Note: Descriptions are shown in the official language in which they were submitted.
CA 2,905,251
CPST Ref: 79909/00017
TITLE:
[0001] TOPOGRAPHICAL FEATURES AND PATTERNS ON A SURFACE OF A
MEDICAL DEVICE AND METHODS OF MAKING THE SAME
BACKGROUND OF THE INVENTION
100021 The invention relates to methods and apparatus for manufacturing
medical devices,
wherein the medical device has a surface treated to promote the migration of
cells onto the
surface of the medical device.
[0003] Various types of intravascular stents have been used in recent years.
An intravascular
stent generally refers to a device used for the support of living tissue
during the healing phase,
including the support of internal structures. Intravascular stents, or stents,
placed intraluminally,
as by use of a catheter device, have been demonstrated to be highly
efficacious in initially
restoring 'latency to sites of vascular occlusion. Intravascular stents, or
stents, may be of the
balloon-expandable type, such as those of U.S. Pat. Nos. 4,733,665; 5,102,417;
or 5,195,984,
which are distributed by Johnson & Johnson Interventional Systems, of Warren,
N.J., as the
PalmazTM and the PaImazSchatzTM balloon-expandable stents or balloon
expandable stents of
other manufacturers, as are known in the art. Other types of intravascular
stents are known as
self-expanding stents, such as Nitinol coil stents or self-expanding stents
made of stainless steel
wire formed into a zigzag tubular configuration.
[0004] Intravascular stents are used, in general, as a mechanical means to
solve the most
common problems of percutaneous balloon angioplasty, such as elastic recoil
and intimal
dissection. One problem intraluminal stent placement shares with other
revaseularization
procedures, including bypass surgery and balloon angioplasty, is restenosis of
the artery. An
important factor contributing to this possible reocclusion at the site of
stent placement is injury
to, and loss of, the natural nonthrombogenic lining of the arterial lumen, the
endothelium Loss
of the endothelium, exposing the thrombogenic arterial wall matrix proteins,
along with the
generally thrombogenic nature of prosthetic materials, initiates platelet
deposition and activation
of the coagulation cascade. Depending on a multitude of factors, such as
activity of the
fibrinolytic system, the use of anticoagulants, and the nature of the lesion
substrate, the result of
this process may range from a small mural to an occlusive thrombus. Secondly,
loss of the
endothelium at the interventional site may be critical to the development and
extent of eventual
intimal hyperplasia at the site. Previous studies have demonstrated that the
presence of an intact
Date Recue/Date Received 2022-02-28
CA 02905251 2015-09-10
WO 2014/158550 PCT/US2014/018079
endothelial layer at an injured arterial site can significantly inhibit the
extent of smooth muscle
cell-related intimal hyperplasia. Rapid re-endothelialization of the arterial
wall, as well as
endothelialization of the prosthetic surface, or inner surface of the stent,
are therefore critical for
the prevention of low-flow thrombosis and for continued patency. Unless
endothelial cells from
.. another source are somehow introduced and seeded at the site, coverage of
an injured area of
endothelium is achieved primarily, at least initially, by migration of
endothelial cells from
adjacent arterial areas of intact endothelium.
[0005] Although an in vitro biological coating to a stent in the form of
seeded endothelial cells
on metal stents has been previously proposed, there are believed to be serious
logistic problems
related to live-cell seeding, which may prove to be insurmountable. Thus, it
would be
advantageous to increase the rate at which endothelial cells from adjacent
arterial areas of intact
endothelium migrate upon the inner surface of the stent exposed to the flow of
blood through the
artery. At present, most intravascular stents are manufactured of stainless
steel and such stents
become embedded in the arterial wall by tissue growth weeks to months after
placement. This
favorable outcome occurs consistently with any stent design, provided it has a
reasonably low
metal surface and does not obstruct the fluid, or blood, flow through the
artery. Furthermore,
because of the fluid dynamics along the inner arterial walls caused by blood
pumping through
the arteries, along with the blood/endothelium interface itself, it has been
desired that the stents
have a very smooth surface to facilitate migration of endothelial cells onto
the surface of the
stent. In fact, it has been reported that smoothness of the stent surface
after expansion is crucial
to the biocompatibility of a stent, and thus, any surface topography other
than smooth is not
desired. Christoph Hehriein, et. al., Influence of Surface Texture and Charge
On the
Biocompatibility of Endovascular Stents, Coronary Artery Disease, Vol. 6,
pages 581-
586(1995) After the stent has been coated with serum proteins, the endothelium
grows over the
fibrin-coated metal surface on the inner surface of the stent until a
continuous endothelial layer
covers the stent surface, in days to weeks. Endothelium renders the
thrombogenic metal surface
protected from thrombus deposition, which is likely to form with slow or
turbulent flow. At
present, all intravascular stents made of stainless steel, or other alloys or
metals, are provided
with an extremely smooth surface finish, such as is usually obtained by
electropolishing the
metallic stent surfaces. Although presently known intravascular stents,
specific including the
PalmazTM and Pa1mazSchatzTM balloon-expandable stents have been demonstrated
to be
2
CA 02905251 2015-09-10
WO 2014/158550 PCT/US2014/018079
successful in the treatment of coronary disease, as an adjunct to balloon
angioplasty,
intravascular stents could be even more successful and efficacious, if the
rate and/or speed of
endothelial cell migration onto the inner surface of the stent could be
increased.
[0006] However, known topographical features, e.g., grooves, impart the
greatest benefit when
the features are placed parallel with blood flow across a medical device. No
benefit from the
topographical features is realized when the features are oriented
perpendicular to the flow of
blood.
[0007] Still further, maintaining this optimal orientation of the features can
be problematic for
continuous features, since the final shape and orientation can depend on many
factors. When the
medical device is a stent, the final shape, and expansion size, can vary
depending on the
condition, size, shape, and compliance of the blood vessel where the stent is
implanted. Similar
implantation site factors can affect the orientation of topographical features
on other implanted
medical devices.
[0008] The present invention attempts to solve this problem, and others.
SUMMARY OF THE INVENTION
[0009] In accordance with the embodiments disclosed herein, at least one
noncontiguous pattern
of topographical features is disposed in or on a surface of the device. The
noncontiguous pattern
of topographical features allows for cell migration in more than one
direction, thus permitting
endothelial cells to migrate in the direction of blood flow, regardless of the
final positioning of
the medical device.
