout time period and the end of the
user's duration
of insulin action, the insulin calculator may be programmed to subtract the
user's IOB
from the rapid-acting insulin dosage based upon the current glucose
concentration level to
determine the recommended rapid-acting insulin dosage amount.
[0377] In certain instances, if the difference between the current time and
the time the most
recent insulin bolus was administered is greater than the user's duration of
insulin action,
then the insulin calculator will not include insulin on board into the
calculation of a
recommended rapid-acting insulin dosage amount 3088. In the time period after
the user's
duration of insulin action has expired (and before the next dose of insulin is
administered),
the insulin calculator may assume the user's insulin on board is zero. In the
time period
after the user's duration of insulin action has expired (and before the next
dose of insulin
is administered), the insulin calculator may be programmed to determine the
rapid-acting
-78-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
insulin dosage amount based on the determined glucose concentration (without
including
the insulin on board information).
[0378] Regarding FIG. 16B, the Insulin on Board Interface is shown, and begins
with a
prompt screen 3090 prompting the user with a question asking if the user has
forgotten to
log any rapid-acting insulin the user has taken since a prior predetermined
time point. The
prior predetermined time point corresponds to the most recent of either: (a)
the current
time minus the set insulin duration of action, or (b) the time of the last
logged dose of
rapid-acting insulin. The prompt screen 3090 has a "Yes" touchscreen button
3092 and a
"No" touchscreen button 3094 to select a yes or no answer to the prompted
question. If the
"No" touchscreen button 3094 is pressed, then the graphical user interface
displays the
Insulin Calculator Interface 3062 (see FIG. 17). If the "Yes" touchscreen
button is pressed,
then the graphical user interface displays a numerical input screen 3096 in
the Insulin on
Board Interface. On the numerical input screen 3096, the amount of rapid-
acting insulin
that the user has forgotten to log since the prior predetermined time point
may be entered.
The amount (e.g., units) of rapid-acting insulin may be adjusted by pressing
the up arrow
3098 (e.g., "+") touchscreen button or the down arrow 3100 (e.g., "-")
touchscreen button
to increase or decrease, respectively, the amount of rapid acting insulin as
desired. For
example, the amount of rapid acting insulin 3102 may be adjusted by 1 unit
increments by
tapping the up arrow 3098 or the down arrow 3100. In some embodiments, the
amount of
rapid-acting insulin may be adjusted by 0.5 unit increments (not shown) if the
calculator is
set to allow half-unit increments. In some embodiments, the amount increases
or decreases
as appropriate at a slow-rate for the first 2 seconds and at a faster rate
after 2 seconds
when the up or down arrow is pressed and held. Numerical input screen may also
include a
"?" touchscreen button 3104, which, when pressed, provides a help screen 3106
that
includes additional instructions to the user. For example, the help screen
3106 may instruct
the user to enter the sum total of insulin units that the user has taken over
the last X hours
that the user has not already logged, where X is the insulin duration of
action time. The
help screen 3106 may also inform the user that the Reader will use the entered
amount of
rapid-acting insulin to calculate the user's next rapid-acting insulin dose.
The help screen
3106 includes an "OK" touchscreen button 3108, which, when pressed, returns
the display
to the numerical input screen 3096.
[0379] On the numerical input screen 3096, once the desired amount of rapid-
acting insulin
has been entered, the "Next" touchscreen button 3110 may be pressed to advance
the
-79-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
graphical user interface to a time input screen 3114, as shown by reference
path (M) (see
FIG. 16C). Alternatively, on the numerical input screen 3096, the "Back"
touchscreen
button 3112 may be pressed, which returns the graphical user interface to the
prompt
screen 3090.
[0380] Regarding FIG. 16C, on the time input screen 3114, the display presents
a question
asking the user how long ago was the unlogged dose of rapid-acting insulin. On
the time
input screen 3114, the time since the last unlogged rapid-acting insulin dose
may be
adjusted by pressing the up arrow 3116 (e.g., "+") touchscreen button or the
down arrow
3118 (e.g., "-") touchscreen button to increase or decrease, respectively, the
time as
desired. For example, the time 3120 may be adjusted by 15 minute increments by
tapping
the up arrow 3116 or the down arrow 3118. In some embodiments, the amount
increases
or decreases as appropriate at a slow-rate for the first 2 seconds and at a
faster rate after 2
seconds when the up or down arrow is pressed and held. In some instances, the
default
time is 15 minutes (e.g., displayed as "15 minutes or less"). In some cases,
the maximum
time is the time value set for the insulin duration of action. If the user
presses the up arrow
3116 once while the time is at the maximum value, then the time displayed may
be shown
as "[insulin duration] hours or more", and the up arrow 3116 may be displayed
as greyed
out and may be un-selectable by the user.
[0381] The time input screen 3114 also includes a "?" touchscreen button 3122,
which,
when pressed, provides a help screen 3106 that includes additional
instructions to the user
as described above and as indicated by reference path (K). The time input
screen includes
a "Back" touchscreen button 3124, which, when pressed, returns the display to
the
numerical input screen 3096 as shown by reference path (L). Once the desired
time since
the last unlogged rapid-acting insulin dose has been entered on the time input
screen 3114,
the "Next" touchscreen button 3126 may be pressed to advance the graphical
user
interface to the next screen in the Insulin on Board Interface, such as screen
3128. Pressing
the "Next" touchscreen button will save the entered rapid-acting insulin dose
with a time
stamp reflecting the time calculated based on the interval since the dose was
taken as
indicated by the user in the previous time input screen 3114. Screen 3128
informs the user
that the entered dose of rapid-acting insulin will be used in the calculation
of the user's
suggested insulin dose, and that the next screen will allow the user to enter
what the user
plans to eat. Screen 3128 includes a "Back" touchscreen button 3130, which,
when
pressed, returns the display to the time input screen 3114. Screen 3128
includes a "Next"
-80-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
touchscreen button 3132, which, when pressed, causes the graphical user
interface to
display the Insulin Calculator Interface 3062 (see FIG. 17).
Insulin Calculator Interface
[0382] An Insulin Calculator Interface 3062 begins with the display of an
"Enter Carbs"
screen (see FIG. 17B). On the "Enter Carbs" screen, the amount of carbs may be
entered.
The amount of carbs may be entered as grams, servings, or by meal, depending
on the
settings of the Reader.
[0383] For example, the amount of carbs may be entered as grams of carbs on
the "Enter
Carbs" (grams) screen 3200. The "Enter Carbs" (grams) screen 3200 initially
displays no
value (e.g., "- 2 or "0" is displayed), however, the grams of carbs may be
adjusted by
pressing the up arrow 3202 (e.g., "+") touchscreen button or the down arrow
3204 (e.g., "-
") touchscreen button to increase or decrease, respectively, the grams of
carbs as desired,
as shown by reference path (T) (see FIG. 17C). For example, the grams of carbs
3206 may
be adjusted by 1 gram increments by tapping the up arrow 3202 or the down
arrow 3204.
In some cases, the minimum grams of carbs that may be entered is zero carbs,
and the
maximum grams of carbs that may be entered is 200 grams of carbs. In some
embodiments, the amount increases or decreases as appropriate at a slow-rate
for the first
2 seconds and at a faster rate after 2 seconds when the up or down arrow is
pressed and
held. Once the desired amount is entered, the amount may be saved by pressing
the
"Done" touchscreen button 3208, which saves the entered grams of carbs and
then
advances the graphical user interface to the Insulin Calculation Interface
3212, as shown
by reference paths (S and V) (see FIGS. 17B, 17C and 17E). The "Enter Carbs"
(grams)
screen 3200 includes a "Back" touchscreen button 3210, which, when pressed,
returns the
display to the Results screen.
[0384] In certain embodiments, the amount of carbs may be entered as servings
of carbs on
the "Enter Carbs" (servings) screen 3216. The "Enter Carbs" (servings) screen
3216
initially displays no value (e.g., "- 2 or "0" or "0.0" is displayed),
however, the servings
of carbs may be adjusted by pressing the up arrow 3218 (e.g., "+") touchscreen
button or
the down arrow 3220 (e.g., "-") touchscreen button to increase or decrease,
respectively,
the servings of carbs as desired, as shown by reference path (T) (see FIG.
17C). For
example, the servings of carbs 3222 may be adjusted by 0.5 serving increments
by tapping
the up arrow 3218 or the down arrow 3220. In some cases, the minimum servings
of carbs
-81-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
that may be entered is zero servings. In some instances, the serving size may
be set to 10
or 12 grams of carbs per serving and the maximum servings of carbs that may be
entered
is 20 servings. In some instances, the serving size may be set to 15 grams of
carbs per
serving and the maximum servings of carbs that may be entered is 15 servings.
In some
embodiments, the amount increases or decreases as appropriate at a slow-rate
for the first
2 seconds and at a faster rate after 2 seconds when the up or down arrow is
pressed and
held. In some cases, the "Enter Carbs" (servings) screen 3216 may include a
display of the
grams of carbs 3228 that corresponds to the number of servings entered (see
FIG. 17C). In
some cases, the "Enter Carbs" (servings) screen 3216 may include a display of
the energy
(e.g., kcal) of carbs 3230 that corresponds to the number of servings entered
(see FIG.
17C). Once the desired amount is entered, the amount may be saved by pressing
the
"Done" touchscreen button 3224, which saves the entered servings of carbs and
then
advances the graphical user interface to the Insulin Calculation Interface
3212, as shown
by reference paths (S and V) (see FIGS. 17B, 17C and 17E). The "Enter Carbs"
(servings)
screen 3216 includes a "Back" touchscreen button 3226, which, when pressed,
returns the
display to the Results screen.
[0385] In certain embodiments, the amount of carbs may be entered by meal on
the "Enter
Carbs" (meal) screen 3232. The "Enter Carbs" (meal) screen 3232 displays a
list of meals,
such as breakfast, lunch, dinner, or no meal. Each selection includes a
corresponding
touchscreen radio button. For example, to select the "Dinner" meal, the
touchscreen radio
button 3234 associated with the "Dinner" selection may be pressed. The "Enter
Carbs"
(meal) screen 3232 includes a "Back" touchscreen button 3238, which, when
pressed,
returns the display to the Results screen. The "Enter Carbs" (meal) screen
3232 includes a
"Next" touchscreen button 3236, which, when pressed, may advance the graphical
user
interface to the "Double Check" screen 3240 via reference path (Z), as shown
in FIG. 17C.
[0386] The "Double Check" screen 3240 (see FIG. 17C) is displayed if the user
has marked
the same meal since midnight (12:00AM) of the current day. The "Double Check"
screen
3240 displays a warning to the user indicating that the user has already
logged insulin for
the selected meal that day, and asks the user if the user wants to log more
insulin for the
same meal that day. The "Double Check" screen 3240 includes a "No" touchscreen
button,
which, when pressed, returns the graphical user interface to the "Enter Carbs"
(meal)
screen 3232 via reference path (Al). The "Double Check" screen 3240 includes a
"Yes"
-82-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
touchscreen button, which, when pressed, advances the graphical user interface
to the
Insulin Calculation Interface 3212 via reference path (Q) (see FIG. 17E).
[0387] Referring again to FIG. 17B, the "Enter "Carbs" screens (e.g., the
"Enter Carbs"
(grams) screen 3200, and the "Enter Carbs" (servings) screen 3216) both
include a "?"
touchscreen button 3214, which, when pressed, causes the graphical user
interface to
display help screens 3246, as shown by reference path (0) in FIG. 17B, or by
reference
path (N) in FIG. 17C. Help screens 3246 (see FIG. 17A) may display information
to the
user depending on whether the Reader is set to display carbs as grams,
servings by grams
of carbs, or servings by kcal of carbs. For example, a help screen 3248 may be
shown that
displays a set of help information to the user regarding carbohydrates when
the Reader is
set to display carbs as grams. In other instances, the help screen 3250 may be
shown that
displays a different set of help information to the user regarding
carbohydrates as servings
by grams of carbs when the Reader is set to display servings by grams of
carbs. In other
instances, the help screen 3252 may be shown that displays yet a different set
of help
information to the user regarding carbohydrates as servings by kcal of carbs
when the
Reader is set to display servings by kcal of carbs. In certain instances, if
the help
information includes more text than is able to be displayed on a single help
screen, the
help screen may include a down arrow 3254, which, when pressed, causes the
graphical
user interface to display the remaining text on a second help screen 3256. The
second help
screen 3256 includes an up arrow 3258, which, when pressed, returns the
graphical user
interface to the first help screen 3248, 3250 or 3252 depending on the
settings of the
Reader as described above. The help screens 3248, 3250, 3252 and 3256 include
an "OK"
touchscreen button 3260, which, when pressed, returns the graphical user
interface to the
"Enter "Carbs" screen (e.g., the "Enter Carbs" (grams) screen 3200, or the
"Enter Carbs"
(servings) screen 3216) from which the help screen was launched, via reference
path (P).
[0388] Referring to FIG. 17B, once the desired amount of carbs is entered, the
amount may
be saved by pressing the "Done" touchscreen button 3208 or 3224, which saves
the
entered amount of carbs (e.g., grams of carbs or servings of carbs) and then
advances the
graphical user interface to the appropriate Insulin Calculation Interface
3212, as shown by
reference path (S) (see FIG. 17E). The Insulin Calculation Interface 3212
displays the
suggested dose of insulin based on the amount of carbs entered by the user on
the previous
screens as described above and/or the user's insulin on board, if any, as
described above.
The Insulin Calculation Interface 3212 may vary in appearance depending on
whether the
-83-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Reader is set to display whole units of insulin, half units of insulin, or a
decimal point or a
comma between the whole and half units of insulin.
[0389] Referring to FIG. 17E, in embodiments of the Insulin Calculation
Interface 3212
where the Reader is set to display whole units of insulin, the Insulin
Calculation Interface
3212 will appear as Suggested Dose screen 3262. Suggested Dose screen 3262
displays
the suggested dose of insulin 3264 as units of insulin. The suggested dose of
insulin may
be adjusted in 1 insulin unit increments by pressing the up arrow 3266 (e.g.,
"+")
touchscreen button or the down arrow 3268 (e.g., "-") touchscreen button to
increase or
decrease, respectively, the suggested dose of insulin as desired. The minimum
and
maximum values for the suggested dose of insulin are 0 and 99, respectively.
If the
suggested dose of insulin is adjusted by the user from its initial value, the
amount of units
increased or decreased by the user 3270 will be shown below the suggested
insulin dose
3264. If the user adjustment of the suggested dose of insulin is calculated to
reduce the
user's blood glucose below the target range, then the graphical user interface
will display a
warning screen 3292 that displays a caution to the user that the insulin dose
entered may
take the user's blood glucose lower than the user's target range and put the
user at risk for
a low glucose event (see FIG. 17D). The warning screen 3292 includes an "OK"
touchscreen button, which, when pressed, returns the graphical user interface
to the
Suggested Dose screen 3262 via reference path (W). The Suggested Dose screen
3262
includes a "Back" touchscreen button 3272, which, when pressed, returns the
user to the
prior "Enter "Carbs" screen (e.g., the "Enter Carbs" (grams) screen 3200, or
the "Enter
Carbs" (servings) screen 3216) via reference path (R). The Suggested Dose
screen 3262
also includes a "Log dose" touchscreen button 3274, which, when pressed, saves
the
suggested insulin dose in the logbook along with an associated glucose test
result and the
time the test was taken (e.g., the insulin dose is not saved as delivered at
the time the "Log
dose" touchscreen button is pressed) and advances the graphical user interface
to the
Logbook via reference path (Y) (see FIG. 17D).
[0390] Referring to FIG. 17E, in embodiments of the Insulin Calculation
Interface 3212
where the Reader is set to display half unit increments of insulin and a
decimal point
between the whole and half units, the Insulin Calculation Interface 3212 will
appear as
Suggested Dose screen 3276. Suggested Dose screen 3276 displays the suggested
dose of
insulin 3278 as units of insulin in 0.5 unit increments. The suggested dose of
insulin may
be adjusted in 0.5 insulin unit increments by pressing the up arrow 3280
(e.g., "+")
-84-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
touchscreen button or the down arrow 3282 (e.g., "-") touchscreen button to
increase or
decrease, respectively, the suggested dose of insulin as desired. The minimum
and
maximum values for the suggested dose of insulin are 0 and 50, respectively.
If the
suggested dose of insulin is adjusted by the user from its initial value, the
amount of units
increased or decreased by the user 3284 will be shown below the suggested
insulin dose
3278. If the user adjustment of the suggested dose of insulin is calculated to
reduce the
user's blood glucose below the target range, then the graphical user interface
will display a
warning screen 3292 that displays a caution to the user that the insulin dose
entered may
take the user's blood glucose lower than the user's target range and put the
user at risk for
a low glucose event (see FIG. 17D). The warning screen 3292 includes an "OK"
touchscreen button, which, when pressed, returns the graphical user interface
to the
Suggested Dose screen 3276 via reference path (W). The Suggested Dose screen
3276
includes a "Back" touchscreen button 3286, which, when pressed, returns the
user to the
prior "Enter "Carbs" screen (e.g., the "Enter Carbs" (grams) screen 3200, or
the "Enter
Carbs" (servings) screen 3216) via reference path (R). The Suggested Dose
screen 3276
also includes a "Log dose" touchscreen button 3288, which, when pressed, saves
the
suggested insulin dose in the logbook along with an associated glucose test
result and the
time the test was taken (e.g., the insulin dose is not saved as delivered at
the time the "Log
dose" touchscreen button is pressed) and advances the graphical user interface
to the
Logbook via reference path (Y) (see FIG. 17D).
[0391] Referring to FIG. 17E, in embodiments of the Insulin Calculation
Interface 3212
where the Reader is set to display half unit increments of insulin and a comma
between the
whole and half units, the Insulin Calculation Interface 3212 will appear as
Suggested Dose
screen 3290. The functions of Suggested Dose screen 3290 are analogous to
those
described for Suggested Dose screen 3276 above.
[0392] If the insulin calculator of the Reader determines that no insulin dose
is suggested,
the Insulin Calculation Interface 3212 will be displayed as No Suggested Dose
screen
3298, which displays information indicating that based on the user's current
glucose level,
no rapid-acting insulin is being suggested. The No Suggested Dose screen
includes a
"Back" touchscreen button 3300, which, when pressed, returns the user to the
prior "Enter
"Carbs" screen (e.g., the "Enter Carbs" (grams) screen 3200, or the "Enter
Carbs"
(servings) screen 3216) via reference path (R). The No Suggested Dose screen
3298
includes a "Next" touchscreen button 3302, which, when pressed, advances the
graphical
-85-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
user interface to Suggested Dose screen 3304. Suggested Dose screen 3304
begins by
displaying a suggested does of insulin 3306 as 0 units of insulin. The
suggested dose of
insulin may be adjusted in 1 unit or 0.5 insulin unit increments, depending on
the Reader
settings, by pressing the up arrow 3308 (e.g., "+") touchscreen button or the
down arrow
3310 (e.g., "-") touchscreen button to increase or decrease, respectively, the
suggested
dose of insulin as desired. If the suggested dose of insulin is adjusted by
the user from its
initial value, the amount of units increased or decreased by the user will be
shown below
the suggested insulin dose 3306. If the user adjustment of the suggested dose
of insulin is
calculated to reduce the user's blood glucose below the target range, then the
graphical
user interface will display a warning screen 3292 that displays a caution to
the user that
the insulin dose entered may take the user's blood glucose lower than the
user's target
range and put the user at risk for a low glucose event (see FIG. 17D). The
warning screen
3292 includes an "OK" touchscreen button, which, when pressed, returns the
graphical
user interface to the Suggested Dose screen 3304 via reference path (W). The
Suggested
Dose screen 3304 includes a "Back" touchscreen button 3312, which, when
pressed,
returns the user to the prior No Suggested Dose screen 3298. The Suggested
Dose screen
3304 also includes a "Log dose" touchscreen button 3314, which, when pressed,
saves the
suggested insulin dose in the logbook along with an associated glucose test
result and the
time the test was taken (e.g., the insulin dose is not saved as delivered at
the time the "Log
dose" touchscreen button is pressed) and advances the graphical user interface
to the
Logbook via reference path (Y) (see FIG. 17D).
[0393] Suggested Dose screens 3262, 3276, 3290 and 3304 include a "Dose
details"
touchscreen button 3294 (e.g., an "i" touchscreen button), which, when
pressed, causes
graphical user interface to display Dose Details Interface via reference path
(X) (see FIG.
17D).
[0394] Dose Details Interface 3296 is shown in FIGS. 18A and 18B. Dose Details
Interface
begins a Dose Details screen (e.g., 3316, 3318 and 3320) that displays a
summary of the
calculated insulin dose. The Dose Details screen (e.g., 3316, 3318 and 3320)
may have
various appearances depending on the settings of the Reader (e.g., whether the
Reader is
set to display carbs as grams of carbs, servings of carbs, or by meal) and the
data input by
the user or calculated by the Reader (e.g., amount of carbs, insulin on board,
user
adjustment to the suggested dose of insulin, etc.). The Dose Details screen
displays the
amount of carbs by meal 3322, as grams of carbs 3324, or as servings of carbs
3326. If
-86-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
insulin correction is enabled on the Reader, the Dose Details screen will
display the
suggested insulin dose 3328. If insulin correction is disabled on the Reader
(e.g., the Easy
setup sets "no correction insulin") then the suggested insulin dose is not
displayed. If the
Reader determines that insulin on board is present, then the Dose Details
screen displays
the amount of insulin on board 3330. In the Reader determines that no insulin
on board is
present, then the amount of insulin on board is not displayed. If the user
adjusted the
suggested insulin dose, the Dose Details screen will display the amount the
user adjusted
the suggested insulin dose 3332. Based on the above data, the Dose Details
screen displays
the calculated insulin dose 3334.
[0395] The Dose Details screen includes a down arrow touchscreen button 3336,
which,
when pressed, advances the Dose Details screen to the second page of the Dose
Details
screen via reference path (B1) (see FIG. 18B). The information displayed on
the second
page of the Dose Details screen may vary depending on whether insulin on board
is
present and whether the suggested dose of insulin only accounts for carbs in
the user's
meal. If insulin on board is not present, then the second page of the Dose
Details screen
3338 displays information indicating that the suggested dose of rapid-acting
insulin covers
the carbs in the user's meal and corrects for the user's current glucose
level. If insulin on
board is present and the user has taken insulin within the past 2 hours, then
the second
Dose Details screen 3340 displays information indicating that the user has
taken rapid-
acting insulin in the past 2 hours, and the suggested dose of insulin covers
the carbs in the
user's meal but does not correct for the user's current glucose level. If
insulin on board is
present and the time when the user has last taken insulin is more than 2 hours
from the
current time but less than the duration of insulin action, then the second
Dose Details
screen 3342 displays information indicating that the user has taken rapid-
acting insulin in
the past 4 hours, and the suggested dose of insulin covers the carbs in the
user's meal,
corrects for the user's current glucose level, and accounts for insulin that
was previously
logged. The second Dose Details screen (e.g., 3338. 3340 and 3342) include an
up arrow
touchscreen button 3344, which, when pressed, returns the graphical user
interface to the
previous Dose Details screen.
Reminders Interface
[0396] An exemplary embodiment of a graphical user interface which may be
utilized in
connection with a Reader as described herein and which facilitates a Reminders
procedure
-87-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
3400 for setting reminders is provided. This graphical user interface is now
described in
greater detail with reference to FIG. 19.
[0397] The Reminder Interface 3400, shown in FIG. 19A, begins with the display
of a
Home Screen 3402 of the Reader. The Home Screen includes a reminders
touchscreen
button 3404 (e.g., a bell shaped icon, an alarm clock icon, a clock icon,
etc.), which, when
pressed, begins the procedure to set and/or adjust reminders. If a reminder
has been
previously set, then next reminder time 3406 is displayed next to the
reminders
touchscreen button 3404.
[0398] Referring to FIG. 19A, if no reminder has been previously set, then
pressing the
reminders touchscreen button 3404 advances the graphical user interface to the
Remind
Me screen 3408 via reference path (D1) (see FIG. 19B). Remind Me screen 3408
includes
selections for the type of reminder, the schedule for the reminder, and the
time of the
reminder.
[0399] For example, on the Remind Me screen 3408, the type of reminder may be
selected
by pressing the Reminder Type touchscreen button 3410. The reminder selection
touchscreen button 3410 displays the currently selected reminder type, which
has a default
value of "Check Glucose". The Reminder Type touchscreen button 3410, when
pressed,
advances the graphical user interface to the Reminder Type screen 3412 via
reference path
(K1) (see FIG. 19D). The Reminder Type screen 3412 lists the available types
of
reminders that may be set. The Reminder Type screen 3412 displays the 3
default
reminders as the "Check Glucose" touchscreen button 3414, the "Take Insulin"
touchscreen button 3416, the and "Alarm" touchscreen button 3418. The default
types of
reminders can be reordered using the PC interface, but not deleted from the
Reader. Each
default reminder touchscreen button includes text describing the type of
reminder, as
described above, and an icon associated with each different type of default
reminder.
Additional custom reminders can be setup using the PC interface. If additional
custom
reminders have been previously setup, then they will be displayed on the
Reminder Type
screen 3420. The custom reminders can by any type of reminder desired by the
user. The
additional custom reminders can be displayed by pressing the down arrow
touchscreen
button 3422 on reminder Type screen 3420. For example, the list of types of
reminders
may be scrolled through by tapping the down arrow 3422 or the up arrow 3424.
In some
embodiments, the pages scroll page by page by a single press of the up or down
arrow and
at a slow rate after 2 seconds when the up or down arrow is pressed and held.
Once a type
-88-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
of reminder is selected by pressing the desired type of reminder, the
graphical user
interface returns to the Remind Me screen 3408 via reference path (J1) (see
FIG. 19B).
[0400] On the Remind Me screen 3408, the schedule of the reminder may be
selected by
pressing the Repeat touchscreen button 3426. The Repeat touchscreen button
3426
displays the currently selected schedule for the reminder, which has a default
value of
"Daily", indicating that the reminder will be repeated daily at the selected
time. The
Repeat touchscreen button 3426, when pressed, advances the graphical user
interface to
the Reminder Schedule screen 3428 via reference path (Ni) (see FIG. 19C). The
Reminder
Schedule screen 3428 lists the available types of schedules that may be set
for the
reminder. The Reminder Schedule screen 3428 displays 3 types of reminder
schedules as
the "Daily" touchscreen button 3430, the "Once" touchscreen button 3432, the
and
"Countdown Timer" touchscreen button 3434. Pressing the "Daily" touchscreen
button
3430 sets the reminder schedule to daily and returns the graphical user
interface to the
Remind Me screen 3408 via reference path (L1). Pressing the "Once" touchscreen
button
3432 sets the reminder schedule to once (e.g., the reminder will not repeat)
and returns the
graphical user interface to the Remind Me screen 3408 via reference path (M1).
