Note: Descriptions are shown in the official language in which they were submitted.
CA 02902250 2015-08-28
51682-49
NEW PEPPER PLANTS PRODUCING FRUITS
WITH EXTREME DARK GREEN COLOR
FIELD OF THE INVENTION
[0001] This invention is in the field of pepper plants, in particular, the
invention relates to
novel pepper plants producing fruits having an extreme dark green color.
BACKGROUND OF THE INVENTION
[0002] Fruits of plants of the genus Capsicum, like sweet peppers and hot
peppers,
hereafter both types being referred to as peppers, are available in a wide
variety of
different colors like red, yellow, brown, and orange generally for fully
matured fruits and
green, white, lilac, and purple for non-mature "unripe" fruits.
[0003] Chlorophyll is the molecule that is called a photoreceptor. It is
found in the
chloroplasts of green pepper plants, and is what makes pepper fruits green.
The basic
structure of a chlorophyll molecule is a porphyrin ring, coordinated to a
central atom.
This is very similar in structure to the heme group found in hemoglobin,
except that in
heme the central atom is iron, whereas in chlorophyll it is magnesium.
[0004] It is usually easy to tell when a food has significant amounts of
chlorophyll,
because chlorophyll provides the green color that is found in the green parts
of the plants
and in many of the fruits and vegetables that are consumed. These plants and
foods would
not be green without their chlorophyll, since chlorophyll pigments reflect
sunlight at
exact appropriate wavelengths for our eyes to detect them as green. The
chlorophyll a
molecule actually reflects light in a blue-green range (about 685 nanometer
wavelengths),
while chlorophyll b reflects light in a more yellow-green color (about 735
nanometer
wavelengths). The overall effect, however, is a color that one would simply
call "green."
[0005] All green plants contain chlorophyll a, and most vegetables that are
eaten contain
both chlorophyll a and chlorophyll b, while in many of them, there is slightly
more
1
CA 02902250 2015-08-28
51682-49
chlorophyll a than chlorophyll b and this slight edge in favor of chlorophyll
a tends to
decrease as the plant ages. Some vegetables contain particularly high amounts
of total
chlorophyll. Best studied of all the vegetables is spinach containing about
300-600
milligrams per ounce.
[0006] Among vegetables consumed around the world - asparagus, green bell
peppers,
broccoli, Brussels sprouts, green cabbage, celery, collard greens, green
beans, green peas,
kale, leeks, green olives, parsley, romaine lettuce, sea vegetables, spinach,
Swiss chard,
and turnip greens are concentrated sources of chlorophyll.
[0007] Research on the health benefits of chlorophyll has focused on the
area of cancer.
This research got underway when damage to genes by carcinogenic substances
called
aflatoxins, was found to be prevented by chlorophyllin, a derivative of
chlorophyll.
Research studies in humans (Egner et al. 2001 and 2003, Jubert et al. 2009)
have found
that damage to DNA by aflatoxin can be decreased as much as 55% through
supplementation with chlorophyllin at 100 milligrams, three times a day, for
four months.
This amount of chlorophyllin, 300 milligrams per day, is the same amount of
chlorophyll
found in one weighted ounce of spinach (a little over 1/2 cup of chopped raw
spinach).
Although research is still in the early stage, prevention and treatment of
liver cancer, skin
cancer, and colon cancer are all being investigated in relationship to intake
of
chlorophyll-containing vegetables and supplementation with chlorophyllin.
[0008] Another study by Chemomorsky and his colleagues (Chemomorsky et al.
1999)
addressed the preventive effect of chlorophyll and derivatives. The growing
body of
epidemiological and experimental evidence associating diets rich in fruits and
vegetables
with prevention of chronic diseases such as cancer has stimulated interest in
plant food
phytochemicals as physiologically active dietary components. Chlorophyll and
its various
derivatives are believed to be among the family of phytochemical compounds
that are
potentially responsible for such associations. Dietary chlorophyll is
predominantly
composed of lipophilic derivatives including chlorophyll a and b (fresh fruits
and
vegetables), metal-free pheophytins and pyropheophytins (thermally processed
fruits and
2
CA 02902250 2015-08-28
51682-49
vegetables), as well as Zn-pheophytins and Zn-pyropheophytins (thermally
processed
green vegetables). Although the use of chlorophyll derivatives in traditional
medical
applications is well documented, it is perhaps the potential of chlorophyll as
a cancer
preventative agent that has drawn significant attention recently. Biological
activities
attributed to chlorophyll derivatives consistent with cancer prevention
include antioxidant
and antimutagenic activity, mutagen trapping, modulation of xenobiotic
metabolism, and
induction of apoptosis. Recent research efforts have also included
investigation of the
impact of digestive factors on chlorophyll structure and bioaceessibility as a
means to
better understand the extent to which these pigments may be bioavailable in
humans and
therefore may have more systemic impact in the prevention of cancer (Ferruzzi
and
Blakeslee, 2007).
100091 It has been recognized that the perception of food products,
particularly fresh
vegetables, is highly impacted by the color of the said product. In vegetable
products like
pepper or tomato, intensity of red color can be perceived as a sign of intense
flavor while
the green color as seen for salad, broccoli and green pepper is perceived as a
sign of
freshness and healthiness of the product. Indeed, the greener is the product,
the fresher
and healthier it is perceived.
[00010] The plant pigments lutein and zeaxanthin are antioxidants. Good
sources of lutein
and zeaxanthin include a variety of vegetables as well as other foods. Fresh,
raw foods
are best when it comes to getting the most nutrition per serving.
[00011] A study conducted by the Journal of the American College of Nutrition
in 2004
concluded that "There is a continuously growing body of evidence that suggests
that
lutein and zeaxanthin may contribute to the protection against several age-
related
diseases, including cataract and age-related macular degeneration as well as
other
diseases including dementia." Vegetables are by far the greatest source of
lutein and
zeaxanthin. Leafy greens such as romaine, spinach, Swiss chard, turnip greens,
kale,
collard greens, watercress and parsley top the content list. Fresh red and
orange peppers
also offer suitable source of lutein. According to the American Optometric
Association,
3
CA 02902250 2015-08-28
51682-49
both lutein and zeaxanthin are of great benefit to eye health. Along with
helping to
prevent age-related macular degeneration (AMD), they can also improve vision
in those
already afflicted with this disease; they decrease the risk of contracting
cataracts too,
since both of these carotenoids protect and maintain healthy cells in the eye.
Other health
benefits are protection of your heart and brain, and they assist the body in
combating
arthritis as well.
[00012] Violaxanthin maybe a metabolite or precursor of zeaxanthin depending
of sun
light exposure and the amount of the first one may be a good indicator of the
amount of
the second one.
[00013] Peppers represent a valuable source of vitamins and nutrients
associated with their
pigments and fruit color, including various antioxidants, carotenoids as well
as
chlorophyll. In the present trend of consumers looking for fresh and healthy
vegetables,
pepper fruits constitute a product of choice.
[00014] Peppers fruits are generally green when immature and turn generally
red, orange,
or yellow once mature. The color of the pepper fruits is a result of a mixture
of different
color components in the fruit. The color component green is provided by the
presence of
chloroplasts containing an abundant amount of chlorophyll. The color
components red
and yellow are provided by chromoplasts filled with red and yellow
carotenoids,
respectively. Examples of such carotenoids are capsanthin and capsorubin,
lutein, beta
carotcnc, violaxanthin and zeaxanthin. The different possible colors of the
immature and
mature fruits are usually a combination of different ratios between the
different
chlorophyll and carotenoids pigments.
[00015] In some of the markets, peppers are usually harvested green, i.e. at a
non mature
stage. Immature pepper fruits generally exhibit a less sweet taste as compared
to red -
mature- fruits.
[00016] Immature green peppers fruits can exhibit various green color
variations from
very pale green to dark green. Higher intensity of the green pepper color is
considered as
4
CA 02902250 2015-08-28
51682-49
a sign of freshness and quality by consumers as well as perceived as a health
attribute. It
is then a continuous and vigorous trend from consumers to get green pepper
fruits
exhibiting a deep and intense green color.
[00017] It is therefore a need to provide pepper plants that produce pepper
fruits with
enhanced deep and intense green color, associated with enhanced nutritional
value thanks
to enhanced antioxidants, carotenoids and other healthy compounds content as
well as a
green color appearance that would render them attractive to the consumer, with
enhanced
perception of freshness and quality.
SUMMARY OF THE INVENTION
[00018] The present invention includes and provides pepper plants having
extreme dark
green colored pepper fruits at immature harvestable stage that also exhibit
high content in
pigments.
[00019] Indeed, it has been surprisingly found by the inventors of the present
invention
that the increase of the quantity of selected pigments such as chlorophyll A,
chlorophyll
B, lutein, a-carotene, [3-carotene and/or violaxanthin is also accompanied by
a
modification of the external color perception of the pepper fruit at immature
harvestable
stage. The fruits of the pepper plant of the present invention thus exhibit an
extreme dark
green color that could be called extreme dark green or extreme green and that
can be
measured, for example, by colorimeter parameters, image analysis using
computer vision,
or by eye-measurement.
[00020] In representative embodiments, the pepper fruits of the invention are
blocky type
pepper fruits.
[00021] In embodiments, the pepper fruits of the invention are commercially
acceptable
with respect to size, shape, color, yield, and the like.
[00022] In embodiments, the pepper plants of the invention are cultivated.
CA 02902250 2015-08-28
51682-49
[00023] According to the invention, there are also provided novel pepper
cultivars
designated RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B and 16452C.
Thus, the invention encompasses the seeds of pepper cultivars RPP 25822, RPP
26098,
RPP 26105, 8728C, 16452A, 16452B and 16452C, the plants of pepper cultivars
RPP
25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B and 16452C, plant parts of
the
pepper cultivars RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B and
16452C (including fruits, seeds, gametes), methods of producing seed from
pepper
cultivars RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or 16452C,
methods for producing a pepper plant containing in its genetic material one or
more
transgenes, and the transgenic pepper plants produced by that method. The
invention
also relates to methods for producing other pepper plants derived from pepper
cultivars
RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or 16452C and to pepper
plants, parts thereof and seed derived by the use of those methods. The
present invention
further relates to pepper seeds and plants (and parts thereof including
fruits) produced by
crossing pepper cultivars RPP 25822, RPP 26098, or RPP 26105, 8728C, 16452A,
16452B or 16452C with itself or with another pepper plant (e.g., an Fl hybrid
seed or
plant).
[00024] In another aspect, the present invention provides regenerable cells
for use in tissue
culture of pepper cultivars RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A,
16452B
and 16452C. In embodiments, the tissue culture is capable of regenerating
plants having
all or essentially all of the physiological and morphological characteristics
of the
foregoing pepper plant and/or of regenerating plants having the same or
substantially the
same genotype as the foregoing pepper plant. In exemplary embodiments, the
regenerable cells in such tissue cultures are meristematic cells, cotyledons,
hypocotyl,
leaves, pollen, embryos, roots, root tips, anthers, pistils, ovules, shoots,
stems, petiole,
pith, flowers, capsules, fruits and/or seeds as well as callus and/or
protoplasts derived
from any of the foregoing. Still further, the present invention provides
pepper plants
regenerated from the tissue cultures of the invention.
6
CA 02902250 2015-08-28
51682-49
1000251 As a further aspect, the invention provides a method of producing
pepper seed,
the method comprising crossing a plant of pepper cultivar RPP 25822, RPP
26098, RPP
26105, 8728C, 16452A, 16452B or 16452C with itself or a second pepper plant.
Pepper
cultivars RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B and/or 16452C
can be the female and/or male parent. In embodiments, the male parent is
pepper cultivar
8728C. In embodiments, the female parent is pepper cultivar 16452A, 16452B or
16452C. Optionally, the method further comprises collecting the seed, which
encompasses harvesting a fruit containing the seed.
[00026] The invention further provides a method of producing a progeny pepper
plant, the
method comprising crossing a plant of pepper cultivar RPP 25822, RPP 26098,
RPP
26105, 8728C, 16452A, 16452B or 16452C with itself or a second pepper plant to
produce at least a first progeny plant, which may optionally be a selfed plant
or an Fl
hybrid. Pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B
and/or 16452C can be the female and/or male parent. In embodiments, the method
further comprises (a) crossing the at least first progeny plant with itself or
a second plant
to produce a seed of a progeny plant of a subsequent generation; (b) growing a
progeny
plant of a subsequent generation from said seed and crossing the progeny plant
of a
subsequent generation with itself or a second plant to produce a progeny plant
of a
further subsequent generation; and (c) optionally repeating steps (a) and (b)
one or more
time to produce a pepper plant further derived from pepper variety RPP 25822,
RPP
26098, RPP 26105, 8728C, 16452A, 16452B or 16452C, wherein the progeny plant
of a
further subsequent generation of step (b) is used in place of the first
progeny plant in step
(a).
[00027] Another aspect of the invention provides methods for producing hybrids
and other
pepper plants derived from pepper cultivars RPP 25822, RPP 26098, RPP 26105,
8728C,
16452A, 16452B and 16452C. Pepper plants derived by the use of those methods
are
also part of the invention as well as plant parts, seed, gametes and tissue
culture from
such hybrid or derived pepper plants.
7
CA 02902250 2015-08-28
51682-49
[00028] In representative embodiments, a pepper plant derived from pepper
cultivar RPP
25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or 16452C comprises cells
comprising at least one set of chromosomes derived from pepper cultivar RPP
25822,
RPP 26098, RPP 26105, 8728C, 16452A, 16452B or 16452C. In embodiments, a
pepper
plant or population of pepper plants derived from pepper cultivar RPP 25822,
RPP
26098, RPP 26105, 8728C, 16452A, 16452B or 16452C comprises, on average, at
least
about 6.25%, 12.5%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 80%,
85%, 90%, 95%, 96%, 97%, 98% or 99% of its alleles (i.e., theoretical allelic
content;
TAC) from pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A,
16452B or 16452C, e.g., at least about 6.25%, 12.5%, 25%, 30%, 35%, 40%, 45%,
50%,
55%, 60%, 65%, 70%, 80%, 85%, 90%, 95%, 96%, 97%, 98% or 99% of the genetic
complement of pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A,
16452B or 16452C. In embodiments, the pepper plant derived from pepper
cultivar RPP
25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or 16452C is one, two,
three,
four, five or more breeding crosses removed from pepper cultivar RPP 25822,
RPP
26098, RPP 26105, 8728C, 16452A, 16452B or 16452C.
[00029] In embodiments, a hybrid or derived plant from pepper cultivar RPP
25822, RPP
26098, RPP 26105, 8728C, 16452A, 16452B or 16452C comprises a desired added
trait(s). In representative embodiments, a pepper plant derived from pepper
cultivar RPP
25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or 16452C comprises some or
all of the morphological and physiological characteristics of pepper cultivar
RPP 25822,
RPP 26098, RPP 26105, 8728C, 16452A, 16452B or 16452C (e.g., as described
herein,
in particular, in any one or more of Tables 1-9 or Figures 1-30). In
embodiments, the
pepper plant derived from pepper cultivar RPP 25822, RPP 26098, RPP 26105,
8728C,
16452A, 16452B or 16452C comprises essentially all of the morphological and
physiological characteristics of pepper cultivar RPP 25822, RPP 26098, RPP
26105,
8728C, 16452A, 16452B or 16452C (e.g., as described herein, in particular, in
any one or
more of Tables 1-9 or Figures 1-30), with the addition of a desired added
trait(s).
8
CA 02902250 2015-08-28
51682-49
[00030] The invention also relates to methods for producing a pepper plant
comprising in
its genetic material one or more transgenes and to the transgenic pepper plant
produced
by those methods (and progeny pepper plants comprising the transgene). Also
provided
are plant parts, seed and tissue culture from such transgenic pepper plants,
optionally
wherein one or more cells in the plant part, seed, or tissue culture comprises
the
transgene. The transgene can be introduced via plant transformation and/or
breeding
techniques.
[00031] In another aspect, the present invention provides for single gene
converted plants
of pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or
16452C. Plant parts, seed, and tissue culture from such single gene converted
plants are
also contemplated by the present invention. The single transferred gene may be
a
dominant or recessive allele. In representative embodiments, the single
transferred gene
confers such traits as male sterility, herbicide resistance, pest resistance
(e.g., insect
and/or nematode resistance), modified fatty acid metabolism, modified
carbohydrate
metabolism, disease resistance (e.g., for bacterial, fungal and/or viral
disease including
resistance to Xanthomonas campestris pv. Vesicatoria [e.g., race 6]), male
fertility,
enhanced nutritional quality, improved appearance (e.g., color), improved salt
tolerance,
industrial usage, or any combination thereof. The single gene may be a
naturally
occurring pepper gene or a transgene introduced into pepper through genetic
engineering
techniques.
[00032] The invention further provides methods for developing pepper plants in
a pepper
plant breeding program using plant breeding techniques including, for example,
recurrent
selection, backcrossing, pedigree breeding, doubled haploid techniques,
restriction
fragment length polymorphism enhanced selection, genetic marker enhanced
selection
and/or transformation. Seeds, pepper plants, and parts thereof, produced by
such
breeding methods are also part of the invention.
9
CA 02902250 2015-08-28
51682-49
[00033] The invention also provides methods of multiplication or propagation
of pepper
plants of the invention, which can be accomplished using any method known in
the art,
for example, via vegetative propagation and/or seed.
[00034] The invention further provides a method of producing food or feed
comprising (a)
obtaining a pepper plant of the invention, optionally wherein the plant has
been cultivated
to maturity, and (b) collecting at least one pepper plant or part thereof
(e.g., fruits, seeds)
from the plant. In embodiments, obtaining a pepper plant comprises growing the
plant.
[00035] Additional aspects of the invention include harvested products and
processed
products from the pepper plants of the invention. A harvested product can be a
whole
plant or any plant part, as described herein. Thus, in some embodiments, a non-
limiting
example of a harvested product includes a seed or a fruit.
[00036] In representative embodiments, a processed product includes, but is
not limited to:
dehydrated, cut, sliced, chopped, diced, ground, pureed, dried, canned,
jarred, washed,
brined, packaged, refrigerated, frozen, blanched and/or heated fruits and/or
seeds of the
pepper plants of the invention, or any other part thereof. In embodiments, a
processed
product includes a sugar or other carbohydrate, fiber, protein, pigment (e.g.,
a carotenoid)
and/or aromatic compound that is extracted, purified or isolated from a pepper
plant of
the invention. In embodiments, the processed product includes washed and
packaged
fruits (or parts thereof) of the invention, for example, cut, sliced or diced
and in a frozen
form.
[00037] Thus, the invention also provides a method of producing a processed
product
from a plant of the invention, the method comprising (a) obtaining a fruit of
a plant of the
invention; and (b) processing the fruit to produce a processed product. In
embodiments,
processing comprises slicing, cutting, dicing, dehydrating, pureeing,
blanching and/or
freezing.
CA 02902250 2015-08-28
51682-49
[00038] In representative embodiments, the invention provides a seed of a
pepper plant of
the invention. In embodiments, the invention is directed to seed that produces
the pepper
plants of the invention.
[00039] The seed of the invention can optionally be provided as an essentially
homogenous population of seed of a single plant or cultivar. Essentially
homogenous
populations of seed are generally free from substantial numbers of other seed,
e.g., at
least about 90%, 95%, 96%, 97%, 98% or 99% pure.
[00040] As a further aspect, the invention provides a plant of pepper cultivar
RPP 25822,
RPP 26098, RPP 26105, 8728C, 16452A, 16452B or 16452C.
[00041] As an additional aspect, the invention provides a pepper plant, or a
part thereof,
having all or essentially all of the physiological and morphological
characteristics of a
plant of pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A,
16452B or
16452C.
[00042] As another aspect, the invention provides fruits and/or seed of the
pepper plants of
the invention and a processed product from the fruits and/or seed of the
inventive pepper
plants.
[00043] As still another aspect, the invention provides a method of producing
pepper seed,
the method comprising crossing a pepper plant of the invention with itself or
a second
pepper plant. The invention also provides seed produced by this method and
plants
produced by growing the seed.
[00044] As yet a further aspect, the invention provides a method for producing
a seed of a
pepper plant derived from pepper cultivar RPP 25822, RPP 26098, RPP 26105,
8728C,
16452A, 16452B or 16452C, the method comprising: (a) crossing a pepper plant
of
pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or
16452C
with a second pepper plant; and (b) allowing seed of a pepper plant derived
from pepper
cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or 16452C to
11
CA 02902250 2015-08-28
51682-49
form. In embodiments, the method further comprises: (c) growing a plant from
the seed
derived from pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A,
16452B or 16452C of step (b); (d) selfing the plant grown from the pepper seed
derived
from pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or
16452C or crossing it to a second pepper plant to form additional pepper seed
derived
from pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or
16452C and (e) optionally repeating steps (c) and (d) one or more times to
generate
further derived pepper seed from pepper cultivar RPP 25822, RPP 26098, RPP
26105,
8728C, 16452A, 16452B or 16452C, wherein a plant grown from the additional
pepper
seed of step (d) is used in place of the plant grown from the seed derived
from pepper
cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or 16452C of
step
(b). Optionally, the method comprises: (e) repeating steps (c) and (d) one or
more times
(e.g., one to three, one to five, one to six, one to seven, one to ten, three
to five, three to
six, three to seven, three to eight or three to ten times) to generate further
derived pepper
plants and seed. As another option, the method can comprise collecting the
seed, which
includes collecting a fruit comprising the seed. The invention also provides
seed
produced by these methods and plants produced by growing the seed.
[00045] As another aspect, the invention is also directed to a method of
producing a
pepper fruit comprising obtaining a plant according to the instant invention
and
harvesting a fruit from the plant. In embodiments, obtaining a plant of the
invention
comprises growing the plant to produce a fruit.
[00046] Still further, as another aspect, the invention provides a method of
vegetatively
propagating a plant of pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C,
16452A, 16452B or 16452C. In a non-limiting example, the method comprises: (a)
collecting tissue capable of being propagated from a plant of pepper cultivar
RPP 25822,
RPP 26098, RPP 26105, 8728C, 16452A, 16452B or 16452C; (b) cultivating the
tissue to
obtain proliferated shoots; and (c) rooting the proliferated shoots to obtain
rooted
plantlets. Optionally, the invention further comprises growing plants from the
rooted
12
CA 02902250 2015-08-28
51682-49
plantlets. The invention also encompasses the plantlets and plants produced by
these
methods.
1000471 As an additional aspect, the invention provides a method of
introducing a desired
added trait into pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C,
16452A,
16452B or 16452C, the method comprising: (a) crossing a first plant of pepper
cultivar
RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or 16452C with a second
pepper plant that comprises a desired trait to produce Fl progeny; (b)
selecting an Fl
progeny that comprises the desired trait; (c) crossing the selected Fl progeny
with pepper
cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or 16452C to
produce backcross progeny; and (d) selecting backcross progeny comprising the
desired
trait to produce a plant derived from pepper cultivar RPP 25822, RPP 26098,
RPP 26105,
8728C, 16452A, 16452B or 16452C comprising a desired trait. In embodiments,
the
selected progeny has one or more of the characteristics of RPP 25822, RPP
26098, RPP
26105, 8728C, 16452A, 16452B or 16452C (e.g., as described herein, in
particular, in
any one or more of Tables 1-9 or Figures 1-30). In embodiments, the selected
progeny
comprises all or essentially all the morphological and physiological
characteristics of the
first plant of pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A,
16452B or 16452C. Optionally, the method further comprises: (e) repeating
steps (c) and
(d) one or more times to produce a plant derived from pepper cultivar RPP
25822, RPP
26098, RPP 26105, 8728C, 16452A, 16452B or 16452C comprising a desired added
trait
and essentially all of the physiological and morphological characteristics of
the pepper
cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or 16452C,
wherein the selected backcross progeny produced in step (d) is used in place
of the
selected Fl progeny in step (c).
[00048] In representative embodiments, the invention also provides a method of
producing
a plant of pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A,
16452B
or 16452C comprising a desired added trait, the method comprising introducing
a
transgene or locus conferring the desired trait into a plant of pepper
cultivar RPP 25822,
RPP 26098, RPP 26105, 8728C, 16452A, 16452B or 16452C. The transgene can be
13
CA 02902250 2015-08-28
51682-49
introduced by transformation methods (e.g., genetic engineering) or breeding
techniques.
In embodiments, the plant comprising the transgene or locus has one or more of
the
morphological and physiological characteristics of RPP 25822, RPP 26098, RPP
26105,
8728C, 16452A, 16452B or 16452C (e.g., as described herein, in particular, in
any one or
more of Tables 1-9 or Figures 1-30). In embodiments, the plant comprising the
transgene
or locus comprises all or essentially all of the morphological and
physiological
characteristics of pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C,
16452A,
16452B or 16452C.
[00049] The invention also provides pepper plants produced by the methods of
the
invention or a selfed progeny thereof, wherein the pepper plant has the
desired added trait
as well as seed from such pepper plants.
[00050] According to the foregoing methods, the desired added trait can be any
suitable
trait known in the art including, for example, male sterility, male fertility,
herbicide
resistance, insect or pest (e.g., insect and/or nematode) resistance, modified
fatty acid
metabolism, modified carbohydrate metabolism, disease resistance (e.g., for
bacterial,
fungal and/or viral disease including resistance to Xanthomonas eampestris pv.
Vesicatoria [e.g., race 6]), enhanced nutritional quality, increased
sweetness, increased
flavor, reduced cracking, improved ripening control, improved salt tolerance,
industrial
usage, or any combination thereof.
1000511 In representative embodiments, a transgene or locus conferring
herbicide
resistance confers resistance to glyphosate, sulfonylurea, imidazolinone,
dicamba,
glufosinate, phenoxy proprionic acid, L-phosphinothricin, cyclohexone,
cyclohexanedione, triazine, benzonitrile, or any combination thereof.
[00052] In representative embodiments, a transgene or locus conferring pest
resistance
(e.g., insect and/or nematode resistance) encodes a Bacillus thuringiensis
endotoxin.
