Note: Descriptions are shown in the official language in which they were submitted.
PROCESS FOR RECOVERING ALKALI METALS AND SULFUR FROM
ALKALI METAL SULFIDES AND POLYSULFIDES
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Provisional Patent
Application No.
61/781,557, filed March 14, 2013.
GOVERNMENT LICENSE RIGHTS
[0002] This invention was made with government support under Award No. DE-
FE0000408 awarded by the United States Department of Energy. The government
has certain
rights in the invention.
FIELD OF THE INVENTION
[0003] The present invention relates to a process for removing nitrogen,
sulfur, and heavy
metals from sulfur-, nitrogen-, and metal-bearing shale oil, bitumen, heavy
oil, or refinery
streams. More particularly, the invention relates to a method of regenerating
alkali metals
from sulfides (mono- and polysulfides) of those metals. The invention further
relates to the
removal and recovery of sulfur from alkali metal sulfides and polysulfides.
BACKGROUND OF THE INVENTION
[0004] The demand for energy and the hydrocarbons from which that energy is
derived is
continually rising. The hydrocarbon raw materials used to provide this energy,
however,
contain difficult to remove sulfur and metals that hinder their usage. Sulfur
can cause air
pollution, and can poison catalysts designed to remove hydrocarbons and
nitrogen oxide from
motor vehicle exhaust. Similarly, other metals contained in the hydrocarbon
stream can
poison catalysts typically utilized for removal of sulfur through standard and
improved
hydro-desulfurization processes whereby hydrogen reacts under extreme
conditions to break
down the sulfur bearing organo-sulfur molecules.
[0005] Extensive reserves of shale oil exist in the U.S. that will
increasingly play a role in
meeting U.S. energy needs. Over 1 trillion barrels reserves lay in a
relatively small area
known as the Green River Formation located in Colorado, Utah, and Wyoming. As
the price
of crude oil rises, the resource becomes more attractive but technical issues
remain to be
1
Date Recue/Date Received 2020-07-27
CA 02906000 2015-09-11
WO 2014/152393 PCT/US2014/027292
solved. A key issue is addressing the relatively high level of nitrogen
contained in the shale
oil chemistry after retorting as well as addressing sulfur and metals content.
[0006] Shale oil characteristically is high in nitrogen, sulfur, and heavy
metals which
makes subsequent hydrotreating difficult. According to America's Strategic
Unconventional
Fuels, Vol. III ¨ Resource and Technology Profiles, p. 111-25, nitrogen is
typically around
2% and sulfur around 1% along with some metals in shale oil. Heavy metals
contained in
shale oil pose a large problem to upgraders. Sulfur and nitrogen typically are
removed
through treating with hydrogen at elevated temperature and pressure over
catalysts such as
Co-Mo/A1203 or Ni-Mo/A1203. These catalysts are deactivated as the metals mask
the
catalysts.
[0007] Another example of a source of hydrocarbon fuel where the removal of
sulfur
poses a problem is in bitumen existing in ample quantities in Alberta, Canada
and heavy oils
such as in Venezuela. In order to remove sufficient sulfur from the bitumen
for it to be useful
as an energy resource, excessive hydrogen must be introduced under extreme
conditions,
which creates an inefficient and economically undesirable process.
[0008] Over the last several years, sodium has been recognized as being
effective for the
treatment of high-sulfur petroleum oil distillate, crude, heavy oil, bitumen,
and shale oil.
Sodium is capable of reacting with the oil and its contaminants to
dramatically reduce the
sulfur, nitrogen, and metal content through the formation of sodium sulfide
compounds
(sulfide, polysulfide and hydrosulfide). Examples of the processes can be seen
in U.S. Pat.
Nos. 3,785,965; 3,787,315; 3,788,978; 4,076,613; 5,695,632; 5,935,421; and
6,210,564.
[0009] An alkali metal such as sodium or lithium is reacted with the oil at
about 350 C
and 300-2000 psi. For example 1-2 moles sodium and 1-1.5 moles hydrogen may be
needed
per mole sulfur according to the following initial reaction with the alkali
metal:
[0010] R¨ S ¨ R' + 2Na + H2 R-H + R'-H + Na2S
[0011] R,R',R"-N + 3Na + 1.5H2 R-H + R'-H + R"-H + Na3N
[0012] Where R, R', R" represent portions of organic molecules or organic
rings.
[0013] The sodium sulfide and sodium nitride products of the foregoing
reactions may be
further reacted with hydrogen sulfide according to the following reactions:
[0014] Na2S + H2S ¨> 2 NaHS (liquid at 375 C)
[0015] Na3N + 3H2S ¨> 3 NaHS + NH3
[0016] The nitrogen is removed in the form of ammonia which may be vented and
recovered. The sulfur is removed in the form of an alkali hydrosulfide, NaHS,
which is
separated for further processing. The heavy metals and organic phase may be
separated by
2
CA 02906000 2015-09-11
WO 2014/152393 PCT/US2014/027292
gravimetric separation techniques. The above reactions are expressed using
sodium but may
be substituted with lithium.
[0017] Heavy metals contained in organometallic molecules such as complex
porphyrins
are reduced to the metallic state by the alkali metal. Once the heavy metals
have been
reduced, they can be separated from the oil because they no longer are
chemically bonded to
the organic structure. In addition, once the metals are removed from the
porphyiin structure,
the nitrogen heteroatoms in the structure are exposed for further
denitrogenation.