[0010] In one embodiment, there is provided a method of manufacturing a
medical device by
first forming a device having at least one surface; and then forming at least
one noncontiguous
pattern of topographical features in or on the surface of the device. The
device may be any
implantable medical device, such as a stent.
[0011] Any type of cell is encompassed by the present invention, which cell
has a cellular
membrane.
[0012] In accordance with the embodiments disclosed herein, the capacity for
complete cell
coverage of conventional implantable materials, including metals and polymers,
may be
enhanced by imparting a noncontiguous pattern of chemically and/or
physiochemically active
geometric physiologically functional features onto a blood contacting surface
of the implantable
material. The inventive implantable devices may be fabricated of polymers, pre-
existing
3
CA 02905251 2015-09-10
WO 2014/158550 PCT/US2014/018079
conventional wrought metallic materials, such as stainless steel or nitinol
hypotubes.
[0013] In any embodiment, an existing medical device, stent, or other article
may be utilized.
Through the use of an existing structure, it is likely that the regulatory
path may be minimized.
Particular, non-limiting devices include dental implants and hip implants.
100141 The noncontiguous pattern of topographical features, when compared with
presently
known devices and methods for manufacturing such devices, improves the control
of various cell
responses at the surface of the medical device.
BRIEF DESCRIPTION OF THE DRAWINGS
[0015] FIG. 1 is a block diagram illustrating a method of manufacturing a
medical device
having at least one noncontiguous pattern of topographical features created in
or on a surface
thereof.
[0016] FIG. 2 is a block diagram illustrating a method of manufacturing an
intravascular stent
having at least one noncontiguous pattern of topographical features created in
or on the inner
surface thereof
[0017] FIG. 3 is a block diagram illustrating a method of manufacturing a
transparent apparatus
having a surface adapted to mount a medical device thereupon, so as to impart
a photomask
pattern to a surface of the medical device.
[0018] FIG. 4 is a block diagram illustrating a method of manufacturing a
transparent mandrel
for mounting an intravascular stent thereon, so as to impart a photomask
pattern to the inner
surface of the stent.
[0019] FIG. 5a is an illustration of one embodiment of a stent having a
noncontiguous pattern of
topographical features imparted in an inner surface of the stent; FIG. 5b is a
close up view of a
portion of FIG. 5a.
[0020] FIGS. 6a-6b is an illustration of a stent having a pattern of
continuous grooves in an
inner surface of the stent.
[0021] FIGS. 7a-7c are illustrations of different noncontiguous patterns of
topographical
features imparted in a surface of a medical device.
[0022] FIGS. 8a-8f are illustrations of different noncontiguous patterns of
topographical
features imparted in a surface of a medical device.
[0023] FIG. 9 is an illustration of one embodiment of the transparent mandrel
of the present
invention, having a photoresist coated stent mounted thereupon.
4
CA 02905251 2015-09-10
WO 2014/158550 PCT/US2014/018079
[0024] FIG. 10a is an illustration of one embodiment of an implantable medical
device having
surfaces imparted with topographical features; FIG. 10b is an illustration of
one embodiment of
an implantable medical device having surfaces imparted with topographical
features, wherein the
features include directional grooves and dots,/pins.
100251 FIG. ha is an illustration of a dental implant having topographical
features; FIG. lib is
an enlarged view of a portion of the dental implant of FIG. 11a.
[0026] FIG. 12a is an illustration of a hip implant having topographical
features; FIG. 12b is an
enlarged view of a portion of the hip implant of FIG. 12a; and FIG. 12c is an
enlarged view of a
distal portion of the hip implant of FIG. 12a.
[0027] FIG. 13a is an illustration of a heart valve with grooves and
noncontiguous dots; and
FIG. 13b is an enlarged view of a portion of the heart valve of FIG. 13a.
[0028] While the invention will be described in connection with the preferred
embodiment, it
will be understood that it is not intended to limit the invention of that
embodiment. On the
contrary, it is intended to cover all alternatives, modifications, and
equivalents, as may be
included within the spirit and scope of the invention as defined by the
appended claims.
DETAILED DESCRIPTION OF THE INVENTION
[0029] In one embodiment, the formation of the noncontiguous pattern of
topographical features
may be by etching the surface with a chemical process. Preferably, the
chemical process may
comprise the steps of coating the surface of the device with a photosensitive
material; mounting
the device on a mask; irradiating the surface of the device by a source of
exposing radiation;
removing the device from the mask; and etching light exposed areas to produce
at least one
noncontiguous pattern of topographical features in or on the surface of the
device. The mask may
be disposed upon a surface of a transparent apparatus adapted to have the
device mounted
thereupon, and the device is mounted on the transparent apparatus. The source
of exposing
radiation may be an ultraviolet light source, but could be a light source with
any wavelength
compatible with the photosensitive material. Alternatively, the exposing
radiation may be
atomic in nature. The exposing radiation may be transmitted through one edge
of the apparatus,
or transmitted by means of a fiber optic cable inserted within the apparatus
below the mask. If a
fiber optic cable is used, either an end transmitting fiber optic cable may be
translated within the
apparatus to gain even exposures, or a bare (preferably frosted) fiber may be
used to broadcast
the exposing radiation from within the apparatus. After exposure, the device
is removed from
5
CA 02905251 2015-09-10
WO 2014/158550 PCT/US2014/018079
the apparatus. The photosensitive material is developed to reveal the pattern
imparted by the
mask by exposing the base material of the device through the use of
appropriate chemicals. The
exposed base material of the device may then be chemically machined to a
desired depth. The
machining may be accomplished by wet or dry chemical etching or polishing, or
by
electrochemical machining.
[0030] The process will be able to follow the contours of the device by
patterning. For example,
the mask pattern can be created such that the groove pattern is altered to
allow for the expansion
of the stent such that the grooves are parallel to bloodflow after expansion
by accounting for the
deformation pattern of the stent. Alternatively, patterns can be tailored to
steer cells in a
.. particular direction. Any 2D or 3D pattern can be effectively embossed or
debossed (or
combination of both) in the surface. Alternatively, other methods may be used
to create the
mask, including, but not limited to electrical discharge machining, dry
etching,
photodegradation, waterjet, abrasive blasting to create the mask pattern.