[00053] In
representative embodiments, transgenic plants (e.g., using genetic engineering
techniques), single gene converted plants, hybrid plants and pepper plants
derived from
14
CA 02902250 2015-08-28
51682-49
pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or
16452C
have at least 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or more of the morphological and
physiological
characteristics of pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C,
16452A,
16452B or 16452C (e.g., as described herein, in particular, in any one or more
of Tables
1-9 or Figures 1-30), or even all of the morphological and physiological
characteristics of
pepper cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or
16452C, so that said plants are not significantly different for said traits
than pepper
cultivar RPP 25822, RPP 26098, RPP 26105, 8728C, 16452A, 16452B or 16452C as
determined at the 5% significance level when grown in the same environmental
conditions; optionally, with the presence of one or more desired additional
traits (e.g.,
male sterility, disease resistance, pest or insect resistance, herbicide
resistance, and the
like).
[00054] The invention also encompasses plant parts, plant material, pollen,
ovules, leaves,
fruits and seed from the pepper plants of the invention. Also provided is a
tissue culture
of regenerable cells from the pepper plants of the invention, where
optionally, the
regenerable cells are: (a) embryos, meristem, leaves, pollen, cotyledons,
hypocotyls,
roots, root tips, anthers, flowers, pistils, ovules, seed, shoots, stems,
stalks, petioles, pith,
fruits and/or capsules; or (b) callus or protoplasts derived from the cells of
(a). Further
provided are pepper plants regenerated from a tissue culture of the invention.
[00055] The invention also contemplates methods of determining the genotype of
a plant
of the invention, the method comprising obtaining nucleic acids from the plant
and
detecting a plurality of polymorphisms in the nucleic acids. Optionally, the
results are
stored on a computer readable medium.
[00056] In representative embodiments is provided a cultivated Capsicum annuum
plant
producing pepper fruits, particularly a blocky type pepper plant producing
blocky type
pepper fruits exhibiting extreme dark green color at immature harvestable
stage as
described and characterized herein.
CA 02902250 2015-08-28
51682-49
[00057] The present invention also includes and provides methods of
introgressing one or
more (e.g., two), extreme dark green pepper fruit QTL allele(s) into a pepper
plant
comprising:
a) crossing a plant from a first pepper plant as a first parent having one or
more extreme
dark green pepper fruit allele(s) with a second pepper plant as a second
parent aiming to
form a population segregating for dark green immature fruit color,
b) phenotyping the population aiming to select plants with darker immature
fruit color
having one or more extreme dark green fruit QTL allele(s),
c) screening the segregating population for a member having one or more
extreme dark
green pepper fruit QTL allele using (a) nucleic acid molecule(s) capable of
identifying
(an) extreme dark green allele(s),
and
d) selecting a pepper plant that contains the one or more extreme dark green
allele(s) at
(a) QTL for further crossing.
[00058] In representative embodiments, the present invention further includes
and
provides methods of producing a pepper plant bearing extreme dark green pepper
fruits
comprising:
a) providing a pepper plant as a first parent;
b) crossing the first parent with a second pepper plant according to the
present
invention;
c) growing pepper plant seed produced by the cross to yield a progeny
pepper plant
bearing fruits;
d) determining the L* value of the surface and/or flesh, chlorophyll a
content,
chlorophyll b content, lutein content, violaxanthin content, or any
combination of the
foregoing, for the pepper fruits of progeny pepper plants of c;
e) selecting the progeny pepper plant(s) that has extreme dark green pepper
fruits as
indicated by one or more of the parameters in (d) at the immature harvestable
stage as
described herein.
16
CA 02902250 2015-08-28
51682-49
[00059] Still further, in representative embodiments, the invention
provides a method of
providing a pepper plant producing pepper fruits exhibiting an increased
content of
chlorophyll a, chlorophyll b, lutein and/or violaxanthin, comprising the steps
of:
a) providing a pepper population segregating for dark green immature fruit
color,
b) screening the segregating population for a member having an extreme dark
green
pepper fruit trait, wherein said trait can be identified by the presence of
one or
more of the following molecular markers in the genome: SP436, 5P626, SP693
and SP694; and
c) selecting at least one member of the segregating population, wherein
said at least
one member is bearing an extreme dark green pepper fruit trait.
[00060] The present invention also includes and provides a pepper plant
bearing extreme
dark green color fruit at immature harvestable stage, said plant comprising
one or more
(e.g., two) genetic detelininants directing or controlling expression of said
extreme dark
green color in the pepper fruit of the pepper plant, wherein said genetic
determinant(s)
are obtainable from a pepper plant source, particularly from Capsicum annuum,
particularly from Capsicum annuum 8728C, RPP 25822, RPP 26098, RPP 26105,
8728C,
16452A, 16452B or 16452C. In embodiments, the pepper plant is homozygous for
the
genetic determinant(s).
[00061] In representative embodiments, the genetic determinant(s) that
controls the
expression of the extreme dark green color in the pepper fruits also controls
the color and
pigment/nutritional characteristics associated with the extreme dark green
color trait as
described herein.
[00062] In representative embodiments, the present invention further includes
and
provides methods of identifying a pepper plant bearing extreme dark green
color pepper
fruits at immature harvestable stage, the method comprising:
a) providing a population segregating for extreme dark green immature
fruit color,
17
CA 02902250 2015-08-28
51682-49
b) screening the segregating population for a member having an extreme dark
green
pepper fruit trait, wherein said trait can be identified by the presence of
one or more of
the following molecular markers in the genomc: SP436, SP626, SP693 and/or
SP694,
c) selecting at least one member of the segregating population, wherein
said at least
one member is bearing one extreme dark green pepper fruit trait and comprises
the
molecular marker(s) of b).
[00063] As still another aspect, the invention provides methods of producing a
pepper
plant bearing extreme dark green color fruit at immature harvestable stage,
comprising
the step of transferring at least one genetic determinant directing or
controlling
expression of said extreme dark green color in the pepper fruit of the pepper
plant from a
donor pepper plant bearing extreme dark green color fruit at immature
harvestable stage
to a recipient pepper plant that does not bear extreme dark green fruit at
immature
harvestable stage, optionally wherein said transfer of said genetic
determinant is
performed by transformation, by crossing, by protoplast fusion, by a doubled
haploid
technique or by embryo rescue, and wherein said at least one genetic
determinant is
represented by at least one QTL or a functional part thereof that directs or
controls
expression of said dark green immature fruit color in the pepper fruit of the
pepper plant,
wherein the at least one QTL or a functional part thereof is genetically or
physically
linked to a marker locus that co-segregates with the extreme dark green color
and is
selected from one or more of: SP436, SP626, SP693 and/or SP694.
[00064] In embodiments, the method further comprises the steps of: detecting
the at least
one genetic determinant; and selecting a pepper plant that bears extreme dark
green color
fruit at immature harvestable stage and comprises said at least one genetic
determinant.
[00065] In embodiments, the transfer of said genetic detelininant comprises:
crossing said
donor pepper plant with said recipient pepper plant to produce progeny plants;
and
selecting from among the offspring a plant that comprises the at least one
genetic
determinant in its genome.
[00066] In further representative embodiments, there is provided:
18
81790136
[00067] 1. A cell of a seed of pepper cultivar 16452A, a representative
sample of seed
having been deposited under ATCC Accession No PTA-121980.
[00068] 2. A cell of a plant of pepper cultivar 16452A, a representative
sample of
seed having been deposited under ATCC Accession No PTA-121980.
[00069] 3. A cell of an inbred or doubled haploid plant produced from a
plant as
defined in paragraph 2 or a selfed progeny thereof.
[00070] 4. A cell of a plant part of a pepper plant as defined in paragraph
2.
[00071] 5. The cell of paragraph 4, wherein the cell is a fruit cell,
pollen cell, an ovule
cell, an anther cell, a root cell, or a leaf cell.
[00072] 6. A culture of regenerable cells of the plant as defined in
paragraph 2.
[00073] 7. A cell of a pepper plant regenerated from a tissue culture of
regenerable
cells of a plant as defined in paragraph 2 or a selfed progeny of said
regenerated pepper plant,
wherein said regenerated pepper plant comprises all of the physiological and
morphological
characteristics of a plant as defined in paragraph 2.
19
CA 2902250 2018-02-02
81790136
[00074] 8. A non-viable, processed product from a plant as defined in
paragraph 2,
wherein the non-viable, processed product comprises dehydrated, cut, sliced,
chopped,
diced, ground, pureed, dried, canned, jarred, washed, packaged, refrigerated,
frozen,
blanched and/or heated pepper fruits.
[00075] 9. A cell of a pepper seed, said pepper seed produced by crossing a
plant as
defined in paragraph 2 with itself or with pepper cultivar 8728C, a
representative sample
having been deposited under NCIMB Accession No. 41858, and harvesting the
resulting
seed, wherein the pepper seed produces a pepper plant that produces sweet
pepper fruits.
[00076] 10. A cell of a pepper plant, or of a part thereof, produced by
growing a seed
as defined in paragraph 9.
[00077] 11. A cell of an inbred or doubled haploid plant produced from a
pepper plant
as defined in paragraph 10.
CA 2902250 2018-02-02
81790136
[00078] 12. A cell of a seed produced by a method for producing a seed
of a pepper
plant derived from a plant as defined in paragraph 2, the method comprising:
(a) crossing a plant of pepper cultivar 16452A, a representative sample of
seed
having been deposited under ATCC Accession No PTA-121980, with pepper cultivar
8728C,
a representative sample having been deposited under NCIMB Accession No. 41858;
(b) allowing seed to form;
(c) growing a plant from the seed of step (b) to produce a plant derived from
pepper cultivar 16452A; and
(d) selfing the plant of step (c) or crossing it to a second pepper plant to
form
additional pepper seed derived from pepper cultivar 16452A.
[00079] 13. The cell as defined in paragraph 12, wherein the method
further
comprises (e) repeating steps (c) and (d) one or more times to generate
further derived pepper
seed from pepper cultivar 16452A, wherein in step (c) a plant is grown from
the additional
pepper seed of step (d) in place of growing a plant from the seed of step (b).
21
CA 2902250 2018-02-02
81790136
[00080] 14. A cell of a plant, or of a part thereof, produced by growing a
seed as
defined in paragraph 12 or paragraph 13.
[00081] 15. A cell of a plant or plantlet produced by a method of
vegetatively
propagating a plant as defined in paragraph 2, the method comprising:
(a) collecting tissue capable of being propagated from a plant of pepper
cultivar
16452A, a representative sample of seed having been deposited under ATCC
Accession
No PTA-121980;
(b) cultivating the tissue to obtain proliferated shoots; and
(c) rooting the proliferated shoots to obtain rooted plantlets.
[00082] 16. A cell of a pepper plant, or of a selfed progeny thereof,
said pepper
plant having a desired added trait and produced by a method of introducing the
desired added
trait into pepper cultivar 16452A, a representative sample of seed having been
deposited
under ATCC Accession No PTA-121980, the method comprising:
(a) crossing a plant of pepper cultivar 16452A with a pepper plant that
comprises a desired added trait to produce Fl progeny;
(b) selecting an Fl progeny that comprises the desired added trait;
(c) backcrossing the selected Fl progeny with pepper cultivar 16452A to
produce backcross progeny;
(d) selecting backcross progeny comprising the desired added trait; and
(e) repeating steps (c) and (d) three or more times to produce a plant derived
from pepper cultivar 16452A comprising all of the physiological and
morphological
characteristics of the pepper cultivar 16452A with the exception of an
addition of the desired
added trait, wherein in step (c) the selected backcross progeny produced in
step (d) is used in
place of the selected Fl progeny of step (b).
22
CA 2902250 2019-02-07
81790136
[00083] 17. A cell of a seed of a plant having a desired added trait as
defined in
paragraph 16, wherein the seed produces a plant that has the desired added
trait.
[00084] 18. A cell of a seed that produces a plant as defined in paragraph
17.
[00085] 19. A cell of a pepper plant, or of a selfed progeny thereof,
said pepper
plant having a desired added trait and produced by a method of producing a
plant of pepper
cultivar 16452A comprising the desired added trait, a representative sample of
seed of pepper
cultivar 16452A having been deposited under ATCC Accession No PTA-121980, the
method
comprising introducing a transgene conferring the desired trait into a plant
as defined in
paragraph 2, wherein the plant is a plant of pepper cultivar 16452A.
23
CA 2902250 2019-02-07
81790136
[00086] 20. A cell of a seed of a plant having a desired added trait as
defined in
paragraph 19, wherein the seed produces a plant that has the desired added
trait.
[00087] 21. A cell of a seed that produces a plant as defined in paragraph
20.
[00088] 22. A method of producing a pepper fruit, the method comprising:
(a) growing a pepper plant as defined in paragraph 2 to produce a pepper
fruit;
and
(b) harvesting the pepper fruit.
[00089] 23. A method of producing a processed product, the method
comprising
(a) obtaining a fruit of a plant as defined in paragraph 2; and
(b) processing said fruit to produce a processed product.
[00090] 24. Use of a pepper plant as defined in paragraph 2, 3, 9, 10, 11,
14, 15, 16, or
19, for producing pepper fruit.
[00091] 25. Use of a pepper plant as defined in paragraph 2, 3, 9, 10, 11,
14, 15, 16, or
19, for producing seed.
24
CA 2902250 2018-02-02
81790136
[00092] 26. Use of a pepper plant as defined in paragraph 2, 3, 9, 10, 11,
14, 15, 16, or
19, for crossing with another pepper plant.
[00093] 27. Use of a pepper plant as defined in paragraph 2, 3, 9, 10, 11,
14, 15, 16, or
19, as a recipient of a transgene conferring a desired added trait.
[00094] 28. Use of pepper eultivar 16452A, a representative sample of seed
having
been deposited under ATCC Accession No. PTA-121980, for developing a pepper
plant
in a pepper plant breeding program which comprises applying plant breeding
techniques
comprising recurrent selection, backcrossing, pedigree breeding, marker
enhanced
selection, haploid/double haploid production, or transformation to the pepper
plant or its
parts, wherein application of said techniques results in development of a
pepper plant.
[00095] - [00114]
[000115] In addition to the exemplary aspects and embodiments described
above, the
invention is described in more detail in the description of the invention set
forth below.
CA 2902250 2018-02-02
81790136
BRIEF DESCRIPTION OF THE DRAWINGS
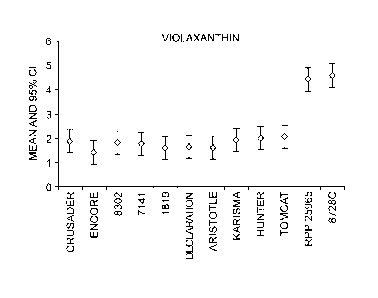
[000116] FIGURE 1 shows the content in violaxanthin in gig of fresh
(average value
with 95 % CI) weight for different entries that were grown in Gilroy,
California in
summer 2010.
[000117] FIGURE 2 shows the content in lutein in p,g/g of fresh (average
value with
95% CI) weight for different entries that were grown in Gilroy, California.
[000118] FIGURE 3 shows the content in chlorophyll A inlig/g of fresh
(average value
with 95 % CI) weight for different entries that were grown in Gilroy,
California.
[000119] FIGURE 4 shows the content in chlorophyll B in gig of fresh
(average value
with 95 % CI) weight for different entries that were grown in Gilroy,
California.
[000120] FIGURE 5 shows the content in beta-carotene in gig of fresh
(average value
with 95 % CI) weight for different entries that were grown in Gilroy,
California.
26
CA 2902250 2018-02-02
CA 02902250 2015-08-28
51682-49
[000121] FIGURE 6 shows the L* value measured by colorimeter for different
entries that
were grown in Gilroy, California.
[000122] FIGURE 7 shows the a* value for different entries that were grown in
Gilroy,
California.
[000123] FIGURE 8 shows the b* value for different entries that were grown in
Gilroy,
California.
[000124] FIGURE 9 shows the content in violaxanthin in gg/g of fresh weight
(average
value with 95 % CI) for non-overgreen (OVG) hybrids, heterozygous OVG hybrids,
homozygous OVG hybrids, OVG inbreds and non-OVG inbreds that were grown under
standard field conditions in Gilroy, California in Summer 2010 (fruit measured
in
August/September 2010). The different entries indicated in the figure are
shown in the
chart below.
Genotype Pepper Entry Variety
Non-OVG Hybrids 1 Crusader
2 Encore
3 8302
4 7141
1819
6 Declaration
7 Aristotle
8 Karisma
Heterozygous OVG 9 Hunter
Hybrids 10 Tomcat
Homozygous OVG 11 RPP 26098
Hybrids 12 RPP 26105
OVG Inbreds 13 87280, OVG male inbred
parent
14 16452A, OVG female
inbred parent of RPP
27
CA 02902250 2015-08-28
51682-49
25822
15 16452A, OVG female
inbred parent of RPP
26098
16 16452C, OVG female
inbred parent of RPP
26105
Non-OVG lnbreds 17 Non-OVG inbred, female
parent of Hunter
18 Non-OVG inbred, female
parent of Tomcat
19 Non-OVG inbred, male
parent of Encore
[000125] FIGURE 10 shows the content in lutein in pg/g of fresh weigh (average
value
with 95 % CI) weight for the different entries shown in Figure 9, which were
all grown
under standard field conditions in Gilroy, California.
[000126] FIGURE 11 shows the content in chlorophyll A in pg/g of fresh weight
(average
value with 95 % CI) for the different entries shown in Figure 9, which were
all grown
under standard field conditions in Gilroy, California.
[000127] FIGURE 12 shows the content in chlorophyll B in gig of fresh weight
(average
value with 95 % CI) for the different entries shown in Figure 9, which were
all grown
under standard field conditions in Gilroy, California.
[000128] FIGURE 13 shows the content in alpha-carotene in gig of fresh weight
(average
value with 95 % CI) for the different entries shown in Figure 9, which were
all grown
under standard field conditions in Gilroy, California.
[000129] FIGURE 14 shows the content in beta-carotene in 1.1g/g of fresh
(average value
with 95 % CI) weight for the different entries shown in Figure 9, which were
all grown
under standard field conditions in Gilroy, California.
[000130] FIGURE 15 shows the L* value measured by image analysis for the
different
entries shown in Figure 9, which were all gown under standard field conditions
in
Gilroy, California.
28
CA 02902250 2015-08-28
51682-49
[000131] FIGURE 16 shows the a* value for the different entries shown in
Figure 9, which
were all grown under standard field conditions in Gilroy, California.
[000132] FIGURE 17 shows the b* value for the different entries shown in
Figure 9, which
were all grown under standard field conditions in Gilroy, California.
[000133] FIGURE 18 shows the content in violaxanthin in j.ig/g of fresh weight
(average
value with 95 % CI) for non-overgreen (OVG) hybrids, heterozygous OVG hybrids,
homozygous OVG hybrids, OVG inbrcds and non-OVG inbreds that were grown under
standard passive greenhouse conditions in El Ejido, Spain in Winter 2010-2011
(fruits
measured in January-February 2011). The pepper entries are the same as in
Figure 9.
[000134] FIGURE 19 shows the content in lutein in g/g of fresh weigh (average
value
with 95 % CI) weight for the different entries shown in Figure 9, which were
grown
under standard passive greenhouse conditions in El Ejido, Spain.
[000135] FIGURE 20 shows the content in chlorophyll A in ptg/g of fresh weight
(average
value with 95 % CI) for the different entries shown in Figure 9, which were
grown under
standard passive greenhouse conditions in El Ejido, Spain.
[000136] FIGURE 21 shows the content in chlorophyll B in ug/g of fresh weight
(average
value with 95 A CI) for the different entries shown in Figure 9, which were
grown under
standard passive greenhouse conditions in El Ejido, Spain.
[000137] FIGURE 22 shows the content in alpha-carotene in ug/g of fresh weight
(average
value with 95 % CI) for the different entries shown in Figure 9, which were
grown under
standard passive greenhouse conditions in El Ejido, Spain.
[000138] FIGURE 23 shows the content in beta-carotene in ug/g of fresh
(average value
with 95 % CI) weight for the different entries shown in Figure 9, which were
grown
under standard passive greenhouse conditions in El Ejido, Spain.
29
CA 02902250 2015-08-28
51682-49
[000139] FIGURE 24 shows the L* value measured by image analysis using
computer
vision for the different entries shown in Figure 9, which were grown under
standard
passive greenhouse conditions in El Ejido, Spain.
[000140] FIGURE 25 shows the a* value for the different entries shown in
Figure 9, which
were grown under standard passive greenhouse conditions in El Ejido, Spain.
[000141] FIGURE 26 shows the b* value for the different entries shown in
Figure 9, which
were grown under standard passive greenhouse conditions in El Ejido, Spain.
[000142] FIGURE 27 shows the L* value measured by image analysis for the
indicated
entries at post-harvest day 1.
[000143] FIGURE 28 shows the L* value measured by image analysis for the
indicated
entries at post-harvest days 1, 5, 8, 12 and 15 (DPH1, DPH5, DPH8, DPH12, and
DPH15).
[000144] FIGURE 29 shows the a* value for the indicated entries at post-
harvest days 1, 5,
8, 12 and 15 (DPH1, DF'H5, DPH8, DPH12, and DPH15).
[000145] FIGURE 30 shows the b* value for the indicated entries at post-
harvest days 1, 5,
8, 12 and 15 (DPH1, DPH5, DPH8, DPH12, and DPH15).
DETAILED DESCRIPTION OF THE INVENTION
[000146] The present invention is based, in part, on the development of novel
pepper
varieties producing fruit having an extreme dark green color. The present
invention also
provides novel pepper cultivars designated RPP 25822, RPP 26098, RPP 26105,
8728C,
16452A, 16452B and 16452C.
[000147] It should be appreciated that the invention can be embodied in
different forms and
should not be construed as limited to the embodiments set forth herein.
Rather, these
CA 02902250 2015-08-28
51682-49
embodiments are provided so that this disclosure will be thorough and
complete, and will
frilly convey the scope of the invention to those skilled in the art.
[000148] Unless otherwise defined, all technical and scientific terms used
herein have the
same meaning as commonly understood by one of ordinary skill in the art to
which this
invention belongs. The teiniinology used in the description of the invention
herein is for
the purpose of describing particular embodiments only and is not intended to
be limiting
of the invention.
[000149] Unless the context indicates otherwise, it is specifically intended
that the various
features and embodiments of the invention described herein can be used in any
combination.
[000150] Moreover, the present invention also contemplates that in some
embodiments of
the invention, any feature or combination of features set forth herein can be
excluded or
omitted. To illustrate, if the specification states that a composition
comprises
components A, B and C, it is specifically intended that any of A, B or C, or a
combination thereof, can be omitted and disclaimed singularly or in any
combination.
[000151] Definitions
[000152] The technical terms and expressions used within the scope of this
application are
generally to be given the meaning commonly applied to them in the pertinent
art of
(molecular) plant breeding and cultivation if not otherwise indicated herein
below.
[000153] As used in this specification and the appended claims, the singular
forms "a",
"an", and "the" include plural referents unless the context clearly dictates
otherwise.
Thus, for example, reference to "a plant" includes one or more plants, and
reference to "a
cell" includes mixtures of cells, tissues, and the like.
31
CA 02902250 2015-08-28
51682-49
[000154] As used herein, "and/or" refers to and encompasses any and all
possible
combinations of one or more of the associated listed items, as well as the
lack of
combinations when interpreted in the alternative ("or").
[000155] The term "about," as used herein when referring to a measurable value
such as a
dosage or time period and the like, is meant to encompass variations of 20%,
10%, 5%,
1%, 0.5%, or even 0.1% of the specified amount.
[000156] The term "comprise," "comprises" and "comprising" as used herein,
specify the
presence of the stated features, integers, steps, operations, elements, and/or
components,
but do not preclude the presence or addition of one or more other features,
integers, steps,
operations, elements, components, and/or groups thereof
[000157] As used herein, the transitional phrase "consisting essentially of'
means that the
scope of a claim is to be interpreted to encompass the specified materials or
steps recited
in the claim "and those that do not materially affect the basic and novel
characteristic(s)"
of the claimed invention. See, In re Herz, 537 F.2d 549, 551-52, 190 U.S.P.Q.
461, 463
(CCPA 1976) (emphasis in the original). Thus, the term "consisting essentially
of' when
used in a claim or the description of this invention is not intended to be
interpreted to be
equivalent to "comprising."
[000158] An "allele" is understood within the scope of the invention to refer
to alternative
or variant forms of various genetic units identical or associated with
different forms of a
gene or of any kind of identifiable genetic element, which are alternative in
inheritance
because they are situated at the same locus in homologous chromosomes. Such
alternative or variant forms may be the result of single nucleotide
polymorphisms,
insertions, inversions, translocations or deletions, or the consequence of
gene regulation
caused by, for example, by chemical or structural modification, transcription
regulation
or post-translational modification/regulation. In a diploid cell or organism,
the two alleles
of a given gene or genetic element typically occupy corresponding loci on a
pair of
homologous chromosomes.
32
CA 02902250 2015-08-28
51682-49
[000159] "Backcrossing". Backcrossing is a process in which a breeder
repeatedly crosses
hybrid progeny back to one of the parents, for example, a first generation
hybrid Fl with
one of the parental genotype of the Fl hybrid.
[000160] As used herein, the teiiii "breeding", and grammatical variants
thereof, refer to
any process that generates a progeny individual. Breeding can be sexual or
asexual, or
any combination thereof. Exemplary non-limiting types of breeding processes
include
crossings, selfing, doubled haploid derivative generation, and combinations
thereof.
[000161] In the context of the present invention, the expression "co-
segregation" refers to
the fact that the allele for the trait and the allele(s) for the marker(s)
tend to be transmitted
together because they are physically close together on the same chromosome
(reduced
recombination between them because of their physical proximity) resulting in a
non-
random association of their alleles as a result of their proximity on the same
chromosome. "Co-segregation" also refers to the presence of two or more traits
within a
single plant of which at least one is known to be genetic and which cannot be
readily
explained by chance.
[000162] "Cotyledon". One of the first leaves of the embryo of a seed plant;
typically one
or more in monocotyledons, two in dicotyledons, and two or more in
gymnosperms.
[000163] A "cultivated pepper" plant is understood within the scope of the
invention to
refer to a plant that is no longer in the natural state but has been developed
by human care
and for human use and/or (commercial) growing purposes and/or consumption.