[0018] The following is a non-limiting description of the foregoing process
of using alkali
metals to treat the petroleum organics. Liquid phase alkali metal is brought
into contact with
the organic molecules containing heteroatoms and metals in the presence of
hydrogen. The
free energy of reaction with sulfur and nitrogen and metals is stronger with
alkali metals than
with hydrogen so the reaction more readily occurs without full saturation of
the organics with
hydrogen. Hydrogen is needed in the reaction to fill in the where heteroatoms
and metals are
removed to prevent coking and polymerization, but alternatively, gases other
than hydrogen
may be used for preventing polymerization. Once the alkali metal compounds are
formed
and heavy metals are reduced to the metallic state, it is necessary to
separate them. This is
accomplished by a washing step, either with steam or with hydrogen sulfide to
form a
hydroxide phase if steam is utilized or a hydrosulfide phase if hydrogen
sulfide is used. At
the same time alkali nitride is presumed to react to form ammonia and more
alkali hydroxide
or hydrosulfide. A gravimetric separation such as centrifugation or filtering
can separate the
organic, upgraded oil, from the salt phase.
[0019] In conventional hydrotreating, instead of forming Na2S to
desulfurize, or forming
Na3N to denitrogenate, H2S and NH3 are formed respectively. The reaction to
form hydrogen
sulfide and ammonia is much less favorable thermodynamically than the
formation of the
sodium or lithium compounds so the parent molecules must be destabilized to a
greater
degree for the desulfurization of denitrogenation reaction to proceed.
According to T. Kabe,
A Ishihara, W. Qian, in Hydrodesulfurization and Hydrodenitrogenation, pp. 37,
110-112,
Wiley-VCH, 1999, this destabilization occurs after the benzo rings are mostly
saturated. To
provide this saturation of the rings, more hydrogen is required for the
desulfurization and
denitrogenation reactions and more severe conditions are required to achieve
the same levels
of sulfur and nitrogen removal compared to removal with sodium or lithium. As
mentioned
above, desulfurizing or denitrogenating using hydrogen without sodium or
lithium is further
complicated with the masking of catalyst surfaces from precipitating heavy
metals and coke.
3
CA 02906000 2015-09-11
WO 2014/152393 PCT/US2014/027292
Since the sodium is in the liquid phase, it can more easily access the sulfur,
nitrogen and
metals where reaction is desirable.
[0020] Once the alkali metal sulfide has been separated from the oil,
sulfur and metals are
substantially removed, and nitrogen is moderately removed. Also, both
viscosity and density
are reduced (API gravity is increased). Bitumen or heavy oil would be
considered synthetic
crude oil (SCO) and can be shipped via pipeline for further refining.
Similarly, shale oil will
have been considerably upgraded after such processing. Subsequent refining
will be easier
since the troublesome metals have been removed.
[0021] Although the effectiveness of the use of alkali metals such as
sodium in the
removal of sulfur has been demonstrated, the process is not commercially
practiced because a
practical, cost-effective method to regenerate the alkali metal has not yet
heretofore been
proposed. Several researchers have proposed the regeneration of sodium using
an electrolytic
cell, which uses a sodium-ion-conductive beta-alumina membrane. Beta-alumina,
however,
is both expensive and fragile, and no significant metal production utilizes
beta-alumina as a
membrane separator. Further, the cell utilizes a sulfur anode, which results
in high
polarization of the cell causing excessive specific energy requirements.
[0022] Metallic sodium is commercially produced almost exclusively in a
Downs-cell
such as the cell described in U.S. Pat. No. 1,501,756. Such cells electrolyze
sodium chloride
that is dissolved in a molten salt electrolyte to form molten sodium at the
cathode and
chlorine gas at the anode. The cells operate at a temperature near 600 C, a
temperature
compatible with the electrolyte used. Unlike the sulfur anode, the chlorine
anode is utilized
commercially both with molten salts as in the co-production of sodium and with
saline
solution as in the co-production of sodium hydroxide.
[0023] Another cell technology that is capable of reducing electrolyte
melting range and
operation of the electrolyzer to less than 200 C has been disclosed by
Jacobsen et al. in U.S.
Pat. No. 6,787,019 and Thompson et al. in U.S. Pat. No. 6,368,486. In those
disclosures, low
temperature co-electrolyte is utilized with the alkali halide to form a low
temperature melting
electrolyte.
[0024] Gordon in US Patent No. 8,088,270 teaches the utilization of
solvents which
dissolve sulfur at a cell operating temperature and dissolving sodium
polysulfide in such
solvents to form an anolyte which when introduced into a cell with an alkali
ion conductive
membrane are electrolyzed to form sulfur at the anode and alkali metal at the
cathode and
where a portion of the anolyte is removed from the cell, allowed to cool until
the sulfur
precipitates out.
4
CA 02906000 2015-09-11
WO 2014/152393 PCT/US2014/027292
[0025] It is an object of the present invention to provide a cost-effective
and efficient
method for the regeneration of alkali metals used in the desulfutization,
denitrogenation, and
demetallation of hydrocarbon streams. As will be described herein, the present
invention is
able to remove contaminants and separate out unwanted material products from
desulfurization / denitrogenation / demetallation reactions, and then recover
those materials
for later use.
[0026] Another objective of the present invention is to teach improvements
in the process
and device for recovering alkali metal from alkali metal sulfide generated by
the sulfur
removal and upgrading process.