Additive methods are
feasible as well where the masking material is added to the translucent
member. An example of
an additive method is inkjet technology to deposit a coating selectively to
create a pattern that
would block the light transmission. Any material that can block the exposure
wavelength can be
used as the mask, including metals, pseudometals, intermetallics, ceramics,
polymers, and the
like.
[0031] Although photolithography methodologies are discussed herein as a
method of forming
the noncontiguous pattern of topographical features, the present invention is
not so limited. Any
methodology to form the noncontiguous pattern of topographical features may be
utilized,
including photolithography, mechanical transfer, electrochemical machining,
laser etching,
electric discharge machining, and/or any other means of applying the pattern
to a surface of the
medical device. Generally, the present invention may comprise forming or
providing a medical
device having at least one surface and forming at least one noncontiguous
pattern of
topographical features in or on said surface.
[0032] Any type of cell is encompassed by the present invention, which cell
has a cellular
membrane. Most distinct cell types arise from a single totipotent cell that
differentiates into
hundreds of different cell types during the course of development.
Multicellular organisms are
composed of cells that fall into two fundamental types: germ cells and somatic
cells. During
development, somatic cells will become more specialized and form the three
primary germ
6
CA 02905251 2015-09-10
WO 2014/158550 PCT/US2014/018079
layers: ectoderm, mesoderm, and endoderm. After formation of the three germ
layers, cells will
continue to specialize until they reach a terminally differentiated state that
is much more resistant
to changes in cell type than its progenitors. The ectoderm differentiates to
form the nervous
system (spine, peripheral nerves and brain), tooth enamel and the epidermis
(the outer part of
integument). It also forms the lining of mouth, anus, nostrils, sweat glands,
hair and nails. The
endoderm forms the gastrointestinal tract cells, the respiratory tract cells,
the endocrine glands
and organ cells, the auditory system cells, and the urinary system cells. The
mesoderm forms
mesenchyme (connective tissue), mesothelium, non-epithelial blood cells and
coelomocytes.
Mesothelium lines coeloms; forms the muscles, septa (cross-wise partitions)
and mesenteries
(length-wise partitions); and forms part of the gonads (the rest being the
gametes).
[0033] In accordance with the embodiments disclosed herein, the capacity for
complete cell
coverage of conventional implantable materials, including metals and polymers,
may be
enhanced by imparting a noncontiguous pattern of chemically and/or
physiochemically active
geometric physiologically functional features onto a blood contacting surface
of the implantable
material. The inventive implantable devices may be fabricated of polymers, pre-
existing
conventional wrought metallic materials, such as stainless steel or nitinol
hypotubes.
[0034] The inventive implantable devices may be intravascular stents, stent-
grafts, grafts, heart
valves, venous valves, filters, occlusion devices, catheters, sheaths, osteal
implants, implantable
contraceptives, implantable antitumor pellets or rods, shunts and patches,
pacemakers, needles,
temporary fixation rods, medical wires or medical tubes for any type of
medical device, or other
implantable medical devices, as will also be hereinafter described. A
pacemaker (or artificial
pacemaker, so as not to be confused with the heart's natural pacemaker) is a
medical device that
uses electrical impulses, delivered by electrodes contacting the heart
muscles, to regulate the
beating of the heart. The electrodes may be covered by tubing or other
material that includes a
surface that may require endothelialization and grooves thereon. Earrings and
other piercings
may benefit from the topographical features, as well as any other implant,
whether the implant is
an organic, inorganic, mechanical, electrical, or biological device.
[0035] Although photolithography methodologies are discussed herein as a
method of forming
the noncontiguous pattern of topographical features, the present invention is
not so limited. Any
methodology to form the noncontiguous pattern of topographical features may be
utilized,
including photolithography, mechanical transfer, electrochemical machining,
laser etching,
7
CA 02905251 2015-09-10
WO 2014/158550 PCT/US2014/018079
electric discharge machining, and/or any other means of applying the pattern
to a surface of the
medical device. Generally, the present invention may comprise forming or
providing a medical
device having at least one surface and forming at least one noncontiguous
pattern of
topographical features in or on said surface.
100361 Adding topographical or groove features to the surface of a stent has
been shown to
accelerate the migration rate of cells. However, topographical or groove
features impart the
greatest benefit when the topographical or groove features are placed parallel
with fluid flow,
and provide little to no benefit when the topographical or groove features are
oriented
perpendicular to the fluid flow. This perpendicular orientation can be
problematic for continuous
topographical or groove features, since the final shape or orientation of the
features can vary
depending on the condition, size, shape, and/or compliance of the blood
vessel, lumen, or tissue
where the device is implanted.
[0037] The device design itself may also not be well suited for continuous
topographical or
groove features. Some geometries do not allow for cell migration across all
areas of the device,
without the cells traveling over the vessel or lumen wall. One such example of
a continuous
groove feature can be seen in FIG. 6a. If evenly spaced, some topographical or
groove features
may only allow for a very small distance of travel, as seen in region 600 of
FIG. 6a. Still further,
a stent with a large expansion ratio can result in more grooves losing their
proper orientation
with blood flow. FIG. 6b illustrates a similar stent geometry to FIG. 6a,
however a
noncontiguous pattern of topographical features 650 is imparted to a surface
of the stent. As can
be seen, regardless of the orientation of the stent, cells may migrate across
all areas of the stent,
unlike with the continuous grooves in FIG. 6a.
[0038] A noncontiguous topographical pattern 520 on at least one surface 510
of a medical
device 500 allows for cell migration in more than one direction, as shown in
FIGS. 5a-b. The
noncontiguous pattern allows the cells to migrate in the direction of blood
flow, regardless of the
final positioning of the surfaces or structures of the medical device. In one
embodiment, the
noncontiguous topographical pattern 520 includes a plurality of grooves 530
forming a triangular
shape 540 on the surface 510 of the device 500. The triangular shapes 540
alternate in facing
one direction and then the opposite direction, as to form a row of alternating
facing triangular
shapes 540.