"Cultivated pepper plants" are further understood to exclude those wild-type
species
which comprise the trait being subject of this invention as a natural trait
and/or part of
their natural genetics. Cultivated pepper plants also typically display
favourable
agronomical and fruit quality characteristics as well as resistance(s) to
Xanthomonas
campestris pv. Vesicatoria, and/or potyvirus, and/or CMV, and/or TMV, and/or
TSWV
virus, whereas non-cultivated plants do not.
33
CA 02902250 2015-08-28
51682-49
[000164] "Detenninate Plant". A determinate plant will grow to a fixed number
of nodes
while an indeterminate plant will continue to grow during the season.
[000165] As used herein, the phrase "diploid individual" refers to an
individual that has two
sets of chromosomes, typically one from each of its two parents. However, it
is
understood that in some embodiments a diploid individual can receive its
"maternal" and
"paternal" sets of chromosomes from the same single organism, such as when a
plant is
selfed to produce a subsequent generation of plants.
[000166] "Doubled haploid line". A stable inbred line achieved by doubling the
chromosomes of a haploid line, e.g., from anther culture. For example, some
pollen
grains (haploid) cultivated under specific conditions develop plantlets
containing in
chromosomes. The chromosomes in these plantlets are then induced to "double"
(e.g.,
using chemical means) resulting in cells containing 2n chromosomes. The
progeny of
these plantlets are termed "doubled haploid" and are essentially non-
segregating (e.g., are
stable). The term "doubled haploid" is used interchangeably herein with
"dihaploid."
[000167] "Essentially all the physiological and morphological
characteristics". A plant
having "essentially all the physiological and morphological characteristics"
means a plant
having the physiological and morphological characteristics of the recurrent
parent, except
for the characteristics derived from the converted or transferred gene(s).
[000168] In the context of the present invention, the expression "established
breeding
population" or "population" refers to a collection of potential breeding
partners produced
by and/or used as parents in a breeding program; e.g., a commercial breeding
program.
The members of the established breeding population are typically well-
characterized
genetically and/or phenotypically. For example, several phenotypic traits of
interest
might have been evaluated, e.g., under different environmental conditions, at
multiple
locations, and/or at different times. Alternatively or in addition, one or
more genetic loci
associated with expression of the phenotypic traits might have been identified
and one or
more of the members of the breeding population might have been genotyped with
respect
34
CA 02902250 2015-08-28
51682-49
to the one or more genetic loci as well as with respect to one or more genetic
markers that
are associated with the one or more genetic loci.
[000169] As used herein, the expression "extreme dark green" or "extreme dark
green
color" or "extreme dark green color trait" (and similar expressions including
grammatical
variants) characterizing the pepper fruits produced by the pepper plant of any
of the
embodiments and optionally associated with any of the following terms "trait",
"locus",
"allele" or "QTL" and the like means that the said fruits are characterized by
a dark green
color and/or an enhanced nutrient content as described herein. In some
instances, the
extreme dark green trait/locus is referred to as the "overgreen" (OVG)
trait/locus.
[000170] In representative embodiments, said fruits having an extreme dark
green color are
characterized by any one or more (in any combination of 2 or all 3) of the
following non-
limiting characteristics at immature (e.g., green) harvestable stage:
a. green outer surface having an L* value (indicating darkness) that is at
least about
2, at least about 3, at least about 4, at least about 5, or more units lower
than an L* value
for a fruit from a suitable control variety,
b. the flesh of the green pepper fruit is characterized by having an L*
value
(indicating darkness) that is at least about 2, at least about 3, at least
about 4, at least
about 5, or more units lower than an L* value of the flesh from a fruit from a
suitable
control variety, and/or
c. the calyx of the green pepper fruit is characterized by having an L*
value
(indicating darkness) that is least about 2, at least about 3, at least about
4, at least about 5
or more units lower than an L* value of a calyx from a fruit from a suitable
control
variety.
[000171] L* values can be determined by any method known in the art (see,
e.g.,
Examples), for (for example, as determined by colorimetry, by image analysis
using
computer vision, etc.).
CA 02902250 2015-08-28
51682-49
10001721 In representative embodiments, the fruits having an extreme dark
green color are
characterized by any one or more (in any combination of 2 or all 3) of the
following non-
limiting characteristics at immature (e.g., green) harvestablc stage:
a. green outer surface having an L* value that is less than about 28, less
than about
27, less than about 26, less than about 25, or less than about 24, e.g., as
determined by
image analysis using computer vision,
b. the flesh of the green pepper fruit is characterized by having an L*
value
(indicating darkness) that is less than about 28, less than about 27, less
than about 26, less
than about 25, or less than about 24, e.g., as determined by image analysis
using
computer vision, and/or
c. the calyx of the green pepper fruit is characterized by having an L*
value
(indicating darkness) that is less than about 28, less than about 27, less
than about 26, less
than about 25, or less than about 24, e.g., as determined by image analysis
using
computer vision.
10001731 In embodiments, using the Munsell Color-Order system and notation,
the outer
surface, flesh and/or calyx of the fruits having an extreme dark green color
are
characterized by having a lower Munsell Value (where 0 indicates pure back and
10
indicates pure white) as compared with a fruit from a suitable control
variety, e.g., the
outer surface, flesh and/or calyx has a Value of about 3 or less, 2.5 or less,
or 2 or less.
[000174] Furthermore, in embodiments, using the Munsell Color-Order system and
notation, the outer surface, flesh and/or calyx of the fruits having an
extreme dark green
color are characterized by having a higher Munsell Hue as compared with a
fruit from a
suitable control variety, e.g., the outer surface, flesh and/or calyx of the
fruits having
extreme dark green color have a Hue of about 5GY (Green-Yellow) or greater,
7.5GY or
greater, or about lOGY or greater.
36
CA 02902250 2015-08-28
51682-49
[000175] In representative embodiments, said fruits having an extreme dark
green color are
characterized by any one or more (in any combination of 2, 3 or all 4) of the
following
non-limiting characteristics at immature (e.g., green) harvestable stage:
a. an increase in the content of violaxanthin of at least about 25%, at
least about
50%, at least about 75%, at least about 100% (doubled), or more as compared
with a
suitable control plant,
b. an increase in the content of lutein of at least about 25%, at least
about 50%, at
least about 75%, at least about 100% (doubled), or more as compared with a
suitable
control plant,
c. an increase in the content of chlorophyll a of at least about 25%, at
least about
50%, at least about 75%, or at least about 100% (doubled), or more as compared
with a
suitable control plant, and/or
d. an increase in the content of chlorophyll b of at least about 25%, at
least about
50%, at least about 75%, at least about 100% (doubled), or more as compared
with a
suitable control plant.
[000176] In representative embodiments, said fruits having an extreme dark
green color are
characterized by any one or more (in any combination of 2, 3 or all 4) of the
following
non-limiting characteristics at immature (e.g., green) harvestable stage:
a. a content of chlorophyll a greater than about 24, particularly greater
than about
25, more particularly greater than about 27, more particularly greater than
about 301,tg/g
of fresh weight,
b. a content of chlorophyll b greater than about 7, particularly greater
than about 8
more particularly greater than about 9, more particularly greater than about
10 gig of
fresh weight,
c. a content of lutein greater than about 5, particularly greater than
about 6,
particularly greater than about 7 pg/g of fresh weight, and/or
d. a content of violaxanthin greater than about 2.2, particularly greater
than about
2.5, more particularly greater than about 3, more particularly greater than
about 3.5 ps/g
of fresh weight.
37
CA 02902250 2015-08-28
51682-49
[000177] In embodiments of the invention, the outer surface of the pepper
fruit, the flesh of
the fruit and/or or the calyx of the pepper fruit has a substantially uniform
extreme dark
green color, e.g., at least about 25%, 35%, 50%, 60%, 70%, 75%, 80%, 85%, 90%,
95%
or more of the outer surface of the fruit, the flesh of the fruit and/or or
the calyx has an
extreme dark green color (e.g., as indicated by L* values).
[000178] In representative embodiments, the extreme dark green pepper fruits
of the
invention are characterized by a slower maturing time, e.g., they retain a
substantially
uniform green surface and/or flesh color for a longer period of time (e.g., at
least about 1,
2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, or 14 days longer or more) as compared
with a fruit
from a suitable control plant with the pepper fruits of the invention. For
example, in
embodiments, the extreme dark green pepper fruits of the invention show no or
essentially no change of the immature extreme dark green color to the mature
color (e.g.,
red), for example, no visible color change or less than about 20%, less than
about a 15%,
less than about a 10% or less than about a 5% loss of the extreme dark green
color of the
fruit surface and/or fruit flesh as a result of fruit ripening. In
embodiments, the ripening
of the extreme dark green pepper fruits of the invention to the mature color
(e.g., red) is
delayed by at least about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, or 14
days longer or more
as compared with a fruit from a suitable control plant.
[000179] Thus, in embodiments, the pepper fruits of the invention
advantageously have a
longer holding time as compared with a fruit from a suitable control pepper
plant, e.g., in
the field, for sale as fresh produce and/or for processing.
[000180] In embodiments, at least about 25%, 35%, 50%, 60%, 70%, 75%, 80%,
85%, 90%
or 95% or more of the fruits produced by the pepper plant, on average, have an
extreme
dark green color. In embodiments, all or essentially all of the fruits
produced by the
pepper plant have an extreme dark green color.
38
CA 02902250 2015-08-28
51682-49
[000181] The extreme dark green pepper fruits of the invention can comprise
any
combination of the foregoing characteristics or any other characteristics
described herein.
[000182] Those skilled in the art will appreciate that the values described
herein generally
represent averages determined from a plurality of pepper fruits, although
individual fruits
may have characteristics that fall outside of these values. In embodiments,
the
characteristics of the pepper fruits having extremely dark green color
described above are
statistically significant as compared with a fruit from a suitable control
pepper plant (e.g.,
p value < 0.1, 0.05 or 0.001).
[000183] A "control" pepper plant or fruit as used herein does not have the
extreme dark
green color trait, e.g., does not comprise QTL1 and/or QTL2 as described
herein and is
generally gown/produced under substantially the same conditions as the extreme
dark
green pepper plants and fruits of the invention. Exemplary control pepper
plants (and
fruits therefrom) include without limitation Crusader, Encore, Declaration,
Aristotle,
Karisma, 8302, 7141 and 1819.
[000184] In representative embodiments, a pepper plant having the extreme dark
green
color trait comprises QTL1 and/or QTL2 or a functional part thereof
[000185] In representative embodiments, QTL1 is genetically or physically
linked to
marker loci SP436 and/or SP626.
[0001861 In embodiments, marker locus SP436 can be identified in an
amplification
reaction (e.g., PCR amplification) of a DNA fragment with the pair of
oligonucleotide
primers including forward primer of SEQ ID NO: 1 and/or reverse primer of SEQ
ID
NO: 2, optionally with the probe of SEQ ID NO 9.
[000187] In embodiments, marker locus SP626 can be identified in an
amplification
reaction (e.g., PCR amplification) of a DNA fragment with the pair of
oligonucleotide
primers including: forward primer of SEQ ID NO: 3 and/or reverse primer of SEQ
ID
NO: 4, optionally with the probe of SEQ ID NO 10.
39
CA 02902250 2015-08-28
51682-49
[000188] In representative embodiments QTL2 is genetically or physically
linked to marker
loci SP693 and/or SP694.
[000189] In embodiments, marker locus SP693 can be identified in an
amplification
reaction (e.g., PCR amplification) of a DNA fragment with the pair of
oligonucleotide
primers including: forward primer of SEQ ID NO: 5 and/or reverse primer of SEQ
ID
NO: 6, optionally with the probe of SEQ ID NO 11.
[000190] In embodiments, marker locus SP694 can be identified in an
amplification
reaction (e.g., PCR amplification) of a DNA fragment with the pair of
oligonucleotide
primers including: forward primer of SEQ ID NO: 7 and/or reverse primer of SEQ
ID
NO: 8, optionally with the probe of SEQ ID NO 12.
[000191] In embodiments, the QTL1 and/or QTL2 is not in the native (e.g.
natural) genetic
background of the pepper plant having the extreme dark green color trait.
[000192] In representative embodiments, the extreme dark green color trait is
derived from
Capsicum annuum inbred line 8728C, inbred line 16452A, inbred line 16452B,
inbred
line 16452C, hybrid RPP 25822, hybrid RPP 26098 and/or hybrid RPP 26105, or in
a
progeny or in an ancestor thereof
[000193] In embodiments, the pepper plants of the invention are homozygous for
the
extreme dark green color trait.
[000194] "Fl hybrid". A first generation progeny from the cross of two non-
isogenic
parent plants.
[000195] "First water date". The date the seed first receives adequate
moisture to
germinate. This can and often does equal the planting date.
[000196] "Gene". As used herein, "gene" refers to a segment of nucleic acid
comprising an
open reading frame. A gene can be introduced into a genome of a species,
whether from
CA 02902250 2015-08-28
51682-49
a different species or from the same species, using transformation or various
breeding
methods.
[000197] "Genetic complement". As used herein, a "genetic complement" refers
to the
total genetic make-up of the plant.
[000198] A "genetic determinant directing or controlling expression" is
understood herein
to refer to a heritable genetic element that is capable of contributing to the
darkness of the
fruit color of the plant by influencing expression of this color trait on the
level of the
DNA itself, on the level of translation, transcription and/or activation of a
final
polypeptide product.
[0001991 "Genetic linkage" or "linkage" or "association" is understood within
the scope of
the invention to refer to an association of characters in inheritance due to
location of
genes in proximity on the same chromosome, measured by percent recombination
between loci (centi-Morgan, cM).
[000200] As used herein, the phrase "genetic marker" refers to a feature of an
individual's
genome (e.g., a nucleotide or a polynucleotide sequence that is present in an
individual's
genome) that is associated with one or more loci of interest. In some
embodiments, a
genetic marker is polymorphic in a population of interest, or the locus
occupied by the
polymorphism, depending on context. Genetic markers include, for example,
single
nucleotide polymorphisms (SNPs), indels (i.e., insertions/deletions), simple
sequence
repeats (SSRs), restriction fragment length polymorphisms (RFLPs), random
amplified
polymorphic DNAs (RAPDs), cleaved amplified polymorphic sequence (CAPS)
markers,
Diversity Arrays Technology (DArT) markers, and amplified fragment length
polymorphisms (AFLPs), among many other examples. Genetic markers can, for
example, be used to locate genetic loci containing alleles on a chromosome
that
contribute to variability of phenotypic traits. The phrase "genetic marker"
can also refer
to a polynucleotide sequence complementary to a genomic sequence, such as a
sequence
of a nucleic acid used as probes. A genetic marker can be physically located
in a position
on a chromosome that is within or outside of to the genetic locus with which
it is
41
CA 02902250 2015-08-28
51682-49
associated (i.e., is intragenic or extragenic, respectively). Stated another
way, whereas
genetic markers are typically employed when the location on a chromosome of
the gene
or of a functional mutation, e.g. within a control element outside of a gene,
that
corresponds to the locus of interest has not been identified and there is a
non-zero rate of
recombination between the genetic marker and the locus of interest, the
presently
disclosed subject matter can also employ genetic markers that are physically
within the
boundaries of a genetic locus (e.g., inside a genomic sequence that
corresponds to a gene
such as, but not limited to a polymorphism within an intron or an exon of a
gene).
10002011 As used herein, the term "genotype" refers to the genetic
constitution of a cell or
organism. An individual's ''genotype for a set of genetic markers" includes
the specific
alleles, for one or more genetic marker loci, present in the individual's
haplotype. As is
known in the art, a genotype can relate to a single locus or to multiple loci,
whether the
loci are related or unrelated and/or are linked or unlinked. In some
embodiments, an
individual's genotype relates to one or more genes that are related in that
the one or more
of the genes are involved in the expression of a phenotype of interest (e.g.,
a quantitative
trait as defined herein). Thus, in some embodiments a genotype comprises a
summary of
one or more alleles present within an individual at one or more genetic loci
of a
quantitative trait. In some embodiments, a genotype is expressed in terms of a
haplotype
(defined herein below).
10002021 As used herein, the tem' "germplasm" refers to the totality of the
genotypes of a
population or other group of individuals (e.g., a species). The tei ___ fll
"germplasm" can also
refer to plant material; e.g., a group of plants that act as a repository for
various alleles.
The phrase "adapted germplasm" refers to plant materials of proven genetic
superiority;
e.g., for a given environment or geographical area, while the phrases "non-
adapted
germplasm," "raw germplasm," and "exotic germplasm" refer to plant materials
of
unknown or unproven genetic value; e.g., for a given environment or
geographical area;
as such, the phrase "non-adapted germplasm" refers in some embodiments to
plant
materials that are not part of an established breeding population and that do
not have a
known relationship to a member of the established breeding population.
42
CA 02902250 2015-08-28
51682-49
[000203] "Heat unit". The amount of heat needed to mature a crop. It is used
to measure
maturity based on the daily accumulated heat produced during the growing
season. The
formula [(daily maximum F 0 ¨ daily minimum FO )-402 is used to calculate heat
units
for peppers.
[000204] "Heterozygous" is understood within the scope of the invention to
refer to unlike
alleles at one or more corresponding loci on homologous chromosomes.
[000205] "Homozygous" is understood within the scope of the invention to refer
to like
alleles at one or more corresponding loci on homologous chromosomes.
[000206] As used herein, the terms "hybrid", "hybrid plant," and "hybrid
progeny" refers to
an individual produced from genetically different parents (e.g., a genetically
heterozygous or mostly heterozygous individual).
[000207] The term "hybridize" as used herein with respect to nucleic acids
refers to
conventional hybridization conditions, preferably to hybridization conditions
at which
5xSSPE, 1% SDS, lxDenhardts solution is used as a solution and/or
hybridization
temperatures are between 35 C and 70 C, preferably 65 C. After hybridization,
washing
is preferably carried out first with 2xSSC, 1% SDS and subsequently with
0.2xSSC at
temperatures between 35 C and 75 C, particularly between 45 C and 65 C, but
especially at 59 C (regarding the definition of SSPE, SSC and Denhardt's
solution; see
Green and Sambrook, Molecular Cloning: A Laboratory Manual, Cold Spring Harbor
Press, 2012). High stringency hybridization conditions as for instance
described in
Sambrook et al, supra, are particularly preferred. Particularly preferred
stringent
hybridization conditions are for instance present if hybridization and washing
occur at
65 C as indicated above. Non-stringent hybridization conditions for instance
with
hybridization and washing carried out at 45 C are less preferred and at 35 C
even less.
[000208] One indication that two nucleic acid sequences are substantially
identical is that
the two molecules hybridize to each other under stringent conditions. The
phrase:
43
CA 02902250 2015-08-28
51682-49
"hybridizing specifically to" refers to the binding, duplexing, or hybridizing
of a
molecule only to a particular nucleotide sequence under stringent conditions
when that
sequence is present in a complex mixture (e.g., total cellular) DNA or RNA.
"Bind(s)
substantially" refers to complementary hybridization between a probe nucleic
acid and a
target nucleic acid and embraces minor mismatches that can be accommodated by
reducing the stringency of the hybridization media to achieve the desired
detection of the
target nucleic acid sequence.
[000209] The expression "immature harvcstable stage" is understood herein to
refer to a
stage in the pepper fruit development where the fruit, having reached
essentially full
physiological development (e.g., cell division and expansion being essentially
complete,
fruit size and pericarp thickness having reached essentially maximum values),
has not yet
gone through the ripening process, e.g., are still an immature green color.
[000210] As used herein, the phrase "inbred line" refers to a genetically
homozygous or
nearly homozygous population. An inbred line, for example, can be derived
through
several cycles of brother/sister breedings or of selfing or in dihaploid
production. In some
embodiments, inbred lines breed true for one or more phenotypic traits of
interest. An
"inbred", "inbred individual", or "inbred progeny" is an individual sampled
from an
inbred line.
[000211] "Internode". The stem segment between nodes.
10002121 As used herein, the term "linkage", and grammatical variants thereof,
refers to the
tendency of alleles at different loci on the same chromosome to segregate
together more
often than would be expected by chance if their transmission were independent,
in some
embodiments as a consequence of their physical proximity.
[000213] "Locus" is understood within the scope of the invention to refer to a
region on a
chromosome, which comprises a gene or any other genetic element or factor
contributing
to a trait.
44
CA 02902250 2015-08-28
=
51682-49
[000214] "Marker-based selection" is understood within the scope of the
invention to refer
to e.g. the use of genetic markers to detect one or more nucleic acids from
the plant,
where the nucleic acid is associated with a desired trait to identify plants
that carry genes
for desirable (or undesirable) traits, so that those plants can be used (or
avoided) in a
selective breeding program.
[000215] As used herein, "marker locus" refers to a region on a chromosome,
which
comprises a nucleotide or a polynucleotide sequence that is present in an
individual's
genome and that is associated with one or more loci of interest, which may
which
comprise a gene or any other genetic element or factor contributing to a
trait. "Marker
locus" also refers to a region on a chromosome, which comprises a
polynucleotide
sequence complementary to a genomic sequence, such as a sequence of a nucleic
acid
used as probes.
[000216] "Microsatellite or SSRs (Simple sequence repeats) Marker" is
understood within
the scope of the invention to refer to a type of genetic marker that consists
of numerous
repeats of short sequences of DNA bases, which are found at loci throughout
the plant's
genome and have a likelihood of being highly polymorphic.
[000217] "Node". A node is the thickened enlargement on a plant. It is where
the stipules,
leaf and peduncle arise.
[000218] "Nodes to 1st flower". The number of nodes to 1st flower is obtained
by counting
the number of nodes from above the point of cotyledon attachment to the node
from
which the first peduncle arises.
[000219] As used herein, the phrase "nucleic acid" refers to any physical
string of monomer
units that can be corresponded to a string of nucleotides, including a polymer
of
nucleotides (e.g., a typical DNA, cDNA or RNA polymer), modified
oligonucleotides
(e.g., oligonucleotides comprising bases that are not typical to biological
RNA or DNA,
such as 2'-0-methylated oligonucleotides), and the like. In some embodiments,
a nucleic
acid can be single-stranded, double-stranded, multi-stranded, or combinations
thereof.
CA 02902250 2015-08-28
51682-49
Unless otherwise indicated, a particular nucleic acid sequence of the
presently disclosed
subject matter optionally comprises or encodes complementary sequences, in
addition to
any sequence explicitly indicated.
[000220] "PCR (Polymerase chain reaction)" is understood within the scope of
the
invention to refer to a method of producing relatively large amounts of
specific regions of
DNA or subset(s) of the genome, thereby making possible various analyses that
are based
on those regions.
[000221] "PCR primer" is understood within the scope of the invention to refer
to relatively
short fragments of single-stranded DNA used in the PCR amplification of
specific
regions of DNA.
[000222] "Pepper". As used herein, the term "pepper" or "pepper plant"
includes any plant
classified as a Capsicum annuum, including C. annuum, C. baccatum, C.
chinense, C.
frutescens and C. pubescens. Pepper plants include varieties, cultivars and
populations
Capsicum. In embodiments, the pepper is a C. annuum. Further, the pepper
plants of the
invention can produce pungent (hot) or sweet (mild) fruits. In embodiments,
the pepper
plant is a sweet pepper plant (e.g., a sweet blocky pepper plant), which
typically produce
immature green fruits that turn red, yellow, red, purple or brown at maturity.
In
embodiments, the pepper plant is not an ancho pepper plant. The fruits can
have any
shape including, e.g., blocky or conical. In embodiments, the fruits are
blocky.
Generally, plants according to the present invention are domesticated (e.g.,
cultivated)
and produce commercially acceptable fruits (e.g., with respect to size, shape,
color, yield,
and the like).
[000223] "Phenotype" is understood within the scope of the invention to refer
to a
distinguishable characteristic(s) of a genetically controlled trait.
[000224] As used herein, the phrase ''phenotypic trait" refers to the
appearance or other
detectable characteristic of an individual, resulting from the interaction of
its genome,
proteome and/or metabolome with the environment.
46
CA 02902250 2015-08-28
51682-49
10002251 "Plant." As used herein, the term "plant" includes plant cells, plant
protoplasts,
plant cell tissue cultures from which plants can be regenerated, plant calli,
plant clumps,
and plant cells that are intact in plants or parts of plants, such as leaves,
pollen, embryos,
cotyledons, hypocotyl, roots, root tips, anthers, pistils, flowers, ovules,
seeds, stems,
fruits, and the like.
[000226] "Plant adaptability". A plant having a good plant adaptability means
a plant that
will perform well in different growing conditions and seasons.
[000227] A ''plant cell" is a structural and physiological unit of a plant,
comprising a
protoplast and a cell wall. The plant cell may be in form of an isolated
single cell or a
cultured cell, or as a part of higher organized unit such as, for example,
plant tissue, a
plant organ, or a whole plant.
[000228] "Plant cell culture" means cultures of plant units such as, for
example, protoplasts,
cell culture cells, cells in plant tissues, pollen, pollen tubes, ovules,
embryo sacs, zygotes
and embryos at various stages of development.
[000229] "Plant Height". Plant height is taken from the top of soil to top
most leaf of the
plant.
[000230] "Plant material". The terms "plant material" and "material obtainable
from a
plant" are used interchangeably herein and refer to any plant material
obtainable from a
plant including without limitation, leaves, stems, roots, flowers or flower
parts, fruits,
pollen, ovules, zygotes, seeds, cuttings, cell or tissue cultures, or any
other part or product
of the plant.
[000231] A "plant organ" is a distinct and visibly structured and
differentiated part of a
plant such as a root, stem, leaf, flower bud, or embryo.
[000232] "Plant part". As used herein, a "plant part" includes any part,
organ, tissue or cell
of a plant including without limitation an embryo, meristem, leaf, pollen,
cotyledon,
47
CA 02902250 2015-08-28
51682-49
hypocotyl, root, root tip, anther, flower, flower bud, pistil, ovule, seed,
shoot, stem, stalk,
petiole, pith, capsule, a scion, a rootstock and/or a fruit including callus
and protoplasts
derived from any of the foregoing.
[000233] "Plant tissue" as used herein means a group of plant cells organized
into a
structural and functional unit. Any tissue of a plant in planta or in culture
is included.
This term includes, but is not limited to, whole plants, plant organs, plant
seeds, tissue
culture and any groups of plant cells organized into structural and/or
functional units. The
use of this term in conjunction with, or in the absence of, any specific type
of plant tissue
as listed above or otherwise embraced by this definition is not intended to be
exclusive of
any other type of plant tissue.