BRIEF SUMMARY OF THE INVENTION
[0027] The present invention provides a process for removing nitrogen,
sulfur, and heavy
metals from sulfur-, nitrogen-, and metal-bearing shale oil, bitumen, heavy
oil, or refinery
streams. The present invention further provides an electrolytic process of
regenerating alkali
metals from sulfides, polysulfides, nitrides, and polynitrides of those
metals. The present
invention further provides an electrolytic process of removing sulfur from a
polysulfide
solution.
[0028] One non-limiting embodiment within the scope of the invention
includes a process
for oxidizing alkali metal sulfides and polysulfides electrochemically. The
process utilizes an
electrolytic cell having an alkali ion conductive membrane configured to
selectively transport
alkali ions, the membrane separating an anolyte compartment configured with an
anode and a
catholyte compartment configured with a cathode. An anolyte solution is
introduced into the
anolyte compartment. The anolyte solution includes an alkali metal sulfide
and/or
polysulfide and an anolyte solvent that partially dissolves elemental sulfur
and alkali metal
sulfide and polysulfide. A catholyte solution is introduced into the catholyte
compartment.
The catholyte solution includes alkali metal ions and a catholyte solvent. The
catholyte
solvent may include one of many non-aqueous solvents such as tetraethylene
glycol dimethyl
ether (tetraglyme), diglyme, dimethyl carbonate, dimethoxy ether, propylene
carbonate,
ethylene carbonate, diethyl carbonate. The catholyte may also include an
alkali metal salt
such as an iodide or chloride of the alkali metal. Applying an electric
current to the
electrolytic cell oxidizes sulfide and/or polysulfide in the anolyte
compartment to form higher
level polysulfide and causes high level polysulfide to oxidize to elemental
sulfur. The
electric current further causes alkali metal ions to pass through the alkali
ion conductive
membrane from the anolyte compartment to the catholyte compartment, and
reduces the
alkali metal ions in the catholyte compartment to form elemental alkali metal.
CA 02906000 2015-09-11
WO 2014/152393 PCT/US2014/027292
[0029] Sulfur may be recovered in the liquid form when the temperature
exceeds the
melting point of sulfur and the sulfur content of the anolyte exceeds the
solubility of the
solvent. Most of the anolyte solvents have lower specific gravity compared to
sulfur so the
liquid sulfur settles to the bottom. This settling may occur within a settling
zone in the cell
where the sulfur may be drained through an outlet. Alternatively a portion of
the anolyte
solution may be transferred to a settling zone out of the cell where settling
of sulfur may
occur more effectively than in a cell.
[0030] The melting temperature of sulfur is near 115 C so the cell is best
operated above
that temperature, above 120 C. At that temperature or above, the alkali metal
is also molten
if the alkali metal is sodium. Operation near a higher temperature, such as in
the 125-150 C
range, allows the sulfur to fully remain in solution as it is formed from the
polysulfide at the
anode, then when the anolyte flows to a settling zone, within or external to
the cell where the
temperature may be 5-20 C cooler, the declining solubility of the sulfur in
the solvent results
in a sulfur liquid phase forming which is has higher specific gravity and
settles from the
anolyte. Then when the anolyte flows back toward the anodes where sulfur is
forming
through electrochemical oxidation of polysulfide, the anolyte has solubility
has the capacity
to dissolve the sulfur as it is formed, preventing fouling and polarization at
the anodes or at
membrane surfaces.
[0031] In one non-limiting embodiment within the scope of the invention, a
cell for
electrolyzing an alkali metal sulfide or polysulfide is provided where the
cell operates at a
temperature above the melting temperature of the alkali metal and where the
cathode is
wholly or partially immersed in a bath of the molten alkali metal with a
divider between an
anolyte compartment and a catholyte compartment. In this case the catholyte
essentially
comprises molten alkali metal but may also include solvent and alkali metal
salt. The divider
may be permeable to alkali metal cations and substantially impermeable to
anions, solvent
and dissolved sulfur. The divider comprises in part an alkali metal conductive
ceramic or
glass ceramic. The divider may be conductive to alkali ions which include
lithium and
sodium.
[0032] In another non-limiting embodiment, a cell for electrolyzing an
alkali metal
polysulfide is provided with an anolyte compaitment and a catholyte
compartment where the
anolyte solution comprises a polar solvent and dissolved alkali metal
polysulfide. The
anolyte solution comprises a solvent that dissolves to some extent elemental
sulfur. The
anolyte may comprise a solvent where one or more of the solvents includes: N,N-
dimethylaniline, quinoline, tetrahydrofuran, 2-methyl tetrahydrofuran,
benzene,
6
CA 02906000 2015-09-11
WO 2014/152393 PCT/US2014/027292
cyclohexane, fluorobenzene, thrifluorobenzene, toluene, xylene, tetraethylene
glycol
dimethyl ether (tetraglyme), diglyme, isopropanol, ethyl propional, dimethyl
carbonate,
dimethoxy ether, dimethylpropyleneurea , formamide, methyl formamide, dimethyl
formamide, acetamide, methyl acetamide, dimethyl acetamide, triethylamine,
diethyl
acetamide, ethanol and ethyl acetate, propylene carbonate, ethylene carbonate,
and diethyl
carbonate.
[0033] In one
non-limiting embodiment, a method for oxidizing sulfides and polysulfides
electrochemically from an anolyte solution at an anode is disclosed where the
anolyte
solution comprises in part an anolyte solvent that dissolves to some extent
elemental sulfur.