Pressing
the "Countdown Timer" touchscreen button 3434 sets the reminder schedule to a
countdown timer and advances the graphical user interface to the Timer
Duration screen
3436 (see FIG. 19C). The countdown timer may be set using the Timer Duration
screen
3436. For example, the Timer Duration screen 3436 displays the current amount
of time
left on the countdown timer in hours and minutes. The default value for the
countdown
timer is 15 minutes. The amount of minutes may be adjusted by pressing the up
arrow
3438 (e.g., "+") touchscreen button or the down arrow 3440 (e.g., "-")
touchscreen button
to increase or decrease, respectively, the amount of minutes as desired. For
example, the
amount of minutes may be adjusted by 1 minute increments by tapping the up
arrow 3438
or the down arrow 3440. In some embodiments, the amount increases or decreases
as
appropriate at a slow-rate for the first 2 seconds and at a faster rate after
2 seconds when
the up or down arrow is pressed and held. The amount of hours may be adjusted
by
pressing the up arrow 3442 (e.g., "+") touchscreen button or the down arrow
3444 (e.g., "-
") touchscreen button to increase or decrease, respectively, the amount of
minutes as
desired. For example, the amount of hours may be adjusted by 1 hour increments
by
tapping the up arrow 3442 or the down arrow 3444. In some embodiments, the
amount
increases or decreases as appropriate at a slow-rate for the first 2 seconds
and at a faster
-89-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
rate after 2 seconds when the up or down arrow is pressed and held. Once the
desired
amount of time for the countdown timer is entered, the amount may be saved by
pressing
the "OK" touchscreen button 3446, which saves the entered amount of time and
then
returns the graphical user interface to the Remind Me screen 3448, as shown by
reference
path (Q1) (see FIG. 19B). From the Remind Me screen 3448 (see FIG. 19B), the
amount
of time for the countdown timer may be adjusted by pressing the Time
touchscreen button
3464, which, when pressed, advances the graphical user interface to the Timer
Duration
screen 3436 via reference path (R1) (see FIG. 19C). On the Timer Duration
screen 3436,
the time for the countdown timer may be adjusted as described above.
[0401] On the Remind Me screen 3408, the time of the reminder may be selected
by
pressing the Time touchscreen button 3450. The Time touchscreen button 3450
displays
the currently set time for the reminder, which has a default value of 12:00
am, indicating
that the reminder will be activated at the selected time. The Time touchscreen
button 3450,
when pressed, advances the graphical user interface to the Reminder Time
screen 3452 via
reference path (P1) (see FIG. 19C). The Reminder Time screen 3452 allows the
user to set
the time when the reminder will be activated. For example, the Reminder Time
screen
3452 displays the currently set time for the reminder in hours and minutes.
The reminder
time may be adjusted by pressing the minutes up arrow 3454 (e.g., "+")
touchscreen
button or the minutes down arrow 3456 (e.g., "-") touchscreen button to
increase or
decrease, respectively, the minutes as desired, and the hours up arrow 3458
(e.g., "+")
touchscreen button or the hours down arrow 3460 (e.g., "-") touchscreen button
to increase
or decrease, respectively, the hours as desired. In some embodiments, the
hours and
minutes increases or decreases as appropriate at a slow-rate for the first 2
seconds and at a
faster rate after 2 seconds when the up or down arrow is pressed and held.
Once the
desired time for the reminder is entered, the time may be saved by pressing
the "OK"
touchscreen button 3462, which saves the entered time and then returns the
graphical user
interface to the Remind Me screen 3408, as shown by reference path (01) (see
FIG. 19B).
[0402] Referring to FIG. 19B, after the desired reminder type, reminder
schedule and
reminder time has been selected as desired, the reminder settings may be saved
by
pressing the "Save" touchscreen button 3466, which, when pressed, advances the
graphical user interface to the Reminder List screen 3468 via reference path
(El) (see FIG.
19A). Referring back to FIG. 19B, the reminder settings may be discarded by
pressing
"Cancel" touchscreen button 3470. If no previous reminder have been set, then
pressing
-90-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
"Cancel" touchscreen button 3470 returns the graphical user interface to the
Home Screen
3402 via reference path (Cl) (see FIG. 19A). If one or more reminders has been
previously set, then pressing "Cancel" touchscreen button advances the
graphical user
interface to the Reminders List screen 3468 via reference path (H1) or (I1)
(see FIG. 19A).
In certain embodiments, if the settings for a previously set reminder are
being adjusted
from the Remind Me screen 3408, then "Cancel" touchscreen button 3470 may be
displayed as a "Delete" touchscreen button.
[0403] Referring to FIG. 19A, from the Home Screen 3402, if one or more
reminders has
been previously set, then pressing the reminders touchscreen button 3404 or,
in some
instances, the next reminder time 3406 advances the graphical user interface
to the
Reminders List screen 3468. The Reminders List screen 3468 displays a list of
the
previously set reminders. The list of previously set reminders displays the
reminder time
3472 for each previously set reminder and a corresponding "On"/"Off' toggle
touchscreen
button 3474 for each previously set reminder. Pressing the reminder time 3472
advances
the graphical user interface to the Remind Me screen 3408 via reference path
(G1), from
which the reminder settings may be adjusted as described above. Pressing the
"On"/"Off'
toggle touchscreen button 3474 turns the corresponding reminder on or off.
[0404] The Reminders List screen includes an "Add New" touchscreen button
3476, which,
when pressed, advances the graphical user interface to the Remind Me screen
3408 via
reference path (F1), from which a new reminder may be setup as desired, as
described
above. The Reminders List screen includes a "Done" touchscreen button 3478,
which,
when pressed, returns the graphical user interface to the Home Screen 3402.
Receive Reminders Interface
[0405] An exemplary embodiment of a graphical user interface which may be
utilized in
connection with a Reader as described herein and which facilitates a Receive
Reminder
procedure 3500 for receiving reminders is provided. This graphical user
interface is now
described in greater detail with reference to FIG. 20.
[0406] The Receive Reminder Interface 3500, shown in FIG. 20B, begins when a
reminder
(e.g., a reminder that has been set using the Reminders Interface 3400
described above) is
activated, such as when the time or triggering event of a scheduled reminder
is reached. A
scheduled reminder should be displayed when the time / triggering event of the
reminder
is reached even if the Reader display is off In certain embodiments, a
scheduled reminder
-91-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
is not displayed on the Reader if the time / triggering event of the reminder
is reached: (1)
During a blood glucose test using a strip - instead the reminder is presented
after the
results have been displayed; (2) When the battery is critically low - instead
the reminder is
presented when the battery is sufficiently charged, and the Reader is powered
on; (3)
During error conditions - instead the reminder is presented the next time the
Reader is
powered on; or (4) When the Reader is connected to a computer and data
transfer is in
process - instead the reminder is presented when the data transfer is
completed.
[0407] When the time / triggering event of the reminder is reached, a Reminder
screen is
3502 displayed (FIG. 20B). The Reminder screen 3502 includes display of the
reminder
with a reminder icon 3504 representing the type of reminder. For example, the
reminder
icon may be an "Alarm" reminder icon 3504 (e.g., a picture of a bell or alarm
clock, etc.),
indicating that the type of reminder is an alarm. In some instances, the
reminder icon is a
"Take Insulin" reminder icon 3506 (e.g., a picture of a syringe) (see FIG.
20A), indicating
that the type of reminder is a reminder to the user to take insulin. In some
instances, the
reminder icon is a "Check Glucose" reminder icon 3508 (e.g., a picture of a
glucose
Reader) (see FIG. 20A), indicating that the type of reminder is a reminder to
the user to
check their glucose level. Other types of icons and reminders are possible.
[0408] If the Reminder sound is set to on, a beep sounds with appearance of
any reminders
screen. If a reminder is ignored, the reminder will appear on the screen with
the next
power on (whether by hardware button or strip insertion). If multiple
reminders are active,
the reminder screens will stack up with the most recent reminder showing
first. The
reminder screens will require dismissal one by one. If daily repeated
reminders have been
missed, once a new day's reminder is current, the previous day's reminder for
that time is
no longer active and is not part of the stack-up of reminder screens.
[0409] The Reminder screen 3502 includes a "Snooze 15 min" touchscreen button
3510,
which, when pressed, sets the active reminder to re-active in 15 minutes, and
returns the
graphical user interface to the next active reminder or, if there are no other
active
reminders, to the previously displayed screen before the reminder was
activated. The
Reminder screen 3502 also includes an "OK" touchscreen button 3512, which,
when
pressed cancels the active reminder and returns the graphical user interface
to the next
active reminder or, if there are no other active reminders, returns the
graphical user
interface to the Home Screen. In the Reader powers off automatically due to a
timeout
-92-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
while displaying the Reminder screen, the reminder that was active when the
Reader
timed-out will be displayed again the next time the Reader is powered on.
Reader Summaries
[0410] In some aspects of the present disclosure, the analyte monitoring
device may be
programmed with software to provide summaries of information and data related
to obtain
readings. The software provides an interface to view and manage features
related to
generated reports. Different types of summaries may be generated. For example,
FIGS.
21A-D illustrate various types of interfaces for displaying summaries on the
analyte
monitoring device, according to certain embodiments. It should be appreciated
that the
summary screens illustrated are exemplary and should not be interpreted as
limiting.
[0411] An exemplary embodiment of a graphical user interface which may be
utilized in
connection with a reader as described herein and which functions to provide
the user with
summaries of information and data related to obtained readings.
Summaries Menu Interface
[0412] FIG. 21A illustrates an exemplary interface for providing a summaries
menu on the
analyte monitoring device. History Menu01 screen 622 is shown in FIG. 21A and
provides
a menu of summary options that the user can select. Option 622a takes the user
to a
Logbook screen for viewing logged data, as shown by block 626. Option 622b
takes the
user to a Daily Graph screen which provides a summary of data in a daily graph
format.
Option 622c provides a screen for averages for glucose readings obtained for a
period of
time. If more options are available that can fit on a single screen shot, then
a trigger
element 622h (e.g., an arrow symbol) for scrolling or otherwise viewing the
remaining
options may be selected. When arrow 622h is selected, the user is taken to
screen 624
wherein the remaining options are displayed. For example, option 624 provides
a
summary screen of low glucose events for a period of time. Option 622d
provides a screen
showing a summary of time that obtained readings were in the target zone.
Option 622f
provides a screen showing summarizing information related to the user of the
reader
device. Option 622g provides a summary screen of daily patterns that have been
determined or identified based on reading acquired for a period of time.
Trigger element
622i takes the user back to screen 622.
[0413] When one of the options are selected, it is determined if sensor data
is present, as
shown at block 628. If no data is present, a No Sensor Data screen 630 is
displayed to
-93-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
indicate to the user that no sensor data is available for the summary. In one
embodiment,
the all summaries except for logbook include sensor data only (e.g., glucose
data obtained
from the sensor). If the device includes only strip data and insufficient
sensor data, then
the No Sensor Data screen is still provided when a summary option is selected.
[0414] If sensor data is provided, then the corresponding screen for the
selected summary
option is displayed, as represented by reference path Ti. From the selected
summary
screen, the user can navigate back to the menu options screens 622,624 to
select another
menu option if desired, as shown by reference path Si.
[0415] FIG. 21B illustrates a Daily Graph screen 632 for showing a daily graph
of sensor
readings obtained over a single day or 24 hour time period. For example, the
daily graph
632 illustrates the readings obtained on Wednesday, February 22. The
horizontal axis of
graph 632 represents time throughout the day, while the vertical axis
represents sensor
reading values. A target zone 632d may also be provided on graph 632 to
represent the
target zone for readings. Graph 632 may also include a time change icon 632a
to inform
the user of a time change. Also shown, are event indicators 632b which
indicate various
events with symbols or icons on the graph 632. For example, the needle icon
represents a
logged insulin event indicating that an insulin dose was taken, and the apple
icon
represents a logged food event indicating that food was eaten. More details
may be stored
or logged for the given event, and in one embodiment, the event indicators
632b may be
selected by the user to provide additional details regarding the event.
[0416] In certain embodiments, event indicators may only be applied to glucose
values after
a glucose reading has been taken, either via the glucose sensor or a test
strip reading.
Event details may be input logged or added as notes associated with glucose
values or
glucose events; such as high or low glucose levels. In certain embodiments,
event details
may be imported from another source (e.g., electronic diary or log) and stored
in a
memory device.
[0417] In the embodiment shown, trigger elements 632c (e.g., left and right
arrows) are also
provided to enable the user to navigate forwards and backwards to another day
or 24-hour
time period. For example, the user could navigate to the next day after
Wednesday using
the right arrow, or go to the previous day by selecting the left arrow.
[0418] FIG. 21C illustrates an exemplary Average Glucose Summary interface for
providing a graph of sensor readings obtained over a time period that is
summarized with
respect to a predetermined time period such as single day or 24 hour time
period as
-94-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
shown. For example, Average Glucose (GLUC) 01 screen provides a bar graph 634a
for
sensor readings taken over the last 7 days. The bar graph 634a includes bars
for various
increments of time throughout a single day. Each bar represents the average
glucose
reading that was obtained at that increment of time of day for the last 7
days. For example,
the average of all the sensor readings obtained between 12am and 6am over the
last 7
days, is 121 mg/dL. For 6am to 12, the average glucose reading was 152 mg/dL,
etc.
[0419] Screen 634 also includes an average glucose value 634b for the time
period of data.
For example, the average glucose reading for all readings obtained over the
last 7 days
was 119 mg/dL. Screen 634 also includes a trigger element 634c (e.g., an arrow
icon) for
changing the different time period of obtained data. For example, if the user
selects the
right arrow icon 634c, the user is taken to AVG GLUCO2 screen 636, which
similarly
displays a bar graph 636a and average glucose value 636b, but for a different
time period,
such as the last 14 days as shown. Similarly, screen 636 also includes trigger
elements
634c for again increasing the time period. In this way, the user can change to
AVG
GLUCO3 screen 638, which similarly displays a bar graph 638a and average
glucose value
638b, but for a different time period, such as the last 30 days as shown.
Similarly, the user
can change to AVG GLUCO4 screen 640, which similarly displays a bar graph 640a
and
average glucose value 640b, but for a different time period, such as the last
90 days as
shown. Trigger elements 634c enable the user to navigate forwards and
backwards
between screens 634,636,638,640 to change the time periods as desired. From
any of the
screens, user confirmation (e.g., by selecting the "ok" button) will take the
user back to the
options menu interface 622,624.
[0420] FIG. 21D illustrates an exemplary screen for showing the percentage of
time the
sensor readings were within a target zone. Time In Target screen 642 displays
a
representation 642a of the percentage of time within a target zone, above a
target zone,
and below a target zone, for sensor readings obtained for a period of time
(e.g., 7 days in
the embodiment shown). Trigger element 642c is provided to enable the user to
change the
time period - e.g., similarly as described in FIG. 21c. Representation 642a
also includes
bar graphs for the associated percentages.
Summaries Menu - Masked Mode Interface
[0421] FIG. 21E illustrates an exemplary screen 644 for showing a graph 644a
of the
number of events associated with sensor readings obtained over a time period,
wherein the
events are summarized with respect to a predetermined time period, such as
single day or
-95-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
24 hour time period as shown. For example, screen 644 indicates a graph 644a
for sensor
readings obtained over the last 7 days, wherein 1 event occurred between 12am
and 6am, 0
events occurred between 6am and 12pm, 3 events between 12pm and 6am, etc. In
the
embodiment shown, the event corresponds to a low glucose reading - e.g., with
respect to
a target zone. Other events may also be implemented - e.g., high glucose
readings, insulin
dosages, food intake events, etc. Trigger element 644c is provided to enable
the user to
change the time period.
[0422] FIG. 21F illustrates an exemplary screen 648 for indicating information
associated
with the use of the sensor over a time period. For example, screen 648
indicates the
average scans per day, and the number of days with sensor data, for sensor
readings
obtained over the last 7 days. Similarly, trigger element 648c is provided to
enable the user
to change the time period.
[0423] FIG. 21G illustrates an example interface for providing a summaries
menu on the
analyte monitoring device, when the device is in masked mode. History Menu
Masked
screen 620 is shown provides a menu of summary options that the user can
select. Option
620a takes the user to a Logbook screen for viewing logged data. Option 620b
provides a
screen showing summarizing information related to the use of the reader
device. Since the
device is in masked mode and does not permit the user from viewing sensor
readings,
summary screens related to obtained readings are also not available.
Logbook Interface
[0424] An exemplary embodiment of a graphical user interface which may be
utilized in
connection with a Reader as described herein and which facilitates a Logbook
procedure
3600 for displaying, adding and/or editing logbook entries is provided. This
graphical user
interface is now described in greater detail with reference to FIG. 22.
[0425] The Logbook Interface 3600, shown in FIG. 22A, may be accessed from the
Consecutive Scans Interface (see FIG. 9), the Insulin Calculator Interface
(see FIG. 17),
and the Reader Summaries Interface (see FIG. 21). From the Reader Summaries
Menu
screen (see FIG. 21), pressing the Logbook touchscreen button advances the
graphical
user interface into the Logbook Interface 3600 (see FIG. 22). If there are no
entries in the
logbook, the Logbook Empty screen 3602 is displayed, indicating that there is
no data to
report in the logbook (see FIG. 22A). If there are one or more entries in the
logbook, then
the Logbook List screen 3604 is displayed via reference path (V1) (see FIG.
22B). FIG.
-96-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
22B shows various examples of Logbook List screens and the type of logbook
entries that
may be displayed. Each logbook entry displays the date 3606 and time 3608
associated
with its corresponding logbook entry. Three logbook entries may be displayed
per screen.
The Logbook List screen includes a down arrow 3610, and in some embodiments an
up
arrow (not shown) that may be pressed to scroll down or up, respectively,
through the
logbook entries in the Logbook List screens. The Logbook List screen may be
scrolled
page by page by pressing the up arrow (not shown) touchscreen button or the
down arrow
3610 touchscreen button to scroll through the Logbook List screens as desired.
For
example, the Logbook List screens may be scrolled page by page by tapping the
up arrow
(not shown) or the down arrow 3610. In some embodiments, the pages scroll at a
slow rate
for the first 2 seconds and at a faster rate after 2 seconds when the up or
down arrow is
pressed and held.
[0426] Referring to FIG. 22B, various examples of Logbook List screens and the
type of
logbook entries that may be displayed are shown. For example, the Logbook List
screen
may display a logged glucose level 3612. The logged glucose level 3612 may be
displayed
as a number corresponding to the user's glucose level in mg/dL. The glucose
level may
have an upward trend arrow or a downward trend arrow associated with the
glucose level,
indicating a rising trend in glucose readings or a decreasing trend in glucose
readings,
respectively. In some cases, if the logged glucose reading was above a maximum
threshold
level, "HI" may be displayed instead of a numerical glucose level, indicating
that the
glucose level exceeded a maximum threshold level. Similarly, if the logged
glucose
reading was below a minimum threshold level, "LO" may be displayed instead of
a
numerical glucose level, indicating that the glucose level was below a minimum
threshold
level. In some instances, the glucose reading may be associated with a Note
icon (e.g., a
picture of a pencil), indicating that a note was entered with the logged
glucose level.
[0427] In some instances, the Logbook List screen may display a logged ketone
level 3614.
The logged ketone level 3614 may be displayed as a number corresponding to the
user's
ketone level in mmol/L. In some instances, the Logbook List screen may display
a logged
glucose control solution test level 3616. The logged glucose control solution
test level
3616 may be displayed as a number corresponding to the glucose control
solution level in
mg/dL. The glucose control solution test level 3616 may be associated with a
corresponding control solution icon (e.g., a picture of a bottle of control
solution). In some
instances, the Logbook List screen may display a logged ketone control
solution test level
-97-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
3704. The logged ketone control solution test level 3704 may be displayed as a
number
corresponding to the ketone control solution level in mmol/L. The ketone
control solution
test level 3704 may be associated with a corresponding control solution icon
(e.g., a
picture of a bottle of control solution). In some instances, the Logbook List
screen may
display a logged temperature error 3618. The temperature error may be a low
temperature
error associated with a low temperature error icon (e.g., a picture of a blue
thermometer),
or a high temperature error associated with a high temperature error icon
(e.g., a picture of
a red thermometer). In some instances, the Logbook List screen may display a
logged
masked reading 3620. The logged masked reading may be associated with a masked
reading icon (e.g., a checkmark icon). The Logbook List screen 3604 includes
an "OK"
touchscreen button 3622, which, when pressed, returns the graphical user
interface to the
previous screen displayed before entering the Logbook Interface 3600 (e.g.,
the
Consecutive Scans Interface (see FIG. 9), the Insulin Calculator Interface
(see FIG. 17),
or the Reader Summaries Interface (see FIG. 21)). For example, pressing "OK"
touchscreen button 3622 may cause the graphical user interface to return to
the Reader
Summaries Interface (see FIG. 21) via reference path (U1) (see FIG. 22A).
[0428] Each logbook entry on the Logbook List screen 3604 is a touchscreen
button.
Pressing a logbook entry on the Logbook List screen 3604 advances the
graphical user
interface to the Individual Logbook Entry screen 3624 associated with the
selected
logbook entry via reference path (W1) (see FIGS. 22C and 22D). Various
examples of
Individual Logbook Entry screens 3624 are shown in FIGS. 22C and 22D.
[0429] If a logged glucose level is selected from the Logbook List screen
3604, the glucose
level individual logbook entry screen 3626 is displayed. The glucose level
individual
logbook entry screen 3626 includes one or more of the following information:
the date and
time 3628 of the logged glucose level; the glucose reading 3630 in mg/dL; the
amount of
carbs 3632 (if any); an meal icon 3634 indicating whether the logged glucose
reading was
pre-meal or post-meal (e.g., a whole apple icon for pre-meal readings or a
eaten apple icon
for post-meal readings); a suggested insulin dose 3636 (if any) and/or a
suggested insulin
dose icon 3638 (e.g., a syringe icon); notes 3640 that were associated with
the logged
glucose reading (if any); a glucose level trend arrow 3642 (e.g., an upward or
a downward
trend arrow), as appropriate; an non-actionable icon 3644 indicating that the
glucose
reading is non-actionable; a high glucose level warning 3646 (as text and/or a
warning
icon); a low glucose level warning 3648 (as text and/or a warning icon); an
masked icon
-98-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
3650 indicating that the glucose reading is masked (e.g., a checkmark icon);
suggested
insulin dose details (e.g., amount of carbs 3652, suggested insulin dose 3654,
IOB 3656,
user adjustments to the suggested insulin dose 3658); a low temperature error
warning
3660 (e.g., as text and/or a low temperature warning icon 3666, such as a blue
thermometer icon); a high temperature error warning 3662 (e.g., as text and/or
a high
temperature warning icon 3668, such as a red thermometer icon); an icon
indicating that
the glucose reading was obtained via test strip or via sensor (e.g., a drop of
blood icon
3664 for readings obtained via test strip); a sensor low temperature error
warning 3670
(e.g., as text and/or a sensor low temperature warning icon 3672, such as a
blue
thermometer); and a sensor high temperature error warning 3674 (e.g., as text
and/or a
sensor high temperature warning icon 3676, such as a blue thermometer icon).
[0430] If notes are associated with the logged glucose reading and the notes
will not all fit
on one screen, the Individual Logbook Entry screen 3626 may include a down
arrow 3678
(see FIG. 22C), and in some embodiments an up arrow 3680 (see FIG. 22D) that
may be
pressed to scroll down or up, respectively, through the notes. The Individual
Logbook
Entry screen 3626 may include an "Add or edit notes" touchscreen button 3682,
which,
when pressed, causes graphical user interface to display the Add to Logbook
screen 3684.
The Add to Logbook screen 3684 includes a list of several different user
selectable notes
that may be entered into the Logbook. The list of user selectable notes may
include one or
more of the following: long-acting insulin 3686, exercise 3688, medication
3690, and the
like. In certain embodiments, 3 user-selectable notes are displayed on the
touchscreen at
once. If there are more than 3 user-selectable notes, the list of notes may be
scrolled as
necessary to view the list using scroll touchscreen buttons, such as down
arrow (e.g., down
triangle) touchscreen button 3692 and up arrow (e.g., up triangle) touchscreen
button (not
shown). One or more user-selectable notes may be selected by touching the
touchscreen
checkbox adjacent the note desired to be selected. Touching a touchscreen
checkbox will
toggle the checkbox from a checked to unchecked state indicating whether the
associated
not is selected or not selected, respectively. For instance, the long-acting
insulin note may
be selected by touching the touchscreen checkbox 3694, which then displays a
check mark
in the touchscreen checkbox to indicate that the rapid-acting insulin note has
been
selected. The other user-selectable notes may be selected or unselected (e.g.,
checked or
unchecked) as desired in an analogous manner. The selection of notes may be
saved by
pressing the "OK" touchscreen button 3696, which saves the selection of user-
selectable
-99-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
notes and returns the graphical user interface to the previous individual
logbook entry
screen 3626.
[0431] If a logged ketone level is selected from the Logbook List screen 3604,
the ketone
level individual logbook entry screen 3698 is displayed, which displays the
logged ketone
reading in mmol/L. If a logged glucose control solution test is selected from
the Logbook
List screen 3604, the glucose control solution test level individual logbook
entry screen
3700 is displayed, which displays the logged glucose control solution test
reading in
mg/dL. If a logged ketone control solution test is selected from the Logbook
List screen
3604, the ketone control solution test level individual logbook entry screen
3702 is
displayed, which displays the logged ketone control solution test reading in
mmol/L.
[0432] Additional Individual Logbook Entry screens include an unlogged dose of
rapid-
acting insulin, FIG. 22E, and a time change, FIG. 22F. The logbook entry of an
unlogged
dose of rapid acting insulin may include the dose amount information, time of
dose
information and time logged information. The logbook entry of a time change
entry may
include the before and after time change information. Time change logs may
additionally
include a warning that other historical options may be affected by the time
change.
Further, in certain embodiments, the user may be prompted to with a query
regarding
whether the user forgot to log any insulin dose since a set time (based on the
duration of
the insulin action, for example). If the user responds in the affirmative, the
user is
prompted further to enter the unlogged insulin dose information so that
previously
unlogged dose is logged in the logbook.
[0433] The Individual Logbook Entry screen 3626 includes an "OK" touchscreen
button
3706, which, when pressed, returns the graphical user interface to the Logbook
List screen
3604 via reference path (X1).
Strip/Hardware Errors Interface
[0434] An exemplary embodiment of a graphical user interface which may be
utilized in
connection with a Reader as described herein and which facilitates a procedure
for
displaying strip/hardware errors 3710 is provided. This graphical user
interface is now
described in greater detail with reference to FIG. 23.
[0435] The Strip/Hardware Errors Interface 3710, shown in FIGS. 23A and 23B,
may
display strip and/or hardware errors as necessary as any relevant errors
occur. A variety of
different error messages may be displayed. For example, an "Error 1" screen
3712 may be
-100-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
displayed if the Reader detects a temperature error. The "Error 1" screen may
display a
message indicating that the Reader may be too hot or too cold, and that the
user should
move the Reader and strips to the appropriate environment and check glucose
again with a
new strip. "Error 1" screen may also display a message indicating that if this
message
appears again, the user should call Customer Service.
[0436] In certain embodiments, the Strip/Hardware Errors Interface 3710 may
display an
"Error 2" screen 3714 if the Reader may not be functioning properly. The
"Error 2" screen
3714 may display a message indicating that the Reader may not be functioning
properly
and that the user should turn off the Reader and try again. The "Error 2"
screen 3714 may
also display a message indicating that if this message appears again, the user
should call
Customer Service.
[0437] In certain embodiments, the Strip/Hardware Errors Interface 3710 may
display an
"Error 3" screen 3716 if the test strip may not be working properly or if the
user's glucose
may be too low. The "Error 3" screen 3716 may display a message indicating
that the test
strip may not be working properly or the user's glucose may be too low. The
"Error 3"
screen 3716 may also display a message indicating that low glucose can be
dangerous and
that the user should check their glucose again with a new strip and treat as
recommended
by the user's health care professional. The "Error 3" screen 3716 may also
display a
message indicating that if this message appears again, the user should call
Customer
Service.
