Note: Descriptions are shown in the official language in which they were submitted.
BONE REGENERATION USING BIODEGRADABLE POLYMERIC
NANOCOMPOSITE MATERIALS AND APPLICATIONS OF THE SAME
HELL)
The present disclosure relates generally to a biocompatible structure for hone
and
tissue regeneration, and more particularly to a biodegradable and
bioresorbable
nanocomposite incorporating polymer, nanostructured hydroxyapatite and
optionally
other beneficial factors.
BACKGROUND
The background description provided herein is for the purpose of generally
presenting the context of the disclosure. Work of the presently named
inventors, to the
extent it is described in this background section, as well as aspects of the
description that
may not otherwise qualify as prior art at the time of filing, are neither
expressly nor
impliedly admitted as prior art against the present disclosure.
Skeletal deficiencies from trauma, tumors and bone diseases, or abnormal
development frequently require surgical procedures to attempt to restore
normal bone
function. Although most of these treatments arc successful, they all have
problems and
Therefore, a heretofore unaddressed need exists in the art to address the
aforementioned deficiencies and inadequacies.
1
CA 2905816 2017-07-31
SUMMARY
Certain aspects of the present disclosure are directed to a biocompatible
structure.
The biocompatible structure is biodegradable and bioresorbable.
In certain embodiments, the biocompatible structure includes polymer layers
CA 2905816 2017-07-31
stacked to have a predetermined shape, bone particle layers disposed between
each of the
two neighboring polymer layers, a coating surrounding the polymer layers and
bone
particle layers; and bone particles attached to an outer surface of the
coating. Each of the
polymer layers is formed with a polymer and Inv tissue forming nanoparticles.
A weight
percentage of the first tissue forming nanoparticles to the polymer is about
0.05- 50%.
In certain embodiment, the weight percentage of the first tissue Ihrming
nanoparticles to the polymer film is about 25%.
In certain embodiment, the polymer includes at least one of a synthetic
biodegradable polymer and a biodegradable polymer derived from natural source.
In certain embodiment, the synthetic biodegradable polymer includes at least
one
of polyurethane, polylactide (PLA), polyglycolide (PGA), poly(lactide-co-
glycolide)
(PLGA), poly(c-caprolactone), polydioxanone, polyanhydride, trimethylene
carbonate,
poly(P-hydroxybutyrate), poly(g-ethyl glutamate), poly(DT1-1 iminocarbonatc),
poly(bisphenol A iminocarbonate), poly(ortho ester), polycyanoaerylatc, and
polyphosphazene.
In certain embodiment, the biodegradable polymer derived from natural source
includes at least one of modi fled polysaccharides (cellulose, chitin,
dextran), and
modified proteins (fibrin, casein).
In certain embodiment, the first tissue forming nanoparticles includes at
least one
of nanoparticles of hydroxypatites (HAP), tricalcium phosphates, mixed calcium
phosphates and calcium carbonate, bone particles of zenograftxenogratl,
allogafts,
autografts, and alloplastic grafts.
In certain embodiment, the second tissue forming particles includes at least
one of
nano-sized bone particles and micro-sized bone particles
In certain embodiment, the biocompatible structure further includes a third
tissue
forming material.
In certain embodiment, the third tissue I-brining material includes at least
one of a
bioactiye material and cells.
3
CA 2905816 2017-07-31
In certain embodiment, the bioactivc material includes at least one of
proteins.
enzymes, growth factors, amino acids, bone morphogenie proteins, platelet
derived
growth factors. and vascular endothelial growth factors.
In certain embodiment, the cells includes at least one of epithelial cells,
neurons,
glial cells, astroeytes, podocytes, mammary epithelial cells, islet cells.
endothelial cells,
mesenchymal cells, stem cells, ostcoblast, muscle cells, striated muscle
cells, fibroblasts,
hepatocytes, ligament fibroblasts, tendon fibroblasts, and ehondrocytes.
In certain embodiment, the biocompatible structure is formed with a shape
conforming to a shape of an implant site.
In certain embodiment, at least one of the polymer layers has a length of
about
0.05-20 centimeter (cm), a width of about 0.02-5 cm, and a thickness of about
0.01-50
millimeter (mnm), and the biocompatible structure is in a cylindrical shape, a
rectangular
shape, or a spherical shape,
In certain embodiment, the biocompatible structure is plasma treated.
1 5 Certain aspects of the present disclosure arc directed to a method of
producing a
biocompatible structure for bone and tissue regeneration.
In certain embodiments, the method includes dissolving a polymer in a solvent
to
form a first solution; adding a first tissue Moiling, nanopartilces to the
first solution to
form a second solution wherein a weight percentage of the first tissue forming
nanoparticles to the polymer is about 0.05-50%; applying the second solution
to a surface
to form a polymer film on the surface: dividing the polymer film into a
plurality of strips;
and forming a layered biocompatible structure by the strips, the second
solution and a
second tissue forming particle materials. The second tissue forming particles
are placed
between two of the strips.
In certain embodiments, the method further includes stirring the first
solution to
uniformly distribute the polymer in the first solution.
In certain embodiments, the method further includes sonicating the second
solution to uniformly distribute the polymer and the first tissue forming
nanoparticles in
the second solution.
4
CA 2905816 2017-07-31
In certain embodiments, the method further includes drying the second solution
on the surface to form the polymer film on the surface.
In certain embodiments, the operation of forming the biocompatible structure
includes constructing a scaffold by stacking the strips to form polymer layers
and adding
bone particles between the polymer layers: applying the second solution to the
scaffold to
form a coated scaffold: and adding the second tissue forming particles to the
coated
scaffold to firm the biocompatible structure.
In certain embodiments, the scaffold is formed by stacking the strips and
layers of
the bone-forming particles alternatively.
0 In certain embodiments, the method further includes, after adding the
second
tissue forming particles to the coated scaffold, plasma treating the coated
scaffold.
In certain embodiments, the weight percentage of the first tissue forming
nanoparticles to the polymer is about 25%.
In certain embodiments, the polymer includes at least one of a synthetic
biodegradable polymer and a biodegradable polymer derived from natural source.
In certain embodiments, the synthetic biodegradable polymer includes at least
one
of polyurethane. polylactide (PIA), polyglycolide (PGA), poly(lactide-co-
glycolide)
(PLOA), poly(e-caprolactonc), polydioxanone, polyanhydride, trimethylene
carbonate,
poly(P-hydroxybutyrate), poly(g-ethyl glutamate), poly(DTH iminocarbonate),
poly(bisphenol A iminocarbonate), poly(ortho ester), polycyanoacrylate, and
polyphosphazene.
In certain embodiments, the biodegradable polymer derived from natural source
includes at least one of modified polysaccharides (cellulose, chitin,
dextran), and
modified proteins (fibrin, casein).
In certain embodiments, the first tissue forming nanoparticles includes at
least
one of nanoparticles of hydroxypatites. tricalcium phosphates, mixed calcium
phosphates
and calcium carbonate, bone particles of zenograftxenooTaft, allografts,
autografts, and
alloplastic grafts.
In certain embodiments, the surface is a polytetralluoroethylenc (PTFE)
surface.
5
CA 2905816 2017-07-31
CA 02905816 2015-09-11
WO 2014/143131 PCT/US2013/051520
In certain embodiments, the second tissue forming particles includes at least
one
of nano-sized bone particles and micro-sized bone particles.
In certain embodiments, the method further includes adding a third tissue
forming
material to the biocompatible structure.