In the method, the anolyte solvent that dissolves to some extent elemental
sulfur is one or
more of the following: N,N-dimethylaniline, quinoline,
tetrahydrofuran, 2-methyl
tetrahydrofuran, benzene, cyclohexane, fluorobenzene, thrifluorobenzene,
toluene, xylene,
tetraethylene glycol dimethyl ether (tetraglyme), diglyme, isopropanol, ethyl
propional,
dimethyl carbonate, dimethoxy ether, dimethylpropyleneurea , formamide, methyl
formamide, dimethyl formamide, acetamide, methyl acetamide, dimethyl
acetamide,
triethylamine, diethyl acetamide, ethanol and ethyl acetate, propylene
carbonate, ethylene
carbonate, and diethyl carbonate.
[0034] In
another non-limiting embodiment, a cell for electrolyzing an alkali metal
monosulfide or a polysulfide is provided with an anolyte compartment and a
catholyte
compartment where the anolyte solution comprises a polar solvent and dissolved
alkali metal
monosulfide or a polysulfide. The anolyte solution comprises a solvent that
dissolves to some
extent elemental sulfur. The anolyte may comprise a solvent where one or more
of the
solvents includes: N,N-dimethylaniline, quinoline, tetrahydrofuran, 2-methyl
tetrahydrofuran,
benzene, cyclohexane, fluorobenzene, thrifluorobenzene, toluene, xylene,
tetraethylene
glycol dimethyl ether (tetraglyme), diglyme, isopropanol, ethyl propional,
dimethyl
carbonate, dimethoxy ether, dimethylpropyleneurea, formamide, methyl
formamide, dimethyl
formamide, acetamide, methyl acetamide, dimethyl acetamide, triethylamine,
diethyl
acetamide, ethanol and ethyl acetate, propylene carbonate, ethylene carbonate,
and diethyl
carbonate.
[0035] In one non-limiting embodiment, a method for oxidizing monosulfide or
polysulfides electrochemically from an anolyte solution at an anode is
disclosed where the
anolyte solution comprises in part an anolyte solvent that dissolves to some
extent elemental
sulfur. In the method, the anolyte solvent that dissolves to some extent
elemental sulfur is one
or more of the following: N,N-dimethylaniline, quinoline, tetrahydrofuran, 2-
methyl
7
CA 02906000 2015-09-11
WO 2014/152393 PCT/US2014/027292
tetrahydrofuran, benzene, cyclohexane, fluorobenzene, thrifluorobenzene,
toluene, xylene,
tetraethylene glycol dimethyl ether (tetraglyme), diglyme, isopropanol, ethyl
propional,
dimethyl carbonate, dimethoxy ether, dimethylpropyleneurea, formamide, methyl
formamide,
dimethyl formamide, acetamide, methyl acetamide, dimethyl acetamide,
triethylamine,
diethyl acetamide, ethanol and ethyl acetate, propylene carbonate, ethylene
carbonate, and
diethyl carbonate.
[0036] In one non-limiting embodiment, the anolyte solvent comprises from
about 60-100
vol. % polar solvent and 0-40 vol. % apolar solvent. A blend of different
anolyte solvents
may help optimize the solubility of elemental sulfur and the solubility of
sulfide and
polysulfide.
[0037] Another non-limiting embodiment discloses a method for removal of
dissolved
elemental sulfur from a solvent/alkali metal polysulfide mixture includes
cooling, reducing
the solubility of sulfur in the solvent and causing a second liquid phase to
form comprising
elemental sulfur, and then separating the liquid phase sulfur from the liquid
phase solvent
mixture. The separation of liquid phase sulfur from liquid phase anolyte
includes one or more
of the following: gravimetric, centrifugation. The alkali metal polysulfide is
of the class
including sodium polysulfide and lithium polysulfide.
[0038] The present invention may provide certain advantages, including but
not limited to
the following:
100391 Removing an alkali metal continuously or semi-continuously in liquid
form from
the cell.
[0040] Removing sulfur continuously or semi-continuously in liquid form
from the cell.
[0041] Removing high alkali metal polysulfides and dissolved sulfur
continuously or
semi-continuously from the electrolytic cell, thereby reducing polarization of
the anode by
sulfur.
[0042] Separating sulfur continuously or semi-continuously from a stream
containing a
mixture of solvent, sulfur, and alkali metal polysulfides such that the
solvent and alkali metal
polysulfides are substantially recovered such that they can be returned back
to an electrolytic
process.
[0043] Operating the electrolytic cells at temperatures and pressures, so
that the
electrolytic cell materials of construction can include materials which would
not tolerate high
elevated temperature.
[0044] Reference throughout this specification to features, advantages, or
similar language
does not imply that all of the features and advantages that may be realized
with the present
8
CA 02906000 2015-09-11
WO 2014/152393 PCT/US2014/027292
invention should be or are in any single embodiment of the invention. Rather,
language
referring to the features and advantages is understood to mean that a specific
feature,
advantage, or characteristic described in connection with an embodiment is
included in at
least one embodiment of the present invention. Thus, discussion of the
features and
advantages, and similar language, throughout this specification may, but do
not necessarily,
refer to the same embodiment, but may refer to every embodiment.
[0045] Furthermore, the described features, advantages, and characteristics
of the
invention may be combined in any suitable manner in one or more embodiments.
One skilled
in the relevant art will recognize that the invention may be practiced without
one or more of
the specific features or advantages of a particular embodiment. In other
instances, additional
features and advantages may be recognized in certain embodiments that may not
be present in
all embodiments of the invention.