[0438] In certain embodiments, the Strip/Hardware Errors Interface 3710 may
display an
"Error 4" screen 3718 if the test strip may not be working properly or if the
user's
glucose/ketones may be too high. The "Error 4" screen 3718 may display a
message
indicating that the test strip may not be working properly or the user's
glucose/ketones
may be too high. The "Error 4" screen 3718 may also display a message
indicating that
high glucose/ketones can be dangerous and that the user should check their
glucose or
ketones again with a new strip and treat as recommended by the user's health
care
professional. The "Error 4" screen 3718 may also display a message indicating
that if this
message appears again, the user should call Customer Service.
[0439] In certain embodiments, the Strip/Hardware Errors Interface 3710 may
display an
"Error 5" screen 3720 if blood may have been applied to the test strip too
soon or the test
strip may have already been used. The "Error 5" screen 3720 may display a
message
indicating that blood may have been applied to the test strip too soon or the
test strip may
-101-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
have already been used. The "Error 5" screen 3720 may also display a message
indicating
that the user should check their glucose again with a new strip. The "Error 5"
screen 3720
may also display a message indicating that if this message appears again, the
user should
call Customer Service.
[0440] In certain embodiments, the Strip/Hardware Errors Interface 3710 may
display an
"Error 7" screen 3722 if the test strip may be damaged, used, or
unrecognizable be the
Reader. The "Error 7" screen 3722 may display a message indicating that the
test strip
may be damaged, used, or unrecognizable be the Reader. The "Error 7" screen
3722 may
also display a message indicating that the user should check their glucose
again with a new
strip. The "Error 7" screen 3722 may also display a message indicating that if
this message
appears again, the user should call Customer Service.
[0441] In certain embodiments, the Strip/Hardware Errors Interface 3710 may
display an
"Error 9" screen 3724 if the Reader is not working properly. The "Error 9"
screen 3724
may display a message indicating that the Reader is not working properly. The
"Error 9"
screen 3724 may also display a message indicating that the user should turn
off the Reader
and try again. The "Error 9" screen 3724 may also display a message indicating
that if this
message appears again, the user should call Customer Service.
[0442] The error screens include an "OK" touchscreen button 3726, which, when
pressed,
returns the graphical user interface to the Home Screen. In certain
embodiments, pressing
the "OK" touchscreen button 3726 from either the "Error 2" screen 3714 or the
"Error 9"
screen 3724 will cause the Reader display to turn off The Reader may be
activated by
inserting a test strip or by pressing the hardware power button.
System Time Loss
[0443] Upon restart of the device after a system time loss, a time recovery
display screen
may be displayed. FIG. 23C illustrates steps to recover the date and time
following a
system time loss. After a device power off, shown at block 3726, due to one or
more
errors, the user presses the home button or inserts a test strip to power on
the device 3728.
Upon power on, the device navigates to a time recovery flow of display
screens, beginning
with recover date 3730, clock style set 3732 and recover time 3734. The steps
to recover
date and time following a system time loss are similar to those described
herein below
with respect to first setup of the device. After the current date and time are
entered into the
device, pressing the "next" button on the final recover screen will navigate
the device back
to the home screen, as shown at block 3736.
-102-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Setup
[0444] An exemplary embodiment of a graphical user interface which may be
utilized in
connection with a reader as described herein and which facilitates a Setup
procedure 2000
is provided. This graphical user interface is now described in greater detail
with reference
to FIGS. 24-27 and 29-33.
First Start Interface
[0445] A graphical user interface which facilitates a Setup procedure 2000 of
the reader
may include a First Start Interface 2001. First Start Interface 2001 begins
with the display
of an introduction screen 2002, e.g., for approximately 3 seconds. This
introduction screen
2002 may include text and/or graphics designed to identify the manufacturer of
the reader
and/or the graphical user interface, e.g., the introduction screen 2002 may
include the
FreeStyle0 butterfly trademark depicted in FIG. 24.
Language Selection
[0446] Following display of the introduction screen 2002, one or more Language
Selection
Screens 2003 are provided. In one embodiment, there is no default selection
and the "OK"
touch-screen button 2004 appears only after a language selection has been
made, e.g., by
touching the empty circle 2006 next to the language to be selected. The
language selection
options may be displayed in alphabetical order. If the list of language
selection options
includes more than 4 languages, the list may be scrolled as necessary to view
the list using
scroll touch-screen buttons 2005. The language list may be minimized by region
and the
order of language selection may be modified at a later time.
Date Selection
[0447] Once the language selection has been made by pressing an empty circle
2006 next to
a language to be selected followed by the "OK" touch-screen button 2004, a
First Date
Selection Screen 2008 is displayed. This is depicted in FIG. 24 by reference
path (Y1)
2007. At this stage, a user may return to the one or more Language Selection
Screens via
reference path (Z1) 2009 by pressing touch-screen "back" button 2010 if
desired. First
Date Selection Screen 2008 provides a prompt 2011 to enter the current date
along with a
sample date 2012 which can be adjusted to the current date by the user. First
Date
Selection Screen 2008 also includes touchscreen up-arrow 2013 and touchscreen
down-
arrow 2014 by which the sample date 2012 may be adjusted by the user. For
example, the
-103-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
sample date 2012 may be adjusted by 1 day increments by tapping the up arrow
2013 or
the down arrow 2014. In one embodiment, the date increases or decreases as
appropriate at
a slow-rate for the first 2 seconds and at a faster rate after 2 seconds when
the up or down
arrow is pressed and held. The date format is set for SKU by region (U.S.
format depicted
in FIG. 24), which applies to all screens showing date.
Clock Style Selection
[0448] The user may move to the next screen in the graphical user interface
Setup
procedure 2000 by pressing touchscreen "next" button 2015, which causes First
Clock
Style Selection Screen 2016 to be displayed. At this stage, a user may return
to the First
Date Selection Screen 2008 by pressing touch-screen "back" button 2018 if
desired. First
Clock Style Selection Screen 2016 provides a prompt 2017 to select a clock
style, e.g., 12-
hour (am/pm) or 24-hour by touching the empty circle 2019 next to the clock
style to be
selected. Note that FIG. 24 depicts a selection of 12-hour (am/pm) as the
clock style.
Time Selection
[0449] The user may move to the next screen in the graphical user interface
Setup
procedure 2000 by pressing touchscreen "next" button 2020, which causes First
Time
Selection Screen 2021 to be displayed. At this stage, a user may return to the
First Clock
Style Selection Screen 2016 by pressing touch-screen "back" button 2022 if
desired. First
Time Selection Screen 2021 provides a prompt 2023 to enter the current time.
As depicted
in FIG. 24, First Time Selection Screen 2021 includes a first touchscreen up-
arrow 2024
and a first touchscreen down-arrow 2025 for adjusting the hour increments of
time 2026.
First Time Selection Screen 2021 also includes a second touchscreen up-arrow
2027 and a
second touchscreen down-arrow 2028 for adjusting the minute increments of time
2026.
The initial time format (12 or 24 hour) is displayed as set for the
appropriate region. Using
the relevant up and down-arrows Time adjusts by 1 minute or 1 hour increments
with
arrow click. When pressing and holding the up or down-arrows, the appropriate
time
increments scroll at slow rate for the first 2 seconds and at faster rate
after 2 seconds.
Target Glucose Range Selection
[0450] Once the current time has been entered on First Time Selection Screen
2021, the
user can move to the next screen in the Setup procedure 2000 by pressing
touchscreen
"next" button 2029, which causes First Target Range Selection Screen 2033 to
be
displayed. This is depicted in FIG. 24 by reference path (A2) 2030. At this
stage, the user
-104-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
may return to the First Time Selection Screen 2021 via reference path (B2)
2031 by
pressing touchscreen "back" button 2032 if desired. First Target Range
Selection Screen
2033 provides a first touchscreen up-arrow 2034 and a first touchscreen down-
arrow 2035
for adjusting the low end (e.g., 80 mg/dL) of target glucose range 2036. First
Target Range
Selection Screen 2033 also provides a second touchscreen up-arrow 2037 and a
second
touchscreen down-arrow 2038 for adjusting the high end (e.g., 140 mg/dL) of
target
glucose range 2036. First Target Range Selection Screen 2033 also provides a
touchscreen
button "?" 2039, which, when pressed, provides a Target Range Details (DTL)
screen
2040, which may display additional information related to the target glucose
range 2036.
Target Range Details (DTL) screen 2040 includes a touchscreen "OK" button
2041,
which, when pressed, returns the user to the First Target Range Selection
Screen 2033.
First Home Button
[0451] Once the target glucose range has been entered on First Target Range
Selection
Screen 2033, the user can move to the next screen in the Setup procedure 2000
by pressing
touchscreen "next" button 2042, which causes First Home Button (BTTN) Screen
2043 to
be displayed. The First Home Button (BTTN) Screen 2043 includes a prompt
describing
the function of the home button of the reader. For example, the prompt may be
a text
prompt which states "While using the Reader, press the button to return to the
home
screen" or the equivalent. This text may be provided with a graphical
depiction of the
location of the home button on the reader as shown in FIG. 24. At this stage,
the user may
return to the First Target Range Selection Screen 2033 by pressing touchscreen
"back"
button 2044 if desired. No "back" button will be displayed if the user arrives
at the First
Home Button Screen 2043 from the Settings Menu (discussed in greater detail
below) If
the reader powers off after this screen is viewed, the next power on event
will not result in
display of the First Start Interface startup sequence described above. If this
screen has not
been displayed, the First Start Interface startup sequence will start at the
beginning, and
any settings made are NOT retained.
Arrow Description Screen
[0452] Pressing the touchscreen "next" button 2045 displayed on the First Home
Button
Screen 2043 results in the display of Arrow Description Screen 2046, which
includes a
description of various trending arrows utilized by the graphical user
interface to convey
glucose trend information. For example, the Arrow Description Screen 2046 may
display a
text prompt which states "When you scan your Sensor an arrow will indicate
your recent
-105-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
glucose trend" or the equivalent. This text prompt may be followed by various
arrows and
associated descriptions of the trending information conveyed thereby. For
example, a first
arrow 2047 pointing straight up may indicate that the user's glucose level is
"Rising
quickly", a second arrow 2048 pointing up and to the right at an approximately
45 degree
angle may indicate that the user's glucose level is "Rising" or the
equivalent, a third arrow
2049 pointing straight to the right may indicate that the user's glucose level
is "Stable" or
the equivalent, a fourth arrow 2050 pointing down and to the right at an
approximately 45
degree angle may indicate that the user's glucose level is "Falling" or the
equivalent, and a
fifth arrow 2051 may indicate that the user's glucose level is "Falling
quickly" or the
equivalent. At this stage, the user may return to the First Home Button Screen
2043 by
pressing touchscreen "back" button 2052 if desired.
Charge Description Screen
[0453] Pressing the touchscreen "next" button 2053 displayed on Arrow
Description Screen
2046 results in the display of Charge Description Screen 2054. Charge
Description Screen
2054 provides a text prompt reminding the user to recharge the reader on a
regular basis.
For example, Charge Description Screen 2054 may display the text prompt
"Recharge the
Reader regularly" or the equivalent. Charge Description Screen 2054 may also
display a
graphic demonstrating to the user how to connect the reader to a power source
for
recharging purposes. At this stage, the user may return to the Arrow
Description Screen
2046 by pressing touchscreen "back" button 2055 if desired. Charge Description
Screen
2054 also includes a touchscreen "done" button 2056, which, when pressed,
completes the
First Start Interface 2001. At this point, a sensor activation procedure may
be
implemented.
Settings Interface
[0454] A graphical user interface which facilitates a Setup procedure 2000 may
include a
Settings Interface 2057 which includes a Settings Menu 2058 which may be
displayed on
the reader. Settings Menu 2058 includes a first settings screen 2059, a second
settings
screen 2060, and a third settings screen 2061. First settings screen 2059
includes the
following menu items: "Sounds", "Target Range", and "Control Solution Test."
Each of
these menu items is represented by a corresponding touchscreen button
(touchscreen
buttons 2062, 2063 and 2064 respectively). Second settings screen 2060
includes the
following menu items: "Time & Date", "Display Brightness", and "Language".
Each of
-106-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
these menu items is represented by a corresponding touchscreen button
(touchscreen
buttons 2065, 2066 and 2067 respectively). Finally, third settings screen 261
includes the
following menu items: "System Status", "Calculator Settings", "Reader Basics",
and
"Professional Options". Each of these menu items is represented by a
corresponding
touchscreen button (touchscreen buttons 2068, 2069, 2070 and 2071
respectively).
Touchscreen scroll buttons 2072, 2073, 2074 and 2075 may be used as
appropriate to
scroll between the first, second and third settings screens.
Sounds
[0455] Pressing touchscreen button 2062 ("Sounds") results in the display of
sound settings
screen 2076.This is depicted in FIG. 25 by reference path (C2) 2077. Sound
settings
screen 2076 includes touchscreen toggle settings for "Volume" 2078,
"Notification Tone"
2079, "Notification Vibrate" 2080, and "Button Tone" 2081. Sounds and
vibration can be
toggled on or off with these settings. Button sounds include, e.g., key
presses and
interactive notifications (e.g., result ready). The first two options impact
screens that
broadcast Notification, Confirmation and Reminder signals. Default button
sound is Off.
Default notification sound is On while vibration is Off. For hold-down
scrolling, a sound
is made for the initial touch only. Once the desired sound settings selections
have been
made using the touchscreen toggle settings, the settings can be accepted by
touching
touchscreen "OK" button 2082.
Target Range
[0456] Pressing touchscreen button 2063 ("Target Range") results in the
display of Target
Range settings screen 2083.This is depicted in FIG. 25 by reference path (D2)
2084.
Target Range settings screen 2083 provides a first touchscreen up-arrow 2085
and a first
touchscreen down-arrow 2086 for adjusting the low end (e.g., 70 mg/dL) of
target glucose
range 2089. Target Range settings screen 2083 also provides a second
touchscreen up-
arrow 2087 and a second touchscreen down-arrow 2088 for adjusting the high end
(e.g.,
130 mg/dL) of target glucose range 2089. At this stage, the user may return to
first settings
screen 2059 via reference path (D2) 202084 by pressing touchscreen "back"
button 2093 if
desired. The current settings can be selected by pressing touchscreen "done"
button 2094.
Target Range settings screen 2083 also provides a touchscreen button "?" 2090,
which,
when pressed, provides a Target Range Details (DTL) screen 2091, which may
display
additional information related to the target glucose range 2089. Target Range
Details
-107-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
(DTL) screen 2091 includes a touchscreen "OK" button 2092, which, when
pressed,
returns the user to the Target Range settings screen 2083.
Display Brightness
[0457] Pressing touchscreen button 2066 ("Display Brightness") results in the
display of
Display Brightness settings screen 2095.This is depicted in FIG. 25 by
reference path (G2)
2096. Display Brightness settings screen 2095 provides settings for "High",
"Medium",
and "Low" display brightness, which can be selected by touching one of empty
circles
2097. Note that FIG. 25 shows the "High" brightness setting selected. Once the
desired
display brightness has been selected by pressing one of the empty circles
2097, the setting
can be accepted by touching touchscreen "OK" button 2098.
Control Solution Test
[0458] Pressing touchscreen button 2064 ("Control Solution Test") results in
the display of
an "Insert Test Strip" (or the equivalent) prompt 2099 and initiates a Control
Solution Test
protocol as shown in flow-diagram 2100.This is depicted in FIG. 25 by
reference path
(E2) 2101. Generally, a test strip is inserted into the reader (Step 2102).
Depending on
whether the test strip is a blood glucose (BG) or ketone test strip, a
corresponding
Precision Control Solution animation (Step 2103) or a Ketone Control Solution
animation
(Step 2104) is displayed. See, e.g., FIG. 35 for respective Precision Control
Solution
animation and Ketone Control Solution animation. Once the appropriate control
solution is
applied (Step 2105), a waiting animation is displayed (Step 2106) while the
results are
processed. See, e.g., FIG. 34, described earlier in the Blood Glucose Strip
Test and Ketone
Strip Test section earlier. Once the results are ready (Step 2107), the blood
glucose control
results (Step 2108) or the ketone results (Step 2109) are provided. These
results may be
displayed or stored, e.g., in a log-book application of the reader.
[0459] FIG. 35 illustrates an example animation interface 2430 to instruct the
user to apply
control solution, according to one embodiment. Screens 2432, 2434, and 2436
display an
image of a control solution dropper and an analyte monitoring device with test
strip
inserted into the strip port. The dropper and test strip move closer as the
sequence of
screens 2432, 2434, and 2436 progress to animate the application of the
dropper on the test
strip. Animation interface 2430 may be displayed, for example, when the
analyte
monitoring device is ready to receive the control solution.
[0460] FIG. 35 also illustrates an example animation interface 2438 to
instruct the user to
apply a ketone control solution, according to one embodiment. Similar to
interface 2430,
-108-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
screens 2440, 2442, and 2444 display an image of a control solution dropper
and an
analyte monitoring device with test strip inserted into the strip port. The
dropper and test
strip move closer as the sequence of screens 2440, 2442, and 2444 progress to
animate the
application of the dropper on the test strip. Animation interface 2438,
however, includes
an indication that a Ketone test is being performed¨e.g., displaying the
words, "Ketone
Test".
Time & Date
[0461] Pressing touchscreen button 2065 ("Time & Date") results in the display
of Time &
Date settings screen 2110.This is depicted in FIG. 25 by reference path (F2)
2111. Time &
Date settings screen 2110 provides a touchscreen time button 2112 and a
touchscreen date
button 2113.
[0462] Pressing touchscreen time button 2112 results in the display of a Set
Time 12 2114
or a Set Time 24 2115 screen. The Set Time 12 2114 screen includes a first
touchscreen
up-arrow 2118 and a first touchscreen down-arrow 2119 for adjusting the hour
increments
of time 2122. Set Time 12 screen 2114 also includes a second touchscreen up-
arrow 2120
and a second touchscreen down-arrow 2121 for adjusting the minute increments
of time
2122. The Set Time 24 screen 2115 includes a first touchscreen up-arrow 2123
and a first
touchscreen down-arrow 2124 for adjusting the hour increments of time 2127.
Set Time
24 screen 2115 also includes a second touchscreen up-arrow 2125 and a second
touchscreen down-arrow 2126 for adjusting the minute increments of time 2127.
The user
can toggle between the Set Time 12 2114 and the Set Time 24 2115 screen by use
of
touchscreen toggle buttons 2115 and 2116 as appropriate. In addition, the Set
Time 12
2114 and the Set Time 24 2115 screens include touchscreen "OK" buttons 2128
and 2129
respectively for accepting the entered time.
[0463] Pressing touchscreen date button 2113 results in the display of Set
Date screen 2130.
Set Date screen 2130 includes a touchscreen up-arrow button 2131 and a
touchscreen
down-arrow button 2132 for adjusting the day of the factory set date 2133. Set
Date screen
2130 also includes touchscreen "OK" button 2134 for accepting the entered
time.
[0464] Time & Date settings screen 2110 also includes touchscreen "done"
button 2135,
which, when pressed, returns the user to the Settings Menu 2058 via reference
path (H2)
2136.
-109-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Language Settings
[0465] Pressing touchscreen button 2067 ("Language") results in the display of
one or more
Language Setting screens 2137. This is depicted in FIG. 26 by reference path
(U.) 2138.
The one or more Language Setting screens 2137 include language options which
can be
selected by pressing the empty circle next to the corresponding language
choice. Note that
FIG. 26 shows the English language option selected. The user may scroll
between the
language setting screens using the displayed up or down-arrows 2139 as
appropriate. The
Language Setting screens 2137 also include touchscreen "OK" button 2140 for
accepting
the selected language.
Calculator Settings
[0466] Pressing touchscreen button 2069 ("Calculator Settings") results in the
display of
Calculator Setting screens 2141. This is depicted in FIG. 26 by reference path
(K2) 2142.
The Calculator Setting screens 2141 include, e.g., an Insulin Calculator
Settings ¨ Easy
Mode screen 2142 and an Insulin Calculator Settings ¨ Advanced Mode screen
2143.
Insulin Calculator Settings ¨ Easy Mode screen 2142 includes, e.g., insulin
unit
measurements of 18u associated with breakfast, 20u associated with lunch, 24u
associated
with dinner, a correction target of 70 to 130 mg/dL and a correction factor of
10 mg/dL.
Insulin Calculator Settings ¨ Advanced Mode screen 2143 includes, e.g., a
carbohydrate
(Carb) ratio of lu for 10g, a correction target of 70 to 130 mg/dL, and a
correction factor
of lu for 10 mg/dL. The Calculator Setting screens 2141 also include
touchscreen "OK"
button 2144 for accepting the indicated calculator settings.
[0467] Pressing touchscreen button 2070 ("Reader Basics") results in the
display of the
First Start Interface First Home Button screen 2043 discussed previously
herein. This is
depicted in FIG. 26 by reference path (L2) 2145.
Professional Options
[0468] As shown in FIG. 26, pressing touchscreen button 2071 ("Professional
Options")
results in the display of a HCP Ask screen 2146. This is depicted in FIG. 26
by reference
path (J2) 2147. The HCP Ask screen 2146 displays a prompt asking whether the
user is a
health care professional. This question can be answered by touching the empty
circle 2148
next to either the "Yes" or "No" option. Note that FIG. 26 shows the "Yes"
option has
been selected. No default selection is provided and touchscreen "next" button
2149 is not
displayed until an option is selected. HCP Ask screen 2146 also includes a
touchscreen
"back" button 2150, which, when pressed, returns the user to the Settings Menu
2058 via
-110-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
reference path (J2) 2147. As indicated in FIG. 26, if the "No" option is
selected a Not
HCP screen 2151 is displayed which indicates, e.g., that "Professional Options
can only be
used by a health care professional." The Not HCP screen 2151 also includes a
touchscreen
"done" button 2152 which, e.g., returns the user to the Settings Menu 2058 via
reference
path (J2) 2147. If the "Yes" option is selected, a passcode entry screen 2153
is displayed
which prompts the user to enter a passcode. Entry of an incorrect passcode
will result in,
e.g., an "Incorrect Code" prompt. Entry of the correct passcode allows the
user access to
the Professional Options screen 2154. Professional Options screen 2154
includes
touchscreen button options; a System Reset 2155 option, a Masked Mode 2156
option, a
Dose Increment 2157 option, and an Insulin Calculator 2159 option. Pressing
the
touchscreen button for the System Reset option results in the display of a
Reset System
(SYS) screen 2155. Selecting the "reset" option from this screen restores all
reader
settings to the factory defaults and deletes all patient data stored in the
reader. The user is
then returned to the First Start Interface 2001.
[0469] Pressing the touchscreen button for the Masked Mode option initiates a
Masked
Mode interface 2156 which is discussed elsewhere herein.
[0470] Pressing the touchscreen button for the Dose Increment option initiates
a Dose
Increment interface 2157. The Dose Increment interface 2157 allows the HCP to
change
the dose increment setting. The dose increment setting applies to both rapid-
acting and
long-acting insulin dosages. In one embodiment, the default value is 0.5 unit.
However,
the any other suitable increment value such as 0.1 unit, 0.2 unit, or some
other suitable
increment, for example, can be used. The dose increment is the minimum amount
of
insulin that can be logged, which may be displayed on the device as shown at
2158.
Pressing the "done" button returns to the Professional Options screen 2154.
[0471] Pressing the touchscreen button for the Insulin Calculator option
initiates an Insulin
Calculator Start interface 2159 which is discussed in greater detail below.
[0472] Professional Options screen 2154 also includes a touchscreen "OK"
button 2159,
which, when pressed, returns the user to the Settings Menu 2058 via reference
path (J2)
2147.
-111-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
System Status
[0473] Pressing touchscreen button 2068 ("System Status") initiates a System
Status
Interface 2160. This aspect of the graphical user interface is discussed in
greater detail
below.
System Status Interface
[0474] A graphical user interface which facilitates a Setup procedure 2000 may
include a
System Status Interface 2160 which includes a System Status menu 2161, which
may be
displayed on the reader. System Status menu 2161 includes the following items:
"System
Info", "Self-Test", "Touchscreen Test", and "Error Log", which can be selected
by
touching corresponding touchscreen buttons 2162, 2163, 2164, and 2165
respectively.
System Status menu 2161 includes a touchscreen "OK" button 2169, which, when
pressed,
returns the user to the Settings Interface 2057.
[0475] Pressing the touchscreen button 2162 for System Info results in display
of System
Info screen 2166, which may display information such as reader serial no.,
reader software
no., reader hardware no., sensor ID, sensor software no., sensor count, strip
count, date of
last scan, last reset, and the like. This is depicted in FIG. 27 by reference
path (N2) 2167.
System Info screen 2166 includes a touchscreen "OK" button 2168, which, when
pressed,
returns the user to the System Status menu 2161 via reference path (M2) 2170.
[0476] Pressing the touchscreen button 2163 for Self-Test results in display
of Self-Test
screen 2171 and initiation of a self-diagnostics protocol. This is depicted in
FIG. 27 by
reference path (02) 2172. The self-diagnostics protocol can also be initiated
by a press of
the power button and a double-tap anywhere on the screen. Self-Test screen
2171 includes
a touchscreen "LCD Test" button 2173. Pressing touchscreen "LCD Test" button
2173
results in display of an LCD Test Intro screen 2174A. LCD Test Intro screen
2174A
includes, e.g., a prompt indicating "On the next screen check for missing
pixels. Press
'start' to begin the test." LCD Test Intro screen 2174A also includes a
touchscreen "start"
button 2175 and a touchscreen "back" button 2176. When pressed, the
touchscreen "back"
button 2176 returns the user to Self-Test screen 2171. Pressing the
touchscreen "start"
button 2175 initiates an LCD test and results in the display of an LCD Test
Results screen
2174B. The LCD Test Results screen 2174B may include a prompt indicating that
the
LCD test has been completed. The LCD Test Results screen 2174B may also
include a
touchscreen "done" button 2177, which, when pressed returns the user to Self-
Test screen
2171 or the System Status menu 2161 via reference path (M2) 2170. Self-Test
screen 2171
-112-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
also includes a touchscreen "OK" button 2168, which, when pressed, returns the
user to
the System Status menu 2161 via reference path (M2) 2170.
[0477] Pressing the touchscreen button for Sound Test results in display of a
Sound Test
screen. Sound Test screen may display a prompt indicating, e.g., "If you do
not hear a
series of tones and vibrations, contact Customer Service." Sound Test screen
may include
a "next" button which, when pressed, may navigate to the next settings test.
Sound Test
screen may include a "back" button which, when pressed, returns the user to
the System
Status menu 1261.
[0478] Pressing the touchscreen button 2164 for Touchscreen Test results in
display of
Touch Sense Test screen 2178. This is depicted in FIG. 27 by reference path
(P2) 2179.
Touch Sense Test screen 2178 may display a prompt indicating, e.g., "Press
start button to
test display touch zone grid and test all the touch zones. Press the Power
button in the
touch sense grid to navigate back to the Diagnostic Menu." Touch Sense Test
screen 2178
includes a touchscreen "start" button 2180, which, when pressed, results in
display of a
touch sense grid 2181 for testing the responsiveness of the various zones of
the touch
sense grid 2181. Touch Sense Test screen 2178 also includes a touchscreen
"back" button
which, when pressed, returns the user to the System Status menu 2161 via
reference path
(M2) 2170.