[000234] As used herein, the term "plurality" refers to more than one. Thus, a
"plurality of
individuals" refers to at least two individuals. In some embodiments, the term
plurality
refers to more than half of the whole. For example, in some embodiments a
"plurality of a
population" refers to more than half the members of that population.
[000235] "Polymorphism" is understood within the scope of the invention to
refer to the
presence in a population of two or more different fauns of a gene, genetic
marker, or
inherited trait or a gene product obtainable, for example, through alternative
splicing,
DNA methylation, etc.
[000236] As used herein, the tetin "population" means a genetically
heterogeneous
collection of plants sharing a common genetic derivation.
[000237] "Probe" as used herein refers to a group of atoms or molecules which
is capable
of recognising and binding to a specific target molecule or cellular structure
and thus
allowing detection of the target molecule or structure. Particularly, "probe"
refers to a
DNA or RNA sequence which is labelled and which can be used to detect the
presence of
and to quantitate a complementary sequence by molecular hybridization.
48
CA 02902250 2015-08-28
51682-49
[000238] As used herein, the term "progeny" refers to the descendant(s) of a
particular
cross. Typically, progeny result from breeding of two individuals, although
some species
(particularly some plants and hermaphroditic animals) can be selfed (i.e., the
same plant
acts as the donor of both male and female gametes). The descendant(s) can be,
for
example, of the Fl, the F2, or any subsequent generation.
[000239] As used herein, the phrase "qualitative trait" refers to a phenotypic
trait that is
controlled by one or a few genes that exhibit major phenotypic effects.
Because of this,
qualitative traits are typically simply inherited. Examples in plants include,
but are not
limited to, flower color, and several known disease resistances such as, for
example,
Bacterial spot resistance or Tomato Mosaic Virus resistance.
[000240] As used herein, the phrase "quantitative trait" refers to a
phenotypic trait that can
be described numerically (i.e., quantitated or quantified). A quantitative
trait typically
exhibits continuous variation between individuals of a population; that is,
differences in
the numerical value of the phenotypic trait are slight and grade into each
other.
Frequently, the frequency distribution in a population of a quantitative
phenotypic trait
exhibits a bell-shaped curve (i.e., exhibits a normal distribution between two
extremes).
[000241] "Quantitative Trait Loci". Quantitative Trait Loci (QTL) refers to
genetic loci
that control to some degree, numerically representable traits that are usually
continuously
distributed. As used herein, the terms "quantitative trait locus" (QTL) and
"marker trait
association" refer to an association between a genetic marker and a
chromosomal region
and/or gene that affects the phenotype of a trait of interest. Typically, this
is determined
statistically; e.g., based on one or more methods published in the literature.
A QTL can
be a chromosomal region and/or a genetic locus with at least two alleles that
differentially
affect a phenotypic trait (either a quantitative trait or a qualitative
trait).
10002421 "Regeneration". Regeneration refers to the development of a plant
from tissue
culture.
49
CA 02902250 2015-08-28
51682-49
[000243] "Resistance". As used herein the terms "resistance" and "tolerance"
(and
grammatical variations thereof) are used interchangeably to describe plants
that show
reduced or essentially no symptoms to a specific biotic (e.g., a pest,
pathogen or disease)
or abiotic (e.g., exogenous or environmental, including herbicides) factor or
stressor. In
some embodiments, "resistant" or "tolerant" plants show some symptoms but are
still
able to produce marketable product with an acceptable yield, e.g., the yield
may still be
reduced and/or the plants may be stunted as compared with the yield or growth
in the
absence of the biotic and/or abiotic factor or stressor. Those skilled in the
art will
appreciate that the degree of resistance or tolerance may be assessed with
respect to a
plurality or even an entire field of plants. A pepper plant may be considered
"resistant" or
"tolerant" if resistance/tolerance is observed over a plurality of plants
(e.g., an average),
even if particular individual plants may be susceptible to the biotic or
abiotic factor or
stressor.
[000244] The "ripening process" is associated with chloroplasts changing to
chromoplasts,
with chlorophyll degradation, carotenoid biosynthesis, seed maturation, and
changes in
the carbohydrate content of the pericarp.
[000245] "Selective breeding" is understood within the scope of the invention
to refer to a
program of breeding that uses plants that possess or display desirable traits
as parents.
[000246] "Sequence Homology or Sequence Identity" is used herein
interchangeably. The
terms "identical" or percent "identity" in the context of two or more nucleic
acid or
protein sequences, refer to two or more sequences or subsequences that are the
same or
have a specified percentage of amino acid residues or nucleotides that are the
same, when
compared and aligned for maximum correspondence, as measured using one of the
following sequence comparison algorithms or by visual inspection. If two
sequences
which are to be compared with each other differ in length, sequence identity
preferably
relates to the percentage of the nucleotide residues of the shorter sequence
which are
identical with the nucleotide residues of the longer sequence. As used herein,
the percent
identity/homology between two sequences is a function of the number of
identical
CA 02902250 2015-08-28
51682-49
positions shared by the sequences (i.e., % identity = # of identical
positions/ total # of
positions x 100), taking into account the number of gaps, and the length of
each gap,
which need to be introduced for optimal alignment of the two sequences. The
comparison
of sequences and determination of percent identity between two sequences can
be
accomplished using a mathematical algorithm, as described herein below. For
example,
sequence identity can be determined conventionally with the use of computer
programs
such as the Bestfit program (Wisconsin Sequence Analysis Package, Version 8
for Unix,
Genetics Computer Group, University Research Park, 575 Science Drive Madison,
WI
53711). Bestfit utilizes the local homology algorithm of Smith and Waterman,
Advances
in Applied Mathematics 2 (1981), 482-489, in order to find the segment having
the
highest sequence identity between two sequences. When using Bestfit or another
sequence alignment program to determine whether a particular sequence has for
instance
95% identity with a reference sequence of the present invention, the
parameters are
preferably so adjusted that the percentage of identity is calculated over the
entire length
of the reference sequence and that homology gaps of up to 5% of the total
number of the
nucleotides in the reference sequence are permitted. When using Bestfit, the
so-called
optional parameters are preferably left at their preset ("default") values.
The deviations
appearing in the comparison between a given sequence and the above-described
sequences of the invention may be caused for instance by addition, deletion,
substitution,
insertion or recombination. Such a sequence comparison can preferably also be
carried
out with the program "fasta20u66" (version 2.0u66, September 1998 by William
R.
Pearson and the University of Virginia; see also W.R. Pearson (1990), Methods
in
Enzymology 183, 63-98, appended examples and http://workbench.sdsc.edu/). For
this
purpose, the "default" parameter settings may be used.
10002471 As used herein, the phrases "sexually crossed" and "sexual
reproduction" in the
context of the presently disclosed subject matter refers to the fusion of
gametes to
produce progeny (e.g., by fertilization, such as to produce seed by
pollination in plants).
A "sexual cross" or "cross-fertilization" is in some embodiments fertilization
of one
individual by another (e.g., cross-pollination in plants). The term "selfing"
refers in some
51
CA 02902250 2015-08-28
51682-49
embodiments to the production of seed by self-fertilization or self-
pollination; i.e., pollen
and ovule are from the same plant.
[000248] "Single gene converted". A single gene converted or conversion plant
refers to a
plant that is developed by plant breeding techniques (e.g., backcrossing) or
via genetic
engineering wherein essentially all of the desired morphological and
physiological
characteristics of a line are recovered in addition to the single gene
transferred into the
line via the plant breeding technique or via genetic engineering.
[000249] A single nucleotide polymorphism (SNP), a variation at a single site
in DNA, is
the most frequent type of variation in the genome. A single-nucleotide
polymorphism
(SNP) is a DNA sequence variation occurring when a single nucleotide __ A, T,
C, or G
¨ in the genome (or other shared sequence) differs between members of a
biological
species or paired chromosomes in an individual. For example, two sequenced DNA
fragments from different individuals, AAGCCTA to AAGCTTA, contain a difference
in
a single nucleotide. In this case there are two alleles: C and T. The basic
principles of
SNP array are the same as the DNA microarray. These are the convergence of DNA
hybridization, fluorescence microscopy, and DNA capture. The three components
of the
SNP arrays are the array that contains nucleic acid sequences (i.e. amplified
sequence or
target), one or more labeled allele-specific oligonucleotide probes and a
detection system
that records and interprets the hybridization signal.
[000250] "Stringent hybridization conditions" and "stringent hybridization
wash
conditions" in the context of nucleic acid hybridization experiments such as
Southern and
Northern hybridizations are sequence dependent, and are different under
different
environmental parameters. Longer sequences hybridize specifically at higher
temperatures. An extensive guide to the hybridization of nucleic acids is
found in Tijssen
(1993) Laboratory Techniques in Biochemistry and Molecular Biology-
Hybridization
with Nucleic Acid Probes part I chapter 2 "Overview of principles of
hybridization and
the strategy of nucleic acid probe assays" Elsevier, New York. Generally,
highly stringent
hybridization and wash conditions are selected to be about 5 C lower than the
thermal
52
CA 02902250 2015-08-28
51682-49
melting point for the specific sequence at a defined ionic strength and pH.
Typically,
under "stringent conditions" a probe will hybridize to its target subsequence,
but to no
other sequences. The thermal melting point is the temperature (under defined
ionic
strength and pH) at which 50% of the target sequence hybridizes to a perfectly
matched
probe. Very stringent conditions are selected to be equal to the melting
temperature (Tm)
for a particular probe. An example of stringent hybridization conditions for
hybridization
of complementary nucleic acids which have more than 100 complementary residues
on a
filter in a Southern or northern blot is 50% formamide with 1 mg of heparin at
42 C.,
with the hybridization being carried out overnight. An example of highly
stringent wash
conditions is 0.1 5M NaCl at 72 C for about 15 minutes. An example of
stringent wash
conditions is a 0.2 times SSC wash at 65 C for 15 minutes (see, Sambrook,
infra, for a
description of SSC buffer). Often, a high stringency wash is preceded by a low
stringency
wash to remove background probe signal. An example medium stringency wash for
a
duplex of, e.g., more than 100 nucleotides, is 1 times SSC at 45 C for 15
minutes. An
example low stringency wash for a duplex of, e.g., more than 100 nucleotides,
is 4-6
times SSC at 40 C for 15 minutes. For short probes (e.g., about 10 to 50
nucleotides),
stringent conditions typically involve salt concentrations of less than about
1.0M Na ion,
typically about 0.01 to 1.0 M Na ion concentration (or other salts) at pH 7.0
to 8.3, and
the temperature is typically at least about 30 C. Stringent conditions can
also be achieved
with the addition of destabilizing agents such as formamide. In general, a
signal to noise
ratio of 2 times (or higher) than that observed for an unrelated probe in the
particular
hybridization assay indicates detection of a specific hybridization. Nucleic
acids that do
not hybridize to each other under stringent conditions are still substantially
identical if the
proteins that they encode are substantially identical. This occurs, e.g. when
a copy of a
nucleic acid is created using the maximum codon degeneracy permitted by the
genetic
code.
[000251] "Substantially equivalent characteristic". A characteristic that,
when compared,
does not show a statistically significant difference (e.g., p=0.05) from the
mean.
53
CA 02902250 2015-08-28
51682-49
[000252] "Transgene". A nucleic acid of interest that can be introduced into
the genome of
a plant by genetic engineering techniques (e.g., transformation) or breeding.
The
transgene can be from the same or a different species. If from the same
species, the
transgene can be an additional copy of a native coding sequence or can present
the native
sequence in a form or context (e.g., different genomic location and/or in
operable
association with exogenous regulatory elements such as a promoter) than is
found in the
native state. The transgene can comprise an open reading frame encoding a
polypeptide
or can encode a functional non-translated RNA (e.g., RNAi).
[000253] As used herein, the term "variety" or "cultivar'' means a group of
similar plants
that by structural features and performance can be identified from other
varieties within
the same species.
[000254] Embodiments
[000255] In representative embodiments, the invention relates to a pepper
plant, particularly
a cultivated pepper plant, more particularly a cultivated blocky fruit type
pepper plant,
more particularly a cultivated sweet blocky fruit type pepper plant bearing
extreme dark
green color fruit at immature harvestable stage, said plant comprising one
more (e.g.,
two) genetic determinants directing or controlling expression of said extreme
dark green
color in the pepper fruit of the pepper plant.
[000256] The tetins "extreme dark green," "extreme dark green color" and
"extreme dark
green color trait" are defined and described in more detail herein.
[000257] In particular, in specific embodiments, said one or more genetic
determinants are
represented by one or more QTL (e.g., two QTL) or a functional part thereof
capable of
directing or controlling expression of said dark green immature fruit color in
the pepper
fruit of the pepper plant.
[000258] In representative embodiments, a pepper plant according to the
invention is
provided, particularly a cultivated pepper plant, wherein one or more (e.g.,
two) QTLs or
54
CA 02902250 2015-08-28
51682-49
a functional part thereof are genetically or physically linked to marker loci,
which co-
segregate with the extreme dark green color and comprise one or more of SP436,
SP626
SP693 and/or SP694.
[000259] In representative embodiments, the one or more QTLs comprise QTL1,
which is
linked to marker loci SP436 and/or SP626 and/or QTL2, which is linked to
marker loci
SP693 and/or SP694.
[000260] In representative embodiments, a pepper plant, particularly a
cultivated pepper
plant, according to any of the preceding embodiments is provided, wherein said
QTL1 or
a functional part thereof is genetically linked to marker loci SP436 and SP626
and
wherein:
i. marker locus SP436 can be identified by amplification (e.g., PCR
amplification)
of a DNA fragment with the pair of oligonucleotide primers: forward primer of
SEQ ID NO: 1 and reverse primer of SEQ ID NO: 2, optionally using probe SEQ
ID NO 9,
marker locus SP626 can be identified in by amplification (e.g., PCR
amplification) of a DNA fragment with the pair of oligonucleotide primers:
forward primer of SEQ ID NO: 3 and reverse primer of SEQ ID NO: 4,optionally
using probe SEQ ID NO 10.
[000261] In representative embodiments, a pepper plant, particularly a
cultivated pepper
plant, according to any of the preceding embodiments is provided, wherein said
QTL2 or
a functional part thereof is genetically linked to marker loci SP693 and
SP694, and
wherein:
i. marker locus SP693 can be identified by amplification (e.g., PCR
amplification)
of a DNA fragment with the pair of oligonucleotide primers: forward primer of
SEQ ID NO: 5 and reverse primer of SEQ ID NO: 6, optionally with probe SEQ
ID NO 11,
CA 02902250 2015-08-28
51682-49
marker locus SP694 can be identified by amplification (e.g., PCR
amplification)
of a DNA fragment with the pair of oligonucleotide primers: forward primer of
SEQ ID NO: 7 and reverse primer of SEQ ID NO: 8, optionally using probe SEQ
ID NO 12.
[000262] In representative embodiments, the extreme dark green color trait is
derived from
Capsicum annuum line 8728C, line 16452A, line 16452B, line 16452C, RPP 25822,
RPP
26098 and/or RPP 26105, or from a progeny or from an ancestor thereof
10002631 In representative embodiments, the invention relates to a pepper
plant, particularly
a cultivated pepper plant, according to any of the preceding embodiments,
comprising
alleles at the one or more QTLs in the pepper genome contributing to extreme
dark green
color in the pepper fruit of the pepper plant, which are complementary to the
corresponding alleles present in Capsicum annuum line 8728C, line 16452A, line
16452B, line 16452C, RPP 25822, RPP 26098 or RPP 26105, or in a progeny or in
an
ancestor thereof, and genetically linked to marker loci in the genome of
Capsicum
annuum line 8728C, line 16452A, line 16452B, line 16452C, RPP 25822, RPP 26098
or
RPP 26105, or in a progeny or in an ancestor thereof, which marker loci co-
segregate
with the Extreme dark green color and can be identified in the genome of
Capsicum
annuum line 8728C, line 16452A, line 16452B, line 16452C, RPP 25822, RPP 26098
or
RPP 26105, and comprise one or more of SP436, SP626, SP693 and/or SP694.
[000264] In representative embodiments of the invention, a pepper plant,
particularly a
cultivated pepper plant, according to any of the embodiments herein is
provided, wherein
QTL1 is obtainable from a donor plant having the genetic background of
Capsicum
annuum line 8728C, line 16452A, line 16452B, line 16452C. RPP 25822, RPP 26098
or
RPP 26105, or from a progeny or an ancestor thereof, comprising said QTL1 or
an
Extreme dark green color-conferring part thereof
[000265] In representative embodiments of the invention, a pepper plant,
particularly a
cultivated pepper plant, according to any of the preceding embodiments is
provided,
56
CA 02902250 2015-08-28
51682-49
wherein QTL2 is obtainable from a donor plant having the genetic background of
Capsicum annuum line 8728C, line 16452A, line 16452B, line 16452C, RPP 25822,
RPP
26098 or RPP 26105, or from a progeny or an ancestor thereof, comprising said
QTL2 or
an Extreme dark green color-conferring part thereof.
[000266] In representative embodiments, a pepper plant, particularly a
cultivated pepper
plant, according to any of the embodiments described herein is provided,
wherein said
genetic determinant(s) are obtainable from Capsicum annuum line 8728C, line
16452A,
line 16452B, line 16452C, RPP 25822, RPP 26098 and/or RPP 26105.
[000267] In representative embodiments of the invention, the pepper plant is a
plant
according to any of the embodiments described herein, which plant is a pepper
plant of
the genus Capsicum, particularly a cultivated pepper plant, particularly a
haploid, a
doubled haploid, an inbred or a hybrid.
[000268] In one embodiment, the invention provides a pepper plant according to
any of the
embodiments described herein, which is a hybrid pepper plant, particularly a
cultivated
pepper plant, comprising QTL1 and/or QTL2 or an Extreme dark green color-
conferring
part thereof and producing pepper fruits having the color and/or pigment
characteristics
associated with the extreme dark green color trait at the immature harvestable
stage as
described herein, wherein QTL1 and/or QTL2 are genetically linked to marker
loci co-
segregating with the Extreme dark green color, wherein said QTL1 and/or QTL2
are
obtainable from a donor plant having the genetic background of Capsicum annuum
line
8728C, line 16452A, line 16452B, line 16452C, RPP 25822, RPP 26098 or RPP
26105,
or in a progeny or an ancestor thereof, comprising said QTL1 and/or QTL2 or an
Extreme
dark green color-conferring part thereof and wherein the marker loci comprises
one or
more of SP436, SP626, SP693 and/or SP694.
[000269] In representative embodiments the pepper plant, particularly a
cultivated pepper
plant, of the invention is a plant according to any of the embodiments
described herein,
which grows fruits selected from the group consisting of blocky type peppers.
57
CA 02902250 2015-08-28
51682-49
[000270] The present invention further relates to seed of a pepper plant,
particularly a
cultivated pepper plant, according to any of the embodiments herein, which is
capable of
growing a pepper plant bearing extreme dark green color fruit according to the
invention.
[000271] In representative embodiments, a kit for the detection of the Extreme
dark green
color locus in a pepper plant, particularly a cultivated pepper plant, is
herein provided,
wherein said kit comprises at least one oligonucleotide primer pair,
optionally with a
probe, selected from:
a. primer pair 1 represented by a forward primer of SEQ ID NO 1 and a
reverse
primer of SEQ ID NO 2, and optionally the probe of SEQ ID NO 9 or;
b. primer pair 2 represented by a forward primer of SEQ ID NO 3 and a
reverse
primer of SEQ ID NO 4, and optionally the probe of SEQ ID NO 10, or;
c. primer pair 3 represented by a forward primer of SEQ ID NO 5 and a
reverse
primer of SEQ ID NO 6, and optionally the probe of SEQ ID NO 11, and/or;
d. primer pair 4 represented by a forward primer SEQ ID NO 7 and reverse
primer
of SEQ ID NO 8, and optionally the probe of SEQ ID NO 12, and/or
another primer or primer pair representing an adjacent markers that is
statistically
correlated and thus co-segregates with the Extreme dark green color.
[000272] In further embodiments, the present invention relates also to the use
of some or all
of these DNA markers / marker sequences according to the invention for
diagnostic
selection and/or genotyping of a pepper plant of the Extreme dark green color
locus or
loci in a pepper plant, particularly a cultivated pepper plant, particularly
of the Extreme
dark green color locus or loci, particularly in a pepper plant according to
the invention.
[000273] In other embodiments, the present invention further contemplates the
use of some
or all of these DNA markers for identifying in a pepper plant, particularly a
cultivated
pepper plant, particularly a pepper plant according to the invention, the
presence of the
Extreme dark green color locus or loci and/or for monitoring the introgressing
of the
58
CA 02902250 2015-08-28
51682-49
Extreme dark green color locus or loci in a pepper plant, particularly a
cultivated pepper
plant, particularly a pepper plant according to the invention and as described
herein.
[000274] In representative embodiments, the invention relates to the
polynucleotide
(amplification product) obtainable in an amplification reaction (e.g., PCR
amplification)
involving at least one oligonucleotide primer selected from the group
consisting of SEQ
ID NO 1; SEQ ID NO 2; SEQ ID NO 3; SEQ ID NO 4; SEQ ID NO 5; SEQ ID NO 6,
SEQ ID NO 7; SEQ ID NO 8 or a pair of oligonucleotide primers (e.g., PCR
oligonucleotide primers), and reacting with probes optionally selected from
the group
comprising SEQ ID NO 9, SEQ ID NO 10, SEQ ID NO 11 and SEQ ID NO 12, selected
from
a. primer pair 1 represented by a forward primer of SEQ ID NO 1 and a
reverse
primer of SEQ ID NO 2, and optionally the probe of SEQ ID NO 9 or;
b. primer pair 2 represented by a forward primer of SEQ ID NO 3 and a
reverse
primer of SEQ ID NO 4, and optionally the probe of SEQ ID NO 10 or;
c. primer pair 3 represented by a forward primer of SEQ ID NO 5 and a
reverse
primer of SEQ ID NO 6, and optionally the probe of SEQ ID NO 11 and/or;
d. primer pair 4 represented by a forward primer of SEQ ID NO 7 and a
reverse
primer of SEQ ID NO 8, and optionally the probe of SEQ ID NO 12 and/or
by another primer representing an adjacent marker that is statistically
correlated and thus
co-segregates with the Extreme dark green color or with one of the markers
disclosed,
which amplification product corresponds to an amplification product obtainable
from
Capsicum annuum line 8728C, line 16452A, line 16452B, line 16452C, RPP 25822,
RPP
26098 or RPP 26105 in an amplification reaction (e.g., PCR amplification) with
identical
primers or primer pairs provided that the respective marker locus or loci is
still present in
said pepper plant and/or can be considered an allele thereof
[000275] Also contemplated herein is a polynucleotide that has at least about
80%,
particularly at least about 85%, particularly at least about 90%, particularly
at least about
95%, particularly at least about 96%, particularly at least about 97%,
particularly at least
59
CA 02902250 2015-08-28
51682-49
about 98%, particularly at least about 99% sequence identity with the sequence
of said
amplification product and/or a polynucleotide exhibiting a nucleotide sequence
that
hybridizes to the nucleotide sequences of said amplification product
obtainable in the
above amplification reaction (e.g., PCR amplification reaction).
[000276] The amplification product according to the invention and described
herein above
can then be used for generating or developing new primers and/or probes that
can be used
for identifying the Extreme dark green color locus.
[000277] The present invention therefore further relates in one embodiment to
derived
markers, particularly to derived primers or probes, developed from an
amplification
product according to the invention and as described herein above by methods
known in
the art, which derived markers are genetically linked to the Extreme dark
green color
locus, particularly the Extreme dark green color locus.
[000278] The identification of a pepper plant with extreme dark green color
may be done
by measuring and selecting plants bearing fruits with the color and/or
pigment/nutritional
characteristics associated with the extreme dark green color trait at the
immature
harvestable stage as described herein.
[000279] In representative embodiments, the invention relates to a method for
producing a
pepper plant, particularly a cultivated pepper plant, exhibiting extreme dark
green color
in the pepper fruit of the pepper plant, comprising the steps of:
a.
selecting a pepper plant of the genus Capsicum annuum, which exhibits Extreme
dark green color, wherein said color related trait is associated with QTL1
and/or QTL 2,
or a functional part thereof capable of directing or controlling expression of
said extreme
dark green color in the pepper fruit of the pepper plant, wherein said QTL1
and/or QTL 2
or a functional part thereof are genetically linked to at least one marker
locus, selected
from marker loci SP436, SP626, SP693 and/or SP694 or any adjacent marker that
is
CA 02902250 2015-08-28
51682-49
statistically correlated and thus co-segregates with the Extreme dark green
color; or with
any of the disclosed marker loci,
b. crossing said plant of step a), which exhibits Extreme dark green color,
with a
pepper plant, particularly a cultivated pepper plant, which is not extreme
dark green
color, and
c. selecting progeny pepper plant from said cross which exhibits extreme
dark green
color and demonstrates association with said marker locus or loci of step a)
and bears
extreme dark green color fruits at the immature harvestable stage as described
herein.
[000280] In further embodiments, the invention provides a method for obtaining
pepper
fruits with extreme dark green color, particularly blocky pepper fruits,
comprising the
steps of
i. sowing a seed of a plant according to any one of the preceding
embodiments or
obtained in a method according to any of the preceding embodiments; and
growing said plant in order to produce fruit and harvesting the fruits
produced by
said plant.
[000281] The present invention further includes and provides methods of
identifying a
pepper plant bearing extreme dark green pepper fruits comprising:
a. providing a population segregating for dark green immature fruit color,
b. screening the segregating population for a member having an extreme dark
green
pepper fruit trait, wherein said trait can be identified by the presence of
the following
molecular markers in the genome: SP436, SP626, SP693 and/or SP694
c. selecting at least one member of the segregating population, wherein
said at least
member is bearing an extreme dark green pepper fruit trait.