[0046] These features and advantages of the present invention will become
more fully
apparent from the following description and appended claims, or may be learned
by the
practice of the invention as set forth hereinafter.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
[0047] In order that the manner in which the above-recited and other
features and
advantages of the invention are obtained will be readily understood, a more
particular
description of the invention briefly described above will be rendered by
reference to specific
embodiments thereof that are illustrated in the appended drawings.
Understanding that these
drawings depict only typical embodiments of the invention and are not
therefore to be
considered to be limiting of its scope, the invention will be described and
explained with
additional specificity and detail through the use of the accompanying drawings
in which:
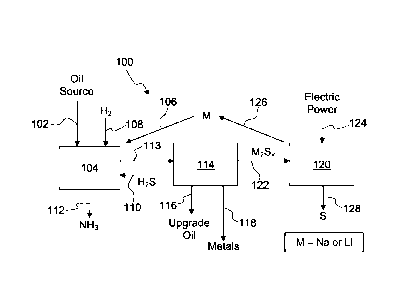
[0048] Figure 1 shows an overall process for removing nitrogen, sulfur, and
heavy metals
from sulfur-, nitrogen-, and metal-bearing oil sources using an alkali metal
and for
regenerating the alkali metal.
[0049] Figures 2A and 2B show schematic processes for converting alkali
metal
hydrosulfide to alkali metal polysulfide and recovering hydrogen sulfide.
[0050] Figure 3 shows a schematic cross-section of an electrolytic cell
which utilizes
many of the features within the scope of the invention.
[0051] Figure 4 shows a schematic of several electrolytic cells operated in
series to extract
alkali metal and oxidize alkali metal sulfide to polysulfide and low
polysulfide to high
polysulfide and high polysulfide to sulfur.
9
CA 02906000 2015-09-11
WO 2014/152393 PCT/US2014/027292
DETAILED DESCRIPTION OF THE INVENTION
[0052] The present embodiments of the present invention will be best
understood by
reference to the drawings, wherein like parts are designated by like numerals
throughout. It
will be readily understood that the components of the present invention, as
generally
described and illustrated in the figures herein, could be arranged and
designed in a wide
variety of different configurations. Thus, the following more detailed
description of the
embodiments of the methods and cells of the present invention, as represented
in Figures 1
through 4, is not intended to limit the scope of the invention, as claimed,
but is merely
representative of present embodiments of the invention.
[0053] The overall process is shown schematically in Figure 1 of one non-
limiting
embodiment for removing nitrogen, sulfur, and heavy metals from sulfur-,
nitrogen-, and
metal-bearing oil sources using an alkali metal and for regenerating the
alkali metal. In the
process 100 of Fig. 1, an oil source 102, such as high-sulfur petroleum oil
distillate, crude,
heavy oil, bitumen, or shale oil, is introduced into a reaction vessel 104. An
alkali metal (M)
106, such as sodium or lithium, is also introduced into the reaction vessel,
together with a
quantity of hydrogen 108. The alkali metal and hydrogen react with the oil and
its
contaminants to dramatically reduce the sulfur, nitrogen, and metal content
through the
formation of sodium sulfide compounds (sulfide, polysulfide and hydrosulfide)
and sodium
nitride compounds. Examples of the processes are known in the art,
including but not
limited to, U.S. Patent Nos. 3,785,965; 3,787,315; 3,788,978; 4,076,613;
5,695,632;
5,935,421; and 6,210,564.
[0054] The alkali metal (M) and hydrogen react with the oil at about 350 C
and 300-2000
psi according to the following initial reactions:
[0055] R ¨ S ¨ R' + 2M + H2 --> R-H + R'-H + M2S
[0056] R,R',R"-N + 3M + 1.5H2 ¨> R-H + R'-H + R"-H + M3N
[0057] Where R, R', R" represent portions of organic molecules or organic
rings.
[0058] The sodium sulfide and sodium nitride products of the foregoing
reactions may be
further reacted with hydrogen sulfide 110 according to the following
reactions:
[0059] M2S + H2S ¨> 2 MHS (liquid at 375 C)
[0060] M3N + 3H2S ¨> 3 MHS + NH3
[0061] The nitrogen is removed in the form of ammonia 112, which may be vented
and
recovered. The sulfur is removed from the oil source in the form of an alkali
hydrosulfide
(MHS), such as sodium hydrosulfide (NaHS) or lithium hydrosulfide (LiHS). The
reaction
products 113, are transferred to a separation vessel 114. Within the
separation vessel 114, the
CA 02906000 2015-09-11
WO 2014/152393 PCT/US2014/027292
heavy metals 116 and upgraded oil organic phase 118 may be separated by
gravimetric
separation techniques.
[0062] The alkali hydrosulfide (MHS) is separated for further processing.
The alkali
hydrosulfide stream may be the primary source of alkali metal and sulfur from
the process of
the present invention. When the alkali hydrosulfide is reacted with a medium
to high
polysulfide (i.e. M2Sx; 4<x<6) then hydrogen sulfide will be released and the
resulting
mixture will have additional alkali metal and sulfide content where the sulfur
to alkali metal
ratio is lower. The hydrogen sulfide 110 can be used in the washing step
upstream where
alkali sulfide and alkali nitride and metals need to be removed from the
initially treated oil.
[0063] A schematic representation of this process is shown in Fig. 2A. For
example, in
the case of sodium the following reaction may occur:
[0064] Na2Sx + 2NaHS ¨> H2S + 2[Na2S(x+i)/2i
[0065] Where x:y represent the average ratio of sodium to sulfur atoms in
the solution. In
the process shown in Fig. 2A, an alkali polysulfide with high sulfur content
is converted to an
alkali polysulfide with a lower sulfur content.