[0479] Pressing the touchscreen button 2165 for Error Log results in display
of an Error
Log Empty screen 2182 or an Error Log screen 2184. This is depicted in FIG. 27
by
reference paths (Q2) 2183 and (R2) 2185 respectively depending on whether any
error
entries are present in the logbook. Error Log screen 2184 may include one or
more down
or up-arrows to scroll between pages of the error log if necessary.
Masked Mode Interface
[0480] An exemplary embodiment of a graphical user interface which may be
utilized in
connection with a reader as described herein and which functions to enable the
operation
of the analyte monitoring device in a masked mode.
[0481] FIG. 28 illustrates a Professional Options screen 2502 that displays a
menu of
options for customizing the operation of the device. For example, the menu of
options may
be geared more for a physician or other health care professional to set up or
otherwise
configure the device for the patient. In one embodiment, the Professional
Options screen
2502 may require a code or password to access, which the physician has but the
patient
-113-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
does not. In another embodiment, the Professional Options screen 2502 may not
be restrict
access and the patient may also change the configuration.
[0482] Professional Options screen 2502 includes a Masked Mode option 2504 for
navigating to a Masked Mode interface 2510 that enables activation or
deactivation of the
Masked Mode. Examples of other options include an Insulin Calculator option
2508 for
navigating to an interface for activating or deactivating the insulin
calculator. A System
Reset Option 2502 is also included to reset the option to a default setting.
[0483] Masked Mode interface 2510 includes an activation icon, symbol, trigger
element,
etc., that may be selected to activate the Masked Mode. Masked Mode interface
2510 may
also provide additional information about the Masked Mode to inform the user
of its use.
Masked Mode interface 2510 also includes trigger element 2514a for navigating
back to
the Professional Options screen 2502. Trigger element 2514b takes the user to
a screen for
setting a reminder to take a sensor reading, as shown by reference path T2.
[0484] Upon selection of trigger element 2514b, Masked Rem screen 2516 is
displayed to
enable setting the device to provide reminders to the user to perform a
reading. For
example, element 2518 may be selected to activate or deactivate the reminder
feature on
the device. Additional information may also be provided to inform the user of
the
reminder option. If the reminder is "off' and the user selects trigger element
2520a takes
the user back to the Professional Options screen 2502. If the reminder is "on"
and the user
selects trigger element 2520b, then a Masked REM ¨ Time screen 2522 is
displayed to
enable the user to set times for initiating a reminder. Once the time is set,
the user can
select trigger element 2522b to trigger the Masked Complete (Comp) screen 2524
which
indicates that the Masked Mode is activated. In some embodiments, the Masked
Mode
reminder is set for a predetermined time period, e.g., a number of hours,
e.g., 8 hours, 12
hours, 24 hours, etc. In some embodiments, if the user runs a manual scan
prior to the
upcoming reminder, the reminder timer period automatically resets. In certain
embodiments, the device includes a reminder delay option, such that upon
activation of the
reminder in Masked Mode, the user has the option of delaying the reminder for
a preset
length of time. Upon user confirmation of the trigger element 2524, the device
navigates
back to the Professional Options screen 2502.
-114-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Calculator Start Interface
[0485] A graphical user interface which facilitates a Setup procedure 2000 may
include a
Calculator Start Interface 2186 as mentioned previously herein. Calculator
Start Interface
2186 may be initiated by pressing the touchscreen button for the Insulin
Calculator 2157
option located on the Professional Options screen 2154. Pressing the
touchscreen button
for the Insulin Calculator 2157 option results in a calculator On/Off status
determination.
If the calculator is On, reference path (X2) 2187 is initiated, which results
in display of a
Calculation Edit screen 2188. Calculation Edit screen 2188 includes a
touchscreen "Turn
Off Calculator" button 2189. Pressing the "Turn Off Calculator" button 2189
results in
display of a Calculation Off screen 2190, which provides a prompt indicating
that the
insulin calculator is turned off. In this case, the calculator button will no
longer be
available when checking glucose levels. The Calculation Off screen 2190
includes a
touchscreen "done" button, which, when pressed, initiates reference path (V2)
2192,
which returns the user to the Professional Options screen 2154.
[0486] Calculation Edit screen 2188 also includes a touchscreen "Change
Calculator
Settings" button 2193. Pressing the touchscreen "Change Calculator Settings"
button 2193
results in initiation of reference path (B3) 2194 discussed in greater detail
below.
Calculation Edit screen 2188 also includes a touchscreen "back" button 2195,
which,
when pressed, initiates reference path (W2) 2196 which returns the user to the
Professional Options screen 2154.
[0487] As discussed above, pressing the touchscreen button for the Insulin
Calculator 2157
option results in a calculator On/Off status determination. If the calculator
is Off, a I Take
screen 2197 is displayed. The I Take screen 2197 includes a prompt, "Does your
patient
take rapid-acting (short acting) insulin at meals?" The I Take screen 2197
also includes
empty circles 2198 which may be pressed so as to indicate a Yes or No answer
to the
above prompt. Note that FIG. 29 shows the Yes option selected. The I Take
screen 2197
also includes a touchscreen "next" button 2199, which initiates a reference
path (Z2) 2200
(discussed in greater detail below) in the event the Yes option was selected.
[0488] Reference path (B3) 2194 and reference path (Z2) 2200 result in display
of
Calculation Type 01 screen 2201 or a Calculation Type 02 screen 2202 depending
on
whether an "Easy" or "Advanced" Setup Option is selected respectively using
touchscreen
toggle element 2203. The "Easy" Setup Option is utilized for patients who
start with a
fixed dose of rapid-acting insulin at meals while the "Advanced" Setup Option
is utilized
-115-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
for patients who count carbs (in grams or servings) to adjust their rapid-
acting insulin dose
at meals. Calculation Type 01 screen 2201 and Calculation Type 02 screen 2202
each
include a touchscreen "back" button 2204 and a touchscreen "next" button 2205.
Pressing
"back" button 2204 returns the user to I Take 2197 via reference path (Y2)
2206 if I Take
2197was the previously viewed screen. Pressing "back" button 2204 returns the
user to
Calculation Edit 2188 via reference path (A3) 2207 if Calculation Edit 2188
was the
previously viewed screen. Touching touchscreen "next" button 2205 initiates
reference
path D3 2208 or E3 2209 depending on whether the Easy or Advanced Setup option
is
selected respectively.
[0489] Reference path D3 2208 results in display of a Calculation Steps EZ
screen 2210,
including the following prompts: "This setup has two parts: 1) Enter each of
your
patient's meal-time insulin doses; and 2) Enter your patient's correction
settings."
Calculation Steps EZ screen 2210 includes a touchscreen "back" button 2211 and
a
touchscreen "next" button 2212. Pressing touchscreen "back" button 2211
returns the user
to Calculation Type 01 screen 2201 and Calculation Type 02 screen 2202 via
reference
path (C3) 2213. Pressing touchscreen "next" button 2212 results in initiation
of an Easy
Calculation Setup interface 2214 described in greater detail below.
[0490] Reference path E3 2209 results in display of a Calculation Steps
Advanced screen
2215, including the following prompts: "This setup has two parts: 1) Enter
your patient's
meal-time insulin settings; and 2) Enter your patient's correction settings."
Calculation
Steps Advanced screen 2215 includes a touchscreen "back" button 2216 and a
touchscreen
"next" button 2217. Pressing touchscreen "back" button 2216 returns the user
to
Calculation Type 01 screen 2201 and Calculation Type 02 screen 2202 via
reference path
(C3) 2213. Pressing touchscreen "next" button 2217 results in display of a
Food Units 01
screen 2218. Food Units 01 screen 2218 includes a prompt "Enter food by:" and
includes
two options "Grams of carbs" and "Servings" which can be selected by pressing
one of
corresponding empty circles 2219. Note that FIG. 29 shows the "Grams of carbs"
option
selected. Food Units 01 screen 2218 includes a touchscreen "back" button 2220
and a
touchscreen "next" button 2221. Pressing touchscreen "back" button 2220
returns the user
to Calculation Steps Advanced screen 2215. Pressing touchscreen "next" button
2221
results in initiation of an Advanced Calculation Setup interface 2222,
discussed in greater
detail below. Food Units 01 screen 2218 also includes a touchscreen "?"
button, which,
when pressed, initiates reference path (F3) 2223, which provides additional
information
-116-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
regarding the selection of "Grams of carbs" or "Servings" as shown in FIG. 29
with
reference to screens H01 2224, H02 2225 and H03 2226.
Easy Calculation Setup Interface
[0491] Easy Calculation Setup interface 2214 is described with reference to
FIG. 30. Three
Meal Calculation screens are provided, one each for Breakfast 2227, Lunch
2228, and
Dinner 2229. Each of these screens includes up and down-arrows 2230 for
adjusting the
displayed units of insulin associated with each meal. Each of these screens
also includes
touchscreen "back" and "next" buttons for navigating between screens of the
Easy
Calculation Setup interface 2214. Finally, each of these screens includes a
touchscreen "?"
button 2231, which, when pressed, initiates reference path G3 2232. Reference
path G3
2232 results in display of a screen 2233 including the prompt: "Enter the
units of rapid-
acting insulin you recommended to cover this meal." Pressing the touchscreen
"next"
button from the Dinner 2229 screen results in display of a Correction Target
screen 2234.
[0492] Correction Target screen 2234 includes first up and down-arrows (2235
and 2236)
for adjusting a low target, e.g., 70 mg/dL, of the target glucose range 2237;
and second up
and down-arrows (2238 and 2239) for adjusting a high target, e.g., 130 mg/dL
of the target
glucose range 2237. Correction Target screen 2234 includes a touchscreen "?"
button
2240, which, when pressed, initiates a reference path (H3) 2241. Reference
path H3 2241
results in display of a screen 2242 including the prompt: "Target is the
desired glucose
value if extra rapid-acting insulin is needed to correct high glucose
reading." Pressing the
touchscreen "next" button from the Correction Target screen 2234 results in
display of a
Correction Factor screen 2243. Correction Factor screen 2243 includes up and
down-
arrows 2244 for adjusting the insulin correction factor 2245. If the set
Correction Factor
scrolls below 1, a No Correction screen 2249 is displayed with the prompt "No
correction
insulin". Correction Factor screen 2243 includes a touchscreen "?" button
2246, which,
when pressed, initiates a reference path (13) 2247. Reference path 13 2247
results in
display of a screen 2248 including the prompt: "The Reader uses this value to
determine
how many units of rapid-acting insulin is needed to lower high glucose to a
target value or
range." Pressing the touchscreen "next" button from the Correction Factor
screen 2243
screen results in initiation of an Insulin On Board (I0B) Setup interface
2250, which is
described in greater detail below.
-117-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Advanced Calculation Setup Interface
[0493] Advanced Calculation Setup interface 2222 is described with reference
to FIG. 31.
Initiation of Advanced Calculation Setup interface 2222 with "By grams of
carbs" selected
on screen 2218 results in display of a Set Carb Ratio screen 2251. Set Carb
Ratio screen
2251 includes touchscreen up and down-arrows 2252 for adjusting correction
factor 2253.
Set Carb Ratio screen 2251 also includes a touchscreen "by time of day" button
2254.
[0494] Pressing the touchscreen "by time of day" button 2254 results in
display of a CR
Time List screen 2255. CR Time List screen 2255 includes touchscreen buttons
2256
which may be used to select a time period, e.g., morning, midday, evening, or
night for the
correction factor 2253. Pressing one of touchscreen buttons 2256 results in
display of a CR
Time screen 2257, which includes touchscreen up and down-arrows 2258 for
adjusting
correction factor 2253 by time of day. CR Time screen 2257 includes a
touchscreen "OK"
button 2259, which, when pressed, results in selection of the displayed
correction factor
2253 by time of day and returns the user to the CR Time List screen 2255. Time
List
screen 2255 includes a touchscreen "back" button 2260 for returning to the Set
Carb Ratio
screen 2251 and a touchscreen "done" button 2261 for indicating completion of
the
correction factor 2253 by time of day setup and, in some embodiments, for
initiating the
Correction Setup interface 2268, discussed in greater detail below.
[0495] Set Carb Ratio screen 2251 and CR Time List screen 2255 each include a
touchscreen "?" button 2264, which, when pressed, results in display of a Carb
Ratio
information screen 2265. Carb Ratio information screen 2265 displays a prompt
indicating
"Carbohydrate ratio is the amount of carbohydrates that 1 unit of insulin will
cover."
Pressing the touchscreen "OK" button 2266 from Carb Ratio information screen
2265
returns the user to the Set Carb Ratio screen 2251 or the CR Time List screen
2255 as
appropriate.
[0496] Set Carb Ratio screen 2251 includes a touchscreen "back" button 2262
and a
touchscreen "done" button 2263. Pressing touchscreen "back" button 2262
returns the user
to the Calculation Start interface 2186 as described previously herein via
reference path
(J3) 2267. Pressing touchscreen "done" button 2263 initiates a Correction
Setup interface
2268 via reference path (K3) 2269.
[0497] Initiation of Advanced Calculation Setup interface 2222 with "By
servings" selected
on screen 2218 results in display of a Servings Definition screen 2270.
Servings Definition
screen 2270 includes touchscreen up and down-arrows 2271 for adjusting
servings
-118-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
definition 2272 (e.g., 1 serving = 10.0 grams carbs). Servings Definition
screen 2270 also
includes a touchscreen "back" button 2273 and a touchscreen "next" button
2274. Pressing
touchscreen "back" button 2273 returns the user to the Calculation Start
interface 2186 as
described previously herein. Pressing touchscreen "next" button 2274 results
in display of
a Set Carb Ratio Servings screen 2275. Set Carb Ratio Servings screen 2275
includes
touchscreen up and down-arrows 2276 for adjusting servings ratio 2277 (e.g.,
For 1
serving: 1.5 units insulin). Set Carb Ratio Servings screen 2275 also includes
a
touchscreen "by time of day" button 2278.
[0498] Pressing the touchscreen "by time of day" button 2278 results in
display of a CR
Servings Time List screen 2279. CR Servings Time List screen 2279 includes
touchscreen
buttons 2280 which may be used to select a time period, e.g., morning, midday,
evening,
or night for the servings ratio 2277. Pressing one of touchscreen buttons 2280
results in
display of a CR Servings Time screen 2281, which includes touchscreen up and
down-
arrows 2282 for adjusting servings ratio 2277 by time of day. CR Servings Time
screen
2281 includes a touchscreen "OK" button 2283, which, when pressed, results in
selection
of the displayed servings ratio 2277 by time of day and returns the user to
the CR Servings
Time List screen 2279. CR Servings Time List screen 2279 includes a
touchscreen "back"
button 2284 for returning to the Set Carb Ratio Servings screen 2275 and a
touchscreen
"done" button 2285 for initiating the Correction Setup interface 2268,
discussed in greater
detail below.
[0499] Set Carb Ratio Servings screen 2275 includes a touchscreen "back"
button 2286 and
a touchscreen "next" button 2287. Pressing touchscreen "back" button 2286
returns the
user to the Servings Definition screen 2270. Pressing touchscreen "next"
button 2287
results in initiation of the Correction Setup interface 2268, discussed in
greater detail
below.
[0500] Servings Definition screen 2270, Set Carb Ratio Servings screen 2275,
and CR
Servings Time List screen 2279 each include a touchscreen "?" button 2288,
which, when
pressed, results in display of a Servings Ratio information screen 2289 via
reference path
(L3) 2290. Servings Ratio information screen 2289 displays a prompt indicating
"Servings
ratio is the amount of insulin that will cover 1 serving" or the equivalent.
Pressing the
touchscreen "OK" button 2291 from Servings Ratio information screen 2289
returns the
user to the Servings Definition screen 2270, Set Carb Ratio Servings screen
2275, or CR
Servings Time List screen 2279 as appropriate.
-119-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Correction Setup Interface
[0501] Correction Setup interface 2268 is described with reference to FIG. 32.
Initiation of
Correction Setup interface 2268 results in display of Target Type screen 2292.
Target
Type screen 2292 includes a prompt "How does your patient correct their
glucose" or the
equivalent. Target Type screen 2292 includes the following two options or
their
equivalent: 1) To a single target; and 2) To a target range, one of which may
be selected
by pressing one of the corresponding empty circles 2293 associated with the
options. Note
that FIG. 32 shows the "To a single target" options selected. Target Type
screen 2292 also
includes a touchscreen "back" button 2294 and a touchscreen "next" button
2295. Pressing
touchscreen "back" button 2294 returns the user to the previously viewed
screen (Set Carb
Ratio screen 2251, CR Time List screen 2255, Set Carb Ratio Servings screen
2275, or CR
Servings Time List screen 2279.
[0502] When the "To a single target" option is selected on screen 2292,
pressing the
touchscreen "next" button 2295 results in display of a Correction Target
screen 2296.
Correction Target screen 2296 includes touchscreen up and down-arrows 2297 for
adjusting correction target 2298 (e.g., 100 mg/dL). Correction Target screen
2296 also
includes a touchscreen "by time of day" button 2299. Pressing the touchscreen
"by time of
day" button 2299 results in initiation of reference pathway (P3) 2300,
discussed in greater
detail below. Correction Target screen 2296 also includes touchscreen "back"
button 2301
and touchscreen "next" button 2302. Pressing touchscreen "back" button 2301
returns the
user to Target Type screen 2292. Pressing touchscreen "next" button 2302
results in
initiation of reference path (03) 2303, discussed in greater detail below.
[0503] When the "To a target range" option is selected on screen 2292,
pressing the
touchscreen "next" button 2295 results in display of a Correction Target Range
screen
2304. Correction Target Range screen 2304 includes first touchscreen up and
down-
arrows 2305A for adjusting the low end (e.g., 70 mg/dL) of correction target
range 2306.
Correction Target Range screen 2304 also includes second touchscreen up and
down-
arrows 2305B for adjusting the high end (e.g., 130 mg/dL) of correction target
range 2306.
Correction Target Range screen 2304 also includes a touchscreen "by time of
day" button
2307. Pressing the touchscreen "by time of day" button 2307 results in
initiation of
reference pathway (M3) 2308, discussed in greater detail below. Correction
Target Range
screen 2304 also includes touchscreen "back" button 2309 and touchscreen
"next" button
-120-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
2310. Pressing touchscreen "back" button 2309 returns the user to Target Type
screen
2292. Pressing touchscreen "next" button 2310 results in initiation of
reference path (03)
2303, discussed in greater detail below. Target Type screen 2292, includes a
touchscreen
"?" button 2311, which, when pressed, displays a target information screen
2312,
including a prompt indicating that "The calculator can determine how much
insulin is
needed to bring the glucose to either a single number or within a range" or
the equivalent.
Pressing the touchscreen "OK" button 2313 returns the user to the Target Type
screen
2292.
[0504] Each of Correction Target screen 2296, and Correction Target Range
screen 2304
includes a touchscreen "?"button 2314, which, when pressed, displays a target
information screen 2315, including a prompt indicating that "The correction
target setting
allows you to set a glucose target that will adjust your patient's insulin
dose if their
glucose readings is above or below the target" or the equivalent. Pressing the
touchscreen
"OK" button 2316 returns the user to the Correction Target screen 2296 or the
Correction
Target Range screen 2304 as appropriate.
[0505] As discussed above, pressing the touchscreen "by time of day" button
2299 on
screen 2296 results in initiation of reference pathway (P3) 2300. Initiation
of reference
pathway (P3) 2300 results in display of Target Time List screen 2317, which
includes
touchscreen buttons 2318 for selecting a time of day (e.g., morning, midday,
evening or
night) for correction target 2298. Pressing one of touchscreen buttons 2318
results in
display of a Target Time screen 2319. Target Time screen 2319 includes
touchscreen up
and down-arrows 2320 for adjusting correction target 2298 for the selected
time of day.
Target Time screen 2319 also includes touchscreen "OK" button 2320 for
accepting the
adjusted correction target 2298 for the selected time of day and returning the
user to
Target Time List screen 2317. Target Time List screen 2317 includes a
touchscreen
"back" button 2321 and a touchscreen "done" button 2322. Pressing touchscreen
"back"
button 2321 initiates reference pathway (Q3) 2323, which returns the user to
the
Correction Target screen 2296. Pressing touchscreen "done" button 2322
initiates
reference path (S3) 2324, discussed in greater detail below.
[0506] As discussed above, pressing the touchscreen "by time of day" button
2307 on
screen 2304 results in initiation of reference pathway (M3) 2308. Initiation
of reference
pathway (M3) 2308 results in display of Target Range Time List screen 2325,
which
includes touchscreen buttons 2326 for selecting a time of day (e.g., morning,
midday,
-121-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
evening or night) for correction target range 2306. Pressing one of
touchscreen buttons
2326 results in display of a Target Range Time screen 2327. Target Range Time
screen
2327 includes first touchscreen up and down-arrows 2328 for adjusting the low
end of
correction target range 2306 for the selected time of day. Target Range Time
screen 2327
includes second touchscreen up and down-arrows 2329 for adjusting the high end
of
correction target range 2306 for the selected time of day. Target Range Time
screen 2327
also includes touchscreen "OK" button 2330 for accepting the adjusted
correction target
range 2306 for the selected time of day and returning the user to Target Range
Time List
screen 2325. Target Range Time List screen 2325 includes a touchscreen "back"
button
2331 and a touchscreen "done" button 2332. Pressing touchscreen "back" button
2331
initiates reference pathway (N3) 2333, which returns the user to the
Correction Target
Range screen 2304. Pressing touchscreen "done" button 2332 initiates reference
path (S3)
2324, discussed in greater detail below.
[0507] Each of Target Time List screen 2317 and Target Range Time List screen
2325
includes a touchscreen "OK" button 2334, which, when pressed, results in
display of
target information screen 2315, including a prompt indicating that "The
correction target
setting allows you to set a glucose target that will adjust your patient's
insulin dose if their
glucose readings is above or below the target" or the equivalent. Pressing the
touchscreen
"OK" button 2316 returns the user to the Target Time List screen 2317 or the
Target
Range Time List screen 2325 as appropriate.
[0508] As discussed above, pressing touchscreen "next" button 2302 or 2310
initiates
reference path (03) 2303. Similarly, pressing "done" button 2322 or 2332
initiates
reference path (S3) 2324. Reference paths (03) 2303 and (S3) 2324 both result
in display
of a Set Correction Factor screen 2335. Set Correction Factor screen 2335
includes
touchscreen up and down-arrows 2336 for adjusting insulin correction factor
2337 (e.g., 1
u insulin for 10 mg/dL). Set Correction Factor screen 2335 also includes a
touchscreen
"By time of day" button 2338, which, when pressed, results in display of a
Correction
Factor Time List screen 2339. Correction Factor Time List screen 2339 includes
touchscreen buttons 2340 for selecting a time of day (e.g., morning, midday,
evening or
night) for correction factor 2337.
[0509] Pressing one of touchscreen buttons 2340 results in display of a
Correction Factor
by Time screen 2341. Correction Factor by Time screen 2341 includes
touchscreen up and
down-arrows 2342 for adjusting correction factor 2337 for the selected time of
day.
-122-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Correction Factor by Time screen 2341 also includes a touchscreen "OK" button
2343 for
accepting the adjusted correction factor 2337 for the selected time of day and
returning the
user to Correction Factor Time List screen 2339.
[0510] Correction Factor Time List screen 2339 includes a touchscreen "back"
button 2344
and a touchscreen "done" button 2345. Pressing touchscreen "back" button 2344
returns
the user to the Set Correction Factor screen 2335. Pressing touchscreen "done"
button
2345 initiates an Insulin On Board (I0B) interface 2346, discussed in greater
detail below.
[0511] Set Correction Factor screen 2335 includes a touchscreen "back" button
2347 and a
touchscreen "next" button 2348. Pressing touchscreen "back" button 2347
initiates
reference path (T3) 2348 or (R3) 2347, respectively, depending on whether the
optional
"target by time of day" format has been selected or not. Pressing touchscreen
"next"
button 2348 initiates the Insulin On Board (I0B) interface 2346. Reference
path (R3) 2347
returns the user to the Correction Target Range screen 2304 or the Correction
Target
screen 2296 as appropriate. Reference path (T3) 2348 returns the user to the
Target Range
Time List screen 2325 or the Target Time List screen 2317 as appropriate.
[0512] Each of Set Correction Factor screen 2335 and Correction Factor Time
List screen
2339 includes a touchscreen "?" button 2349, which, when pressed, results in
display of
informational screen 2350. Informational screen 2350 displays a prompt
indicating "The
Reader uses this value to determine how many units of rapid-acting insulin is
needed to
lower high glucose to a target value or range" or the equivalent.
Informational screen 2350
also includes a touchscreen "OK" button 2351 for returning the user to Set
Correction
Factor screen 2335 or Correction Factor Time List screen 2339 as appropriate.
Insulin On Board (I0B) Setup Interface
[0513] The Insulin On Board (I0B) Setup interface 2346 is described with
reference to
FIG. 33. Initiation of the IOB Setup interface 2346 results in display of Set
Insulin
Duration screen 2352. Set Insulin Duration screen 2352 includes touchscreen up
and
down-buttons 2353 for adjusting insulin duration (e.g., 4hrs:30min). Set
Insulin Duration
screen 2352 also includes touchscreen "back" button 2355 and touchscreen
"next" button
2356. Pressing touchscreen "back" button 2355 returns the user to the
previously viewed
screen, e.g. Set Correction Factor screen 2335 or Correction Factor Time List
screen 2339.
Pressing touchscreen "next" button 2356 results in display of IOB Ask screen
2357. Set
Insulin Duration screen 2352 also includes touchscreen "?" button 2358, which,
when
-123-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
pressed, results in display of insulin duration description screen 2359.
Insulin duration
description screen 2359 displays a prompt indicating "Insulin duration is the
amount of
time rapid-acting insulin remains active in a patient's body" or equivalent.
"The Reader
uses the insulin duration value and the time of your patient's last dose when
it calculates
an insulin dose" or equivalent" or equivalent. Insulin duration description
screen 2359
includes a touchscreen "OK" button which, when pressed, returns the user to
Set Insulin
Duration screen 2352.
[0514] IOB Ask screen 2357 displays a prompt indicating "Do you want the
Active Insulin
symbol to be displayed on the Home screen?" or equivalent. IOB Ask screen 2357
includes a Yes and a No option, one of which may be selected by pressing one
of the
corresponding empty circles 2361 associated with the option. Note that FIG. 33
shows the
Yes option selected. IOB Ask screen 2357 includes a touchscreen "back" button
2362 and
a touchscreen "next" button 2363. Pressing touchscreen "back" button 2362
returns the
user to Set Insulin Duration screen 2352. Pressing touchscreen "next" button
2363 results
in display of a Calculation Done screen 2364. IOB Ask screen 2357 also
includes a
touchscreen "?" button 2365, which, when pressed, results in display of an
insulin
calculator description screen 2366. Insulin calculator description screen 2366
displays a
prompt indicating that "The insulin calculator estimates the amount of rapid-
acting insulin
still in your patients body" or equivalent and "If you select yes, this
estimate will be
shown on the Home screen as a symbol" or equivalent. Note the symbol 2367
depicted in
FIG. 33. The insulin calculator description screen 2366 includes a touchscreen
"OK"
button, which, when pressed, returns the user to the IOB Ask screen 2357.
