Note: Descriptions are shown in the official language in which they were submitted.
CA 02906941 2015-09-14
WO 2014/146049 PCMJS2014/030925
SPECIFICATION
TO ALL WHOM IT MAY CONCERN:
BE IT KNOWN THAT We, Charles Francis Palmer, Jr. a resident of Greer, South
Carolina and a citizen of USA, and Lester A. Haney, II have invented certain
new and useful
improvements in
AQUEOUS ALKYD RESIN EMULSIONS CONTAINING NON-FUGITIVE,
REACTIVE EMULSIFYING SURFACTANTS
of which the following is a specification.
1
AQUEOUS ALKYD RESIN EMULSIONS CONTAINING NON-FUGITIVE,
REACTIVE EMULSIFYING SURFACTANTS
FIELD OF THE INVENTION
The subject invention pertains to aqueous alkyd resin emulsions containing non-
fugitive,
reactive emulsifying surfactants. The invention also relates to alkyd resin
emulsions containing
non-fugitive, reactive emulsifying surfactants for topcoats and to a process
for their preparation.
BACKGROUND OF THE INVENTION
While no longer the largest volume vehicles in coatings, alkyd coatings are
still of major
importance since they are the most commonly used resin or binder system in oil-
based and
solvent-based coatings. Alkyd coatings are relatively inexpensive and perform
well, often with
fewer film defects than other coatings. They are used in many industrial and
architectural
applications. The hydrophobic nature of the alkyd polymer makes them good
choices when water
repellency is important.
Alkyd resins are polyesters generally prepared from a polyol, phthalic
anhydride, and
unsaturated vegetable fatty acids such as linseed, soy, or tung oil (see
Figure 1 below). The
inclusion of the fatty acid confers a tendency to form a flexible coating.
Alkyds are often
categorized as long, medium, or short oil based on the amount of vegetable oil
in the alkyd; long
2
Date Recue/Date Received 2020-08-27
CA 02906941 2015-09-14
WO 2014/146049 PCT/US2014/030925
oil alkyds have more fatty acid content than short oils.
Preferred fatty acids are those known as drying oils with multiple double
bonds since
they will air cure to give a hard coating. This curing reaction crosslinks the
oligomcric alkyd
chains to build molecular weight and improve durability and other properties.
Alkyds are
sometimes modified with other radical reactive monomers or polymers for a
number of reasons.
These are included to speed curing, to improve water compatibility or
solubility, or to lower
viscosity.
Solvents are employed in traditional alkyd manufacture to reduce the viscosity
of the
oligomeric polyester and often to help remove byproduct water formed in the
synthesis. These
solvents include xylene or ketones. However, these solvents are classified as
VOC, volatile
organic compounds. In recent years, the U.S. EPA has passed stringent
regulations mandating
significant reductions of VOCs in alkyd coatings. Additional restrictions of
VOC in coatings will
be enacted in the U.S. The European Community mandated that solvent borne
alkyd coatings be
limited to 50 g/1 VOC by 2010, thus effectively eliminating solvent borne
alkyd coatings in that
area.
There are several types of alkyds. The main classification is into oxidizing
and
nonoxidizing types. This invention is mainly concerned with the oxidizing
types. Oxidizing
alkyds cross-link by the same mechanisms as drying oils crosslink, that is,
cross-linking through
double bonds and preferably through conjugated double bonds.
A number of new technologies have been recently developed to render solvent
borne
alkyd coatings more environmentally acceptable by replacing some or all of the
solvent with
water. Alkyd resins are converted into useable waterborne products by one of
two methods. One
method is to graft an alkyd resin onto a latex polymer. This gives the coating
properties of both
3
CA 02906941 2015-09-14
WO 2014/146049 PCT/US2014/030925
polymer types. The latex polymer and the surfactant additives that are used to
manufacture the
latex render the latex/alkyd polymer dispersible in water, while the grafted
alkyd renders alkyd-
type properties such as toughness and resistance to various chemicals to the
coating.
Additional processing steps are needed to make these hybrid products. The
alkyd
polymer needs to be of a particular type and structure in order to make a
viable alkyd/latex
coating. In some cases, the durability of these products is superior to those
of latex polymeric
coatings, but the alkyd resin chemistry must be altered to maximize the
benefits of the grafted
alkyd resin onto the latex backbone. These additional processing steps add
cost to the product.
This process also employs additives such as coalescing solvents to improve
properties such as
the gloss and flexibility of the coating.