[0066] Alternatively, rather than reacting the alkali metal hydrosulfide
with an alkali
metal polysulfide, the alkali metal hydrosulfide can be reacted with sulfur. A
schematic
representation of this process is shown in Fig. 2B. For example, in the case
of sodium the
following reaction may occur:
100671 YS + 2NaHS H2S + Na2S(y+i)
[0068] Where Y is a molar amount of sulfur added to the sodium hydrosulfide.
[0069] The alkali metal polysulfide may be further processed in an
electrolytic cell to
remove and recover sulfur and to remove and recover the alkali metal. One
electrolytic cell
120 is shown in Fig. 1. The electrolytic cell 120 receives alkali polysulfide
122. Under the
influence of a source electric power 124, alkali metal ions are reduced to
form the alkali
metal (M) 126, which may be recovered and used as a source of alkali metal
106. Sulfur 128
is also recovered from the process of the electrolytic cell 120. A detailed
discussion of one
possible electrolytic cell that may be used in the process within the scope of
the present
invention is given with respect to Fig. 3. A more detailed discussion relating
to the recovery
of sulfur 128 is given with respect to Fig. 4, below.
[0070] The vessel where the reaction depicted in Figures 2A and 2B occurs
could be the
anolyte compaitment of the electrolytic cell 120 depicted in Figure 1, the
thickener 312
depicted in Figure 4, or in a separate vessel conducive to capturing and
recovering the
11
CA 02906000 2015-09-11
WO 2014/152393 PCT/US2014/027292
hydrogen sulfide gas 110 generated. Alternatively, sulfur generated in the
process depicted
in Figure 1 could be used as an input as depicted in Figure 2B.
[0071] Fig= 3 shows a schematic sectional view of an electrolytic cell 300
which utilizes
many of the features within the scope of the invention. The cell is comprised
of a housing
310, which typically is an electrical insulator and which is chemically
resistant to solvents
and sodium sulfide. A cation conductive membrane 312, in this case in the form
of a tube,
divides the catholyte compartment 314 from the anolyte compartment 316. Within
the
catholyte compartment is a cathode 324. The cathode 324 may be configured to
penetrate the
housing 310 or have a lead 326 that penetrates the housing 310 so that a
connection may be
made to negative pole of a DC electrical power supply (not shown). Within the
anolyte
compartment 316 is an anode 326 which in this case is shown as a porous mesh
type
electrode in a cylindrical form which encircles the membrane tube 312. A lead
328
penetrates the housing so that a connection may be made with a positive pole
of the DC
power supply. An anolyte solution flows through an anolyte inlet 330. The
anolyte is
comprised of a mixture of solvents and alkali metal sulfides. As anolyte flows
in through the
inlet 330 anolyte also flows out of the outlet 332. In some cases a second
liquid phase of
molten sulfur may also exit with the anolyte. A second outlet may be provided
from the
anolyte compartment at a location lower than the anolyte outlet 332. The
second, lower outlet
may be used more for removal of molten sulfur that has settled and accumulated
at the cell
bottom. The space between the cathode 324 and the membrane 312 is generally
filled with
molten alkali metal. As the cell operates, alkali metal ions pass through the
membrane 312
and reduce at the cathode 324 to form alkali metal in the catholyte
compartment 314 resulting
in a flow of alkali metal through the catholyte outlet 334.
[0072] A cell may have multiple anodes, cathodes, and membranes. Within a
cell the
anodes all would be in parallel and the cathodes all in parallel.
[0073] Referring to Figure 3, electrolytic cell housing 310 is preferably
an electrically
insulative material such as most polymers. The material also is preferably
chemically
resistant to solvents. Polytetrafluoroethylene (PTFE) is particularly
suitable, as well as
Kynar polyvinylidene fluoride, or high density polyethylene (HDPE). The cell
housing 310
may also be fabricated from a non insulative material and non-chemically
resistant materials,
provided the interior of the housing 310 is lined with such an insulative and
chemically
resistant material. Other suitable materials would be inorganic materials such
as alumina,
silica, alumino-silicate and other insulative refractory or ceramic materials.
12
CA 02906000 2015-09-11
WO 2014/152393 PCT/US2014/027292
[0074] The cation conductive membrane 312 preferably is substantially
permeable only to
cations and substantially impermeable to anions, polyanions, and dissolved
sulfur. The
membrane 312 may be fabricated in part from an alkali metal ion conductive
material. If the
metal to be recovered by the cell is sodium, a particularly well suited
material for the divider
is known as NaSICON which has relatively high ionic conductivity at room
temperature. A
typical NaSICON composition substantially would be Nai+.Zr2SixP3_x012 where
0<x<3.
Other NaSICON compositions are known in the art. Alternatively, if the metal
to be
recovered in the cell is lithium, then a particularly well suited material for
the divider would
be lithium titanium phosphate (LTP) with a composition that is substantially,
Li(l-Fx+4y)AixTi(l-
x_y)(PO4)3 where 0<x<0.4, 0<y<0.2. Other suitable materials may be from the
ionically
conductive glass and glass ceramic families such as the general composition
Lii+xAlxGe2_
xPO4. Other lithium conductive materials are known in the art. The membrane
312 may have
a portion of its thickness which has negligible through porosity such that
liquids in the
anolyte compartment 316 and catholyte compartment 314 cannot pass from one
compartment
to the other but substantially only alkali ions (Mt), such as sodium ions or
lithium ions, can
pass from the anolyte compartment 316 to the catholyte compartment 314. The
membrane
may also be comprised in part by an alkali metal conductive glass-ceramic such
as the
materials produced by Ohara Glass of Japan.