8
CA 02905251 2015-09-10
WO 2014/158550 PCT/US2014/018079
[0039] The pattern itself could be any noncontiguous shape that promotes a
favorable cell
response, as further discussed below in relation to FIGS. 7a-7b. The shapes
could be of any size,
number, height, or depth required to issue a proper cell response. The limit
of the distance for the
noncontiguous pattern may be across a particular length or depth. In one
embodiment, the
noncontiguous pattern includes a continuous groove for at least 0.1-5.0
microns in length,
alternatively, at least 10.0 microns in length, alternatively, at least 1.0-
100.0 microns in length.
Then noncontiguous groove is placed at an angle thereafter, preferably between
about 10-150
degrees, alternatively, between about 20-120 degrees, alternatively, between
about 30-100
degrees. In one embodiment, the noncontiguous pattern may take place after 5-
10 microns of
groove length. In one embodiment, the noncontiguous pattern may be sinusoidal
pattern 720 of
curved lines, as shown in FIG. 7C. The sinusoidal pattern 720 of curved lines
may have a
specific wavelength and amplitude, may have a constant amplitude and
wavelength, or may a
discontinuous amplitude and wavelength that varies along the length of the
groove. The
wavelength and the amplitude of the sinusoidal pattern 720 may be at least 0.1-
5.0 microns in
length, alternatively, at least 10.0 microns in length, alternatively, at
least 1.0-100.0 microns in
length
[0040] Alternatively, as shown in FIG. 8A, the noncontiguous shapes may
include intermatched
shaped pattern 800, such as alternating octagonal grooved features 802
displaced with square
grooved features 804. Alternatively, the noncontiguous pattern may be
alternating diagonal
groove pattern with alternating diagonal groove 812 and 814 displaced at an
angle., as shown in
FIG. 8B. Alternatively, the noncontiguous pattern may be boomerang like shape
820 with three
prongs 822 stemming from curved portions 824, wherein the end of each prong
820 abuts an
adjacent curved portion 824, as shown in FIG. 8C. Alternatively, the
noncontiguous pattern may
be zig-zag like grooved feature 830, where the every fourth zig-zag groove 832
includes a depth
greater than the previous grooved features 834, as shown in FIG. 8D.
Alternatively, the
noncontiguous pattern may include general hexagonal pattern 840 with at least
three alternating
diagonal groove features 842 disposed within each hexagonal pattern 830 and
the plurality of
diagonal groove features 842 terminate at an angle into a length of an
adjacent diagonal groove
feature 842, as shown in FIG. 8E. Alternatively, the noncontiguous pattern may
include open
hexagonal grooved features 850 whereby an inner hexagonal groove 852 encircles
an outer
hexagonal groove 854 about at least one point, and the inner hexagonal groove
852 may be a
9
CA 02905251 2015-09-10
WO 2014/158550 PCT/US2014/018079
different width or depth than the outer hexagonal groove 854, as shown in FIG.
8F.
[0041] These features could be added to surfaces of articles other than
stents. The term "stent" is
used throughout this application to simplify the explanation, but is not
intended to be a limiting
description. As stated above, the inventive implantable devices may be
intravascular stents,
stent-grafts, grafts, heart valves, venous valves, filters, occlusion devices,
catheters, sheaths,
steal implants, implantable contraceptives, implantable antitumor pellets or
rods, shunts and
patches, pacemakers, needles, temporary fixation rods, medical wires or
medical tubes for any
type of medical device, or other implantable medical devices, as will also be
hereinafter
described.
[0042] Using photolithography, mechanical machining, micromachining, laser
machining, or
other means to transfer the pattern, a pattern of multiple non-contiguous
shapes can be produced
in or on the surface of an implantable medical device to promote healing, by
allowing for cell
migration in the direction of blood flow regardless of alignment of the device
after implantation.
The noncontiguous pattern of topographical features may be created through
photolithography,
mechanical transfer, electrochemical machining, or any other means of applying
the pattern to a
surface of the device. These new techniques embodied in the present invention
described herein
provide the opportunity to apply not just grooved features, but any
conceivable pattern of shapes.
[0043] Additionally, not only may these patterns be utilized for cell
migration, but also to allow
for cells to spread quickly to the sides once the path in the direction of
blood flow is occupied by
existing cells. This may be particularly useful for specific implantable
medical devices, such as
heart valves.
[0044] In still further embodiments, the noncontiguous pattern of
topographical features can be
used to promote other cell responses, such as demoting cell proliferation,
pinning cells in place,
thwarting tissue growth, enhancing osteoblast formation, and/or the like
Surface modification
could include geometric features, charge distribution, alternative chemistry
for the patterns,
coatings on the patterns, oxides on the patterns, nitrides on the patterns,
and the like.
Pattern Shape
[0045] The pattern itself could be any noncontiguous shape that promotes a
favorable cell
response. The shapes could be of any size, number, height, or depth required
to issue a proper
cell response. Illustrations of exemplary patterns are shown in FIGS. 7-8.
FIG. 7A depicts a
hexagonal noncontiguous pattern of topographical features 700. FIG. 7B depicts
a triangular
CA 02905251 2015-09-10
WO 2014/158550 PCT/US2014/018079
noncontiguous pattern of topographical features 800. FIG. 8 depicts an
intermatched shaped
pattern 800, such as alternating octagonal grooved features 802 displaced with
square grooved
features 8040.
[0046] Additionally, in further embodiments, the noncontiguous pattern of
topographical
features could be placed anywhere on a surface of the device, could be used on
external surfaces
of the device to prevent cell migration, or could be used for drug delivery.
For example,
considering a disposable device such as a needle used with an insulin pump, it
may be
advantageous to thwart tissue growth to case removal of the temporary device.
Other similar
devices may include temporary fixation rods used for knee, shoulder, or elbow
repair, and/or the
like. Devices with a noncontiguous pattern of topographical features may also
be useful for
promoting healing at closure sites, or for bone mending (such as the
breastplate after open heart
surgery).
[0047] In some embodiments, multiple noncontiguous patterns of topographical
features may be
imparted to a single device, such as on different surfaces or different
portions of a surface, to
achieve different cell responses for different objectives. For example,
considering a heart valve,
a first noncontiguous pattern of topographical features could be incorporated
in the anchoring
portion of the heart valve and a second noncontiguous pattern of topographical
features
incorporated near the leaflets of the valve to prevent tissue growth on the
leaflets.