[0515] Calculation Done screen 2364 includes a prompt indicating that setup is
complete
and may include a prompt indicating "When checking glucose, the insulin
calculator will
now be available" or equivalent. Calculation Done screen 2364 also includes a
touchscreen "back" button 2368 and a touchscreen "done" button 2369. Pressing
touchscreen "back" button 2368 returns the user to IOB Ask screen 2357.
Pressing
touchscreen "done" button 2369 returns the user to the reader Home Screen as
described
herein.
Save Changes Interface
[0516] A Save Changes interface 2370 operates to remind the user to save
changes in the
event a strip is inserted 2371 into the reader or the Home button is pressed
2372 before a
-124-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
sequence is completed. In the event a strip is inserted 2371 into the reader,
reference path
(U3) 2373 is initiated. Reference path (U3) 2373 results in display of a
reminder prompt
2374 to the user. A Save Changes screen 2375 is displayed which includes a
prompt
indicating "Do you want to save your changes?" or equivalent. Save Changes
screen 2375
includes a touchscreen "Yes" button 2376 and a touchscreen "No" button 2377.
If the
"No" button 2377 is pressed or a selection is not made in 10 seconds the
settings return to
their previous values. If the "Yes" button 2376 is pressed, the new settings
are saved. In
either case, either a Blood Glucose Strip Test interface or a Ketone Strip
Test interface is
initiated as appropriate based on the identity of the inserted test strip.
[0517] Reference path (V3) 2378 results in display of a reminder prompt 2379
to the user.
A Save Changes screen 2380 is displayed which includes a prompt indicating "Do
you
want to save your changes?" or equivalent. Save Changes screen 2380 includes a
touchscreen "Yes" button 2381 and a touchscreen "No" button 2382. If the "No"
button
2382 is pressed, the settings return to their previous values. If the "Yes"
button 2381 is
pressed, the new settings are saved. In either case, the user is returned to
the Home screen
as described herein.
Additional Information Regarding Data Management Software
[0518] Additional information for the Auto Assist Software is provided in the
following
paragraphs and figures. It should be appreciated that the exemplary interface
flows are
exemplary and should not be interpreted as limiting.
Exemplary Interface Flows
Application Startup
[0519] FIG. 36 illustrates a method 4000 when starting up the RD software,
according to
one embodiment. At block 4002, the Reader is coupled to a remote processing
device,
such as the user's computer. At block 4004 it is determined whether the
software is
installed on the remote processing device. If not, then the user is prompted
to run an
installer file stored on the reader, as shown at block 4006. If the user
accepts, a default
browser is launched with the data management software (RD software) install
website, as
shown at block 4008. The user may elect to start the installation of the RD
software and an
installer application is downloaded to the remote device to install the
complete installer
application, as shown by blocks 1010 and 1012. The standard operation system
(OS)
-125-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
installer application is run and the RD software is launched, as shown at
block 4012 and
4014.
[0520] Referring back to block 4004, if the RD software is already installed
on the remote
device, then it is determined if the RD software is currently running. If so,
then the user is
taken to the Reader Landing screen at block 4026.
[0521] If the RD software is not currently running, then it is determined if
an auto-launch is
enabled to automatically launch the RD software if the analyte monitoring
device is
coupled to the remote device, as shown at block 4020. If the auto-launch is
enabled, then
the RD software is launched on the remote device. If the auto-launch is not
enabled, then
the user may manually launch the software when desired, as shown at block
4022.
[0522] When the RD software is launched, the software may automatically
perform or ask
to determine if updates to the software are available, as represented by block
4034. The
RD software may, for example, access a server via the intern& to determine
what updates
are currently available, and then compare the version of the software and any
previous
updates to see if any additional updates are missing. If new updates are not
available, then
the RD software application continues with the Data Management Startup process
to
enable the user to use the RD software, as represented at block 4036.
[0523] If new updates are available, then the Reader Landing screen is
displayed, as shown
at block 4026. If the user elects not to run the update routines at this time,
the RD software
application continues with the Data Management Startup process to enable the
user to use
the RD software, as represented at block 4036. If the user elects to install
the updates, then
the update routines are run, as shown at block 4024, before continuing on with
the Data
Management Startup process at block 4036
[0524] If instead of starting at block 4002, the user launches RD software
already installed
on a remote device, as shown at block 4028, then it is determined if the
analyte monitoring
device is coupled to the remote device, as shown at block 4030. If the reader
device is not
connected, then a Reader Welcome screen is displayed to assist the user as the
RD
software is running, as shown at block 4032. If the reader is coupled to the
remote device,
then it is determined if any updates are available as shown and discussed for
block 4034.
[0525] FIG. 37 illustrates a flowchart for the Data Management Startup process
4036,
according to one embodiment. As the RD software starts up, it is determined if
there is
date (e.g., sensor readings) on the reader device, as shown at block 4042. If
there is no
data on the reader device, then it is determined if the reader device has
already been set up,
-126-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
as shown at block 4044. If not, then the user is taken to a Guided Reader
Setup ¨ Welcome
screen for guiding the user through the reader setup process, as shown at
block 4046. If the
reader device has been setup before, then it is determined if the reader
device and remote
device are out of sync (e.g., the time on the Reader device and the remote
device are
different) If the reader device is out of sync, then a Reader Out of Sync
screen is provided,
as shown at block 4052, to inform the user and to enable synchronization. If
the reader
device is not out of sync, then the Reader Landing Screen is displayed, as
shown at block
4050.
[0526] Referring back to block 4042, if sensor reading data is on the reader
device, then the
data may be downloaded to the remote device, either automatically or upon user
confirmation, as shown by block 4054. At block 4054, it is determined if the
reader device
and remote device are out of sync. If so, then the Reader Out of Sync screen
is displayed.
If no out of sync, then it is determined if it is the first time creating
reports on the remote
device, as shown at block 4058., If so, then a Guided Reports Setup ¨ Welcome
screen is
displayed, as shown at block 4060, to assist the user with setting up reports.
If it is not the
first time creating reports on the remote device, then it is determined if a
quick print
feature is enabled to allow quick display and/or printing of predetermined or
pre-
customized reports. If the quick printing feature is not enabled, then the
user is taken to a
Generate Reports screen to enable the user to generate reports, as shown at
block 4068.
[0527] FIG. 38 illustrates an example Welcome screen, according to one
embodiment. The
Welcome screen 5000, may be shown after the user launches the software prior
to
connecting the Reader device, for example, to inform the user that the system
does not
currently recognize a connected Reader and prompts them to connect one. Screen
5000
includes a Reader tab 5002 and a Reports tab 5004 that corresponds to a Reader
Mode and
a Reports mode, respectively, of the RD software. The tabs 5002,5004 are
maintained on
other screens for the RD software. In one embodiment, the Reader mode and
Reports
mode are not accessible to the user if a Reader is not connected to the remote
device.
Reader Mode
[0528] FIG. 39 illustrates a flowchart for a method 4080 of navigating through
the Reader
mode for accessing setting and functions that are used to setup and control
the Reader
device. Reader setting are directly read form or saved back to the Reader
device via
functions primarily originating from the Reader mode and collection of
screens.
-127-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0529] Block 4082 represents a Reader Landing screen, wherein details
regarding the
Reader may be accessed. FIG. 40 illustrates an example screen displayed when
the Reader
device is connected, according to one embodiment. Screen 5050 includes the
update
notification 5056, as well as, a quick synopsis of the profile of the Reader
that is
connected. The screen 5050 also includes a menu 5058 of trigger elements for
initiating
other Reader Mode screens, such as a Profile screen, Settings screen, Custom
Notes
screen, Reminders screen, Professional Options screen, and a Backups screen,
which are
all discussed further later.
[0530] FIG. 41 illustrates an example screen that is displayed to indicate
that the Reader
device is out of sync, according to one embodiment. Screen 5050 displays an
Out of Sync
content notification 5080 that informs the user that the Reader device may be
out of sync,
and may further provide additional details about the sync (e.g., the time on
both the
Reader and the remote device) and enable the user to sync the Reader device.
For
example, trigger element 5081 is provided to enable the user to elect to sync
the Reader
device.
[0531] As shown in FIG. 39, from the Reader Landing screen at block 4082, the
user may
select from a menu 5058 of trigger elements for initiating other Reader Mode
screens,
such as a Profile screen at block 4084, Settings screen at block 4086, Custom
Notes screen
at block 4088, Reminders screen at block 4090, Professional Options screen at
block 4092,
and a Backups screen at block 4094, are accessible from the Reader Landing
screen.
[0532] At block 4084, the user is taken to a Profile screen. FIG. 42
illustrates a Profile
screen 5086, according to one embodiment. As shown, Profile screen 5086
provides a
section 5058 in which the user can set a name and patient ID, for example, to
be associated
with the Reader device. As shown, the menu 5058 of trigger elements for
initiating various
screens is maintained in the Profile screen 5086.
[0533] Referring back to FIG. 39, at block 4084, the user is taken to a
Settings screen. The
Settings screen provides screens for enabling the user to adjust general
settings - e.g., as
time, date, clock, style, language, sound and vibration options, sync settings
- as well as
target zone settings (e.g., glucose target zone settings.
[0534] At block 4084, the user is taken to a Custom Notes screen where the
user can view,
edit, and/or delete notes from the Reader device. These notes can be default
notes or
customized notes by the user.
-128-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0535] At block 4086, the user is taken to a Reminders screen where the user
can view, edit,
and/or delete reminders from the Reader device. The reminders may be provided
to
remind the user to check glucose readings, take insulin, etc.
[0536] At block 4088, the user is taken to a Professional Options screen where
the user can
access restricted features that should only be accessed by trained health care
professionals
(HCP). A password or code only given to the HCP's may be required to access
the
settings. Example features that may be restricted are the activation and
setting of an insulin
calculation feature, a masked mode operation of the device, the resetting of
the system
and/or settings on the device, etc.
Insulin Calculator Setup Interface:
[0537] An exemplary embodiment of a graphical user interface which may be
utilized in
connection with Health Management Software for a Reader as described herein
and which
facilitates a procedure for inputting the insulin calculator settings via the
Health
Management Software is provided. This graphical user interface is now
described in
greater detail with reference to FIGS. 43-46.
[0538] In some cases, the Health Management Software for the Reader may
include
programming for two or more types of medication dosage calculators. During
setup of the
Health Management Software, the Health Management Software may prompt the user
and/or the health care professional to select a type of medication dosage
calculator (e.g.,
insulin bolus calculator). The initial selection of the type of medication
dosage calculator
may be changed as desired by the user or the health care professional. In
certain
embodiments, the two or more types of medication dosage calculators include
two types of
bolus calculators. For instance, the two types of bolus calculators can
include an easy
bolus calculator and an advanced bolus calculator.
[0539] By "easy calculator", "easy bolus calculator", "simple bolus
calculator", "easy
insulin calculator" or "simple insulin calculator" is meant a bolus calculator
that includes
basic features for determining a recommended medication dosage amount, such as
a
recommended insulin dosage amount. For example, an easy bolus calculator may
include
algorithms configured to determine a recommended medication dosage amount
based on a
fixed medication dosage amount. In these instances, the easy bolus calculator
may be
appropriate for a user that administers a fixed medication dosage amount
(e.g., a fixed
insulin dosage amount) for each meal. In some embodiments, the easy bolus
calculator
only takes into account the fixed medication dosage amount when recommending
the
-129-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
medication dosage amount to the user, and thus functions as a reminder and/or
log for the
fixed medication dosage amount.
[0540] The insulin calculator setup procedure begins on the Insulin Calculator
Interface
Setup screen 3800, where the user can select an Insulin Calculator On/Off
toggle button
3802 to turn the insulin calculator on or off. When the Insulin Calculator
On/Off toggle
button is selected into the "On" position, the insulin calculator is activated
and may be set
up as described below. The desired type of insulin calculator (e.g., easy or
advanced
calculator) can be selected by selecting the insulin calculator selection box
3804, which
allows the selection of "Easy" to activate the easy insulin calculator, and
"Advanced" to
activate the advanced insulin calculator.
[0541] If the user selects the "Easy" selection in the insulin calculator
selection box 3804,
the Insulin Calculator Setup Interface screen 3800 displays the set up options
for the easy
bolus calculator. Set up for the easy bolus calculator is shown in FIG. 43.
[0542] In certain embodiments, the easy bolus calculator may determine a
recommended
medication dosage amount (e.g., a recommended rapid-acting insulin dosage
amount)
based on information, such as, but not limited to, a fixed medication dosage
amount, a
target blood glucose range (e.g., correction target), and an insulin
sensitivity (e.g.,
correction factor). In some instances, the easy bolus calculator may also
include
information, such as the patient's insulin on board, in the determination of a
recommended
medication dosage amount. For example, a fixed medication dosage amount may be
entered by meal (e.g., breakfast, lunch and dinner).
[0543] The Insulin Calculator Setup Interface screen 3800 includes amount
entry boxes for
each meal. A fixed medication dosage amount may be entered into the breakfast
amount
entry box 3806, the lunch amount entry box 3808 and the dinner amount entry
box 3810 as
units of insulin. In some embodiments, the correction target range may be
entered. The
Insulin Calculator Setup Interface screen 3800 includes correction target
range amount
entry boxes for the minimum target range value 3812 and the maximum target
range value
3814. In some embodiments, the Insulin Calculator Setup Interface screen 3800
includes a
correction factor amount entry box 3816 in which the insulin sensitivity
(e.g., correction
factor) may be entered as 1 unit per X mg/dL, where X is the amount entered
for the
correction factor. In some embodiments, the Insulin Calculator Setup Interface
screen
3800 includes radio buttons for enabling or disabling insulin calculator trend
correction by
-130-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
selecting either the trend correction enabled radio button 3818 or the trend
correction
disabled radio button 3820, respectively.
[0544] If the user selects the "Advanced" selection in the insulin calculator
selection box
3824, the Insulin Calculator Setup Interface screen 3822 displays the set up
options for the
advanced bolus calculator. Set up for the advanced bolus calculator is shown
in FIG. 44,
which shows advanced bolus calculator settings available when the insulin
calculator is set
to count carbs by grams of carbs.
[0545] By "advanced calculator", "advanced bolus calculator" or "advanced
insulin
calculator" is meant a bolus calculator that includes additional information,
such as, but
not limited to, the amount of carbohydrates consumed, the carbohydrate ratio,
a target
blood glucose range (e.g., correction target), and an insulin sensitivity
(e.g., correction
factor), in determining a recommended medication dosage amount (e.g., a
recommended
insulin dosage amount). For example, rather than using a fixed medication
dosage amount
for each meal as in the easy calculator, the advanced bolus calculator may use
dose
determination information entered by the user, such as the amount of
carbohydrates
consumed, to determine a recommended medication dosage amount. The advanced
bolus
calculator may also include additional dose determination information into the
determination of the recommended medication dosage amount, such as but not
limited to,
a patient's the current blood glucose level, an amount of exercise, a target
analyte
concentration (e.g., a target blood glucose range), an insulin sensitivity
(e.g., correction
factor), a duration of insulin action, a carbohydrate ratio, and insulin on
board information,
such as an administered medication dose time information, an administered dose
frequency information over a predetermined time period, and an administered
medication
dose amount.
[0546] The Insulin Calculator Setup Interface screen 3822 includes an "Enter
Food By"
selection box 3826, which, when selected, allows the user to set the insulin
calculator to
enter food by grams of carbs or by servings. If "Grams of Carbs" is selected
in the "Enter
Food By" selection box 3826, then Insulin Calculator Setup Interface screen
3822 displays
the set up options for the advanced bolus calculator by grams of carbs.
[0547] In some embodiments, the carbohydrate ratio may be entered. The Insulin
Calculator
Setup Interface screen 3822 includes a carbohydrate ratio amount entry box
3828 for
entering the amount of the user's carbohydrate ratio as 1 unit per X grams of
carbs, where
X is the amount entered. In some embodiments, the correction target range may
be
-131-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
entered. The Insulin Calculator Setup Interface screen 3822 includes
correction target
range amount entry boxes for the minimum target range value 3832 and the
maximum
target range value 3834. In some instances, the correction target may be
entered as a single
target value rather than a target range by selecting "Single Target" (not
shown) from the
correction target selection box 3830. In some embodiments, the Insulin
Calculator Setup
Interface screen 3822 includes a correction factor amount entry box 3836 in
which the
insulin sensitivity (e.g., correction factor) may be entered as 1 unit per X
mg/dL, where X
is the amount entered for the correction factor. In some embodiments, the
Insulin
Calculator Setup Interface screen 3822 includes radio buttons for enabling or
disabling
insulin calculator trend correction by selecting either the trend correction
enabled radio
button 3838 or the trend correction disabled radio button 3840, respectively.
[0548] In certain instances, the carbohydrate ratio, the target range, and/or
the correction
factor may be entered by time of day, as shown in FIG. 45. To enable the
carbohydrate
ratio time of day settings, the carbohydrate ratio "By Time of Day" checkbox
3842 may be
selected. To enable the target range time of day settings, the target range
"By Time of
Day" checkbox 3844 may be selected. To enable the correction factor time of
day settings,
the correction factor "By Time of Day" checkbox 3846 may be selected.
[0549] If the carbohydrate ratio "By Time of Day" checkbox 3842 is selected,
Insulin
Calculator Setup Interface screen 3848 displays the time of day settings for
the
carbohydrate ratio (see FIG. 45A). The carbohydrate ratio may be set to the
same or
different values at 4 different times of day, such as morning (e.g., 4am-
10am), midday
(e.g., 10am-4pm), evening (e.g., 4pm-lOpm), and night (e.g., lOpm-4am). The
morning
carbohydrate ratio may be entered in the morning carbohydrate ratio amount
entry box
3850, the midday carbohydrate ratio may be entered in the midday carbohydrate
ratio
amount entry box 3852, the evening carbohydrate ratio may be entered in the
evening
carbohydrate ratio amount entry box 3854, and the night carbohydrate ratio may
be
entered in the night carbohydrate ratio amount entry box 3856.
[0550] If the correction target range "By Time of Day" checkbox 3844 is
selected, Insulin
Calculator Setup Interface screen 3848 displays the time of day settings for
the target
range (see FIG. 45B). The target range may be set to the same or different
values at 4
different times of day, such as morning (e.g., 4am-10am), midday (e.g., 10am-
4pm),
evening (e.g., 4pm-lOpm), and night (e.g., lOpm-4am). The morning target range
may be
entered in the minimum value morning target range amount entry box 3858 and
the
-132-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
maximum value morning target range amount entry box 3860, the midday target
range
may be entered in the minimum value midday target range amount entry box 3862
and the
maximum value midday target range amount entry box 3864, the evening target
range may
be entered in the minimum value evening target range amount entry box 3866 and
the
maximum value evening target range amount entry box 3868, and the night target
range
may be entered in the minimum value night target range amount entry box 3870
and the
maximum value night target range amount entry box 3872.
[0551] If the correction factor "By Time of Day" checkbox 3846 is selected,
Insulin
Calculator Setup Interface screen 3848 displays the time of day settings for
the correction
factor (see FIG. 45B). The correction factor may be set to the same or
different values at 4
different times of day, such as morning (e.g., 4am-10am), midday (e.g., 10am-
4pm),
evening (e.g., 4pm-lOpm), and night (e.g., lOpm-4am). The morning correction
factor may
be entered in the morning correction factor amount entry box 3874, the midday
correction
factor may be entered in the midday correction factor amount entry box 3876,
the evening
correction factor may be entered in the evening correction factor amount entry
box 3878,
and the night correction factor may be entered in the night correction factor
amount entry
box 3880.
[0552] The Insulin Calculator Setup Interface screen 3882 includes an "Enter
Food By"
selection box 3884, which, when selected, allows the user to set the insulin
calculator to
enter food by grams of carbs or by servings (see FIG. 46A). If "Servings" is
selected in the
"Enter Food By" selection box 3884, then Insulin Calculator Setup Interface
screen 3882
displays the set up options for the advanced bolus calculator by servings of
carbs.
[0553] FIGS. 43-46 show the Insulin Calculator Setup Interface screen 3882
when the
carbohydrate ratio, the target range, and the correction factor are selected
to be entered by
time of day by selecting the carbohydrate ratio "By Time of Day" checkbox
3886, the
target range "By Time of Day" checkbox 3888, and the correction factor "By
Time of
Day" checkbox 3890.
[0554] Insulin Calculator Setup Interface screen 3882 displays the time of day
settings for
the carbohydrate ratio (see FIG. 46A). The carbohydrate ratio may be set to
the same or
different values at 4 different times of day, such as morning (e.g., 4am-
10am), midday
(e.g., 10am-4pm), evening (e.g., 4pm-lOpm), and night (e.g., lOpm-4am). The
morning
carbohydrate ratio may be entered in the morning carbohydrate ratio amount
entry box
3892, the midday carbohydrate ratio may be entered in the midday carbohydrate
ratio
-133-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
amount entry box 3894, the evening carbohydrate ratio may be entered in the
evening
carbohydrate ratio amount entry box 3896, and the night carbohydrate ratio may
be
entered in the night carbohydrate ratio amount entry box 3898. The number of
grams of
carbs per 1 serving may be selected from the servings selection box 3900.
[0555] Insulin Calculator Setup Interface screen 3882 displays the time of day
settings for
the target range (see FIG. 46B). The target range may be set to the same or
different values
at 4 different times of day, such as morning (e.g., 4am-10am), midday (e.g.,
10am-4pm),
evening (e.g., 4pm-lOpm), and night (e.g., lOpm-4am). As shown in FIG. 46B,
the target
range is selected to be entered as a "Single Target" rather than a target
range, as shown in
the correction target selection box 3902. The morning target value may be
entered the
morning target value amount entry box 3904, the midday target value may be
entered in
the midday target value amount entry box 3906, the evening target value may be
entered
in the evening target value amount entry box 3908, and the night target value
may be
entered in the night target value amount entry box 3910.
[0556] Insulin Calculator Setup Interface screen 3882 displays the time of day
settings for
the correction factor (see FIG. 46B). The correction factor may be set to the
same or
different values at 4 different times of day, such as morning (e.g., 4am-
10am), midday
(e.g., 10am-4pm), evening (e.g., 4pm-lOpm), and night (e.g., lOpm-4am). The
morning
correction factor may be entered in the morning correction factor amount entry
box 3912,
the midday correction factor may be entered in the midday correction factor
amount entry
box 3914, the evening correction factor may be entered in the evening
correction factor
amount entry box 3916, and the night correction factor may be entered in the
night
correction factor amount entry box 3918.
Masked Mode Setup Interface:
[0557] The Professional Options screen also enables the Masked Mode setup to
be viewed
and set on the remote device. The setup screen provided functions similar to
the Masked
Mode setup discussed earlier, except that the setup takes place via the RD
software
application.
[0558] FIG. 47 illustrates a Masked Mode setup screen 5090, according to one
embodiment. As shown, Masked Mode setup screen 5090 provides a section 5092 in
which the user can activate the Masked Mode and set reminders to check glucose
at preset
intervals. For example, the reminder is reset every time the sensor is
scanned. As shown,
-134-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
the menu 5058 of trigger elements for initiating various screens is maintained
in the
Profile screen 5086.
Reset System:
[0559] The Professional Options screen also enables the user reset settings of
the Reader
device. The Reset System interface may permit reset of all settings at once,
and/or permit
the user to selectively reset specific setting on the device.
[0560] Referring back to FIG. 39, at block 4094, the user is taken to a
Backups screen
where the user can save a backup file of a current Reader setting. In one
embodiment, the
backup files do not save glucose results or other Reader logbook entries.
[0561] Thus, from the menu 5058 of trigger elements for initiating various
Reader Mode
screens, which remains on the various Reader Mode screens to enable quick
reference and
access to those screens, the user is able to navigate to the desired Reader
Mode screen.
Once the settings are viewed, edited, or deleted, the user can save the
setting to the reader,
as shown by reference path X3. At block 4096, a Reader Status screen is
displayed to
indicate to the user that a save is in progress. In block 4098, a Progress
screen is shown to
indicate a save to the reader, and after the save the user is taken to a
Reader Landing ¨
Settings saved screen to indicate that the save was successful, as shown by
block 4100.
[0562] If changes to any settings in menu 5058 are cancelled, as shown by
reference path
Y3, or the user navigates away, as shown by reference path A4, then the user
is taken to a
warming Alert screen to alert the user that changes will be lost, as shown at
4102 via
reference path Z3. If changes are cancelled, then the user is navigated back
to the Reader
Landing screen 4082. If the user elects to save the changes, then to blocks
4096 and 4098
as previous described. If the user elects to not save the settings, then the
user is taken to
the Reader Landing ¨ Setting saved screen 4100.
[0563] From the Backups screen, the user can save a backup file, as shown at
block 4104.
Progress screen 4106 is displayed while the save is in progress. If the save
should be
cancelled before complete, then the user is taken back to the Backups screen
4108.
[0564] If the save is determined to be not valid, as shown at block 4105, then
the user is
taken to an Alert screen 4110 to indicate to the user that a save is not
valid. For example, if
the filename already exists, then the user can elect to either save it and
overwrite the
previous file, and will be taken to the Progress screen 4106. If the user
elects not to save it,
then the user is then back to the Backups screen 4112.
-135-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0565] From the Backups screen at block 4094, the user can select a backup
file to restore,
as shown by block 4114 and reference path C4. Once the backup file is selected
the user is
taken to a Progress screen 4116 that indicates that the backup file is being
processed. If the
processing of the backup file is determined to not be valid or encounters an
error, then the
Alert screen 4120 is displayed to indicate that there was an error with the
backup file (e.g.,
that the file is damaged). The user is then taken back to the Backups screen,
as shown by
block 4122. If the processing of the backup file is valid, then the Restore
Reader Setting is
displayed to indicate that the Reader settings are being restored to the
settings on the
backup file. If the restore should be cancelled, then the user is taken back
to the Backups
screen, as shown by block 4126. If the restore is not cancelled, then the user
is taken to
either a Reader Status screen 4128, or to a Progress screen 4130 and Backups
screen 4132,
similarly as described above.
Out of Sync Flow
[0566] FIG. 48 illustrates a method 5100 for a flow for syncing the Reader
device when the
Reader is coupled to the remote device and determined to be out of sync with
the remote
device. For example, when out of sync, the time on the Reader is different
than the time on
the remote device.
[0567] If the Reader is coupled to the remote device and determined to be out
of sync with
the remote device, then the Reader Out of Sync screen at 5102 is initiated, as
shown by
block 5102. An Alert screen is displayed to alert the user of the sync and ask
if the user
wishes to synch the Reader with the remote device. The user is also taken to
the Alert
screen at 5106 from the General Reader Settings screen when it is determined
that the two
devices are out of sync, as shown by block 5104.
[0568] If the user elects to sync the two devices, then a Reader Status screen
at 5108 and
Progress screen at 5110 are displayed while the update is in progress. When
the update is
complete, the user is taken back to the Reader Landing ¨ Reader updated
screen, as shown
at block 5112.
Guided Reader Setup Flow
[0569] When a new Reader that has never been used is connected to the remote
device, the
user is guided through the initial setup of that Reader in a step-by-step
fashion. Along the
way, basic Reader configuration settings such as name, patient ID, date, time,
and
language are collected for the purpose of initializing the Reader. In one
embodiment, the
-136-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
patient name and ID are optional settings while the date, time, and language
options must
be set to complete the setup process.