[0075] The anode 326 is located within the anolyte compartment 316. It may
be
fabricated from an electrically conductive material such as stainless steel,
nickel, iron, iron
alloys, nickel alloys, and other anode materials known in the art. The anode
326 is connected
to the positive terminal of a direct current power supply. The anode 326 may
be a mesh,
monolithic structure or may be a monolith with features to allow passage of
anolyte through
the anode structure. Anolyte solution is fed into the anolyte compartment
through an inlet
330 and passes out of the compartment through and outlet 332. The electrolytic
cell 300 can
also be operated in a semi-continuous fashion where the anolyte compartment is
fed and
partially drained through the same passage.
[0076] The electronically conductive cathode 324 is in the form of a strip,
band, rod, or
mesh. The cathode 324 may be comprised of most electronic conductors such as
steel, iron,
copper, or graphite. A portion of the cathode may be disposed within the
catholyte
compartment 314 and a portion outside the catholyte compartment 314 and cell
housing 310
for electrical contact. Alternatively, a lead 325 may extend from the cathode
outside the cell
housing 310 for electrical contact.
13
CA 02906000 2015-09-11
WO 2014/152393 PCT/US2014/027292
[0077] Within
the catholyte compartment 314 is an alkali ion conductive liquid which
may include a polar solvent. Non-limiting examples of suitable polar solvents
are as
tetraethylene glycol dimethyl ether (tetraglyme), diglyme, dimethyl carbonate,
dimethoxy
ether, propylene carbonate, ethylene carbonate, diethyl carbonate and such. An
appropriate
alkali metal salt, such as a chloride, bromide, iodide, perchlorate,
hexafluorophosphate or
such, is dissolved in the polar solvent to form that catholyte solution. Most
often the
catholyte is a bath of molten alkali metal.
[0078] One non-
limiting example of the operation of the electrolytic cell 300 is described
as follows: Anolyte solution is fed into the anolyte compartment 316. The
electrodes 324,
326 are energized such that there is an electrical potential between the anode
326 and the
cathode 324 that is greater than the decomposition voltage which ranges
between about 1.8V
and about 2.5V depending on the composition. Concurrently, alkali metal ions,
such as
sodium ions, pass through the membrane 312 into the catholyte compartment 314,
sodium
ions are reduced to the metallic state within the catholyte compai __ tment
314 with electrons
supplied through the cathode 324, and sulfide and polysulfide is oxidized at
the anode 326
such that low polysulfide anions become high polysulfide anions and/or
elemental sulfur
forms at the anode. While sulfur is formed it is dissolved into the anolyte
solvent in entirety
or in part. On sulfur saturation or upon cooling, sulfur may form a second
liquid phase of
that settles to the bottom of the anolyte compartment 316 of the electrolytic
cell. The sulfur
may be removed with the anolyte solution to settle in a vessel outside of the
cell or it may be
directly removed from a settling zone 336 via an optional sulfur outlet 338,
as shown in Fig.
3.
[0079] A mode
of operation may be to have the anolyte of one electrolytic cell flow into a
second cell and from a second cell into a third cell, and so forth where in
each successive cell
the ratio of sodium to sulfide decreases as the polysulfide forms become of
higher order.
Figure 4 is non-limiting schematic of four electrolytic cells, 402, 404, 406,
408 operated in
series to extract alkali metal and oxidize alkali metal sulfide to low alkali
metal polysulfide,
to oxide low alkali metal polysulfide to higher alkali metal polysulfide, and
to oxide higher
alkali metal polysulfide to high alkali metal polysulfide, and to oxide high
alkali metal
polysulfide to sulfur. The electrolytic cells 402, 404, 406, and 408 may be
operated such that
only in the final cell is sulfur produced but where alkali metal is produced
at the cathode of
all of them.
[0080] In a non-
limiting example, an alkali metal monosulfide, such as sodium sulfide
(Na2S) may be introduced into the first electrolytic cell 402. Under the
influence of a DC
14
CA 02906000 2015-09-11
WO 2014/152393 PCT/US2014/027292
power supply, sodium ions are transported from the anolyte compartment to the
catholyte
compat tinent where the alkali ions are reduced to form alkali metal.
Sulfide is oxidized in
the anolyte compartment to form a low polysulfide, such as Na2S4. The low
alkali metal
polysulfide is transported to the anolyte compartment of a second electrolytic
cell 404.
Under the influence of a DC power supply, sodium ions are transported from the
anolyte
compartment to the catholyte compartment where the alkali ions are reduced to
form alkali
metal. The low polysulfide is oxidized in the anolyte compartment to form a
higher
polysulfide, such as Na2S6. The higher alkali metal polysulfide is transported
to the anolyte
compartment of a third electrolytic cell 406. Under the influence of a DC
power supply,
sodium ions are transported from the anolyte compartment to the catholyte
compartment
where the alkali ions are reduced to form alkali metal. The higher polysulfide
is oxidized in
the anolyte compartment to form a high polysulfide, such as Na2S8. The high
alkali metal
polysulfide is transported to the anolyte compartment of a fourth electrolytic
cell 408. Under
the influence of a DC power supply, sodium ions are transported from the
anolyte
compartment to the catholyte compartment where the alkali ions are reduced to
form alkali
metal. High polysulfide is oxidized in the anolyte compartment to form sulfur,
which is
subsequently removed from the anolyte compat tment and recovered.