Emulsification of the alkyd resin into water is the other method to remove
some or all of
the VOCs in the emulsified product. One of the main advantages of the
emulsification process is
that the alkyd resin used in this application does not necessarily need to be
altered to prepare the
emulsion, as long as the proper surfactant and emulsification process is used
to make the
product. The proper surfactant is one with the proper molecular weight,
structure, and HLB
(hydrophile/lipophile balance).
A number of emulsification processes are known. U.S. Patent 6,780,910 Bouvy et
al.
describes methods to prepare alkyd emulsions. In Zukert et al. U.S. Patent
3,979,346, it is
proposed to prepare aqueous dispersions of alkyd resins by the use of a
hydrophilic
polyoxyethylene non-ionic emulsifier containing two or more unsaturated fatty
alcohol or fatty
acid groups, together with an anionic surfactant containing carboxylic acid
groups prepared from
a drying oil and maleic anhydride, which is hydrolyzed in the process. The
properties of such
dispersions are far from optimal, and coatings prepared therefrom may absorb
water and
4
CA 02906941 2015-09-14
WO 2014/146049 PCT/US2014/030925
hydrolyze. McNamee et al. in U.S. published application US 2007/0299228
disclose the use of
branched polyoxyalkylene surfactants modified by reaction with an unsaturated
fatty acid to
contain more than one unsaturated fatty acid group. Preferred arc fatty acid
reaction products of
polyoxyethylated sugars such as sorbitol. Due to the hydrophilic nature of the
surfactant, water
resistance of coatings prepared therefrom may be compromised.
There are a number of technical difficulties and limitations with the
emulsification
process as described in the patent literature. Usually an invert emulsion
process is preferred since
it is less capital intensive because it does not require high shear mixing
equipment and is also
easier to process since the inversion process produces less foam than shearing
into water.
In the invert emulsion process, the neat alkyd with or without solvent is
heated to a high
enough temperature to reduce its viscosity to a manageable level. The
surfactant package of
choice is then added to the molten alkyd, followed by the gradual addition of
hot water. As the
water is added the mixture forms a water-in-oil emulsion, but as the water
content increases and
the emulsion nears the inversion point, "flipping" from a water-in-oil
emulsion to an oil-in-water
emulsion, the viscosity often becomes unmanageably high. The temperature is
maintained as
high as possible to reduce the viscosity of the emulsion, but this can cause
problems since
nonionic surfactants have lower water solubility as the temperature increases.
Once the inversion
point is crossed, the viscosity drops.
The solvent used to make the alkyd is often removed prior to emulsion
preparation since
if it is left in the emulsion it contributes to VOCs in the coating based on
the waterborne alkyd.
Also, if it is left in with the alkyd in emulsion preparation its later
removal is often difficult or
impossible since the emulsion stability is negatively affected. However, the
solvent often must
be left in with the alkyd emulsion to keep the viscosity at a manageable level
throughout the
CA 02906941 2015-09-14
WO 2014/146049 PCT/US2014/030925
inversion process. With solvent present through the emulsion process the alkyd
does not have to
be heated to as high a temperature to keep it fluid or from solidifying. Thus
an emulsification
method that allowed the alkyd solvent to remain through the inversion process
so that lower
viscosity could be achieved at lower temperatures and thus shorten production
times and reduce
cost, but be able to be later removed to produce a low or no VOC emulsion
would be desirable.
These alkyd emulsions contain a few percent of surfactants that remain in the
formulation
and thus are present in the coating after application. There they can cause a
number of problems.
If the surfactant molecules remain unbound and free to migrate, when the dried
coating later is
exposed to water some of the surfactant molecules can dissolve into the water.
This reduces the
surface tension of the water, improves its wetting of the coating surface, and
promotes its
penetration through the coating. When surfactant-coated micelle spheres stack
on a surface
during the drying process and start to coalesce, the concentration of
surfactant molecules at the
intersection of micelles can become relatively high. If these surfactant
molecules are not bound
to the alkyd resin, upon exposure to water extraction of the surfactant takes
place leading to
pitting and degradation of the integrity of the coating. Damage to the
substrate to be protected
becomes much more likely. On metal substrates, this can lead to corrosion and
loss of adhesion.
In addition, free surfactants in the coating can also plasticize the alkyd and
prevent its reaching
maximum hardness upon curing.
Many of the problems that are caused by extractable surfactants can be
mitigated if the
surfactant can co-cure into the alkyd coating. This locks it into place so
that it cannot be
extracted by water. Therefore reactive surfactants that can co-cure with the
alkyd in the air
autoxidation process to become locked into the coating and avoid the above
mentioned problems
caused by free surfactants would be desirable.