[000282] In still other embodiments, the invention relates to an Extreme dark
green color-
conferring QTL or an Extreme dark green color-conferring part thereof, which
is
associated with at least a 1st DNA marker represented by a 1st pair of
oligonucleotide
primers (e.g., PCR oligonucleotide primers) comprising forward primer of SEQ
ID NO 1,
61
CA 02902250 2015-08-28
51682-49
reverse primer of SEQ ID NO 2, and optionally the probe of SEQ ID NO 9, and/or
at
least a 2nd DNA marker represented by a 2nd pair of oligonucleotide primers
(e.g., PCR
oligonucleotide primers) comprising forward primer of SEQ ID NO 3, reverse
primer of
SEQ ID NO 4, and optionally the probe of SEQ ID NO 10, particularly said QTL
or a
functional part thereof is associated with said 1st and 2nd DNA marker.
[000283] In still further embodiments, the invention relates to an Extreme
dark green color-
conferring QTL or an Extreme dark green color-conferring part thereof, which
and is
associated with at least a 1st DNA marker represented by a 1st pair of
oligonucleotide
primers (e.g., PCR oligonucleotide primers) comprising forward primer of SEQ
ID NO 5,
reverse primer of SEQ ID NO 6 and optionally the probe of SEQ ID NO 11, and a
2nd
pair of oligonueleotidc primers (e.g., PCR oligonucleotide primers) comprising
forward
primer of SEQ ID NO 7, reverse primer of SEQ ID NO 8 and optionally the probe
of
SEQ ID NO 12, particularly said QTL or a functional part thereof is associated
with said
1st and 2nd DNA marker.
[000284] The present invention also relates to the use of extreme dark green
color
propagating material obtainable from a pepper plant according to any of the
preceding
embodiments for growing a pepper plant in order to produce extreme dark green
color
fruit and harvest said extreme dark green color fruits wherein the said fruits
are
characterized, at immature harvestable stage, by the color and/or pigment
characteristics
associated with the dark green color trait as described herein.
[000285] It is further contemplated by the present invention to provide a
method for
increasing the pigment content of pepper fruits of pepper plant selected from
the group
comprising chlorophyll a, chlorophyll b, lutein and/or violaxanthin,
comprising the steps
of:
a. selecting a plant of the genus Capsicum, which exhibits Extreme dark
green color,
wherein said color related trait is associated with one or more QTLs or a
functional
part thereof capable of directing or controlling expression of said extreme
dark green
62
CA 02902250 2015-08-28
51682-49
color in the pepper fruit of the pepper plant, wherein said trait can be
identified by the
presence of one or more of the following molecular markers loci in the genome:
SP436, SP626, SP693 and/or SP694 or by any adjacent marker that is
statistically
correlated and thus co-segregates with the Extreme dark green color;
b. crossing said plant of step a), which exhibits Extreme dark green color,
with a pepper
plant, particularly a cultivated pepper plant, which does not exhibit extreme
dark
green color, and
c. selecting progeny from said cross which exhibit the extreme dark green
color trait and
demonstrates association of the extreme dark green color with said marker loci
of step
a).
[000286] Further embodiments of the present invention provide a method for
providing
pepper plant producing pepper fruits exhibiting the extreme dark green pepper
trait as
described herein, comprising the steps of:
a. selecting a plant of the genus Capsicum, which exhibits Extreme dark
green color,
wherein said trait is associated with QTL1 and/or QTL2, or a functional part
thereof capable of directing or controlling expression of said extreme dark
green
color in the pepper fruit of the pepper plant, wherein said QTL1 or a
functional
part thereof is genetically linked to marker loci SP436 and/or SP626 and
wherein
QTL2 or a functional part thereof is genetically linked to marker loci SP693
and/or SP694, which co-segregate with the Extreme dark green color and can be
identified in an amplification reaction (e.g., PCR amplification reaction) by
i. forward primer of SEQ ID NO 1, reverse primer of SEQ ID NO 2 and
optionally the probe of SEQ ID NO 9 for marker locus SP436;
ii. forward primer of SEQ ID NO 3, reverse primer of SEQ ID NO 4 and
optionally the probe of SEQ ID NO10, for marker locus SP626;
iii. forward primer of SEQ ID NO 5, reverse primer of SEQ ID NO 6 and
optionally the probe of SEQ ID NO 11 for marker locus SP693;
iv. forward primer of SED ID NO 7, reverse primer of SEQ ID NO 8 and
optionally the probe of SEQ ID NO 12 for marker locus SP694
63
CA 02902250 2015-08-28
51682-49
v. or by any adjacent marker that is statistically correlated and thus co-
segregates with the Extreme dark green color.
[000287] In representative embodiments, the pepper plant according to the
present
invention, as described in any of the previous embodiments, is homozygous at
QTL1
and/or QTL2.
[000288] Based on the description of the present invention, the skilled person
who is in
possession of Capsicum annuum line 8728C, line 16452A, line 16452B, line
16452C,
RPP 25822, RPP 26098 or RPP 26105, or a progeny or ancestor thereof containing
the
genetic determinants capable of directing or controlling the extreme dark
green color
trait, as described herein, has no difficulty to transfer the said genetic
determinant(s) of
the present invention to other pepper plants of various types using breeding
techniques
well-known in the art with the support of the QTL(s) and marker loci herein
disclosed.
[000289] Botanical Description of Pepper Cultivars RPP 25822, RPP 26098 and
RPP
26105.
[000290] Pepper cultivars RPP 25822, RPP 26098 and RPP 26105 are suitable for
the fresh
and processor markets.
[000291] OVG hybrids RPP 26098, RPP 25822 and RPP 26105 produce an open, erect
bush with medium vigor. The fruit is blocky, extremely dark green in color and
glossy.
These hybrids are particularly well-adapted to production conditions in the
Southeastern
United States. Yield and marketable fruit characteristics are within
commercially
acceptable limits for non-OVG hybrids.
[000292] Additional physiological and morphological description of OVG hybrids
RPP
26098, RPP 25822 and RPP 26105 as well as male inbred parent 8728C is provided
below in Table 1.
64
CA 02902250 2015-08-28
51682-49
TABLE 1:
Hybrid RPP 26098
TRAIT NAME VALUE
Seedling: anthocyanin coloration of hypocotyl Present
Plant: shortened internodes (in upper part) Absent (indeterminant)
Plant: vigor Medium
Plant: height Medium
Flower: anthocyanin coloration in anther Present
Fruit: color (before maturity) Green
Fruit: intensity of color (before maturity) Very dark
Fruit: length Medium
Fruit: diameter Medium
Fruit: shape in longitudinal section Square
Fruit: color (at maturity) Red
Fruit: intensity of color (at maturity) Very dark
Fruit: number of locules Equally three and four
Fruit: length (cm) 8
Fruit: diameter (cm) 8
Fruit: capsaicin in placenta Absent
Time of maturity Medium
Ripening can be compared with variety... Crusader
Plant setting ability cold Normal
Plant setting ability hot Normal
Resistances:
Pepper Mottle Virus Highly resistant
Xanthomonas cam pestris pv. vesicatoria, race 1 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 2 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 3 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 4 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 5 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 6 Susceptible
Xanthomonas campestris pv. vesicatoria, race 7 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 8 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 9 Highly resistant
CA 02902250 2015-08-28
51682-49
Hybrid RPP 25822
TRAIT NAME VALUE
Seedling: anthocyanin coloration of hypocotyl Present
Plant: shortened internodes (in upper part) Absent (indeterminant)
Plant: vigor Medium
Plant: height Medium
Flower: anthocyanin coloration in anther Present
Fruit: color (before maturity) Green
Fruit: intensity of color (before maturity) Very dark
Fruit: length Medium
Fruit: diameter Medium
Fruit: shape in longitudinal section Square
Fruit: color (at maturity) Red
Fruit: intensity of color (at maturity) Very dark
Fruit: number of locules Equally three and four
Fruit: length (cm) 8
Fruit: diameter (cm) 8
Fruit: capsaicin in placenta Absent
Time of maturity Medium
Ripening can be compared with variety... Crusader
Plant setting ability cold Normal
Plant setting ability hot Normal
Resistances:
Pepper Mottle Virus Highly resistant
Xanthomonas cam pestris pv. vesicatoria, race 1 Highly resistant
Xanthomonas cam pestris pv. vesicatoria, race 2 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 3 Highly resistant
Xanthomonas cam pestris pv. vesicatoria, race 4 Highly resistant
Xanthomonas cam pestris pv. vesicatoria, race 5 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 6 Susceptible
Xanthomonas cam pestris pv. vesicatoria, race 7 Highly resistant
Xanthomonas cam pestris pv. vesicatoria, race 8 Highly resistant
66
CA 02902250 2015-08-28
51682-49
Xanthomonas campestris pv. vesicatoria, race 9 Highly resistant
Hybrid RPP 26105
TRAIT NAME VALUE
Seedling: anthocyanin coloration of hypocotyl Present
Plant: shortened internodes (in upper part) Absent (indeterminant)
Plant: vigor Medium
Plant: height Medium
Flower: anthocyanin coloration in anther Present
Fruit: color (before maturity) Green
Fruit: intensity of color (before maturity) Very dark
Fruit: length Medium
Fruit: diameter Medium
Fruit: shape in longitudinal section Square
Fruit: color (at maturity) Red
Fruit: intensity of color (at maturity) Very dark
Fruit: number of locules Equally three and four
Fruit: length (cm) 8
Fruit: diameter (cm) 8
Fruit: capsaicin in placenta Absent
Time of maturity Medium
Ripening can be compared with variety... Crusader
Plant setting ability cold Normal
Plant setting ability hot Normal
Resistances:
Pepper Mottle Virus Highly resistant
Xanthomonas cam pestris pv. vesicatoria, race 1 Highly resistant
Xanthomonas cam pestris pv. vesicatoria, race 2 Highly resistant
Xanthomonas cam pestris pv. vesicatoria, race 3 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 4 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 5 Highly resistant
Xanthomonas cam pestris pv. vesicatoria, race 6 Susceptible
Xanthomonas cam pestris pv. vesicatoria, race 7 Highly resistant
67
CA 02902250 2015-08-28
,
,
51682-49
1 Xanthomonas campestris pv. vesicatoria, race 8 Highly resistant
_
Xanthomonas campestris pv. vesicatoria, race 9 Highly resistant
Inbred line 8728C
TRAIT NAME VALUE
Seedling: anthocyanin coloration of hypocotyl Present
Plant: length of internodes Medium-Long
Plant: intensity of anthocyanin Medium
Plant: vigor Strong
Plant: hairiness of the plant Very weak
Plant: height Medium-high
Fruit: color before maturity Green
Fruit: attitude of the fruits Drooping
Fruit: shape in longitudinal section Square
Fruit: color at maturity Red
Fruit: speed of fruit color change to mature Slow
Fruit: type Sweet-blocky
Predominant number of locules Three and four
Thickness of pericarp of the fruit Medium-thick
Capsaicin in placenta Absent
Fruit: pungency level No pungency
Fruit: sensitivity for cracking Medium
Fruit: Size at harvest Medium
Sensitivity for BER Isolated BER
Sensitivity for blind and fork Not sensitive
Tendency for parthenocarpy Yes
Flower expression of male sterility No
Balance of the plant Vegetative
Seeds pre-germinate in fruits No
Resistances:
Pepper Mottle Virus Susceptible
Phytophthora capsici Susceptible
Tobacco Etch Virus Highly resistant
68
CA 02902250 2015-08-28
51682-49
Tobacco Mosaic Virus Susceptible
Xanthomonas campestris pv. vesicatoria, race 1 Susceptible
Xanthomonas campestris pv. vesicatoria, race 2 Susceptible
Xanthomonas campestris pv. vesicatoria, race 3 Susceptible
Xanthomonas campestris pv. vesicatoria, race 4 Highly resistant
Xanthomonas cam pestris pv. vesicatoria, race 5 Highly resistant
Xanthomonas cam pestris pv. vesicatoria, race 6 Susceptible
Inbred Line 16452A
TRAIT NAME VALUE
Seedling: anthocyanin coloration of hypocotyl Present
Absent
Plant: shortened internodes (in upper part)
(indeterminant)
Plant: vigor Medium
Plant: height Medium
Flower: anthocyanin coloration in anther Present
Fruit: color (before maturity) Green
Fruit: intensity of color (before maturity) Very dark
Fruit: length Medium
Fruit: diameter Medium
Fruit: shape in longitudinal section Square
Fruit: color (at maturity) Red
Fruit: intensity of color (at maturity) Very dark
Equally three
Fruit: number of locules and four
Fruit: length (cm) 3.5"
Fruit: diameter (cm) 3.5"
Fruit: capsaicin in placenta Absent
Time of maturity Medium
Ripening can be compared with variety... Crusader
Plant setting ability cold Normal
Plant setting ability hot Normal
Resistances:
69
CA 02902250 2015-08-28
51682-49
Pepper Mottle Virus Highly resistant
Xanthomonas campestris pv. vesicatoria, race 1 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 2 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 3 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 4 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 5 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 6 Susceptible
Xanthomonas campestris pv. vesicatoria, race 7 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 8 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 9 Highly resistant
Inbred Line 16452B
TRAIT NAME VALUE
Seedling: anthocyanin coloration of hypocotyl Present
Absent
Plant: shortened internodes (in upper part)
(indeternninant)
Plant: vigor Medium
Plant: height Medium
Flower: anthocyanin coloration in anther Present
Fruit: color (before maturity) Green
Fruit: intensity of color (before maturity) Very dark
Fruit: length Medium
Fruit: diameter Medium
CA 02902250 2015-08-28
51682-49
Fruit: shape in longitudinal section Square
Fruit: color (at maturity) Red
Fruit: intensity of color (at maturity) Very dark
Equally three
Fruit: number of locules
and four
Fruit: length (cm) 3.5"
Fruit: diameter (cm) 3.5"
Fruit: capsaicin in placenta Absent
Time of maturity Medium
Ripening can be compared with variety... Crusader
Plant setting ability cold Normal
Plant setting ability hot Normal
Resistances:
Pepper Mottle Virus Highly resistant
Xanthomonas cam pestris pv. vesicatoria, race 1 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 2 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 3 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 4 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 5 Highly resistant
Xanthomonas cam pestris pv. vesicatoria, race 6 Susceptible
Xanthomonas campestris pv. vesicatoria, race 7 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 8 Highly resistant
Xanthomonas campestris pv. vesicatoria, race 9 Highly resistant
Inbred Line 16452C
71
CA 02902250 2015-08-28
51682-49
TRAIT NAME VALUE
Seedling: anthocyanin coloration of hypocotyl Present
Plant: shortened internodes (in upper part) Absent
(indeterminant)
Plant: vigor Medium
Plant: height Medium
Flower: anthocyanin coloration in anther Present
Fruit: color (before maturity) Green
Fruit: intensity of color (before maturity) Very dark
Fruit: length Medium
Fruit: diameter Medium
Fruit: shape in longitudinal section Square
Fruit: color (at maturity) Red
Fruit: intensity of color (at maturity) Very dark
Equally three
Fruit: number of locules and four
Fruit: length (cm) 3.5"
Fruit: diameter (cm) 3.5"
Fruit: capsaicin in placenta Absent
Time of maturity Medium
Ripening can be compared with variety... Crusader
Plant setting ability cold Normal
Plant setting ability hot Normal
Resistances:
Pepper Mottle Virus Highly resistant
72
CA 02902250 2015-08-28
,
, ,
51682-49
Xanthomonas cam pestris pv. vesicatoria, race 1 Highly
resistant
Xanthomonas cam pestris pv. vesicatoria, race 2 Highly
resistant
Xanthomonas campestris pv. vesicatoria, race 3 Highly
resistant
Xanthomonas cam pestris pv. vesicatoria, race 4 Highly
resistant
Xanthomonas cam pestris pv. vesicatoria, race 5 Highly
resistant
Xanthomonas campestris pv. vesicatoria, race 6 Susceptible
Xanthomonas cam pestris pv. vesicatoria, race 7 Highly
resistant
Xanthomonas cam pestris pv. vesicatoria, race 8 Highly
resistant
Xanthomonas cam pestris pv. vesicatoria, race 9 Highly
resistant
73
CA 02902250 2015-08-28
51682-49
[000293] Breeding Methods.
[000294] This invention is also directed to methods for producing a pepper
plant by
crossing a first parent pepper plant with a second parent pepper plant wherein
the first or
second parent pepper plant is a plant of pepper cultivar 8728C, 16452A,
16452B,
16452C, RPP 25822, RPP 26098 or RPP 26105. Further, both first and second
parent
pepper plant can be selected from pepper cultivar 8728C, 16452A, 16452B,
16452C, RPP
25822, RPP 26098 or RPP 26105. Thus, any of the following exemplary methods
using
pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP
26105
are part of this invention: selling, backcrosses, hybrid production, crosses
to populations,
doubled haploid production, and the like. All plants produced using pepper
cultivar
8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105 as at least
one
parent are within the scope of this invention, including those developed from
pepper
plants derived from pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822,
RPP
26098 or RPP 26105. Advantageously, pepper cultivar 8728C, 16452A, 16452B,
16452C, RPP 25822, RPP 26098 or RPP 26105 can be used in crosses with other,
different, pepper plants to produce the first generation (F1) pepper hybrid
seeds and
plants with desirable characteristics. The pepper plants of the invention can
also be used
for transformation where exogenous transgenes are introduced and expressed by
the
plants of the invention. Genetic variants created either through traditional
breeding
methods or through transformation of the cultivars of the invention by any of
a number of
protocols known to those of skill in the art are intended to be within the
scope of this
invention.
[000295] The following describes exemplary breeding methods that may be used
with
pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP
26105
in the development of further pepper plants. One such embodiment is a method
for
developing pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098
or
RPP 26105 progeny pepper plants in a pepper plant breeding program comprising:
obtaining a plant, or a part thereof, of pepper cultivar 8728C, 16452A,
16452B, 16452C,
RPP 25822, RPP 26098 or RPP 26105, utilizing said plant or plant part as a
source of
74
CA 02902250 2015-08-28
51682-49
breeding material, and selecting a pepper cultivar 8728C, 16452A, 16452B,
16452C, RPP
25822, RPP 26098 or RPP 26105 progeny plant with molecular markers in common
with
pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP
26105
and/or with some, all or essentially all morphological and/or physiological
characteristics
of pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP
26105 (e.g., as described herein, in particular, in any one or more of Tables
1-9 or
Figures 1-30). In representative embodiments, the progeny plant has at least
1, 2, 3, 4, 5,
6, 7, 8, 9, 10 or more of the morphological and physiological characteristics
of pepper
cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105
(e.g.,
as described herein, in particular, in any one or more of Tables 1-9 or
Figures 1-30), or
even all of the morphological and physiological characteristics of pepper
cultivar 8728C,
16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105 so that said progeny
pepper plant is not significantly different for said traits than pepper
cultivar 8728C,
16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105, as determined at
the
5% significance level when grown in the same environmental conditions;
optionally, with
the presence of one or more desired additional traits (e.g., male sterility,
disease
resistance, pest or insect resistance, herbicide resistance, and the like).
Breeding steps
that may be used in the breeding program include pedigree breeding,
backcrossing,
mutation breeding and/or recurrent selection. In conjunction with these steps,
techniques
such as RFLP-enhanced selection, genetic marker enhanced selection (for
example, SSR
markers) and/or and the making of doubled haploids may be utilized.
[000296] Another representative method involves producing a population of
pepper cultivar
8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105 progeny
plants, comprising crossing pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP
25822, RPP 26098 or RPP 26105 with another pepper plant, thereby producing a
population of pepper plants that, on average, derives at least about 6.25%,
12.5%, 25%,
30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 80%, 85%, 90%, 95%, 96%, 97%,
98% or 99% of its alleles (i.e., theoretical allelic content; TAC) from pepper
cultivar
8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105, e.g., at
least
about 6.25%, 12.5%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 80%,
CA 02902250 2015-08-28
51682-49
85%, 90%, 95%, 96%, 97%, 98% or 99% of the genetic complement of pepper
cultivar
8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105. One
embodiment of this invention is the pepper plant produced by this method and
that has
obtained at least 6.25%, 12.5%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%,
70%,
80%, 85%, 90%, 95%, 96%, 97%, 98% or 99% of its alleles from pepper cultivar
8728C,
16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105. A plant of this
population may be selected and repeatedly selfed or sibbed with a pepper plant
resulting
from these successive filial generations. Another approach is to make doubled
haploid
plants to achieve homozygosity.
[000297] One of ordinary skill in the art of plant breeding would know how to
evaluate the
traits of two plant varieties to determine if there is no significant
difference between the
two traits expressed by those varieties. For example, see Fehr and Walt,
Principles of
Cultivar Development, pp. 261-286 (1987). In embodiments, the invention
encompasses
progeny plants having a combination of at least 2, 3, 4, 5, 6, 7, 8, 9, 10 or
more of the
characteristics as described herein (e.g., in any one or more of Tables 1-9 or
Figures 1-
30) for pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or
RPP 26105, so that said progeny pepper plant is not significantly different
for said traits
than pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or
RPP
26105, as determined at the 5% significance level when grown in the same
environmental
conditions. Using techniques described herein and those known in the art,
molecular
markers may be used to identify said progeny plant as progeny of pepper
cultivar 8728C,
16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105. Mean trait values
may be used to determine whether trait differences are significant, and
optionally the
traits are measured on plants grown under the same environmental conditions.
[000298] Progeny of pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822,
RPP
26098 or RPP 26105 may also be characterized through their filial relationship
with
pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP
26105, as for example, being within a certain number of breeding crosses of
pepper
cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105. A
76
CA 02902250 2015-08-28
51682-49
breeding cross is a cross made to introduce new genetics into the progeny, and
is
distinguished from a cross, such as a self or a sib cross or a backcross to
8728C, 16452A,
16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105 as a recurrent parent, made
to
select among existing genetic alleles. The lower the number of breeding
crosses in the
pedigree, the closer the relationship between pepper cultivar 8728C, 16452A,
16452B,
16452C, RPP 25822, RPP 26098 or RPP 26105 and its progeny. For example,
progeny
produced by the methods described herein may be within 1, 2, 3, 4, 5 or more
breeding
crosses of pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098
or
RPP 26105.
[000299] In representative embodiments, a pepper plant derived from pepper
cultivar
8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105 comprises at
least one set of chromosomes derived from pepper cultivar 8728C, 16452A,
16452B,
16452C, RPP 25822, RPP 26098 or RPP 26105.
[000300] In embodiments, the pepper plant or population of pepper plants
derived from
pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP
26105
comprises, on average, at least about 6.25%, 12.5%, 25%, 30%, 35%, 40%, 45%,
50%,
55%, 60%, 65%, 70%, 80%, 85%, 90%, 95%, 96%, 97%, 98% or 99% of its alleles
(i.e.,
theoretical allelic content; TAC) from pepper cultivar 8728C, 16452A, 16452B,
16452C,
RPP 25822, RPP 26098 or RPP 26105, e.g., at least about 6.25%, 12.5%, 25%,
30%,
35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 80%, 85%, 90%, 95%, 96%, 97%, 98% or
99% of the genetic complement of pepper cultivar 8728C, 16452A, 16452B,
16452C,
RPP 25822, RPP 26098 or RPP 26105.
[000301] In embodiments, the pepper plant derived from pepper cultivar 8728C,
16452A,
16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105 is one, two, three, four,
five or
more breeding crosses removed from pepper cultivar 8728C, 16452A, 16452B,
16452C,
RPP 25822, RPP 26098 or RPP 26105.
77
CA 02902250 2015-08-28
51682-49
[000302] In representative embodiments, a plant derived from pepper cultivar
8728C,
16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105 is a doubled haploid
plant, a hybrid plant or an inbred plant.
[000303] In embodiments, a hybrid or derived plant from pepper cultivar 8728C,
16452A,
16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105 comprises a desired added
trait.
In representative embodiments, a pepper plant derived from pepper cultivar
8728C,
16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105 comprises all of the
morphological and physiological characteristics of pepper cultivar 8728C,
16452A,
16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105 (e.g., as described herein,
in
particular, in any one or more of Tables 1-9 or Figures 1-30). In embodiments,
the
pepper plant derived from pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP
25822,
RPP 26098 or RPP 26105 comprises all or essentially all of the morphological
and
physiological characteristics of pepper cultivar 8728C, 16452A, 16452B,
16452C, RPP
25822, RPP 26098 or RPP 26105 (e.g., as described herein, in particular, in
any one or
more of Tables 1-9 or Figures 1-30), with the addition of a desired added
trait.
[000304] In embodiments, a hybrid or derived plant from pepper cultivar 8728C,
16452A,
16452B, 16452C, RPP 25822, RPP 26098 or RPP 26105 comprises QTL1 and/or QTL2
as described herein. In embodiments, the QTL1 and/or QTL2 is not in the native
(e.g.
natural) genetic background of the pepper plant having the extreme dark green
color trait.
In embodiments, the hybrid or derived plant comprises one or more of marker
loci:
SP436, SP626, SP693 and/or SP694. In embodiments, the hybrid or derived plant
is
homozygous for QTL1 and/or QTL2.
[000305] Further Embodiments of the Invention.
[000306] With the advent of molecular biological techniques that have allowed
the isolation
and characterization of genes that encode specific protein products,
scientists in the field
of plant biology developed a strong interest in engineering the genome of
plants to
contain and express foreign nucleic acids including additional or modified
versions of
78
CA 02902250 2015-08-28
51682-49
native (endogenous) nucleic acids (optionally driven by a non-native promoter)
in order
to alter the traits of a plant in a specific manner. Any nucleic acid
sequences, whether
from a different species or from the same species, which are introduced into
the genome
using transformation or various breeding methods, are referred to herein
collectively as
"transgenes.'' Over the last fifteen to twenty years, several methods for
producing
transgenie plants have been developed, and in particular embodiments the
present
invention also relates to transformed versions of pepper plants disclosed
herein.
[000307] Genetic engineering techniques can be used (alone or in combination
with
breeding methods) to introduce one or more desired added traits into plant,
for example,
pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or RPP
26105
or progeny or pepper plants derived thereof
[000308] Plant transformation generally involves the construction of an
expression vector
that will function in plant cells. Optionally, such a vector comprises one or
more nucleic
acids comprising a coding sequence for a polypeptide or an untranslated
functional RNA
under control of, or operatively linked to, a regulatory element (for example,
a promoter).
In representative embodiments, the vector(s) may be in the form of a plasmid,
and can be
used alone or in combination with other plasmids, to provide transformed
pepper plants
using transformation methods as described herein to incorporate transgenes
into the
genetic material of the pepper plant.