[0570] FIG. 49 illustrates a method 5100 for a flow for the Guided Reader
Setup interface,
according to one embodiment. When a new Reader that has never been used is
connected
to the remote device, the New Reader Welcome screen is displayed to start the
guided
setup process, as shown at block 5116. The Start/Patient info screen is
initiated to receive
patient info, as shown at block 5118. The Confirm Date/Time/Language screen is
initiated
to receive the appropriate entries from the user, as shown at block 5118. Once
entered, the
Ready to Save screen is initiated to indicate to the user that the entries are
to be saved. As
the save is in progress, the Reader Status screen at 5130 or the Progress
screen at 5128 is
displayed. From the Progress screen at block 5128, the Reader Landing ¨ Reader
ready
screen at block 5132 is displayed when the save is complete.
[0571] If the setup process is interrupted or exited from any of blocks
5118,5120,5122, then
the Alert screen at block 5124 is displayed to alert the user that any changes
will be lost if
not saved. After the Alert screen is displayed at block 5124, the user is
taken to the New
Reader Welcome screen so that the initial setup can be completed, as shown by
blocks
5116,5126.
[0572] FIG. 50 illustrates a single screen of the Guided Reader setup
interface, according to
one embodiment. The initial screen of the Guided Reader setup interface
includes
information regarding the guided setup and provides a trigger element 5136
that enables
the user to begin the guided setup.
Reader Mode
Reports Mode Flow
[0573] FIG. 51 illustrates a flowchart for a method 4080 of navigating through
the Reports
mode of the RD software, according to one embodiment. The reports mode of the
application provides access to settings and functions for creating, viewing,
saving, and
printing reports. In the examples shown, the Reports mode is provided as a tab
on the
interface screens for the RD software, along with the tab for the Reader mode.
[0574] At block 5140, the Reports Landing screen is displayed on the remote
device when
the Reports tab is selected. The user is provided with the option to generate
reports or to
view or edit Report Preferences¨e.g., the user may select, for example,
between
-137-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
corresponding trigger elements on the Landing Screen to navigate to the
Generate Reports
interface or the Report Preferences interface.
[0575] From the Generate Reports screen at block 5142, the user can select the
parameter of
the particular Report to be generated. The Choose screen at block 5146 enables
the user to
set the destination of the directory for auto-saving. When a report is
generated, the
Progress screen at block 5144 is displayed while the report is being
generated. When
complete, the View reports screen at block 5148 displays the generated report,
or provides
a menu to select from various reports generated. The Reports can be saved via
a Save
window screen at block 5152 and the progress screen at block 5154 will be
displayed
when the save is in progress. After the save is complete, the user is taken
back to the View
Reports screen at block 5148.
[0576] If the View Reports screen at block 5148 is closed, then the user is
taken back to the
Generate Reports screen at block 5142. The user may also print one or more
Reports from
the View Reports screen 5148 via Print screen at block 5150.
[0577] From the Reports Landing screen at block 5140, the user may elect to
navigate to the
Report Set screen at block 5156. The Set Reports screen at block 5156 enable
the user to
pre-select reports to be generated each time the user generates reports with
the data
management software. Example reports may include, a Snapshot, Calendar,
Average Day,
Logbook, Daily Statistics, Mealtime Averages, and Reader Settings, as will be
discussed
further later. The various reports are selectable and will be set as the
default preferences
for the creation of reports from the Reader. The Calendar may default to a
predetermined
time period, such as 3 months for example. If the user selects the Mealtime
Averages
report they are presented with an overly that allows them to set the default
pre and post
meal target ranges.
[0578] From the Reports Landing screen at block 5140, the user may also elect
to navigate
to: Timeframe screen at block 5158 to enable the user to establish default
timeframes used
when reports are generated; Glucose Targets screen at block 5160 to enable the
user to
establish the default setting for the glucose target range and hypoglycemia
threshold to be
applied to generated reports; Auto-Save Options screen at block 5162 to enable
the user to
activate and set the auto save feature, as well as, choose the file name
format, and save
location (as shown at block 5174) that will used during report creation; Print
Color screen
at block 5164 that enables the user to choose default print color options; and
Quick Print
screen at block 5166 which allows the user to enable or disable the quick
print feature,
-138-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
which causes the software to immediately generate reports once a Reader with
data is
connect to the computer.
[0579] Settings or changes made to the screens 5156, 5158, 5160, 5162, 5164,
and 5166 can
be saved, at which point the Progress screen at block 5172 is displayed during
the save. If
the user cancels or navigates away from any of screens 5156, 5158, 5160, 5162,
5164, and
5166, it is determined if any changes were made, as shown at block 5168. If
not, the user
is able to navigate away to the desired screen. If changes were made, then an
Alert screen
is displayed to alert the user that changes may be lost and to provide the
user with the
option to save, as represented by the Progress screen at block 5172.
[0580] FIG. 52 illustrates an example Reports landing screen that is displayed
when the
user first connects a Reader with stored data, according to one embodiment. As
shown,
Reports landing screen 5220 includes trigger elements 5222 and 5224 to
initiate the
Generate Reports interface and Report Preferences interface, respectively.
[0581] FIG. 53 illustrates an example Generate Reports screen, according to
one
embodiment. Generates Reports screen 5230 is shown providing information
regarding the
current settings for creation of reports, as well as providing the user with
navigation
options (e.g., trigger elements) to make changes to the current settings¨e.g.,
patient
information 5232, timeframe 5234, reports 5236 selected to print or view,
glucose targets
5238, and auto-save options 5240. Screen 5230 also provides trigger elements
5240,5242
to print the reports or view them, respectively.
[0582] FIG. 54 illustrates an example Logbook Report screen when viewed from
the
Reports Mode, according to one embodiment. As show, Logbook Report screen
includes a
table 5252 of the data in the Logbook Report as well as trigger elements
5254,5256 for
printing and saving the report, respectively.
Guided Reports Setup Interface
[0583] The first time a user accesses the printing features of the
application, they are guided
through the reports setup and creation process in a step-by-step fashion by a
Guided
Reports Setup interface. Along the way, the RD software collects default
reports
preferences such as patient information, timeframe, report set, glucose
targets, and auto-
save options, as well as prepares the first set of reports for viewing,
saving, and printing.
[0584] FIG. 55 illustrates a flowchart for a Guided Reports Setup interface,
according to
one embodiment. The first time a user accesses the printing features of the
application,
user is taken to the First-Use Reports Welcome screen to begin the guided
setup. The
-139-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Start/Patient info screen is initiated to receive patient info, as shown at
block 5182. Then
the user is navigated through the following screens: Timeframe screen at block
5186 to
enable the user to establish default timeframes used when reports are
generated; Set
Reports screen at block 5188 enable the user to pre-select reports to be
generated each
time the user generates reports with the data management software; Glucose
Targets
screen at block 5190 to enable the user to establish the default setting for
the glucose
target range and hypoglycemia threshold to be applied to generated reports;
Auto-Save
Options screen at block 5192 to enable the user to activate and set the auto
save feature, as
well as, choose the file name format, and save location that will used during
report
creation; and Ready to Generate Reports screen at block 5194 which allows the
user to
generate the selected reports and either view them or print them.
[0585] When printing the selected reports, the Progress screen at block 5196
is displayed
while the report is being generated. When complete, the View Reports screen at
block
5198 displays the generated report, or provides a menu to select from various
reports
generated. The Reports are then immediately printed via a Print window screen
at block
5200. If auto save is set, once the reports are generated at block 5196, the
selected reports
are automatically saved to the selected destination, as shown at block 5204.
If the creation
at block 5196 is interrupted or cancelled, then the user is taken back to the
Generate
Reports screen at block 5202.
[0586] When electing to view the selected reports form block 5194, the
Progress screen at
block 5206 is displayed while the report is being generated. When complete,
the View
Reports screen at block 5210 displays the generated report, or provides a menu
to select
from various reports generated. If auto save is set, once the reports are
generated at block
5206, the selected reports are automatically saved to the selected
destination, as shown at
block 5204. If the creation at block 5206 is interrupted or cancelled, then
the user is taken
back to the Generate Reports screen at block 5208.
Export Reader Flow
[0587] The RD software provides a function for users to export data from a
Reader coupled
to the computer as a tab-delimited file or other spreadsheet-compatible
format, for
example. The Export function may be accessed, for example, via the "File" menu
of the
application..
[0588] FIG. 56 illustrates a method for exporting Reader data, according to
one
embodiment. At block 5252 of Export Meter Data interface 5250, it is
determined if the
-140-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Reader device is coupled to the remote device. If not coupled, then the Export
data feature
is disabled (e.g., in the File menu) as shown at block 4254. If the Reader
device is coupled
to the remote device, then it is determined if the Reader device has sensor
data stored, as
shown at block 5256. If the Reader has no data stored, then the Export data
feature is
disabled at block 4254. If the Reader does have data, then it is determined if
the Reader is
downloading data. If so, then the Export data feature is disabled at block
4254. If the
Reader is not downloading data, then the Export data feature is enabled (e.g.,
in the File
menu).
[0589] Once enabled, the user can select to export data from the Reader, as
represented by
block 5262. Once selected, the meter export file is saved as shown at block
5264. If the
save is determined to be valid, then the Progress screen 5272 is shown to
indicate that the
process of exporting data is in progress. If no error occurs, the user is
taken back to the
originating screen as shown at block 5276. If an error occurs, an Alert screen
is provided
to notify the user of the failed export, as shown at block 5274.
[0590] If at block 5266, it is determined that the saving of the meter export
file was not
valid, then an Alert screen is provided to notify that the save was not valid
(e.g., the file
already exists), as shown at block 5268. The user is provided with the option
to overwrite
the existing file, which navigates the user to the Progress screen at block
5272. The user is
also provided the option to not save the invalid file, in which case the user
is taken back to
the originating screen, as shown at block 5270.
Reports
[0591] In some embodiments, the RD software provides a user interface to
manage and/or
control features related to reports. For example, the RD software provides a
reports mode
for creating, editing, viewing, printing, and for performing any other
functions associated
with report generation and management.
[0592] Different types of reports may be generated. For example, FIGS. 45-53
illustrate
various types of reports, according to certain embodiments. It should be
appreciated that
the reports illustrated are exemplary and should not be interpreted as
limiting. Further
details regarding various reports that may be implemented with the software is
described
in US Patent Application No. 11.146,897, filed on June 6, 2005, and US
Provisional
Application Nos. 61/451,488, filed March 10, 2011; and 60/577,064, filed Jun.
4, 2004,
the entireties of which are incorporated herein by reference.
-141-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0593] As stated above, reports may be generated and communicated to a remote
device -
e.g., for display on the remote device and/or printing on the remote device.
The remote
device may be, for example, a desktop computer, laptop, cell phone, etc. For
example, the
remote device may be a personal computer accessed by the user, enabling the
user to view
and/or printout the reports. In other instances, the remote device may be a
computer
accessed by another party, such as a physician or health care professional.
The user may,
for example, bring the device to their physician so that the physician could
transfer the
data to his or her computer for display and/or printing of the reports.
[0594] The analyte monitoring device may communicate the reports to the remote
device
using any variety of wired (e.g., USB, FireWire, SPI, SDIO, RS-232 port, etc.)
or wireless
technologies (e.g., radio frequency (RF) communication, Zigbee0 communication
protocols, WiFi, infrared, wireless Universal Serial Bus (USB), Ultra Wide
Band (UWB),
Bluetooth0 communication protocols, and cellular communication, such as code
division
multiple access (CDMA) or Global System for Mobile communications (GSM), etc).
[0595] In some instances, the analyte monitoring device may include software
that is loaded
onto the remote device - e.g., the first time connecting to the remote device.
In other
instances, the software may be loaded to the remote device via the intern& or
storage
device (e.g., CD-ROM, FLASH memory drive, etc.).
[0596] In some instances, the reports may be communicated to a remote device
and
thereafter communicated to another remote device. For example, the user may
download
the data to his own computer and thereafter transmit the data to the physician
for further
analysis. Upon receipt, the physician could view and download reports to
assess the
activities and events of the user.
Exemplary Reports
[0597] In the following paragraphs, example reports are provided and
described. The
various reports may include general identification information for the
associated patient
(e.g., name of the patient, identification number, etc.) and/or associated
device (e.g., name
of the device; model of the device, etc.).
Snapshot:
[0598] In some aspects of the present disclosure, a Snapshot report is
provided. The
Snapshot report captures the overall condition of the patient's health
management (e.g.,
diabetes management). For instance, the report may highlight the key metrics
for the
user's activities over a specific time period. In some embodiments, the
Snapshot report
-142-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
provides significant pieces of information related to one or more of the
following:
utilization, glucose levels, events, and notes.
[0599] FIG. 57 illustrates an exemplary Snapshot report for a specific time
frame (e.g., a
two week period as shown), according to certain embodiments. The Snapshot
report 5300
includes key metrics associated with the user's history over the two week
period of time.
For example, Snapshot report includes: section 5302 displaying metrics 5308
regarding
the user's glucose, insulin, and carbohydrate intake are provided for the
given two-week
time period; section 5304 displaying metrics 5312 regarding low glucose events
for the
two-week time period; section 5306 displaying metrics 5316 regarding glucose
readings
for the two-week time period; and section 5307 displaying the estimated Alc
percentage
for the two week period.
[0600] In the embodiment shown, metrics 5308 includes average glucose value;
time above,
in, and below the target zone, average carb intake per day; average rapid-
acting insulin
intake per day; and average long-acting insulin intake per day. Section 5302
also includes
a graph 5310 of glucose values for the two week period that have been averaged
with
respect to specific times throughout the day. Graph 5310 also indicates the
range of
glucose readings for the specific time throughout the day.
[0601] Metrics 5312 includes the total number of low glucose events, the
average duration
of a low glucose event, and the low glucose threshold. Section 5304 also
includes a graph
5314 of a summary of low glucose events for the two week period that have been
averaged
with respect to specific times throughout the day.
[0602] Metrics 5316 includes the average number of scans per day and the
percentage of
available sensor data. Section 5306 also includes a graph 5318 of the
percentage high
glucose readings of sensor data recorded for the two week period, categorized
with respect
to specific times throughout the day.
[0603] Snapshot Screen 5300 also includes a Comments section 5320 that
indicates any
comments that the user has logged. In some embodiments, the comments section
provides
software generated comments generated from analysis of the sensor data.
Snapshot Screen
5300 also includes a section for identifying the Report, the time period
applicable to the
reports, and the target range.
-143-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Calendar:
[0604] In some aspects of the present disclosure, a Calendar report is
provided. The
Calendar report provides an overview of the patient's involvement and
highlights points of
concern (e.g., hypoglycemic events).
[0605] For example, FIG. 58 illustrates a Calendar report that highlights the
key metrics for
the user's activities in calendar format, according to certain embodiments.
The events of
each day of the selected month are detailed in calendar-format. The Calendar
report is
provided for a one-month period, e.g., March, with the following events
indicated: average
glucose readings for the given day 5352; number of scans per the given day
5354; and the
occurrence of low glucose events for the given day. For example, on March 7,
the average
glucose reading was 171 mg/dL; there were 7 scans; and 2 low glucose events.
Daily Patterns:
[0606] In some aspects of the present disclosure, a Daily Patterns report is
provided. A
Daily Patterns report communicates the trend in glucose levels for the given
time period,
with respect to times throughout the day.
[0607] FIG. 59 illustrates a Daily Patterns report, according to one
embodiment. Daily
Patterns Report 5360 includes a graph 5362 displaying the median glucose
values 5364
with respect to time periods throughout a day; the range of glucose values
associated with
the 25th to 75th percentile of readings 5366;and the range of glucose values
associated
with the 10th to 90th percentile of readings 68.
[0608] Below graph 5362, and aligned with respect to the time periods, is a
graph of carbs
taken and logged 5370, as represented above the horizontal axis, and of rapid-
acting
insulin taken and logged 5372, as represented below the horizontal axis.
[0609] Daily Patterns Report 5360 also includes sections 5374, 5376, and 5378
next to
graph 5362 and aligned with respect to time periods throughout the day.
Section 5374
indicates the daily average glucose value for each time period throughout the
day. Section
5376 indicates the daily average carb intake per time period, as well as the
number of
related notes taken. Section 5378 indicates the daily averages for intake of
rapid-acting
insulin and long-acting insulin for each time period throughout the day, as
well as the
number of related notes.
-144-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Mealtime Patterns:
[0610] In some aspects of the present disclosure, a Mealtime Patterns report
is provided. A
Mealtime Patterns report communicates the rise and fall in glucose levels
relative to
meals.
[0611] FIG. 60 illustrates a Meal Patterns report, according to one
embodiment. The report
includes data for a given time period 5414 (e.g., two weeks) and includes
plots of the
glucose values before and after specific meals of the day.
[0612] The report includes plots 5402, 5404, and 5406 for three different meal
events¨e.g.,
meals occurring at different time periods 5416a, 5416b, 5416c of the day
(e.g., Morning,
Midday, and Evening, respectively). The number of notes logged for food intake
and
insulin intake are also provided at sections 5418a, 5418b, and 5418c.
Furthermore, the
average carbs taken and logged 5420a, 5420b, and 5420c for the respective time
period is
also shown. The average insulin taken and logged 5422a, 5422b, and 5422c for
the
respective time period is also shown.
[0613] The meal time reference points 5412a, 5412b, and 5412c indicate the
time at which
the meal was taken. One hour incremental time periods before and after the
reference
points are provided. Median glucose plots 5408a, 5408b, and 5408c are
displayed for the
respective periods. The 10th to 90th Percentile range 5410a, 5410b, and 5410c
are also
provided on plots 5402, 5404, and 5406, respectively. The average glucose
values 5426a,
5426b, and 5426c for the respective incremental time periods are also provided
below and
aligned with the respective plots.
Daily Statistics:
[0614] In some aspects of the present disclosure, a Daily Statistics report is
provided. The
Daily Statistics report highlights glucose readings for days within the given
time period
(e.g., 2 weeks). The data may be used to assist in the identification of
causes of
hypoglycemic events and other abnormalities, for example.
[0615] FIG. 61 illustrates the first page of a Daily Statistics report 5500
for a given time
period (e.g., two week period). The first page includes the daily statistics
for the first seven
days of the given two-week time period. Plots 5502, 5504, 5506, 5508, 5510,
5512, and
5514 of glucose values for each of the seven days are displayed.
[0616] The day is broken up into incremental time periods (e.g., two hour
periods as
shown) and event information may be indicated on the chart. For example, a
carb intake
-145-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
event 5502b is indicated at the corresponding incremental time period in which
it
occurred.
[0617] Rapid and long acting insulin intake events 5502c and 5502e,
respectively, are
indicated at the corresponding incremental time period in which it occurred.
[0618] Events associated with food intake and insulin logged without a value
5502f and
5502i, respectively, are indicated at the corresponding incremental time
period in which it
occurred.
[0619] User Time change event 5502g is indicated at the corresponding
incremental time
period in which it occurred. For example, if the user changes the time on the
Reader
device, this event would be indicated at the appropriate time and day.
[0620] Sensor scan event 5502h is indicated at the corresponding incremental
time period in
which it occurred. In this way, any discontinuities in glucose readings are
also displayed,
as represented as breaks in the glucose plot.
[0621] Furthermore, Daily Totals section 5516 displays daily totals of
additional glucose
related data. For example, section 5516 provides daily totals for the first
day - March 17th.
Section 5516 includes the average glucose for the day, the total number of
carbs for the
day, the amount of rap-acting insulin taken for the day, and the amount of
long-acting
insulin taken from the day.
Logbook:
[0622] In some aspects of the present disclosure, a Logbook report is
provided. A Logbook
report provides a detailed look at obtained sensor readings and, in some
cases, other
relevant data - e.g., insulin dosages, meal events, notes, strip glucose
measurements, and
ketone events - categorized by time period (e.g., by day).
[0623] FIG. 62 illustrates an exemplary Logbook report, according to one
embodiment.
The exemplary Logbook report 5520 illustrates the first page of the Logbook
report 5520
and shows glucose related data in tables 5522, 5524, and 5526 for first 3 days
of the two
week time period. Glucose readings, carb intake, insulin data (e.g., intake
for rapid and
long acting insulin) are provided for time periods (e.g., hourly time periods
as shown)
throughout the day. Various events are also represented in the table (e.g.,
via shading,
symbols, icons, etc.), such as a low glucose readings 5428, high glucose
readings 5530,
food intake 5532 and insulin intake 5534 that was logged without a value.
Notes are also
indicated in the table - e.g., notes indicating exercise or other relevant
events.
-146-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0624] Graphs 5540, 5542, and 5544 of the glucose values throughout the
associated day
are also provided for each day shown. The daily average is also indicated for
each day.
[0625] FIG. 63 illustrates another exemplary Logbook report, according to one
embodiment. The exemplary Logbook report 5550 is similar to the Logbook report
5520
in FIG. 62, and similar features will not be repeated for FIG. 63 for the sake
of clarity and
brevity. Logbook report 5550 includes strip glucose measurements 5552 and
ketone
measurements 5554 in the associated tables at the associated time and day.
Reader Settings:
[0626] In some aspects of the present disclosure, a Reader Settings Report is
provided. A
Reader Settings Report provides a summary of settings that are currently set
for the
Reader device.
[0627] FIG. 64 illustrates an example Reader Settings Report, according to one
embodiment. Reader Settings Report 5600 includes a Profile section 5602, which
displays
profile settings, such as the patients name and ID. Reader Settings Report
5600 includes a
Settings section 5604, which displays general settings on the Reader, such as
date, time,
clock style, notification sound, button tone, vibration, and target glucose
range. Reader
Settings Report 5600 also includes a Notes section 5606, which displays Notes
settings,
such as which categories of notes are available. Example categories of notes
may relate to
rapid acting insulin, long acting insulin, food, exercise, medication, control
solution,
stress, etc. Reader Settings Report 5600 also includes a Reminders section
5608, which
displays Reminder settings, such as alarms to check glucose and to take
insulin dosages.
Reader Settings Report 5600 also includes a Changes section 5610, which
displays any
changes to the Reader settings within a given time period (e.g., the last 30
days as shown).
[0628] If insulin calculation or masked mode operation are available on the
Reader device,
the Reader Setting Report may also include summary sections for these settings
as well.
For example, FIG. 65 illustrates a Reader Settings Report 5620 including an
Insulin
Calculator section 5622, which displays Insulin Calculator settings - e.g.,
whether rapid or
long acting insulin calculator is on, a calculator mode (e.g., advanced or
easy as discussed
earlier), carbohydrate ration, correction target, and correction factors.
Reader Settings
Report 5620 also includes a Masked Mode section 5624, which displays Masked
Mode
settings, such as whether the Masked Mode is activated, whether the check
glucose
reminder is activated, and the frequency or time settings for the reminder,
etc.
-147-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
Data Management Software
[0629] In certain embodiments, the data management software may include a data
management software version. Information for the data management software is
provided
in the following described embodiments and associated figures. It should be
appreciated
that the example interfaces and flows are exemplary and should not be
interpreted as
limiting.
Home Screens
[0630] FIG. 66 illustrates a home screen for the data management software when
the
Reader device is not connected to the PC on which the data management software
resides.
In certain embodiments, the home screen of FIG. 66, i.e., a 'no Reader
connected' screen
6600, is displayed prior to a Reader device being connected, and the software
will return
to the home screen upon disconnection of the Reader device. As shown in the
figure, the
'no Reader connected' screen includes an image 6610 representative of the
state of the
Reader device, that is, no Reader connected. As can be seen, the image 6610
depicts a
Reader device and a connection cable, wherein the Reader device is not
connected to the
connection cable. The 'no Reader connected' screen 6600 also includes a text
notification
of the status of the Reader, as seen by notification 6620. The home screen
further includes
two main menu icons: Generate Reports 6630 and Change Reader Settings 6640. In
the
'no Reader connected' home screen 6600, both the Generate Reports 6630 and
Change
Reader Settings 6640 menu are disabled, represented by the menus being shown
in as a
dimmed output.
[0631] FIG. 67A illustrates a home screen for the data management software
when an
unconfigured Reader device is connected to the PC on which the data management
software resides. In certain embodiments, the home screen of FIG. 67A, i.e.,
an
`unconfigured Reader connected' screen 6700, is displayed when a Reader that
has not yet
been configured for a user, is connected. As shown in the figure, the
`unconfigured Reader
connected' screen includes an image 6710 representative of the connected
Reader device.
In certain embodiments, the data management software may be compatible with a
plurality
of different Reader device models, and the image 6710 may automatically
display an
image of the particular model currently connected. The `unconfigured Reader
connected'
screen 6700 also includes text information 6720 associated with the connected
Reader. On
the `unconfigured Reader connected' screen, the text information includes a
serial number
-148-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
of the Reader and the date and time information as stored on the Reader for
current date
and time.
[0632] The text information, in certain embodiments, includes a menu 6750, to
add a reader
profile associated with the Reader device. When selected, the add a reader
profile menu
6750 launches a pop-up screen 6760 to enter a name and/or patient ID of the
user of the
Reader, as illustrated in FIG. 67B. The pop-up screen allows the user of the
Reader to
associate their information, including name and patient ID. After entry of the
name and
patient ID, the 'save' button is selected to save the information and
associate it with the
connected Reader. Once reader profile information is associated with a Reader,
the next
time it is connected to the software, the user information will load with the
Reader device.
[0633] Returning to FIG. 67A, the home screen further includes two main menu
icons:
Generate Reports 6730 and Change Reader Settings 6740. In the `unconfigured
Reader
connected' home screen 6700, both the Generate Reports 6730 and Change Reader
Settings 6740 menu are shown in full brightness, indicating the Generate
Reports 6730
and Change Reader Settings 6740 menus are active. In certain embodiments,
prior to entry
and association of a name and patient ID, selection of either the Generate
Reports 6730 or
Change Reader Settings 6740 menu will activate the pop-up screen to enter the
name and
patient ID, thus not allowing the running of reports or changing of settings
until after the
Reader is configured with user information.
[0634] FIG. 68 illustrates a home screen for the data management software when
the
Reader device is connected to the PC on which the data management software
resides. In
certain embodiments, the home screen of FIG. 68, i.e., a 'Reader connected'
screen 6800,
is displayed when a Reader is successfully connected. As shown in the figure,
the 'Reader
connected' screen includes an image 6810 representative of the state of the
Reader device,
that is, Reader successfully connected. In certain embodiments, the data
management
software may be compatible with a plurality of different Reader device models,
and the
image 6810 representative of the state of the Reader device may automatically
display an
image of the particular model currently connected. The 'Reader connected'
screen 6800
also includes text information 6820 for the connected Reader. In certain
embodiments, the
information included may be the name of the user of the Reader, a patient ID
number of
the user, a serial number of the Reader, and the date and time information as
stored on the
Reader for current date and time. The home screen further includes two main
menu icons:
Generate Reports 6830 and Change Reader Settings 6840. In the 'Reader
connected' home
-149-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
screen 6800, the Generate Reports and Change Reader Settings 6840 menu are
both
enabled, represented by the menus being shown in full brightness.
[0635] FIG. 69A illustrates a home screen for the data management software
when the time
and date of connected Reader device isn't synchronized with the time of the
computer on
which the software is loaded. In certain embodiments, when the time stored on
the Reader
device is off from the time of the computer by more than a predetermined
length of time -
e.g., five minutes or more - an alert 6910 may be displayed in place of the
time
information for the connected Reader. The alert 6910 includes an 'update'
button which,
when pressed, launches an update time and date pop-up window 6920, as shown in
FIG.