In certain embodiments, the third tissue forming material includes at least
one of a
bioactive material and cells.
In certain embodiments, the cells include epithelial cells, neurons, glial
cells,
astrocytes, podocytes, mammary epithelial cells, islet cells, endothelial
cells,
mesenchymal cells, stem cells, osteoblast, muscle cells, striated muscle
cells, fibroblasts,
hepatocytes, ligament fibroblasts, tendon fibroblasts, and chondrocytes.
In certain embodiments, the bioactive material comprises proteins, enzymes,
growth factors, amino acids, bone morphogenic proteins, platelet derived
growth factors,
and vascular endothelial growth factors.
In certain embodiments, the biocompatible structure is formed with a shape
conforming to a shape of an implant site
In certain embodiments, the strip has a length of about 0.05-20 cm, a width of
about 0.02-5 cm, and a thickness of about 0.01-50 mm, and the biocompatible
structure is
in a cylindrical shape, a rectangular shape, or a spherical shape.
Certain aspects of the present disclosure are directed to a method of treating
bone
deficiencies. The method includes applying a biocompatible structure to an
implant
surgical site. The biocompatible structure includes polymer layers stacked to
have a
predetermined shape, bone particle layers disposed between each of the two
neighboring
polymer layers; a coating surrounding the polymer layers and bone particle
layers; and
bone particles attached to an outer surface of the coating. Each of the
polymer layers is
formed with a polymer and first tissue forming nanoparticles. The
predetermined shape
of the biocompatible structure is configured to conform to the implant
surgical site. A
weight percentage of the first tissue forming nanoparticles to the polymer is
about 5-50%
such that a resorption rate of the biocompatible structure substantially
matches a rate of
tissue generation in the biocompatible structure.
6
CA 02905816 2015-09-11
WO 2014/143131 PCT/US2013/051520
BRIEF DESCRIPTION OF THE DRAWINGS
The accompanying drawings illustrate one or more embodiments of the disclosure
and, together with the written description, serve to explain the principles of
the
disclosure. The same reference numbers may be used throughout the drawings to
refer to
the same or like elements in the embodiments.
FIG. lA illustrates a biocompatible structure according to certain embodiments
of
the present disclosure;
FIG. 1B illustrates a part of a biocompatible structure according to certain
embodiments of the present disclosure;
FIG. 2 schematically shows a Scanning Electron Microscopy image of a
biocompatible structure at a low resolution according to certain embodiments
of the
present disclosure;
FIGs. 3A-3C schematically show Scanning Electron Microscopy images of a
biocompatible structure at a high resolution according to certain embodiments
of the
present disclosure;
FIG. 4 schematically shows procedures for producing a biocompatible structure
according to certain embodiments of the present disclosure;
FIGs. 5A and 5B schematically show a pull test set up for measuring maximum
load and maximum stress of polymer films according to certain embodiments of
the
present disclosure;
FIG. 6 schematically shows maximum load of the polymer films according to
certain embodiments of the present disclosure; and
FIG. 7 schematically shows maximum stress of the polymer films according to
certain embodiments of the present disclosure.
DETAILED DESCRIPTION
The present disclosure will now be described more fully hereinafter with
reference to the accompanying drawings, in which exemplary embodiments of the
7
CA 02905816 2015-09-11
WO 2014/143131 PCT/US2013/051520
disclosure are shown. This disclosure may, however, be embodied in many
different
forms and should not be construed as limited to the embodiments set forth
herein.
Rather, these embodiments are provided so that this disclosure will be
thorough and
complete, and will fully convey the scope of the disclosure to those skilled
in the art.
Like reference numerals refer to like elements throughout. As used in the
description
herein and throughout the claims that follow, the meaning of "a," "an," and
"the"
includes plural reference unless the context clearly dictates otherwise. Also,
as used in
the description herein and throughout the claims that follow, the meaning of
"in" includes
"in" and "on" unless the context clearly dictates otherwise. Moreover, titles
or subtitles
may be used in the specification for the convenience of a reader, which has no
influence
on the scope of the disclosure. Additionally, some terms used in this
specification are
more specifically defined below.
Typically, terms such as "first", "second", "third", and the like are used for
distinguishing various elements, members, regions, layers, and areas from
others.
Therefore, the terms such as "first", "second", "third", and the like do not
limit the
number of the elements, members, regions, layers, areas, or the like. Further,
for
example, the term "first" can be replaced with the term "second", "third", or
the like.
Typically, terms such as "about," "approximately," "generally,"
"substantially,"
and the like unless otherwise indicated mean within 20 percent, preferably
within 10
percent, preferably within 5 percent, and even more preferably within 3
percent of a
given value or range. Numerical quantities given herein are approximate,
meaning that
the term "about," "approximately," "generally," or "substantially" can be
inferred if not
expressly stated.
Typically, "nanoscopic-scale," "nanoscopic," "nanometer-scale," "nanoscale,"
the
"nano-" prefix, and the like refers to elements or articles having widths or
diameters of
less than about 1 um, preferably less than about 100 nm in some cases.
Specified widths
can be smallest width (i.e. a width as specified where, at that location, the
article can have
a larger width in a different dimension), or largest width (i.e. where, at
that location, the
article's width is no wider than as specified, but can have a length that is
greater), unless
8
CA 02905816 2015-09-11
WO 2014/143131 PCT/US2013/051520
pointed out otherwise.
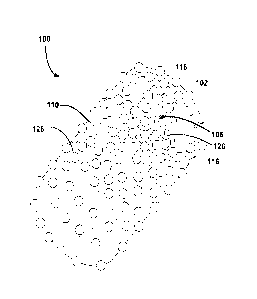
FIG. lA schematically shows structure of a biocompatible structure 100
according to certain embodiments of the present disclosure. The biocompatible
structure
100 can be in any shape that conforms to a shape of an implant site. For
example, the
biocompatible structure can have a cylindrical shape, a rectangular shape, or
a spherical
shape.
The biocompatible structure includes two or more modified polymer layers 102
stacked together. As will be described below, the modified polymer layers 102
each have
nanoparticles 112 dispersed in a polymer matrix 114. In certain embodiments,
the
nanoparticles 112 are hydroxypatite (HAP) nanoparticles. Further, as shown in
FIGs. lA
and 1B, spacer particles 116 are located in between any two of the layers 102
and can
function as spacer layer 106 between the polymer layers 102. In certain
embodiments,
the spacer particles 116 each have a diameter of about 2-100 ium. In certain
embodiments, the spacer particles 116 are partially embedded, or trapped, in
the surface
portion of the polymer layers 102. In certain embodiments, the spacer
particles 116 are
formed as layers 106, and each spacer layer 106 can have a thickness between
approximately 0.001 mm and approximately 50 mm, but are typically less than 3
mm.
The layers can be mechanically stacked or applied in situ on top of one
another. In
certain embodiments, the spacer particles 116 can be bone particles or
composite
particulates as described below. In certain embodiments, the spacer particles
116 can be
HAP particles as described below. In certain embodiments, a portion of a
polymer layer
102 can contact a portion of an adjacent polymer layer 102. In certain
embodiments,
those contacted portions can cross-link with each other. In certain
embodiments, a
polymer coating 110 encloses the stacked polymer layers 102 and spacer layers
106.
Further, the surface of the coating 110 can have trapped spacer particles 116.
In certain
embodiments, the spacer particles 116 can form a layer and cover a substantial
portion of
the entire coating 110.