[000309] Additional methods include, but are not limited to, expression
vectors introduced
into plant tissues using a direct nucleic acid transfer method, such as
microprojectile-
mediated delivery (e.g., with a biolistic device), DNA injection,
Agrobacterium-mediated
transformation, electroporation, and the like. Transformed plants obtained
from the
plants (and parts and tissue culture thereof) of the invention are intended to
be within the
scope of this invention.
79
CA 02902250 2015-08-28
51682-49
[000310] Expression Vectors for Plant Transformation ¨ Selectable markers.
[000311] Expression vectors typically include at least one nucleic acid
comprising or
encoding a selectable marker, operably linked to a regulatory element (for
example, a
promoter) that allows transformed cells containing the marker to be either
recovered by
negative selection, e.g., inhibiting growth of cells that do not contain the
selectable
marker, or by positive selection, e.g., screening for the product encoded by
the selectable
marker. Many commonly used selectable markers for plant transformation are
well
known in the transformation art, and include, for example, nucleic acids that
code for
enzymes that metabolically detoxify a selective chemical agent which may be an
antibiotic or an herbicide, or nucleic acids that encode an altered target
which is
insensitive to the inhibitor. Positive selection methods are also known in the
art.
[000312] One commonly used selectable marker for plant transformation is a
neomycin
phosphotransferase H (nptII) coding sequence, for example, isolated from
transposon
Tn5, which when placed under the control of plant regulatory signals confers
resistance
to kanamycin. Fraley, et al., PNAS, 80:4803 (1983). Another commonly used
selectable
marker is hygromycin phosphotransferase, which confers resistance to the
antibiotic
hygromycin. Vanden Elzen, et al., Plant Mol. Biol., 5:299 (1985).
[000313] Additional selectable markers of bacterial origin that confer
resistance to
antibiotics include gentamycin acetyl transferase, streptomycin
phosphotransferase,
aminoglycoside-3'-adenyl transferase, the bleomycin resistance determinant.
Hayford, et
al., Plant Physiol., 86:1216 (1988); Jones, et al., Mol. Gen. Genet., 210:86
(1987); Svab,
et al., Plant Mol. Biol., 14:197 (1990); Hille, et al., Plant Mol. Biol.,
7:171 (1986). Other
selectable markers confer resistance to herbicides such as glyphosate,
glufosinate, or
bromox)mil. Comai, etal., Nature, 317:741-744 (1985); Gordon-Kamm, et al.,
Plant Cell,
2:603-618 (1990); and Stalker, et al., Science, 242:419-423 (1988).
[000314] Selectable markers for plant transfoiniation that are not of
bacterial origin include,
for example, mouse dihydrofolate reductase, plant 5-enolpyruvylshikimate-3-
phosphate
CA 02902250 2015-08-28
51682-49
synthase, and plant acetolactate synthase. Eichholtz, et al., Somatic Cell
Mol. Genet.,
13:67 (1987); Shah, et al., Science, 233:478 (1986); and Charest, et al.,
Plant Cell Rep.,
8:643 (1990).
[000315] Another class of selectable marker for plant transformation involves
screening of
presumptively transformed plant cells rather than direct genetic selection of
transformed
cells for resistance to a toxic substance such as an antibiotic. These
selectable markers are
particularly useful to quantify or visualize the spatial pattern of expression
of a transgene
in specific tissues and are frequently referred to as a reporter gene because
they can be
fused to transgene or regulatory sequence for the investigation of nucleic
acid expression.
Commonly used reporters for screening presumptively transformed cells include
alpha-
glucuronidase (GUS), alpha-galactosidase, luciferase and chloramphenicol,
acetyltransferase. Jefferson, R. A., Plant Mol. Biol., 5:387 (1987); Teen, et
al., EMBO J.,
8:343 (1989); Koncz, et al., PNAS, 84:131 (1987); and DeBlock, et al., EMBO
J., 3:1681
(1984).
[000316] In vivo methods for visualizing GUS activity that do not require
destruction of
plant tissues are available. Molecular Probes, Publication 2908, IMAGENE
GREEN, pp.
1-4 (1993) and Naleway, et al., J. Cell Biol., 115:151a (1991).
[000317] Green Fluorescent Protein (GFP) is also utilized as a marker for
nucleic acid
expression in prokaryotic and eukaryotic cells. Chalfie, et al., Science,
263:802 (1994).
GFP and mutants of GFP may be used as screenable markers.
[000318] Expression Vectors for Plant Transformation¨Promoters.
[000319] Transgenes included in expression vectors are generally driven by a
nucleotide
sequence comprising a regulatory element (for example, a promoter). Numerous
types of
promoters arc well known in the transformation arts, as are other regulatory
elements that
can be used alone or in combination with promoters.
81
CA 02902250 2015-08-28
51682-49
[000320] As used herein, "promoter" includes reference to a region of DNA
upstream from
the start of transcription and involved in recognition and binding of RNA
polymerase and
other proteins to initiate transcription. A "plant promoter" is a promoter
capable of
initiating transcription in plant cells.
[000321] Examples of promoters under developmental control include promoters
that
preferentially initiate transcription in certain tissues, such as leaves,
roots, seeds, fibers,
xylem vessels, tracheids, or sclerenchyma. Such promoters are referred to as
"tissue-
preferred." Promoters that initiate transcription only in certain tissue are
referred to as
"tissue-specific." A "cell type" specific promoter preferentially drives
expression in
certain cell types in one or more organs, for example, vascular cells in roots
or leaves.
An "inducible" promoter is a promoter that is under environmental control.
Examples of
environmental conditions that may affect transcription by inducible promoters
include
anaerobic conditions or the presence of light. Tissue-specific, tissue-
preferred, cell type
specific, and inducible promoters constitute the class of "non-constitutive"
promoters. A
"constitutive" promoter is a promoter that is active under most environmental
conditions.
[000322] A. Inducible Promoters:
[000323] An inducible promoter is operably linked to a nucleic acid for
expression in a
plant. Optionally, the inducible promoter is operably linked to a nucleotide
sequence
encoding a signal sequence which is operably linked to a nucleic acid for
expression in
the plant. With an inducible promoter, the rate of transcription increases in
response to an
inducing agent.
[000324] Any inducible promoter can be used in the instant invention. See
Ward, et al.,
Plant Mol. Biol., 22:361-366 (1993). Exemplary inducible promoters include,
but are not
limited to, that from the ACEI system which responds to copper (Melt, et al.,
PNAS,
90:4567-4571 (1993)); promoter from the In2 gene from maize which responds to
benzenesulfonamide herbicide safeners (Hershey, et al., Mol. Gen. Genet.,
227:229-237
(1991) and Gatz, et al., Mol. Gen. Genet., 243:32-38 (1994)) or Tet repressor
from Tn10
82
CA 02902250 2015-08-28
51682-49
(Gatz, et al., Mol. Gen. Genet., 227:229-237 (1991)). A representative
inducible promoter
is a promoter that responds to an inducing agent to which plants do not
normally respond.
An exemplary inducible promoter is the inducible promoter from a steroid
hormone gene,
the transcriptional activity of which is induced by a glucocorticosteroid
hormone. Schena,
et al., PNAS, 88:0421 (1991).
[000325] B. Constitutive Promoters:
[000326] A constitutive promoter is operably linked to a nucleic acid for
expression in a
plant or the constitutive promoter is operably linked to a nucleotide sequence
encoding a
signal sequence which is operably linked to a nucleic acid for expression in a
plant.
[000327] Many different constitutive promoters can be utilized in the instant
invention.
Exemplary constitutive promoters include, but are not limited to, the
promoters from
plant viruses such as the 35S promoter from CaMV (Odell, et al., Nature,
313:810-812
(1985)) and the promoters from such genes as rice actin (McElroy, et al.,
Plant Cell,
2:163-171 (1990)); ubiquitin (Christensen, et al., Plant Mol. Biol., 12:619-
632 (1989) and
Christensen, et al., Plant Mol. Biol., 18:675-689 (1992)); pEMU (Last, et al.,
Theor.
Appl. Genet., 81:581-588 (1991)); MAS (Velten, etal., EMBO J., 3:2723-2730
(1984))
and maize H3 histone (Lepetit, et al., Mol. Gen. Genet., 231:276-285 (1992)
and
Atanassova, et al., Plant J., 2 (3):291-300 (1992)). The ALS promoter,
XbaI/Ncol
fragment 5' to the Brassica napus ALS3 structural gene (or a nucleotide
sequence
similarity to said XbaI/NcoI fragment), represents a particularly useful
constitutive
promoter. See PCT Application No. WO 96/30530.
[000328] C. Tissue-Specific or Tissue-Preferred Promoters:
[000329] A tissue-specific promoter is operably linked to a nucleic acid for
expression in a
plant. Optionally, the tissue-specific promoter is operably linked to a
nucleotide sequence
encoding a signal sequence which is operably linked to a nucleic acid for
expression in a
plant. Plants transformed with a nucleic acid of interest operably linked to a
tissue-
83
CA 02902250 2015-08-28
51682-49
specific promoter transcribe the nucleic acid of interest exclusively, or
preferentially, in a
specific tissue.
[000330] Any tissue-specific or tissue-preferred promoter can be utilized in
the instant
invention. Exemplary tissue-specific or tissue-preferred promoters include,
but are not
limited to, a root-preferred promoter, such as that from the phaseolin gene
(Murai, et al.,
Science, 23:476-482 (1983) and Sengupta-Gopalan, et al., PNAS, 82:3320-3324
(1985));
a leaf-specific and light-induced promoter such as that from cab or rubisco
(Simpson, et
al., EMBO J., 4(11):2723-2729 (1985) and Timko, et al., Nature, 318:579-582
(1985));
an anther-specific promoter such as that from LAT52 (Twell, et al., Mol. Gen.
Genet.,
217:240-245 (1989)); a pollen-specific promoter such as that from Zml3
(Guerrero, et
al., Mol. Gen. Genet., 244:161-168 (1993)) or a microspore-preferred promoter
such as
that from apg (Twell, et al., Sex. Plant Reprod., 6:217-224 (1993)).
[000331] Signal Sequences for Targeting Proteins to Subcellular Compartments.
[000332] Transport of polypeptides produced by transgenes to a subcellular
compartment
such as the chloroplast, vacuole, peroxisome, glyoxysome, cell wall, or
mitochondrion, or
for secretion into the apoplast, is generally accomplished by means of
operably linking a
nucleotide sequence encoding a signal sequence to the 5' and/or 3' region of a
nucleic
acid encoding the polypeptide of interest. Signal sequences at the 5' and/or
3' end of the
coding sequence target the polypeptide to particular subcellular compartments.
[000333] The presence of a signal sequence can direct a polypeptide to either
an
intracellular organelle or subcellular compartment or for secretion to the
apoplast. Many
signal sequences are known in the art. See, for example, Becker, et al., Plant
Mol. Biol.,
20:49 (1992); Close, P. S., Master's Thesis, Iowa State University (1993);
Knox, C., et
al., "Structure and Organization of Two Divergent Alpha-Amylase Genes from
Barley,"
Plant Mol. Biol., 9:3-17 (1987); Lerner, et al., Plant Physiol., 91:124-129
(1989); Fontes,
et al., Plant Cell, 3:483-496 (1991); Matsuoka, et al., PNAS, 88:834 (1991);
Gould, et al.,
J. Cell. Biol., 108:1657 (1989); Creissen, et al., Plant J, 2:129 (1991);
Kalderon, et al., A
84
CA 02902250 2015-08-28
51682-49
short amino acid sequence able to specify nuclear location, Cell, 39:499-509
(1984); and
Steifel, et al., Expression of a maize cell wall hydroxyproline-rich
glycoprotein gene in
early leaf and root vascular differentiation, Plant Cell, 2:785-793 (1990).
[000334] Foreign Polypeptide Transgenes and Agronomic Transgenes.
[000335] With transgenic plants according to the present invention, a foreign
protein can be
produced in commercial quantities. Thus, techniques for the selection and
propagation of
transformed plants, which are well understood in the art, yield a plurality of
transgenic
plants which are harvested in a conventional manner, and a foreign polypeptide
then can
be extracted from a tissue of interest or from total biomass. Protein
extraction from plant
biomass can be accomplished by known methods which are discussed, for example,
by
Heney and On, Anal. Biochem., 114:92-6(1981).
[000336] According to a representative embodiment, the transgenic plant
provided for
commercial production of foreign protein is a pepper plant of the invention.
In another
embodiment, the biomass of interest is seed. For the relatively small number
of
transgenic plants that show higher levels of expression, a genetic map can be
generated,
for example via conventional RFLP, PCR, and SSR analysis, which identifies the
approximate chromosomal location of the integrated DNA molecule. For exemplary
methodologies in this regard, see Methods in Plant Molecular Biology and
Biotechnology, Glick and Thompson Eds., 269:284, CRC Press, Boca Raton (1993).
Map
information concerning chromosomal location is useful for proprietary
protection of a
subject transgenic plant. If unauthorized propagation is undertaken and
crosses made with
other germplasm, the map of the integration region can be compared to similar
maps for
suspect plants, to determine if the latter have a common parentage with the
subject plant.
Map comparisons can involve hybridizations, RFLP, PCR, SSR, and sequencing,
all of
which are conventional techniques.
[000337] Likewise, by means of the present invention, agronomic transgenes and
other
desired added traits can be expressed in transformed plants (and their
progeny, e.g.,
CA 02902250 2015-08-28
51682-49
produced by breeding methods). More particularly, plants can be genetically
engineered
to express various phenotypes of agronomic interest or other desired added
traits.
Exemplary nucleic acids of interest in this regard conferring a desired added
trait(s)
include, but are not limited to, those categorized below:
[000338] A. Transgenes that Confer Resistance to Pests or Disease:
[000339] 1. Plant disease resistance transgenes. Plant defenses are often
activated by
specific interaction between the product of a disease resistance gene (R) in
the plant and
the product of a corresponding avirulence (Avr) gene in the pathogen. A plant
line can be
transformed with a cloned resistance transgene to engineer plants that are
resistant to
specific pathogen strains. See, for example, Jones, et al., Science, 266:789
(1994)
(cloning of the tomato Cf-9 gene for resistance to Cladosporium fulvum);
Martin, et al.,
Science, 262:1432 (1993) (tomato Pto gene for resistance to Pseudomonas
syringae pv.
tomato encodes a protein kinase); and Mindrinos, et al., Cell, 78:1089 (1994)
(Arabidopsis RSP2 gene for resistance to Pseudomonas syringae).
[000340] 2. A Bacillus thuringiensis protein, a derivative thereof, or a
synthetic
polypeptide modeled thereon. See, for example, Geiser, et al., Gene, 48:109
(1986), who
disclose the cloning and nucleotide sequence of a Bt delta-endotoxin gene.
Moreover,
DNA molecules encoding delta-endotoxin transgenes can be purchased from
American
Type Culture Collection, Manassas, Va., for example, under ATCC Accession Nos.
40098, 67136, 31995, and 31998.
[000341] 3. A lectin. See, for example, the disclosure by Van Damme, et
al., Plant
Mol. Biol., 24:25 (1994), who disclose the nucleotide sequences of several
Clivia miniata
mannose-binding lectin transgenes.
[000342] 4. A vitamin-binding protein such as avidin. See, e.g., PCT
Application No.
US 93/06487. The application teaches the use of avidin and avidin homologues
as
larvicides against insect pests.
86
CA 02902250 2015-08-28
51682-49
[000343] 5. An enzyme inhibitor, for example, a protease or proteinase
inhibitor, or an
amylase inhibitor. See, for example, Abe, et al., J. Biol. Chem., 262:16793
(1987)
(nucleotide sequence of rice cysteine proteinase inhibitor); Huub, et al.,
Plant Mol. Biol.,
21:985 (1993) (nucleotide sequence of cDNA encoding tobacco proteinase
inhibitor I);
and Sumitani, et al., Biosci. Biotech. Biochem., 57:1243 (1993) (nucleotide
sequence of
Streptomyces nitrosporeus alpha-amylase inhibitor).
[000344] 6. An insect-specific hoinione or pheromone, such as an
ecdysteroid and
juvenile hormone, a variant thereof, a mimetic based thereon, or an antagonist
or agonist
thereof. See, for example, the disclosure by Hammock, et al., Nature, 344:458
(1990), of
baculovirus expression of cloned juvenile hormone esterase, an inactivator of
juvenile
hormone.
[000345] 7. An insect-specific peptide or neuropeptide which, upon
expression,
disrupts the physiology of the affected pest. For example, see the disclosures
of Regan, J.
Biol. Chem., 269:9 (1994) (expression cloning yields DNA coding for insect
diuretic
hormone receptor) and Pratt, et al., Biochem. Biophys. Res. Comm., 163:1243
(1989) (an
allostatin is identified in Diploptera puntata). See also, U.S. Pat. No.
5,266,317 to
Tomalski, et al., who disclose transgenes encoding insect-specific, paralytic
neurotoxins.
[000346] 8. An insect-specific venom produced in nature, by a snake, a
wasp, etc. For
example, see Pang, et al, Gene, 116:165 (1992), for disclosure of heterologous
expression
in plants of a transgene coding for a scorpion insectotoxic peptide.
[000347] 9. An enzyme responsible for a hyper-accumulation of a
monoterpene, a
sesquiterpene, a steroid, hydroxamic acid, a phenylpropanoid derivative, or
another non-
protein molecule with insecticidal activity.
[000348] 10. An enzyme involved in the modification, including the post-
translational
modification, of a biologically active molecule; for example, a glycolytic
enzyme, a
87
CA 02902250 2015-08-28
51682-49
proteolytic enzyme, a lipolytic enzyme, a nuclease, a cyclase, a transaminase,
an esterase,
a hydrolase, a phosphatase, a kinase, a phosphorylase, a polymerase, an
elastase, a
chitinase, and a glucanase, whether natural or synthetic. See PCT Application
No. WO
93/02197 in the name of Scott, et al., which discloses the nucleotide sequence
of a callase
transgene. DNA molecules which contain chitinase-encoding sequences can be
obtained,
for example, from the ATCC under Accession Nos. 39637 and 67152. See also,
Kramer,
et al., Insect Biochem. Mol. Biol., 23:691 (1993), who teach the nucleotide
sequence of a
cDNA encoding tobacco hornworm chitinase, and Kawalleck, et al., Plant Mol.
Biol.,
21:673 (1993), who provide the nucleotide sequence of the parsley ubi4-2
polyubiquitin
transgene.
[000349] 11. A molecule that stimulates signal transduction. For example,
see the
disclosure by Botella, et al., Plant Mol. Biol., 24:757 (1994), of nucleotide
sequences for
mung bean calmodulin cDNA clones, and Griess, et al., Plant Physiol., 104:1467
(1994),
who provide the nucleotide sequence of a maize calmodulin cDNA clone.
10003501 12. A hydrophobic moment peptide. See PCT Application No. WO
95/16776
(disclosure of peptide derivatives of tachyplesin which inhibit fungal plant
pathogens)
and PCT Application No. WO 95/18855 (teaches synthetic antimicrobial peptides
that
confer disease resistance).
10003511 13. A membrane permease, a channel former, or a channel blocker.
For
example, see the disclosure of Jaynes, et al., Plant Sci., 89:43 (1993), of
heterologous
expression of a cecropin-beta, lytic peptide analog to render transgenic
tobacco plants
resistant to Pseudomonas solanacearum.
10003521 14. A viral-invasive protein or a complex toxin derived therefrom.
For
example, the accumulation of viral coat proteins in transformed plant cells
imparts
resistance to viral infection and/or disease development effected by the virus
from which
the coat protein transgene is derived, as well as by related viruses. See
Beachy, et al.,
Ann. Rev. Phytopathol., 28:451 (1990). Coat protein-mediated resistance has
been
88
CA 02902250 2015-08-28
51682-49
conferred upon transformed plants against alfalfa mosaic virus, cucumber
mosaic virus,
tobacco streak virus, potato virus X, potato virus Y, tobacco etch virus,
tobacco rattle
virus, and tobacco mosaic virus. Id.
[000353] 15. An insect-specific antibody or an immunotoxin derived
therefrom. Thus,
an antibody targeted to a critical metabolic function in the insect gut would
inactivate an
affected enzyme, killing the insect. See Taylor, et al., Abstract #497,
Seventh Intl
Symposium on Molecular Plant-Microbe Interactions, Edinburgh, Scotland (1994)
(enzymatic inactivation in transgenic tobacco via production of single-chain
antibody
fragments).
[000354] 16. A virus-specific antibody. See, for example, Tavladoraki, et
al., Nature,
366:469 (1993), who show that transgenic plants expressing recombinant
antibody
transgenes are protected from virus attack.
[000355] 17. A developmental-arrestive protein produced in nature by a
pathogen or a
parasite. Thus, fungal endo-alpha-1,4-D-polygalacturonases facilitate fungal
colonization
and plant nutrient released by solubilizing plant cell wall homo-alpha-1,4-D-
galacturonase. See Lamb, et al., Bio/technology, 10:1436 (1992). The cloning
and
characterization of a transgene which encodes a bean endopolygalacturonase-
inhibiting
protein is described by Toubart, et al., Plant J., 2:367 (1992).
[000356] 18. A developmental-arrestive protein produced in nature by a
plant. For
example, Logemann, et al., Bio/technology, 10:305 (1992), have shown that
transgenic
plants expressing the barley ribosome-inactivating transgene have an increased
resistance
to fungal disease.
[000357] 19. A lettuce mosaic potyvirus (LMV) coat protein transgene
introduced into
Lactuca sativa in order to increase its resistance to LMV infection. See
Dinant, et al.,
Mol. Breeding, 3:1, 75-86 (1997).
89
CA 02902250 2015-08-28
51682-49
[0003581 Any disease or present resistance transgenes, including those
exemplified above,
can be introduced into a pepper plant of the invention through a variety of
means
including but not limited to transformation and breeding.
[000359] B. Transgenes that Confer Resistance to an Herbicide:
[000360] Exemplary polynucleotides encoding polypeptides that confer traits
desirable for
herbicide resistance include acetolactate synthase (ALS) mutants that lead to
herbicide
resistance such as the S4 and/or Hra mutations ((resistance to herbicides
including
sulfonylureas, imidazolinones, triazolopyrimidines, pyrimidinyl
thiobenzoates);
glyphosate resistance (e.g., 5-enol-pyrovyl-shikimate-3-phosphate-synthase
(EPSPS)
transgene, including but not limited to those described in U.S. Pat. Nos.
4,940,935,
5,188,642, 5,633,435, 6,566,587, 7,674,598 as well as all related application;
or the
glyphosate N-acetyltransferase (GAT) transgene, described in Castle et al.,
Science,
2004, 304:1151-1154; and in U.S. Patent Application Publication Nos.
20070004912,
20050246798, and 20050060767)); glufosinate resistance (e.g., BAR; see e.g.,
U.S. Pat.
No. 5,561,236); 2,4-D resistance (e.g., aryloxy alkanoate dioxygenase or AAD-
1, AAD-
12, or AAD-13), HPPD resistance (e.g., Pseudomonas HPPD) and PPO resistance
(e.g.,
fomesafen, acifluorfen-sodium, oxyfluorfen, lactofen, fluthiacet-methyl,
saflufenacil,
flumioxazin, flumiclorac-pentyl, carfentrazone-ethyl, sulfentrazone,); a
cytochrome P450
or variant thereof that confers herbicide resistance or tolerance to, inter
alia, HPPD-
inhibiting herbicides, PPO-inhibiting herbicides and ALS-inhibiting herbicides
(U.S.
Patent Application Publication No. 20090011936; U.S. Pat. Nos. 6,380,465;
6,121,512;
5,349,127; 6,649,814; and 6,300,544; and PCT International Publication No. WO
2007/000077); dicamba resistance (e.g., dicamba monoxygenase), and traits
desirable for
processing or process products such as high oil (e.g., U.S. Pat. No.
6,232.529); modified
oils (e.g., fatty acid desaturase transgenes (U.S. Pat. No. 5,952.544; PCT
International
Publication No. WO 94/11516)); modified starches (e.g., ADPG
pyrophosphorylases
(AGPase), starch synthases (SS), starch branching enzymes (SBE), and starch
debranching enzymes (SDBE)); and polymers or bioplastics (e.g., U.S. Pat. No.
5,602,321; beta-ketothiolase, polyhydroxybutyrate synthase, and acetoacetyl-
CoA
CA 02902250 2015-08-28
51682-49
rcductase (Schubert et al., J. Bacteriol., 1988, 170:5837-5847) facilitate
expression of
polyhydroxyalkanoates (PHAs)).
[000361] In embodiments, the polynucleotide encodes a polypcptide conferring
resistance
to an herbicide selected from glyphosate, sulfonylurea, imidazolinone,
dicamba,
glufosinate, phenoxy proprionic acid, L-phosphinothricin, cyclohexone,
cyclohexanedione, triazine, and benzonitrile.
[000362] Any transgene conferring herbicide resistance, including those
exemplified above,
can be introduced into the pepper plants of the invention through a variety of
means
including, but not limited to, transfoimation (e.g., genetic engineering
techniques) and
crossing.
[000363] C. Transgenes that Confer or Contribute to a Value-Added Trait:
[000364] 1. Increased iron content of the pepper, for example, by introducing
into a plant a
soybean ferritin transgene as described in Goto, et al., Acta Horticulturae.,
521, 101-109
(2000).
[000365] 2. Decreased nitrate content of leaves, for example, by introducing
into a pepper
a transgene coding for a nitrate reductase. See, for example, Curtis, et al.,
Plant Cell
Rep., 18:11, 889 896 (1999).
[000366] 3. Increased sweetness of the pepper by introducing a transgene
coding for
monellin that elicits a flavor 100,000 times sweeter than sugar on a molar
basis. See
Penarrubia, et al., Bio/technology, 10:561-564 (1992).
[000367] 4. Modified fatty acid metabolism, for example, by introducing
into a plant
an antisense sequence directed against stearyl-ACP desaturase to increase
stearic acid
content of the plant. See Knultzon, et al., PNAS, 89:2625 (1992).
91
CA 02902250 2015-08-28
51682-49
[000368] 5. Modified carbohydrate composition effected, for example, by
introducing
into plants a transgene coding for an enzyme that alters the branching pattern
of starch.