6
CA 02906941 2015-09-14
WO 2014/146049 PCT/US2014/030925
A number of patent applications claim reactive surfactants that can do this.
They usually
are based on fatty acid alkoxylates in which the fatty acid has a number of
double bonds similar
to the drying oil fatty acids. However, most of the known ones have some
significant drawbacks.
They are nonionic surfactants and so have lower solubility in hot water, which
limits their ability
to make inverse emulsions with a manageable viscosity. Nonionics also cannot
take advantage of
charge stabilization mechanisms to reduce particle size and improve emulsion
stability. Most
known nonionics for alkyd emulsions have long ethoxylate chains to give them a
high HLB
which is required to make a stable emulsion. These nonionics have a strong
tendency to slow
drying, likely due to complexation of metal ion drying agents. Long chain
nonionics also have a
tendency to plasticize alkyd coatings, reducing their hardness.
The previous alkyd reactive surfactants contain hydrophobes designed to have
good
compatibility with the fatty acid chains of the alkyd resin; the surfactants
of the instant invention
were designed to have good compatibility with the aromatic groups of the
polyester portion of
most alkyd resins.
BRIEF DESCRIPTION OF THE FIGURES
Figure 1 illustrates outlines of water drop on alkyd coating drawdown on a
steel panel.
SUMMARY OF THE INVENTION
It has now been surprisingly discovered that improved waterborne alkyd
coatings can be
produced from aqueous emulsions or dispersions of alkyd resins, where the
surfactant is a
nonionic or anionic surfactant reactive with the alkyd resin in conventional
coating processes.
The surfactants are polyoxyalkylene polyethers according to structures I or II
below prepared by
7
CA 02906941 2015-09-14
WO 2014/146049 PCT/US2014/030925
polyoxyalkylating a polystyrenated phenol that has previously been reacted
with one or more
ally! glycidyl ether groups, and that after polyoxyalkylation may be
optionally be converted to an
anionic surfactant such as a phosphate ester or a sulfate. The ally! groups
pendant to the
polyether chain on the surfactant provide reactive sites for participation in
the alkyd
autooxidative drying process.
Formula (I) is an anionic surfactant of structure:
(i) 0 \
0
(I)
where R ¨ CH3, CH2C143, C6H5, or C141429; n ¨ 1,2,3 ; x is 1-10, y is 0-200, z
is 4-200, more
preferably from about 5 to 60, and most preferably from about 5 to 40; Z can
be either SO3 or
P032-, and M is Nat, K, NH 4 , or an alkanolamine.
The present invention is further directed towards the emulsion alkyd polymers
in the
presence of a nonionic surfactant of formula (II)
8
CA 02906941 2015-09-14
WO 2014/146049 PCT/US2014/030925
0
(II)
where R = CH3. CH2CH3, C6H5, or C14H29; n = 1,2,3; x is 1-10, y is 0-200, z is
4-200, more
preferably from about 5 to 60, and most preferably from about 5 to 40.
The present invention is further directed toward a process for emulsifying
solvent borne
alkyd polymers comprising the addition of one or more of surfactants of
formula I or II to a
solventbome alkyd, adding water and emulsifying through an inversion process,
followed by
solvent removal from the aqueous emulsion.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT(S)
The present invention thus involves synthesizing a special surfactant or
emulsifier
preferably using styrenated phenol as the hydrophobe. Styrenated phenol is
commercially
obtained as a mixture of mono-, di-, and tristyrenated phenols in various
ratios. Styrenated
phenol is a polyaromatic hydrophobe, so these surfactants have good affinity
for the aromatic
groups in the alkyd. They are also inherently lower foaming than most linear
surfactants.
The mixture of styrenated phenols are converted into reactive surfactants by
first reaction
with one or more equivalents of allyl glycidyl ether. This attaches pendant
allyl groups for
subsequent curing through fatty acid double bonds on the alkyd. These
substituted styrenated
9
CA 02906941 2015-09-14
WO 2014/146049 PCT/US2014/030925
phenols are oxyalkylated with ethylene oxide (EO), optionally together with
propylene oxide
(PO) or butylene oxide (BO). Other alkylene oxides may also be used, for
example long chain
ex-olefin oxides, but EO, and PO or BO are preferred, EO and PO being most
preferred. The
surfactants are designed by selecting the EO/PO or BO architecture to produce
a product that
emulsifies, disperses and stabilizes the alkyd resin or hybrid latex/alkyd
resin, without conferring
hydrophilic properties and to minimize its affinity for chelating metal ions
used in the alkyd
drying process.