The polymer layers 102 can have different sizes and shapes as desired. In
certain
embodiments, the polymer layers 102 can be made as strips. For example, the
polymer
9
CA 02905816 2015-09-11
WO 2014/143131 PCT/US2013/051520
strips 102 each can have a length of 0.005-50 cm, a width of 0.002-50 cm, and
a
thickness of 0.001-50 mm. The size of the entire structure 100 can vary in
order to match
the size of the bone defect that needs to be regenerated.
In certain embodiments, the polymer matrix 114 of the modified polymer layer
102 can be polyurethane. The particles 112 dispersed in the polymer matrix 114
can be
hydroxypatite (HAP) nanoparticles. The weight percentage of the nanoparticles
112 in
the polymer film/layer 102 is defined as the total weight (e.g., grams) of the
nanoparticles
112 divided by the total of the weight of the nanoparticles 112 (grams) and
the weight of
the solid polymers 114 (grams) used for the preparation of the polymer film
102. For
example, a total of A grams of nanoparticles 112 and a total of B grams of
polymers 114
are used to manufacture a polymer film 102. The weight percentage of the
nanoparticles
112 in the polymer film 102 is calculated as A/(A+B). In certain embodiments,
the
weight percentage of HAP nanoparticles 112 in the polymer layer 102 is about
0.05-95%.
In certain embodiments, the weight percentage of HAP nanoparticles 112 in the
polymer
layer 102 is about 20%.
In certain embodiment, the nanoparticles 112 dispersed in the polymer layer
102
are Hydroxylapatite nanoparticles and can have a dimensional range between 1-
100
nanometer (nm). Hydroxylapatite, also called hydroxyapatite (HA or HAP), is a
naturally occurring mineral form of calcium apatite with the formula
Ca5(PO4)3(OH), but
is usually written Caio(PO4)6(OH)2to denote that the crystal unit cell
comprises two
entities. Hydroxylapatite is the hydroxyl endmember of the complex apatite
group. The
Off ion can be replaced by fluoride, chloride or carbonate, producing
fluorapatite or
chlorapatite. It crystallizes in the hexagonal crystal system. Pure
hydroxylapatite powder
is white. Naturally occurring apatites can, however, also have brown, yellow,
or green
colorations, comparable to the discolorations of dental fluorosis. Up to 50%
of bone by
weight is a modified form of hydroxylapatite (known as bone mineral). In
certain
embodiments, the HAP nanoparticles dispersed in the polymer layer can be
composed of
pure HAP, having significant crystallinity and very good dispensability due to
the
presence of oxygen groups on the surface.
CA 02905816 2015-09-11
WO 2014/143131 PCT/US2013/051520
The presence of HAP nanoparticles 112 in the polymer film 114, among other
things, contributes to the pore size and the strength of the polymer film 114.
In addition,
the concentration of HAP nanoparticles 112 is also related to the degradation
rate of the
polymer film 114 when the polymer film 114 is used as implant material.
In certain embodiments, the HAP nanoparticles 112 can enhance
bone/mineralization in bone cells. The HAP nanoparticles 112, together with
other
nanomaterials, have the ability to increase the osteogenesis and
mineralization in bone
cells.
In certain embodiment, the spacer particles 116 between the polymer layers 102
of the present disclosure are bone particles. The bone particles 116 can be
autografts,
allografts, xenografts (usually bovine) or alloplastic bone grafts (synthetic,
such as
tricalcium phosphate). In certain embodiment, the bone particles 116 are
treated with
bone mineral products, or composite particles. Bones from slaughtered animals
are an
inexpensive raw material available in large quantities to produce bone
mineral. Bones
typically contain 50 to 60% of very fine crystallites of a form of modified
hydroxylapatite, which is bonded by collagenic tissue and contains significant
qualities of
proteinaceous and other matter as well as associated fat and muscle tissues.
Such a
modified hydroxylapatite, in a pure state and has its essential crystal
structure, represents
a highly biocompatible remodeling bone implant material.
In certain embodiments, the bone particles 116 include hydroxyapatite like
crystallites with a particular degree of crystallinity, habit, and size
(irregular platelike
morphology, 5-10 nm in thickness 10-50 nm in length). The specific surface
chemistry
of the bone particles 116 results from the calcium to phosphate ratio (37.5-
38.0% calcium
and 15.5-19.0% phosphorus). The inorganic phase of the bone particle 116
contains
porosity including ultrastructural interstices (10-100 nm) between the
crystallites
occurring naturally and produced by removal of the organic phase, and
microscopic
spaces (1-20ium) including osteocyte lacunae, canaliculi, vascular channels,
volkman's
canals, and the canals of haversian systems (100-500 nm). The specific surface
area,
which is a measure of porosity is in the range 50 to100 m2/gm as determined by
mercury
11
CA 02905816 2015-09-11
WO 2014/143131 PCT/US2013/051520
porosimetry. The crystallinity of the bone particle 116 can be characterized
by X-ray
diffraction and the porosity and crystallite morphology and size by electron
microscopy.
In certain embodiment, the bone particles 116 of the present disclosure are
demineralized bone particles 116 purchased from Geistlich BioOss, INC. The
bone
particles 116 can be of bovine origin and treated such that only the inorganic
structure is
left, while the organic materials are removed. The bone particles 116 are
composed of
powder particles with a diameter of 0.01-100 micrometer (gm).
In certain embodiments, the spacer particles 116 can be large particles of HAP
that, e.g., are produced in the lab, or composite particles (polymer and
inorganic
particles).
In certain embodiments, the biocompatible structure 100 can include bioactive
materials 126. In certain embodiments, the bioactive materials 126 can be
sprayed on the
surface of the biocompatible structure 100, and/or incorporated in the polymer
structures
102 to promote bone growth.
The bioactive materials 126 can be proteins/peptides, HA, drugs, growth
factors,
antibiotics (such as tetracycline), and bone morphogenic proteins. Preferred
bioactive
agents 126 are those that enhance tissue regeneration and/or tissue adhesion.
Illustrative
examples include growth factors, antibiotics, immuno-stimulators, and immuno-
suppressants. In one embodiment, the bioactive agent 126 may be a bone
morphogenic
protein such as bone morphogenetic proteins (BMP). In another embodiment, the
bioactive agent 126 may be a growth factor such as fibroblast growth factors
(FGF) or an
agent which promotes the generation of connective tissue.
In certain embodiments, tissue can also be grown in vivo by implanting the
biocompatible structure 100 and stem cells or other types of suitable cells
(liver cells for
the growth of liver tissue; myocardial cells, muscle cells for
replacing/restoring damaged
heart tissue; epithelial cells, connective tissue cells for skin grafts;
osteblasts for bone
generation) to an implant site. Alternatively, tissue can be grown in vitro on
the
biocompatible structure 100 and then implanted (for example, for growth of
connective
tissue/coronary vessels for arterial grafts).