See Shiroza, et al., J. Bacteria, 170:810 (1988) (nucleotide sequence of
Streptococcus
mutants fructosyltransferase transgene); Steinmetz, et al., Mol. Gen. Genet.,
20:220
(1985) (nucleotide sequence of Bacillus subtilis levansucrase transgene); Pen,
et al.,
Bio/technology, 10:292 (1992) (production of transgenic plants that express
Bacillus
lichenifonnis alpha-amylase); Elliot, et al., Plant Mol. Biol., 21:515 (1993)
(nucleotide
sequences of tomato invertase transgenes); Sogaard, et al., J. Biol. Chem.,
268:22480
(1993) (site-directed mutagenesis of barley alpha-amylase transgene); and
Fisher, et al.,
Plant Physiol., 102:1045 (1993) (maize endosperm starch branching enzyme II).
[000369] 6. Delayed and/or attenuated symptoms to Bean Golden Mosaic
Geminivirus
(BGMV), for example by transforming a plant with antisense genes from the
Brazilian
BGMV. See Arago et al., Molecular Breeding. 1998, 4: 6, 491-499.
[000370] 7. Increased methionine content by introducing a transgene coding for
a
methionine-rich storage albumin (2S-albumin) from the Brazil nut, e.g., as
described in
Arago et al., Genetics and Molecular Biology. 1999, 22: 3, 445-449.
[000371] Any transgene that confers or contributes a value-added trait,
including those
exemplified above, can be introduced into the pepper plants of the invention
through a
variety of means including, but not limited to, transformation (e.g., genetic
engineering
techniques) and crossing.
[000372] D. Transgenes that Control Male-Sterility:
[000373] I. Introduction of a deacetylase transgene under the control of a
tapetum-
specific promoter and with the application of the chemical N-Ac-PPT. See,
e.g.,
International Publication WO 01/29237.
92
CA 02902250 2015-08-28
51682-49
[000374] 2. Introduction of various stamen-specific promoters. See, e.g.,
International
Publications WO 92/13956 and WO 92/13957.
[000375] 3. Introduction of the bamase and the barstar transgenes. See,
e.g., Paul, et
al., Plant Mol. Biol., 19:611-622 (1992).
[000376] Any transgene that controls male sterility, including those
exemplified above, can
be introduced into the pepper plants of the invention through a variety of
means
including, but not limited to, transformation (e.g., genetic engineering
techniques) and
crossing.
[000377] Those skilled in the art will appreciate that any of the traits
described above with
respect to plant transformation methods can be introduced into a plant of the
invention
(e.g., pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822, RPP 26098 or
RPP
26105 and hybrid pepper plants and other pepper plants derived therefrom)
using
breeding techniques.
[000378] Methods for Plant Transformation.
[000379] Numerous methods for plant transformation have been developed,
including
biological and physical, plant transformation protocols. See, for example,
Miki, et al.,
"Procedures for Introducing Foreign DNA into Plants" in Methods in Plant
Molecular
Biology and Biotechnology, Glick and Thompson Eds., CRC Press, Inc., Boca
Raton, pp.
67-88 (1993). In addition, expression vectors and in vitro culture methods for
plant cell or
tissue transformation and regeneration of plants are available. See, for
example, Gruber,
et al., "Vectors for Plant Transformation" in Methods in Plant Molecular
Biology and
Biotechnology, Glick and Thompson Eds., CRC Press, Inc., Boca Raton, pp. 89-
119
(1993).
93
CA 02902250 2015-08-28
51682-49
[000380] A. .. Agrobacterium-Mediated Transfoimation.
[000381] One method for introducing an expression vector into plants is based
on the
natural transformation system of Agrobacterium. See, for example, Horsch, et
al.,
Science, 227:1229 (1985); Curtis, et al., Journal of Experimental Botany,
45:279, 1441-
1449 (1994); Torres, et al., Plant Cell Tissue and Organ Culture, 34:3, 279-
285 (1993);
and Dinant, et al., Molecular Breeding, 3:1, 75-86 (1997). A. tumefaciens and
A.
rhizogenes are plant pathogenic soil bacteria which genetically transform
plant cells. The
Ti and Ri plasmids of A. tumefaciens and A. rhizogenes, respectively, carry
genes
responsible for genetic transformation of the plant. See, for example, Kado,
C. I., Crit.
Rev. Plant Sci., 10:1 (1991). Descriptions of Agrobacterium vector systems and
methods
for Agrobacterium-mediated transgene transfer are provided by Gruber, et al.,
supra,
Miki, et al., supra, and Moloney, et al., Plant Cell Rep., 8:238 (1989). See
also, U.S. Pat.
No. 5,591,616 issued Jan. 7,1997.
[000382] B. Direct Transgene Transfer.
[000383] Several methods of plant transformation collectively referred to as
direct
transgene transfer have been developed as an alternative to Agrobacterium-
mediated
transformation. A generally applicable method of plant transformation is
microprojectile-
mediated transformation wherein DNA is carried on the surface of
microprojectiles
measuring 1 micron to 4 micron. The expression vector is introduced into plant
tissues
with a biolistic device that accelerates the microprojectiles to speeds of 300
m/s to 600
m/s which is sufficient to penetrate plant cell walls and membranes. Russell,
D. R., et al.,
Plant Cell Rep., 12 (3, Jan.), 165-169 (1993); Aragao, F. J. L., et al., Plant
Mol. Biol., 20
(2, Oct.), 357-359 (1992); Aragao, F. J. L., et al., Plant Cell Rep., 12 (9,
July), 483-490
(1993); Aragao, Theor. Appl. Genet., 93:142-150 (1996); Kim, J., Minamikawa,
T., Plant
Sci., 117:131-138 (1996); Sanford, et al., Part. Sci. Technol., 5:27 (1987);
Sanford, J. C.,
Trends Biotech., 6:299 (1988); Klein, et al., Bio/technology, 6:559-563
(1988); Sanford,
J. C., Physiol. Plant, 7:206 (1990); Klein, et al., Bio/technology, 10:268
(1992).
94
CA 02902250 2015-08-28
51682-49
[000384] Another method for physical delivery of DNA to plants is sonication
of target
cells. Zhang, et al., Bio/technology, 9:996 (1991). Alternatively, liposome
and
spheroplast fusion have been used to introduce expression vectors into plants.
Deshayes,
et al., EMBO J., 4:2731 (1985) and Christou, et al., PNAS, 84:3962 (1987).
Direct uptake
of DNA into protoplasts using CaCl<sub>2</sub> precipitation, polyvinyl alcohol, or
poly-L-
ornithine has also been reported. Hain, et al., Mol. Gen. Genet., 199:161
(1985) and
Draper, et al., Plant Cell Physiol., 23:451 (1982). Electroporation of
protoplasts and
whole cells and tissues have also been described. Saker, M., Kuhne, T.,
Biologia
Plantarum, 40(4):507-514 (1997/98); Donn, et al., In Abstracts of VIlth
International
Congress on Plant Cell and Tissue Culture IAPTC, A2-38, p. 53 (1990);
D'Halluin, etal.,
Plant Cell, 4:1495-1505 (1992); and Spencer, et al., Plant Mol. Biol., 24:51-
61 (1994).
See also Chupean, et al., Bio/technology, 7:5, 503-508 (1989).
[000385] Following transformation of plant target tissues, expression of the
above-
described selectable marker transgenes allows for preferential selection of
transformed
cells, tissues and/or plants, using regeneration and selection methods now
well known in
the art.
[000386] The foregoing methods for transformation would typically be used for
producing
a transgenic pepper line. The transgenic pepper line could then be crossed
with another
(non-transformed or transformed) line in order to produce a new transgenic
pepper line.
Alternatively, a genetic trait that has been engineered into a particular
plant cultivar using
the foregoing transformation techniques could be introduced into another line
using
traditional breeding (e.g., backcrossing) techniques that are well known in
the plant
breeding arts. For example, a backcrossing approach could be used to move an
engineered trait from a public, non-elite inbred line into an elite inbred
line, or from an
inbred line containing a foreign transgene in its genome into an inbred line
or lines which
do not contain that transgene.
[000387] Gene Conversions.
CA 02902250 2015-08-28
51682-49
[000388] When the term "pepper plant" is used in the context of the present
invention, this
term also includes any gene conversions of that plant or variety. The term
''gene
converted plant" as used herein refers to those pepper plants that are
developed, for
example, by backcrossing, genetic engineering and/or mutation, wherein
essentially all of
the desired morphological and physiological characteristics of a variety
(e.g., as described
herein, in particular, in any one or more of Tables 1-9 or Figures 1-30) are
recovered in
addition to the one or more genes transferred into the variety. To illustrate,
backcrossing
methods can be used with the present invention to improve or introduce a
characteristic
into the variety. The term ''backcrossing" as used herein refers to the
repeated crossing of
a hybrid progeny back to the recurrent parent, e.g., backcrossing 1, 2, 3, 4,
5, 6, 7, 8, 9, or
more times to the recurrent parent. The parental plant that contributes the
gene for the
desired characteristic is termed the "nonrecurrent" or ''donor parent." This
terminology
refers to the fact that the nonrecurrent parent is generally used one time in
the breeding
e.g., backcross) protocol and therefore does not recur. The gene that is
transferred can be
a native gene, a mutated native gene or a transgene introduced by genetic
engineering
techniques into the plant (or ancestor thereof). The parental plant into which
the gene(s)
from the nonrecurrent parent are transferred is known as the "recurrent"
parent as it is
used for multiple rounds in the backcrossing protocol. Poehlman & Sleper
(1994) and
Fehr (1993). In a typical backcross protocol, the original variety of interest
(recurrent
parent) is crossed to a second variety (nonrecurrent parent) that carries the
gene(s) of
interest to be transferred. The resulting progeny from this cross are then
crossed again to
the recurrent parent and the process is repeated until a plant is obtained
wherein
essentially all of the desired morphological and physiological characteristics
of the
recurrent parent are recovered in the converted plant in addition to the
transferred gene(s)
and associated trait(s) from the nonrecurrent parent.
[000389] Many gene traits have been identified that can be improved by
backcrossing
techniques. Gene traits may or may not be transgenic. Examples of these traits
include,
but are not limited to, male sterility, modified fatty acid metabolism,
modified
carbohydrate metabolism, herbicide resistance, pest or disease resistance
(e.g., resistance
to bacterial, fungal, or viral disease such as resistance to Xanthomonas
campestris pv.
96
CA 02902250 2015-08-28
51682-49
Vesicatoria [e.g., race 6]), insect resistance, enhanced nutritional quality,
increased
sweetness, increased flavor, improved ripening control, improved salt
tolerance,
industrial usage, yield stability, and yield enhancement. These genes are
generally
inherited through the nucleus.
[000390] Tissue Culture.
[000391] Further reproduction of pepper plants variety can occur by tissue
culture and
regeneration. Tissue culture of various tissues of pepper and regeneration of
plants
therefrom is well known and widely published. For example, reference may be
had to
Teng, et al., HortScience, 27:9, 1030-1032 (1992); Teng, et al., HortScience,
28:6, 669-
1671 (1993); Zhang, et al., Journal of Genetics and Breeding, 46:3, 287-290
(1992);
Webb, et al., Plant Cell Tissue and Organ Culture, 38:1, 77-79 (1994); Curtis,
et al.,
Journal of Experimental Botany, 45:279, 1441-1449 (1994); Nagata, et al.,
Journal for the
American Society for Horticultural Science, 125:6, 669-672 (2000); and
Ibrahim, et al.,
Plant Cell Tissue and Organ Culture, 28(2), 139-145 (1992). It is clear from
the literature
that the state of the art is such that these methods of obtaining plants are
routinely used
and have a very high rate of success. Thus, another aspect of this invention
is to provide
cells which upon growth and differentiation produce pepper plants having
desired
characteristics of pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822,
RPP
26098 or RPP 26105 (e.g., as described herein, in particular, in any one or
more of Tables
1-9 or Figures 1-30). Optionally, pepper plants can be regenerated from the
tissue culture
of the invention comprising all or essentially all of the physiological and
morphological
characteristics of pepper cultivar 8728C, 16452A, 16452B, 16452C, RPP 25822,
RPP
26098 or RPP 26105.
[000392] As used herein, the term "tissue culture" indicates a composition
comprising
isolated cells of the same or a different type or a collection of such cells
organized into
parts of a plant. Exemplary types of tissue cultures are protoplasts, calli,
meristematic
cells, and plant cells that can generate tissue culture that are intact in
plants or parts of
plants, such as leaves, pollen, embryos, roots, root tips, anthers, pistils,
flowers, seeds,
97
CA 02902250 2015-08-28
51682-49
petioles, suckers, fruits and the like. Means for preparing and maintaining
plant tissue
culture are well known in the art. By way of example, a tissue culture
comprising organs
has been used to produce regenerated plants. U.S. Pat. Nos. 5,959,185,
5,973,234, and
5,977,445 describe certain techniques.
[000393] DEPOSIT INFORMATION
[000394] Applicants have made a deposit of at least 2500 seeds of pepper
cultivars RPP
25822, RPP 26098, RPP 26105, 16452A, 16452B and 16452C with the American Type
Culture Collection (ATCC), 10801 University Boulevard, Manassas, Va., 20110-
2209
U.S.A. on January 30, 2015 under ATCC Deposit Nos. PTA-121983, PTA-121984, PTA-
121982, PTA-121980, PTA-121981 and PTA-121985, respectively.
[000395] In addition, a seed deposit of Capsicum annuum 8728C was made with
the
NCIMB, Ferguson Building, Craibstone Estate, Bucksburn, Aberdeen AB21 9YA,
Scotland, UK on July 29th, 2011 under the provisions of the Budapest Treaty
under
NCIMB Deposit No. 41858.
[000396] The foregoing invention has been described in detail by way of
illustration and
example for purposes of clarity and understanding. However, it will be
apparent that
certain changes and modifications such as single gene modifications and
mutations,
somaclonal variants, variant individuals selected from large populations of
the plants of
the instant inbred and the like may be practiced within the scope of the
invention.
[000397] EXAMPLE 1
Identification of Extreme Dark Green Color Trait
[000398] GERMPLASM AND POPULATION DEVELOPMENT
[000399] A breeding strategy was designed to identify the extreme dark green
fruit color
from pungent ancho chile peppers into sweet blocky peppers. An initial hybrid
cross was
made by crossing aneho 'San Luis' (extreme dark green source) with a blocky
breeding
98
CA 02902250 2015-08-28
51682-49
line. The Fl from this cross was used to generate a segregating F2 population.
F2
Individuals with the extreme dark green fruit color and blocky shape were
selected based
on visual appearance, followed by selfing and conventional pedigree selection
for the
extreme dark green color trait for seven generations, to create stable fixed
inbred lines.
Stable extreme dark green inbred lines were testcrossed to one another to
develop
extreme dark green hybrid varieties. Isolation of the genetic factors
responsible for
extreme dark green was simultaneously pursued following a quantitative trait
loci
discovery approach. A mapping population was generated by crossing an extreme
dark
green inbred with a non-extreme dark green inbred. The resulting hybrid was
used to
generate a dihaploid population via anther culture. The segregating dihaploid
population
was phenotyped for the extreme dark green color trait using a categorical
scale to classify
lines as extreme dark green or non-extreme dark green, and using colorimetry
to obtain
quantitative and objective color data for each line (see below material &
methods) as well
as using pigments content measurement. The categorical scale was based on
visually
matching fruit with color reference cards labeled from I (light) to 9 (dark),
where 9
represented the extreme dark green phenotype.
[000400] An extreme dark green pepper plant Capsicum annuum, 8728C, produces
fruit
which at immature harvestable stage exhibit the extreme dark green color
trait.
[000401] Plant 8728C was crossed with inbred line 16452, which also produces
extreme
dark green fruits, to produce hybrid RPP 25965. Hybrid RPP 25964 also produces
extreme dark green pepper fruits.
10004021 Further characterization and definition of the trait was accomplished
by screening
a panel of geimplasm ranging in phenotype from regular green to extreme dark
green.
[000403] To this end, several green pepper varieties available on the market
were used and
evaluated by visual ranking with color reference, but also by colorimetry
measurement
and by measuring the content of select pigments.
99
CA 02902250 2015-08-28
51682-49
[000404] The pepper varieties available on the market that were tested were:
Crusader
(Rogers), Encore (Rogers), Declaration (Harris Moran), Aristotle (Harris
Seeds), Karisma
(Harris Moran), Hunter (Rogers), Tomcat (Rogers), 8302 (Seminis), 7141
(Seminis) and
1819 (Seminis).
[000405] It has been determined that the color measurement by objective means
with a
colorimeter correlates with chlorophyll A&B, lutein and violaxanthin content.
That is to
say that the deeper the color is, the higher the content of the pepper fruits
in those
pigments.
[000406] Table 2 shows the correlation between colorimetry values and pigment
content of
the pepper fruits analyzed according to the present invention.
[000407] This clearly shows that the pigment content of the pepper fruits
according to the
present invention bearing the extreme dark green color trait relates to dark
green color as
evaluated by either subjective eye-visual perception or objective coloiimetry.
[000408] Table 2 also shows the correlation between pigment contents and
colorimeter
values measured for pepper fruits of pepper plants according to the present
invention
grown in different areas, i.e. Gilroy (California, USA), Naples (Florida, USA)
and El
Ejido (Spain).
[000409] From Table 2 it appears that the values of the different measured
parameters are
not affected by environmental conditions and thus shows that the phenotype of
extreme
dark green color, either evaluated by colorimeter measurement or by pigments
contents is
controlled by genetic determinants contained in the genome of the said plants.
[000410] PHENOTYPING
[000411] Fruit color and metabolite (pigment) measurements of a germplasm
panel
representing pepper varieties with and without the extreme dark green color
trait.
100
CA 02902250 2015-08-28
51682-49
[000412] Thirty six C. annuum varieties comprised of inbred lines and Fl
hybrids were
grown in summer 2010 in Gilroy, California under standard field conditions, in
autumn
2010/winter 2011 in El Ejido, Spain under standard passive greenhouse
conditions, and in
spring 2011 in Naples, Florida under standard field conditions. This gennplasm
panel
was assembled to allow direct comparisons of pepper geimplasm with and without
the
extreme dark green color trait and demonstrate that pepper plants with the
extreme dark
green color trait are darker/more intense in green color with higher fruit
pigment levels.
Multiple trial locations were used to demonstrate the behavior of the trait
across
environments and growing conditions.
[000413] A randomized and replicated block design was used for laying out
experimental
plots, and a plot was treated as the experimental unit, with 5 random fruit
selected to
represent the plot.
[000414] Samples from the 5 fruit were pooled for pigment measurements, and
color data
for the 5 fruit were summarized to provide a representative data set for the
variety and
replicate.
[000415] Materials and Methods
[0004161 Environmental conditions, including light source, object size, color
background,
and angle of vision or illumination can affect how a colored object appears to
a human
observer. In order to overcome this subjectivity, color measurement systems
and
instruments have been developed for quantifying colors and expressing colors
in terms of
three variables which completely and uniquely describe the color. A complete
discussion
of color measurement theory can be found in: Berns, R. 2000. Billmeyer and
Saltzman's
principles of color technology. Wiley. Malacara, D. 2002. Color vision and
colorimetry
theory and applications. SPIE Press.
101
CA 02902250 2015-08-28
51682-49
[000417] To measure fruit color, two experimental methodologies were used,
colorimetry/
spectrophotometry, and image analysis via a computer vision system. To measure
fruit
pigment contents, reversed phase liquid chromatography and UV DAD detection
were
used.
[000418] Colorimetry/spectrophotometry
10004191 A Konica Minolta colorimeter model CR-400 was used in California and
Florida
to generate color data on pepper fruits, using the CIELAB L*a*b* color space,
C
illuminant, and 2 degree angle of observer. The colorimeter was operated using
the 8mm
aperture measuring head and Spectramagic NX software version 1.9. A Konica
Minolta
hand-held spectrophotometer model CM-2500d with 8mm measurement aperture was
used in Spain to generate color data on pepper fruits, using the CIELAB L*a*b*
color
space, C and D65 illuminant, and 10 degree angle of observer. The
spectrophotometer
was operated using Spectramagic NX software version 3.6. In all three
locations, the
instrument was calibrated prior to and during use according to the
manufacturer's
instructions. Fruit samples were harvested from each plot at immature (e.g.
unripe, fully
physiologically developed) stage and brought to the lab for further
processing. The
surface of each fruit was cleaned by gently wiping with a damp paper towel to
remove
any dirt or debris. The measurement aperture was then held in tight connection
against
the surface of the fruit, to ensure no infiltration of external sources of
light, and three
measurements were taken, moving the aperture to three random representative
spots on
the fruit surface between measurements.
[000420] The CIELAB color scale is used widely by the food industry to measure
color and
demonstrate differences in color. The scale includes 3 data variables, L*, a*,
and b*. L*
indicates darkness/lightness on a 0 to 100 scale, where 0 is black and 100 is
white. The
variables a* and b* indicate the amount of red, green, blue, and yellow color:
+a* is the
red direction, -a* is the green direction, +b* is the yellow direction, and
¨b* is the blue
direction. Differences in color between two samples can be expressed in terms
of the
change in L*, a*, and/or b*.
102
CA 02902250 2015-08-28
51682-49
[000421] Image analysis
[000422] Computer vision systems (CVS) can be used to measure the whole
distribution of
color on a fruit surface, by translating the RGB pixel colors of a digital
sample image into
CIELAB L*a*b* values. Algorithms used by the image analysis software correct
for the
cameras' own color characteristics, fruit glossiness and fruit curvature, and
calibrate the
system.
[000423] CVS systems were used in California, Florida, and Spain to generate
color data on
pepper fruits. The systems consisted of the following hardware and software
components:
= Camera, Canon EOS Digital Rebel XT (Florida and California); EOS 450D
(Spain)
= Standardized lighting system, Westcott SpiderliteTD5 Location Kit with
Westcott
27W/110V Daylt FLOU lamps
= X-Rite Digital Color Checker SG card for color calibration
= Lastolite EZY grey balance card, for white balance calibration
= Photoflex medium LiteRoom table top shooting white tent
= Flocked paper (Savage) and velvet fabric photographic background
= Image version (1.45)
= Color transformer plug-in for ImageJ; Macro programs to automate image
processing
[000424] Photography was conducted in a room with no windows to ensure that
the lighting
system was the only source of illumination. Lamps were placed at equal
distances from
the sample platform and equal heights from the floor to center of the back of
the light
head. Lamp heads were angled at 45 degrees to the sample platfon-n. Prior to
taking
photographs, lamps were turned on and allowed to warm up for at least 10
minutes. The
103
CA 02902250 2015-08-28
51682-49
background material was placed on the sample platfonn and the white tent
placed on top
of the background, with zipper opening facing out. The camera was mounted on a
stand
with the lens pointing down and positioned over the tent, at a fixed distance
from the top
of the table.
[0004251 The camera was manually calibrated for white balance (uniformity of
illumination
within the photo field) and color at the beginning of each sample evaluation
day. The
same fruit samples used for collection of color data via
colorimeter/spectrophotometer
were used for the CVS and were placed inside the tent on the background.
Fruits were
spaced evenly in the photo field, not touching each other and not in the
shadows of one
another, and the tent was closed. The photographs were taken through an
opening in the
top of the tent.
[0004261 Photographs were processed using the ImageJ software program, version
1.95,
and a proprietary macro developed for automation of the processing. Pixels in
the digital
photographs were identified by the software as either background or sample,
and the
RGB color values of each sample pixel were translated by the software into
CIELAB
L*a*b* color values using a color calibration algorithm. The macro then
calculated
summary statistics for all the color values of the sample pixels in each
photograph of fruit
samples.
[0004271 Reversed phase liquid chromatography and UV DAD detection
The same fruit sample used for color analysis via
colorimetry/spectrophotometry and image
analysis was further analyzed for pigment content. Each of the five fruits
comprising a sample
was cut into pieces, removing and discarding the peduncle, seeds, and
placental tissue, leaving
only the pericarp. Pericarp pieces were combined in a blender (Waring model
61BL30) and
processed to form a homogenous puree. An antifoaming agent (Silicone Anti-foam
SAG 1572),
p,1_, to 100 g pepper, was added to the blended puree to prevent splitting
into aqueous and
foam layers and to keep the pepper puree homogeneous during subsampling.1.5 mL
aliquots of
the homogenized pepper pericarp were sub-sampled into 3.5 mL cryo vials, which
were flash-
104
CA 02902250 2015-08-28
51682-49
frozen in liquid nitrogen and stored at -80 C until analysis. Frozen puree
samples were freeze
dried and milled, then extracted twice with tert-buthyl-methyl-ether (TBME)
and twice with
methanol. Extracts were analyzed and separated into pigment components using
an Agilent 1100
HPLC with binary pump and YMC C30 column. The injection volume was 10 1.1L
(standard
injection) and eluent flow was 0.5 mL/minute. Detection was via UV DAD.
10004281 Data processing
[0004291 Chromatograms were processed using Agilent Chemstatione software, to
integrate and identify peaks. UV spectra were checked for each peak against
that of
library spectra before the identification was accepted. For quantitation the
response factor
of the calibrated reference compounds was calculated and used for the non-
calibrated
peaks.
SEQUENCES
SEQ ID NO: 1
5' AGATATTCCCTCCCTCTTCATTATTCCT 3'
SEQ ID NO: 2
5' GAGGCTGCACGAACAGATCA 3'
SEQ ID NO: 3
5' GTGAAGGAAGCGTGATGAATGG 3'
SEQ ID NO: 4
5' CCTAACAGCACTTCAGGTGCAA 3'
105
CA 02902250 2015-08-28
51682-49
SEQ ID NO: 5
5' ACGAGGATGCAACTGACTCAAAA 3'
SEQ ID NO: 6
5' CCCAAGTCACTAGGTTGTTGATTCT 3'
SEQ ID NO: 7
5' TCTTATTGGAGCAAAGAATAACTGGGTTAT 3'
SEQ ID NO: 8
5' TGCACTCTATGTGTTTGATATTTTGTCTCA 3'
SEQ ID NO: 9
5' CTGGAGTTACCAGTTTATA 3'
SEQ ID NO: 10
5' TAGTACGGTGTGCCAACAA 3'
SEQ ID NO: 11
5' ATGATGCGAATGGTCA 3'
SEQ ID NO: 12
5' TGTAGCTTCAATCTATTTGTTC 3'
106
CA 02902250 2015-08-28
51682-49
[000430] EXAMPLE 2
Genotyping and OTL Discovery
[000431] A bi-parental population of 188 fixed lines was developed for the
purpose of QTL
mapping. The population was genotyped with a set of genome wide markers. The
QTL
analysis following standard practice was done with QTL Cartographer software.