A method for making ionic surfactants is to make them anionic by the addition
of an
anionic group such as a sulfate or a phosphate group onto the terminus of a
polyoxyalkylated
fatty acid or alcohol. The anionic character gives it the ability to charge
stabilize alkyd
emulsions.
In general the surfactants may be envisioned as having at least two portions;
a first
portion which is hydrophobic and which will promote formation of a clear
coating during
coalescence of alkyd resin from aqueous dispersion, and a second portion which
is hydrophilic.
At least one of these two portions, generally the hydrophobic portion, must
contain unsaturation,
which is reactive with alkyd resins during cure. It is well known and accepted
that nonionic
surfactants are excellent products to emulsify and disperse a wide range of
hydrophobic
compounds including alkyd resins. Nonionic surfactants outperform anionic
surfactants in
making stabilizing emulsions as demonstrated by improved water sensitivity,
better colloidal
stability, and lower foam profile when compared to an emulsion made with an
anionic surfactant.
In some cases, those skilled in the art of making emulsions, a small amount of
anionic surfactant
is used in conjunction with the nonionic surfactant.
CA 02906941 2015-09-14
WO 2014/146049 PCT/US2014/030925
Alkyd resins are synthesized with drying oils as a major part of the
formulation. Drying
oils are liquid vegetable or fish oils that react with oxygen to form solid
films. Drying oils are
raw materials for binders such as alkyd resins and epoxy esters. When these
films are exposed to
air, such as when the coating is curing, an autoxidative cross-linking
reaction takes place. When
a film is applied to a substrate, internal, naturally present hydroperoxides
decompose to form free
radicals. Hydrogen molecules on methylene groups between double bonds are
particularly
susceptible to abstraction, yielding a resonance-stabilized free radical that
reacts with oxygen to
give predominantly conjugated peroxy free radicals. The peroxy free radicals
can abstract
hydrogen molecules from other methylene groups between double bonds to form
additional
hydroperoxides and generate free radicals. Thus, a cross-linking chain
reaction is established,
resulting from autoxidation and the coating is cured.
EXAMPLES
The following examples are intended to demonstrate the usefulness of the
compositions
of the present invention and should not be construed to limit the scope of the
invention in
anyway.
EXAMPLE 1
Distyrenated phenol (DSP) (694 g, 1 equivalent) was added to a stainless steel
autoclave
along with allyl glycidyl ether (AGE) (494 g, 2 equivalents) and potassium
hydroxide KOH (2.3
g) and the autoclave sealed and heated to 105 C. When all of the AGE was
consumed, the
reaction mass was cooled, and the product discharged. This is AGE 2 DSP
adduct.
11
1680 g of this AGE 2 DSP adduct (1 equivalent) was added to another autoclave
and heated to
105 C. Ethylene oxide (2026 g, 15 equivalents) was then added over the course
of several hours.
After all of the EO was consumed, the reaction mass was cooled and the
catalyst neutralized with
the addition of a small amount of acid. This material is Example 1 (aka ERS
01617Tm).
EXAMPLE 2
Example 1 ethoxylate was sulfated with sulfamic acid in a glass reactor
equipped with
a stirrer, thermometer, and reflux condenser by heating to 120 C until the %
sulfate was > 90%.
The product, Example 2 (aka ERS 01618Tm), was isolated as the ammonium salt.
EXAMPLE 3
Example 1 ethoxylate was phosphated with phosphoric anhydride (P205) following
standard methods to produce Example 3 in its acid form as a viscous liquid.
EXAMPLE 4
Emulsion preparation procedure with solvent stripping
CP-14 Emulsion
Material % By Weight Amount Used
short oil alkyd, 75% solids in methyl propyl
ketone solvent (available as Beckosol 6422- 49.4% 741g
K3-75 TM from Reichhold)
Example 2 4.0% 60g
Ammonium hydroxide 0.74% 11.1g
Deionized water 45.86% 687.9g
41% solids
Charge short oil alkyd, Example 2, and ammonium hydroxide to a reactor flask
equipped
12
Date Recue/Date Received 2020-08-27
CA 02906941 2015-09-14
WO 2014/146049 PCT/US2014/030925
with nitrogen sparge, thermocouple, overhead mixing, and a condenser and start
stirring. Once
the mixture is homogenous, begin adding ambient temperature water to the
mixture in a slow
stream so that the water is incorporated evenly into the mixture. As the water
is added the
viscosity will rise until the inversion point is met, then the viscosity will
begin to drop. When all
of the water is charged, allow the emulsion to mix for several minutes and
then check the particle
size. This emulsion was 41% solids. The average particle size of this emulsion
was 0.227
microns with a D50 of 0.209 microns.