[0048] With reference to FIG. I, the method of creating a noncontiguous
pattern of
topographical features on a surface of a medical device 100 is illustrated.
First, a medical device
is provided 105. In a preferred embodiment, the medical device is metallic in
nature, but need
only be suitable for chemical machining. Photoresist is then applied to the
device and treated
appropriately to make the photoresist photosensitive 110. A positive or
negative photoresist may
be used. In a preferred embodiment, the photoresist is an electrodeposited
positive photoresist
(InterViTm 3D-P Photoresist PEPR-2400) from MicroChem. Alternative
photoresists are
contemplated by and within the scope of this disclosure, including negative
photoresist. By
electrodepositing the photoresist, all surfaces of the device are easily
coated with a uniform layer
of resist, as compared to traditional photoresist application methods. It is
important to attain
sufficient control over coating thickness on especially the inner diameter of
the stent. In
alternative embodiments, the device may be coated with photoresist by dipping,
spraying,
spinning, electrodeposition, or any other typical means of applying
photoresist. Once the device
11
CA 02905251 2015-09-10
WO 2014/158550 PCT/US2014/018079
is coated, the device is mounted on a photomasked transparent apparatus 115.
The method of
creating the photomasked transparent apparatus is discussed further below, in
relation to FIG. 3.
In mounting the device on the photomasked apparatus, it is preferable to
maintain intimate
contact between the device and apparatus, to aid in pattern transfer. In one
embodiment, an
external force is applied to the device to obtain this intimate contact. In
another embodiment, an
interference fit between the apparatus and the device can be used to obtain
the intimate contact.
In embodiments where the device is nitinol-based, the interference fit may be
obtained by shape
memory. Once the device is mounted on the apparatus, the photoresist coating
on the device is
exposed to exposing radiation through the photomasked apparatus 120. In a
preferred
embodiment, the exposing radiation is an ultraviolet light source, though the
light source could
have any wavelength that is compatible with the particular photoresist
utilized by the inventive
method. One such source is a light guide or an internal 0.7mm fiber with UV
radiation provided
by a 200W Lesco SuperSpot Max-HP source. In an alternative embodiment, the
exposing
radiation may be atomic in nature. The exposing radiation may be transmitted
through one edge
of the apparatus, or transmitted by means of a fiber optic cable inserted
within the apparatus
below the photomask. If a fiber optic cable is used, either an end
transmitting fiber optic cable
may be translated within the apparatus to gain even exposures, or a bare
(preferably frosted) fiber
may be used to broadcast the exposing radiation from within the apparatus.
After exposure, the
now exposed device is removed from the apparatus 125. The exposed photoresist
is then
developed to reveal the noncontiguous pattern imparted by the photomask 130.
In one
embodiment, a rinse process may then be employed on the exposed photoresist to
enhance
pattern coverage and give rise to about 100% pattern coverage. Some metals may
a rinse of
warmed deionized water, while other metals may not require the rinse step. In
the preferred
embodiment, using a positive photoresist, developing exposes the base material
of the device in
the exposed portions of the photoresist through the use of appropriate
chemicals. In the preferred
embodiment, the appropriate chemicals are those recommended by the
manufacturer of the
photoresist, including InterViaTM 3D-P Developer, InterViaTM 3D-P Remover,
InterViaTM 3D-P
Solvent, and InterViaTM 3D-P TC. The exposed base material of the device may
then be
chemically machined to a desired depth 1350. The machining may be accomplished
by wet or
dry chemical etching or polishing, or by electrochemical machining. In one
embodiment, the
electrochemical methods are carried out in a phosphoric acid bath. Once the
machining is
12
CA 02905251 2015-09-10
WO 2014/158550 PCT/US2014/018079
complete, the remaining photoresist may be removed from the device 140, by
appropriate means.
Appropriate means may include chemical or mechanical removal of the remaining
photoresist.
The result is a medical device having a noncontiguous pattern of topographical
features created
on at least one surface of the device.
100491 In a further embodiment, after the machining is complete, the
patterning and machining
process can be repeated using additional transparent apparatuses, having
distinct photomask
patterns, to achieve multiple-depth noncontiguous patterns of topographical
features on the
surface of the device. Alternatively, the patterning and machining process can
be repeated to
impart distinct noncontiguous patterns of topographical features to different
portions or surfaces
of the device, having the same or different depths, patterns, shapes, etc.
[0050] With reference to FIG. 2, the method of creating a noncontiguous
pattern of
topographical features on the inner diameter surface of an intravascular stent
200 is illustrated.
First, an intravascular stent is provided 205. In a preferred embodiment, the
intravascular stent is
metallic in nature, but the material of the intravascular stent need only be
suitable for chemical
machining. Photoresist is then applied to the stent and treated appropriately
to make the
photoresist photosensitive 210. In a preferred embodiment, the photoresist is
an electrodeposited
positive photoresist (InterViaTM 3D-P Photoresist PEPR-2400) from MicroChem.
Alternative
photoresists are contemplated by and within the scope of this disclosure,
including negative
photoresist. If a negative photoresist is used, additional steps are required
to expose the masked
portions of the stent and then expose the remaining surfaces. By
electrodepositing the
photoresist, all surfaces of the stent are easily coated with a uniform layer
of resist, as compared
to traditional photoresist application methods. It is important to attain
sufficient control over
coating thickness on especially the inner diameter of the stmt. In alternative
embodiments, the
stent may be coated with photoresist by dipping, spraying, spinning,
electrodeposition, or any
other typical means of applying photoresist. Once the stent is coated, the
stent is mounted on a
photomasked transparent mandrel 215. The method of creating the photomasked
transparent
mandrel is discussed further below, in relation to FIG. 4. In mounting the
stent on the
photomasked mandrel, it is preferable to maintain intimate contact between the
stent and the
mandrel, to aid in pattern transfer. In one embodiment, an external force is
applied to the stent to
obtain this intimate contact. In another embodiment, an interference fit
between the mandrel and
the stent can be used to obtain the intimate contact. In embodiments where the
stent is nitinol-
13
CA 02905251 2015-09-10
WO 2014/158550 PCT/US2014/018079
based, the interference fit may be obtained by shape memory. Once the stent is
mounted on the
mandrel, the photoresist coating on the stent is exposed to exposing radiation
through the
photomasked mandrel 220. In a preferred embodiment, the exposing radiation is
an ultraviolet
light source, though the light source could have any wavelength that is
compatible with the
particular photoresist utilized by the inventive method. One such source is a
light guide or an
internal 0.7mm fiber with UV radiation provided by a 200W Lesco SuperSpot Max-
HIP source.