12
CA 02905816 2015-09-11
WO 2014/143131 PCT/US2013/051520
Suitable living cells can be placed in the biocompatible structure before
implantation or implanted together with the biocompatible structure 100 into a
body. The
living cells include epithelial cells (e.g., keratinocytes, adipocytes,
hepatocytes), neurons,
glial cells, astrocytes, podocytes, mannnary epithelial cells, islet cells,
endothelial cells
(e.g., aortic, capillary and vein endothelial cells), and mesenchymal cells
(e.g., dermal
fibroblasts, mesothelial cells, osteoblasts), smooth muscle cells, striated
muscle cells,
ligament fibroblasts, tendon fibroblasts, chondrocytes, fibroblasts, and any
of a variety of
stem cells. Also suitable for use in the biocompatible structure 100,200 are
genetically
modified cells, immunologically masked cells, and the like. Appropriate
extracellular
matrix proteins (ECM) may be added to the biocompatible structure to further
promote
cell ingrowth, tissue development, and cell differentiation within the
scaffold. ECM
proteins can include one or more of fibronectin, laminin, vitronectin,
tenascin, entactin,
thrombospondin, elastin, gelatin, collagen, fibrillin, merosin, anchorin,
chondronectin,
link protein, bone sialoprotein, osteocalcin, osteopontin, epinectin,
hyaluronectin,
undulin, epiligrin, and kalinin.
Additional bioactive agent 126 incorporated in the biocompatible structure
100,
among other things, includes biologically active macromolecules helpful for
cell growth,
morphogenesis, differentiation, and tissue building, include growth factors,
proteoglycans, glycosaminoglycans and polysaccharides. These compounds are
believed
to contain biological, physiological, and structural information for
development or
regeneration of tissue structure and function.
In certain embodiments, the biocompatible structure 100 can be plasma-
treated/activated/electro-sprayed to functionalize the surface of the
biocompatible
structure 100. Surface treatment can improve the hydrophilicity of the
biocompatible
structure 100 and promote the colonization of cells and the adhesion of bone
particles to
the surface and pores of the biocompatible structure 100. The surface can also
be
functionalized by electron or ion bombardment, laser irradiation and/or by any
other
physical or chemical surface reaction that affects the bonds near the surface.
These
processes can also help in sterilization of the implant. Plasma treatment
breaks the
13
CA 02905816 2015-0V1
WO 2014/143131 PCT/US2013/051520
surface bonds of the polymer. After plasma treatment, oxygen atoms "attach" to
the
surface, changing the surface energy of the surface such that the surface
becomes more
hydrophilic and has oxygen and nitrogen rich functional groups.
The biocompatible structure 100 of the present disclosure is highly porous,
biocompatible, and allows for vascular ingrowth for bone/tissue regeneration.
The
surface typically does not inhibit any biological entity from interacting and
to be
hydrophilic or potentially become hydrophilic under different conditions or
processes.
Suitable materials for building structures for tissue/bone engineering and
regeneration are
certain polymers, ceramics, carbon- based materials and metals and metal
composites. In
certain embodiments, the polymer layers 102 of the biocompatible structure 100
of the
present disclosure are formed from polyurethane. In certain embodiments, the
biocompatible structure 100 has a layered structure composed of a polymeric
material
that may contain other substances, such as bioactive substances or substances
promoting
the generation of tissue growth. Those substances can be formed inside a
polymer layer
102 or on the surface of a polymer layer 102. Some of the bioresorbable
polymers may
or may not require enzymes in order to degrade. The layered, porous design
gives this
structure a very high surface area for neovascularization and the growth of
cells
necessary for tissue regeneration. In addition, stem cells, osteoblasts, and
other types of
suitable cells can be incorporated into the system to aid in tissue
generation. The
biocompatible structure 100 can assume different shapes and dimensions as may
be
required for a particular application. The biocompatible structure 100 can be
properly
positioned in the surgical site directly or with medical pins, screws, or
other devices.
The biocompatible structure 100 is configured such that the degradation rate
or
the resorption rate of the biocompatible structure 100 is substantially
matching a rate of
tissue generation in the biocompatible structure 100. The controllable
degradation rate of
the biocompatible structure 100 can also provide controllable release of the
bioactive
substance or cells formed in the biocompatible structure 100. The polymer may
have a
different degradation rate than that of the biocompatible structure 100, but
it contributes
significantly to the degradation rate of the biocompatible structure 100.
Accordingly, a
14
CA 02905816 2015-09-11
WO 2014/143131 PCT/US2013/051520
polymer with suitable degradation property is chosen to produce the
biocompatible
structure 100 of the present disclosure.
The polymer layers 102 can be degraded by several mechanisms. The most
common mechanism is diffusion. Further, the bioactive substances (agent) of
the
biocompatible structure can diffuse in various manners. The bioactive agent
(drug) can
have a core surrounded by an inert diffusion barrier, which can be membranes,
capsules,
microcapsules, liposomes, and hollow fibers. Alternatively, the active agent
can be
dispersed or dissolved in an inert polymer. Drug diffusion through the polymer
matrix is
the rate-limiting step, and release rates are determined by the choice of
polymer and its
consequent effect on the diffusion and partition coefficient of the drug to be
released. By
adjusting the diffusion method of the bioactive agent or cells, and components
of the
biocompatible structure component, suitable rate of bioactive agent or cells
is achieved.
In certain embodiments, after implantation the biocompatible structure 100 can
be
eventually absorbed by the body, for example, by conversion of a material that
is
insoluble in water into one that is water/liquid-soluble, and thus need not be
removed
surgically.
In certain embodiments, the polymer layers 102 in the biocompatible structure
100 are biocompatible, processable, sterilizable, and capable of controlled
stability or
degradation in response to biological conditions. The reasons for designing a
biocompatible structure 100 that degrades over time often go beyond the
obvious desire
to eliminate the need for retrieval. For example, the very strength of a rigid
metallic
implant used in bone fixation can lead to problems with "stress shielding,"
whereas a
bioresorbable implant can increase ultimate bone strength by slowly
transferring load to
the bone as it heals. For drug delivery, the specific properties of various
degradable
systems can be precisely tailored to achieve optimal release kinetics of the
drug or active
agent.
An ideal biodegradable polymer layer 102 for medical applications typically
has
adequate mechanical properties to match the application (strong enough but not
too
strong), does not induce inflammation or other toxic response, may be fully
metabolized
CA 02905816 2015-09-11
WO 2014/143131 PCT/US2013/051520
once it degrades, and is sterilizable and easily processed into a final end
product with an
acceptable shelf life. In general, polymer degradation is accelerated by
greater
hydrophilicity in the backbone or end groups, greater reactivity among
hydrolytic groups
in the backbone, less crystallinity, greater porosity, and smaller finished
device size.
A wide range of synthetic biodegradable polymers can be used to form the
polymer matrix 102 of the present disclosure, including polylactide (PLA),
polyg-
lycolide (PGA), poly(lactide-co-glycolide) (PLGA), poly(e- caprolactone),
polydioxanone, polyanhydride, trimethylene carbonate, poly(13-
hydroxybutyrate), poly(g-
ethyl glutamate), poly(DTH iminocarbonate), poly(bisphenol A iminocarbonate),
poly(ortho ester), polycyanoacrylate, and polyphosphazene. There are also a
number of
biodegradable polymers derived from natural sources such as modified
polysaccharides
(cellulose, chitin, dextran) or modified proteins (fibrin, casein) that can be
used to form
the polymer matrix of the present disclosure.
Other materials can be tyrosine-derived polycarbonate poly(DTE-co-DT
carbonate), in which the pendant group via the tyrosine-an amino acid-is
either an ethyl
ester (DTE) or free carboxylate (DT). Through alteration of the ratio of DTE
to DT, the
material's hydrophobic/hydrophilic balance and rate of in vivo degradation can
be
manipulated. It was shown that, as DT content increases, pore size decreases,
the
polymers become more hydrophilic and anionic, and cells attach more readily.