[0081] It will be understood that the foregoing examples of different
polysulfides are
given as representative examples of the underlying principle that that higher
order
polysulfides may be formed by and the combination of oxidizing the polysulfide
and
removing sodium ions.
[0082] The multi-cell embodiment described in relation to Figure 4 enables
alkali metal
and sulfur to be formed more energy efficiently compared to a single cell
embodiment. The
reason for the energy efficiency is because it requires less energy to oxidize
lower
polysulfides compared to higher polysulfides. The voltage required to oxidize
polysulfides to
sulfur is about 2.2 volts, whereas monosulfide and low polysulfide may be
oxidized at a
lower voltage, such as 1.7 volts.
[0083] In the case of the alkali metal being sodium, the following typical
reactions may
occur in the electrolytic cell 300:
[0084] At the Cathode:
[0085] Na+ + e- ¨> Na
[0086] At the Anode:
[0087] 1) Na2Sx ¨> Na+ + e + v2 Na2s(2.)
[0088] 2) Na2S, ¨> Na+ + e- + Na2S8 + x/16 S8
CA 02906000 2015-09-11
WO 2014/152393 PCT/US2014/027292
[0089] Where x ranges from 0 to about 8.
[0090] Most sodium is produced commercially from electrolysis of sodium
chloride in
molten salt rather than sodium polysulfide, but the decomposition voltage and
energy
requirement is about half for polysulfide compared to chloride as shown in
Table 1.
[0091] Table 1. Decomposition voltage and energy (watt-hour/mole) of sodium
and
lithium chlorides and sulfides
NaC1 Na2S LiC1 Li2S
V 4.0 <2.1 4.2 2.3
Wh/mole 107 <56 114 60
[0092] The open circuit potential of a sodium/polysulfide cell is as low as
1.8V when a
lower polysulfide, Na2S3 is decomposed, while the voltage rises with rising
sulfur content.
Thus, it may be desirable to operate a portion of the electrolysis using
anolyte with lower
sulfur content. In one embodiment, a planar NaSICON or Lithium Titanium
Phosphate
(LTP) membrane is used to regenerate sodium or lithium, respectively. NaSICON
and LTP
have good low temperature conductivity as shown in Table 2. The conductivity
values for
beta alumina were estimated from the 300 C conductivity and activation energy
reported by
May. G. May, J. Power Sources,3,1 (1978).
[0093] Table 2. Conductivities of NaSICON, LTP, Beta alumina at 25 C, 120 C
Conductivity mS/cm
Temperature C NaSICON LTP Beta alumina (est)
25 0.9 0.9 0.7
120 6.2 1.5 7.9
[0094] It may be beneficial to operate 2 or more sets of cells, a non-
limiting example of
which is shown in Figure 4. Some cells would operate with lower order sulfide
and
polysulfides in the anolyte while another set of cells operate with higher
order polysulfide. In
the latter, free sulfur would become a product requiring removal.
[0095] The following example is provided below which discusses one specific
embodiment within the scope of the invention. This embodiment is exemplary in
nature and
should not be construed to limit the scope of the invention in any way.
[0096] An electrolytic flow cell utilizes a 1" diameter NaSICON membrane
with
approximately 3.2 cm2 active area. The NaSICON is sealed to a scaffold
comprised of a non-
conductive material that is also tolerant of the environment. One suitable
scaffold material is
alumina. Glass may be used as the seal material. The flow path of electrolytes
will be
through a gap between electrodes and the membrane. The anode (sulfur
electrode) may be
16
CA 02906000 2015-09-11
WO 2014/152393 PCT/US2014/027292
comprised of aluminum. The cathode may be either aluminum or stainless steel.
It is within
the scope of the invention to configure the flow cell with a bipolar
electrodes design.
Anolyte and catholyte solutions will each have a reservoir and pump. The
anolyte reservoir
will have an agitator. The entire system will preferably have temperature
control with a
maximum temperature of 150 C and also be configured to be bathed in a dry
cover gas. The
system preferably will also have a power supply capable of delivering to 5 VDC
and up to
100 mA/ cm2.
[0097] As much as possible, materials will be selected for construction
that are corrosion
resistant with the expected conditions. The flow cell will be designed such
that the gap
between electrodes and membrane can be varied.
[0098] In view of the foregoing, it will be appreciated that the disclosed
invention
includes one or more of the following advantages:
[0099] Removing an alkali metal continuously or semi-continuously in liquid
form from
the cell.
[00100] Removing sulfur continuously or semi-continuously in liquid form from
the cell.
[00101] Removing high alkali metal polysulfides and dissolved sulfur
continuously or
semi-continuously from the electrolytic cell, thereby reducing polarization of
the anode by
sulfur.
[00102] Separating sulfur continuously or semi-continuously from a stream
containing a
mixture of solvent, sulfur, and alkali metal polysulfides such that the
solvent and alkali metal
polysulfides are substantially recovered such that they can be returned back
to an electrolytic
process.
[00103] Operating the electrolytic cells at temperatures and pressures, so
that the
electrolytic cell materials of construction can include materials which would
not tolerate high
elevated temperature.
[00104] While specific embodiments of the present invention have been
illustrated and
described, numerous modifications come to mind without significantly departing
from the
spirit of the invention, and the scope of protection is only limited by the
scope of the
accompanying claims.
17