In an alternative embodiment, the exposing radiation may be atomic in nature.
The exposing
radiation may be transmitted through one end of the mandrel, or transmitted by
means of a fiber
optic cable inserted within the mandrel below the photomask. If a fiber optic
cable is used,
either an end transmitting fiber optic cable may be translated within the
mandrel to gain even
exposures, or a bare (preferably frosted) fiber may be used to broadcast the
exposing radiation
from within the mandrel. In one embodiment, certain light guide with high
optical numerical
aperture (NA) produces the pattern definition of the noncontiguous pattern.
Light guides and
fibers preferably emit optical radiation radially. The translated fibers may
include a conical tip as
the optical radiation exits at a perpendicular angle to the mask. The methods
of illuminating the
mask may include: 1) end lighting, which relies on internal reflection and
transmission through
the mask; 2) a diffuse internal light that broadcasts over an area large
enough to expose the entire
article (or multiple articles) without having to move the light relative to
the mask; and 3) an end
lit internal fiber that is translated inside the mask to expose the article
one section at a time in a
continuous manner. The third method allows for very long lengths to be
exposed. In addition, the
exposure can be varied or interrupted, if desired. The second method can also
work using
translation to expose longer length articles.
[0051] After exposure, the now exposed stent is removed from the mandrel 225.
The exposed
photoresist is then developed to reveal the noncontiguous pattern imparted by
the photomask
230. In the preferred embodiment, using a positive photoresist, developing
exposes the base
material of the stent in the exposed portions of the photoresist through the
use of appropriate
chemicals. In the preferred embodiment, the appropriate chemicals are those
recommended by
the manufacturer of the photoresist, including Inter\7iaTM 3D-P Developer,
InterViaTM 3D-P
Remover, InterViaTM 3D-P Solvent, and InterViaTM 3D-P TC. The exposed base
material of the
stent may then be chemically machined to a desired depth 235. The machining
may be
accomplished by wet or dry chemical etching or polishing, or by
electrochemical machining. In
14
CA 02905251 2015-09-10
WO 2014/158550 PCT/US2014/018079
one embodiment, the electrochemical methods are carried out in a phosphoric
acid bath. Once
the machining is complete, the remaining photoresist may be removed from the
stent 240, by
appropriate means. Appropriate means may include chemical or mechanical
removal of the
remaining photoresist. The result is an intravascular stent having a
noncontiguous pattern of
topographical features created on an inner diameter surface of the stent.
[0052] With reference to FIG. 3, the method of manufacturing a photomasked
transparent
apparatus 3000 is illustrated. First, a transparent apparatus is provided 305.
In a preferred
embodiment, the transparent apparatus is comprised of quartz, glass, or any
other material
capable of transmitting an exposing radiation through a photomask onto a
photoresist coated
medical device. The transparent apparatus has at least one surface adapted to
mount a medical
device thereupon. The at least one surface of the transparent apparatus is
then coated with an
opaque layer 310. In one embodiment, the opaque layer is a thin wall material
on the top or
bottom of the at least one surface. In another embodiment, the opaque layer
may be a metal, a
polymer, a composite, a ceramic, or any other material that sufficiently
blocks the transmission
of the exposing radiation. The opaque layer may be deposited by several
methods, including:
dipping, spraying, vapor deposition, plating, or painting. Once coated,
portions of the opaque
layer may be selectively removed from the transparent apparatus by appropriate
means 315, so as
to form a photomask pattern on the surface of the apparatus. The appropriate
means may include
laser ablation, mechanical means, photolithography, etching, or engraving,
and/or the like. With
portions of the opaque layer removed, an exposing radiation is able to be
transmitted through the
now photomasked surface of the transparent apparatus.
[0053] With reference to FIG. 4, the method of manufacturing a photomasked
transparent
apparatus 400 is illustrated. First, a transparent mandrel is provided 405. In
a preferred
embodiment, the transparent mandrel is comprised of quartz, glass, or any
other material capable
of transmitting an exposing radiation through a photomask onto a photoresist
coated
intravascular stent. In one embodiment, the mandrel is a cylindrical tube or
rod. In alternative
embodiments, the mandrel may be tapered, have an elliptical cross section, or
have a polygonal
cross section. The transparent mandrel has at least one surface adapted to
mount an intravascular
stent thereupon. In one embodiment, the mandrel has at least one open end,
within which a fiber
optic cable may be inserted for transmittal of the exposing radiation from
within the mandrel
through a photomask on the exterior of the mandrel. The at least one surface
of the transparent
CA 2,905,251
CPST Ref: 79909/00017
mandrel is then coated with an opaque layer 410. In one embodiment, the opaque
layer is a thin
wall tube disposed against the inner or outer surface of the cylindrical
mandrel. In another
embodiment, the opaque layer may be a metal, a polymer, a composite, a
ceramic, or any other
material that sufficiently blocks the transmission of the exposing radiation.
The opaque layer
may be deposited by several methods, including: dipping, spraying, vapor
deposition, plating, or
painting. In the preferred embodiment, a metallic coating is deposited by
physical vapor
deposition on the outer surface of a cylindrical quart tube. Once coated,
portions of the opaque
layer may be selectively removed from the transparent mandrel by appropriate
means 415, so as
to form a photomask pattern on the surface of the mandrel. The appropriate
means may include
laser ablation, mechanical means, photolithography, etching, or engraving,
and/or the like. In the
preferred embodiment, the opaque layer is removed by laser ablation, utilizing
a femtosecond
laser cutting system. With portions of the opaque layer removed, an exposing
radiation is able to
be transmitted through the now photomasked surface of the transparent mandrel.
Another
method of producing the transparent mandrel is through the use of
photolithography and
chemical etch processes, which includes a photosensitive polymer coated on the
mandrel and UV
is applied selectively (e-beam or UV projection method).