These materials are subject to both hydrolysis (via ester bonds) and oxidation
(via
ether bonds). Degradation rate is influenced by PEO molecular weight and
content, and
the copolymer with the highest water uptake degrades most rapidly.
These polymeric materials 102 can also be developed in such a way that they
are
stable in the biological environment, and degrade only under specific
enzymatic
conditions (plasmin, etc.). These materials can also include partially
expressed fragments
of human or animal fibrin such that the system degrades only in contact with
plasmin.
The polymer 114 is preferably in solution mixed with a suitable solvent, and
other
substances can be added to the solution, for example, collagen, drugs,
proteins, pep tides,
hydroxyapctitc crystals (HA), and antibiotics, depending on the type of tissue
to be
16
CA 02905816 2015-09-11
WO 2014/143131 PCT/US2013/051520
grown. The solution can be sonicated to promote mixing of the constituents.
By chosen a suitable polymer 114, the biocompatible structure 100 can achieve
controllable supply of therapeutic, analgesic and/or antibacterial substances,
growth
factors, proteins, peptides, drugs, tissue subcomponents including but not
limited to bone
particles and hydroxyappetite, which promote growth, prevent infections and
the like.
FIG. 2 schematically shows a Scanning Electron Microscopy image of a
biocompatible structure 100 at a low resolution according to certain
embodiments of the
present disclosure. The biocompatible structure 100 has bone particles 116
over porous
polymer membrane matrix and a hollow interior to promote cellular growth and
blood
flow. In FIG. 2, bioactive materials 126 are shown on the surface of the
biocompatible
structure 100. In certain embodiments, the bioactive materials 126 can be
sprayed on the
surface of the biocompatible structure 100, and/or incorporated in the polymer
structures
102 to promote bone growth.
FIGs. 3A-3C schematically shows Scanning Electron Microscopy images of a
biocompatible structure 100 at high resolutions according to certain
embodiments of the
present disclosure. As shown in FIGs. 3A-3C, the surface of the biocompatible
structure
100 made from polyurethane polymer and hydroxyapatite nanoparticles can be
very
rough and can have one or more polymeric pores 304. The polymeric pores 304
typically
are large in size. The size of the polymeric pores 304 can be from about 0.001
gm up to
about 10 mm. The nanostructural hydroxyapatatite 308 at the surface of the
biocompatible structure 300 can have a size of about 1 nm to about 500 nm, and
the
majority of the nanostructural hydroxyapatite 308 can have a size of about 2
nm to about
300 nm. Inside the biocompatible structure 100 is semi-empty due to the
spacing
between the layers offered by the bone particles. The pore size should vary
both in the
range of nanometer (nm) and the range of micrometer (gm).
When placed in an implant site, new tissue of a patient can grow across the
pores
on the surface of the biocompatible structure, and inside the hollow interior
of the
biocompatible structure.
In certain embodiments, the biocompatible structure 100 useful for bone and
17
CA 02905816 2015-09-11
WO 2014/143131 PCT/US2013/051520
tissue regeneration can be produced by the following procedures: A polymer 114
is
dissolved in a solvent to form a first solution. HAP nanoparticles 112 are
added to the
first solution to form a second solution. The second solution is applied to a
surface to
form a polymer film on the surface. A weight percentage of the first tissue
forming
material to the polymer is about 0.5-95%. The polymer film is cut into a
plurality of
strips 102. The biocompatible structure is formed by stacking the strips 102
and placing
bone particle layers 106 in between the strips 102. Then the structure is
coated by a
coating 110 formed from the second solution, and bone particles 116 are then
added onto
the surface of the coating 110.
(1) Dissolving a polymer in a solvent to form a first solution.
In certain embodiments, a polymer 114 is dissolved in a solvent to form a
first
solution. The polymer 114 can be a synthetic biodegradable polymer, a
biodegradable
polymer derived from natural source, or their mixture. In certain embodiment,
suitable
synthetic biodegradable polymer may include polyurethane, polylactide (PLA),
polyglycolide (PGA), poly(lactide-co-glycolide) (PLGA), poly(e-caprolactone),
polydioxanone, polyanhydride, trimethylene carbonate, po1y(13-
hydroxybutyrate), poly(g-
ethyl glutamate), poly(DTH iminocarbonate), poly(bisphenol A iminocarbonate),
poly(ortho ester), polycyanoacrylate, polyphosphazene, or their mixture. In
certain
embodiments, the biodegradable polymer derived from natural source may include
modified polysaccharides (cellulose, chitin, dextran), modified proteins
(fibrin, casein),
or their mixture.
In certain embodiment, the polymer 114 is an ester-type hydrophilic
polyurethane
with a linear expansion of 50-65 %. The water uptake of the polymer 114 varies
with its
composition, anywhere from 30-90%. The polymer 114 is thermoplastic.
Alternatively,
a thermosetting polymer 114 may work equally well. In certain embodiment, the
polymer 114 may be mixed with other polymers to control its degradation rate.
In certain
embodiment, the polymer is a powder with particles having a diameter of about
0.02-50
mm.
The solvent can be methanol or ethanol or any solvent of the polymer used. In
18
certain embodiment. other organic or inorganic solvent (polar aprotic and
prone) may
also be used. In certain embodiments, the solvent is at least one of acetone,
methyl ethyl
ketone, nitromethane, n-propanol, n-butanol, isopropanol, propylene carbonate,
dymethil
sulfoxide, acetonitrile dimethylformamide, ethyl acetate, and tetrahydrofuran,
dichloromethane,
The polymer 114 is evenly distributed in the first solution. In certain
embodiment, low power heating can be used to help the dissolvation of the
polymer in
the solvent. In certain embodiments, stirring is used to accelerate the
uniform
distribution of the polymer in the first solution. In certain embodiment,
afier complete
dissolvation of the solid polymer in the solvent, the first solution has a low
viscosity,
(2) Adding a first tissue forming material 112 to the first solution to form a
second solution.
The first tissue forming material 112 is then added to the first solution to
form a
second solution. In certain embodiments, the first tissue forming material 112
may
include nanoparticles of hydroxyapatitc (HAP), tricalcium phosphates, mixed
calcium
phosphates and calcium carbonate, bone particles of xenograft, allografts,
autografts,
alloplastic grafts, or a mixture thereof.
In certain embodiment. the HAP nanoparticles 112 have a dimensional range
between 1-100 inn. The HAP nanoparticiels 112 can be composed of pure HAP,
having
significant crystallinity, and having very good dispensability due to the
presence of
oxygen groups on the surface.
The polymer 114 and the first tissue forming material 112 are evenly
distributed
in the second solution. In certain embodiments, sonication is used to
accelerate the
homogenization of the polymer 114 and the first tissue forming material 112 in
the
second solution.
The weight percentage of the polymer 114 to the first tissue forming material
112
in the second solution is about 20: 1 to 2: 1. The ratio is related with the
characteristics of
the produced biocompatible structure 100. The characteristics of the
biocompatible
structure 100 include resistance to load and stress, porosity, degradation
rate, etc. In
19
CA 2905816 2017-07-31
certain embodiments, the ratio of the polymer 114 to the first tissue forming
material 112
can be adjusted to meet requirement of the condition of a patient, including
the bone
implant position, size, and metabolic rate of the patient.
In certain embodiment, the first polymer 114 is polyurethane and the first
tissue
= 5 forming material 112 is HAP nanopowder containing HAP
nanoparticles. The weight
ratio of the added dry HAP nanopowder to the dry mass of the added polymer
varies
according to the purpose of use.