The temperature of the emulsion was then raised by heating to 70 C with a
nitrogen
sparge. The temperature was then gradually increased until vapors were
detected in the
condenser. The emulsion was then stirred until all of the solvent was removed.
After cooling, the
particle size, percentage solids, and viscosity were checked. This product
emulsion was 48%
solids and contained 0.4% residual solvent. The viscosity was not measured,
but was quite low.
The particle size of the solvent-stripped emulsion was 0.214 microns with a
D50 of 0.198
microns.
As the particle size data shows, the particle size of the alkyd emulsion
remained very
low, actually decreasing from the solvent-containing emulsion. The solids
content was
significantly higher. This result shows that the use of the anionic
surfactants of this invention can
overcome the viscosity increase problem inherent with the invert emulsion
procedure by leaving
the solvent in the mixture and then removing it after emulsion formation. The
anionic sulfate has
sufficient water solubility to perform as an effective emulsifier even at the
elevated temperatures
needed for practical solvent stripping. This procedure will allow coating
manufacturers to
convert existing low cost solventborne alkyds to waterborne products
containing little or no
13
VOC. This process also produces a product alkyd emulsion with higher solids
(active ingredient)
than the solvent-containing waterborne emulsion.
EXAMPLE 5 AND COMPARATIVE EXAMPLE
Comparison of coating properties between reactive and non-reactive surfactant
Two waterborne emulsions of a solventbome long oil alkyd (Beckosol 10539TM LOA
from Reichhold) were prepared using the procedure of Example 4 with the
amounts of each
ingredient shown in Table 1 below. The only difference was the emulsifier.
Both emulsifiers
(Example 2 and POE 20 DSP) are styrenated phenol based, have nearly the same
ethoxylate
chain length, and both are sulfates. The only significant difference between
them is that Example
2 is reactive, while POE 20 DSP contains no reactive allyl groups.
After the emulsions were prepared, a cobalt-based drying catalyst package was
added to
the emulsion and four mil drawdowns were made on steel Q panels. After two
weeks the
adhesion, pencil hardness, gloss, and water contact angle were checked.
Results are in Table 2.
Table 1.
Example 5 Amount Comparative Example 5 Amount
Long oil alkyd (Beckosol 10- 38.2g Long oil alkyd (Beckosol 10- 38.2g
539TM) 539TM)
Example 2 1.8g POE 20 styrenated phenol 1.8g
Ammonium Hydroxide 0.6g Ammonium Hydroxide 0.6g
Water 58.8g Water 58.8g
Drier package 0.6g Drier package 0.6g
14
Date Recue/Date Received 2020-08-27
CA 02906941 2015-09-14
WO 2014/146049 PCT/US2014/030925
Table 2.
Property Example 5 Comparative Example 5
Cross-cut adhesion OB (100% fail) OB (100% fail)
Pencil Hardness F 2B
Gloss (60 ) 117 113
Water contact angle 84.5 790
Review of the data in Table 2 shows that Example 5, the alkyd coating with the
reactive
surfactant, has a higher water contact angle than Comparative Example 5, the
alkyd coating with
the non-reactive surfactant. This indicates that the Example 5 coating is more
water repellent,
evidence that the reactive surfactant is cured into the alkyd and not
available to dissolve into the
water and reduce its surface tension.
The Example 5 coating with the reactive surfactant is also significantly
harder than the
one with non-reactive surfactant. Presumably, this is due to the
plasticization of the coating by
the unbound surfactant.
The gloss of the coating is also higher with the reactive surfactant. This
suggests that the
surface has fewer defects.
Water drops were placed on each of these drawdown panels and allowed to stand
covered
so that they would not evaporate. The water drop on the Comparative Example 5
coating with
the non-reactive surfactant had significantly wet the coating and had spread
out to cover a much
larger area than the drop on the reactive surfactant coating. After two days,
rust on the panel was
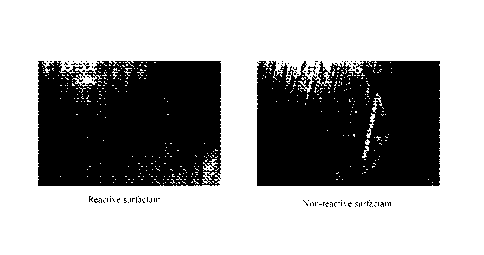
clearly visible on the panel with non-reactive surfactant (Figure 1). The
coating with the reactive
surfactant resisted the ingress of water and protected the steel much better
than did the non-
reactive.