Raw
phenotypic data was used in the analysis.
[000432] Two QTL (QTL1 and QTL2) were identified at <0.01% significance with
markers, SP436 & SP626 on the one hand and SP693 & SP694 on the other hand,
showing the highest association (linkage) with QTL1 and QTL2 respectively.
QTL 1
Marker locus SP 436
Forward primer: 5' AGATATTCCCTCCCTCTTCATTATTCCT 3' (SEQ ID NO: 1)
Reverse primer: 5' GAGGCTGCACGAACAGATCA 3' (SEQ ID NO: 2)
Extreme dark green allele specific probe:
5' CTGGAGTTACCAGTTTATA 3' (SEQ ID NO 9)
Probe sequence was labelled with FM at the 5' end and with MGB-NFQ at the 3'
end
Marker locus SP626
Forward primer: 5' GTGAAGGAAGCGTGATGAATGG 3' (SEQ ID NO: 3)
Reverse primer: 5' CCTAACAGCACTTCAGGTGCAA 3' (SEQ ID NO: 4)
Extreme dark green allele specific probe:
5' TAGTACGGTGTGCCAACAA 3' (SEQ ID NO10).
Probe sequence was labelled with VC at the 5' end and with MGB-NFQ at the 3'
end.
107
CA 02902250 2015-08-28
51682-49
QTL2
Marker locus SP693
Forward primer: 5' ACGAGGATGCAACTGACTCAAAA 3' (SEQ ID NO: 5)
Reverse primer: 5' CCCAAGTCACTAGGTTGTTGATTCT 3' (SEQ ID NO: 6)
Extreme dark green allele specific probe:
5' ATGATGCGAATGGTCA 3' (SEQ ID NO 11)
Probe sequence was labelled with VC at the 5' end and with MGB-NFQ at the 3'
end.
Marker locus SP694
Forward primer: 5' TCTTATTGGAGCAAAGAATAACTGGGTTAT 3' (SEQ ID NO: 7)
Reverse primer: 5' TGCACTCTATGTGTTTGATATTTTGTCTCA 3' (SEQ ID NO: 8)
Extreme dark green allele specific probe:
5' TGTAGCTTCAATCTATTTGTTC 3' (SEQ ID NO 12)
Probe sequence was labelled with VC at the 5' end and with MGB-NFQ at the 3'
end.
10004331 Table 3 shows the content in violaxanthin, Chlorophyll A and
Chlorophyll B,
lutein and beta-carotene for the various pepper plants chosen for the trials
as well as plant
8728C and hybrid RPP25965. This table also shows the different colorimeter
values of
those plants. It clearly shows that the plant according to the inventions are
different from
existing blocky pepper varieties available on the market and do clearly
differentiate from
those.
[000434] Table 4 also shows a QTL validation of the extreme dark green color
trait
according to the present invention. Various individuals, including doubled
haploid plant
derived from the population generated for QTL discovery, with different
genetic profiles,
108
CA 02902250 2015-08-28
51682-49
either none of the QTL according to the present invention, only one, or both
of QTL1 and
QTL2 of the present invention.
[000435] Results of Table 4 clearly show the contribution of both QTL1 and
QTL2 to the
pigment content. It appears that both QTL lead to the increased content of
violaxanthin,
lutein, chlorophyll A and Chlorophyll B.
109
CA 02902250 2015-08-28
=
51682-49
TABLE 2
Viola Chloro Chloro b-caro
xanthin - Lutein - phyll A - phyll B - tene - L*(C) - a*(C) - b*(C) -
Gilroy Gilroy Gilroy Gilroy Gilroy Gilroy
Gilroy Gilroy
Violaxanthin - Gilroy 1
Lutein - Gilroy 0.95 1
Chlorophyll A - Gilroy 0.96 0.99 1
Chlorophyll B- Gilroy 0.96 1.00 1.00 1
b-carotene - Gilroy 0.97 0.97 0.99 0.98 1
I(C) - Gilroy -0.79 -0.80 -0.76 -0.79 -0.73 1
a*(C) - Gilroy 0.75 0.87 0.82 0.85 0.77 -0,76 1
b*(C) - Gilroy -0.74 -0.86 -0.80 -0.83 -0.76 0.77 -
0.99 " 1
Violaxanthin - El Ejido 0.93 0.93 0.94 0.94 0.93 -0.76
0.75 -0.72
Lutein - El Ejido 0.90 0,95 0.93 0.94 0.90 -0.84 0.85
-0.84
Chlorophyll A - El Ejido 0.92 0.96 0.95 0.96 0.93 -0.82
0.84 -0.82
Chlorophyll B - El Ejido 0.91 095 0.94 095 091 -0.84
0.84 -0.83
b-carotene - El Ejido 0.93 095 0.96 096 0.95 -0.77 0.79
-0.77
L*(C) - El Ejido -0.66 -0.77 -0.72 -0.75 -0.67 0.82 -
0.87 0.88
a*(C) - El Ejido 070 079 0.73 077 068 -0.81 0.92
-0.92
b*(C) - El Ejido -0.69 -0.78 -0.72 -0.75 -0.67 0.80 -
0.92 0.92
Violaxanthin - Naples 0.97 0.94 0.95 0.95 0.96 -0.76 0.75
-0.73
Lutein- Naples 0.96 0.98 0.98 0.99 0.97 -0.81 0.85
-0.84
Chlorophyll A - Naples 0.97 0.98 0.99 0.98 0.98 -0.78
0.80 -0.78
Chlorophyll B - Naples 0.97 098 0.99 0.99 0.98 -0.80
0.81 -0.80
b-carotene - Naples 0.97 0.95 0.97 0.96 0.98 . -0.75
0.76 -0.74 .
L*(C) - Naples -0.69 -0.80 -0.75 -0.78 -0.69 0.84 -
0.82 0.82
a*(C). Naples 0.80 0.81 0.78 0.81 0.74 -0.87 0.87
-0.86
b*(C) - Naples -0.79 -0.81 -0.77 -0.80 -0.74 0.88 -
0.88 0.88
Viola Chloro Chloro b-caro
xanthin - Lutein - phyll A - phyll B - tene - El L*(C) - El a*(C) - El 13*(C) -
El
El Ejido El Ejido El Ejido El Ejido Ejido
Ejido Ejido Ejido
Violaxanthin -El Ejido 1
Lutein - El Ejido 0.96 1
Chlorophyll A- El Ejido 0.98 0.99 1
Chlorophyll B- El Ejido 0.97 1.00 1.00 1
b-carotene - El Ejido 0.99 0.97 0.99 0.97 1
L*(C) - El Ejido -0.70 -0.81 -0.78 -0.79 -0.73 1
a*(C) - El Ejido 0.73 0.84 0.82 0.84 0.75 -0.89 1
b*(C) - El Ejido -0.71 -0.82 -0.80 -0.82 -0.74 0.88 -
0.99 1
Violaxanthin - Naples 0.91 0.88 0.91 0.90 0.92 -0.65 0.69
-0.68
Lutein - Naples 0.92 0.93 0.96 0.95 0.94 -0.74 0.78
-0,77
Chlorophyll A - Naples 0.94 0.92 0.95 0.94 0.95 -0.70
0.73 -0.72
Chlorophyll B - Naples 095 0.94 096 095 096 -0.72
a 75 -0.74
b-carotene - Naples 091 088 0 92 090 0.93 -0.65 0.68
-0.68 -
L*(C) - Naples -0.73 -0.83 -0 79 -0.81 -0.74 077
-077 076
a*(C) - Naples 0.77 0.84 0.83 0.85 0.77 -0.82 0.90
-0.89
b*(C) - Naples -0.76 -0.84 -0.82 -0.84 -0.76 0.83
-0.91 0.91.
110
CA 02902250 2015-08-28
51682-49
TABLE 2 - continued
Viola Chloro Chloro b-caro
xanthin - Lutein - phyll A - phyll B- tene - L*(C) - a*(C) - b*(C)
-
Naples Naples Naples Naples Naples Naples Naples Naples
Violaxanthin - Naples 1
Lutein - Naples 0.97 1
Chlorophyll A - Naples 0.97 0.99 1
Chlorophyll 8- Naples 0.97 0.99 1.00 1
b-carotene - Naples 0.98 0.98 0.99 0.98 1
L*(C) - Naples -0.70 -0.79 -0.73 -0.76 -0.69 1
a*(C) - Naples 0.79 0.84 0.80 0.82 0.77 -0.76 1
b*(C) - Naples -0.78 -0.84 -0.79 -0.81 -0.76 0.78 -
0.99 1
111
CA 02902250 2015-08-28
,
51682-49
TABLE 3
Entry Violaxanthin Lutein Chlorophyll A
Chlorophyll B b-carotene
Crusader 1.87 4.08 20.6 5.54 1.20
Encore 1.42 3.51 17.6 4.67 1.07
8302 1.80 4.74 21.7 6.09 1.25
7141 1.77 5.20 24.6 6.83 1.44
1819 1.57 4.30 20.5 5.69 1.23
Declaration 1.65 3.60 17.9 4.85 1.11
Aristotle 1.61 3.45 17.5 4.70 1.12
Karisma 1.92 4.28 19.5 5.43 1.18
Hunter 1.98 4.24 21.0 5.89 1.17
Tomcat 2.04 4.27 21.1 5.89 1.23
RPP 25965 4.37 8.04 33.8 10.50 1.76
8728C 4.53 9.81 42.8 13.34 2.19
Standard
Deviation 0.41 0.63 3.0 0.82 0.17
F-test Probability 0.0% 0.0% 0.0% 0.0% 0.0%
5% LSD 0.67 1.03 4.8 1.34 0.28
Entry L*(C) a*(C) b*(C)
Crusader 38.3 -10.6 13.5
Encore 40.7 -11.7 16.2
8302 37.6 -7.6 8.2
7141 38.2 -8.2 8.6
1819 40.7 -9.5 11.3
Declaration 40.7 -9.7 11.7
Aristotle 40.9 -10.1 12.4
Karisnna 40.4 -9.8 12.0
Hunter 36.5 -8.9 10.3
Tomcat 37.8 -9.9 12.3
RPP 25965 34.4 -5.1 4.3
8728C 33.7 -4.4 3.3
Standard
Deviation 0.8 0.6 1.1
F-test Probability 0.0% 0.0% 0.0%
5% LSD 1.4 1.0 1.7
112
CA 02902250 2015-08-28
=
51682-49
TABLE 4
Entry Violaxanthin Lutein Chlorophyll A Chlorophyll B
b-carotene
8728C, (QTL1 +
QTL2) 3.01 7.03 40.97 12.80 2.09
11498, (no QTL) 1.09 1.97 14.23 3.92 0.92
DH 16, (QTL1 +
QTL2) 2.40 6.53 37.38 11.70 1.88
DH54, (QTL1) 2.20 4.91 30.43 8.71 1.74
DH69, (QTL2) 1.91 4.59 27.88 7.98 1.64
DH 11, (no QTL) 1.16 3.12 20.48 5.66 1.24
Standard Deviation 0.37 0.72 3.98 1.15 0.25
F-test Probability 0.0% 0.0% 0.0% 0.0% 0.0%
5% LSD 0.59 1.17 6.47 1.88 0.40
Entry L*(C) a*(C) b*(C)
8728C,
(QTL1+QTL2) 36.99 -4.74 3.84
11498, (no QTL) 43.33 -9.76 13.13
DH 16,
(QTL1+QTL2) 37.45 -4.34 3.51
DH54, (QTL1) 38.26 -6.83 6.54
DH69, (QTL2) 40.45 -8.39 9.93
DH 11, (no QTL) 42.35 -9.64 12.18
Standard Deviation 1.28 0.88 1.68
F-test Probability 0.0% 0.0% 0.0%
5% LSD 2.09 1.44 2.73
[0004361 EXAMPLE 3
Pigment Content of OVG Hybrids
[000437] Immature green fruits from a panel of OVG and non-OVG inbred and
hybrid
peppers were grown under standard field conditions in Gilroy California in
Summer 2010
and harvested in late summer for measurement of pigment content essentially as
described above in Example 1. Levels of violaxanthin (Figure 9), lutein
(Figure 10),
113
CA 02902250 2015-08-28
51682-49
chlorophyll A (Figure 11), chlorophyll B (Figure 12), alpha-carotene (Figure
13), and
beta-carotene (Figure 14) were determined.
[000438] The different entries shown in the figures are indicated in Table 5
below.
Table 5
Genotype Pepper Entry Variety
Non-OVG Hybrids 1 Crusader
2 Encore
3 8302
4 7141
1819
6 Declaration
7 Aristotle
8 Karisma
Heterozygous OVG 9 Hunter
Hybrids 10 Tomcat
Homozygous OVG 11 RPP 26098
Hybrids 12 RPP 26105
OVG Inbreds 13 8728C, OVG male inbred
parent
14 16452A, OVG female
inbred parent of RPP
25822
16452B, OVG female
inbred parent of RPP
26098
16 16452C, OVG female
inbred parent of RPP
26105
Non-OVG Inbreds 17 Non-OVG inbred, female
parent of Hunter
18 Non-OVG inbred, female
parent of Tomcat
19 Non-OVG inbred, male
parent of Encore
114
CA 02902250 2015-08-28
51682-49
[000439] The results demonstrate that fruits from homozygous OVG hybrids and
OVG
inbred lines have higher levels of pigments (violaxanthin, lutein, chlorophyll
a,
chlorophyll b, alpha-carotene, and beta-carotene) as compared with non-OVG
fruit.
[000440] Similar results were also observed in a field trial conducted in
Spring 2010 in
Naples, Florida under standard field conditions.
[000441] EXAMPLE 4
Pigment Content of OVG Hybrids RPP 26105, RPP 26098, RPP 25822
[000442] In a further study, pigment content was assessed in immature green
fruits from
homozygous OVG Hybrids RPP 26105, RPP 26098, RPP 25822 in comparison with
control varieties and varieties heterozygous for the extreme dark green trait.
[000443] Fruits were collected from a field trial conducted in Gilroy, CA in
Summer 2013.
sample of ten fruits of each variety were collected and evaluated. Briefly,
fruits were
harvested from a field trial consisting of three replication of 20 plants per
replication. For
each harvest, the fruits from each of the field replications per variety were
pooled, and
then the fruit samples were taken.
Table 6
Non- Non- Non- OVG
RPP RPP RPP OVG OVG OVG Hetero-
Value 26105 26098 25822 Hybrid Hybrid Hybrid zygote
13-carotene
(IU/100g) 345 394 364 240 262 220 278
Lutein
( g/g) 62.7 41.0 49.3 19.3 23.8 19.2 24.1
[000444] These data confirm the results observed in Example 3 above that
immature green
fruit from homozygous OVG hybrids have an increased content of beta-carotene
and
lutein as compared with immature fruit from non-OVG hybrids or OVG
heterozygotes.
115
CA 02902250 2015-08-28
51682-49
[000445] EXAMPLE 5
Analysis of Surface Color of OVG Hybrids
[000446] The pepper fruits collected in Example 3 were also evaluated for
surface color.
Colors were assessed by image analysis using the CIELAB scale (L, a*, b*) as
described
above in Example 1. Results are shown in Figure 15 (L*), Figure 16 (a*) and
Figure 17
(b*).
[000447] In general, the homozygous OVG hybrids and inbreds had a lower L*
value
(darker), a higher a* value (greener), and lower b* (bluer) value as compared
with the
other fruits evaluated.
[000448] Similar results were observed in a separate field trial conducted in
Spring 2010 in
Naples, Florida under standard field conditions.
[000449] EXAMPLE 6
Greenhouse Trial Conducted in El Eiido, Spain
[000450] A third trial was conducted under standard passive greenhouse
conditions in El
Ejido, Spain in Winter 2010-2011 (fruit measurements were done in January-
February of
2011), using the same varieties and measurements described in Example 3 for
the Gilroy
open field trial.
[000451] Violaxanthin (Figure 18), lutein (Figure 19), chlorophyll A (Figure
20),
chlorophyll B (Figure 21), alpha-carotene (Figure 22), and beta-carotene
(Figure 23)
content were determined.
[000452] In addition, the pepper fruits were evaluated for surface color.
Colors were
assessed by image analysis using the CIELAB scale (L, a*, b*) essentially as
described
above in Example 1. Results are shown in Figure 24 (L*), Figure 25 (a*), and
Figure 26
(b*).
116
CA 02902250 2015-08-28
51682-49
[000453] These results were consistent with those observed in the field trials
described in
Examples 3 to 5 above.
[000454] EXAMPLE 7
Color Analysis of Peduncle, Interior Flesh, and Calyx of OVG Hybrid Fruits
[000455] Immature green pepper fruits were harvested from two trials in
Naples, Florida
(winter 2013) for color analysis of the peduncle, interior flesh and calyx.
The first trial
included 3 field replications (20 plants of each variety) and was harvested at
3 different
times. The second trial included 2 replications (10 plants of each variety),
also harvested
at 3 different times. Fruits were selected in good condition from each harvest
and
replication for the color analysis (10 fruits for peduncle, 5 for calyx, and
10 for flesh for
each variety). For each harvest, the fruits from each of the field
replications per variety
were pooled, and then the samples were taken.
[000456] Peduncle, interior flesh and calyx color were evaluated by
colorimeter as
described in Example 1, with the additional determination of the corresponding
Munsell
Color chip based on the L*, a* and b* values. Results of the colorimeter
analysis are
shown in Table 7 below.
TABLE 7
Peduncle
Munsell Color
Variety L*(C) a*(C) b*(C) Chip
Non-OVG
hybrid 46.514 -15.182 25.618
5GY4/6
Non-OVG
hybrid 50.136 -15.706 26.952
5GY4/8
Heterozygous
OVG hybrid 47.388 -15.008 24.976 5GY4/6
RPP 25822 46.1 -14.368 23.372 5GY4/6
RPP 26098 46.212 -14.202 22.212
5GY4/6
RPP 26105 44.75 -13.826 21.992
5GY3/6
117
CA 02902250 2015-08-28
51682-49
Interior Flesh
Munsell Color
Variety L*(C) a*(C) b*(C) Chip
Non-OVG
hybrid 39.866 -13.164 23.788 5GY3/4
Non-OVG
hybrid 41.398 -15.328 30.454 5GY3/6
Heterozygous
OVG 38.802 -13.36 24.192 2.5G3/6
RPP 25822 37.246 -12.638 21.36
1OGY2/4
RPP 26098 33.984 -13.186 21.924
2.5G2/4
RPP 26105 35.952 -12.17 19.892
1OGY2/4
Calyx
Munsell Color
Variety L*(C) a*(C) b*(C) Chip
Non-OVG
hybrid 40.502 -17.18 29.948 7.5GY3/4
Non-OVG
hybrid 45.556 -17.528 29.956 2.5GY4/4
Heterozygous
OVG hybrid 39.792 -16.382 27.582 5GY3/4
RPP 25822 31.462 -13.748 19.516
2.5G2/4
RPP 26098 32.384 -15.992 24.404
2.5G2/4
RPP 26105 32.61 -14.282 20.21 2.5G2/4
10004571 Based on these data, the three homozygous OVG hybrids RPP 25822, RPP
26098
and RPP 26105 have darker interior flesh color (lower L* values) than the
other hybrids
evaluated. In addition, based on a* and b* ehroma values, the interior flesh
of the
homozygous OVG hybrids have more of a bluish hue (lower b* values). With
respect to
the calyx color, the homozygous OVG hybrids are darker (L*) as well as greener
(a*) and
bluer (b*) in hue than the other hybrids. In this study, no distinctive
differences were
noted with respect to peduncle color.
118
CA 02902250 2015-08-28
51682-49
10004581 EXAMPLE 8
Post-Harvest Study
1000459] A post-harvest study was conducted in Fall 2011 in Naples, Florida to
evaluate
post-harvest color retention, handling and quality performance of immature
green OVG
pepper fruits, e.g., how fruits perform when subjected to standard commercial
harvest,
handling and storage practices.
[000460] A panel of 15 pepper varieties/lines was assessed in a study with 2
replicates of
20 plants for each variety/line. Yield data were collected. From each
variety/line, 50
pepper fruits of good quality, unifolin size and free of scratching or damage
were
selected. Fruits were washed by dipping in 70 F water bath, rinsed with tap
water and
wiped dry with a paper towel. Fruits were labeled with a marker, and fruits
from each
plot carefully packed into black plastic crates. The crated fruits were then
stored at
50 5 F to replicate commercial handling conditions.
10004611 On each of post-harvest days 1, 5, 8, 12 and 15 (DPH1, DPH5, DPH8,
DPH12
and DPH15), 10 fruits per variety/line were evaluated for the following
parameters:
= Digital images were captured for Image Analysis as described above in
Example
1.
= Weight (g) of fruit
= Marketability (acceptable appearance; yes/no)
= Hand firmness rating on 1-3 scale (1/soft; 2/average; 3/firm)
= Visual Observations: bruising, silvering, micro-cracking, discoloration,
etc.
10004621 A summary of the results of image analysis of surface fruit color (L*
values) at
post-harvest day 1 (DPH1) is shown in Figure 27. The values for L* (Figure
28), a*
(Figure 29) and b* (Figure 30) at post-harvest days 1, 5, 8, 12 and 15 (DPH1,
DPH5,
DPH8, DPH12, and DPH15) are also shown. A summary of the day 1 post-harvest
results are shown below in Table 8.
119
CA 02902250 2015-08-28
51682-49
Table 8: Day 1 Post Harvest
Genotype Variety L value a value b value
8302 28.78 -16.03 21.53
2815 31.22 -17.93 24.55
Vanguard 32.14 -18.61 26.09
Non-OVG Aristotle 31.65 -18.36 25.74
Hybrid nn024272 28.40 -14.91 20.61
nn024226 28.69 -16.00 21.55
nn029991 31.61 -17.30 24.06
nn029869 30.69 -17.38 23.92
Heterozygous
Tomcat 28.35 -15.50 21.44
OVG Hybrid
nn025822 24.97 -9.41 12.89
Homozygous
nn026098 24.57 -10.03 13.89
OVG Hybrid
nn026105 24.25 -10.34 14.36
[0004631 The fruits from the three homozygous OVG hybrids retained their
darker color
over the course of 15 days post-harvest. In addition, no discoloration was
observed (e.g.,
no darkening of the color, the integrity of the color was maintained in
storage, and the
like), and no susceptibility to post-harvest disorders was observed.
10004641 With respect to the other parameters that were assessed in this
study, it was
determined that during storage: (1) all varieties lost moisture and freshness,
(2) all
varieties/lines decreased fruit weight at a linear rate, (3) post-harvest
quality of fruits
from homozygous OVG hybrids RPP 26098, RPP 25822 and RPP 26105 was similar to
commercial checks. In addition, yield was within normal commercial limits for
total
yield and size distribution of fruits for the three OVG hybrids.
[000465] No significant differences were observed between the two replicates
of this study.
120
CA 02902250 2015-08-28
'
51682-49
[000466] EXAMPLE 9
Sensory Evaluation of OVG Hybrids
[000467] Trained panelists performed a sensory evaluation in which 4 varieties
of immature
green pepper fruits were rated for various attributes and quality factors (see
Table 9).
Homozygous OVG hybrids RPP 25822, RPP 26098 and RPP 26105 and one non-OVG
hybrids were included in the panel.
TABLE 9. Sensory Evaluation
LABEL RPP 25822 RPP 26098 RPP 26105 Non-OVG
Hybrid
Temperature Avg. (F) 63.8 67.1 65.6 66.1
Total Soluble Solids Avg ( /0) 4.2 4.0 4.5 4.2
ATTRIBUTES**
Bell Pepper Intensity 7.8 5.1 6.8 6.2
Green/Grassy 4.6 2.2 2.8 2.8
Musty/Fermented 0.0 0.0 0.0 0.0
Chemical 0.0 0.0 0.0 0.0
Capsicum-Chile Odor 1.1 0.4 0.5 0.6
Sweetness 3.0 3.2 3.9 2.7
Sourness 0.2 0.1 0.1 0.8
Bitterness 1.3 0.8 0.8 0.8
Heat 0.4 0.1 0.1 0.3
Juiciness 7.6 7.6 7.8 7.7
Pulp Firmness/ Crunchiness 9.8 9.4 9.4 9.1
Graininess/Mealyness 0.0 0.0 0.0 0.0
Sliminess 0.0 0.0 0.0 0.0
External Qualities 13.0 14.0 14.0 12.0
External Appearance Defects 0.0 0.0 0.0 0.0
Skin Color 14.0 14.0 14.0 12.1
Flesh Color 13.0 12.9 12.9 11.5
Internal Qualities 13.3 13.1 13.3 11.9
QUALITY FACTOR**
Appearance 112 13.4 13.0 12.0
Sweet/Taste Balance 12.3 12.4 12.4 10.9
Texture 12.8 12.6 12.1 11.9
Aroma 12.4 11.7 11.5 11.4
OVERALL QUALITY** 12.5 12.5 12.8 11.4
**All ratings on a scale of 0 to 15
121
CA 02902250 2015-08-28
51682-49
[000468] The OVG hybrids have higher Overall Quality ratings than the non-OVG
hybrid
primarily due to higher Appearance scores, increased Sweetness and lower
scores for
Sourness, resulting in higher Sweet/Taste Balance scores. In general, the
sweetness
scores were ranked on the low side of the scale for all varieties due to
strong bell pepper
aroma/flavor intensity in the samples, which masked the sweetness.
[000469] The Appearance scores for all varieties were rated in the excellent
range. The
Aroma scores for all varieties were rated in the good to excellent range.
[000470] This sensory evaluation was repeated on materials harvested during a
different
growing season and similar results were observed.
[000471] The foregoing invention has been described in detail by way of
illustration and
example for purposes of clarity and understanding. However, it will be
apparent that
certain changes and modifications such as single gene modifications and
mutations,
somaclonal variants, variant individuals selected from large populations of
the plants of
the invention and the like may be practiced within the scope of the invention.
122