In certain embodiment, as described below in connection with FIGs. 6-7, if the
weight ratio of the dry HAP nanopowders to the dry mass of polyurethane is
below 25%
(i.e., the weight percentage of dry HAP nanopowder in the total weight of dry
HAP
nanopower and dry mass of polymer is about 20%), the produced polymer film 102
as
described below is strong and hard. If the weight percentage of the dry RAP
nanopowden to the polyurethane is above 40 %, the produced the polymer film as
described below is weak and breaks easily. In certain embodiment, the HAP
nanoparticles 112 do not allow a good crosslinking of the polymer strands.
Therefore the
polymer film produced with a high ratio of HAP nanoparticles 112 is very
powdery and
breaks very easily.
(3) Applying the second solution to a surface to form a polymer film on the
surface.
In certain embodiment, the polymer film is fbirned by applying the second
solution to a surface, and allowing it to dry. In certain embodiment, the
second solution
can be dried at a room temperature (e.g.. 25 C). In certain embodiment, the
second
solution is mildly heated to form the polymer film on the surface, for
example, at a
temperature higher than room temperature (e.g., 25'C) and lower than 80 C. In
certain
embodiment, the drying process is under a vacuum condition. In certain
embodiment, the
surface is a Teflon Tm surface. In certain embodiment, the surface is a
polytetrafiuoroethylene (PTFE) surface. In certain embodiment, the second
solution can
be dried on a PTFE surface under vacuum and under mild heat t'or less than 24
hours to
form the polymer film, The thickness of the polymer film can be about 2-10 mm.
20
CA 2905816 2017-07-31
CA 02905816 2015-09-11
WO 2014/143131 PCT/US2013/051520
(4) Cutting the polymer film into a plurality of strips.
In certain embodiments, the formed polymer film is cut into the plurality of
strips.
The strips can be any suitable shape and size to produce a biocompatible
structure with a
predetermined shape and size. In certain embodiment, each of the strips 102 is
identical
to other strips. In certain embodiment, each of the strips has a length of
about 0.002-50
cm, a width of about 0.002-50 cm, and a thickness of about 0.001-50 mm.
(5) Forming the biocompatible structure 100 by the strips, the second
solution,
and a second tissue forming material.
In certain embodiment, the biocompatible structure 100 is formed from the
strips,
the second solution, and a second tissue forming material and the following
operations:
(a) Constructing a scaffold by stacking the strips to form polymer layers 102
and
adding bone particle layers 106 between the polymer layers. In certain
embodiments, a
strip is disposed on a surface as the first polymer layer 102. A first layer
of bone
particles 106 is then applied on the first polymer layer 102. A second strip
is then used to
cover the first bone particle layer 106 to form the second polymer layer 102.
By
alternatively disposing polymer layers 102 and bone particle layers 106, the
scaffold with
a predetermined shape and size is constructed. The scaffold structure composed
of
polymer layer 102 containing HAP nanoparticles 112, bone particle layer 106,
polymer
layer 102 containing HAP nanoparticles 112, bone particle layer 106
alternatively. In
certain embodiments, at least one polymer layer 102 is located as one of the
outside
layers of the scaffold. In certain embodiment, at least one bone particle
layer 106 is
located as one of the outside layers of the scaffold. In order for the entire
structure to
stay together, methanol or other solvent of the polymer is added by, for
example
pipetting, to superficially liquefy the polymer layers 102, such that the bone
particles 116
can be "trapped" in the polymer layers 102 when the structure dries. The bone
particles
116 can be partially embedded in the polymer layers 102. After the polymer
layers 102
re-solidifies, the bone particle layers 106 are connected with the polymer
layers 102.
(b) Applying the second solution to the scaffold to form a coated scaffold. In
certain embodiments, the scaffold built as described above is then coated by
covering
21
CA 02905816 2015-09-11
WO 2014/143131 PCT/US2013/051520
with a polymer film that is in a liquid form. In certain embodiment, the
second solution
is a sticky solution before applying to the scaffold. In certain embodiment,
part of the
second solution poured on the surface of the scaffold penetrates to the inside
of the
scaffold. The poured second solution forms a coat 110 on the surface of the
scaffold and
helps to hold the components of the scaffold together.
(c) In certain embodiment, the forming operation further includes adding the
second tissue forming material to the coated scaffold to form the
biocompatible structure
100. In certain embodiment, the second tissue forming material can be nano-
sized bone
particles, micro-sized bone particles, or a mixture thereof The structure is
then allowed
to dry overnight under vacuum and mild heat to form the biocompatible
structure
according to the present disclosure.
The biocompatible structure 100 can be any shape and size such that the
biocompatible structure matches the size of the bone defect that needs to be
regenerated.
In certain embodiment, the biocompatible structure has a cylindrical shape or
a spherical
shape. In certain embodiment, the length of the biocompatible structure is
about 2.5 cm
(1 inch) and the diameter is about 0.1-1 cm, which matches the diameter of the
bone that
needs to be replaced.
In certain embodiment, the method further includes subjecting the
biocompatible
structure 100 to plasma treatment. For example, once completely dried, the
biocompatible structure 100 is placed into glass vials for storage. The
biocompatible
structure 100 is plasma treated by a radio frequency (RF) plasma discharge
device, under
an environment of oxygen, nitrogen or a mixture of oxygen and nitrogen. In
certain
embodiment, the RF plasma treatment time is about 1-3 minutes. In certain
embodiment,
the plasma treated biocompatible structure 100 is sterilized and sent for
animal studies.
The purpose of the plasma treatment is to break the surface bonds of the
polymer. After
plasma treatment, oxygen atoms "attach" to the surface, changing the surface
energy of
the surface such that the surface becomes more hydrophilic and has oxygen and
nitrogen
rich functional groups.
In certain embodiment, the method of manufacturing the biocompatible structure
22
CA 02905816 2015-09-11
WO 2014/143131 PCT/US2013/051520
100 further includes adding a third tissue forming material to the
biocompatible structure
100. In certain embodiment, the third tissue forming material includes a
bioactive
material, cells, or a mixture thereof The bioactive material includes
proteins, enzymes,
growth factors, amino acids, bone morphogenic proteins, platelet derived
growth factors,
vascular endothelial growth factors, or a mixture thereof The cells includes
epithelial
cells, neurons, glial cells, astrocytes, podocytes, mammary epithelial cells,
islet cells,
endothelial cells, mesenchymal cells, stem cells, osteoblast, muscle cells,
striated muscle
cells, fibroblasts, hepatocytes, ligament fibroblasts, tendon fibroblasts,
chondrocytes, or a
mixture thereof
The biocompatible structure 100 can be any shape, size and weight to fit with
an
implant site. In certain embodiment, long bones were surgically removed from
the tibia
of goats, and biocompatible structures conform to the implant sited of the
goats according
to the present disclosure are used for bone regeneration of the goats.
In certain embodiment, when the biocompatible structure 100 is used in dental
applications for bone generation, the concentration of HAP nanoparticles can
be much
higher than the concentration of HAP nanoparticles in the biocompatible
structure for
some other bone regeneration, for example, tibia regeneration. In certain
embodiment,
the biocompatible structure for dental applications can be crumbled and forms
a lot of
particles with high surface area.