EXAMPLE 6
In another test of adhesion, 4 mil drawdowns were made of Example 4 aqueous
short oil
alkyd emulsion prepared with Example 2 difunctional reactive surfactant and of
Synaqua
4804TM, a commercially available waterborne short oil alkyd (available from
Arkema) assumed
not to contain a reactive surfactant. Both alkyd emulsions were catalyzed
prior to drawdown with
the same drier package. After 24 hours of drying, the adhesion of the coatings
was measured by
ASTM Method D 3359-08 "Measuring Adhesion by Tape Test." A crosscut pattern
was scribed
onto the alkyd surfaces. The attempt to scribe the crosscut pattern on the
commercial alkyd
coating failed, while it was successful on the alkyd emulsified with Example 2
reactive
surfactant.
EXAMPLES 4,7 AND COMPARATIVE EXAMPLE
In another test of adhesion to metal, two short oil alkyd emulsions were
prepared
following the method of Example 4. One was a repeat of Example 4, while
Example 7 used a
mixture of Example 2 and Example 3 as emulsifiers. More water was required to
prepare the
emulsion of Example 7 to produce a suitable viscosity. After Example 4 and
Example 7
emulsions were made they were heated to 80-90 C and the solvent was removed
until the solvent
content was less than 0.5%. A commercially available metal catalyst drier
package was added
to each emulsion prior to drawdown. The recipes for each example and their
particles sizes after
solvent removal are given in Table 3.
16
Date Recue/Date Received 2020-08-27
Table 3.
Example 4 Example 7 Comparative commercial
waterborne short oil alkyd
Short oil alkyd 49.4% 39.5% Synaqua 4804TM (Arkema)
(Beckosol 6422-K3-
75TM)
Example 2 4.0% 3.16% 0
Example 3 0 0.8% 0
Ammonium Hydroxide 0.74% 1.2% 0
DI H20 45.86% 55.34% 0
Dura Chemicals DriCAT 0.5% on 0.5% on Batch 0.5% on Batch Weight
SO7TM Batch Weight Weight
pH 7.5 7.5 7.5
Particle Size
D10 0.154 0.317 0.180
D50 0.198 2.418 0.226
D90 0.280 4.580 0.296
Two aluminum and two steel panels were prepared prior to making drawdowns of
each
emulsion. One of each panel was wiped clean with acetone and one of each was
scuffed with an
abrasive plastic pad. A 4 mil drawdown was cast on each panel and allowed to
air dry at room
temperature. The 60 gloss on all panels for each emulsion was >100.
After one week the adhesion of the coating to the metal panel was tested
following the
ASTM Method D 3359-08 "Measuring Adhesion by Tape Test." All of the drawdowns
of
Examples 4 and 7 passed the adhesion test as did the comparative example on
the scuffed steel
panel. The comparative waterborne alkyd failed the adhesion test when applied
to the acetone
cleaned aluminum panel.
17
Date Recue/Date Received 2020-08-27
EXAMPLE 8
A short oil alkyd emulsion was prepared following the procedure and materials
of
Example 4 but without the solvent removal step. Another emulsion of the same
alkyd was
prepared in identical fashion except that another reactive surfactant, a
commercially available
alkoxylate of linseed fatty acid, was used instead of the Example 2 styrenated
phenol surfactant.
Equal amounts of the same metal drier catalyst package (DriCat 507TM) were
added to each
emulsion.
The drying time of these two catalyzed emulsions were measured by drawing down
films
of each on a Leneta chart and allowing them to air dry at room temperature.
The alkyd emulsion
with the Example 2 reactive surfactant cured tack- free after 5.5 hours, while
the linseed
alkoxylate coating was not tack-free even after eight hours.
While the many embodiments of the invention have been disclosed above and
include
presently preferred embodiments, many other embodiments and variations are
possible within
the scope of the present disclosure and in the appended claims that follow.
Accordingly, the
details of the preferred embodiments and examples provided are not to be
construed as limiting.
The scope of the claims should not be limited by the preferred embodiments set
forth in the
examples, but should be given the broadest interpretation consistent with the
description as a
whole.
18
Date Recue/Date Received 2020-08-27