69B. In certain embodiments, the update time and date pop-up window 6920 is an
automatic update, whereby the Reader device will be synchronized with the time
of the
computer or synchronizes with a time obtained via the internet. In other
embodiments, the
update time and date pop-up window is a manual update, such that the user
manually
adjusts the time shown on the pop-up window via entry of numbers corresponding
to the
hour and minute of the time, or by selecting arrows to increase or decrease
the shown
time. In certain embodiments, when an update of the time is required, the
Generate
Reports and/or Change Reader Settings menus are unavailable to the user. In
other
embodiments, the Generate Reports and Change Reader Settings menus are
available
despite the unsynchronized clock.
[0636] FIGS. 70A-70D illustrate a home screen for the data management software
when an
update to the software is available. In certain embodiments there may be two
types of
software updates, wherein a first type of update requires immediate attention,
and a second
type of update which can be applied at application shutdown. FIG. 70A shows a
pop-up
window 7010 associated with an update that requires immediate attention. As
can be seen
in the figure, window 7010 includes a text notification to the user that an
update is
required and the software is unavailable until the update is applied. The
window 7010 also
includes two options for the user to choose: 'close' and 'update now'. If
'close' is chosen,
the software may shut down. If 'update now' is chosen, a download of the
update is
initiated. While the download is in progress, as illustrated in FIG. 70B, a
notification 7020
is shown on the screen indicating the download is in progress which prevents
any user
interaction with the software while the download is in progress. Upon
completion of the
download of the update, an updater may automatically launch and guide the user
through
-150-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
installation of the update. In certain embodiments, updates requiring
immediate action
may include updates to the software or firmware of the Reader device itself
[0637] FIG. 70C shows a pop-up window 7030 associated with an update that can
be
applied immediately or at application shutdown. As can be seen in the figure,
window
7030 includes a text notification to the user than an update is available and
may be applied
immediately or at application shutdown. The window 7030 also includes two
options for
the user to choose 'update at shutdown' or 'update now'. If 'update now' is
chosen, a
download of the update is initiated as described above, and a download in
progress
notification is shown. If 'update at shutdown' is chosen, the update is
downloaded in the
background of the software, and a passive notification 7040, as illustrated in
FIG. 70D,
may be displayed at the bottom of the home screen informing the user when the
download
has been completed. This passive notification 7040 would not preclude normal
use of the
software, and would display a reminder to the user that an update has been
downloaded
and will be applied upon shutdown of the software. In certain embodiments,
disconnection
of the Reader device may trigger a software shutdown and the update
installation. In
certain embodiments, updates that may be delayed until application shutdown
may include
updates to the data management software.
Generate Reports
[0638] FIG. 71 illustrates a generate reports screen for the data management
software in
certain embodiments. The generate reports screen 7100 may be accessed after
selection of
the 'Generate Reports' menu on the home screen as described above. The
generate reports
screen 7100 may include a 'Home' button to navigate the software back to the
home
screen. In certain embodiments, the generate reports screen 7100 may include
multiple
frames. Such frames include a select reports section 7120, a reader profile
section 7130,
and a set report parameters section 7140.
[0639] In certain embodiments, the reader profile section 7130 may include
patient and
Reader information, such as patient name, patient ID, Reader serial number and
Reader
current time and date information.
[0640] The set report parameters section 7140 may include settings associated
with reports
to be run, such as the timeframe for the reports to be run, which can be set
as a number of
days or weeks, or by selecting a particular date range. The report parameters
section may
additionally include an option to set the target glucose range for certain
reports, as
described below. The set report parameters section 7140 may also include
report specific
-151-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
settings, such as settings applicable only to the advanced daily patterns
report 7150 as
shown in the exemplary embodiment of FIG. 71.
[0641] In certain embodiments, the select reports section 7120 includes a
plurality of report
types. The report types may be represented as icons, text, or combinations of
both. The
plurality of report types may include a snapshot report, a daily patterns
report, an
advanced daily patterns report, a mealtime patterns report, a monthly summary
report, a
weekly summary report, a daily log report and a Reader details report. In
certain
embodiments, multiple reports may be selected by selecting a selection box
next to each
report in the reports section. In certain embodiments, the selection of
certain reports
automatically triggers the selection of other reports (for example, selection
of the
advanced daily patterns report automatically triggers the selection of the
daily patterns
report. In this manner, multiple reports may be selected simultaneously, such
that a single
command can instruct the software to run multiple reports at once. Reports are
then
available for the user to view on screen or print, by use of the 'View
Reports' 7170 and
'Print Reports' 7160 buttons. In one embodiment, reports are printed in a
preset order -
e.g. snapshot, daily patterns, advanced daily patterns, mealtime patterns,
monthly
summary, weekly summary, daily log, and reader details.
[0642] FIG. 72 illustrates an exemplary snapshot report. As can be seen in the
figure, the
snapshot report may include the time period - e.g. 14 days - to which the
report applies, an
indication of average glucose level, including indications of the percentage
of time the
glucose level was above the target glucose level, a percentage of time the
glucose level
was within the target, and a percentage of time the glucose level was below
the target, and
a number of low (or high) glucose events during the time period, including an
indication
of the average duration of such events. The report may further include a
percentage of the
available sensor data that was captured by the Reader and a count of the
number of daily
scans that were taken during the time period. Further, the report may include
carbohydrate
and insulin information, such as the average daily intake of carbs per day and
the amount
of units of insulin (rapid acting and long acting) taken per day. The snapshot
report may
also include comments associated with the snapshot results, such as comments
related to
increase or decrease in number of daily glucose tests from the current time
period
compared to the past time period, possible sources of error in the snapshot
data, or
comparisons of the current time period results to the past time period.
-152-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0643] FIGS. 73A and 73B illustrate exemplary daily patterns reports. FIG. 73A
illustrates
a daily pattern report including a user's ambulatory glucose profile and FIG.
73B
illustrates a daily pattern report including indications of glucose
measurement values. As
can be seen in the figures, the daily pattern report shows a 1 day, i.e., 24
hour, time period
graphical representation of glucose measurement values organized by time of
day for a
period of days - e.g., 14 days. The glucose measurement values may be
displayed as
individual points, or may be averaged into a gradient pattern representative
of density of
measurement values within particular ranges. The graph may include a
representation of
the target glucose range for the user, as well as a median over time,
represented by an
average line, and lines representing the 10 percentile, 25 percentile, 75
percentile and 90
percentiles. In certain embodiments, the daily pattern report may include
numerical
averages for shorter periods throughout the day, such as for every two hours,
as is shown
in FIGS. 73A and 73B. In some embodiments, the time of day period with the
highest
average glucose level may be highlighted for the user's attention. In certain
embodiments,
bins with average glucose above a predetermined threshold (e.g., 240 mg/dL for
example,
or below a pre-determined threshold (e.g., 70mg.dL, for example) are
highlighted. In
other embodiments, what is outside the target range or a fixed level outside
or beyond the
target range is highlighted. The daily pattern report may also have an
indication of the
daily average glucose level. In addition to measured glucose levels, the daily
pattern report
may include other average daily information, such as carbohydrates, rapid-
acting insulin,
and long-acting insulin. As shown in the figures, daily information can be
displayed as
numerical daily averages, and also as average ingestion amounts by time of
day.
[0644] FIGS. 74A and 74B illustrate exemplary advanced daily pattern reports.
Similar to
the daily pattern report, the advanced daily pattern report may include a
graphical
representation of glucose measurement values and the average distribution of
glucose
measurement values over a period of time. The graph may additionally include a
median
goal glucose level line and a low threshold glucose level line. In certain
embodiments, the
low threshold glucose level line is representative of a level of glucose low
enough to risk
health problems for the user, such as is described above herein. The advanced
daily pattern
report may further include an indication system for notifying the user of
variability and
warnings with respect to the averaged measured glucose levels. As shown in the
figures,
the advanced daily patterns report may include indications of the likelihood
of low
glucose, comparison between the median glucose level and the glucose level
goal, and
-153-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
indications of the variability of measured glucose levels below the median. An
analysis of
the glucose measurement values and variability factors may result in a
notification to the
user of a likely situation with respect to their level of glucose control. In
the example of
FIGS. 74A and 74B, the user's glucose variability below the median is high. In
certain
aspects, the notification to the user may include warnings and possible
factors contributing
to the determined issue or problem.
[0645] FIG. 75 illustrates an exemplary mealtime pattern report. As can be
seen in the
figure, the mealtime pattern report may include graphical and numerical
representations of
glucose level information with respect to particular times of the day that may
be associated
with meals, i.e., morning (breakfast), midday (lunch), evening (dinner), and
nighttime
(bedtime). As illustrated in the figures, a time indication is displayed in
terms of hours
before and after the corresponding meal. Further, the representations may
include
numerical indications of the average current glucose level at the time of the
meals'
ingestion and peak glucose levels. In certain embodiments, a target glucose
level line is
shown on the graphs, as well as a low limit glucose level line. In certain
other
embodiments, the two lines shown on the graph represent the target range. In
addition to
the graphs, a representation of the average amount of carbohydrates ingested
and insulin
taken may also be displayed.
[0646] FIG. 76 illustrates an exemplary monthly summary report. As can be seen
in the
figure, the monthly summary report includes a calendar display of the current
month, as
well as numerical indications of average glucose level, number of scans per
day, and
indications of low glucose events. In the example shown in the figures, the
5th day of the
month had a low average glucose level of 64mg/dL, had 3 scans in the day, and
had a
plurality of low glucose event notifications. In certain embodiments, the days
with
average glucose above a predetermined threshold (e.g., 240 mg/dL) or below a
predetermined threshold (e.g., 70 mg/dL) are highlighted. In other
embodiments, outside
of the target range or a fixed level beyond the target range are highlighted.
[0647] FIG. 77 illustrates an exemplary weekly summary report. As can be seen
in the
figure, the weekly summary report includes a representation of the 7 days of
the week. For
each day of the week, a graphical representation of the 15 minute historical
glucose data
during the day is displayed. Each graphical representation may also include an
indication
of when a sensor scan was taken, and in certain embodiments, the specific data
associated
with the scan, including glucose level, carbohydrates, and current insulin on
board. As also
-154-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
shown in the figure, a target glucose range may be shown in a differentiated
color to
highlight good glucose control. In some embodiments the graphical
representations
include icons representative of glucose related events, including injection of
rapid-acting
insulin, ingestion of carbohydrates, time changes, and insertion or activation
of a new
sensor.
[0648] Still referring to FIG. 77, each daily information line may include
numerical and
iconic representations of information, including average glucose, carbohydrate
intake,
rapid-acting insulin, and long-acting insulin.
[0649] FIG. 78 illustrates an exemplary daily log report. In certain
embodiments, the daily
log report may comprise a combination of graphical, numerical, and text
information
related to the user's glucose management. Referring to the figure, the daily
log may
include a graphical representation of the glucose level over time for the
selected day,
including specific indications of when sensor data was received and icons
representative
of carbohydrate intake and insulin injection. Also shown in the exemplary
embodiment of
a daily log report are numerical indications of specific glucose measurements
taken during
a time period, amount of carbohydrate intake for a period, amount of rapid-
acting and
long-acting insulin for a period, measured ketone levels for a time period,
and any notes
associated with the time period, such as an indication of medication ingested
or exercise
performed. In certain embodiments, low or high or dangerous glucose level
measurements
may be highlighted for particular attention by the user. In certain
embodiments, the daily
log report is configured to present to the user all the real-time value
including sensor
reading or blood glucose test strip measurements overlayed over the historical
data, and
providing real time results.
[0650] FIGS. 79A and 79B illustrate an exemplary reader details report. In
certain
embodiments, the reader details report may include user information,
configurable
settings, change logs, and notes. For example, as shown in the figures, the
reader details
report may include user profile information including patient name and ID,
settings,
including date and time, clock style, and sound/vibration settings, available
notes,
currently set reminders and alarms, insulin calculator settings and masked
mode settings.
In certain embodiments, settings changes for a past period of time, such as 30
days, may
be displayed in a footnote style, such that settings changes are indicated to
the user for
review.
-155-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0651] Referring again to FIG. 79A, also shown is Reader time change
information where
the user can configure the Reader to modify the time setting of the reader,
and upon saving
the time change in the Reader, such change is shown as presented in FIG. 79A
with the
date and time of the Reader time change, as well as what the time change was
(e.g., "The
time was set ahead 2 hours"). A corresponding icon is also provided under
Settings shown
in FIG. 79A corresponding to "Reader Date & Time".
[0652] FIG. 80 illustrates an exemplary frame of the generate reports menu for
setting
report parameters. As can be seen in the figure, the set report parameters
frame 7140
includes an option to set a timeframe associated with the reports to be
executed. The
timeframe may be chosen as a length of time, i.e., 2 weeks as shown in the
figure, or by
choosing a date range. In certain embodiments, the length of time timeframe
will apply to
the length of time preceding the current date. The set report parameters frame
7140 also
includes an option for setting the target glucose range to display on the
executed reports,
including the snapshot report, the daily patterns report, the mealtime
patterns report, the
weekly summary report, and the daily log report.
[0653] Also shown in FIG. 80 are settings associated with a specific report,
i.e., the
advanced daily patterns report 7150. In certain embodiments, the settings for
the advanced
daily patterns report cannot be changed from the main set report parameters
frame, but
instead requires selection of an 'edit' button to enter a screen for editing
the advanced
daily patterns report settings. When the 'edit' button is selected, the data
management
software may launch a sub-menu to edit the advanced daily patterns report
settings, such
as shown in FIG. 81A. As shown in the figure, the advanced daily patterns
settings screen
8110 includes options to change settings for times associated with daily
events or meals,
such as breakfast time, lunch time, dinner time and bedtime. The screen 8110
also includes
options to change the median goal glucose level and a low glucose allowance
parameter.
[0654] Referring still to FIG. 81A, in certain embodiments, each section of
the settings
screen 8110 may include a help or details button, represented by a "?'
indication as shown.
The daily events settings help menu 8120 may include an explanation of the
purpose of the
daily events, the definition of the daily events for the purposes of
calculation and
suggestions, and the rules associated with the daily events. For example, the
time between
bedtime and breakfast must be 12 hours or less. The setting median goal help
menu 8130
may include an explanation of what a median goal is, and what it is utilized
for in the
advanced daily patterns report. The low glucose allowance help menu 8140
includes an
-156-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
explanation of the available settings. For example, the menu 8140 includes an
explanation
to the user that increasing the low glucose allowance parameter increases the
amount of
allowable low glucose readings (below a preset parameter, such as 70mg/dL). In
certain
embodiments, a small low glucose allowance parameter translates to
approximately 2% of
readings at 50mg/dL, or 4% of readings at 60mg/dL, a medium low glucose
allowance
parameter translates to approximately 4% of readings at 50mg/dL, or 8% of
readings at
6mg/dL, and a large low glucose allowance parameter translates to
approximately 10% of
readings at 50mg/dL, or 20% of readings at 60mg/dL.
[0655] Still referring to FIG. 81A, the advanced daily patterns parameters
menu also
includes a 'save' and a 'cancel' button. When the 'save' button is selected,
any changes
made to the settings are kept, and when the 'cancel' button is selected, any
changes made
to the settings are discarded. In certain embodiments, when 'cancel' is
chosen, a
notification may pop-up on the screen requesting confirmation from the user to
discard
changes. In certain embodiments, when any settings or parameters are
configured with
invalid values, the data management software will not allow the user to save
the changes
made to the parameters and settings. In certain embodiments, when such
situation occurs,
the software will display a notification 8150 informing the user that the
settings are
invalid, as illustrated in FIG. 81B. The notification 8150 may include an
explanation of
which settings are invalid, and the corresponding requirements associated with
the
settings, and allow the user an opportunity to correct the settings. In some
embodiments,
an icon, such as a `!' symbol, are displayed next to the particular setting
that is out of
range or is invalid. Once all invalid settings are corrected, the
configurations may be saved
by the user clicking the 'save' button.
Reader Settings
[0656] FIGS. 82-86 illustrate exemplary screens for adjusting settings of the
Reader device
in certain embodiments. FIG. 82 is an example of a general settings screen.
General
settings include settings including time and date, clock style, sound and
vibration settings,
and language settings. In certain embodiments the time and date may be
automatically
updated based on the current time and date settings of the computer to which
the Reader is
connected, or via the internet. As shown, an 'update' button is made
available, whereby
the Reader time and date is updated to match the computer time and date. In
certain
embodiments, updating the time and date does not affect data recorded on the
Reader prior
to the time and date change. Another available setting, in certain
embodiments, includes a
-157-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
clock style setting. The Reader device may be configured to display the time
in 12-hour
(am/pm) format or in 24-hour format. Further, sound and vibration settings may
also be
adjustable. Such settings include volume level (high/low) and whether to
include
tones/vibrations for items such as notifications and touches.
[0657] FIG. 83A is an exemplary reader profile settings screen, wherein a
reader profile
has not yet been stored on the Reader device. As previously described above, a
reader
profile may include information including a patient name and patient ID. The
settings
screen when a reader profile is not yet stored includes fillable fields for
reader profile
information including name and patient ID. Once completed, as illustrated in
FIG. 83B,
the settings screen includes available buttons to 'discard changes' or 'save
to reader'.
Selection of 'discard changes' will cancel the entered reader profile
information, while
'save to reader' will save the changes to the Reader and thus next time the
Reader is
connected to the data management software, the reader profile information will
be
available. Once 'save to reader' is chosen, a 'saving reader settings'
notification is
displayed to inform the user that the settings are being saved, and to
preclude the user
from making any additional modifications while the save is in progress, as
shown in FIG.
83C. In certain embodiments, the notification may be an animated notification.
In certain
embodiments, as illustrated in FIG. 83D, when the Reader is disconnected from
the data
management software prior to the saving of any settings modifications, the
software may
launch a pop-up notification to inform the user of the error. In certain
embodiments, as
illustrated in FIG. 83E, when there is an error between the Reader and the
data
management software prior to the saving of any settings modifications, the
software may
launch a pop-up notification to inform the user of the error. In certain
embodiments, upon
confirmation of the error notification, the data management software may
revert back to
the home screen. In other embodiments, reconnection of the Reader to the data
management software may automatically confirm the error notification, and
return back to
the settings screen to allow the user to reattempt a 'save to reader' option.
[0658] FIG. 84 illustrates a target glucose range settings screen. The target
glucose settings
screen allows the user to choose the target glucose range associated with
glucose graphs
and the Reader's calculator for managing glucose levels. The Reader will also
utilize the
target glucose range to determine percentage or amount of time within target.
In certain
embodiments, the target glucose settings screen will include a warning to the
user to
consult with a health care professional prior to determining and entering a
target glucose
-158-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
range. In certain embodiments, when a modification is made to the target
glucose range,
the software enables the 'discard changes' and 'save to reader' options. As
described
above, upon selection of the 'save to reader' option, a 'saving reader
settings' notification
is displayed to inform the user that the settings are being saved, and to
preclude the user
from making any additional modifications while the save is in progress.
[0659] FIGS. 85A and 85B illustrate a notes screen, wherein custom notes can
be
configured by the user. FIG. 85A illustrates a screen when no custom notes
have yet been
configured. As can be seen in the figure, in certain embodiments, some
standard notes may
be pre-stored on the Reader device, including rapid-acting insulin, long-
acting insulin,
food, exercise, and medication notes. To add a custom note, an 'add note'
button is made
available to the user. Selecting the 'add note' button will make available a
line item for a
custom note, such as shown on the screen of FIG. 85B. FIG. 85B illustrates a
notes screen
with custom notes ready for configuration. In certain embodiments, any custom
notes
already saved on the Reader device will be shown in the software. In certain
embodiments,
all custom notes are always available for editing, including changing the name
of the note
and the priority of the note (position in the list from top to bottom).
Additionally, an 'x'
option is provided to delete a custom note. Upon modification to any custom
note, the
software enables the 'discard changes' and 'save to reader' options. As
described above,
upon selection of the 'save to reader' option, a 'saving reader settings'
notification is
displayed to inform the user that the settings are being saved, and to
preclude the user
from making any additional modifications while the save is in progress.
Changes to the
notes are not applied to the Reader until the 'save to reader' option is
selected.
[0660] FIG. 86 illustrates a reminders screen. As can be seen in the figure,
all configured
reminders are displayed and available for editing by the user. Each reminder
includes an
option to turn on and off the reminder, the ability to change the time of
execution of the
reminder, an option related to the occurrence of the reminder (repeating
daily, weekly, etc.
or once only), and a selection of the type of alarm, such as a check glucose
alarm, a take
insulin alarm, or a general alarm. Additionally, an 'x' option is provided to
delete a
reminder. New reminders can be added by selecting the 'add reminder' button,
whereby a
new reminder line will appear and be ready for configuration. Upon
modification to any
reminder, the software enables the 'discard changes' and 'save to reader'
options. As
described above, upon selection of the 'save to reader' option, a 'saving
reader settings'
notification is displayed to inform the user that the settings are being
saved, and to
-159-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
preclude the user from making any additional modifications while the save is
in progress.
Changes to the reminders are not applied to the Reader until the 'save to
reader' option is
selected.
[0661] FIGS. 87A-87E illustrate screens associated with professional options
of the Reader
device. As described above, professional options are options made available
only to a
healthcare provider or other professional associated with the Reader device,
and are not
made readily available to the user. As shown in FIG. 87A, the professional
options menu
choice includes a 'locked' icon, in certain embodiments represented by an icon
of a
padlock. Upon selection of the professional options menu, a pop-up menu is
initiated
requesting an access code in order to gain access to the professional options
menu. Upon
entry of a valid access code, the software navigates to the masked mode
options screen. In
certain embodiments, upon entry of a valid access code, the professional
options menu is
made available without the need to reenter an access code. In such
embodiments, the
professional options menu choice is then displayed without the locked icon, or
with an
alternate unlocked icon.
[0662] FIG. 87B illustrates a masked mode options screen, which is part of the
professional
options as described above. The masked mode settings screen, in certain
embodiments,
includes two components: enabling the masked mode feature and adjustment of
the check
glucose reminder. In certain embodiments, the check glucose reminder settings
are
unavailable while the masked mode setting is off. Once the masked mode is
enabled, the
reminder to check glucose settings are made available, which includes an
option to turn
on/off the reminder and set the time for the reminders, as shown in FIG. 87C.
[0663] The professional options additionally include a system reset screen, as
shown in
FIG. 87D, whereby the Reader can be reset to factory defaults. Activation of
the system
reset will restore all Reader settings to factory defaults, delete all patient
data stored in the
Reader, and end any sensor paired with the Reader. In certain embodiments, a
system reset
may take up to 2 minutes or more to complete. Selection of the system reset
button, in
certain embodiments, will activate a confirmation notification as shown in
FIG. 87E,
which will require confirmation prior to proceeding with the reset. The
notification may
include a warning to the user that all patient data will be deleted, the
settings will be reset
to factory defaults, and the paired sensor, if applicable, will be ended. The
notification also
includes an option to cancel the system reset before it is initiated.
-160-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0664] FIG. 88 illustrates options available in main systems menus of the data
management
software. Such menus include a 'File' menu and a 'Help' menu. The 'File' menu
may
include options such as auto-launch, auto-save, auto-save options, and exit.
Selection of
the auto-launch option will cause the data management software to launch
automatically
upon detection by the computer of a connected Reader device. Selection of the
auto-save
option will cause the software to automatically save a copy, such as a PDF
copy, of any
reports run in the data management software. Auto-save includes settings
configurable by
the user, including an option to enable auto-save, a file name format option,
and a file save
location option, as shown in FIG. 89.
Exemplary Embodiments
[0665] In some aspects of the present disclosure, methods of operating an
analyte
monitoring device are provided that include receiving an indication for
powering on an
analyte monitoring device; powering on the analyte monitoring device;
providing power to
an RF reader element within the analyte monitoring device; activating a scan
state for
scanning an analyte sensor; receiving an indication of a predetermined event;
and
powering off the RF reader and maintaining power to the analyte monitoring
device.
[0666] In one embodiment, the scan state comprises displaying a prompt to scan
the analyte
sensor on a display of the analyte monitoring device.
[0667] In one embodiment, the predetermined event is a lapse of a
predetermined period of
time without performing a scan. In some instances, the predetermined event is
a
deactivation of the scan state. In some instances, the predetermined event is
a user-
initiated change from a scan prompt screen to a home screen. In some
instances, the
predetermined event is an occurrence of a scan error or failed scan.
[0668] In one embodiment, the methods comprise: receiving an indication to
perform a
sensor scan; repowering the RF reader; and reactivating the scan state for
scanning the
analyte sensor.
[0669] In one embodiment, the methods comprise: detecting the analyte sensor;
and
scanning the analyte sensor to perform an analyte reading.
[0670] In one embodiment, the analyte is glucose or a ketone body.
[0671] In some aspects of the present disclosure, analyte monitoring devices
are provided.
The analyte monitoring devices include a processor; and memory operably
coupled to the
processor, wherein the memory includes instructions stored therein for
operating an
-161-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
analyte monitoring device, the instructions comprising instructions for
performing the
previously described methods.
[0672] In some aspects of the present disclosure, methods of operating an
analyte
monitoring device are provided that include performing a first scan of an
analyte sensor;
displaying a reading resulting from the first scan; preventing performance of
a second scan
for a predetermined period of time; and enabling performance of a second scan
after lapse
of the predetermined period of time.
[0673] In one embodiment, the methods include powering the analyte monitoring
device off
after the reading is displayed; and powering on the analyte monitoring device
before the
lapse of the predetermined period of time.
[0674] In one embodiment, the methods include receiving an indication of an
attempt of a
second scan before the lapse of the predetermined period of time; and
indicating that the
second scan cannot be performed. In some instances, the methods include
indicating an
estimated time remaining before performance of a second scan is enabled.
[0675] In one embodiment, the methods include exiting a screen displaying the
reading; and
indicating that any results displayed before the lapse of the predetermined
period of time is
for the first scan.
[0676] In one embodiment, the methods include performing the second scan of
the analyte
sensor after the lapse of the predetermined period of time.
[0677] In one embodiment, the predetermined period of time is between 1 and 5
minutes.
[0678] In one embodiment, the analyte is glucose or a ketone body.
[0679] In some aspects of the present disclosure, analyte monitoring devices
are provided.
The analyte monitoring devices include a processor; and memory operably
coupled to the
processor, wherein the memory includes instructions stored therein for
operating an
analyte monitoring device, the instructions comprising instructions for
performing the
previously described methods.
[0680] In some aspects of the present disclosure, methods of operating an
analyte
monitoring device are provided that include performing a first scan of an
analyte sensor;
displaying a screen for calculating a suggested insulin dose based on a result
of the first
scan; exiting the screen for calculating a suggested dose before logging the
suggested dose
calculation; enabling logging of the suggested dose calculation for a
predetermined period
of time; and preventing logging of the suggested dose calculation for the
first scan after a
lapse of the predetermined period of time.
-162-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0681] In one embodiment, the methods include powering the device off and then
back on
after displaying the screen for calculating the suggest dose and before the
lapse of the
predetermined period of time; wherein the exiting of the screen results from
powering the
analyte monitoring device off In some instances, the screen for calculating
the suggested
dose is displayed when the device is powered back on, and wherein the screen
for
calculating the suggested dose enables the user to log the suggested dose
calculation.