In certain embodiment, instead of manufacturing the biocompatible structure
100
and then using it as implant material, the biocompatible structure 100 can
also be formed
in situ. For example, a first polymer layer is air sprayed at an implant site
or a bone
defect area, a first layer of bone particles is then added to the polymer
layer and deposits
on the polymer layer. After that, a second polymer layer is air sprayed on the
first bone
particle layer, followed by adding a second layer of bone particles. The
process is
repeated until the biocompatible structure, including alternating polymer
layers and bone
particle layers, matches the implant site or mimics the bone defect that needs
to be
replaced.
In certain embodiment, a Doctor of Medicine (MD) can take a 3D computer axial
23
tomography scan (CAT) of a patient and sent the result for example by emailing
the CAT
scan file to a manufacturer. The manufacturer then can build the implant
according to the
present disclosure to perfectly match the actual bone defect.
FIG. 4 illustrates an example of preparing a biocompatible structure according
to
certain embodiments of the present disclosure.
In operation 402, 500 ml methanol is added to a I L beaker. The beaker is
placed
on a magnetic stirrer and a magnetic stir bar is used for mixing. 80 grams -
polyurethane
114 is then added to the methanol in the beaker. The solution is mixed by the
stirring bar
to completely dissolve the polyurethane in the methanol solvent and uniformly
distributed the polyurethane 114 in the solution. The mixing and dissolving of
polyurethane is at room temperature. In certain embodiment, the solution can
be heated
to accelerate the process.
In operation 406,20 gram HAP nanoparticles 112 (e.g., Berkeley Advanced
Biomaterials, Inc.) is then added to the solution. Sonication is applied to
guarantee the
evenly distribution of the HAP nanoparticles 112 in the solution.
In operation 410, 10 ml of the solution is pipetted from the beaker and
applied to
PTFE surface. A thin layer of solution is formed on the PTEF surface. The thin
layer of
solution is allowed to dry at room temperature for variable times to form a
polymer film.
Alternatively, the layer of solution on the PTFE surface can be placed in an
oven to heat
or low pressure for a period of time to accelerate the formation of the
polymer film. In
certain embodiment, the temperature can be about30-70cC, and the period of
time for the
heating is about 2-1500 minutes. In certain embodiment, the second solution is
allowed
to dry on a PTFE surface under vacuum under mild heat for less than 24 hours
to form
the polymer film. The thickness of the polymer film can be about 0.01-50 mm.
In operation 414, the polymer film is then cut into identical strips with a
length of
about 0,05-20 cm, a width of about 0.02-5 cm, and a thickness of about 0.01-50
mm. In
certain embodiment, the polymer film can be cut into strips with varies shape
and size.
In operation 418, a first strip is placed on the PTFE surface to form a first
polymer layer 102. A first layer of bone particles 106 is added on the surface
of the first
24
CA 2905816 2017-07-31
polymer layer 102. A second strip is placed onto the first bone particle layer
106 to form
a second polymer layer 102. Then a second bone particle layer 106 is formed on
the
second polymer layer 102. By alternatively disposing the strips and the bond
particle
layers, a three-dimensional scaffold is formed with a predetermined shape and
size.
In order for the entire structure to stay together, methanol or other solvent
of the
polymer is added by, for example pipetting. to superficially liquefy the
polymer layers
102, such that the bone particles 116 can be "trapped" in the polymer layers
102 when
the structure dries. The bone particles 116 can he partially embedded in the
polymer
layers 102. After the polymer layers 102 re-solidifies, the bone particle
layers 106 are
connected with the polymer layers 102. Alternatively, after adding each bone
particle
layer 106, the methanol or other solvent can be added to trap or embed the
bone particles
116 in the corresponding polymer layers 102.
Next, l ml of the methanoltpolyurethane/HAP nanoparticle solution is added to
the surface of the three-dimensional scaffold and allowed to dry. Accordingly,
a coating
110 is formed on the surface of the three-dimensional scaffold. ln certain
embodiment,
the coating 110 not only covers the outside of the three-dimensional scaffold,
but also
can penetrate to the inside of the three-dimensional scaffold.
Further, bone particles 116 or other suitable particles may be added to the
surface
of the coating 110.
In operation 422 and operation 426, the structure is then dried under vacuum
overnight. En certain embodiment, the structure is further subjected to plasma
treatment.
A series of biocompatible structures 100 is produced according to the above
example by varying the HAP concentration. The HA.P concentration in the
polymer film
is closely related with the characters of the produced biocompatible structure
100.
FIGs. 5A and 5B show a pull test system 500 used to measure the maximum load
and maximum stress of polymer films 550 with various concentrations of
polyurethane
and HAP nanopartiele in accordance with certain embodiments of the present
disclosure.
In one example, the mechanical behavior of the composites was analyzed using
an
A DM EIThi 7600 EXPERT single-column, universal, electromechanical testing
machine.
25
CA 2905816 2017-07-31
The instrument performs a "pull test" by stretching the polymer film in its
axial direction
and instantaneously produces a "csv" file using the eP2 Digital Controller and
Gauge
Safe Basic Testing Software. The pull test system 500 includes a pull test
structure 510, a
digital controller 530 and, optionally, a computer 540. The pull test
structure 510 has a
base 511, a column513 fixed to and perpendicular to the base 511, a bottom
head 515
connected with two bottom grips 517a and 517h facing each other, a top head
521
connected with two top grips 519a and 519b facing each other, a scale 523
attached to
the column 513, and a rail 525 placed in the column 513. At least one of the
top head 521
and the bottom head 515 is connected with the rail 525 and is movable along
the rail 525.
In this embodiment. the top head 521 is connected through a chain or a cable
to a motor
(not shown) and the chain or the cable pulls/drives the top head 521 along the
rail 525.
The top grips 519a/519b move together and at the same speed with the top head
521.
Polymer films 550 were prepared and tested. In certain embodiment, the polymer
films 550 contain various concentrations of polyurethane and HAP
nanoparticles. In one
embodiment, the weight percentage of the HAP nanoparticles in the polymer
.films are
0%, 0.5%, 1%, 2%, 3%. 5%, 10%, 15%, 20% and 30% respectively. As described
above,
the weight percentage of the HAP nanoparticles is defined as the weight of the
HAP
nanopartiele powder (in gram) used for preparing the polymer film divided by
the total
weight of HAP nanoparticle powder (in gram) and solid polymers (in gram) used
for
preparing the polymer film 550. The polymer films 550 used in the test have
predetermined dimensions. In certain embodiments, the size of the polymer
films 550 is
6 cm x 1.5 cm x 0.02 cm. In certain embodiment, polymer films with the same
concentration of HAP nanoparticles are prepared with different sizes for
testing.
During the maximum load and maximum stress testing process, the top grips
521a152Ib and the bottom grips 517a15 17h clip two ends of the polymer film
550 in the
longtitudial direction of the polymer film 550. The dimension of the polymer
film 550
and the parameters of the force to be used are entered into the digital
controller 530. In
certain embodiments, the length of the polymer film used in the calculation is
an
effective length, for example, measured by the scale, from the bottom edges of
the top
grips
26
CA 2905816 2017-07-31
519a/519b to the top edges of the bottom grips 517w517b. In certain
embodiments, if the
polymer film 550 clipped between the top grips 51911b and the bottom grips
517eb has a
dog bone shape, the length used for calculation is the narrow portion of the
dog bone
shape. When the testing starts. the motor moves at least one of the top head
521 and the
bottom head 515, for example. the top head 521. The top grips 519a1519b move
together
and at the same speed with the top head 521 to pull the polymer film 550 at a
predetermined speed. In certain embodiment, the speed can he 0.01-2.5 mm per
minute.