[00541 With reference to FIG. 9, one embodiment of the photomasked transparent
mandrel
having a photoresist coated stent is depicted. The transparent mandrel 900 is
has a photoresist
coated intravascular stent 905 mounted on the outer surface of the mandrel.
The outer surface of
the mandrel is coated with an opaque layer 910. Portions of the opaque layer
910 have been
selectively removed to form a mask pattern, the mask pattern comprising
openings 915 where the
opaque layer has been removed.
[0055] In another embodiment of the present invention, the machined pattern
may be used to
enhance bone formation by enhancing osteoblast production for devices such as,
but without
limitation to, orthopedic or dental devices.
[0056] Referring to FIG. 10A, a structural member 1006 includes a luminal
surface 1036 as well
as a leading edge 1014 and a trailing edge 1016 relative to the direction 1010
of blood flow. Any
or all of the luminal surface 1036, the leading edge 1014, and the trailing
edge 1016 may include
topographical features disposed therein or thereon. For example, in one
embodiment, the
topographical features of luminal surface 1036 may be grooves 1018 disposed
therein, and is
noncontiguous by virtue of the edge of the structural member. The grooves 1018
may be
16
Date Recue/Date Received 2020-08-31
CA 2,905,251
CPST Ref: 79909/00017
oriented in any direction relative to the direction 1010 of blood flow;
however, orientation of the
grooves 1018 parallel to the direction 1010 of blood flow, as illustrated in
FIG. 10A, exposes EC
within the grooves 1018 to shear stress caused by the blood flow. As noted
hereinabove, such
exposure of EC to shear stress increases the rate of migration of the EC.
[0057] The leading edge 1014 of the structural member 1006, in one embodiment,
may have
topographical features such grooves 1020 disposed therein or thereon. The
grooves 1020 may be
oriented in any direction relative to the direction 1010 of blood flow and is
noncontiguous by
virtue of the edge of the structural member. In one embodiment as illustrated
in FIG. 10A, the
grooves 1020 are oriented such that a component of blood flow along the
leading edge 1014
exposes EC within the grooves 1020 to shear stress caused by the blood flow.
Similarly, the
trailing edge 1016 of the structural member 1006, in one embodiment, may have
topographical
features such as grooves 1022 disposed therein or thereon. The grooves 1022
may be oriented in
any direction relative to the direction 1010 of blood flow. In one embodiment
as illustrated in
FIG. 10A, the grooves 1022 are oriented such that a component of blood flow
along the trailing
edge 1016 exposes EC within the grooves 1022 to shear stress caused by the
blood flow.
[0058] It should be noted that the topographical features on one or more of
the surfaces 1036,
1014, 1016, may take any of a variety of forms, and are not limited to the
grooves discussed
above. For example, any or all of the grooves 1018, 1020, 1022 illustrated in
FIG. 10A may
alternatively be dots, divots, pores, holes, complex geometries, and/or the
like.
[0059] Any of the geometrically functional features or recesses may also be
included in the
trailing edge, leading edge, or surface regions to enhance the endothelial
migration and
attachment to such surfaces.
[0060] An implantable device may include problematic surfaces that may be
resistant to
endothelialization or may otherwise be relatively slow to endothelialize. The
problematic
surfaces may be disadvantaged for cell adhesion because of, for example,
hemodynamic reasons
such as disruption via turbulence or low shear stress (which may occur in
thick stents, for
example, greater than about 100 um) or chemical reasons such as anti-mitotic
and/or anti-
inflammatory drugs. The problematic surfaces could be, for example, stent
bridges disposed at
various angles against the blood flow.
[0061] Referring to FIG. 10b, it is contemplated that a combination of
properly oriented grooves
may facilitate EC migration to the problematic surfaces and/or promote cell
stability thereon.
17
Date Recue/Date Received 2020-08-31
CA 2,905,251
CPST Ref: 79909/00017
For example, in one embodiment, a main highway 1000 of the grooves 1018 may be
disposed in
the luminal surface 1036 of the structural member 1006 and oriented generally
parallel to the
direction 1010 of blood flow, as illustrated in FIG. 10b. The main highway
1000 could provide
an abundance of migrating EC, which could be diverted therefrom to a
problematic surface, for
example, a surface 1002 on a transversely disposed structural member 1007 of
the implantable
device. For example, the main highway 1000 may be diverted to groove endpoints
1054 on the
transversely disposed structural member 1007 of the implantable device.
[0062] It is further contemplated that diversion of migrating EC from the main
highway 1000
could be applied to surfaces having a specific function, and is noncontiguous
by virtue of the
diversion, which may or may not otherwise be conducive to EC migration. In
some
embodiments, the machined pattern may include features which pin or demote
cell proliferation,
so as to stop cell proliferation in a particular location. These patterns may
be used to steer cells to
control a directionality of healing response. In some embodiments, and without
limitation, these
features may be pores, holes, divots, and/or the like. FIG. 10b illustrates
one embodiment of a
surface with directional and pinning topographical features created thereupon.
For example,
referring to FIG. 10B, the structural member 1007 may include surfaces
including a plurality of
pores 1008 as might be found, for example, in a drug eluting stent. The
plurality of pores may
act to pin cell proliferation in the location of the pores 1008, and demote
proliferation beyond the
location of pores 1008.
[0063] In another embodiment of the present invention, the machined pattern
may include
features which pin or demote cell proliferation. These patterns may be used to
steer cells to
control a directionality of healing response. FIG. 10b illustrates one
embodiment of a surface
with directional topographical features created thereupon.
[0064] In one embodiment, a first pattern may be applied to a first surface of
a dental implant,
and a second pattern may be applied to a second surface of the dental implant.
The first surface
may serve to promote adhesion and healing of the implant in the bony part of
the jaw, while the
second surface may serve to stop proliferation of bone into the gum line.
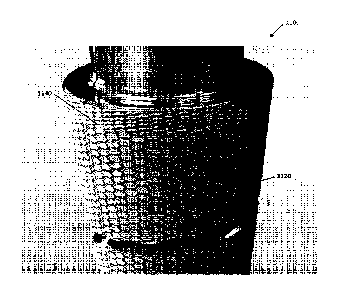
[0065] Additional applications where it may be advantageous to demote healing
include, without
limitation, temporary implants such as a vena cava filter or an insulin pump
needle.