[0682] In one embodiment, the methods include receiving an indication to log
the suggested
dose calculation; and associating the suggested dose calculation with the
first scan and
with a time the first scan was performed; wherein a countdown for an estimated
amount of
insulin remaining in-body starts at a time of the logging of the suggested
dose calculation.
[0683] In one embodiment, the methods include powering the device off after
displaying
the screen for calculating the suggest dose and before lapse of the
predetermined period of
time, wherein the exiting of the screen results from powering the analyte
monitoring
device off; and powering the device back on after the lapse of the
predetermined period of
time.
[0684] In one embodiment, the methods include displaying a prompt to scan the
analyte
sensor for a second scan when the device is powered back on.
[0685] In one embodiment, the analyte is glucose or a ketone body.
[0686] In some aspects of the present disclosure, analyte monitoring devices
are provided.
The analyte monitoring devices include a processor; and memory operably
coupled to the
processor, wherein the memory includes instructions stored therein for
operating an
analyte monitoring device, the instructions comprising instructions for
performing the
previously described methods.
[0687] In some aspects of the present disclosure, methods are provided that
include
performing consecutive scans of an analyte sensor; displaying, on a display of
the analyte
monitoring device, a resulting reading for each of the consecutive scans;
displaying a
graph on the display of the analyte monitoring device, the graph displaying
resulting
readings for a first predetermined period of time and tracking subsequent
resulting
readings during a second predetermined period of time following the first
predetermined
period of time; and after the subsequent resulting readings are tracked for
the entire second
predetermined period of time, shifting the subsequent resulting readings into
the first
predetermined period of time and continuing to track subsequent resulting
readings during
-163-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
the second period of time; and repeatedly shifting the subsequent resulting
readings after
tracking occurs for the entire second period of time.
[0688] In one embodiment, the graph is displayed on the display after
resulting readings are
obtained for the first predetermined period of time.
[0689] In one embodiment, before resulting readings are obtained for the first
predetermined period of time, the graph is displayed without any resulting
readings; and
the resulting readings for the first predetermined period of time are
displayed on the graph
after the resulting readings are obtained for the first predetermined period
of time. In some
instances, the first predetermined period of time is a multiple of the second
predetermined
period of time. In some instances, the first predetermined period of time is 8
hours and the
second predetermined period of time is 1 hour.
[0690] In one embodiment, the analyte is glucose or a ketone body.
[0691] In some aspects of the present disclosure, analyte monitoring devices
are provided.
The analyte monitoring devices include a processor; and memory operably
coupled to the
processor, wherein the memory includes instructions stored therein, the
instructions
comprising instructions for performing the previously described methods.
[0692] In some aspects of the present disclosure, methods of displaying
analyte sensor
readings on an analyte monitoring device are provided that include obtaining
sensor
readings from an analyte sensor; and displaying on a display on the analyte
monitoring
device, a graph of sensor readings obtained over a prior 24-hour period.
[0693] In one embodiment, the methods include displaying a trigger element for
shifting the
graph forward or backward by 24 hours; receiving an indication that the
trigger element
was initiated by a user; and shifting the graph forward or backward by 24
hours.
[0694] In some aspects of the present disclosure, analyte monitoring devices
are provided.
The analyte monitoring devices include a processor; and memory operably
coupled to the
processor, wherein the memory includes instructions stored therein for
displaying analyte
sensor readings on an analyte monitoring device, the instructions comprising
instructions
for performing the previously described methods.
[0695] In some aspects of the present disclosure, methods of displaying
analyte sensor
readings on an analyte monitoring device are provided that include obtaining
sensor
readings from an analyte sensor; and displaying on a display on the analyte
monitoring
device, a summary of average sensor readings for a prior predetermined period
of time,
-164-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
wherein the average sensor readings include an average sensor reading for a
plurality of
divisions within a day.
[0696] In one embodiment, the prior predetermined period of time is a 7 day
period, and the
average sensor readings include an average sensor reading for four divisions
within a day.
[0697] In one embodiment, the methods include displaying a total average of
each average
sensor reading for the plurality of divisions within a day.
[0698] In one embodiment, the methods include displaying a trigger element for
shifting the
graph forward or backward by the predetermined period of time; receiving an
indication
that the trigger element was triggered by a user; and shifting the graph
forward or
backward by the predetermined period of time.
[0699] In some aspects of the present disclosure, analyte monitoring devices
are provided.
The analyte monitoring devices include a processor; and memory operably
coupled to the
processor, wherein the memory includes instructions stored therein for
displaying analyte
sensor readings on an analyte monitoring device, the instructions comprising
instructions
for performing the previously described methods.
[0700] In some aspects of the present disclosure, analyte monitoring devices
are provided.
The analyte monitoring devices include a processor; and memory operably
coupled to the
processor, wherein the memory includes instructions stored therein for
displaying analyte
sensor readings on an analyte monitoring device, the instructions including
instructions for
instructions for obtaining sensor readings from an analyte sensor; and
instructions for
displaying on a display on the analyte monitoring device, a summary of average
sensor
readings for a prior predetermined period of time, wherein the average sensor
readings
include an average sensor reading for a plurality of divisions within a day.
[0701] In one embodiment, the prior predetermined period of time is a 7 day
period, and the
average sensor readings include an average sensor reading for four divisions
within a day.
[0702] In one embodiment, the instructions include instructions for displaying
a total
average of each average sensor reading for the plurality of divisions within a
day.
[0703] In one embodiment, the instructions include instructions for displaying
a trigger
element for shifting the graph forward or backward by the predetermined period
of time;
instructions for receiving an indication that the trigger element was
triggered by a user;
and instructions for shifting the graph forward or backward by the
predetermined period of
time.
-165-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0704] In some aspects of the present disclosure, methods of displaying
analyte sensor
readings on an analyte monitoring device are provided that include obtaining
sensor
readings from an analyte sensor; and displaying on a display on the analyte
monitoring
device, a summary of average events associated with the sensor readings
obtained for a
prior predetermined period of time, wherein the average events include an
average event
for a plurality of divisions within a day; wherein the summary is displayed
upon user-
selection.
[0705] In one embodiment, the prior predetermined period of time is a 7 day
period, and the
average event include an average event for four divisions within a day.
[0706] In one embodiment, the methods include displaying a total average of
each average
event for the plurality of divisions within a day.
[0707] In one embodiment, the methods include displaying a trigger element for
shifting the
summary forward or backward by the predetermined period of time; receiving an
indication that the trigger element was triggered by a user; and shifting the
summary
forward or backward by the predetermined period of time.
[0708] In one embodiment, the event is a low glucose reading.
[0709] In some aspects of the present disclosure, analyte monitoring devices
are provided.
The analyte monitoring devices include a processor; and memory operably
coupled to the
processor, wherein the memory includes instructions stored therein for
displaying analyte
sensor readings on an analyte monitoring device, the instructions comprising
instructions
for performing the previously described methods.
[0710] In some aspects of the present disclosure, analyte monitoring devices
are provided.
The analyte monitoring devices include a processor; and memory operably
coupled to the
processor, wherein the memory includes instructions stored therein for
displaying analyte
sensor readings on an analyte monitoring device, the instructions including
instructions for
obtaining sensor readings from an analyte sensor; and instructions for
displaying on a
display on the analyte monitoring device, a summary of average events
associated with the
sensor readings obtained for a prior predetermined period of time, wherein the
average
events include an average event for a plurality of divisions within a day;
wherein the
summary is displayed upon user-selection.
[0711] In one embodiment, the prior predetermined period of time is a 7 day
period, and the
average event include an average event for four divisions within a day.
-166-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0712] In one embodiment, the analyte monitoring devices include instructions
for
displaying a total average of each average event for the plurality of
divisions within a day.
[0713] In one embodiment, the analyte monitoring devices include instructions
for
displaying a trigger element for shifting the summary forward or backward by
the
predetermined period of time; instructions for receiving an indication that
the trigger
element was triggered by a user; and instructions for shifting the summary
forward or
backward by the predetermined period of time.
[0714] In one embodiment, the event is a low glucose reading.
[0715] In some aspects of the present disclosure, methods of displaying
analyte sensor
readings on an analyte monitoring device are provided that include obtaining
sensor
readings from an analyte sensor; and displaying on a display on the analyte
monitoring
device, a summary of sensor readings obtained for a prior predetermined period
of time,
wherein the summary of sensor readings include one or more numbers or
percentages of
sensor readings with respect to a target range; wherein the summary is
displayed upon
user-selection.
[0716] In one embodiment, a number or percentage of sensor readings above,
below, and
within a target range are included in the summary.
[0717] In some aspects of the present disclosure, analyte monitoring devices
are provided.
The analyte monitoring devices include a processor; and memory operably
coupled to the
processor, wherein the memory includes instructions stored therein for
operating an
analyte monitoring device, the instructions comprising instructions for
performing the
previously described methods.
[0718] In some aspects of the present disclosure, methods are provided that
include
obtaining sensor readings from an analyte sensor; and displaying on a display
on the
analyte monitoring device, a summary of data associated with use of the sensor
for a prior
predetermined period of time, wherein the use of the sensor includes an
average number of
scans per day; wherein the summary is displayed upon user-selection.
[0719] In one embodiment, the use of the sensor includes a number of days
having sensor
data within the prior predetermined period of time.
[0720] In some aspects of the present disclosure, analyte monitoring devices
are provided.
The analyte monitoring devices include a processor; and memory operably
coupled to the
processor, wherein the memory includes instructions stored therein for
displaying analyte
sensor readings on an analyte monitoring device, the instructions comprising
instructions
-167-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
for obtaining sensor readings from an analyte sensor; and instructions for
displaying on a
display on the analyte monitoring device, a summary of data associated with
use of the
sensor for a prior predetermined period of time, wherein the use of the sensor
includes an
average number of scans per day; wherein the summary is displayed upon user-
selection.
[0721] In one embodiment, the use of the sensor includes a number of days
having sensor
data within the prior predetermined period of time.
[0722] In some aspects of the present disclosure, methods are provided that
include
obtaining sensor readings from an analyte sensor; and displaying on a display
on the
analyte monitoring device, a graph of daily patterns for a prior predetermined
period of
time, wherein the graph of daily patterns includes an average sensor reading
for a plurality
of divisions within a day; wherein the graph is displayed upon user-selection.
[0723] In one embodiment, the prior predetermined period of time is a 7 day
period, and the
graph of daily patterns includes an average sensor reading for four divisions
within a day.
[0724] In one embodiment, the chart of daily patterns indicates a range of
average sensor
readings for the plurality of divisions within a day.
[0725] In one embodiment, the methods include displaying a trigger element for
shifting the
graph forward or backward by the predetermined period of time; instructions
for receiving
an indication that the trigger element was triggered by a user; and shifting
the graph
forward or backward by the predetermined period of time.
[0726] In one embodiment, the analyte is glucose or a ketone body.
[0727] In some aspects of the present disclosure, analyte monitoring devices
are provided.
The analyte monitoring devices include a processor; and memory operably
coupled to the
processor, wherein the memory includes instructions stored therein for
displaying analyte
sensor readings on an analyte monitoring device, the instructions including
instructions for
obtaining sensor readings from an analyte sensor; and instructions for
displaying on a
display on the analyte monitoring device, a graph of daily patterns for a
prior
predetermined period of time, wherein the graph of daily patterns includes an
average
sensor reading for a plurality of divisions within a day; wherein the graph is
displayed
upon user-selection.
[0728] In one embodiment, the prior predetermined period of time is a 7 day
period, and the
graph of daily patterns includes an average sensor reading for four divisions
within a day.
[0729] In one embodiment, the chart of daily patterns indicates a range of
average sensor
readings for the plurality of divisions within a day.
-168-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0730] In one embodiment, the instructions include instructions for displaying
a trigger
element for shifting the graph forward or backward by the predetermined period
of time;
instructions for receiving an indication that the trigger element was
triggered by a user;
and instructions for shifting the graph forward or backward by the
predetermined period of
time.
[0731] In one embodiment, the analyte is glucose or a ketone body.
[0732] In some aspect of the present disclosure, methods of operating an
analyte monitoring
device are provided that include receiving an indication to operate in a
masked mode;
performing scans of an analyte sensor; and storing sensor readings obtained
from the scans
without displaying the sensor readings on a display of the analyte monitoring
device.
[0733] In one embodiment, the methods include receiving an indication to
operate in a non-
masked mode; performing scans of an analyte sensor; and displaying sensor
readings on a
display of the analyte monitoring device.
[0734] In one embodiment, the methods include transmitting the stored sensor
readings to a
remote device.
[0735] In one embodiment, the methods include the analyte is glucose or a
ketone body.
[0736] In some aspects of the present disclosure, analyte monitoring devices
are provided.
The analyte monitoring devices include a processor; and memory operably
coupled to the
processor, wherein the memory includes instructions stored therein for
operating an
analyte monitoring device, the instructions comprising instructions for
performing the
previously described methods.
[0737] In some aspect of the present disclosure, methods of operating an
analyte sensor are
provided that include communicating between an analyte sensor and a first
analyte
monitoring device; establishing a pairing with the first analyte monitoring
device to enable
the first analyte monitoring device to perform analyte readings with the
analyte sensor;
receiving an identification code for the first analyte monitoring device from
the first
analyte monitoring device; and storing the device identification code in
memory to
indicate the established pairing with the first analyte monitoring device.
[0738] In one embodiment, the methods include receiving a request for the
device
identification form a second analyte monitoring device; and transmitting the
device
identification code for the first analyte monitoring device to the second
analyte monitoring
device.
-169-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0739] In some aspects of the present disclosure, analyte monitoring devices
are provided.
The analyte monitoring devices include a processor; and memory operably
coupled to the
processor, wherein the memory includes instructions stored therein for
operating an
analyte monitoring device, the instructions comprising instructions for
performing the
previously described methods.
[0740] In some aspects of the present disclosure, methods for operating an
analyte
monitoring device are provided that include communicating between a first
analyte
monitoring device and a first sensor; determining, with a processor of the
first analyte
monitoring device, that the first sensor is not paired with any analyte
monitoring device;
determining, with the processor, that the first analyte monitoring device is
not paired with
any analyte sensor; and pairing the first analyte monitoring device with the
first sensor to
enable the first analyte monitoring device to perform analyte readings with
the first sensor;
and transmitting an identification code for the first analyte monitoring
device to the first
sensor to indicate the pairing.
[0741] In some aspects of the present disclosure, analyte monitoring devices
are provided.
The analyte monitoring devices include a processor; and memory operably
coupled to the
processor, wherein the memory includes instructions stored therein for
operating an
analyte monitoring device, the instructions comprising instructions for
performing the
previously described methods.
[0742] In some aspects of the present disclosure, methods of operating an
analyte
monitoring device are provided that include communicating between a first
analyte
monitoring device and a first sensor; determining, with a processor of the
first analyte
monitoring device, that the first sensor is paired with a second analyte
monitoring device;
and preventing, with the processor, the first analyte monitoring device from
performing
analyte readings with the first sensor; wherein the determining that the first
sensor is
paired comprises receiving, with the processor, an indication that the first
sensor contains
an identification (ID) code for the second analyte monitoring device that is
paired to the
first sensor.
[0743] In one embodiment, the methods include visually indicating on a display
on the first
analyte monitoring device that the first sensor cannot be used.
[0744] In one embodiment, the methods include communicating between the first
analyte
monitoring device and a second sensor; determining, with the processor, that
the second
sensor is not paired with any analyte monitoring device; determining, with the
processor,
-170-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
that the first analyte monitoring device is not paired with any analyte
sensor; pairing the
first analyte monitoring device with the second sensor to enable the first
analyte
monitoring device to perform analyte readings with the second sensor; and
transmitting an
identification code for the first analyte monitoring device to the second
sensor for storage
on the second sensor, the identification code indicating a pairing of the
first analyte
monitoring device with the second sensor.
[0745] In one embodiment, the methods include communicating between the first
analyte
monitoring device and a third sensor; determining, with the processor, that
the third sensor
is not paired with any analyte monitoring device; and preventing, with the
processor, the
first analyte monitoring from performing analyte readings with the second
sensor.
[0746] Certain embodiments include a reader device for receiving analyte data
from an
analyte sensing device, the reader device comprising a housing, a display
mounted on the
housing, a processor mounted in the housing, and memory storing instructions
which,
when executed by the processor, causes the processor to navigate from a
current user
interface screen on the display to a home screen in response to an input from
a user,
wherein navigating from the current user interface screen to the home screen
including
checking whether the analyte sensing device is expired and, if the analyte
sensing device is
expired, displaying a sensor expired notification, calculate a time remaining
until the
analyte sensing device expires, display the home screen, wherein the home
screen includes
an indication of the remaining time until the analyte sensing device expires,
receive
analyte information from the analyte sensing device, and display the received
analyte
information on the display.
[0747] In some embodiments, the sensor expired notification requires
confirmation prior to
navigating to the home screen.
[0748] In some embodiments, the indication of the time remaining before
expiration of the
analyte sensing device includes an indication of no active sensor, and the
time remaining
is displayed as a number of days when the time remaining is greater than 1
day, as a
number of hours when the time remaining is greater than 1 hour and less than 1
day, and
as a number of minutes when the time remaining is less than 1 hour.
[0749] In some embodiments, the home screen includes an indication of an
estimated
insulin-on-board amount.
-171-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0750] In some embodiments, the indication of the estimated insulin-on-board
amount
includes a person-shaped icon, wherein the estimated insulin-on-board amount
is
represented by a fill percentage of the person-shaped icon.
[0751] In some embodiments, the displayed analyte information includes a
numerical
current analyte level, a graphical representation of past analyte levels, and
an arrow
representing a trend of the numerical current analyte level.
[0752] Certain embodiments include instructions causing the processor to
determine
whether a current analyte level is above a high analyte level threshold, below
a low
analyte level threshold, above a high projected analyte level threshold or
below a low
projected analyte level threshold.
[0753] In some embodiments, the high analyte level threshold is 240mg/dL, the
low analyte
level threshold is 70mg/dL, the high projected analyte level threshold is
240mg/dL within
the next 15 minutes, and the low projected analyte level threshold is 70mg/dL
within the
next 15 minutes.
[0754] In some embodiments, the graphical representation of past analyte
levels includes 8
hours of past analyte data.
[0755] Certain embodiments include instructions causing the processor to not
allow
consecutive analyte level scans within a predetermined period of time.
[0756] Certain embodiments include instructions causing the processor to not
allow more
than one analyte level scan within a predetermined period of time.
[0757] In some embodiments, the predetermined period of time is 3 minutes.
[0758] Certain embodiments include a method of operating a reader device of an
analyte
monitoring system, comprising scanning for a new sensor with the reader
device, wherein
scanning includes waiting a predetermined length of time before timing out the
scanning
for the new sensor, checking whether the new sensor has previously been paired
with a
different reader device, not allowing a pairing of the reader device with the
new sensor if
the sensor has already been paired with the different reader device, waiting a
predetermined length of time for a paired sensor to warm up, displaying a home
screen
after waiting the predetermined length of time, wherein the home screen
includes an
indication of the remaining time until the paired sensor expires, starting a
glucose scan
using the paired sensor and displaying results of the glucose scan, a display
including a
simultaneous display of a numerical current glucose level, a graphical
representation of
-172-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
historical glucose levels for a past predetermined time period, and an arrow
representation
of a current glucose trend.
[0759] Certain embodiments include displaying a sensor expiring soon
notification when
the new sensor expires within a predetermined length of upcoming time.
[0760] In some embodiments, the predetermined length of upcoming time includes
3 days.
[0761] In some embodiments, the sensor expiring soon notification is displayed
after first
scan of the day for the last 3 days before sensor expiration and after every
scan for the last
8 hours before sensor expiration.
[0762] In some embodiments, a time remaining notification is contextual and
units of
display vary based on how much time remaining before sensor expiration.
[0763] In some embodiments, when the time remaining before sensor expiration
is more
than 3 days, the time remaining is displayed in units of days, when the time
remaining
before sensor expiration is between 1 hour and 1 day, the time remaining is
displayed in
units of hours, and when the time remaining before sensor expiration is less
than 1 hour,
the time remaining is displayed in units of minutes.
[0764] In some embodiments, when the time remaining before sensor expiration
reaches
zero, the reader device automatically navigates back to the home screen.
[0765] Certain embodiments include displaying a high or low glucose value
indication.
[0766] In some embodiments, the high glucose value indication is 240mg/dL and
the low
glucose value indication is 70mg/dL.
[0767] Certain embodiments include selecting the high or low glucose value
indication and
navigating to a high or low glucose notification screen, wherein the high or
low glucose
notification screen includes an option to set a reminder for a next glucose
scan.
[0768] Certain embodiments include displaying a high or low projected glucose
value
indication.
[0769] In some embodiments, the high projected glucose value indication is
240mg/dL
within the next 15 minutes and the low projected glucose value indication is
70mg/dL
within the next 15 minutes.
[0770] Certain embodiments include selecting the high or low projected glucose
value
indication and navigating to a high or low projected glucose notification
screen, wherein
the high or low projected glucose notification screen includes an option to
set a reminder
for a next glucose scan.
-173-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0771] In some embodiments, the graphical representation of historical glucose
levels
includes the prior 8 full clock hours and minutes of the current clock hour.
[0772] Certain embodiments include a method of operating a reader device in a
masked
mode, the method comprising, entering a masked mode, wherein the masked mode
precludes display of sensor data on a display of the reader device.
[0773] In some embodiments, the masked mode can only be activated or
deactivated by a
health care professional.
[0774] In some embodiments, notifications of completed sensor scans are
displayed without
results on the display of the reader device.
[0775] Certain embodiments include setting reminders to scan for current
sensor data after a
predetermined time interval.
[0776] In some embodiments, a next reminder is reset upon a manual scan.
[0777] Certain embodiments include a glucose monitoring system comprising a
glucose
sensor, a reader device paired with the glucose sensor and a reader software
executable on
a personal computer, the reader software configured to analyze data saved on
the reader
device and configure settings associated with the reader device, wherein the
reader
software automatically launches upon connection of the reader device to the
personal
computer, wherein the reader software is configured to automatically update
the reader
device firmware or the reader software upon detection of an available update
and wherein
the reader software includes a plurality of reports related to glucose data
saved on the
reader device executable by the reader software.
[0778] Certain embodiments include a glucose monitoring system comprising a
glucose
sensor, a reader device paired with the glucose sensor and including firmware
and a reader
software executable on a personal computer, the reader software configured to
analyze
data saved on the reader device and configure settings associated with the
reader device,
wherein the reader software automatically launches upon connection of the
reader device
to the personal computer, wherein the reader software is configured to
automatically
update the reader device firmware or the reader software upon detection of an
available
update and wherein the reader software includes a plurality of reports related
to glucose
data saved on the reader device executable by the reader software.
[0779] In some embodiments, the reader device must be configured prior to use,
wherein
configuring the reader device includes associating a user profile with the
reader device.
-174-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0780] In some embodiments, the reader device software requires a user to
configure the
reader device prior to use, wherein configuring the reader device includes
associating a
user profile with the reader device.
[0781] In some embodiments, the user profile includes a patient name and a
patient ID.
[0782] In some embodiments, reader software options are unavailable until the
reader
device is associated with the user profile.
[0783] In some embodiments, the reader software automatically discovers the
user profile
associated with the reader device when the reader device is connected to the
personal
computer.
[0784] In some embodiments, the reader software checks a time and date
information stored
on the reader device upon connection of the reader device to the personal
computer, and
wherein the time and date information are checked versus current time and date
information stored on the personal computer.
[0785] In some embodiments, the time and date information of the reader device
are
synchronized automatically or manually with the current time and date
information stored
on the personal computer.
[0786] In some embodiments, the time and date information of the reader device
are
synchronized with the current time and date information stored on the personal
computer.
[0787] In some embodiments, the reader software is unavailable to the user
when a software
or firmware update is in progress.
[0788] In some embodiments, the reader software executes one or more of the
plurality of
reports simultaneously.
[0789] In some embodiments, the results of the executed plurality of reports
are saved in a
PDF format.
[0790] In some embodiments, the plurality of reports include a snapshot
report, a daily
patterns report, a mealtime patterns report, a monthly summary report, a
weekly summary
report, and a daily log report.
[0791] In some embodiments, the one or more of the plurality of reports
includes graphical
information and numerical information.
[0792] In some embodiments, the reader software includes customizable notes
for events
associated with the glucose data.
[0793] In some embodiments, the reader software includes customizable
reminders
configurable for execution on the reader device.
-175-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
[0794] In some embodiments, the reader software includes authorized access
only options.
[0795] In some embodiments, the authorized access only options require an
access code for
access.
[0796] In some embodiments, the authorized access only options include an
option to
disable or enable a masked mode, wherein the masked mode configures the reader
device
to not display the glucose data on a display of the reader device.
[0797] In some aspects of the present disclosure, analyte monitoring devices
are provided.
The analyte monitoring devices include a processor; and memory operably
coupled to the
processor, wherein the memory includes instructions stored therein for
operating an
analyte monitoring device, the instructions comprising instructions for
performing the
previously described methods.
[0798] It should be understood that techniques introduced herein can be
implemented by
programmable circuitry programmed or configured by software and/or firmware,
or they
can be implemented entirely by special-purpose "hardwired" circuitry, or in a
combination
of such forms. Such special-purpose circuitry (if any) can be in the form of,
for example,
one or more application-specific integrated circuits (ASICS), programmable
logic devices
(PLDs), field-programmable gate arrays (FPGAs), etc.
[0799] Software or firmware implementing the techniques introduced herein may
be stored
on a machine-readable storage medium and may be executed by one or more
general-
purpose or special-purpose programmable microprocessors. A "machine-readable
medium" ", as the term is used herein, includes any mechanism that can store
information
in a form accessible by a machine (a machine may be, for example, a computer,
network
device, cellular phone, personal digital assistant (PDA), manufacturing took,
any device
with one or more processors, etc.). For example, a machine-accessible medium
includes
recordable/non-recordable media (e.g., read-only memory (ROM); random access
memory
(RAM); magnetic disk storage media; optical storage media; flash memory
devices; etc.),
etc. The term "logic", as used herein, can include, for example, special
purpose hardwired
circuitry, software and/or firmware in conjunction with programmable
circuitry, or a
combination thereof
[0800] The preceding merely illustrates the principles of the present
disclosure. It will be
appreciated that those skilled in the art will be able to devise various
arrangements which,
although not explicitly described or shown herein, embody the principles of
the present
disclosure and are included within its spirit and scope. Furthermore, all
examples and
-176-
CA 02902234 2015-08-20
WO 2014/145049 PCT/US2014/029698
conditional language recited herein are principally intended to aid the reader
in
understanding the principles of the invention and the concepts contributed by
the inventors
to furthering the art, and are to be construed as being without limitation to
such
specifically recited examples and conditions. Moreover, all statements herein
reciting
principles, aspects, and aspects of the present disclosure as well as specific
examples
thereof, are intended to encompass both structural and functional equivalents
thereof
Additionally, it is intended that such equivalents include both currently
known equivalents
and equivalents developed in the future, i.e., any elements developed that
perform the
same function, regardless of structure. The scope of the present disclosure,
therefore, is not
intended to be limited to the exemplary aspects shown and described herein.
Rather, the
scope and spirit of present disclosure is embodied by the appended claims.
-177-