The top grips 519a1519b move along the rail 525 at a predetermined speed to
pull the
polymer film 550 until the polymer film 550 breaks. The original dimensions of
the
polymer film 550, the moving speed of the top gips 519a1519b. the length of
the
polymer film 5.50 immediately before it breaks are recorded. The maximum load
and the
maximum stress are calculated. In certain embodiments, the calculation is
performed by a
processor (not shown) in the computer 540. The maximum load is the pull force
(newton)
applied to the polymer film 550 when the polymer film breaks. The maximum
stress
(KPa) is the pull force applied to the polymer film 550 when the polymer film
550 breaks
divided by the cross-sectional area of the polymer film 550 (the original
width times the
original thickness of the polymer film 550).
The load and stress tests are performed for the polymer films 550 made
according
to the present disclosure. In certain embodiments, the polymer films contain
various
. 20 concentrations of polyurethane and HAP nanoparticles.
FIG. 6 is a load graph of the polymer films 550 in a two dimensional
coordinate
system, which shows a functional relationship between the weight percentage of
the HAP
nanoparticles in a polymer film and a maximum load of that polymer film. The X-
axis of
the coordinate system is the weight percentage of the HAP nanopaitieles and
the Y-axis
of the coordinate system is the maximum load of the polymer film. As shown in
FIG. 6,
the maximum load (in newton) for the polymer films 550 containing 0%, 0.5%,
1%. 2%,
3%, 5%, 10%, 20%) and 30%> of HAP nanoparticles are measured and calculated.
The
maximum load increases sharply from about 20 newton (N) to about 44 N when the
HAP
concentration increases from 0% to about 1%. Then the maximum load drops to
about 31
27
CA 2905816 2017-07-31
N when the HAP concentration increases from I % to around 10%. After that, the
maximum load increases again to about 41 N at around 20% HAP concentration and
drops to about 38 N at around 30% HAP concentration. Thus. the load graph has
two
peaks corresponding to 1% and around 20% of HAP concentration. In certain
embodiment, the second peak at around 20% HAP concentration in the load graph
is
named load peak.
FIG. 7 is a stress graph of the polymer films 550 in a two dimensional
coordinate
system, which shows a functional relationship between the weight percentage of
the HAP
nanoparticles in a polymer film and a maximum load of that polymer film. The X-
axis of
the coordinate system is the weight percentage of the 11AP nanoparticles and
the Y-axis
of the coordinate system is the maximum stress of the polymer film 550. As
shown in
FIG. 7, the maximum stress (in KPa) Ibr the polymer films containing 0%, 0.5%,
1%,
2%>, 3%, 5%>, 10%), 20%) and 30%> of HAP nanoparticles are measured and
calculated. The maximum stress increases from about 11,000 KPa to about 15,000
KPa
when the HAP concentration increases from 0% to about 1%. Then the maximum
stress
decreases to about 13,600 KPa when the HAP concentration increases from 1% to
about
3%. After that, the maximum stress increases to about 22,000 Kra when the HAP
concentration increases from about 3% to about 20%. Further increasing HAP
concentration in the polymer films from about 20% to 30% can result in
decreasing of
the maximum stress from 22,000 to about 20,800 KPa. Thus. the stress graph has
two
peaks corresponding to 1% and 20% of HAP concentration. In certain embodiment,
the
second peak at 20% HAP concentration in the stress graph is named stress peak.
In certain embodiments, a computer 540 can be used to calculate optimal weight
percentage of HAP in the polymer film 550 according to the above load and
stress graphs
of a series of polymer films 550. The computer 540, utilizing one or more
CPUs, can
receive the data from the pull test structure 510 and the digital controller
530, run a
calculation software, and then present the result on a monitor.
An optimal weigh percentage of HAP in the polymer film 550 is determined
based on the results from the load graph and the stress graph by the computer
540. In
28
CA 2905816 2017-07-31
CA 02905816 2015-09-11
WO 2014/143131 PCT/US2013/051520
certain embodiments, both the load graph and the stress graph have at least
two peaks.
The first peak 604 in the load graph corresponding to a lower HAP
concentration, and the
second peak 608 in the load graph corresponding to a higher HAP concentration.
The
first peak 704 in the stress graph corresponding to a lower HAP concentration,
and the
second peak 708 in the stress graph corresponding to a higher HAP
concentration. The
second peak 608 in the load graph is named load peak 608, and the second peak
708 in
the stress graph is named stress peak 708. The peak values from the load peak
608 and
the stress peak 708 are extracted. In this example, both of the load peak 608
and the
stress peak 708 correspond to a HAP weight percentage (HAP concentration) of
20%.
The maximum value and the minimum value of the load peak 608 and the stress
peak 708
are determined. In this example, both the maximum value and the minimum value
are
20%. The optimal concentration range has an upper limit value and a lower
limit value.
The upper limit value is the maximum value plus a first predetermined value.
The lower
limit value is the minimum value minus a second predetermined value. Each of
the first
predetermined value and the second predetermined value can be, for example,
10%, 5%,
or 0%. Accordingly, in this example, the optimal concentration range of the
HAP in the
polymer film is 10%-30%, preferably 15%-25%, and more preferably 20%.
In another example, the load peak 608 and the stress peak 708 have different
values. For example, the load peak may be at 17.5% and the stress peak may be
at
22.5%. Accordingly, the maximum value is 22.5% and the minimum value is 17.5%.
With the first and second predetermined values at about 10%, preferably 5%,
and more
preferably 0%, the optimal concentration ranges of the HAP weight percentage
in the
polymer film are 7.5%-32.5%, preferably 12.5%-27.5%, and more preferably 17.5%-
22.5%. In other embodiments, the first and second predetermined values can be
different
values.
In certain embodiments, according to the results shown in FIGs. 6 and 7, the
polymer film with 20% HAP concentration shows good structure stability and
strength.
In certain embodiments, the biocompatible structure 100 prepared according to
the present disclosure for the treatment of animals and/or humans. In certain
29
CA 02905816 2017-01-11
embodiment, long bones were surgically removed from the tibia of goats. For
generating long bones of these goats, biocompatible structures of a weight
about 1.0-
2.5 grams (g) were used. For example, 10 implants with the weight of 2.39 g,
2.34 g,
2.11 g, 1.86 g, 2.135 g, 2.18 g, 1.55 g, 2.5 g, 1.22 g, and 1.69 g,
respectively, were
used to generate long bones for the goats with surgically removed tibia part.
For the
above 10 examples, the biocompatible structure was made by using 4.52 g of
polymer
(polyurethane), 0.45 g of HAP nanoparticles, and 15 g of bone particles.
The bone growth using the biocompatible structures 100 according to
embodiments of the present disclosure has maturity and integrity.
The foregoing description of the exemplary embodiments of the disclosure has
been presented only for the purposes of illustration and description and is
not
intended to be exhaustive or to limit the disclosure to the precise forms
disclosed.
Many modifications and variations arc possible in light of the above teaching.
The embodiments are chosen and described in order to explain the principles
of the disclosure and their practical application so as to activate others
skilled in the
art to utilize the disclosure and various embodiments and with various
modifications
as are suited to the particular use contemplated. Alternative embodiments will
become apparent to those skilled in the art to which the present disclosure
pertains.
Accordingly, the scope of the present disclosure is defined by the appended
claims
rather than the foregoing description and the exemplary embodiments described
therein.