[0066] In any embodiment of the present invention, an existing medical device,
stent, or other
article may be utilized. Through the use of an existing structure, it is
likely that the regulatory
18
Date Recue/Date Received 2020-08-31
CA 02905251 2015-09-10
WO 2014/158550 PCT/US2014/018079
path may be minimized.
[0067] Particular, non-limiting examples of medical devices that may be worked
upon by the
inventive method disclosed herein include dental implants, hip implants, and
valves. Other
devices may also be worked upon, as previously discussed above.
[0068] FIG. ha depicts one embodiment of a textured dental implant 1100 having
topographical features created thereupon. The dental implant 1100 has a
portion imparted with a
noncontiguous texture 1120 to promote bone growth in the jaw bone and a
portion imparted with
a dotted texture 1140 to pin the cells so they don't proliferate into the
gums. In the depicted
embodiment, the noncontiguous texture is alternating triangular grooved
features that run along
.. the length of the implant 1100, to provide directional migration of cells
and thereby promote
bone growth along and into the portion of the implant 1100 that is installed
into the jaw bone of a
patient. The portion of the implant 1100 having a dotted texture 1140 serves
to halt the
proliferation of cell growth such that the bone growth does not continue into
the gums of the
patient. The ideal texture for the bone growth may be a crosshatch to add an
anchoring effect to
the dental implant 1100. FIG. lib is an enlarged view of the noncontiguous
portion 1120
having displaced hexagonal grooved features and dotted portion 1140 of the
dental implant 1100.
In alternative embodiments, the features of the grooved portion 1120 may have
different
arrangements and/or noncontiguous shapes, such as noncontiguous grooves that
run diagonally,
noncontiguous grooves that run helically, complex geometries that promote bone
growth along
the length of the implant, features having multiple depths, and/or the like.
The portion of the
implant 1100 having a dotted texture 1140 may comprise divots, pores, holes,
wells, and/or the
like, serving to pin cells in place and thereby demote cell proliferation
beyond the dotted portion
1140. In alternative embodiments, the features of the dotted portion 1140 may
have different
arrangements and/or shapes, or the portion may have greater or lesser width or
height.
[0069] FIG. 12a depicts one embodiment of a textured hip implant 1200 having
topographical
features created thereupon. The hip implant 1200 has a portion imparted with a
noncontiguous
grooved texture 1220 to promote bone growth and a portion imparted with a
dotted texture 1240
to pin the cells so they don't proliferate beyond the dotted portion. In the
depicted embodiment,
the grooves are an alternating hexagonal pattern that run along the length of
the implant 1200, to
provide multi-directional migration of cells and thereby promote bone growth
along and into the
portion of the implant 1200 that is installed into the bone of a patient. The
portion of the implant
19
CA 02905251 2015-09-10
WO 2014/158550 PCT/US2014/018079
1200 having a dotted texture 1240 serves to halt the proliferation of cell
growth such that the
bone growth does not continue into the joint of the patient. The ideal texture
for the bone growth
may be a crosshatch to add an anchoring effect to the hip implant 1200. FIG.
12b is an enlarged
view of the grooved portion 1220 that includes an noncontiguous triangular
features and dotted
portion 1240 of the hip implant 1200. In alternative embodiments, the features
of the
noncontiguous grooved portion 1220 may have different arrangements and/or
shapes, such as
noncontiguous grooves that run diagonally, noncontiguous grooves that run
spirally, complex
geometries of features that promote bone growth along the length of the
implant, features having
multiple depths, and/or the like. The portion of the implant 1200 having a
dotted texture 1240
may comprise divots, pores, holes, wells, and/or the like, serving to pin
cells in place and thereby
demote cell proliferation beyond the dotted portion 1240. In alternative
embodiments, the
features of the dotted portion 1240 may have different arrangements and/or
shapes, or the portion
may have greater or lesser width or height. FIG. 12C shows the noncontiguous
grooved portion
1220 on the distal portion including a first direction of the triangular
grooves and a second
direction of the triangular grooves 1220a in direction generally at an angle
to the first direction of
the grooves on the side of the implant.
[0070] FIG. 13a depicts one embodiment of a textured heart valve 1300 having
topographical
features created thereupon. The heart valve 1300 has a portion imparted with a
grooved texture
1320 to promote cell growth where the heart valve 1300 is anchored to the
tissue, and a portion
imparted with a dotted texture or noncontiguous elliptical pattern 1340 to pin
the cells so they
don't proliferate into the valve portion of the heart valve 1300. In the
depicted embodiment, the
grooves run along the length of the struts on the heart valve 1300, to provide
directional
migration of cells and thereby promote cell growth along and into the portion
of the implant
1300 that is anchored into the heart of a patient The portion of the heart
valve 1300 having a
noncontiguous elliptical pattern 1340 serves to halt the proliferation of cell
growth such that the
cell growth does not continue into the valve portion. The ideal texture for
the cell growth may
be a crosshatch to add an anchoring effect to the heart valve 1300. FIG. 13b
is an enlarged view
of the grooved portion 1320 and dotted portion 1340 of the heart valve 1300.
In alternative
embodiments, the features of the grooved portion 1320 may have different
arrangements and/or
shapes, such as grooves that run diagonally, grooves that run helically,
complex geometries that
promote cell growth along the length of the implant, features having multiple
depths, and/or the
CA 2,905,251
CPST Ref: 79909/00017
like. The portion of the heart valve 1300 having a dotted texture 1340 may
comprise divots,
pores, holes, wells, and/or the like, serving to pin cells in place and
thereby demote cell
proliferation beyond the dotted portion 1340. In alternative embodiments, the
features of the
dotted portion 1340 may have different arrangements and/or shapes, or the
portion may have
greater or lesser width or height.
[0071] In still further alternative embodiments of the present invention, the
devices modified
could be more "industrial" in nature, rather than being medical devices. One
such example is an
earring post or stem (or other piercing articles), which may have its surface
modified with a
noncontiguous pattern of topographical features to prevent hole closure,
infection, etc.
[0072] It is to be understood that the invention is not limited to the exact
details of construction,
operation, exact materials, or embodiments shown and described, as obvious
modifications and
equivalents will be apparent to one skilled in the art. Accordingly, the
invention is therefore to be
limited only by the scope of the appended claims.
21
Date Recue/Date Received 2020-08-31